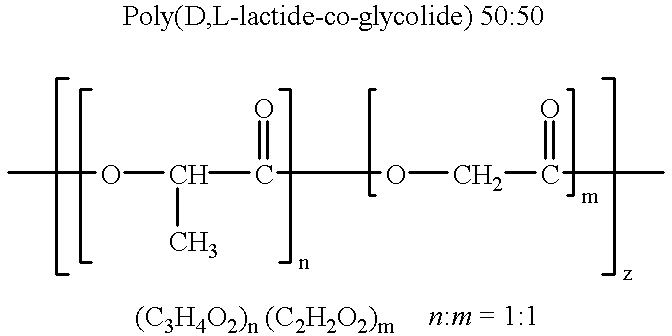Patents
Literature
42860 results about "Active agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
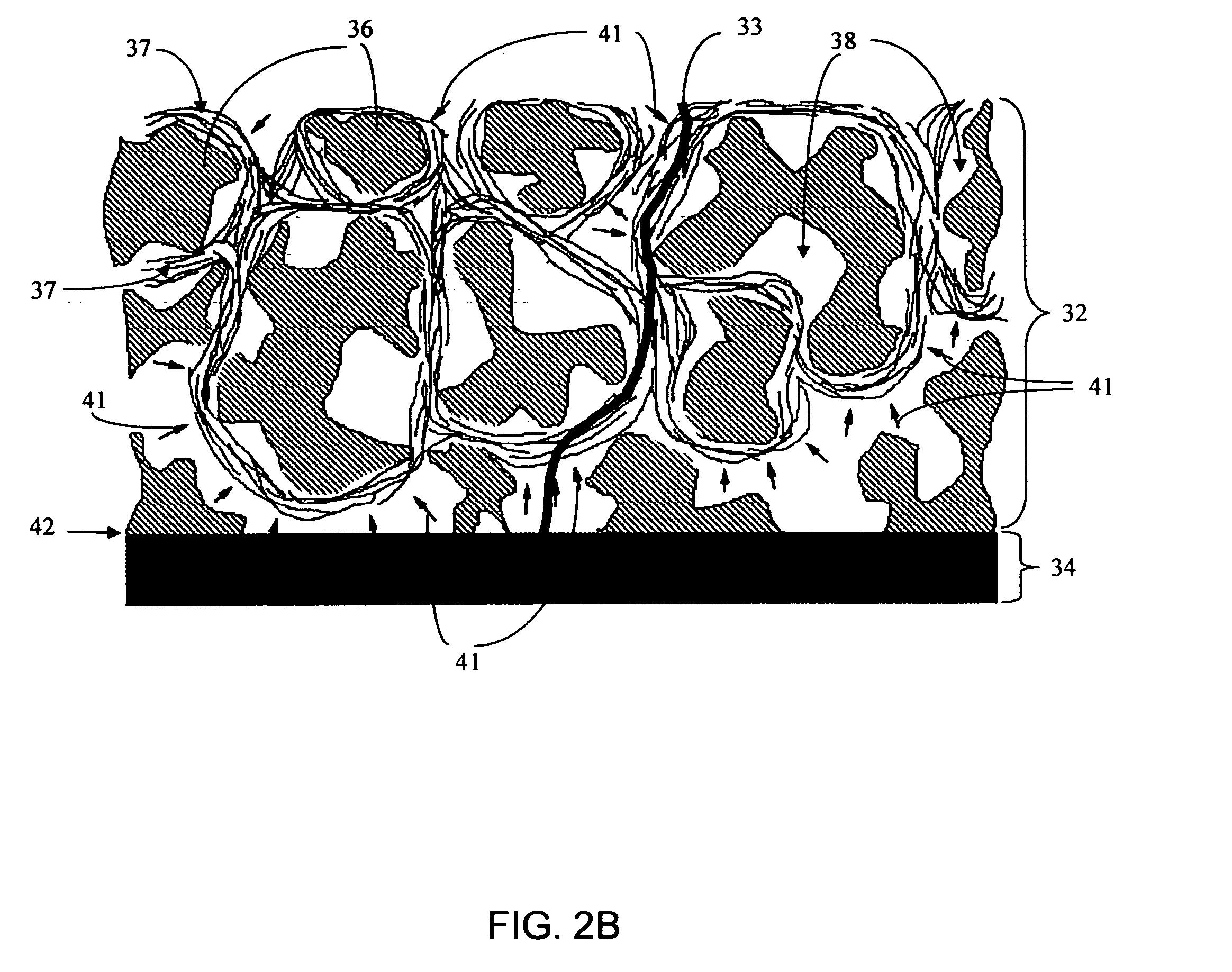
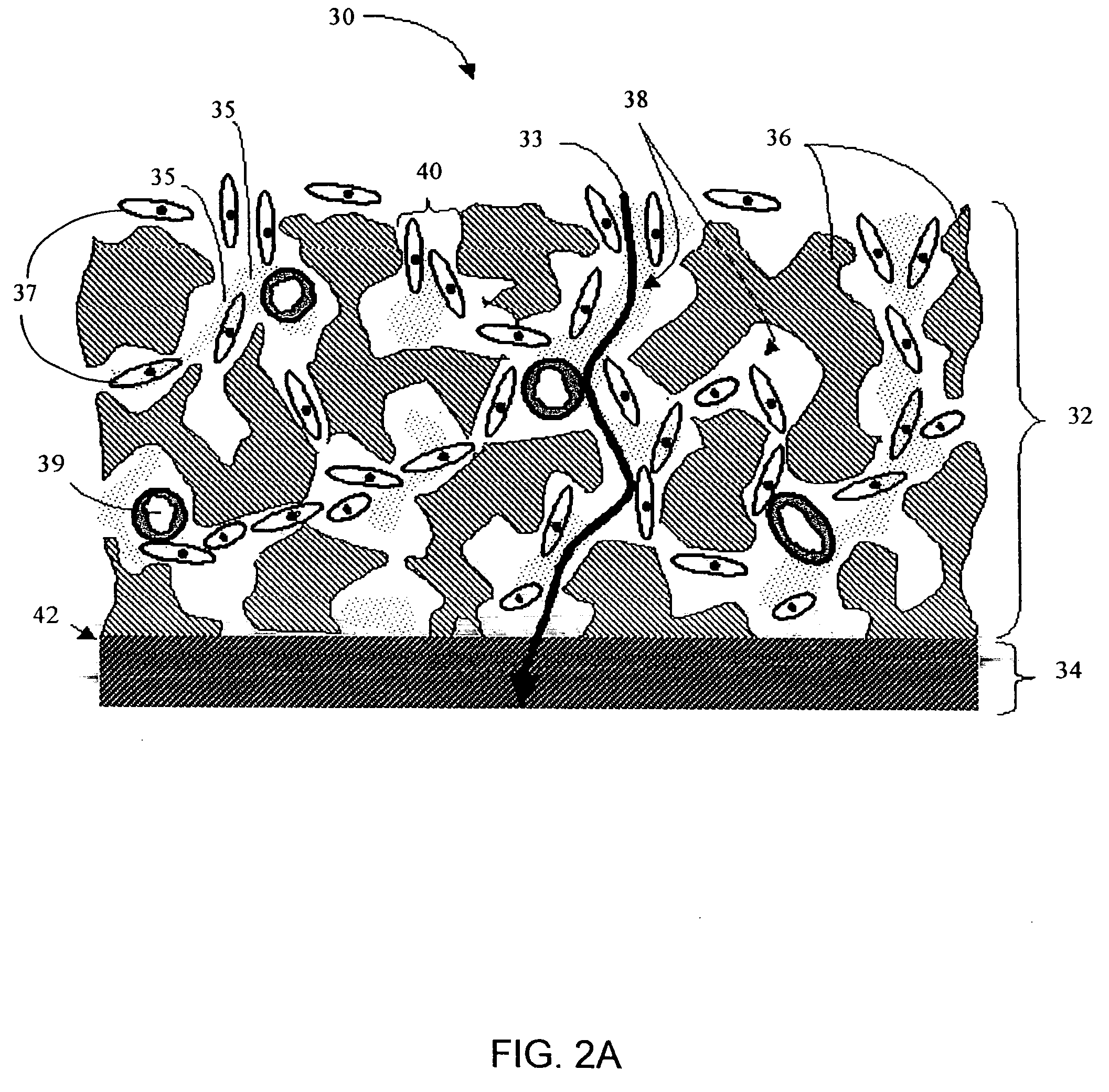
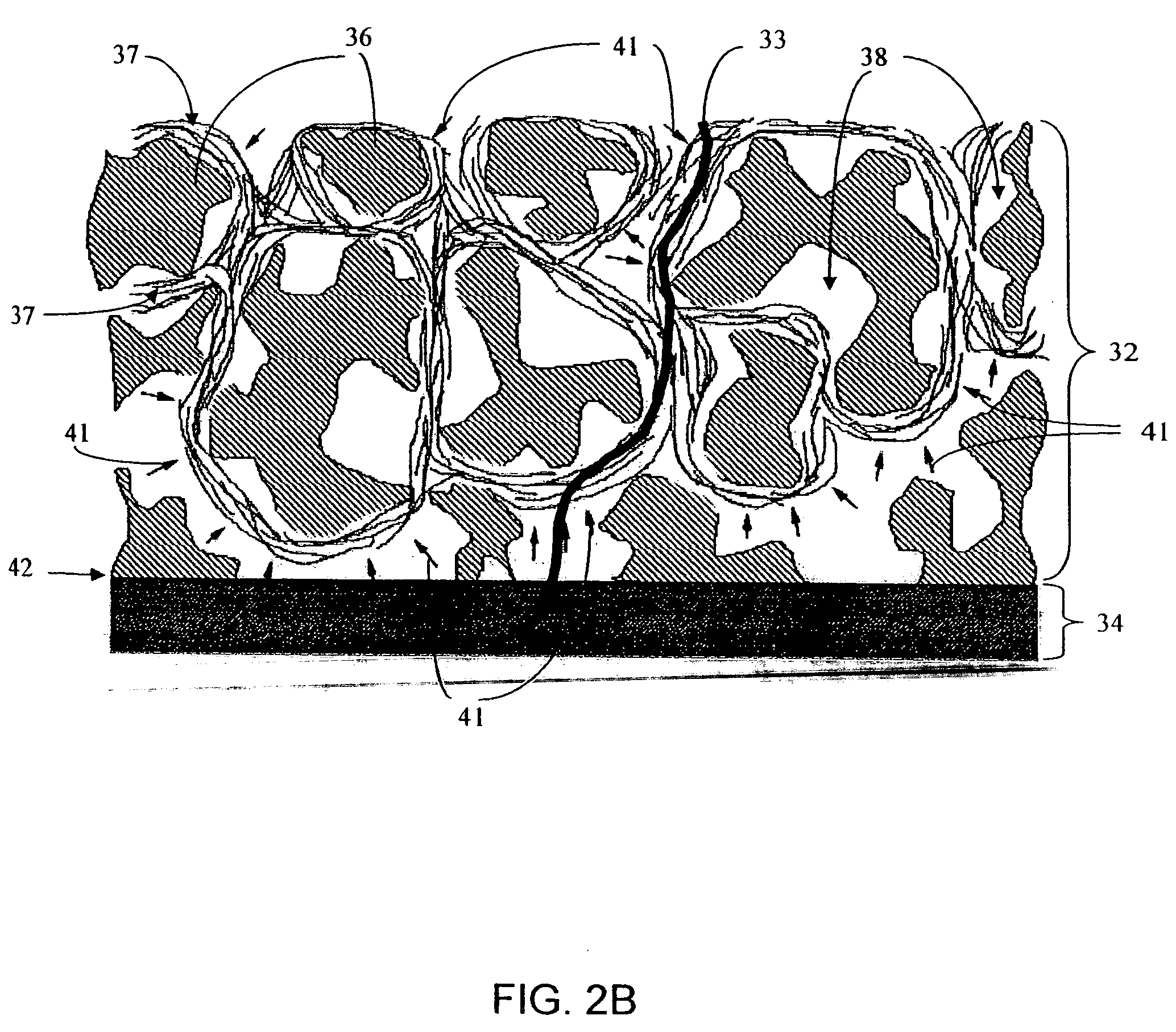
Biointerface membranes incorporating bioactive agents
InactiveUS20050031689A1Improve performancePowder deliveryAdditive manufacturing apparatusBiointerfaceActive agent
A biointerface membrane for an implantable device including a nonresorbable solid portion with a plurality of interconnected cavities therein adapted to support tissue ingrowth in vivo, and a bioactive agent incorporated into the biointerface membrane and adapted to modify the tissue response is provided. The bioactive agents can be chosen to induce vascularization and / or prevent barrier cell layer formation in vivo, and are advantageous when used with implantable devices wherein solutes are transported across the device-tissue interface.
Owner:DEXCOM
Neural regeneration peptides and methods for their use in treatment of brain damage
InactiveUS7563862B2High expressionEasy SurvivalPeptide/protein ingredientsGenetic material ingredientsNervous systemInjury brain
The invention discloses a family of peptides termed NRP compounds or NRPs that can promote neuronal migration, neurite outgrowth, neuronal proliferation, neural differentiation and / or neuronal survival, and provides compositions and methods for the use of NRPs in the treatment of brain injury and neurodegenerative disease. NRP compounds can induce neurons and neuroblasts to proliferate and migrate into areas of damage caused by acute brain injury or chronic neurodegenerative disease, such as exposure to toxins, stroke, trauma, nervous system infections, demyelinating diseases, dementias, and metabolic disorders. NRP compounds may be administered directly to a subject or to a subject's cells by a variety of means including orally, intraperitoneally, intravascularly, and directly into the nervous system of a patient. NRP compounds can be formulated into pharmaceutically acceptable dose forms for therapeutic use. Methods for detecting neural regeneration, neural proliferation, neural differentiation, neurite outgrowth and neural survival can be used to develop other neurally active agents.
