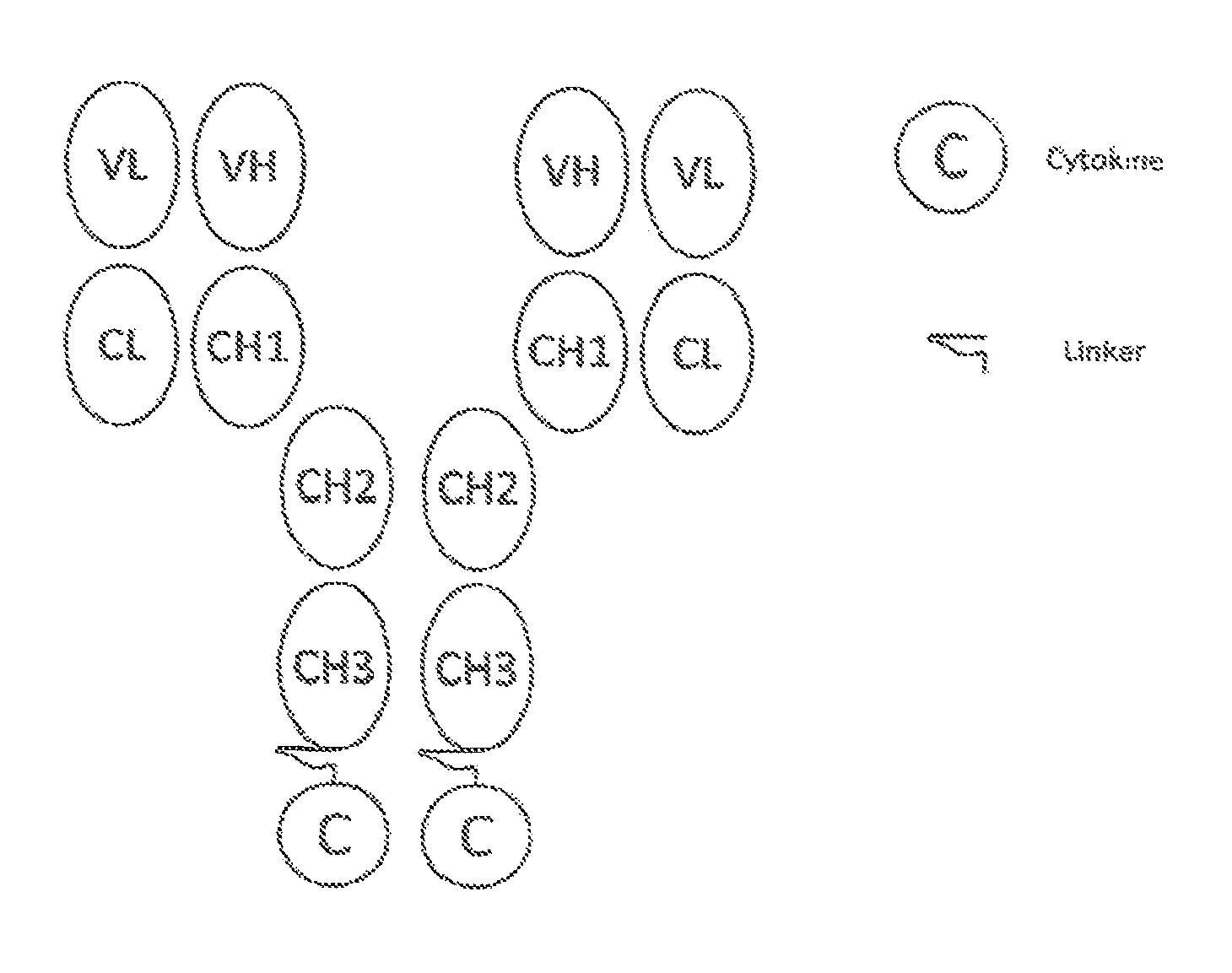
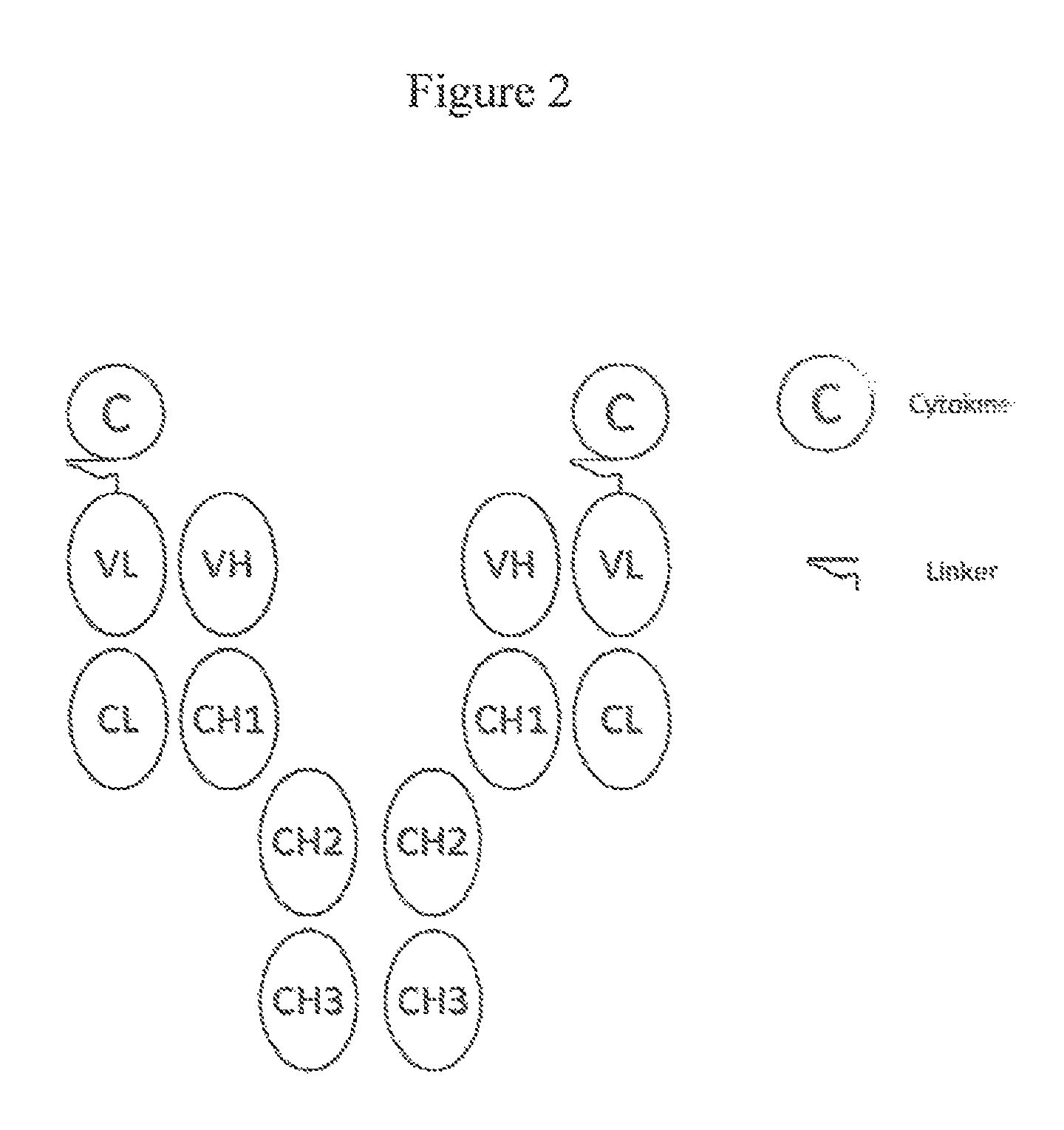
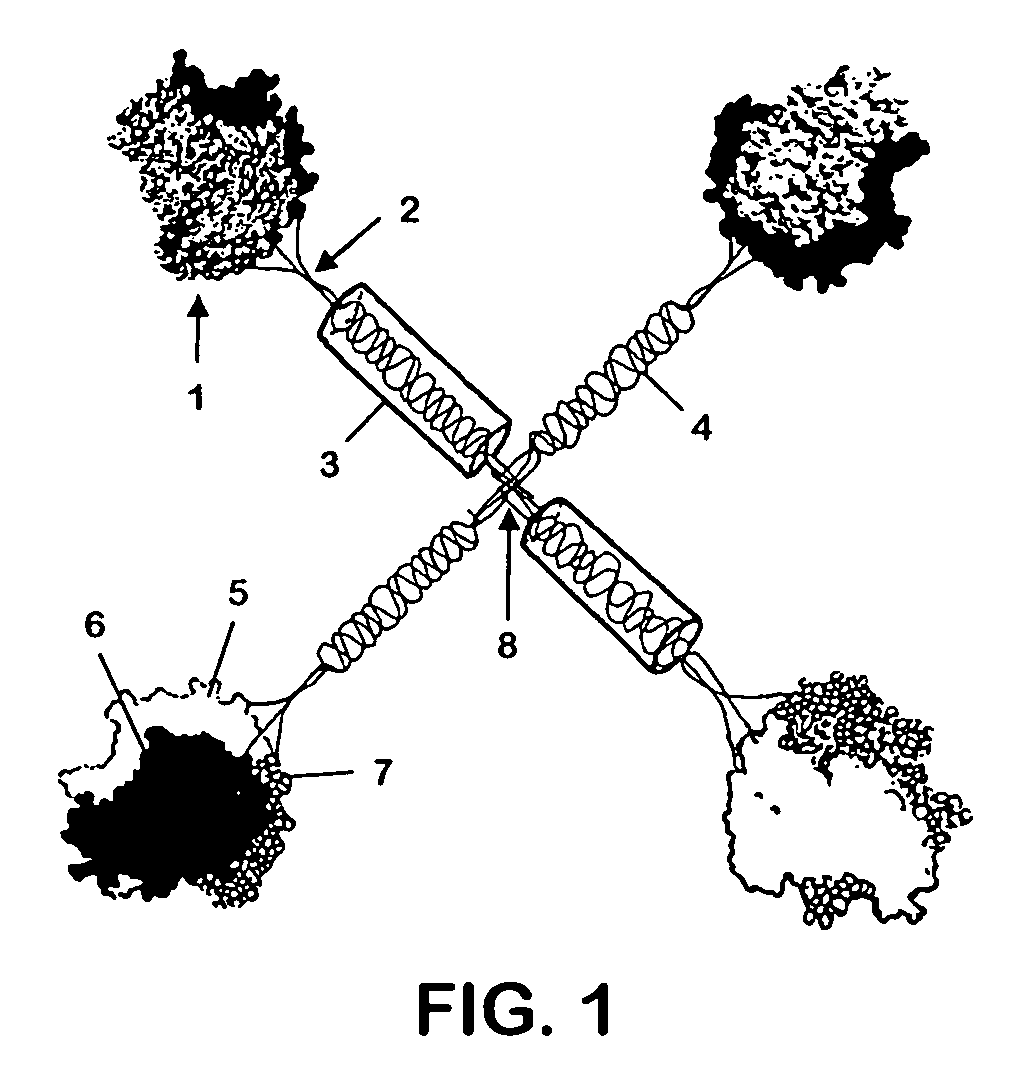
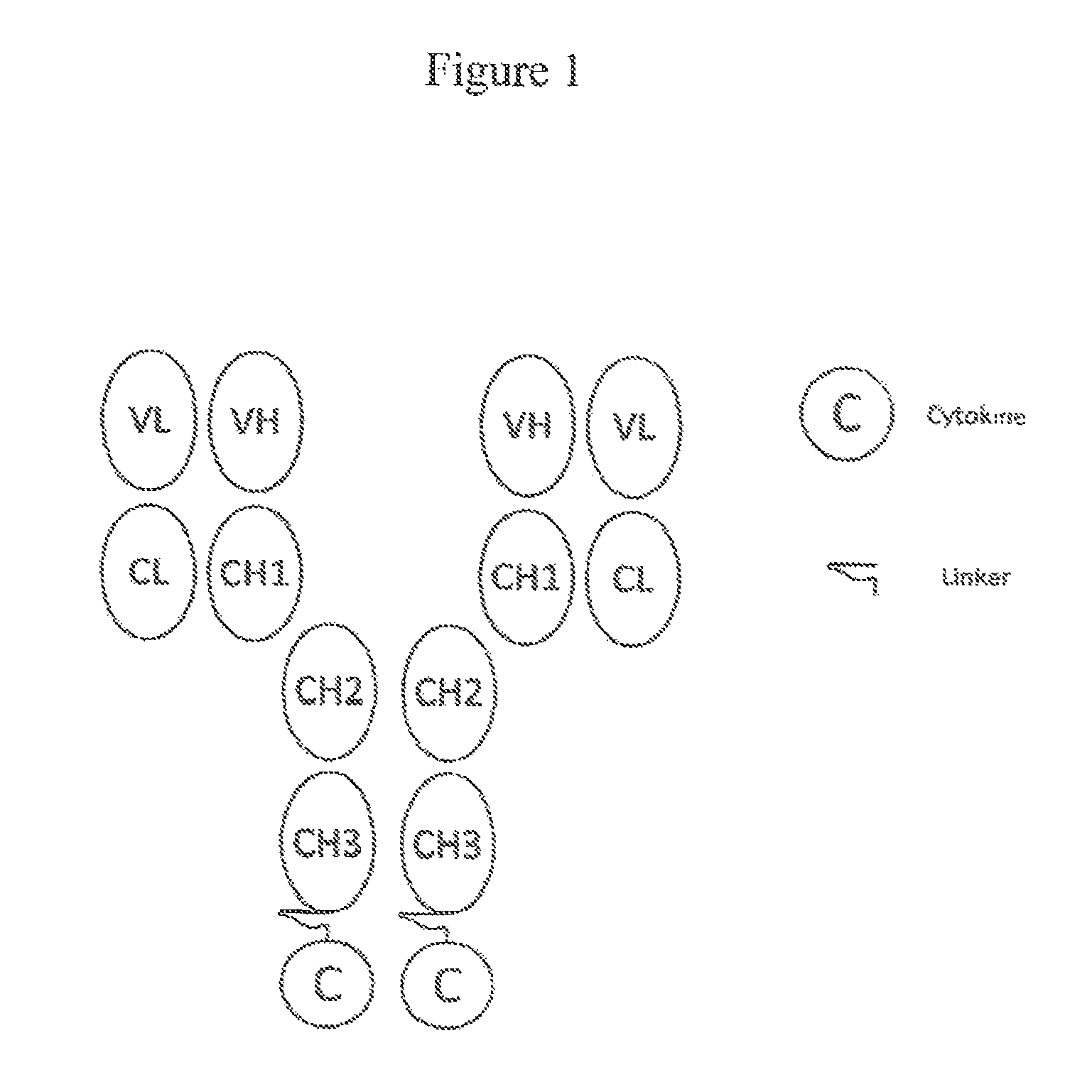Patents
Literature
225 results about "Systemic toxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition Systemic Effects or Systemic Toxicity Toxic effects as a result of absorption and distribution of a toxicant to a site distant from its entry point. ... Most chemicals that produce systemic toxicity do not cause a similar degree of toxicity in all organs, but usually demonstrate major toxicity to one or two organs. These are referred to as the target organs of toxicity for that chemical.
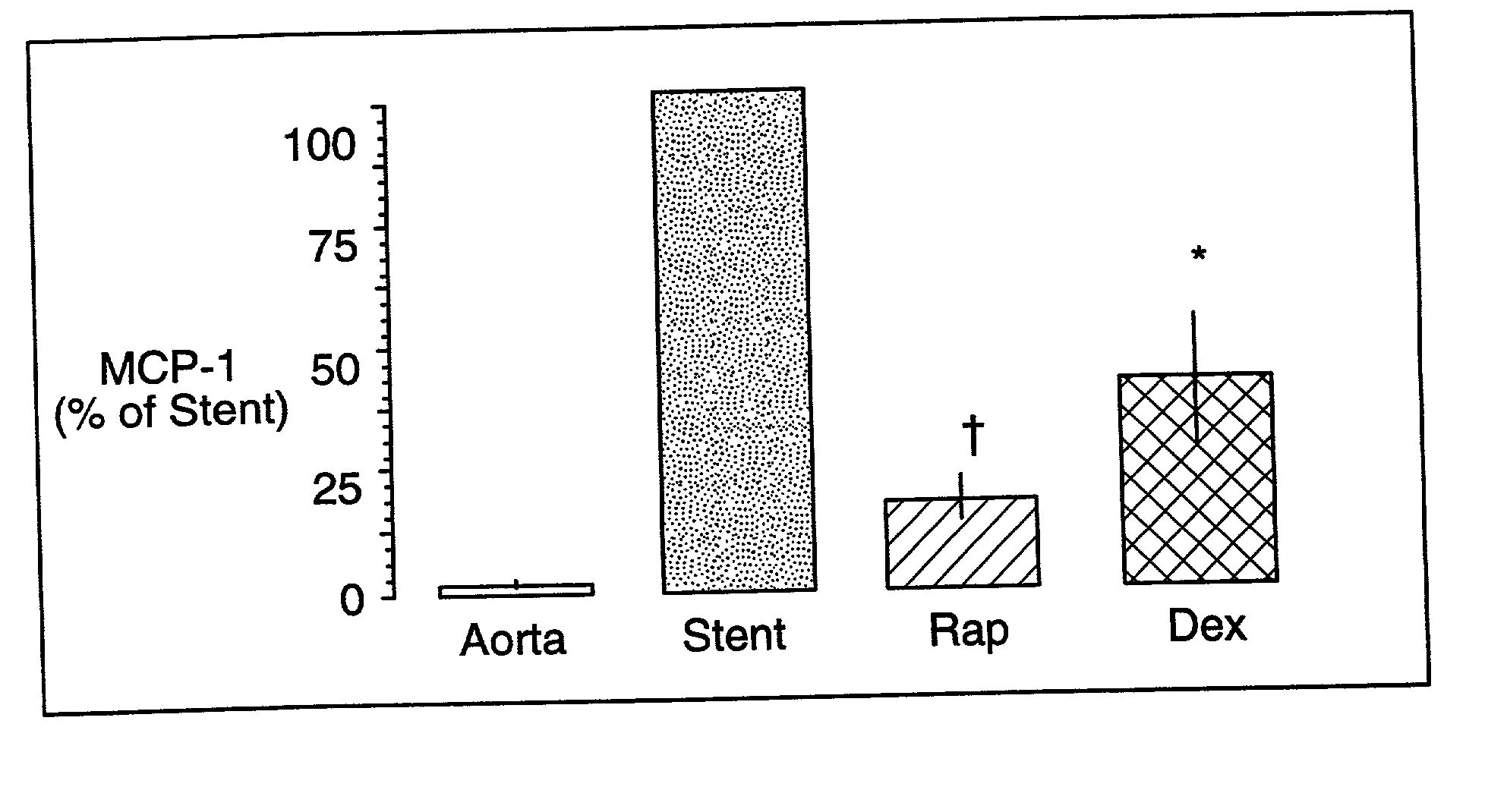
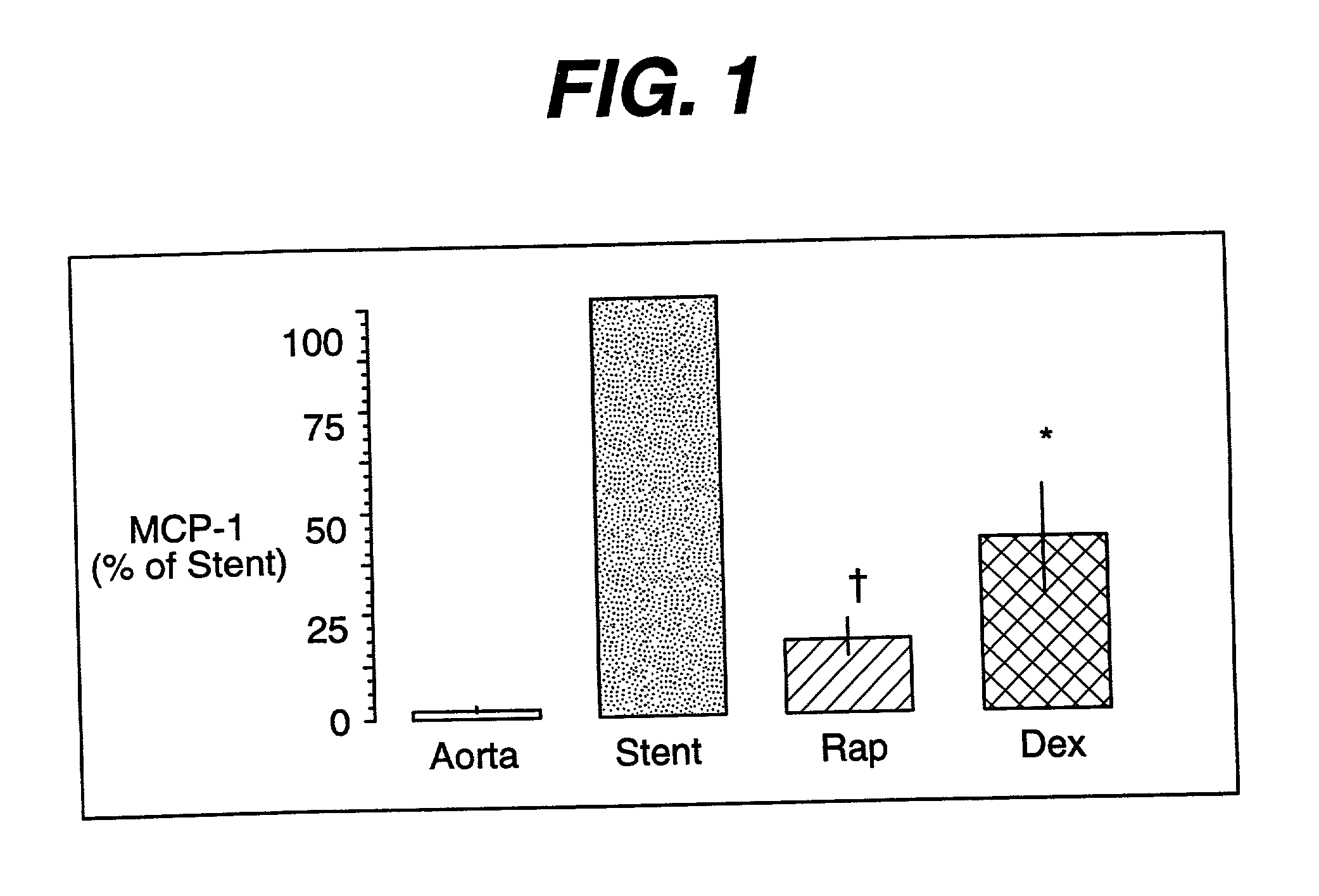
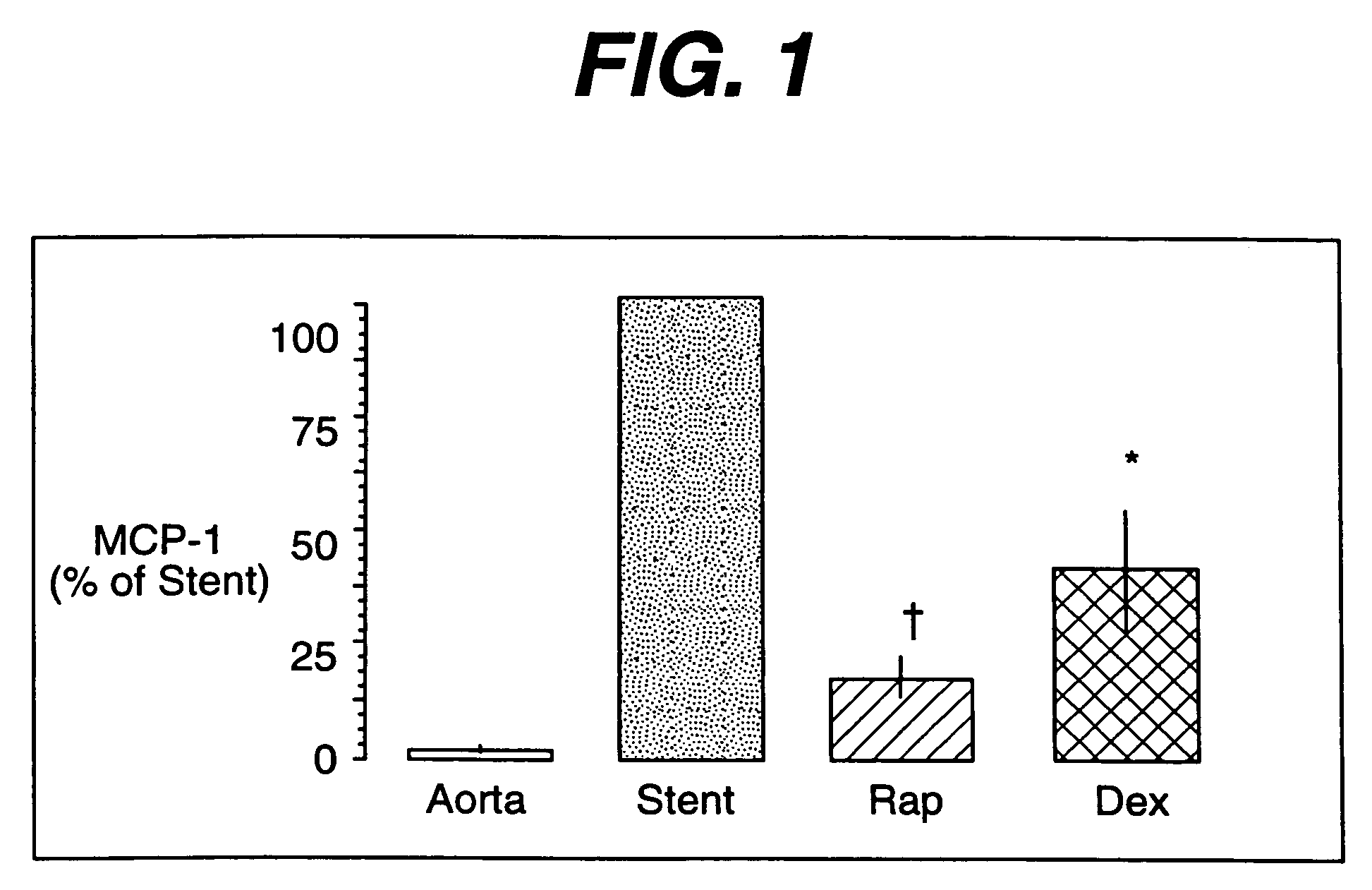
