Patents
Literature
198 results about "Acid phosphatase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acid phosphatase (EC 3.1.3.2, acid phosphomonoesterase, phosphomonoesterase, glycerophosphatase, acid monophosphatase, acid phosphohydrolase, acid phosphomonoester hydrolase, uteroferrin, acid nucleoside diphosphate phosphatase, orthophosphoric-monoester phosphohydrolase (acid optimum)) is a phosphatase, a type of enzyme, used to free attached phosphoryl groups from other molecules during digestion. It can be further classified as a phosphomonoesterase. Acid phosphatase is stored in lysosomes and functions when these fuse with endosomes, which are acidified while they function; therefore, it has an acid pH optimum. This enzyme is present in many animal and plant species.
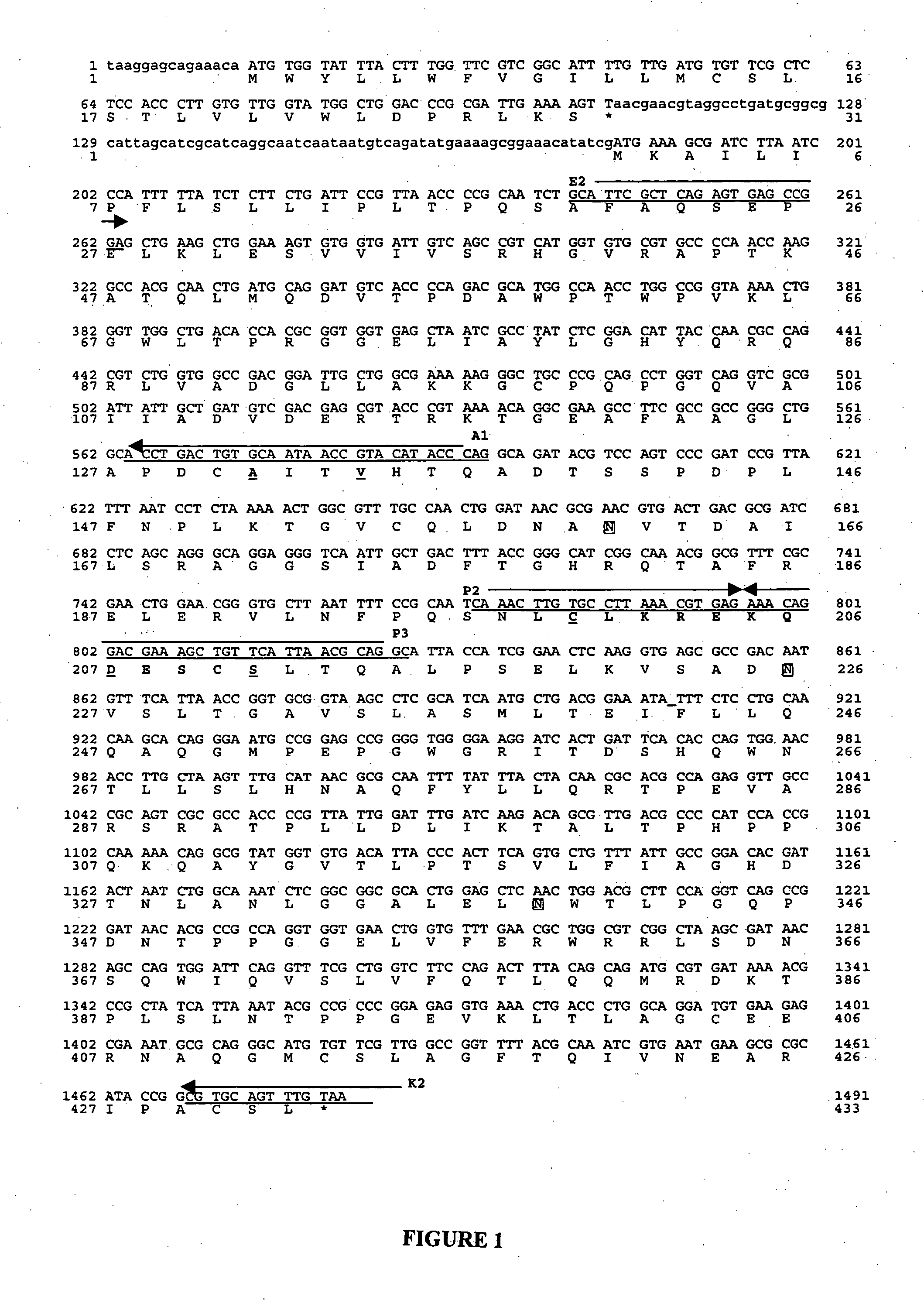
Site-directed mutagenesis of Escherichia coli phytase
InactiveUS20050095691A1Good enzymatic propertiesHigh catalytic efficiencyFungiSugar derivativesEscherichia coliPhytase
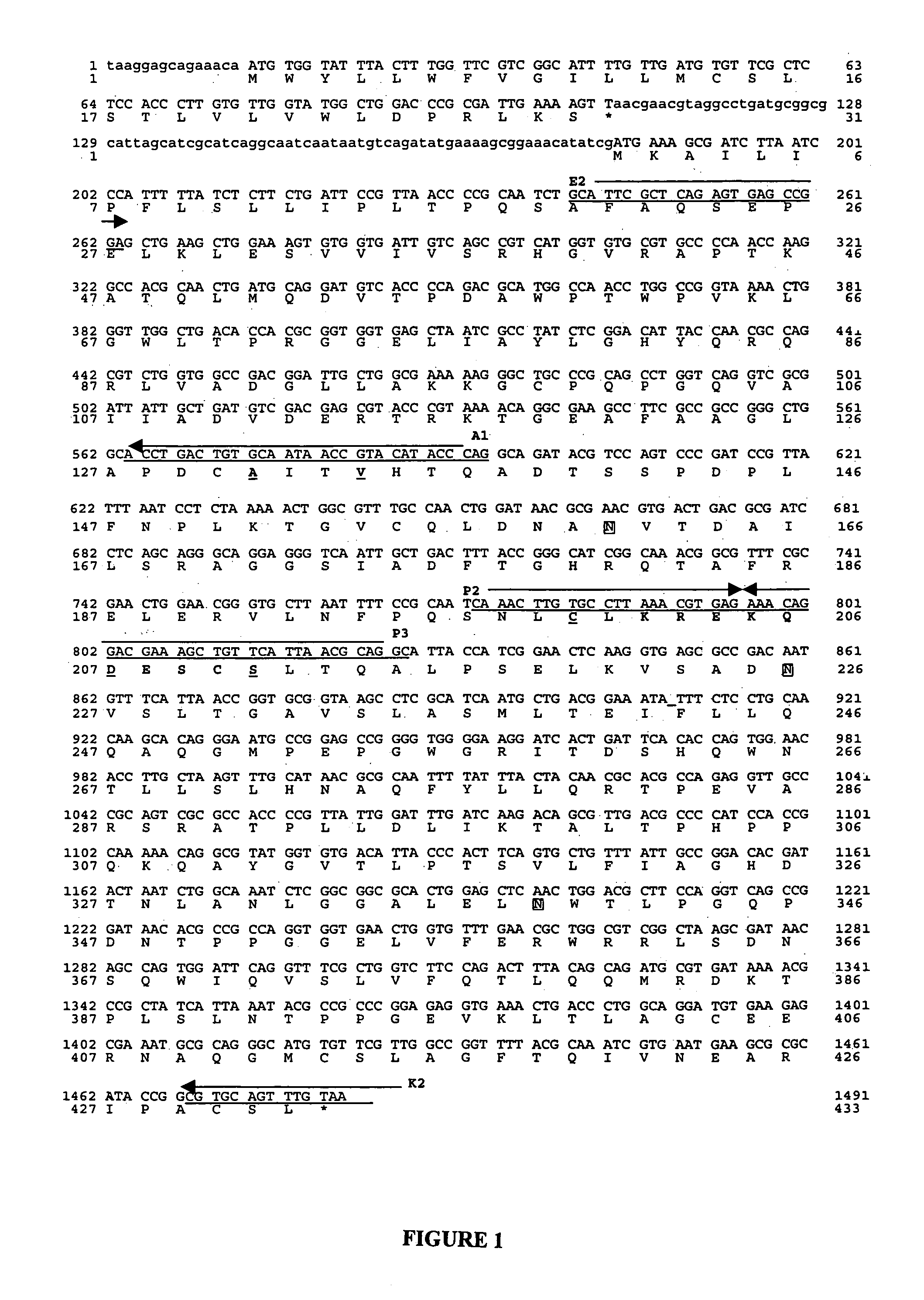
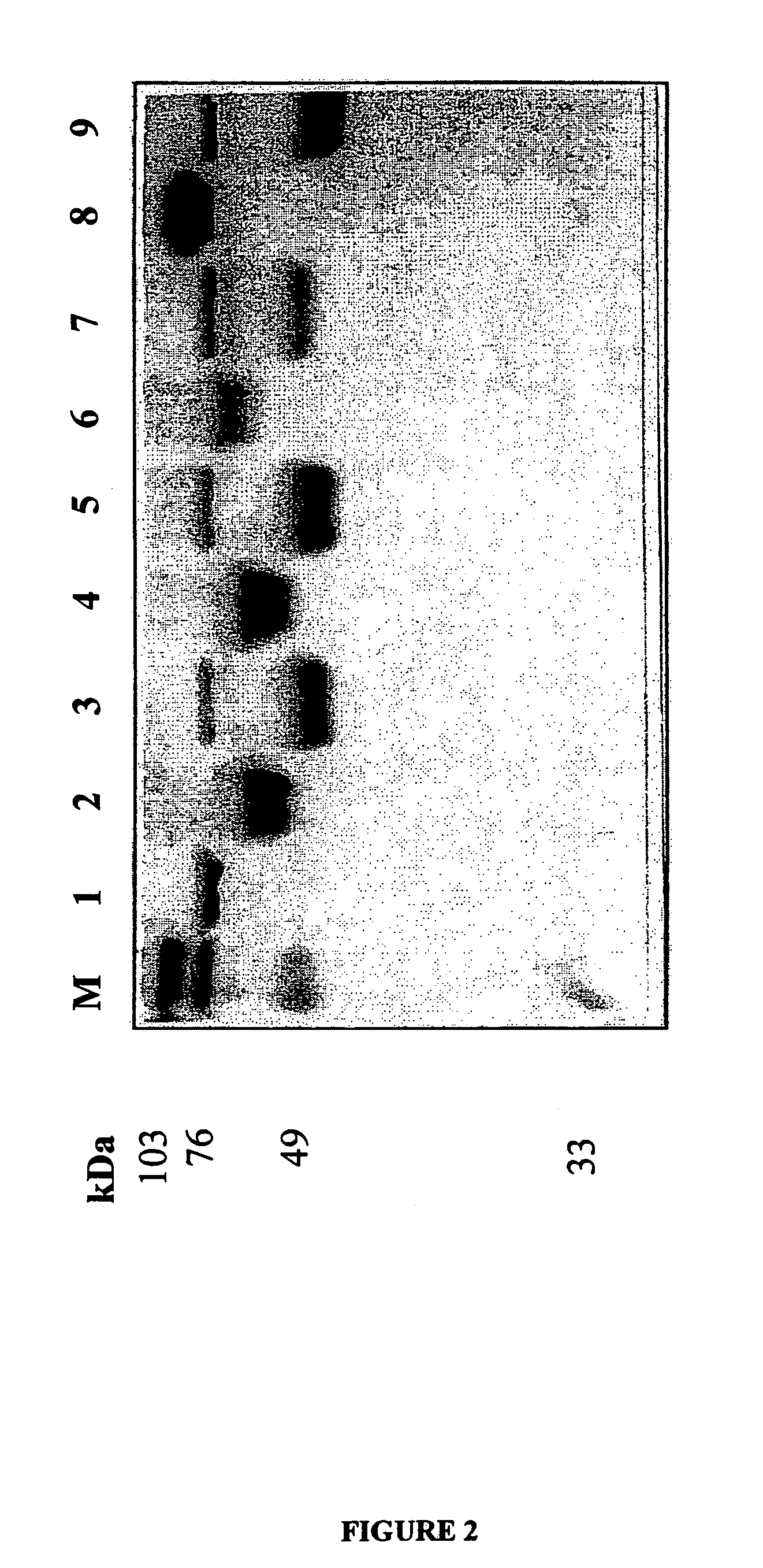
The present invention relates to an isolated mutant acid phosphatase / phytase with improved enzymatic properties. The mutant acid phosphatase / phytase composition is particularly useful in animal feed compositions.
Owner:CORNELL RES FOUNDATION INC
Plants with increased phosphorous uptake
The invention provides plant acid phosphatase coding sequences. Also provided are constructs comprising these sequences, plants transformed therewith and methods of use thereof. In certain aspects of the invention, transgenic plants are provided exhibiting improved phosphorous utilization.
Owner:NOBLE RES INST LLC
Oleanane-type triadic compound and its preparation method and medical application
The invention discloses an oleanane-type triad compound, and also provides its preparation method and medical application. Acid phosphatase 1B activity of oleanane-type (oleanane-type) triad compounds, structure: R1 is hydroxyl, acetoxy, R2 is carboxyl, hydroxymethylene, R3 is methyl, carboxyl, R4 For carboxyl, methyl. Multiple in vitro protein tyrosine phosphatase 1B inhibition experiments show that the compound has obvious PTP1B inhibitory activity and can be used in the preparation of medicines for diabetes, obesity and complications.
