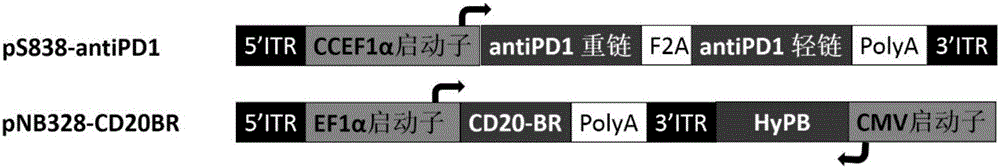
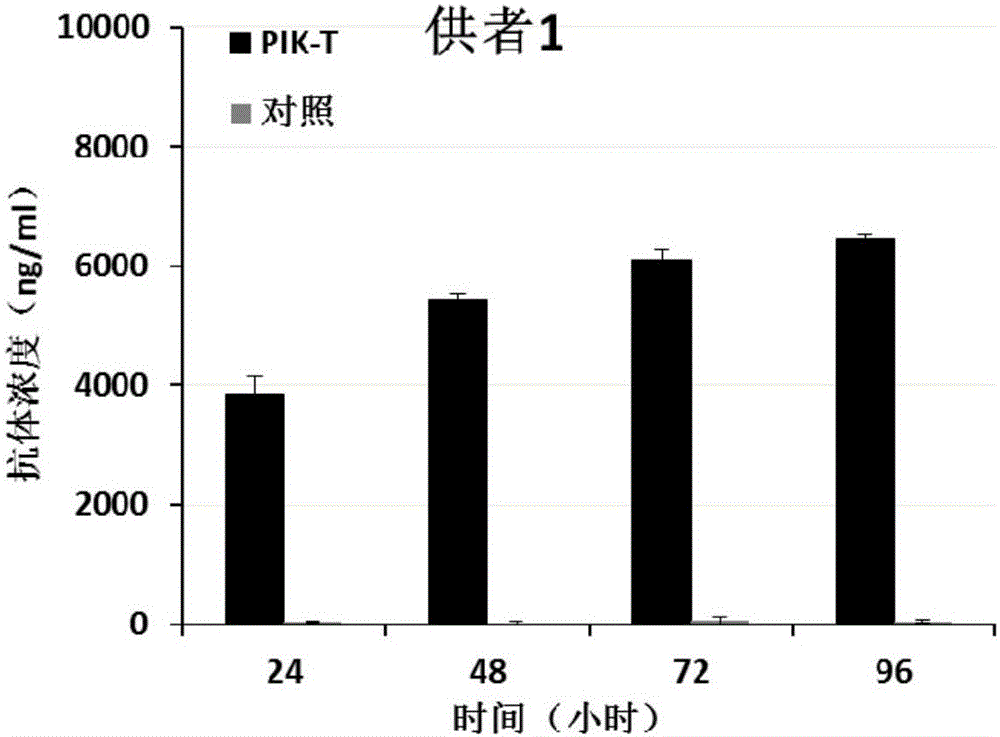
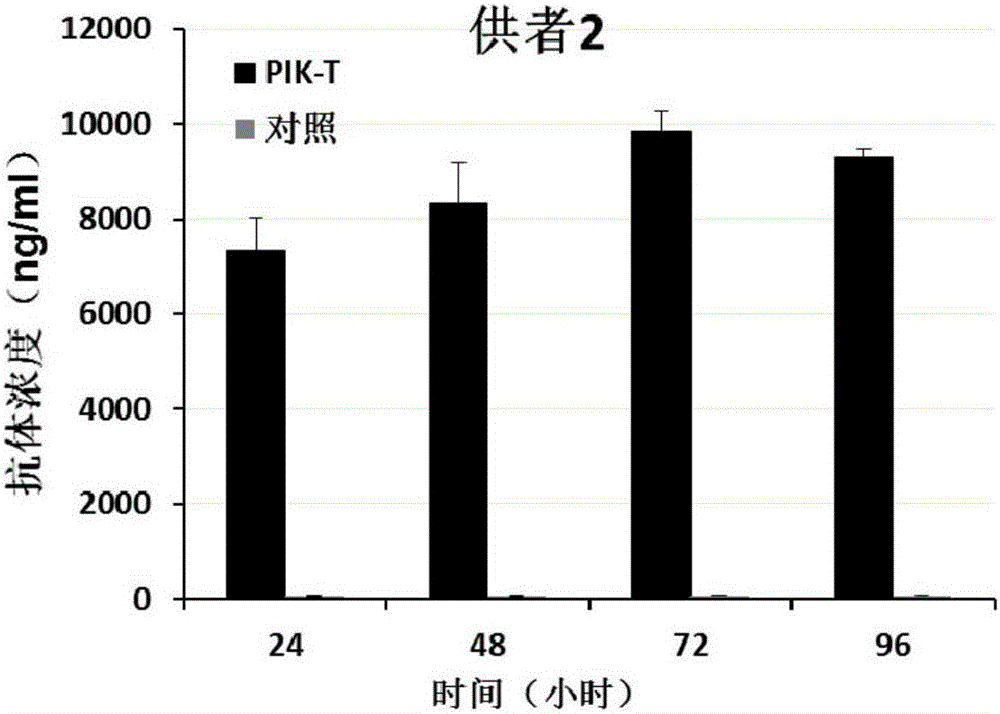
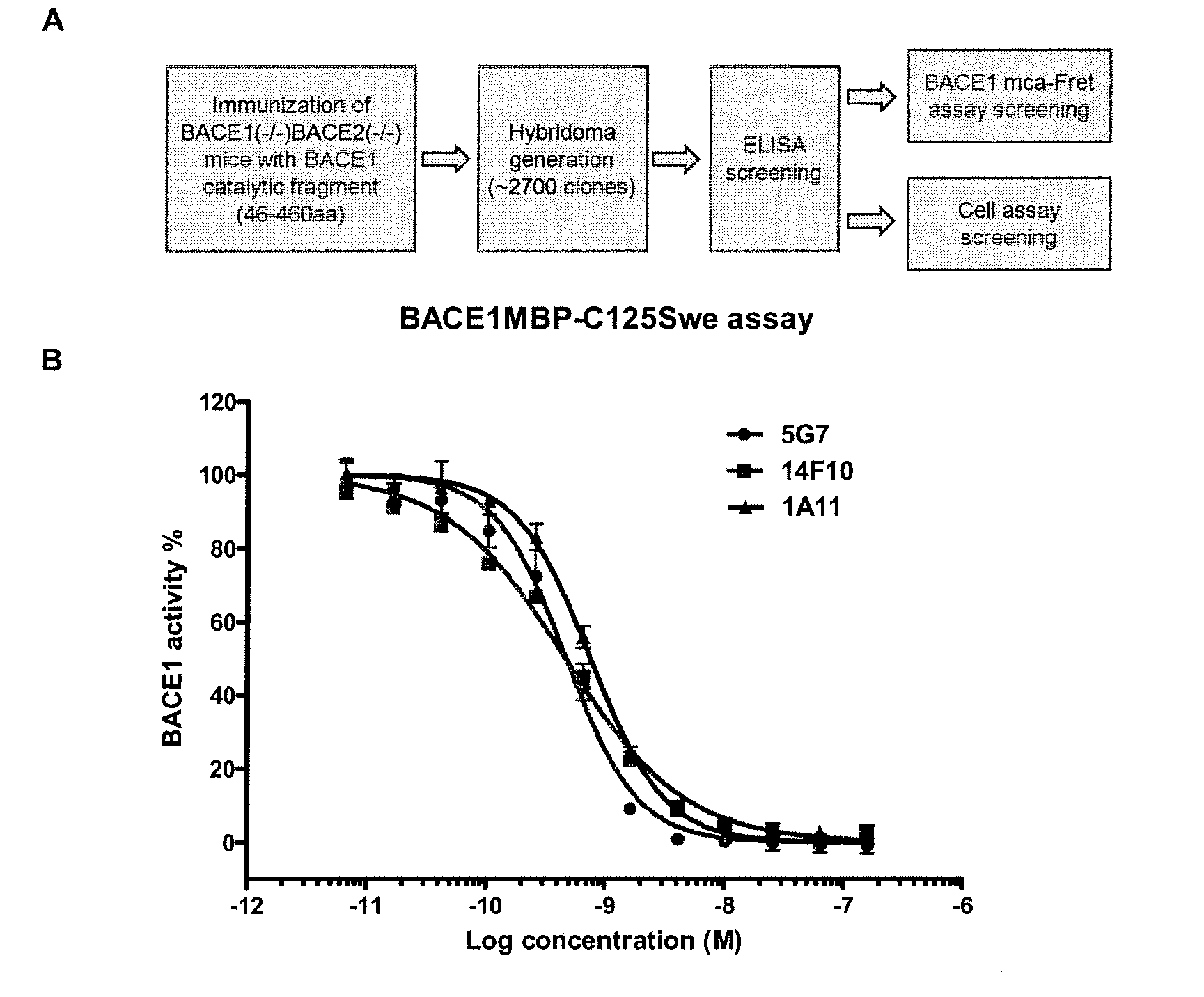
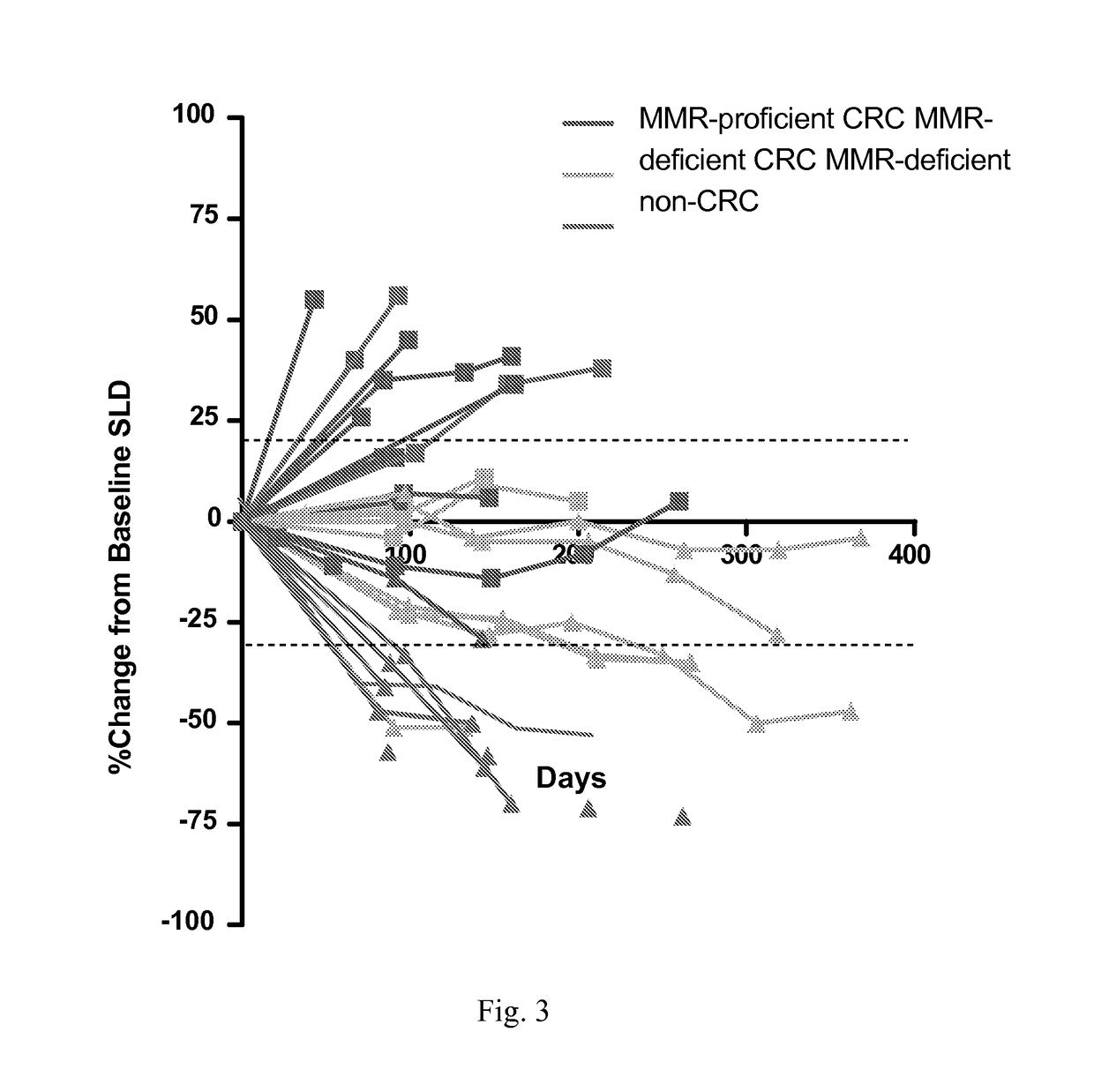
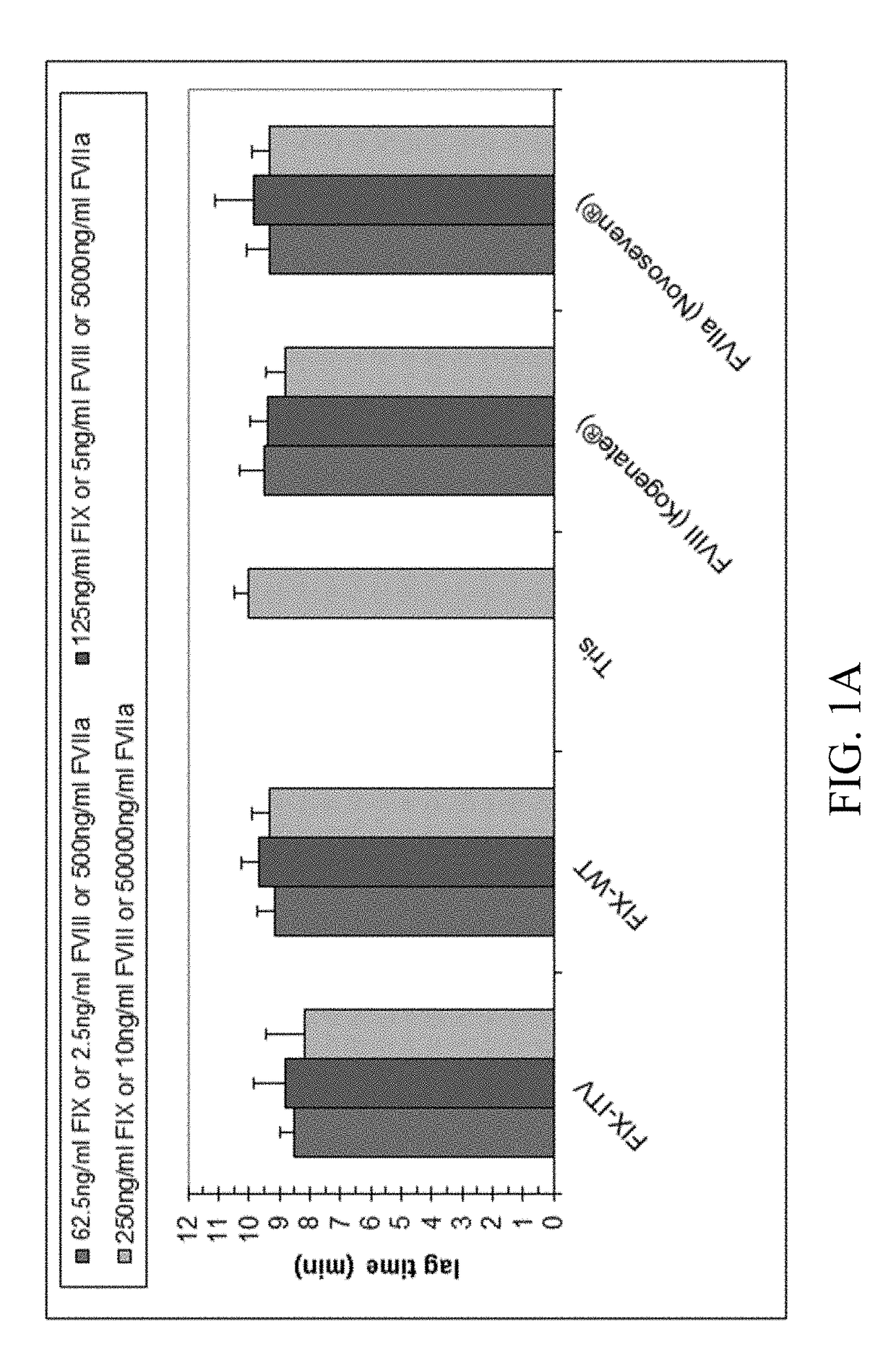
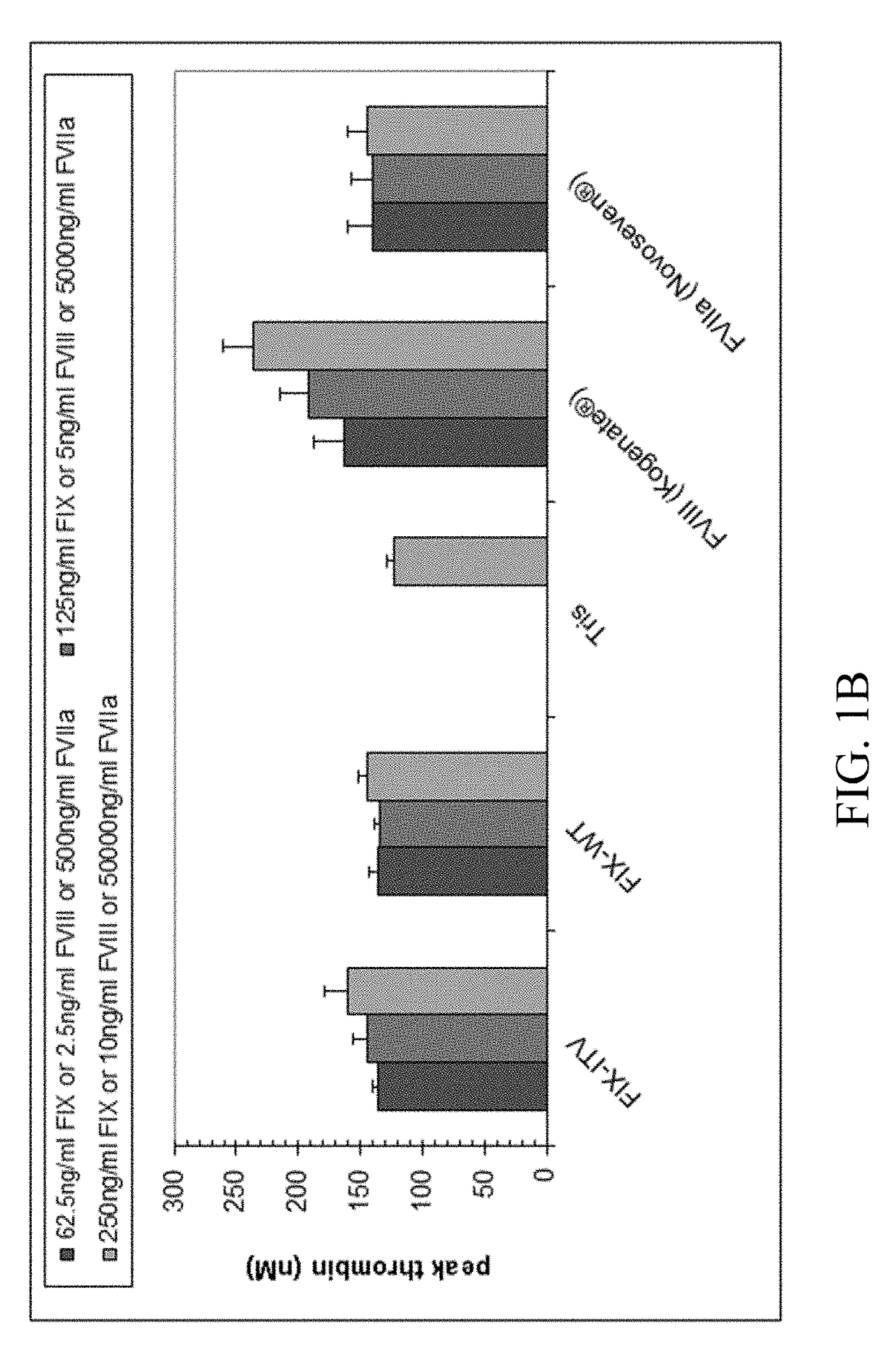
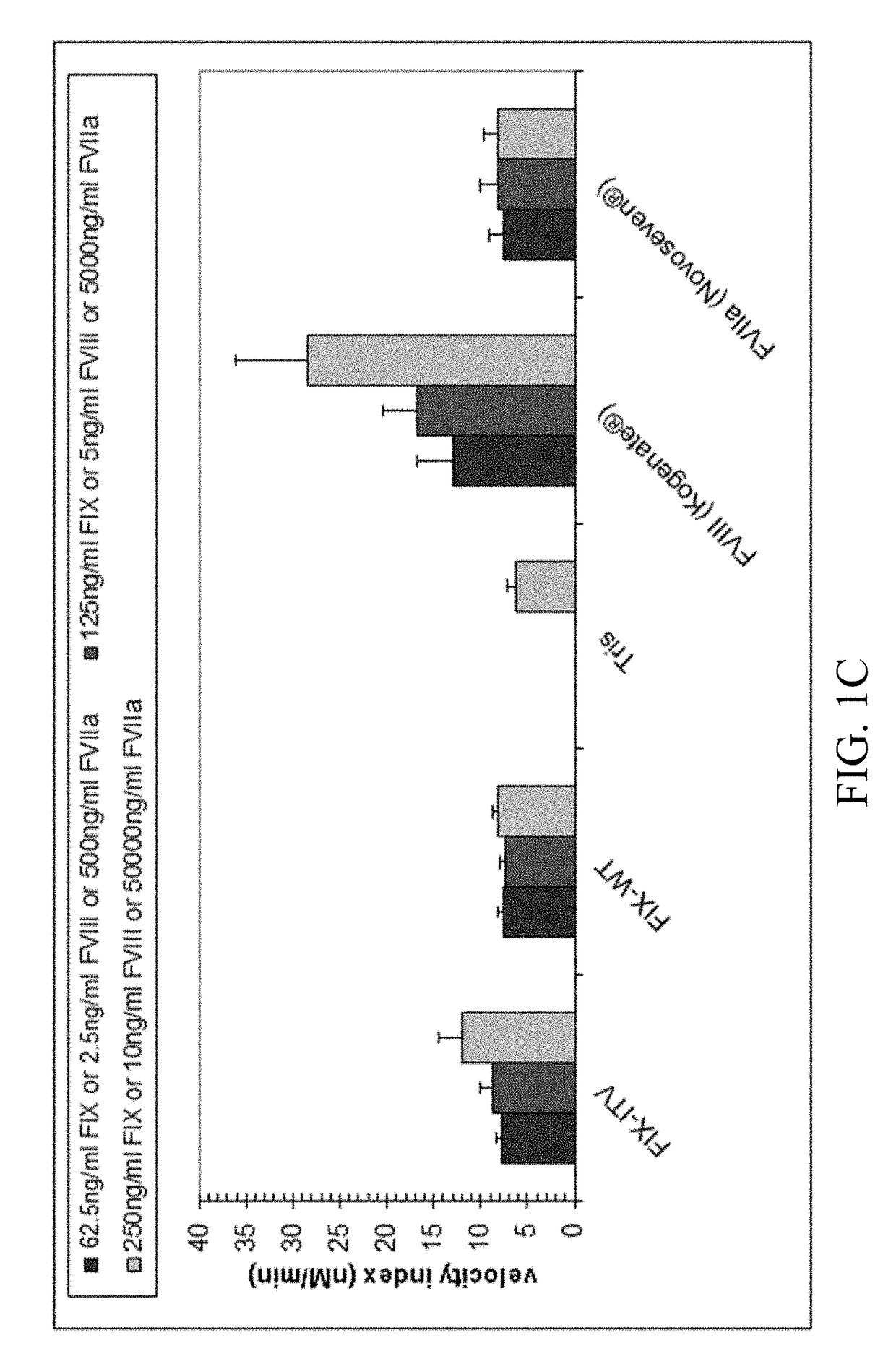
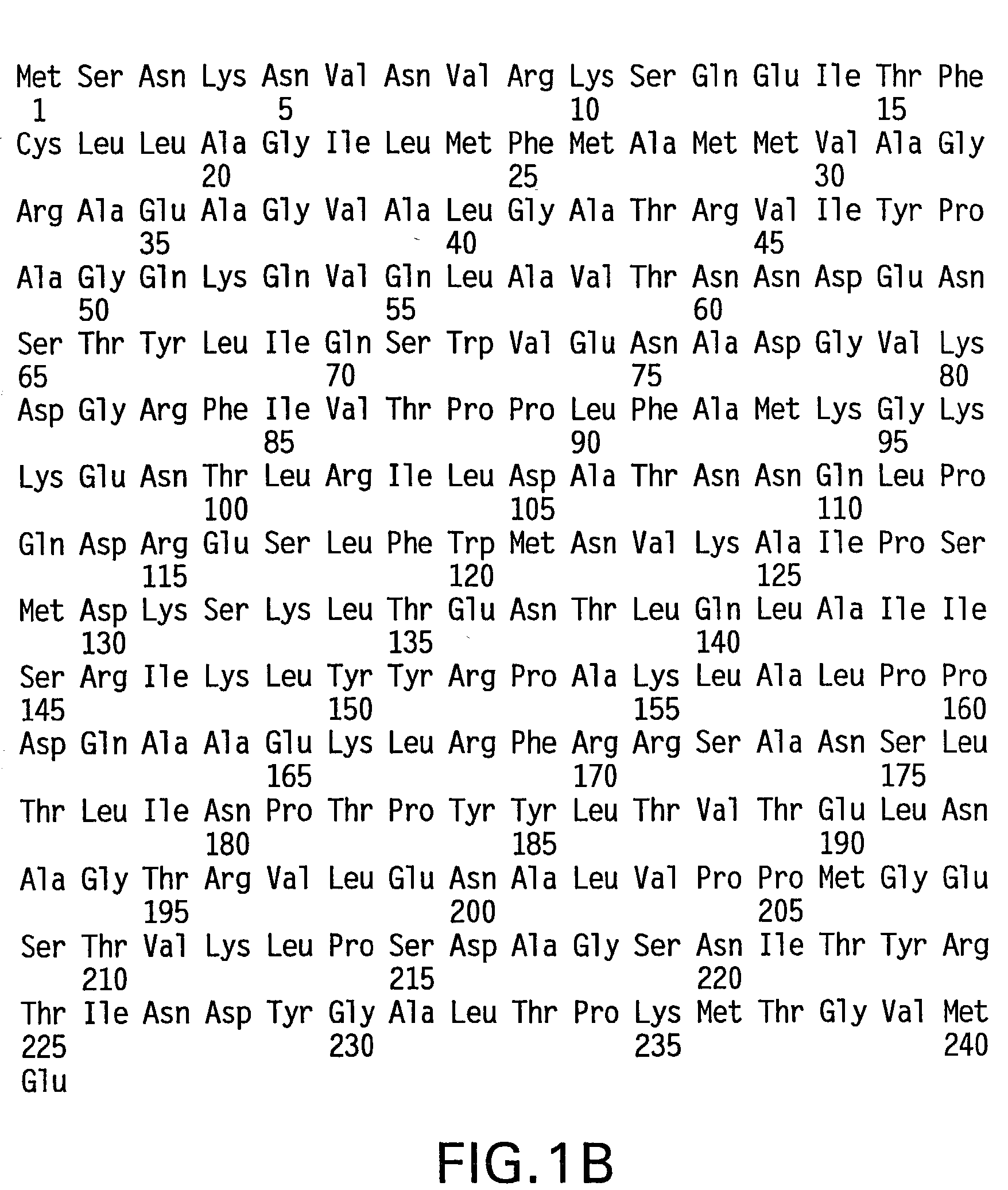Patents
Literature
79 results about "Inhibitory antibodies" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
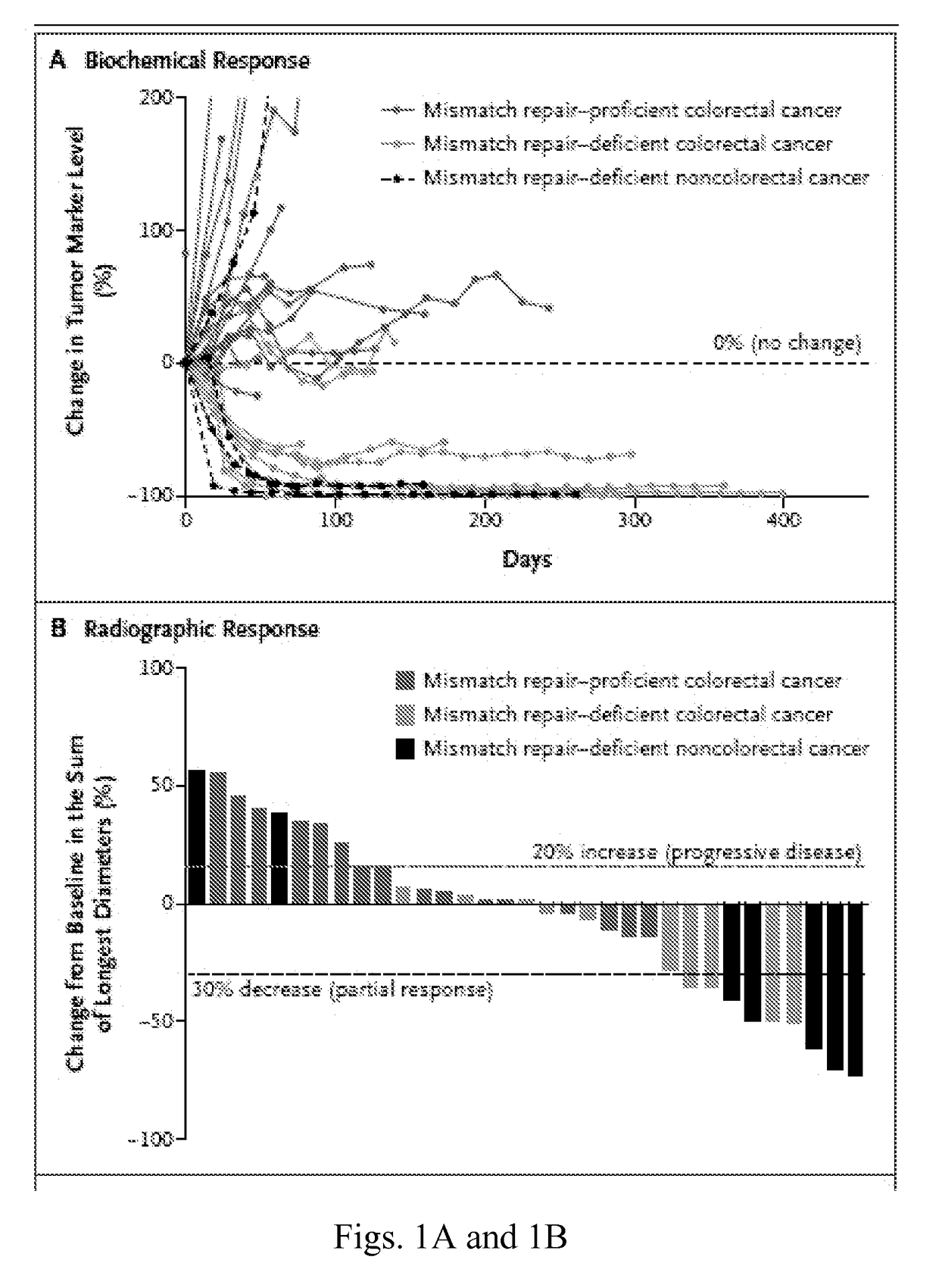
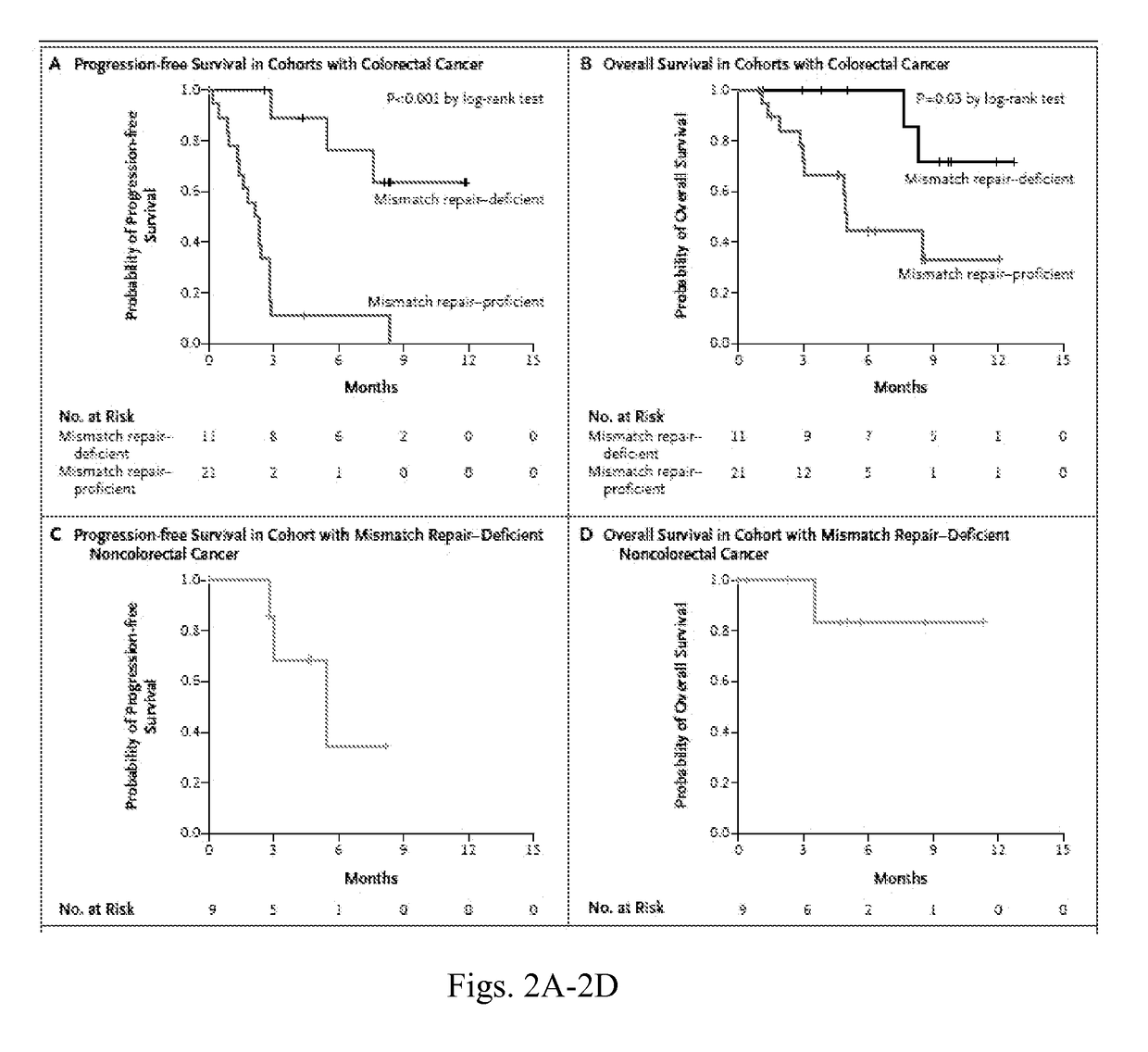
MCE Inhibitory Antibodies are monoclonal antibodies (mAbs) that have been extensively used in the hottest research areas, such as cancer, immunology and infection. These monoclonal antibodies (mAbs) bind monospecifically to certain cells or proteins, thus stimulating the immune system to attack the malignant tumor cells...
