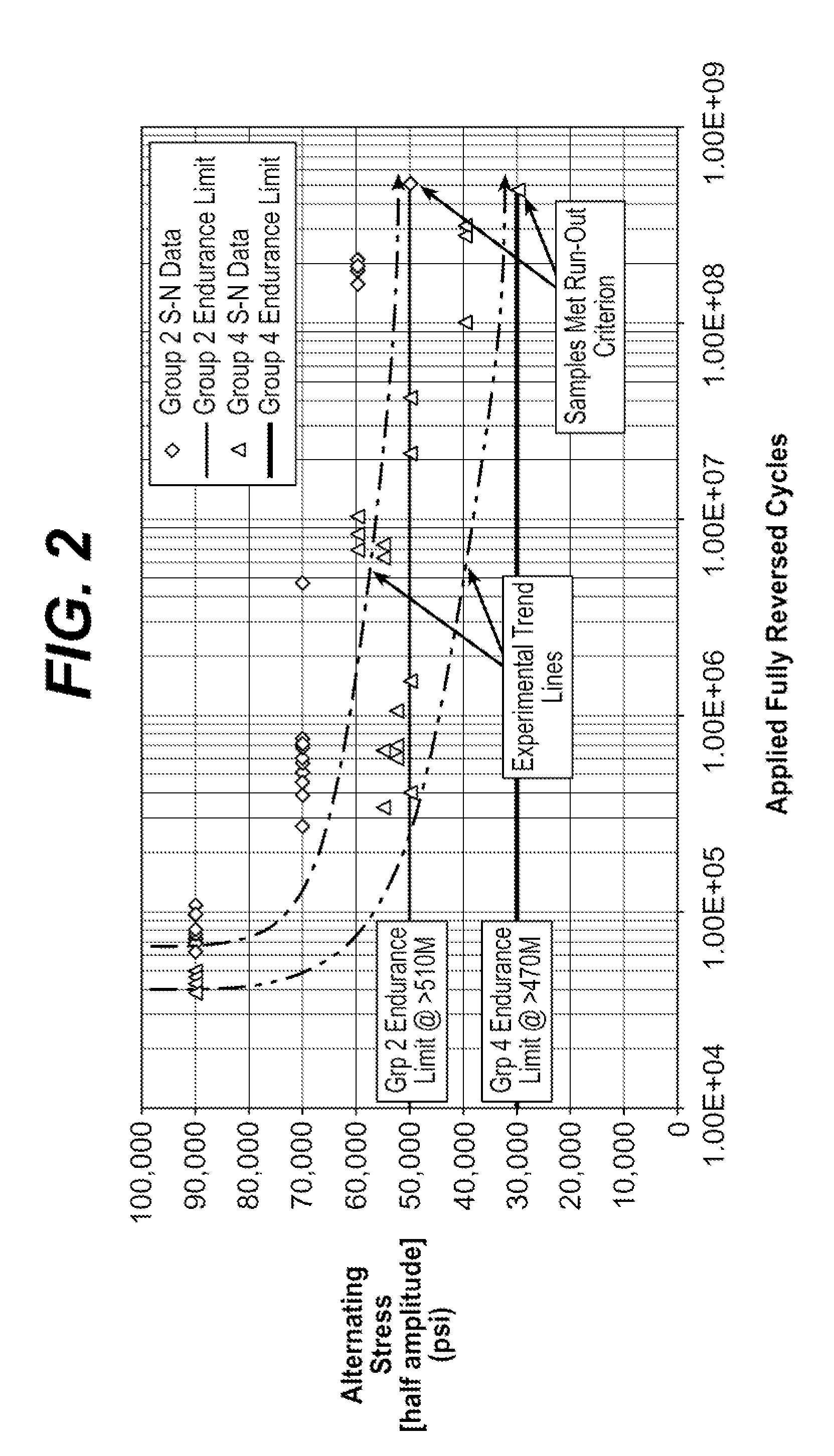
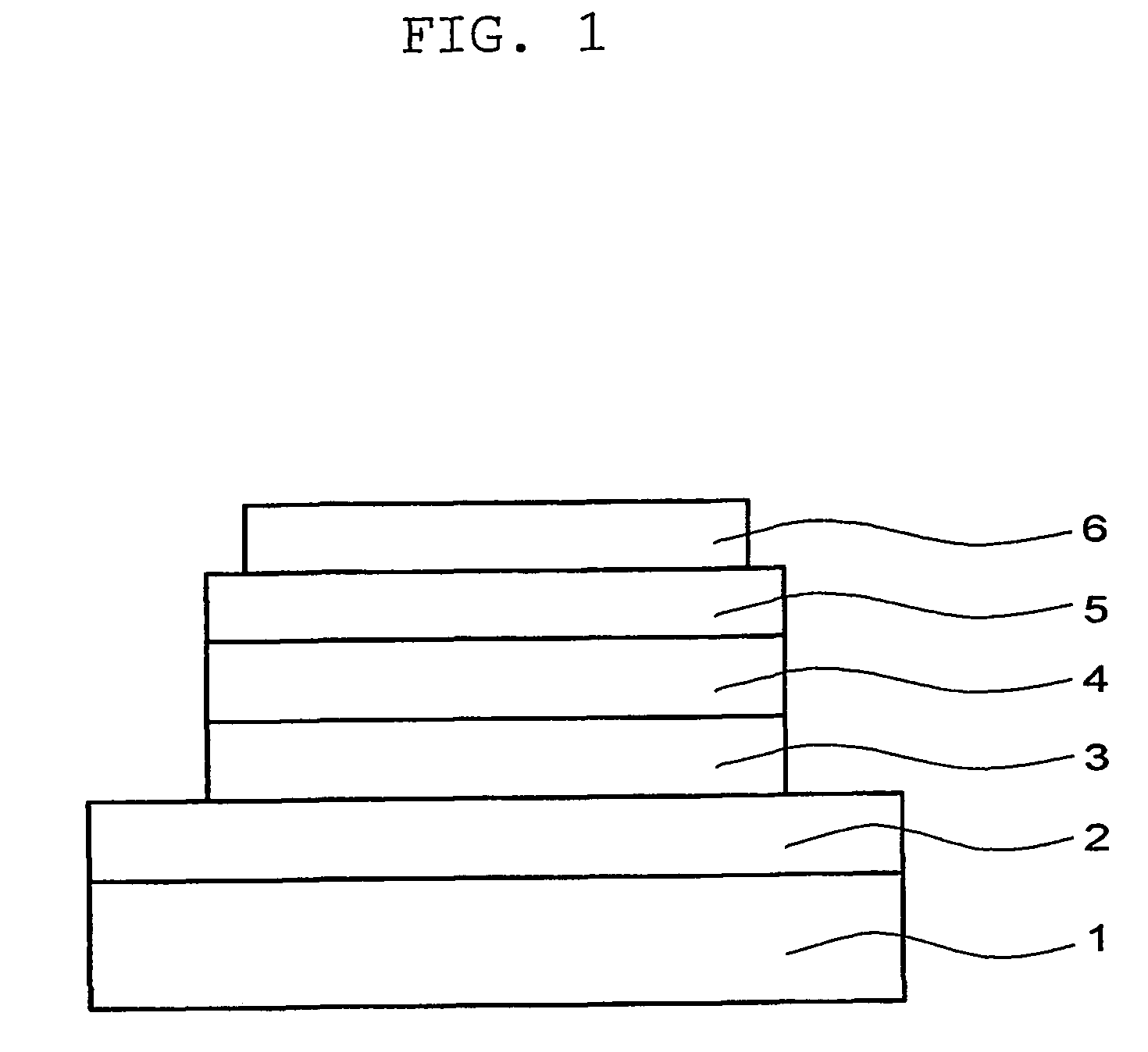
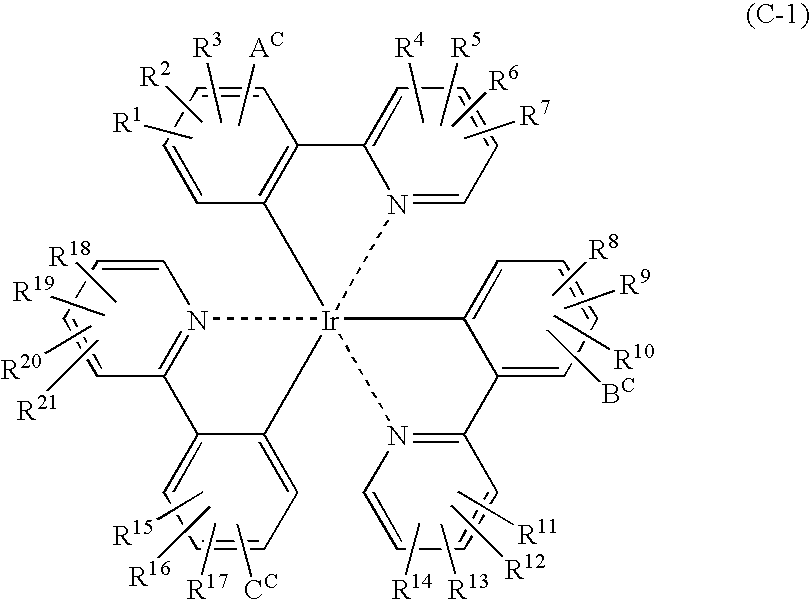
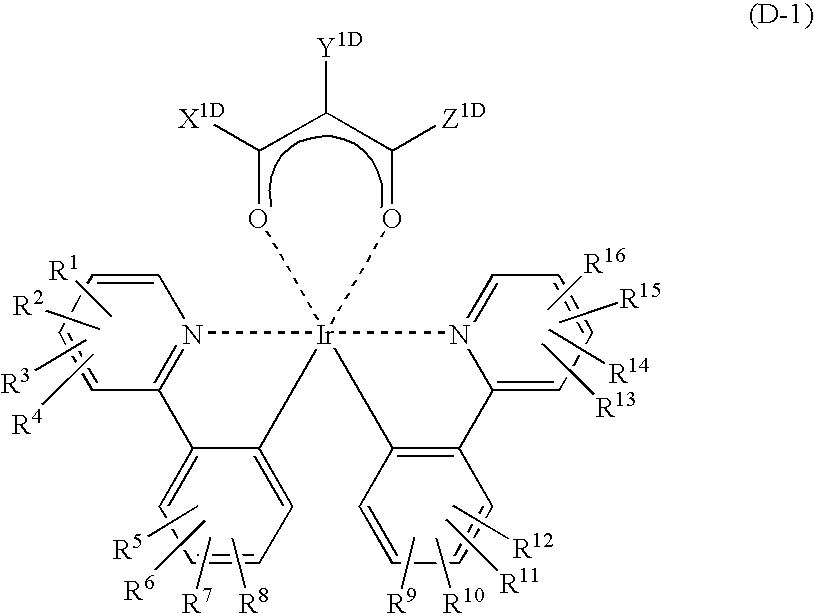
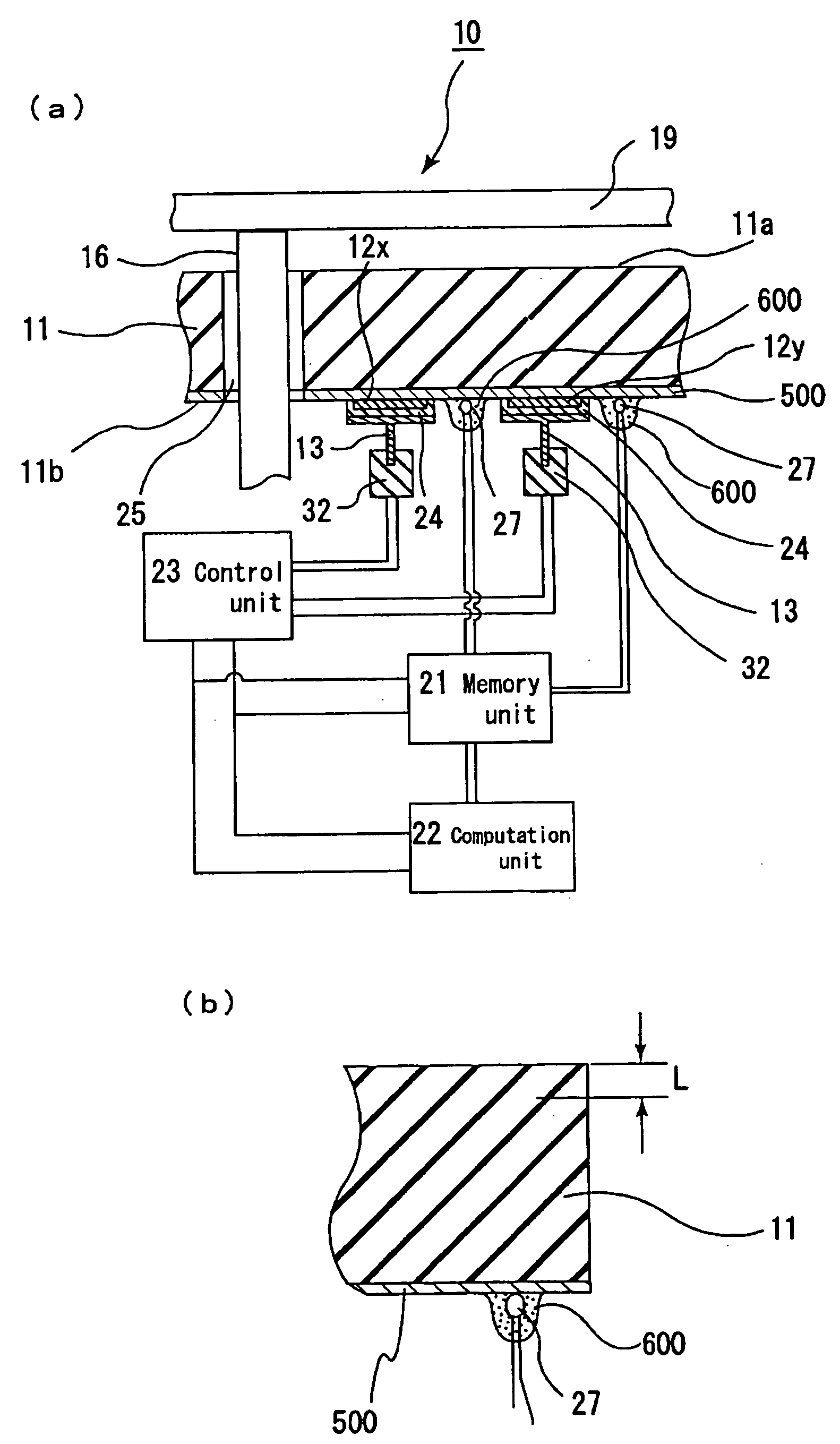
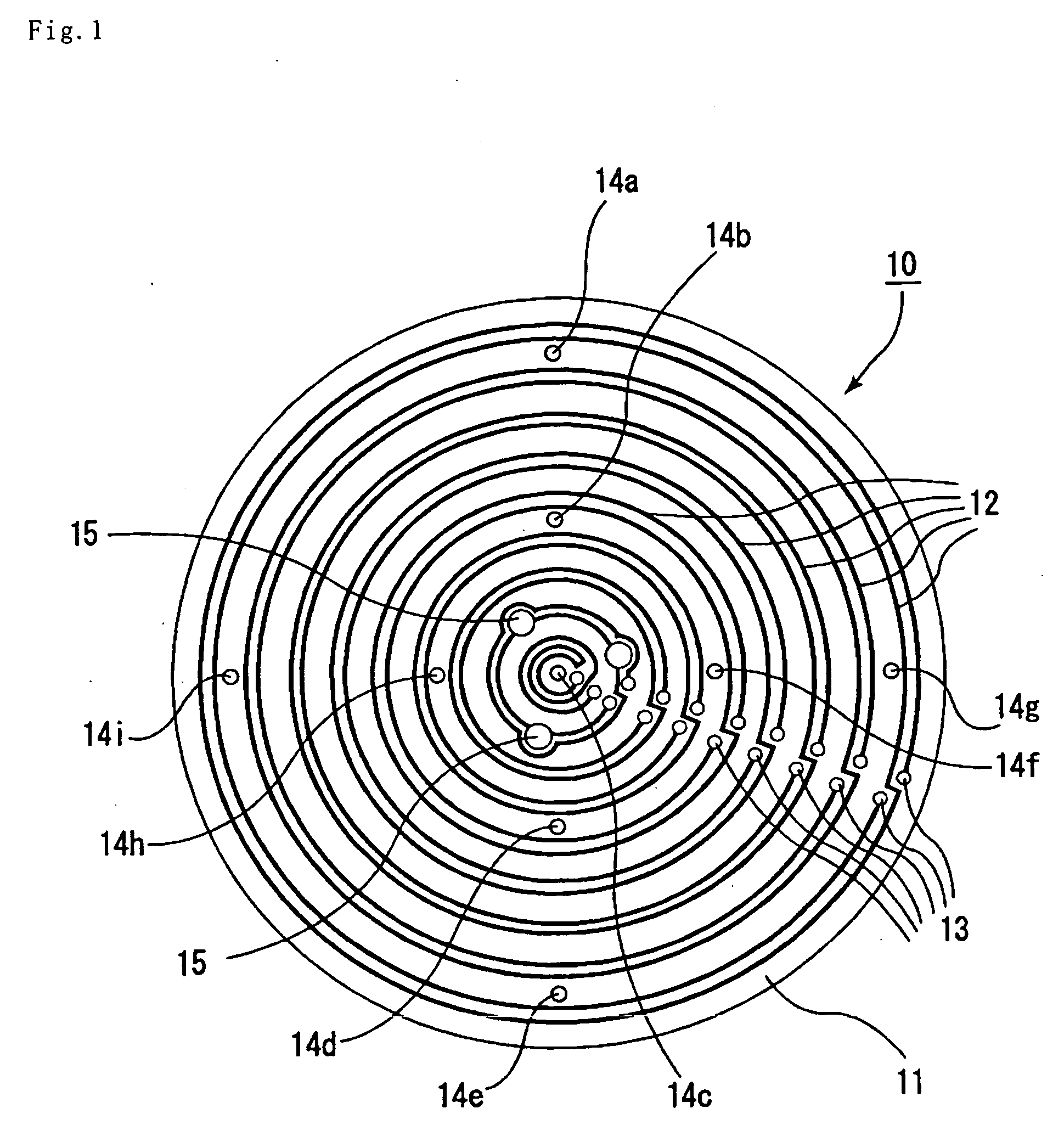
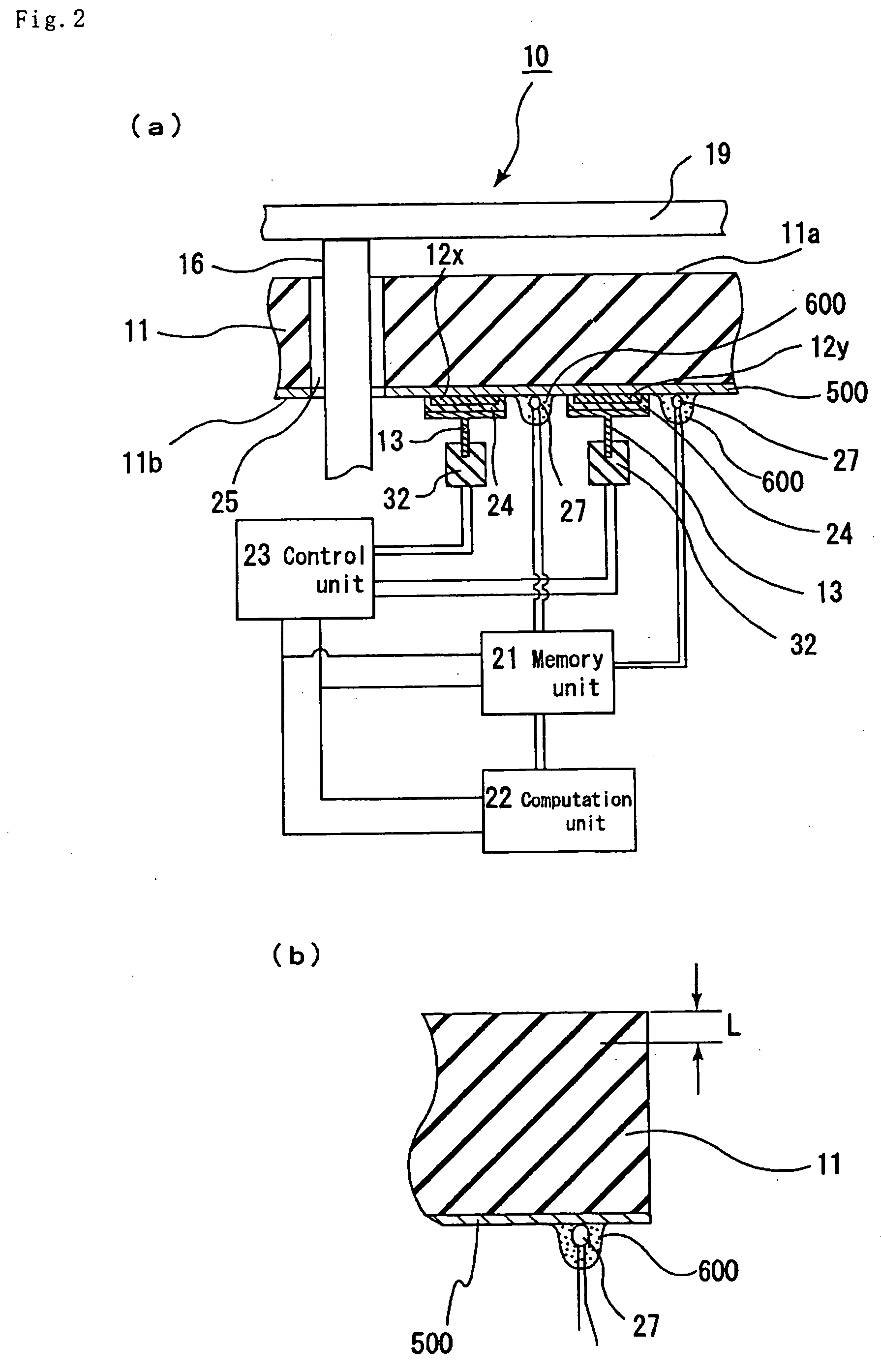
Patents
Literature
57272results about How to "Increased durability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
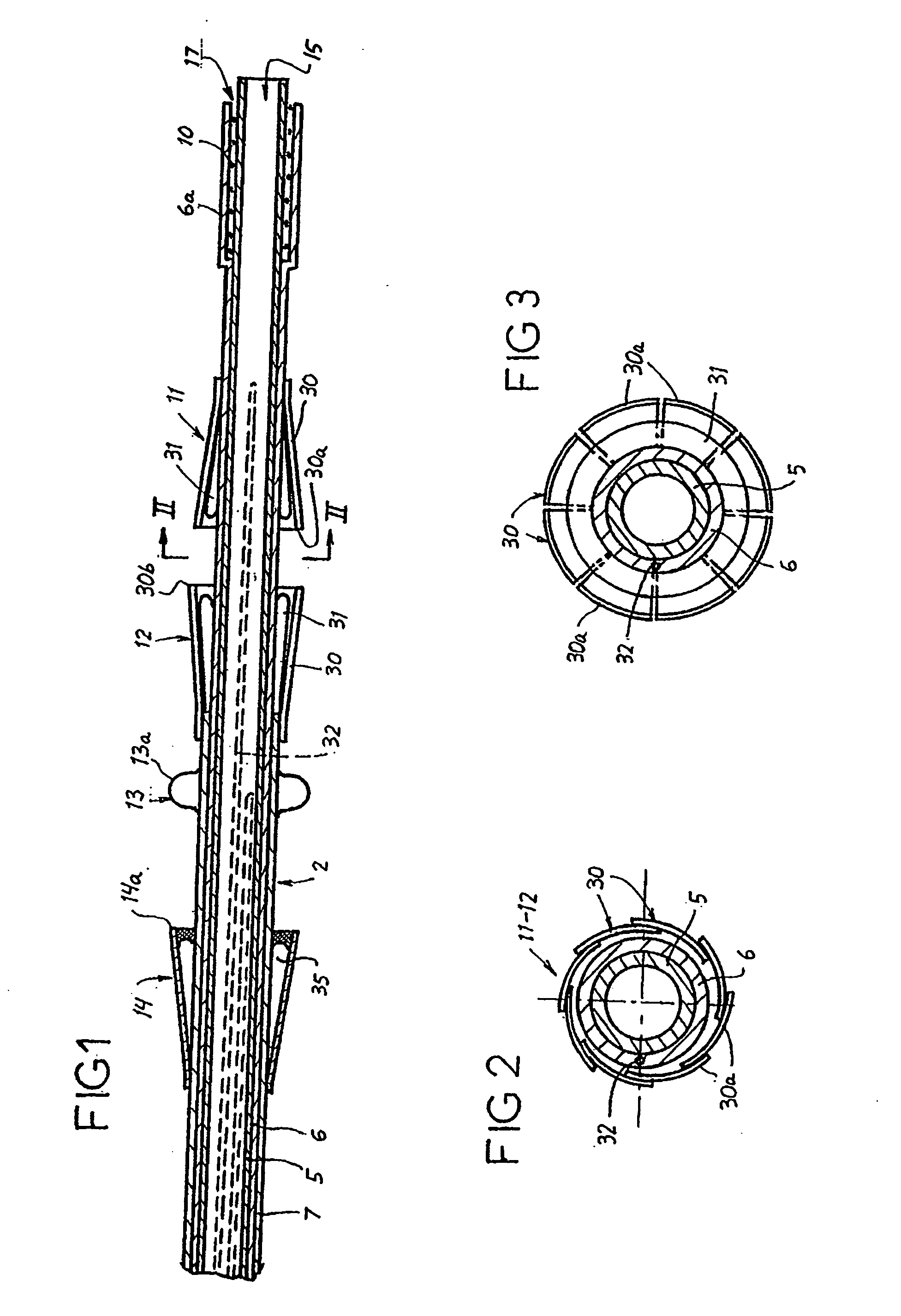
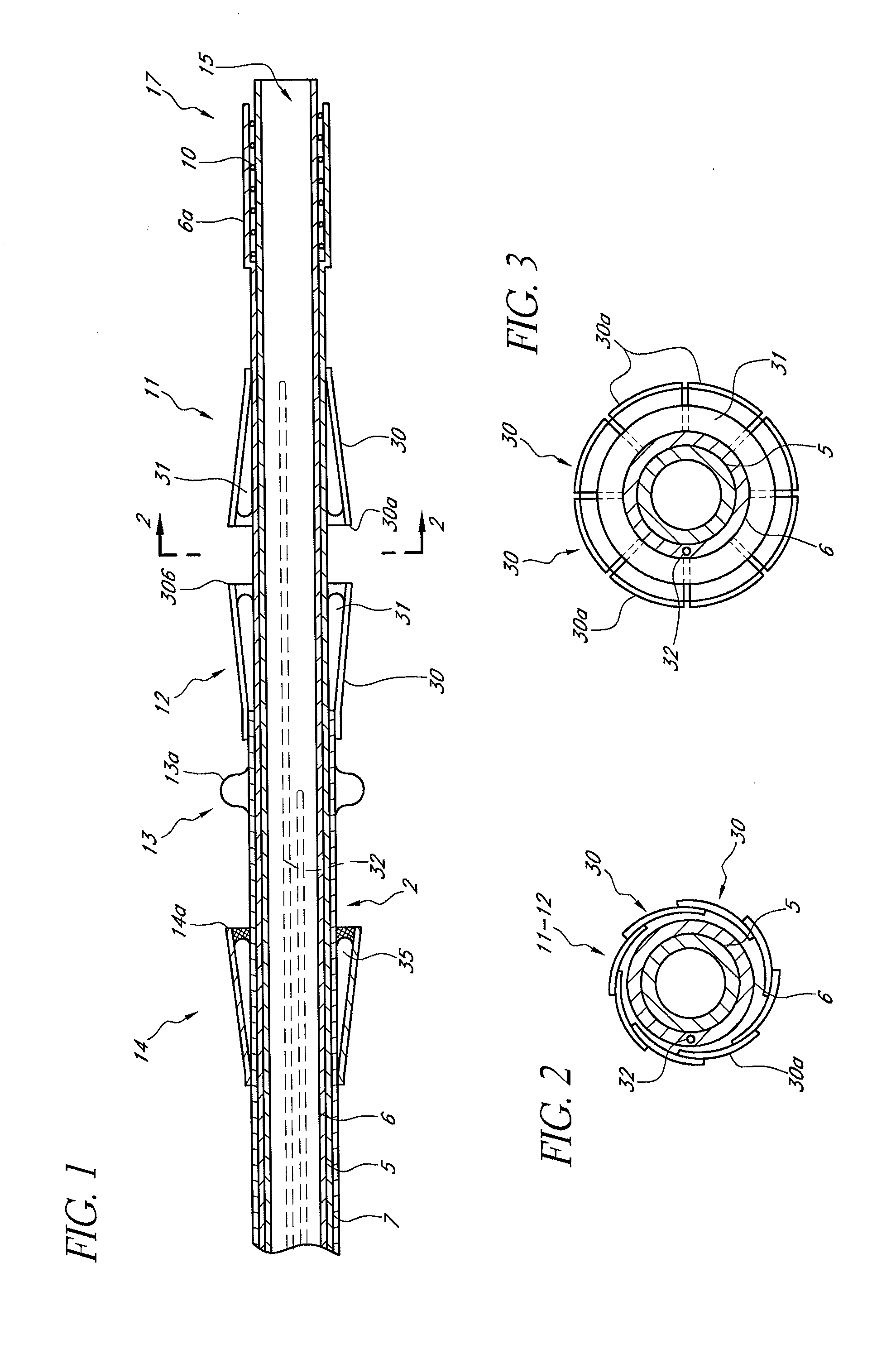
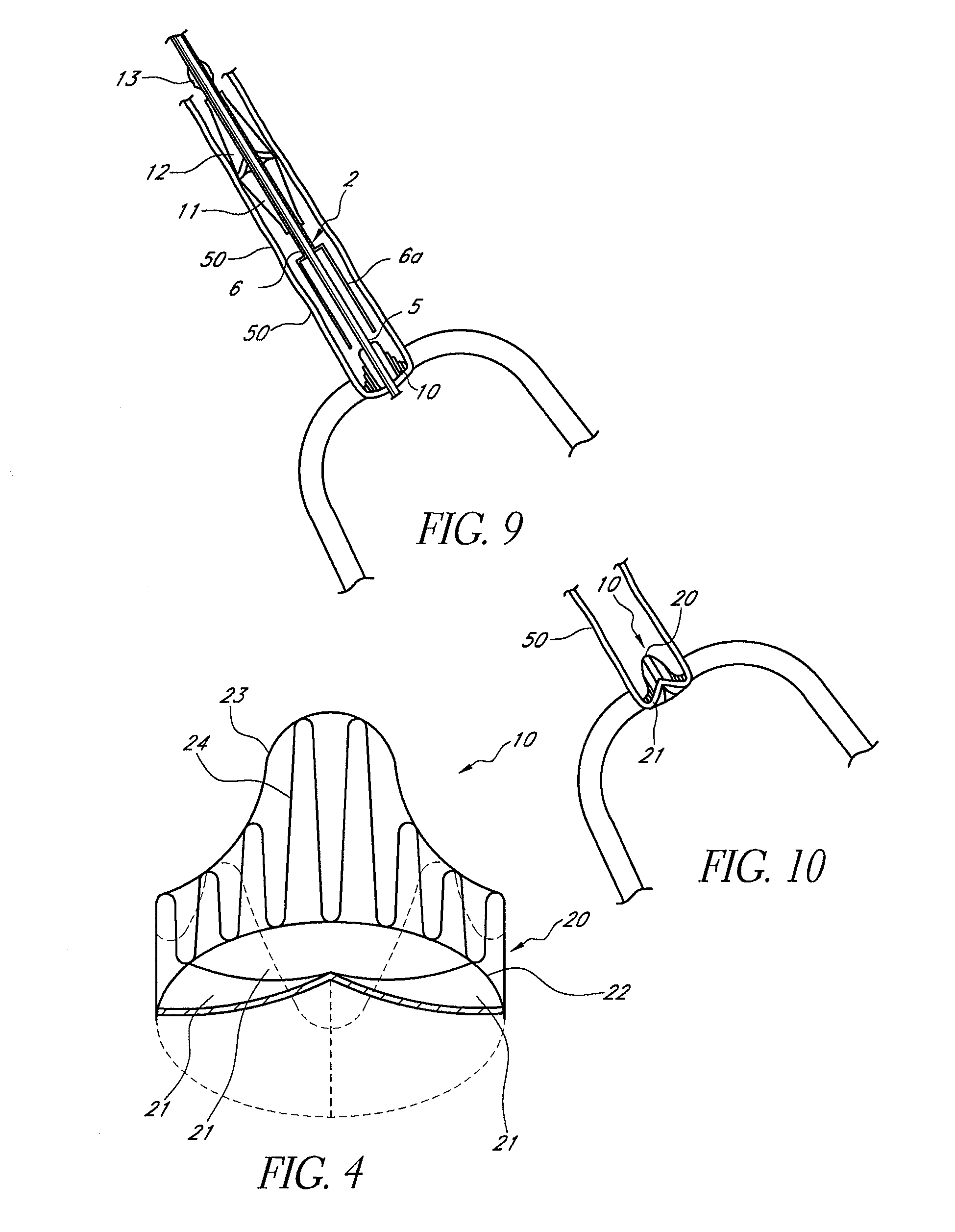
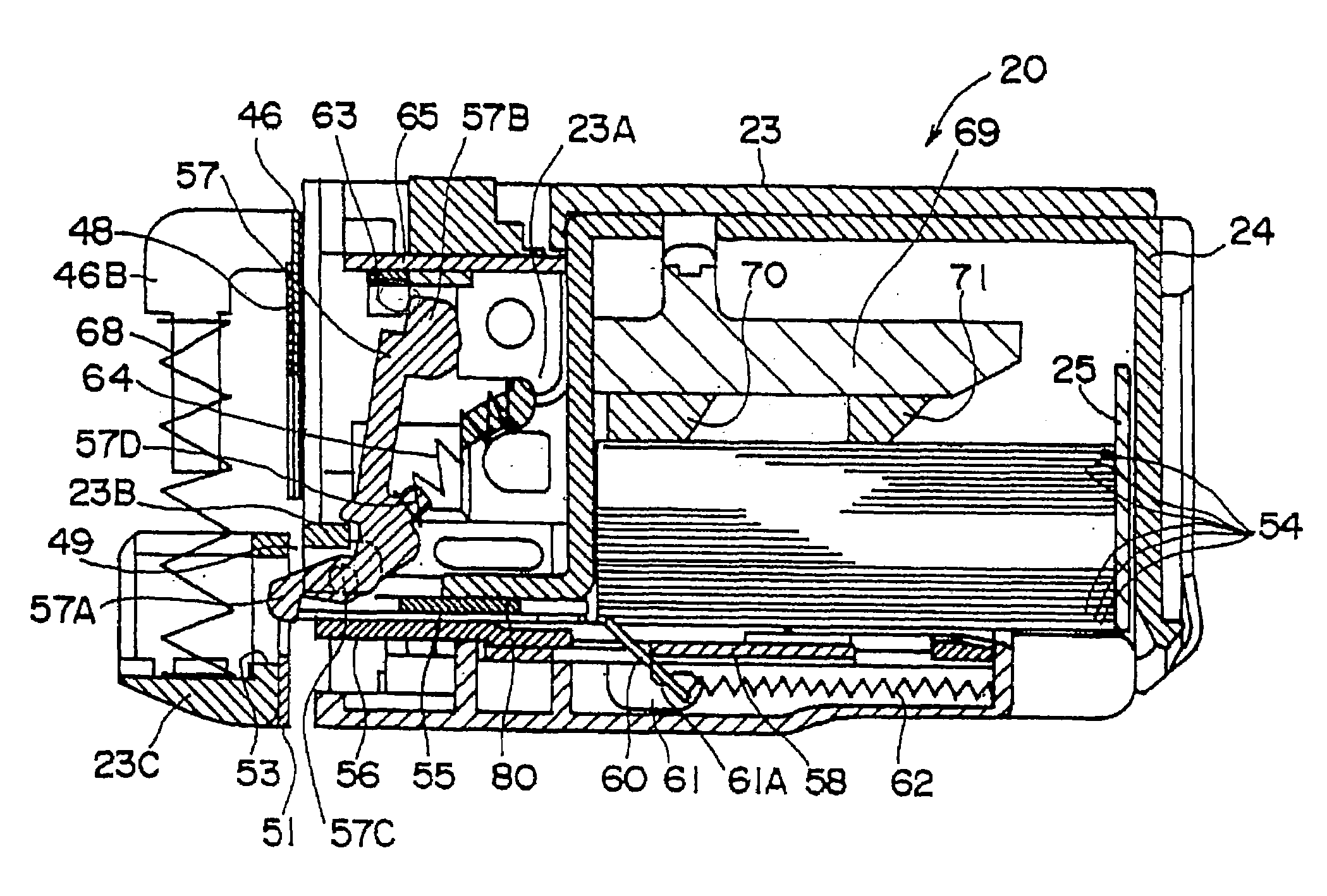
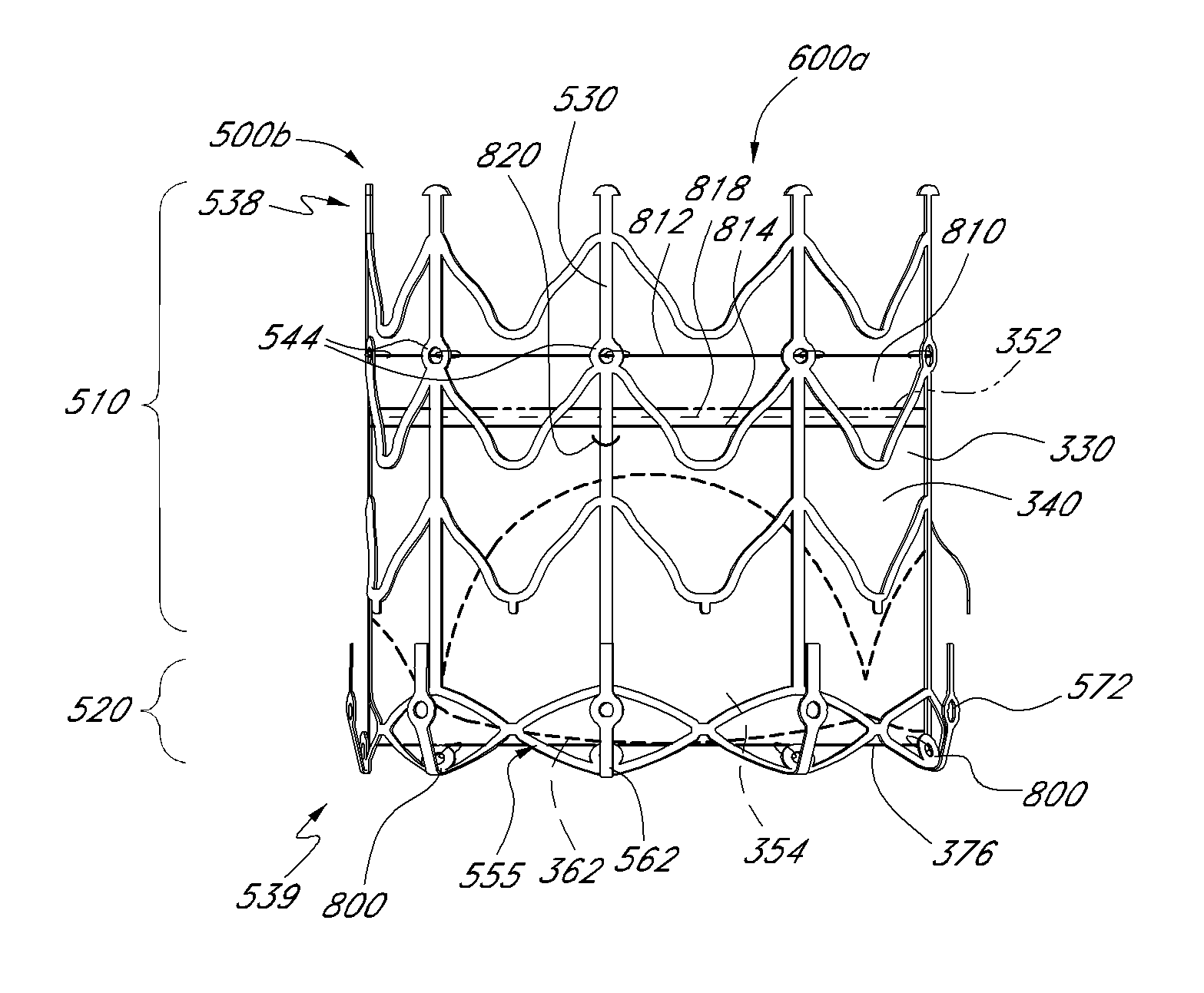
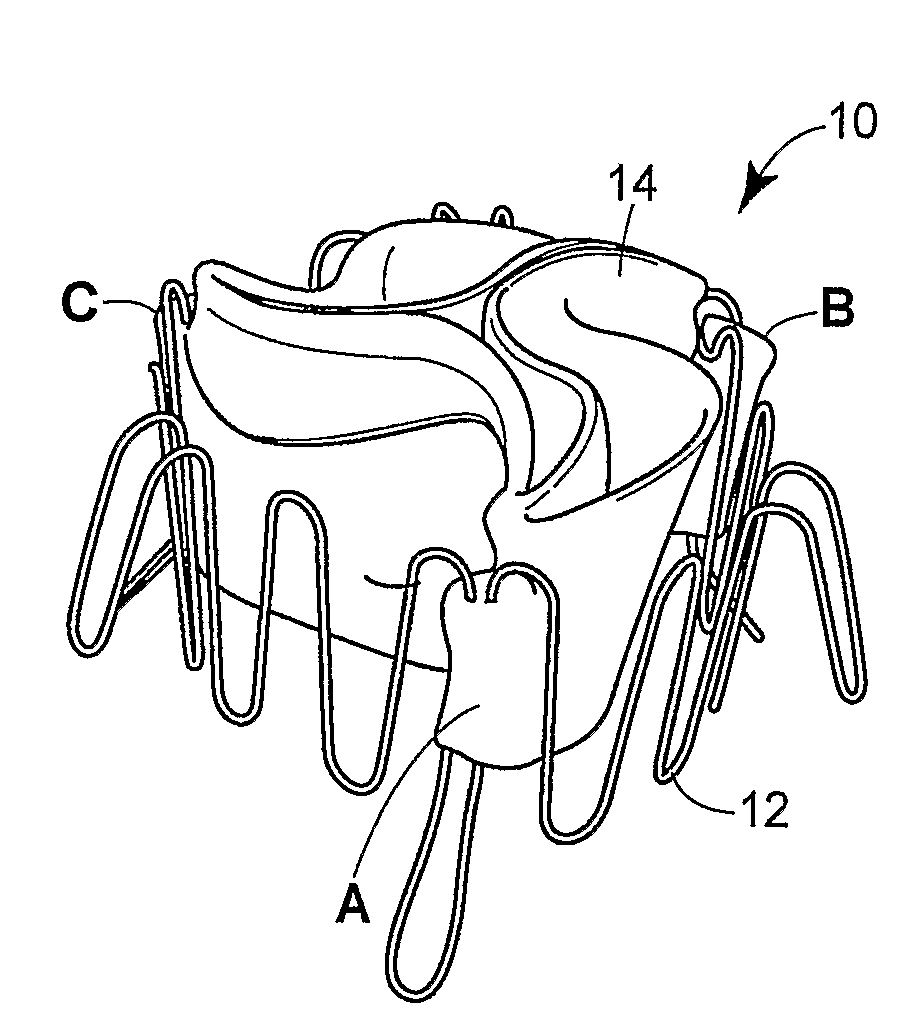
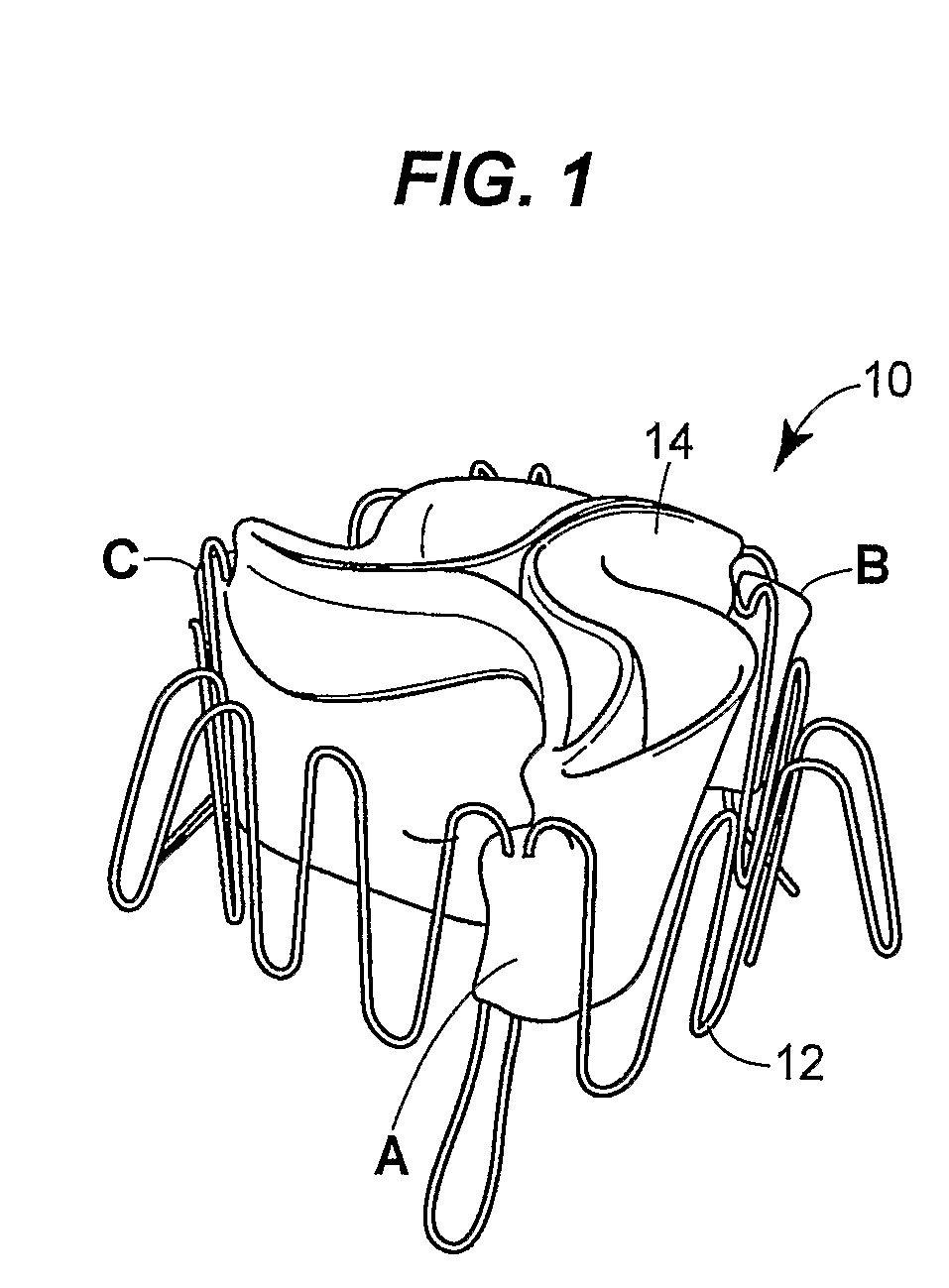
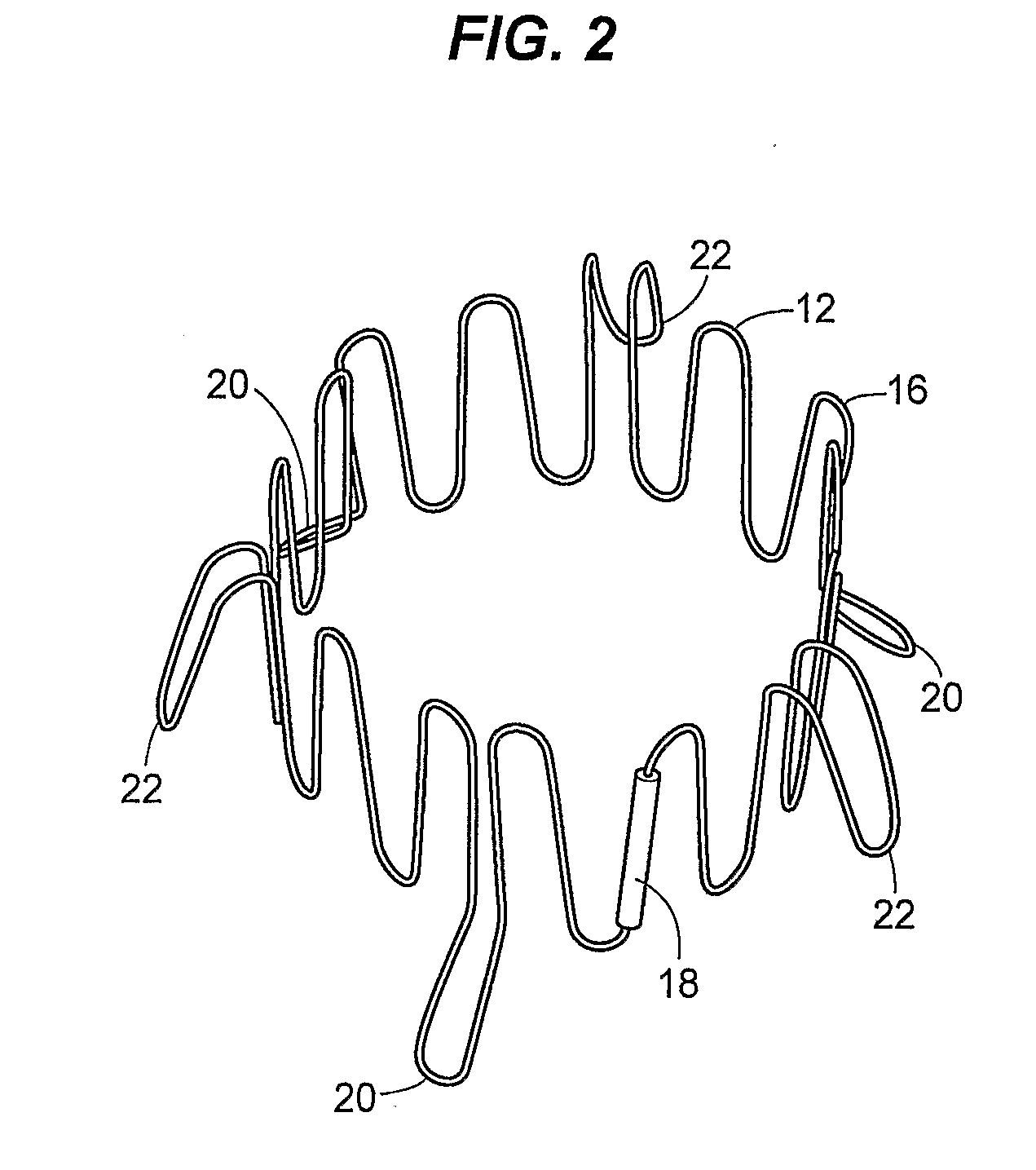
Prosthetic valve for transluminal delivery
InactiveUS7018406B2Preventing substantial migrationEliminate the problemBalloon catheterHeart valvesProsthesisCommissure
A prosthetic valve assembly for use in replacing a deficient native valve comprises a replacement valve supported on an expandable valve support. If desired, one or more anchor may be used. The valve support, which entirely supports the valve annulus, valve leaflets, and valve commissure points, is configured to be collapsible for transluminal delivery and expandable to contact the anatomical annulus of the native valve when the assembly is properly positioned. The anchor engages the lumen wall when expanded and prevents substantial migration of the valve assembly when positioned in place. The prosthetic valve assembly is compressible about a catheter, and restrained from expanding by an outer sheath. The catheter may be inserted inside a lumen within the body, such as the femoral artery, and delivered to a desired location, such as the heart. When the outer sheath is retracted, the prosthetic valve assembly expands to an expanded position such that the valve and valve support expand within the deficient native valve, and the anchor engages the lumen wall.
