Patents
Literature
350 results about "Clotting factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of clotting factor. : any of several plasma components (such as fibrinogen, prothrombin, thromboplastin, and factor VIII) that are involved in the clotting of blood. — called also coagulation factor.
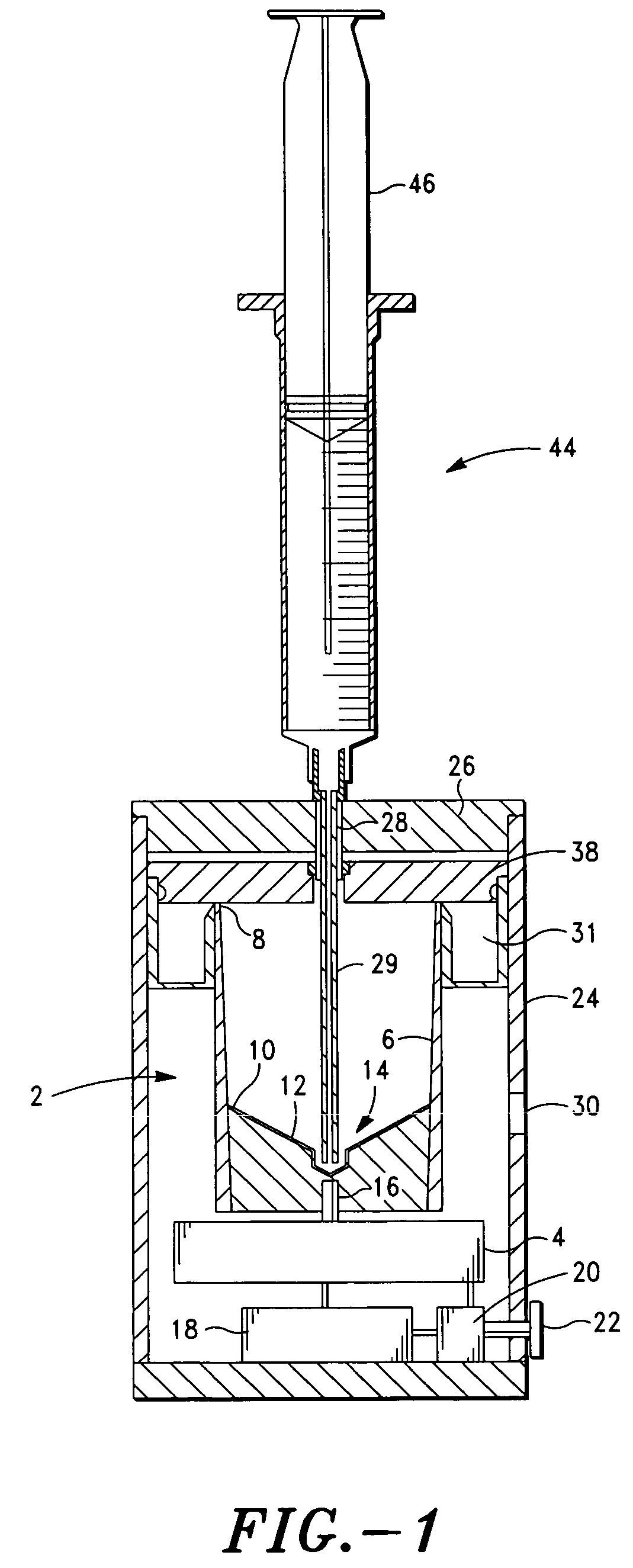
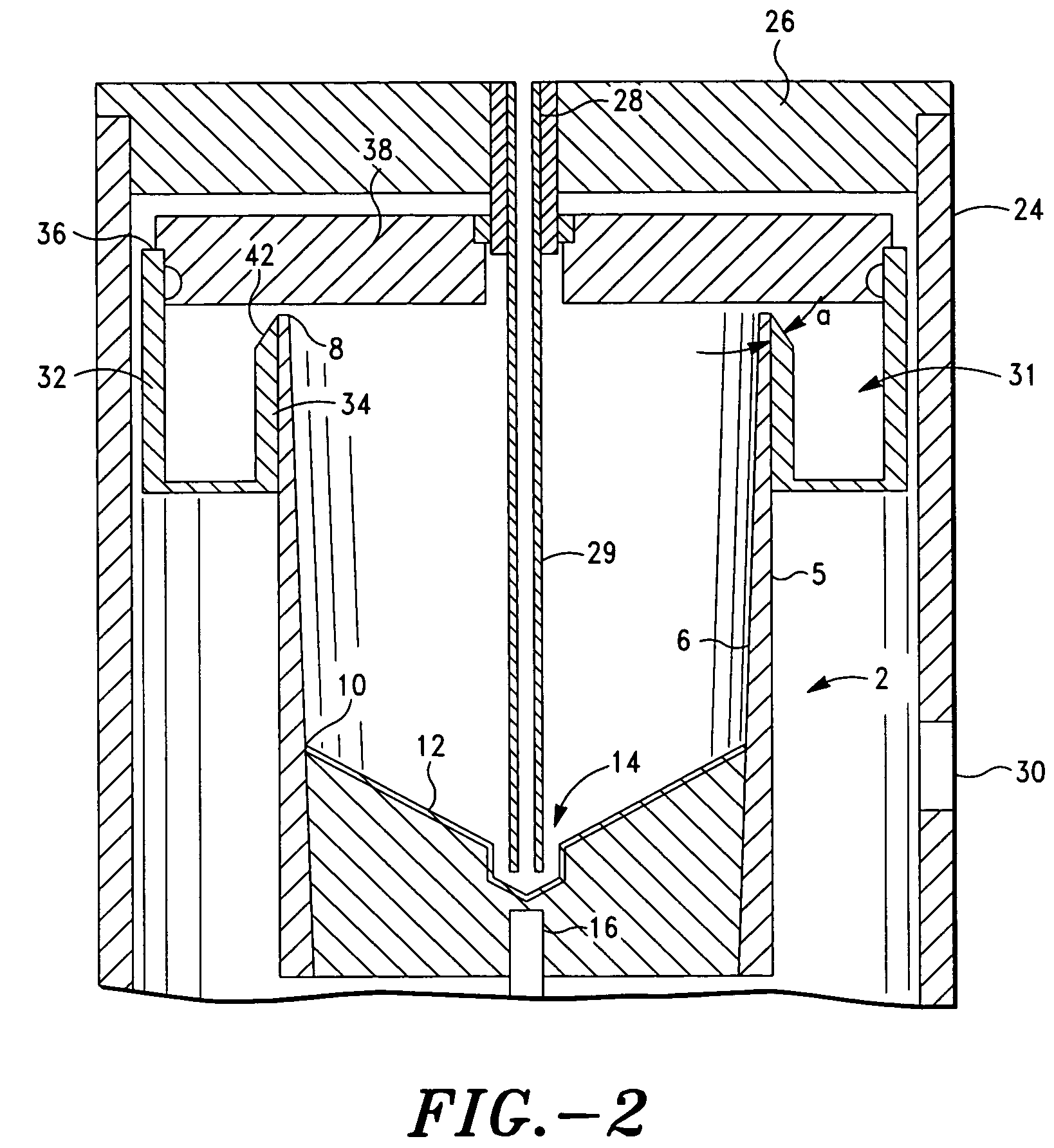
Method and apparatus for preparing platelet rich plasma and concentrates thereof
ActiveUS20060175242A1Shaking/oscillating/vibrating mixersTransportation and packagingFiberRed blood cell
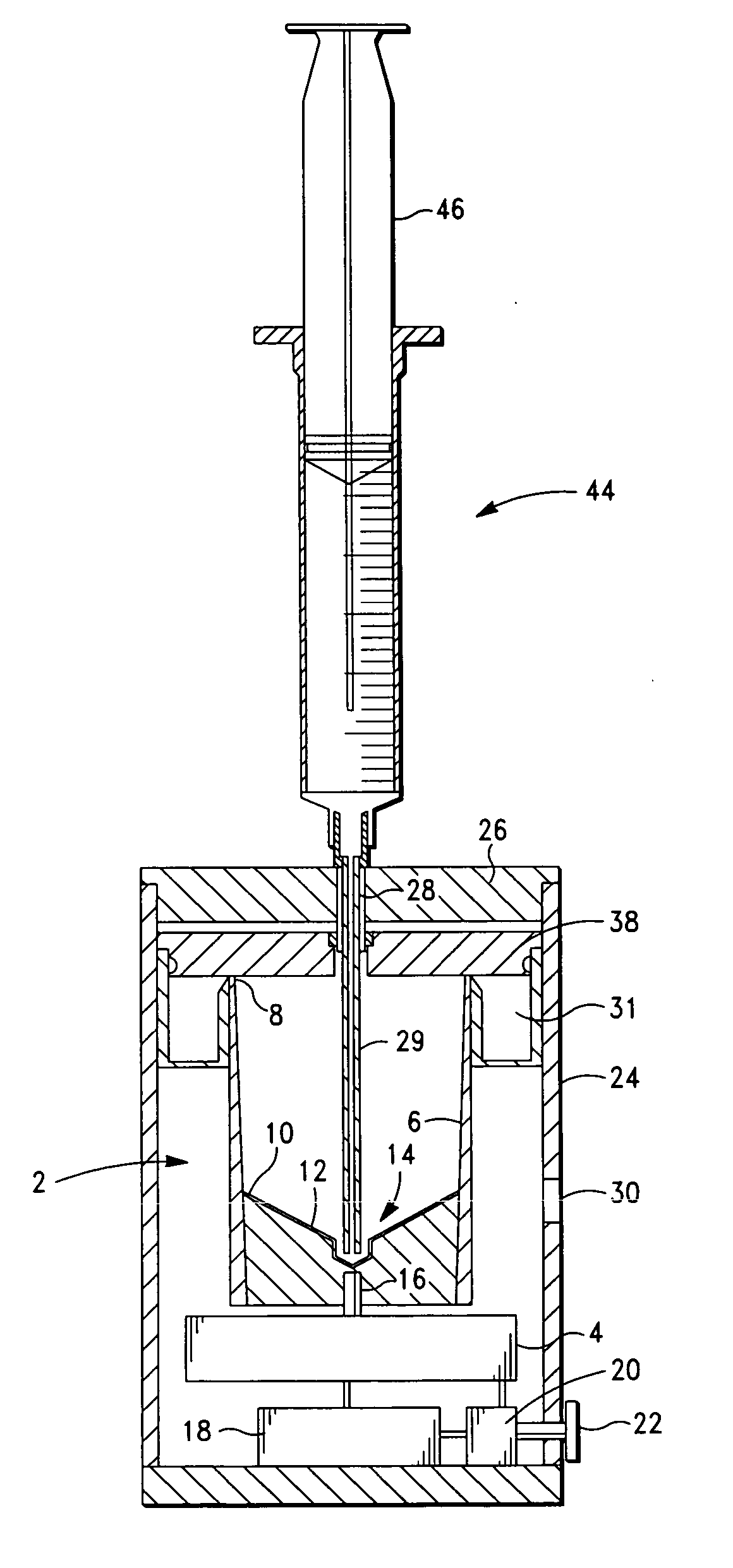
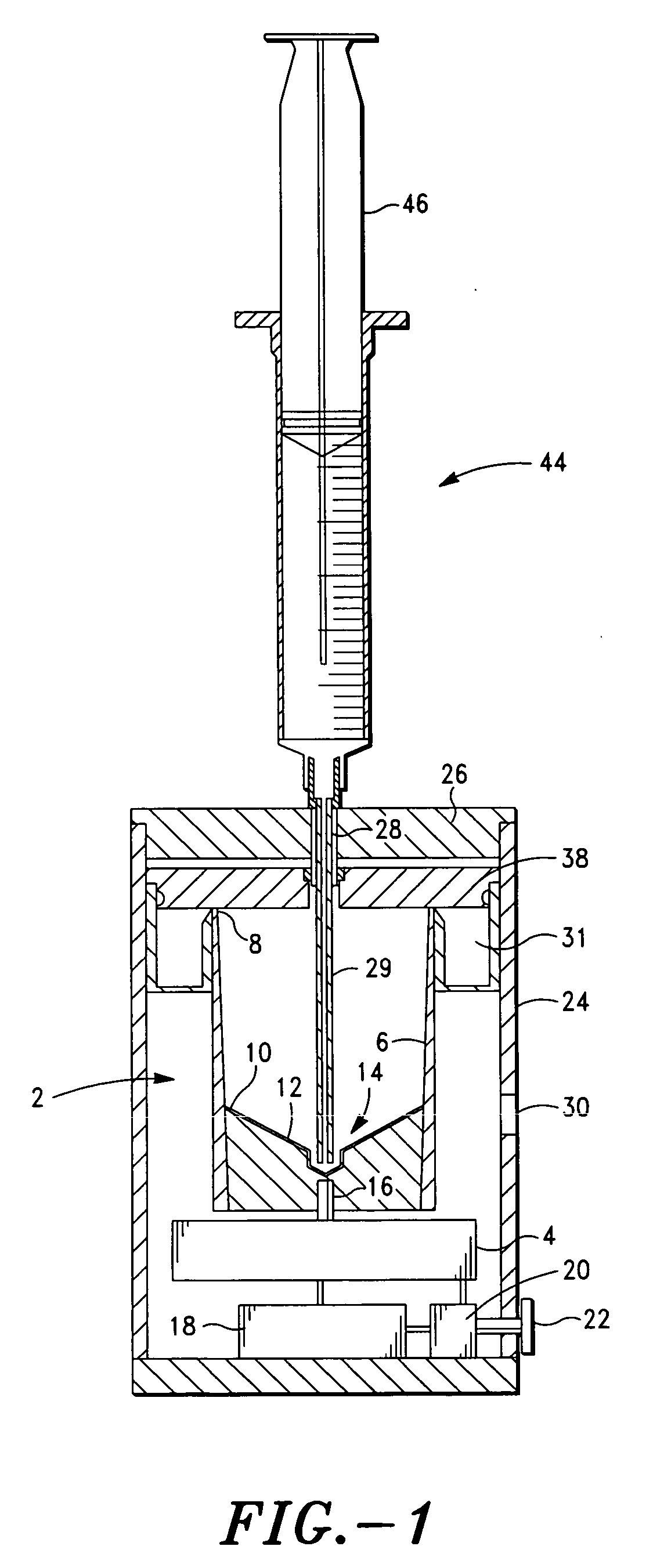
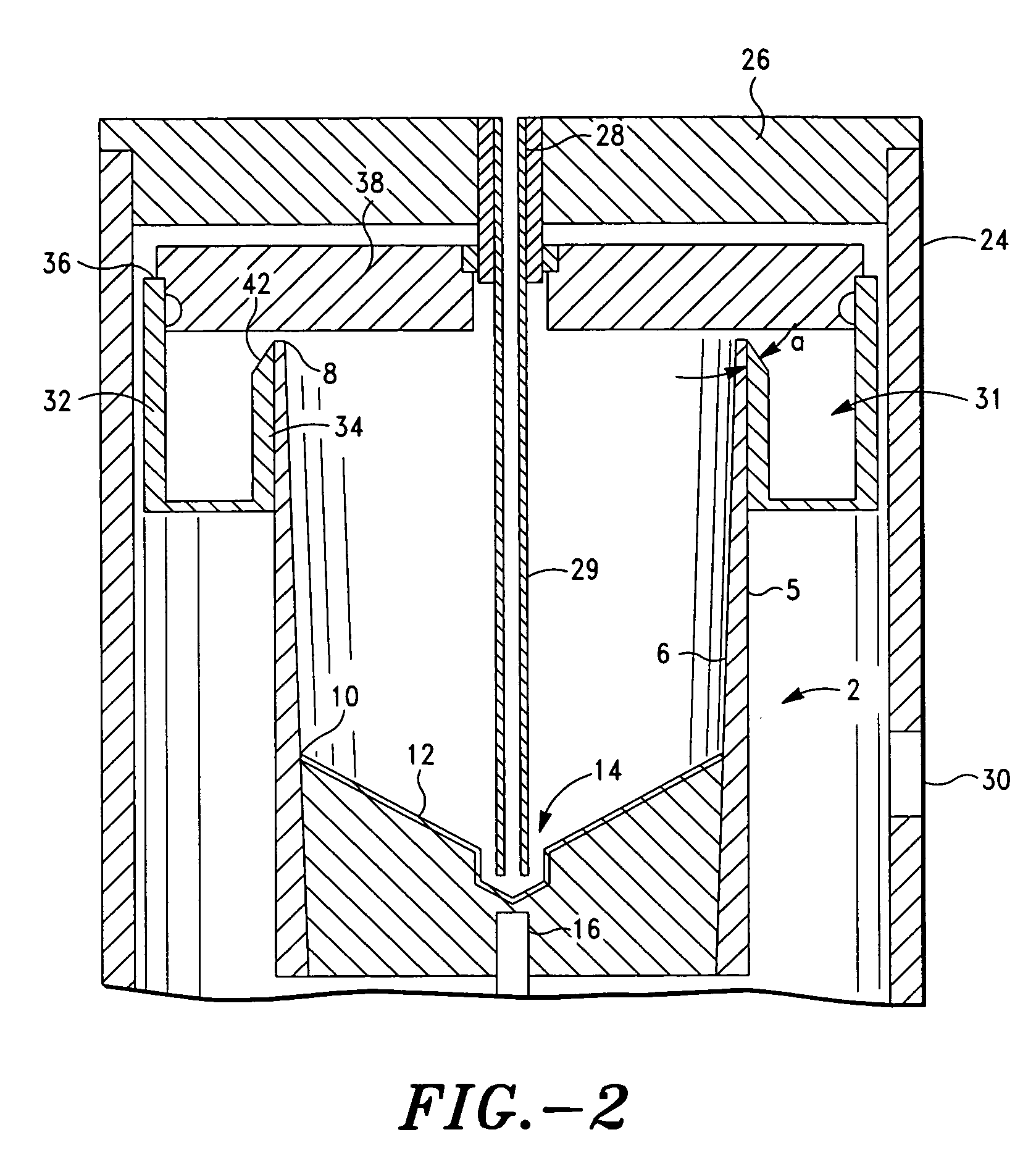
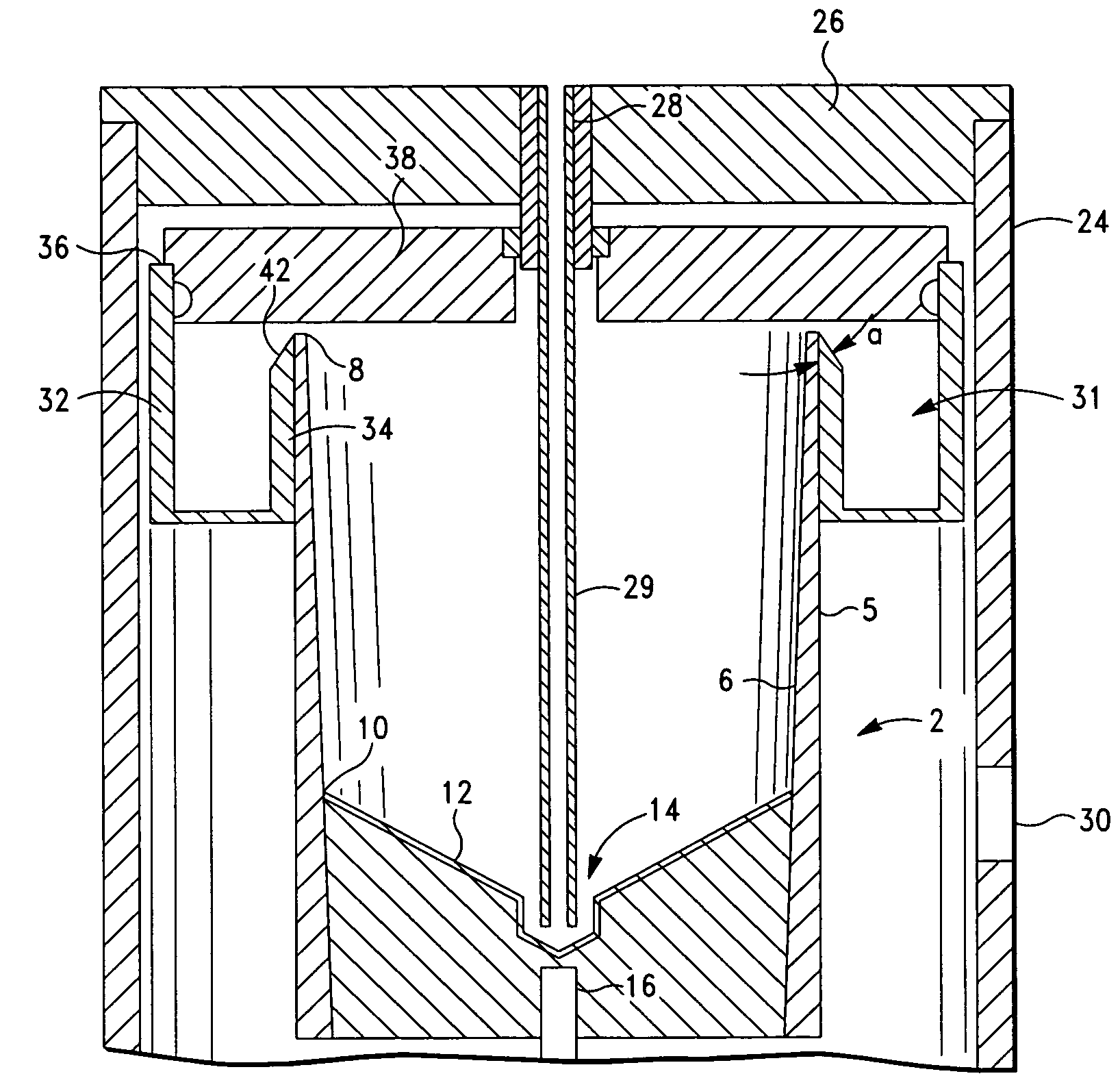
The PRP separator-concentrator of this invention is suitable for office use or emergency use for trauma victims. The PRP separator comprises a motorized centrifugal separation assembly, and a concentrator assembly. The centrifugal separator assembly comprises a centrifugal drum separator that includes an erythrocyte capture module and a motor having a drive axis connected to the centrifugal drum separator. The concentrator assembly comprises a water-removal module for preparing PRP concentrate. The centrifugal drum separator has an erythrocyte trap. The water removal module can be a syringe device with water absorbing beads or it can be a pump-hollow fiber cartridge assembly. The hollow fibers are membranes with pores that allow the flow of water through the fiber membrane while excluding flow of clotting factors useful for sealing and adhering tissue and growth factors helpful for healing while avoiding activation of platelets and disruption of any trace erythrocytes present in the PRP.