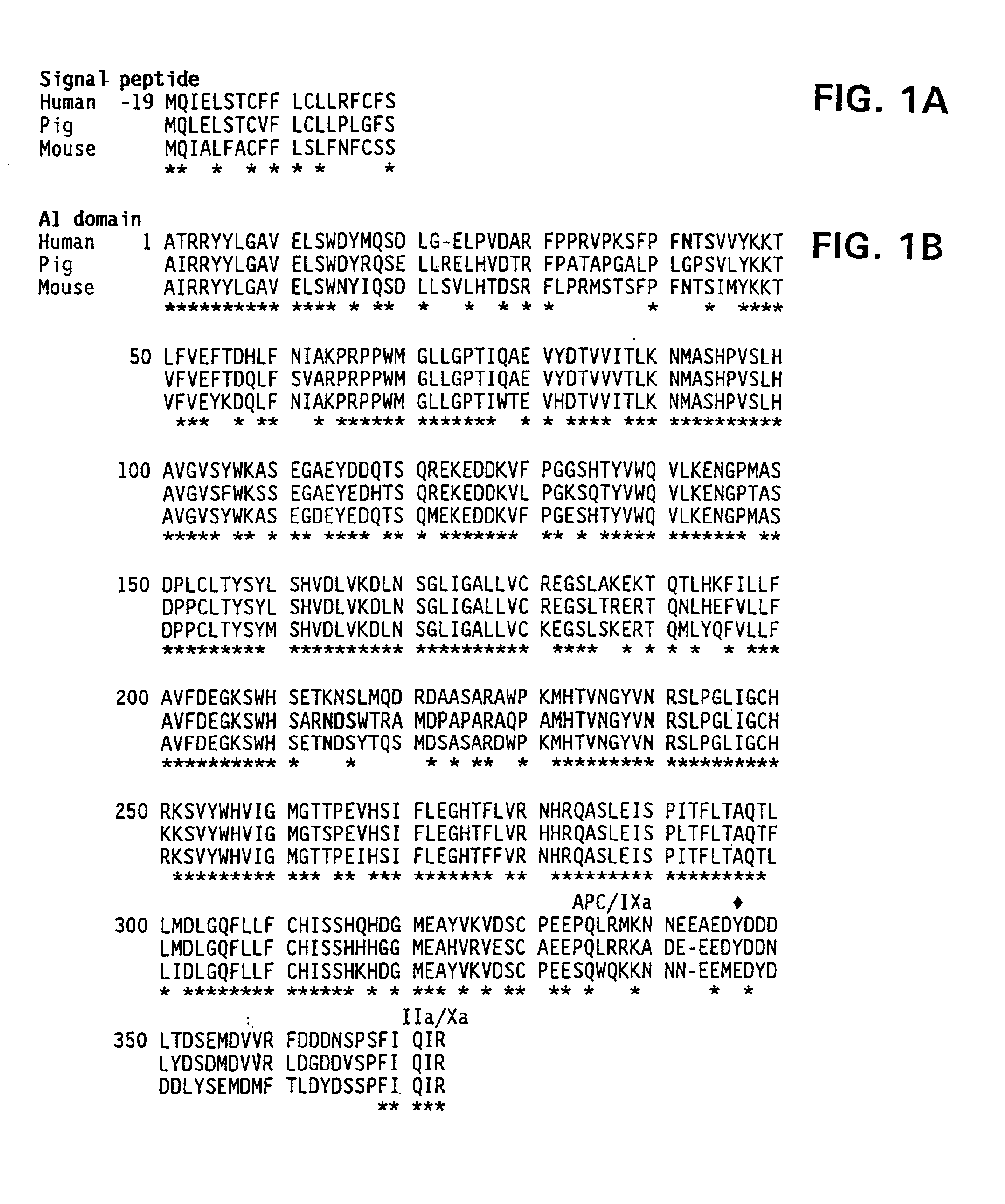
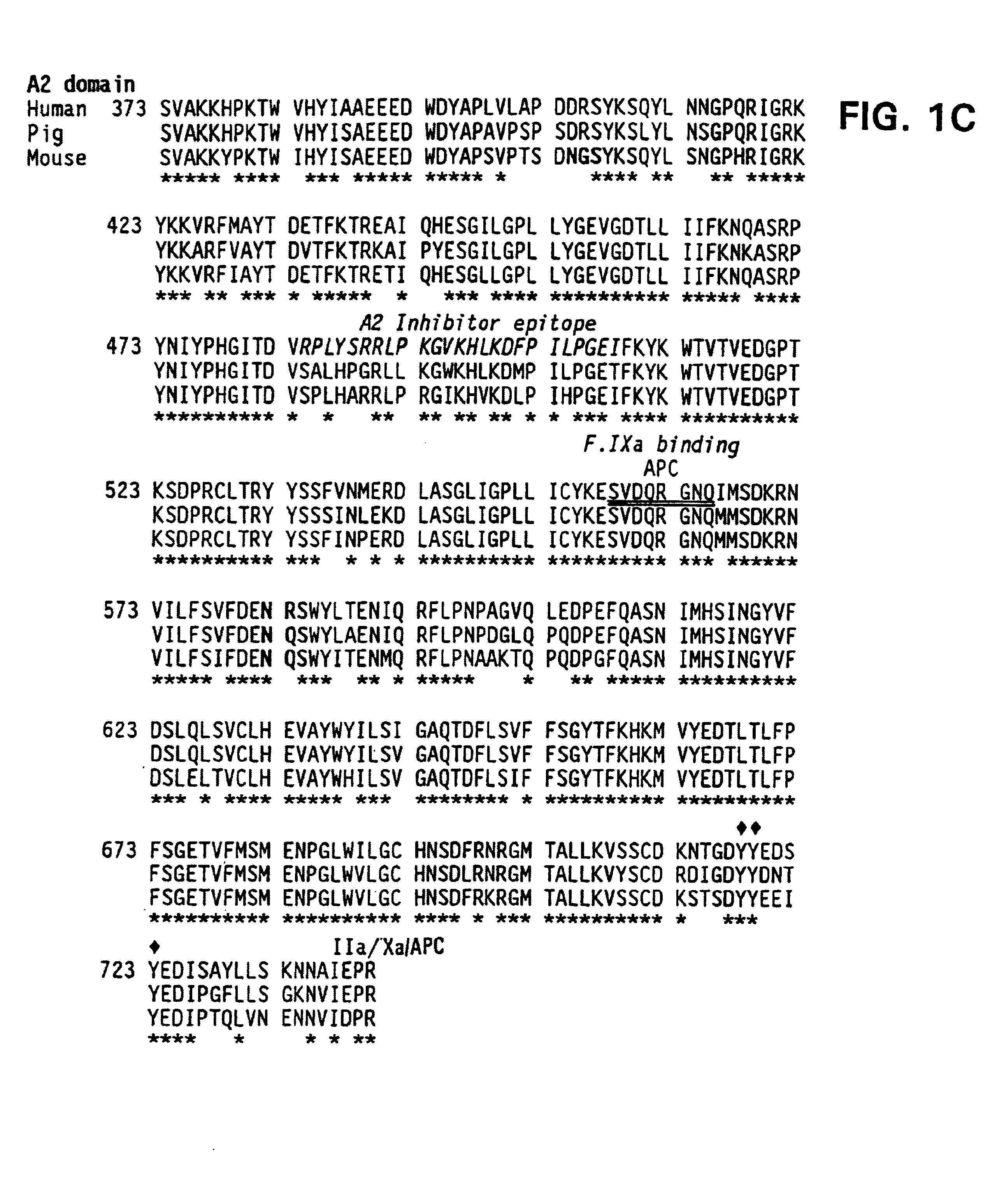
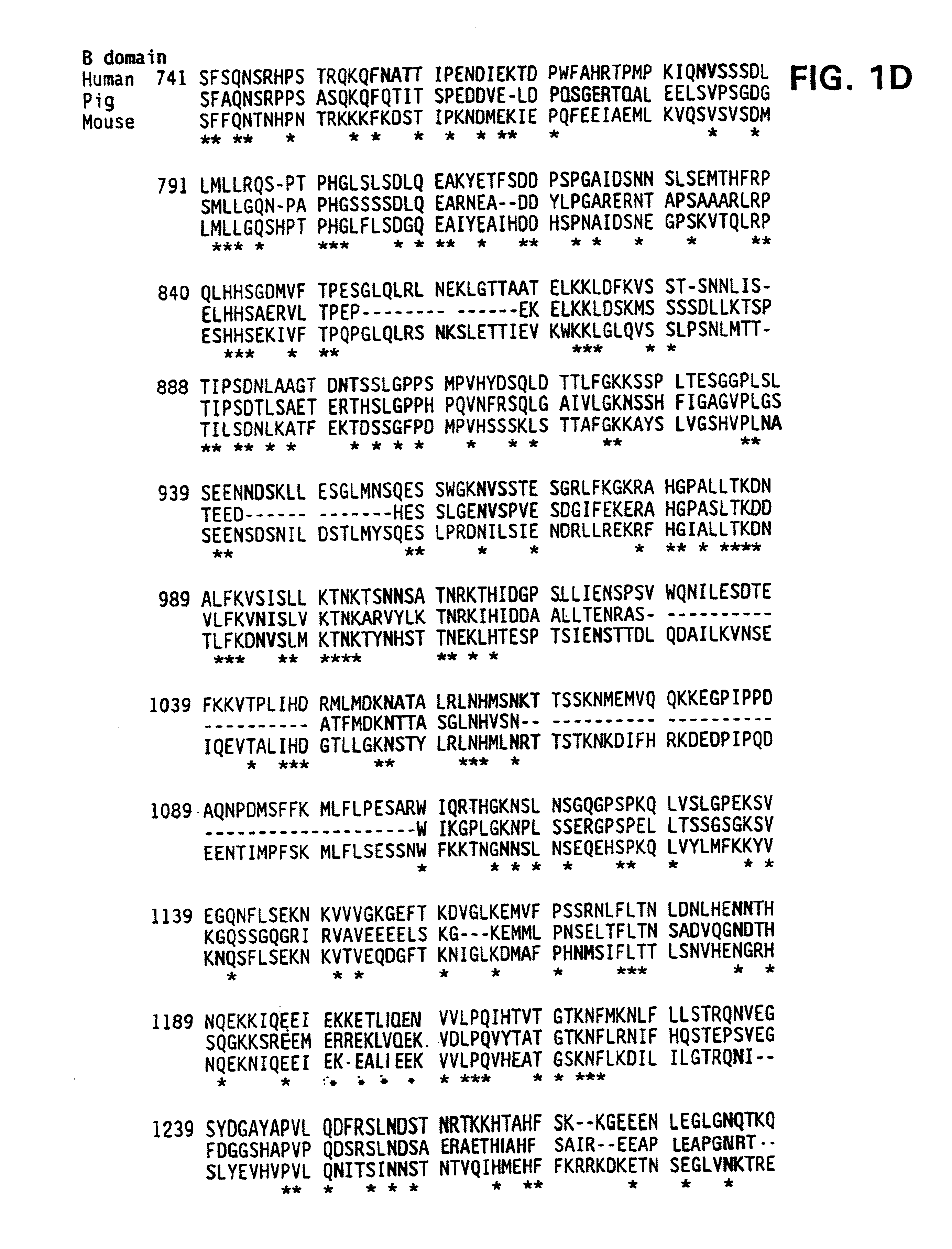
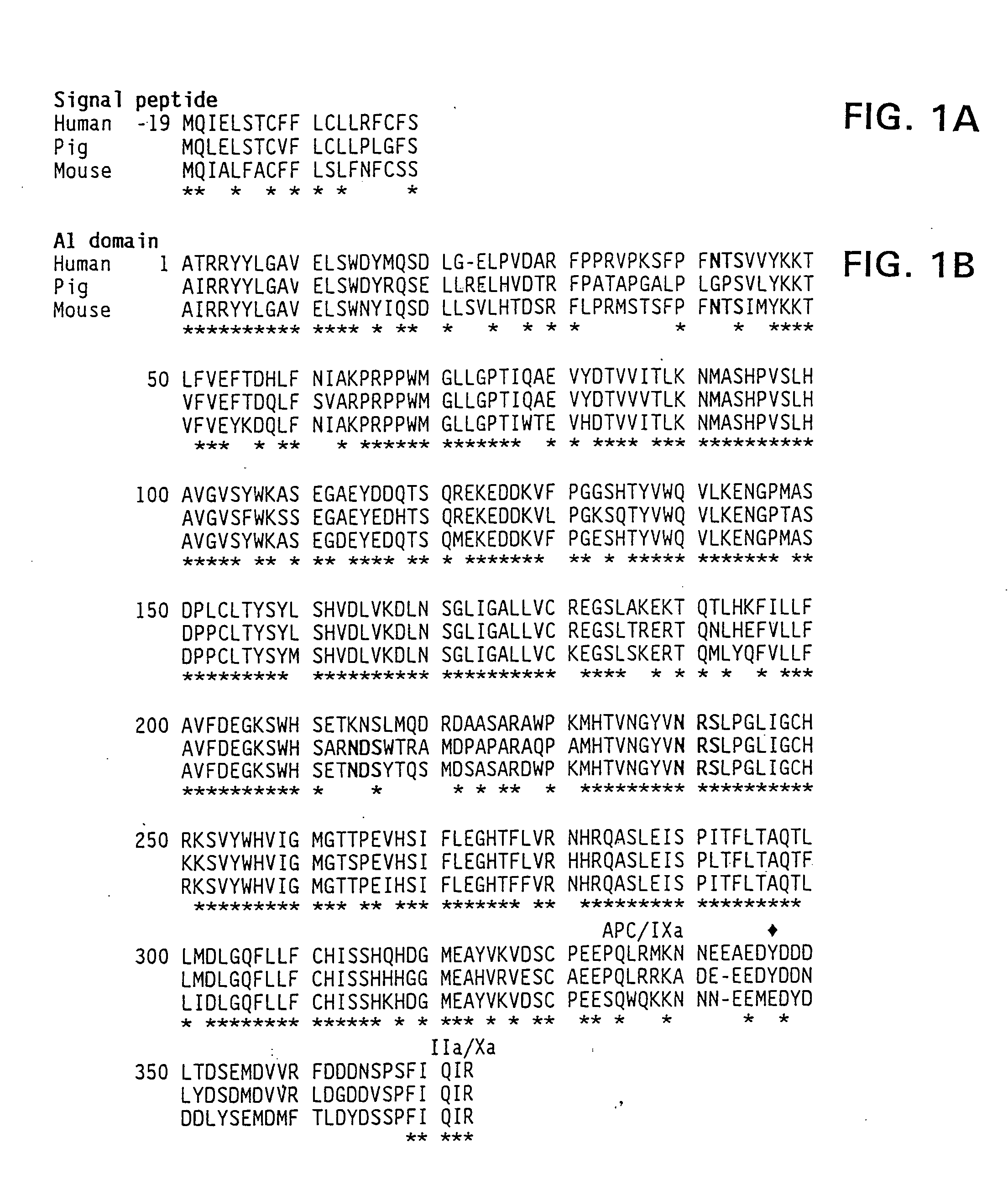
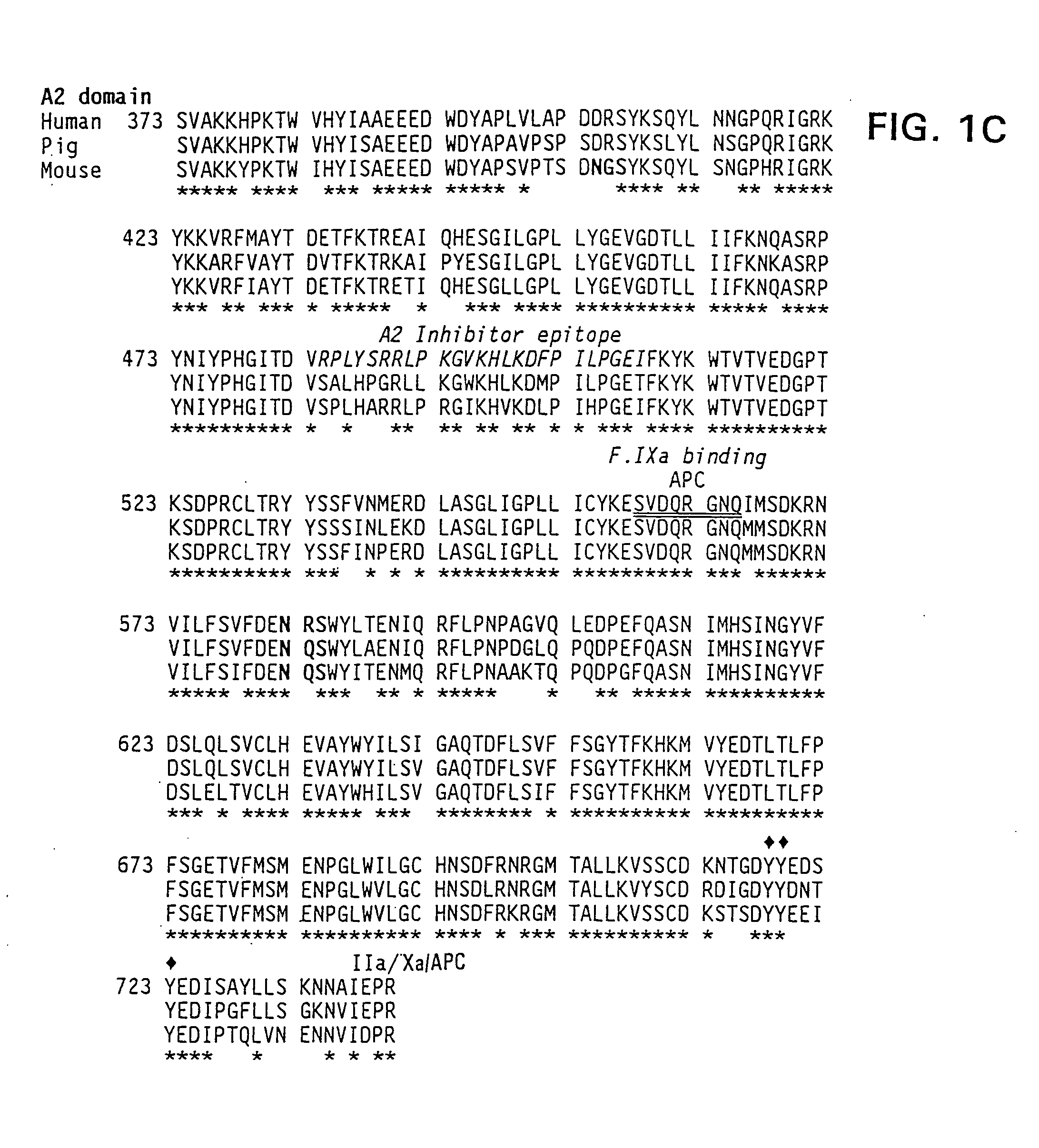
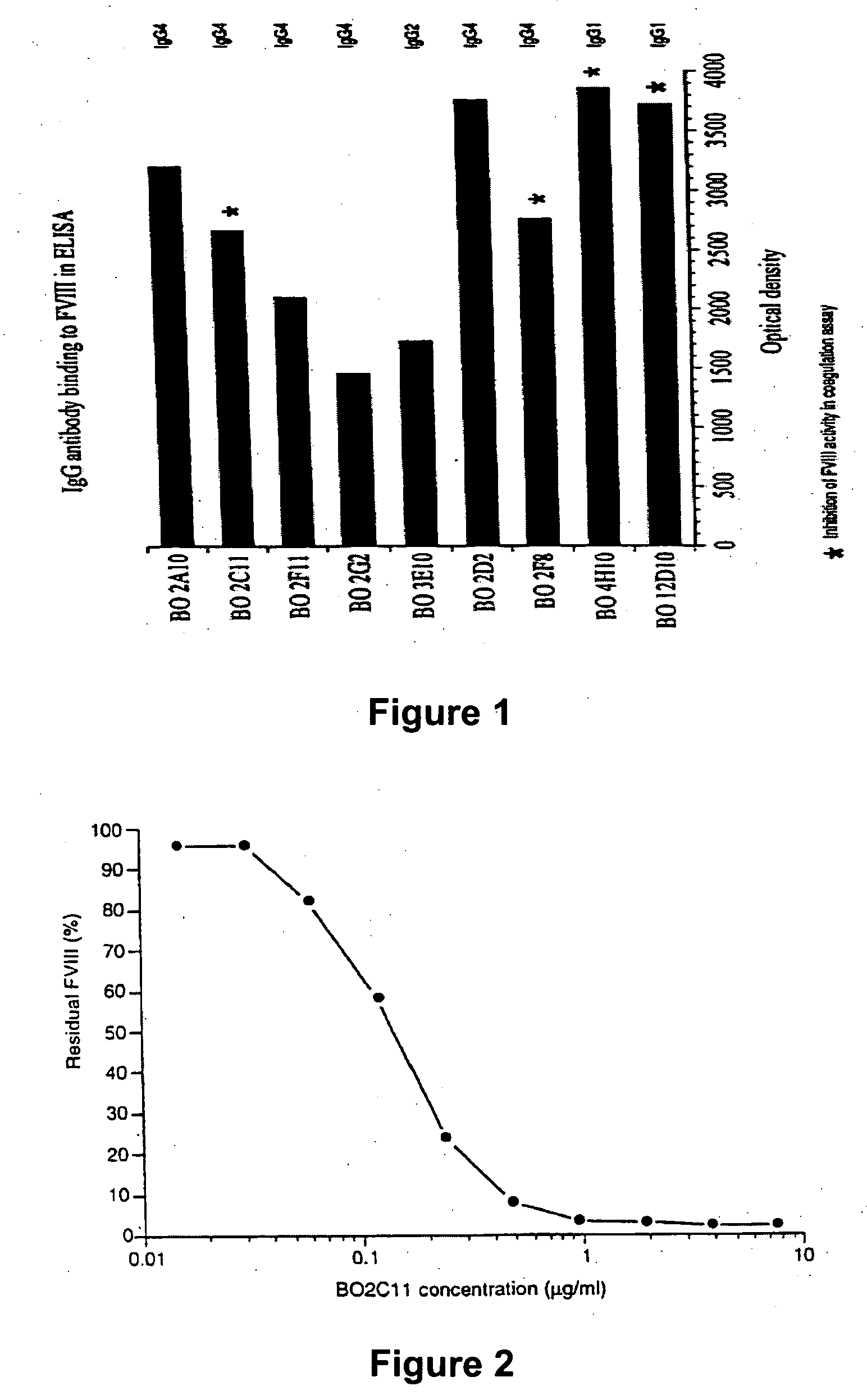
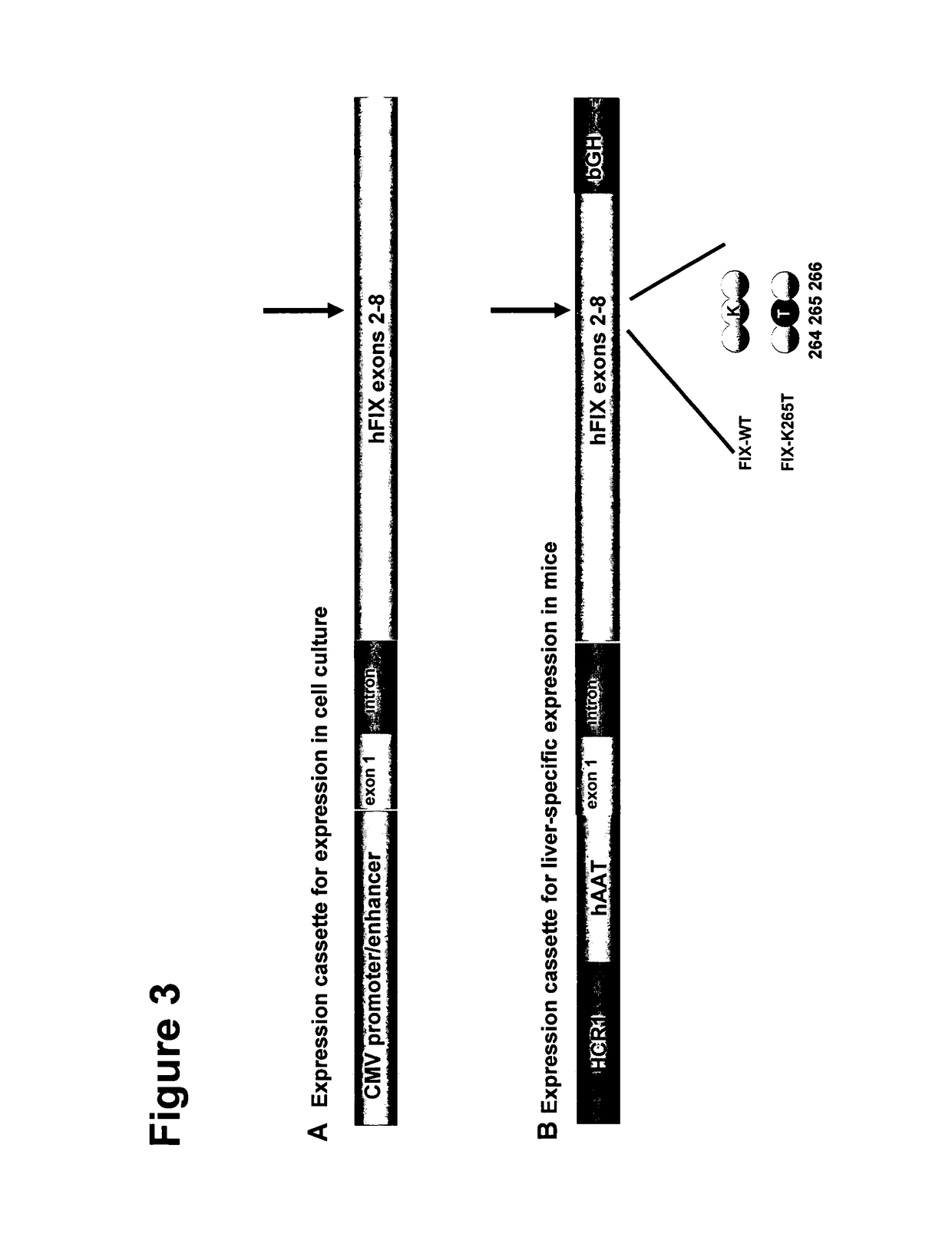
Modified fVIII having reduced immunogenicity through mutagenesis of A2 and C2 epitopes
InactiveUS20050123997A1Low immunogenicityImmunoglobulins against blood coagulation factorsFactor VIIEpitopeVaccine Immunogenicity
Specific amino acid loci of human fVIII interact with inhibitory antibodies of hemophilia patients after being treated with fVIII. Modified fVIII is disclosed in which the amino acid sequence is changed by multiple substitutions in human fVIII A2 and C2 domains. The modified fVIII is useful for hemophiliacs, either to avoid or prevent the action of inhibitory antibodies.