Owner:MEDTRONIC COREVALVE
Non-cylindrical prosthetic valve system for transluminal delivery
InactiveUS20070043435A1Preventing substantial migrationEliminate the problemBalloon catheterHeart valvesCoronary arteriesProsthesis
A prosthetic valve assembly for use in replacing a deficient native valve comprises a replacement valve supported on an expandable prosthesis frame. If desired, one or more expandable anchors may be used. The prosthesis frame, which entirely supports the valve annulus, valve leaflets, and valve commissure points, is configured to be collapsible for transluminal delivery and expandable to contact the anatomical annulus of the native valve when the assembly is properly positioned. Portions of the prosthesis frame may expand to a preset diameter to maintain coaptivity of the replacement valve and to prevent occlusion of the coronary ostia. The prosthesis frame is compressible about a catheter, and restrained from expanding by an outer sheath. The catheter may be inserted inside a lumen within the body, such as the femoral artery, and delivered to a desired location, such as the heart. When the outer sheath is retracted, the prosthesis frame expands to an expanded position such that the valve and prosthesis frame expand at the implantation site and the anchor engages the lumen wall. The prosthesis frame has a non-cylindrical configuration with a preset maximum expansion diameter region about the valve opening to maintain the preferred valve geometry. The prosthesis frame may also have other regions having a preset maximum expansion diameter to avoid blockage of adjacent structures such as the coronary ostia.
Owner:MEDTRONIC COREVALVE
Bioabsorbable Polymer, Bioabsorbable Composite Stents
InactiveUS20080249608A1Simple and inexpensive to manufactureVarying of ductilityStentsSurgeryMetallic materialsMedical device
Biocompatible materials may be configured into any number of implantable medical devices including intraluminal stents. The biocompatible material may comprise metallic and non-metallic materials in hybrid structures. In one such structure, a device may be fabricated with one or more elements having an inner metallic core that is biodegradable with an outer shell formed from a polymeric material that is biodegradable. Additionally, therapeutic agents may be incorporated into the microstructure or the bulk material.
Owner:CORDIS CORP
Light emitting material and organic light-emitting device
InactiveUS7396598B2High efficiency of light emissionKeep energy smallDischarge tube luminescnet screensGroup 8/9/10/18 element organic compoundsFluorescenceTriplet state
Owner:SAMSUNG ELECTRONICS CO LTD
Electroluminescent (EL) devices
InactiveUS6225467B1Improve efficiencyIncreased durabilitySilicon organic compoundsElectroluminescent light sourcesArylHalogen
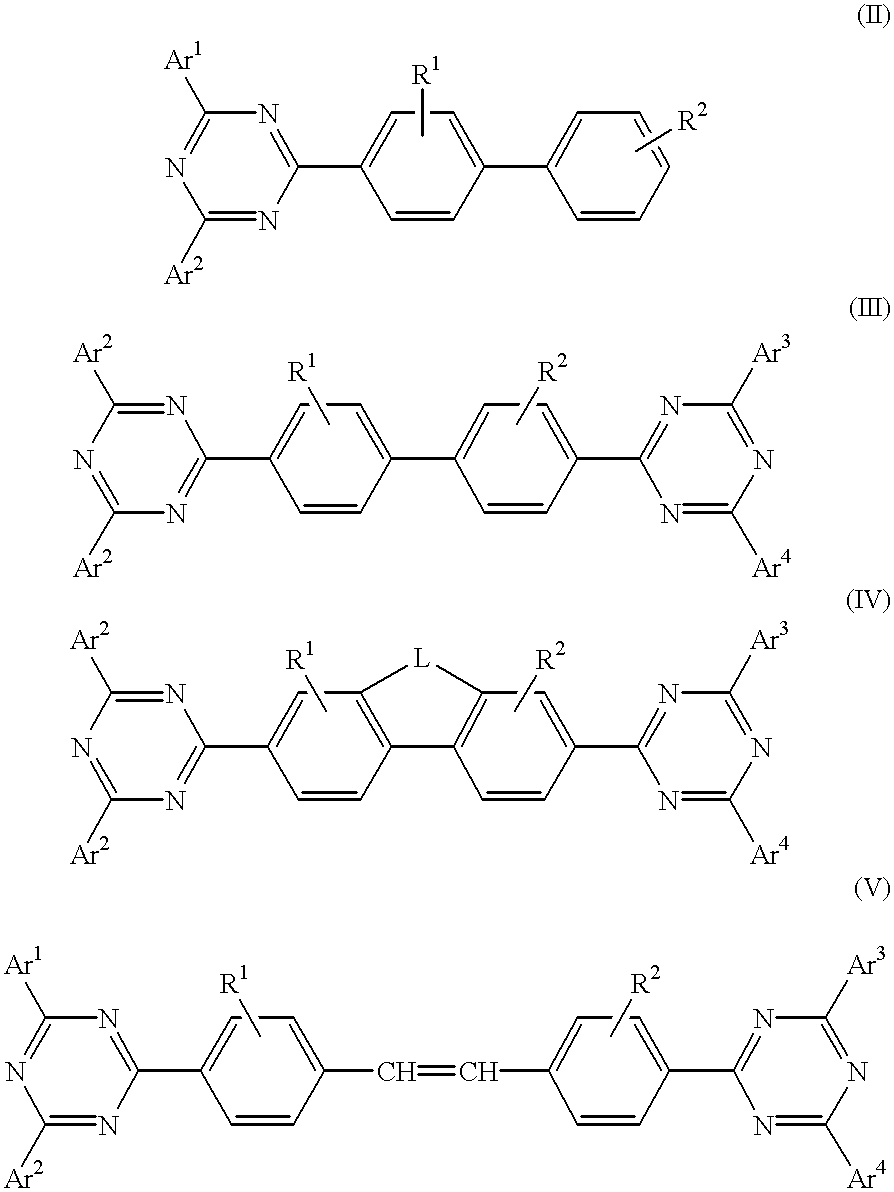
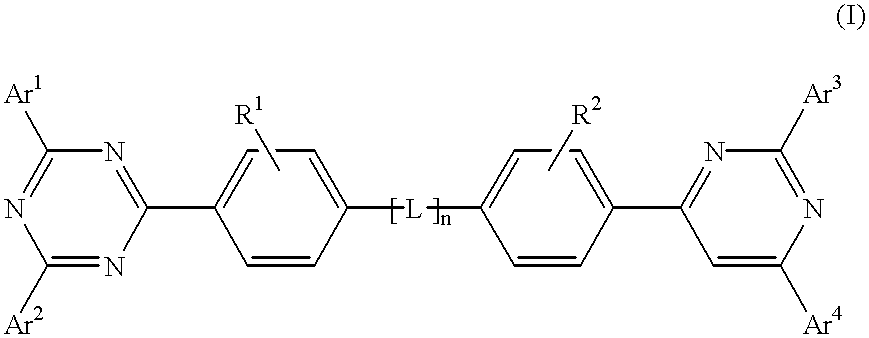
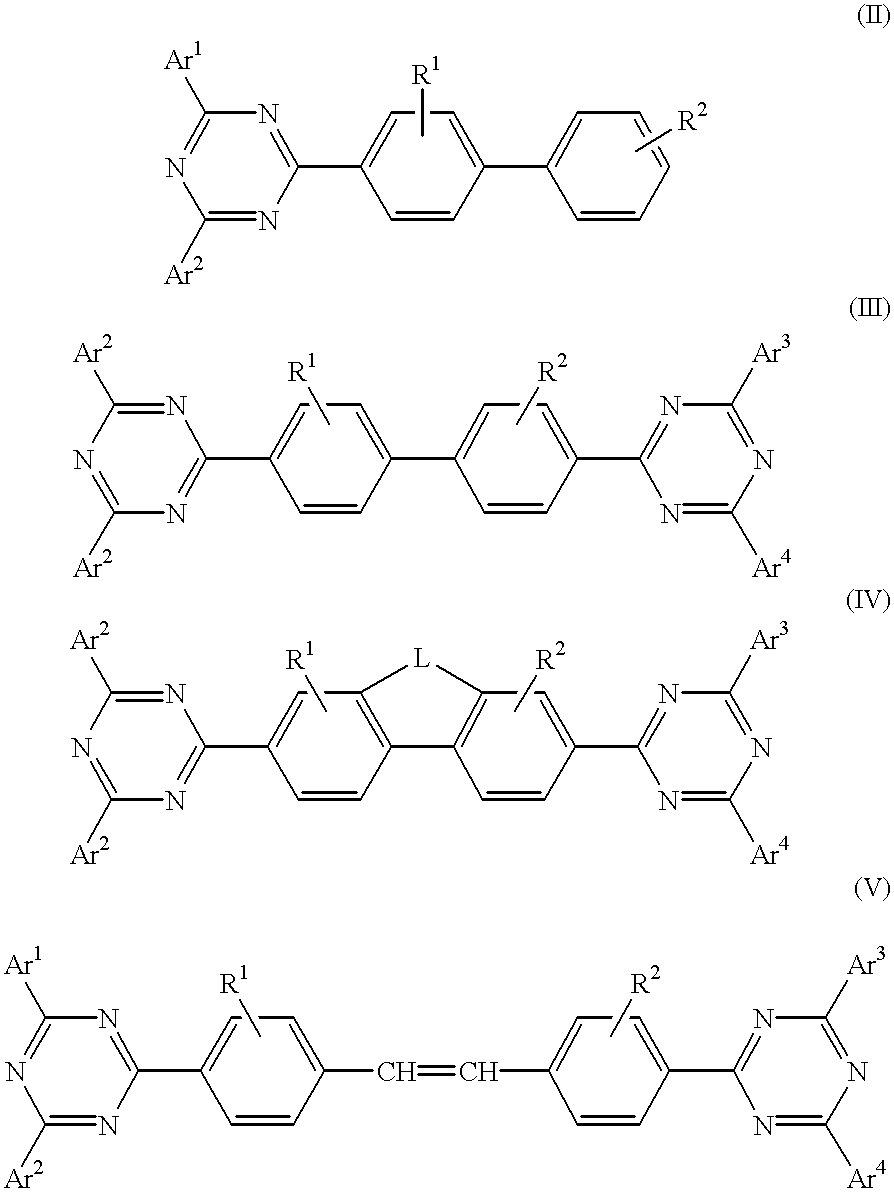
The triazinewherein Ar1, Ar2, Ar3, and Ar4 are each independently an aryl; R1 and R2 are substituents selected from the group consisting of hydrogen, an alkyl, an aryl, an alkoxy, a halogen atom, and a cyano; R3 and R4 are each a divalent group L selected from the group consisting of -C(R'R'')-, alkylene, an oxygen atom, a sulfur atom, and -Si(R'R'')-, wherein R' and R'' are selected from the group consisting of hydrogen, alkyl, alkoxy, and aryl.
Owner:LG DISPLAY CO LTD
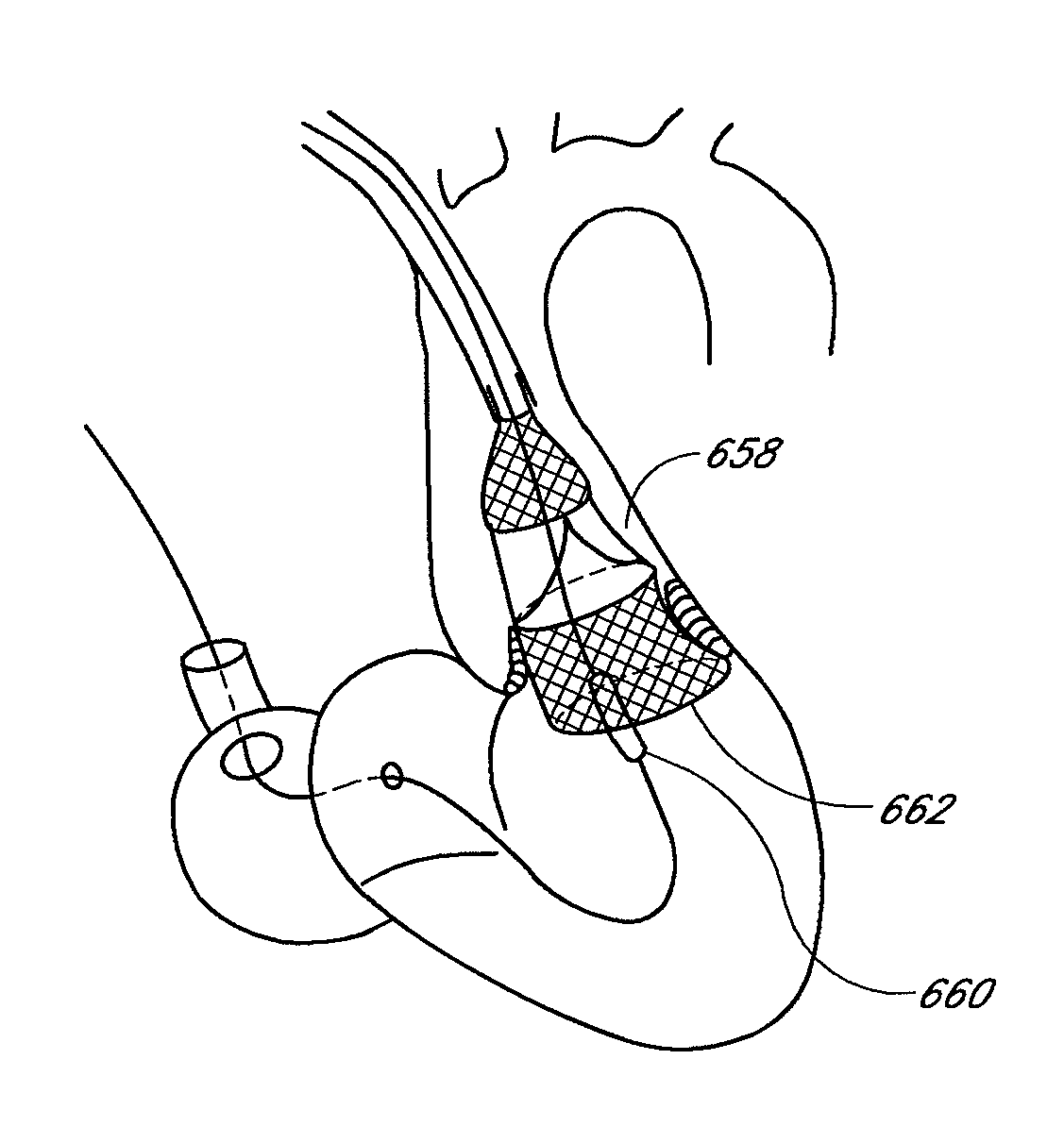
System and method for transapical delivery of an annulus anchored self-expanding valve
ActiveUS20080140189A1Preventing substantial migrationEliminate the problemStentsBalloon catheterLimited accessCardiac muscle
A prosthetic valve assembly for use in replacing a deficient native valve comprises a replacement valve supported on an expandable prosthesis frame. The valve may be delivered transluminally or transmyocardially using a thorascopic or other limited access approach using a delivery catheter. Preferably, the initial partial expansion of the valve is performed against the native valve annulus to provide adequate anchoring and positioning of the valve as the remaining portions of the valve expand. The valve may be delivered using a retrograde or antegrade approach. When delivered using a retrograde approach, a delivery catheter with a pull-back sheath may be used, while antegrade delivery is preferably performed with a delivery catheter with a push-forward sheath that releases the proximal end of the valve first.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
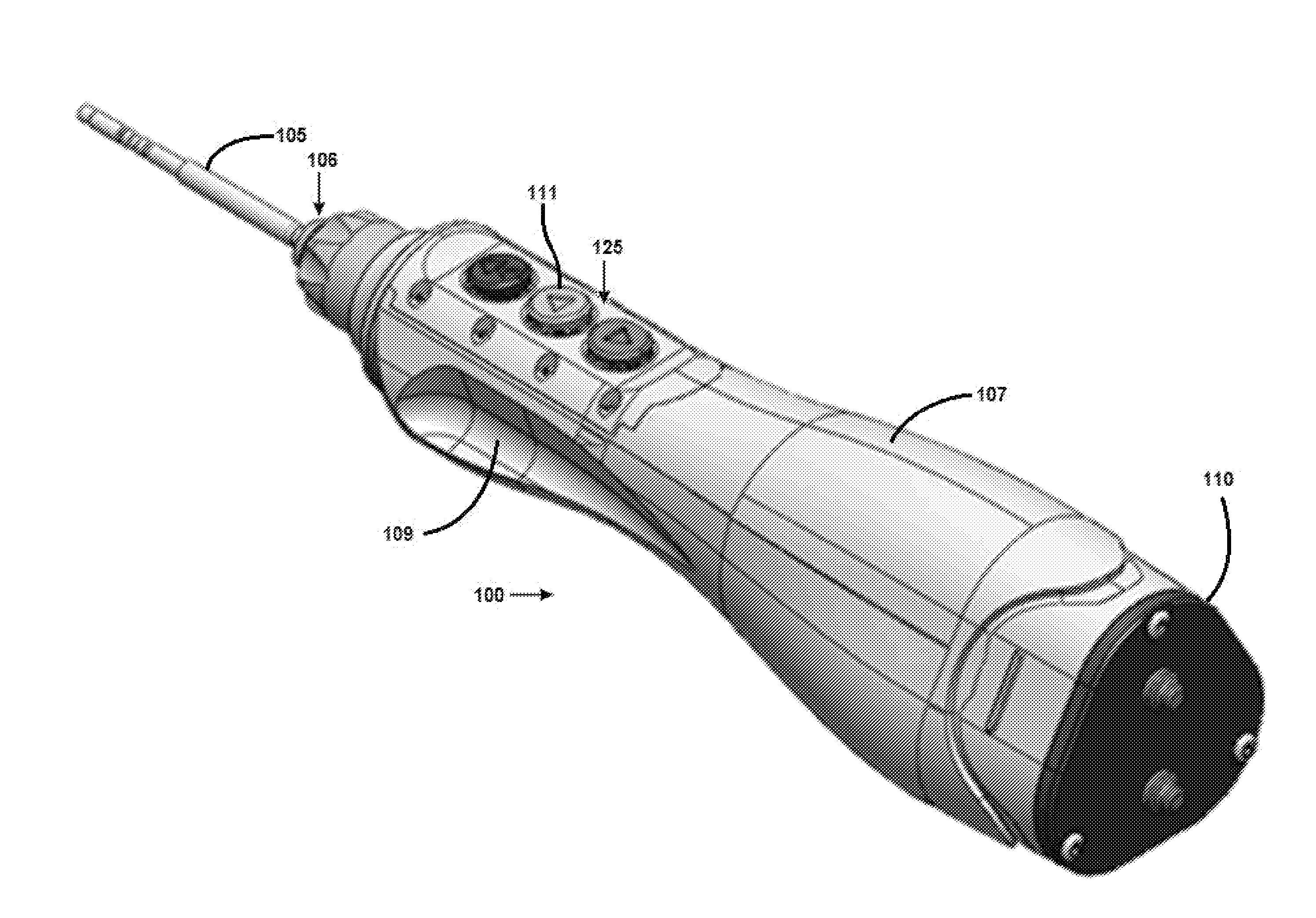
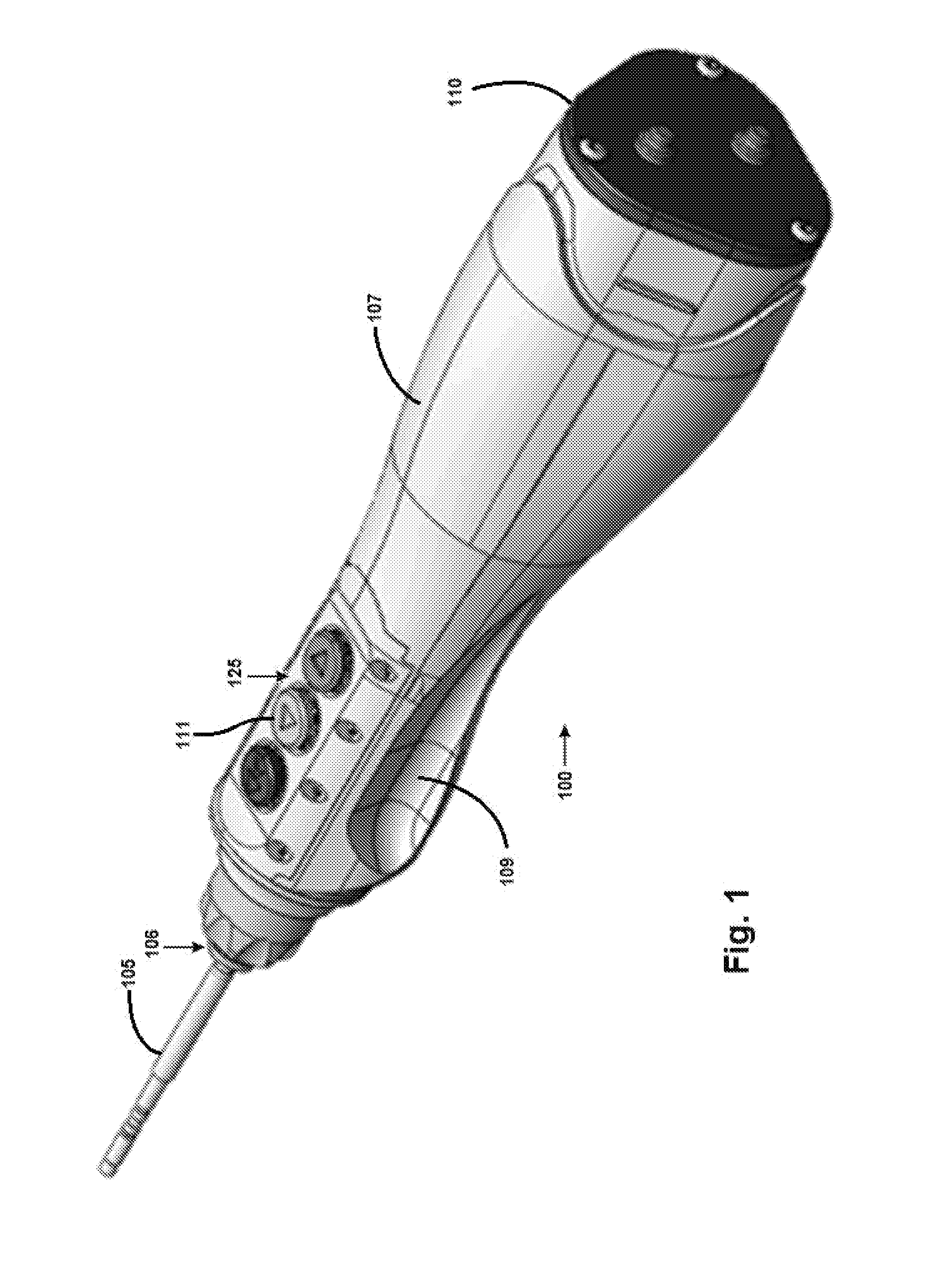
Surgical Handpiece
InactiveUS20150201918A1Improve operationIncreased durabilityDental implantsDiagnosticsSurgical instrumentationEngineering
Embodiments of the present invention provide a unique surgical handpiece having improved operation, durability and reliability. In one embodiment, the present invention provides a motorized handheld surgical instrument having one or more sensors for sensing motion, position, pressure, humidity, and various other environmental conditions relevant to the operation and maintenance of the surgical instrument.
