Industrial preparation method of dexmedetomidine hydrochloride
A technology for dexmedetomidine hydrochloride and dexmedetomidine, which is applied in the field of medicine, can solve problems such as corrosion, rust, toxicity and low yield in workshops and equipment, and achieves avoiding health hazards, reducing process steps, and reducing yield. High rate and purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
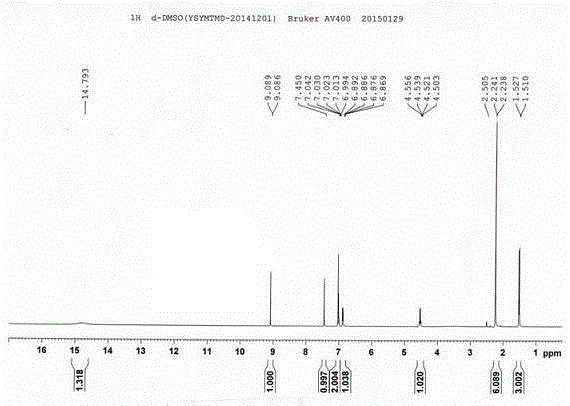
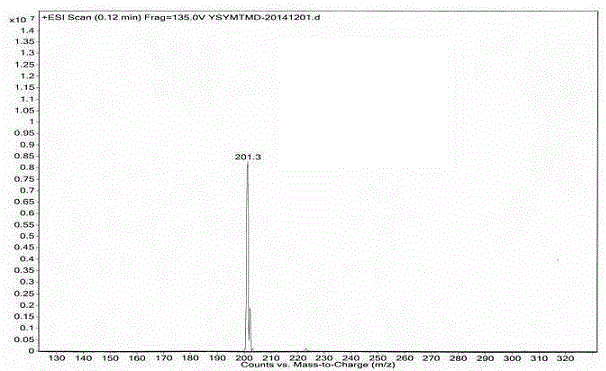
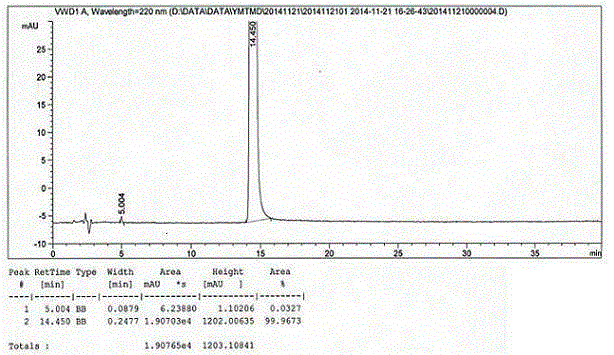
Image
Examples
Embodiment 1
[0045] (1) Preparation of medetomidine
[0046] Into a 20L dry glass reactor, add 1.86kg of trimethylsilimidazole and 9.85L of dichloromethane in sequence, stir for 10 minutes, start to add 2.52kg of titanium tetrachloride dropwise, control the internal temperature at 20-30°C, continue stirring after dropping After 30 minutes, continue to add 1.33 kg of 1-(2,3-dimethylbenzene) ethanol dropwise under the temperature adjustment. After the drop is completed, continue to stir and react for 18 hours, and take samples for TLC detection or HPLC detection. After the reaction is completed, add the reaction solution into 10L of ice water, stir for 20min, let stand to separate layers, separate the aqueous phase and the organic phase, discard the organic phase, adjust the pH value of the aqueous phase to 10 with sodium hydroxide, add 12L of dichloromethane for extraction , separated the organic phase, added 4L dichloromethane to the aqueous phase for extraction, and combined the organic p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com