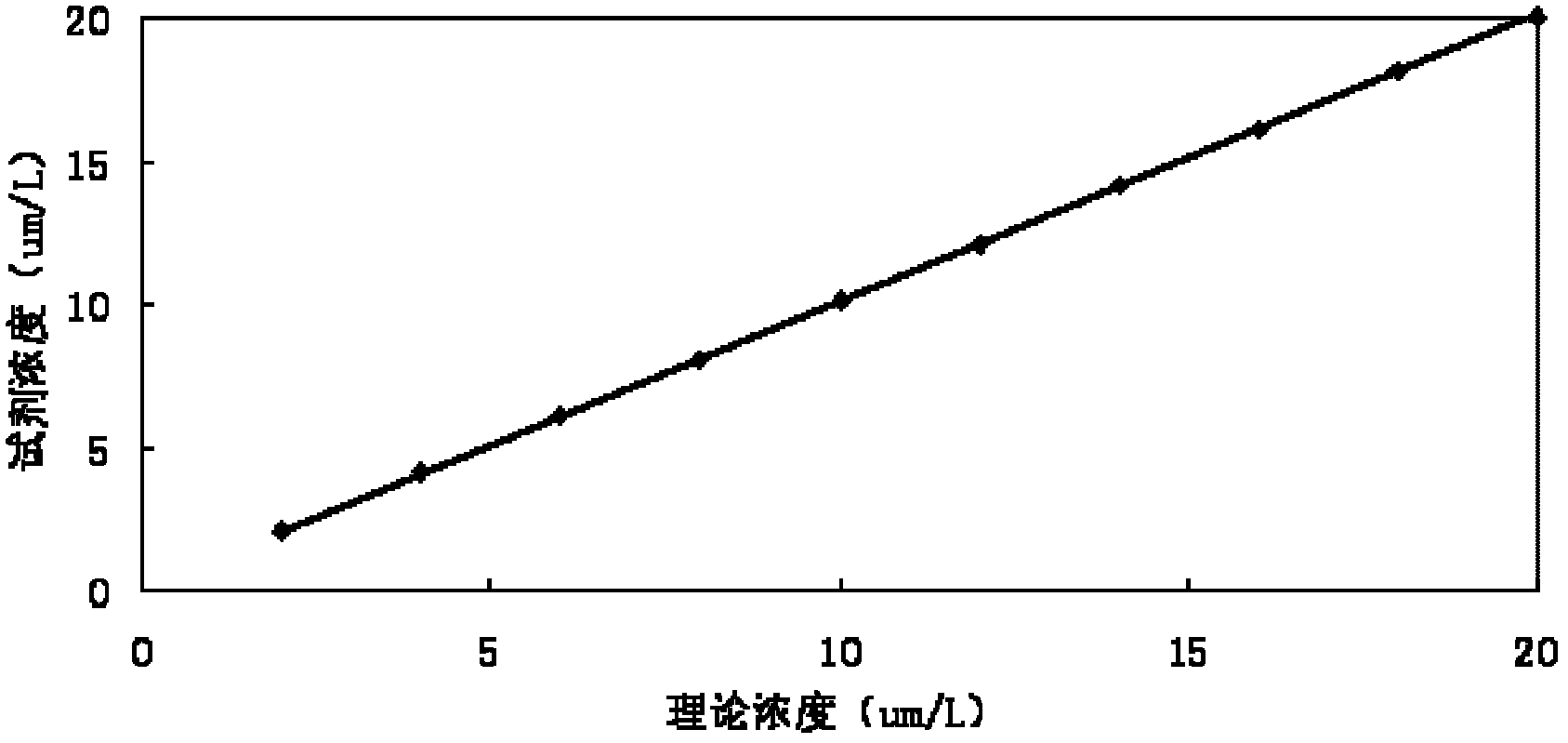
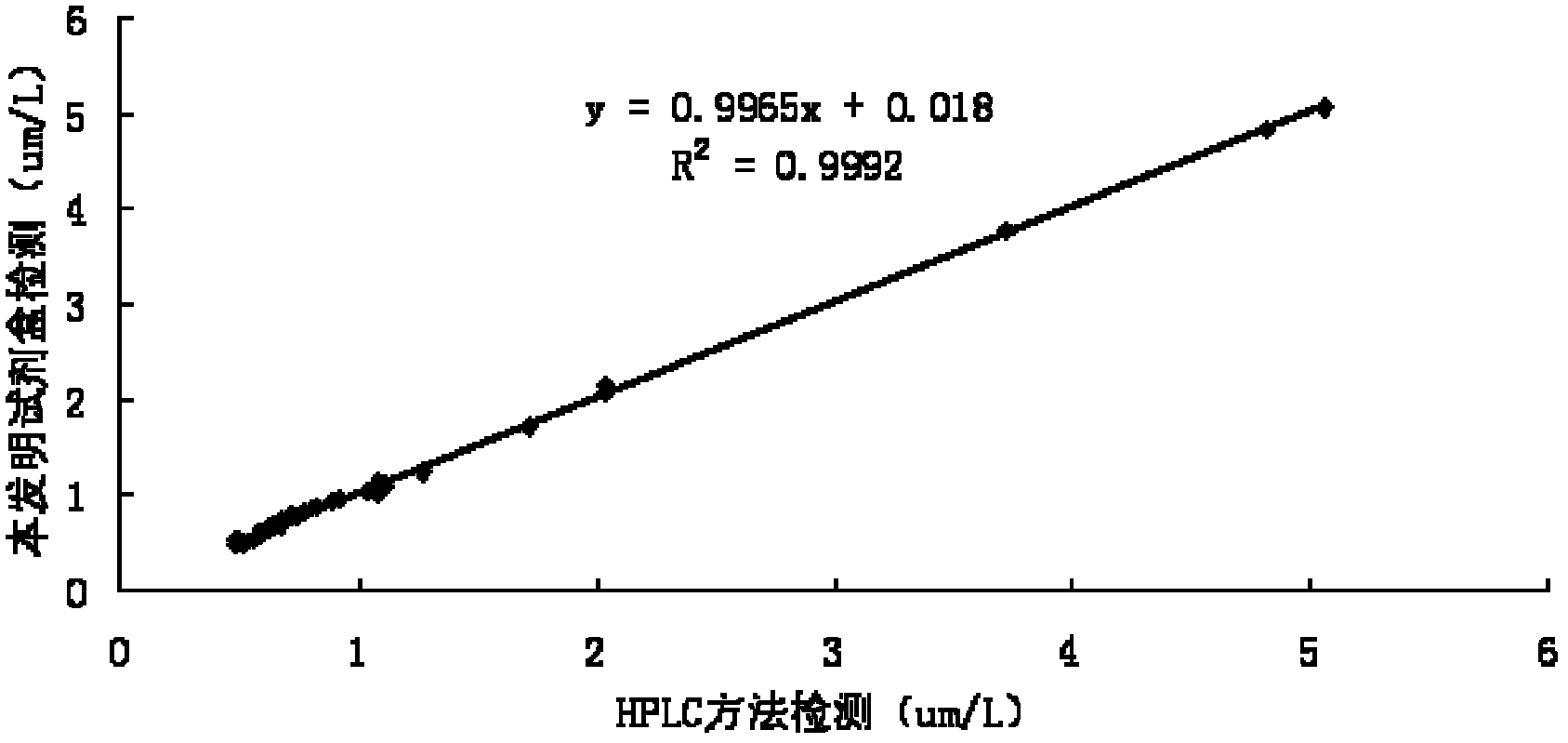
Patents
Literature
1526 results about "Human blood" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human Blood. Human blood is mainly composed of three cell types known as red blood cells (aka RBC or Erythrocytes), white blood cells (aka WBC or Leukocytes), and thrombocytes (Platelets). These blood cells are present in the medium of the liquid plasma.
Pumping cartridge having an integrated filter and method for filtering a fluid with the cartridge
InactiveUS6905479B1Positive displacement pump componentsOther blood circulation devicesParticulatesEngineering
The present invention involves, in some embodiments, reusable pump drive systems, which are coupled to removable, and preferably disposable, pumping cartridges. The invention provides, in some embodiments, novel pumping cartridges for use in pumping systems. One such cartridge includes an integrated filter element therein for filtering fluids, for example fluids pumped to the body of a patient in a medical procedure. In some embodiments, the integrated filter is utilized as a blood clot removal filter and in other embodiments is used to remove particulates liquids infused to a patient. The filter element includes a filter with pores therein that are preferably sized to permit essentially unrestricted flow of individual human blood cells therethrough and to block or collect blood clots, cell clumps, particulates etc. with sizes substantially larger than the average size of a human blood cell.
Owner:DEKA PROD LLP
Measuring device and methods for use therewith
ActiveUS20050265094A1Good flexibilityElectrolysis componentsPhotography auxillary processesMeasurement deviceEngineering
Abstract of the DisclosureThe ability to switch at will between amperometric measurements and potentiometric measurements provides great flexibility in performing analyses of unknowns. Apparatus and methods can provide such switching to collect data from an electrochemical cell. The cell may contain a reagent disposed to measure glucose in human blood.
Owner:AGAMATRIX INC
Method and device for removal of radiocontrast media from blood
ActiveUS7163520B2Accurate detectionImprove conductivityOther blood circulation devicesSolvent extractionCoronary sinusBlood pump
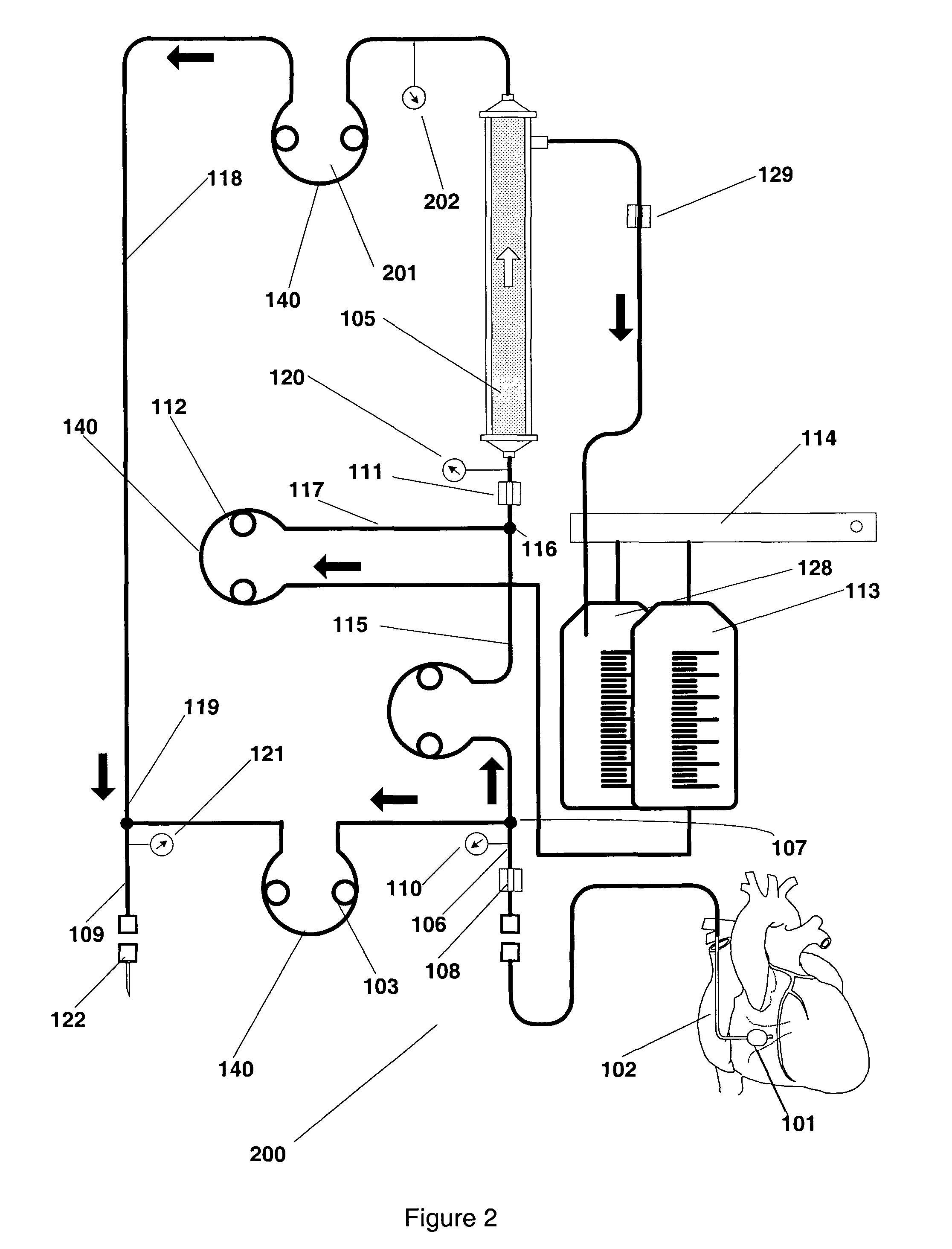
An extracorpeal blood circuit for removal of contrast from human blood using a filter and withdrawal filter pump and a by-pass pump. The withdrawal filter blood pump operates when a contrast bolus has been detected. Otherwise the bypass blood pump maintains physiological blood flow from the coronary sinus preventing the need for deflation and re-inflation of the balloon catheter. When contrast is detected blood the bypass blood pump is stopped to prevent contrast from leaking back into the patients circulatory system via the bypass pump and CS blood flow is maintained at its physiological blood flow resulting in the heart being oblivious to the transition by the prefilter blood pump.
Owner:GAMBRO LUNDIA AB
Device for measuring human blood sugar levels
InactiveUS6885881B2Accurate measurementEasy to useCatheterDiagnostic recording/measuringMeasurement pointGlycemic
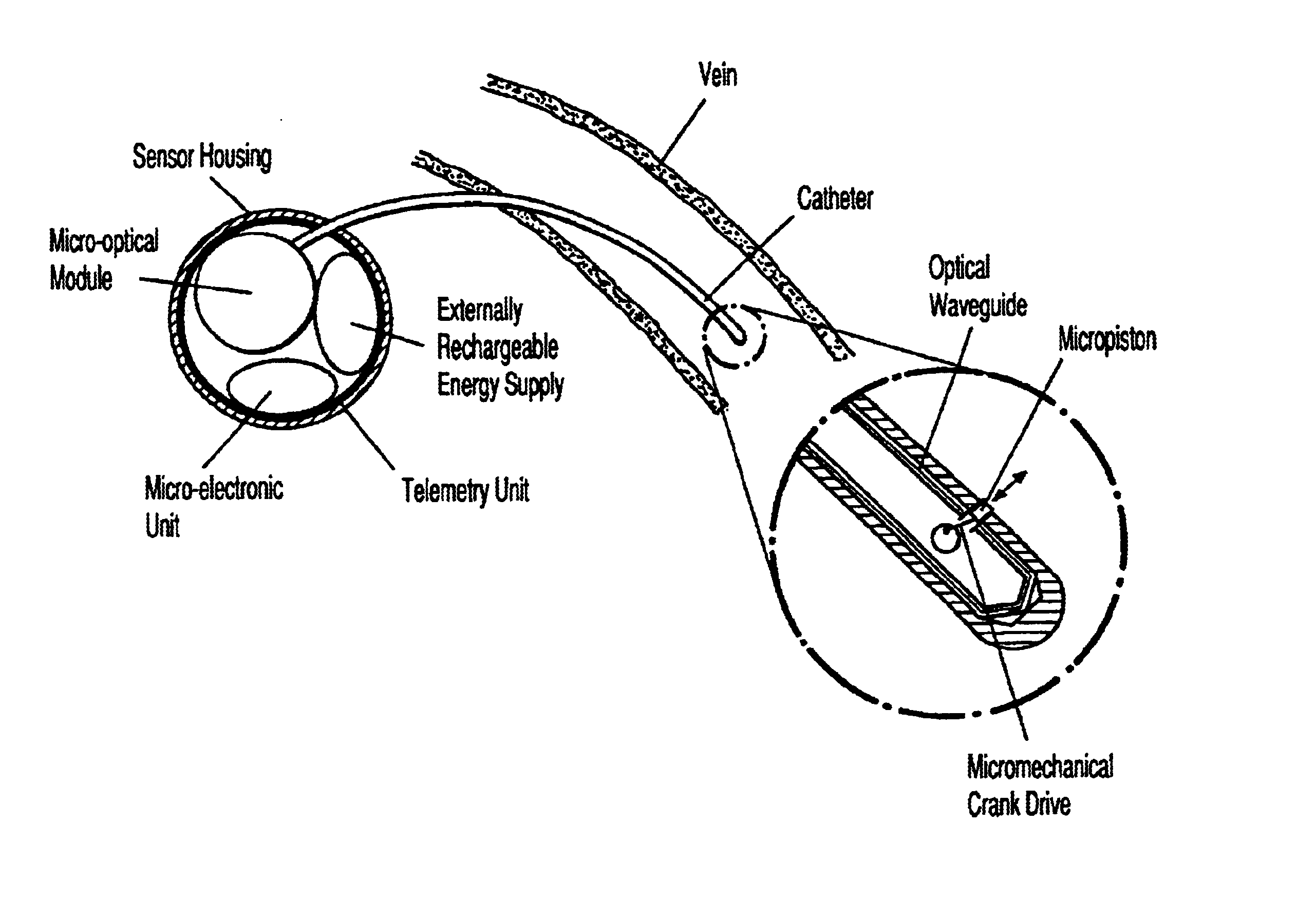
Device for measuring human blood sugar levels with a catheter, the free end of which is positioned in a blood vessel, wherein the catheter consists of at least one optical waveguide comprising a light source for coupling light into the at least one optical waveguide, a measurement point at the free end of the catheter at which point the light is emitted from the at least one optical waveguide, wherein the light is dispersed by the blood and / or transmitted by the blood and wherein the dispersed and / or transmitted light is coupled again into the minimum of one return optical waveguide, a detector to receive the light which is returned, and a computer unit for analysing the light received by the detector. Provision is made for a cleansing device to be located at the point of measurement for removing the tissue particles deposited from the blood in order to provide a device which is as accurate as possible and which delivers constant measurement values over time for blood sugar levels which can be used as the basis for further data analysis.
Owner:LEONHARDT STEFFEN
Measuring device and methods for use therewith
InactiveUS20050276133A1Good flexibilityDigital storageMaterial electrochemical variablesMeasurement deviceEngineering
The ability to switch at will between amperometric measurements and potentiometric measurements provides great flexibility in performing analyses of unknowns. Apparatus and methods can provide such switching to collect data from an electrochemical cell. The cell may contain a reagent disposed to measure glucose in human blood.
Owner:AGAMATRIX INC
Methods and devices for monitoring the integrity of a fluid connection
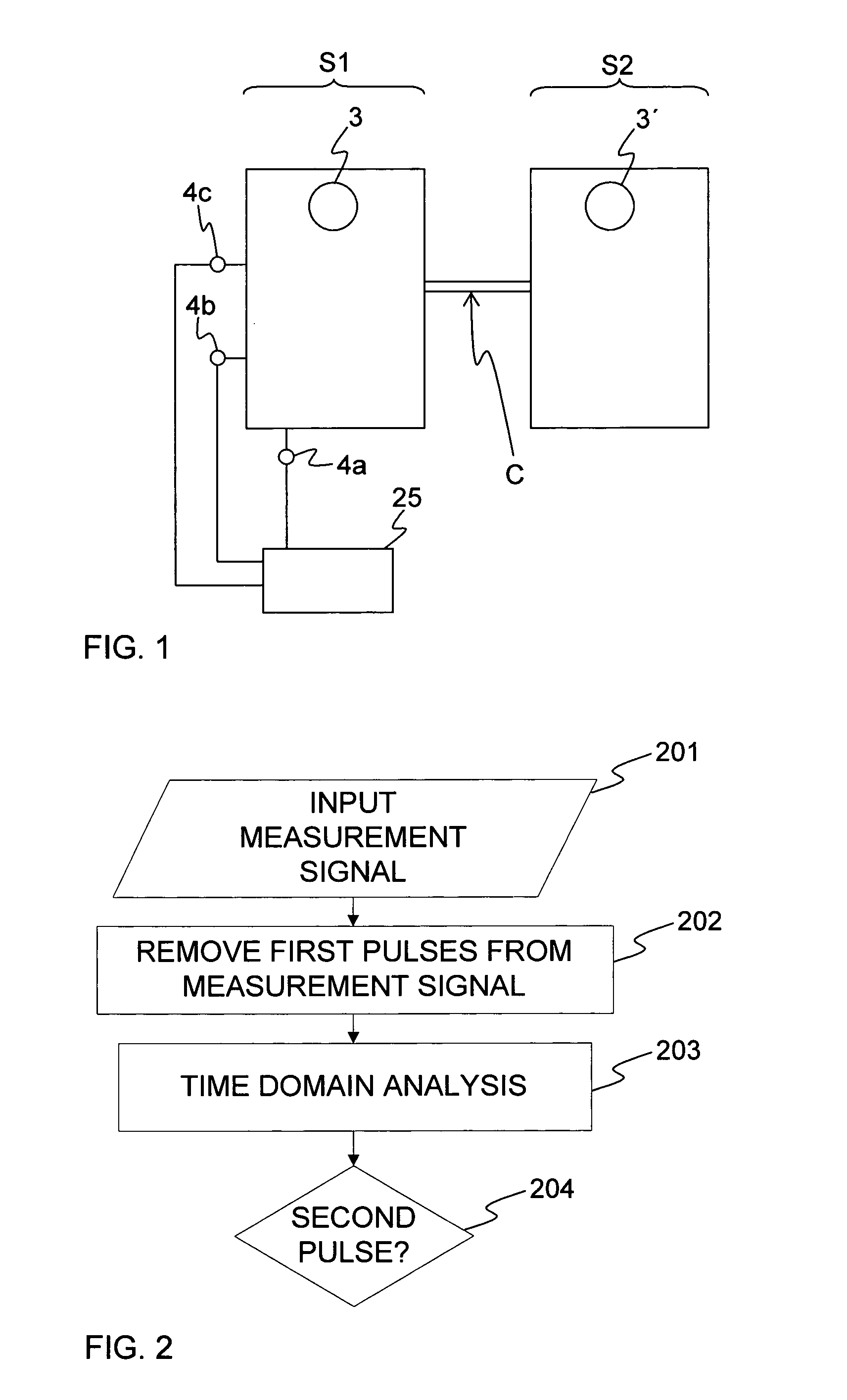
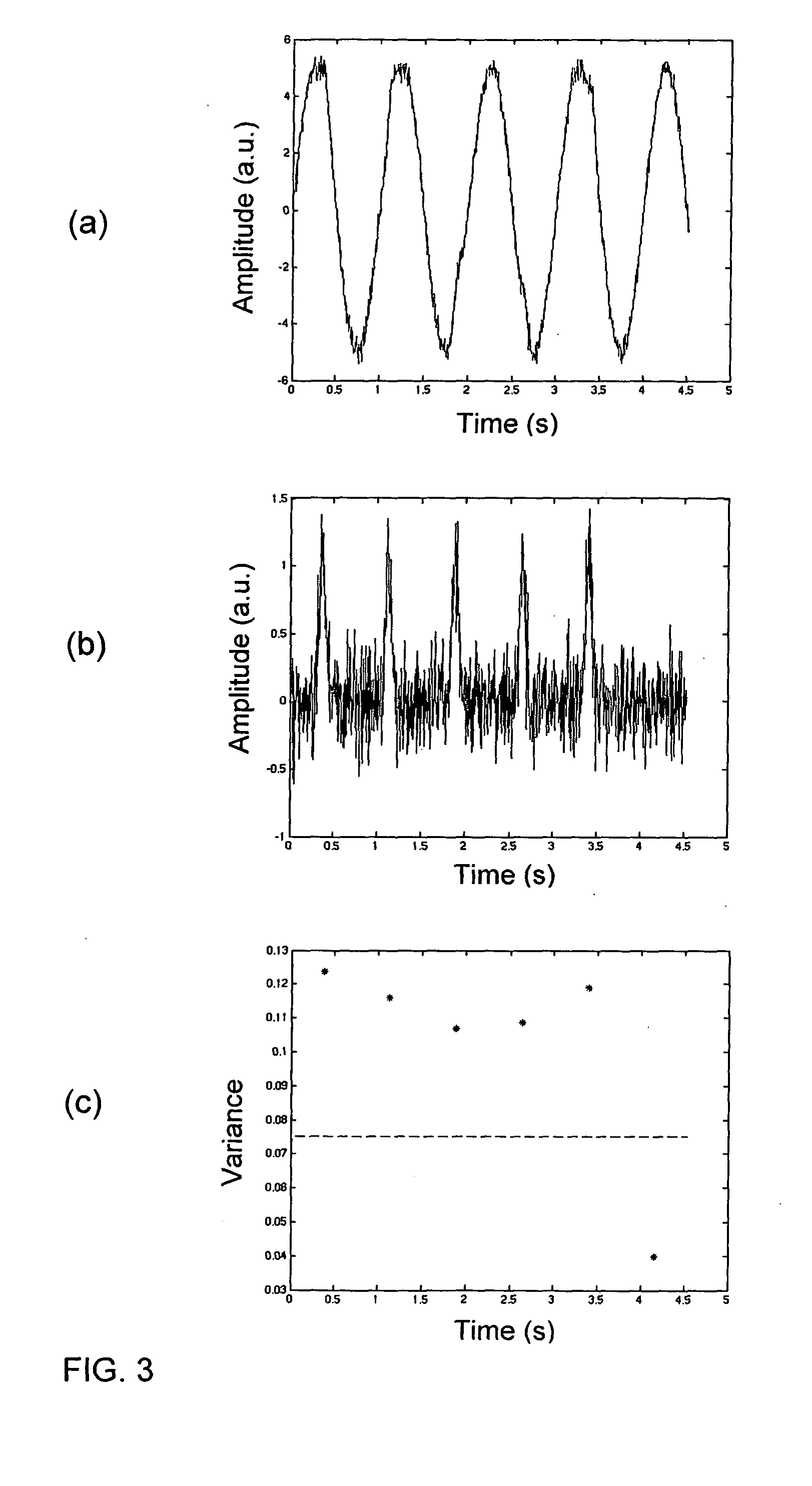
ActiveUS20110106466A1Improve robustnessImprove certaintyMeasurement of fluid loss/gain rateOther blood circulation devicesTime domainBlood flow
A surveillance device monitors the integrity of a fluid connection between first and second fluid containing systems based on at least one time-dependent measurement signal from a pressure sensor in the first fluid containing system. The first fluid containing system comprises a first pulse generator, and the second fluid containing system comprises a second pulse generator. The pressure sensor is arranged to detect first pulses originating from the first pulse generator and second pulses originating from the second pulse generator. The integrity of the fluid connection is determined based on the presence of second pulses in the measurement signal. The second pulses may be detected by analysing the measurement signal in the time domain and / or by using timing information indicative of the timing of the second pulses in said at least one measurement signal. The analysis may be based on a parameter value that represents a distribution of signal values within a time window of the measurement signal. The parameter value may be calculated as a statistical dispersion measure of the signal values, or may result from a matching of the signal values within the time window to a predicted temporal signal profile of a second pulse. The fluid connection may be established between a human blood system and an extracorporeal blood flow circuit.
