Killing cell for high-efficiency and stable expression of antibodies and use thereof
A cell-killing and lethal technology, applied in the fields of cell biology and oncology, can solve problems such as insufficiency, unsustainable expression, loss, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
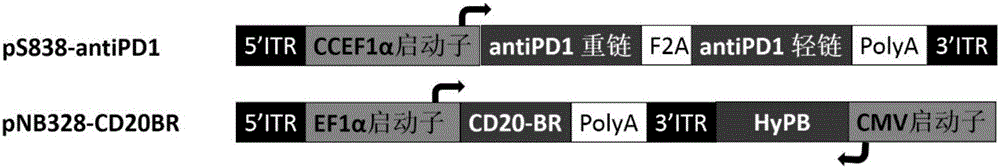
[0137] Example 1: Construction of recombinant plasmids pS838-AntiPD1 and pNB328-CD20BR
[0138] Entrusted Shanghai Jereh Biological Company to synthesize two DNA sequences, the sequences are as follows:
[0139] Seq1: CGATAGGACGCTGATCTTAAT (SEQ ID NO: 1)
[0140] Seq2: TACCTGCGACTAGAAT (SEQ ID NO:2)
[0141] Denaturation at 98°C for 5 minutes, followed by natural cooling to form double-stranded DNA adapters with ClaI and PacI sticky ends at the upstream and downstream respectively.
[0142] The pNB vector was double-digested with CalI and PacI (refer to CN201510638974.7 for the construction process), and loaded with the above double-stranded DNA to obtain the pS vector.
[0143] According to the CCEF promoter sequence published by CN201510021408.1, Shanghai Jereh Biological Co., Ltd. was commissioned to synthesize it, and introduced XbaI and EcoRI restriction sites in the upstream and downstream, respectively, and loaded it into the pS vector that was double-digested with ...
Embodiment 2
[0151] Example 2: Genetic modification of peripheral blood T lymphocytes
[0152] Prepare 1×10 7 Freshly isolated peripheral blood mononuclear cells (PBMC) were co-transfected with pNB328-CD20BR and pS838-antiPD1 into the nuclei with a Lonza 2b-Nucleofector instrument at a ratio of 1:2, and placed at 37°C for 5 %CO 2 Culture in an incubator; transfer to a 6-well plate containing 30ng / mL anti-CD3 