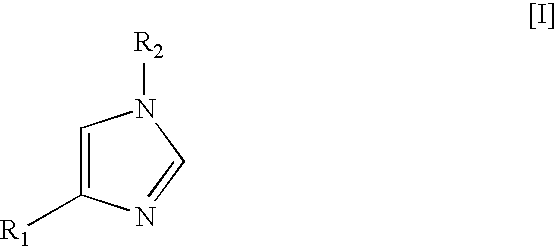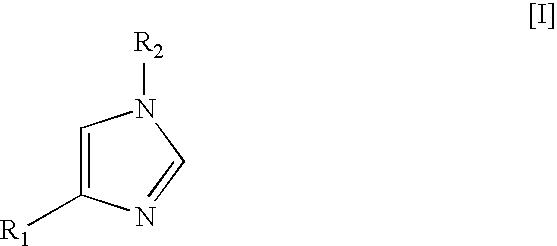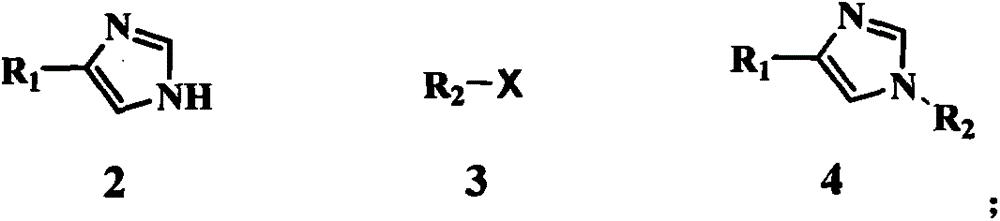Patents
Literature
92 results about "Medetomidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
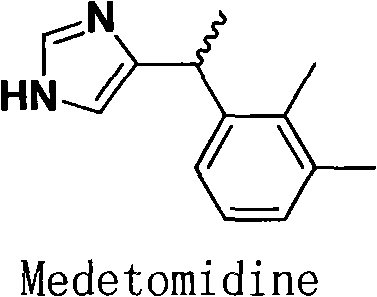
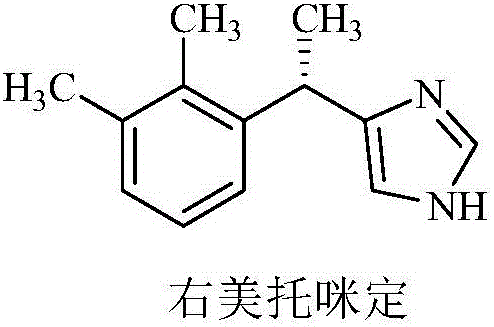
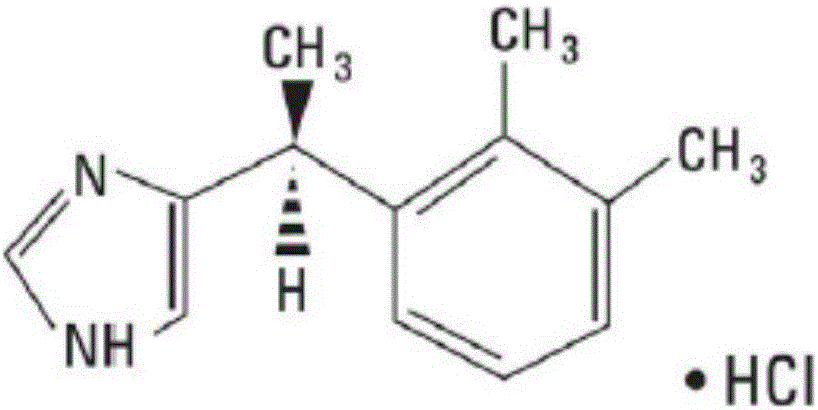
Medetomidine is a synthetic drug used as both a surgical anesthetic and analgesic. It is often used as the hydrochloride salt, medetomidine hydrochloride, a crystalline white solid. It is an α2 adrenergic agonist that can be administered as an intravenous drug solution with sterile water.
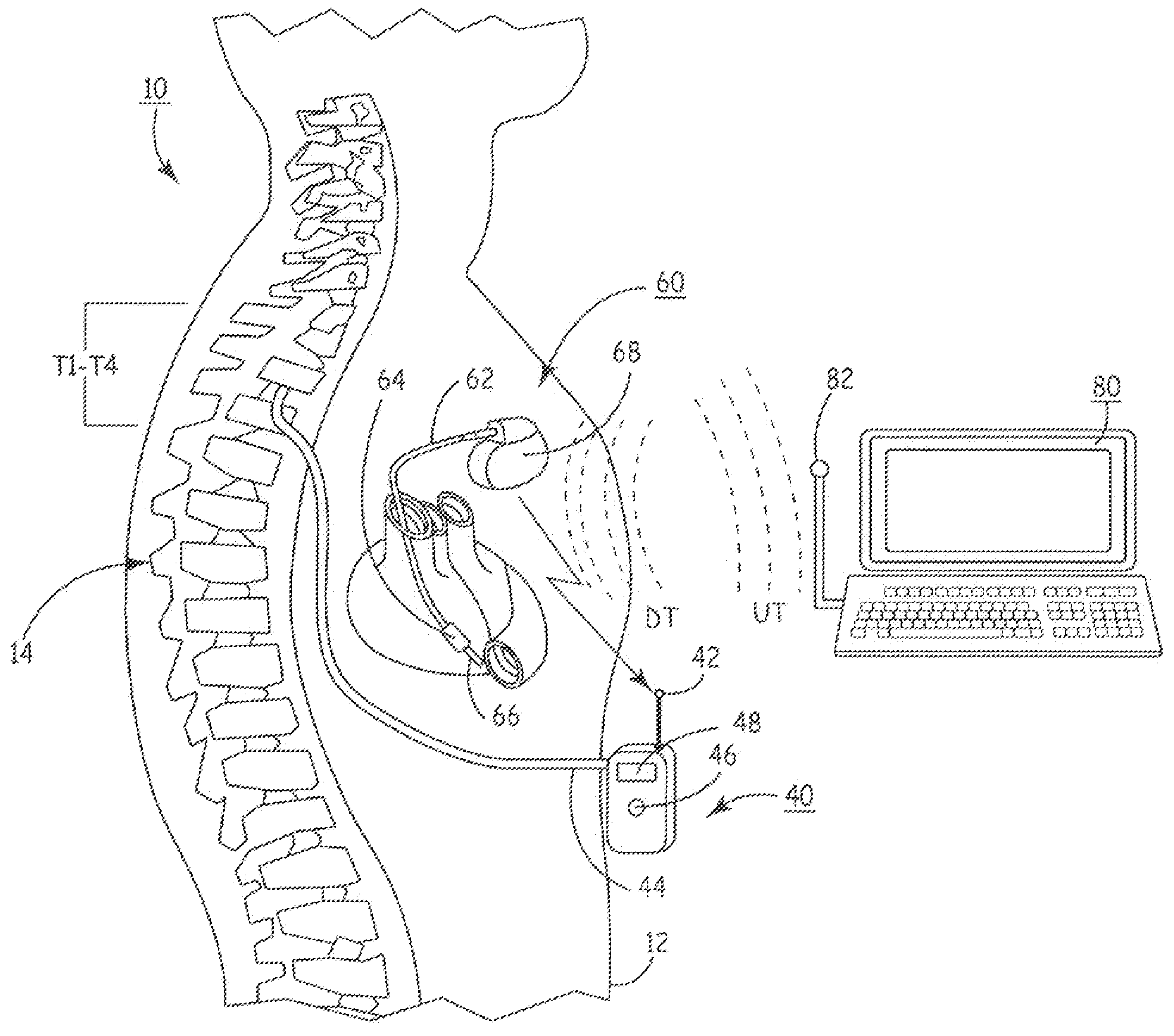