Owner:GAMBRO LUNDIA AB
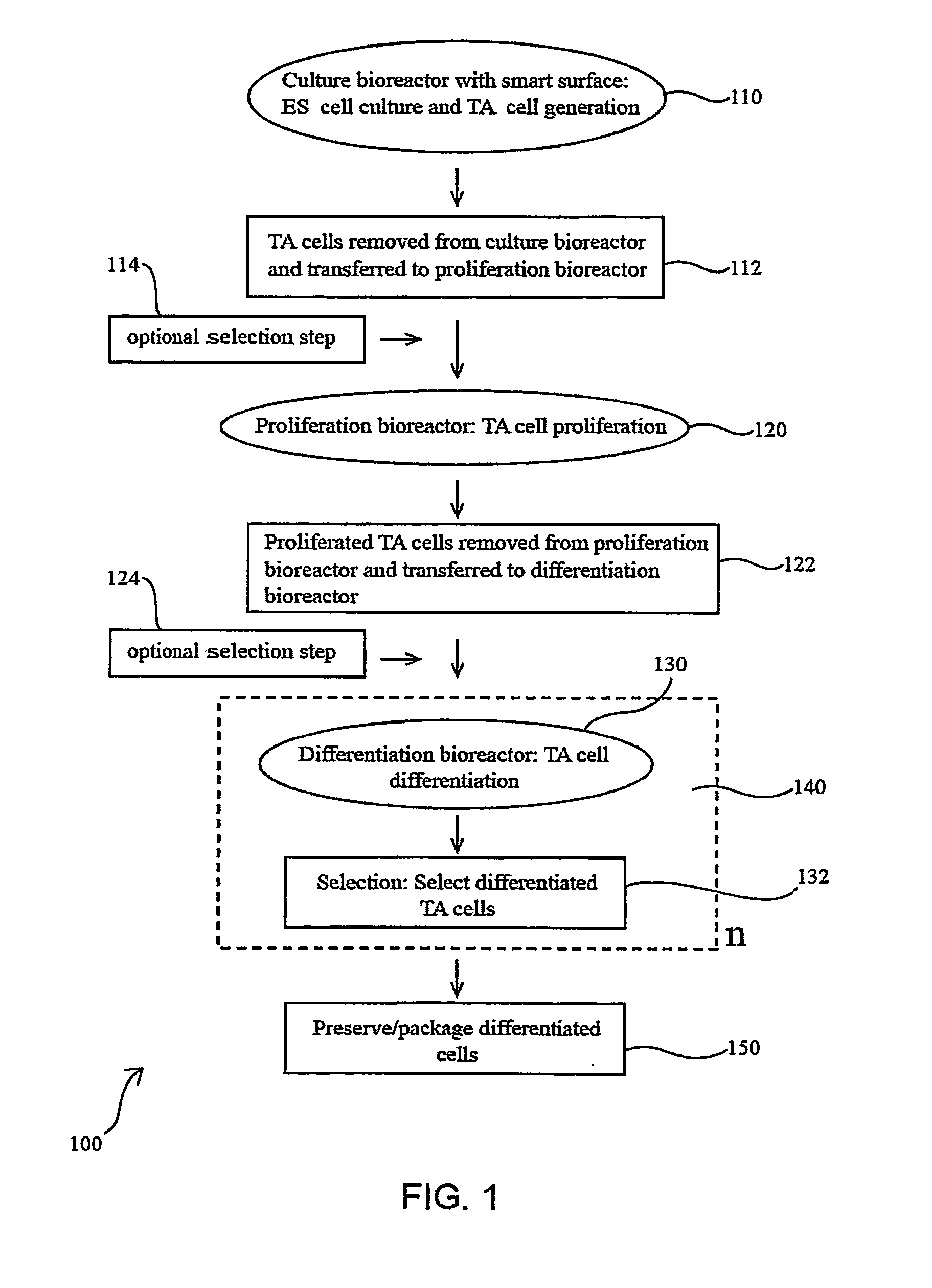
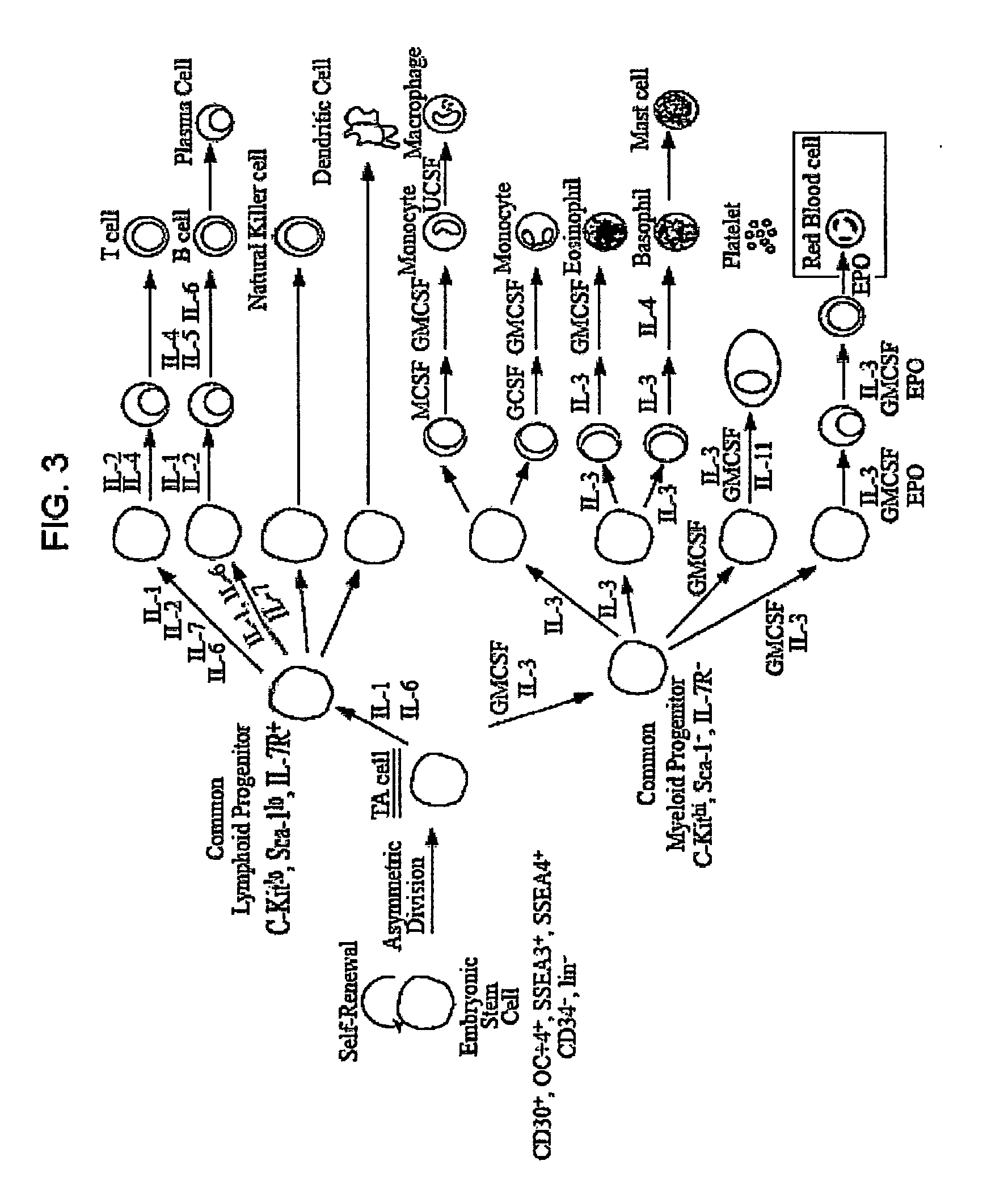
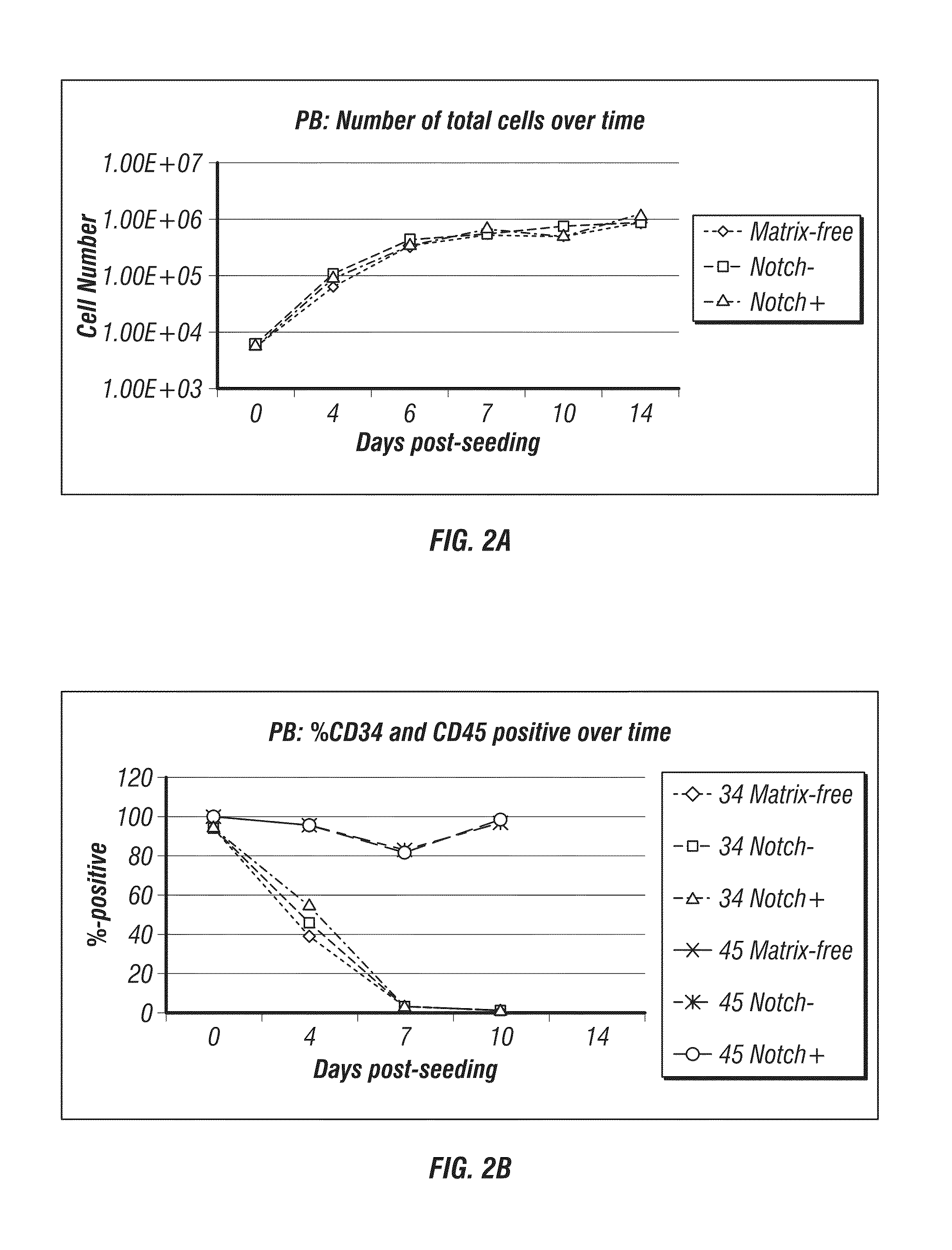
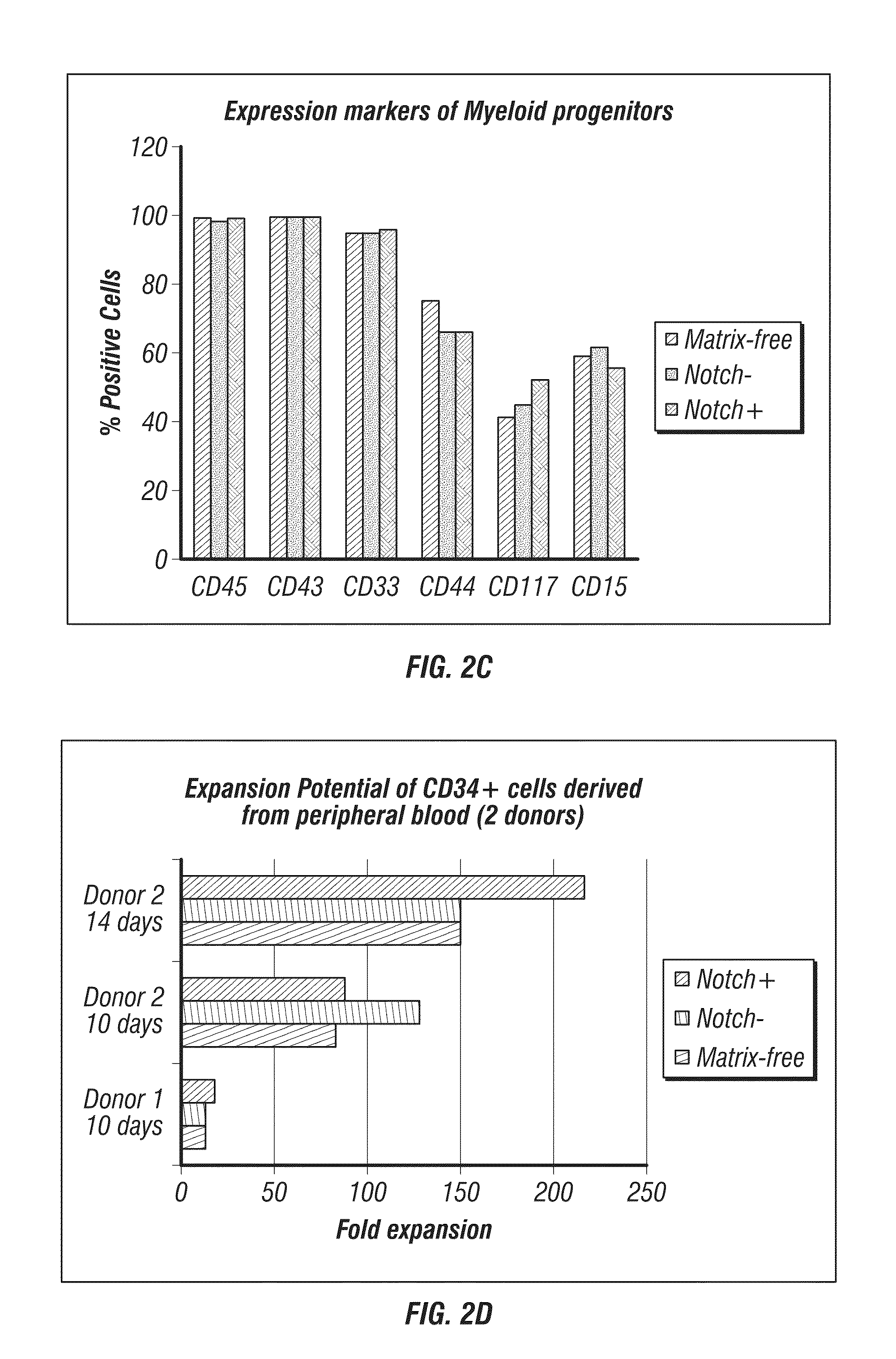
Methods for producing blood products from pluripotent cells in cell culture
The present invention provides methods for in vitro production of clinically useful quantities of differentiated human blood cells. In various embodiments of the present invention, immortal pluripotent cells are used to produce differentiated blood cell populations using a cell production device. In a specific embodiment, the device is a sequential series of bioreactors utilizing growth media containing specific combinations of maintenance-, proliferation- or differentiation-promoting factors that maintain, expand and promote the maturation and differentiation of the desired cell types. The immortal pluripotent cells can optionally be genetically modified so as to remove histcompatibility or blood group antigens.
