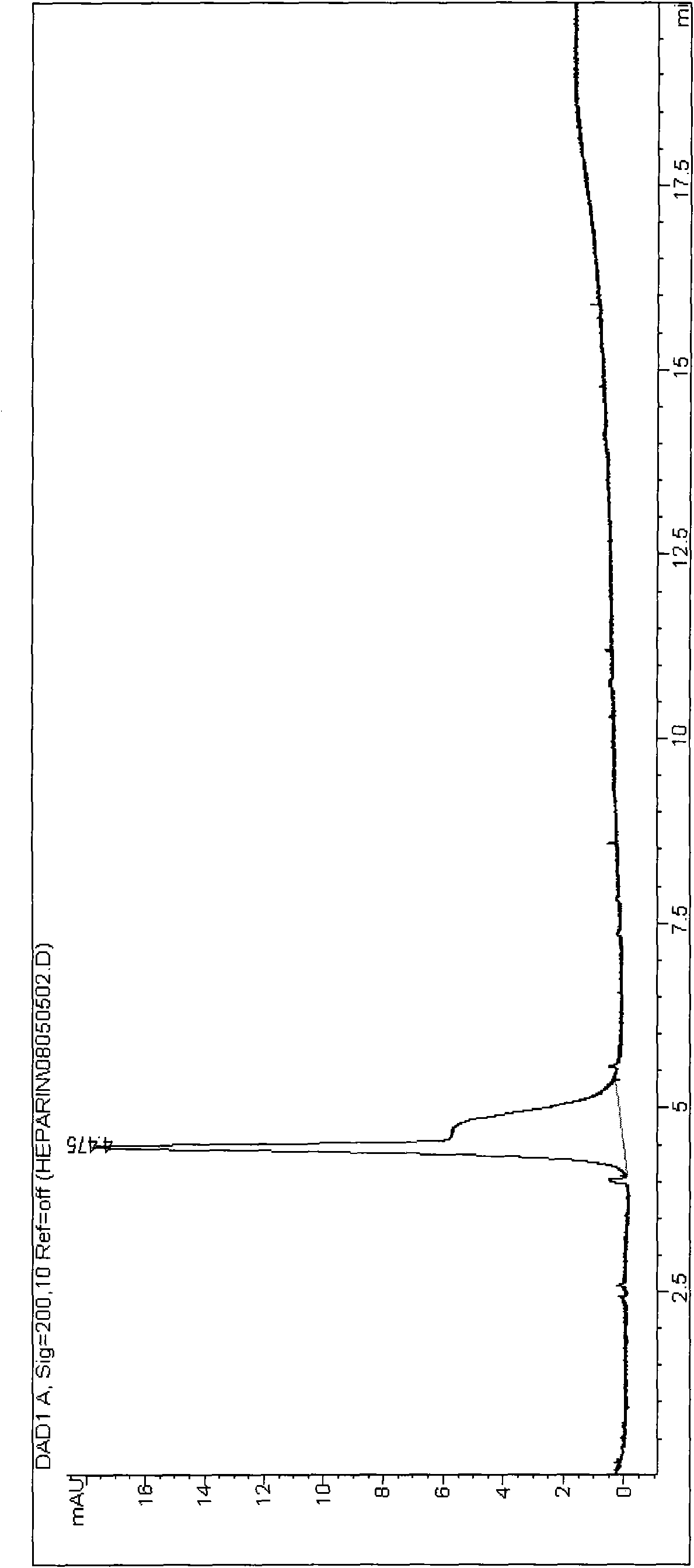
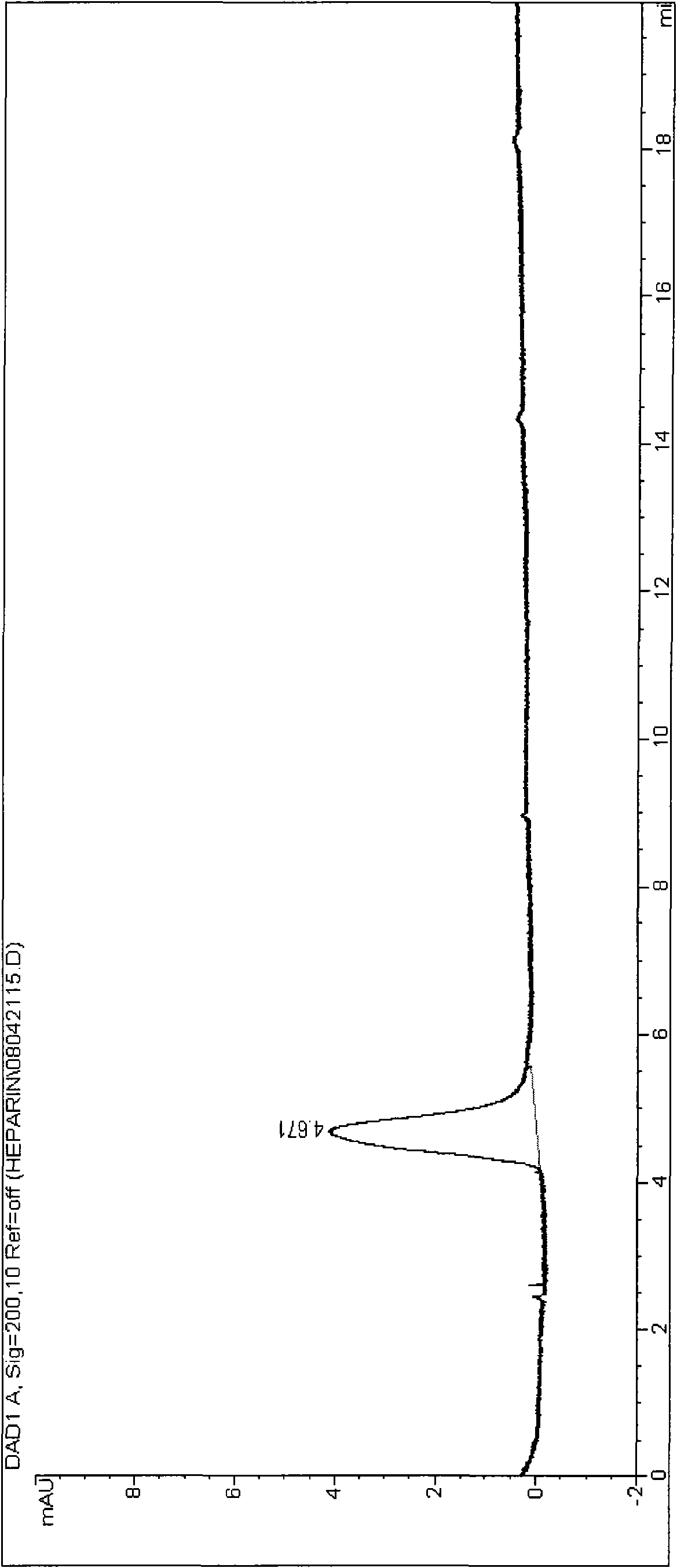
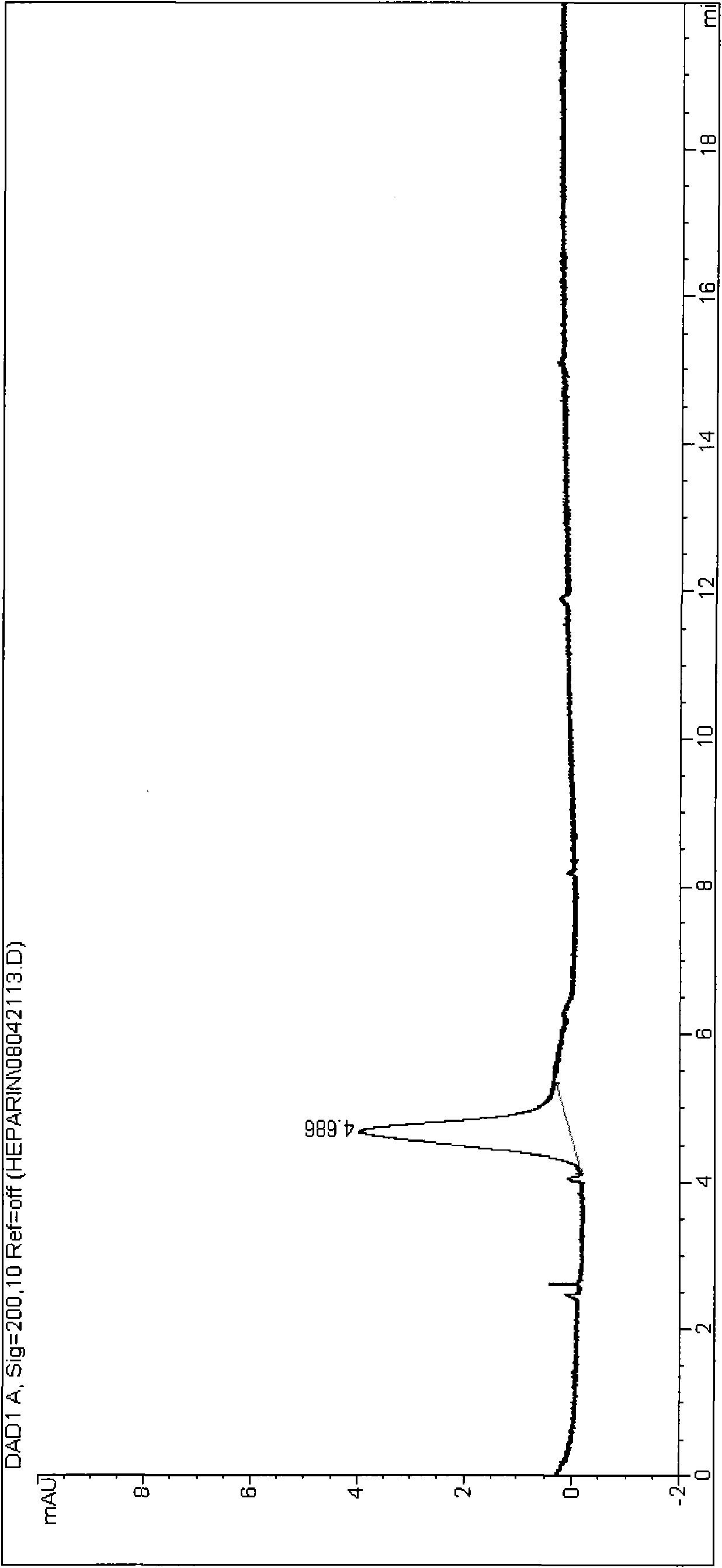
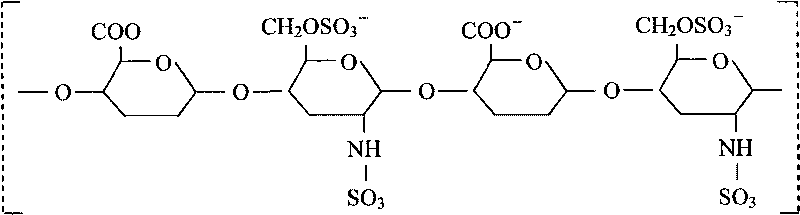
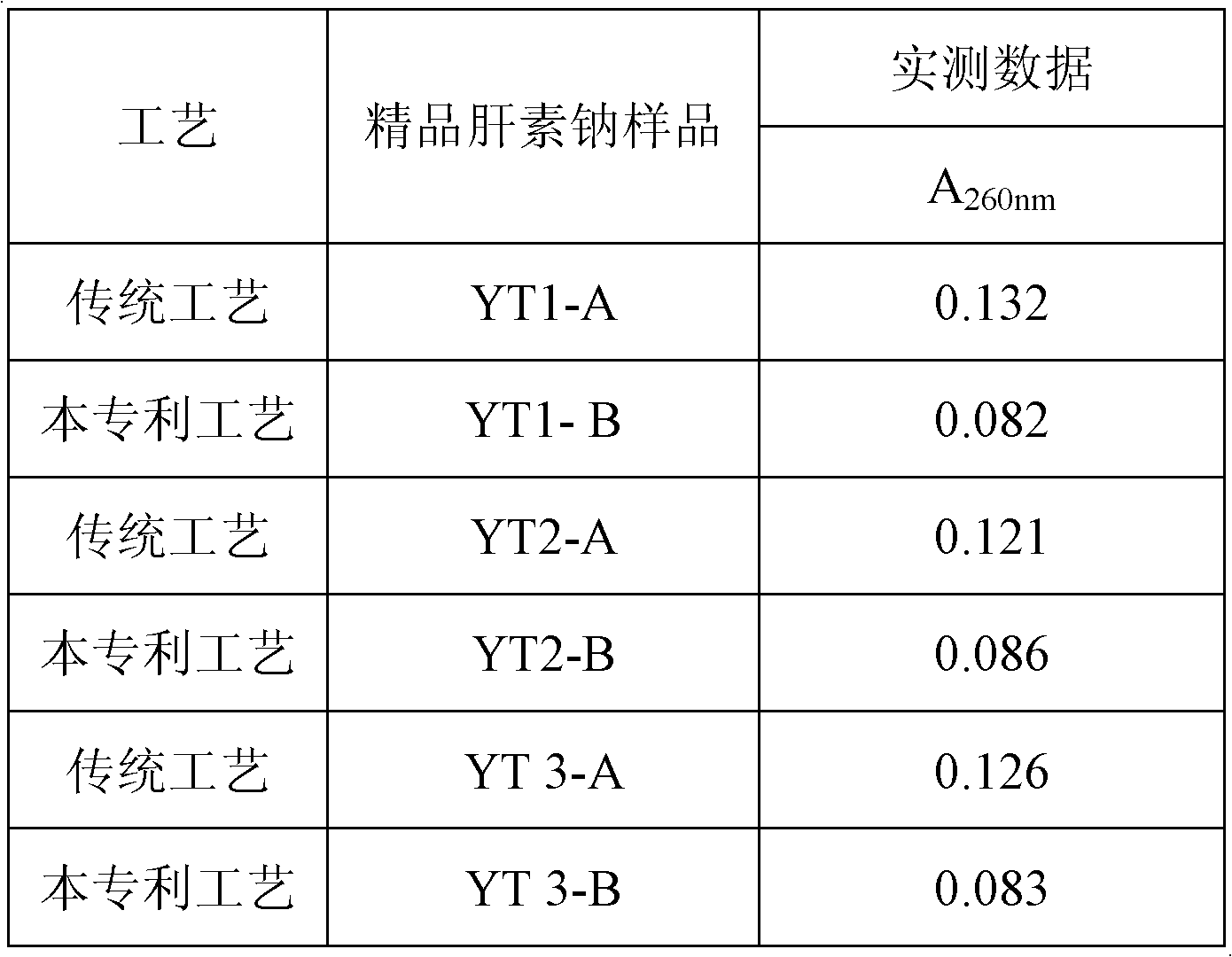
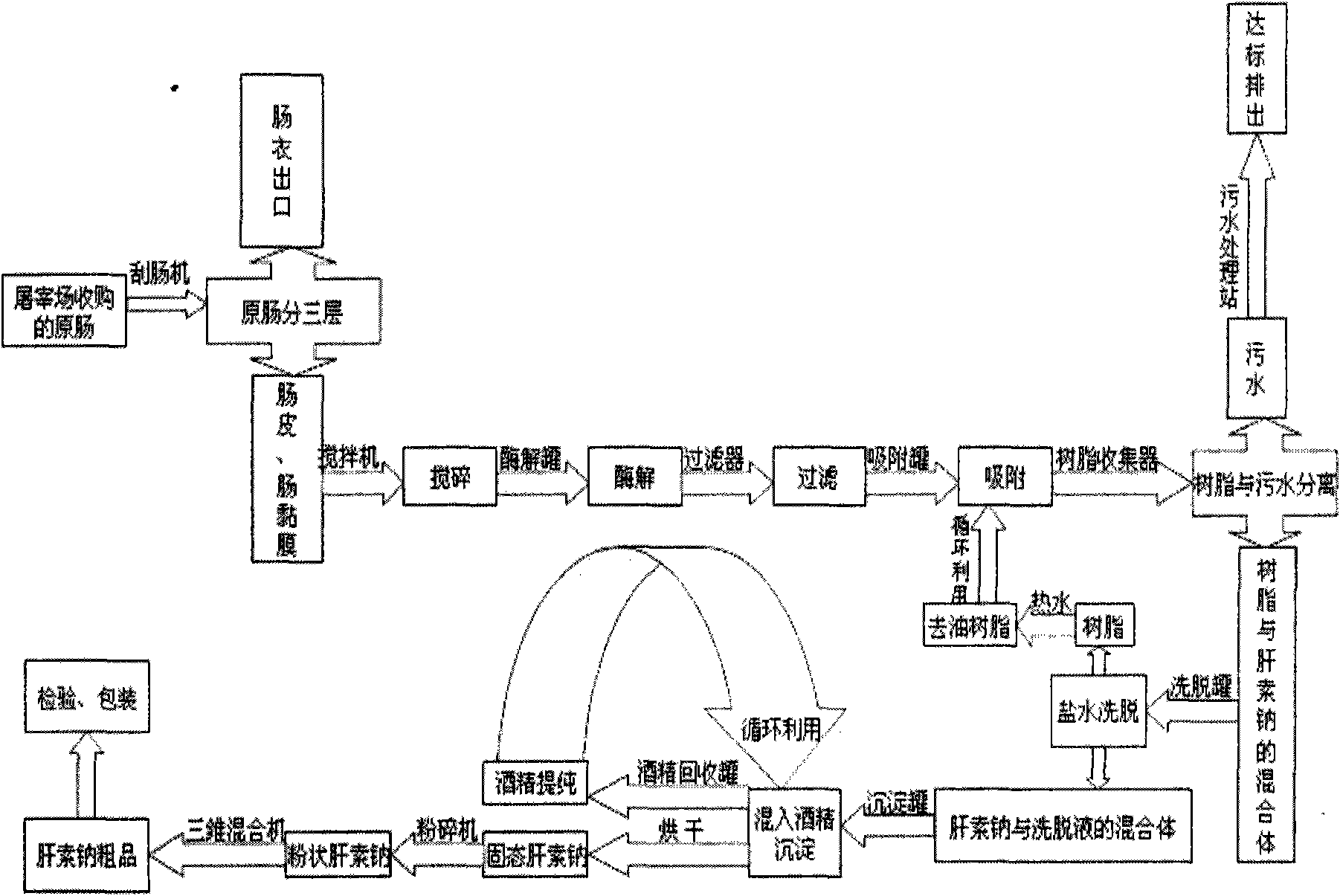
Patents
Literature
605 results about "Heparin sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
DOSAGE AND ADMINISTRATION. Heparin sodium is not effective by oral administration and should be given by intermittent intravenous injection, after dilution in 50 or 100 mL of 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP, or by intravenous infusion.
Technique for producing ultra-low molecular heparin sodium (calcium)
InactiveCN101519459AImprove securityGood and long-lasting antithrombotic effectPulmonary artery embolismDisease
Aiming at the conditions that heparin has severe bleeding side effects in clinical practice and clinical application thereof is restricted, the invention discloses a technique for producing ultra-low molecular heparin sodium (calcium). The technique comprises the following steps of: taking heparin sodium solution, adding sodium nitrite solution for cracking; adjusting the lysis buffer by using alkaline; absorbing impurities by using an anion-exchange column; washing for obtaining ultra-low molecular heparin calcium; carrying out filtration by using an ultrafiltration membrane and obtaining a precipitate by using alcohol; and after desalting, dehydration, re-precipitation, cooling and drying, obtaining a finished product of ultra-low molecular heparin