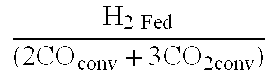Patents
Literature
12798 results about "Production rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Production rate, in terms of manufacturing, refers to the number of goods that can be produced during a given period of time. Alternatively, production rate is also the amount of time it takes to produce one unit of a good. In construction, this is the rate at which workers are expected to complete a certain segment, such as a road or building.
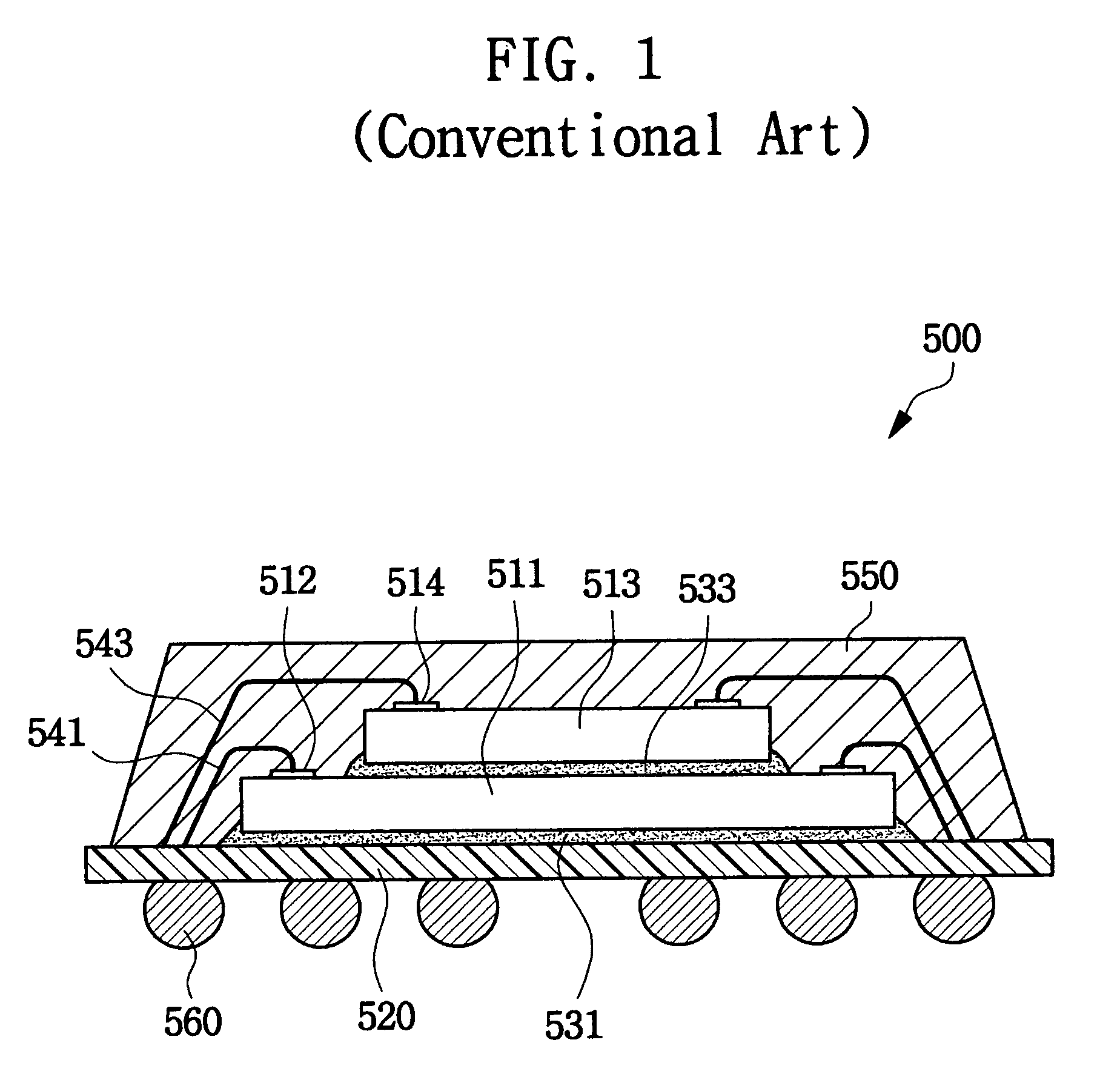
Suture reinforcement material for automatic suturing device
Owner:GUNZE LTD
Code symbol reading system supporting operator-dependent system configuration parameters
InactiveUS20120193423A1Easy loadingEasy to modifySensing detailsCharacter and pattern recognitionProduction rateWorking environment
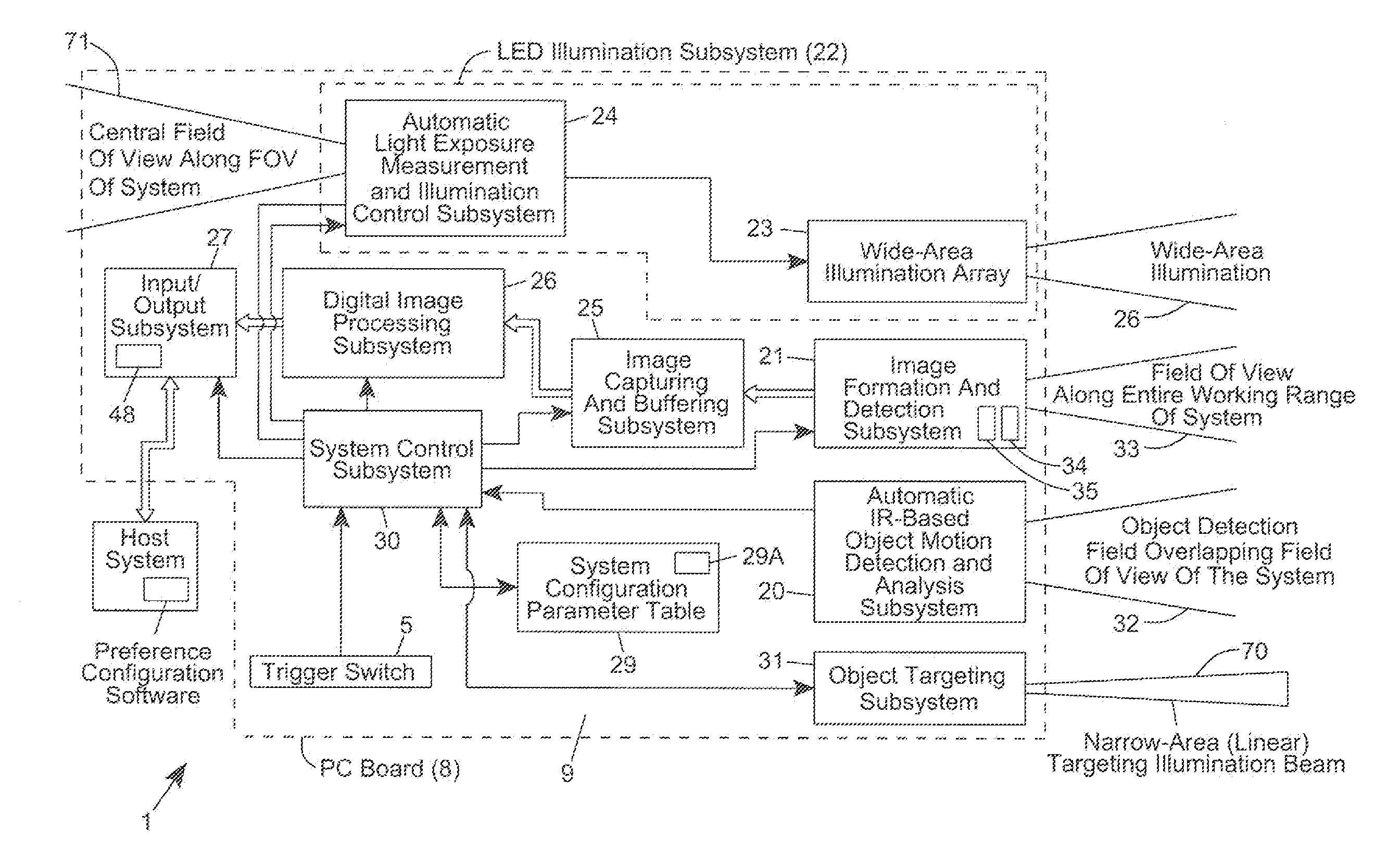
A method of and system for setting and switching user preferences between system operators, to provide a higher return on investment (ROI) and a more satisfying work environment. The system allows operators to easily select and implement particular customizable system configuration parameters (SCPs) in a code symbol reading system, based on personal preferences of the system operator, which can lead to more effective scanning performance. A different set of customizable SCPs are programmably stored in system memory (e.g. EPROM) for each system operator / user registered to use the system, to improve the quality of the working environment and increase worker productivity.
Owner:METROLOGIC INSTR
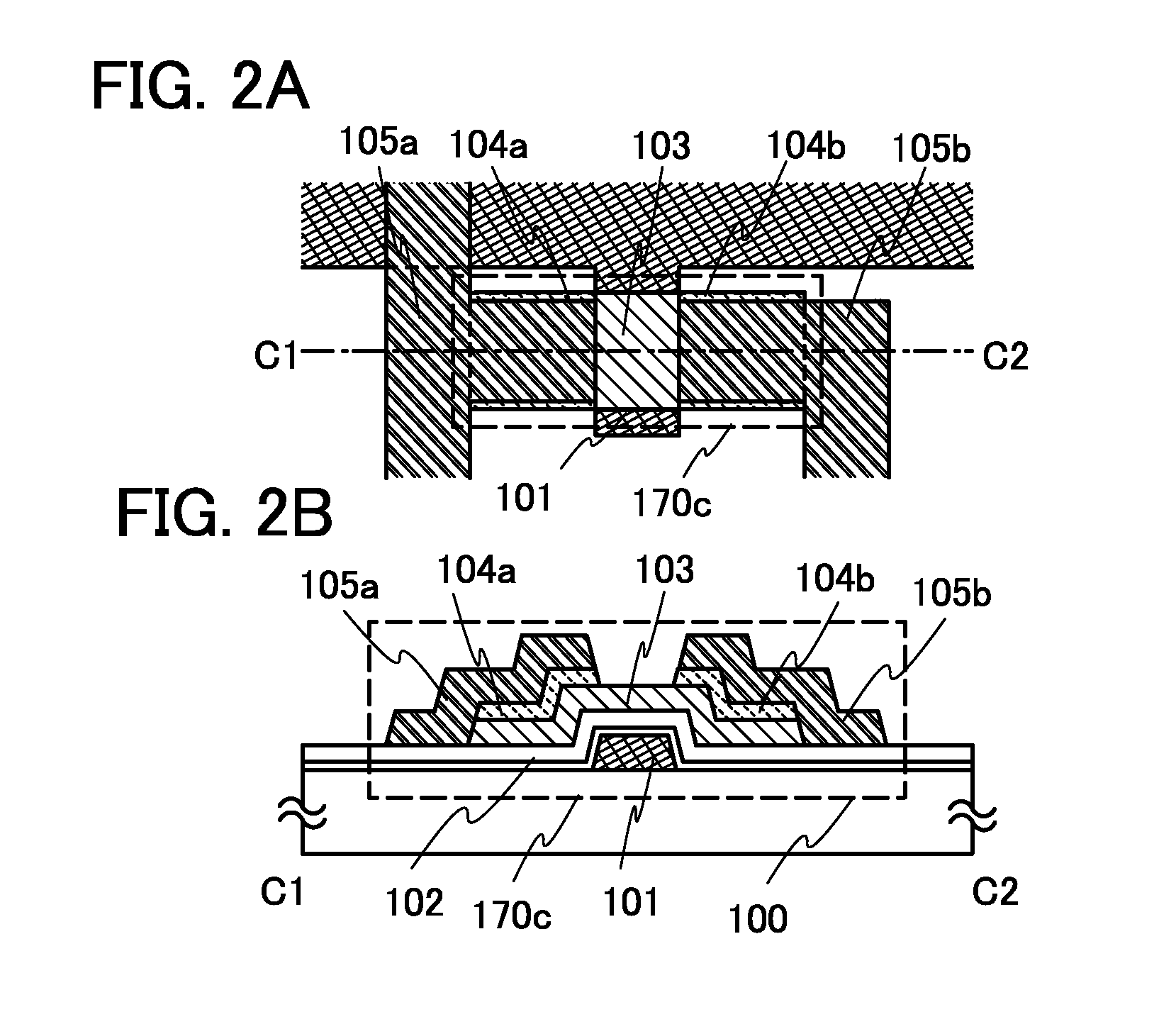
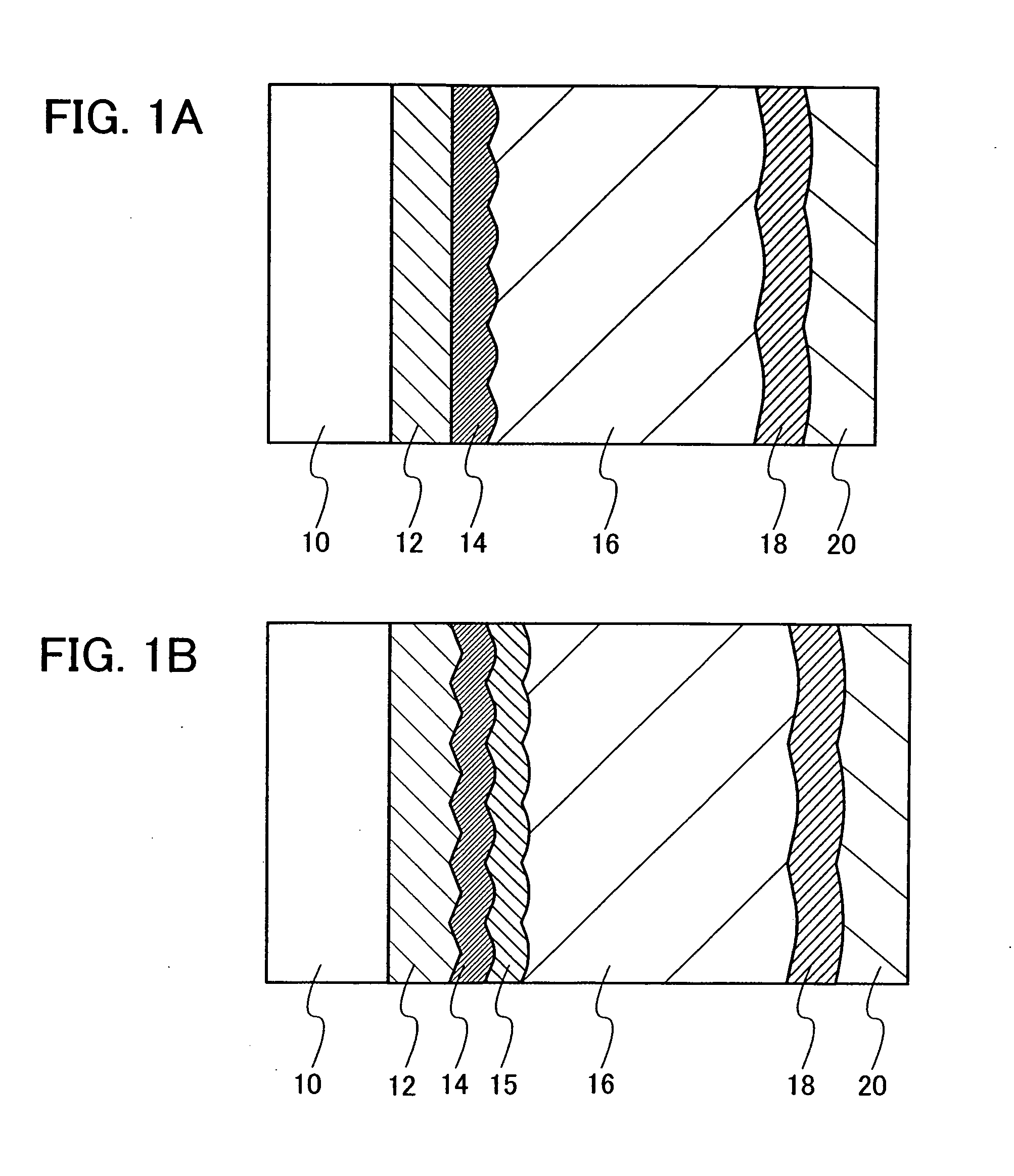
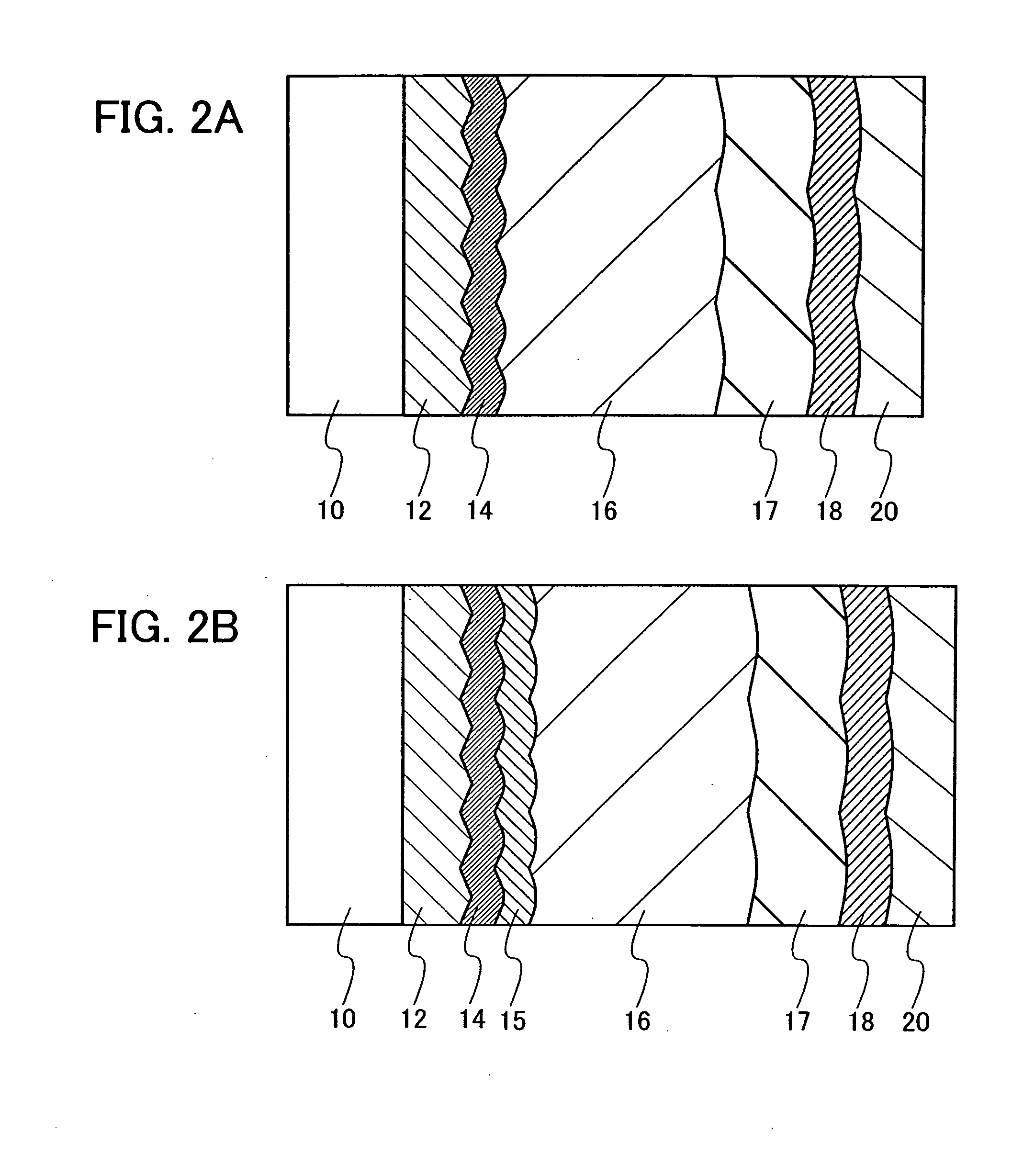
Semiconductor device and method for manufacturing the same
ActiveUS20100025678A1Comparatively-easy manufacturing processRun at high speedTransistorSolid-state devicesProduction rateOhmic contact
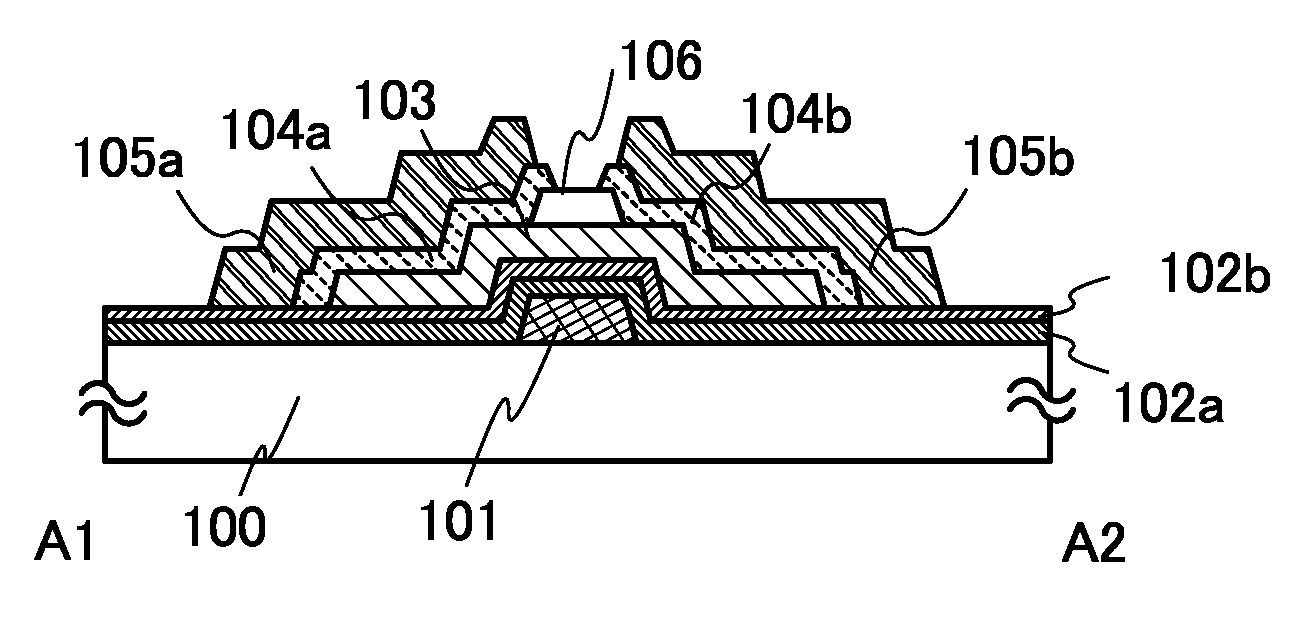
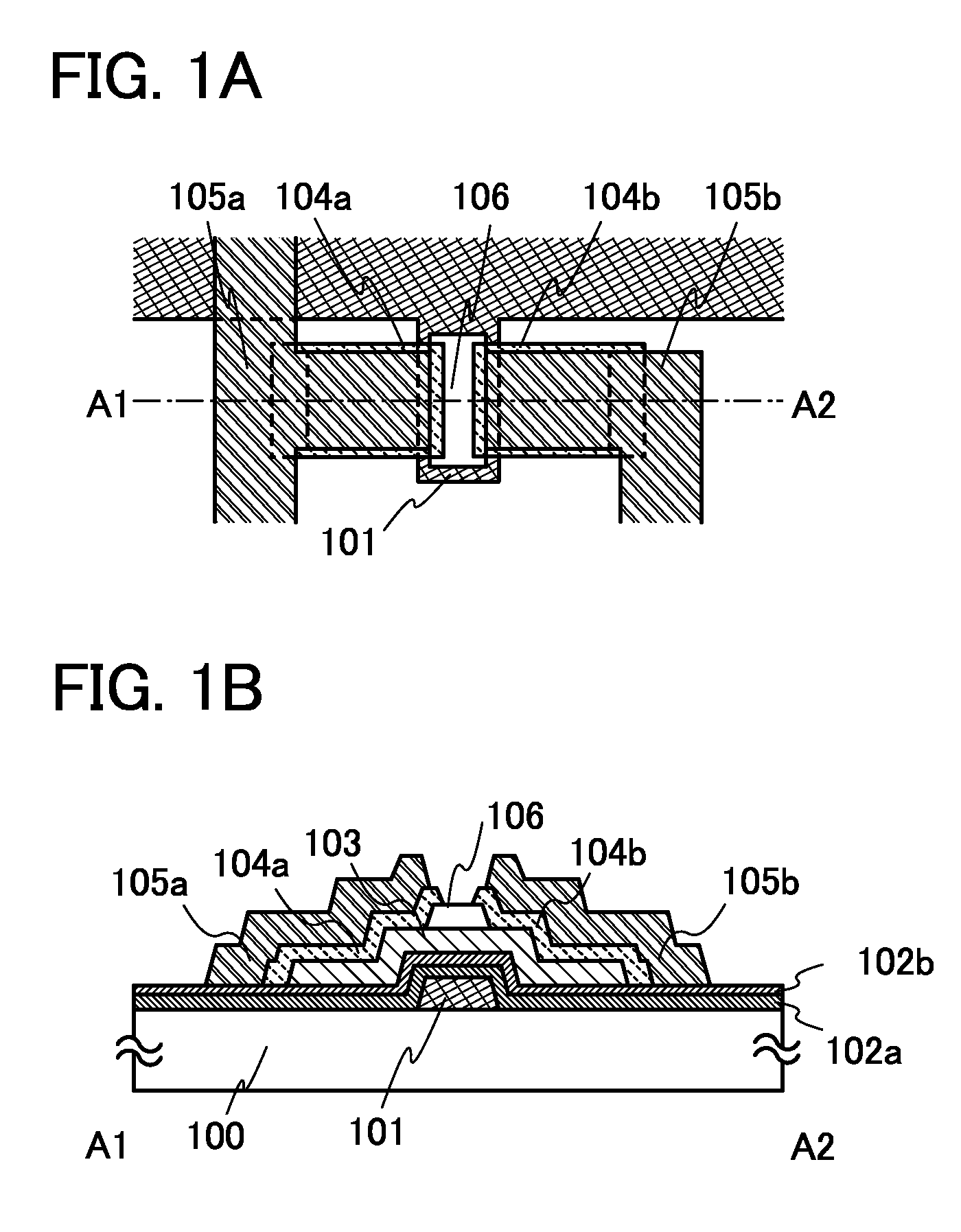
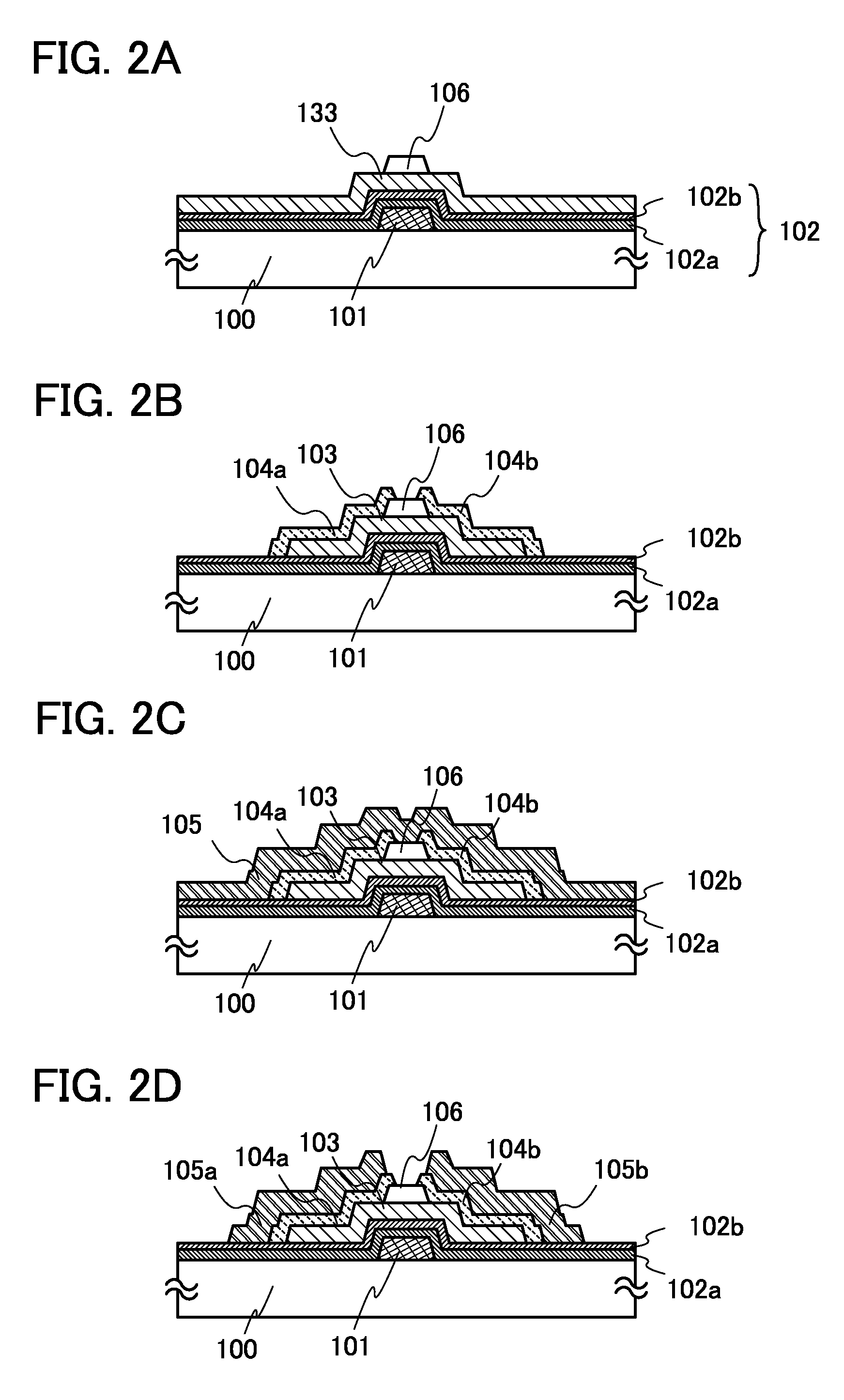
It is an object to provide a semiconductor device including a thin film transistor with favorable electric properties and high reliability, and a method for manufacturing the semiconductor device with high productivity. In an inverted staggered (bottom gate) thin film transistor, an oxide semiconductor film containing In, Ga, and Zn is used as a semiconductor layer, and a buffer layer formed using a metal oxide layer is provided between the semiconductor layer and a source and drain electrode layers. The metal oxide layer is intentionally provided as the buffer layer between the semiconductor layer and the source and drain electrode layers, whereby ohmic contact is obtained.
