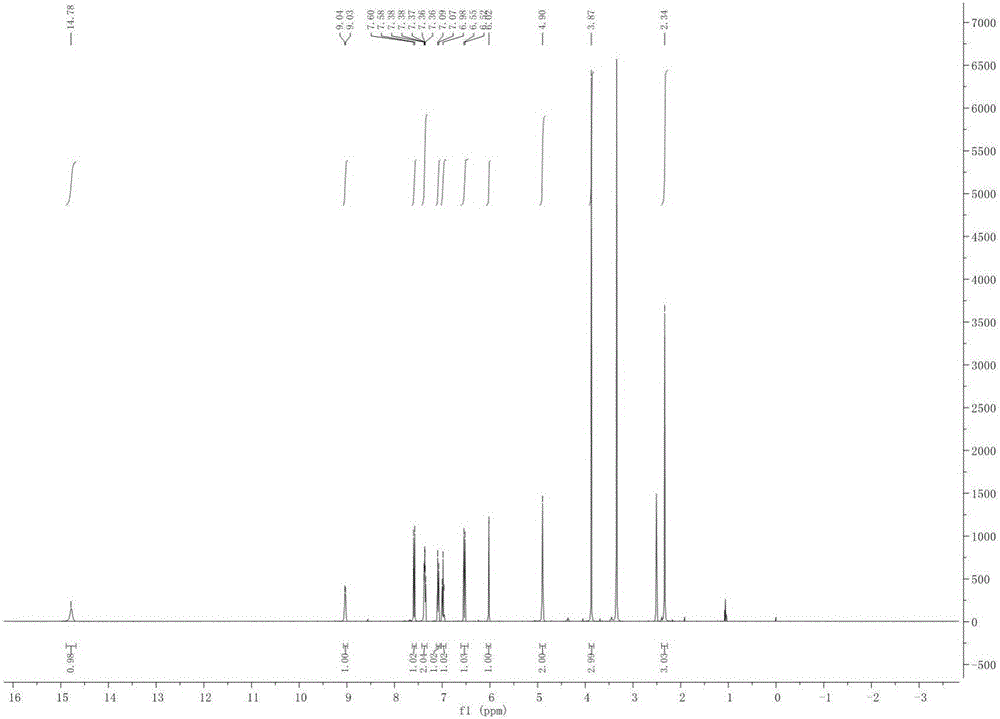
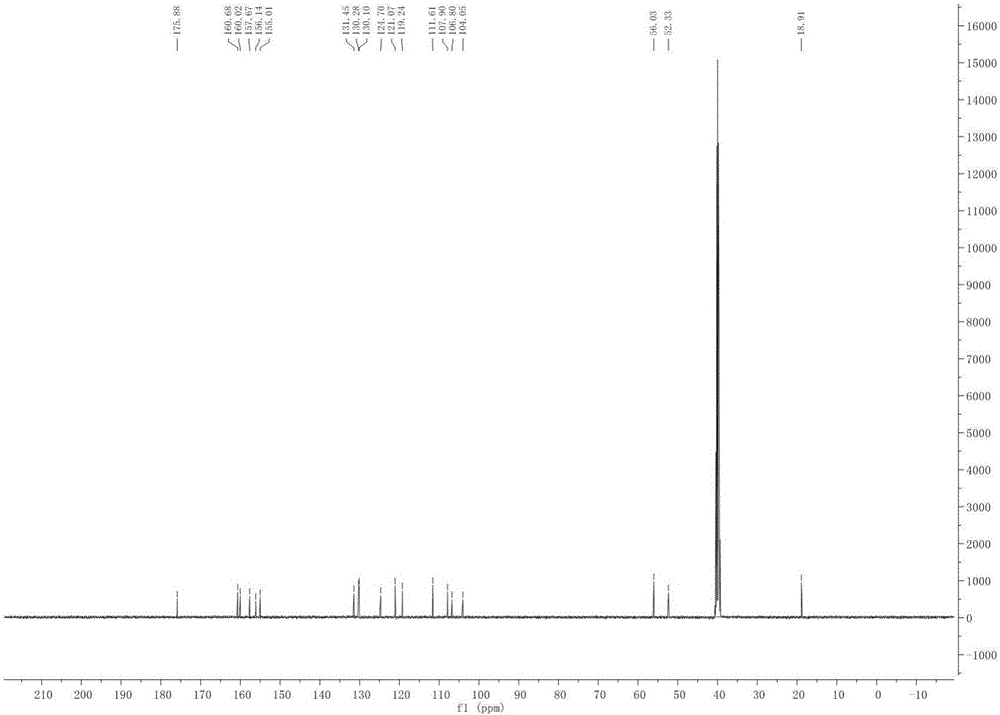
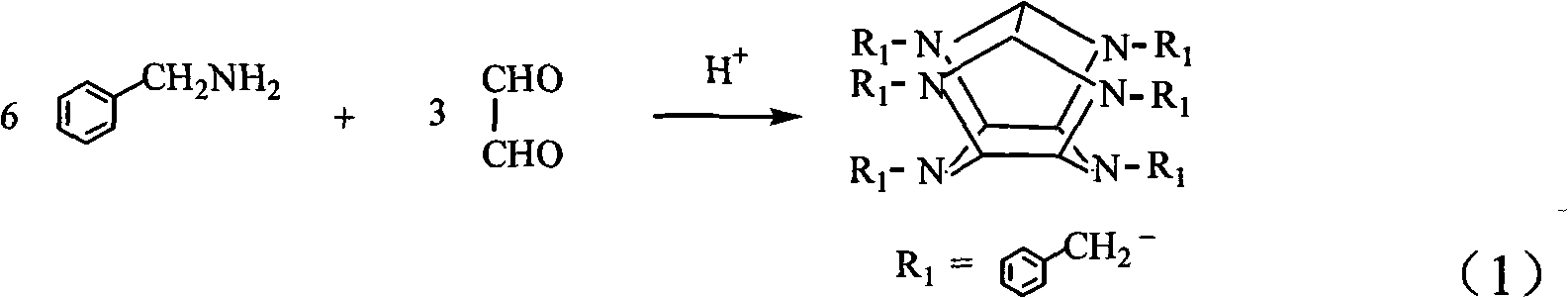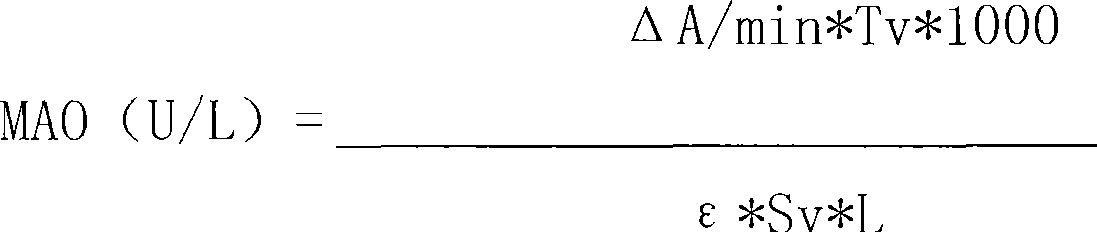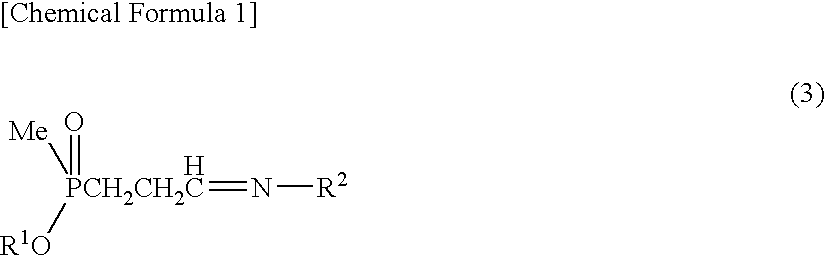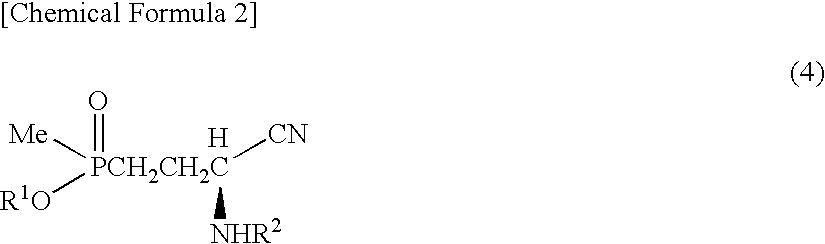Patents
Literature
490 results about "Benzylamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Benzylamine is an organic chemical compound with the condensed structural formula C₆H₅CH₂NH₂ (sometimes abbreviated as PhCH₂NH₂ or BnNH₂). It consists of a benzyl group, C₆H₅CH2, attached to an amine functional group, NH₂. This colorless liquid is a common precursor in organic synthesis and used in the industrial production of many pharmaceuticals. The hydrochloride salt was used to treat motion sickness on the Mercury-Atlas 6 mission in which NASA astronaut John Glenn became the first American to orbit the Earth.
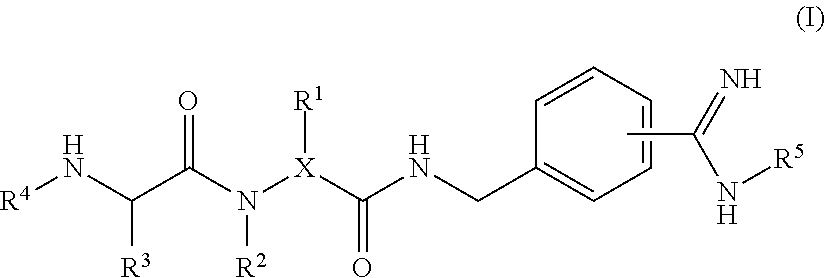
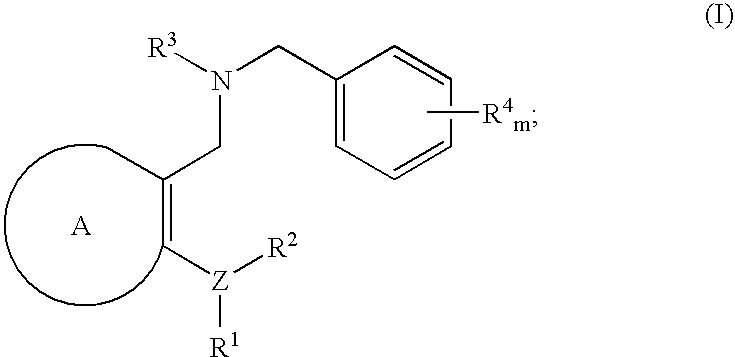
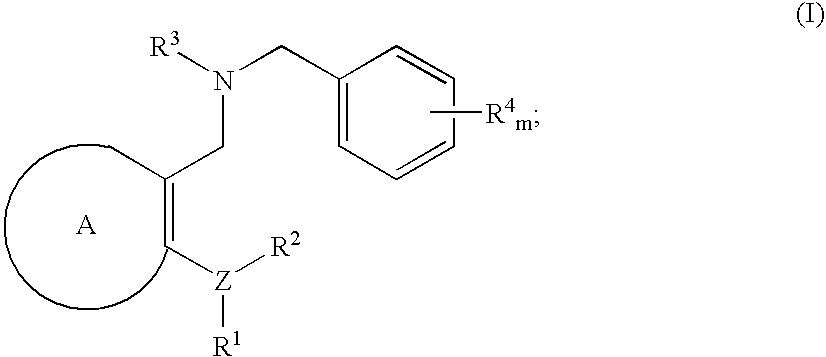
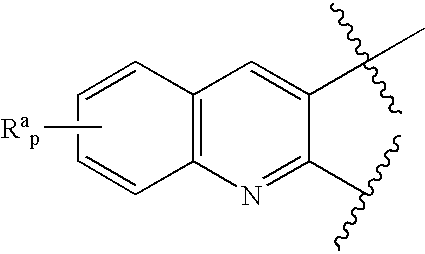
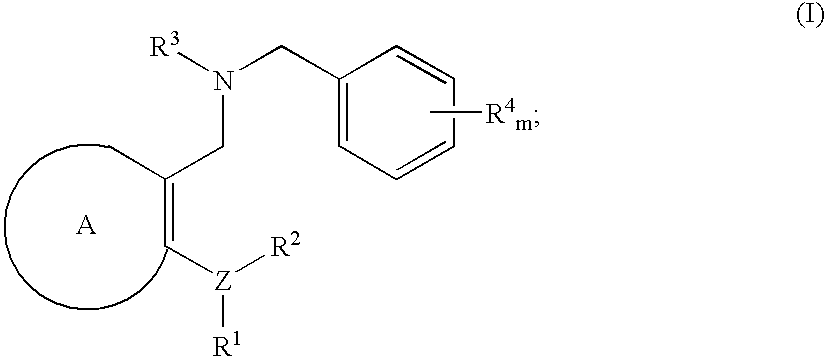
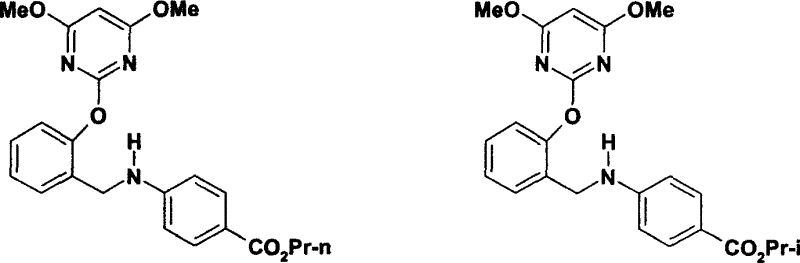
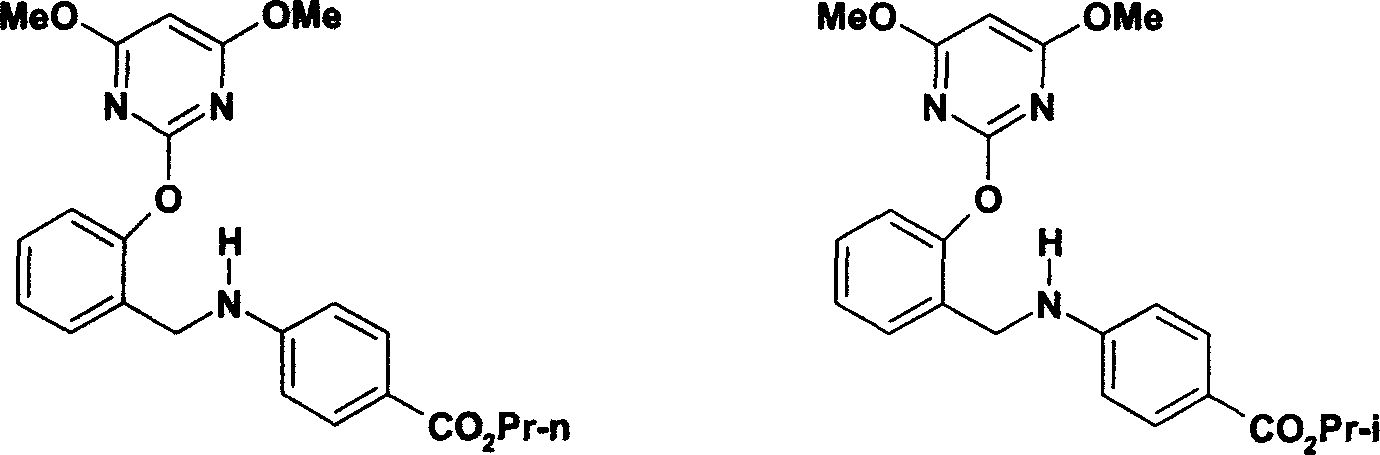
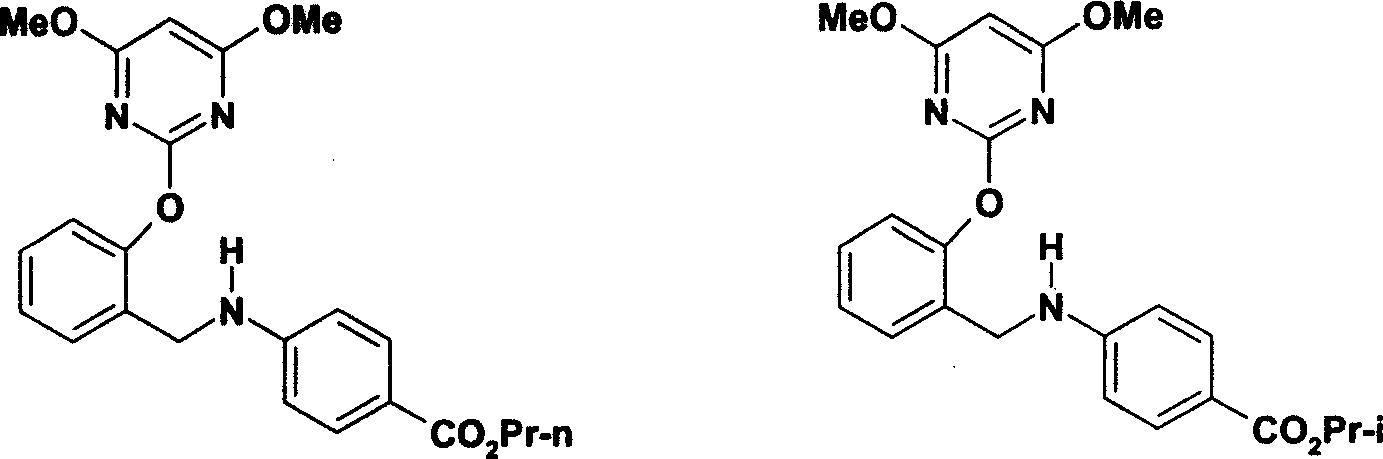
Novel benzylamine derivatives and their utility as cholesterol ester-transfer protein inhibitors
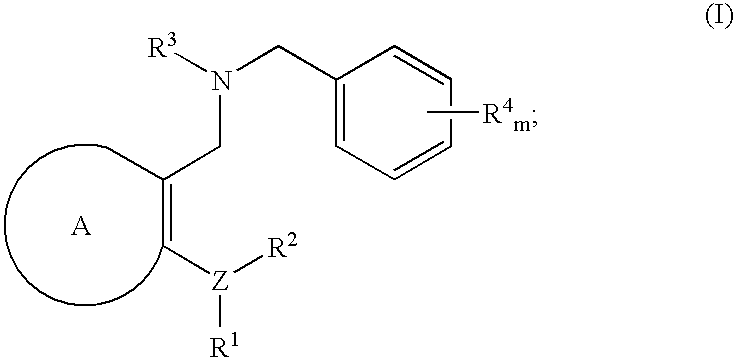
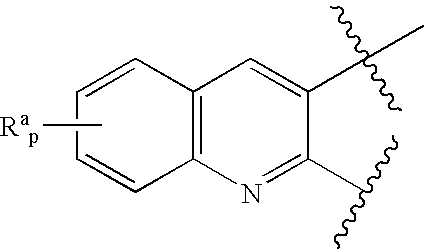
The present invention provides, among other things, new benzylamine compounds, compositions comprising benzylamine compounds, methods of making benzylamine compounds, and methods of using benzylamine compounds for treating or preventing a variety of conditions or diseases associated with lipoprotein metabolism.