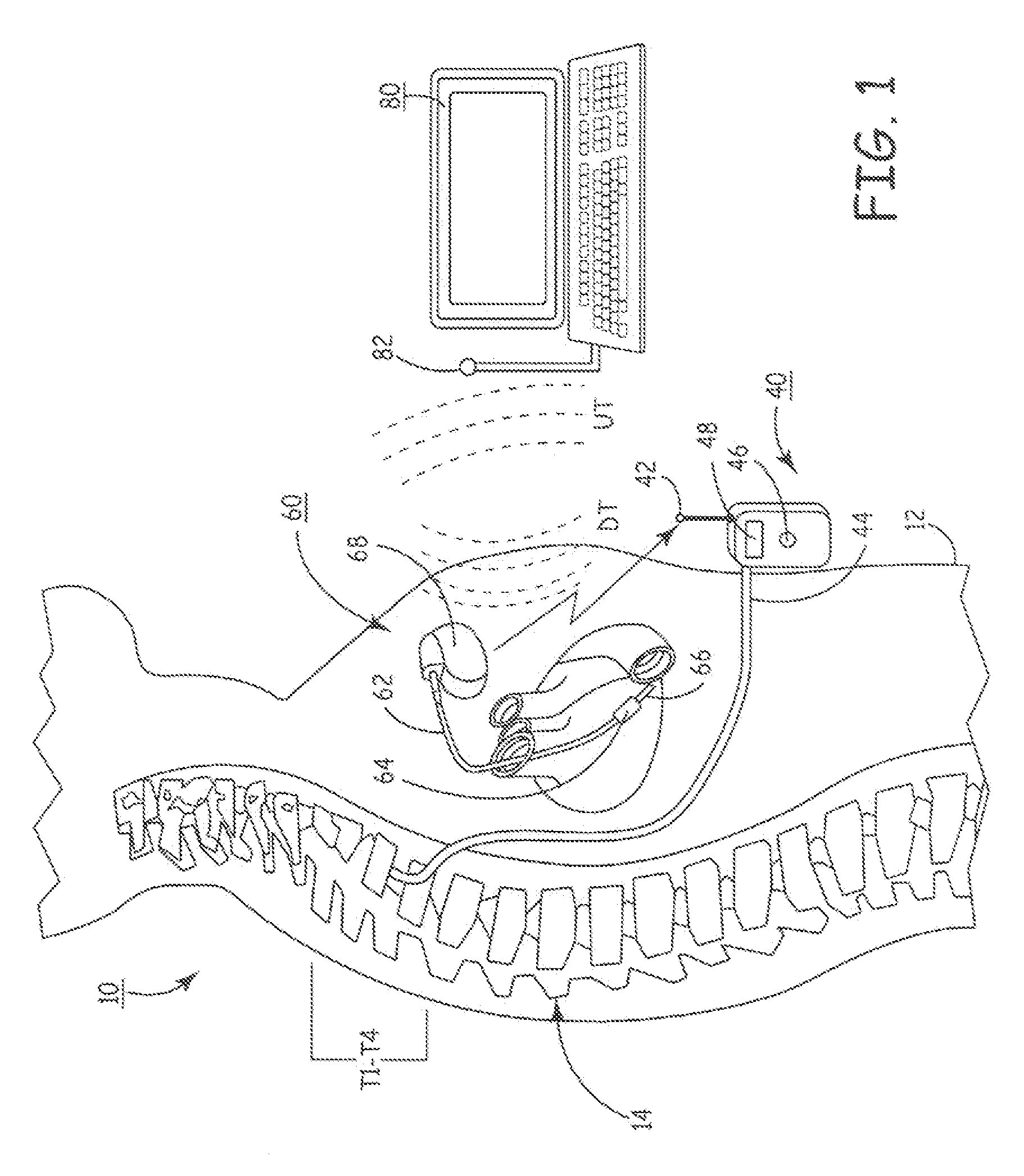
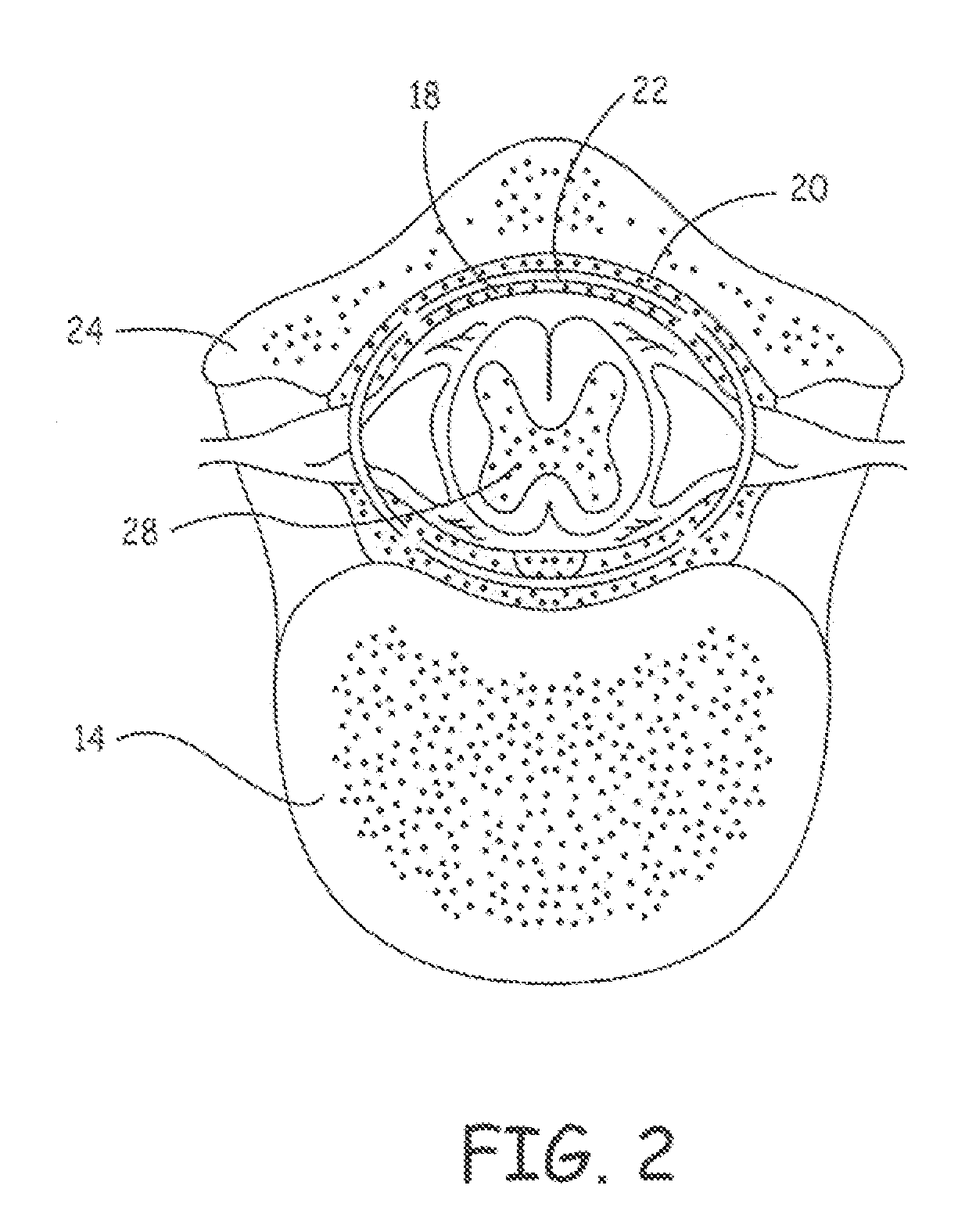
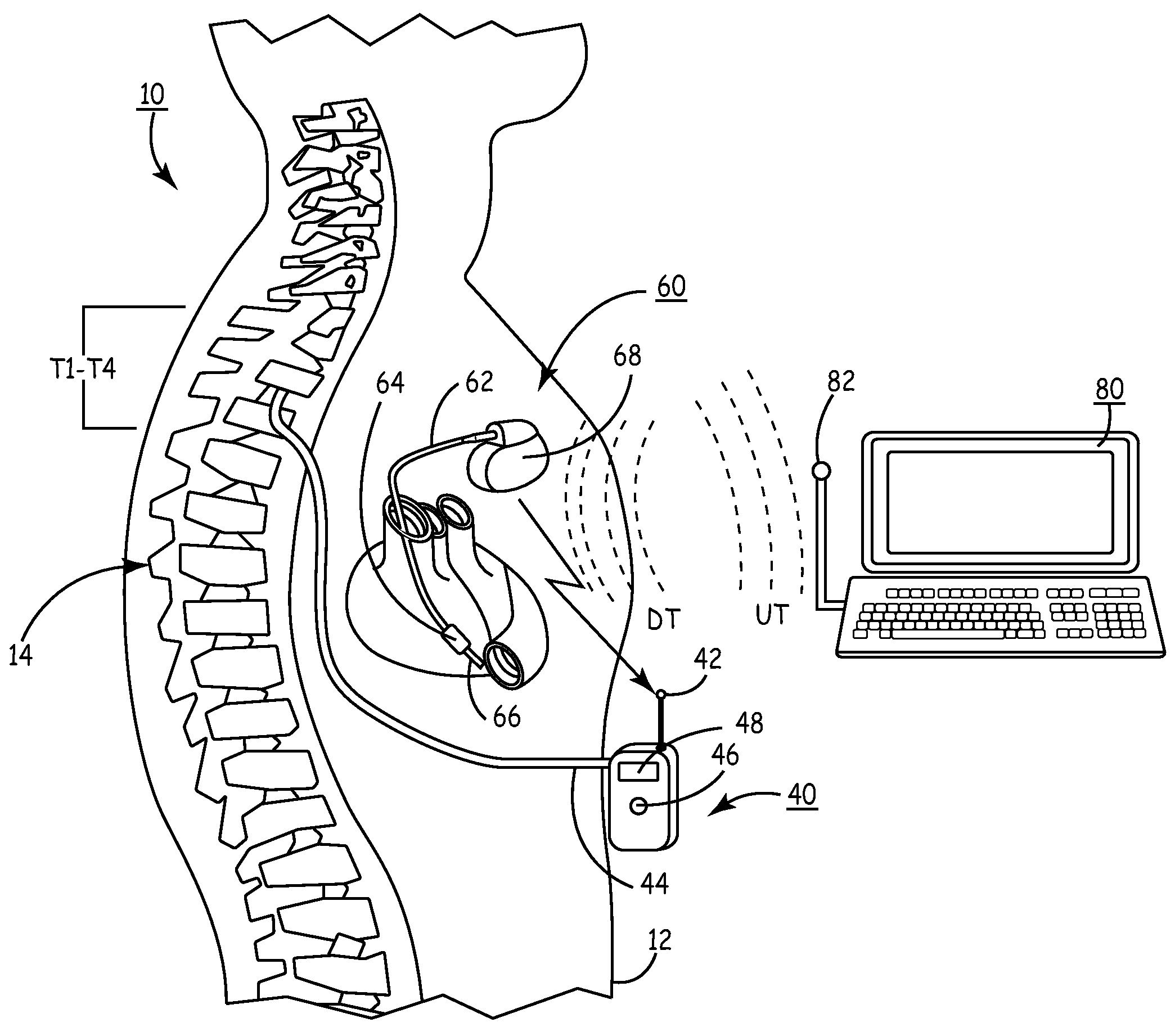
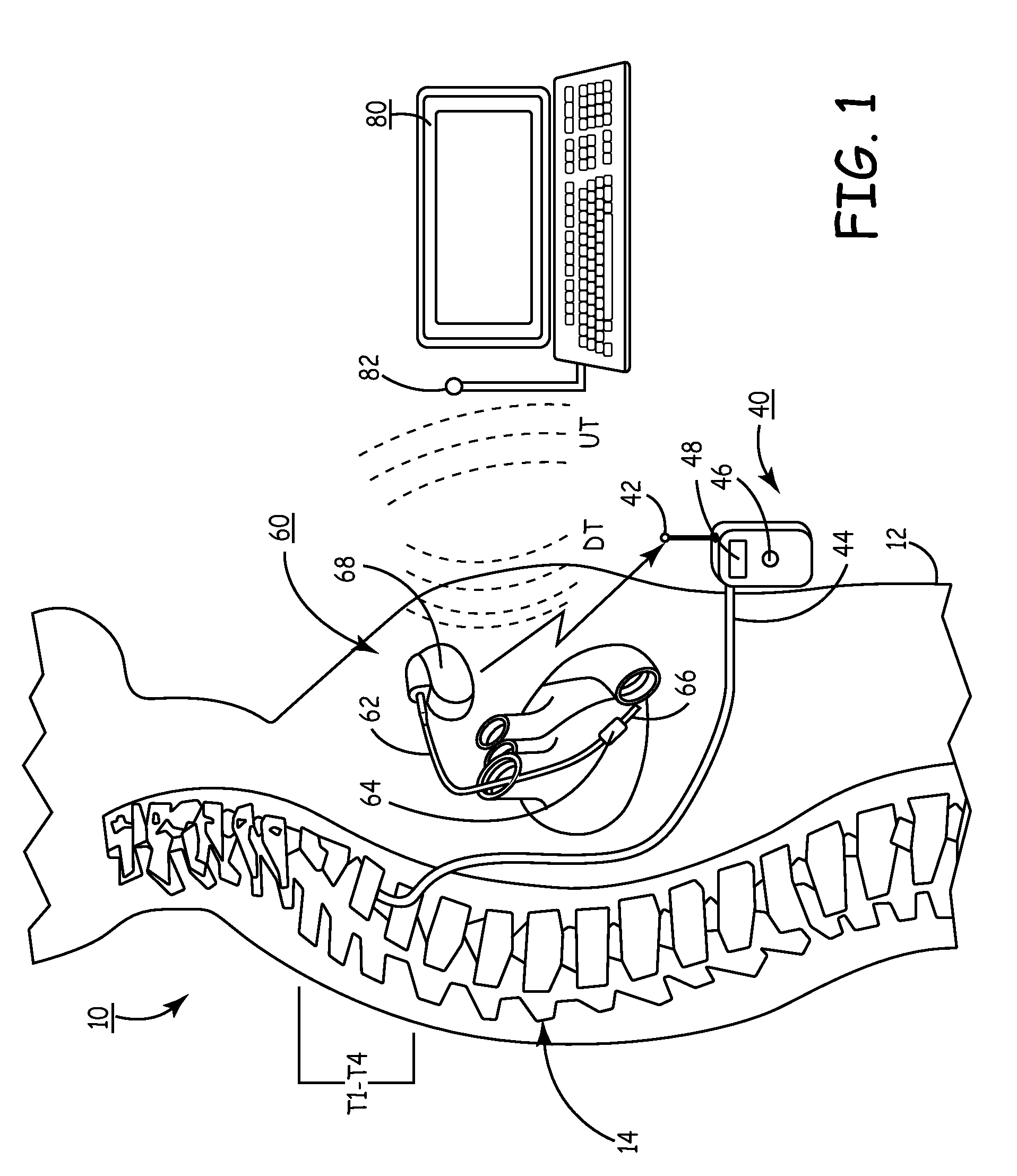
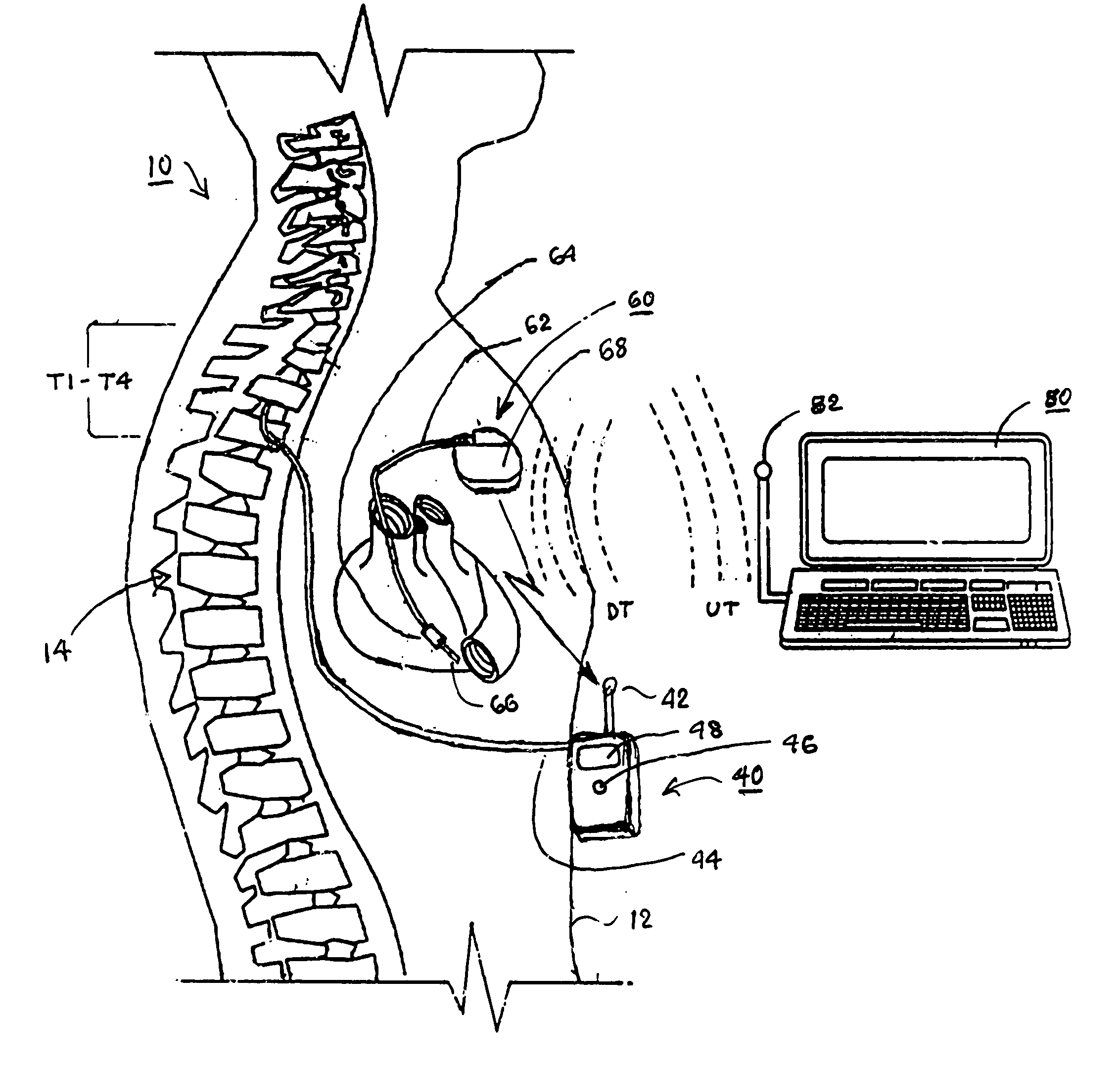
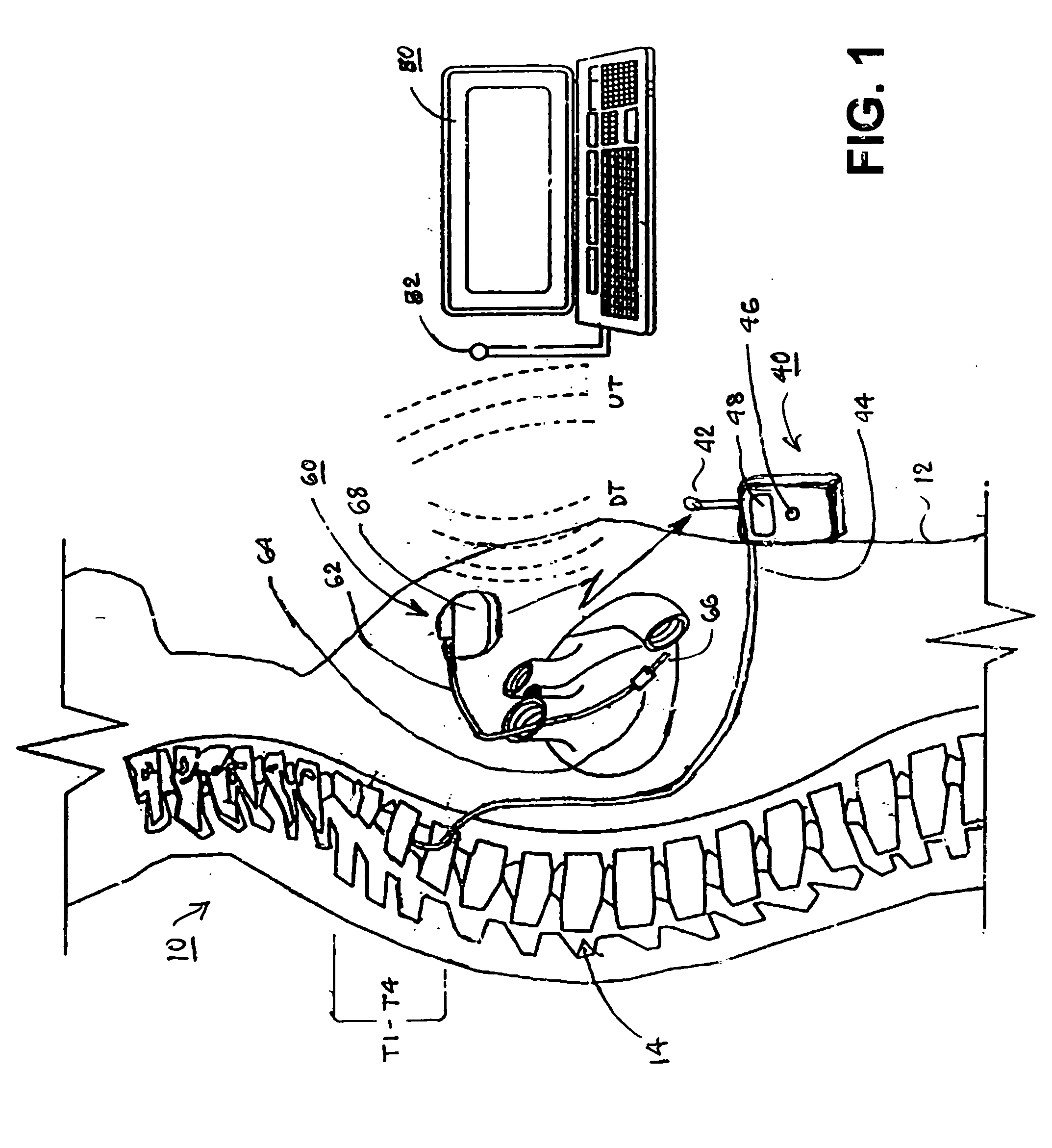
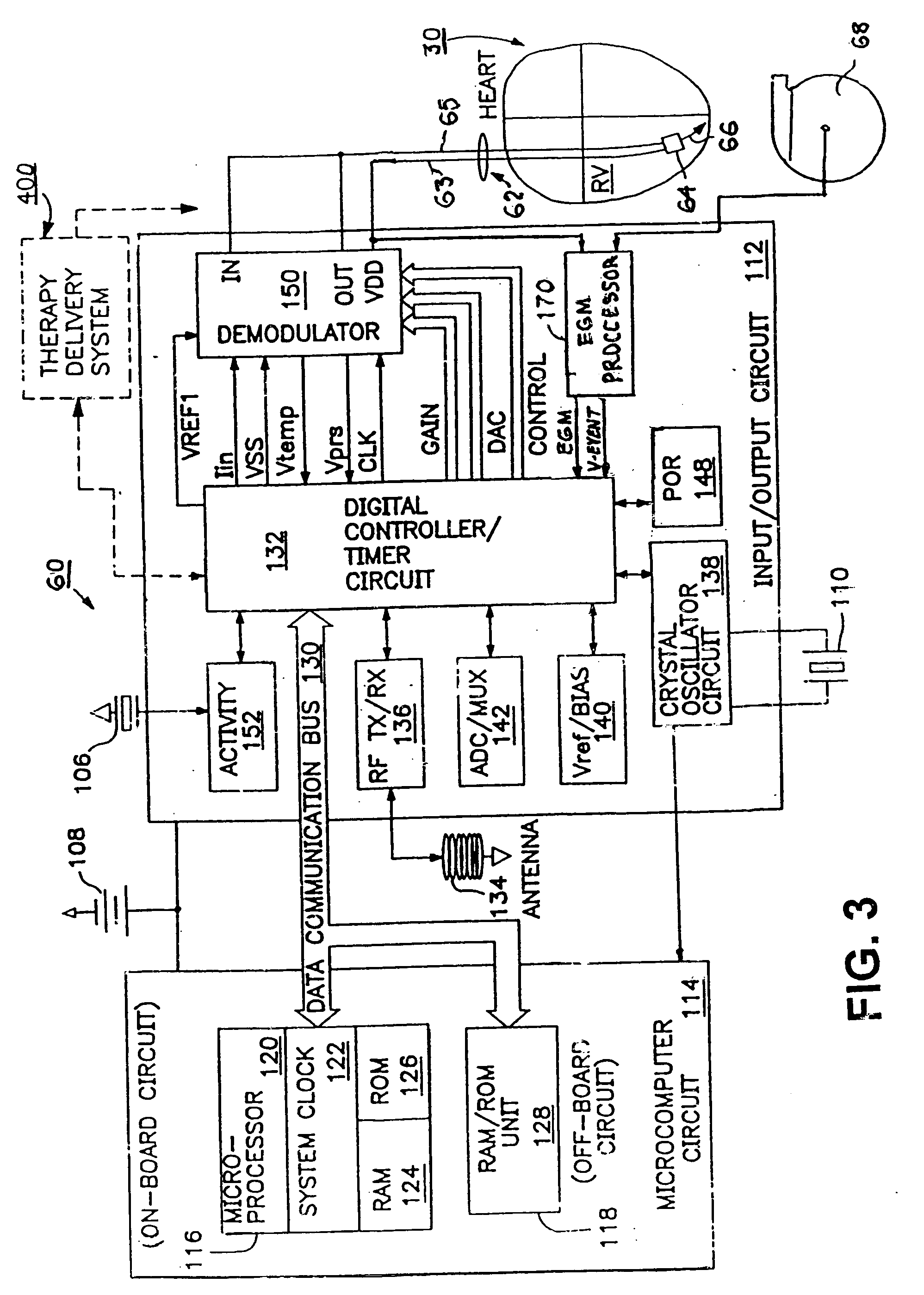
Delivery of a sympatholytic cardiovascular agent to the central nervous system
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms of acute or chronic cardiac insult or impaired cardiac performance. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with cardiac insult or impaired cardiac performance and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist (e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine).