Owner:HANUMAN +1
Method and apparatus for preparing platelet rich plasma and concentrates thereof
ActiveUS7708152B2Shaking/oscillating/vibrating mixersTransportation and packagingFiberRed blood cell
The PRP separator-concentrator of this invention is suitable for office use or emergency use for trauma victims. The PRP separator comprises a motorized centrifugal separation assembly, and a concentrator assembly. The centrifugal separator assembly comprises a centrifugal drum separator that includes an erythrocyte capture module and a motor having a drive axis connected to the centrifugal drum separator. The concentrator assembly comprises a water-removal module for preparing PRP concentrate. The centrifugal drum separator has an erythrocyte trap. The water removal module can be a syringe device with water absorbing beads or it can be a pump-hollow fiber cartridge assembly. The hollow fibers are membranes with pores that allow the flow of water through the fiber membrane while excluding flow of clotting factors useful for sealing and adhering tissue and growth factors helpful for healing while avoiding activation of platelets and disruption of any trace erythrocytes present in the PRP.
Owner:HANUMAN +1
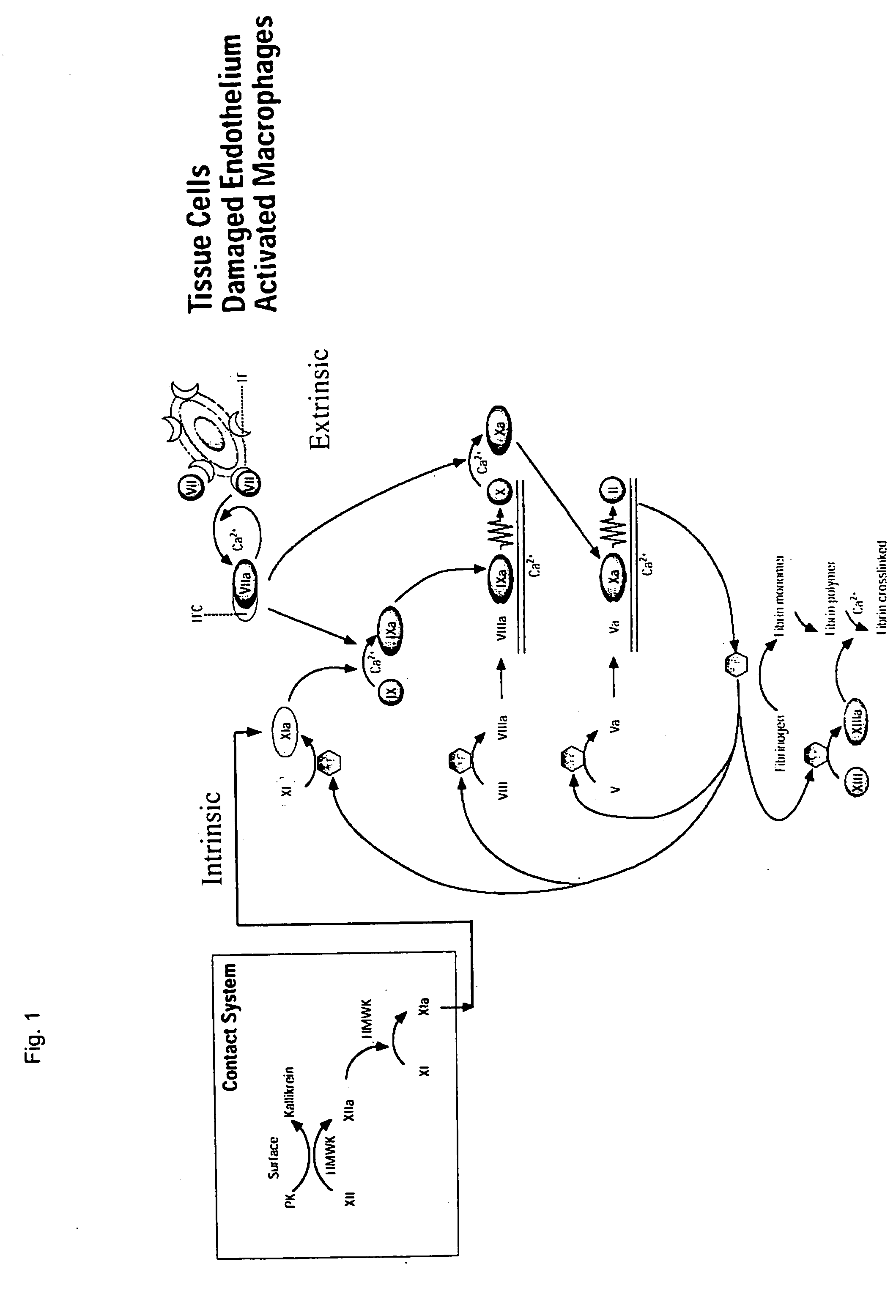
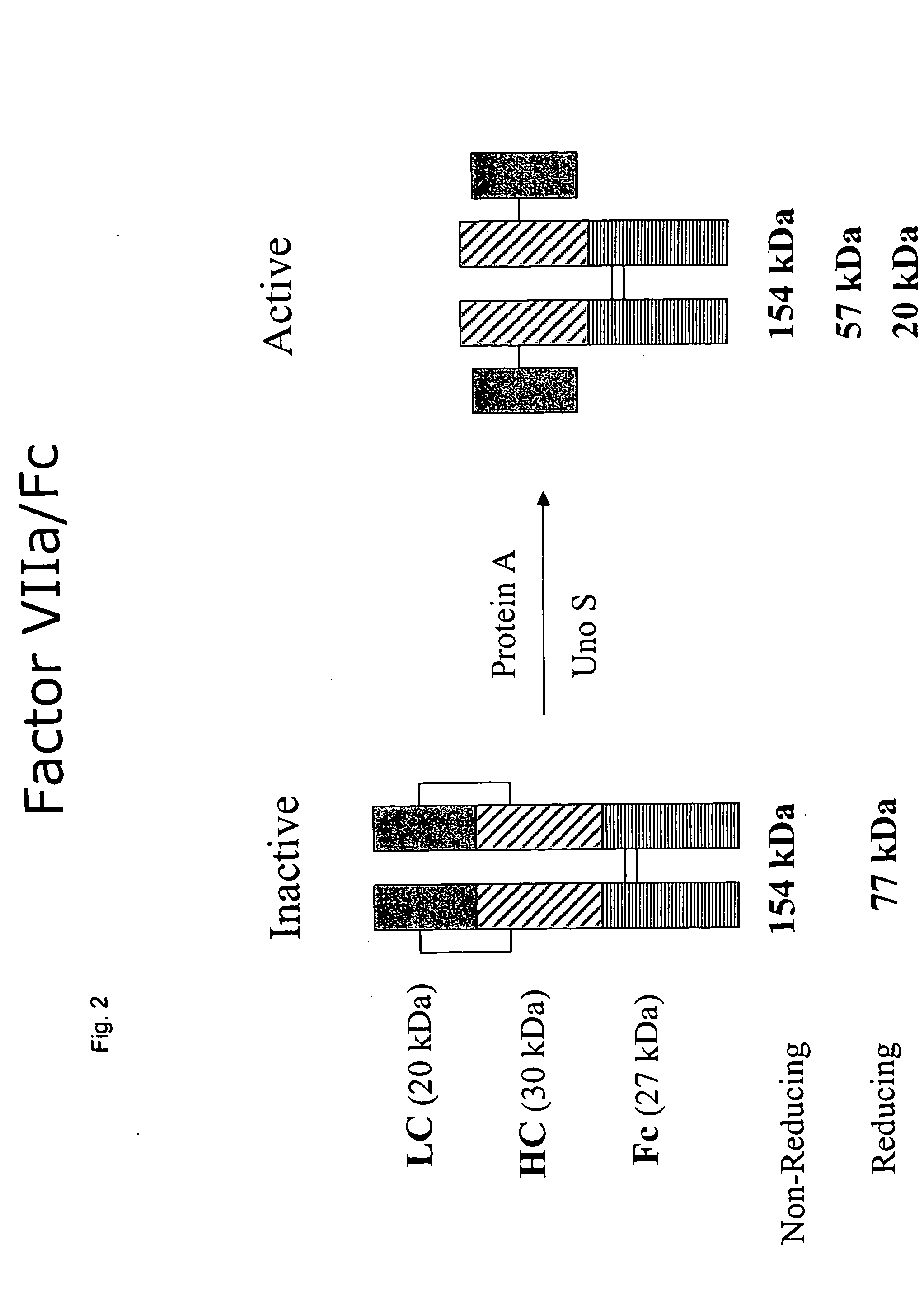
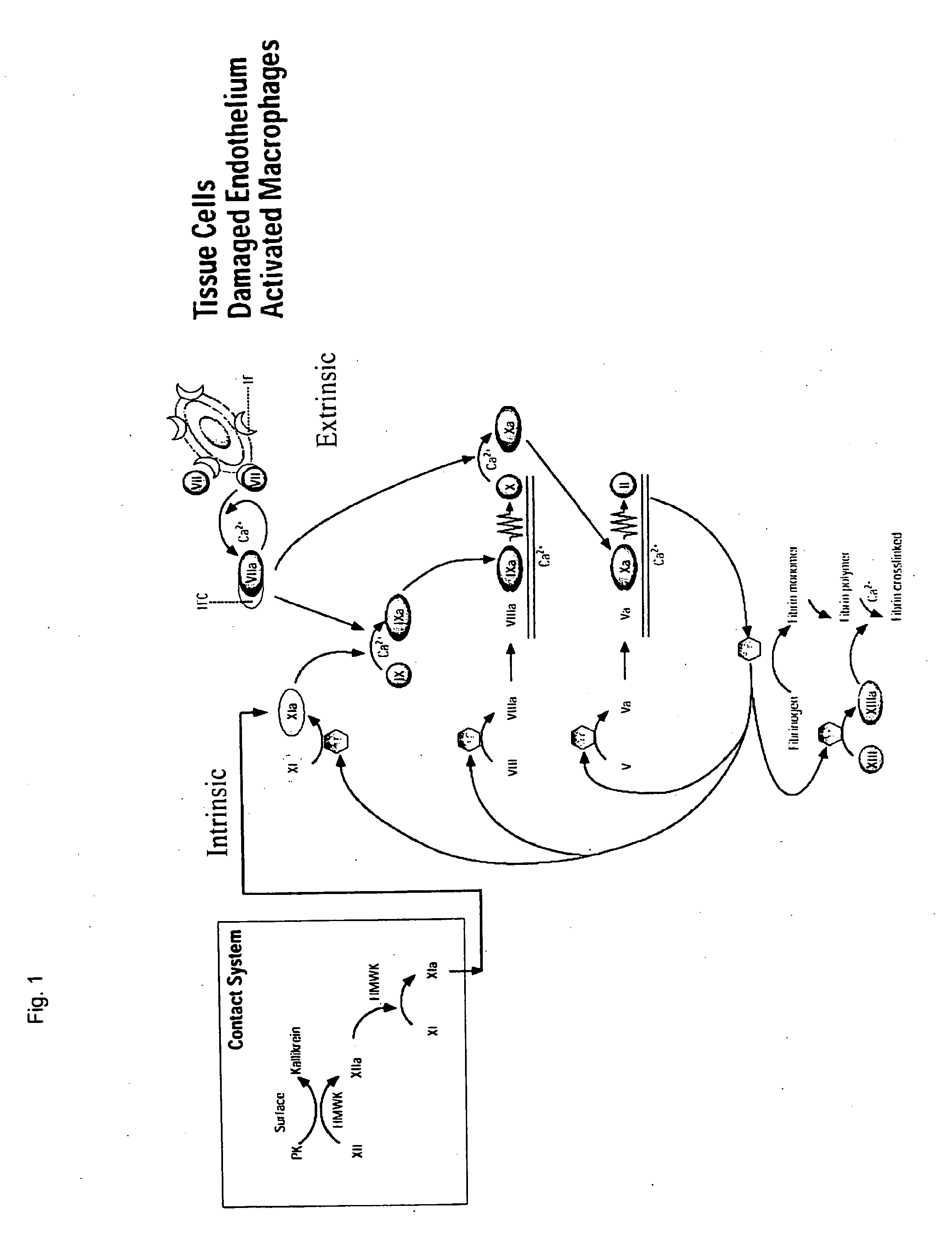
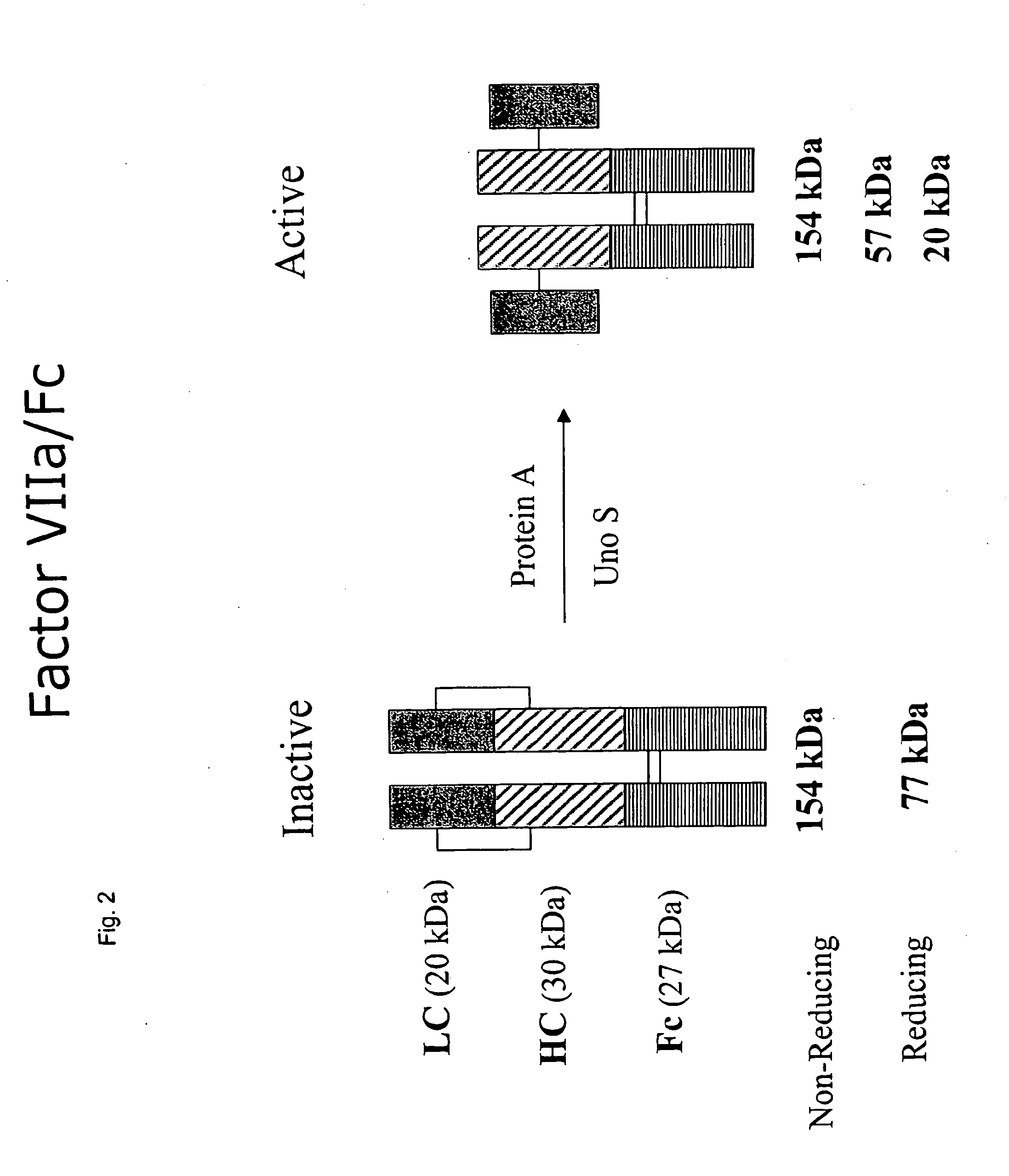
Clotting factor-Fc chimeric proteins to treat hemophilia
InactiveUS20050147618A1Peptide/protein ingredientsAntibody mimetics/scaffoldsHemostatic DisordersChimera Protein
The invention relates to a chimeric protein comprising at least one clotting factor and at least a portion of an immunoglobulin constant region. The invention relates to a method of treating a hemostatic disorder comprising administering a therapeutically effective amount of a chimeric protein wherein the chimeric protein comprises at least one clotting factor and at least a portion of an immunoglobulin constant region.
Owner:BIOVERATIV THERAPEUTICS INC
Fibrin glue without fibrinogen and biosealant compositions and methods
InactiveUS6168788B1Stop blood flowPrevents unwantedSurgical adhesivesPeptide/protein ingredientsFibrin glueClot formation
The invention is a fibrin glue that avoids the use of fibrinogen and thus eliminates the need for premixing and premature clot formation. The fibrin glue of the invention comprises thrombin, thromboplastin and calcium and may have clotting Factors, VII, IX and X, and the like. The invention also comprises a biosealant for use with the fibrin glue without fibrinogen or for use alone. The biosealant is a two component mixture of gelatin / resorcinol and glyoxal / glutaraldehyde / 4-(p-maleimidophenyl) butyric acid. The two components are mixed on use.
Owner:WORTHAM LEON
Human Coagulation Factor VII Polypeptides
InactiveUS20090055942A1Prolong half-life in vivoPeptide/protein ingredientsFermentationFactor VIIClotting factor
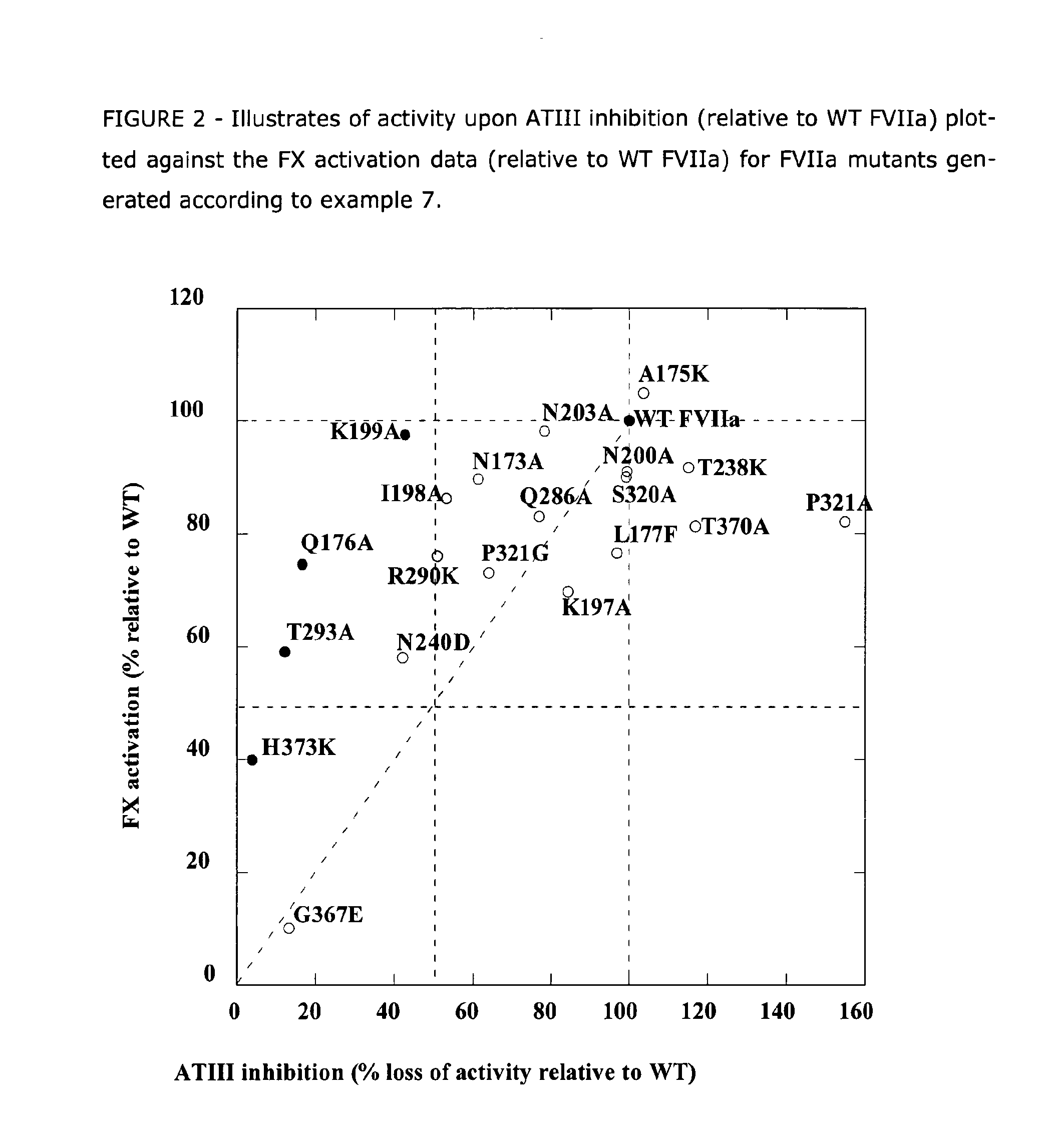
The present invention relates to novel human coagulation Factor Vila variants having substitutions of one or more amino acids at a position selected from the group consisting of position 172, 173, 175, 176, 177, 196, 197, 198, 199, 200, 203, 235, 237, 238, 239, 240, 286, 287, 288, 289, 290, 291, 292, 293, 294, 295, 297, 299, 319, 320, 321, 327, 341, 363, 364, 365, 366, 367, 370, 373 corresponding to amino acid positions of SEQ ID NO:1 and wherein said Factor VII polypeptide exhibits increased resistance to inactivation by an endogenous inhibitor of said FVII polypeptide relative to wild-type human FVIIa.