Owner:BEIHUA UNIV
Cervical acid phosphatase - papanicolaou (CAP-PAP) test kit, method and accesories, processes for producing and using the same
InactiveUS20040137551A1Improves cell defense mechanismMicrobiological testing/measurementMaterial analysisGynecologyCervical cancer screening
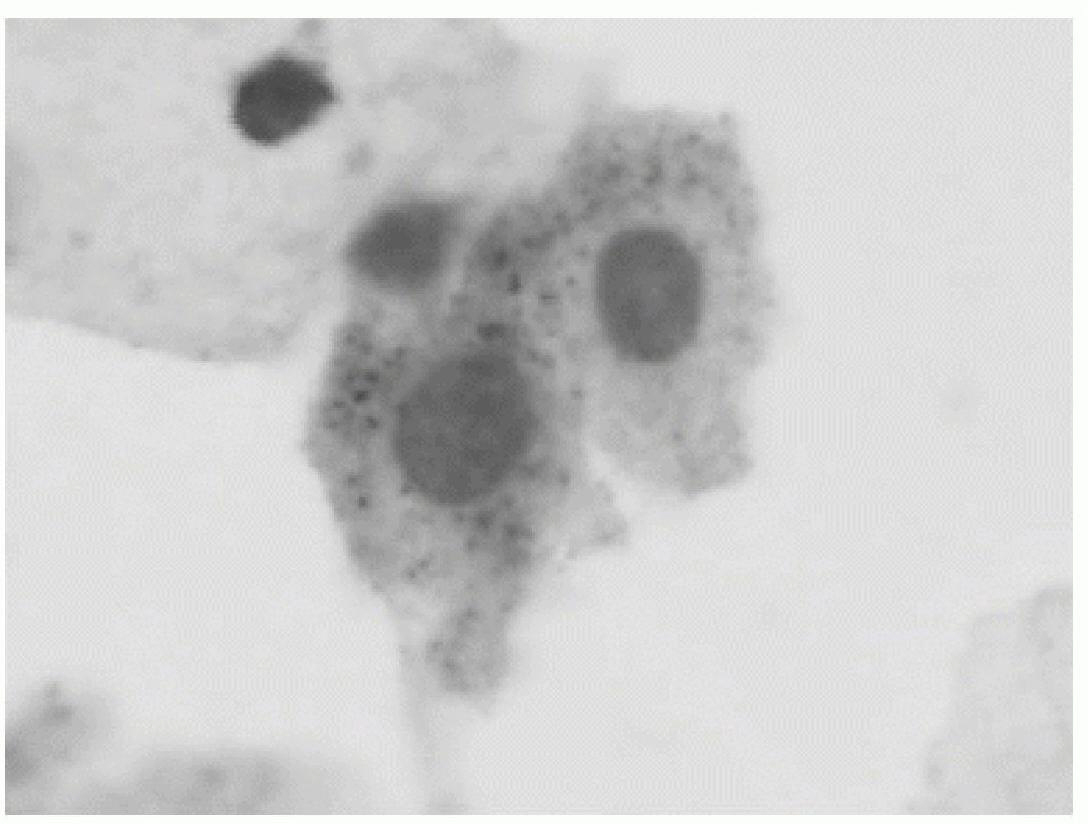
Cervical Acid Phosphatase-Papanicolaou Test Kit (CPK) is an assembly of reagents, controls and instructions for visualization of cervical acid phosphatase on smears or monolayers of cervical specimens, and for performing the CAP-PAP Test (CPT). CPT is a single-slide, double-staining method for demonstration of cervical acid phosphatase activity inside abnormal cervical cells on Papanicolaou stained smears, and a set of criteria for using this test for cervical cancer screening. In previous clinical trials this method was found to enable Pap test screeners to improve test sensitivity (detection of abnormal cells) for more than 10% (from 0.8 to 0.9), and to reduce false negative readings (missing abnormal cells) for more than 50% (from 0.1 to 0.02). Due to better accuracy and the low cost, when approved, CPT may begin to replace current technologies for cervical cancer screening. CPK is designed to meet requirements for testing large series of specimens on regular basis-the usual practice in cytopathology laboratories performing the Pap test. CPK brings consistency for staining and interpretation, makes internal and external controls easier, and improves the test accuracy for lower cost, while increases laboratory productivity for less liability.
Owner:MARKOVIC NENAD S +1
Preparing method and application of dual-response sandwich-type immunosensor based on TiO2 mesomorphic nanomerter material
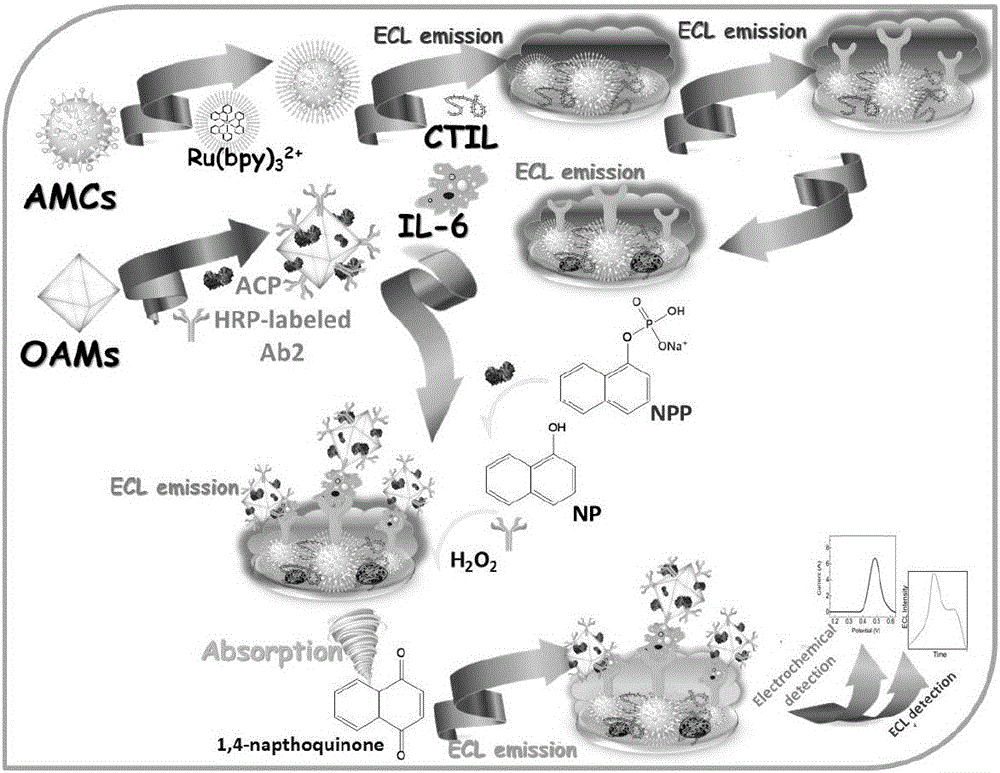
The invention discloses a preparing method and application of a dual-response sandwich-type immunosensor based on TiO2mesomorphic nanomerter material. The invention is characterized in that a novel dual-response sandwich-type immunosensor is prepared by combining electrochemistry and an electrochemical luminance method on the basis of two kinds of TiO2 mesomorphic nanomerter materials, and the dual-response sandwich-type immunosensor is applicable to detection of interleukin-6. Anatase type TiO2 mesocages materials and ionic liquid are used for fixing Ru(bpy)<3>2+ and an IL-6 antibody respectively, and the Ru(bpy)<3>2+ and the IL-6 antibody are used as a signal probe and a molecular recognition probe; octahedron anatase type TiO2 mesocages materials are used for fixing IL-6 second antibody and acid phosphatase which are marked by horse radish peroxidase, and the IL-6 second antibody and acid phosphatase are subjected to a typical sandwich-type immunoreaction to conduct self-assembly on surface of the electrode to prepare the IL-6 sandwich-type immunosensor. The prepared sandwich-type immunosensor can produce electrochemistry signals and electrochemical luminance signals, wherein signal values and IL-6 concentration present linear states within a range of 10<-6>-90 pg / ml and a range of 10<-8>-90 pg / ml respectively.
Owner:FUJIAN NORMAL UNIV
Acidic phosphatase, its encoding gene and application
InactiveCN102206617AImprove efficiencyReduce pollutionHydrolasesMicroorganismsGene engineeringAcid phosphatase
The invention discloses an acidic phosphatase, its encoding gene and an application. The protein provided in the invention is a following protein as a) or b): a) a protein composed by an amino acid sequence which is described as SEQ ID NO:1; b) a protein with acidic phosphatase activity derived from a) that the amino acid sequence is conducted a substitution and / or deficiency and / or addition through one or a plurality of amino acid residue which is described as SEQ ID NO:1. According to the invention, a gene resource is provided to the novel crops varieties capable of efficiently using soil nutrition by employing a gene engineering approach, which is applied to cultivate the novel crops varieties capable of efficiently using soil nutrition.
