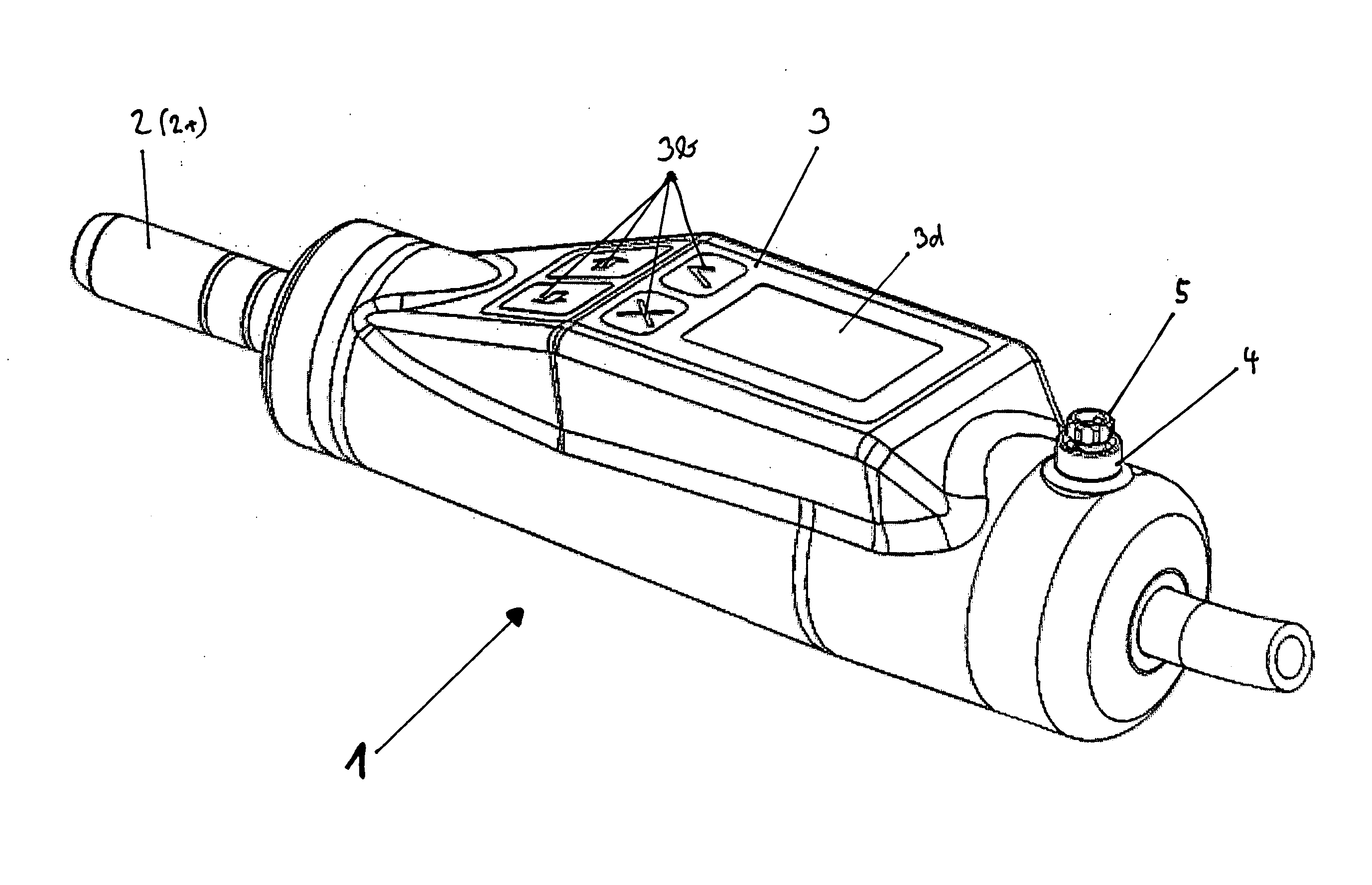
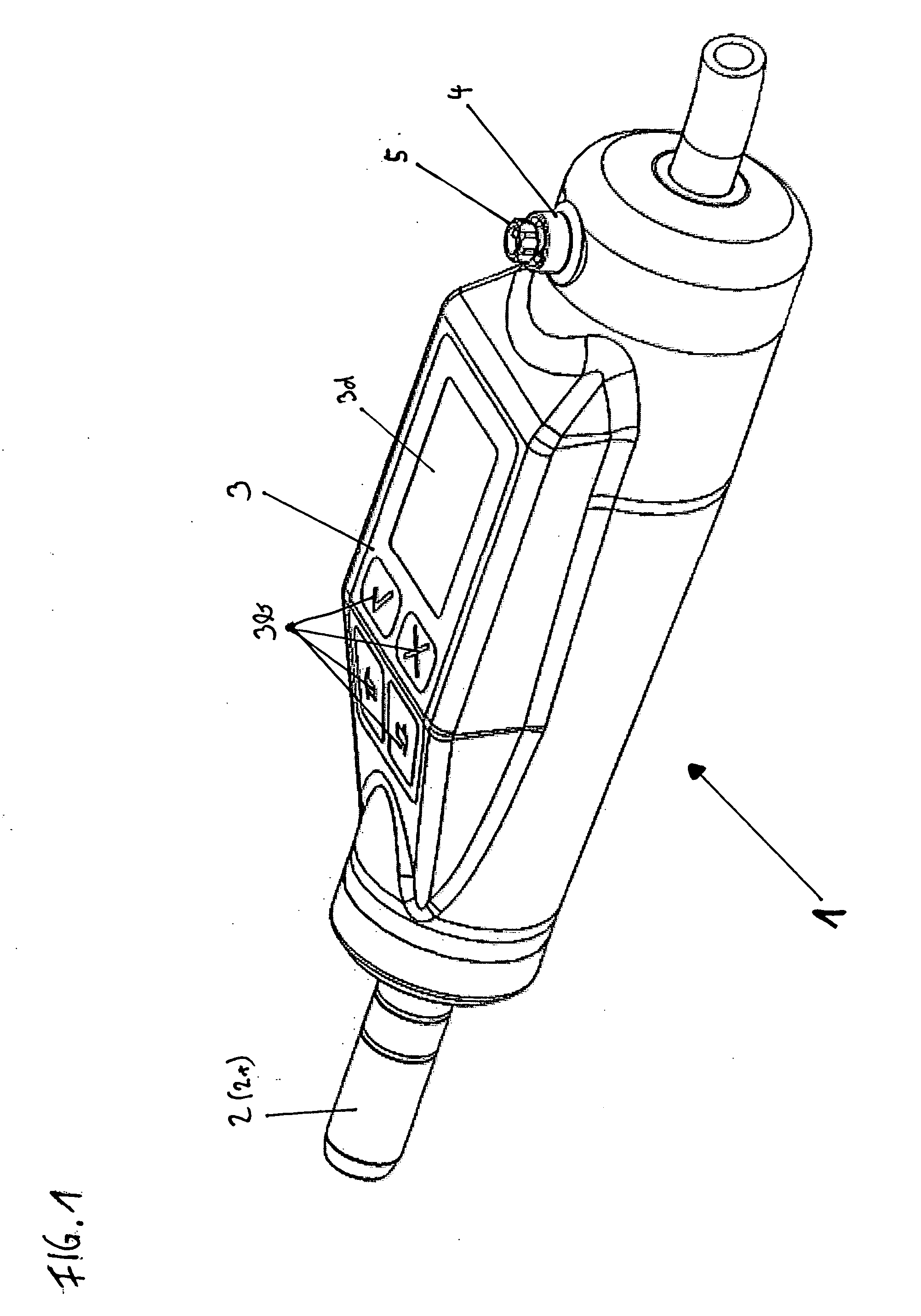
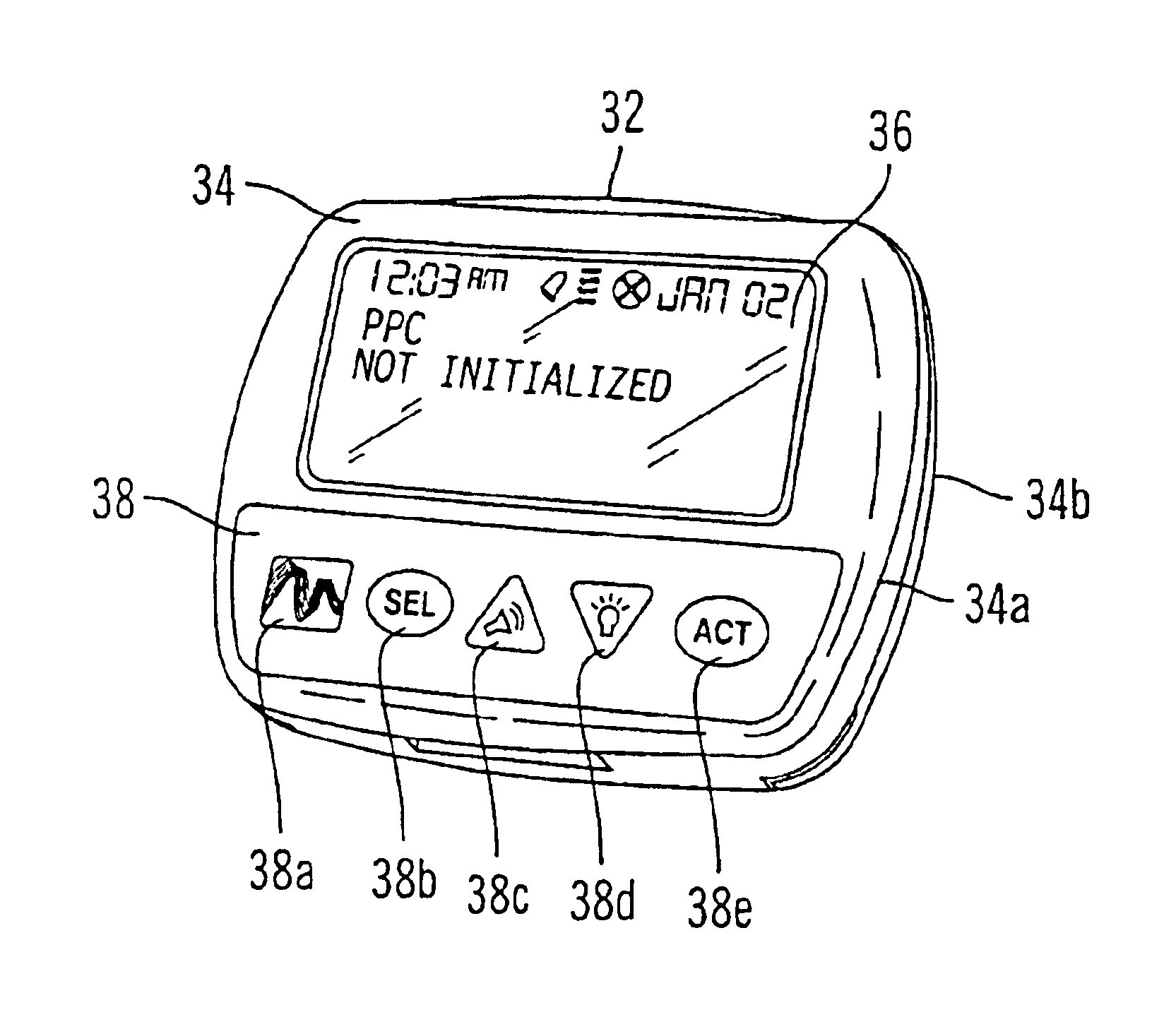
Patents
Literature
28043 results about "Medical device" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical device. A medical device is an instrument, apparatus, implant, in vitro reagent, or similar or related article that is used to diagnose, prevent, or treat disease or other conditions, and does not achieve its purposes through chemical action within or on the body.
Adaptive pattern recognition based control system and method
InactiveUS6400996B1Minimize timeEasy to implementError preventionFrequency-division multiplex detailsData streamSmart house
An adaptive interface for a programmable system, for predicting a desired user function, based on user history, as well as machine internal status and context. The apparatus receives an input from the user and other data. A predicted input is presented for confirmation by the user, and the predictive mechanism is updated based on this feedback. Also provided is a pattern recognition system for a multimedia device, wherein a user input is matched to a video stream on a conceptual basis, allowing inexact programming of a multimedia device. The system analyzes a data stream for correspondence with a data pattern for processing and storage. The data stream is subjected to adaptive pattern recognition to extract features of interest to provide a highly compressed representation that may be efficiently processed to determine correspondence. Applications of the interface and system include a video cassette recorder (VCR), medical device, vehicle control system, audio device, environmental control system, securities trading terminal, and smart house. The system optionally includes an actuator for effecting the environment of operation, allowing closed-loop feedback operation and automated learning.
Owner:BLANDING HOVENWEEP
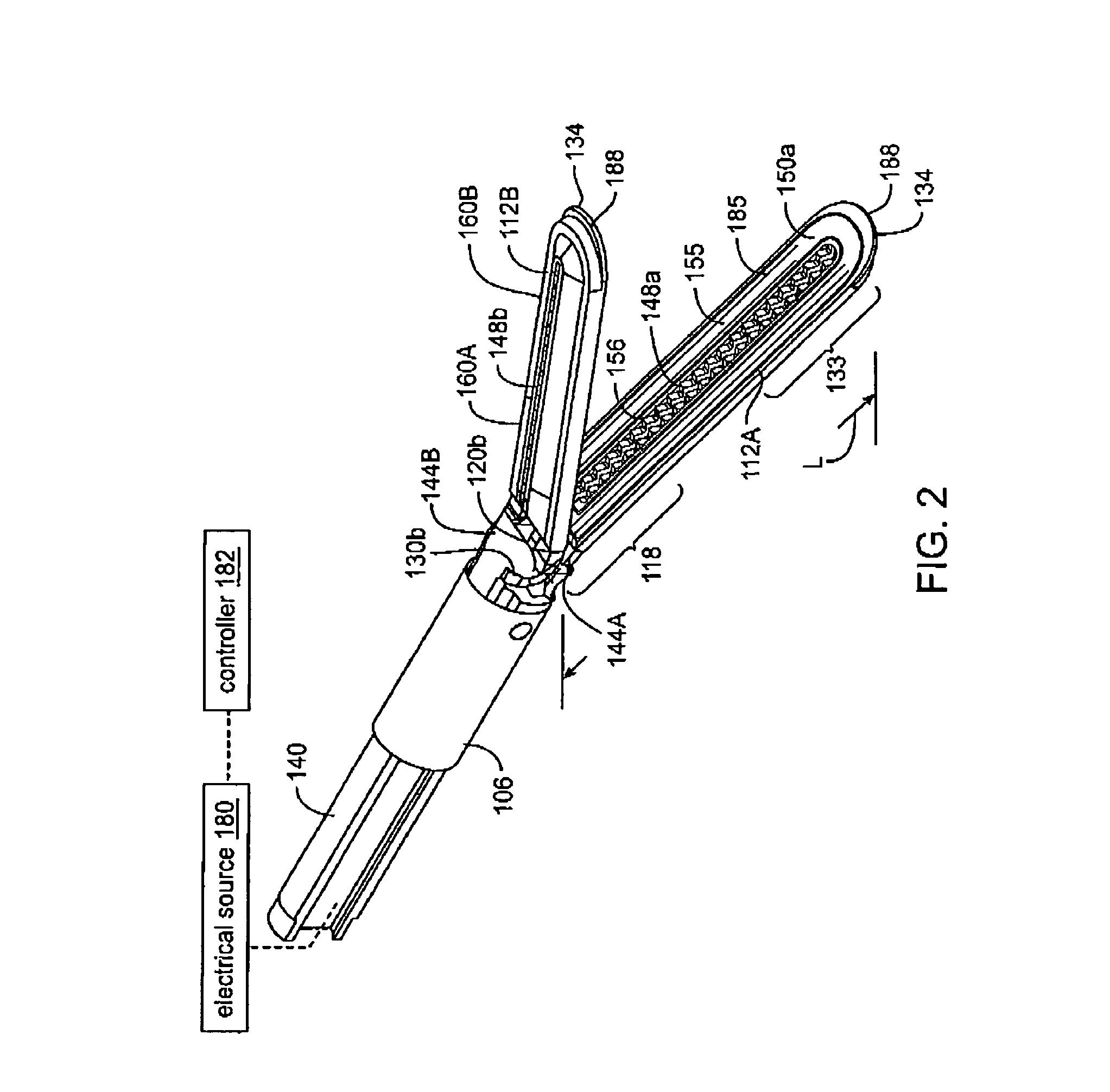
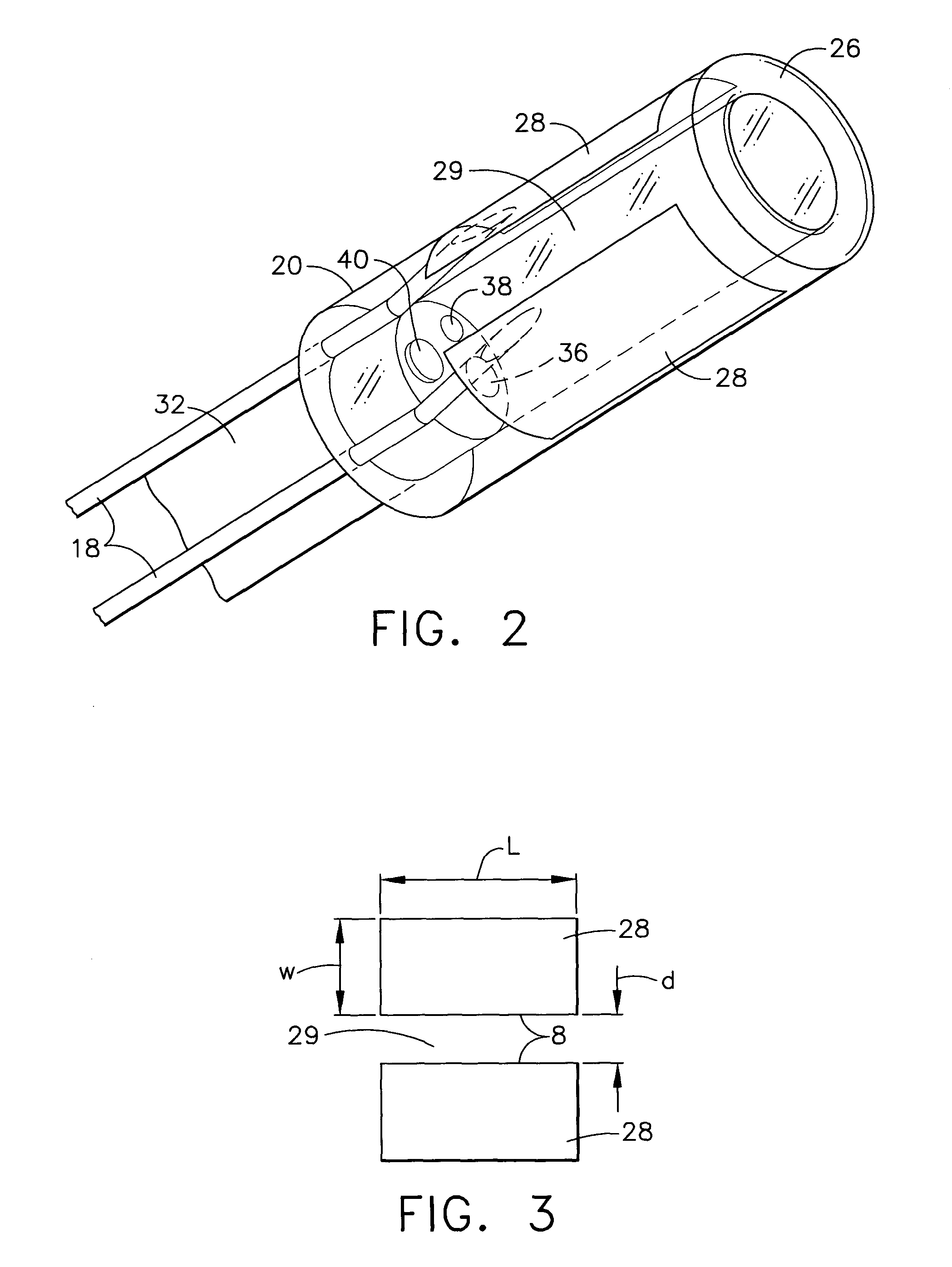
Electrosurgical working end with replaceable cartridges
InactiveUS7041102B2Reduce conductancePrevent any substantial dehydrationSurgical instruments for heatingCoatingsEngineeringMedical device
An electrosurgical medical device and method for creating thermal welds in engaged tissue that carries the electrosurgical components in a disposable cartridge. The instrument also can carry a blade member in a disposable cartridge, thus making the instrument system inexpensive to use. In another embodiment, the electrical leads of the cartridge have a surface coating of a thermochromic material to provide the physician with a visual indicator of operational parameters when applying energy to tissue.