Owner:NOVO NORDISK AS
Long-acting coagulation factors and methods of producing same
ActiveUS8476234B2Prolong lifeImproving area under curve (AUC)Peptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideClotting factor
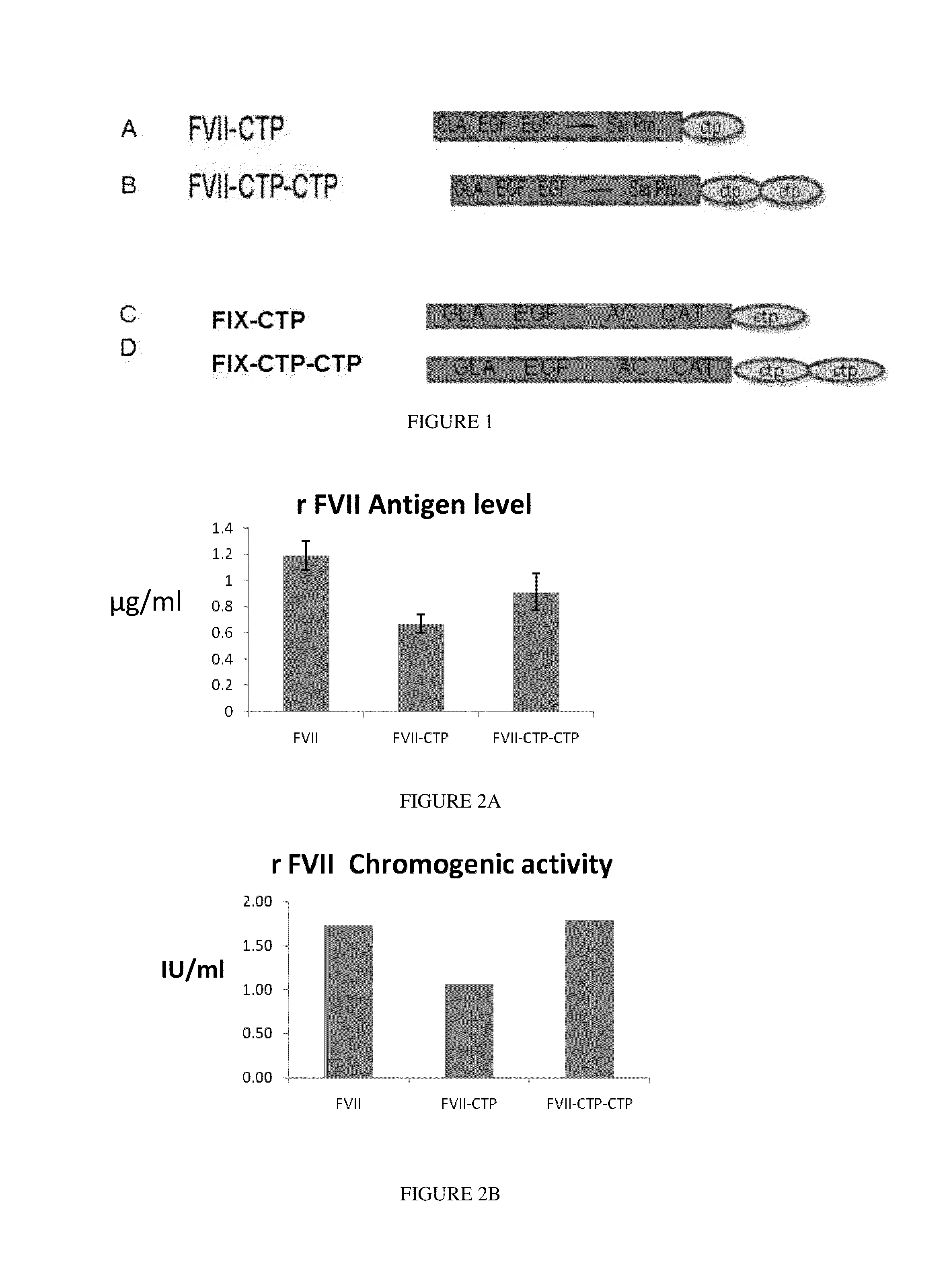
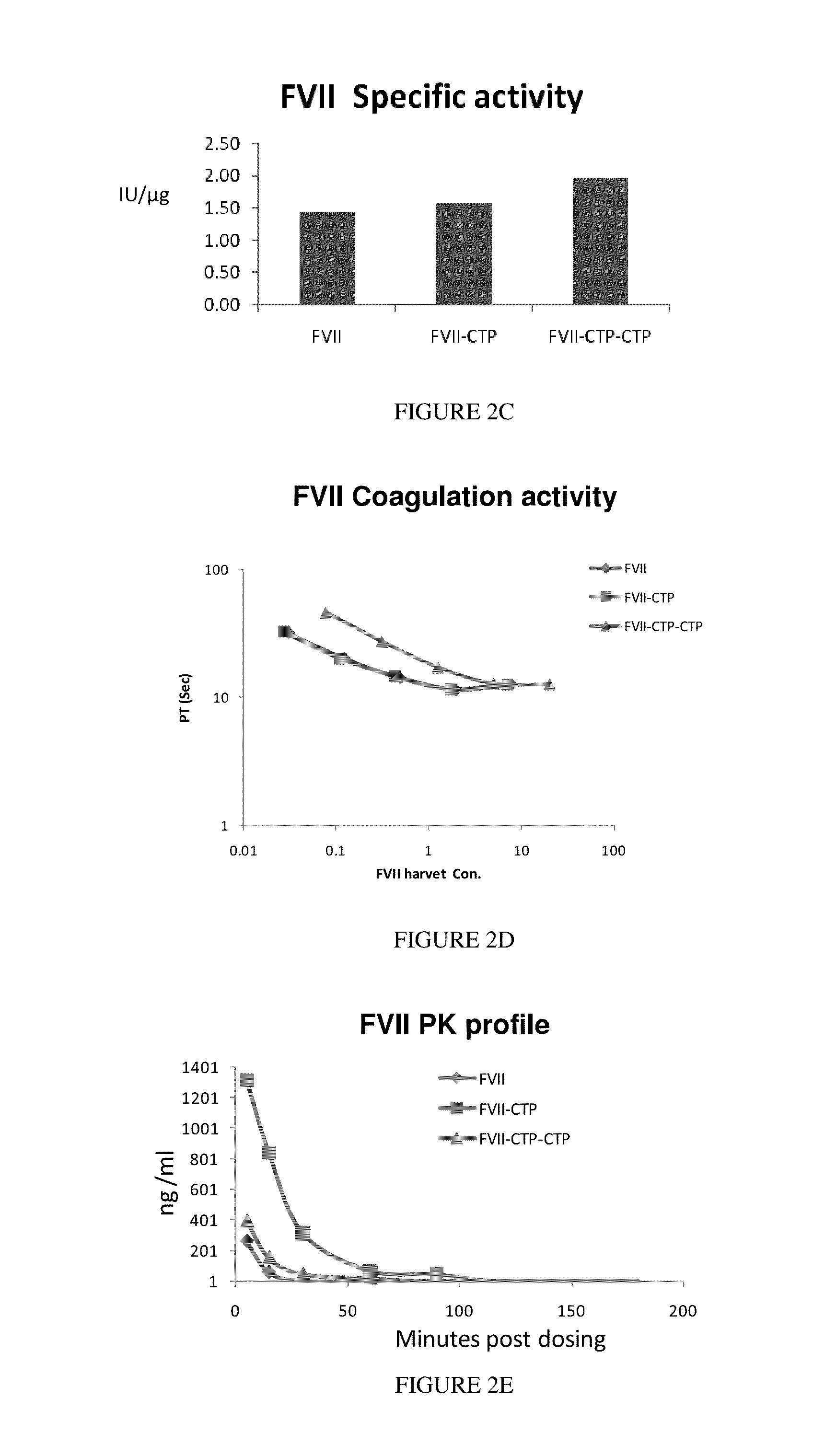
Polypeptides and polynucleotides encoding same comprising at least one carboxy-terminal peptide (CTP) of chorionic gonadotrophin attached to a carboxy terminus of a coagulation factor and not to an amino terminus are disclosed. Pharmaceutical compositions comprising the polypeptides and polynucleotides of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
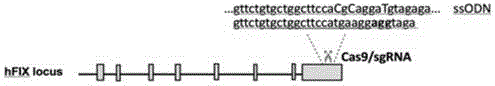
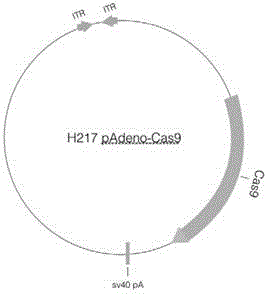
Site specific repairing carrier system and method of blood coagulation factor genetic mutation
The invention discloses a method for carrying out in-situ repairing on blood coagulation factor F8 / F9. The method comprises the following steps: in a target genome sequence, selecting the mutation sites of blood coagulation factor as the gene sites for in-situ repairing; designing the binding sites of nuclease of sgRNA sequence of a CRISPR / Cas system; designing a homologous recombinant repairing donor sequence for in-situ repairing; delivering nuclease protein and / or sgRNA and the nucleotide sequence of the homologous recombinant repairing donor to the gene sites of in-situ repairing by a delivering carrier; generating damages to the genome DNA by the nuclease on the gene in-situ repairing sites; and inserting the homologous recombinant repairing donor sequence into the gene in-situ repairing sites so as to repair the gene or supplement the expression of gene. The in-situ repairing of mutation sites of blood coagulation factor can be applied to the clinic, and the method has the advantages of precise induction, safer and controllable process, and definite target.
Owner:EAST CHINA NORMAL UNIV
Modified coagulation factors with prolonged in vivo half-life
InactiveUS20100120664A1Increase productionHigh expressionFactor VIIPeptide/protein ingredientsHalf-lifeNucleic acid sequencing
The present invention relates to nucleic acid sequences coding for modified coagulation factors, preferably coagulation factor VIII, and their derivatives; recombinant expression vectors containing such nucleic acid sequences; host cells transformed with such recombinant expression vectors; and recombinant polypeptides and derivatives coded for by said nucleic acid sequences, whereby said recombinant polypeptides and derivatives have biological activities and prolonged in vivo half-lives compared to the unmodified wild-type proteins. The invention also relates to corresponding sequences that result in improved in vitro stability. The present invention further relates to processes for the manufacture of such recombinant proteins and their derivatives. The invention also relates to a transfer vector for use in human gene therapy, which comprises such nucleic acid sequences.
Owner:CSL BEHRING GMBH
Optimised coding sequence and promoter
ActiveUS20130024960A1Shorten the timeImprove the level ofFactor VIIOrganic active ingredientsLipid storageLipid storage disease
An optimized coding sequence of human blood clotting factor eight (VIII) and a promoter may be used in vectors, such as rAAV, for introduction of factor VIII, and / or other blood clotting factors and transgenes. Exemplary of these factors and transgenes are alpha-1-antitrypsin, as well as those involved in the coagulation cascade, hepatocye biology, lysosomal storage, urea cycle disorders, and lipid storage diseases. Cells, vectors, proteins, and glycoproteins produced by cells transformed by the vectors and sequence, may be used in treatment.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC +2
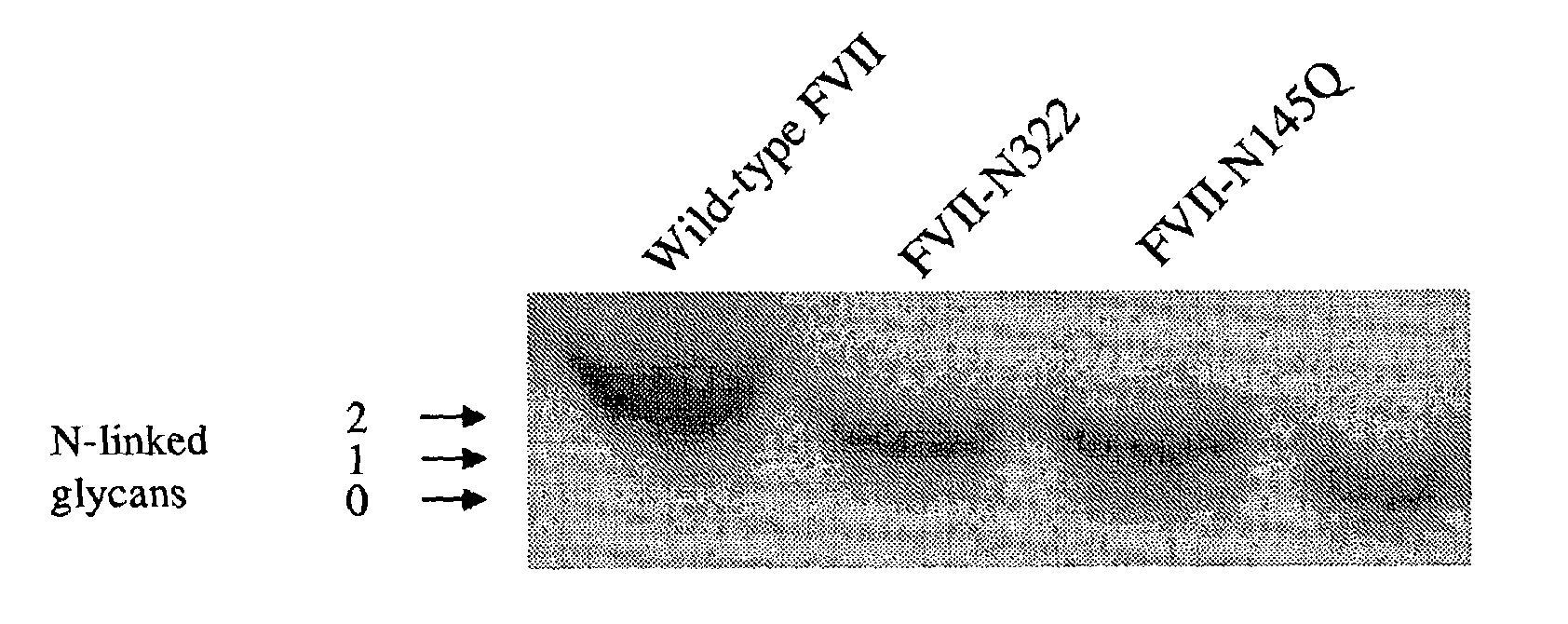
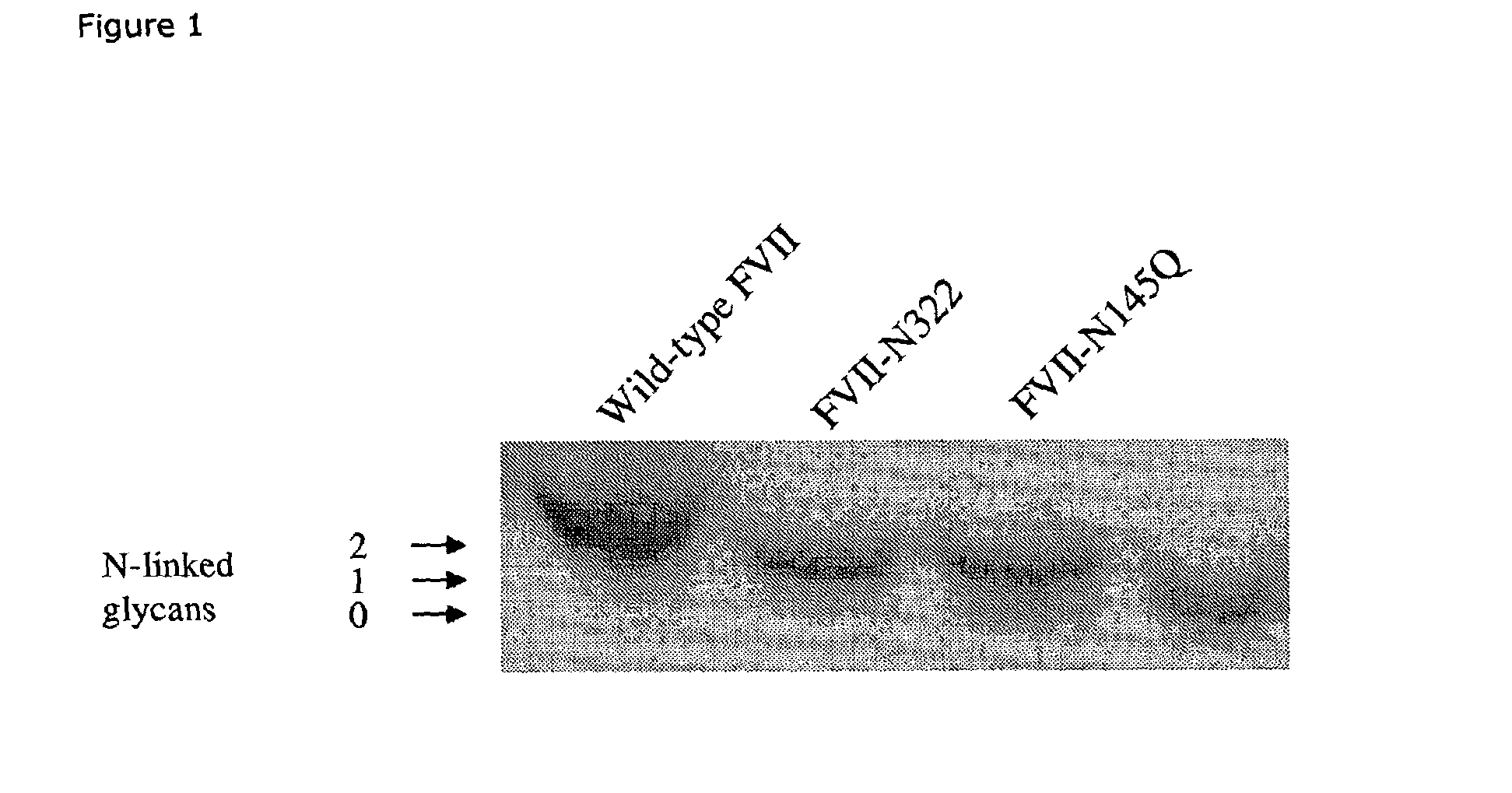
Glycosylation-Disrupted Factor VII Variants
InactiveUS20080058255A1Improve biological activityRapid clearancePeptide/protein ingredientsSurgical drugsNucleotideFactor VII
The present invention relates to human coagulation Factor VII polypeptides, as well as polynucleotide constructs encoding such polypeptides, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions comprising Factor VII polypeptides, uses and methods of treatment; and any additional inventive features related thereto.
Owner:NOVO NORDISK AS
Method And Apparatus For Preparing Platelet Rich Plasma And Concentrates Thereof
ActiveUS20100206798A1Shaking/oscillating/vibrating mixersTransportation and packagingHollow fibreFiber
The PRP separator-concentrator of this invention is suitable for office use or emergency use for trauma victims. The PRP separator comprises a motorized centrifugal separation assembly, and a concentrator assembly. The centrifugal separator assembly comprises a centrifugal drum separator that includes an erythrocyte capture module and a motor having a drive axis connected to the centrifugal drum separator. The concentrator assembly comprises a water-removal module for preparing PRP concentrate. The centrifugal drum separator has an erythrocyte trap. The water removal module can be a syringe device with water absorbing beads or it can be a pump-hollow fiber cartridge assembly. The hollow fibers are membranes with pores that allow the flow of water through the fiber membrane while excluding flow of clotting factors useful for sealing and adhering tissue and growth factors helpful for healing while avoiding activation of platelets and disruption of any trace erythrocytes present in the PRP.