Owner:MEDTRONIC INC
Delivery of a sympatholytic cardiovascular agent to the central nervous system to counter heart failure and pathologies associated with heart failure
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms and otherwise treat heart failure (HF) and pathologies associated with HF. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with HF (or pathologies associated with HF) and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist, e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine.
Owner:MEDTRONIC INC
Delivery of a sympatholytic cardiovascular agent to the central nervous system to counter heart failure and pathologies associated with heart failure
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms and otherwise treat heart failure (HF) and pathologies associated with HF. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with HF (or pathologies associated with HF) and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist, e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine.
Owner:MEDTRONIC INC
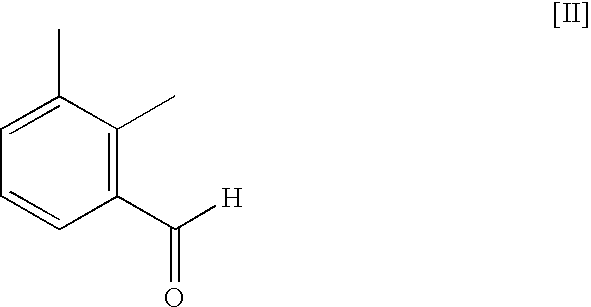
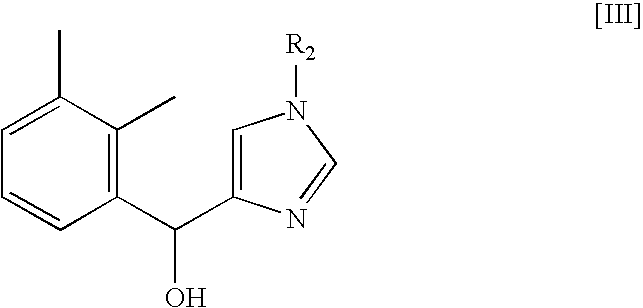
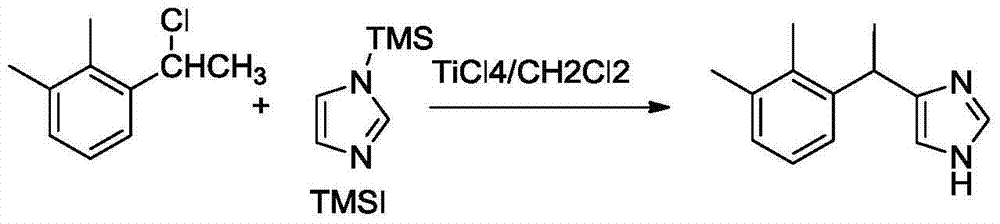
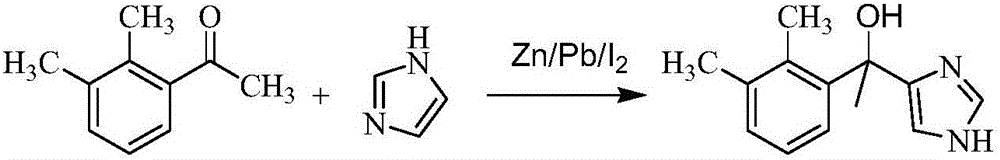
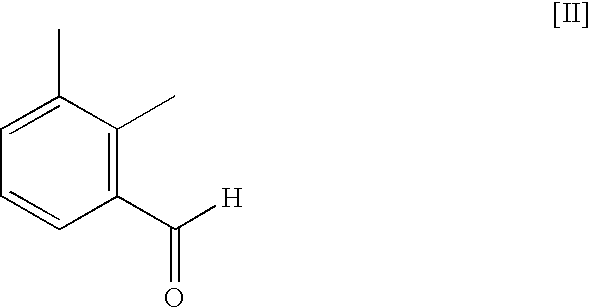
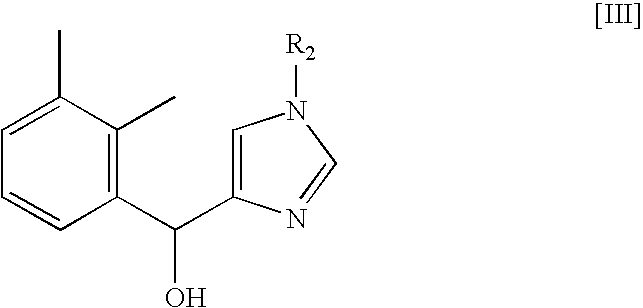
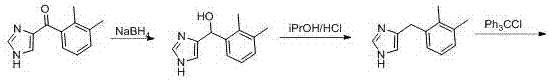
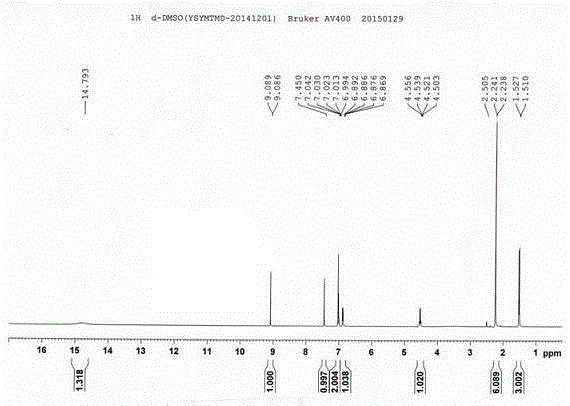
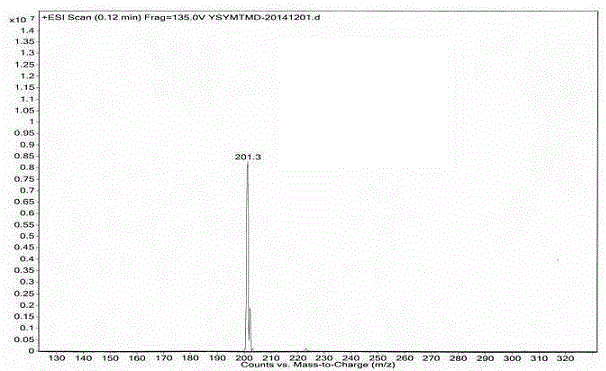
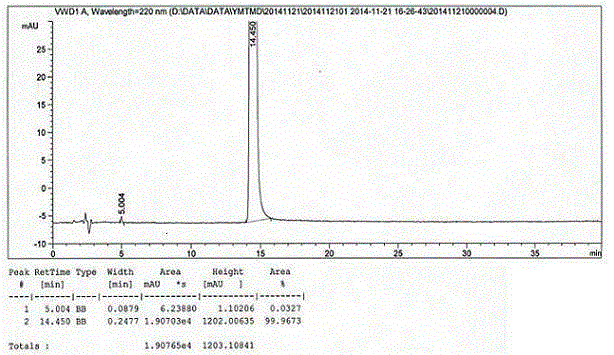
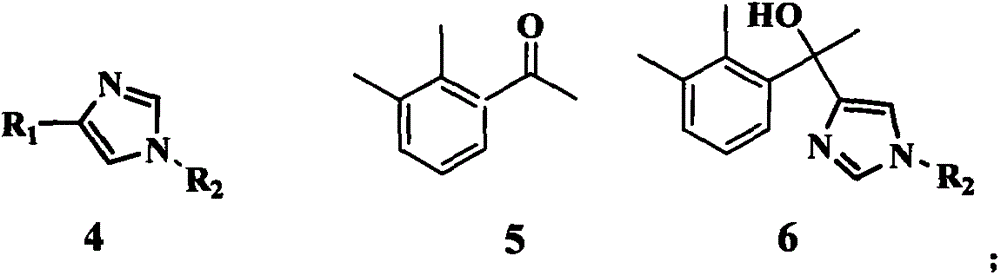
Method for preparing medetomidine and its salts.
ActiveUS20100048915A1Increase productionHigh yieldOrganic active ingredientsOrganic chemistryGrignard reagentMedetomidine
The invention provides an improved, highly efficient method for preparing Medetomidine, and its salts, in particular its pharmaceutically acceptable salts. The method utilizes the high reactivity of halogenated imidazoles towards transmetalation with Grignard reagents and the subsequent reaction with 2,3-dimethylbenzaldehyde.
Owner:GRINDEKS
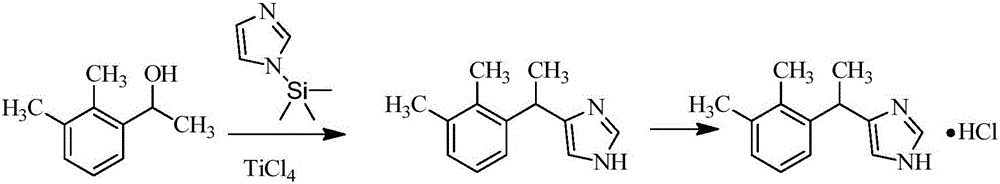
New method for preparing dexmedetomidine hydrochloride
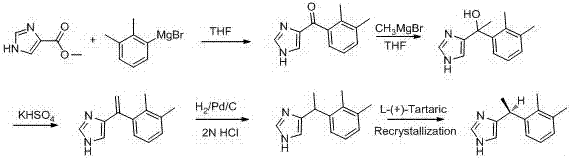
The invention provides a new method for preparing dexmedetomidine hydrochloride. According to the method, Lewis acid is used as a catalyst, and racemized medetomidine is prepared by a Friedel-Crafts reaction; and the racemized medetomidine is resolved by (+)-di-p-toluoyl-tartaric acid [(+)-DDTA] to obtain dexmedetomidine. The dexmedetomidine is salified in hydrochloric acid to obtain dexmedetomidine hydrochloride.
Owner:北京华禧联合科技发展有限公司
Delivery of a sympatholytic cardiovascular agent to the central nervous system
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms of acute or chronic cardiac insult or impaired cardiac performance. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with cardiac insult or impaired cardiac performance and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist (e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine).
Owner:MEDTRONIC INC
Premix formulation for parenteral use and packaging thereof
InactiveUS20170128421A1Minimizes extractables/leachablesEasy to storeOrganic active ingredientsPharmaceutical containersPerioperative careMedicine
The present invention relates to stable premix formulations for parenteral use and packaging for such formulations. The present invention relates to use of a single layer or multilayer film bags comprising polypropylene for packing premixed injectable formulations which remain stable during the storage period.The present invention relates to use of a single layer or multilayer film bags comprising polypropylene for packing dexmedetomidine premixed formulations. Such stable premixed formulations packed into a single layer or multilayer film bags comprising polypropylene are highly advantageous since they are ready-to-use, for example, in perioperative care of a subject in need thereof for sedation.
Owner:SURA SIVA PRASAD REDDY +5
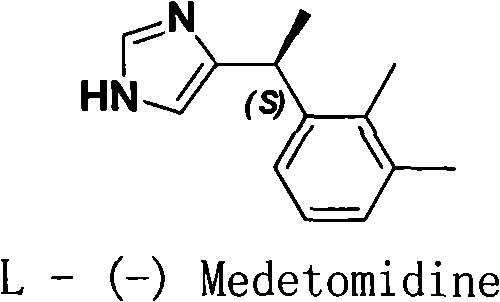
Method for resolution of levorotatory enantiomer and dexiotropic enantiomer of medetomidine
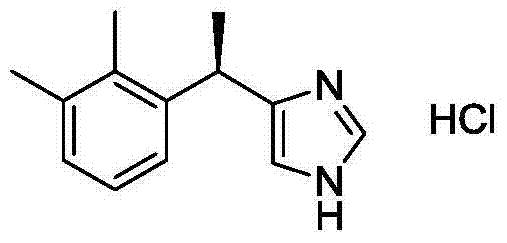
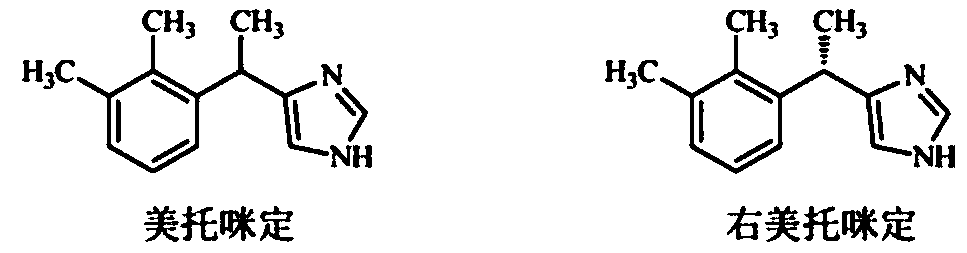
The invention discloses a method for obtaining levorotatory enantiomer and dexiotropic enantiomer by separation from medetomidine (chemical name: 4-(1-(2,3-dimethylphenyl)-ethyl)-1H-imidazole) of racemate, which comprises the steps of reacting medetomidine racemate with optically active acid so that medetomidine racemate can be converted into salts of diastereoisomer, and then separating the saltsof the diastereoisomer by using a half-dose resolution method so as to obtain single enantiomer with particular optical purity.
Owner:北京华禧联合科技发展有限公司
Method for preparing dexmedetomidine hydroch
ActiveCN105175339ALow costShort synthetic routeOptically-active compound separationOrganic racemisationAlcoholDexmedetomidine
The invention relates to a method for preparing dexmedetomidine hydroch. The method particularly includes the following steps that 1, dispinner medetomidine is dissolved in methyl alcohol and mixed with a right-handed resolving agent according to the molar ratio of 1:0.4-0.6, stirring is conducted for 2-3 hours at the heating temperature of 60-80 DEG C, diethyl ether is added, cooling, still standing, crystallization and filtering are conducted, a mother solution and a left-handed resolving agent are mixed according to the molar ratio of 1:0.4-0.6, stirring and crystallization are conducted for 24 hours, and dexmedetomidine is obtained after filtering is completed; 2, obtained dexmedetomidine is placed in HCl / diethyl ether, and the end product dexmedetomidine hydroch is formed. Compared with the prior art, dexmedetomidine hydroch is prepared at low cost; the synthetic route is short, dexmedetomidine hydroch with high purity can be prepared, isomers are not detected, quality meets the newest standard of the pharmacopeia, and industrial production is facilitated. Adopted raw materials are low in cost, reaction conditions are moderate, and the method can be applied to actual production.
Owner:CISEN PHARMA
Method for preparing dexmedetomidine and hydrochloride thereof
ActiveCN109912508AOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDexmedetomidineDouble bond
The invention provides a method for preparing dexmedetomidine and a hydrochloride thereof. Specifically, a composition of a carbon-carbon hydrogenation reduction catalyst and (R,S)-Duanphos is used asa catalyst for chiral catalytic reduction of a double bond, and the dexmedetomidine with an enantiomeric excess being 99.9% can be directly obtained. The preparation method provided by the inventionis short in synthetic route, and the product does not need chiral resolution and is high in yield.
Owner:SHANGHAI TIANCI INT PHARMA
Medetomidine industrial splitting method
The invention discloses a medetomidine industrial splitting method which takes L-(-) camphorsulfonic acid as a chiral reagent and C1-C5 alcohol as a chiral auxiliary. According to the method disclosed by the invention, the splitting yield reaches up to over 40% and the splitting optical purity reaches over 99.5%. The method is simple in industrialized operation and can be used for industrialized and commercialized production.
