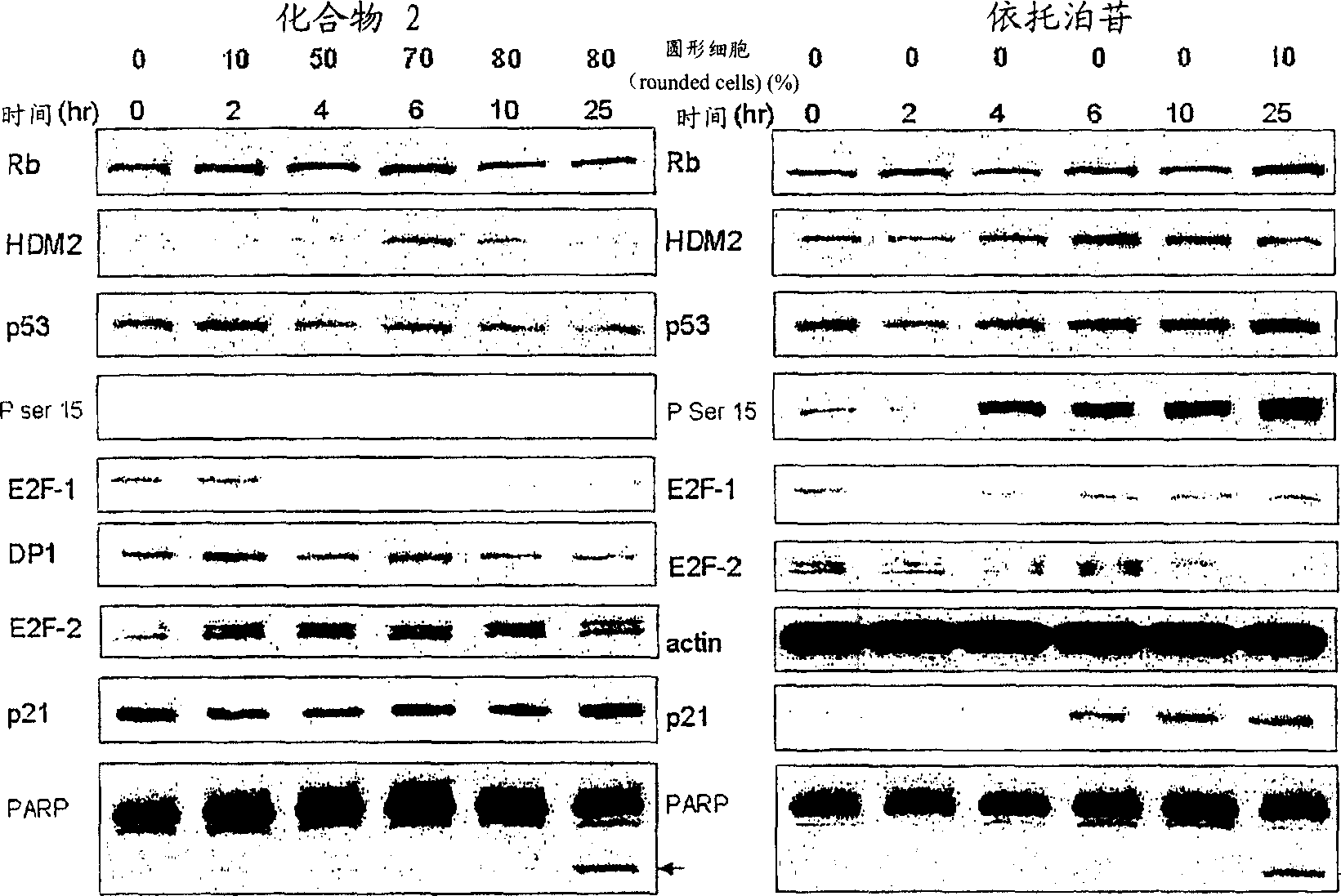
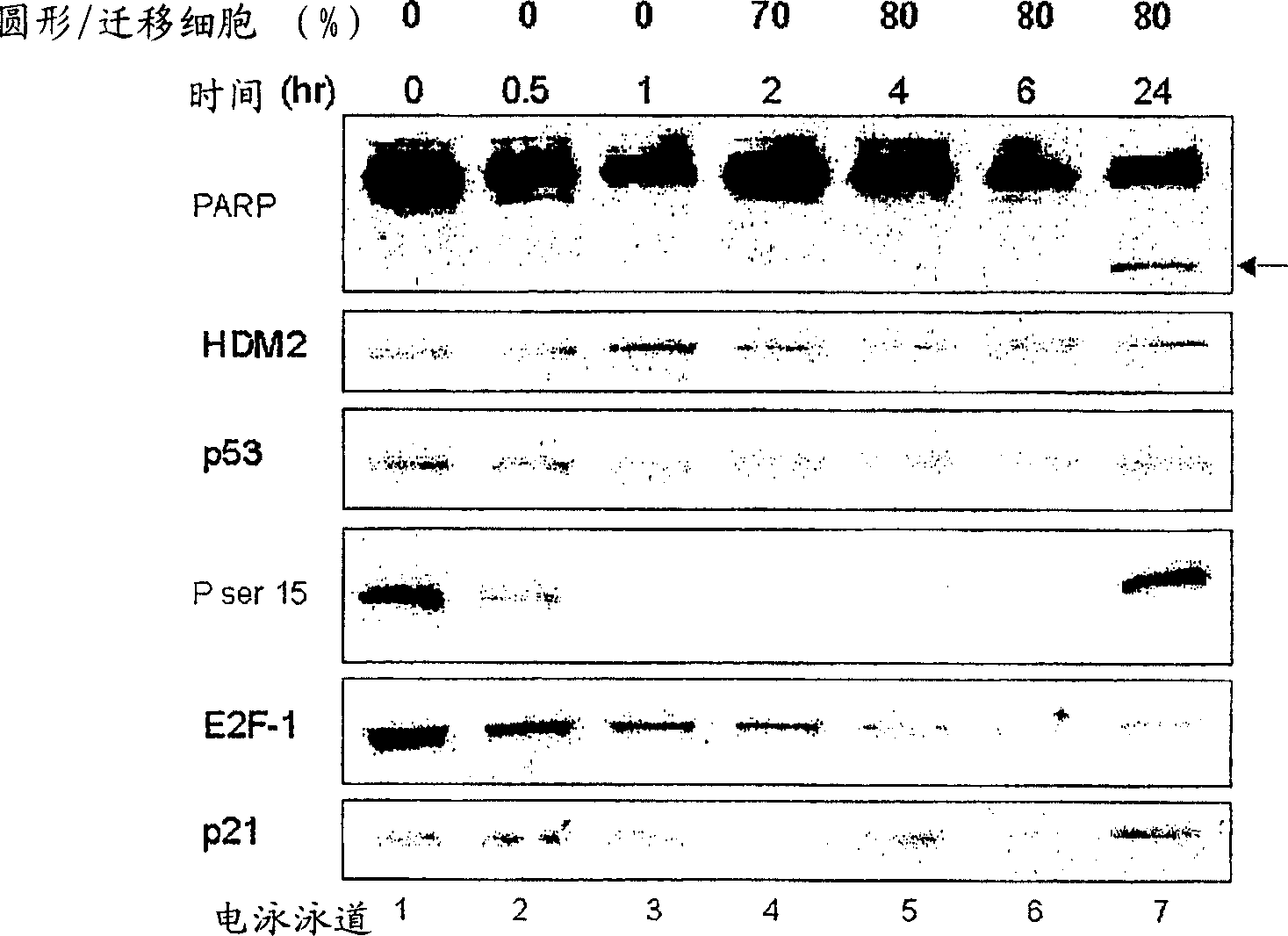
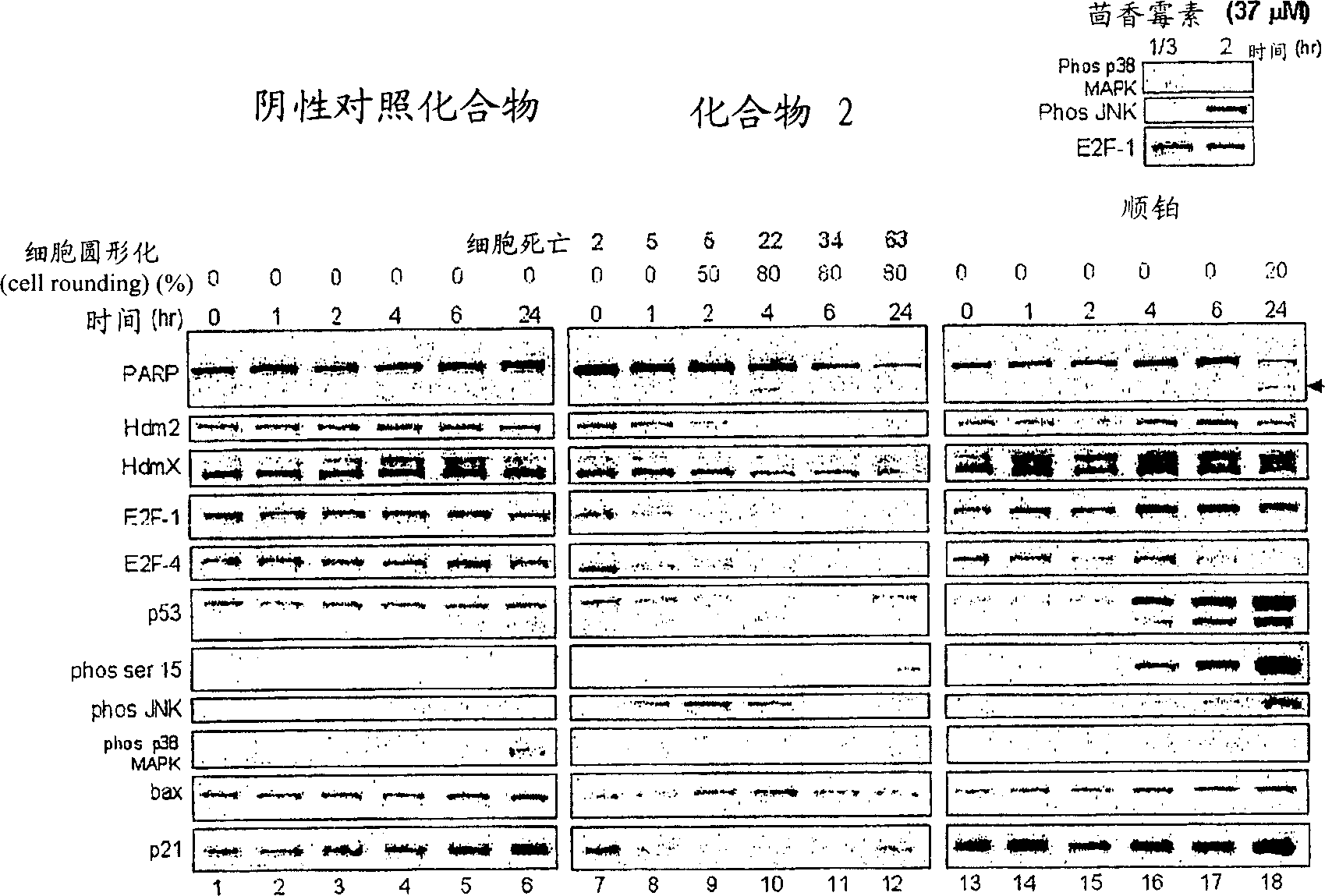
Bisarylsulfonamide compounds and their use in cancer therapy
A compound and application technology, applied in the field of bisaryl sulfonamide compounds, can solve problems such as low affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0835] General method for the preparation of primary bisarylsulfonamides
[0836] The appropriate sulfonyl chloride (1.0 mol equiv) was suspended in dichloromethane. The suspension was cooled to 0 °C. While the reaction mixture is being stirred, a suitable amino component (suitably such as aniline, benzylamine, etc.) (1.1 molar equivalent) and pyridine (1.5 molar equivalent) are added. The reaction mixture was slowly warmed to room temperature. Stirring was continued until TLC [heptane:ethyl acetate (2:1)] indicated that the reaction was complete. The product was isolated and purified according to the following procedure: the reaction mixture was diluted with dichloromethane, then washed successively with dilute aqueous hydrochloric acid, saturated NaHCO 3 Aqueous, water