Owner:EMORY UNIVERSITY
Mutant proteins, high potency inhibitory antibodies and fimch crystal structure
InactiveUS20030199071A1Great functional inhibitory activityStrong inhibitory activityHydrolasesImmunoglobulins against bacteriaPassive ImmunizationsMutated protein
The present invention provides bacterial immunogenic agents for administration to humans and non-human animals to stimulate an immune response. It particularly relates to the vaccination of mammalian species, especially human patients, with variants of the E. coli FimCH protein that elicit antibodies that have better functional inhibitory activity than antibodies raised against wild type protein. In particular, such variants include mutations that promote a more open confirmation of the FimH protein, particularly in regions involved in mannose binding, to expose regions previously poorly exposed and mutations that abolish a significantly reduce mannose binding. In another aspect, the invention provides antibodies against such proteins and protein complexes that may be used in passive immunization to protect or treat pathogenic bacterial infections. The present invention also provides machine readable media embedded with the three-dimensional atomic structure coordinates of FimCH bound to mannose, and subsets thereof, and methods of using the crystal structure to provide candidate amino acid residues for mutation.
Owner:WASHINGTON UNIV IN SAINT LOUIS +1
Modified factor VIII
Owner:EMORY UNIVERSITY
Methods for treating cancer using GRM8 inhibitors
ActiveUS10683352B1Decreasing number and activityReduce tumor volumeOrganic active ingredientsPeptide/protein ingredientsAntiendomysial antibodiesOncology
The present invention provides methods for treating cancer using mGluR8 inhibitors, such as mGluR8 inhibitory antibodies and small molecules. The invention also features compositions containing mGluR8 inhibitors, methods of diagnosing patients with mGluR8-associated cancer, and methods of predicting the response of cancer in a subject to treatment with mGluR8 inhibitors.
Owner:FLAGSHIP PIONEERING INNOVATIONS V INC
Anti-cancer vaccine composition
ActiveUS20100196394A1Increases magnitudeSimple methodAntibacterial agentsOrganic active ingredientsAntigenSingle-Chain Antibodies
Combinations of anti-cancer vaccines and inhibitory antibodies to CD223 overcome immune suppression in cancer patients. The vaccines may be isolated antigens, groups of antigens, or whole tumor cells. The inhibitory antibodies may be generated in an animal by injection of fragments of CD223. Antibodies may be monoclonal antibodies or single chain antibodies or humanized antibodies.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Pharmaceutical compositions and methods for fabrication of solid masses comprising tnf-inhibiting antibodies
ActiveUS20150329631A1Minimize adverse effectsReduce volatilityPeptide/protein ingredientsMetabolism disorderIntestinal wallsWhite blood cell
Embodiments of the invention provide shaped masses comprising one or more drugs such as proteins or polypeptides and methods for forming such shaped masses. One embodiment provides a shaped mass comprising a drug such as a protein or polypeptide having a biological activity in the body of a mammal. The shaped mass is formed by compression of a precursor material comprising the drug wherein an amount of biologically active drug in the mass is a preserved above a minimum level. Drugs which may be incorporated into the shaped mass may include one or more glucose regulating proteins such as insulin, incretins; and immunoglobulins such as TNF-inhibiting antibodies or interleukin neutralizing antibodies. Embodiments of the shaped mass may be incorporated into a tissue penetrating member which is inserted into the intestinal wall allowing for the oral delivery of proteins and peptides which would otherwise be degraded in the intestinal tract.
Owner:RANI THERAPEUTICS
Nucleic acid molecules encoding modified factor VIII proteins, expression products, and methods of making the same
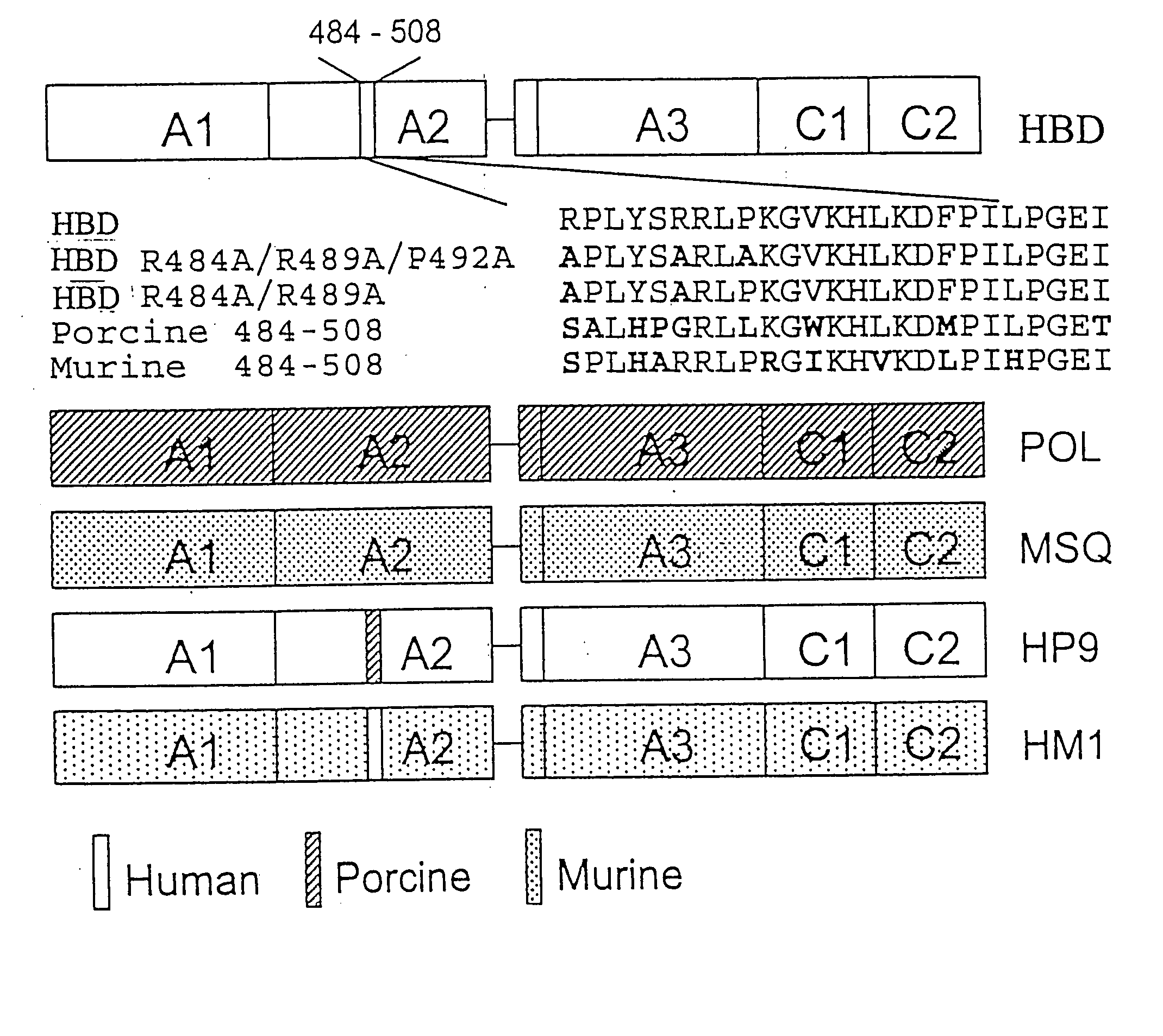
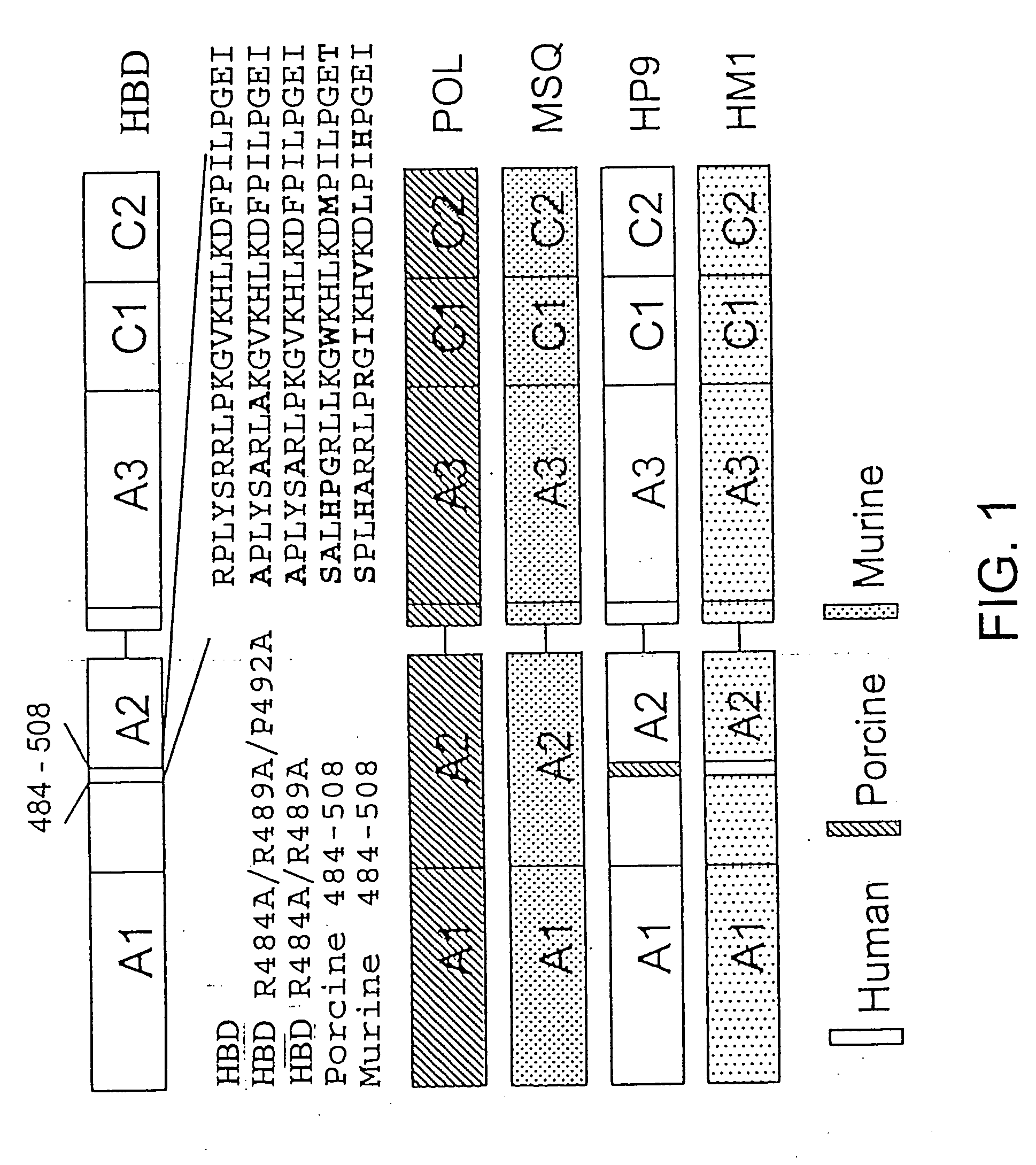
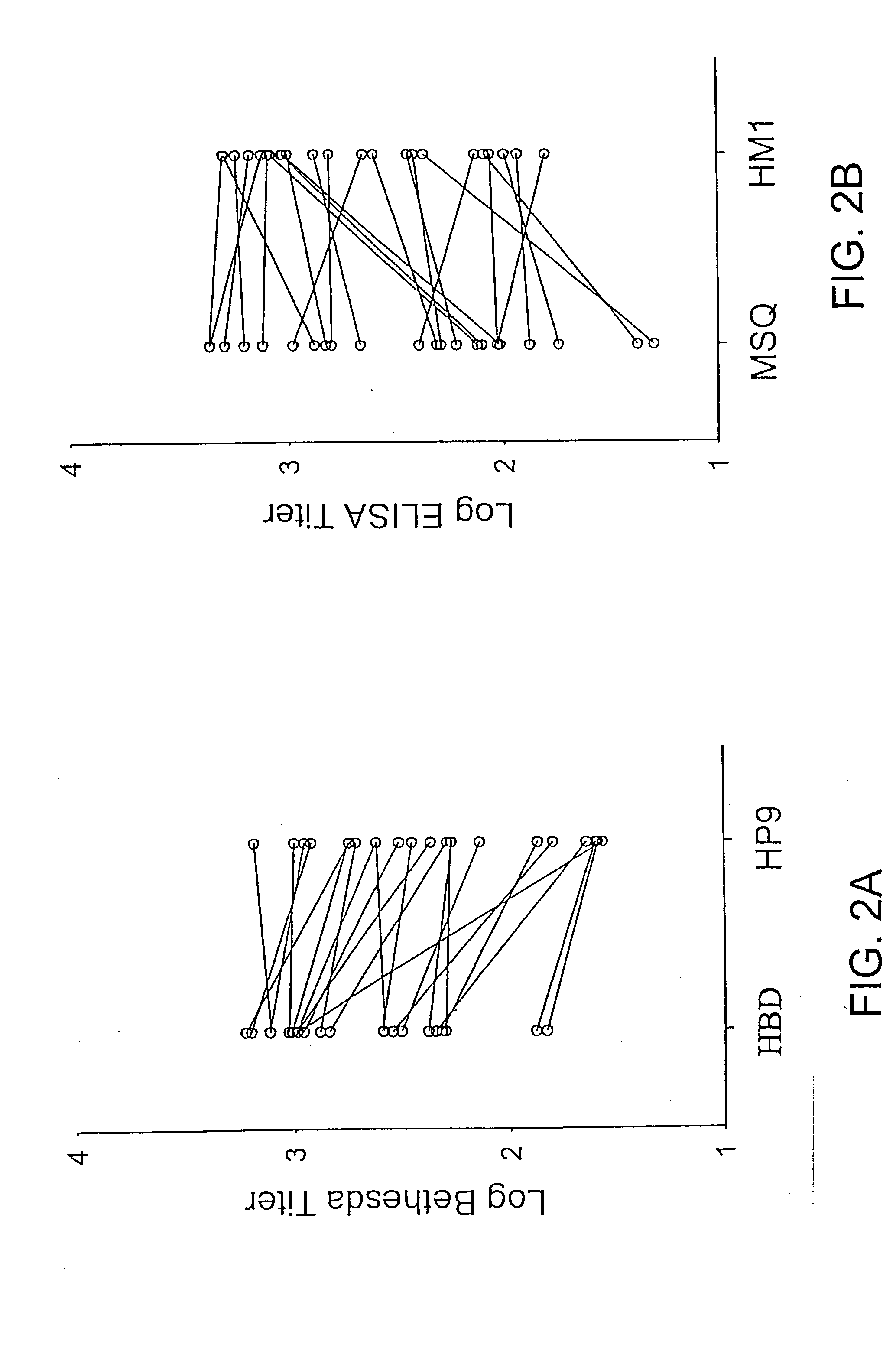
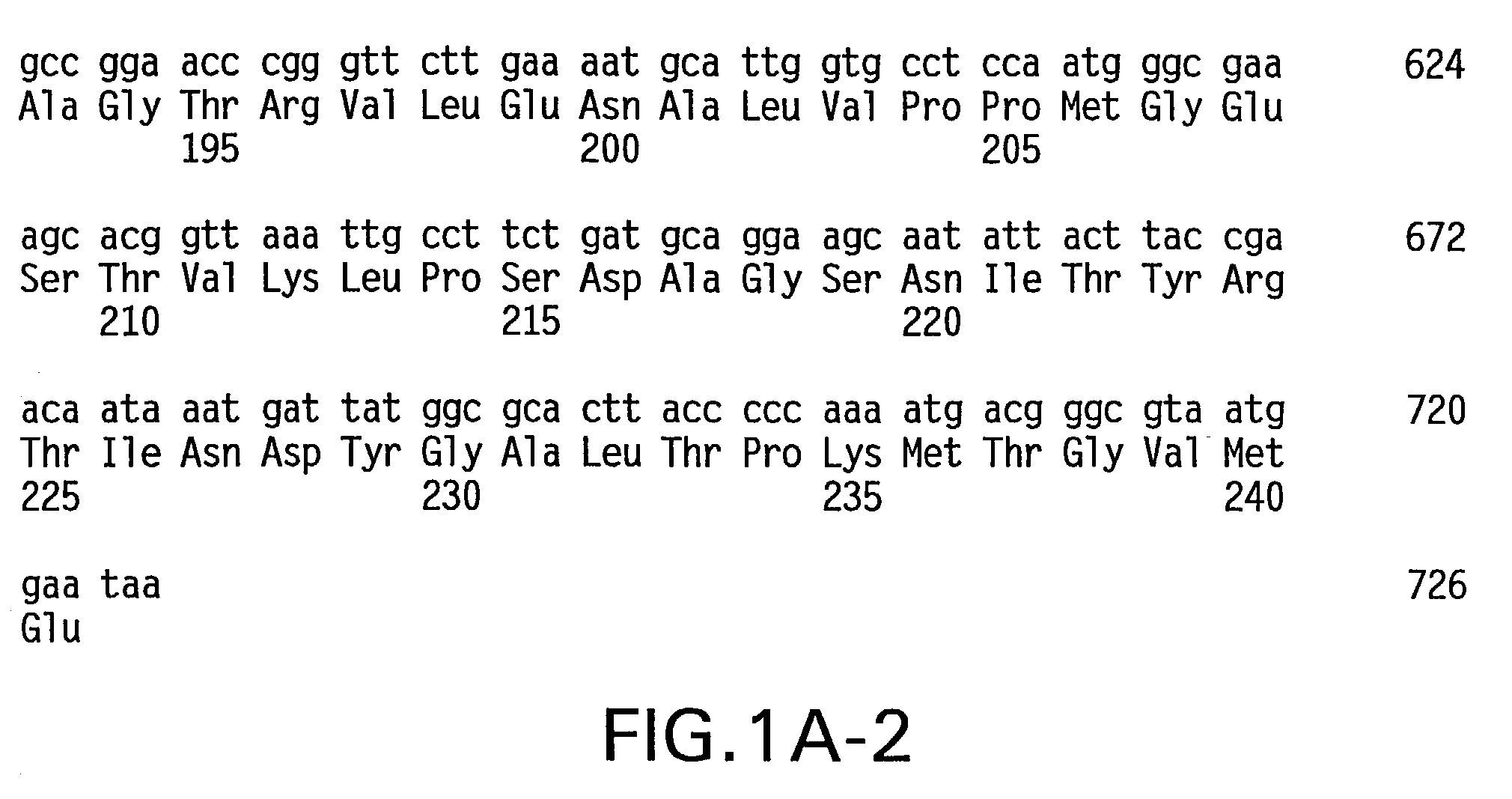
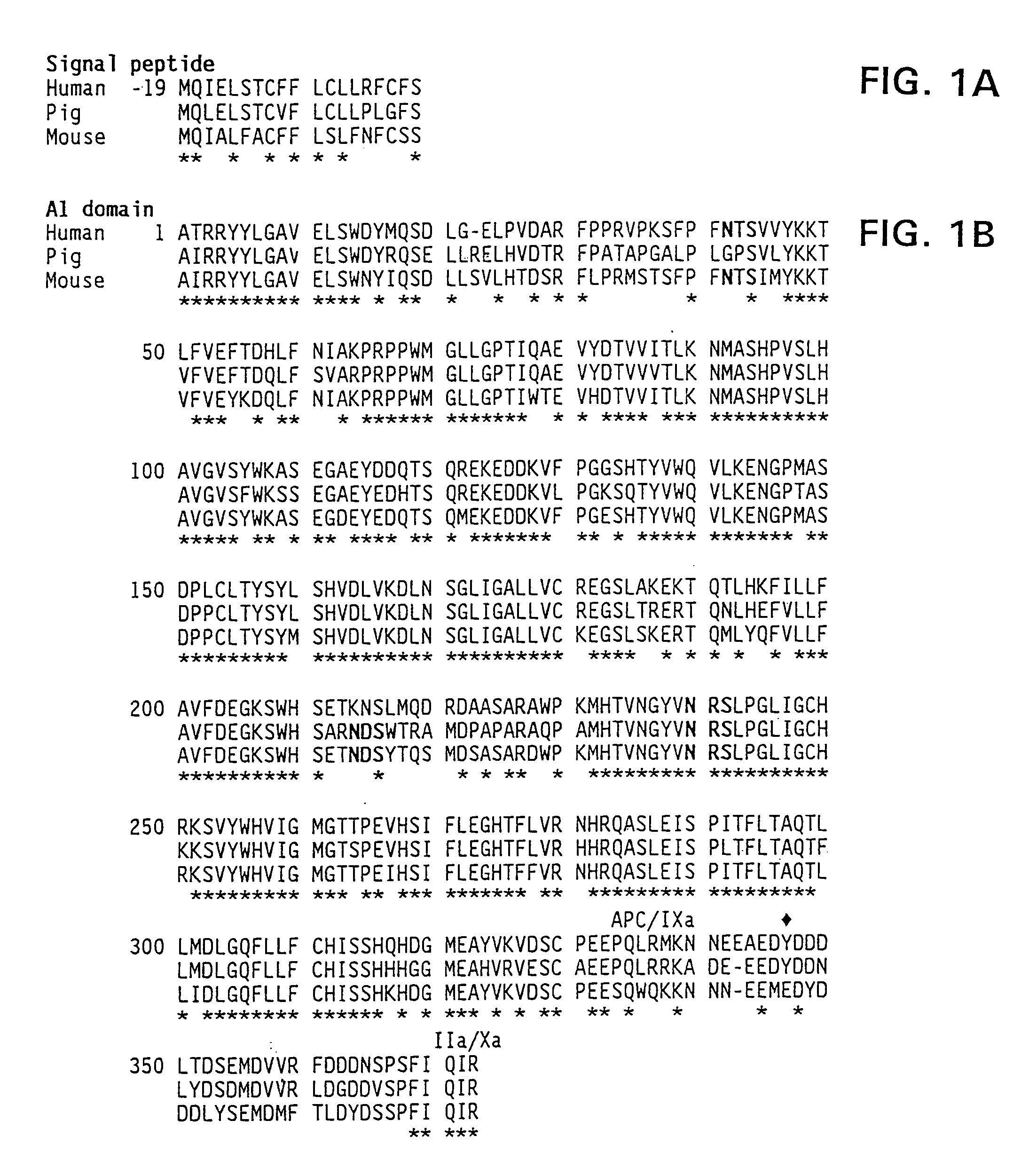
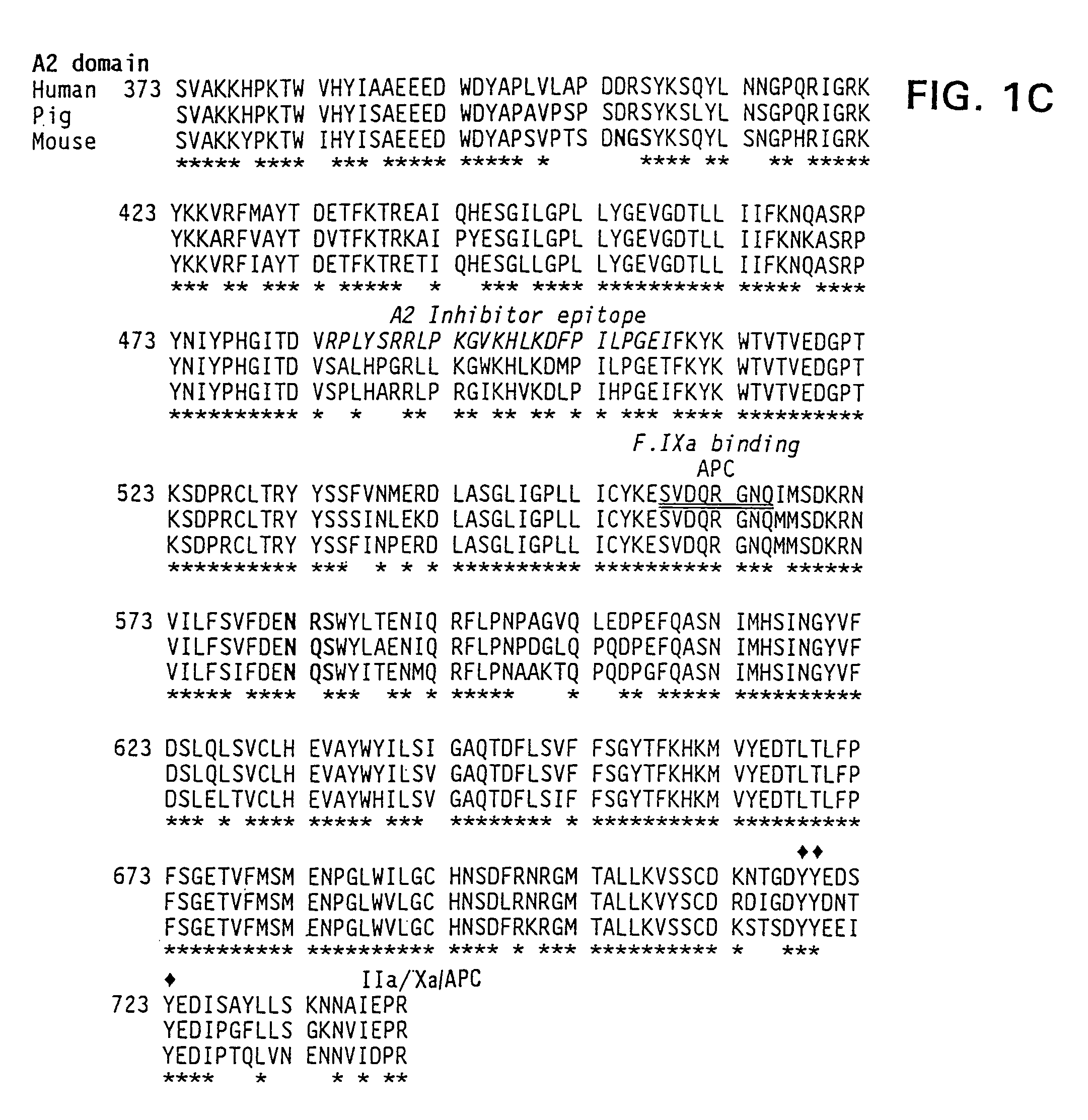
Specific amino acid loci of human factor VIII interact with inhibitory antibodies of hemophilia patients who have developed such antibodies after being treated with factor VIII. Modified factor VIII is disclosed in which the amino acid sequence is changed by a substitution at one or more amino acids of positions 484-508 of the A2 domain. The modified factor VIII is useful as a clotting factor supplement for hemophiliacs.