Owner:安庆生命科技园发展有限公司
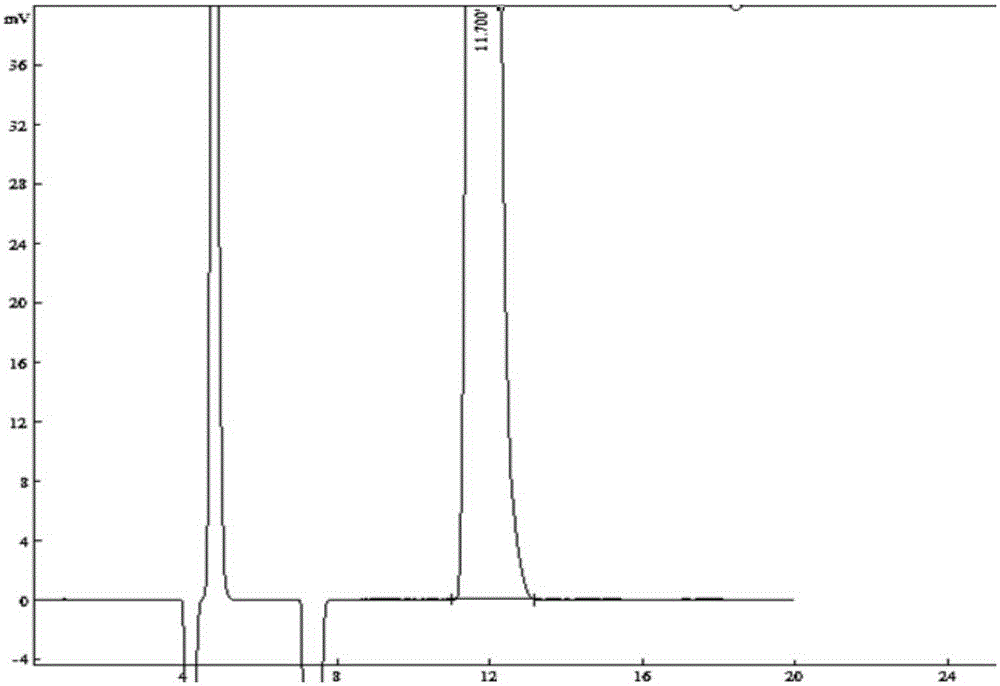
Method of measuring content of related substance in dexmedetomidine hydrochloride active ingredient
InactiveCN107402262AAccurately measure the content of substancesImprove quality controlComponent separationBULK ACTIVE INGREDIENTDrug product
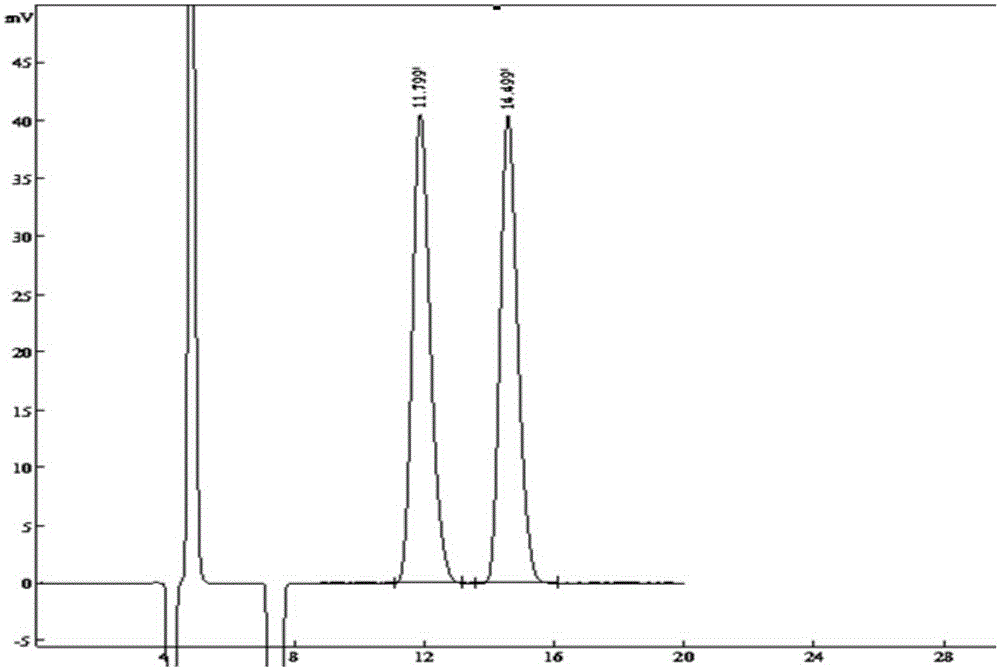
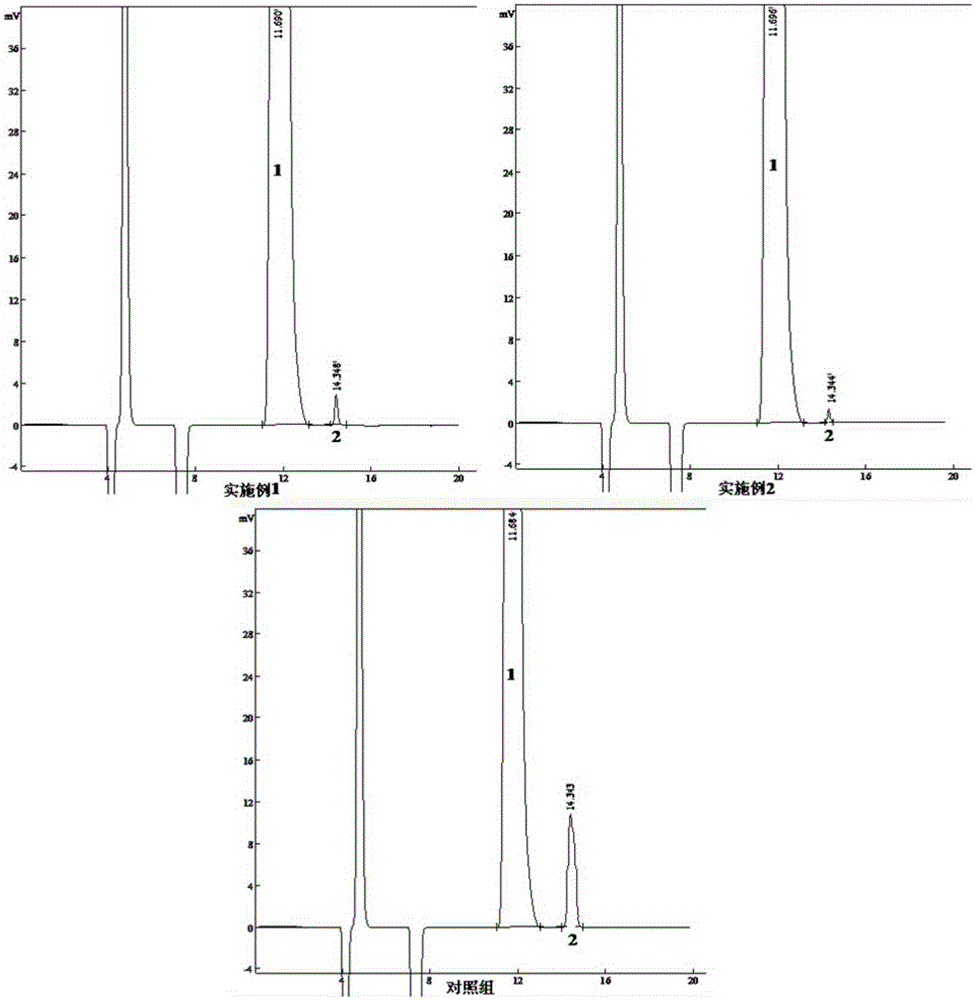
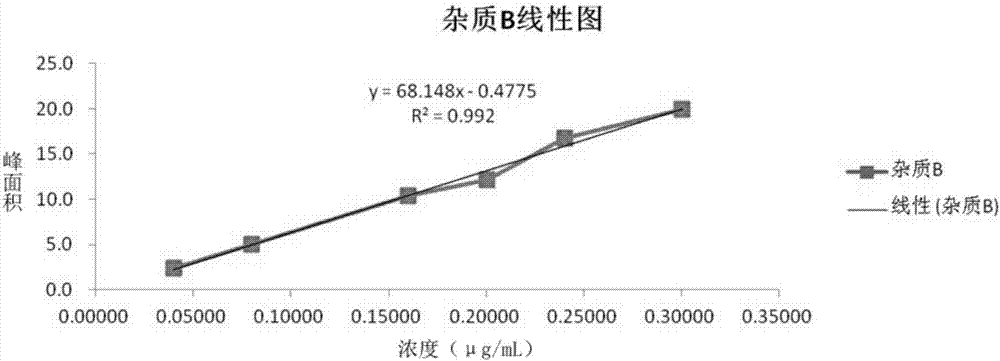
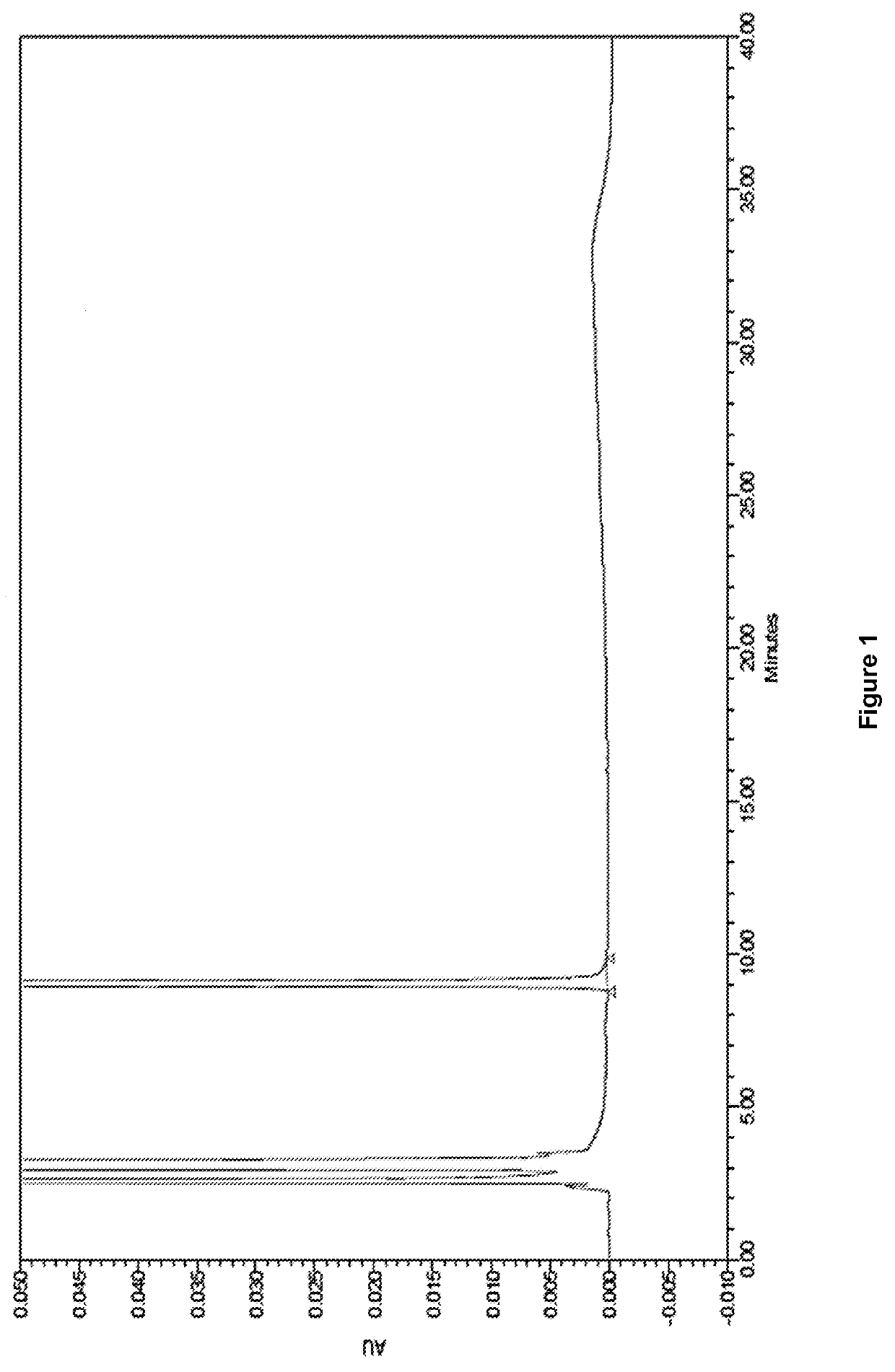
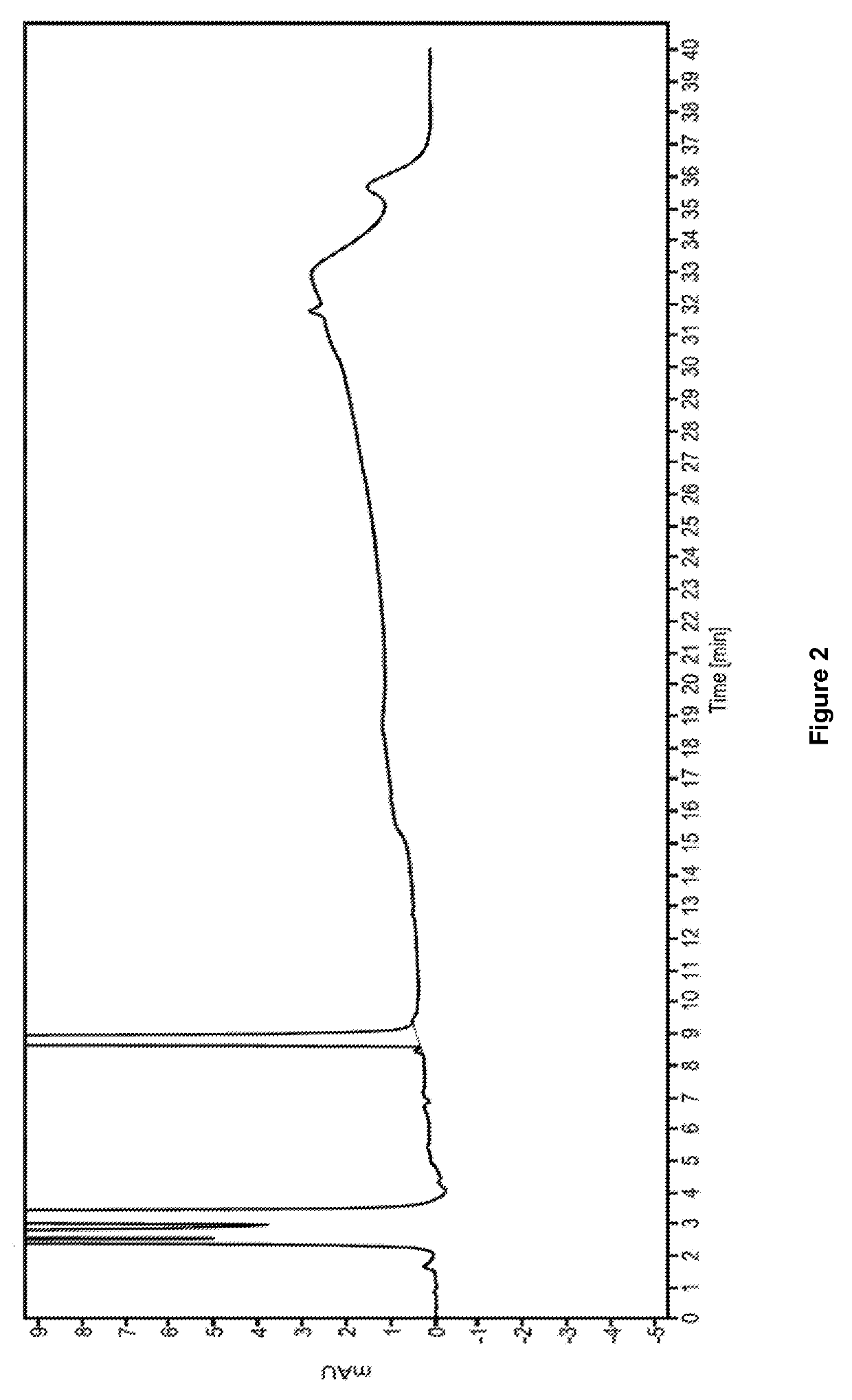
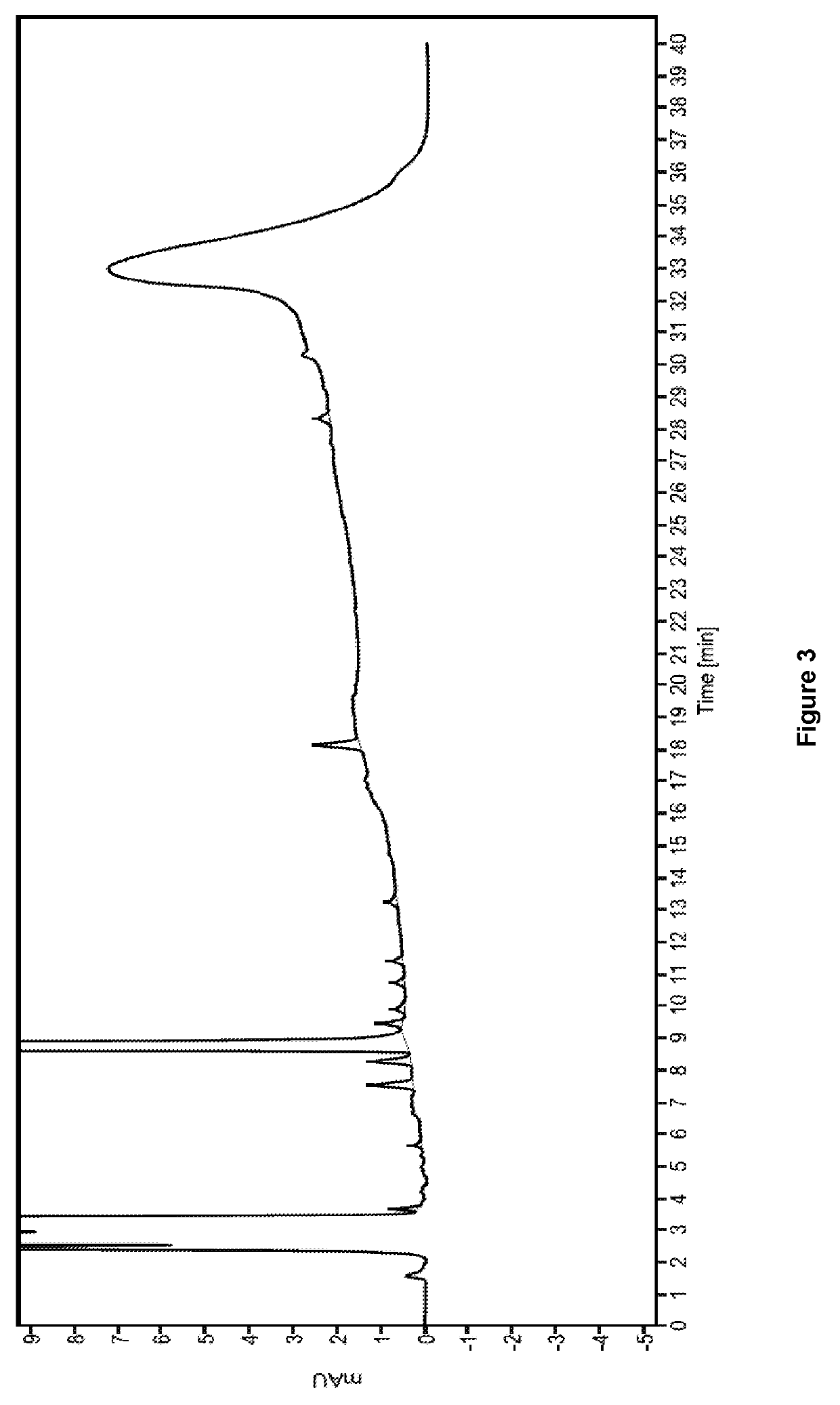
The invention provides a method of measuring the content of related substance in dexmedetomidine hydrochloride active ingredient. The method comprises the following steps: analyzing the dexmedetomidine hydrochloride active ingredient by means of liquid chromatography to obtain a chromatogram conveniently; and determining the content of related substance in the dexmedetomidine hydrochloride active ingredient based on the chromatogram. The method can effectively measure impurities, initial materials and intermediates probably introduced in a synthetic process of dexmedetomidine hydrochloride and effectively control the quality of a dexmedetomidine hydrochloride drug. The method has the characteristics of effectiveness, sensitivity, specificity and accuracy.
