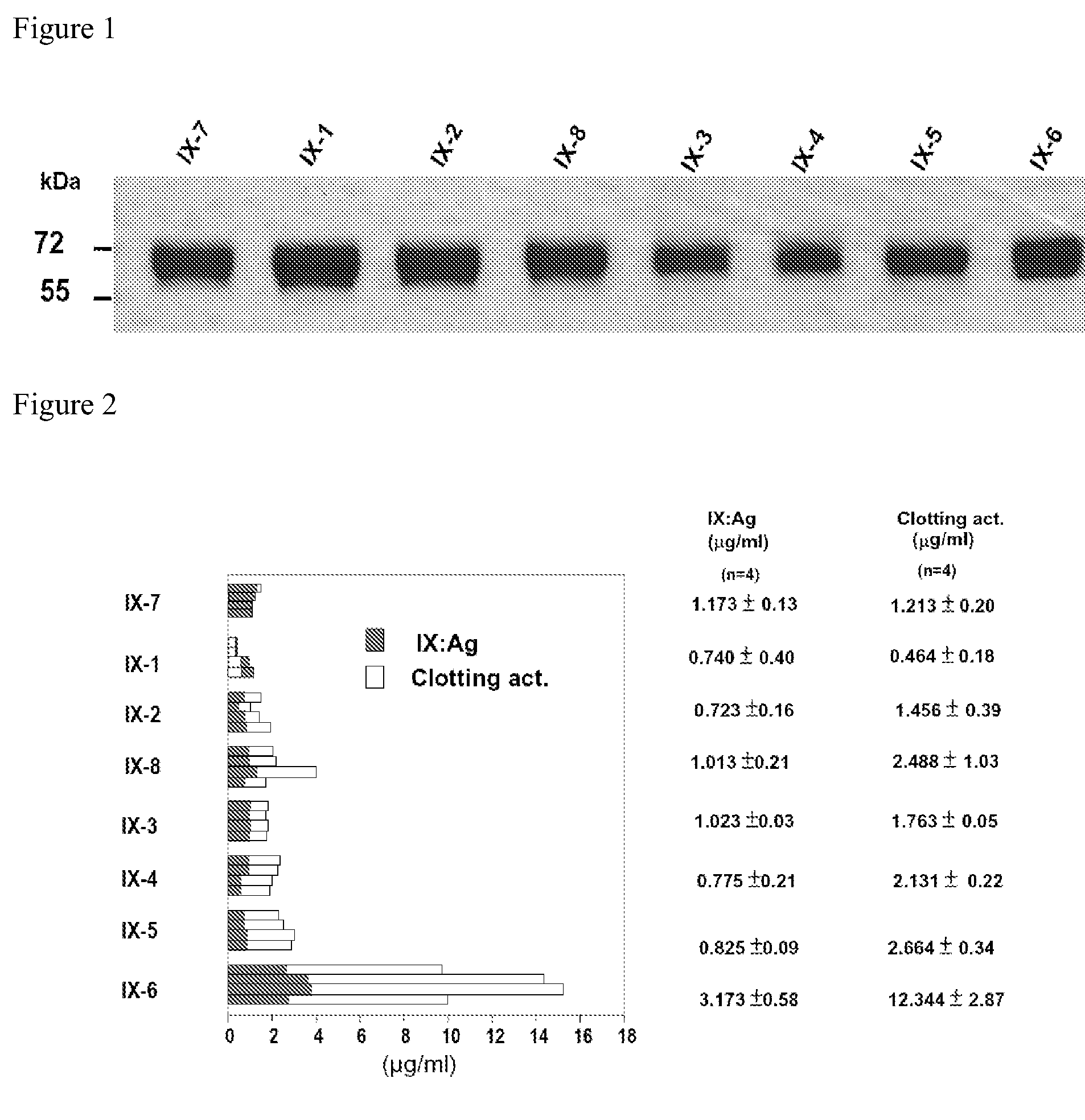
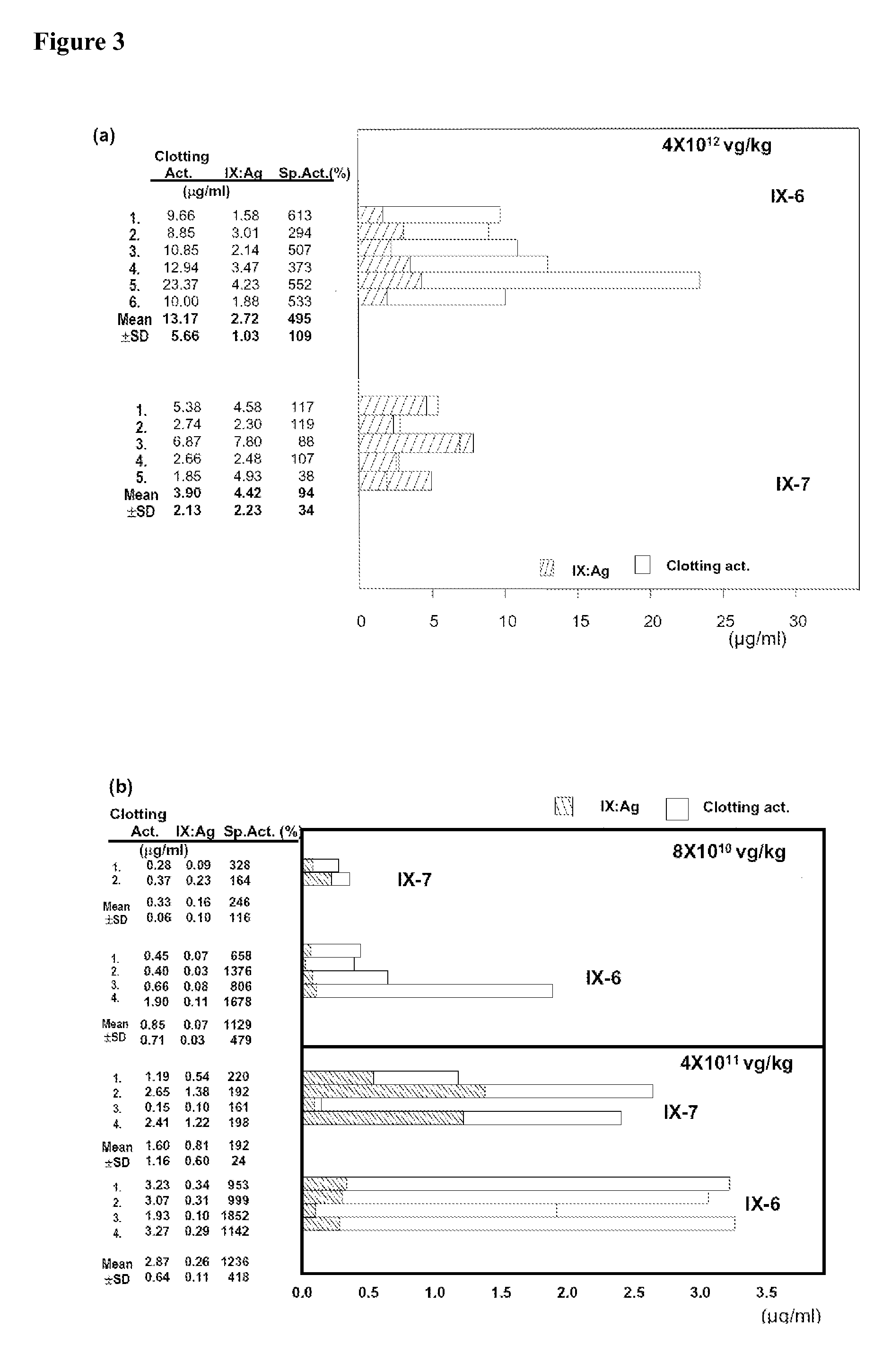
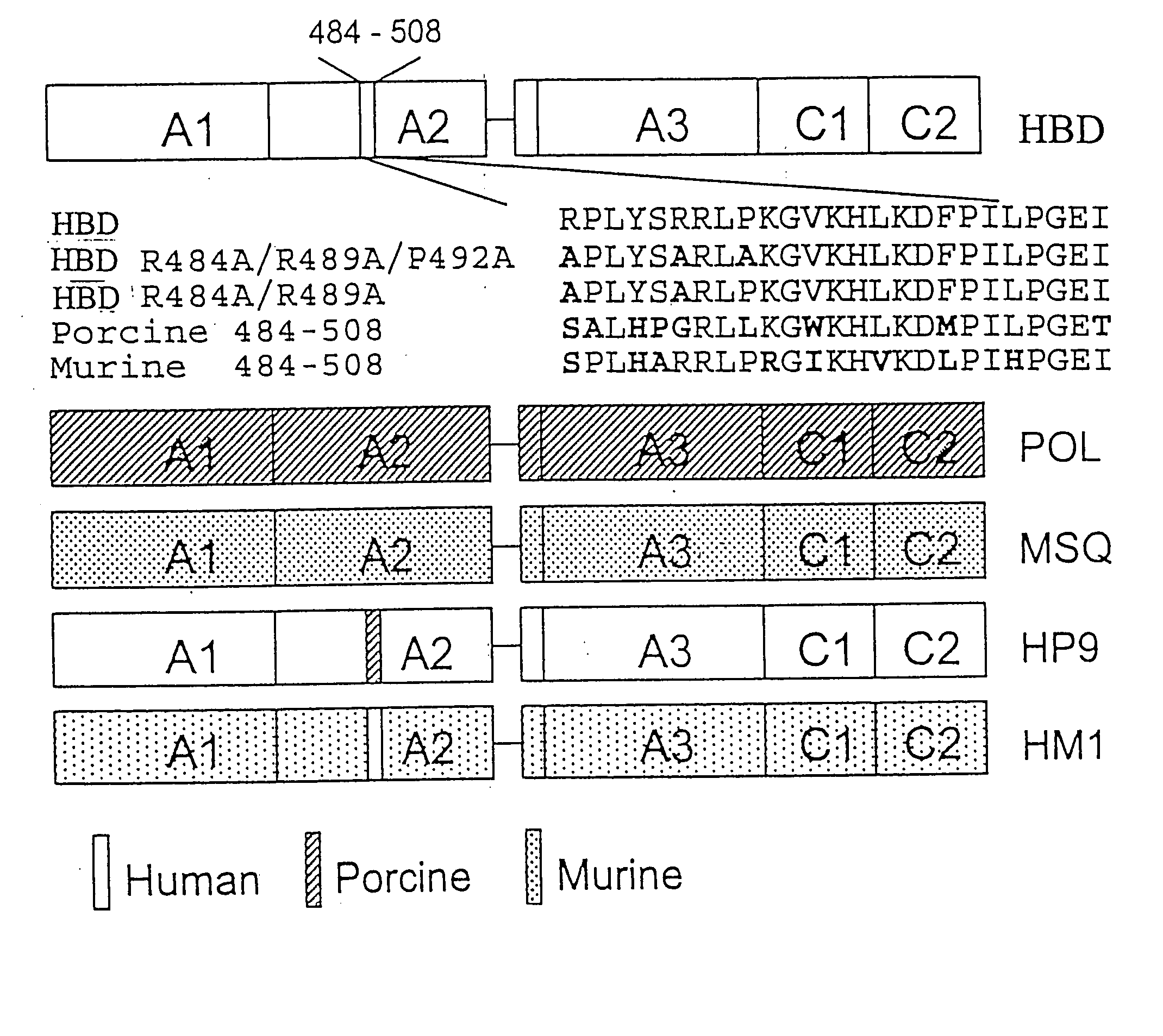
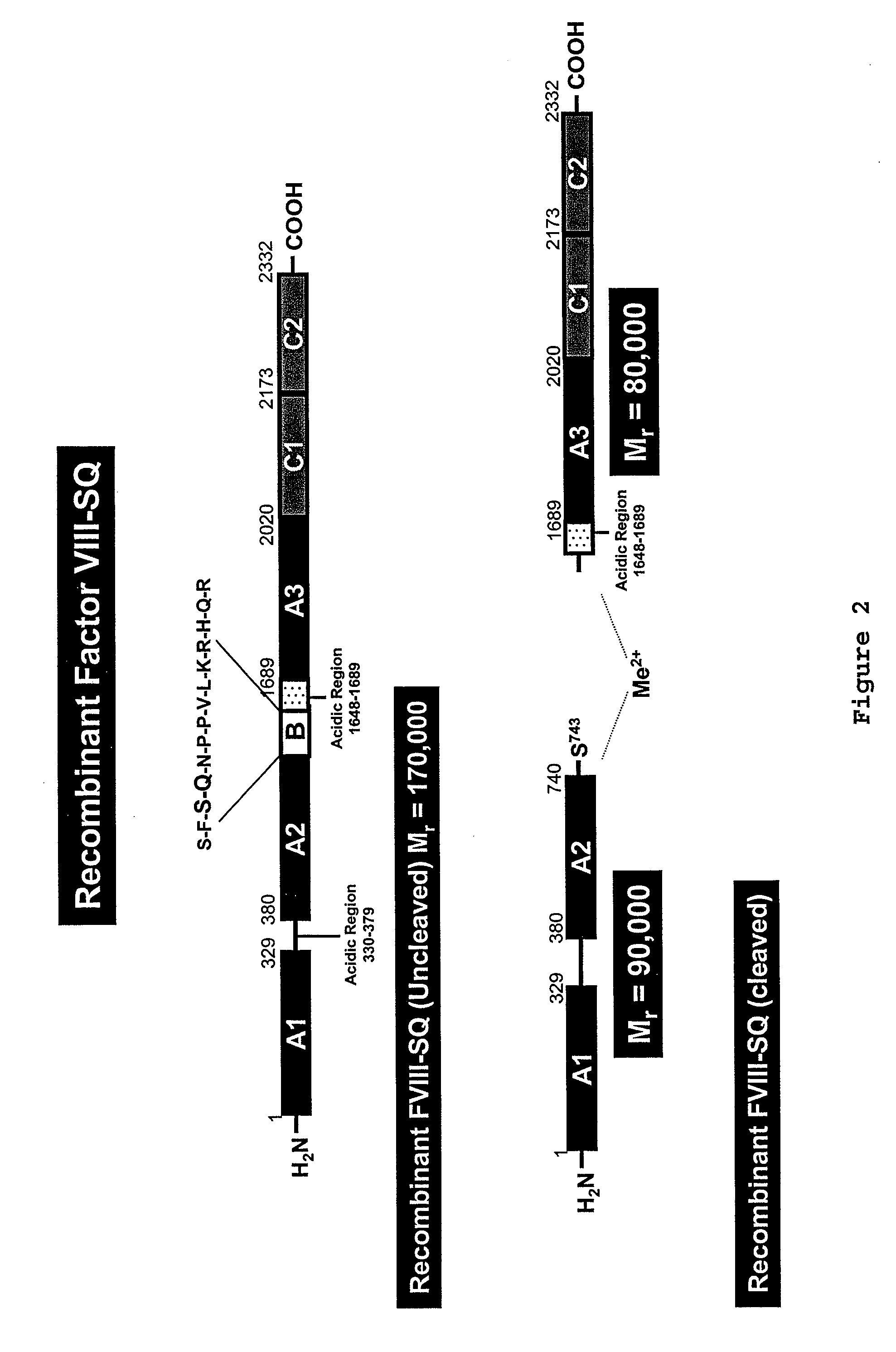
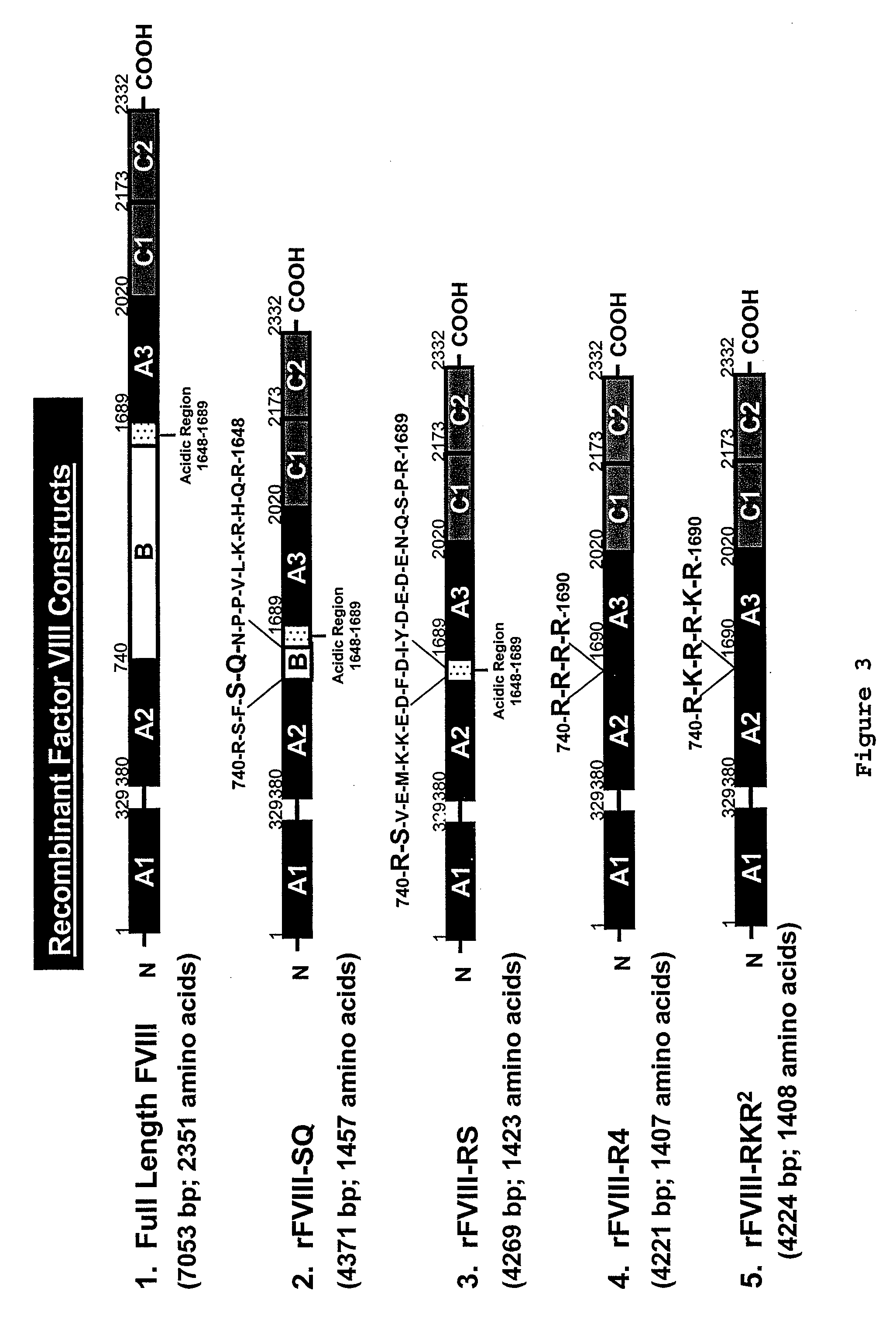
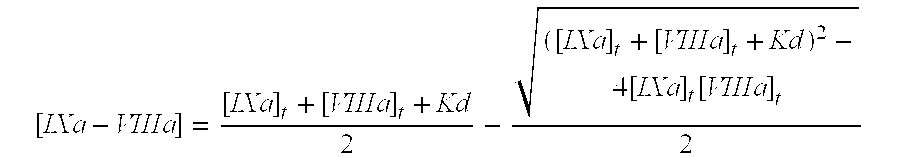Patents
Literature
209 results about "Hemophilias" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A rare, hereditary blood disorder marked by a tendency toward excessive bleeding
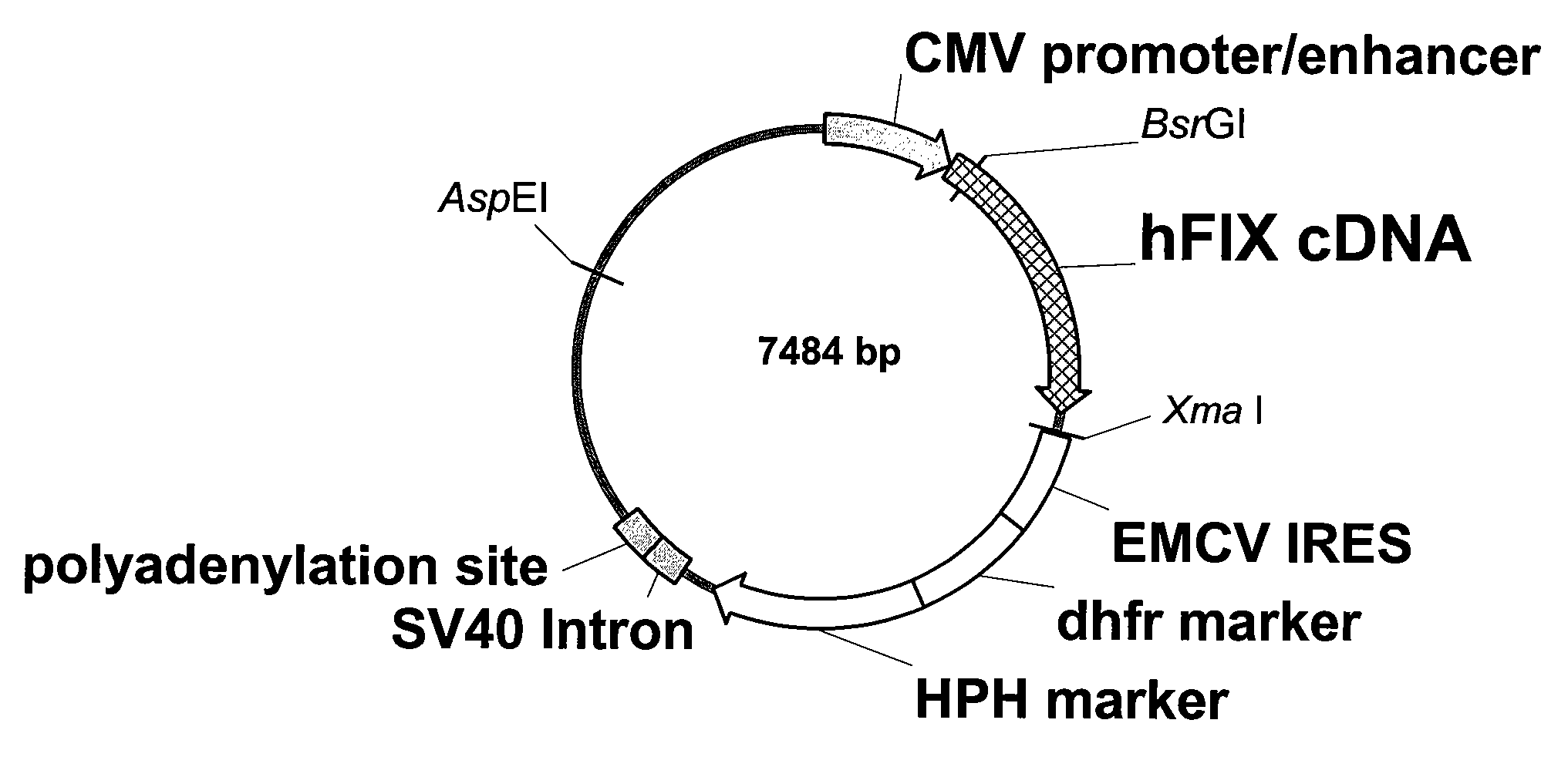
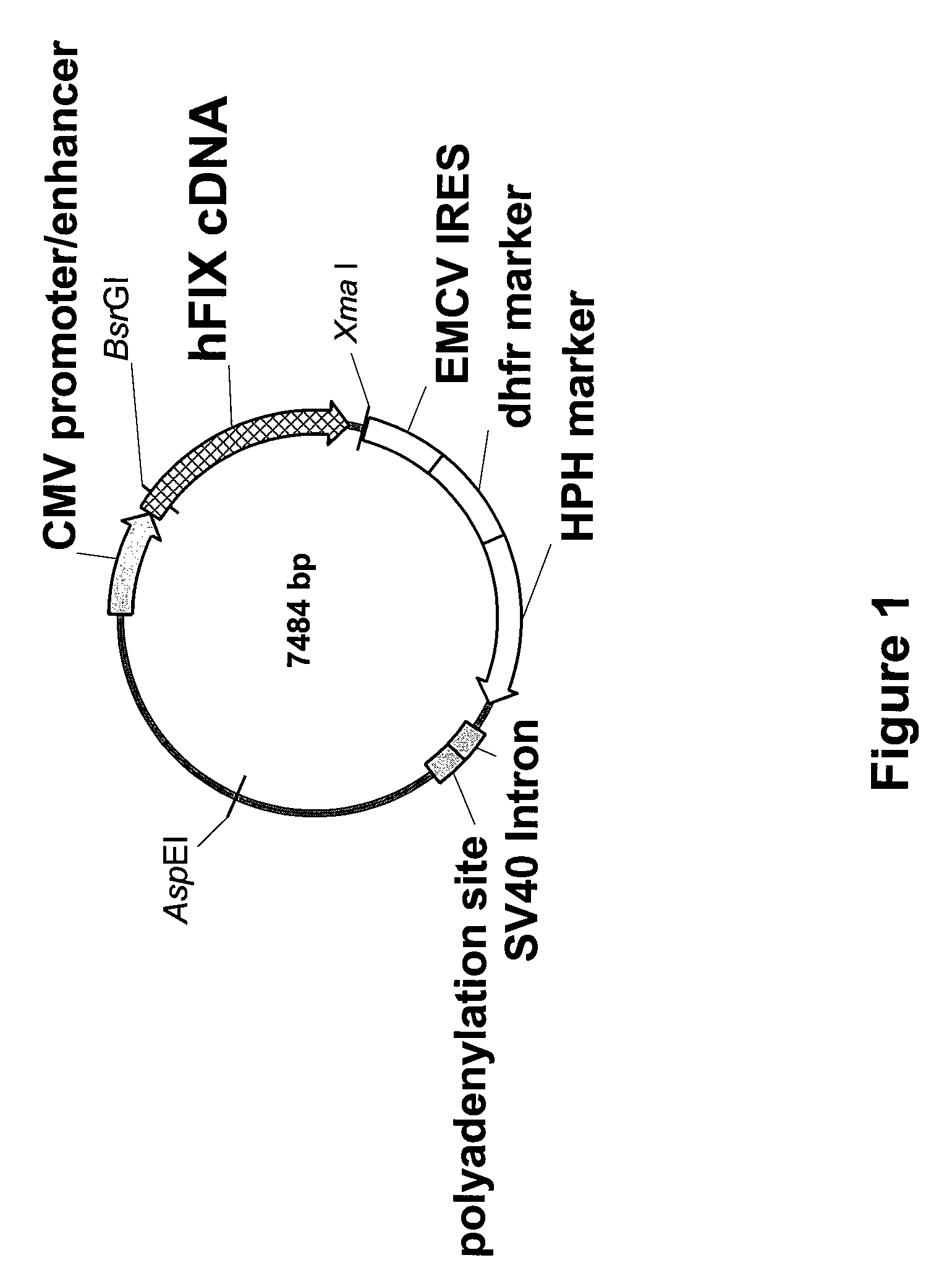
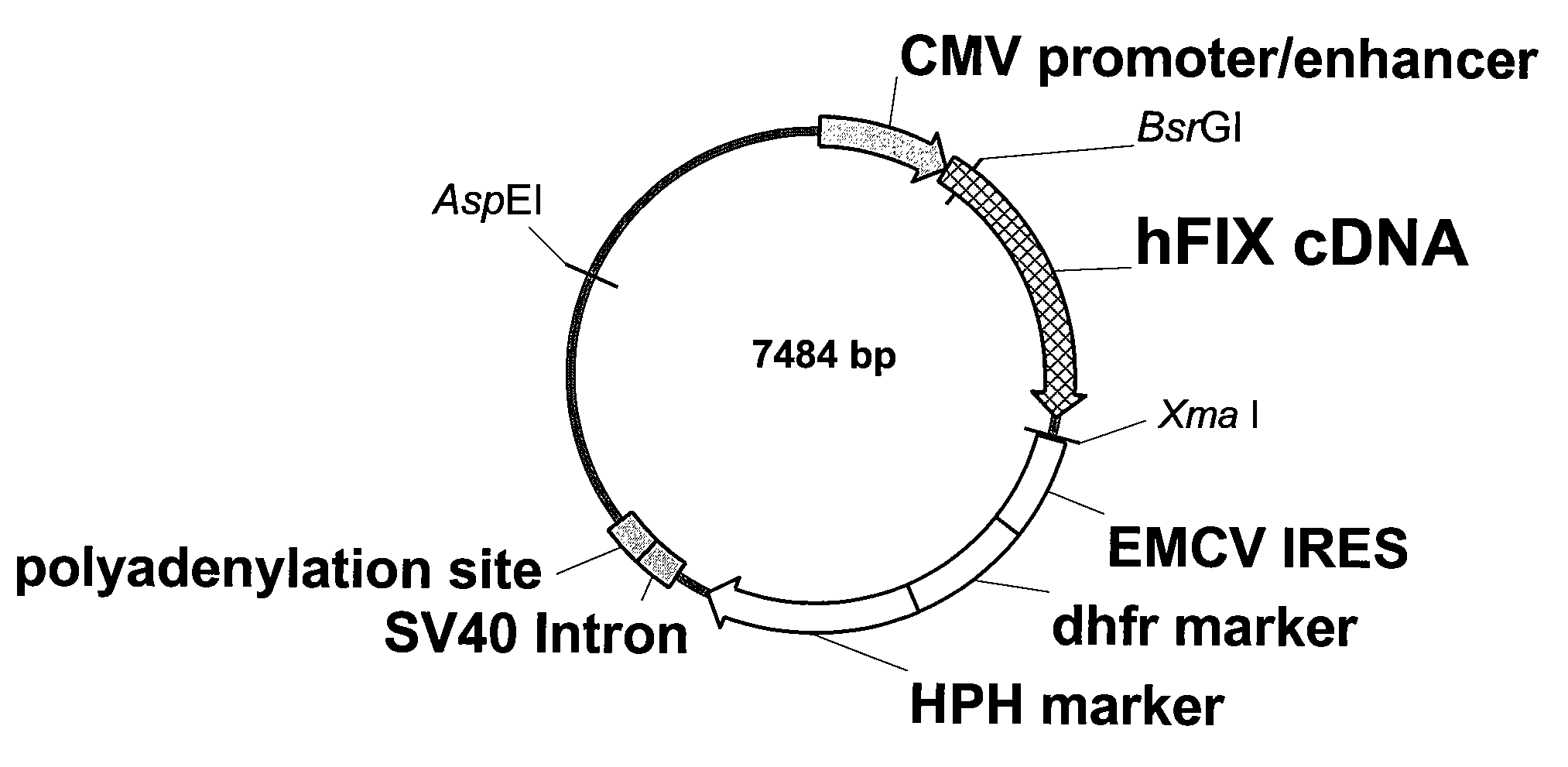
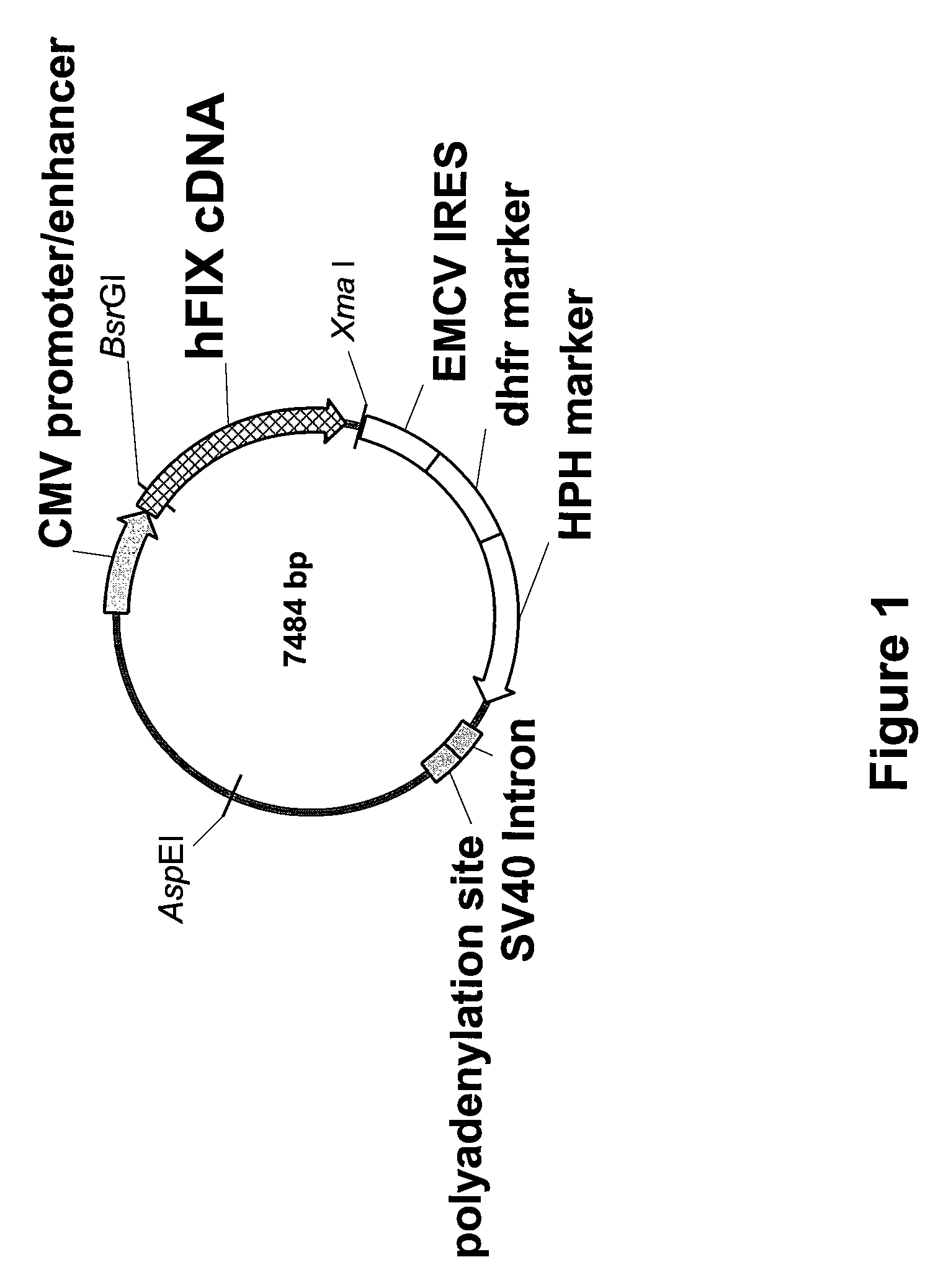