Owner:CURONZ HLDG
Cyclodextrin polymers for carrying and releasing drugs
This invention discloses methods for preparing compositions of cyclodextrin polymers for carrying drugs and other active agents. Methods are also disclosed for preparing cyclodextrin polymer carriers that release drugs under controlled conditions. The invention also discloses methods for preparing compositions of cyclodextrin polymer carriers that are coupled to biorecognition molecules for targeting the delivery of drugs to their site of action. The advantages of the water soluble (or colloidal) cyclodextrin polymer carrier are: (1) Drugs can be used that are designed for efficacy without conjugation requirements. (2) It will allow the use of drugs designed solely for efficacy without regard for solubility. (3) Unmodified drugs can be delivered as macromolecules and released within the cell. (4) Drugs can be targeted by coupling the carrier to biorecognition molecules. (5) Synthesis methods are independent of the drug to facilitate multiple drug therapies.
Owner:KOSAK KENNETH M
Analyte measuring device
InactiveUS20050033132A1Additive manufacturing apparatusMicrobiological testing/measurementAnalyteCell layer
An implantable analyte-measuring device including a membrane adapted to promote vascularization and / or interfere with barrier cell layer formation. The membrane includes any combination of materials, architecture, and bioactive agents that facilitate analyte transport to provide long-term in vivo performance of the implantable analyte-measuring device.
Owner:DEXCOM
Laundry detergent compositions with efficient hueing dye
ActiveUS7208459B2Counter undesirable yellowingSimple compositionOrganic detergent compounding agentsNon-surface-active detergent compositionsActive agentLaundry
Laundry detergent compositions comprise (a) surfactant, and (b) a hueing dye, wherein the hueing dye exhibits a hueing efficiency of at least 10 and a wash removal value in the range of from about 30% to about 85%.