Owner:HANUMAN +1
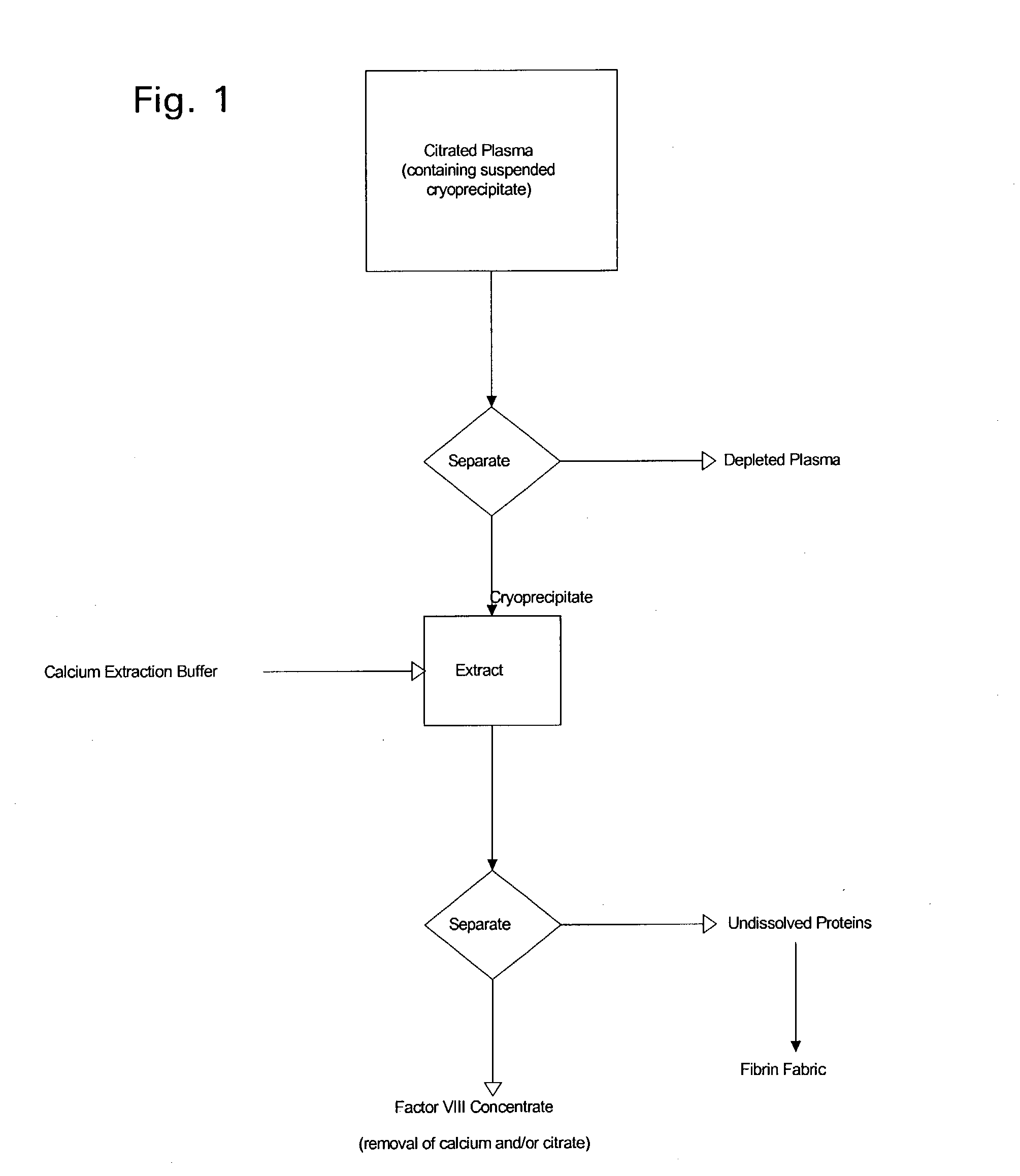
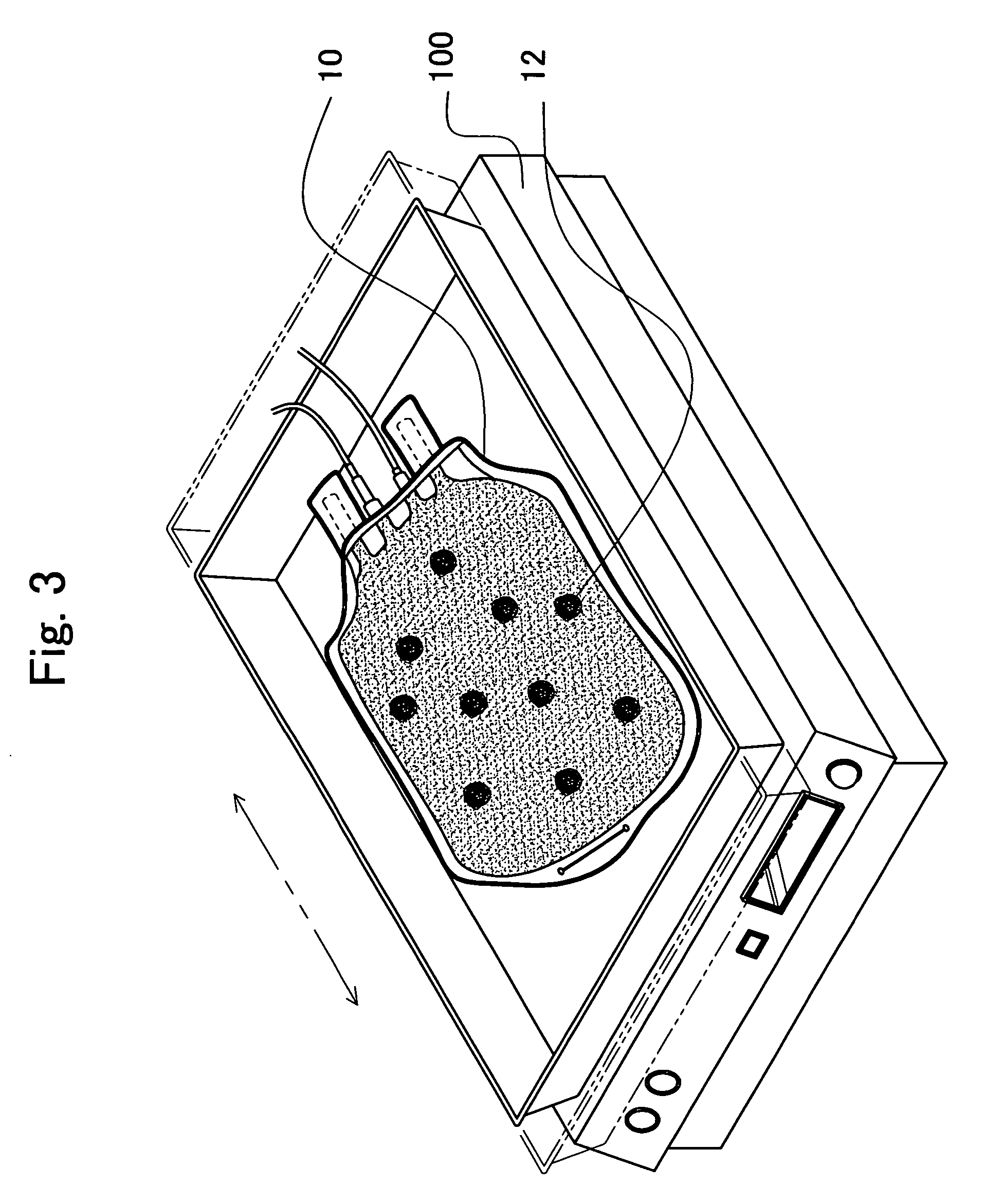
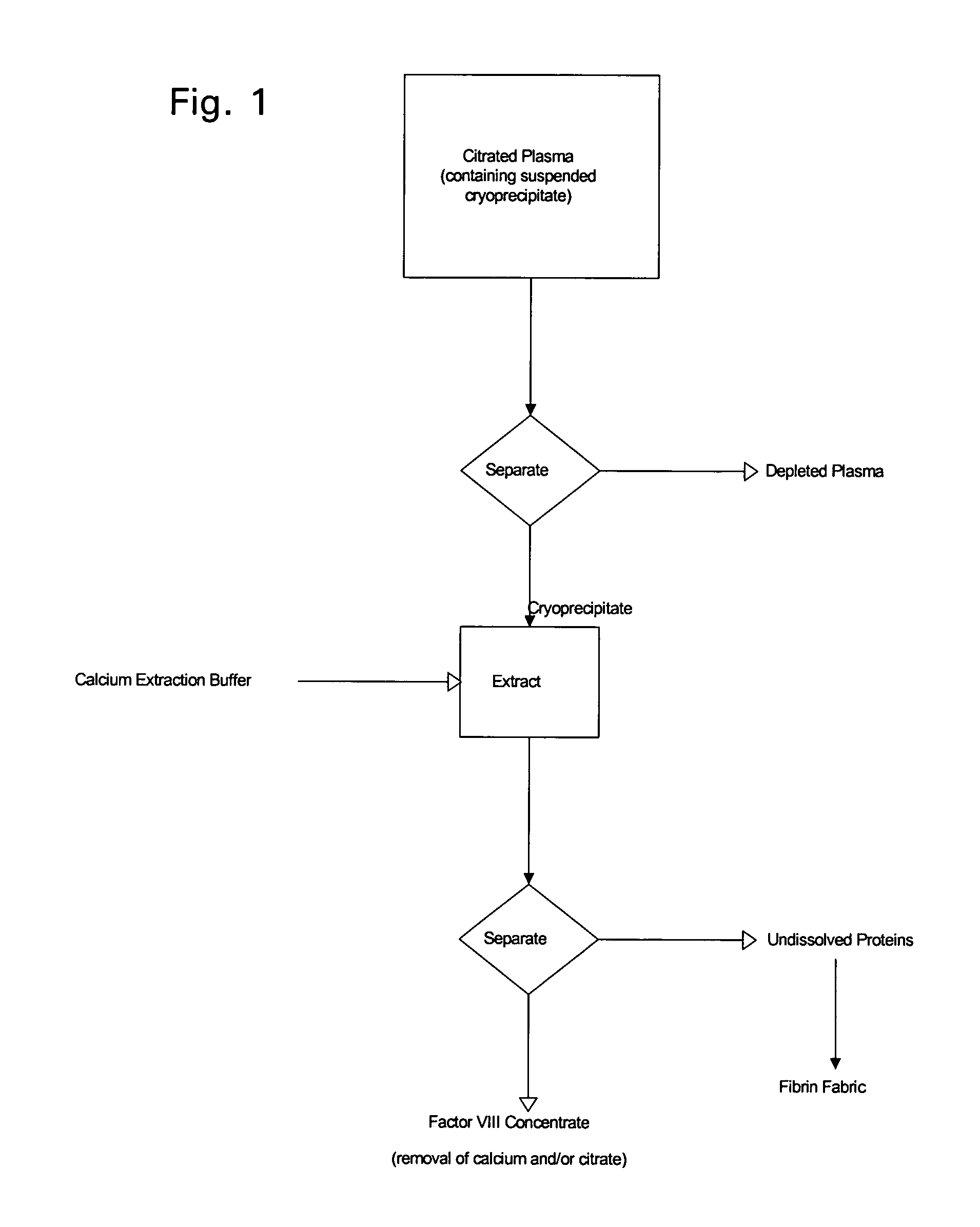
Enhanced production of blood clotting factors and fibrin fabric
The blood collection, processing and transfer by separation of discrete components containing additional citrate (at least about trisodium citrate 9% w / v) in one or other of collection or processing bag provides for enhanced yield and purity of cryoprecipitate. Inhibiting the activation or denaturation of blood components including blood cells and plasma proteins and with the removal of the activated and denatured components thereby improving safety and efficacy of end products. The inventive process is particularly suited to an improved extraction process to yield concentrated clotting factors from single donors or limited pools without use of chromatography. Following extraction the remaining cryoprecipitate can advantageously be formed into a fibrin fabric used in surgeries and in the treatment of wounds
Owner:SHANBROM TECH
Container for serum production and method of regenerative medicine using the same
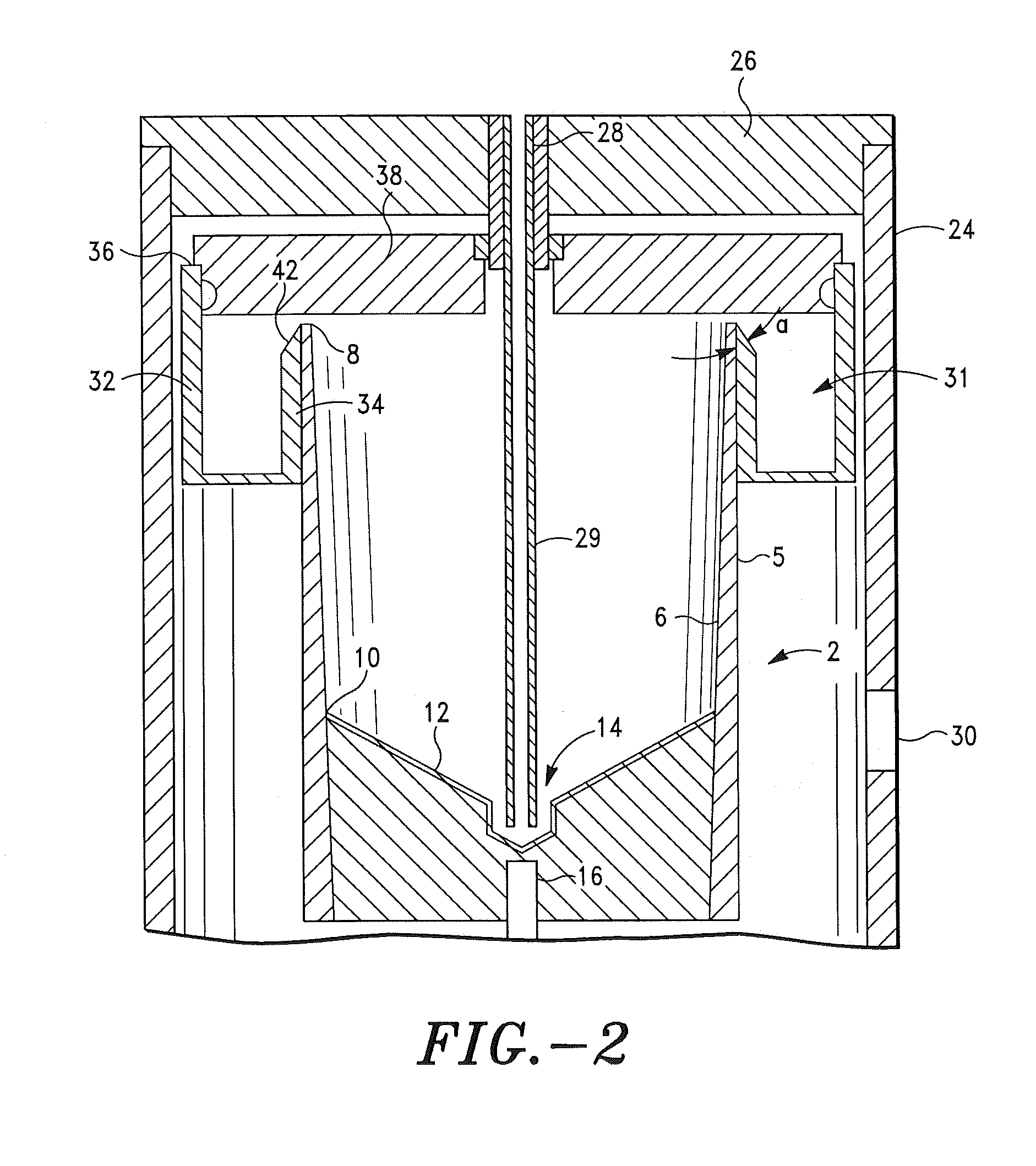
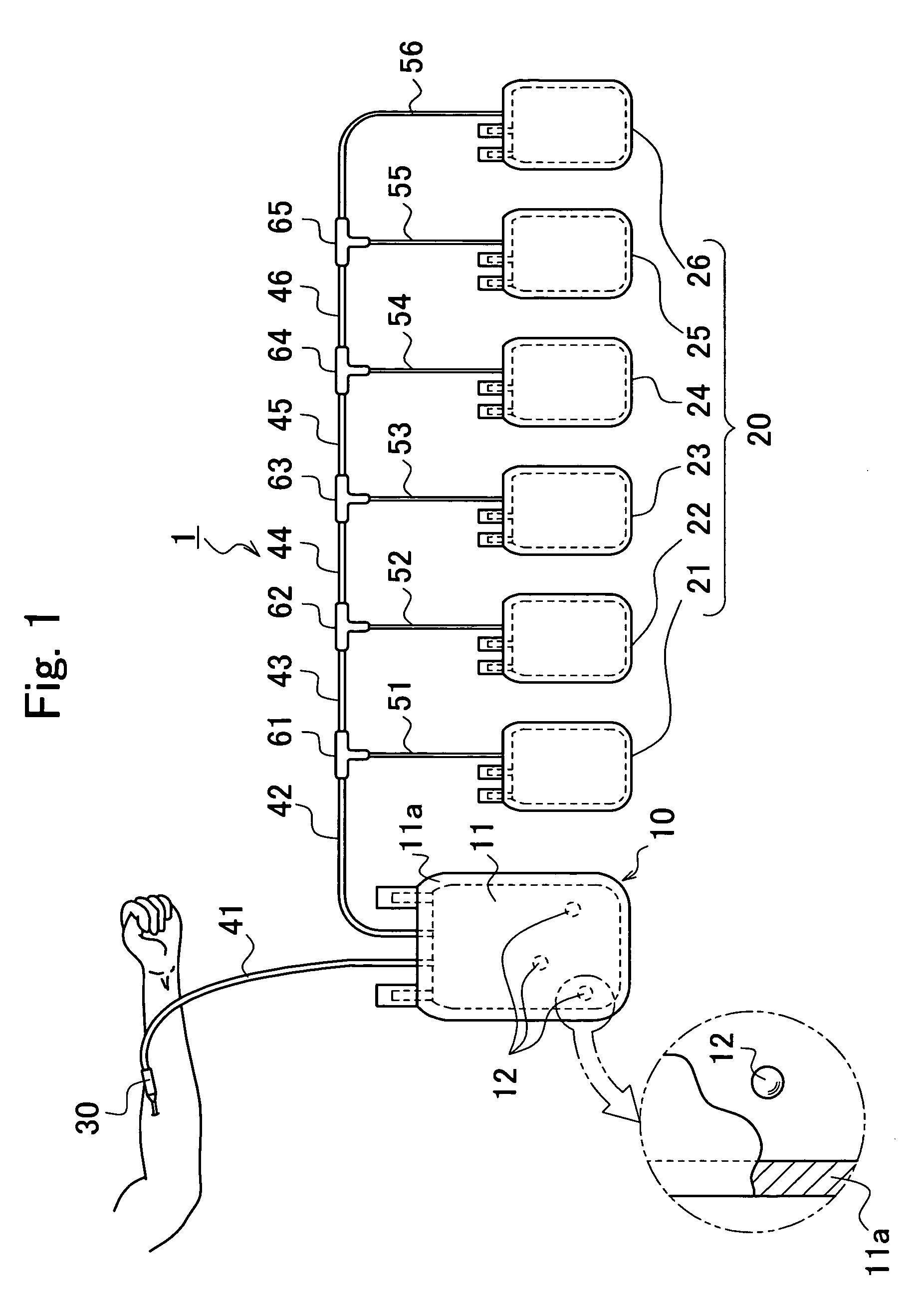
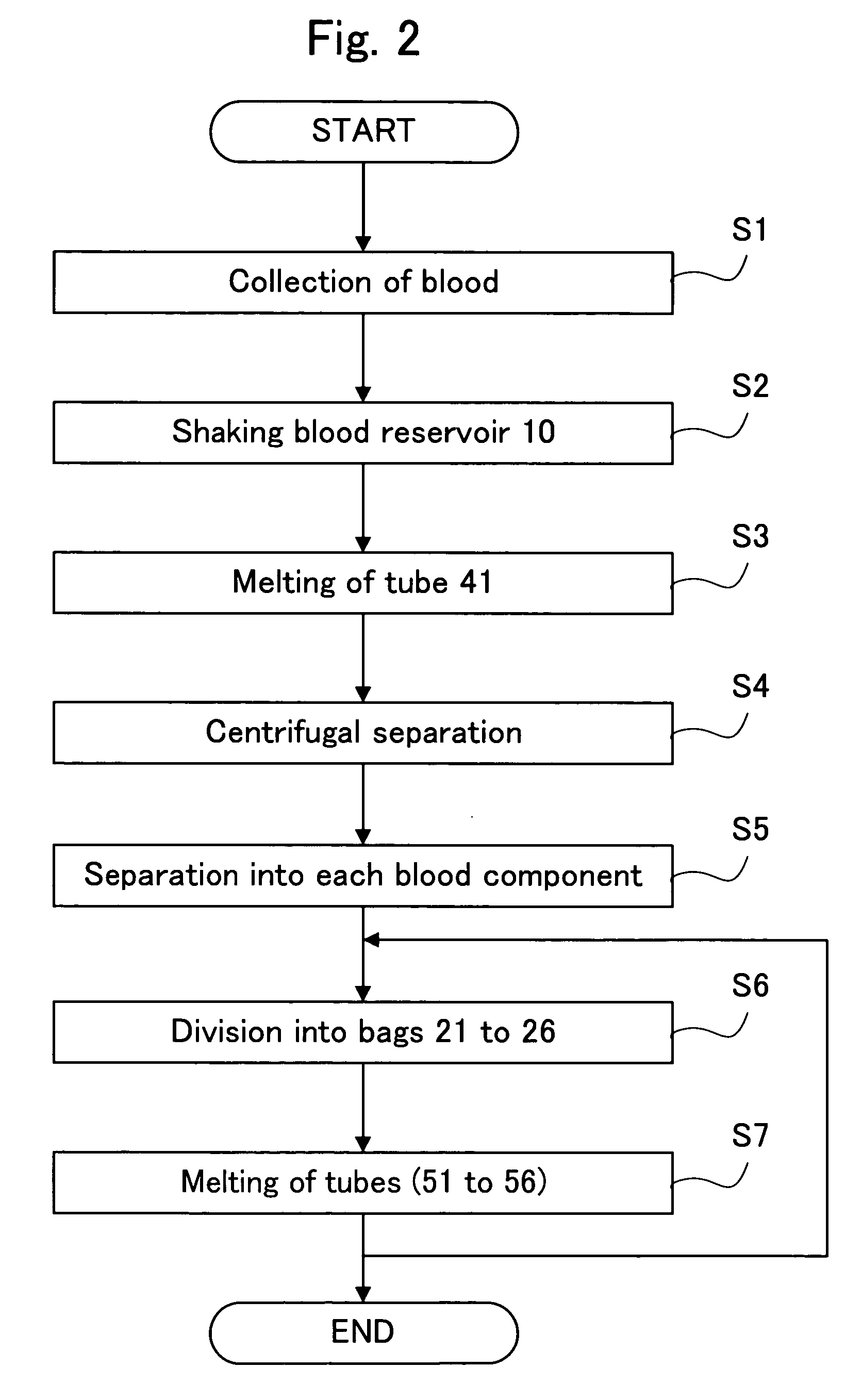
ActiveUS20060251622A1Regenerated safelyQuick and efficient production of a large amount of serumBioreactor/fermenter combinationsBiocideBlood component separatorClotting factor
A blood storage container suitable for quick and efficient production of a large amount of serum while ensuring high safety, and a method of separating blood and a regenerative medical process using the same are provided. In a blood component separator 1 for separating collected blood into a plurality of blood components and preserving them, the separator 1 including a blood reservoir 10 for reserving the blood and a component storage part 20 aseptically connected in an air-tight manner to this blood reservoir 10, to the aforementioned blood reservoir 10 being imparted a serum producing function to remove coagulation factors from the blood to an extent enabling use in practical applications as a serum, and the aforementioned component storage part 20 storing each blood component generated by separation of the blood in the blood reservoir 10.
