Patents
Literature
119 results about "Drugs infusion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
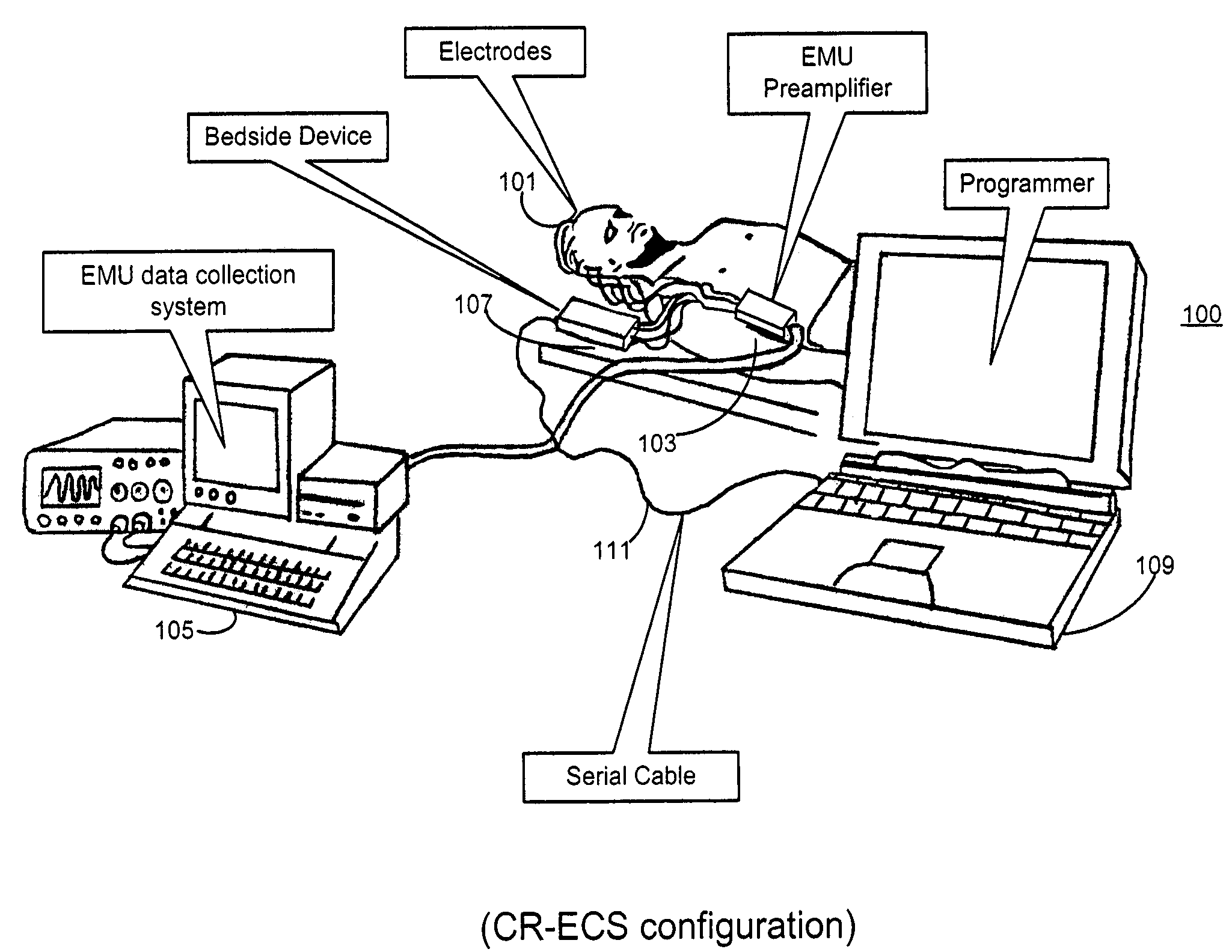
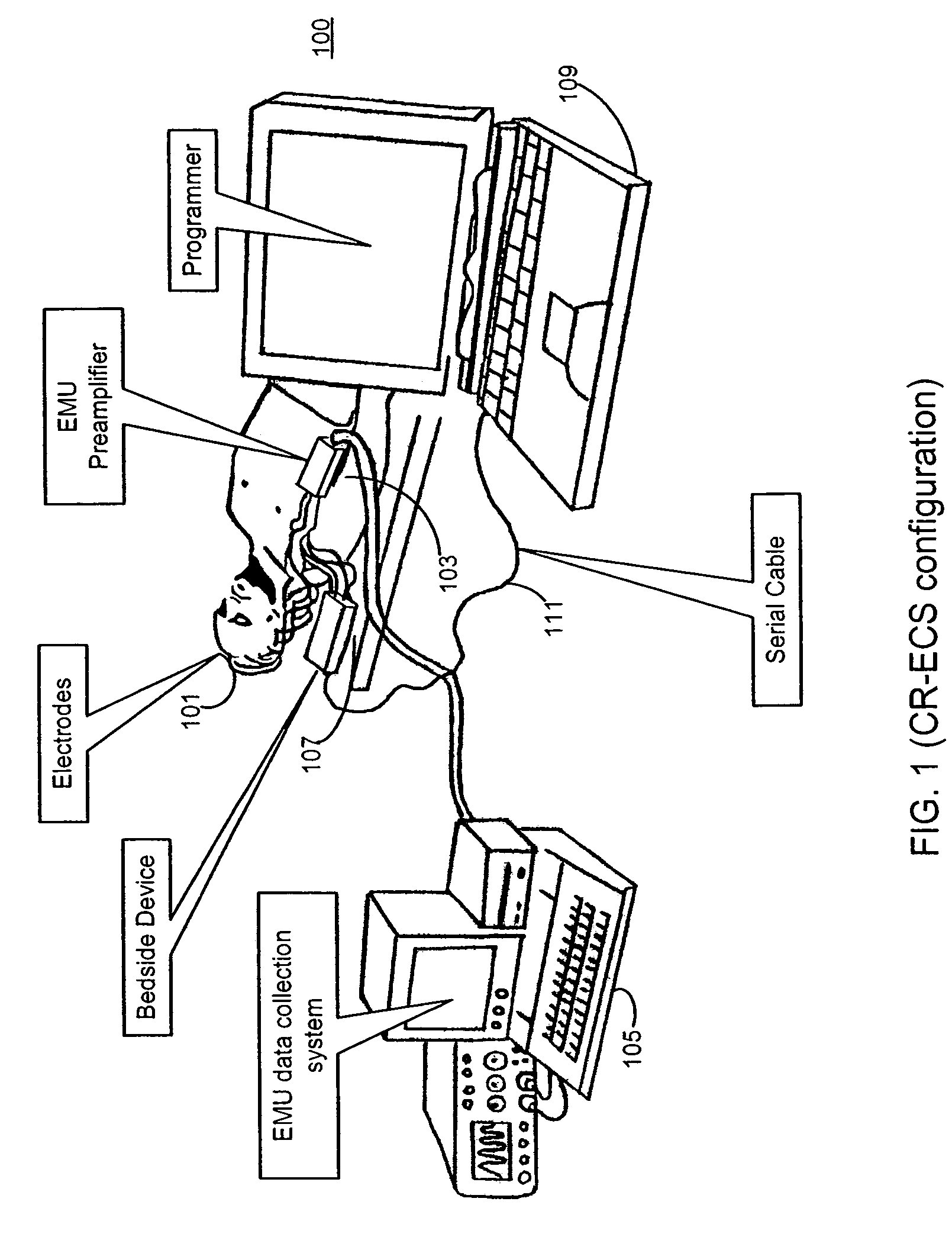
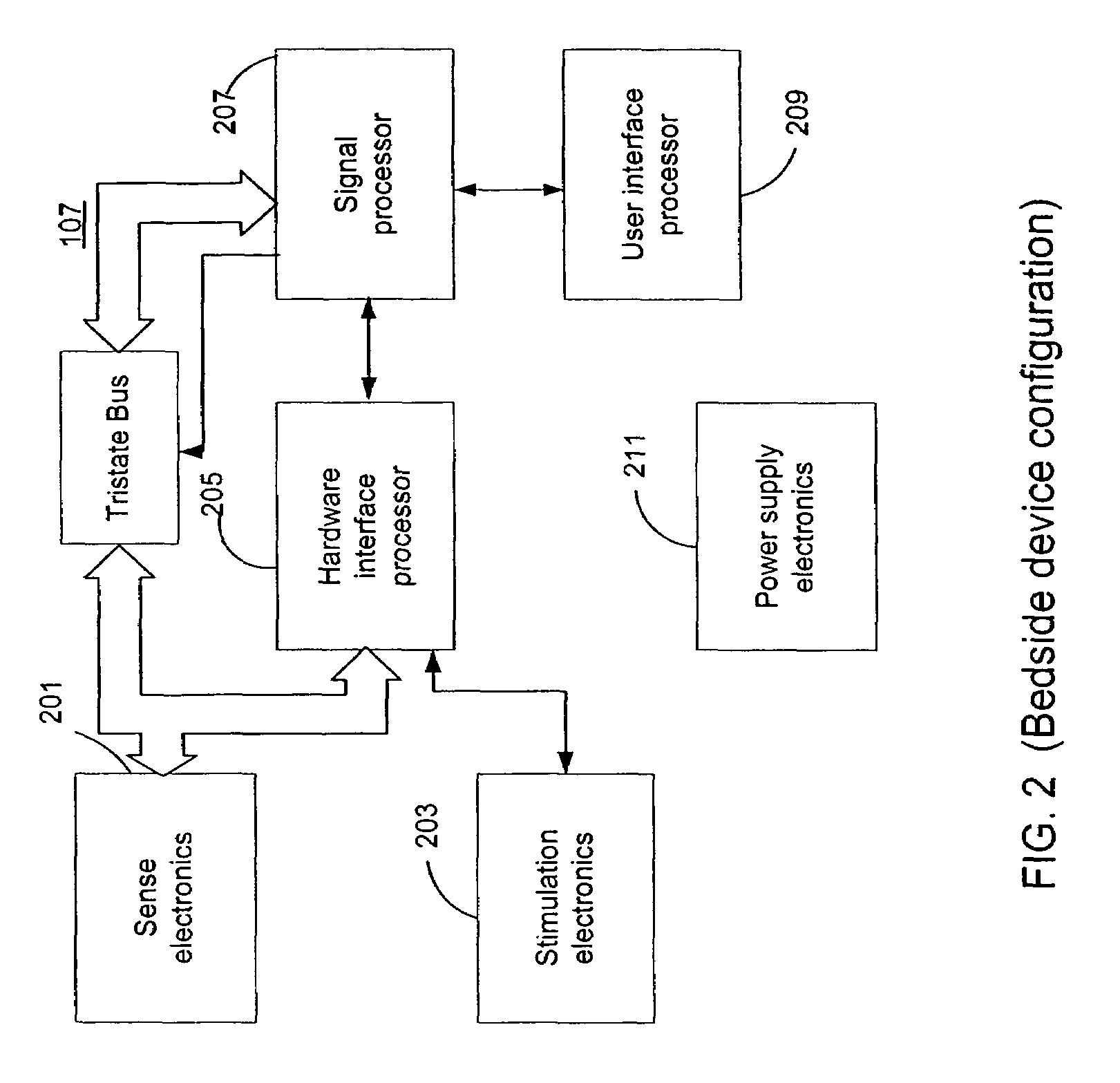
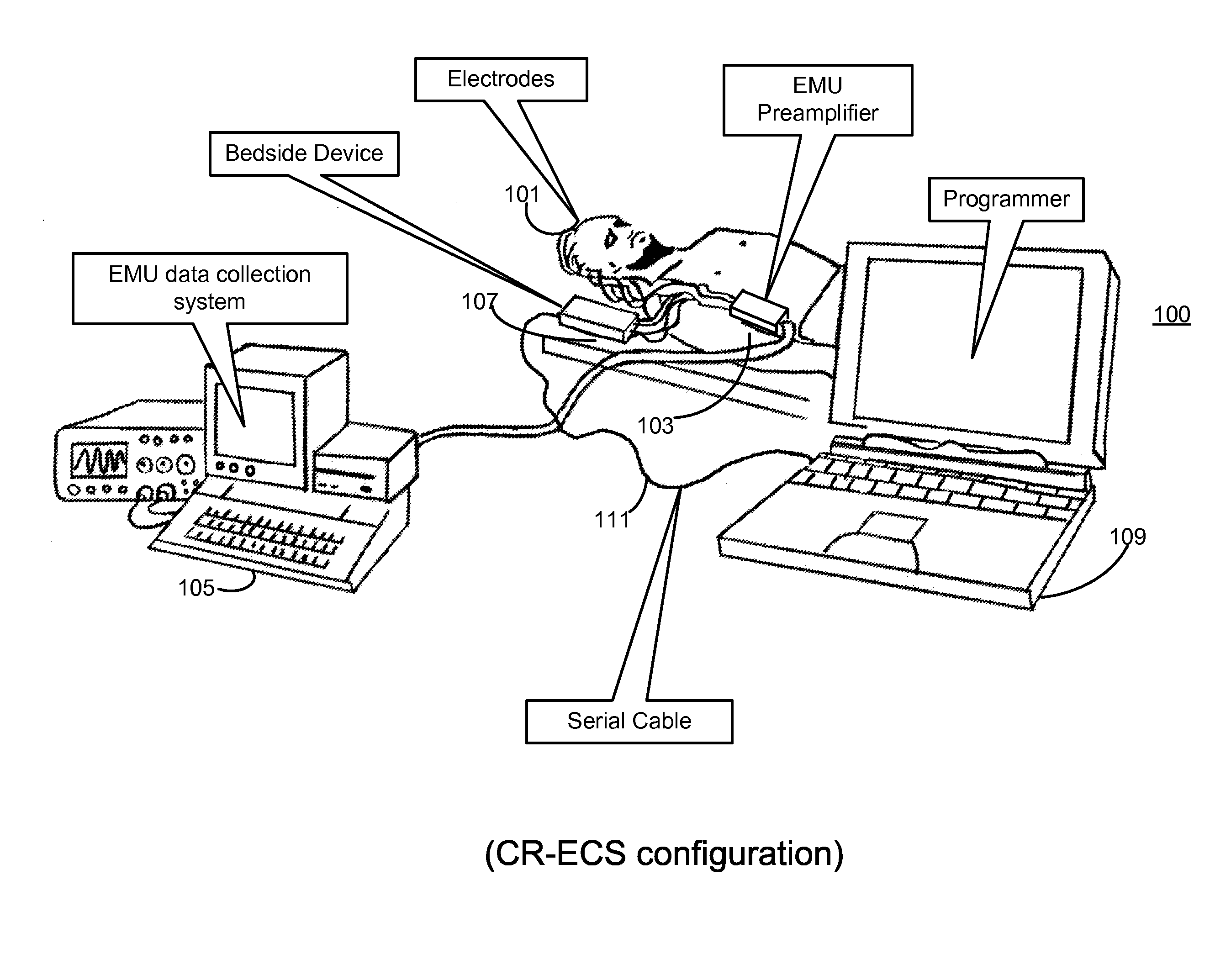
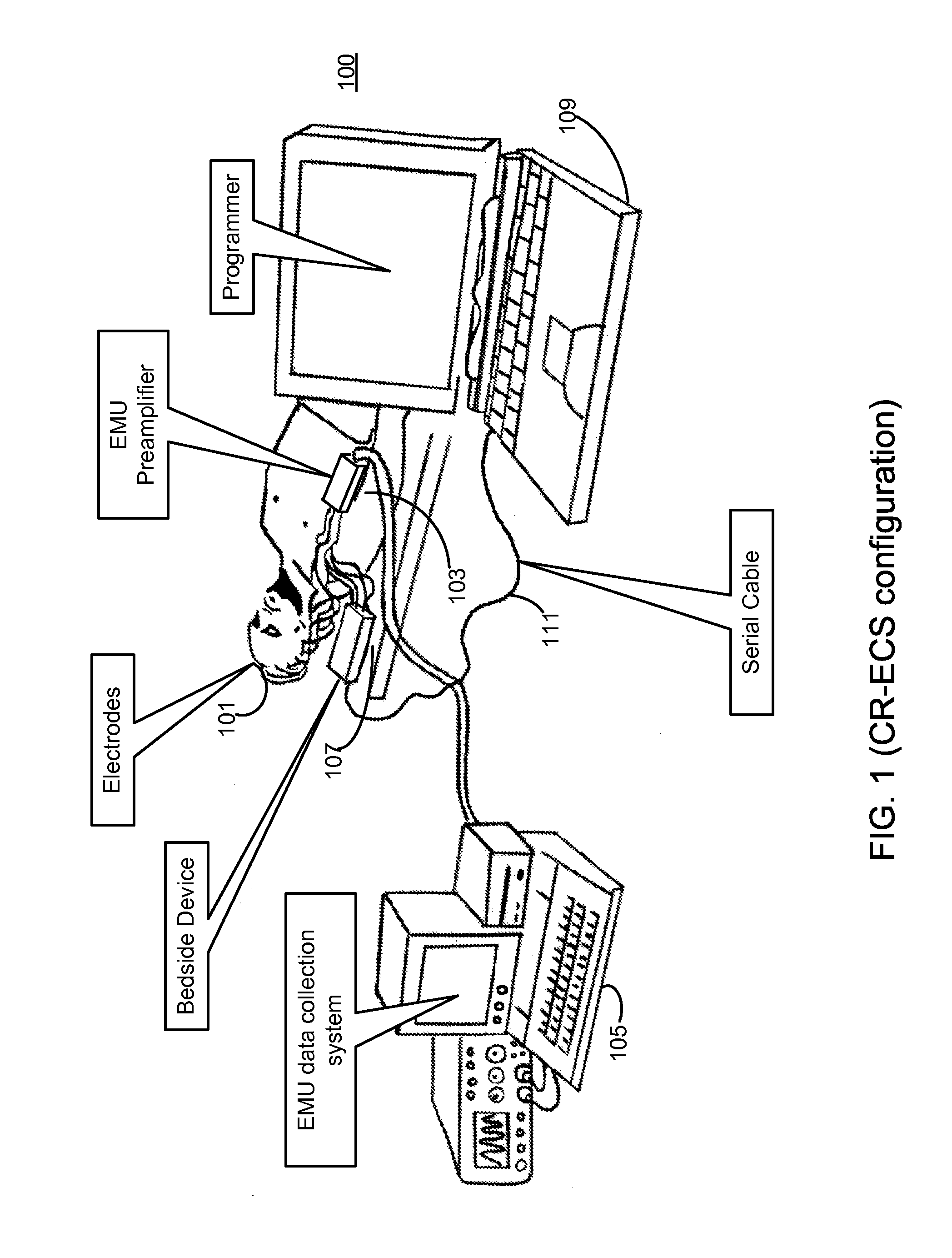
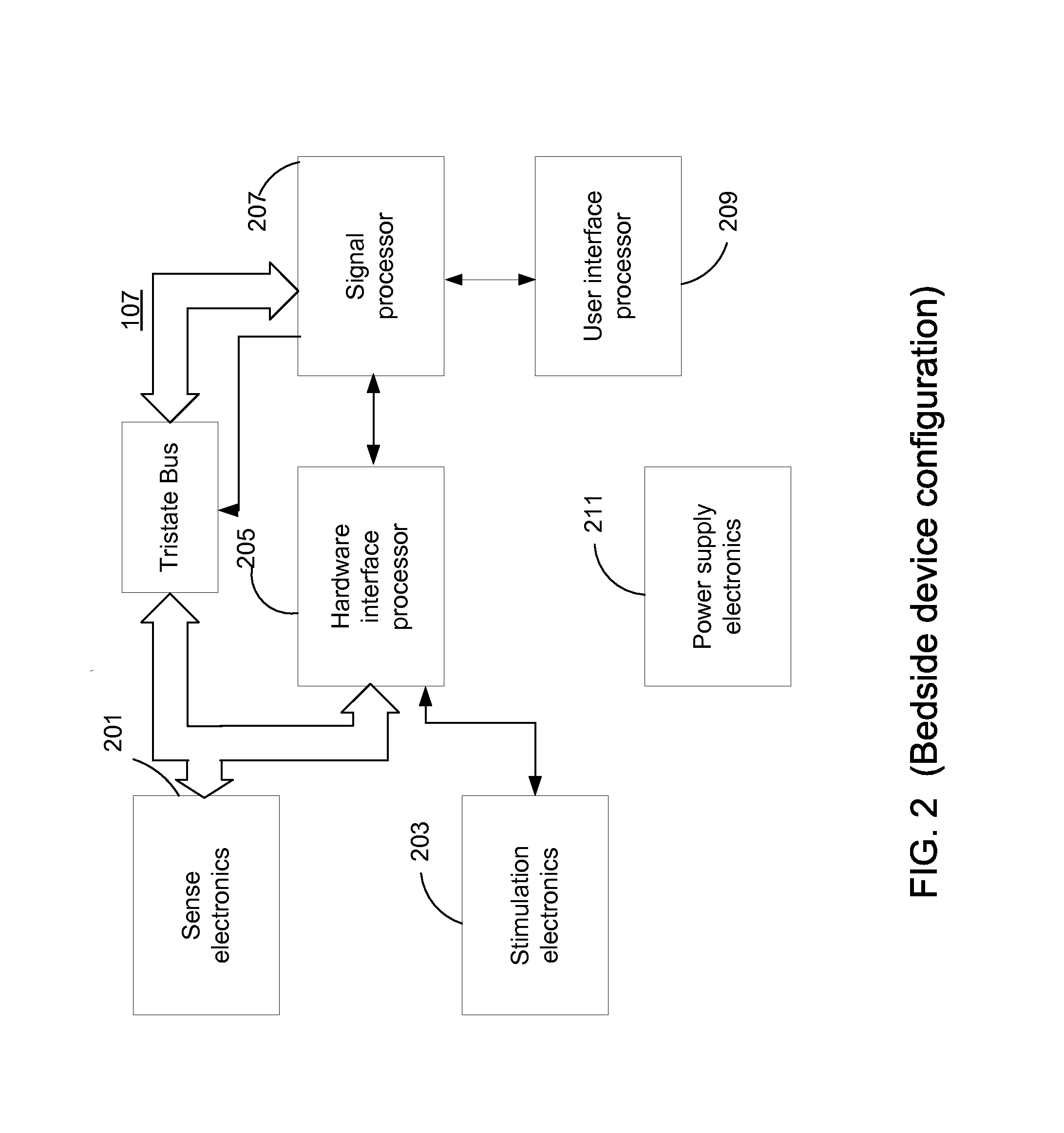
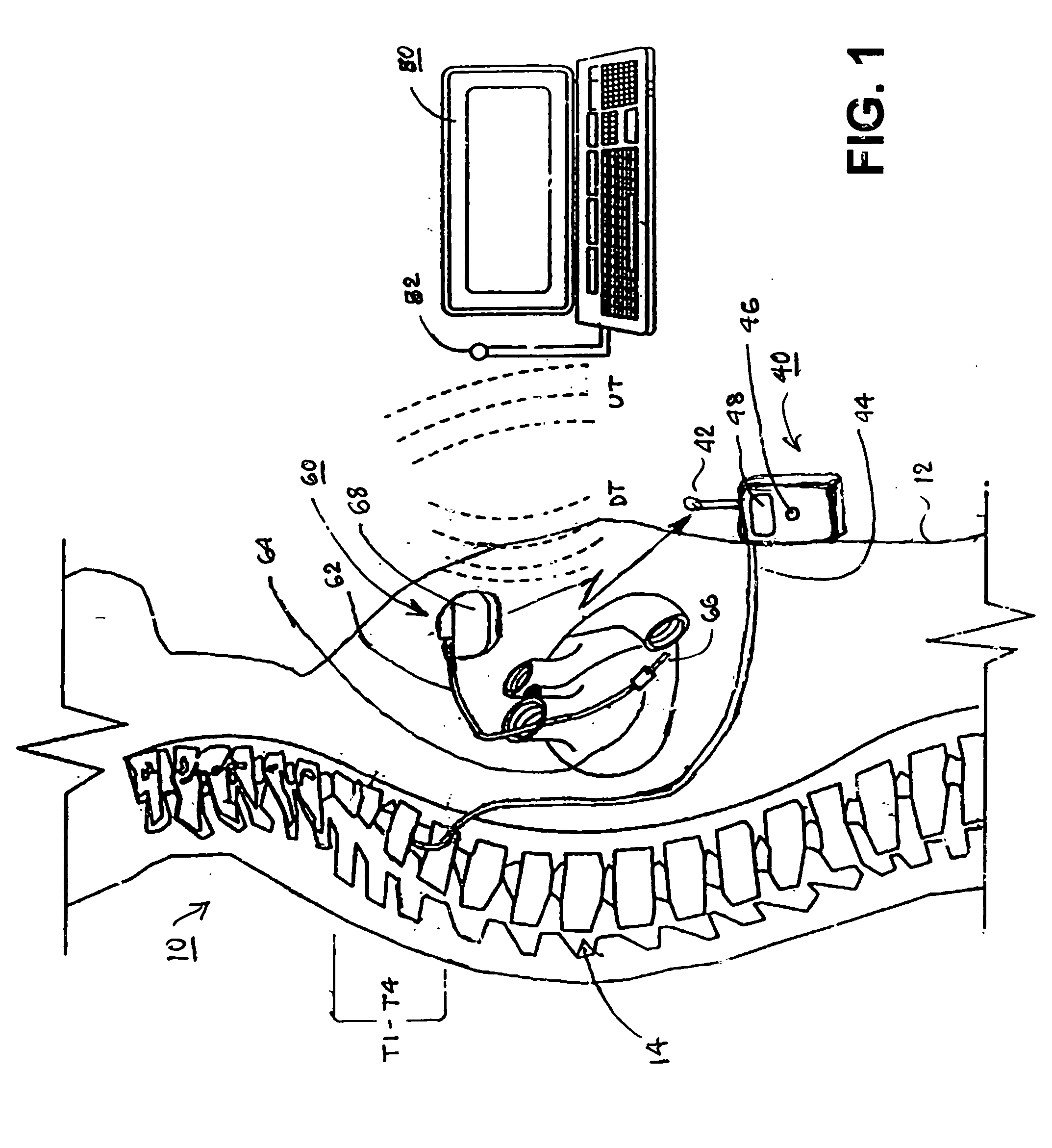
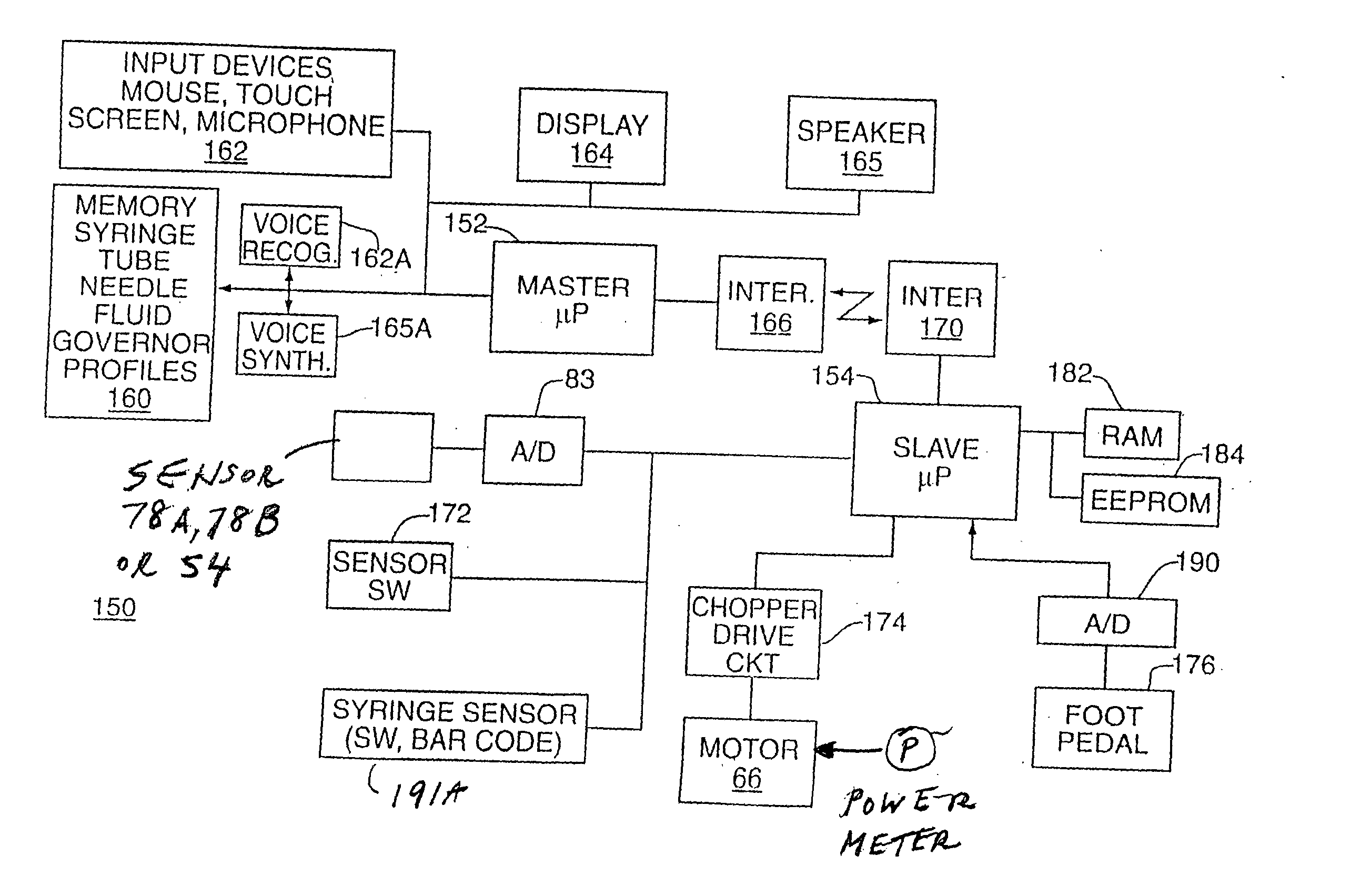
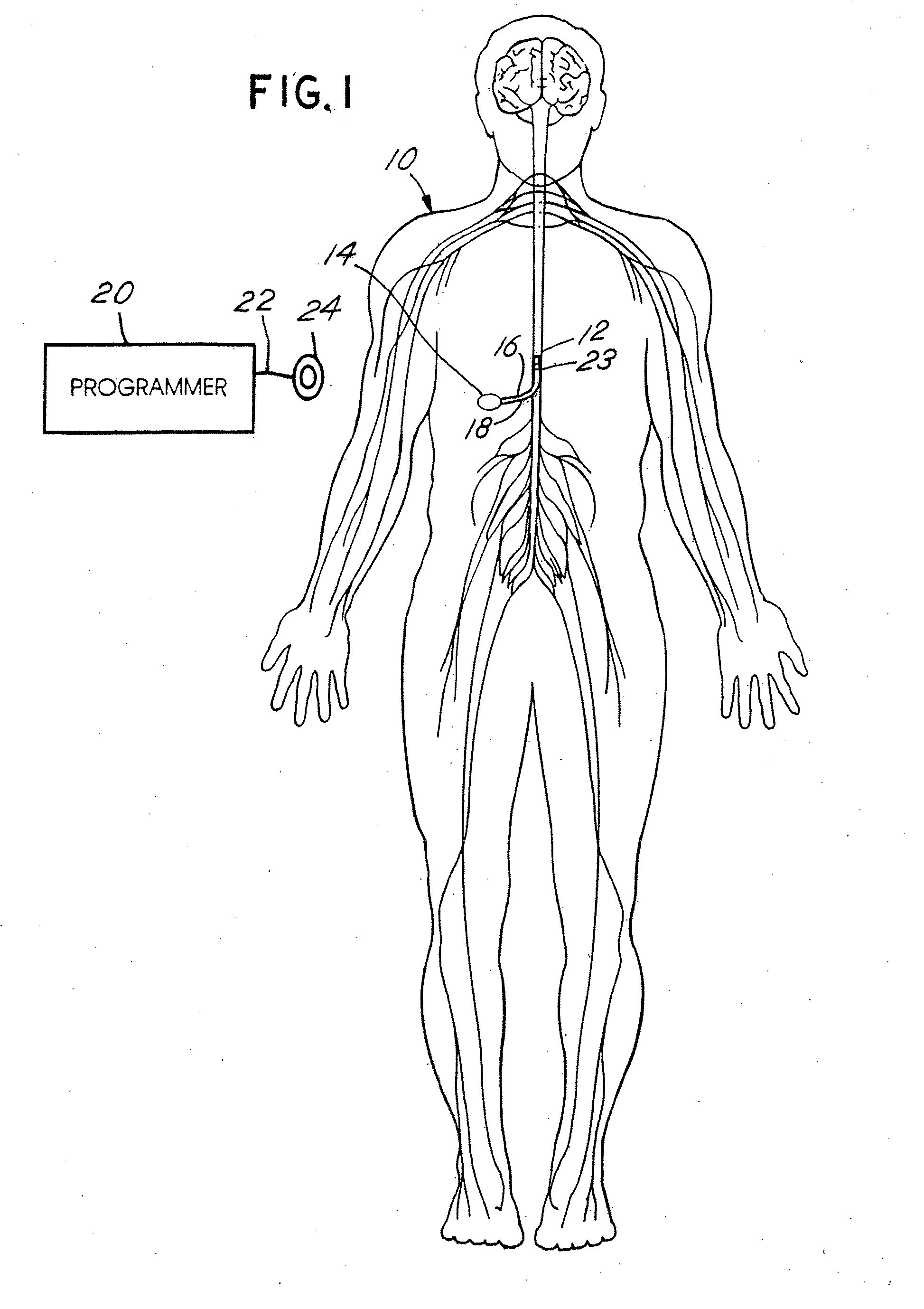
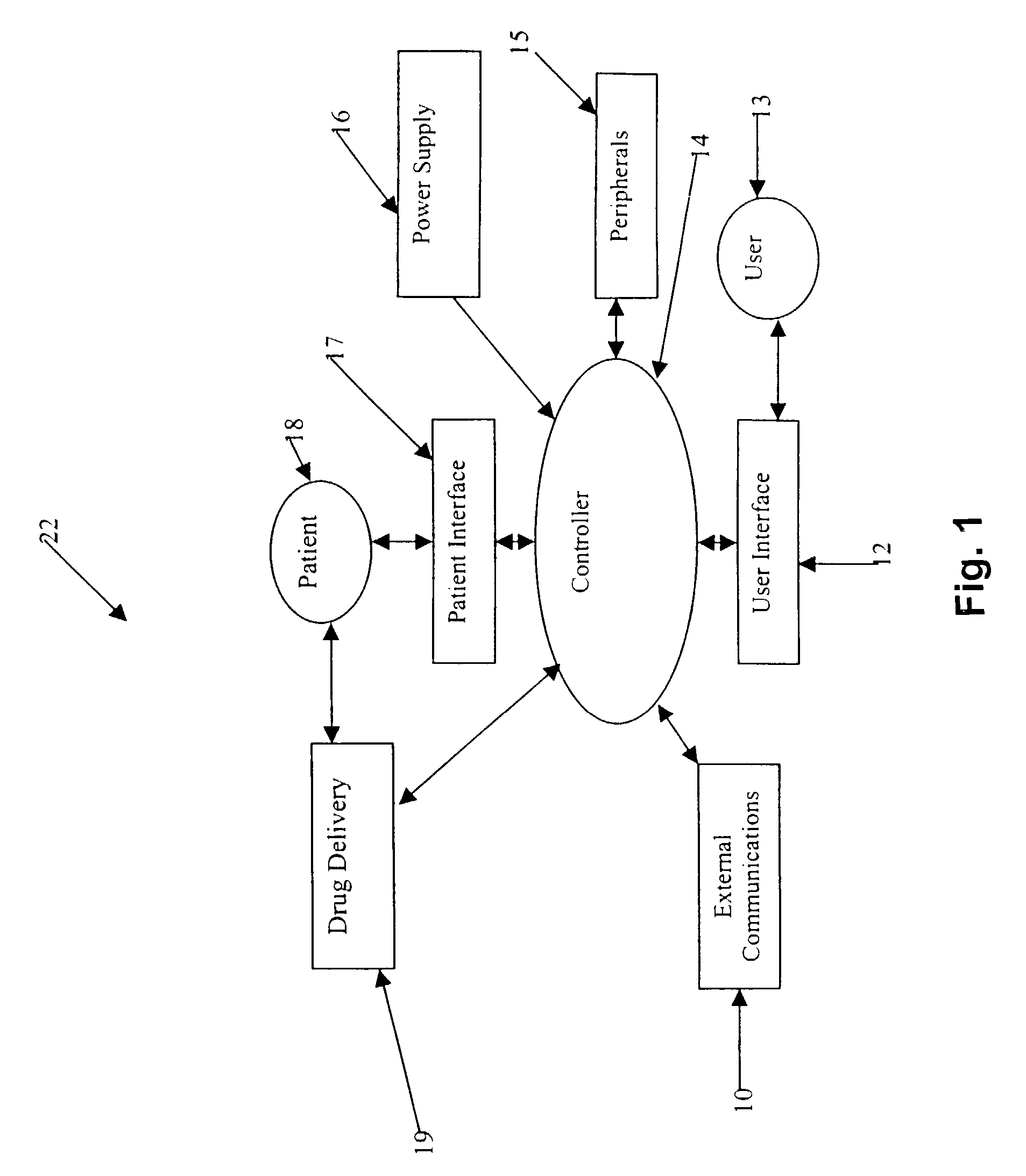
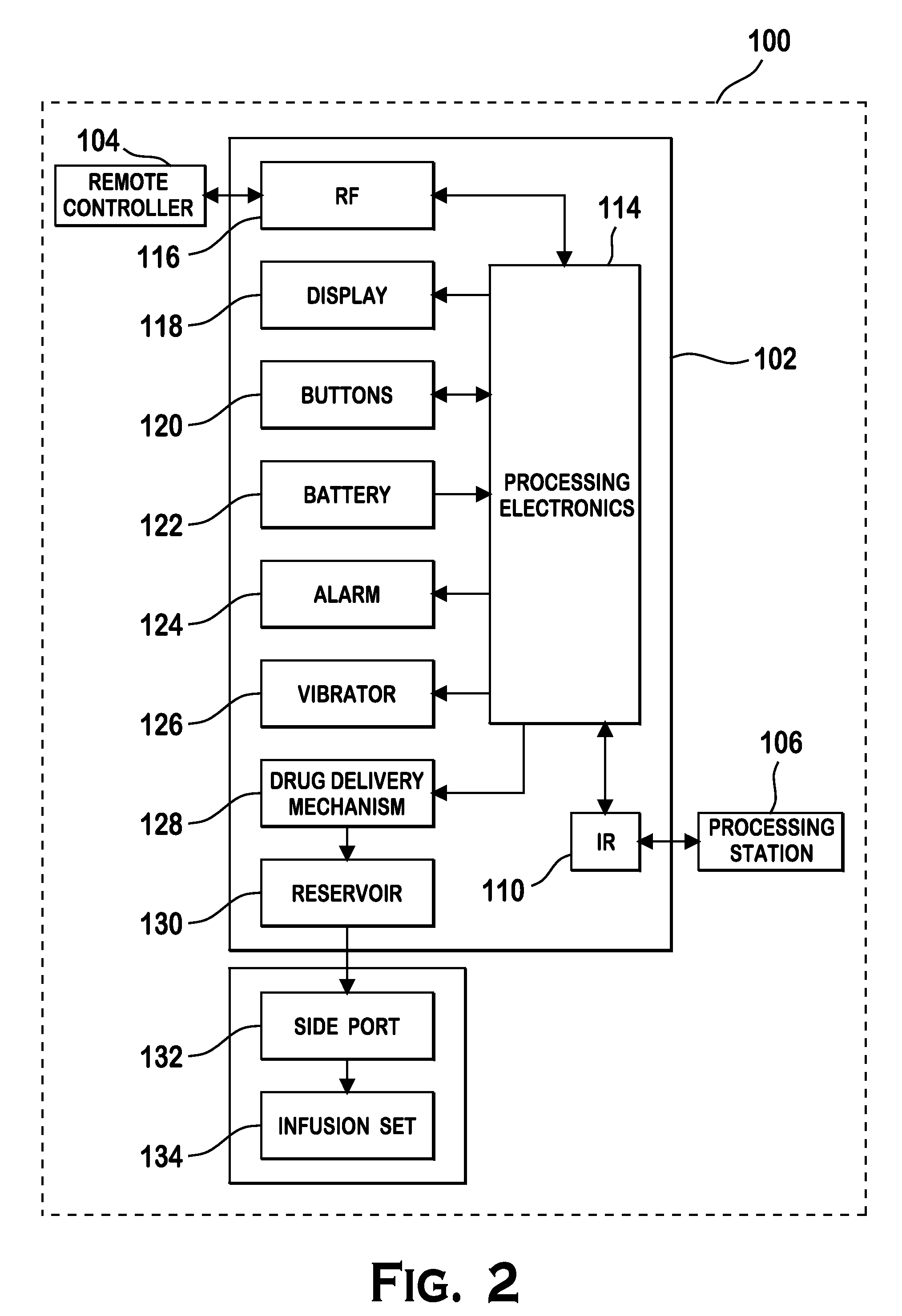
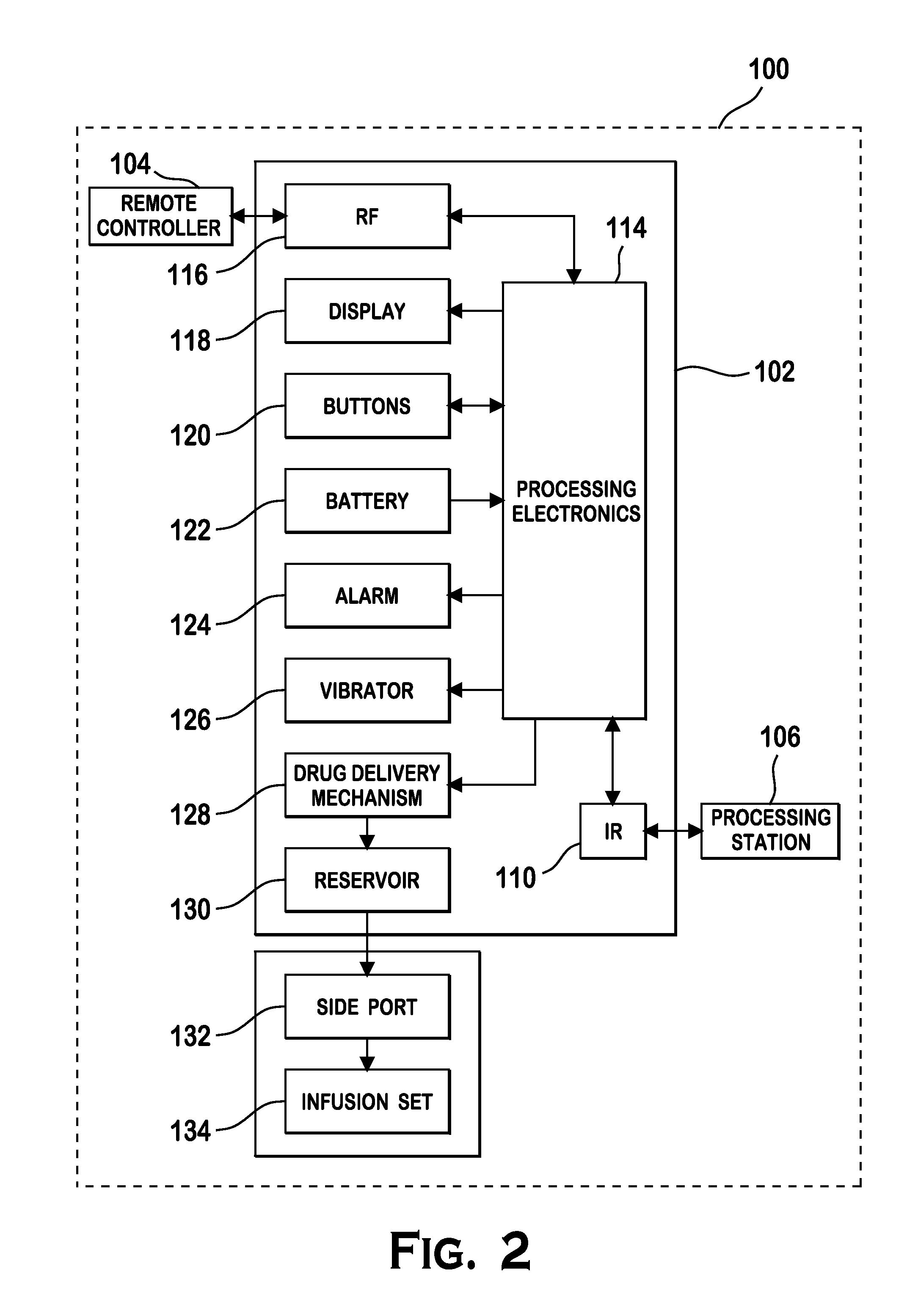
Microprocessor controlled ambulatory medical apparatus with hand held communication device
InactiveUS6873268B2Enhance user interfaceReduce system sizeEnergy efficient ICTElectrotherapyDrugs infusionHand held
An implantable infusion pump possesses operational functionality that is, at least in part, controlled by software operating in two processor ICs which are configured to perform some different and some duplicate functions. The pump exchanges messages with an external device via telemetry. Each processor controls a different part of the drug infusion mechanism such that both processors must agree on the appropriateness of drug delivery for infusion to occur. Delivery accumulators are incremented and decremented with delivery requests and with deliveries made. When accumulated amounts reach or exceed, quantized deliverable amounts, infusion is made to occur. The accumulators are capable of being incremented by two or more independent types