Owner:AUSTRALIAN STEM CELL CENT
Catheter For Aspirating, Fragmenting And Removing Material
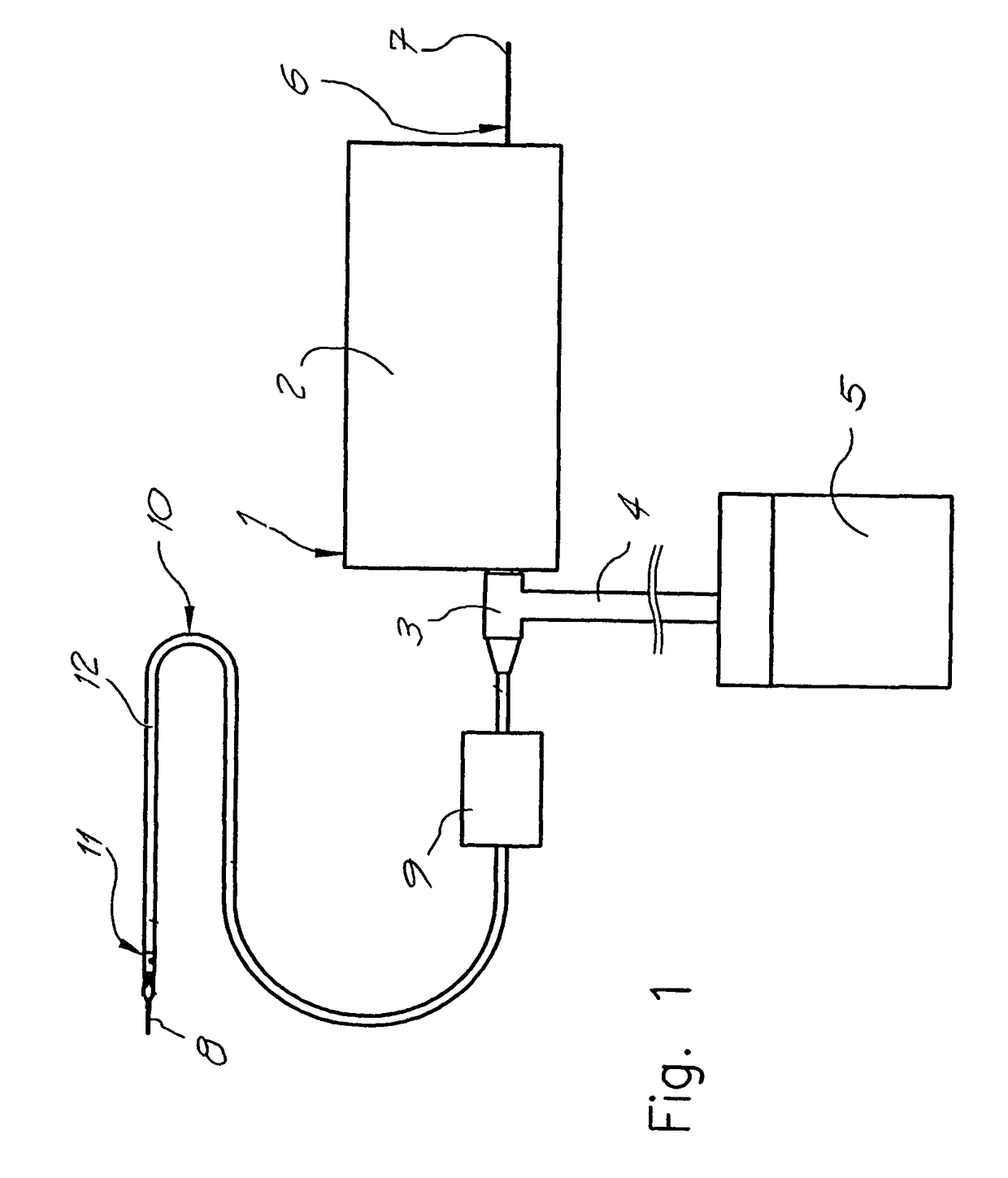
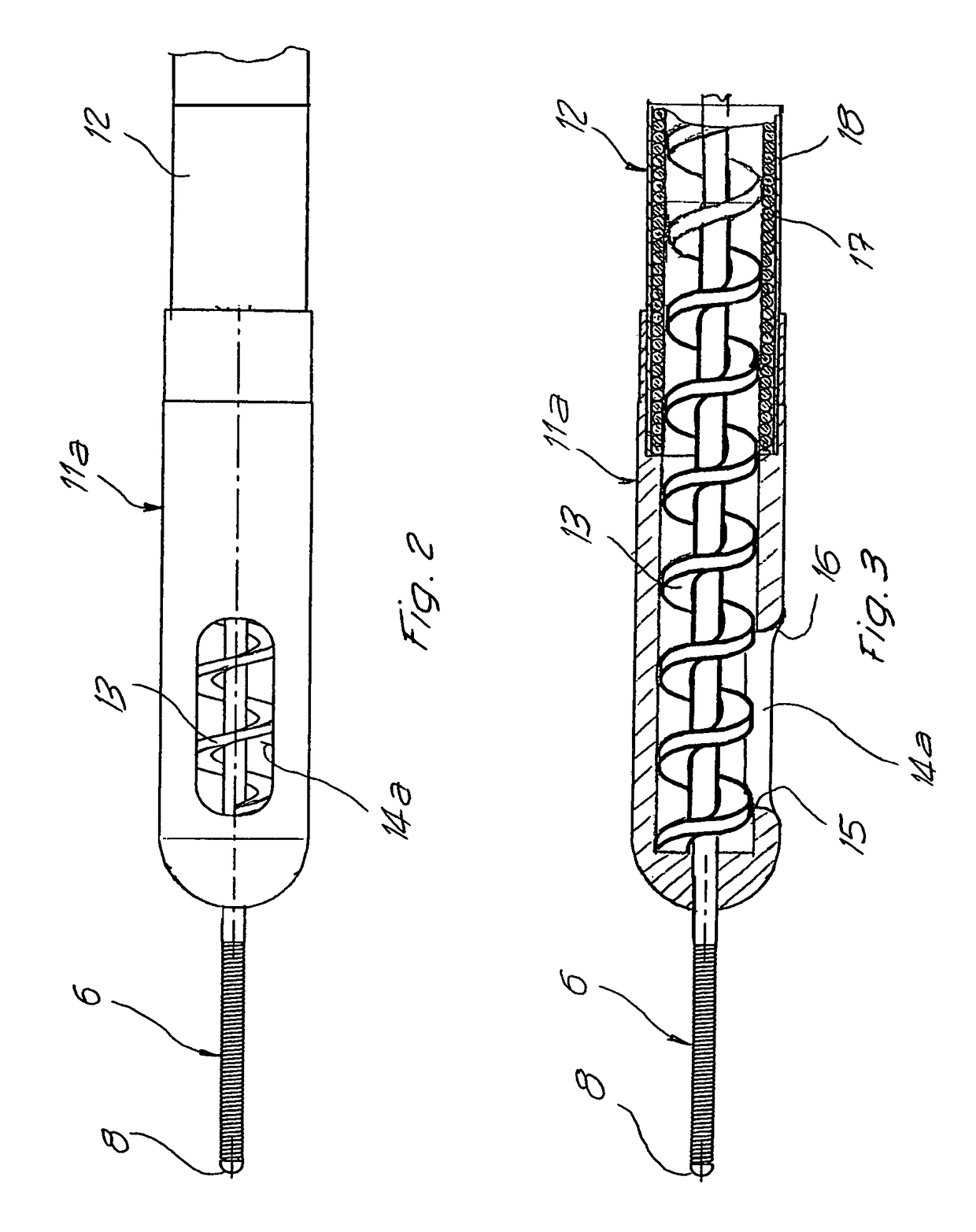
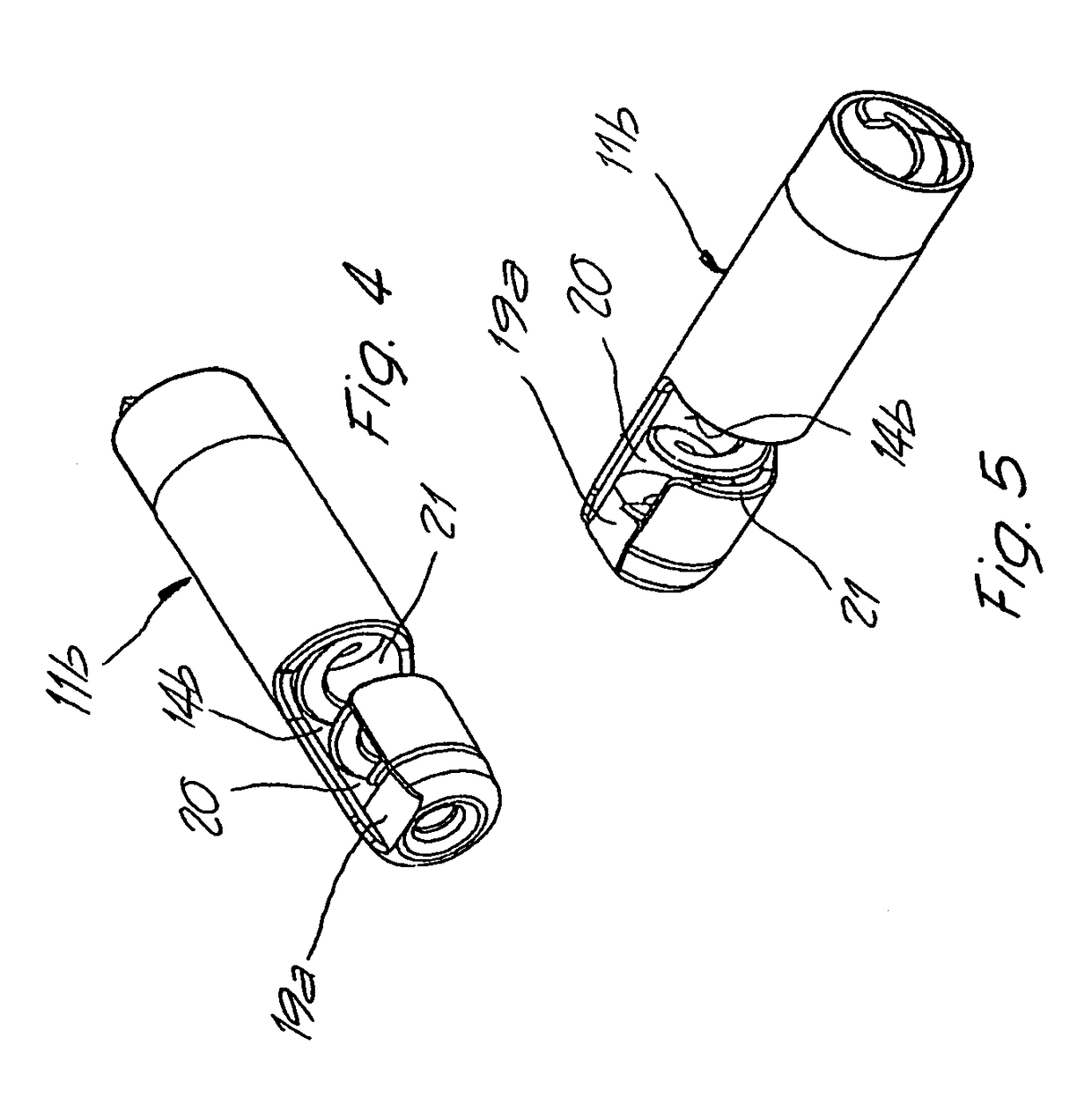
ActiveUS20070219484A1Resistant to tensionPressure resistanceCannulasExcision instrumentsThrombusEngineering
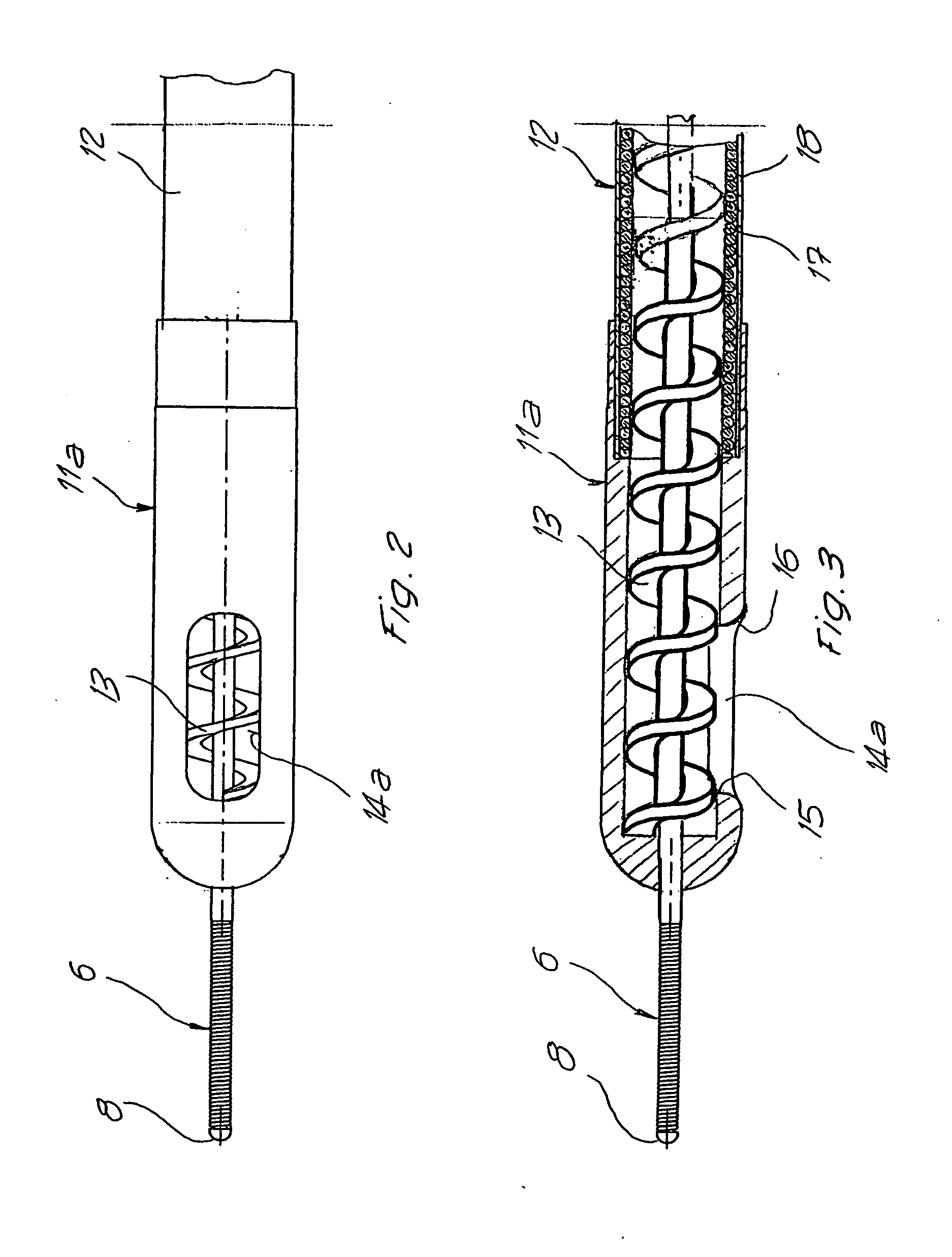
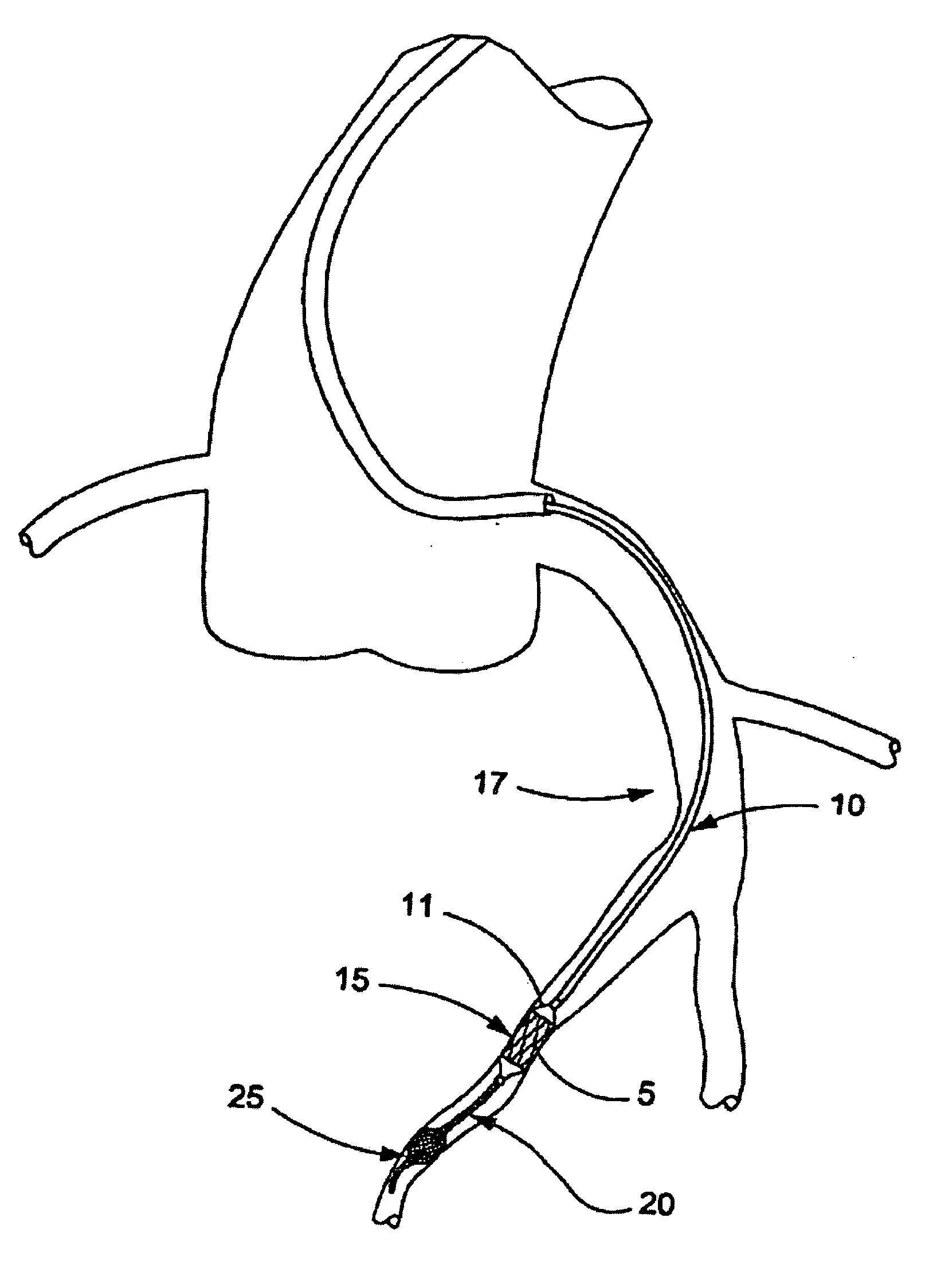
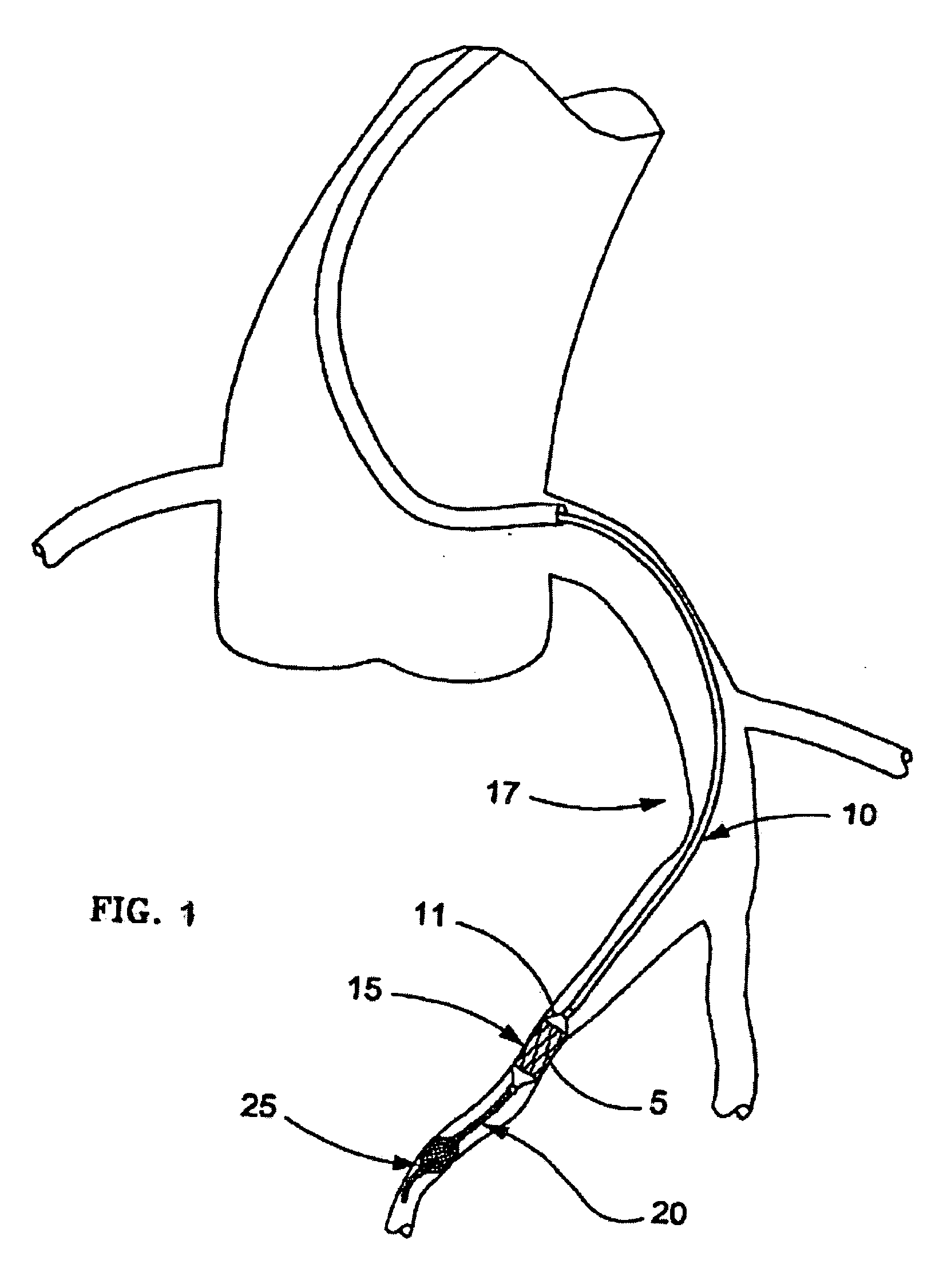
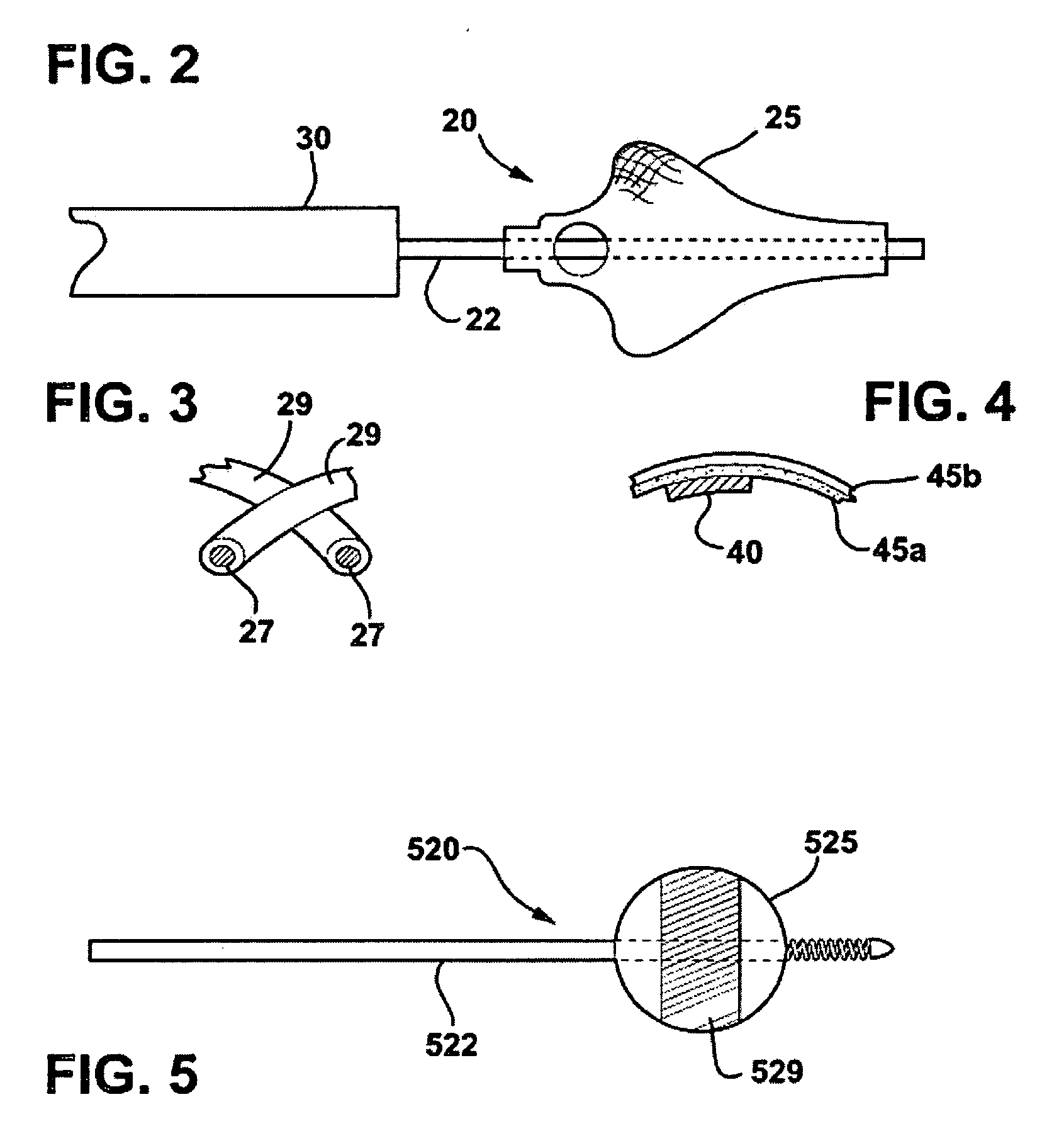
Catheter for aspiration, fragmentation and removal of removable material from hollow bodies, in particular of thrombi and emboli from human blood vessels, comprising a working head, a flexible transport screw, a flexible tube, and a cutting tool. The working head is independently displaceable along a guide wire, is arranged at the distal end of the catheter, and has at least one lateral opening. The flexible transport screw can be rotated at a distance by means of a rotary drive of a drive unit. The flexible tube surrounds the transport screw, is connected to the working head, and is intended for removing the detached thrombi and emboli fragments. The transport screw forms a shearing cutting tool in cooperation with the lateral opening of the working head, in order to comminute the detached thrombi and emboli between peripheral borders of the transport screw and borders of the lateral opening.