Owner:ETHICON ENDO SURGERY INC
Jaw structure for electrosurgical instrument and method of use
InactiveUS7011657B2Improve the immunityEfficient weldingElectrotherapySurgical instruments for heatingTissue heatingVolumetric Mass Density
An electrosurgical medical device and technique for creating thermal welds in engaged tissue that provides very high compressive forces. In one exemplary embodiment, at least one jaw of the instrument defines a tissue engagement plane carrying first and second surface portions that comprise (i) an electrically conductive material and (ii) a positive temperature coefficient (PTC) material having a selected increased resistance that differs at each selected increased temperature over a targeted treatment range. One type of PTC material is a doped ceramic that can be engineered to exhibit a selected positively sloped temperature-resistance curve over about 37° C. to 100° C. The 70° C. to 100° C. range can bracket a targeted “thermal treatment range” at which tissue welded can be accomplished. The engineered resistance of the PTC matrix at the upper end of the temperature range will terminate current flow through the matrix. In one mode of operation, the engagement plane cause ohmic heating within tissue from Rf energy delivery tissue PTC matrix is heated to exceed the treatment range. Thereafter, energy density in the engaged tissue will be modulated as the conductivity of the second portion hovers within the targeted treatment range to thereby provide optical tissue heating for purposes of tissue welding.
Owner:ETHICON ENDO SURGERY INC
Circular stapler buttress combination
A combination medical device comprising a circular stapler instrument and one or more portions of preformed buttress material adapted to be stably positioned upon the staple cartridge and / or anvil components of the stapler prior or at the time of use. Positioned buttress material(s) are delivered to a tissue site where the circular stapler is actuated to connect previously severed tissue portions. The buttress material is retained and provides an improved seal between the joined tissue sections. The buttress material is made up of two regions, one of which serves primarily to secure the buttress material to the stapler prior to actuation, and one of which serves primarily to form the improved seal. The former region is severed and discarded upon activation of the circular stapler to form an anastomoses. Methods of use and preparation of the buttress material are also described.
Owner:SYNOVIS LIFE TECH
Electrosurgical instrument and method of use
ActiveUS7087054B2Reduce conductancePrevent any substantial dehydrationElectrotherapySurgical instruments for heatingEngineeringMedical device
An electrosurgical medical device and method for creating thermal welds in engaged tissue. In one embodiment, at least one jaw of the instrument defines a tissue engagement plane that carries a recessed central portion. In another embodiment, the controller coupled to the Rf source is adapted to switch from a power control operational mode to a voltage controlled operational mode at a selected transition impedance level.
Owner:ETHICON ENDO SURGERY INC
Electrosurgical instrument and method of use
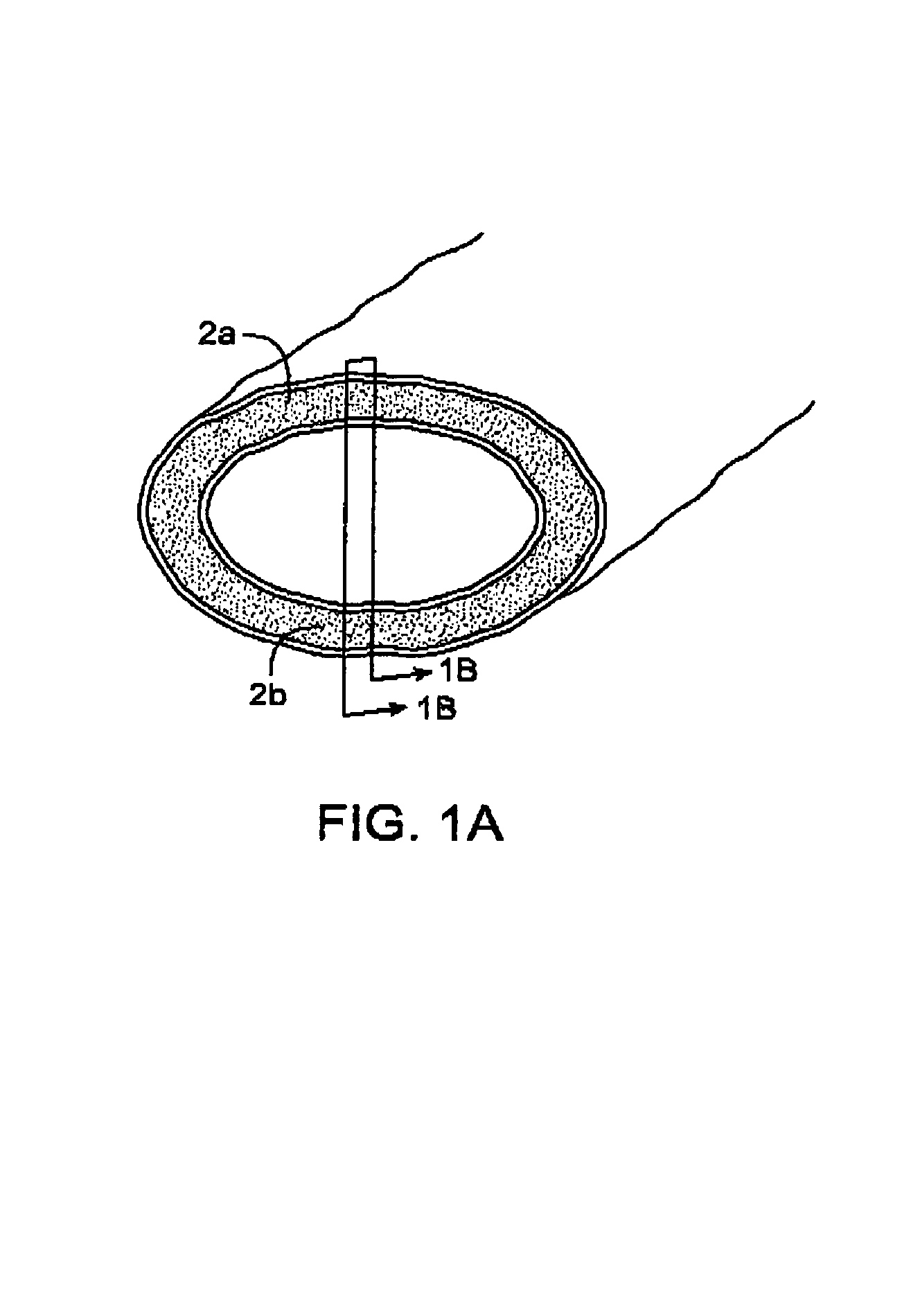
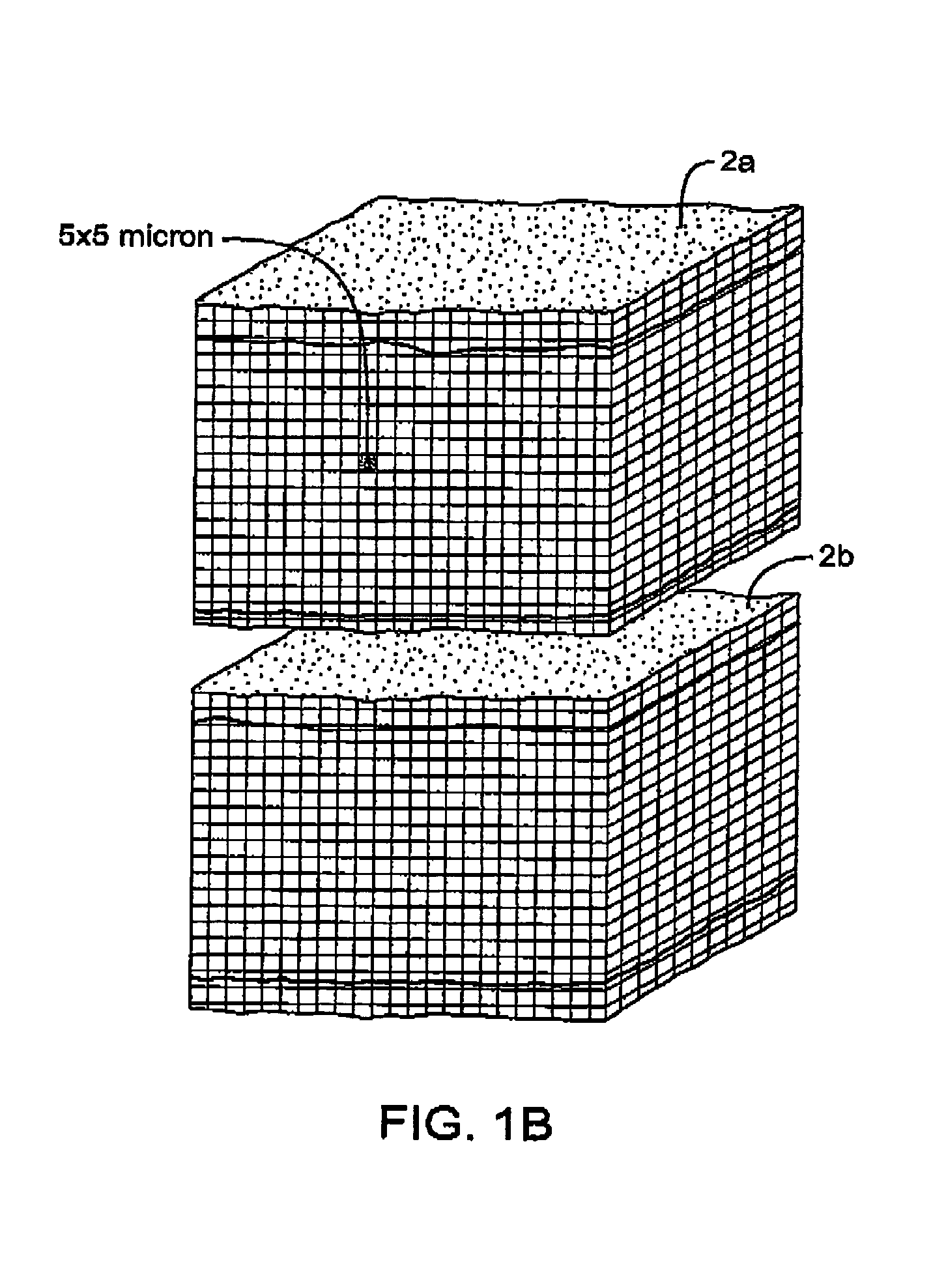
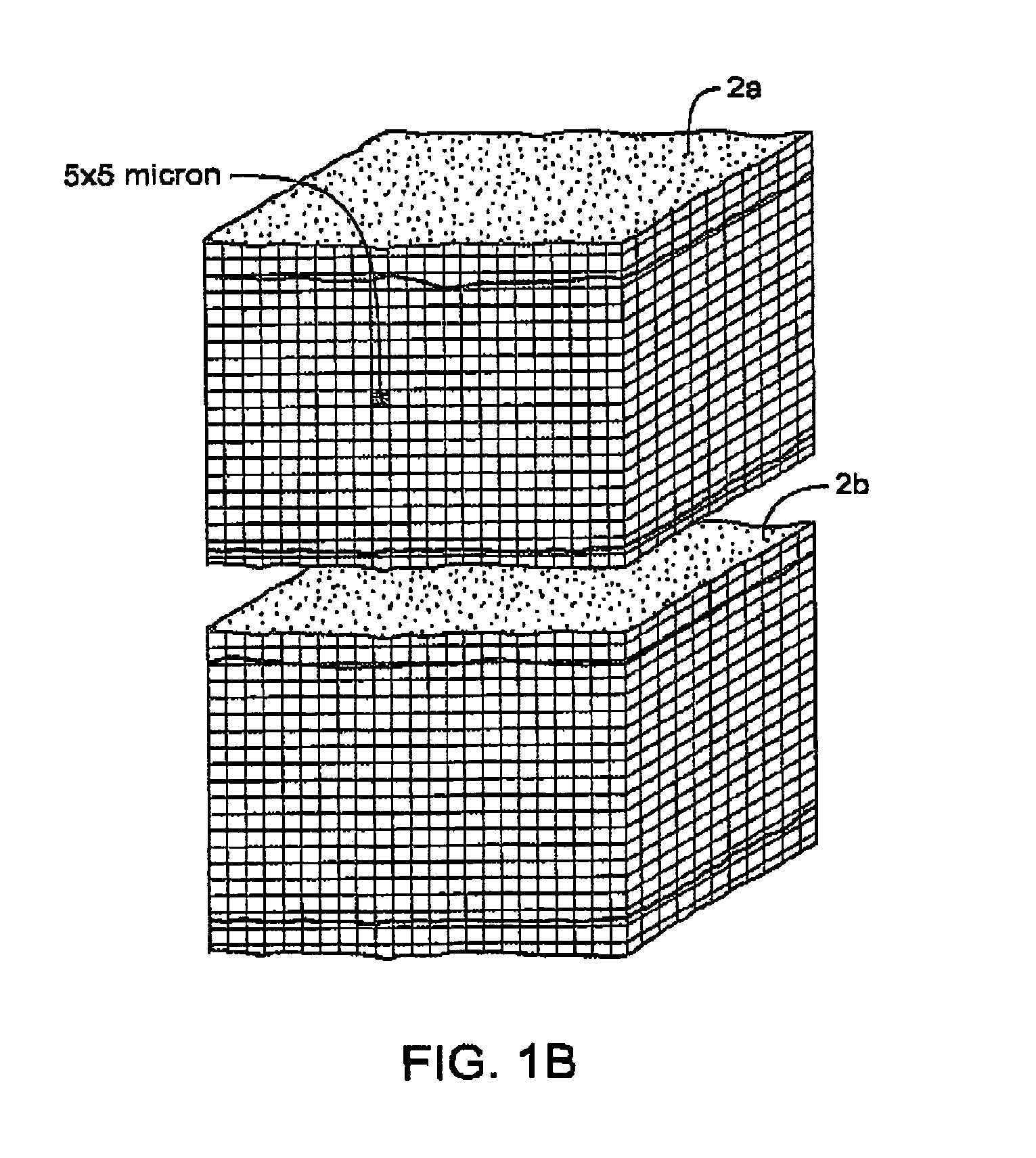
InactiveUS7083619B2Reduce conductancePrevent any substantial dehydrationSurgical instruments for heatingCoatingsMicron scaleElastomer
An electrosurgical medical device and method for creating thermal welds in engaged tissue. In one embodiment, at least one jaw of the instrument defines a tissue engagement plane carrying a conductive-resistive matrix of a conductively-doped non-conductive elastomer. The engagement surface portions thus can be described as a positive temperature coefficient material that has a unique selected decreased electrical conductance at each selected increased temperature thereof over a targeted treatment range. The conductive-resistive matrix can be engineered to bracket a targeted thermal treatment range, for example about 60° C. to 80° C., at which tissue welding can be accomplished. In one mode of operation, the engagement plane will automatically modulate and spatially localize ohmic heating within the engaged tissue from Rf energy application—across micron-scale portions of the engagement surface.
Owner:ETHICON ENDO SURGERY INC
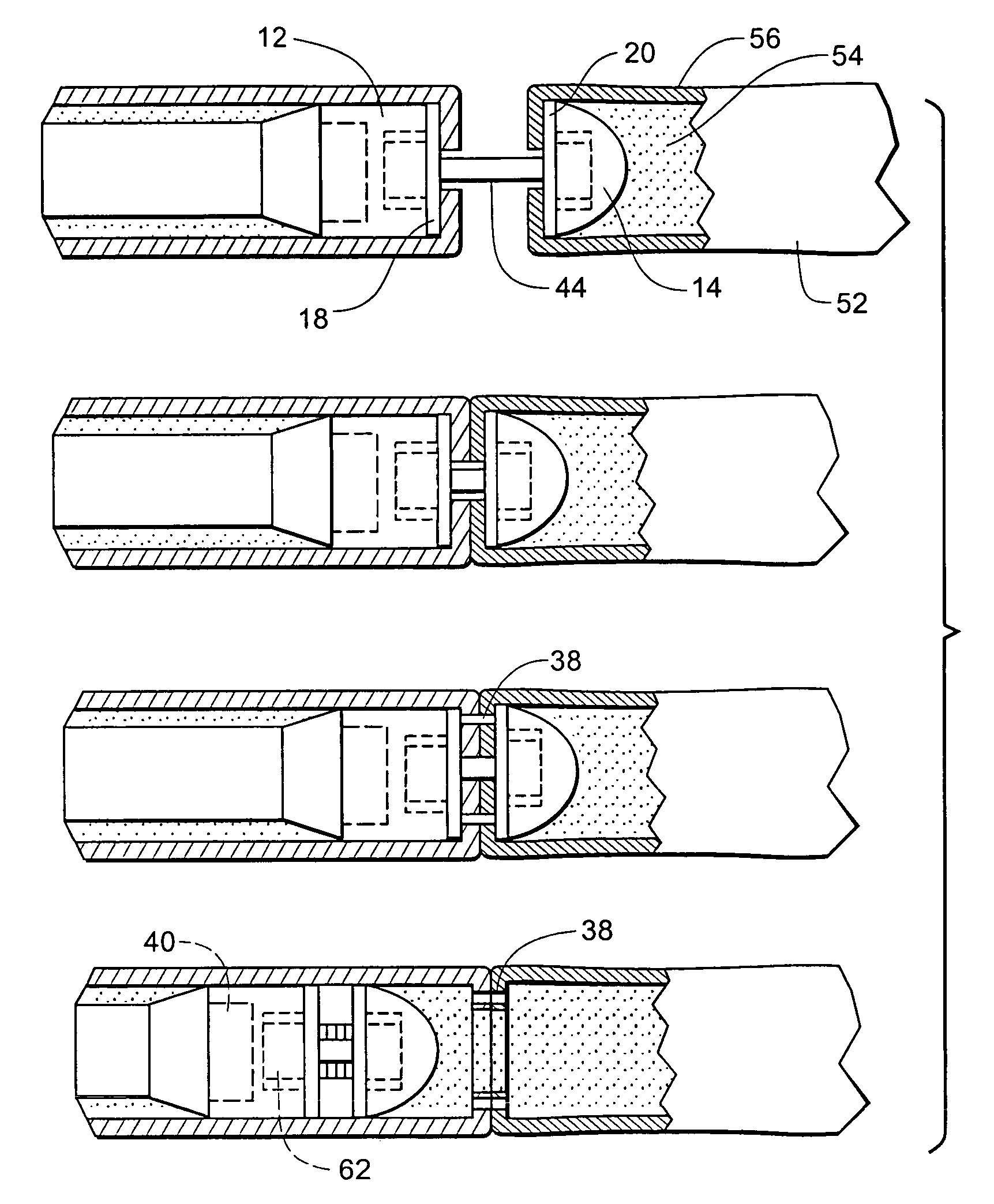
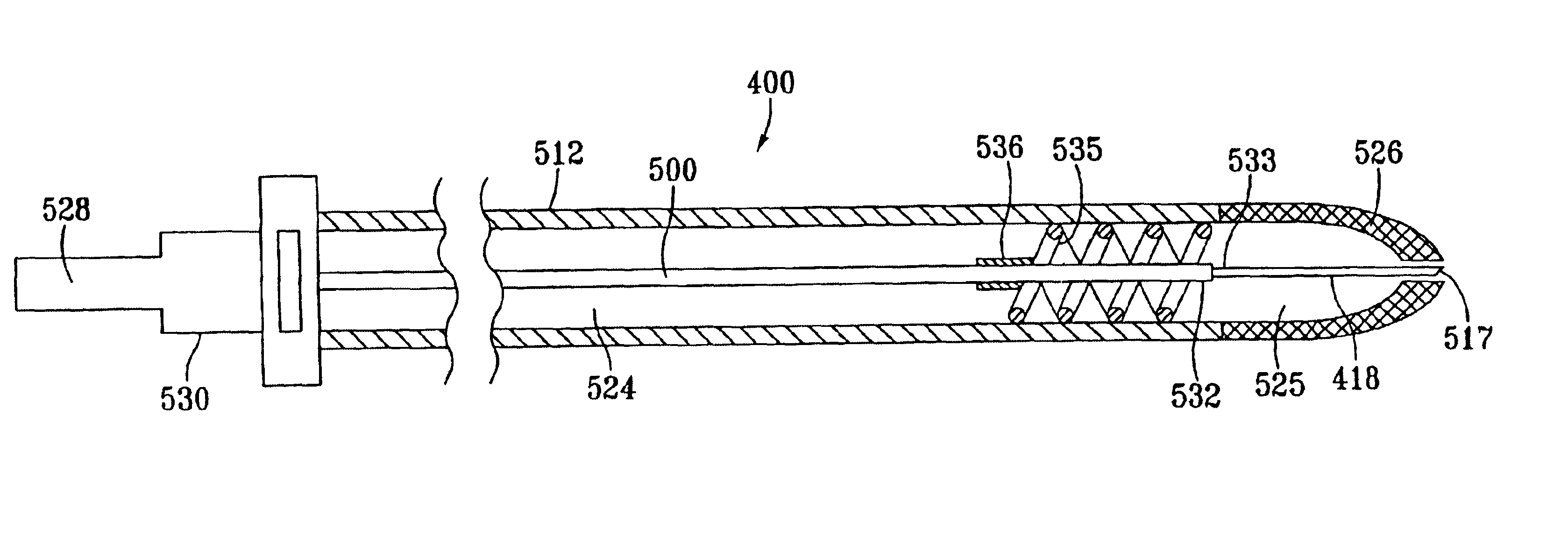
Fluid-assisted medical devices, systems and methods
Surgical devices, systems and methods for treating tissue are provided. An exemplary surgical device comprises a tip portion including first and second jaws each having a tissue grasping surface, at least one of the jaws being movable toward the other jaw. The tissue grasping surface of each jaw has includes an electrically insulative surface. The device also includes first and second electrodes connectable to different terminals of an RF generator to generate electrical current flow therebetween, with each of the electrodes having an electrode surface. One of the electrode surfaces is located on one of the jaws separated from one edge of the tissue grasping surface, and the other of the electrode surfaces is located on one or the other of the jaws separated from the other edge of the tissue grasping surface. The device also includes at least one fluid passage being connectable to a fluid source.
