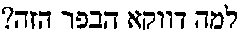Patents
Literature
13132 results about "Imidazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
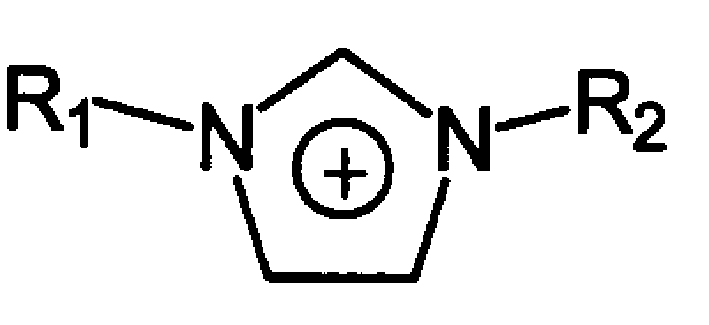
Imidazole is an organic compound with the formula C₃N₂H₄. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms.
Use of 8-amino-aryl-substituted imidazopyrazines as kinase inhbitors
The present invention relates to 8-amino-aryl-substituted imidazopyrazines which modulate the activity of protein kinases ("PKs"). The compounds of this invention are therefore useful in treating disorders related to abnormal PK activity. Pharmaceutical compositions comprising these compounds, methods of treating diseases utilizing pharmaceutical compositions comprising these compounds and methods of preparing them are also disclosed.
Owner:SUGEN INC
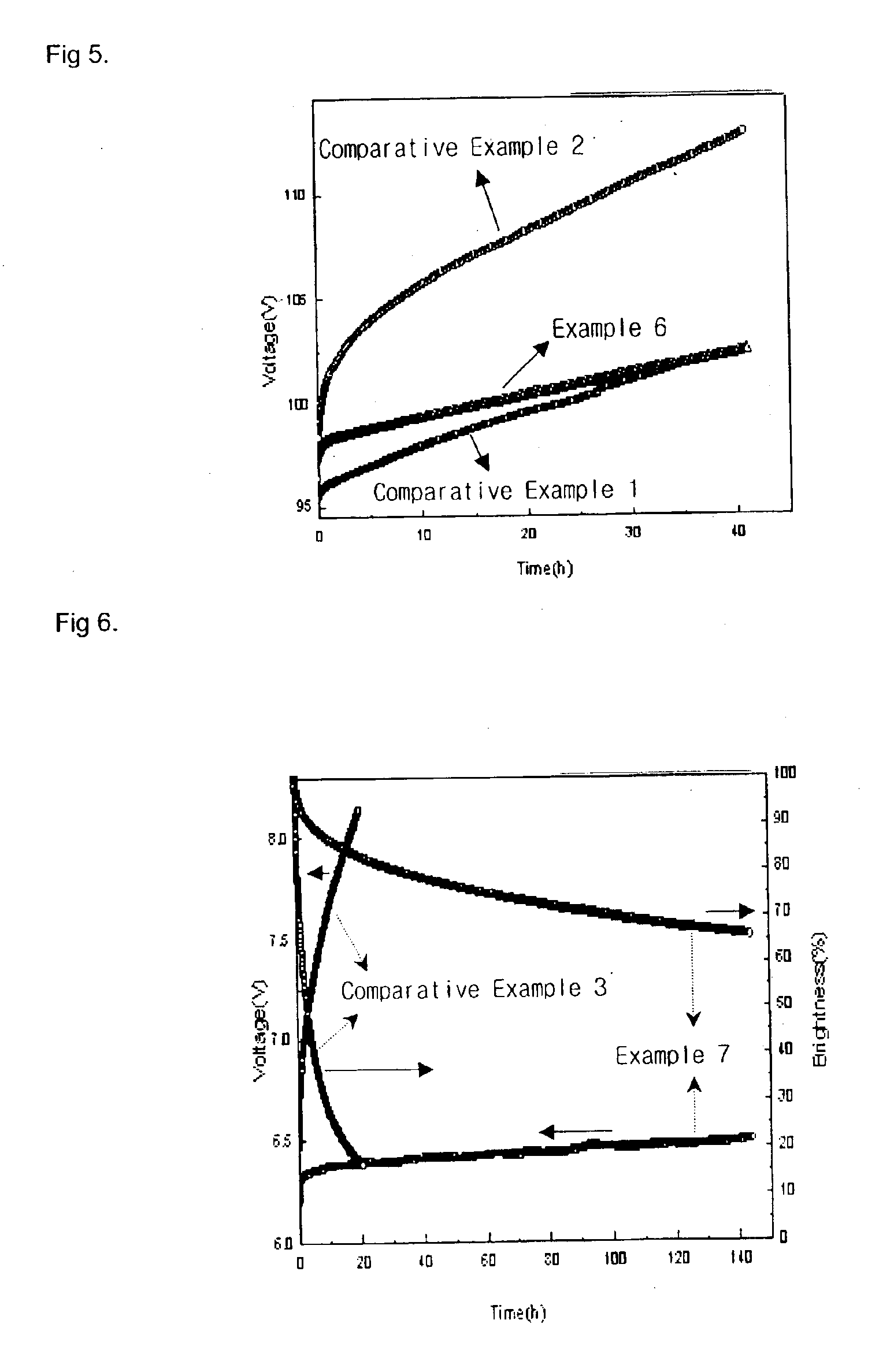
Material for transporting electrons and organic electroluminescent display using the same
InactiveUS6878469B2Improve the display effectImprove efficiencyOrganic chemistryDischarge tube luminescnet screensAnthraceneElectron injection
Novel materials for electron injection / transportation and emitting layers can greatly improve the stability of an organic electroluminescent display. Electroluminescent displays incorporating these materials produce blue light at low voltage levels. These novel organic materials include compounds in which 1 to 2 imidazole functional groups are introduced in the 2 or 2,6-site of 9,10 substituted anthracene. An organic electroluminescent display with an organic compound layer of these materials has high efficiency, thermal stability, operationally stability and maintains driving voltage before and after operation.
Owner:LG CHEM LTD
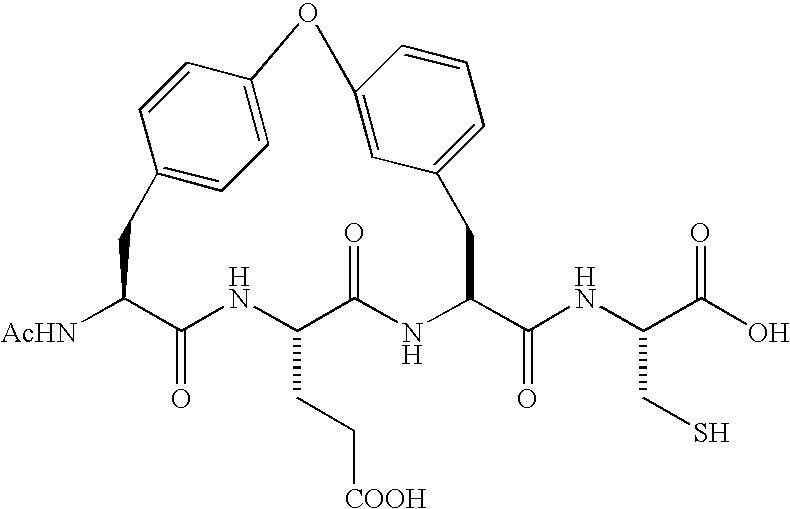
Imidazolidinones as NS3-serine protease inhibitors of hepatitis C virus
InactiveUS6838475B2Reduce frictionReduce wearBiocideOrganic active ingredientsSerine Protease InhibitorsHepacivirus
The present invention discloses novel imidazolidinones which have HCV protease inhibitory activity as well as methods for preparing such compounds. In another embodiment, the invention discloses pharmaceutical compositions comprising such imidazolidinones as well as methods of using them to treat disorders associated with the HCV protease.
Owner:SCHERING CORP
Cinnamide compound
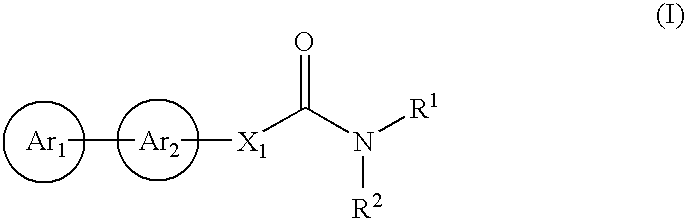
The present invention relates to a compound represented by Formula (I): (wherein Ar1 represents an imidazolyl group which may be substituted with 1 to 3 substituents; Ar2 represents a pyridinyl group, a pyrimidinyl group, or a phenyl group which may be substituted with 1 to 3 substituents; X1 represents (1) —C≡C— or (2) a double bond etc. which may be substituted; R1 and R2 represent, for example, a C1-6 alkyl group or C3-8 cycloalkyl group which may be substituted) or a pharmacologically acceptable salt thereof and to the use thereof as pharmaceutical agents. The object of the present invention is to find a therapeutic or preventive agent for diseases caused by Aβ. According to the present invention, a therapeutic or preventive agents for diseases caused by Aβ can be provided.
Owner:EISIA R&D MANAGEMENT CO LTD
Pharmaceutical co-crystal compositions
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphonic acid, phosphinic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, sp2 amine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, s-heterocyclic ring, thiophene, n-heterocyclic ring, pyrrole, o-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES +2
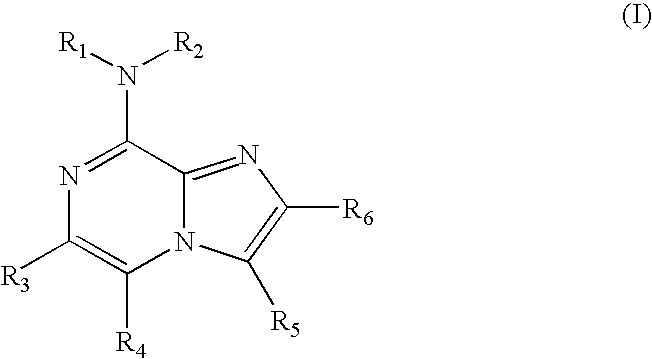
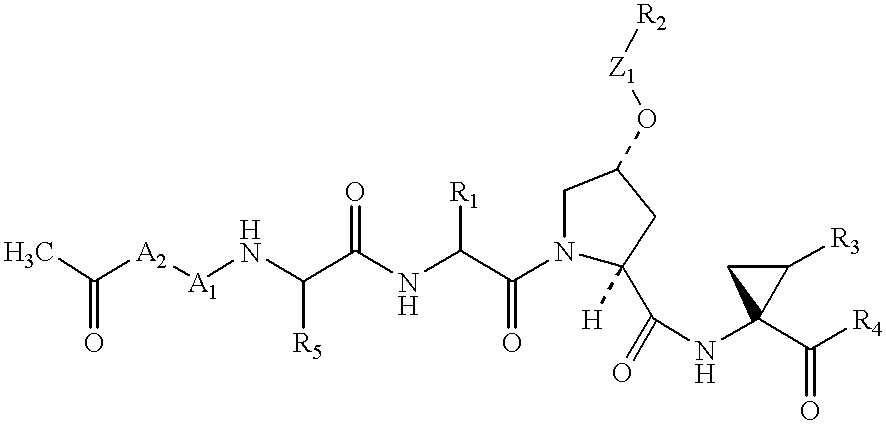
Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds
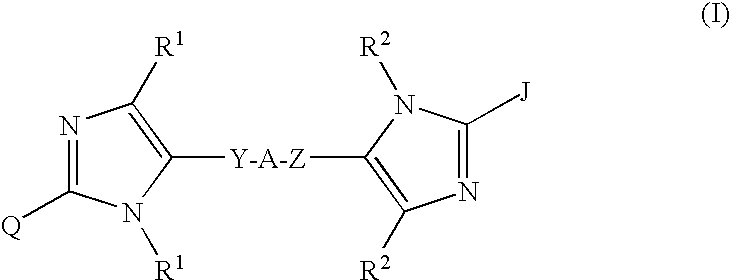
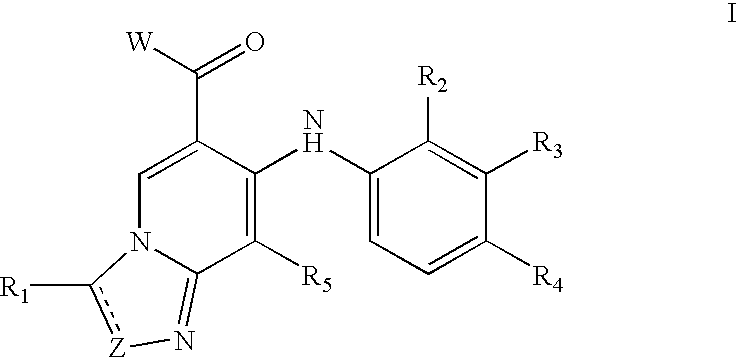
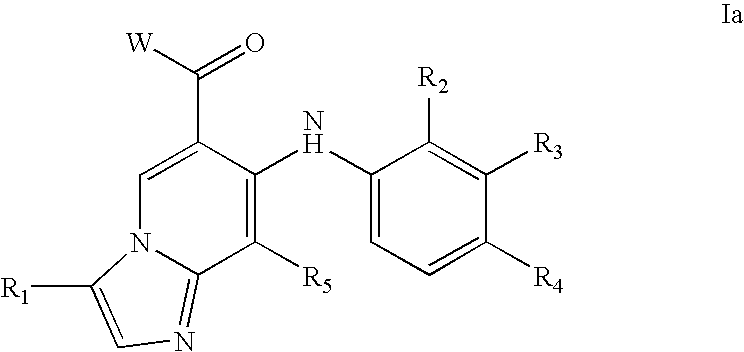
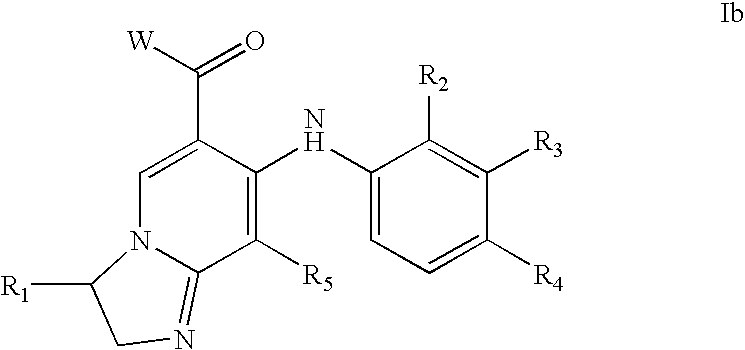
Compounds of Formula I-a and all pharmaceutically-acceptable forms thereof, are described herein. The variables R1, R2, R3, Z1, Q, and A shown in Formula I-a are defined herein. Pharmaceutical compositions containing one or more compounds of Formula I-a, or a pharmaceutically acceptable form of such compounds, and one or more pharmaceutically acceptable carriers, excipients, or diluents are provided herein. Methods of treating patients suffering from certain diseases responsive to inhibition of tyrosine kinase activity are also given. In certain embodiments the diseases are responsive to inhibition of Btk activity and / or B-cell proliferation. Such methods comprise administering to such patients an amount of a compound of Formula I-a effective to reduce signs or symptoms of the disease. These diseases include cancer, an autoimmune and / or inflammatory disease, or an acute inflammatory reaction. Thus methods of treatment include administering a sufficient amount of a compound or salt as provided herein to decrease the symptoms or slow the progression of these diseases. Other embodiments include methods of treating other animals, including livestock and domesticated companion animals, suffering from a disease responsive to inhibition of kinase activity. Methods of treatment include administering a compound of Formula I-a as a single active agent or administering a compound of Formula I-a in combination with one or more other therapeutic agent. A method for determining the presence of Btk in a sample, comprising contacting the sample with a compound or form thereof of Formula I-a under conditions that permit detection of Btk activity, detecting a level of Btk activity in the sample, and therefrom determining the presence or absence of Btk in the sample.
