Patents
Literature
47 results about "Imidazothiazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Imidazothiazoles are bicyclic heterocycle consisting of an imidazole ring fused to a thiazole ring;it contains three hetero atom in structure two nitrogen(one nitrogen in as bridge and one in ring)and one sulphur atom. It shows a variety of activity. I.e: anti-inflammatory, antioxidant, antimicrobial, antifungal and anthelmintic. Any derivative of this compound, like in anthelmintics such as butamisole or levamisole.
Topical formulations comprising 1-N-arylpyrazole derivatives and amitraz
The present invention provides for, inter alia, novel topical formulations comprising at least one 1-N-arypyrazole derivative and amitraz and to methods for treating, controlling, or preventing parasite infestations on mammals or birds The inventive formulations include spot-on, pour-on or spray formulations and may include a further ectoparasiticide, such as an IGR compound, an avermectin or milbemycin derivative, or a pyrethroid insecticides, and anthelmintics, such as benzimidazoles and imidazothiazoles. The inventive formulation provides a larger duration of parasite control at a faster rate of control. The inventive formula remains effective up to three months from the first application. Moreover, the inventive formulations prevent tick attachment to the animal, thereby providing protection against tick borne diseases. The ectoparasites which may be controlled, treated or prevented by the present invention includes ticks, fleas, mites, mange, lice, mosquitoes, flies and cattle grubs.
Owner:MERIAL INC
Topical formulations comprising 1-N-arylpyrazole derivatives and amitraz
ActiveUS20050137244A1Treating and controlling and preventing parasite infestationLong durationBiocideDead animal preservationDiseaseMammal
The present invention provides for, inter alia, novel topical formulations comprising at least one 1-N-arypyrazole derivative and amitraz and to methods for treating, controlling, or preventing parasite infestations on mammals or birds The inventive formulations include spot-on, pour-on or spray formulations and may include a further ectoparasiticide, such as an IGR compound, an avermectin or milbemycin derivative, or a pyrethroid insecticides, and anthelmintics, such as benzimidazoles and imidazothiazoles. The inventive formulation provides a larger duration of parasite control at a faster rate of control. The inventive formula remains effective up to three months from the first application. Moreover, the inventive formulations prevent tick attachment to the animal, thereby providing protection against tick borne diseases. The ectoparasites which may be controlled, treated or prevented by the present invention includes ticks, fleas, mites, mange, lice, mosquitoes, flies and cattle grubs.
Owner:MERIAL INC
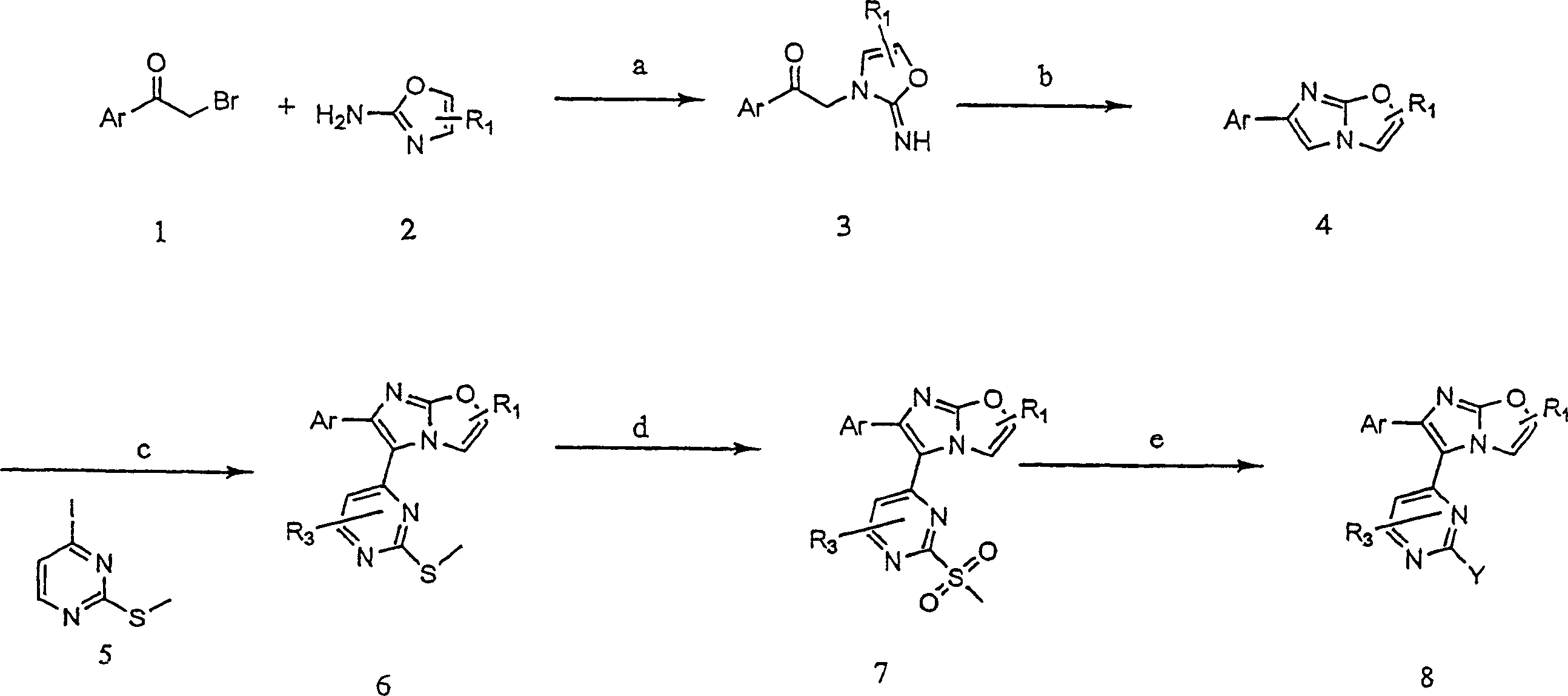
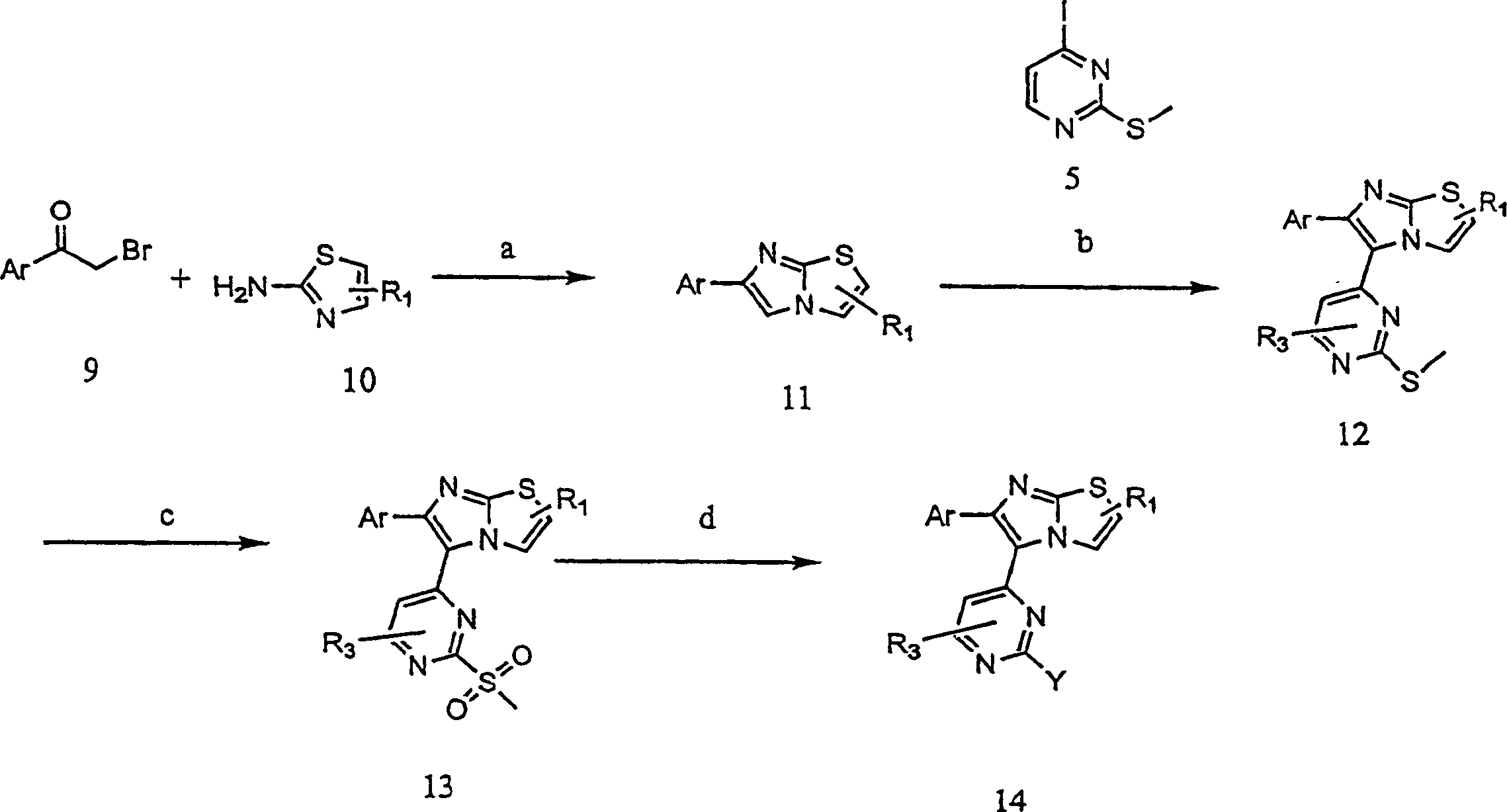
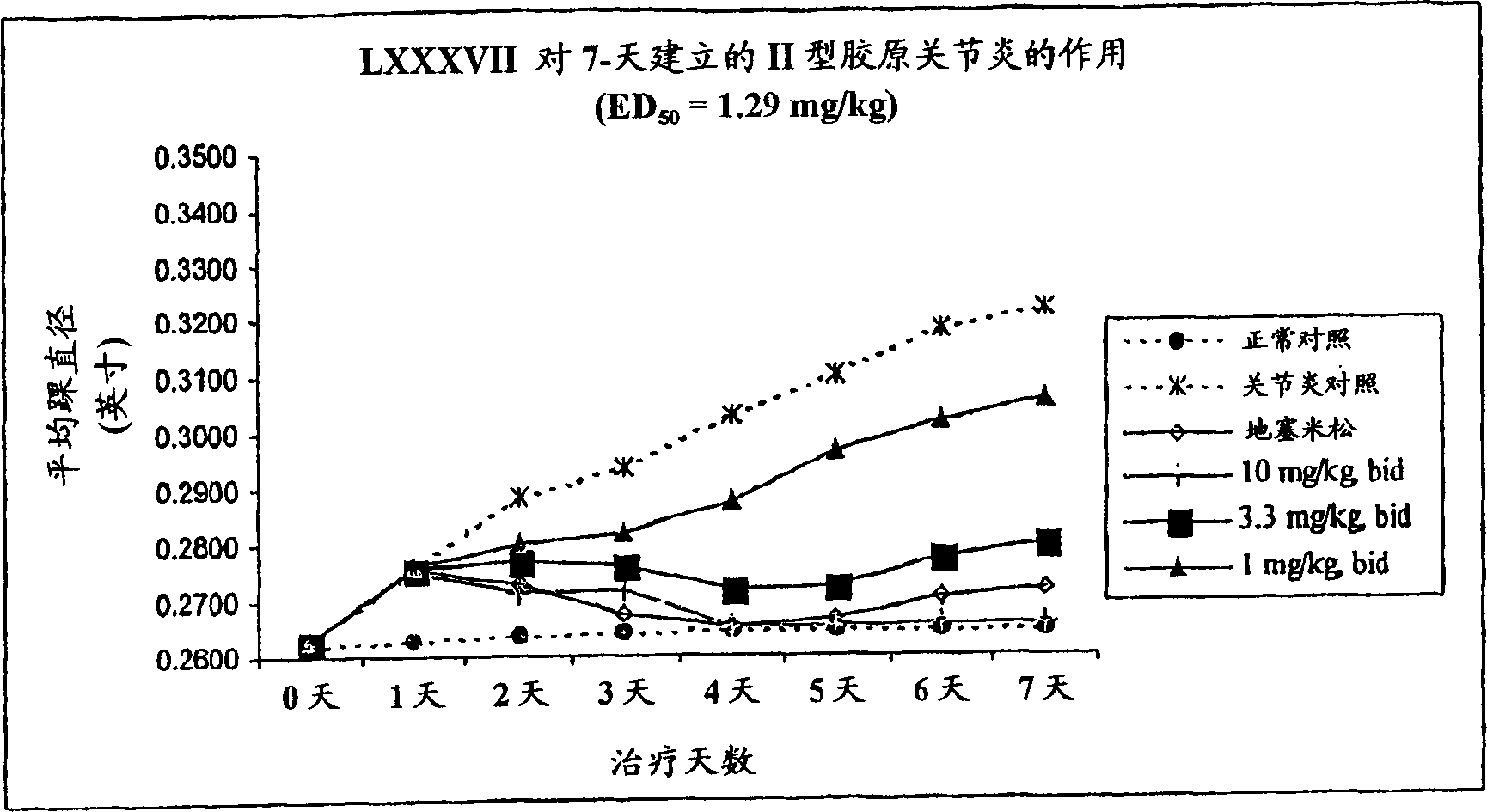
RAF inhibitors and their uses
The present invention provides imidazooxazole and imidazothiazole compounds and their synthesis. The compounds of the present invention are capable of inhibiting the activity of RAF kinase, such as B-RAFV600E. The compounds are useful for the treatment of cell proliferative disorders such as cancer.
Owner:ARQULE INC
Ligands for aggregated tau molecules
Provided are certain benzothiazole, imidazothiazole, imidazopyrimidine and imidazopyridine compounds, including, for example: formula (I) and pharmaceutically and physiologically acceptable salts, hydrates, and solvates thereof. Such compounds can be used as diagnostic ligands or labels of tau protein and PHF.