Owner:STRAUB MEDICAL AG
Catheter for aspirating, fragmenting and removing material
Owner:STRAUB MEDICAL AG
Cuff-free portable device for monitoring human physiological parameters and method
InactiveCN101828908AReal-time displayEliminate discomfortDiagnostic recording/measuringSensorsMicrocontrollerEngineering
The invention discloses a cuff-free portable device for monitoring a human physiological parameters and a method. An optical signal receiver inside a shell is connected with a signal amplifying module, a signal filtering module, an A / D conversion module and a singlechip in turn; the singlechip is externally connected with a data storage; a shell connecting strip is tied on the human wrist; and the surface of the shell is provided with a liquid crystal screen. A photoelectric detection module is arranged on the back of the shell and contacted with the body surface skin corresponding to the radial artery; a chip temperature sensor is arranged in the middle of the back of the shell and contacted with the wrist skin; the photoelectric detection module calculates the acquired human body pulse wave light volume description signals to acquire the parameter values of the human blood pressure, blood oxygen saturation, heart rate and respiratory frequency through the extraction of the signal characteristic time and calculation of a signal power spectrum; and a human body surface temperature signal is acquired by the temperature sensor. The cuff-free portable device for monitoring the human physiological parameters can non-invasively, comfortably, accurately display a plurality of human physiological parameters in real time.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Optimised coding sequence and promoter
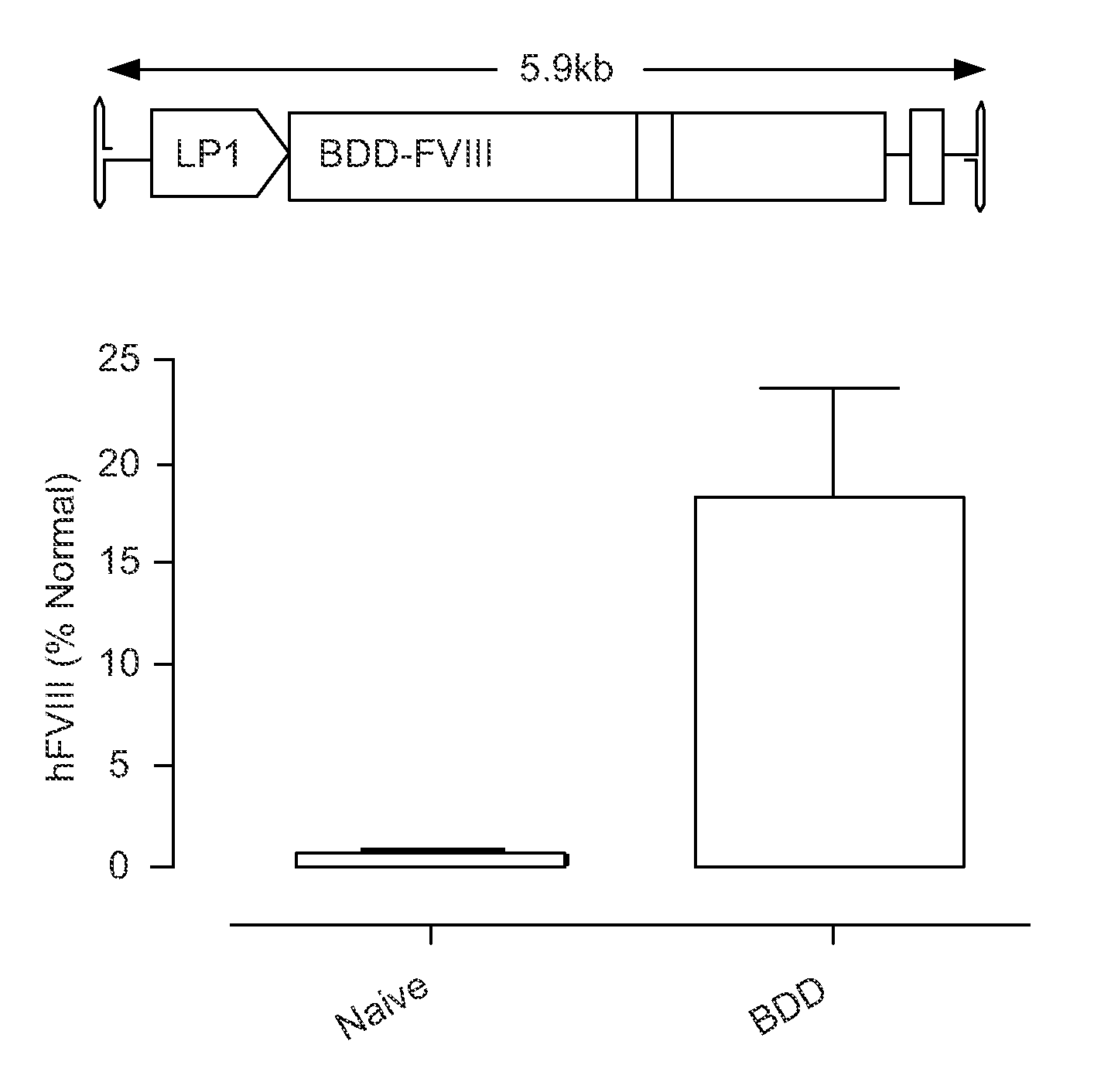
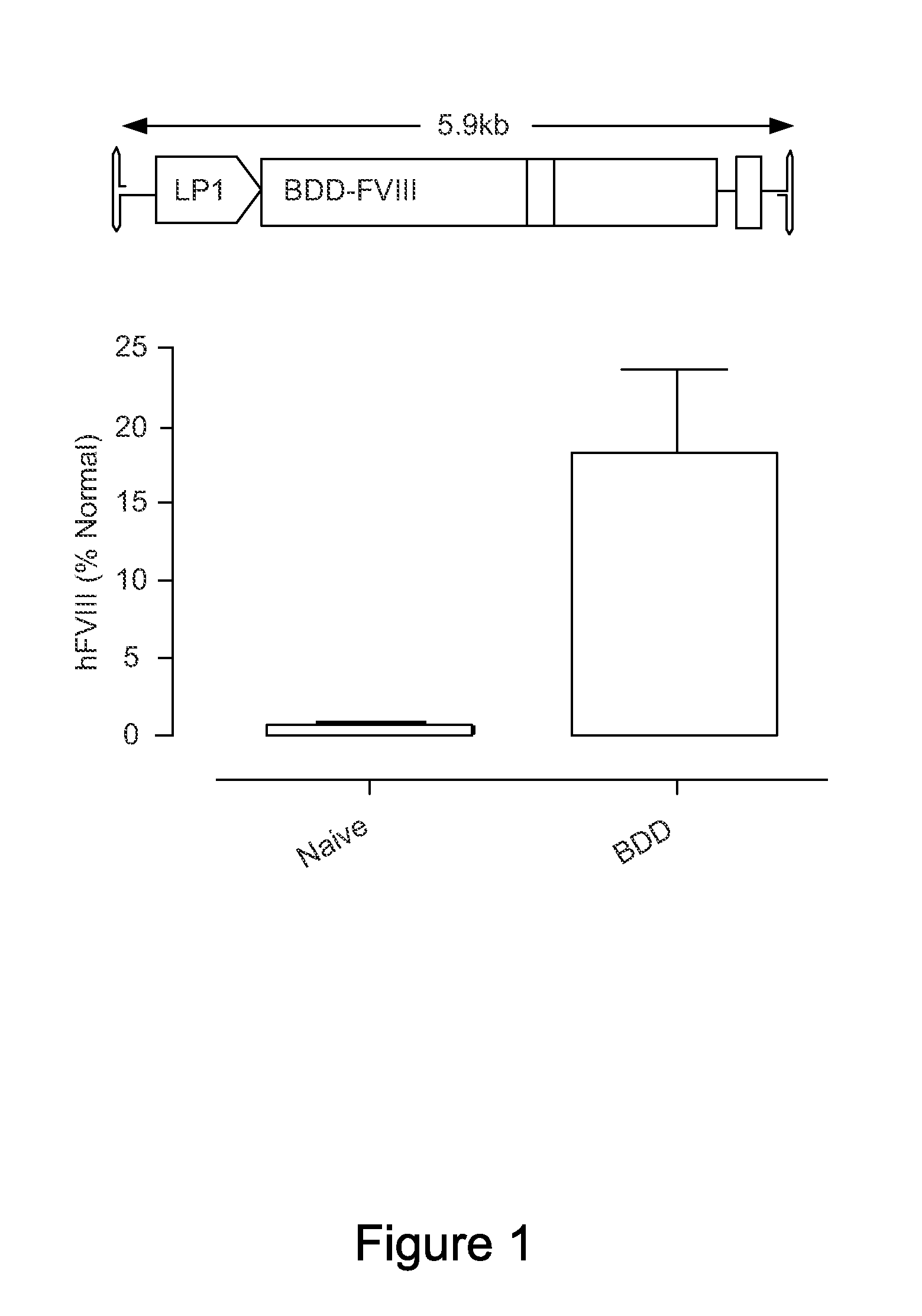
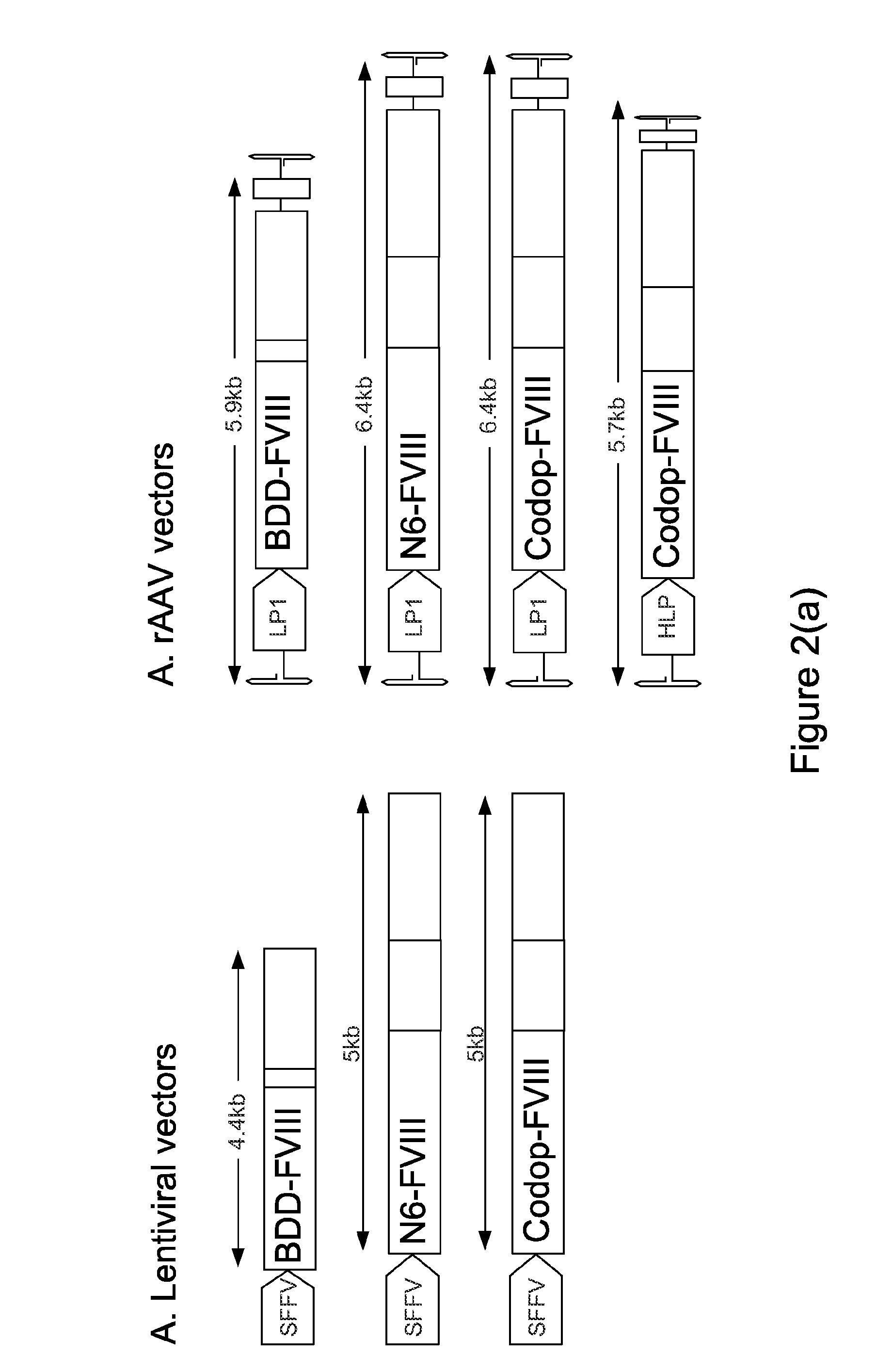
ActiveUS20130024960A1Shorten the timeImprove the level ofFactor VIIOrganic active ingredientsLipid storageLipid storage disease
An optimized coding sequence of human blood clotting factor eight (VIII) and a promoter may be used in vectors, such as rAAV, for introduction of factor VIII, and / or other blood clotting factors and transgenes. Exemplary of these factors and transgenes are alpha-1-antitrypsin, as well as those involved in the coagulation cascade, hepatocye biology, lysosomal storage, urea cycle disorders, and lipid storage diseases. Cells, vectors, proteins, and glycoproteins produced by cells transformed by the vectors and sequence, may be used in treatment.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC +2
Functional-material-based glucose biosensor and manufacturing method thereof
InactiveCN102507693AStrong specificityImprove analysis accuracyMaterial analysis by electric/magnetic meansConcentrations glucoseBlood Glucose Measurement
The invention relates to a functional-material-based glucose biosensor. A working electrode of the glucose biosensor is made from a graphene nanomaterial, the glucose biosensor is formed by using the working electrode made from the graphene nanomaterial in the assembling of the glucose biosensor, belongs to a quantitative test technology and shows excellent linearity and resolution, and the detection sensitivity, the detection range and the detection speed are increased; and particularly in the concentration range of human blood glucose, the amplitude of a response current can be increased by 50%, and the resolution can be increased by over twice. The functional-material-based glucose biosensor and a preparation method thereof have the advantages that: immobilized enzyme of a nano-functional membrane prepared from the graphene nanomaterial can be continuously analyzed for over 1,000 times, so that the cost for measurement is low, the accuracy of analysis is higher than that of other methods, the relative error reaches about 1%, the response time is shortened to 20 seconds, the service life is also greatly prolonged, the precise timing measurement of the concentration of glucose can be achieved; and the functional-material-based glucose biosensor has the characteristics of high specificity, short-time performance, low-cost analysis, no special requirements on analyzing substances, safety and simplicity and convenience in operation, and convenience for on-site measurement and the like and can be applied to the daily measurement of blood glucose of diabetes patients.
Owner:GUILIN MEDICAL UNIVERSITY
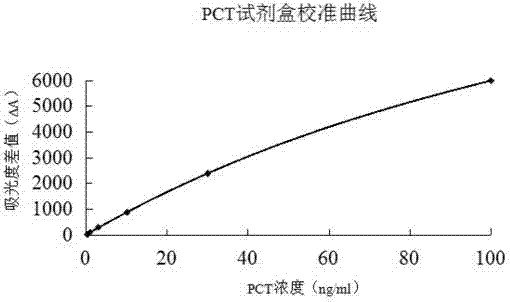
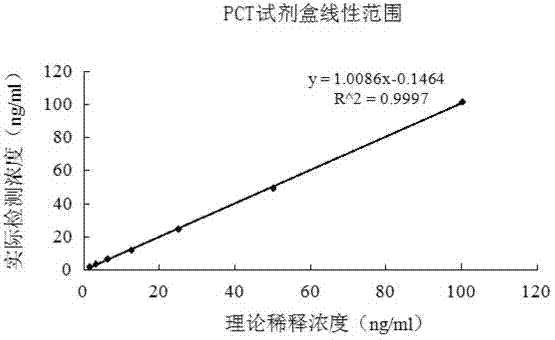
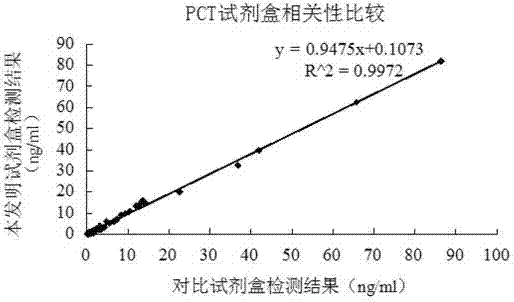
Latex enhanced turbidimetric immunoassay kit of quantitatively detecting procalcitonin PCT
ActiveCN102759631AHigh detection sensitivityMeet the needs of clinical applicationsMaterial analysis by observing effect on chemical indicatorBiotin-streptavidin complexTurbidimetry
The invention relates to a latex enhanced turbidimetric immunoassay kit of quantitatively detecting procalcitonin PCT. The kit comprises an R1 reagent, an R2 reagent and a calibrator, wherein the R1 reagent comprises a protecting agent, a reaction enhancing agent, a preservative and buffer solution; the R2 reagent comprises a protecting agent, a preservative, buffer solution and anti-human PCT antibody coated sensitization polystyrene latex particles; the calibrator comprises a protecting agent, a preservative, buffer solution and PCT recombinant protein; the human PCT antibody in the R2 reagent is linked with polystyrene latex particles through streptavidin-biotin; and the particle diameter of the latex particles in the R2 reagent is 40-500 nm. The kit can be used on a biochemical analyzer and a scatter turbidimetry analyzer for quantitatively detecting the PCT content in human blood. The invention provides the PCT detection kit which has the advantages of convenience, quickness, high sensitivity, strong specificity and accurate quantification; and the kit has high instrument compatibility, is low in detection cost and meets the requirements on PCT turbidimetric products in clinical use.