of delivery requests. Operational modes of the infusion device are changed automatically in view of various system errors that are trapped, various system alarm conditions that are detected, and when excess periods of time lapse between pump and external device interactions.
Owner:MEDTRONIC MIMIMED INC
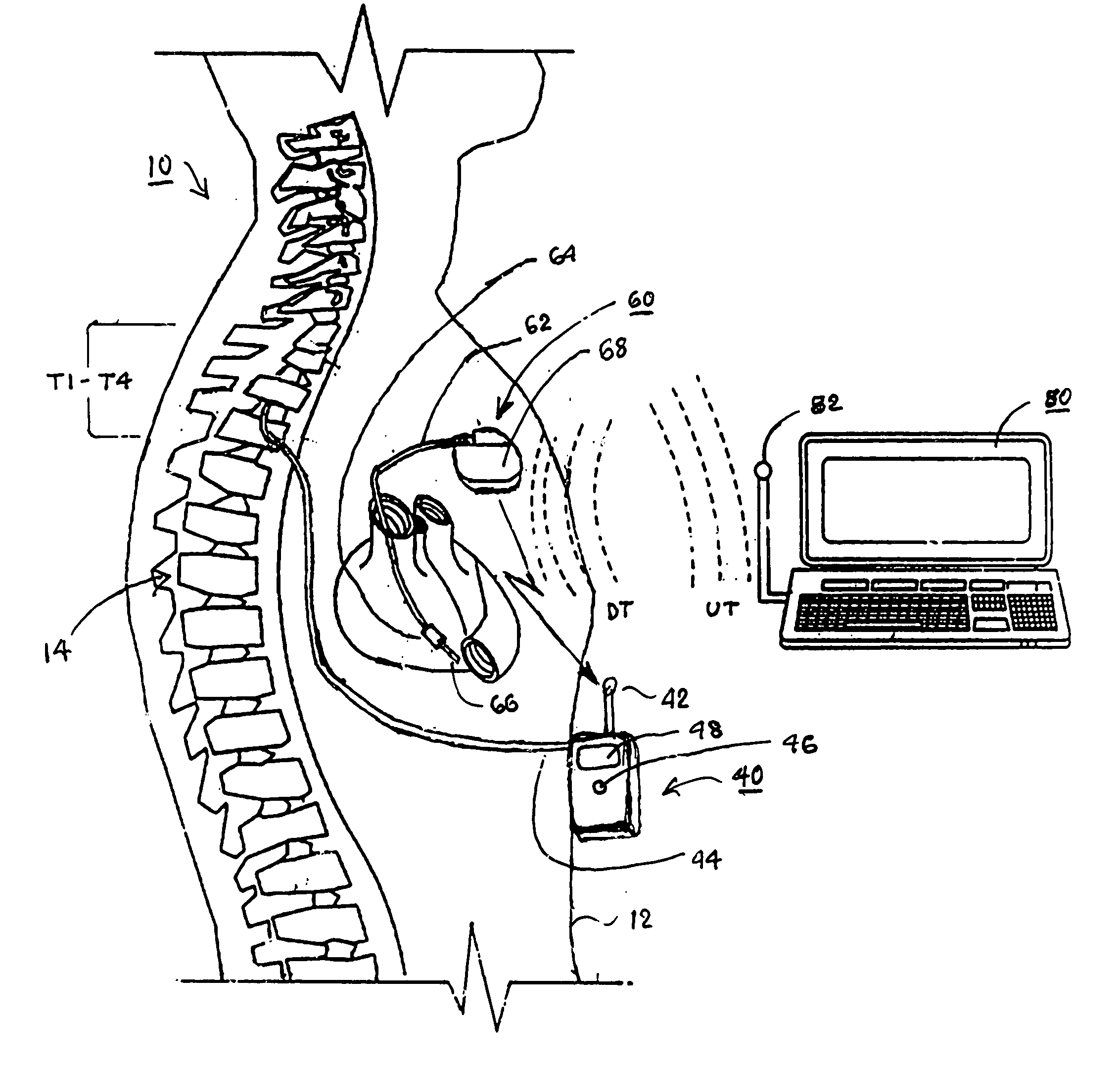
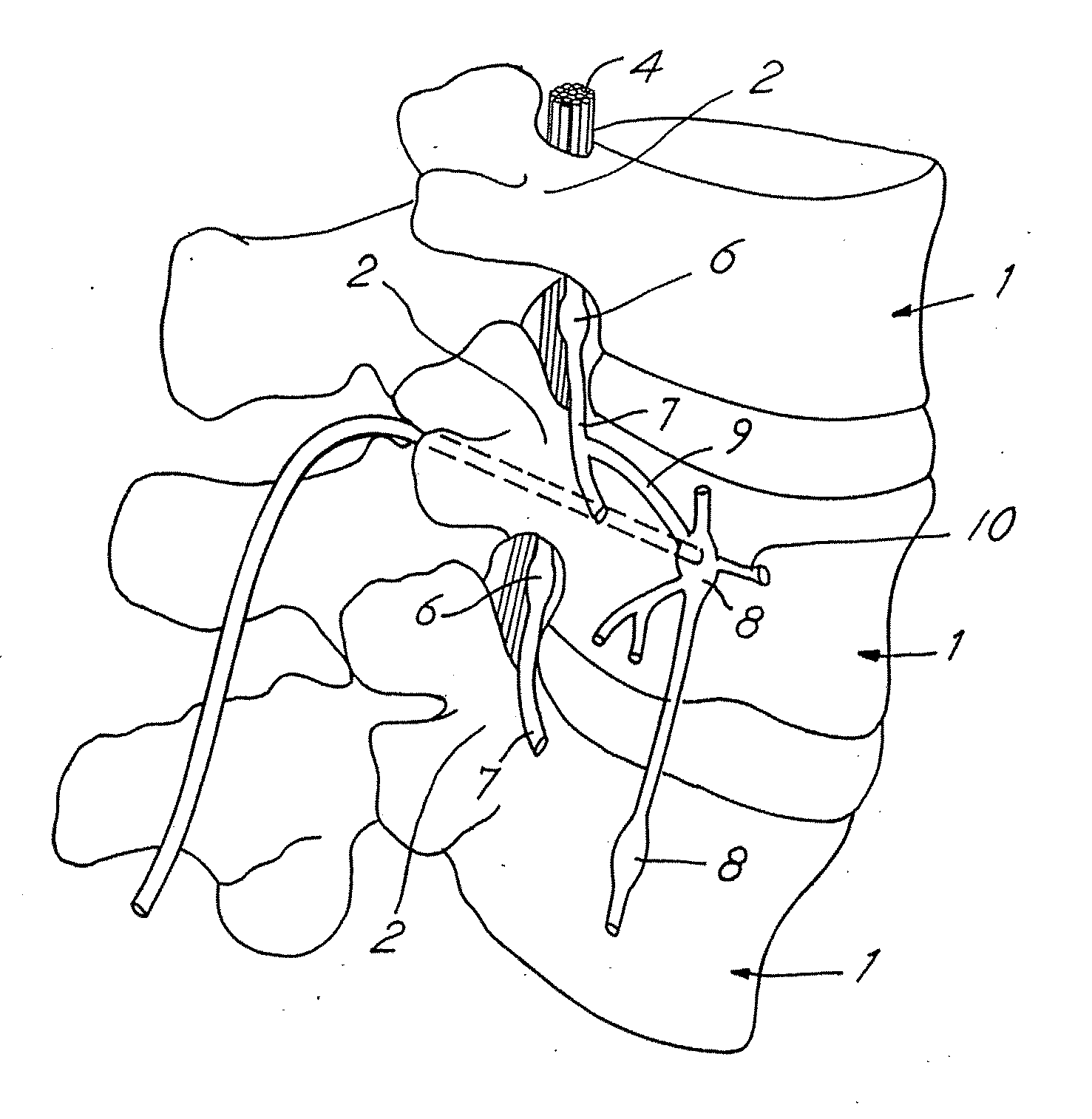
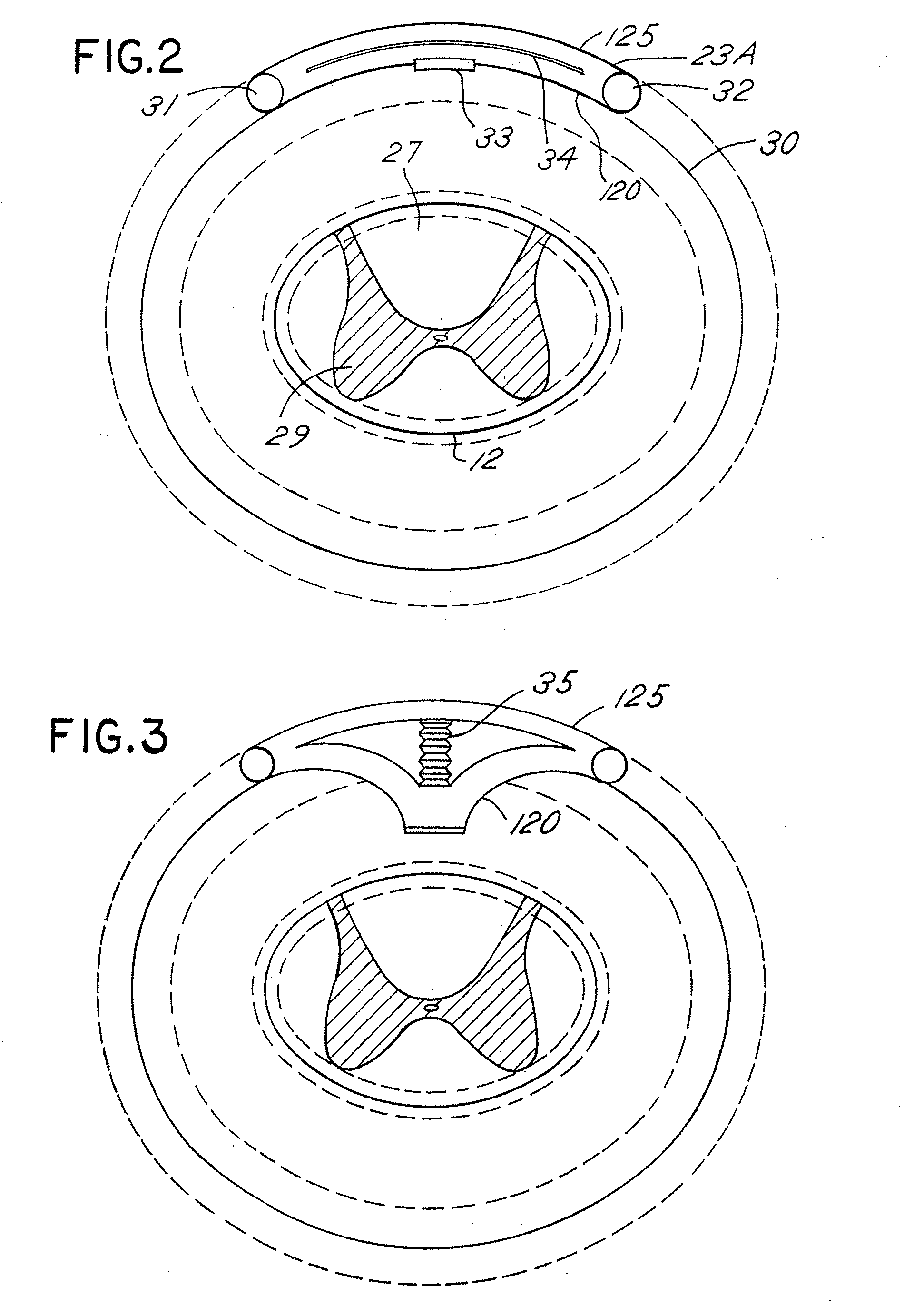
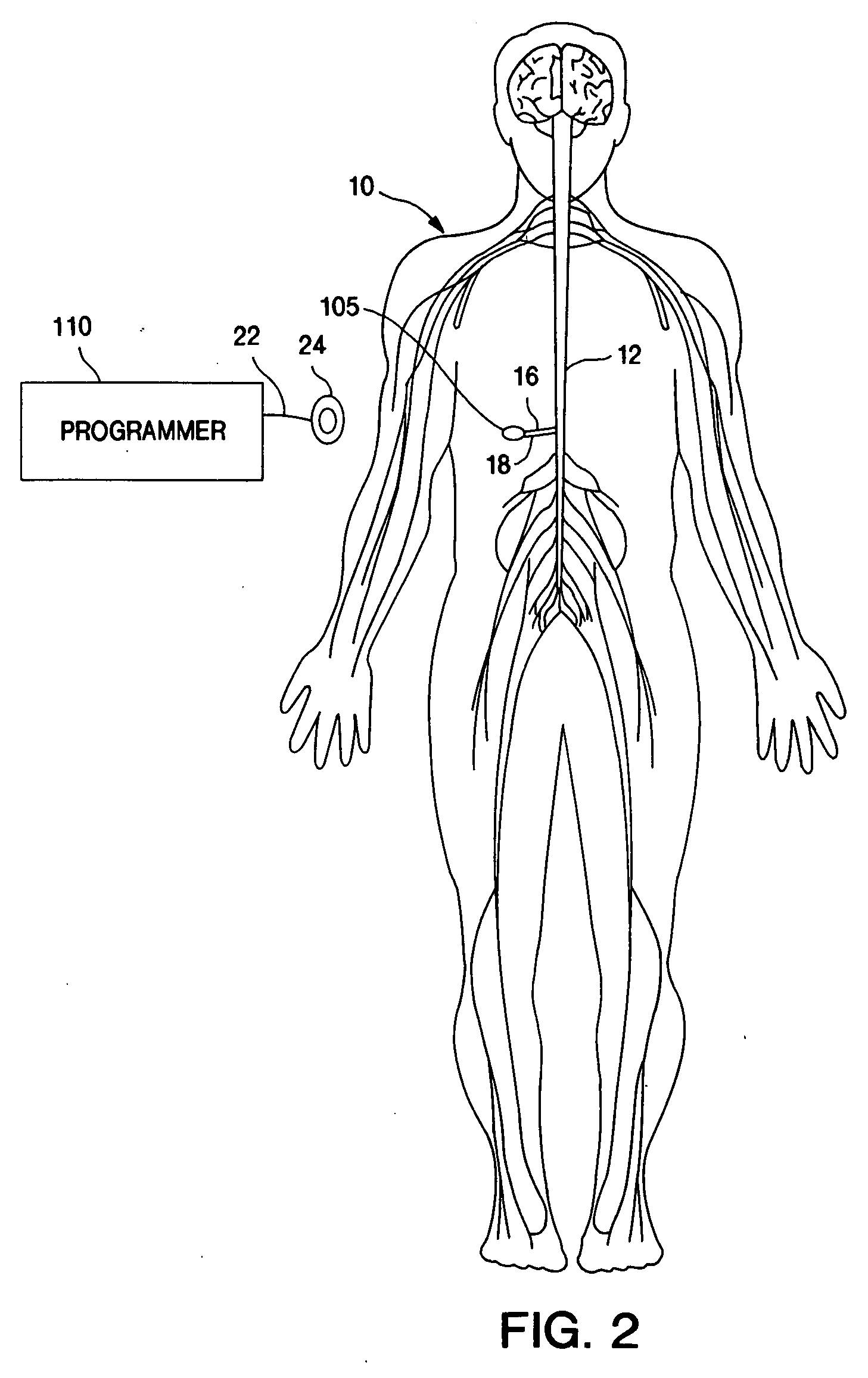
Techniques for positioning therapy delivery elements within a spinal cord or brain
Apparatus and techniques to address the problems associated with lead migration, patient movement or position, histological changes, neural plasticity or disease progression. Disclosed are techniques for implanting a lead having therapy delivery elements, such as electrodes or drug delivery ports, within a vertebral or cranial bone so as to maintain these elements in a fixed position relative to a desired treatment site. The therapy delivery elements may thereafter be adjusted in situ with a position control mechanism and / or a position controller to improve the desired treatment, such as_electrical stimulation and / or drug infusion to a precise target. The therapy delivery elements may be positioned laterally in any direction relative to the targeted treatment site or toward or away from the targeted treatment site. A control system maybe provided for open- or closed-loop feedback control of the position of the therapy delivery elements as well as other aspects of the treatment therapy.