Owner:TSINGHUA UNIV
Exfoliated cell preservation solution and preparation method thereof
InactiveCN102113481AHigh retention rateScatter tilingDead animal preservationBenzoic acidHeparin sodium
The invention relates to exfoliated cell preservation solution and a preparation method thereof. The preservation solution comprises the following components in percentage by weight: 0.6 percent of sodium chloride, 0.06 percent of potassium chloride, 2 percent of glucose, 20 percent of ethanol, 3 percent of 1-3 propylene glycol, 2 percent of glycerin, 0.15 percent of glycine, 0.1 percent of mucolytic agent, 0.2 percent of cell protective agent, 0.1 percent of heparin sodium and 0.05N of sodium benzoate-benzoic acid buffer solution. A pH value is adjusted by 1 percent sodium hydroxide or hydrochloric acid solution to ensure that the pH of the preservation solution is 5.8. By the exfoliated cell preservation solution, cells can be well preserved and the preservation time is more than 10 days; cell nucleuses are clear, and cytoplasm is stretched; red blood cells and mucus are effectively removed; the cells are dispersed and flatly laid and an acid phosphatase (ACP) enzyme is well preserved simultaneously; and the exfoliated cell preservation solution is suitable for ACP enzyme staining.
Owner:XIAMEN MAIWEI BIOTECH
Improved histiocyte staining method and application thereof
The invention relates to an improved cervical squamous epithelial cell staining method, a cervical squamous epithelial cell staining kit and application of the method and the kit to diagnosis of individual cervical squamous epithelial cell cancers. The improved cervical squamous epithelial cell staining method comprises the steps of staining cervical squamous epithelial cells by using cell acid phosphatase staining reagent and staining the cervical squamous epithelial cells by using Pap staining reagent. The cervical squamous epithelial cell staining kit comprises the cell acid phosphatase staining reagent and the improved Pap staining reagent. The invention additionally relates to a kit for diagnosing individual cervical squamous epithelial cell cancers or precancerous lesions.
Owner:ANHUI NEW MARK SCI TECH
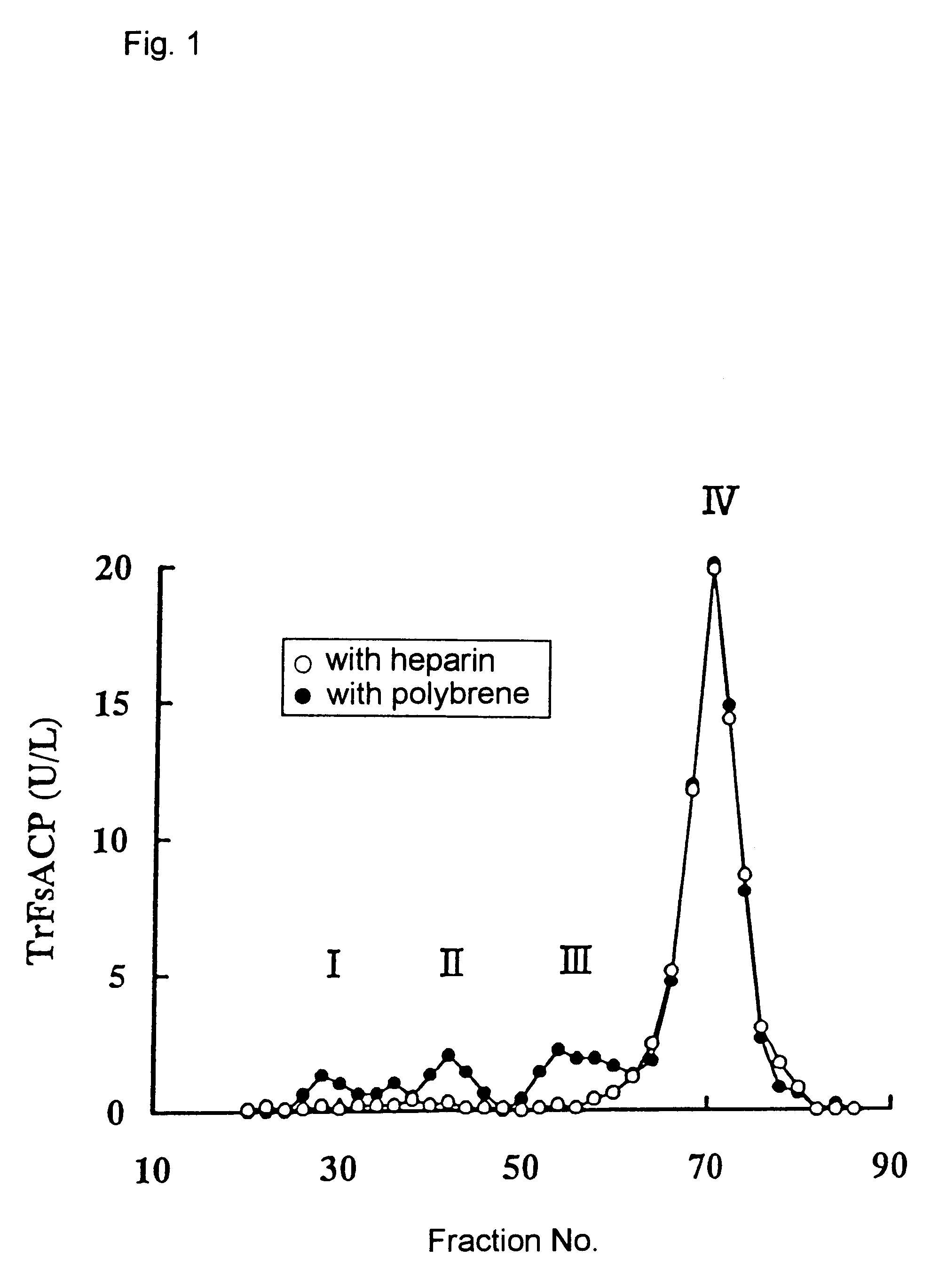
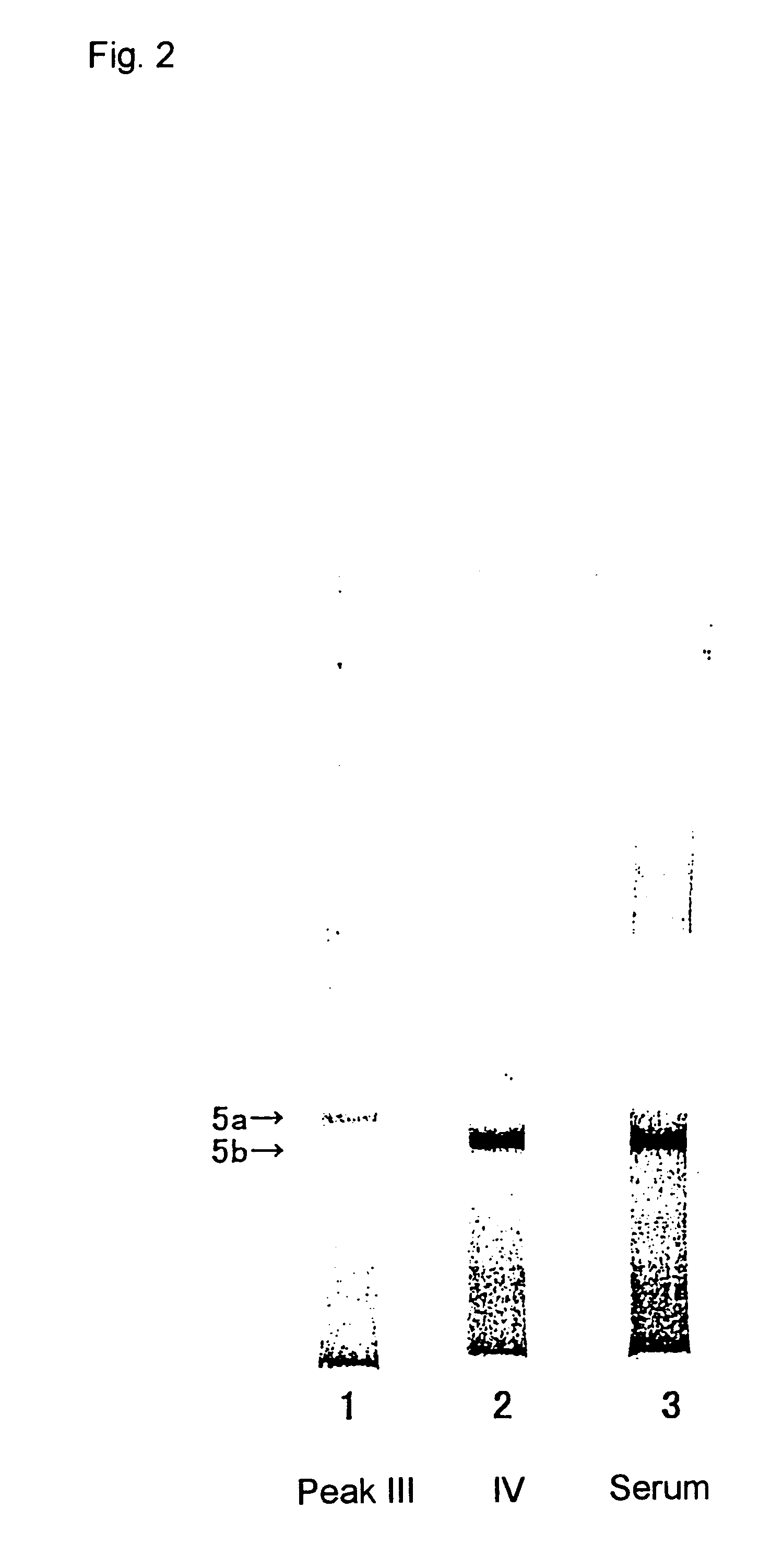
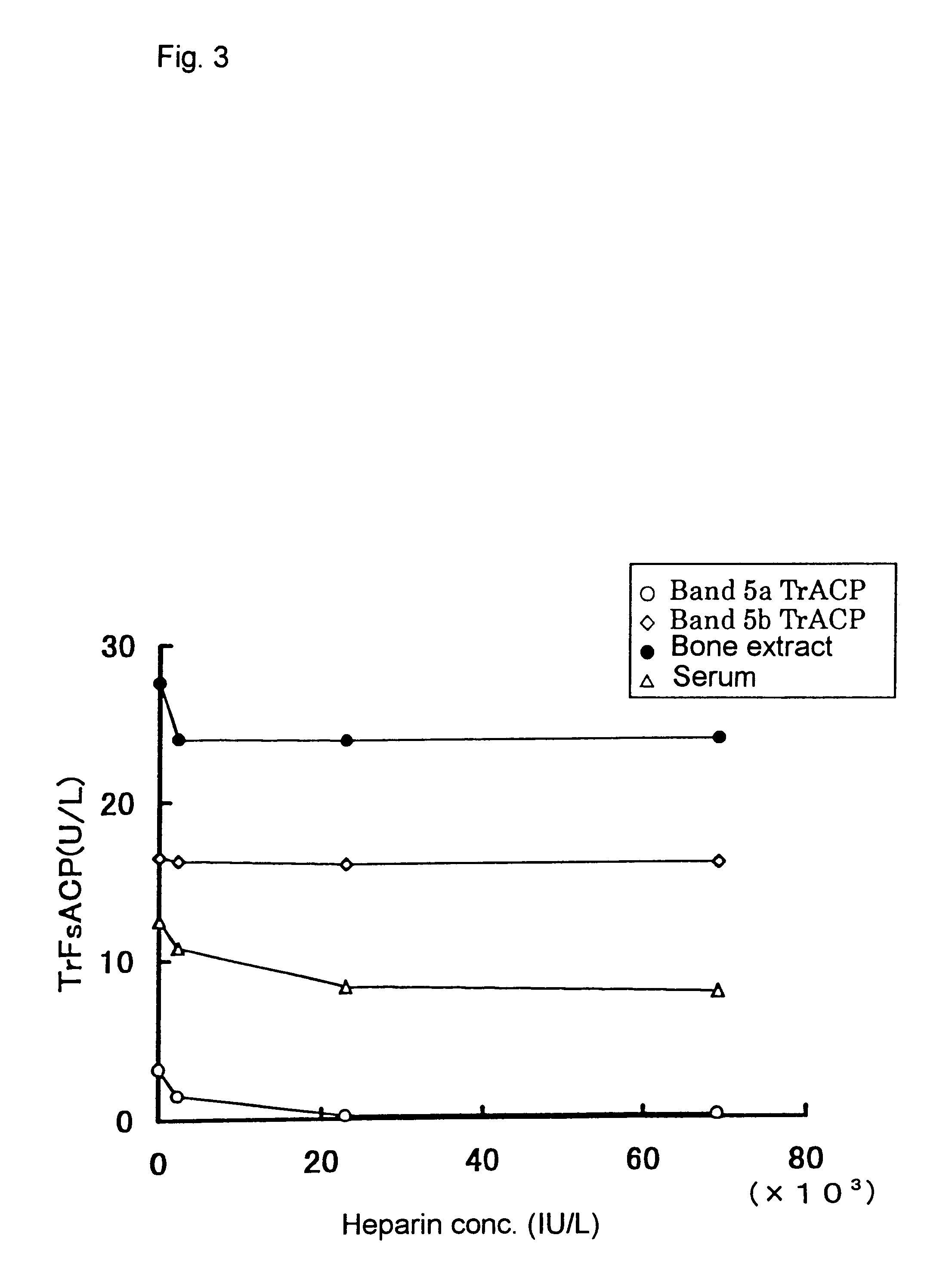
Method for specific measurement of acid phosphatase derived from osteoclasts
InactiveUS6448027B1Economical and simpleMicrobiological testing/measurementBiological testingAcid phosphataseStereochemistry
A method for measuring activity of osteoclast-derived acid phosphatase located at Band 5b of Band 5 in polyacrylamide gel electrophoresis of a sample which is characterized by using an inhibitor to acid phosphatases located at Band 5 other than Band 5b; and a composition and a kit for using in the method.
Owner:FALCO BIOSYST
Target cell staining kit for displaying proliferative activity of cell and use method thereof
ActiveCN101781675AGood precisionIncreased sensitivityMicrobiological testing/measurementCervical cellsCell sensitivity
The invention discloses a target cell staining kit for displaying the proliferative activity of a cell and a use method thereof. The kit comprises I type and II type which respectively comprise an enzyme substrate, test paper, enzyme stationary liquid, phosphate buffer solution, Mayer hematoxylin and the like. The staining method comprises the steps of preparing pieces, fixing, staining and the like. The method can display acid phosphatase in exfoliative cells on smears such as exfoliative cervical cells, esophagus brush sheet cells and the like and search abnormal proliferated cells according to the target. Compared with the traditional Pasteur smear method, the method has the advantages of higher sensitivity for detecting cancer and precancerous lesions and similar specificity, therefore, the invention can be used for cytology screening of cervical carcinoma, esophageal cancer, lung cancer and other malignant tumors.