Owner:JMS CO LTD
Enhanced production of blood clotting factors and fibrin fabric
The blood collection, processing and transfer by separation of discrete components containing additional citrate (at least about trisodium citrate 9% w / v) in one or other of collection or processing bag provides for enhanced yield and purity of cryoprecipitate. Inhibiting the activation or denaturation of blood components including blood cells and plasma proteins and with the removal of the activated and denatured components thereby improving safety and efficacy of end products. The inventive process is particularly suited to an improved extraction process to yield concentrated clotting factors from single donors or limited pools without use of chromatography. Following extraction the remaining cryoprecipitate can advantageously be formed into a fibrin fabric used in surgeries and in the treatment of wounds.
Owner:SHANBROM TECH
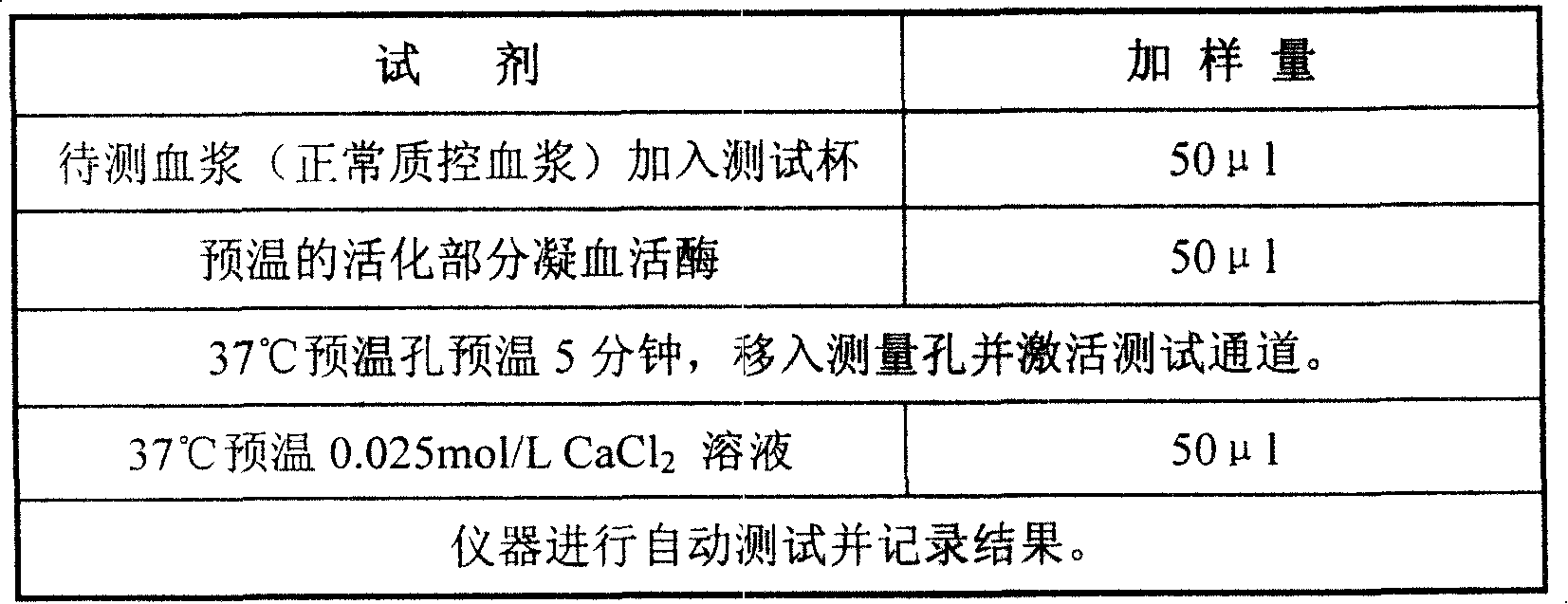
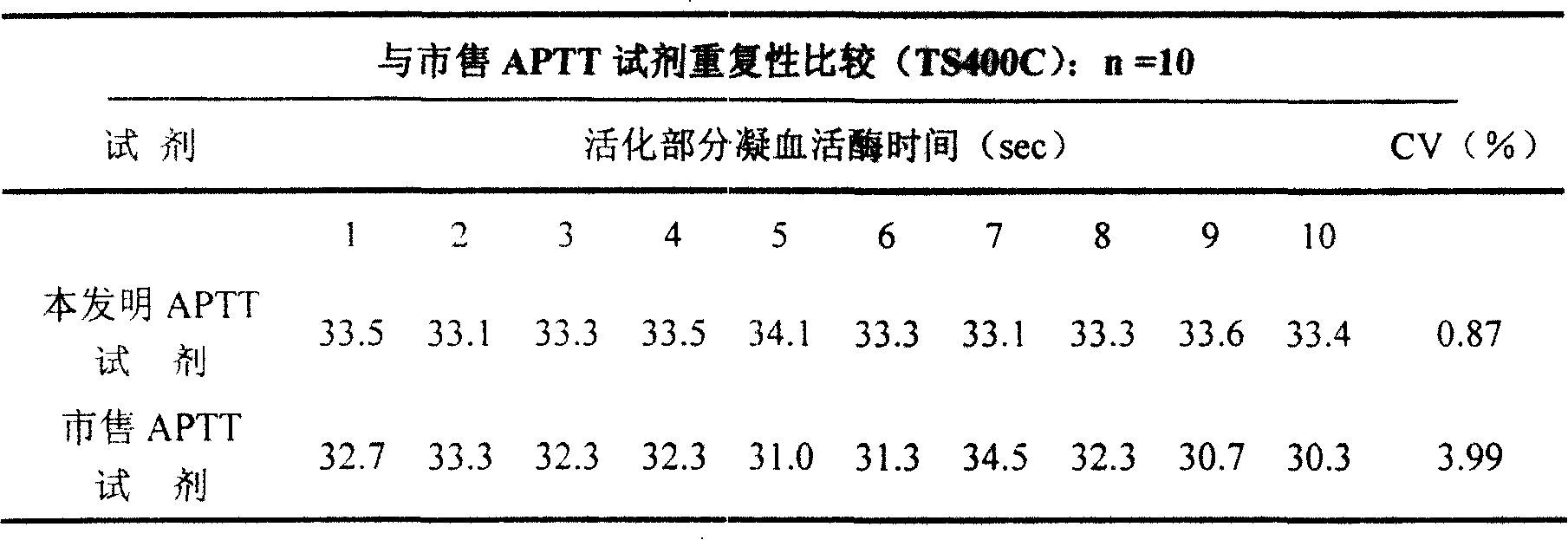
External diagnostic reagent kit used for measuring activated partial thromboplastin time
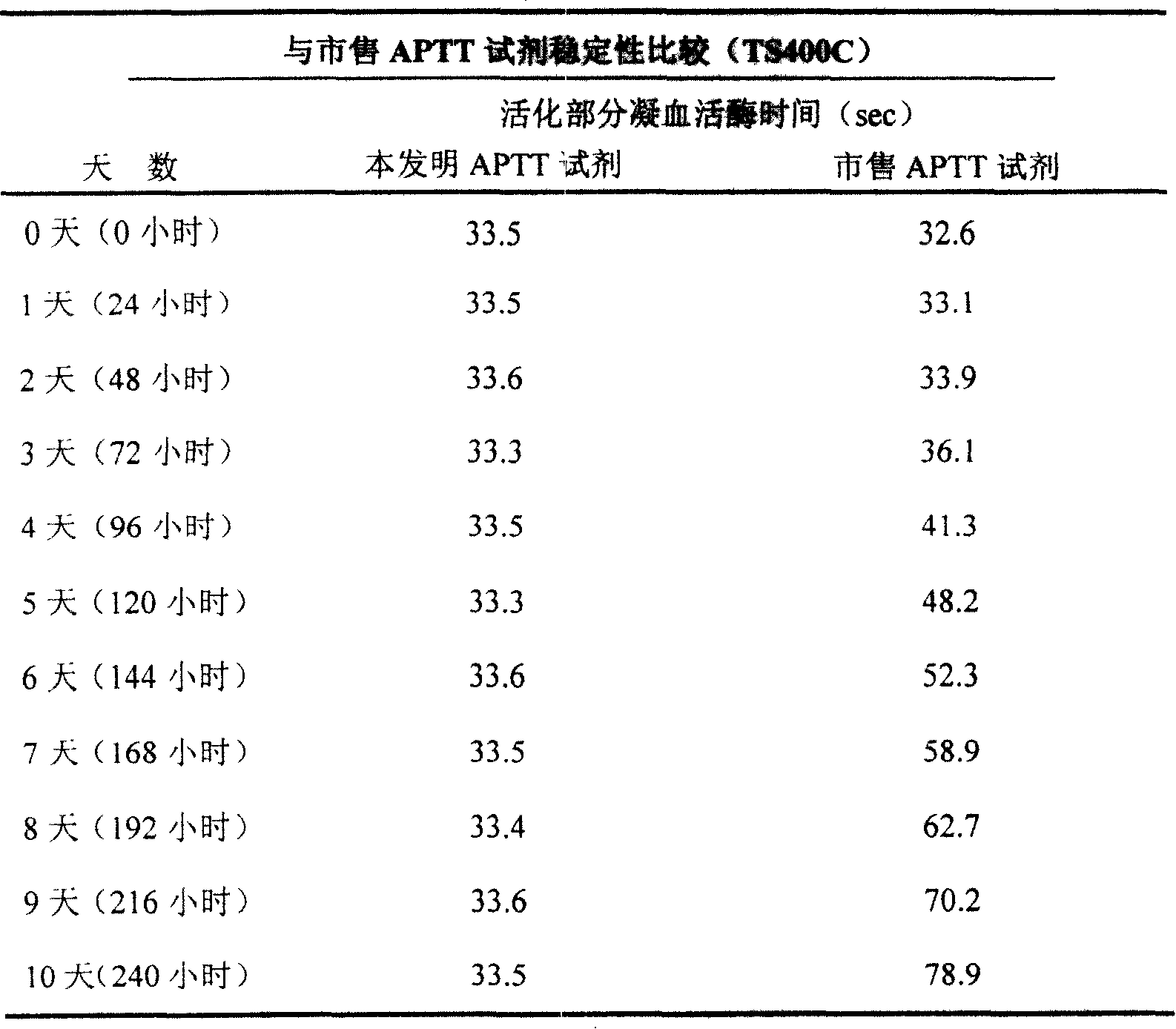
ActiveCN101221189AImprove stabilityGood repeatabilityMicrobiological testing/measurementBiological testingDisease causeAPTT - reference
The invention relates to an in vitro diagnostic kit for the determination of activated partial thromboplatin time (APTT) in clinical test. The invention consists of a partial thromboplatin reagent and a calcium chloride solution, which is used for the detection of the defects of the intrinsic coagulation pathway factors and the screening test of the related inhibitors, and the invention is also a primary means for the current coagulation factor and heparin anticoagulant treatment and the detection of lupus anticoagulant. The invention has the advantages of long stability time and good repeatability of the partial thromboplatin reagent after a re-dissolution, at the same time, the invention has better consistency of the measurement results of a blood coagulation analyzer by using an optical method and a magnetic bead method, therefore, the invention is applicable to large, medium and small hospitals, and the test results of different hospitals have comparability, therefore the invention has important meaning for implementing the one general report of test reports, provides the reliable experimental data for the clinical diagnosis and the treatment of diseases and improves the efficiency and value of the basic studies of thrombosis and hemostasis.
Owner:SHANGHAI SUNBIO TECH
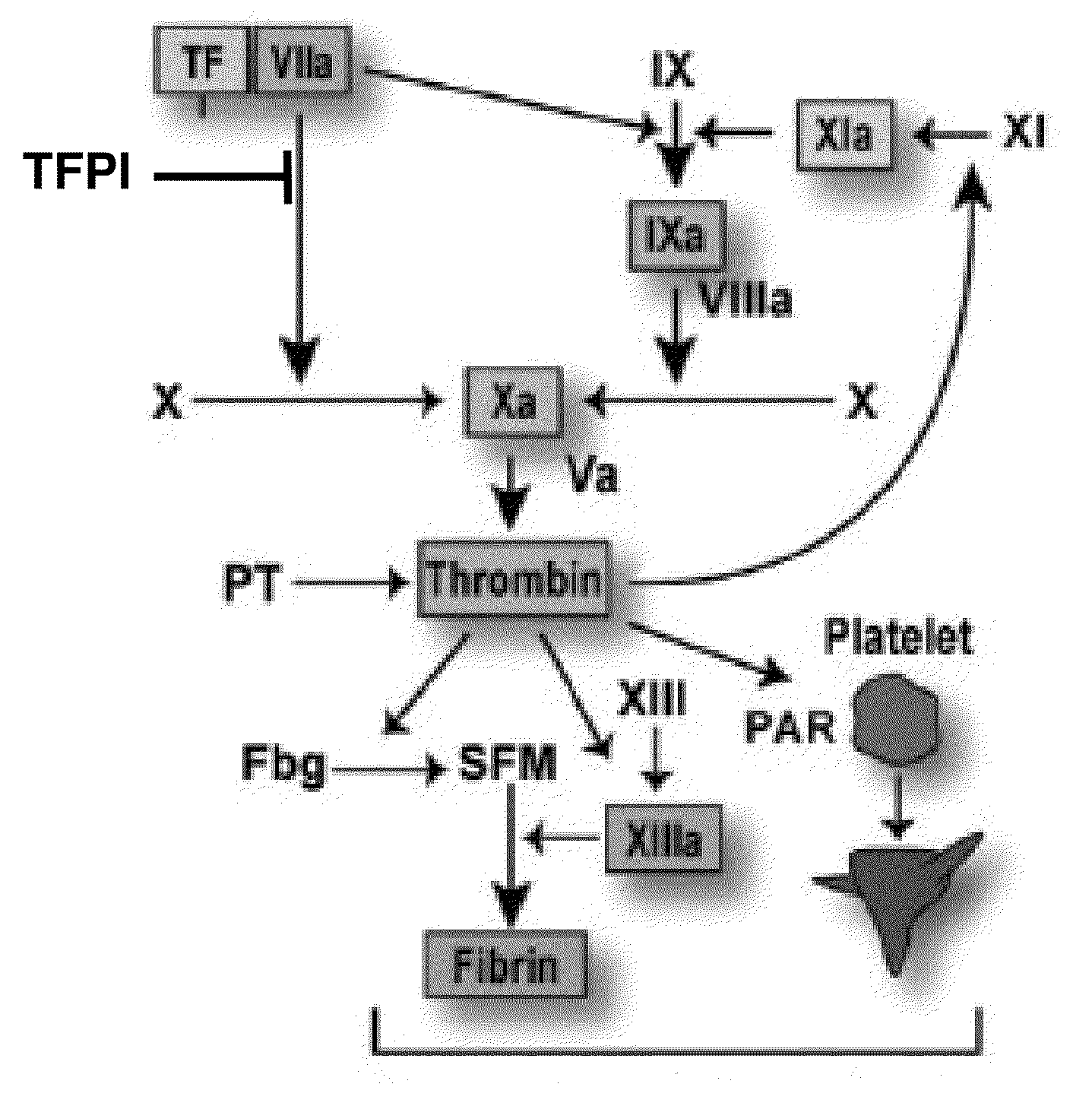
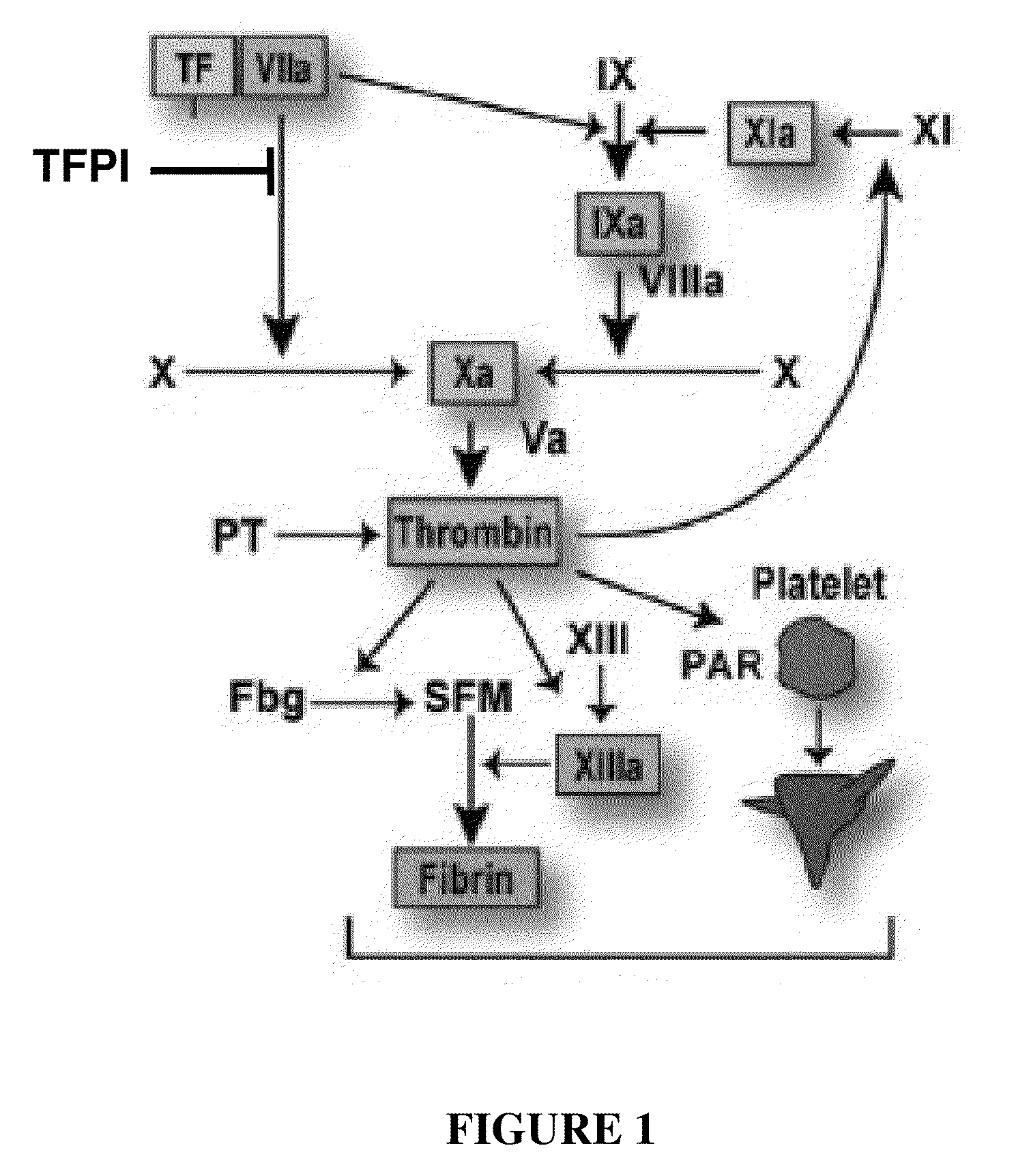
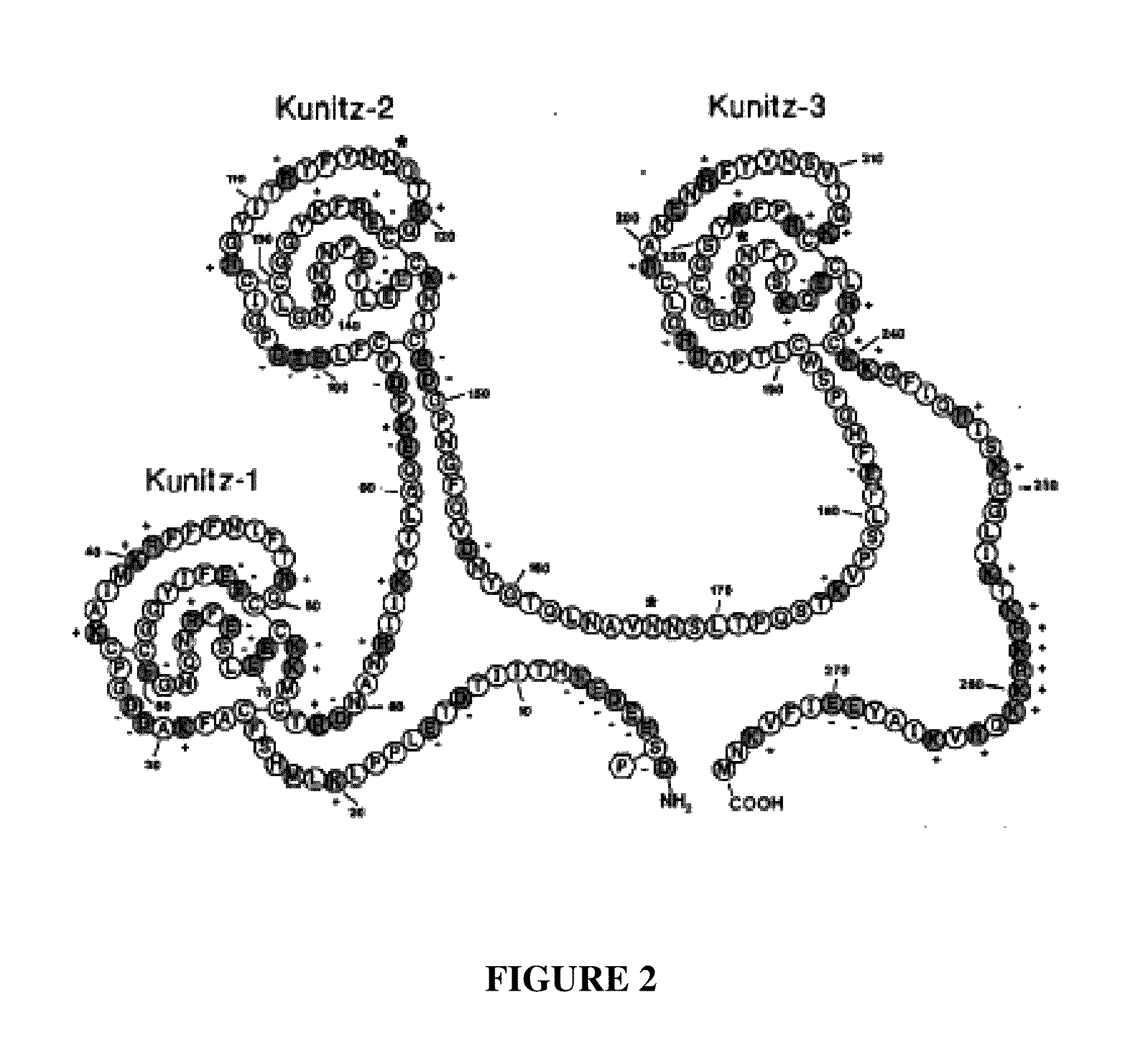
TFPI inhibitors and methods of use
ActiveUS20100173847A1Improves TFPI-regulated thrombin generationFactor VIIPeptide-nucleic acidsMedicineThrombin activity
The invention provides peptides that bind Tissue Factor Pathway Inhibitor (TFPI), including TFPI-inhibitory peptides, and compositions thereof. The peptides may be used to inhibit a TFPI, enhance thrombin formation in a clotting factor-deficient subject, increase blood clot formation in a subject, and / or treat a blood coagulation disorder in a subject.