Owner:WISTA LAB LTD
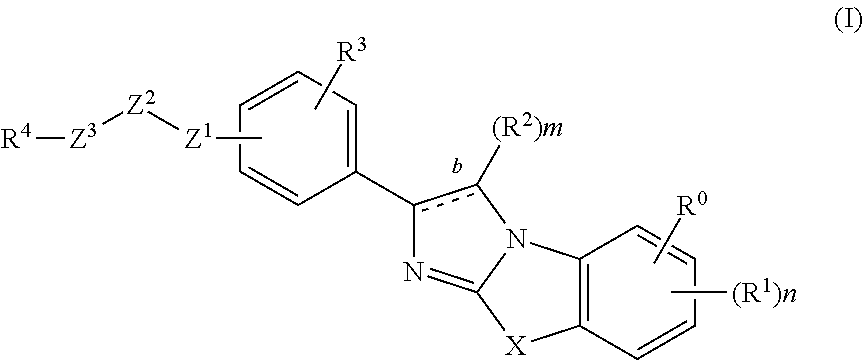
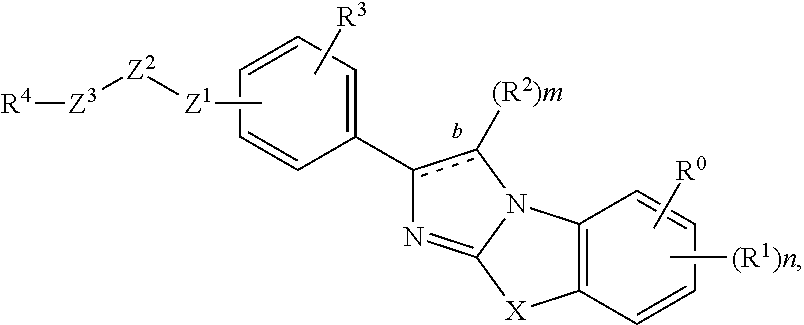
Novel imidazothiazoles and imidazoxazoles
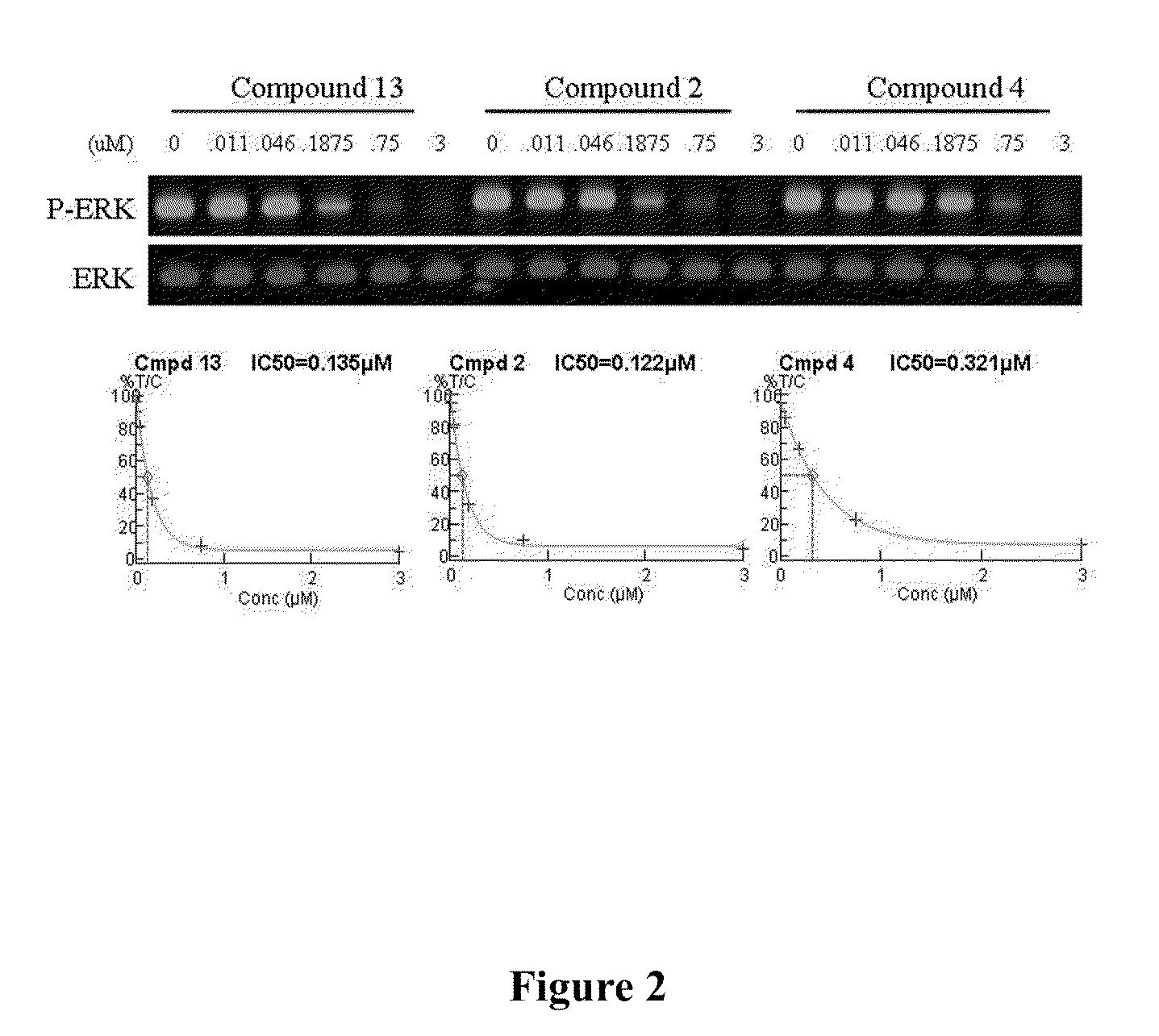
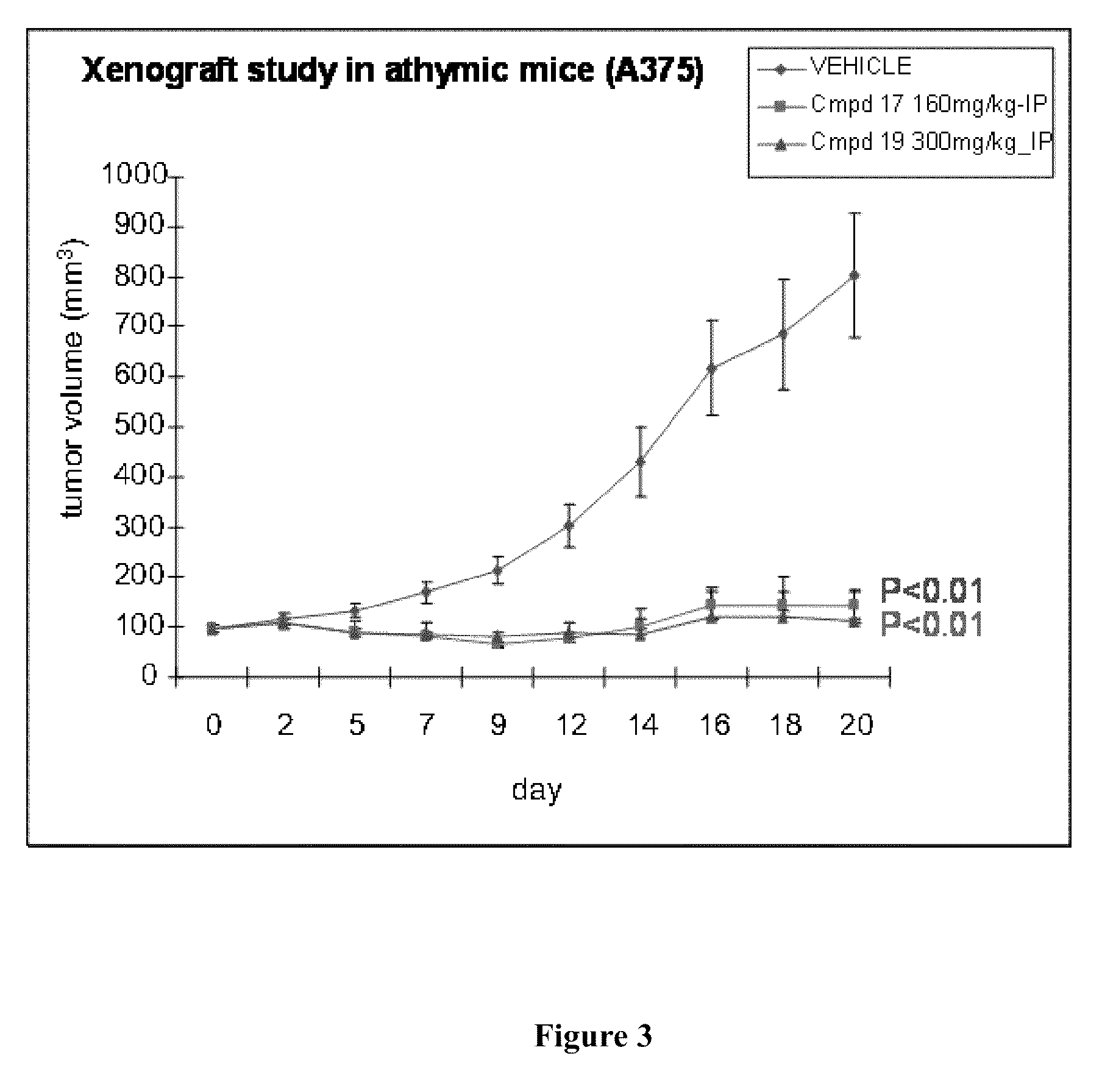
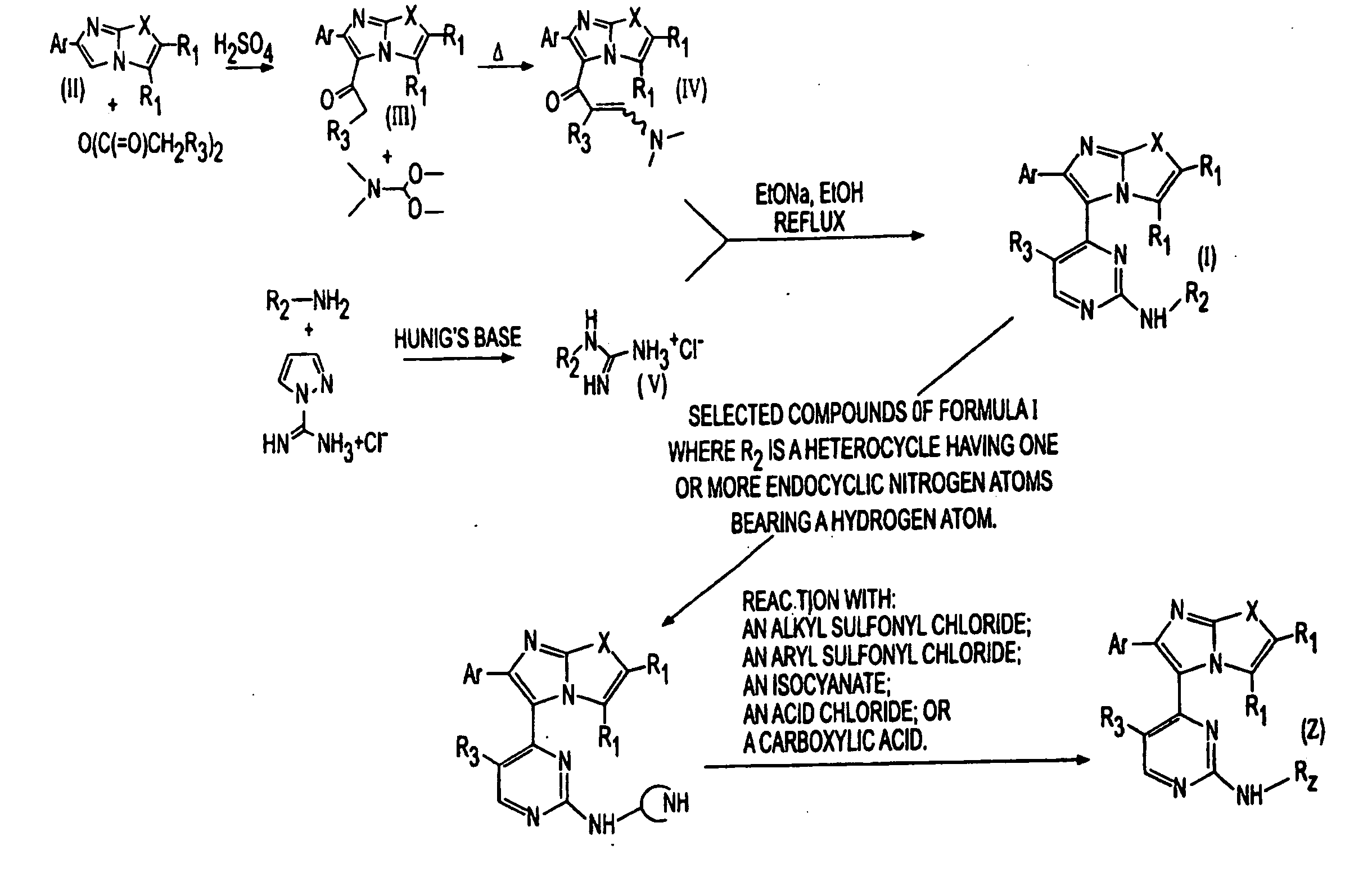
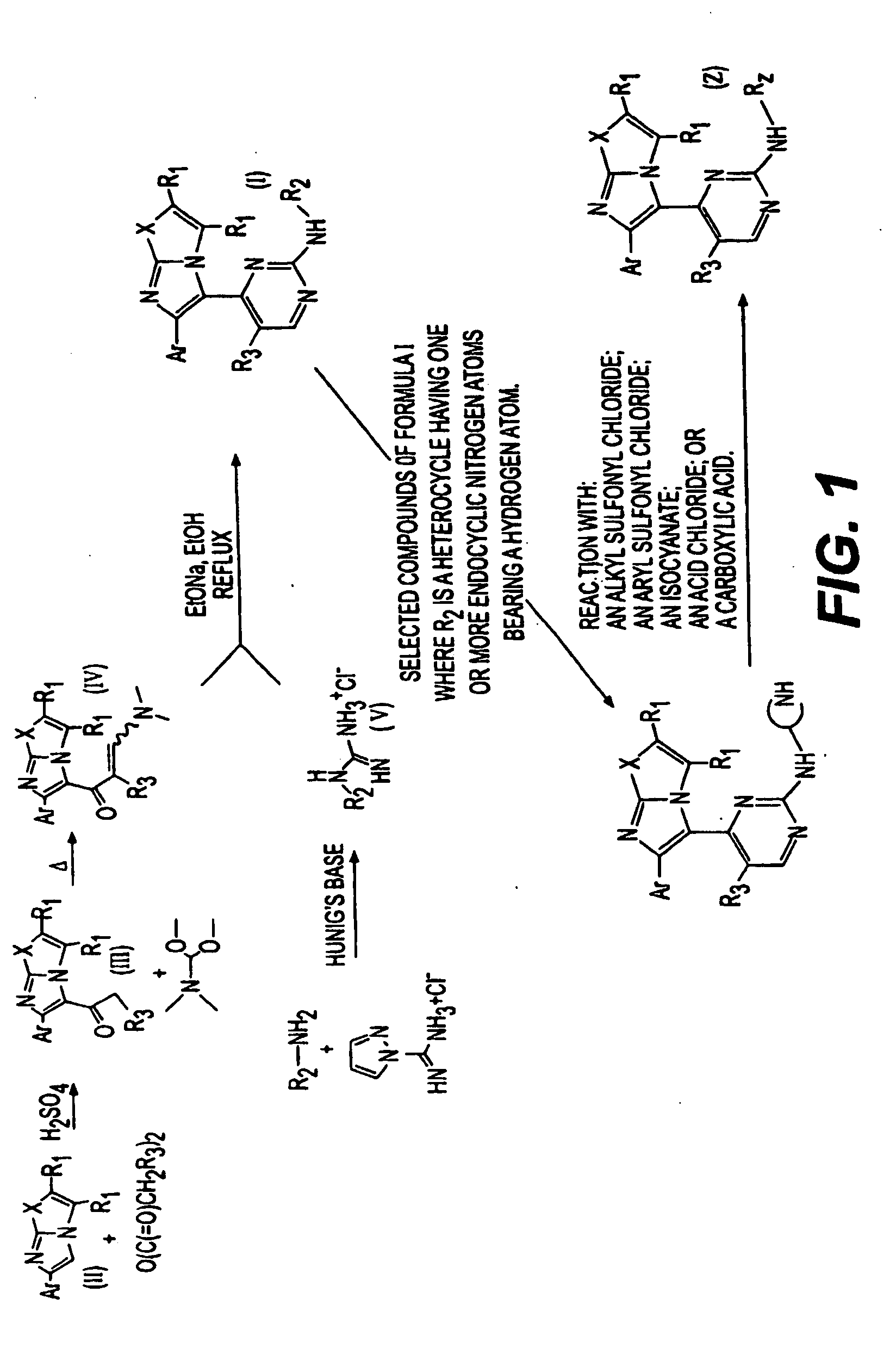
The present invention is directed to novel compounds of formula (I) wherein the variables are as defined herein. The compounds of formula (I) are useful as kinase inhibitors and as such would be useful in treating certain conditions and diseases, especially inflammatory conditions and diseases and proliferative disorders and conditions, for example, cancers.
Owner:ABBOTT LAB INC
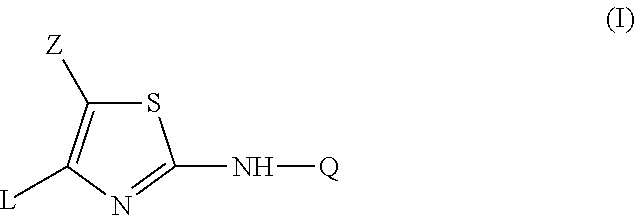
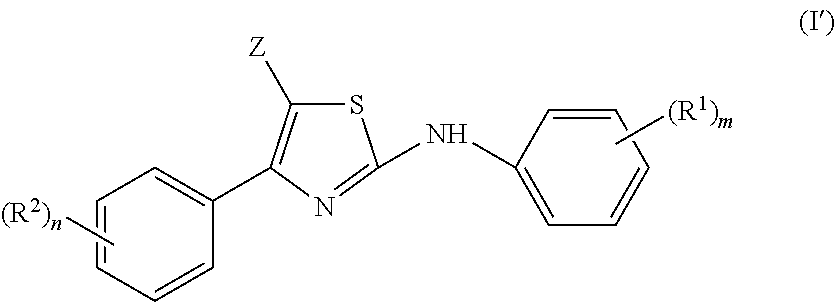
2-aniline-4-aryl substituted thiazole derivatives
This invention concerns the use of a compound of formula (I)a N-oxide, a pharmaceutically acceptable addition salt, a quaternary amine and a stereochemically isomeric form thereof, whereinZ is hydrogen, halo, C1-6alkyl, Het1, HO—C1-6alkyl-, cyano-C1-6alkyl-, amino-C(═O)—C1-6alkyl-, formylamino-C1-6alkyl-, C1-6alkyl-C(═O)—NH—C1-6alkyl-, mono- or di(C1-6alkyl)amino-C(═O)—C1-6alkyl-, phenyl-C1-6alkyl-, or Het4-C1-6alkyl-;Q is phenyl, pyridyl, benzofuranyl, 2,3-dihydro-benzofuranyl, pyrazolyl, isoxazolyl or indazolyl wherein each of said ring systems is optionally being substituted with up to three substituents each independently selected from halo, cyano, C1-6alkyl, C1-6alkyl-O—, C1-6alkylthio, Ar or polyhaloC1-6alkyl;L is phenyl, pyridyl, pyrimidazolyl, 8-Azapyrimidazolyl, pyridazinyl, imidazothiazolyl or furanyl wherein each of said ring systems may optionally be substituted with one or two or more substituents, each substituent independently being selected from halo, hydroxy, amino, cyano, C1-6alkyl or C1-6alkyl-O—;Het1 represents morpholinyl; pyrazolyl or imidazolyl;Het4 represents morpholinyl, pyrazolyl or imidazolyl;Ar represents phenyl optionally substituted with halo, C1-6alkyl, C1-6alkyl-O— or polyhaloC1-6alkyl; for the manufacture of a medicament for the prevention or the treatment or prophylaxis of psychotic disorders, intellectual impairment disorders or diseases or conditions in which modulation of the α7 nicotinic receptor is beneficial.
Owner:JANSSEN PHARMA NV
RAF Inhibitors and Their Uses
The present invention provides imidazooxazole and imidazothiazole compounds and their syntheses. The compounds of the present invention are capable of inhibiting the activity of RAF kinase, such as B-RAFV600E. The compounds are useful for the treatment of cell proliferative disorders such as cancer.
Owner:ARQULE INC
Synthesis of imidazooxazole and imidazothiazole inhibitors of p38 map kinase
An efficient route for the synthesis is of formula (I) of imidazooxazole and imidazothiazole inhibitors of the p38 MAP kinase pathway, useful as therapeutics for disease conditions including inflammation and auto-immune responses is described.