Owner:MEDTRONIC ADVANCED ENERGY
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Drug releasing anastomosis devices and methods for treating anastomotic sites
ActiveUS7108701B2Reduce drug toxicityGood curative effectSuture equipmentsSurgical needlesBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH
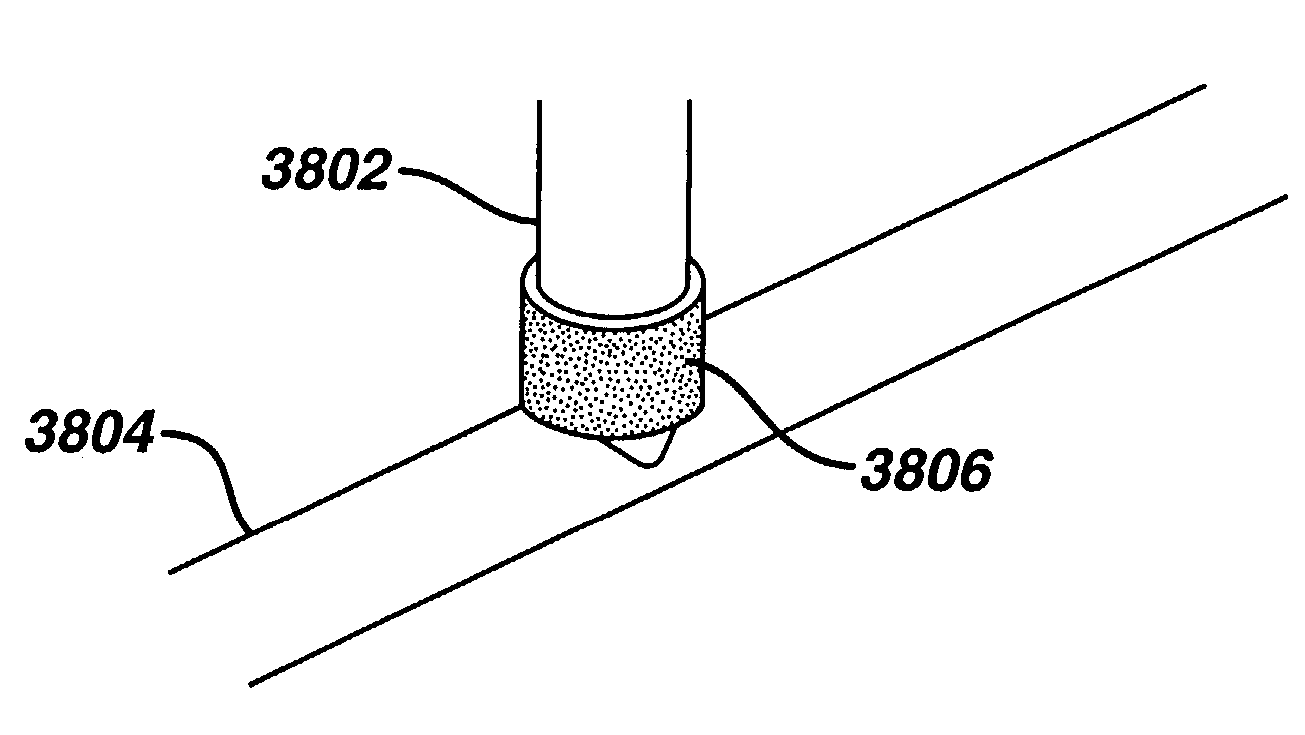
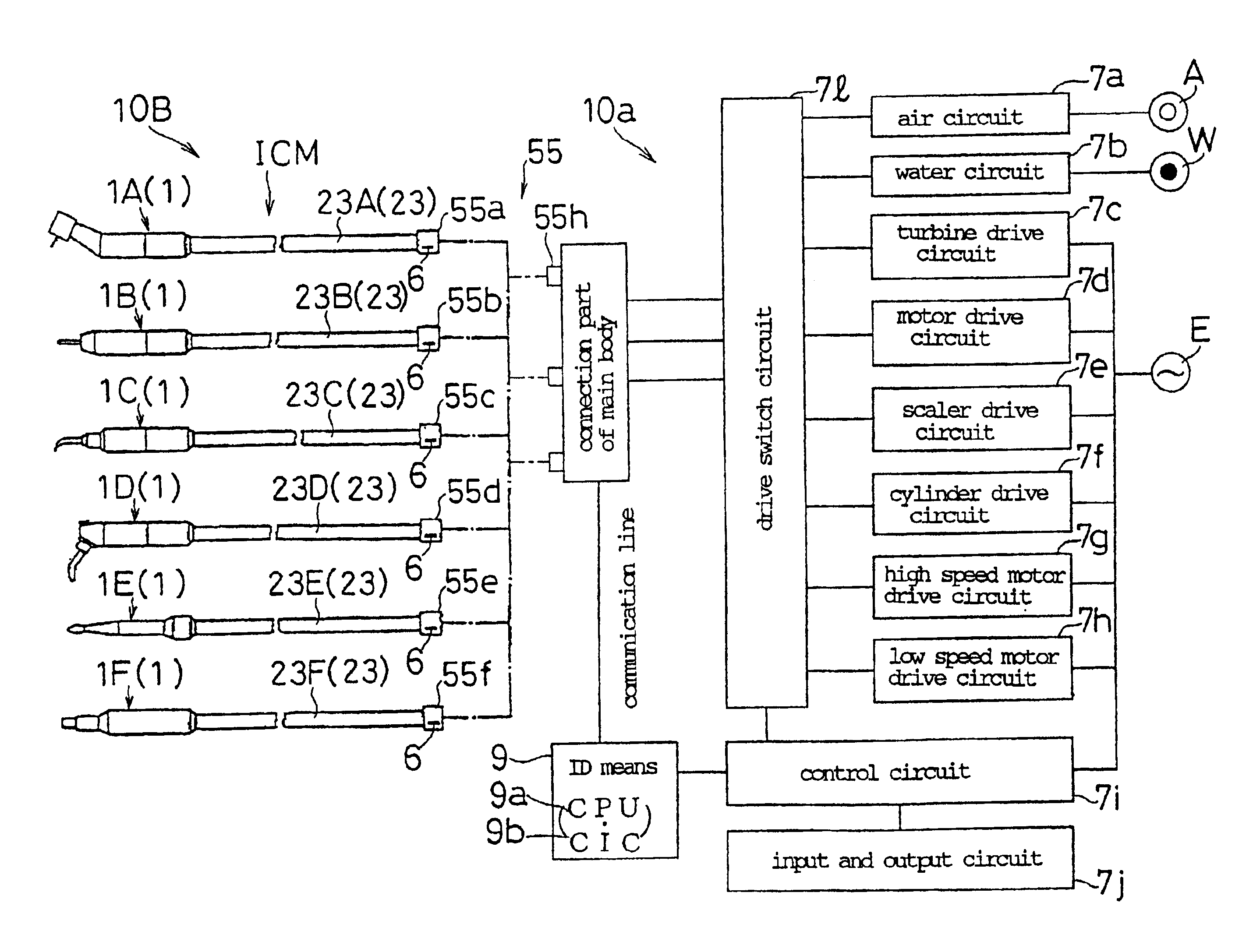
Identification type instrument assembly, identification type adapter, identification type tube, and medical apparatus using them
InactiveUS6899538B2Easily and surely specifiedImprove the problemDiagnosticsSurgeryMedical deviceBiomedical engineering
An identification type instrument assembly detachably connected to a main body of a medical apparatus for use in diagnosis and treatment. The instrument assembly is provided with identification signal output means for actively outputting unique self-identification signals prepared in advance under a predetermined procedure, and the connected instrument assembly can be specified in the main body by the identification signals outputted from the identification signal output means when the instrument assembly is connected to the main body.
Owner:MORITA MFG CO LTD
Method and system for integrated medical tracking
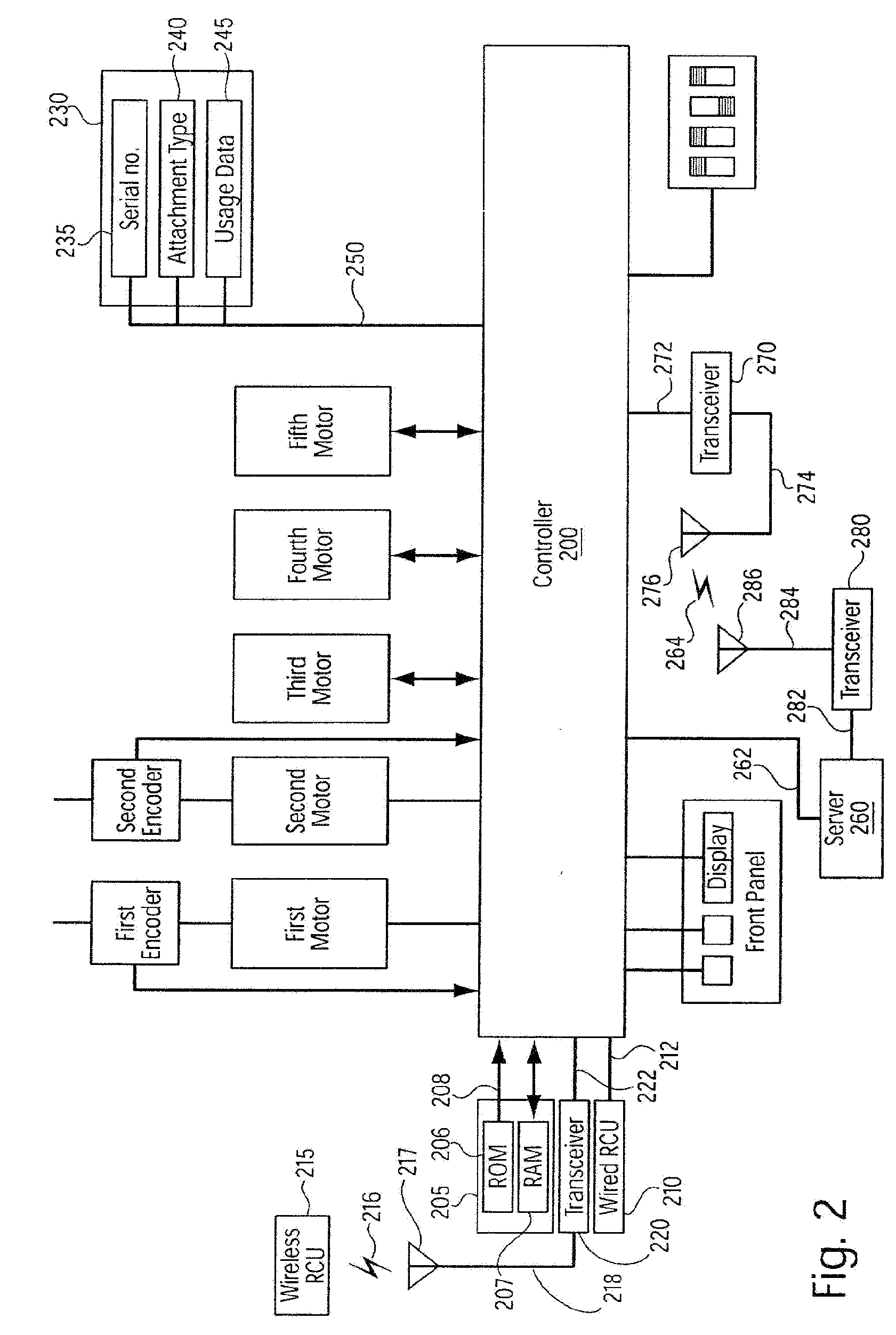
InactiveUS20090099876A1Thorough and integrated and accurate data collectionAccurate data collectionMechanical/radiation/invasive therapiesDiagnostic recording/measuringPatient dataComputer science
A system for processing data provided by medical devices. The system includes a medical device having a memory device configured to store medical device information. The system also includes an information system connected to the medical device. The information system includes at least a server configured to receive at least a portion of the medical device information from the memory device of the medical device. The server is also configured to employ the medical device information to process at least one of patient data, prescription data and inventory / ordering data. The medical device information may include serial number data, device identifier data, operation data and / or usage data for the medical device. The patient data may include at least one of patient tracking data, patient recordkeeping data and patient billing data. The prescription data may include prescription issuance data, prescription filling data and prescription tracking data. The inventory / ordering data may include at least one of maintenance and replacement schedule data, administrator notification data, and automatically-generated medical device order data.
Owner:TYCO HEALTHCARE GRP LP
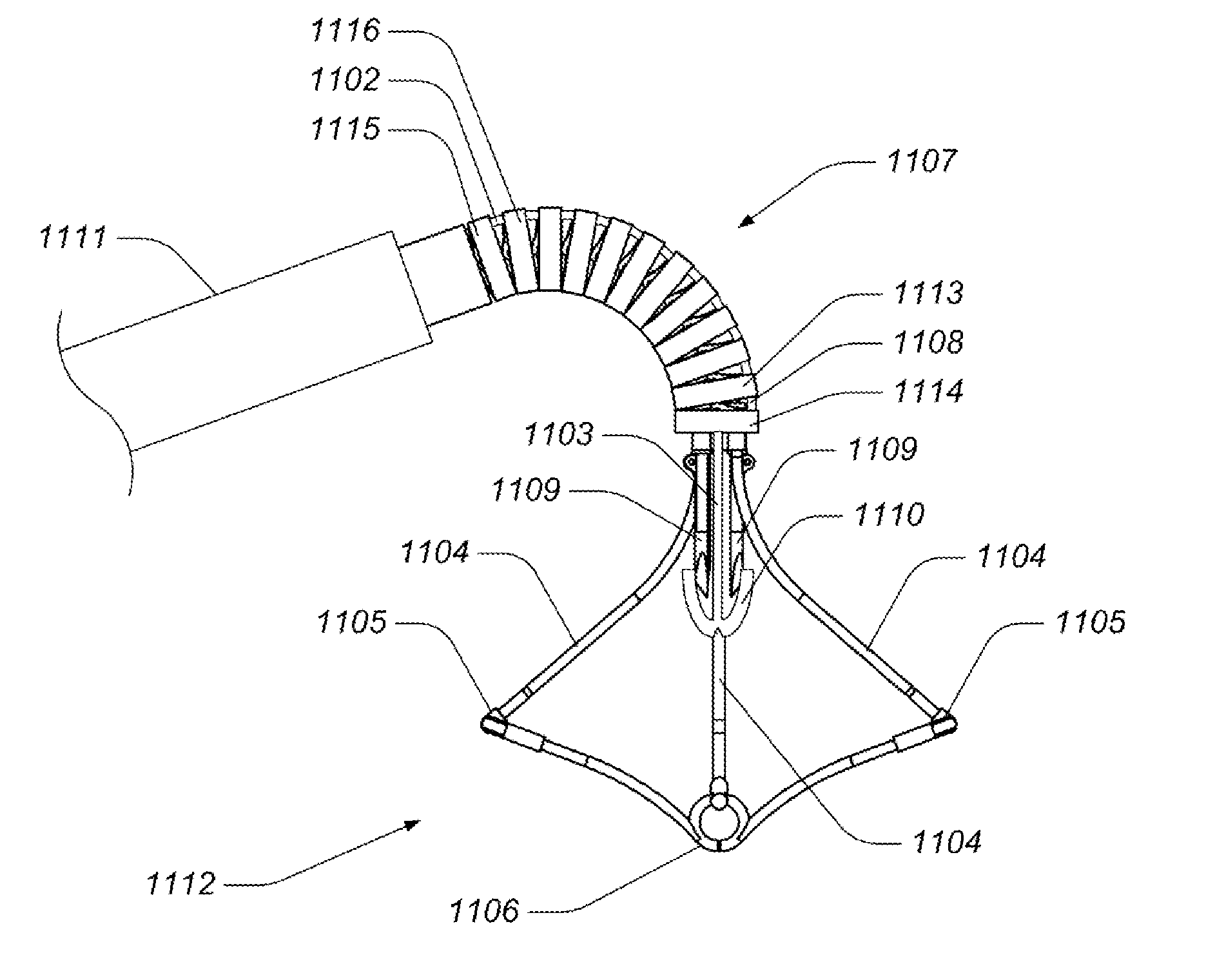
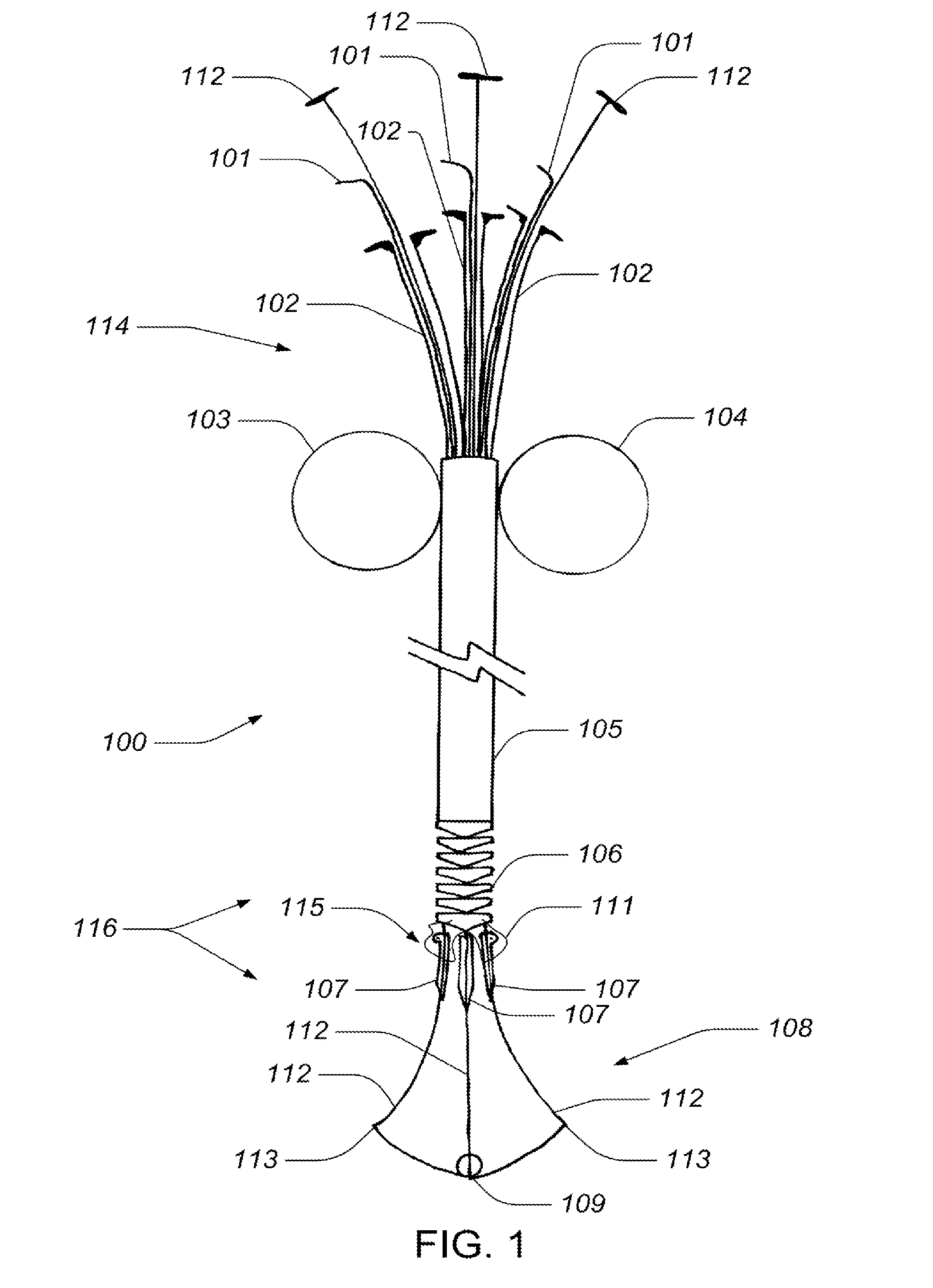
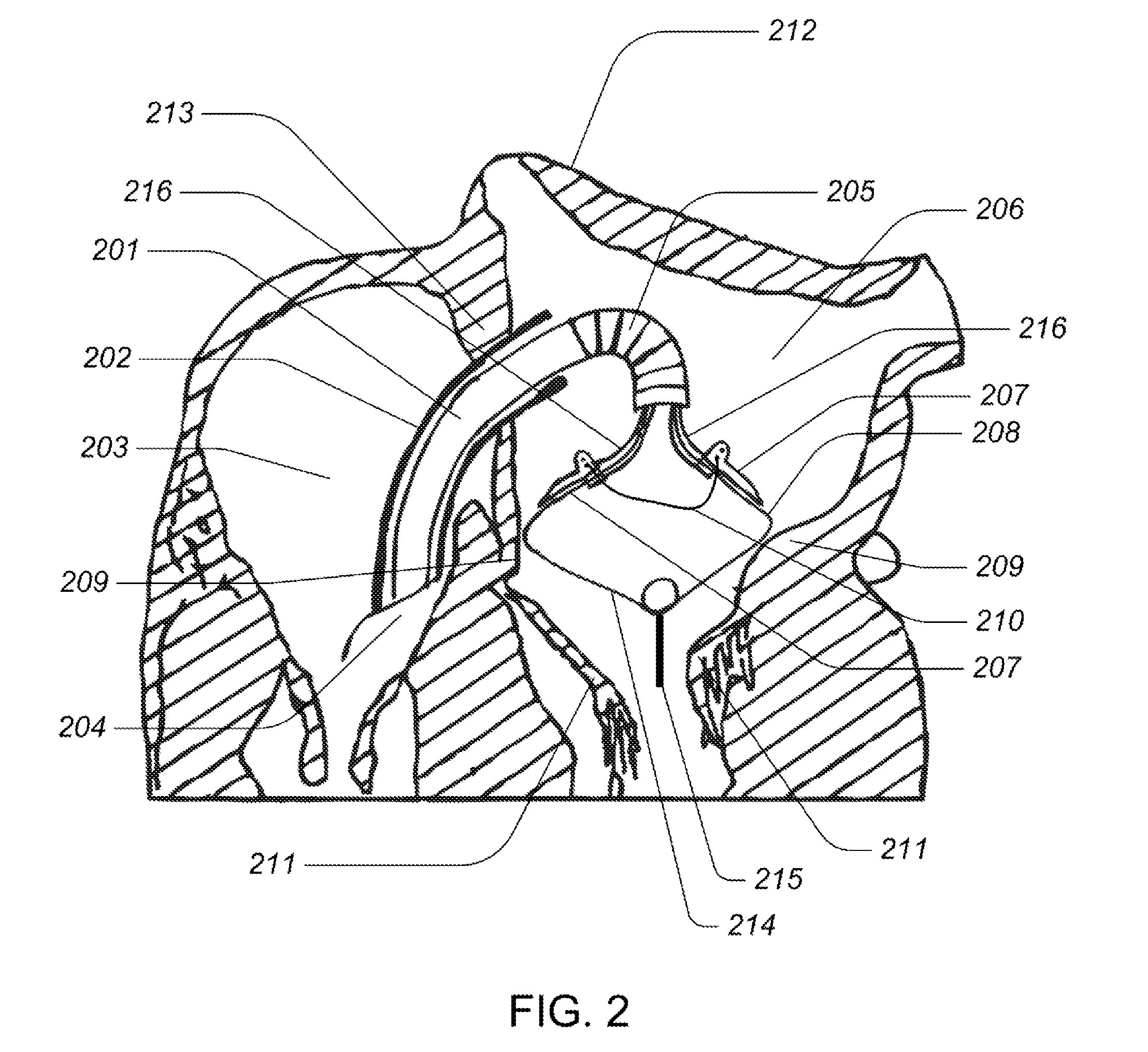
Medical device, kit and method for constricting tissue or a bodily orifice, for example, a mitral valve
InactiveUS20110082538A1Prevent retreatSuture equipmentsBone implantPosterior leafletAnnuloplasty rings
A device, kit and method may include or employ an implantable device (e.g., annuloplasty implant) and a tool operable to implant such. The implantable device is positionable in a cavity of a bodily organ (e.g., a heart) and operable to constrict a bodily orifice (e.g., a mitral valve). The tissue anchors may be guided into precise position by an intravascularly or percutaneously deployed anchor guide frame of the tool and embedded in an annulus of the orifice. Constriction of the orifice may be accomplished via a variety of structures, for example by cinching a flexible cable or via a anchored annuloplasty ring, the cable or ring attached to the tissue anchors. The annuloplasty ring may be delivered in an unanchored, generally elongated configuration, and implanted in an anchored generally arch, arcuate or annular configuration. Such may approximate the septal and lateral (clinically referred to as anterior and posterior) annulus of the mitral valve, to move the posterior leaflet anteriorly and the anterior leaflet posteriorly, thereby improving leaflet coaptation to eliminate mitral regurgitation.