Owner:GILEAD CONNENTICUT INC
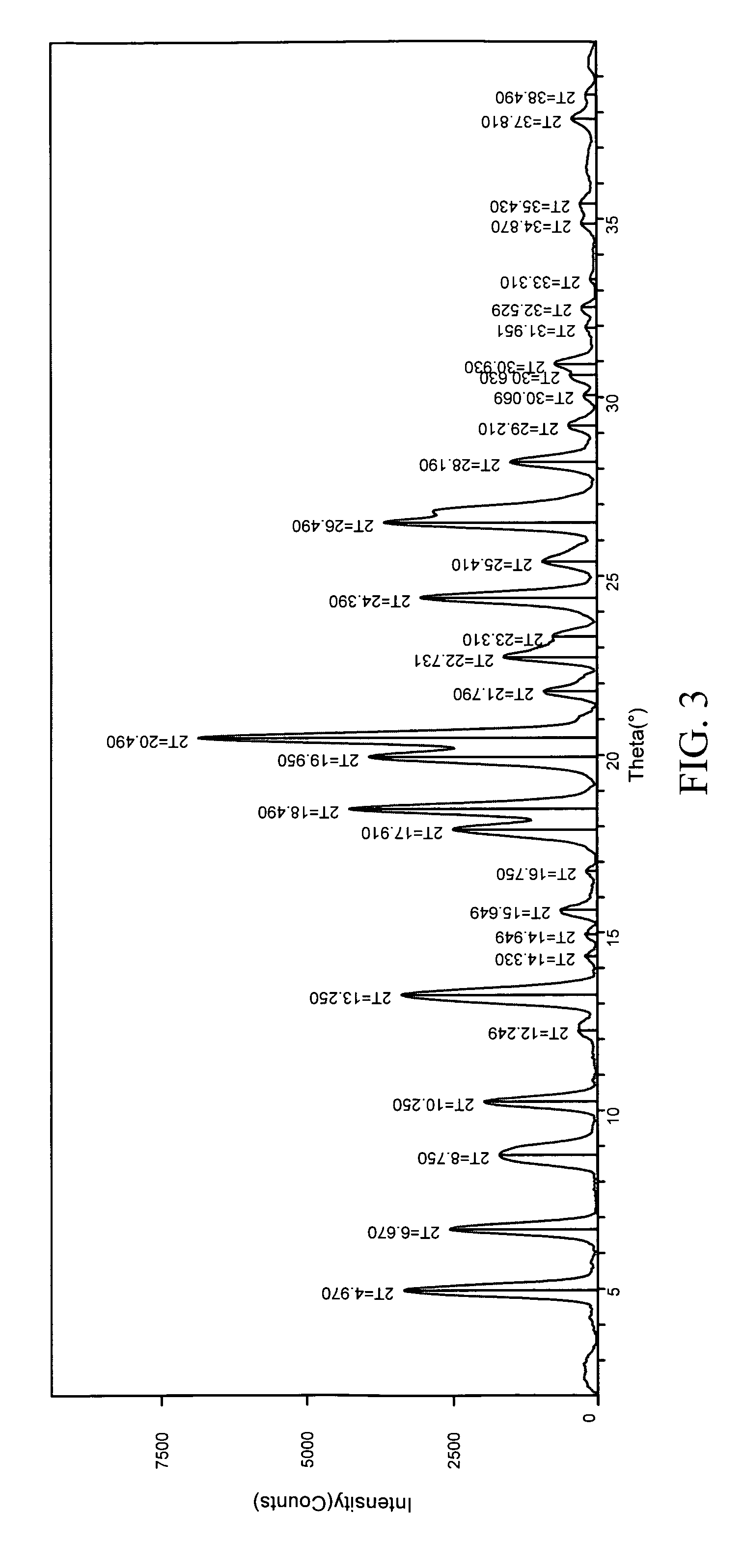
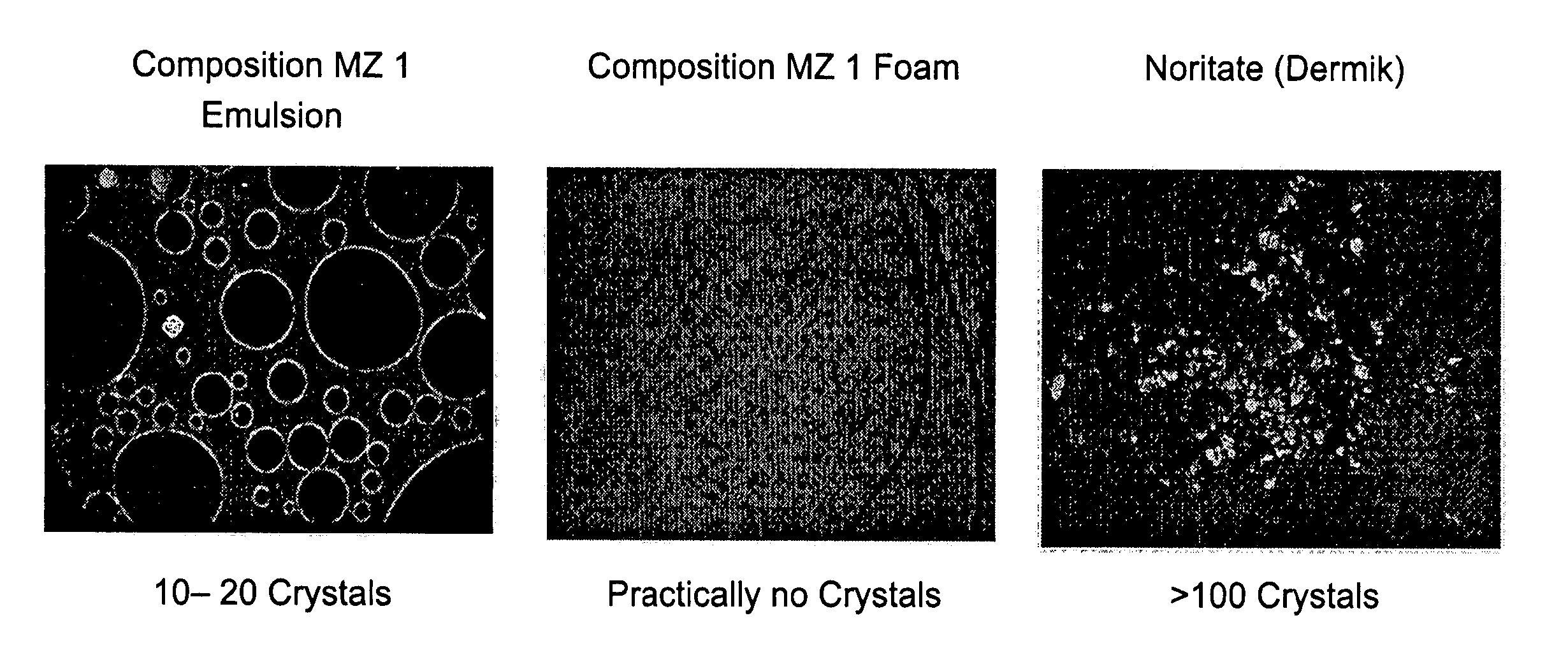
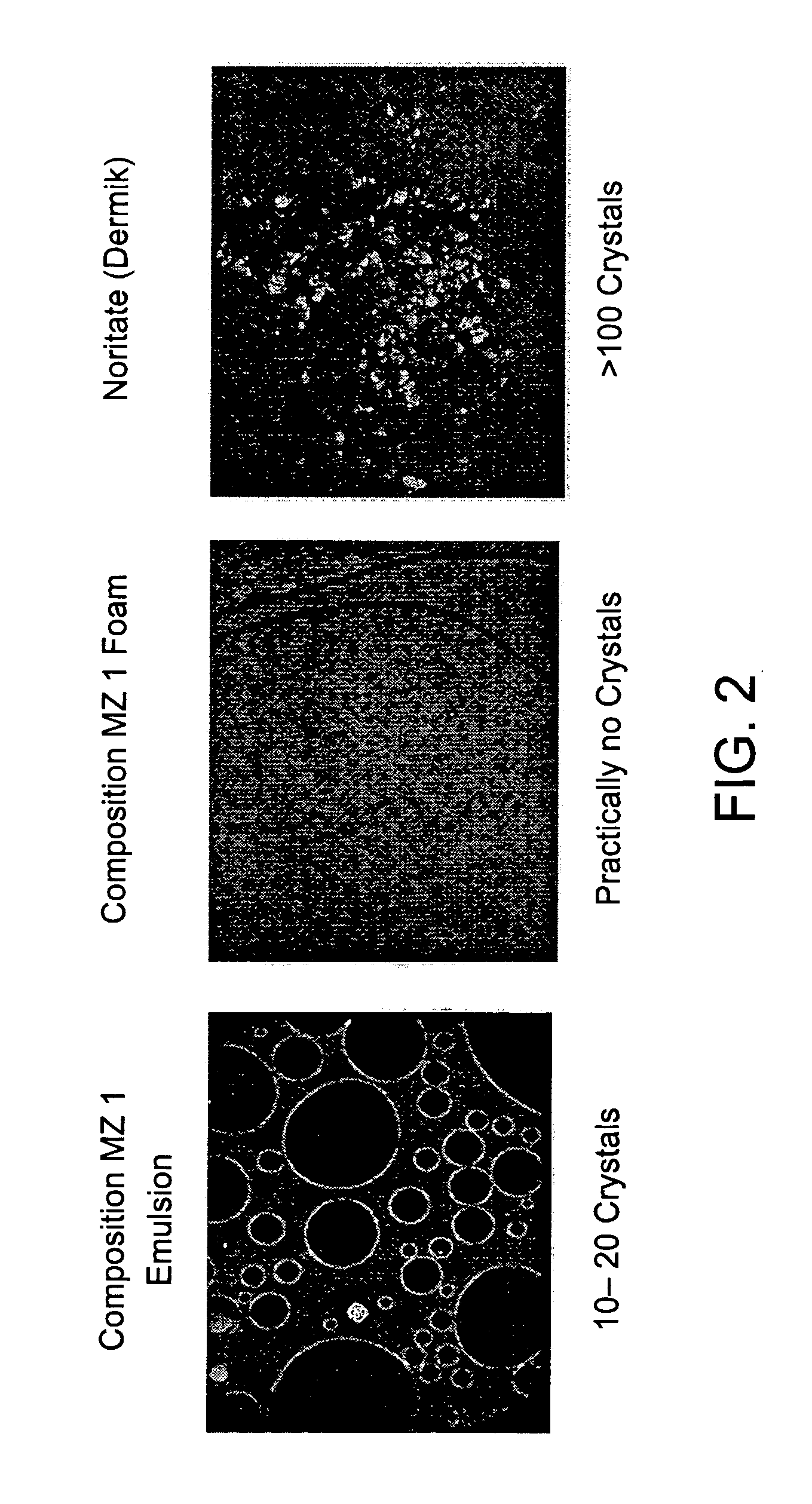
Kit and composition of imidazole with enhanced bioavailability
A composition and therapeutic kit provide a therapeutic azole with increased solubility. The kit includes an aerosol packaging assembly containing a container accommodating a pressurized product and an outlet capable of releasing the pressurized product as a foam. The pressurized product includes a foamable composition including: i. a therapeutic azole, wherein the solubility of the azole in the composition before foaming is less than the solubility of the azole in the composition after foaming; ii. at least one organic carrier selected from the group consisting of a hydrophobic organic carrier, a co-solvent, an emollient and mixtures thereof, at a concentration of about 2% to about 50% by weight; iii. a surface-active agent; iv. about 0.01% to about 5% by weight of at least one polymeric additive selected from the group consisting of a bioadhesive agent, a gelling agent, a film forming agent and a phase change agent; v. water; and vi. liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition.
