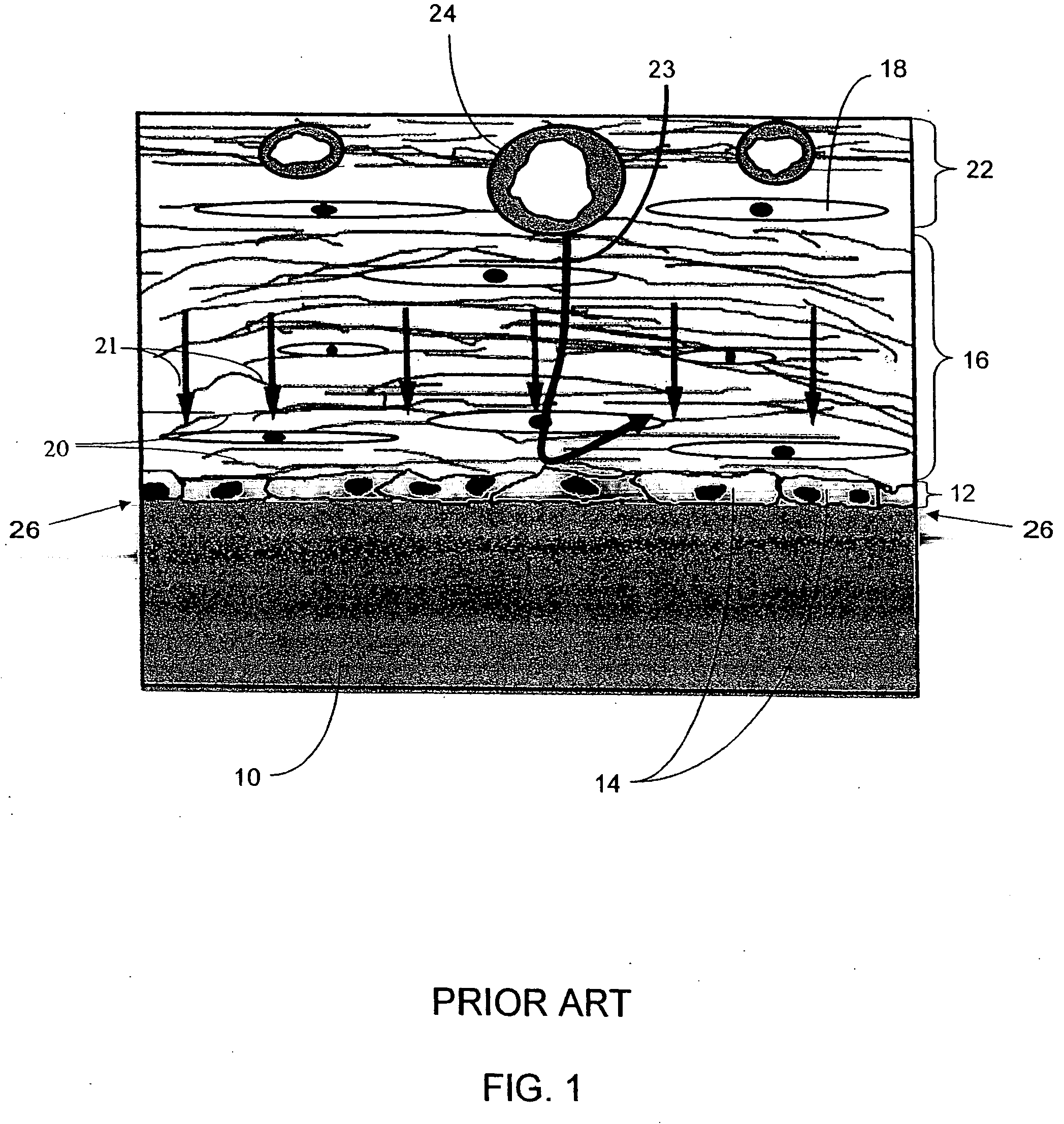
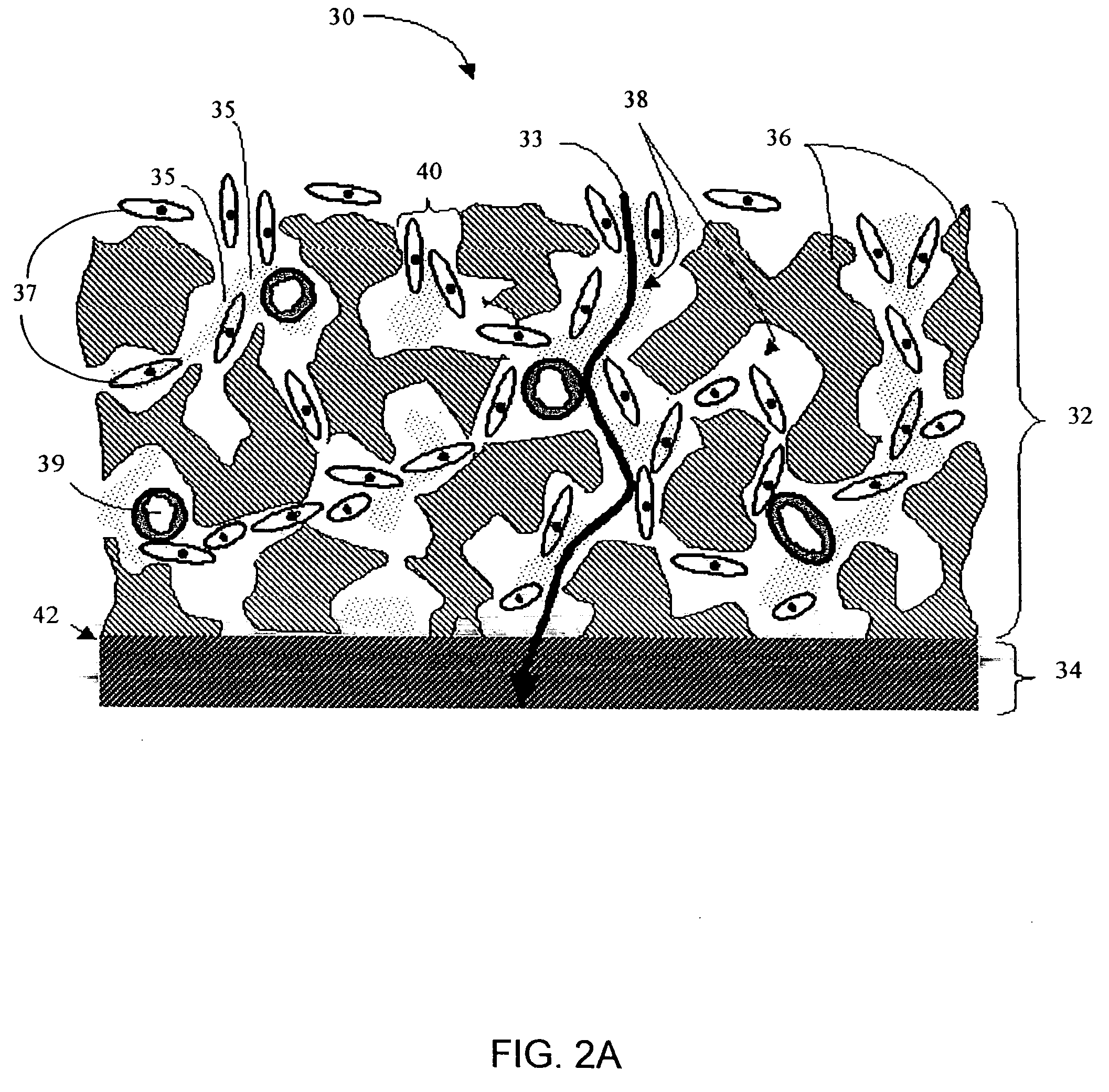
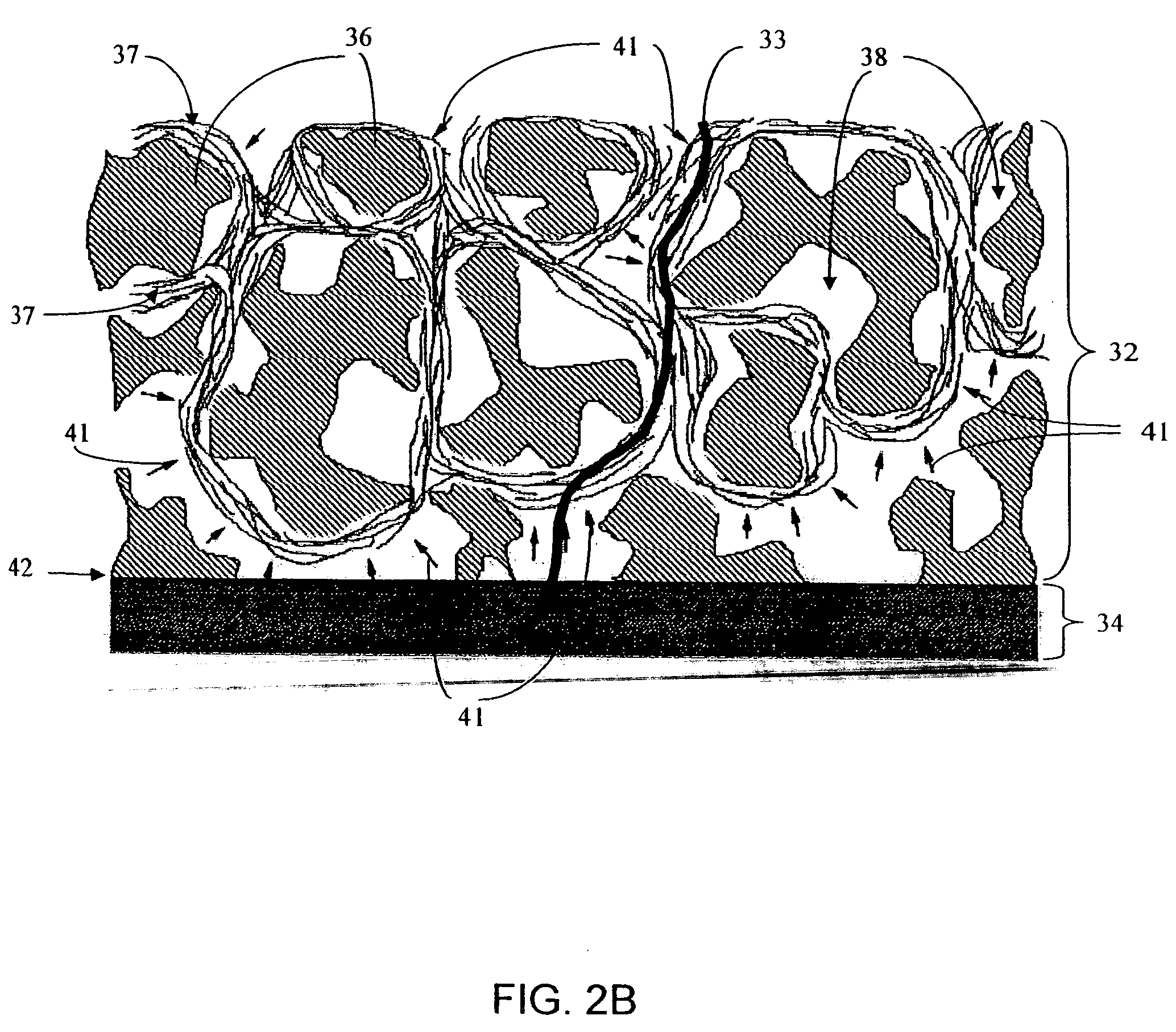
Biointerface membranes incorporating bioactive agents
a bioactive agent and biointerface technology, applied in the field of biointerface membranes, can solve the problems of loss of function, inability to provide data safely and reliably for long periods of time, and current use devices are unable to achieve the effect of improving the performance of blood-contacting surfaces
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Biointerface Membrane with Porous Silicone
[0184] A porous silicone cell disruptive (first) domain was prepared by mixing approximately 1 kg of sugar crystals with approximately 36 grams of water for 3-6 minutes. The mixture was then pressed into a mold and baked at 80° C. for 2 hours. The silicone was vacuumed into the mold for 6 minutes and cured at 80° C. for at least 2 hours. The sugar was dissolved using heat and deionized water, resulting in a flat sheet, porous membrane. Different architectures were obtained by varying the crystal size (crystals having an average diameter of about 90, 106, 150, 180, and 220 μm) and distribution within the mold that the silicone was cast from. After removal of silicone from the mold, the resulting membranes were measured for material thickness.