Owner:SEMICON ENERGY LAB CO LTD
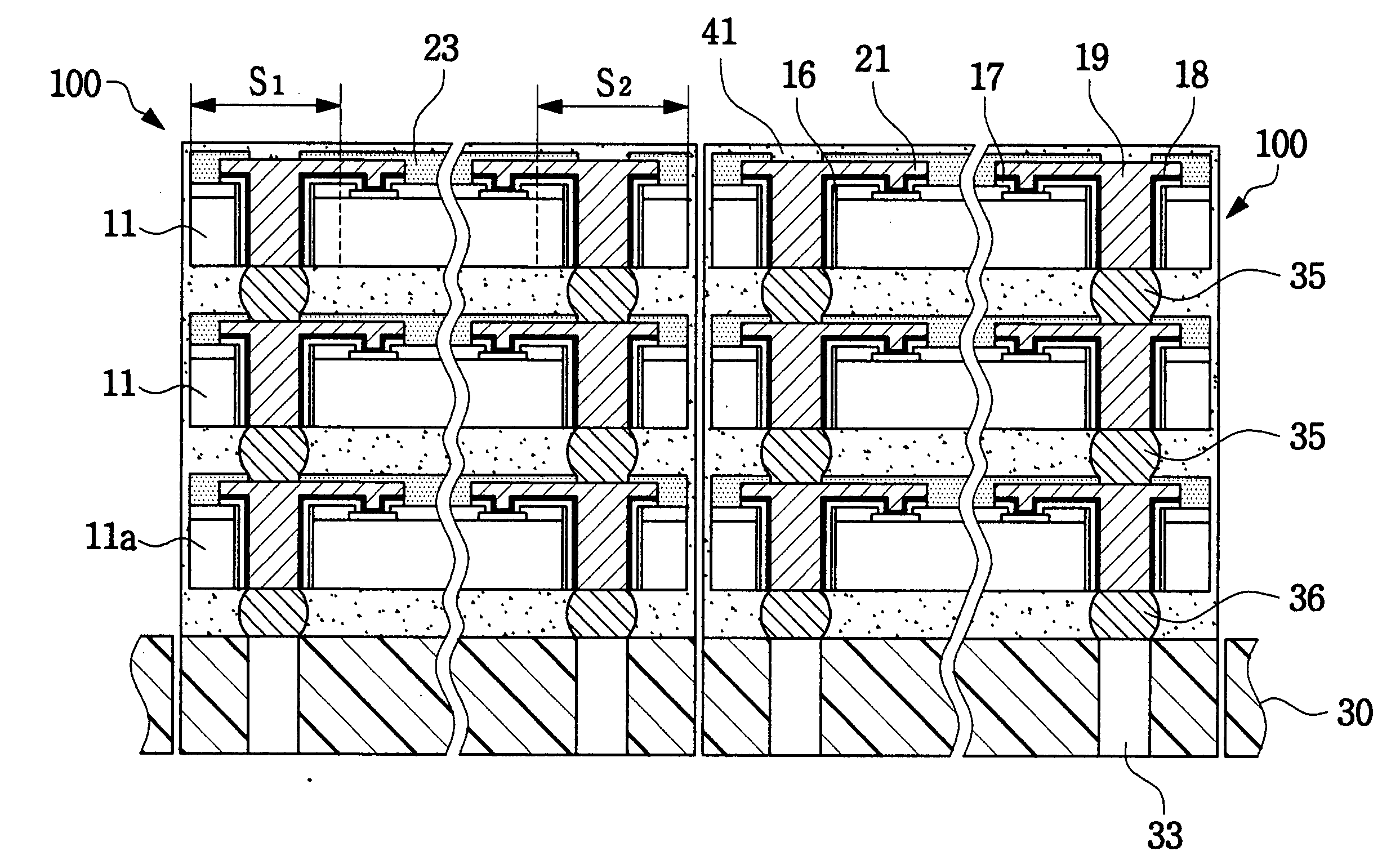
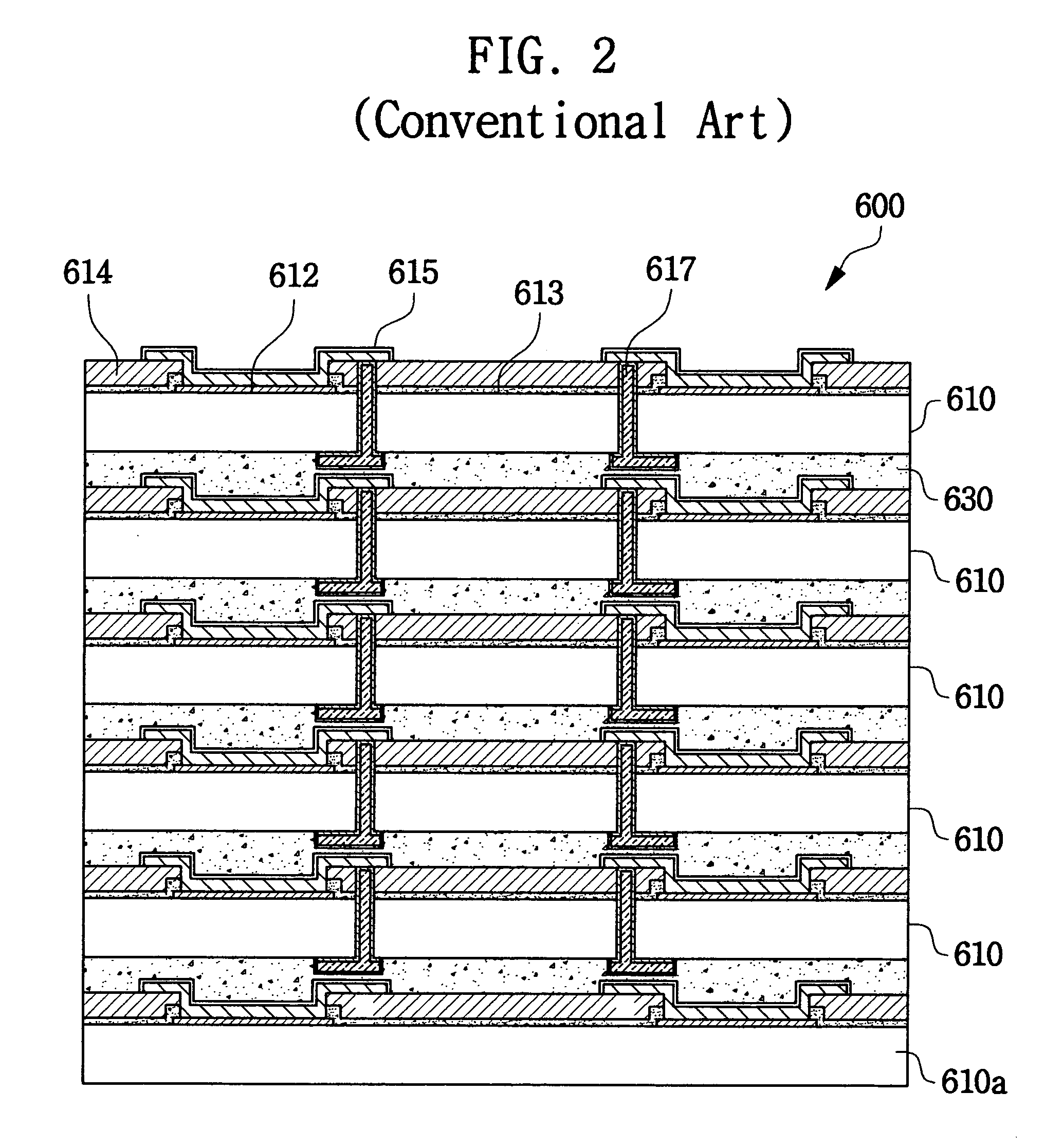
Chip stack package and manufacturing method thereof
ActiveUS20050046002A1Reduce manufacturing costReduce time costSemiconductor/solid-state device detailsSolid-state devicesProduction rateElectrical connection
A chip stack package is manufactured at a wafer level by forming connection vias in the scribe lanes adjacent the chips and connecting the device chip pads to the connection vias using rerouting lines. A lower chip is then attached and connected to a substrate, which may be a test wafer, and an upper chip is attached and connected to the lower chip, the electrical connections being achieved through their respective connection vias. In addition to the connection vias, the chip stack package may include connection bumps formed between vertically adjacent chips and / or the lower chip and the substrate. The preferred substrate is a test wafer that allows the attached chips to be tested, and replaced if faulty, thereby ensuring that each layer of stacked chips includes only “known-good die” before the next layer of chips is attached thereby increasing the production rate and improving the yield.
Owner:SAMSUNG ELECTRONICS CO LTD
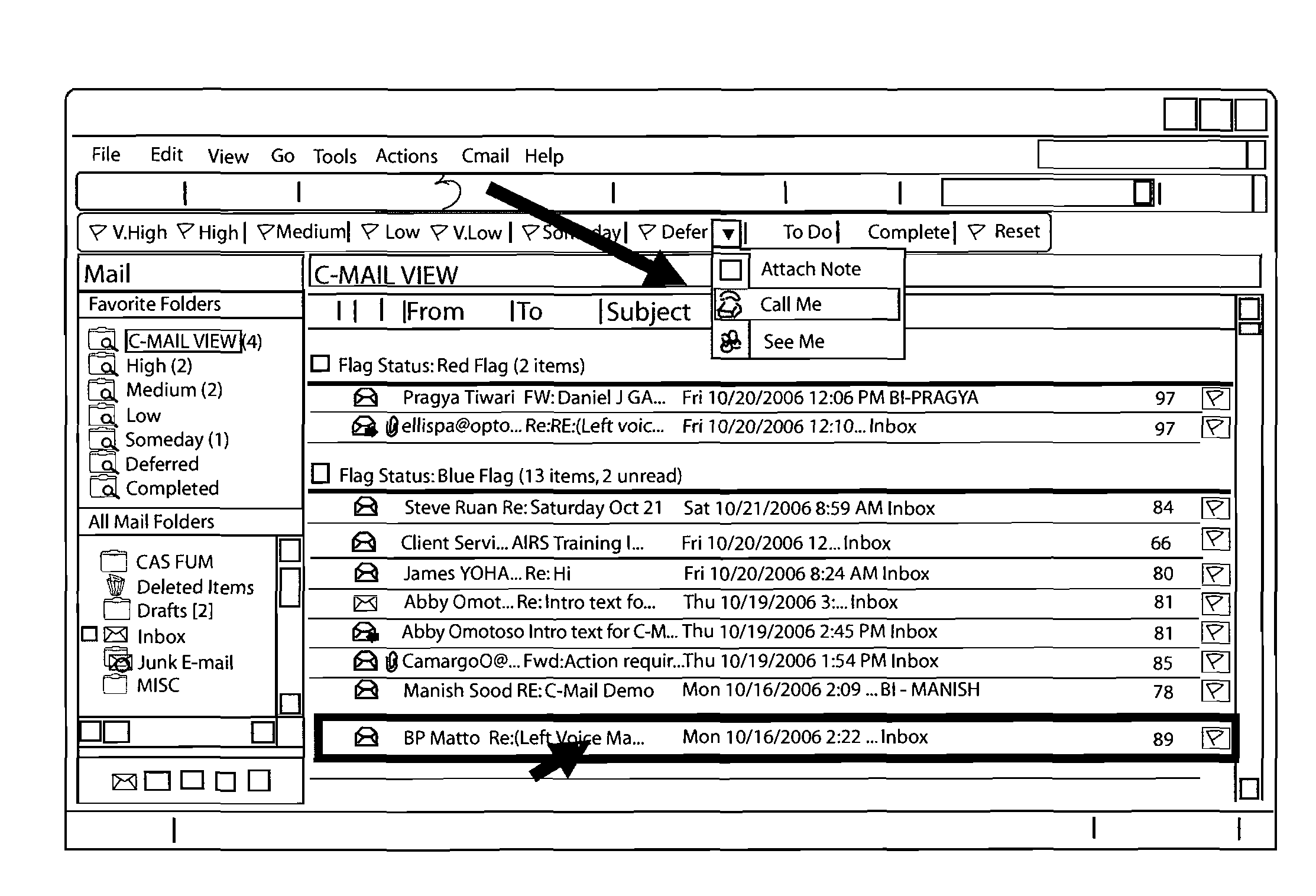
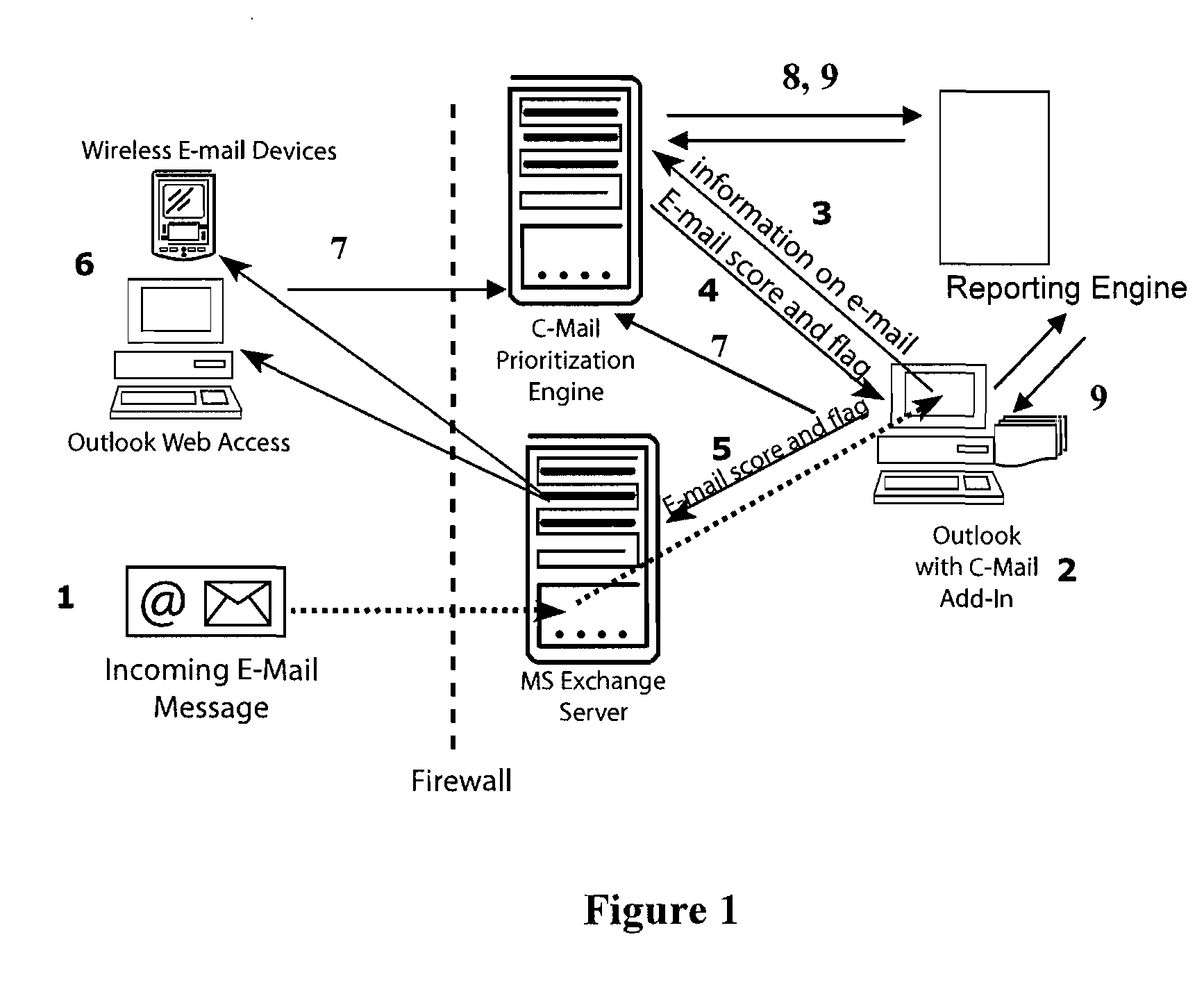
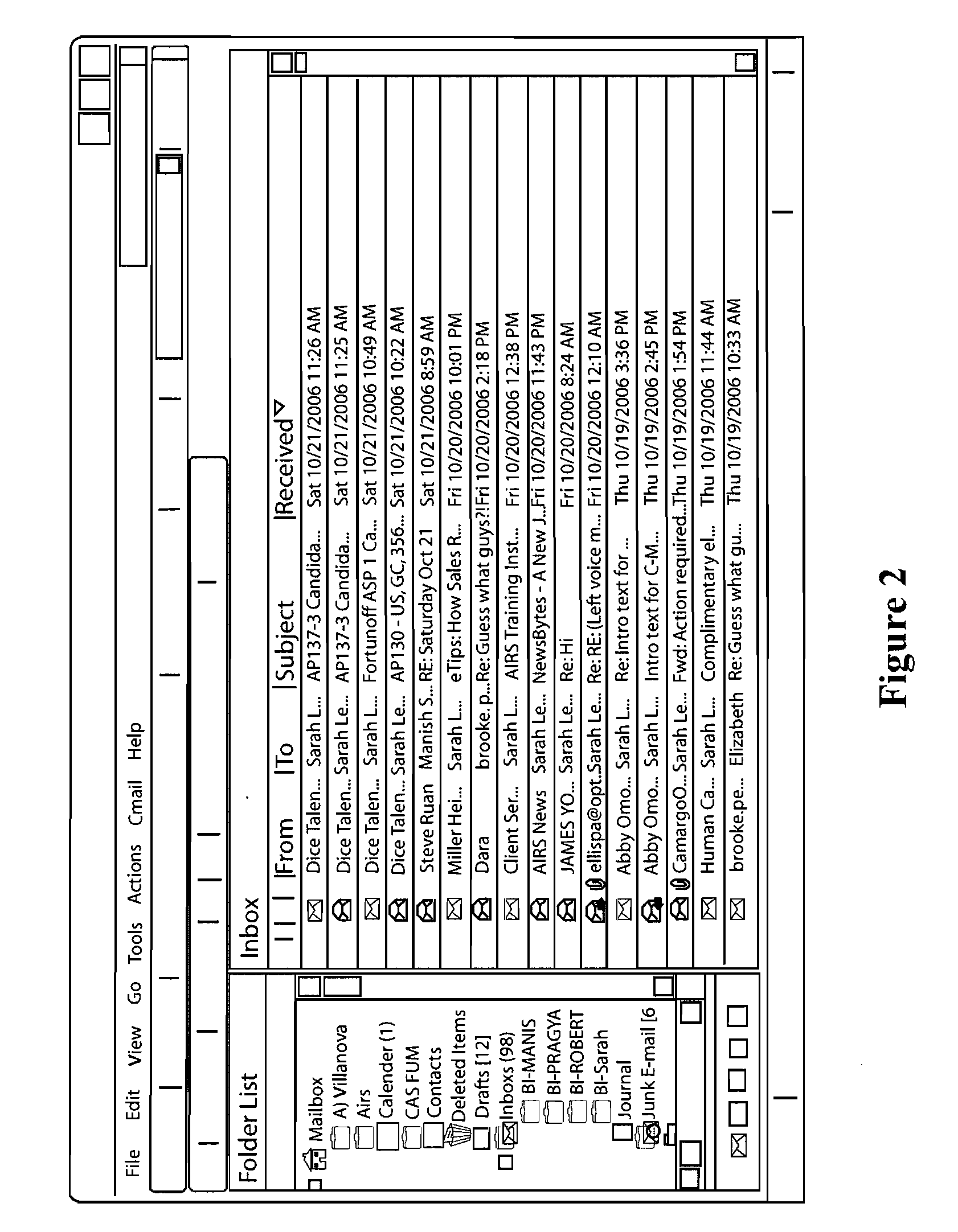
System and method of dynamically prioritized electronic mail graphical user interface, and measuring email productivity and collaboration trends
InactiveUS20080126951A1Efficient use ofEfficient managementTransmissionInput/output processes for data processingGraphicsProduction rate
A system and method for managing electronic communications more effectively utilizes electronic communications. The method assigns a prioritization score and category to each electronic communication so that a user can more effectively manage the communications. The user interacts with a graphical user interface to effectively manage electronic communications. The system arranges and displays the electronic communications according to prioritization scores and categories, and includes interactive modules to override a system assigned prioritization scores and assign any score or category the user selects. The method also measures productivity of users as a function of at least three different metrics, a decision-making metric; a communication metric; and a processing metric, and takes into account prioritization scores and the amount of time it takes users to effectively utilize the electronic communications. The method also generates reports of the productivity of individual users, and the productivity of relationships between multiple users of electronic communications.
Owner:MESSAGEMIND LLC
Chip stack package and manufacturing method thereof
ActiveUS7276799B2Low costShorten the timeSemiconductor/solid-state device detailsSolid-state devicesProduction rateElectrical connection
A chip stack package is manufactured at a wafer level by forming connection vias in the scribe lanes adjacent the chips and connecting the device chip pads to the connection vias using rerouting lines. A lower chip is then attached and connected to a substrate, which may be a test wafer, and an upper chip is attached and connected to the lower chip, the electrical connections being achieved through their respective connection vias. In addition to the connection vias, the chip stack package may include connection bumps formed between vertically adjacent chips and / or the lower chip and the substrate. The preferred substrate is a test wafer that allows the attached chips to be tested, and replaced if faulty, thereby ensuring that each layer of stacked chips includes only “known-good die” before the next layer of chips is attached thereby increasing the production rate and improving the yield.