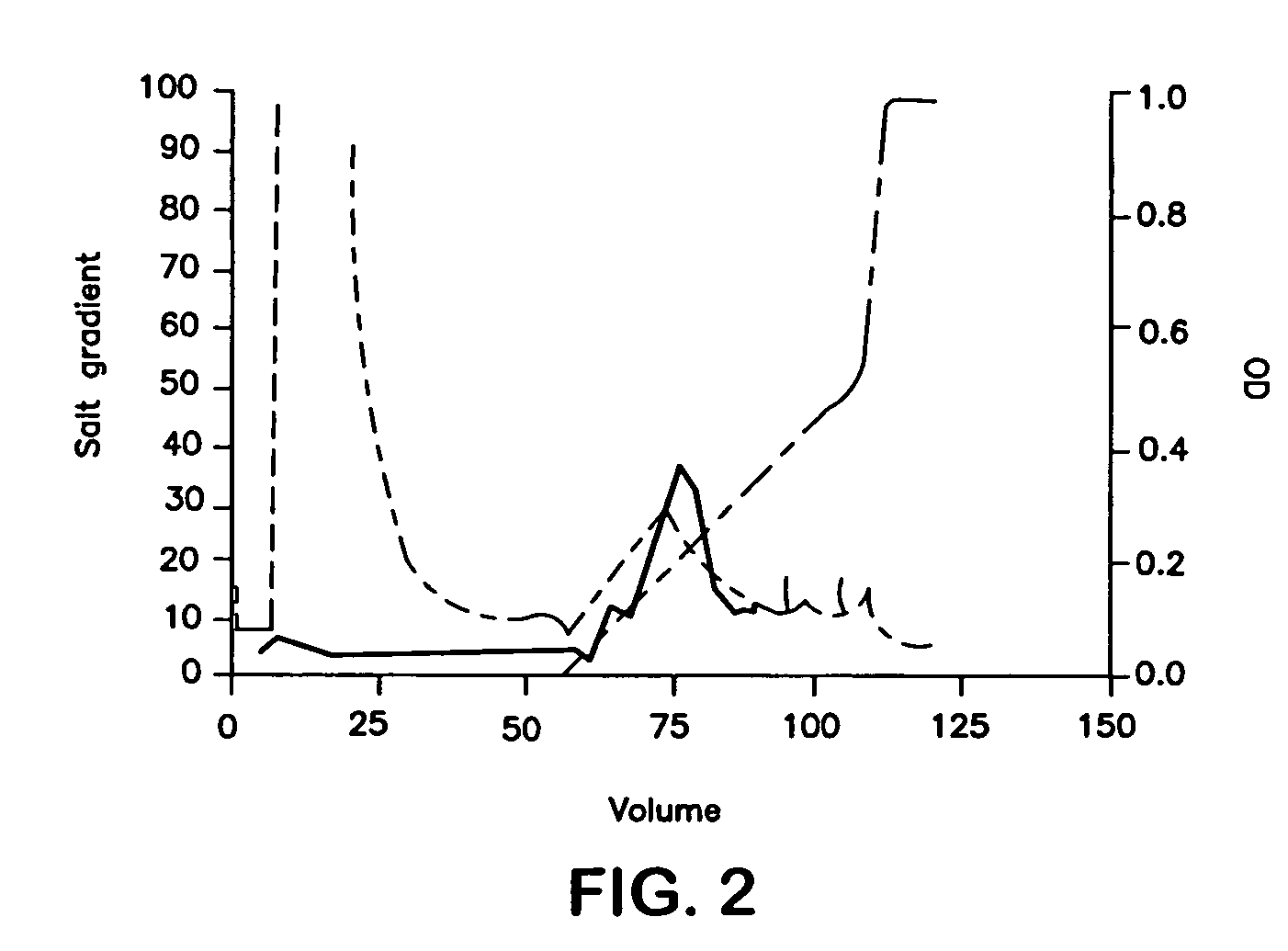
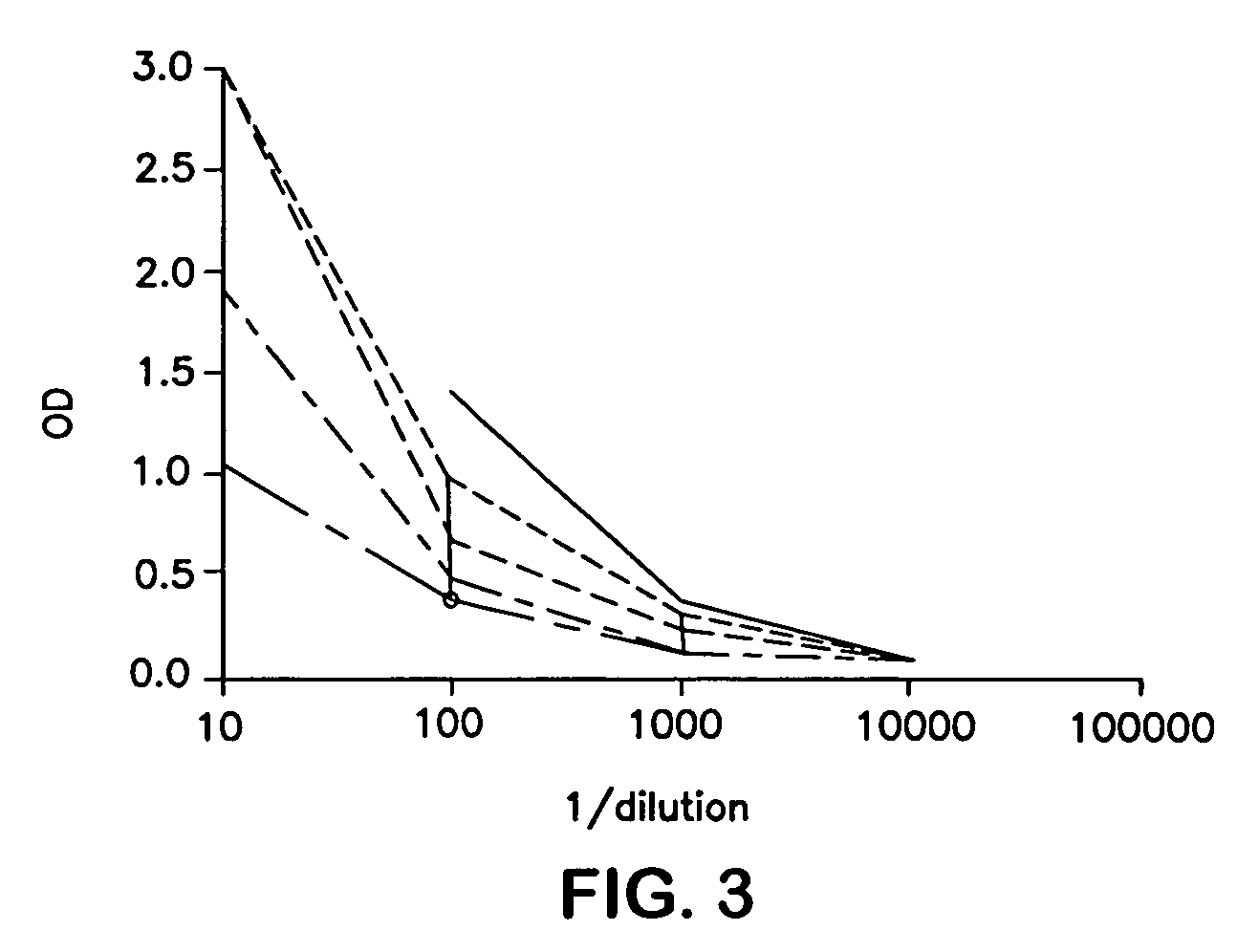
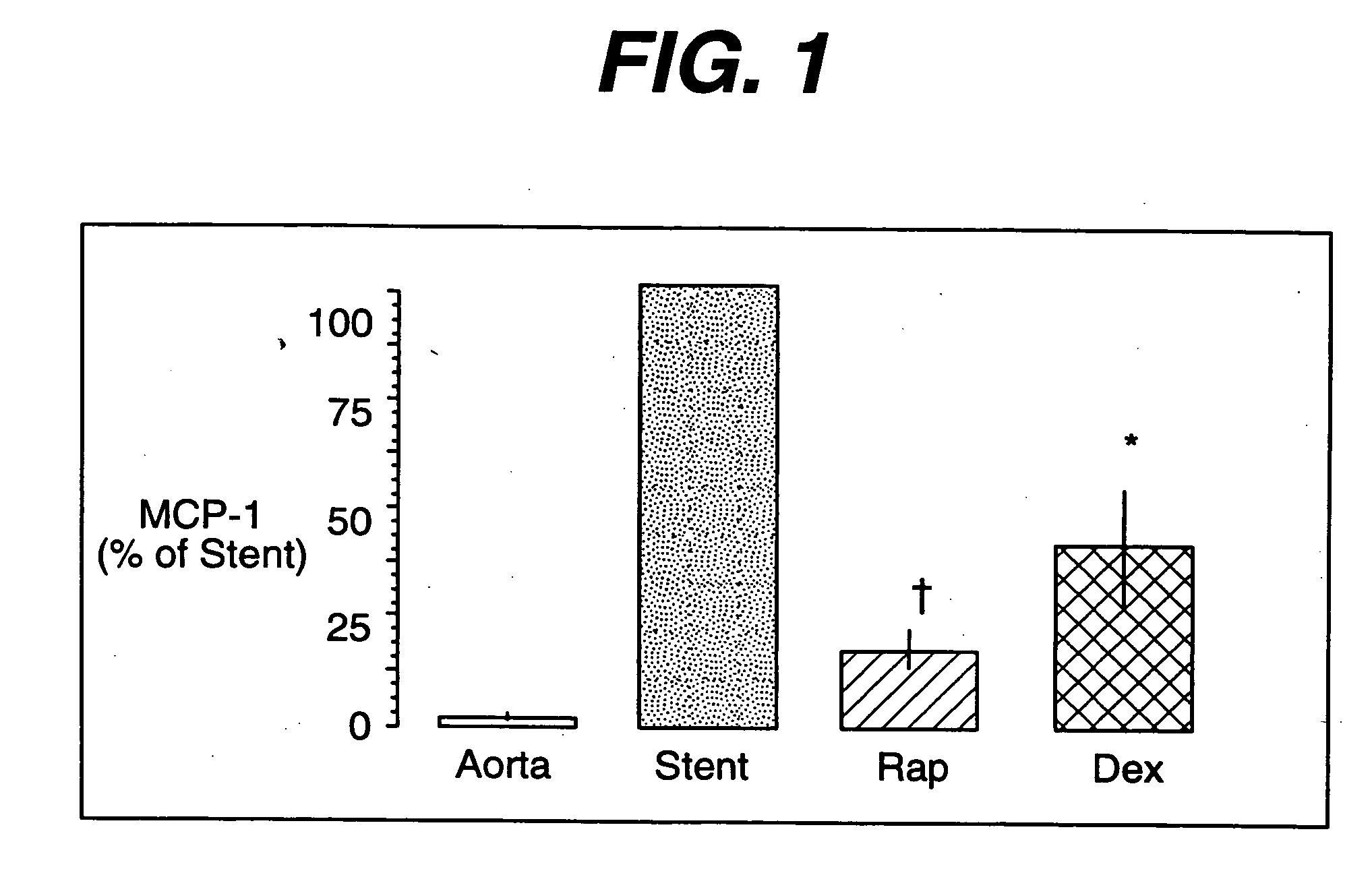
Drug/drug delivery systems for the prevention and treatment of vascular disease
InactiveUS20020007215A1Prevent proliferationGood effectOrganic active ingredientsStentsVascular diseaseWhole body
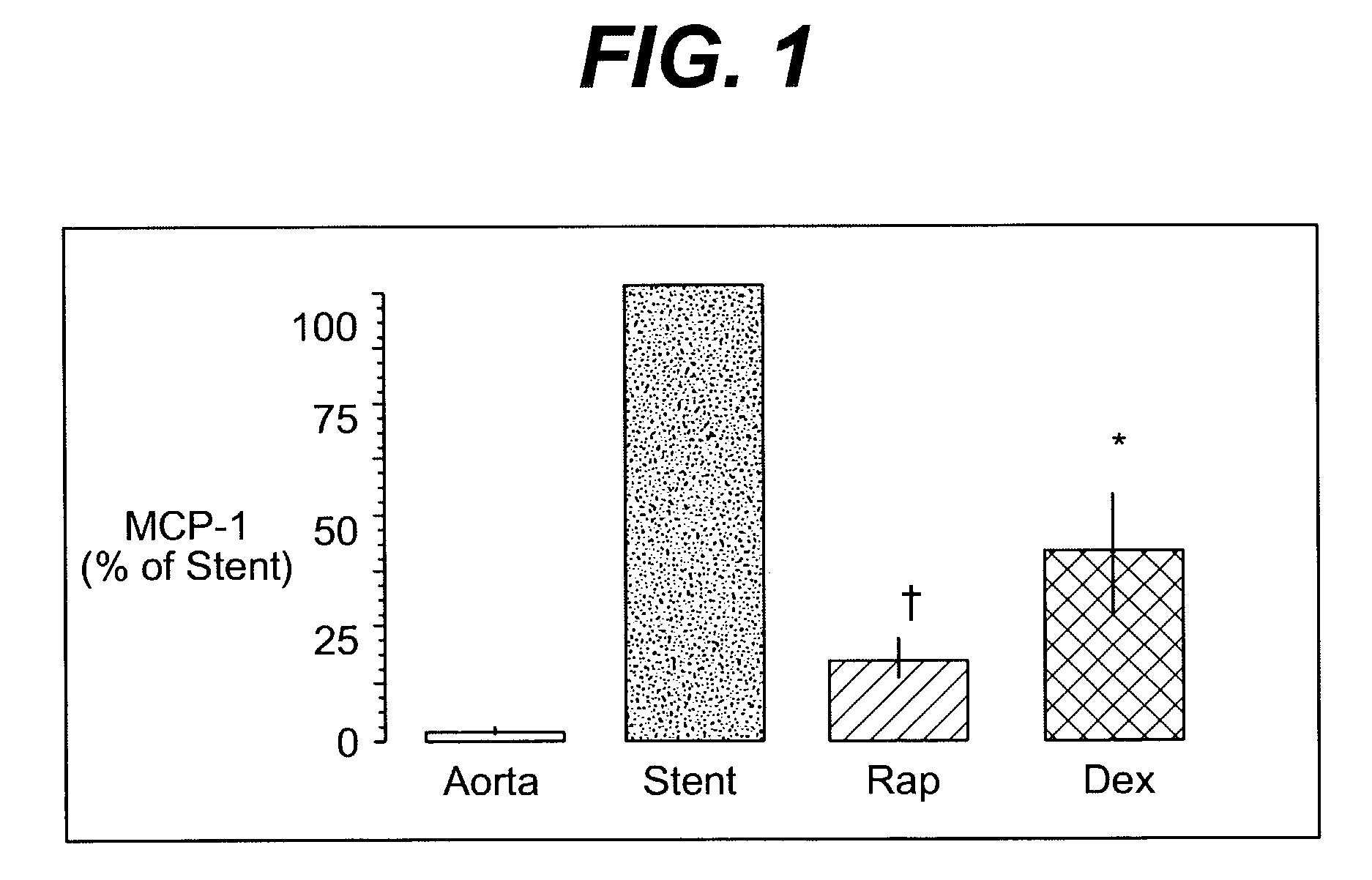
A drug and drug delivery system may be utilized in the treatment of vascular disease. A local delivery system is coated with rapamycin or other suitable drug, agent or compound and delivered intraluminally for the treatment and prevention of neointimal hyperplasia following percutaneous transluminal coronary angiography. The local delivery of the drugs or agents provides for increased effectiveness and lower systemic toxicity.