Owner:MEDTRONIC INC
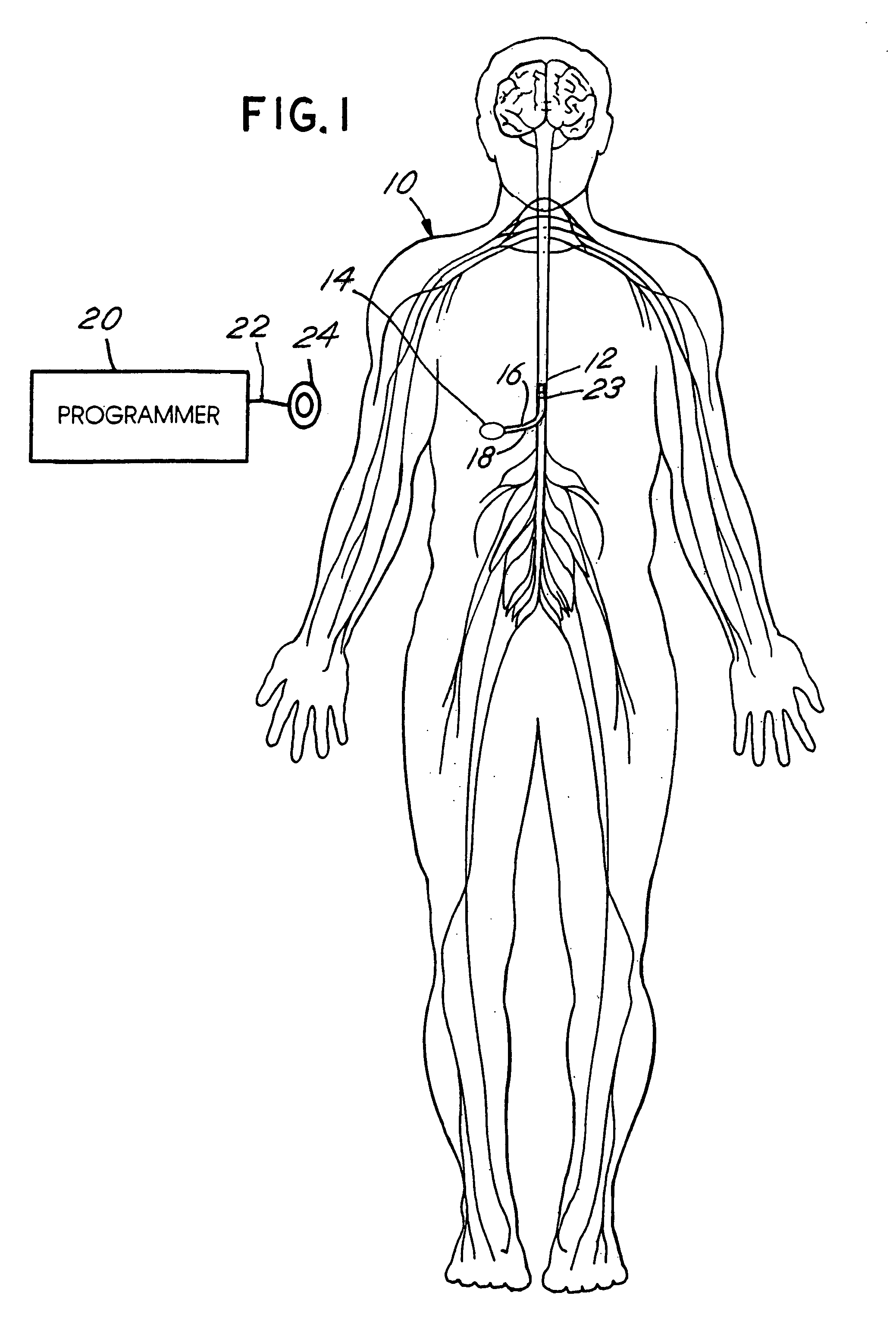
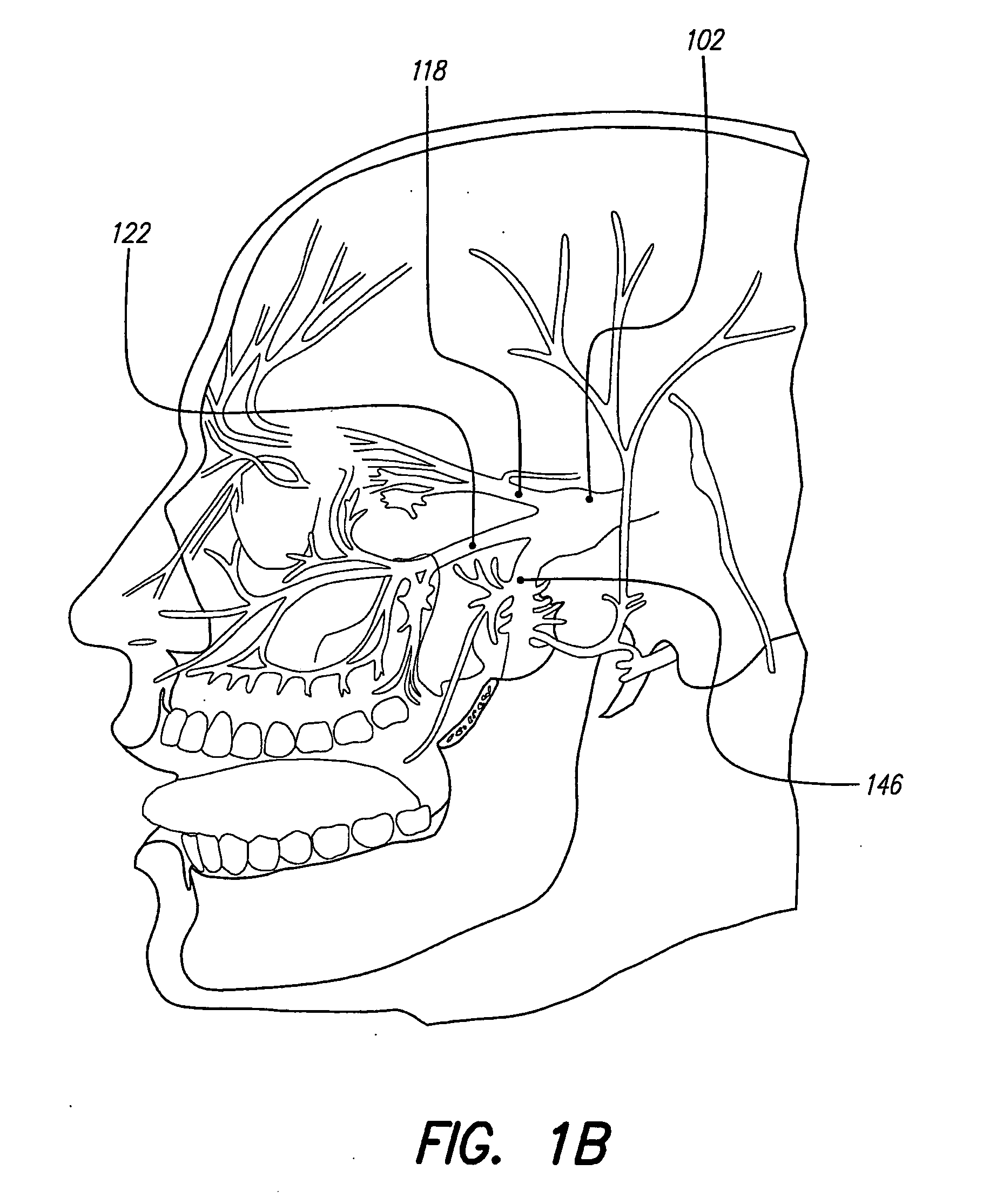
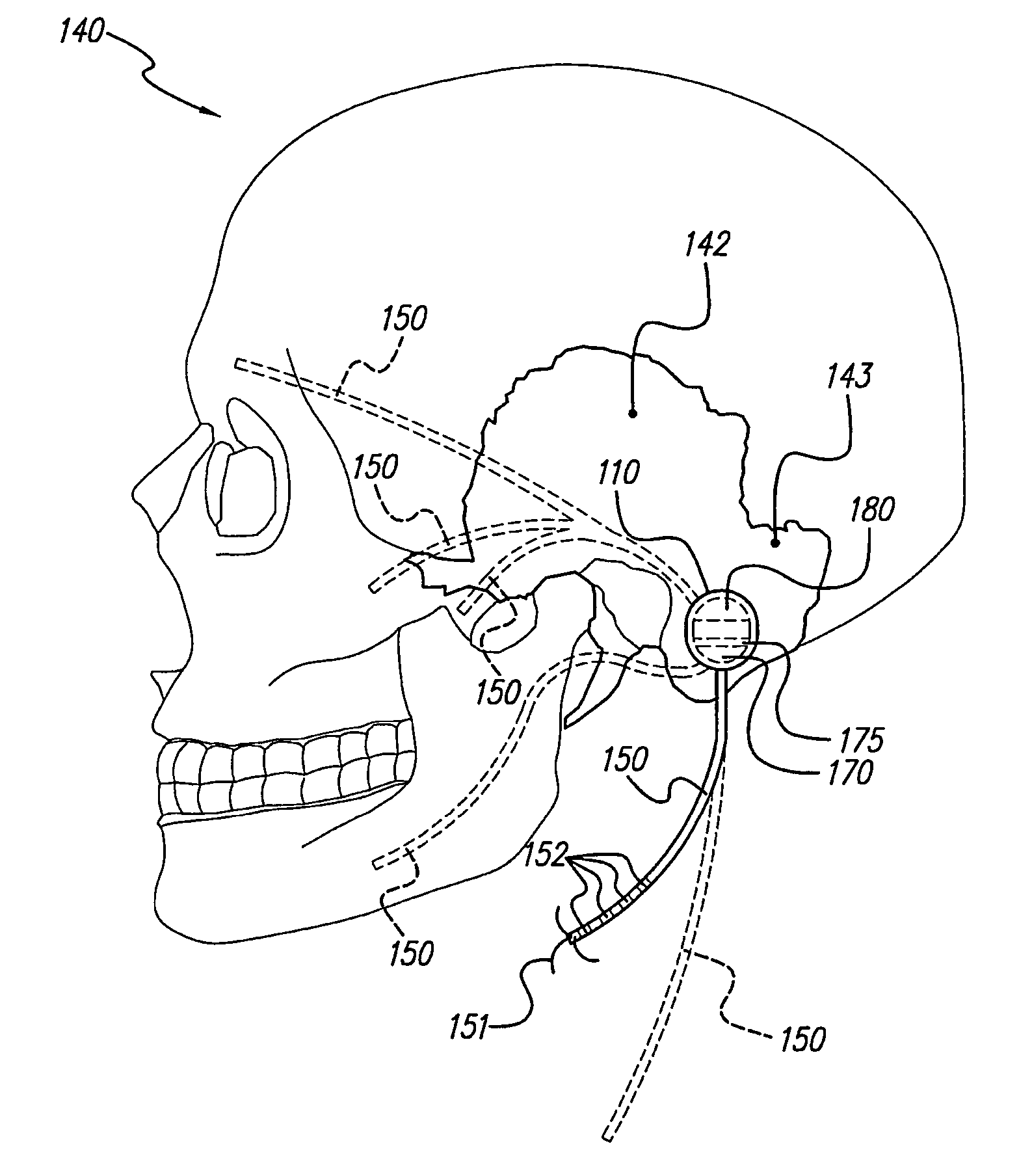
Skull-mounted electrical stimulation system and method for treating patients
ActiveUS20060293723A1Extension of timeMinimal discomfortHead electrodesMedical devicesDrugs infusionClosed loop
A system and method for applying electrical stimulation or drug infusion to nervous tissue of a patient to treat epilepsy, movement disorders, and other indications uses at least one implantable system control unit (SCU) (110), including an implantable signal / pulse generator (IPG) and one or more electrodes (152, 152′). The IPG is implanted in the mastoid area (143) of the skull (140) and communicates with at least one external appliance (230), such as a Behind-the-Ear (BTE) unit (100). In a preferred embodiment, the system is capable of open- and closed-loop operation. In closed-loop operation, at least one SCU includes a sensor, and the sensed condition is used to adjust stimulation parameters.
Owner:BOSTON SCI NEUROMODULATION CORP
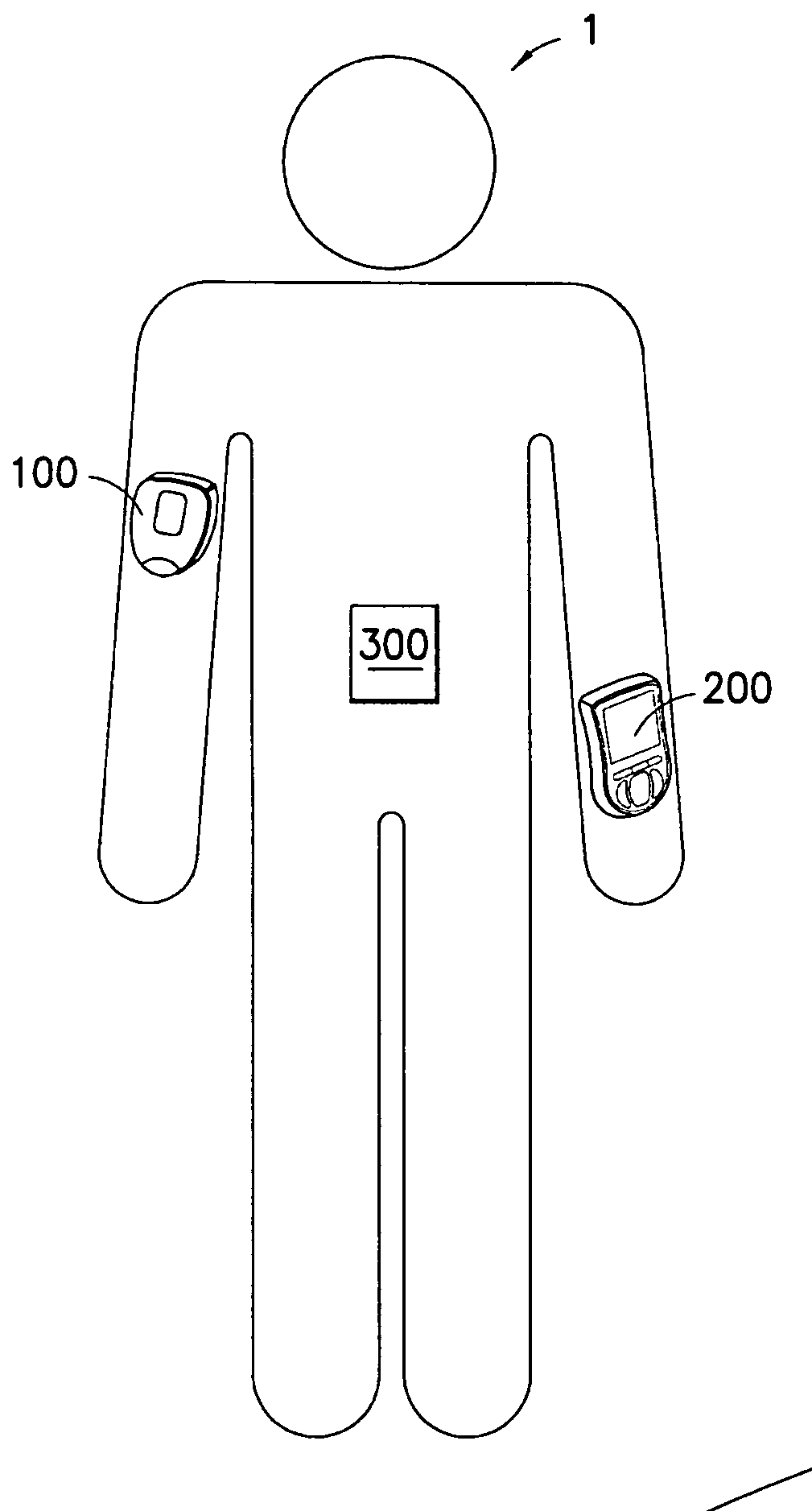
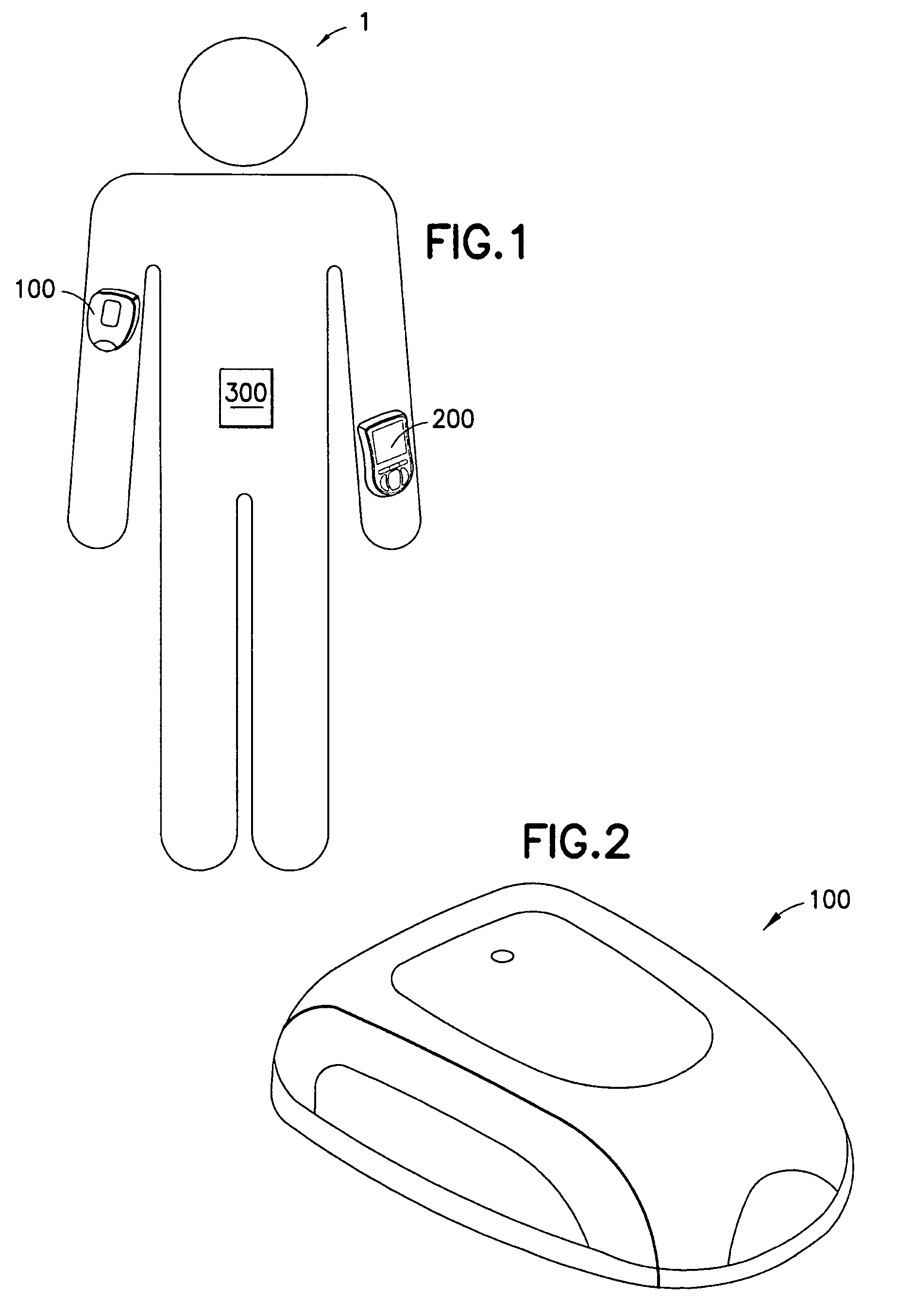
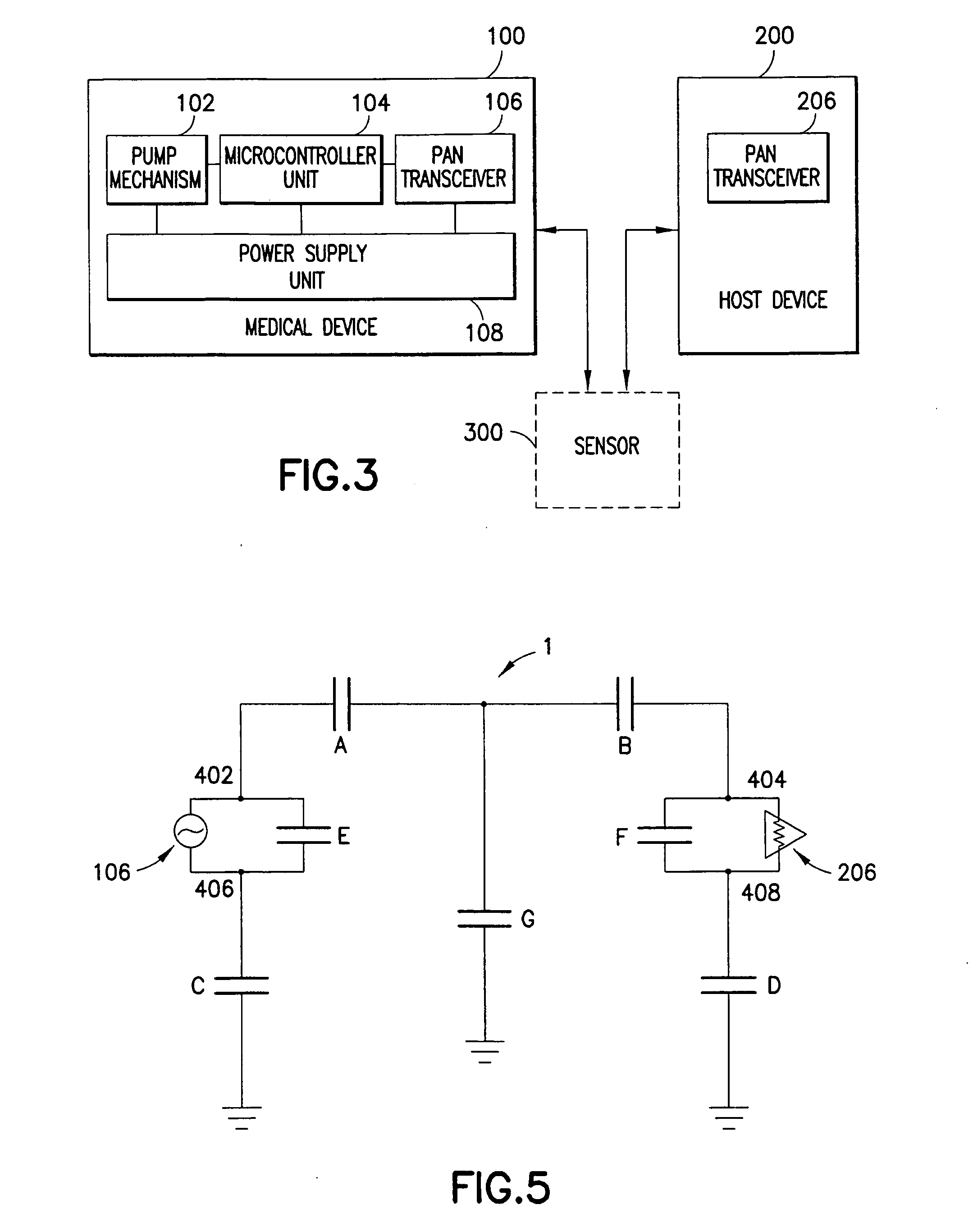
Medical device having capacitive coupling communication and energy harvesting
ActiveUS20110022025A1Low component requirementsImprove functionalityMedical devicesPressure infusionCapacitanceDrugs infusion
Provided is a wearable, self-contained drug infusion or medical device capable of communicating with a host controller or other external devices via a personal area network (PAN). The medical device utilizes a PAN transceiver for communication with other devices in contact with a user's body, such as a physiological sensor or host controller, by propagating a current across the user's body via capacitive coupling. The wearable nature of the medical device and the low power requirements of the PAN communication system enable the medical device to utilize alternative energy harvesting techniques for powering the device. The medical device preferably utilizes thermal, kinetic and other energy harvesting techniques for capturing energy from the user and the environment during normal use of the medical device. A system power distribution unit is provided for managing the harvested energy and selectively supplying power to the medical device during system operation.
Owner:BECTON DICKINSON & CO
Clustering of recorded patient neurological activity to determine length of a neurological event
Apparatus and method detect a detection cluster that is associated with a neurological event, such as a seizure, of a nervous system disorder and update therapy parameters that are associated with a treatment therapy. The occurrence of the detection cluster is detected when the maximal ratio exceeds an intensity threshold. If the maximal ratio drops below the intensity threshold for a time interval that is less than a time threshold and subsequently rises above the intensity threshold, the subsequent time duration is considered as being associated with the detection cluster rather than being associated with a different detection cluster. Consequently, treatment of the nervous system disorder during the corresponding time period is in accordance with one detection cluster. Treatment therapy may be provided by providing electrical stimulation, drug infusion or a combination. Therapy parameters may be updated for each mth successive group of applications of the treatment therapy or for each nth detection cluster.