Owner:KARDIUM
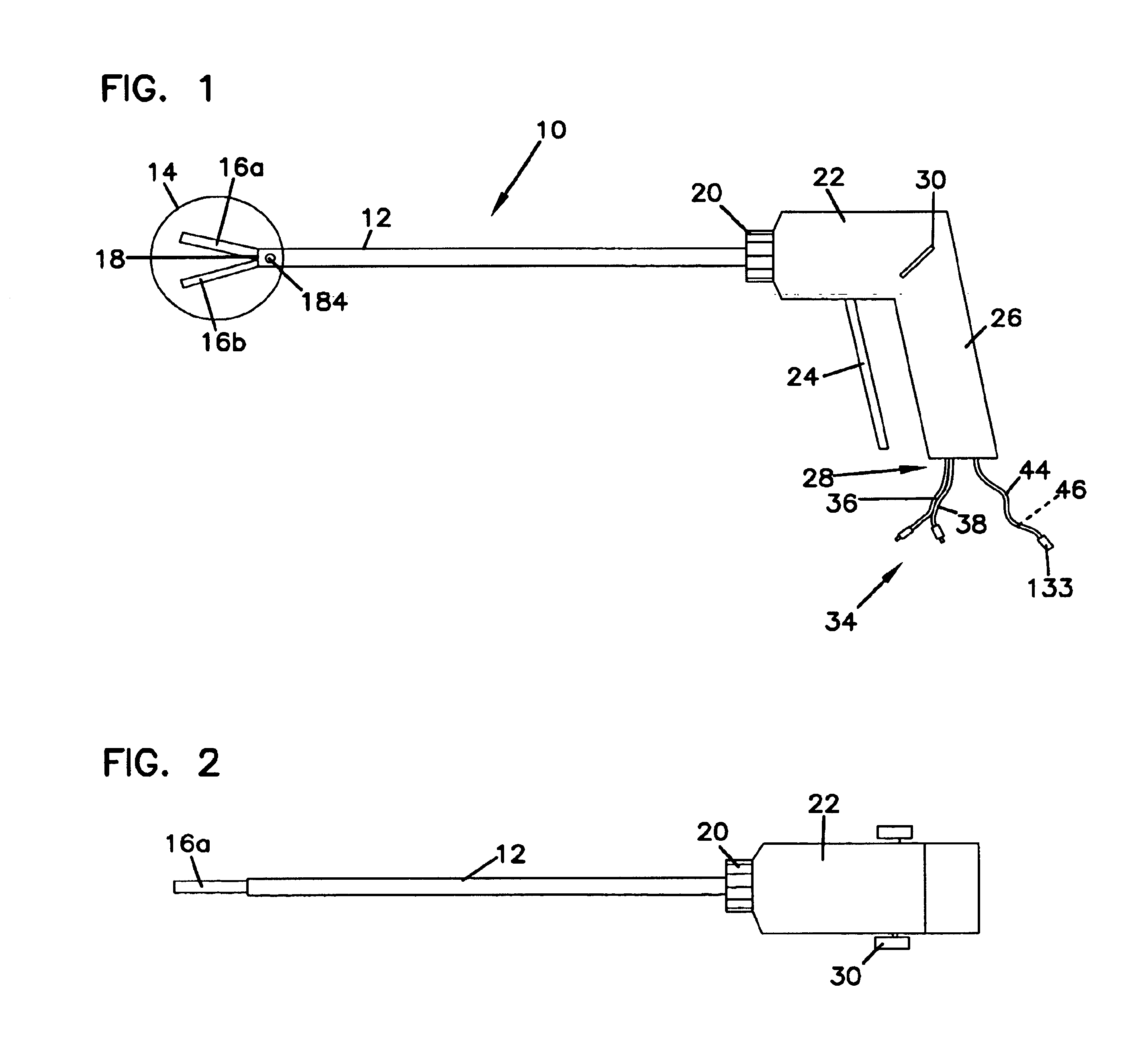
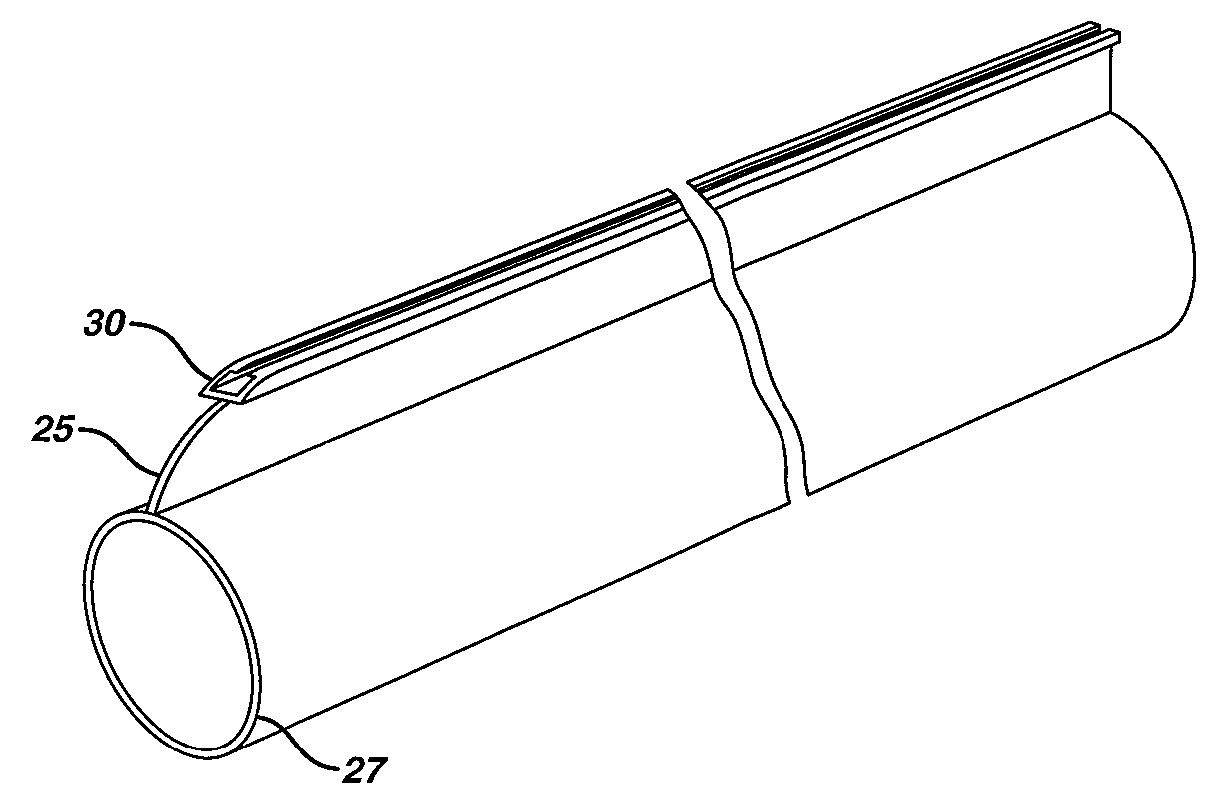
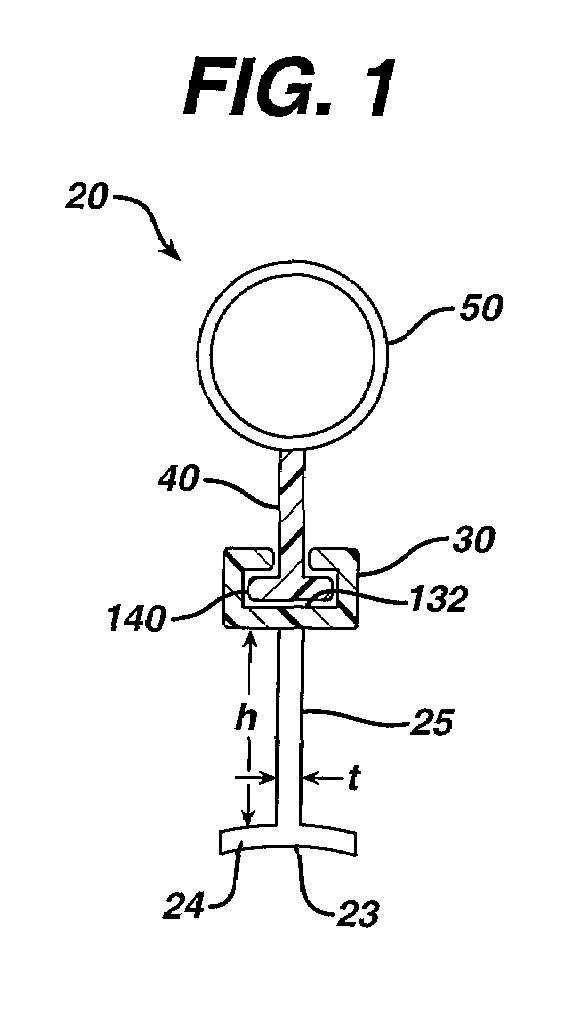
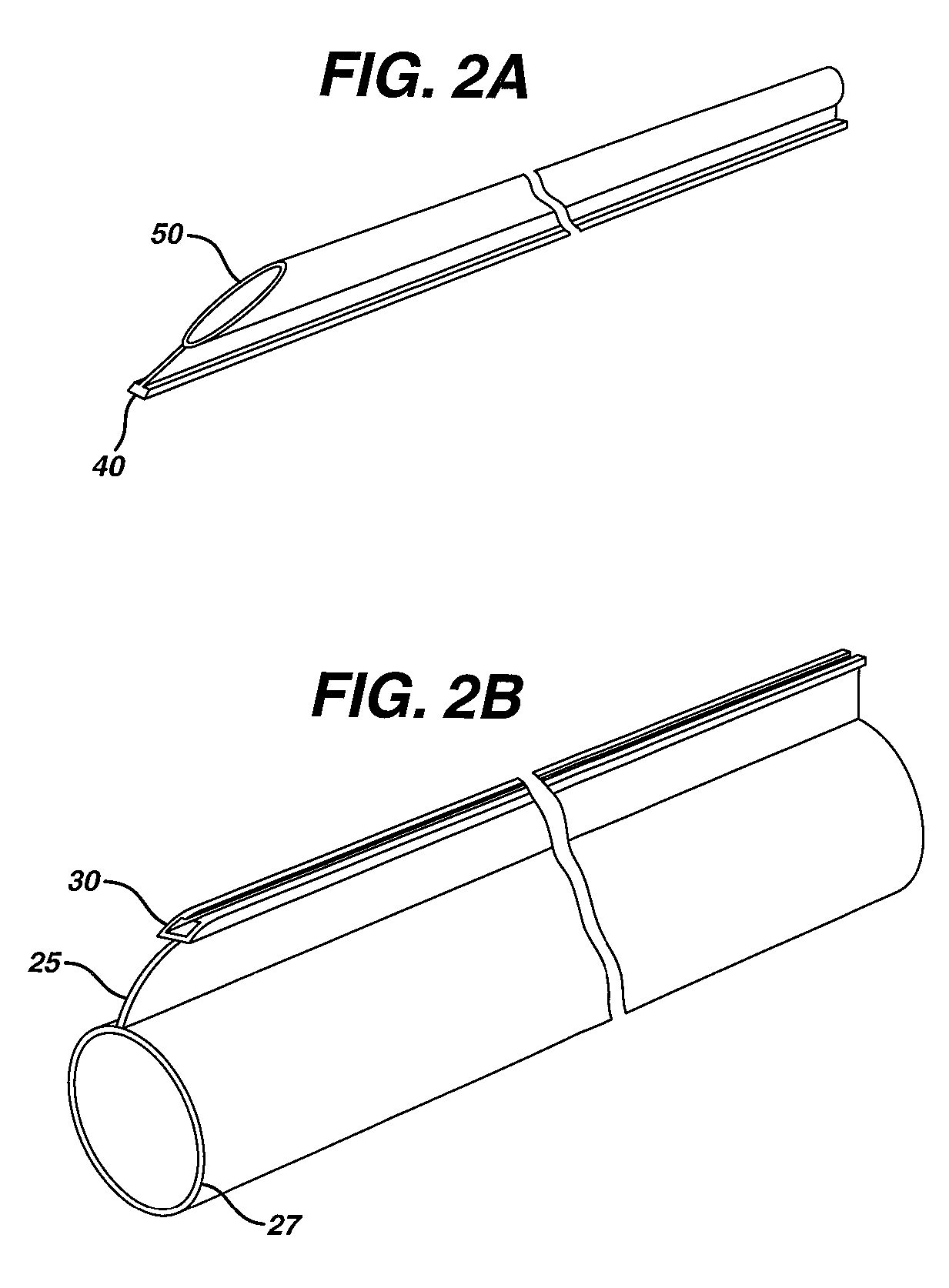
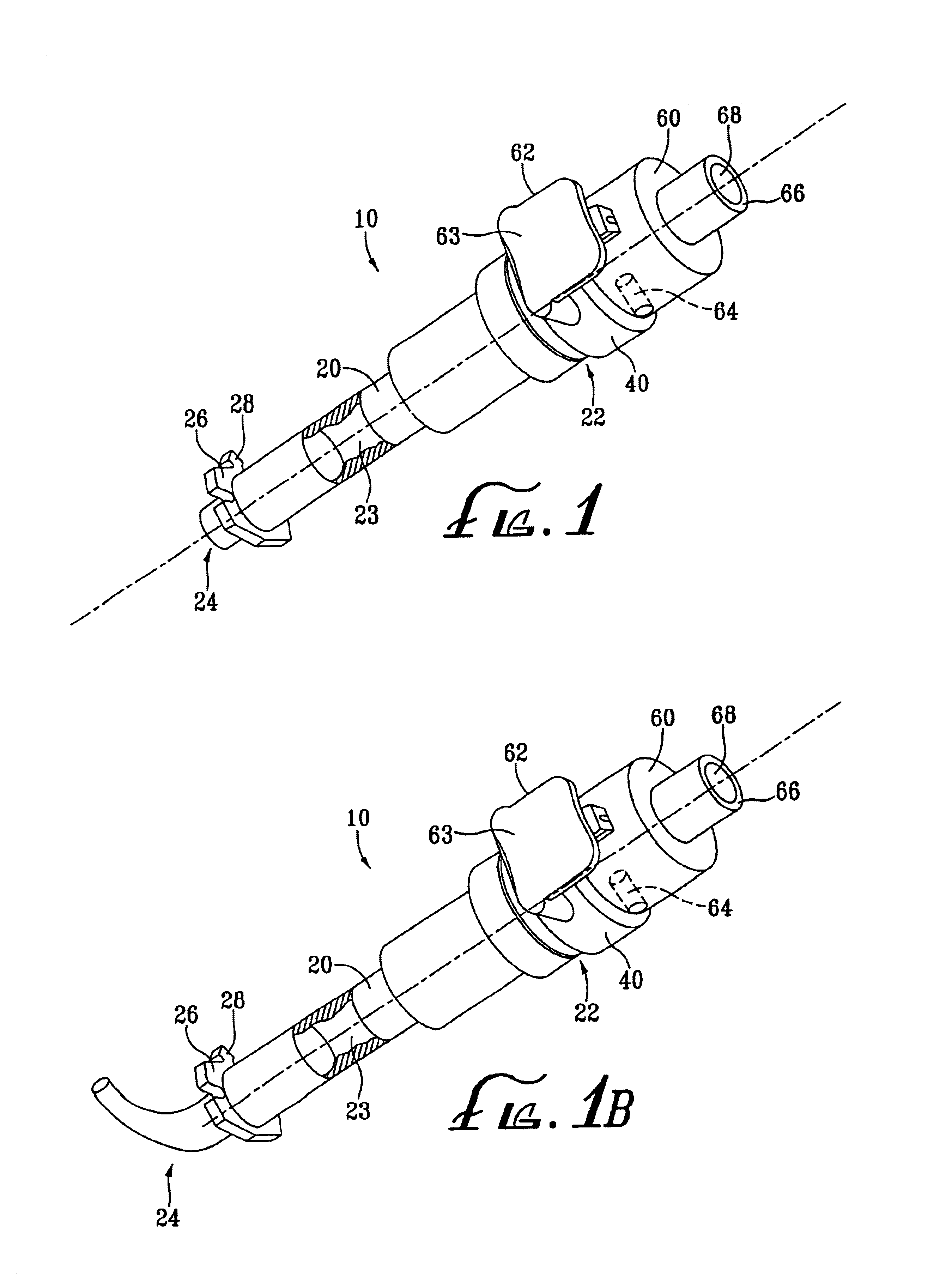
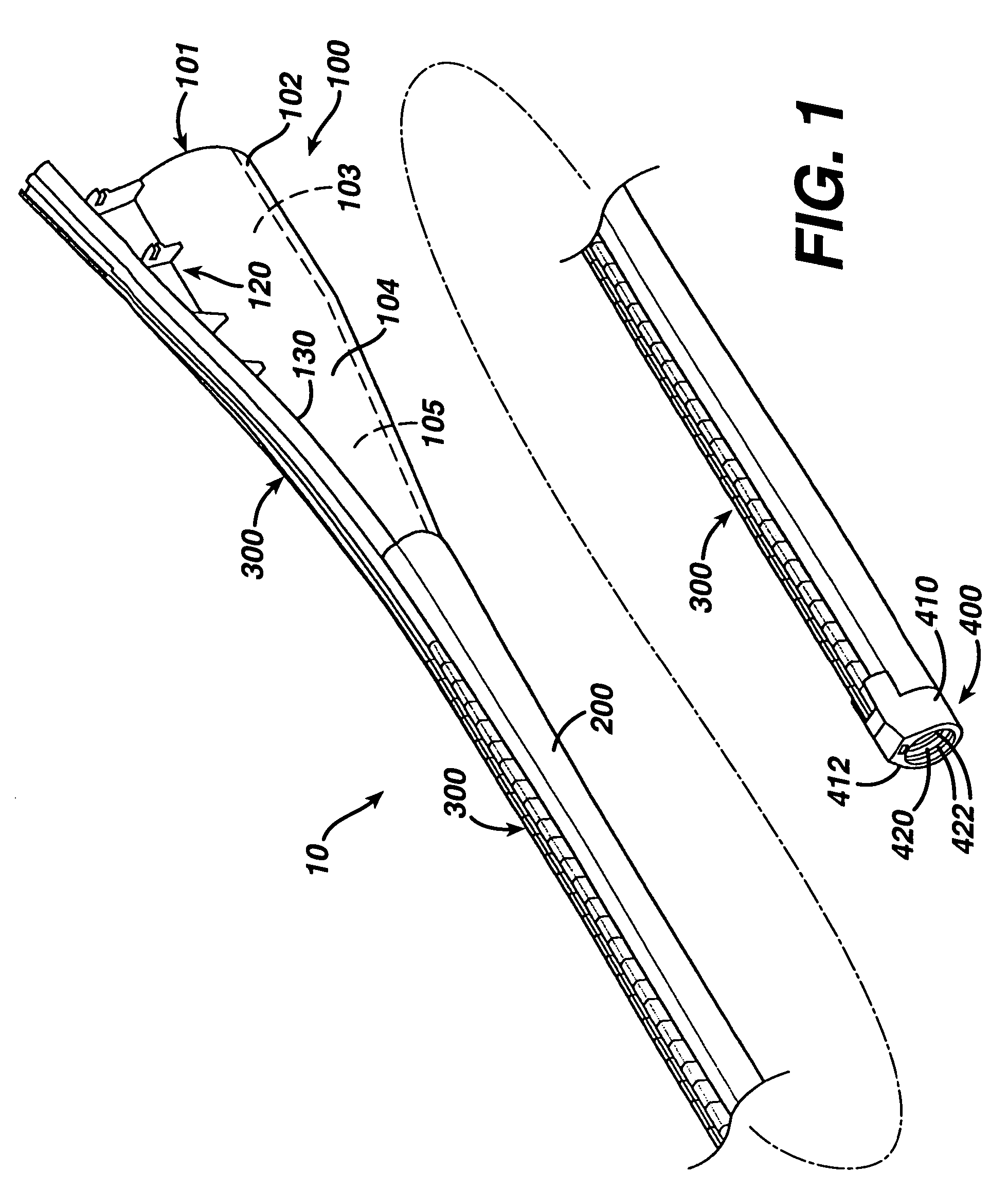
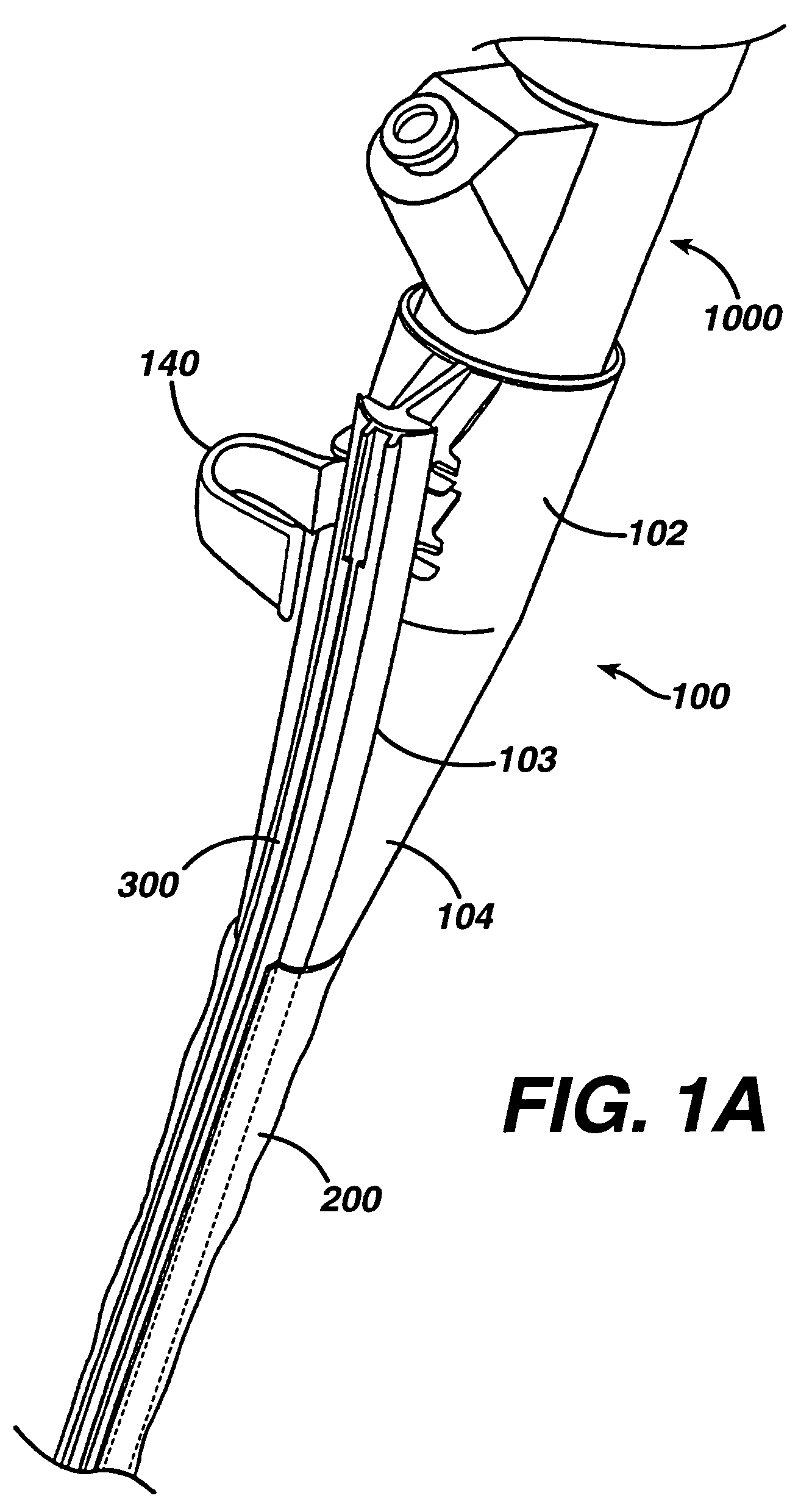
Method of guiding medical devices
ActiveUS7431694B2Change flexibilityFlexible and/or relatively short accessory medical instrumentsSurgeryEndoscopesEndoscopeGuide tube
A guide system for use with an endoscope, and a method of use is disclosed. The guide system can include a track, in the form of a rail, and a mating member for engaging the rail. The guide system can also include an accessory, such as an accessory guide tube through which a medical instrument can be carried external of the endoscope. An end cap can be provided to support the track relative to the distal end of the endoscope.
Owner:ETHICON ENDO SURGERY INC
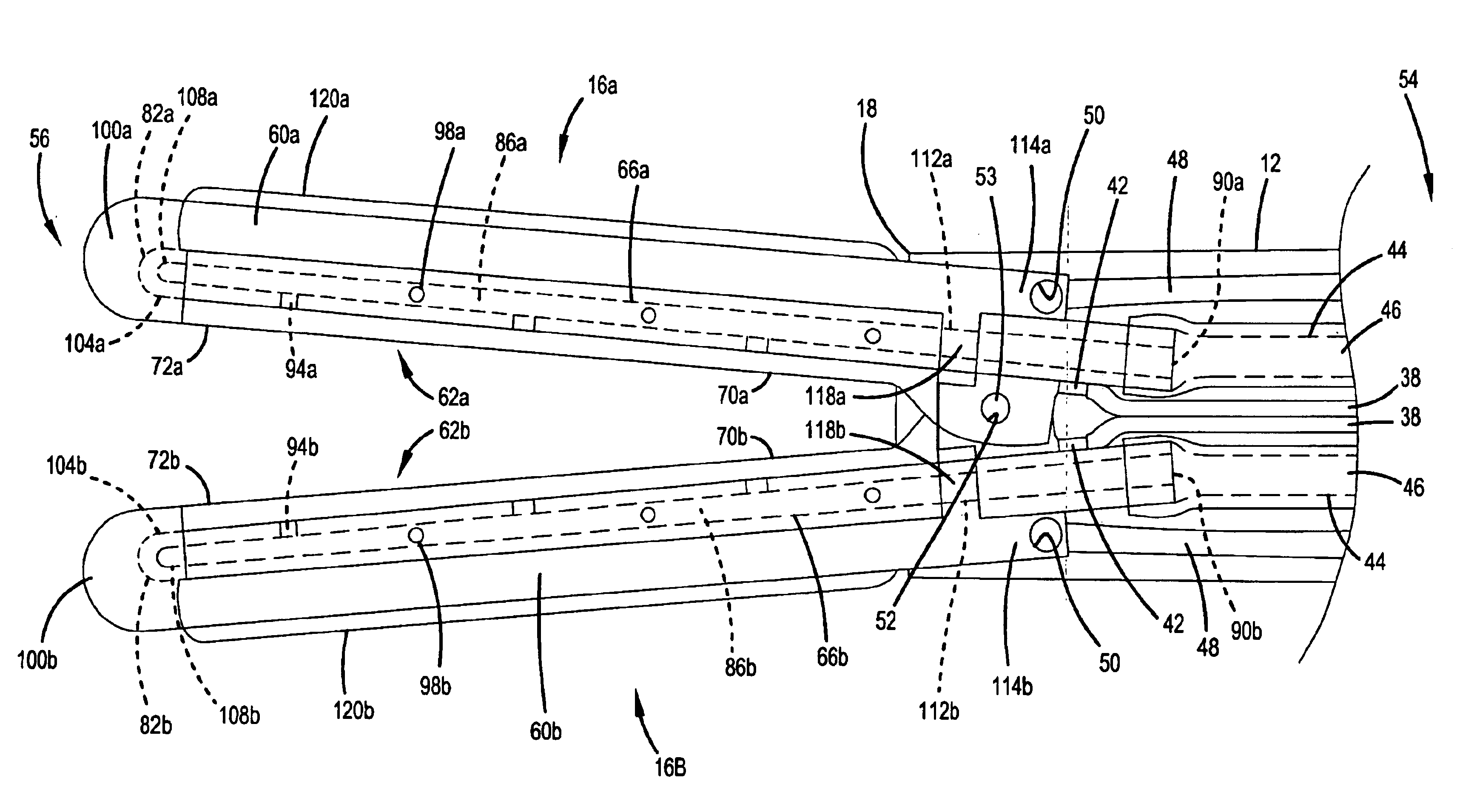
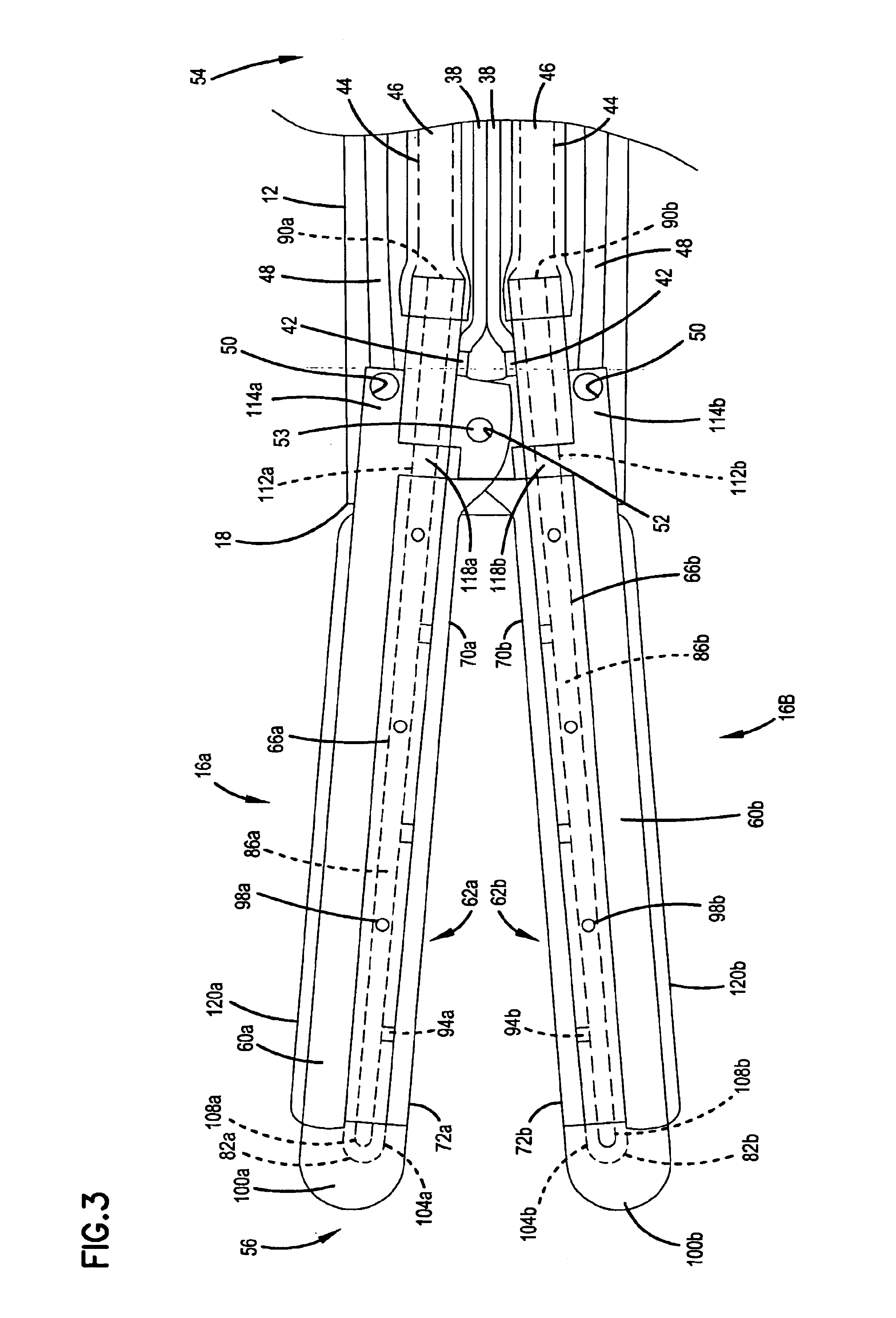
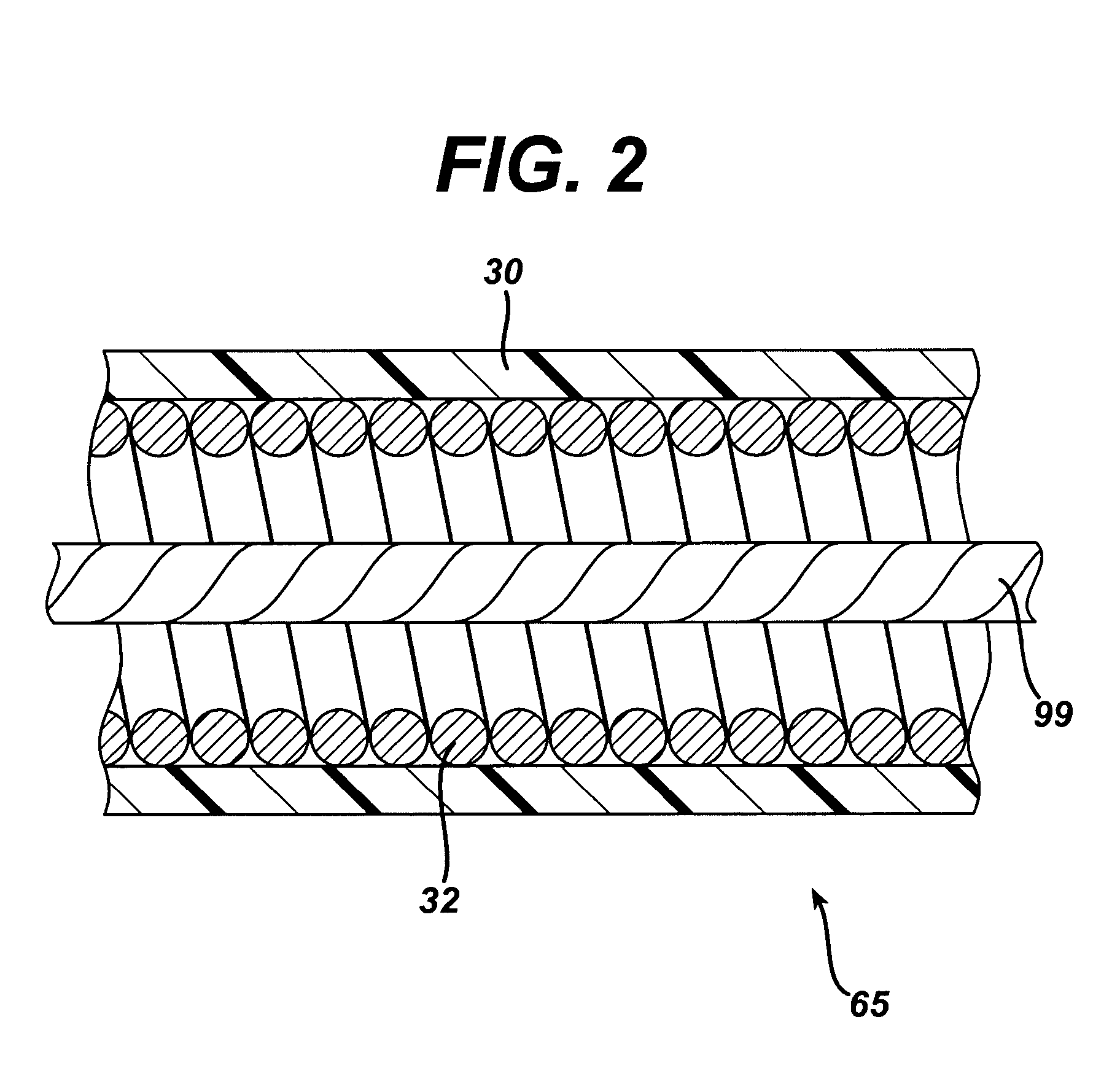
Articulation mechanism for medical devices
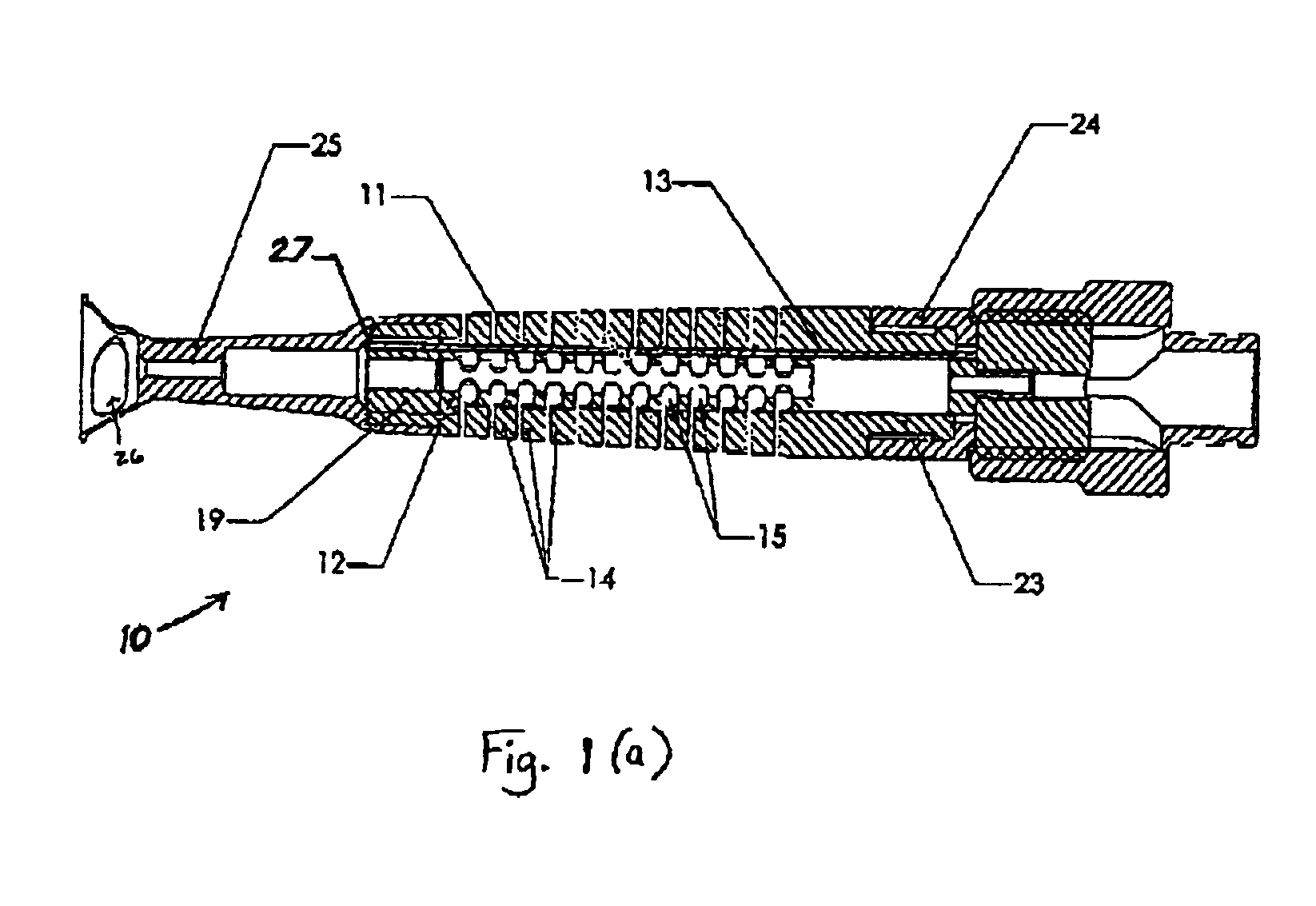
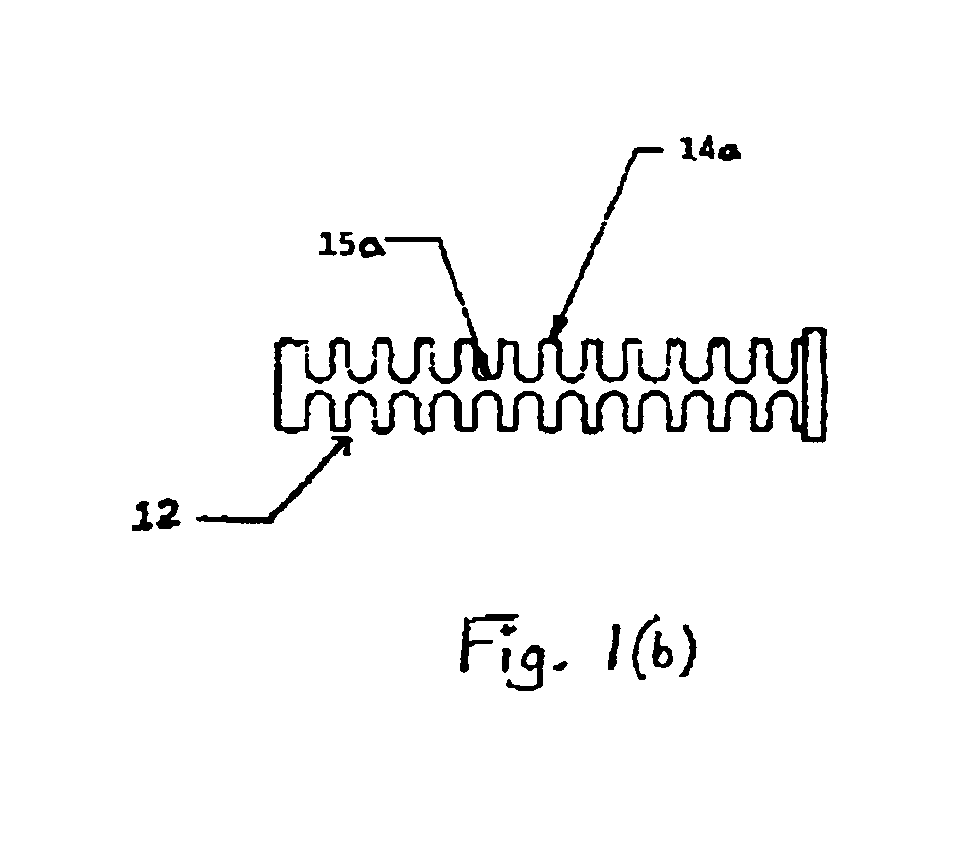
An improved articulating device for use with a medical insertion instrument comprising in part a first tube having a plurality of ribs defining a plurality of bending segments, a second tube axially disposed within the first tube, and