Owner:PROCTER & GAMBLE CO
Apparatus and method for utilizing a meniscus in substrate processing
InactiveUS20050217703A1Process environment can be powerfully controlled and managedMore processedLiquid surface applicatorsSemiconductor/solid-state device manufacturingActive agentEngineering
An apparatus for processing a substrate is provided which includes a proximity head proximate to a surface of the substrate when in operation. The apparatus also includes an opening on a surface of the proximity head to a cavity defined in the proximity head where the cavity delivers an active agent to the surface of the substrate through the opening. The apparatus further includes a plurality of conduits on the surface of the proximity head that generates a fluid meniscus on the surface of the substrate surrounding the opening.
Owner:LAM RES CORP
Compressed high density fibrous polymers suitable for implant
An embodiment of the present invention may be made by the following steps: providing a mixture comprising a plurality of fibers, a lubricant, and a suspension fluid, with the suspension fluid filling a void space between said fibers and subjecting said mixture to at least one compressive force. The compressive force causes the migration and alignment of said fibers; and may remove substantially all of the suspension fluid from said mixture. The mixture may further comprise a biologically active agent, or a reinforcing agent.
Owner:DSM IP ASSETS BV
Methods and compositions useful for administration of chemotherapeutic agents
InactiveUS6096331AReduce morbidityLow toxicityPowder deliveryEchographic/ultrasound-imaging preparationsActive agentIn vivo
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of a pharmaceutically active agent, wherein the agent is associated with a polymeric biocompatible material.
Owner:ABRAXIS BIOSCI LLC