Owner:SAMSUNG ELECTRONICS CO LTD
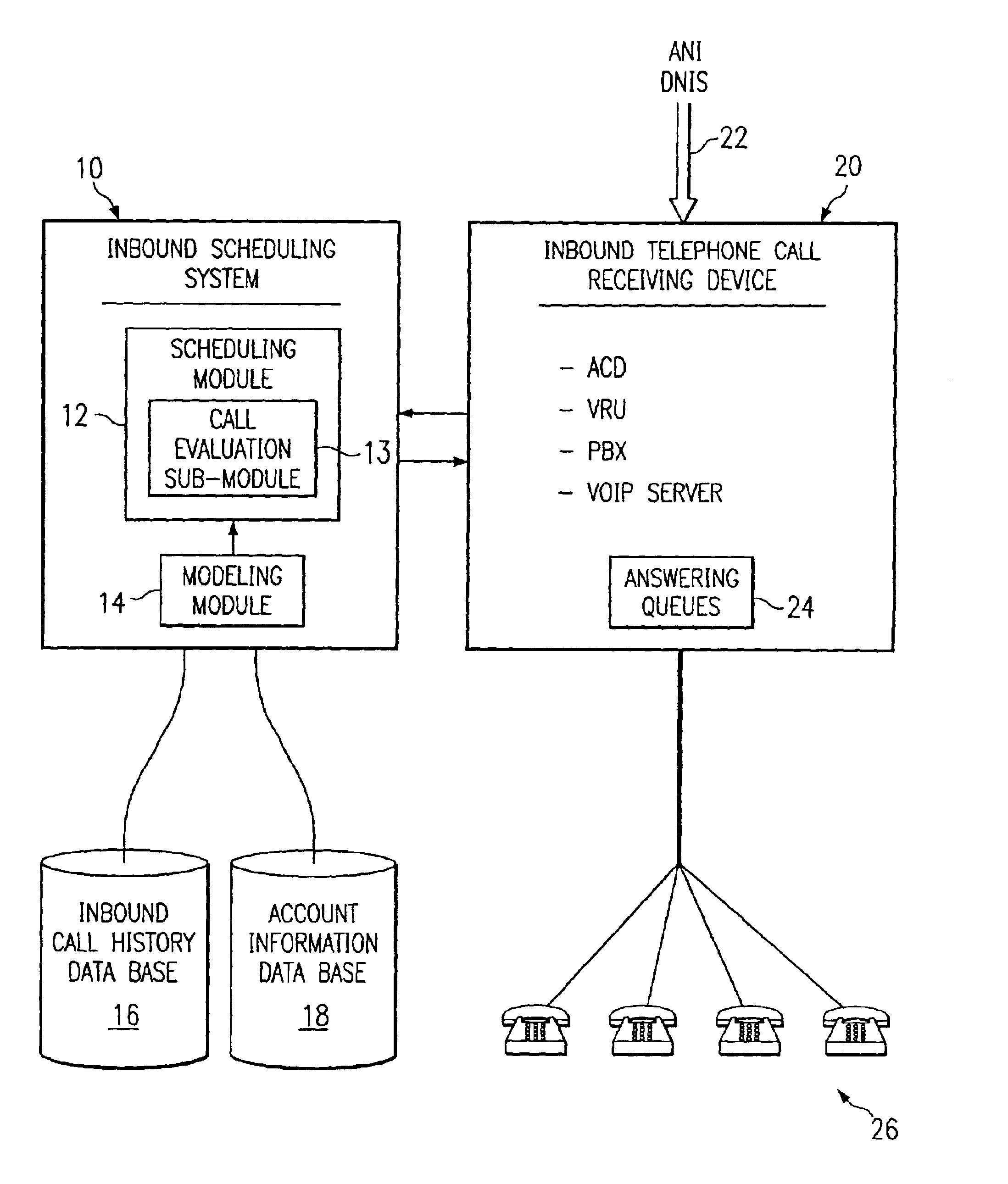
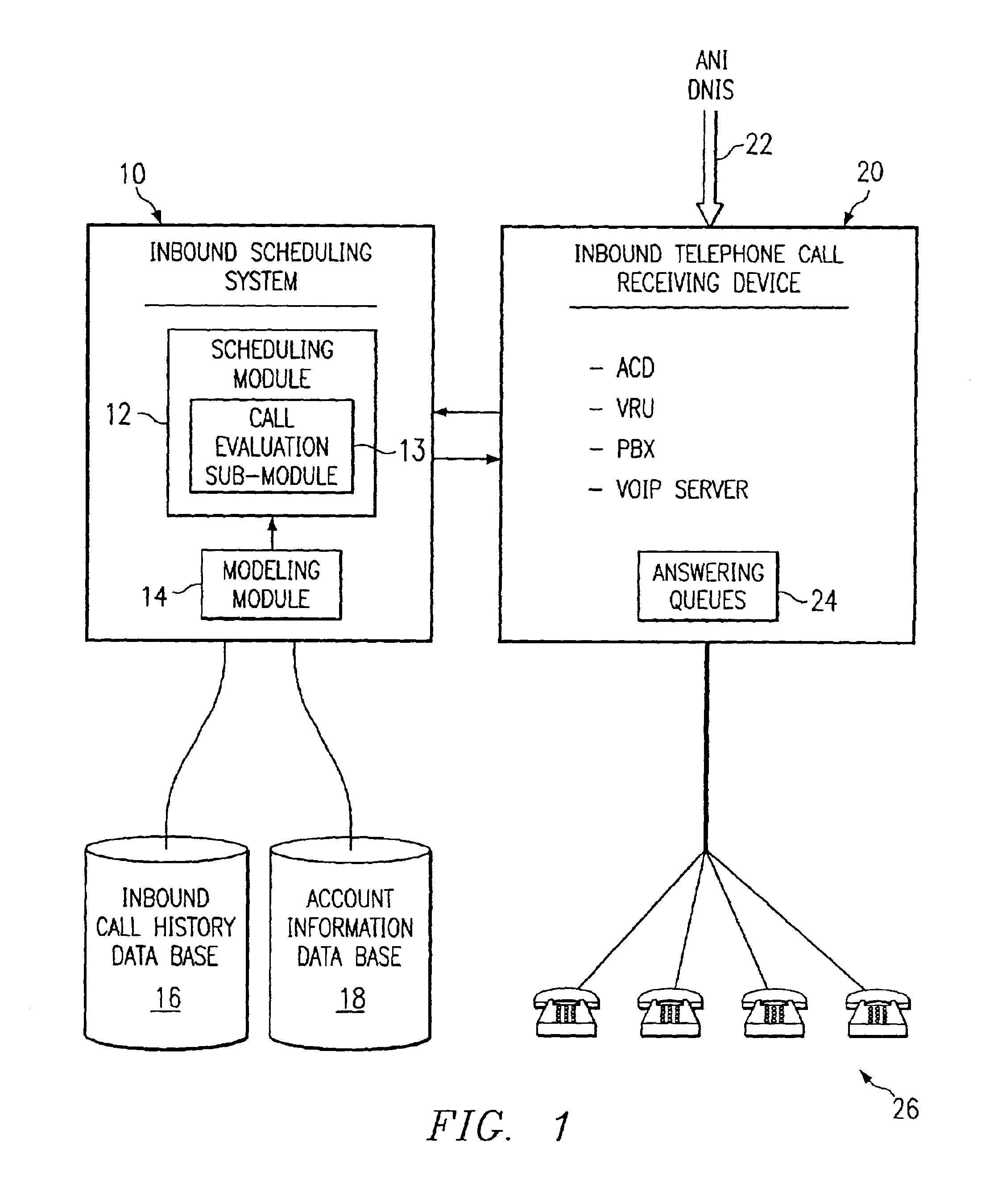
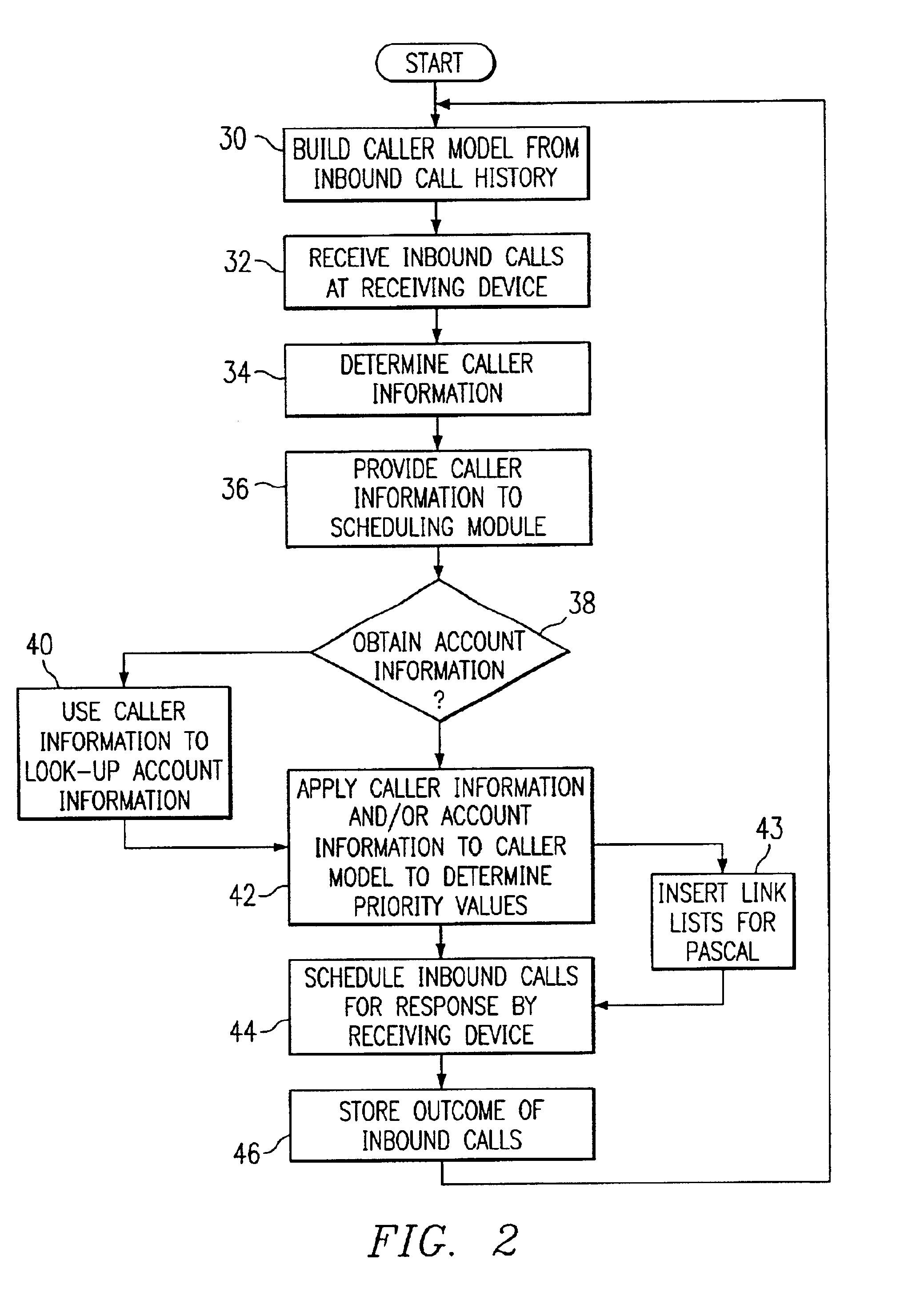
Method and system for self-service scheduling of inbound inquiries
InactiveUS6859529B2Accurate modelingMaximize useSpecial service for subscribersManual exchangesProduction rateRegression analysis
A method and system schedules inbound inquiries, such as inbound telephone calls, for response by agents in an order that is based in part on the forecasted outcome of the inbound inquiries. A scheduling module applies inquiry information to a model to forecast the outcome of an inbound inquiry. The forecasted outcome is used to set a priority value for ordering the inquiry. The priority value may be determined by solving a constrained optimization problem that seeks to maximize an objective function, such as maximizing an agent's productivity to produce sales or to minimize inbound call attrition. A modeling module generates models that forecast inquiry outcomes based on a history and inquiry information. Statistical analysis such as regression analysis determines the model with the outcome related to the nature of the inquiry. Operator wait time is regulated by forcing low priority and / or highly tolerant inbound inquiries to self service.
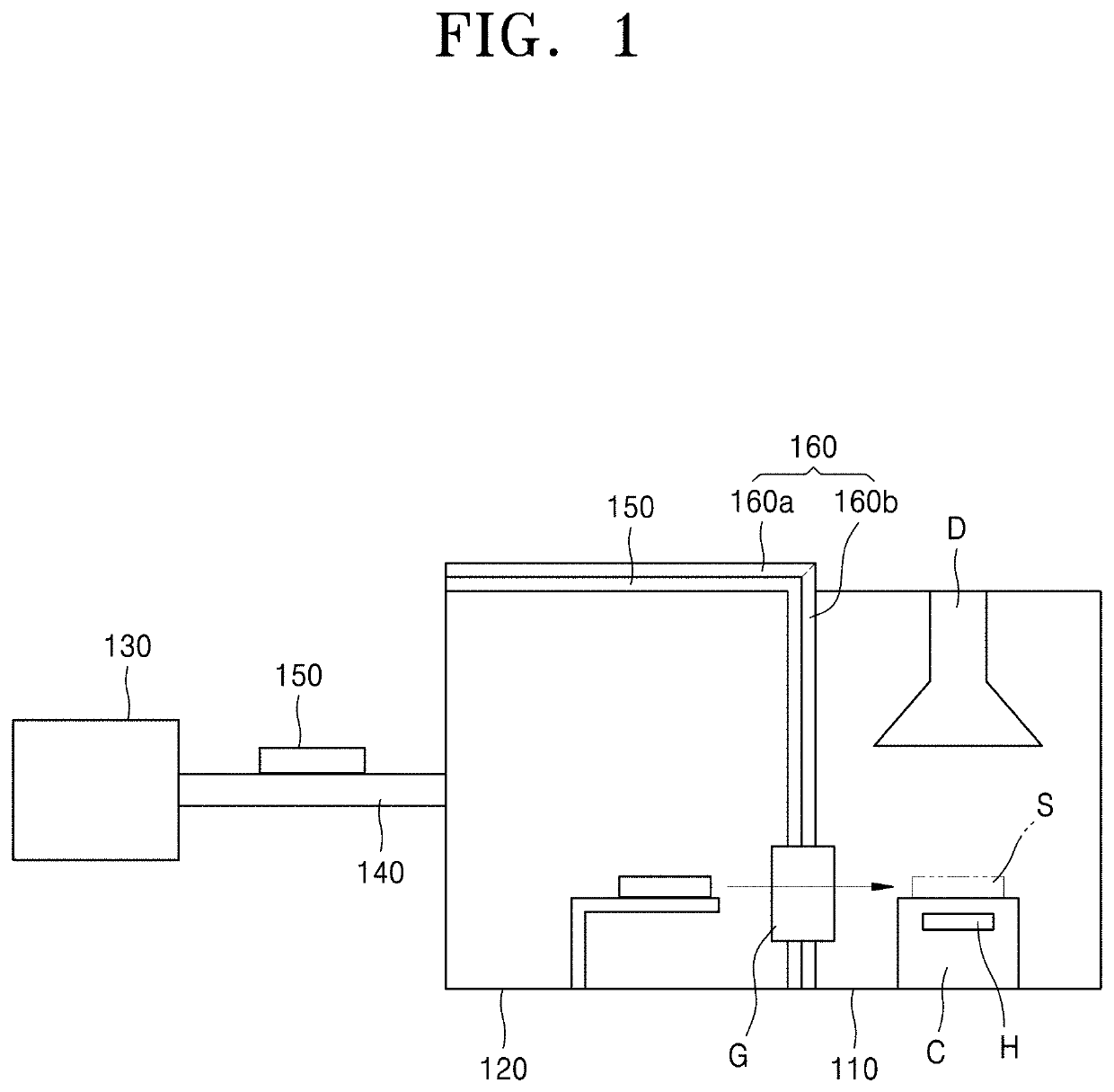
Apparatus for processing a substrate including a heating apparatus
InactiveUS20030066826A1Drying solid materials with heatMuffle furnacesProduction rateDevice material
An apparatus for heating a substrate of a semiconductor device includes a hot plate, on which a semiconductor substrate is placed, and a heater for heating the hot plate. The hot plate is preferably a composite plate including a plurality of plates having different thermal conductivities from each other. For example, a first plate adjacent to the heater can be made of aluminum, which has a relatively high thermal conductivity. A second plate, laminated on top of the first plate, can be made of titanium or stainless steel, which both have a thermal conductivity lower than aluminum. A composite hot plate as disclosed herein is better able to maintain a constant temperature and a uniform temperature distribution in order to more uniformly heat a substrate and to reduce an amount of energy required for the heating process. In addition, the reliability and productivity of the semiconductor device manufactured by the apparatus can be improved.
Owner:SAMSUNG ELECTRONICS CO LTD
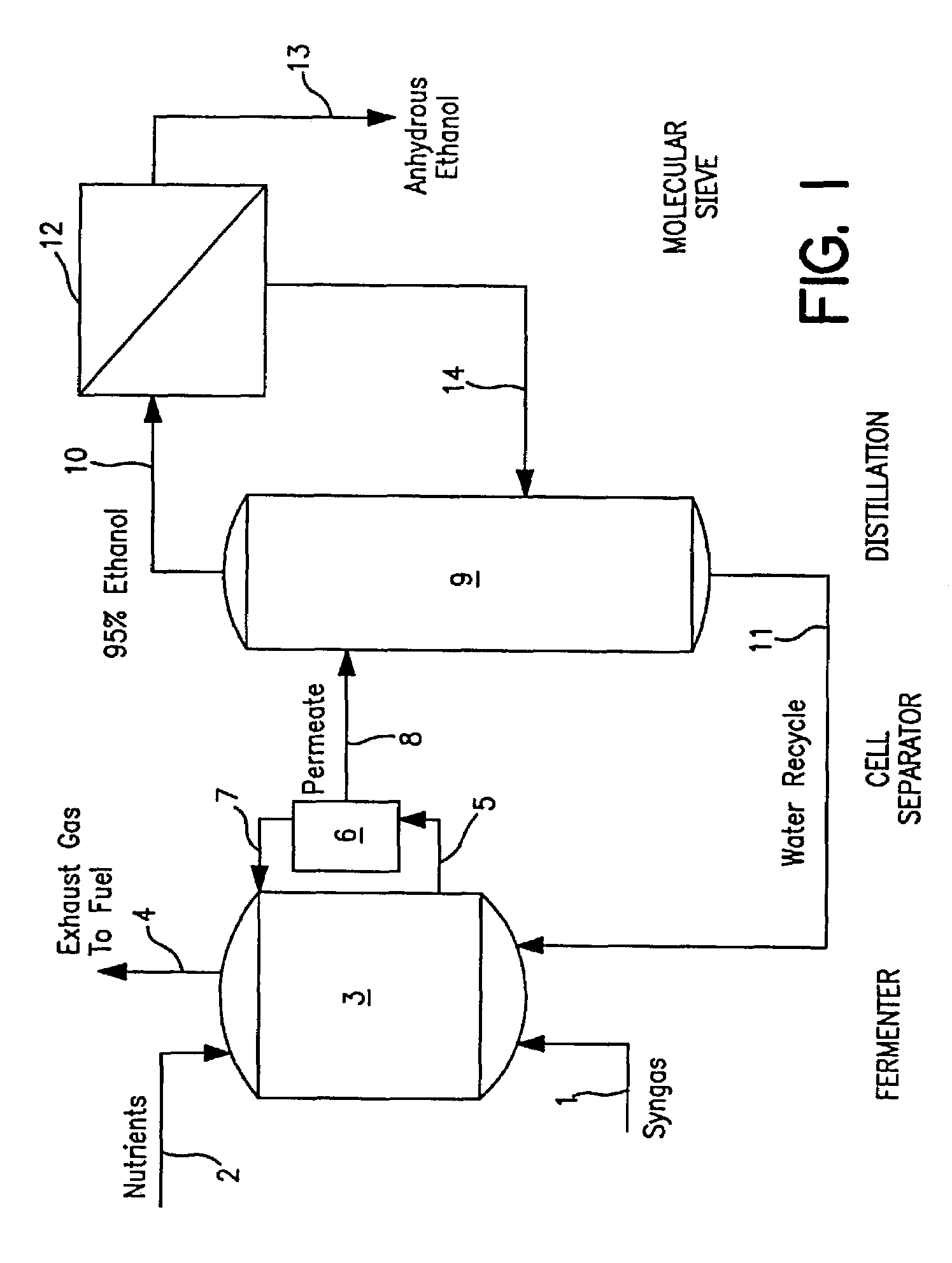
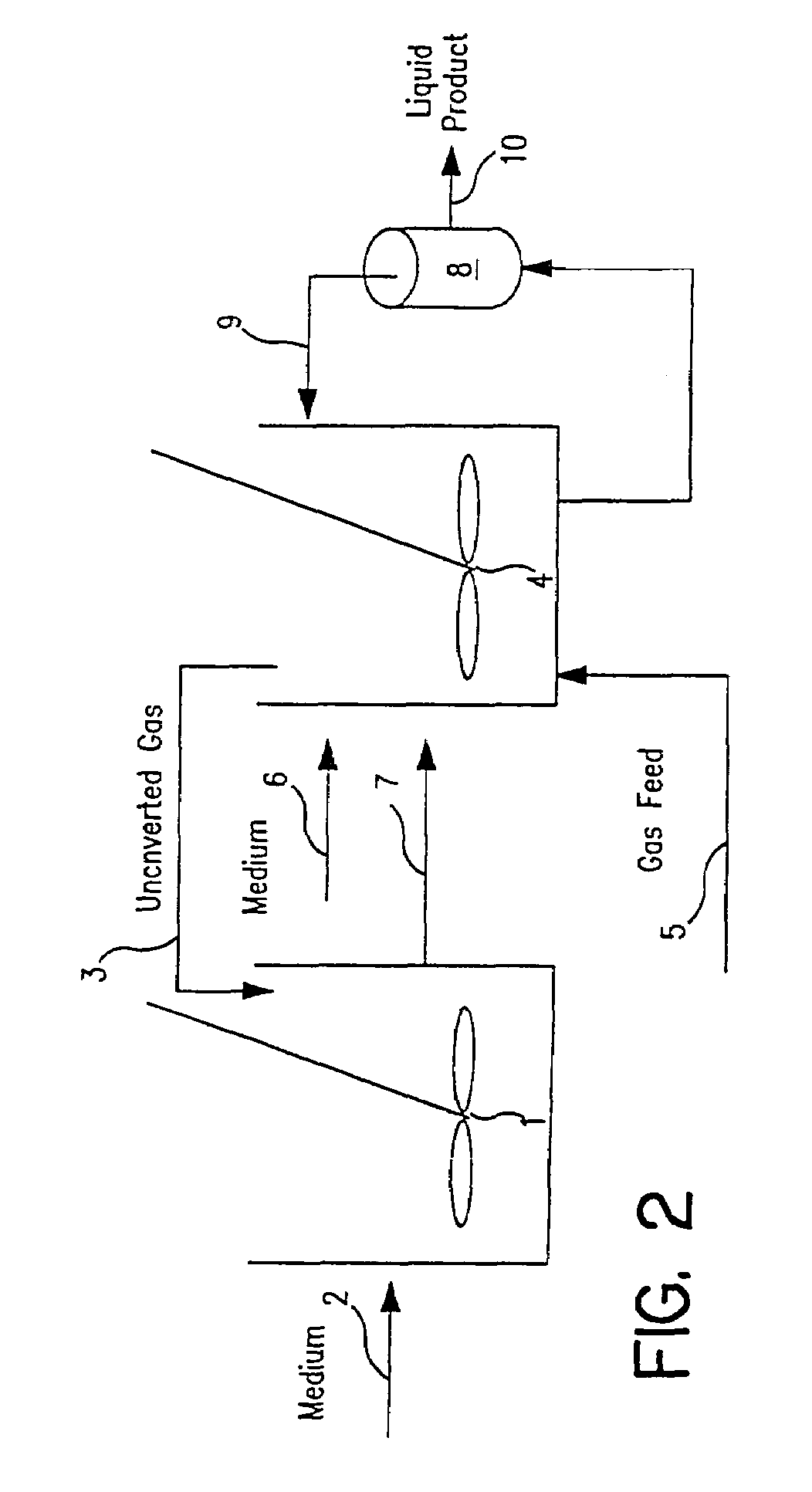
Methods for increasing the production of ethanol from microbial fermentation
InactiveUS7285402B2Good culture stabilityPermit growthBioreactor/fermenter combinationsSolid waste disposalBioreactorNutrient
A stable continuous method for producing ethanol from the anaerobic bacterial fermentation of a gaseous substrate containing at least one reducing gas involves culturing a fermentation bioreactor anaerobic, acetogenic bacteria in a liquid nutrient medium; supplying the gaseous substrate to the bioreactor; and manipulating the bacteria in the bioreactor by reducing the redox potential, or increasing the NAD(P)H TO NAD(P) ratio, in the fermentation broth after the bacteria achieves a steady state and stable cell concentration in the bioreactor. The free acetic acid concentration in the bioreactor is maintained at less than 5 g / L free acid. This method allows ethanol to be produced in the fermentation broth in the bioreactor at a productivity greater than 10 g / L per day. Both ethanol and acetate are produced in a ratio of ethanol to acetate ranging from 1:1 to 20:1.
Owner:JUPENG BIO HK LTD
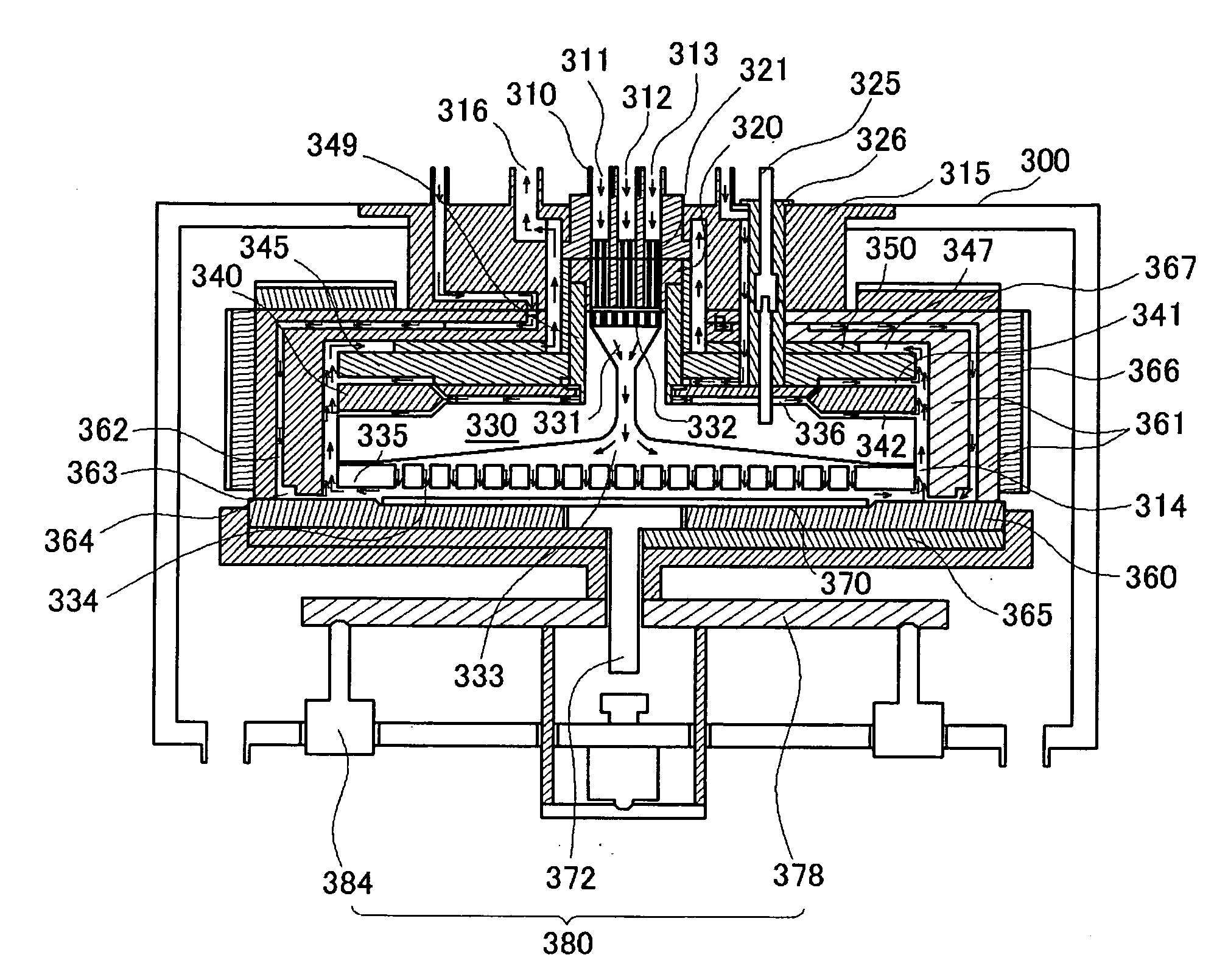
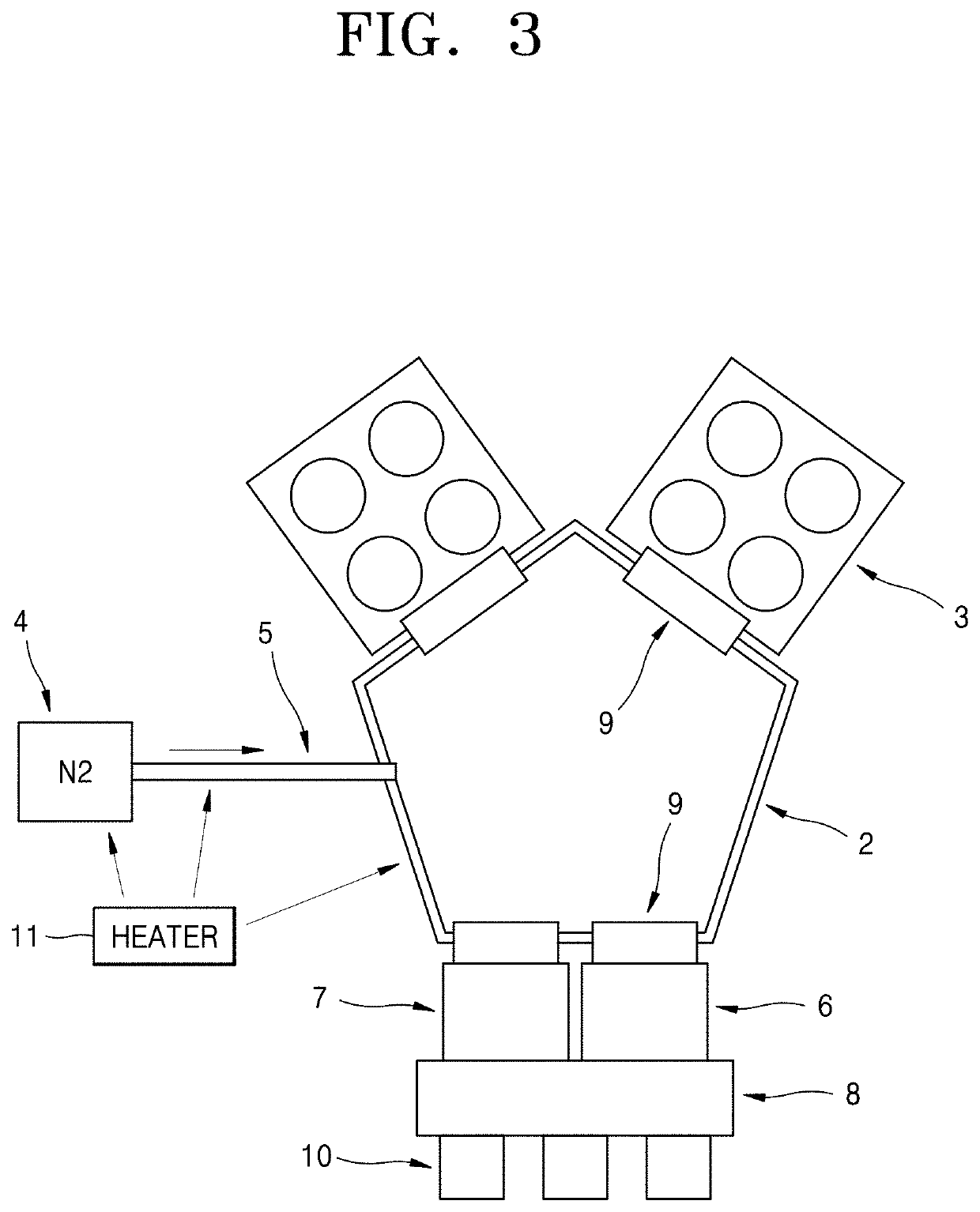
Atomic layer deposition apparatus
InactiveUS20060137608A1Uniform thicknessEasy to controlChemical vapor deposition coatingEngineeringAtomic layer deposition
An atomic layer deposition (ALD) apparatus is, suitable for thermal ALD and plasma-enhanced ALD of conductive and non-conductive films. The ALD apparatus can maintain electrical insulation of a gas dispersion structure, such as a showerhead assembly, which acts as an RF electrode to generate plasma inside a reaction chamber while depositing electrically conductive films in the reaction chamber. Fine tubules of micro-feeding tube assembly prevents plasma generation in them and reactive gases each have separate flow paths through the micro-feeding tube assembly. Process gases out of the micro-feeding tube assembly enter narrow grooves of a helical flow inducing plate and form helical flows which mix well each other. Symmetrically mounted pads on showerhead assembly and flow guiding plate maintain a symmetrical gap through which an inert gas flows continuously to keep reactive gases outside the gap and unwanted film deposition in the gap. Longer operating time before maintenance (cleaning) and thus higher productivity can be achieved.