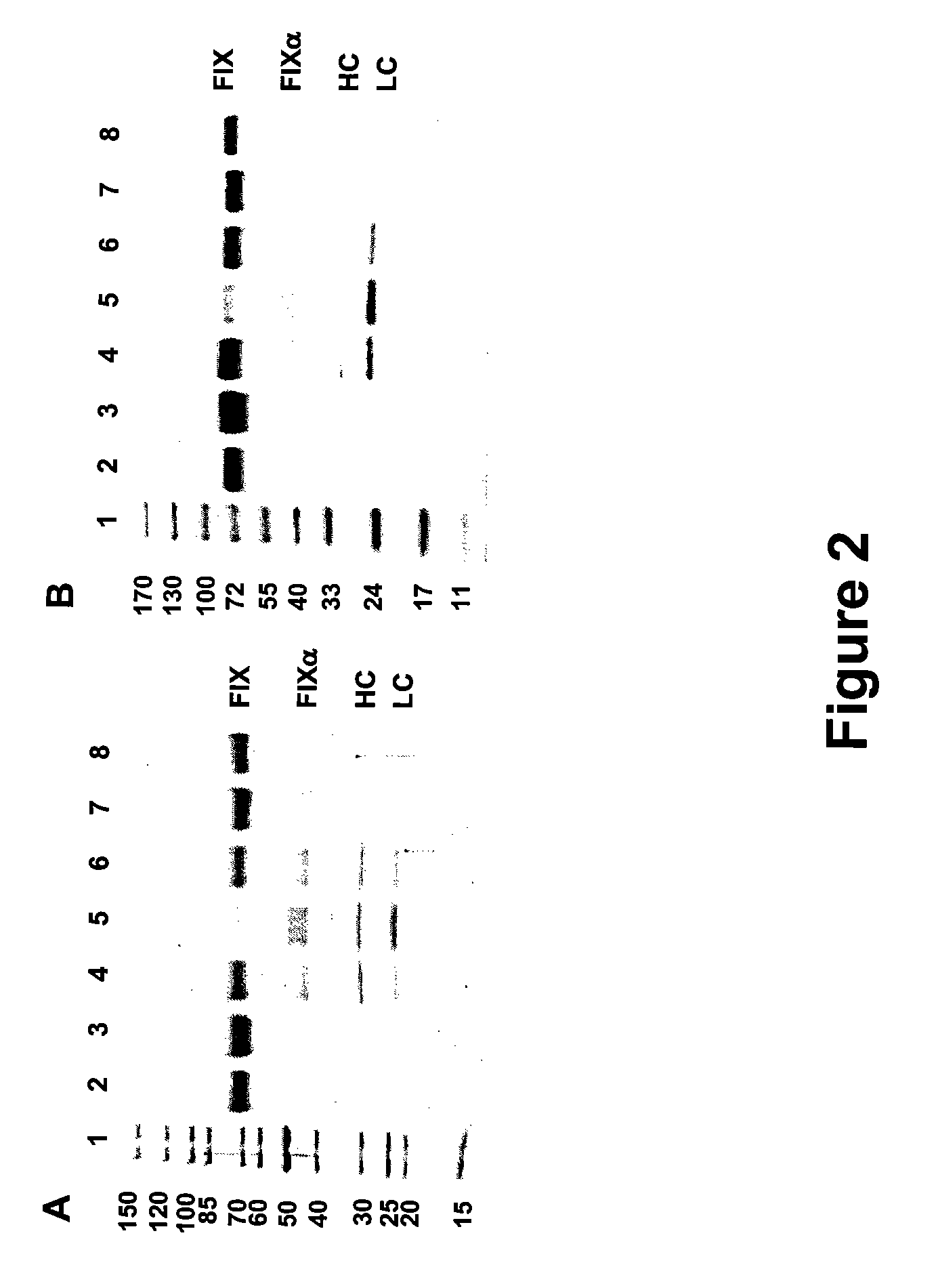
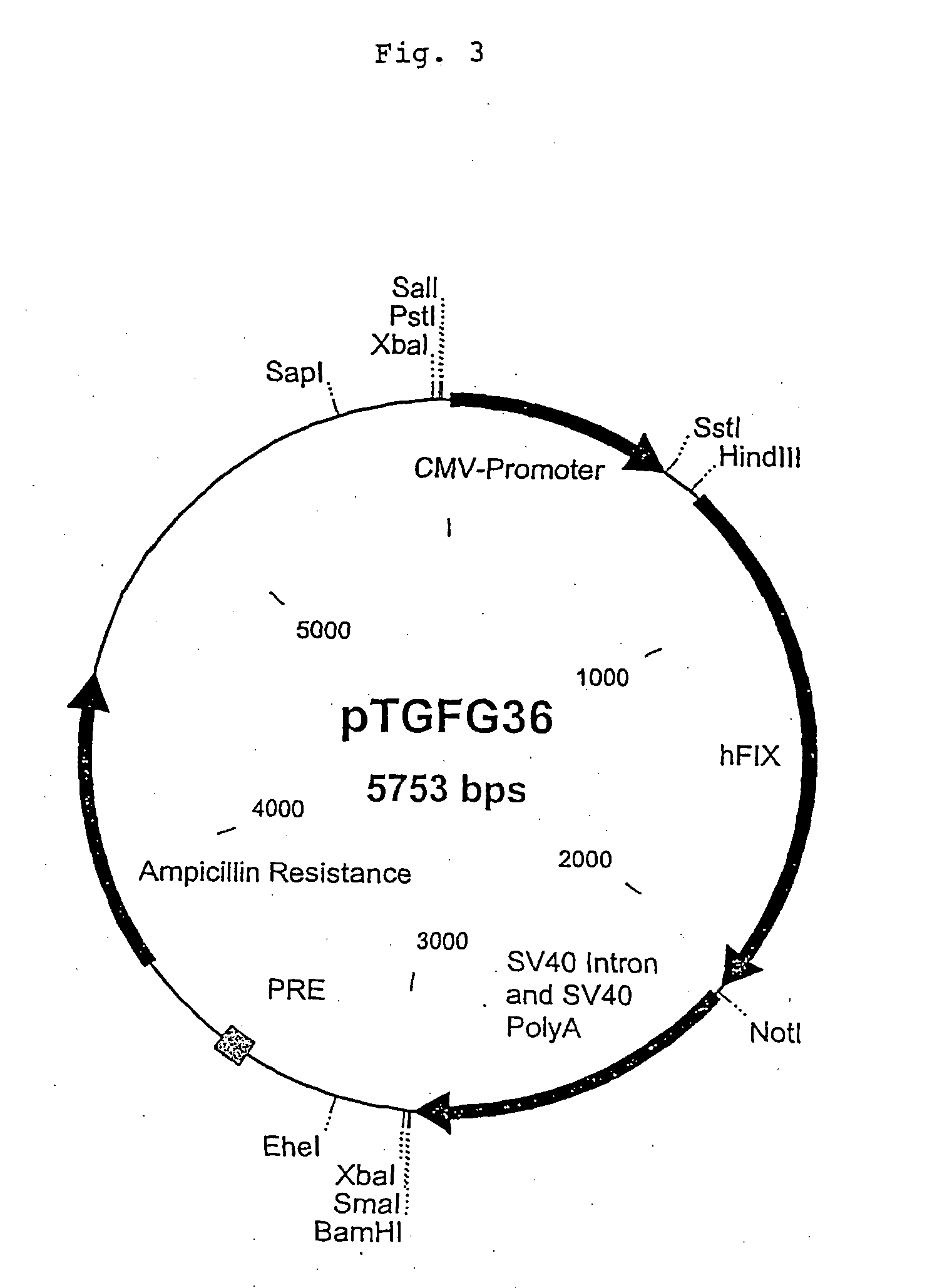
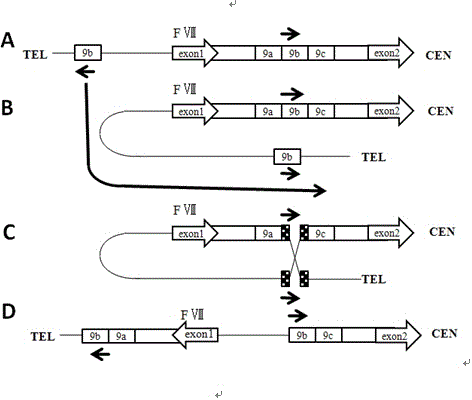
Recombinant human factor ix and use thereof
ActiveUS20080167219A1Easy to adaptPeptide/protein ingredientsGenetic material ingredientsWild typeFactor ii
The present invention aims at converting factor IX into a molecule with enhanced activity which provides an alternative for replacement therapy and gene therapy for hemophilia B. Using recombinant techniques, factor IX with replacement at positions 86, 277, and 338 exhibits better clotting activity than recombinant wild type factor IX.