Owner:泰普生物科学(中国)有限公司
Site-directed mutagenesis of Escherichia coli phytase
The present invention relates to an isolated mutant acid phosphatase / phytase with improved enzymatic properties. The mutant acid phosphatase / phytase composition is particularly useful in animal feed compositions.
Owner:CORNELL RES FOUNDATION INC
Combined detection kit for seminal plasma
ActiveCN103760331AComprehensive detection indicatorsThe detection method is simpleMicrobiological testing/measurementBiological testingWhite blood cellInfertility
The invention discloses a combined detection kit for seminal plasma. The nine major indexes of pH value, leukocyte esterase, lecithin body, citric acid, zinc, acid phosphatase, elastase, fructose and neutral alpha-glucosidase in the seminal plasma can be rapidly, accurately, simply, conveniently and practically detected at one time in a combined way, functions of glands such as the prostate, the epididymis and the vesicula seminalis can be comprehensively evaluated, detailed related information is provided for the clinical diagnosis and treatment of caused related diseases such as prostatitis, reproductive system inflammation, sexual dysfunction and sterility infertility, a market gaps are filled, and the combined detection kit is easy to clinically popularize.
Owner:AUTOBIO DIAGNOSTICS CO LTD
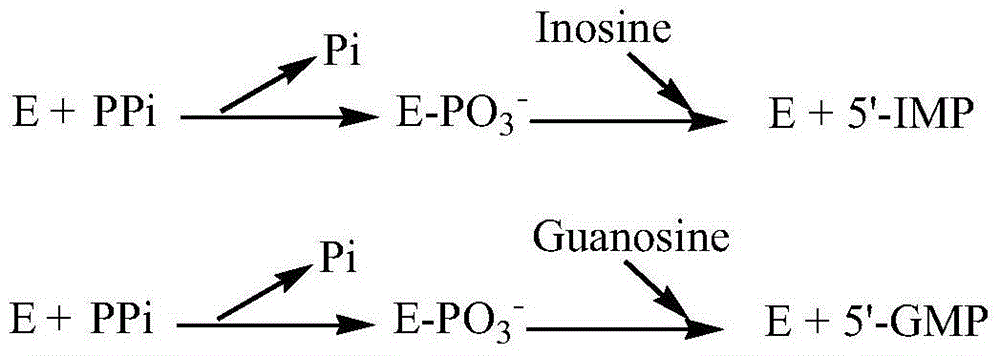
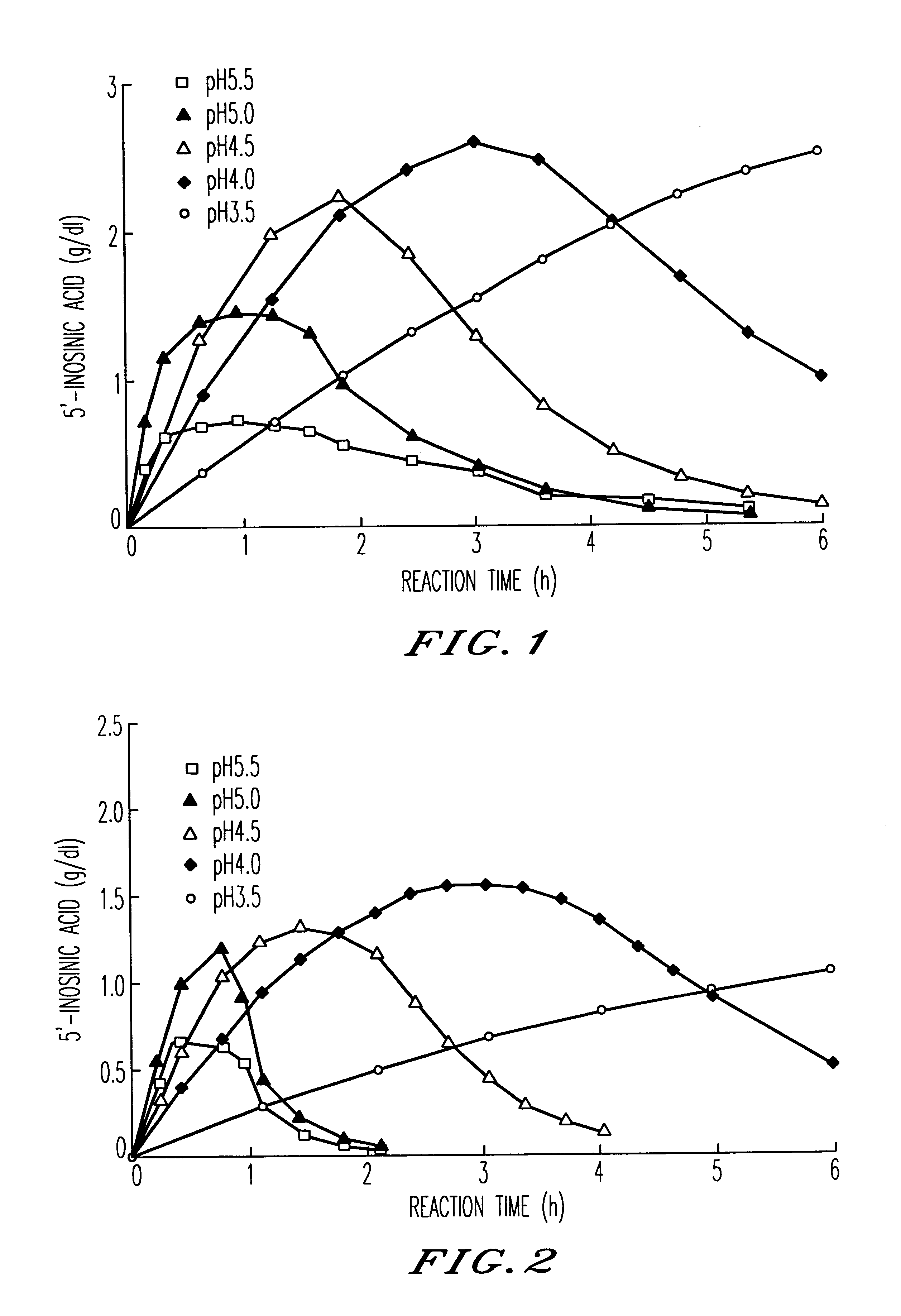
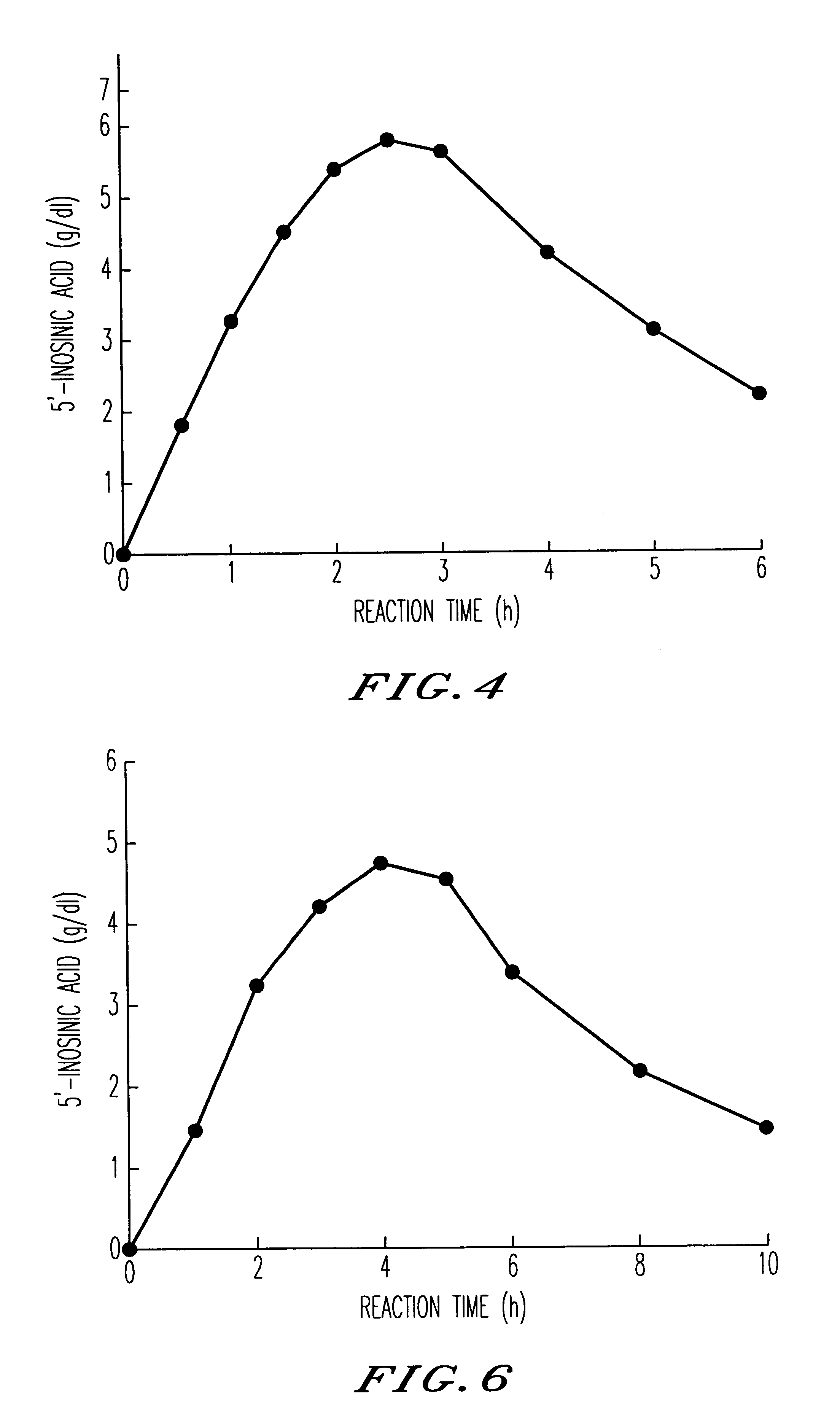
Method for producing nucleoside-5'-phosphate ester
InactiveUS6015697AInexpensively and efficiently producingHigh reaction yieldSugar derivativesBacteriaPhosphateAcid phosphatase
A method for producing nucleoside-5'-phosphate esters inexpensively and in high yields by phosphorylating a nucleoside with a phospahte group donor using an acid phosphatase having an increased affinity for the nucleoside and / or an increased temperature stability at a pH of pH 3.0 to 5.5, to produce a nucleoside-5'-phosphate ester. Mutant acid phosphatases having increased affinity for nucleosides and / or an enhanced temperature stability are also provided.
Owner:AJINOMOTO CO INC
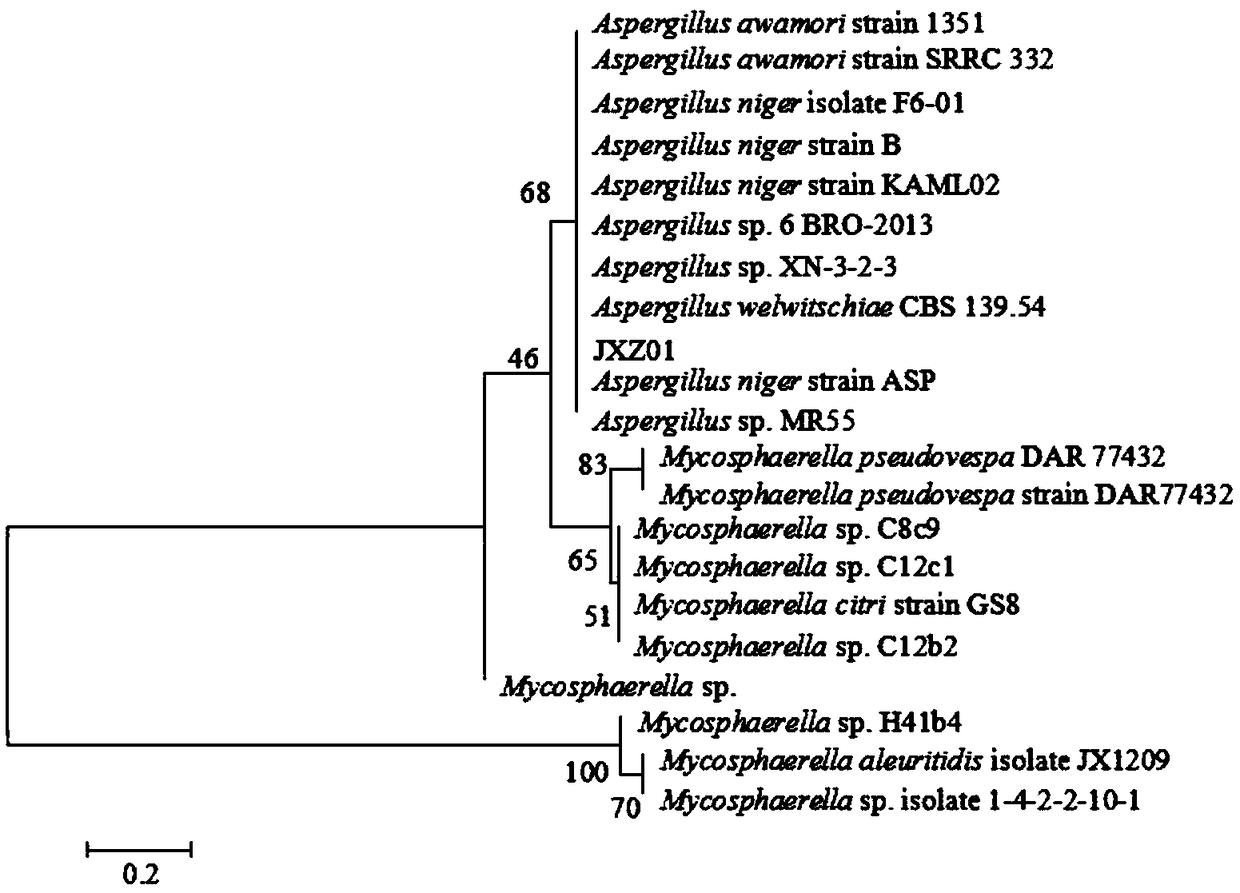
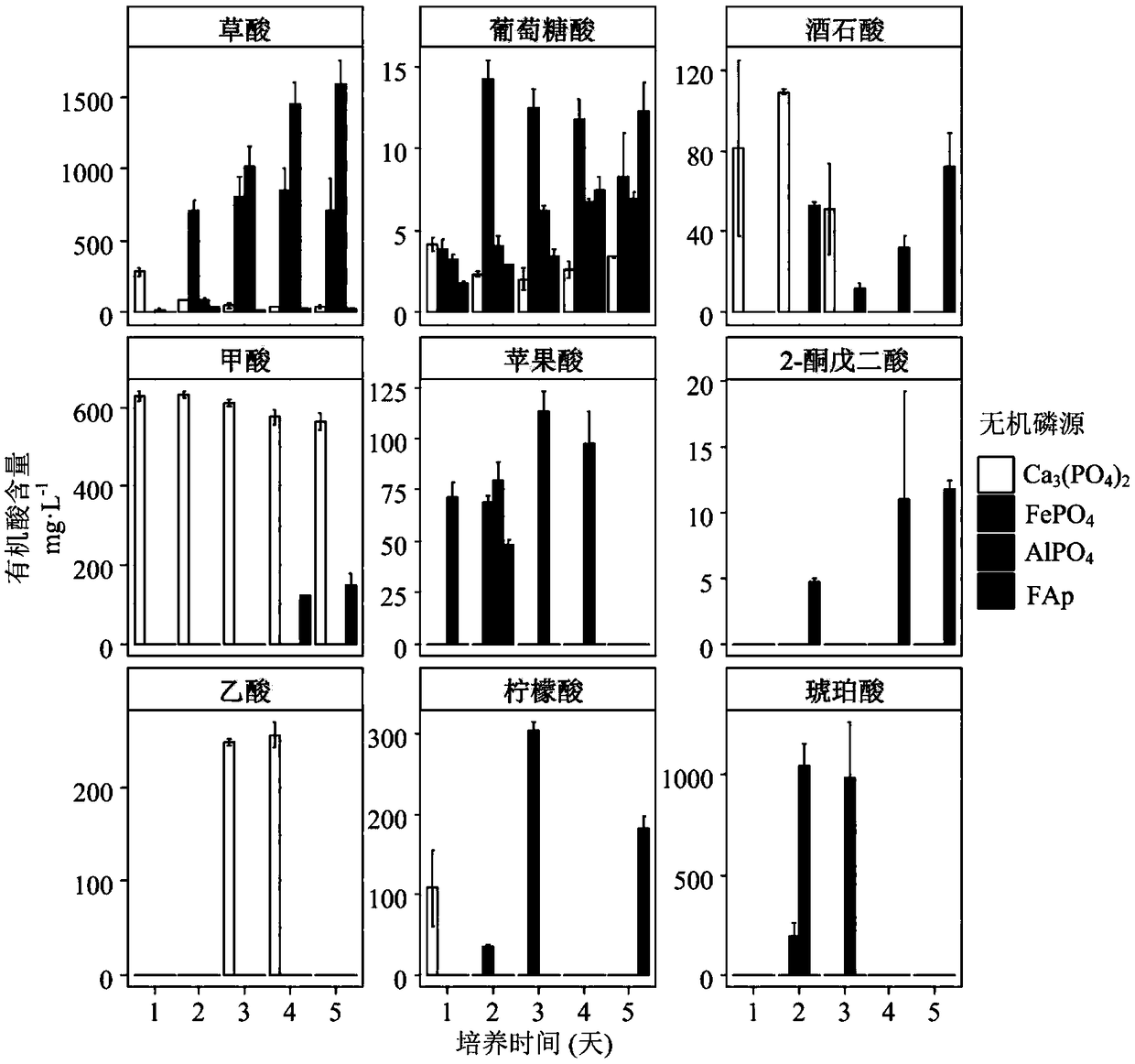
Separation and application of aspergillus niger JXZ01 with decomposition capability of various difficult-to-dissolve phosphorous sources
ActiveCN109182136AImprove toleranceImprove repair effectFungiContaminated soil reclamationPhytaseDecomposition
The invention belongs to the field of agricultural microbiology and discloses a fungus with a good phosphorous decomposition capability and application thereof to heavy metal pollution remediation. Aclassified name of the fungus is aspergillus niger with the strain number of JXZ01. The strain is preserved in China General Microbiological Culture Collection Center (CGMCC) on July 19, 2018 and thepreservation number is CGMCC_No. 15994. The strain not only can secrete various organic acids to realize the aim of decomposing tricalcium phosphate, iron phosphate, aluminum phosphate and rock phosphate and releasing soluble phosphate, also can be used for secreting acidic phosphatase and phytase to realize the effects of decomposing lecithin and calcium phytate and simultaneously releasing the soluble phosphate. The strain has good tolerance capability and repairing effect on heavy metal including Cu, Pb, Cr, Zn and the like, and has a potential of being applied to the field of the heavy metal pollution remediation of soil.