Owner:DR REDDYS LAB LTD
Novel benzylamine derivatives as CETP inhibitors
The present invention provides, among other things, new benzylamine compounds, compositions comprising benzylamine compounds, methods of making benzylamine compounds, and methods of using benzylamine compounds for treating or preventing a variety of conditions or diseases associated with lipoprotein metabolism.
Owner:DR REDDYS LAB LTD
Herbicide composition used for rape field contg. propyl-ester nitorfen and iso-propyl-ester nitrofen
ActiveCN1513321ABroad herbicidal spectrumStrong targetingBiocideDead animal preservationMetolachlorBenzylamine
A composite herbicide for rape field contains the pyrimidine benzylamine kind of herbicide, at least one of quizalofop-ethyl, fluazifop-p-butyl, haloxyfop, fenoxaprop and sethoxydim, acetochlor, alachlor, napropamide, ehaprochlor, metolachlor, ethamet sulfuron and benazolin. Its advantages are high effect, broad spectrum, low dosage and high safety.
Owner:ZHEJIANG RES INST OF CHEM IND CO LTD +1
Methods of treating alzheimer's disease and pharmaceutical compositions thereof
ActiveUS20140073681A1Good effectImprove and augment effectBiocideNervous disorderCholinesterase inhibitionBenzylamine
The present invention describes methods of treating dementia comprising administering an effective daily dose of N-(2-(6-fluoro-1H-indol-3-yl)ethyl-(2,2,3,3-tetrafluoropropoxy)benzylamine to improve or augment the effect of an acetylcholinesterase inhibitor.
Owner:H LUNDBECK AS
Methods of treating alzheimer's disease and pharmaceutical compositions thereof
ActiveUS9375418B2Nervous disorderPharmaceutical delivery mechanismCholinesterase inhibitionBenzylamine
Owner:H LUNDBECK AS
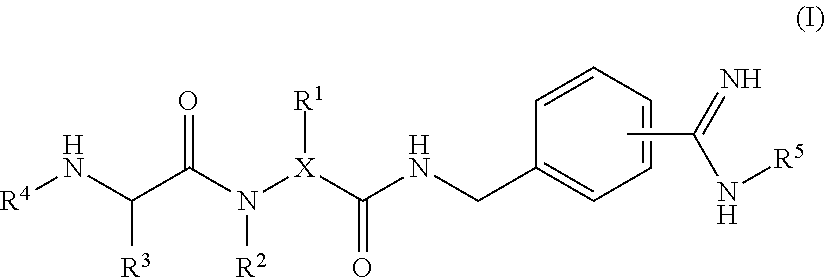
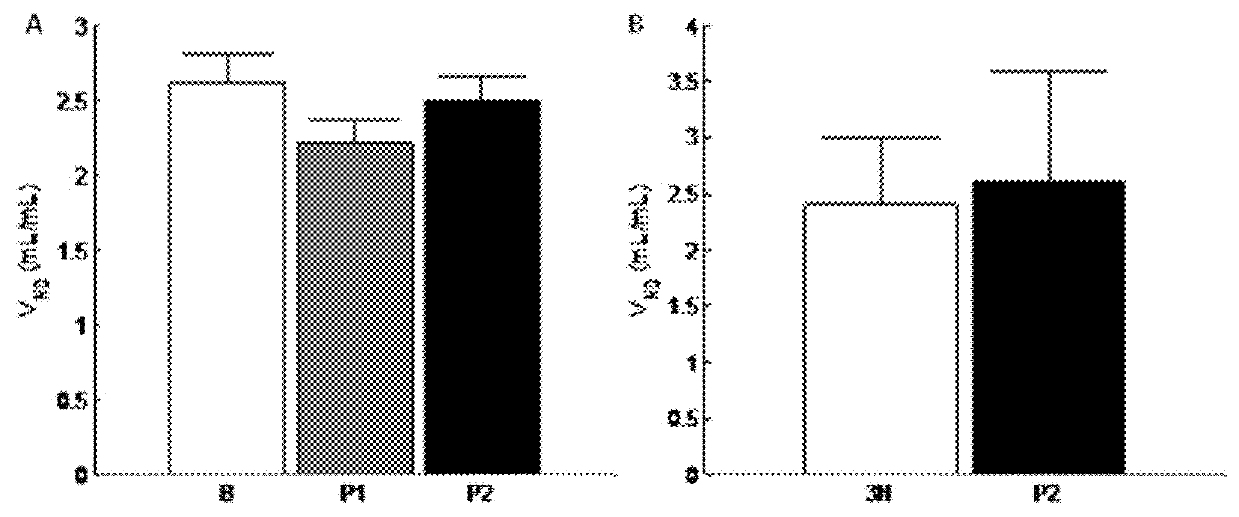
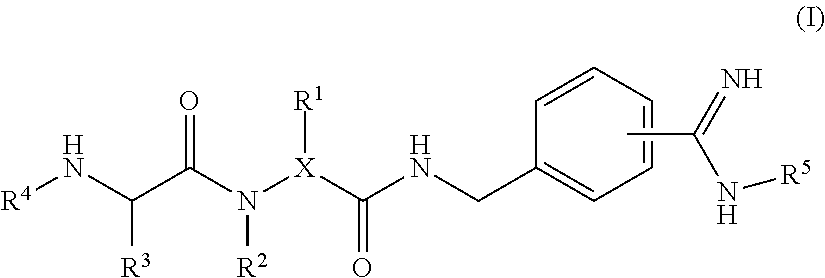
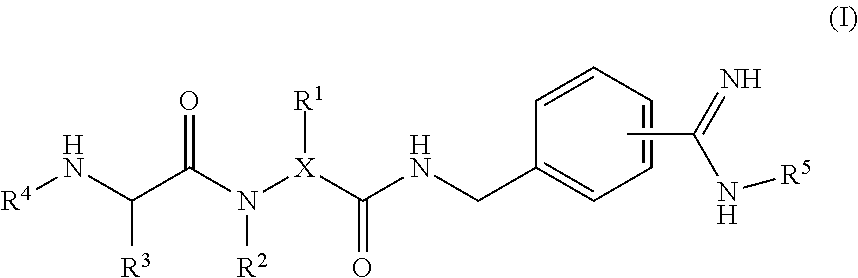
4-amidino benzylamines for cosmetic and/or dermatological use
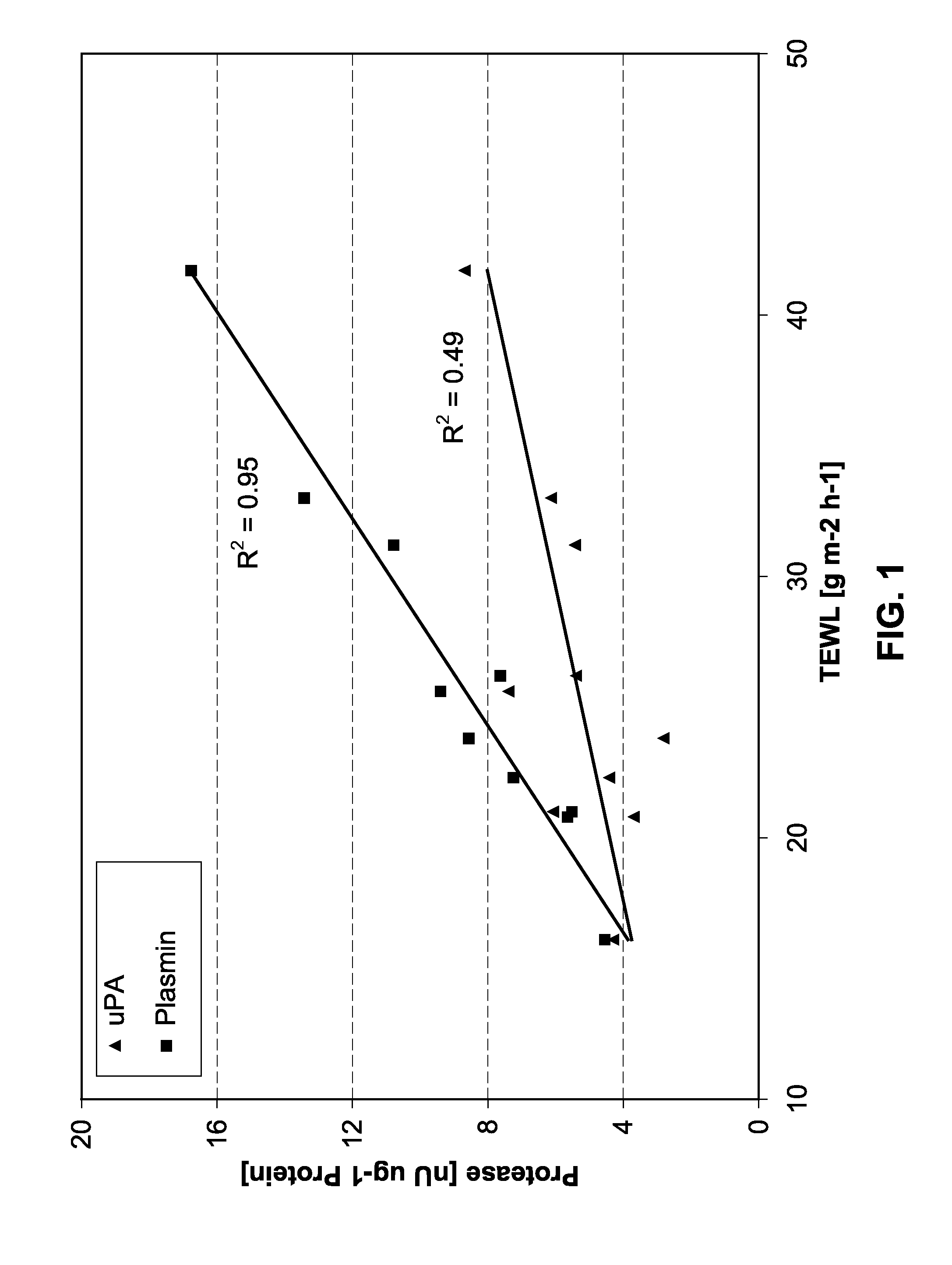
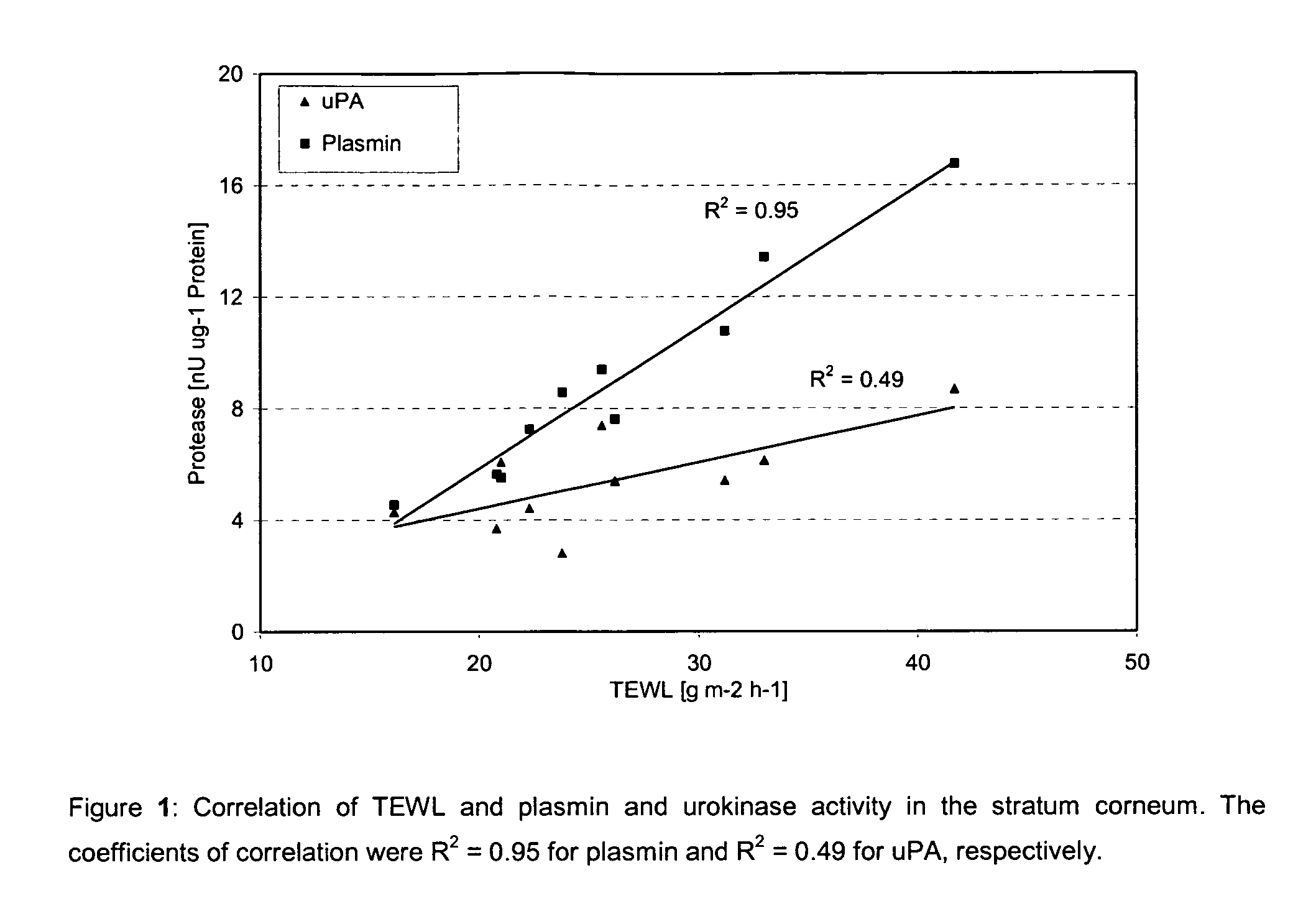
This invention relates to the use of 4-amidino benzylamine derivatives as cosmetic ingredients and to cosmetic compositions, as well as to non-therapeutic methods for the cosmetic treatment of the skin and the scalp. Said derivatives and compositions can be used as urokinase inhibitors to prevent and restore damage of the epidermal barrier. Barrier abnormalities and disruptions respectively are often the starting point of a dry skin state, of itching, of dandruff and of the perception of sensitive skin. These 4-amidino benzylamine derivatives can be used for topical skin and scalp care applications in form of creams, lotions, gels, shampoos and the like.