Owner:FOAMIX PHARMACEUTICALS LIMITED
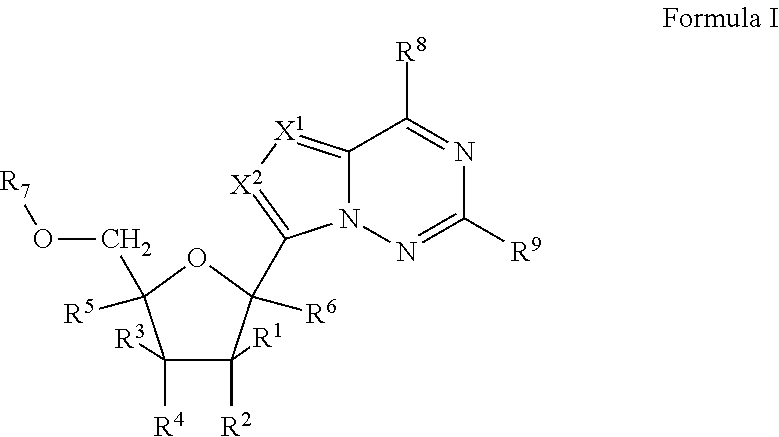
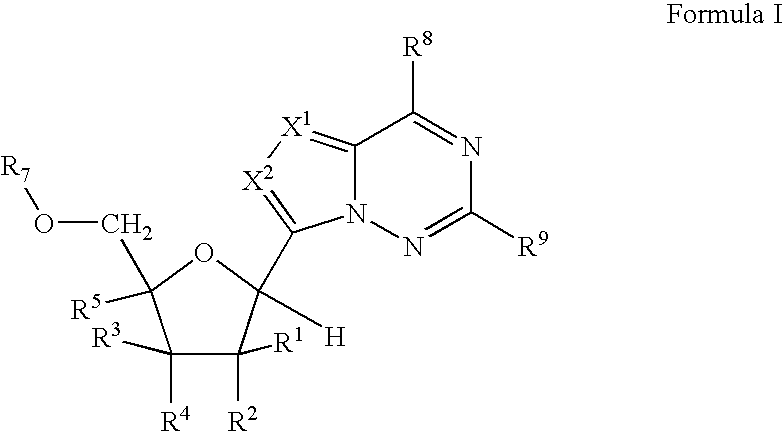
2'-fluoro substituted carba-nucleoside analogs for antiviral treatment
Provided are select imidazo[1,2-f][1,2,4]triazinyl nucleosides, nucleoside phosphates and prodrugs thereof, wherein the 2′ position of the nucleoside sugar is substituted with halogen and carbon substituents. The compounds, compositions, and methods provided are useful for the treatment of Flaviviridae virus infections, particularly hepatitis C infections caused by both wild type and mutant strains of HCV.
Owner:GILEAD SCI INC
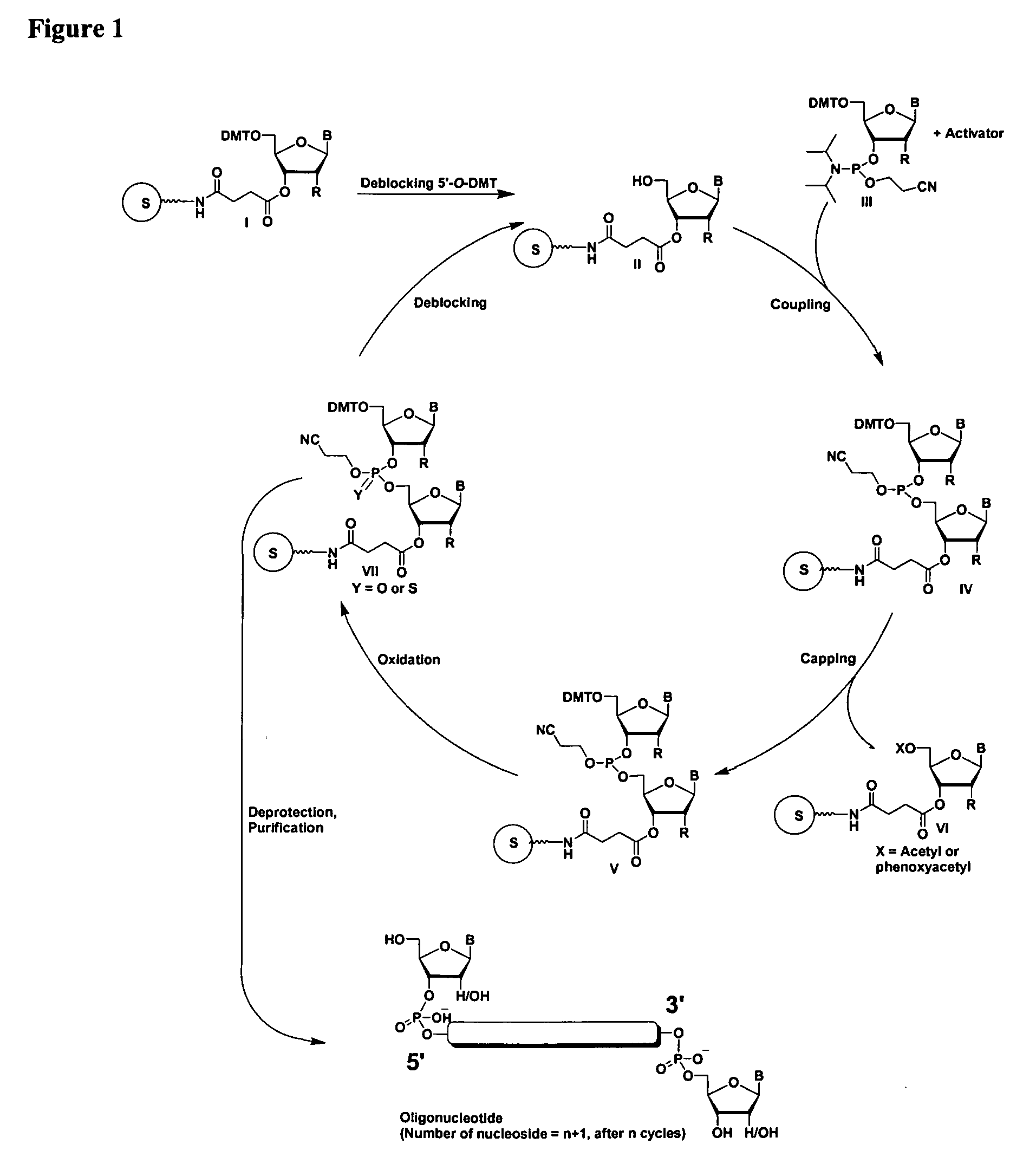
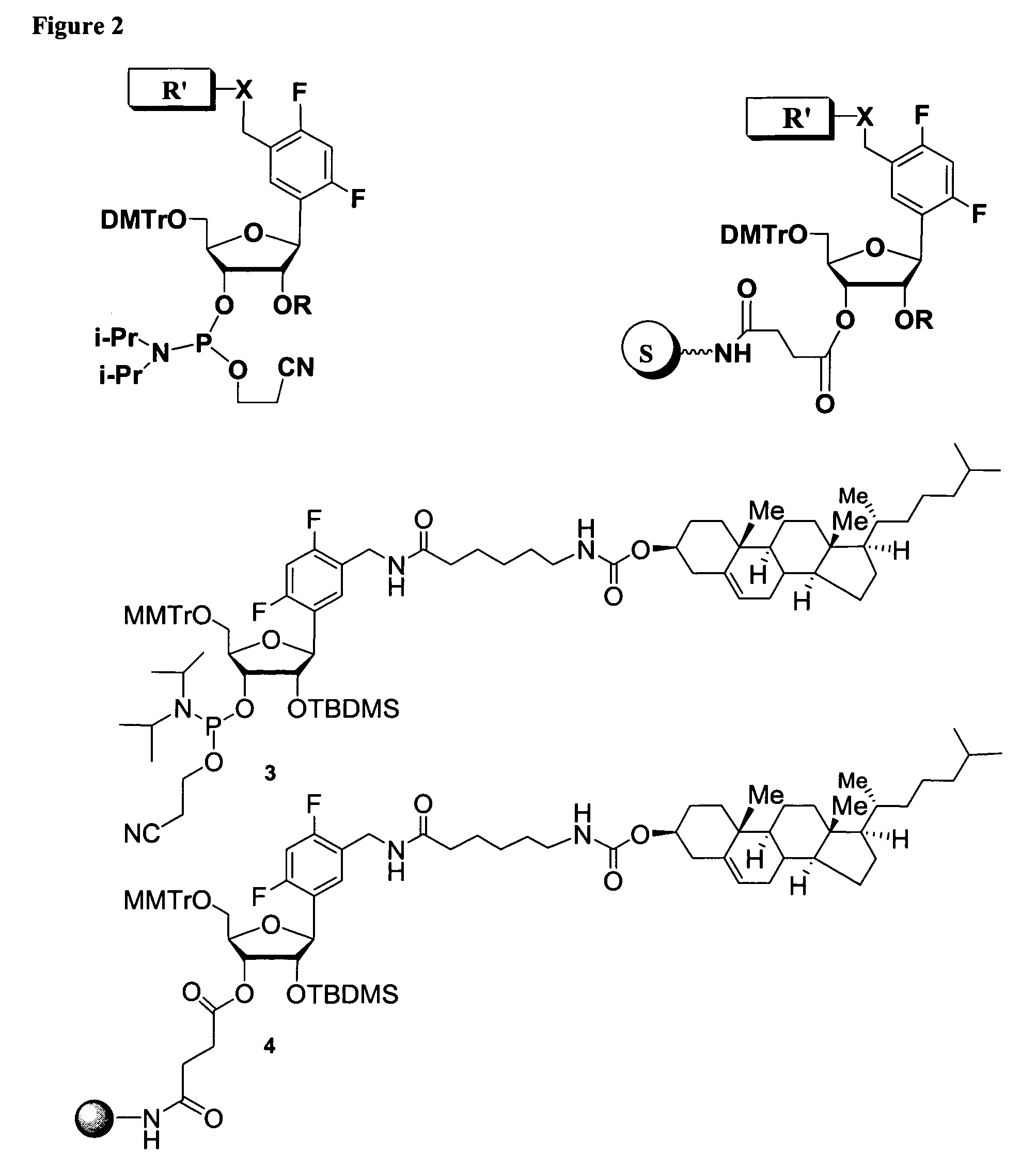
Oligonucleotides comprising a ligand tethered to a modified or non-natural nucleobase
ActiveUS20070054279A1Organic active ingredientsSugar derivativesDouble strandedSingle stranded oligonucleotides
One aspect of the present invention relates to a double-stranded oligonucleotide comprising at least one ligand tethered to an altered or non-natural nucleobase. In certain embodiments, the non-natural nucleobase is difluorotolyl, nitropyrrolyl, or nitroimidazolyl. In certain embodiments, the ligand is a steroid or aromatic compound. In certain embodiments, only one of the two oligonucleotide strands comprising the double-stranded oligonucleotide contains a ligand tethered to an altered or non-natural nucleobase. In certain embodiments, both of the oligonucleotide strands comprising the double-stranded oligonucleotide independently contain a ligand tethered to an altered or non-natural nucleobase. In certain embodiments, the oligonucleotide strands comprise at least one modified sugar moiety. Another aspect of the present invention relates to a single-stranded oligonucleotide comprising at least one ligand tethered to an altered or non-natural nucleobase. In certain embodiments, the non-natural nucleobase is difluorotolyl, nitropyrrolyl, or nitroimidazolyl. In certain embodiments, the ligand is a steroid or aromatic compound. In certain embodiments, the ribose sugar moiety that occurs naturally in nucleosides is replaced with a hexose sugar, polycyclic heteroalkyl ring, or cyclohexenyl group. In certain embodiments, at least one phosphate linkage in the oligonucleotide has been replaced with a phosphorothioate linkage.