Owner:NANJING AGRICULTURAL UNIVERSITY
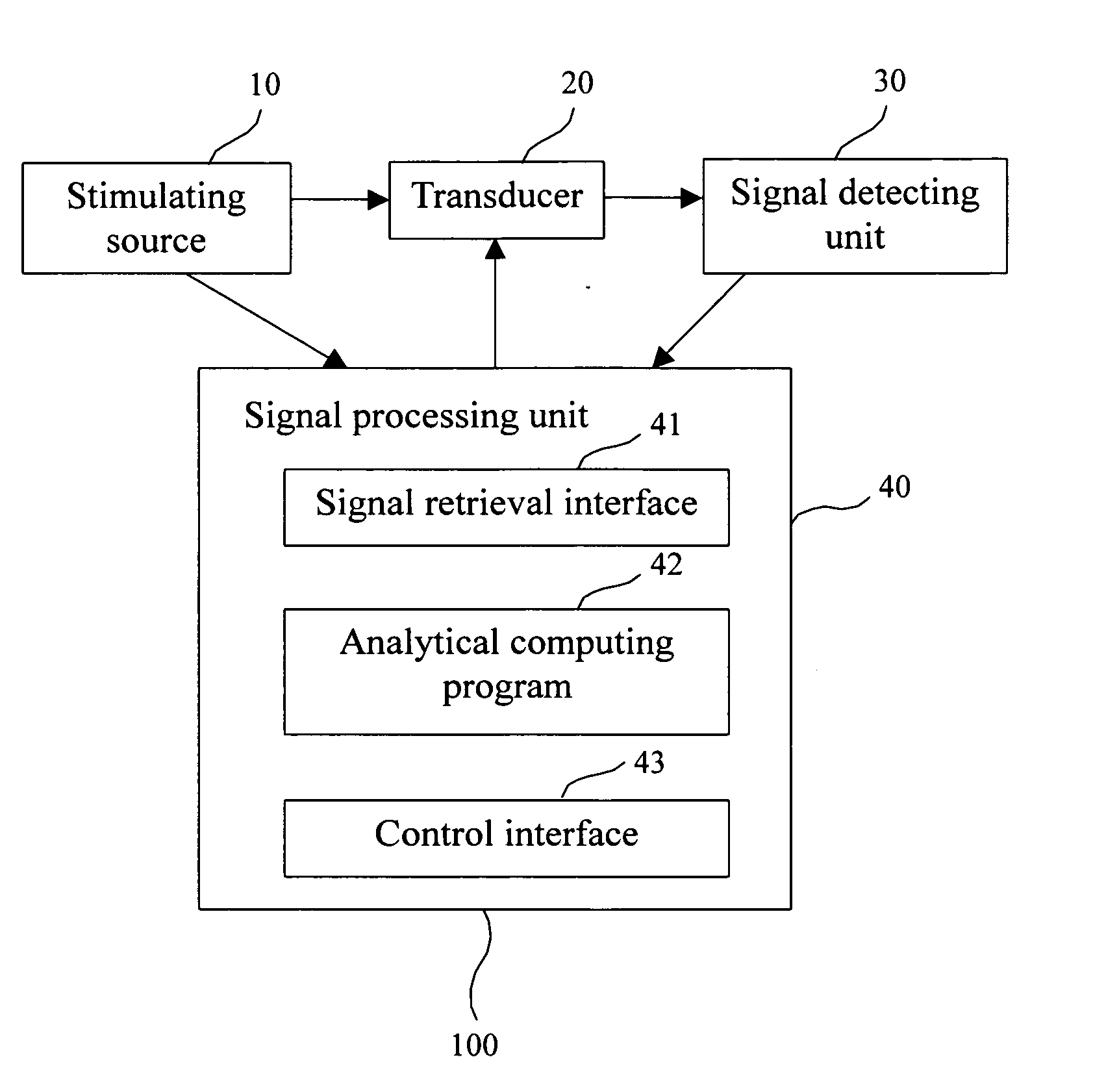
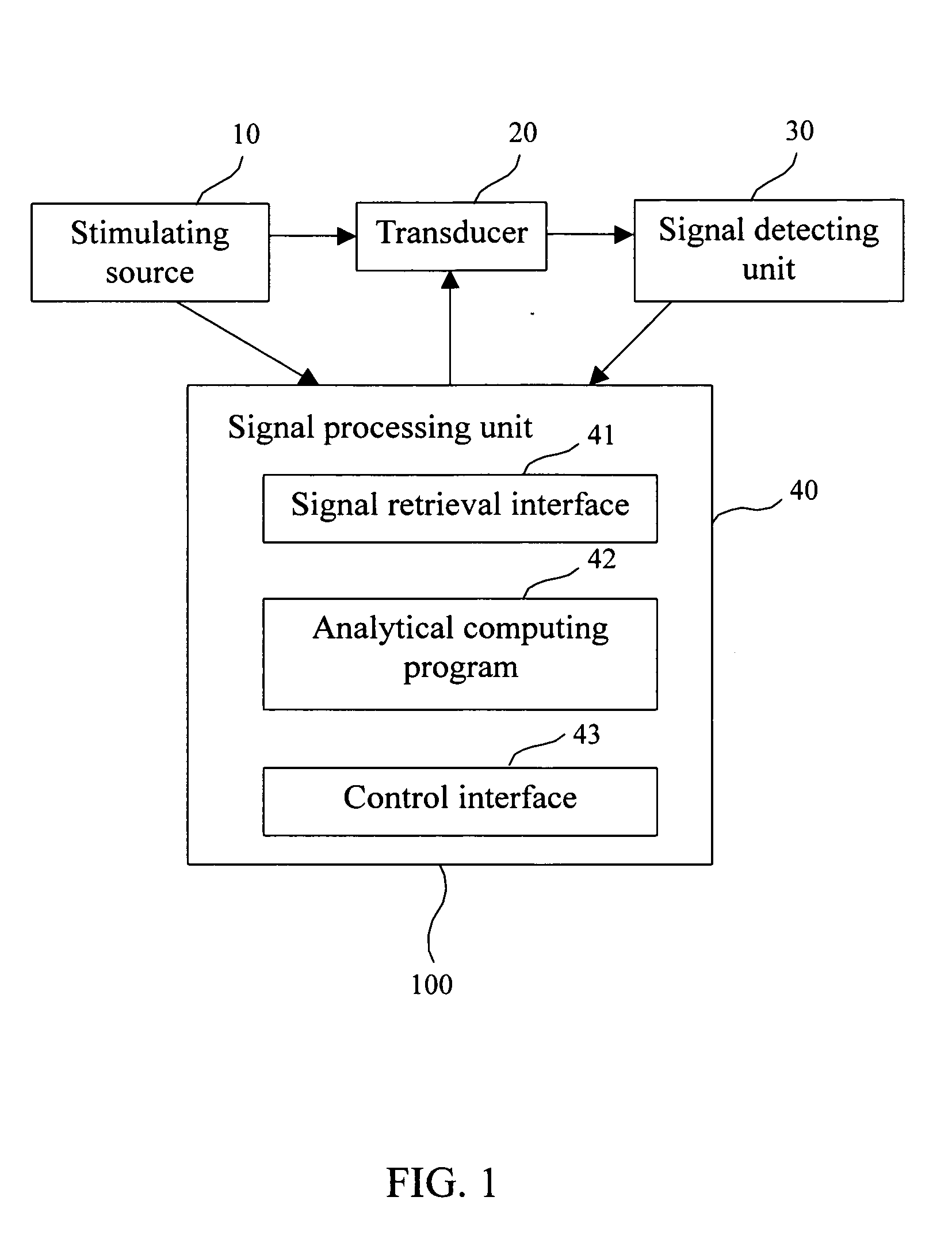
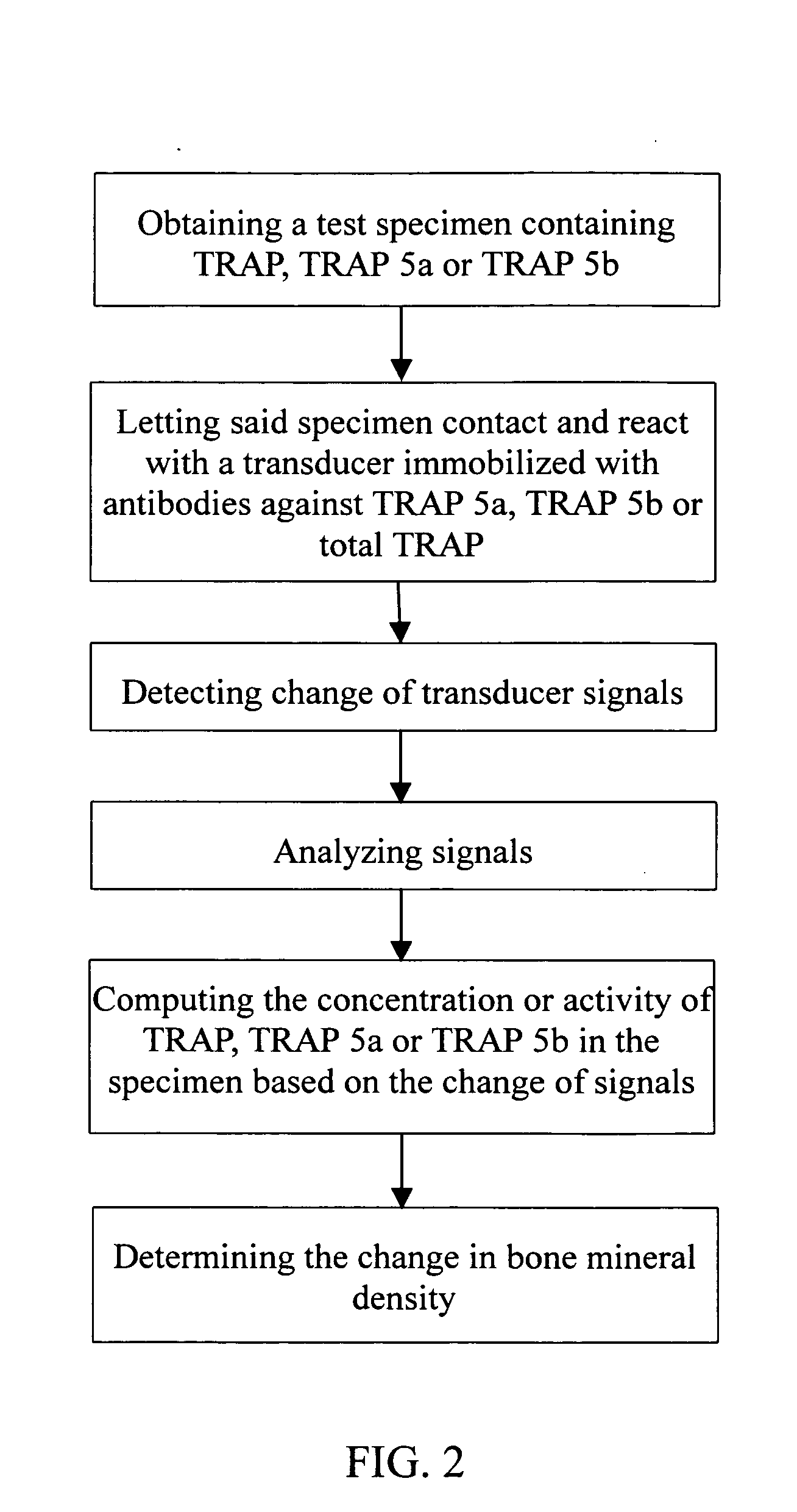
Biosensor and method for bone mineral density measurement
InactiveUS20050059875A1High sensitivityLow priceAnalysing fluids using sonic/ultrasonic/infrasonic wavesMaterial nanotechnologyTransducerVolumetric Mass Density
This invention is related a biosensor for bone mineral density measurement, comprising a stimulating source; a transducer having antibodies against TRAP 5a, TRAP 5b or total TRAP (i.e. TRAP 5a+TRAP 5b) immobilized thereon; a signal detecting unit; and a signal processing unit; wherein the TRAP refers to tartrate-resistant acid phosphatase (TRAP). The method for bone mineral density measurement disclosed in this invention is detecting the concentration or activity of TRAP, TRAP 5a and TRAP 5b in blood by using the biosensor described above. Accordingly, the method can monitor changes of the bone mineral density to prevent osteoporosis.
Owner:IND TECH RES INST
Enzyme-labeling-liquid-based cytology staining kit for screening bladder cancer
ActiveCN102260731AReduce workloadGuaranteed accuracyMicrobiological testing/measurementLiquid base cytologyLiquid-based cytology
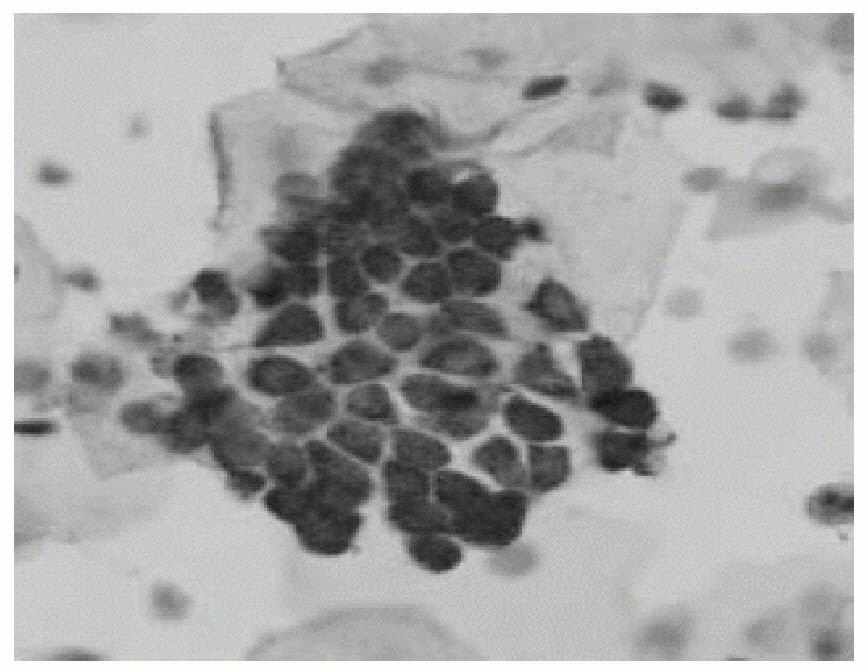
The invention relates to application of an enzyme-labeling-liquid-based cytology technology to the screening of bladder cancer. In the technology, a cell sample which is tabletted by a liquid-based tabletting method is treated by an acid phosphatase chemical staining method, and a bright red precipitate is formed in cytoplasm of abnormal cells by specific staining to ensure that the abnormal cells have remarkable marks. The invention provides a kit for the detection. The kit mainly comprises an enzyme-labeling-liquid-based cytology staining reagent, enzyme-labeling-liquid-based cytology preserving fluid and settlement buffer solution, and can provide high detection specificity and a good staining effect.