Owner:YICHANG HUMANWELL PHARMA
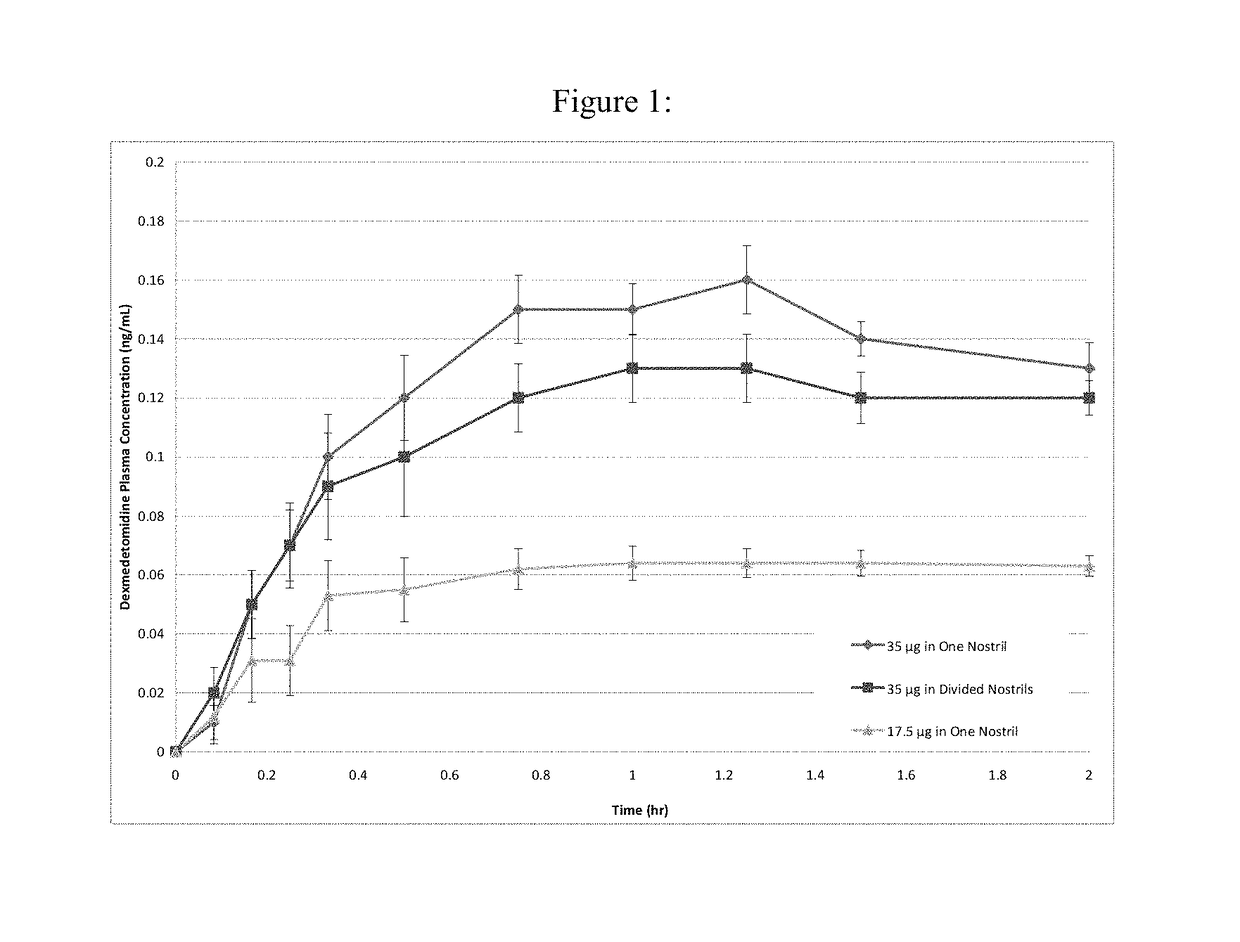
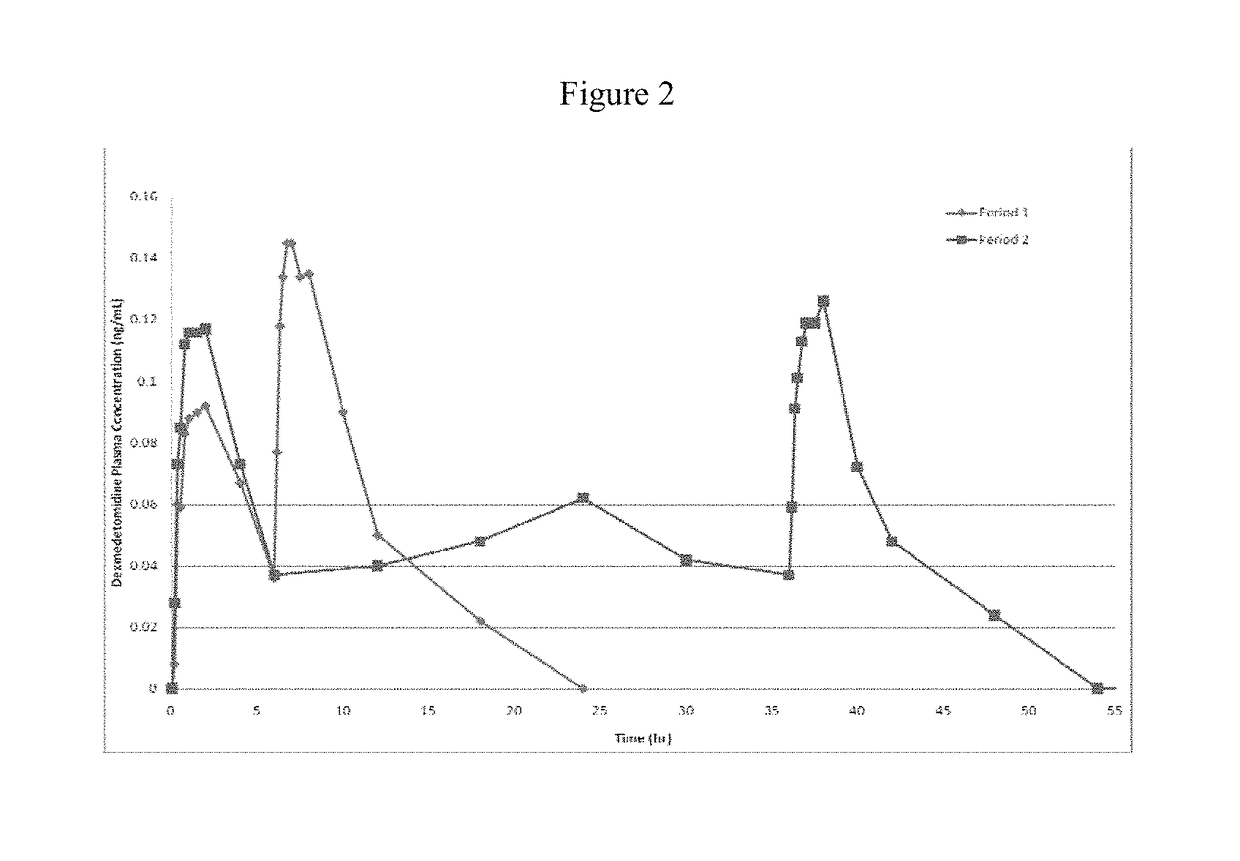
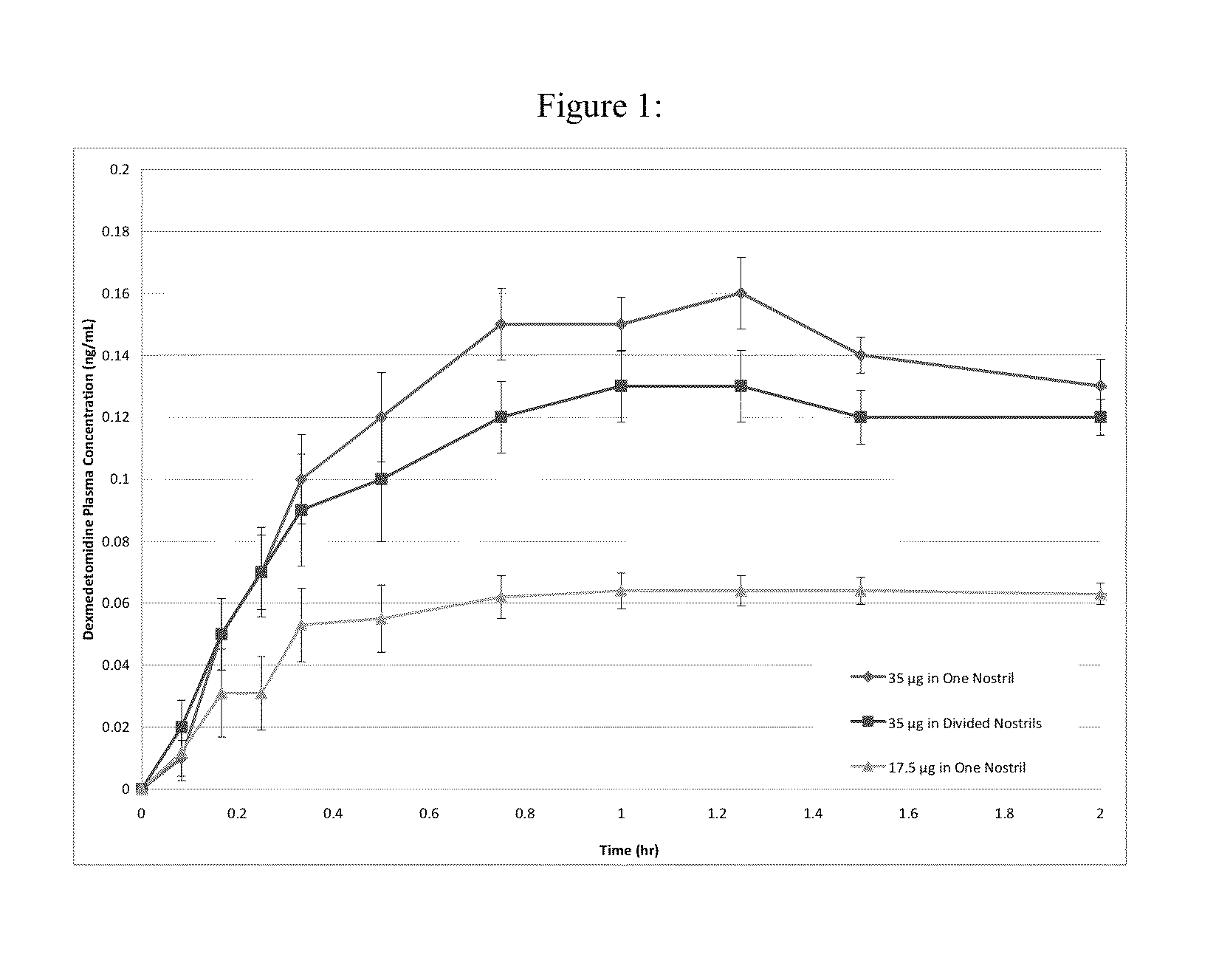
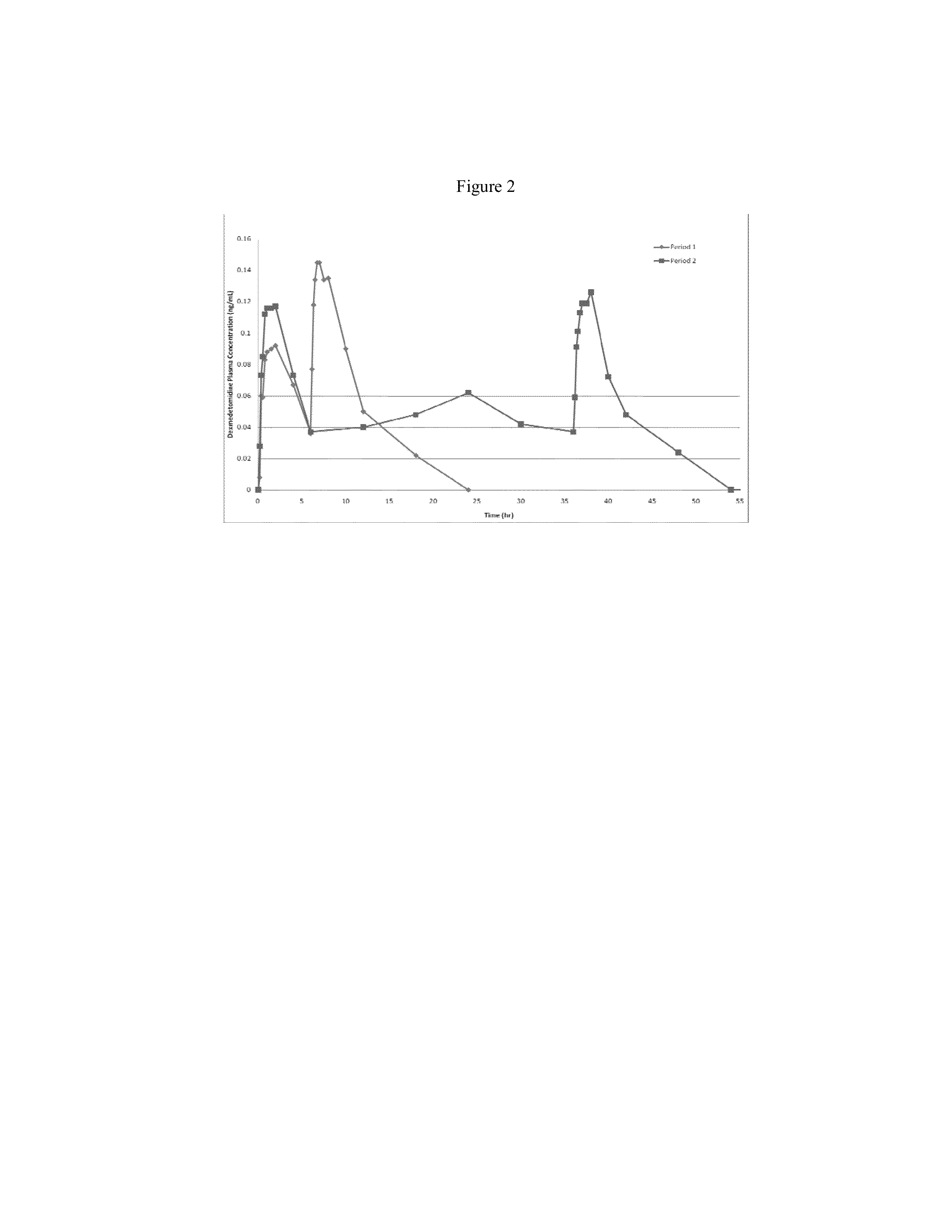
Intranasal dexmedetomidine compositions and methods of use thereof
The present invention provides intranasal formulations comprising dexmedetomidine, or a pharmaceutically acceptable salt thereof, and uses thereof.