Owner:ASM GENITECH KOREA
High productivity plasma processing chamber
InactiveUS20050229849A1Maximizing allowable timeReduce probabilityElectric discharge tubesSemiconductor/solid-state device manufacturingTemperature controlProduction rate
Embodiments of the present invention are generally directed to apparatus and methods for a plasma-processing chamber requiring less maintenance and downtime and possessing improved reliability over the prior art. In one embodiment, the apparatus includes a substrate support resting on a ceramic shaft, an inner shaft allowing for electrical connections to the substrate support at atmospheric pressure, an aluminum substrate support resting on but not fixed to a ceramic support structure, sapphire rest points swaged into the substrate support, and a heating element inside the substrate support arranged in an Archimedes spiral to reduce warping of the substrate support and to increase its lifetime. Methods include increasing time between in-situ cleans of the chamber by reducing particle generation from chamber surfaces. Reduced particle generation occurs via temperature control of chamber components and pressurization of non-processing regions of the chamber relative to the processing region with a purge gas.
Owner:APPLIED MATERIALS INC
Atomic layer deposition apparatus
ActiveUS20080069955A1Shorten the timeVacuum evaporation coatingSputtering coatingProduction ratePlanar substrate
The present invention relates to an ALD apparatus, and particularly relates to an ALD apparatus that is suitable for rapidly depositing a thin film on a substrate having an actual area that is larger than a planar substrate. In the reaction chamber of the ALD apparatus according to an exemplary embodiment of the present invention, more gas is supplied to a portion where more gas is required by having differences in the space for gas to flow rather than supplying the gas in a constant flux and a constant flow velocity such that the time required for supplying reactant gases and waste of reactant gases may be minimized to increase productivity of the ALD apparatus. The ceiling of the reaction space is shaped to provide a nonuniform gap over the substrate.
Owner:ASM KOREA LTD
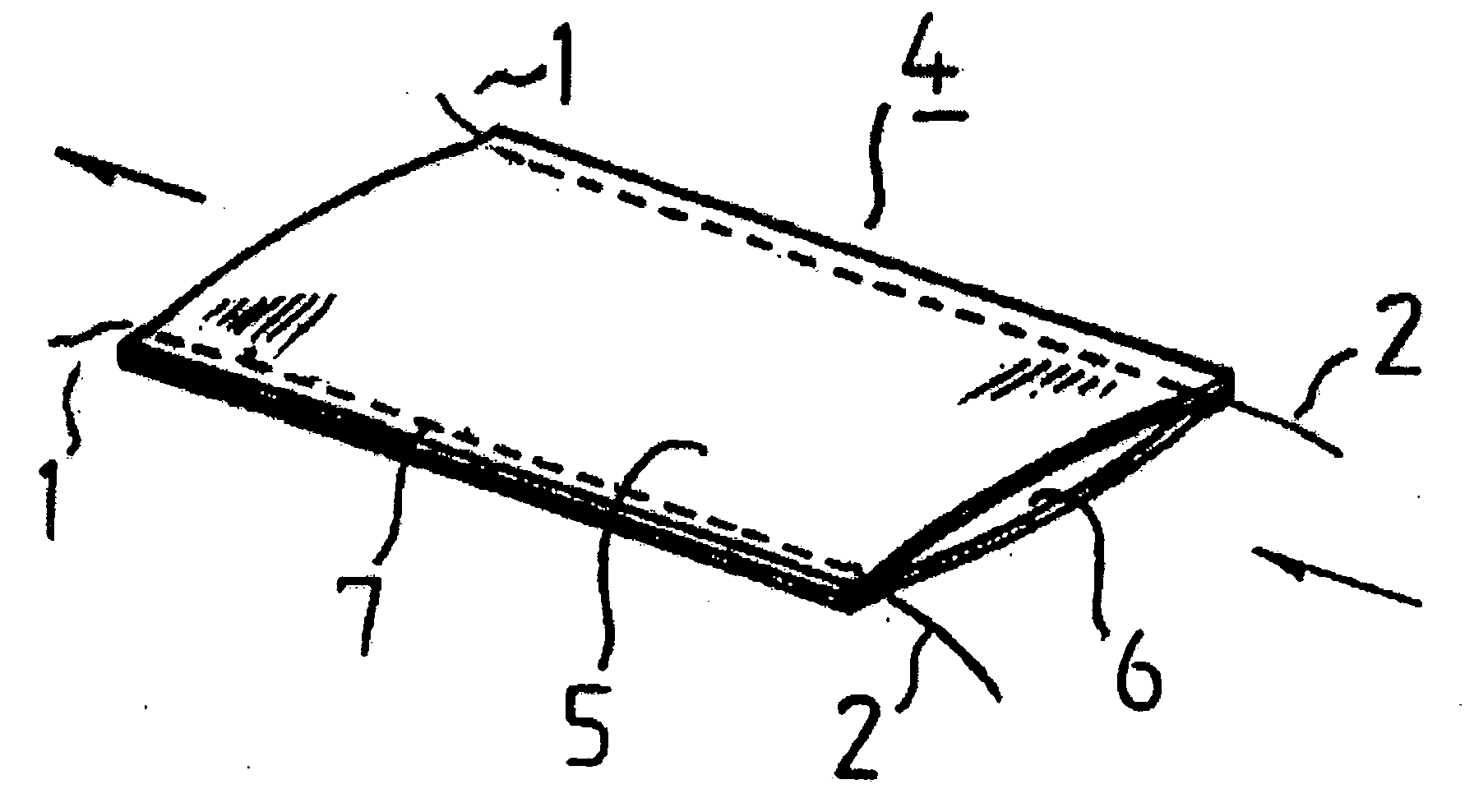
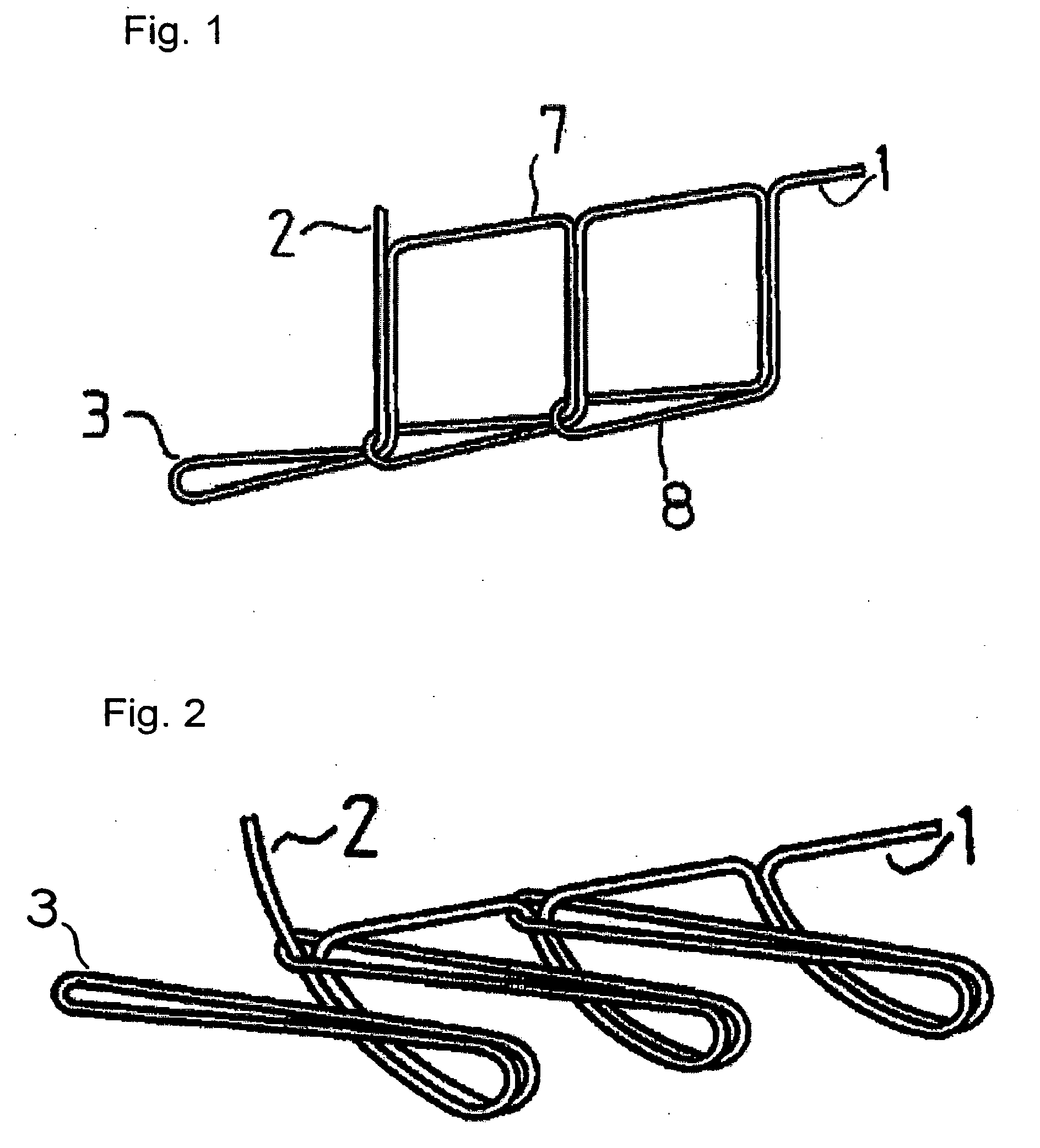
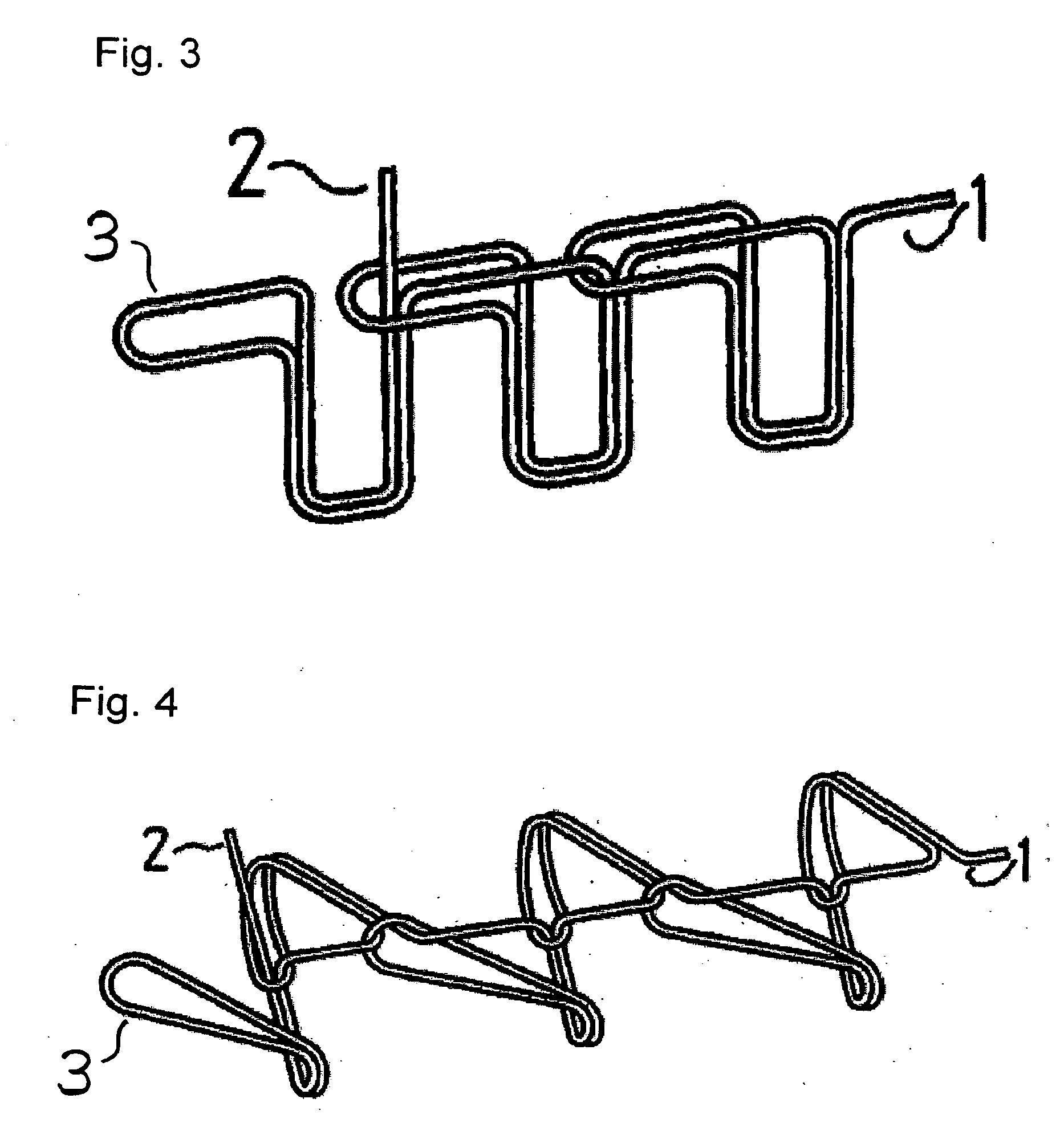
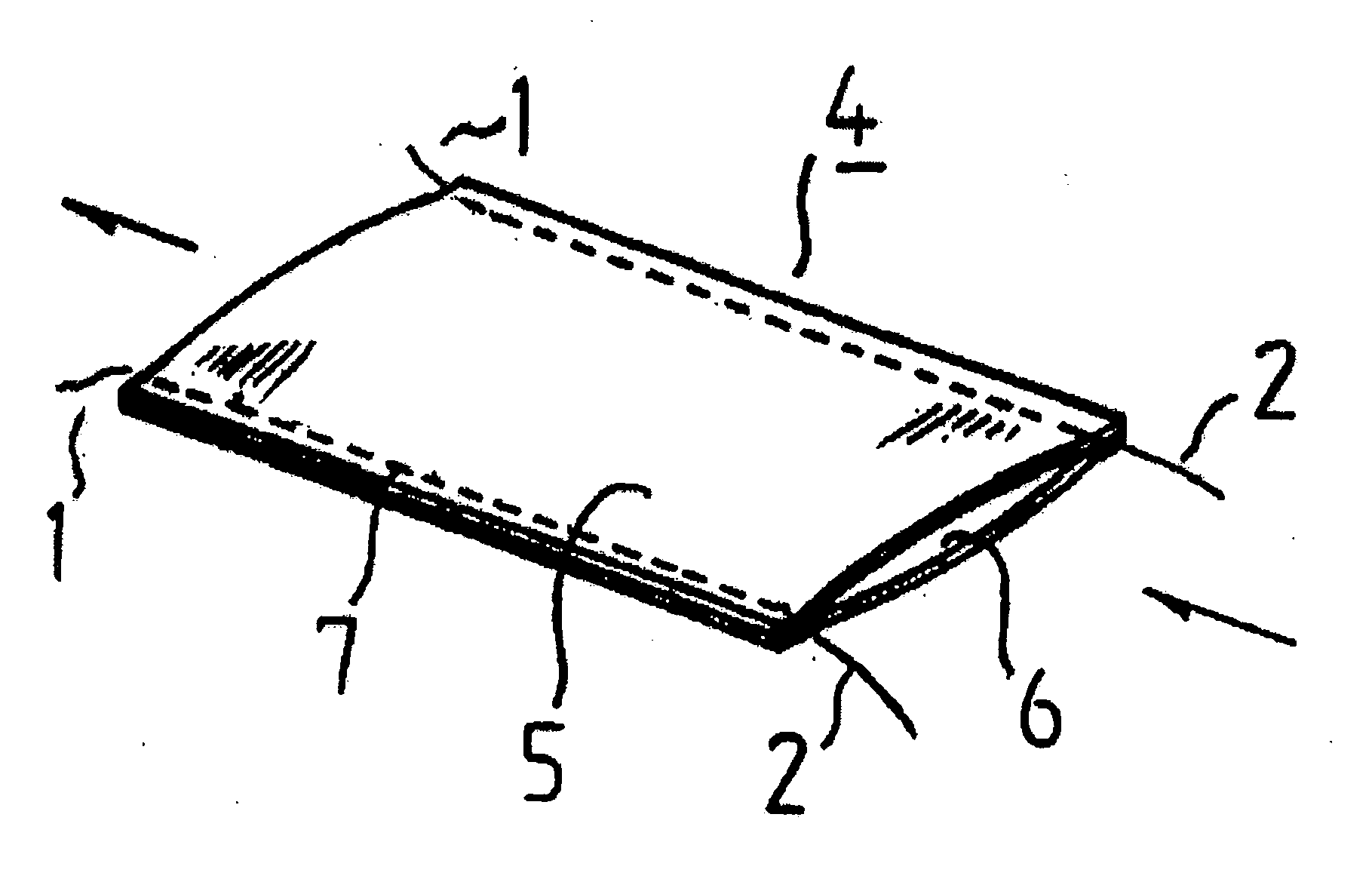
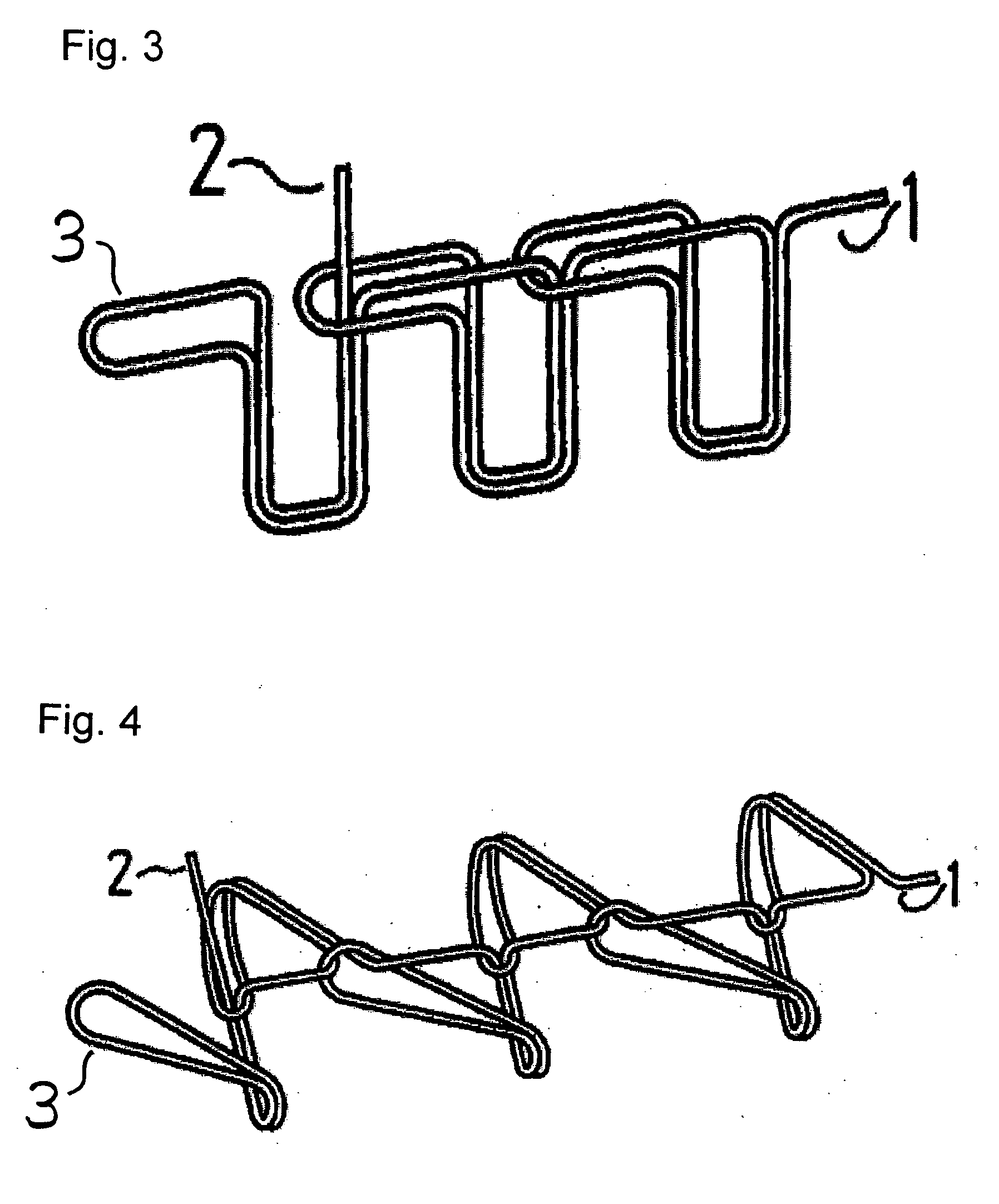
Suture reinforcement material for automatic suturing device
ActiveUS20070049953A2Smoothly removedPreventing a thread from unravelingSuture equipmentsSurgical needlesProduction rateSurgical operation
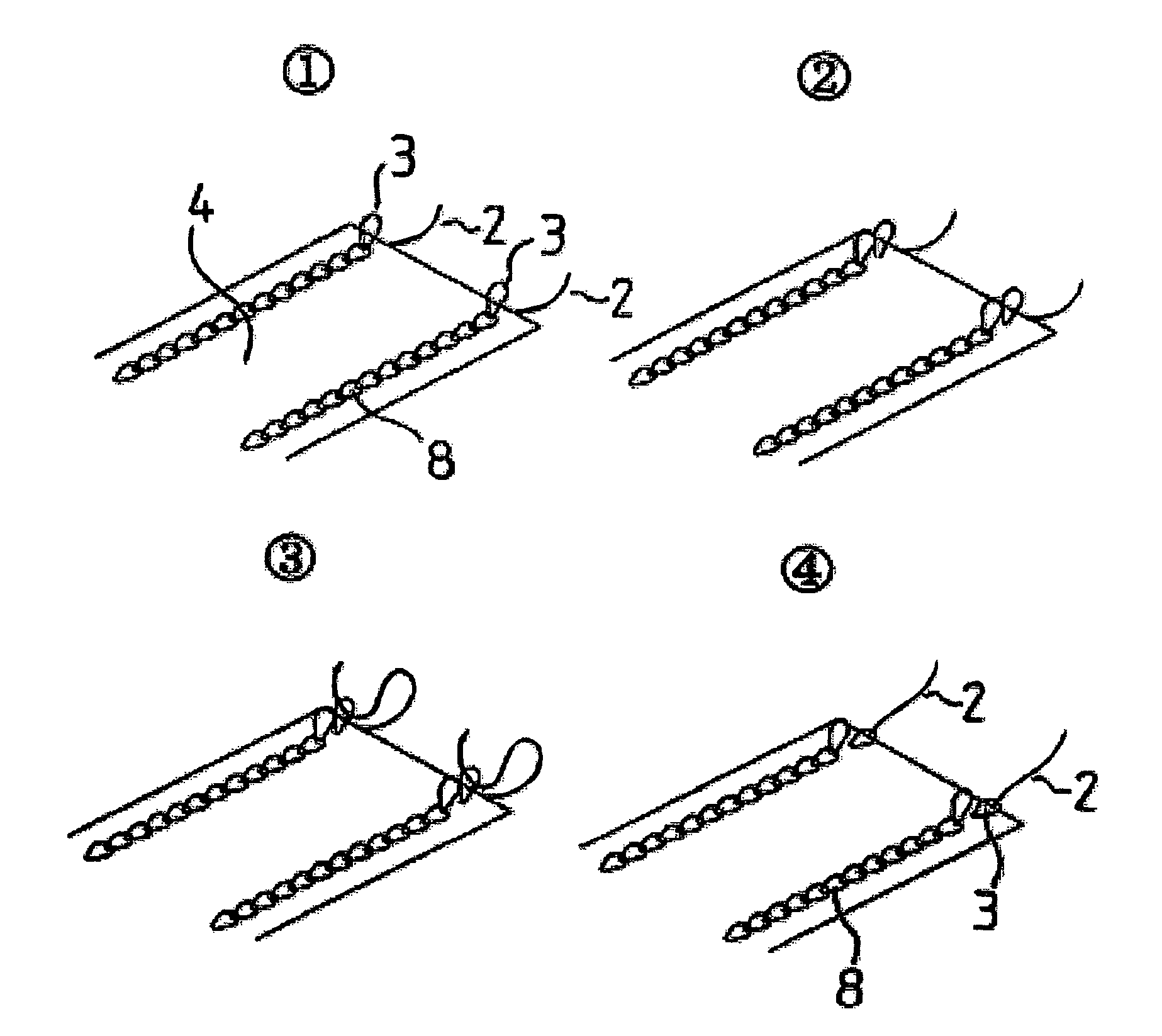
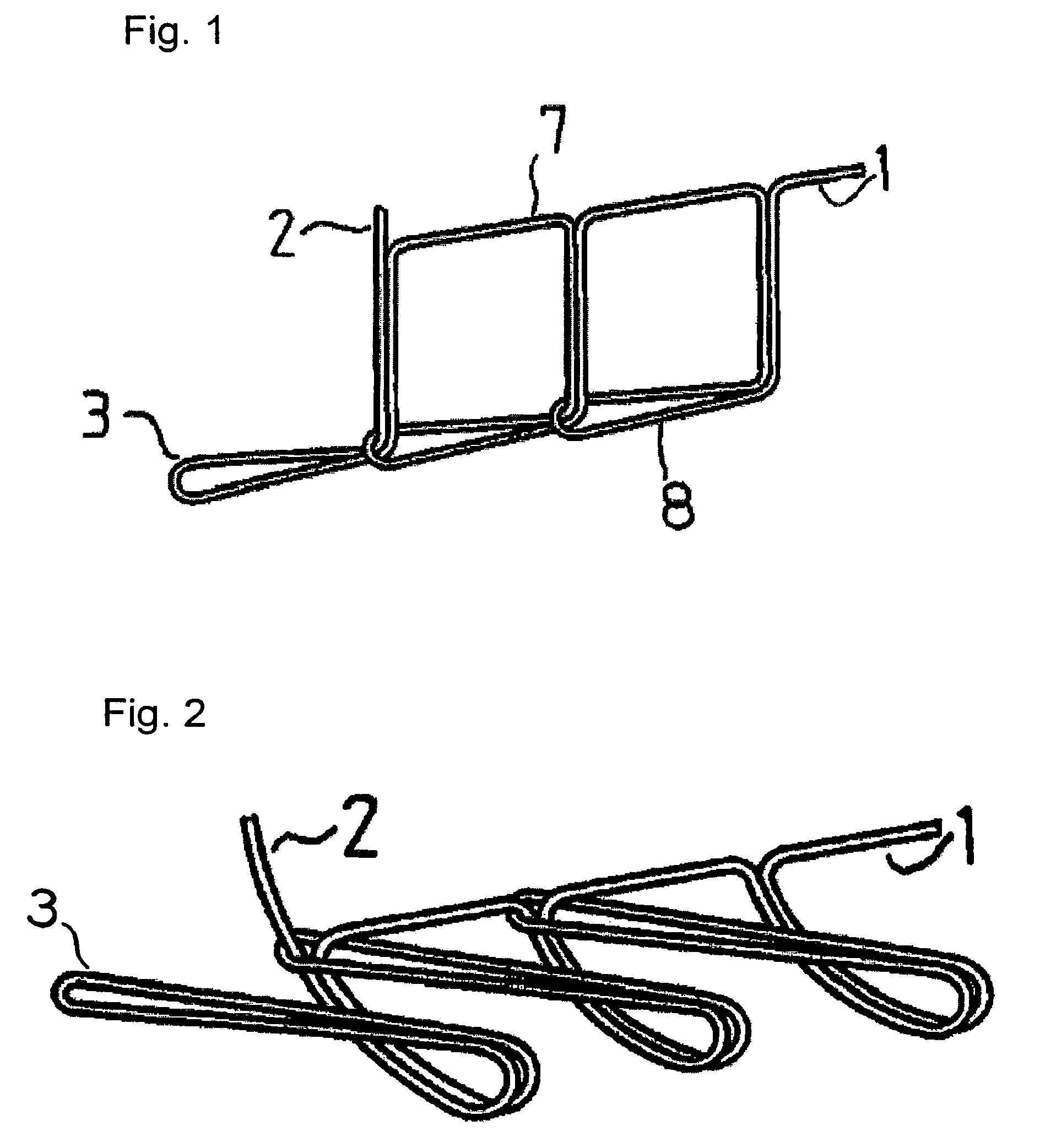
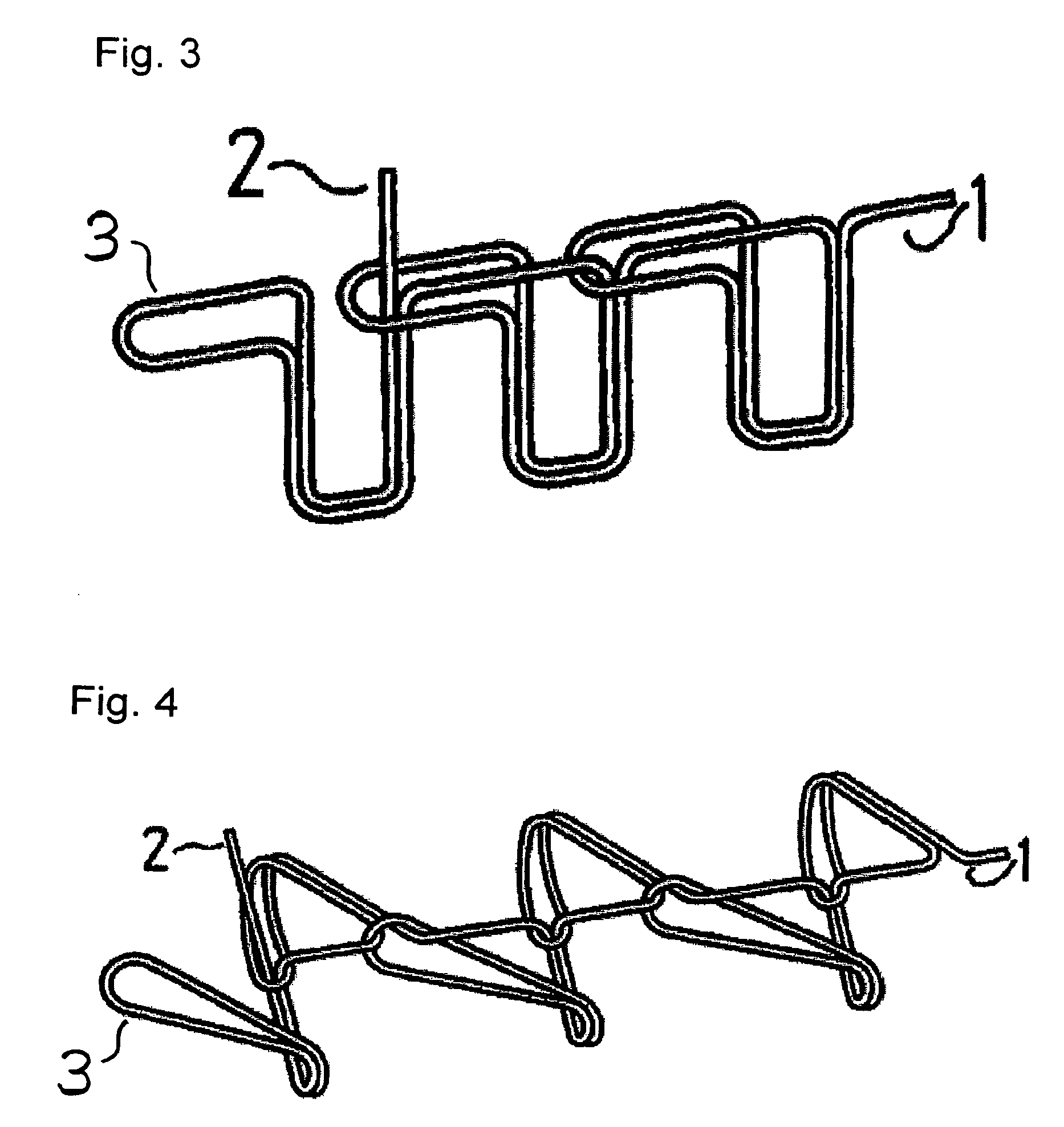
The present invention relates to a tubular suture reinforcement material suitable for use in automatic suturing devices that are widely used in surgical operations, etc. More specifically, the present invention relates to a tubular suture reinforcement material with which a tubular shape is easily obtained to thereby increase its productivity, and removal of thread is easily achieved. The present invention aims to overcome drawbacks such that sheet-like materials joined by the conventional temporary adhesion easily separate from each other, and the tubular suture reinforcement material manufactured using a running stitch requires a lot of time and skill, and removal of the thread is difficult depending on the kind of sheet-like material, sewing pitch, etc. More specifically, the present invention relates to a tubular suture reinforcement material for an automatic suturing device, wherein both ends of one or two sheet-like materials are sewed using a chain stitch (intralooping stitch) with a single thread to form a tubular shape, and one or two thread ends at one or two sewing ends are suitably extended.