calcium. The product has better and safer antithrombotic effect under low level of anticoagulation, and can be widely used for preventing and treating diseases such as deep vein thrombosis, pulmonary embolism, disseminated intravascular coagulation, and the like.
Owner:SUZHOU FAST BIOLOGICAL PHARMACY TECH
Method for separating chondroitin polysulfate from heparin sodium by extraction method
ActiveCN101575385APromote pig productionLiquid solutions solvent extractionFermentationCentrifugationDissolution
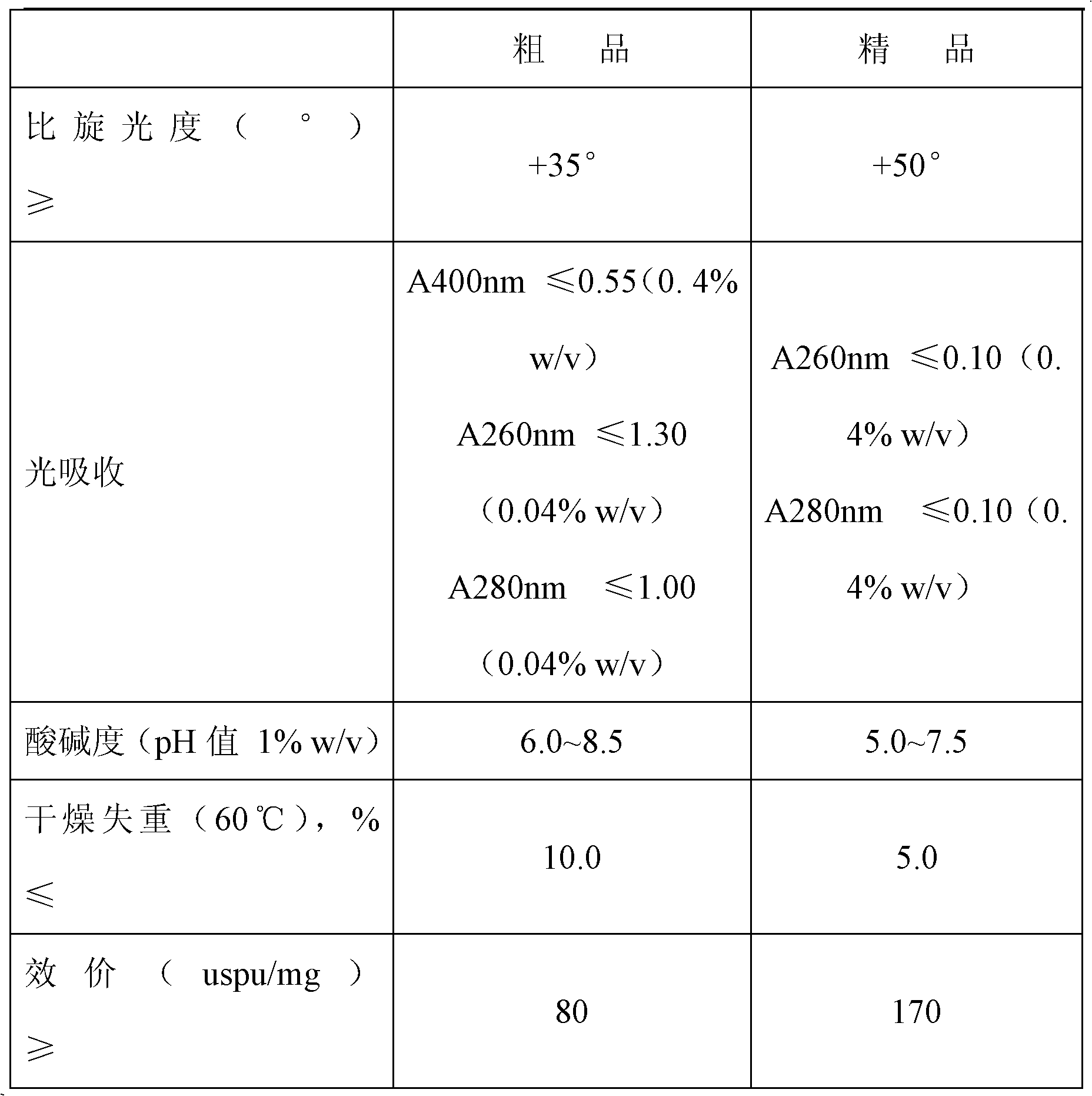
The invention discloses a method for separating chondroitin polysulfate from heparin sodium by an extraction method, breaking through the conclusion drawn by domestic and international experts, i.e., the chondroitin polysulfate in the heparin sodium is inseparable. The technical scheme is as follows: an acetone extraction method is adopted to remove the chondroitin polysulfate in the heparin sodium and thoroughly separate the chondroitin polysulfate from the heparin sodium by the refinement steps of crude product dissolution, enzymolysis, rapid heating, impurity centrifugation, a plurality of precipitation and oxidation so that the refined product rate of the heparin sodium reaches over 83 percent, the activity valence is over 180 usp / mg and over 200 iu / mg by being converted into the WHO international standard, and the quality standard accords with various indexes specified by Chinese pharmacopoeias, America pharmacopoeias, English pharmacopoeias and Western European pharmacopoeias.