Owner:GENERAL ELECTRIC CO +1
Killing cell for high-efficiency and stable expression of antibodies and use thereof
PendingCN107523545AAntibacterial agentsAntibody mimetics/scaffoldsAntigen Binding FragmentAutoimmune disease
The invention relates to a killing cell for high-efficiency and stable expression of antibodies and a use thereof. Specifically, the invention provides the transgenic killing cell; a genome of the killing cell is stably integrated with an expression cassette containing an encoding sequence of human Fc segment-containing antibodies, or containing an encoding sequence of chimeric antigen receptors and inhibitory antibodies or activated type antibodies, and both ends of the expression cassette contain inverted terminal repeated sequences of a transposon. The killing cell can stably express the human Fc segment-containing antibodies or the chimeric antigen receptors and antigen-binding fragments derived from interested antibodies with high level while maintaining cell killing toxic effect. In addition, for preventing systemic toxicity and autoimmune diseases caused by over expression of antibodies due to in-vivo continuous proliferation of immune cells for stable expression of the antibodies, a molecular braking system is also introduced. With use of monoclonal antibody agents appearing on the market, the killing cell integrated with the antibody expression cassette can be quickly eliminated, and the safety of treatment is effectively improved.
Owner:SHANGHAI CELL THERAPY RES INST +1
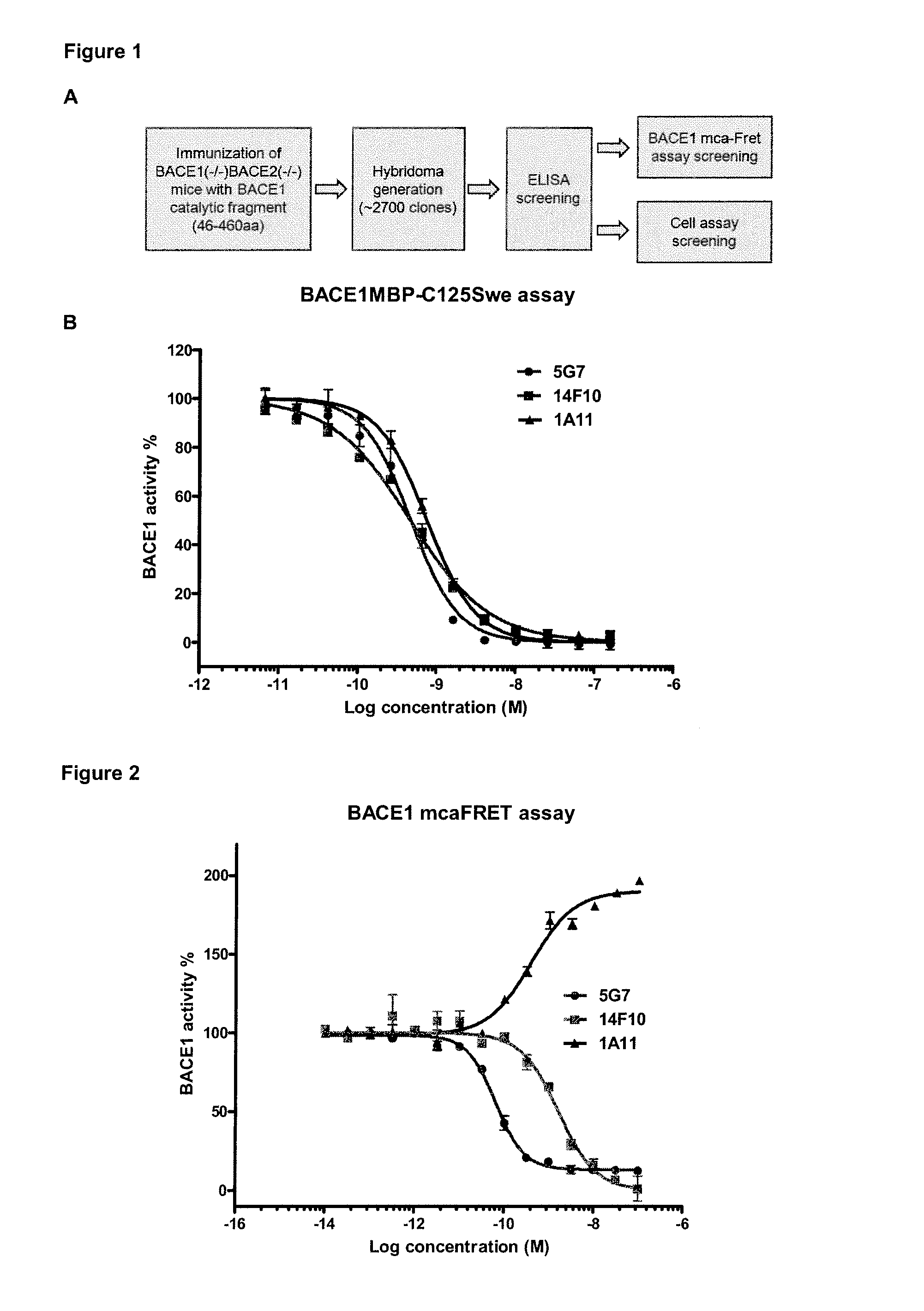
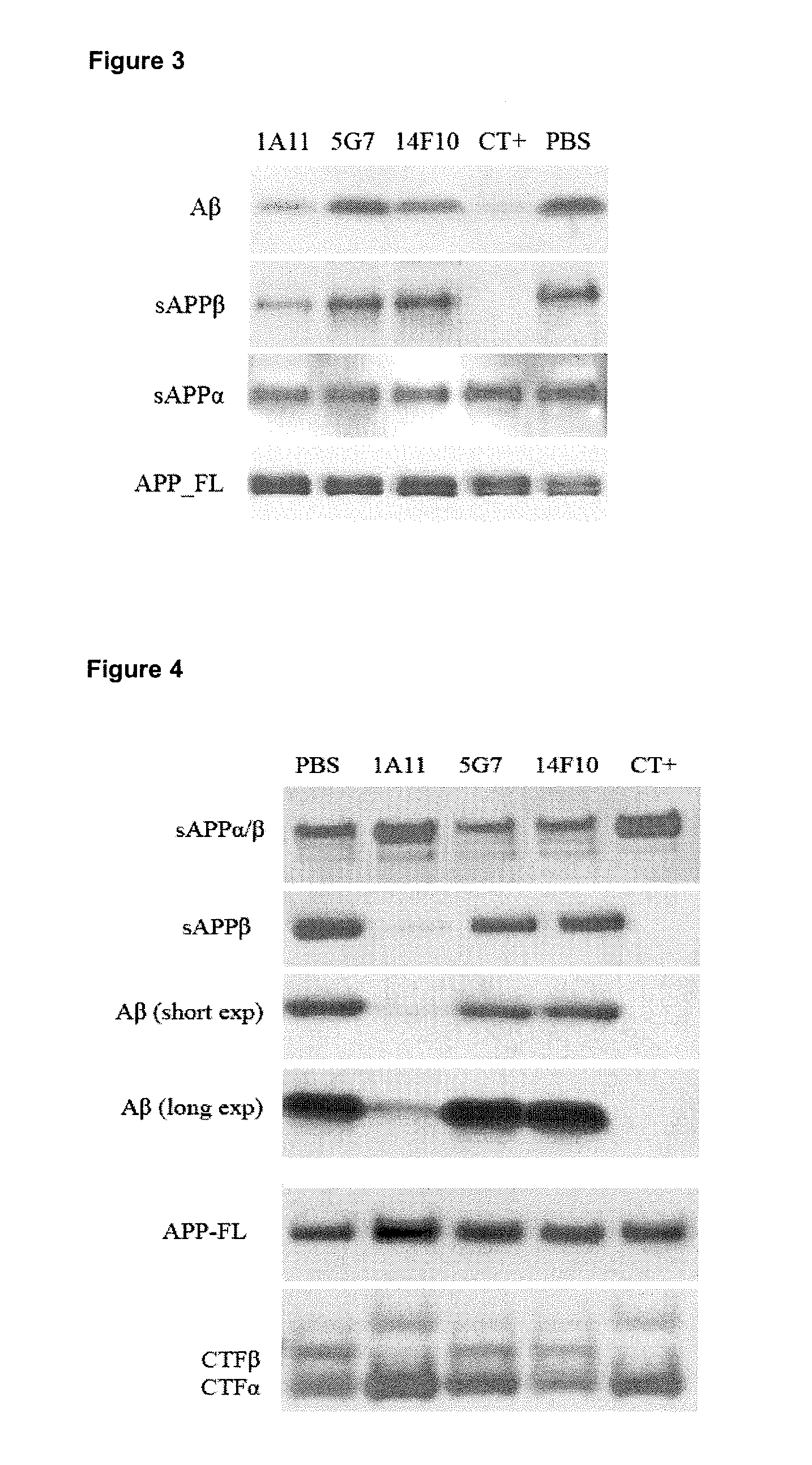
Bace1 inhibitory antibodies
The present invention relates to antibodies with a specificity for BACE1. More specifically, the invention provides monoclonal antibodies that bind to BACE1 and are capable of inhibiting the activity of BACE1 and methods producing these antibodies. The antibodies can be used for research and medical applications. Specific applications include the use of BACE1-specific antibodies for the treatment of Alzheimer's disease.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +1
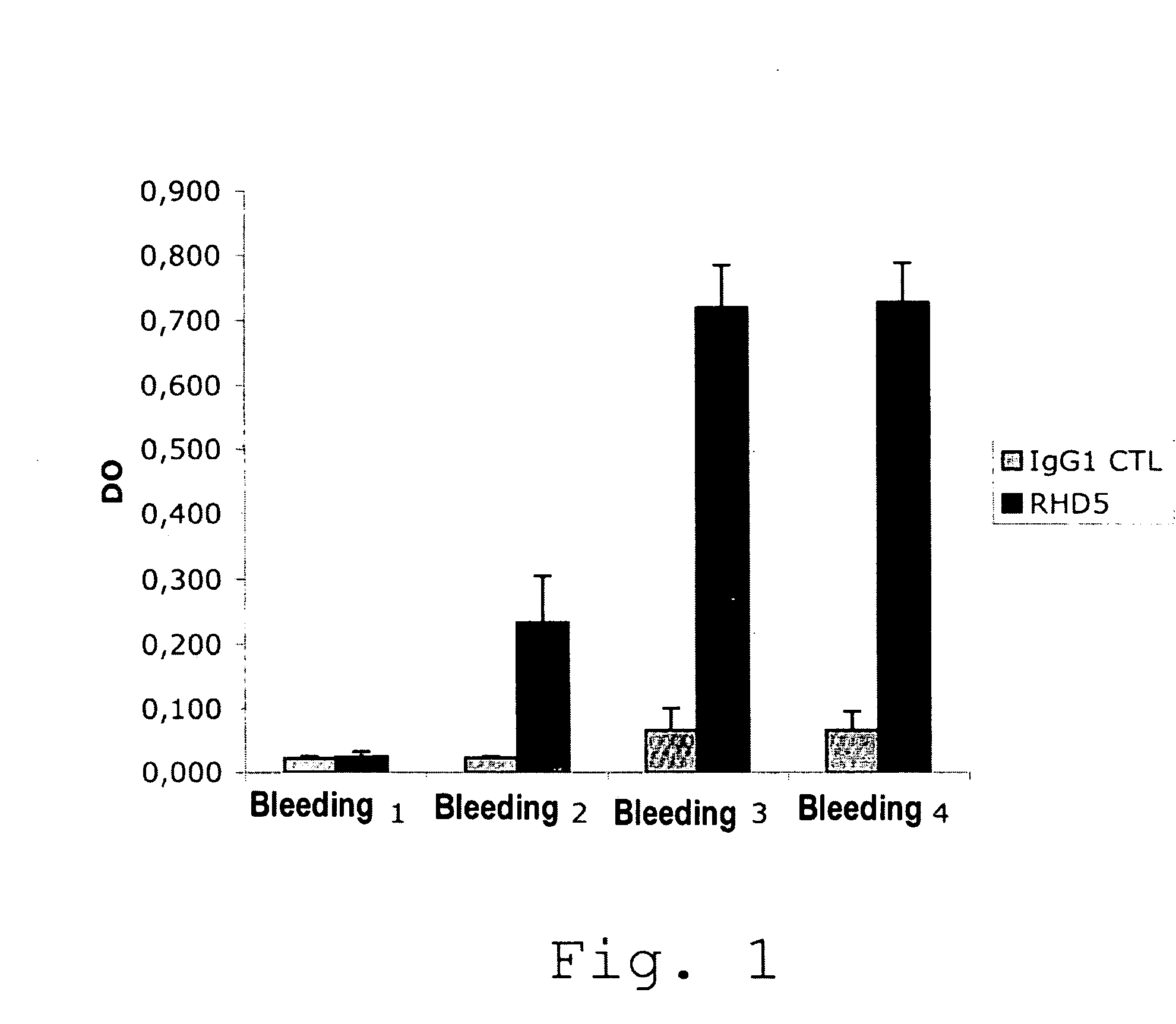
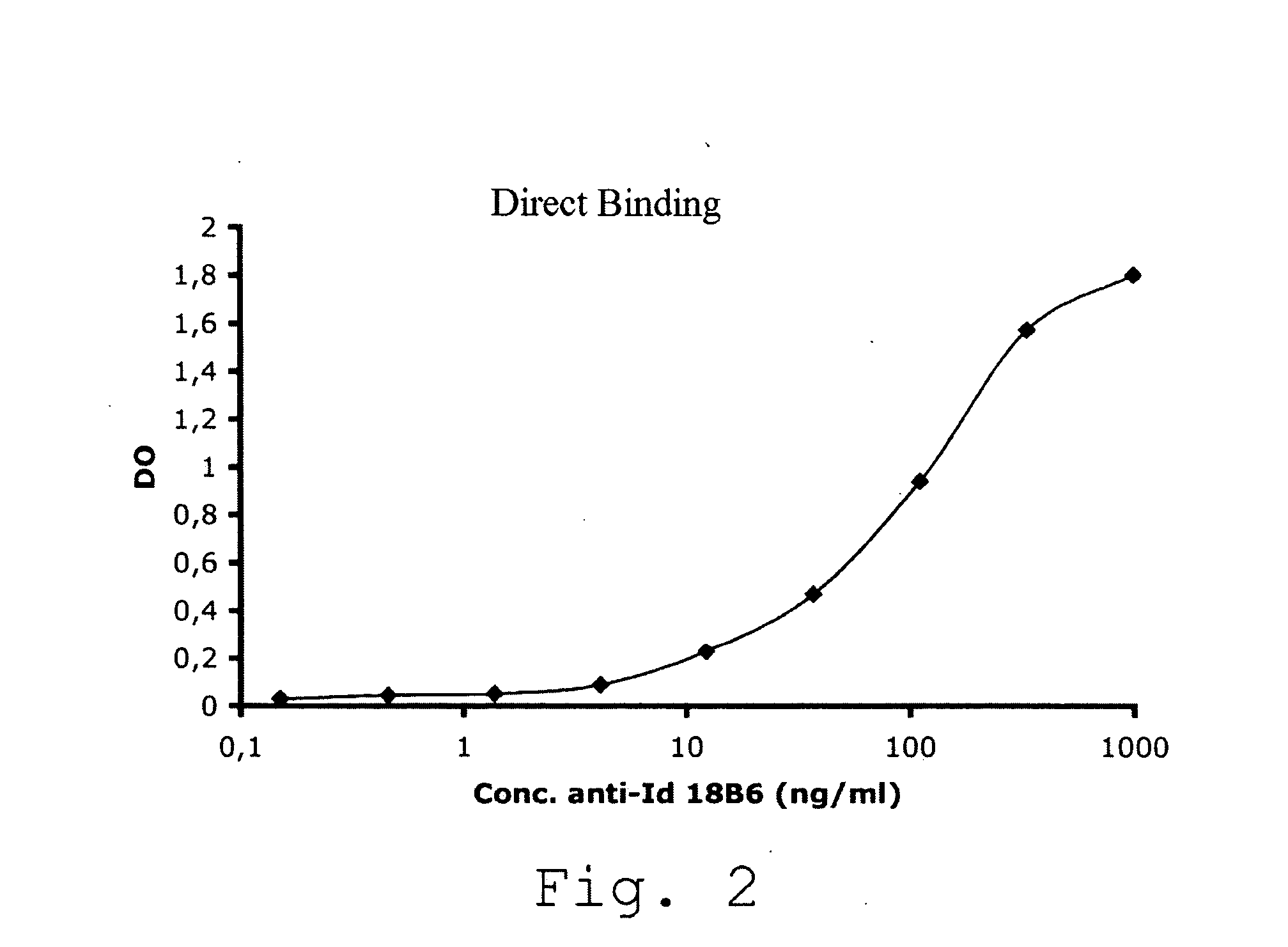
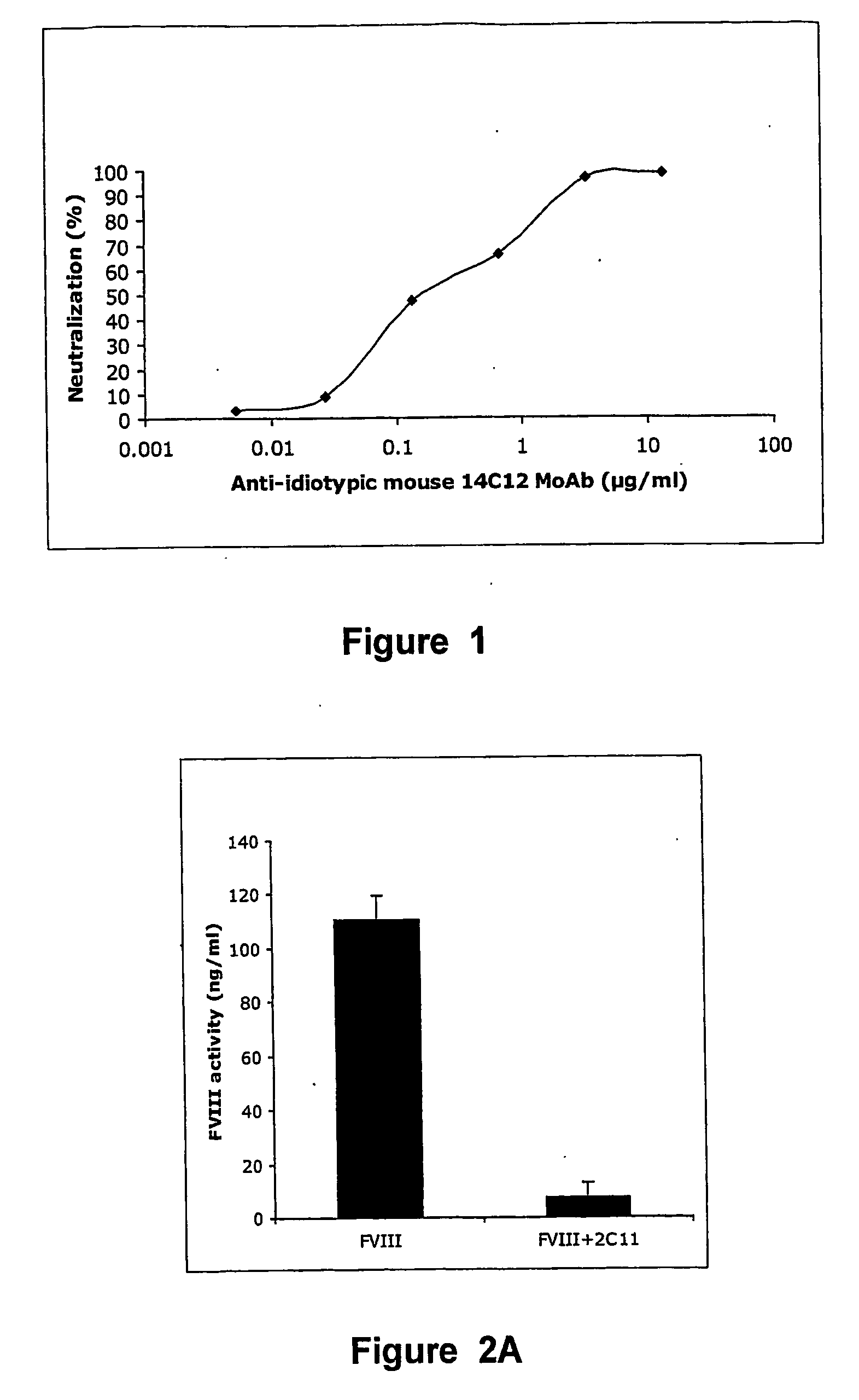
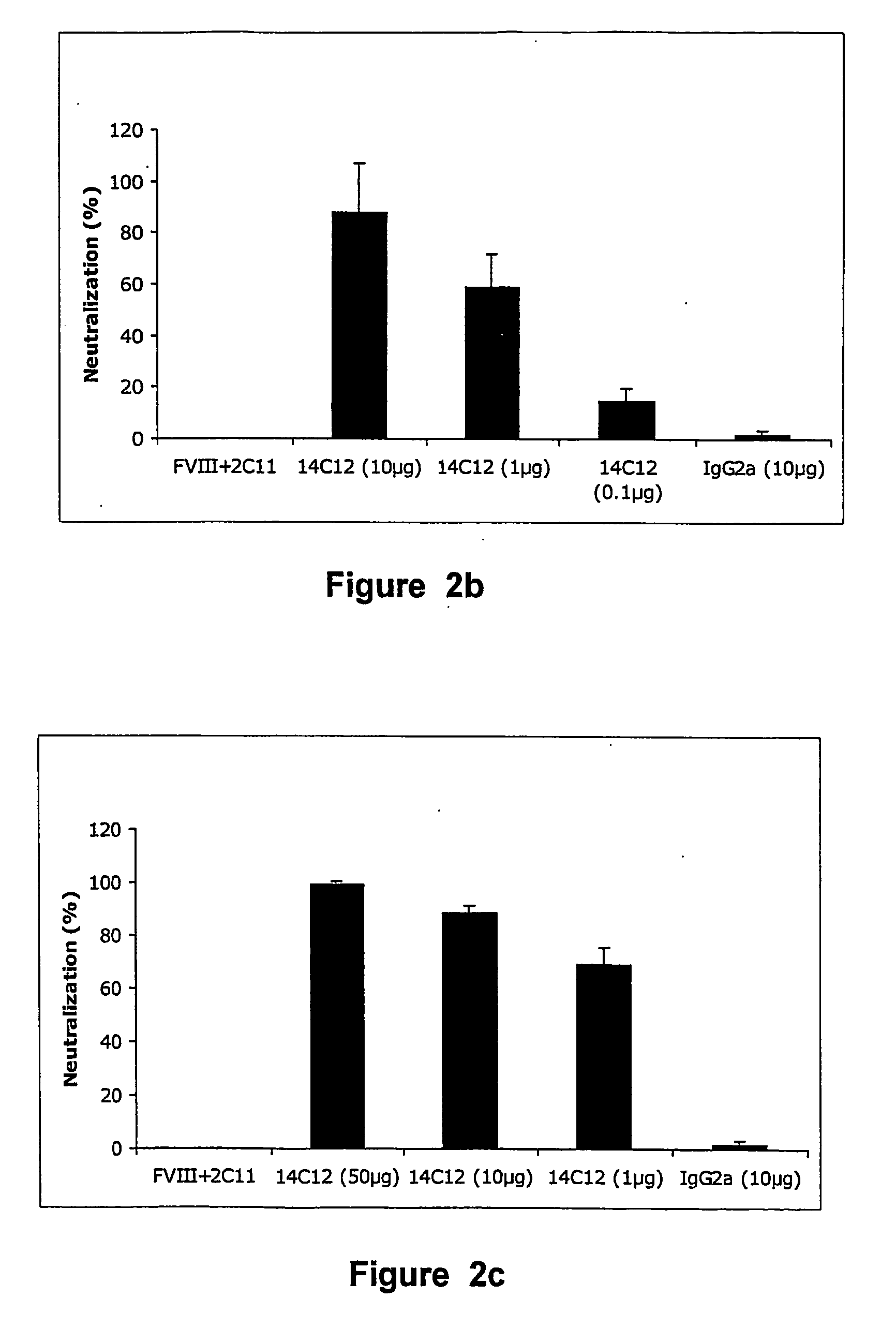
Anti-idiotypic antibodies neutralizing the inhibitory activity of an inhibitory antibody directed against the c1 domain of factor viii
The present invention is related to a monoclonal anti-idiotypic antibody directed against a Factor VIII inhibitor antibody binding to the C1 domain of Factor VIII, as well as to a cell line producing this monoclonal anti-idiotypic antibody, to the use of this monoclonal anti-idiotypic antibody as medicament, and more particularly to the use thereof for manufacturing a medicament intended for the treatment of haemophilia A.