Owner:DSM IP ASSETS BV
Synthetizing method of lacosamide
InactiveCN103113256AHighlight substantive featuresSignificant progressOrganic compound preparationCarboxylic acid amides preparationPtru catalystAmmonium chloride mixture
The invention provides a synthetizing method of lacosamide. The method comprises the steps of: based on D-serine as a raw material, performing an acylation reaction with acetic anhydride and then performing a condensation reaction with benzylamine; and finally, performing a methylation reaction with dimethyl sulfate, thereby obtaining lacosamide, wherein N,N' dicyclohexylcarbodiimide (DCC) or N,N' carbonyl diimidazole (CDI) is used as a catalyst in the condensation reaction; and a phase transfer catalyst including triethyl benzyl ammonium chloride (TEBA), tetrabutylammonium chloride (TBAC), tetrabutylammonium bromide (TBAB) or tetrabutylammonium hydrogen sulfate (TBAHS) is adopted in the methylation reaction. The method has the advantages of being simple in synthetizing process, moderate in reaction condition, simple in after-treatment, high in yield and high in product purity.
Owner:SUZHOU HONGRUI MEDICAL TECH
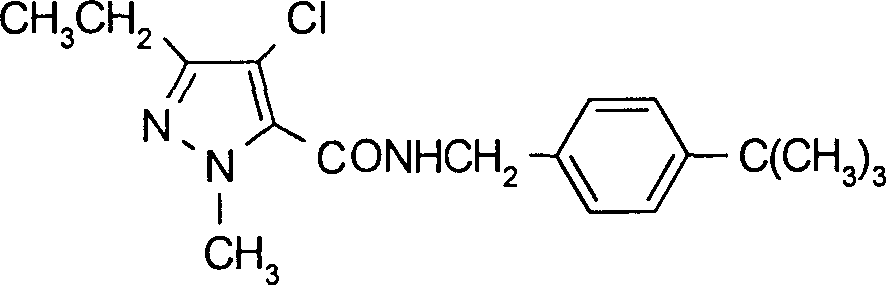
Method for synthesizing tolfenpyrad
The invention provides a method for synthesizing tolfenpyrad, relates to a preparation method of the tolfenpyrad, and the method is used for solving the problems such as tedious process and poor product purity of an existing tolfenpyrad synthesis method. The method comprises the following steps of: 1, synthesizing ethyl propionyl pyruvate; 2, synthesizing ethyl 3-ethyl-5-pyrazolecarboxylate; 3, synthesizing ethyl 1-methyl-3-ethyl-5-pyrazolecarboxylate; 4, synthesizing ethyl 1-methyl-3-ethyl-4-chloro-5-pyrazolecarboxylate; 5, synthesizing 4-(4-methyl phenoxy) cyanophenyl; 6, synthesizing 4-(4-methyl phenoxy) benzylamine; and 7, synthesizing the tolfenpyrad. Since sodium ethoxide is replaced by sodium hydroxide, the method provided by the invention has the characteristics of short reaction time, no generation of isomer and high purity of the product; and the obtained product has high purity and does not need re-crystallization.
Owner:HEILONGJIANG UNIV
Intermediate of pemetrexed disodium, preparation method thereof and method for preparing pemetrexed disodium thereby
ActiveCN101560206AHigh yieldReduce manufacturing costOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsBenzoic acidGlutaric acid
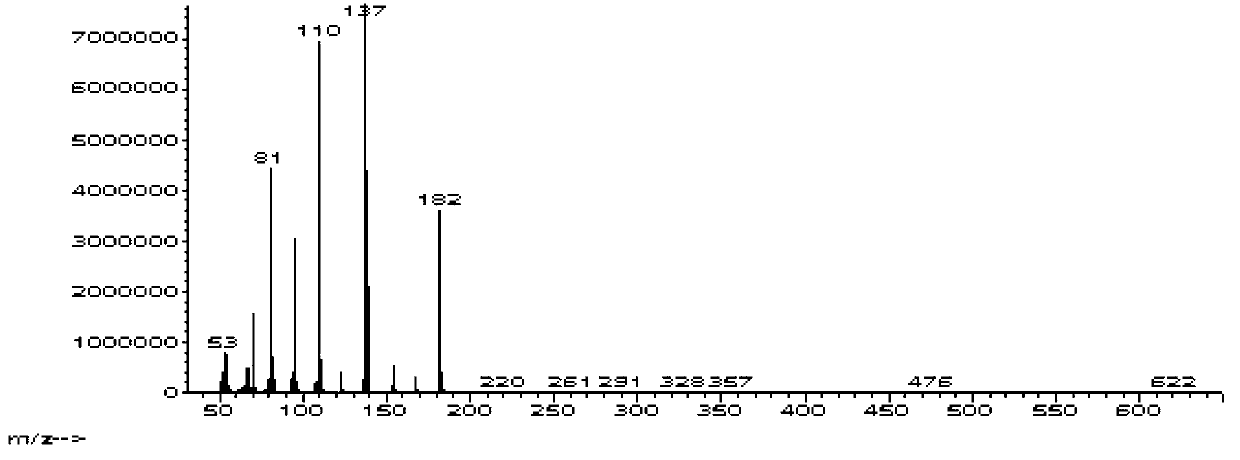
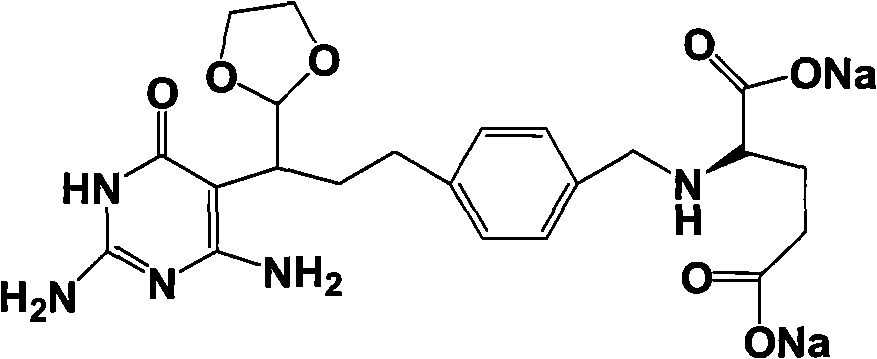
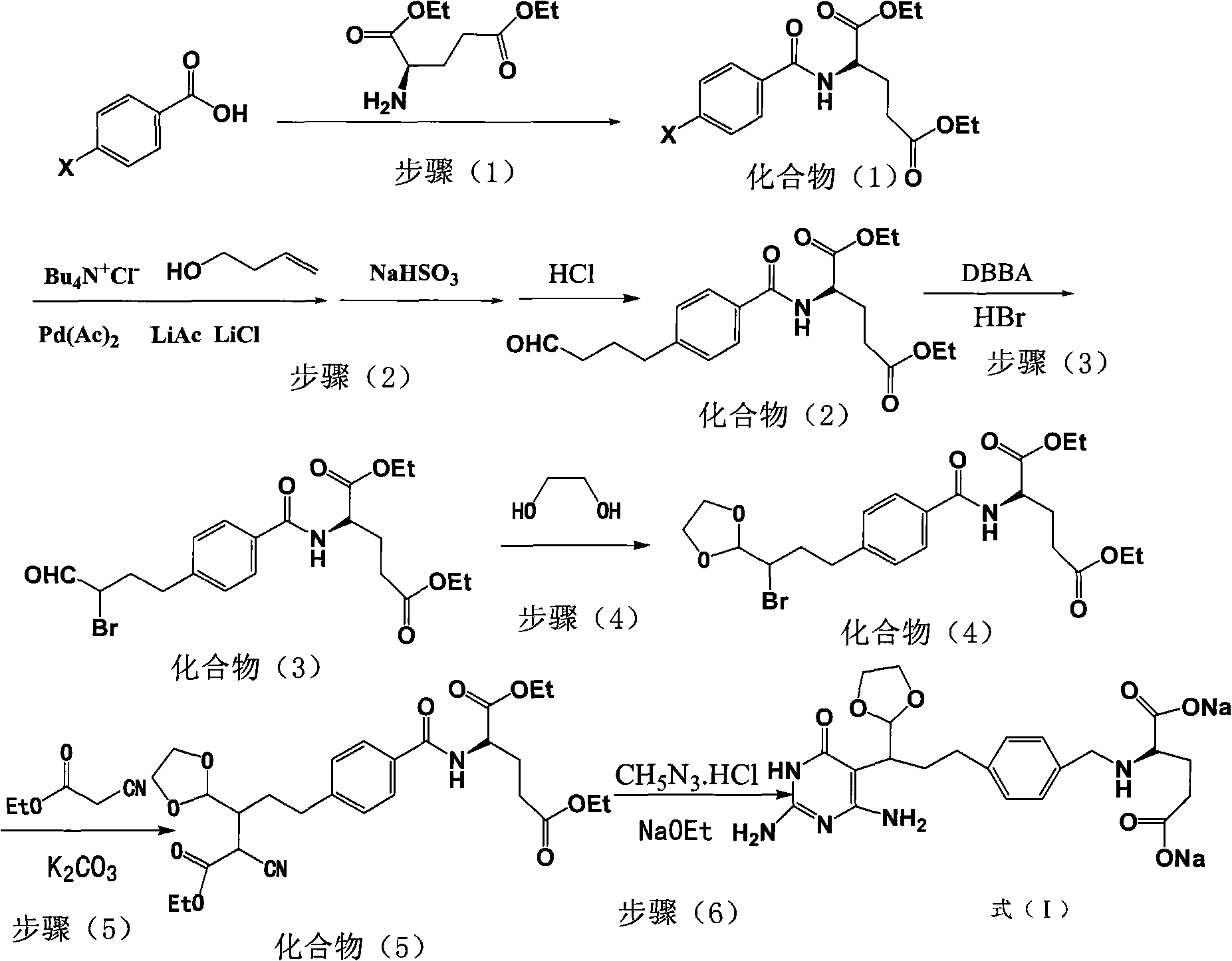
The invention relates to an intermediate of pemetrexed disodium, a preparation method thereof and a method for preparing pemetrexed disodium thereby; and the intermediate is (2-(4-(3-(2,4-diamino-6-oxy-1,6-dihydro-pyridine-5-group)-3-(1,3)dioxolane-2-group-propyl) benzylamine)sodium glutaric acid. The synthesis of the intermediate comprises the following steps: firstly, condensation reaction is conducted on 4-bromobenzoic acid or 4-iodobenzoic acid and L-glutamate diethylester, then Hack reaction is conducted, 4-bromo is replaced and 4-butyraldehyde is formed, then selective bromo replacement is conducted and the 4-butyladehyde is converted into 2-bromobutyraldehyde, and then condensation reaction of aldehyde and ethylene glycol is utilized for protecting the aldehyde, and pyrimidine ring is further synthesized, and finally the intermediate is obtained. Acid hydrolysis ring-closing reaction and sodium hydroxide salification are respectively conducted for once on the intermediate so as to obtain the pemetrexed disodium. The method for preparing pemetrexed disodium in the invention has high yield, low cost and easy operation and is applicable to industrialized production.
Owner:山东立新制药有限公司
Process for preparing 1,3-dibenzyl imidazoline-2-ketone-cis-4, 5-dicarboxylic acid (Ôàá)
InactiveCN1434039AImprove responseSave raw materialsOrganic compound preparationAmino-carboxyl compound preparationElectrophilic additionPotassium hydroxide
The present invention provides a method for preparing 1,3-dibenzylimidazoline-2-ketone-cis-4,5- dicarboxylic acid, and is characterized by that it utilizes chlorine gas to make fumaric acid produce electrophilic addition reaction to obtain meso-2,3-dichlorosuccinic acid, in the pressence of phase transfer catalyst the meso-2,3-dichlorosuccinic acid and benzylamine are undergone the processes of benzylamination reaction in the solvent so as to synthesize cis-2,3-dibenzylamine succinic acid, the latter and solid carbonyl chloride and undergone the process of phase transfer catalytic ring-closing reaction in the potassium hydroxide solution so as to obtain the invented product. Its total yield rate is up to 74%.