Owner:MEDTRONIC INC
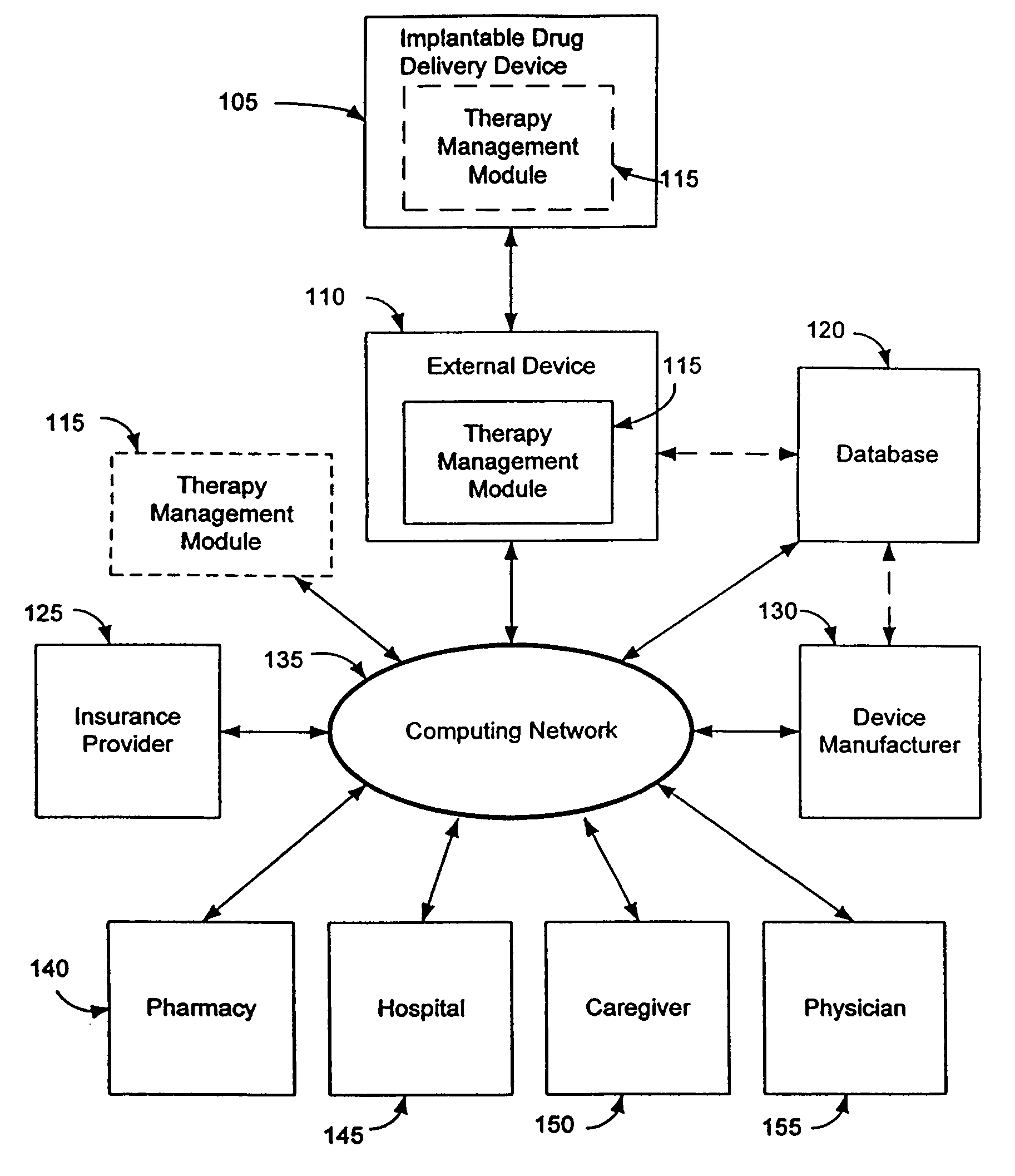
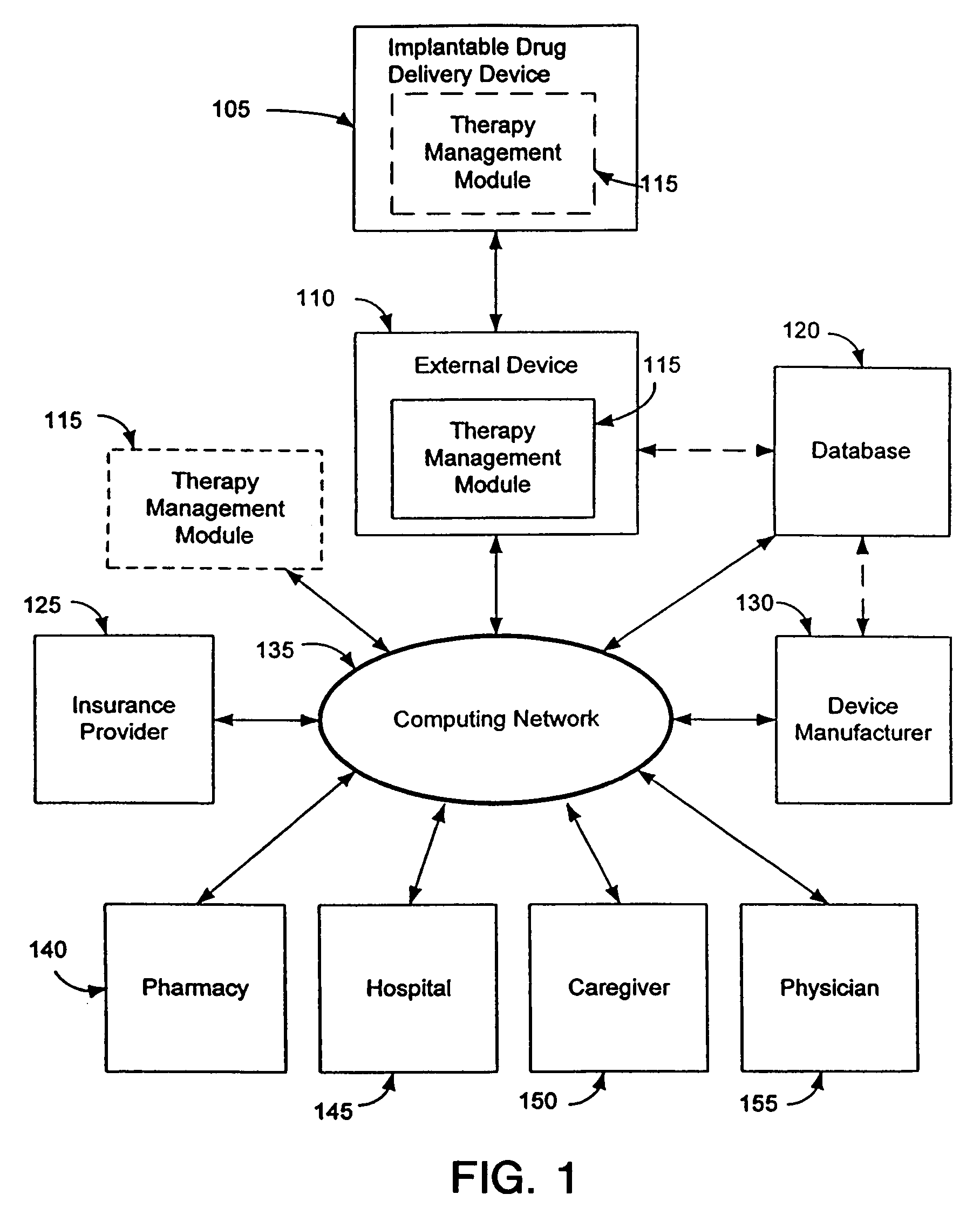
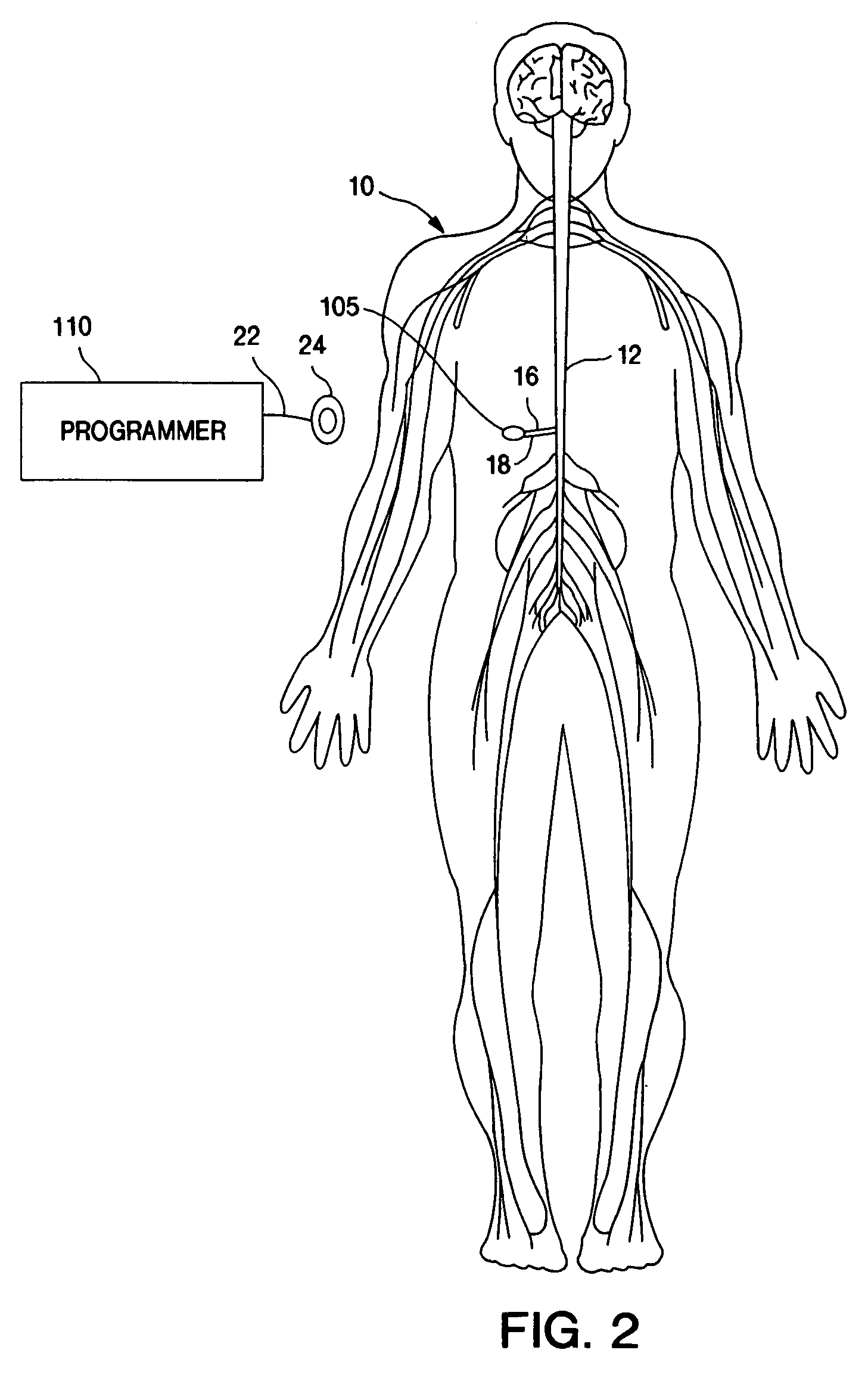
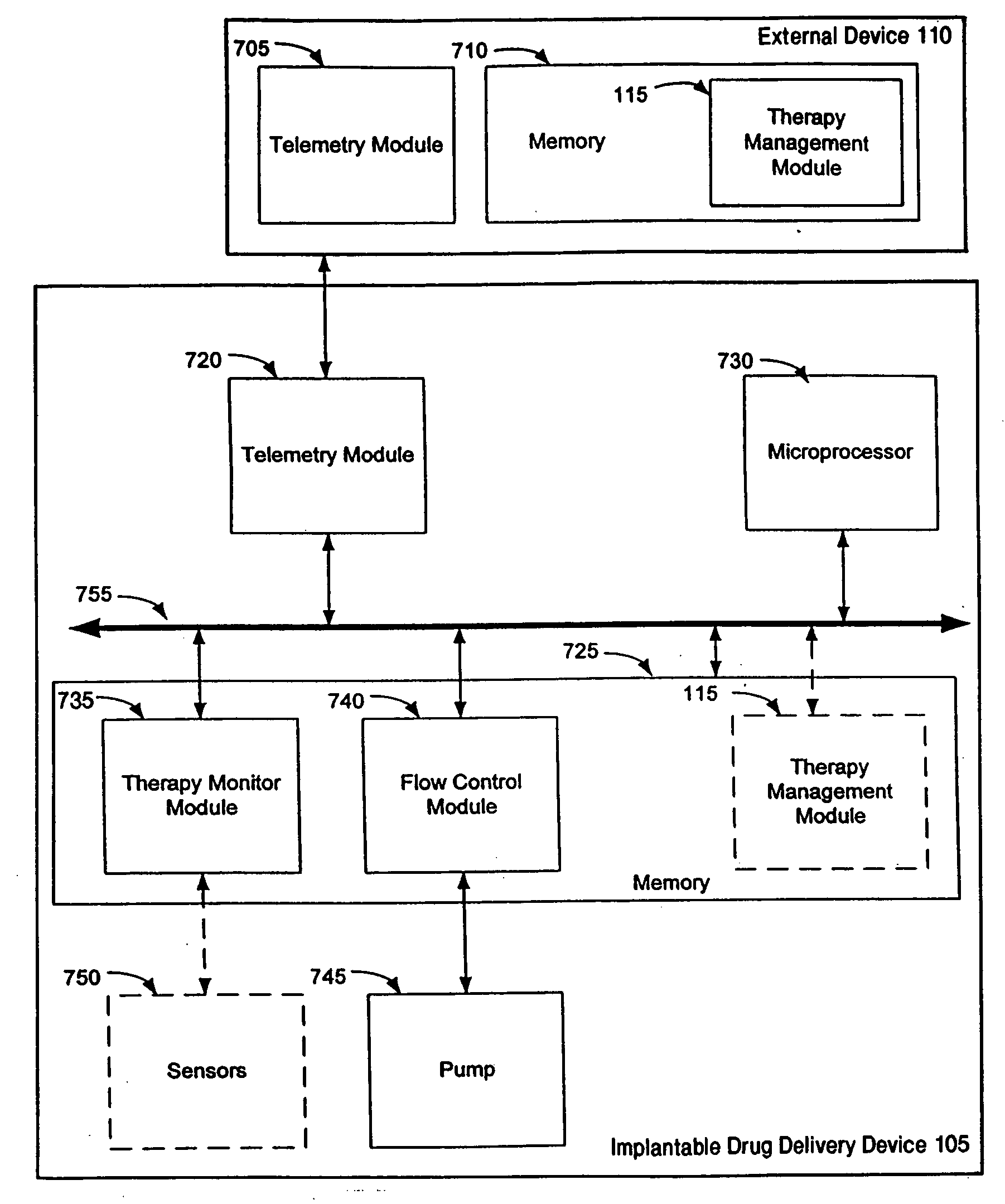
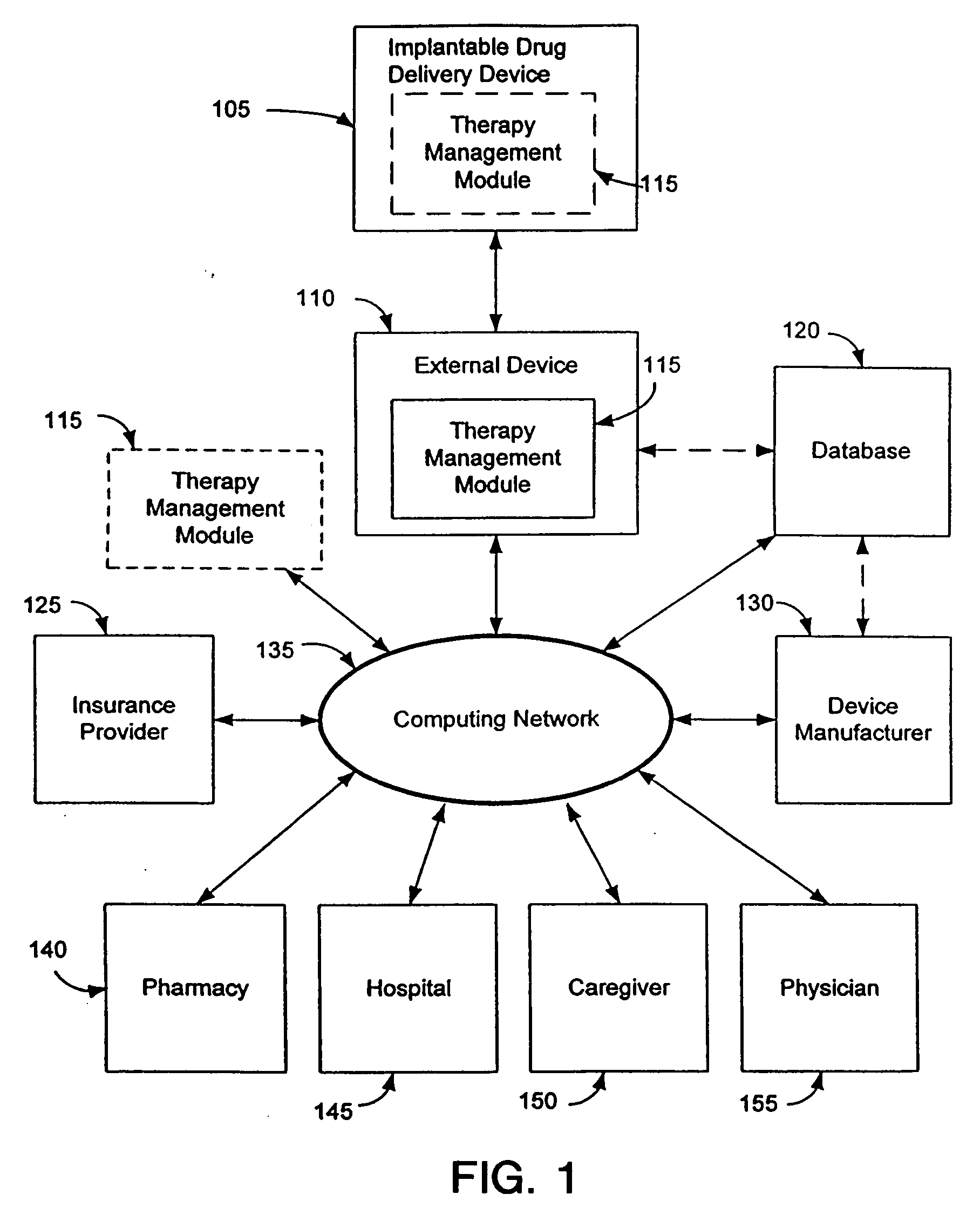
Therapy management techniques for an implantable medical device
Disclosed is a method and apparatus for automatically adjusting drug infusion rate to optimize treatment therapy. The implantable medical device can communicate with a database or algorithm controlled by a caregiver or physician. Thus, the caregiver may request a therapy change (e.g., infusion rate versus time, pump clock settings, etc.) or a therapy management module may automatically activate the therapy change at some future time for convenience, or for technical or clinical reasons.
Owner:MEDTRONIC INC
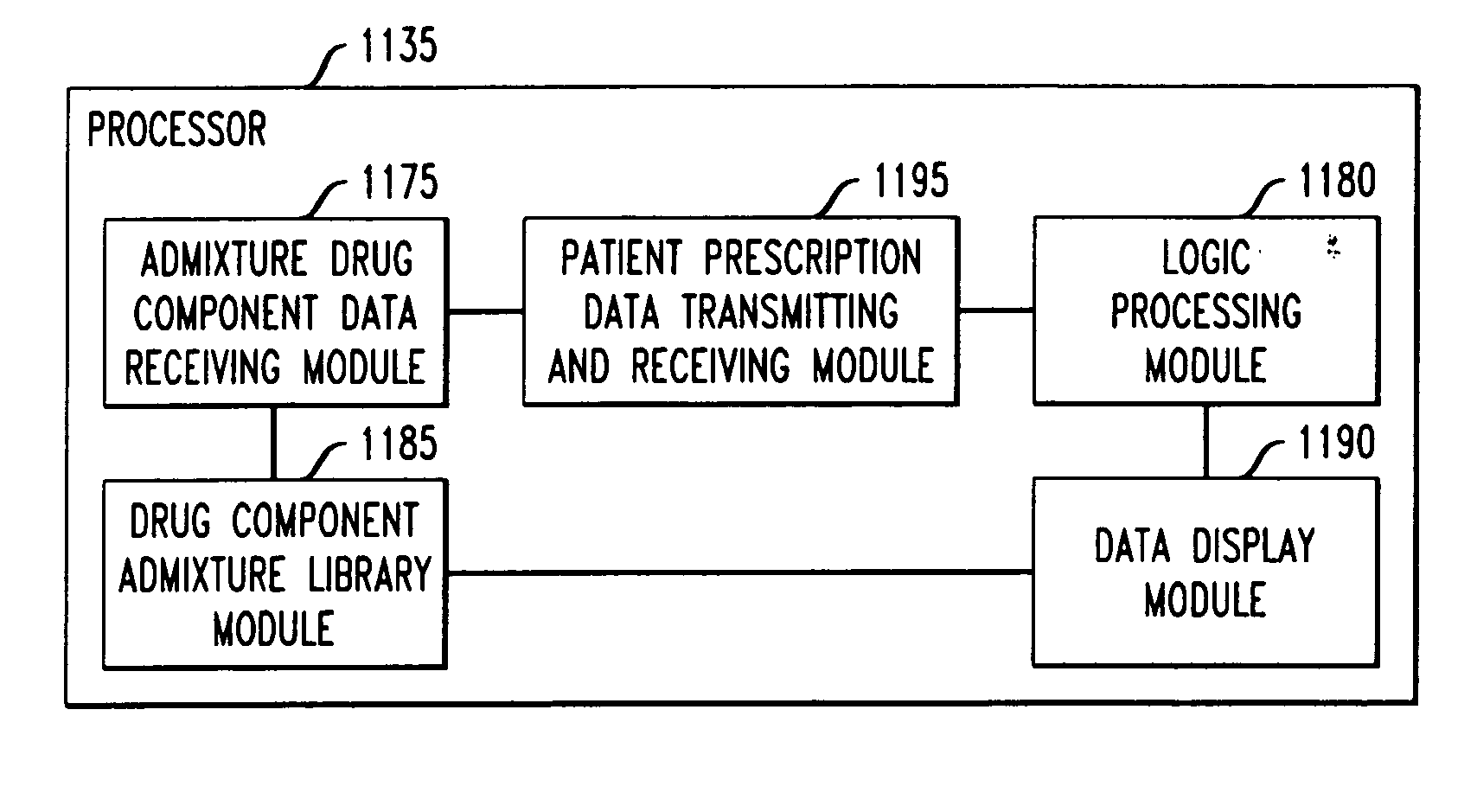
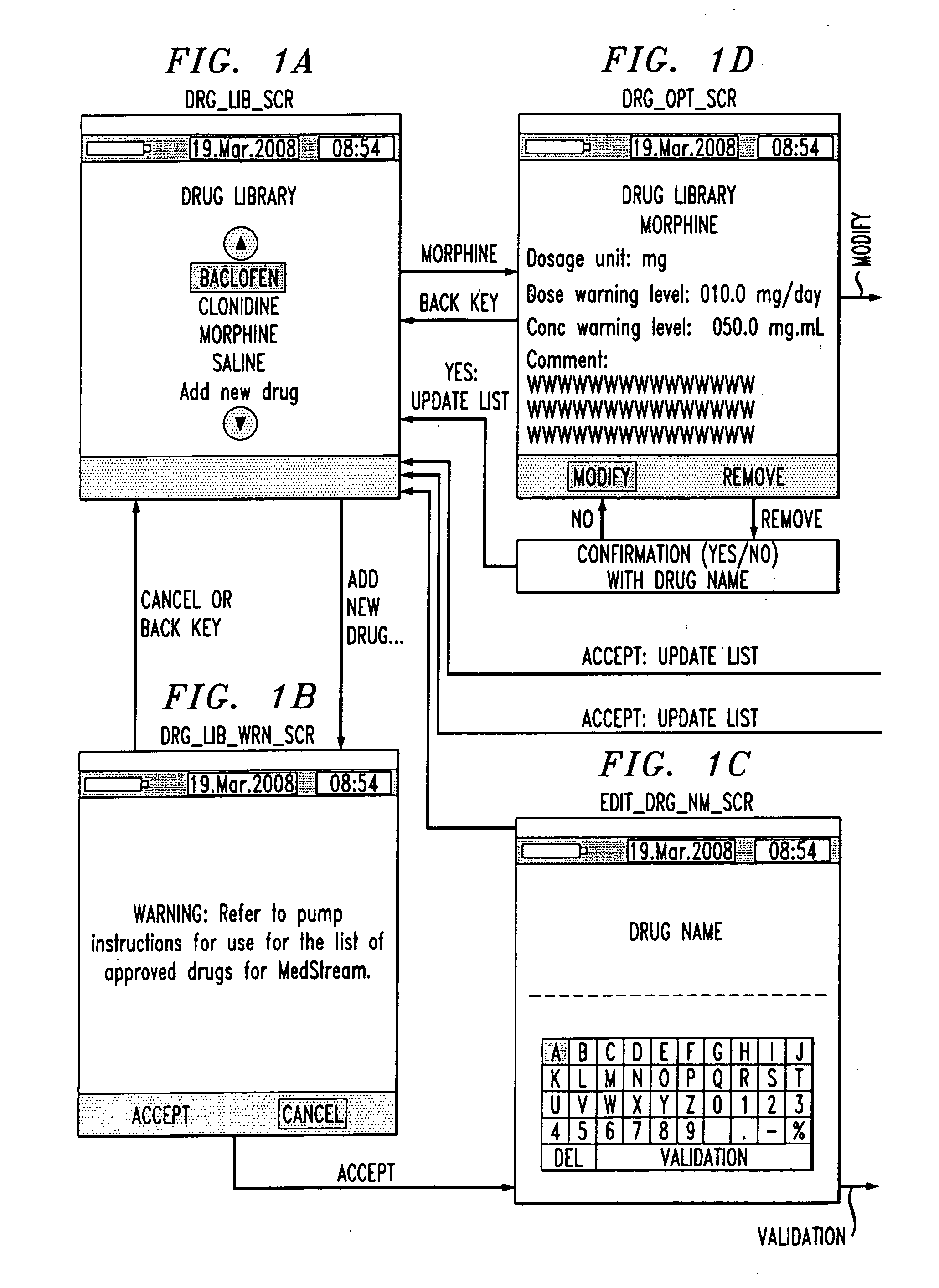
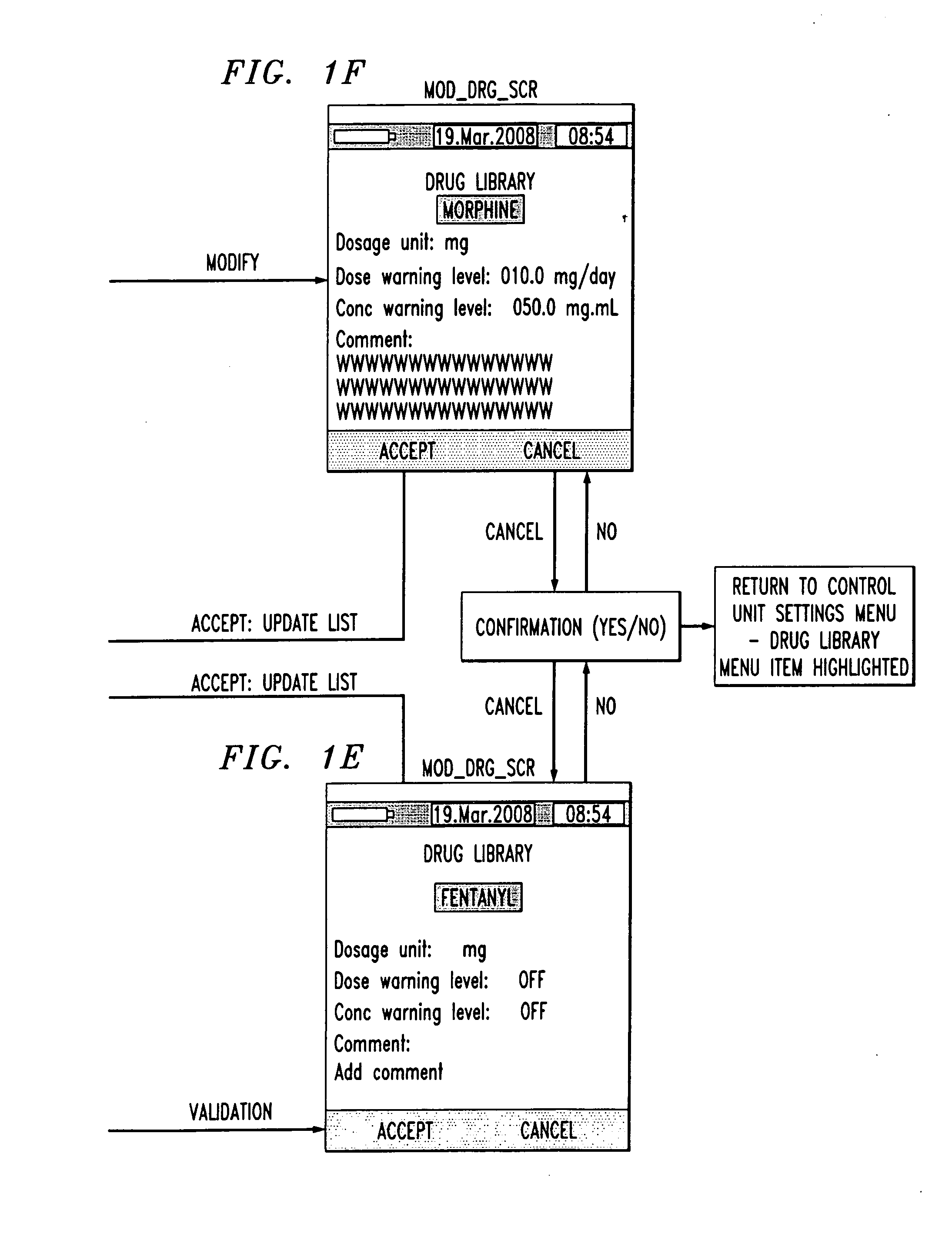
Drug component admixture library for a drug infusion delivery system
InactiveUS20110320049A1Shorten the timeReduce generationLevel controlControlling ratio of multiple fluid flowsDrugs infusionDelivery system
Minimizing improper dosage of a drug admixture (including a single primary drug component and at least one second drug component). For each drug component in the drug admixture, receiving a name of the drug component along with its dosage unit, a maximum dose warning level and a maximum concentration warning level. Receiving a concentration for each of the single primary drug component and the at least one secondary drug component; and a dose setting of only the primary drug component. Automatically calculating a dose of each of the at least one secondary drug component. Generating an alert when: (i) the received dose setting of the primary drug component or calculated dose setting of the at least one secondary drug component exceeds the dose warning level; or (ii) the received concentration of the primary drug component or the at least one secondary drug component exceeds the concentration warning level.
Owner:MEDOS INT SARL
Skull-mounted electrical stimulation system and method for treating patients
ActiveUS7769461B2Extension of timeMinimal discomfortHead electrodesMedical devicesDrugs infusionElectricity
A system and method for applying electrical stimulation or drug infusion to nervous tissue of a patient to treat epilepsy, movement disorders, and other indications uses at least one implantable system control unit (SCU) (110), including an implantable signal / pulse generator (IPG) and one or more electrodes (152, 152′). The IPG is implanted in the mastoid area (143) of the skull (140) and communicates with at least one external appliance (230), such as a Behind-the-Ear (BTE) unit (100). In a preferred embodiment, the system is capable of open- and closed-loop operation. In closed-loop operation, at least one SCU includes a sensor, and the sensed condition is used to adjust stimulation parameters.
Owner:BOSTON SCI NEUROMODULATION CORP
Clustering of recorded patient neurological activity to determine length of a neurological event
Apparatus and method detect a detection cluster that is associated with a neurological event, such as a seizure, of a nervous system disorder and update therapy parameters that are associated with a treatment therapy. The occurrence of the detection cluster is detected when the maximal ratio exceeds an intensity threshold. If the maximal ratio drops below the intensity threshold for a time interval that is less than a time threshold and subsequently rises above the intensity threshold, the subsequent time duration is considered as being associated with the detection cluster rather than being associated with a different detection cluster. Consequently, treatment of the nervous system disorder during the corresponding time period is in accordance with one detection cluster. Treatment therapy may be provided by providing electrical stimulation, drug infusion or a combination. Therapy parameters may be updated for each mth successive group of applications of the treatment therapy or for each nth detection cluster.
Owner:MEDTRONIC INC
Delivery of a sympatholytic cardiovascular agent to the central nervous system to counter heart failure and pathologies associated with heart failure
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms and otherwise treat heart failure (HF) and pathologies associated with HF. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with HF (or pathologies associated with HF) and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist, e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine.
Owner:MEDTRONIC INC
Drug infusion device with tissue identification using pressure sensing
An automatic injection device includes a drive mechanism and a sensor used to determine an internal characteristic such as a force or internal pressure generated during an injection process. This characteristic is then used as a control parameter by a microprocessor or controller to determine the exit pressure of the fluid expelled by the device. This exit pressure is then used to identify the kind of tissue in which the injection is being introduced.
Owner:MILESTONE SCIENTIFIC INC
Techniques for positioning therapy delivery elements within a spinal cord or brain
Apparatus and techniques to address problems associated with lead migration, patient movement or position, histological changes, neural plasticity or disease progression. Disclosed are techniques for implanting a lead having therapy delivery elements, such as electrodes or drug delivery ports, within a vertebral or cranial bone so as to maintain these elements in a fixed position relative to a desired treatment site. The therapy delivery elements may thereafter be adjusted in situ with a position control mechanism and / or a position controller to improve the desired treatment, such as electrical stimulation and / or drug infusion to a precise target. The therapy delivery elements may be positioned laterally in any direction relative to the targeted treatment site or toward or away from the targeted treatment site. A control system maybe provided for open- or closed-loop feedback control of the position of the therapy delivery elements as well as other aspects of the treatment therapy.