Owner:OSSEODYNE SURGICAL SOLUTIONS LLC
Coating composition for multiple hydrophilic applications
InactiveUS20030203991A1Tough and durable and printable surfaceImprove wettabilityOther chemical processesSynthetic resin layered productsColloidWear resistance
A coating composition is disclosed which comprises an aqueous polymeric matrix, a hydrophilic polymer, a colloidal metal oxide and a crosslinker. The coating composition when applied on medical devices is hydrophilic, shows improved lubricity, abrasion resistance and substrate adhesion on metallic or plastic substrates. The coating also shows improved water sheeting thus providing the coated substrates with anti-fog properties. The coating absorbs aqueous dye or stain solutions making the substrate suitable for printing.
Owner:HYDROMER INC
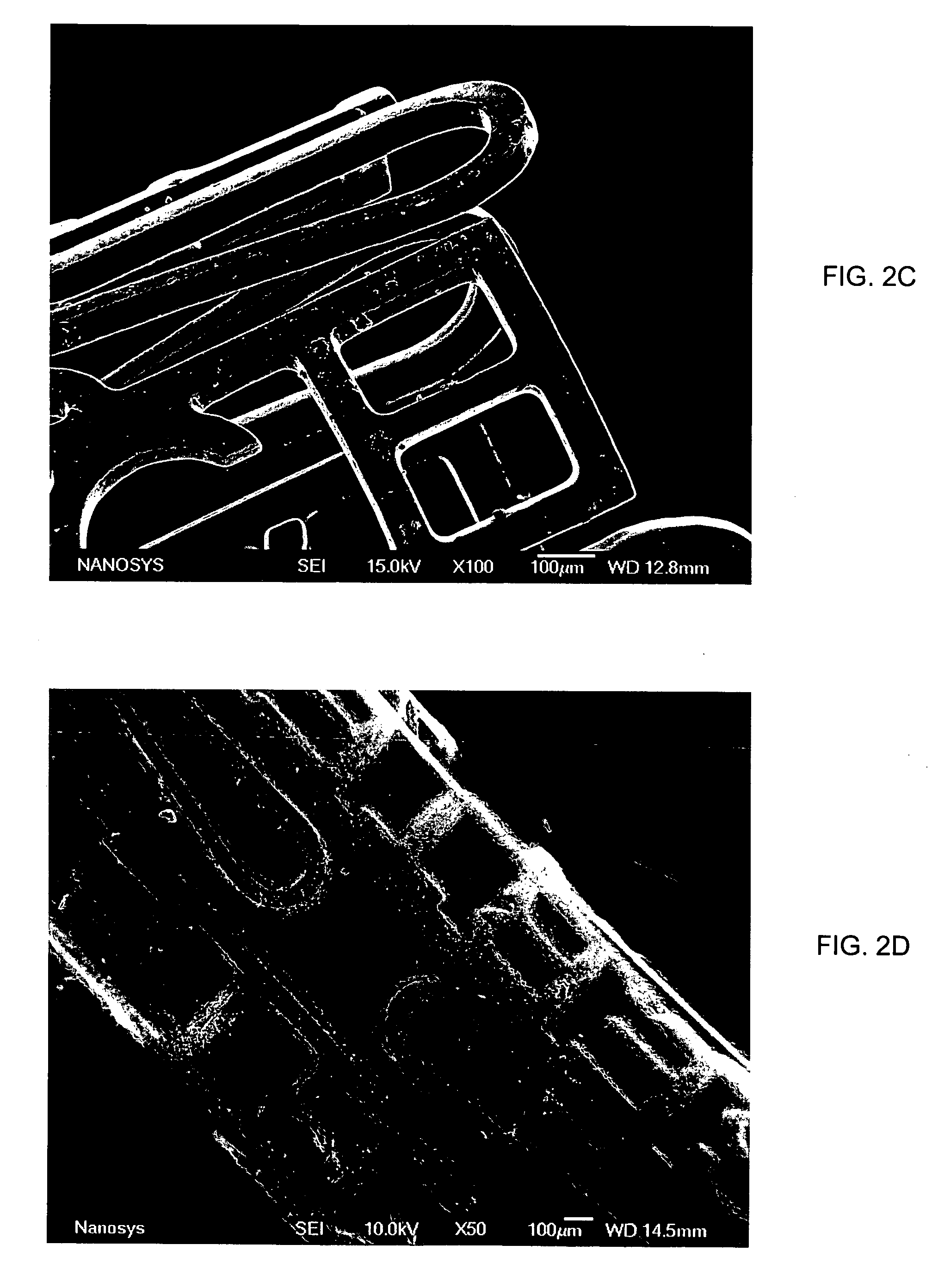
Medical device applications of nanostructured surfaces
InactiveUS20050038498A1Fine surfacePrevent/reduce bio-foulingAntibacterial agentsMaterial nanotechnologyFiberNanofiber
This invention provides novel nanofiber enhanced surface area substrates and structures comprising such substrates for use in various medical devices, as well as methods and uses for such substrates and medical devices.
Owner:NANOSYS INC
Staple detecting mechanism of electric stapler
InactiveUS7048165B2Improve detection accuracyIncreased durabilityStapling toolsNailing toolsMiniaturizationEngineering
A staple sheet-detecting mechanism of an electric stapler having improved detection accuracy and miniaturization has a passage for feeding a staple sheet composed of straight forward staples arranged in parallel, and a forming plate and driver arranged above an anvil of the passage. A staple is inserted into papers located under the staple by moving the forming plate and the driver to a side of the staple. Above the anvil, where the forming plate waits, is a sensor (rocking member) of which one end contacts a tip edge of the staple sheet in a feeding direction, while the other end turns on or off an interrupter (detecting element). A rocking fulcrum of the sensor is provided biased to a side of the staple sheet in the passage. The forming plate and the driver are provided with recessed portions and, respectively, for allowing the sensor to rock.
Owner:MAX CO LTD
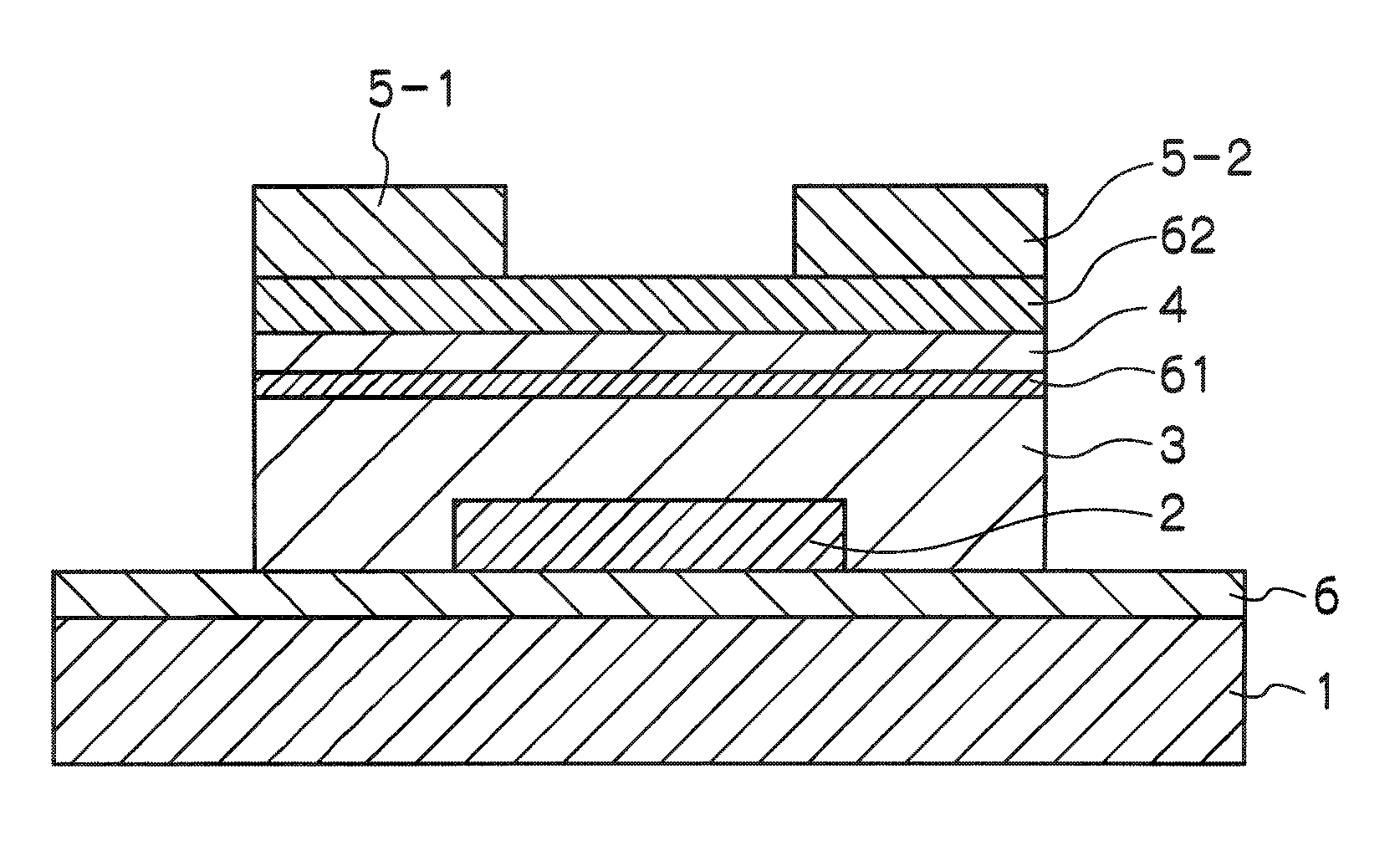
Thin film field effect transistor
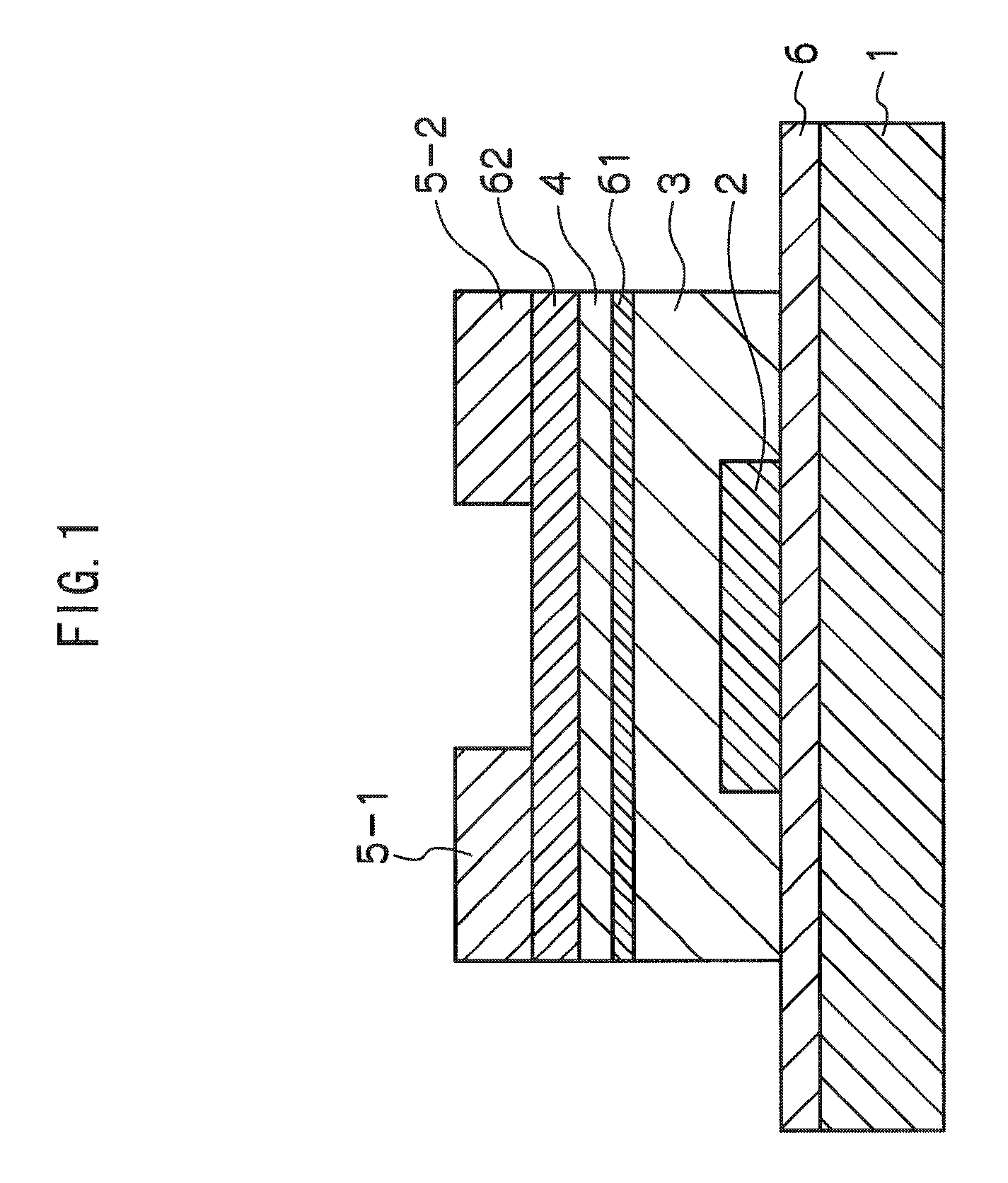
ActiveUS8203143B2Increased durabilityImprove stabilitySemiconductor devicesInterface layerField-effect transistor
A thin film field effect transistor has at least a gate electrode 2, a gate insulating layer 3, an active layer 4, a source electrode 5-1 and a drain electrode 5-2 on a substrate 1. The active layer includes an amorphous oxide semiconductor including at least In and Zn, a first interface layer 61 is disposed between the gate insulating layer and the active layer such that it is adjacent to at least the active layer, and a second interface layer is disposed on the opposite side of the active layer with respect to the first interface layer such that it is adjacent to the active layer. A content of Ga or Al in the amorphous oxide semiconductor of each of the first interface layer and the second interface layer is higher than a content of Ga or Al in the amorphous oxide semiconductor of the active layer.
Owner:SAMSUNG DISPLAY CO LTD
Medical device applications of nanostructured surfaces
InactiveUS20060204738A1Improve adhesionIncrease frictionBiocideMaterial nanotechnologyOsteoblastNanofiber
This invention provides novel nanofiber enhanced surface area substrates and structures comprising such substrates for use in various medical devices, as well as methods and uses for such substrates and medical devices. In one particular embodiment, methods for enhancing cellular functions on a surface of a medical device implant are disclosed which generally comprise providing a medical device implant comprising a plurality of nanofibers (e.g., nanowires) thereon and exposing the medical device implant to cells such as osteoblasts.