Owner:BAUDAX BIO INC
Method for preparing dexmedetomidine and intermediate thereof
ActiveCN105884691AAvoid introducingShort synthetic routeOrganic chemistry methodsBulk chemical productionMedetomidineMagnesium
The invention provides a method for preparing dexmedetomidine and an intermediate thereof. Specifically speaking, the method comprises the steps that a compound shown as a formula II (please see the formula in the description) reacts with metal magnesium to prepare a metal magnesium Grignard reagent, and then the metal magnesium Grignard reagent reacts with a compound shown as a formula III (please see the formula in the description). The process has the advantages of being few in step, high in yield, easy to operate, high in product purity and the like and is quite suitable for large-scale industrialized production.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
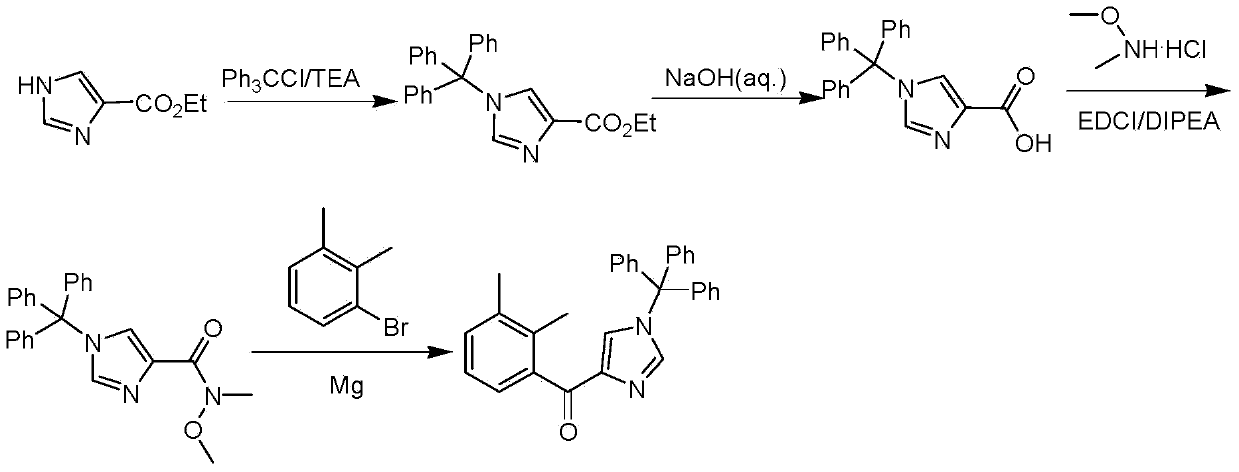
Preparation method for medetomidine intermediate
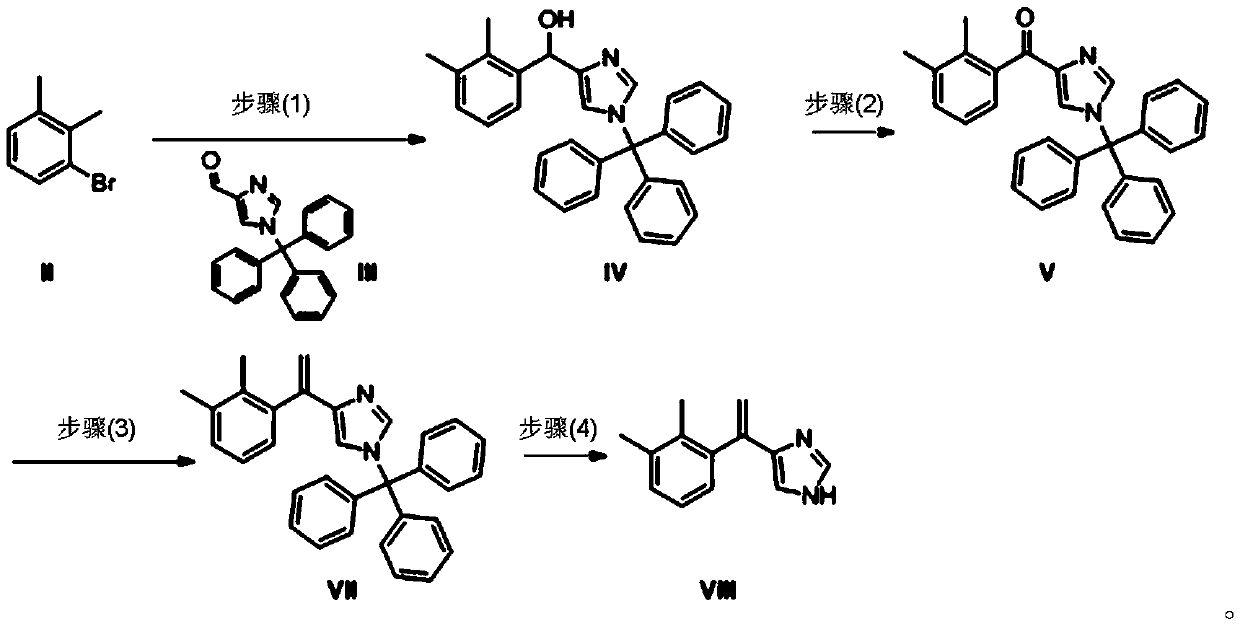
ActiveCN103588711ALow priceEasy to operateOrganic chemistryGrignard reagentHydroxylamine Hydrochloride
The invention discloses a preparation method for 2,3-dimethylphenyl-1-triphenylmethyl-imidazole-4-one. The method comprises the following steps: with imidazole-4-ethyl formate as a raw material, carrying out amino protection by using triphenylchloromethane and then carrying out basic hydrolysis so as to obtain 1-triphenylmethyl-1H-imidazole-4-formic acid; subjecting 1-triphenylmethyl-1H-imidazole-4-formic acid and N,O-dimethyl hydroxylamine hydrochloride so as to obtain N-methoxy-N-methyl-1-triphenylmethyl-1H-imidazole-4-methanamide; and subjecting N-methoxy-N-methyl-1-triphenylmethyl-1H-imidazole-4-methanamide and a Grignard reagent prepared through a reaction of 2,3-dimethylbromobenzene with magnesium metal to a Grignard reaction so as to produce the target product 2,3-dimethylphenyl-1-triphenylmethyl-imidazole-4-one. Compared with reported preparation methods in the prior art, the preparation method provided by the invention is easier and more convenient to operate and is beneficial for industrial production.
Owner:TIANJIN WEIJIE PHARMA
Intranasal dexmedetomidine compositions and methods of use thereof
The present invention provides intranasal formulations comprising dexmedetomidine, or a pharmaceutically acceptable salt thereof, and uses thereof.
Owner:BAUDAX BIO INC
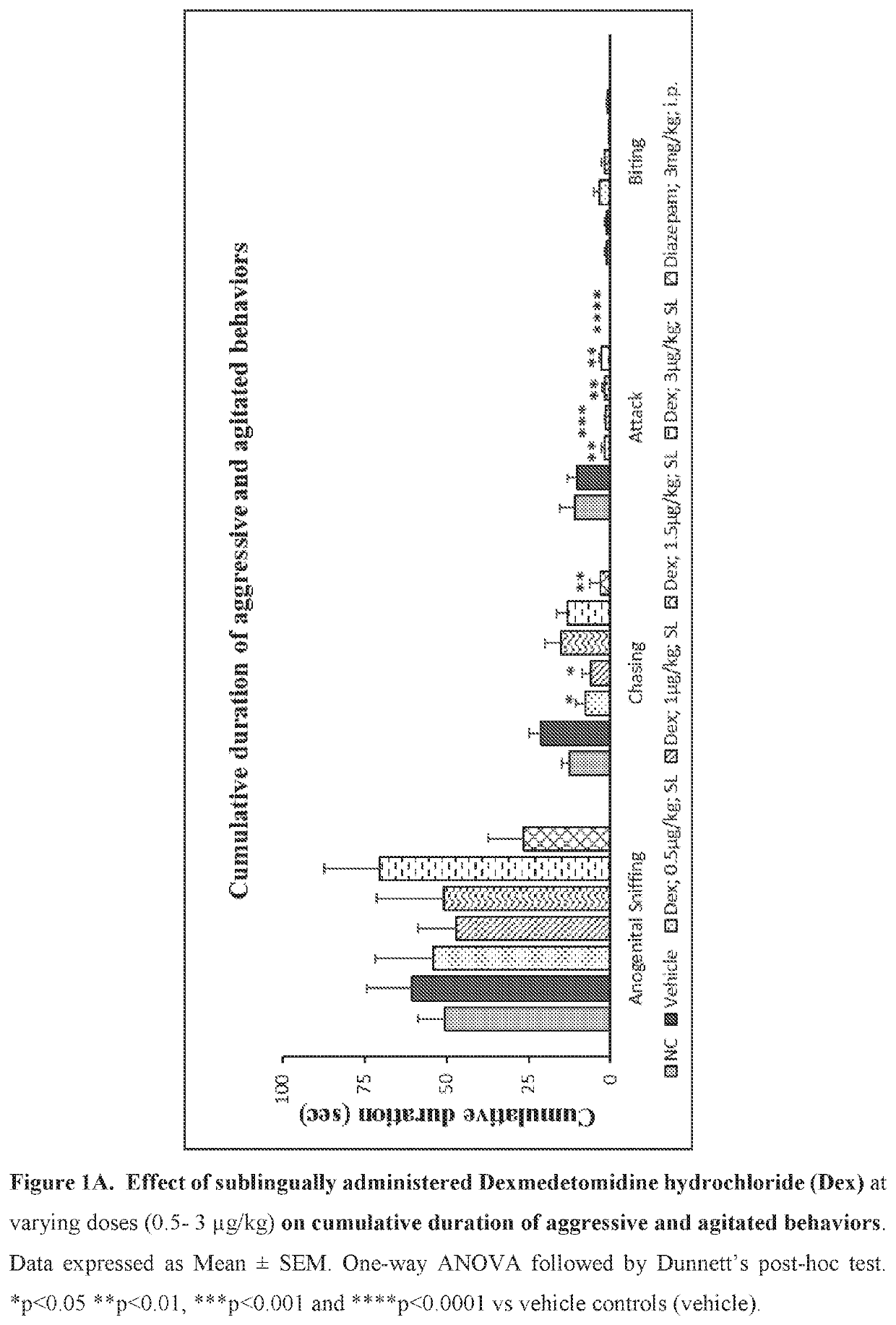
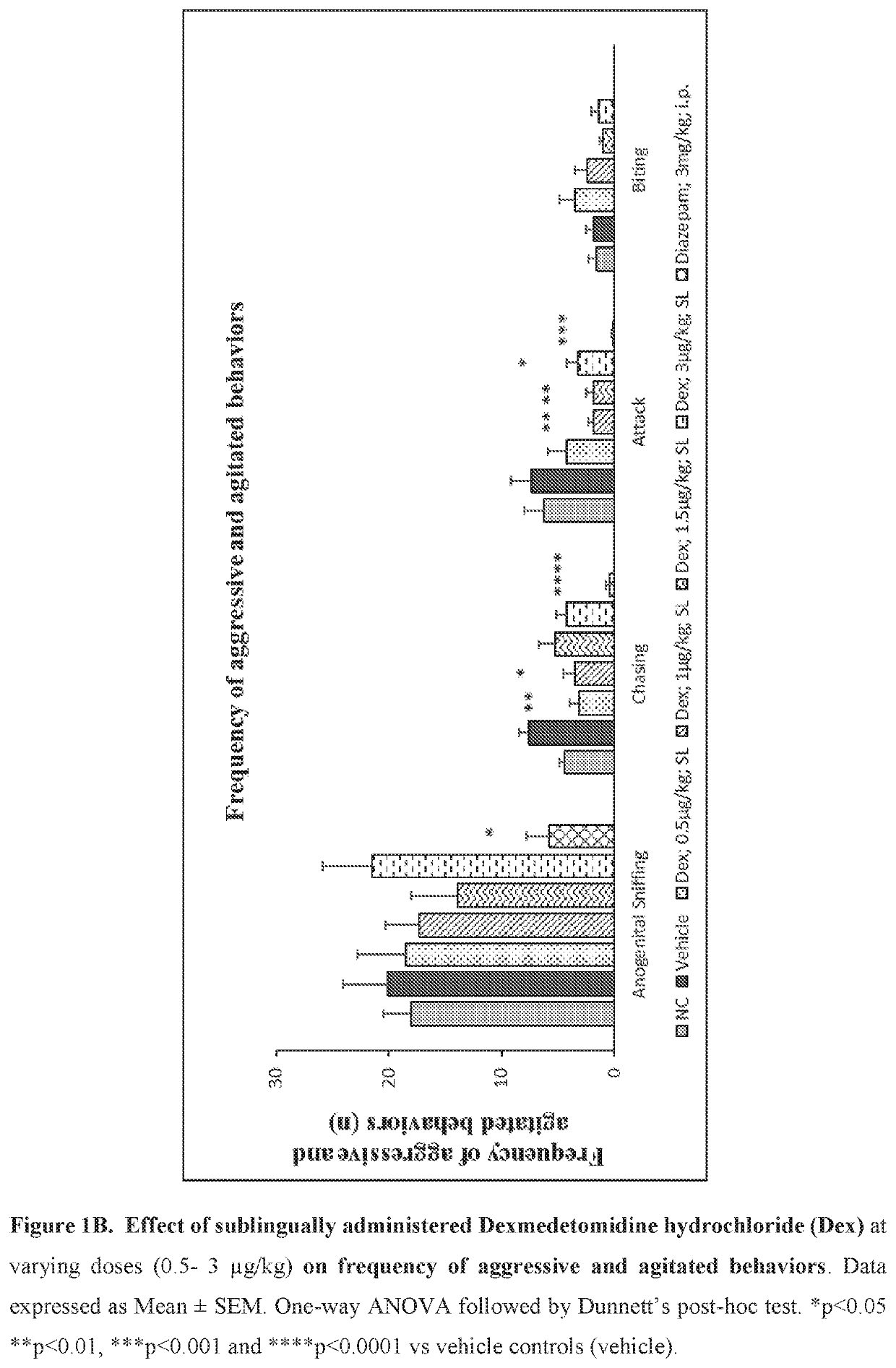
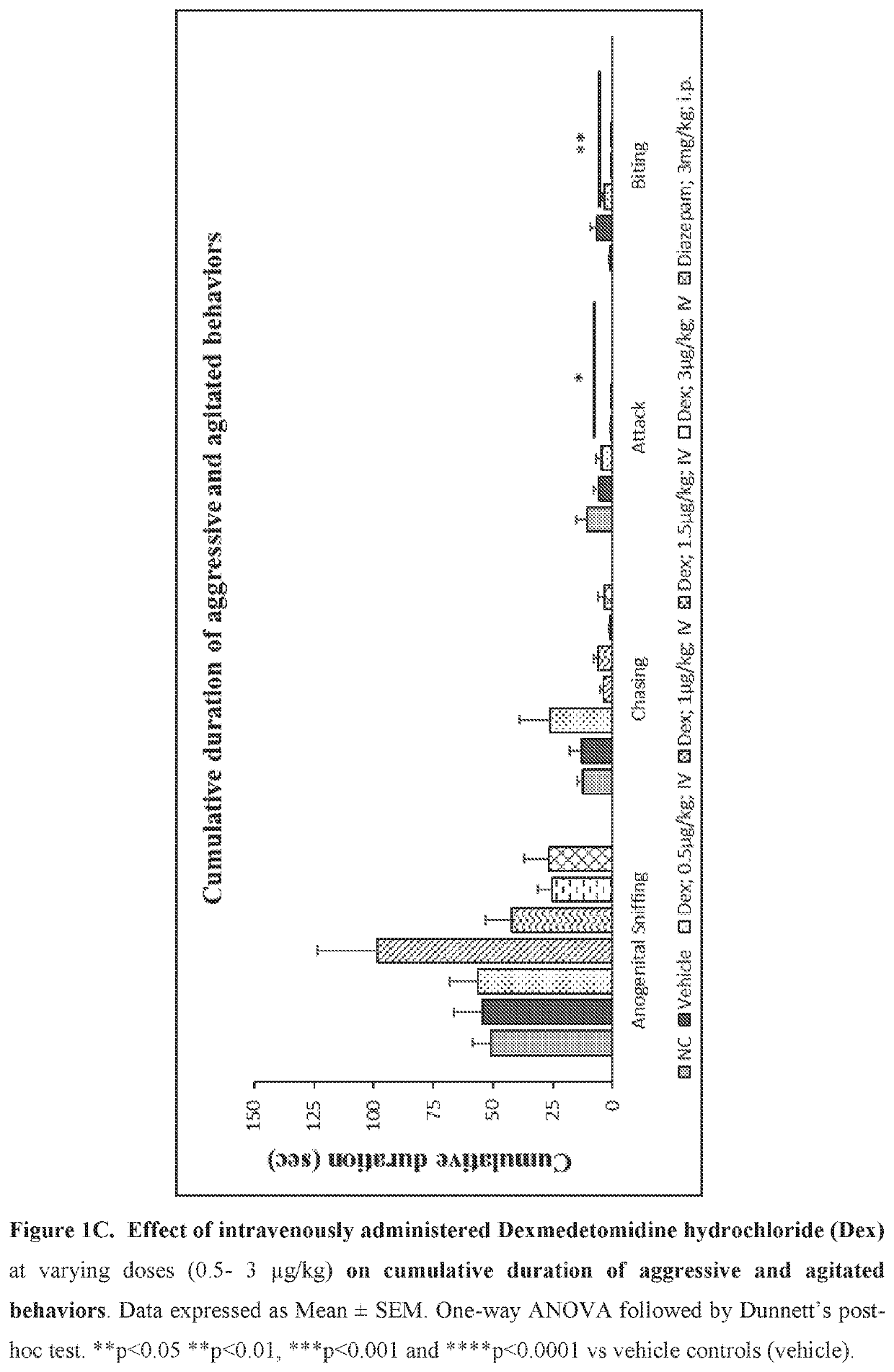
Use of sublingual dexmedetomidine for the treatment of agitation
PendingUS20190365715A1Preventing and reducing signOrganic active ingredientsNervous disorderNeuropsychiatric diseaseSublingual administration
The present invention discloses a method of treating agitation or the signs of agitation in a subject comprising the sublingual administration of an effective amount of an alpha-2 adrenergic agonist, more particularly Dexmedetomidine, or a pharmaceutically acceptable salt thereof. The method is particularly suitable for the treatment of agitation associated with neurodegenerative and / or neuropsychiatric diseases. The present invention also discloses the sublingual administration of an alpha-2 adrenergic agonist, more particularly Dexmedetomidine or a pharmaceutically acceptable salt thereof at a dose that is effective to treat agitation or the signs of agitation in a subject, but does not cause significant sedation.