antibody and 3000IU / mL IL-2 (purchased from Novoprotein Company) after 6 hours, and place at 37°C, 5% CO 2 Incubator culture. After the cells grow healthily, the pluripotent T cells expressing PD1 antibody, referred to as PIK-T, are obtained. Untransfected PBMC were spread on a culture plate containing 30ng / mL anti-CD3 antibody and 3000IU / mL IL-2 (purchased from Novoprotein Company) at 37°C and 5% CO 2 cultured in an incubator as a control. Since only the pNB328 vector contains the transposase required for exogenous gene integration, and the pS838 vector only contains the ITR el...
Embodiment 3
[0153] Example 3: Quantitative detection of PD1 antibody expression in PIK-T cells
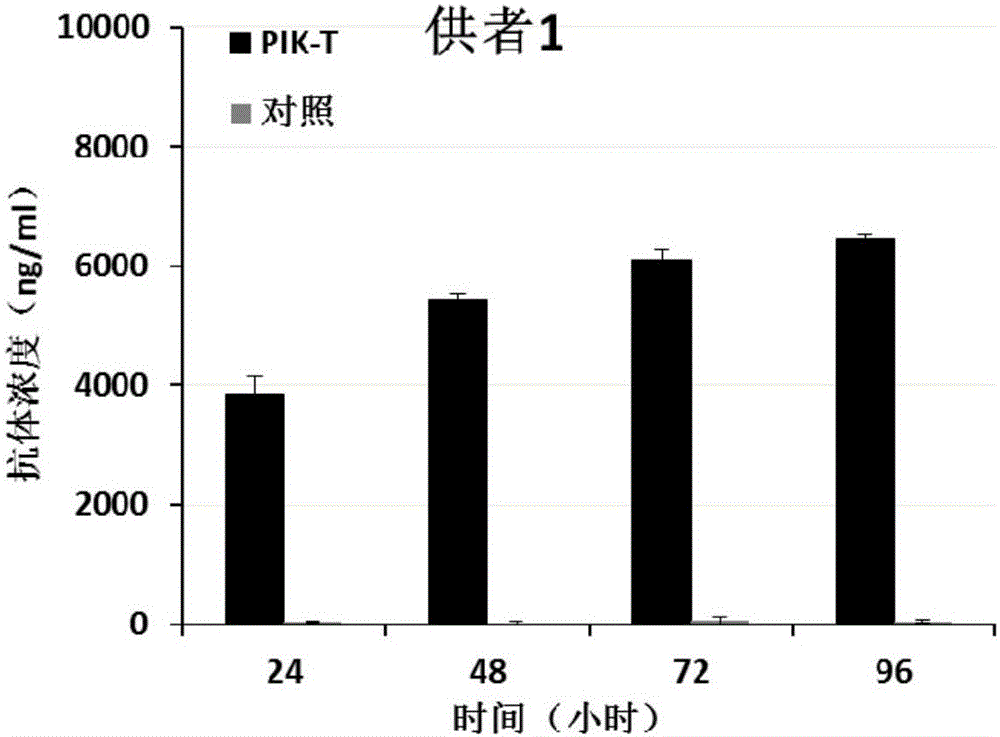
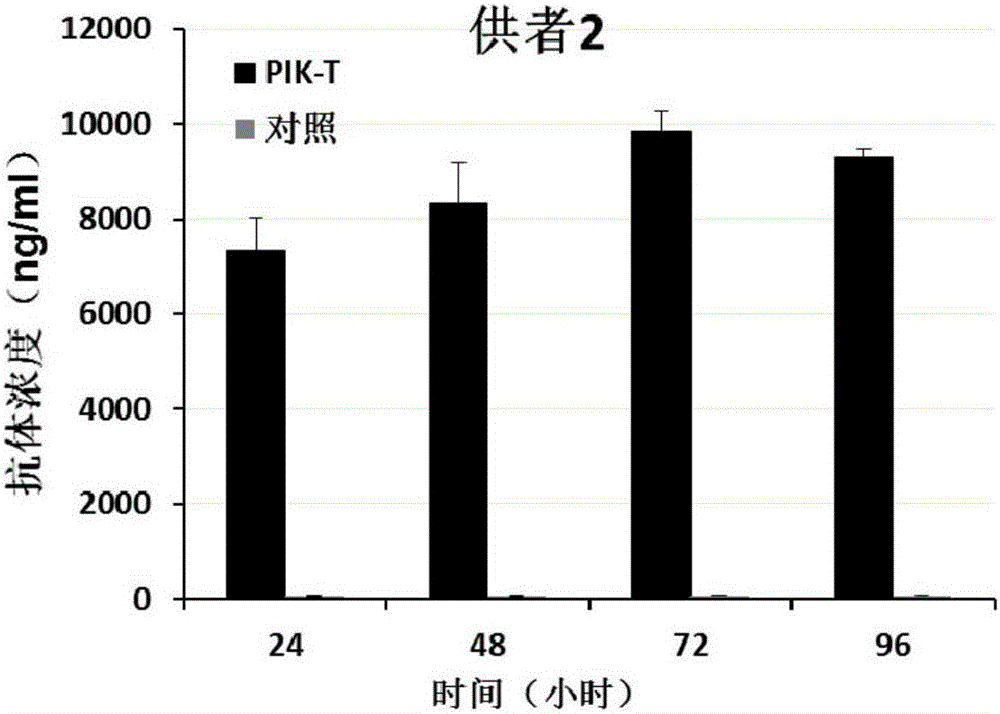
[0154] The PIK-T and control T cells prepared in Example 2 were subcultured at a dilution ratio of 1:3, and two weeks later, 1.0×10 6 Cells / well were spread in a 6-well plate with 4ml of AIM-V medium (purchased from Gibco), placed at 37°C, 5% CO 2 Culture in an incubator, and collect 800 μl of cell supernatant after 24 hours, 48 hours, 72 hours, and 96 hours of culture, and store at -20°C for future use. Human PD1 recombinant protein was used to coat the microtiter plate (purchased from SinoBiological Company), and HRP-labeled mouse anti-human IgG mAb (purchased from Abcam Company) was used for detection, and commercialized anti-PD1 antibody (purchased from Merck Company) was used as For standard products, the expression level of PD1 antibody was detected by double-sandwich ELISA method.
[0155] It was found that PIK-T cells from three different donors were able to stably express PD1 anti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com