Owner:ARQULE INC
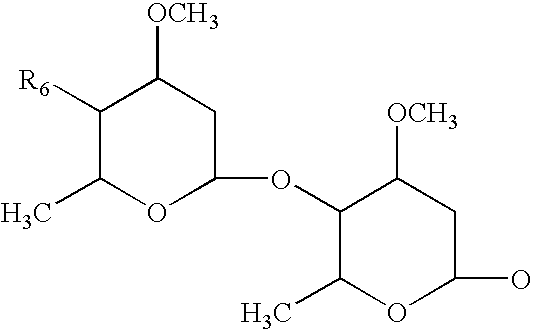
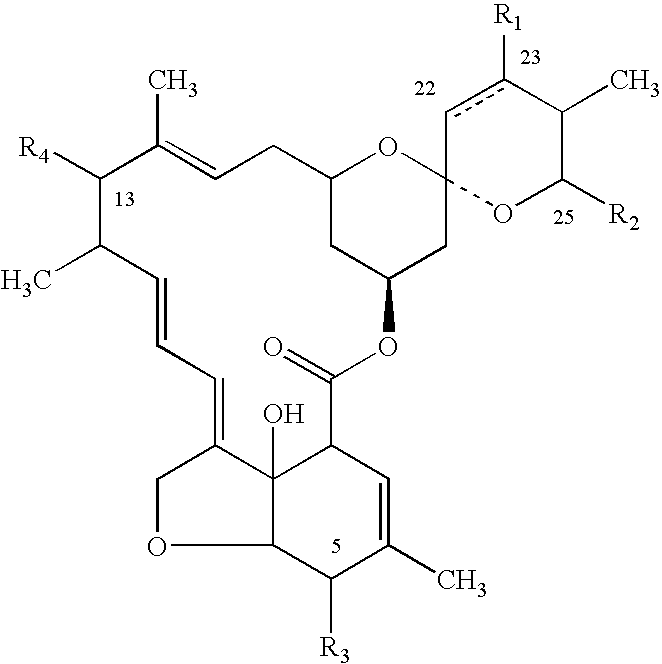
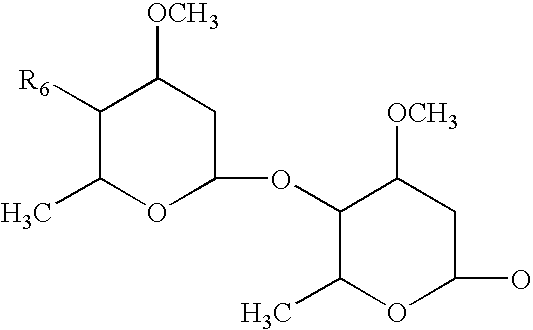
Stable veterinary combination formulations of macrocyclic lactones and imidazothiazoles
ActiveUS20160051524A1Improve stabilityImprove solubilityBiocidePharmaceutical delivery mechanismAntiparasite agentControl parasites
The present invention is directed to stabilized compositions comprising at least one macrocyclic lactone, or derivative thereof, in combination with levamisole, and an amino sugar stabilizing agent, optionally an additional antiparasitic agent, and a method for treating or controlling a parasitic infection or infestation in an animal by administering said composition.
Owner:ZOETIS SERVICE LLC
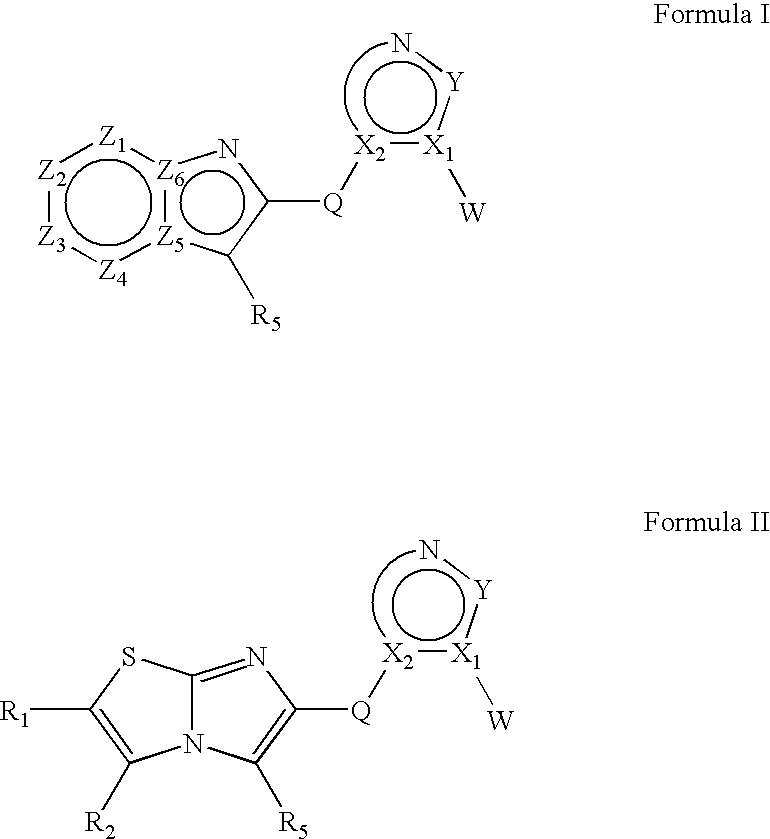
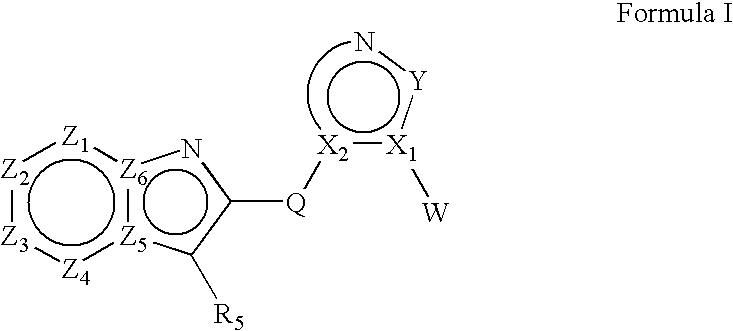
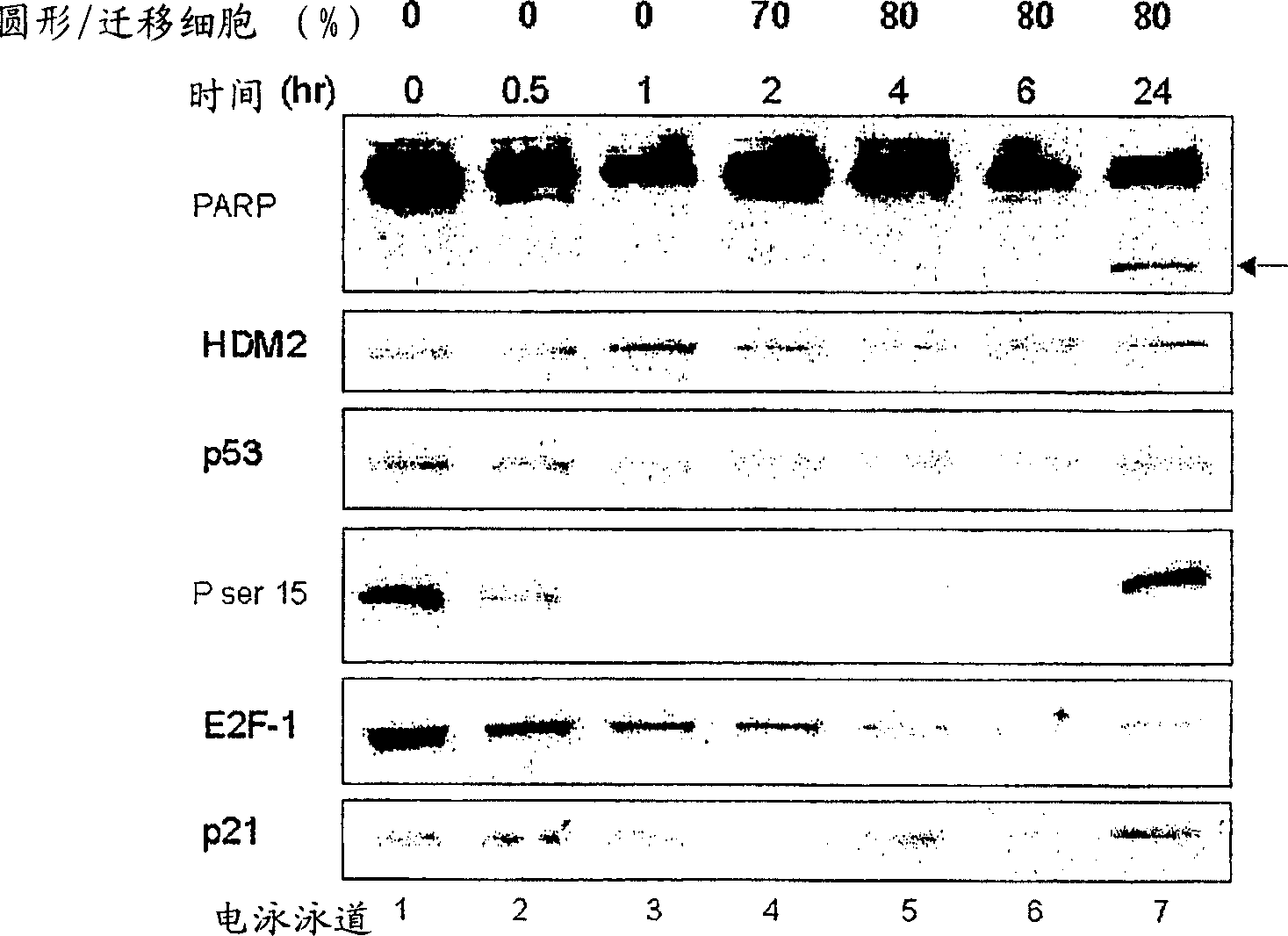
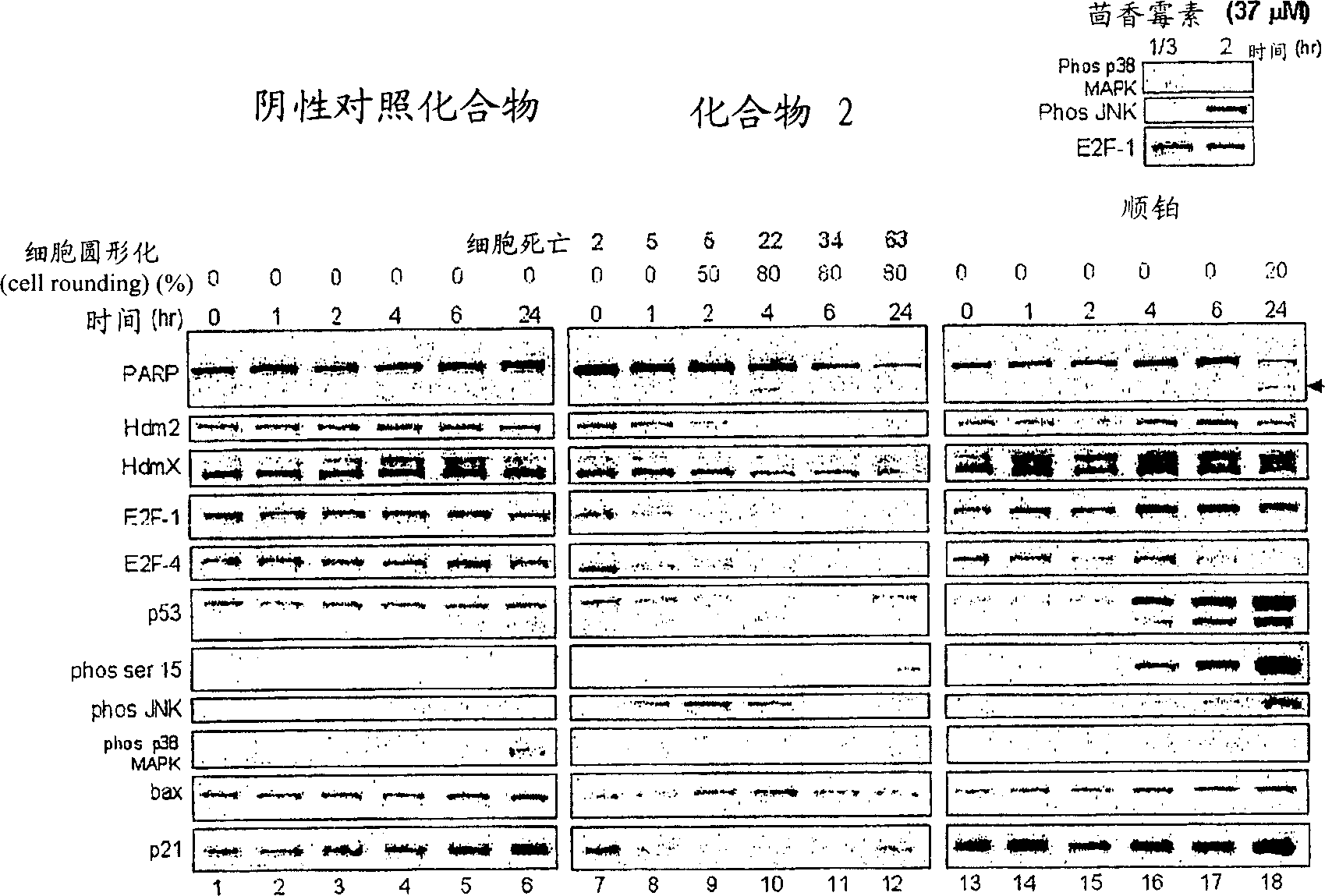
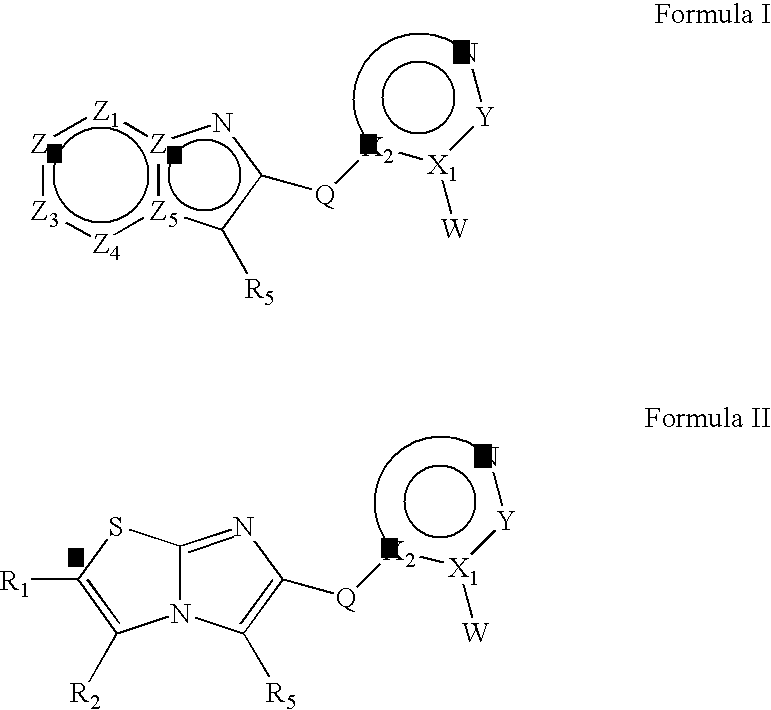
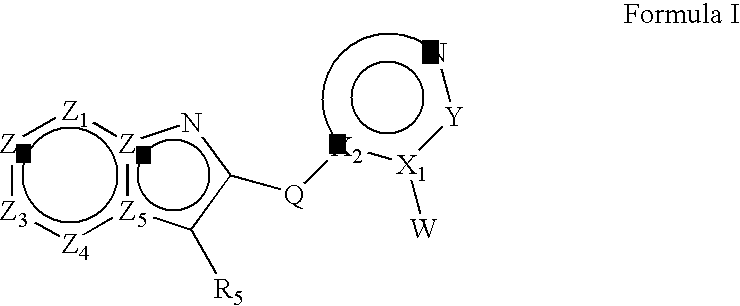
Heteroaryl substituted fused bicyclic heteroaryl compound as GABAA receptor ligands
This invention relates to heteroaryl substituted fused bicyclic heteroaryl compounds, such as heteroaryl substituted imidazopyridines, imidazopyrazines, imidazopyridizines, imidazopyrimidines, and imidazothiazoles, which may be described by Formula I or Formula II: The invention is particularly related to such compounds that bind with high selectivity and high affinity to the benzodiazepine site of GABAA receptors. This invention also relates to pharmaceutical compositions comprising such compounds and to the use of such compounds in treatment of certain central nervous system (CNS) diseases. Processes for preparing compounds of Formula I and Formula II are disclosed.This invention also relates to the use of benzimidazoles, pyridylimidazoles and related bicyclic heteroaryl compounds of Formula I or Formula II in combination with one or more other CNS agents to potentiate the effects of the other CNS agents. Additionally this invention relates to the use such compounds as probes for the localization of GABAA receptors in tissue sections.
Owner:NEUROGEN
RAF Inhibitors and Uses Thereof
The present invention provides imidazooxazole and imidazothiazole compounds and their synthesis. The compounds of the present invention are capable of inhibiting the activity of RAF kinase, such as B-RAFV600E. The compounds are useful for the treatment of cell proliferative disorders such as cancer.
Owner:ARQULE INC
Bisarylsulfonamide compounds and their use in cancer therapy
The present invention relates to the use of bisarylsulfonamide compounds of formula (I) wherein W is a CI-5 branched or unbranched alkyl group or a C2-5 alkenyl group; nis0or1; R<1> is H, a C, 1-8 branched or unbranched alkyl group, a C2-8 alkenyl group, or an aryl or aralkyl group; Ar<1> is a substituted thienyl, furyl, pyrrolyl, imidazothiazolyl, thiazolyl, pyridyl or phenyl group; and Ar<2> is a substituted phenyl, indolyl or benzoimidazolyl group; in the preparation of a medicament for treating proliferative disorders. Further aspects of the invention relate to compounds of formula (I), pharmaceutical compositions thereof, and an assay for determining binding to HDM2.