Owner:QINGDAO JIULONG BIO PHARMA
Unit dosage forms for the treatment of herpes simplex
InactiveUS7351715B2Increase successSafe and effective and inexpensiveBiocidePeptide/protein ingredientsDiseaseCell membrane
The components of this invention are chosen because of their complementarity for the prevention or treatment of diseases caused by the herpes simplex virus. L-Lysine favorably increases the physiologic immunomodulation necessary for defense against this virus. Zinc improves and maintains a normal immune response. 2-Deoxy-2-D-glucose and heparin sodium alter the surface interaction between the herpes virus and the cell, preventing fusion and infectivity. N-Acetyl-L-cysteine increases glutathione levels thereby creating a thiol redox barrier to the virus at the cell membrane. Quercetin reduces intraoellular replication of the herpes virus and viral infectivity. Ascorbate, in concert with copper and D-α-tocopherol, provides an antioxidant defense against the herpes virus, which tends to lose latency during period of oxidative, free radical excess. Selenium and quercetin also participate in reducing various oxidative stresses. Together the components of this invention provide the potential for improved resistance to, improved recovery from, and a decreased frequency of recurrence of herpes simplex virus infection.
Owner:CHRONORX
Preparation method of biodegradable medicine composite macromolecular scaffold material
InactiveCN1367023AAvoid combiningPrevent embolismSurgeryPharmaceutical containersPolymer scienceFreeze-drying
A preparation method of biodegradable medicine-compounded macromolecular scaffolding material includes the following steps: dissolving macromolecular polylactic acid, polycaprolactone and restenosis-resisting medicine in the solvent, pouring the prepared solution into a container for film-forming, making the said film into filament, dipping the said filament in the mixed solution prepared with L-lactic acid and diglycolide copolymer, solvent and restenosis-resisting medicine and drying in the air or freeze-drying, then soaking the said filament in anticoagulative solution, drying in the air, making filament wind round the mould, thermosetting and forming so as to obtain the invented product. The described solvent is chloroform, 1,4-dioxane and dimethyl sulfoxide, the restenosis-resisting medicine is taxol, taxadter, arotinoid ethylester, probucol, dectan and cilomosi, and the anticoagulative solution is prepared with carboxylated sulfurnic aid esterified chitin aqueos solution or heparin sodium aqueos solution and acetone through the process of mixing and solvation reaction.
Owner:TSINGHUA UNIV +1
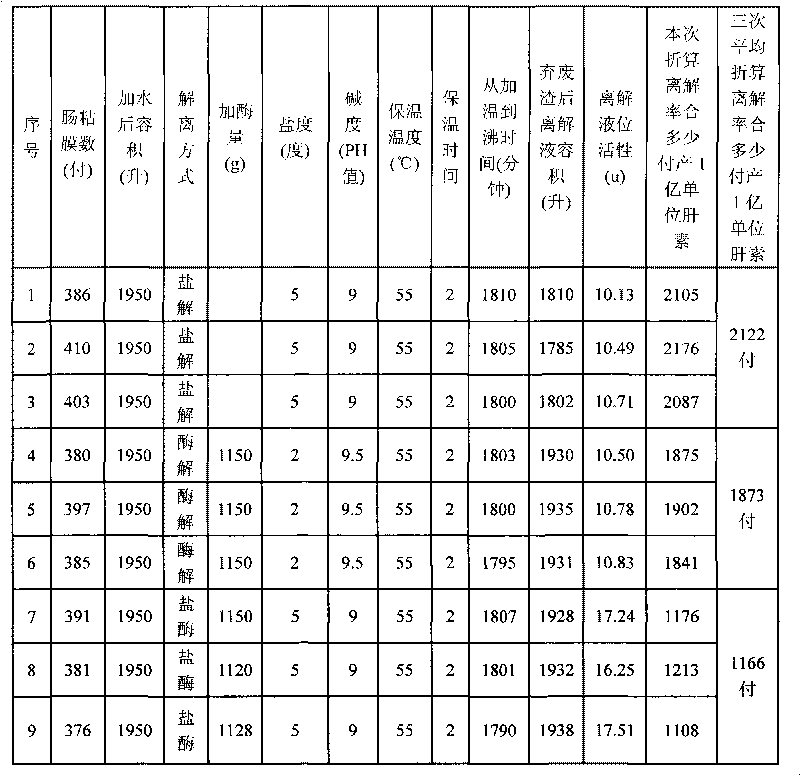
Method for preparing heparin sodium by combining enzymolysis and salt decomposition
The invention relates to a method for preparing heparin sodium by combining enzymolysis and salt decomposition, comprises the following steps: 1) dissociation: according to the weight ratio of 4-5:1 between water and chatterling mucosa, adding the water into the chatterling mucosa, and adding salt to adjust the solution salinity to be 5; adding alkali to adjust the pH value to be 9, and stirring and heating to 55 DEG C; carrying out heat preservation for 30min, and then adding alkali protease 2709 into a reaction tank according to the weight ratio of 2000-3000:1 between the chatterling mucosa and the alkali protease 2709; and carrying out heat preservation for 2h, heating to 85 DEG C and filtering; and 2) absorbing, eluting, precipitating, filtering and drying filtrate obtained in the step 1), and finally obtaining the heparin sodium. By adopting the technique combining enzymolysis and salt decomposition, the method leads heparin to be thoroughly dissociated from the chatterling mucosa, thus completing full extraction. On the basis of the prior art, the method increases about one third of unit production value and controls the increased cost not more than 1% of the increased production value.
Owner:叶青理
Nanofiber vascular prostheses and preparation method
ActiveCN101708344AImprove bindingCombine firmly and evenlyFilament/thread formingProsthesisFiberN dimethylformamide
The invention discloses a nanofiber vascular prostheses and a preparation method. The preparation method is as follows: mixing the solution of gelatin and glacial acetic acid with the aqueous solution of a crosslinker evenly to pre-crosslink the solution of gelatin and glacial acetic acid, adding heparin sodium aqueous solution to prepare spinning dope and collecting the formed fibers on a collecting roll by using the electrospinning process to form a nanofiber nonwoven membrane tube; dissolving polyurethane into the mixed solvent of tetrahydrofuran and N,N-dimethylformamide to prepare polyurethane spinning dope and continuously collecting the formed fibers on the collecting roll which has collected the nanofiber nonwoven membrane tube by using the electrospinning process; and taking off the nanofiber nonwoven membrane tube covered by the polyurethane fiber nonwoven membrane tube structure from the collecting roll and then soaking the nanofiber nonwoven membrane tube into the aqueous solution of a post-crosslinker to carry out post-crosslinking treatment, thus preparing the nanofiber vascular prostheses. The inner layer of the vascular prostheses of the invention can improve the blood compatibility and the outer layer has biological stability and can improve the physical and mechanical properties.