Owner:GUNZE LTD
Method for manufacturing photoelectric conversion device
InactiveUS20090029503A1Quality improvementReduce deterioration rateFinal product manufactureSemiconductor/solid-state device manufacturingProduction rateMicrowave
To form a microcrystalline semiconductor with high quality which can be directly formed at equal to or less than 500° C. over a large substrate with high productivity without decreasing a deposition rate. In addition, to provide a photoelectric conversion device which employs the microcrystalline semiconductor as a photoelectric conversion layer. A reactive gas containing helium is supplied to a treatment chamber which is surrounded by a plurality of juxtaposed waveguides and a wall, the pressure in the treatment chamber is maintained at an atmospheric pressure or a subatmospheric pressure, microwave is supplied to a space sandwiched between the juxtaposed waveguides to generate plasma, and a photoelectric conversion layer of a microcrystalline semiconductor is deposited over a substrate which is placed in the treatment chamber.
Owner:SEMICON ENERGY LAB CO LTD
Suture prosthetic material for automatic sewing device
ActiveUS20060178683A1Stable removalOvercomes drawbackSuture equipmentsSurgical needlesProduction rateSurgical operation
The present invention relates to a tubular suture reinforcement material suitable for use in automatic suturing devices that are widely used in surgical operations, etc. More specifically, the present invention relates to a tubular suture reinforcement material with which a tubular shape is easily obtained to thereby increase its productivity, and removal of thread is easily achieved. The present invention aims to overcome drawbacks such that sheet-like materials joined by the conventional temporary adhesion easily separate from each other, and the tubular suture reinforcement material manufactured using a running stitch requires a lot of time and skill, and removal of the thread is difficult depending on the kind of sheet-like material, sewing pitch, etc. More specifically, the present invention relates to a tubular suture reinforcement material for an automatic suturing device, wherein both ends of one or two sheet-like materials are sewed using a chain stitch (intralooping stitch) with a single thread to form a tubular shape, and one or two thread ends at one or two sewing ends are suitably extended.
Owner:GUNZE LTD
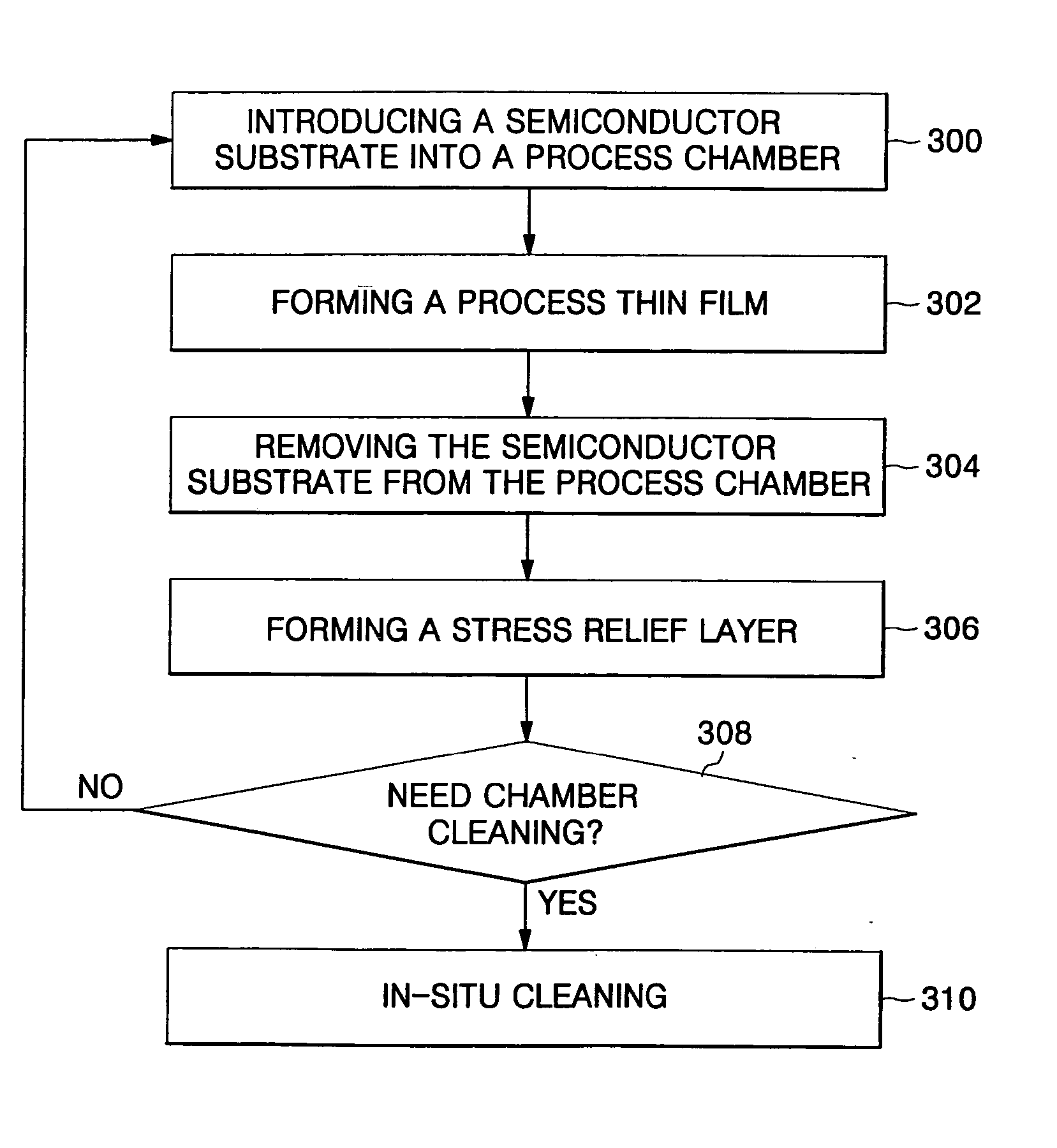
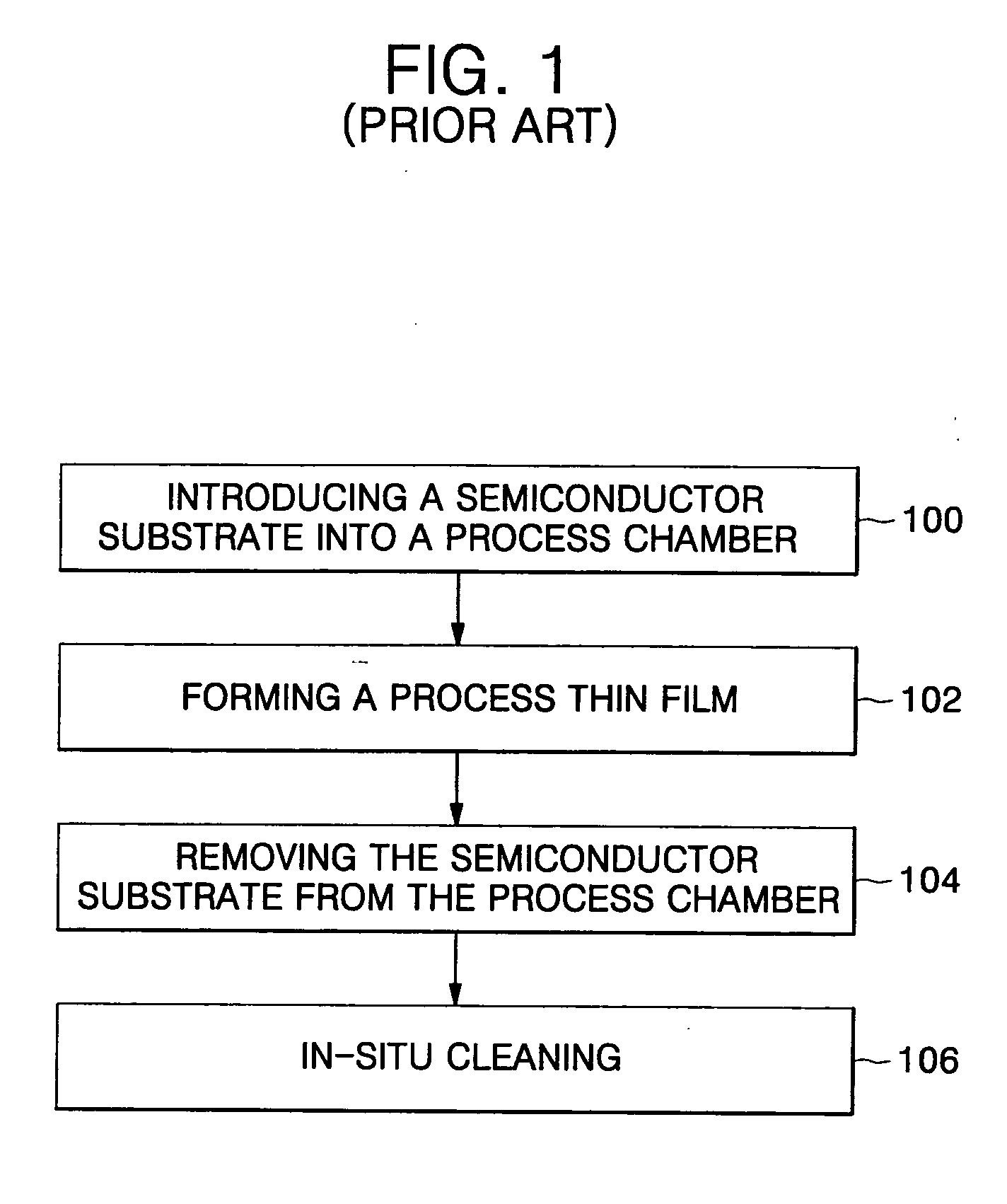
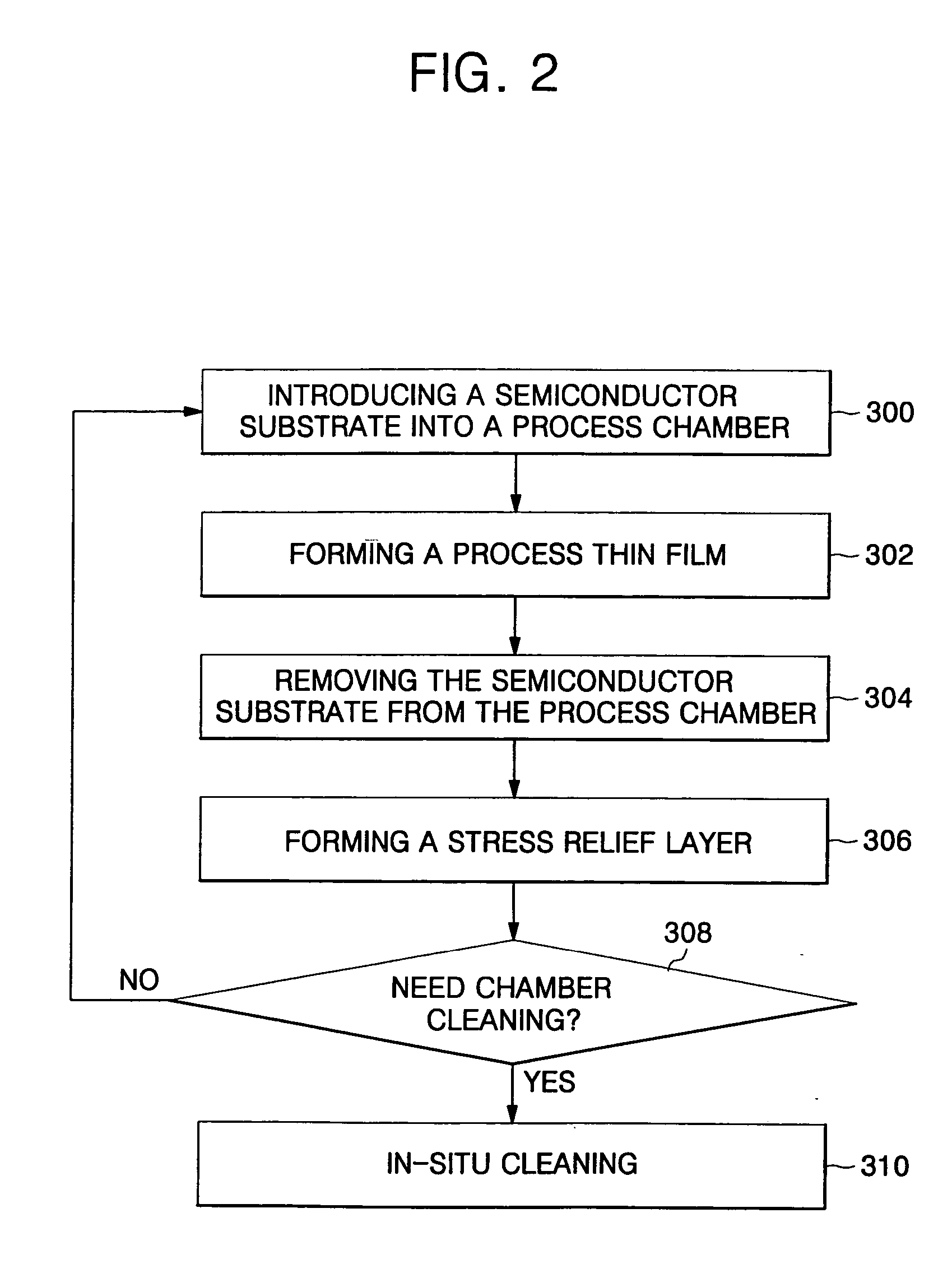
Method of forming thin film for improved productivity
ActiveUS20050130427A1Extended maintenance periodReduce processing timeElectrostatic cleaningSemiconductor/solid-state device manufacturingProduction rateStress relief
There is provided a method of forming a thin film for providing improved fabrication productivity. The method includes introducing a semiconductor substrate into a process chamber. A process thin film is formed on the semiconductor substrate, in which a chamber coating layer is formed on inner walls of the process chamber while the process thin film is formed. The semiconductor substrate is removed from the process chamber. A stress relief layer is formed on the chamber coating layer. After all of the above operations are repeatedly performed at least one time, an in-situ cleaning is performed on the chamber coating layer and the stress relief layer, which are alternately formed in stack on the inner walls of the process chamber.