Owner:合肥科久盛生物医药有限公司
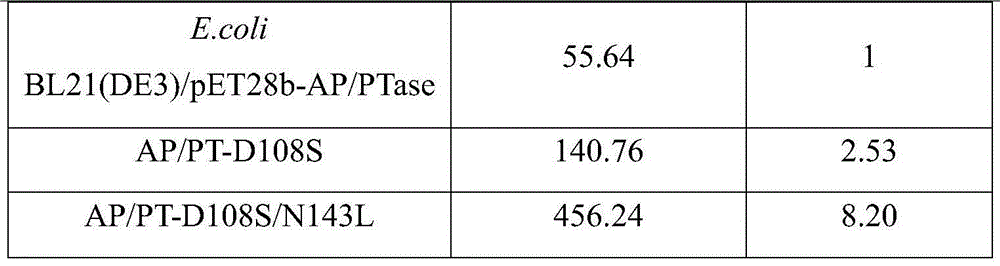
Acid phosphatase mutant, encoding gene, vector and application
ActiveCN103555687AIncrease enzyme activityHigh utility valueHydrolasesFermentationTert-leucineBiology
The invention provides an acid phosphatase mutant, an encoding gene thereof, a vector containing the gene and application. The mutant is obtained through point mutation of an amino acid sequence shown as SEQ ID No. 1, and the point mutation comprises mutation of 108th site into serine and / or mutation of 143rd site into leucine. The beneficial effects mainly comprise that by employing a half-rationality design method, the acid phosphatase (EB-AP / PTase) gene is subjected to multi-round site saturation mutagenesis for obtaining acid phosphatase mutants AP / PT-D108S and AP / PT-D108S / N143L with improved enzyme activity, and the acid phosphatase mutants AP / PT-D108S and AP / PT-D108S / N143L have relatively high practical value and wide market application prospect.
Owner:ZHEJIANG UNIV OF TECH
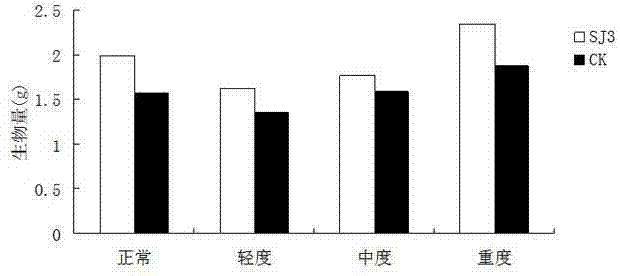
Endophytic fungus for promoting biomass growth of aleurites montana in low-phosphorus environment
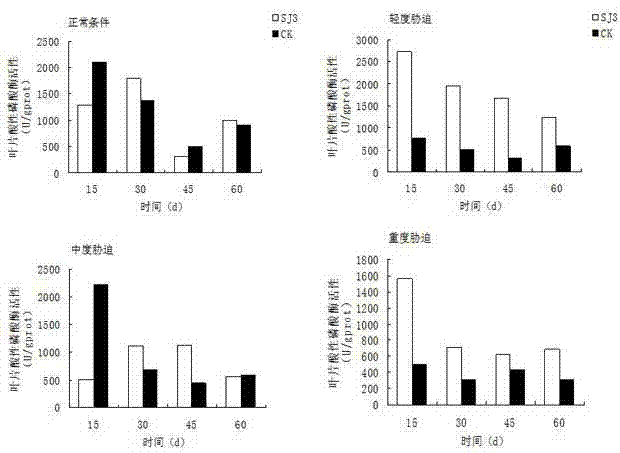
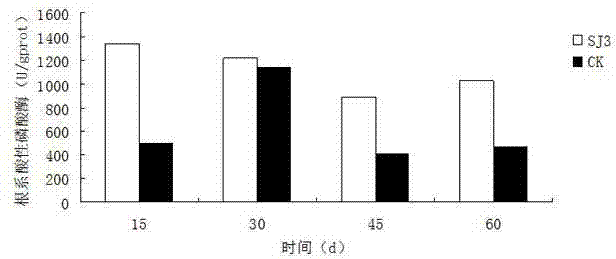
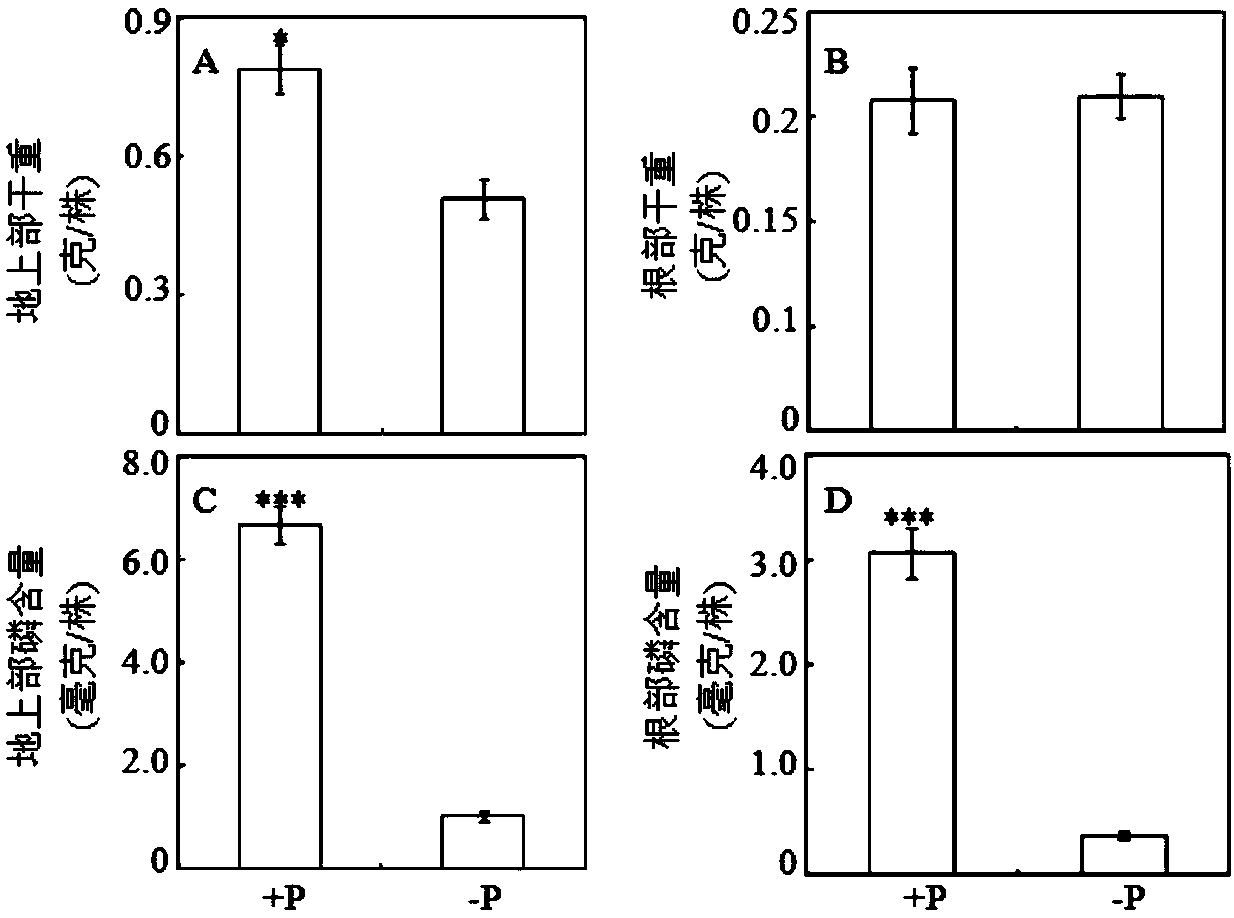
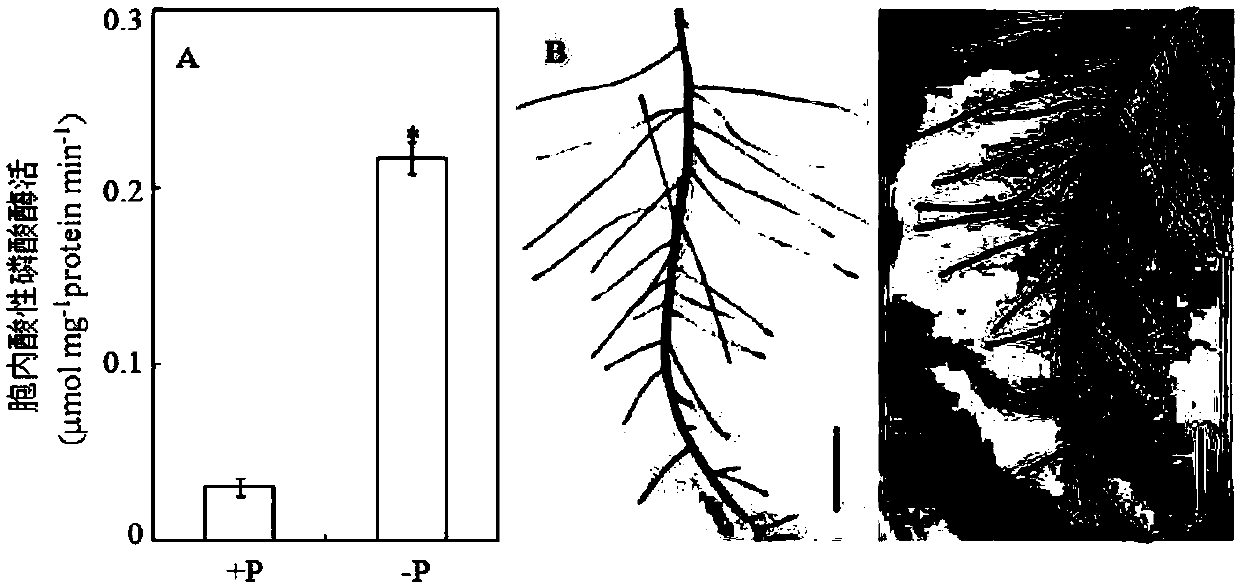
The invention relates to an endophytic fungus for promoting biomass growth of aleurites montana in a low-phosphorus environment. The endophytic fungus is Penicillium sp. SJ3, has been registered and collected in the General Microbial Center of China Committee for Culture Collection of Microorganisms on January 30, 2015, and has a collection number of CGMCC No.10468. An aleurites montana endophytic fungus SJ3 is inoculated into an aleurites montana soil culture seedling; and by measuring the biomass of plants, the acid phosphatase activity of leaves and the acid phosphatase activity of roots, results show that the biomass, the leaf acid phosphatase activity and the root acid phosphatase activity of the plants inoculated with a strain are basically higher than that of comparisons to a relatively high extent, and show that the aleurites montana endophytic fungus SJ3 has a great effect on promoting the biomass growth of the plants in the low-phosphorus environment so as to further verify that the endophytic fungus can be used for promoting the biomass growth of aleurites montana plants in the low-phosphorus environment.
Owner:FUJIAN AGRI & FORESTRY UNIV
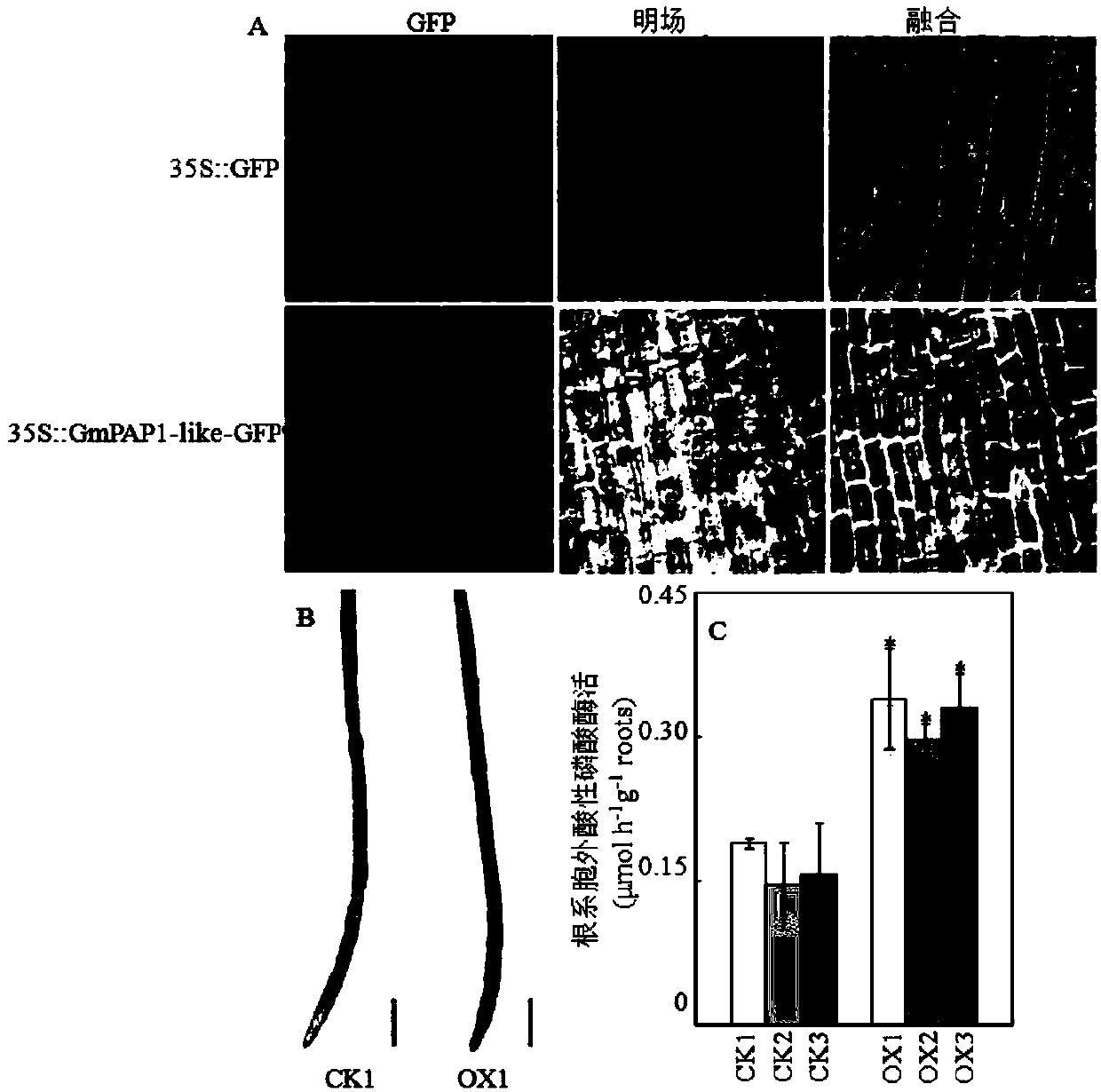
Acid phosphatase GmPAP1-like protein gene and application thereof
ActiveCN108048474AAffect activityImprove the ability to tolerate low phosphorusBacteriaHydrolasesNucleotideStrong acids
The invention discloses an acid phosphatase GmPAP1-like protein gene from the cell wall of soybeans and application thereof. The acid phosphatase GmPAP1-like protein gene has a nucleotide sequence asshown in SEQ ID No. 1 and an amino acid sequence as shown in SEQ ID No. 2. The acid phosphatase GmPAP1-like protein gene provided by the invention is capable of participating in activation and utilization of extracellular organic phosphorus, eventually improves the low-phosphorous resistance of plants and has strong acid phosphatase activity. Overexpression of the acid phosphatase protein gene inthe roots of crops can substantially improve the organic-phosphorus activation and utilization capabilities of transgenic roots; and thus, soybean roots can be improved in adaptive capacity to low-phosphorous stress of acid soil through gene transformation, and the acid phosphatase protein gene has good application prospects in construction of transgenic soybeans with resistance to low-phosphorousstress.