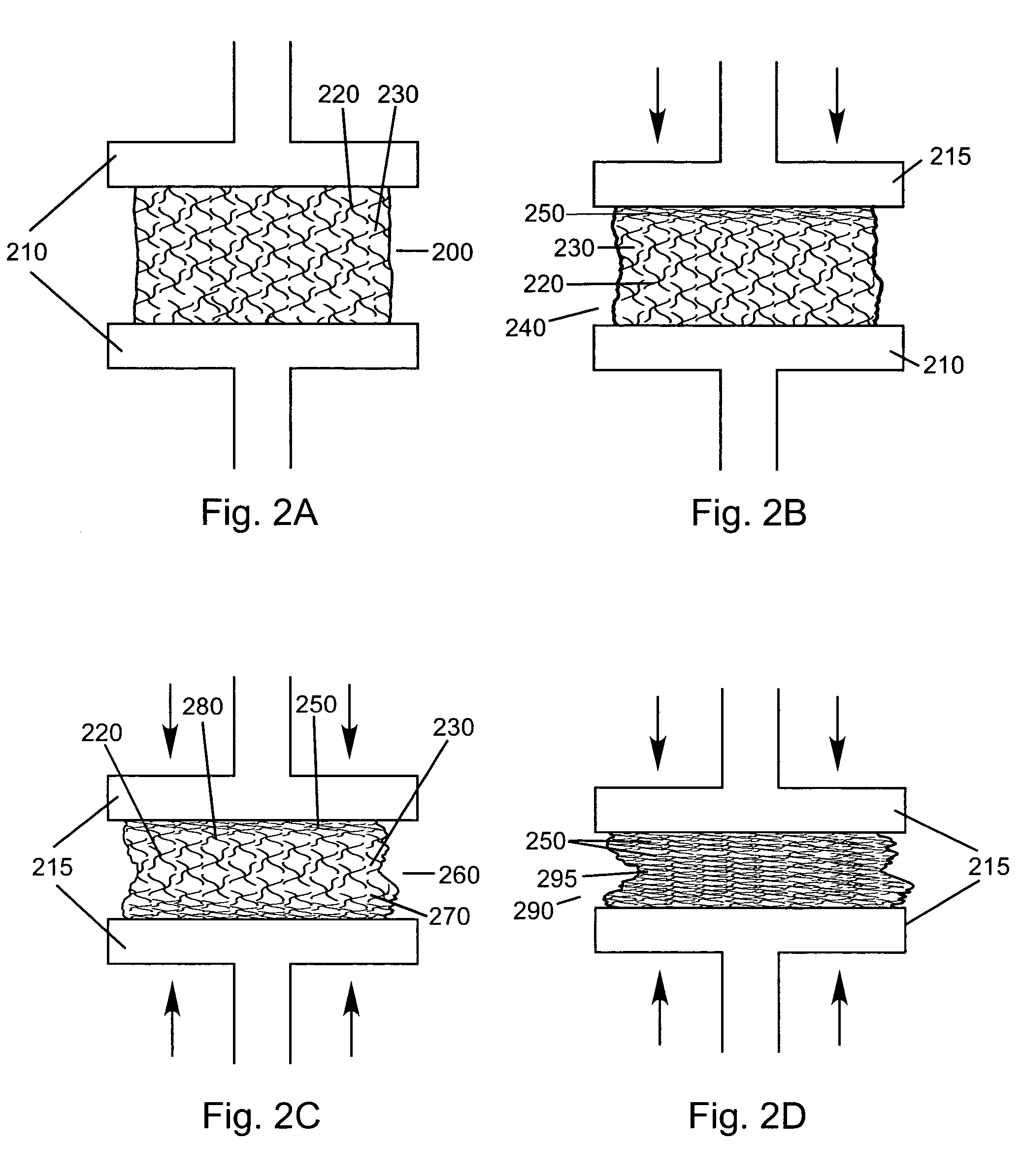
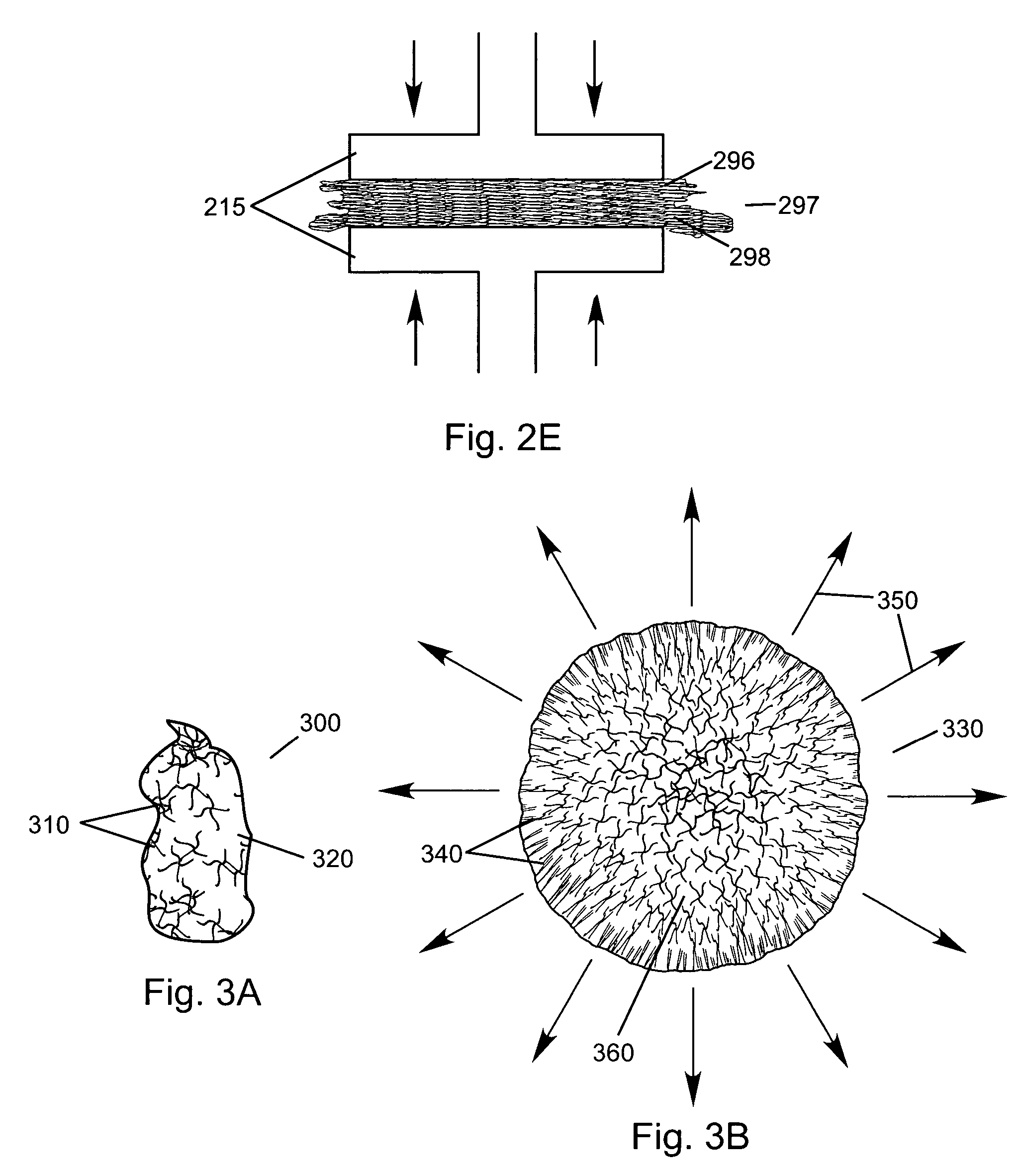
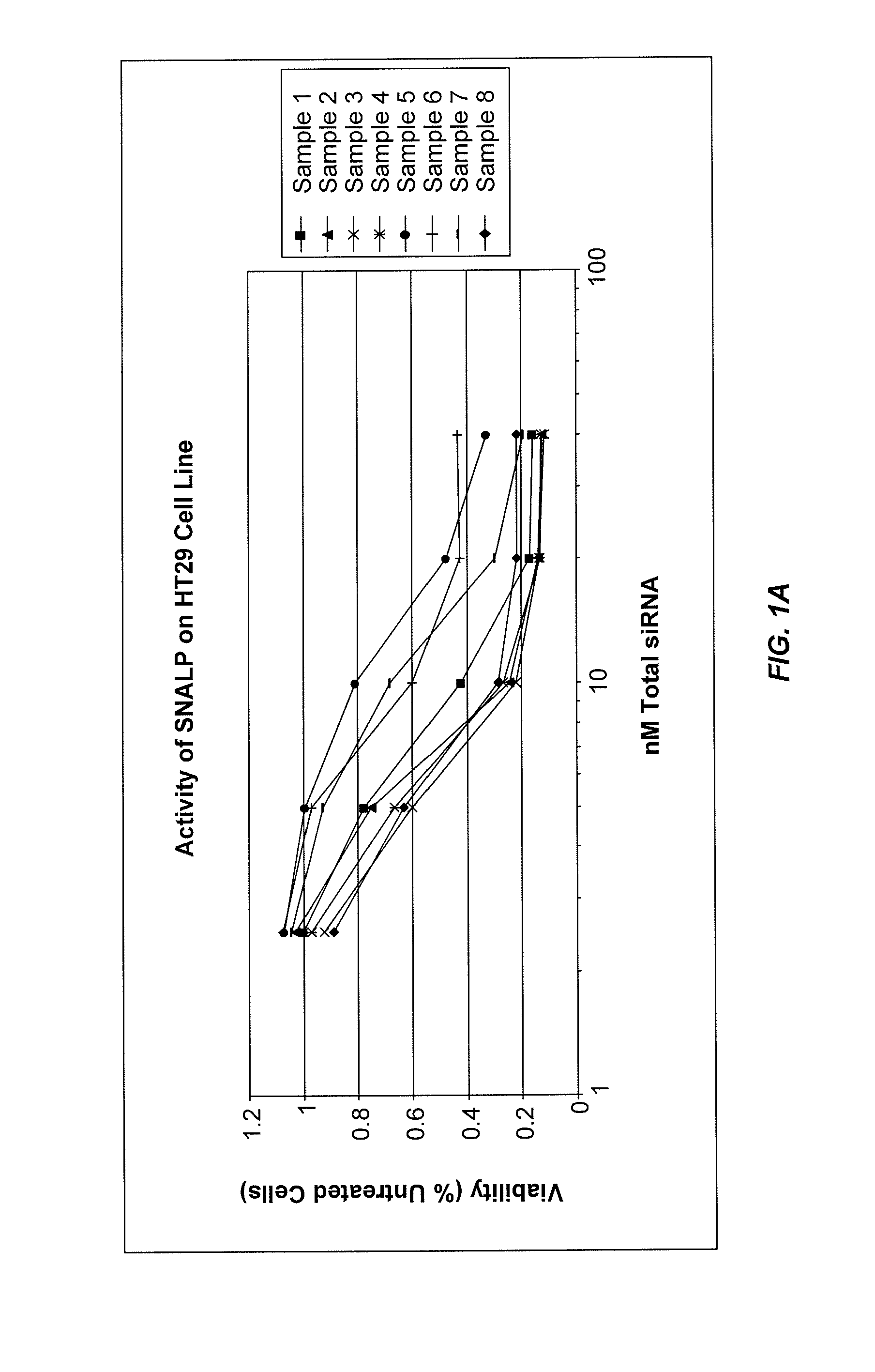
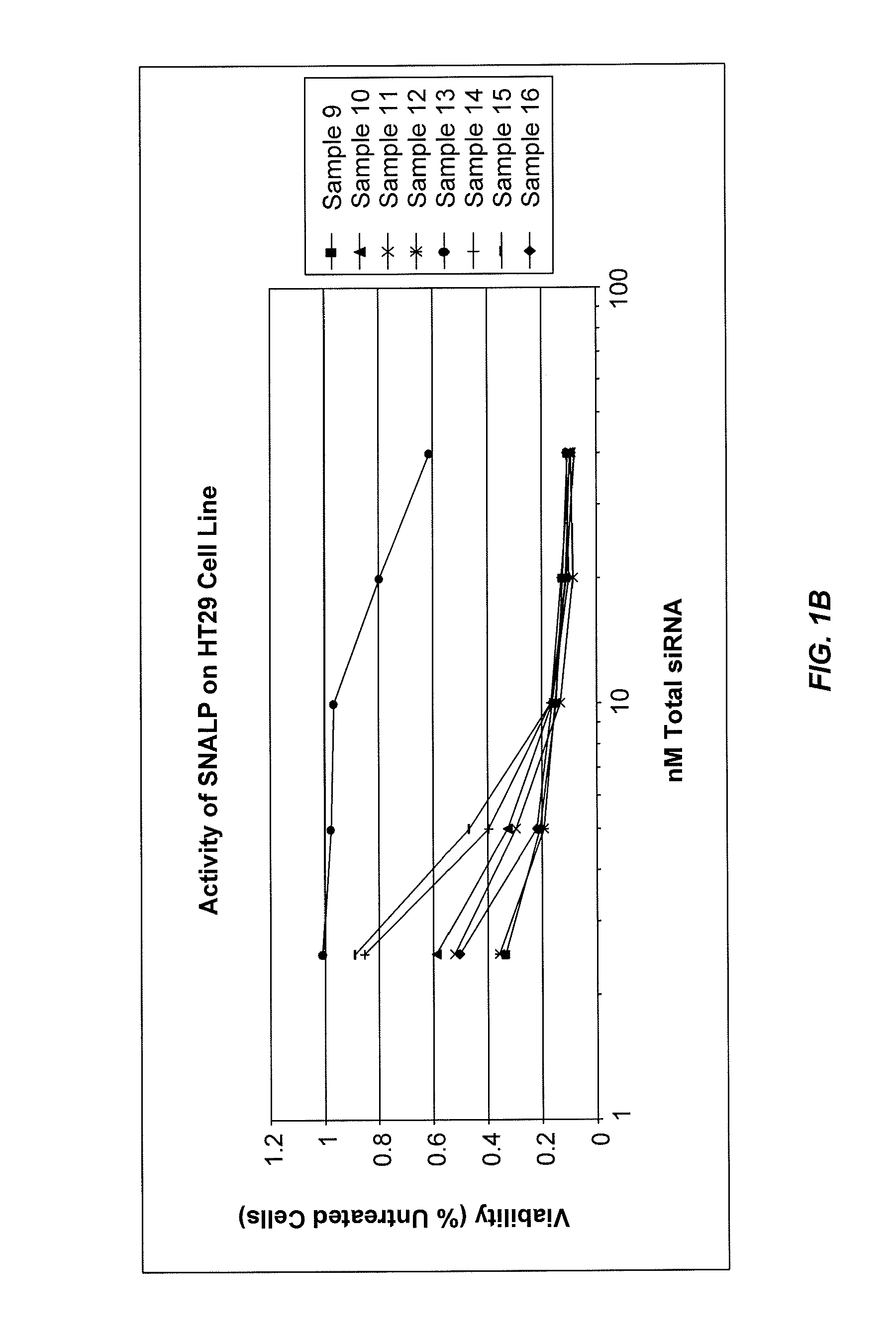
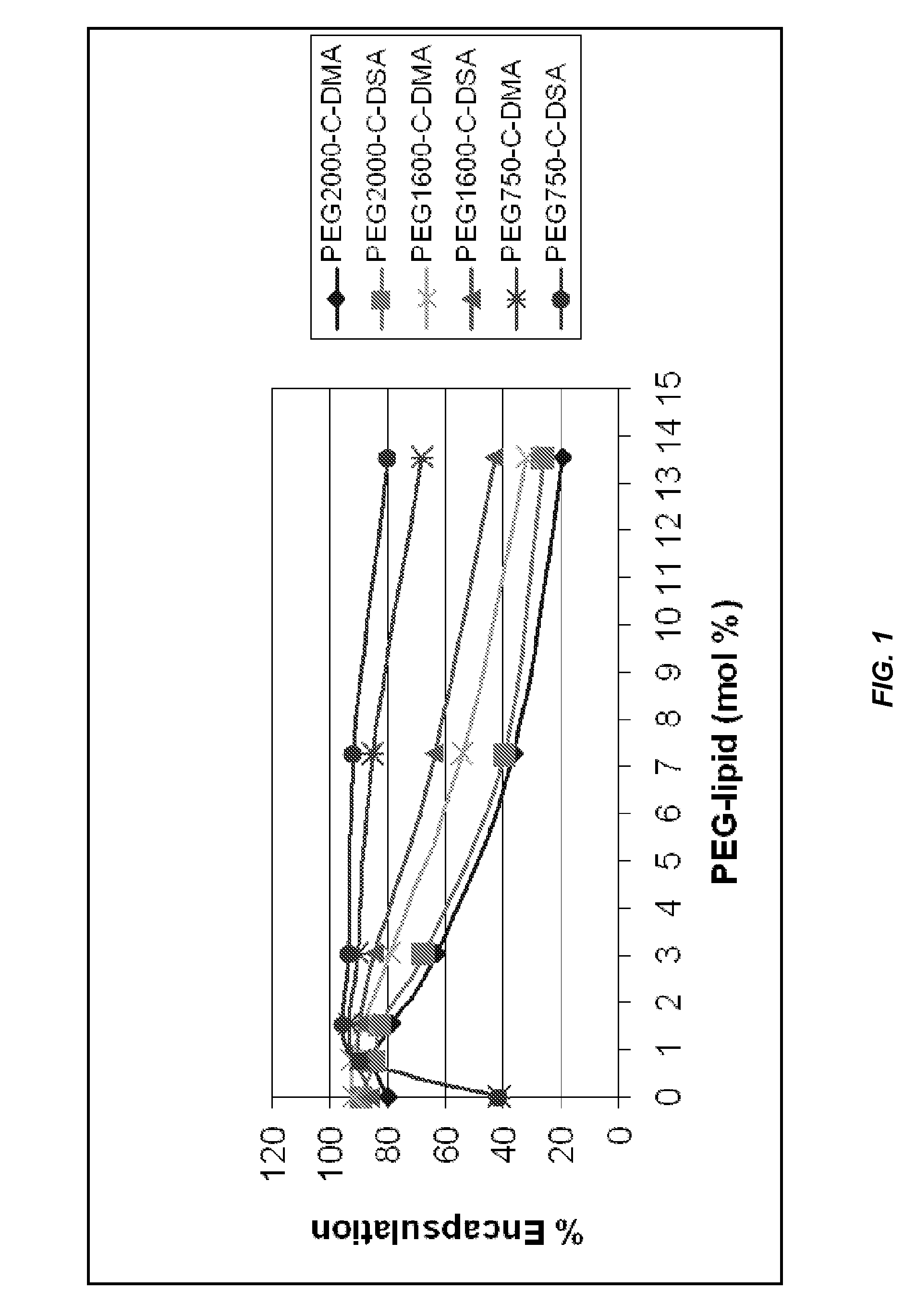
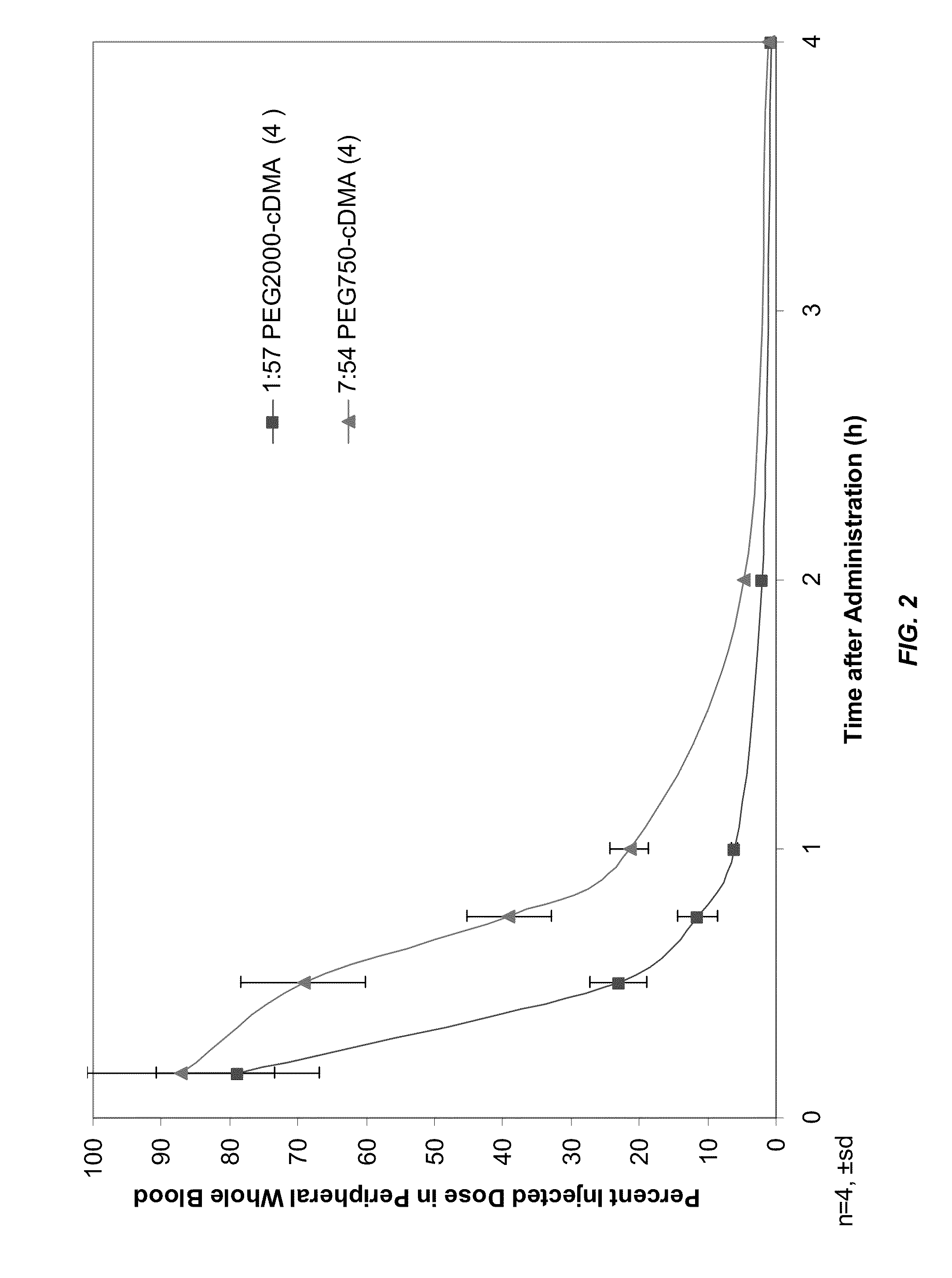
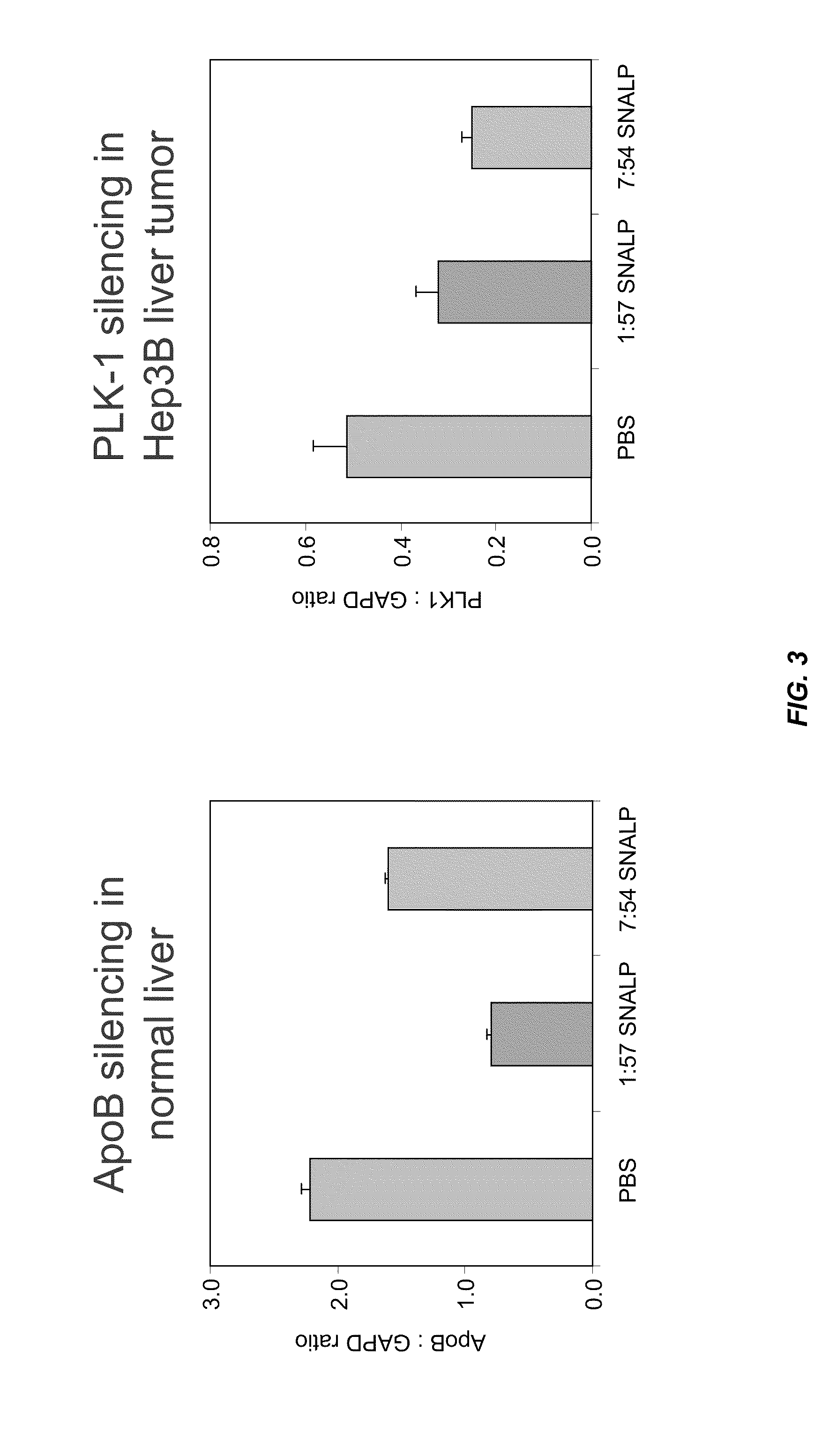
Lipid formulations for nucleic acid delivery
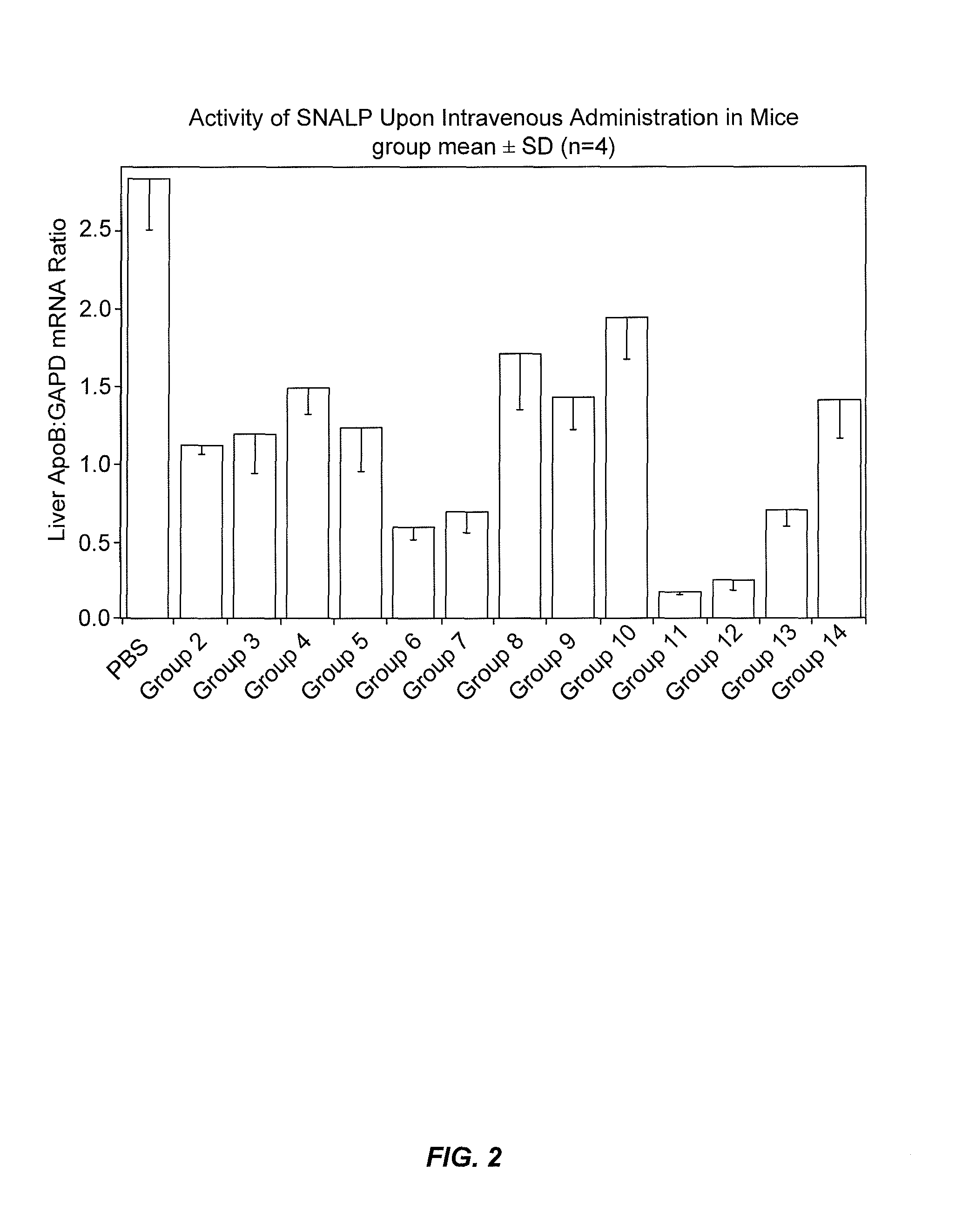
The present invention provides novel, stable lipid particles comprising one or more active agents or therapeutic agents, methods of making the lipid particles, and methods of delivering and / or administering the lipid particles. More particularly, the present invention provides stable nucleic acid-lipid particles (SNALP) comprising a nucleic acid (such as one or more interfering RNA), methods of making the SNALP, and methods of delivering and / or administering the SNALP.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
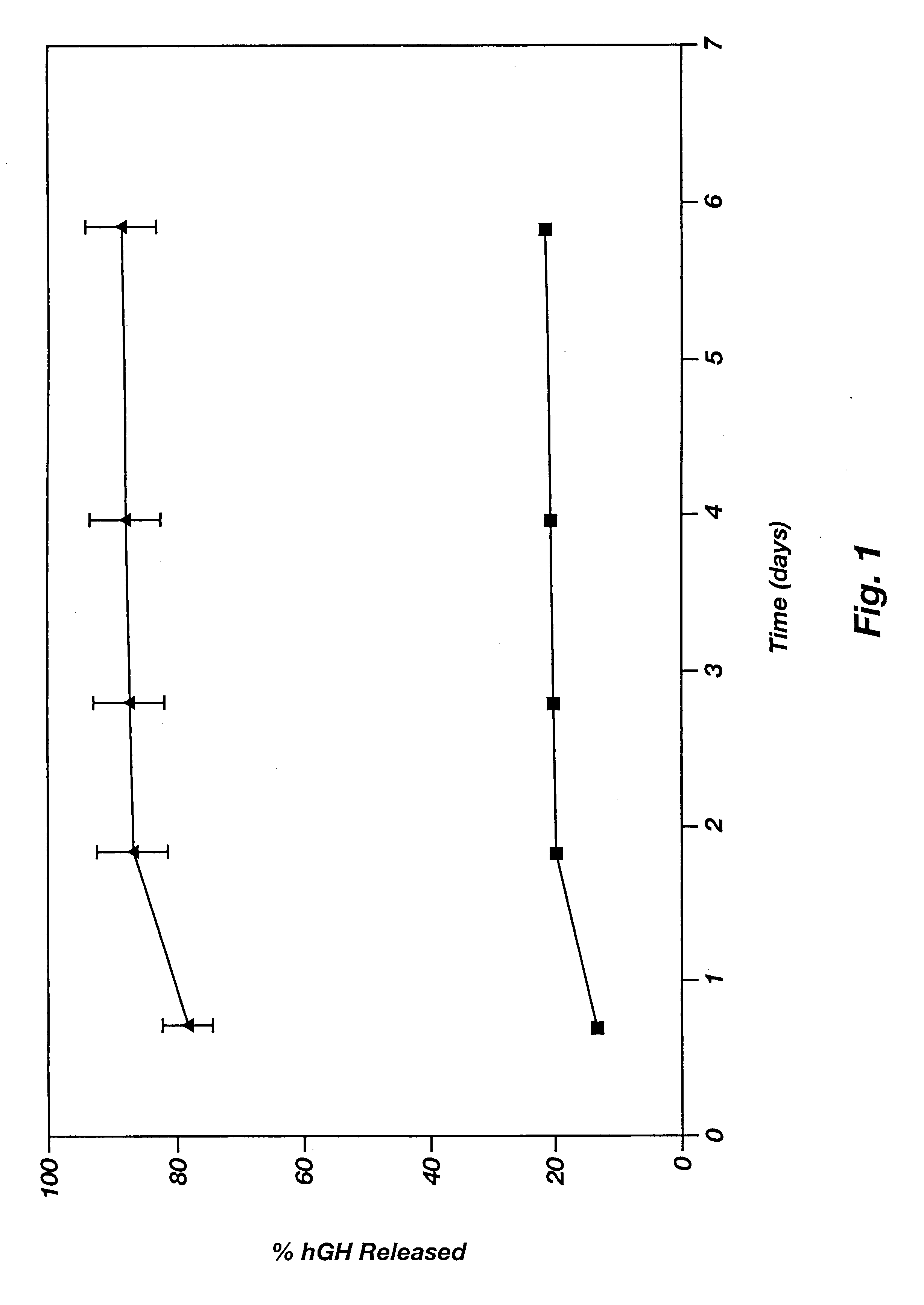
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Protein stabilized pharmacologically active agents, methods for the preparation thereof and methods for the use thereof
InactiveUS6749868B1Low toxicityLong half-lifePowder deliveryEchographic/ultrasound-imaging preparationsSuspended particlesFree protein
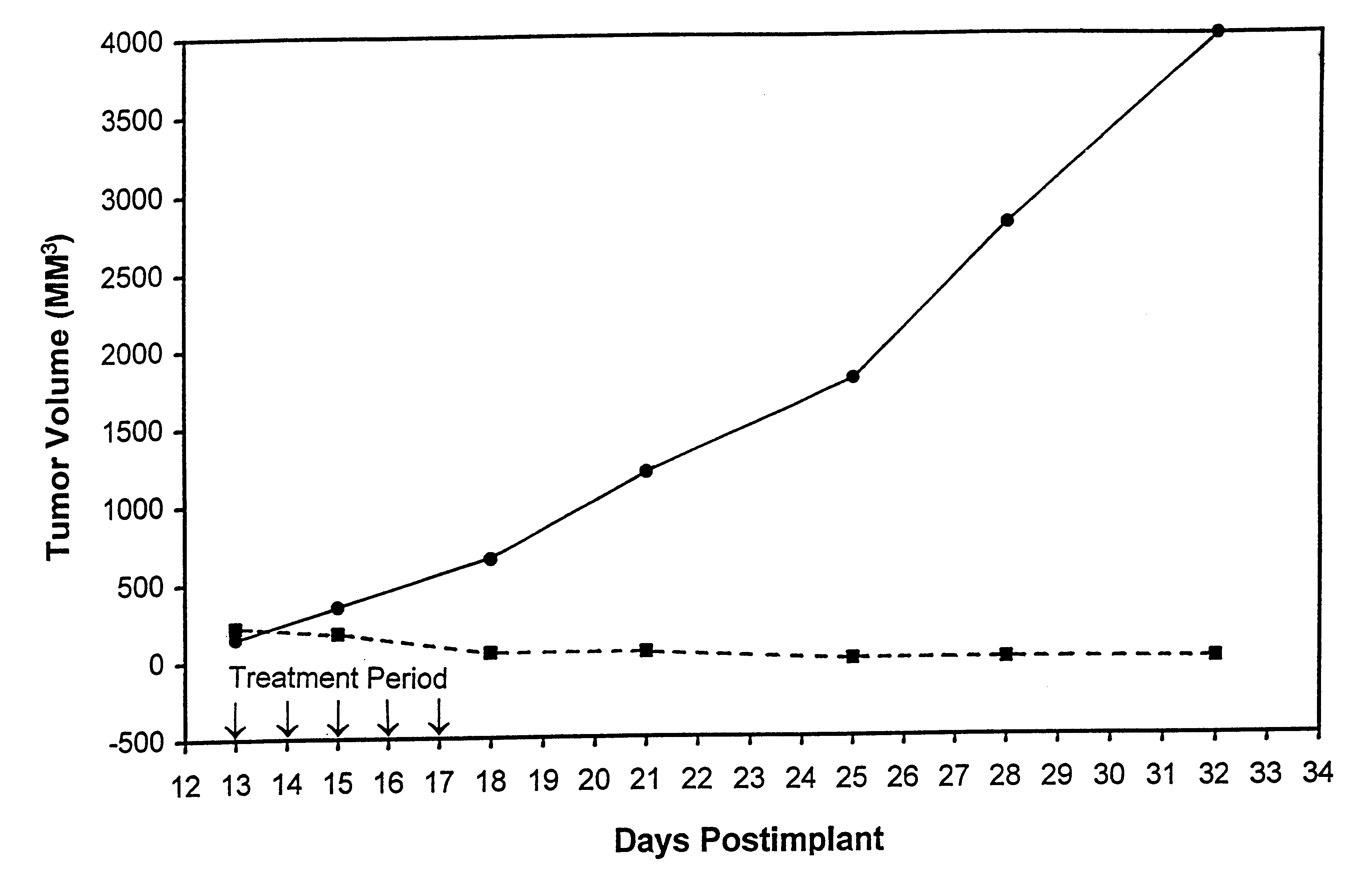
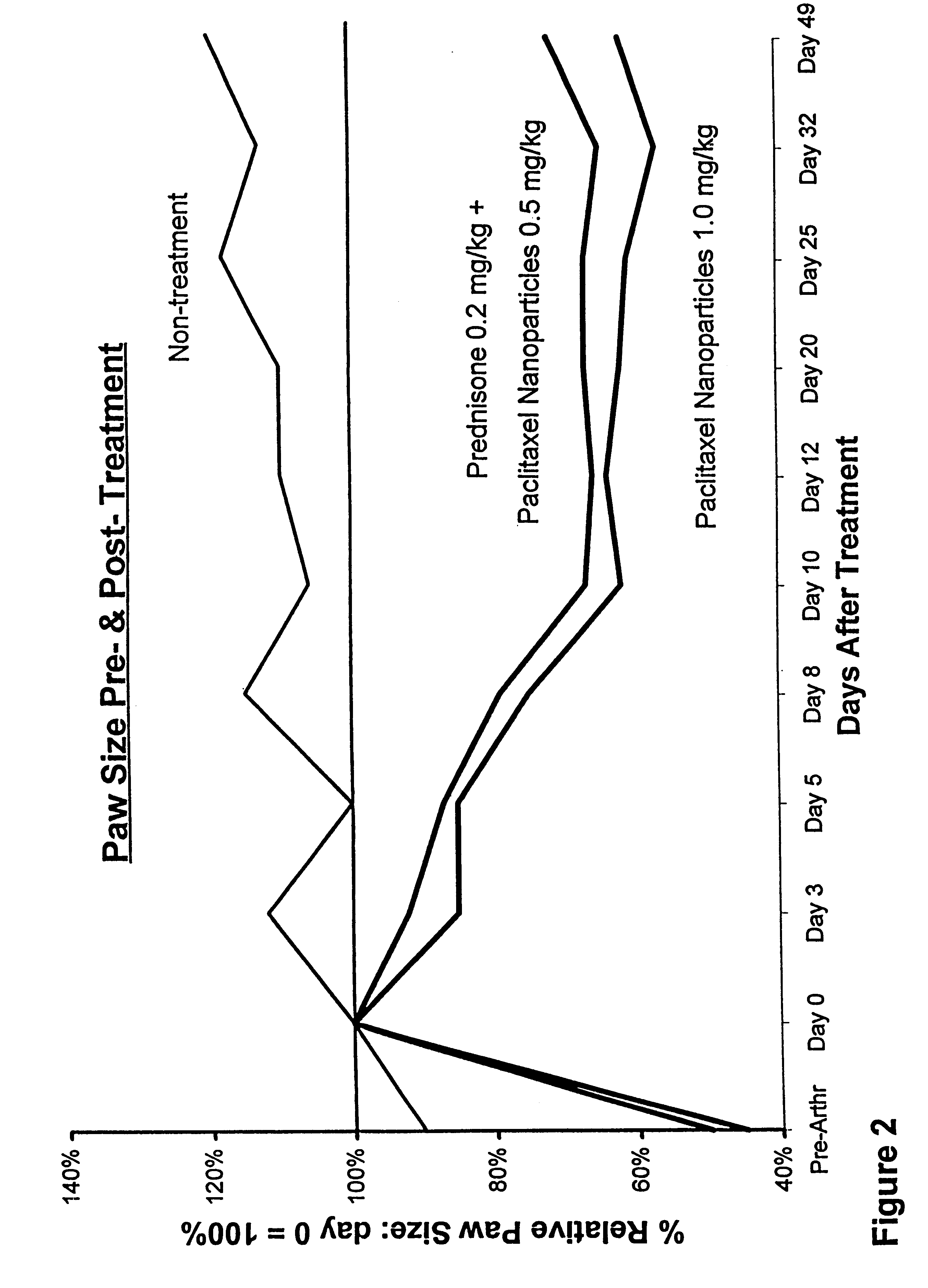
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful election of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Bioactive agent delivering system comprised of microparticles within a biodegradable to improve release profiles
InactiveUS6589549B2Improve stabilityPowder deliveryPeptide/protein ingredientsActive agentEngineering
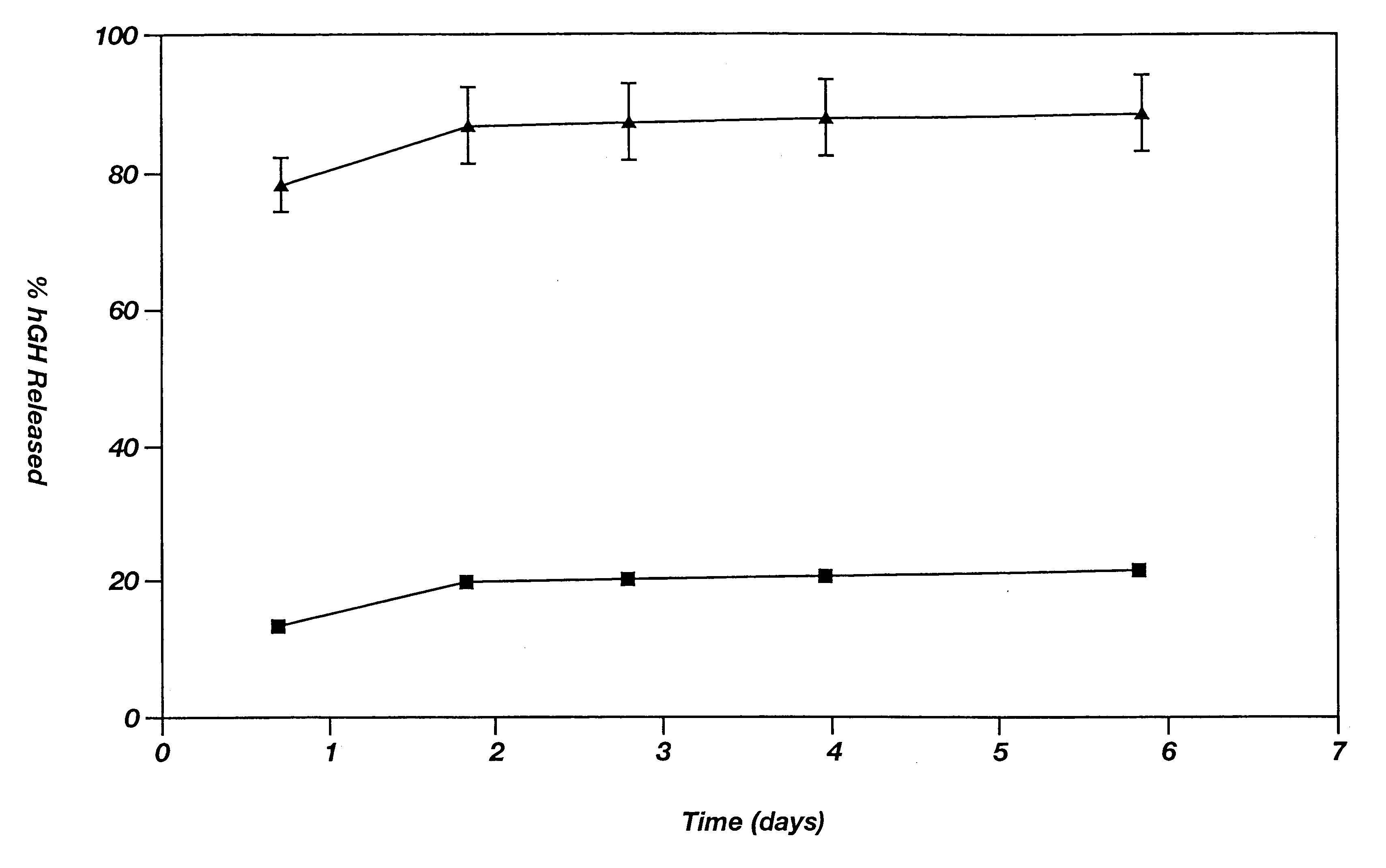