Owner:南通双辉医疗器械科技有限公司
Method for directly producing enoxaparin sodium from crude product heparin sodium
ActiveCN102603925AControl impurity contentReduce intermediate environmentOrganic solventDepolymerization
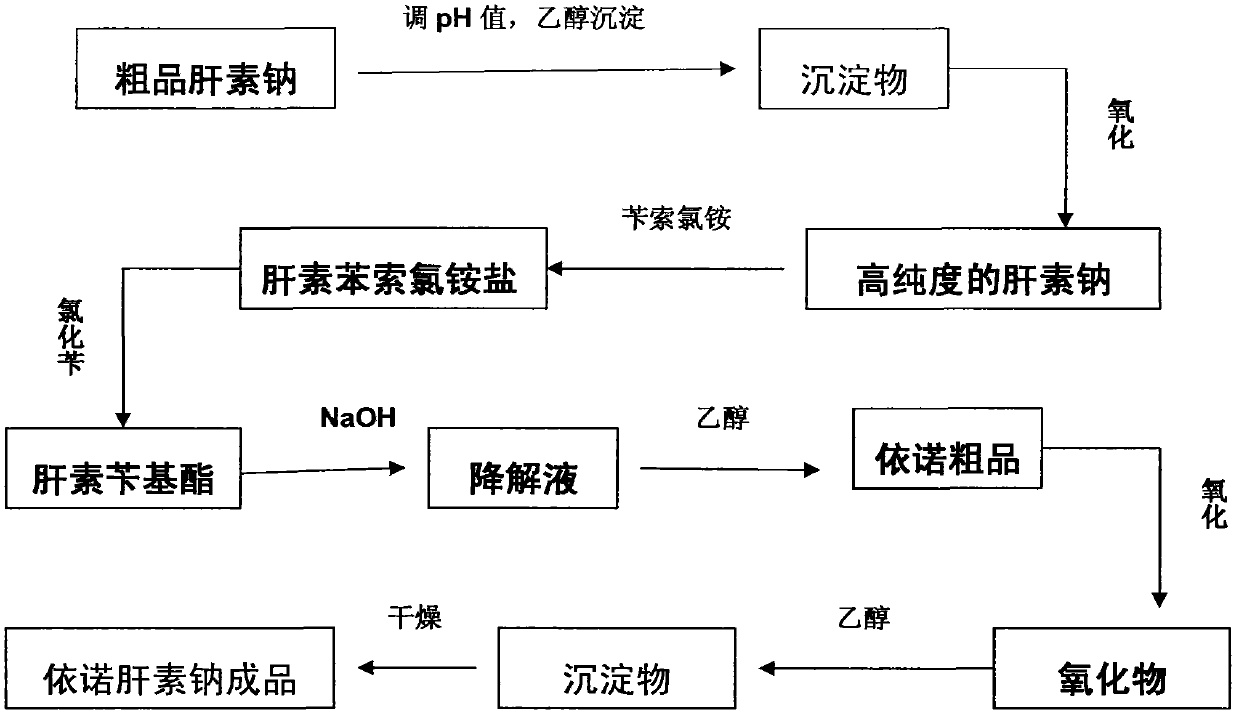
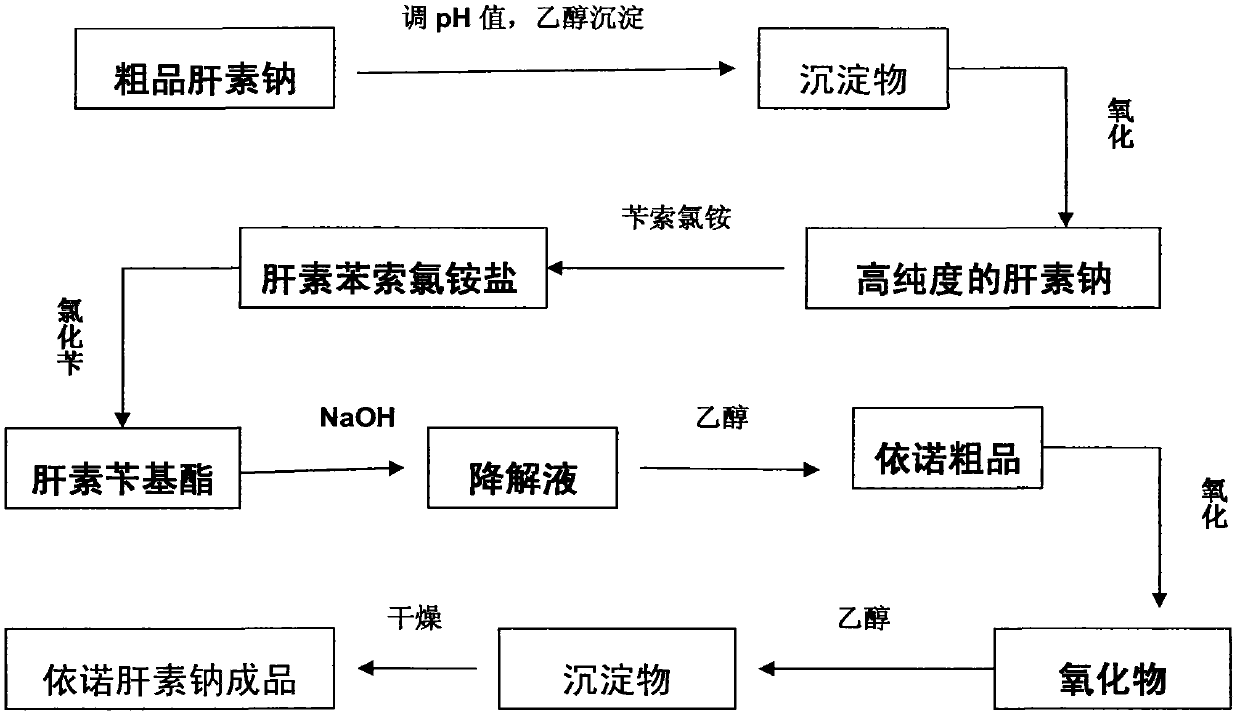
The invention relates to a preparation method for directly producing enoxaparin sodium from crude product heparin sodium. The preparation method comprises the following steps of: taking the crude product heparin sodium as a raw material, performing fractionated precipitation through an organic solvent to remove most of impurities in the crude product heparin sodium, and then removing part of residual impurity proteins, pigments and other impurities by oxidation through hydrogen peroxide so as to get the high-purity heparin sodium which is in line with the production requirements of the enoxaparin sodium; and taking the high-purity heparin sodium as an intermediate product, preparing a heparin quaternary ammonium salt, preparing heparin benzyl ester, performing alkaline depolymerization on the heparin benzyl ester, neutralizing with an acid, performing alcohol precipitation, refining, decoloring, dehydrating and drying to get an enoxaparin sodium finished product. By adopting the method disclosed by the invention, the use of the organic solvent is greatly reduced, the production efficiency is improved, the influences on the environment are reduced, the enoxaparin sodium finished product which achieves or is better than European Pharmacopoeia 7.0 version is obtained, and the method is simple to operate and can realize industrialized production.
Owner:DONGYING TIANDONG PHARM CO LTD
Antibacterial and antitumor orthopaedic implantation material and preparation method thereof
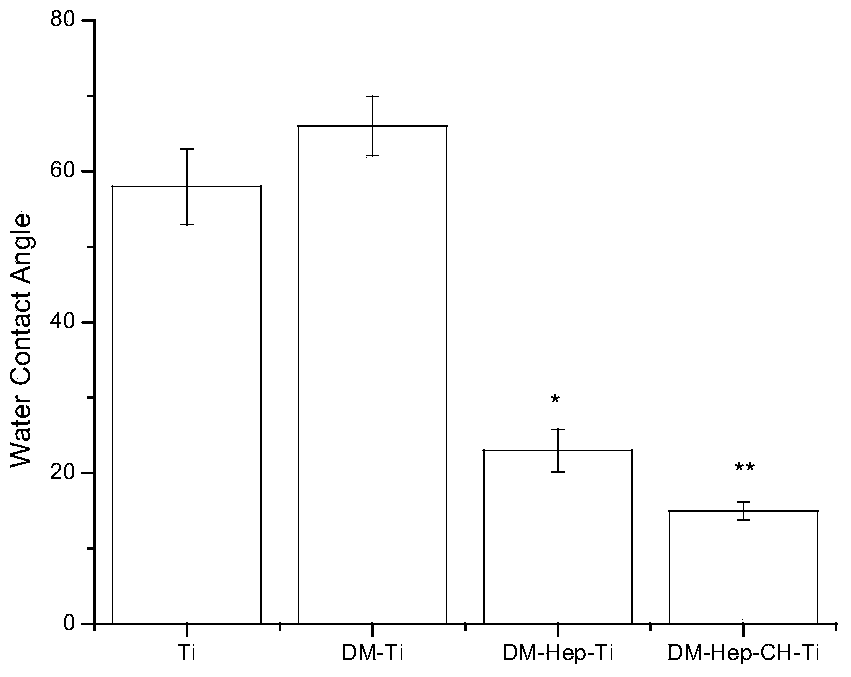
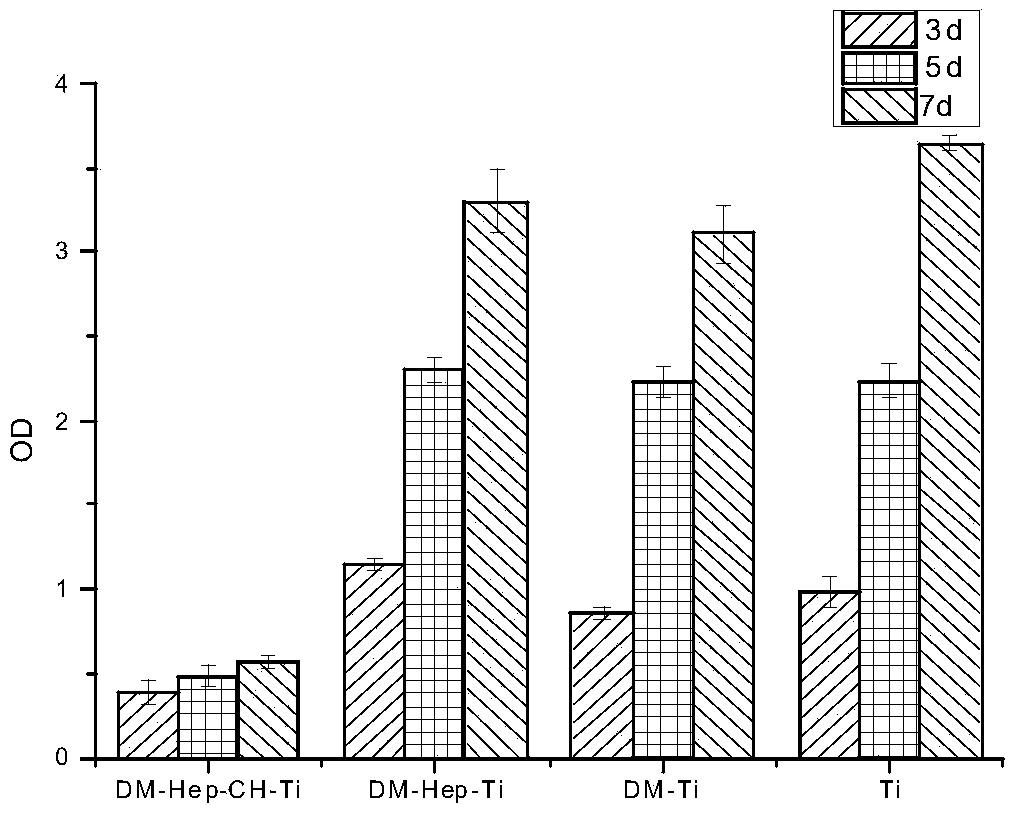
The invention discloses an antibacterial and antitumor orthopaedic implantation material and a preparation method thereof. The implantation material is a medical pure titanium sheet with the surface modified by chitosan, methotrexate, heparin sodium, polylysine and dopamine. According to the material, dopamine serves as a bridge to allow polylysine and a heparin sodium particle to be bonded on the surface of titanium; heparin, as a good anticoagulant, can improve biocompatibility between the material and blood, and is bonded with chitosan and methotrexate with antibacterial and antitumor effects through an electrostatic effect. Physiological-biochemical characteristics of drugs are utilized ingeniously to form an orderly and uniform antibacterial and antitumor coating with high biocompatibility on the surface of the titanium, and the prepared implantation material has good antibacterial and antitumor activity, and can effectively prevent bacterial adhesion and reproduction, as well as recurrence and transfer of a cancer.
Owner:GUANGZHOU GENERAL HOSPITAL OF GUANGZHOU MILITARY COMMAND
Method for improving enzymolysis efficiency of crude heparin sodium extraction technology
The invention relates to a method for improving enzymolysis efficiency of a crude heparin sodium extraction technology. The method can effectively improve enzymolysis efficiency, decompose impurity proteins, improve crude product quality, improve a yield and increase economic benefits. The method comprises the following steps of 1, dissolution: preparing mucous membrane water, 2, acid protease catalysis: heating the mucous membrane water, adding acid protease into the mucous membrane water based on the number of small intestines of the pig, adjusting a pH, adding metal ions into the mixed solution based on the number of small intestines of the pig, adjusting salinity by sodium chloride, and carrying out a thermal insulation reaction process, 3, alkaline protease catalysis: carrying out heating, adding two alkaline proteases into the reaction produce based on the number of small intestines of the pig, adding a pH value and salinity, carrying out a thermal insulation reaction process, carrying out heating and carrying out a thermal insulation reaction process, and 4, filtration on the enzymolysis mother liquor by a combined filter cloth, introduction of the filtered enzymolysis mother liquor into an adsorption tank, and follow-up processes.