Owner:LFB BIOTECH
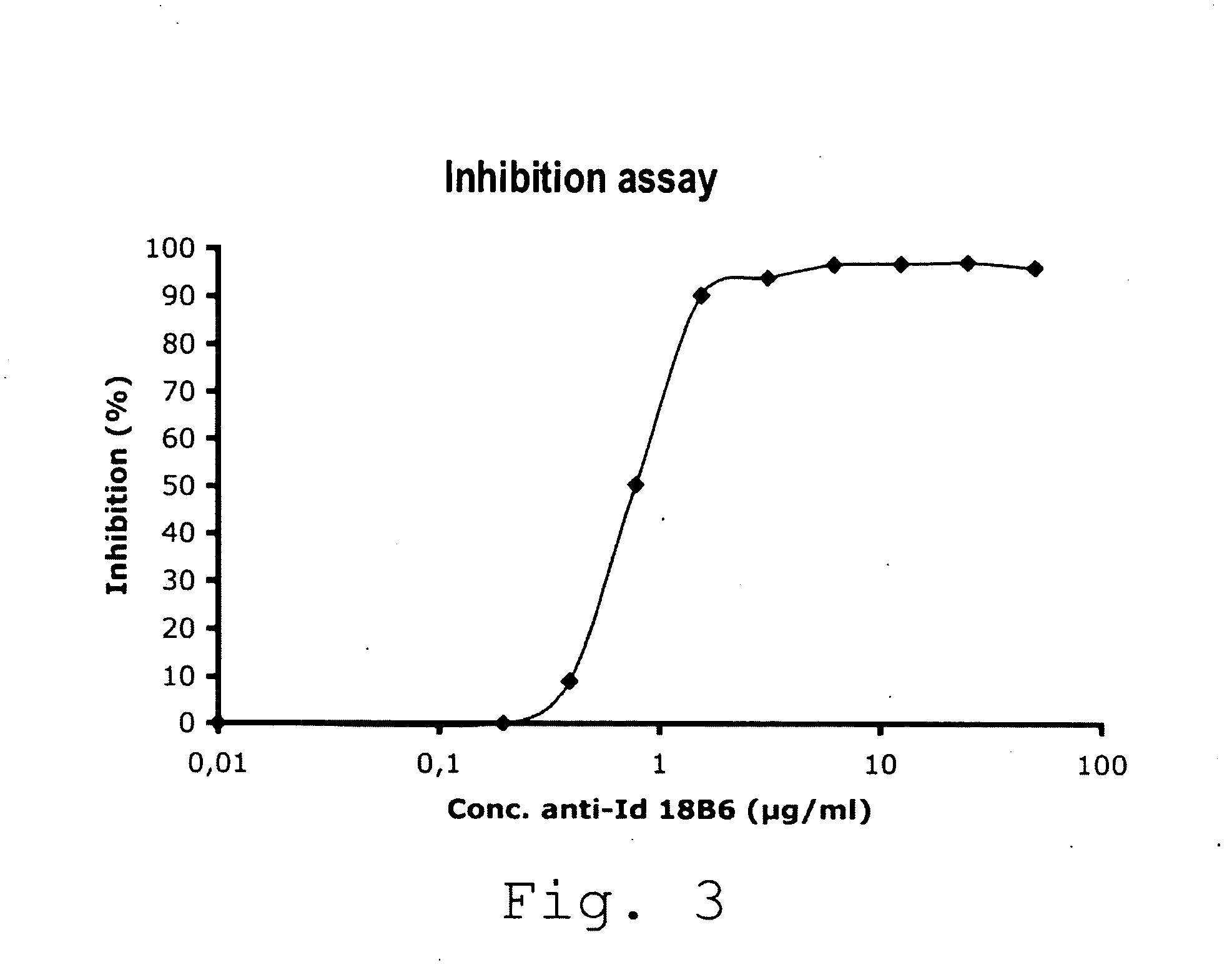
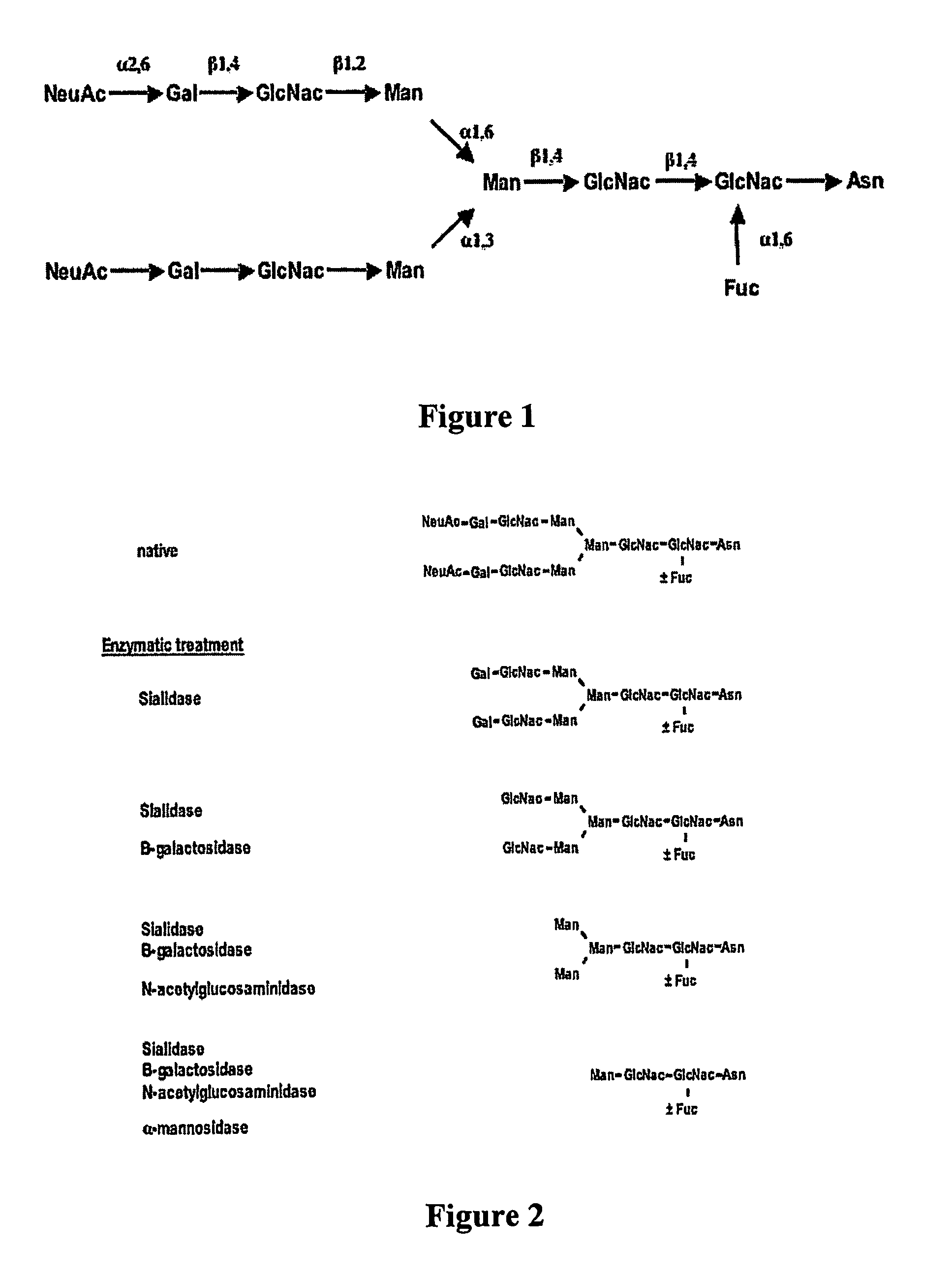
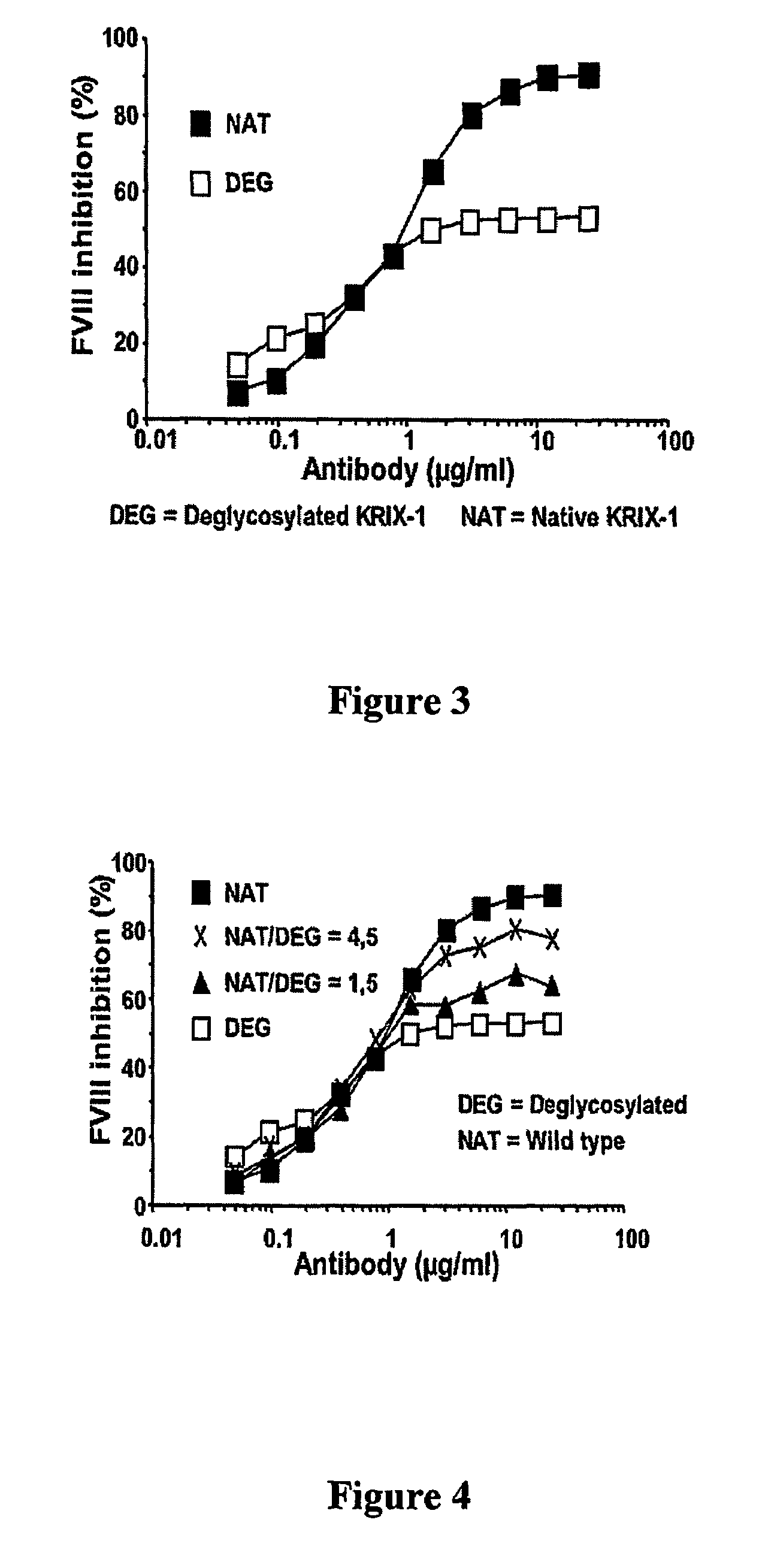
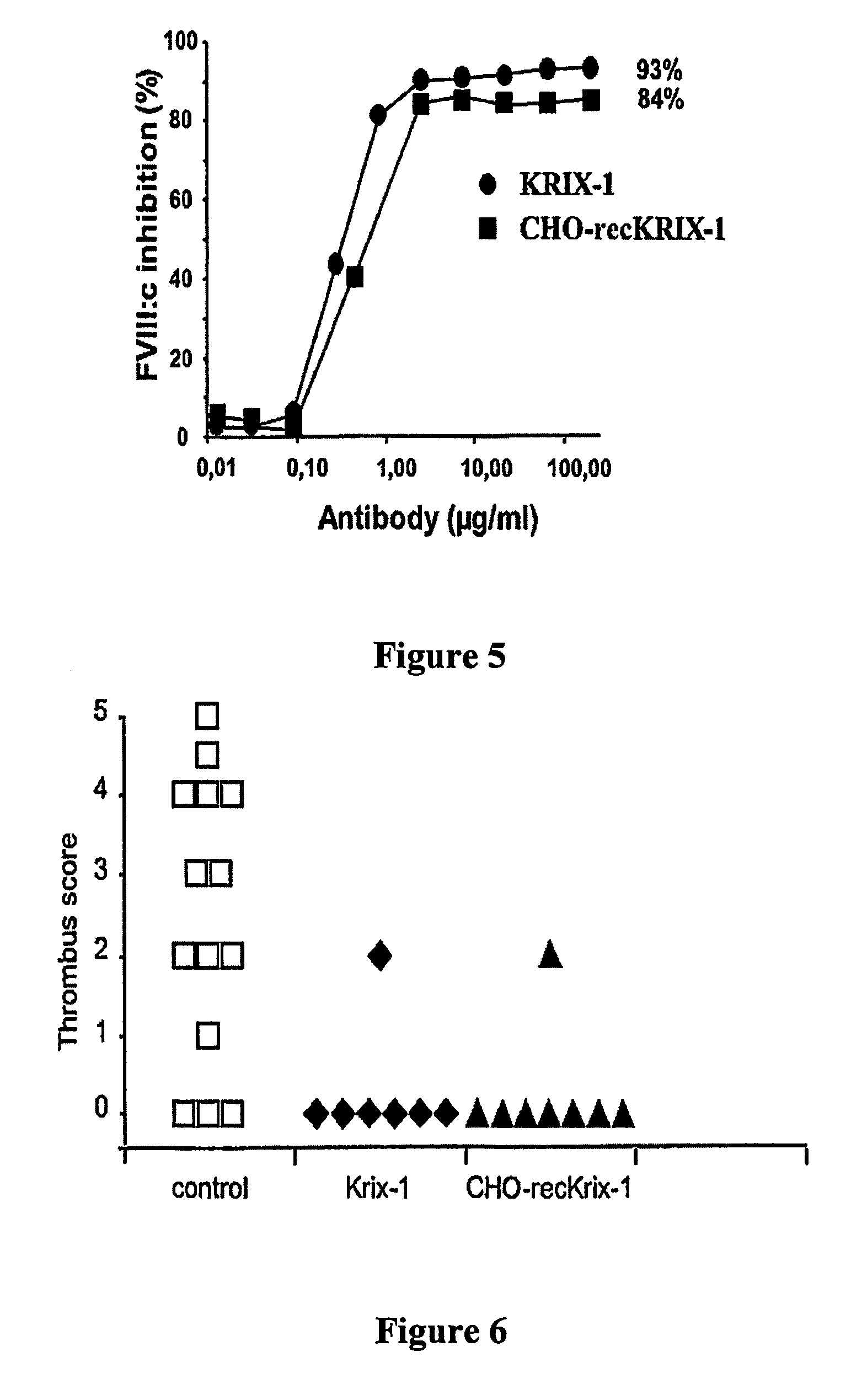
Factor VIII inhibitory antibodies with reduced glycosylation
InactiveUS7785594B2Reduce bleeding riskEffective anti-thromboticImmunoglobulins against blood coagulation factorsAntibacterial agentsFactor iiSite-directed mutagenesis
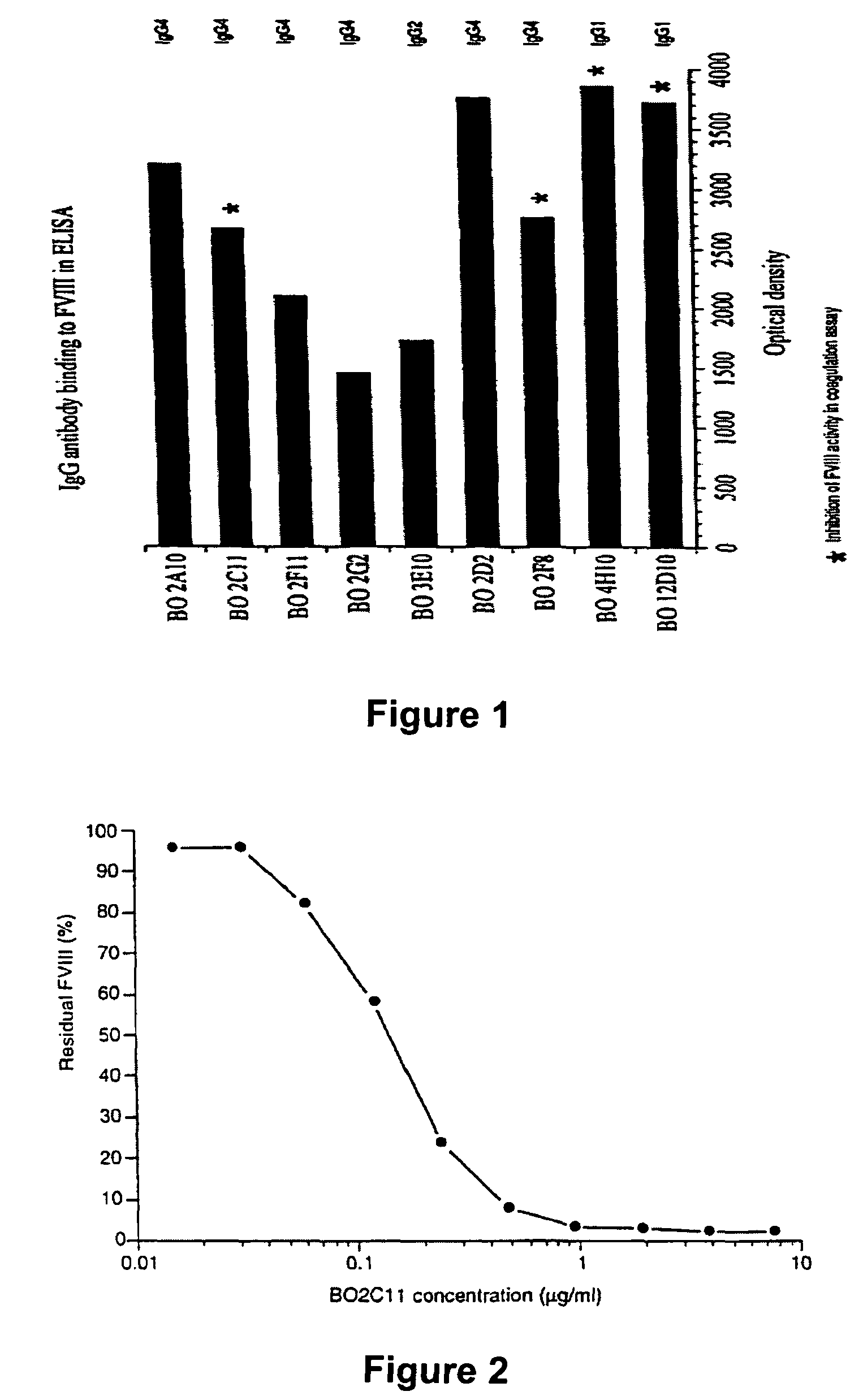
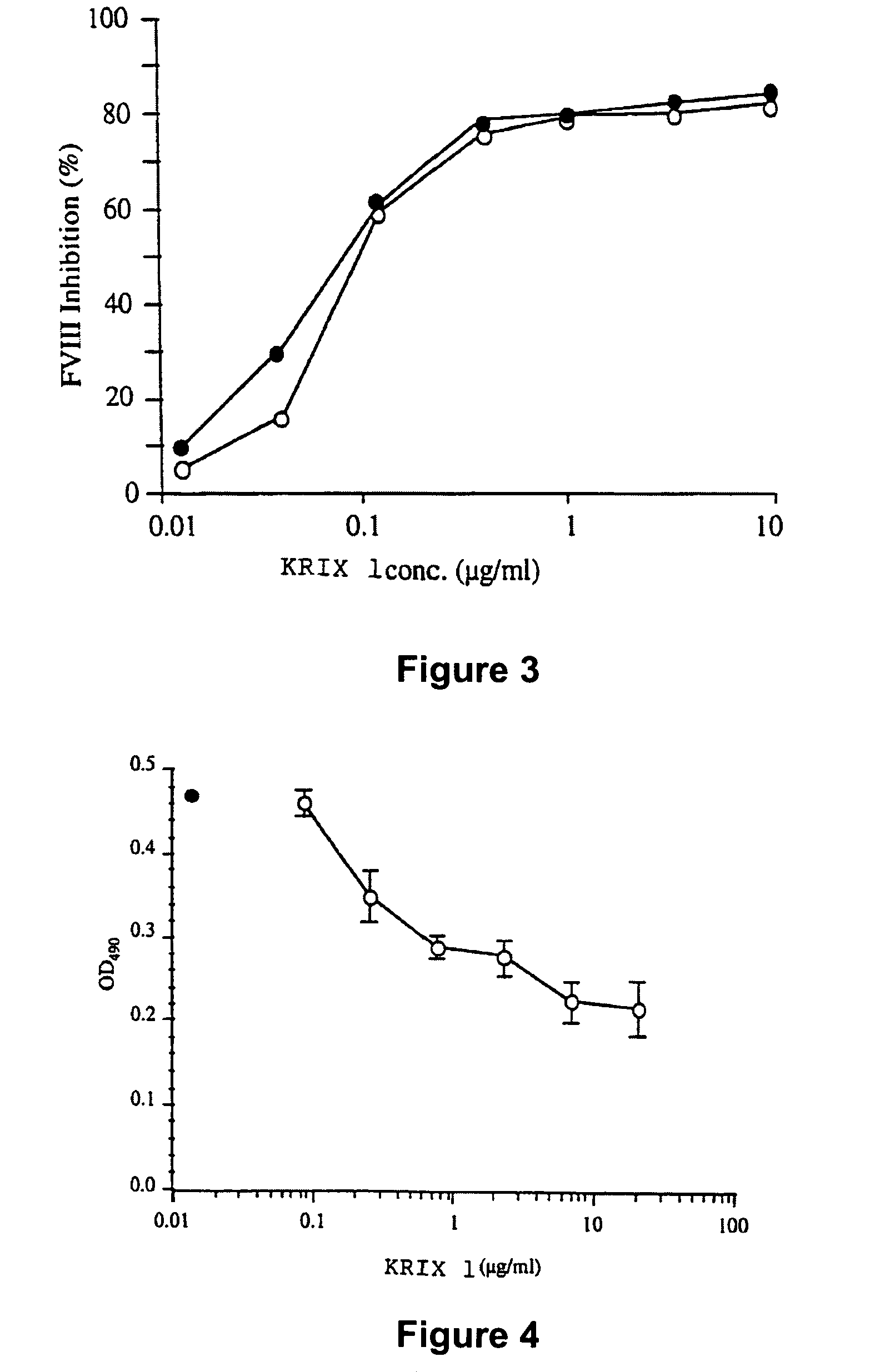
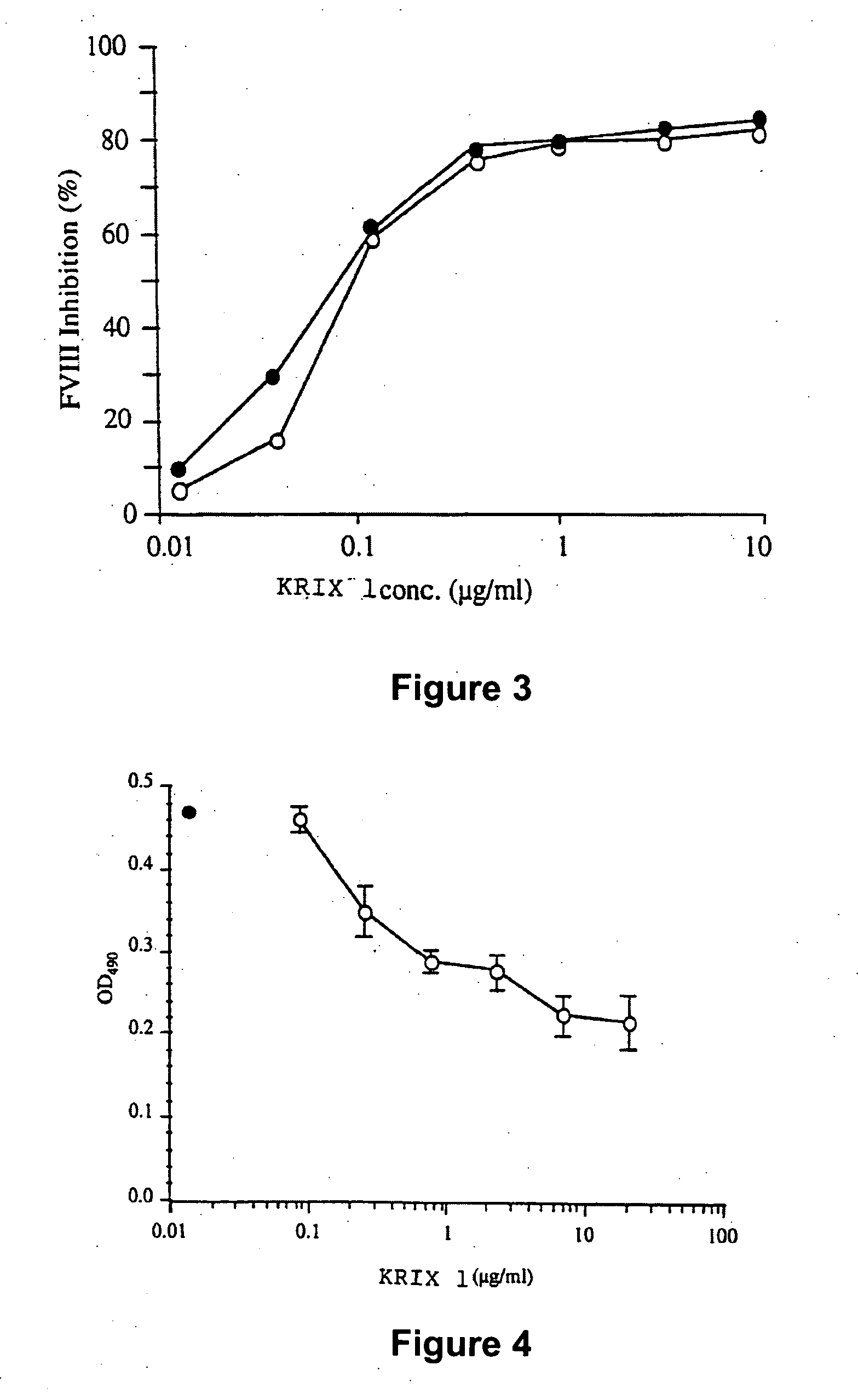
The present invention discloses inhibitory antibodies against Factor VIII with modified glycosylation, either by enzymatic deglycosylation or by site directed mutagenesis. Said antibodies with modified glycosylation have equal affinity for FVIII but show different inhibiting properties. The use of one or a mixture of said antibodies allow modulation of the inhibition of factor VII to levels between 40 and 95%. The present invention further disclosed pharmaceutical compositions comprising inhibitory antibodies against Factor VIII with modified glycosylation, combinations of these antibodies and methods for treating haemostasis disorders using said antibodies and antibody mixtures.