A composition and method for releasing a bio-active agent or a drug within a biological environment in a controlled manner is disclosed. The composition is a dual phase polymeric agent-delivery composition comprising a continuous biocompatible gel phase, a discontinuous particulate phase comprising defined microparticles and an agent to be delivered. A microparticle containing a bio-active agent is releasably entrained within a biocompatible polymeric gel matrix. The bioactive agent release may be contained in the microparticle phase alone or in both the microparticles and the gel matrix. The release of the agent is prolonged over a period of time, and the delivery may be modulated and / or controlled. In addition, a second agent may be loaded in some of the microparticles and / or the gel matrix.
Owner:BTG INT LTD
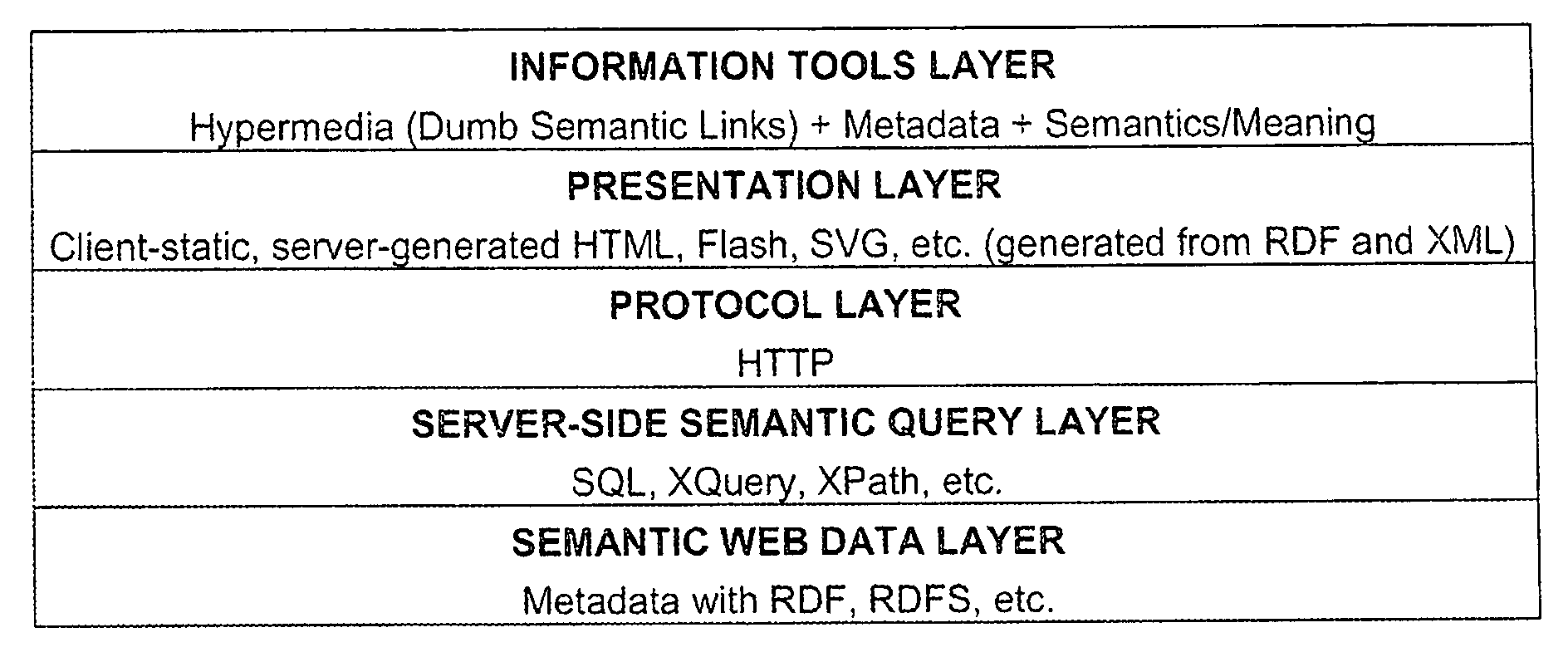
System and method for knowledge retrieval, management, delivery and presentation
InactiveUS20070038610A1Efficient queryWebsite content managementSpecial data processing applicationsAction CodeActive agent
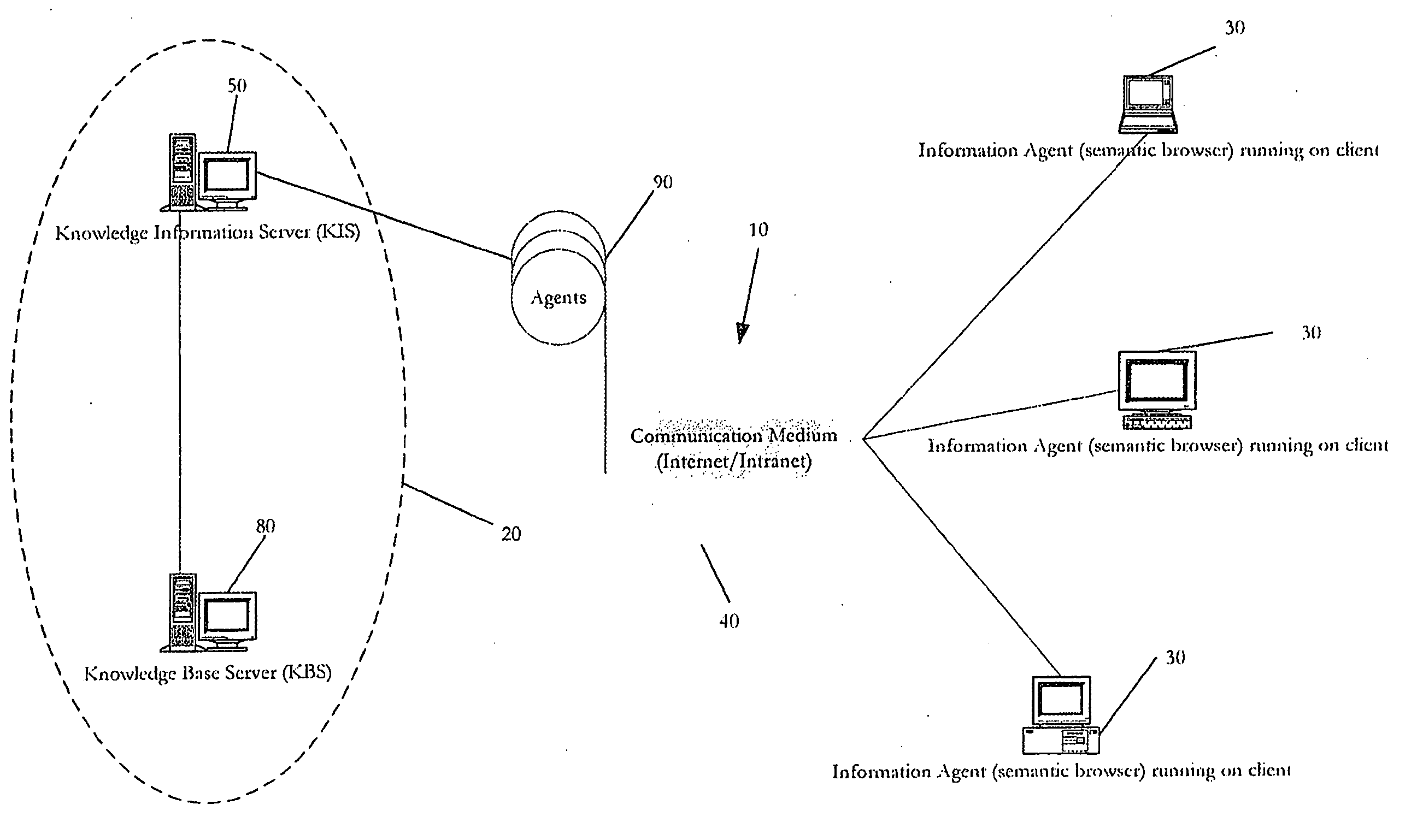
The present invention is directed to an integrated implementation framework and resulting medium for knowledge retrieval, management, delivery and presentation. The system includes a first server component that is responsible for adding and maintaining domain-specific semantic information and a second server component that hosts semantic and other knowledge for use by the first server component that work together to provide context and time-sensitive semantic information retrieval services to clients operating a presentation platform via a communication medium. Within the system, all objects or events in a given hierarchy are active Agents semantically related to each other and representing queries (comprised of underlying action code) that return data objects for presentation to the client according to a predetermined and customizable theme or “Skin.” This system provides various means for the client to customize and “blend” Agents and the underlying related queries to optimize the presentation of the resulting information.