Owner:GLO TECH LLC
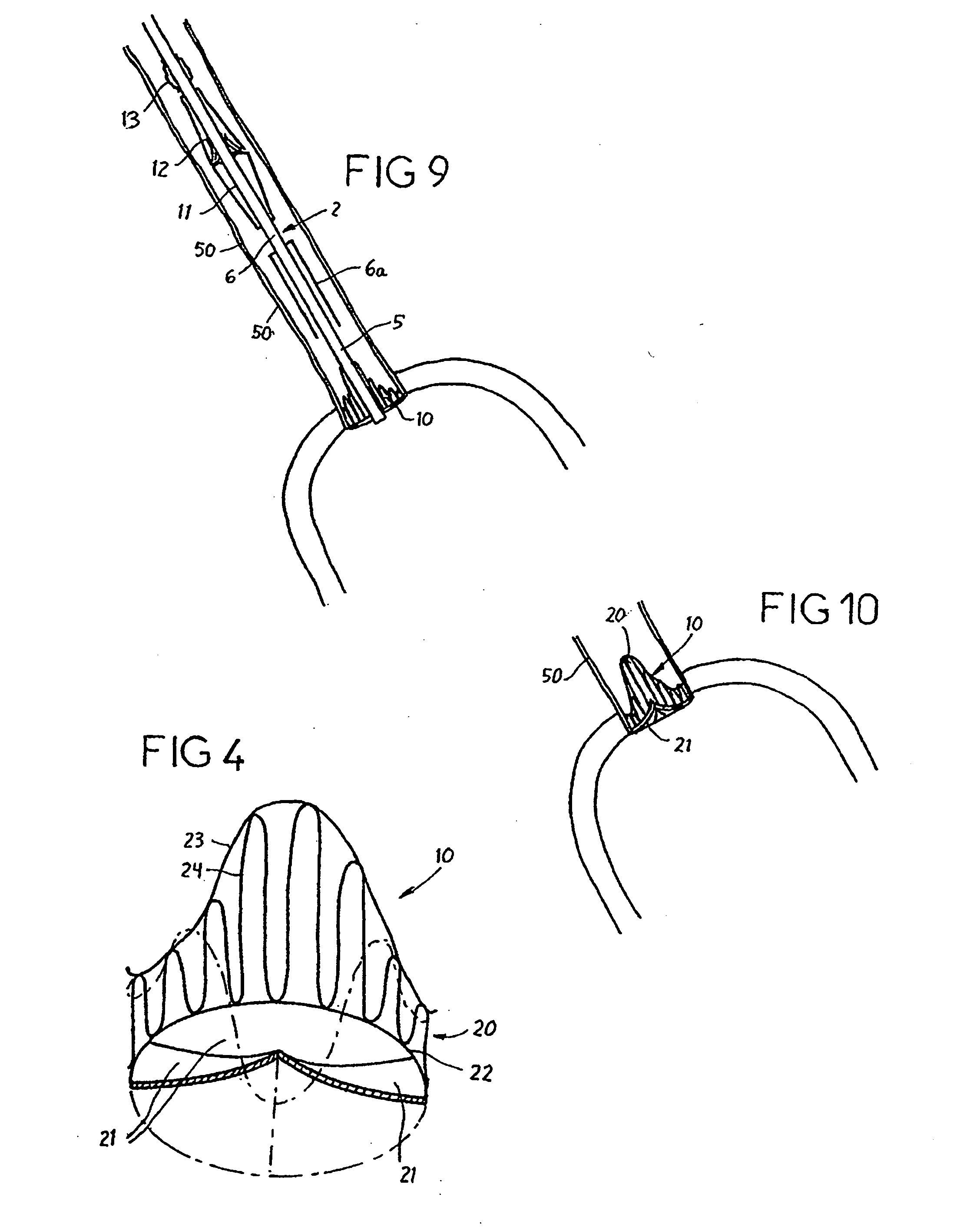
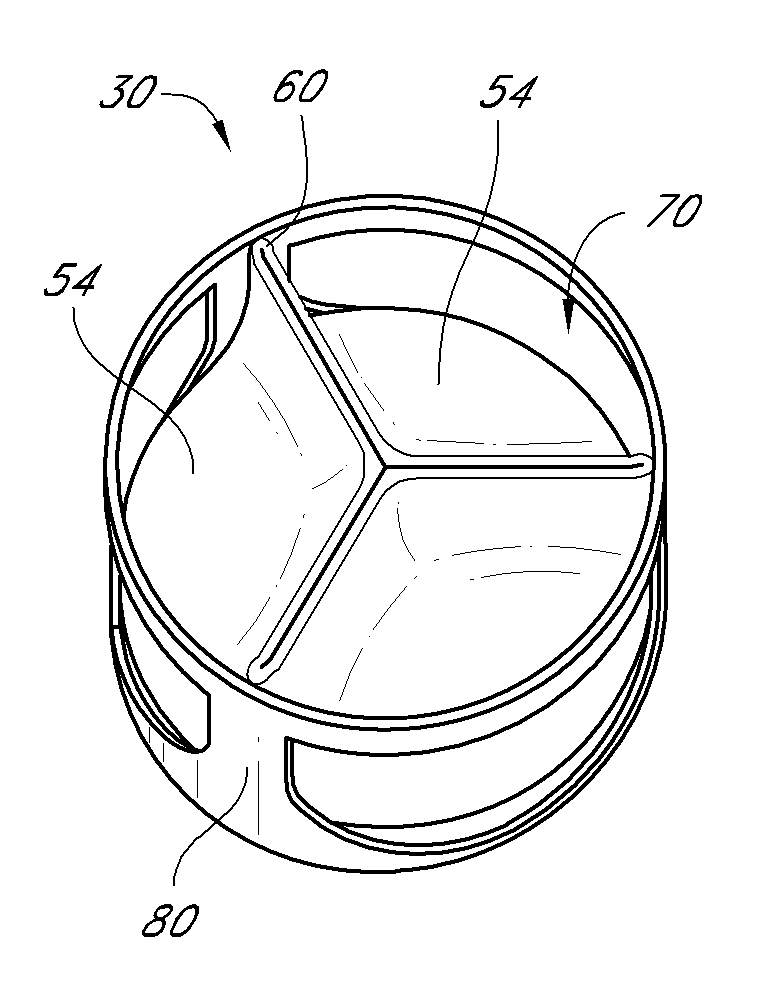
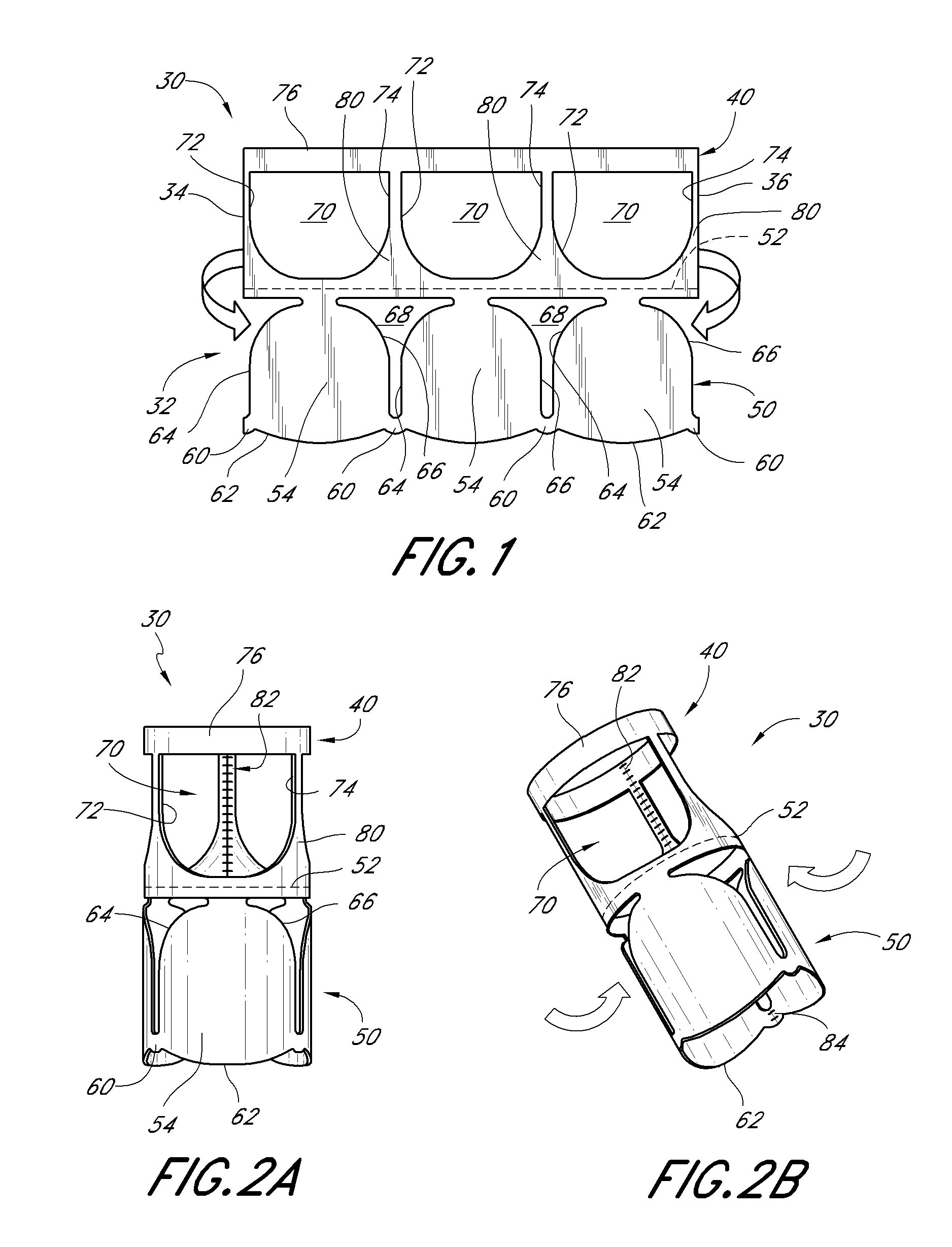
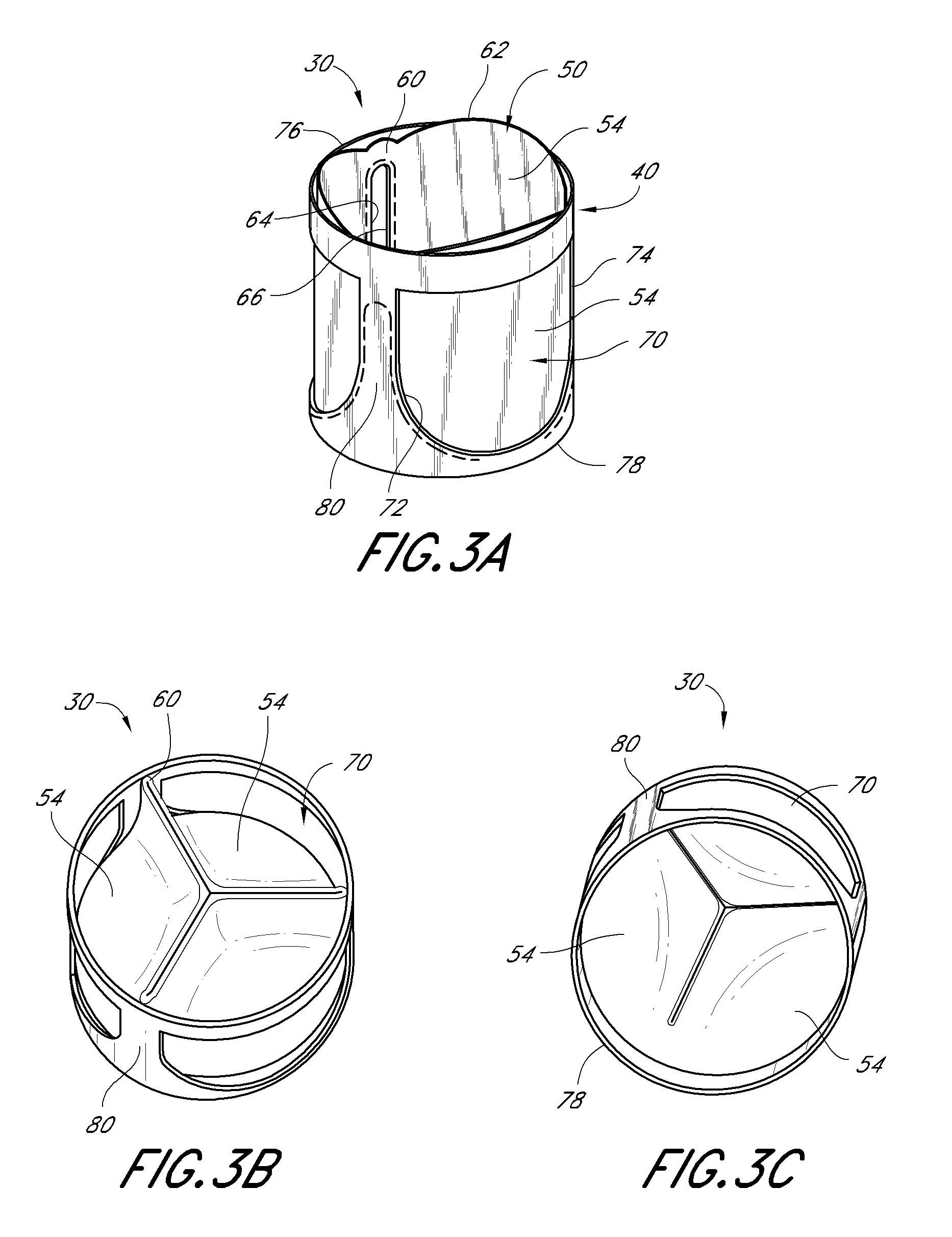
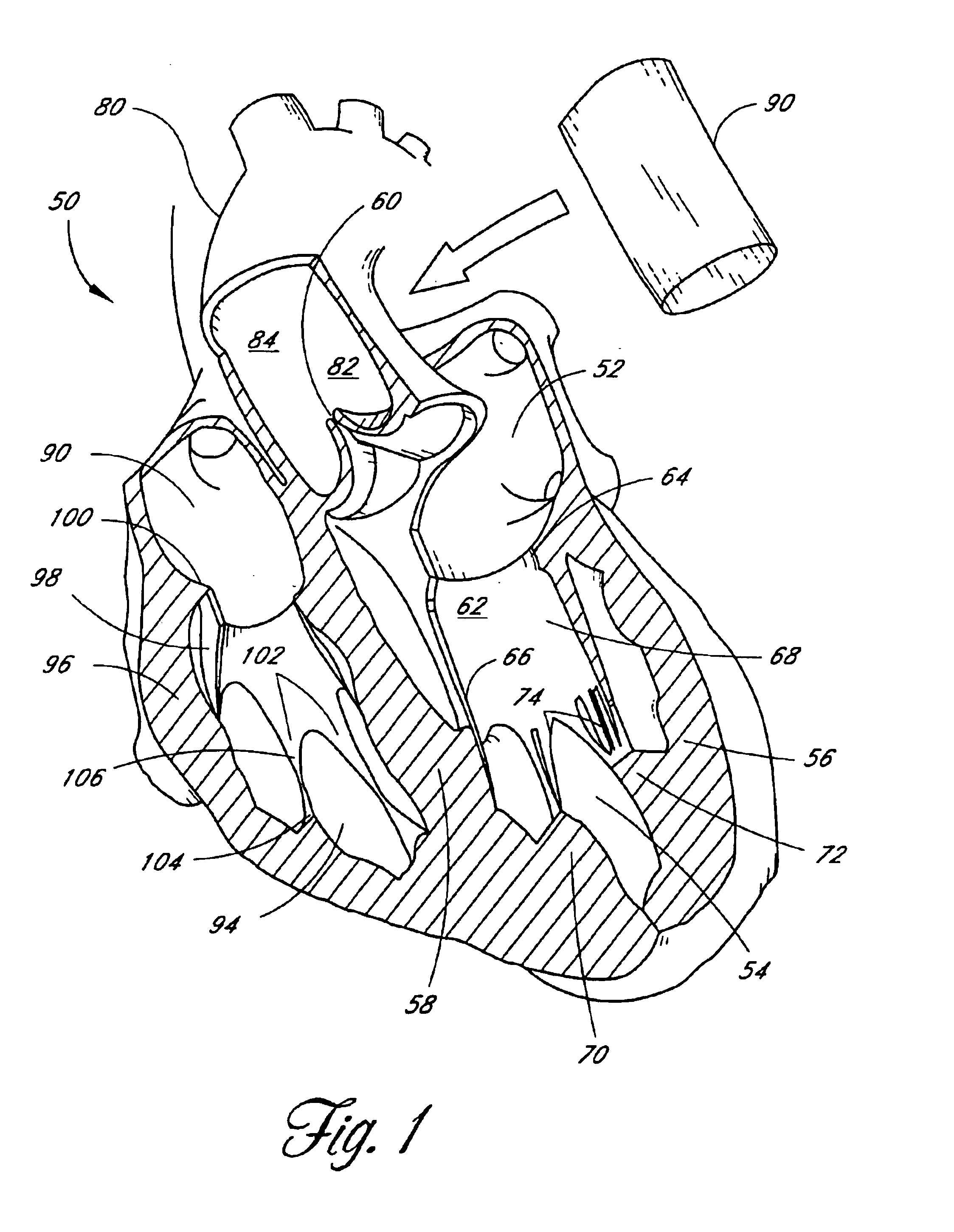
Heart valve
A heart valve includes a valve body made of a flexible material such as pericardium. The valve body is made of two layers of material, an outer layer, and an inner layer that defines a plurality of leaflets. The leaflets of the inner layer are attached to the outer layer. In some embodiments the valve body is made by cutting a single piece of flat source tissue, folding the cut tissue and forming it into a tubular pattern having the inner and outer layers. The multi-layer valve body can be mounted on a stent for delivery within a patient's heart.
Owner:EDWARDS LIFESCI CARDIAQ
Heart valve
A replacement heart valve can comprise a valve body and an elongate frame. The valve body can define a plurality of leaflets adapted to move between an open state and a coapted state. The elongate frame can be configured to move between a radially compacted state and a radially expanded state, the frame comprising a plurality of struts. Stitches attach the valve body to the plurality of struts. At least one of the stitches can be a loose stitch configured to freely slide over the corresponding strut when the frame is moved between the radially compacted and radially expanded states.
Owner:EDWARDS LIFESCI CARDIAQ
Implants for replacing cartilage, with negatively-charged hydrogel surfaces and flexible matrix reinforcement
ActiveUS9314339B2Strong and durableStrong and secure anchoringFinger jointsWrist jointsFiberChemical agent
A permanent non-resorbable implant allows surgical replacement of cartilage in articulating joints, using a hydrogel material (such as a synthetic polyacrylonitrile polymer) reinforced by a flexible fibrous matrix. Articulating hydrogel surface(s) are chemically treated to provide a negative electrical charge that emulates the negative charge of natural cartilage, and also can be treated with halogenating, cross-linking, or other chemical agents for greater strength. For meniscal-type implants, the reinforcing matrix can extend out from the peripheral rim of the hydrogel, to allow secure anchoring to soft tissue such as a joint capsule. For bone-anchored implants, a porous anchoring layer enables tissue ingrowth, and a non-planer perforated layer can provide a supportive interface between the hard anchoring material and the softer hydrogel material.
Owner:FORMAE
Metal Complex, Light-Emitting Device, and Image Display Apparatus
InactiveUS20080210930A1Improve efficiencyIncrease brightnessIndium organic compoundsSolid-state devicesLight emitting deviceCoordination complex
Owner:CANON KK
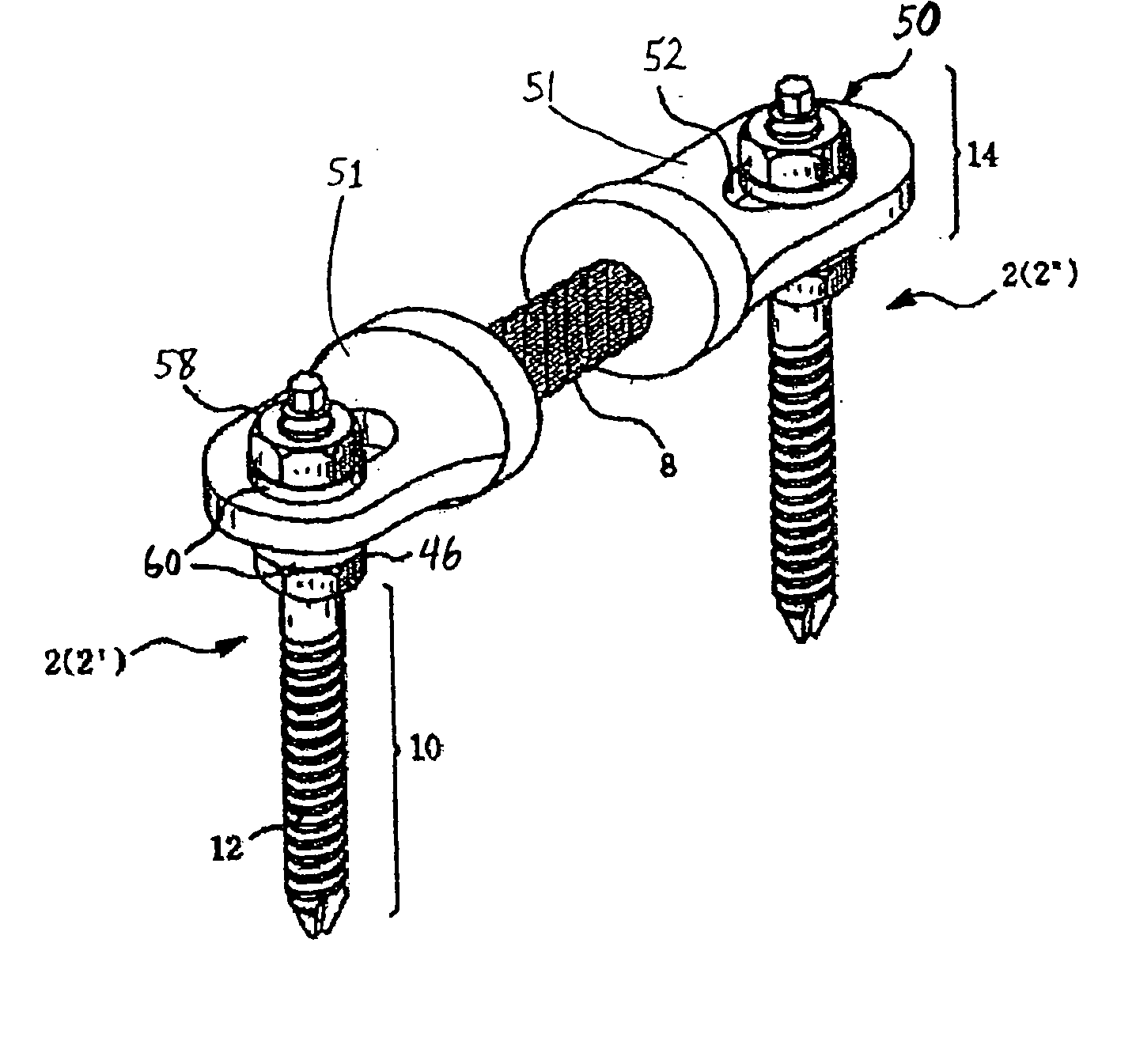
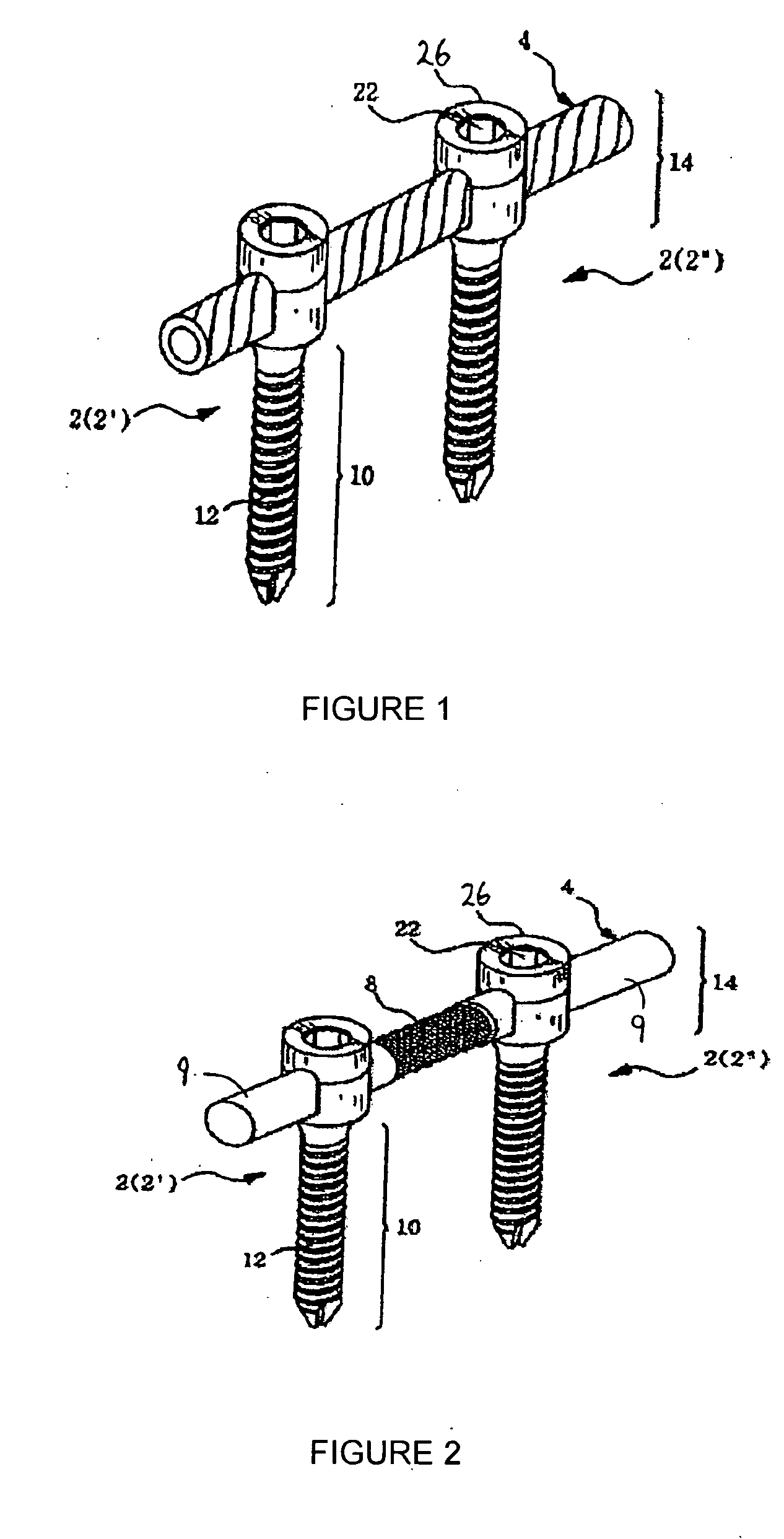
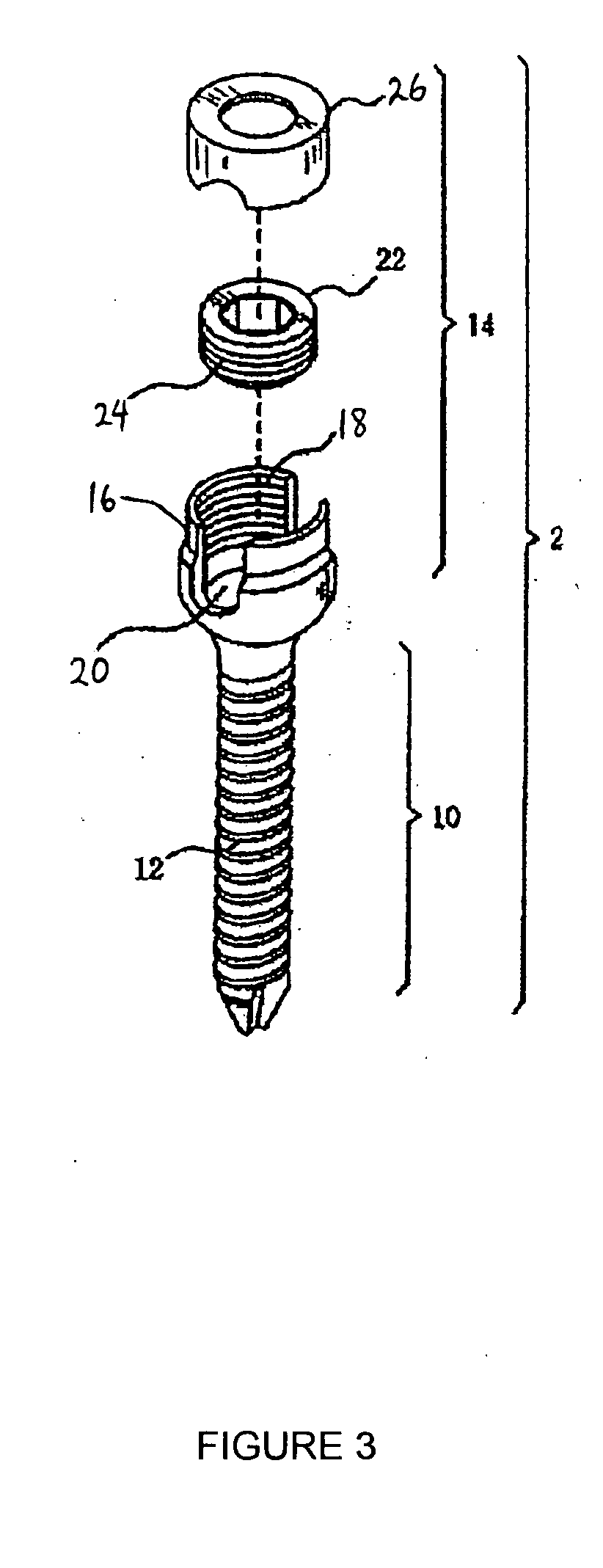
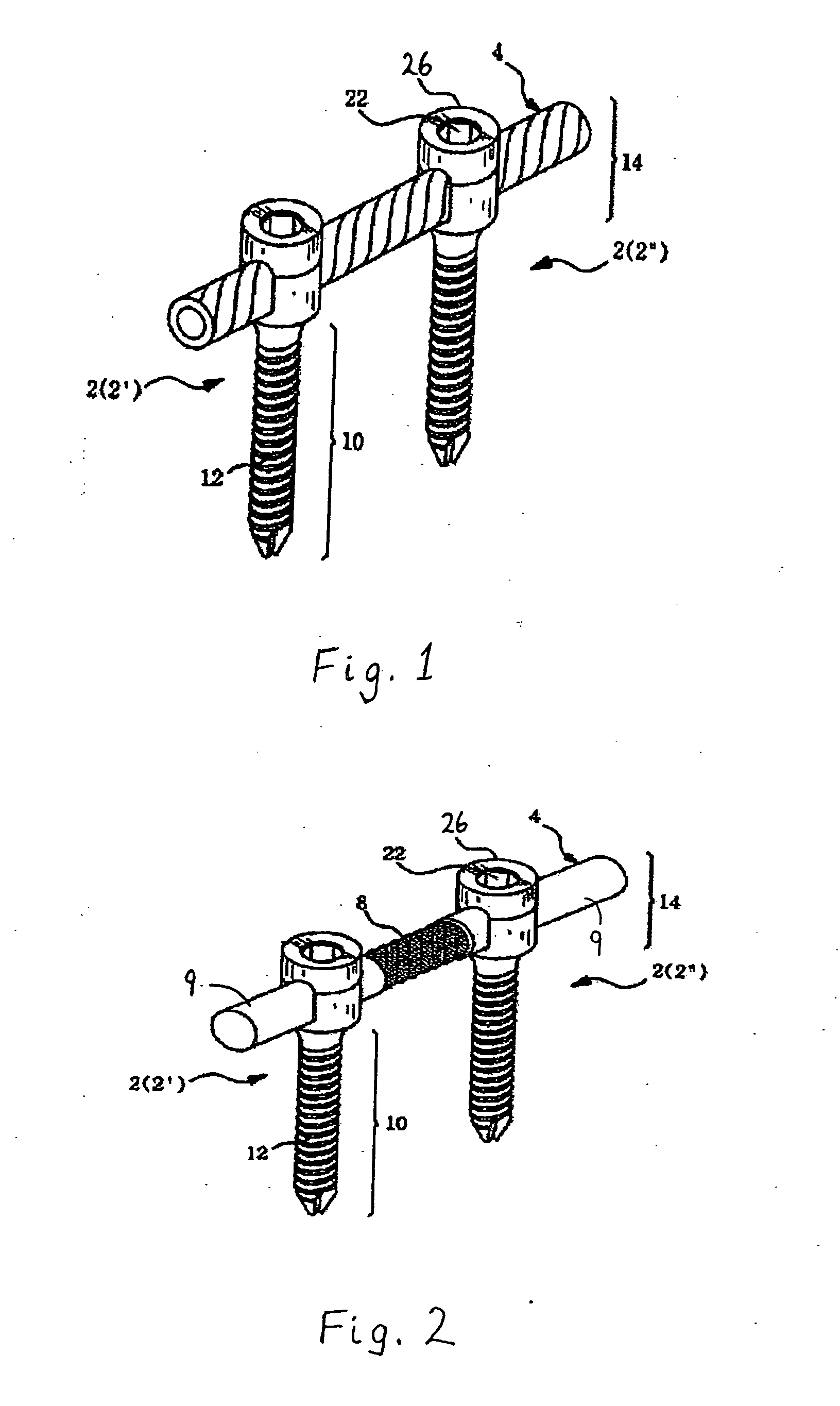
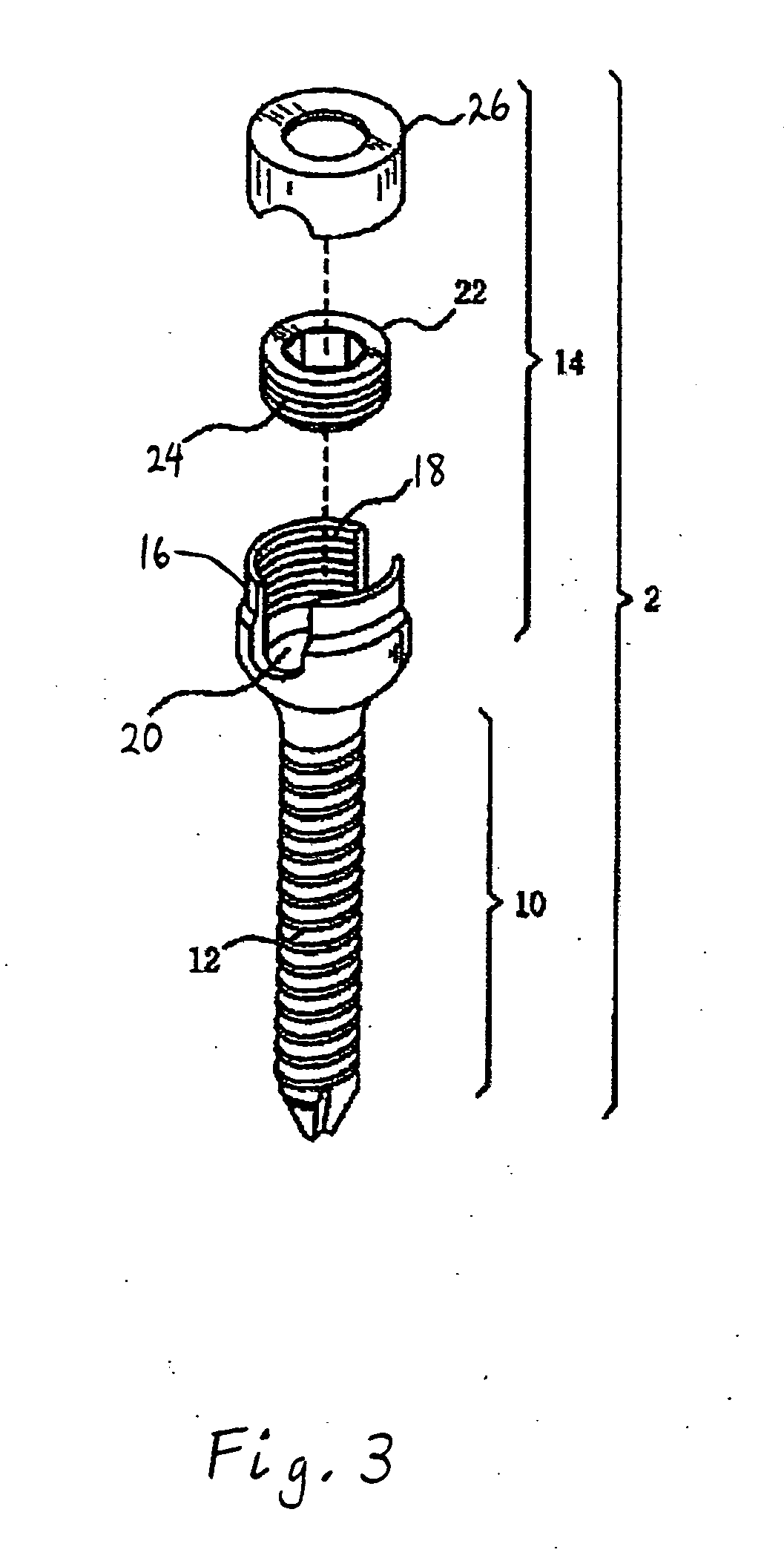
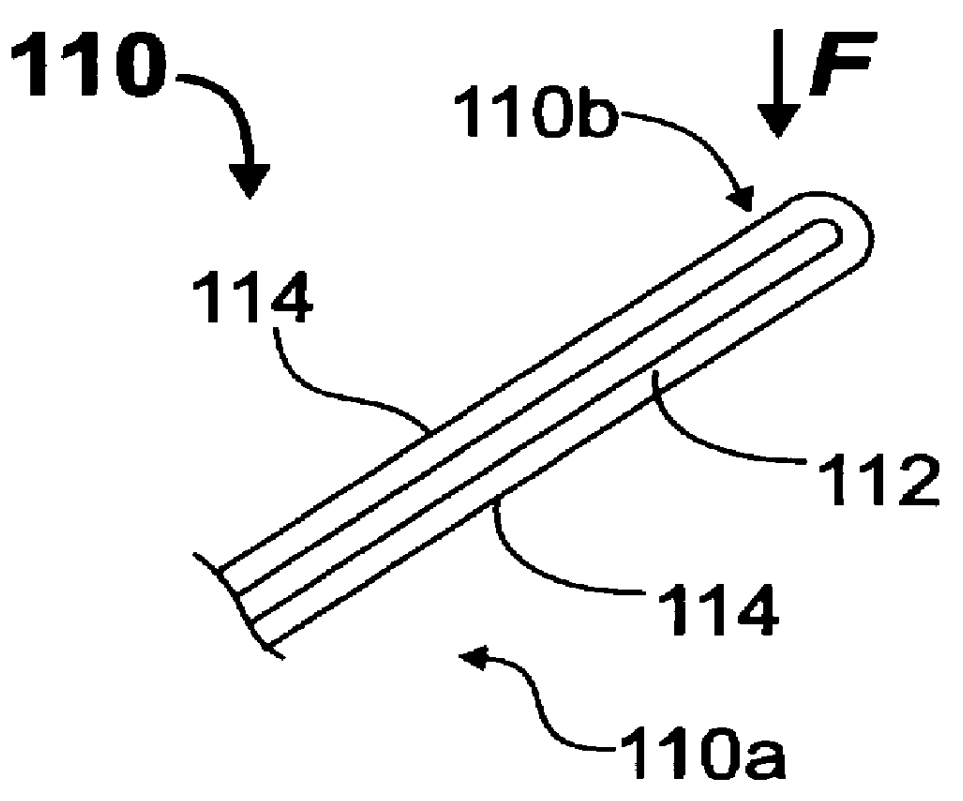
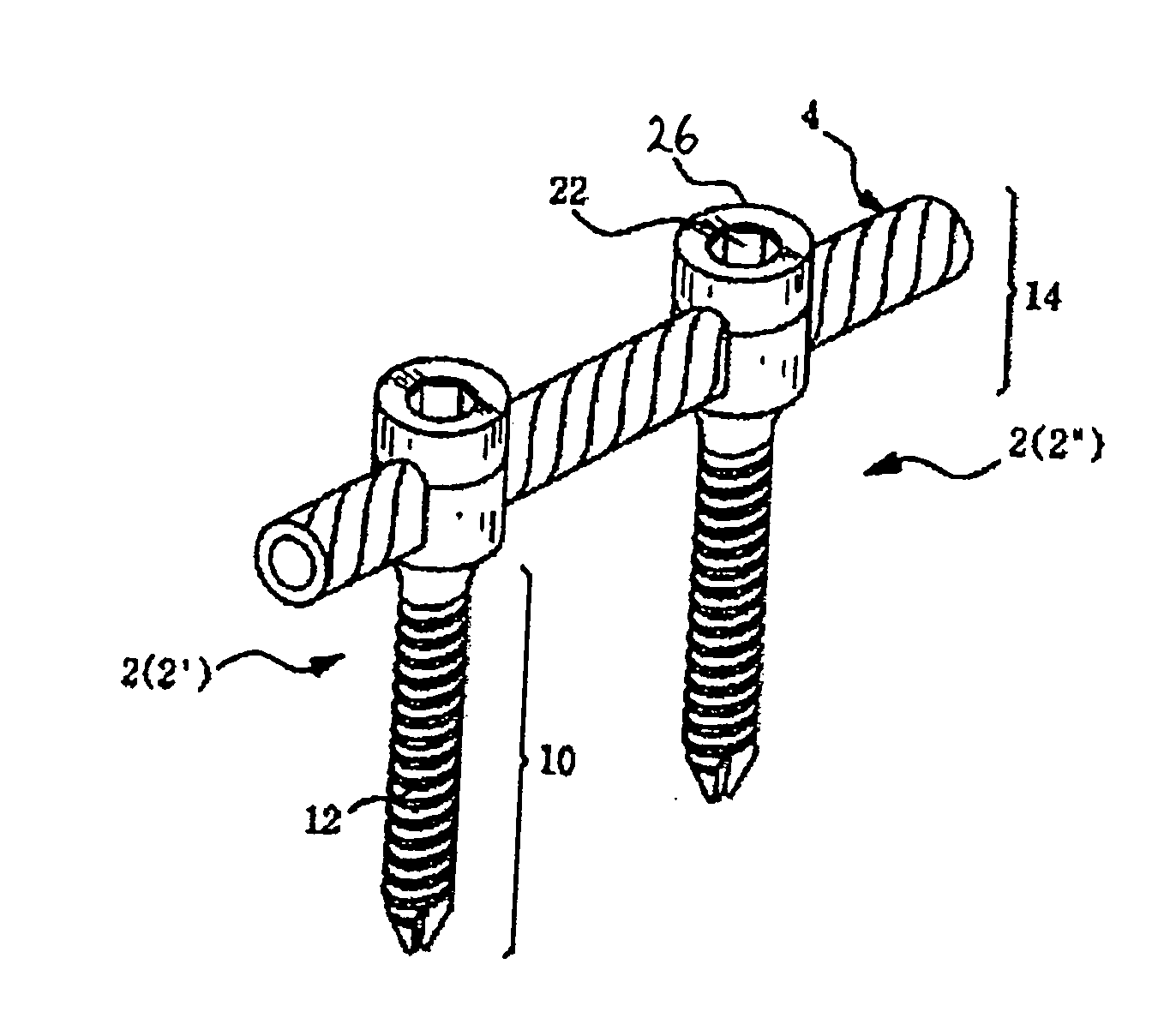
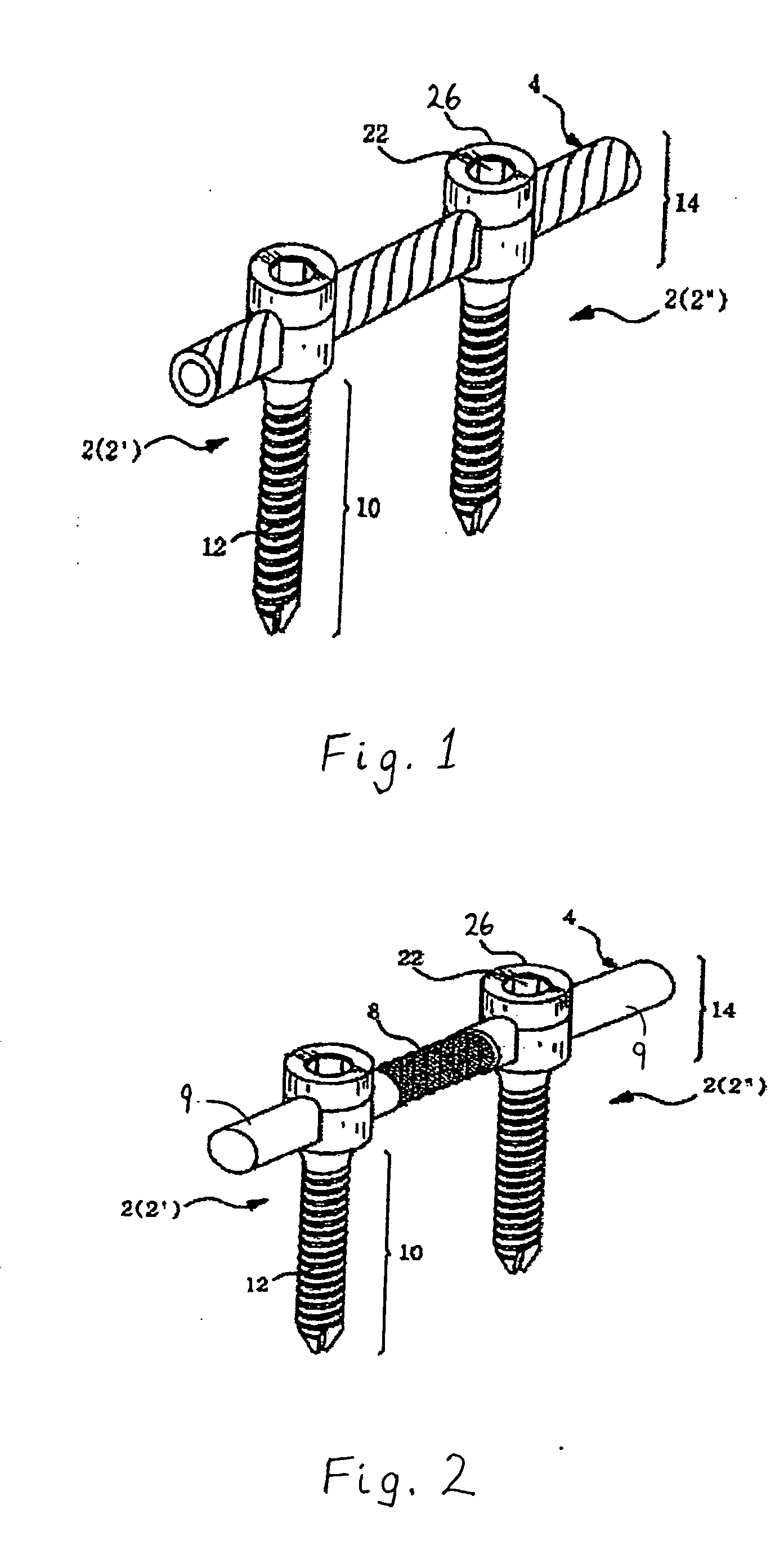
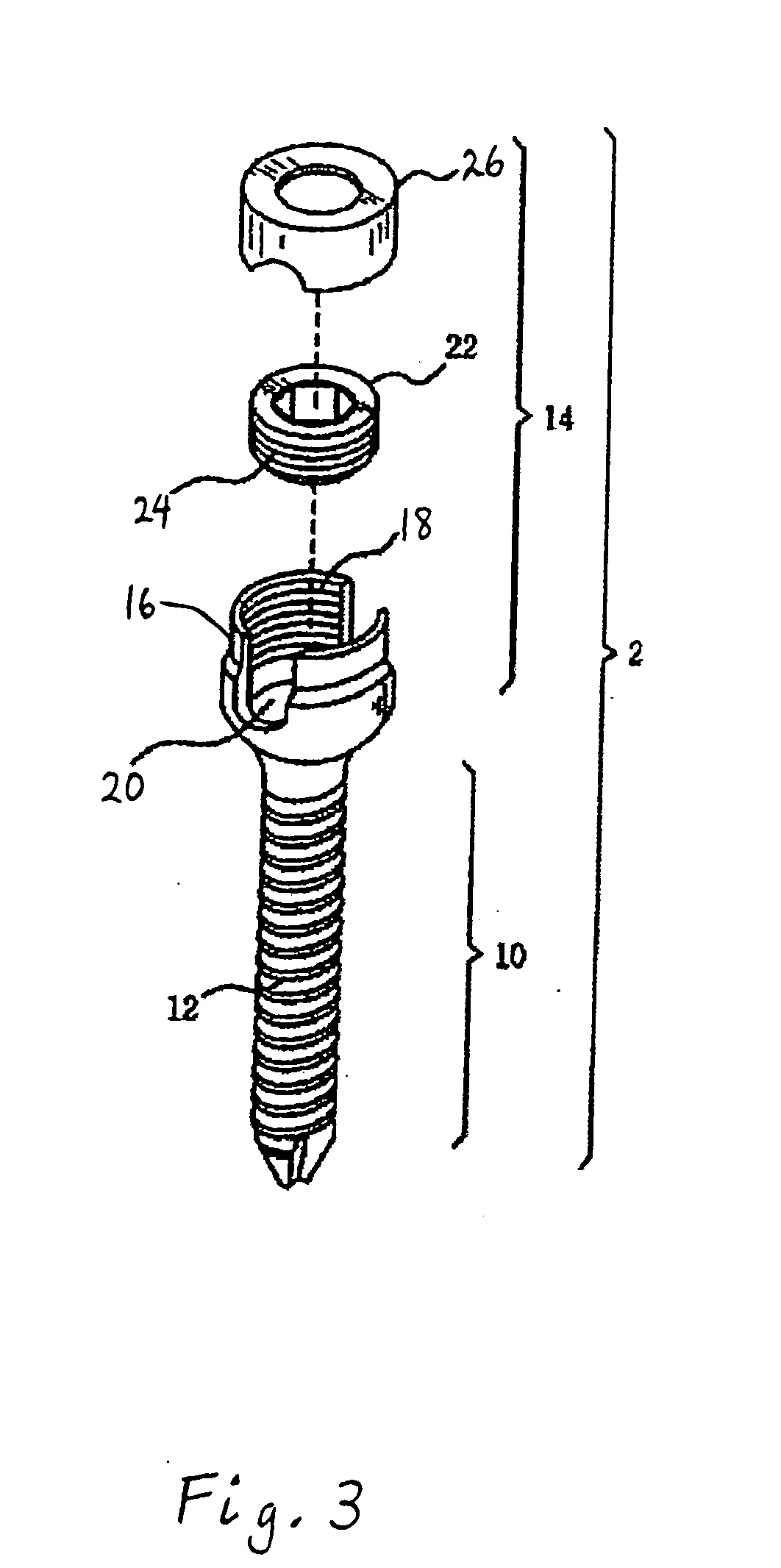