Owner:MEDTRONIC INC
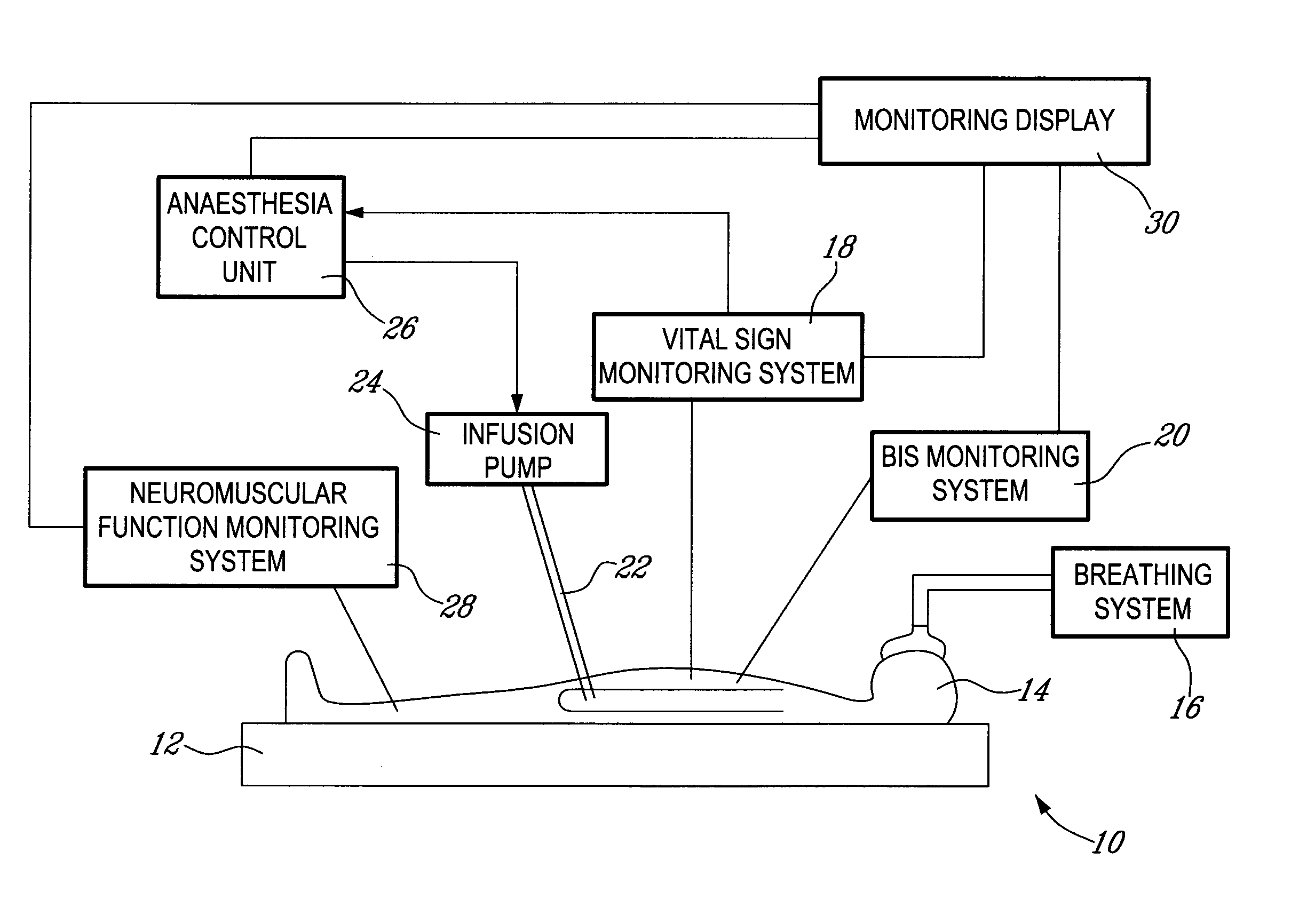
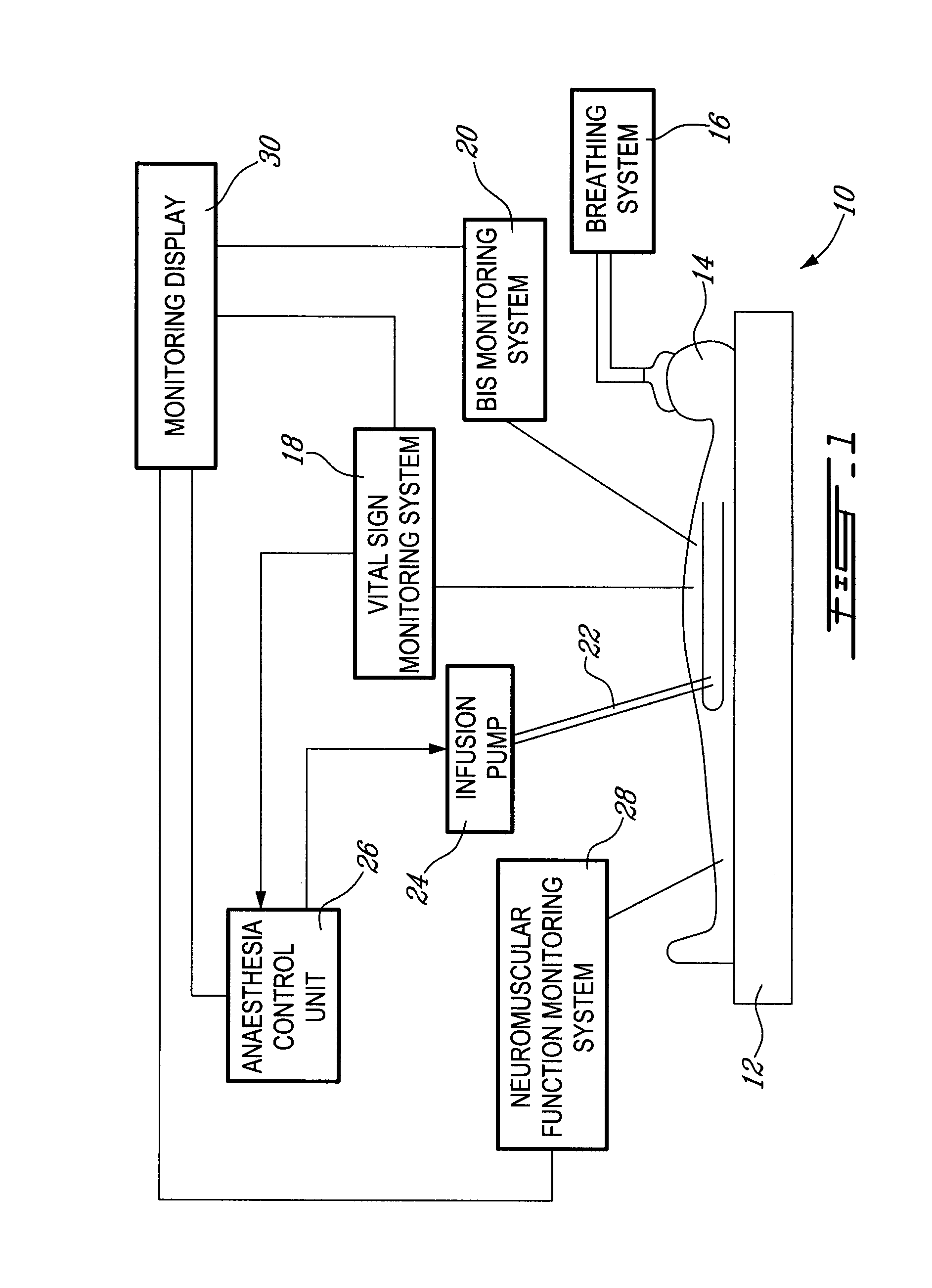
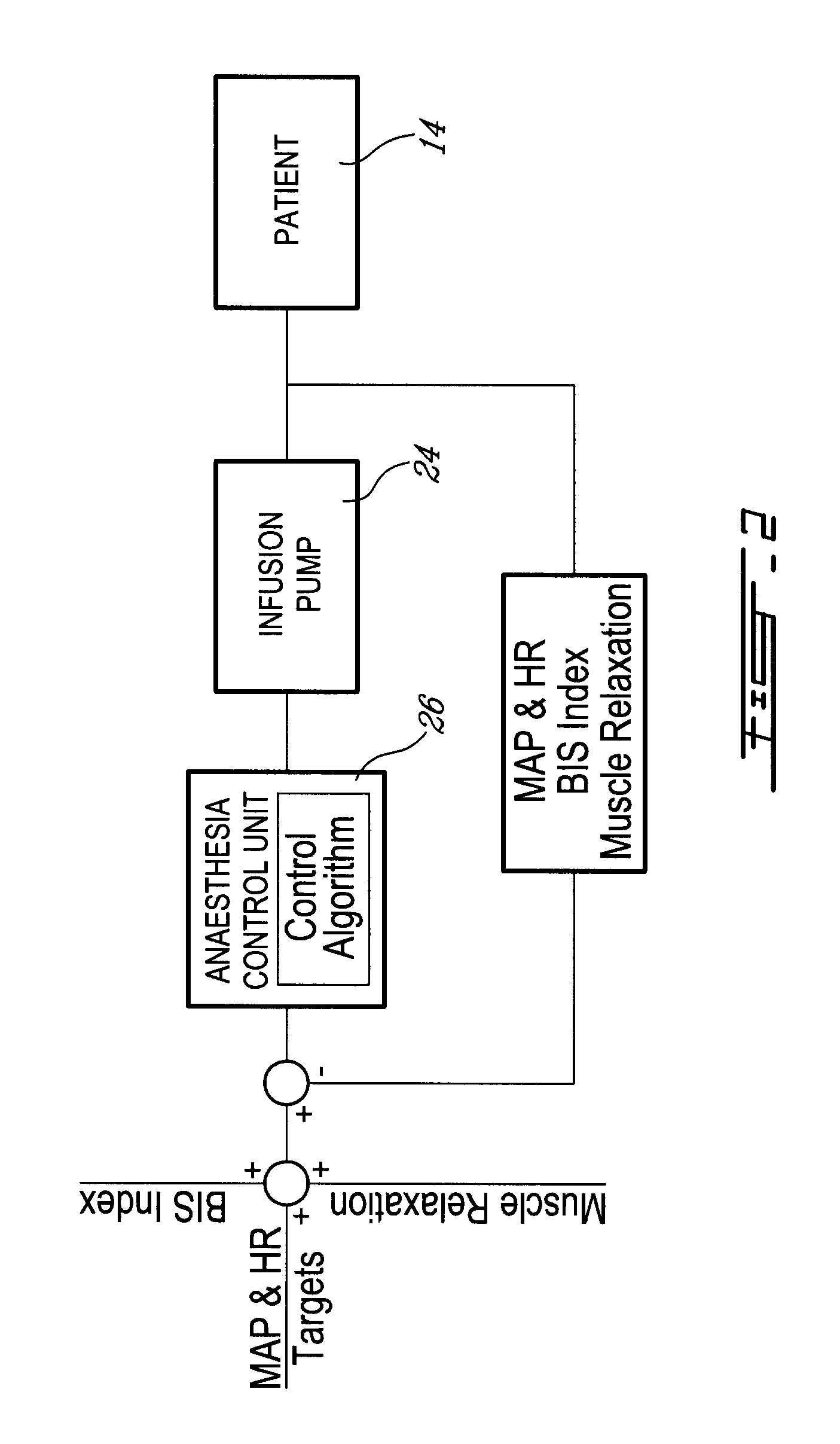
Method and system for administering an anaesthetic
A method and system for objectively scoring intra-operative pain during general anaesthesia based on the patient's mean arterial pressure and heart rate. The index is used for closed-loop control of the intra-operative analgesia through adjustment of the drug infusion level according to fuzzy logic. It is further displayed along with other components of anaesthesia and important patient data on a monitoring display for presentation to medical staff.
Owner:HEMMERLING THOMAS +4
Therapy management techniques for an implantable medical device
Disclosed is a method and apparatus for automatically adjusting drug infusion rate to optimize treatment therapy. The implantable medical device can communicate with a database or algorithm controlled by a caregiver or physician. Thus, the caregiver may request a therapy change (e.g., infusion rate versus time, pump clock settings, etc.) or a therapy management module may automatically activate the therapy change at some future time for convenience, or for technical or clinical reasons.
Owner:MEDTRONIC INC
Dosage sensing unit with tactile feedback
ActiveUS20100114026A1Pharmaceutical delivery mechanismMedical devicesDrugs infusionBiological activation
A drug infusion assembly comprises a drug delivery device arranged to adhere to a patient's skin and includes a reservoir that holds the drug, a cannula that delivers the drug to the patient, and a pump that causes the drug to flow to the cannula. The assembly further includes a monitor device for providing information about the operation of a drug delivery device. The monitor device includes a housing arranged to be attached to and detached from the drug delivery device, a sensor that senses the operation of the drug delivery device and generates an activation signal, a clock mechanism that generates a time signal, a memory that receives and stores the activation signal and the time signal and creates an information packet coordinating the time signal and the activation signal, an interrogator that interrogates the memory such that the memory generates a memory signal in response thereto, and a responder that receives the memory signal and generates a response.
Owner:CALIBRA MEDICAL
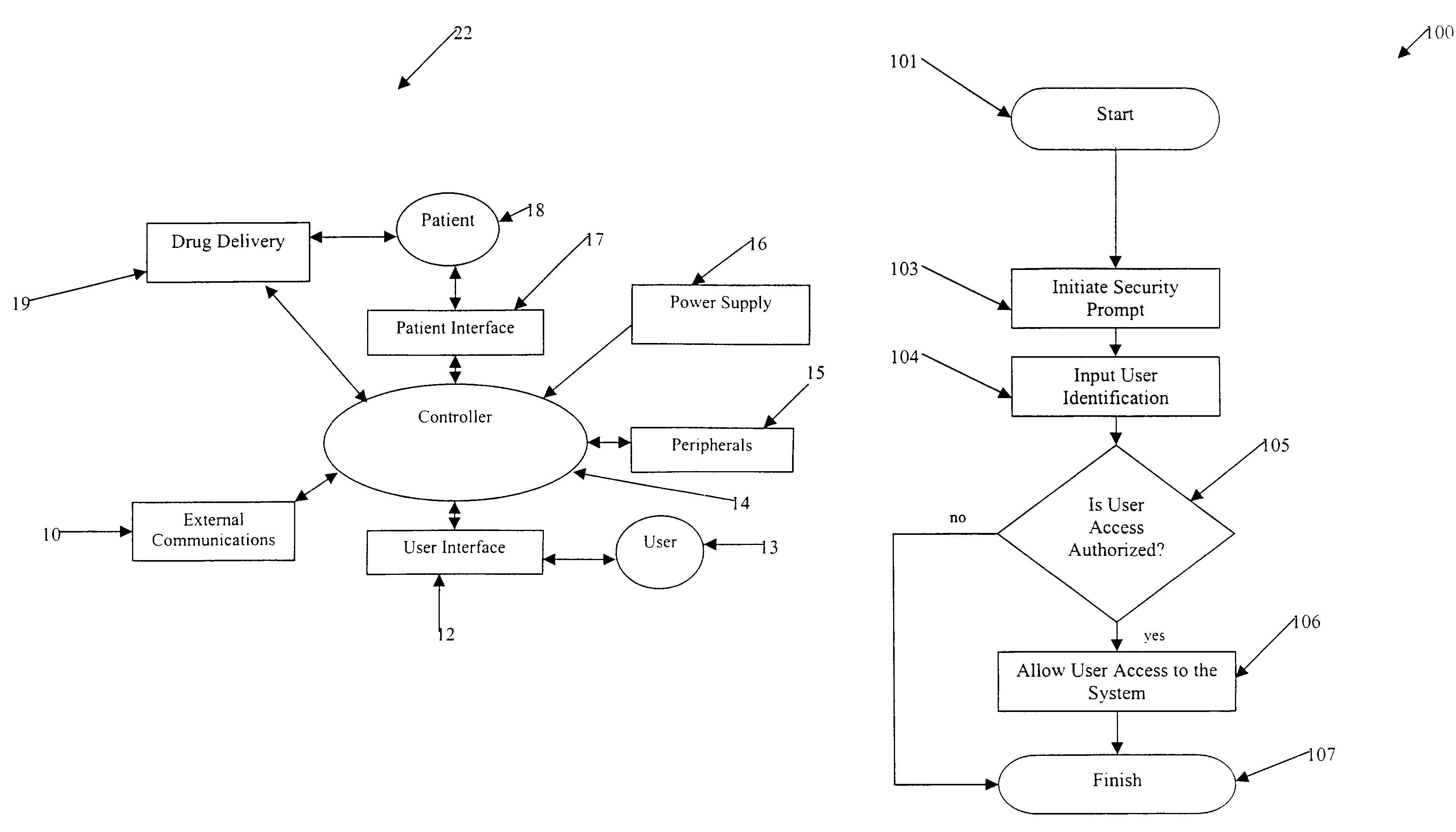
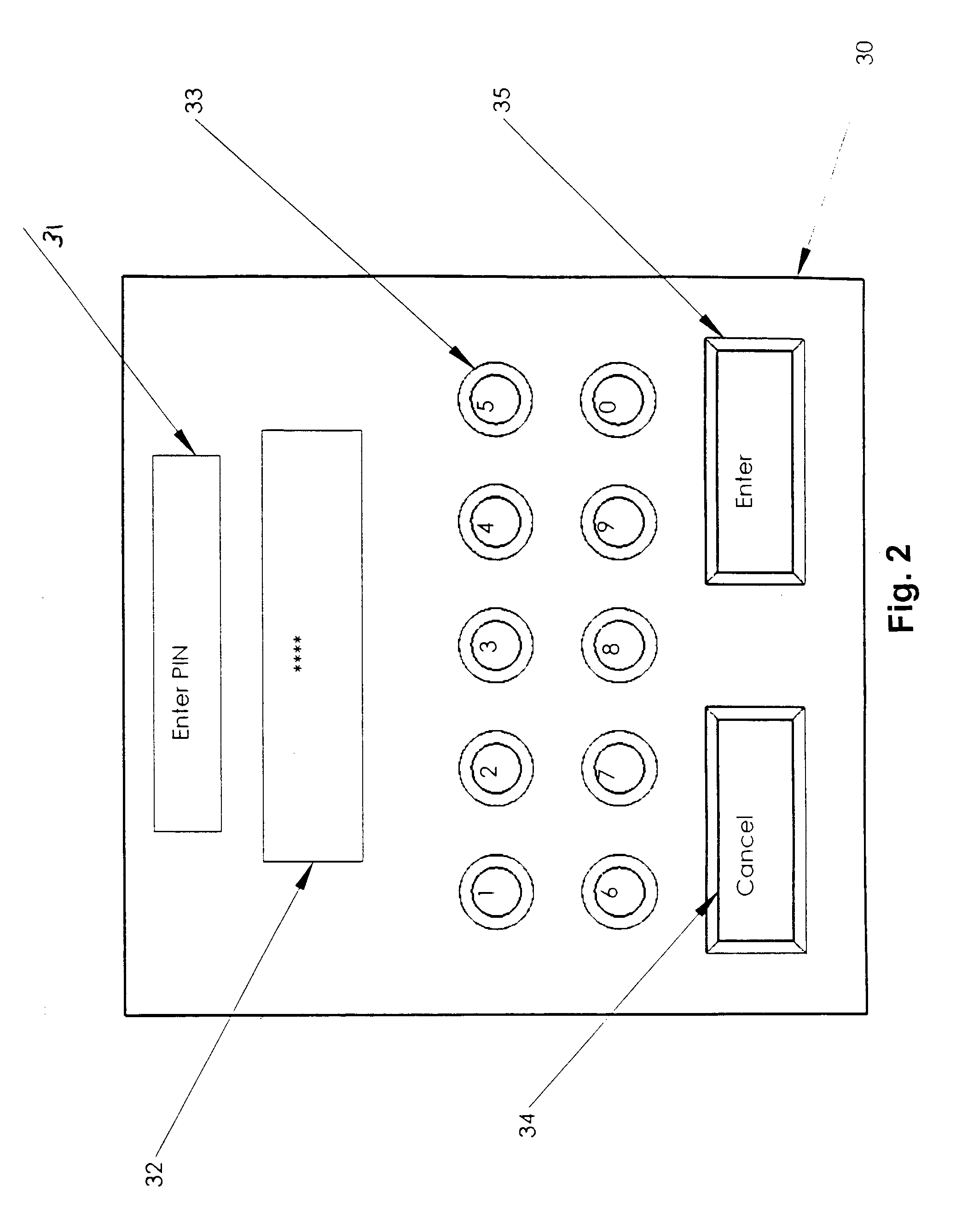
User authorization system and method for a sedation and analgesia system
InactiveUS7578802B2Improve the level ofPrevent and improper useData processing applicationsDrug and medicationsDrugs infusionInternet privacy
The present invention comprises a security system integral with a sedation and analgesia system. The invention includes a computer assisted IV drug infusion administration device coupled with a secure user interface, or other security means, that can restrict and monitor user access to prevent unauthorized or improper use of the device. The system can also provide varying levels of access to the device so that different users may have different levels of access to system operations. The variety of access levels may prevent accidental or intentional misuse of the drug delivery system, while still permitting access to the required functionality. Data, such as usage statistics and procedural events associated with drug delivery, may be recorded in association with a user's personal identification information to help identify training needs and to identify possible misuse of access information.
Owner:SCOTT LAB
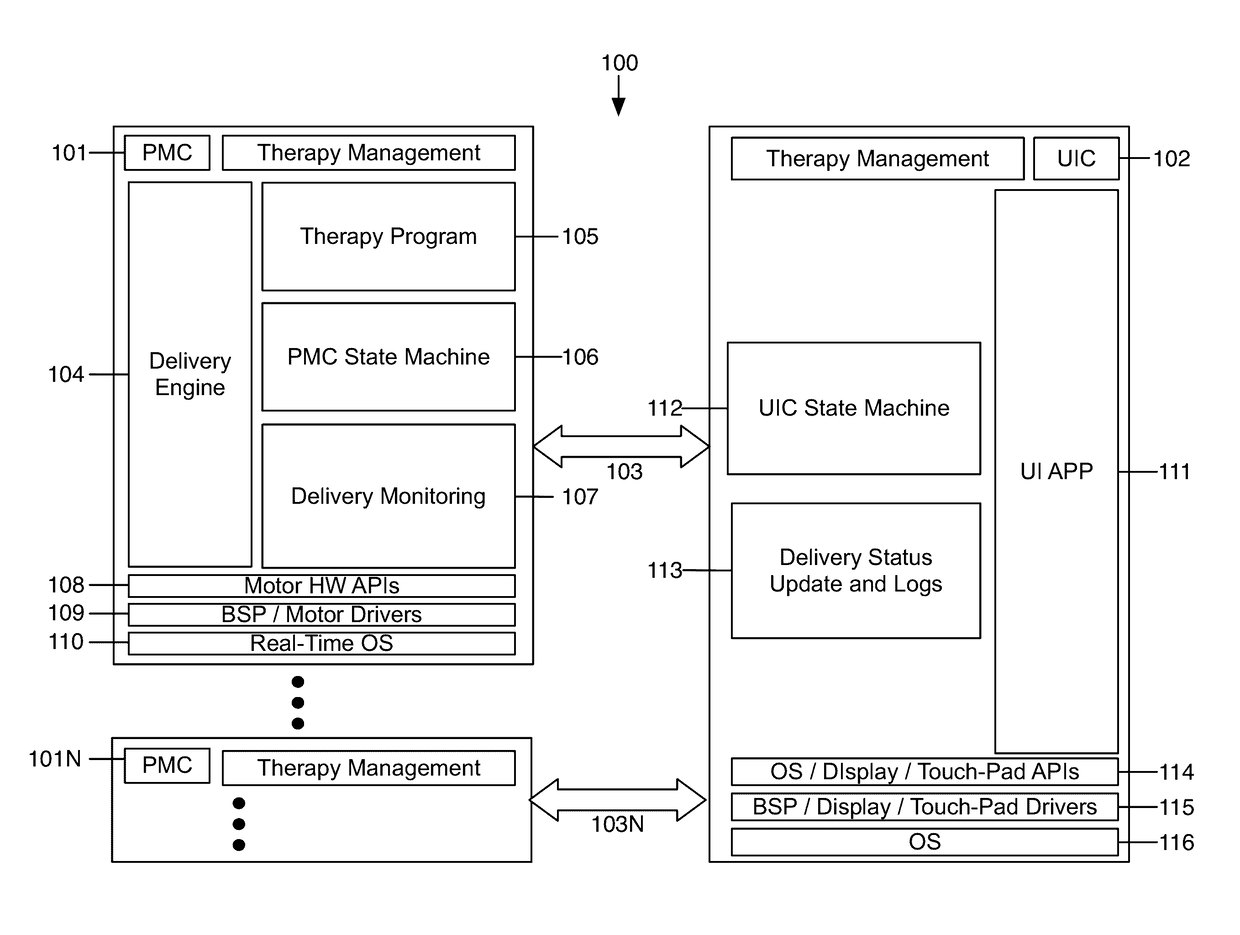
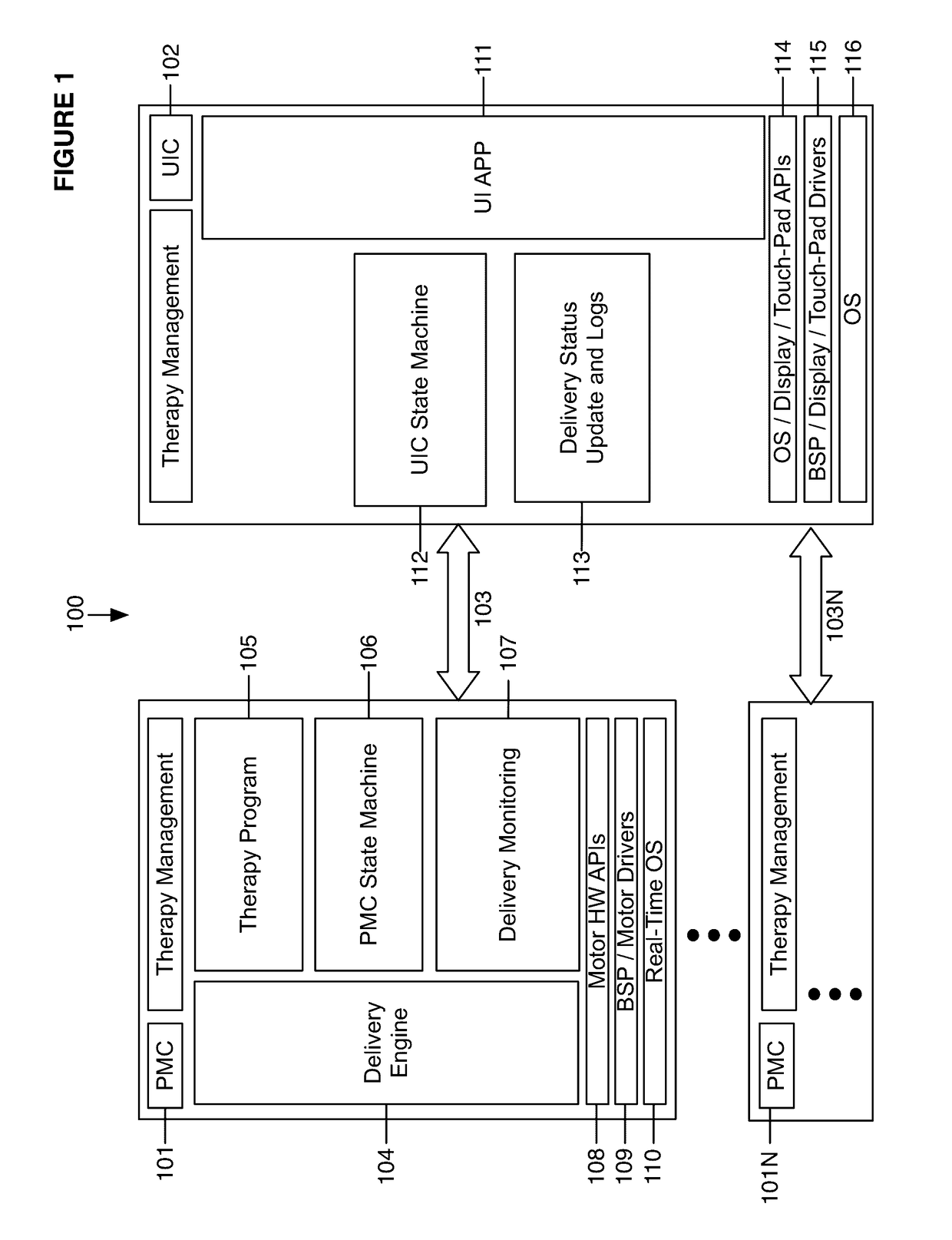
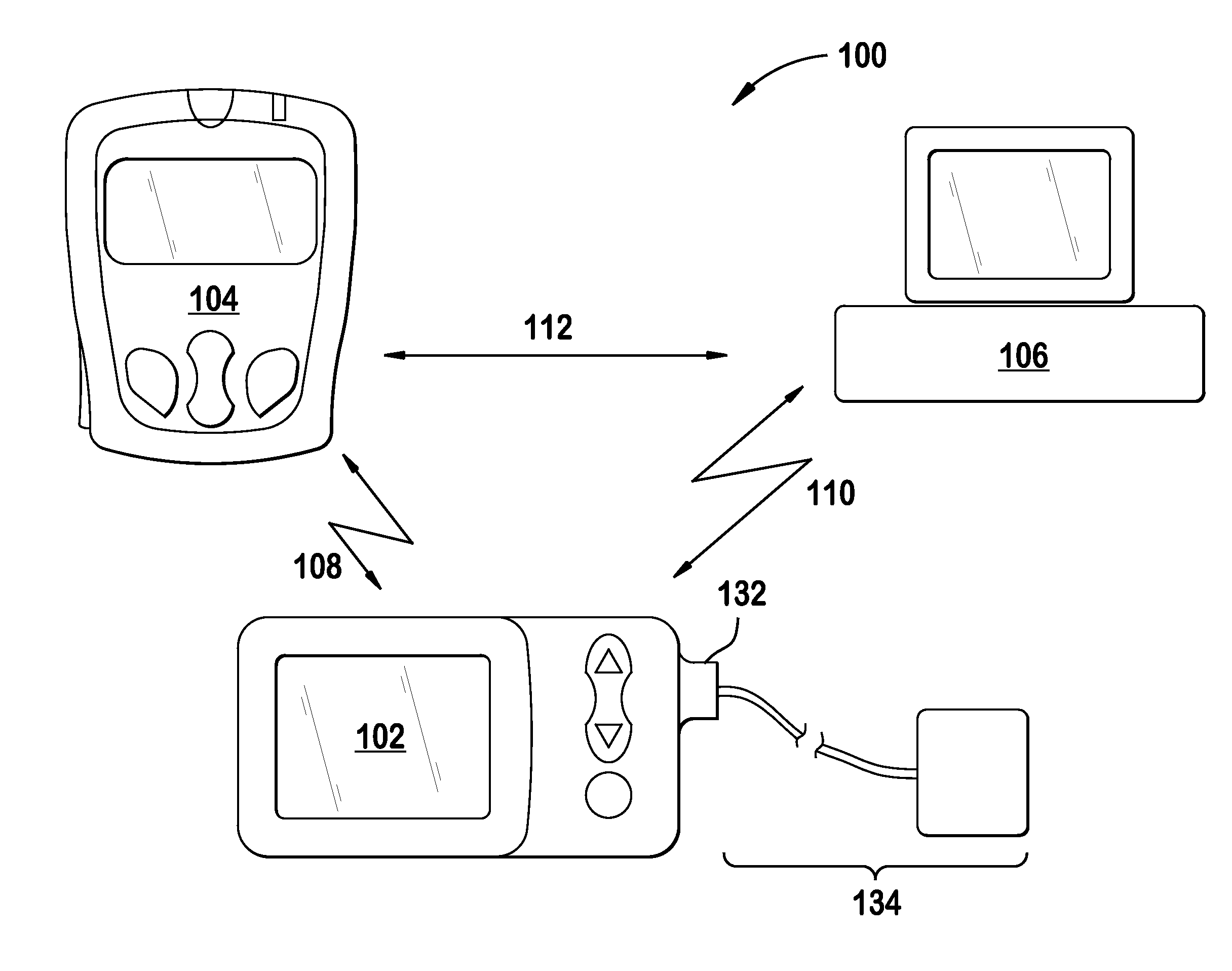
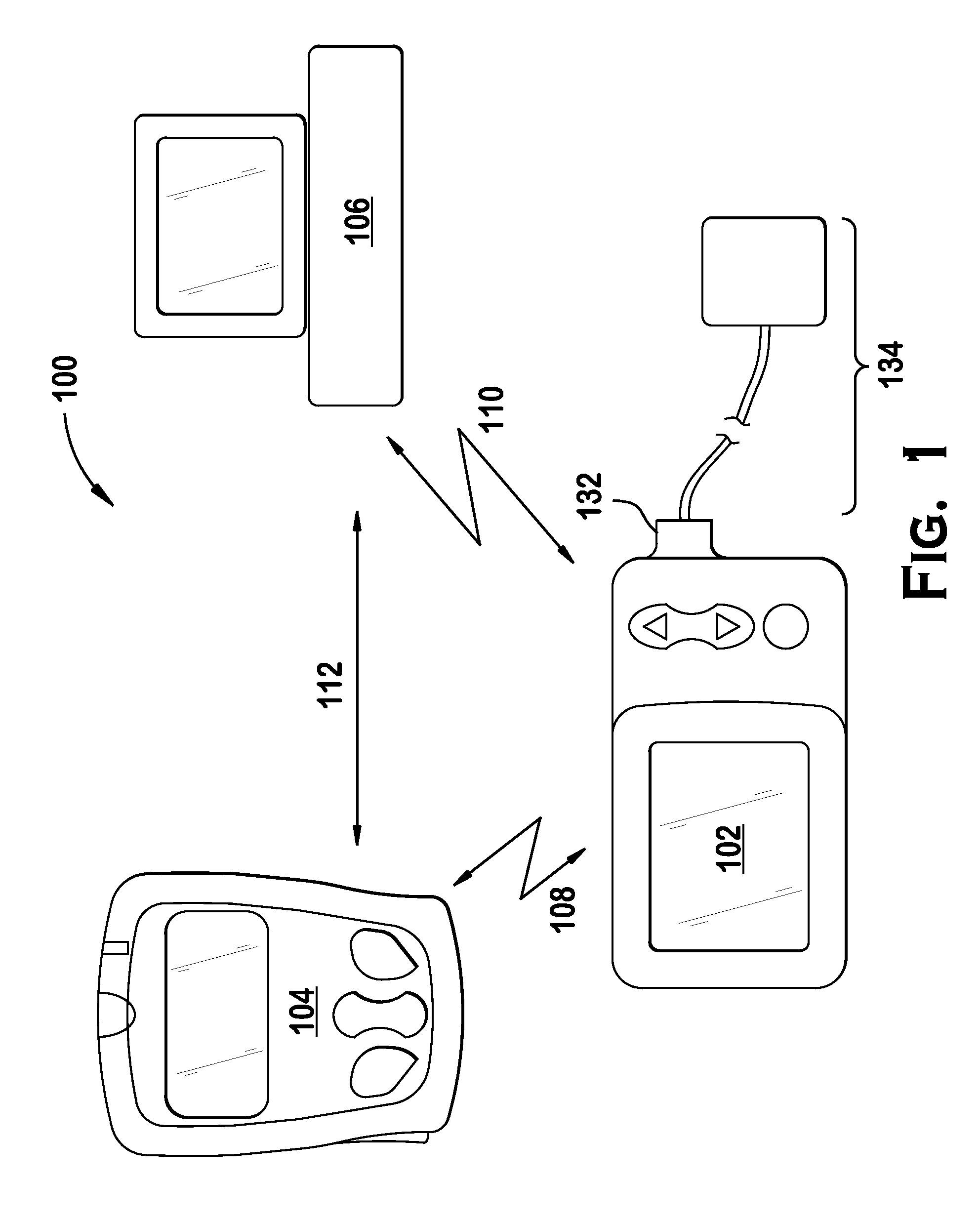
Fail-safe drug infusion therapy system
A fail-safe drug infusion system, including a user interface controller (UIC) and at least one pump motor controller (PMC), with protocols that enable the PMC to operate therapy delivery for a limited amount of time if the UIC fails or the communication link between the UIC and the PMC is interrupted. Includes synchronization methods to synchronize the delivery information back to the UIC after the UIC reboots or after the communication link is restored. The PMC may apply intelligent fail-safe drug infusion therapy by temporarily displaying therapy information, for example information normally displayed by the UIC, while taking control of alarm signaling and providing minimal user control of the therapy until the UIC restores itself, the infusion completes normally, or the user stops the infusion. If the PMC becomes inoperable, the UIC may wait for the PMC to reboot, or attempt to switch infusion channels to provide robust drug infusion.