Owner:OMOIGUI NOSA
Apparatus for preparing a solid phase microparticulate composition
InactiveUS6042792ALow costImproved substantivityCosmetic preparationsComponent separationWaxMicroparticle
Described are controlled, time-release microparticulate active and bioactive compositions (including perfuming compositions) for targeted delivery to services such as skin, hair and fabric and the environment proximate thereto, where the active and bioactive materials have a calculated log10P values of between 1 and 8 (P being the n-octanol-water partition coefficient). Such compositions include the active or bioactive material in single phase, solid solution in a wax or polymer matrix also having coated thereon and / or containing a compatible surfactant. Also described are processes and apparatus for preparing such compositions and processes for using same. Furthermore, certain component(s) of the aforementioned compositions in combination with one another are novel, and other components have novel uses in increasing fragrance substantivity.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
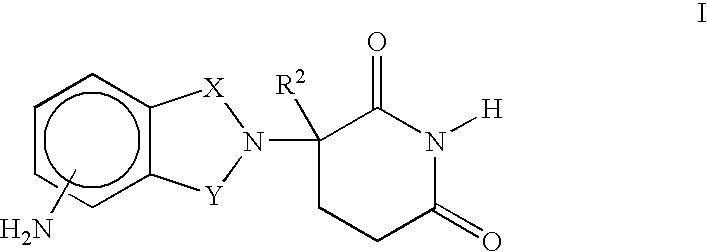
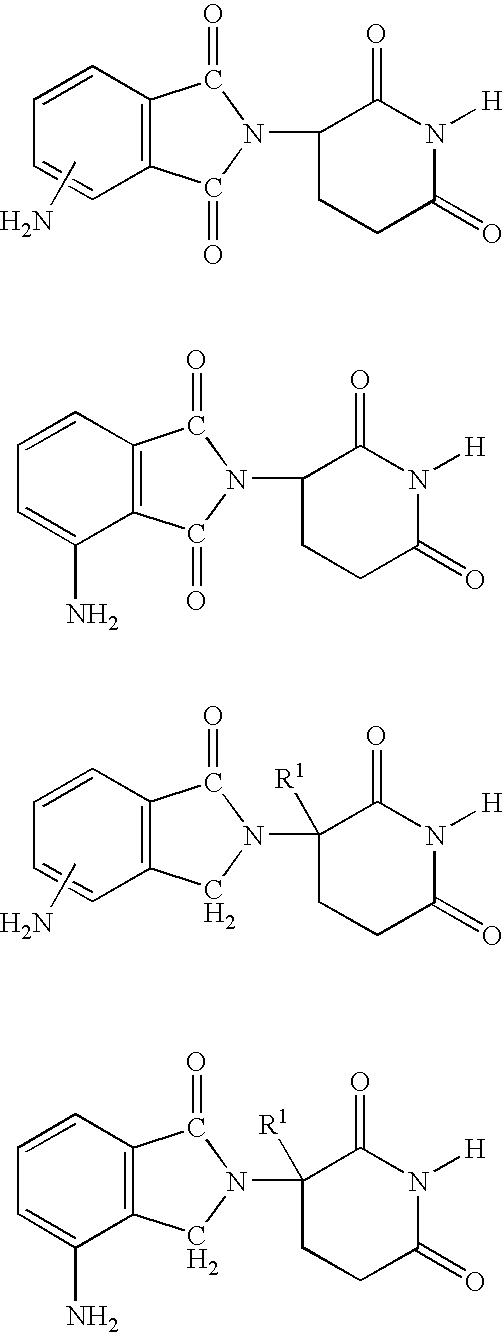
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of myeloproliferative diseases
InactiveUS20040087546A1Reduce adverse effectsImprove toleranceBiocideNervous disorderActive agentMyeloproliferative disease
Methods of treating, preventing and / or managing a myeloproliferative disease are disclosed. Specific methods encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent, and / or the transplantation of blood or cells. Particular second active agents are capable of suppressing the overproduction of hematopoietic stem cells or ameliorating one or more of the symptoms of a myeloproliferative disease. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Wound treatment apparatus and method
ActiveUS8529548B2Promote wound healingReduce bacterial loadCannulasInfusion devicesFlow stressThermal energy
An apparatus and method for aspirating, irrigating and / or cleansing wounds is provided. The apparatus and method include one or more of the following: simultaneous aspiration and irrigation of the wound, supplying of thermal energy to fluid circulated through the wound; supplying physiologically active agents to the wound; a biodegradable scaffold in contact with the wound bed; and application of stress or flow stress to the wound bed.
Owner:SMITH & NEPHEW INC
Method for forming a photoresist pattern
InactiveUS20070163625A1Inhibition formationSurface-active detergent compositionsNon-surface-active detergent compositionsResistPhotoresist
A photoresist cleaning solution and method for forming photoresist patterns using the same. More specifically, disclosed are a photoresist cleaning solution comprising H2o and an ionic surfactant represented by Formula 1, and a method for forming a photoresist pattern using the same. By spraying the cleaning solution of the present invention over photoresist film before and / or after exposing step, pattern formation in an undesired region caused by ghost images can be removed.
Owner:SK HYNIX INC
Drug delivery device
Drug delivery devices, and methods of delivering pharmaceutically active agents to a target tissue within a body using such devices, are disclosed. One drug delivery device includes a body having an internal surface for placement proximate a target tissue and a well having an opening to the internal surface. An inner core comprising a pharmaceutically active agent is disposed in the well.
Owner:NOVARTIS AG
Biointerface membranes incorporating bioactive agents
InactiveUS20060198864A1Improve performanceAdditive manufacturing apparatusSurgeryBiointerfaceActive agent
A biointerface membrane for an implantable device including a nonresorbable solid portion with a plurality of interconnected cavities therein adapted to support tissue ingrowth in vivo, and a bioactive agent incorporated into the biointerface membrane and adapted to modify the tissue response is provided. The bioactive agents can be chosen to induce vascularization and / or prevent barrier cell layer formation in vivo, and are advantageous when used with implantable devices wherein solutes are transported across the device-tissue interface.
Owner:DEXCOM INC
Methods of using and compositions comprising immunomodulatory compounds for treatment and management of macular degeneration
InactiveUS20040091455A1Extension of timeUseful in treatingBiocideSenses disorderActive agentSurgical procedures
Methods of treating, preventing and / or managing macular degeneration are disclosed. Specific embodiments encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent and / or surgery. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
High molecular wegiht polymers, devices and method for making and using same
Anhydride polymers that release active or activatable agent(s) have pre-selected properties such as molecular weight, flexibility, hardness, adhesiveness, and other valuable properties. The polymers are suitable for use in compositions, formulations, coatings, devices, and the like that benefit from the controlled release of an agent(s) over a period of time. The polymers are prepared by a process involving various alternative and sequential steps that allow the design a priori of products with specific characteristics. The polymers are suitable as delivery systems, either by themselves, as compositions, formulations or devices.
Owner:RUTGERS THE STATE UNIV
Lipid formulations for nucleic acid delivery
ActiveUS8283333B2Improve effectivenessHigh activityOrganic active ingredientsSugar derivativesLipid particleActive agent
The present invention provides novel, serum-stable lipid particles comprising one or more active agents or therapeutic agents, methods of making the lipid particles, and methods of delivering and / or administering the lipid particles. More particularly, the present invention provides serum-stable nucleic acid-lipid particles (SNALP) comprising a nucleic acid (e.g., one or more interfering RNA molecules), methods of making the SNALP, and methods of delivering and / or administering the SNALP (e.g., for the treatment of cancer). In particular embodiments, the present invention provides tumor-directed lipid particles that preferentially target solid tumors. The tumor-directed formulations of the present invention are capable of preferentially delivering a payload such as a nucleic acid to cells of solid tumors compared to non-cancerous cells.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Implantable or insertable medical device resistant to microbial growth and biofilm formation
InactiveUS6887270B2Prevent preferential partitioningPrevent chemical modificationAntipyreticAnalgesicsActive agentMicrobial adhesion
Disclosed are implantable or insertable medical devices that provide resistance to microbial growth on and in the environment of the device and resistance to microbial adhesion and biofilm formation on the device. In particular, the invention discloses implantable or insertable medical devices that comprise at least one biocompatible matrix polymer region, an antimicrobial agent for providing resistance to microbial growth and a microbial adhesion / biofilm synthesis inhibitor for inhibiting the attachment of microbes and the synthesis and accumulation of biofilm on the surface of the medical device. Also disclosed are methods of manufacturing such devices under conditions that substantially prevent preferential partitioning of any of said bioactive agents to a surface of the biocompatible matrix polymer and substantially prevent chemical modification of said bioactive agents.
Owner:BOSTON SCI SCIMED INC
Compositions for rapid and non-irritating transdermal delivery of pharmaceutically active agents and methods for formulating such compositions and delivery thereof
InactiveUS6444234B1Effective therapeutic doseReduced barrier effectBiocideCosmetic preparationsSolventActive agent
Pharmaceutical compositions for the transdermal administration of a medicament or other active agent by topical application of the composition to the skin of humans or other animals are described. Methodology for formulating such compositions which provide for very rapid uptake of the medicament and transmigration into and through the skin to either fatty tissues or the vascular system, while minimizing irritation to the skin and / or immunological response, is based on a transdermal delivery system (TDS) wherein the medicament is modified to form a true solution in a complex formed from particular solvents and solvent and solute modifiers in combination with skin stabilizers. Uptake of the medicament is further facilitated and made more rapid by including Forskolin or other source of cellular energy, namely induction of cAMP or cGMP. Selection of specific solvents and solvent and solute modifiers and other functional ingredients and the amounts thereof are chosen such that there is a balance between the sum of the mole-moments [(molar amount of each individual ingredient)x(dipole moment of that ingredient)] of the delivery system and the sum of the moler moments of the composition in which the medicament is dissolved. Preferably, the van der Waals forces of the delivery system is also similarly matched to the van der Waals forces of the total composition, namely, delivery system plus active agent.