Owner:杭州惠顺生物科技有限公司
Method for extracting heparin sodium by utilizing pork lungs
The invention relates to a method for extracting heparin sodium by utilizing pork lungs, which has the following steps of: grinding fresh pork lungs into pork lung paste; adding deionized water, lysis agent and preservative; reacting to obtain pork lung serous fluid; adding deionized water and sodium chloride; reacting to obtain pork lung alkaline hydrolysis liquid; slowly heating the pork lung alkaline hydrolysis liquid and adding heparin sodium protamex; after heat preservation and reaction, slowly heating and continuously carrying out heat preservation to obtain pork lung enzymolysis liquid; replenishing sodium chloride after cooling the pork lung enzymolysis liquid; reacting to obtain pork lung salt hydrolysis liquid; heating and carrying out heat preservation on the pork lung salt hydrolysis liquid; adding composite protein precipitation agent; stirring; collecting clear liquid after static placing; concentrating the clear liquid and then adding ethanol; precipitating overnight to obtain precipitate; and dehydrating and drying to obtain a crude product of the heparin sodium. According to the invention, the production cost of the heparin sodium can be reduced, the product quality and the yield can be improved, the large-scale production can be easily realized, meanwhile, the usage amount of chemical reagents is reduced, the emission of waste is reduced, and no waste gas orwaste water is discharged. The economic benefit and the social benefit are obvious.
Owner:TOPROBIO MICRO BIOTECH
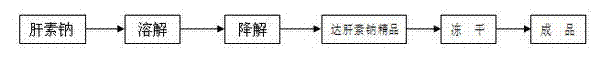
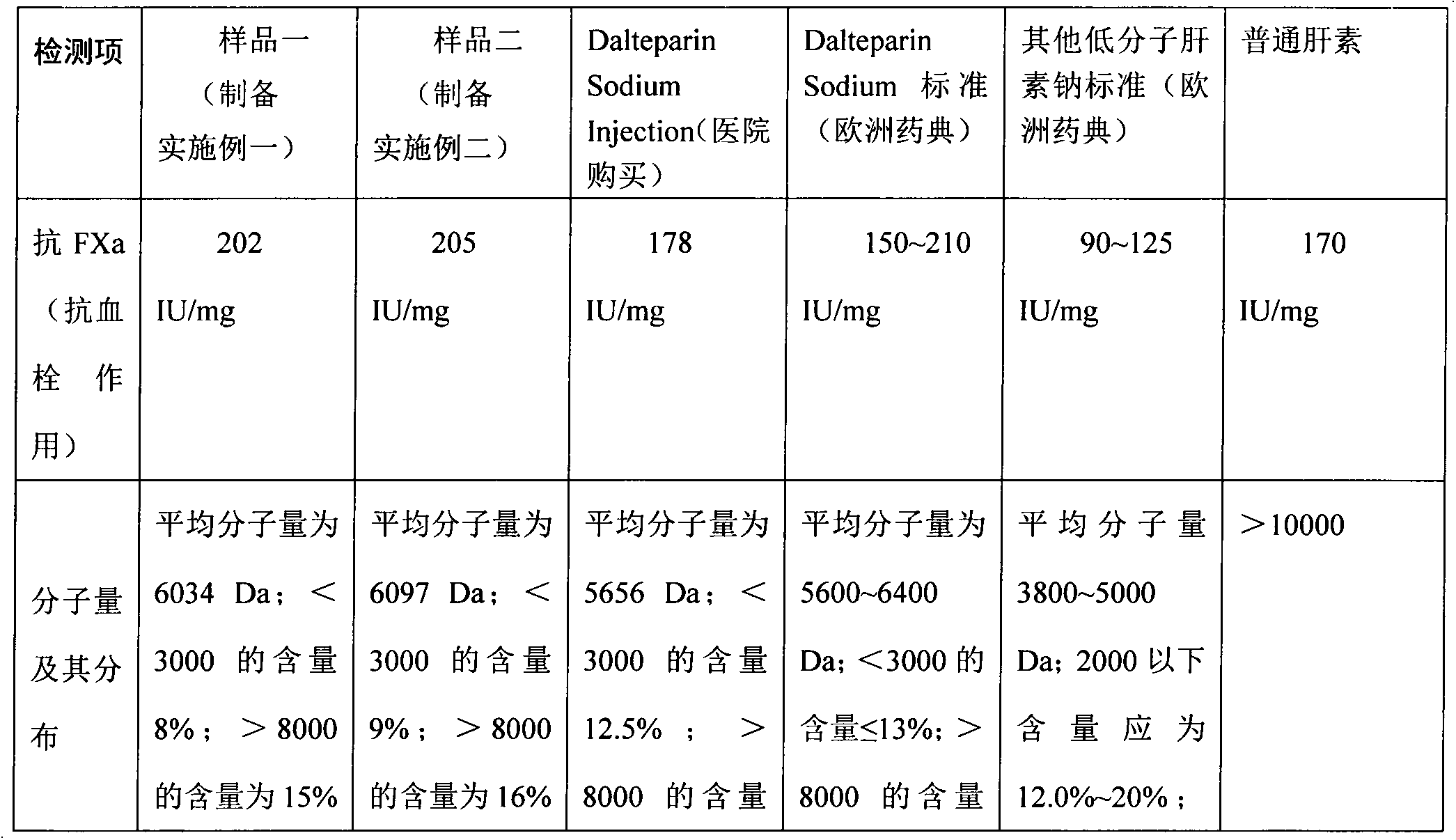
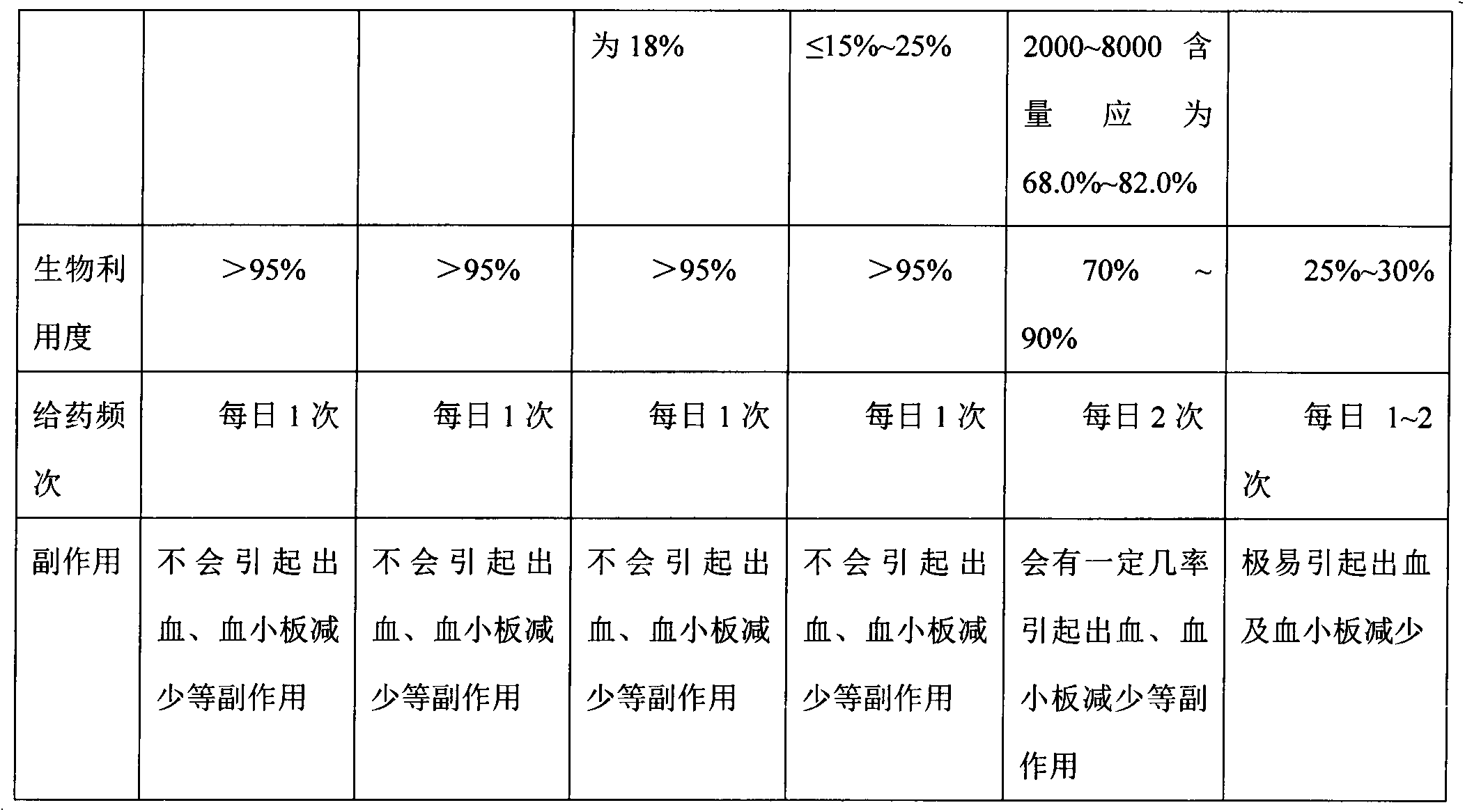
Preparation process of dalteparin sodium
ActiveCN102558393APrevent embolismPrevent venous thromboembolismOrganic active ingredientsAntipyreticExtracorporeal circulationFreeze-drying
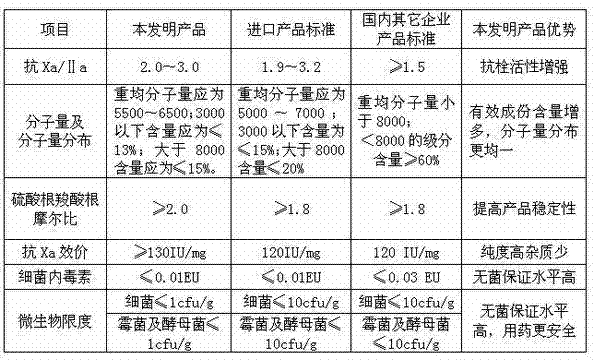
The invention discloses a preparation process of dalteparin sodium. The preparation process comprises the following steps: preparing a heparin sodium solution, a heparin degradation fluid, a reducing solution and a crude product, refining, freeze-drying and the like. The average molecular weight of the obtained product is 5,500 to 6,500, the peak molecular weight is 3,500 to 6,000, a component with the molecular weight of less than 3,000 is not greater than 13% , a component with the molecular weight of greater than 8,000 is not greater than 15%, anti-Xa activity is more than or equal to 130IU / mg. The invention has the advantages of rich source of raw materials, high yield, stable and reliable quality, high purity, low cost, simple process, easiness in operation and no waste discharge. The dalteparin sodium has the anticoagulant, antithrombotic, anti-tumor, anti-inflammatory, anti-allergy and blood lipid regulating effects, thereby having a significant curative effect. The dalteparin sodium can be used for preventing preoperative and postoperative thrombosis of general surgery, orthopedic surgery and neurosurgery, effectively preventing venous thromboembolism of ischemic stroke patients, greatly reducing the risk of stroke, effectively preventing the solidification caused by extracorporeal circulation of blood, effectively preventing the instable coronary heart disease, and having a wide usable range.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Method for separating purified heparin sodium
The invention relates to a method for separating purified heparin sodium. Heparin sodium obtained through extracting porcine intestinal mucosa contains a great amount of protein, nucleic acid and other impurities, so that certain problems are brought to refining and purifying of heparin sodium. The traditional process method is that residual impurities are removed through a salt resolving process or an enzymolysis process, which has the disadvantages that the requirement on extraction quality of heparin sodium is high, the removal efficiency is relatively low, the follow-up oxidation precipitation refining time is long, the consumption of oxidant (hydrogen peroxide) is high, and the yield is low. According to the method provided by the invention, residual impurities in heparin sodium are removed with precipitant through enzymolysis, so that the process design is reasonable, the removal efficiency is high, the follow-up refining time is shortened, the product yield is improved, and the cost is lowered.