Owner:FUDAN UNIV
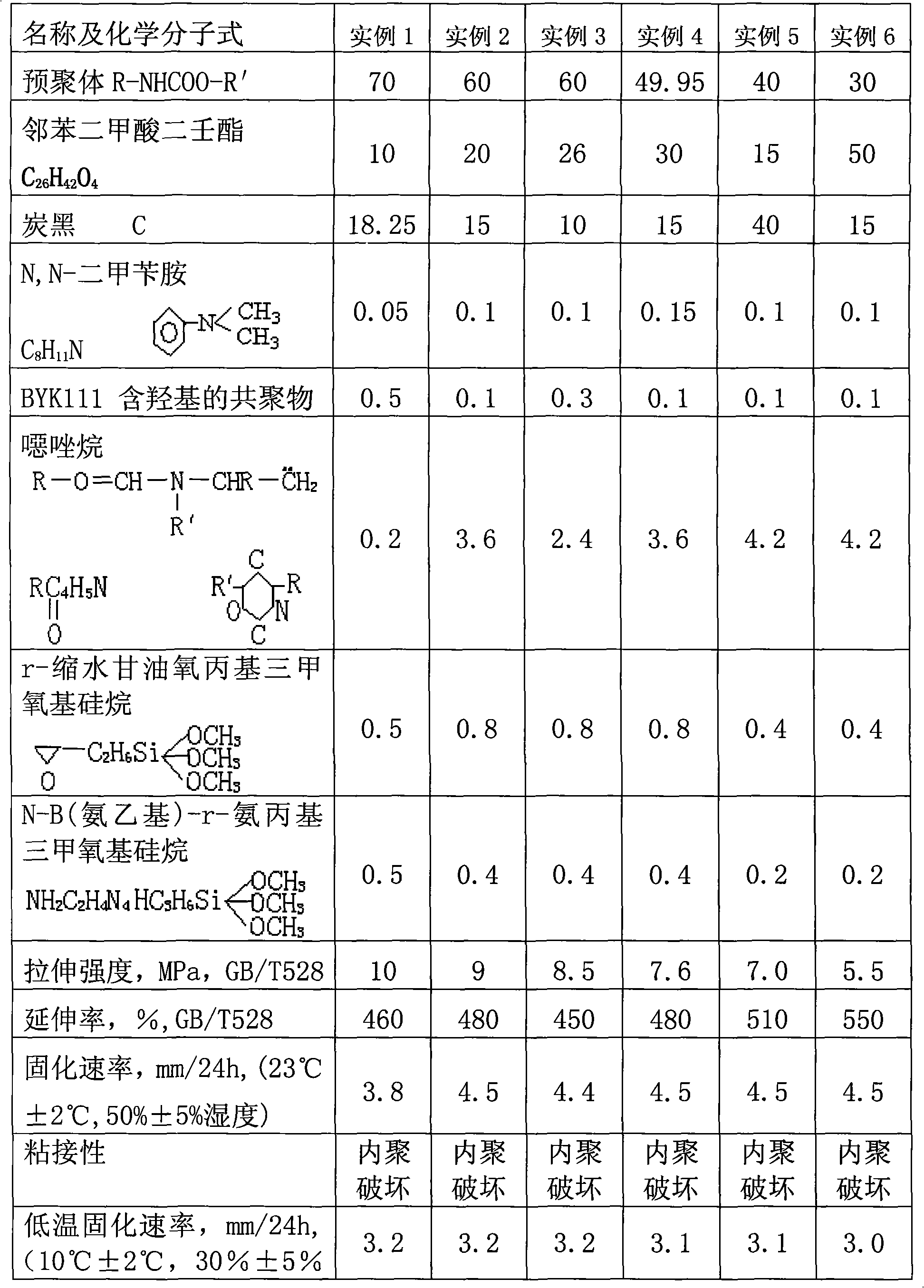
Polyurethane sealant and preparation method thereof
InactiveCN101580699AImprove cure rateOther chemical processesPolyureas/polyurethane adhesivesJet aeroplaneElastomer
The invention discloses a polyurethane sealant and a preparation method thereof. The polyurethane sealant comprises 30-70 weight percent of performed polymer, dinonyl phthalate, carbon soot, N,N-dimethyl benzylamine, byk-111, oxazolidine, epoxy silicone hydride and amino-group silicone hydride, wherein the performed polymer is obtained through the reaction of polyoxyalkylene dihydric alcohol, polyoxypropylene trihydric alcohol, hexamethylene glycol and 4,4'-diphenylmethane diisocyanate by taking dibutyl tin laurate as a catalyst. After being solidified, the polyurethane sealant has over 8MPa of tensile strength of elastomer and over 450 percent of elongation at break, not only has better solidifying speed at normal temperature, but also has very high solidifying speed at low temperature and low humidity. The polyurethane sealant is suitable for elastic splicing with high mechanical property, such as the elastic splicing during the installation of glass of automobiles, trains, ships, airplanes, cable cars, and the like and is suitable for the rapid splicing at low temperature and low humidity and other elastic splicing with high mechanical property.
Owner:北京森聚柯高分子材料有限公司 +1
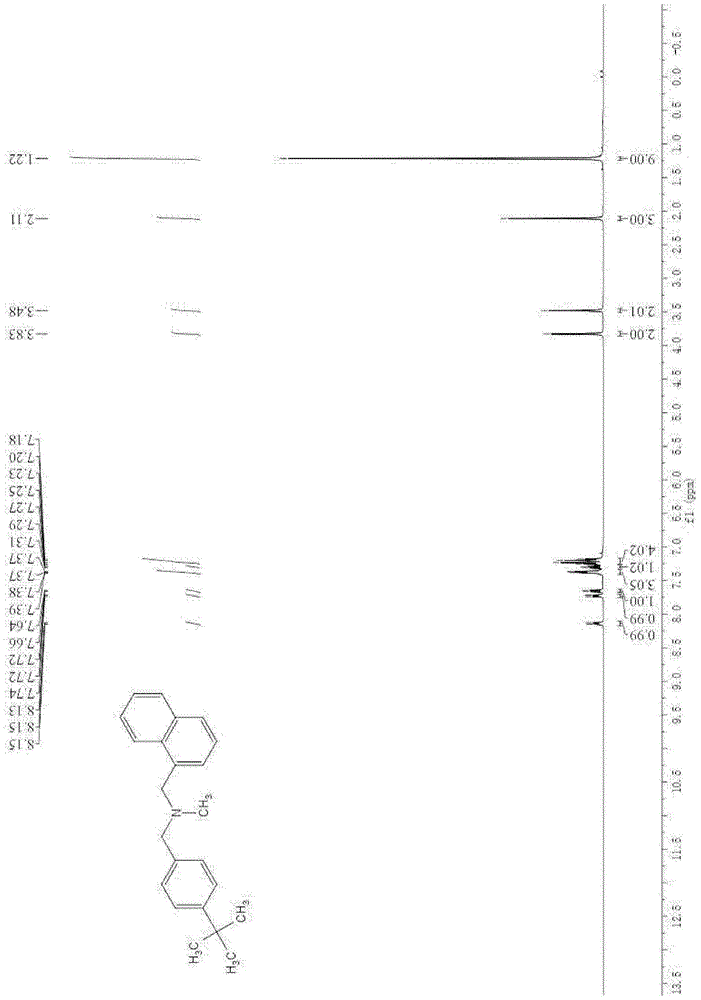
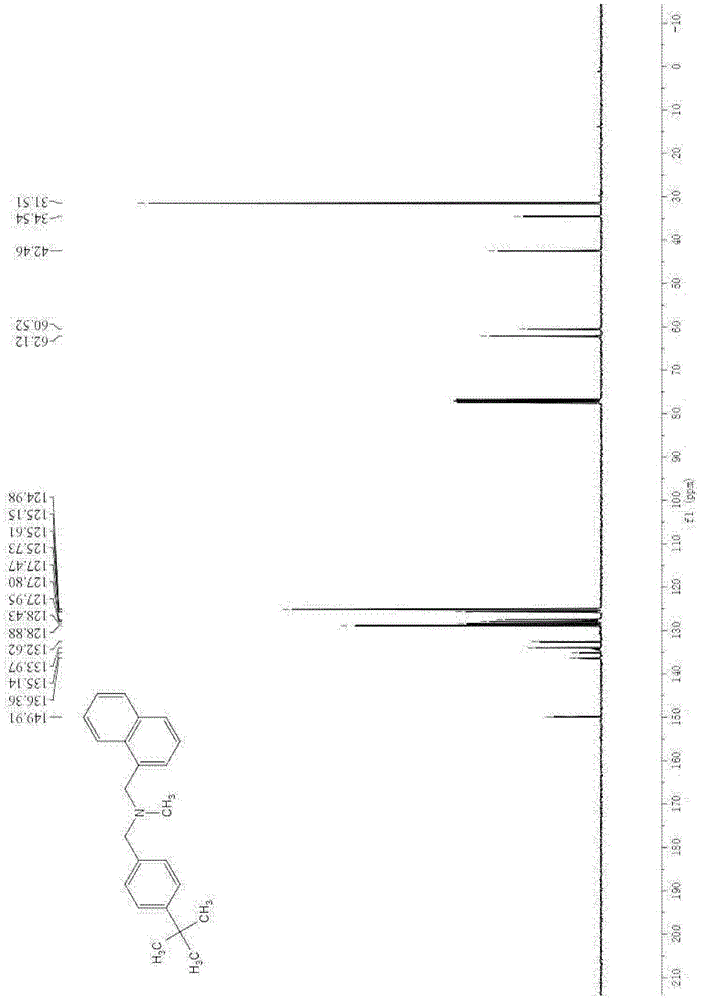
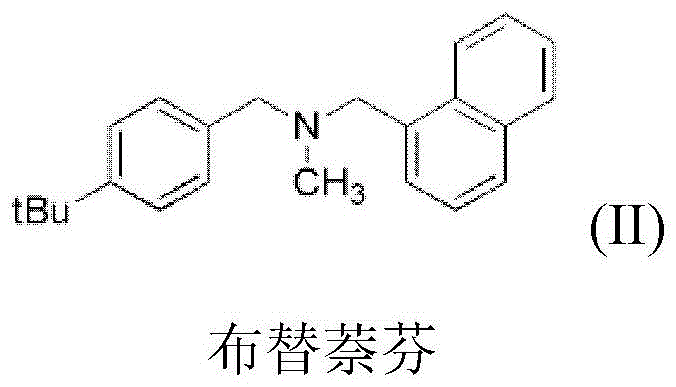
A kind of method of synthesizing butenafine
InactiveCN105130823BWide variety of sourcesAvoid residueOrganic compound preparationAmino compound preparationSynthesis methodsTwo step
The present invention discloses a butenafine synthesis method, the butenafine is shown as formula II, a desired product is prepared from 4-tert-butyl-benzylamine, 1-naphthoyl chloride, and formic acid as starting materials by amidation and reductive methylation two-step method by use of a non-metallic boron compound as a catalyst and an organic silane compound as a reducing agent, the butenafine synthesis method is simple, easy to operate, wide in source of raw materials, and low in cost; and a metal catalyst is not required to participate in the synthesis, the metal residues in the medicine can be avoided, and the method is safe, environmentally-friendly, and in line with the requirements of green chemistry.
Owner:UNIV OF SCI & TECH OF CHINA
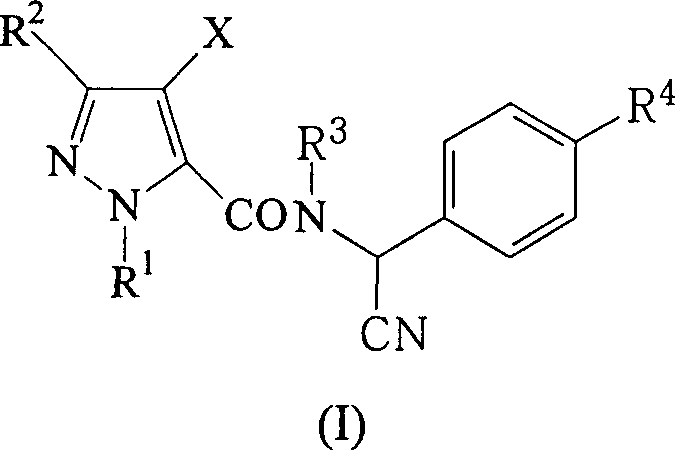
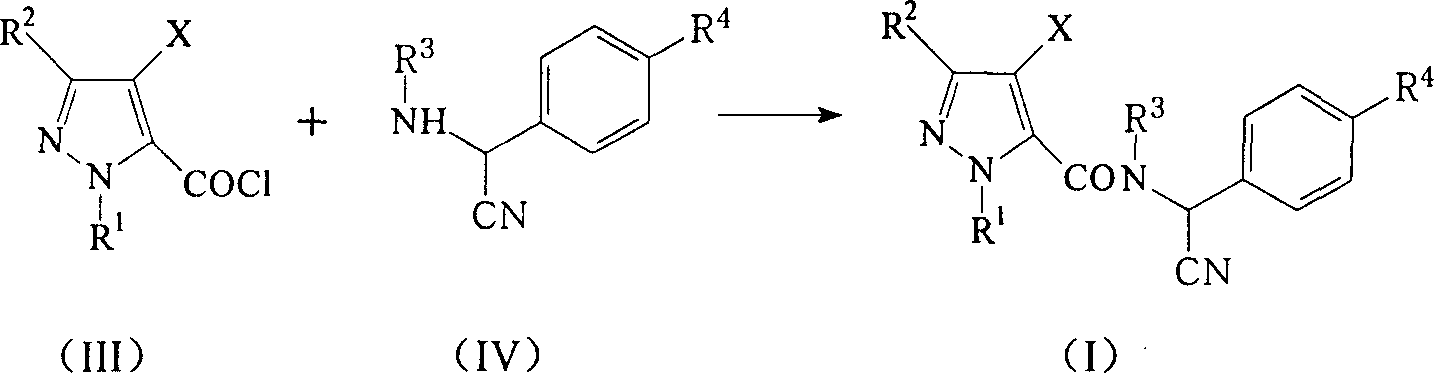
Alpha-cyano-N-benzylpyrazoamide compound, preparation method and pest control agent thereof as active component
The invention discloses an alpha-cyano-N-benzyl pyrazole amide compound and preparing method and pest prevention agent, which is characterized by the following: using organic solvent to react pyrazole formyl chloride and alpha-cyano benzylamine under -10-60 deg.c with general formula as (I); separating (I); adopting the compound or its acid salt as active to prepare pest prevention agent.