Owner:LIFE SCI RES PARTNERS VZW
Glycosylated, low antigenicity, low immunogenicity factor VIII
InactiveUS20050009148A1Factor VIIMammal material medical ingredientsBlood coagulation factor VIIIVaccine Immunogenicity
The development of inhibitory antibodies to blood coagulation factor VIII (fVIII) results in a severe bleeding tendency. These antibodies arise in patients with hemophilia A (hereditary fVIII deficiency) who have been transfused with fVIII. They also occur in non-hemophiliacs, which produces the condition acquired hemophilia. We describe a method to construct and express novel recombinant fVIII molecules which escape detection by existing inhibitory antibodies (low antigenicity fVIII) and which decrease the likelihood of developing inhibitory antibodies (low immunogenicity fVIII). In this method, fVIII is glycosylated at sites that are known to be antibody recognition sequences (epitopes). This produces the desired properties of low antigenicity fVIII and low immunogenicity fVIII. The mechanism is similar to one used by viruses such as the AIDS virus, which glycosylates its surface proteins to escape detection by the immune system.
Owner:LOLLAR JOHN S
Methods of treating hemostasis disorders using antibodies binding the C1 domain of factor VIII
InactiveUS7829085B2Reduce bleeding riskTreatment safetyImmunoglobulins against blood coagulation factorsAntibody ingredientsFactor iiThrombus
The invention, in general, features a method of treatment and / or prevention of a thrombotic pathological condition, in a mammal, which includes administering to the mammal in need of such treatment a therapeutically effective amount of a composition including an antibody directed against the C1 domain of Factor VIII, which is a partially inhibitory antibody of Factor VIII.
Owner:LIFE SCI RES PARTNERS VZW
Checkpoint Blockade and Microsatellite Instability
InactiveUS20170267760A1High mutational burdenMicrobiological testing/measurementDigestive systemProgrammed deathImmune tolerance
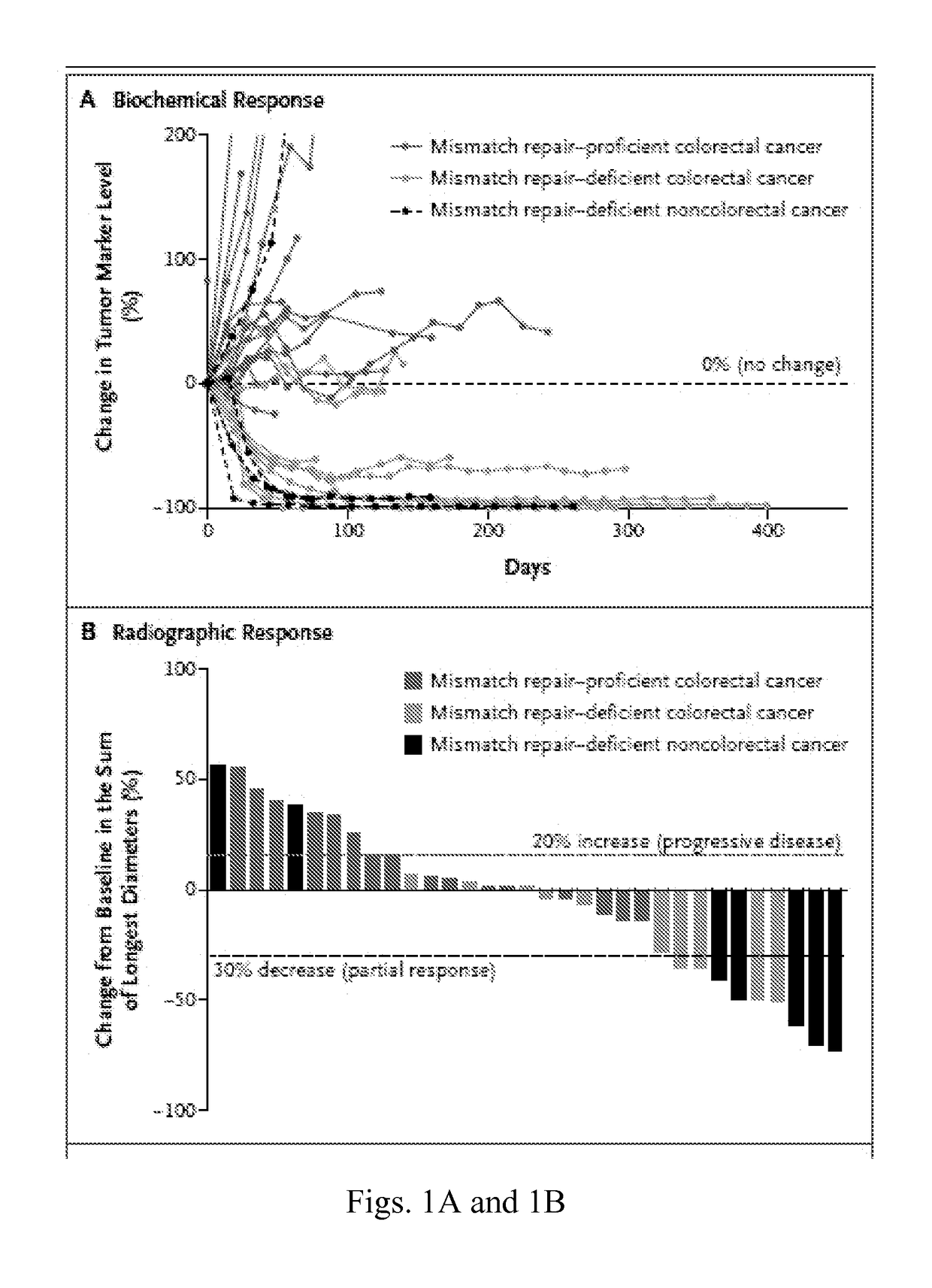
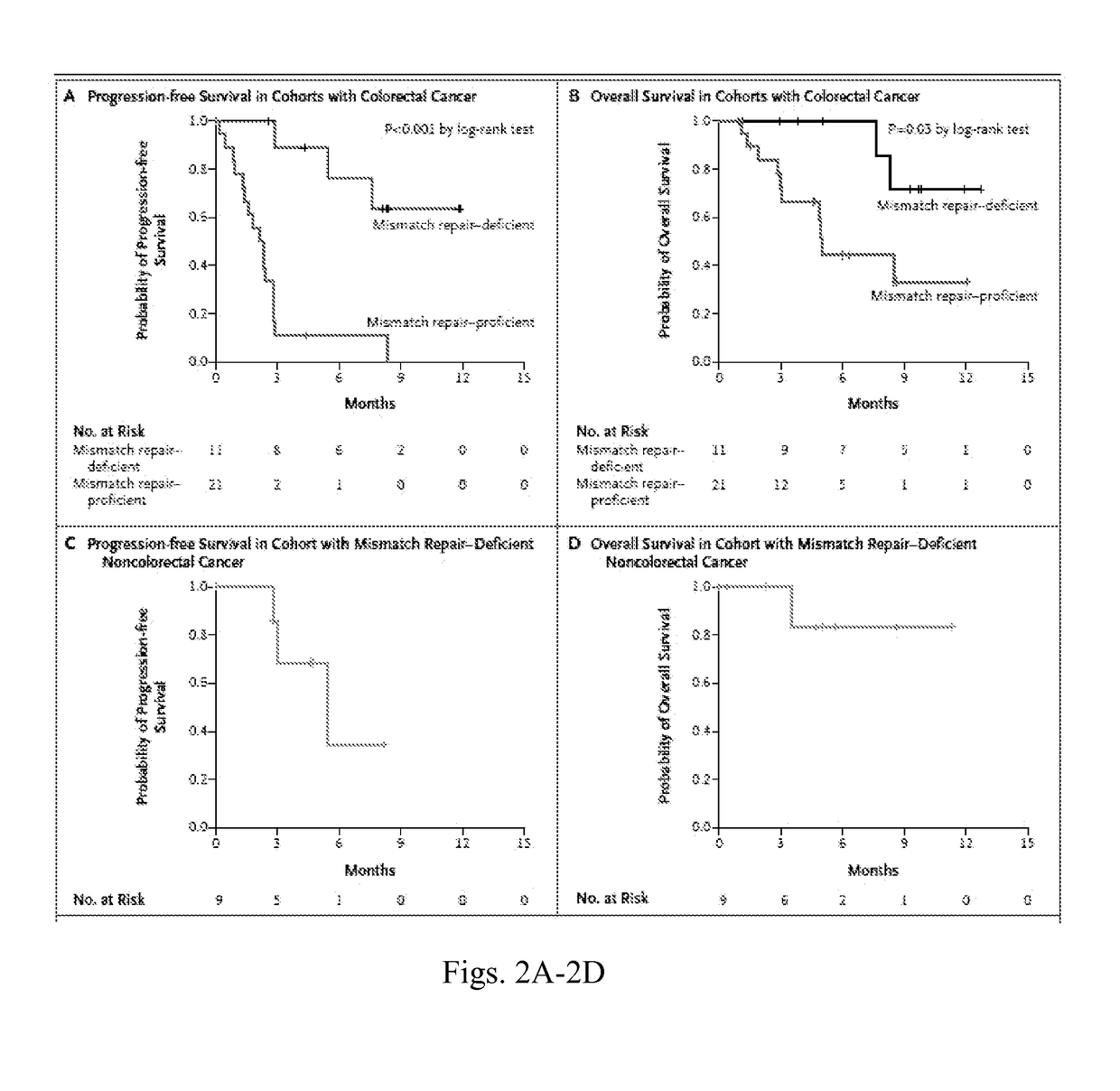
Blockade of immune checkpoints such as cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1) shows promise in patients with cancer. Inhibitory antibodies directed at these receptors have been shown to break immune tolerance and promote anti-tumor immunity. These agents work particularly well in patients with a certain category of tumor. Such tumors may be particularly susceptible to treatment because of the multitude of neoantigens which they produce.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Modified factor VIII
InactiveUS20050079584A1Superior coagulant activityFactor VIIPeptide/protein ingredientsEpitopeInhibitory antibodies
Specific amino acid loci of human factor VIII interact with inhibitory antibodies of hemophilia patients who have developed such antibodies after being treated with factor VIII. Modified factor VIII is disclosed in which the amino acid sequence is changed by a substitution at one or more of the specific loci. The modified factor VIII is not inhibited by inhibitory antibodies against the A2 or C2 domain epitopes. The modified factor VIII is useful for hemophiliacs, either to avoid or prevent the action of inhibitory antibodies.
Owner:EMORY UNIVERSITY
Methods for reducing proteinuria in a human subject suffering from immunoglobulin a nephropathy
InactiveUS20180105604A1Reduce and prevent proteinureaPrevent and reduce damageAntibody mimetics/scaffoldsAntibody ingredientsHuman ARDSBiological activation
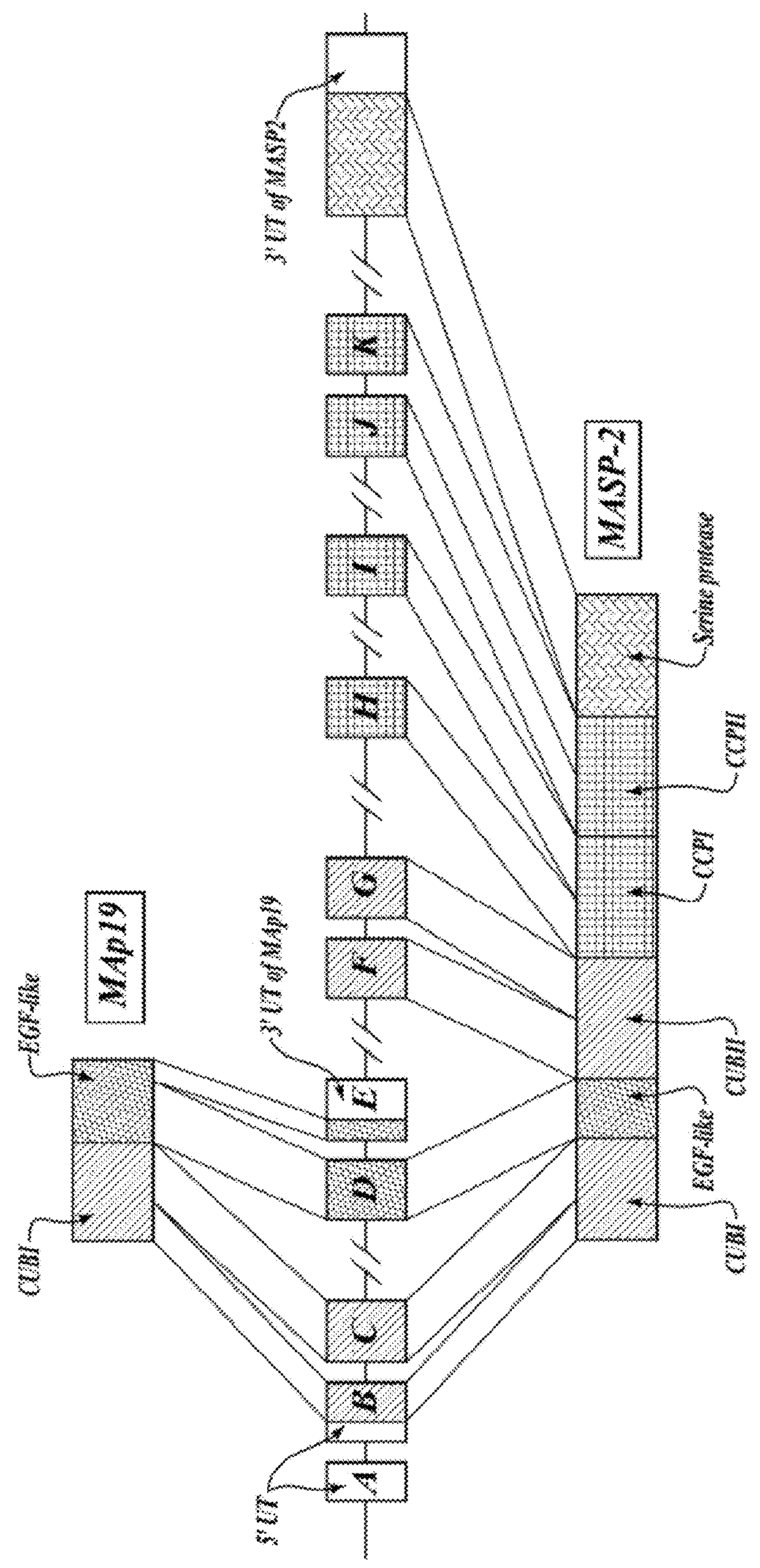
In one aspect, the invention provides methods for reducing proteinuria in a human subject suffering, or at risk of developing Immunoglobulin A Nephropathy (IgAN). The methods comprise the step of administering, to a subject in need thereof, an amount of a MASP-2 inhibitory antibody effective to inhibit MASP-2-dependent complement activation.
Owner:UNIVERSITY OF LEICESTER +1
Ligands for use in therapeutic compositions for the treatment of hemostasis disorders
InactiveUS20060115474A1Reduce bleeding riskTreatment safetyImmunoglobulins against blood coagulation factorsAntibody ingredientsInhibitory antibodiesThrombosis
The invention, in general, features a method of treatment and / or prevention of a thrombotic pathological condition, in a mammal, which includes administering to the mammal in need of such treatment a therapeutically effective amount of a composition including an antibody directed against the C1 domain of Factor VIII, which is a partially inhibitory antibody of Factor VIII.
Owner:LIFE SCI RES PARTNERS VZW
Checkpoint Blockade and Microsatellite Instability
InactiveUS20170313775A1Microbiological testing/measurementDigestive systemProgrammed deathImmune tolerance
Blockade of immune checkpoints such as cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1) shows promise in patients with cancer. Inhibitory antibodies directed at these receptors have been shown to break immune tolerance and promote anti-tumor immunity. These agents work particularly well in patients with a certain category of tumor. Such tumors may be particularly susceptible to treatment because of the multitude of neoantigens which they produce.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
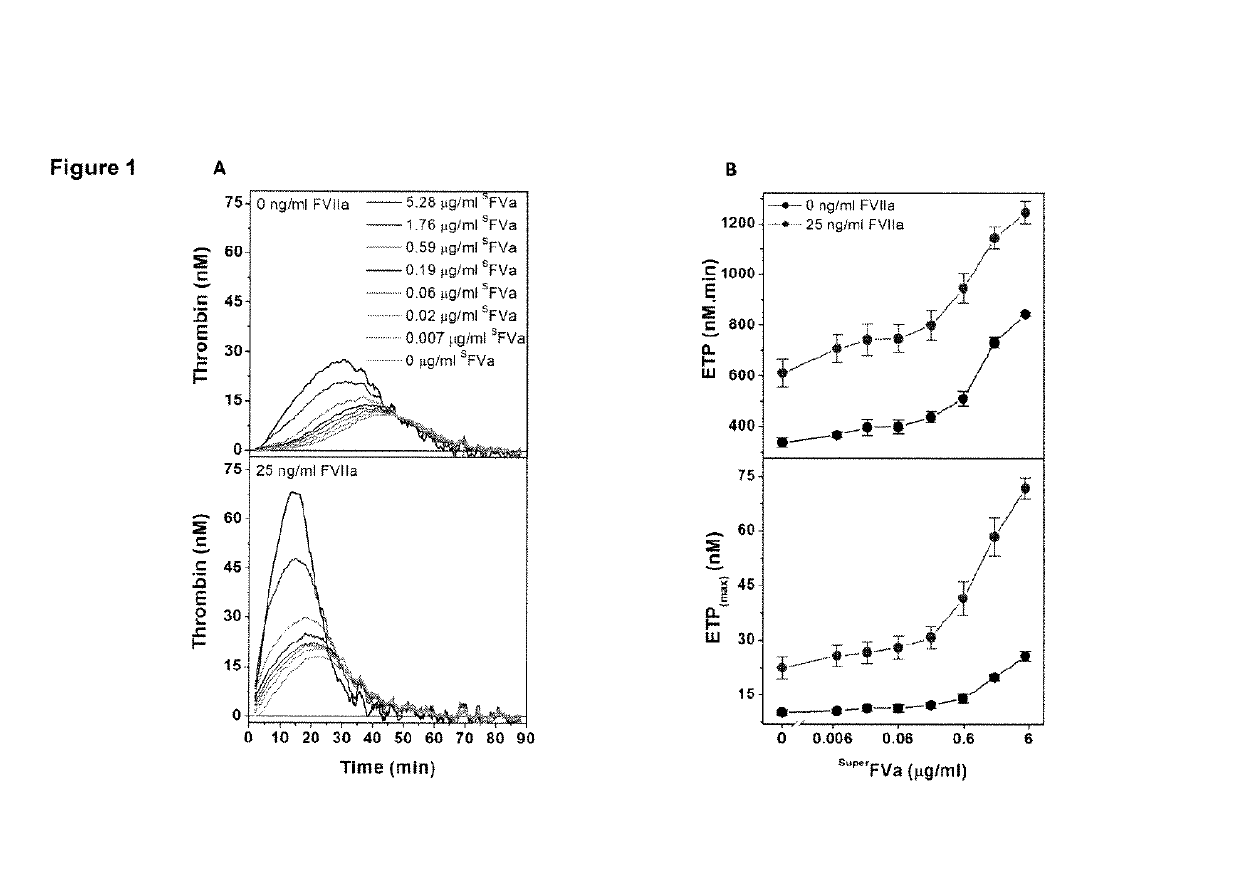
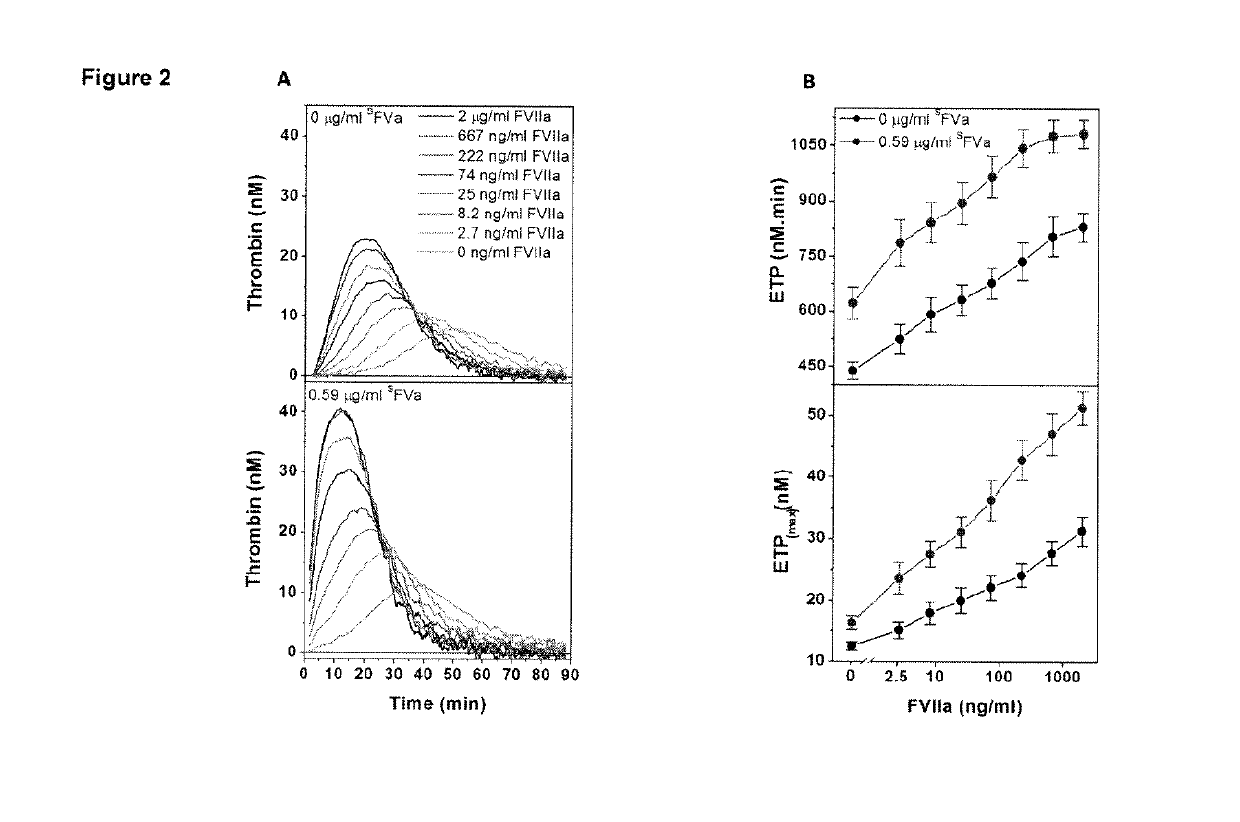
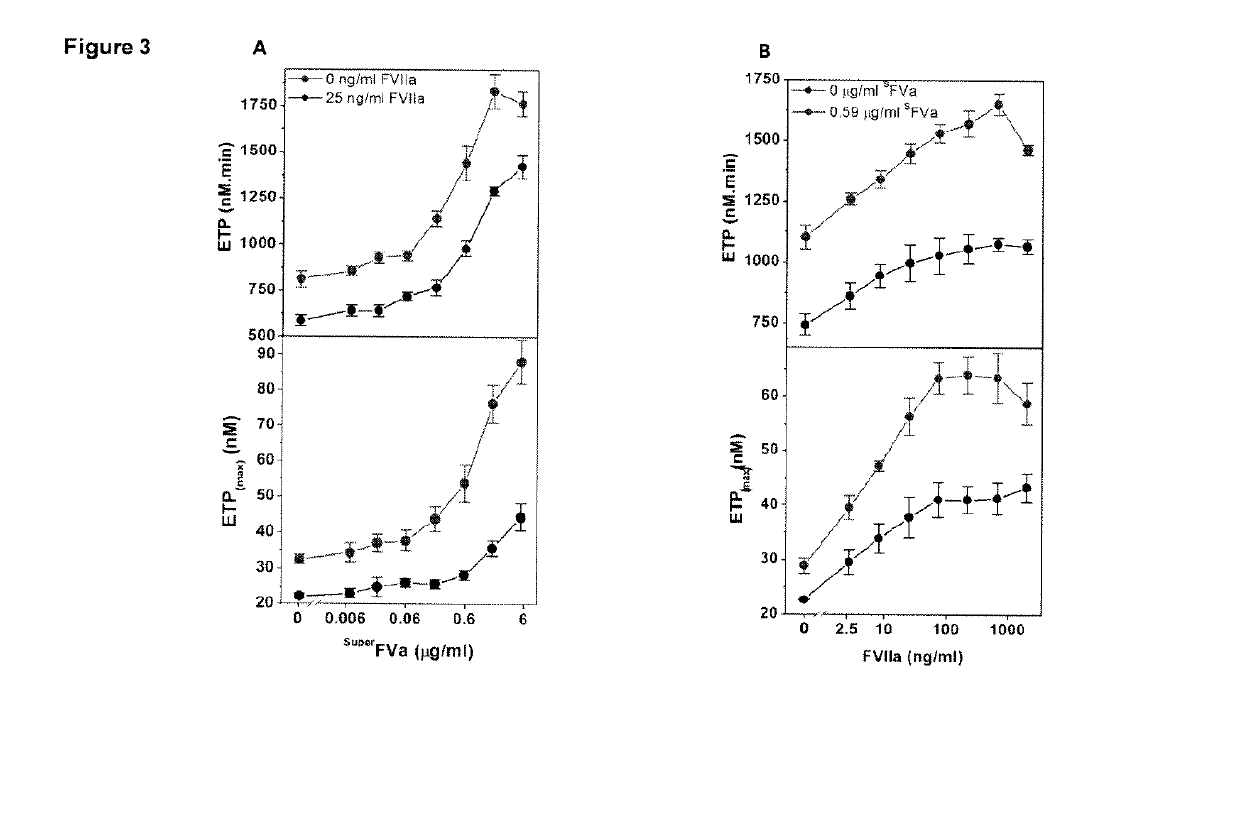
Factor IX variants with clotting activity in absence of their cofactor and/or with increased F.IX clotting activity and their use for treating bleeding disorders
The present invention relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has clotting activity in absence of its cofactor. The present invention furthermore relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has increased F.IX clotting activity compared to wildtype. The present invention furthermore relates to the use of these variants for the treatment and / or prophylaxis of bleeding disorders, in particular hemophilia A and / or hemophilia B or hemophilia caused or complicated by inhibitory antibodies to F.VIII. The present invention also relates to further variants of factor IX (F.IX) which have desired properties and can, thus be tailored for respective specific therapeutic applications.