Method and apparatus for flexible fixation of a spine
InactiveUS20050065516A1Easy constructionSimple designInternal osteosythesisEar treatmentSpinal columnCoupling
A flexible spinal fixation device having a flexible metallic connection unit for non-rigid stabilization of the spinal column. In one embodiment, the fixation device includes at least two securing members configured to be inserted into respective adjacent spinal pedicles, each securing member each including a coupling assembly. The fixation device further includes a flexible metal connection unit configured to be received and secured within the coupling assemblies of each securing member so as to flexibly stabilize the affected area of the spine.
Owner:DEPUY SYNTHES PROD INC
Collapsible Heart Valve with Polymer Leaflets
InactiveUS20090112309A1Reduced invasivenessIncreased durabilityHeart valvesDiagnosticsBase heartNon functional
A Catheter Based Heart Valve (CBHV) is described herein which replaces a non functional, natural heart valve. The CBHV significantly reduces the invasiveness of the implantation procedure by being inserted with a catheter as opposed to open heart surgery. Additionally, the CBHV is coated with a biocompatible material to reduce the thrombogenic effects and to increase durability of the CBHV. The CBHV includes a stent and two or more polymer leaflets sewn to the stent. The stent is a wire assembly coated with Polystyrene-Polyisobutylene-Polystyrene (SIBS). The leaflets are made from a polyester weave as a core material and are coated with SIBS before being sewn to the stent. Other biocompatible materials may be used, such as stainless steel, Titanium, Nickel-Titanium alloys, etc.
Owner:FLORIDA INTERNATIONAL UNIVERSITY
Method and apparatus for flexible fixation of a spine
ActiveUS20050124991A1Easy constructionSimple designInternal osteosythesisCannulasSpinal columnCoupling
A flexible spinal fixation device having a flexible metallic connection unit for non-rigid stabilization of the spinal column. In one embodiment, the fixation device includes at least two securing members configured to be inserted into respective adjacent spinal pedicles, each securing member each including a coupling assembly. The fixation device further includes a flexible metal connection unit configured to be received and secured within the coupling assemblies of each securing member so as to flexibly stabilize the affected area of the spine.
Owner:N SPINE INC
Component for semicondutor processing apparatus and manufacturing method thereof
InactiveUS20090194233A1Increased durabilityLiquid surface applicatorsSemiconductor/solid-state device manufacturingTectorial membranePorosity
A component (10) for a semiconductor processing apparatus includes a matrix (10a) defining a shape of the component, and a protection film (10c) covering a predetermined surface of the matrix. The protection film (10c) consists essentially of an amorphous oxide of a first element selected from the group consisting of aluminum, silicon, hafnium, zirconium, and yttrium. The protection film (10c) has a porosity of less than 1% and a thickness of 1 nm to 10 μm.