Owner:CYCLACEL
Heteroaryl substituted fused bicyclic heteroaryl compounds as GABAA receptor ligands
This invention relates to heteroaryl substituted fused bicyclic heteroaryl compounds, such as heteroaryl substituted imidazopyridines, imidazopyrazines, imidazopyridizines, imidazopyrimidines, and imidazothiazoles, which may be described by Formula I or Formula II: The invention is particularly related to such compounds that bind with high selectivity and high affinity to the benzodiazepine site of GABAA receptors. This invention also relates to pharmaceutical compositions comprising such compounds and to the use of such compounds in treatment of certain central nervous system (CNS) diseases. Processes for preparing compounds of Formula I and Formula II are disclosed. This invention also relates to the use of benzimidazoles, pyridylimidazoles and related bicyclic heteroaryl compounds of Formula I or Formula II in combination with one or more other CNS agents to potentiate the effects of the other CNS agents. Additionally this invention relates to the use such compounds as probes for the localization of GABAA receptors in tissue sections.
Owner:NEUROGEN
Ligands for aggregated tau molecules
Provided are certain benzothiazole, imidazothiazole, imidazopyrimidine and imidazopyridine compounds, including, for example:formula (I) and pharmaceutically and physiologically acceptable salts, hydrates, and solvates thereof. Such compounds can be used as diagnostic ligands or labels of tau protein and PHF.
Owner:WISTA LAB LTD
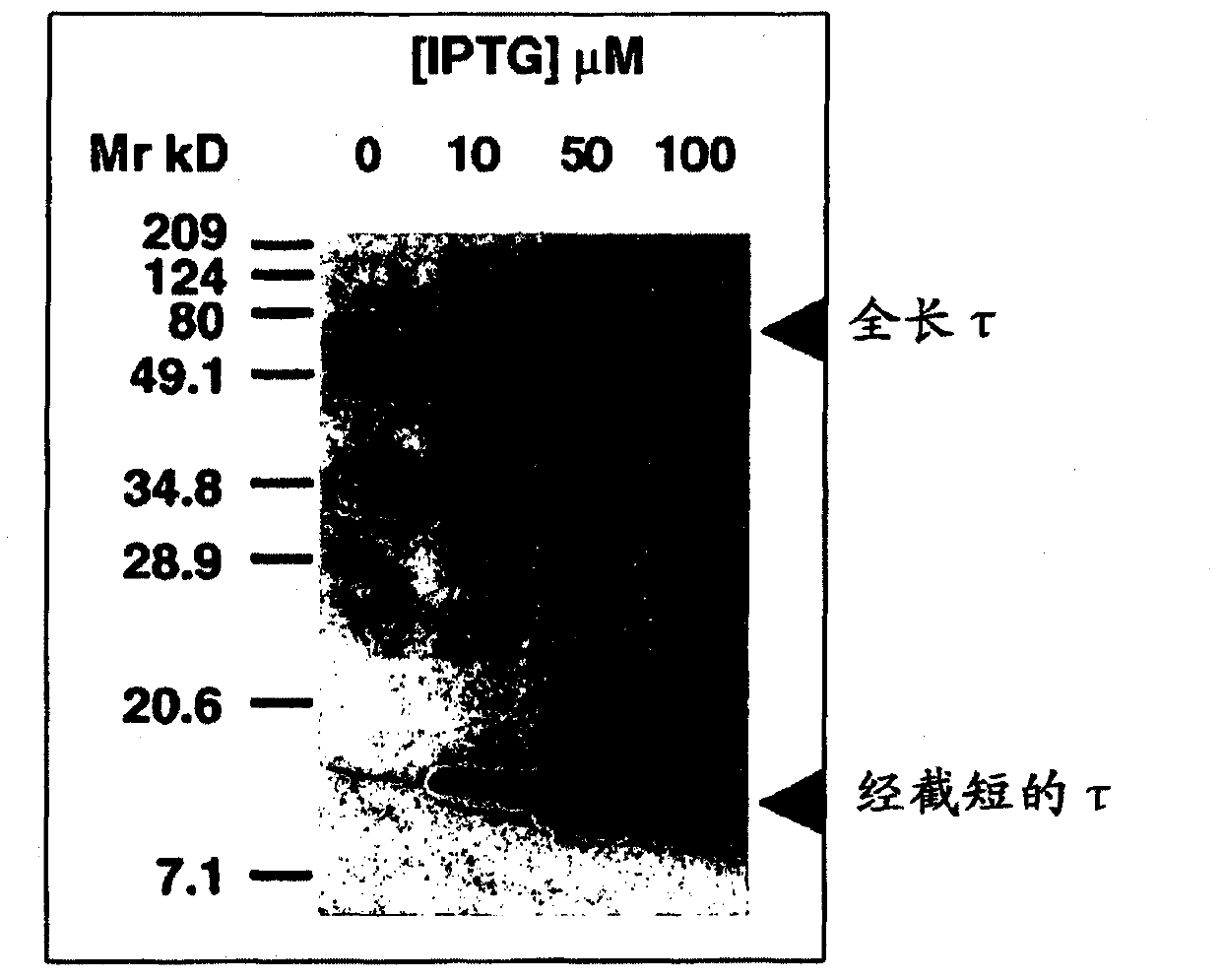
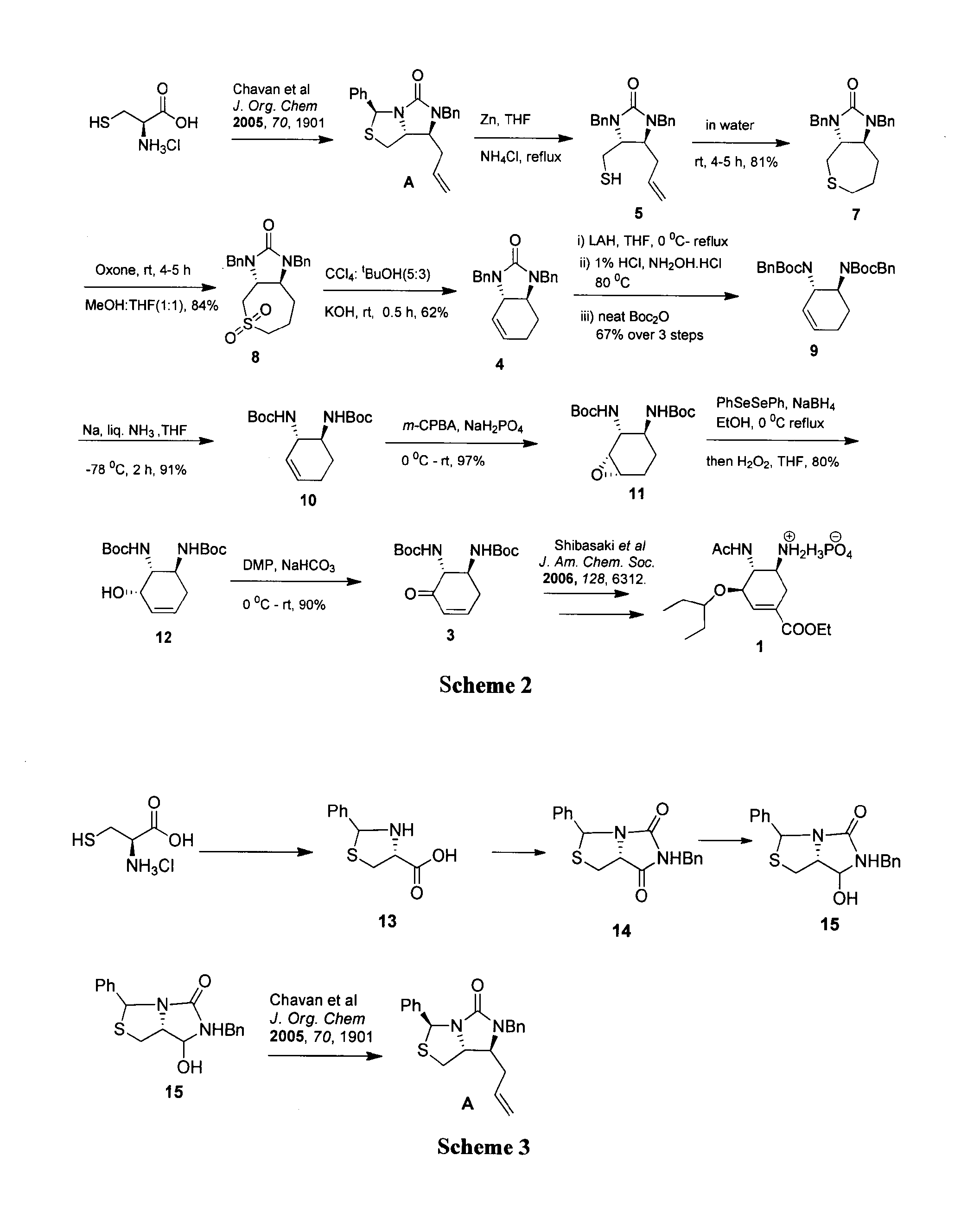