Owner:LIN SHU WHA
Modified fVIII having reduced immunogenicity through mutagenesis of A2 and C2 epitopes
InactiveUS20050123997A1Low immunogenicityImmunoglobulins against blood coagulation factorsFactor VIIEpitopeVaccine Immunogenicity
Specific amino acid loci of human fVIII interact with inhibitory antibodies of hemophilia patients after being treated with fVIII. Modified fVIII is disclosed in which the amino acid sequence is changed by multiple substitutions in human fVIII A2 and C2 domains. The modified fVIII is useful for hemophiliacs, either to avoid or prevent the action of inhibitory antibodies.
Owner:EMORY UNIVERSITY
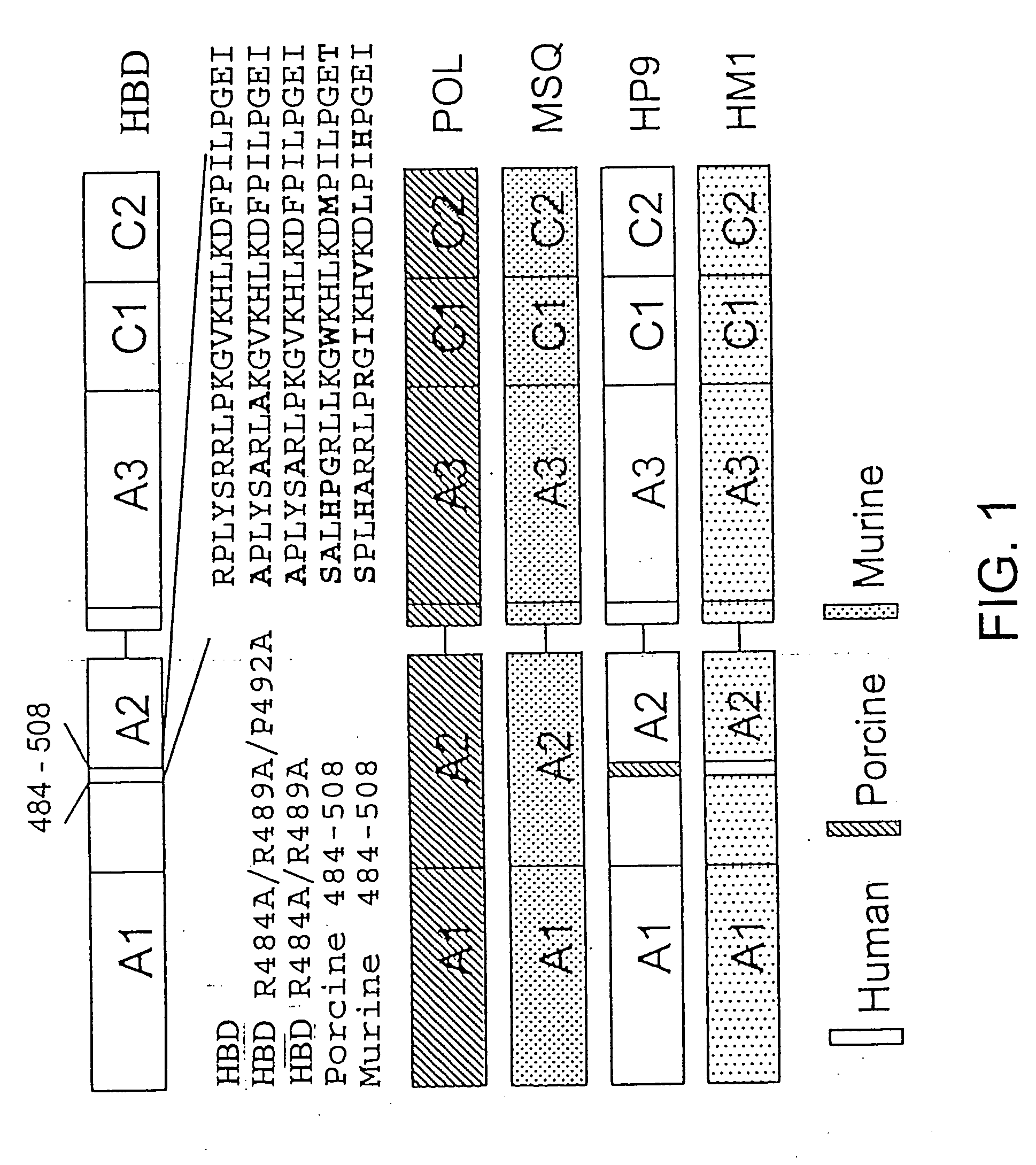
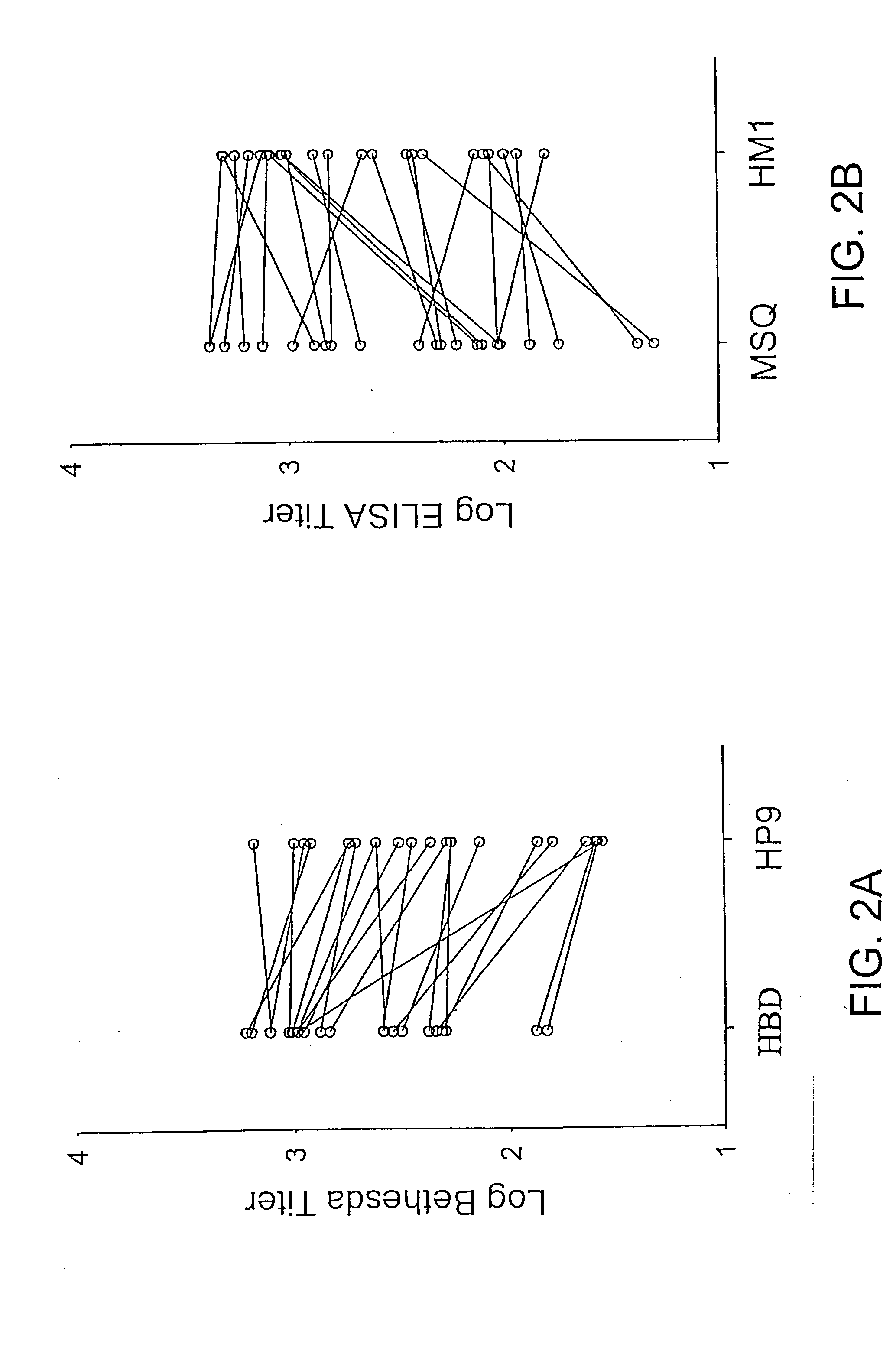
Mutant human factor IX with an increased resistance to inhibition by heparin
InactiveUS7125841B2Improve the immunityShorten clotting timePeptide/protein ingredientsMammal material medical ingredientsFactor iiBiology
The present invention is related to a novel composition of matter and methods of using the same. More particularly, the invention describes mutant human factor IX which has an increased resistance to inhibition by heparin. Methods of making and using this composition for the therapeutic intervention of hemophilia are disclosed.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS20120190051A1Eliminate needPeptide/protein ingredientsTransferrinsErythropoietin receptorFactor VIII vWF
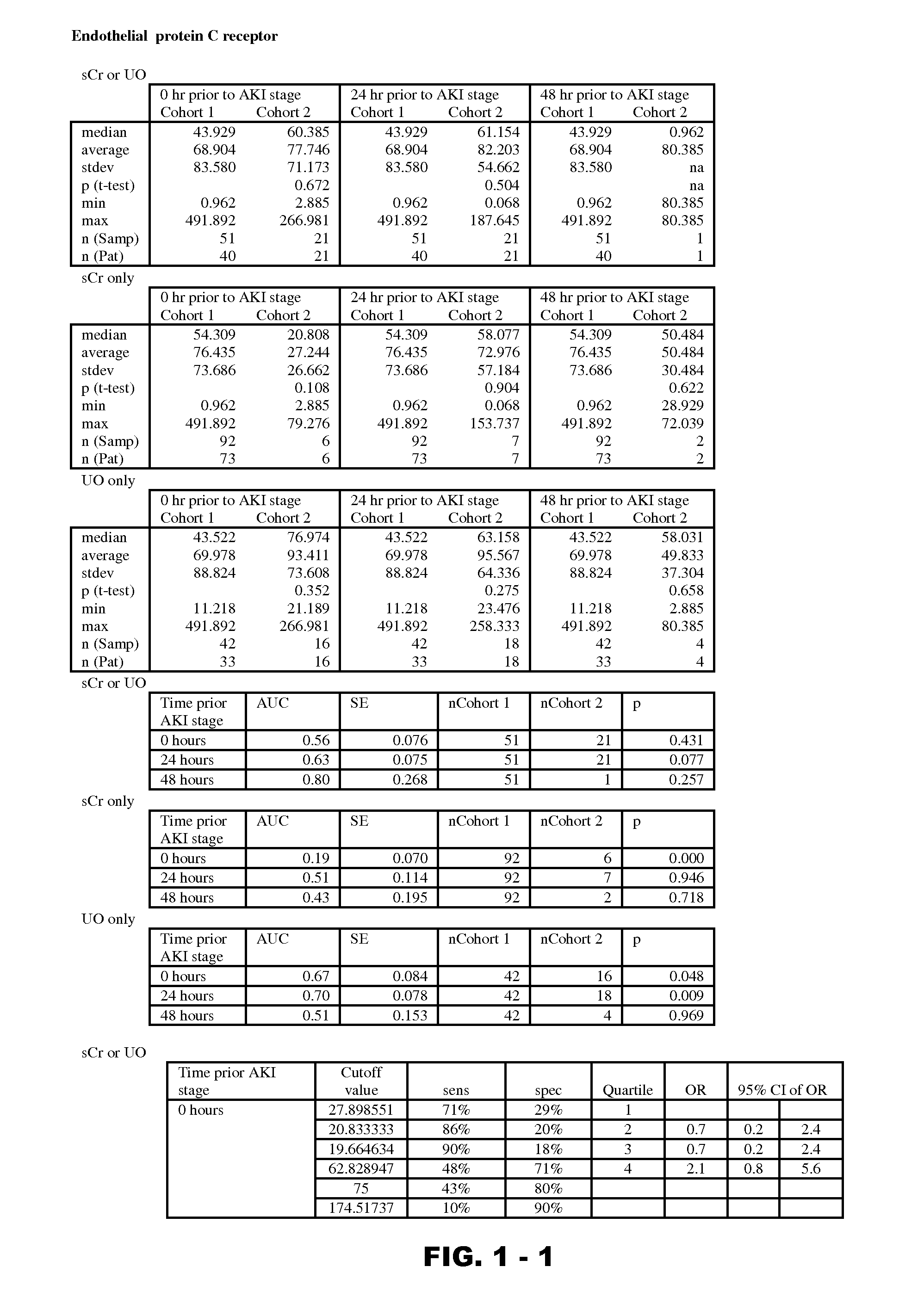
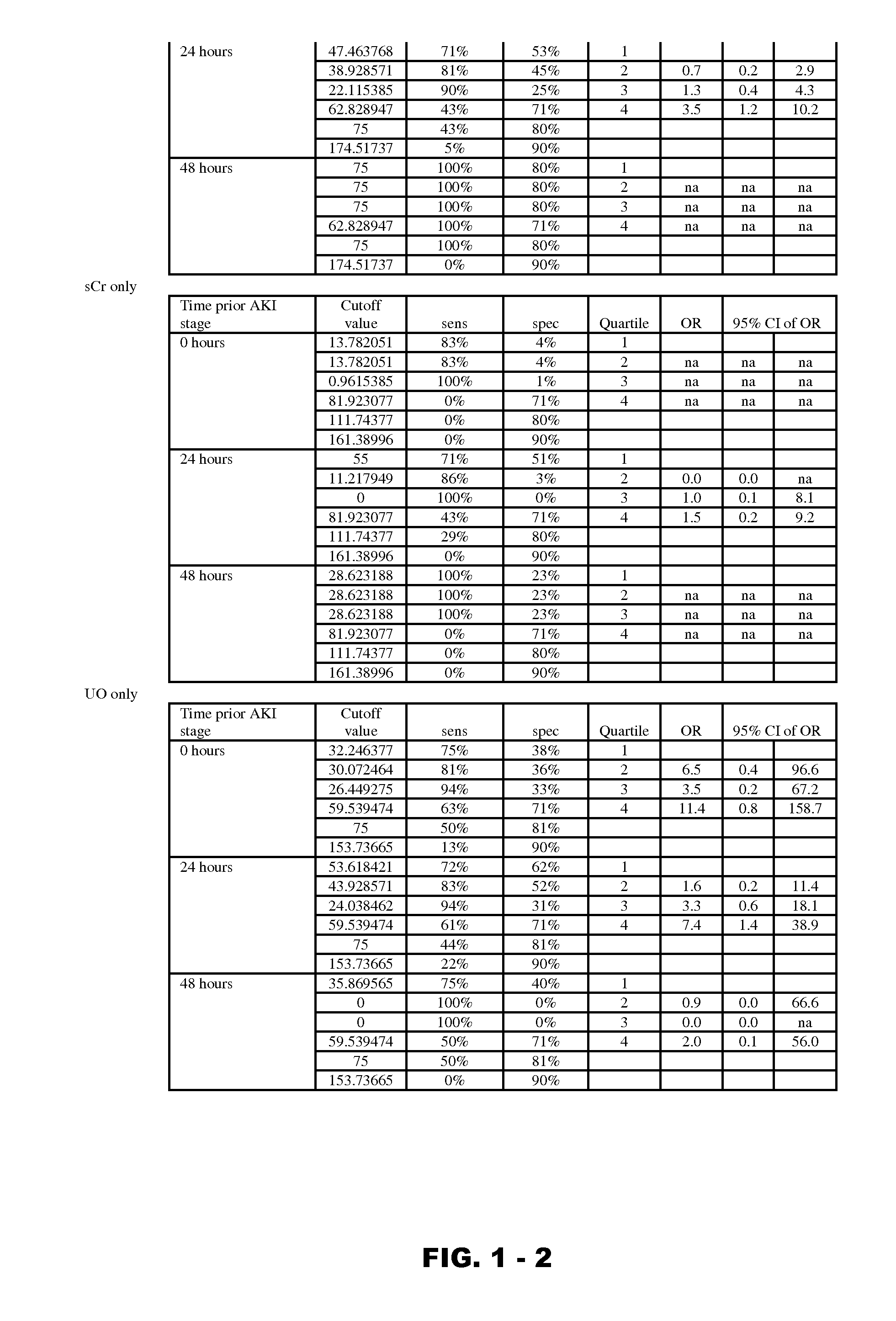
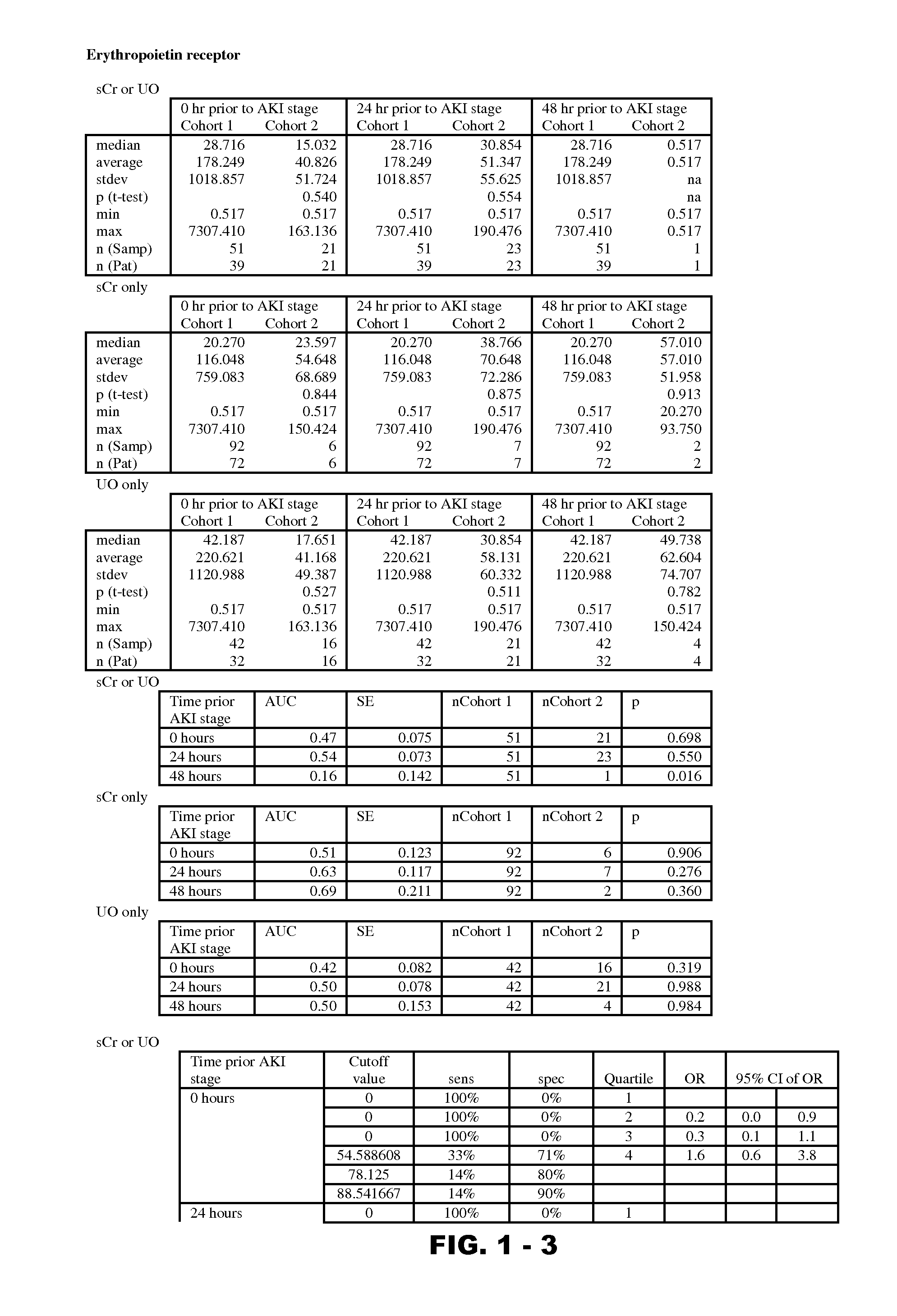
Disclosed are methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, disclosed are assays that detect one or more markers selected from the group consisting of Prostatic acid phosphatase, Lactotransfenin, Soluble erythropoietin receptor, Von Willebrand factor, Soluble endothelial protein C receptor, and Beta-2-glycoprotein 1 as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Recombinant blood clotting factors
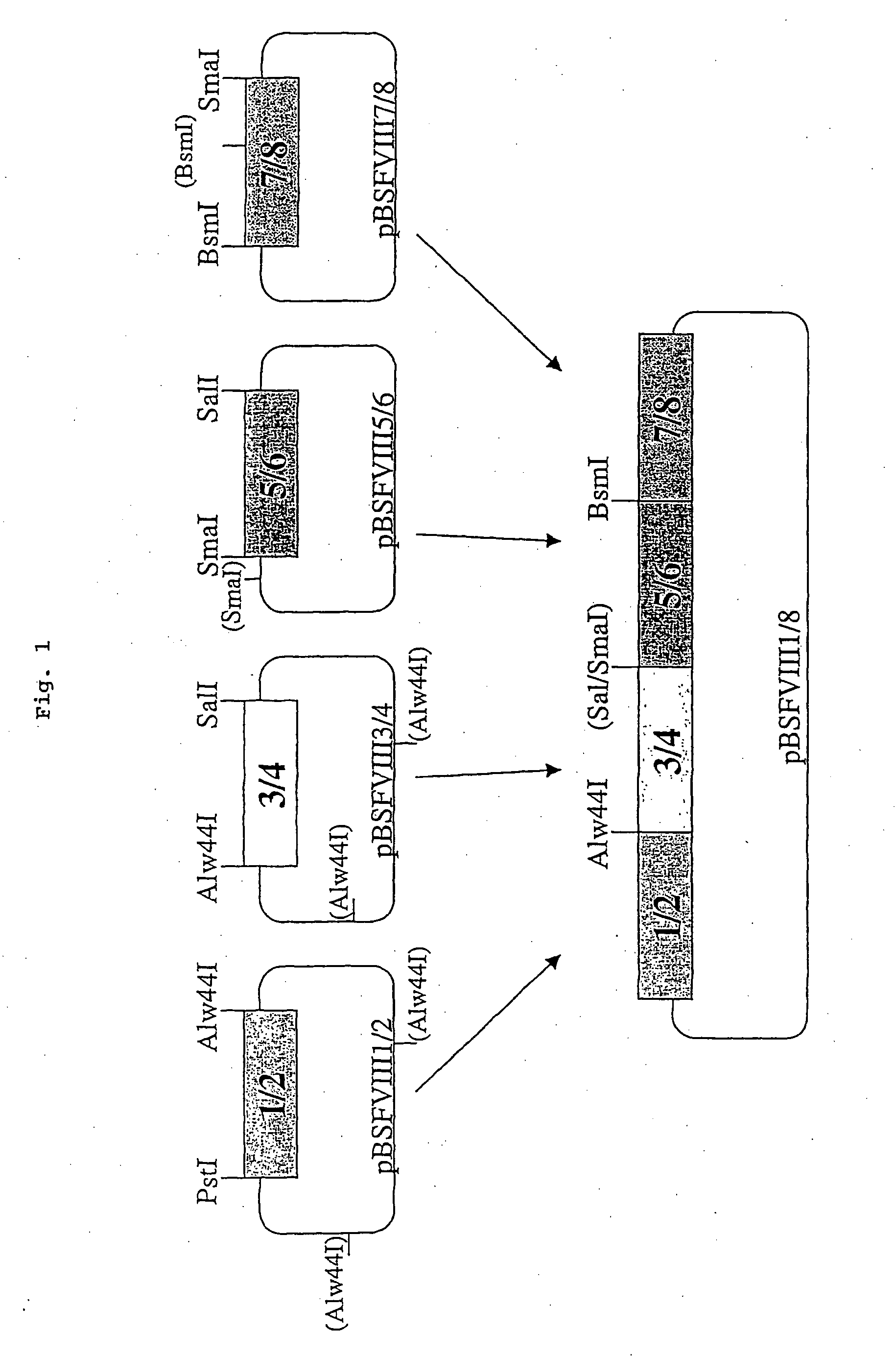
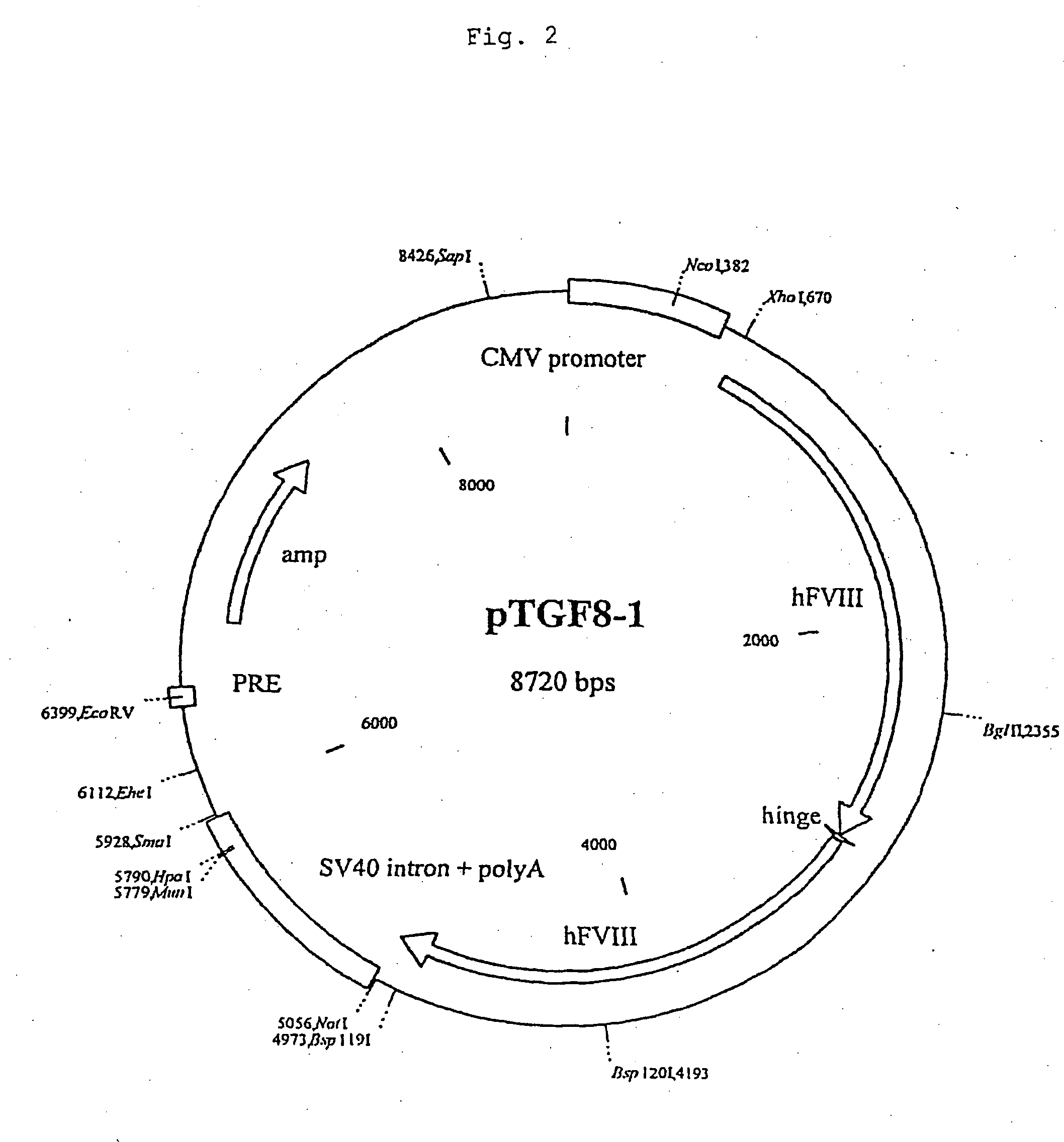
