Patents
Literature
145 results about "Internal ribosome entry site" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
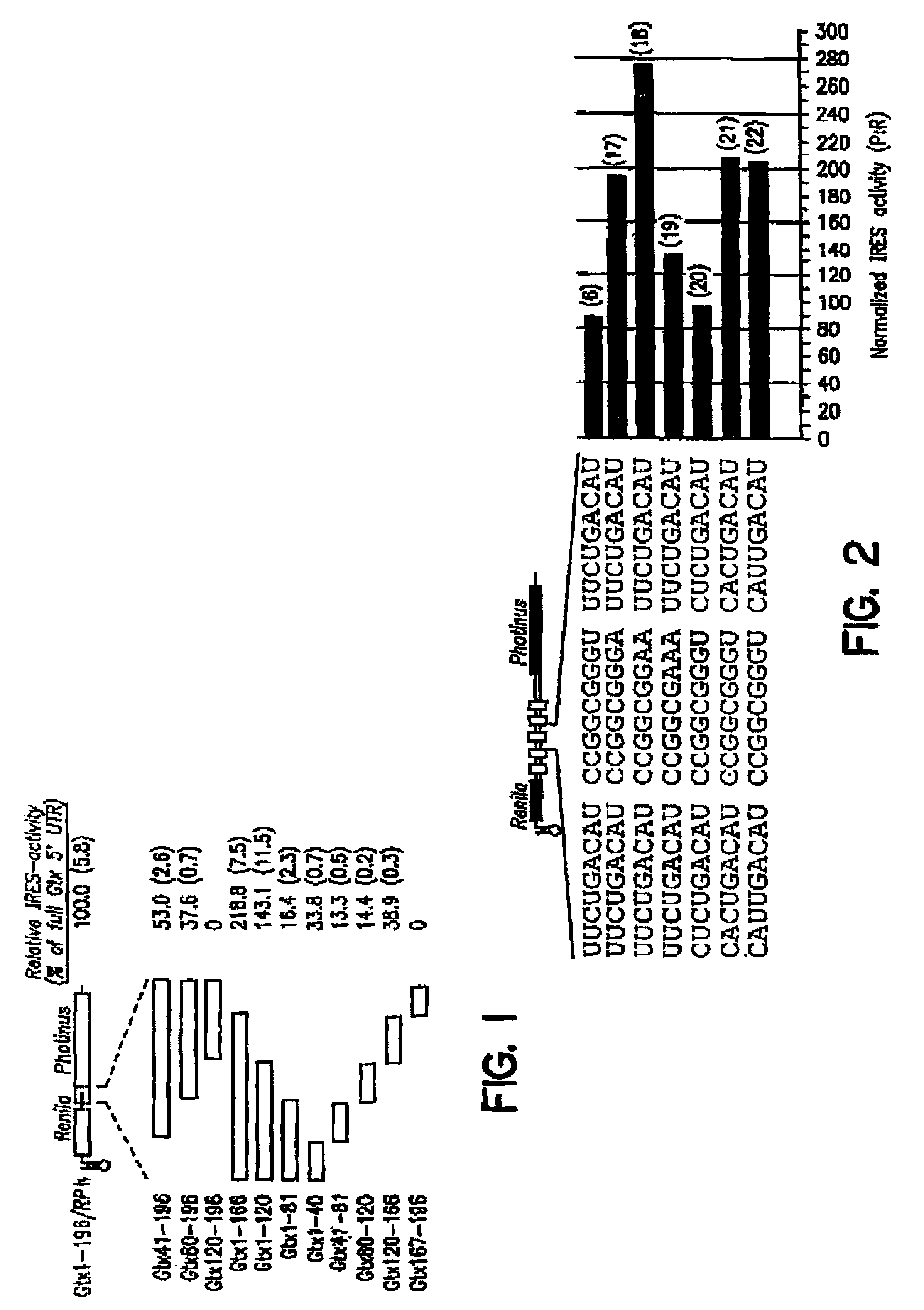
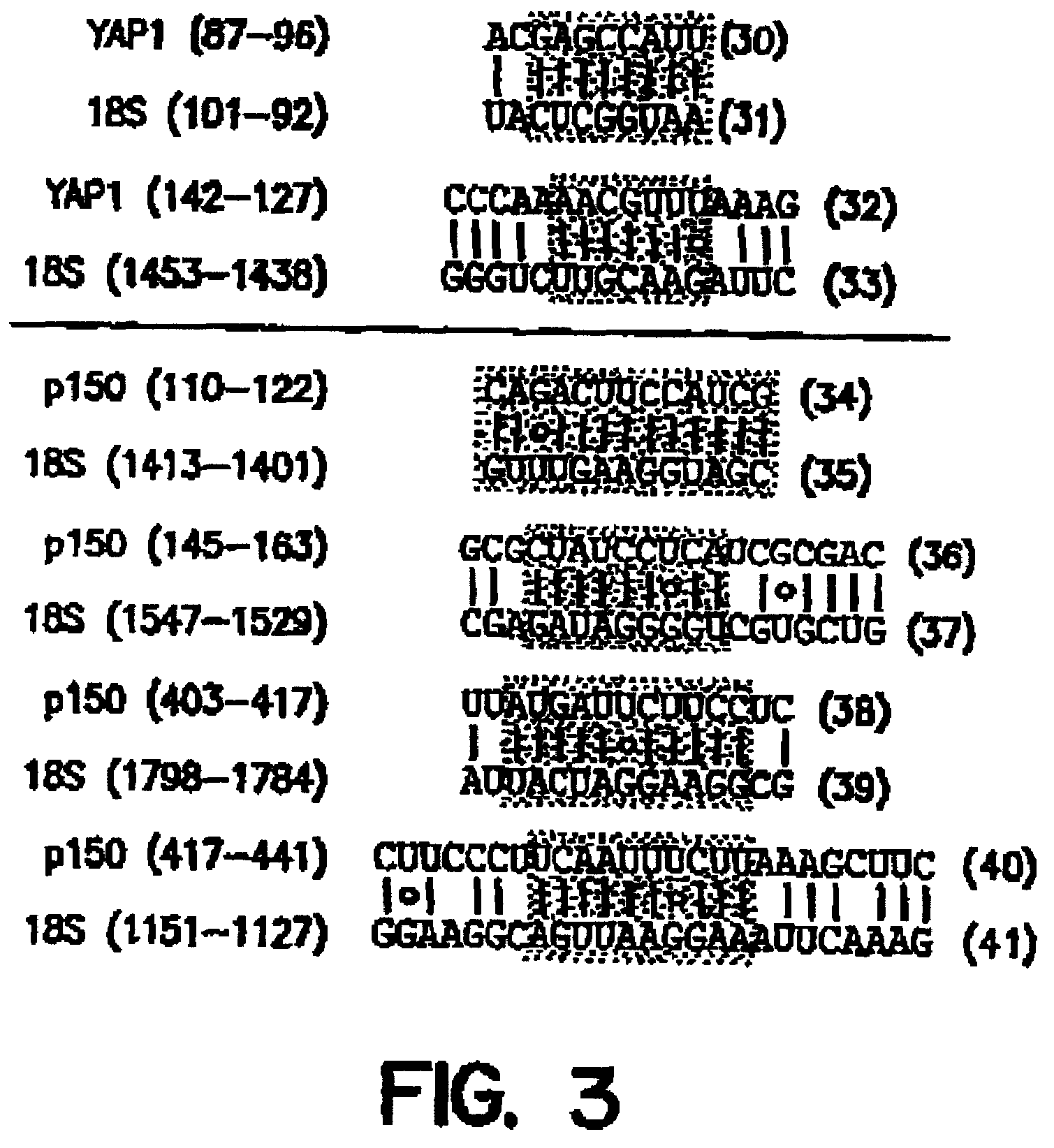
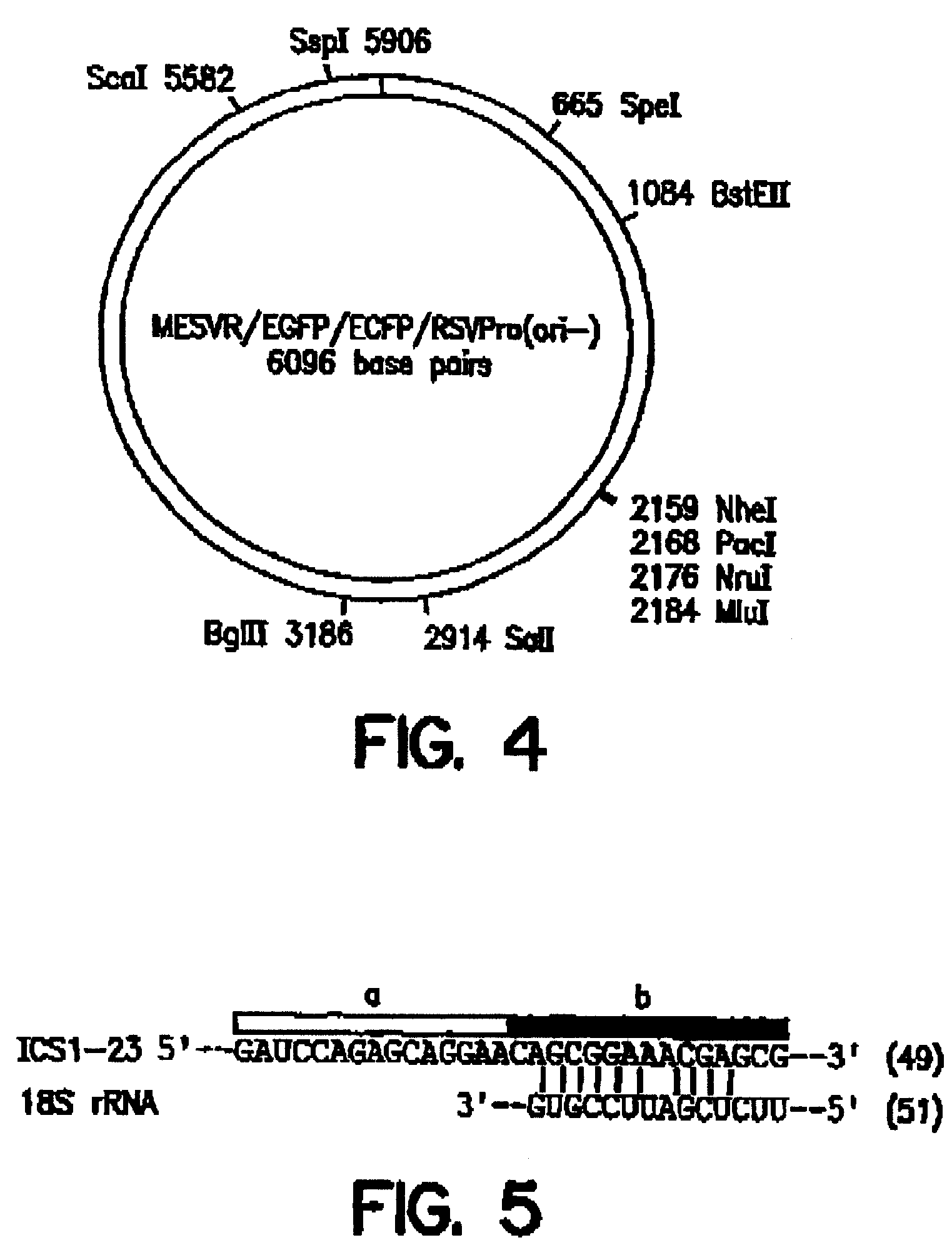
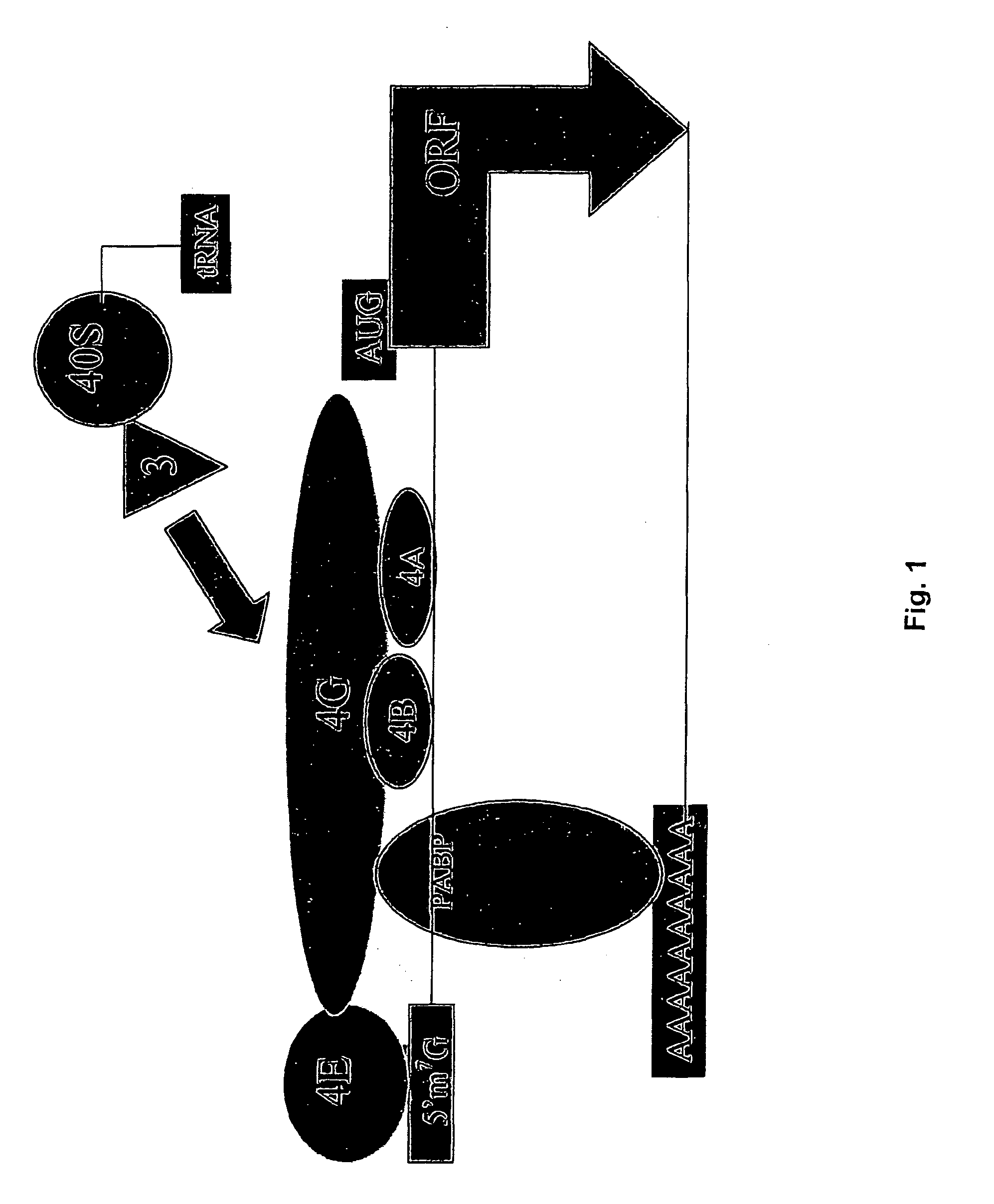
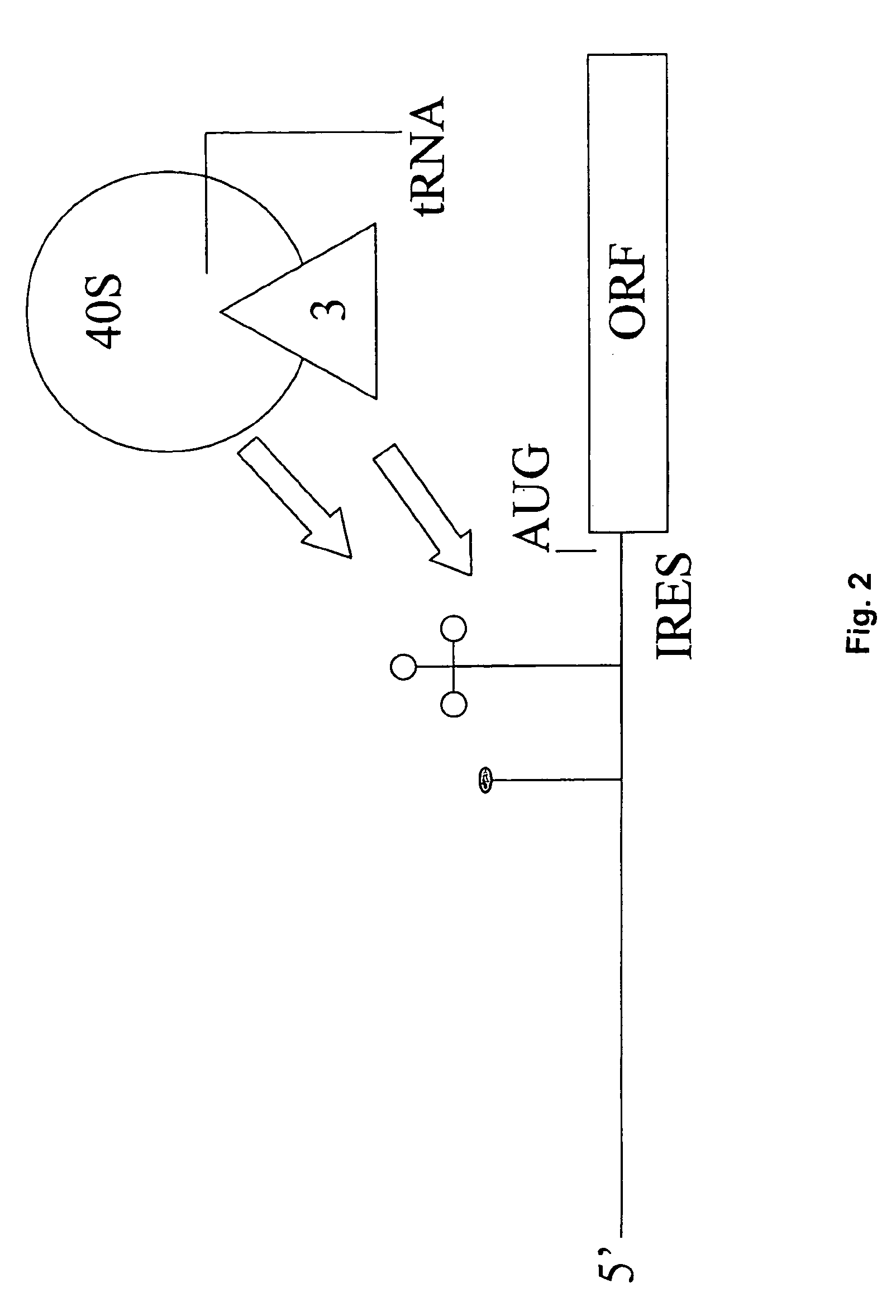
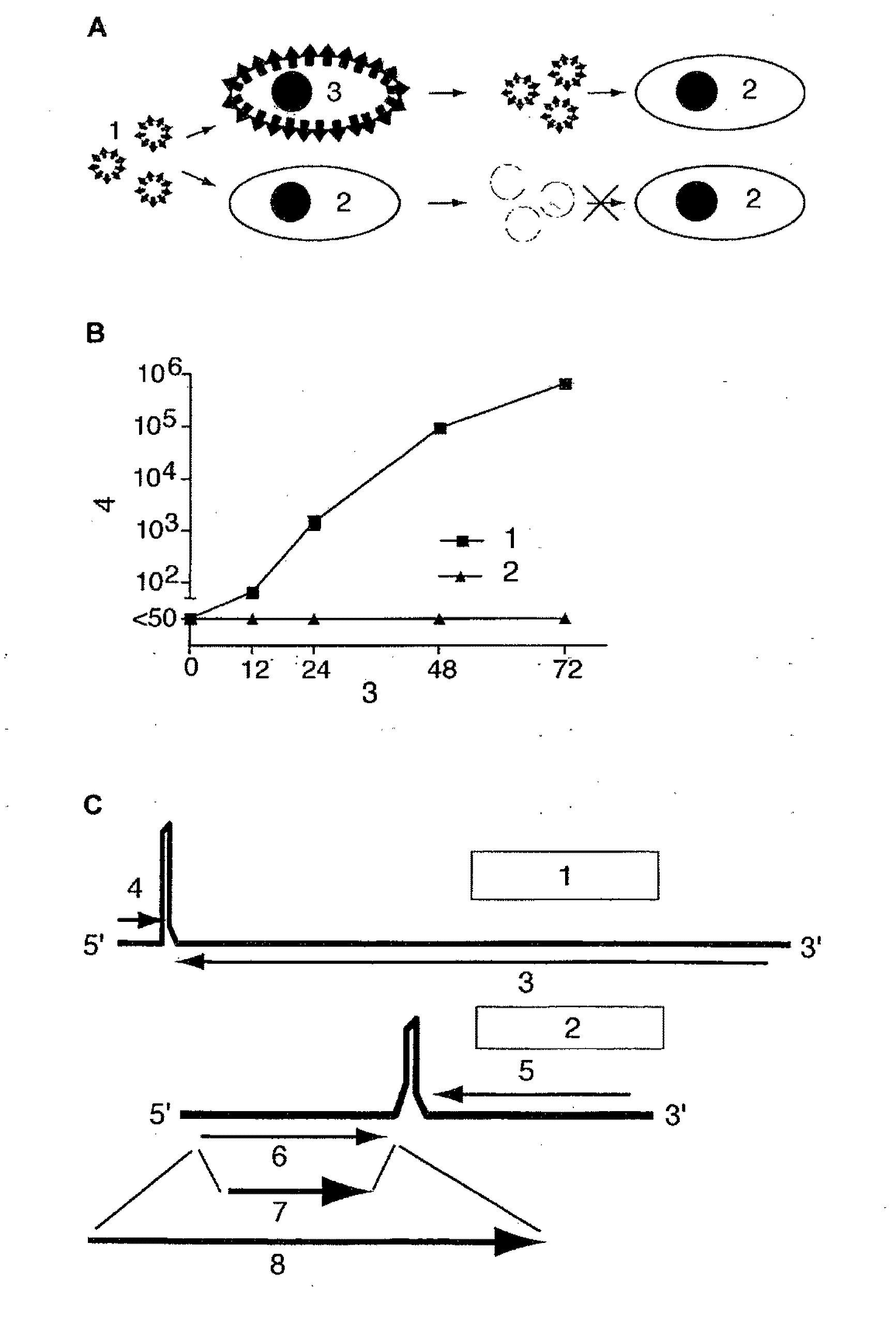
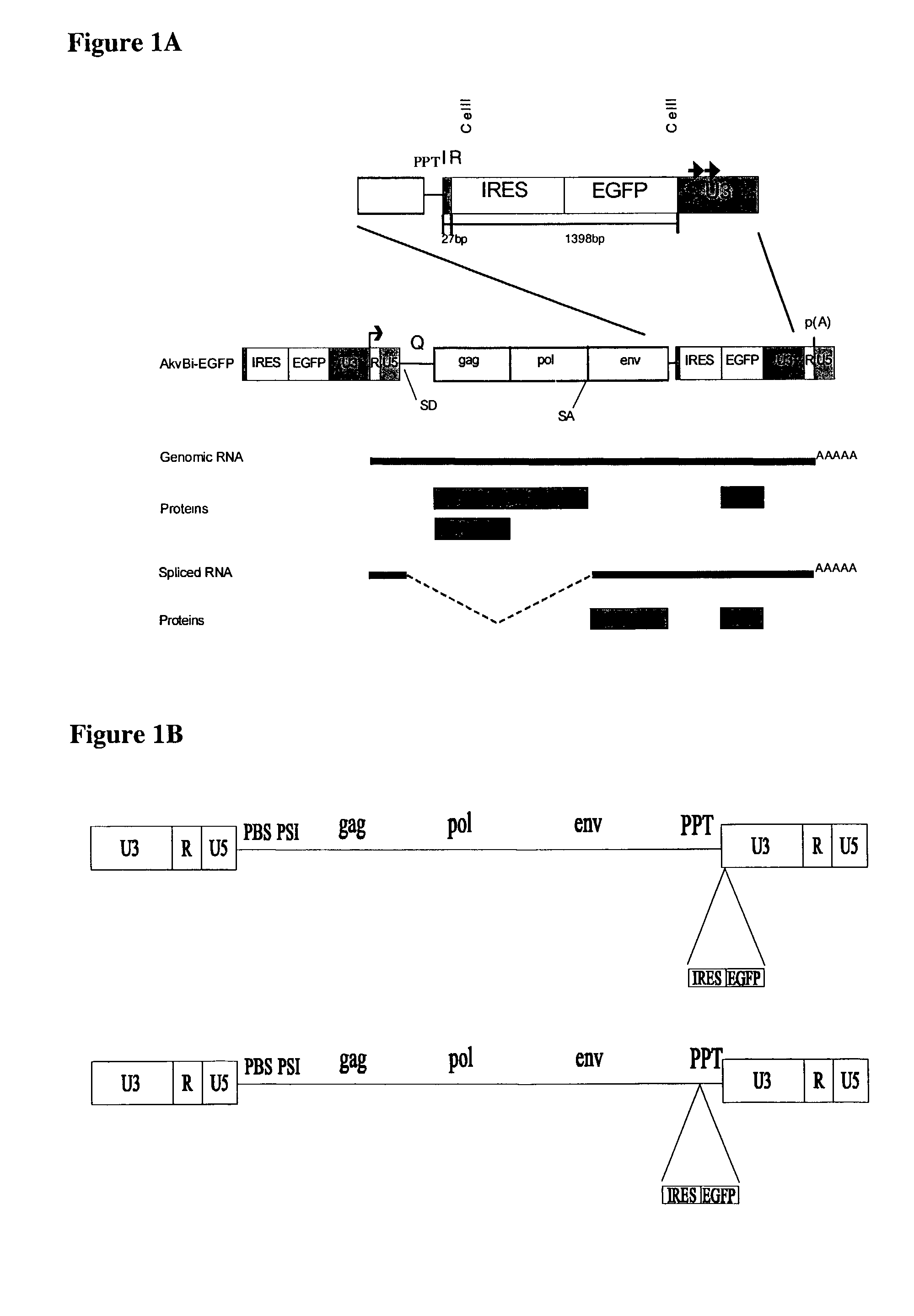
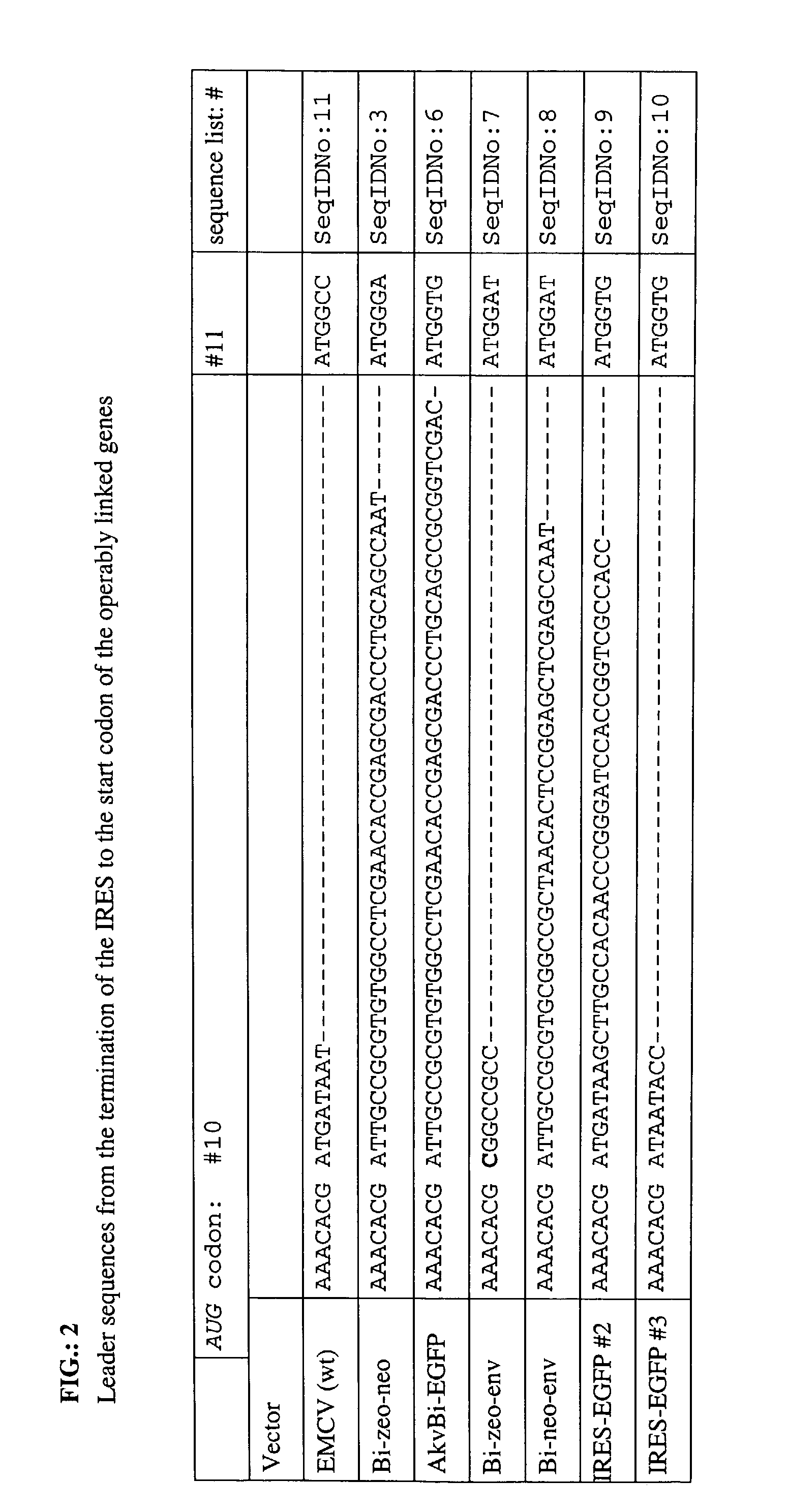
An internal ribosome entry site, abbreviated IRES, is an RNA element that allows for translation initiation in a cap-independent manner, as part of the greater process of protein synthesis. In eukaryotic translation, initiation typically occurs at the 5' end of mRNA molecules, since 5' cap recognition is required for the assembly of the initiation complex. The location for IRES elements is often in the 5'UTR, but can also occur elsewhere in mRNAs.