Owner:TAKEDA PHARMA CO LTD
CLOTTING FACTOR-Fc CHIMERIC PROTEINS TO TREAT HEMOPHILIA
ActiveUS20110182896A1Reduce riskEffective treatmentPeptide/protein ingredientsHydrolasesHemostatic DisordersImmunoglobulin IgE
Owner:BIOVERATIV THERAPEUTICS INC
Recombinant blood clotting factors
The present invention relates to an improved method for the production of recombinant human blood clotting factors, in particular of factor VIII and factor IX. An immortalized human cell line can be used to stably express viral transcription activator proteins and carrying a vector having a promoter functionally linked to a DNA sequence coding for a blood coagulating factor, provided that said promoter is not a viral promoter which is stimulated by said viral transcription activator proteins. The invention further relates to an immortalized human cell line carrying said vector, factor VIII muteins particularly suitable for the above production method; pharmaceutical compositions comprising such factor VIII muteins, and the use of such factor VIII muteins for preparing a medicament for treating hemophilia.
Owner:OCTAPHARMA +1
Modulators of coagulation factors
ActiveUS20060040881A1Improved nucleic acid ligands for anticoagulantMaintain good propertiesOrganic active ingredientsAntipyreticCoronary Artery BypassesSurgery procedure
The invention provides improved nucleic acid ligands that inhibit coagulation and improved modulators of the nucleic acids to provide ideal modulators of coagulation. These improved nucleic acids and modulators are particularly useful for inhibiting coagulation in a host undergoing a therapeutic regime such as surgery or coronary artery bypass.
Owner:TOBIRA THERAPEUTICS
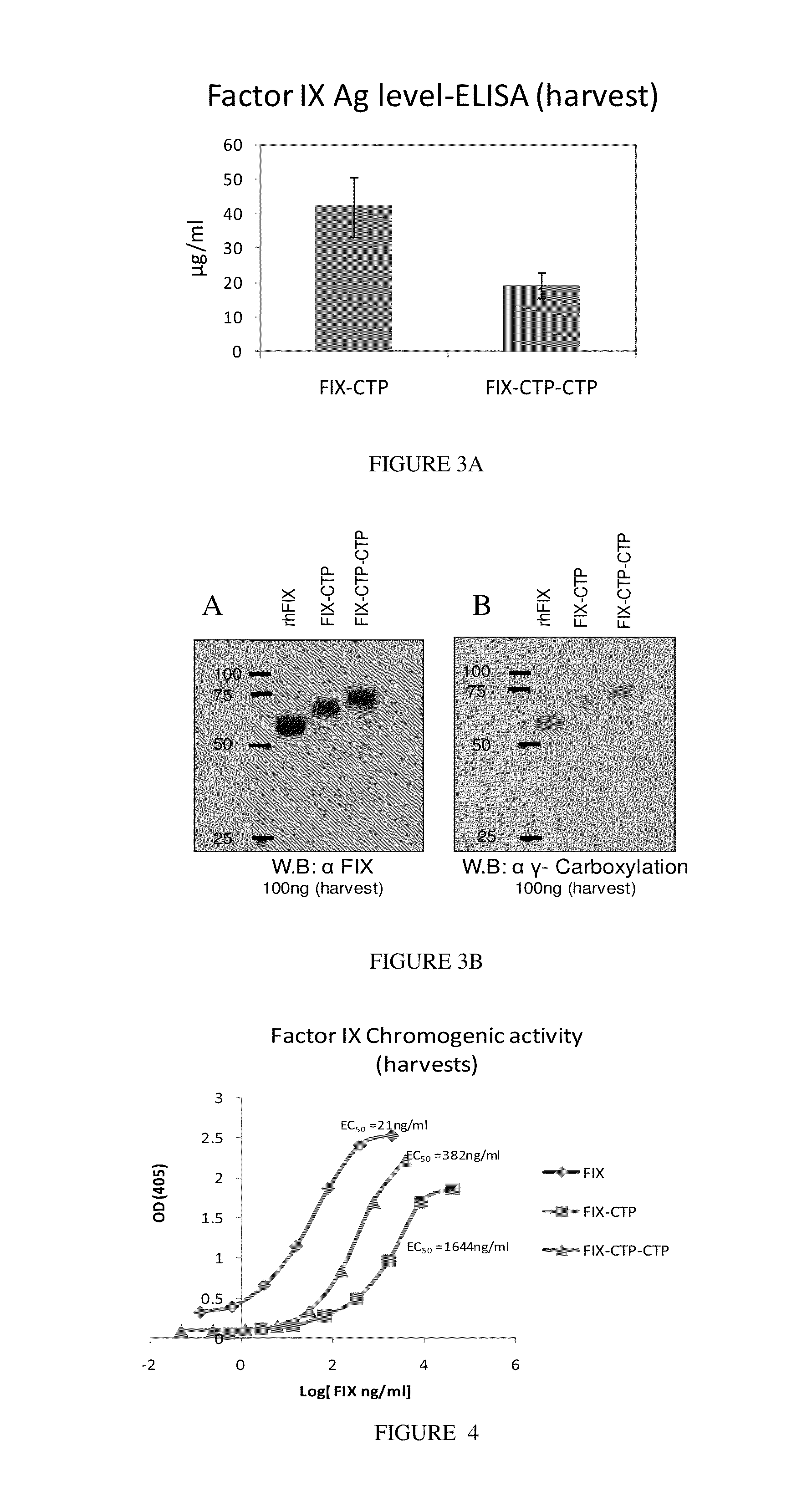
Enhanced gamma-carboxylation of recombinant vitamin K-dependent clotting factor
The invention relates to methods of optimizing gamma carboxylation of a vitamin K-dependent protein, methods of generating fully gamma carboxylated vitamin K-dependent protein, and compositions comprising chimeric nucleic acids and proteins for use in treatment of vitamin K-dependent disease states.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Clotting factor preparations for delivery into tissue of the intestinal tract using a swallowable drug delivery device
ActiveUS20190133937A1Improved long-term tolerance to and efficacyProcess controlPeptide/protein ingredientsPressure infusionIntestinal wallsTherapeutic effect
Embodiments provide devices, preparations and methods for delivering therapeutic agents (TAs) such as clotting factors (CFs, e.g., Factor 8) within the GI tract. Many embodiments provide a swallowable device e.g., a capsule for delivering TAs into the intestinal wall (IW). Embodiments also provide TA preparations configured to be contained within the capsule, advanced from the capsule into the IW and / or surrounding tissue (ST) and degrade to release the TA into the bloodstream to produce a therapeutic effect (e.g., improved clotting). The preparation can be operably coupled to delivery means having a first configuration where the preparation is contained in the capsule and a second configuration where the preparation is advanced out of the capsule into the IW or ST (e.g., the peritoneal cavity). Embodiments are particularly useful for delivery of CFs for treatment of clotting disorders (e.g., hemophilia) where such CFs are poorly absorbed and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
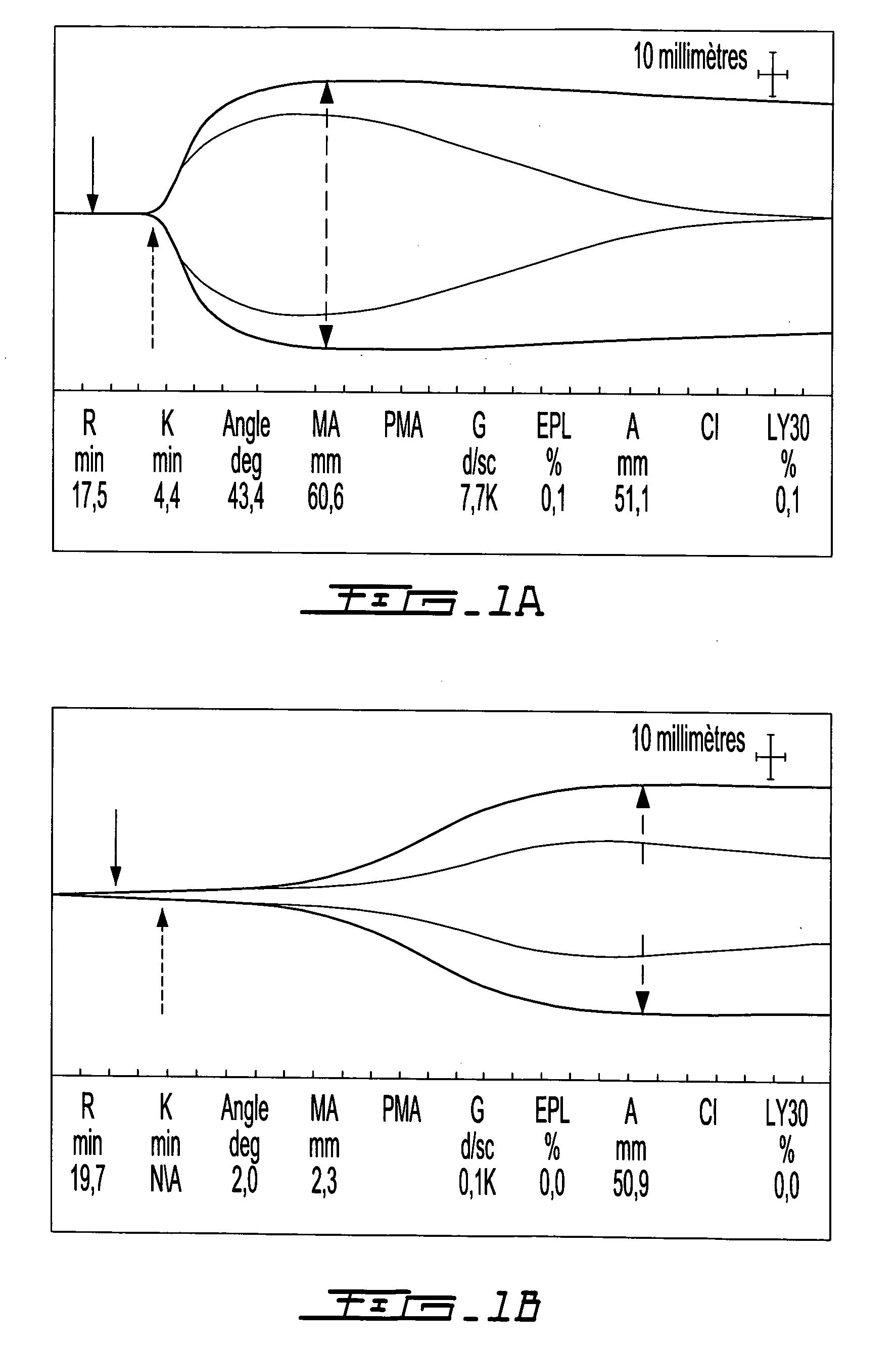
Kit for measuring the thrombin generation in a sample of a patient's blood or plasma
InactiveUS20050221414A1Simple and efficient and fast and reproducible assayConvenient typeMicrobiological testing/measurementBiological material analysisTissue factorThrombin activity
The invention provides a kit for measuring the thrombin generation in a sample of a patient's blood or plasma, or in a sample of clotting factors. The kit contains lyophilized tissue factor / phospholipid-complex and a lyophilized mixture containing a thrombin-substrate and CaCl2. The invention also provides processes for preparing the reagents for the kit. The kit can be used in a method for measuring the thrombin generation in a sample, wherein it is possible to detect changes in thrombin generation kinetics, for example after administration of inhibitor bypassing agents to a patient who has developed inhibitors to an exogenous clotting factor such as Factor VIII.
Owner:BAXTER INT INC +1
Inhibition of colony stimulating factor-1 receptor signaling for the treatment of brain cancer
InactiveUS20150119267A1Compound screeningApoptosis detectionAfter treatmentColony-stimulating factor
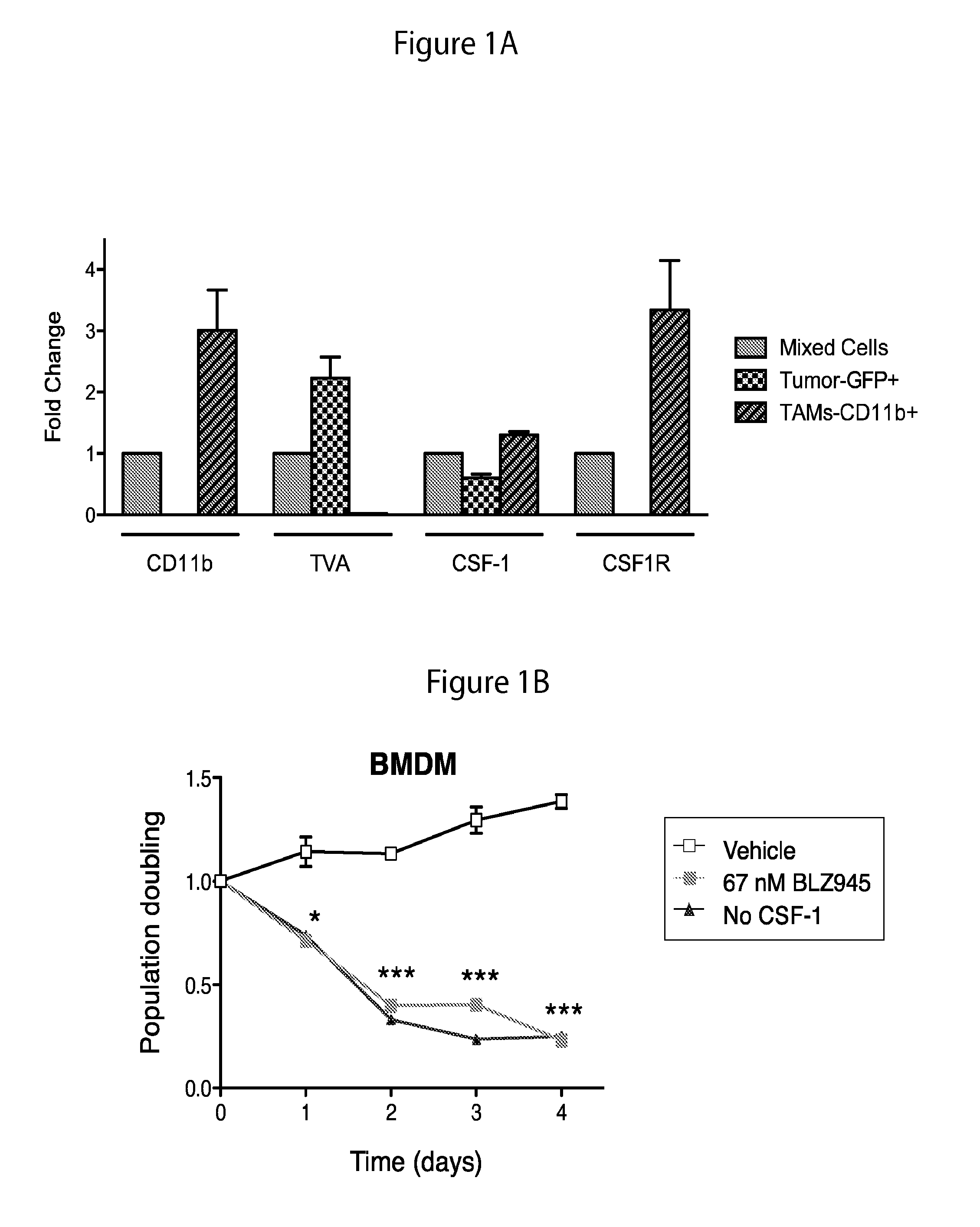
The present invention provides a method of screening brain tumor patients for treatment with inhibitor of CSF-1R, based on differential gene expression including adrenomeduUin (ADM), arginase 1 (ARG1), clotting factor F13A1, mannose receptor C type 1 (MRC1 / CD206), and protease inhibitor SERPINB2 after treatment with the inhibitor. Based on the same differential gene expression profile, the present invention also provides a method of screening a compound to treat brain cancer.