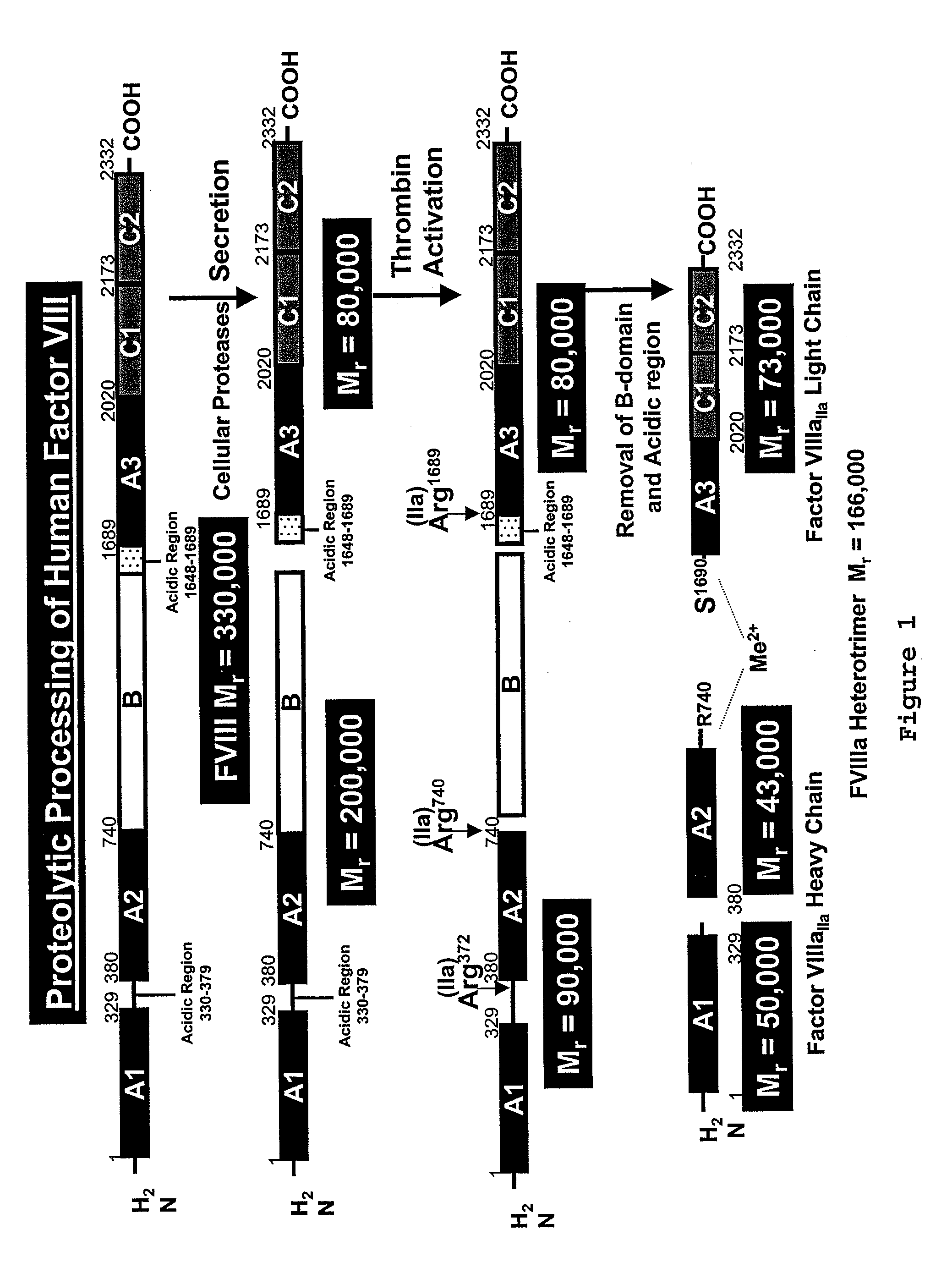
The present invention relates to an improved method for the production of recombinant human blood clotting factors, in particular of factor VIII and factor IX. An immortalized human cell line can be used to stably express viral transcription activator proteins and carrying a vector having a promoter functionally linked to a DNA sequence coding for a blood coagulating factor, provided that said promoter is not a viral promoter which is stimulated by said viral transcription activator proteins. The invention further relates to an immortalized human cell line carrying said vector, factor VIII muteins particularly suitable for the above production method; pharmaceutical compositions comprising such factor VIII muteins, and the use of such factor VIII muteins for preparing a medicament for treating hemophilia.
Owner:OCTAPHARMA +1
Hemophilia treatment by inhalation of coagulation factors
Hemophilia treatment by the inhalation of coagulation factors. Dry powder Factor IX is aerosolized to a mass median aerodynamic diameter of 4 μm or less, with at least 90% monomer content, at least 80% activity level, and 10% water or less. The aerosol is slowly, and deeply inhaled into the lung, and followed by a maximal exhale.
Owner:WYETH LLC +1
Recombinant factor viii having enhanced stability following mutation at the a1-c2 domain interface
InactiveUS20120065136A1Enhance inter-domain binding affinityIncreased stability parameterFactor VIIFungiFactor iiHaemophilia A
The invention relates to a recombinant factor VIII that includes one or more mutations at an interface of A1 and C2 domains of recombinant factor VIII. The one or more mutations include substitution of one or more amino acid residues with either a cysteine or an amino acid residue having a higher hydrophobicity. This results in enhanced stability of factor VIII. Methods for making the recombinant factor VIII, pharmaceutical compositions containing the recombinant factor VIII, and use of the recombinant factor VIII for treating hemophilia A are also disclosed.