Owner:ICU MEDICAL INC
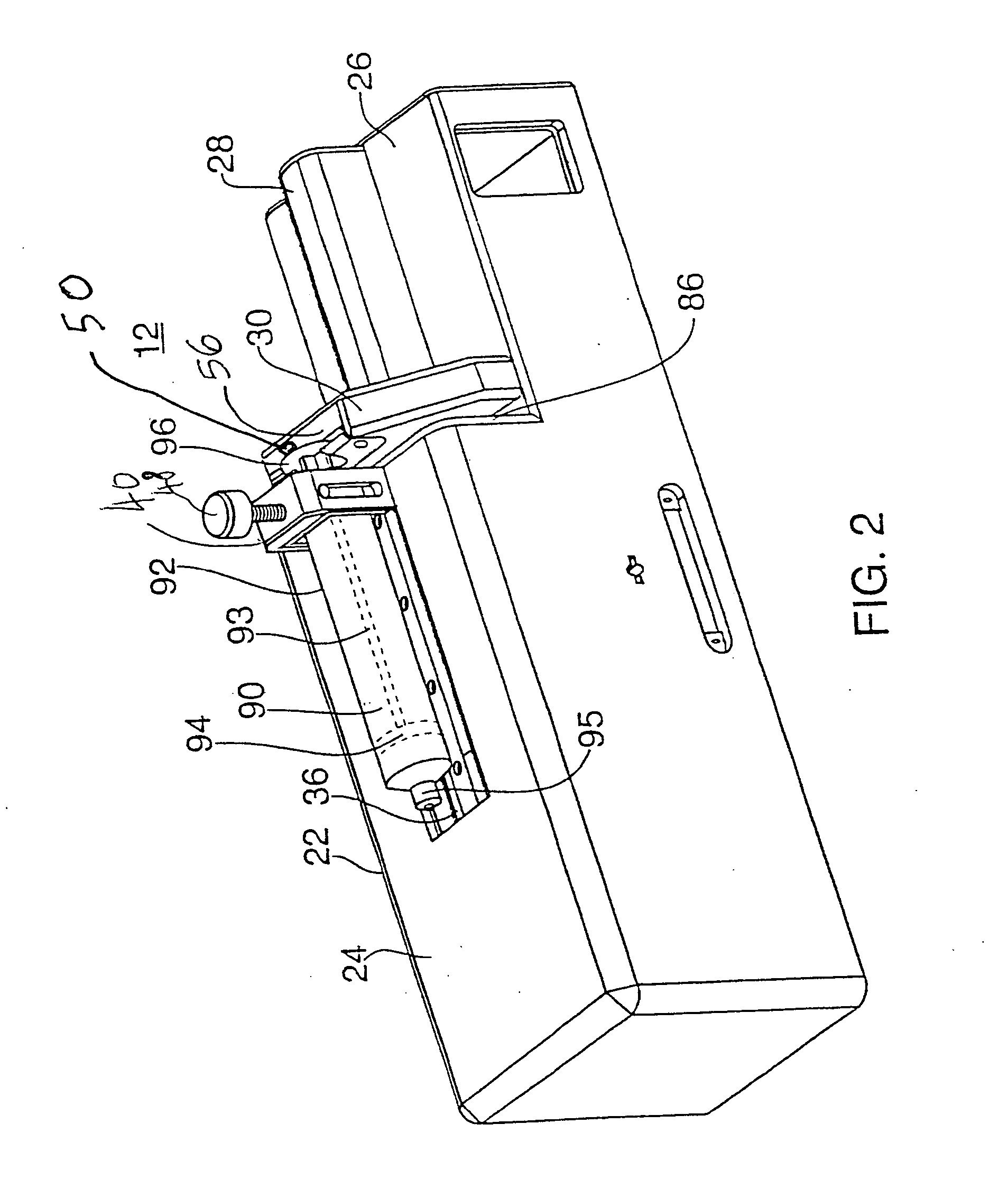
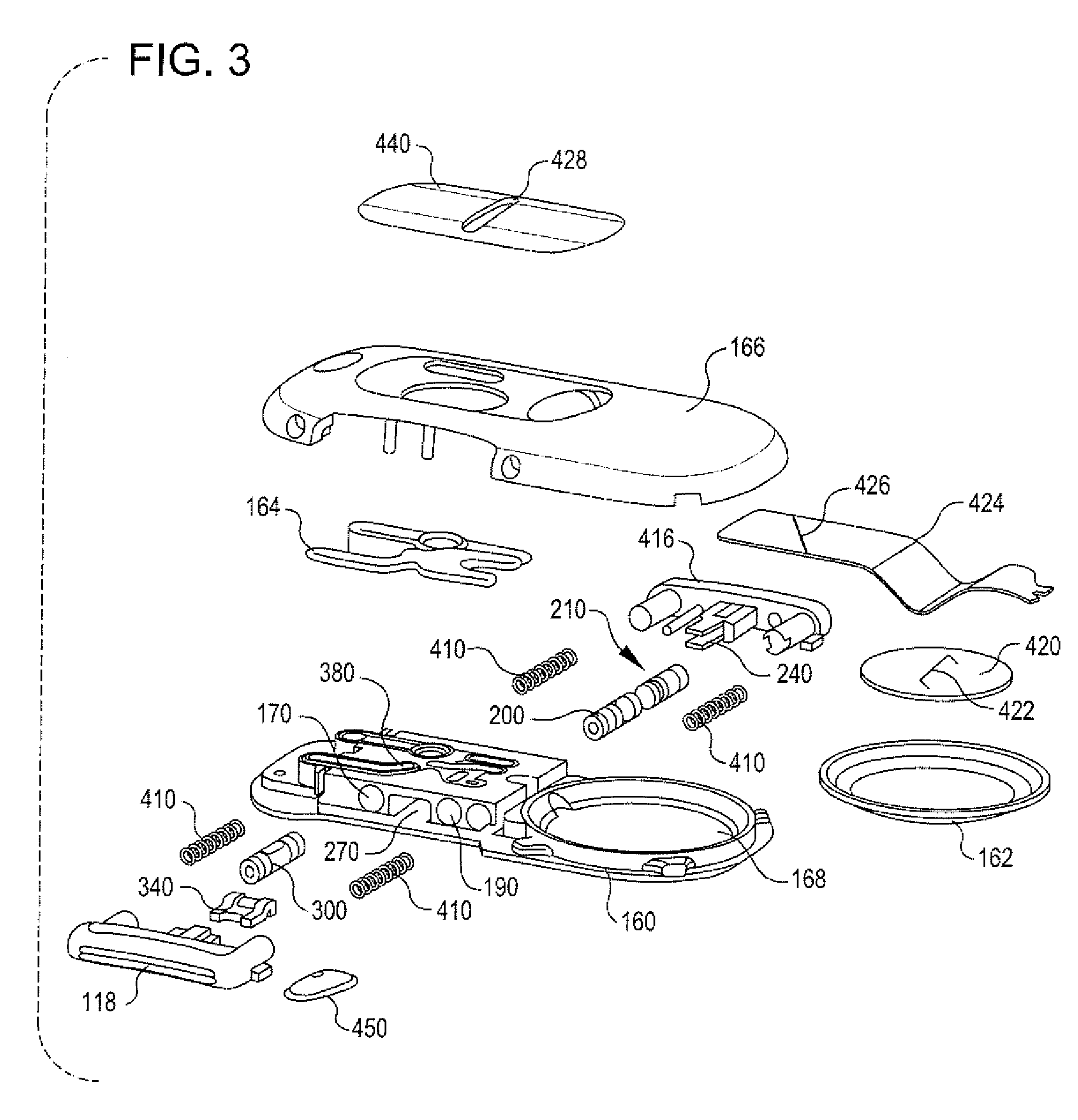
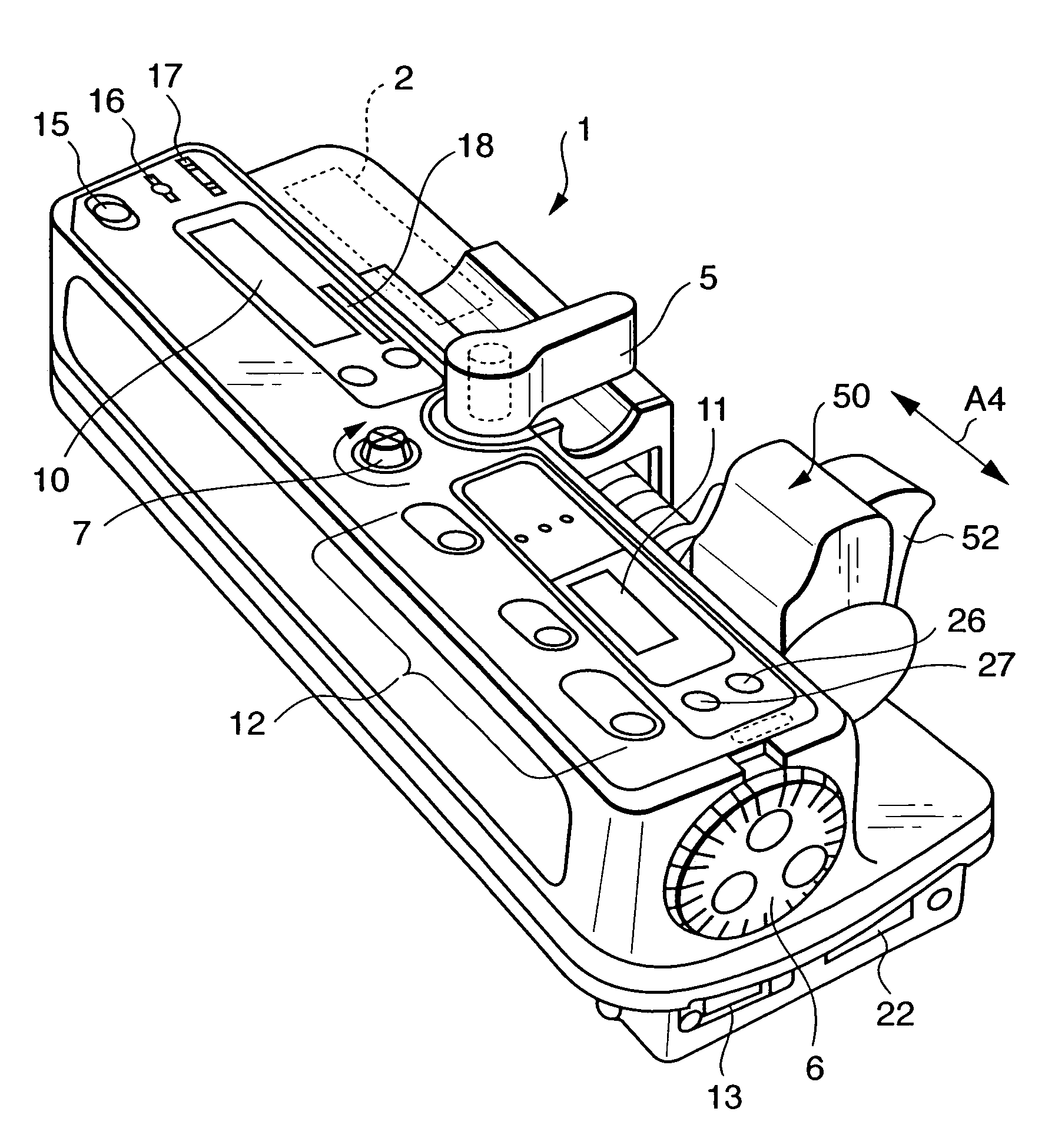
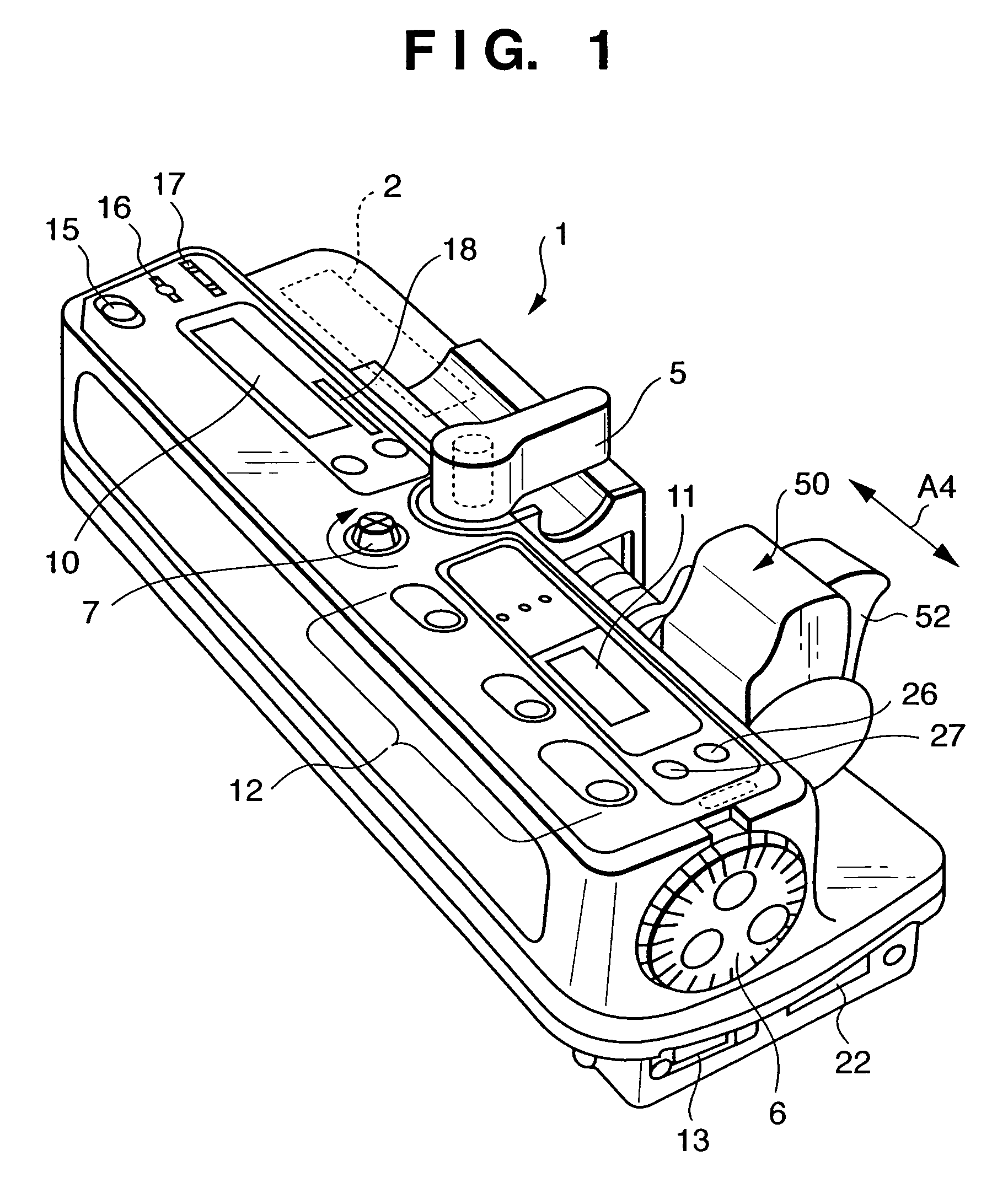
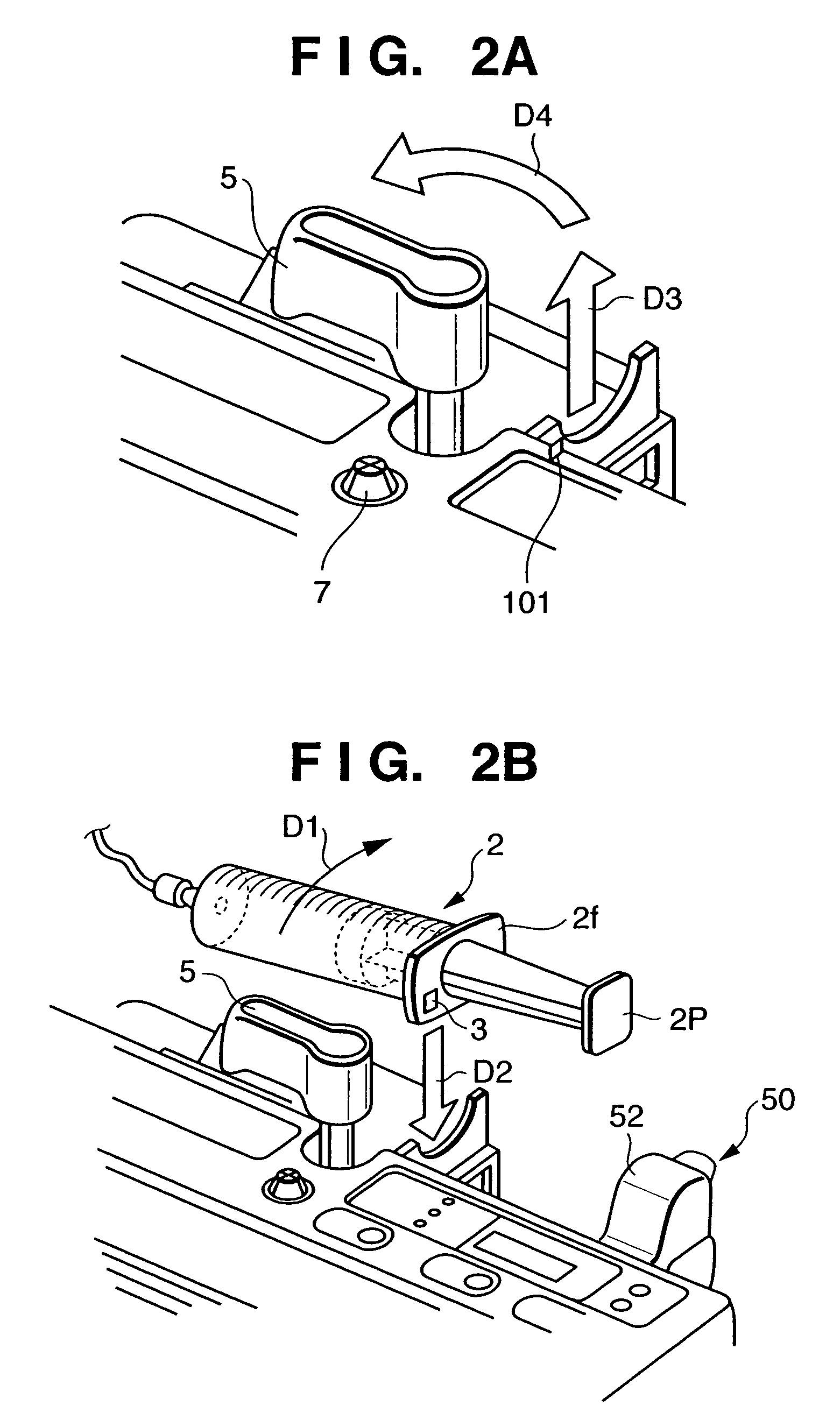
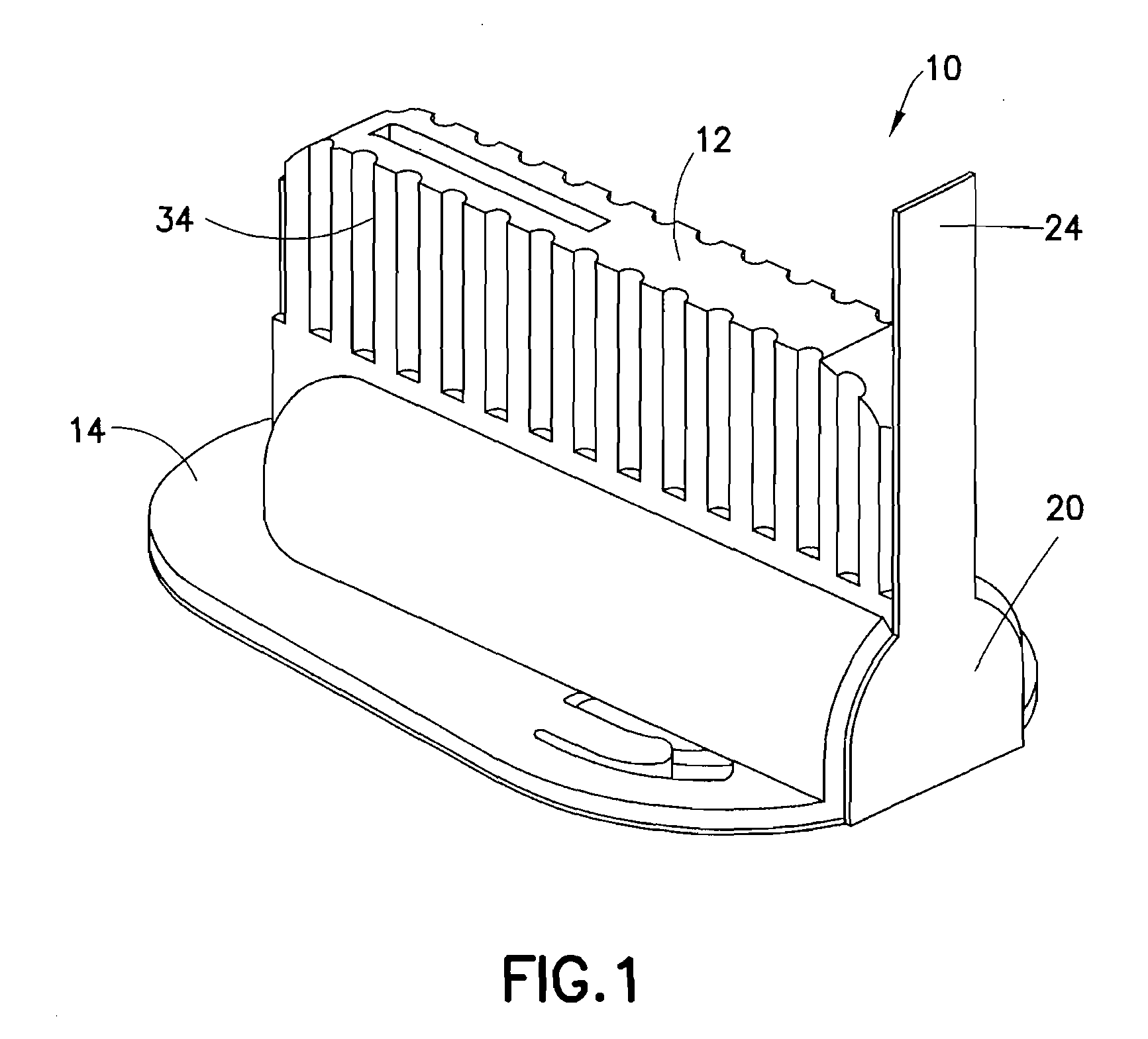
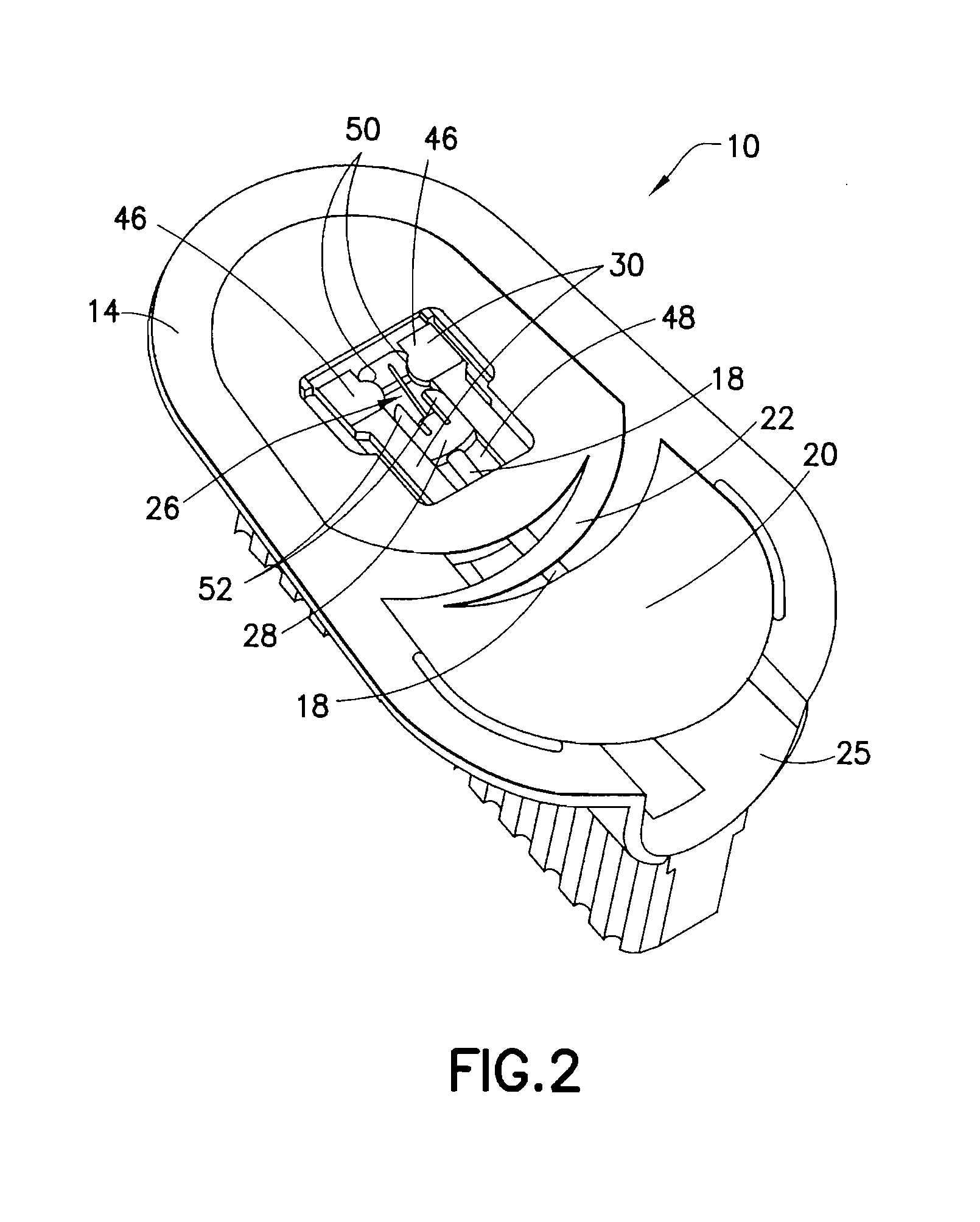
Integrated spring-activated ballistic insertion for drug infusion device
ActiveUS8795234B2Maintaining degree of comfortPromote absorptionInfusion syringesInfusion needlesDrugs infusionHigh rate
An infusion set has an integrated ballistic inserter that can insert a needle at a controlled high rate of speed to a depth to deliver content to the upper 3 mm of skin surface, and a skin securing, adhesive layer to secure the skin surface at the insertion site such that the inserter that can insert a needle without a risk of tenting of the skin surface. A driving spring of the ballistic inserter is captured within the ballistic inserter, and can be released by user operation, to insert a needle at such a controlled rate of speed.