Owner:QINGDAO JIULONG BIO PHARMA
Rapid hemostatic dressing and preparation method thereof
ActiveCN103599558AImprove cohesionAccelerate solidificationAbsorbent padsBandagesCollagenanHeparin sodium
The invention provides a rapid hemostatic dressing, which comprises the following raw materials in parts by weight: 30 to 100 parts of chitosan, 2 to 30 parts of heparin sodium, 10 to 80 parts of collagen, and 10 to 80 parts of aloin. The rapid hemostatic dressing provided by the invention can fulfill the requirements on rapid and large-amount hemostasis, and the hemostatic speed of the rapid hemostatic dressing is faster than that of conventional hemostatic dressing. The invention also provides a preparation method of the rapid hemostatic dressing. The rapid hemostatic dressing which is prepared through a foaming processing method has a faster hemostatic speed.
Owner:湖北仙明医疗器械有限公司
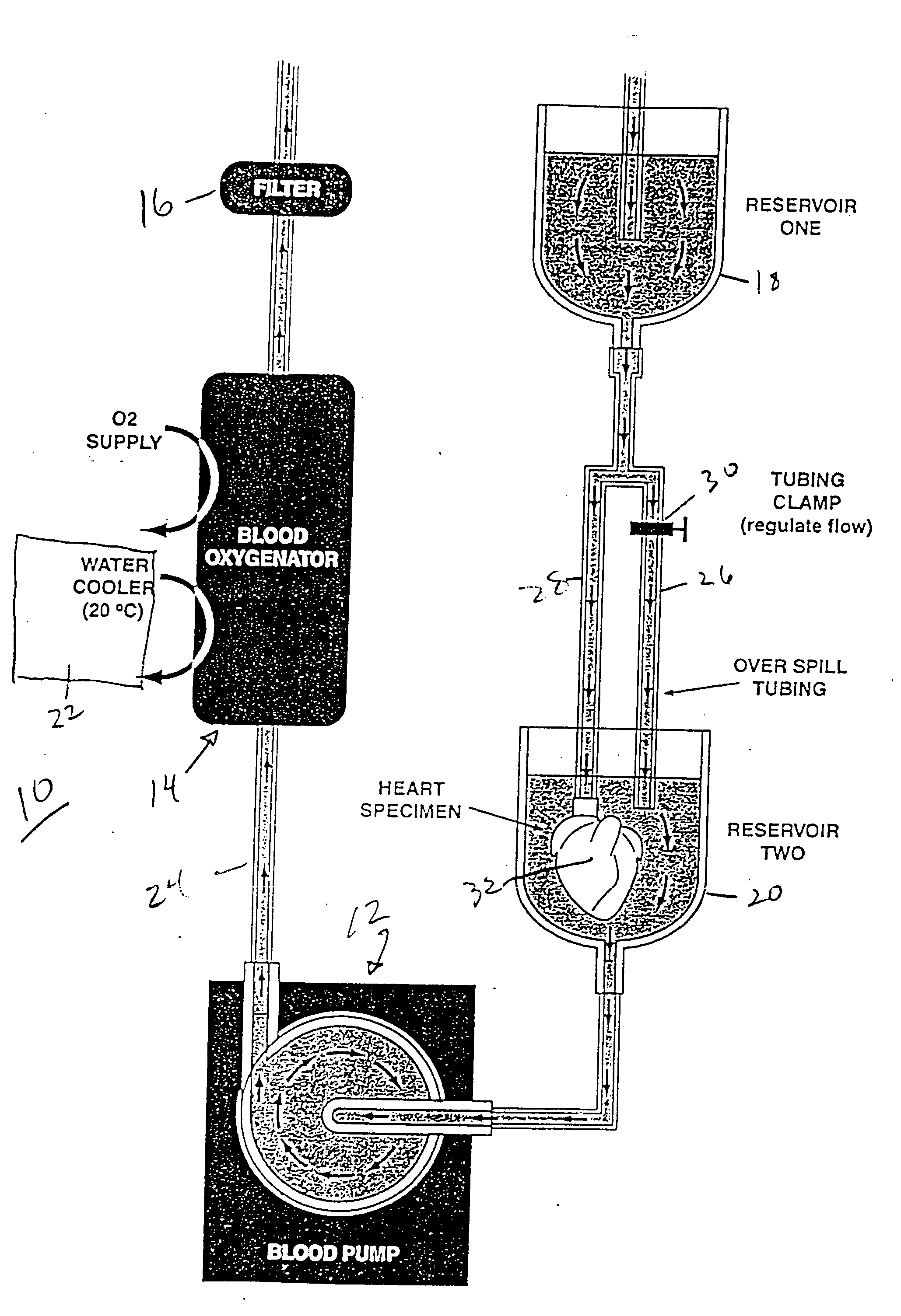
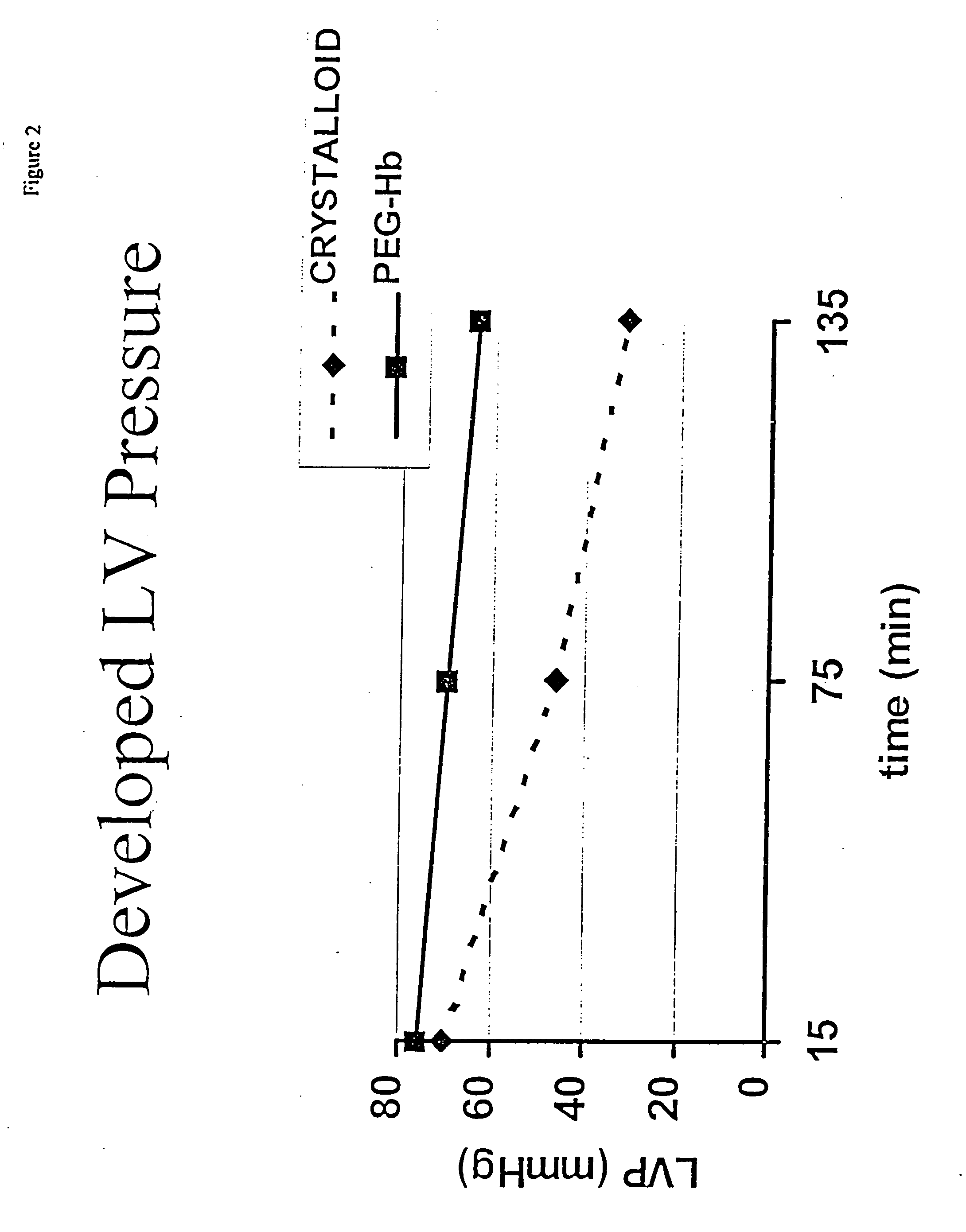
Continuous cardiac perfusion preservation with PEG-HB for improved hypothermic storage
InactiveUS20070243518A1Extended storage timeEasy to storeDead animal preservationHuman albuminPotassium
Efforts to extend myocardial preservation for transplantation by perfusion with prior crystalloid based solutions have been limited by edema and compromised function. Hypothermic perfusion preservation with a polyethylene glycol (PEG) conjugated hemoglobin solution extends preservation times. The polyethylene glycol (PEG) conjugated hemoglobin solution comprises PEG-Hb, and at least one of the constituents selected from the group of human albumin, dextrose, heparin sodium, lidocaine HCl, MgSO4, KCl, CaCl2, Tromethamine (THAM) solution, NaCl, NaHCO3, and Na2HPO4 / NaH2PO4. Comparison of cardiac function after continuous perfusion using a hypocalcemic normokalemic crystalloid perfusate is made with and without the addition of PEG-Hemoglobin (Hb).