Owner:UNIVERSITY OF ROCHESTER
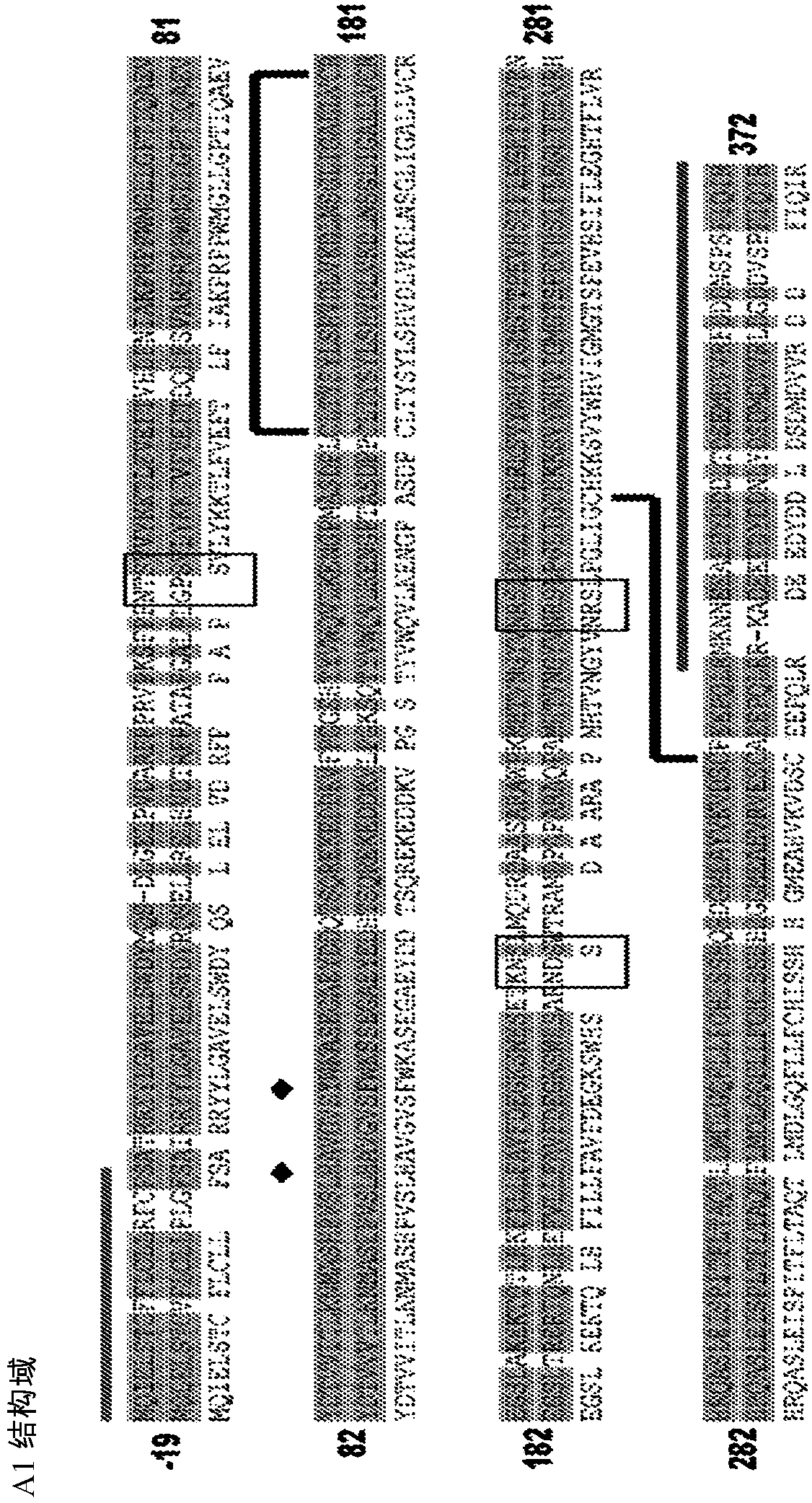
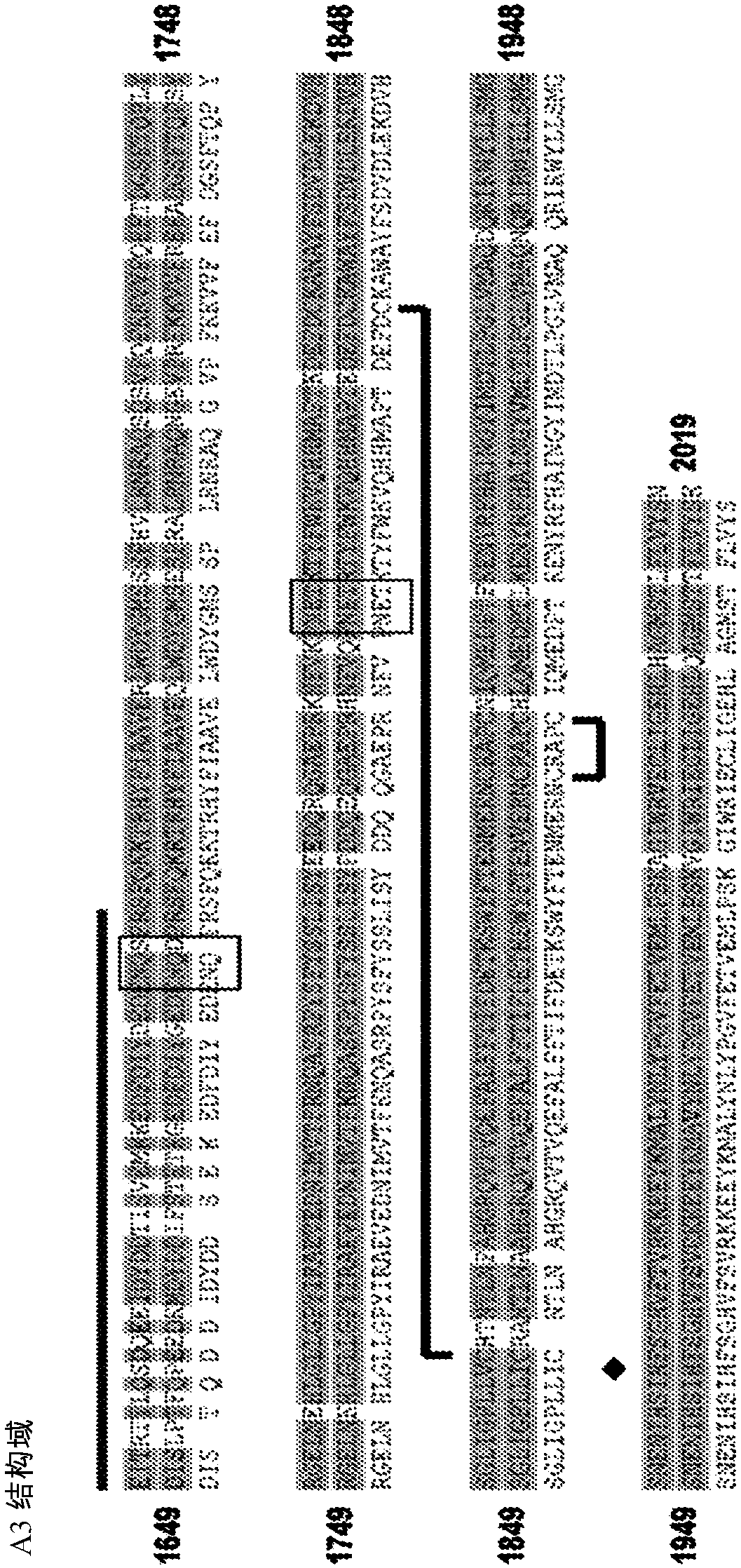
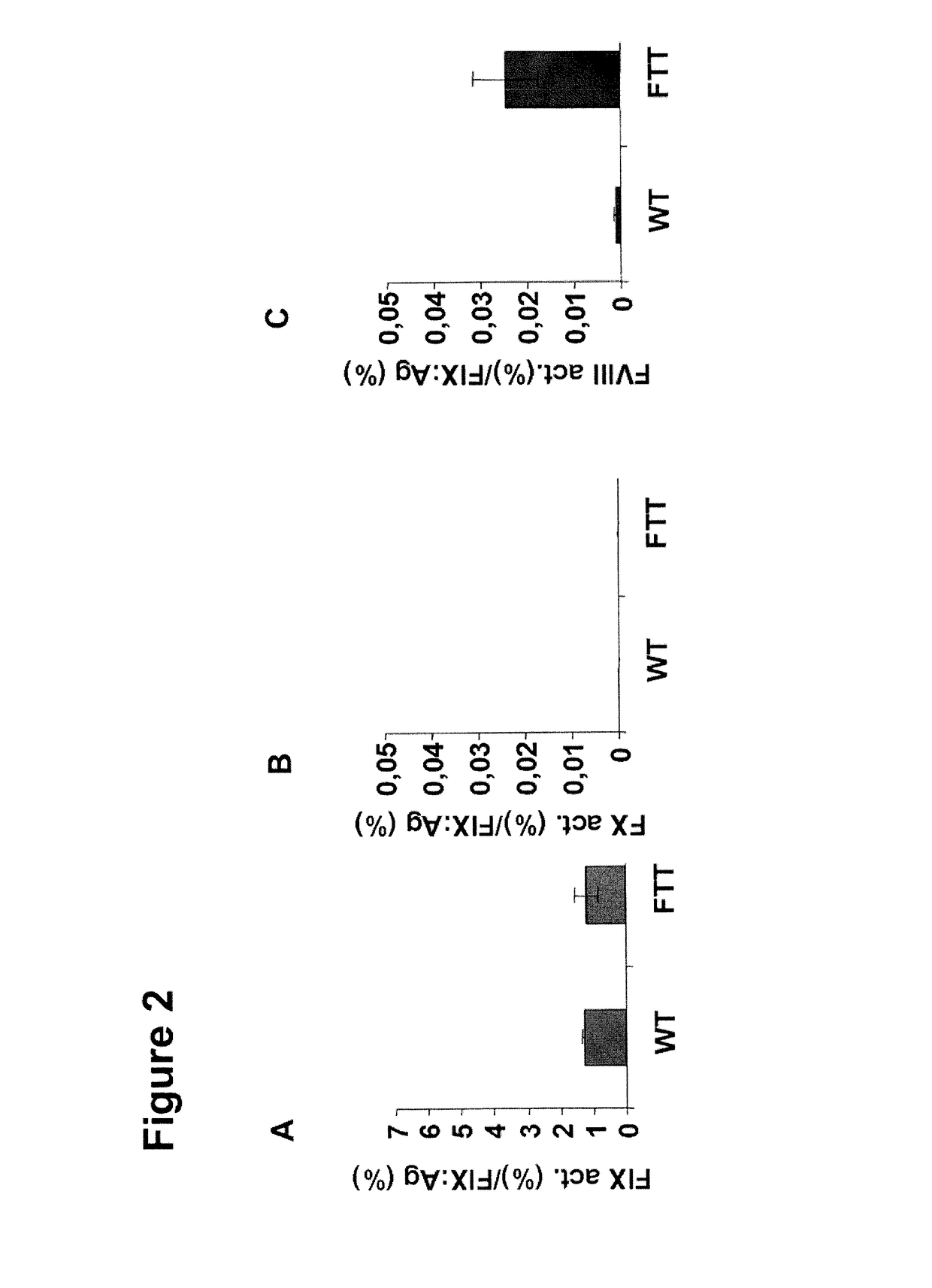
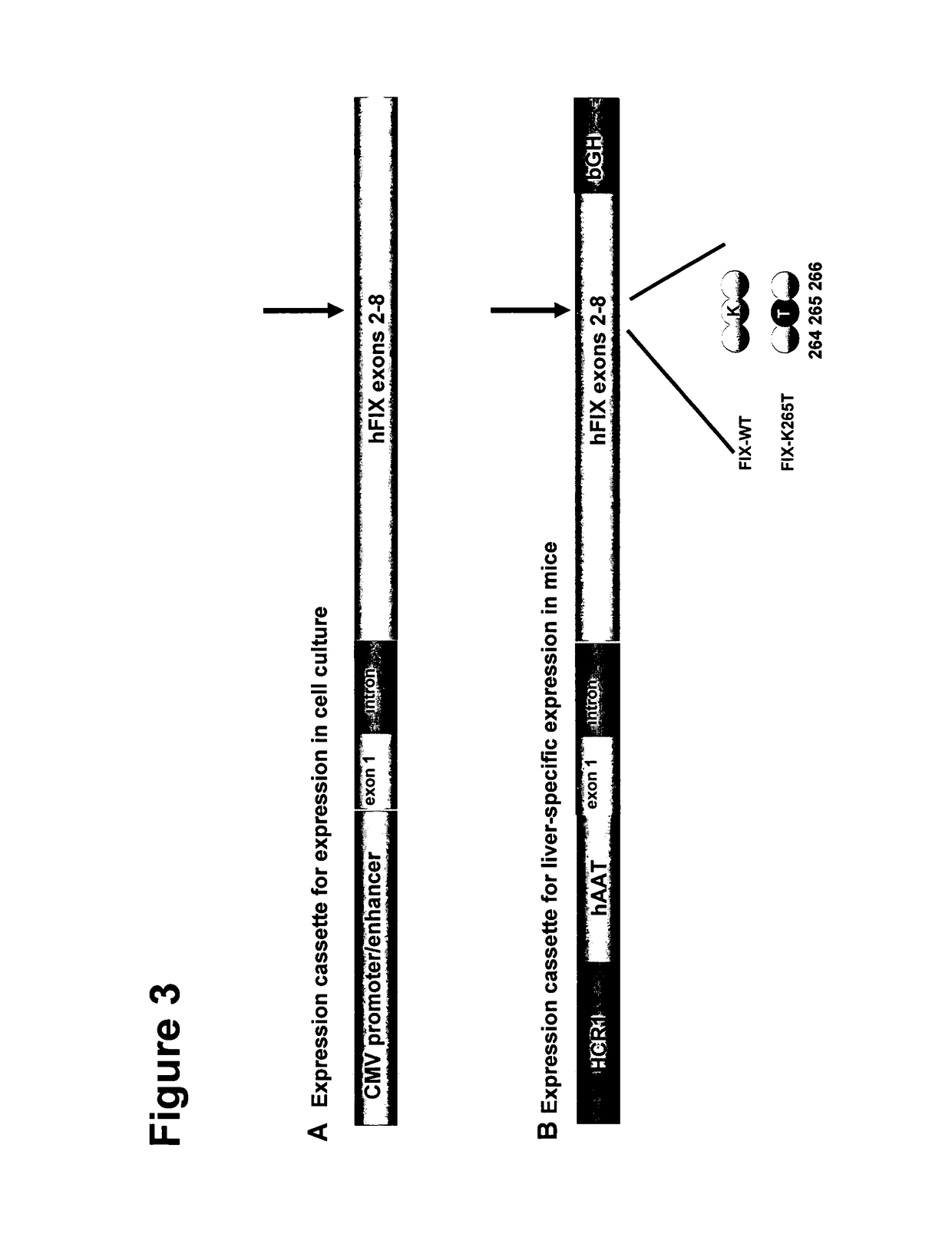
Vectors for Liver-Directed Gene Therapy of Hemophilia and Methods and Use Thereof
ActiveUS20150283267A1Improve efficiencyImprove securityVectorsPeptide/protein ingredientsFactor iiFhit gene
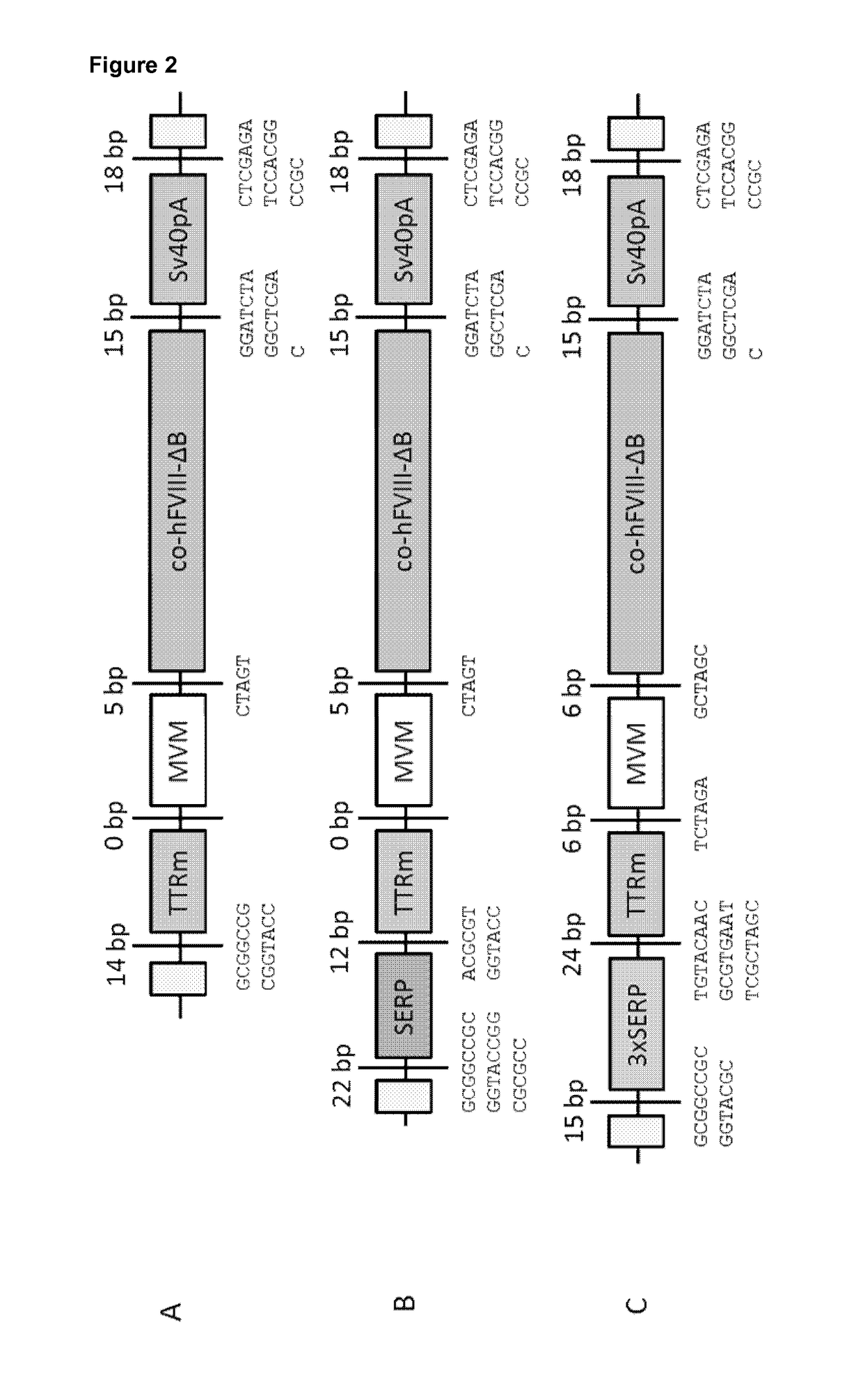
The present invention relates to vectors containing liver-specific regulatory sequences and codon-optimized factor IX or factor VIII genes, methods employing these vectors and uses of these vectors. Expression cassettes and vectors containing these liver-specific regulatory elements and codon-optimized factor IX or factor VIII genes are also disclosed. The present invention is particularly useful for applications using gene therapy, in particular for the treatment of hemophilia A and B.