Owner:ALNYLAM PHARM INC
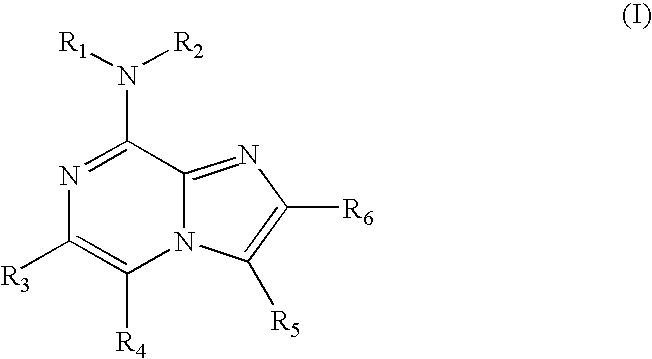
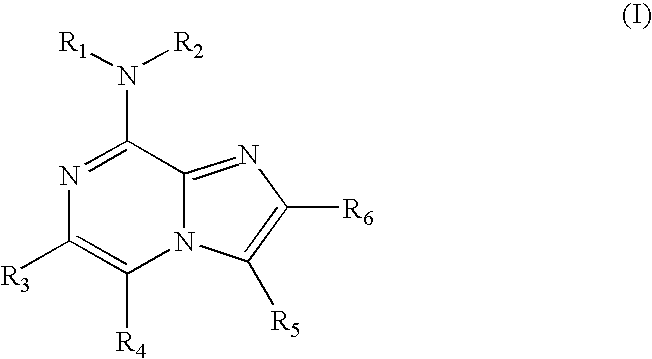
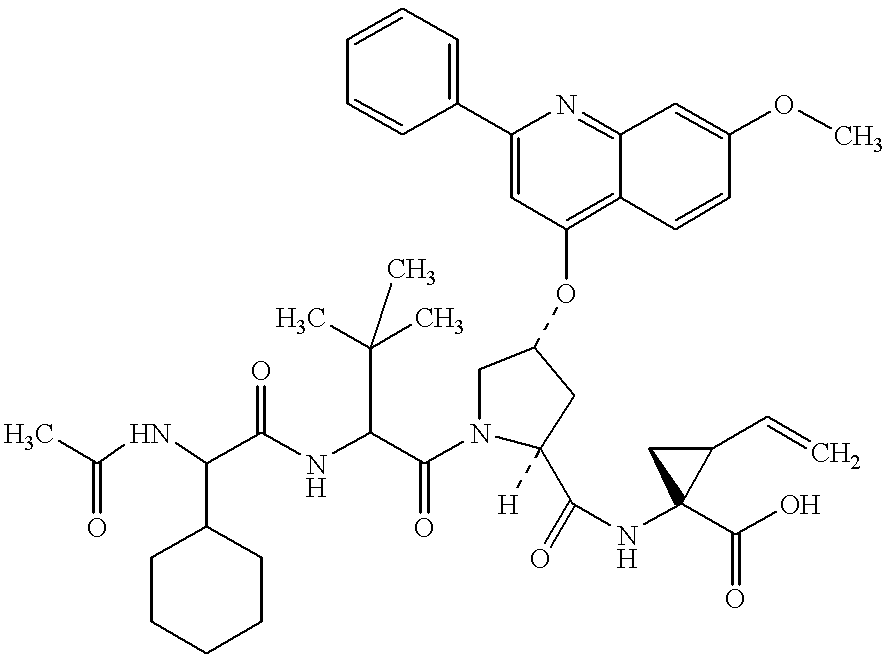
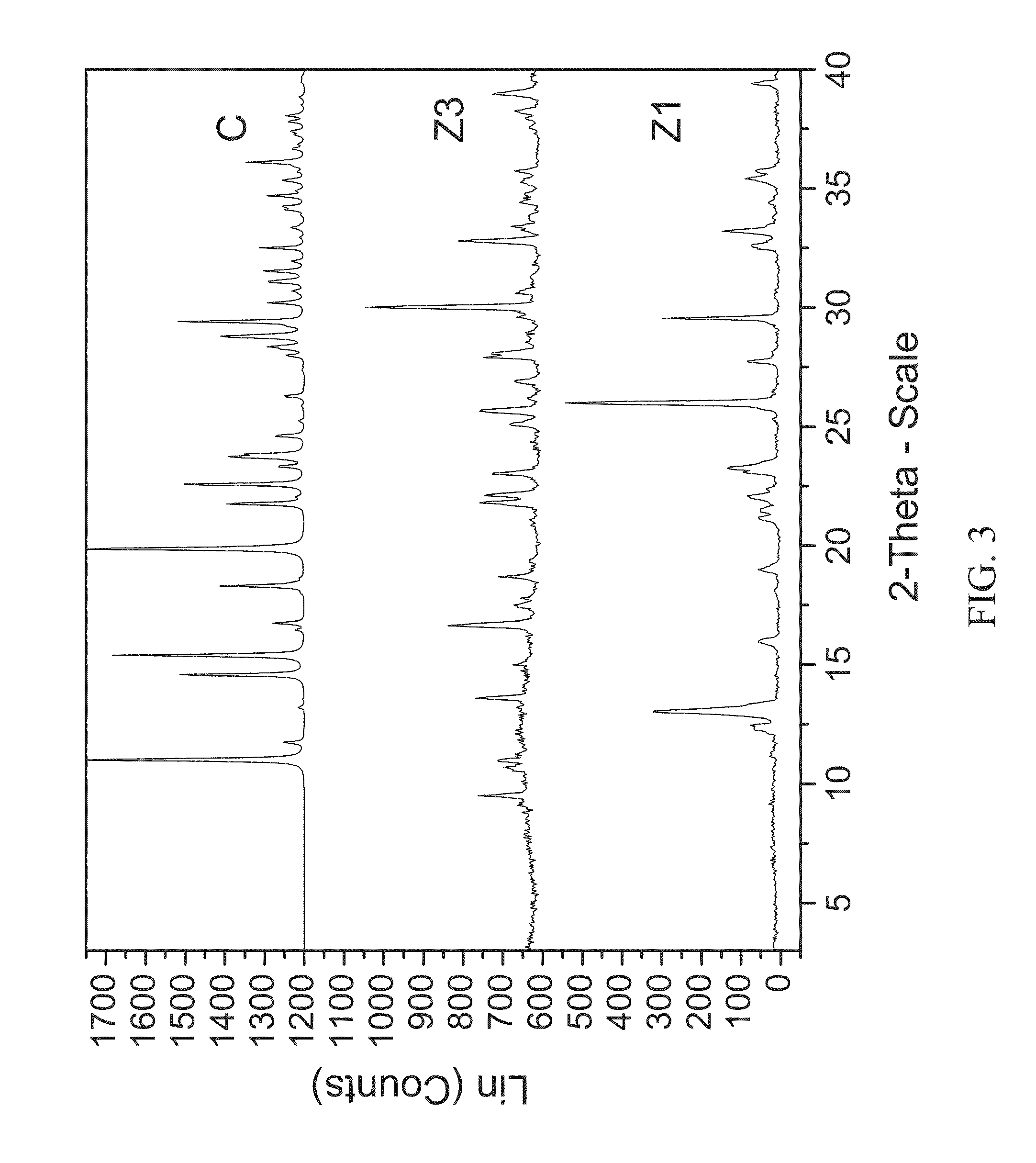
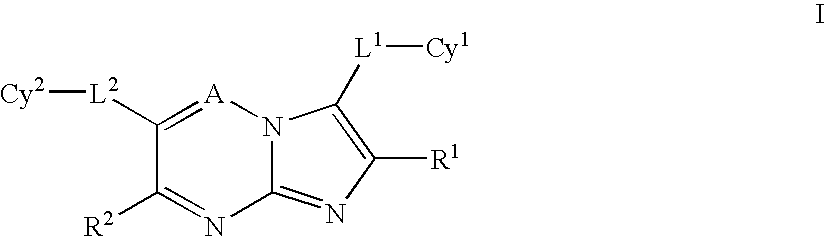
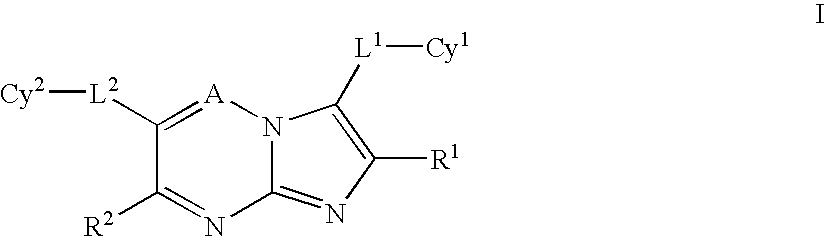
Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds
ActiveUS7393848B2Prevent proliferationInhibit the activity of BtkBiocideOrganic chemistryActive agentBruton's tyrosine kinase
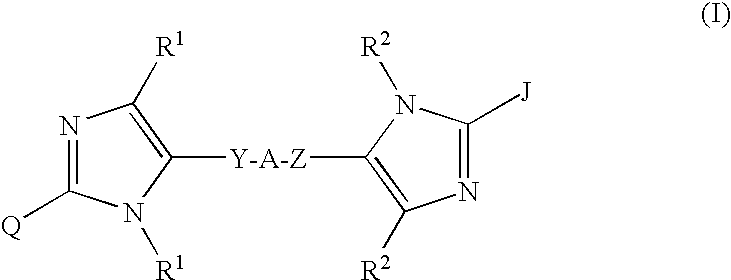
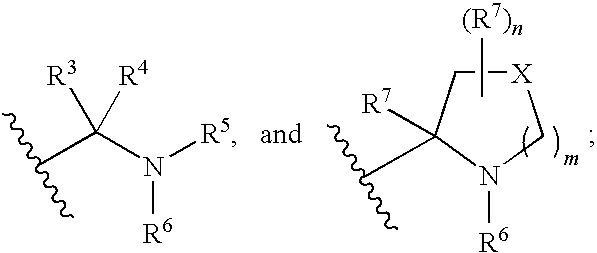
Compounds of Formula Iand all pharmaceutically acceptable forms thereof, are described herein.The variables R1, R2, R3, Z2, and Q, shown in Formula I are defined herein.Pharmaceutical compositions containing one or more compounds of Formula I, or a pharmaceutically acceptable form of such compounds, and one or more pharmaceutically acceptable carriers, excipients, or diluents are provided herein.Methods of treating patients suffering from certain diseases responsive to inhibition of tyrosine kinase activity are also given. In certain embodiments the diseases are responsive to inhibition of Btk activity and / or B-cell proliferation. Such methods comprise administering to such patients an amount of a compound of Formula I effective to reduce signs or symptoms of the disease. These diseases include cancer, an autoimmune and / or inflammatory disease, or an acute inflammatory reaction. Thus methods of treatment include administering a sufficient amount of a compound or salt as provided herein to decrease the symptoms or slow the progression of these diseases.Other embodiments include methods of treating other animals, including livestock and domesticated companion animals, suffering from a disease responsive to inhibition of kinase activity.Methods of treatment include administering a compound of Formula I as a single active agent or administering a compound of Formula I in combination with one or more other therapeutic agent.A method for determining the presence of Btk in a sample, comprising contacting the sample with a compound or form thereof of Formula I under conditions that permit detection of Btk activity, detecting a level of Btk activity in the sample, and therefrom determining the presence or absence of Btk in the sample.
Owner:GILEAD CONNENTICUT INC
Imidazole derivatives having an inhibitory activity for farnesyl transferase and process for preparation thereof
InactiveUS6268363B1Inhibition is effectiveInhibitory activityOrganic chemistryUrinary disorderFarnesyl Protein TransferaseBULK ACTIVE INGREDIENT
The present invention relates to a novel imidazole derivative represented by formula (1) which shows an inhibitory activity against farnesyl transferase or pharmaceutically acceptable salts or isomers thereof, in which A, n1 and Y are defined in the specification; to a process for preparation of the compound of formula (1); to intermediates which are used in the preparation of the compound of formula (1); and to a pharmaceutical composition comprising the compound of formula (1) as an active ingredient.