Owner:WYETH LLC
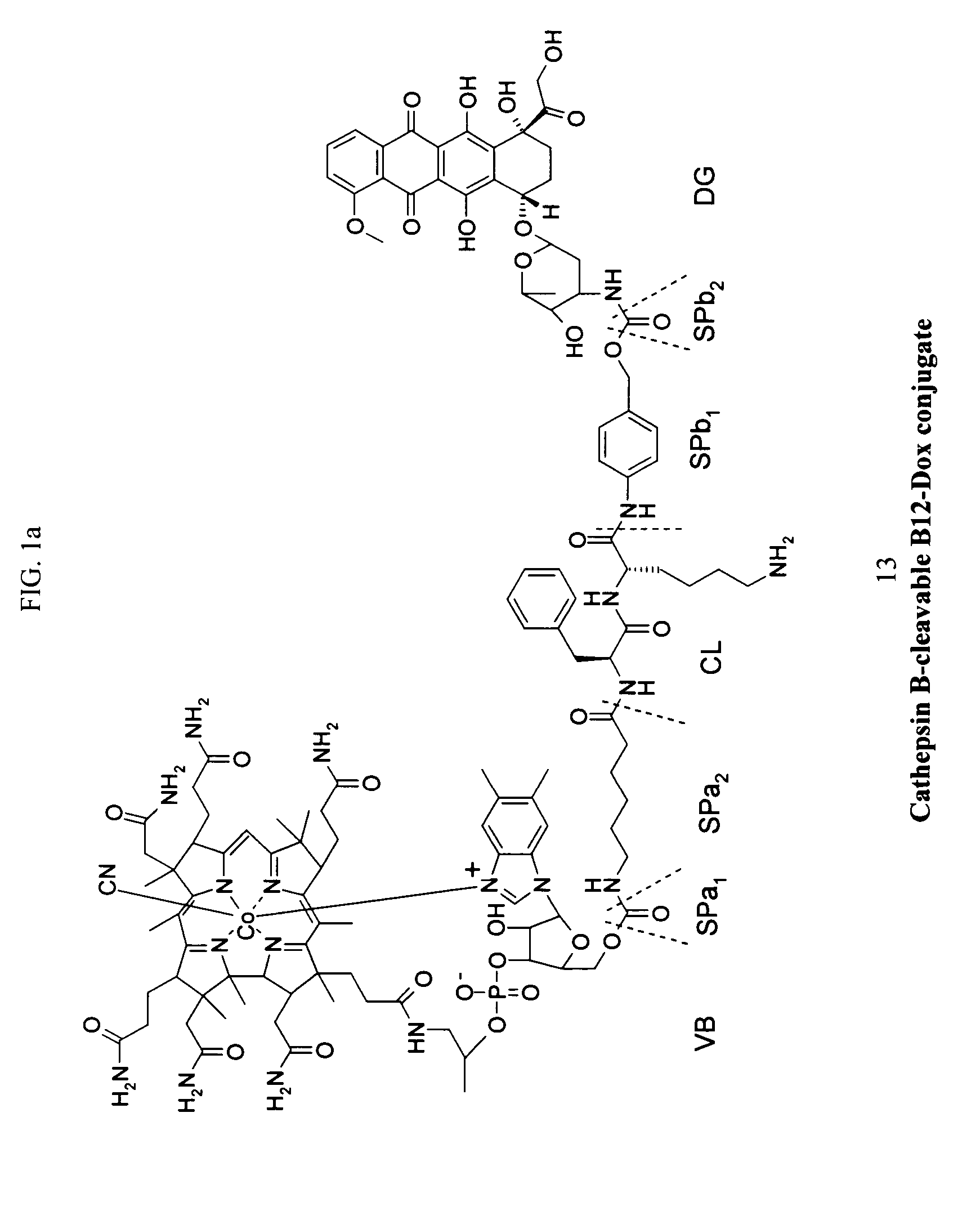
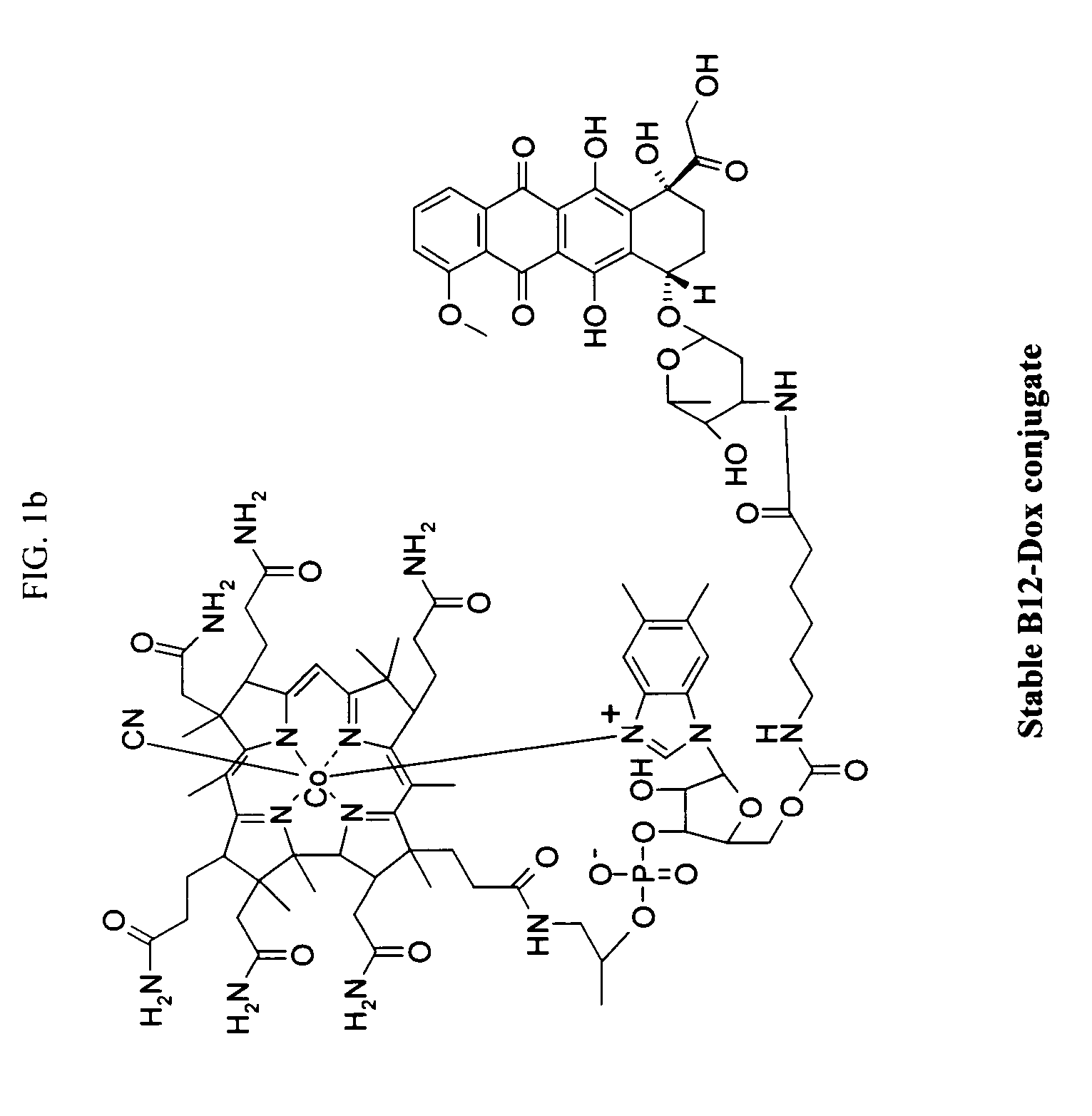
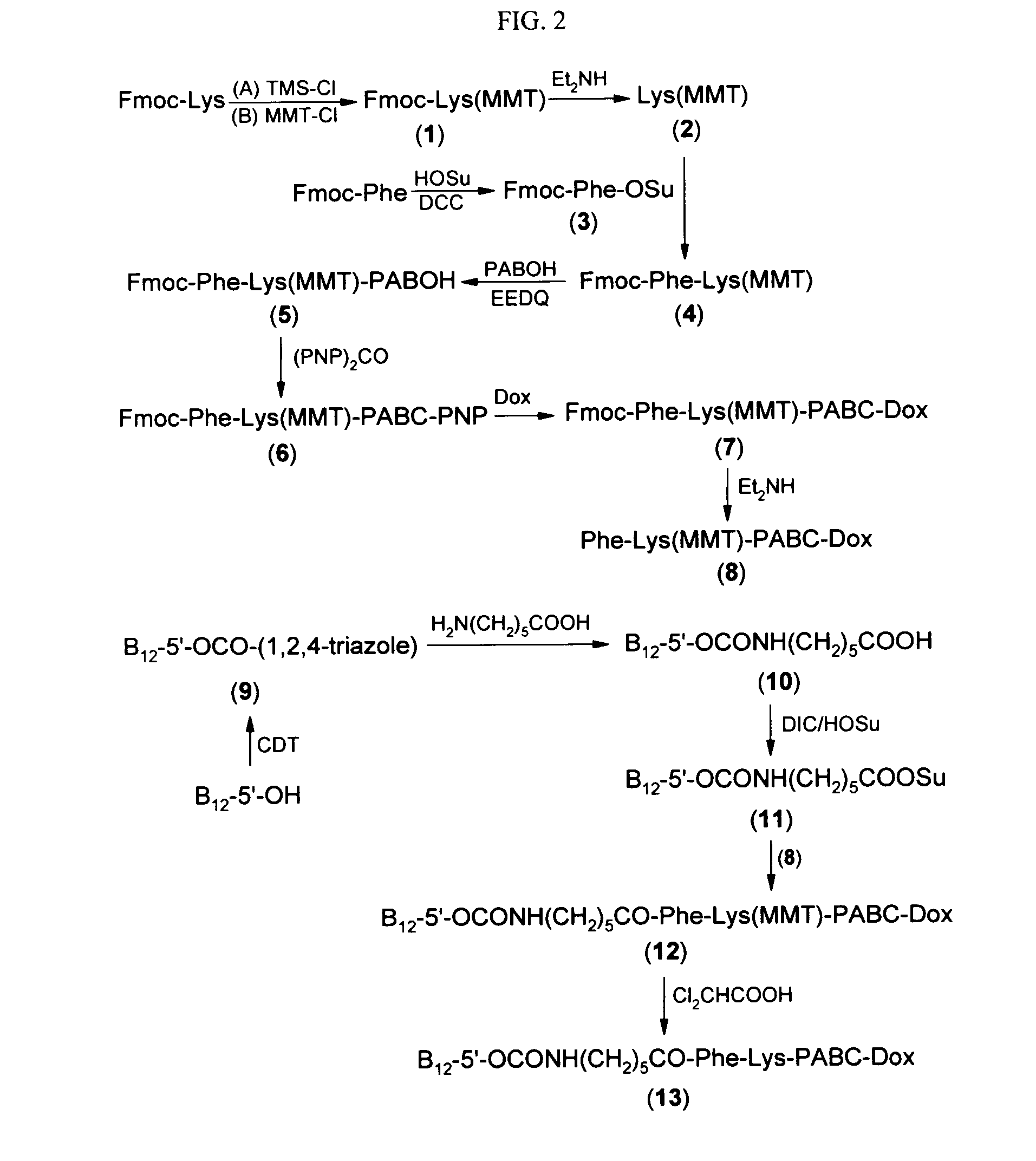
Cobalamin conjugates for anti-tumor therapy
The present invention provides a cobalamin-drug conjugate suitable for the treatment of tumor related diseases. Cobalamin is indirectly covalently bound to an anti-tumor drug via a cleavable linker and one or more optional spacers. Cobalamin is covalently bound to a first spacer or the cleavable linker via the 5′-OH of the cobalamin ribose ring. The drug is bound to a second spacer of the cleavable linker via an existing or added functional group on the drug. After administration, the conjugate forms a complex with transcobalamin (any of its isoforms). The complex then binds to a receptor on a cell membrane and is taken up into the cell. Once in the cell, an intracellular enzyme cleaves the conjugate thereby releasing the drug. Depending upon the structure of the conjugate, a particular class or type of intracellular enzyme affects the cleavage. Due to the high demand for cobalamin in growing cells, tumor cells typically take up a higher percentage of the conjugate than do normal non-growing cells. The conjugate of the invention advantageously provides a reduced systemic toxicity and enhanced efficacy as compared to a corresponding free drug.
Owner:INFLABLOC PHARMA
Inhibitor which is deactivatable by a reagent produced by a target cell
ActiveUS8809504B2Undesirable effectModulating responseNGF/TNF-superfamilyAntibody ingredientsActive agentWhole body
The invention relates to molecules inhibiting biologically active compounds and further comprising moieties specifically cleavable by a reagent produced by a target cell. The invention relates to inhibitors that bind, inhibit, suppress, neutralize, or decrease activity of a biologically active agent. Inhibitors comprise at least one moiety that bind, inhibit, suppress, neutralize, or decrease activity of a biologically active agent and at least one moiety that can be cleaved specifically by a reagent produced by target cells. The cleavage deactivates the inhibitor. Following cleavage, the active agent is liberated into the local environment. Administration of the inhibitor alone or together with the active agent suppress the compound's activity until it reaches the proximity of a target cell. Targeted specific release enables the agent concentration in specific site to reach levels that have desired therapeutic effects without systemic toxicity.
Owner:VYTACERA BIO LLC
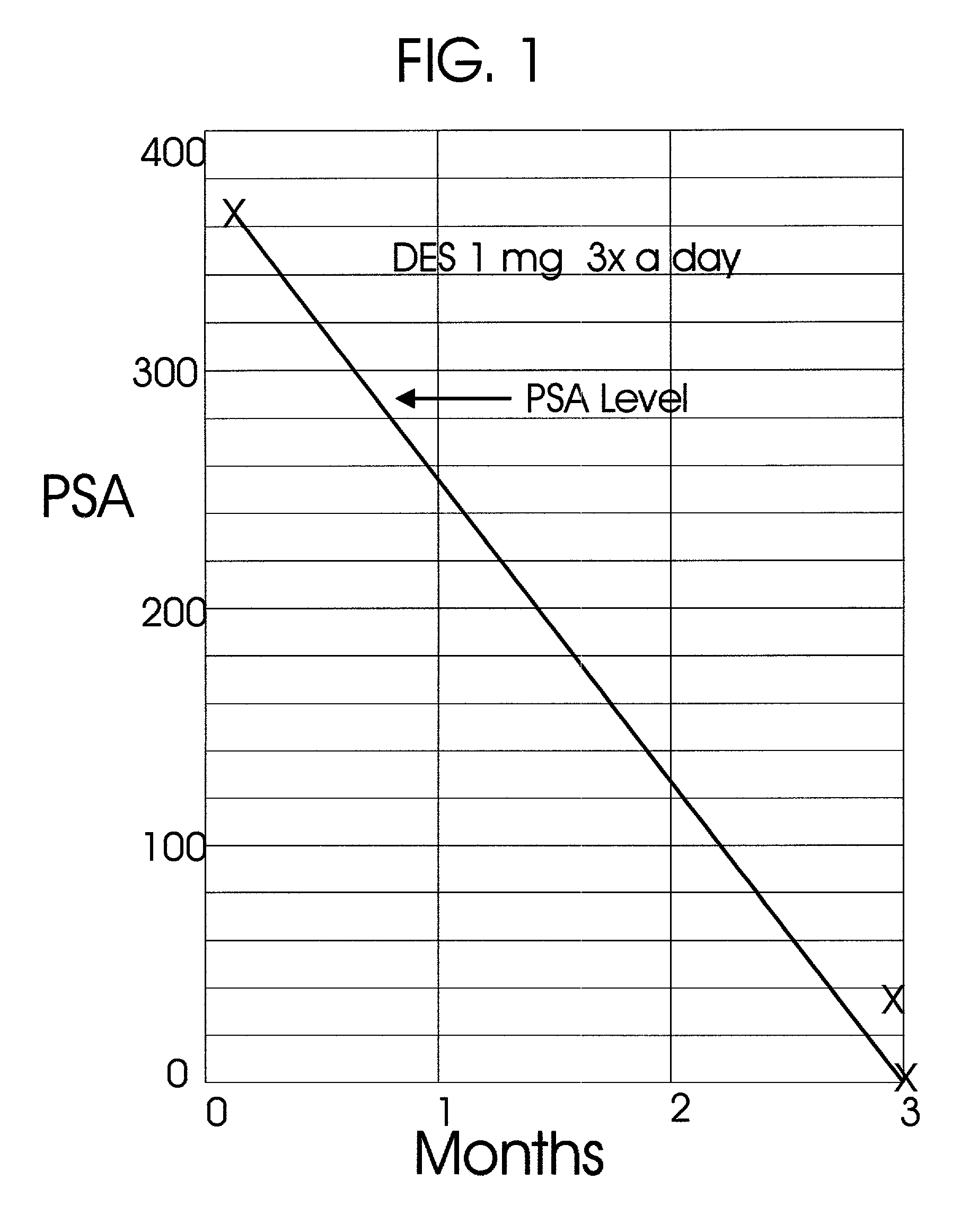
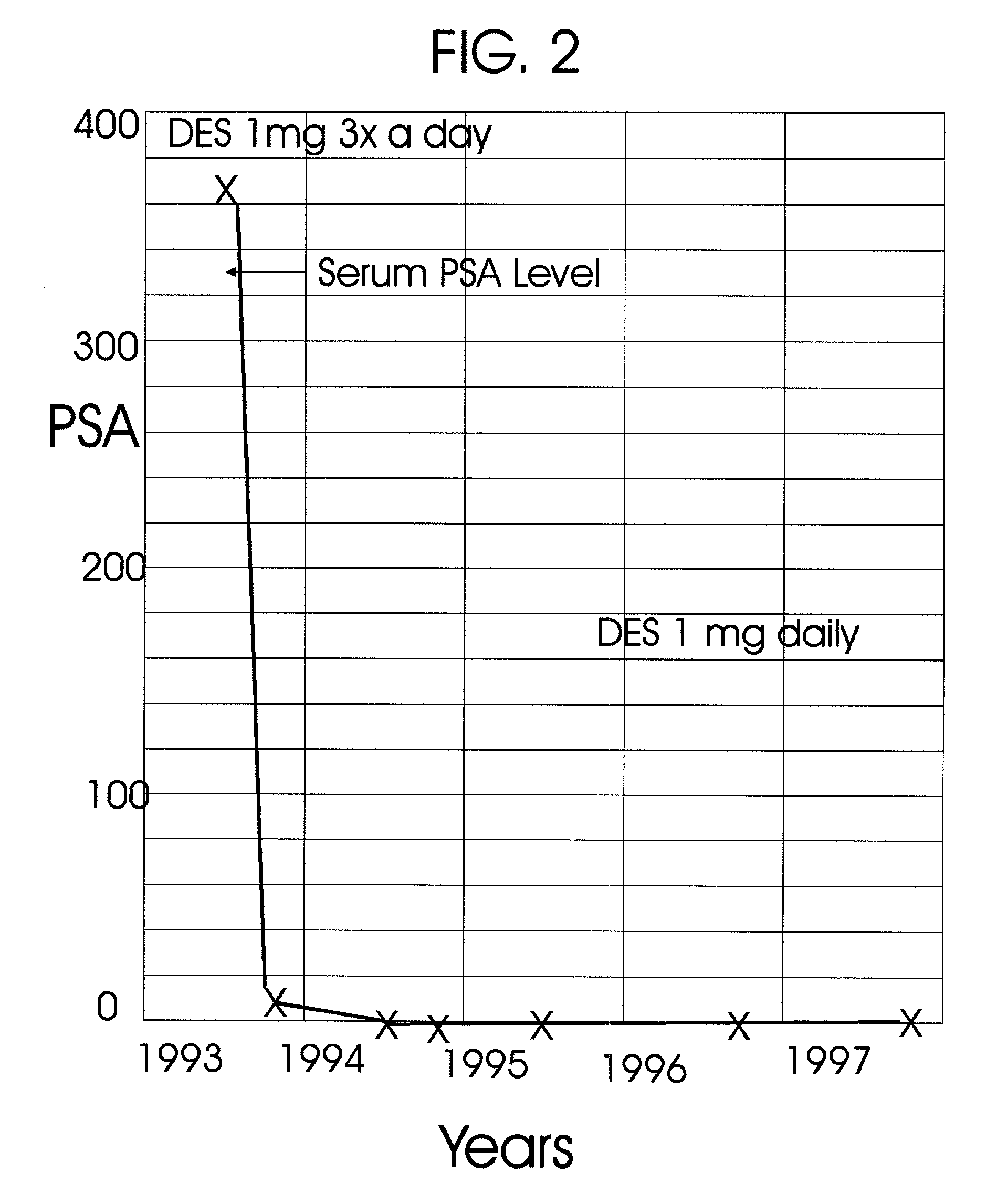
Prostatic hormonal implants treatment of prostate cancer
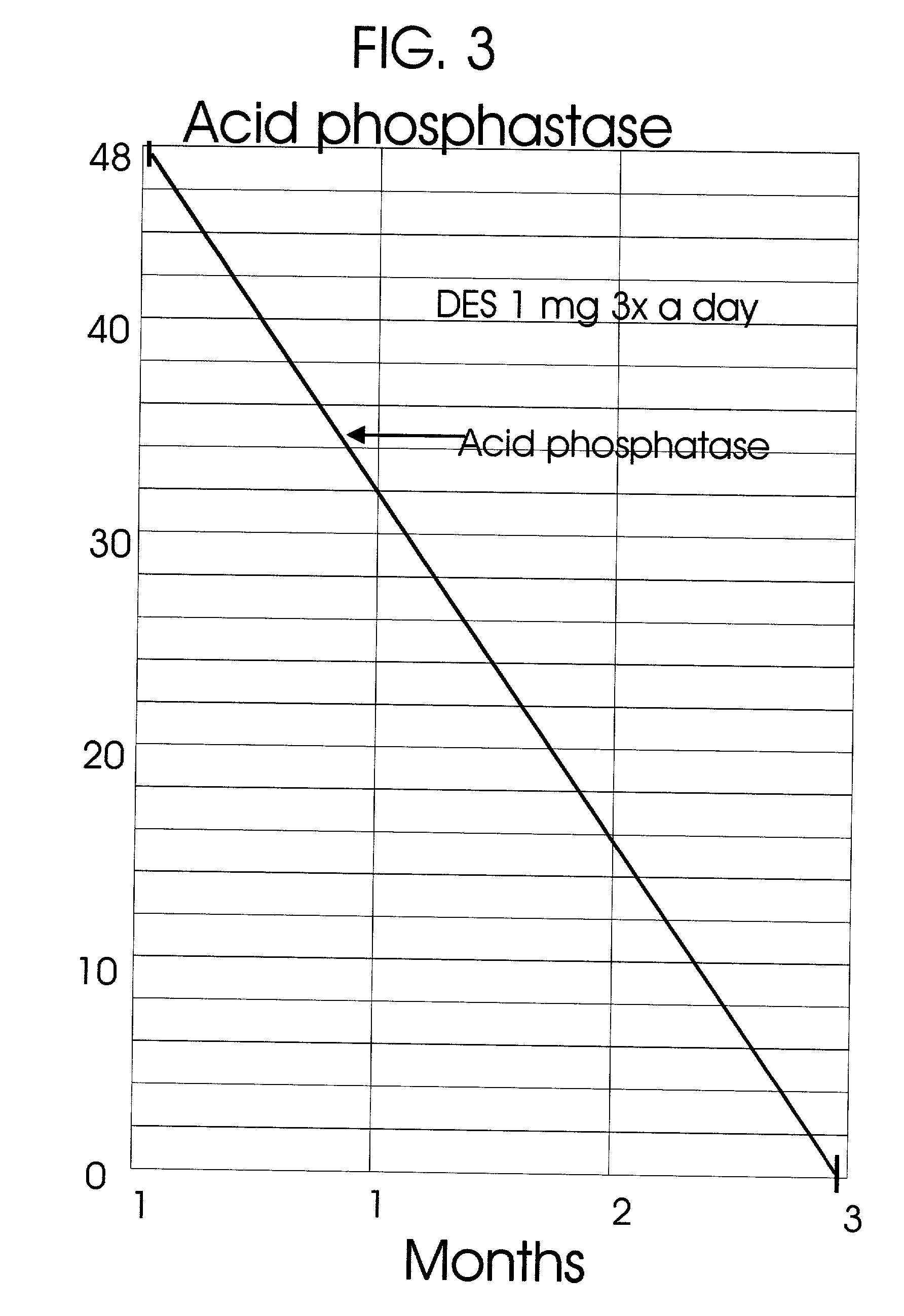
An improved method and products for the primary hormonal treatment of early stage, low and intermediate risk prostate cancers by prostatic implants of androgen suppressive drugs formulated as fused with a lipoid carrier or encapsulated in microcapsules or in Silastic capsules is provided. Such prostatic implants renders a constant slow-release of their contents to the prostate for extended periods by biodegradation and diffusion. It facilitates higher prostatic and lower systemic concentrations of androgen suppressive hormones. Because of their high prostatic and lower systemic concentrations, tumor control is much improved and the their systemic toxicity is minimized. Tumor control after such primary hormonal implant treatment is followed by clinical examinations and the biochemical tumor control is followed by periodic estimations of serum levels of PSA and acid phosphatase. More complex and expensive surgery or radiation therapy for this group of good prognostic early stage prostate cancer is reserved for those patients failing to this primary hormonal treatment. It will preserve potency more than by surgery or radiation therapy. Furthermore, it would reduce the cost of treatment for early stage prostate cancer significantly. Androgen suppressive hormonal implants to the prostate before, during or after lower dose conventional radiation therapy would also facilitate equal or better cure rates of localized prostate cancer as compared to the more complex and toxic higher dose radiation therapy.
Owner:SAHADEVAN VELAYUDHAN
Topical anesthetic formulation
The topical medicament gel formulation of the present invention includes an anesthetic, an anti-microbial, an oxidant, a nutrient, a diuretic, an opioid, an anti-emetic, an anti-seizure drug, and a non-steroidal anti-inflammatory drug (NSAID), USP in a molecular, as opposed to a salt form, as the active ingredient. Additional constituents illustratively include a skin penetration enhancer and a gelling agent. This invention deals with problems commonly associated with topical application of local medicaments such as: slow onset of action; need for occlusion; and rapid loss of effect due to rapid systemic dispersion. The invention permits enhanced penetration of the medicament and thereby allows for a lesser total dosage of pharmaceutically active ingredient. The use of a lesser total dosage also decreases systemic toxicity.
Owner:WEPFER SCOTT
Coated medical devices for the prevention and treatment of vascular disease
InactiveUS7419678B2Reduce systemic toxicityEasy to manageOrganic active ingredientsSurgeryVascular diseaseWhole body
A drug and drug delivery system may be utilized in the treatment of vascular disease. A local delivery system is coated with rapamycin or other suitable drug, agent or compound and delivered intraluminally for the treatment and prevention of neointimal hyperplasia following percutaneous transluminal coronary angiography. The local delivery of the drugs or agents provides for increased effectiveness and lower systemic toxicity.