Owner:NANJING NORMAN BIOLOGICAL TECH
Methods for using dendritic cells to activate gamma/delta-T cell receptor-positive T cells
InactiveUS6821778B1Control progressIncrease the number ofArtificial cell constructsBlood/immune system cellsAdoptive cellular immunotherapyDendritic cell
This invention relates to methods of using human dendritic cells to present antigens for the induction of antigen-specific T cell-mediated immune responses. In particular, it relates to the isolation of dendritic cells from human blood, exposing the cells to antigens, co-culturing the antigen-pulsed dendritic cells with gammadelta-T cell receptor-positive-T cells (gammadelta-TCR<+> T cells) obtained from unprimed or weakly primed individuals for the stimulation of antigen-specific T cell proliferative and cytotoxic activities. The dendritic cell antigen presentation system described herein has a wide range of applications, including but not limited to, activation and expansion of large numbers of antigen-specific major histocompatibility complex-unrestricted T cells for use in adoptive cellular immunotherapy against infectious diseases and cancer.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Method of selectively assaying adiponectin multimers
ActiveUS20070042424A1Accurate assessmentPeptide/protein ingredientsMicrobiological testing/measurementElisa kitFiltration
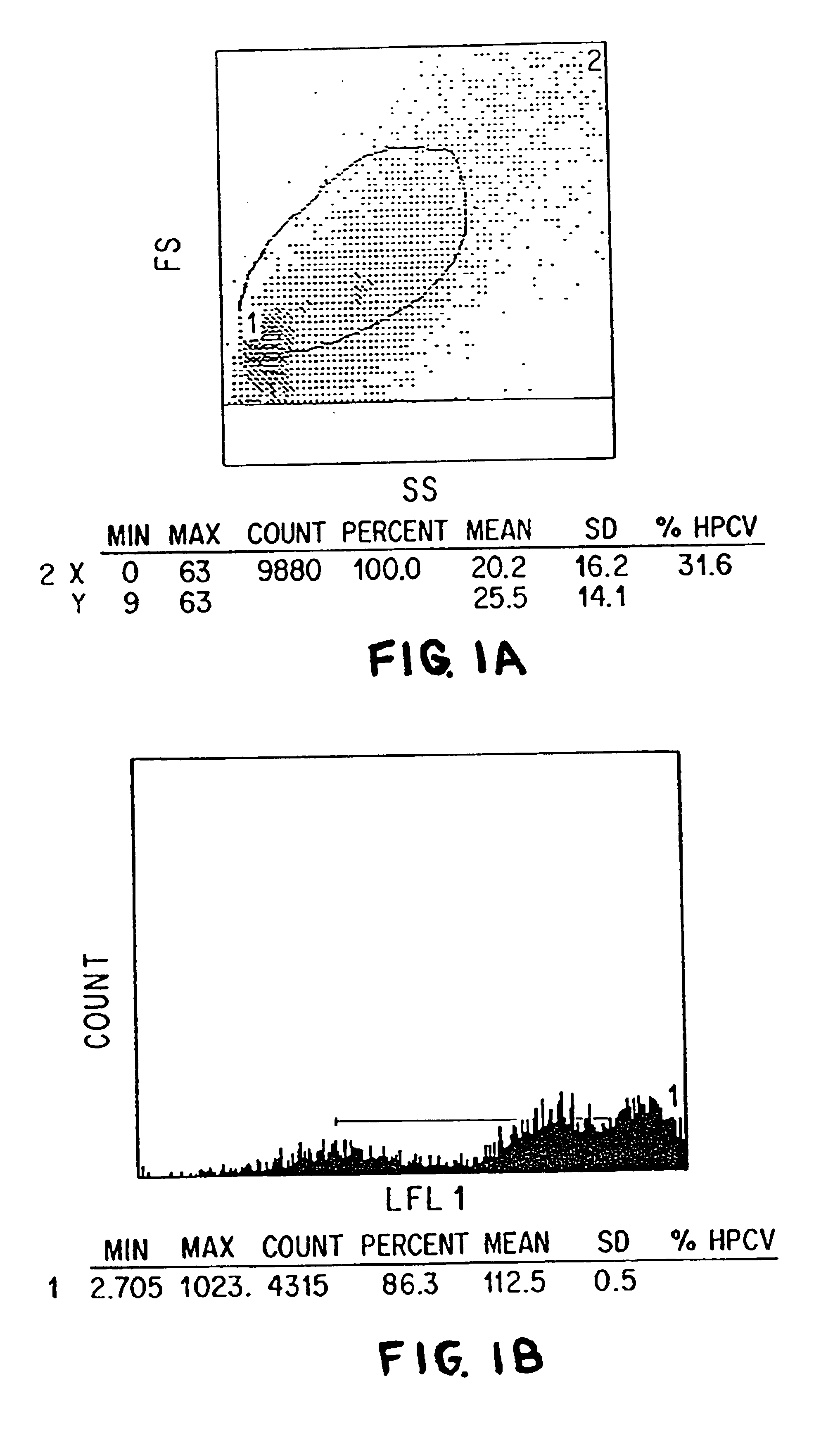
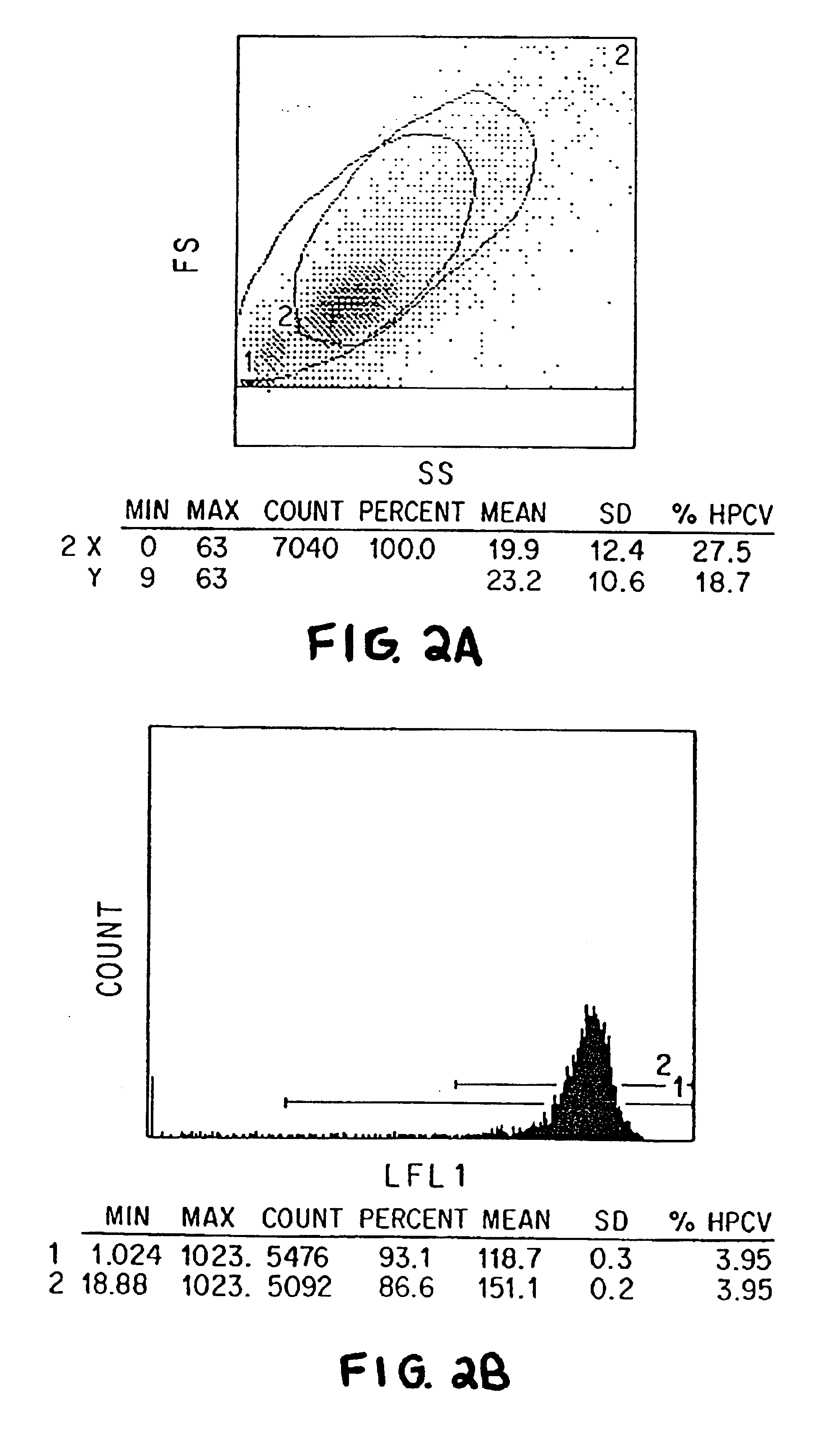
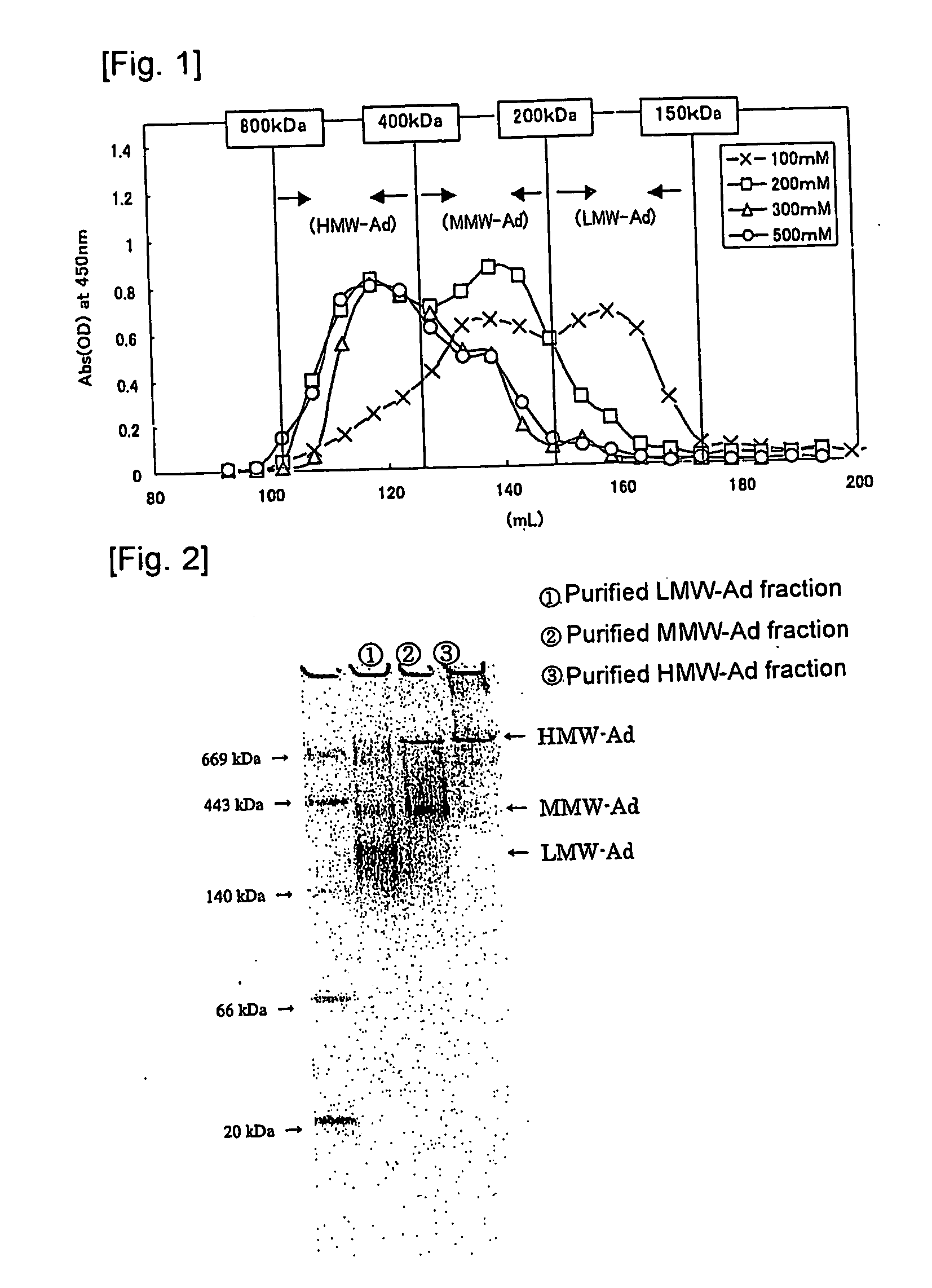
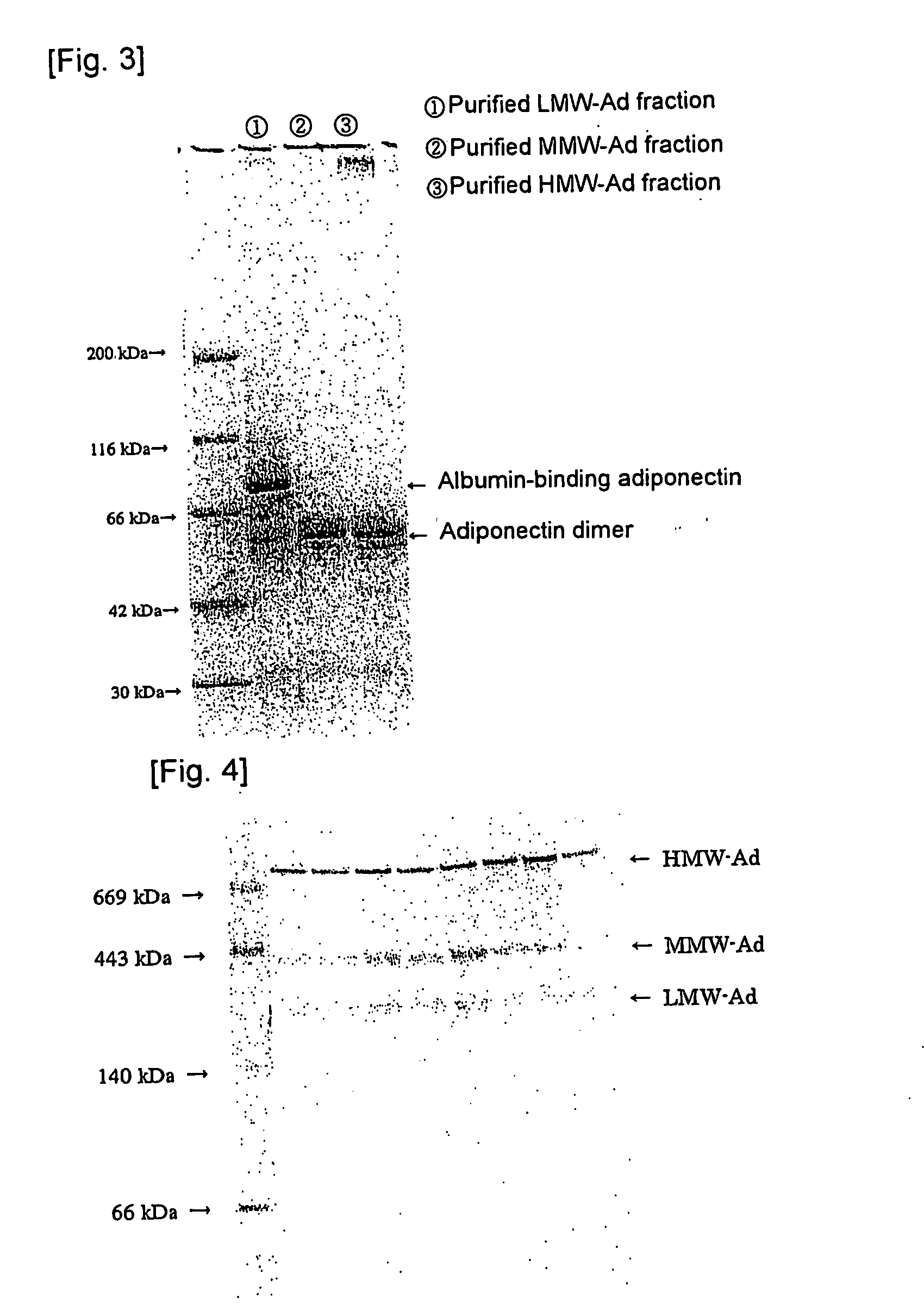
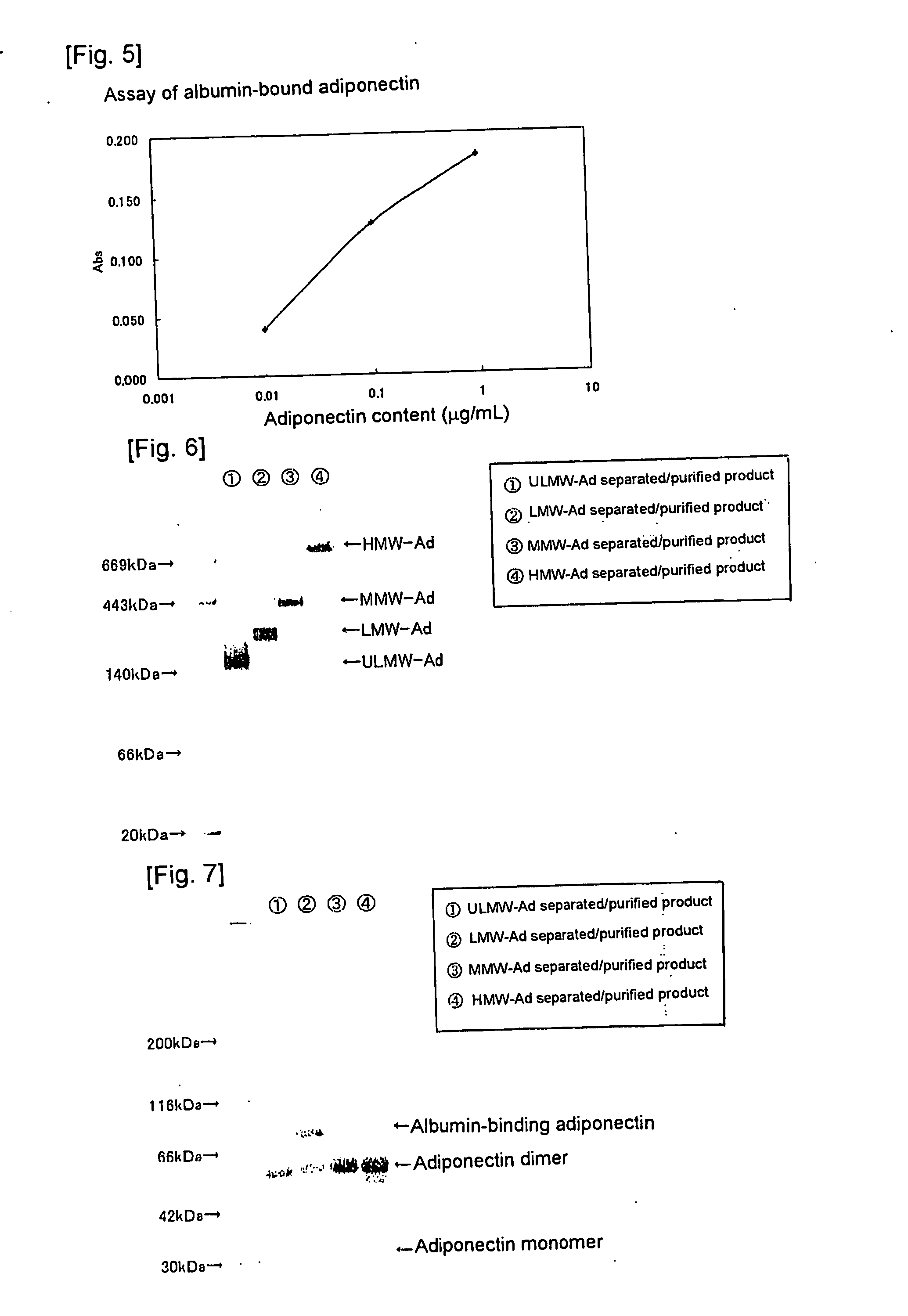
We have discovered a “human adiponectin ELISA kit”, which can specifically measure adiponectin in human blood serum (or blood plasma) or in extracted fluid from lipocytes or culture supernatant fluid. After fractioning human serum by use of gel filtration column, each fraction was measured by the present kit, and as a result, adiponectin immunity activity was detected as a plurality of peaks at above 670 kD of molecular weight. From the results, it is presumed that adiponectin is present in the blood as a polymer or forms a complex with polymer proteins.
Owner:SEKISUI MEDICAL CO LTD +1
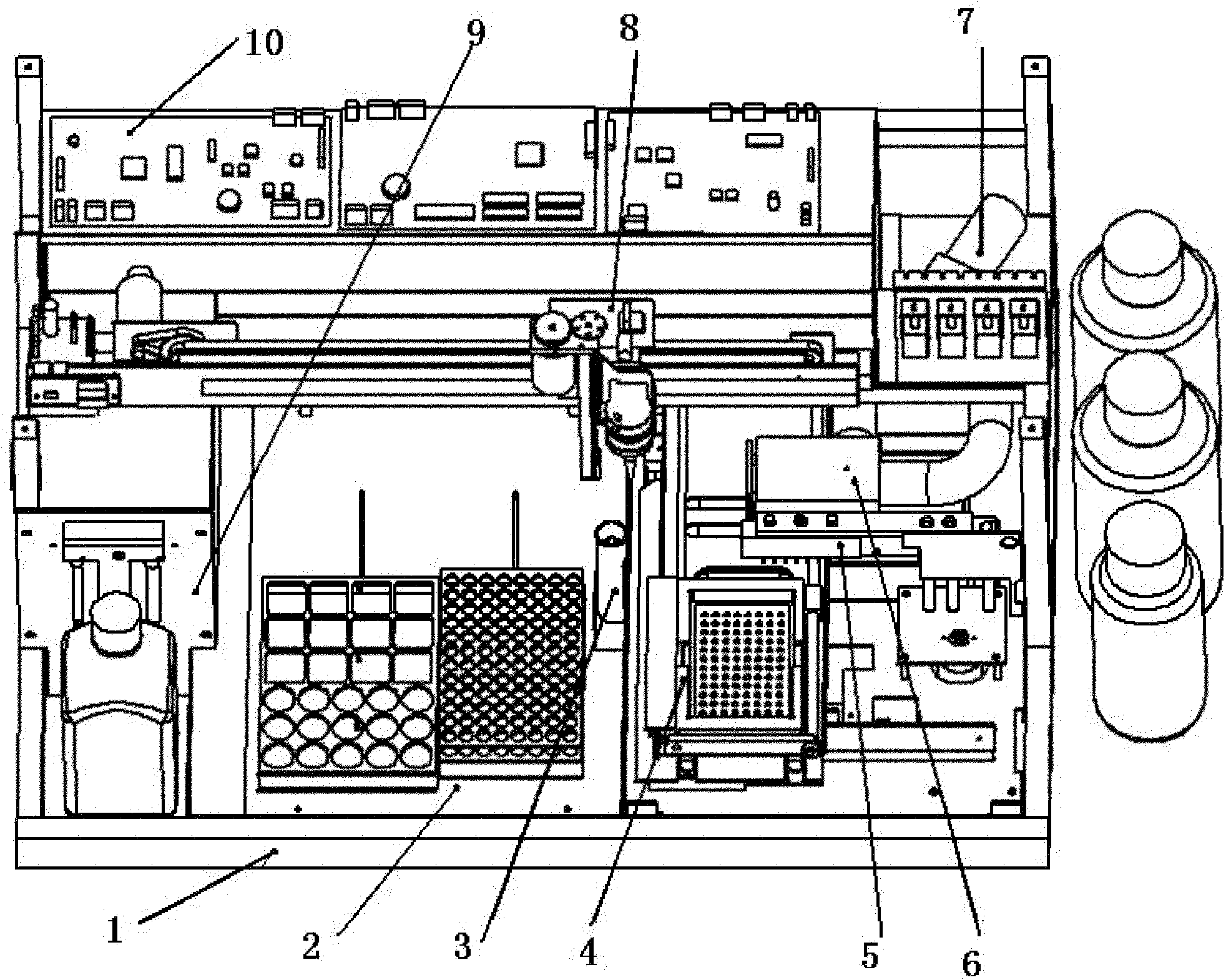
Fully automatic biochemical immune analyzer
ActiveCN102147405AReasonable design layoutFully automatedMaterial analysis by optical meansBiological testingHuman bloodPesticide
The invention provides a fully automatic biochemical immune analyzer which comprises a rack, and a sample support, a reagent shelf assembly, an injection assembly, a washer, a reaction plate assembly, a washing assembly, an optical measuring assembly, a water channel and gas channel system and a circuit control system which are arranged on the rack. The fully automatic biochemical immune analyzeris mainly used for fully automatic quantitative measurement or qualitative detection of immune and biochemical indexes of human blood and other body fluid, concentration of therapeutic drugs and general ingredients, harmful additives, harmful and toxic elements, residues of pesticides and veterinary drugs, microbial toxins and the like in the food safety field.