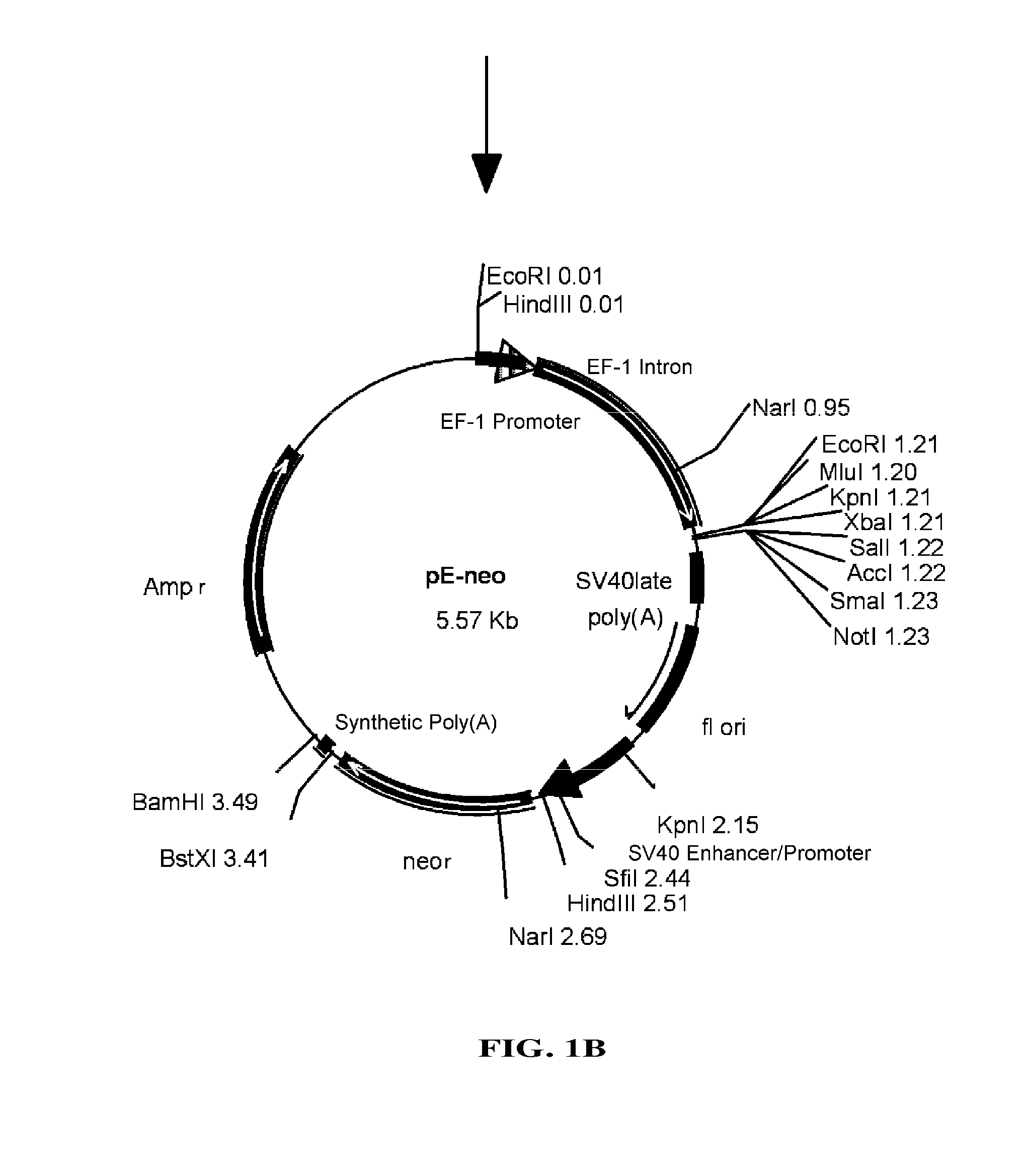
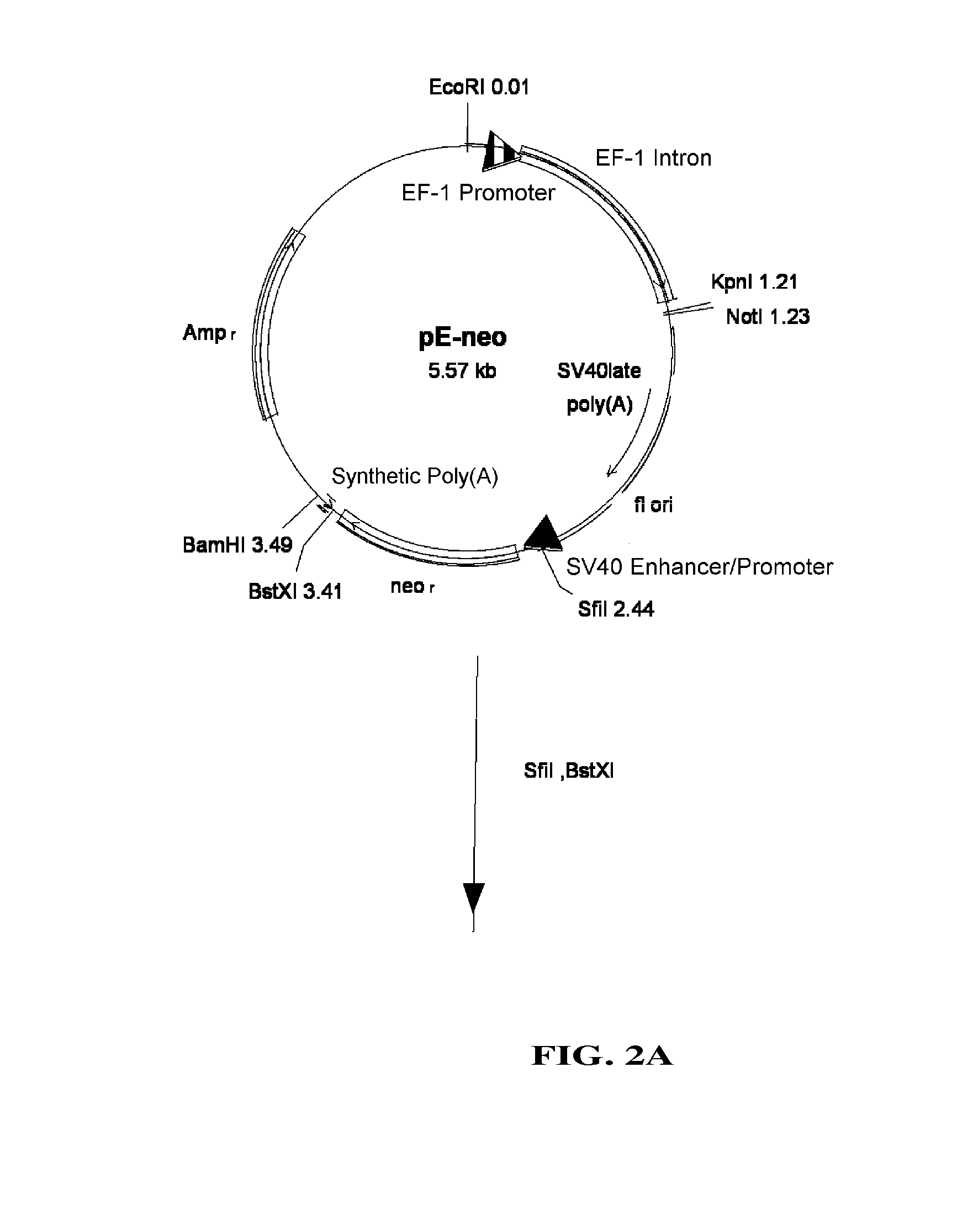
Vector system
InactiveUS7259015B2Eliminate the effects ofInhibition of activationFungiNervous disorderVector systemTyrosine
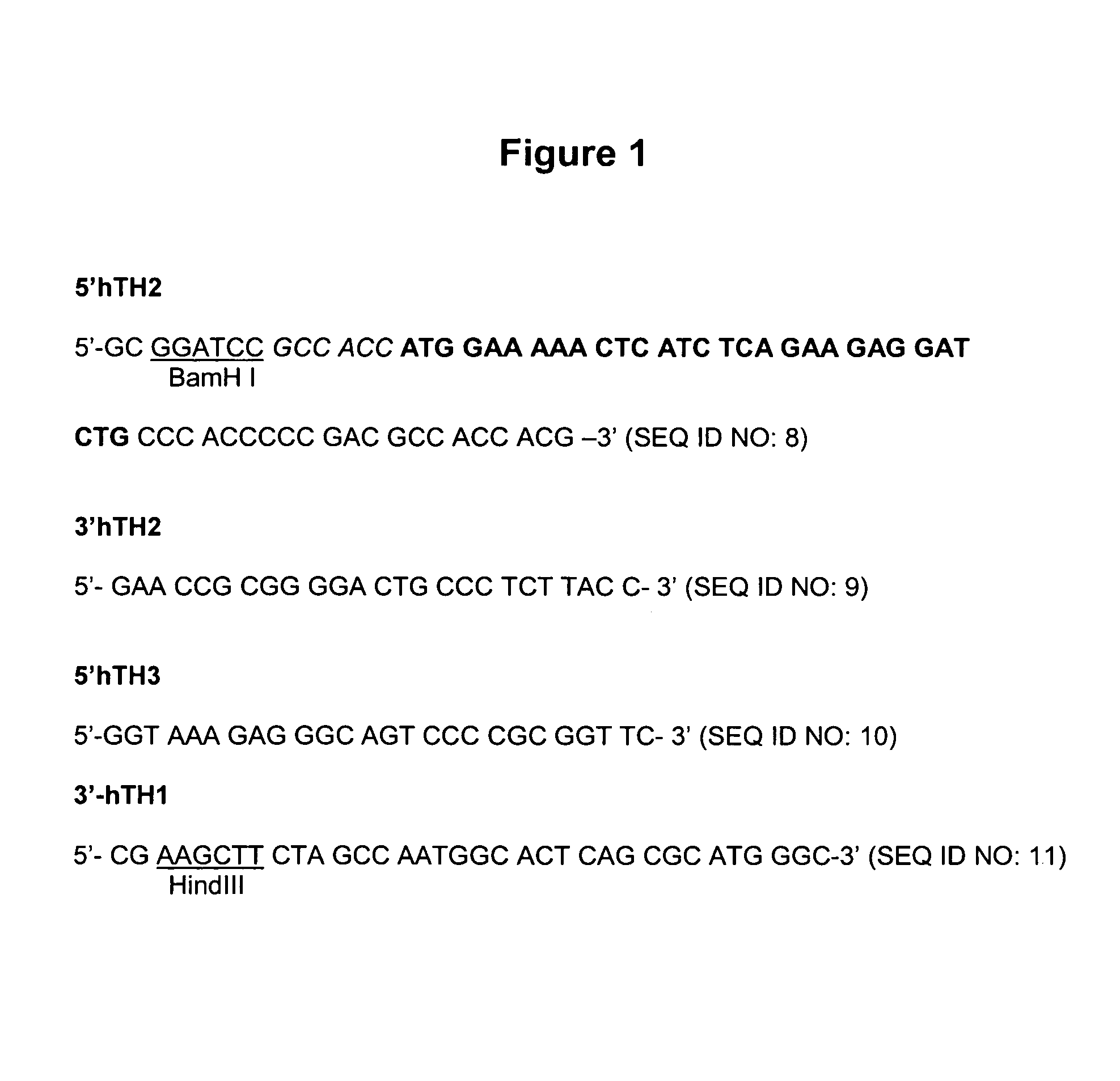
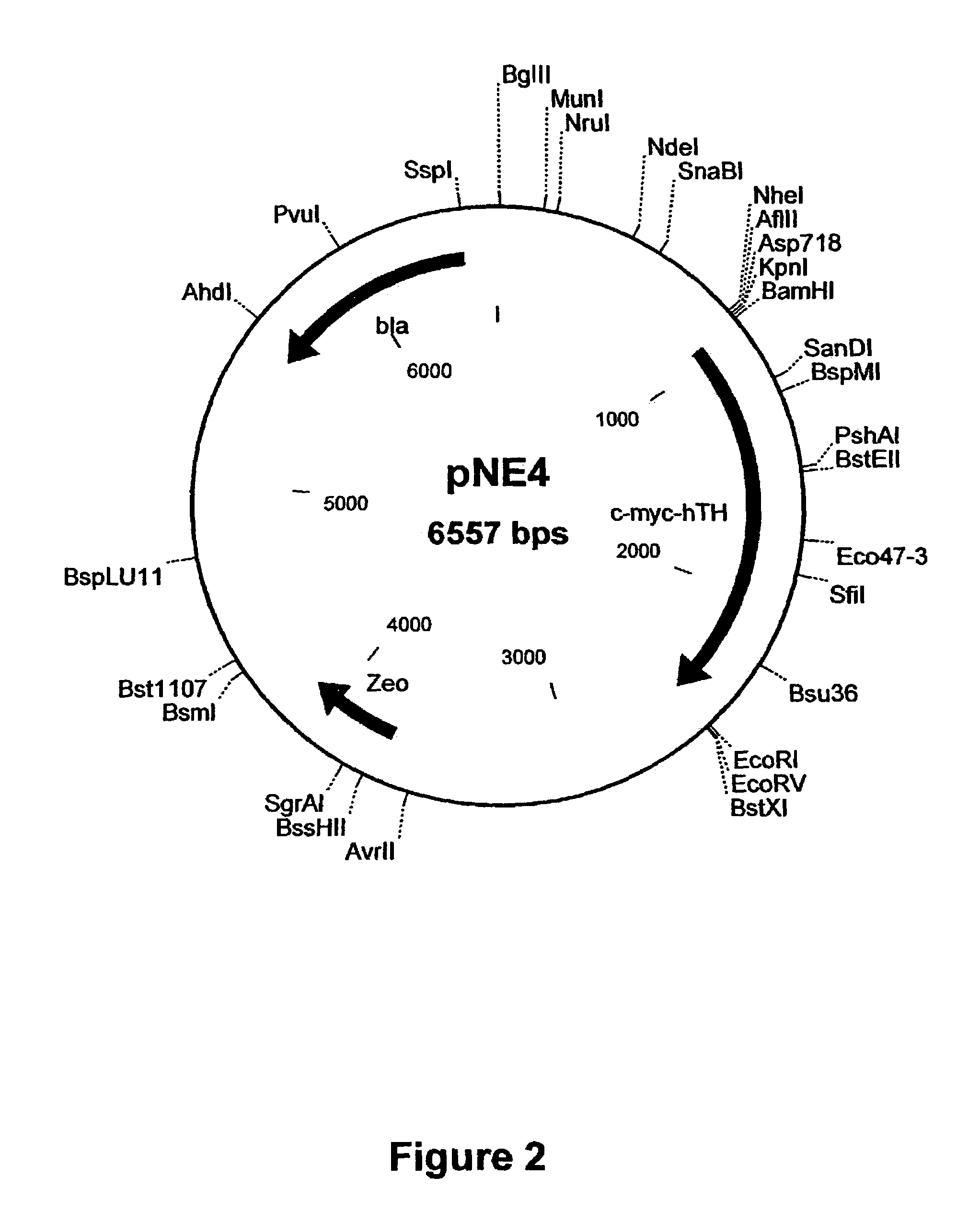
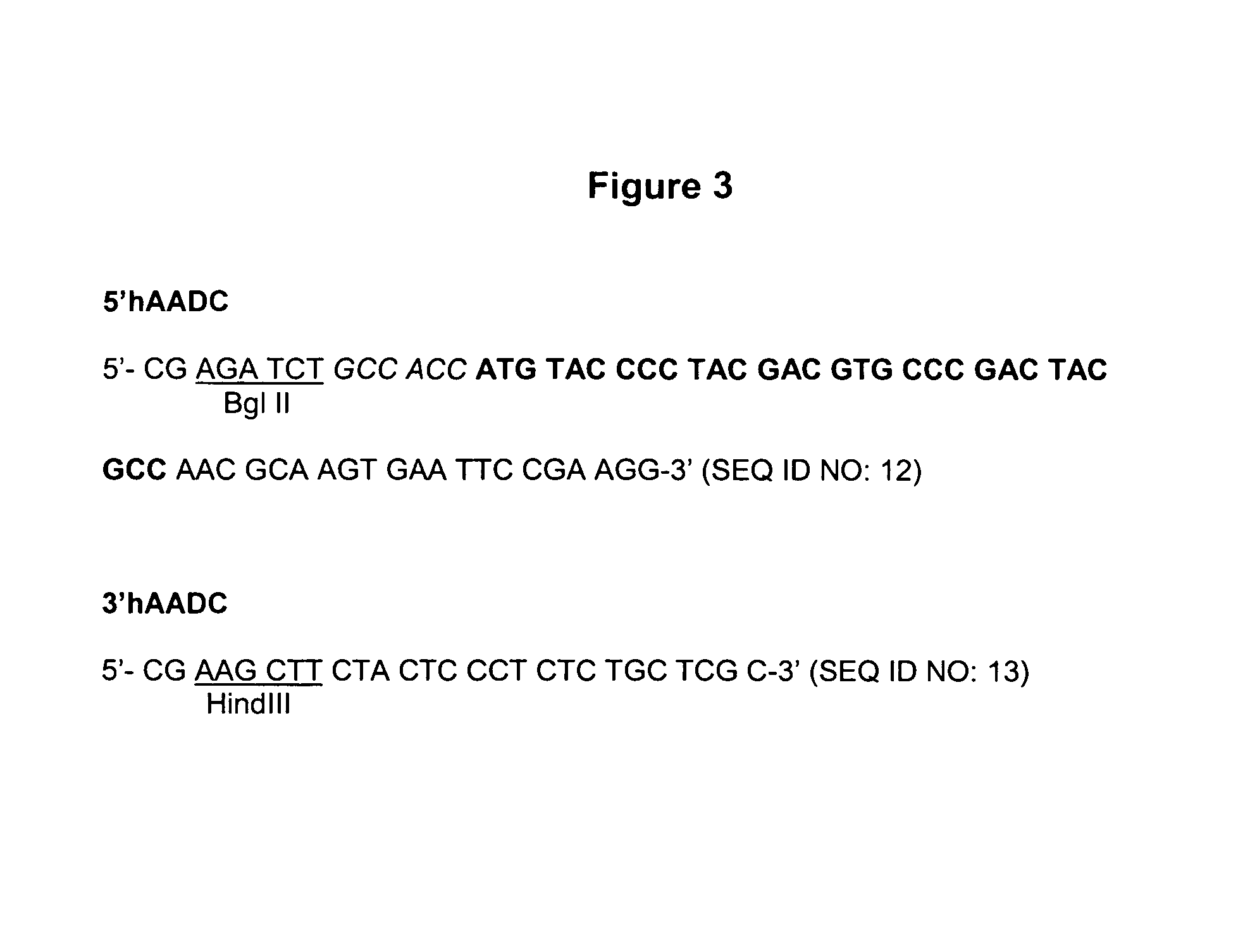
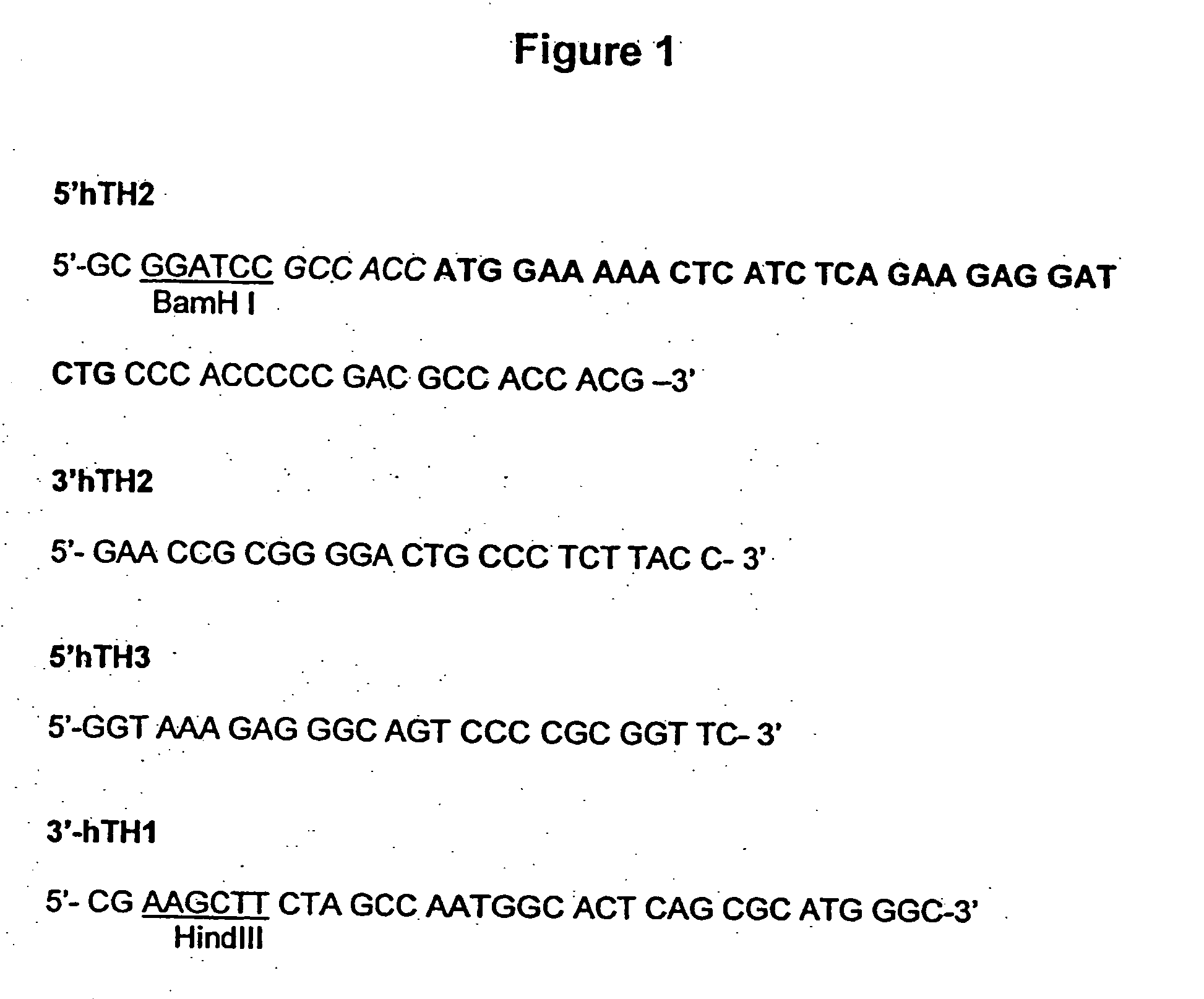
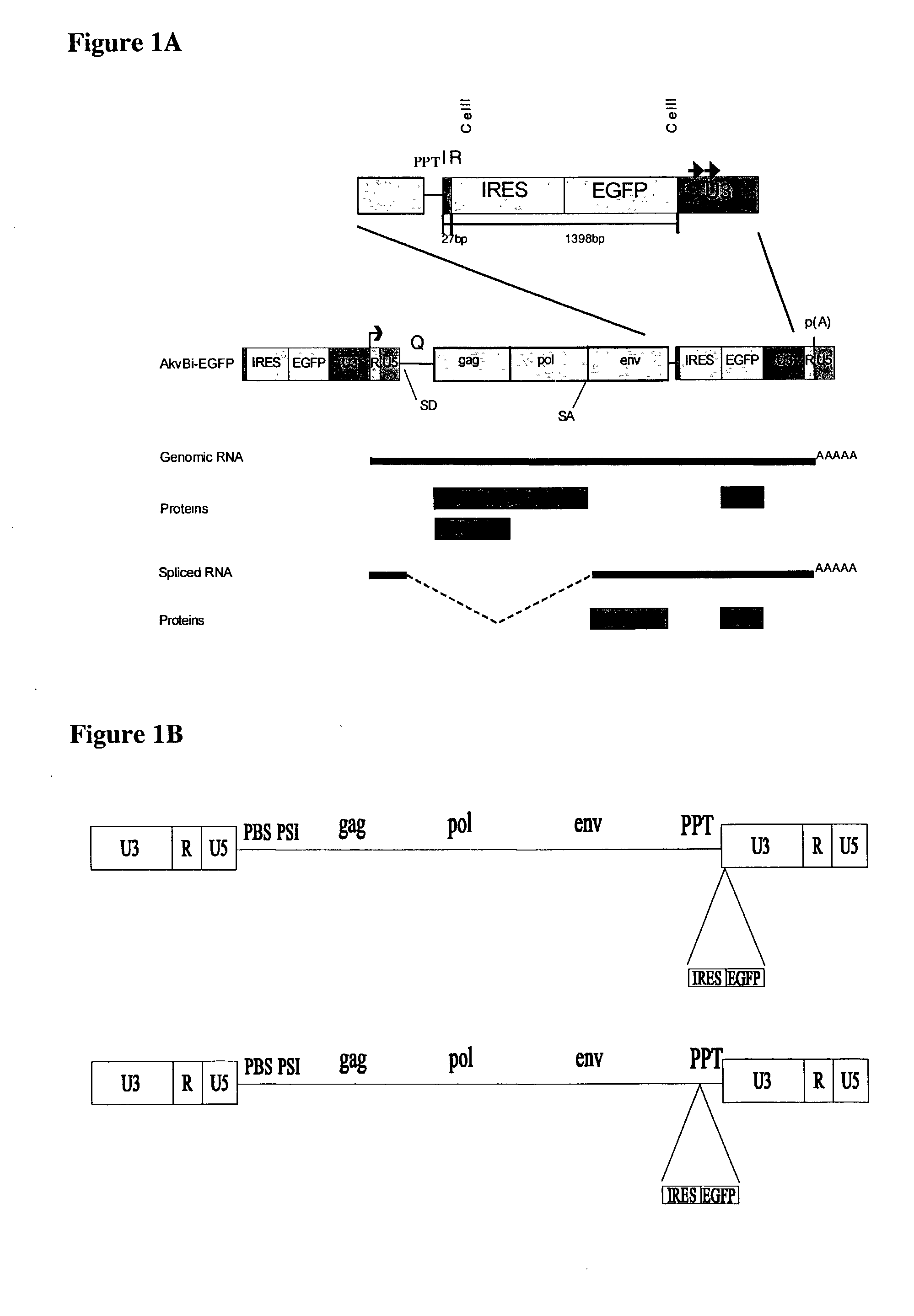
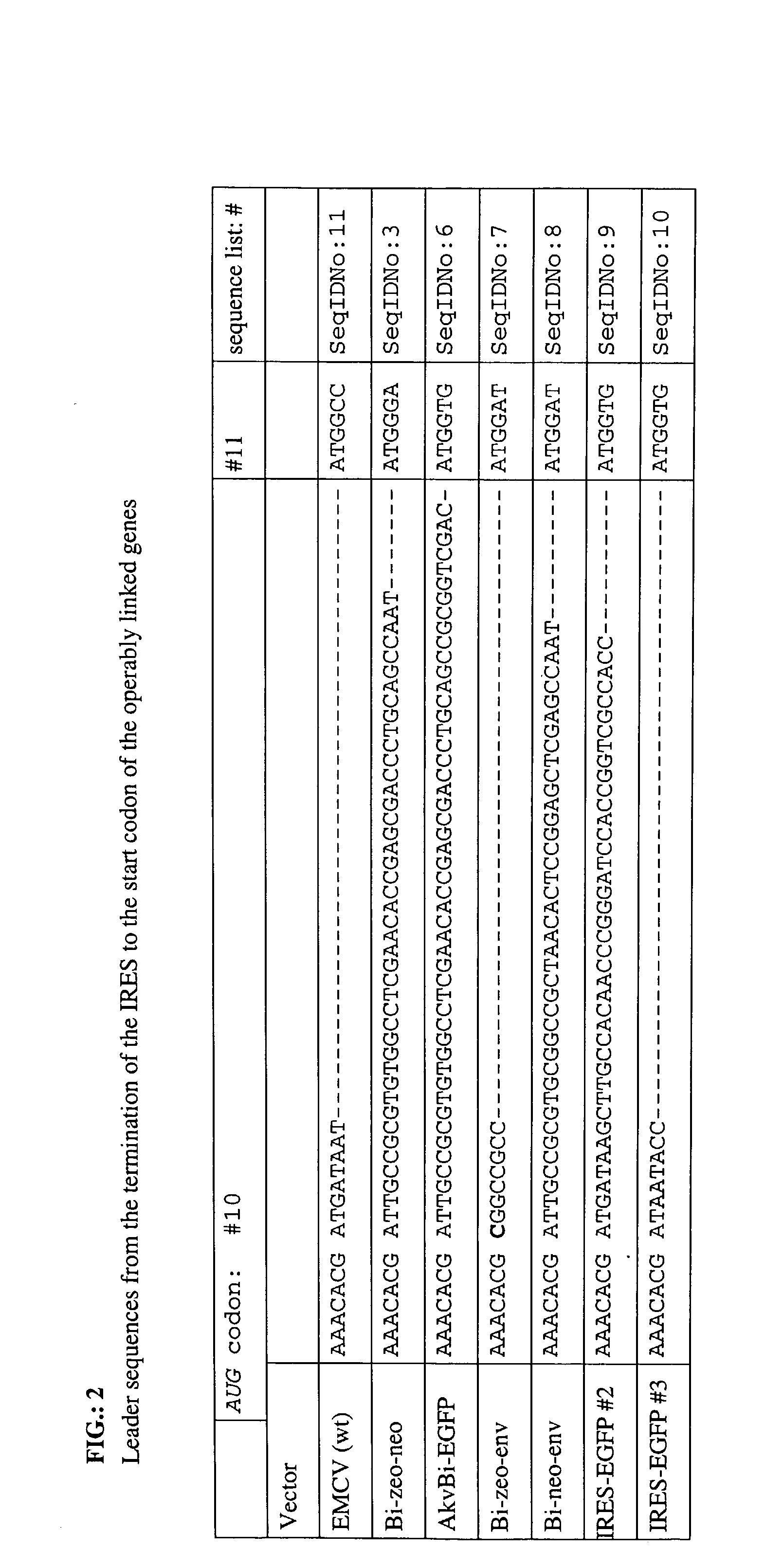
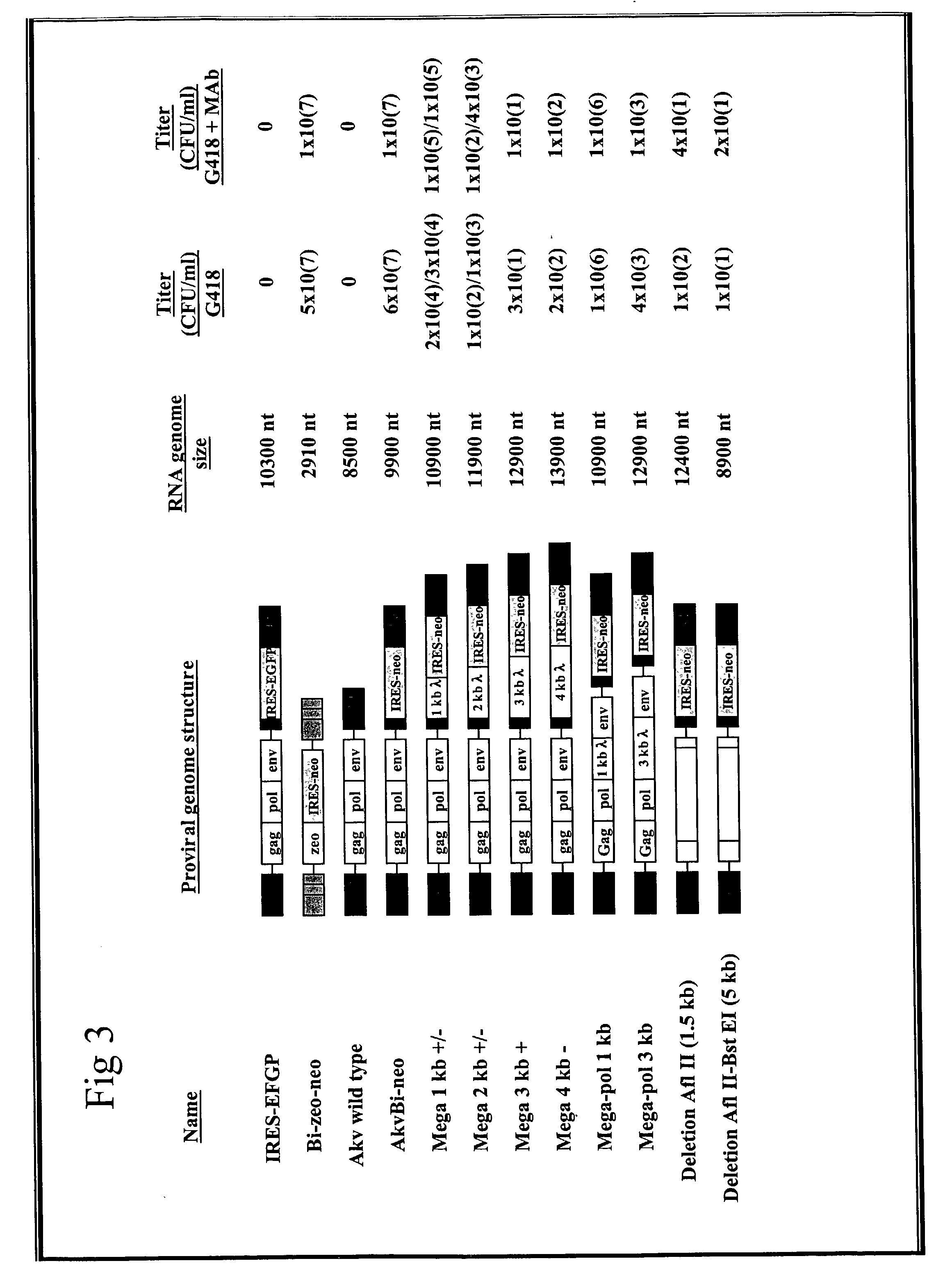
Provided are retroviral vector genomes and vector systems comprising the genomes. In particular, a retroviral vector genome comprising two or more NOIs, operably linked by one or more Internal Ribosome Entry Site(s); a lentiviral vector genome comprising two or more NOIs suitable for treating a neurodegenerative disorder; and a lentiviral vector genome which encodes tyrosine hydroxylase, GTP-cyclohydrolase I and, optionally, Aromatic Amino Acid Dopa Decarboxylase are provided.