Owner:TOKYO ELECTRON LTD
Phosphorescent material, and organic electroluminescent device and image display apparatus using same
InactiveUS8067099B2Improve efficiencyIncreased durabilityIndium organic compoundsDischarge tube luminescnet screensOrganic electroluminescenceOrganic compound
Provided is an organic electroluminescent device which has a high efficiency and high durability. The organic electroluminescent device includes an anode and a cathode; and a layer including an organic compound interposed between the anode and the cathode, in which the layer includes a phosphorescent material including an Ir complex or Pt complex having at least one ligand represented by any one of the following general formulae (1) to (4):
Owner:CANON KK
3D interconnect with protruding contacts
ActiveUS7262495B2Easy to assembleImprove electrical contact characteristicsSemiconductor/solid-state device detailsSolid-state devicesEngineeringSemiconductor
This invention relates to a semiconductor having protruding contacts comprising, a first semiconductor substrate having at least one interconnect located substantially within the first substrate, and a second semiconductor substrate having at least one protruding contact point that substantially contacts at least one interconnect.
Owner:HEWLETT-PACKARD ENTERPRISE DEV LP +1
Marking and guidance method and system for flexible fixation of a spine
ActiveUS20050065515A1Easy constructionSimple designInternal osteosythesisEar treatmentSpinal columnCylindrical channel
A method and system for marking and guiding the insertion of securing members (e.g., pedicle screws) of a spinal fixation device. In one embodiment, the marking and guidance method and system includes the use of a guide tube configured to be inserted into a patient's back until a first end reaches an entry point on or near a vertebral bone of the patient's spinal column, wherein the guide tube includes a hollow cylindrical channel along its longitudinal center axis; a penetrating device configured to be positioned within the cylindrical channel of the guide tube and having a sharp tip configured to protrude outwardly from the first end of the guide tube so as to allow the first end of the guide tube to penetrate through the patient's back muscle and tissue and reach the vertebral bone at the entry point; a marking pin configured to be inserted through the cylindrical channel of the guide tube, after removal of the penetrating device, until a first end of the marking pin having a sharp tip reaches the entry point; and a pushing device configured to be inserted through the cylindrical channel of the guide tube and provide a driving force at a second end of the marking pin, opposite the first end, so as to drive and secure the first end of the marking pin into the vertebral bone, wherein the marking pin identifies the location of the entry point on the vertebral bone for subsequent implantation of a securing member of a spinal fixation device.
Owner:N SPINE INC
Composite interconnection element for microelectronic components, and method of making same
InactiveUS6029344AEliminate needImprove featuresSemiconductor/solid-state device testing/measurementFinal product manufactureMetal foilSolderability
Interconnection elements for electronic components, exhibiting desirable mechanical characteristics (such as resiliency, for making pressure contacts) are formed by shaping an elongate element (core) of a soft material (such as gold) to have a springable shape (including cantilever beam, S-shape, U-shape), and overcoating the shaped elongate element with a hard material (such as nickel and its alloys), to impart a desired spring (resilient) characteristic to the resulting composite interconnection element. A final overcoat of a material having superior electrical qualities (e.g., electrical conductivity and / or solderability) may be applied to the composite interconnection element. The elongate element may be formed from a wire, or from a sheet (e.g., metal foil). The resulting interconnection elements may be mounted to a variety of electronic components, including directly to semiconductor dies and wafers (in which case the overcoat material anchors the composite interconnection element to a terminal (or the like) on the electronic component), may be mounted to support substrates for use as interposers and may be mounted to substrates for use as probe cards or probe card inserts. In one embodiment, a hybrid composite interconnection element is formed by mounting a core to an end of an flat elongate element formed from a sheet, and overcoating at least the core, the flat elongate element providing a "floating" support for the overcoated core, capable of absorbing non-planarities (tolerances) of an electronic component. Methods of fabricating interconnection elements on sacrificial substrates are described. Methods of fabricating tip structures and contact tips at the end of interconnection elements are described.
Owner:FORMFACTOR INC
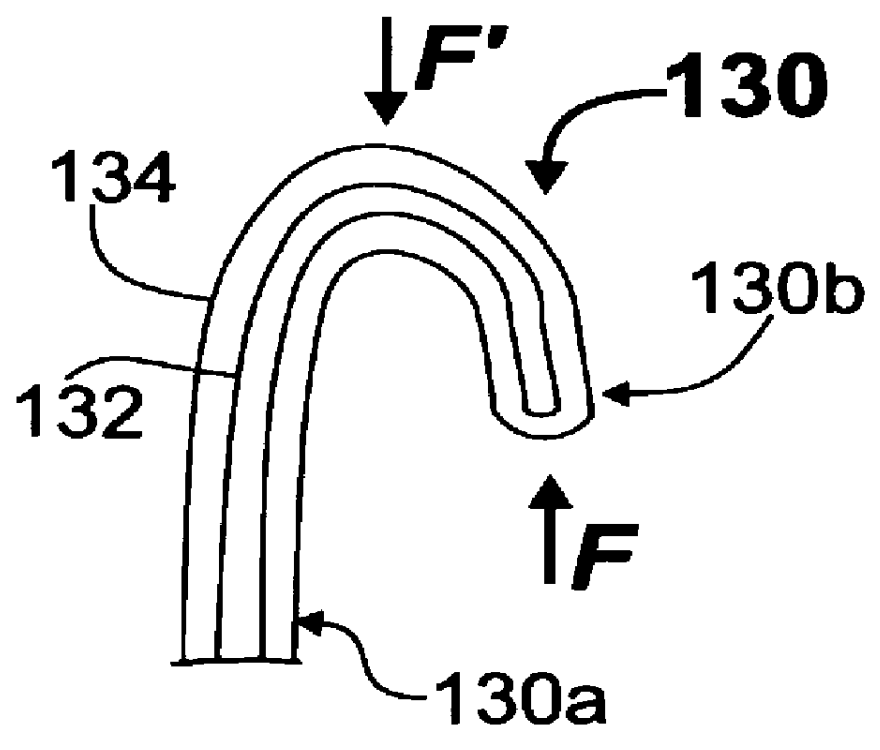
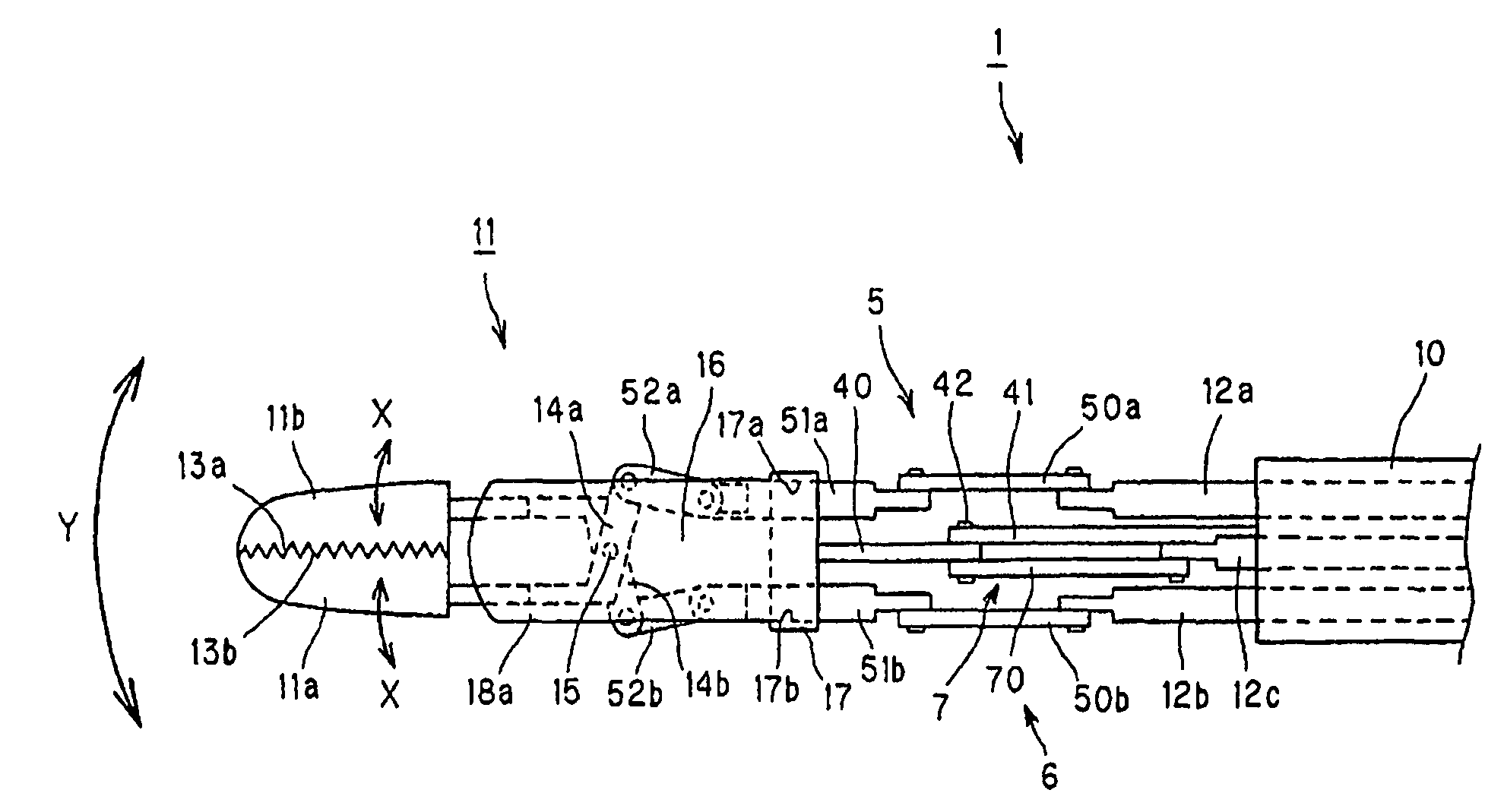
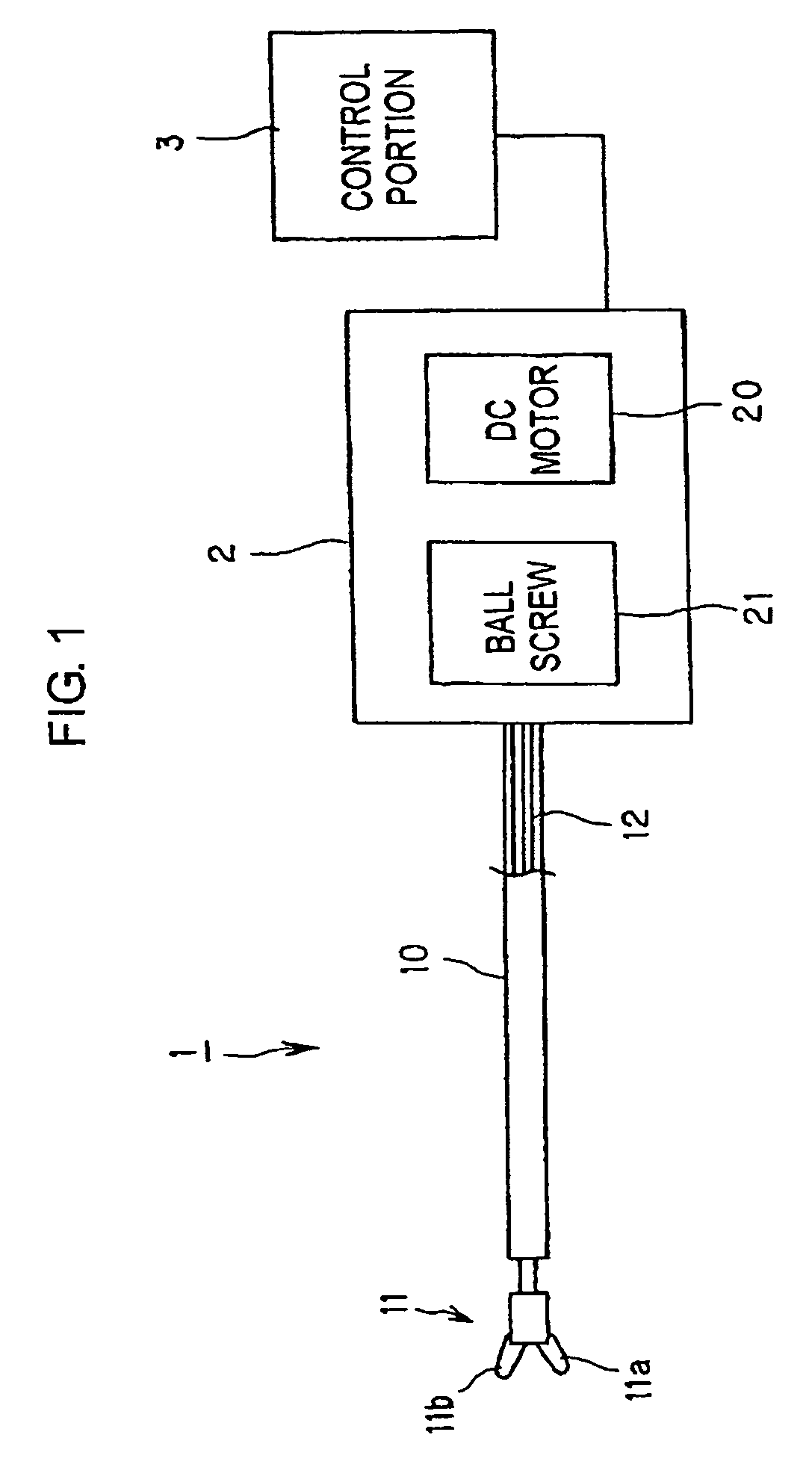
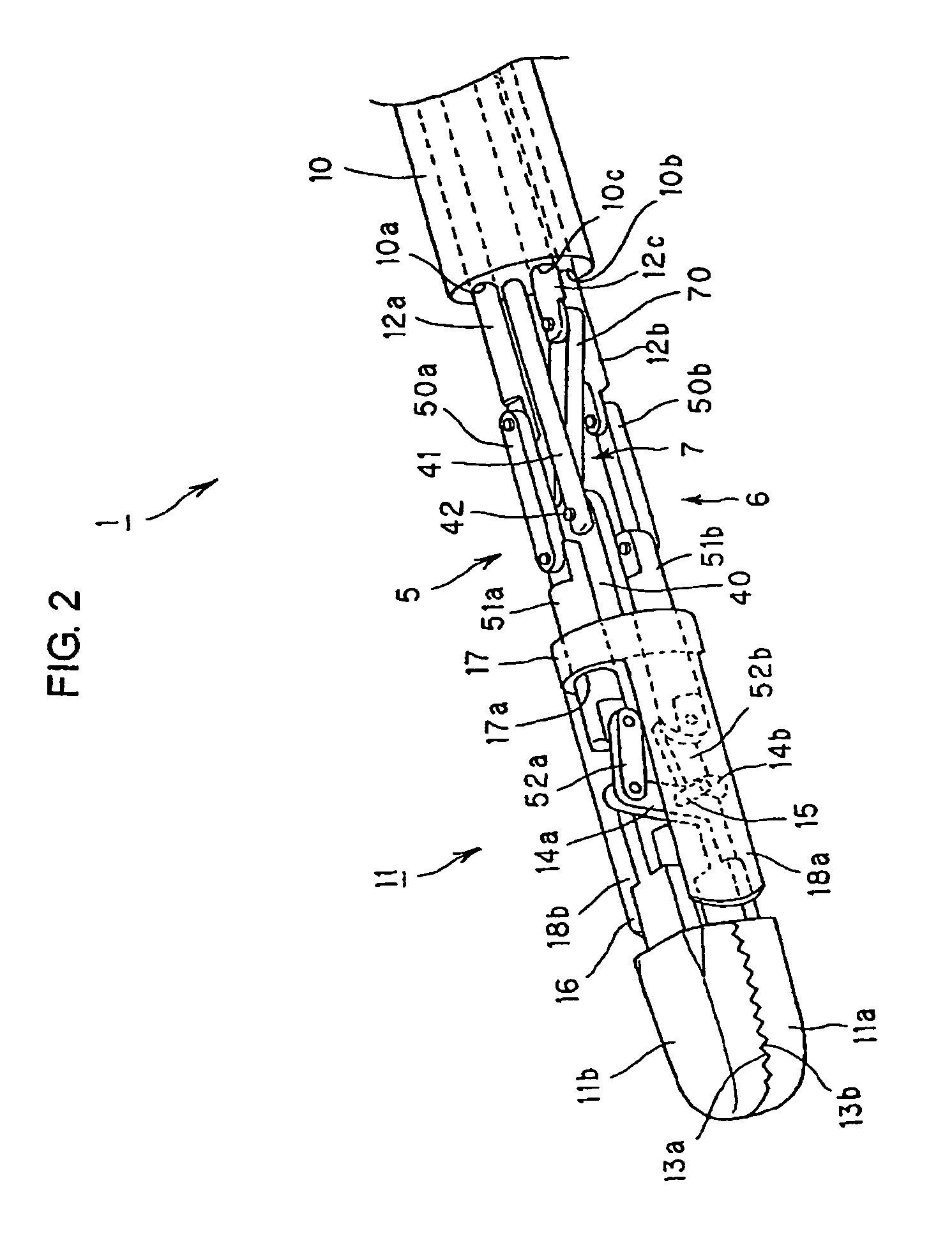
Manipulator with multiple degrees of freedom
ActiveUS7819894B2Increased durabilityImprove accuracyJointsDiagnosticsThree degrees of freedomDegrees of freedom
A bending forceps 1 has at least three degrees of freedom including relative opening / closing of a pair of gripping members 11a, 11b, rotation of both the gripping members around a first axis 15 and rotation of both the gripping members around a second axis 42 existing on an imaginary plane substantially perpendicular to the first axis 15. A drive power from an actuator is converted to each motion of the opening / closing of the gripping members, rotation thereof around the first axis and rotation thereof around the second axis by first to third link mechanisms 5, 6 and 7. The present invention enables the durability and control accuracy to be raised by employing a link mechanism as a drive power transmitting means. Further, the present invention facilitates sterilization, cleaning and attachment / detachment to / from a driving means.
Owner:MITSUISHI MAMORU +1
Method and apparatus for flexible fixation of a spine
ActiveUS20050177157A1Improved construction and designIncreased durabilityInternal osteosythesisEar treatmentMechanical engineering
A flexible connection unit for use in a spinal stabilization device, comprising a longitudinal member having first and second end portions and a flexible portion located between the end portions, wherein the flexible portion comprises at least one spacer and a flexible member located in a longitudinal axial channel of the at least one spacer, wherein the flexible member comprises a biocompatible metal material and the end portions maintain the at least one spacer in a substantially fixed longitudinal axial position with respect to the flexible member.
Owner:N SPINE INC
Prosthetic heart value
InactiveUS6911043B2Increased durabilityImprove performanceHeart valvesBlood vesselsDistal portionProsthesis
A tubular prosthetic semilunar or atrioventricular heart valve is formed by cutting flat, flexible leaflets according to a pattern. The valve is constructed by aligning the side edges of adjacent leaflets so that the leaflet inner faces engage each other, and then suturing the leaflets together with successive stitches along a fold line adjacent the side edges. The stitches are placed successively from a proximal in-flow end of each leaflet toward a distal out-flow end. During operation, when the leaflets open and close, the leaflets fold along the fold line. Distal tabs extend beyond the distal end of each leaflet. The successive stitches terminate proximal of the distal tab portion so that no locked stitches are placed along the distal portion of the fold line. The tab portions of adjacent leaflets are folded over each other and sewn together to form commissural attachment tabs. The commissural tabs provide commissural attachment points to accommodate sutures and the like in order to secure the tab to a vessel wall, if a semilunar valve, and papillary muscles and / or chordae tendineae if an atrioventricular valve.
Owner:MEDTRONIC 3F THERAPEUTICS
Prosthetic valve for transluminal delivery
InactiveUS8016877B2Preventing substantial migrationEliminate the problemBalloon catheterHeart valvesProsthesisCommissure
A method for deploying a prosthetic valve assembly is provided. The prosthetic valve assembly replaces a deficient native valve and comprises a replacement valve supported on an expandable valve support. The valve support, which entirely supports the valve annulus, valve leaflets, and valve commissure points, is configured to be collapsible for transluminal delivery and expandable to contact the anatomical annulus of the native valve when the assembly is properly positioned. Portions of the valve support may expand to a preset diameter to maintain coaptivity of the replacement valve and to prevent occlusion of the coronary oslia. The prosthetic valve assembly is compressible about a catheter, and restrained from expanding by an outer sheath. The catheter may be inserted inside a lumen within the body, such as the femoral artery, and delivered to a desired location, such as the heart.