Owner:SHENZHEN YHLO BIOTECH
Affinity purified human polyclonal antibodies and methods of making and using them
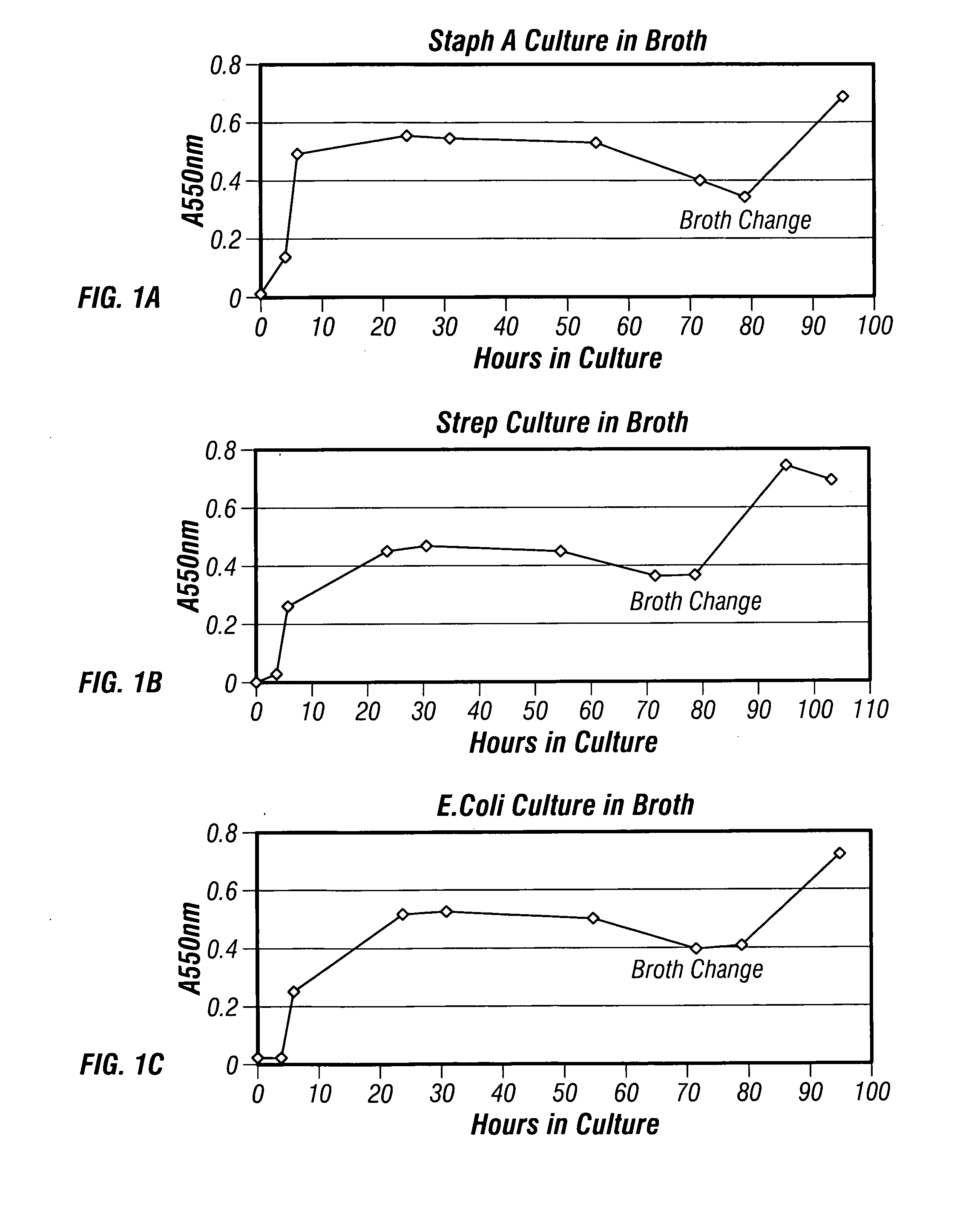
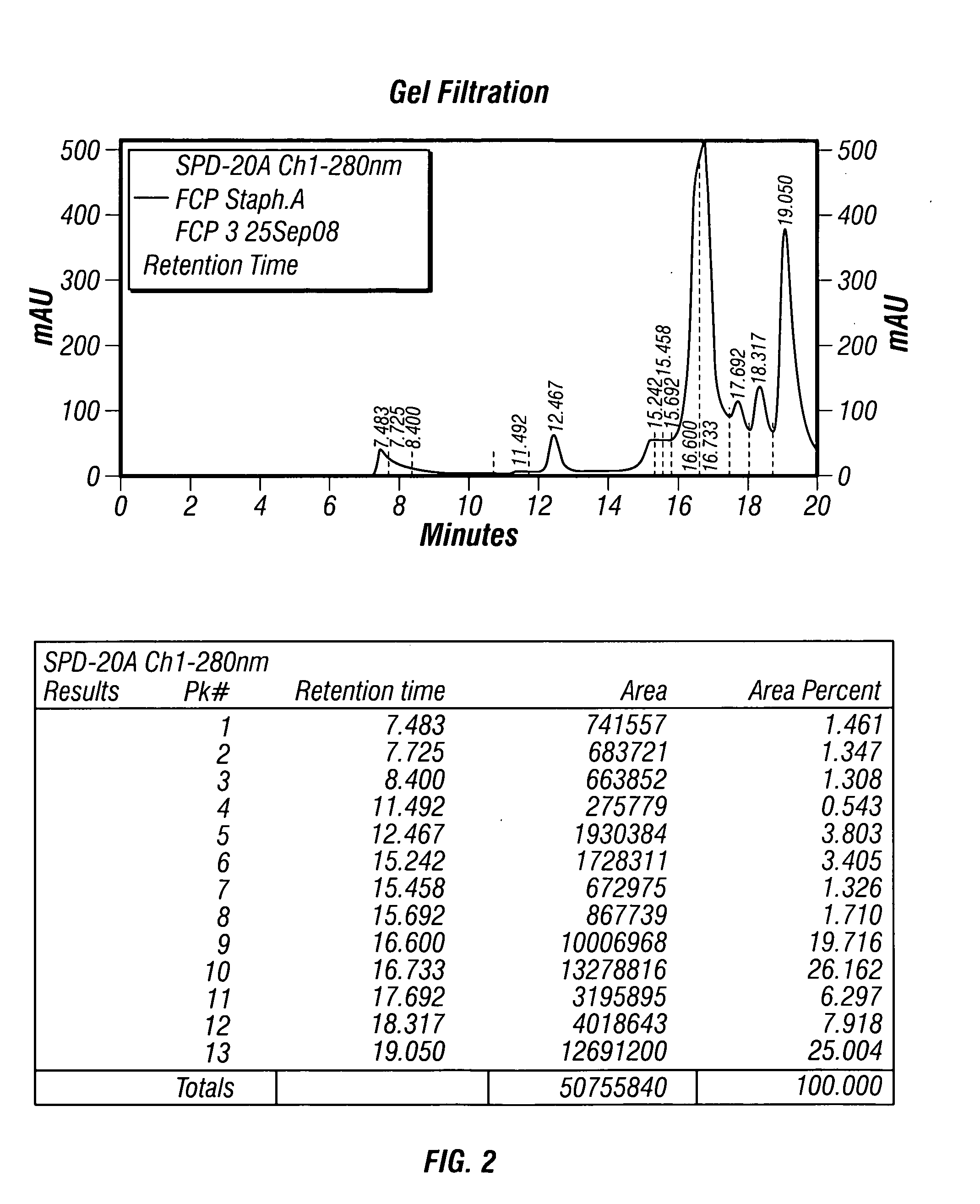
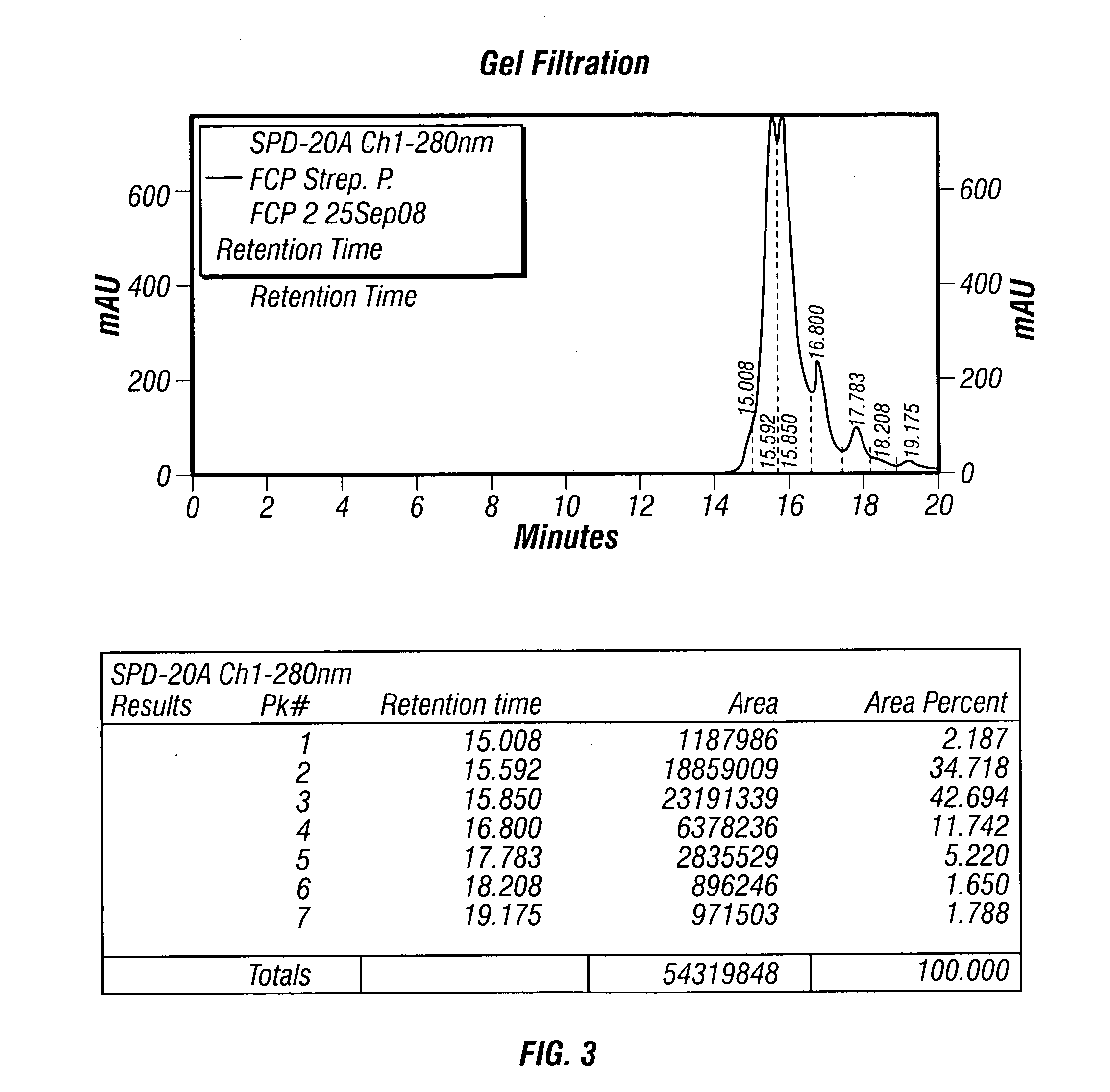
The present invention describes a method for treating, removing or preventing a bacterial infection, which method comprises administering to a human suffering, suspected of suffering or at risk of suffering from Staphylococcus aureus (S. aureus) infection, a Streptococcus infection, Escherichia coli (E. coli) infection, Pseudomonas aeruginosa (P. aeruginosa) infection, Acinetobacter baumannii (A. baumannii) infection, Enterococcus faecium (E. faecium) infection and / or Clostridium difficile (C. difficile) infection, an effective amount of human polyclonal antibodies affinity purified from a human blood sample with an antigenic preparation comprising cellular and / or secreted antigen(s) from bacterial cells selected from S. aureus, a Streptococcus, E. coli, P. aeruginosa, A. baumannii, E. faecium, C. difficile or a combination thereof, and optionally, wherein said affinity purified human polyclonal antibodies are purified (e.g., as made more concentrated as compared to the starting or unpurified material) relative to the same human polyclonal antibodies in the unpurified or non-affinity-purified human blood sample, e.g., intravenous immunoglobulin (IVIG) sample, and / or also optionally, wherein said affinity purified human polyclonal antibodies are specific for the bacterial antigens used in the affinity purification, and / or further optionally wherein the affinity purified human polyclonal antibodies are substantially free of human antibodies that specifically bind to non-bacterial antigens in the human blood sample. Pharmaceutical compositions for treating bacterial infections, comprising an effective amount of human polyclonal antibodies affinity purified from a human blood sample with an antigenic preparation comprising cellular and / or secreted antigen(s) from S. aureus, Streptococcus, E. coli, P. aeruginosa, A. baumannii, E. faecium, C. difficile or a combination thereof, are also provided.
Owner:SCANTIBODIES LAB
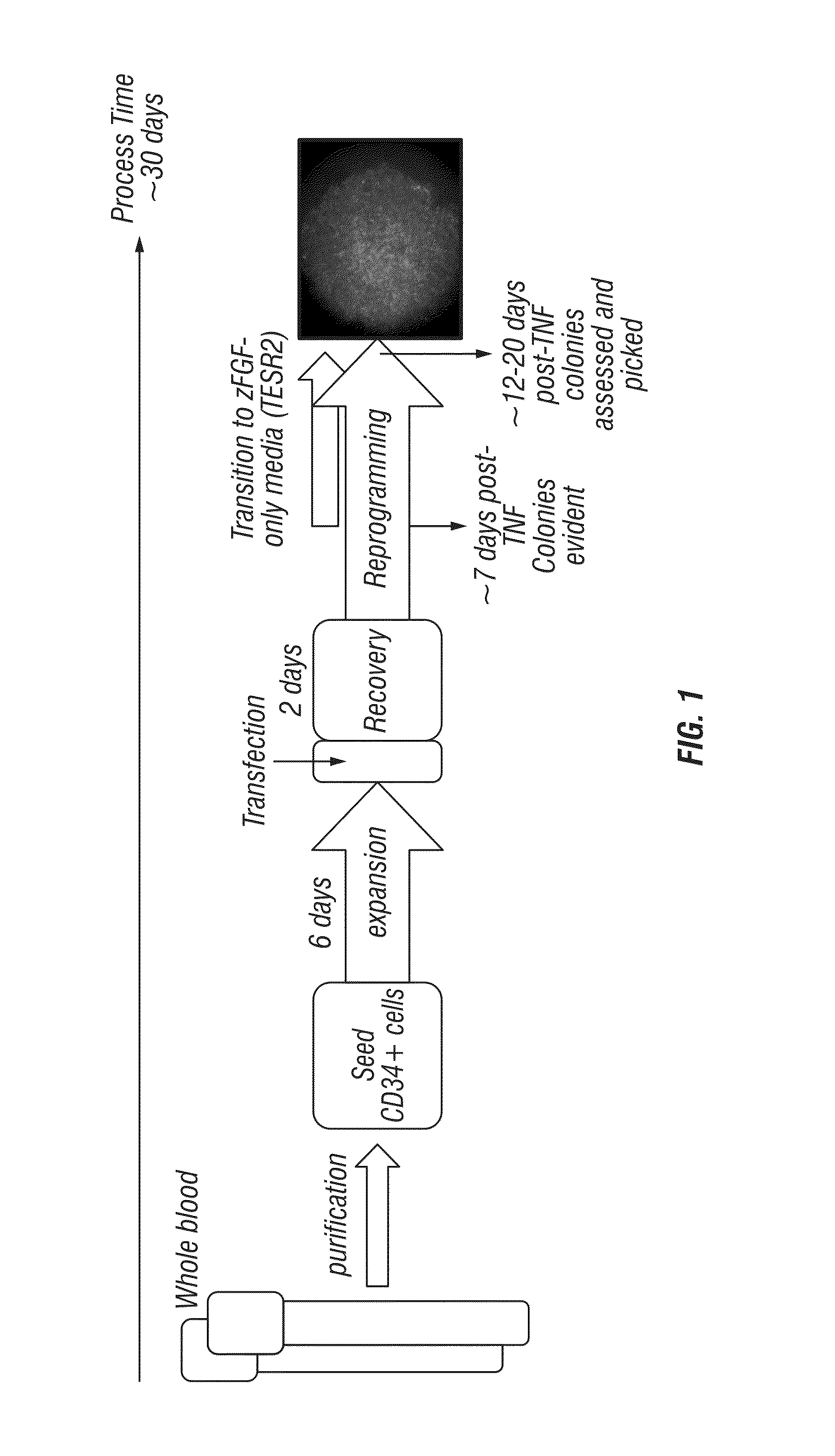
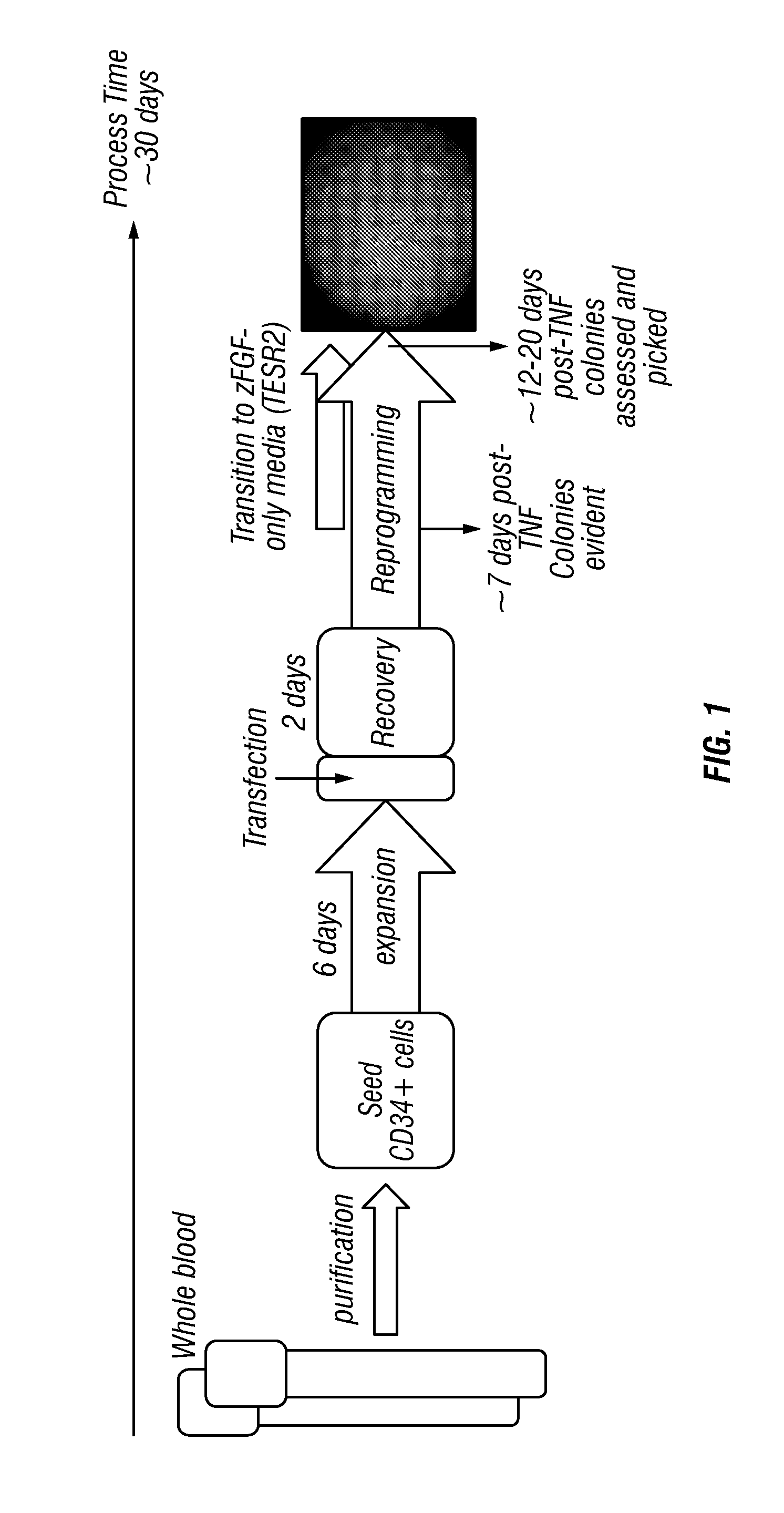
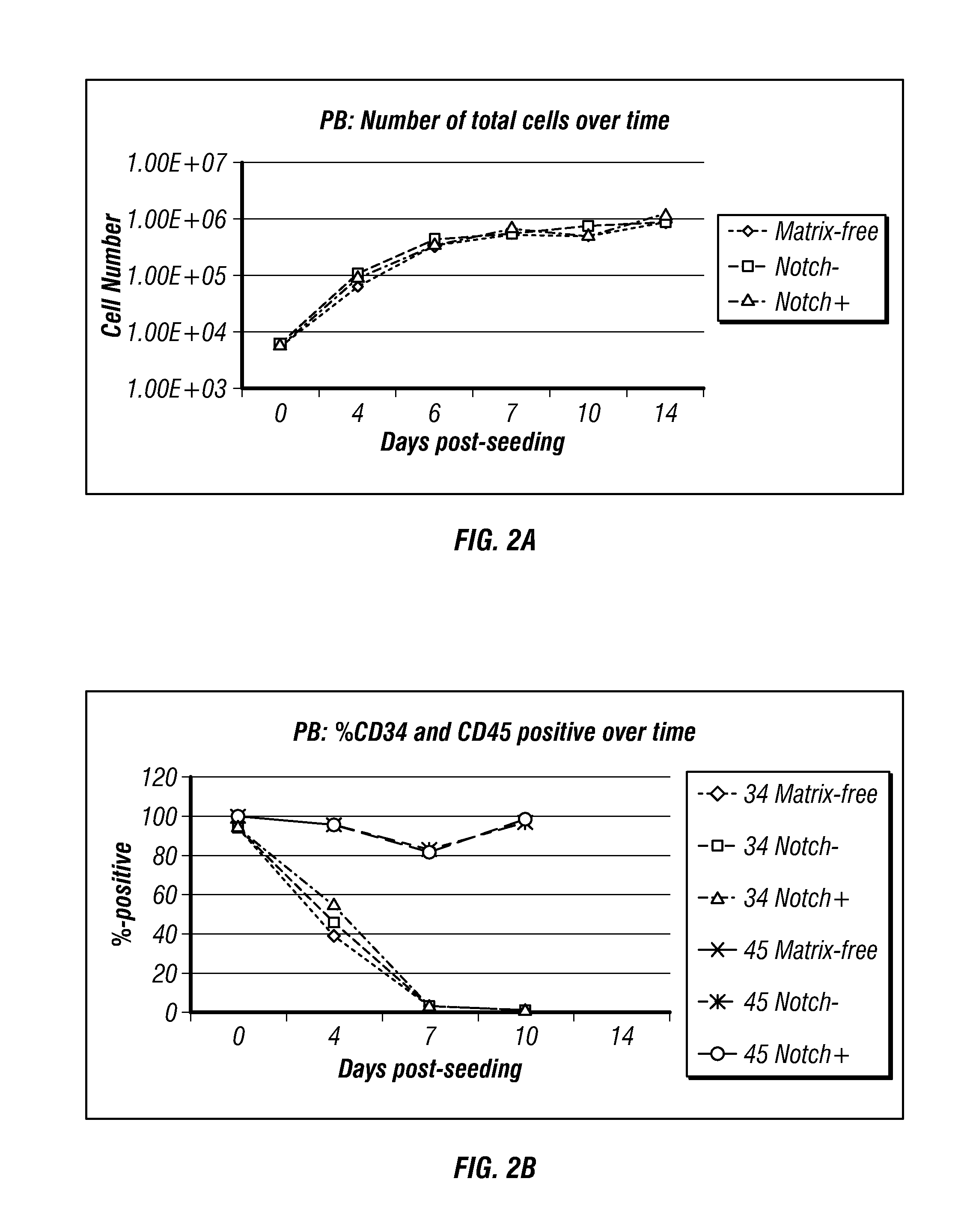
Generation of induced pluripotent stem cells from small volumes of peripheral blood
ActiveUS8691574B2Improve efficiencyLower the volumeGenetically modified cellsCulture processProgenitorReprogramming
Owner:FUJIFILM CELLULAR DYNAMICS INC
High-simulation laparoscopic surgery simulated training device
A high-simulation laparoscopic surgery simulated training device comprises a simulated human abdomen unit, an animal liver and a simulated canal system, wherein the simulated human abdomen unit is used for simulating the human abdomen skin through silica gel and composed of anterior abdominal wall skin and back skin which can be separated from each other, the anterior abdominal wall skin and the back skin are connected through sealing buckles, and thus the seal performance of the whole simulated human abdomen is guaranteed; the animal liver is arranged in the simulated human abdomen unit; the simulated canal system is arranged in the simulated human abdomen unit and connected with the animal liver. According to the high-simulation laparoscopic surgery simulated training device, the abdominal skin is simulated through sealing silica gel, inflation is performed through a poking clip to form the pneumoperitoneum, the human blood circulation in the physiological state is achieved through a pumping set, the real animal liver is anastomosed with the postcava, the portal vein, the hepatic artery and the biliary tract of a human simulator training system through the postcava, the portal vein, the hepatic artery and the biliary tract, and thus various simple or complex laparoscopic surgery training items can be carried out.