Owner:OXFORD BIOMEDICA (UK) LTD
Vector system
InactiveUS20070025970A1Eliminate the effects ofInhibition of activationBiocideFungiVector systemTyrosine hydroxylase
The present invention relates to retroviral vector genomes and to vector systems comprising such genomes. In particular the present invention relates to a retroviral vector genome comprising two or more NOIs operably linked by one or more Internal Ribosome Entry Site(s); a lentiviral vector genome comprising two or more NOIs suitable for treating a neurodegenerative disorder; and a lentiviral vector genome which encodes tyrosine hydroxylase, GTP-cyclohydrolase I and optionally Aromatic Amino Acid Dopa Decarboxylase.
Owner:OXFORD BIOMEDICA (UK) LTD
Transient Transfection with RNA
ActiveUS20080260706A1Lymphocyte transfectabilitySimilar efficiencyBiocideGenetic material ingredientsGene deliveryDNA construct
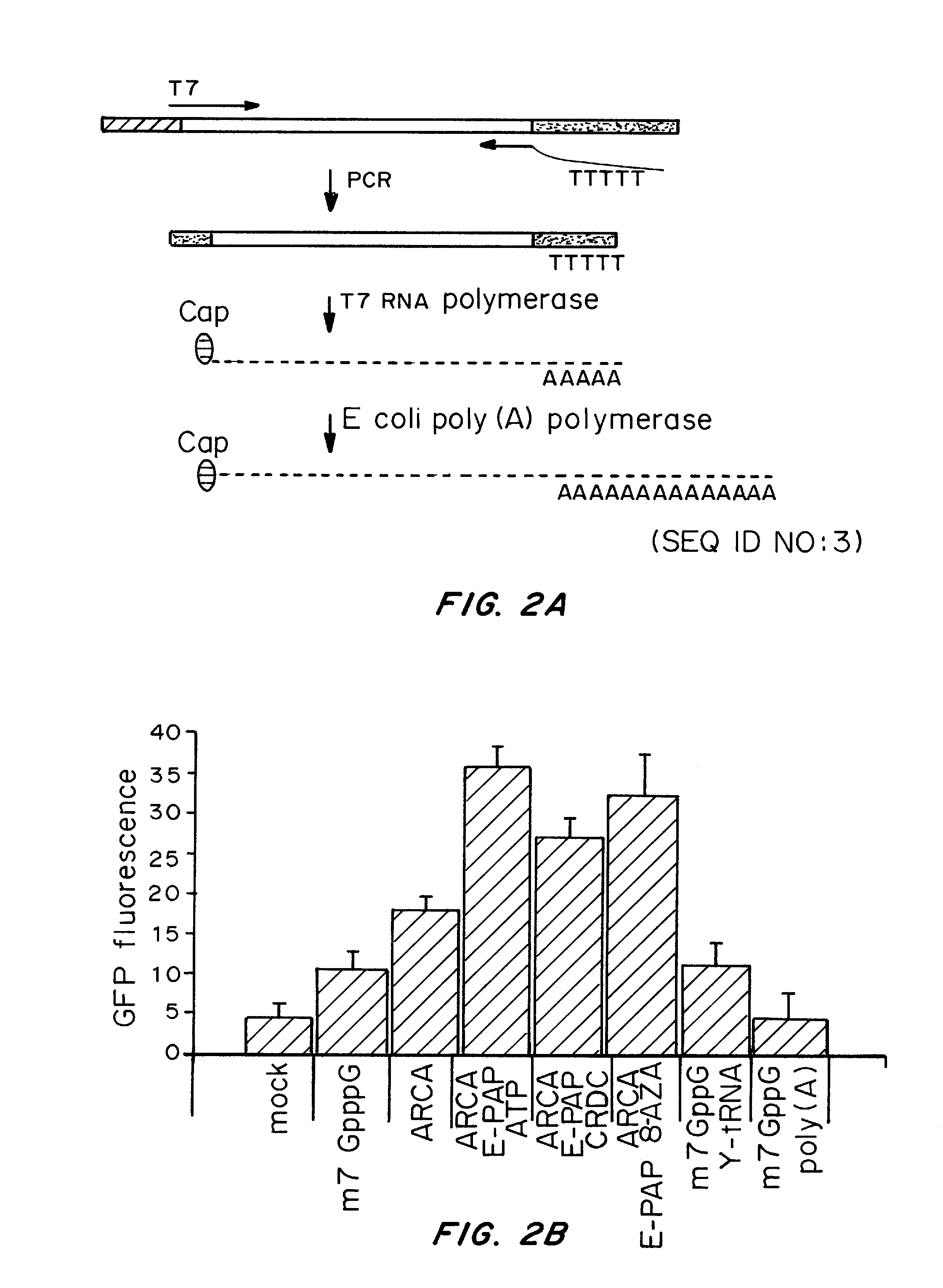
A method of mRNA production for use in transfection is provided, that involves in vitro transcription of PCR generated templates with specially designed primers, followed by polyA addition, to produce a construct containing 3′ and 5′ untranslated sequence (“UTR”), a 5′ cap and / or Internal Ribosome Entry Site (IRES), the gene to be expressed, and a polyA tail, typically 50-2000 bases in length. This RNA can efficiently transfect different kinds of cells. This approach results in increased efficiency (fidelity and productivity) of mRNA synthesis and is less time consuming because it does not require cloning, and also consequently eliminates the unwanted errors and effects related to RNA made on DNA templates obtained with cloning techniques. The results of transfection of RNAs demonstrate that RNA transfection can be very effective in cells that are exceedingly difficult to transfect efficiently with DNA constructs. Further, the levels of gene expression following mRNA transfection are consistent from cell to cell in an experiment and these levels can be controlled over a wide range simply by changing the amount of mRNA that is transfected, and without obvious cytotoxic effects due to the levels of RNA per se. Due to high efficiency the cells can be simultaneously transfected with multiple genetic constructs. The method can be used to deliver genes into cells not- or only poorly transfectable for DNA, in vitro and in vivo.
Owner:YALE UNIV
Nucleic acid transfer vector for the introduction of nucleic acid into the DNA of a cell
Owner:RGT UNIV OF MINNESOTA
Synthetic internal ribosome entry sites and methods of identifying same
InactiveUS7468275B2High expressionStrong therapeutic activitySugar derivativesHydrolasesInternal ribosome entry siteOligonucleotide
Synthetic and isolated translational regulatory elements, including oligonucleotides that have translational enhancing activity, internal ribosome entry site (IRES) activity, or translational inhibitory activity, and multimers of such translational regulatory elements are provided. In addition, compositions that include such translational regulatory elements are provided, as are methods of using the translational regulatory elements.
Owner:THE SCRIPPS RES INST
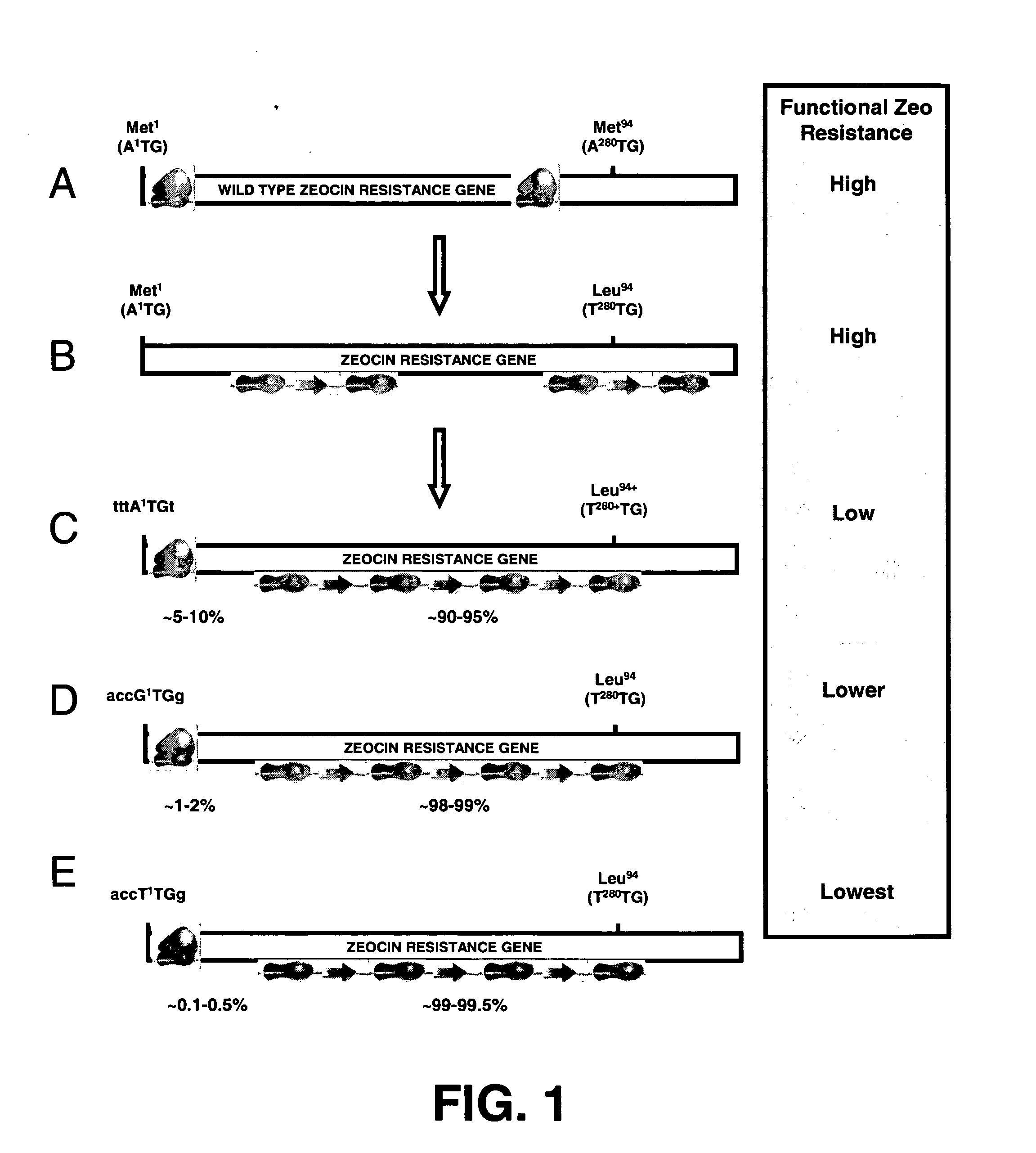
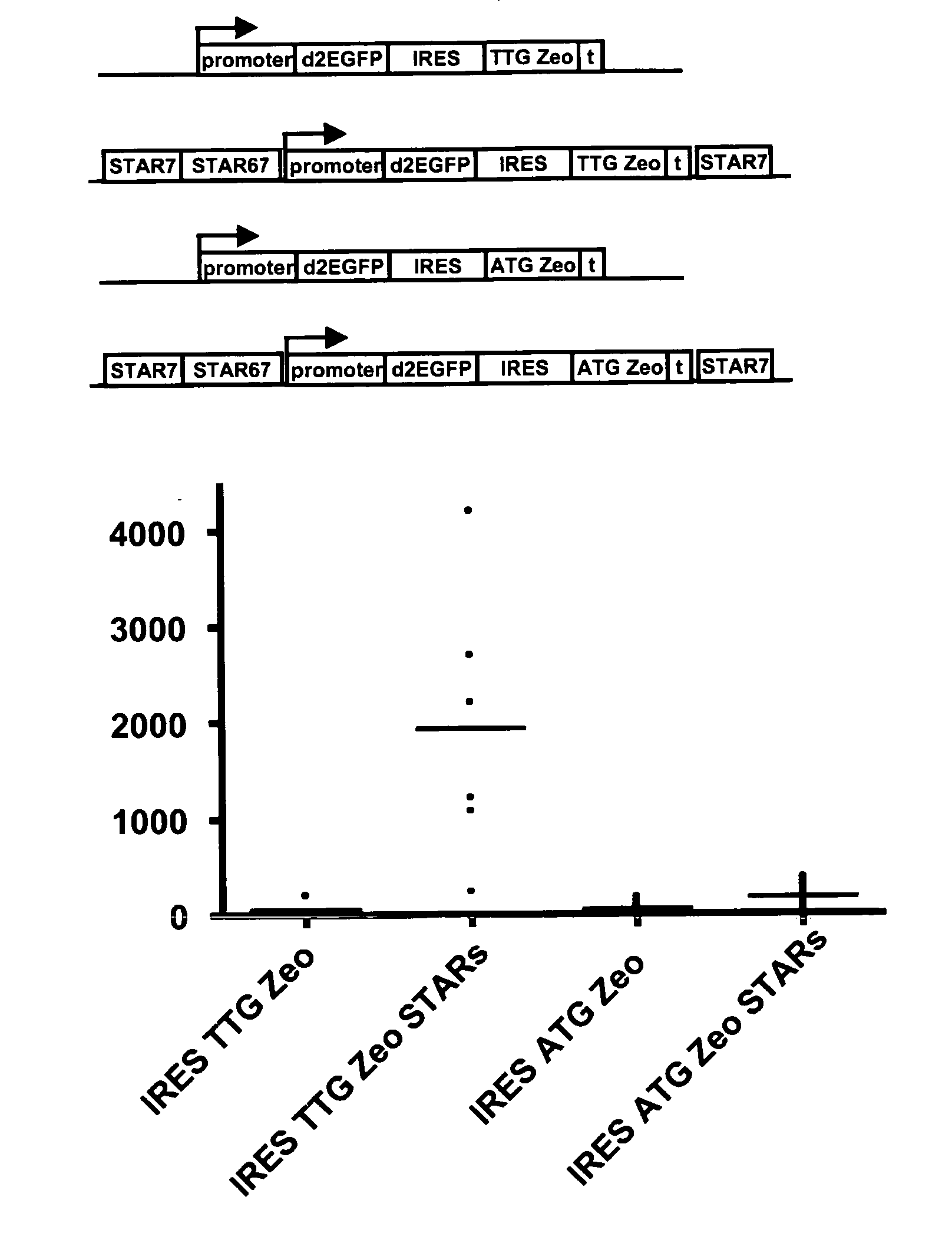
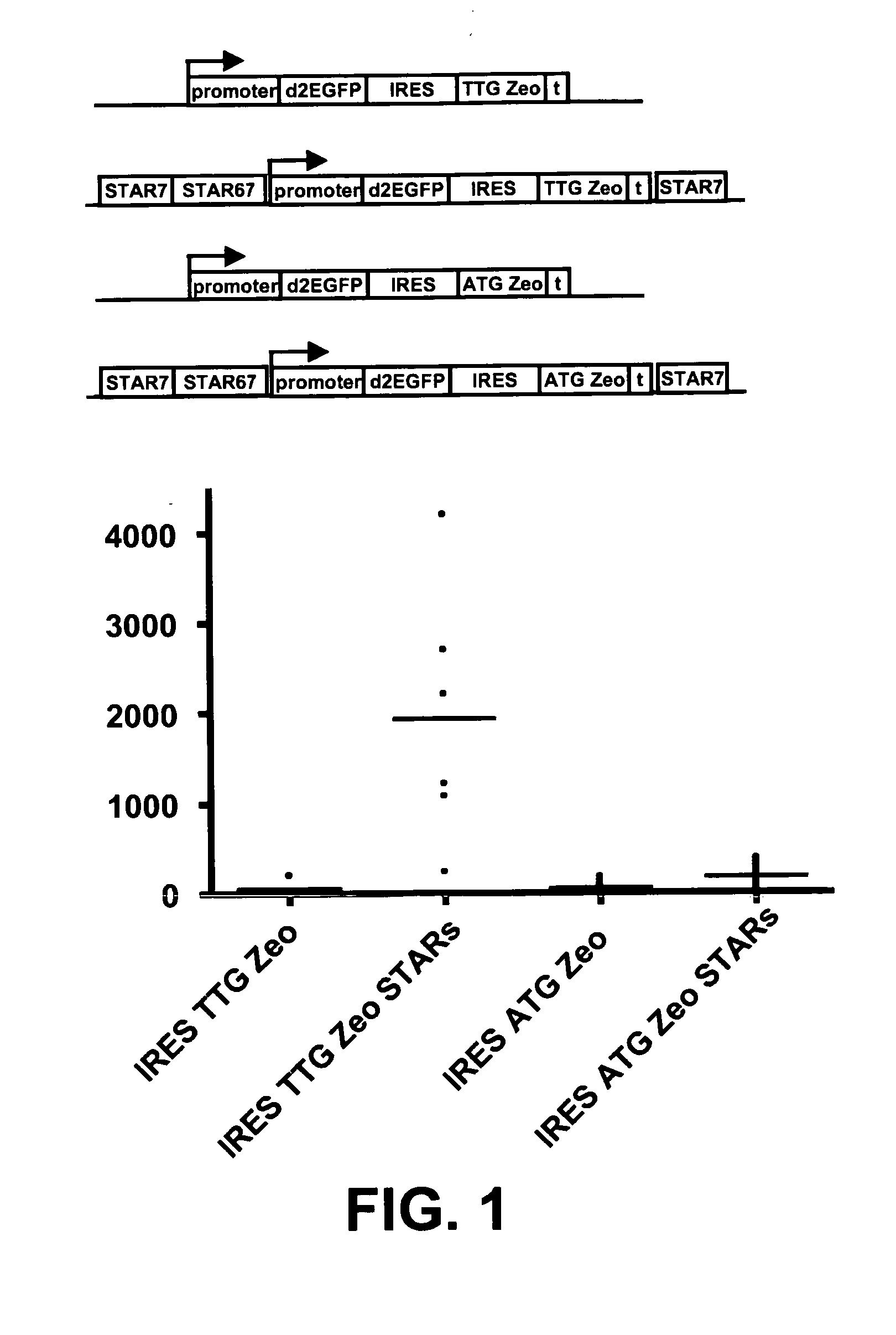
Selection of host cells expressing protein at high levels
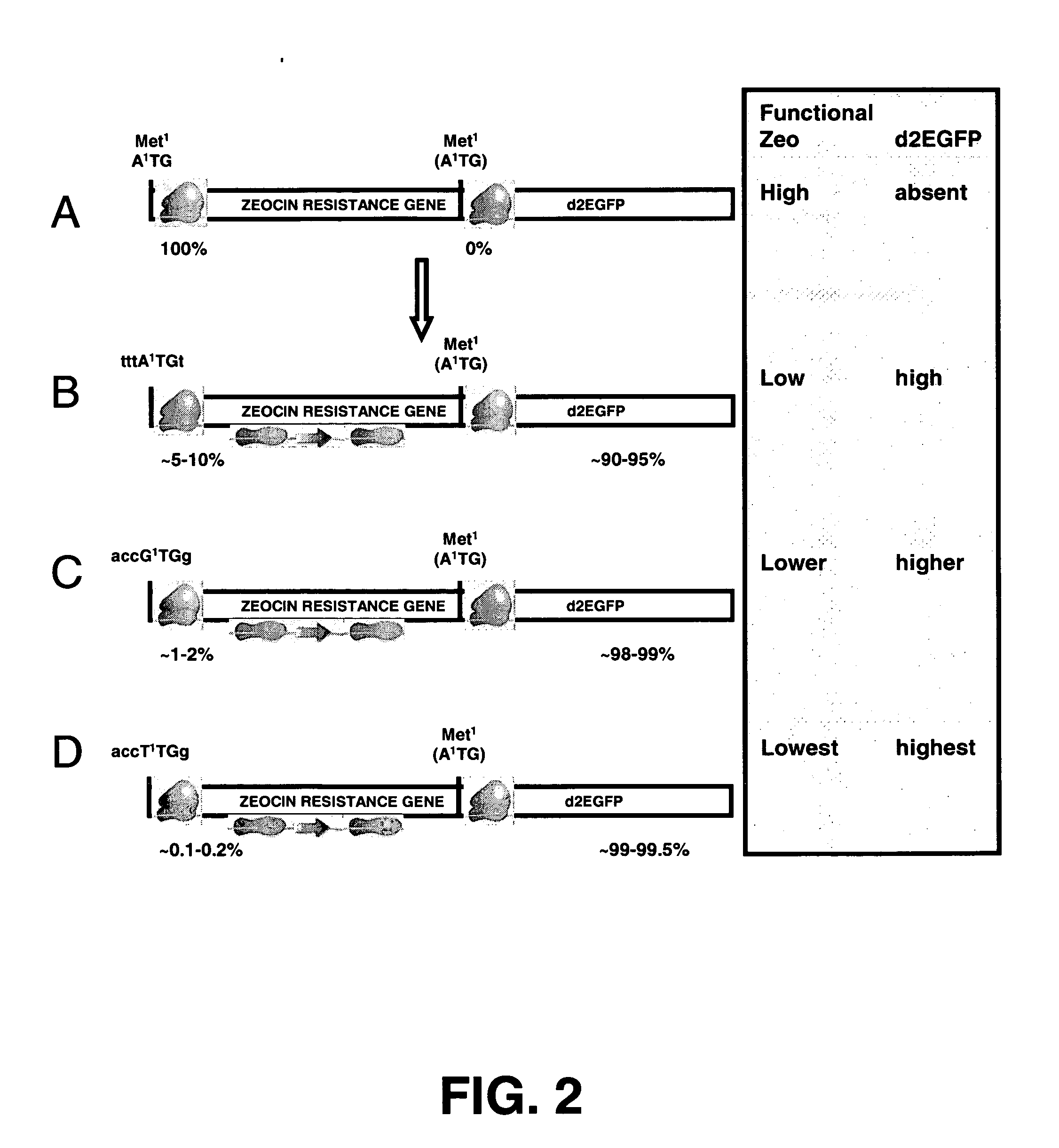
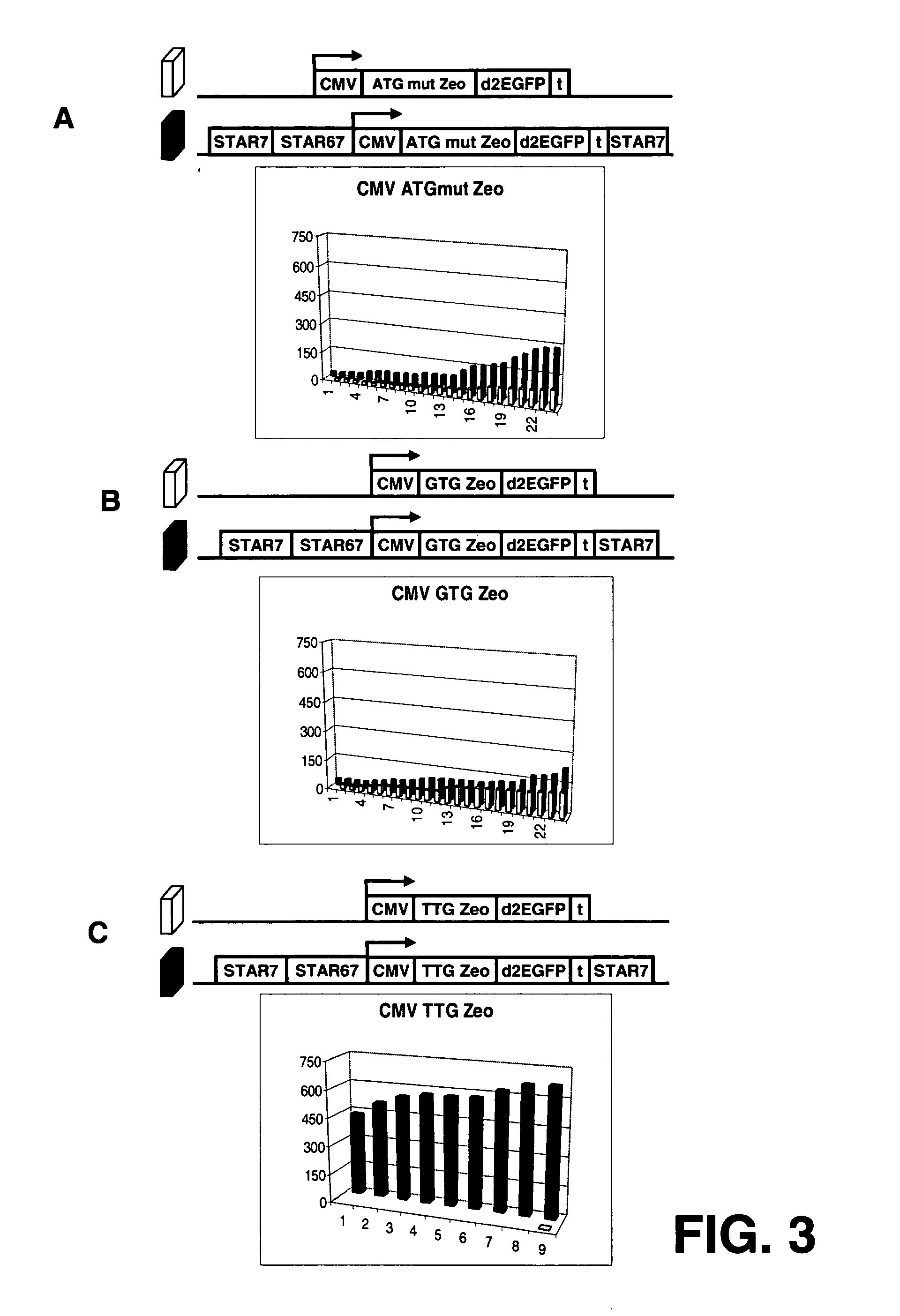
InactiveUS20060141577A1Decreases translation initiation efficiencyVectorsCell receptors/surface-antigens/surface-determinantsStart codonA-DNA
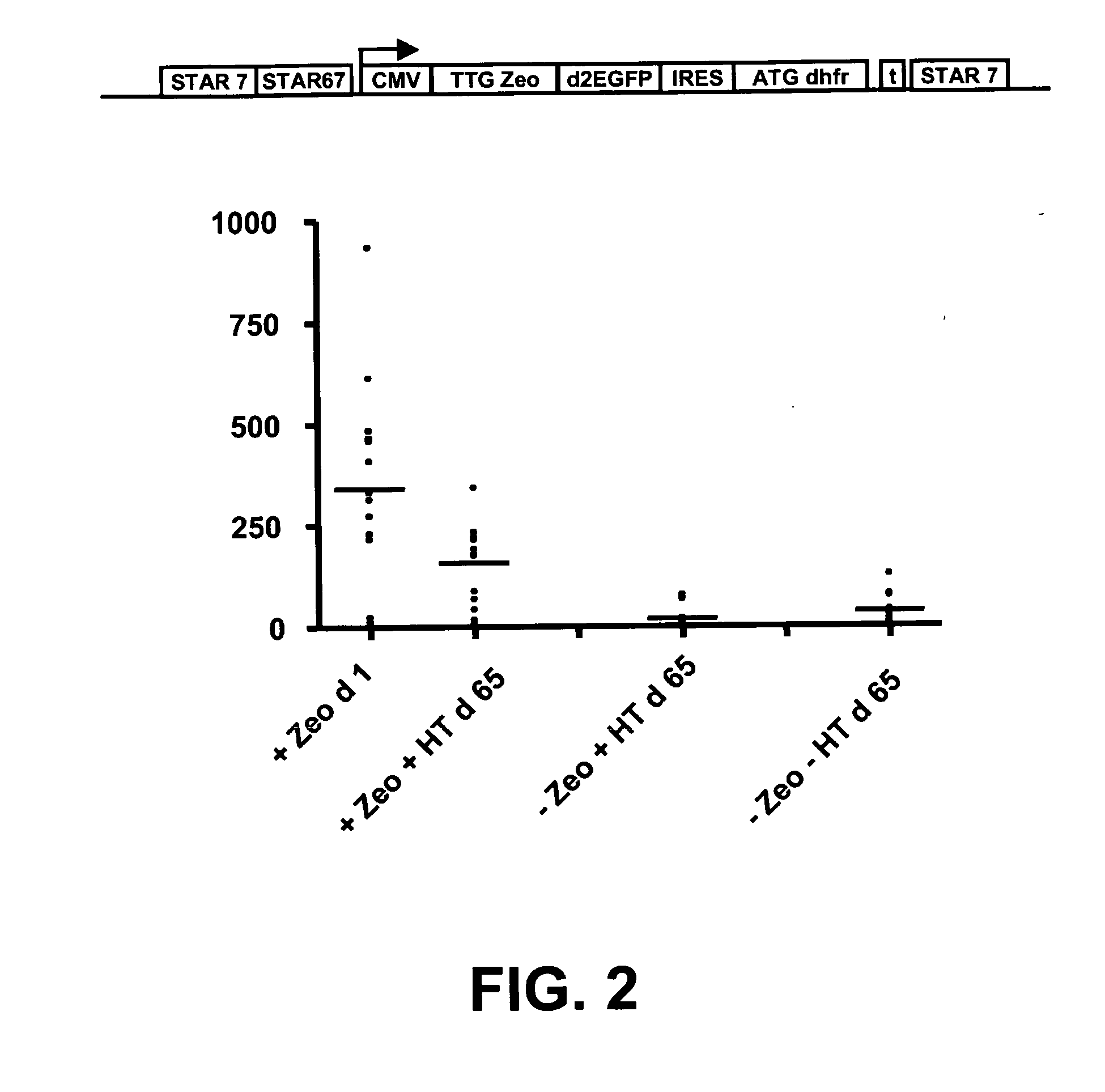
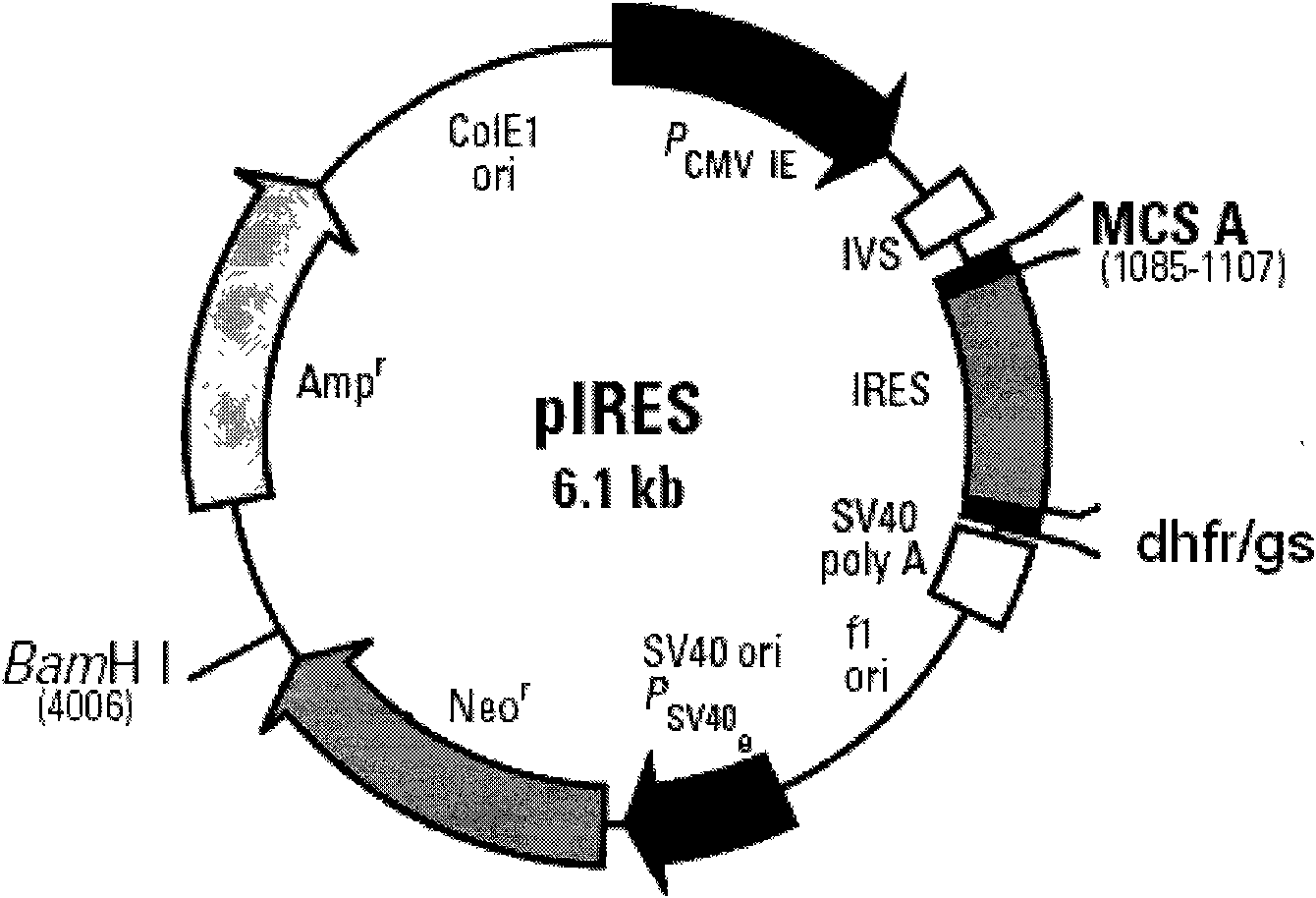
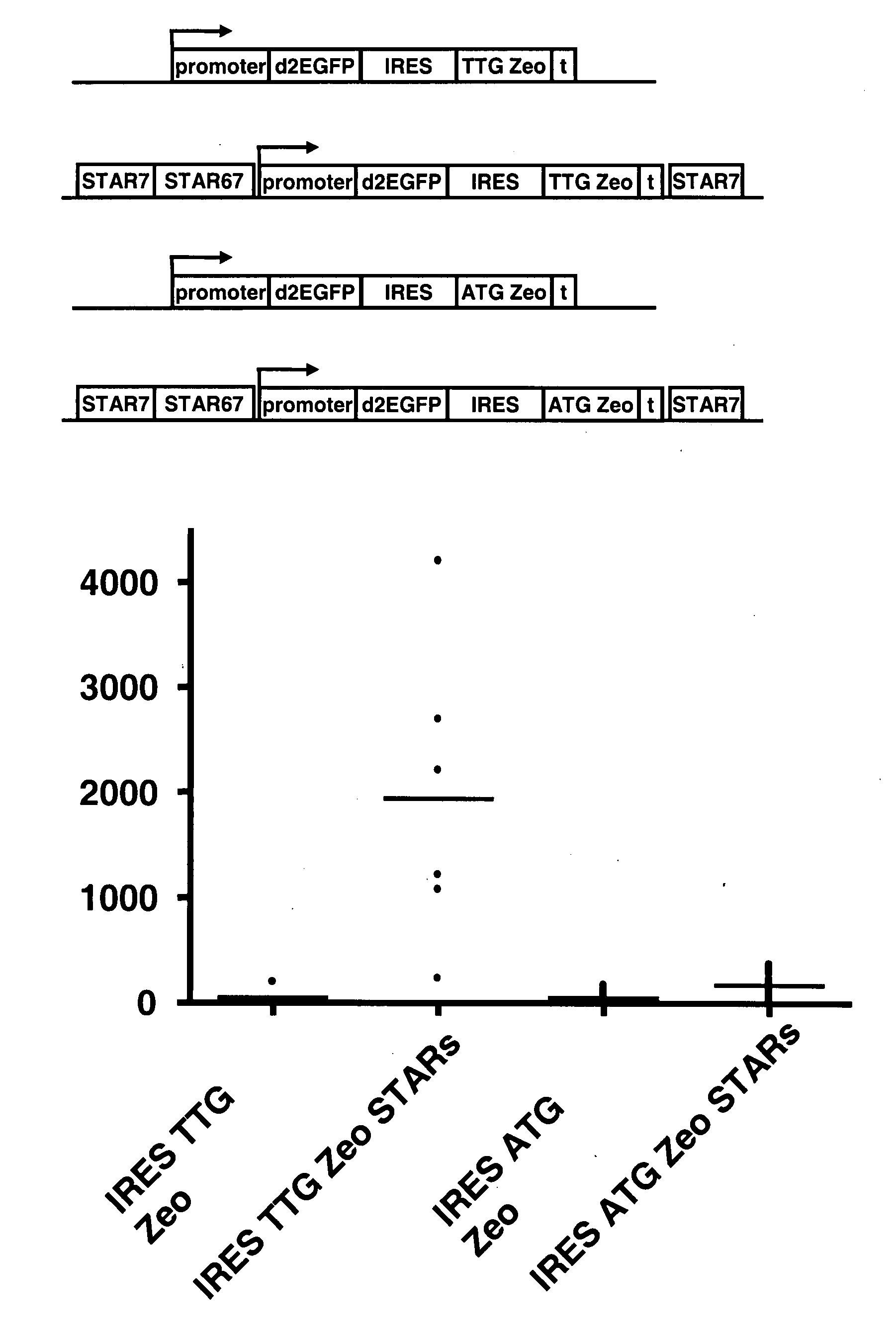
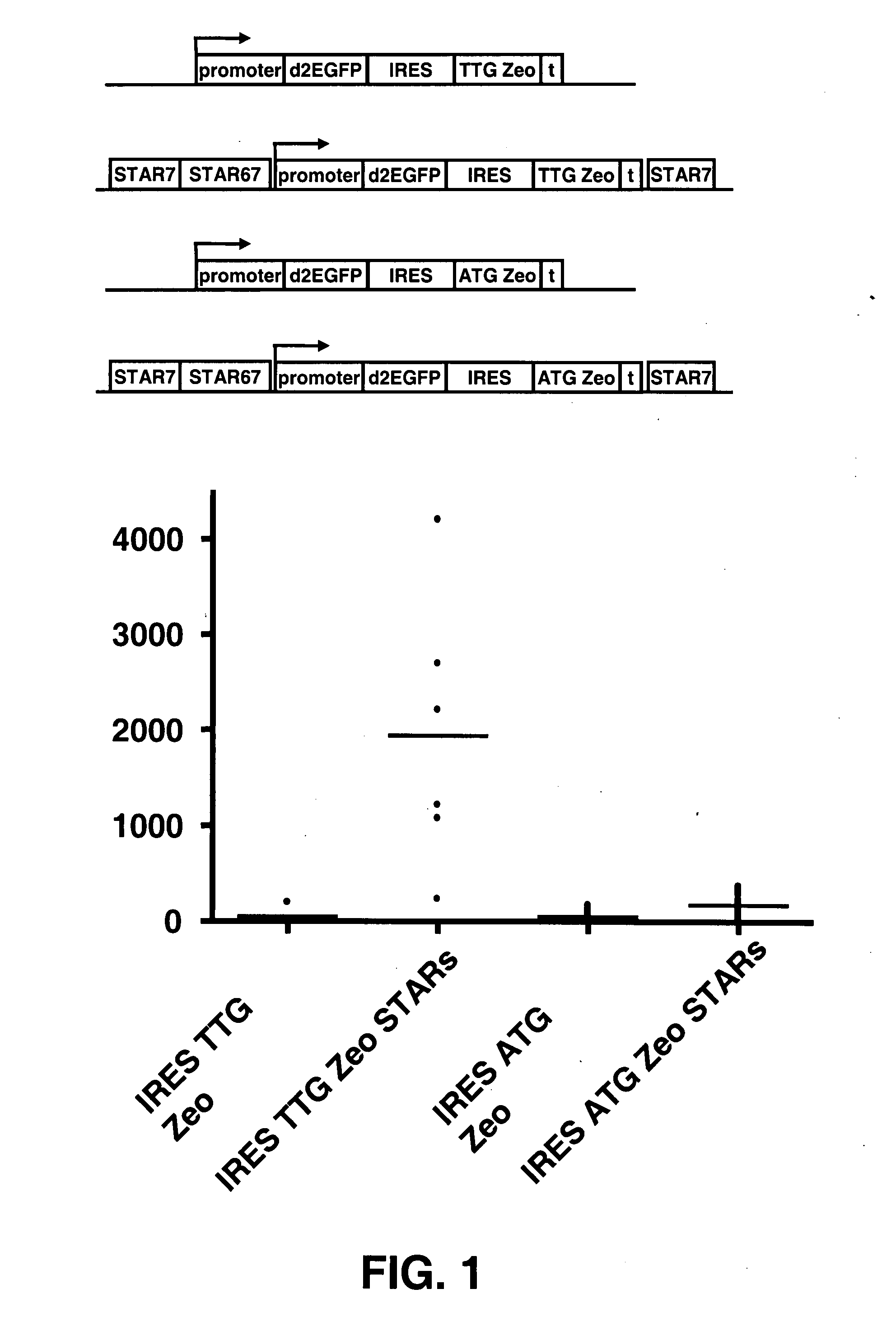
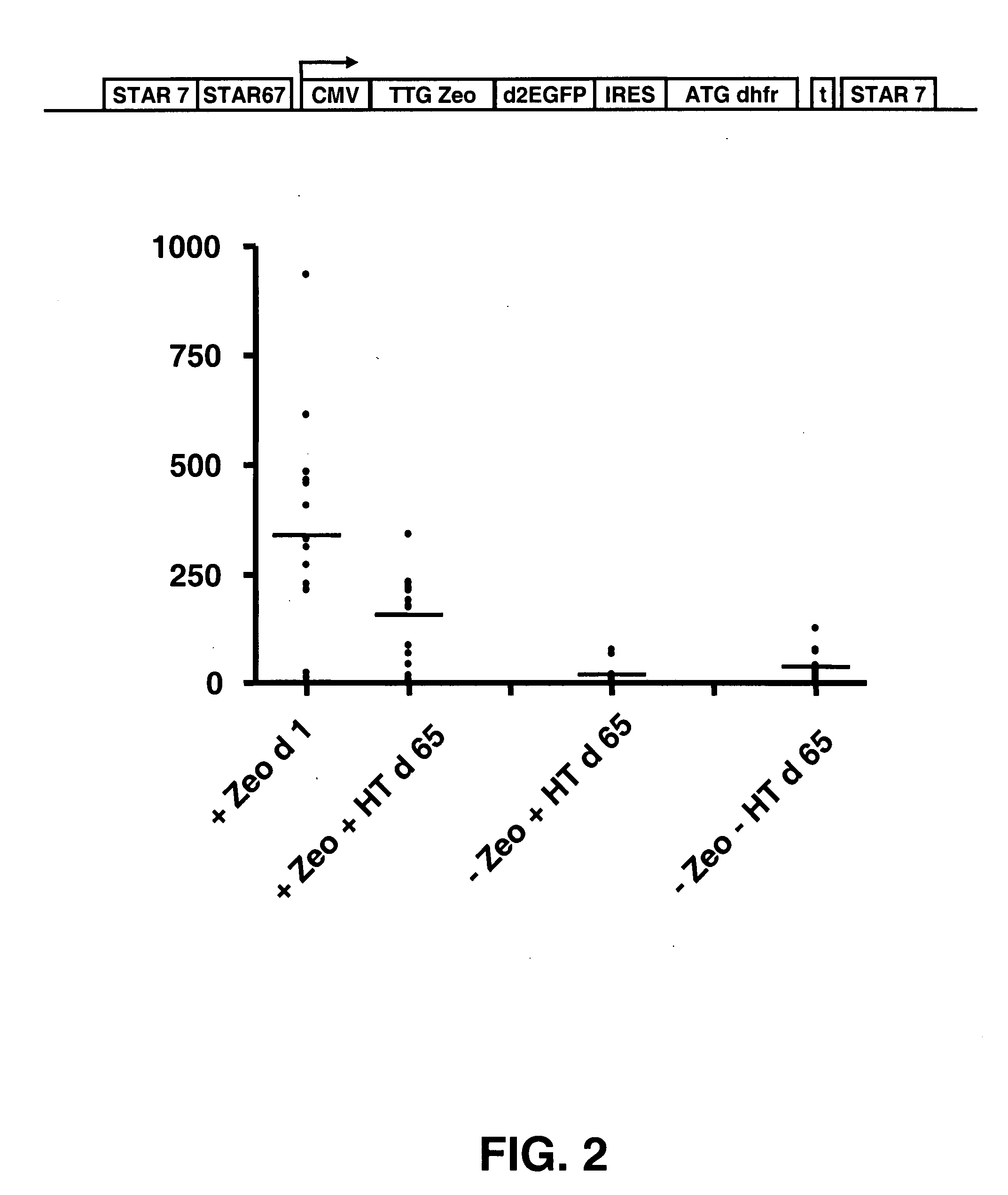