Owner:BIOXCEL THERAPEUTICS INC
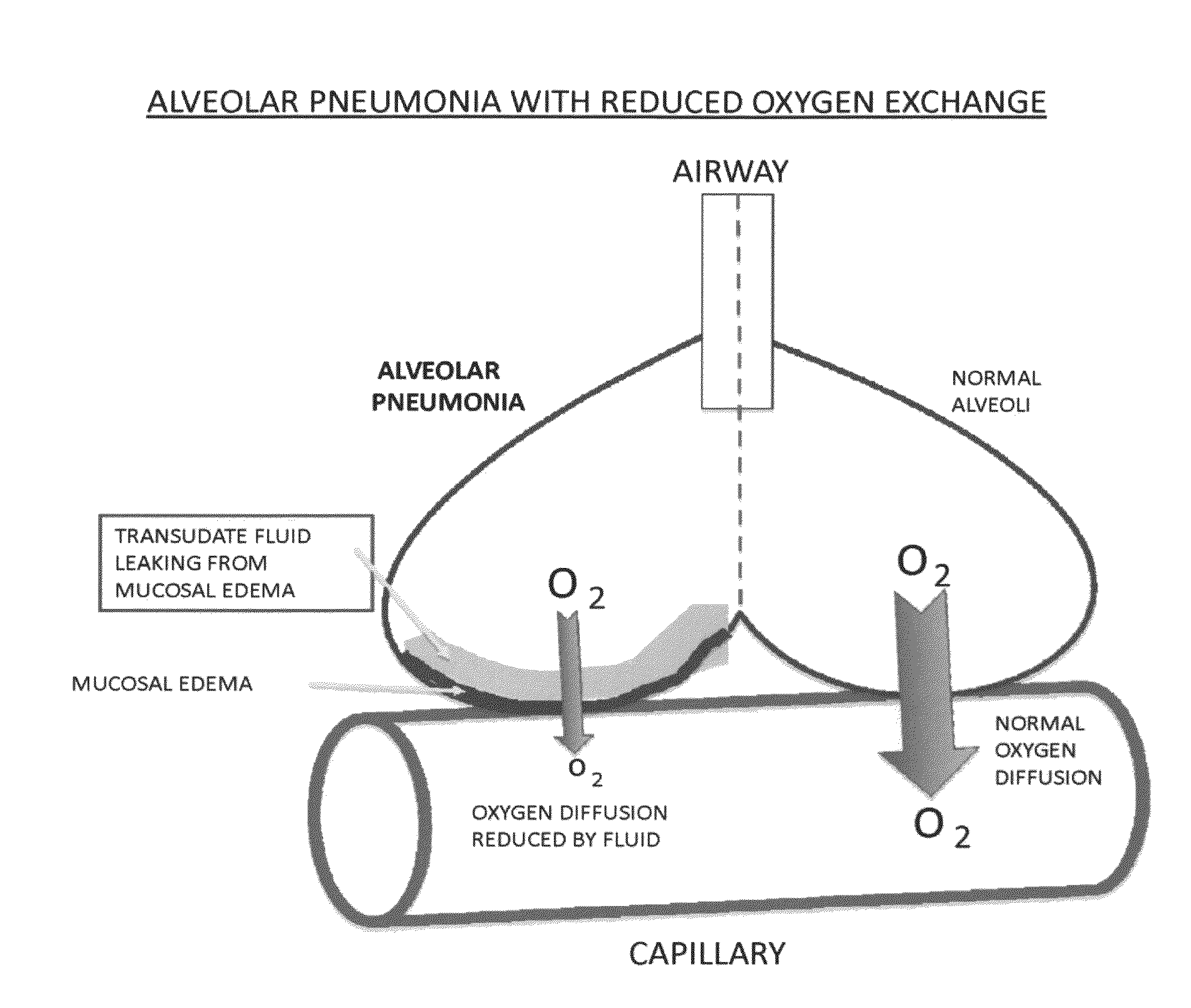
Compositions and methods for treatment of pulmonary diseases and conditions
InactiveUS20110244058A1Promote absorptionImprove permeabilityBiocideOrganic chemistryDiseaseAdrenergic receptor agonists
The invention provides compositions and methods for treating pulmonary diseases and conditions. The provided compositions and methods utilize either low concentrations of selective α-2 adrenergic receptor agonists having a binding affinity of 300 fold or greater for α-2 over α-1 adrenergic receptors or ketamine at specific pH. The compositions preferably comprise brimonidine and / or dexmedetomidine and / or ketamine.
Owner:EYE THERAPIES
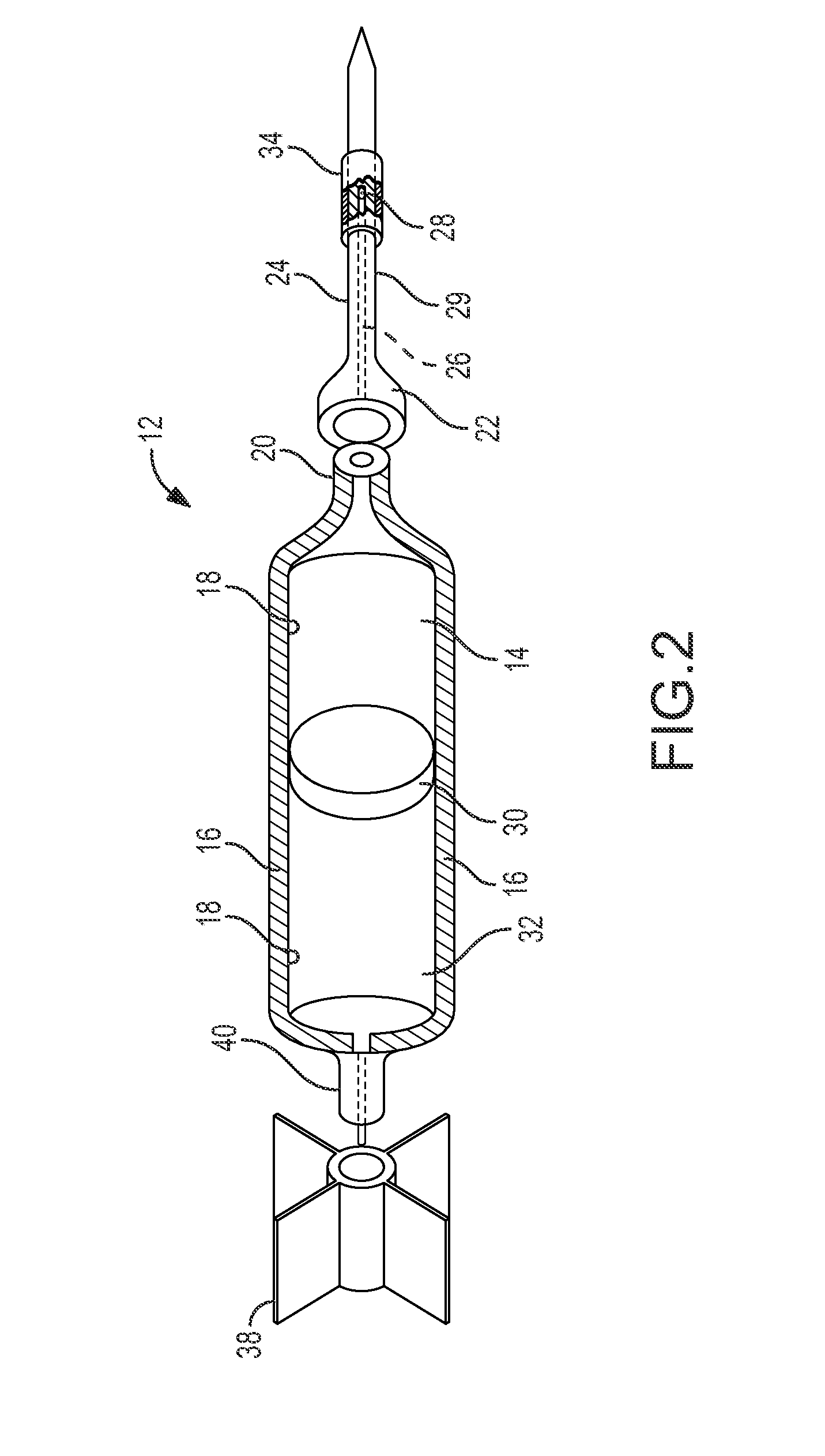
Pharmaceutical Combination for and Method of Anesthetizing and Immobilizing Non-Domesticated Mammals
ActiveUS20100010006A1Reduce amountShorten driving distanceBiocidePharmaceutical delivery mechanismMedicineBULK ACTIVE INGREDIENT
Non-domesticated mammalian animals are anesthetized and immobilized by injecting an effective amount of an anesthetizing and immobilizing drug comprising pharmaceutically active ingredients of butorphanol tartrate, azaperone tartrate and medetomidine hydrochloride (BAM) into the animal from a dart. The BAM combination is preferably initially formed as lyophilized powder of the pharmaceutically active ingredients, and then reconstituted before injection as an injectable liquid in the environment of the animal.
Owner:GREAT WESTERN BANK
Method for preparing medetomidine and its salts
ActiveUS7902377B2Increase productionHigh yieldBiocideOrganic active ingredientsGrignard reagentMedetomidine
The invention provides an improved, highly efficient method for preparing Medetomidine, and its salts, in particular its pharmaceutically acceptable salts. The method utilizes the high reactivity of halogenated imidazoles towards transmetalation with Grignard reagents and the subsequent reaction with 2,3-dimethylbenzaldehyde.
Owner:GRINDEKS
Dexmedetomidine hydrochloride injection composition
ActiveCN107412152AFor long-term storageSafe for clinical useOrganic active ingredientsNervous disorderForeign matterSide effect
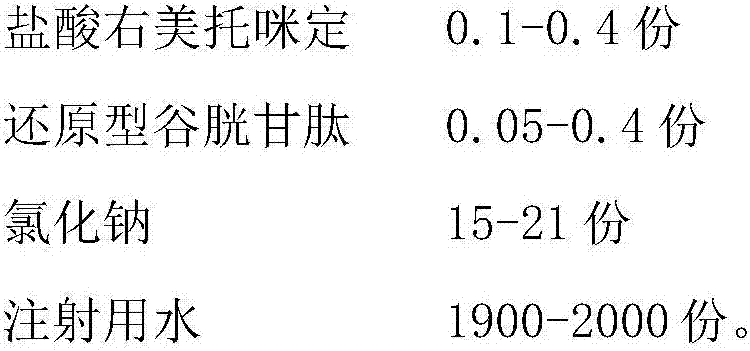
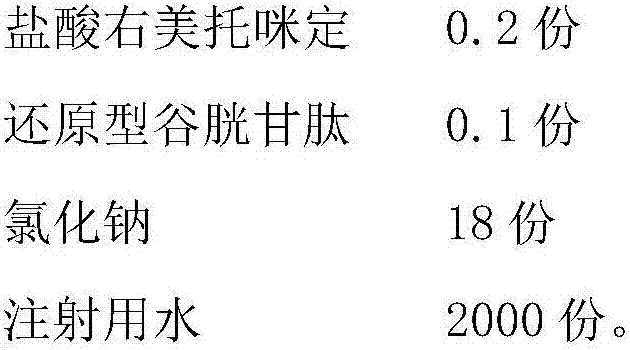
The invention belongs to the technical field of medicines and particularly relates to a dexmedetomidine hydrochloride injection composition which includes: dexmedetomidine hydrochloride, reduced glutathione, sodium chloride and water for injection, wherein the mass ratio of the dexmedetomidine hydrochloride to the reduced glutathione in the injection composition is 0.6:1-4:1. Compared with the prior art, the dexmedetomidine hydrochloride injection composition has good photo-stability, is not liable to generate visible foreign matters, is easy to store for long time and is safe to use clinically, has good treatment effect and is low in side effect. In addition, a preparation process of the dexmedetomidine hydrochloride injection composition is simple and low-cost and has convenience in industrial production.
Owner:广东泽盛药业有限公司
Methods for treating agitation using dexmedetomidine hydrochloride
PendingUS20210267944A1Reduce agitationReduce signOrganic active ingredientsNervous disorderIntravenous routePharmaceutical drug
The present disclosure relates to the treatment of agitation or signs of agitation in certain human subjects, including subjects with a neurodegenerative, neuropsychiatric or opioid withdrawal disorder, by administering dexmedetomidine hydrochloride by the intravenous route.