Owner:JIANGSU PESTICIDE RES INST
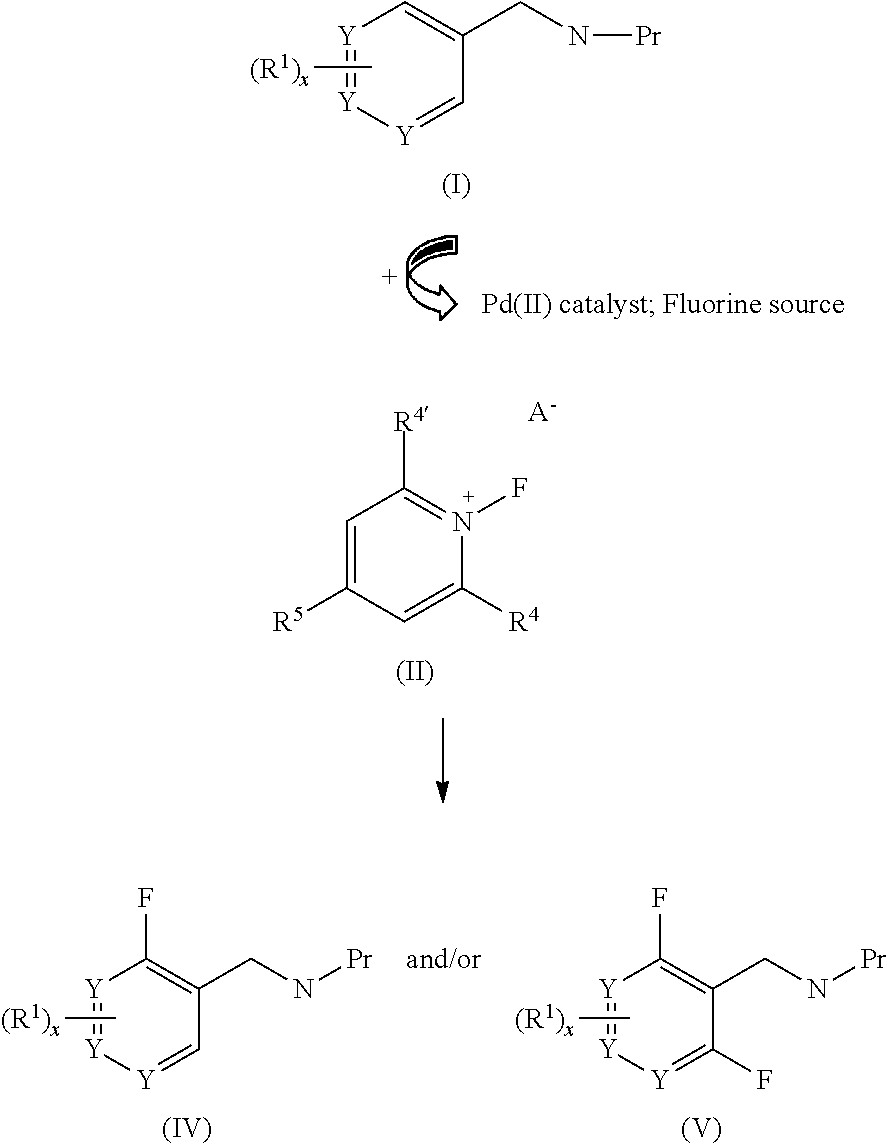
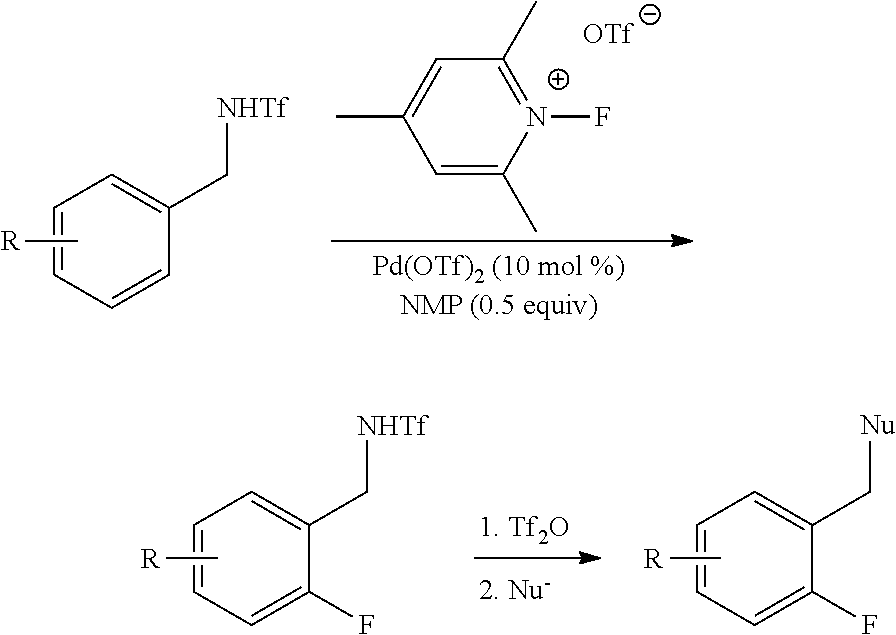
Palladium-catalyzed ortho-fluorination
InactiveUS20120059179A1Improve versatilityIncrease displacementCarboxylic acid nitrile preparationOrganic compound preparationArylOrtho position
A new method of ortho-fluorination where an aryl C—H bond is directly replaced by an aryl C-F bond in a palladium-catalyzed reaction is provided. The method includes the ortho-fluorination of a triflamide protected benzylamine, a palladium catalyst, such as Pd(OTf)2, a fluorinating reagent such as N-fluoro-2,4,6-trimethylpyridinium triflate, and a ligand to promote the reaction such as N-methylpyrrolidinone (NMP).
Owner:THE SCRIPPS RES INST
Liquid-phase synthesis method of dipeptide diaminobutyroyl benzylamide diacetate
InactiveCN107936108ASimple processLow costPeptide preparation methodsAnimals/human peptidesSolubilitySynthesis methods
The invention discloses a liquid-phase synthesis method of dipeptide diaminobutyroyl benzylamide diacetate. The method comprises the following steps of enabling Boc-Beta-Ala-OH, N-ethyl-5-phenylisoxazole-3'-sulfonic acid inner salt and H-Pro-OMe.HCl to react to obtain Boc-Beta-Ala-Pro-OMe, and enabling the Boc-Beta-Ala-Pro-OMe to react with LiOH to obtain Boc-Beta-Ala-Pro-OH; afterwards, synthesizing Boc-Beta-Ala-Pro-DAB(Boc)-OH with the Boc-Beta-Ala-Pro-OH and H-DAB(Boc)-OMe.HCl by adopting the same method; next, enabling the Boc-Beta-Ala-Pro-DAB(Boc)-OH, 1-hydroxybenzotriazole, N,N-diisopropylcarbodiimide and benzylamine to react to obtain Boc-Beta-Ala-Pro-DAB(Boc)-NH-Bzl, finally, removing a Boc protecting group by using trifluoroacetic acid, separating and purifying, so as to obtain the dipeptide diaminobutyroyl benzylamide diacetate with purity being more than 95 percent. According to the liquid-phase synthesis method, amino acid methyl ester which is low-cost and is easily obtained is used as a raw material; a by-product generated and synthesized by a peptide bond in each step has water solubility and is easily separated, and the liquid-phase synthesis method which has a simple and convenient process and lower cost is provided for the synthesis of the dipeptide diaminobutyroyl benzylamide diacetate.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
Preparation method of ultrathin tungsten selenide nanometer slices
ActiveCN107601443AEasy to prepareThe detection method is simpleMaterial nanotechnologyBinary selenium/tellurium compoundsReaction temperatureOil phase
The invention discloses a preparation method of ultrathin tungsten selenide nanometer slices, and particularly relates to an oil phase method for preparing tungsten selenide. The material has the property of catalyzing benzylamine to produce imine through coupling. The method provided by the invention belongs to the technical field of material preparation and application. A Schlenk line route is used; oleylamine, oleic acid, octadecene and N-dodecyl mercaptan are used as solvents; a selenium source and a tungsten source at a certain ratio are used as reactants for preparation at a certain reaction temperature to obtain the feather-shaped ultrathin nanometer slices with the thickness being smaller than 2nm; the nanometer slices have the effect of efficiently catalyzing the benzylamine to produce N-benzylamine through coupling; in cyclohexane and acetonitrile solvents, the yield of N-benzylamine through benzylamine coupling respectively reaches 95 percent and 96 percent. The catalyst hasthe advantages of great specific surface area and high catalysis efficiency; noble metal is not used; the cost is low. The preparation process is simple; the operation is easy; the use of an organictemplate and surfactant is not needed; the preparation method is suitable for industrial production; the prepared tungsten selenide phase is pure; the specific surface area is large; the repeated utilization performance is high.
Owner:ANHUI UNIVERSITY
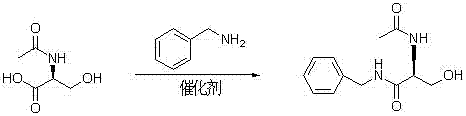
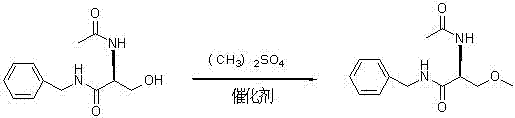
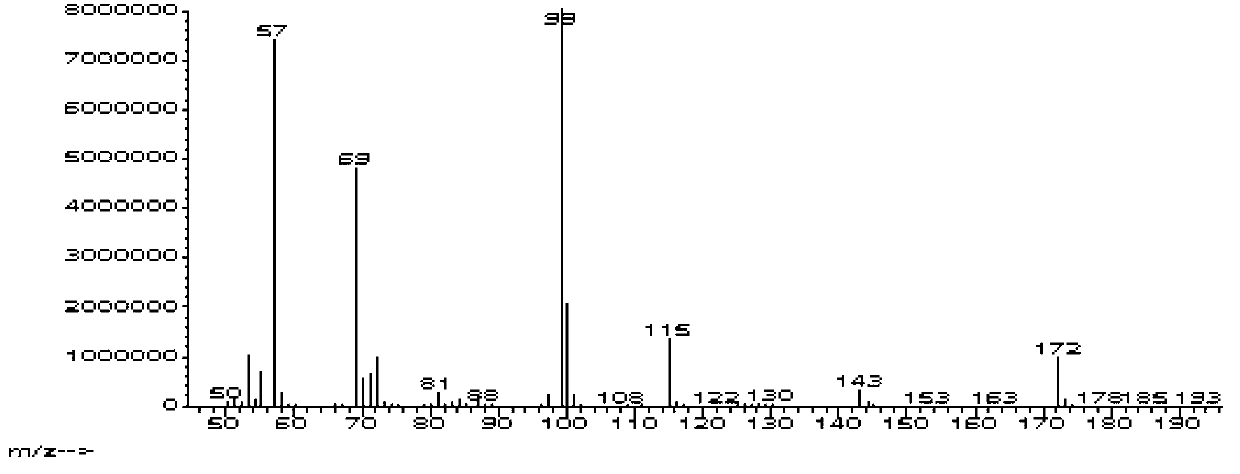
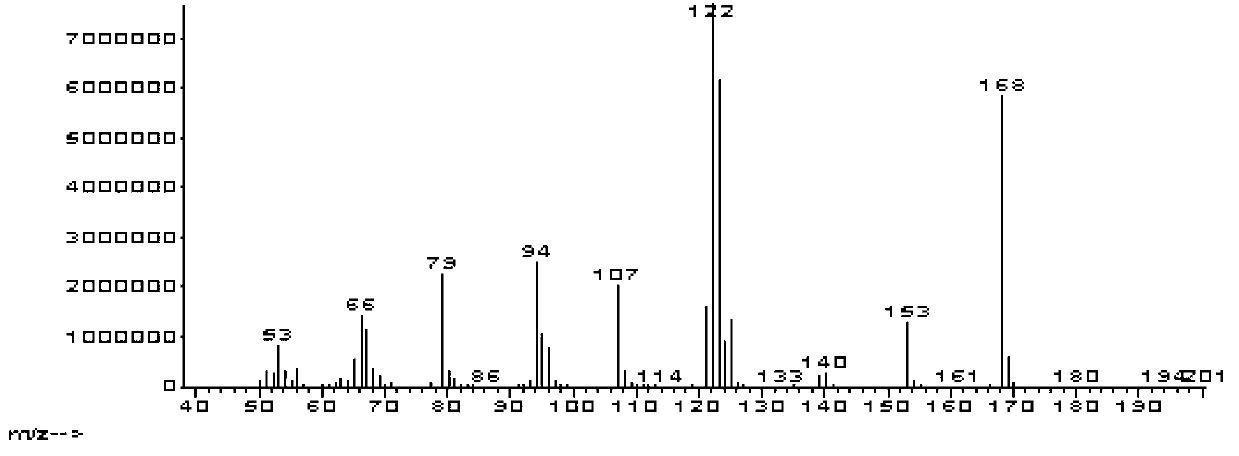
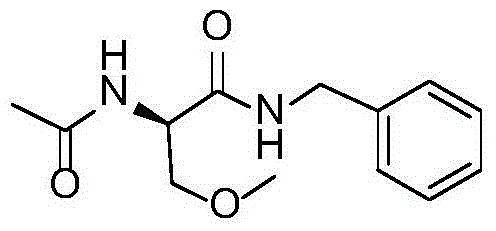
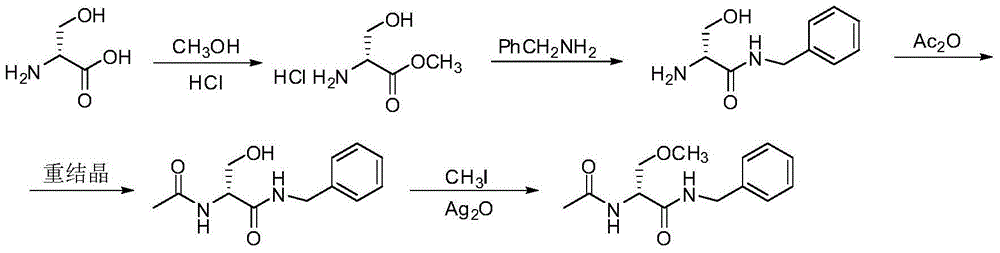
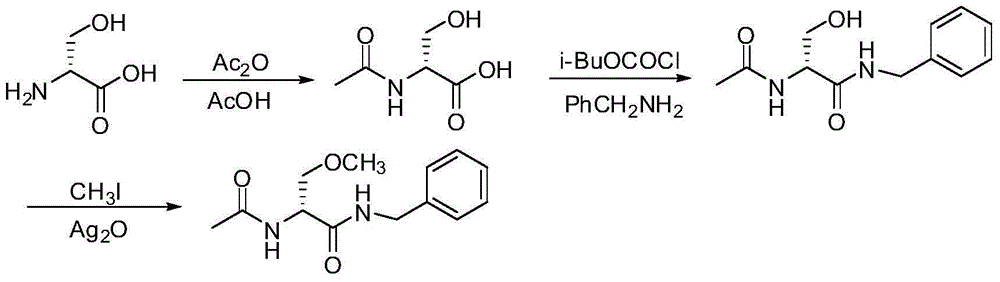
A preparing process of lacosamide
ActiveCN104030943AHigh yieldSimple and fast operationOrganic compound preparationCarboxylic acid amides preparationBenzylamineSerine
A preparing process of lacosamide is disclosed. D-serine is adopted as an initial raw material. The method includes: performing amino protection, performing methylation, condensing with benzylamine under a condition of existence of a carboxyl activator, removing an amino protection group, and performing amidation to obtain the lacosamide. The total yield is higher than 66%. The method is high in yield, simple and convenient to operate, high in product purity and especially suitable for industrial production.