Owner:LG CHEM LTD
Pharmaceutical co-crystal compositions
InactiveUS20070026078A1Improve solubilityLow hygroscopicityBiocidePowder deliveryThioketoneHydroxamic acid
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphonic acid, phosphinic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, sp2 amine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, s-heterocyclic ring, thiophene, n-heterocyclic ring, pyrrole, o-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES +2
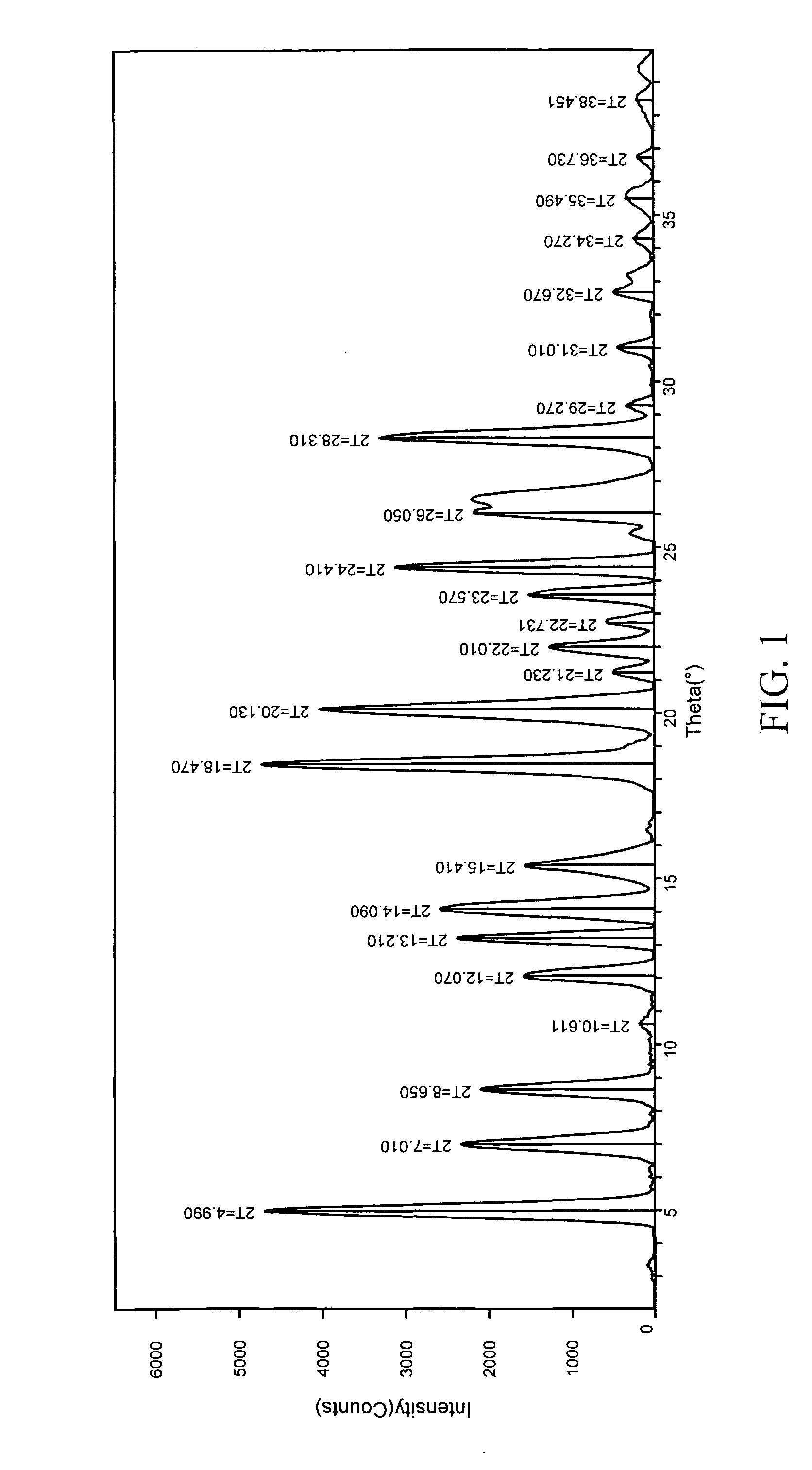
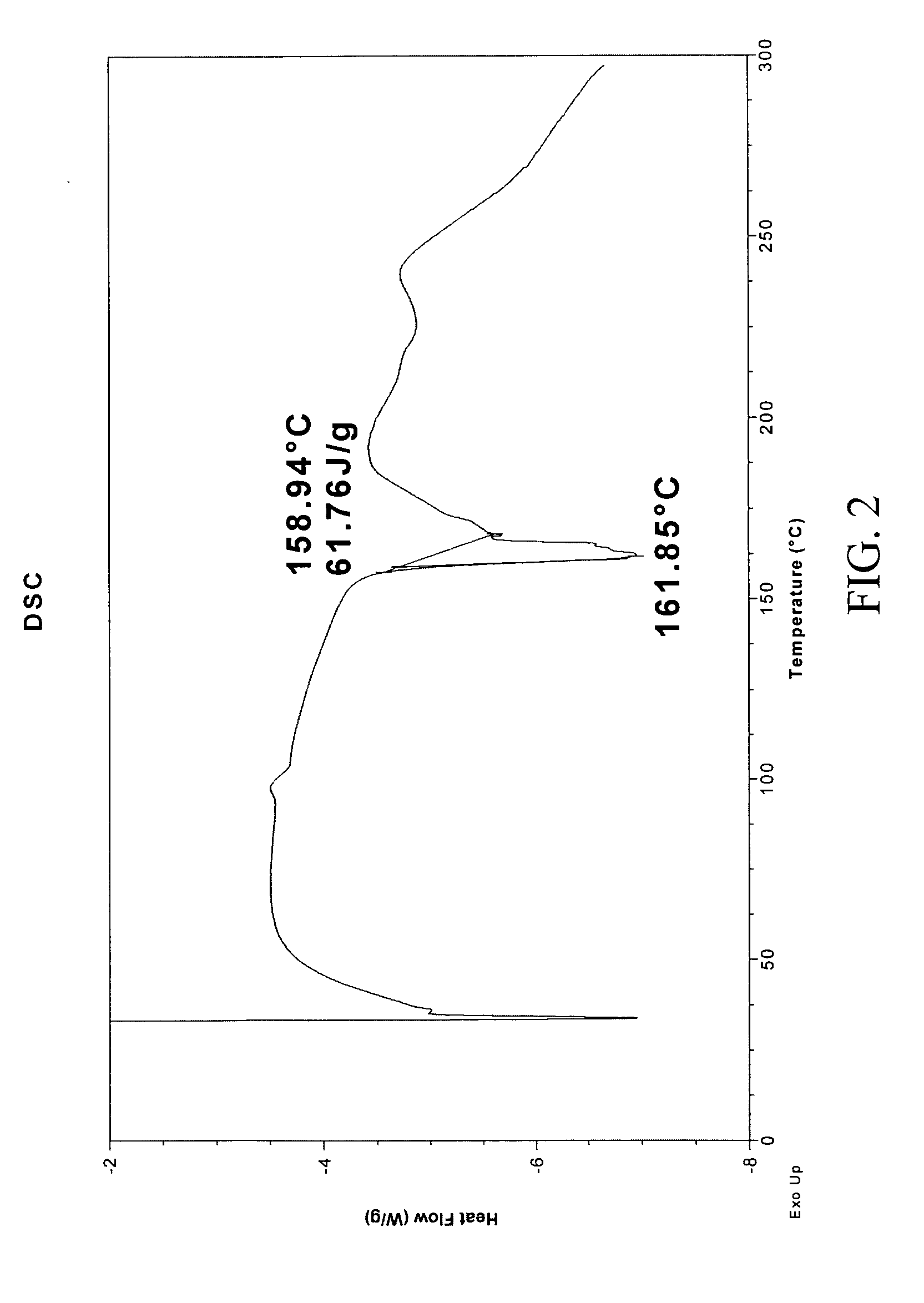
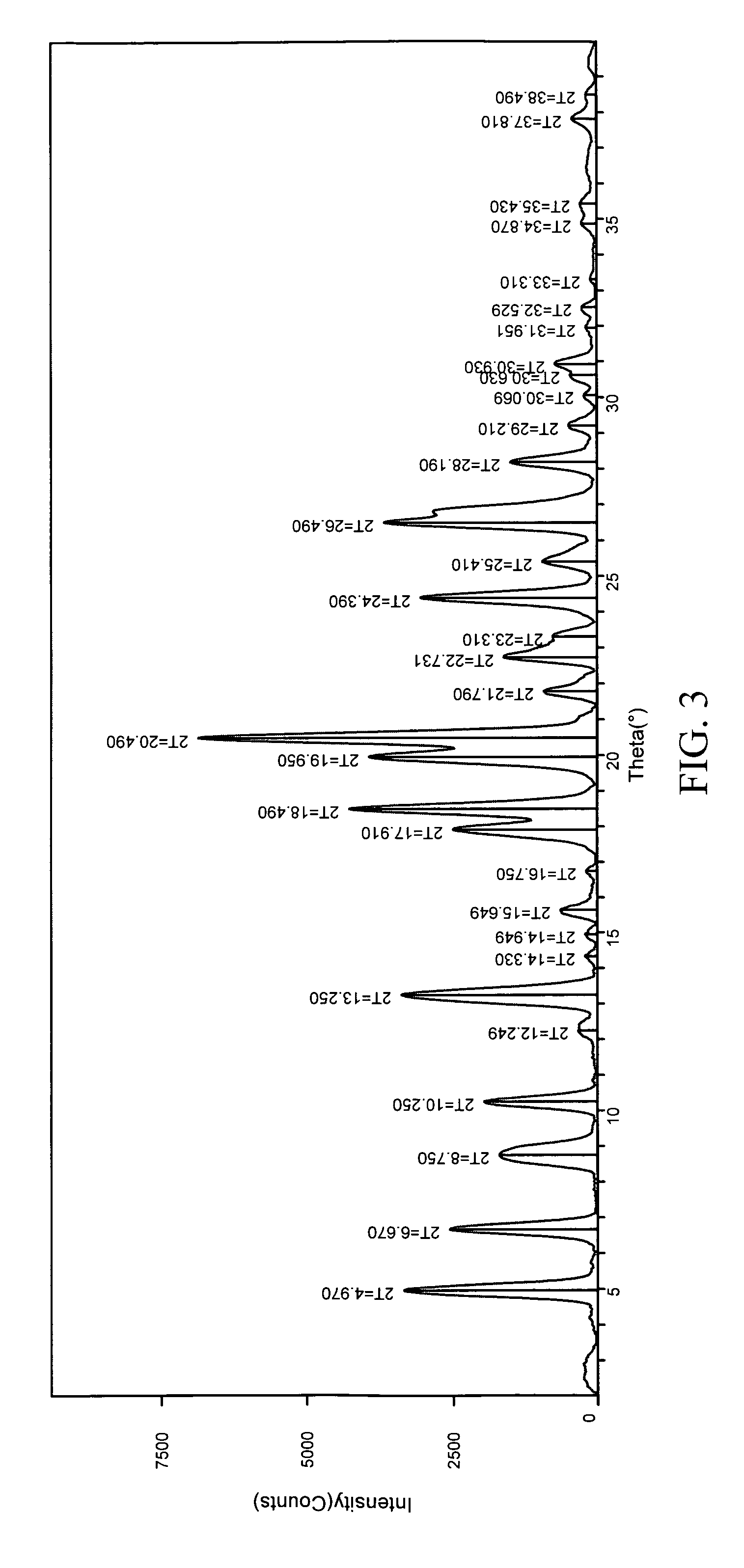
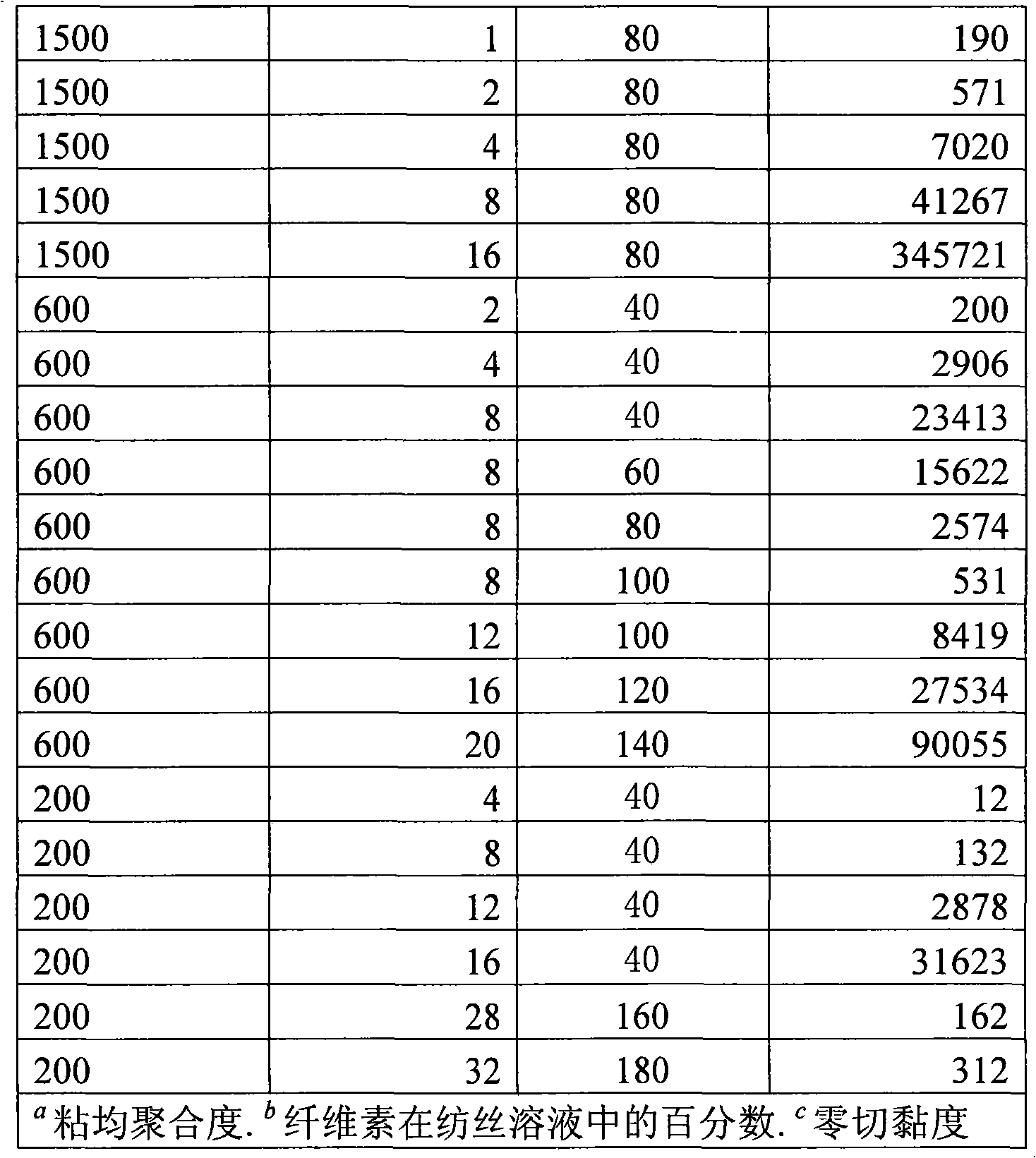
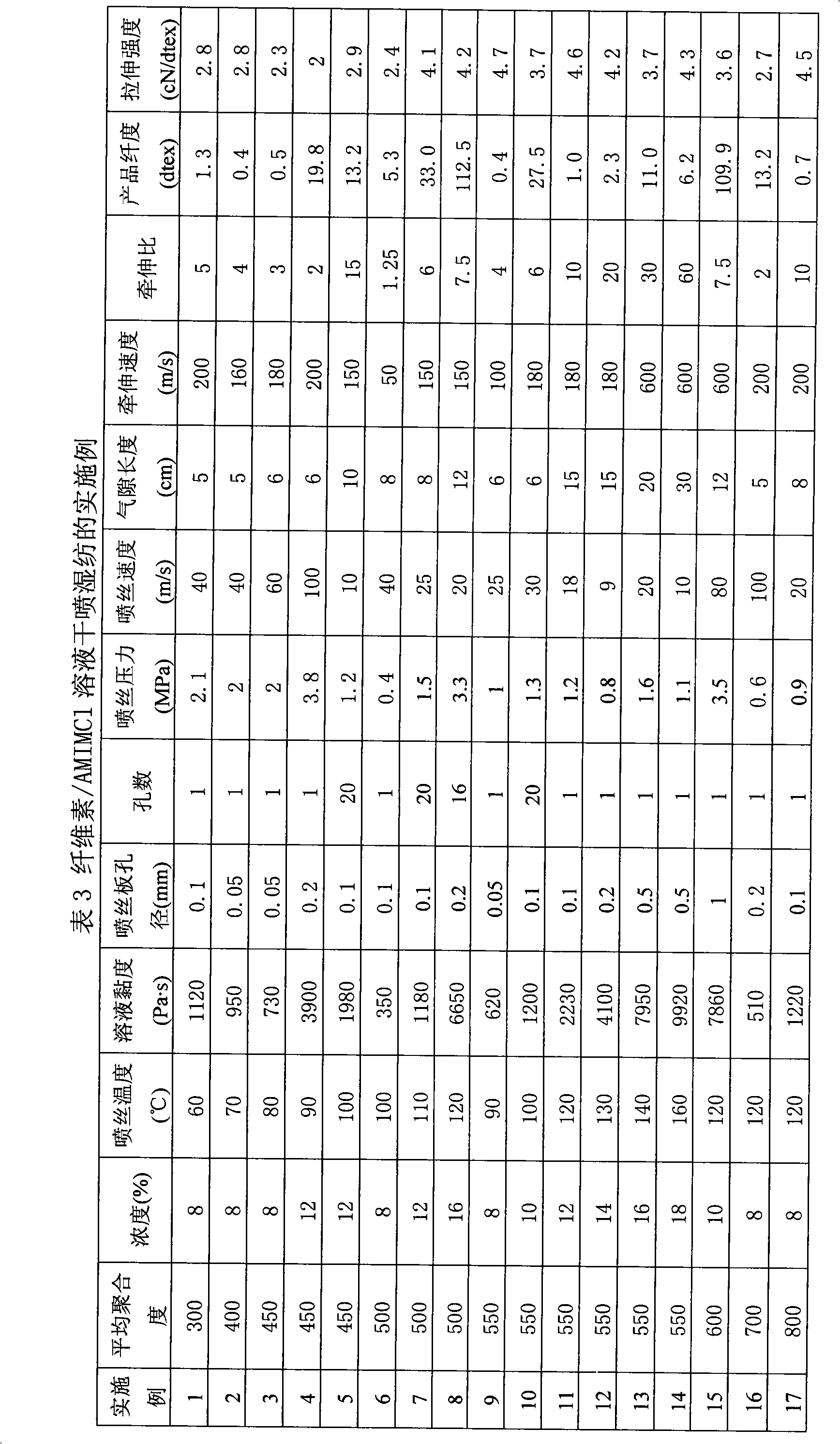
Method for continuously preparing regenerated cellulose fibre
InactiveCN101328626AReduce manufacturing costReduce the temperatureArtificial filament recoveryFibre treatmentPolymer scienceTetrafluoroborate
The invention discloses a method for continuously preparing regenerated cellulose fibers through the solvent method, comprising the following steps that: a cellulose raw material is dissolved into an ion liquid to prepare a spinning liquid; gel type regenerated cellulose fibers are obtained through spinning; and the regenerated cellulose fibers are obtained through cleaning, rear draft and drying, wherein, the ion liquid is selected from one or a plurality among the following ion liquids: a). an ion liquid with 1, 3-dialkyl imidazole as a cation and formiate radical, radical vinegar or propionate radical as an anion; and b). an ion liquid with 1-R1-3-R2- dialkyl imidazole as the cation and chlorine, bromine, iodine, formiate radical, radical vinegar, sulfate radical, nitrate radical, tetrafluoroborate radical, thiocyanate radical, hexafluorophosphate radical, p-toluenesulfonate radical or trifluoromethanesulfonic acid radical as the anion. The method has the advantages of wide technological range, mild temperature condition, adequate pressure, quick spinning speed and so on, can prepare the regenerated cellulose fibers with superior performance and complete specifications, and has low production cost, high production efficiency and wide application prospect.