Owner:SLOAN KETTERING INST FOR CANCER RES
Human coagulation factor VII polypeptides
InactiveUS20060166915A1High activityPromote formationPeptide/protein ingredientsGenetic material ingredientsNucleotidePolynucleotide
The present invention relates to novel human coagulation Factor VIIa variants having coagulant activity as well as polynucleotide constructs encoding such variants, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK HEALTH CARE AG
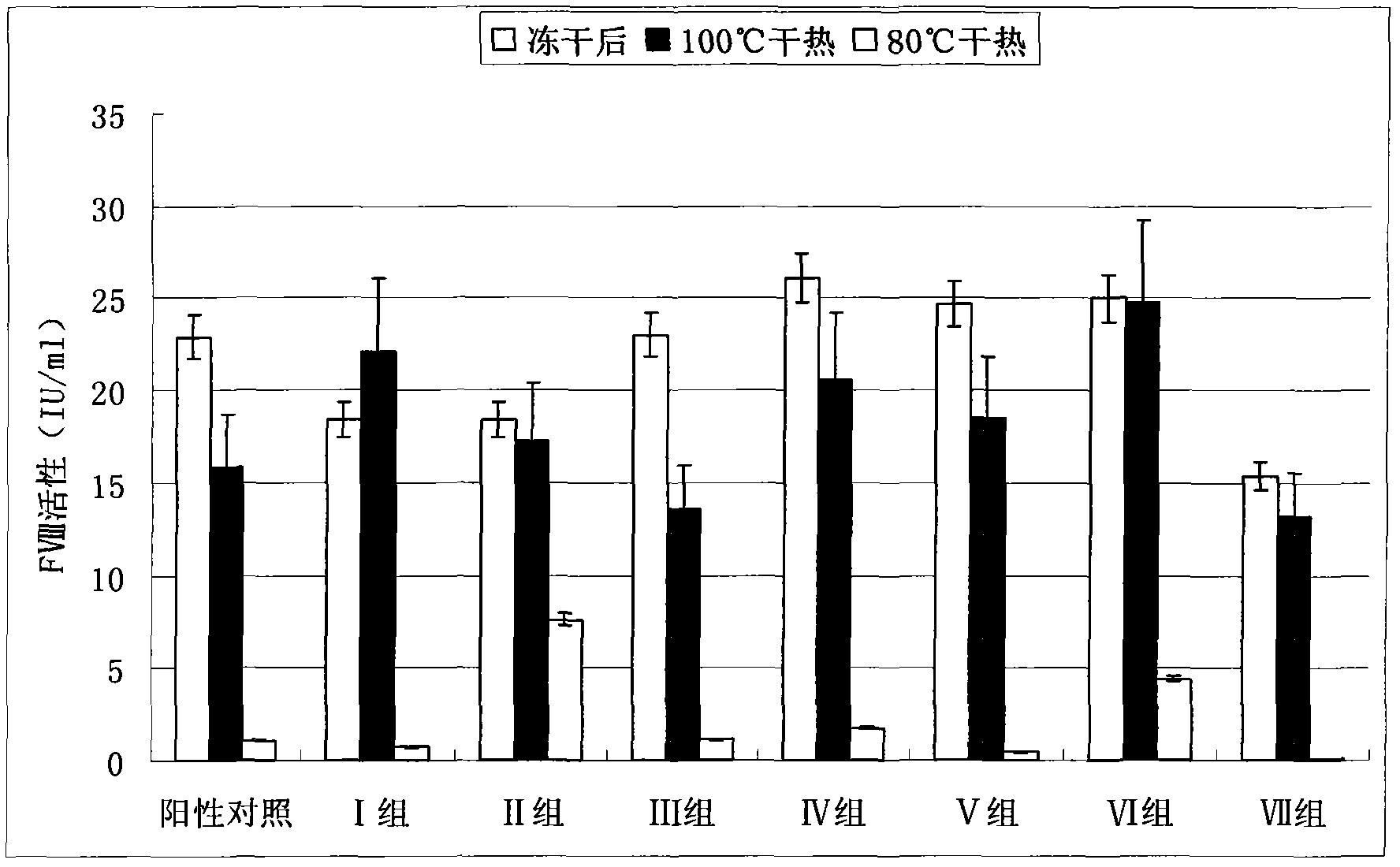
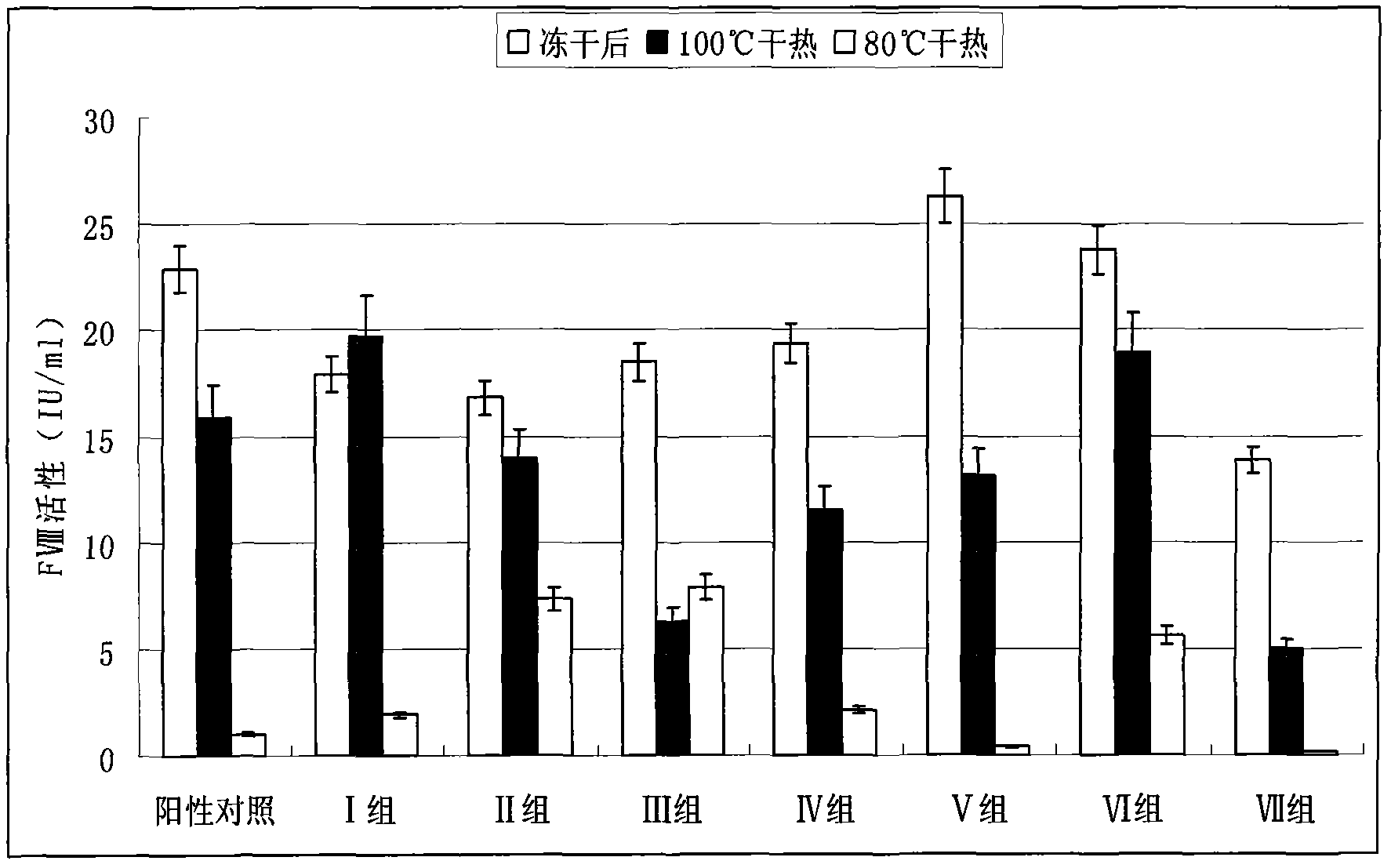
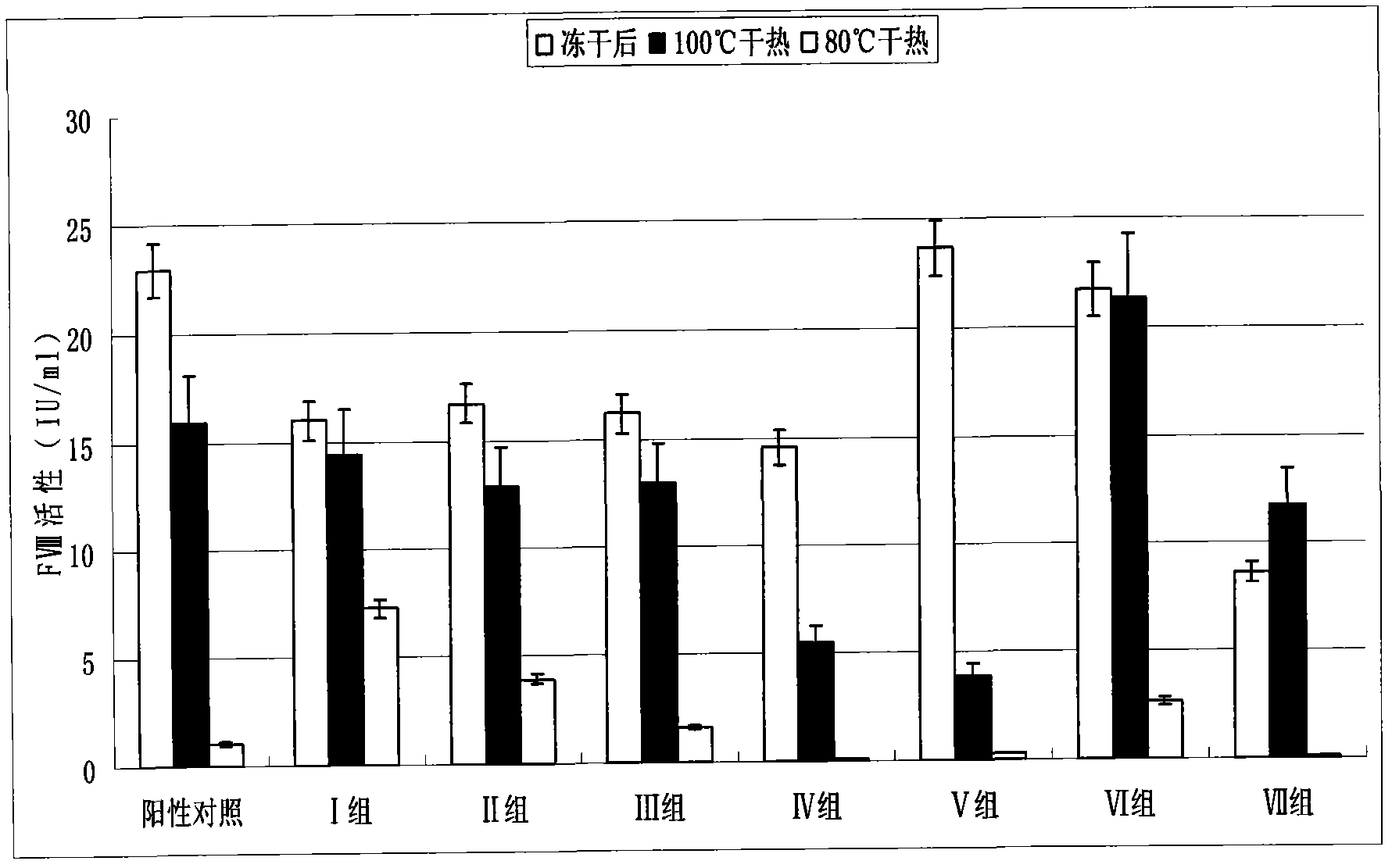
Dry heat treatment stabilizing agent for human coagulation factor VIII and vWF (von willebrand factor) compound or human coagulation factor VIII preparation
ActiveCN102580062ALittle loss of activityIncreased loss of activityPeptide/protein ingredientsPharmaceutical non-active ingredientsSucroseFreeze-drying
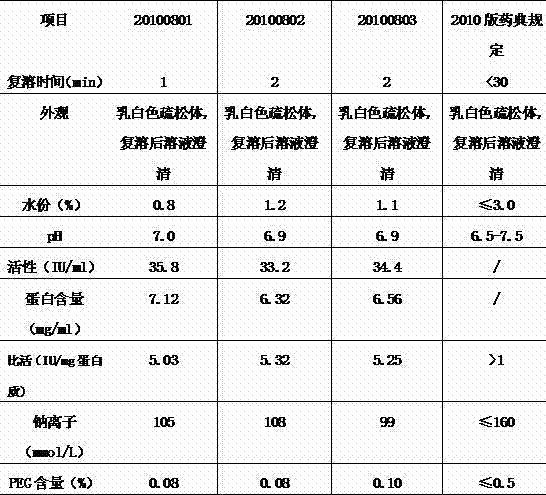
The invention discloses a dry heat treatment stabilizing agent for a human coagulation factor VIII and vWF compound or a human coagulation factor VIII preparation. The stabilizing agent of the invention comprises histidine or its salt, arginine or its salt, lysine or its salt, mannitol, mycose, and sucrose, and also can comprise one or several of common glycine, sucrose, common salt, calcium chloride, sodium citrate, and heparins. Experiments prove that the human coagulation factor VIII and vWF compound or the human coagulation factor VIII preparation contains 0.1-10% of histidine or its salt, 0.1-10% of arginine or its salt, and one or several of 0.1-10% of lysine or its salt, 0.1-10% of glycine, 0.1-10% of mannitol, 0.1-10% of sucrose, and 0.1-10% of mycose, so the human coagulation factor VIII and vWF compound or the human coagulation factor VIII preparation can effectively inactivate viruses under a 80-100DEG C dry heat environment, can effectively protect the activity of the human coagulation factor VIII, and has a qualified freeze-drying appearance and a redissolving appearance. So the stabilizing agent of the invention can be used as the dry heat treatment stabilizing agent for the human coagulation factor VIII and vWF (von willebrand factor) compound or the human coagulation factor VIII preparation.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI
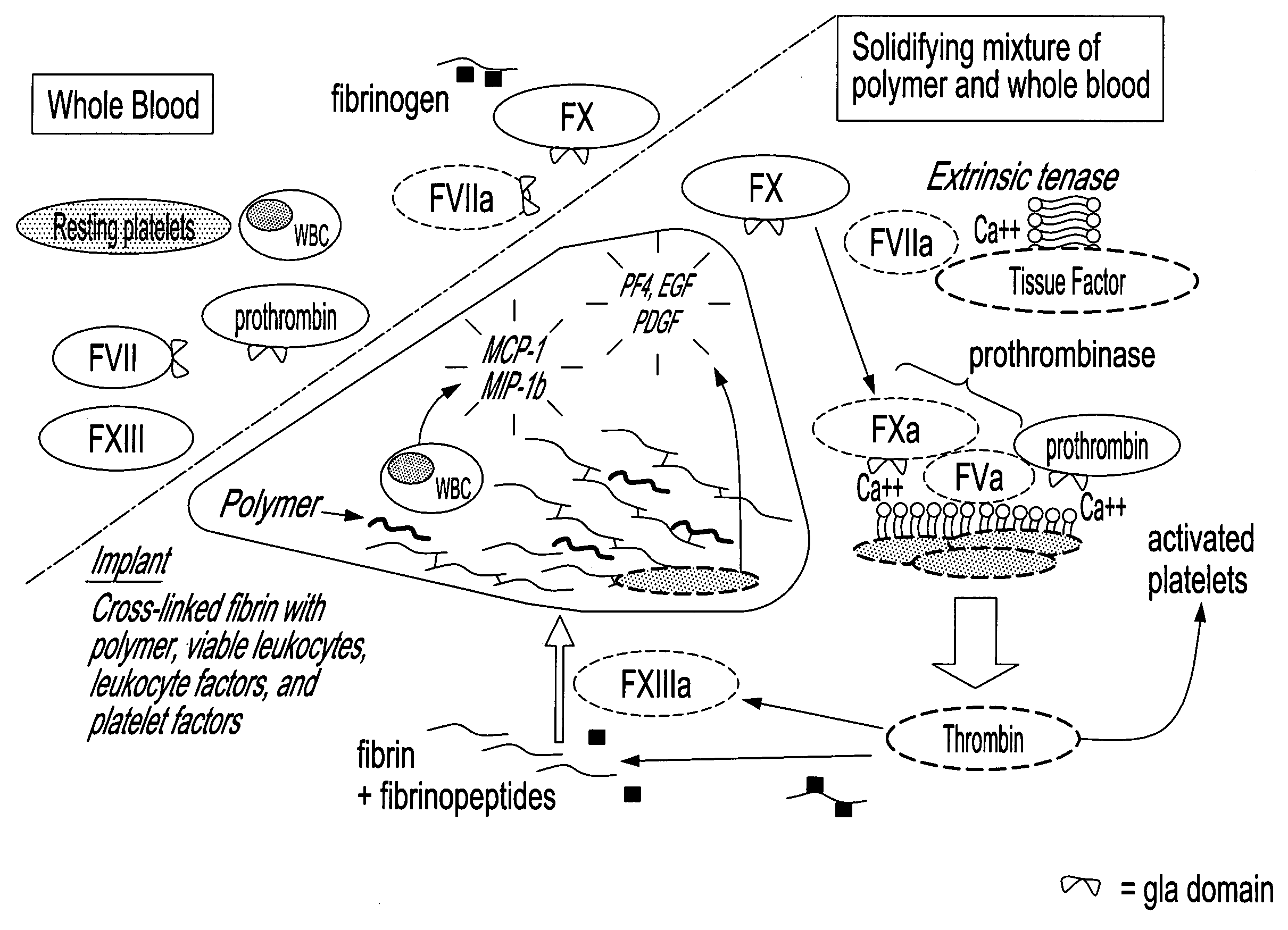
Method for in situ solidification of blood-polymer compositions for regenerative medicine and cartilage repair applications
InactiveUS20100178355A1Improve responsePeptide/protein ingredientsSkeletal disorderFactor iiLigament structure
The present invention relates to a method for repairing or regenerating tissues in a patient such as cartilage, meniscus, ligament, tendon, bone, skin, cornea, periodontal tissues, abscesses, resected tumors, cardiac tissues and ulcers. The method comprises the step of administering simultaneously or sequentially a pro-coagulant factor and an effective amount of a polymer composition comprising a biocompatible polymer and blood or a component thereof. When the polymer composition is in contact with the pro-coagulant factor it is converted into a non-liquid state such that the polymer composition will adhere to the site in need of repair to effect repair of the tissue and / or regeneration thereof.
Owner:PIRAMAL HEALTHCARE CANADA
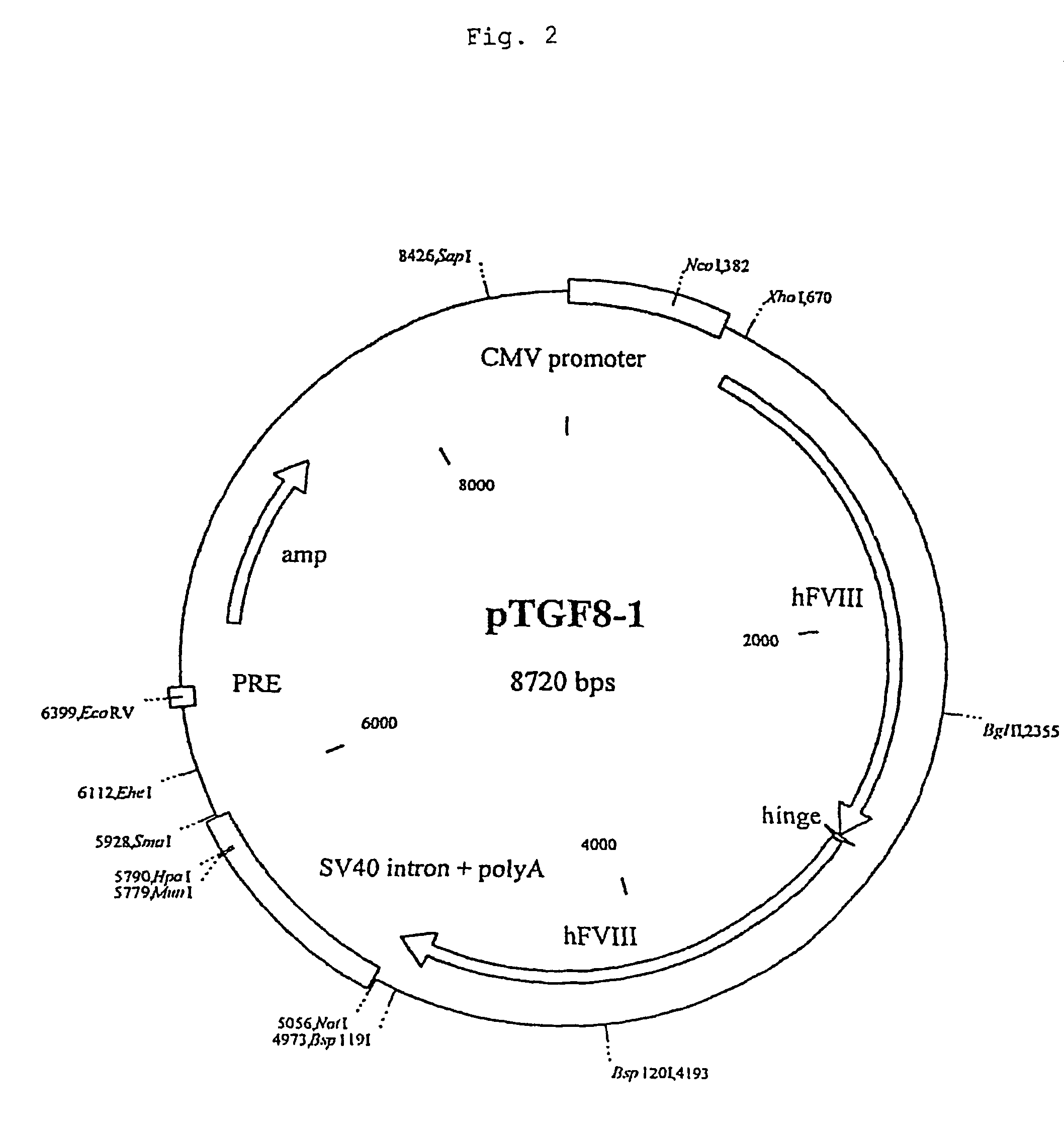
Recombinant expression of factor VIII in human cells
InactiveUS20060099685A1SsRNA viruses negative-senseMicrobiological testing/measurementBlood coagulation factor VIIIHeterologous
The invention discloses a process for recombinant production of blood coagulation Factor VIII in an immortalized human embryonic retina cell, said cell expressing at least an adenoviral E1A protein and comprising a nucleic acid sequence encoding said Factor VIII, said nucleic acid sequence being under control of a heterologous promoter, said process comprising culturing said cell and expressing the Factor VIII in said cell, and harvesting the expressed Factor VIII. Cells that can be used in the process of the invention are also provided.