Owner:MEDTRONIC COREVALVE
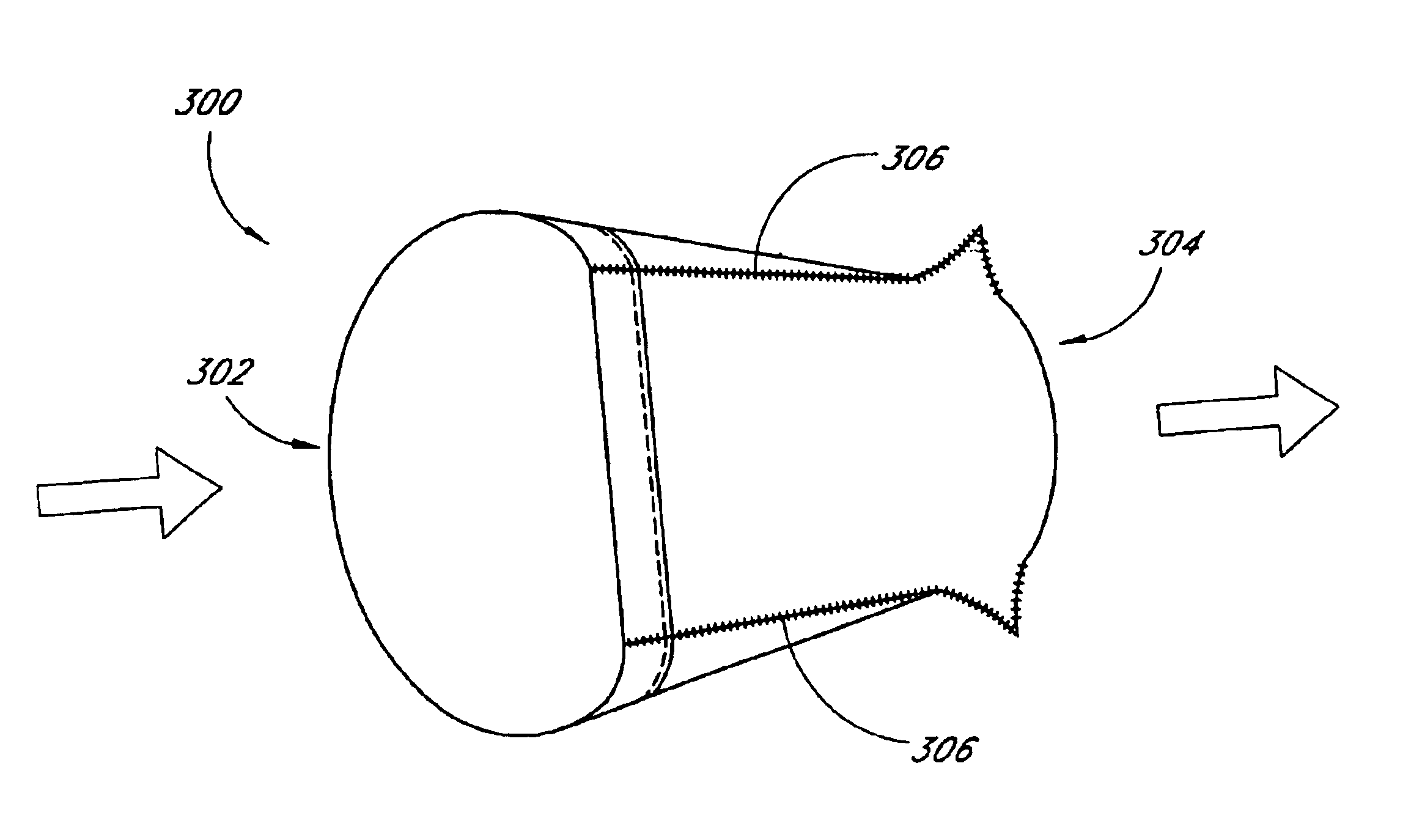
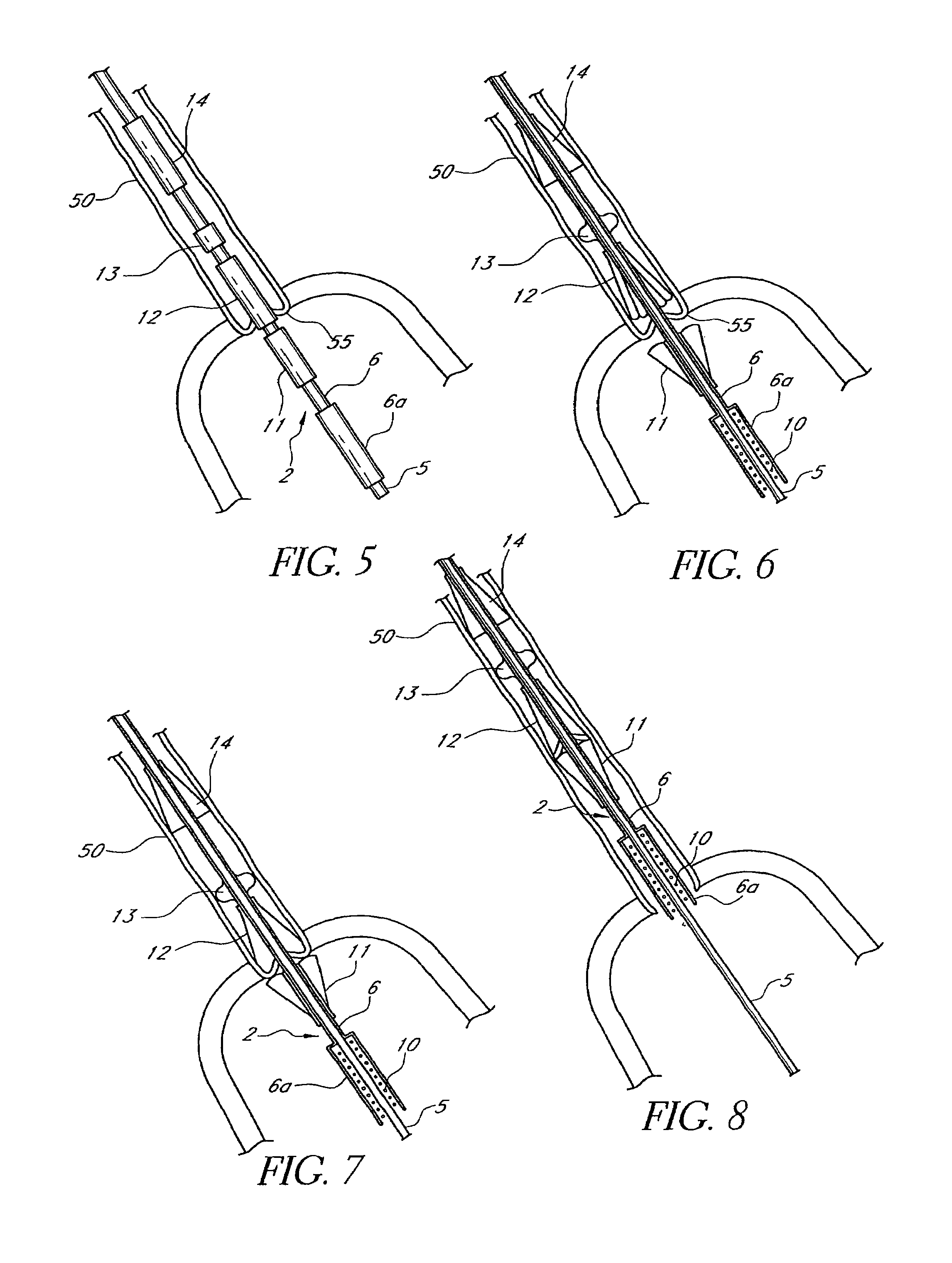
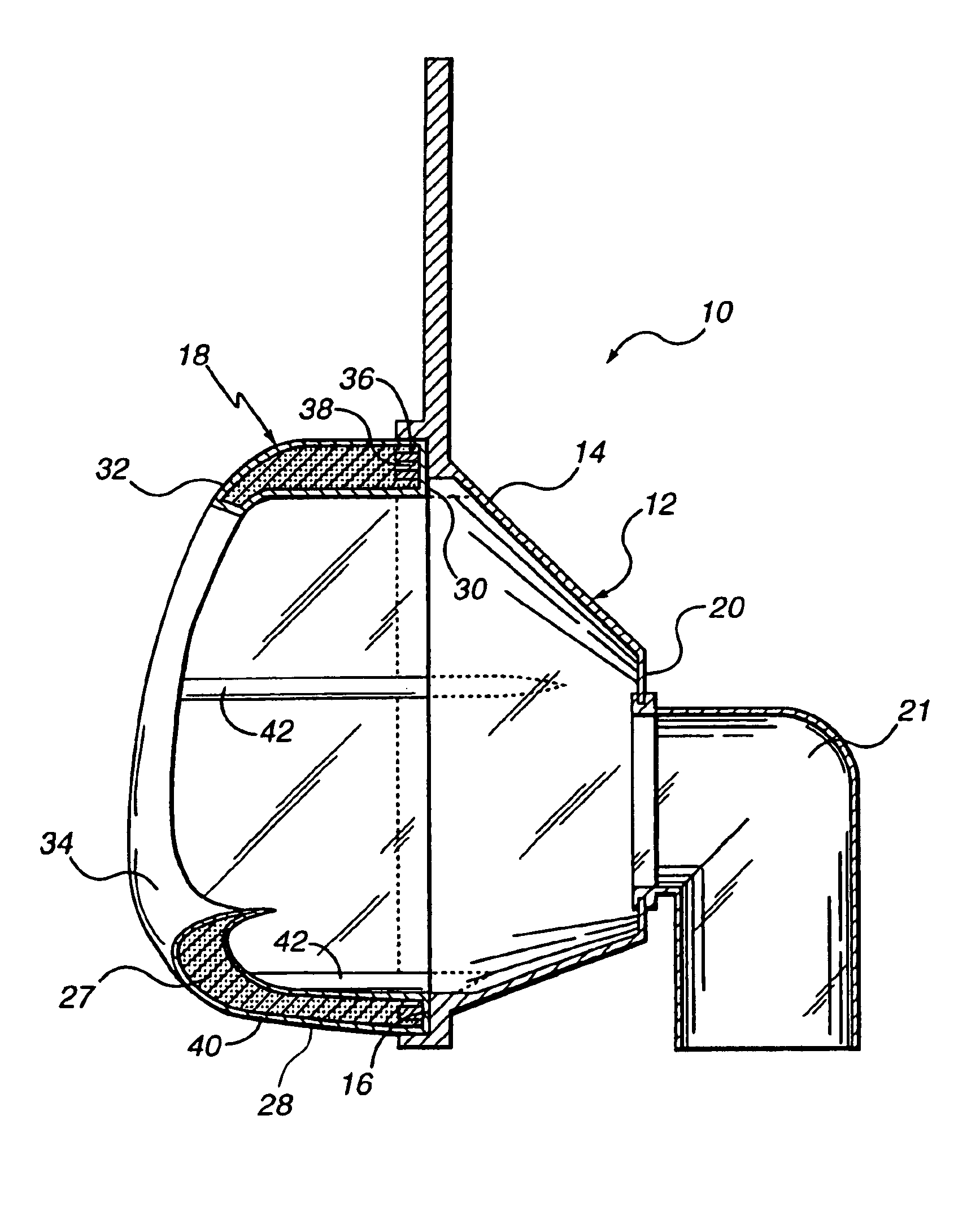
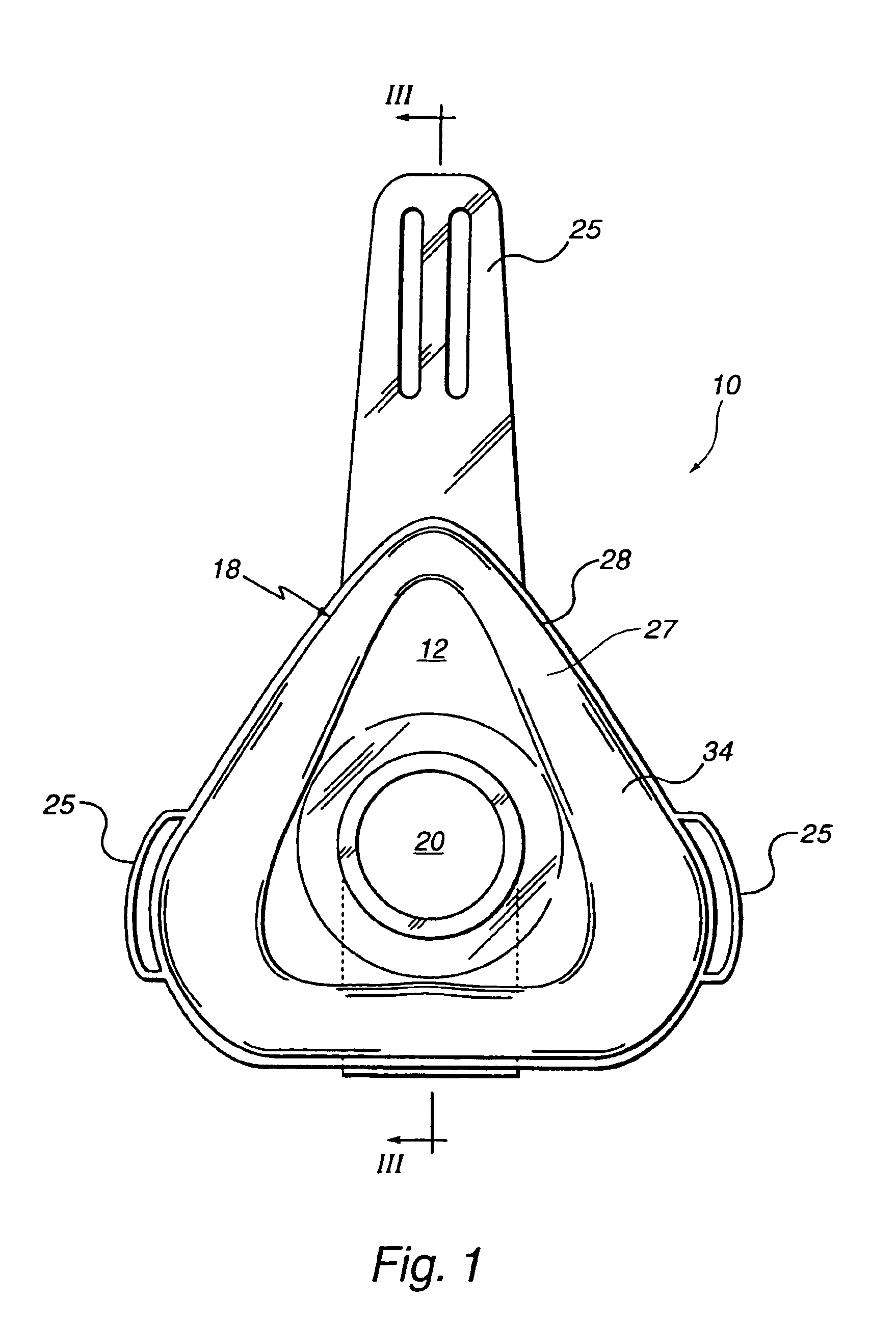
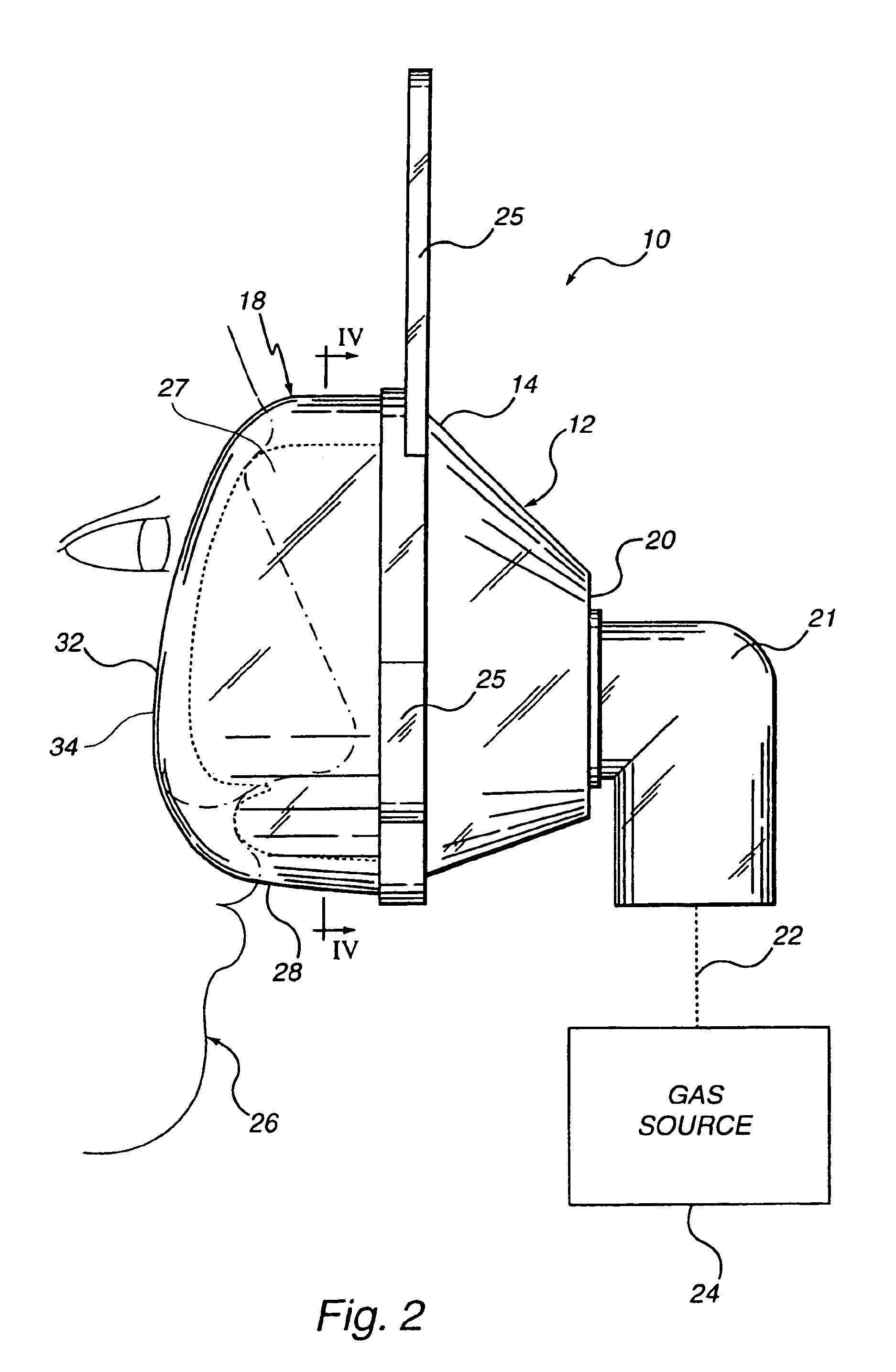
Customizable seal, mask with customizable seal and method of using such a seal
InactiveUS6895965B2Overcomes shortcomingReadily conformsRespiratory masksBreathing masksEngineeringMechanical engineering
A seal and a mask having a seal adapted for confronting engagement with a surface of a user to form an interface therewith. The seal includes a first portion defined by a gel substance and a second portion associated with the first portion. The second portion includes a selectively formable substance adapted to be molded from a first pattern into a second pattern and to retain the second pattern responsive to being so molded. The seal and mask having the seal is tailored to patient by causing the formable portion of the seal to be placed in a malleable state, applying the seal to the patient while the formable portion is in the malleable state, and causing the formable portion to be placed in a fixed state to retain a shape generally conforming to the portion of the patient underlying the seal.
Owner:RIC INVESTMENTS LLC
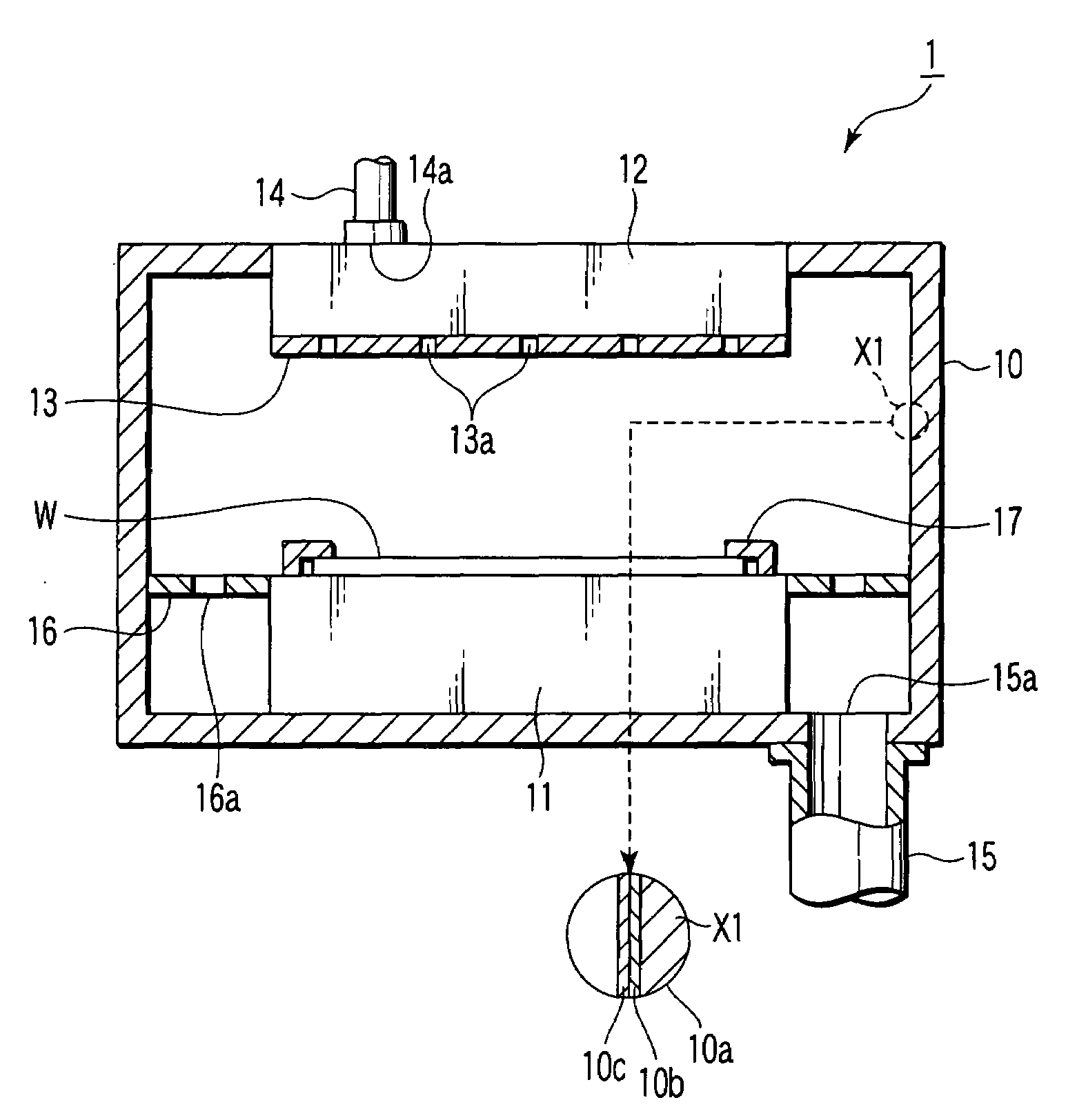
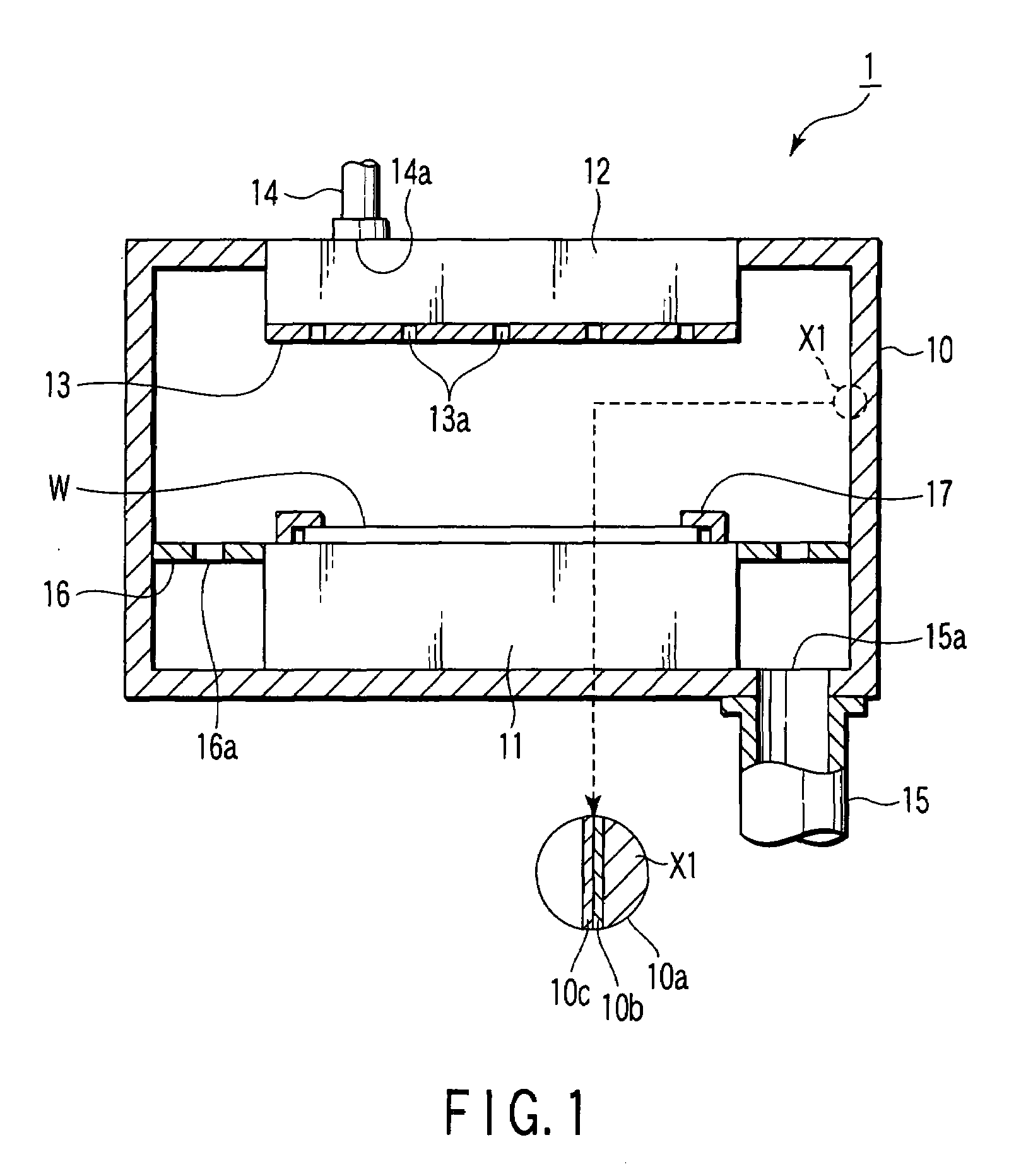
Ceramic heater for semiconductor manufacturing and inspecting equipment
InactiveUS20050092733A1Improve responseCausing temperature distributionMuffle furnacesSemiconductor/solid-state device manufacturingMetallurgySemiconductor
A ceramic heater for a semiconductor producing / examining device including a ceramic substrate having a heating surface for receiving a semiconductor wafer, a heating device for generating heat sufficient for producing / examining the semiconductor wafer and formed on the heating surface or inside the ceramic substrate, and a temperature measuring device for measuring a temperature of the heating surface and pressed against the ceramic substrate.
Owner:IBIDEN CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com