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
Imidazopyridines and triazolopyridines
The present invention relates to imidazopyridine and triazolopyridine derivatives, pharmaceutical compositions and methods of use thereof.
Owner:WARNER-LAMBERT CO
Novel Imidazo [4,5-b] Pyridine Derivatives as Inhibitors of Glycogen Synthase Kinase 3 for Use in the Treatment of Dementia and Neurodegenerative Disorders
Owner:ASTRAZENECA AB
Dihydrospiro[dibenzo[a,d][7]annulene-5,4'-imidazol] compounds for the inhibition of beta-secretase
Owner:WYETH
Carba-nucleoside analogs for antiviral treatment
Provided are imidazo[1,5-f][1,2,4]triazinyl, imidazo[1,2-f][1,2,4]triazinyl, and [1,2,4]triazolo[4,3-f][1,2,4]triazinyl nucleosides, nucleoside phosphates and prodrugs thereof. The compounds, compositions, and methods provided are useful for the treatment of Flaviviridae virus infections, particularly hepatitis C infections.
Owner:GILEAD SCI INC
Imidazo[1,2-beta]pyridazine and pyrazolo[1,5-alpha]pyrimidine derivatives and their use as protein kinase inhibitors
ActiveUS7750007B2Inhibitory activityBiocideOrganic chemistryProtein kinase inhibitor activityPTK Inhibitors
The present invention provides protein kinase inhibitors comprising imidazo[1,2-b]pyridazine and pyrazolo[1,5-a]pyrimidine compounds of the following structure (I) and (II):or a stereoisomer, prodrug or pharmaceutically acceptable salt thereof, wherein R, R1, R2 and X are as defined herein. Compositions and methods for using the same in the treatment of cancer and other Pim kinase-associated conditions are also disclosed.
Owner:SUMITOMO PHARMA ONCOLOGY INC
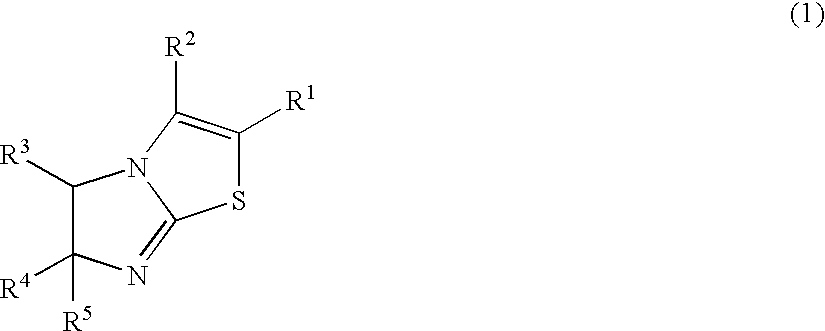
Imidazothiazole derivatives
There is provided a novel compound that inhibits interaction between murine double minute 2 (Mdm2) protein and p53 protein and exhibits anti-tumor activity. The present invention provides an imidazothiazole derivative represented by the following formula (1) having various substituents that inhibits interaction between Mdm2 protein and p53 protein and exhibits anti-tumor activity:wherein R1, R2, R3, R4, and R5 in the formula (1) each has the same meaning as defined in the specification.
Owner:DAIICHI SANKYO CO LTD
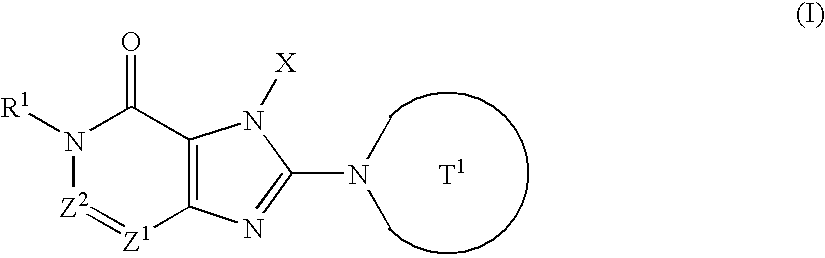
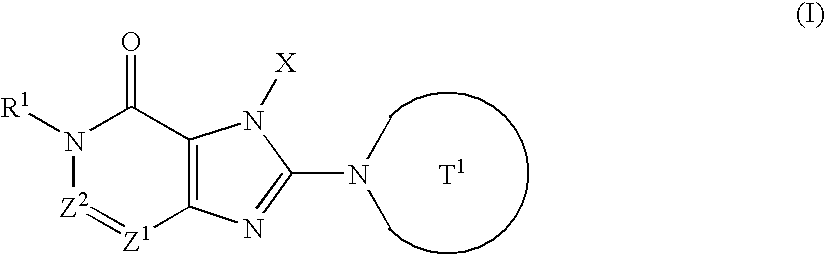
Novel condensed imidazole derivatives
The present invention is related to compounds represented by the following formula, or salts or hydrates thereof wherein, T1 represents a 4- to 12-membered heterocyclic group containing one or two nitrogen atoms in the ring, which is a monocyclic or bicyclic structure that may have one or more substituents; X represents a C1-6 alkyl group which may have one or more substituents, or such; Z1 and Z2 each independently represent a nitrogen atom or a group represented by the formula —CR2—; R1 and R2 independently represent a hydrogen atom, a C1-6 alkyl group which may have one or more substituents, or a C1-6 alkoxy group which may have one or more substituents, or such. These are novel compounds that exhibit an excellent
Owner:EISIA R&D MANAGEMENT CO LTD
Multi-cyclic cinnamide derivatives
InactiveUS20070219181A1Improve efficacyInhibit productionBiocideNervous disorderDiseaseCompound (substance)
The present invention provides a compound represented by the formula (I): or a pharmacologically acceptable salt thereof, wherein Ar1 represents an imidazolyl group that may be substituted with a C1-6 alkyl group, or the like, Ar2 represents a phenyl group that may be substituted with a C1-6 alkoxy group, or the like, X1 represents a double bond or the like, and Het represents an imidazolyl group that may be substituted with a C1-6 alkyl group, or the like, which is effective as a therapeutic or prophylactic agent for a disease caused by Aβ.
Owner:EISIA R&D MANAGEMENT CO LTD
Room temperature ionic liquid containing unsaturated double bond and its prepn and application
InactiveCN1417407AGroup 5/15 element organic compoundsPulping with organic solventsSulfate radicalsTriflic acid
The room temperature ionic liquid containing unsaturated double bond has the general expressino of A+B-, where A+ contains R1 being hydroxyl with 1-4 carbon atoms and R2 containing 2-20 carbon atoms and at least one double bond; and B- is one of anions, including chlorate radical, bromate radical, iodate radical, acetate radical, sulfate radical, nitrate radical, tetrafluorobromate radical, etc. Its preparation is to mixture and react olefin halide R1X and N-alkyl imidazole to obtain ionic liquid dialkyl imidazolium halide. The present invention also relates to the application of the ionic liquid in dissolving cellulose and preparing cellulose derivative.