and brine washes. MgSO for organic part 4 Drying and concentration under reduced pressure gave the crude sulfonamide. Column chromatography [column (Isolute SI; Jones Chromatography), heptane:ethyl ace...
Embodiment 2
[0952] General method for the preparation of N-alkylated primary bisarylsulfonamides
[0953] The primary bisarylsulfonamide (1.0 mol equivalent) of Example 1 was dissolved in anhydrous acetone, the solution was cooled to 0° C., and then triethylamine (5.0 mol equivalent) was slowly added. After 30 minutes, the alkylating agent (alkyl or aralkyl halide; 5.0 mol equiv.) was added slowly and the solution was stirred until TLC [heptane:ethyl acetate (2:1)] indicated complete reaction. The reaction mixture was then concentrated, diluted with water and extracted with ethyl acetate. After concentration of the extract, the residue was subjected to column chromatography [column (Isolute SI; Jones Chromatography), heptane:ethyl acetate (12:1→3:1)] to give the desired alkylated sulfonamide.
[0954] The N-alkylated bisarylsulfonamide compounds of the present invention are prepared as described above. The specific analysis results of representative compounds are as follows:
[0955]...
Embodiment 3
[0966] Fluorescence polarization competitive binding assay
[0967] The assay was done using a 96-well microtiter (Costar). TBS-BSA buffer solution (50 mM Tris pH7.4, 150 mM NaCl, and 0.1% BSA) of recombinant HDM2 (1.5 μg / well) was incubated at room temperature in the presence of serially diluted test compounds (at a final concentration of 5% DMSO in TBS-BSA buffer solution) for 5 minutes. Add a fluorescently labeled p53-derived peptide (Fluorescein-Met-Pro-Arg-Phe-Met-Asp-Tyr-Trp-Glu-Gly-Leu-Asn-NH 2 , 0.2 μM), and then the plate was incubated at room temperature for 45 minutes. The fluorescence polarization of the peptide was measured (excitation 485 nm, emission 520 nm). Calculate IC from dose-response curve 50 value.
[0968] ELISA-type competitive binding assay
[0969] Streptavidin-coated 96-well plates (Pierce Chemical Co., St. Louis, MO, USA) were washed with TBS / BSA (25 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.05% Tween 20, 0.1% BSA). Biotinylated p53-derived pep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com