Owner:JANSSEN VACCINES & PREVENTION BV
Method for preparing freeze-dried human blood coagulation factor VIII
ActiveCN102228683AEasy to cleanGuaranteed stabilityPowder deliveryPeptide/protein ingredientsUltrafiltrationFreeze-drying
The invention discloses a method for preparing a freeze-dried human blood coagulation factor VIII. The method comprises the following process of: dissolving by taking water for injection, comprising 3-10 IU (International Unit) / ml of heparin, as a heparin sodium solution and cryoprecipitating; performing PEG (Polyethylene Glycol) precipitation and taking supernatant; performing centrifugal filtration; performing S / D (Solvent / Detergent) inactivation at the temperature of 24-26 DEG C; performing DEAE (Diethylaminoethyl) Sepharose Fast Flow chromatographic column balance, adsorption, washing andelution; performing molecular membrane ultrafiltration; preparing, removing bacteria, sub-packaging, freeze-drying and capping; and dry-heating at the temperature of 99.5-100.5 DEG C and inactivating. In the invention, the process method of combining the PEG precipitation and an ion exchange chromatography technology is adopted; the method is easy and convenient to operate; the F VIII active recovery rate is increased; miscellaneous proteins can be removed on a large scale; the product yield reaches over 60 percent; and the specific activity of the product reaches 5 IU / mg and is obviously greater than a value which is not less than 1 IU / mg stipulated in the pharmacopeia. Meanwhile, the PEG residue is 0.08g / L which is obviously less than the value which is less than or equal to 0.5 IU / mg stipulated in the pharmacopeia, so that Al<3+> residues in the final preparation of an Al(OH)3 gel method are avoided; the product has high purity and high safety; and the quality of the final product is obviously improved.
Owner:NANYUE BIOPHARMING
Method for adsorbing human prothrombin complex from plasma
ActiveCN104109202AHigh yieldHigh activityPeptide preparation methodsPeptidasesProthrombin complex concentrateCellulose
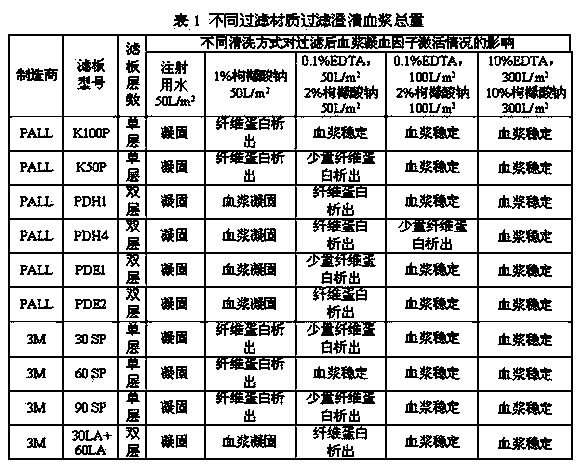
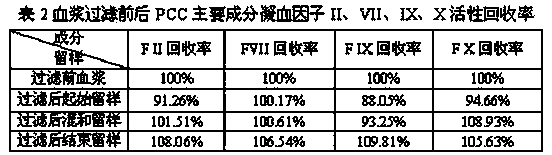
The invention relates to a production method for adsorbing a human complex from plasma by a fixed bed column chromatography technique, which comprises the following steps: (1) cryoprecipitation plasma removal: filtering by using a cellulose deep filter plate which is cleaned by an EDTA (ethylene diamine tetraacetic acid) solution and a sodium citrate solution; (2) filtering the plasma subjected to deep filtration through a 0.2 mu m filter element membrane while fixed bed loading; (3) balancing 2-5 column volumes in a fixed bed chromatographic column filled with anion exchange gel Capto DEAE by using a buffer solution A at the plasma loading flow rate of 60-120 cm / hour, washing the chromatographic column with a buffer solution B, and eluting the chromatographic column with a buffer solution C to obtain a PCC (prothrombin complex concentrate) product. When the calculation is based on coagulation factor IX, the yield of the PCC can reach 75-90%, and the specific activity can reach 5.5 IU / mg above.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
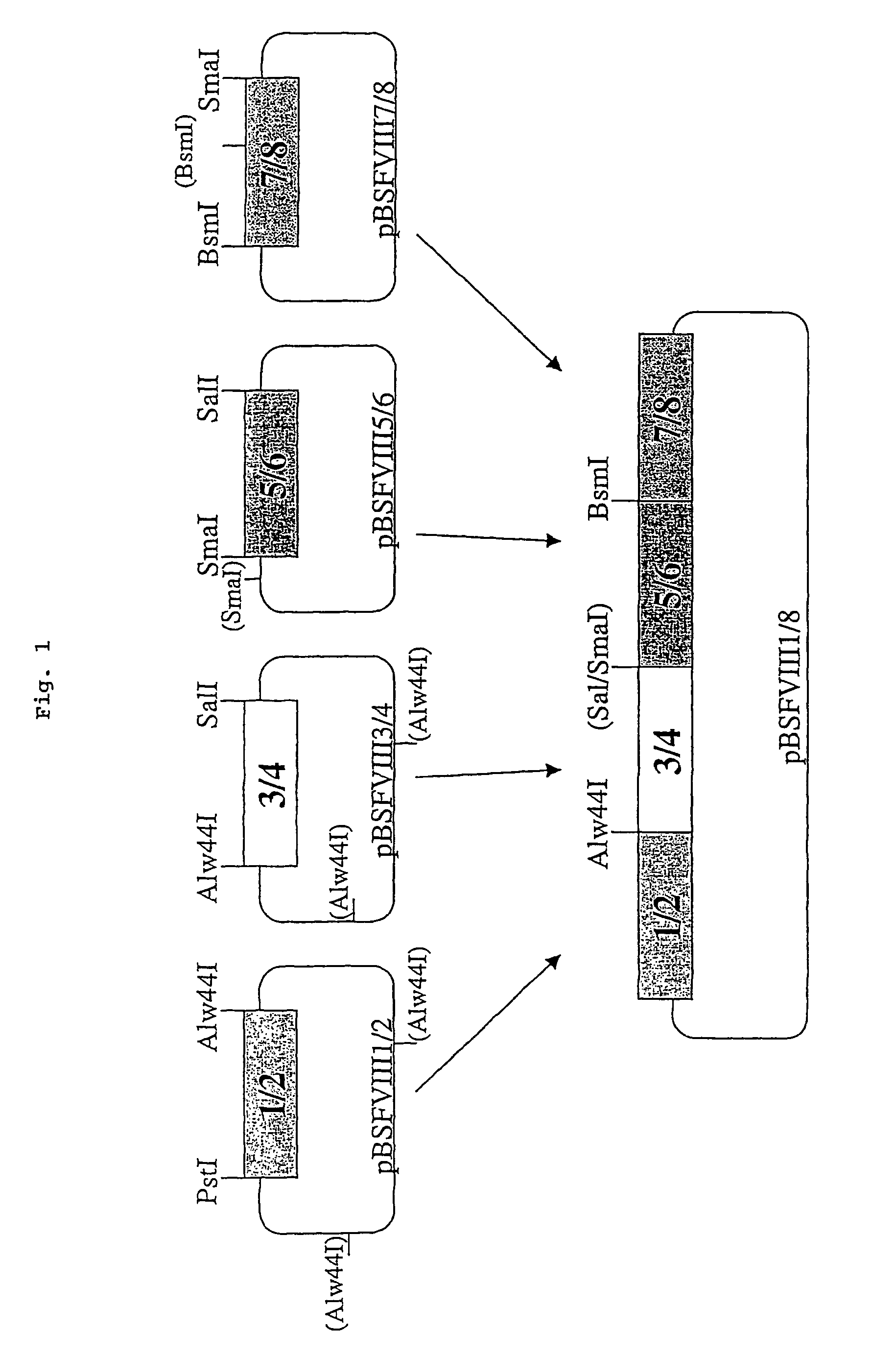
Nucleic acid molecules encoding modified factor VIII proteins, expression products, and methods of making the same
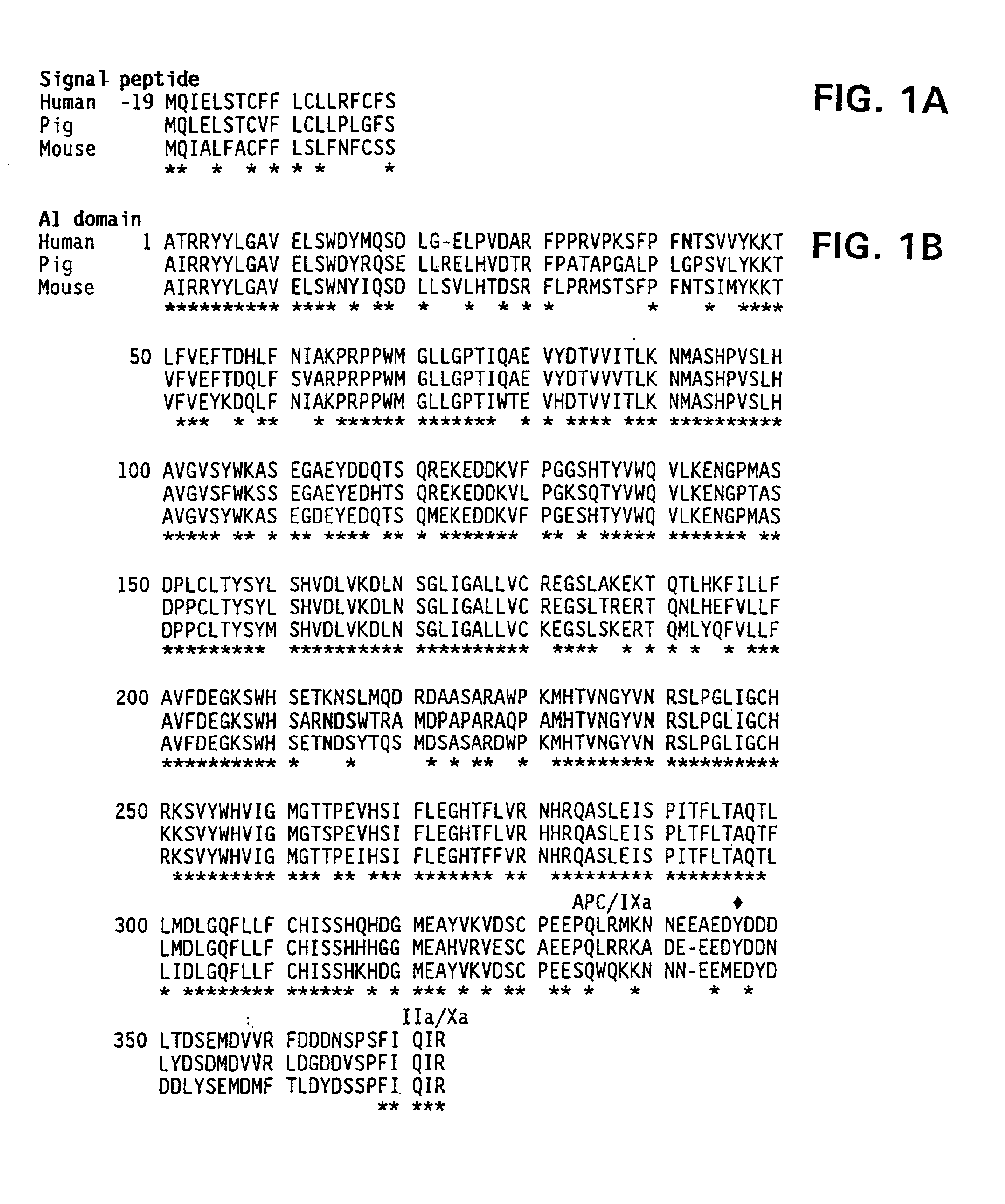
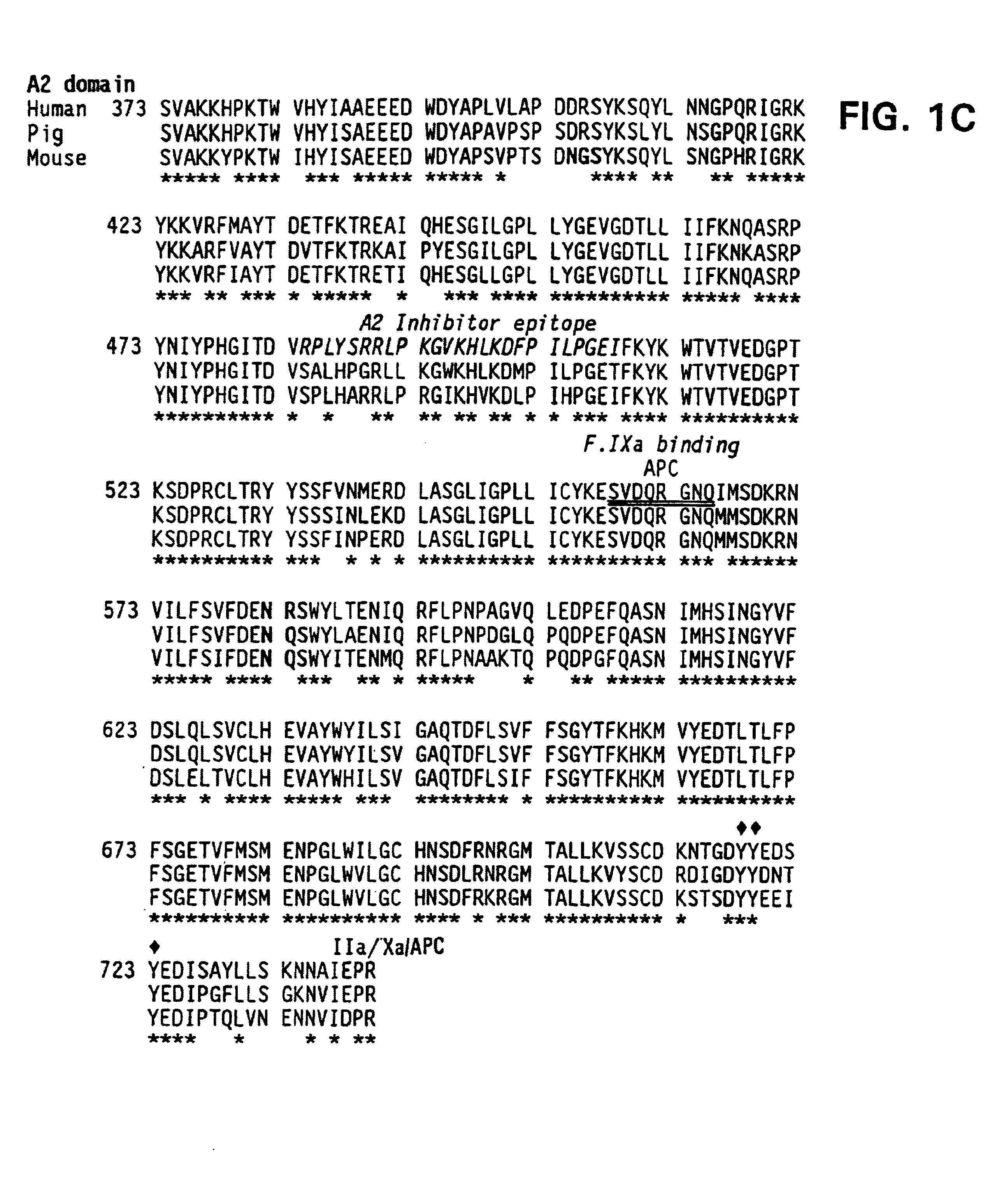
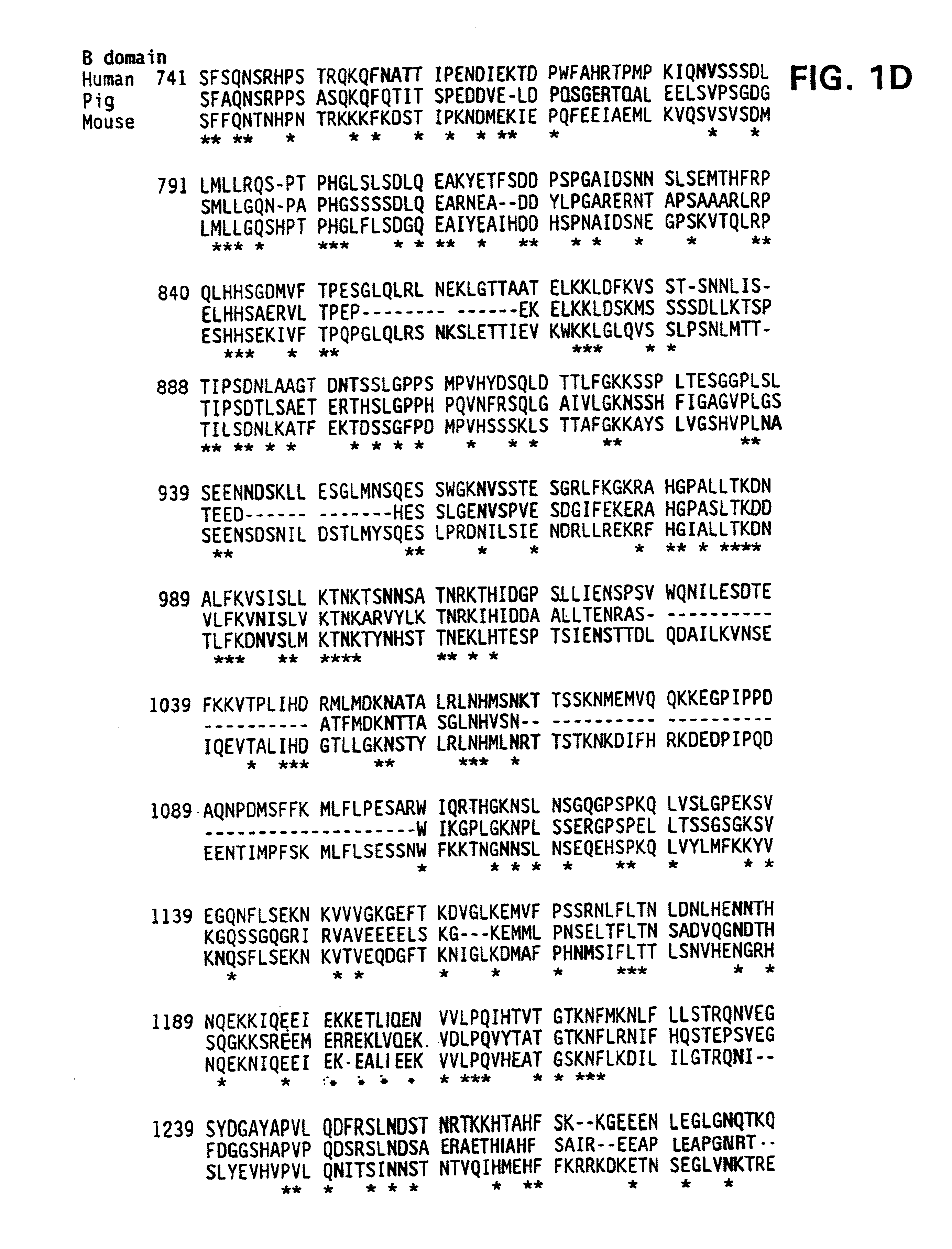
Specific amino acid loci of human factor VIII interact with inhibitory antibodies of hemophilia patients who have developed such antibodies after being treated with factor VIII. Modified factor VIII is disclosed in which the amino acid sequence is changed by a substitution at one or more amino acids of positions 484-508 of the A2 domain. The modified factor VIII is useful as a clotting factor supplement for hemophiliacs.
Owner:GENERAL ELECTRIC CO +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com