Owner:SOUTH CHINA AGRI UNIV
Quantitative detection agent and detection for conical acid phosphate enzyme
InactiveCN1746665AGood technical effectMonitor credibilityMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsSemen samplePhosphate
A reagent for detecting acid phosphatase of seminal plasma quantitatively consists of substrate, termination solution, reference material and quality control solution containing acid phosphatase. Its detecting method includes incubating substrate in standard tube synchronously with quality control tube and analysis tube, reacting on quality control solution with substrate and reacting on semen sample in analysis tube with substrate, terminating reacting in quality control tube and in analysis tube, calculating out concentration value of seminal plasma sample according to absorbance of each tube.
Owner:深圳华康生物医学工程有限公司
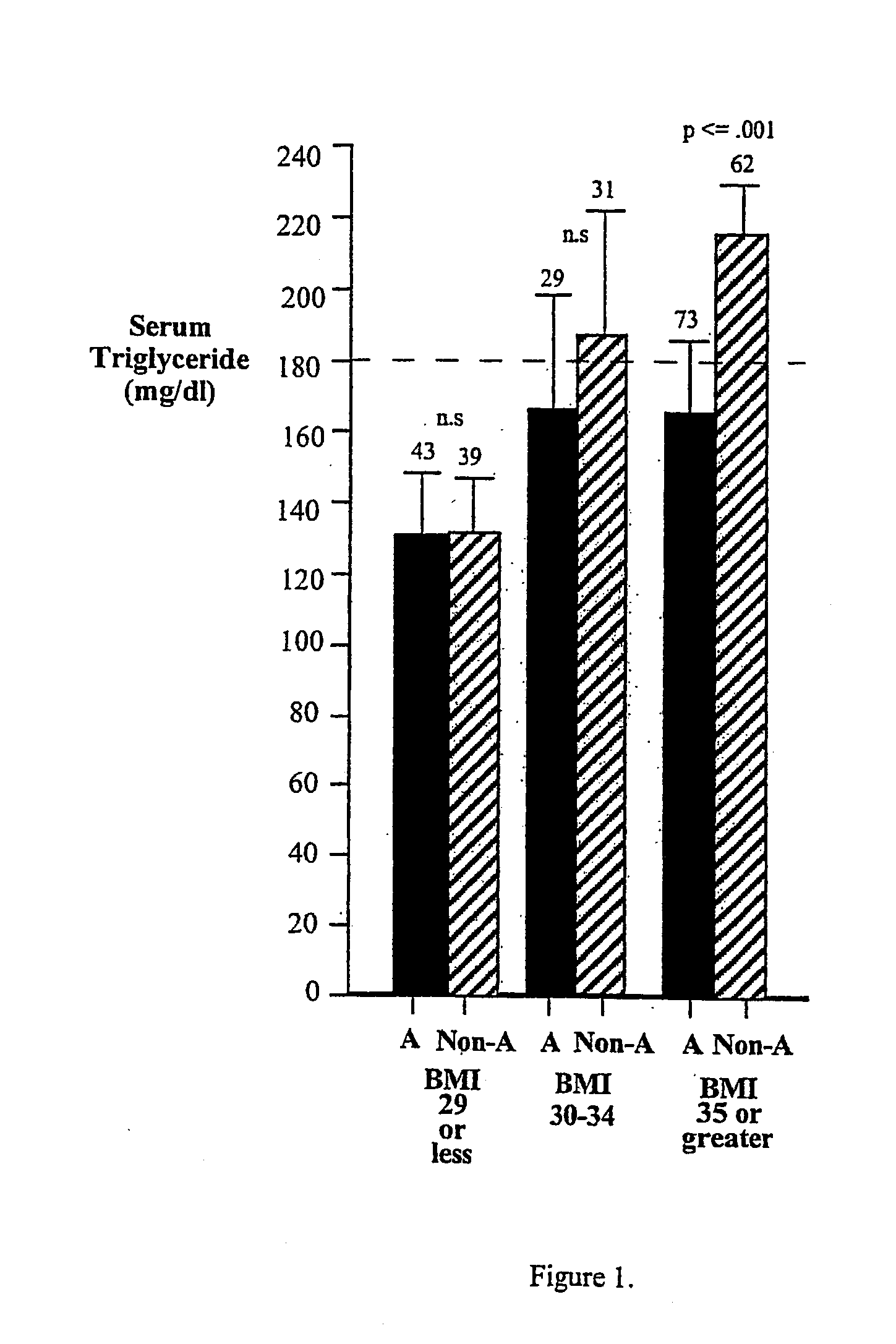
Acid phosphatase (acp1)gene as a susceptibility locus for hyperlipidemia
InactiveUS20050055732A1Reduce probabilityMetabolism disorderMicrobiological testing/measurementSecondary hyperlipidemiaSusceptibility locus
The ACP1 *A allele provides a means for diagnosing susceptability of a human subject to hyperlipidemia, especially hyperlipidemia associated with metabolic syndrome, a means for treating, or preventing the onset of, hyperlipidemia and metabolic syndrome, and a means for screening and identifying drugs suitable for use in treating or preventing hyperlipidemia, especially hyperlipidemia associated with metabolic syndrome. Diagnostic kits are also provided.
Owner:BURNHAM INST FOR MEDICAL RES +1
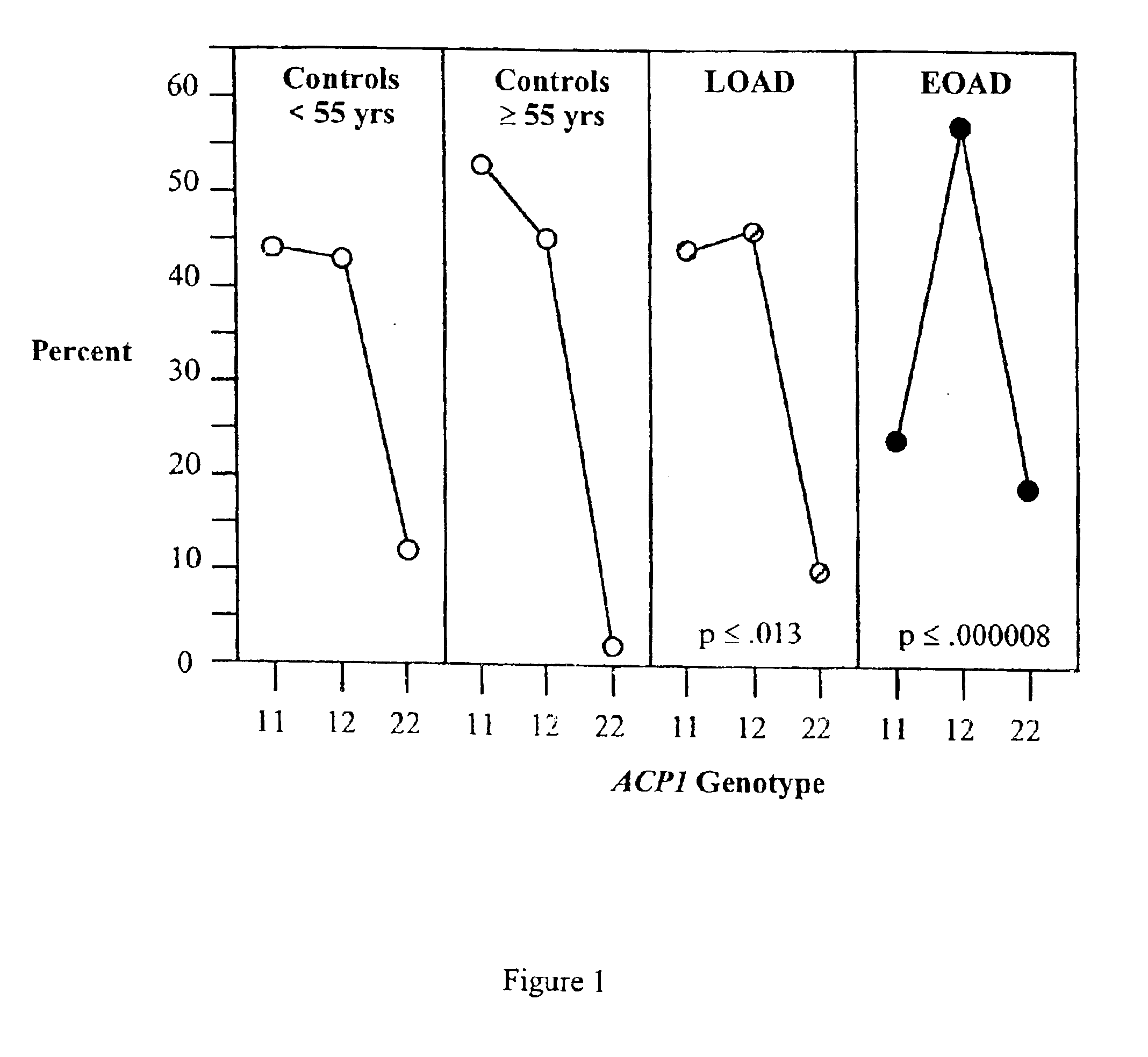
Association between the acid phosphatase (ACP1) gene and Alzheimer's disease
Alzheimer's disease is characterized by the presence of senile plaques formed from beta amyloid (Abeta), and neurofibrillary tangles (NTFs) formed from paired helical filaments consisting of hyperphosphorylated tau. A number of studies have shown that the NTFs correlate better with the duration and severity of Alzheimer's disease than senile plaques. However, a criticism of the primary etiological role of NTFs in Alzheimer's disease is the absence of variants of kinases or phosphatases associated with Alzheimer's disease. Acid phosphatase, a product of the ACP 1 gene, is a ubiquitous low molecular weight protein tyrosine phosphatase. A common allele, ACP 1*A, is associated with a lower activity of acid phosphatase. It is due to an Arg 105 Gln substitution of the ACP1 locus and detected as a Taq I polymorphism. We report a significant association of the low activity 2 allele with sporadic early onset Alzheimer's disease (EOAD). These findings support the possibility that other variants of kinase and genes may be associated with sporadic Alzheimer's disease.
Owner:CITY OF HOPE
Soil conditioner for tobacco field acidic soil, and preparation method thereof
InactiveCN110184066AImprove use valueRaise the pHAgriculture tools and machinesOther chemical processesDecompositionPotassium
The invention discloses a soil conditioner for tobacco field acidic soil, wherein the soil conditioner comprises, by weight, 40-80 parts of shells, 20-60 parts of potassium feldspar, 10-50 parts of limestone, and 15-40 parts of dolomite. According to the present invention, the soil conditioner can neutralize the active acid and the exchangeable acid in the soil, obviously increase the pH value ofthe soil, increase the soil plough layer exchangeable Ca<2+> and Mg<2+> concentrations, improve the soil microbial environment, increase the numbers of soil bacteria, actinomycetes and aerobic cellulose decomposition bacteria, improve the activities of urease, acid phosphatase, catalase and cellulase, passivate the heavy metals in the soil, reduce the heavy metals in tobacco, reduce the occurrenceof tobacco root diseases, improve the aroma quality, the aroma amount and the concentration of tobacco leaves, and reduce the irritation, the miscellaneous gas and the like of tobacco leaves.
Owner:青岛农特生物科技有限责任公司 +1
Method for producing nucleoside-5'-phosphate ester
InactiveUS6207435B1Inexpensively and efficiently producingHigh reaction yieldBacteriaHydrolasesPhosphateAcid phosphatase
A method for producing nucleoside-5'-phosphate esters inexpensively and in high yields by phosphorylating a nucleoside with a phospahte group donor using an acid phosphatase having an increased affinity for the nucleoside and / or an increased temperature stability at a pH of pH 3.0 to 5.5, to produce a nucleoside-5'-phosphate ester. Mutant acid phosphatases having increased affinity for nucleosides and / or an enhanced temperature stability are also provided.
Owner:AJINOMOTO CO INC
Prostatitis joint-detection kit
ActiveCN102866153AAvoid missing detectionMaterial analysis by observing effect on chemical indicatorLeukocyte esteraseDiluent
The invention discloses a prostatitis joint-detection kit which comprises a sample diluent, a chromogenic reagent and a detection card body provided with six reaction holes, wherein the six reaction holes of the detection card body are internally and respectively provided with solid reagents used for detecting lecithin bodies, leukocyte esterase, citric acid, zinc, acid phosphatase and pH value. The prostatitis joint-detection kit has the advantages of being capable of detecting six representative indexes of the prostatitis together with no need of the microscopic examination. The prostatitis joint-detection kit can judge whether the six indexes are normal or not according to the color changes of the samples in the six reaction holes, efficiently assist the auxiliary diagnosis and the preliminary parting of the prostatitis, and avoid the detection omission caused by the visual fatigue of detection staffs. The prostatitis joint-detection kit is easy to popularize in the clinical application.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Kit for screening abnormal cervical cells, and enzyme-labeled liquid based cell preservation solution
ActiveCN103262838AShorten the timeReduce ambiguityMicrobiological testing/measurementDead animal preservationCervical cellsMedicine
The invention relates to the technical field of a medicine preparation, and particularly relates to a kit for screening abnormal cervical cells, and enzyme-labeled liquid based cell preservation solution. The kit for screening abnormal cervical cells has excellent sensitivity and good specificity; and the enzyme-labeled liquid based cell preservation solution disclosed by the invention can well keep acid phosphatase activity of cervical exfoliated cells and the cellular morphology, and has the functions of preventing corrosion, removing impurities and dissolving red blood cells; and the effects of tabletting and dyeing the stored cells are superior to the effect of the traditional preservation solution during the usage period.