Owner:VRIJE UNIV BRUSSEL
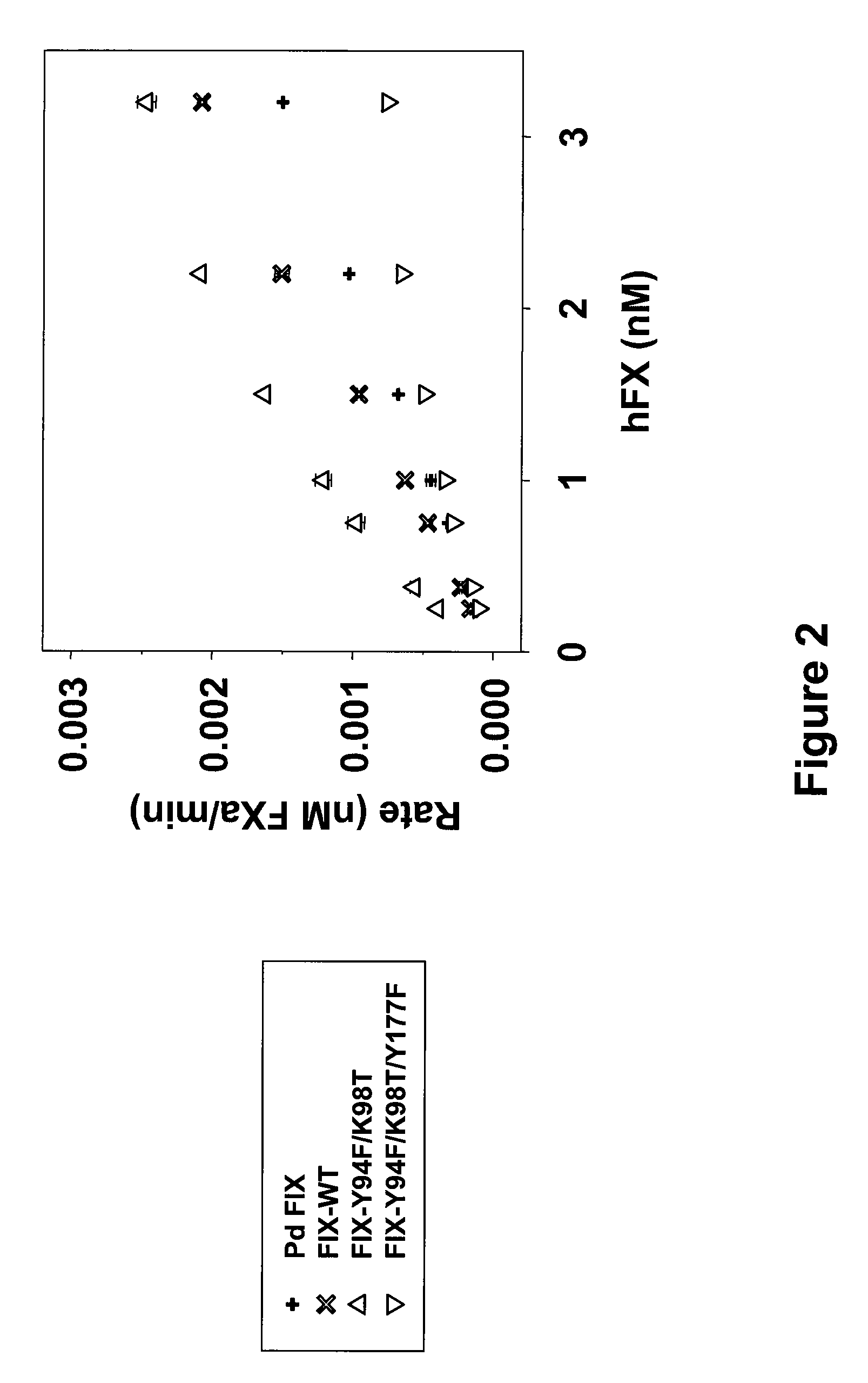
FIX-Mutant Proteins for Hemophilia B Treatment
InactiveUS20080214462A1Improved clot activityHigh activityPeptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsDisease
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having improved FIX clotting activity. Three full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assays in FIX-depleted plasma. Two mutant proteins had increased specific FIX activity. Furthermore, a pre-activated FIX protein had an increased activity in FIX-depleted plasma. Therefore these FIX mutants can be used for the treatment of FIX associated bleeding disorders.
Owner:BAXTER INT INC +1
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS9229010B2Peptide/protein ingredientsMammal material medical ingredientsFactor VIII vWFErythropoietin receptor
Disclosed are methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, disclosed are assays that detect one or more markers selected from the group consisting of Prostatic acid phosphatase, Lactotransfenin, Soluble erythropoietin receptor, Von Willebrand factor, Soluble endothelial protein C receptor, and Beta-2-glycoprotein 1 as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
FVIII-Independent FIX-Mutant Proteins for Hemophilia A Treatment
ActiveUS20080214461A1Peptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsMutated protein
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having factor VIII (FVIII) independent factor X (FX) activation potential. Five full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assay in FVIII-depleted plasma as well as in FVIII-inhibited patient plasma. In FVIII-depleted plasma functional activity of the FIX mutants was calculated as increased FVIII equivalent activity. The mutant proteins had increased FVIII equivalent activity. In FVIII-inhibited patient plasma the FEIBA equivalent activity was calculated for analysis of FVIII independent FX activation potential. The proteins had also increased FEIBA equivalent activity. Furthermore, the pre-activated FIX proteins had an increased activity in FIX-depleted plasma containing FVIII inhibitors. Therefore these FIX mutants are alternatives as bypassing agents for treatment of FVIII inhibitor patients.
Owner:TAKEDA PHARMA CO LTD
Method for detecting whole-blood sample coagulation item using magnetic-bead method
InactiveCN1936580AEasy to masterAccurate reflectionBiological testingMagnetic beadBlood coagulations
One kind blood samples detection method using magnetic beads, completed with the following steps: (1) Get fresh clinical samples; (2)Put these specimen on coagulation analyzer, use magnetic beads and special adjustment reagents aiming at the whole blood specimens, measure four coagulations: Activated partial thromboplastin time (APTT), Prothrombin time (PT), Prothrombin time (TT), Fibrinogen (FIB). The advantages of this invention are: 1.The method is simple, time-saving and easy to technical staff. 2. Reduce costs and save centrifuges and other equipment, reduce errors; 3. It is efficient and suitable for the battlefield, natural disasters and remote mountainous areas, medical teams. Particularly, it's suitable for hemorrhagic diseases and natural disasters (such as hemophilia, etc.). 4. Save specimen volume. 5. Reflect the level of specimens of blood coagulation preciously and accurately.
Owner:MEIDE TAIPINGYANG PRECISION INSTR MFG
Production of recombinant blood clotting factors in human cell lines
ActiveUS20040023333A1Increased activationStable against proteolytic inactivationFactor VIIFungiHuman cellA-DNA
The present invention relates to an improved method for the production of recombinant human blood clotting factors, in particular of factor VIII and factor IX, utilizing an immortalized human cell line stably expressing viral transcription activator proteins and carrying a vector having a promoter functionally linked to a DNA sequence coding for a blood coagulating factor, provided that said promoter is not a viral promoter which is stimulated by said viral transcription activator proteins; an immortalized human cell line carrying said vector, factor VIII muteins particularly suitable for the above production method; pharmaceutical compositions comprising such factor VIII muteins and the use of such factor VIII muteins for preparing a medicament for treating hemophilia.
Owner:OCTAPHARMA +1
Vesicle and application thereof
PendingCN113136362AMicrobiological testing/measurementSkeletal/connective tissue cellsMedicineTherapeutic effect
The invention belongs to the field of biological medicine, and relates to a vesicle and application thereof. The source of the vesicle comprises stem cells or somatic cells, the marker of the vesicle comprises Syntaxin 4, and the vesicle is an induced vesicle. Compared with exosomes in MSCs, the vesicle provided by the invention can specifically express Syntaxin 4, and can be used for distinguishing MSCs-derived vesicles from characteristic markers of Exosomes. The method for preparing the vesicle has the advantages that the yield is high, 300-2000 vesicles can be produced by single MSCs, the preparation process is simple, the consumed time is short, the requirements on reagents and equipment are low, and the treatment effect is good. The vesicle can play a significant role in coagulation promotion in vitro, can significantly improve the bleeding tendency of hemophilia mice after being injected in vivo, and can be used for treatment for improving the bleeding tendency of hemophilia. Moreover, the vesicle can be discharged through skin and hair and is safe in vivo, so that the vesicle has a good application prospect.
Owner:EV CELL BIOTECH GUANGZHOU CO LTD
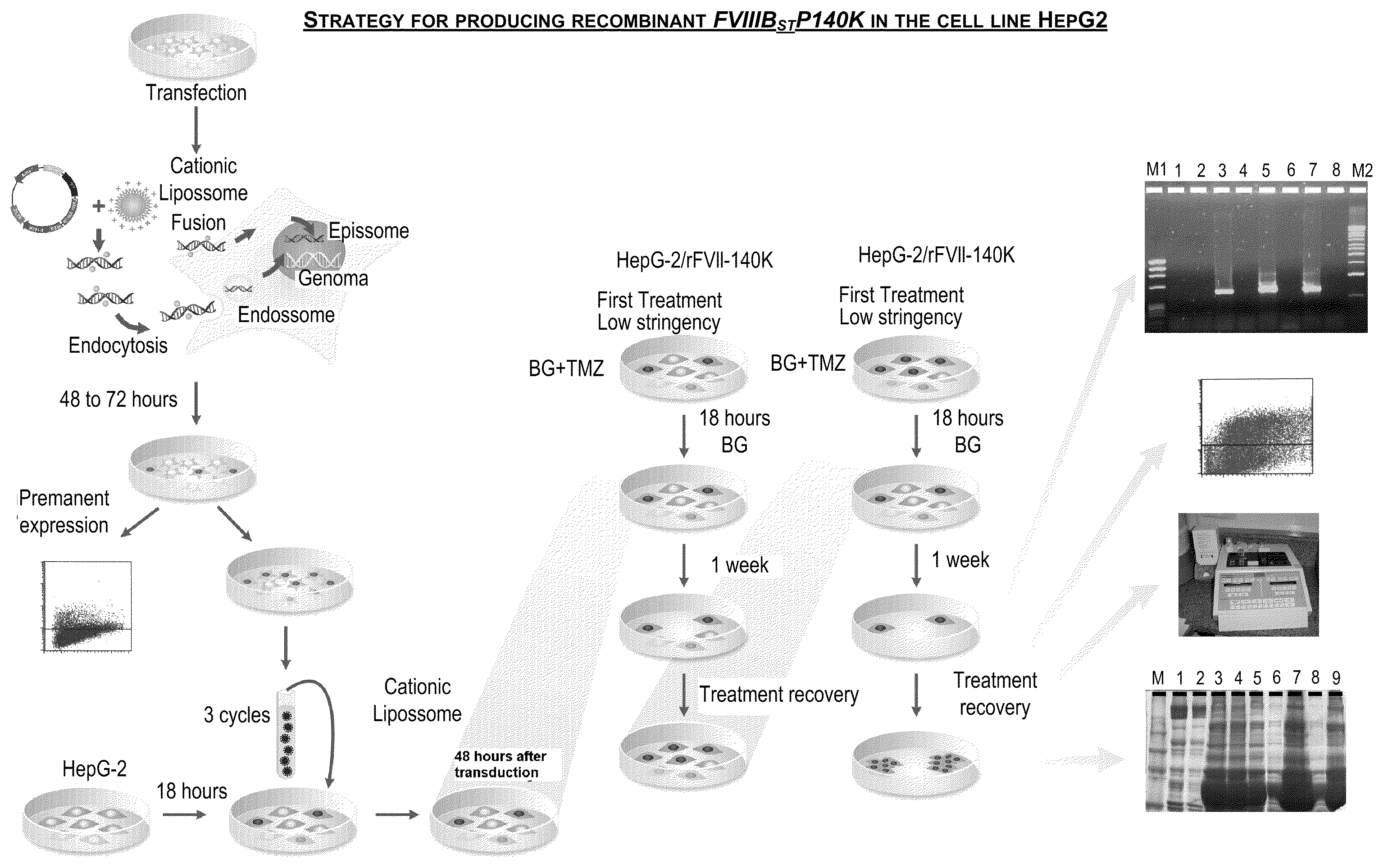
Recombinant human blood coagulation factor VIII protein, composition, use of a recombinant factor VIII protein, use of a composition, method of obtaining a recombinant human blood coagulation factor VIII protein and use thereof
InactiveUS20100172891A1Improve biological activityImprove the level ofFactor VIIPeptide/protein ingredientsHemophiliasHuman Blood Coagulation Factor
The present invention refers to a recombinant human blood coagulation factor VIII protein and a composition containing it. The present invention also refers to the use of the protein or composition of the invention for manufacturing a medicine for treating hemophilia A. Additionally, the present invention refers to the method of obtaining a recombinant human blood coagulation factor VIII protein. A further object of the present invention is a recombinant protein obtained by the method described herein, and its use in the preparation of a medicine for the treatment of hemophilia A.
Owner:FUNDACAO HEMOCENT DE RIBEIRAO PRETO +1
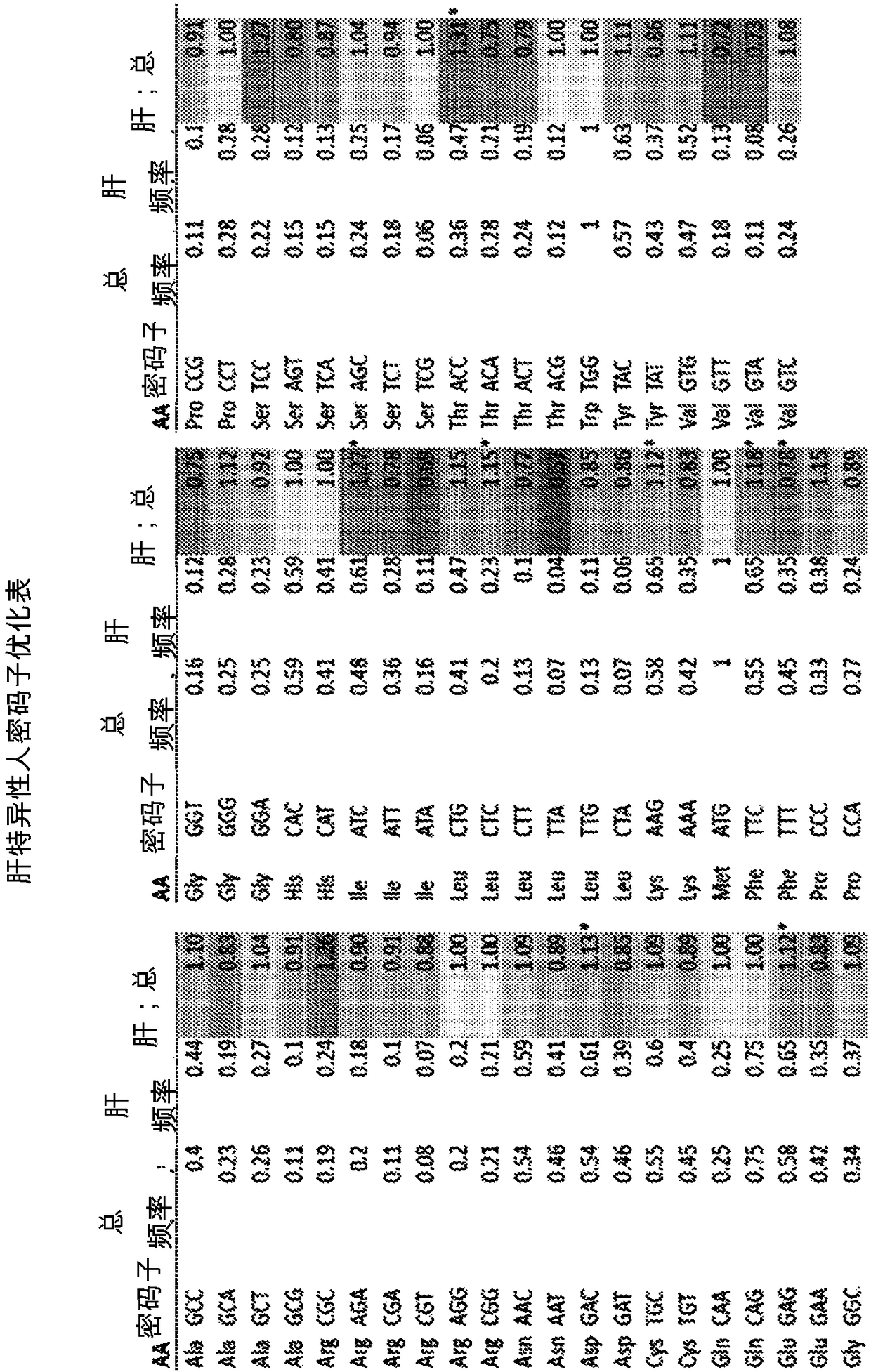
Optimized Liver-Specific Expression Systems for FVIII and FIX
The present invention relates to nucleic acid expression cassettes and vectors containing liver-specific regulatory elements and codon-optimized factor IX or factor VIII transgenes, methods employing these expression cassettes and vectors and uses thereof. The present invention is particularly useful for applications using liver-directed gene therapy, in particular for the treatment of hemophilia A and B.