Owner:福安药业集团重庆博圣制药有限公司 +2
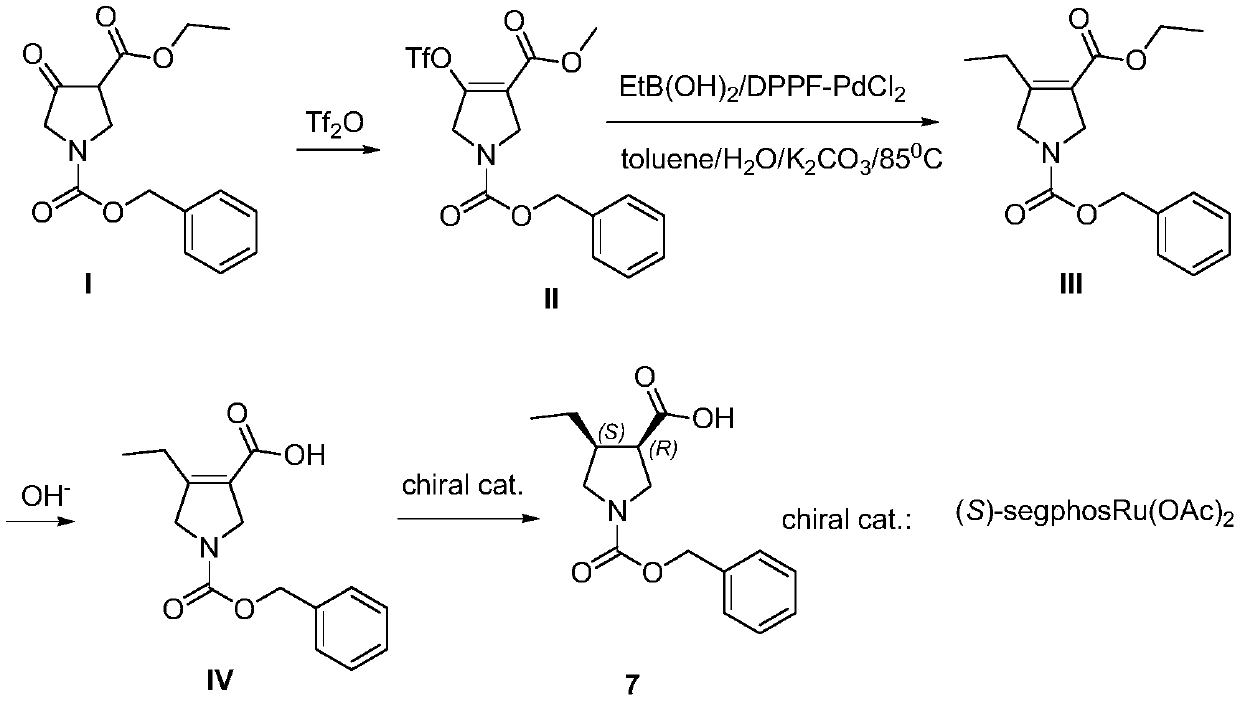
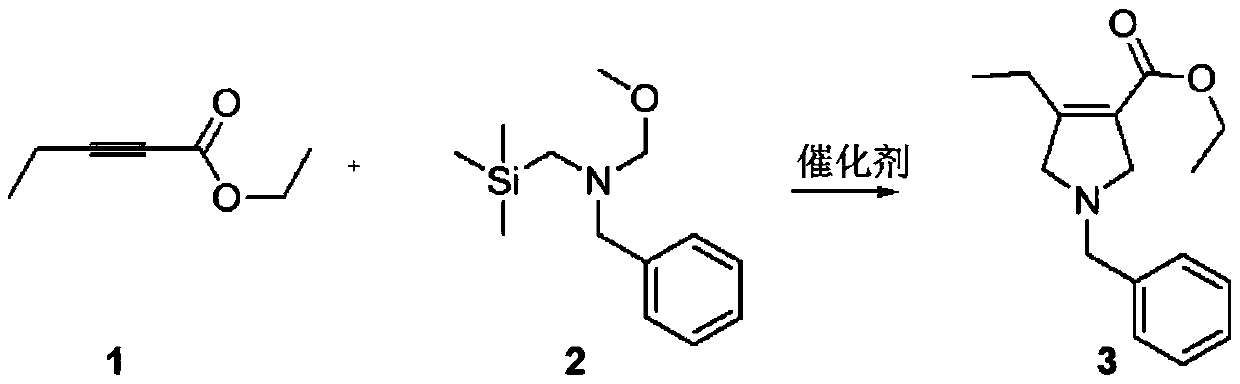
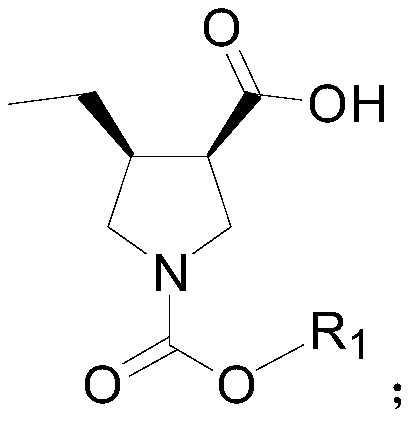
Method for synthesizing upadacitinib intermediate and the intermediate
The invention discloses a method for synthesizing upadacitinib intermediate. The method includes performing a ring-forming reaction with 2-pentynoate and N-(methoxymethyl)-N-(trimethylsilylmethyl)benzylamine under a catalyst A to prepare a compound (3), by which the compound (7) or the compound (8) can be obtained in various manners. The method especially the first and the third technical schemes,is high in yield and purity and is high in overall yield. The method is simple in post-treatment and is suitable for industrial large-scale production.
Owner:ZHEJIANG NORMAL UNIVERSITY
Preparation method of (S, S)-8H-6H-pyrrolo [3, 4-b] pyridine
ActiveCN103030638ASimple methodHigh yieldOrganic chemistryBulk chemical productionHigh pressureBenzylamine
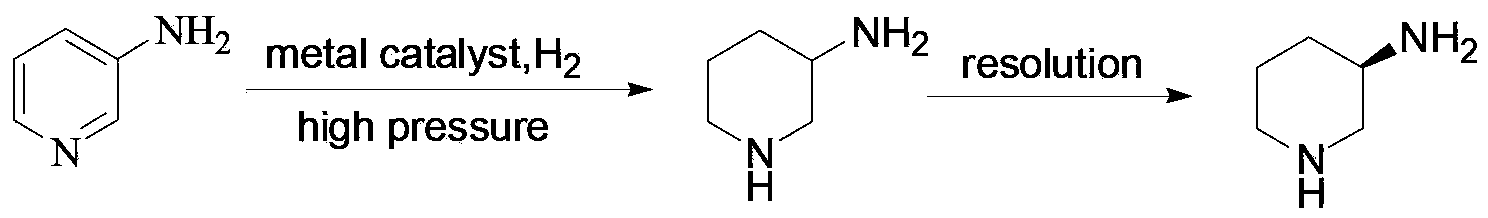
The invention discloses a method for enantioselectively synthetizing (S, S)-8H-6H-pyrrolo [3,4-b] pyridine by using (R)-2-phenylglycinol as a chiral inducing reagent. The method comprises the following steps: by taking (R)-2-phenylglycinol as a raw material, carrying out one-step Michael addition reaction and ester exchange reaction to synthetize a intermediate 2; carrying out condensation on the intermediate 2 and acryloyl chloride to obtain a double-ring intermediate 3; carrying out hydrogenation reduction to obtain an intermediate 4 in high stereoselectivity; reacting the intermediate 4 with benzylamine through one-step amine-ester exchange to obtain an intermediate 5; hydrolyzing the intermediate 5 to obtain an intermediate 6; and carrying out ring closing reaction, reducing, removing protecting group on nitrogen to obtain a final product II. The method has the characteristics of favorable stereoselectivity and high yield, and is simple to operate; compared with the traditional method, for no need of hydrogenating and reducing pyridine ring under high pressure, no need of resolution and the like, the preparation method disclosed by the invention is low in cost, safe and reliable, the yield can achieve 20%-30%; and the method is suitable for industrial production.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Preparation method of amino protection (R)-3-amino piperidine
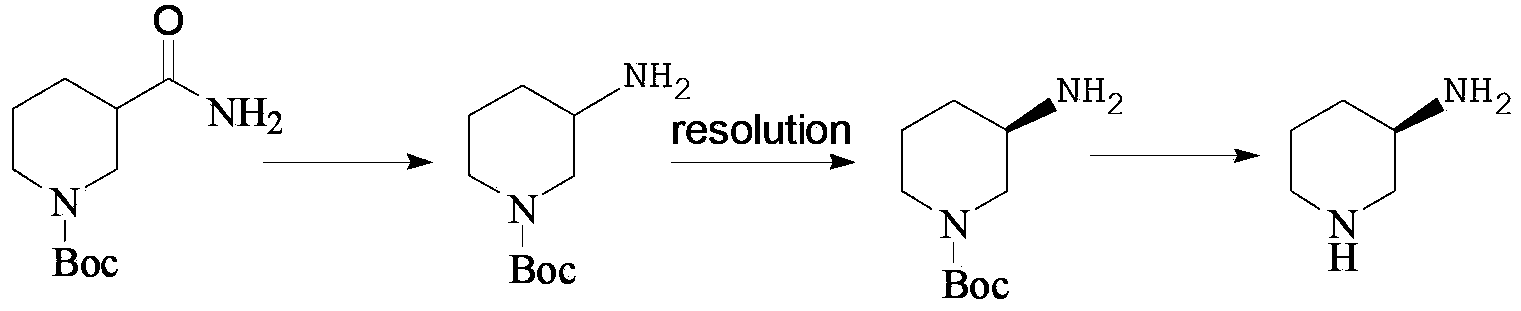
ActiveCN103980186AImprove protectionHigh optical purityOrganic chemistryBulk chemical productionChemical compoundMetal catalyst
The invention discloses a preparation method of amino protection (R)-3-amino piperidine, and the preparation method comprises the following steps: (1) in the presence of a first solvent or absence of a solvent, reacting a compound of formula V with benzylamine to obtain a compound of formula VI; (2) in the presence of a second solvent, performing catalytic hydrogenation of the compound of formula VI to remove benzyl to obtain the amino protection (R)-3-amino piperidine. According to the method, the defects of use of expensive metal catalysts and use of high-pressure hydrogenation and various splitting for synthesis of a chiral amino piperidine ring can be avoided, at the same time, the synthetic route is short, the process is simple, the yield is high, and the method is suitable for industrial mass production.
Owner:SHANGHAI VIWIT PHARMA CO LTD
Multi-substituted fluorine-containing pyridine
The invention relates to multi-substituted pyridine, in particular to multi-substituted fluorine-containing pyridine which is obtained by performing the reaction of alkynyl imine and benzylamine for 1 to 30h at 60 to 100 DEG C in an organic solvent in the presence of alkali. Various multi-substituted 3-hydrogen, 3-fluorine and 3-trifluoromethyl pyridine derivatives can be synthesized through a cascade reaction of fluoroalkyl alkynyl imine and methylamine. Substituent groups on pyridine rings can be introduced step by step from a very beginning raw material preparation step, and various compounds with bioactivities synthesized by adopting the method can be used for synthesizing insecticides and antibacterial drugs.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
4-amidino benzylamines for cosmetic and/or dermatological use
Owner:DSM IP ASSETS BV
Synthetic method and application of MACAmide
ActiveCN104513171AHigh purityEasy to getOrganic compound preparationCarboxylic acid amides preparationBenzylamineFatty acid
The invention relates to a synthetic method of MACAmide. The method includes following steps: with a fatty acid and benzylamine or m-methoxybenzylamine as reaction raw materials, mixing the raw materials in a dichloromethane solution in which HOAt, EDC.HCl and DIPEA are dissolved; performing a reaction with stirring; washing a reaction product with water; and drying a substance being undissolved in water to obtain the MACAmide. The method is simple in processes and the raw materials are easy to obtain. Operation conditions of the method are easy to control. The reaction product can reach a purity of 95% without purification. The invention provides basis for industrialized synthesis of the MACAmide. In addition, the MACAmide has effects of enhancing male reproductive ability and treating male sexual dysfunction. The invention provides market prospects to application of the MACAmide.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Preparation method of stable alkali-free setting accelerator
Belonging to the technical field of concrete additive preparation, the invention relates to a preparation method of a stable alkali-free setting accelerator. The method includes: firstly conducting gelatinization treatment on black fungus chaff powder, them performing mixing heating with an acrylic monomer, ammonium persulfate and other substances to obtain fungus chaff water-absorbent resin, then subjecting the fungus chaff water-absorbent resin and a self-made gelatin gel solution to mixing grinding so as to obtain mixed water-absorbent resin powder, then subjecting the mixed water-absorbent resin powder and calcium carbonate powder to mixing pressing, and performing ball milling to obtain calcium carbonate resin composite powder, then using aluminum sulfate and benzylamine as the raw materials to prepare an organic amine dispersion solution, and finally heating the organic amine dispersion solution, calcium carbonate resin composite powder and other substances, and then performing cooling. The stable alkali-free setting accelerator prepared by the method provided by the invention has good stability, does not hydrolyze easily, enhances the setting time to a initial setting time of less than 2min and a final setting time of less than 4min, is free of alkali component, does not cause harm to human body, also is environment-friendly, and improves the concrete strength, thus being widely applicable to bridges, underground engineering and other fields.