A process for the preparation of intermediate for the preparation of oseltamivir phosphate
ActiveUS20160222029A1Carbamic acid derivatives preparationOrganic compound preparationCysteine thiolatePhosphate
Owner:COUNCIL OF SCI & IND RES
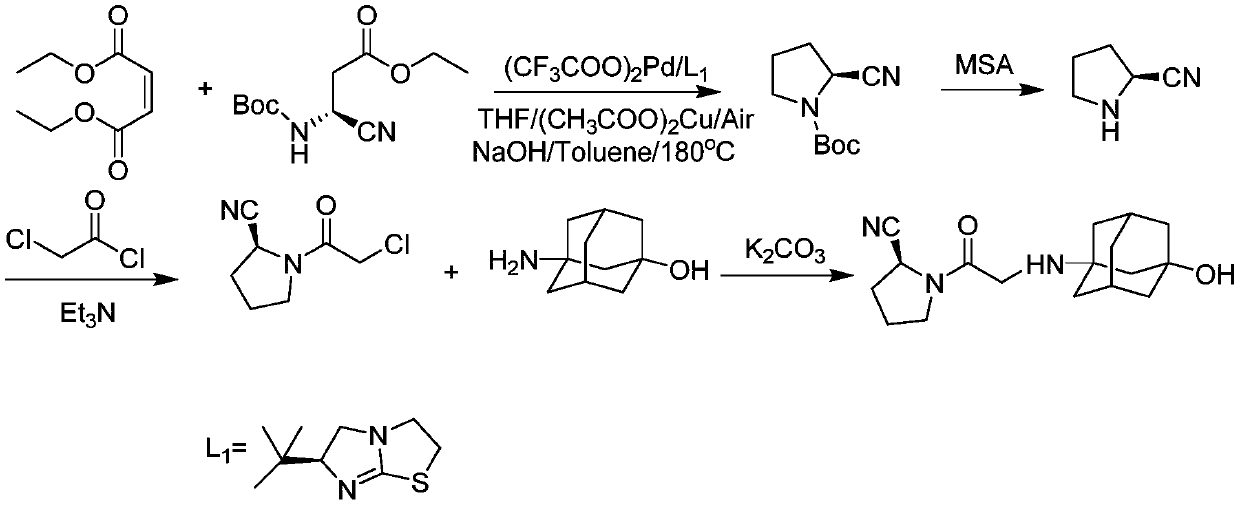
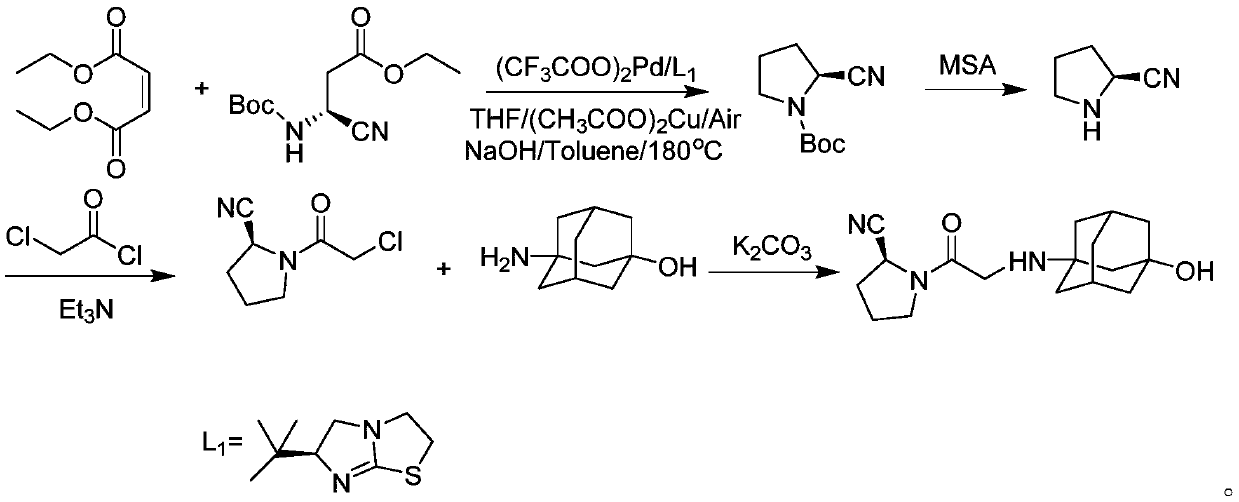
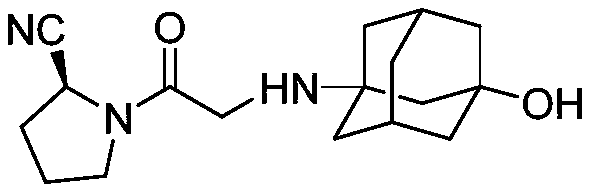
Preparation method for vildagliptin
The invention provides a preparation method for vildagliptin. Through the presence of imidazo thiazole as a chiral ligand and a palladium catalyst, a vildagliptin product with high yield and high purity can be obtained by taking diethyl fumarate and amino protected (S)-3-amino-cyano-ethyl propanoate as initial raw materials through steps such as cyclization, deprotection and coupling; and the preparation method is short in reaction route, high in yield and less in by-product, so that production costs can be reduced, and industrial production can be realized.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
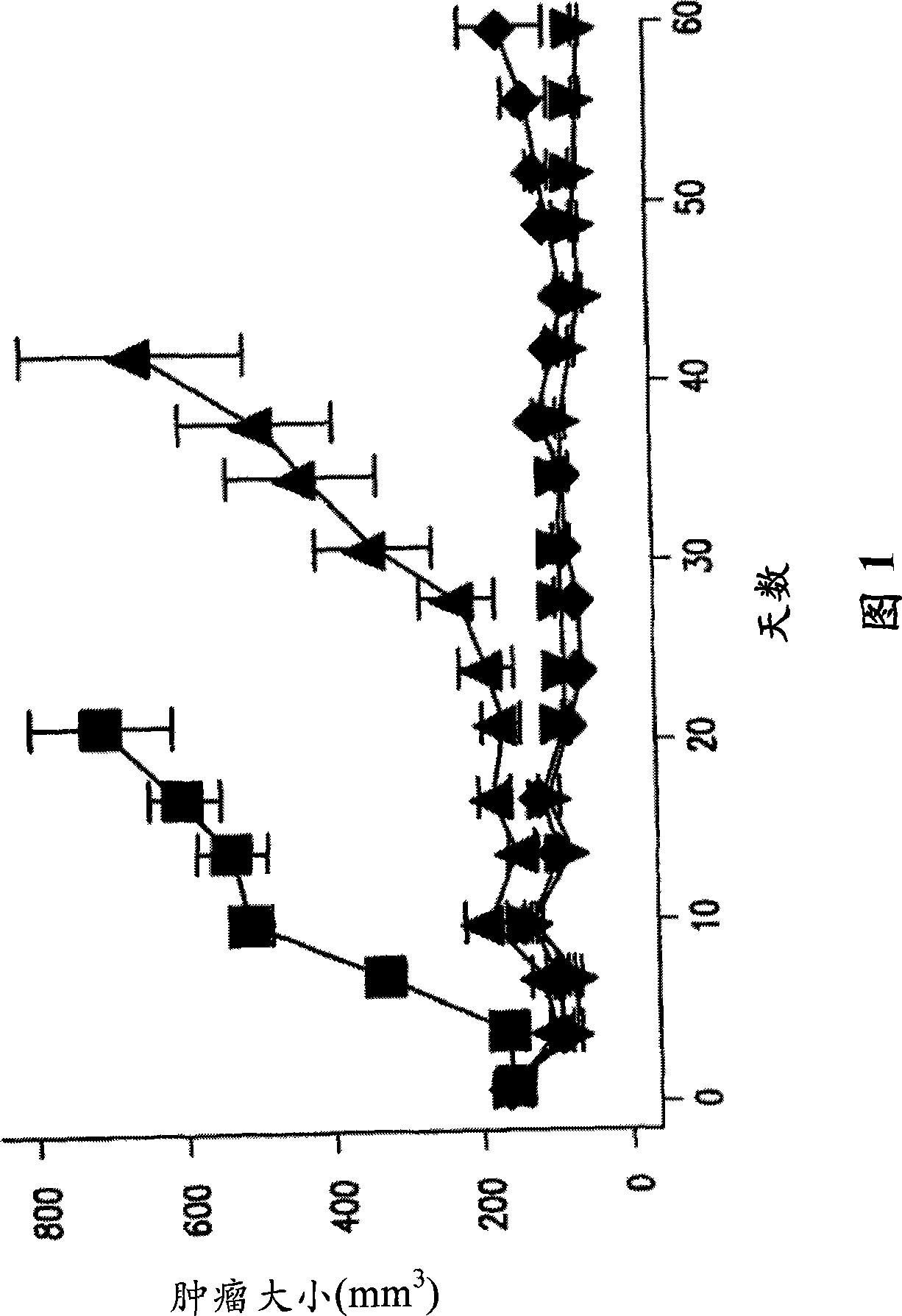
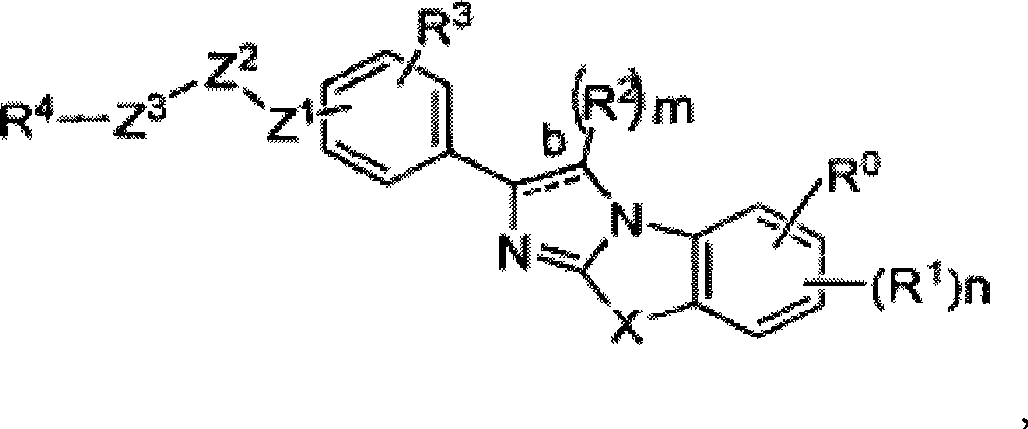
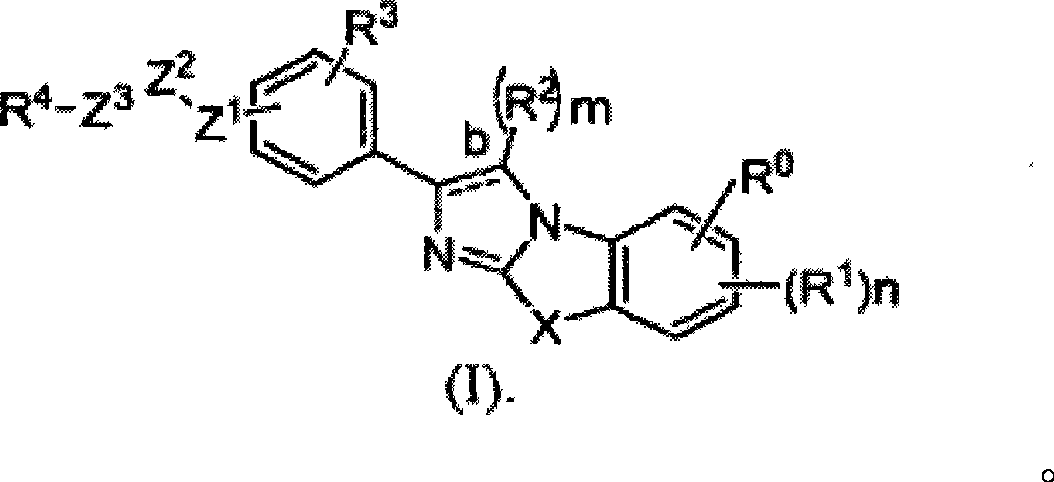
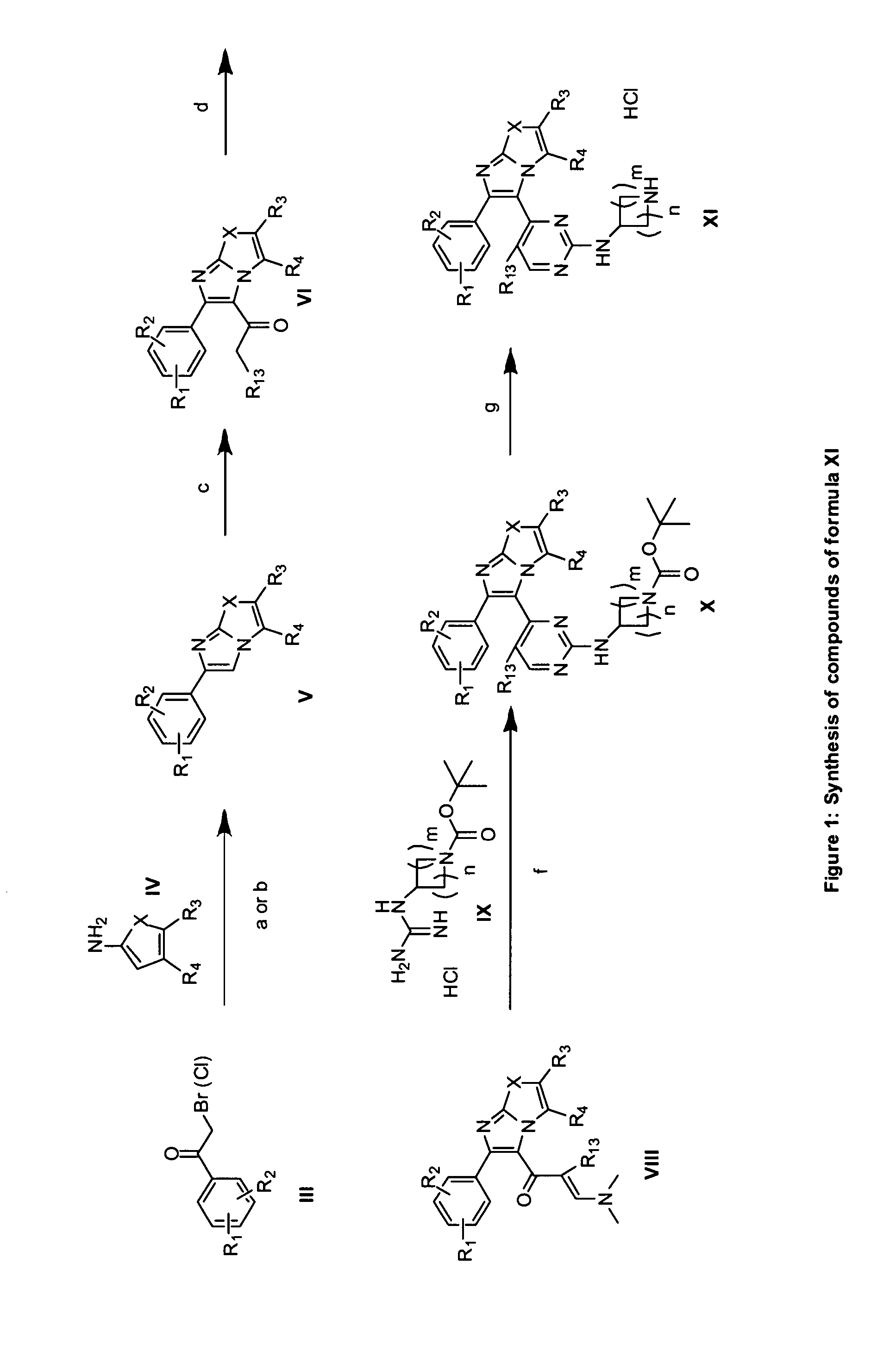
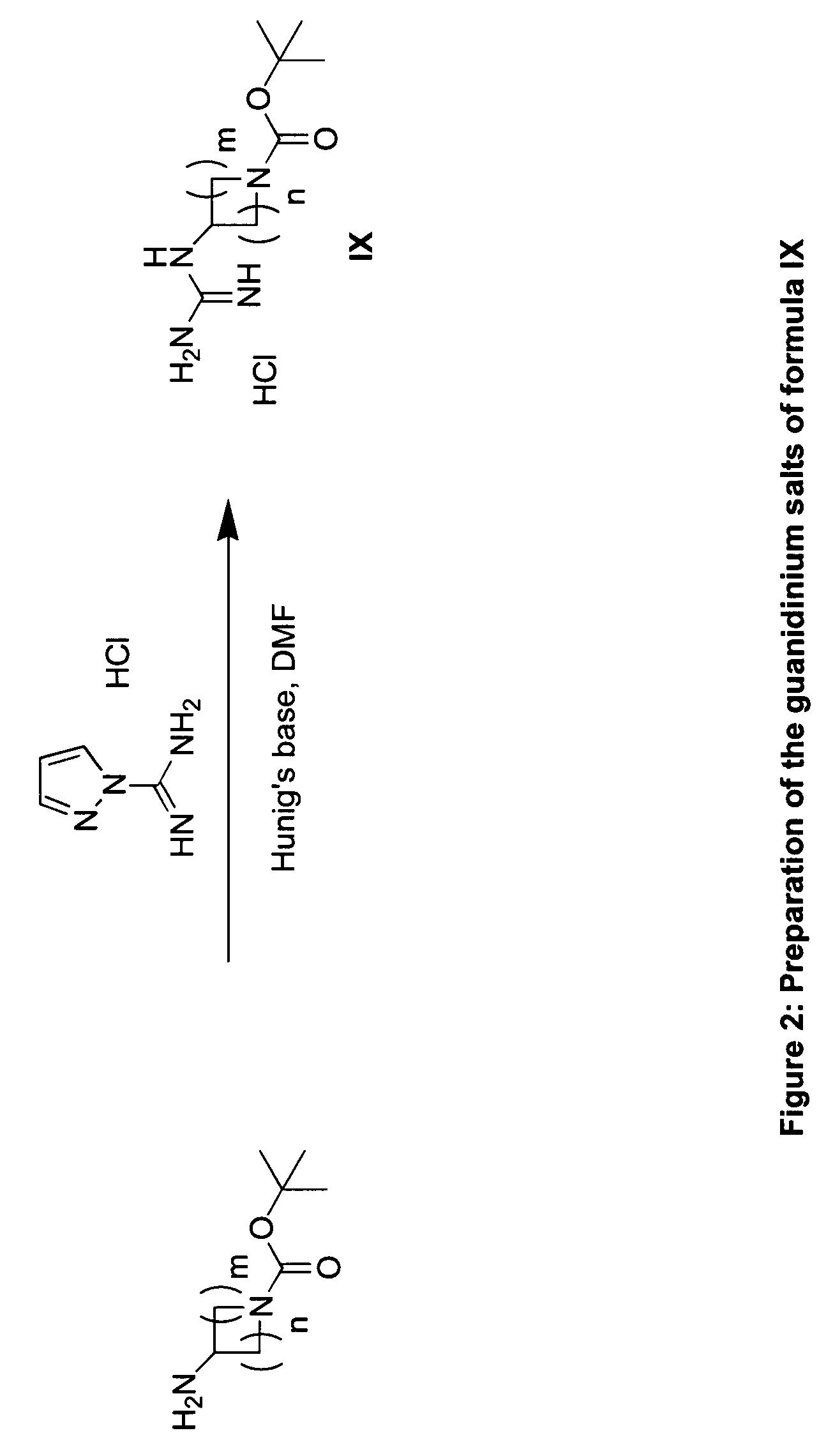
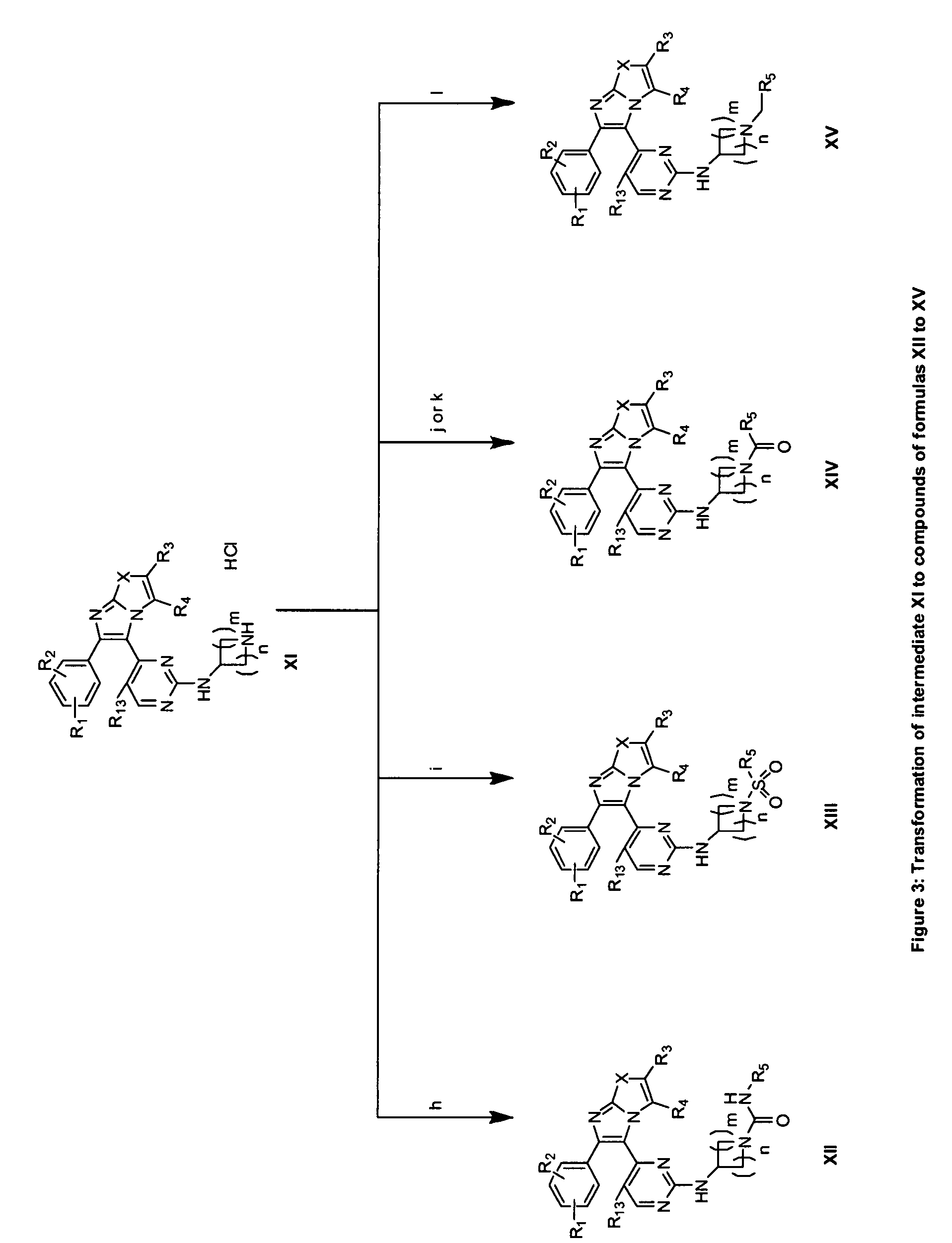
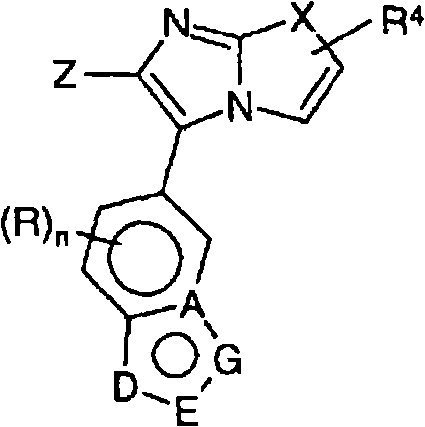
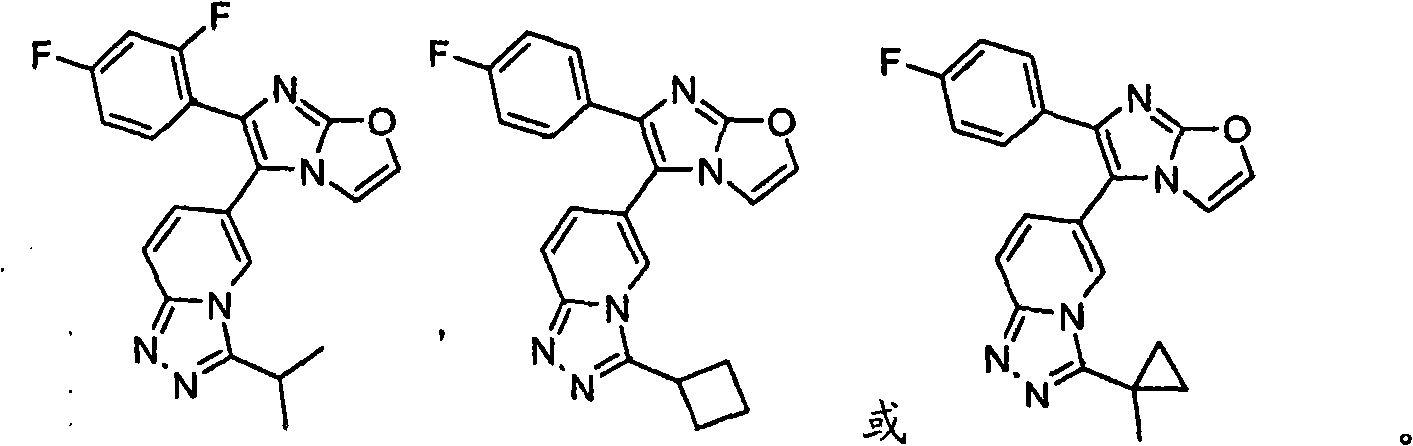
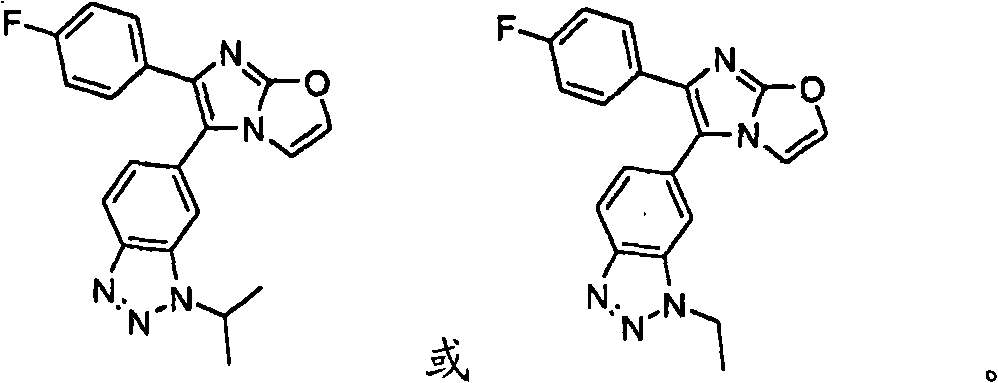
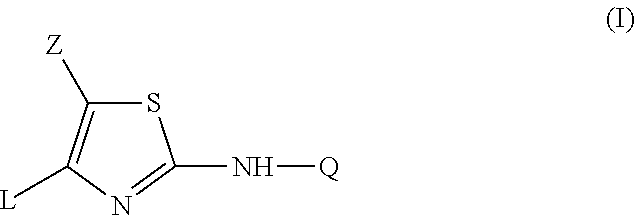
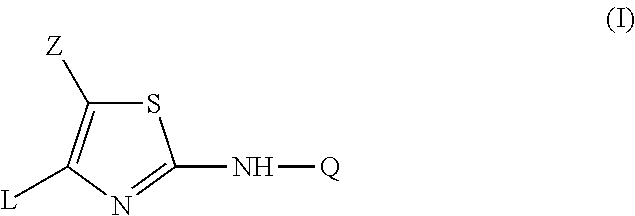
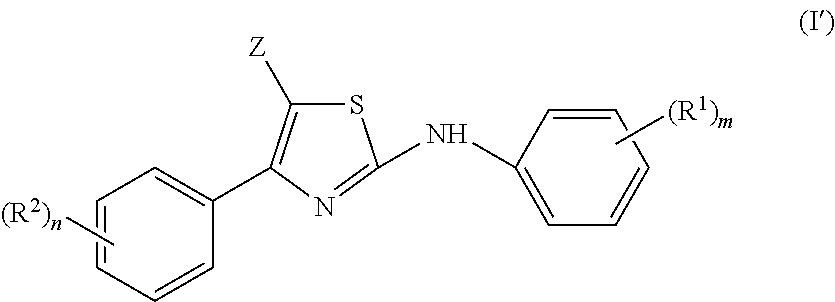
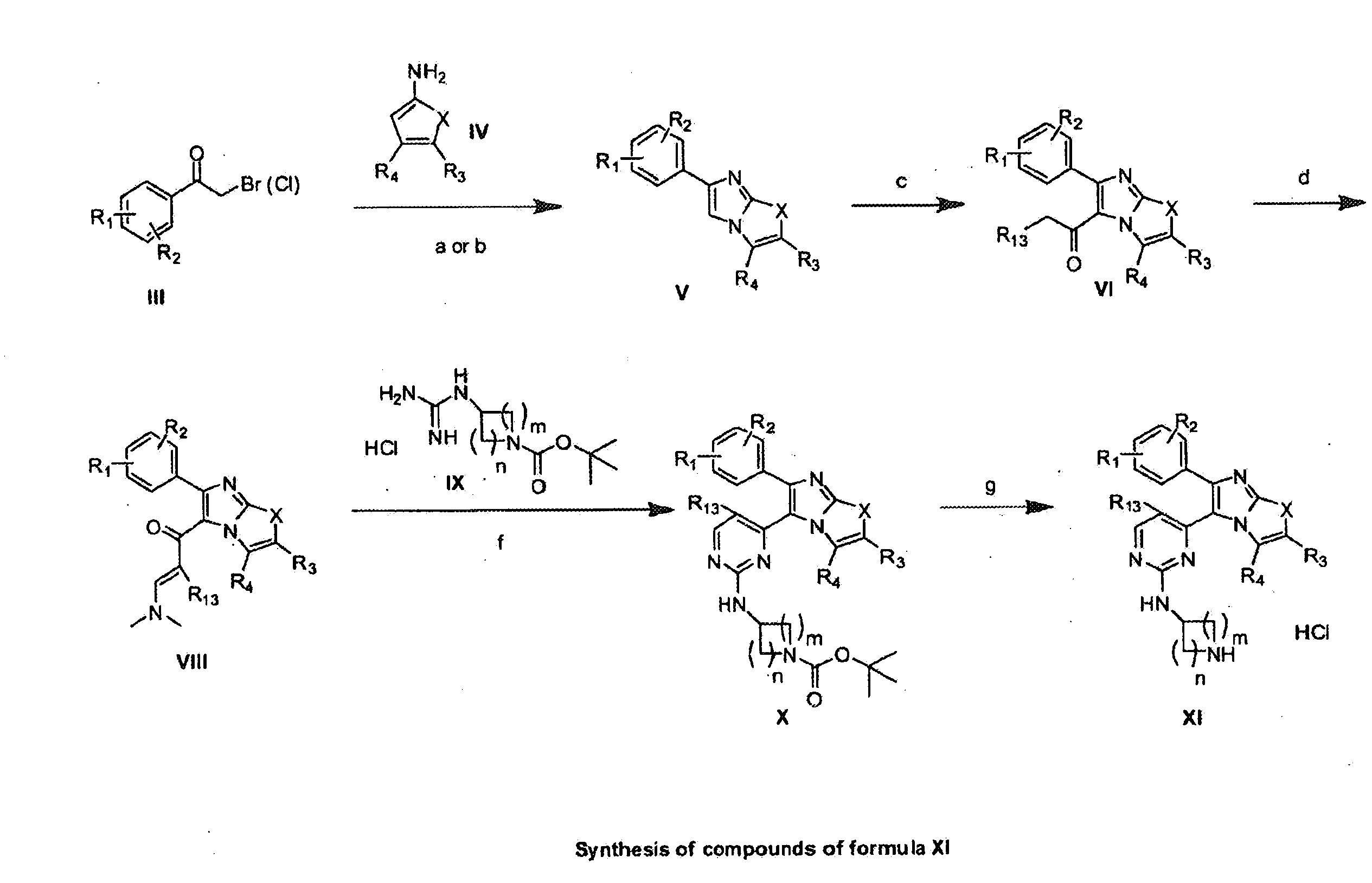
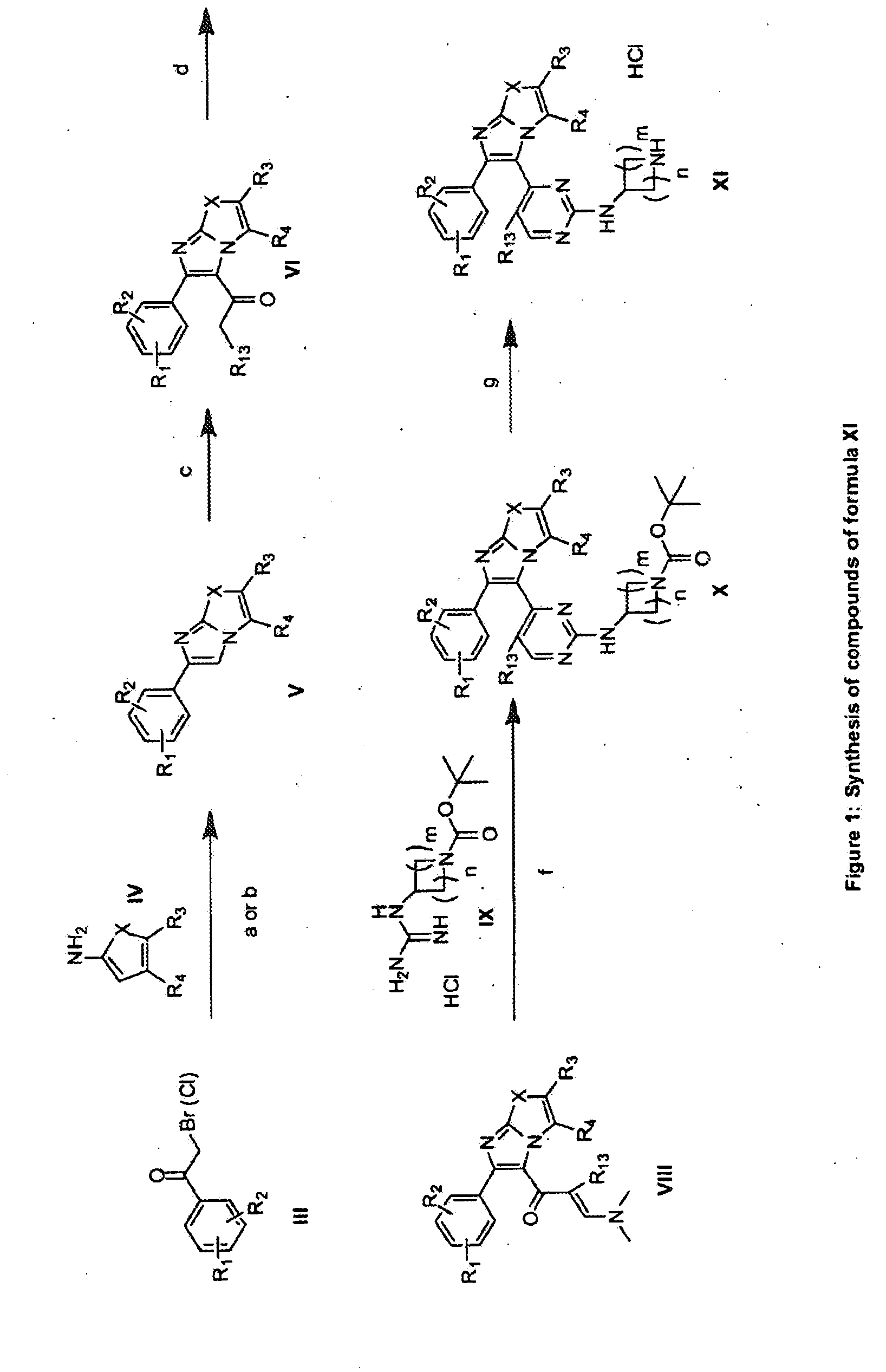
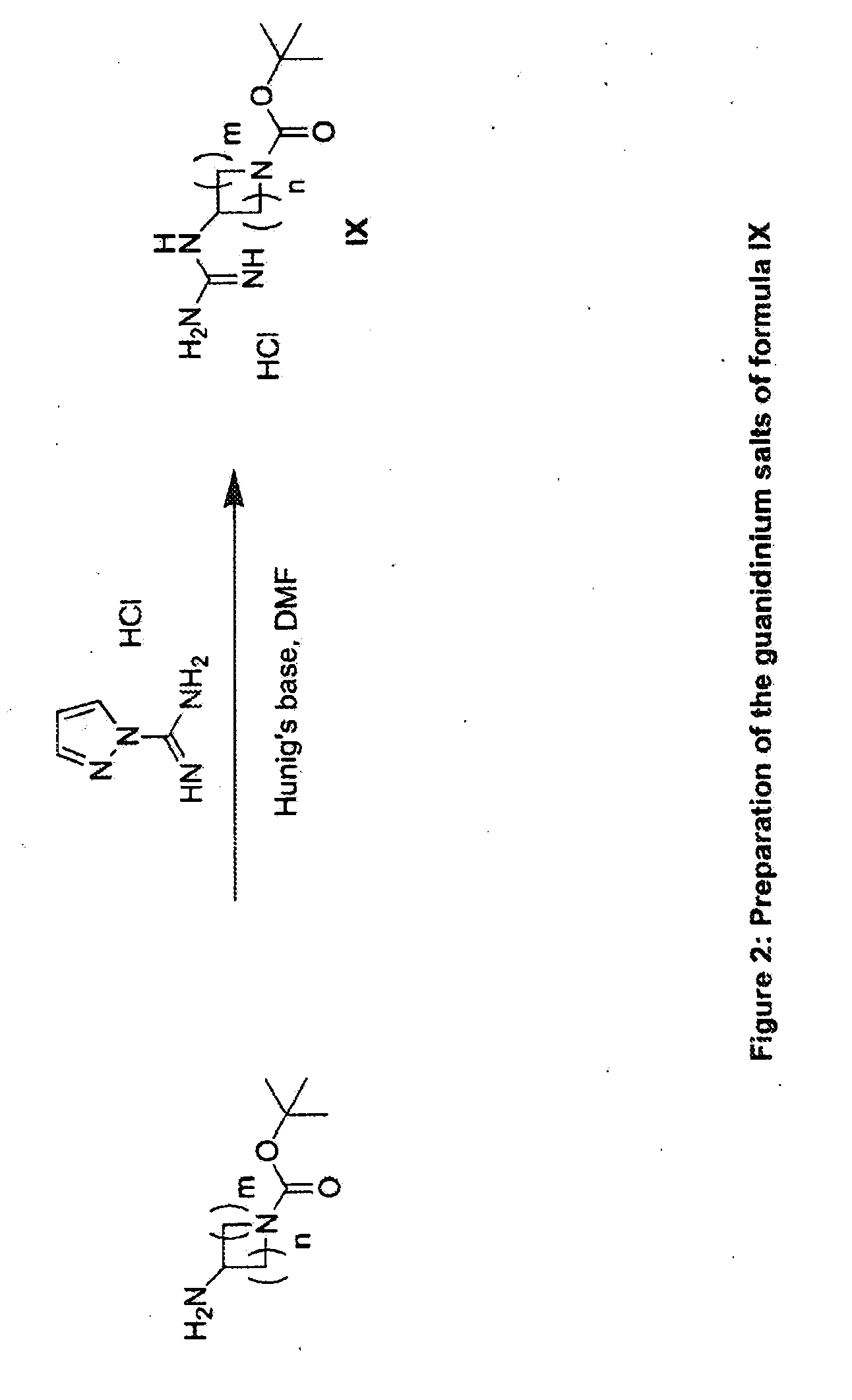
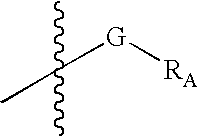
Substituted Imidazo[2,1-b]thiazole Compounds and Uses Thereof