The invention provides a DNA molecule comprising a multicistronic transcription unit coding for i) a polypeptide of interest, and for ii) a selectable marker polypeptide functional in a eukaryotic host cell, wherein the polypeptide of interest has a translation initiation sequence separate from that of the selectable marker polypeptide, and wherein the coding sequence for the polypeptide of interest is upstream from the coding sequence for the selectable marker polypeptide in said multicistronic transcription unit, and wherein an internal ribosome entry site (IRES) is present downstream from the coding sequence for the polypeptide of interest and upstream from the coding sequence for the selectable marker polypeptide, and wherein the nucleic acid sequence coding for the selectable marker polypeptide in the coding strand comprises a GTG or a TTG startcodon. The invention also provides methods for obtaining host cells expressing a polypeptide of interest, said host cells comprising the DNA molecules of the invention. The invention further provides the production of polypeptides of interest, comprising culturing host cells comprising the DNA molecules according to the invention.
Owner:CHROMAGENICS BV
Creation of artificial internal ribosome entry site (ires) elements
A method of creating a nucleic acid sequence having an adenine-rich nucleic acid block of at least 25 nucleotides in length is provided, whereby said nucleic acid sequence is capable of causing translation by internal ribosome entry (IRES element). Said adenine-rich nucleic acid block comprises from 40 to 100 mol-% adenine.
Owner:ICON GENETICS
Replication-defective arenavirus vectors
ActiveUS20100297172A1Enhance protein expressionHigh expressionAntibacterial agentsSsRNA viruses negative-senseAntigenDisease
The invention relates to an infectious arenavirus particle that is engineered to contain a genome with the ability to amplify and express its genetic information in infected cells but unable to produce further infectious progeny particles in normal, not genetically engineered cells. One or more of the four arenavirus open reading frames glycoprotein (GP), nucleoprotein (NP), matrix protein Z and RNA-dependent RNA polymerase L are removed or mutated to prevent replication in normal cells but still allowing gene expression in arenavirus vector-infected cells, and foreign genes coding for an antigen or other protein of interest or nucleic acids modulating host gene expression are expressed under control of the arenavirus promoters, internal ribosome entry sites or under control of regulatory elements that can be read by the viral RNA-dependent RNA polymerase, cellular RNA polymerase I, RNA polymerase II or RNA polymerase III. The modified arenaviruses are useful as vaccines and therapeutic agents for a variety of diseases.
Owner:UNIV ZURICH
Internal ribosome entry sites for recombinant protein expression
InactiveUS20050112095A1Efficiently translatedConstant ratioBiocideSsRNA viruses positive-senseInternal ribosome entry siteEnterovirus 71
The invention describes compositions and methods for recombinant protein expression in a wide range of cell types, including mammalian, insect, and bacterial cells. The compositions comprise a viral IRES sequence selected from enterovirus 71 (EV71), hepatitis C virus (HCV), or encephalomyocarditis virus (EMCV), or a variant or fragment thereof, or alternatively, a homolog of a viral IRES selected from EV71, HCV, or EMCV, or a variant or fragment thereof. Methods of using the compositions are also described.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
Crustacean Expression Vector
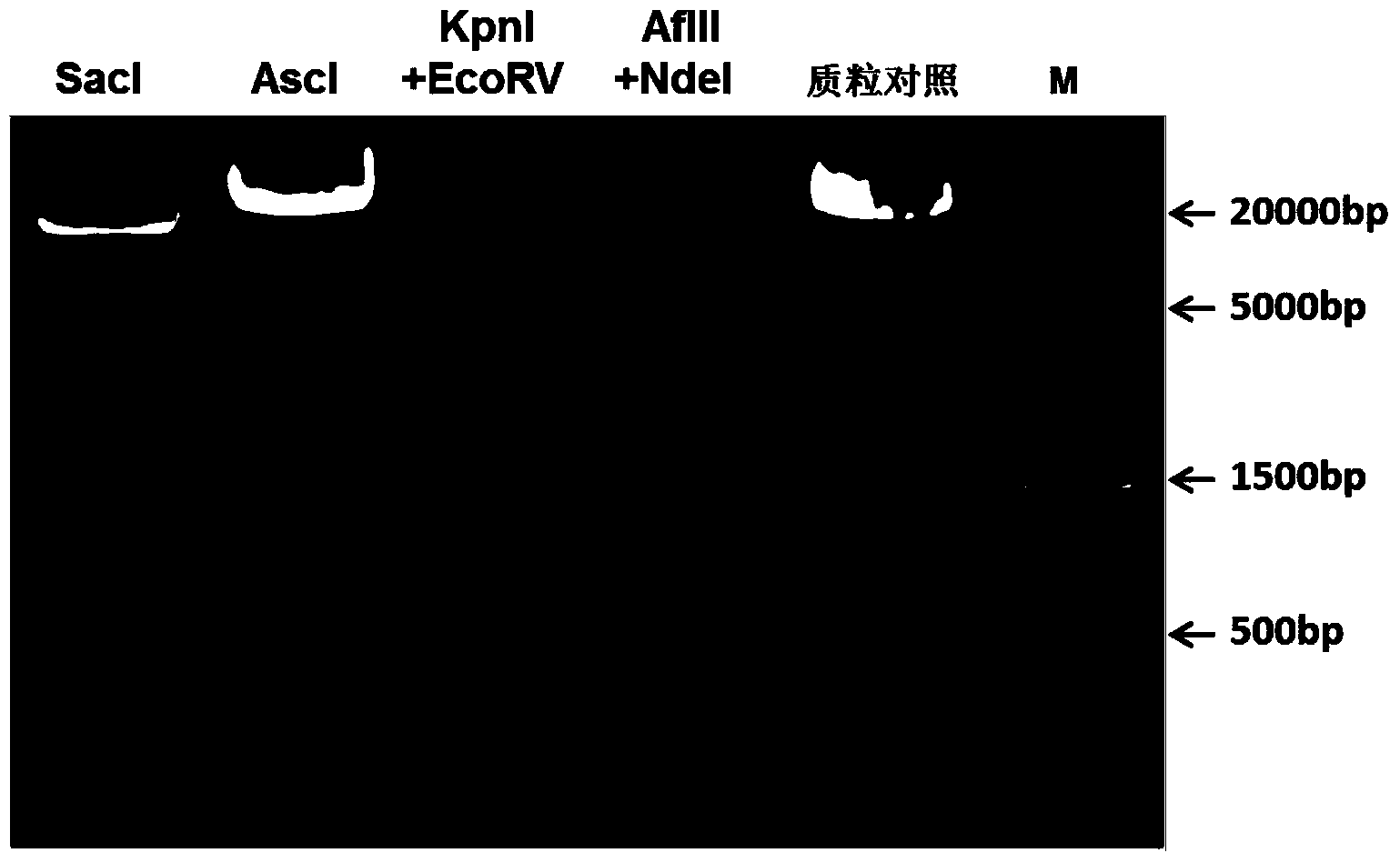
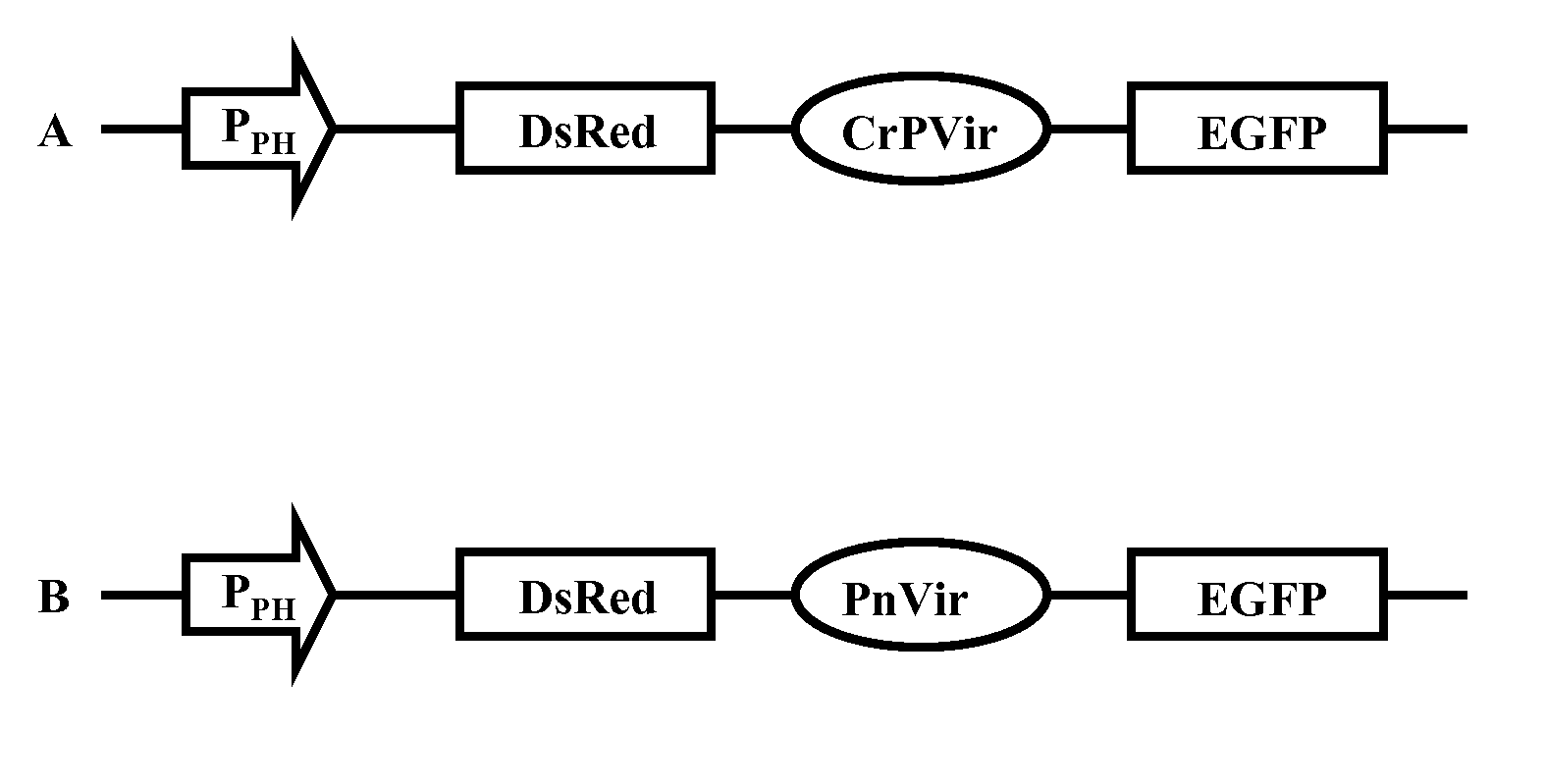
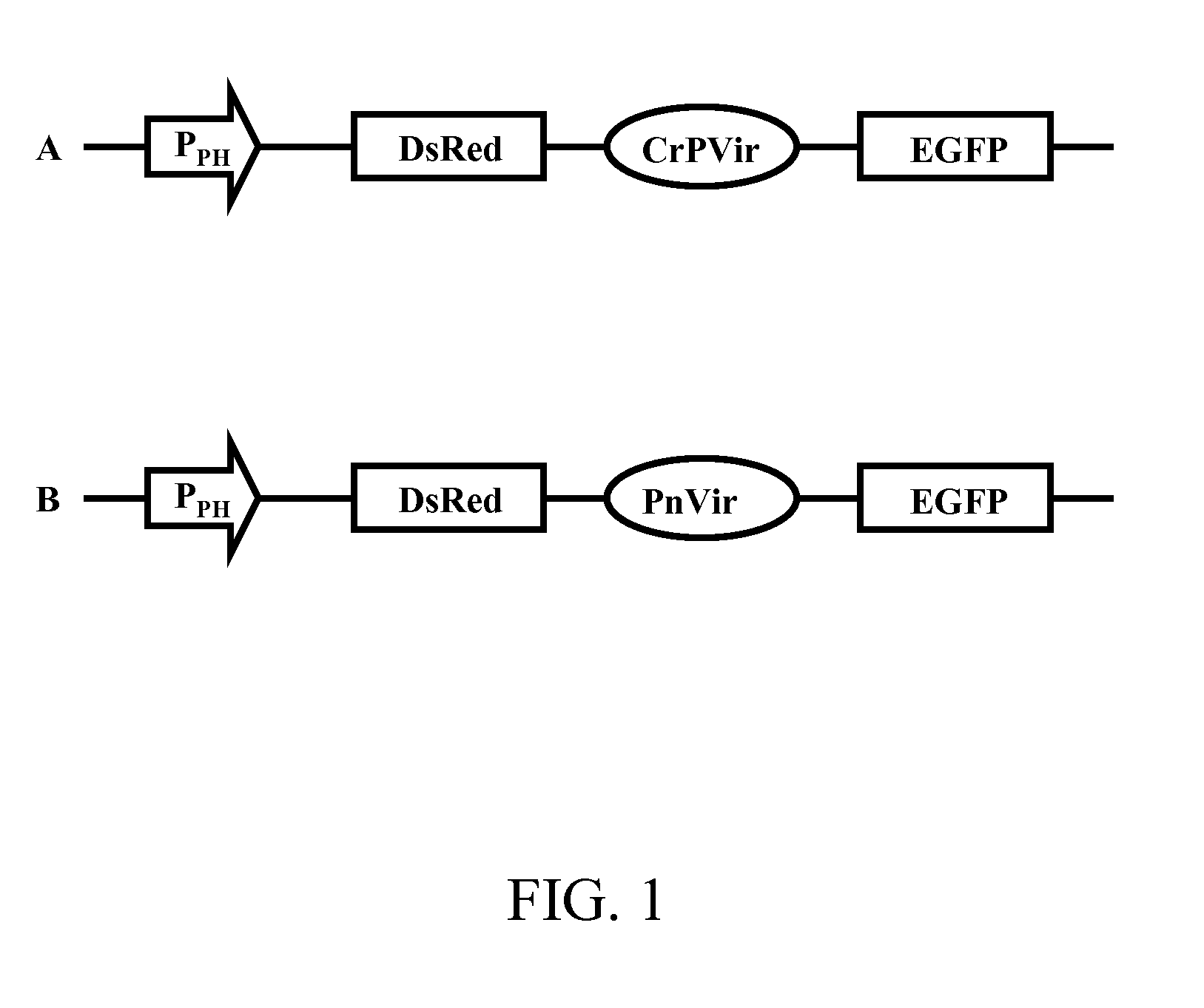
Methods and constructs for genetic manipulation of one or more of shrimp, shellfish, mollusks, and fish are disclosed. The nucleic acid construct includes a promoter and an internal ribosome entry site of an insect picomavirus, such as a cricket paralysis-like picomavirus. One or more open reading frames can be operably associated with one or both of the promoter and the internal ribosome entry site, and one or more proteins or protein subunits can be expressed upon introduction of the construct into a host cell, such as into a shrimp. Method for producing immortalized crustacean cell lines using enhancer elements derived from shrimp and / or shrimp viruses are also described.