Owner:RGT UNIV OF CALIFORNIA
Tissue sample preservative solution and preparation method thereof
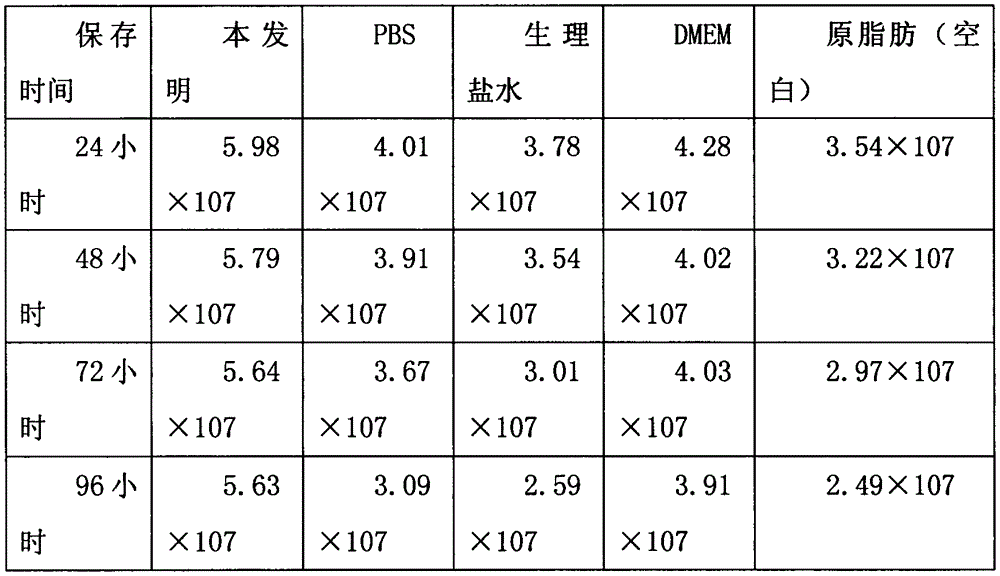
The invention discloses a tissue sample preservative solution used for preserving tissue samples, such as fat, umbilical cords, placentas and the like, after collection and before separation. The tissue sample preservative solution mainly comprises following components: high-glucose DMEM dry-powder culture medium, sodium bicarbonate, DMSO, dexamethasone, insulin, penicillin, streptomycin, amphotericin and a heparin sodium injection. The high-glucose DMEM dry-powder culture medium and the sodium bicarbonate are used for maintaining osmotic equilibrium among cells inside and outside tissue, maintaining a pH value, maintaining a moistening situation of the tissue and providing nutritional components. The DMSO is a freeze-storage protective agent and can prevent freeze-injuries on the tissue samples. The dexamethasone can inhibit immunization and protect activity of stem cells in the tissue sample; the insulin can promote absorption and utilization of the tissue sample to glucose. The penicillin, the streptomycin and the amphotericin can prevent pollution from bacteria and moulds and can eliminate pollution which has occurred. The heparin sodium injection can prevent blood solidification in the tissue samples and increase a yield of the stem cells. The tissue sample preservative solution is simple in components, is low in cost, is convenient to use, can maintain activities of the stem cells in the tissue samples, such as fat, umbilical cords, placentas and the like, and can greatly reduce a time limit from collection to preparation of the tissue samples.
Owner:上海鑫曙医疗科技有限公司
Process for preparing high-purity low-molecular heparin sodium
The invention discloses a process for preparing high-purity low-molecular heparin sodium and belongs to the field of bioengineering. The process mainly comprises the following steps of: salting-out and impurity removal, supplementary material warming and oxidization, gel elution, ion exchange, ethanol precipitation, vacuum drying and the like. The problems that the existing heparin sodium structure analog is hard to remove, the purity can not achieve the requirements for medicinal use, the preparation process is complicated, and the like are solved. The process has the beneficial effects that by adopting a supplementary material warming and oxidization method, the preparation and the decolorization of the low-molecular heparin sodium are realized, and the heparin sodium structure analogue is effectively removed by using weakly-basic anion exchange resin; and the process has the advantages of shortened production period, saved cost, simple process, easiness in operation and is suitable for industrial production.
Owner:NANJING XINBAI PHARMA +1
Process for producing heparin sodium
InactiveCN101891842ASolve problems such as polluting the environmentIncrease added valuePre treatmentIon-exchange resin
The invention belongs to the field of preparation for medicaments, and in particular relates to a process for producing heparin sodium. In the process, the skin of small intestines of a pig from which mucosae is removed serves as a raw material, and the heparin sodium product is obtained by preprocessing, adsorbing, washing, eluting, precipitating and drying sequentially, wherein in the preprocessing step, the skin of the small intestines is crushed into paste, and then salt special for sausage casing is added into the paste for pickling; and in the adsorbing step, acrylic acid strongly basic anion exchange resin, namely rohm and haas FPA98 resin is adopted. The production process can effectively solve the problems of environmental pollution and the like caused by a large amount of waste intestine residue of the pig (the skin of the small intestines primarily), further increases the added value of the small intestines of the pig, and reduces the production cost; and the purity of the product is over 120 titers / mg.
Owner:HANGZHOU LONGYANG BIOTECH
Crude heparin sodium purification technology
The invention relates to a heparin sodium purification technology. The crude heparin sodium purification technology sequentially comprises the following steps: 1, dissolving crude heparin sodium in a sodium chloride solution, and adding an alkaline protease for enzymatic hydrolysis; 2, inactivating, adding diatomite, carrying out high speed centrifugation, and removing insoluble impurities; 3, adding polysilicate, carrying out high speed centrifugation, and removing insoluble impurities; 4, adding alcohol for precipitating, and removing supernatant alcohol; 5, dissolving in the sodium chloride solution, adding hydrogen peroxide for oxidizing, carrying out high speed centrifugation after oxidation, and removing insoluble impurities; 6, adding alcohol for precipitating, and removing supernatant alcohol; and 7, filtering, and carrying out vacuum lyophilizing to obtain refined heparin sodium. The crude heparin sodium purification technology has the advantages of low content of proteins in the refined heparin sodium, and high yield of the refined heparin sodium.
Owner:PUJIANG CAREX BIOTECH
Bone marrow stem cell protection solution and preparation method thereof
InactiveCN107668024AEffective protectionExpand the scope of clinical applicationDead animal preservationCell activityInosamycins
The present invention provides a bone marrow stem cell protection solution, which is mainly prepared by dissolving a hypoxic protection agent, heparin sodium and an aminoglycoside antibiotic in a DMEM / F12 culture medium aqueous solution, wherein per mL of the DMEM / F12 culture medium aqueous solution can respectively dissolve 5-10 mg of the hypoxic protection agent, 0.01-0.05 U of the heparin sodium and 50-200 U of the aminoglycoside antibiotic, and the concentration of the DMEM / F12 culture medium aqueous solution is 30-40 mg / ml. According to the present invention, the bone marrow stem cell protection solution has advantages of stable performance, safety and no toxicity, can effectively protect bone marrow stem cells, can make bone marrow stem cells be preserved for a long time and can maintain the cell activity in vitro, can maintain the original multidirectional differentiation ability, and can expand the clinical application of bone marrow stem cells, wherein the bone marrow stem cells can be transported to most domestic cities and neighboring countries by using the existing transportation tools.
Owner:CENTURY BIOSTRENGTH BEIJING PTY LTD
Method for preparing anticoagulant vascular stent
InactiveCN101927037AImprove bindingNot easy to fall offPharmaceutical containersMedical packagingMedicineDistilled water
The invention discloses a method for preparing an anticoagulant vascular stent, which comprises the following steps of: A, depositing a plasma-polymerized allyl amine functional film on the surface of the vascular stent by using a pulse plasma polymerization method; B, preparing heparin sodium mixed solution; and C, precipitating heparin sodium, namely soaking the vascular stent on which the plasma-polymerized allyl amine functional film is deposited prepared in the step A into the heparin sodium mixed solution prepared in the step B, reacting at the temperature of between 4 and 20 DEG C for 12 to 48 hours, and after the reaction, fully rinsing by using distilled water, and drying to obtain the anticoagulant vascular stent. The vascular stent prepared by the method has the advantages of high binding force between an anticoagulant film layer on the surface of the vascular stent and the vascular stent, and excellent tissue compatibility and blood compatibility.
Owner:CHENGDU SOUTHWEST JIAOTONG UNIV SCI & TECH GARDEN MANAGEMENT
Novel sacculus dilating catheter
The present invention provides a new type balloon dilation catheter which includes ballon and medication material coated on stent. Said medication material comes from one or two and more than two mixtures of heparin sodium, fiber degrading enzyme, serine proteinase, batroxobin, aspirin, genistein, hirudin and its recombined product, colchicine, sirolimus, biolimus, zotarolimus, tracrolimus, pimecrolimus, simvastatin, atorvastatin, pravastatin, ciclosporin, Anti-CD34, dexamethasone, bleomycin, plicamycin, daunomycin, mitomycin C, actinomycin D, taxol, celastrol, methopterin, 5-fluorouracil, cytarabine and 6-purinethol. The balloon is made of macromolecule nylon material, and the stimulation to blood vessel is far lower than the stent with metal structure.
Owner:上海赢生医疗科技有限公司
Method for extracting crude product of heparin sodium
The invention relates to a method for extracting a crude product of heparin sodium, which comprises the following steps of: firstly, taking the gut skin and the intestinal mucosa of a primitive gut as raw materials for extracting the heparin sodium, putting sodium chloride, sodium hydroxide and proteinase into an enzymolysis tank simultaneously according to a certain proportion and heating to carry out enzymolysis on the raw materials to form a liquid state; filtering, putting filtrate into an adsorption tank, eluting resin by brine after adsorbing the filtrate by adopting the higher resin, enabling the heparin sodium to enter eluent, putting the eluent into a depositing tank and depositing by alcohol; and drying and pulverizing. The invention has high efficiency, high stability, resource saving and high product yield and purity, is easy for large-scale production, can meet the requirements of markets at home and abroad to a crude product of heparin and has extensive application prospect.
Owner:喻延安
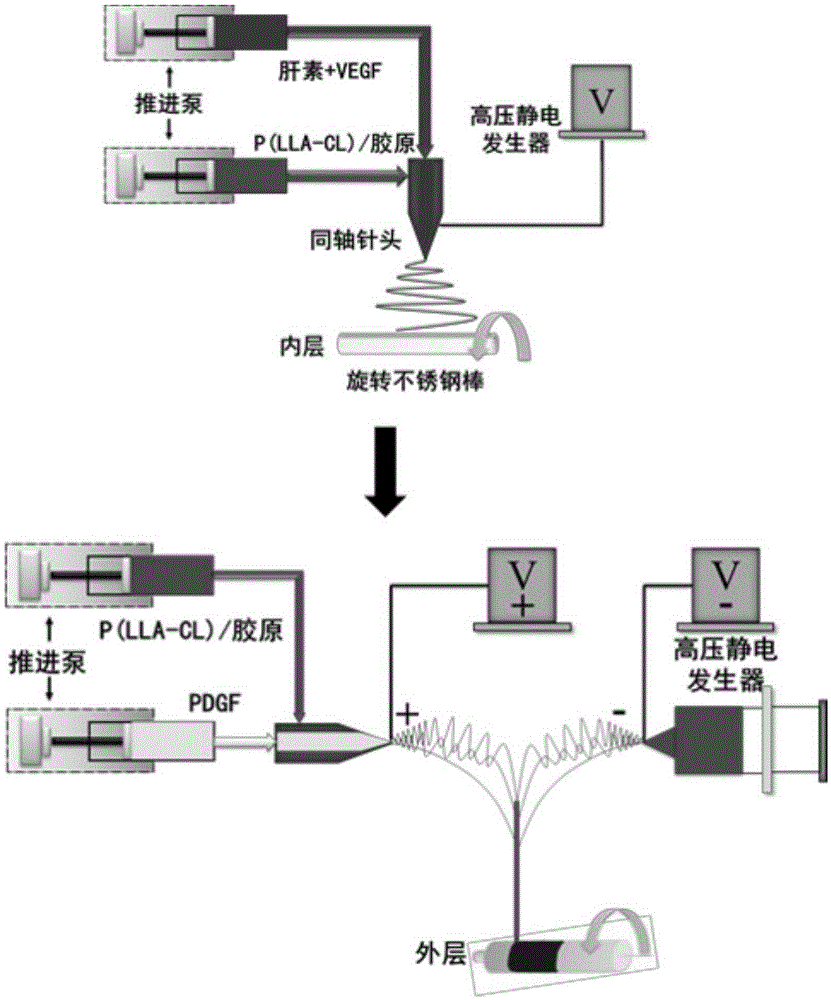
Preparation method of heparin and twin factor synergistically regulated P(LLA-CL)/collagen bilayer intravascular stent
ActiveCN105233339ARapid endothelializationPromote growthStentsPharmaceutical containersCross-linkBlood Vessel Tissue
The invention relates to a preparation method of a heparin and twin factor synergistically regulated P(LLA-CL) / collagen bilayer intravascular stent. The method comprises the following steps: uniformly mixing P(LLA-CL) with collagen in a solvent to obtain a composite spinning solution; dissolving heparin sodium and VEGF in a dilution solution to obtain an internal layer supported medicine solution; dissolving PDGF in the dilution solution to obtain an external layer supported medicine solution; carrying out coaxial electrostatic spinning with the internal layer supported medicine solution as a core layer and the spinning solution as a shell layer to obtain a intravascular stent internal layer; and carrying out bidirectional conjugate electrostatic spinning with the external layer loaded medicine solution as a core layer and the spinning solution as a shell layer to obtain an intravascular stent external layer, continuously receiving the intravascular stent external layer at the outer side of the intravascular stent internal layer to obtain a bilayer intravascular stent, and cross-linking to obtain the heparin and twin factor synergistically regulated P(LLA-CL) / collagen bilayer intravascular stent. The intravascular stent provided by the invention has excellent mechanical performances and biocompatibility, has natural blood vessel simulating components, structure and functions, is in favor of realizing in situ regeneration of blood vessel tissues and reconstruction of a multilayer structure, and has important applications in the blood vessel tissue engineering.