Substituted imidazo[2,1-b]thiazole compounds corresponding to formula I,a method for producing them, pharmaceutical compositions containing them, and the use thereof for regularing mGluR5 receptors, or for treating or inhibiting disorders or disease states at least partially mediated by mGluR5 receptor such as pain, anxiety attacks, drug or alcohol dependency, and others.
Owner:PACESETTER INC +1
6-phenylimidazol[2, 1-b]thiazole-3-amide derivative, its preparation method and application
ActiveCN103204862AGood antitumor activityThe structure is correctOrganic active ingredientsOrganic chemistryMorpholineStructural formula
The invention discloses a 6-phenylimidazol[2, 1-b]thiazole-3-amide derivative, its preparation method and application. The structural formula of the compound is shown as formula I, wherein R1 is H, or one or more of the following mono-substituted or multi-substituted groups on a benzene ring: fluorine, chlorine, bromine, methyl, methoxyl, hydroxyl, nitro, amino, trifluoromethyl and cyano; R2 is hydrogen, fluorine, chlorine, bromine, methyl, methoxyl, hydroxyl, mercapto, amino, methylamino, ethylamino, morpholine, piperazine, methyl piperazine, ethyl piperazine, benzyl piperazine, p-methoxylbenzyl piperazine or p-chlorobenzyl piperazine; and n is 0 or 1 or 2. The raw materials for preparation of the derivative are easily available, the reaction is simple, and the synthesis steps are simple and are easy to operate. The compound provided in the invention has good anti-tumor activity, and has important practical value and application prospects in the field of antitumor medicine preparation. (Formula I).