Owner:INTERVET INC
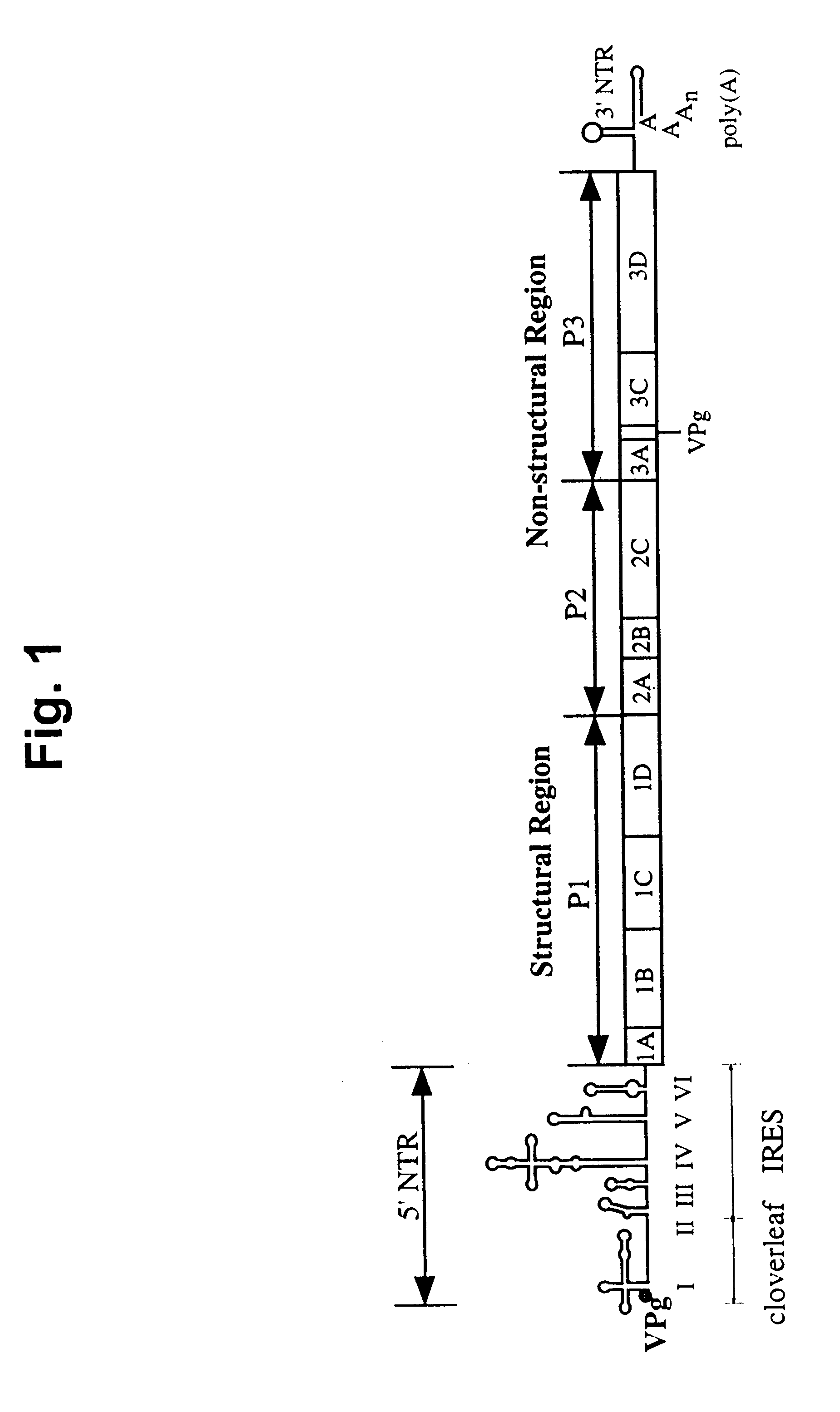
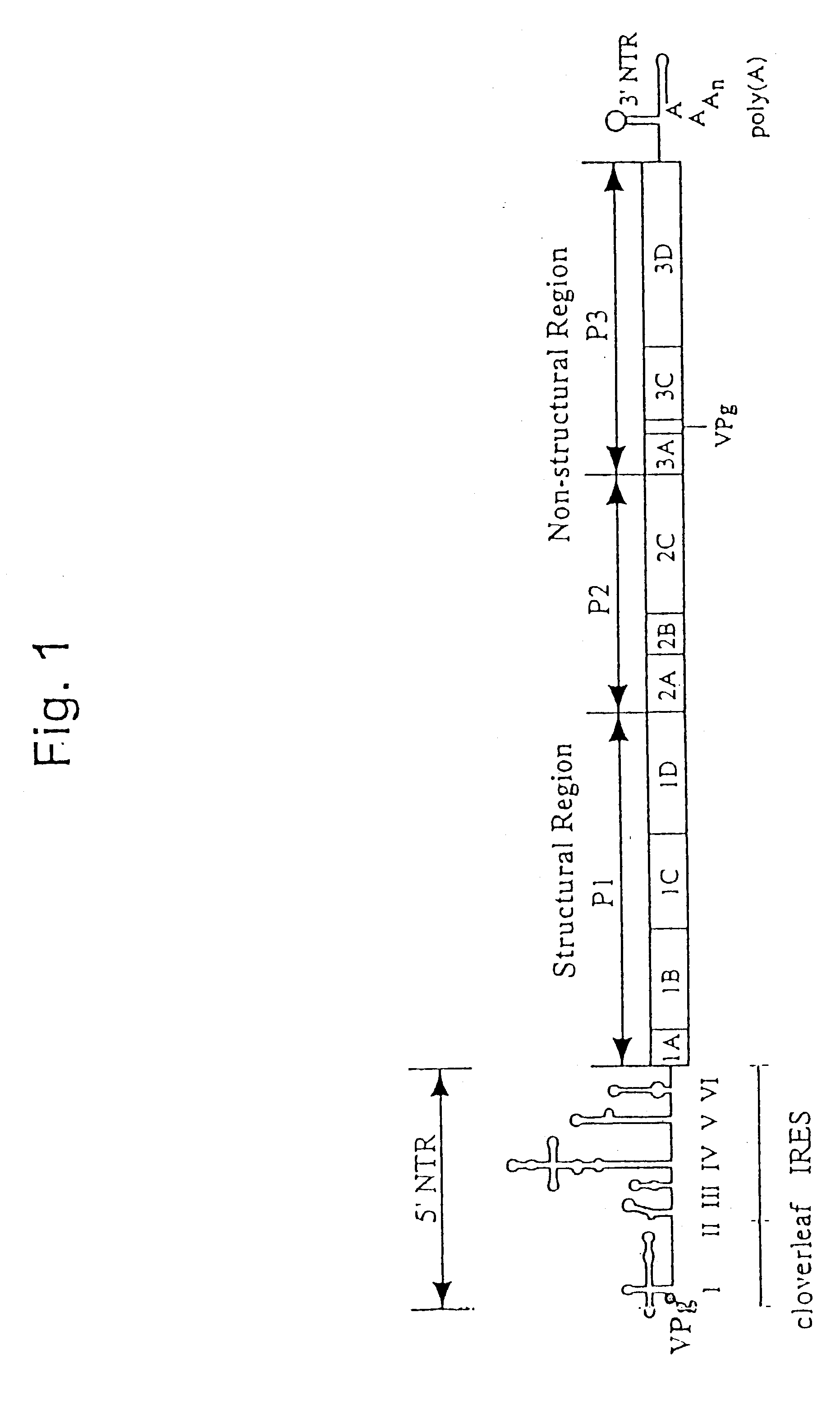
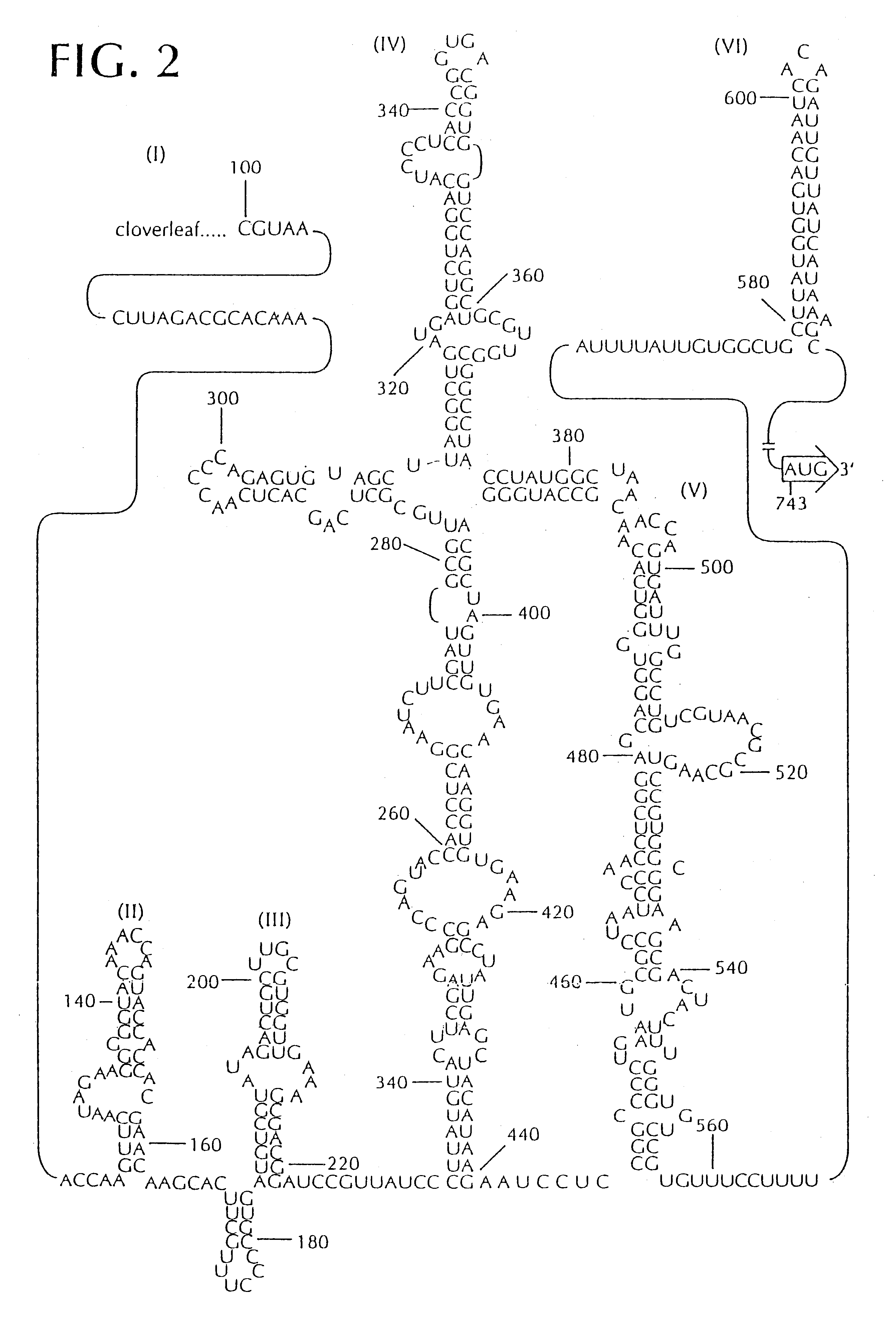
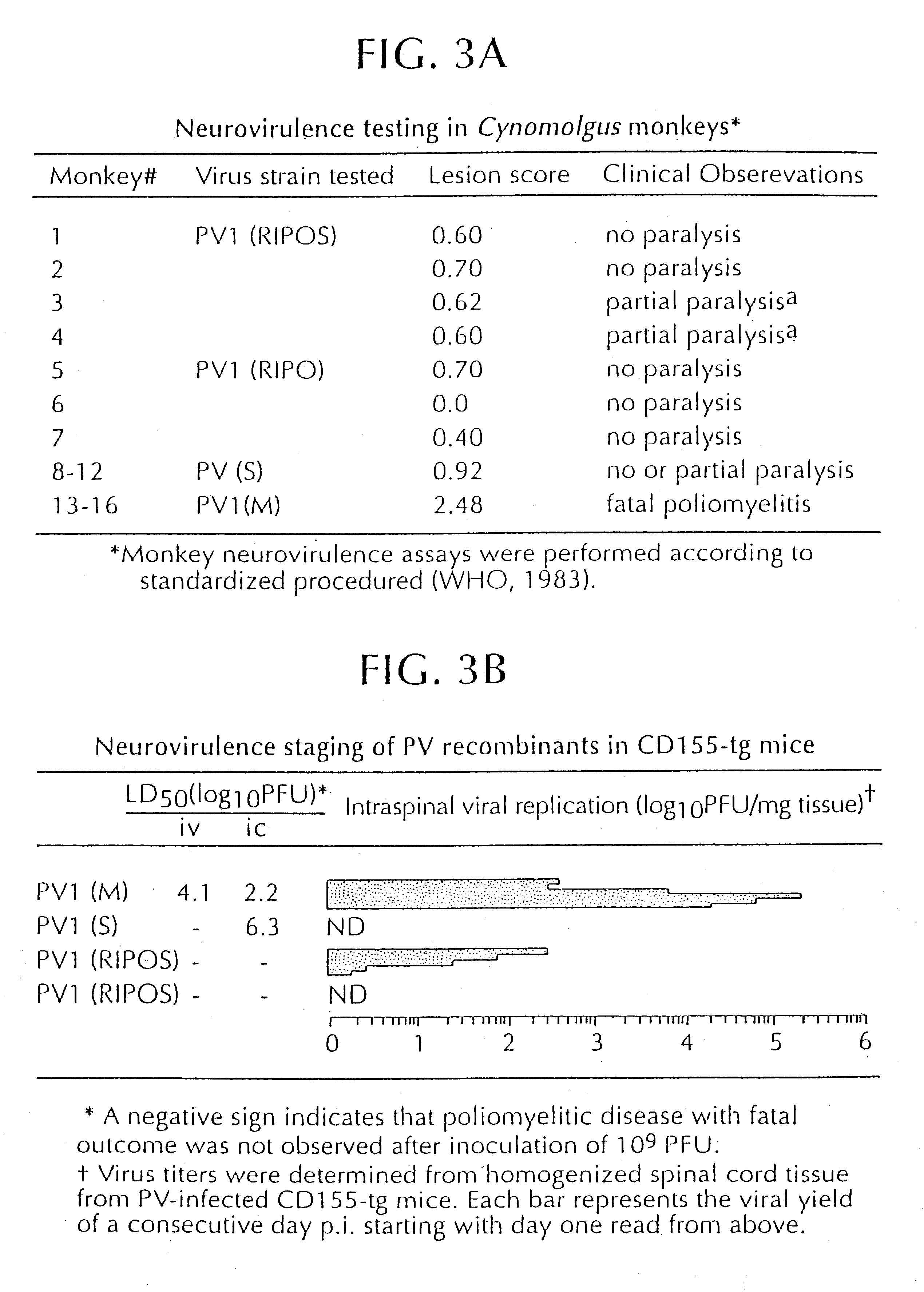
Recombinant poliovirus for the treatment of cancer
The present invention is directed to non-pathogenic, oncolytic, recombinant polioviruses for the treatment of various forms of malignant tumors. The recombinant polioviruses of the invention are those in which the internal ribosomal entry site (IRES) of the wild type poliovirus was exchanged with the IRES of other picornaviruses, and optionally P1, P3 or the 3'NTR thereof was exchanged with that of poliovirus Sabin type. More particularly, the present invention is directed to the administration of the non-pathogenic, oncolytic, recombinant poliovirus to the tumor directly, intrathecally or intravenously to cause tumor necrosis. The method of the present invention is particularly useful for the treatment of malignant tumors in various organs, such as: breast, colon, bronchial passage, epithelial lining of the gastrointestinal, upper respiratory and genito-urinary tracts, liver, prostate and the brain. Astounding remissions in experimental animals have been demonstrated for the treatment of malignant glioblastoma multiforme, an almost universally fatal neoplasm of the central nervous system.
Owner:NEW YORK UNIV OF RES FOUND OF THE
Replication-defective arenavirus vectors
Owner:UNIV ZURICH
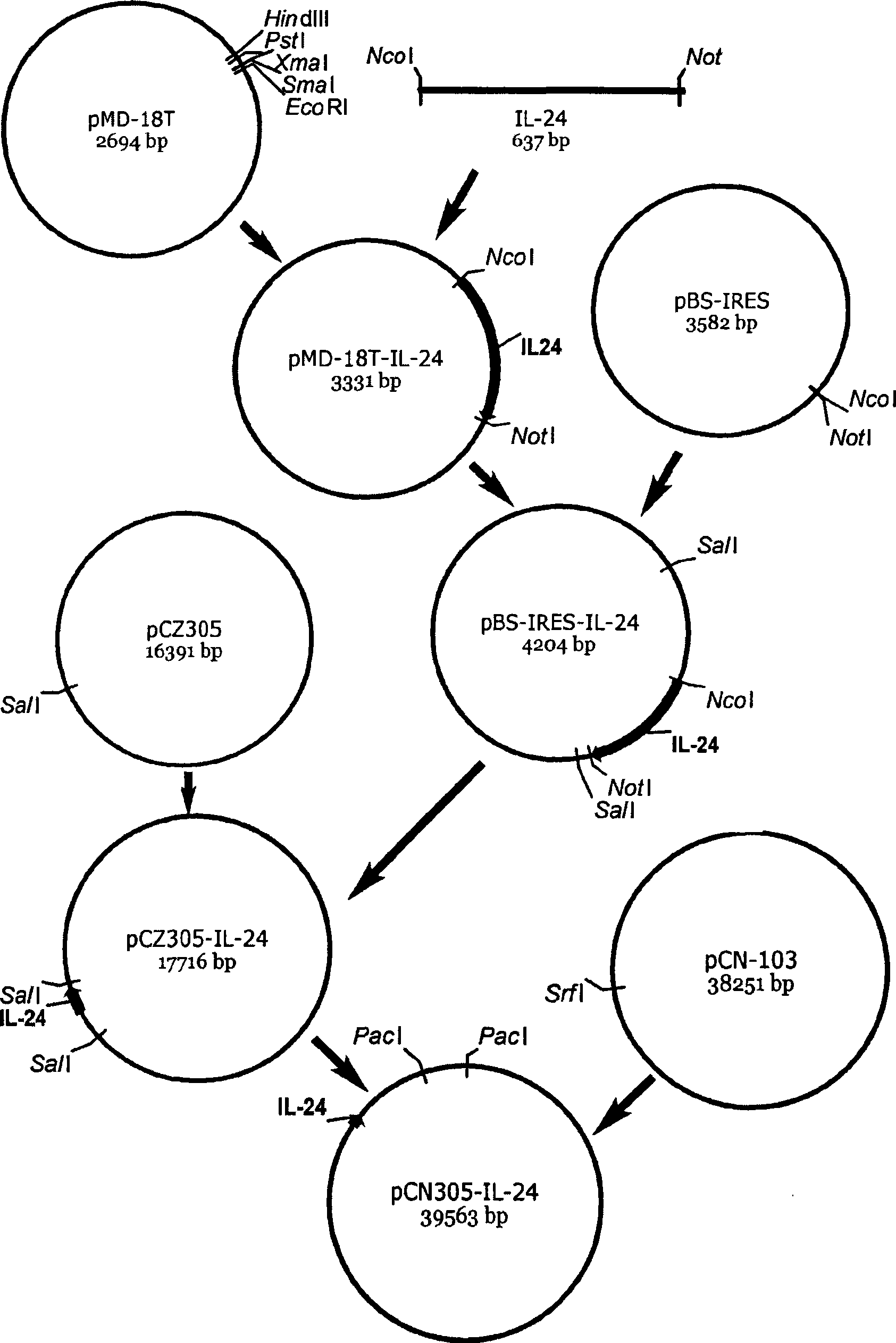
Late promoter targeting regulation oncolytic adenovirus pCN305 vector and construction method and application thereof
InactiveCN101381742AIncrease lethalityEffective combinationPeptide/protein ingredientsGenetic material ingredientsOncolytic adenovirusMultiple cloning site
The invention discloses a late promoter targeting adjustment and control oncolytic adenovirus pCN305 vector, a construction method and application thereof. The vector is established on a pCN103 vector with targeting property; all exogenous genes for killing and inhibiting various cancers, such as IL-24, TRAIL, SOCS3, cpp-SOCS3, SOCS1, cpp-SOCS1and so on, are adjusted, controlled and expressed by late promoters of viruses through induction of internal ribosome into IRES sites and polyclonal sites; and the pCN305 vector has good anticancer effect through experiment in vivo and vitro. All double-target replicating oncolytic adenovirus established by application of the pCN305 vector, such as AdCN305-IL-24, AdCN305-TRAIL, AdCN305-SOCS3, AdCN305-cpp-SOCS3, AdCN305-SOCS1, AdCN305-cpp-SOCS1 and so on, can be used for treating various tumors. Moreover, the invention can develop a novel virus-gene anticancer medicine for effectively treating the tumors.
Owner:ZHEJIANG SCI-TECH UNIV
Recombinant poliovirus for the treatment of cancer
The present invention is directed to non-pathogenic, oncolytic, recombinant polioviruses for the treatment of various forms of malignant tumors. The recombinant polioviruses of the invention are those in which the internal ribosomal entry site (IRES) of the wild type poliovirus was exchanged with the IRES of other picornaviruses, and optionally P1, P3 or the 3'NTR thereof was exchanged with that of poliovirus Sabin type. More particularly, the present invention is directed to the administration of the non-pathogenic, oncolytic, recombinant poliovirus to the tumor directly, intrathecally or intravenously to cause tumor necrosis. The method of the present invention is particularly useful for the treatment of malignant tumors in various organs, such as: breast, colon, bronchial passage, epithelial lining of the gastrointestinal, upper respiratory and genito-urinary tracts, liver, prostate and the brain. Astounding remissions in experimental animals have been demonstrated for the treatment of malignant glioblastoma multiforme, an almost universally fatal neoplasm of the central nervous system.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
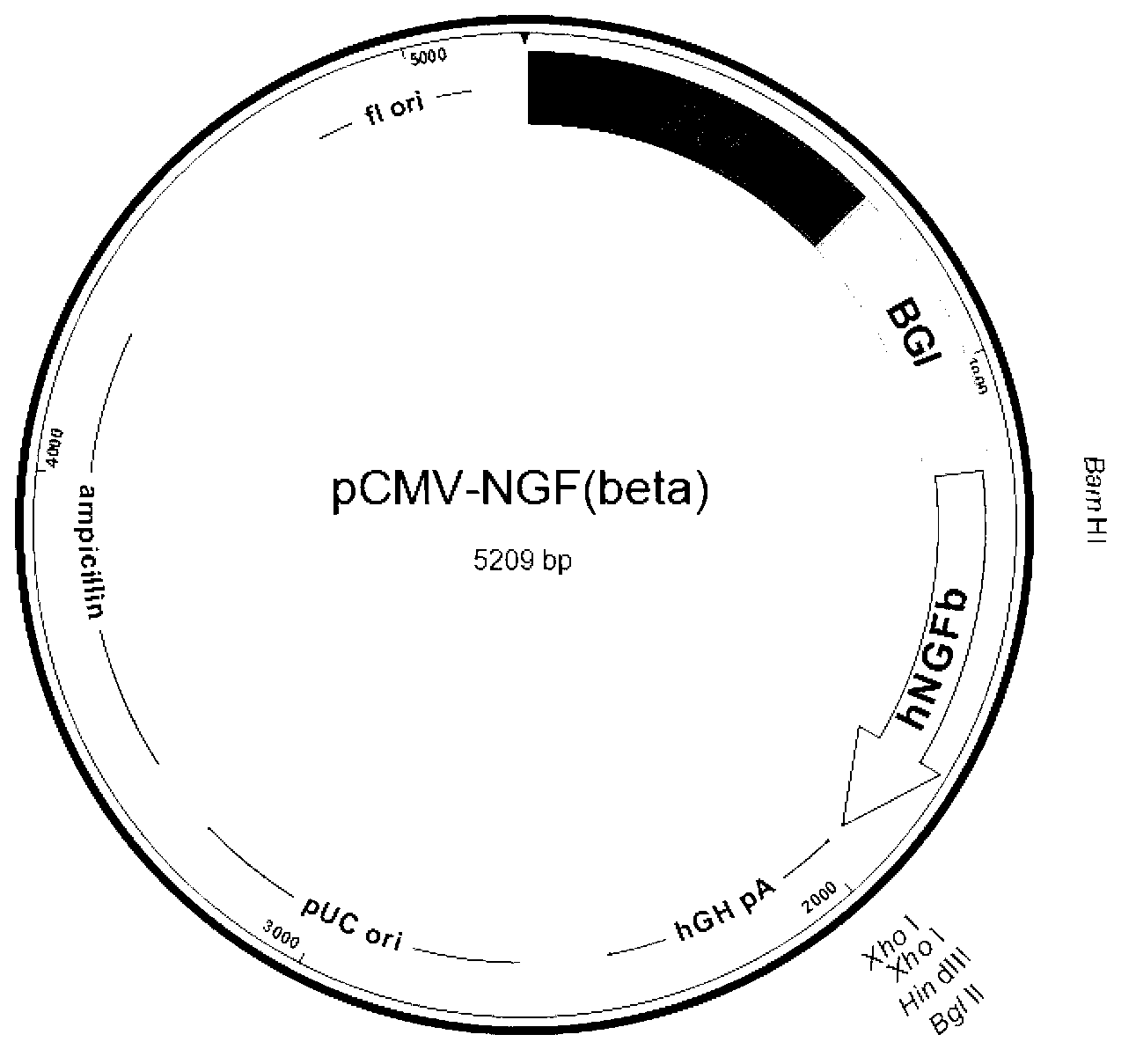
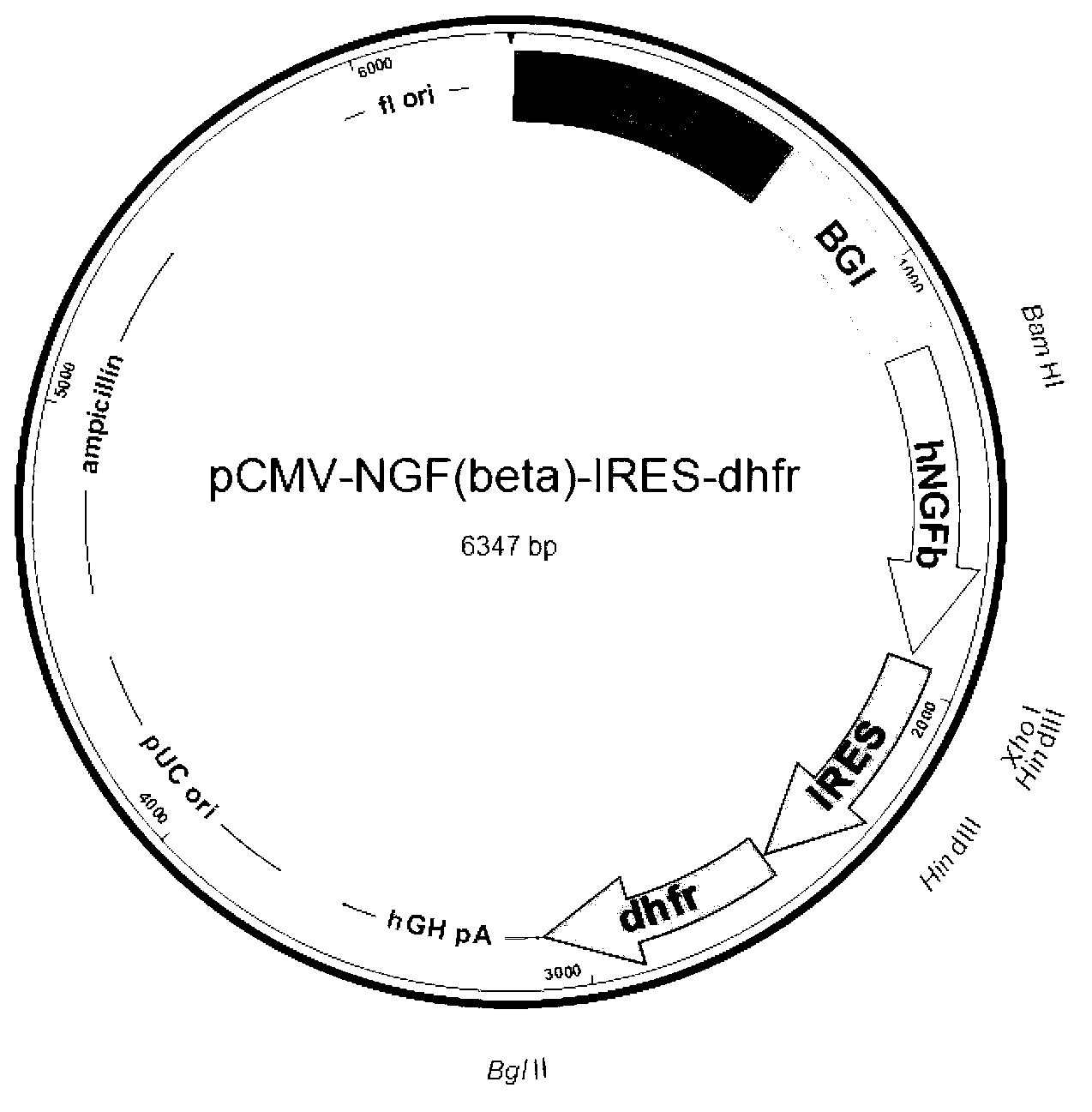
Recombinant expression vector for human beta-NGF and recombinant cell strain containing same
ActiveCN103074374AUniform drug resistanceUniform expressionAnimals/human peptidesVector-based foreign material introductionBiotechnologyIntein
The invention discloses a recombinant expression vector for stably and efficiently expressing a human beta-NGF (Nerve Growth Factor), a recombinant cell strain containing the recombinant expression vector and a method for producing a recombinant human beta-NGF. The recombinant expression vector comprises a promoter, a beta globin gene intron and a human beta-NGF gene, an internal ribosome entry site sequence and a selective marker gene and has higher integration and expression efficiency in a host cell. A recombinant eukaryotic cell containing the recombinant expression vector expresses the recombinant human beta-NGF equivalent with a natural beta-NGF in activity and still can stably express after multiple times of passage; and the expressed beta-NGF is secreted into a culture medium and is easy to separate and purify, the production downstream difficulty and the production downstream cost are greatly reduced, and the clinical application prospect and the commercial value are good.
