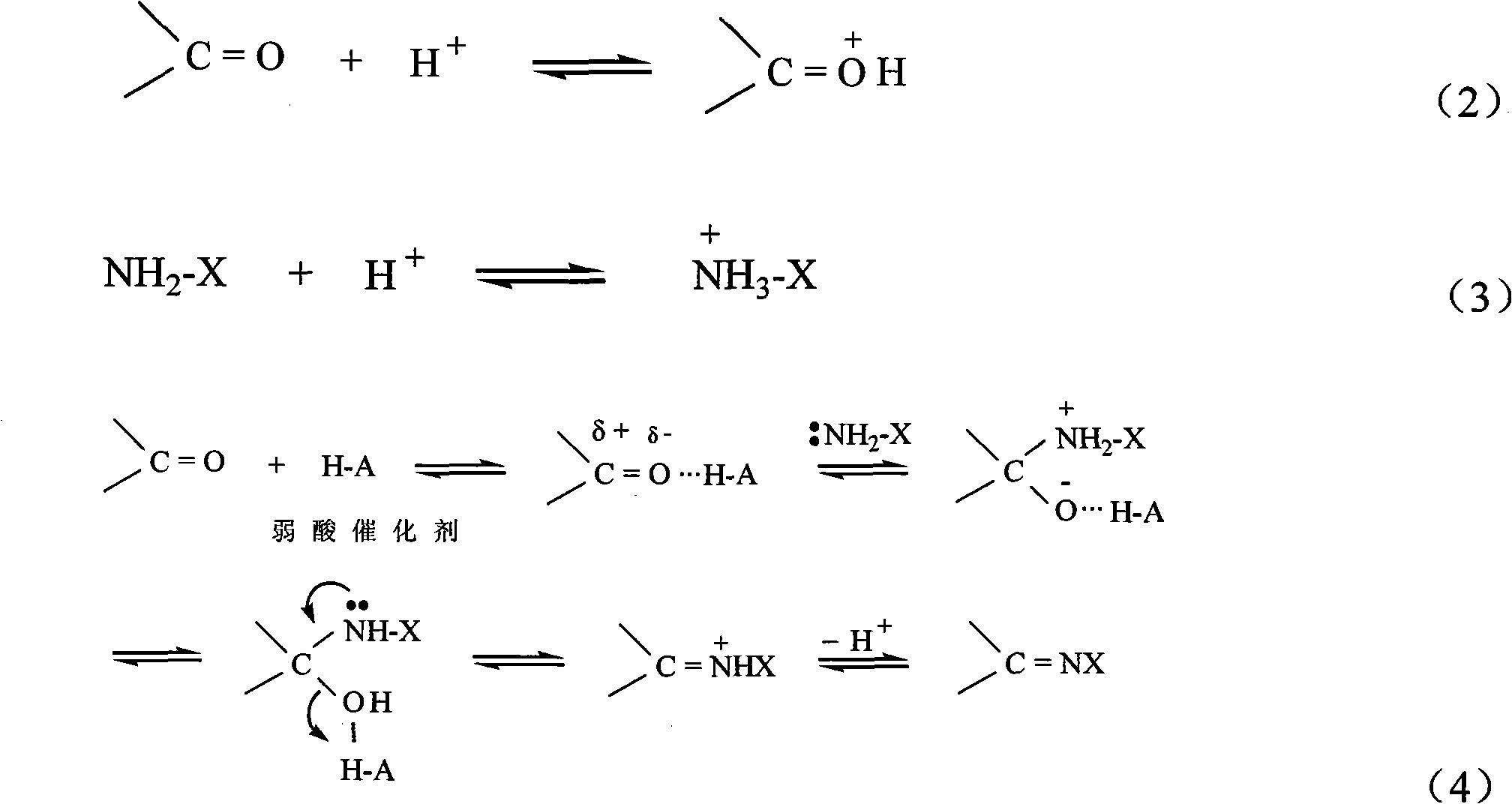
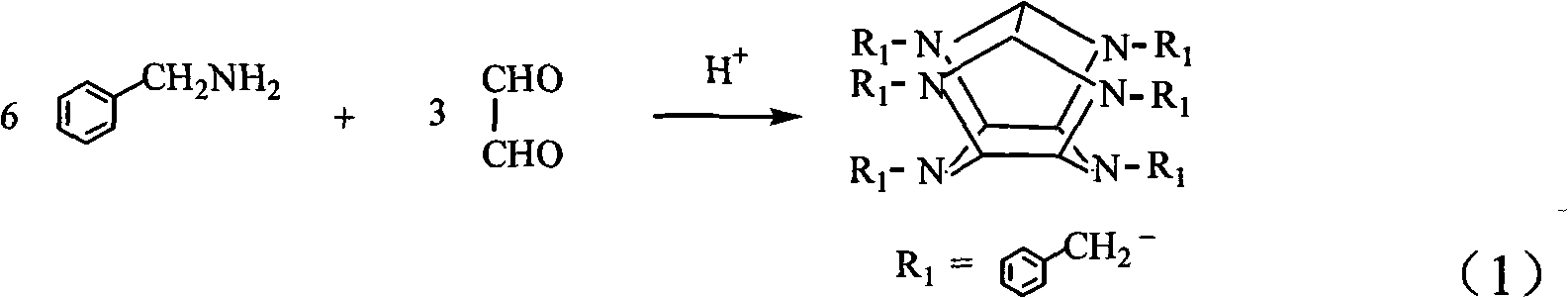Synthetic method of hexabenzylhexaazaisowurtzitane
A technology of hexabenzyl hexaazide and isowurtzitane is applied in the field of synthesis of hexabenzyl hexaaz isowurtzitane, and can solve the problems of affecting product quality and yield, loss of amino nucleophilic ability, etc. The effect of reducing side reactions and improving application efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
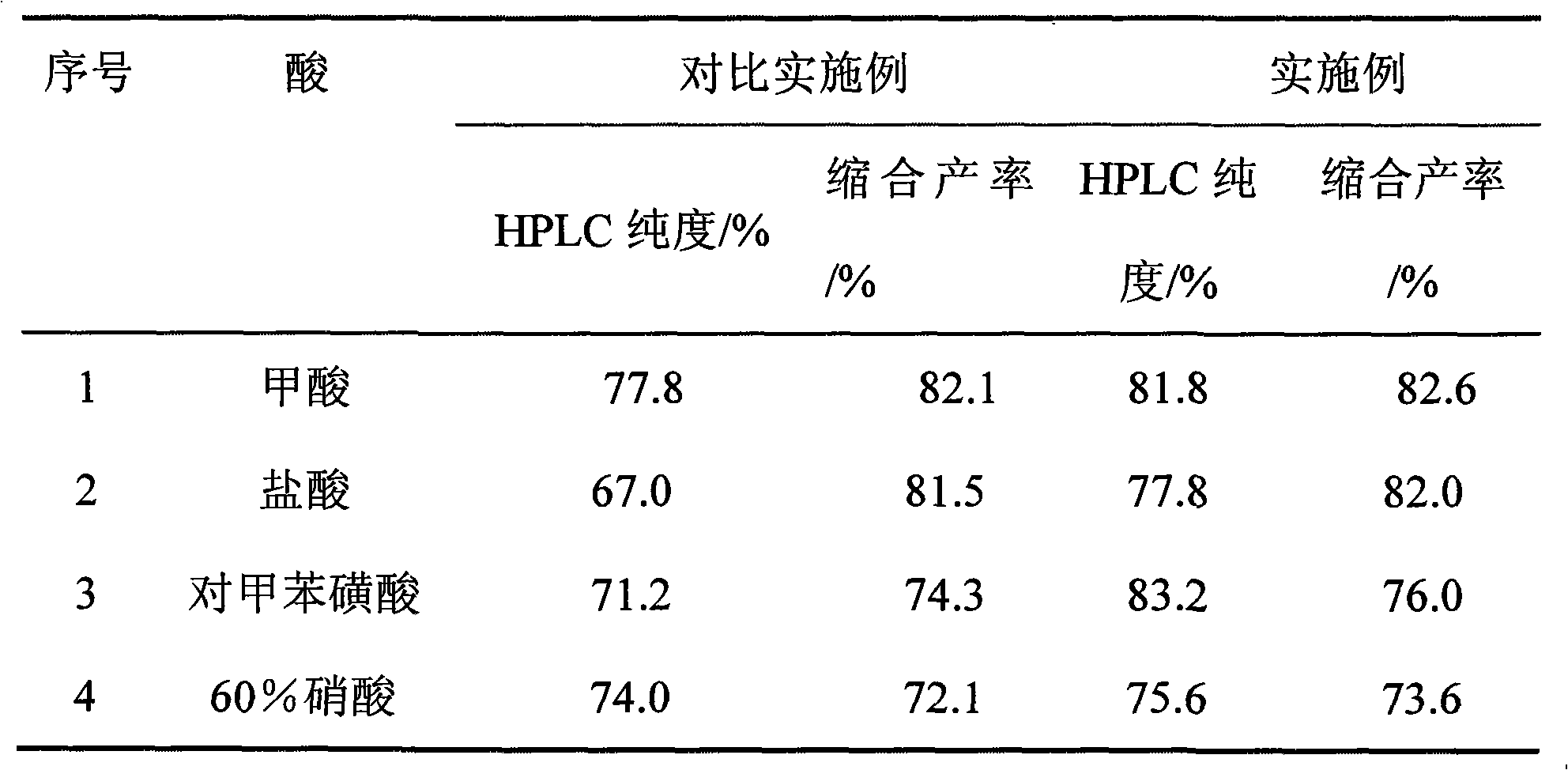
[0026] 4.4 liters of acetonitrile, 0.44 liters of distilled water, and 480 g of benzylamine were added to a 12-liter reaction vessel, and stirred. 292 grams of 40% glyoxal aqueous solution and 23.2 g of formic acid (88% by weight) were thoroughly mixed, and slowly added to the reaction system with a constant pressure dropping funnel for 2.5 hours. The constant pressure dropping funnel was washed with distilled water, and the washing water was also added to the reaction flask. After the addition was complete, the stirring reaction was continued at room temperature for 24 h, and finally the reactant was filtered. The HBIW crude product has a purity of 81.1% (HPLC purity), and a yield of 82.6% by weight (the ratio of the weight of the product actually obtained to the theoretical amount calculated from the amount of glyoxal).
Embodiment 2
[0030] Method is the same as Example 1, except that the acid catalyst used is hydrochloric acid.
Embodiment 3
[0034] Method is the same as Example 1, except that the acid catalyst used is p-toluenesulfonic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com