means for transmitting an axial deflecting pull load. Such device has improved controlled for positioning an end of the device at a selected position within the body. The articulating device has a generally constant moment of inertia and a polar moment of inertia that generally decreases from its proximal to distal end.
Owner:EDWARDS LIFESCIENCES CORP
Method of operating an endoscopic device with one hand
ActiveUS7094202B2Minimize the potential for miscommunicationsAvoid insufficient lengthEndoscopesVaccination/ovulation diagnosticsEngineeringActuator
An endoscopic accessory medical device is provided. The device can include a handle, a flexible shaft, and an end effector. The handle can include an actuator for operating the end effector through a wire or cable pulling member that extends through the flexible shaft. The handle and actuator can be operable with a single hand, such that the operation of the end effector can be accomplished with the same hand that is used to hold the handle and advance the end effector through an endoscope. The handle can include an actuation mechanism that is decoupled from operation of the end effector when the actuator is in a first open position, which becomes operatively coupled to the end effector when the actuator is moved to a second position, such as by squeezing the actuator, and which operates the end effector when the actuator is moved further to a third position.
Owner:ETHICON ENDO SURGERY INC
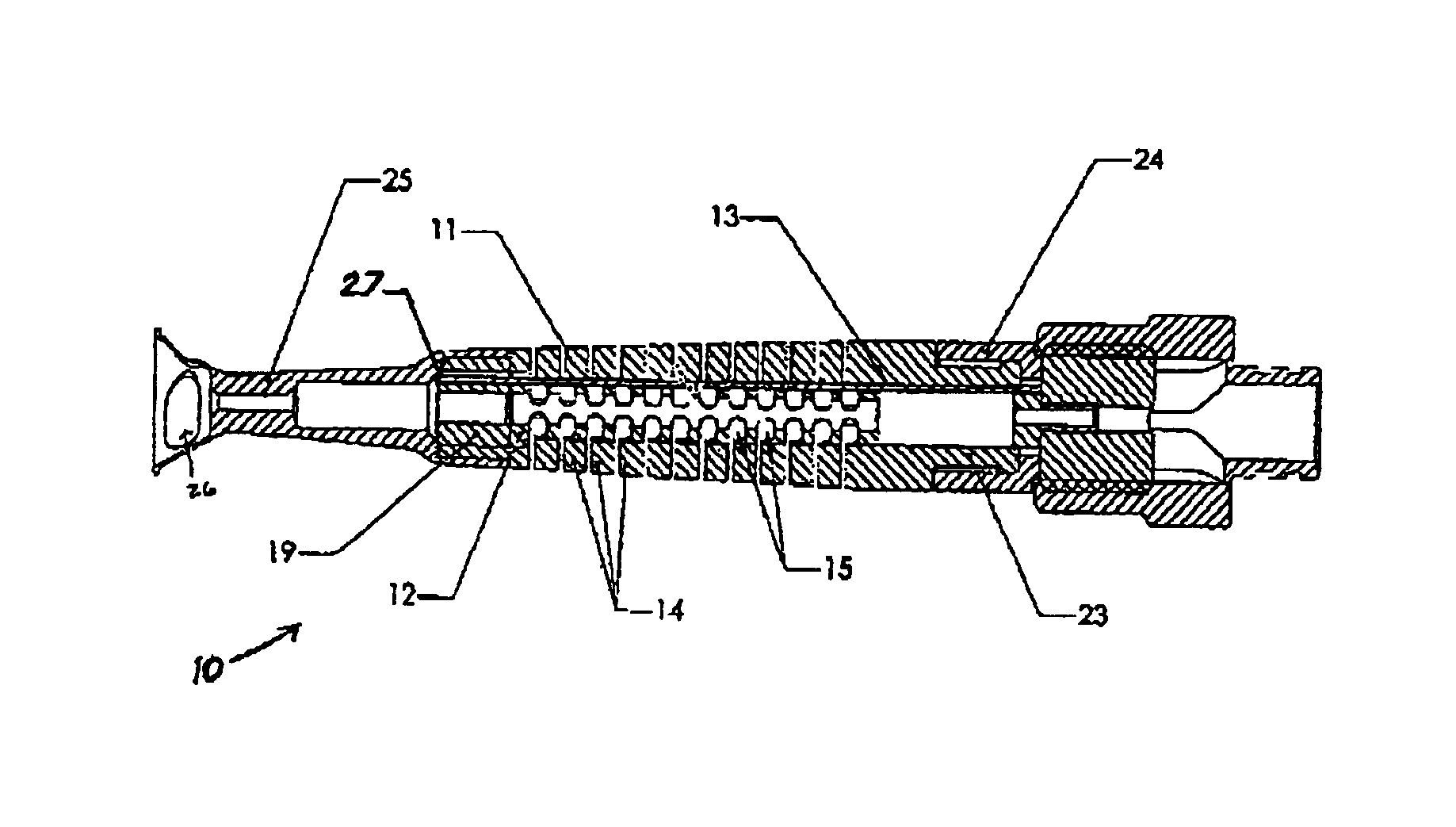
Medical device introducer and obturator
An introducer having an elongate tubular member and a device connector releasably attached to a proximal end of the tubular member. The introducer allows exchange of medical instruments, such as a blood filter and cardioplegia catheter, through a single lumen. An obturator having a retractable blade for making incision on a tissue is insertable through the lumen of the introducer. Methods of using the obturator and the introducer for introducing medical device(s) into body cavity, such as a vessel or cardiac tissue, are also disclosed.
Owner:EDWARDS LIFESCIENCES CORP
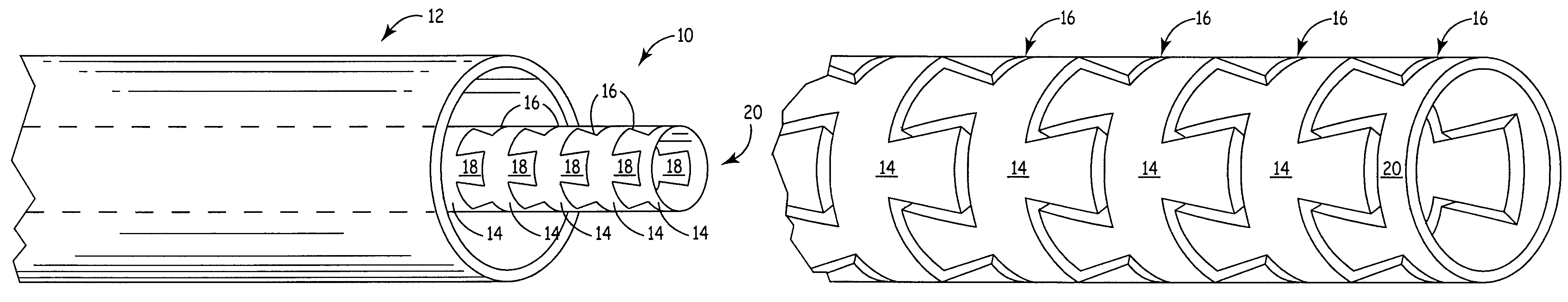
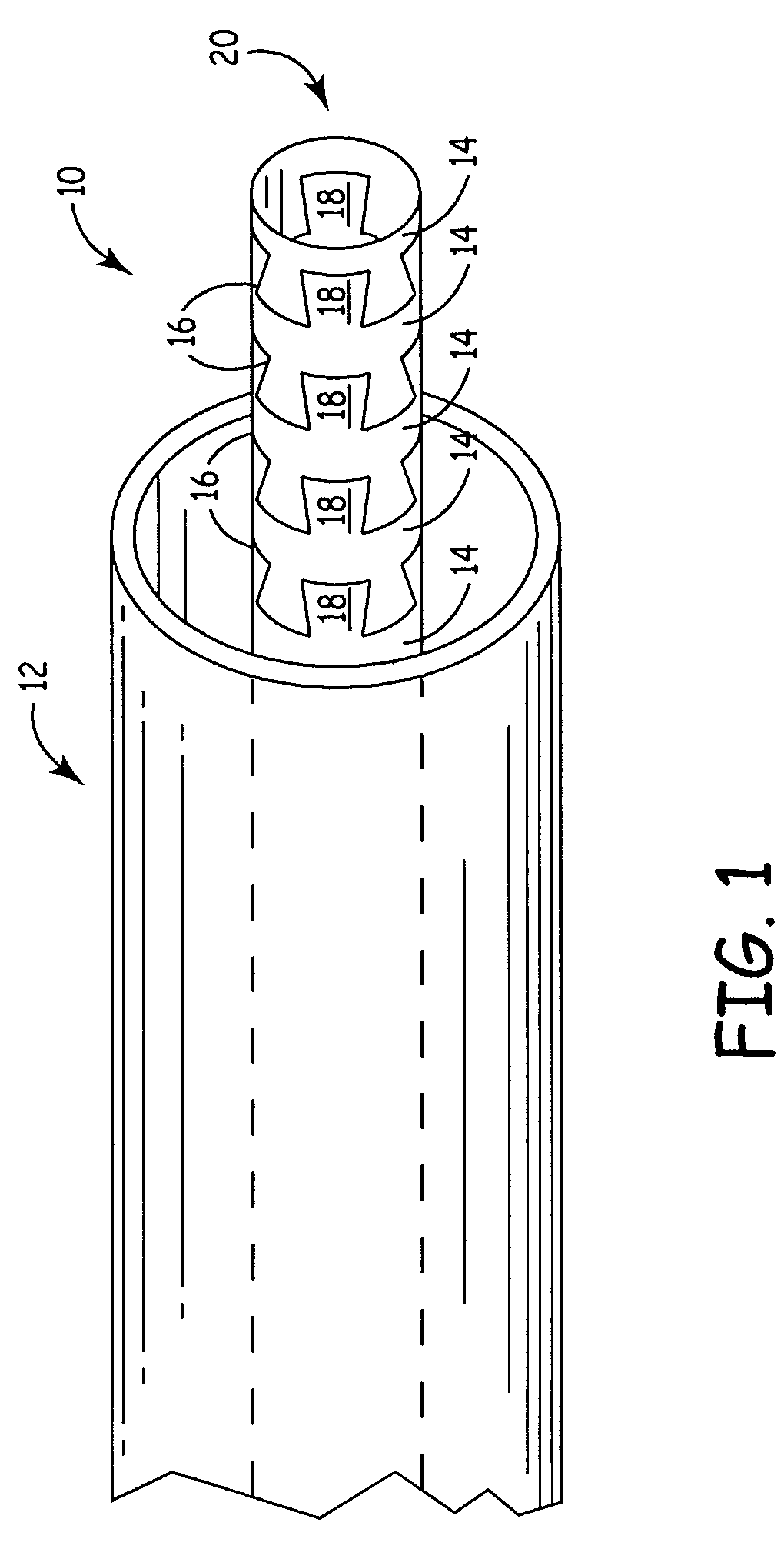
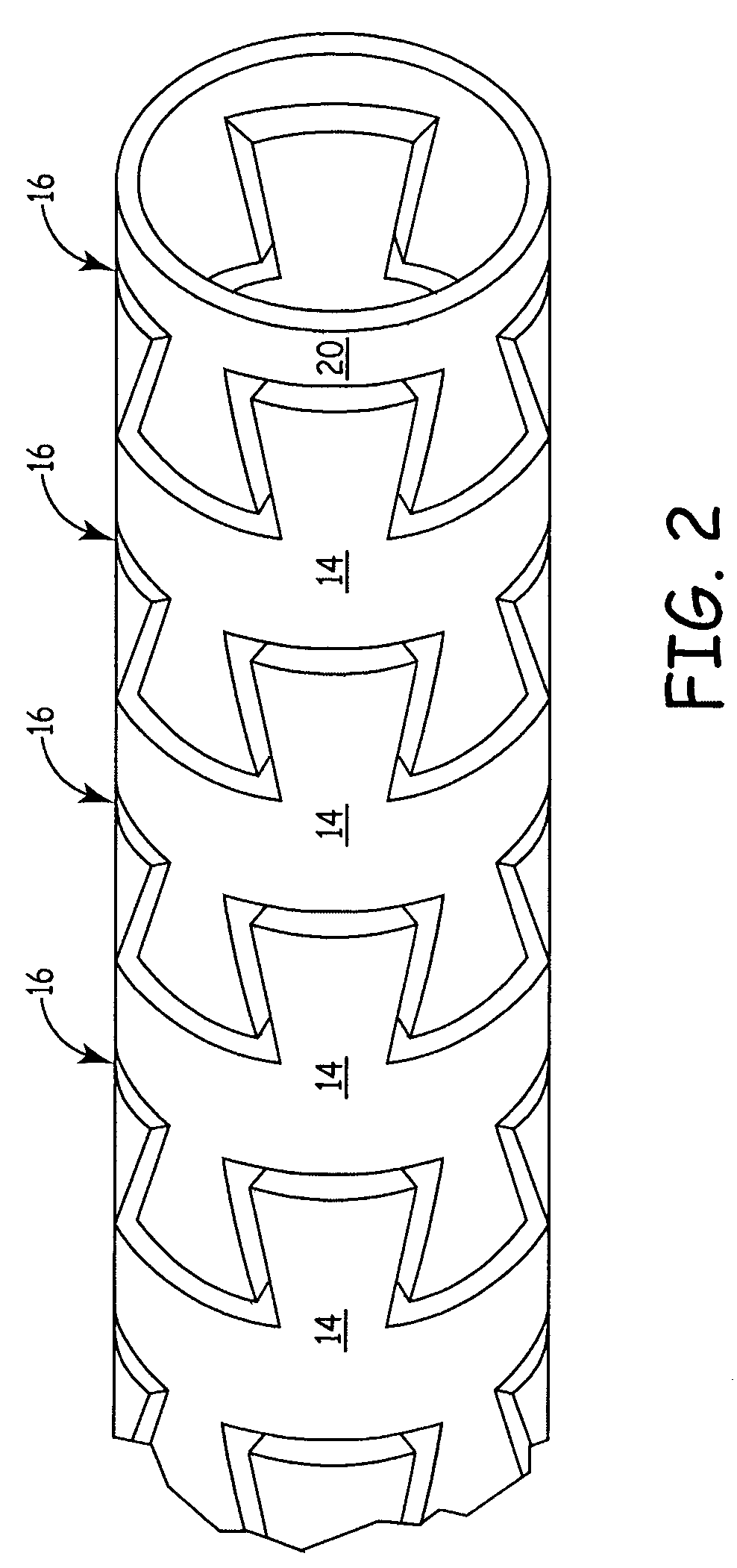
Flexible medical device
InactiveUS7413563B2Improved torque and flexure characteristicCatheterSurgical forcepsMedical deviceBiomedical engineering
This invention relates to flexible medical device. The flexible medical device may comprise interlocking portions formed of a continuous channel. When formed of a continuous channel, the medical device comprises interconnected interlocking portions, rather than independent interlocking portions. The flexible medical device may also be designed so that different portions of the device have different flexibility characteristics.