Owner:深圳市中科深研生物科技有限公司
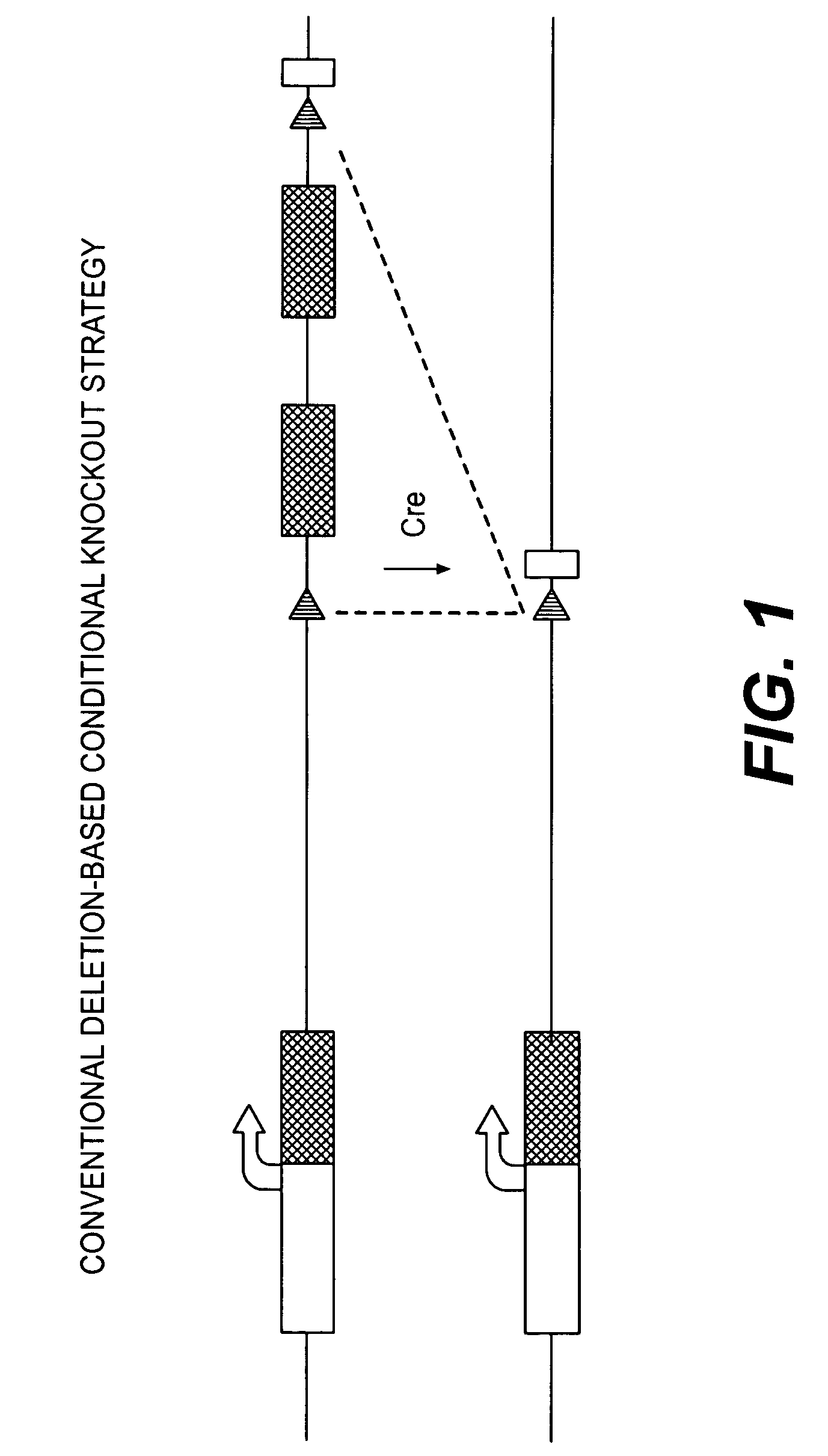
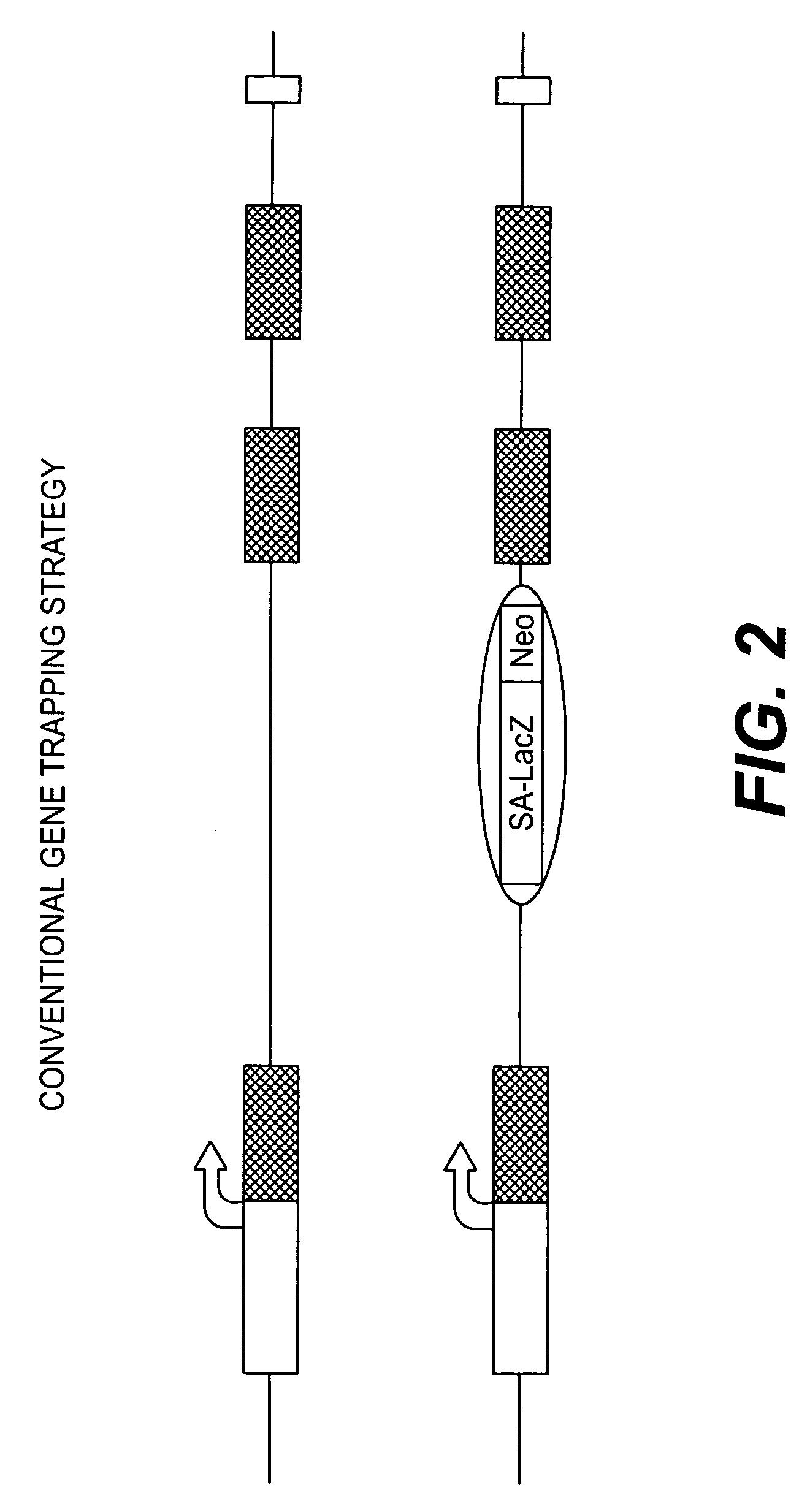
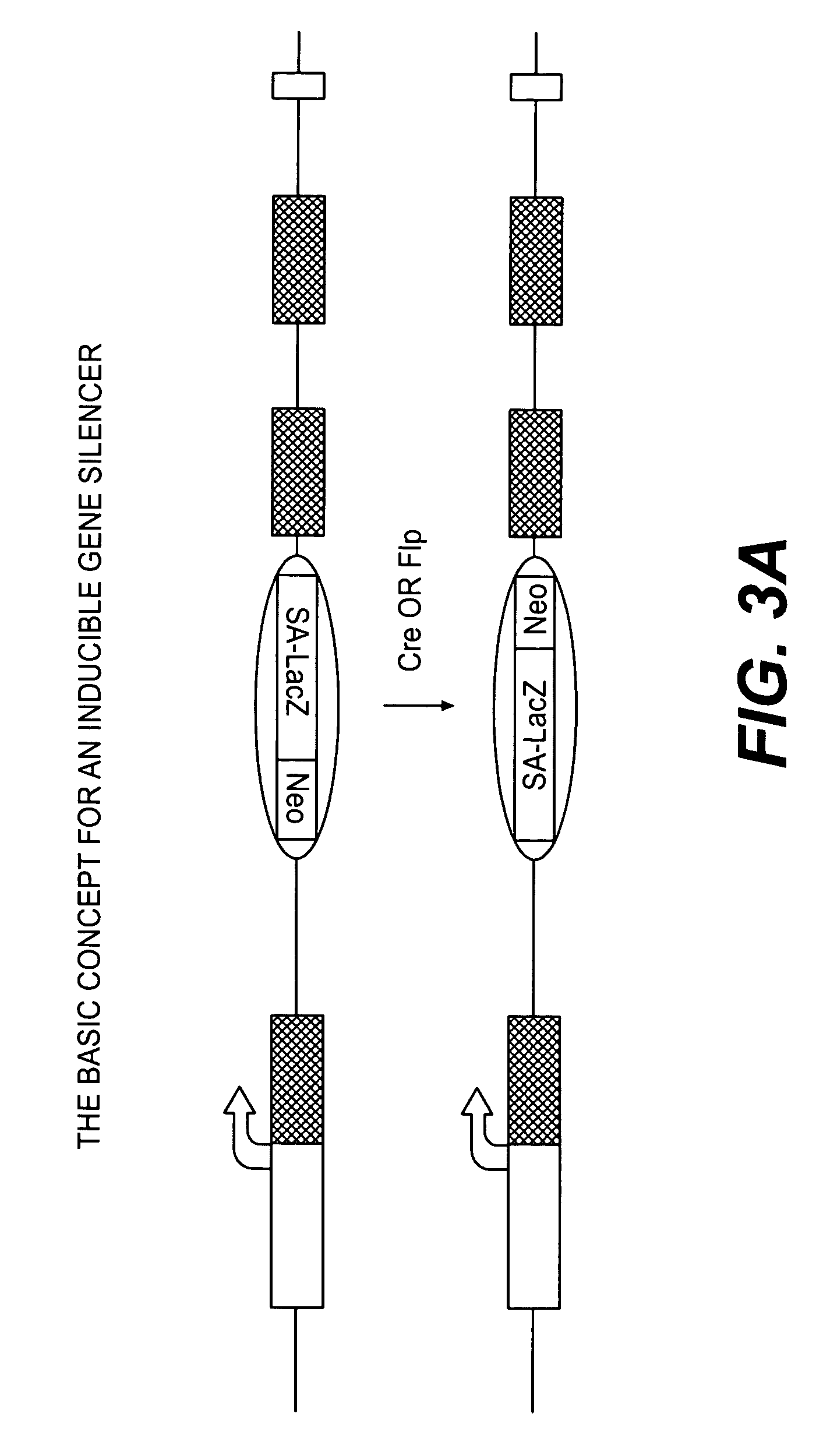
Conditional knockout method for gene trapping and gene targeting using an inducible gene silencer
InactiveUS7625755B2Less genetic manipulationEasy constructionSugar derivativesNucleic acid vectorPoly-A RNANucleotide
A method for conditionally knocking out and altering gene function and genetic sequences that can be used in such methods, for use in gene trapping and gene targeting. Specifically, the genetic sequence is a inducible gene silencer comprising: (a) a splice acceptor sequence; (b) an internal ribosomal entry site (IRES) sequence; (c) a nucleotide sequence coding for a reporter protein; (d) a polyadenylation sequence; and (e) a pair of oppositely oriented recombination site sequences, which cause single cycle inversions in the presence of a suitable recombinase enzyme, flanking elements (a) through (d).
Owner:WYETH
Selection of Host Cells Expressing Protein at High Levels
InactiveUS20100136616A1Impaired translationImprove translationAnimal cellsVectorsHigh level expressionA-DNA
The invention provides a DNA molecule comprising a multicistronic transcription unit coding for i) a polypeptide of interest, and for ii) a selectable marker polypeptide functional in a eukaryotic host cell, wherein the polypeptide of interest has a translation initiation sequence separate from that of the selectable marker polypeptide, and wherein the coding sequence for the polypeptide of interest is upstream from the coding sequence for the selectable marker polypeptide in said multicistronic transcription unit, and wherein an internal ribosome entry site (IRES) is present downstream from the coding sequence for the polypeptide of interest and upstream from the coding sequence for the selectable marker polypeptide, and wherein the nucleic acid sequence coding for the selectable marker polypeptide in the coding strand comprises a GTG or a TTG startcodon. The invention also provides methods for obtaining host cells expressing a polypeptide of interest, said host cells comprising the DNA molecules of the invention. The invention further provides the production of polypeptides of interest, comprising culturing host cells comprising the DNA molecules according to the invention.
Owner:CHROMAGENICS BV
Method for enhancing expression quantity of gene recombinant human coagulation factor 8
InactiveCN101597616ASimplify the screening processImprove screening efficiencyFermentationVector-based foreign material introductionMammalInternal ribosome entry site
The invention establishes a novel expression carrier containing a weakened internal ribosome entry site (IRES for short) sequence and a double-sieving mark by a molecular biology technique. The carrier can be used for the recombinant expression of a human coagulation factor 8, and can effectively enhance the expression quantity of the coagulation factor 8 in the cells of mammals by the comprehensive sieving of a stable expression strain.
Owner:SHANGHAI TONEKER BIOTECH
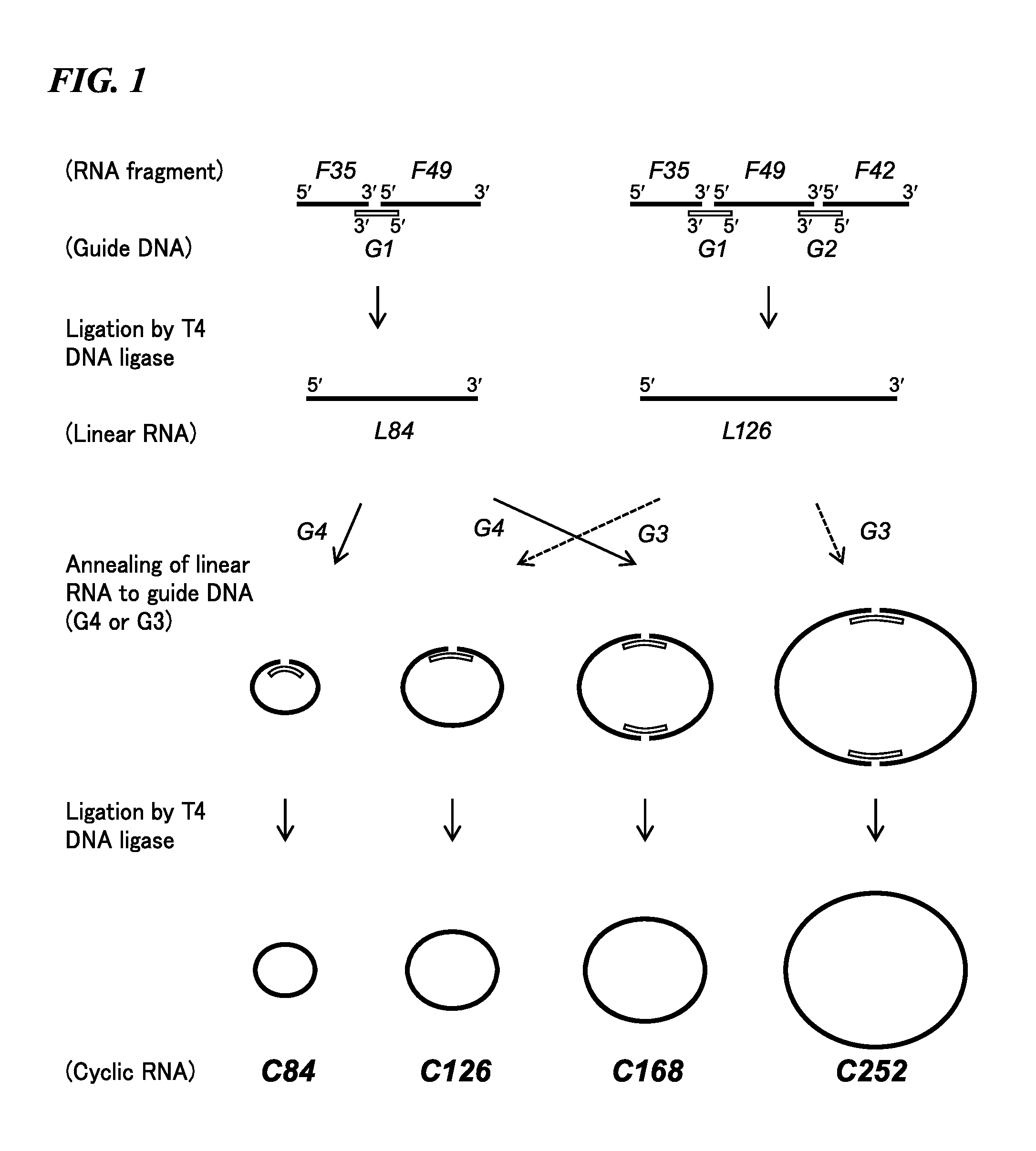
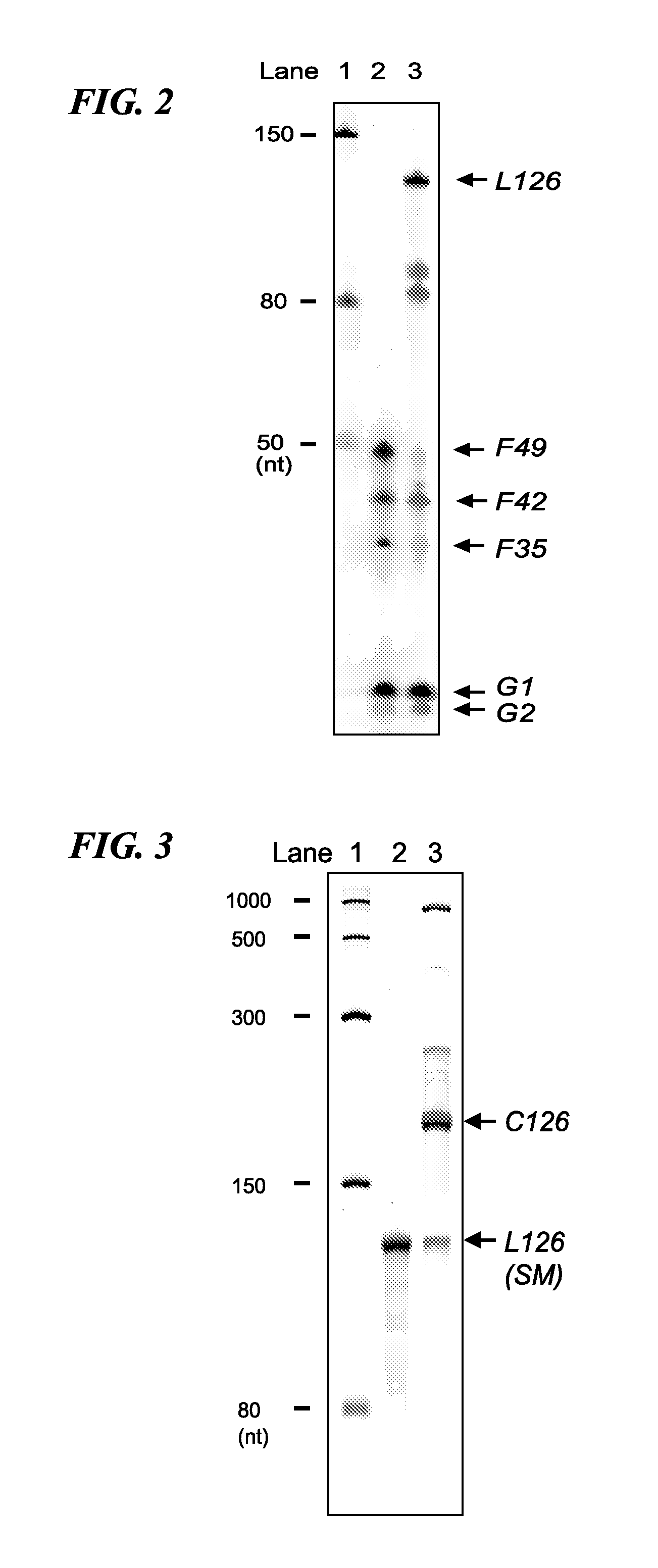
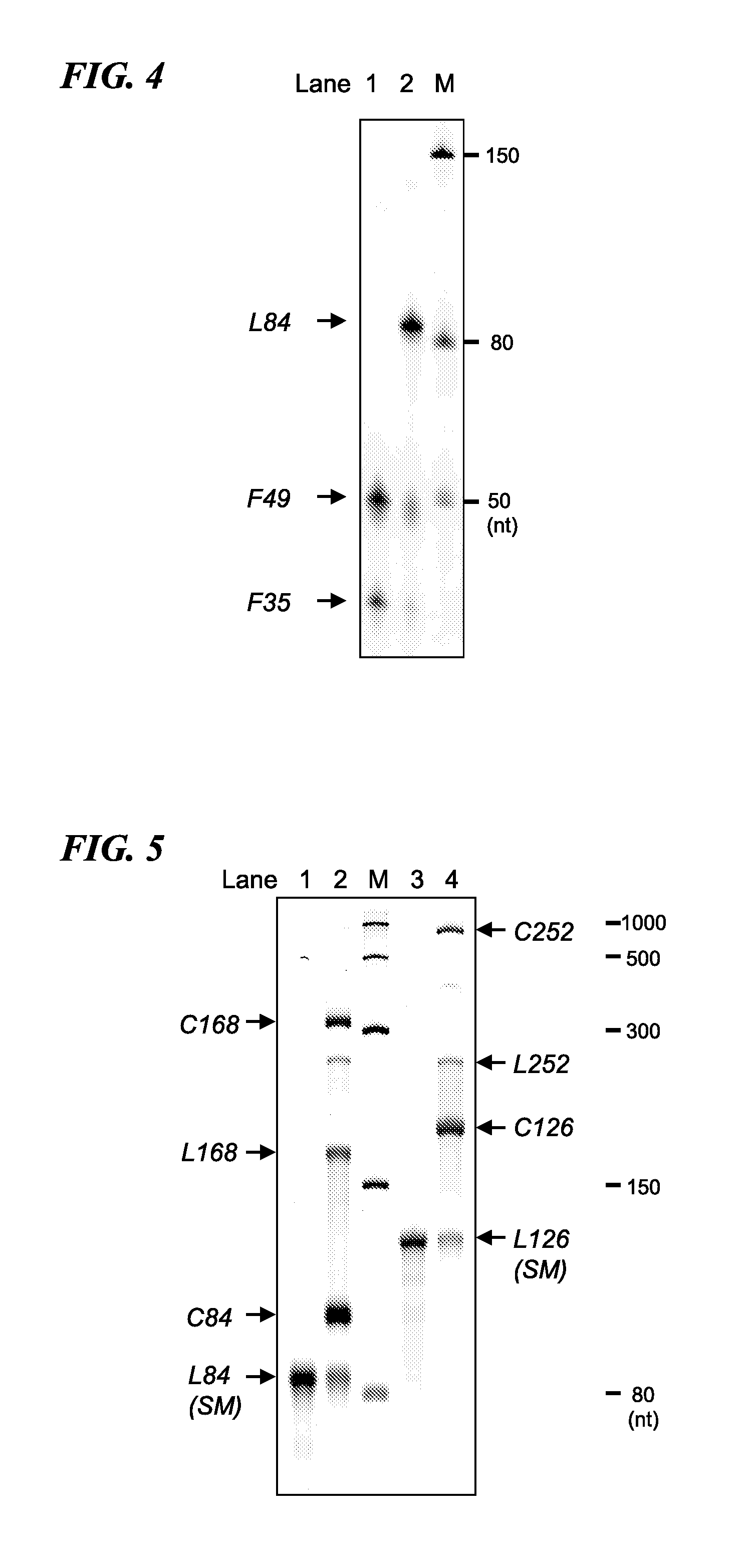
Cyclic RNA and protein production method
The present invention provides a cyclic RNA preferable for carrying out rotary protein translation in which translation domains other than that of the target protein are sufficiently short and translation efficiency is high, and a method for producing protein that uses this cyclic RNA as template. More specifically, the present invention provides a cyclic RNA that encodes a protein, has a full-length number of bases that is equal to or greater than 102 and is a multiple of 3, has at least one start codon, does not have a stop codon in the same reading frame as the start codon, and does not contain an internal ribosome entry site (IRES). In addition, the present invention provides a method for producing protein in a eukaryotic cell expression system that consists of using the aforementioned cyclic RNA as template and expressing a protein encoded by that cyclic RNA.
Owner:RIKEN
Selection of host cells expressing protein at high levels
The invention provides a DNA molecule comprising a multicistronic transcription unit coding for i) a polypeptide of interest, and for ii) a selectable marker polypeptide functional in a eukaryotic host cell, wherein the polypeptide of interest has a translation initiation sequence separate from that of the selectable marker polypeptide, and wherein the coding sequence for the polypeptide of interest is upstream from the coding sequence for the selectable marker polypeptide in the multicistronic transcription unit, and wherein an internal ribosome entry site is present downstream from the coding sequence for the polypeptide of interest and upstream from the coding sequence for the selectable marker polypeptide, and wherein the nucleic acid sequence coding for the selectable marker polypeptide in the coding strand comprises a GTG or a TTG start codon. Also provided are methods for obtaining host cells expressing a polypeptide of interest, such host cells comprising DNA molecules of the invention. Further provided is the production of polypeptides of interest, comprising culturing host cells comprising the DNA molecules of the invention.
Owner:CHROMAGENICS BV
Compositions and methods for regulating mRNA transcription and translation
InactiveUS20050019808A1Significant changeSsRNA viruses positive-senseSugar derivativesCell freeInternal ribosome entry site
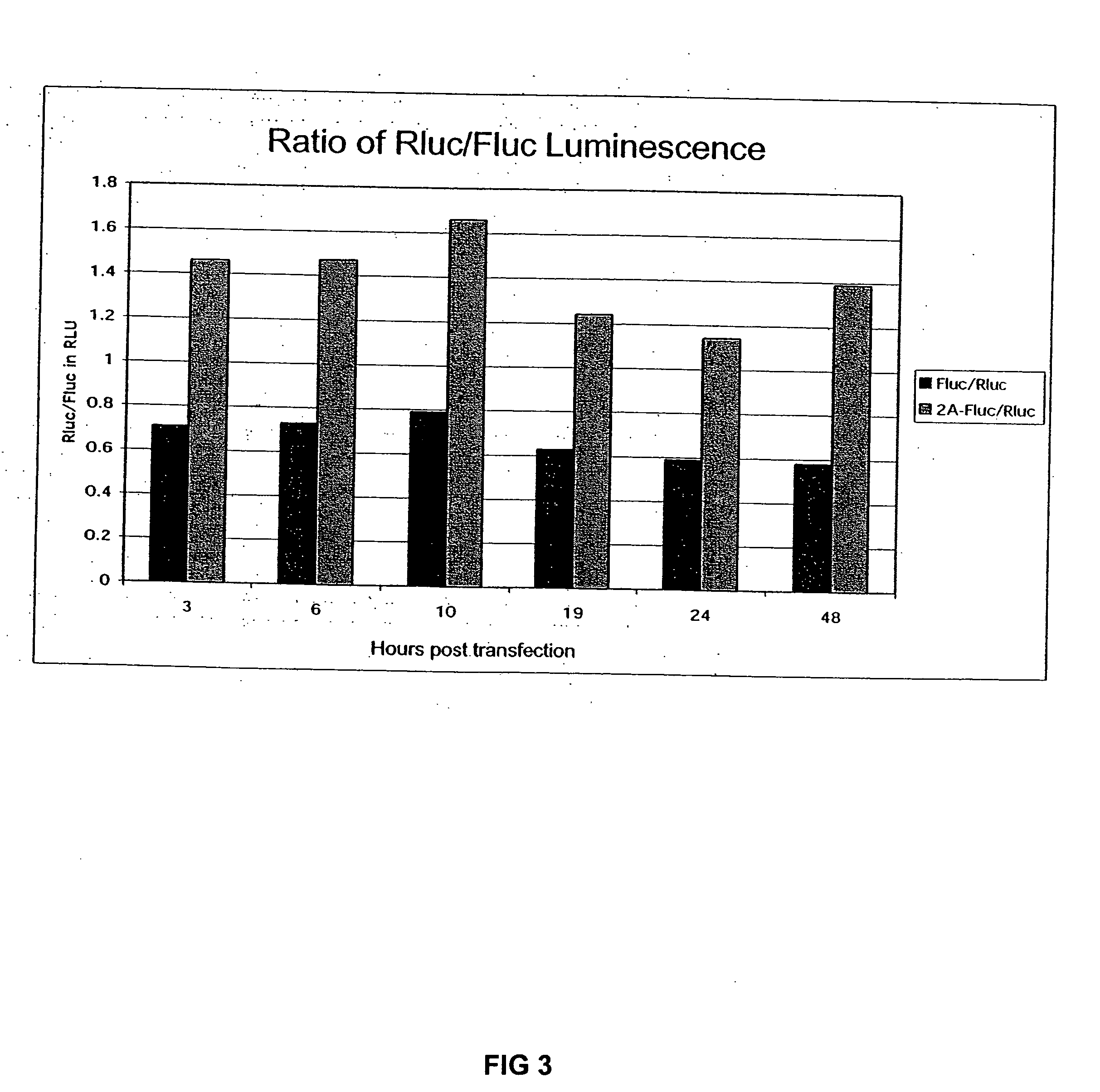
The invention relates to compositions, specifically novel nucleic acid constructs encoding a cardiovirus 2A polypeptide operably linked to suitable promoters. Also, disclosed are methods whereby the nucleic acid constructs are introduced into cells or cell free systems to regulate cellular mRNA transcription and cap-dependent or internal ribosomal entry site (IRES)-dependent mRNA translation.
Owner:WISCONSIN ALUMNI RES FOUND
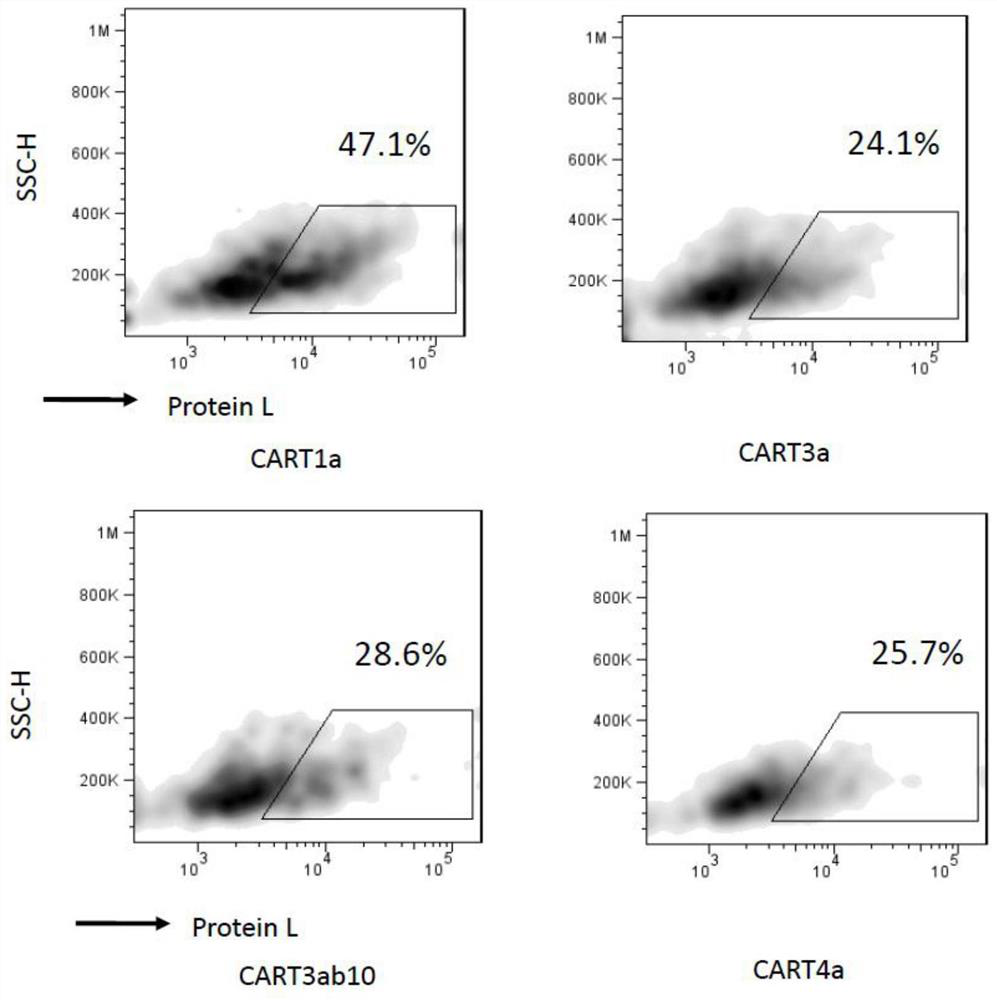
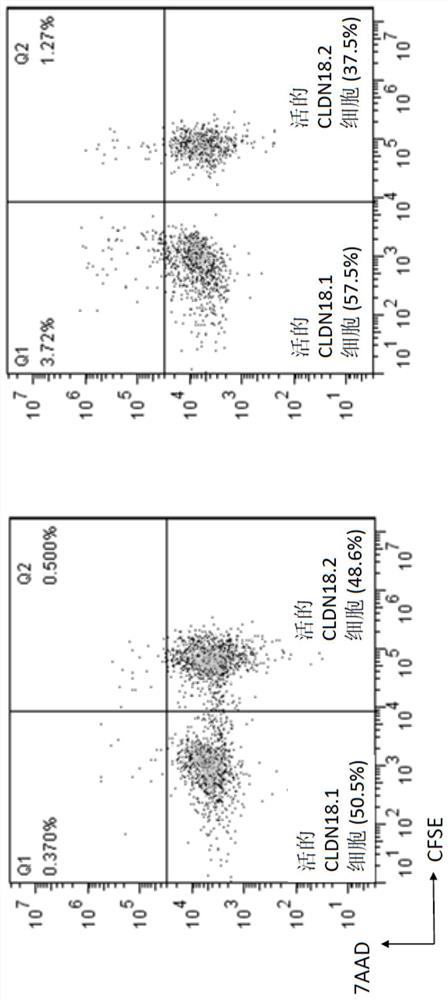
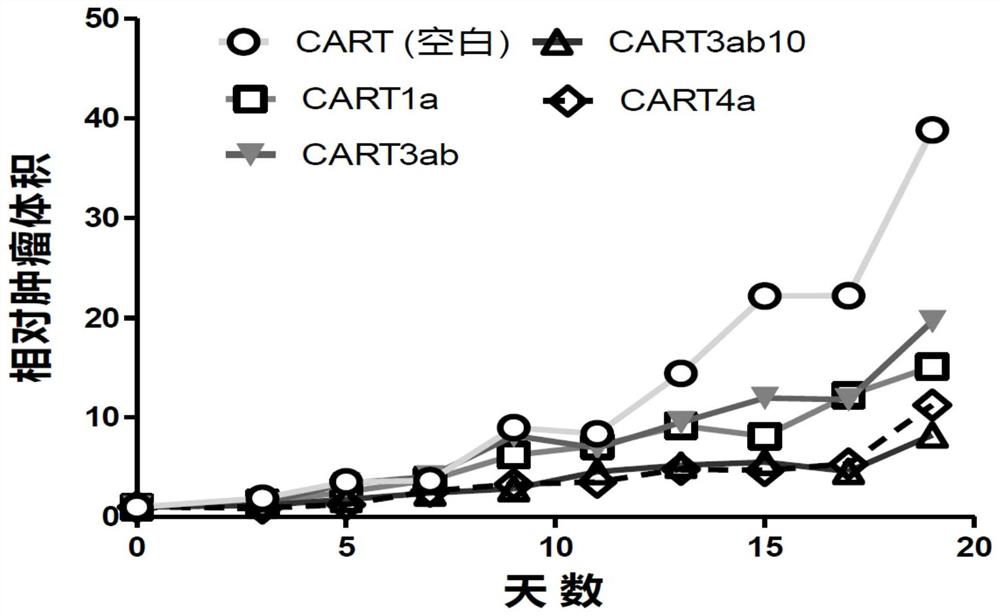
Claudin 18.2-targeting CAR molecule, immune cell modified by Claudin 18.2-targeting CAR molecule and application of Claudin 18.2-targeting CAR molecule
PendingCN111848809AHigh activityHigh affinityImmunoglobulin superfamilyImmunoglobulins against cell receptors/antigens/surface-determinantsTransmembrane domainIntracellular domain
The present invention discloses a nucleic acid construct. The nucleic acid construct is characterized in that the nucleic acid construct has a structure as shown in the formula car-[(IRES)-f]<m>, wherein the IRES is a sequence of an internal ribosome entry site, f encodes a functional protein F, m is 0 or a non-0 natural number, car encodes a CAR, the CAR comprises (a) an extracellular binding domain that specifically recognizes CLDN18.2, (b) a hinge domain, (c) a transmembrane domain, (d) a costimulating intracellular domain, and (e) a signal transduction domain, the extracellular binding domain comprises scFv, and the amino acid sequences and functions of the extracellular binding structural domain are described in the specification. The CAR molecule provided by the invention has excellent safety and effectiveness and shows a very good tumor inhibition effect.