Owner:江苏宇辉新材料集团有限公司
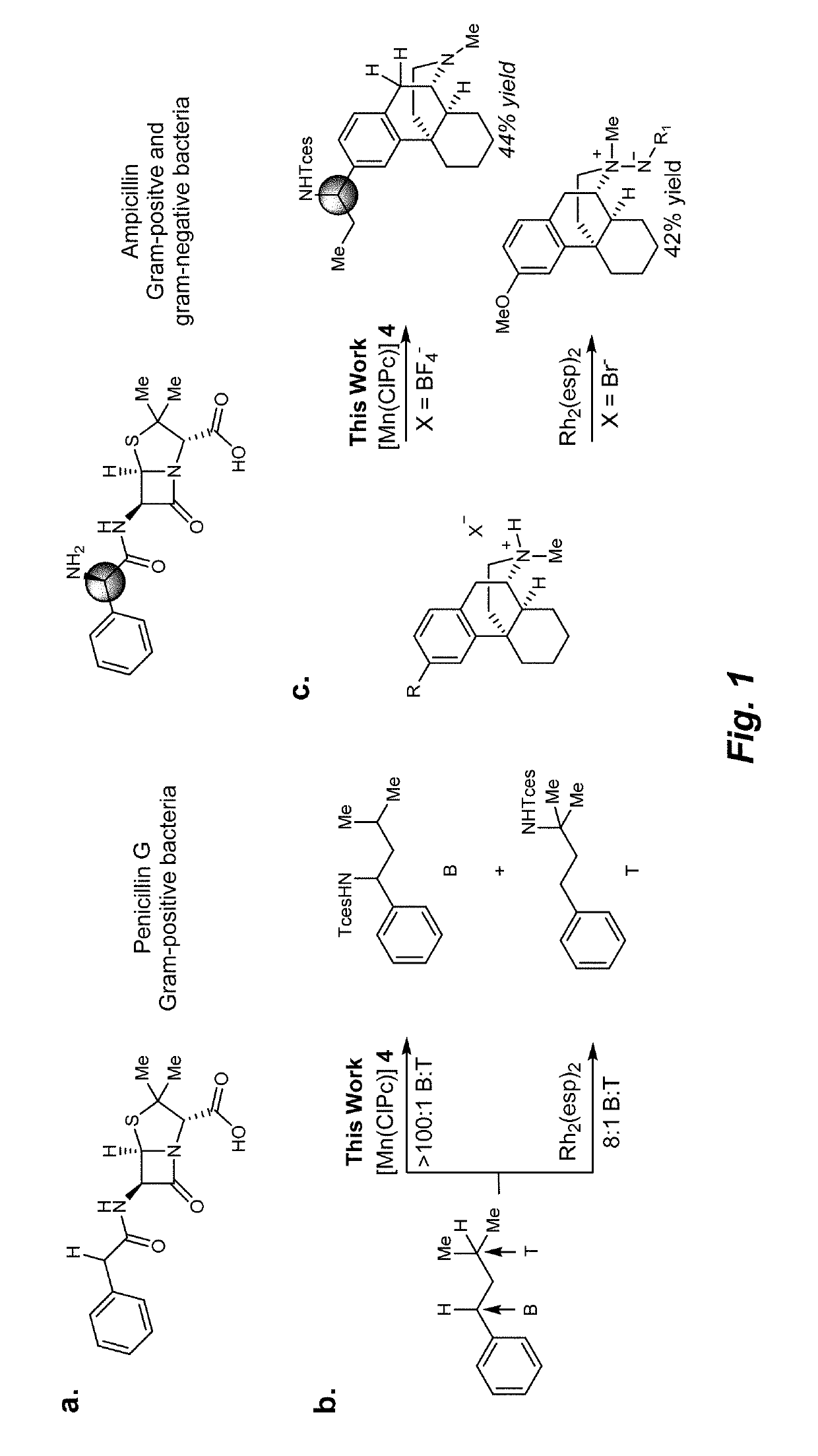
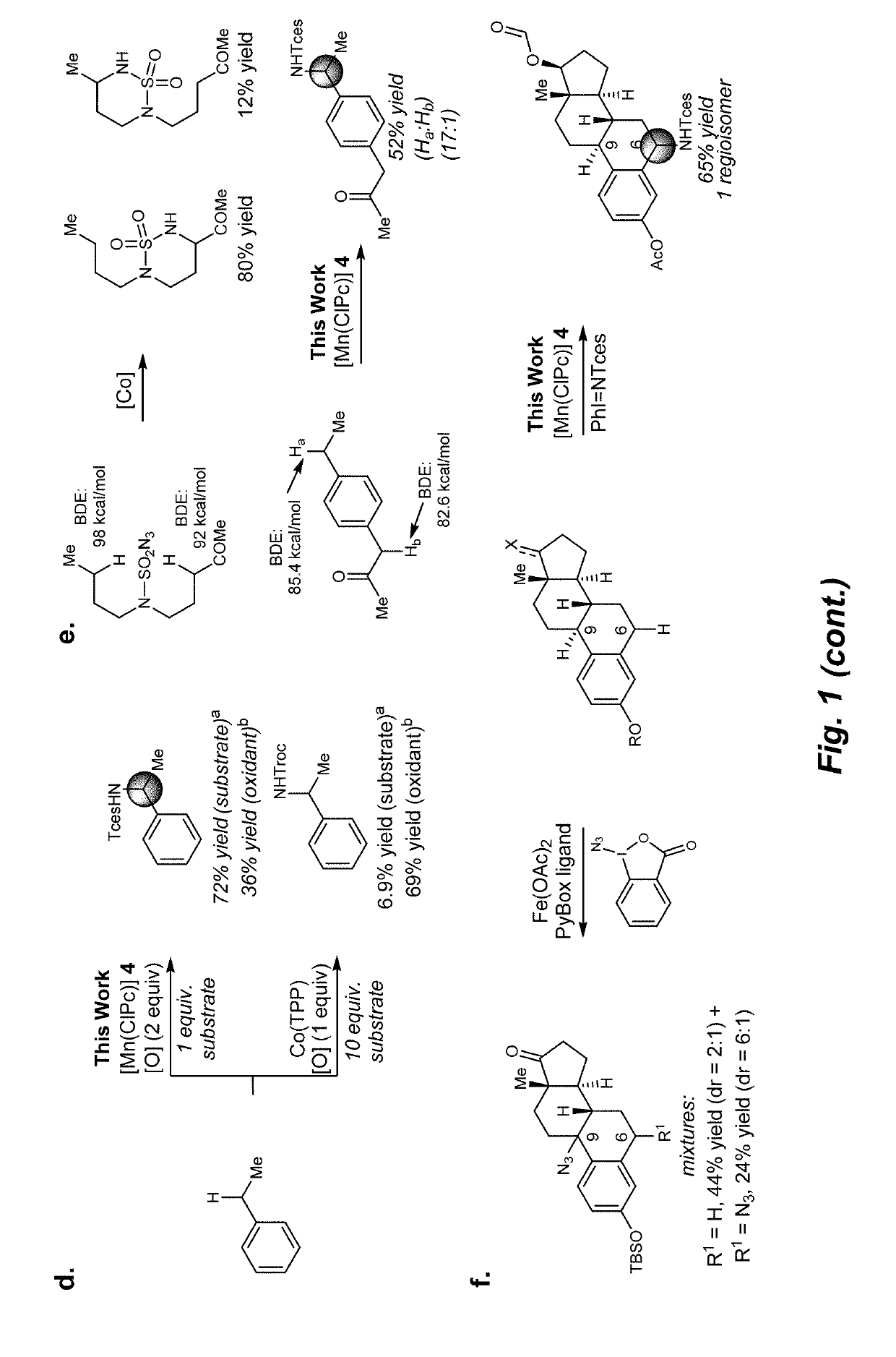
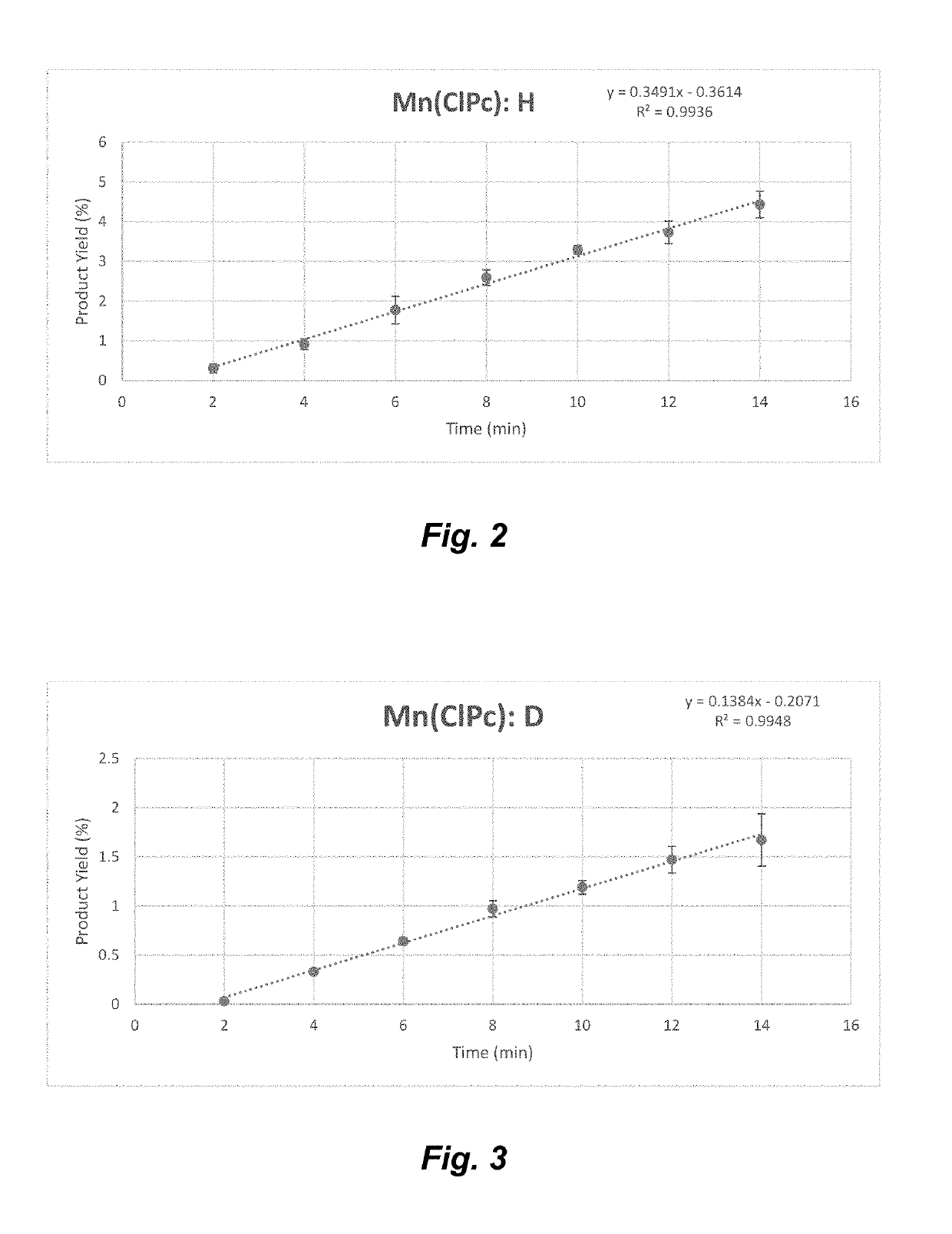
Manganese (III) catalyzed c--h aminations
ActiveUS20190106448A1Organic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsRate-determining stepSite selectivity
Reactions that directly install nitrogen into C—H bonds of complex molecules are significant because of their potential to change the chemical and biological properties of a given compound. Selective intramolecular C—H amination reactions that achieve high levels of reactivity, while maintaining excellent site-selectivity and functional-group tolerance is a challenging problem. Herein is reported a manganese perchlorophthalocyanine catalyst [MnIII(ClPc)] for intermolecular benzylic C—H amination of bioactive molecules and natural products that proceeds with unprecedented levels of reactivity and site-selectivity. In the presence of Brønsted or Lewis acid, the [MnIII(ClPc)]-catalyzed C—H amination demonstrates unique tolerance for tertiary amine, pyridine and benzimidazole functionalities. Mechanistic studies indicate that C—H amination proceeds through an electrophilic metallonitrene intermediate via a stepwise pathway where C—H cleavage is the rate-determining step of the reaction. Collectively these mechanistic features contrast previous base-metal catalyzed C—H aminations.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Synthetic method of hexabenzylhexaazaisowurtzitane
The invention relates to a preparation method of initial intermediate hexabenzylhexaazaisowurtzitane of a high energy density compound HNIW, comprising the following steps: (1) mixing a solvent with benzylamine and placing the mixture into a reactor; (2) mixing acid catalysts with a glyoxal water solution to prepare an aldehydic acid mixed solution; (3) slowly adding the prepared aldehydic acid mixed solution into the reactor and stirring for reaction; and (4) after dripping the aldehydic acid mixed solution, continuously stirring for reaction until the reaction is completely finished, and then separating to obtain hexabenzylhexaazaisowurtzitane solid products.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Palladium/carbon nanotube catalyst and preparation and application thereof
InactiveCN104248950AEasy to operateSmall sizeCarboxylic preparation by ozone oxidationMetal/metal-oxides/metal-hydroxide catalystsPalladium catalystCarboxylic acid
The invention relates to a palladium / carbon nanotube catalyst used for selectively supporting palladium nanoparticles in carbon nanotube chamber or outside the nanotube chamber in alpha, beta unsaturated carboxylic acid hydrogenation reaction, which takes carbon nanotube as a carrier, the palladium nanoparticles can be selectively dispersed in the carbon nanotube chamber or outside the nanotube chamber, the size is uniform, and the particle size is 2-5nm. The catalyst can be used for hydrogenation and asymmetric hydrogenation of asymmetric carboxylic acid. The catalyst appears higher activity than that of the commercialized palladium catalysts in an alpha-phenylcinnamic acid hydrogenation reaction, and the highest reaction activity can reach 564mmol h<-1> g<-1>. When a chiral modification agent cinchonidine and an auxiliary agent benzylamine are added in a reaction system, the enantiomer selectivity of alpha-phenylcinnamic acid asymmetric hydrogenation product can reach more than 75%, the activity is increased to 133mmol h<-1> g<-1>, which is the highest intrinsic reaction activity of a heterogeneous catalyst to the asymmetric hydrogenation reaction of alpha-phenylcinnamic acid.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
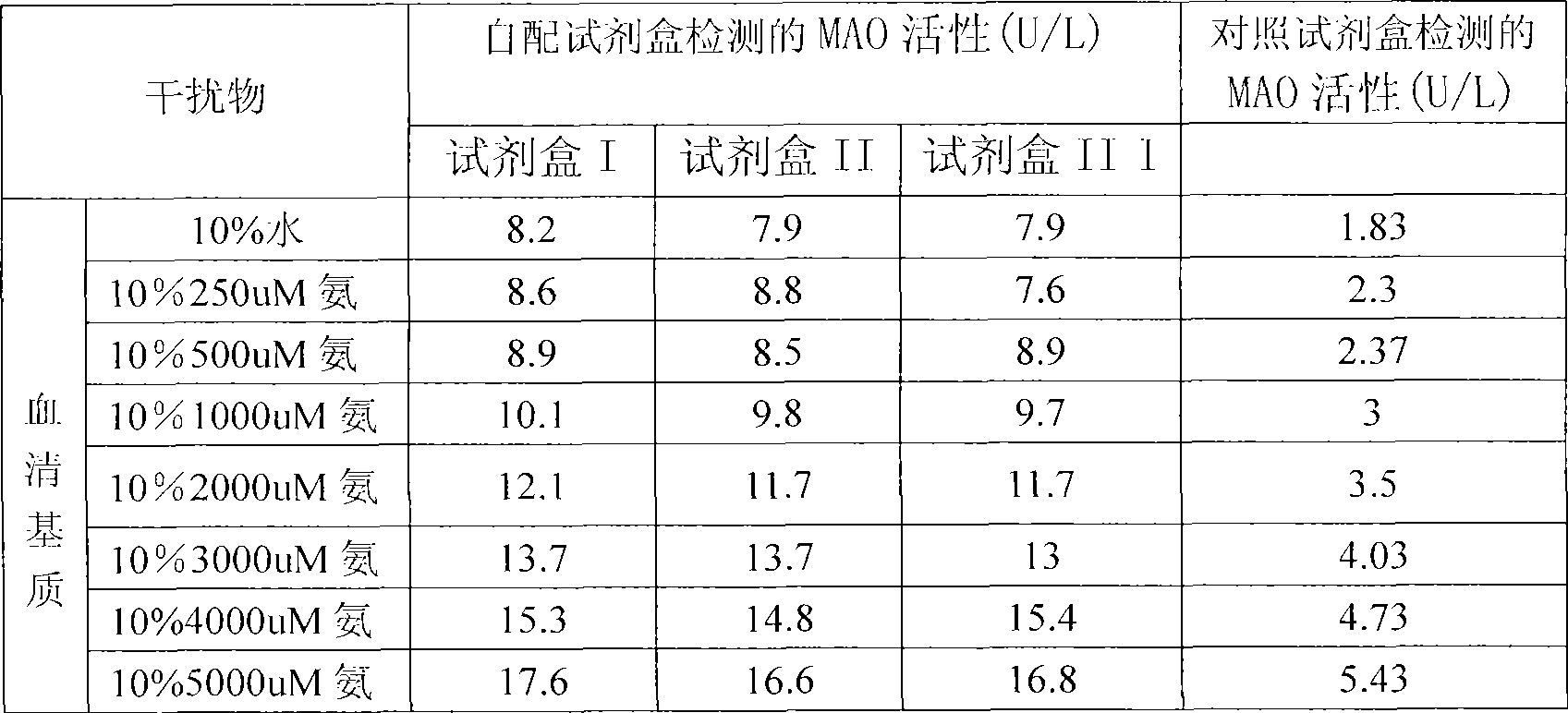
Reagent kit for monoamine oxidase MAO single-reagent measurement
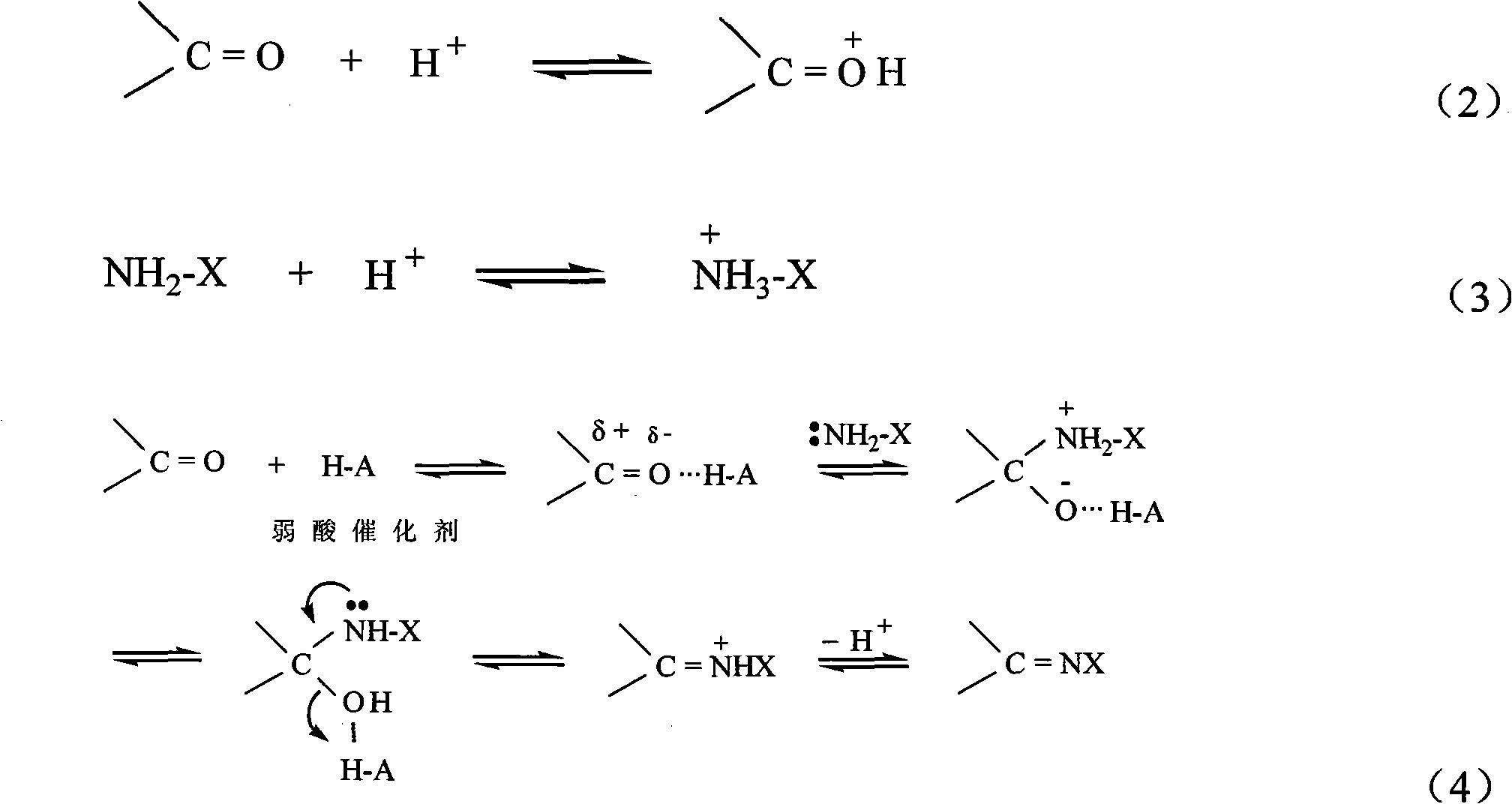
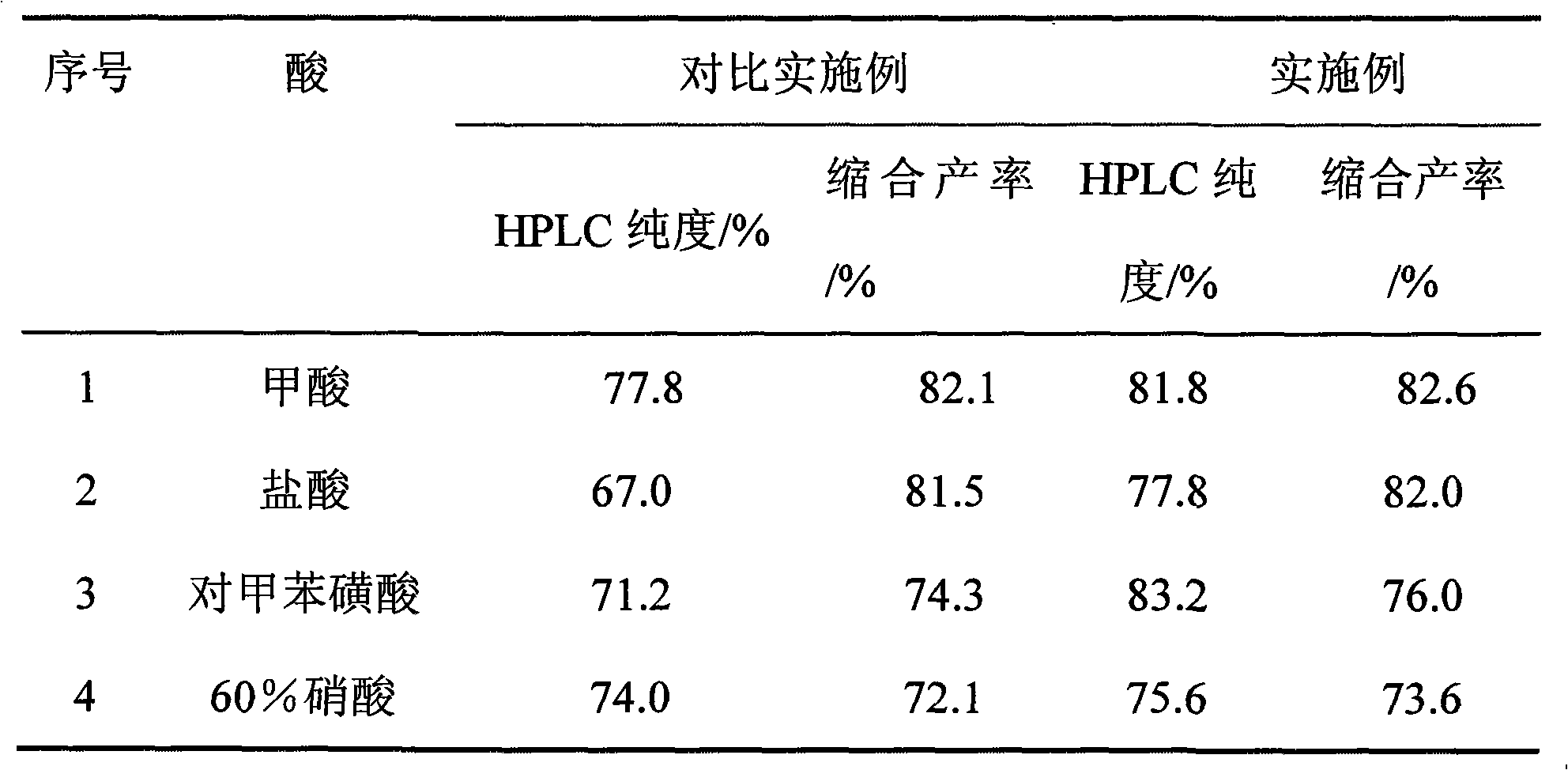
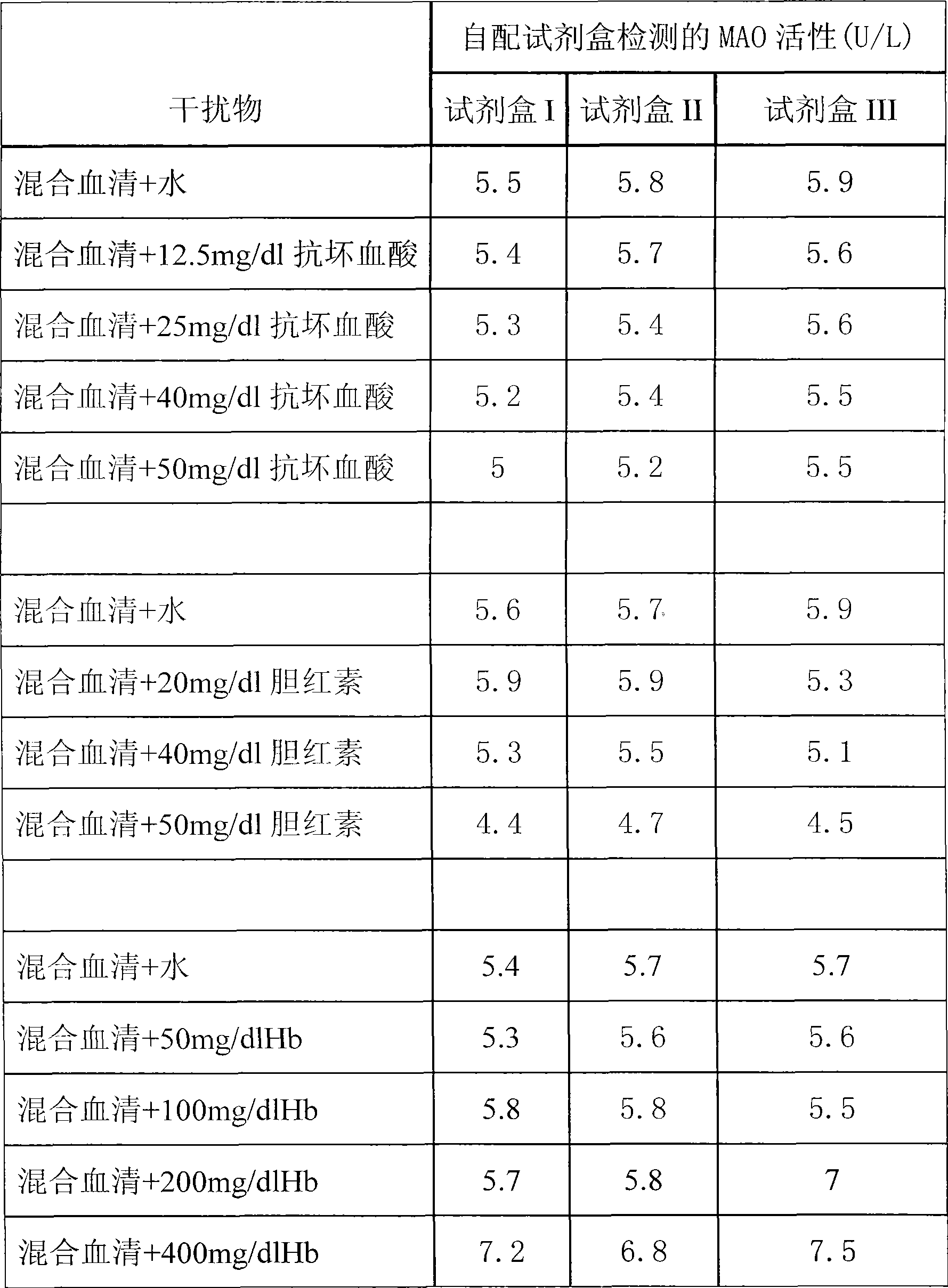