Owner:山东中科恒联生物基材料有限公司
Functionalized nanoparticles and method
ActiveUS20100283014A1Material nanotechnologyPolycrystalline material growthSemiconductor materialsChemical reaction
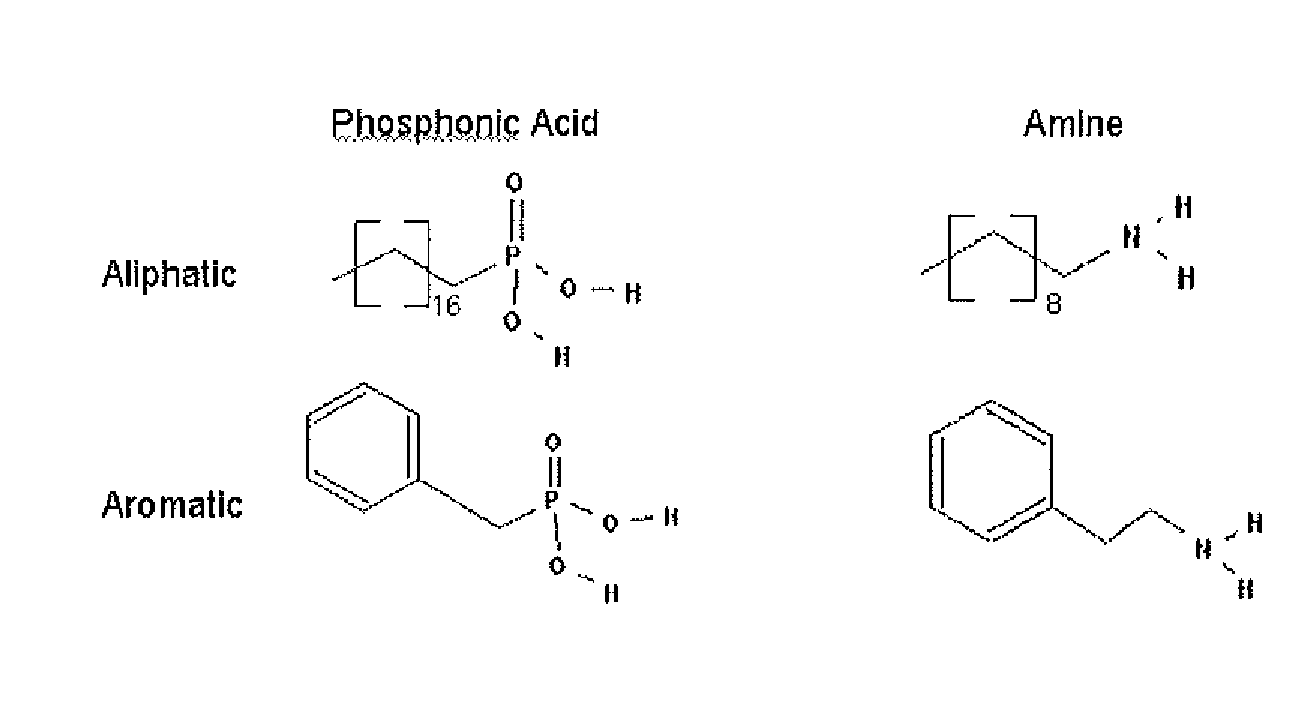
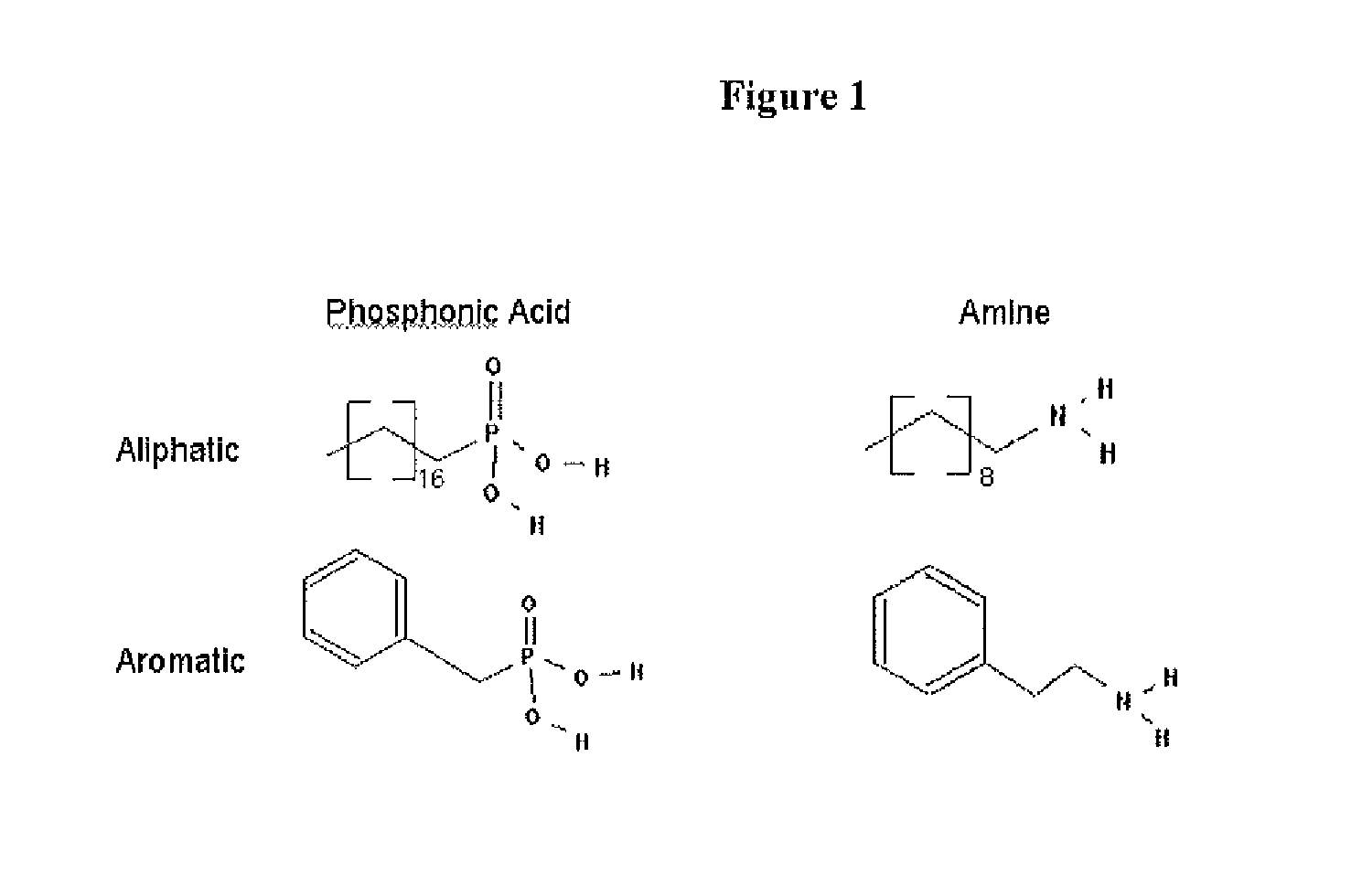
A nanoparticle including an inorganic core comprising at least one metal and / or at least one semi-conductor compound comprising at least one metal includes a coating or shell disposed over at least a portion of a surface of the core. The coating can include one or more layers. Each layer of the coating can comprise a metal and / or at least one semiconductor compound. The nanoparticle further includes a ligand attached to a surface of the coating. The ligand is represented by the formula: X-Sp-Z, wherein X represents, e.g., a primary amine group, a secondary amine group, a urea, a thiourea, an imidizole group, an amide group, a phosphonic or arsonic acid group, a phosphinic or arsinic acid group, a phosphate or arsenate group, a phosphine or arsine oxide group; Sp represents a spacer group, such as a group capable of allowing a transfer of charge or an insulating group; and Z represents: (i) reactive group capable of communicating specific chemical properties to the nanocrystal as well as provide specific chemical reactivity to the surface of the nanocrystal, and / or (ii) a group that is cyclic, halogenated, or polar a-protic. In certain embodiments, at least two chemically distinct ligands are attached to an surface of the coating, wherein the at least two ligands (I and II) are represented by the formula: X-Sp-Z. In ligand (I) X represents a phosphonic, phosphinic, or phosphategroup and in ligand (II) X represents a primary or secondary amine, or an imidizole, or an amide; In both ligands (I) and (II) Sp, which can be the same or different in the two compounds, represents a spacer group, such as a group capable of allowing a transfer of charge or an insulating group; Z, which can be the same or different in the two compounds, is a group chosen from among groups capable of communicating specific chemical properties to the nanoparticle as well as provide specific chemical reactivity to the surface of the nanoparticle. In preferred embodiments, the nanoparticle includes a core comprising a semiconductor material.
Owner:SAMSUNG ELECTRONICS CO LTD
7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy
Owner:ONCOCEUTICS INC
Foamable compositions containing nitro-imidazoles, processes for preparing same and methods of treatment utilizing same
A pharmaceutical or cosmeceutical foamable composition for topical application of nitroimidazoles such as Metronidazole and a process of manufacturing the same is disclosed. A method of treatment of skin and scalp disorders, especially of rosacea, acne and foul smelling lesions, by dispensing nitroimidazoles such as Metronidazole in foamable composition is also disclosed.
Owner:AGIS INDUSTRIES (1983) LTD
Linked diimidazole derivatives
The present invention discloses compounds of Formula (I), or pharmaceutically acceptable salts, esters, or prodrugs thereof:which inhibit RNA-containing virus, particularly the hepatitis C virus (HCV). Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
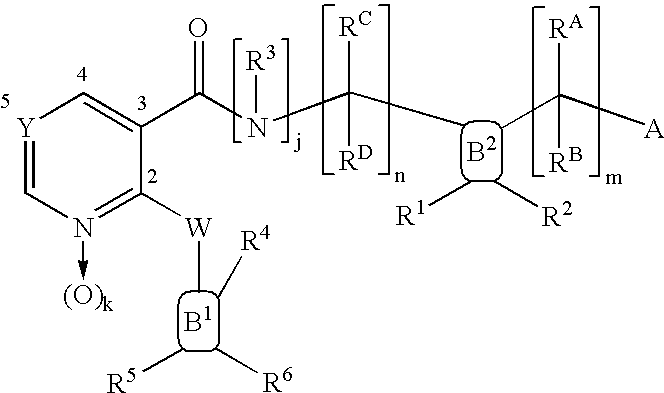
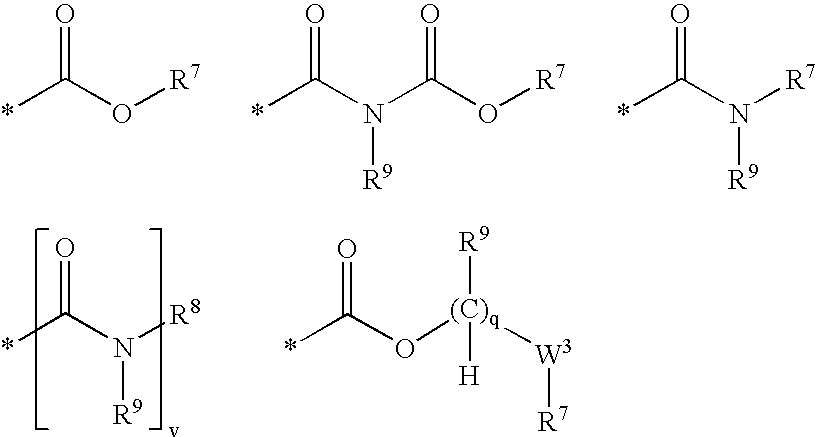
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

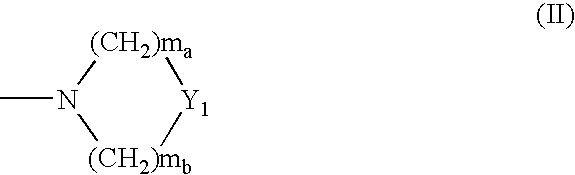
![Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ead034c7-c473-4c4b-9966-87a09aefc546/US20050090499A1-20050428-C00001.png)
![Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ead034c7-c473-4c4b-9966-87a09aefc546/US20050090499A1-20050428-C00002.png)
![Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ead034c7-c473-4c4b-9966-87a09aefc546/US20050090499A1-20050428-C00003.png)
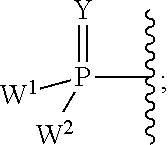
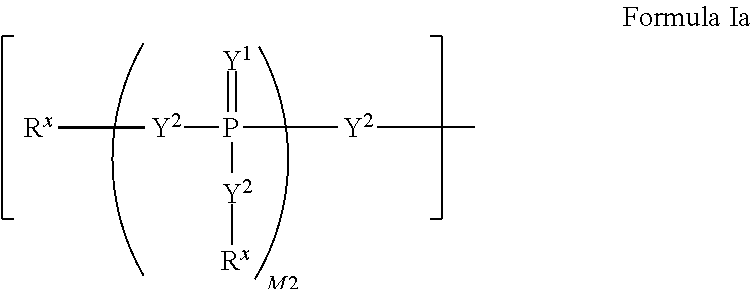
![Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ebbaacdc-083b-4261-bc4a-f1b6b1a1c3a3/US07393848-20080701-C00001.png)
![Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ebbaacdc-083b-4261-bc4a-f1b6b1a1c3a3/US07393848-20080701-C00002.png)
![Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ebbaacdc-083b-4261-bc4a-f1b6b1a1c3a3/US07393848-20080701-C00003.png)




![Novel Imidazo [4,5-b] Pyridine Derivatives as Inhibitors of Glycogen Synthase Kinase 3 for Use in the Treatment of Dementia and Neurodegenerative Disorders Novel Imidazo [4,5-b] Pyridine Derivatives as Inhibitors of Glycogen Synthase Kinase 3 for Use in the Treatment of Dementia and Neurodegenerative Disorders](https://images-eureka.patsnap.com/patent_img/a948393a-afe0-45f9-9570-e296e0ad6404/US20080255085A1-20081016-C00001.png)
![Novel Imidazo [4,5-b] Pyridine Derivatives as Inhibitors of Glycogen Synthase Kinase 3 for Use in the Treatment of Dementia and Neurodegenerative Disorders Novel Imidazo [4,5-b] Pyridine Derivatives as Inhibitors of Glycogen Synthase Kinase 3 for Use in the Treatment of Dementia and Neurodegenerative Disorders](https://images-eureka.patsnap.com/patent_img/a948393a-afe0-45f9-9570-e296e0ad6404/US20080255085A1-20081016-C00002.png)
![Novel Imidazo [4,5-b] Pyridine Derivatives as Inhibitors of Glycogen Synthase Kinase 3 for Use in the Treatment of Dementia and Neurodegenerative Disorders Novel Imidazo [4,5-b] Pyridine Derivatives as Inhibitors of Glycogen Synthase Kinase 3 for Use in the Treatment of Dementia and Neurodegenerative Disorders](https://images-eureka.patsnap.com/patent_img/a948393a-afe0-45f9-9570-e296e0ad6404/US20080255085A1-20081016-C00003.png)
![Dihydrospiro[dibenzo[a,d][7]annulene-5,4'-imidazol] compounds for the inhibition of beta-secretase Dihydrospiro[dibenzo[a,d][7]annulene-5,4'-imidazol] compounds for the inhibition of beta-secretase](https://images-eureka.patsnap.com/patent_img/ff3938b9-266f-464f-a99f-be2342573144/US20070203116A1-20070830-C00001.png)
![Dihydrospiro[dibenzo[a,d][7]annulene-5,4'-imidazol] compounds for the inhibition of beta-secretase Dihydrospiro[dibenzo[a,d][7]annulene-5,4'-imidazol] compounds for the inhibition of beta-secretase](https://images-eureka.patsnap.com/patent_img/ff3938b9-266f-464f-a99f-be2342573144/US20070203116A1-20070830-C00002.png)
![Dihydrospiro[dibenzo[a,d][7]annulene-5,4'-imidazol] compounds for the inhibition of beta-secretase Dihydrospiro[dibenzo[a,d][7]annulene-5,4'-imidazol] compounds for the inhibition of beta-secretase](https://images-eureka.patsnap.com/patent_img/ff3938b9-266f-464f-a99f-be2342573144/US20070203116A1-20070830-C00003.png)
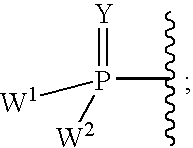
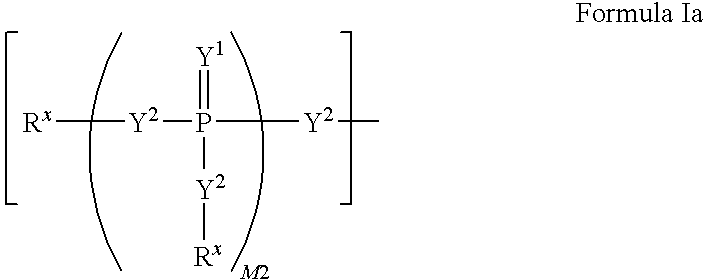
![Imidazo[1,2-beta]pyridazine and pyrazolo[1,5-alpha]pyrimidine derivatives and their use as protein kinase inhibitors Imidazo[1,2-beta]pyridazine and pyrazolo[1,5-alpha]pyrimidine derivatives and their use as protein kinase inhibitors](https://images-eureka.patsnap.com/patent_img/7d3e1e07-4aea-462e-858e-a93a49cbb310/US07750007-20100706-D00001.png)
![Imidazo[1,2-beta]pyridazine and pyrazolo[1,5-alpha]pyrimidine derivatives and their use as protein kinase inhibitors Imidazo[1,2-beta]pyridazine and pyrazolo[1,5-alpha]pyrimidine derivatives and their use as protein kinase inhibitors](https://images-eureka.patsnap.com/patent_img/7d3e1e07-4aea-462e-858e-a93a49cbb310/US07750007-20100706-D00002.png)
![Imidazo[1,2-beta]pyridazine and pyrazolo[1,5-alpha]pyrimidine derivatives and their use as protein kinase inhibitors Imidazo[1,2-beta]pyridazine and pyrazolo[1,5-alpha]pyrimidine derivatives and their use as protein kinase inhibitors](https://images-eureka.patsnap.com/patent_img/7d3e1e07-4aea-462e-858e-a93a49cbb310/US07750007-20100706-D00003.png)
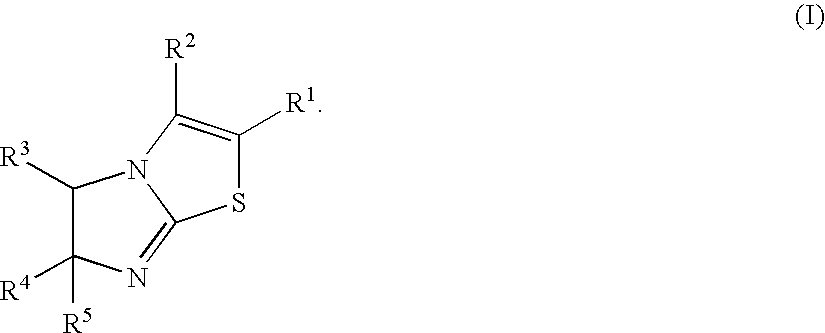
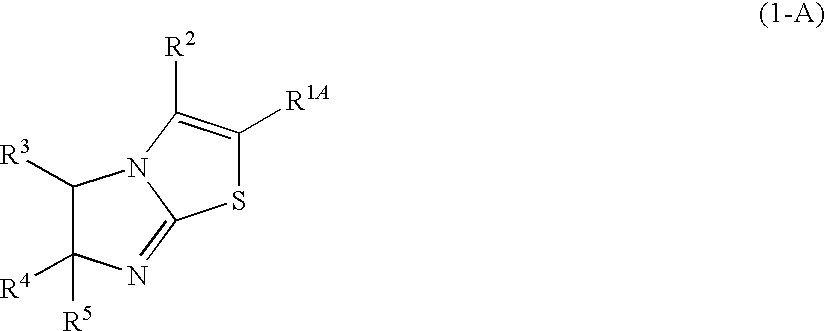
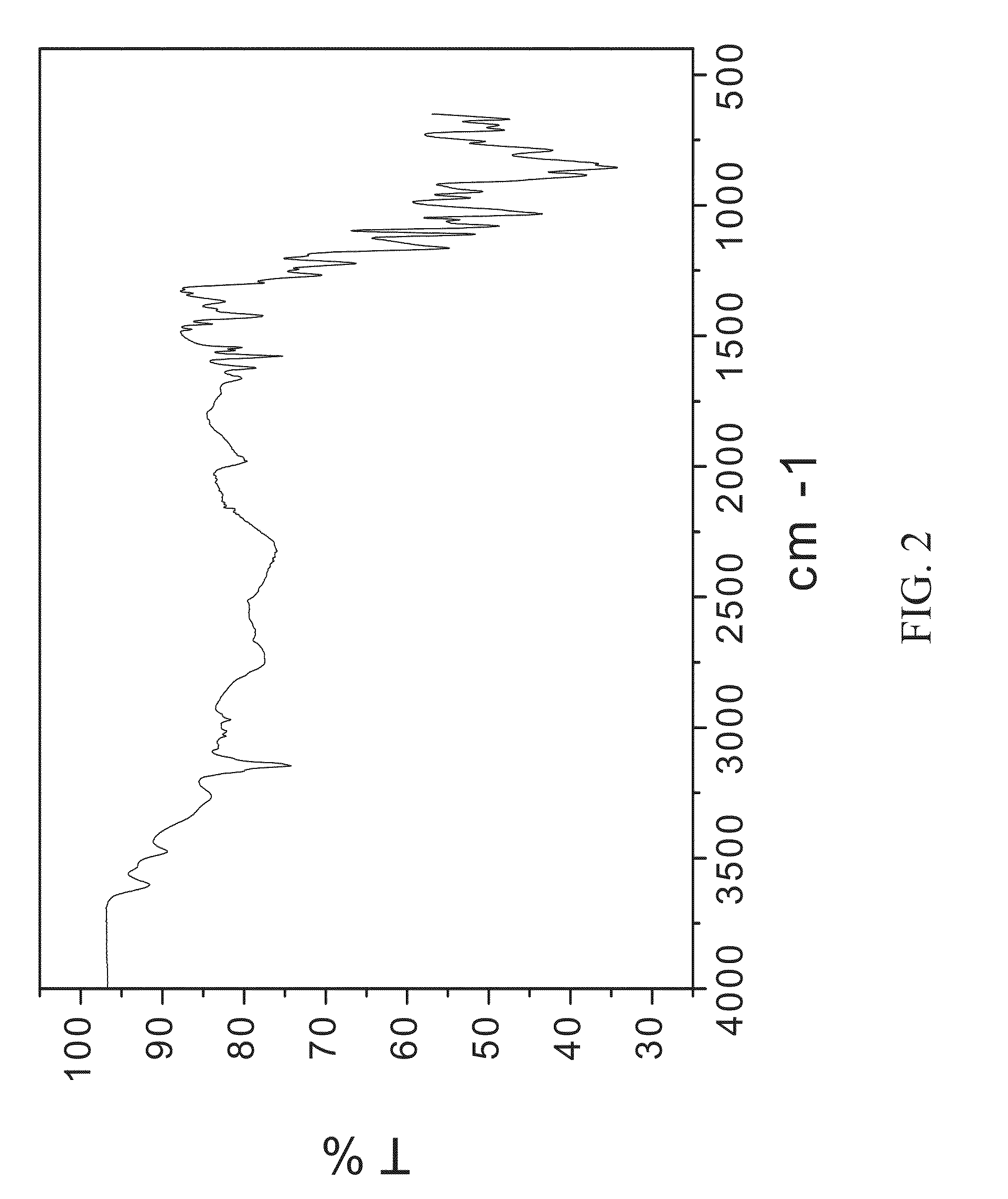



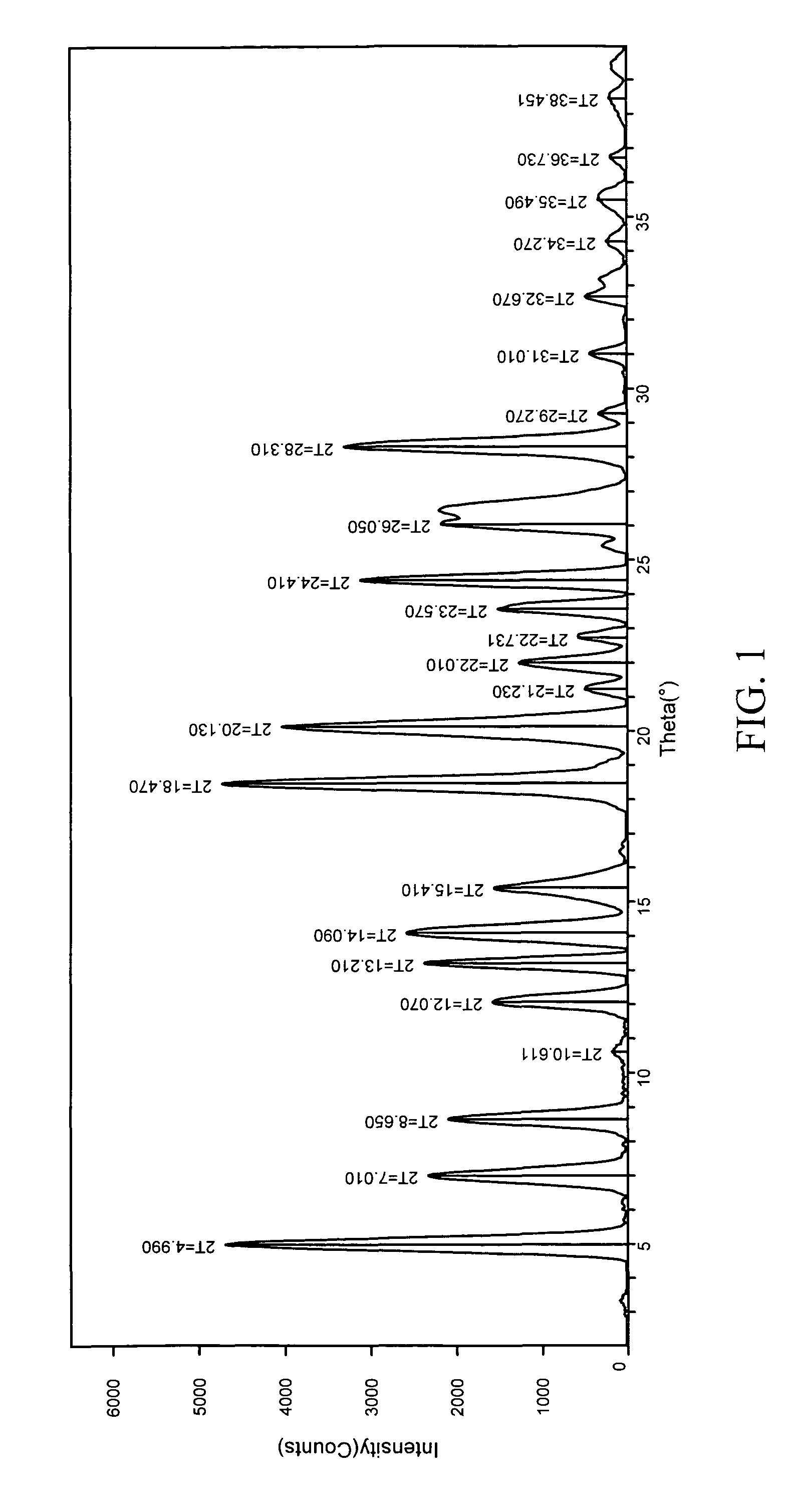
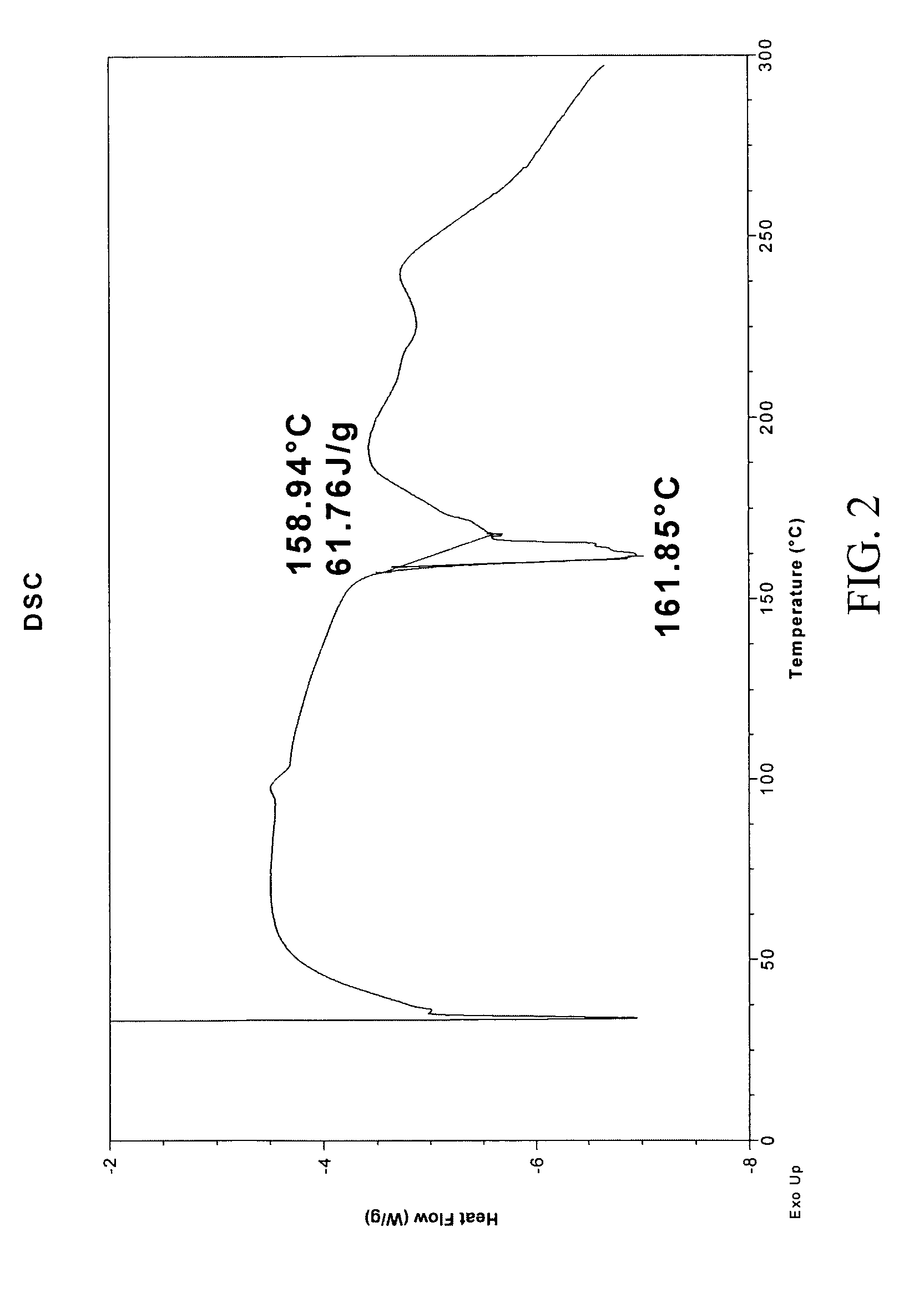
![7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy 7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy](https://images-eureka.patsnap.com/patent_img/b2faab5b-d907-46ea-8973-de72880bc4ce/US20140335048A1-20141113-D00001.png)
![7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy 7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy](https://images-eureka.patsnap.com/patent_img/b2faab5b-d907-46ea-8973-de72880bc4ce/US20140335048A1-20141113-C00001.png)
![7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy 7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy](https://images-eureka.patsnap.com/patent_img/b2faab5b-d907-46ea-8973-de72880bc4ce/US20140335048A1-20141113-C00002.png)