Owner:XIAN MAGNETIC MEDICAL TECH CO LTD
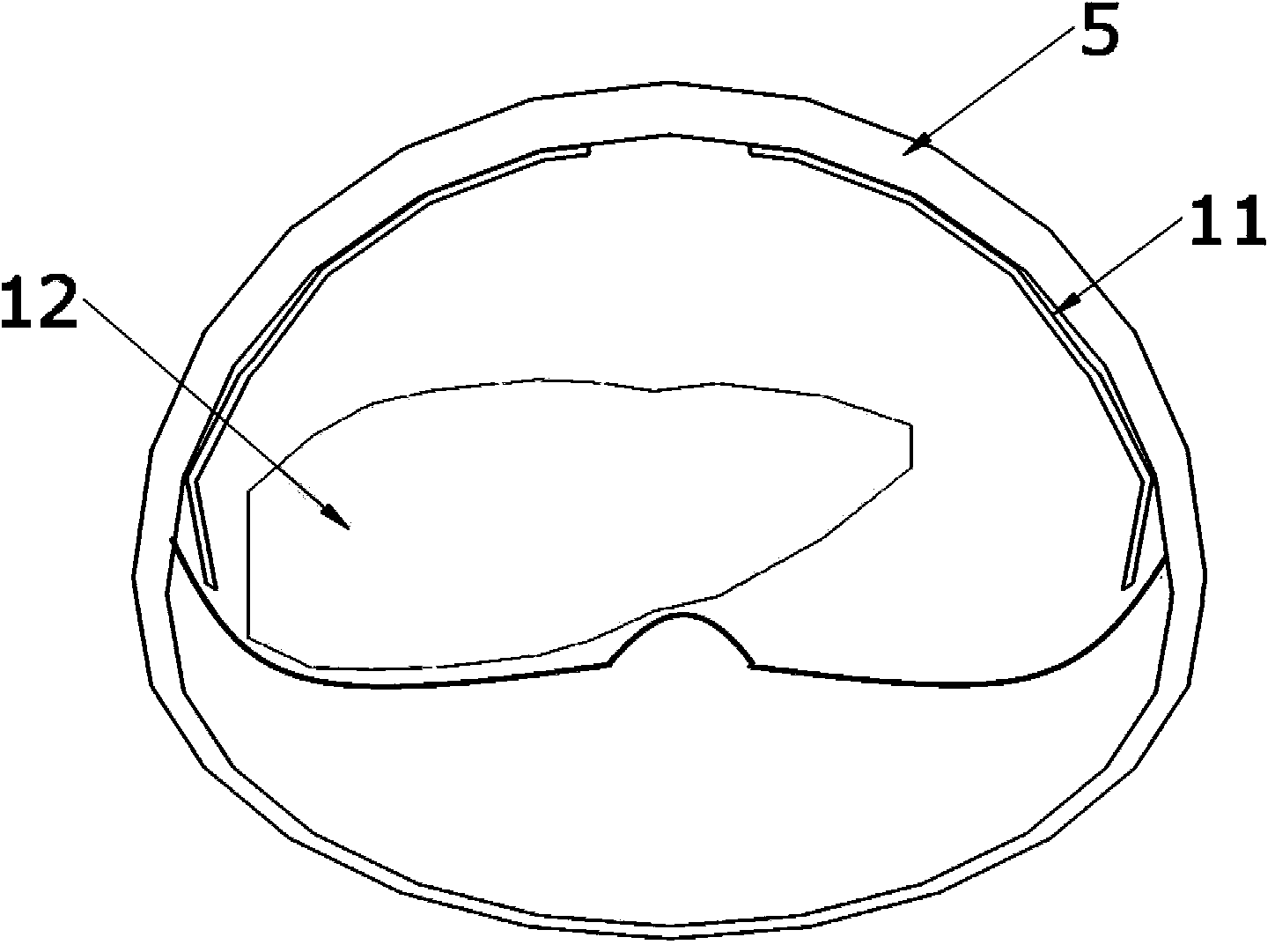
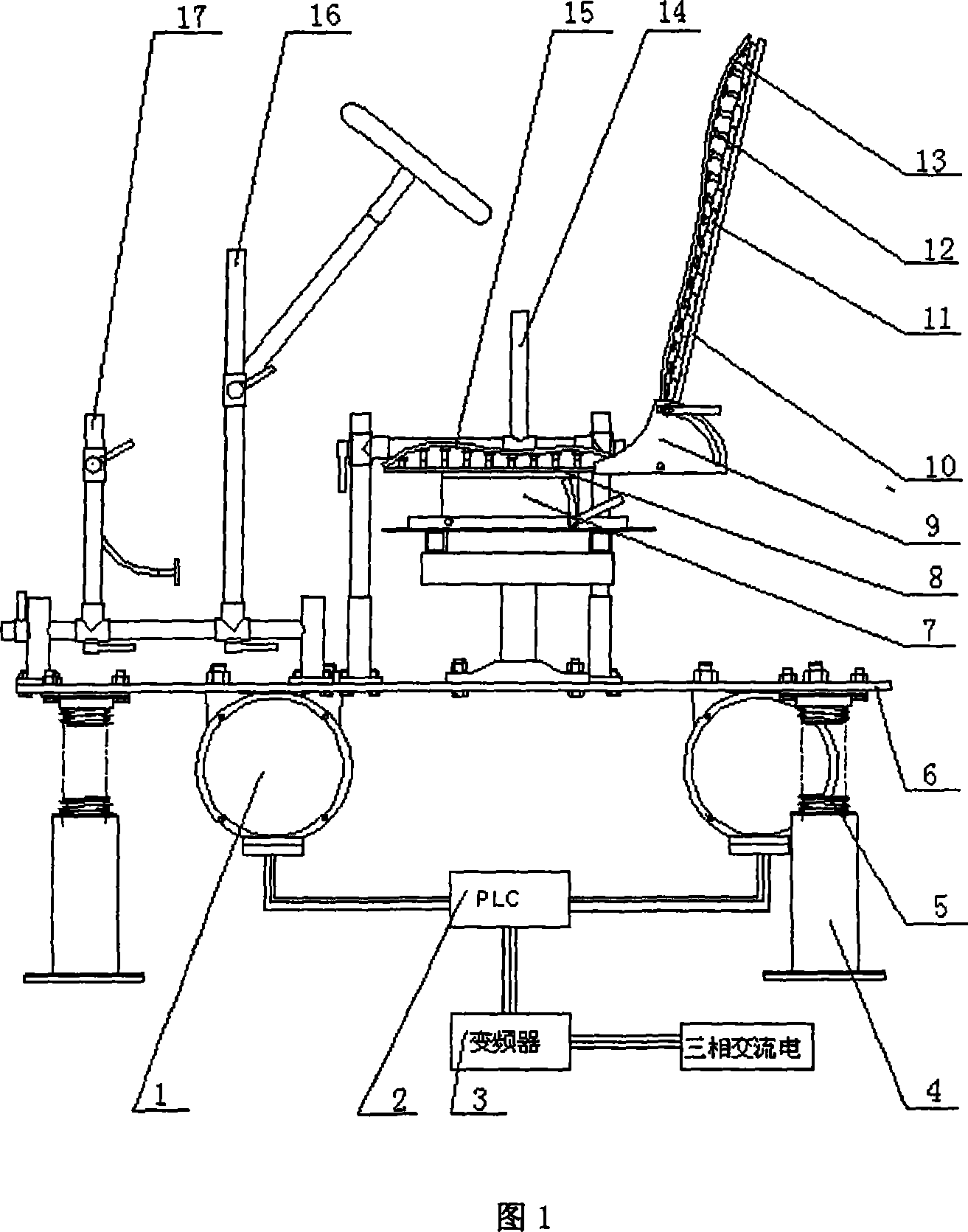
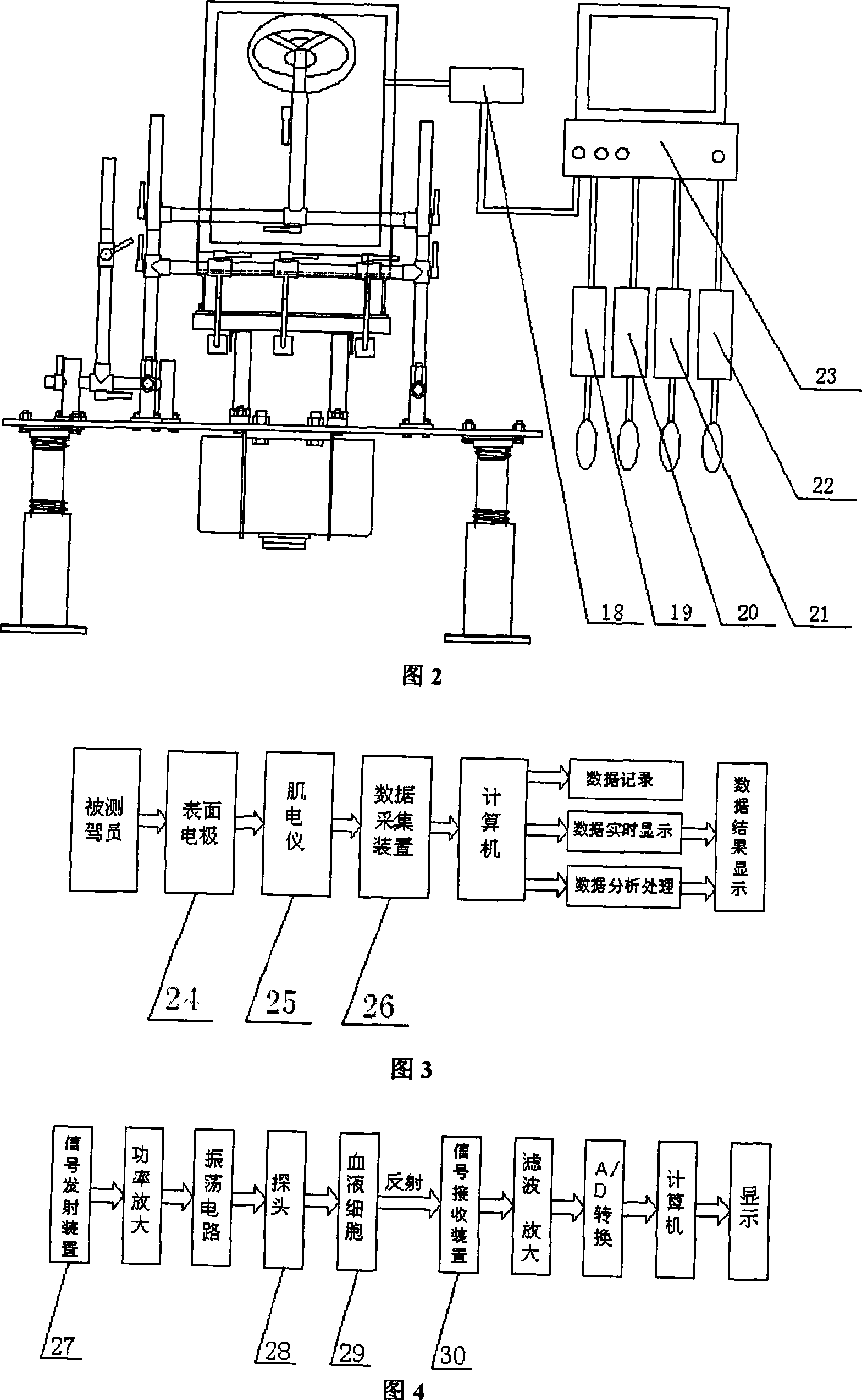
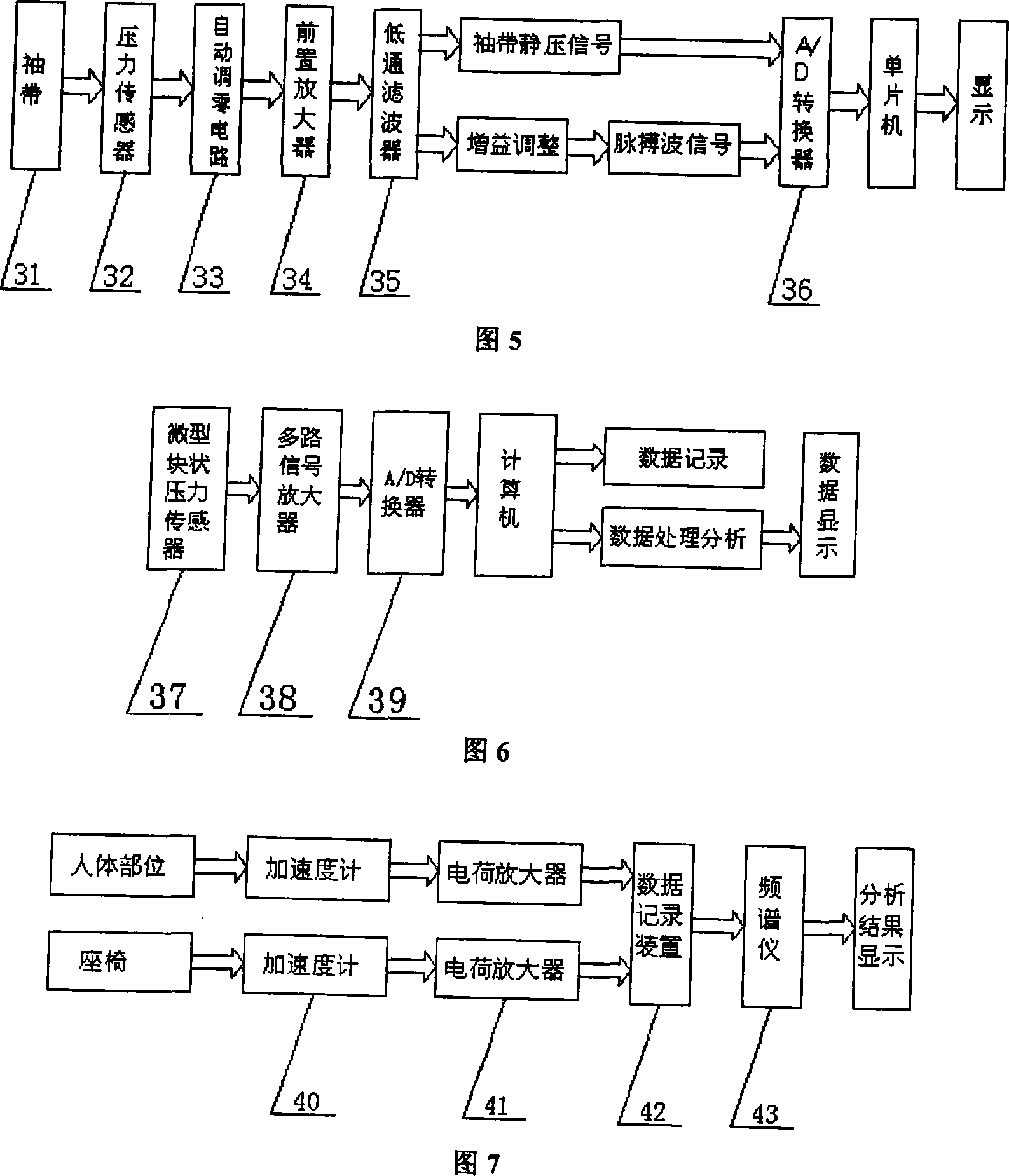
Dynamic riding-driving circumstance human body physiological character and biomechanics testing platform
InactiveCN101214143AEasy to measureEasy to operateVehicle testingDiagnostic recording/measuringHuman bodyButtocks
The invention discloses a dynamic driving environment human physiological characteristics and biomechanics test platform which mainly consists of a dynamic excitation system, a three-dimensional digital adjustable seat system, a three-dimensional adjustable driving mechanism and a human physiological characteristics and biomechanics test system. The invention can simulate the dynamic environment (vibration environment) of automobiles, which means to implement the stepless frequency conversion, the amplitude, the frequency fixation and frequency sweep vibration for the variable acceleration; simulate the posture operation of the automobiles in various driving environment; measure the human physiological character indexes of the human electromyography signals, the human arterial blood flow, the human blood pressure response, the human heartbeat number of a driver, etc., and the vibration response of every human part, the contact stress of a human-chair interface and the body pressure distribution of human buttock and back; study and comprehensively test the human physiological characteristics and the human biomechanics of the driving postures of different populations. Through the stimulation on a dynamic driving environment human physiological characteristics and biomechanics test system, scientific data required by the automobile human-machine interface design for populations with different shapes can be obtained.
Owner:XI AN JIAOTONG UNIV
Embolic protection apparatus with vasodilator coating
InactiveUS20060282114A1Reduce and prevent incidencePrevent embolismDilatorsExcision instrumentsVascular dilatationEmbolic Protection Devices
An apparatus for temporary prevention of embolization in a human blood vessel. The apparatus comprises a body that is transformable between a radially collapsed configuration and a radially expanded configuration sized and shaped for sealing against an inner wall of the vessel to at least partially obstruct fluid flowing there through, the body having a vasodilator coating.
Owner:MEDTRONIC VASCULAR INC
Watch strap human blood pressure non-invasive continuous detection device
InactiveCN101288587AConvenient and comfortable continuous blood pressure measurementEasy to wear and detectEvaluation of blood vesselsAngiographyElectricityHuman body
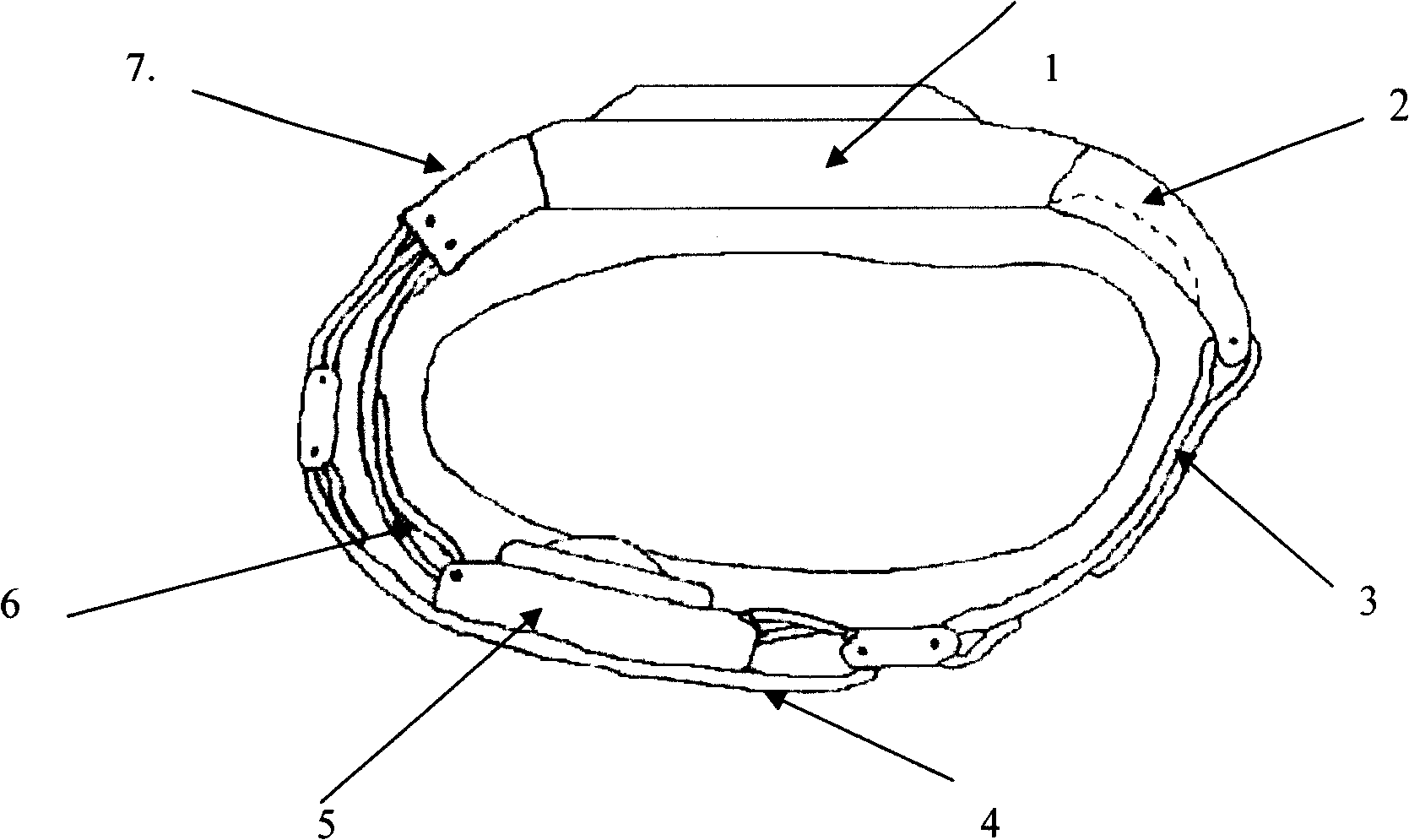
The invention belongs to general electronic blood pressure measuring apparatus, aiming at providing a wristlet typed human body blood pressure trauma-free continuous checkout gear for realizing the convenient and comfortable continuous blood pressure checkup. The technical scheme adopted by the invention is that the wristlet typed human body blood pressure trauma-free continuous checkout gear consists of two parts of a wristwatch and a human body pulse wave checkup gear, and the two parts are connected by a wristlet and an electric signal wire; the human body pulse wave checkup gear consists of a piezoresistive pulse wave sensor and a base of the pulse wave sensor; a pulse wave signal processor unit is attached to the inside of the wristwatch of the wristlet typed human body blood pressure continuous checkout gear; the wristlet consists of a pressing belt of the pulse wave sensor, a sensor location adjustment strip, a length and size regulating mass used for regulating the wristlet length, a camber regulating mass used for regulating the measuring point locating relation between the wristlet and the pulse and a fastening belt. The checkout gear is mainly used for manufacturing blood pressure continuous monitoring and measuring apparatus.
Owner:胡梦辰
Kit for determining glycocholic acid in human blood
ActiveCN102955033ARapid determinationEasy to operateMaterial analysisAntiendomysial antibodiesActive agent
The invention discloses a kit for determining glycocholic acid in human blood. The kit comprises a reagent A, a reagent B and a calibrator for glycocholic acid with known concentration, wherein the mass ratio of the reagent A to the reagent B is (1-5):1; the reagent A comprises a microorganism inhibiting agent, EDTA (Ethylene Diamine Tetraacetic Acid), glycerol, albumin bovine serum, a surface active agent, a reactive reinforcing agent, sodium chloride and a Good's buffer solution with the pH of 6-8; and the reagent B comprises the microorganism inhibiting agent, the EDTA (Ethylene Diamine Tetraacetic Acid), the glycerol, the albumin bovine serum, the surface active agent, the reactive reinforcing agent, sodium chloride, the Good's buffer solution with the pH of 6-8 and nanoparticles crosslinked with a glycocholic acid monoclonal antibody. The kit is simple, convenient and fast in detection and operation, can be used for simultaneously and quickly determining a large amount of samples and has good detection accuracy and sensitivity.
Owner:浙江强盛生物科技有限公司
Latex enhanced immunoturbidimetry kit for detection of asymmetric dimethylarginine content
ActiveCN102628868AThe detection process is fastHigh detection specificityBiological testingCompetitive bindingLatex particle
The invention relates to a latex enhanced immunoturbidimetry kit for the detection of asymmetric dimethylarginine content. Specifically, the invention relates to a latex enhanced immunoturbidimetry kit for the detection of asymmetric dimethylarginine (ADMA) content in human blood samples. The kit comprises a mouse anti-human monoclonal antibody solution, an ADMA-human Serum albumin complex crosslinked latex particle expansion and an ADMA calibrator. According to the method, by the utilization of competitive binding of free ADMA in blood and ADMA crosslinked on the surface of latex particles to ADMA monoclonal antibody, turbidity formed by the reaction between the ADMA-crosslinked latex particles and ADMA monoclonal antibody is reduced. Therefore, the content of ADMA is detected through the reduction degree of turbidity. The kit provided by the invention can be applied in a biochemical analyzer commonly-used in clinic, is convenient and rapid to operate and has strong specificity. Both sensitivity and detection range of the kit can satisfy clinic application needs.
Owner:BEIJING STRONG BIOTECH INC
Centrifugal device and method for fluid component separation
ActiveUS7947186B2Easy to separateEnergeticWater/sewage treatment by centrifugal separationSpecific gravity using centrifugal effectsEngineeringVolumetric Mass Density
A rotor is provided for the centrifugal separation of a composite fluid, such as animal or human blood, into components. An inner surface of the rotor includes at least one hydrophobic area having a first surface energy sufficiently low to provide a flow path for a lower density fluid component to flow to the bottom of a central collection chamber after centrifugal separation. A method of separating a composite fluid into components using such a rotor is also provided. Additionally, a method of manufacturing such a rotor is provided.