Owner:VRIJE UNIV BRUSSEL
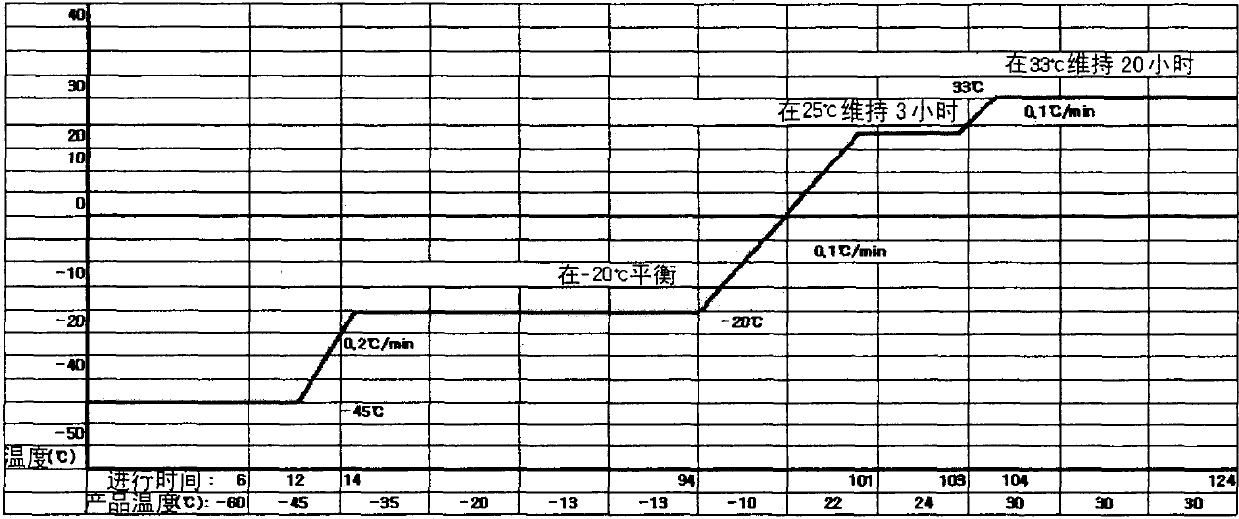
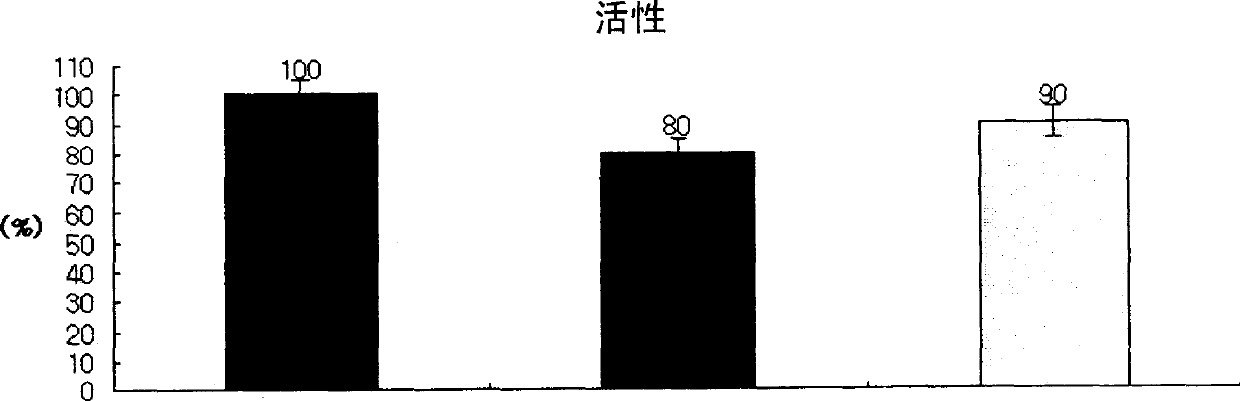
Pharmaceutical preparation of recombinant factor VIII lyophilized without albumin as a stabilizer
Disclosed is a lyophilized preparation of recombinant factor VIII used as a therapeutic preparation of hemophilia A. The lyophilized preparation of recombinant factor VIII is prepared by performing lyophilization using a mixture comprising 6 to 100 mM of L-arginine, 3.5 to 50 mM of L-isoleucine, and 10 to 100 mM of L-glutamic acid as a stabilizer for stabilizing the recombinant factor VIII which exhibits an unstable activity during lyophilization, rather than using human blood derived albumin.
Owner:GREEN CROSS CORP THE
Clotting factor preparations for delivery into tissue of the intestinal tract using a swallowable drug delivery device
ActiveUS10603275B2Improved long-term tolerance to and efficacyProcess controlPeptide/protein ingredientsCatheterIntestinal wallsTherapeutic effect
Owner:RANI THERAPEUTICS
Primers, method and kit for detecting inversion of first intron of hemophilia A clotting factor VIII gene
InactiveCN103820539AImprove efficiencySave human effortMicrobiological testing/measurementDNA/RNA fragmentationMedicineGenetics
The invention discloses a method, primers and a kit for detecting inversion of the first intron of hemophilia A clotting factor VIII gene. The primers comprise primers SEQ ID NO. 1, SEQ ID NO. 2 and SEQ ID NO. 3 for amplifying the Intlh-1 region of the clotting factor VIII gene and primers SEQ ID NO. 1, SEQ ID NO. 3 and SEQ ID NO. 4 for amplifying the Intlh-2 region of the clotting factor VIII gene. The method and the kit can be utilized to simply, efficiently and specifically detect the inversion of the first intron of the hemophilia A clotting factor VIII gene.
Owner:CHANGSHA ADICON CLINICAL LAB
Optimized factor ix gene
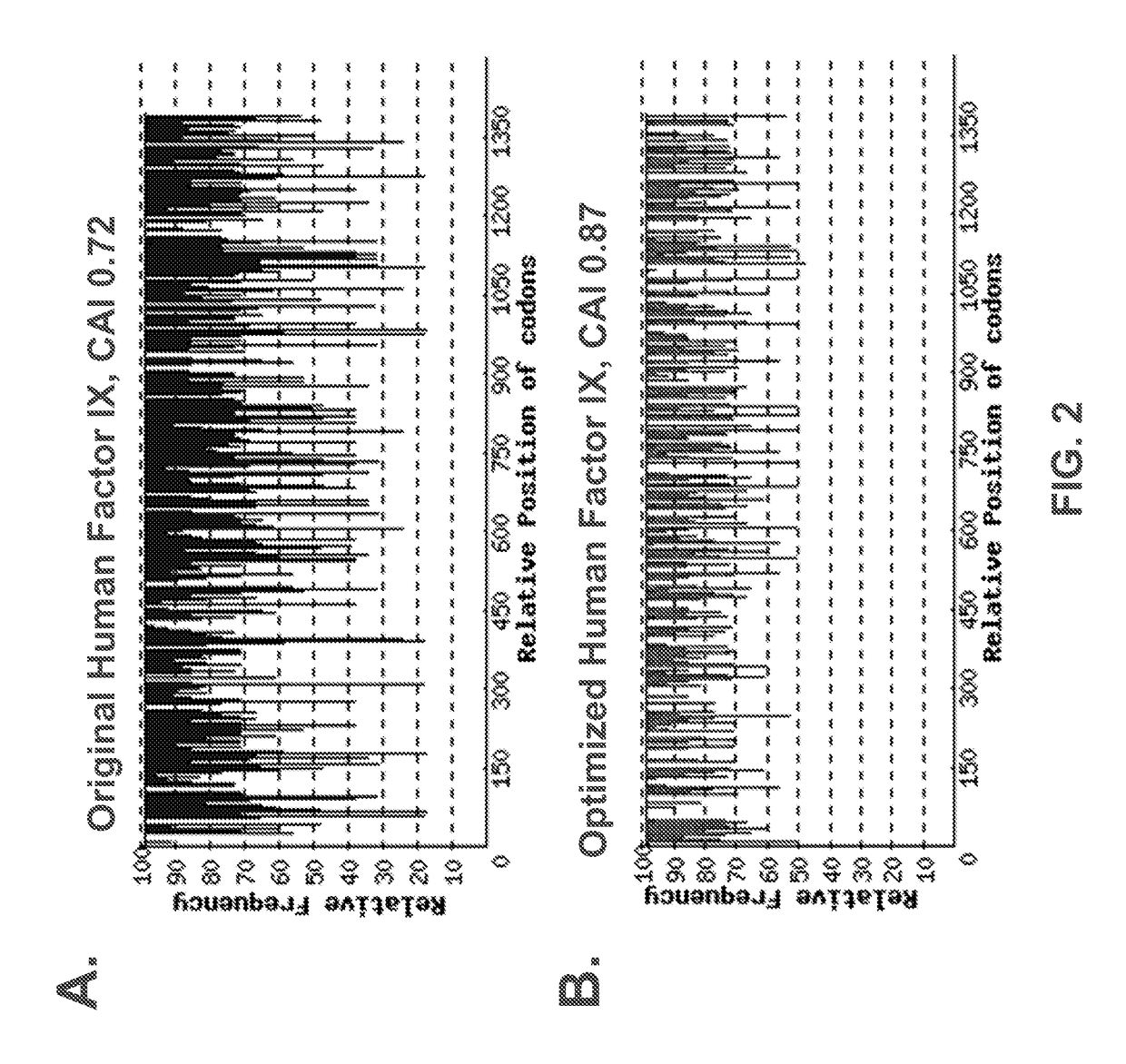
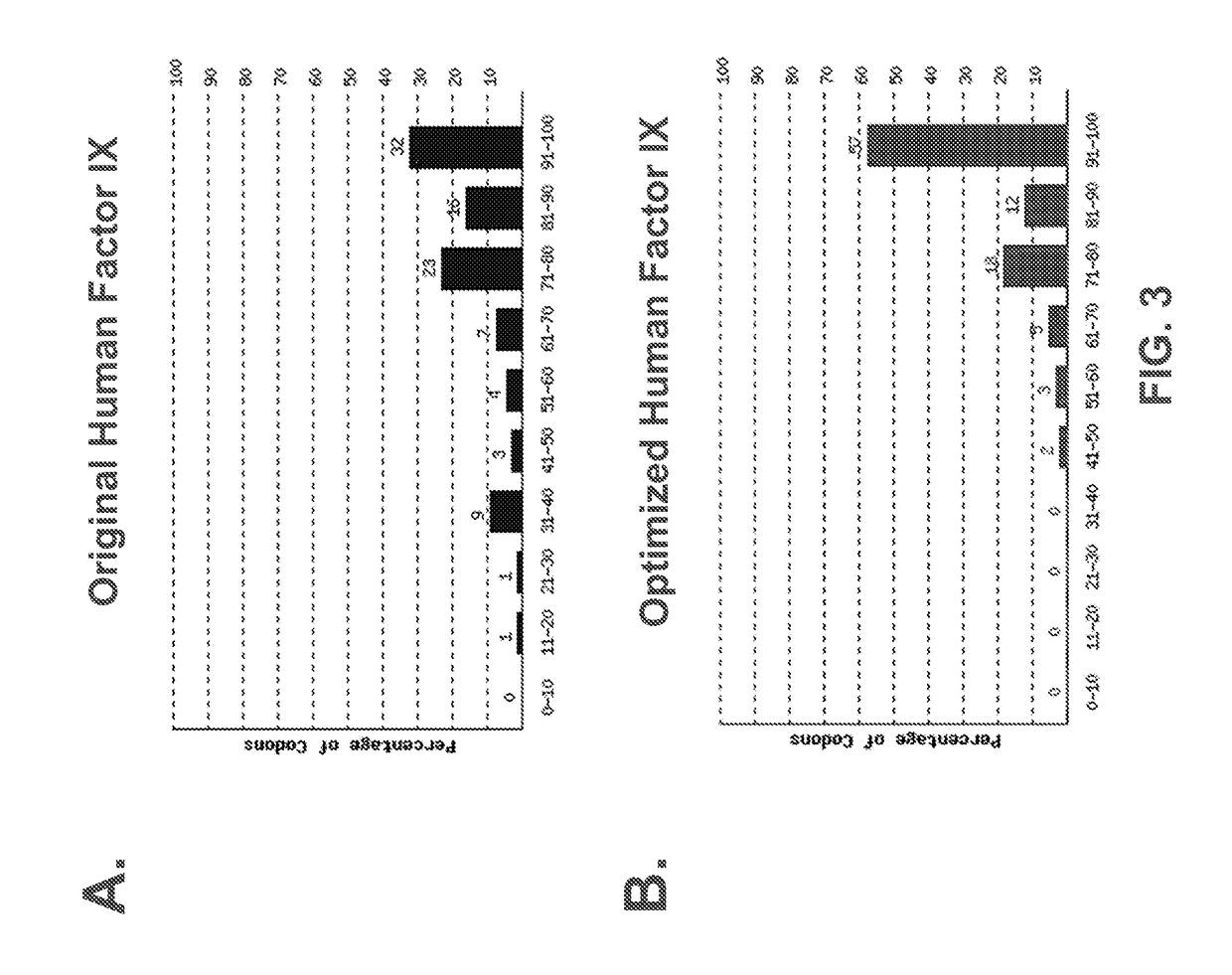
ActiveUS20170260516A1High activityHigh expressionSugar derivativesGenetic material ingredientsFactor iiNucleic acid sequencing
The present invention provides codon optimized Factor IX sequences, vectors and host cells comprising codon optimized Factor IX sequences, polypeptides encoded by codon optimized Factor IX sequences, and methods of producing such polypeptides. The present invention also provides methods of treating bleeding disorders such as hemophilia comprising administering to the subject a codon optimized Factor IX nucleic acid sequence or the polypeptide encoded thereby.
Owner:BIOVERATIV THERAPEUTICS INC
Freeze-dried tranexamine powder injection and its preparing prcess
InactiveCN1390539AReduce volumeReduce weightPeptide/protein ingredientsBlood disorderFreeze-dryingAngioneurotic oedema
A freeze dried tranexamine acid powder used as antihemorrhagic injection for treating various hemorrhagic diseases contains tranexamine acid (0.05-10 wt. portions), freeze drying supporting agent (0-100) and pH regulator.
Owner:于航
Modulation of platelet adhesion based on the surface-exposed beta-switch loop of platelet glycoprotein IB-alpha
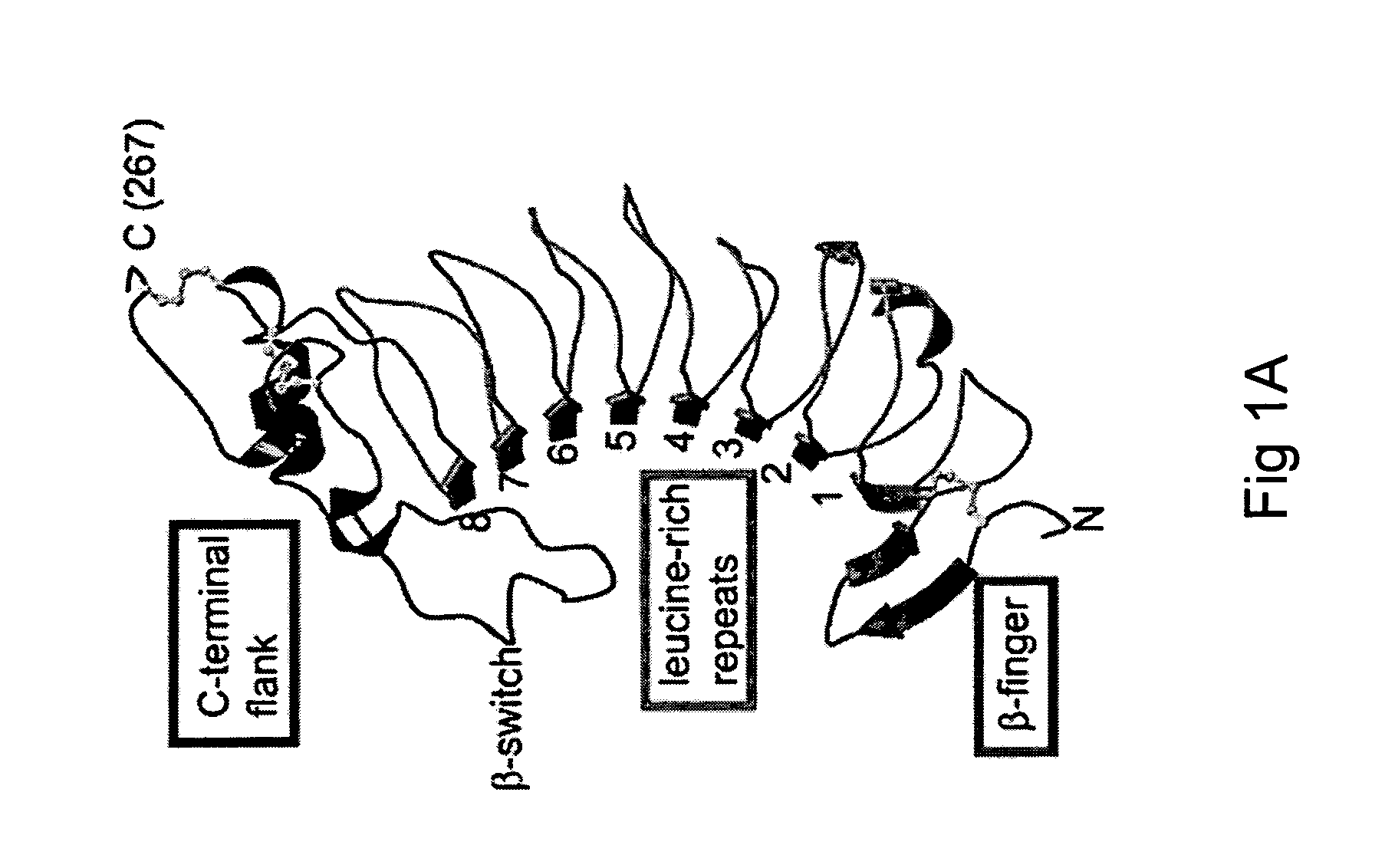
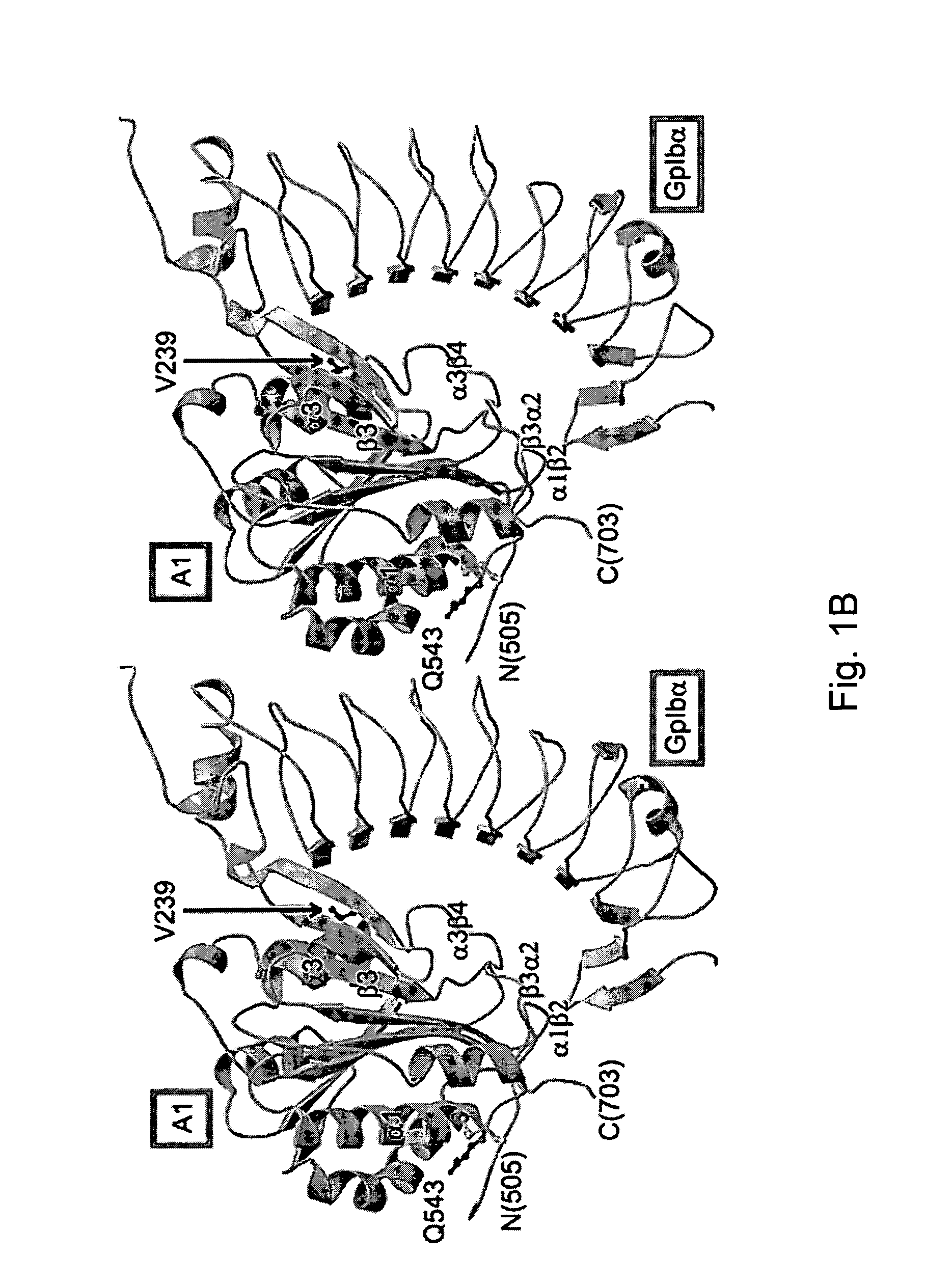
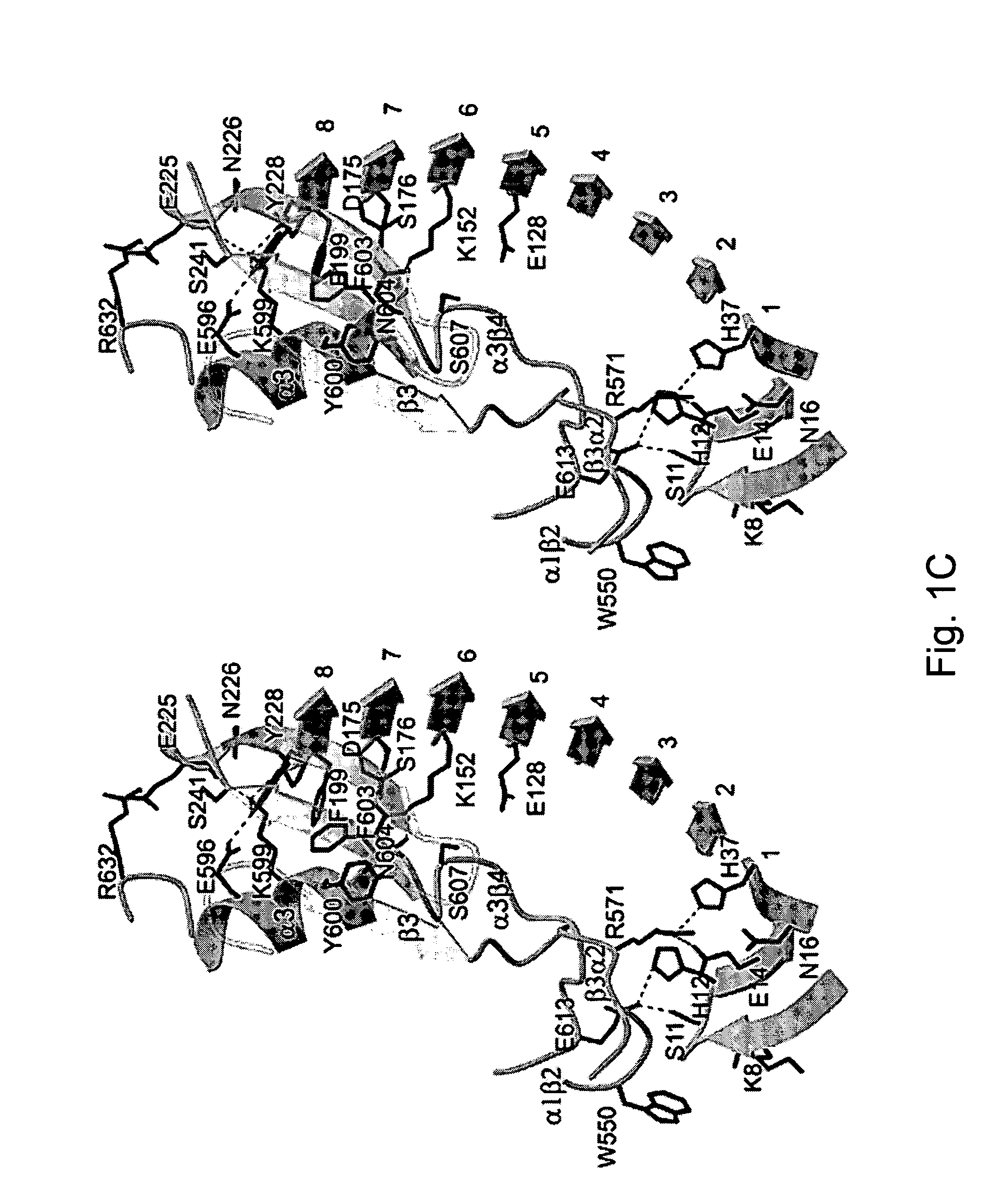
ActiveUS7771724B2Trend downReduce decreaseFactor VIICell receptors/surface-antigens/surface-determinantsGlycoprotein IbAntiendomysial antibodies
The invention relates to the adhesion of platelet GpIbα to strand β3 of domain A1 of von Willebrand factor (vWF), the strand β3 comprising amino acid residues at amino acid position 560-566 and / or a functional part or equivalent thereof, the platelet GpIbα, the GpIbα region comprising an amino acid sequence corresponding to a beta-switch loop of platelet GpIbα, comprising amino acid residues at amino acid position 227-242 and / or a functional part or equivalent thereof. The invention provides a method of interfering with adhesion of blood platelets to vWF that includes modulating adhesion. The invention further provides proteinaceous compounds, antibodies, medicaments and pharmaceutical compositions to that end. The invention also provides means and methods to increase platelet adhesion by topical application of a compound increasing platelet adhesion.
Owner:ABLYNX NV
Compositions and methods for the treatment of hemophilia a
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
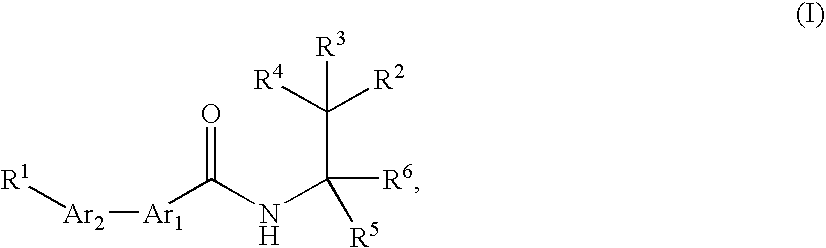
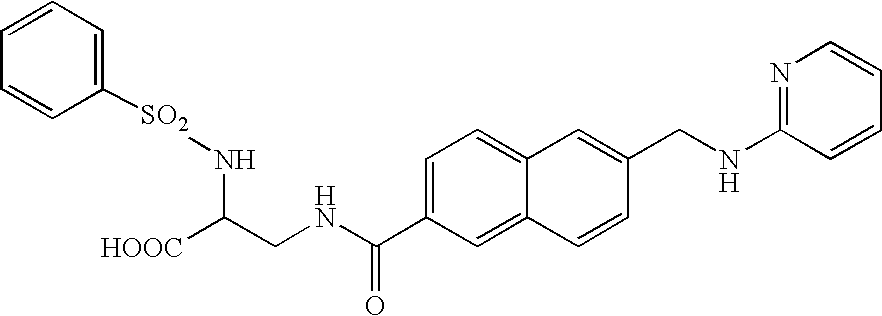
Bis (hetero) aryl carboxamide derivatives for use as PG12 antagonists
InactiveUS20060247260A1High antagonistic activityBiocideNervous disorderChemistryDetrusor instability
This invention relates to aryl or heteroaryl amido alkane derivatives of formula (I) in which Ar1 and Ar2 independently represent phenyl or a 5 or 6-membered heteroaromatic ring, R6 represents carboxyl or tetrazolyl, and the remaining variables are as defined in the text and claims, which are useful as an active ingredient of pharmaceutical preparations. The aryl or heteroaryl amido alkanes of the present invention have PGI2 antagonistic activity, and can be used for the prophylaxis and treatment of diseases associated with PGI2 activity. Such diseases include urological diseases or disorder as follows: bladder outlet obstruction, overactive bladder, urinary incontinence, detrusor hyper-reflexia, detrusor instability, reduced bladder capacity, frequency of micturition, urge incontinence, stress incontinence, bladder hyperreactivity, benighn prostatic hypertrophy (BPH), prostatitis, urinary frequency, nocturia, urinary urgency, pelvic hypersensitivity, urethritis, pelvic pain syndrome, prostatodynia, cystitis, or idiophatic bladder hypersensitivity. The compounds of the present invention are also useful for treatment of pain including, but not limited to inflammatory pain, neuropathic pain, acute pain, chronic pain, dental pain, premenstrual pain, visceral pain, headaches, and the like; hypotension;hemophilia and hemorrhage; and inflammation, since the diseases also relate to PGI2.