InactiveCN101498662AEasy to operateEasy to useMicrobiological testing/measurementColor/spectral properties measurementsSemi automaticOxygen
The invention discloses a kit of single reagent used for diagnosing monoamine oxidase (MAO) in serum. MAO hydrolyzed benzylamine, butyl amine, amyl amine, Beta-phenylethyl amine and other amine compounds are utilized to produce oxygen; the oxygen reacts with glutamate dehydrogenase in a coupling way; oxidized coenzyme is formed by the reaction of reduced coenzyme; and the activity of sample MAO formed by quantitative reaction is calculated through measuring the descending speed of the absorbance of light with a wavelength of 340 nm; in addition, enzyme and substrate enzyme which can slowly generate reduced nicotinoyl coenzyme are added in the reaction process, so that an enzyme-substrate enzyme-nicotinoyl coenzyme slow reaction system is formed in single reagent and the nicotinoyl coenzyme can be compensated slowly and circularly and further the single reagent can be stabilized when the concentration of the single reagent achieves a certain balance. The kit is convenient for use and simple is structure, can be used on a common ultraviolet / visible light analyzer or a semi-automatic / full-automatic biochemical analyzer for rapid measurement without using a special or additional apparatus and has low cost.
Owner:BEIJING STRONG BIOTECH INC
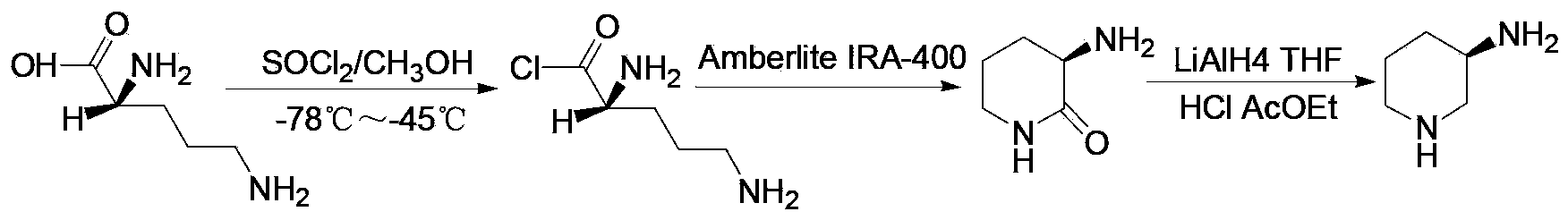
Method for producing α-amino acid including phosphorus and production intermediates thereof
ActiveUS7795464B2High enantiomeric excessEfficient processOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsAcid hydrolysisBenzylamine
Owner:MMAG CO LTD
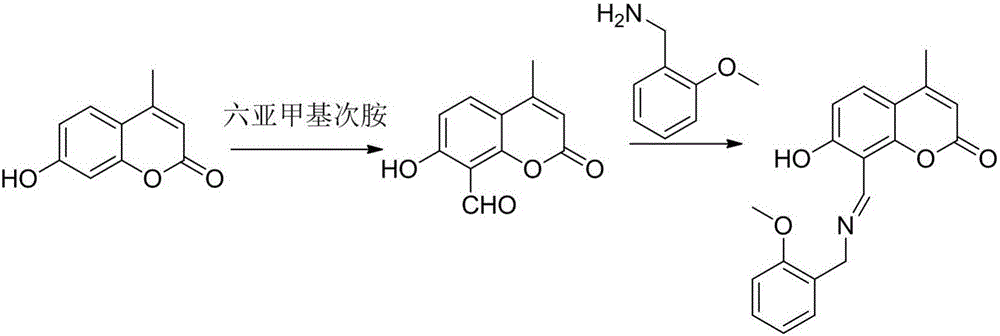
Cupric ion fluorescence enhancement type molecular probe and preparation method as well as application thereof
ActiveCN105949160AEasy to manufactureHigh sensitivityOrganic chemistryFluorescence/phosphorescenceOrtho positionOxygen
The invention discloses a cupric ion fluorescence enhancement type molecular probe and a preparation method as well as application thereof. The molecular formula of the probe is shown in the description; 4-methyl-7-hydroxy coumarin is taken as a fluorescence matrix, and reacts with hexamethylene second-amine; a formyl group is introduced at an ortho-position of hydroxy, and an obtained intermediate reacts with o-ethoxyphenol benzylamine; then nitrogen atoms and oxygen atoms are introduced; meanwhile the fluorescent light of a compound is enabled to close, and a specific reaction with cupric ions can be performed. By introducing the nitrogen atoms and the oxygen atoms for performing specific coordination with the cupric ions in the reaction matrix, a cupric ion sensor can quickly cooperate with the cupric ions in a detection process, so that fluorescent light is increased rapidly, thus realizing detection on the cupric ions and being capable of effectively avoiding the disturbance of other metal ions. The fluorescence probe prepared by the method is a high-selectivity fluorescence enhancement type cupric ion chemosensor simple in structure, cheap and easily available in material, easy to prepare and high in yield.
Owner:XUZHOU UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![Preparation method of (S, S)-8H-6H-pyrrolo [3, 4-b] pyridine Preparation method of (S, S)-8H-6H-pyrrolo [3, 4-b] pyridine](https://images-eureka.patsnap.com/patent_img/5bddebff-bd5c-4554-be69-d72e3113a71c/BDA00002489042500061.png)
![Preparation method of (S, S)-8H-6H-pyrrolo [3, 4-b] pyridine Preparation method of (S, S)-8H-6H-pyrrolo [3, 4-b] pyridine](https://images-eureka.patsnap.com/patent_img/5bddebff-bd5c-4554-be69-d72e3113a71c/BDA00002489042500062.png)
![Preparation method of (S, S)-8H-6H-pyrrolo [3, 4-b] pyridine Preparation method of (S, S)-8H-6H-pyrrolo [3, 4-b] pyridine](https://images-eureka.patsnap.com/patent_img/5bddebff-bd5c-4554-be69-d72e3113a71c/BDA00002489042500071.png)