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
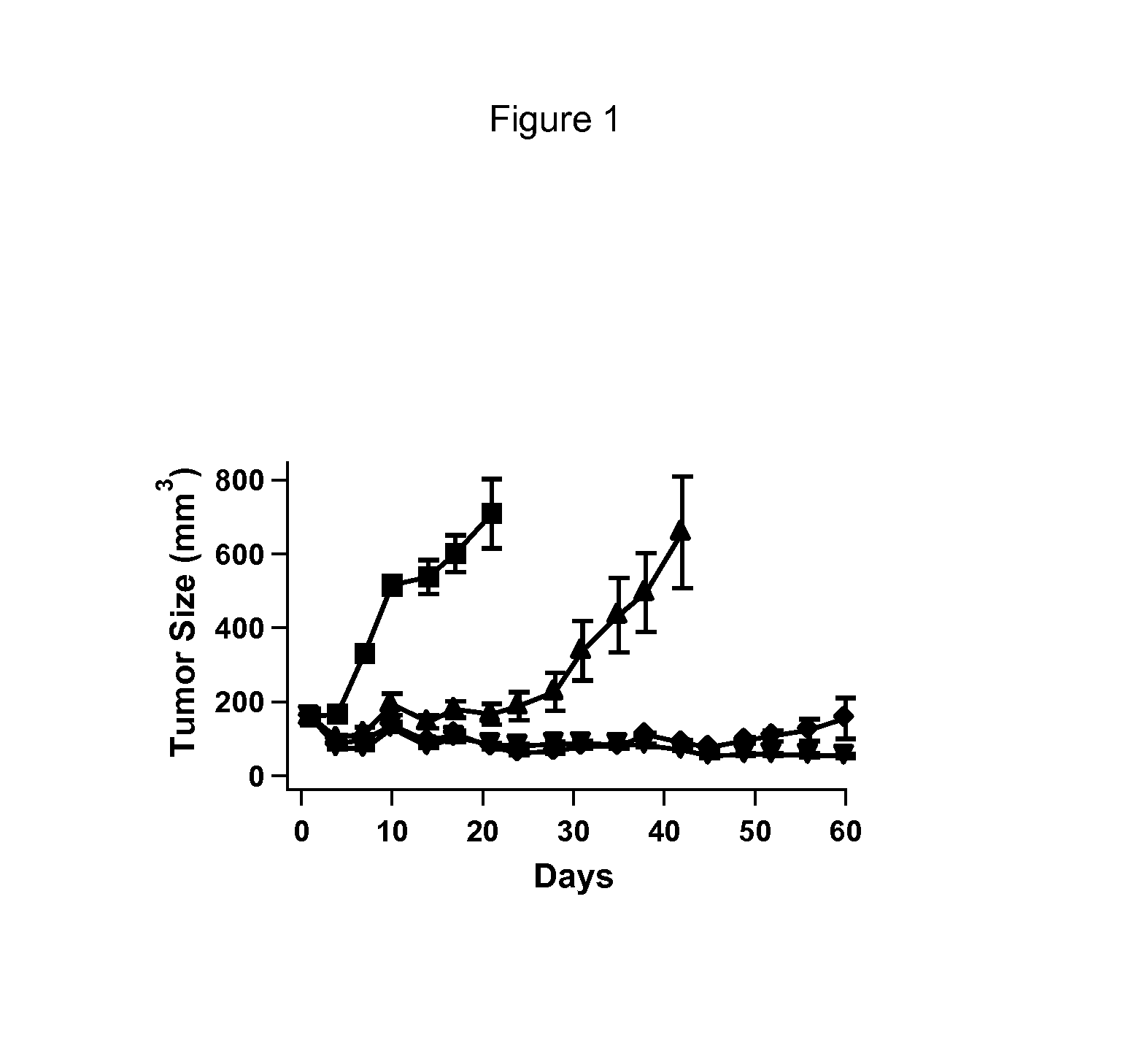
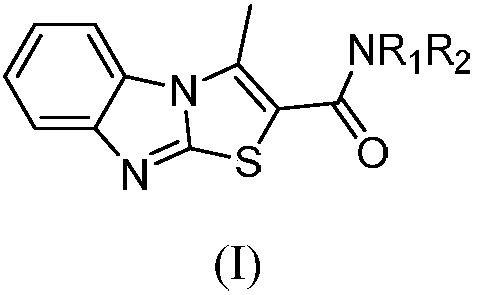
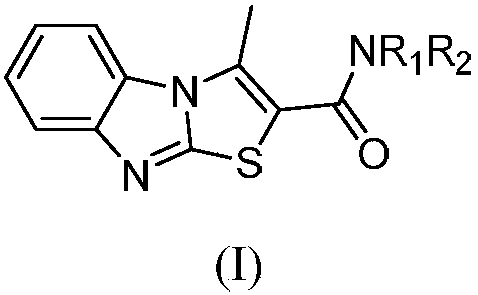
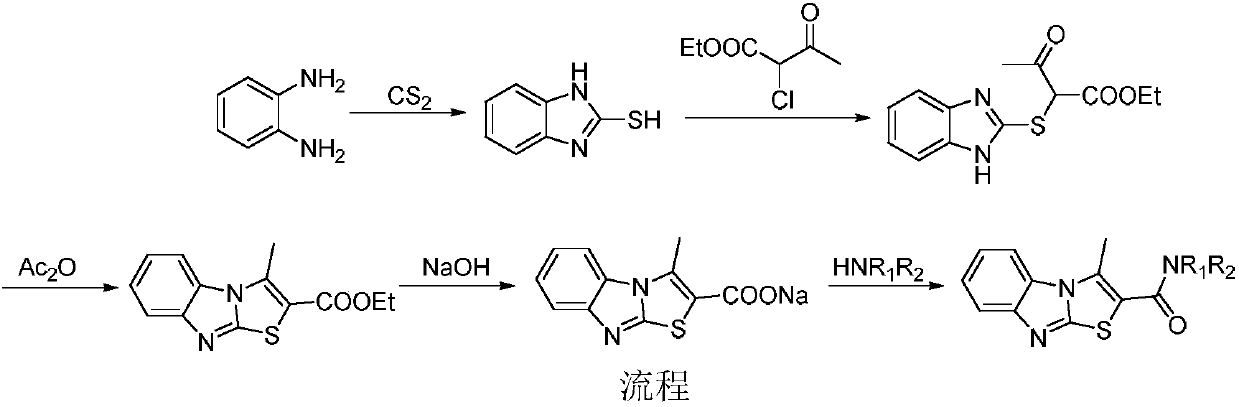
Benzimidazothiazole carboxamide compound and application thereof
The invention relates to the technical field of medicines, and relates to benzimidazothiazole carboxamide compound and application thereof. The benzimidazothiazole carboxamide compound comprises derivatives and pharmaceutically acceptable salts of the benzimidazothiazole carboxamide compound, and the general structure of benzimidazothiazole carboxamide compound is as shown in the description, wherein R1 and R2 are as described in the claims and the description. The benzimidazothiazole carboxamide compound and pharmaceutically acceptable acid addition salts of the benzimidazothiazole carboxamide compound can be combined with existing drugs or used alone as an epidermal growth factor tyrosine kinase inhibitor for treating associated diseases caused by transduction disorder of epidermal growth factor receptor signals such as small cell lung cancer, squamous cell carcinoma, adenocarcinoma, large cell carcinoma, colorectal cancer, mammary cancer, ovarian cancer and renal cell carcinoma.
Owner:SHENYANG PHARMA UNIVERSITY
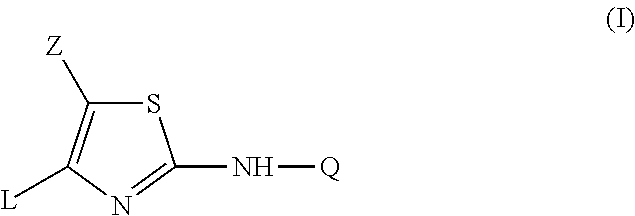
2-aniline-4-aryl substituted thiazole derivatives
This invention concerns the use of a compound of formula (I)a N-oxide, a pharmaceutically acceptable addition salt, a quaternary amine and a stereochemically isomeric form thereof, whereinZ is hydrogen, halo, C1-6alkyl, Het1, HO—C1-6alkyl-, cyano-C1-6alkyl-, amino-C(═O)—C1-6alkyl-, formylamino-C1-6alkyl-, C1-6alkyl-C(═O)—NH—C1-6alkyl-, mono- or di(C1-6alkyl)amino-C(═O)—C1-6alkyl-, phenyl-C1-6alkyl-, or Het4-C1-6alkyl-;Q is phenyl, pyridyl, benzofuranyl, 2,3-dihydro-benzofuranyl, pyrazolyl, isoxazolyl or indazolyl wherein each of said ring systems is optionally being substituted with up to three substituents each independently selected from halo, cyano, C1-6alkyl, C1-6alkyl-O—, C1-6alkylthio, Ar or polyhaloC1-6alkyl;L is phenyl, pyridyl, pyrimidazolyl, 8-Azapyrimidazolyl, pyridazinyl, imidazothiazolyl or furanyl wherein each of said ring systems may optionally be substituted with one or two or more substituents, each substituent independently being selected from halo, hydroxy, amino, cyano, C1-6alkyl or C1-6alkyl-O—;Het1 represents morpholinyl; pyrazolyl or imidazolyl;Het4 represents morpholinyl, pyrazolyl or imidazolyl;Ar represents phenyl optionally substituted with halo, C1-6alkyl, C1-6alkyl-O— or polyhaloC1-6alkyl; for the manufacture of a medicament for the prevention or the treatment or prophylaxis of psychotic disorders, intellectual impairment disorders or diseases or conditions in which modulation of the α7 nicotinic receptor is beneficial.
Owner:JANSSEN PHARMA NV
The preparation method of 5,6-dihydro-6-(2-naphthyl)imidazo[2,1-b]thiazole oxalate
ActiveCN110194774BSimple and efficient operationShort reaction pathOrganic compound preparationCarboxylic acid salt preparationOXALIC ACID DIHYDRATEOxalate
The invention discloses a preparation method of 5,6-dihydro-6-(2-naphthyl)imidazo[2,1-b]thiazole oxalate, comprising step 1: adding 2-vinyl to the solvent Naphthalene is the raw material, hydrogen peroxide and brominated compounds, and a catalyst is added to react in the reaction temperature range of -20°C to 200°C to obtain brominated intermediates. The amount of hydrogen peroxide is 0.5-5 equivalents, and the amount of brominated compounds is 1 ‑10 equivalents; step 2: add 2‑aminothiazole and solvent to the brominated intermediate obtained in step 1, without catalyst, react in the reaction temperature range of ‑20°C-200°C to obtain the thiazole intermediate, the The amount of 2-aminothiazole is 0.8-2 equivalents; Step 3: Add one or both of dihydrate oxalic acid and anhydrous oxalic acid and a solvent to the thiazole intermediate obtained in step 2, at -20°C-300°C Reaction in the reaction temperature range obtains 5,6-dihydro-6-(2-naphthyl) imidazo[2,1-b]thiazole oxalate. The reaction route is short and the pollution is small.
Owner:SUZHOU BAILINGWEI HYPERFINE MATERIAL
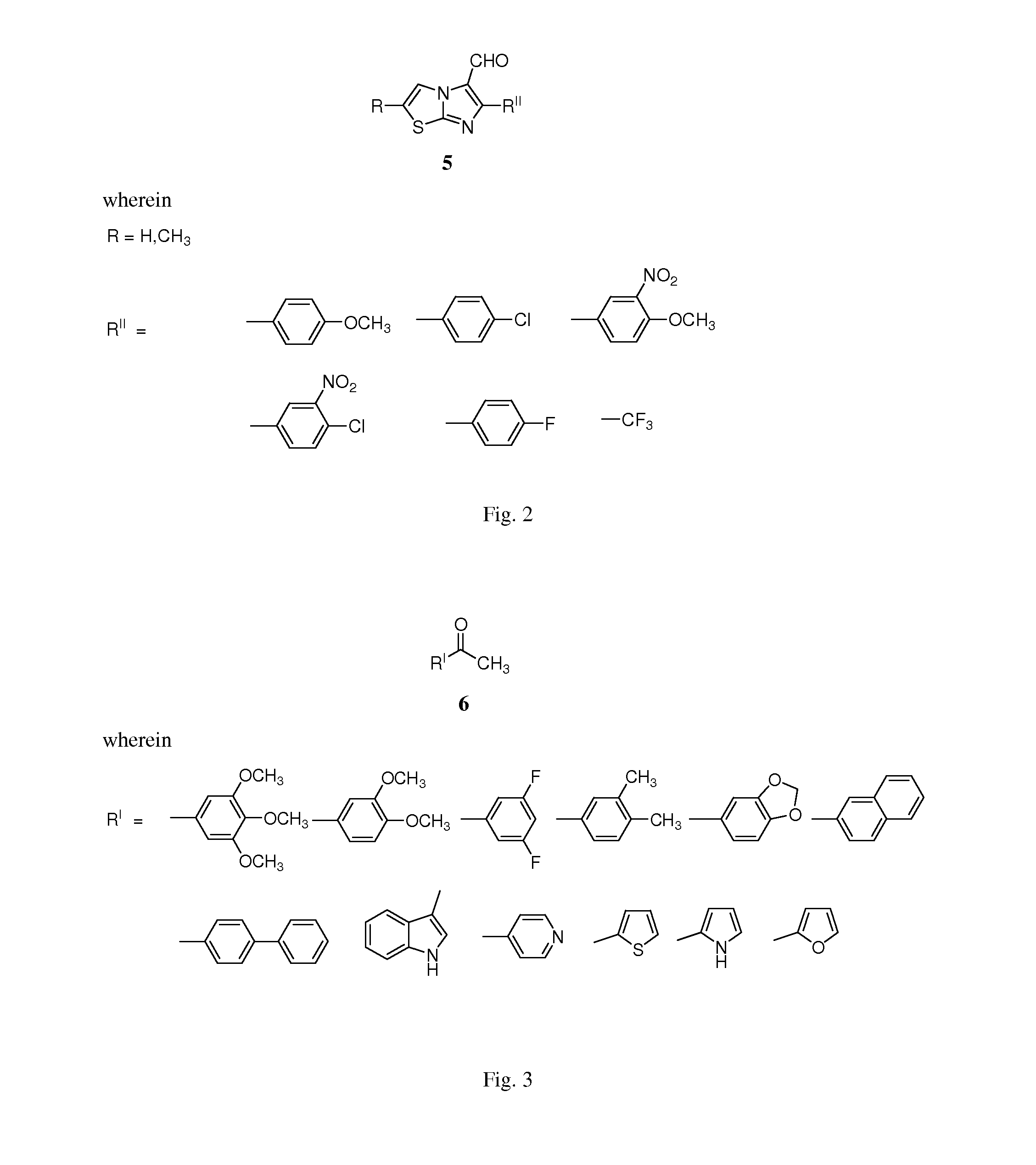
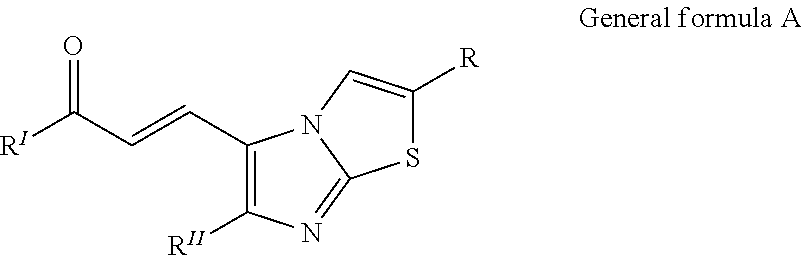
Imidazothiazole-chalcone derivatives as potential anticancer agents and process for the preparation thereof
The present invention provides a compounds 7a-f to 18a-f and 19a-f to 30a-f of general formula A, useful as potential anticancer agents against human cancer cell lines. The present invention further provides a process for the preparation of imidazothiazole-chalcone hybrids 7a-f to 18a-f and 19a-f to 30a-f of general formula Awherein
Owner:COUNCIL OF SCI & IND RES
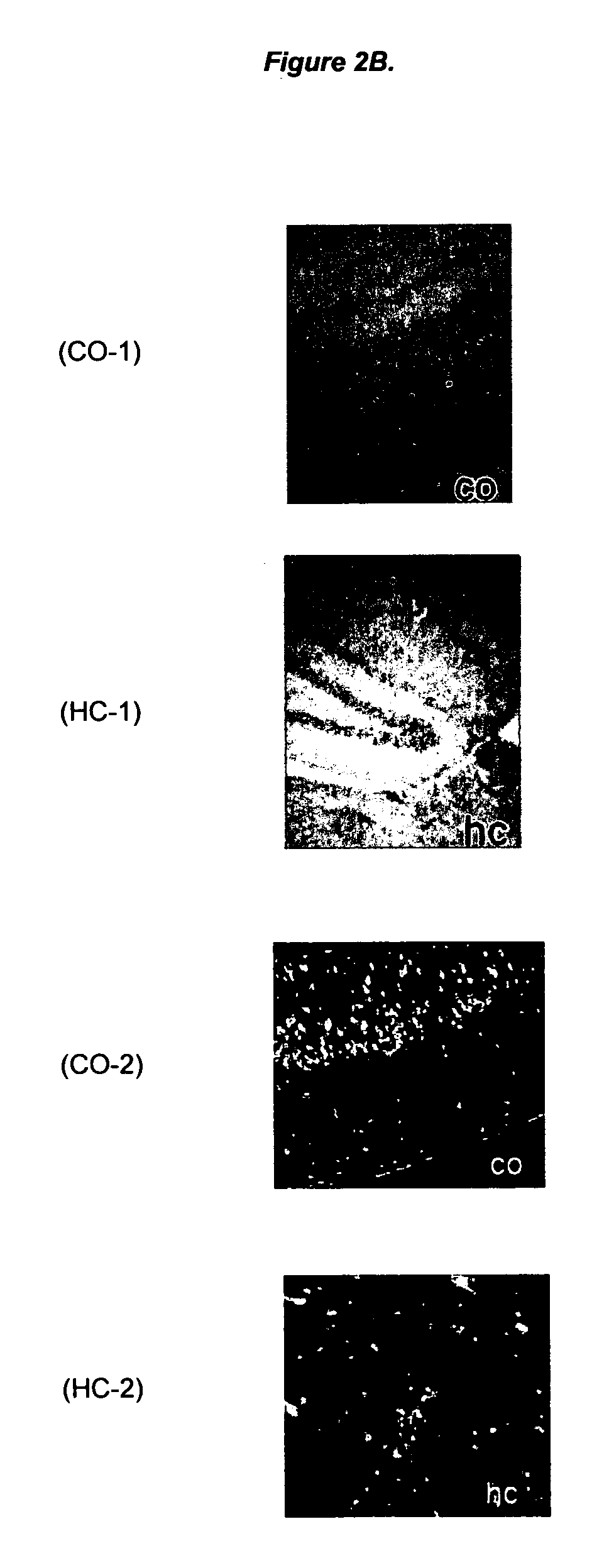
A kind of benzimidazolo[2,1-b]thiazole compound and its application
ActiveCN111362972BStrong inhibitory activityStrong medicineOrganic active ingredientsOrganic chemistryDiseaseTyrosine
Owner:JIANGNAN UNIV
Coumarosone fluorescent probe for detecting Cu < 2 + > as well as preparation method and application of coumarosone fluorescent probe
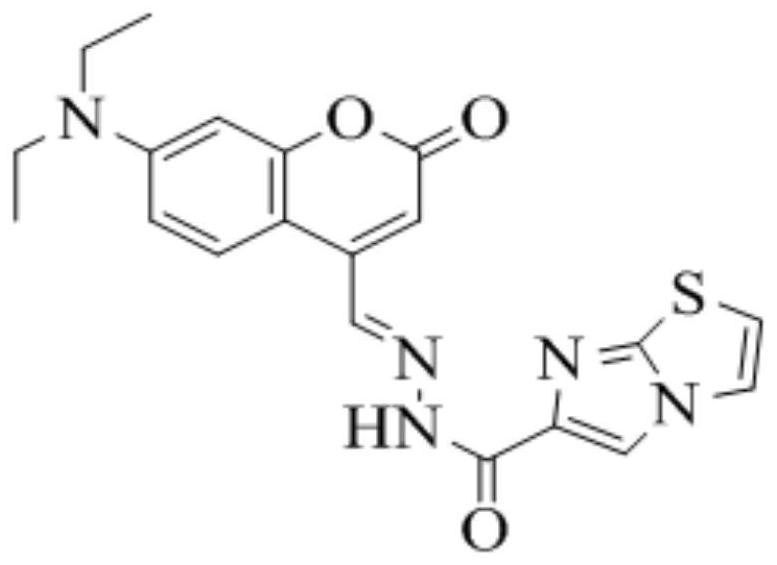
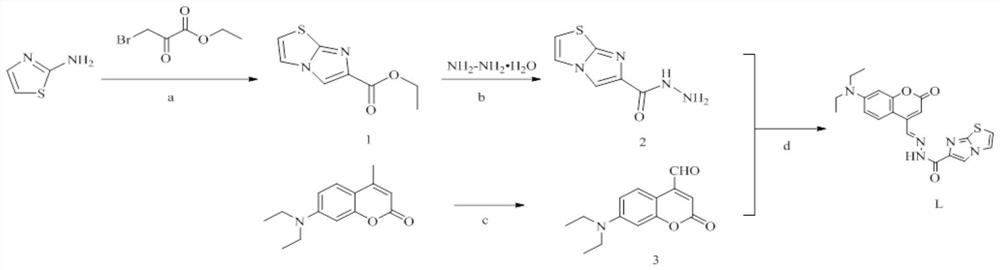
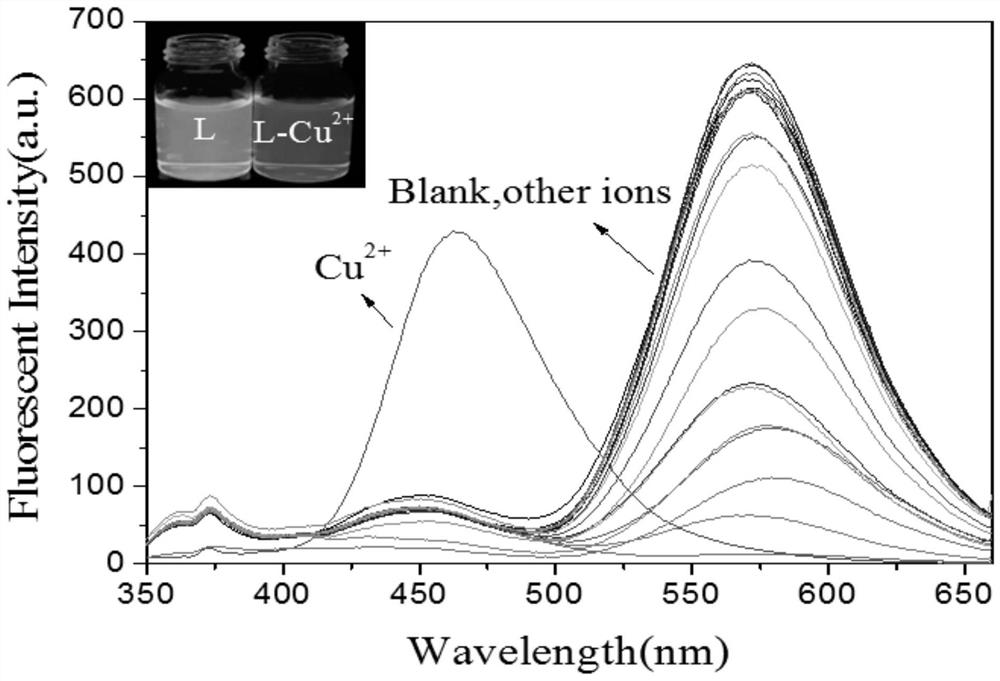
PendingCN114437112AEfficient identificationImprove anti-interference abilityOrganic chemistryFluorescence/phosphorescenceFluoProbesImidazothiazole
The invention relates to the field of chemical analysis, in particular to a coumarin fluorescent probe for detecting Cu < 2 + > as well as a preparation method and application of the coumarin fluorescent probe. The novel fluorescent probe for identifying Cu < 2 + >, namely (E)-N '-(7-(diethylamino)-2-oxo-2H-benzopyran-4-yl methylene) imidazo [2, 1-b] thiazole-6-hydrazide, is successfully prepared by utilizing dehydration condensation of imidazo [2, 1-b] thiazole and coumarin aldehyde, the probe is novel in structure, realizes two-coordinate binding with Cu < 2 + >, accords with an ICT mechanism, and can be used for detecting Cu < 2 + >. And the whole material shows specific recognition on copper ions.