Owner:SAMSUNG ELECTRONICS CO LTD
Heater module for semiconductor production system
InactiveUS20040238523A1Speed up coolingHigh bonding strengthSemiconductor/solid-state device manufacturingHot plates heating arrangementsProduction rateComputer module
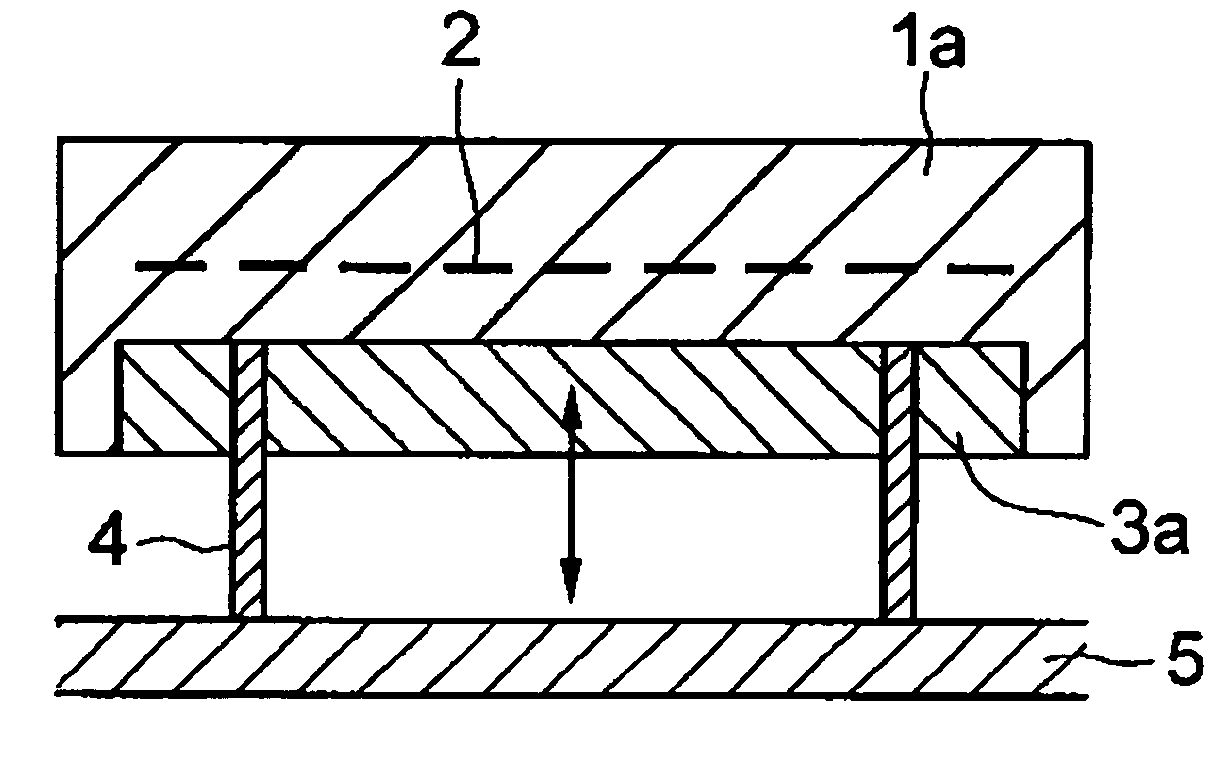
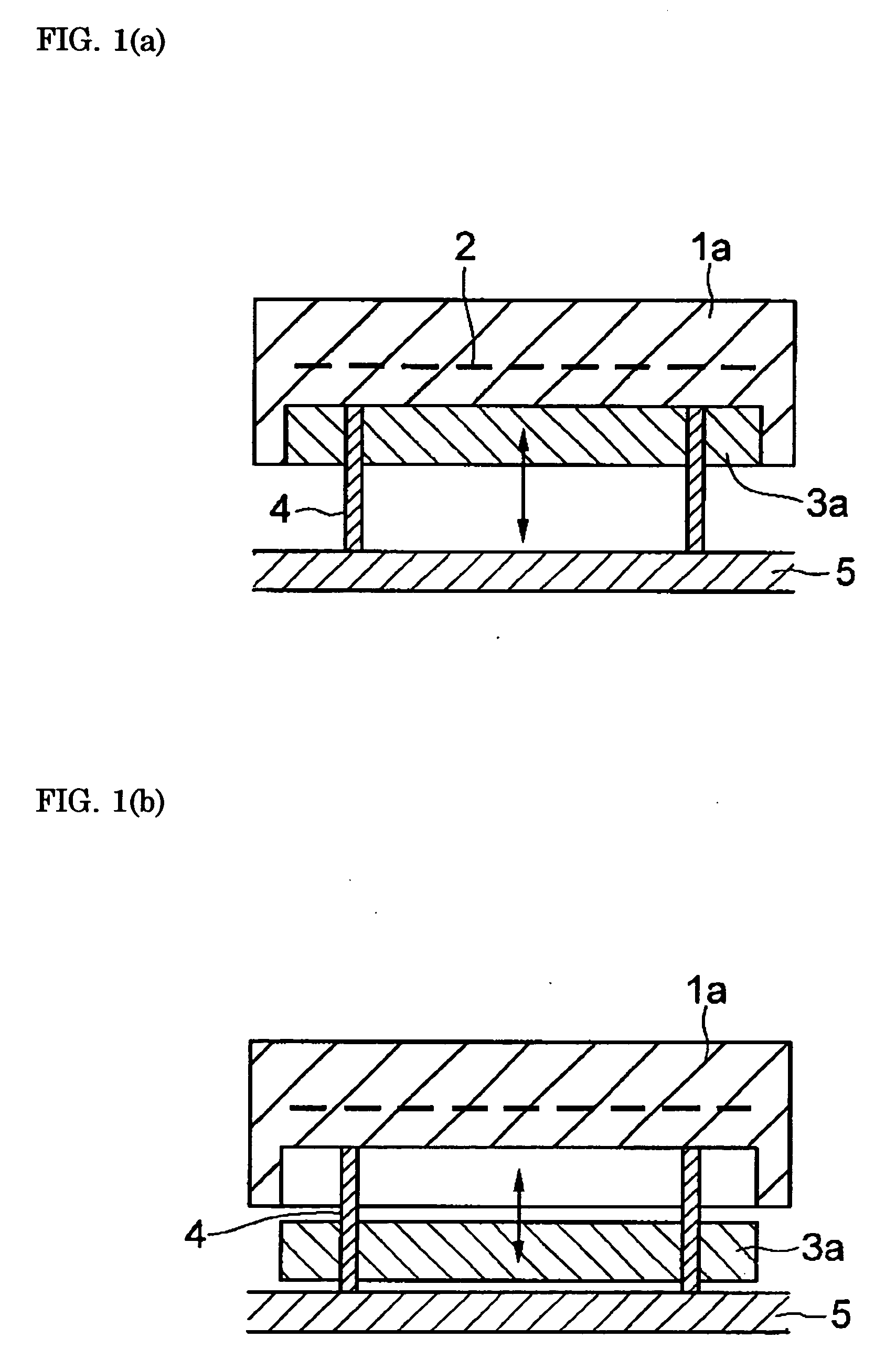
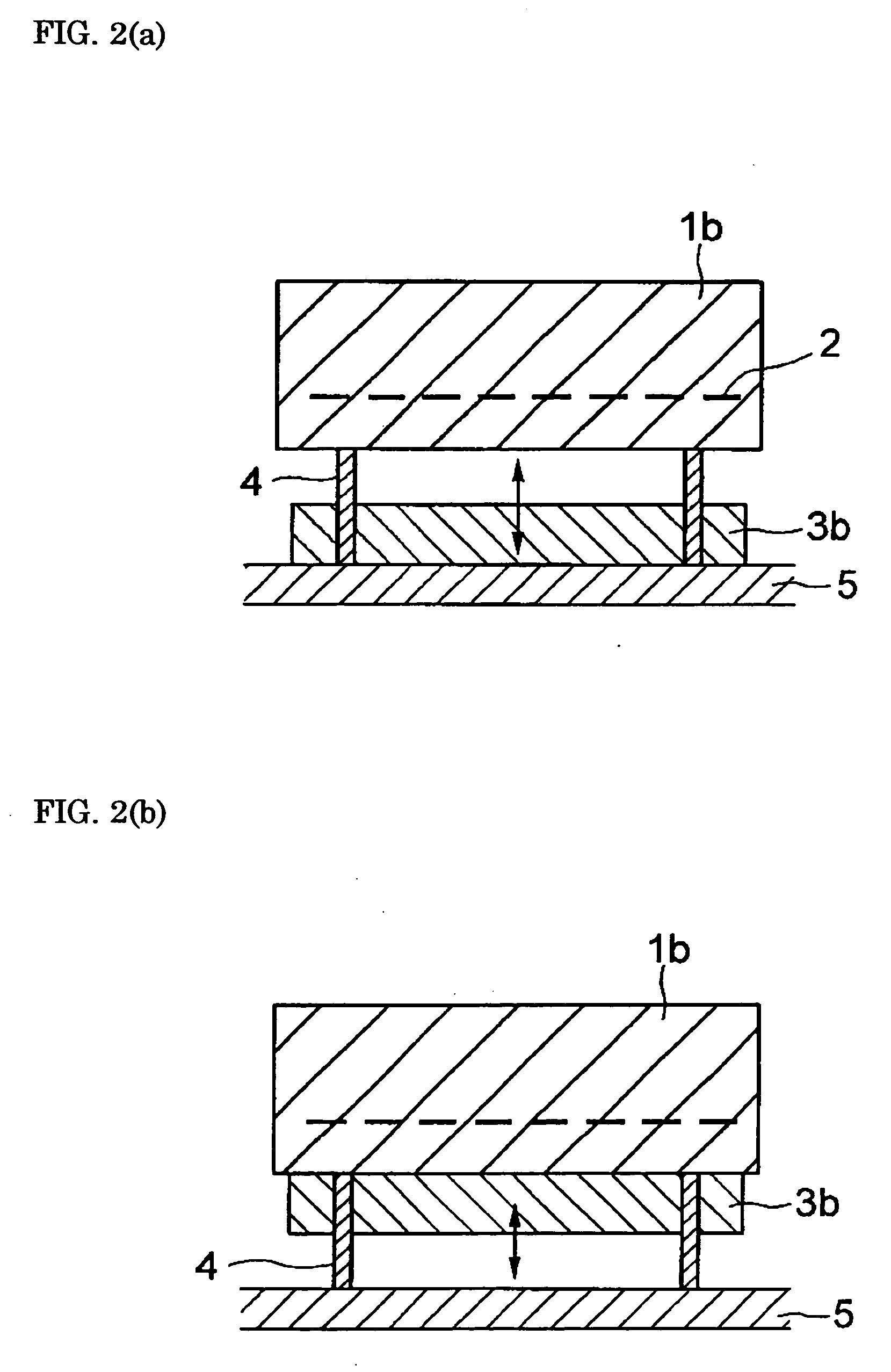
Heater module, and semiconductor manufacturing equipment in which the heater module is utilized, for raising the cooling speed of a post-heating heater markedly more than conventional, and that can contribute toward bettering and improving productivity, without accompanying scaling-up of and cost increases in the semiconductor manufacturing equipment. The heater module is furnished with heater part 1a for controlled heating of a wafer placed on its top face, and block part 3a provided to be shiftable relative to said heater part, for varying heat capacity in total with heater part 1a by abutting on or separating from the reverse surface of heater part 1a. By having the heat capacity of block part 3a be 20% or more of the total heat capacity of heater part 1a and block part 3a, the heater cooling speed can be made 10° C. / min or more.
Owner:SUMITOMO ELECTRIC IND LTD
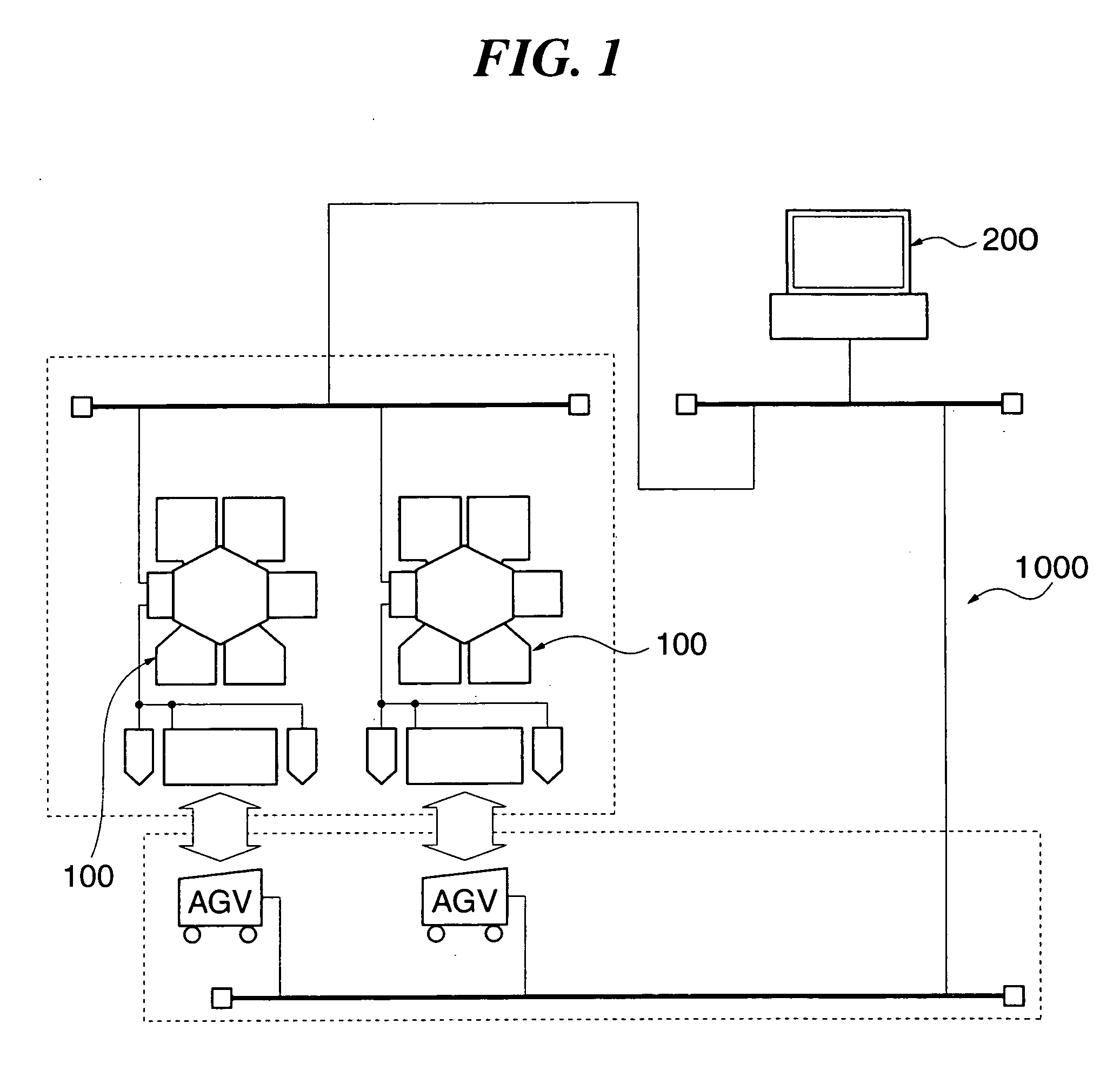
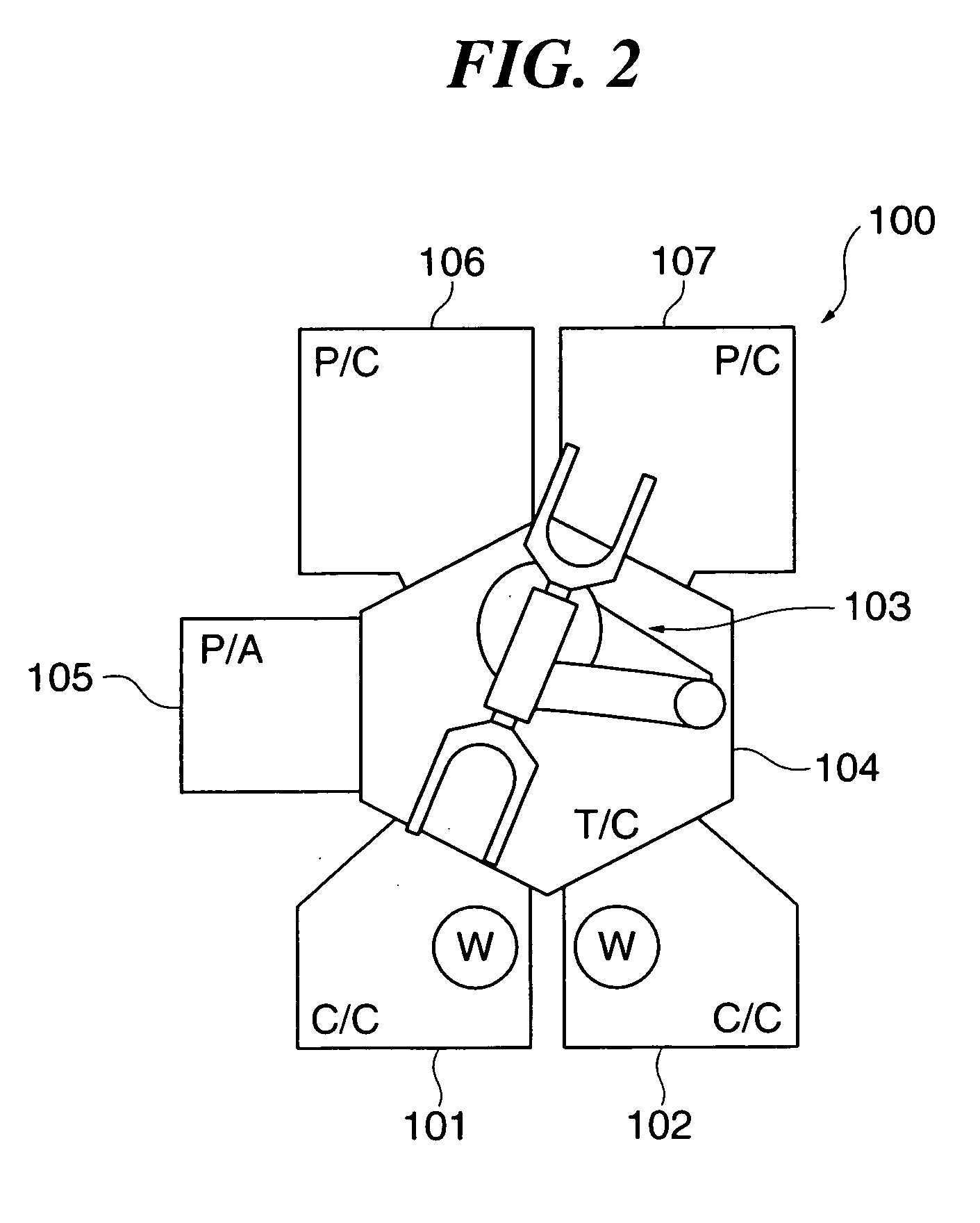
Substrate processing apparatus, substrate processing method, and program for implementing the method
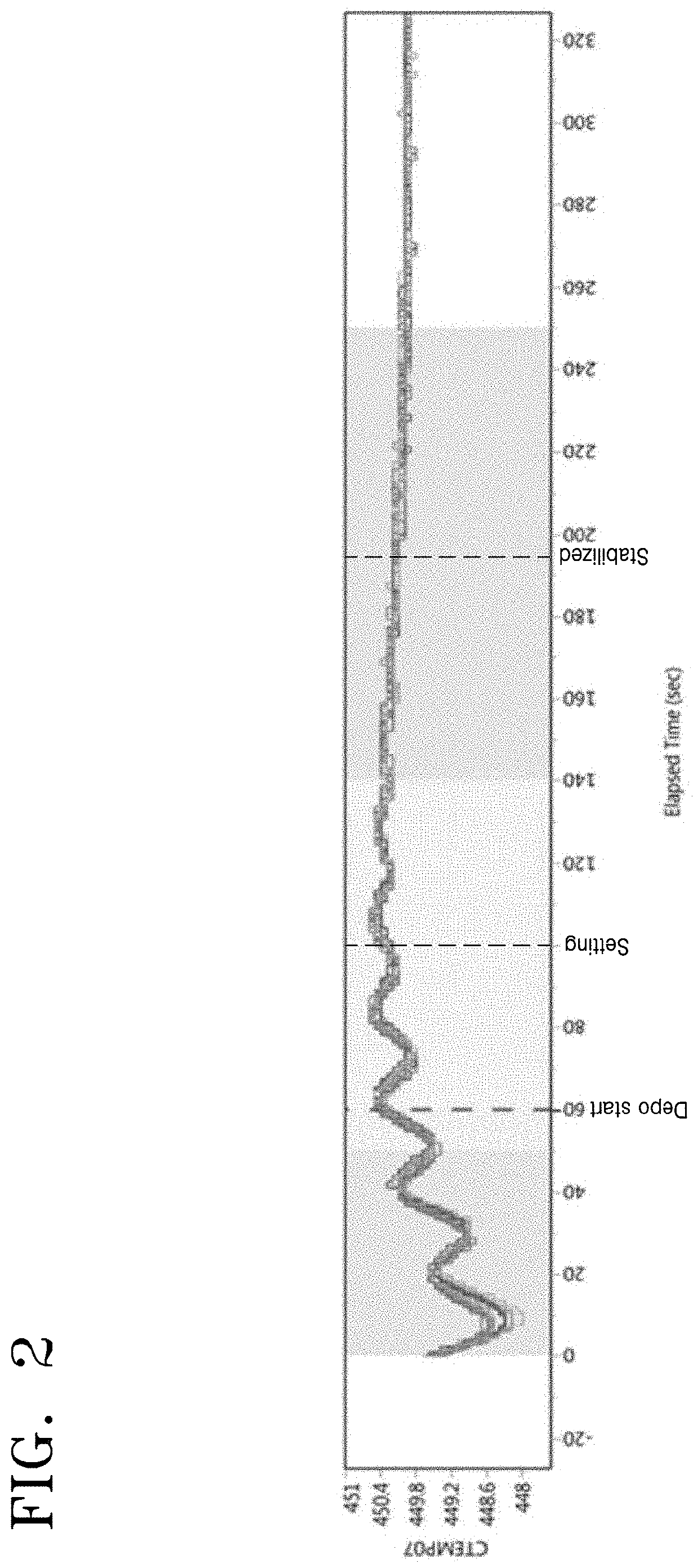
InactiveUS20050233477A1Improve productivityLiquid surface applicatorsSemiconductor/solid-state device testing/measurementProduction rateEtching
A substrate processing apparatus which is capable of enhancing productivity in manufacturing product substrates. In process chambers 106 and 107 of an etching apparatus 100, etching is carried out on a substrate as an object to be processed, and dummy processing is carried out on at least one non-product substrate before execution of the etching. A host computer 200 determines whether or not the dummy processing is to be executed. The host computer 200 determines whether or not the interior of each of the process chambers 106 and 107 is in a stable state, and omits the execution of the dummy processing when it is determined that it is in the stable state.
Owner:TOKYO ELECTRON LTD
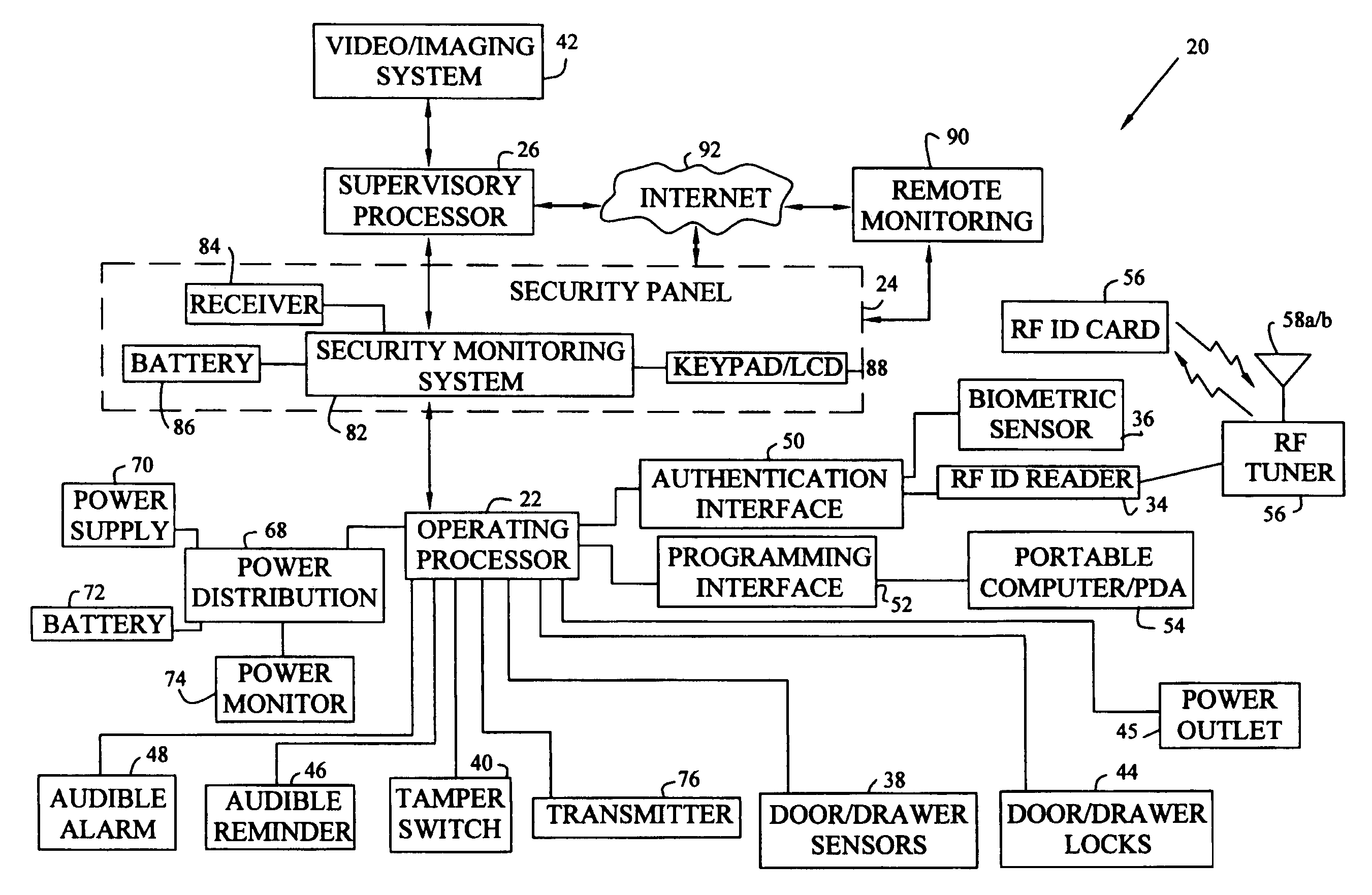
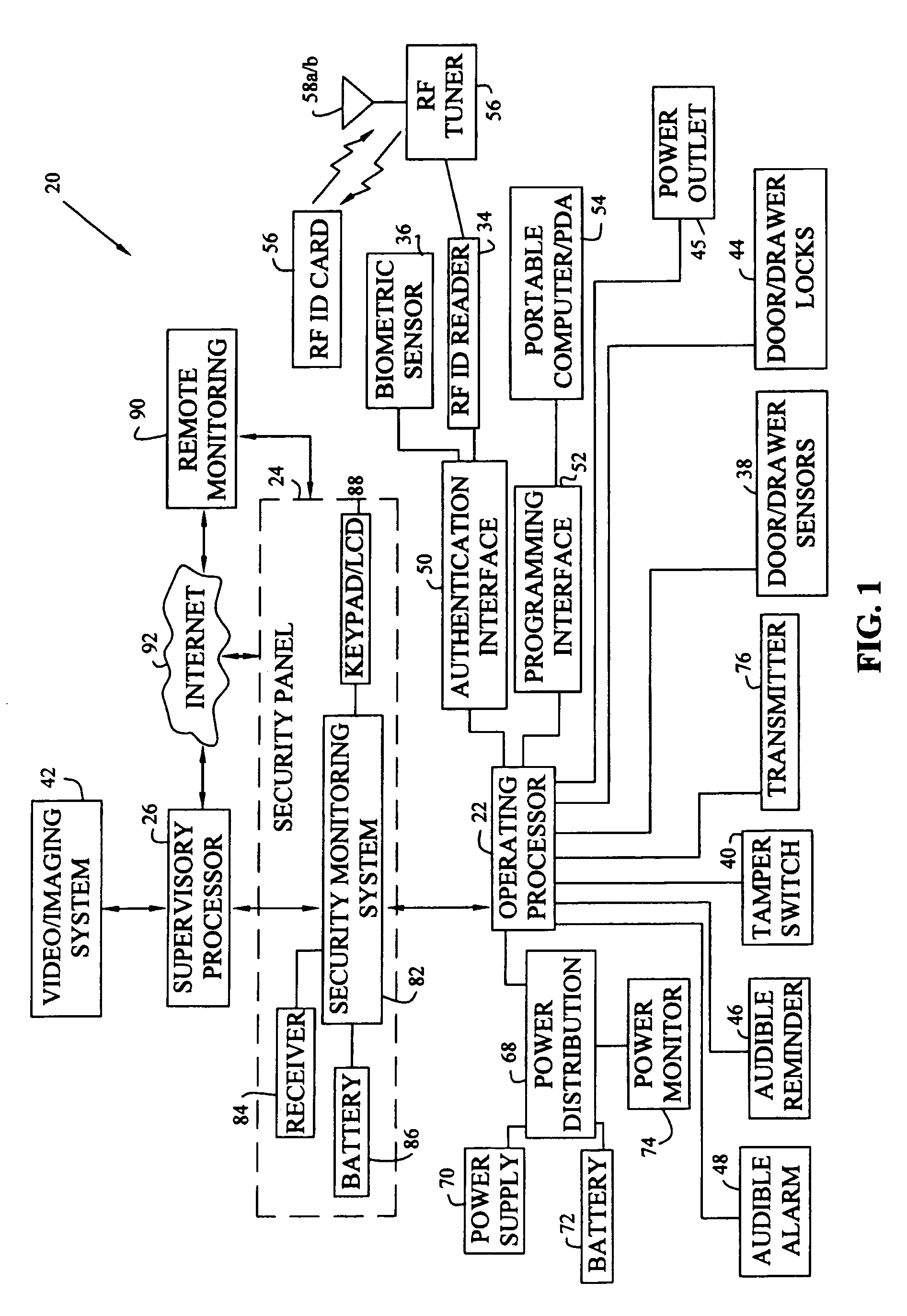
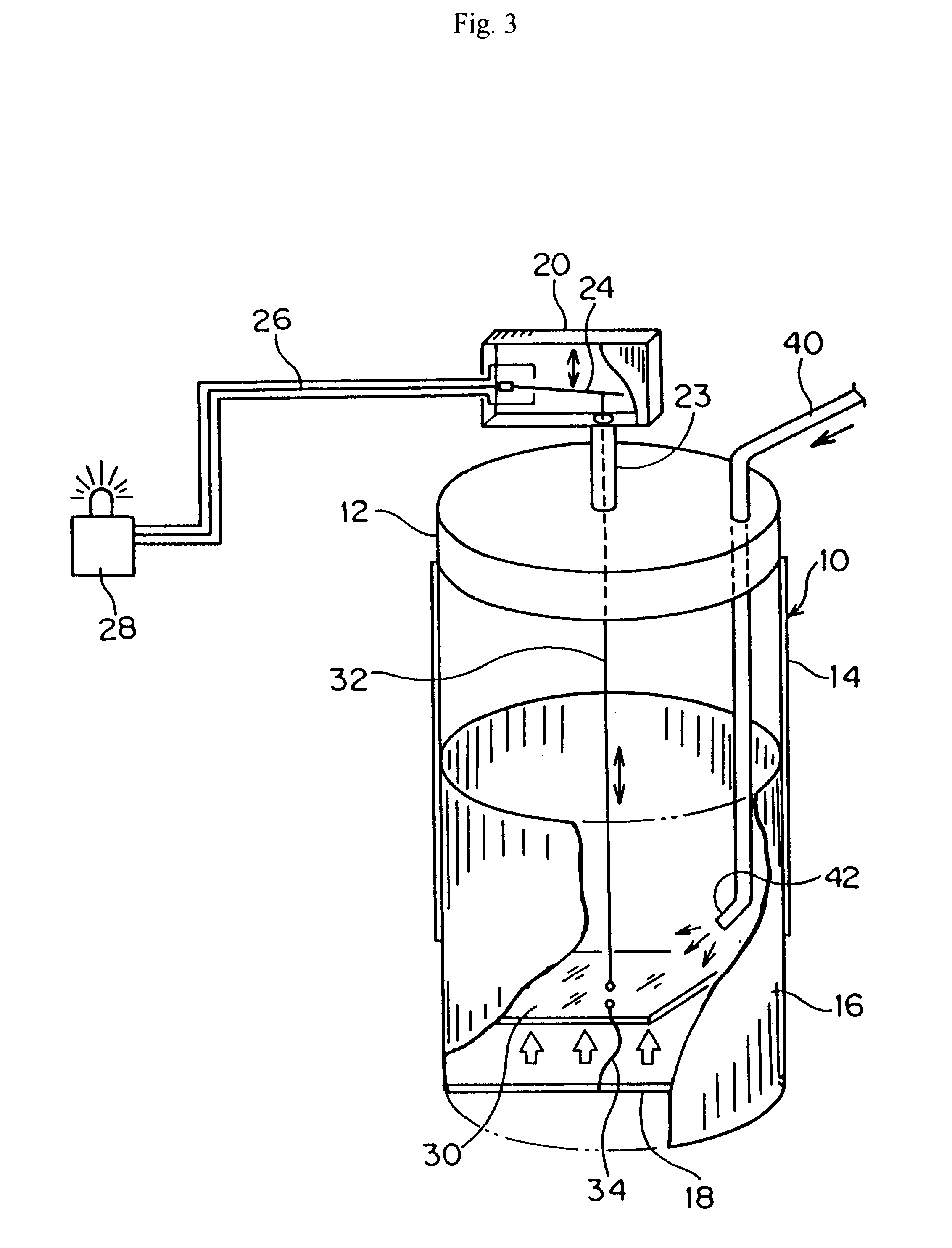
Workspace security system
InactiveUS6965294B1Effectively eliminate most false security alarmsElectric signal transmission systemsMultiple keys/algorithms usageProduction rateWorkspace
A workspace security system having processors, sensors, and actuators for local and remote monitoring, control, and automation of physical and environmental security, safety, efficiency, and productivity. The system includes passive and active access authentication devices and active or passive unlocking and lockdown of office furniture components, work sites, and facilities. The system may include multilevel authentication credentials, automatic lockdown of office furniture and other components based on the proximity of credentials, and video or other imaging systems to detect and record motion.