Owner:CARDIA INC
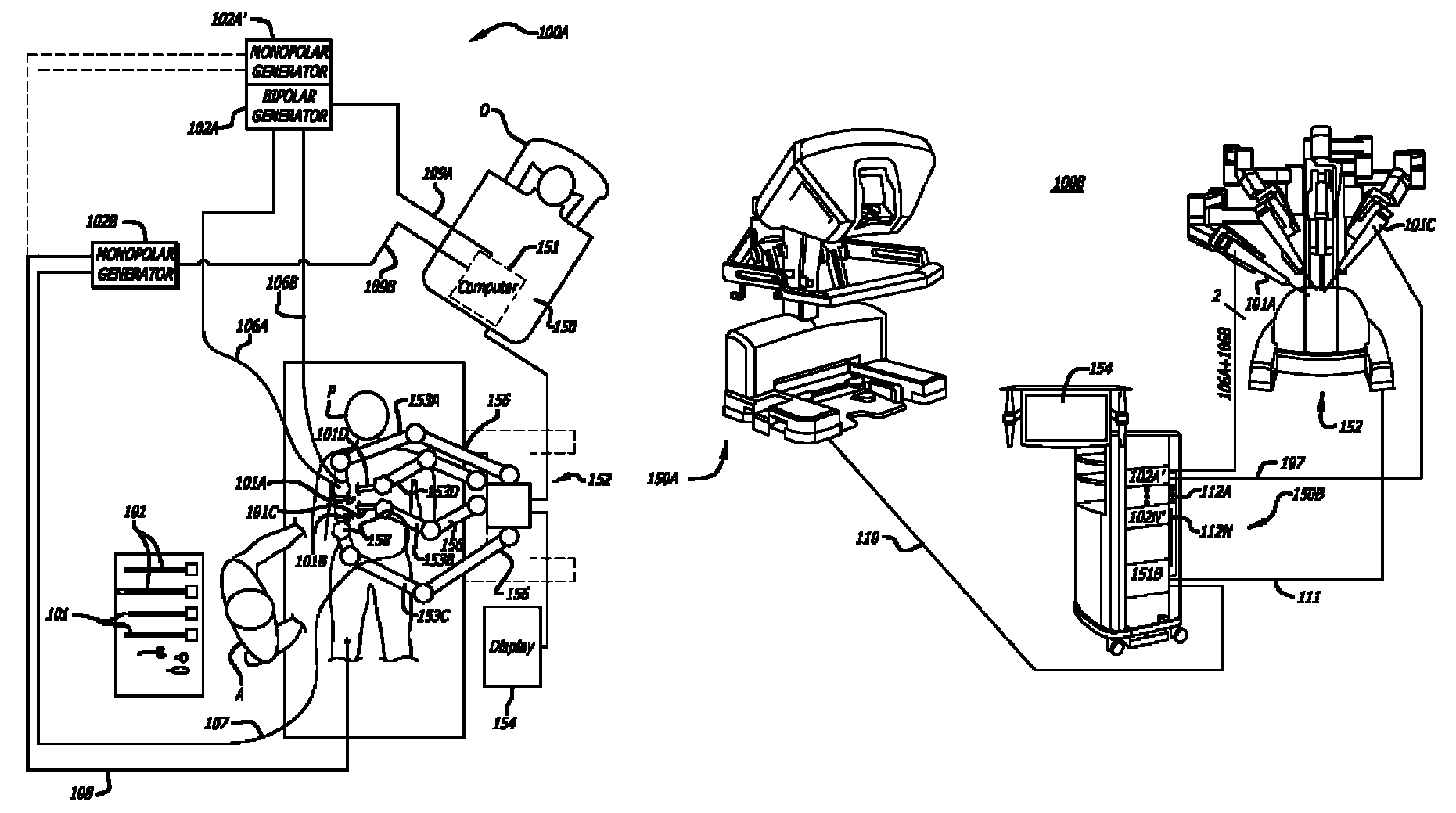
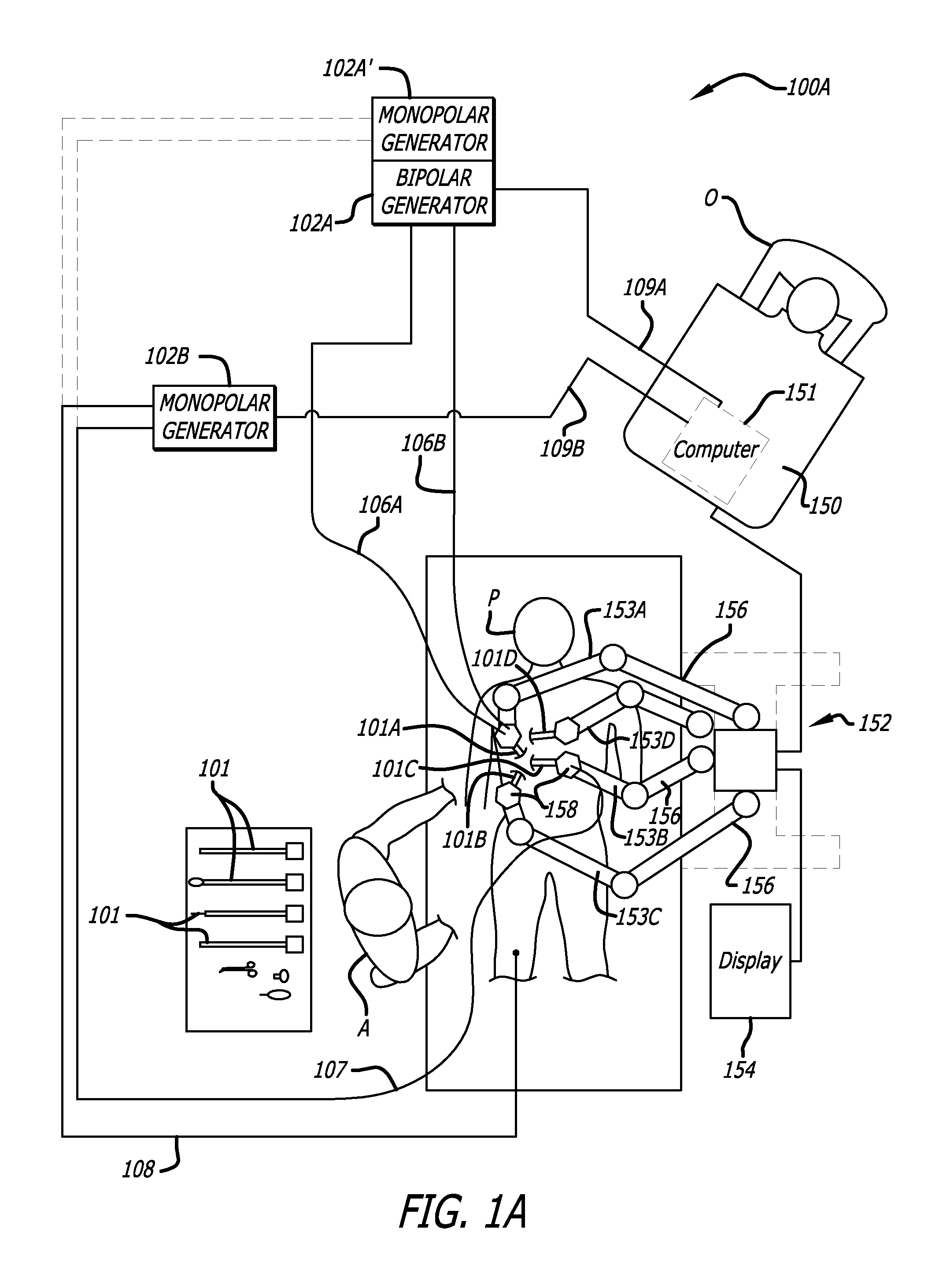
Ergonomic surgeon control console in robotic surgical systems
A control console to remotely control medical equipment is disclosed having a base with an ergonomically adjustable pedal system. The base further has an opening to receive the pedal system. The pedal system includes a moveable pedal tray with a pedal base. The tray includes a first left pedal assembly and a first right pedal assembly, and an upper tier having a second left pedal assembly and a second right pedal assembly respectively in alignment with and elevated above the first left pedal assembly and the first right pedal assembly. Rollers are rotatable coupled to the moveable pedal tray to allow it roll over a floor. A drive assembly is coupled between the moveable pedal tray and the base. The drive assembly applies a force to the to roll the moveable pedal tray over the floor within the opening of the base.
Owner:INTUITIVE SURGICAL +1
Force switch
ActiveUS7479608B2Contact surface shape/structureInternal osteosythesisMedical deviceMedical treatment
Owner:ETHICON ENDO SURGERY INC
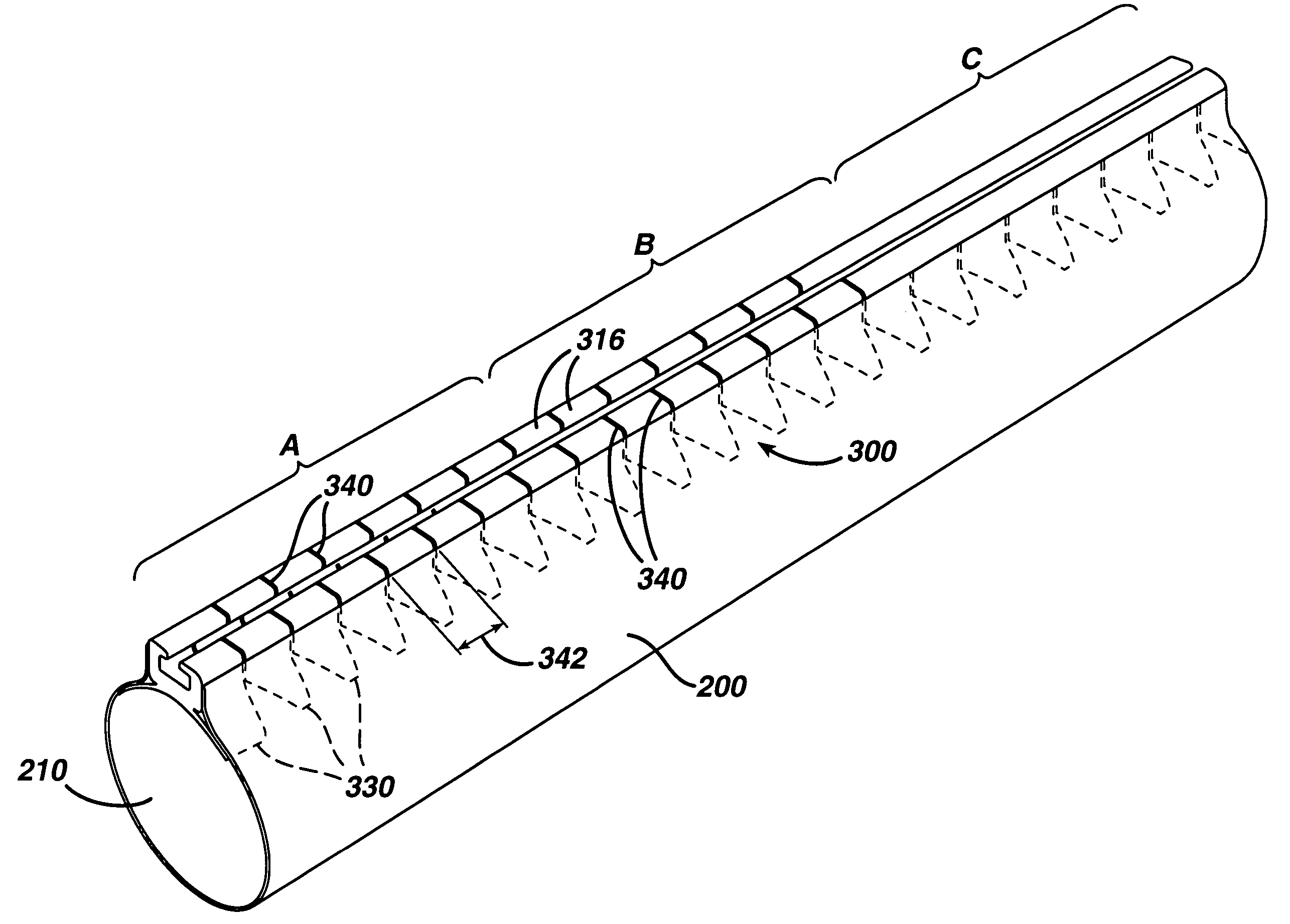
Track for medical devices
ActiveUS7615003B2Reduce in quantityQuickly and consistentlyGastroscopesCannulasMedical deviceFeeding tube
A medical apparatus and method useful for positioning one or more members within the gastro-intestinal tract is disclosed. The medical apparatus can include a track supported on a sheath sized to receive an endoscope, and a carrier slidable with respect to the track. A feeding tube accessory adapted to slidably engage the carrier is disclosed.
Owner:ETHICON ENDO SURGERY INC
Method and apparatus for indicating an encountered obstacle during insertion of a medical device
A technique for detecting and indicating an internal anatomical obstacle encountered during insertion of a medical device into the body of a patient, comprising an elongated member such as a tube, catheter, guidewire, or other device, having a location indicating element, such as a permanent magnet, flexibly coupled to its distal end, and an external detector that tracks and displays the location and orientation of the location indicating element. The flexible coupling has sufficient stiffness to maintain the orientation of the location indicating element against the forces from both gravity and flowing blood within a patient's vasculature, but allows the location indicating element to change orientation if it encounters an obstacle during insertion. The medical caregiver monitors the detector's display and determines encounters with obstacles by observing changes in the orientation of the location indicating element.
Owner:LUCENT MEDICAL SYST
Medical device with improved wall construction
InactiveUS7097644B2Torsional stiffness sufficientComfortably insertedEndoscopesSurgical instruments for heatingMedical deviceBiomedical engineering
A medical device having a laminate elongate hollow member is disclosed. The laminate member provides torsional stiffness for controlling positioning of the distal end of the device, and provides bending flexibility for assisting in insertion of the device in the patient's esophagus or other body lumen.
Owner:ETHICON ENDO SURGERY INC
Feeding tube and track
InactiveUS20060258904A1Reduce in quantityQuickly and consistentlySurgical needlesEndoscopesFeeding tubeMedical device
A medical apparatus and method useful for positioning one or more members within the gastrointestinal tract is disclosed. The medical apparatus can include a track supported on a sheath sized to receive an endoscope, and a carrier slidable with respect to the track. A feeding tube accessory adapted to slidably engage the carrier is disclosed.
Owner:ETHICON ENDO SURGERY INC
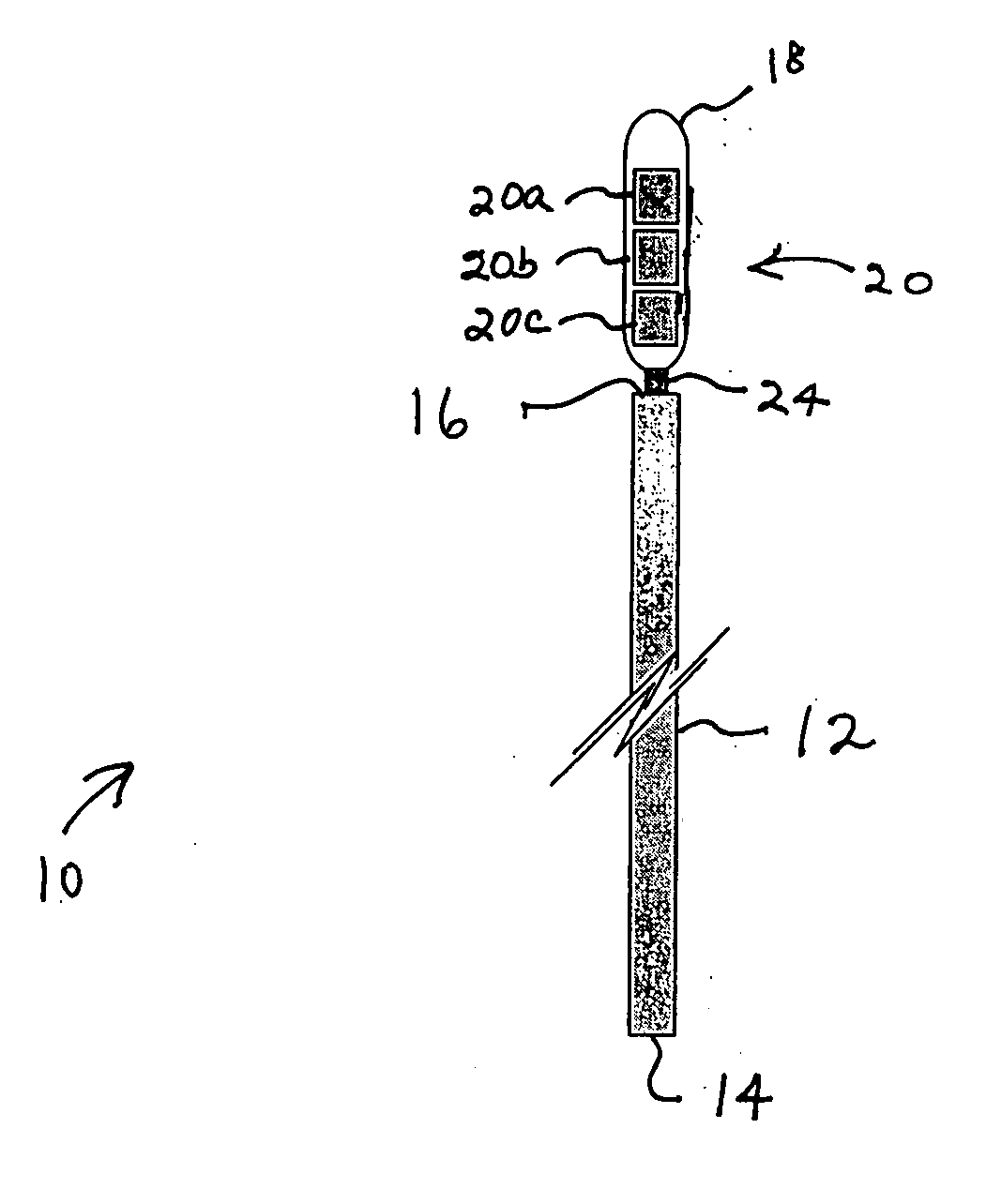
Medical device
A medical device includes a medical instrument with a transponder detachably fixed to the medical device. The transponder serves as a data medium about specific information of the medical instrument. The medical device further includes a data exchange mechanism for exchanging data with the transponder. When a medical treatment is to be performed, the medical instrument is placed to a receiving mechanism for receiving the medical instrument separated from the transponder, while the transponder is placed to the data exchange mechanism which is placed locally apart or locally placed different from the receiving mechanism so that disadvantageous influences of the medical treatment do not affect the transponder.
Owner:SCHMID +2
Microprocessor controlled ambulatory medical apparatus with hand held communication device
InactiveUS6873268B2Enhance user interfaceReduce system sizeEnergy efficient ICTElectrotherapyDrugs infusionHand held
An implantable infusion pump possesses operational functionality that is, at least in part, controlled by software operating in two processor ICs which are configured to perform some different and some duplicate functions. The pump exchanges messages with an external device via telemetry. Each processor controls a different part of the drug infusion mechanism such that both processors must agree on the appropriateness of drug delivery for infusion to occur. Delivery accumulators are incremented and decremented with delivery requests and with deliveries made. When accumulated amounts reach or exceed, quantized deliverable amounts, infusion is made to occur. The accumulators are capable of being incremented by two or more independent types of delivery requests. Operational modes of the infusion device are changed automatically in view of various system errors that are trapped, various system alarm conditions that are detected, and when excess periods of time lapse between pump and external device interactions.