Owner:BIOXCEL THERAPEUTICS INC
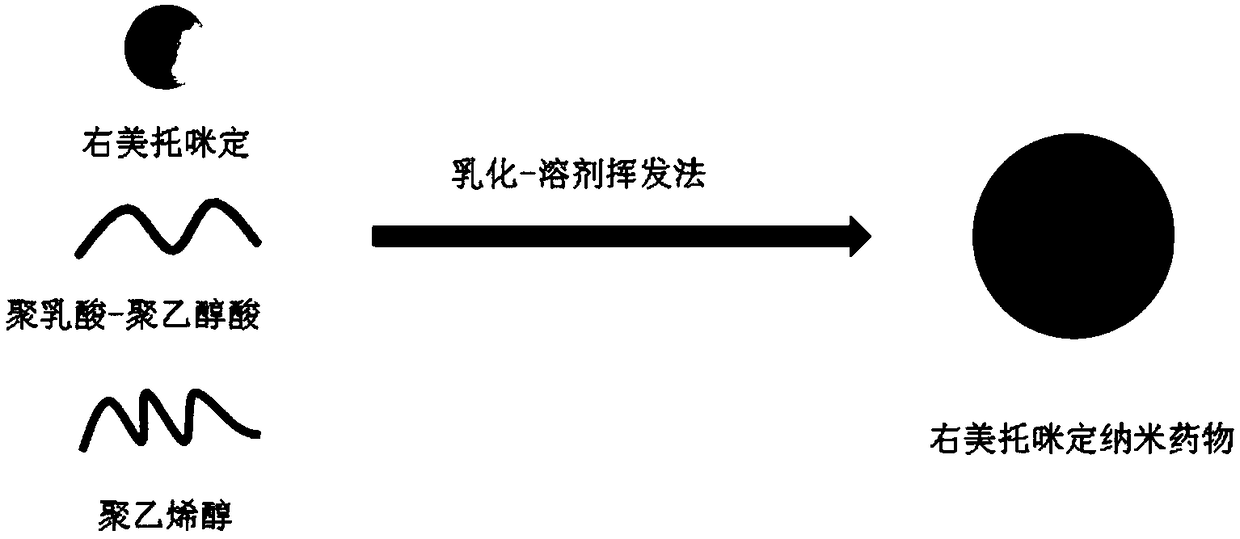
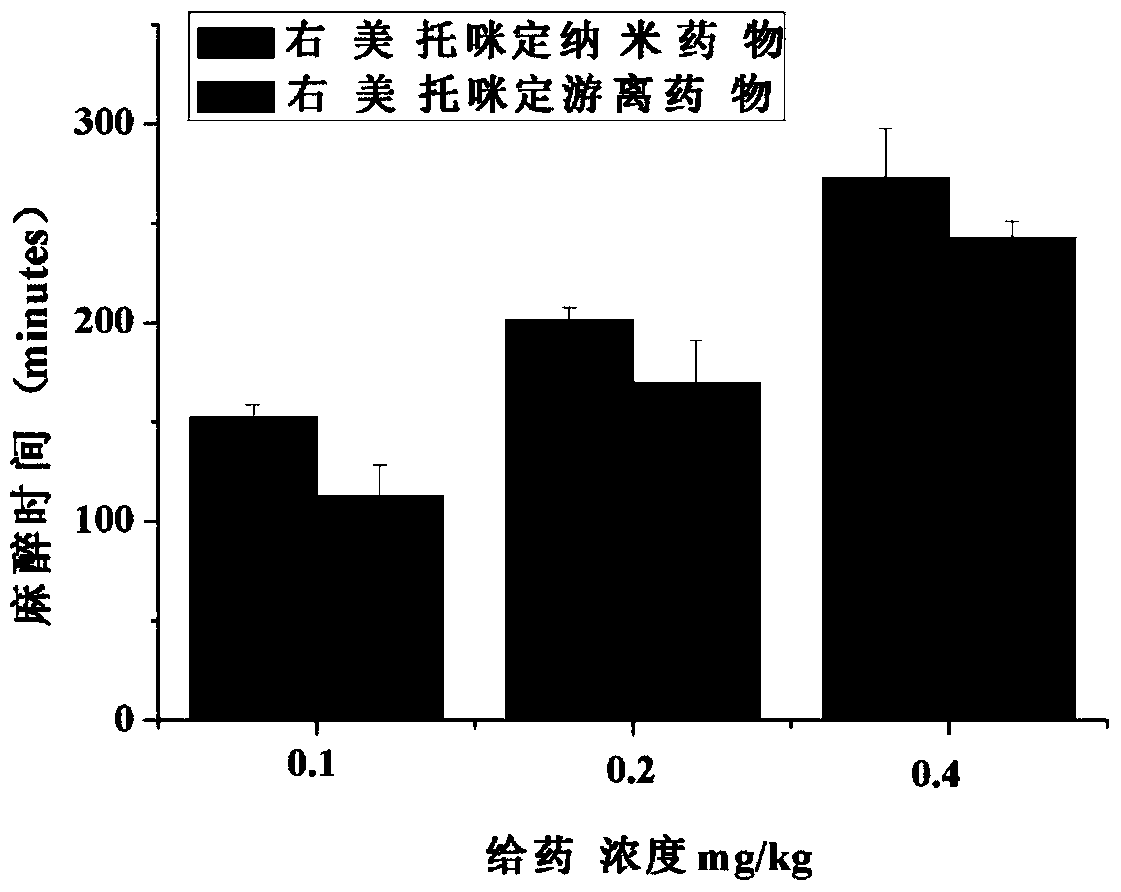
Dexmedetomidine nano preparation and preparation method thereof
InactiveCN109381444AGood dispersionHigh biosecurityOrganic active ingredientsAnaestheticsOrganic solventPolyvinyl alcohol
The invention discloses a dexmedetomidine nano preparation which is prepared by wrapping dexmedetomidine by a polylactic acid-polyglycolic acid copolymer and by taking polyvinyl alcohol as an emulsifier. The nano preparation is of a spherical structure and has a particle size of 250-350 nanometers. A preparation method comprises the following steps: dissolving the dexmedetomidine and the polylactic acid-polyglycolic acid copolymer; slowly putting a 1% polyvinyl alcohol solution into an organic solvent so as to obtain a dexmedetomidine nano preparation suspension; separating the nano preparation suspension in a centrifuge, and washing with water, thereby obtaining a cured dexmedetomidine nano preparation. The dexmedetomidine nano preparation disclosed by the invention has high dispersibility and a good slow release function, the anesthesia time of the dexmedetomidine is effectively prolonged, and a tolerance dosage is increased, so that the application range of the dexmedetomidine is widened. The dexmedetomidine nano preparation is simple in preparation method and easy to popularize.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
Preparation method of dexmedetomidine
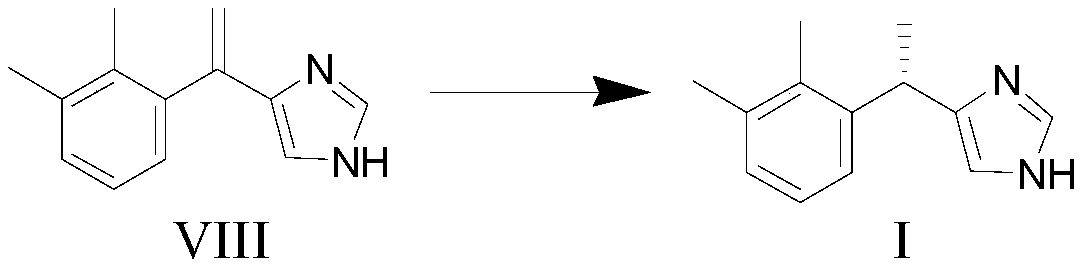
ActiveCN107879979AHigh chemical purityHigh optical purityOrganic chemistryBulk chemical productionDexmedetomidinePhase-transfer catalyst
The invention discloses a preparation method of dexmedetomidine, which includes the steps of: performing a methylation reaction to 5-(2,3-dimethylphenyl)-1-triphenylmethyl-1H-imidazole and MeI under effect of a phase transfer catalyst; removing a triphenylmethyl protective group with concentrated hydrochloric acid to obtain the dexmedetomidine. The method reduces synthesis cost and avoids rigorousreaction conditions. The method employs simple reaction conditions and can produce dexmedetomidine at high chemical and optical purities. The method has excellent processability, is beneficial to industrial production, can reduce environment stress, is simple in synthesis route and can avoid generation of some technical impurities, thus reducing work load of purifying a final product.
Owner:RAFFLES PHAMRMATECH CO LTD
Dexmedetomidine or medetomidine for use in treating separation anxiety in dogs
PendingUS20200069650A1Separation anxietyRelieve symptomsOrganic active ingredientsNervous disorderOral medicationPharmaceutical medicine
The invention relates to a method of treating separation anxiety in companion animals, particularly dogs, comprising administering to a subject in need thereof an effective amount of dexmedetomidine, medetomidine or a pharmaceutically acceptable salt thereof as the active ingredient. The active ingredient is preferably administered oromucosally, e.g. in the form of an oromucosal gel.
Owner:ORION CORPORATION
Premixed preparation for dexmedetomidine
InactiveCN106038538ANo reduction in active ingredient contentLow impurity contentOrganic active ingredientsPharmaceutical containersIntensive care unitDexmedetomidine
The invention relates to a premixed preparation for dexmedetomidine. Specifically, the invention relates to a ready-to-use premixed preparation of dexmedetomidine packaged in a container containing plastic or a pharmaceutically acceptable salt thereof. The ready-to-use premixed preparation can be used for sedation of patients in an intensive care unit or procedural sedation.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Ready-to-use dexmedetomidine compositions
InactiveUS20190262314A1Organic active ingredientsInorganic non-active ingredientsPerioperative careReady to use
The present invention provides stable, ready-to-use pharmaceutical compositions, comprising dexmedetomidine or a pharmaceutically acceptable salt thereof, one or more pharmaceutically acceptable excipients, and a pharmaceutically acceptable liquid vehicle, where the composition is provided in a sealed container that comprises a cyclic olefin polymer. The present invention also provides ready-to-use pharmaceutical compositions comprising dexmedetomidine or a pharmaceutically acceptable salt thereof, a pharmaceutically acceptable liquid vehicle and an antioxidant and / or stabilizer, such as L-methionine. These solutions were found to be stable over the shelf life of the product. Other aspects of the invention relate to methods for making such compositions, as well as methods of using such compositions for perioperative care of a patient or for sedation.
Owner:SLAYBACK PHARMA LLC
Industrial preparation method of dexmedetomidine hydrochloride
The invention discloses an industrial preparation method of dexmedetomidine hydrochloride, and belongs to the field of medicines. The method comprises the following steps: directly carrying out a Friedel-Crafts alkylation reaction on initial raw materials comprising 1-(2,3-dimethylphenyl)ethanol not subjected to a chlorination (thionyl chloride) reaction and protected imidazole under the catalysis of a Lewis acid to obtain racemic dexmedetomidine, carrying out pre-resolution purification on the racemic dexmedetomidine through a chiral acid, carrying out chiral acid resolution and alkali dissociation, and adding a hydrochloric acid organic solvent to form a salt in order to obtain the dexmedetomidine hydrochloride. The method avoids use of the toxic and corrosive regent thionyl chloride, allows the above product with high chiral and chemical purity to be obtained and the yield to be high, and is suitable for industrial production.
Owner:江苏开元医药有限公司
Process for preparing medetomidine
ActiveCN103664788BEfficient preparationEasy to purifyOrganic chemistryHigh volume manufacturingProcess conditions
The invention discloses a method for preparing medetomidine. Medetomidine can be effectively prepared according to the method for preparing medetomidine, and by adopting a cheap 4-imidazole derivative and cheap 1-(2,3-dimethylphenyl)ethanone as raw materials, the medetomidine product is prepared only through three-step reactions. The method has simple process conditions, is easy to control, and is conducive to industrialized mass production; and the yield of medetomidine prepared by the method for preparing medetomidine can reach 76%, and the product purity can reach 99.5%.
Owner:YICHANG HUMANWELL PHARMA +1
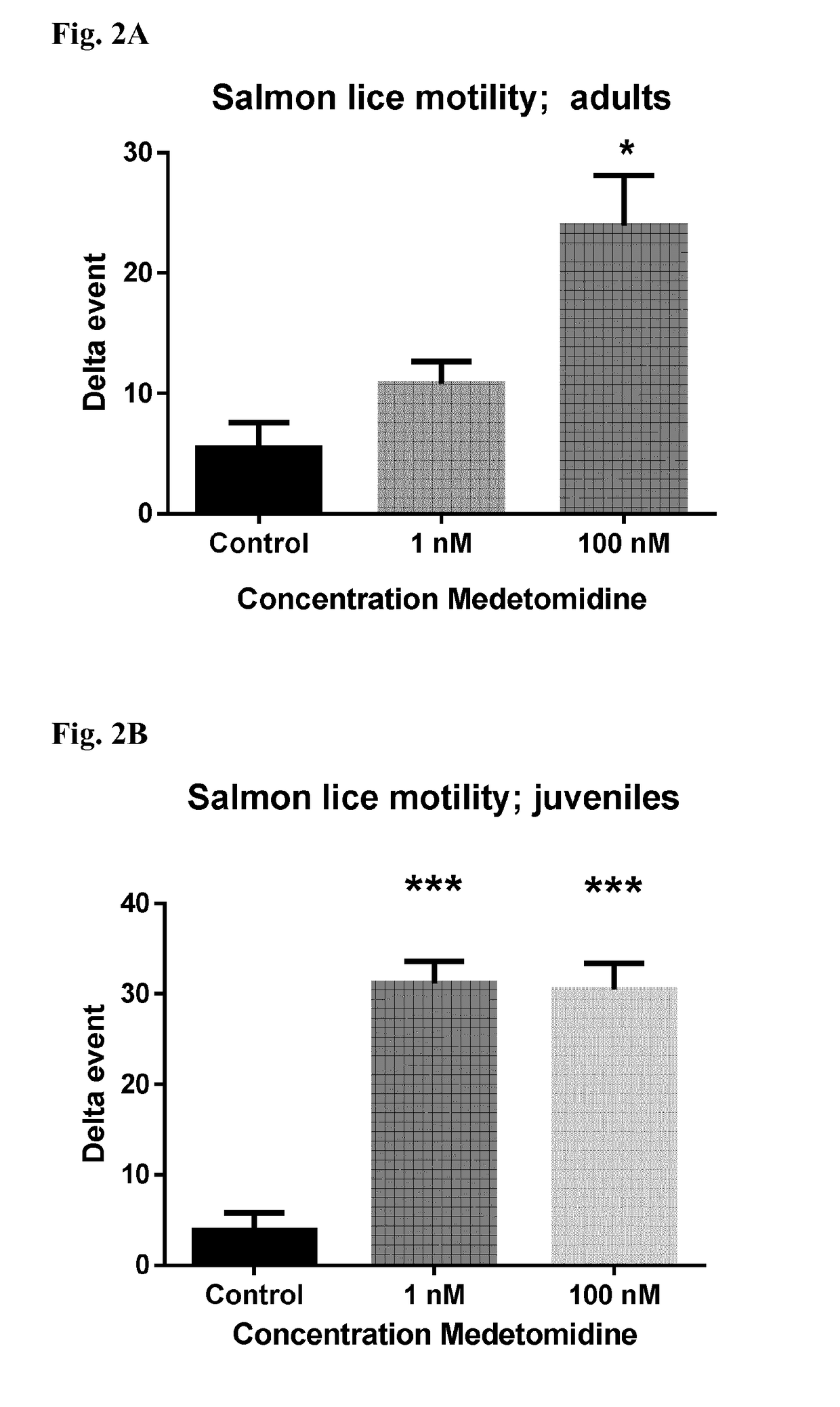
Medetomidine for use in controlling parasitic crustaceans on fish
InactiveUS20180146647A1Reduce and prevent marine biofoulingIncrease water flowBiocideOrganic active ingredientsCrustaceanWater flow
Medetomidine or a salt thereof for use in controlling parasitic crustaceans, such as sea lice, on fish, e.g. salmon. A method of improving water flow into and out of a cage or net for fish farming, by providing said cage or net with a surface coating containing medetomidine or a salt thereof in an amount effective to reduce biofouling of said cage or net. The coating is capable of releasing medetomidine or the salt thereof into the water in the cage or net in an amount effective to reduce or prevent parasitic infestation of the fish in the cage or net.
Owner:I TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com