Owner:宁夏师范学院
Preparation method of 5-(2-oxotetrahydrothienoimidazole-4(2h)-enyl)pentanoic acid compounds
The invention discloses a preparation method of 5-(2-oxytetrahydrothiophene imidazole-4(2H)-alkenyl pentanoic acid compounds. The preparation method comprises the following steps: performing reactionon (3S,7aR)-6-substituted benzyl-7-alkoxy-3-substituted phenyl tetrahydroimidazole[1,5-c]thiazole-5(3H)-ketone and 1-cyclohexene oxytrimethylsilane to generate (3S,7aR)-6-substituted benzyl-7-(2-oxycyclohexyl)-3-substituted tetrahydroimidazole[1,5-c]thiazole-5(3H)-ketone, and sequentially performing ring-opening reaction, reduction ring-opening reaction, cyclization and elimination reaction to obtain the 5-(2-oxytetrahydrothiophene imidazole-4(2H)-alkenyl pentanoic acid compounds. The preparation method has the advantages of little side reaction, high yield, environmental friendliness, mild and easily controlled reaction condition and simplicity in aftertreatment, and is suitable for industrialized production.
Owner:ZHEJIANG NHU CO LTD +2
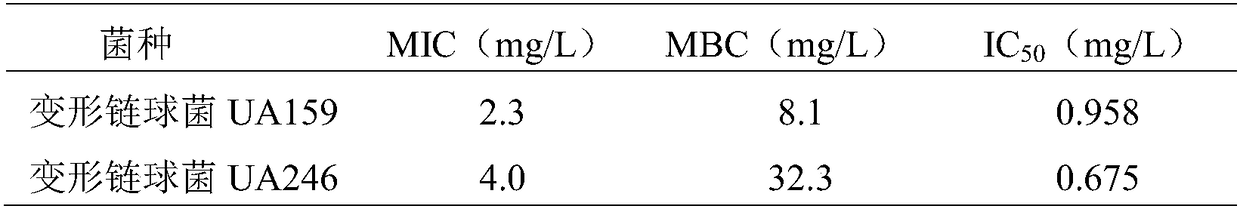
Application of N-(5-bromo-2-methoxyphenyl)benzoimidazothiazole carboxamide
InactiveCN108685912AInhibition formationEnhanced inhibitory effectAntibacterial agentsOrganic active ingredientsBiofilmMicrobiology
The invention discloses an application of N-(5-bromo-2-methoxyphenyl)benzoimidazothiazole carboxamide in preparation of drugs for inhibiting and killing streptococcus mutans type strains or in preparation of drugs for inhibiting formation of a streptococcus mutans type strain biofilm. Experiments prove that the compound shows good bacteriostatic and bactericidal activities against the streptococcus mutans type strains in a floating state, and the inhibition rate of the compound to the streptococcus mutans biofilm reaches 99% or more when the final concentration of the compound in a culture medium reaches 4 mg / L. The compound can be used as a novel lead compound for preventing dental caries, and has broad application prospects in preparation of oral streptococcus mutans biofilm inhibitors.
Owner:SHANDONG UNIV
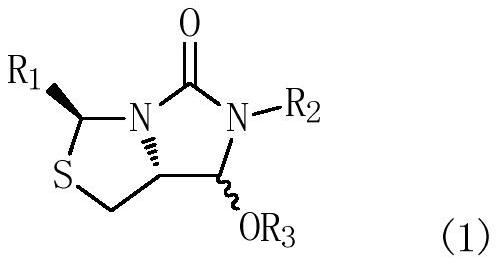
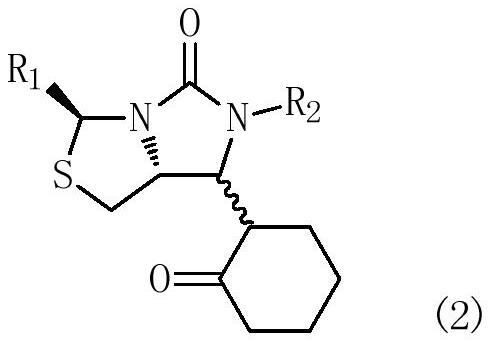
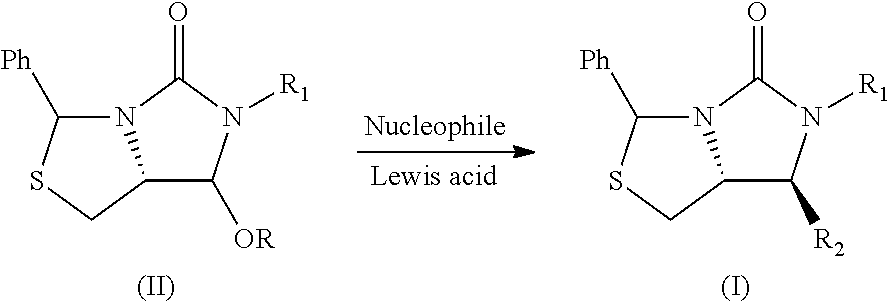
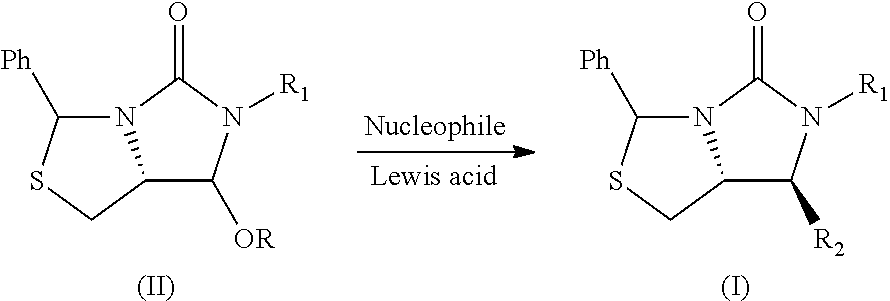
Process for the preparation of a substituted imidazothiazolone compound
PendingUS20210040119A1Cheap and friendly to environmentHigh selectivityOrganic chemistryEngineeringCombinatorial chemistry
The present invention provides a new process for the preparation of a substituted imidazothiazolone compound. The process of the present invention uses a fluoride-free Lewis acid which is cheap and friendly to environment, and provides high selectivity and yield.
Owner:DSM IP ASSETS BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Substituted Imidazo[2,1-b]thiazole Compounds and Uses Thereof Substituted Imidazo[2,1-b]thiazole Compounds and Uses Thereof](https://images-eureka.patsnap.com/patent_img/eff0ab66-7e39-4009-a80c-78048ae07ffc/US20090005399A1-20090101-C00001.png)
![Substituted Imidazo[2,1-b]thiazole Compounds and Uses Thereof Substituted Imidazo[2,1-b]thiazole Compounds and Uses Thereof](https://images-eureka.patsnap.com/patent_img/eff0ab66-7e39-4009-a80c-78048ae07ffc/US20090005399A1-20090101-C00002.png)
![Substituted Imidazo[2,1-b]thiazole Compounds and Uses Thereof Substituted Imidazo[2,1-b]thiazole Compounds and Uses Thereof](https://images-eureka.patsnap.com/patent_img/eff0ab66-7e39-4009-a80c-78048ae07ffc/US20090005399A1-20090101-C00003.png)
![6-phenylimidazol[2, 1-b]thiazole-3-amide derivative, its preparation method and application 6-phenylimidazol[2, 1-b]thiazole-3-amide derivative, its preparation method and application](https://images-eureka.patsnap.com/patent_img/20e66d43-fdcc-4e68-8565-5e6a524d2f55/BDA0000130404760000011.PNG)
![6-phenylimidazol[2, 1-b]thiazole-3-amide derivative, its preparation method and application 6-phenylimidazol[2, 1-b]thiazole-3-amide derivative, its preparation method and application](https://images-eureka.patsnap.com/patent_img/20e66d43-fdcc-4e68-8565-5e6a524d2f55/BDA0000130404760000021.PNG)
![6-phenylimidazol[2, 1-b]thiazole-3-amide derivative, its preparation method and application 6-phenylimidazol[2, 1-b]thiazole-3-amide derivative, its preparation method and application](https://images-eureka.patsnap.com/patent_img/20e66d43-fdcc-4e68-8565-5e6a524d2f55/BDA0000130404760000022.PNG)
![The preparation method of 5,6-dihydro-6-(2-naphthyl)imidazo[2,1-b]thiazole oxalate The preparation method of 5,6-dihydro-6-(2-naphthyl)imidazo[2,1-b]thiazole oxalate](https://images-eureka.patsnap.com/patent_img/164df8da-8d06-43df-9d35-e3dfa0cda118/HDA0002084925820000011.png)
![The preparation method of 5,6-dihydro-6-(2-naphthyl)imidazo[2,1-b]thiazole oxalate The preparation method of 5,6-dihydro-6-(2-naphthyl)imidazo[2,1-b]thiazole oxalate](https://images-eureka.patsnap.com/patent_img/164df8da-8d06-43df-9d35-e3dfa0cda118/HDA0002084925820000021.png)
![The preparation method of 5,6-dihydro-6-(2-naphthyl)imidazo[2,1-b]thiazole oxalate The preparation method of 5,6-dihydro-6-(2-naphthyl)imidazo[2,1-b]thiazole oxalate](https://images-eureka.patsnap.com/patent_img/164df8da-8d06-43df-9d35-e3dfa0cda118/HDA0002084925820000031.png)
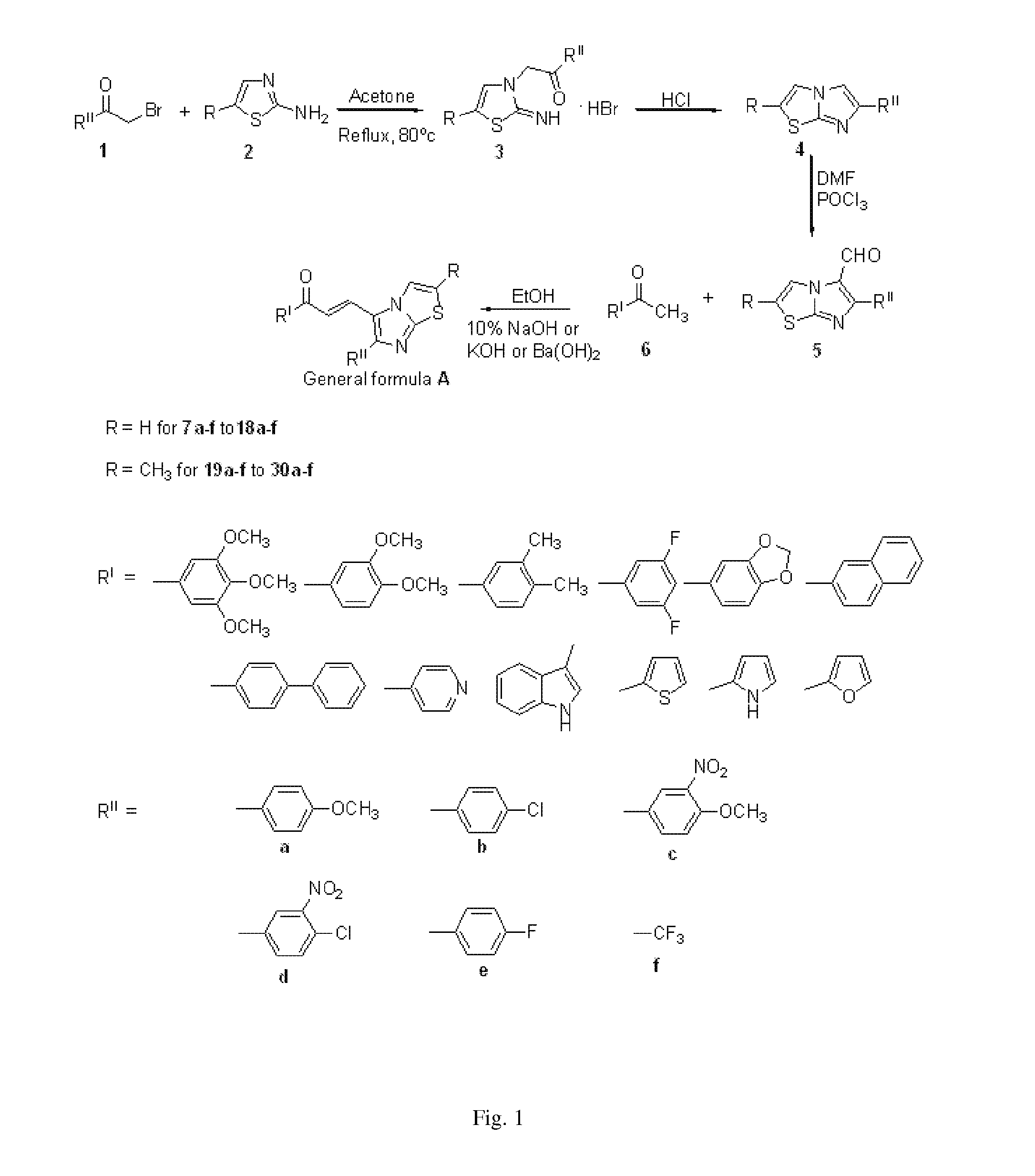
![A kind of benzimidazolo[2,1-b]thiazole compound and its application A kind of benzimidazolo[2,1-b]thiazole compound and its application](https://images-eureka.patsnap.com/patent_img/32a1800f-3294-4538-81c9-73623dd5497e/BDA0002470808370000043.png)
![A kind of benzimidazolo[2,1-b]thiazole compound and its application A kind of benzimidazolo[2,1-b]thiazole compound and its application](https://images-eureka.patsnap.com/patent_img/32a1800f-3294-4538-81c9-73623dd5497e/BDA0002470808370000051.png)
![A kind of benzimidazolo[2,1-b]thiazole compound and its application A kind of benzimidazolo[2,1-b]thiazole compound and its application](https://images-eureka.patsnap.com/patent_img/32a1800f-3294-4538-81c9-73623dd5497e/BDA0002470808370000052.png)