Owner:MEDTRONIC MIMIMED INC
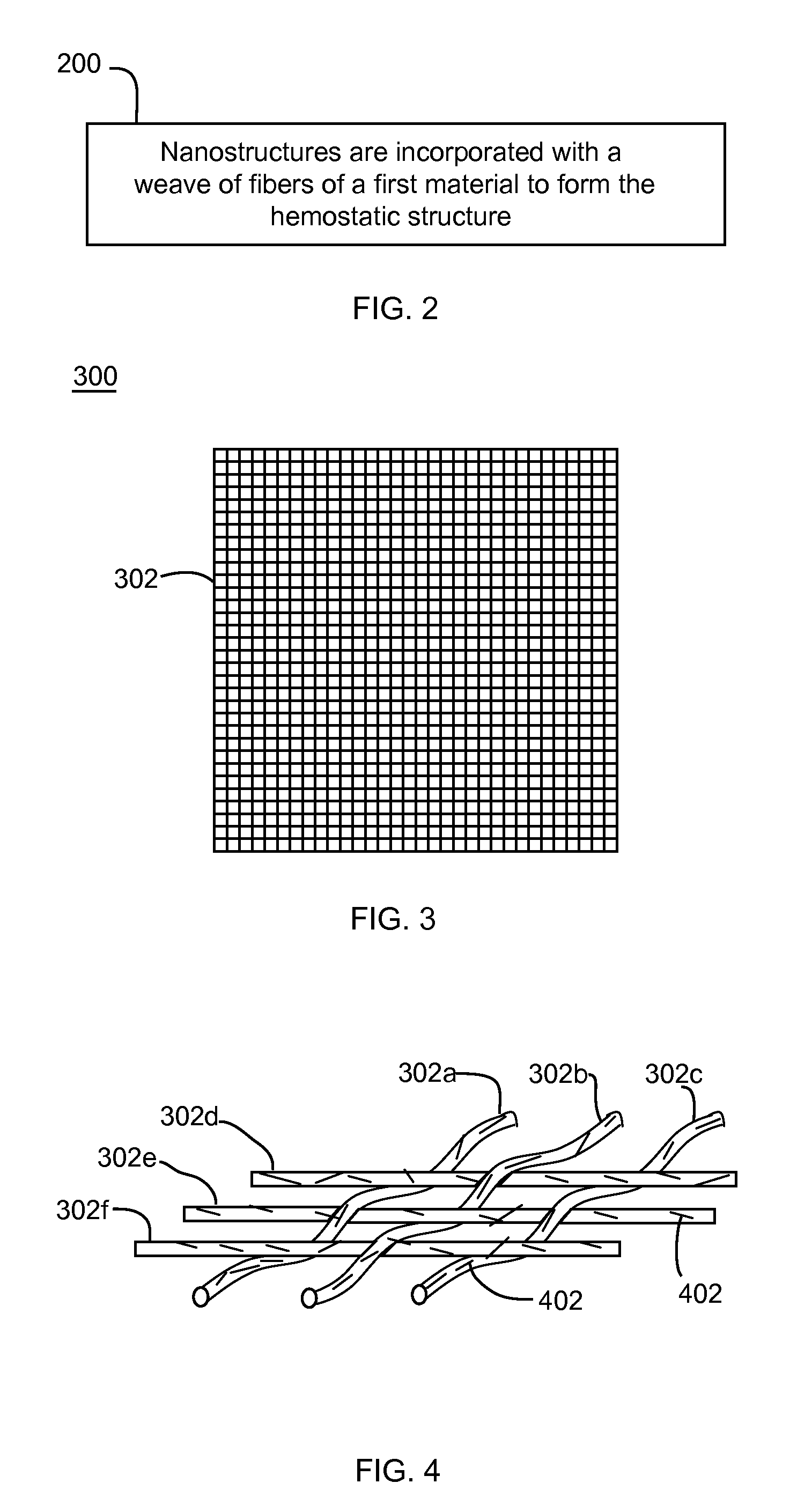
Nanostructure-enhanced platelet binding and hemostatic structures
InactiveUS8319002B2Enhancing overall rate and strengthInduce platelet binding and efficient hemostasisBiocideSurgical adhesivesPlateletNanofiber
Methods, systems, and apparatuses for nanomaterial-enhanced platelet binding and hemostatic medical devices are provided. Hemostatic materials and structures are provided that induce platelet binding, including platelet binding and the coagulation of blood at a wound / opening caused by trauma, a surgical procedure, ulceration, or other cause. Example embodiments include platelet binding devices, hemostatic bandages, hemostatic plugs, and hemostatic formulations. The hemostatic materials and structures may incorporate nanostructures and / or further hemostatic elements such as polymers, silicon nanofibers, silicon dioxide nanofibers, and / or glass beads into a highly absorbent, gelling scaffold. The hemostatic materials and structures may be resorbable.
Owner:NANOSYS INC
Orthopedic medical device with unitary components
InactiveUS20060111723A1Foster innovativeReduce chanceSurgical furnitureDiagnosticsPlastic surgerySurgical department
A cordless medical apparatus suitable for use in orthopedic medical applications is disclosed. The medical device includes a disposable sterile housing having a sleeve, said sleeve being configured to receive an unsterilized motor assembly. The invention further includes a sterile packaging system for enclosing the sterile housing and inserting a non-sterile component in the sterile housing.
Owner:CHAPOLINI ROBERT J +1
Entrapping apparatus and method for use
Medical devices that have a novel mechanical trap(s) on the distal end of a shaft that is used for the removal of material from the body. Further an expandable channel is included to entrap the material that aid with removal or obliteration of tissue or foreign bodies is disclosed.
Owner:ARTEMIS MEDICAL
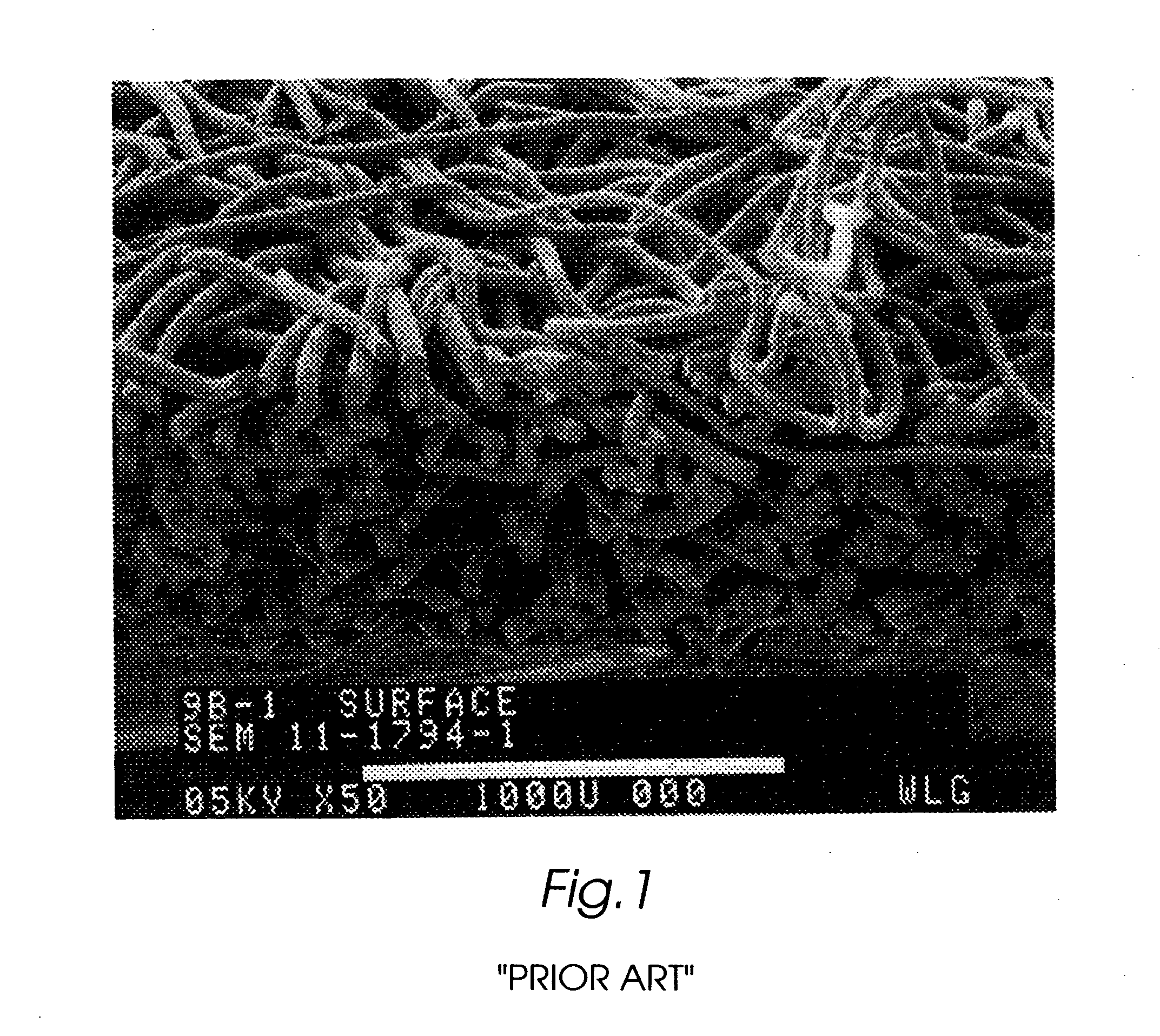
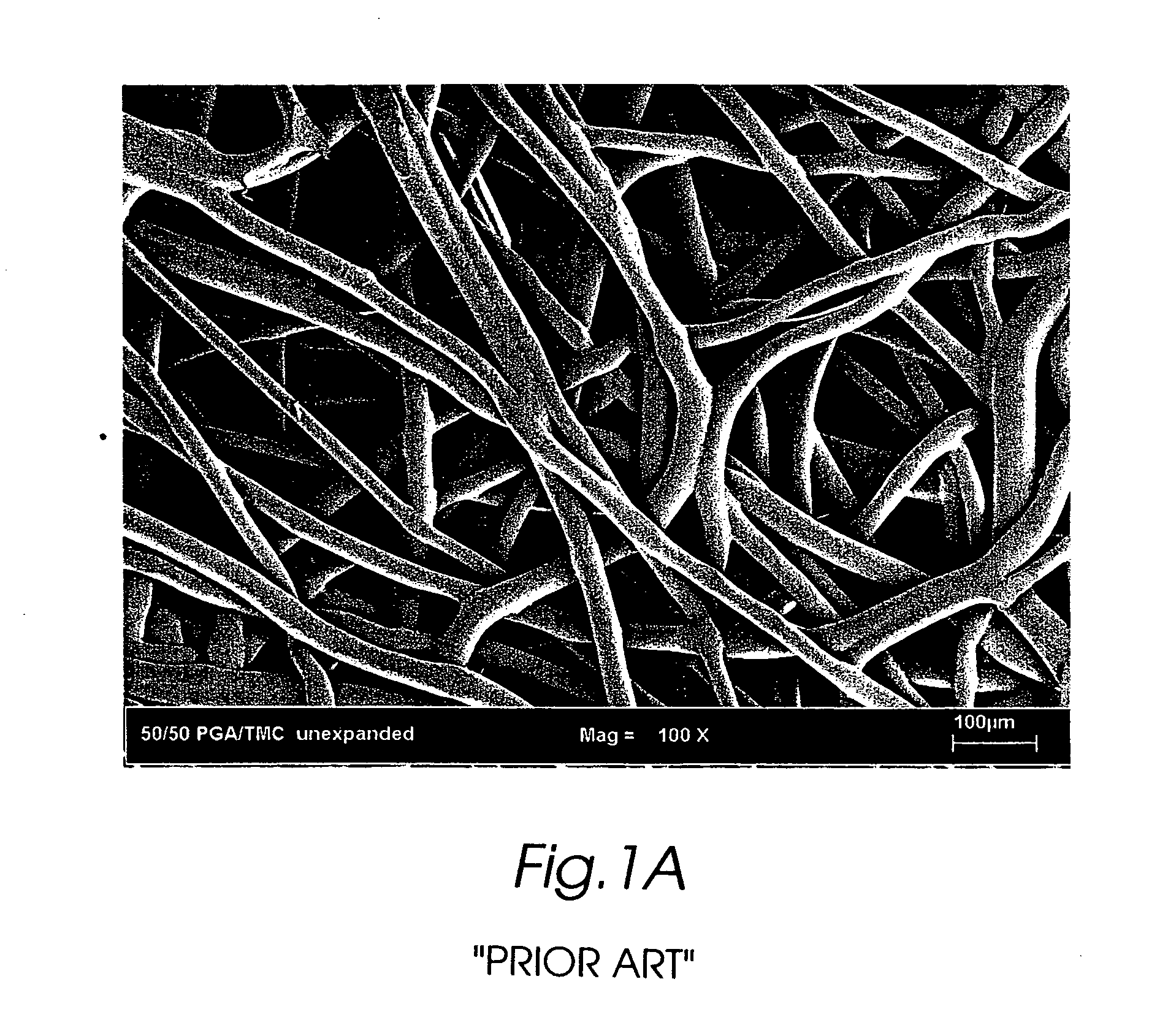
Composite self-cohered web materials
The present invention is directed to implantable bioabsorbable non-woven self-cohered web materials having a high degree of porosity. The web materials are very supple and soft, while exhibiting proportionally increased mechanical strength in one or more directions. The web materials often possess a high degree of loft. The web materials can be formed into a variety of shapes and forms suitable for use as implantable medical devices or components thereof.
Owner:WL GORE & ASSOC INC
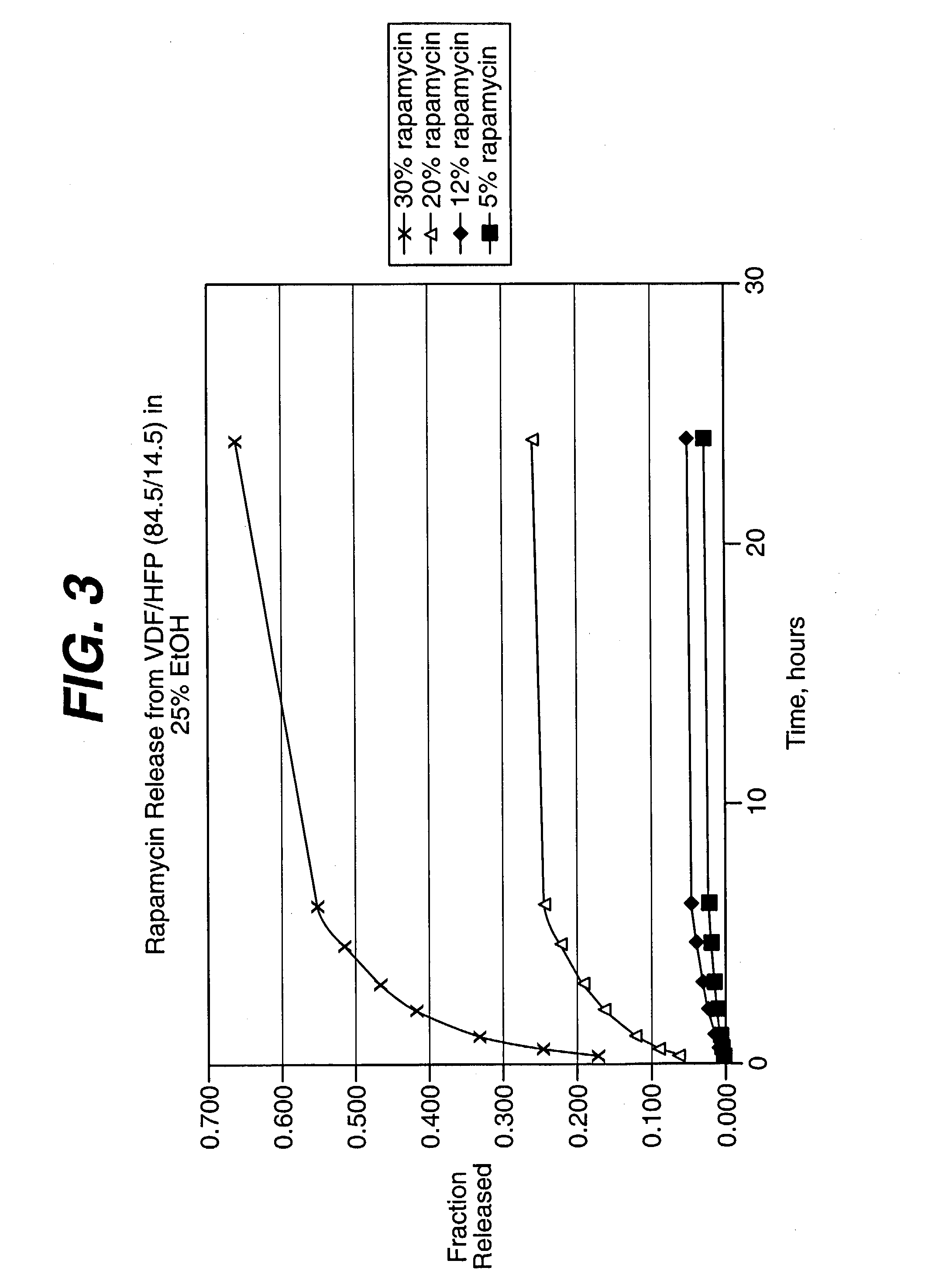
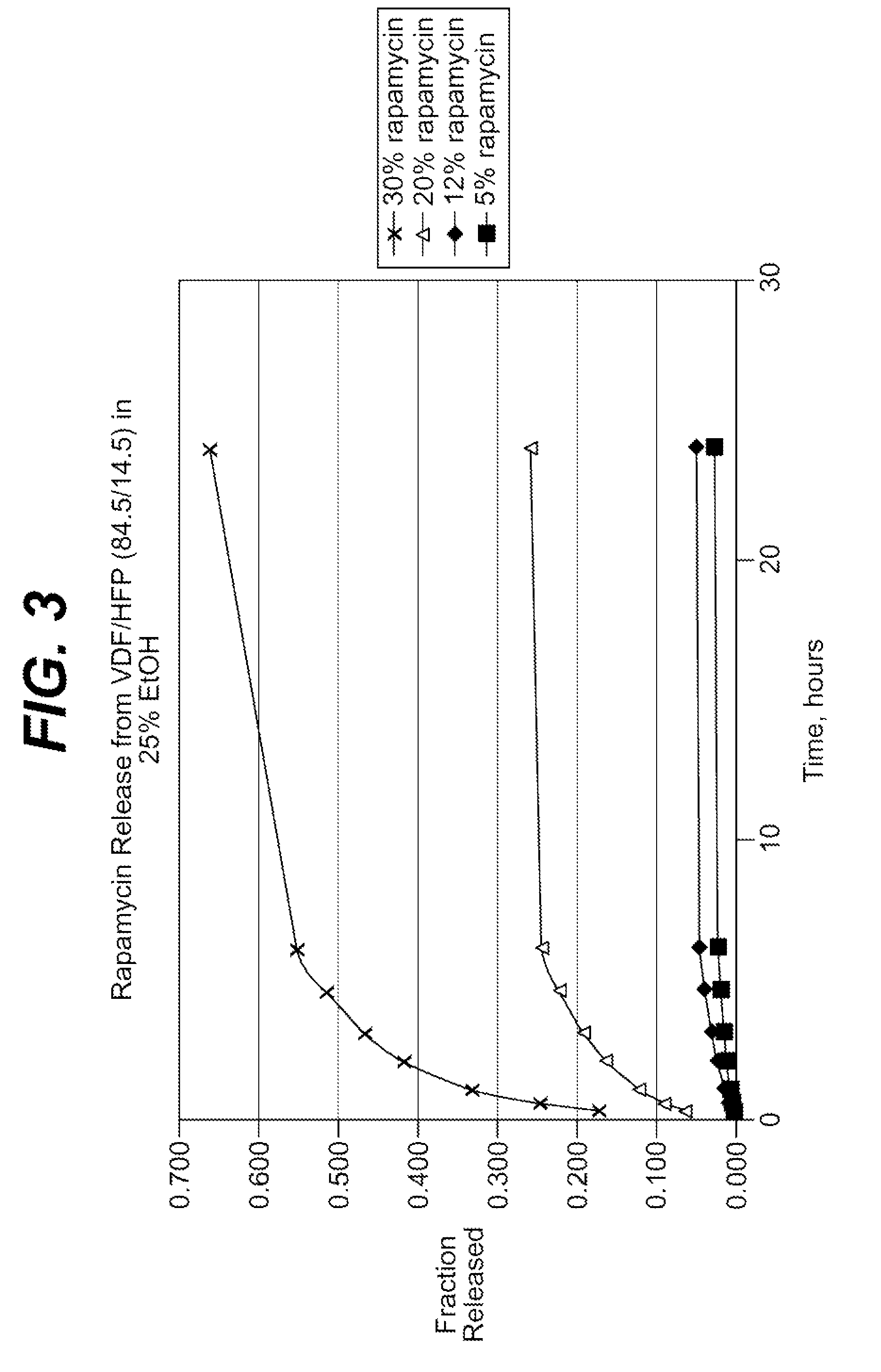
Extraction of solvents from drug containing polymer reservoirs
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com