Owner:WYETH
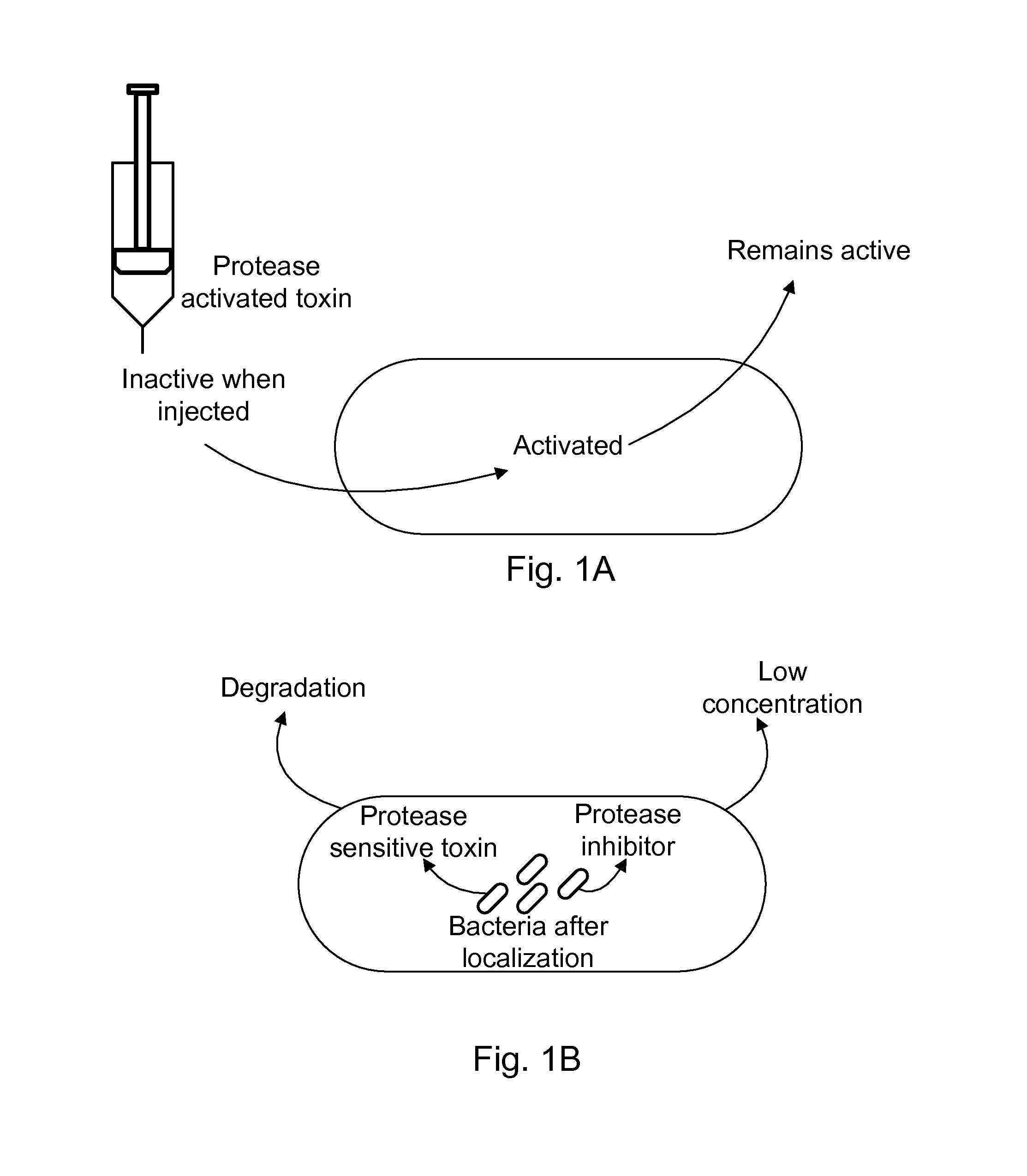
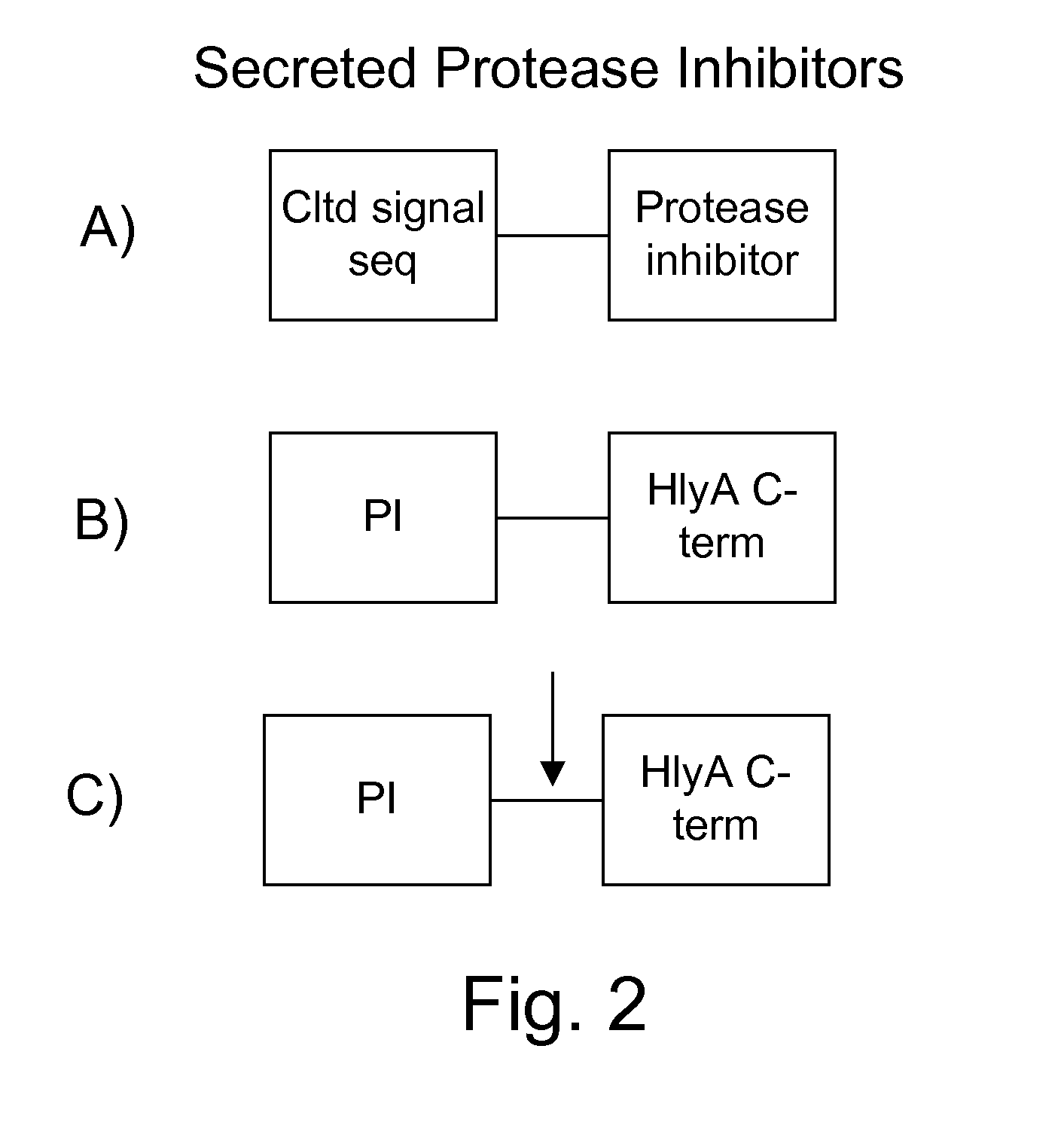
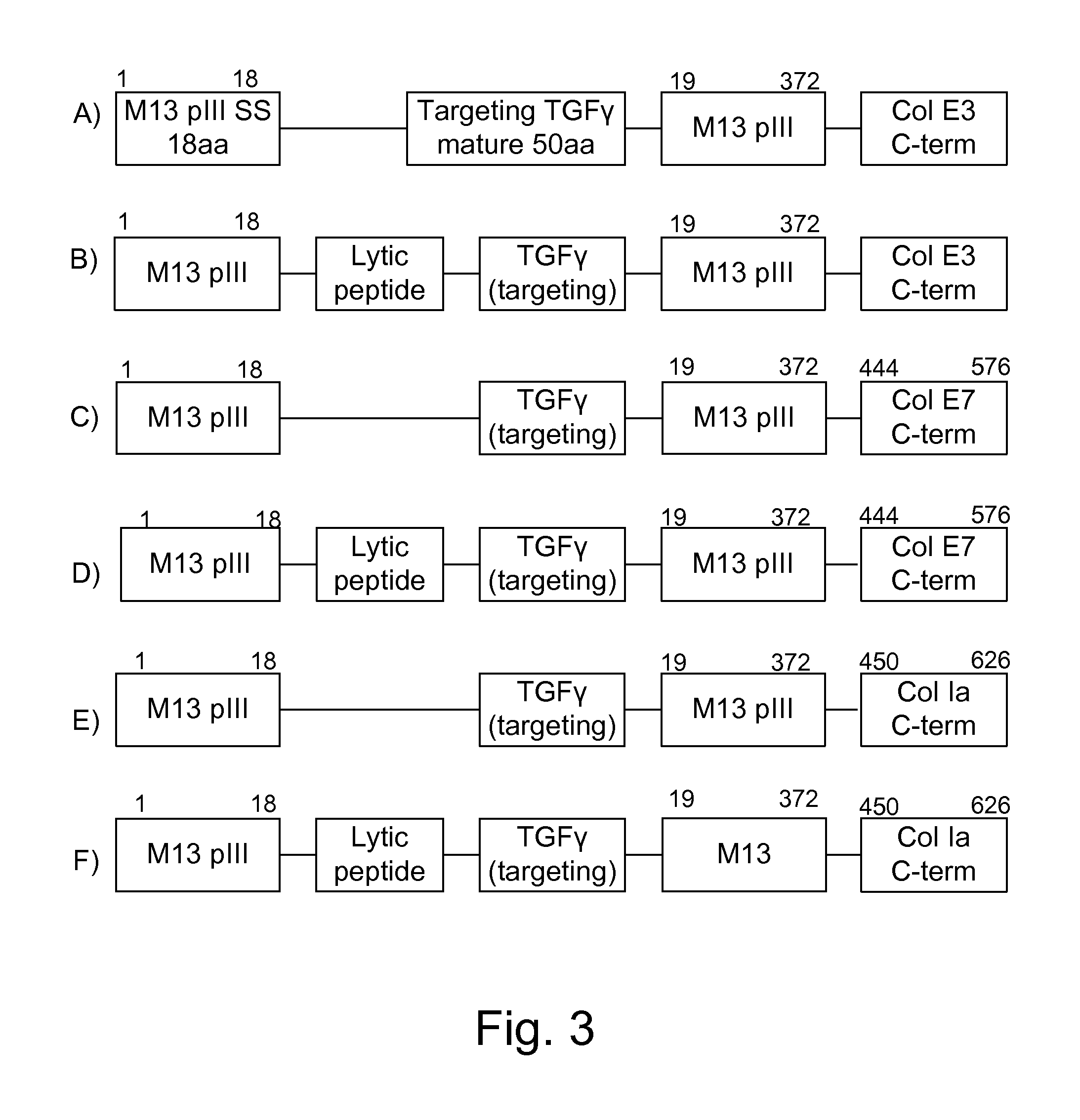
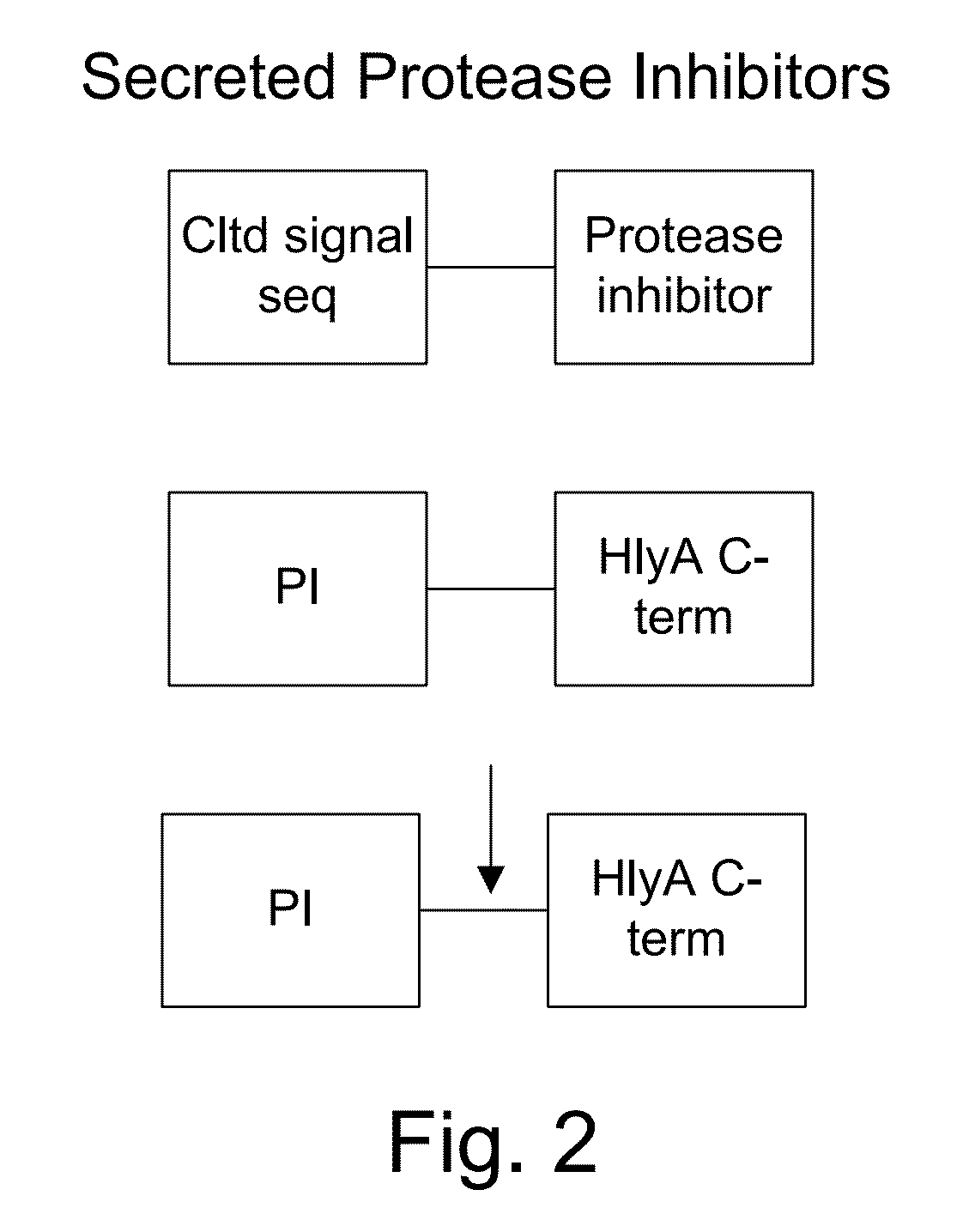
Protease inhibitor: protease sensitivity expression system composition and methods improving the therapeutic activity and specificity of proteins delivered by bacteria
Bacteria which co-express protease inhibitors and protease sensitive therapeutic agents, which are surface displayed, secreted and / or released and result in their localized production and maintenance within a target tissue and inactivation outside of the target tissue, thereby increasing therapeutic activity and reducing the systemic toxicity. The bacteria may be attenuated, non-pathogenic, low pathogenic or a probiotic. Protease sensitivity may be further accomplished by engineering protease degradation sites within the therapeutic agents, further enhancing the inactivation outside of the target tissue while retaining activity within the target tissue through co-expression of a protease inhibitor. Novel chimeric proteins secreted by bacteria, including chimeric toxins targeted to neoplastic cells, tumor matrix cells and cells of the immune system, and combination therapies of these protease inhibitor:chimeric toxin-expressing bacteria together with small-molecule and biologic agents are also described. Non-conjugative bacteria limiting exchange of genetic material, and antibody resistant bacteria are also provided.
Owner:BERMUDES DAVID GORDON
Protease sensitivity expression system
The present invention uses co-expression of protease inhibitors and protease sensitive therapeutic agents that results in their localized production within the target tissue and inactivation outside of the target tissue, thereby increasing therapeutic activity and reducing the systemic toxicity. Inactivation is also accomplished by engineering protease degradation sites within the therapeutic construct for proteases, preferably those that are under-expressed within the target tissue yet present in non-target tissues within the body, resulting in therapeutic activity within the target tissue and inactivation outside of the target tissue. Novel chimeric proteins secreted by bacteria are also described. The chimeric proteins include chimeric toxins targeted to neoplastic cells and cells of the immune system. Novel combination therapies of these protease inhibitor:chimeric toxin-expressing bacteria together with small-molecule and biologic agents are also described. Non-conjugative bacteria capable of delivering phage / phagemids expression cassettes for DNA and RNA-based therapeutics are also described.
Owner:BERMUDES DAVID
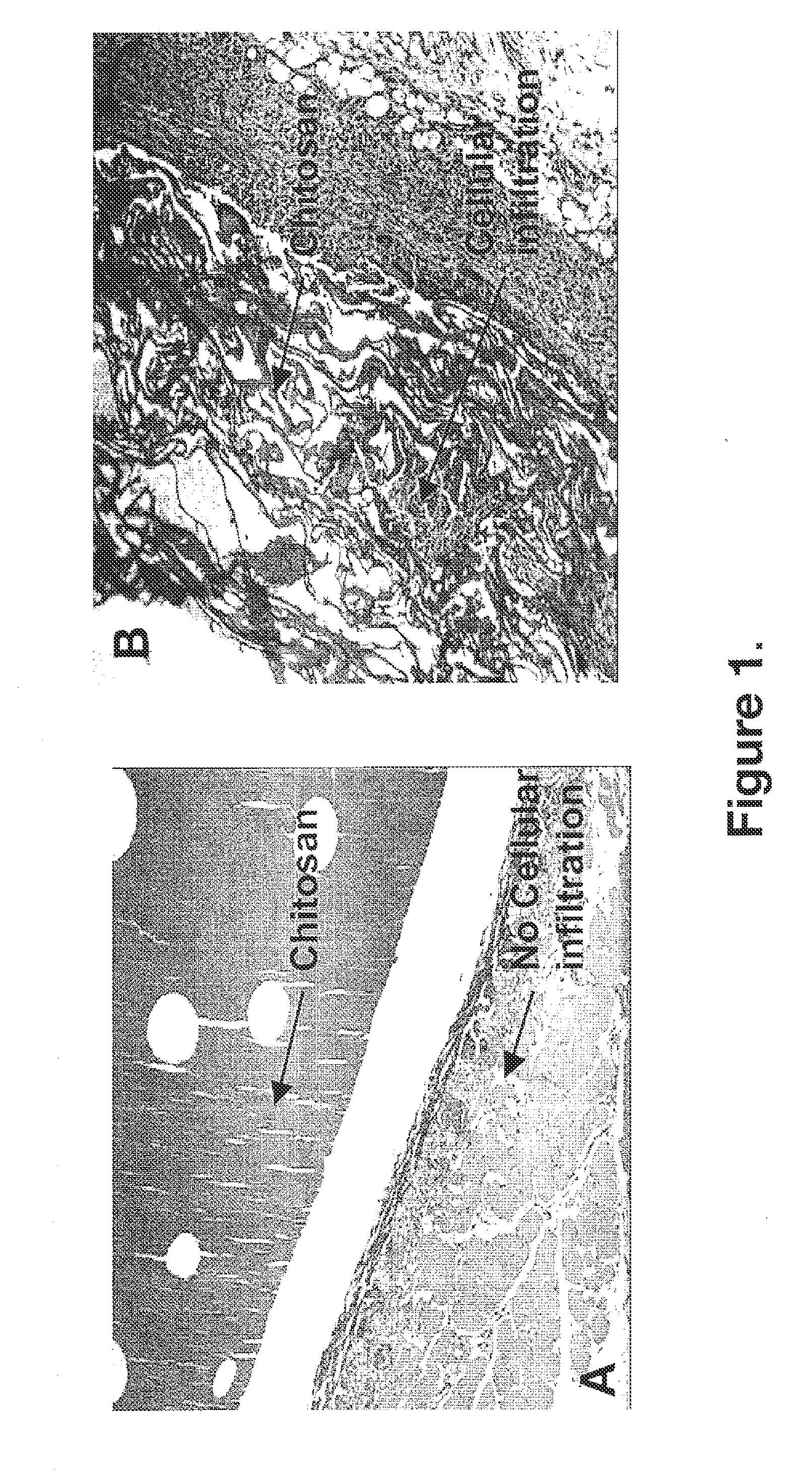
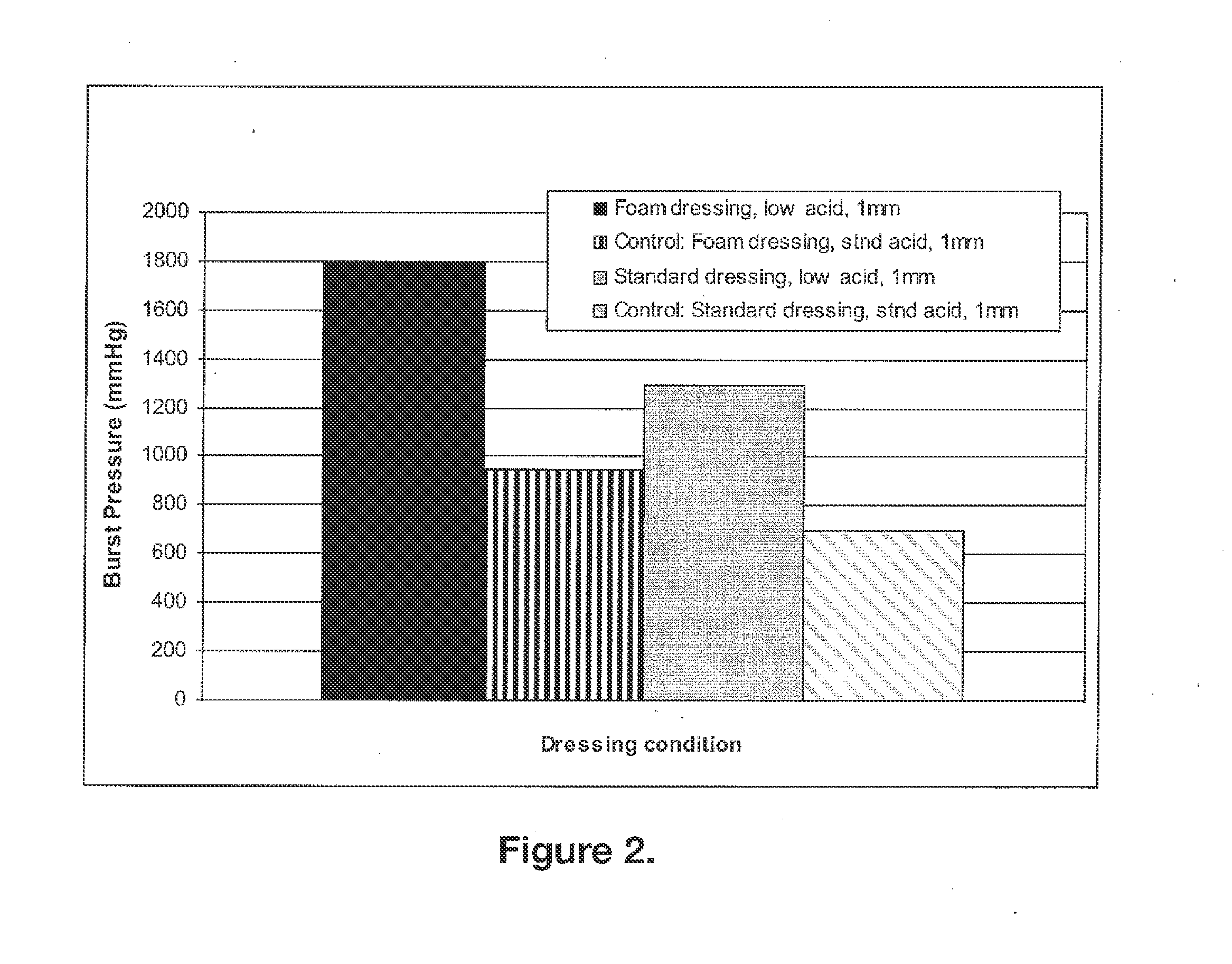
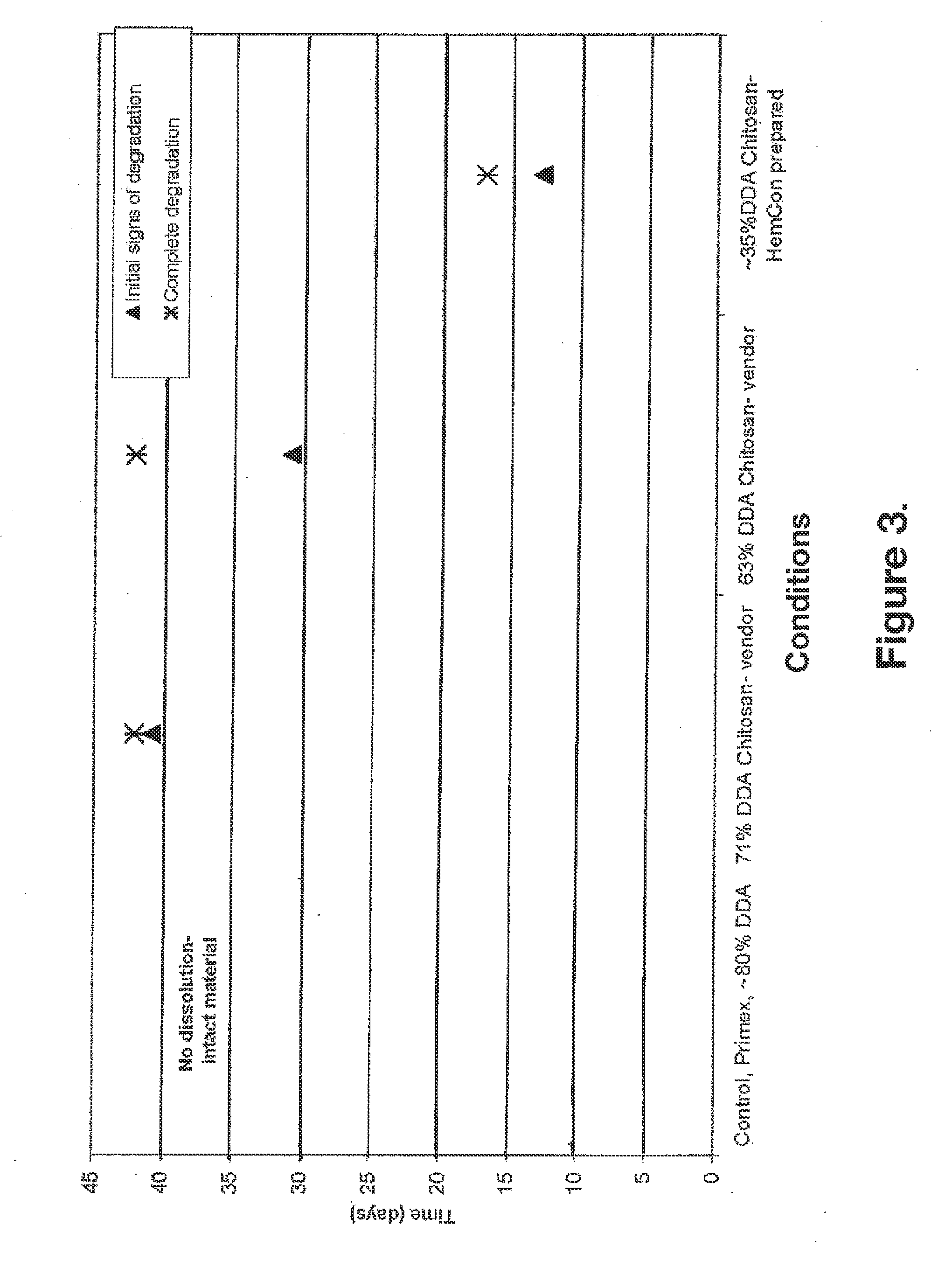
Biocompatible and bioabsorbable derivatized chitosan compositions
ActiveUS20140275291A1Reduces unnecessary riskQuick controlCompounds screening/testingBiocideCrosslinked chitosanChemical composition
The invention relates to biocompatible, bioabsorbable derivatized non-crosslinked chitosan compositions optionally crosslinked to gelatin / collagen by 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC) for biomedical use and methods of making and testing such compositions, including a modified acute systemic toxicity test. The compositions comprise derivatized chitosan reacetylated to a degree of N-deacetylation (DDA) of between about 15% and 40%. The compositions are typically bioabsorbed in about 90 days or less and can be made to bioabsorb at differing rates of speed. The compositions are initially soluble in aqueous solution below pH 6.5. The compositions have an acid content that can be adjusted between about 0% (w / w) and about 8% (w / w) to customize the composition for uses that require and / or tolerate differing levels of cytotoxicity, adhesion, composition cohesion, and cell infiltration into the composition.