Owner:TRANSDERMAL DELIVERY SOLUTIONS
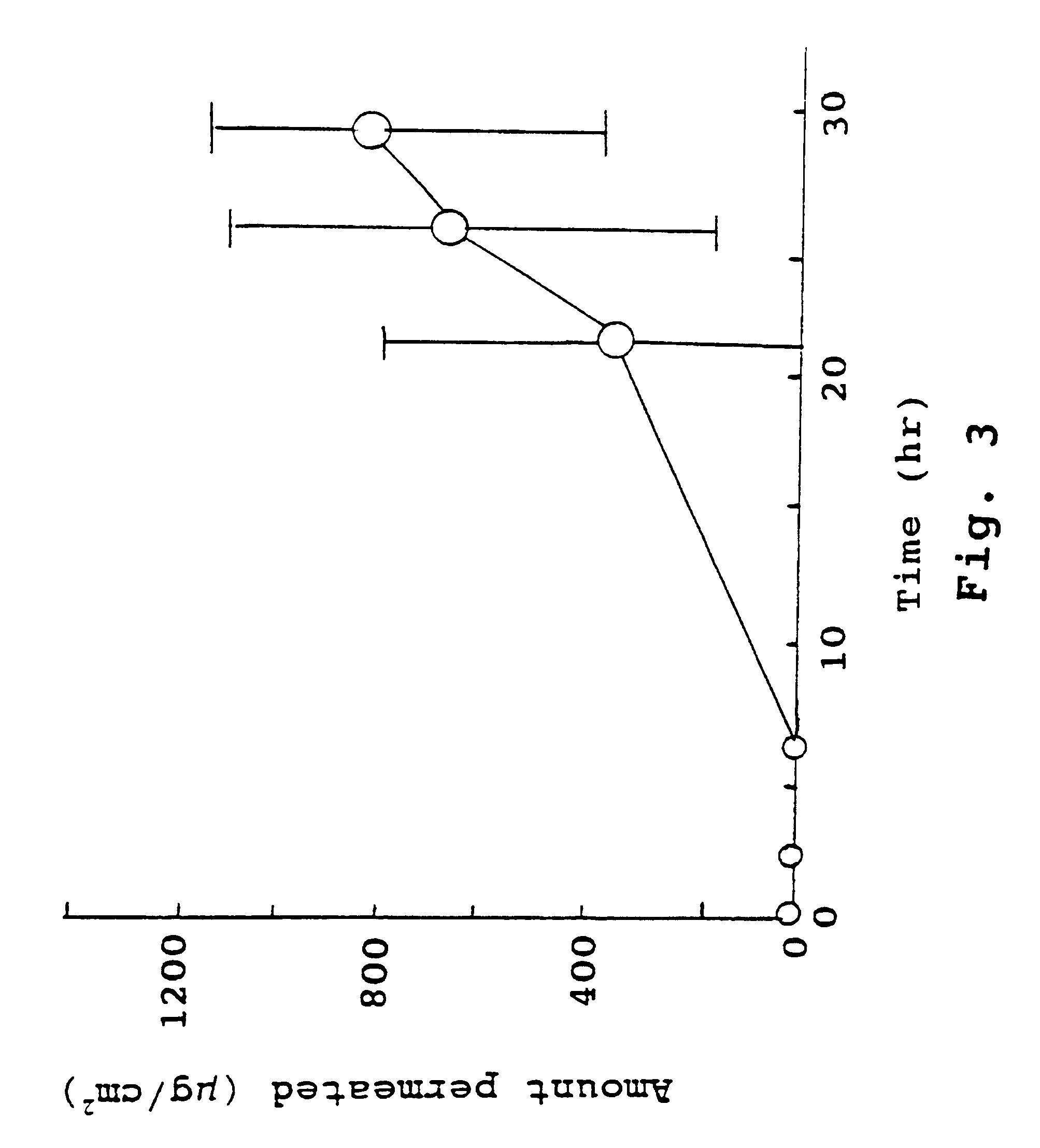
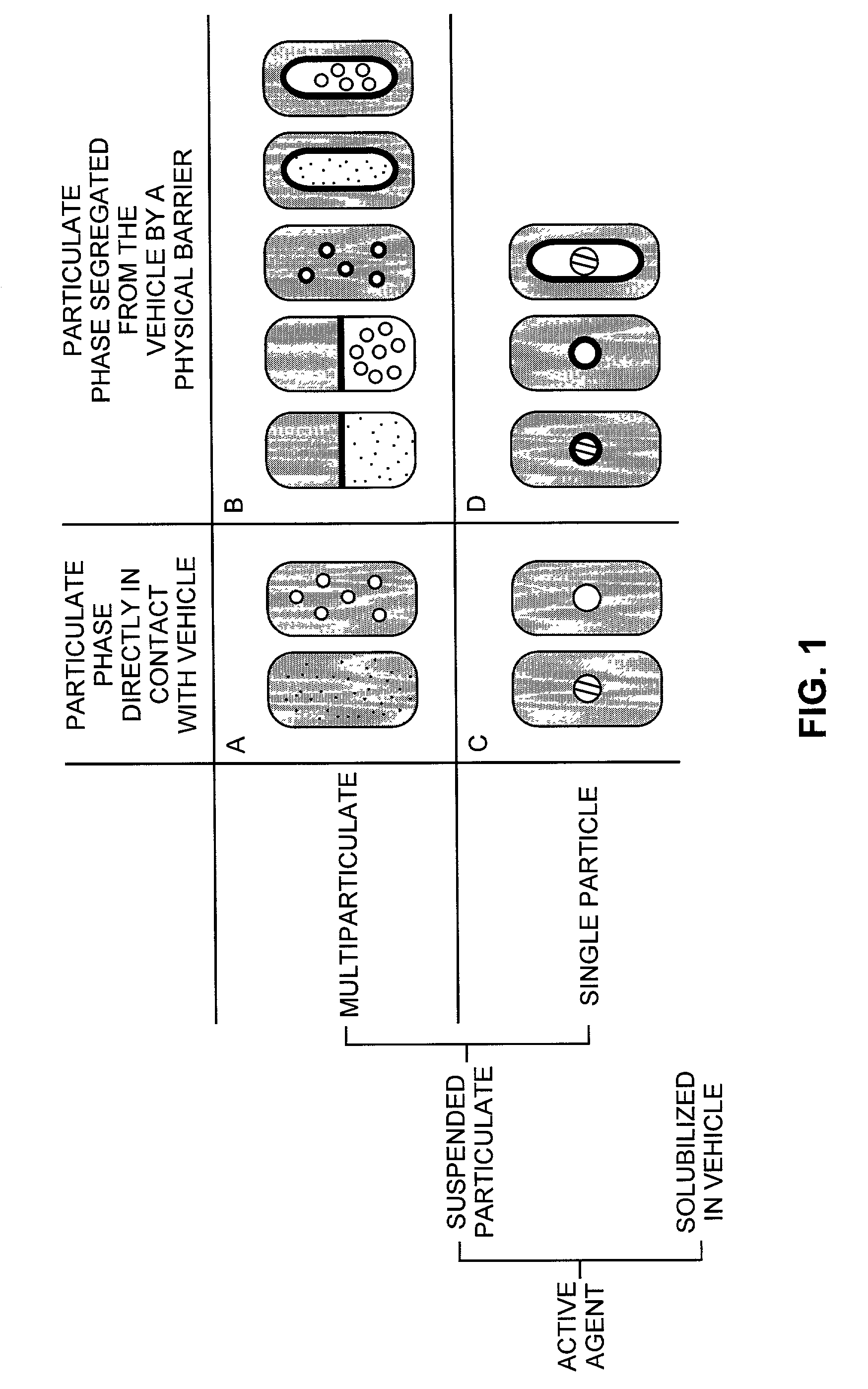
Pharmaceutical formulations and systems for improved absorption and multistage release of active agents
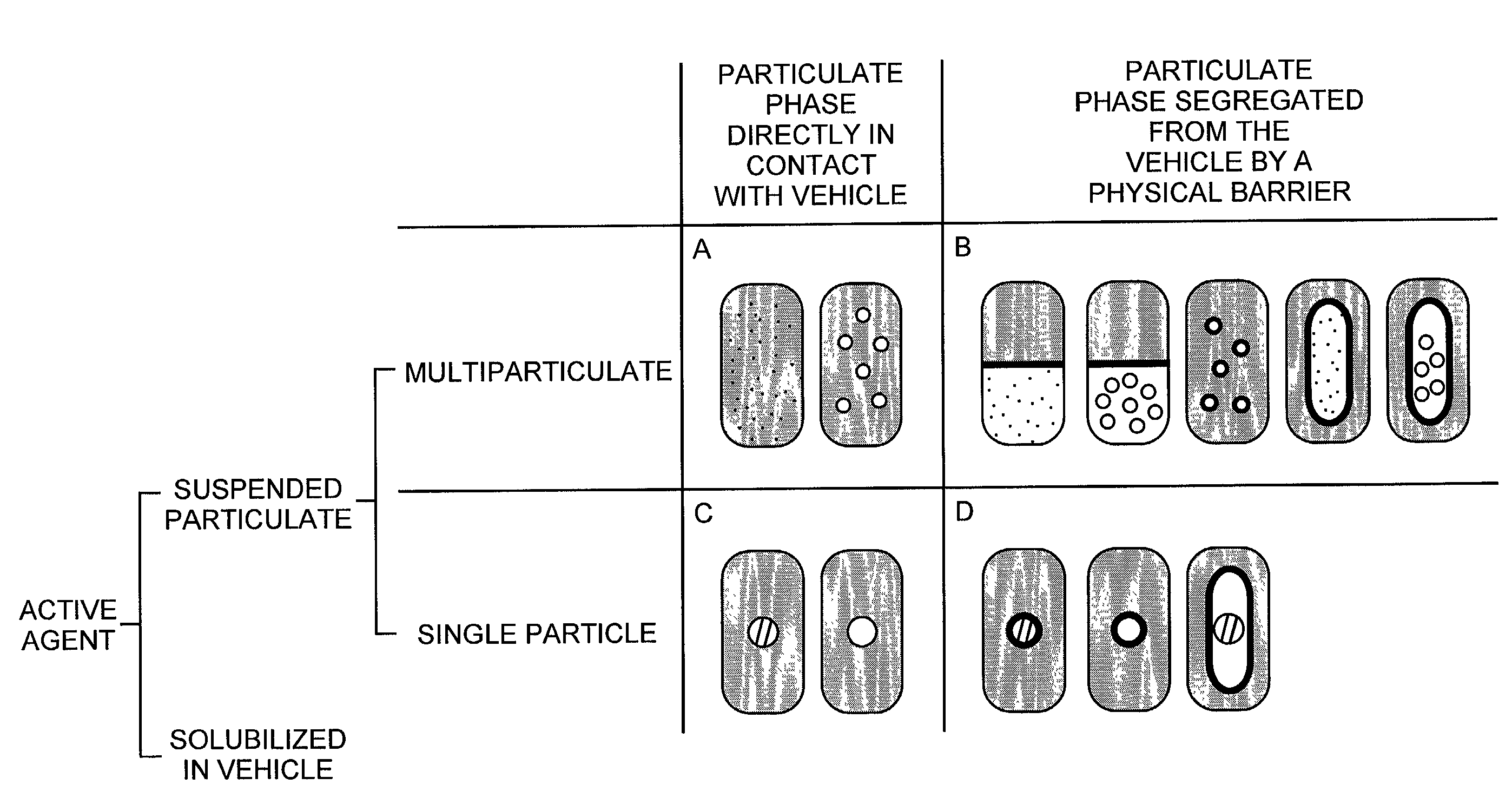
InactiveUS7374779B2Improve bioavailabilityLow variabilityPowder deliveryOrganic active ingredientsActive agentFast release
The present invention pertains to pharmaceutical formulations and systems for delivery of active agents, wherein a first fraction of an active agent is suspended in a vehicle and a second fraction of active agent is solubilized in the vehicle, with the suspended fraction representing about 5 wt. % to about 80 wt. % of the active agent and the second fraction representing about 20 wt. % to about 95 wt. % of the active agent. One or more additional active agents, which may be fully solubilized, partially solubilized, or suspended, may also be present. The first and second fractions of the active agent may or may not have different release profiles. Generally, a significant fraction of the solubilized drug will release rapidly, providing for rapid onset, while the suspended drug may be formulated for delayed and / or sustained release.