Owner:IRIS INT
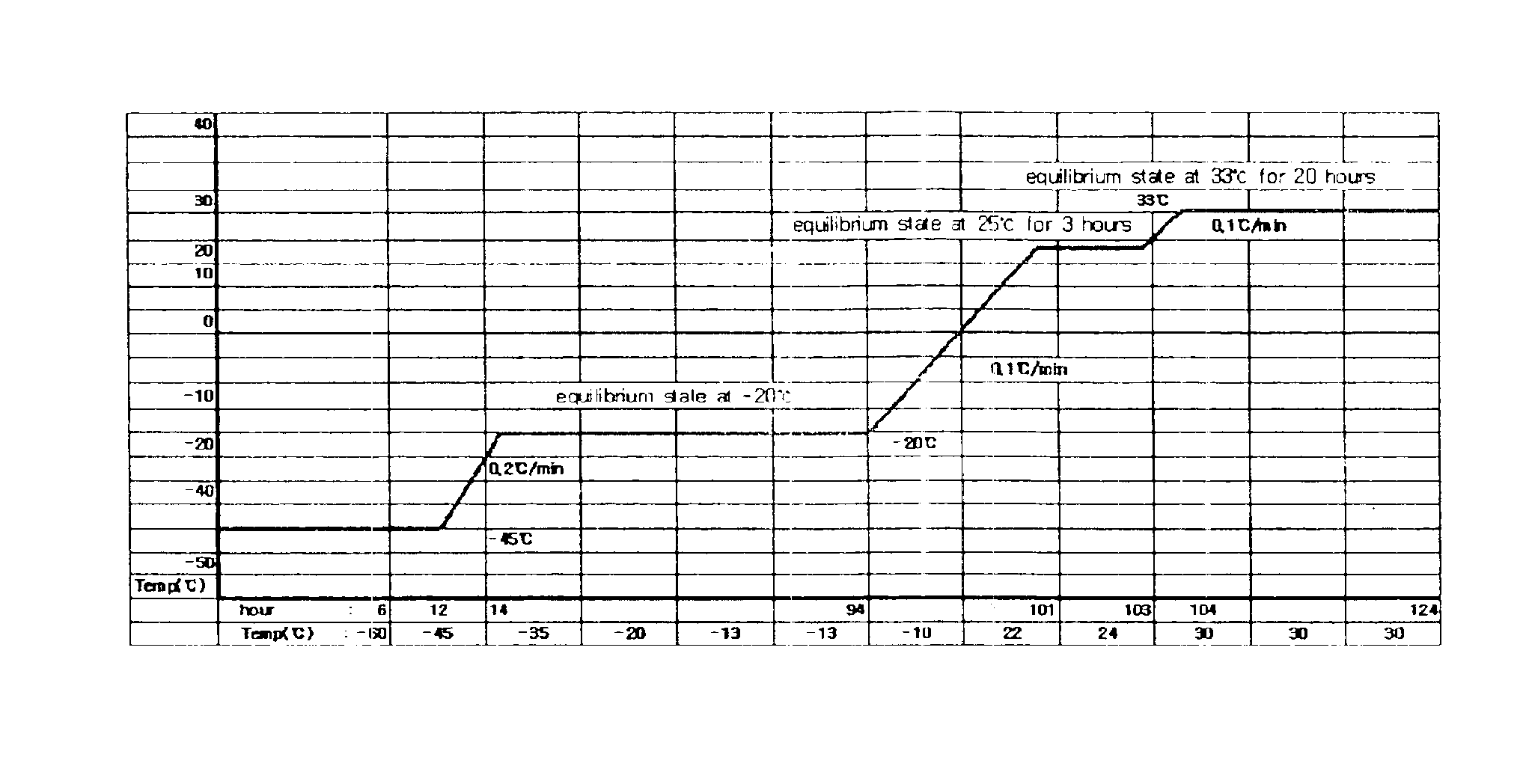
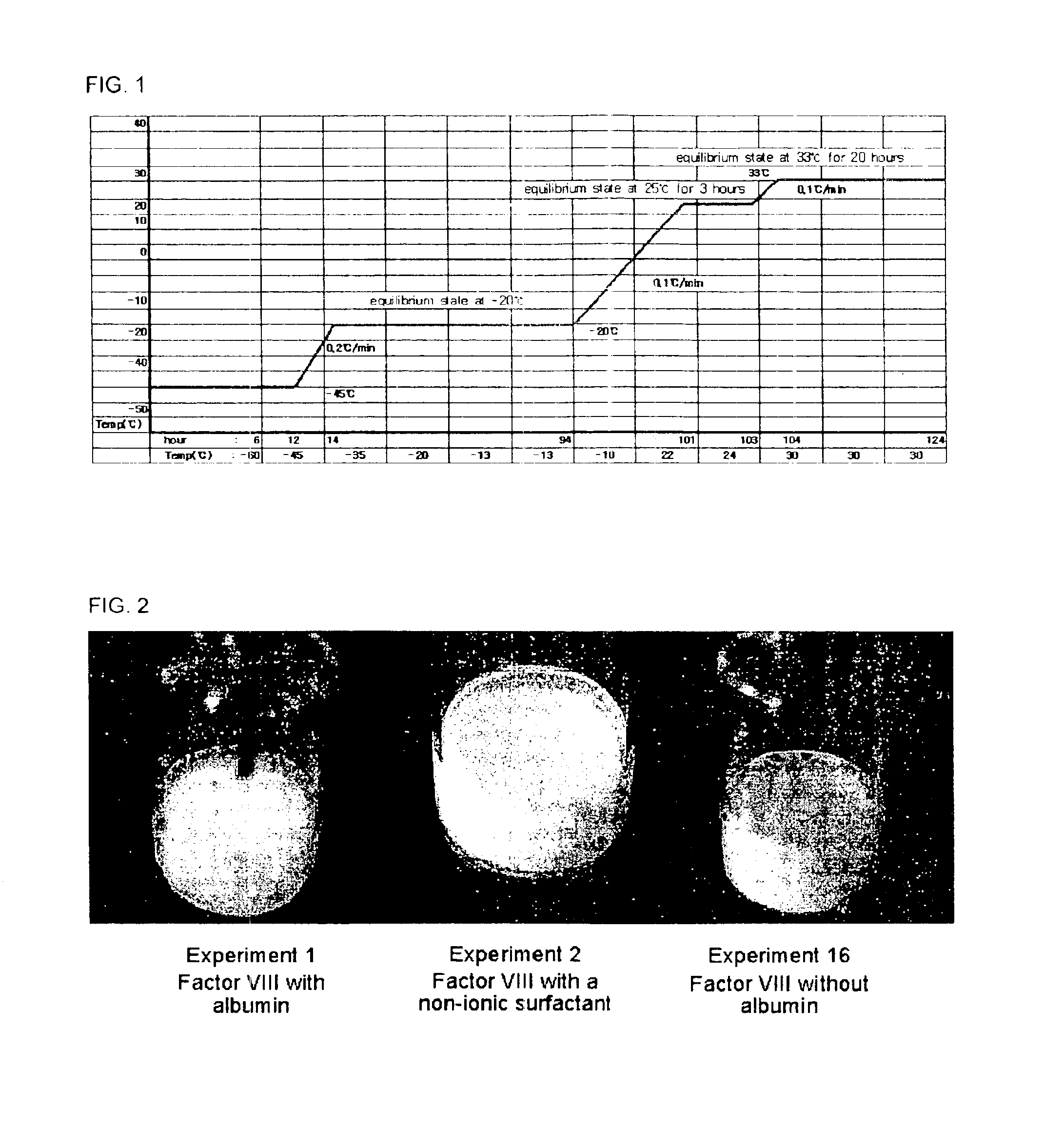
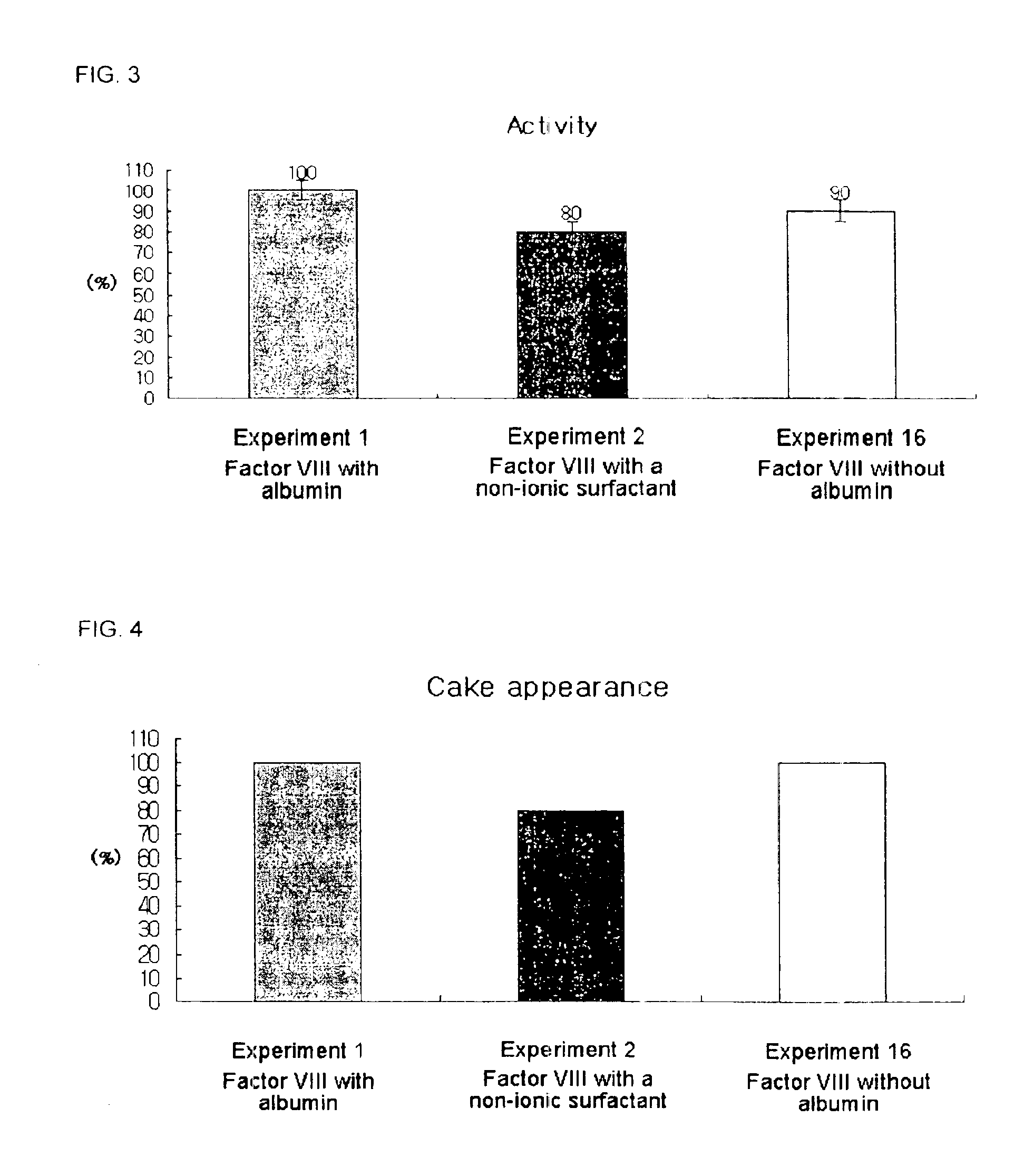
Pharmaceutical preparation of recombinant factor VIII lyophilized without albumin as a stabilizer
ActiveUS6887852B1Same pharmaceutical efficacyAvoid virus infectionFactor VIIPowder deliveryMedicineArginine
Disclosed is a lyophilized preparation of recombinant factor VIII used as a therapeutic preparation of hemophilia A. The lyophilized preparation of recombinant factor VIII is prepared by performing lyophilization using a mixture comprising 6 to 100 mM of L-arginine, 3.5 to 50 mM of L-isoleucine, and 10 to 100 mM of L-glutamic acid as a stabilizer for stabilizing the recombinant factor VIII which exhibits an unstable activity during lyophilization, rather than using human blood derived albumin.
Owner:KOREA GREEN CROSS CORP
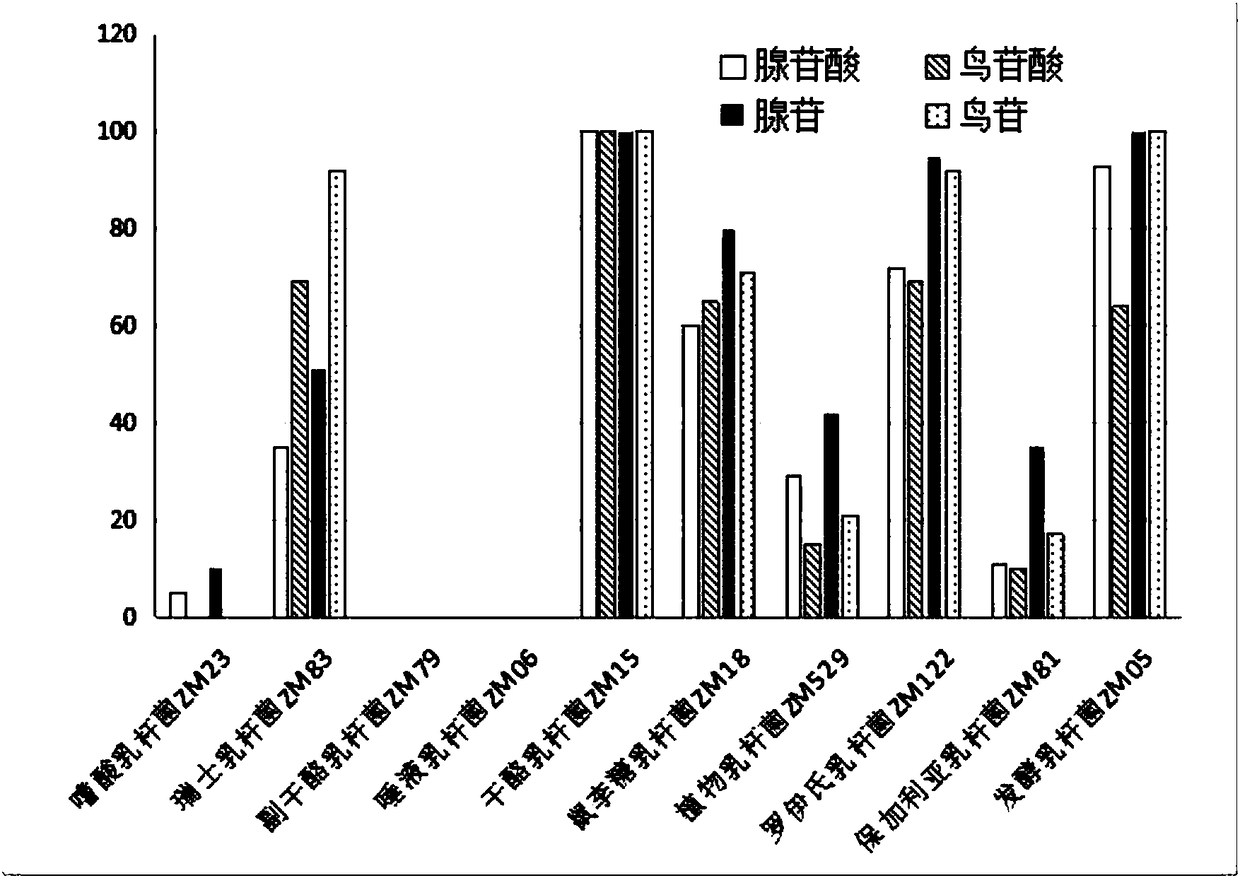
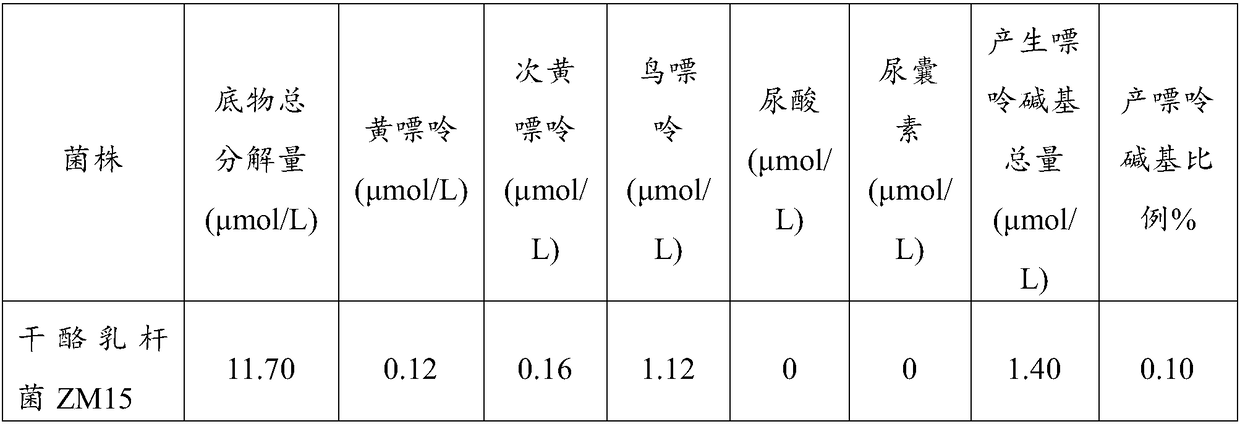
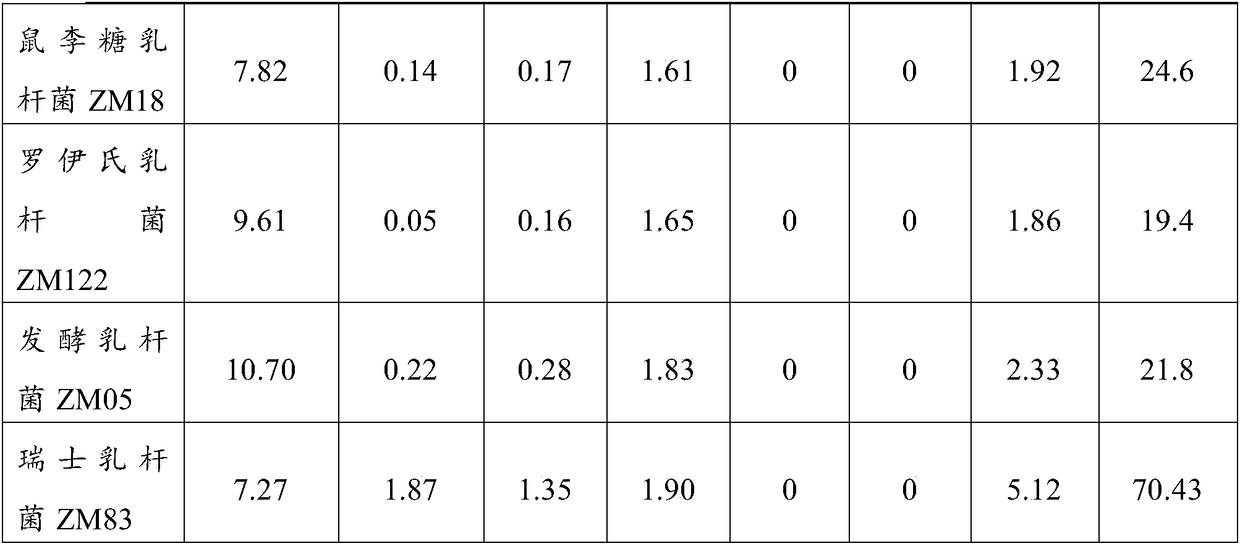
Lactobacillus casei strain and probiotic composition for lowering blood uric acid and application thereof
The invention provides a lactobacillus casei strain and a probiotic composition for lowering blood uric acid and application thereof, and belongs to the technical field of food medicines. The lactobacillus casei strain ZM15 for lowering the blood uric acid has the collection number of CGMCC No.13980. The lactobacillus casei strain ZM15 has the rates of degrading adenylic acid, guanylic acid, adenosine and guanosine as high as 100%; a degraded product does not contain uric acid and allantoin; in addition, the degraded product has a low purine base proportion of only 0.10%, so that the product can be prevented from being further decomposed into the uric acid, and the human blood uric acid content can be effectively reduced. The probiotic composition provided by the invention is obtained by compounding four strains of ZM15, ZM18, ZM122 and ZM05; through mutual coordination of the four strains, the blood uric acid lowering effect of the probiotic composition is significantly better than that during independent application of ZM15, ZM18, ZM122 and ZM05 strains, so that the blood uric acid lowering effect can be further improved.
Owner:JIAXING INNOCUL PROBIOTICS CO LTD
Generation of induced pluripotent stem cells from small volumes of peripheral blood
ActiveUS20120009676A1Improve processing efficiencyLower the volumeGenetically modified cellsCulture processProgenitorReprogramming
Methods and compositions relating to the production of induced pluripotent stem cells (iPS cells) are disclosed. For example, induced pluripotent stem cells may be generated from peripheral blood cells, such as human blood progenitor cells, using episomal reprogramming and feeder-free or xeno-free conditions. In certain embodiments, the invention provides novel methods for improving overall reprogramming efficiency with low number of blood progenitor cells.
Owner:FUJIFILM CELLULAR DYNAMICS INC
Use of human cells of myeloid leukaemia origin for expression of antibodies
ActiveUS20100028947A1High activityIncrease productionPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsProtein moleculesHuman cell
The invention relates to a method for producing a protein molecule composition having a defined glycosylation pattern, comprising (a) introducing in a host cell which is an immortalized human blood cell at least one nucleic acid encoding at least a part of said protein; and (b) culturing said host cell under conditions which permit the production of said protein molecule composition; and (c) isolating said protein molecule composition.
Owner:GLYCOTOPE GMBH
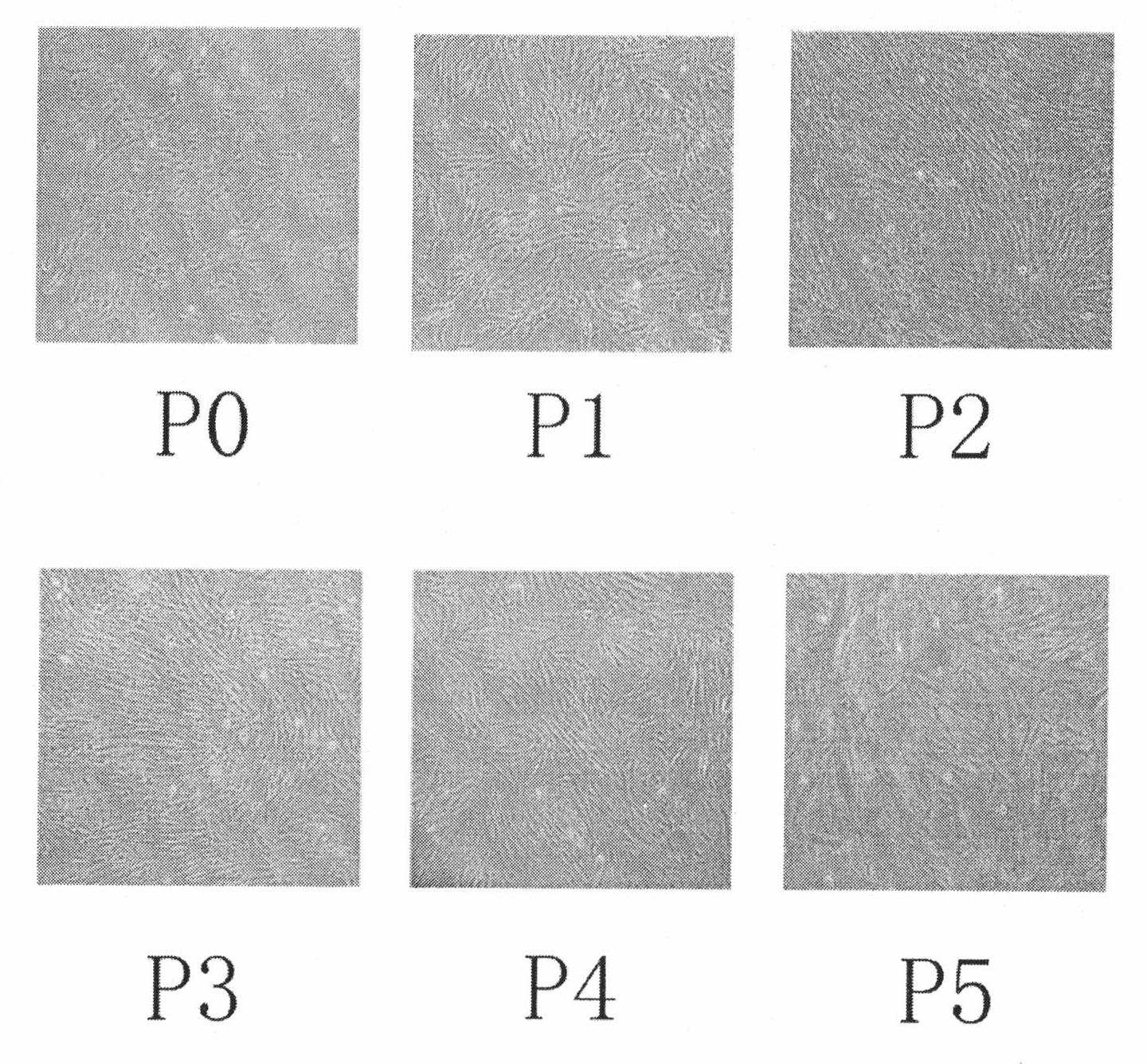
Human amnion mesenchymal stem cell serum-free culture medium and culture method thereof
The invention relates to a human amnion mesenchymal stem cell serum-free culture medium and a culture method thereof. The culture medium is formed by adding human serum albumin, human transferrin, human insulin and sodium selenite into a DMEM / F12 basic culture medium. The culture method for the culture medium comprises the following steps of: digesting human amnion by using trypsin, then digesting the human amnion by using collagenase IV and deoxyribonuclease I, and filtering the mixture to obtain single cell suspension; and adding the human serum albumin, the transferrin, the insulin and the sodium selenite into the DMEM / F12 basic culture medium in a ratio of VDMEM to VF12 of 1:1, and putting human amnion mesenchymal stem cells in a 37 DEG C CO2 incubator with saturated humidity and volume fraction of 5 percent under the serum-free condition, wherein culture in vitro and amplification are realized by solution change and transfer of culture, potentiality of multi-direction differentiation is maintained, and the amplified cells can be induced in vitro to form cartilage cells, osteoblasts and adipocytes. The culture medium and the culture method have the characteristics of no other animal sources, wide source and no limitation of ethics.
Owner:辽宁艾米奥干细胞与再生医学研究院有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com