Owner:TRICOL BIOMEDICAL INC
Protease inhibitor: protease sensitivity expression system composition and methods improving the therapeutic activity and specificity of proteins delivered by bacteria
ActiveUS9068187B1Direct cytotoxicDirect inhibitoryBiocidePeptide/protein ingredientsBacteroidesSurface display
Bacteria which co-express protease inhibitors and protease sensitive therapeutic agents, which are surface displayed, secreted and / or released and result in their localized production and maintenance within a target tissue and inactivation outside of the target tissue, thereby increasing therapeutic activity and reducing the systemic toxicity. The bacteria may be attenuated, non-pathogenic, low pathogenic or a probiotic. Protease sensitivity may be further accomplished by engineering protease degradation sites within the therapeutic agents, further enhancing the inactivation outside of the target tissue while retaining activity within the target tissue through co-expression of a protease inhibitor. Novel chimeric proteins secreted by bacteria, including chimeric toxins targeted to neoplastic cells, tumor matrix cells and cells of the immune system, and combination therapies of these protease inhibitor:chimeric toxin-expressing bacteria together with small-molecule and biologic agents are also described. Non-conjugative bacteria limiting exchange of genetic material, and antibody resistant bacteria are also provided.
Owner:BERMUDES DAVID GORDON
Protease inhibitor: protease sensitivity expression system and method improving the therapeutic activity and specificity of proteins and phage and phagemids delivered by bacteria
The present invention uses co-expression of protease inhibitors and protease sensitive therapeutic agents that results in their localized production within the target tissue and inactivation outside of the target tissue, thereby increasing therapeutic activity and reducing the systemic toxicity. Inactivation is also accomplished by engineering protease degradation sites within the therapeutic construct for proteases, preferably those that are under-expressed within the target tissue yet present in non-target tissues within the body, resulting in therapeutic activity within the target tissue and inactivation outside of the target tissue. Novel chimeric proteins secreted by bacteria are also described. The chimeric proteins include chimeric toxins targeted to neoplastic cells and cells of the immune system. Novel combination therapies of these protease inhibitor:chimeric toxin-expressing bacteria together with small-molecule and biologic agents are also described. Non-conjugative bacteria capable of delivering phage / phagemids expression cassettes for DNA and RNA-based therapeutics are also described.
Owner:BERMUDES DAVID GORDON
Modular organ microphysiological system with integrated pumping, leveling, and sensing
ActiveUS20170227525A1Improve reliabilityLow costBioreactor/fermenter combinationsBiological substance pretreatmentsSource to sinkMulti organ
Fluidic multiwell bioreactors are provided as a microphysiological platform for in vitro investigation of multi-organ crosstalks for an extended period of time of at least weeks and months. The disclosed platform is featured with one or more improvements over existing bioreactors, including on-board pumping for pneumatically driven fluid flow, a redesigned spillway for self-leveling from source to sink, a non-contact built-in fluid level sensing device, precise control on fluid flow profile and partitioning, and facile reconfigurations such as daisy chaining and multilayer stacking. The platform supports the culture of multiple organs in a microphysiological, interacted systems, suitable for a wide range of biomedical applications including systemic toxicity studies and physiology-based pharmacokinetic and pharmacodynamic predictions. A process to fabricate the disclosed bioreactors is also provided.
Owner:MASSACHUSETTS INST OF TECH
Drug/drug delivery systems for the prevention and treatment of vascular disease
InactiveUS7300662B2Prevent proliferationGood effectOrganic active ingredientsOrganic chemistryVascular diseaseWhole body
Owner:WYETH LLC
Tumescent infiltration drug delivery of high subcutaneous drug concentrations with prolonged local and systemic effects and minimal local or systemic toxicity
ActiveUS20170100331A1Increase local drug concentrationReduce blood viscosityInorganic non-active ingredientsPharmaceutical delivery mechanismTherapeutic effectVasoconstrictor Agents
Disclosed are methods of subcutaneous delivery of a drug or a therapeutic agent to a subject comprising administering to said subject a tumescent composition comprising: (a) the drug or the therapeutic agent, wherein a tumescent concentration of the drug is simultaneously: 1) below a threshold for local, subcutaneous tissue toxicity, 2) above a threshold for positive local therapeutic effect, and 3) above a concentration achievable by intravenous (IV), intramuscular (IM) or oral (PO) delivery; (b) a vasoconstrictor; and (c) a pharmaceutically acceptable carrier. Some embodiments relate to a method of treating or preventing sepsis or Systemic Inflammatory Response Syndrome (SIRS) in a subject. Some embodiments relate to a tumescent solution for treating a localized viral infection, e.g., varicella-zoster (shingles), the tumescent solution comprising an antiviral agent.
Owner:HK PHARMA INC
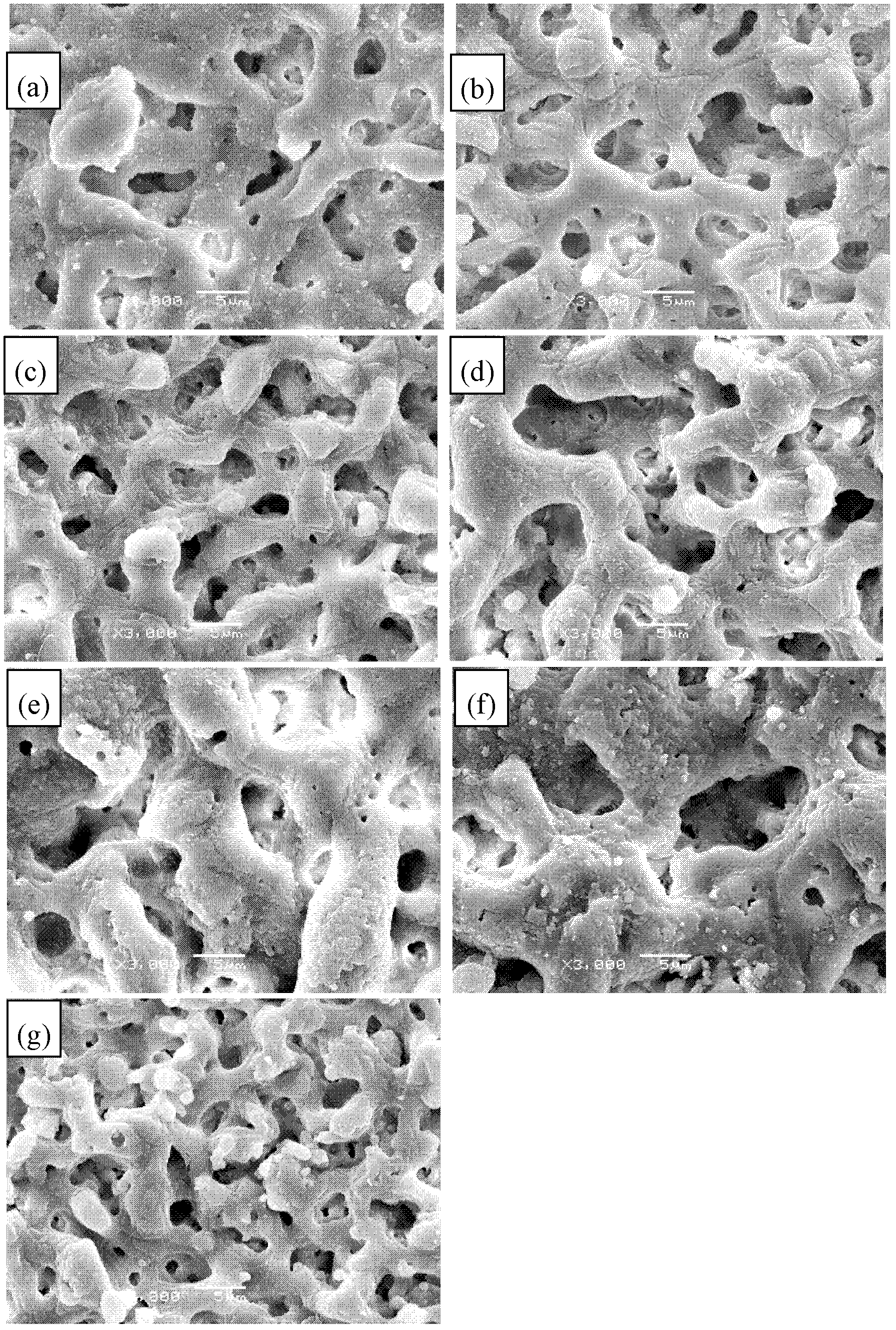
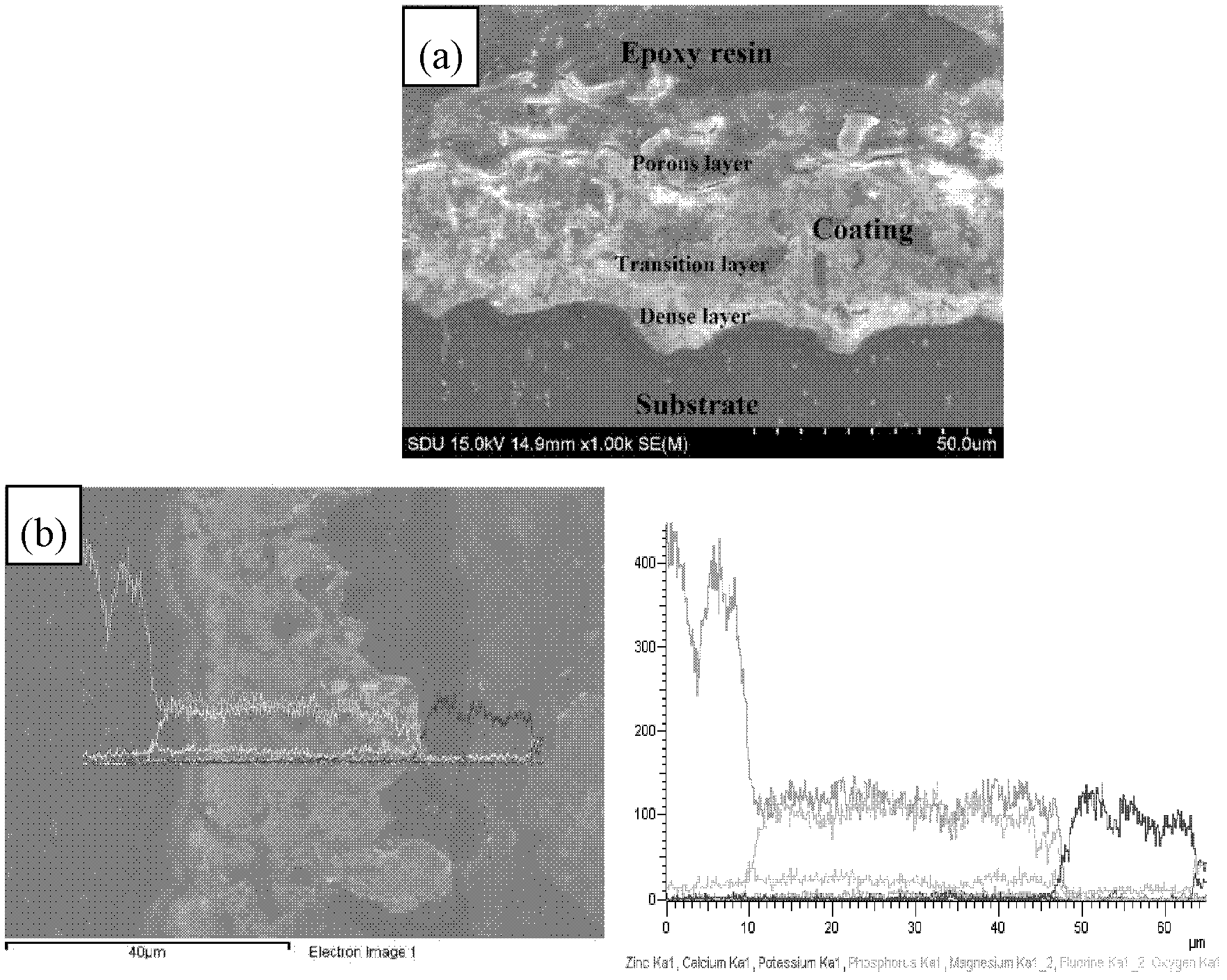
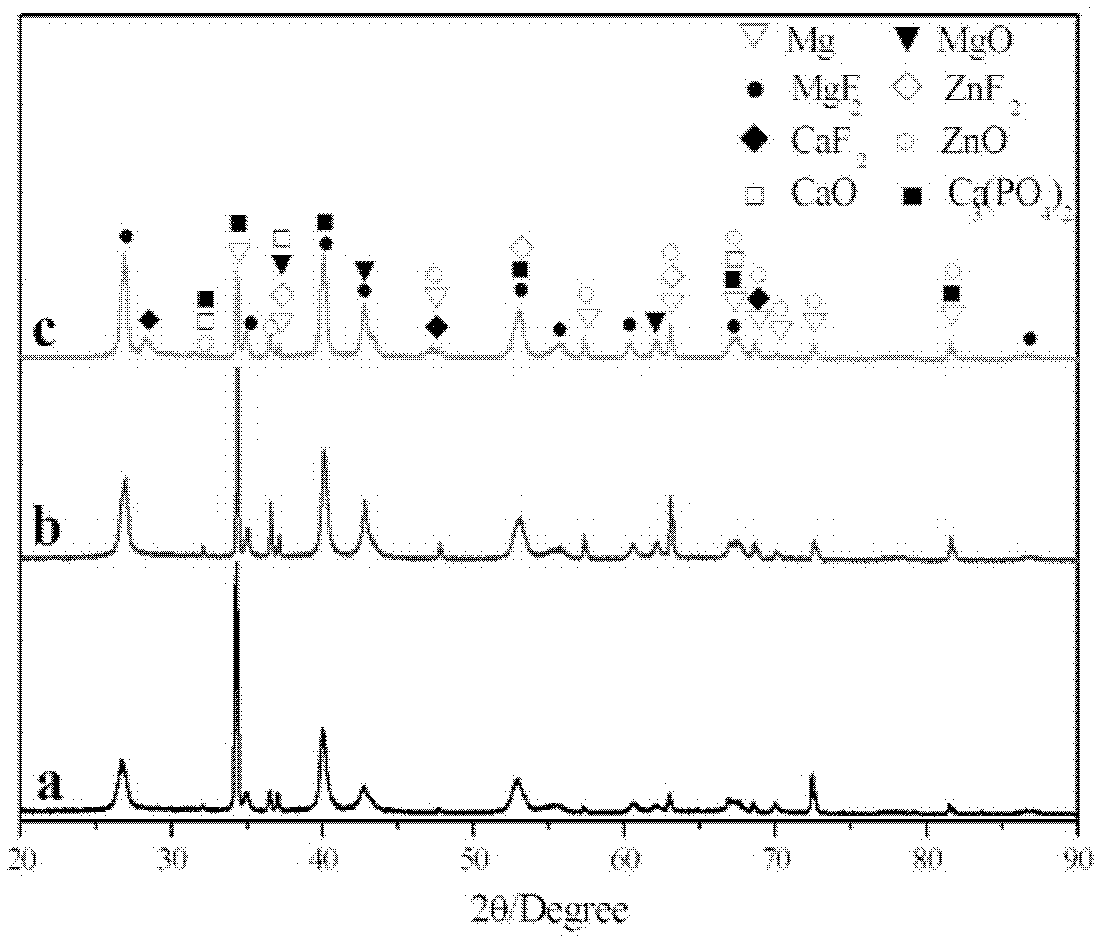
Preparation method of biological ceramic coating rich in calcium and phosphate phases on surface of magnesium alloy
ActiveCN102220620AHigh hardnessHigh densityAnodisationPhosphatisationPlasma electrolytic oxidationMicro arc oxidation
The invention relates to a preparation method of a biological ceramic coating rich in calcium and phosphate phases on the surface of magnesium alloy, comprising the following steps of: adding (C6H5O7)2Ca3.4H2O and Na3PO4 to deionized water, and simultaneously adding KOH, NH4HF2, N(CH2CH2OH)3, C3H8O3 and H2O2 to prepare an electrolyte with a certain concentration ratio of calcium and phosphate; disposing the magnesium alloy in the electrolyte as an anode which is prepared through micro arc oxidation energization reaction. The calcium-phosphate ceramic coating obtained by the preparation methodconsists of three layers of a loose layer, a transition layer and a compact layer, wherein the surface of the loose layer consists of a plurality of uniformly distributed micropores, the transition layer is between the loose layer and the compact layer, and the compact layer and a substrate body form good metallurgical bonding; therefore, the coating has high rigidity, high density, high bonding force and good corrosion resistance and abrasion resistance; simultaneously, the mouse acute systemic toxicity test indicates that the coating has good biocompatibility, and the simulated body fluid soaking test indicates that the coating has good biological activity.
Owner:SHANDONG UNIV
Device, method, system, and program for intelligent in vivo cell-level chemical or genetic material delivery
InactiveUS20060040390A1Easy and cost-effective to manufactureSimple materialMedical devicesOther foreign material introduction processesGenetic MaterialsWhole body
The invention provides a device, method, system and program for intelligent in vivo cell-level chemical or genetic material delivery; wherein multiple injectable biocompatible physical delivery device containers are used to selectively administer medicine, chemical(s) or genetic materials to a target cell in a patient, human or animal, with reduced systemic toxicity; said delivery device container includes an internal contents-to-cell transfer mechanism, usually a syringe; a biological “key” molecule, magnetic device or vibration frequency signature sensor placed on the surface of the delivery device container which is adapted to selectively bind to said target cell directly or indirectly; a “tag” placed on the surface of the delivery device container, usually metallic and biocompatible in nature, which will display to an observer when scanned through external devices such as x-ray, MRI, CT, sound, etc.; and a release mechanism to move the internal contents of the delivery device container into said target cell over a predetermined and specified timed basis.
Owner:MINOR JOHN SCOTT JR +2
Preparation method and application of metal organic framework drug carrier system based on cytosine arabinoside micromolecule prodrug
ActiveCN108619511ASolve the shortcomings of being easily inactivated by metabolismImprove stabilityOrganic active ingredientsPowder deliveryMetal-organic frameworkIn vivo
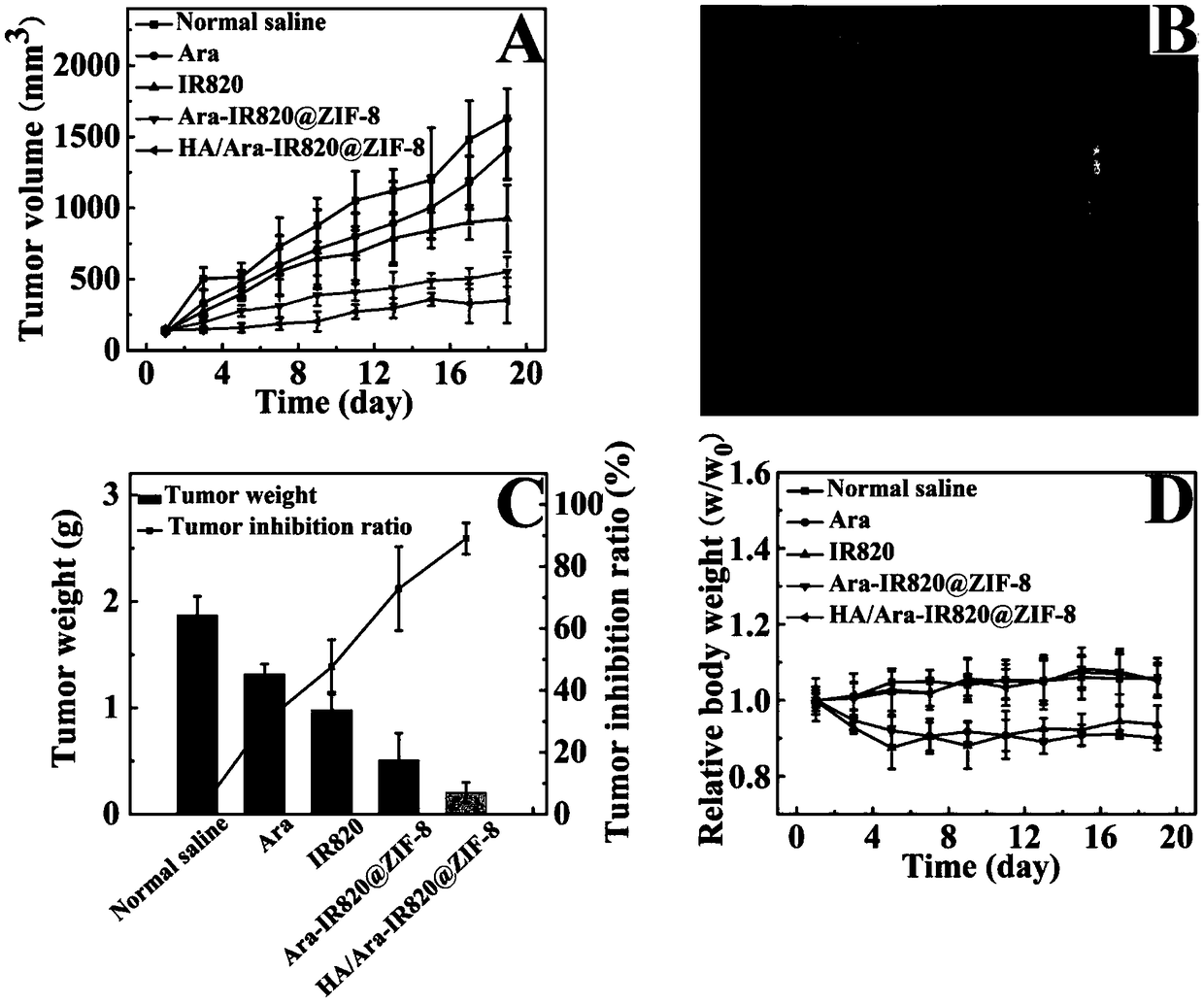
The invention discloses a preparation method and application of metal organic framework drug carrier system based on drug cytosine arabinoside micromolecule prodrug. The preparation method disclosed by the invention comprises the steps: choosing molecules which can generate coordination reaction with metal ions to be covalently combined with cytosine arabinoside to synthesize novel multifunctionalmicromolecule prodrug; carrying the prodrug on the metal organic framework through a simple environment-friendly one-step entrapment method to form medicine carrying nanoparticles; centrifuging, washing and drying to obtain a cytosine arabinoside micromolecule prodrug carrying MOF intravenous injection preparation. Taking synthesized Ara-IR820@ZIF-8 as an example, a mouse in-vivo antitumor pharmacological result shows that HA / Ara-IR820@ZIF-8 nano water solution has a good antitumor effect and lower system toxicity. The intravenous injection preparation has the advantages of high medicine carrying amount, simple and economic preparation process, batch production, small toxicity, good safety and wide application prospect in achieving high medicine carrying amount of the cytosine arabinosidein the metal organic framework and applying intravenous injection dosage to treat solid tumors.