Owner:BECTON DICKINSON & CO
Multi-Frequency Communication System For A Drug Infusion Device
Disclosed is a medical infusion device, such as an externally worn insulin pump, capable of being in remote communication with a controller or data acquisition unit such as a blood glucose meter. The disclosed medical infusion device includes a dual frequency antenna to facilitate communication with the remote device and the antenna is mounted using a spring-design that inhibits transmission of vibration to the antenna.
Drug container and drug infusion device comprising the same
When using a drug container having an identification tag fixed or detachably provided at a predetermined position of the container, the tag having drug data on a kind and a concentration of a drug and both or one of upper and lower limits of a flow rate on a continuous infusion and the upper and lower limits, time and flow rate on a one-shot administration recorded thereon, it is possible to ensure safety by prompting for a stop of injection by a warning when a setting beyond the upper and lower limits is performed and rewrite a liquid infusing time and a flow rate and so on required to be set according to symptoms of a patient.
Owner:TERUMO KK
Dosage sensing unit with tactile feedback
A drug infusion assembly comprises a drug delivery device arranged to adhere to a patient's skin and includes a reservoir that holds the drug, a cannula that delivers the drug to the patient, and a pump that causes the drug to flow to the cannula. The assembly further includes a monitor device for providing information about the operation of a drug delivery device. The monitor device includes a housing arranged to be attached to and detached from the drug delivery device, a sensor that senses the operation of the drug delivery device and generates an activation signal, a clock mechanism that generates a time signal, a memory that receives and stores the activation signal and the time signal and creates an information packet coordinating the time signal and the activation signal, an interrogator that interrogates the memory such that the memory generates a memory signal in response thereto, and a responder that receives the memory signal and generates a response.
Owner:CALIBRA MEDICAL
Drug infusion device with tissue identification using pressure sensing
An automatic injection device includes a drive mechanism and a sensor used to determine an internal characteristic such as a force or internal pressure generated during an injection process. This characteristic is then used as a control parameter by a microprocessor or controller to determine the exit pressure of the fluid expelled by the device. This exit pressure is then used to identify the kind of tissue in which the injection is being introduced.
Owner:MILESTONE SCIENTIFIC INC
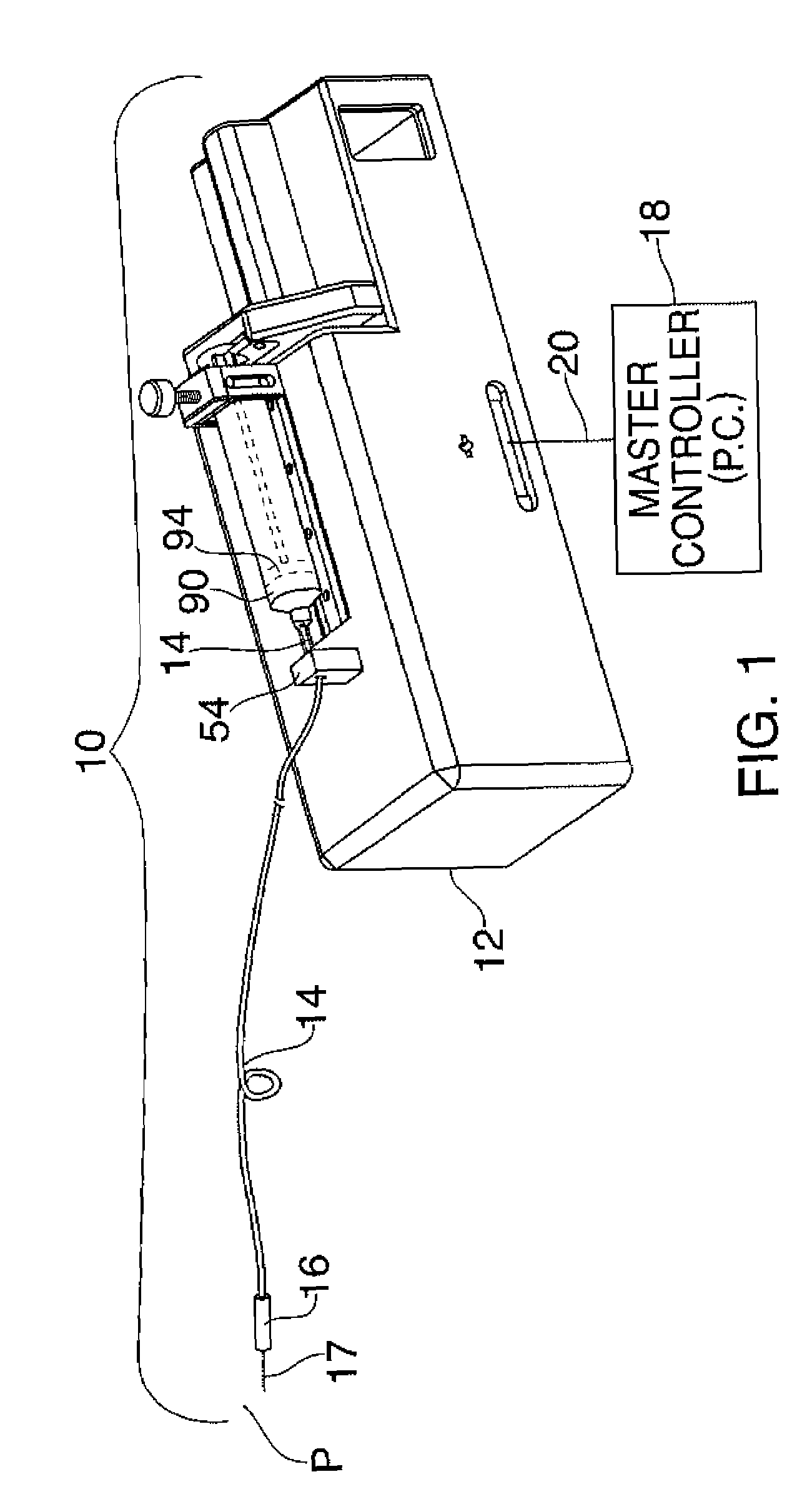
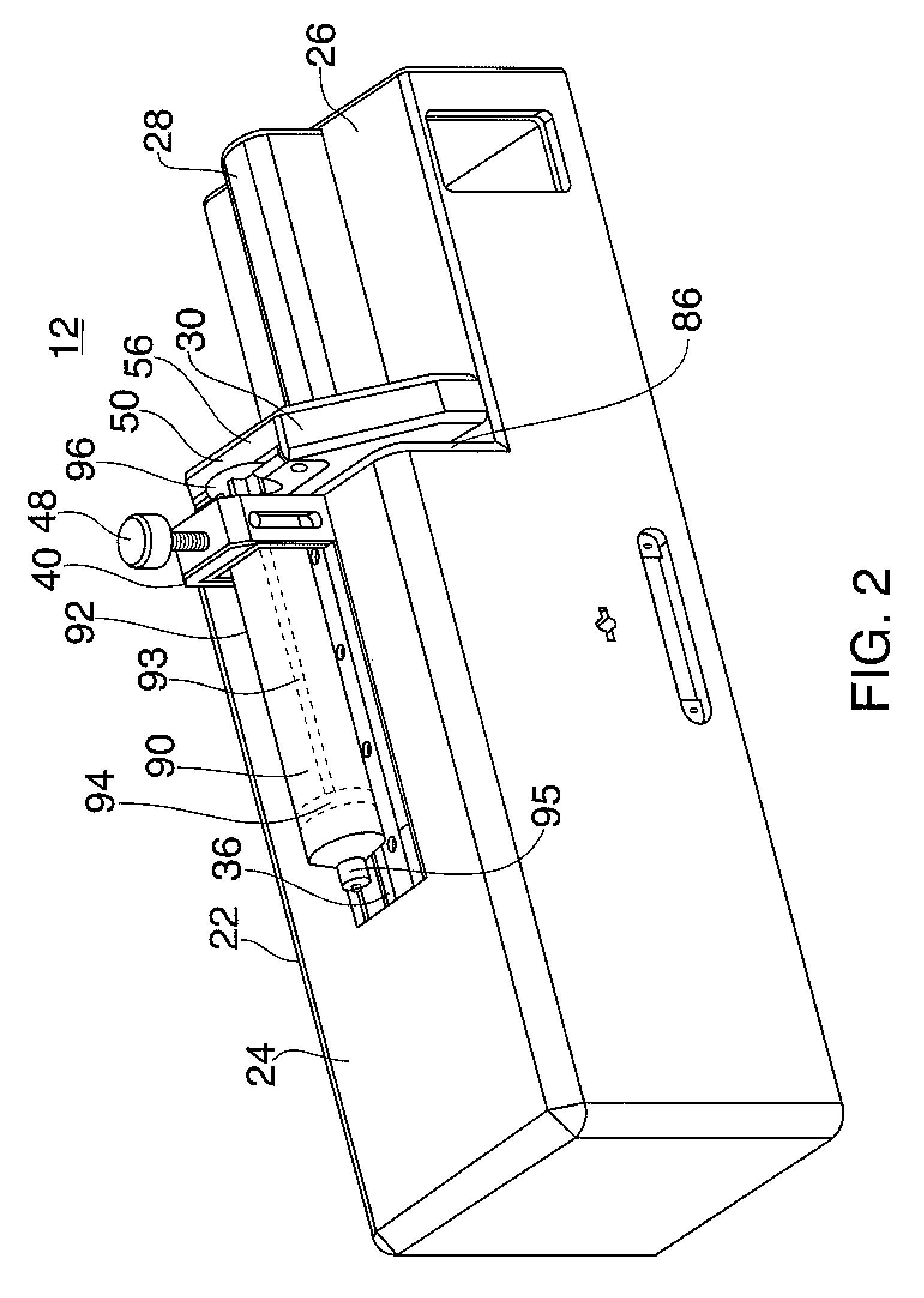
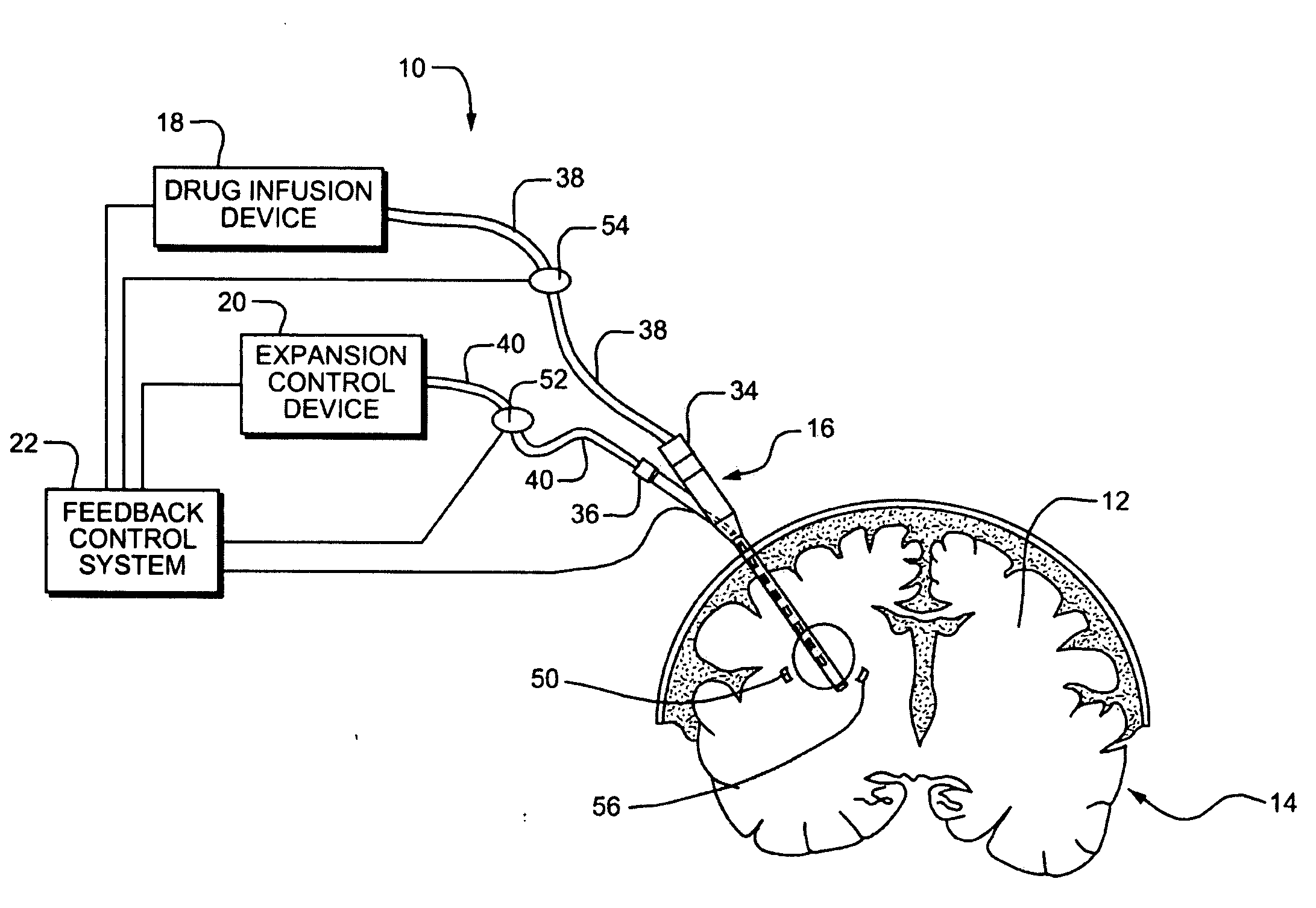
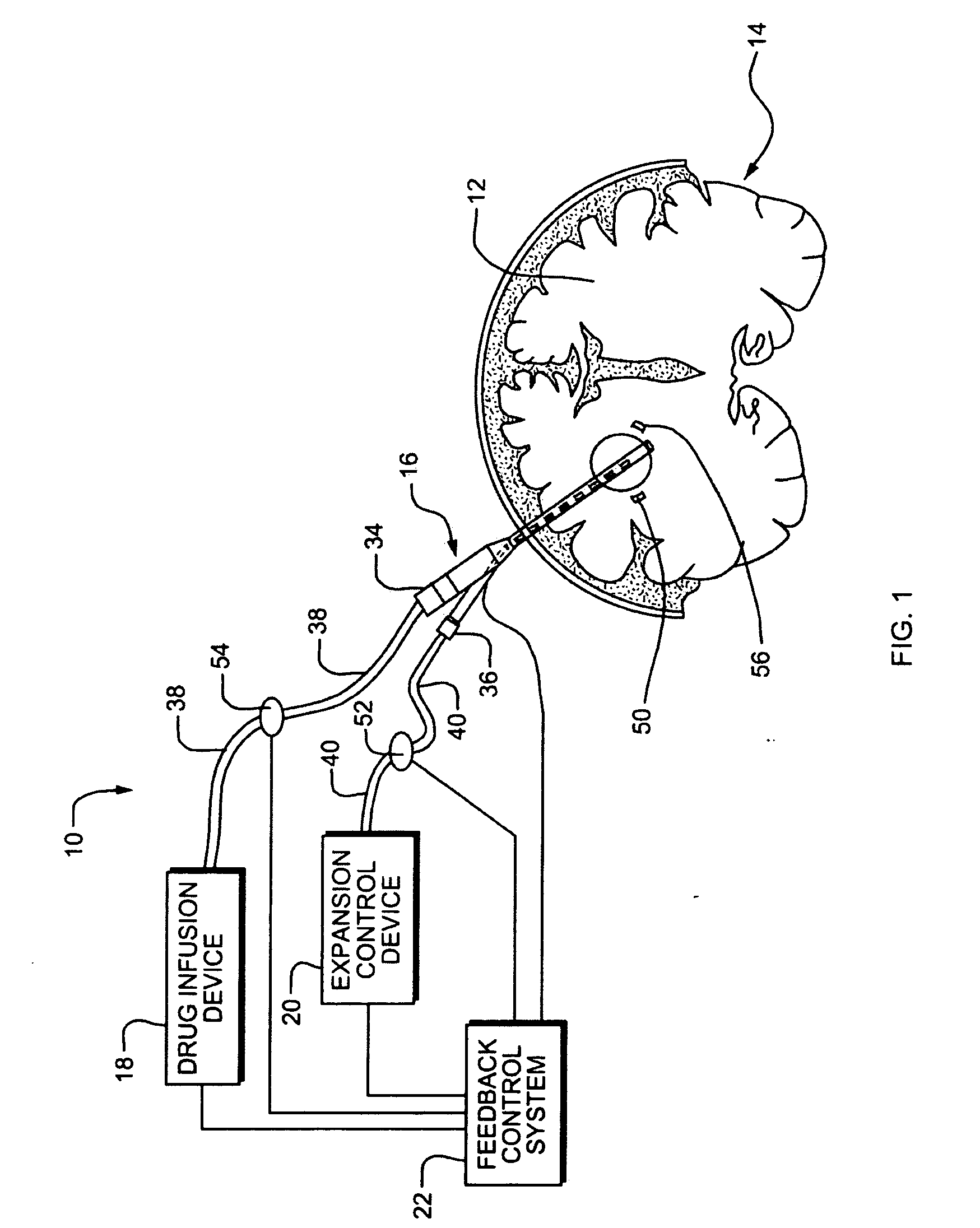
Systems and Methods for Drug Infusion with Feedback Control
A system and method for infusing a drug under continuous positive pressure (such as convection enhanced deliver) to a target tissue to be treated is particularly useful for post-resection anticancer drug therapy. The system comprises a drug infusion catheter having an expandable device which is expanded within the target tissue such that the target tissue conforms to an outer surface of the expandable device, thereby creating a form of seal around the target volume in order to maintain an effective drug pressure gradient within the target tissue. The system further comprises a sensor to measure a parameter which can be correlated to the degree of conformance between the target tissue and the outer surface of the expandable device. The sensor is coupled to a feedback control system to determine whether there is a loss of conformance, and to adjust the expansion of the expandable device in order to maintain good conformance.
Owner:CYTYC CORP
Techniques for positioning therapy delivery elements within a spinal cord or brain
Apparatus and techniques to address problems associated with lead migration, patient movement or position, histological changes, neural plasticity or disease progression. Disclosed are techniques for implanting a lead having therapy delivery elements, such as electrodes or drug delivery ports, within a vertebral or cranial bone so as to maintain these elements in a fixed position relative to a desired treatment site. The therapy delivery elements may thereafter be adjusted in situ with a position control mechanism and / or a position controller to improve the desired treatment, such as electrical stimulation and / or drug infusion to a precise target. The therapy delivery elements may be positioned laterally in any direction relative to the targeted treatment site or toward or away from the targeted treatment site. A control system maybe provided for open- or closed-loop feedback control of the position of the therapy delivery elements as well as other aspects of the treatment therapy.
Owner:MEDTRONIC INC
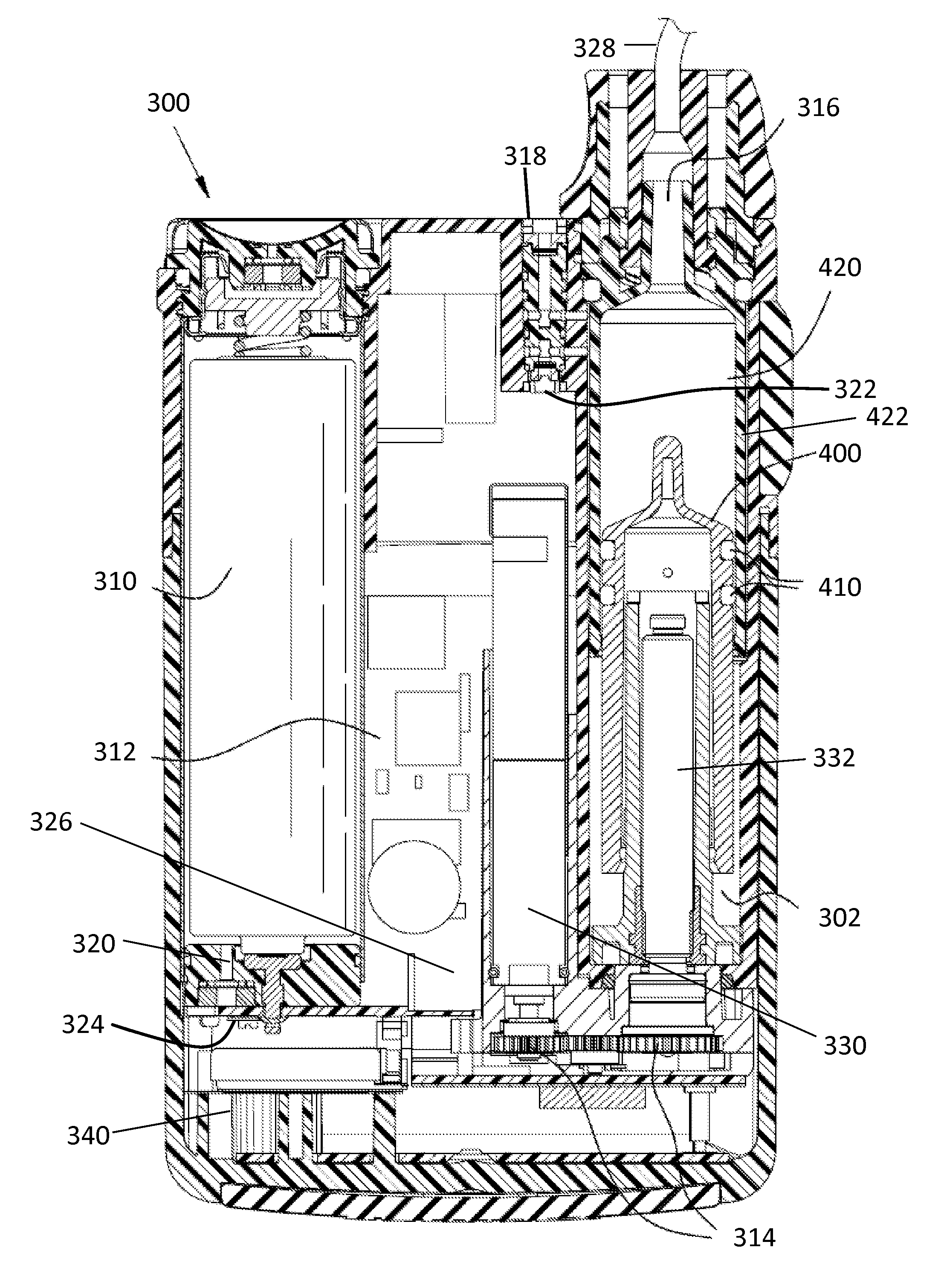
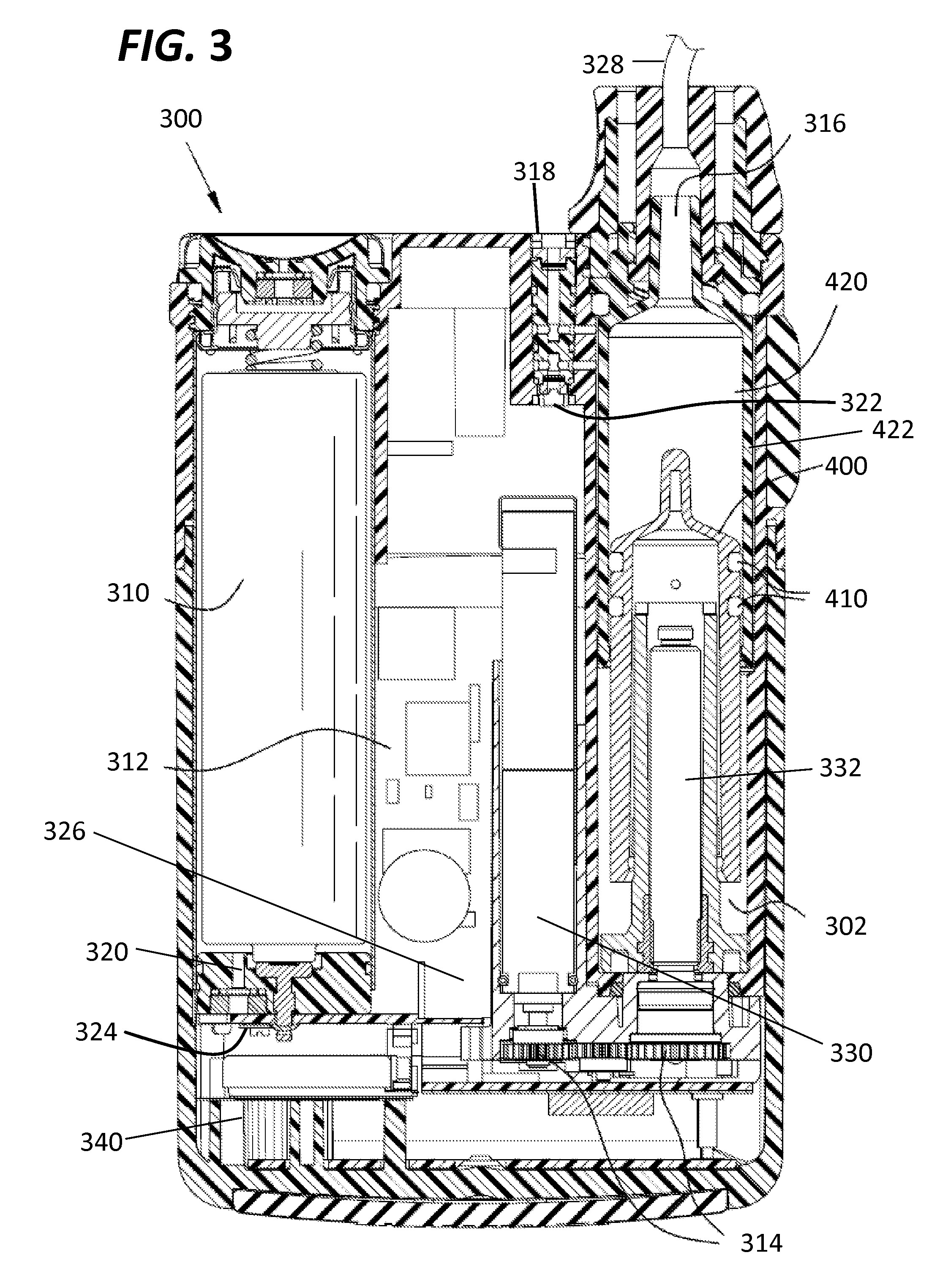
Portable infusion pump with pressure and temperature compensation
InactiveUS20130338576A1Avoid problemsMedical devicesPressure infusionDrugs infusionIntensive care medicine
Described is drug infusion device configure to maintain its intended drug delivery rate by monitoring atmospheric effects. The device may include one or more vents that permit the passage of gas between the exterior and interior of the device's housing. The device may also include one or more pressure and / or temperature sensors, the readings from which may be used to determine malfunctions in the venting of the device and / or changes in pressure that could cause the unintended over-delivery or under-delivery of medication.
Owner:ANIMAS CORP
Slide-Activated Angled Inserter And Cantilevered Ballistic Insertion For Intradermal Drug Infusion
ActiveUS20120136299A1Maintain positionPrevent retractionInfusion syringesSurgical needlesDrugs infusionAdhesive
An infusion set has an adhesively secured main hub, and a slidable top cover, needle hub and angled or cantilevered needle that can be used for performing an intradermal needle insertion precisely targeting the upper 3 mm of skin surface, for example, one that substantially duplicates the Mantoux insertion technique, for injecting into the intradermal layers of skin, while maintaining a degree of comfort to the user. By sliding the top cover, the cantilevered needle is loaded and released or the angled needle is slid into an insertion site, while flexible arms and adhesive are used to stretch and / or flatten a skin surface, or otherwise create skin tension, at the insertion site.