Owner:KIMBALL INTERNATIONAL
Semiconductor device and manufacturing method thereof
ActiveUS20100025677A1Small amount of photocurrentReduce parasitic capacitanceTransistorElectroluminescent light sourcesProduction rateCharge carrier
To provide a semiconductor device including a thin film transistor having excellent electric characteristics and high reliability and a manufacturing method of the semiconductor device with high mass productivity. The summary is that an inverted-staggered (bottom-gate) thin film transistor is included in which an oxide semiconductor film containing In, Ga, and Zn is used as a semiconductor layer, a channel protective layer is provided in a region that overlaps a channel formation region of the semiconductor layer, and a buffer layer is provided between the semiconductor layer and source and drain electrodes. An ohmic contact is formed by intentionally providing the buffer layer having a higher carrier concentration than the semiconductor layer between the semiconductor layer and the source and drain electrodes.
Owner:SEMICON ENERGY LAB CO LTD
Substrate processing system
ActiveUS20190390343A1Improve productivityQuickly securedElectric discharge tubesVacuum evaporation coatingProduction rateTemperature control
Provided is a substrate processing system for improving productivity of processes. In this regard, the substrate processing system includes: a first chamber providing a space where at least one substrate is accommodated; a second chamber configured to transfer at least one substrate to the first chamber; and a temperature control unit configured to change a temperature of a gas in the second chamber.
Owner:ASM IP HLDG BV
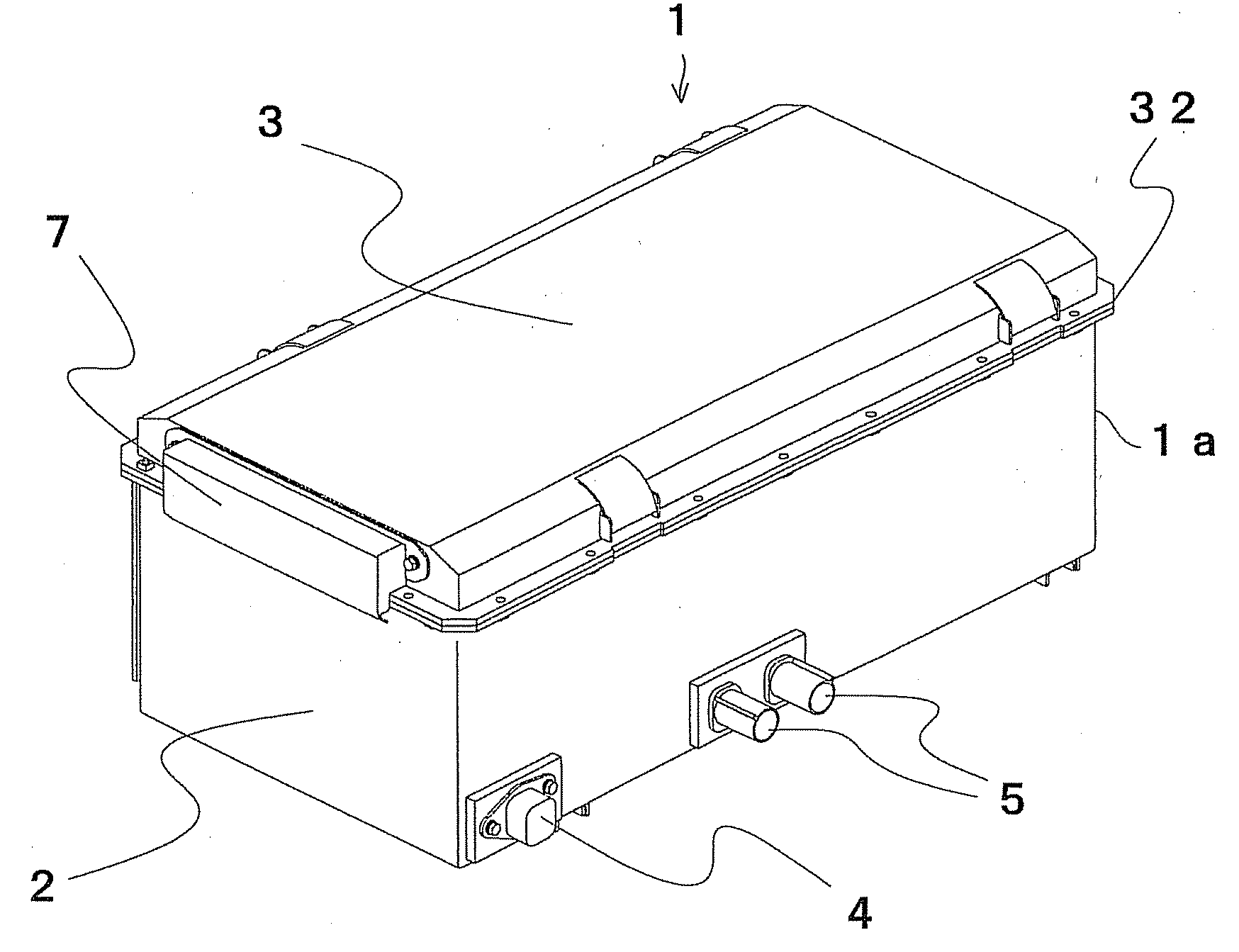
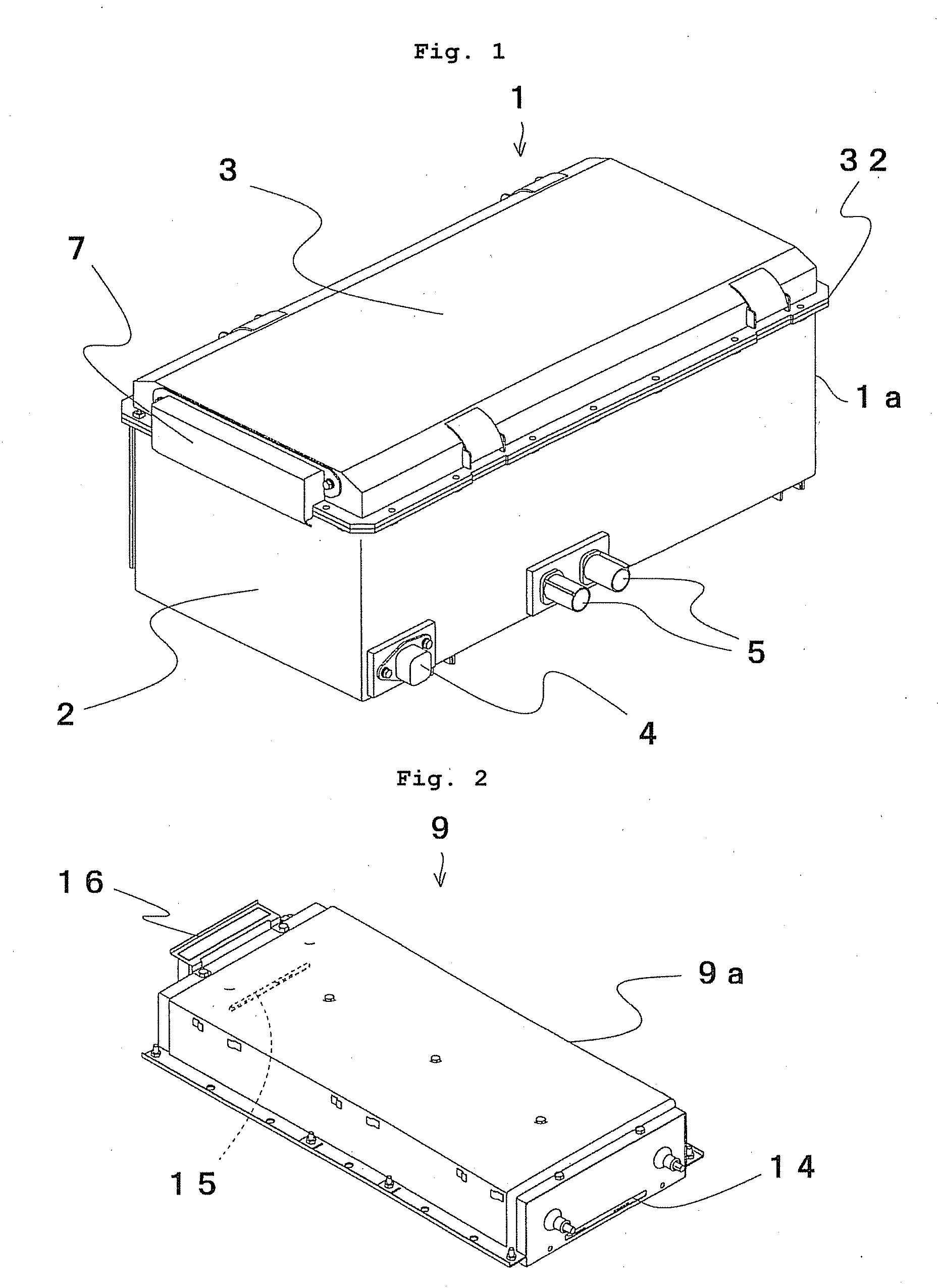
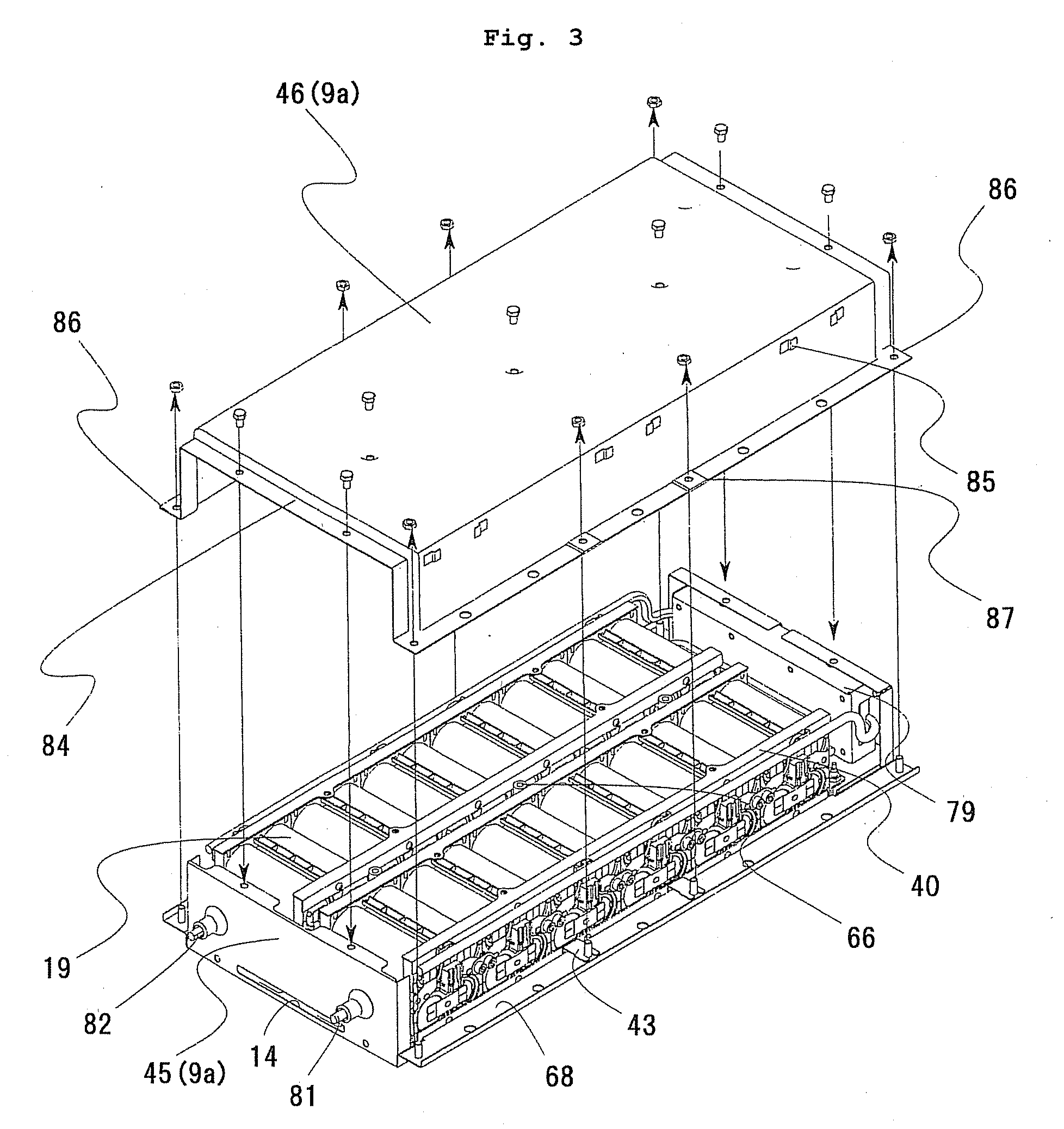
Cell controller, battery module and power supply system
ActiveUS20080050645A1Increase production capacityIncrease productivityBatteries circuit arrangementsCell electrodesProduction ratePotential difference
A cell controller having excellent productivity is provided. A cell-con 80 has 12 ICs IC-1 to IC-2 mounted on a substrate, and these ICs detect voltages of respective cells constituting a cell pack, perform capacity adjustment on the respective cells, and are mounted two by two on rectangular longer sides of a rectangular continuous straight line L-L′ defined on a substrate from the IC-1 on a highest potential side to the IC-12 on a lowest potential side continuously in order of potential differences of the corresponding cell packs. Distances between the rectangular shorter sides of the rectangular continuous straight line L-L′ are the same. On the cell-con 80, between the IC-1 to IC-12 having different ground voltages, each of the ICs has signal output terminals connected to signal input terminals of a lower order IC respectively in an electrically non-insulated state.
Owner:HITACHI ASTEMO LTD
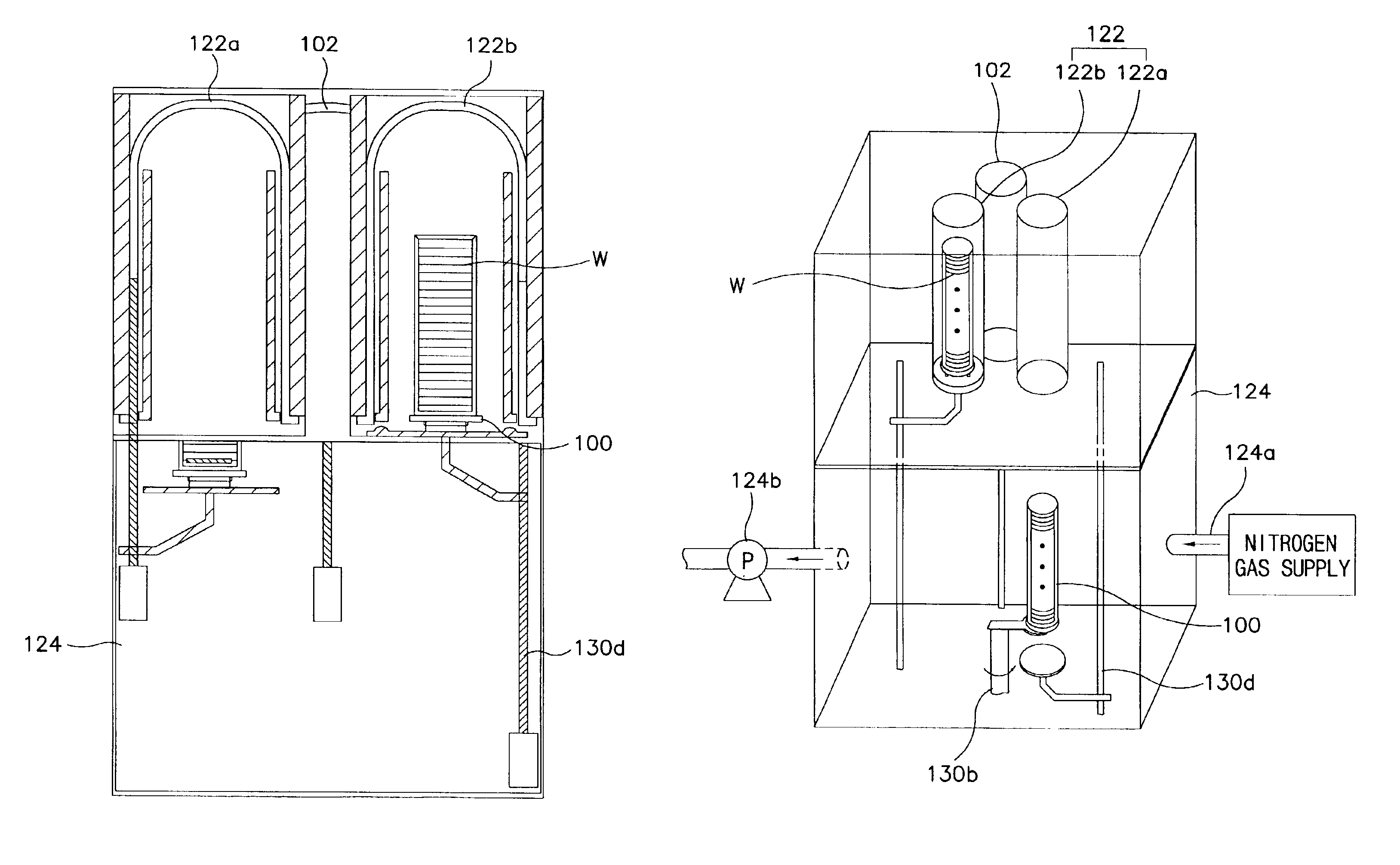
Method of and apparatus for performing sequential processes requiring different amounts of time in the manufacturing of semiconductor devices
InactiveUS6911112B2Without need for significant downtimeHigh productSemiconductor/solid-state device manufacturingCharge manipulationProduction rateDevice material
A method of manufacturing a semiconductor device includes first and second processes, the latter requiring more processing time. An apparatus for performing the semiconductor manufacturing process includes a first reactor, and a plurality of second reactors for each first reactor. A first group of wafers are subjected to the first process within the first reactor, and are then transferred into a second reactor as isolated from the outside air. The first group of wafers is subjected to the second process within the second reactor. At the same time, a second group of wafers are subjected to the first process within the first reactor. After the first process is completed, the second group of wafers is transferred into an unoccupied one of the second reactors as isolated from the outside air. There, the second group of wafers is subjected to the second process. Accordingly, process failures otherwise due to the exposure of the wafers are minimized, and productivity is high despite the difference in the processing times.
Owner:SAMSUNG ELECTRONICS CO LTD
Method for fabricating semiconductor device, and electro-optical device, integrated circuit and electronic apparatus including the semiconductor device
InactiveUS7547589B2High resolutionDecrease productivityTransistorOther printing matterProduction rateEngineering
The invention provides a technique that enables formation of minute patterns on an uneven substrate in volume production without reducing productivity. The method for fabricating a semiconductor device includes: first patterning a semiconductor film on a substrate to form element regions, each of which will be provided with a source / drain region and a channel region, second forming a gate insulating film covering segments of the patterned semiconductor film in the respective element regions, third forming gate electrodes on the gate insulating film at predetermined positions, and fourth forming the source / drain region and the channel region in each element region. At least the gate electrodes are formed by a process including an exposure step through a holographic exposure mask in the third step, and by a process including an exposure step through a projection exposure mask, the element regions are formed in the first step, and the source / drain regions and the channel regions are formed in the fourth step.
Owner:SEIKO EPSON CORP
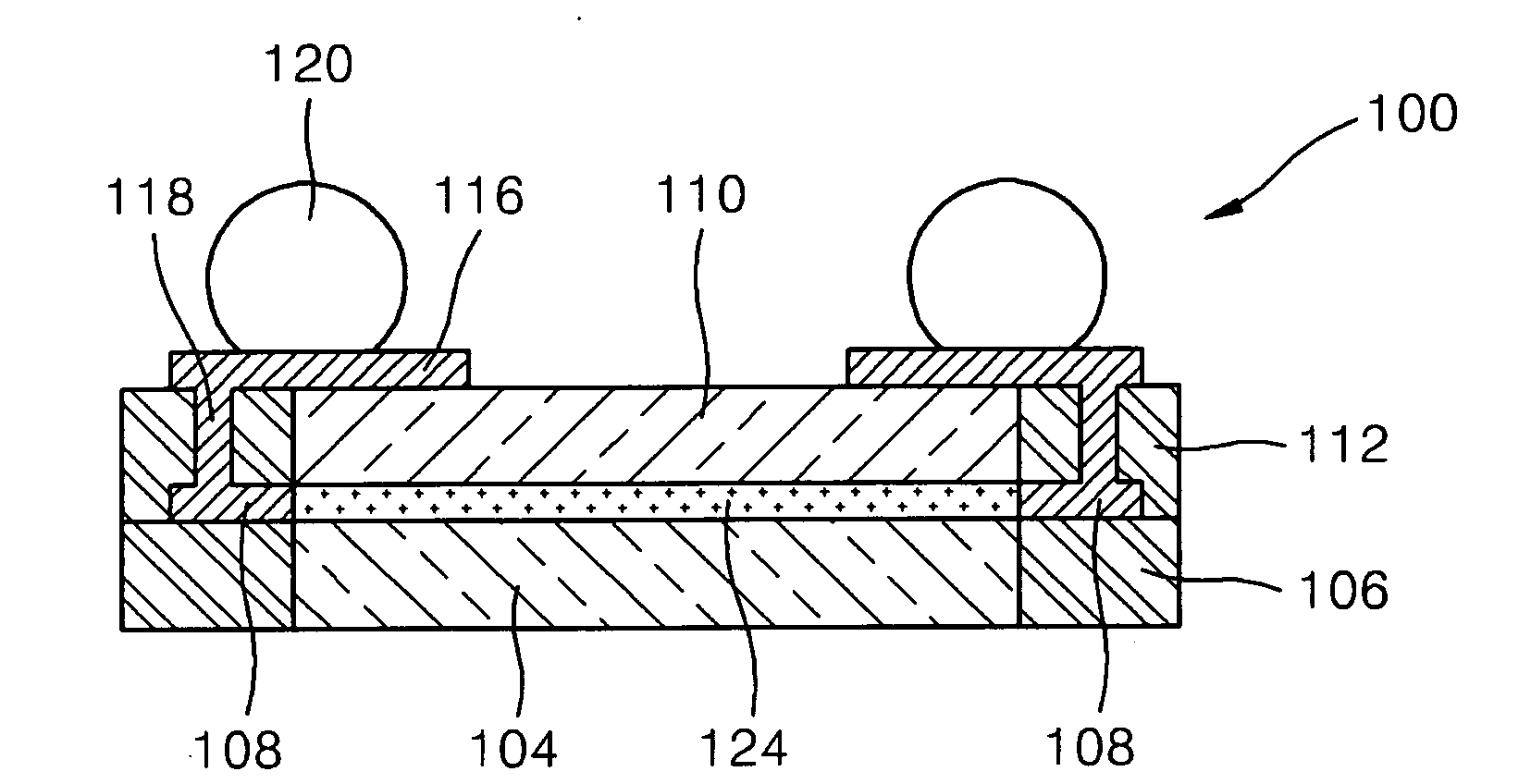
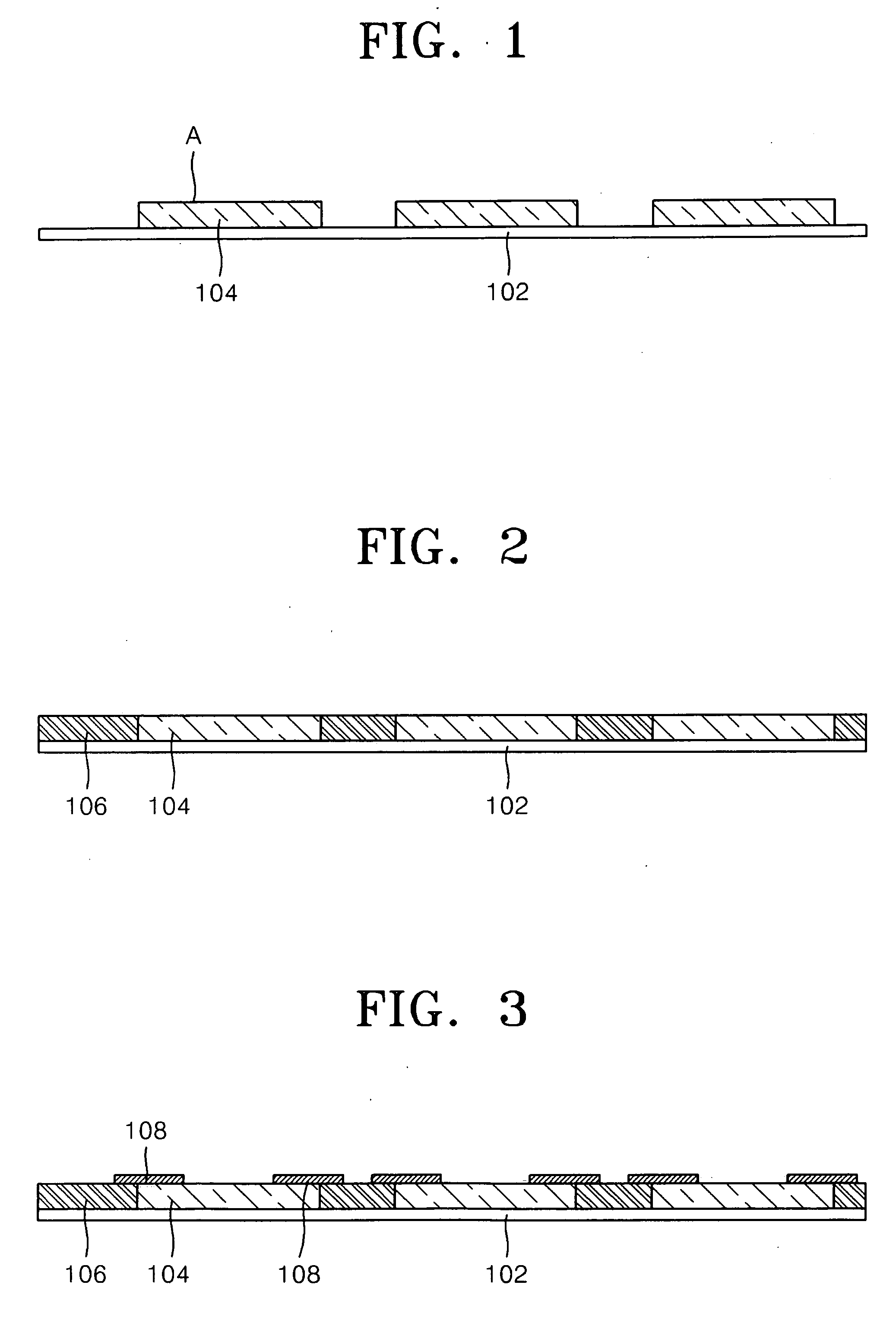
Substrate for mounting semiconductor chips
InactiveUS6281450B1Improve connection reliabilityImprove batch productivitySemiconductor/solid-state device detailsSolid-state devicesProduction rateElectrical conductor
A substrate for mounting a semiconductor chip having bumps using an adhesive thereon, said substrate being, for instance, provided with an insulating coating having an opening in the semiconductor chip mounting area so that the wiring conductors will not be exposed to the substrate surface near the boundary of the semiconductor chip mounting area, is improved in connection reliability and has high mass productivity.