Owner:SHANDONG UNIV
Drug/Drug Delivery Systems for the Prevention and Treatment of Vascular Disease
InactiveUS20070026036A1Reduce systemic toxicityEasy to manageBiocideOrganic chemistryVascular diseaseWhole body
A drug and drug delivery system may be utilized in the treatment of vascular disease. A local delivery system is coated with rapamycin or other suitable drug, agent or compound and delivered intraluminally for the treatment and prevention of neointimal hyperplasia following percutaneous transluminal coronary angiography. The local delivery of the drugs or agents provides for increased effectiveness and lower systemic toxicity.
Owner:WYETH LLC
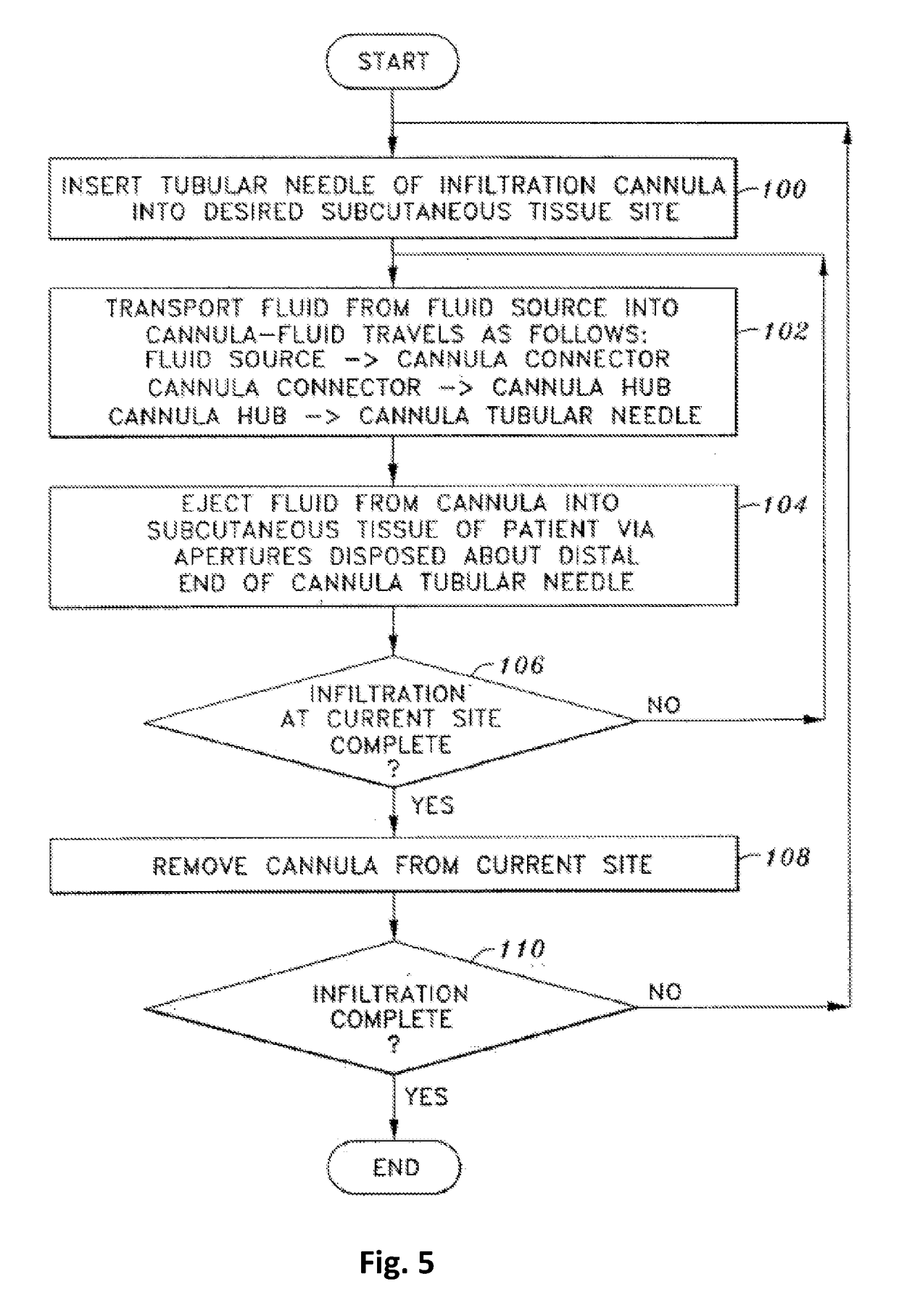
Method for pressure mediated selective delivery of therapeutic substances and cannula
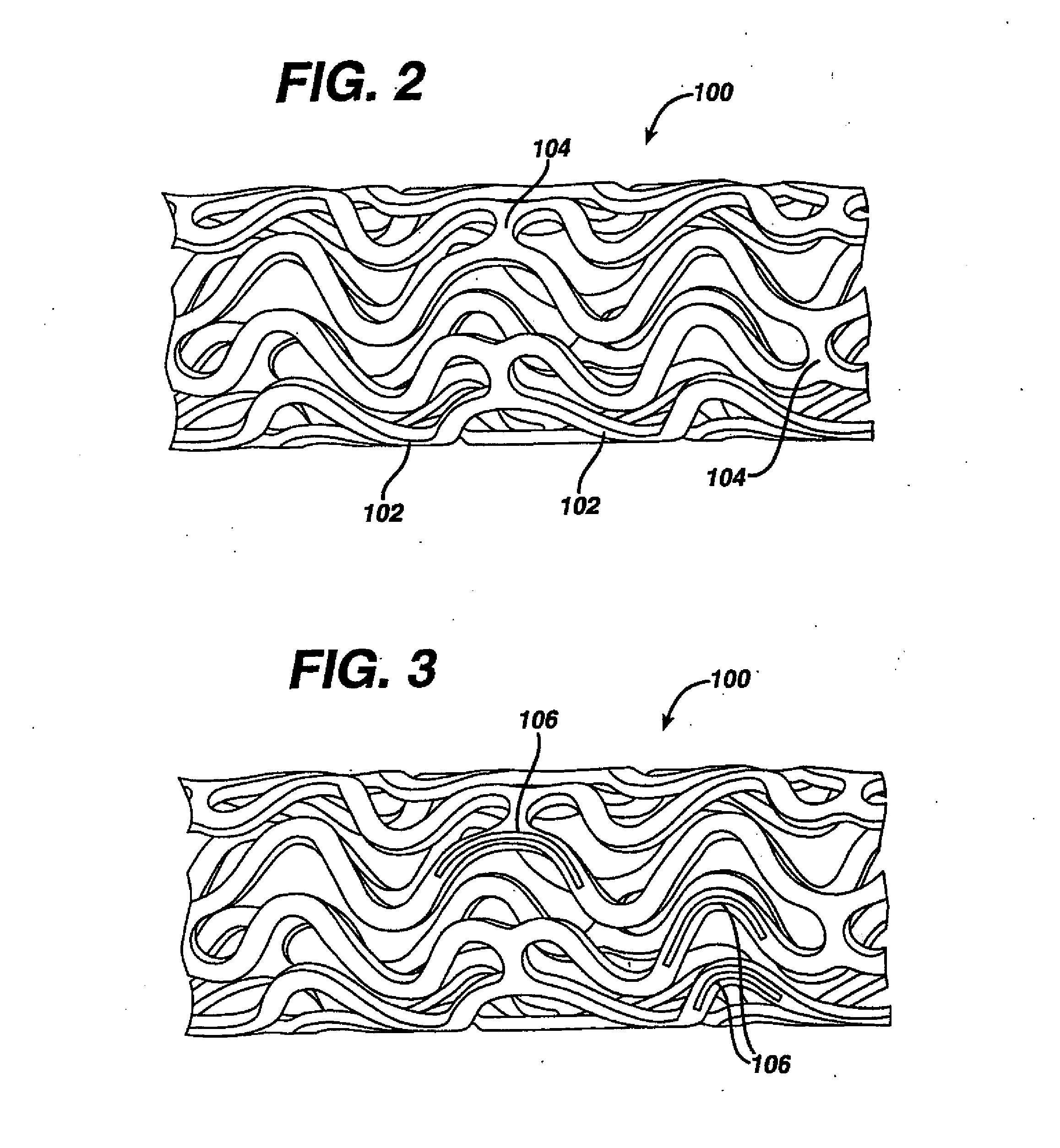
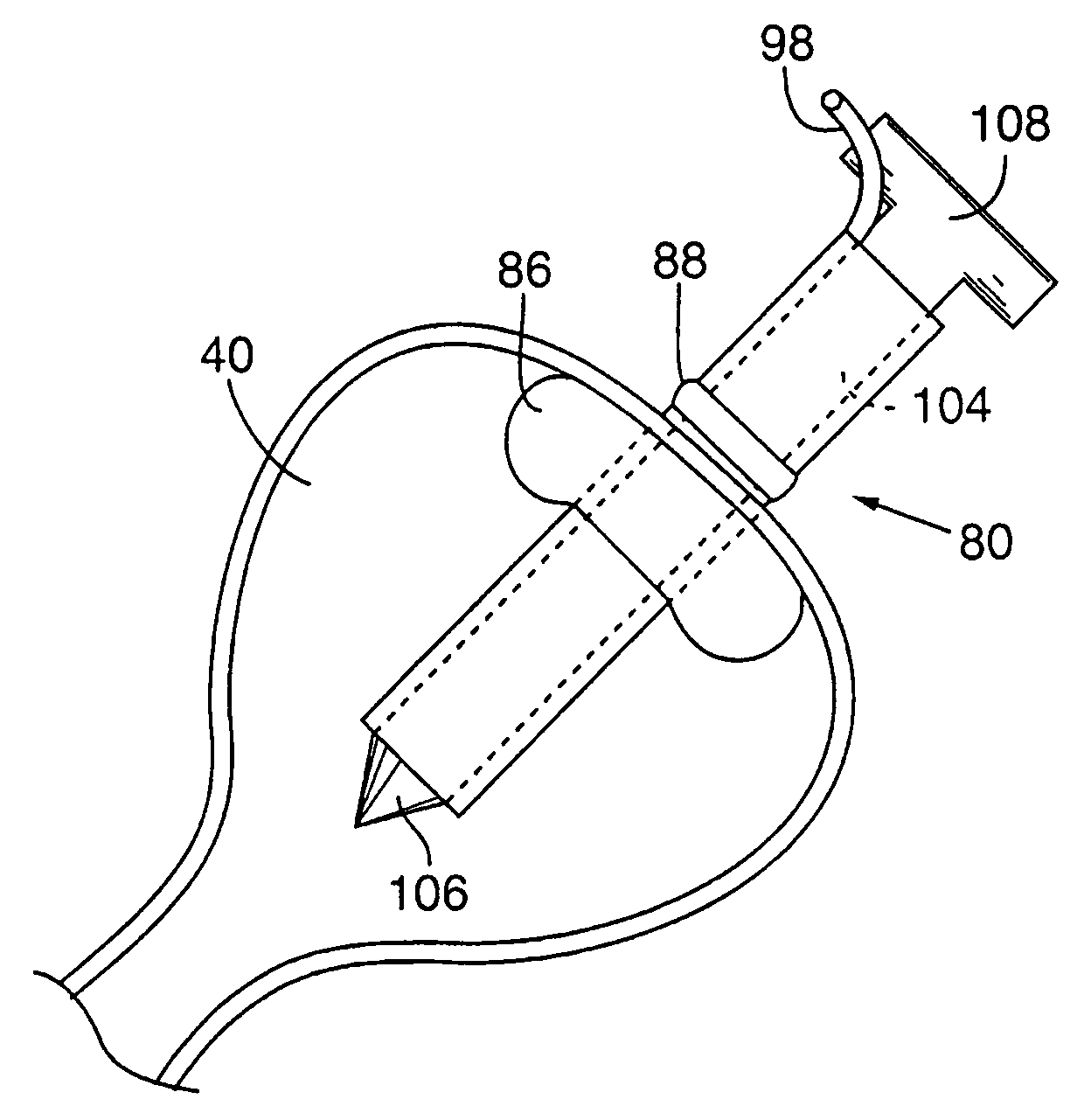
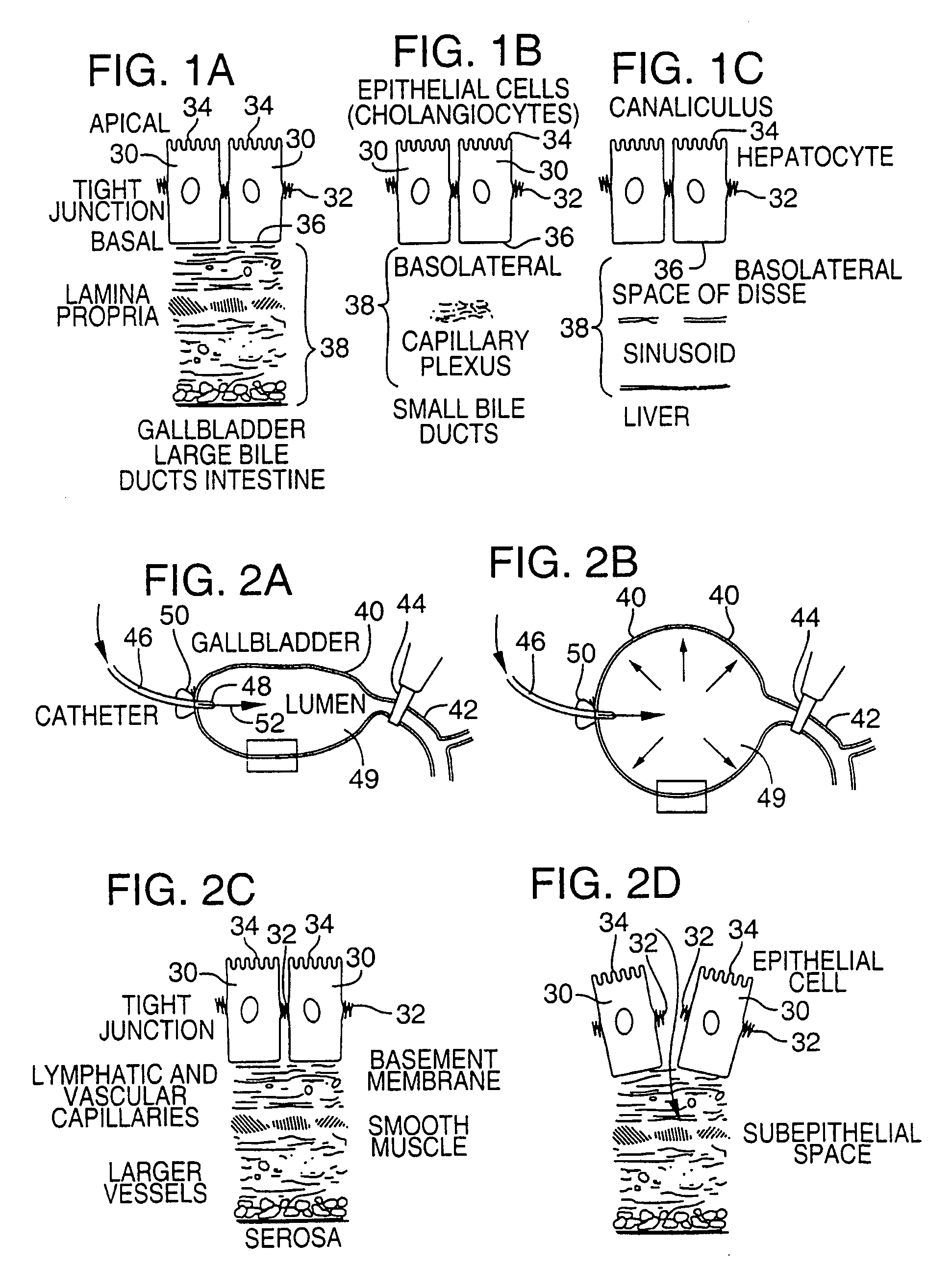
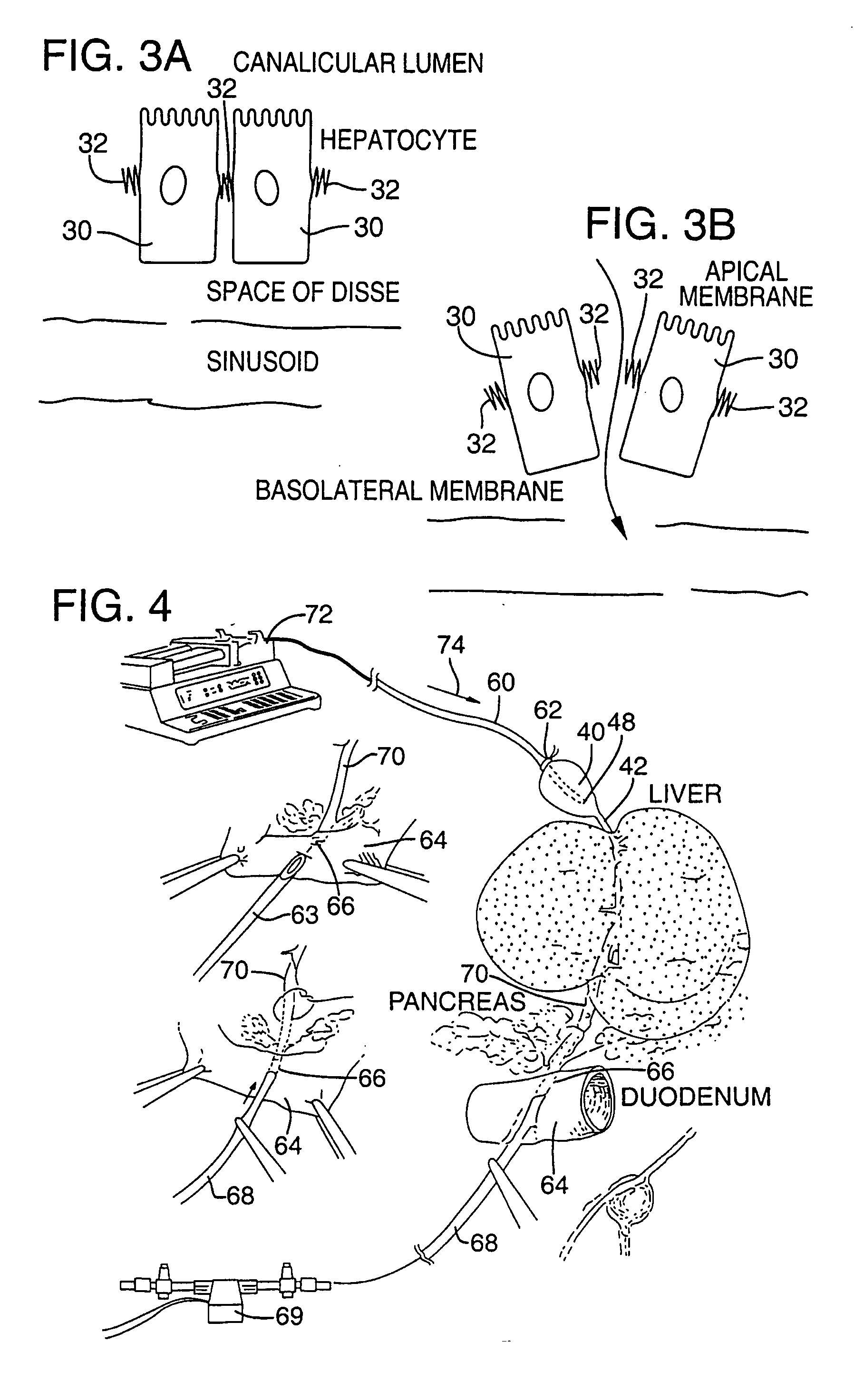
Methods and devices are disclosed for selective delivery of therapeutic substances to specific histologic or microanatomic areas of organs. Introduction of the therapeutic substance into a hollow organ space (such as an hepatobiliary duct or the gallbladder lumen) at a controlled pressure, volume or rate allows the substance to reach a predetermined cellular layer (such as the ephithelium or sub-epithelial space). The volume or flow rate of the substance can be controlled so that the intralumenal pressure reaches a predetermined threshold level beyond which subsequent subepithelial delivery of the substance occurs. Alternatively, a lower pressure is selected that does not exceed the threshold level, so that delivery occurs substantially only to the epithelial layer. Such site specific delivery of therapeutic agents permits localized delivery of substances (for example to the interstitial tissue of an organ) in concentrations that may otherwise produce systemic toxicity. Occlusion of venous or lymphatic drainage from the organ can also help prevent systemic administration of therapeutic substances, and increase selective delivery to superficial epithelial cellular layers. Delivery of genetic vectors can also be better targeted to cells where gene expression is desired. The access device comprises a cannula with a wall piercing tracar within the lumen. Two axially spaced inflatable balloons engage the wall securing the cannula and sealing the puncture site. A catheter equipped with an occlusion balloon is guided through the cannula to the location where the therapeutic substance is to be delivered.
Owner:DEPT OF HEALTH & HUMAN SERVICE THE GOVERNMENT OF THE US SEC
Engineered TAA antibody-TNFSF member ligand fusion molecules
InactiveUS9534056B2Reduce systemic toxicityImprove efficacyPeptide/protein ingredientsAntibody mimetics/scaffoldsWhole bodyApoptosis
Owner:IMMUNGENE
Multimeric fusion proteins of the TNF superfamily ligands
A method for constructing stable bioactive fusion proteins of the difficult to express tumor necrosis factor superfamily (TNFSF), and particularly members CD40L (CD154) and RANKL / TRANCE, with collecting, particularly pulmonary surfactant protein D (SPD) is described. Single trimers of these proteins lack the full stimulatory efficacy of the natural membrane forms of these proteins in many cases. The multimeric nature of these soluble fusion proteins enables them to engage multiple receptors on the responding cells, thereby, mimicking the effects of the membrane forms of these ligands. For CD40L-SPD, the resulting protein stimulates B cells, macrophages, and dendritic cells, indicating its potential usefulness as a vaccine adjuvant. The large size of these fusion proteins makes them less likely to diffuse into the circulation, thereby limiting their potential systemic toxicity. This property may be especially useful when these proteins are injected locally as a vaccine adjuvant or tumor immunotherapy agent to prevent them from diffusing away. In addition, these and other TNFSF-collectin fusion proteins present new possibilities for the expression of highly active, multimeric, soluble TNFSF members.
Owner:RGT UNIV OF CALIFORNIA
Drug/drug delivery systems for the prevention and treatment of vascular disease
InactiveUS20050002986A1Reduce systemic toxicityEasy to manageBiocideOrganic chemistryCoronary arteriesVascular disease
A drug and drug delivery system may be utilized in the treatment of vascular disease. A local delivery system is coated with rapamycin or other suitable drug, agent or compound and delivered intraluminally for the treatment and prevention of neointimal hyperplasia following percutaneous transluminal coronary angiography. The local delivery of the drugs or agents provides for increased effectiveness and lower systemic toxicity.