Owner:合肥科久盛生物医药有限公司
Modified glucoamylase enzymes and yeast strains having enhanced bioproduct production
ActiveUS10364421B2Promote high levelGenerate substantially more ethanolPolypeptide with localisation/targeting motifHydrolasesAmylaseFermentation
Owner:CARGILL INC
Chlorpyrifos degrading bacteria and application thereof
The invention discloses a chlorpyrifos degrading bacteria Erwiniaxishuaiensis SCU-B244<T> = CGMCCNo.1.12772<T> = KCTC42022<T> (Figure) (i). Through the separation from the cricket body surface, screening, physiological and biochemical identification, the bacteria is identified as gram negative and facultative aerobic bacteria, which has cream bacterium colony, and is oxidase negative and catalase positive; the cells mainly comprises fatty acids of C16:0, C16:1delta<9>, C18:1delta<9>, C11:0<3-OH> and C14:0<3-OH>, GC content in cells is 49.3%-49.5%; and the bacteria has alkaline phosphatase, esterase (C4), lipase (C8), acid phosphatase, beta-galactosidase and beta-glucosidase activities. The bacteria has one day degradation rate on chlorpyrifos of 44.64%, and can be widely used in the fields of development of chlopyrifos degrading enzyme and various types of biological engineering enzyme preparations. Figure instructions: the figure is photos of Erwinia xishuaiensis SCU-B244<T> under 30000 times scanning electron microscope.
Owner:SICHUAN UNIV
Kit for detecting activity of acid phosphatase in seminal plasma and detection method
InactiveCN101974612AReduce dosageOmit the incubation stepMicrobiological testing/measurementColor/spectral properties measurementsPhenyl phosphateAcid phosphatase
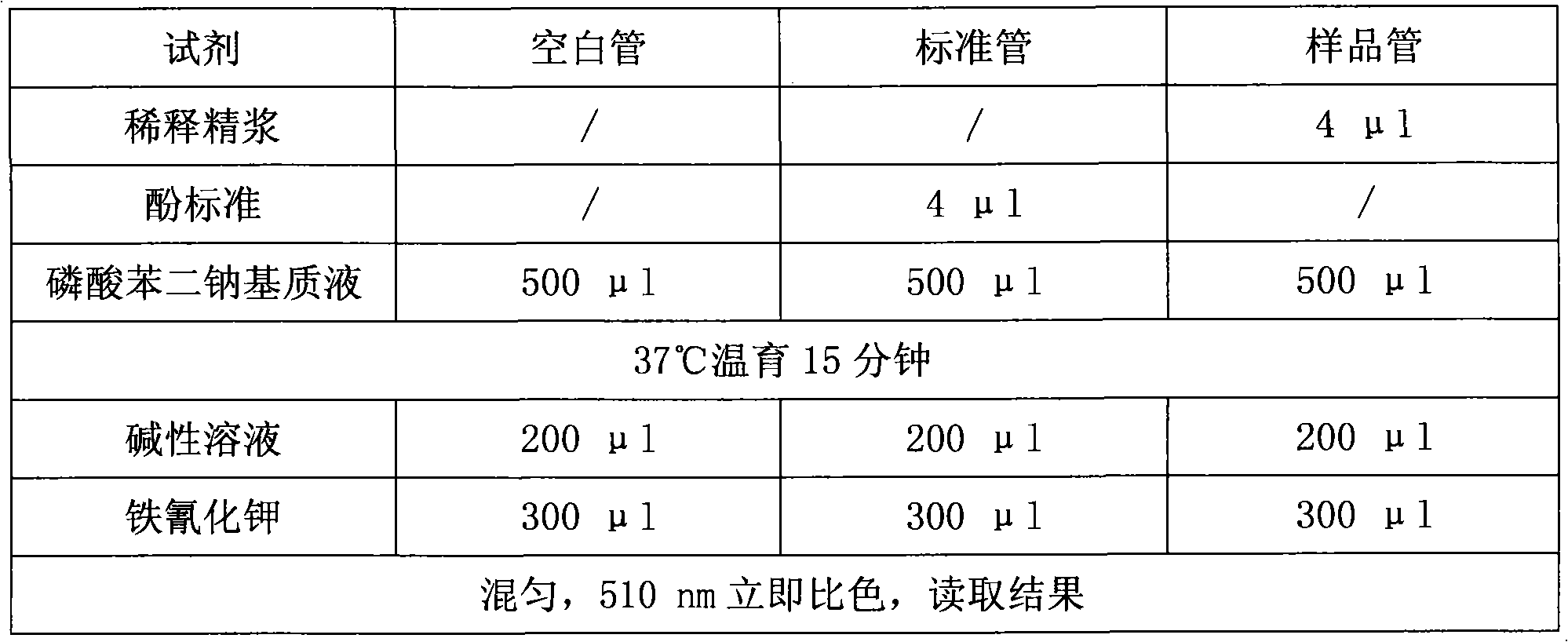
The invention discloses a kit for detecting the activity of acid phosphatase in seminal plasma and a detection method. The kit comprises substrate solution, a standard sample, stop solution and a color development reagent, wherein the substrate solution is disodium phenyl phosphate substrate solution; and the stop solution is alkaline solution. The detection method comprises the following steps of: first setting parameters of a detecting instrument; then adding the disodium phenyl phosphate substrate solution into an empty tube, adding the standard sample and the substrate solution into a standard tube, and adding the dilute seminal plasma and the substrate solution into a sample tube; next simultaneously performing incubation on the empty tube, the standard tube and the sample tube at the temperature of 37 DEG C for 15 minutes; and finally adding the same amount of alkaline solution and color development reagent into the incubated empty tube, the incubated standard tube and the incubated sample tube, performing uniform mixing, and performing color comparison by using the detecting instrument to obtain a result. The kit and the detection method of the invention can make the operation simple, convenient, time-saving and reagent-saving, and can make stable and reliable the result and be applied to laboratories for the department of andrology in various hospitals as synchronous comparison tests can be performed.
Owner:南京欣迪生物药业工程有限责任公司
Quantitative determination method for titer of recombinant insect baculovirus
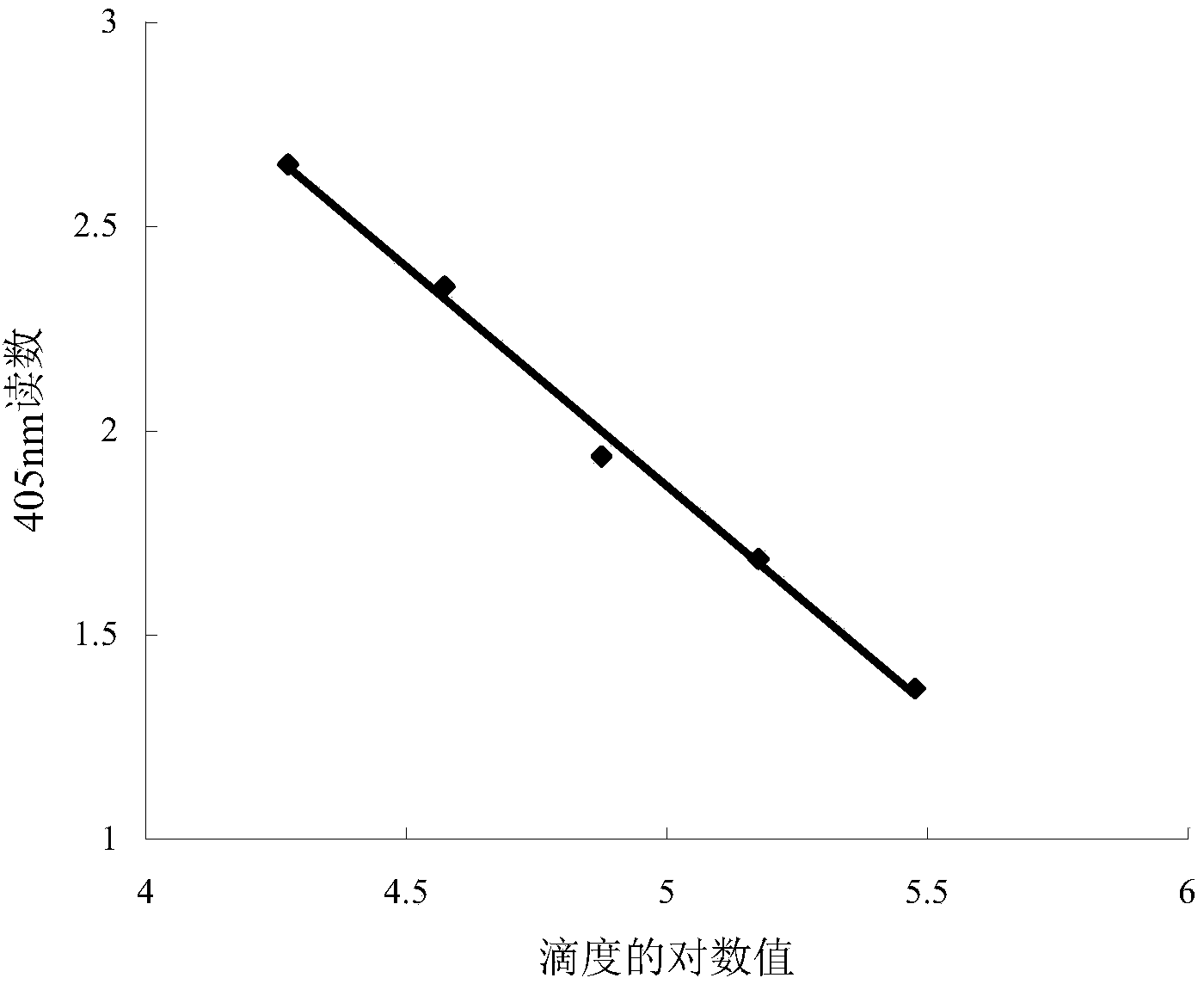
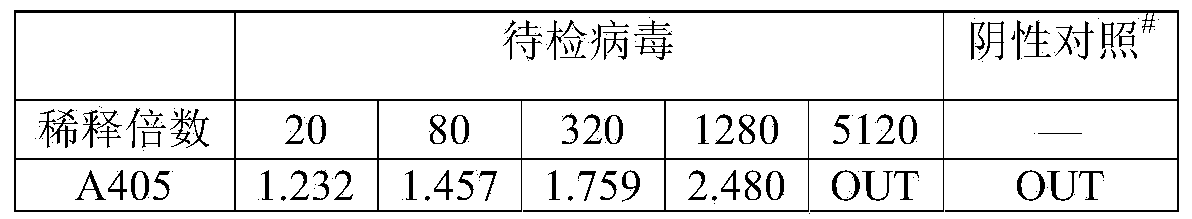
ActiveCN103529205AThe result is accurateHigh sensitivityBiological material analysisColor/spectral properties measurementsQuantitative determinationTiter
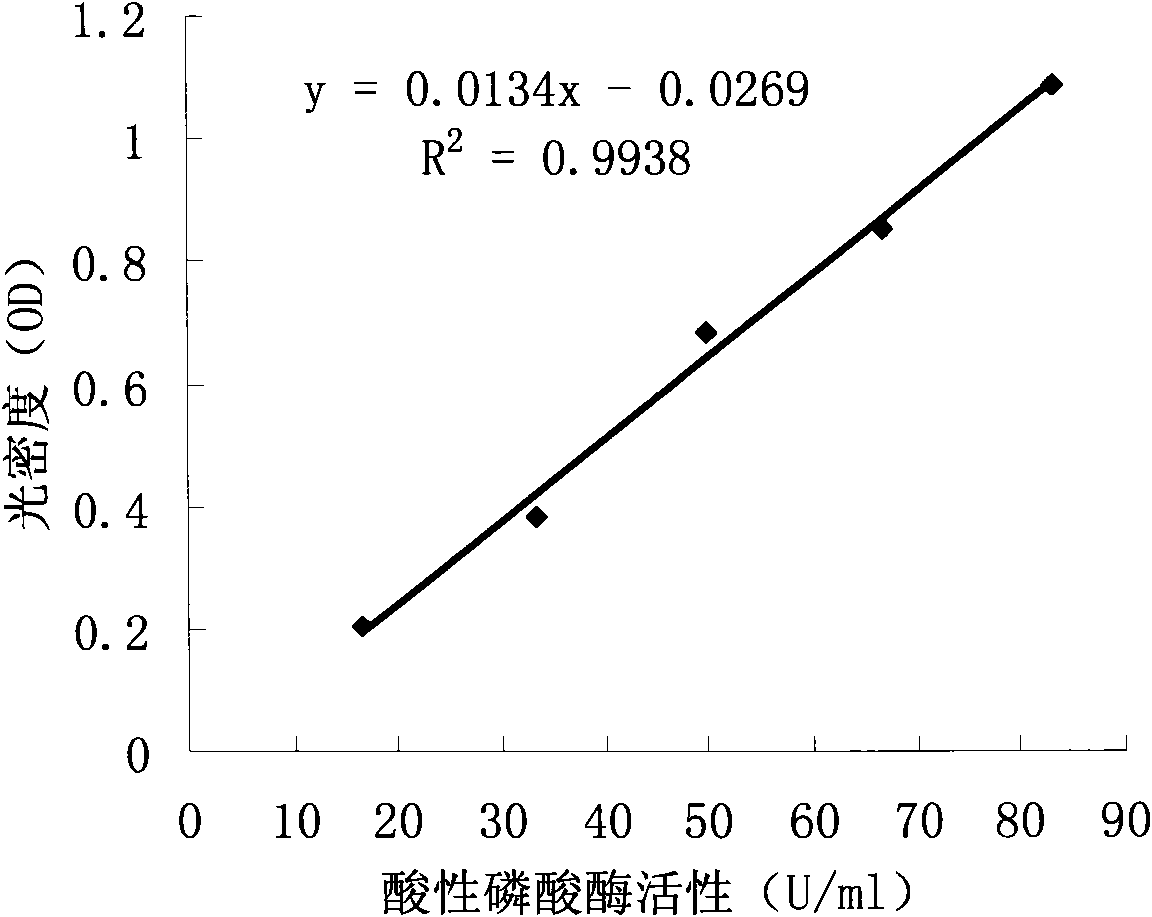
The invention provides a quantitative determination method for the titer of recombinant insect baculovirus. The method utilizes the fact that the recombinant insect baculovirus interferes in growth and duplication of host cells after the recombinant insect baculovirus infects insect cells, and then the method detects the number of the cells by using an acidic phosphatase method so as to realize quantitative determination of the titer of the recombinant insect baculovirus. The method has the advantages of high sensitivity, good accuracy, usage of cheap and easily available reagents, simple and convenient operation, easy mastery of technology, small consumption of time and higher efficiency.
Owner:WATERSTONE PHARMA WUHAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com