Owner:HITACHI CHEM CO LTD
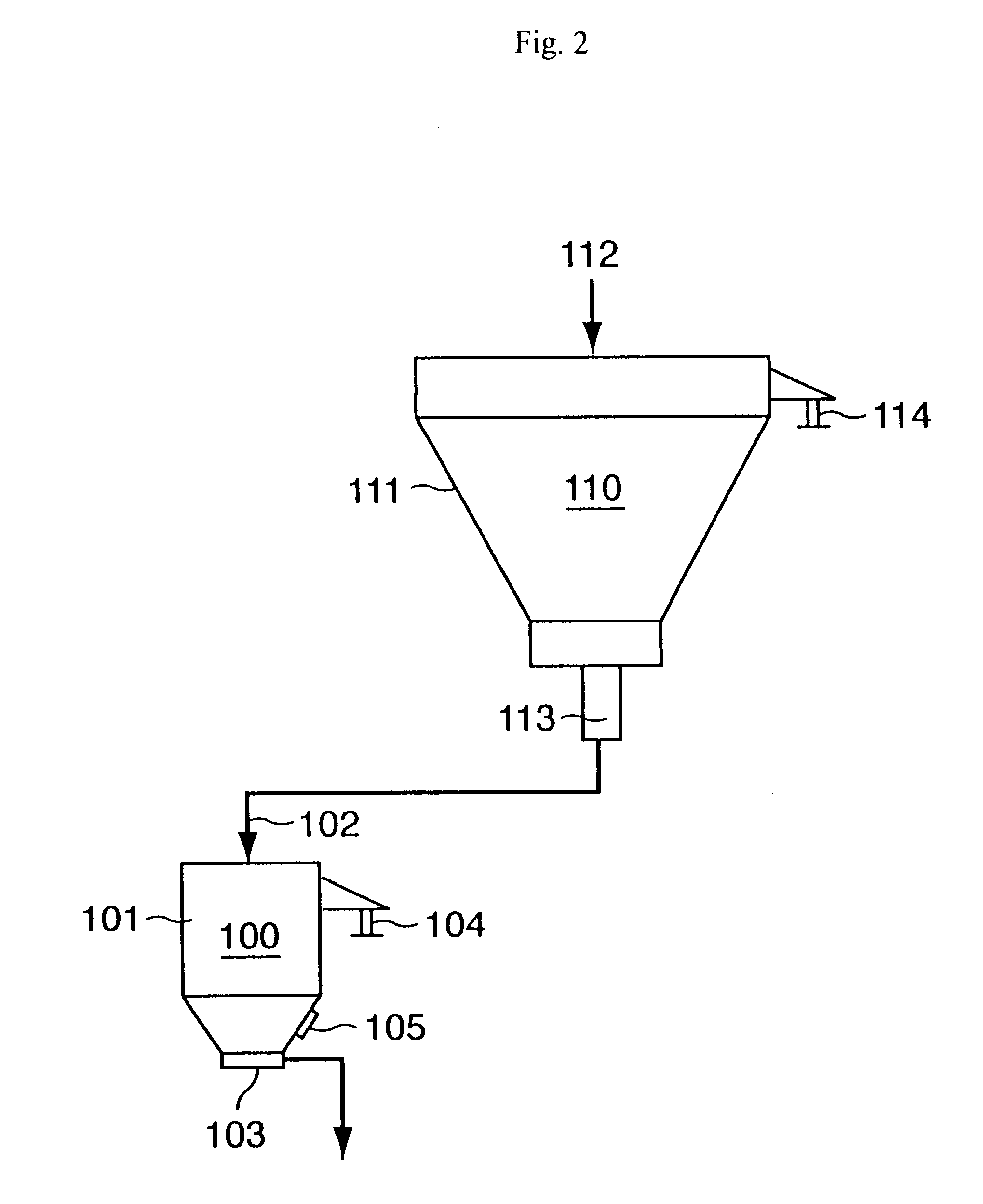
Continuous production process for water-absorbent resin powder and powder surface detector used therefor
A surface-modified water-absorbent resin powder is produced continuously with high productivity in a state where the particle diameter distribution is narrow and where the properties are high by a process comprising a polymerizing step, a drying step, a pulverizing step, a classifying step, and a surface-modifying step, and further, conveying steps of connecting them, wherein the conveying steps include at least two hoppers for storing the water-absorbent resin powder. A powder surface detector used favorably for this process includes a float hung down by a hanging line.
Owner:NIPPON SHOKUBAI CO LTD
Wafer level stacked package having via contact in encapsulation portion and manufacturing method thereof
InactiveUS20090014876A1Semiconductor/solid-state device detailsSolid-state devicesProduction rateSemiconductor chip
Provided are a wafer level stacked package with a via contact in an encapsulation portion, and a manufacturing method thereof. A plurality of semiconductor chips and encapsulation portions may be vertically deposited and electrically connected through a via contact that may be vertically formed in the encapsulation portion. Thus, an effective fan-out structure may be produced, vertical deposition may be available regardless of the type of a semiconductor device, and productivity may be improved.
Owner:SAMSUNG ELECTRONICS CO LTD
Deposition Over Mixed Substrates
<heading lvl="0">Abstract of Disclosure< / heading> Chemical vapor deposition methods are used to deposit silicon-containing films over mixed substrates. Such methods are useful in semiconductor manufacturing to provide a variety of advantages, including uniform deposition over heterogeneous surfaces, high deposition rates, and higher manufacturing productivity. An example is in forming the base region of a heterojunction bipolar transistor, including simultaneous deposition over both single crystal semiconductor surfaces and amorphous insulating regions.
Owner:ASM IP HLDG BV
Transient Transfection with RNA
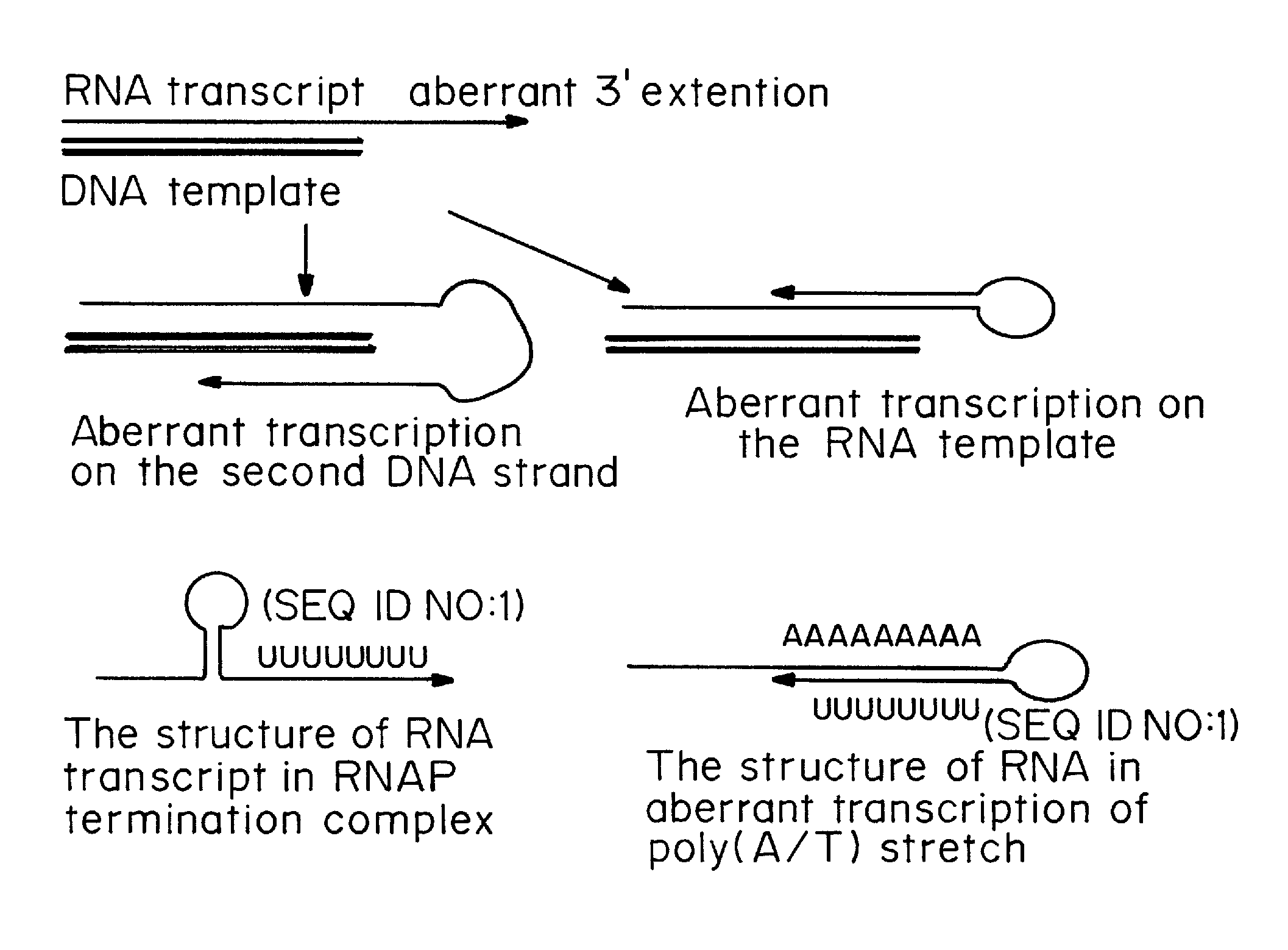
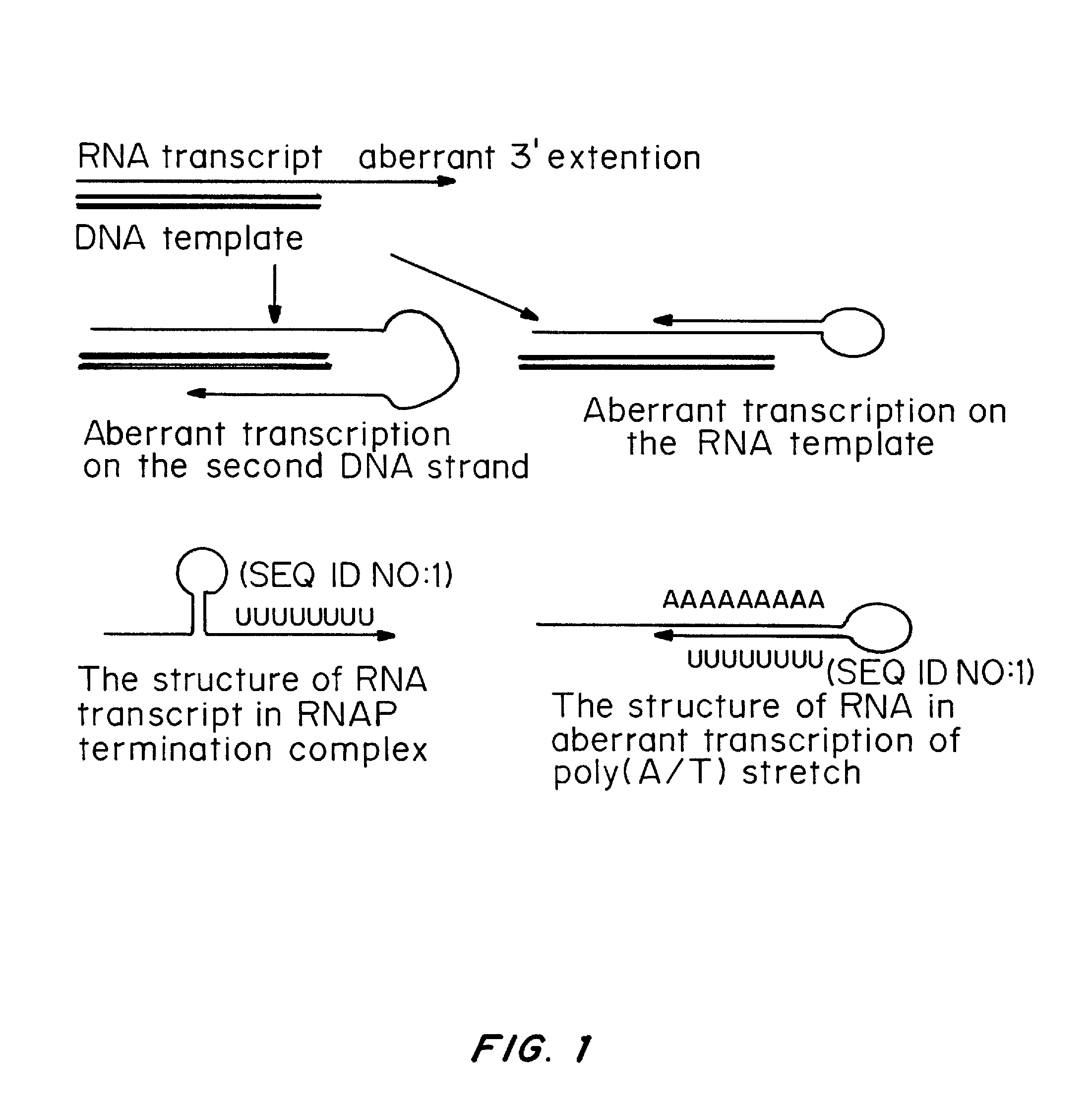
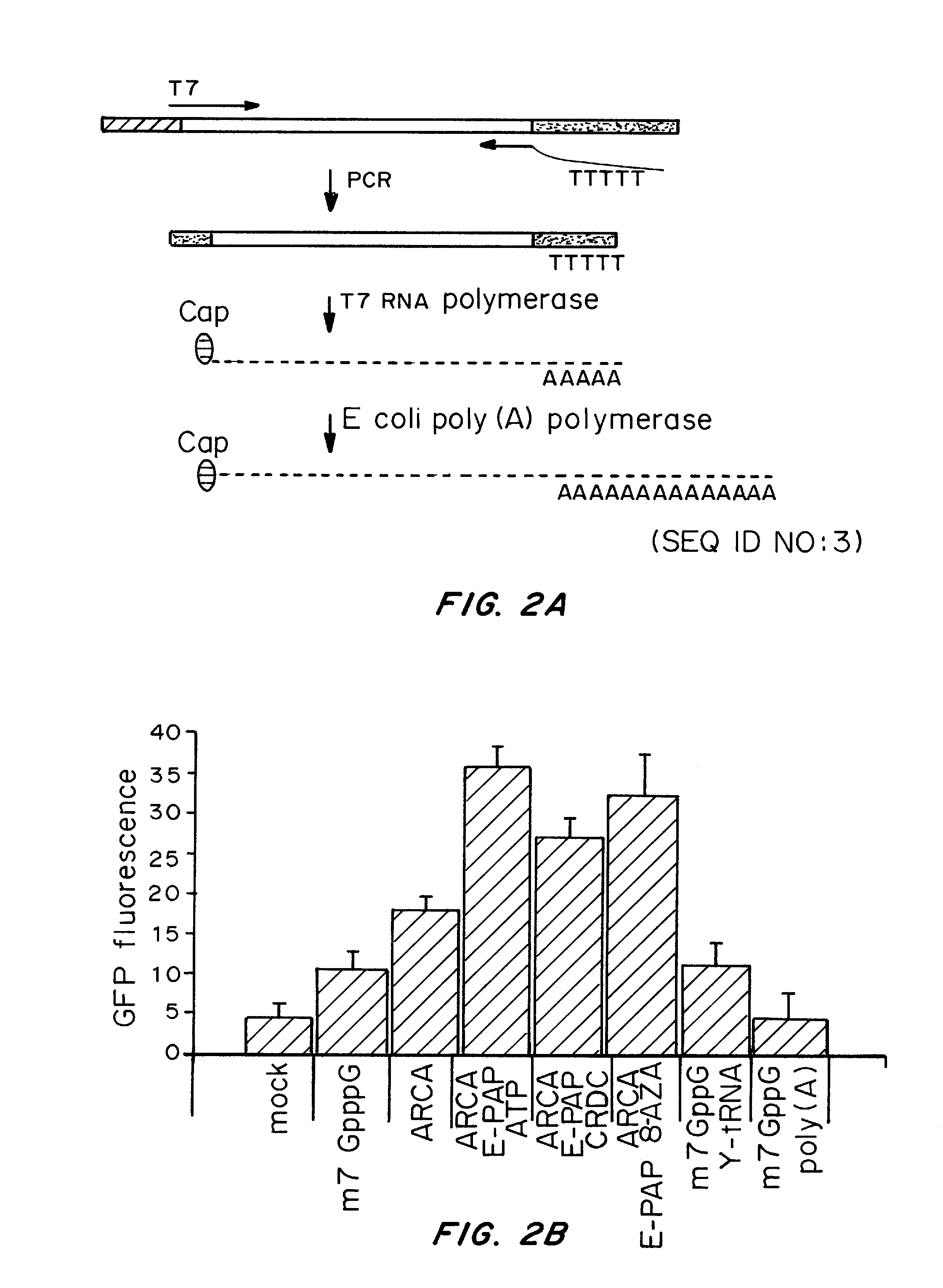
ActiveUS20080260706A1Lymphocyte transfectabilitySimilar efficiencyBiocideGenetic material ingredientsGene deliveryDNA construct
A method of mRNA production for use in transfection is provided, that involves in vitro transcription of PCR generated templates with specially designed primers, followed by polyA addition, to produce a construct containing 3′ and 5′ untranslated sequence (“UTR”), a 5′ cap and / or Internal Ribosome Entry Site (IRES), the gene to be expressed, and a polyA tail, typically 50-2000 bases in length. This RNA can efficiently transfect different kinds of cells. This approach results in increased efficiency (fidelity and productivity) of mRNA synthesis and is less time consuming because it does not require cloning, and also consequently eliminates the unwanted errors and effects related to RNA made on DNA templates obtained with cloning techniques. The results of transfection of RNAs demonstrate that RNA transfection can be very effective in cells that are exceedingly difficult to transfect efficiently with DNA constructs. Further, the levels of gene expression following mRNA transfection are consistent from cell to cell in an experiment and these levels can be controlled over a wide range simply by changing the amount of mRNA that is transfected, and without obvious cytotoxic effects due to the levels of RNA per se. Due to high efficiency the cells can be simultaneously transfected with multiple genetic constructs. The method can be used to deliver genes into cells not- or only poorly transfectable for DNA, in vitro and in vivo.
Owner:YALE UNIV
Engineered adsorbent structures for kinetic separation
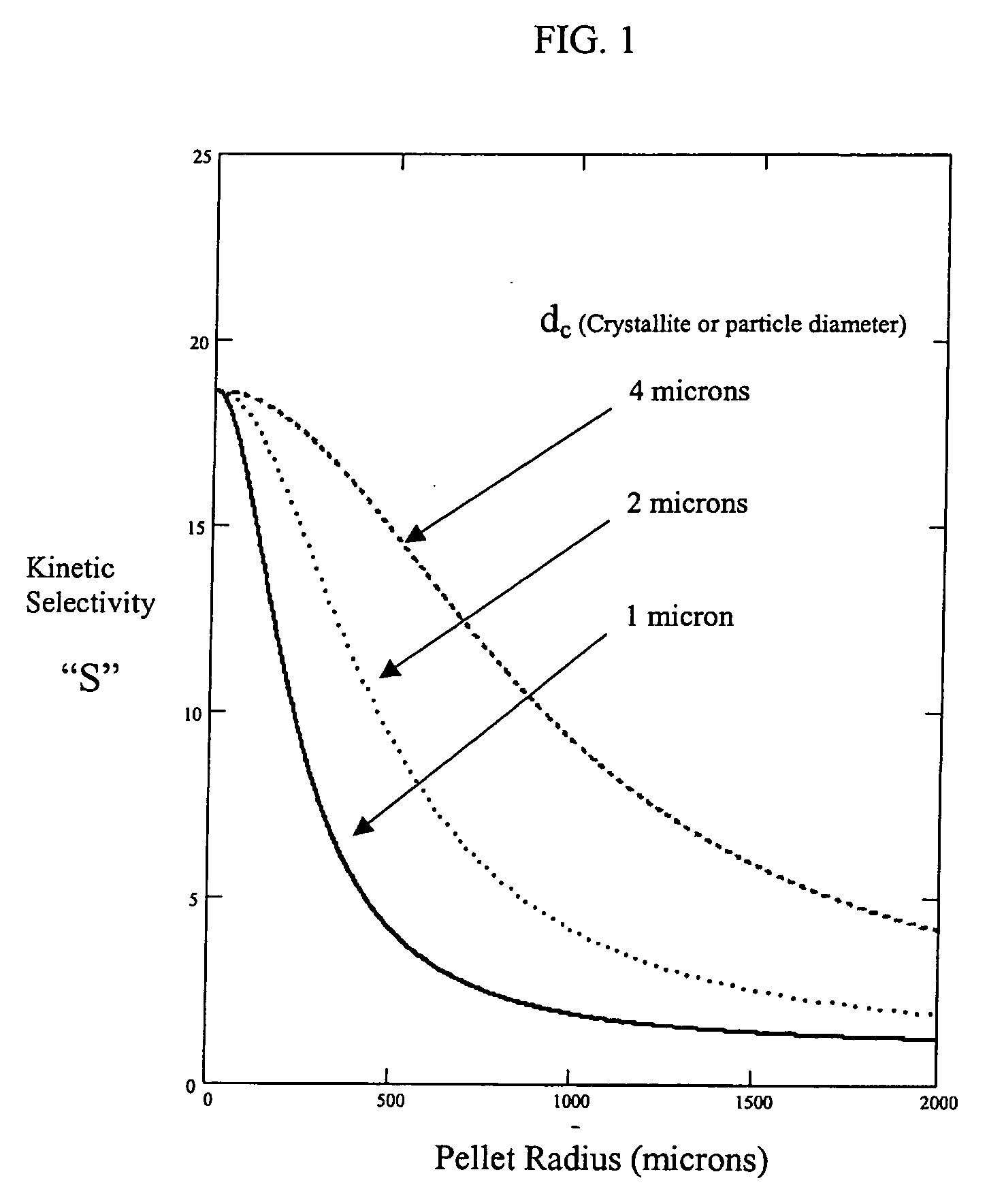
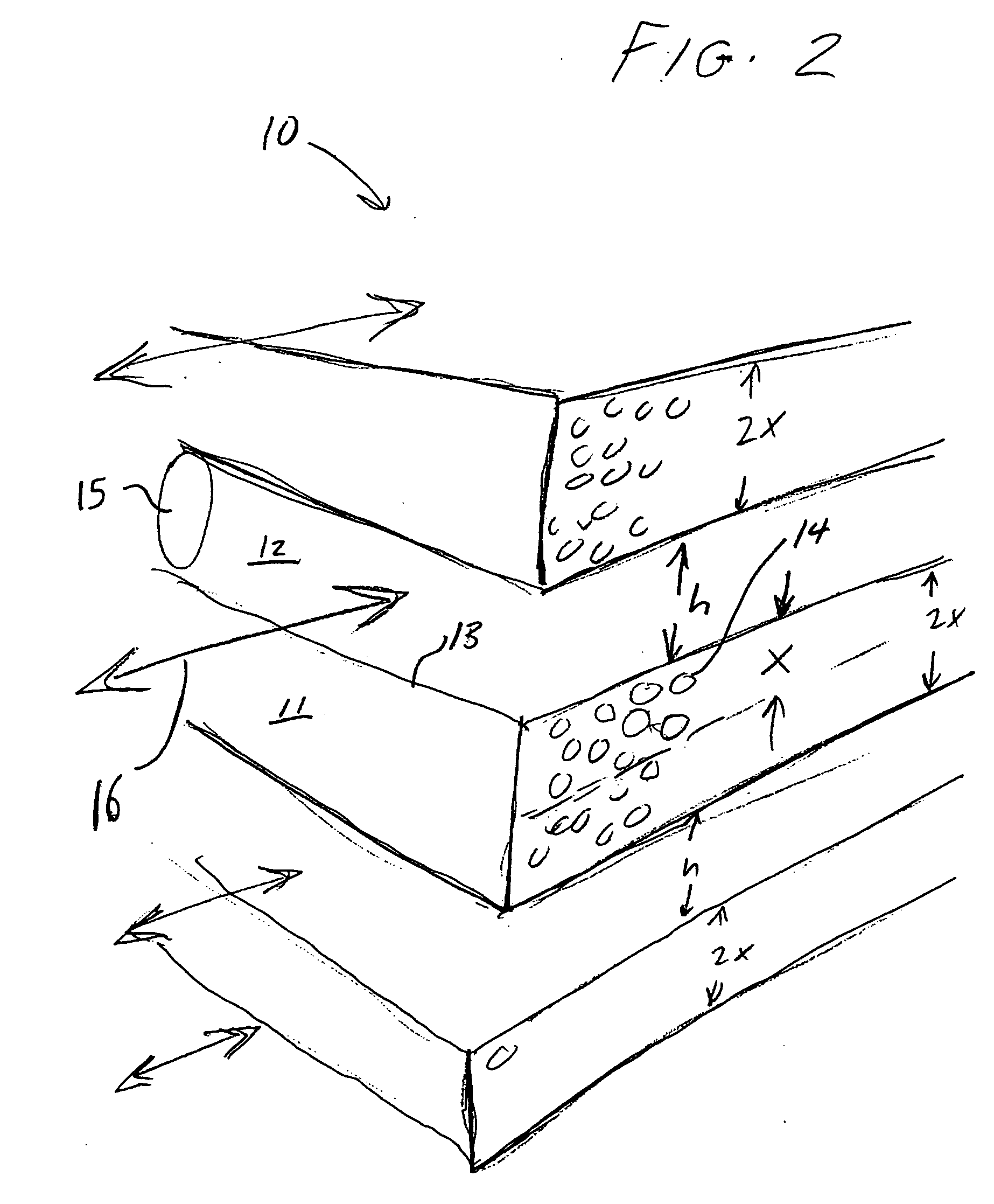
ActiveUS20060169142A1Easy to controlHigh selectivityGas treatmentMethane captureProduction rateSorbent
Improved adsorbent sheet based parallel passage adsorbent structures for enhancing the kinetic selectivity of certain kinetic-controlled adsorption processes, such as PSA, TSA and PPSA processes, and combinations thereof, are provided. The enhancements in kinetic selectivity made possible through the implementation of the present inventive improved adsorbent structures may unexpectedly enable significant intensification of selected kinetic adsorption processes relative to attainable performance with conventional adsorbent materials in beaded or extruded form. Such process intensification enabled by the present inventive adsorbent structures may provide for increased adsorption cycle frequencies, and increased gas flow velocities within the adsorbent beds, which may increase the productivity and / or recovery of a kinetic adsorption system incorporating the inventive adsorbent structures.
Owner:AIR PROD & CHEM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com