Owner:WYETH
Humanized Anti-ceacam5 antibody and uses thereof
InactiveUS20150125386A1Overcome tumorImprove targetingImmunoglobulins against animals/humansRadioactive preparation carriersDrug conjugationWhole body
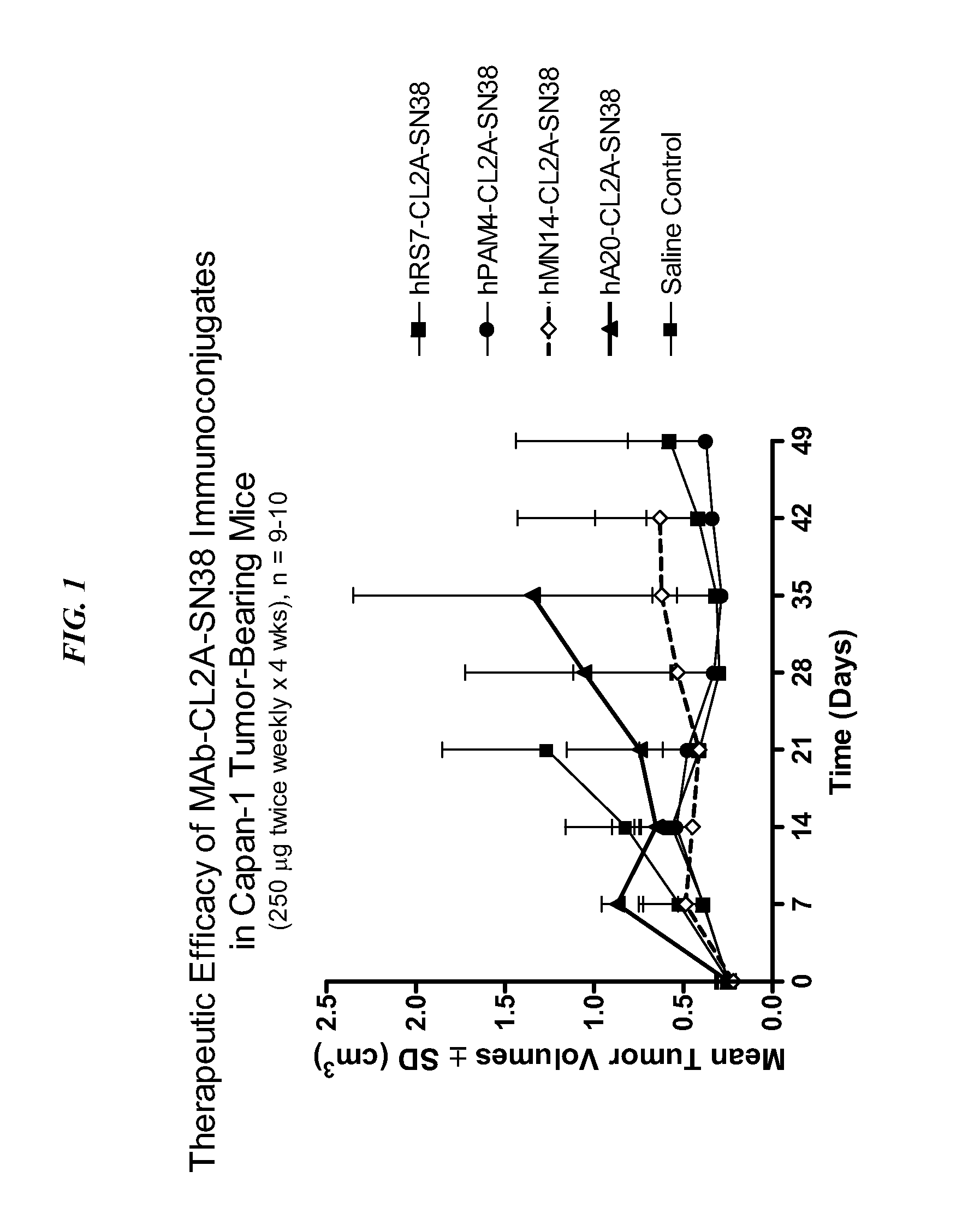
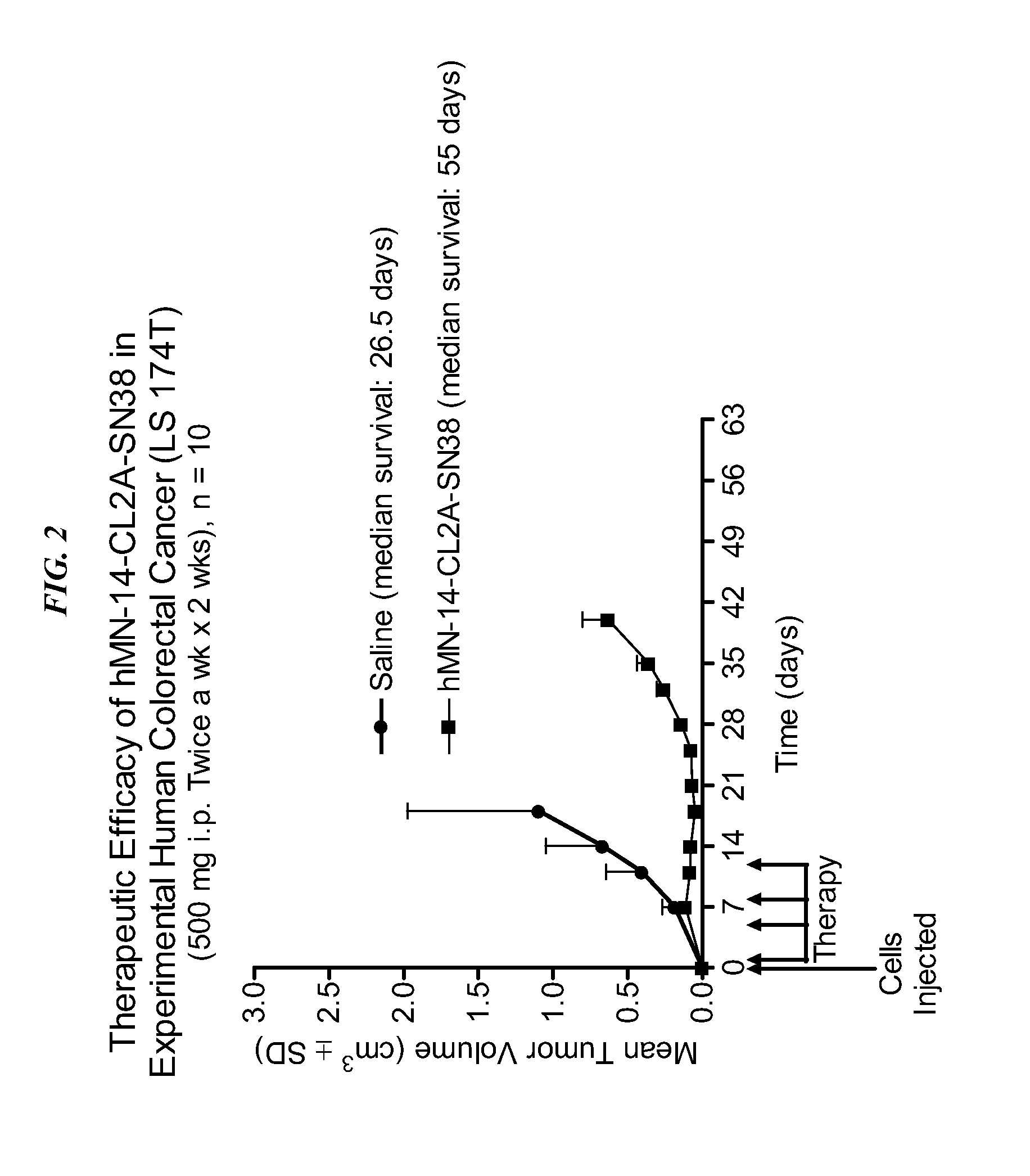
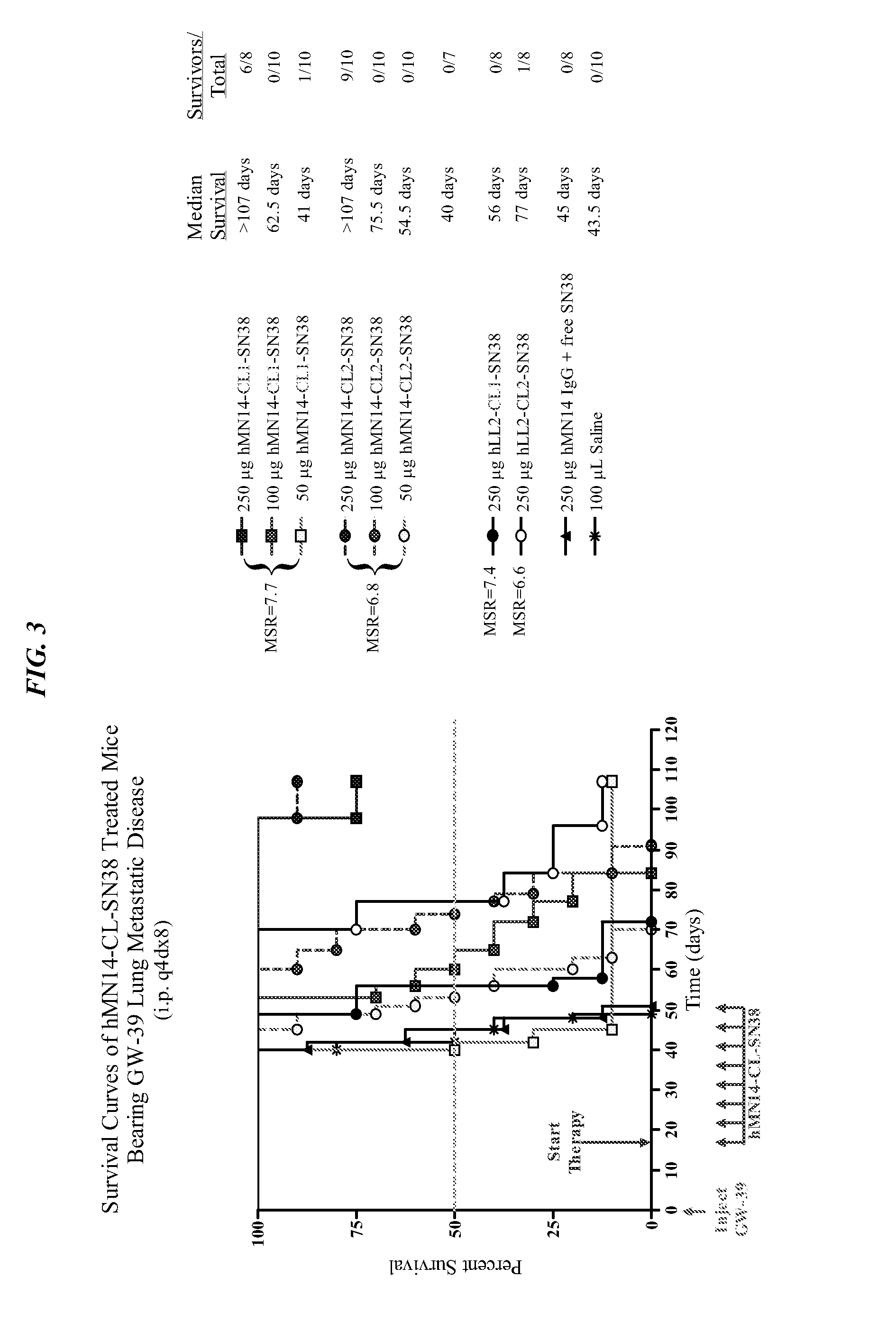
The present invention concerns compositions and methods of use of a humanized Class III anti-CEA antibody, comprising the heavy and light amino acid sequences SEQ ID NO:1 and SEQ ID NO:2. The antibody is effective to treat CEACAM5-expressing tumors, either alone or in combination with one or more therapeutic agents. Drug conjugated Class III anti-CEA antibodies, such as SN-38 or P2PDox immunoconjugates, are particularly efficacious. Surprisingly, the antibody-drug conjugates (ADCs) exhibit high anti-cancer efficacy, while exhibiting low levels of systemic toxicity that are readily treated with standard amelioration techniques. Antibodies and / or immunoconjugates comprising the amino acid sequences SEQ ID NO:1 and SEQ ID NO:2 are surprisingly efficacious for therapy of solid tumors, even when the tumor has proven resistant to standard anti-cancer therapies.
Owner:IMMUNOMEDICS INC
Prodrugs for use as ophthalmic agents
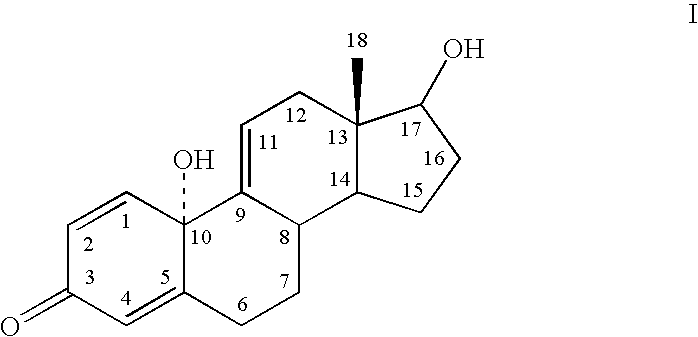
ActiveUS20040171596A1Reduced lipid solubilityImproved transcorneal penetrationOrganic active ingredientsSteroidsSolubilityDisease
The subject invention provides a mechanism by which steroidal quinol compounds confer beneficial ophthalmic effects. The subject compounds possess a lipophilic-hydrophilic balance for transcorneal penetration and are readily reduced into parent phenolic A-ring steroid compounds to provide protection or treatment against various ocular symptoms and disorders. The compounds according to the subject invention appear to be highly advantageous as prodrugs to provide protection and / or treatment against ocular disorders. These prodrugs confer lipid solubility optimal for transocorneal penetration and are readily converted to endogenous reducing agents into active phenolic A-ring steroid compounds. To the extent that these prodrugs have reduced feminizing effects and systemic toxicity, they would be expected to be quite advantageous for protecting or treating the eye against ocular disorders such as cataract or glaucoma without undesired (systemic) side effects).
Owner:UNIV OF FLORIDA RES FOUNDATION INC +2
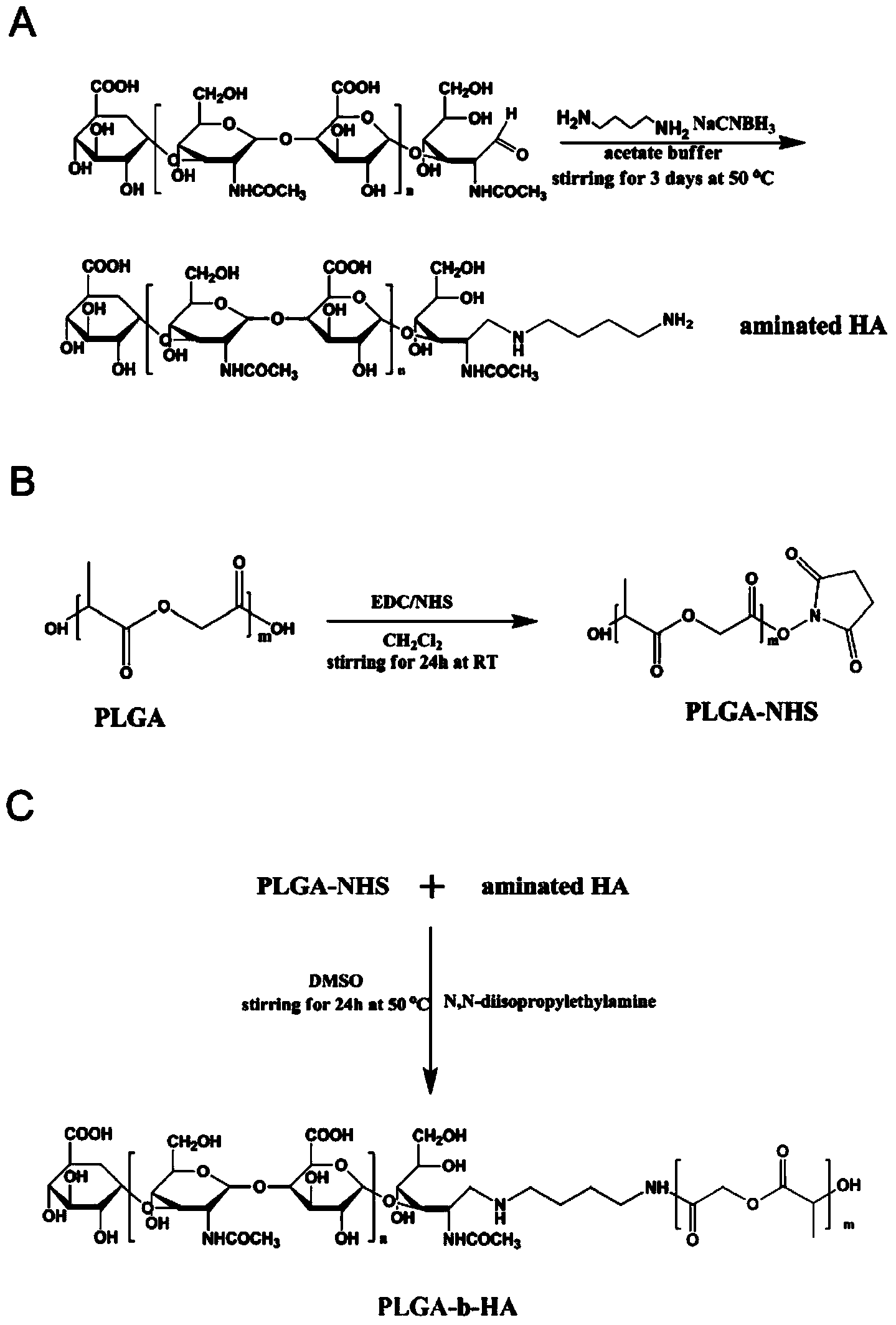
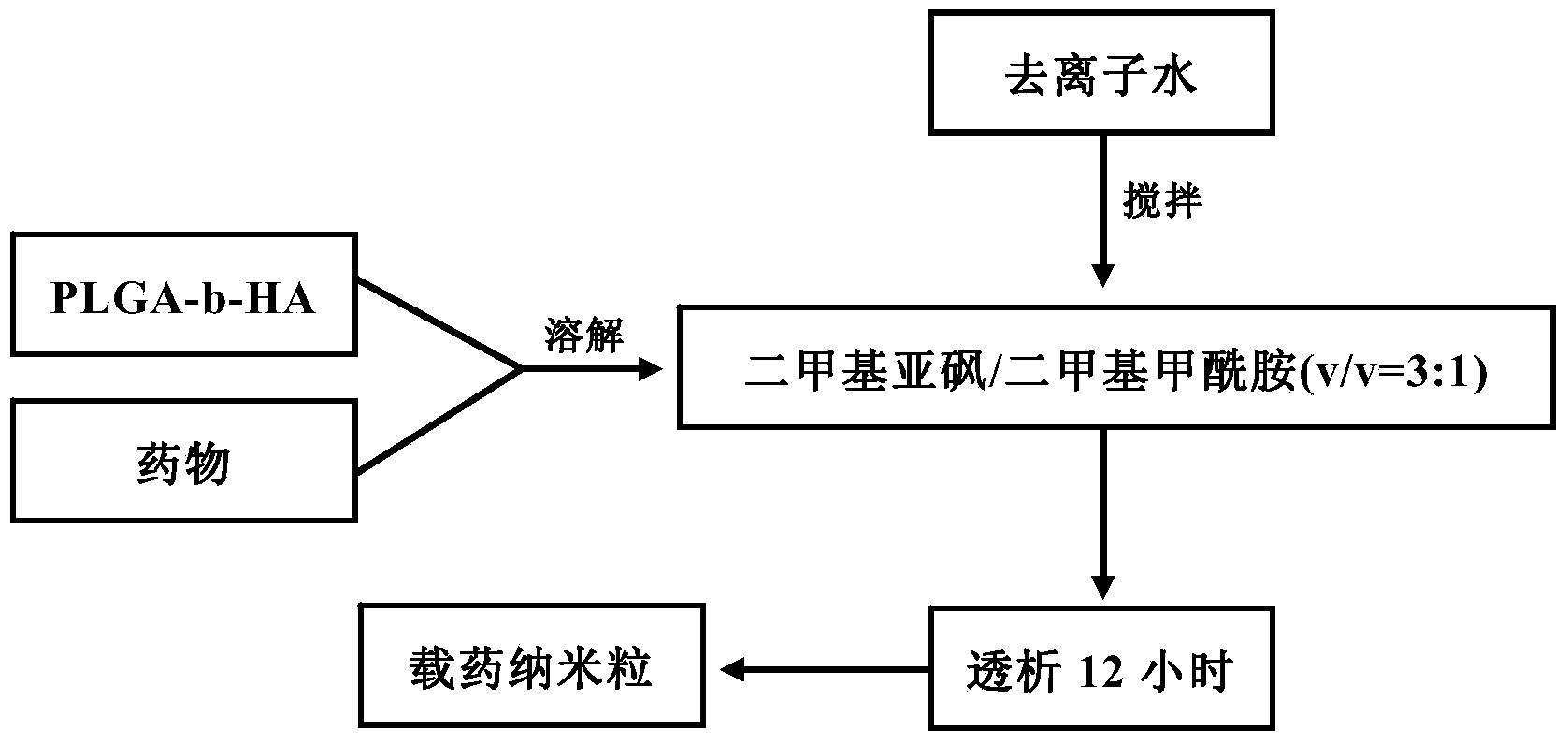
Method for preparing docetaxel and sulforaphane loaded self-assembled nano-particle and application of nano-particle
The invention relates to the technical field of medicines, and provides a docetaxel and sulforaphane loaded hyaluronic acid modified poly(lactic-co-glycolic acid) copolymer self-assembled nano-particle, a preparation method thereof and an application of the self-assembled nano-particle in preparing a medicine for treating breast cancer. The microscopic structure of the nano-particle comprises a hydrophobic PLGA (poly(lactic-co-glycolic acid)) core and a hydrophilic HA (hyaluronic acid) shell, wherein the PLGA core is used for wrapping a medicine DTX (docetaxel) or SFN (sulforaphane), and the hydrophilic shell HA can target a breast cancer stem cell highly expressing a CD44 receptor. In-vitro cytotoxicity tests indicate that the drug-loaded nano-particle can play a good role in resisting differentiation of mammary gland cells and breast cancer stem cells when compared with free medicines; in-vivo tumor inhibition experiments indicate that the drug-loaded medicine is more effective than free medicines and combined free medicines, and has a small systematic toxicity. The nano-particle can play a role in simultaneously performing target differentiating on mammary gland cells and breast cancer stem cells, and a new strategy is provided to treatment of breast cancer.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Topical anesthetic formulation
InactiveUS7273887B1Improve permeabilityReduce systemic toxicityBiocidePharmaceutical non-active ingredientsWhole bodyAdditive ingredient
The topical anesthetic formulation of the present invention is typically a solution that preferably includes lidocaine, USP as the active anesthetic ingredient with benzyl alcohol and isopropyl alcohol. This invention deals with problems commonly associated with topical application of local anesthetics such as: slow onset of action; need for occlusion; messiness of creams, ointments or gels; and rapid loss of effect due to rapid systemic dispersion. The invention permits enhanced penetration of the anesthetic and thereby allows for a lesser total dosage of pharmaceutically active ingredient. The use of a lesser total dosage also decreases systemic toxicity.