Owner:DRK BLUTSPENDEDIENST BADEN WURTTEMBERG HESSEN GGMBH
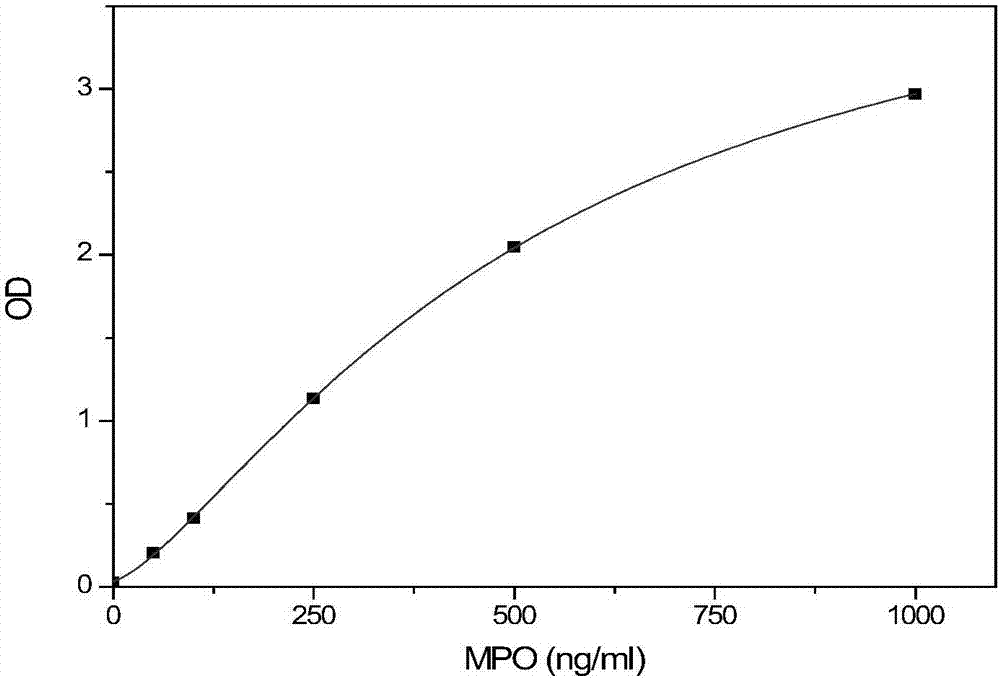
Human myeloperoxidase enzyme activity and total quantity detection kit, detection method and preparation method
The invention discloses a human myeloperoxidase enzyme activity and total quantity detection kit, a detection method and a preparation method. The detection kit comprises a solid-phase carrier, a zymolysis substrate, buffer solution, an enzyme antibody reagent, a plurality of calibration products, cleaning solution, a chromogenic substrate and stop solution, the solid-phase carrier is coated with 200-400ng / hole of anti-MPO (myeloperoxidase) non-restraining antibodies, the zymolysis substrate comprises 0.1-50mmol / L of H2O2, the enzyme antibody reagent contains anti-MPO (myeloperoxidase) restraining antibodies marked by horse radish peroxidase, the usage amount of the antibodies is 1:2000-1:8000, the antigen content of myeloperoxidase of the calibration products is 0-1000ng / ml, the chromogenic substrate at least comprises chromogenic substrate A solution and chromogenic substrate B solution, the chromogenic substrate A solution contains 0.015-0.02% of peroxide, and the chromogenic substrate B solution contains 0.25-0.32g / L of TMB (tetramethylbenzidine). According to the detection kit, the conditions of the myeloperoxidase in human bodies can be accurately responded, and the detection kit provides more accurate basis for clinical diagnosis and early warning of coronary arteries diseases, particularly, and unstable coronary heart diseases.
Owner:海格德生物科技(深圳)有限公司
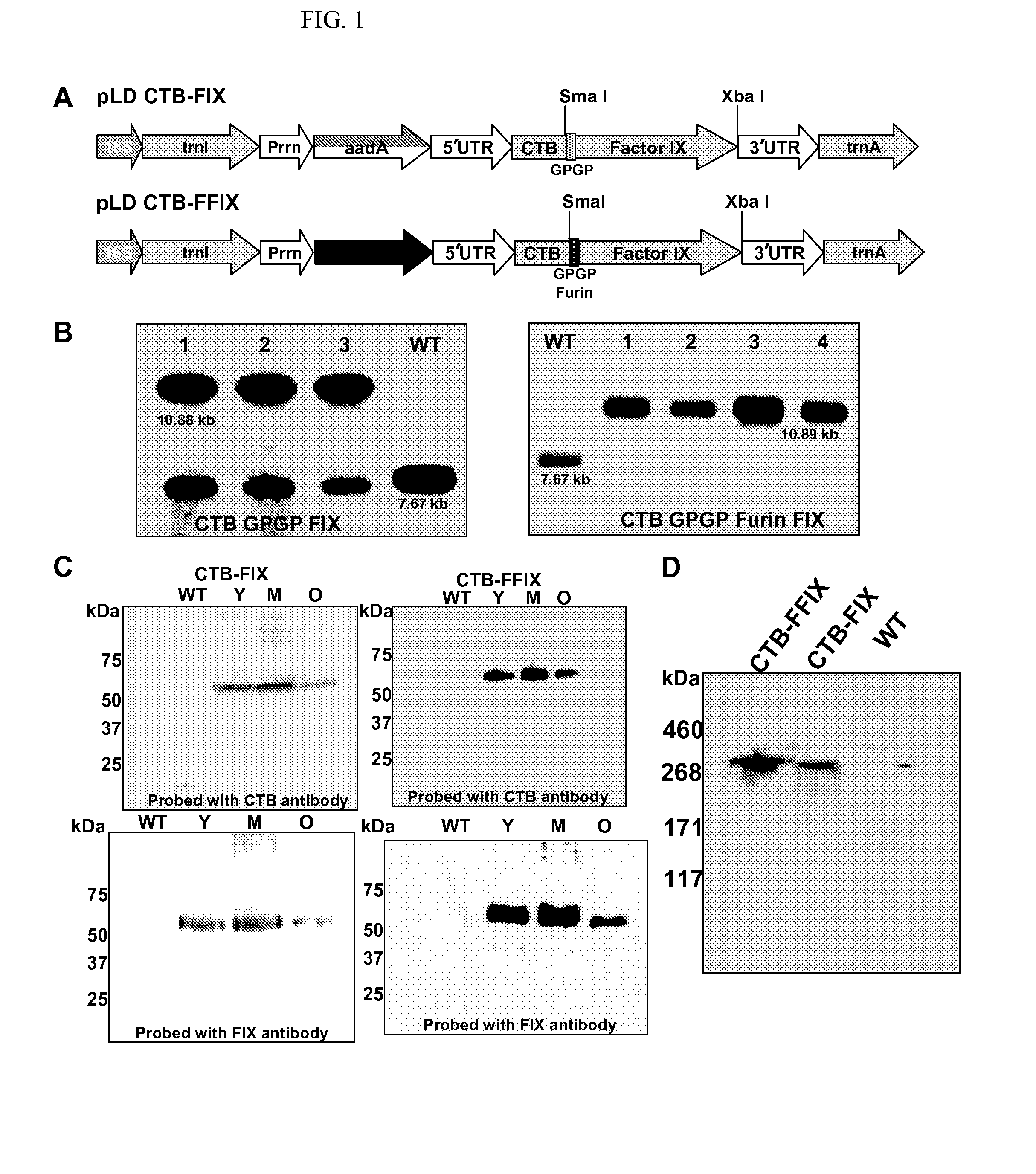
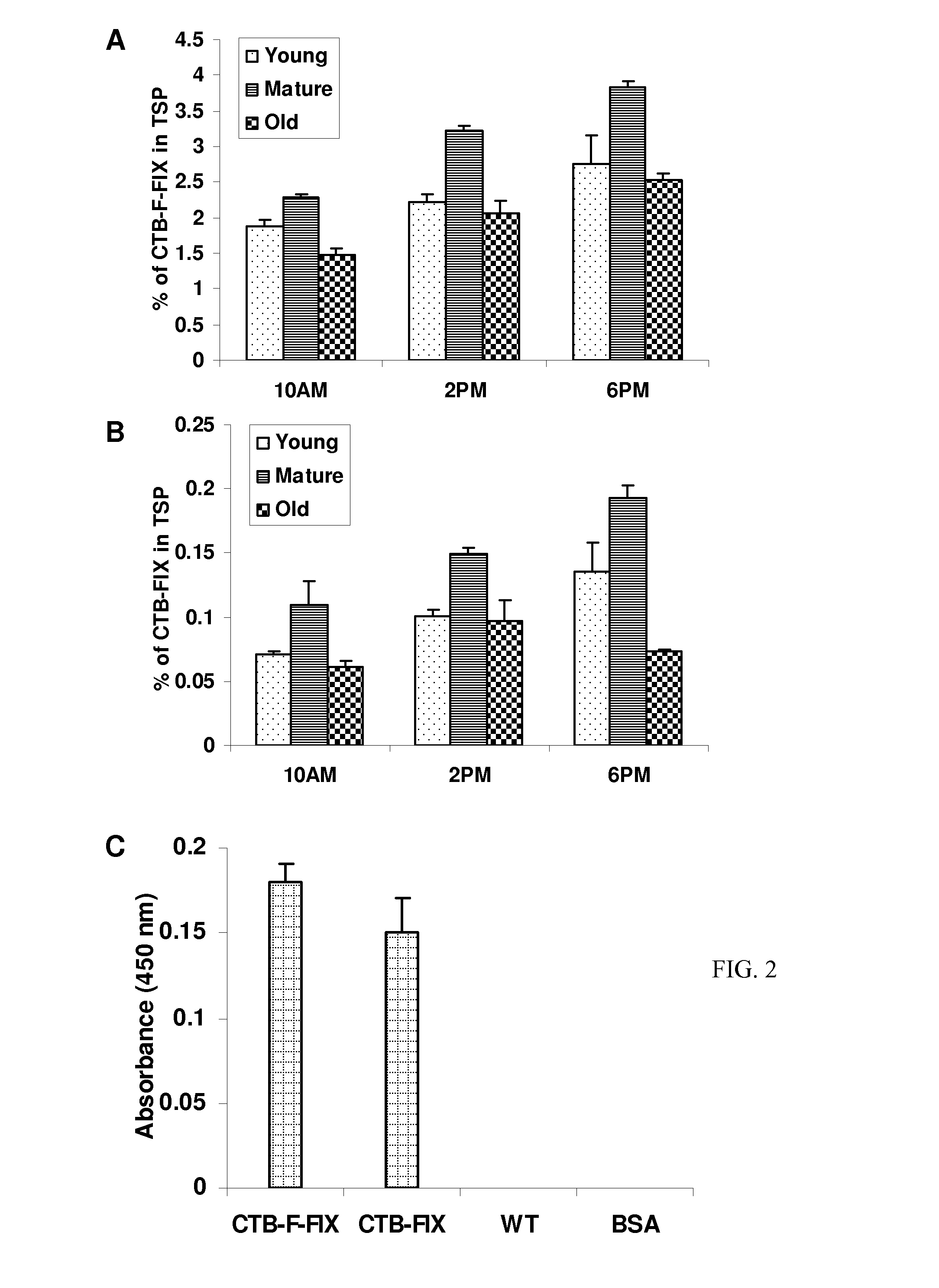
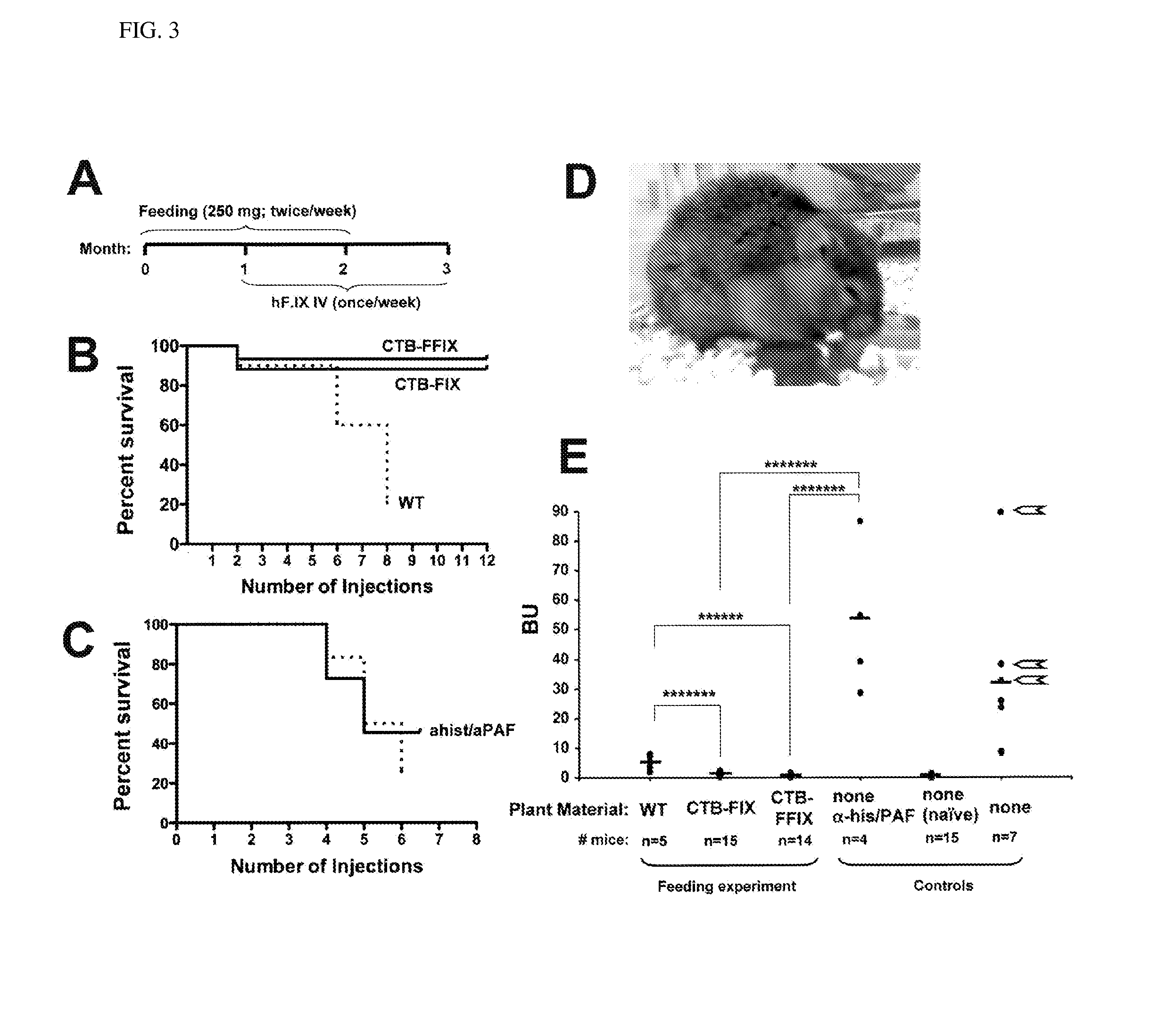
Administration of plant expressed oral tolerance agents
Protein replacement therapy for patients with hemophilia or other inherited protein deficiencies is often complicated by pathogenic antibody responses, including antibodies that neutralize the therapeutic protein or that predispose to potentially life-threatening anaphylactic reactions by formation of IgE. Using murine hemophilia B as a model, we have developed a prophylactic protocol against such responses that is non-invasive and does not include immune suppression or genetic manipulation of the patient's cells. Oral delivery of coagulation factor IX (F. IX) expressed in chloroplasts, bioencapsulated in plant cells, effectively blocked formation of inhibitory antibodies in protein replacement therapy. Inhibitor titers were mostly undetectable and up to 100-fold lower in treated mice when compared to controls. Moreover, this treatment eliminated fatal anaphylactic reactions that occurred after 4 to 6 exposures to intravenous F. IX protein. While only 20-25% of control animals survived after 6-8 F. IX doses, 90-95% of tolerized mice survived 12 injections without signs of allergy or anaphylaxis. This high-responder strain of hemophilia B mice represents the first hemophilic animal model to study anaphylactic reactions. The plant material was effective over a range of oral antigen doses (equivalent to 5-80 μg recombinant F.IX / kg), and controlled inhibitor formation and anaphylaxis long-term, up to 7 months. Oral antigen administration caused a deviant immune response that suppressed formation of IgE and inhibitory antibodies. This cost-effective and efficient approach to oral delivery of protein antigens to the gut should be applicable to several genetic diseases that are prone to pathogenic antibody responses during treatment.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Therapy for treatment or prevention of conditions associated with bleeding or hypocoagulation
ActiveUS10407488B2High specific activityIncrease resistancePowder deliveryPeptide/protein ingredientsHalf-lifeOverproduction
Owner:RGT UNIV OF CALIFORNIA +1
Factor IX Variants with Clotting Activity in Absence of Their Cofactor and/or With Increased F.IX Clotting Activity and Their Use for Treating Bleeding Disorders
The present invention relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has clotting activity in absence of its cofactor. The present invention furthermore relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has increased F.IX clotting activity compared to wildtype. The present invention furthermore relates to the use of these variants for the treatment and / or prophylaxis of bleeding disorders, in particular hemophilia A and / or hemophilia B or hemophilia caused or complicated by inhibitory antibodies to F.VIII. The present invention also relates to further variants of factor IX (F.IX) which have desired properties and can, thus be tailored for respective specific therapeutic applications.
Owner:DRK BLUTSPENDEDIENST BADEN WURTTEMBERG HESSEN GGMBH
Factor IX variants with clotting activity in absence of their cofactor and their use for treating bleeding disorders
The present invention relates to variants of a vitamin K-dependent serine protease of the coagulation cascade, preferably variants of factor IX (F.IX), wherein the variant is characterized in that it has clotting activity in absence of its cofactor. The present invention furthermore relates to the use of these variants for the treatment and / or prophylaxis of bleeding disorders, in particular hemophilia A and / or hemophilia B or hemophilia caused or complicated by inhibitory antibodies to F.VIII. The present invention also relates to further variants of factor IX (F.IX) which have desired properties and can, thus be tailored for respective specific therapeutic applications.
Owner:DRK BLUTSPENDEDIENST BADEN WURTTEMBERG HESSEN GGMBH
Methods and compositions for treating cancer using serotonin receptor inhibitors
ActiveUS11034751B1Reduces expression and activityReduce tumor volumePeptide/protein ingredientsReceptors for cytokines/lymphoines/interferonsAntiendomysial antibodiesTryptamine Receptors
The present invention provides methods for treating cancer using serotonin receptor inhibitors, such as serotonin receptor inhibitory antibodies, among others. The invention also features compositions containing serotonin receptor inhibitors, methods of diagnosing patients with serotonin receptor-associated cancer, and methods of predicting the response of cancer in a subject to treatment with serotonin receptor inhibitors.
Owner:FLAGSHIP PIONEERING INNOVATIONS V INC
Anti-idiotypic antibodies against factor VIII inhibitor and uses thereof
InactiveUS20060239998A1Increase stability and lifetimeEnhanced inhibitory effectAnimal cellsCell receptors/surface-antigens/surface-determinantsFactor VIII inhibitorIn vivo
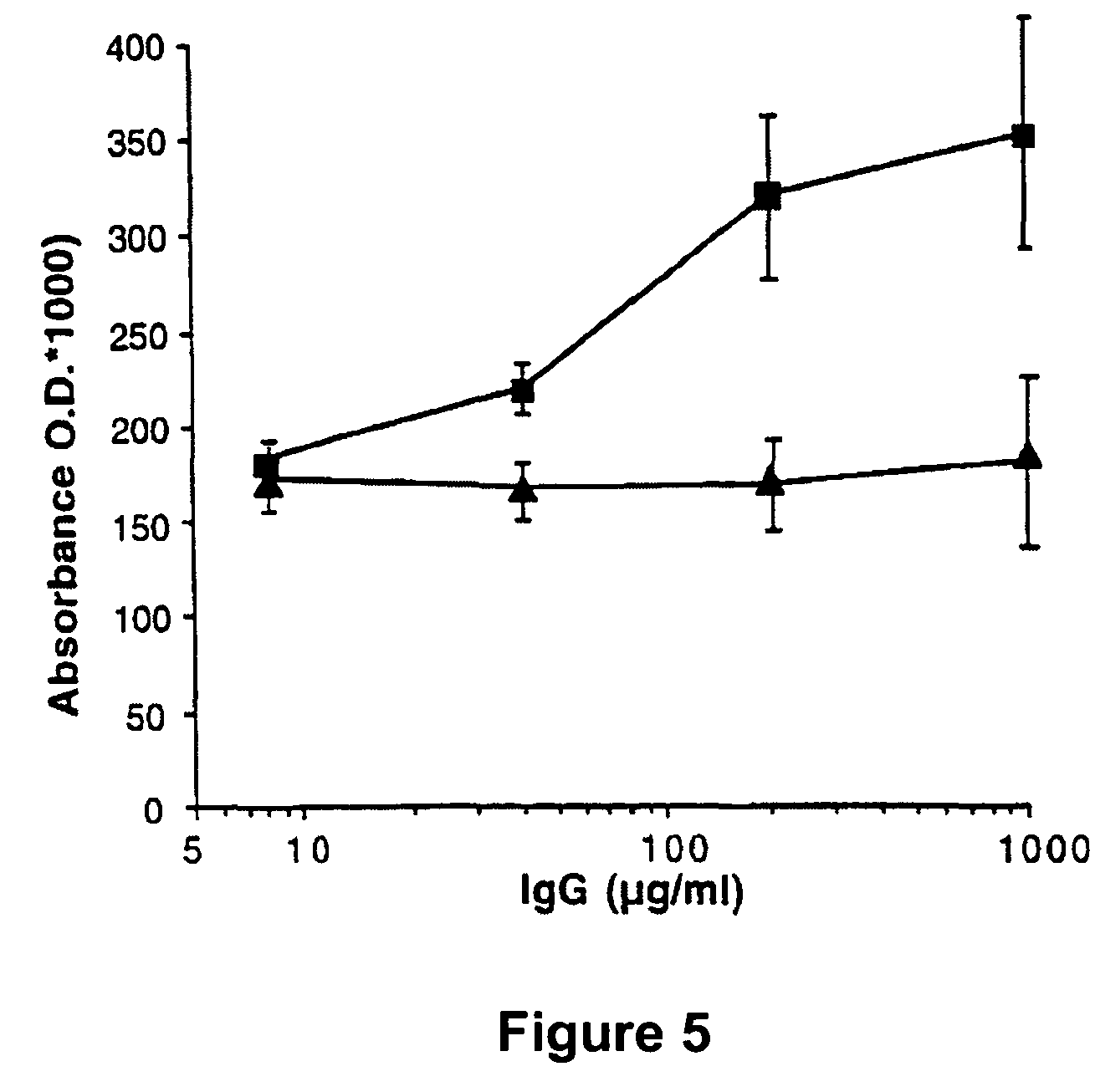
The present invention discloses anti-idiotypic antibodies and fragments thereof against inhibitory Factor VIII anti-bodies, said inhibitory antibodies having an affinity for the C2 domain of Factor VIII. The anti-idiotypic antibodies of the present invention are able to completely neutralise in vitro and in an in vivo mouse model the inhibitory activity of FVIII inhibitors. The anti idiotypic antibodies of the present invention can be applied for the prevention, treatment or reduction of bleeding disorders of hemophilia patients with inhibitory antibody against the C2 domain of Factor VIII.