Owner:BAYER HEALTHCARE AG
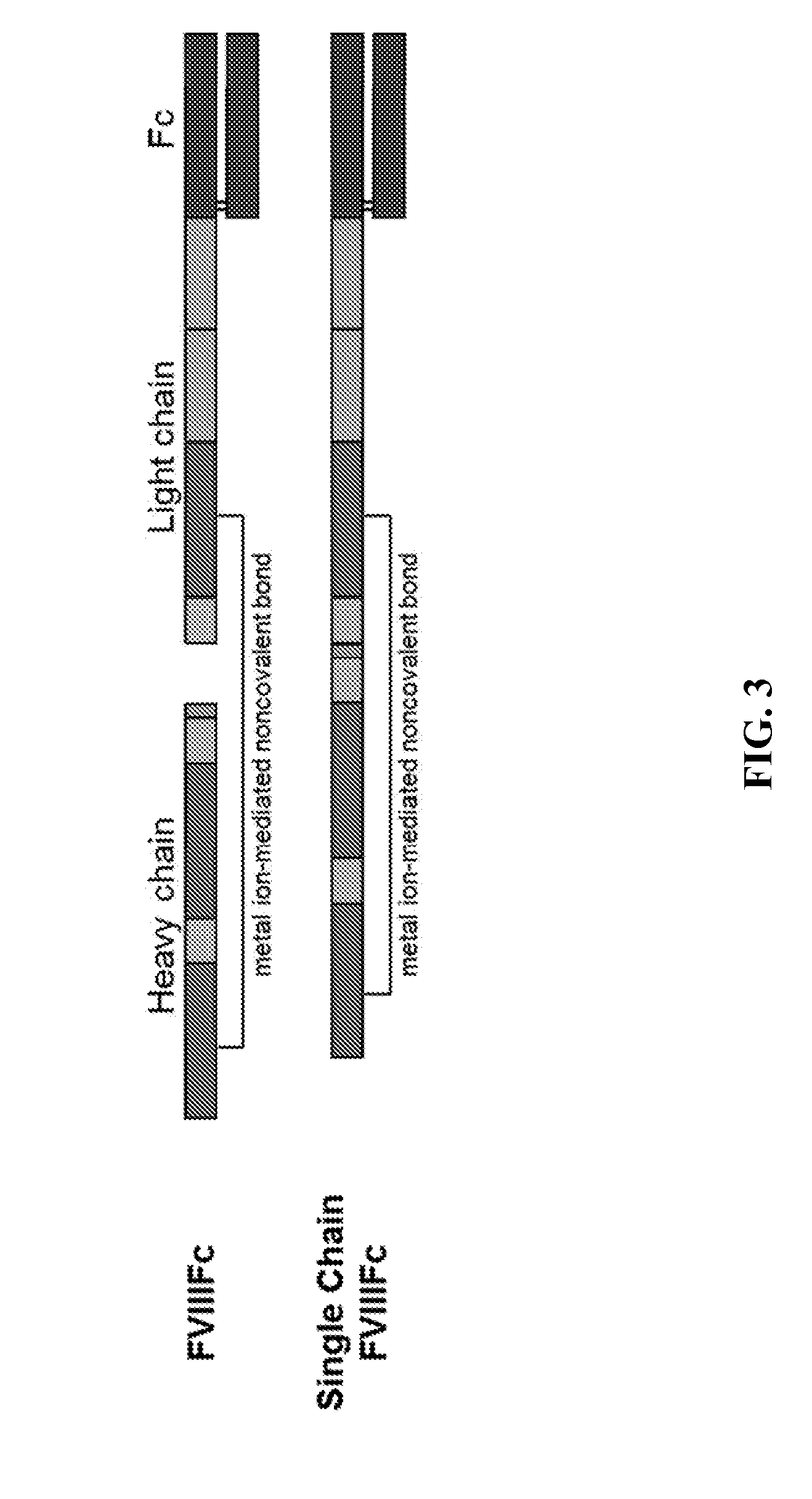
Cell line expressing single chain factor viii polypeptides and uses thereof
The present invention provides cell lines for producing single chain FVIII polypeptides, e.g., chimeric single chain FVIII polypeptides, methods of producing single chain FVIII polypeptides, single chain FVIII polypeptides, and methods of treating Hemophilia A with a single chain Factor VIII polypeptide.
Owner:BIOVERATIV THERAPEUTICS INC
Recombinant promoters and vectors for protein expression in liver and use thereof
Disclosed herein are recombinant viral vectors comprising a liver specific promotor in operable combination with a heterologous nucleic acid sequence encoding a protein, such as a clotting factor. Methods of treating a subject with a clotting disorder, such as hemophilia A or hemophilia B, are also provided.
Owner:EMORY UNIVERSITY +1
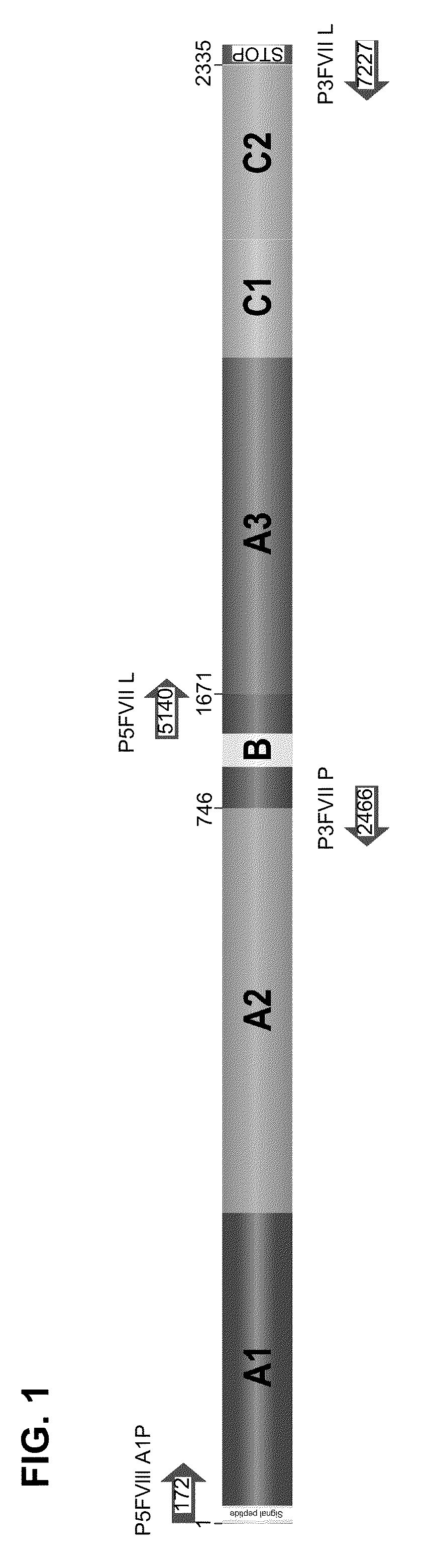
Factor IX variants with clotting activity in absence of their cofactor and their use for treating bleeding disorders
The present invention relates to variants of a vitamin K-dependent serine protease of the coagulation cascade, preferably variants of factor IX (F.IX), wherein the variant is characterized in that it has clotting activity in absence of its cofactor. The present invention furthermore relates to the use of these variants for the treatment and / or prophylaxis of bleeding disorders, in particular hemophilia A and / or hemophilia B or hemophilia caused or complicated by inhibitory antibodies to F.VIII. The present invention also relates to further variants of factor IX (F.IX) which have desired properties and can, thus be tailored for respective specific therapeutic applications.
Owner:DRK BLUTSPENDEDIENST BADEN WURTTEMBERG HESSEN GGMBH
Zone B partially-deleted type recombinant human blood coagulation factor VIII
ActiveCN105017410AConsistent biological activityImprove stabilityFactor VIIPeptide/protein ingredientsBlood coagulation factor VIIIClotting factor
The invention belongs to the field of biological medicines, and in particular relates to a gene recombinant human blood coagulation factor VIII for treating hemophilia A. By performing zone B partially-deleted type mutation on a full-length blood coagulation factor VIII, the obtained novel gene recombinant human blood coagulation factor VIII product has a characteristic that the structure is more stable.
Owner:BEIJING NORTHLAND BIOTECH
F9 gene copy number variation detection kit
ActiveCN104046699AHigh precisionImprove accuracyMicrobiological testing/measurementMedicineHemophilias
The invention discloses an F9 gene copy number variation detection kit which comprises a 2*PCR (Polymerase China Reaction) buffer solution, competitive DNA, PCR primer mixed liquor and Taq DNA polymerase. An AccuCopy technology has high precision, variable coefficient of being less than 5% and high result accuracy. Therefore, according to the F9 gene copy number variation detection kit developed by the AccuCopy technology, about 15 patients suffering hemophilia B with large deletion mutation is detected and found, and the clinical application effect is good.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE +1
Traditional Chinese medicine preparation for treating hemophilia and preparation method thereof
InactiveCN103301338AReduce financial burdenNo side effectsMammal material medical ingredientsBlood disorderMedicinal herbsViola yedoensis
The invention provides a traditional Chinese medicine preparation for treating hemophilia and a preparation method thereof. The traditional Chinese medicine is prepared from the following crude drugs of water chestnut, radix rubiae, buffalo horn, astragalus membranaceus, chickweed, codonopsis pilosula, hairyvein agrimony, herba violae, subprostrate sophora, celastrus aculeatus, dried rehamnnia root, fragrant hair grass, lalang grass rhizome, radix ranunculi ternate, placenta hominis, antelope horn, sweet wormwood, receptaculum nelumbinis, rhizoma bletillae, broken dense flowers and liquorice. The traditional Chinese medicine preparation has the beneficial effects that the traditional Chinese medicine preparation is a pure traditional Chinese medicine preparation, free of toxic and side effects, accurate in effect of bleeding pain, fast to become effective, good in treatment effect, convenient to take, available in materials, and low in drug cost, does not generate the drug dependence after being taken for a long period of time, and can help a sufferer get rid of whole blood and blood coagulation factors; the economical burden on treatment of the sufferer is reduced.
Owner:全清清
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com