Owner:TRANSDERMATECH
Killing cell for high-efficiency and stable expression of antibodies and use thereof
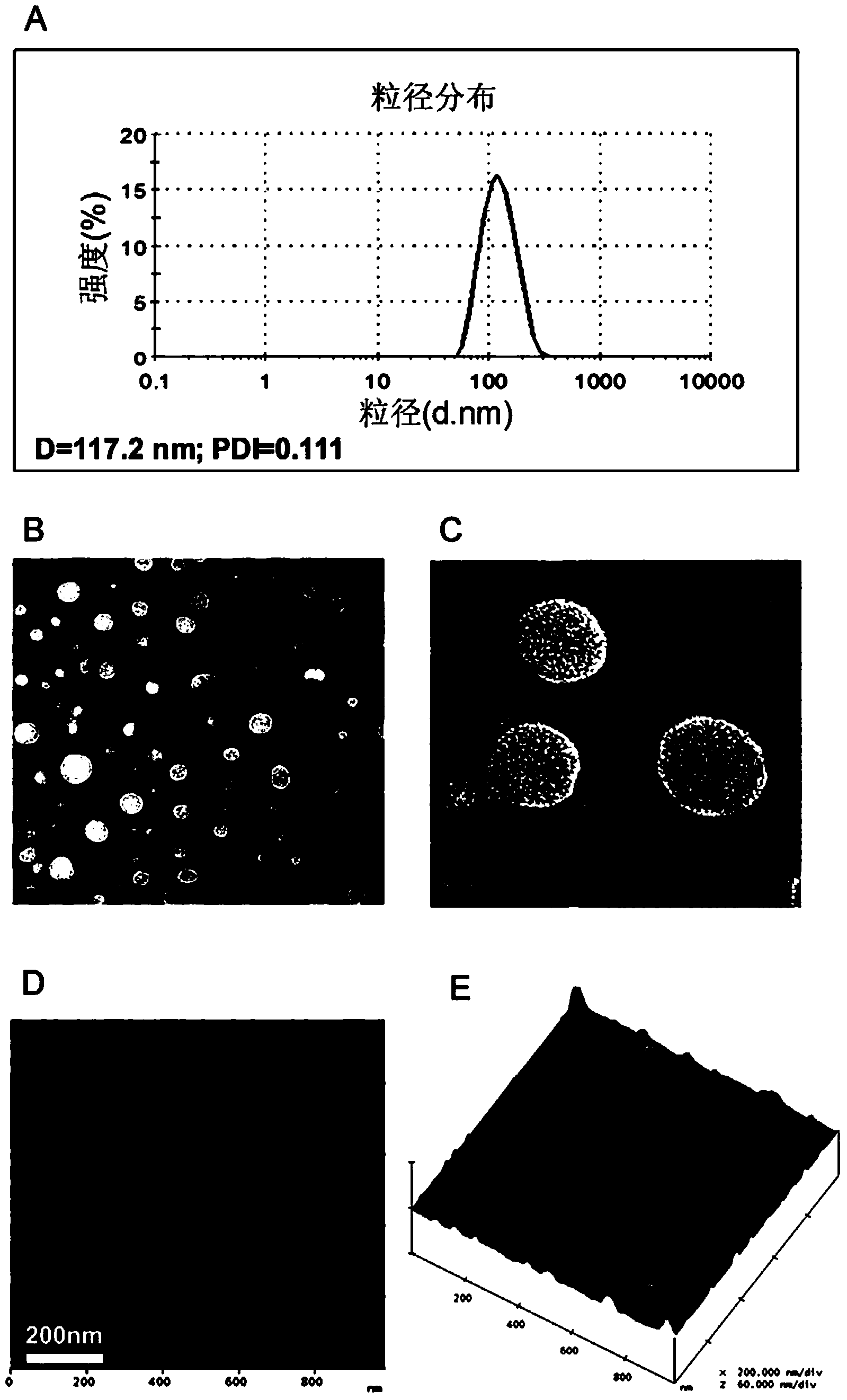
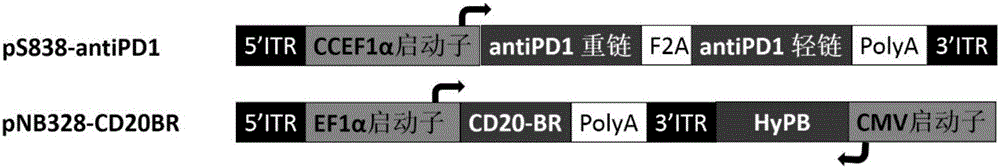
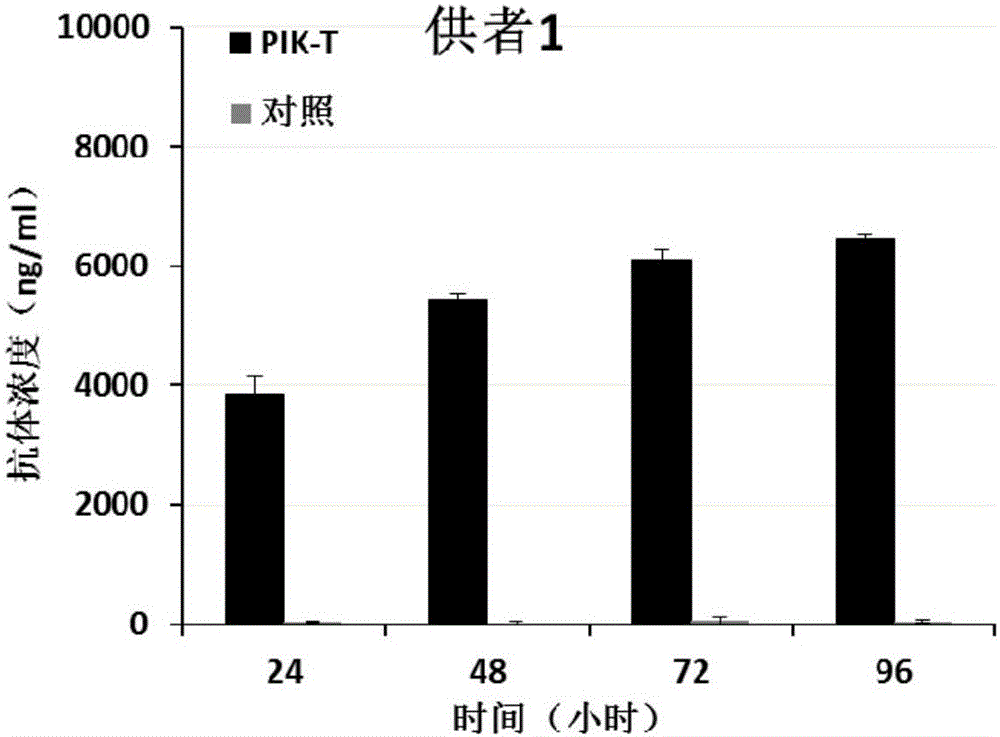
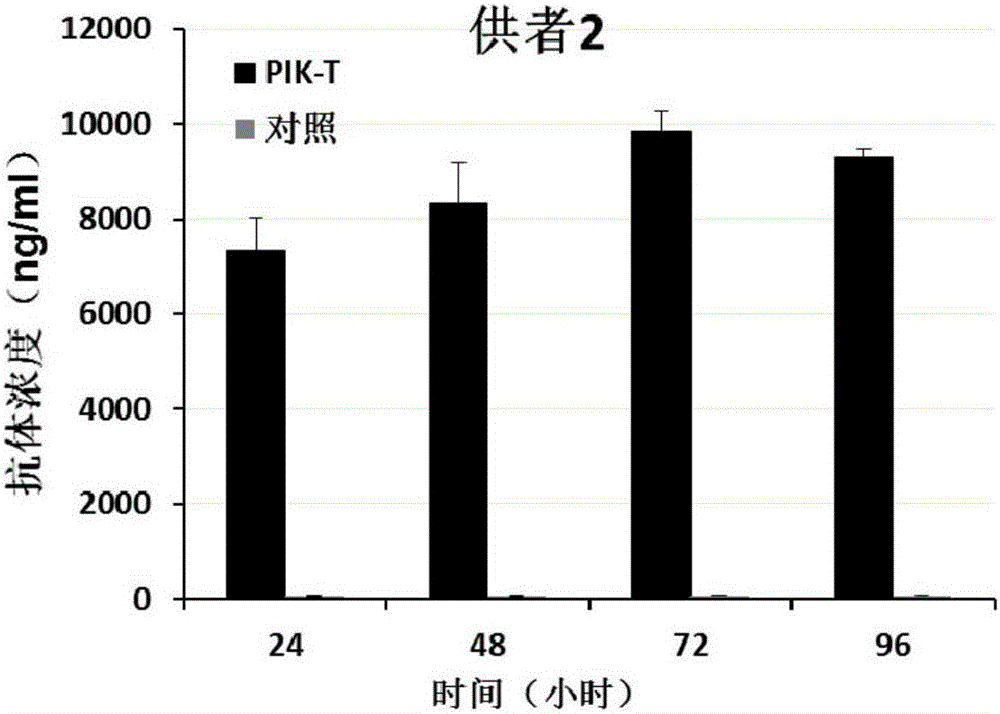
PendingCN107523545AAntibacterial agentsAntibody mimetics/scaffoldsAntigen Binding FragmentAutoimmune disease
The invention relates to a killing cell for high-efficiency and stable expression of antibodies and a use thereof. Specifically, the invention provides the transgenic killing cell; a genome of the killing cell is stably integrated with an expression cassette containing an encoding sequence of human Fc segment-containing antibodies, or containing an encoding sequence of chimeric antigen receptors and inhibitory antibodies or activated type antibodies, and both ends of the expression cassette contain inverted terminal repeated sequences of a transposon. The killing cell can stably express the human Fc segment-containing antibodies or the chimeric antigen receptors and antigen-binding fragments derived from interested antibodies with high level while maintaining cell killing toxic effect. In addition, for preventing systemic toxicity and autoimmune diseases caused by over expression of antibodies due to in-vivo continuous proliferation of immune cells for stable expression of the antibodies, a molecular braking system is also introduced. With use of monoclonal antibody agents appearing on the market, the killing cell integrated with the antibody expression cassette can be quickly eliminated, and the safety of treatment is effectively improved.
Owner:SHANGHAI CELL THERAPY RES INST +1
Inhibitor which is deactivatable by a reagent produced by a target cell
The invention relates to molecules inhibiting biologically active compounds and further comprising moieties specifically cleavable by a reagent produced by a target cell. More specifically, the invention relates to inhibitors that bind, inhibit, suppress, neutralize, or decrease activity of a biologically active agent. Inhibitors comprise at least one moiety that bind, inhibit, suppress, neutralize, or decrease activity of a biologically active agent and at least one moiety that can be cleaved specifically by a reagent produced by target cells. The cleavage deactivates the inhibitor. Following cleavage, the active agent is liberated into the local environment. Administration of the inhibitor alone or together with the active agent suppress the compound's activity until it reaches the proximity of a target cell. This targeted specific release enables the agent concentration in a site to reach levels that have desired therapeutic effects without systemic toxicity. The invention also relates to production and uses of the inhibitors in diagnosis and treatment of a disease.
Owner:VYTACERA BIO LLC
Camptothecine and non-linear polyethyleneglycol prodrug of derivative thereof
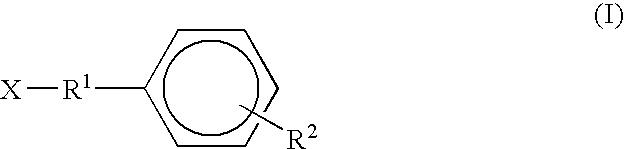
InactiveCN101385860ANo systemic toxicityReduce weightOrganic active ingredientsPharmaceutical non-active ingredientsPositive controlHCT116 Cell
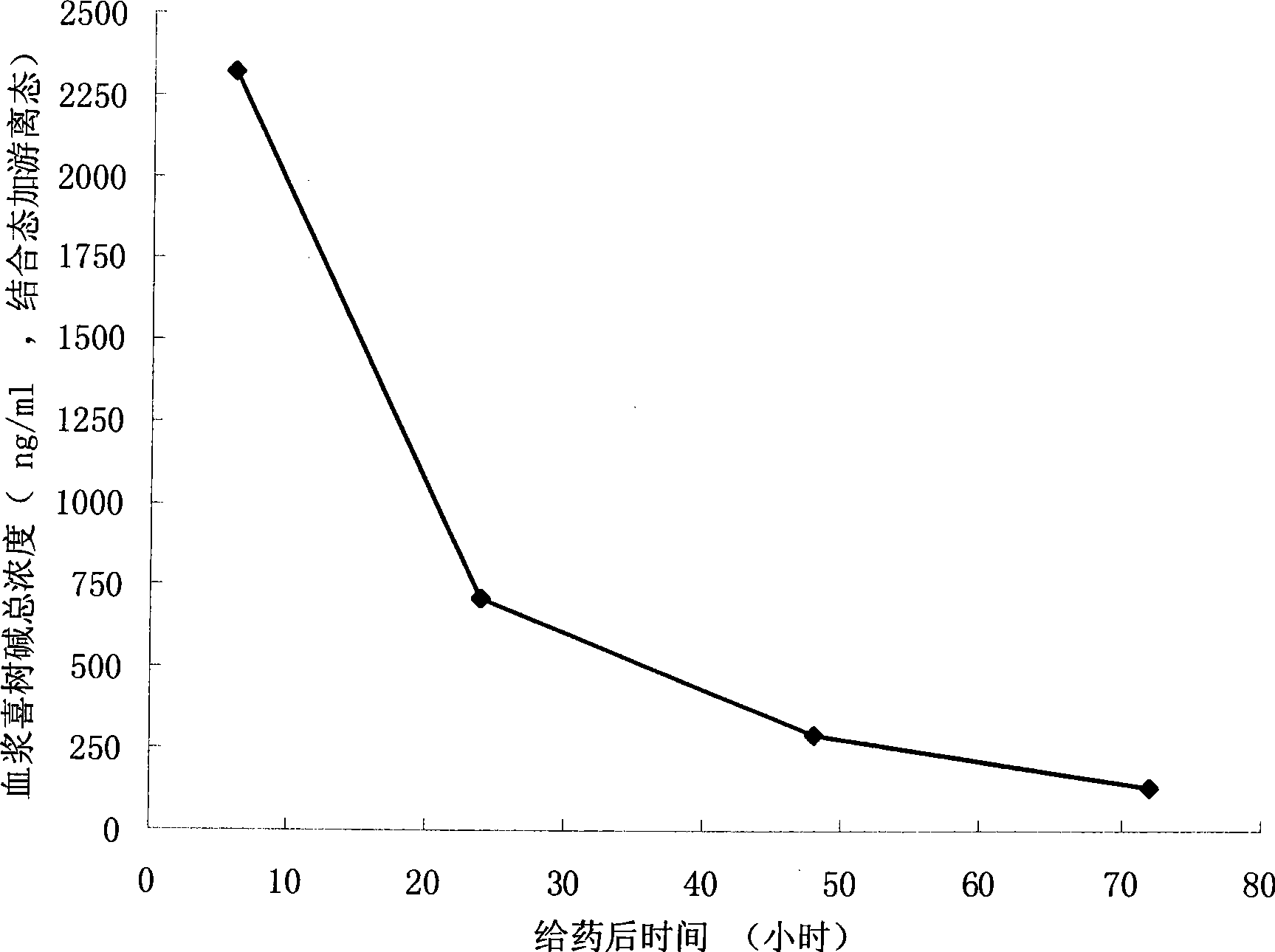
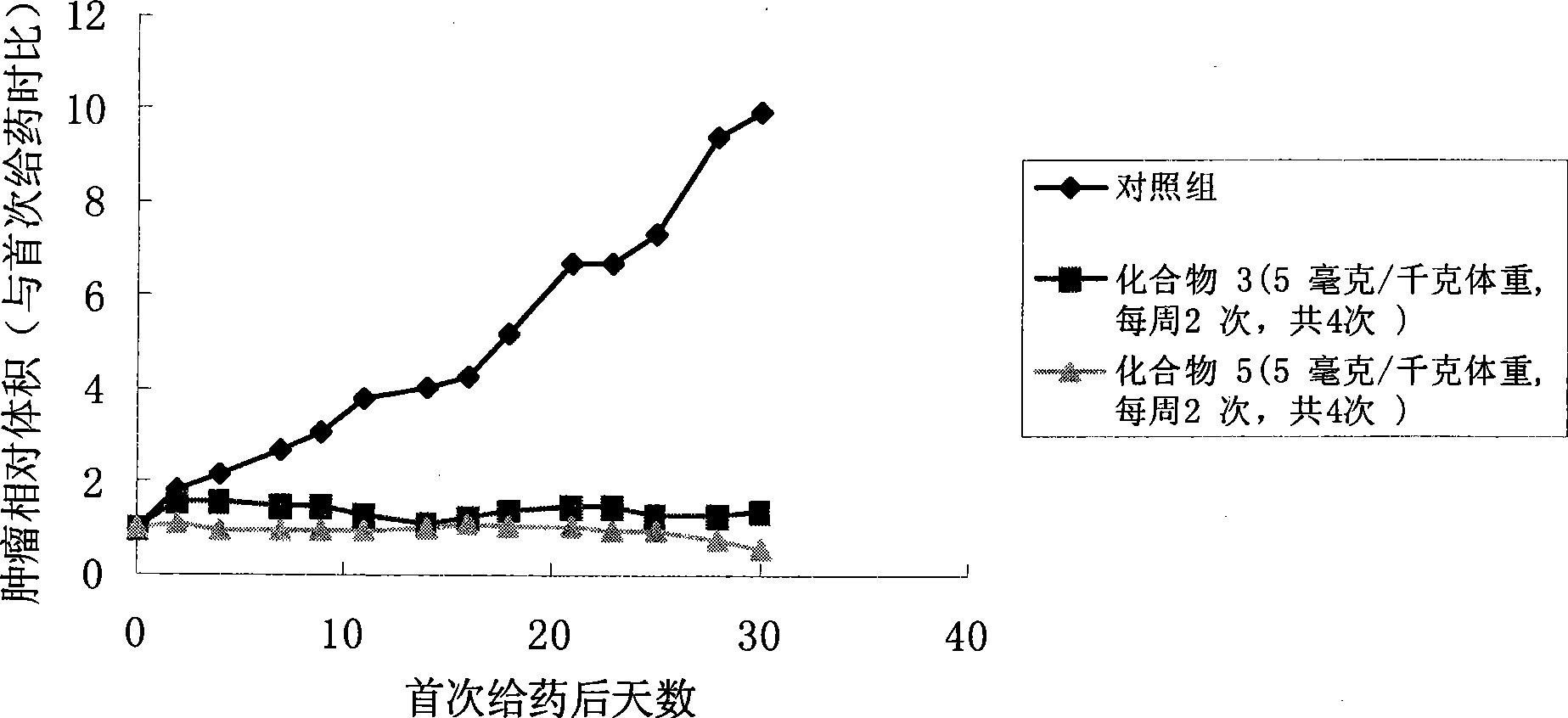
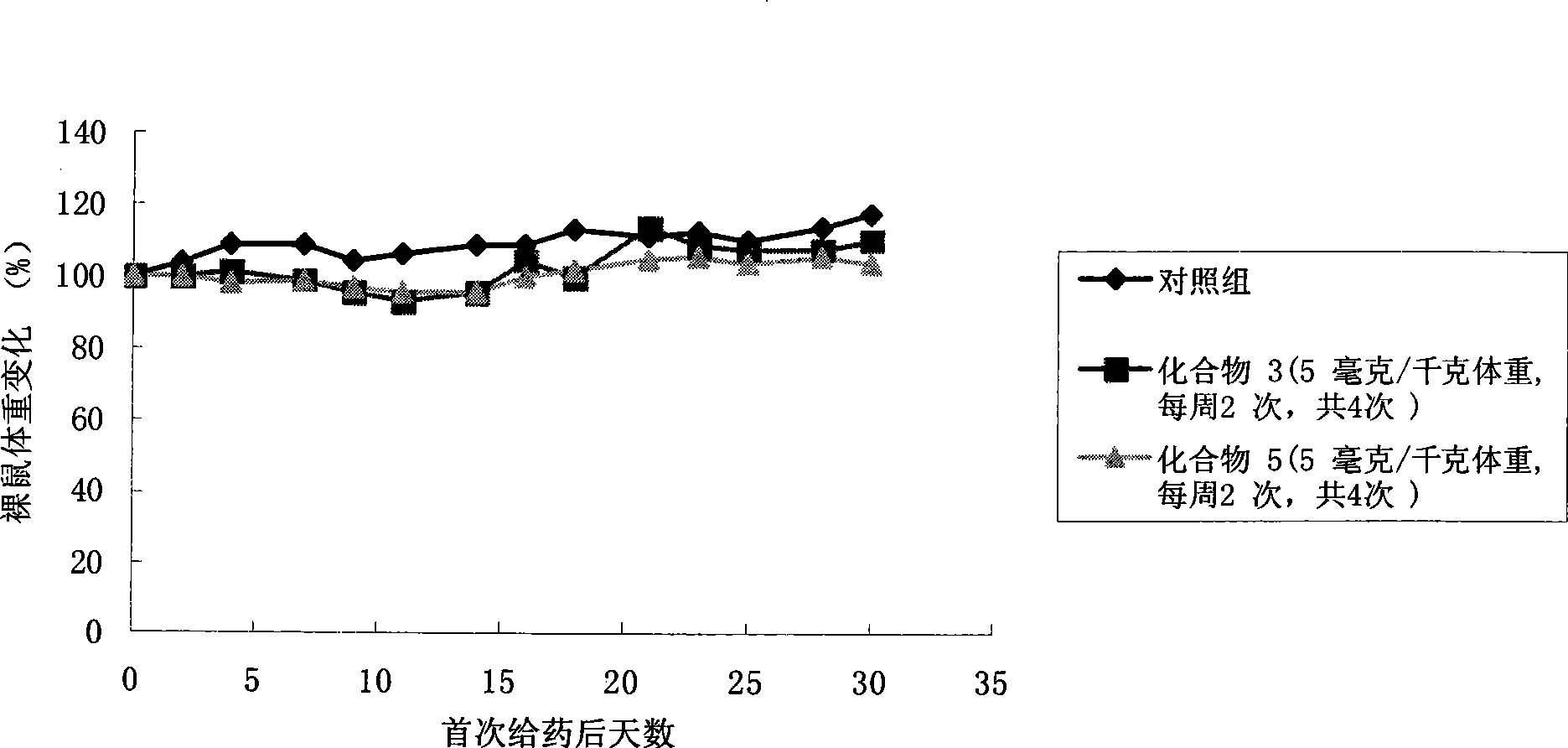
The invention discloses a pro-drug as shown in general formula (I), which is formed by coupling camptothecin or a derivative of the camptothecin with non-linear configuration glycol polyethylene, wherein, the definition for varied groups is available in the specification. A physiological disposition research on nude mice discloses that: the pro-drug has a time-delay plasma concentration - time curve, and the plasma concentration can still be maintained at over 100ng / ml 72 hours after intravenous medication. Evaluation on physiological drug effect shows that the pro-drug has strong growth inhibiting effect against the LOVO HCT116 cell line transplantable tumor inoculated into the nude mice and also has obvious growth inhibitory activity against human lung carcinoma H460 cell line transportable tumor inoculated into the nude mice. The inhibition of the pro-drug is better than that of Irinotecan, a positive control drug and has no obvious systemic toxicity.
Owner:AMERICAN CAOYAOQUAN
Tumor-targeted delivery carrier based on cell-derived micro-vacuoles, preparation method and application
ActiveCN106692984AGood biological stabilityHigh biosecurityEnergy modified materialsGenetic material ingredientsDspe pegCell culture supernatant
The invention discloses a tumor-targeted delivery carrier based on cell-derived micro-vacuoles, a preparation method and an application. The preparation method comprises the following steps of: (A) preparing a conditioned medium: supplementing fetal bovine serum, antibiotics, DSPE-PEG-Biotin and DSPE-PEG-Folate into a basal medium; (B) using the obtained conditioned medium in cell culture, and collecting cell culture supernatant for subsequent separation; (C) carrying out low-speed centrifugation on the obtained culture supernatant to remove cell debris and apoptotic bodies, then adding SA-IONPs, mixing uniformly, incubating, then separating by a magnet, using PBS for re-suspension, eluting for multiple times to obtain the cell-derived micro-vacuoles with membrane surfaces modified by folic acid and iron oxide nano-particles, and freezing for storage; and (D) loading chemotherapeutic drugs or therapeutic genes into the functionalized micro-vacuoles doubly-modified by an electroporation mode, and carrying out re-suspension after separation with the magnet. The tumor-targeted delivery carrier based on cell-derived micro-vacuoles, the preparation method and the application disclosed by the invention are applicable to specific targeting delivery of multiple chemotherapeutic drugs and therapeutic genes, and have the advantages of enhancing the anti-tumor effect, reducing the systemic toxicity and improving the clinical effect of the current therapeutic selection, so that a new hope is brought for clinical therapy of tumors.
Owner:珈泌生物科技(武汉)有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com