Owner:BECTON DICKINSON & CO
Data Transmission System For A Drug Infusion Device
Disclosed is a medical infusion device, such as an externally worn insulin pump, capable of being in remote communication with a controller or data acquisition unit such as a blood glucose meter. The disclosed medical infusion device includes a dual frequency antenna to facilitate communication with the remote device and the antenna is mounted using a spring-design that inhibits transmission of vibration to the antenna.
Owner:ANIMAS CORP
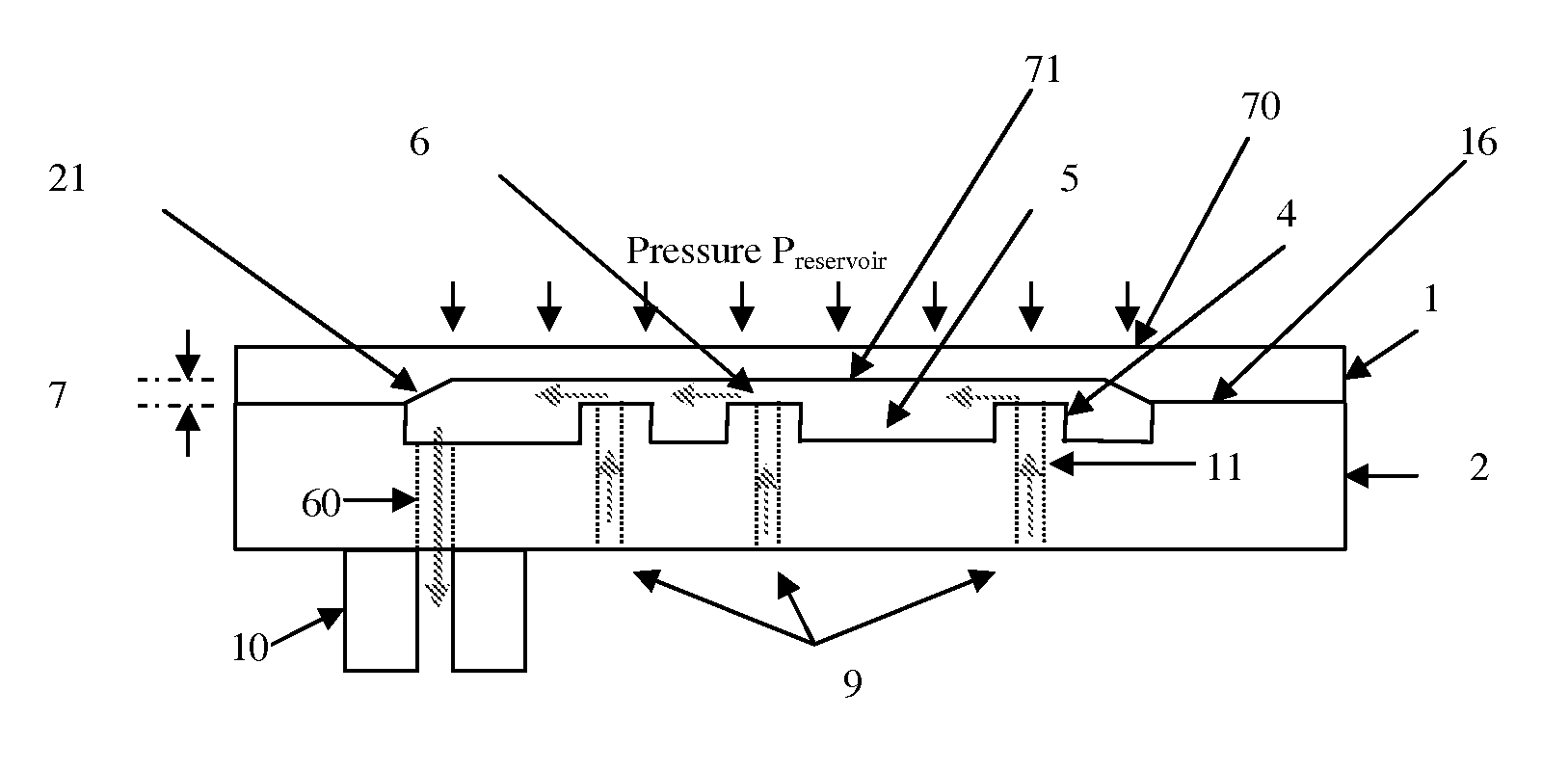
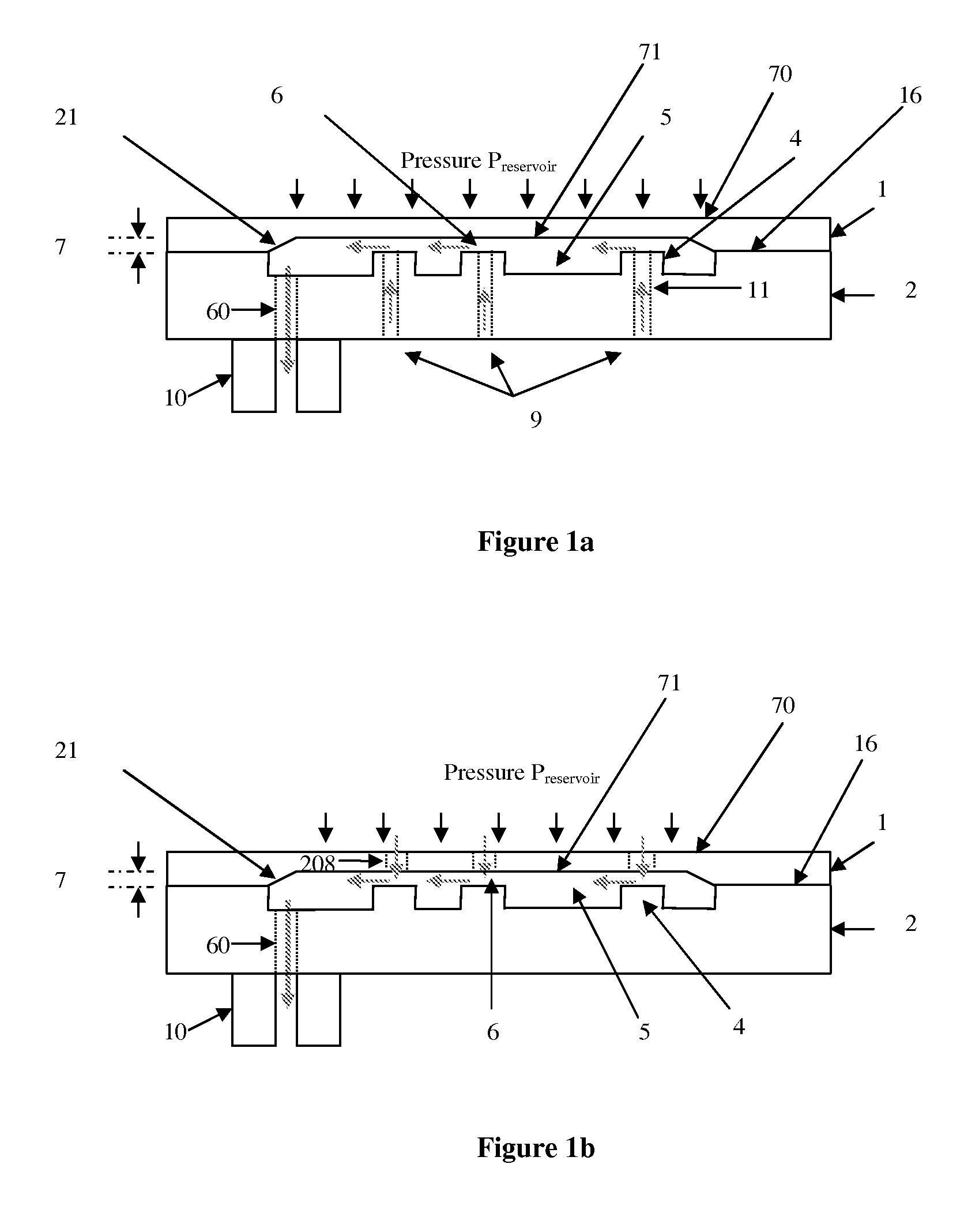
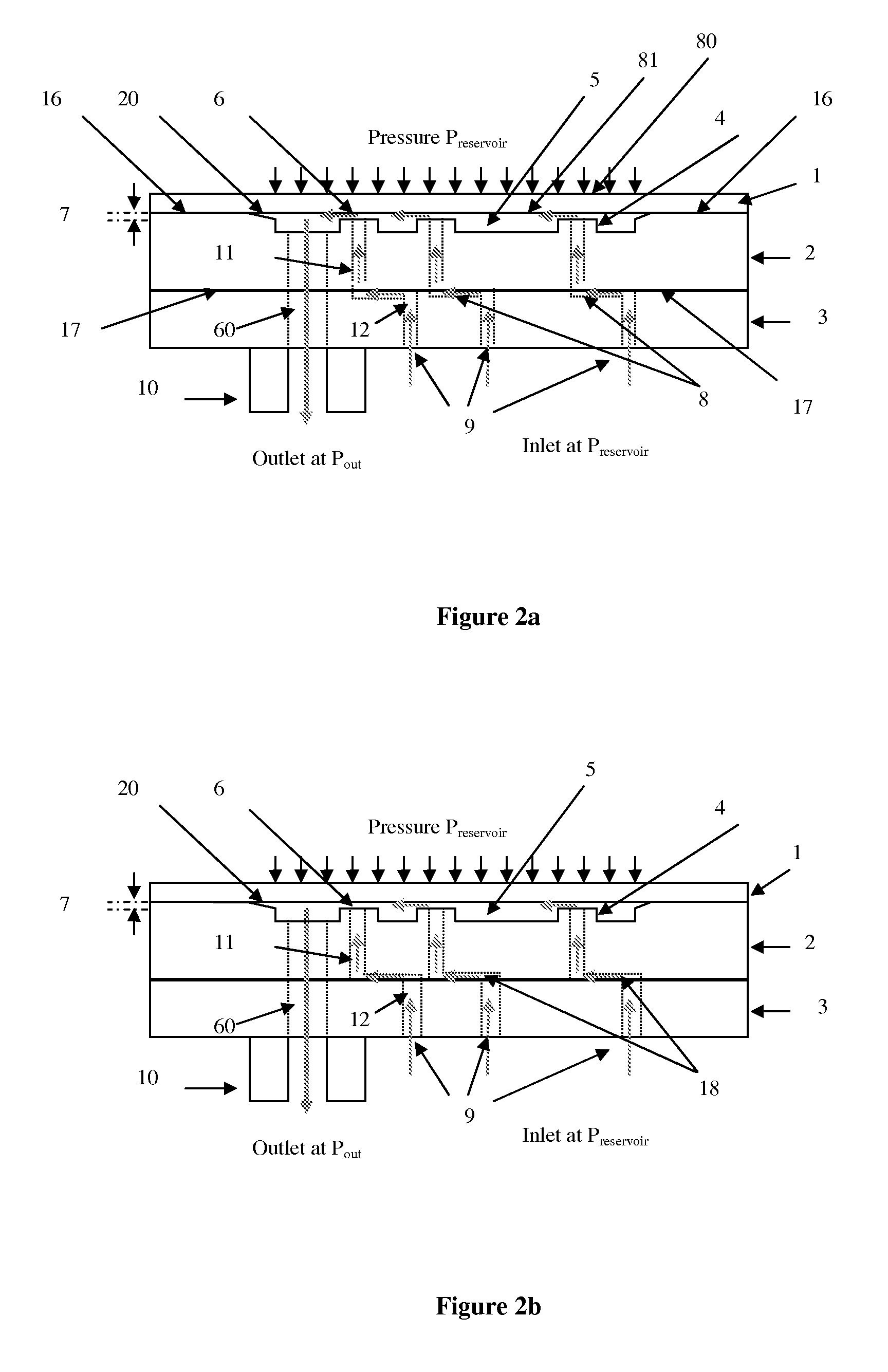
Micromechanic passive flow regulator
ActiveUS20120316492A1Simple and cheap to manufactureAvoid less flexibilityValve arrangementsWound drainsDrugs infusionDrug reservoir
The invention concerns a flow regulator, made of a stack of 3 plates, respectively a top plate including a flexible membrane (1), a middle plate (2) with pillars and through holes and a bottom plate (3) with fluidic ports, micro channels and through holes (8,9,12). The principle is based on the deformation of the membrane due to the pressure of the liquid. The membrane goes in contact with the pillars of the middle plate, obstructing gradually the through holes of the pillars. The device is designed to keep the flow constant in a predefined range of pressure. The device is dedicated to ultra low flow rate up to 1 ml per day or below, typically for drug infusion. Plastic flow regulators comprise preferably several independent valves coupled in parallel. The membrane plate is therefore made of several flexible membranes obstructing gradually the flow by increasing the pressure. Stress limiters are used to avoid plastic deformation of the membrane. For implanted pump, the use of a flow regulator instead of a flow restrictor has several advantages, including the possibility to reduce significantly the reservoir pressure and to generate directly the pressure during the pump filling by using an elastic drug reservoir.
Owner:DEBIOTECH SA
Constant rate fluid delivery device with selectable flow rate and titratable bolus button
InactiveUS8771227B2Precise and variable flow rate controlLess-expensive to manufactureOther blood circulation devicesPharmaceutical delivery mechanismDrugs infusionElectronic component
A wearable, self-contained drug infusion device is disclosed that is capable of achieving the precise flow rate control needed for dose-critical drugs such as insulin. In preferred embodiments of the device, at least two flow channels are utilized in conjunction with a series of valves for providing a user with selectable, constant flow rate control. The device can be made with small dimensions so that it can be worn by the user with a minimum of discomfort and inconvenience. In addition, the simple mechanical nature of the device provides the user with close control over the flow rate, which is required for safe and effective delivery of insulin and other drugs. Also, the absence of electronic components allows the device to be manufactured inexpensively. The device is provided with a first channel that is long and narrow, functioning as a flow restrictor. The first channel is preferably provided in a serpentine pattern. A second channel is also provided that has a larger cross section so that flow is not restricted. A series of valves are used to force the flow of fluid through a selectable portion of the serpentine portion of the first channel before entering the remainder of the second channel and flowing to the delivery cannula. In one embodiment of the device, a needle port is provided in fluid communication with the delivery cannula for delivering bolus injections. In another embodiment, a bolus button is provided for delivering bolus injections. A flow restrictor is preferably included in the bolus button to limit the rate at which the bolus button refills.
Owner:BECTON DICKINSON & CO
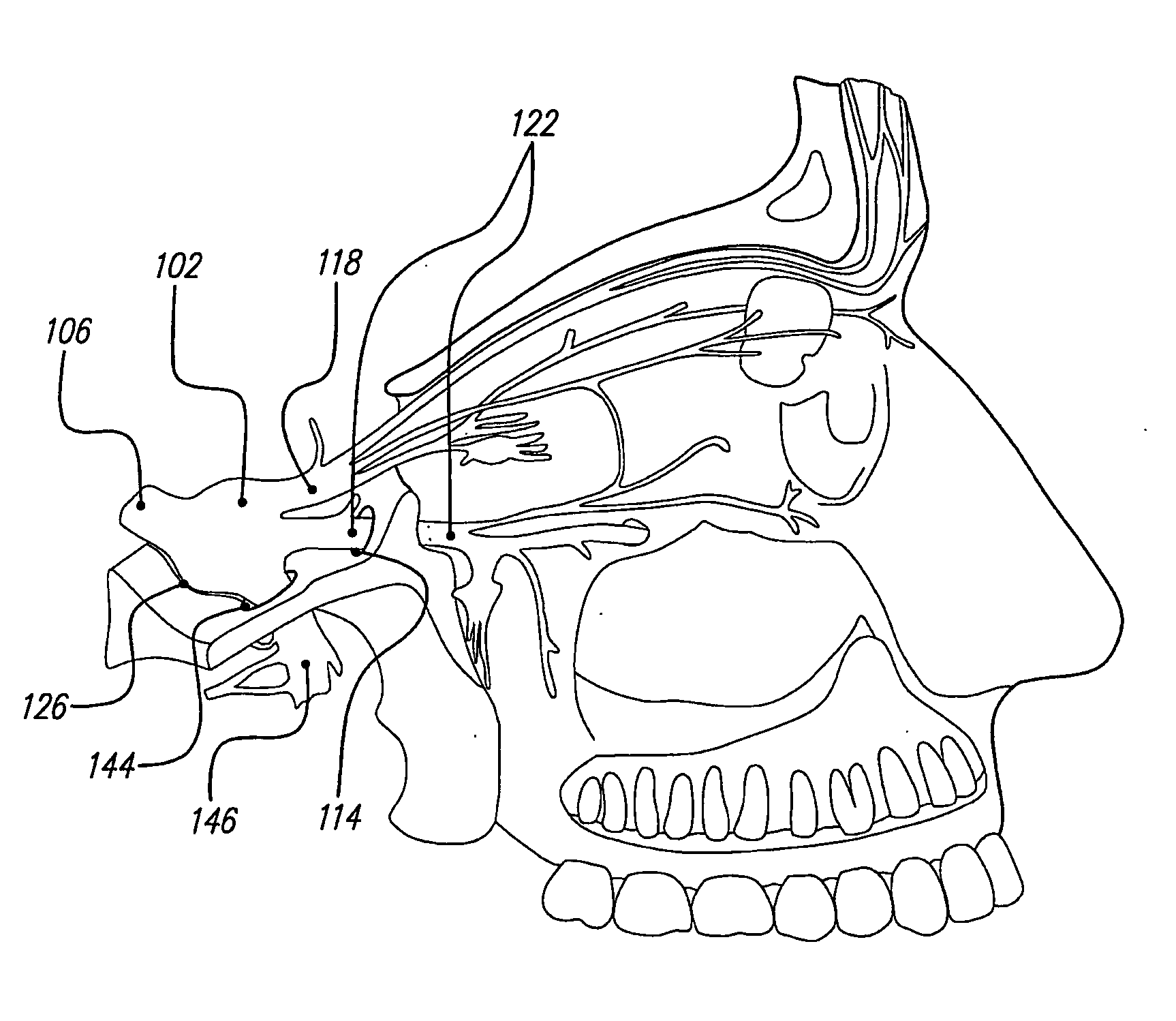
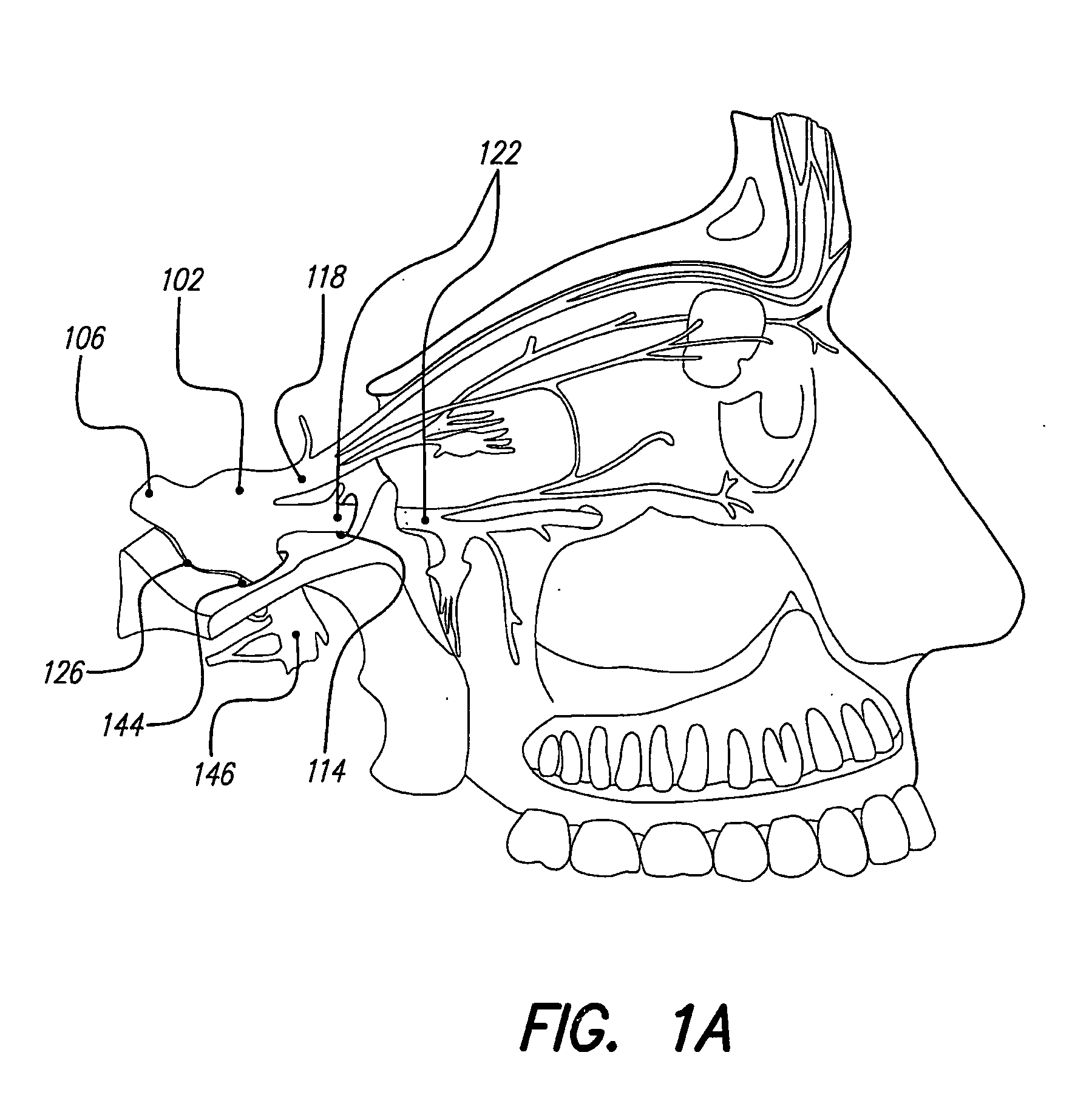
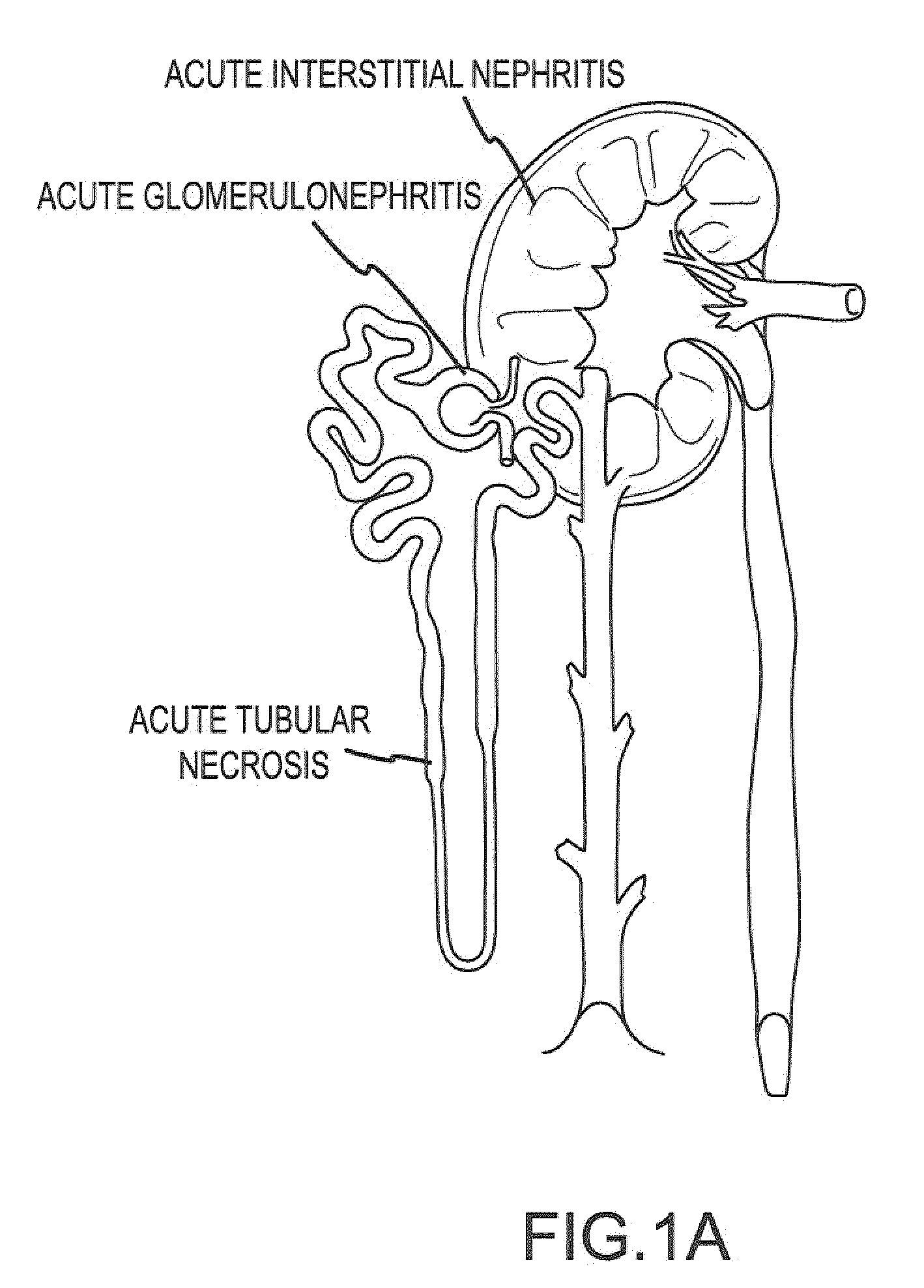
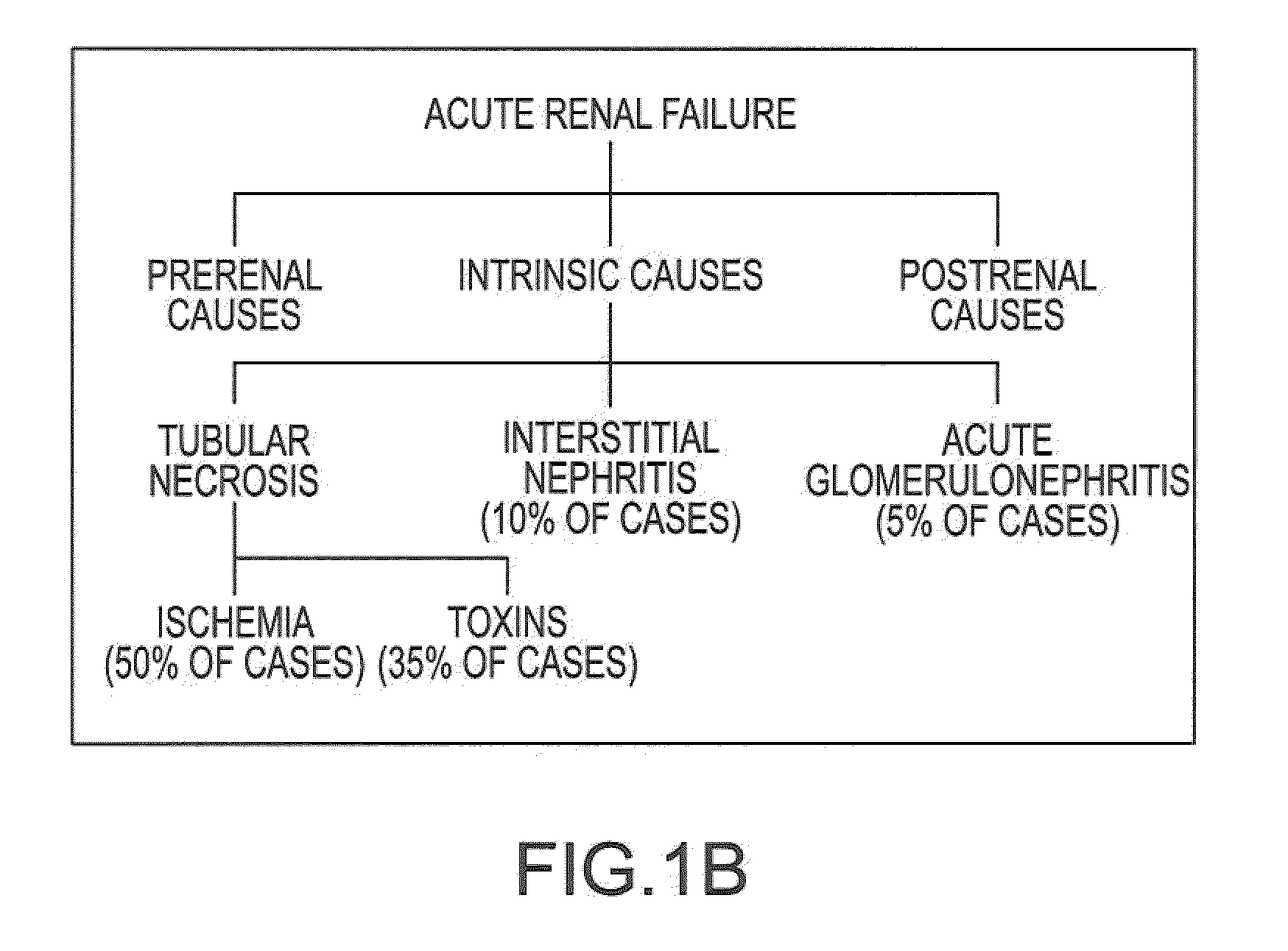
Treatment systems and methods for renal-related diseases
InactiveUS20090306625A1Increase risk of injuryAccurate and reliableOrganic active ingredientsBalloon catheterDiseaseMain vessel
Systems and methods are provided for locally delivering specific prophylactic, regenerative, or therapeutic agents within the body of a patient. Systems and methods can involve direct delivery of prophylactic, regenerative, or therapeutic agents into branch blood vessels or body lumens from a main vessel or lumen, respectively, and in particular into renal arteries extending from an aorta in a patient. Drug infusion techniques encompass specific treatment and prevention regimes for renal diseases including, but not limited to, Acute Kidney Injury, Renal Cell Carcinoma, Polycystic Kidney Disease, and Chronic Kidney Disease.
Owner:ANGIODYNAMICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com