Owner:L&L BIOPHARMA CO LTD
Method for performing genetic modification under a drug-free environment and components thereof
ActiveUS20110099649A1Minimize biasEffective targetingStable introduction of DNANucleic acid vectorGene targetsResistant genes
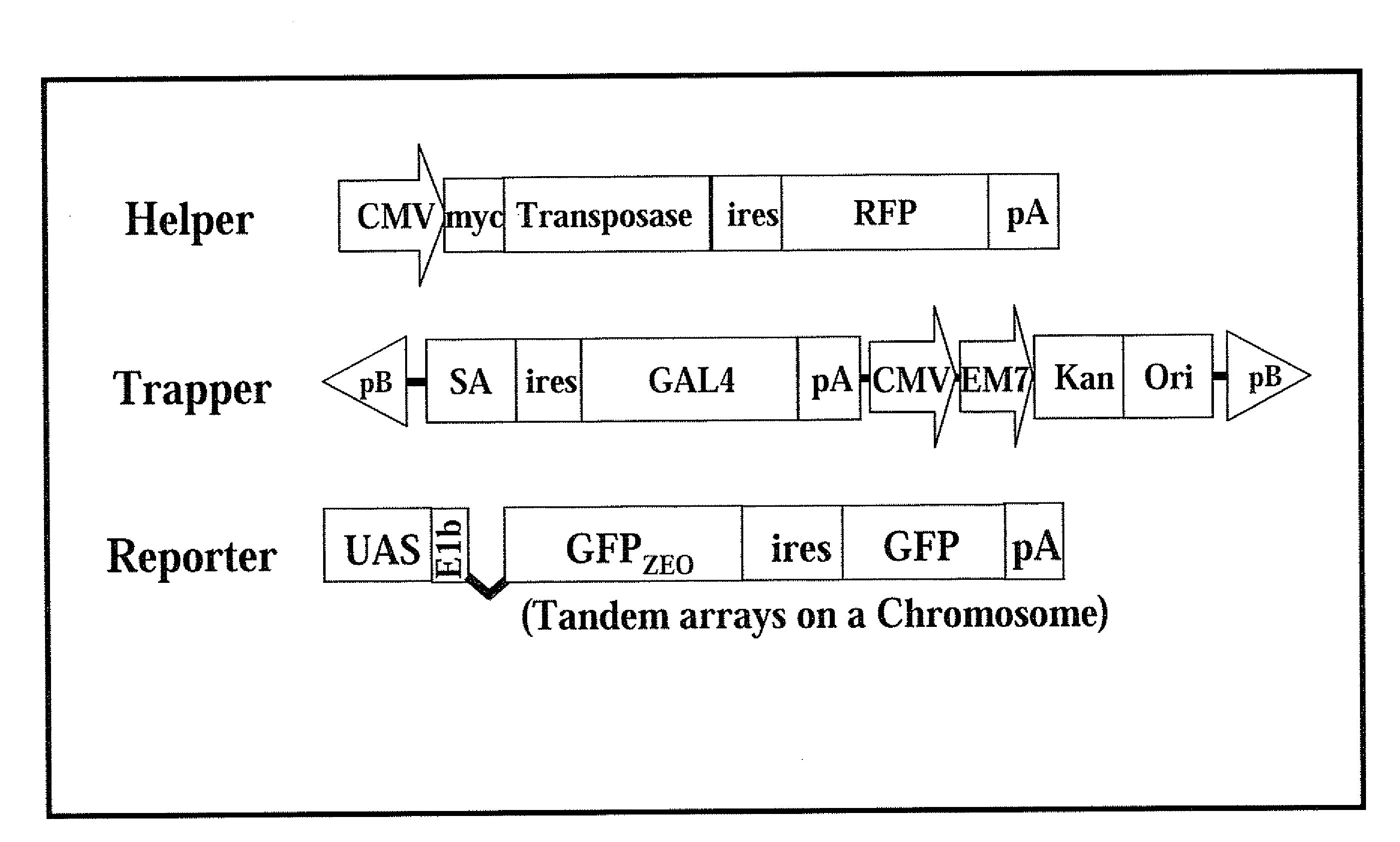
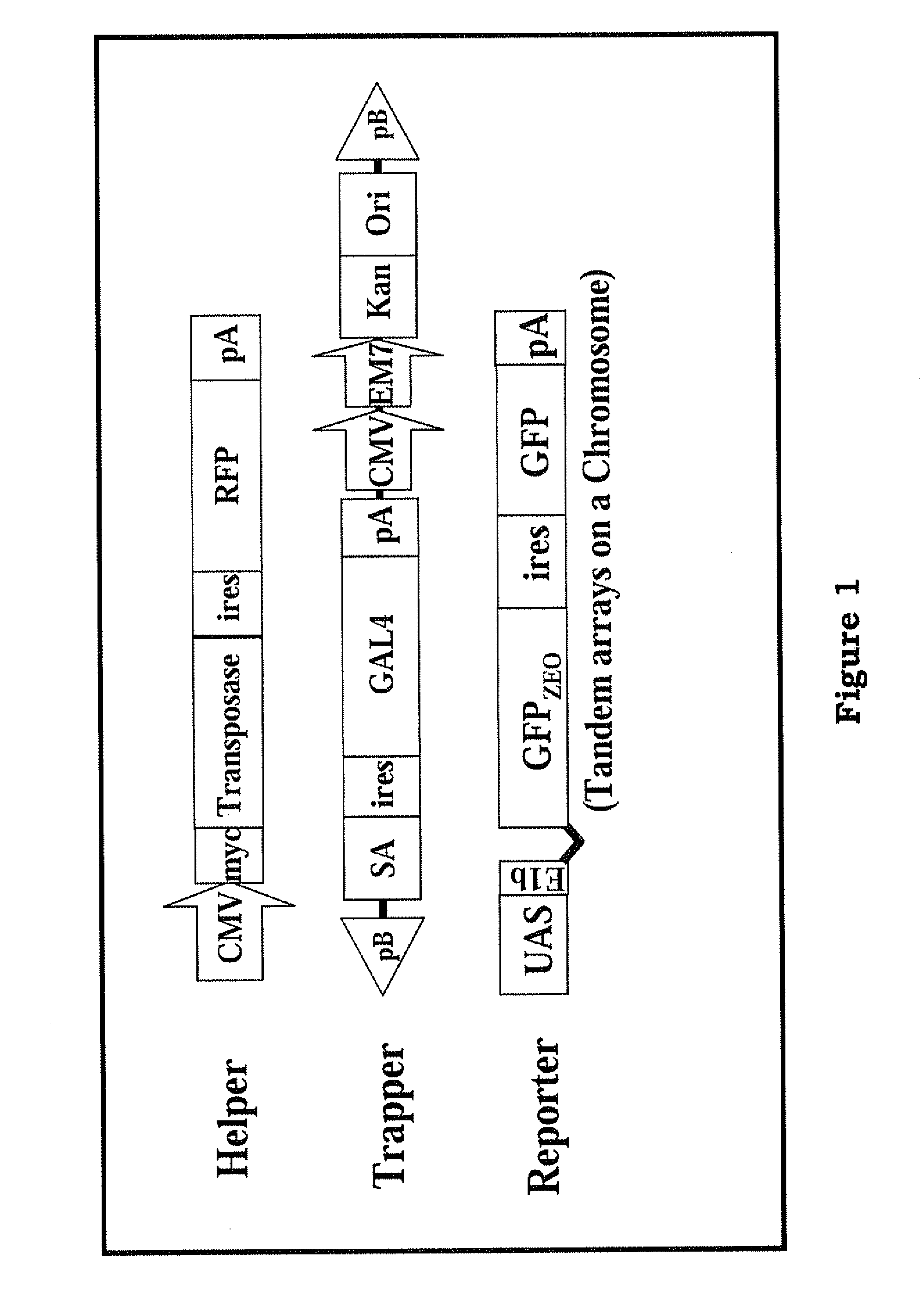
The present invention provides a method and components thereof of performing genetic modification under a drug-free environment. The method comprises the steps of generating a trapped mammalian cell library by trapper constructs (including the element of piggyBac terminal inverted repeats (TIRs)), reporter constructs, and helper constructs (including a sequence of an internal ribosomal entry site (IRES)). The present art allows: (1) to target & identify the silenced loci; (2) to separate genes with low-level expression at certain differentiation stages; (3) to evaluate the efficiency of gene targeting in the silent or repressed loci. The present invention avoids the biased gene targeting observed in the prior arts, and eliminates the needs of introducing antibiotic genes into the host genome which may lead to a potential threat of drifting antibiotic resistant genes into environment.
Owner:CHANG GUNG UNIVERSITY
Gene knock-in recombinant vector and preparation method thereof as well as method for preparing mouse model
ActiveCN103642828AShorten the timeHigh detection sensitivityMicrobiological testing/measurementVector-based foreign material introductionWhite blood cellFluorescence microscope
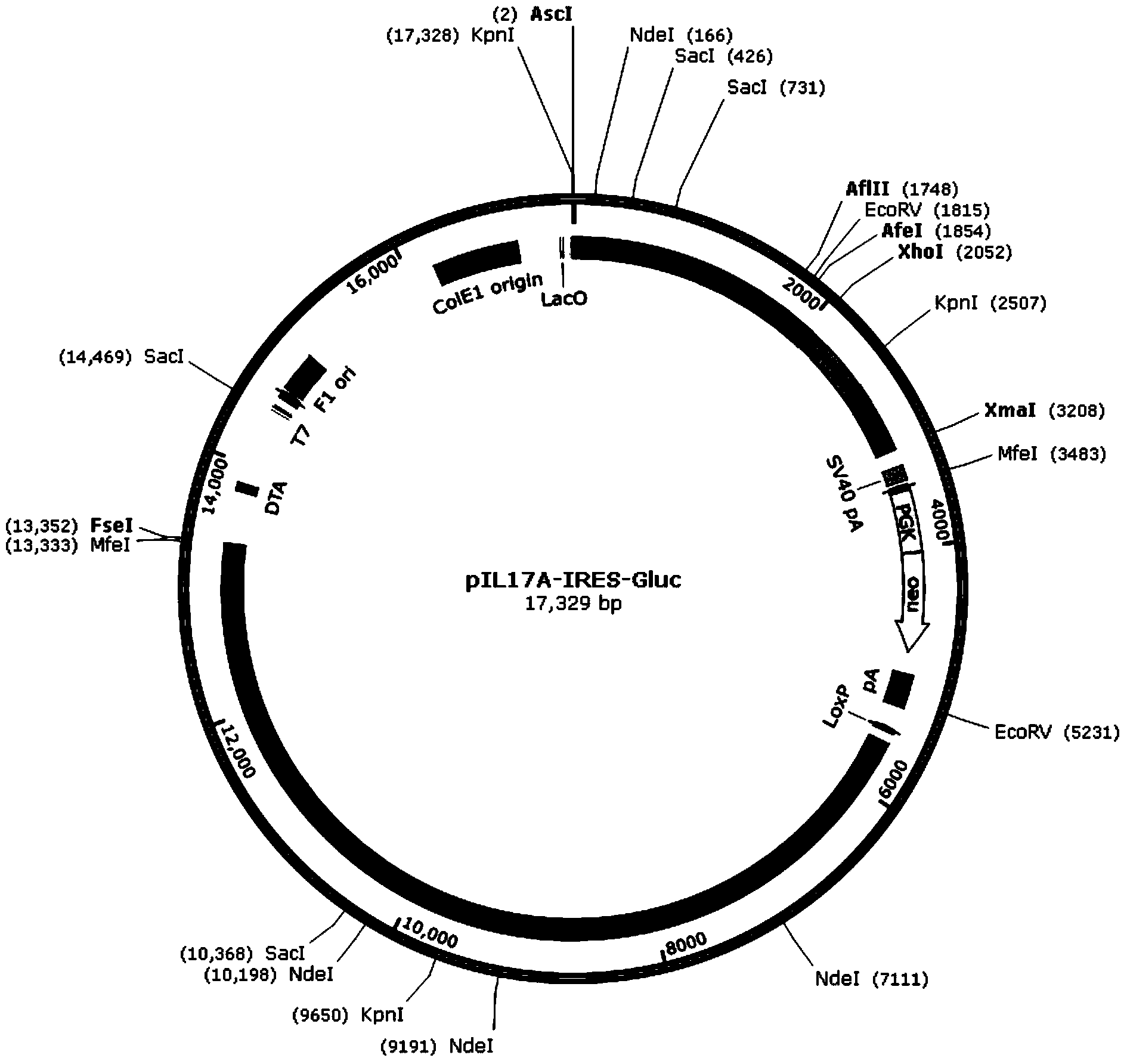
The invention discloses a recombinant vector for gene knock-in. The recombinant vector comprises a DNA (deoxyribonucleic acid) fragment structure IL17A-IRES-luc, wherein IL17A is an encoding gene of interleukin 17A, IRES is internal ribosome entry site, and luc is an encoding gene of secreting luciferase. The invention also provides a method for preparing the gene knock-in recombinant vector and a mouse model prepared by using the recombinant vector, wherein the mouse model can be used for detecting the expression of the IL17A gene by detecting the expression of the secreting luciferase in blood or urine; a fluorescent microscope or other complicated equipment is not needed; the secreting luciferase emits light by virtue of an oxidizing reaction of substrate coelenterazine in the catalysis of luciferase, and laser light is not needed, so that the fluorescent background is far lower than GFP (green fluorescent protein) fluorescence, the detection sensitivity is higher, the background is cleaner, and the experiment results are more accurate.
Owner:BIOCYTOGEN PHARMACEUTICALS (BEIJING) CO LTD
Isolated Polynucleotide Sequence with IRES Activity
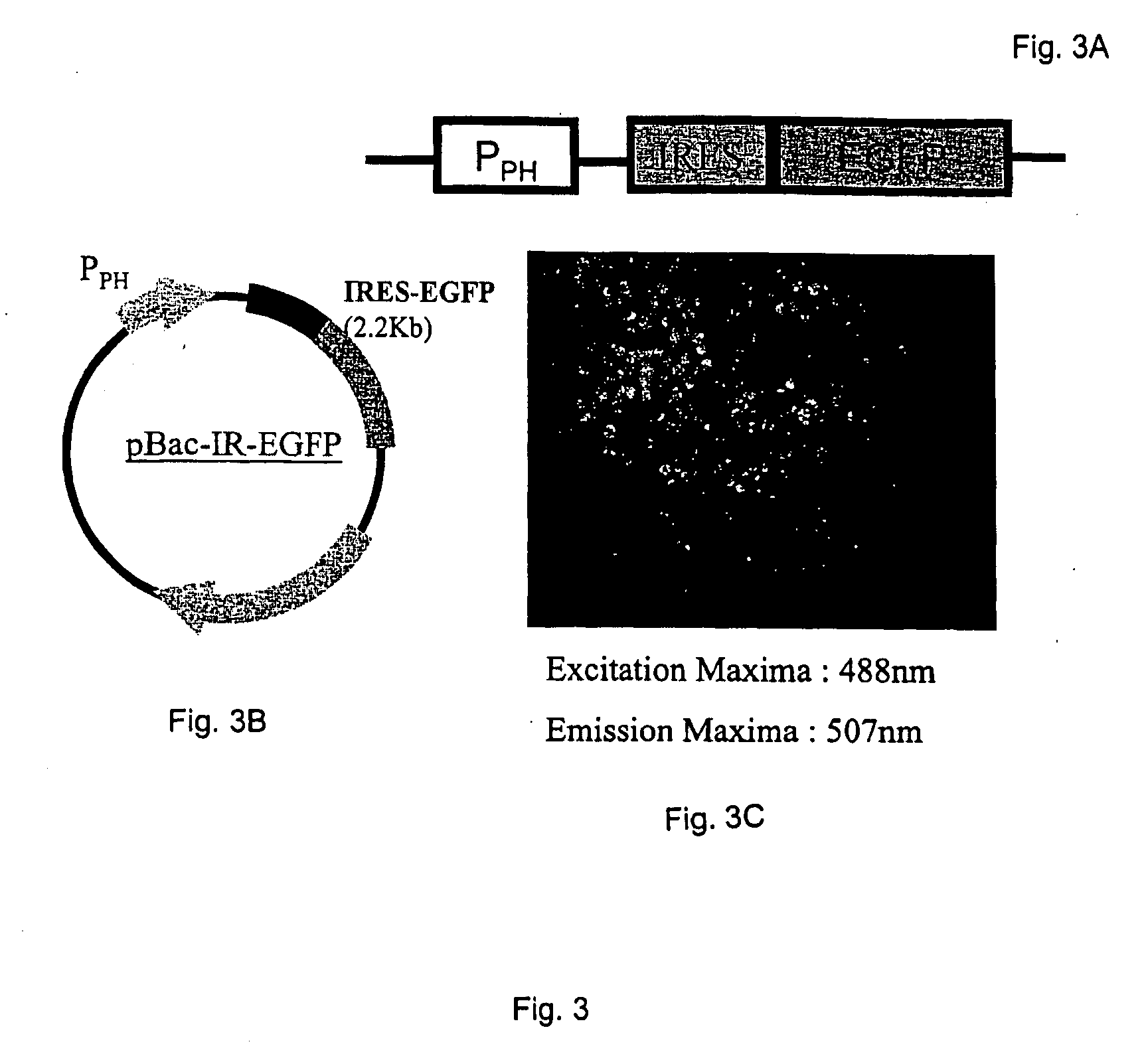
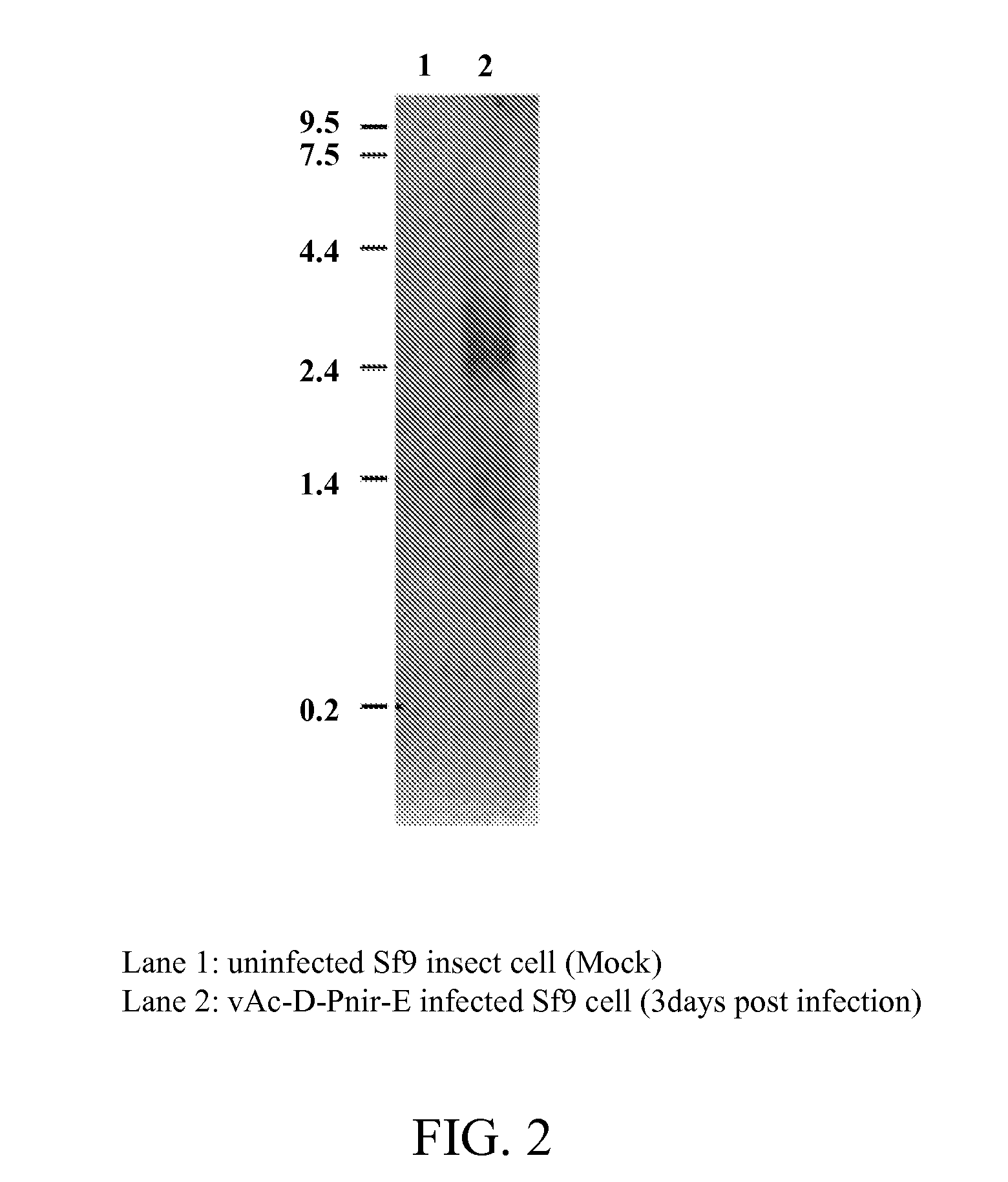
Provided herein is an isolated polynucleotide sequence with internal ribosome entry site (IRES) activity, which directs translation initiation in an insect expression system in a cap-independent manner. In particular, the invention relates to an isolated polynucleotide comprises the 5′ UTR of perina nuda Picorna-like virus (PnV) that possesses IRES activity. Methods of identifying a polynucleotide with IRES activity and methods of expressing at least two polypeptides in an insect system are also disclosed herein.
Owner:CHUNG YUAN CHRISTIAN UNIVERSITY +1
Bicistronic mRNA coexpression gene transporter and preparation method thereof
InactiveCN102994536AAchieve multiple gene co-expressionVector-based foreign material introductionBicistronic mrnaNuclear matrix
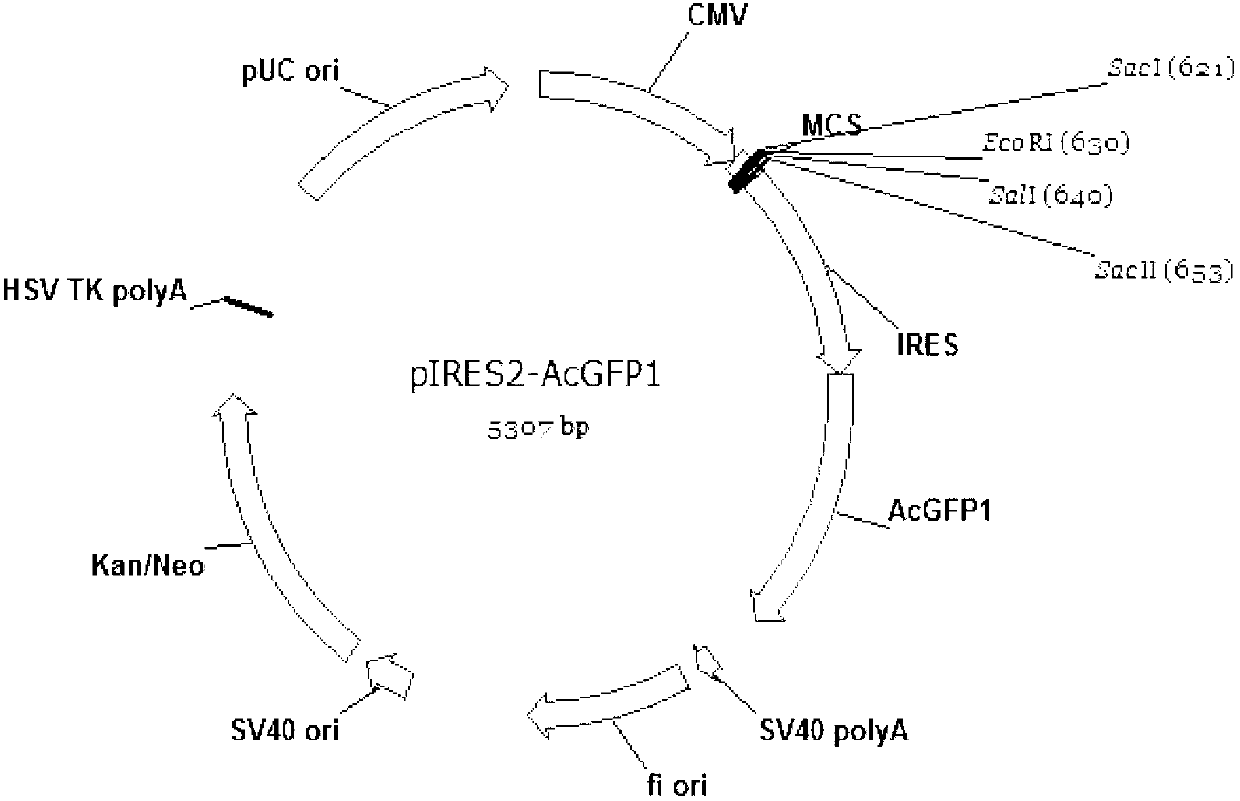
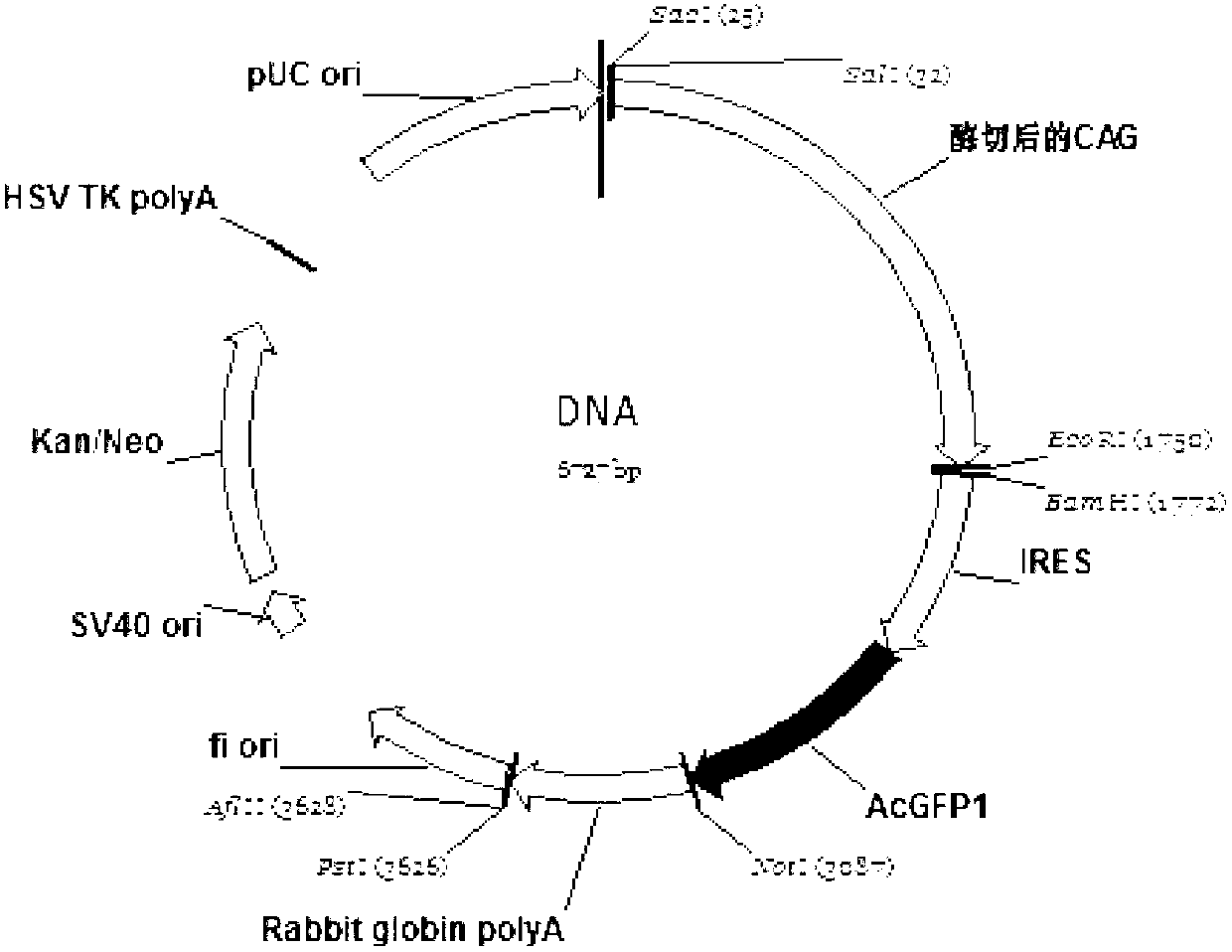
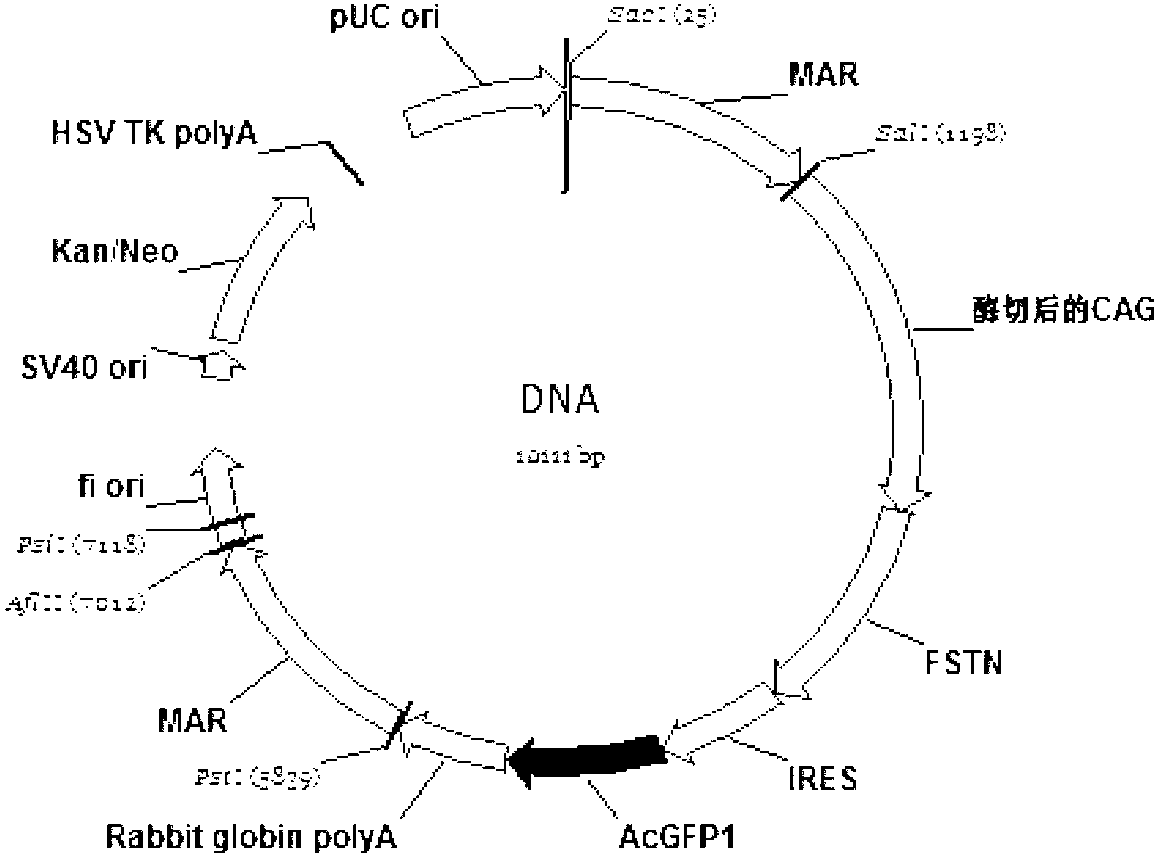
The invention relates to a bicistronic mRNA coexpression gene transporter and a preparation method thereof. The gene order of the gene transporter is SEQ ID No. 15; the gene transporter from 5' end to 3' end comprises bovine Nuclear matrix binding region MAR, artificially constructed combined promoter CAG, Profilin gene FSTN, internal ribosome entry site (IRES), green fluorescent protein AcGFP gene and Rabbit globin poly A signal region; the preparation method comprises the following steps: constructing a carrier pCAG-IRES2-AcGFP1; obtaining FSTN gene and inserting the pCAG-IRES2-AcGFP1 carrier; obtaining the sequence of MAR and inserting the pCAG-IRES2-AcGFP1 carrier; constructing a plasmid carriers so as to obtain the bicistronic mRNA coexpression gene transporter. The gene transporter is not only pure and safe gene transporter but also realizes dual-gene coexpression in any combination, achieves the purpose of multi-gene coexpression through repeated utilization of IRES, and provides new thinking and route for improving the shape of dual or multiple gene control such as economic character.