[0185] The cell-impermeable (second) domain was prepared by placing approximately 706 gm of dimethylacetamide (DMAC) into a 3L stainless steel bowl to which a polycarbonate urethane solut...
example 2
Neovascularizing Agents in Biointerface Membranes
[0188] In a first experiment, disks were employed, which were prepared for three-week implantation into the subcutaneous space of rats to test a neovascularizing agent. Monobutyrin was chosen based on its hydrophobic characteristics and ability to promote neovascularization. This experiment consisted of soaking the porous silicone prepared as described above in the concentrated solution of the bioactive compound at elevated temperature. This facilitated a partitioning of the agent into the porous silicone dependent upon its solubility in silicone rubber. Porous silicone disks were exposed to phosphate buffer mixed with Monobutyrin (500 mg / ml) for four days at 47° C. These disks were then autoclaved in the same solution, then rinsed in sterile saline immediately prior to implant. Disks were implanted into the subcutaneous dorsal space. Rats were euthanized and disks explanted at 3 weeks. Disks were fixed in 10% NBF and histologically ...
example 3
Anti-Inflammatory Agents in Biointerface Membranes
[0190] Dexamethasone was loaded into a porous silicone biointerface membrane by sorption. In this experiment, 100 mg of Dexamethasone was mixed with 10 mL of Butanone (solvent) and the mixture heated to about 70° C.-80° C. to dissolve the Dexamethasone in the solvent. The solution was then centrifuged to ensure solubility. The supernatant was pipetted from the solution and placed in a clean glass vial. Disks of porous silicone were placed in the Dexamethasone solution at 40° C. for 5 days, after which the disks were air-dried. The disks were sprayed with 70% isopropanol to remove trapped air from the porous silicone, attached to glucose sensors, and sterilized in 0.5% glutaraldehyde for 24 hours. After rinsing, the glucose sensors were placed in a 40 mL phosphate buffer solution conical. These conicals were placed on a shaker table with a setting of about 7 or 8. Dexamethasone release in PBS solution was measured daily for the first...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com