Owner:LIPOCINE
Non-invasive systems and methods for in-situ photobiomodulation
InactiveUS20100016783A1High selectivityAntibacterial agentsPowder deliveryDiseaseBiological regulation
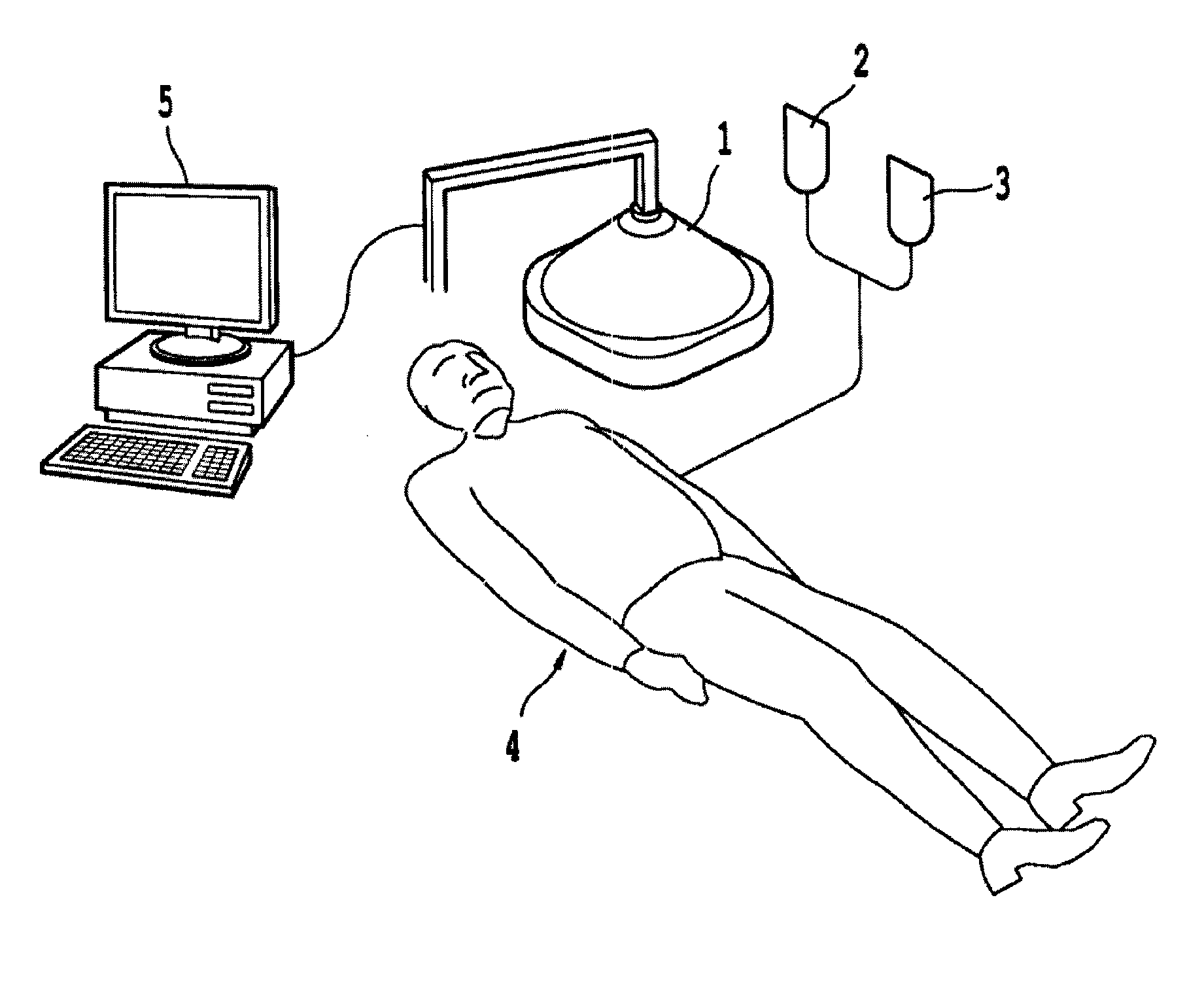
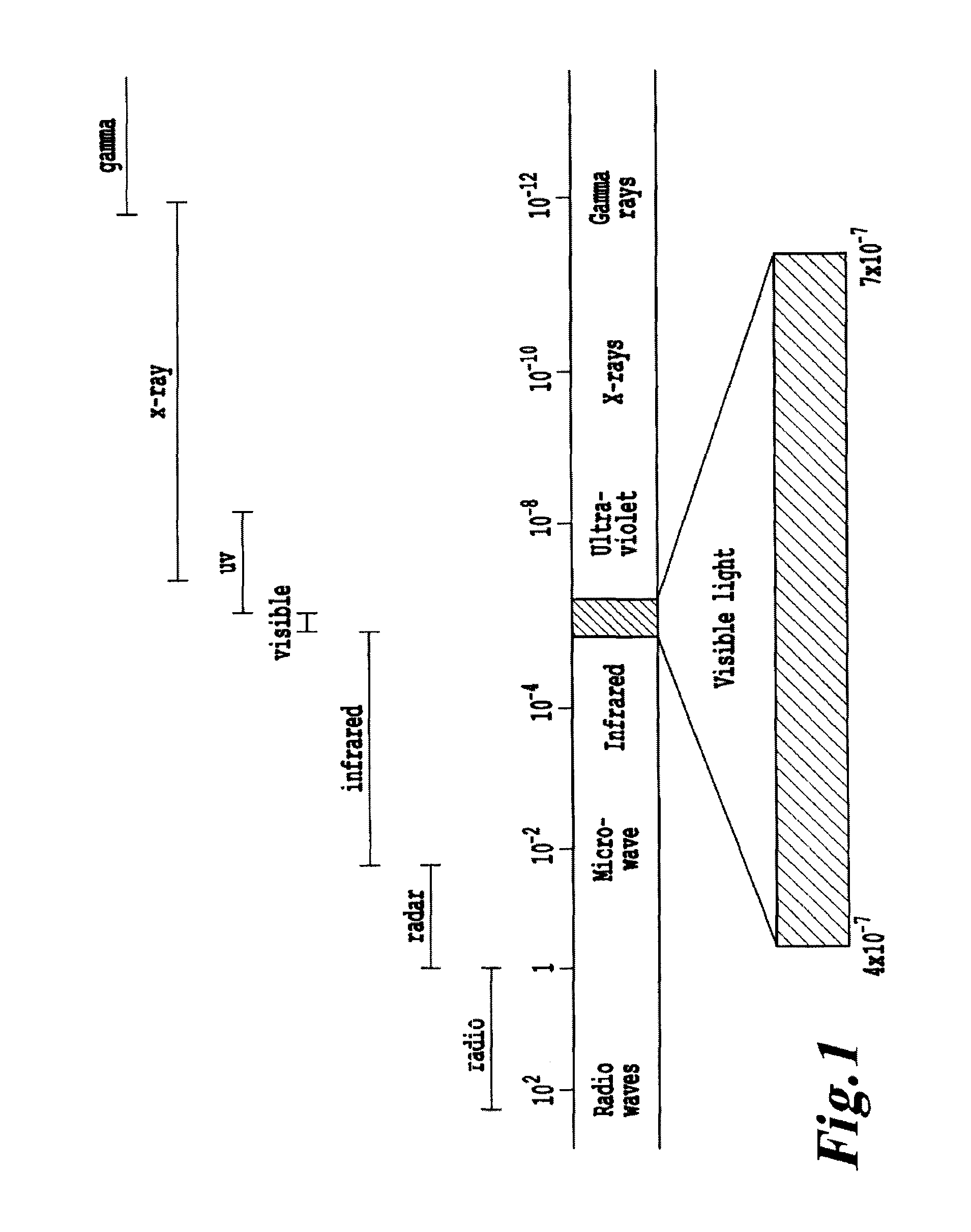
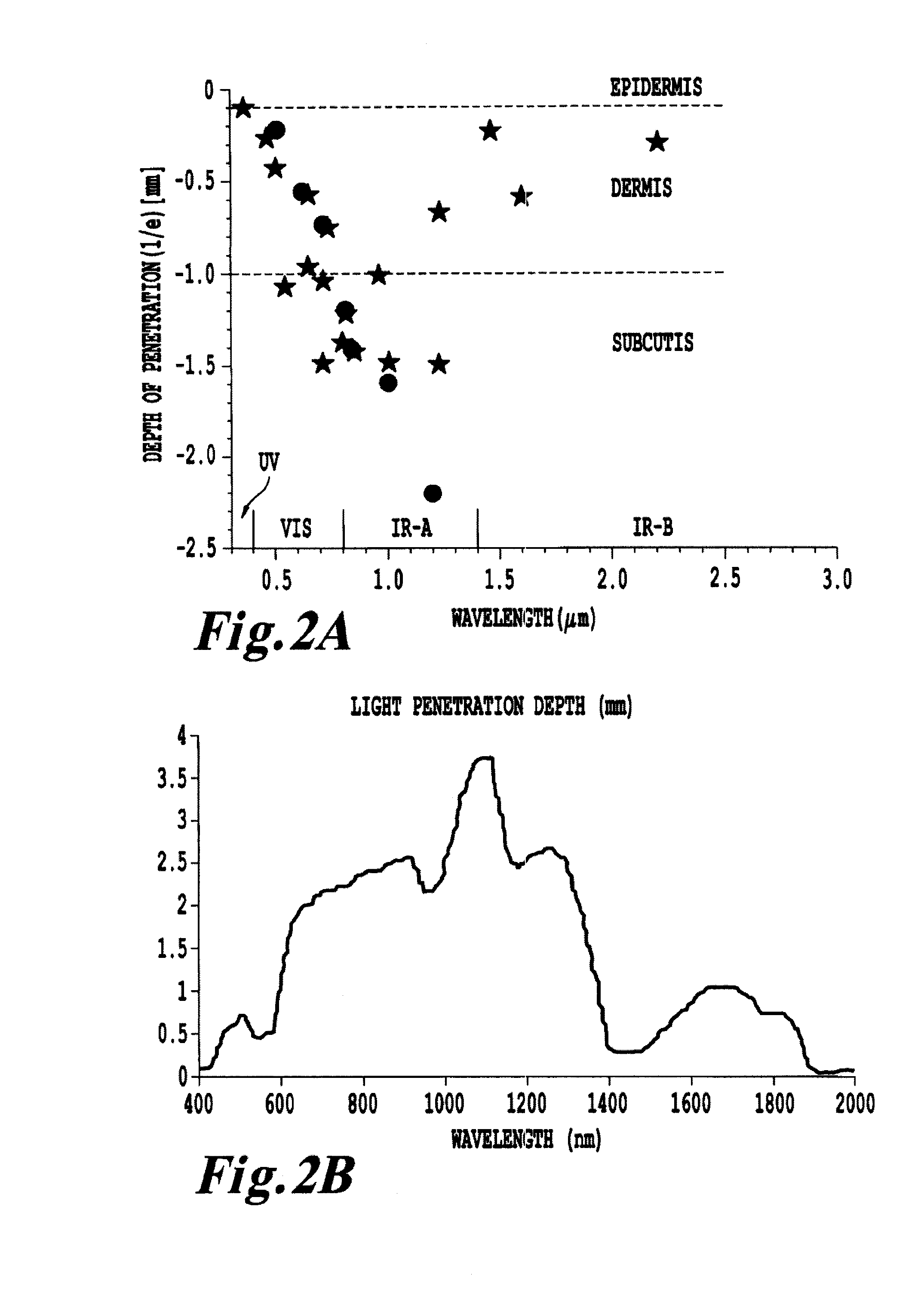
Products, compositions, systems, and methods for modifying a target structure which mediates or is associated with a biological activity, including treatment of conditions, disorders, or diseases mediated by or associated with a target structure, such as a virus, cell, subcellular structure or extracellular structure. The methods may be performed in situ in a non-invasive manner by application of an initiation energy to a subject thus producing an effect on or change to the target structure directly or via a modulation agent. The methods may further be performed by application of an initiation energy to a subject in situ to activate a pharmaceutical agent directly or via an energy modulation agent, optionally in the presence of one or more plasmonics active agents, thus producing an effect on or change to the target structure. Kits containing products or compositions formulated or configured and systems for use in practicing these methods.
Owner:DUKE UNIV +1
System and method for knowledge retrieval, management, delivery and presentation
InactiveUS20080162498A1Website content managementSpecial data processing applicationsAction CodeActive agent
The present invention is directed to an integrated implementation framework and resulting medium for knowledge retrieval, management, delivery and presentation. The system includes a first server component that is responsible for adding and maintaining domain-specific semantic information and a second server component that hosts semantic and other knowledge for use by the first server component that work together to provide context and time-sensitive semantic information retrieval services to clients operating a presentation platform via a communication medium. Within the system, all objects or events in a given hierarchy are active Agents semantically related to each other and representing queries (comprised of underlying action code) that return data objects for presentation to the client according to a predetermined and customizable theme or “Skin.” This system provides various means for the client to customize and “blend” Agents and the underlying related queries to optimize the presentation of the resulting information.
Owner:OMOIGUI NOSA
Microporation of tissue for delivery of bioactive agents
InactiveUS20050165393A1Improve throughputImprove breathabilitySonopheresisUltrasound therapyBiological bodyThermal energy
A method of enhancing the permeability of a biological membrane, including the skin or mucosa of an animal or the outer layer of a plant to a permeant is described utilizing microporation of selected depth and optionally one or more of sonic, electromagnetic, mechanical and thermal energy and a chemical enhancer. Microporation is accomplished to form a micropore of selected depth in the biological membrane and the porated site is contacted with the permeant. Additional permeation enhancement measures may be applied to the site to enhance both the flux rate of the permeant into the organism through the micropores as well as into targeted tissues within the organism.
Owner:ALTEA THERAPEUTIC CORP +1
Complex salt for anti-spotting detergents
InactiveUS20050003983A1Avoid chemical reactionsGood photo-bleaching effectCationic surface-active compoundsDetergent dyesActive agentOrganic chemistry
Provided is a method of using a complex salt as anti-spotting detergents, the complex salt being formed by the reaction of a photo-bleaching component having a water-soluble anionic substituent and a cationic surfactant. The complex salt is water insoluble in a stationary state, such as hand washing or pre-soaking for machine washing, so it effectively suppress the spotting of the photo-bleaching component into the interwoven webs of fabric. In addition, the complex salt can uniformly and rapidly dissolve in an agitating state, such as when machine washing, to allow the photo-bleaching component to absorb into fabric for effective bleaching and enhanced detergency.
Owner:KIM DONG GYU +6
Film forming foamable composition
InactiveUS20060193789A1Improve solubilityReduce deliveryCosmetic preparationsBiocideAlcohol freeFilm-forming agent
A foamable composition, includes (1) about 6% to about 70% by weight of at least one organic carrier; (2) about 0.1% to about 5% by weight of at least one surface-active agent; (3) about 0.01% to about 5% by weight of at least one film forming agent; (4) water; and (5) about 3% to about 25% by weight of the total composition of at least one liquefied or compressed gas propellant. The composition is substantially alcohol free and is used in treating, alleviating or preventing a disorder.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com