Owner:INNER MONGOLIA UNIVERSITY
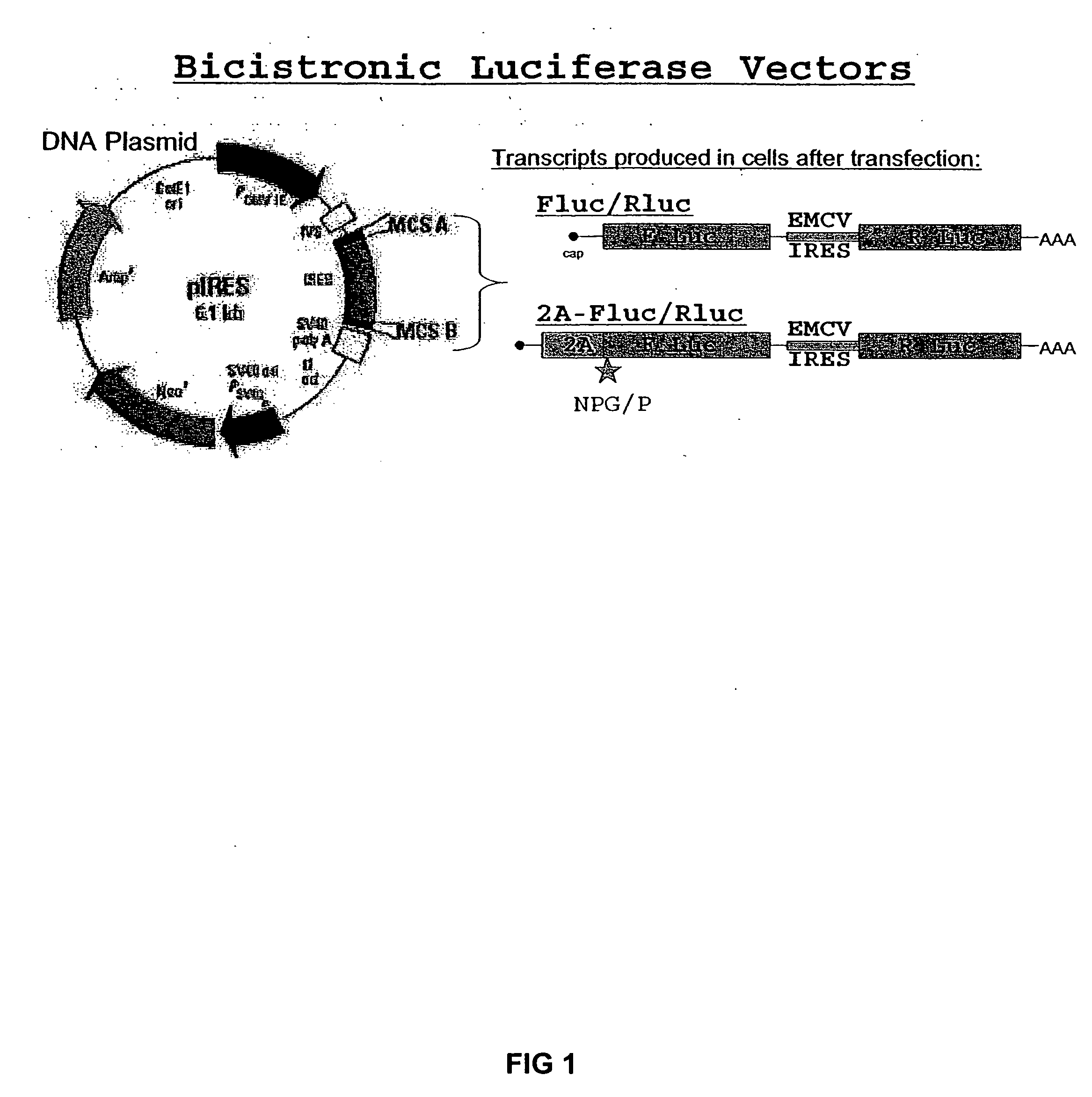
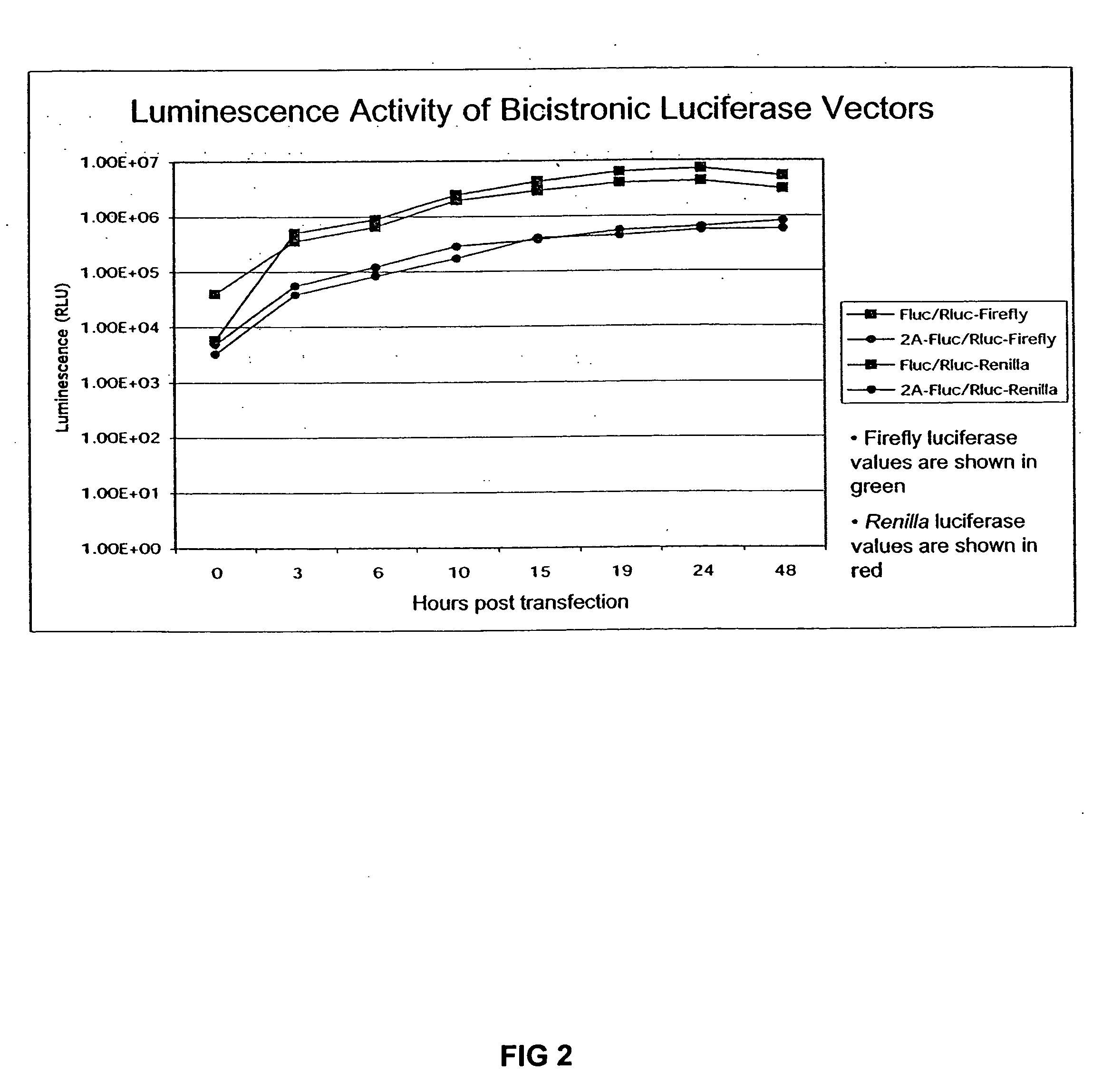
Expression of heterologous genes from an IRES translational cassette in retroviral vectors
InactiveUS20030157718A1Genetic material ingredientsMicroorganism based processesHeterologousInternal ribosome entry site
The present invention relates to a retroviral vector which expresses a gene, e.g. for therapeutic use and / or of viral origin, under the translational control of an internal ribosomal entry site (IRES) resulting in the efficient translation of said gene.
Owner:SKAU VACCINES APS
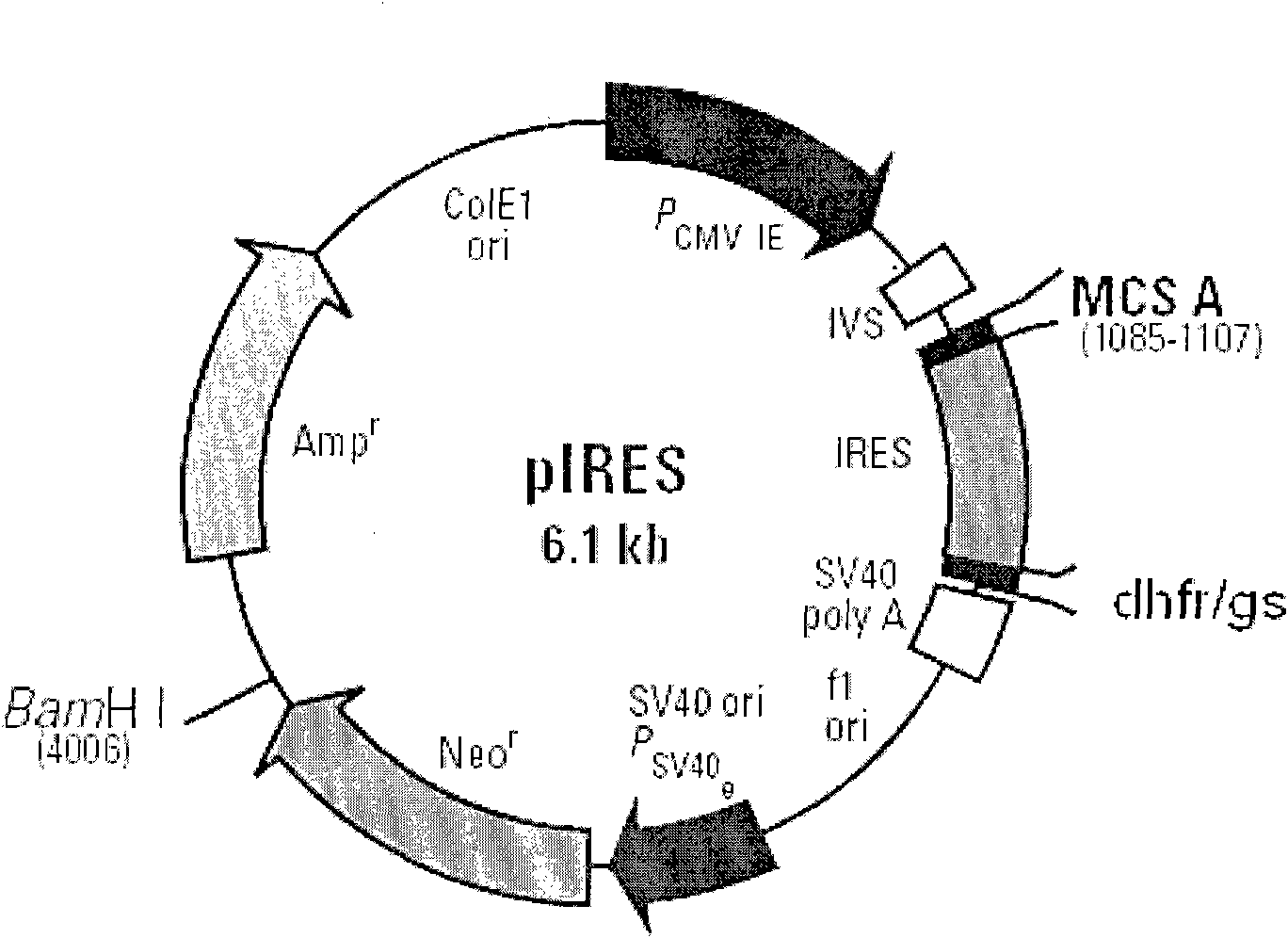
Novel expression vector
InactiveUS20130244231A1Low costEfficient expressionAnimal cellsVectorsInternal ribosome entry siteRegulatory site
Disclosed are a novel expression vector for efficient expression of recombinant proteins in mammalian cells, a mammalian cell transformed with the vector, and a method for production of the mammalian cell. The expression vector includes a gene expression regulatory site, and a gene encoding the protein downstream thereof, and an internal ribosome entry site further downstream thereof, and a gene encoding a glutamine synthetase further downstream thereof.
Owner:JCR PHARMA
Expression of heterologous genes from an IRES translational cassette in retroviral vectors
InactiveUS7056730B2Efficient replicationIncrease probabilityGenetic material ingredientsMicroorganism based processesHeterologousInternal ribosome entry site
Owner:SKAU VACCINES APS
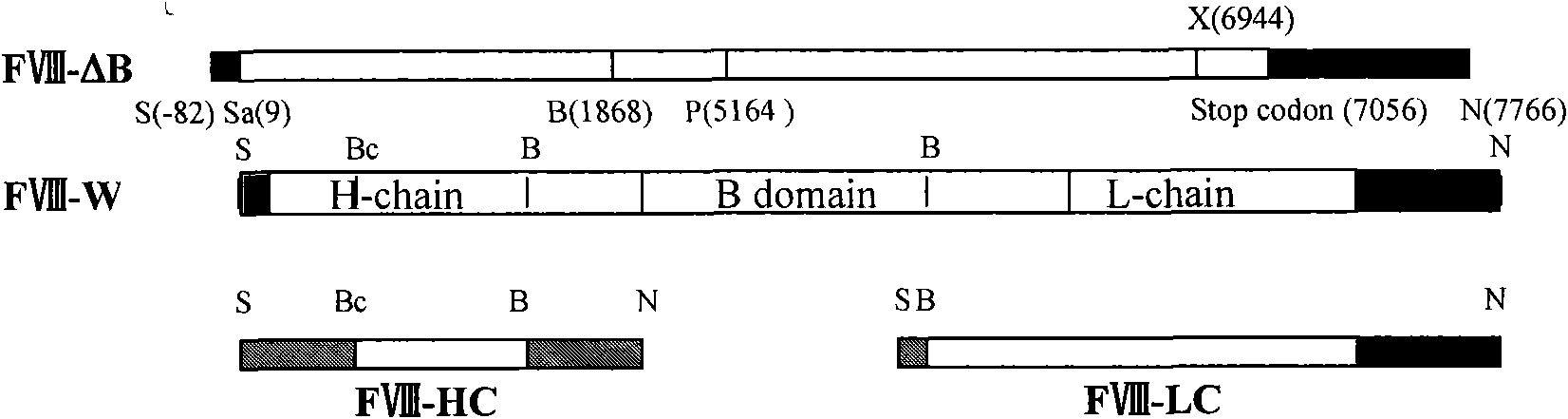
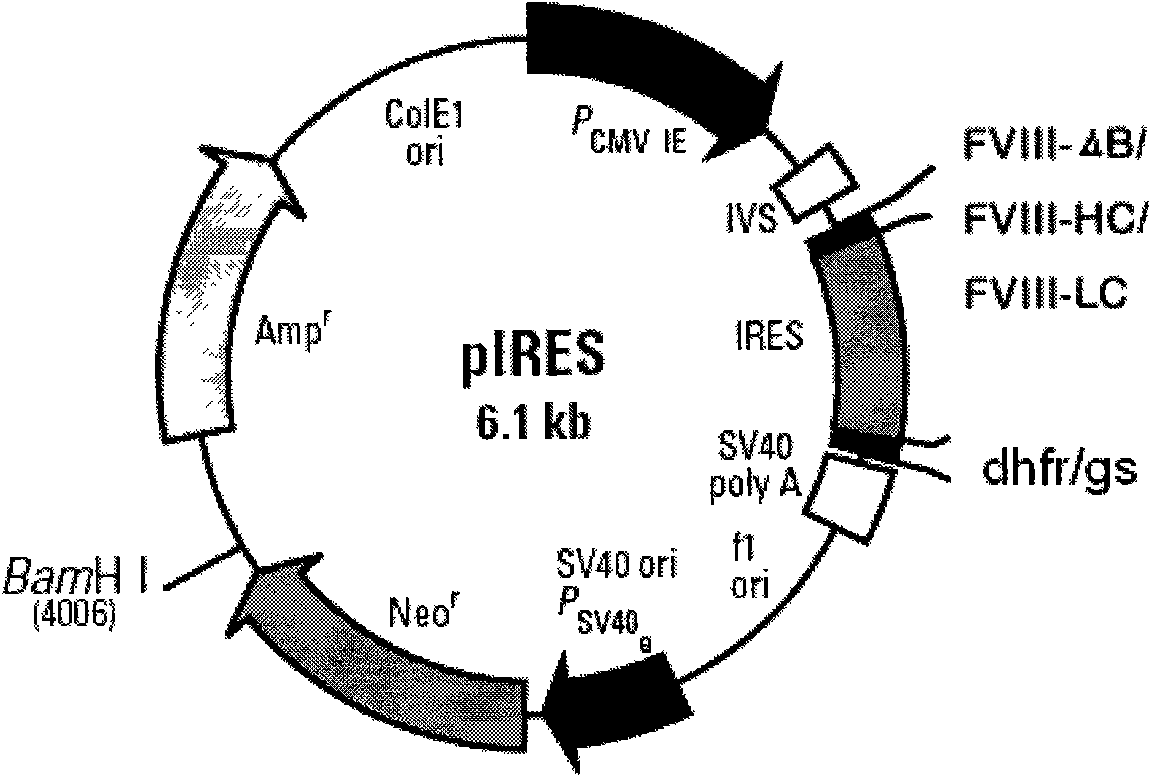
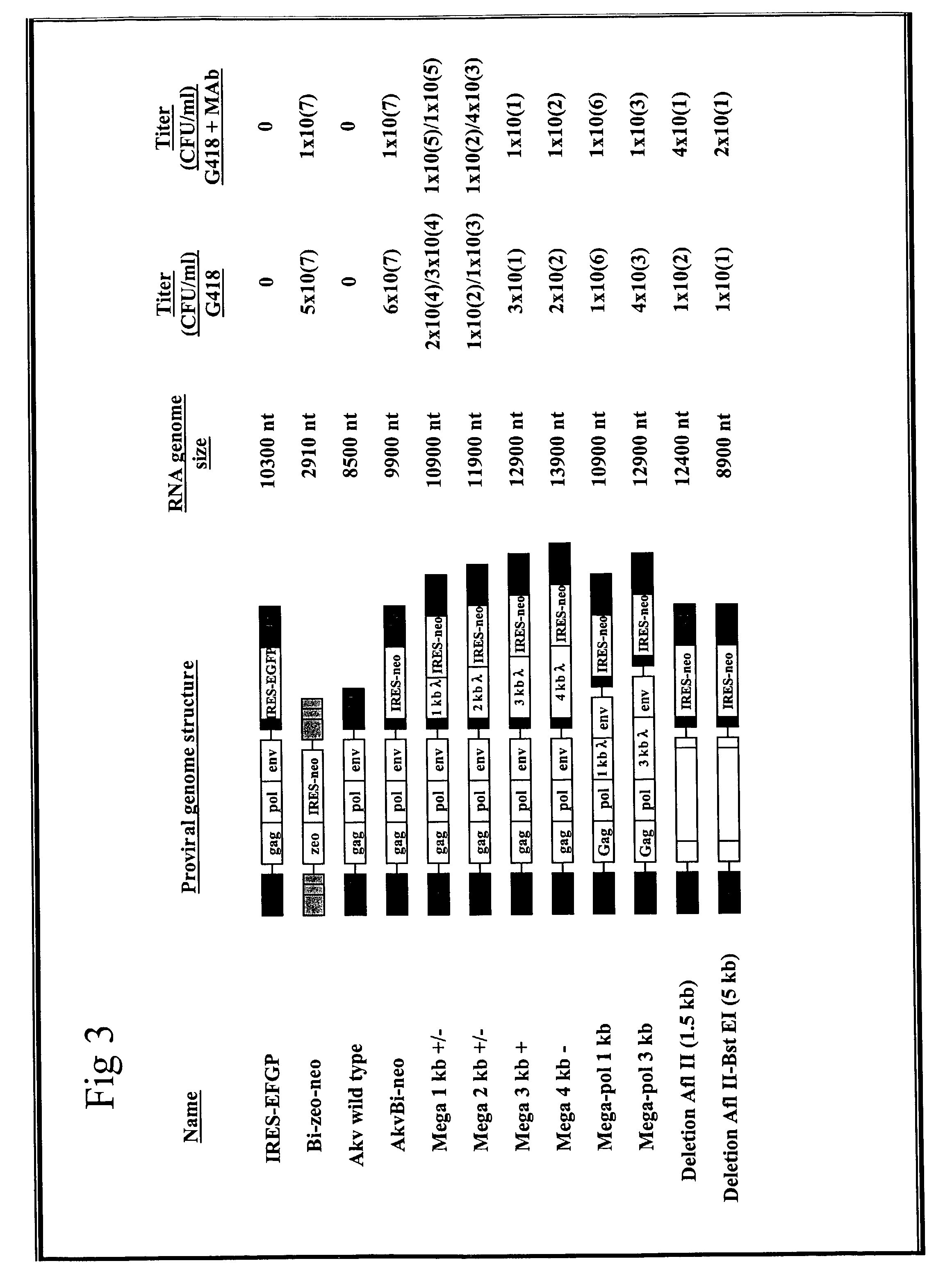
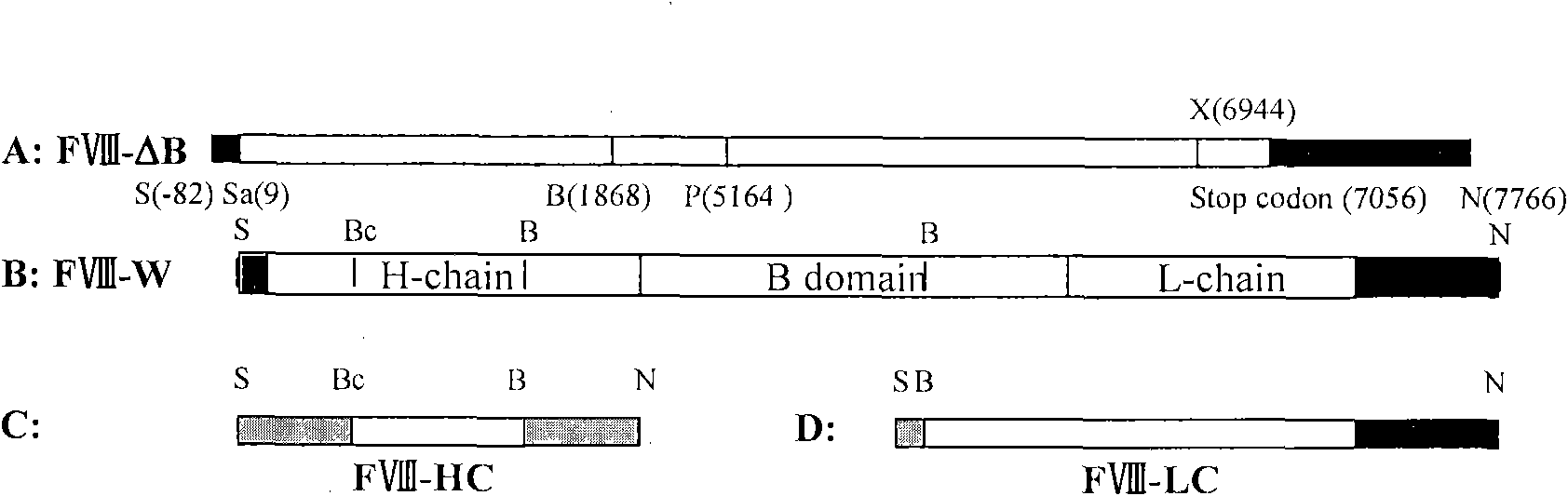
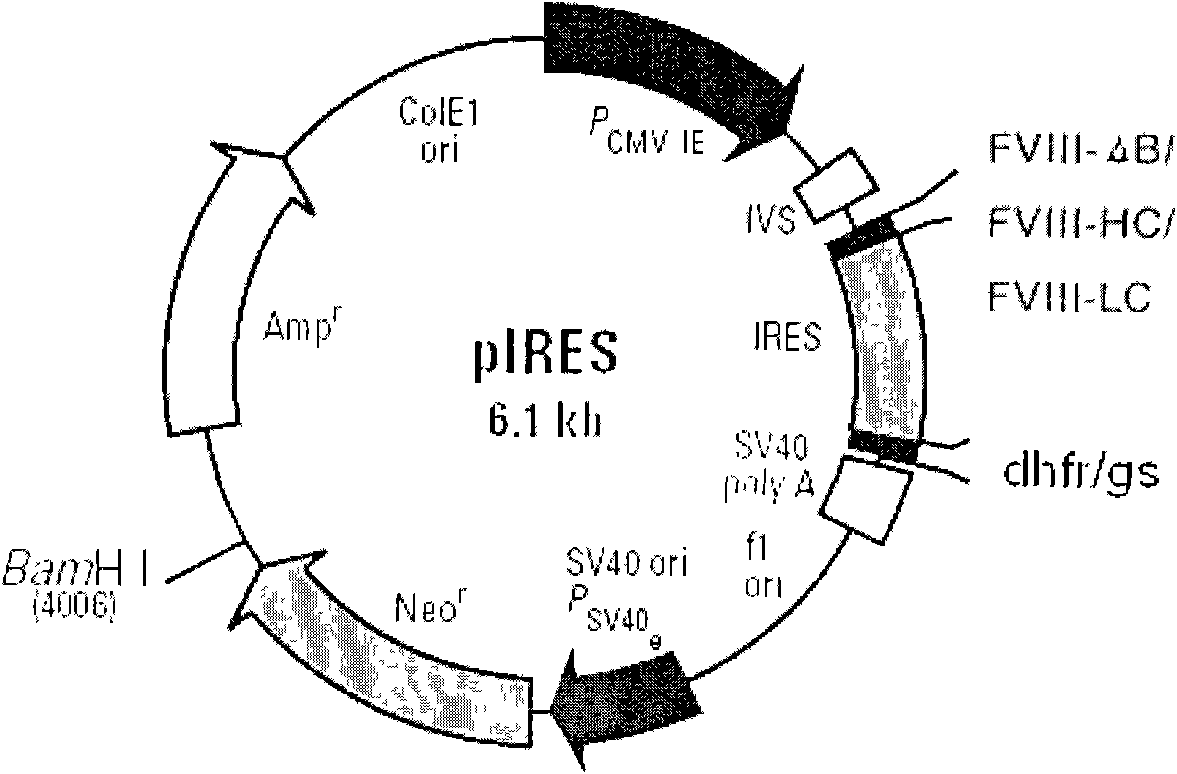
Novel recombinant human blood coagulation factor VIII and production method thereof
InactiveCN102311495AHigh expressionHigh activityFactor VIIFermentationInternal ribosome entry siteClotting factor
The invention provides a novel recombinant human blood coagulation factor VIII and a production method thereof. Particularly, the invention relates to reconstruction of a human blood coagulation factor VIII gene. A novel expression vector containing a weakened internal ribosome entry site (IRES for short) sequence and double screening marks is constructed. The vector can be used for high-efficiency recombinant expression of the human blood coagulation factor VIII, so that the expression of the vector in a mammalian cell expression system is increased.
Owner:SHANGHAI TONEKER BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com