Owner:LIFE SCI RES PARTNERS VZW
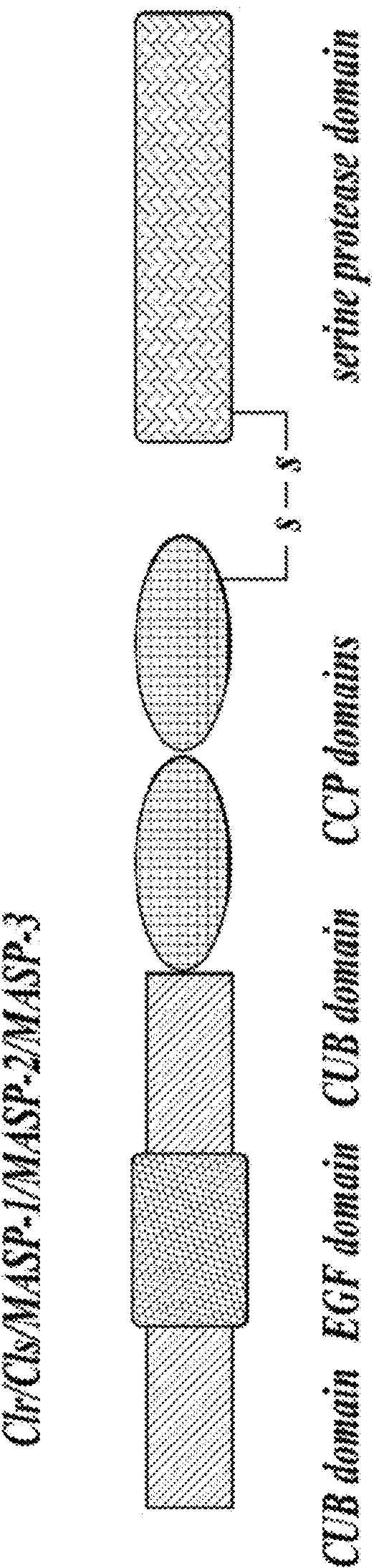
Compositions for inhibiting masp-2 dependent complement acitivation
The present invention relates to anti-MASP-2 inhibitory antibodies and compositions comprising such antibodies for use in inhibiting the adverse effects of MASP-2 dependent complement activation. In one aspect, the invention provides an isolated human monoclonal antibody, or antigen binding fragment thereof, that binds to human MASP-2, comprising:(i) a heavy chain variable region comprising CDR-HI, CDR-H2 and CDR-H3 sequences; and (ii) a light chain variable region comprising CDR-LI, CDR-L2 and CDR-L3, wherein the heavy chain variable region CDR-H3 sequence comprises an amino acid sequence set with factor B in forming additional alternative pathway C3 convertase (C3bBb). The alternative pathway C3 convertase is stabilized by the binding of properdin. Properdin extends the alternative pathway C3 convertase half-life six to ten fold. Addition of C3b to the alternative pathway C3 convertase leads to the formation of the alternative pathway C5 convertase.
Owner:OMEROS CORP
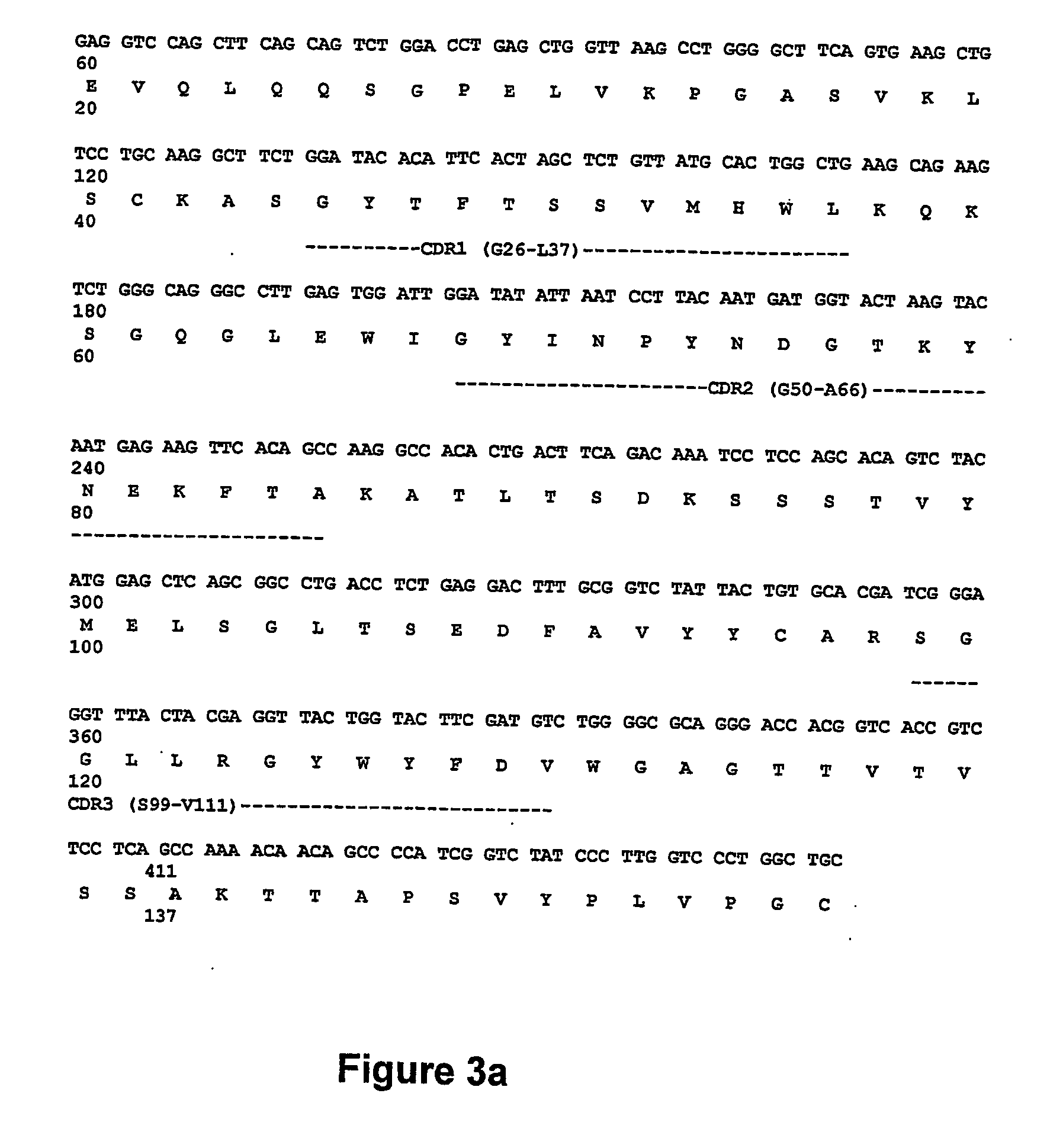
Cytotoxic Antibodies Directed Against Antibodies Inhibiting Factor VIII
InactiveUS20090130094A1Increase the number ofHigh affinityAnimal cellsSugar derivativesCytotoxic antibodyNucleic acid sequencing
The invention relates to an anti-idiotypical antibody targeting an antibody inhibiting the human factor VIII, said inhibiting antibody targeting the C2 region of the human factor VIII, the variable region of each of the light chains thereof being encoded by a sequence of nucleic acids of which at least 70% is identical to the murine sequence of nucleic acids SEQ ID NO: 1, and the variable region of each of the heavy chains thereof being encoded by a sequence of nucleic acids of which at least 70% is identical to the murine sequence of nucleic acids SEQ ID NO: 2, the constant regions of the light chains and the heavy chains being constant regions from a non-murine species. The invention also relates to the use of said antibody for activating the FcγRIII receptors of cytotoxic immune cells, and to the production of a medicament especially for the treatment of haemophilia A.
Owner:LFB BIOTECH
Rna-based compositions and adjuvants for prophylactic and therapeutic treatment
ActiveUS20170175122A1Improving immunogenicityAntineoplastic agentsCarrier-antigen complex architectureAdjuvantNucleotide
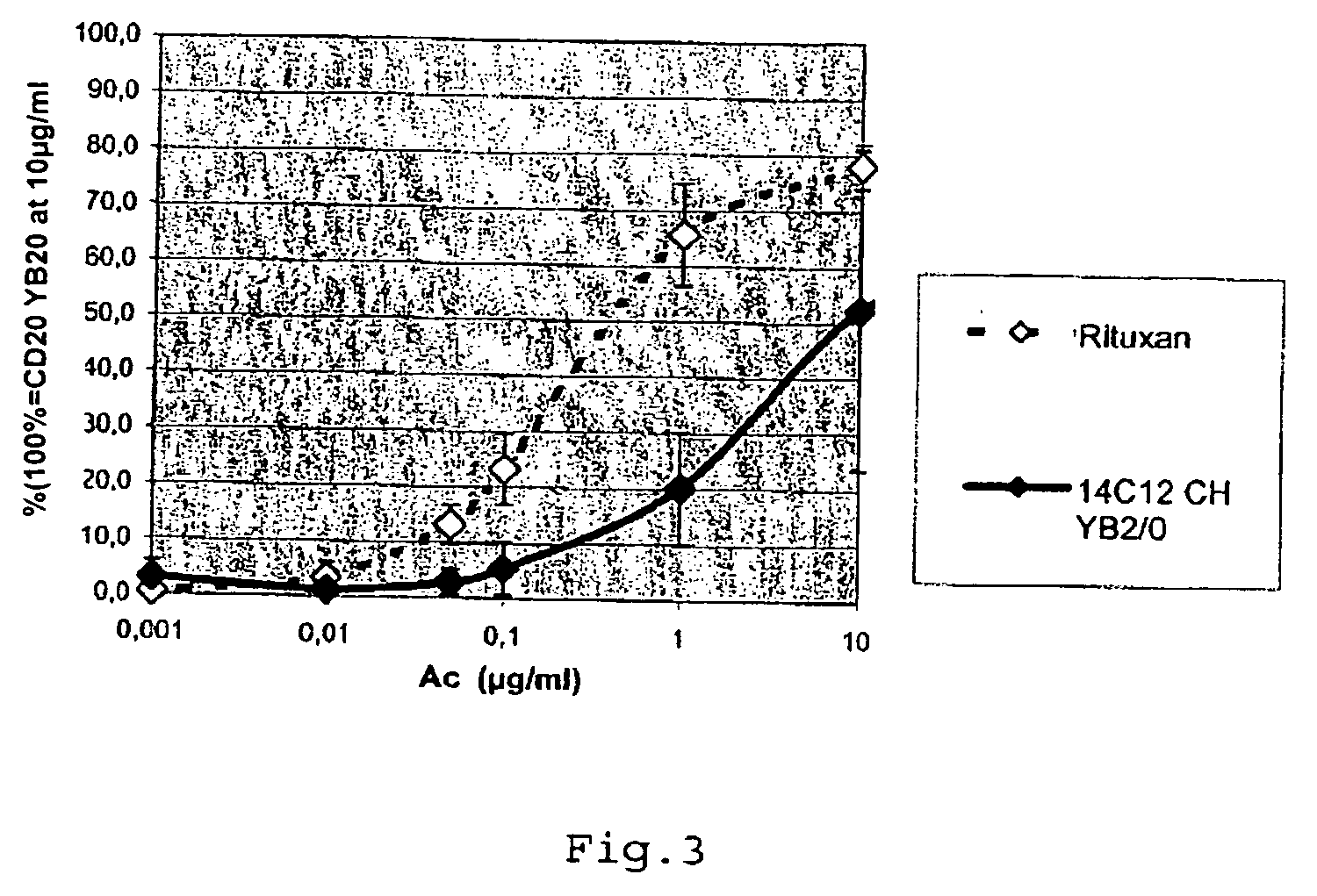
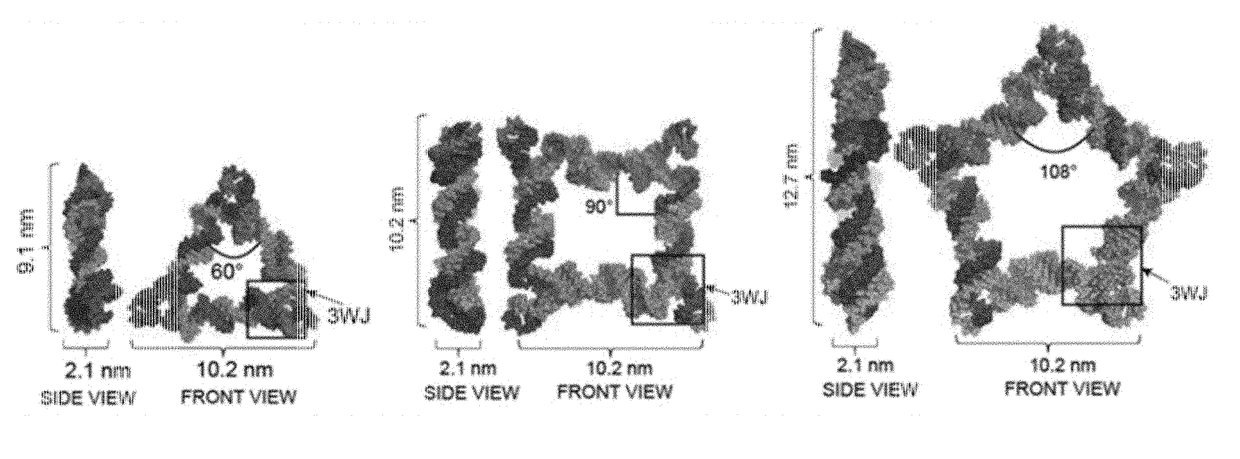
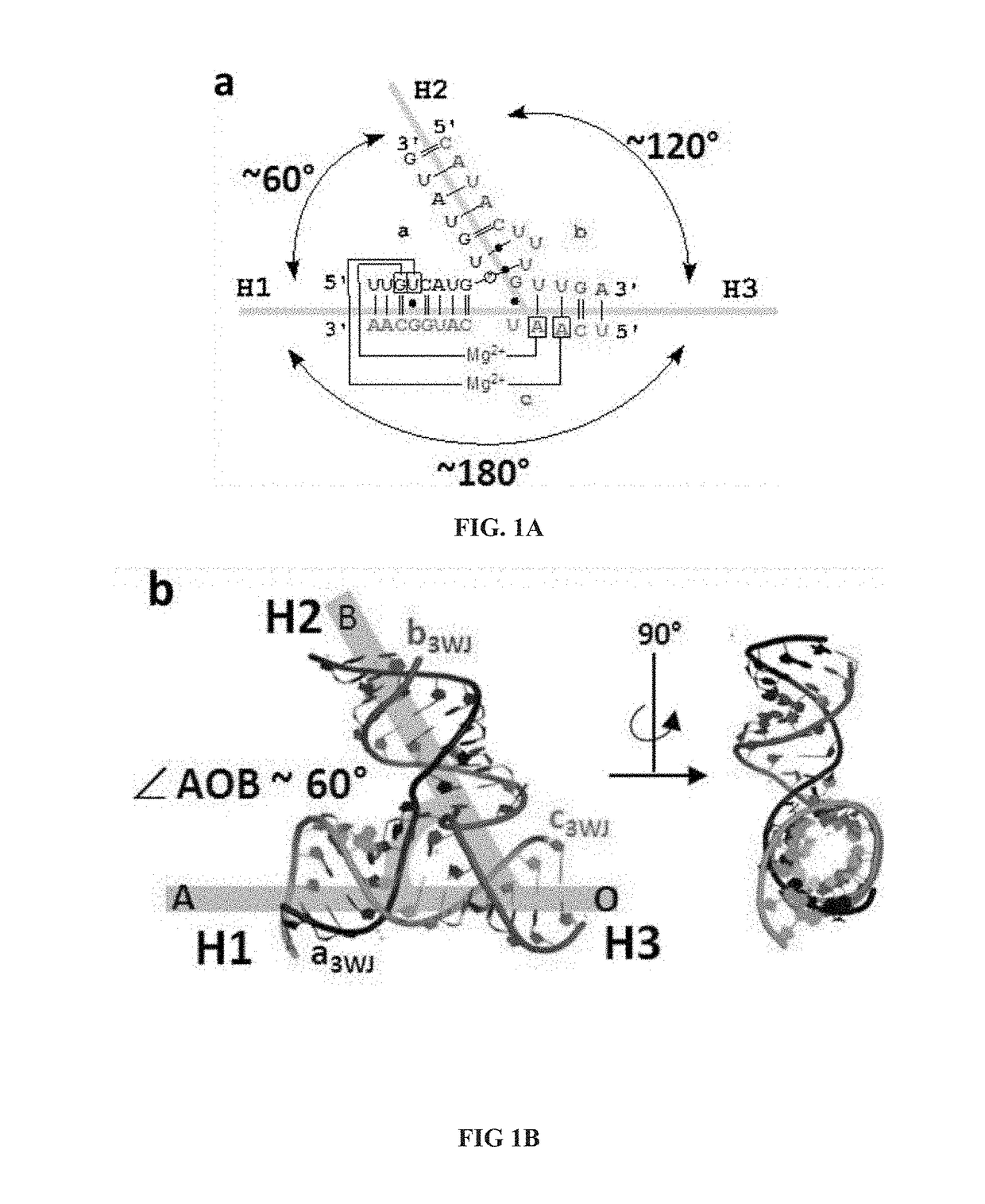
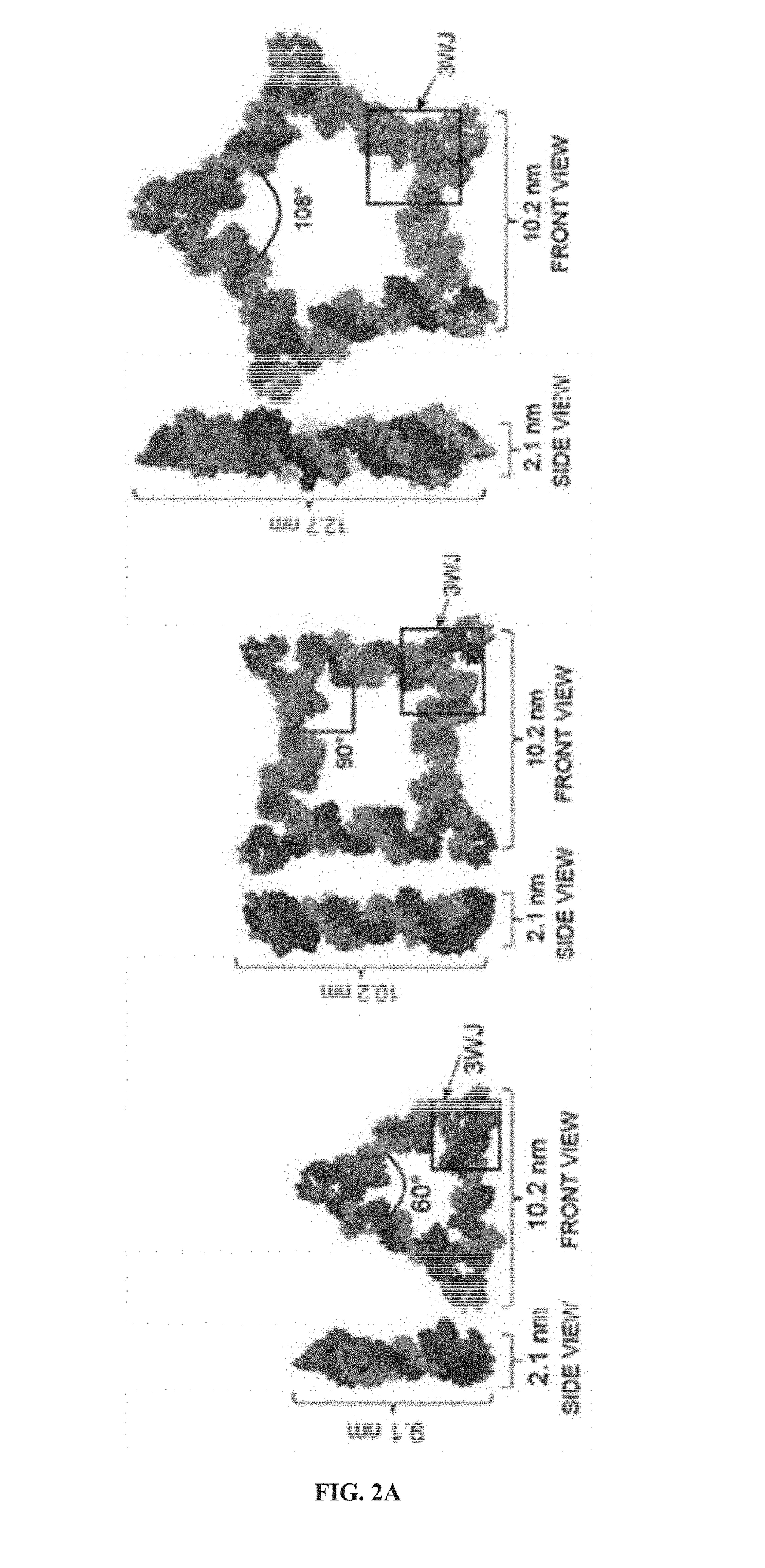
The present invention is directed towards an artificial RNA nanostructure comprising multiple external strands of RNA, each external strand comprising about 40-50 nucleotides; one internal strand of RNA comprising more than about 50 nucleotides; the internal strands and external strands assembled to form a triangle nanostructure, a square nanostructure, or a polygon nanostructure and a pRNA three-way junction (3WJ) motif at each vertex of the nanostructure. Such nanostructure can be provided in a composition together with an adjuvant for use in inducing the production of high affinity neutralizing antibodies or inhibitory antibodies, inducing the production of cytokines, inducing an immune response in a subject, or a combination thereof.
Owner:UNIV OF KENTUCKY RES FOUND
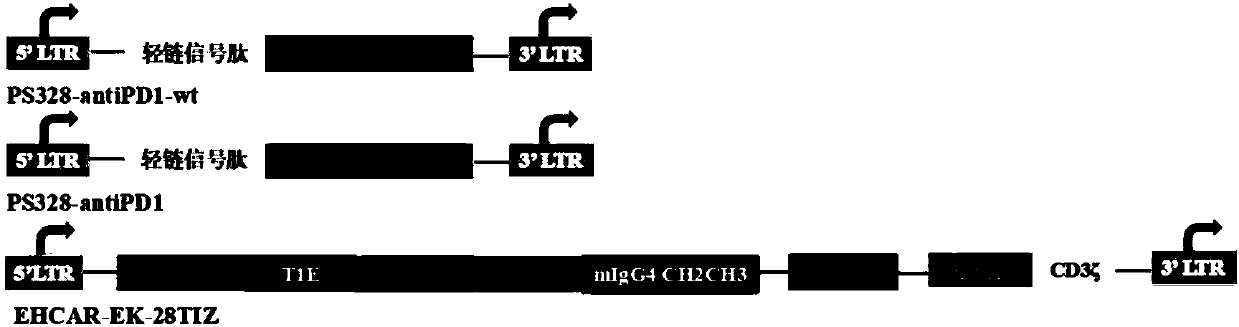
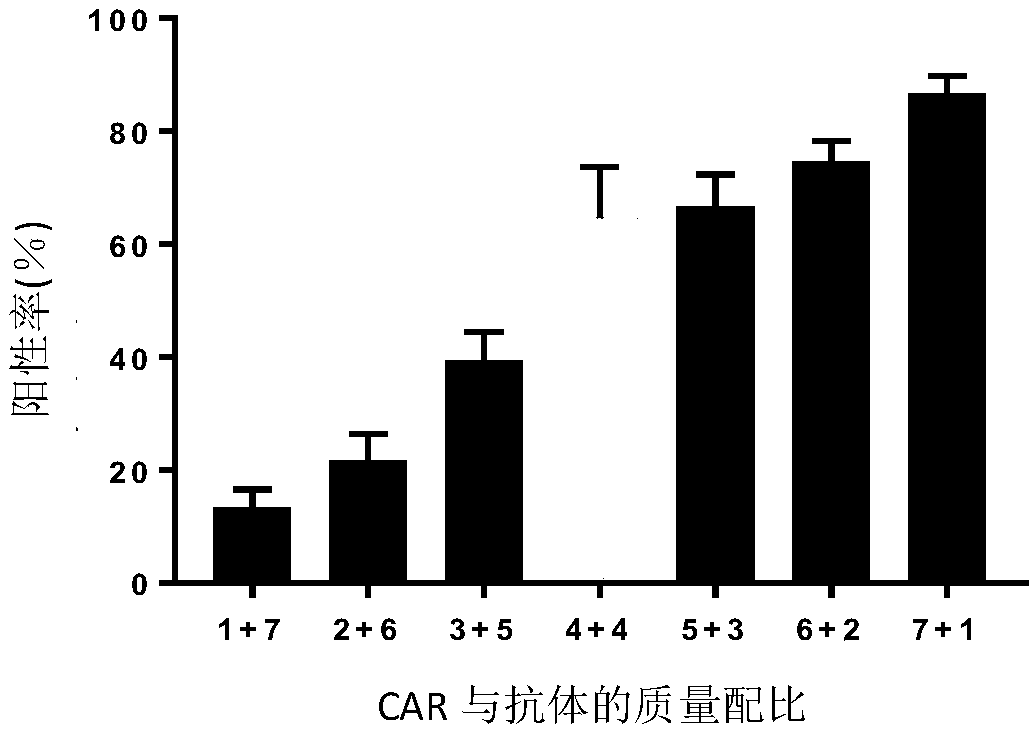
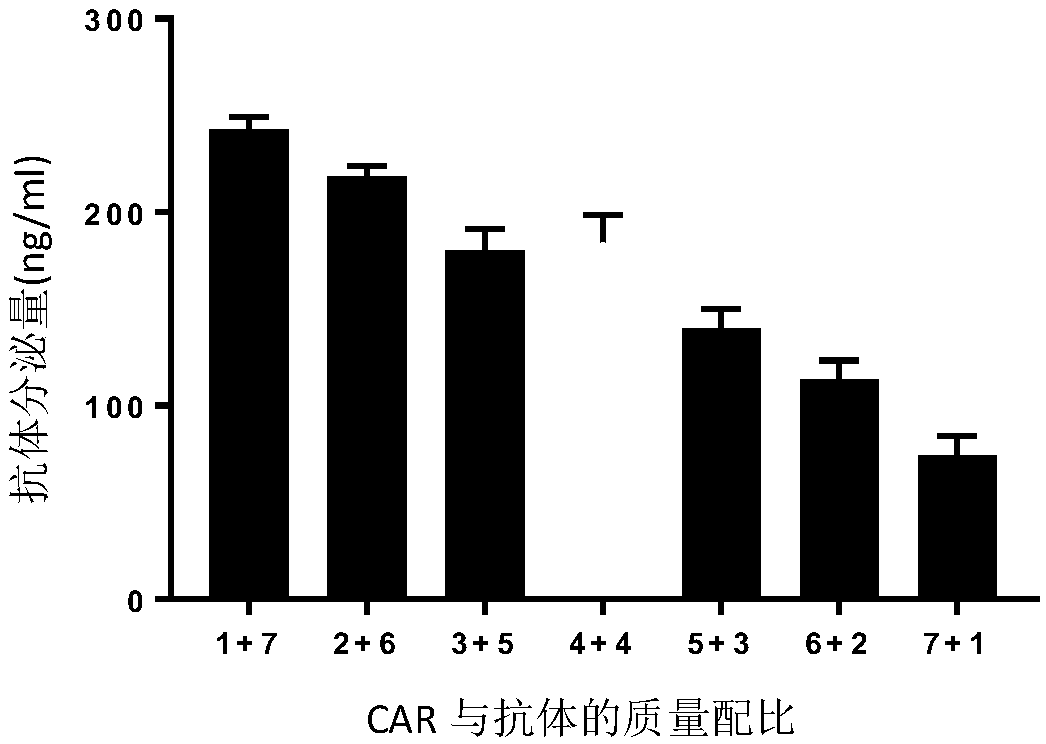
CAR-T cell targeting ErbB receptor family and self-expressing PD-1 antibody and use thereof
PendingCN109971724AMammal material medical ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenApoptosis
The present invention provides a CAR-T cell self-expressing a PD-1 immunological checkpoint inhibitory antibody and targeting a chimeric antigen receptor of ErbB receptor family and a use thereof. TheT cell comprises a coding sequence expressing the chimeric antigen receptor targeting the ErbB receptor family and a coding sequence of the PD1 antibody; and / or expressing the chimeric antigen receptor targeting the ErbB receptor family and the PD1 antibody. The T cell has better proliferation and activation ability than those of a T cell singly targeting the chimeric antigen receptor of the ErbBreceptor family, can overcome inhibition of an immune microenvironment, promotes apoptosis of tumor cells, and exerts an anti-tumor immune response and a tumor cell killing function.
Owner:SHANGHAI CELL THERAPY RES INST +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com