Owner:DONGHUA UNIV
Process for preparing intestinal membrane protein powder by utilizing residual liquid sourced from heparin sodium production
InactiveCN102178050AReduce pollutionReduce production energy consumptionFood processingAnimal feeding stuffIntestinal membraneSubstrate concentration
The invention relates to a process for preparing intestinal membrane protein powder by utilizing residual liquid sourced from heparin sodium production, which comprises the following steps of: (1) raw material pretreatment: filtering heparin sodium residual liquid by nylon cloth of 180 meshes for removing impurities; (2) raw material desalination and dehydration: desalinating and dehydrating raw materials by the heparin sodium residual liquid obtained, after impurity removal, through nanofiltration membrane equipment; (3) enzymolysis: adding composite animal protein hydrolase which is 0.2-0.8 percent by substrate weight into the heparin sodium residual liquid with the substrate concentration of 3-6 percent, adjusting the pH value to 7.0 to 8.5 and carrying out enzymolysis at the constant temperature of 50-60 DEG C for 4-8 hours; (4) carrier addition: adding wheat bran which accounts for 20-50 percent by substrate weight into enzymolysis liquid, completely dissolving and uniformly stirring; and (5) spraying and drying. The method has the advantages of simple process, low cost, enzymolysis thoroughness, high product yield and high product quality, can be used for reducing the environmental pollution, changing the waste materials into valuable things and reducing the production energy consumption and the production cost.
Owner:ANHUI BAODI MEAT FOODS
Neural stem cells medium and method for performing human neural stem cells in-vitro long-term culture and amplification by using neural stem cells medium
ActiveCN105062972AGenetic stabilitySolve the easy differentiation of in vitro cultureNervous system cellsInsulin activityCuticle
The invention relates to a neural stem cells medium and a method for performing human neural stem cells in-vitro long-term culture and amplification by using the neural stem cells medium. The neural stem cells medium comprises the following ingredients by weight proportion: 100-1000 micrograms of heparin sodium, 10-100 micrograms of vitamin E, 5-50 milligrams of insulin human recombinant, 0.5-5 milligrams of putrescine, 2-10 micrograms of sodium selenite, 2-10 milligrams of human transferrin, 2-10 micrograms of progestin, 300 milligrams of L-glutamine, 5.9 grams of 2-[4-(2-Hydroxyethyl)-1-piperazine]ethanesulfonic acid, 10-100 micrograms of recombinant human epidermal growth factors, 10-100 micrograms of recombinant human basic fibroblast growth factors, 20-200 milligrams of vitamin C glucoside and 40,000-400,000 IU (international unit) of gentamicin. By the neural stem cells medium, the technical problems that human neural stem cells are easy to differentiate when cultured in vitro and long-term culture and amplification are difficult to implement are solved.
Owner:ZHEJIANG ORIGIN BIOTECH
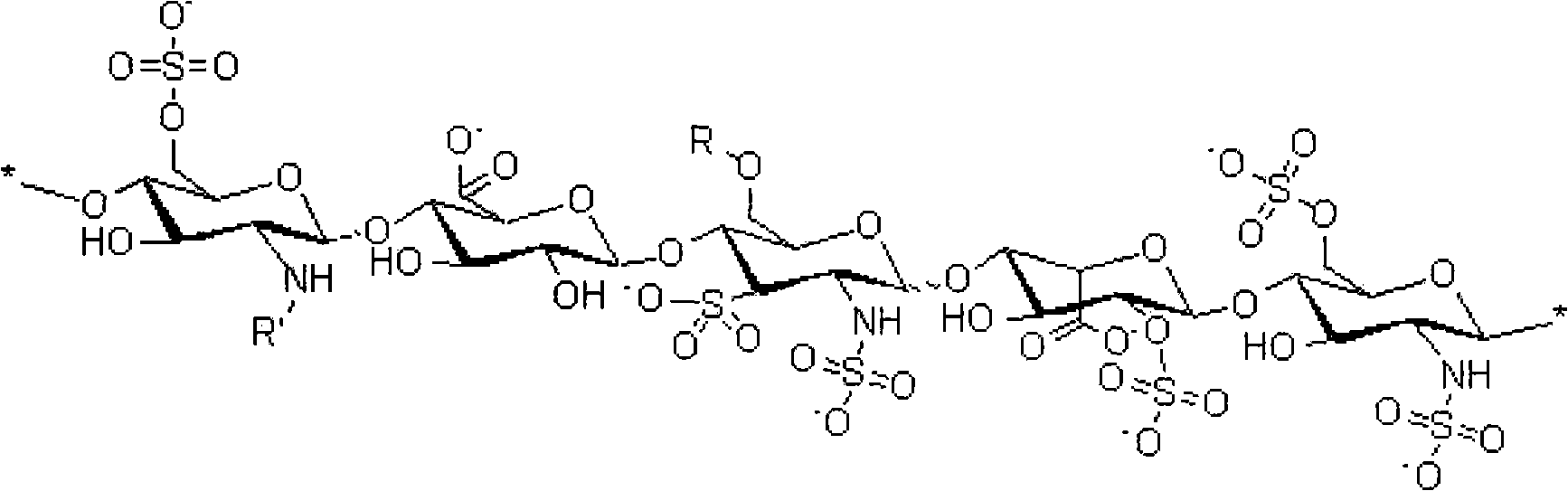
Heparin sodium production process
The invention relates the technology of heparin sodium. The invention overcomes the defects of many procedures, big heparin sodium biological activity damage, little productivity and high residual quantity. The invention comprises the following steps: clearing raw material, extracting, flocculating and setting, ion exchanging, washing, carrying out elution, settling, dewatering and drying. The invention has the advantages of rational technology, improving quality, little active damage, little residual quantity, high purify, low cost, and good economic benefit.
Owner:胡世辉
Preparation method of high-quality low-molecular weight dalteparin sodium
ActiveCN103232558AEfficient degradationAvoid the tedious process of repeated impurity removalBlood disorderExtracellular fluid disorderAlcoholEnzymatic hydrolysis
The invention discloses a preparation method of high-quality low-molecular weight dalteparin sodium, belonging to the field of biomedicines. According to the method, a crude product, namely heparin sodium is taken as a raw material, compound enzymatic hydrolysis and improved nitrite degradation methods are taken as the basis, and then a low-molecular weight dalteparin sodium fine product with specific average molecular weight (5600-6400) is prepared through enzyme hydrolysis, oxidation, impurity removal by ultrafiltration, impurity removal by alcohol precipitation, degradation, reduction, alkali oxygen purification, ultrafiltration refining, freeze drying and other steps; and the preparation method has the characteristics of simple preparation process, good product stability, good anti-thrombotic activity and the like.
Owner:山东辰龙药业有限公司
Exfoliated cell preservation solution and preparation method thereof
InactiveCN102113481AHigh retention rateScatter tilingDead animal preservationBenzoic acidHeparin sodium
The invention relates to exfoliated cell preservation solution and a preparation method thereof. The preservation solution comprises the following components in percentage by weight: 0.6 percent of sodium chloride, 0.06 percent of potassium chloride, 2 percent of glucose, 20 percent of ethanol, 3 percent of 1-3 propylene glycol, 2 percent of glycerin, 0.15 percent of glycine, 0.1 percent of mucolytic agent, 0.2 percent of cell protective agent, 0.1 percent of heparin sodium and 0.05N of sodium benzoate-benzoic acid buffer solution. A pH value is adjusted by 1 percent sodium hydroxide or hydrochloric acid solution to ensure that the pH of the preservation solution is 5.8. By the exfoliated cell preservation solution, cells can be well preserved and the preservation time is more than 10 days; cell nucleuses are clear, and cytoplasm is stretched; red blood cells and mucus are effectively removed; the cells are dispersed and flatly laid and an acid phosphatase (ACP) enzyme is well preserved simultaneously; and the exfoliated cell preservation solution is suitable for ACP enzyme staining.
Owner:XIAMEN MAIWEI BIOTECH
Kit for pancreas decellularized scaffold and preparation and reseeding methods of scaffold
The invention discloses a kit for a pancreas decellularized scaffold and preparation and reseeding methods of the scaffold. The kit comprises perfusion fluid 1, perfusion fluid 2 and perfusion fluid 3, wherein the perfusion fluid 1 comprises 0.25 g / L of EDTA (ethylene diamine tetraacetic acid), 10,000 U / L of heparin sodium and PBS (phosphate buffer solution); the perfusion fluid 2 comprises 1% Triton-X100 and 0.1% ammonia water; the perfusion fluid 3 comprises 0.01 g / L of DNase I (eoxyribonuclease I). The kit has the characteristics that the expense is low, cells are radically removed, a detergent is easily eliminated, the structures of extracellular matrixes and blood vascular systems are well preserved, the reseeding and growth of exogenous and endogenous cells are facilitated, and the exogenous and endogenous cells can be conveniently transplanted to diabetic experiment animals.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
Process for preparing and purifying ultra low molecular weight heparin
InactiveCN102040671ASolve the separation problemNo pollution in the processUltrafiltrationHollow fibreLiquid temperature
The invention discloses a process for preparing and purifying ultra low molecular weight heparin sodium (calcium), which comprises the following steps of: reacting heparin with organic quaternary ammonium salt to generate heparin quaternary ammonium salt, performing nucleophilic substitution to generate heparin benzyl ester, and degrading under the alkaline condition to obtain a low molecular weight heparin fragment; and separating and purifying by an inorganic ceramic ultrafiltration and hollow fiber ultrafiltration combined method to obtain the ultra low molecular weight heparin sodium (calcium) of which the molecular weight distribution is 2,000 to 2,500D and the average molecular weight is 2,200D. The low molecular weight heparin fragment is obtained by controlling reaction conditions in the esterification process and the degradation time of ester hydrolysis; a ceramic membrane and a hollow fiber ultrafiltration membrane are combined to separate and purify the heparin fragment; and by selecting the pore diameter of the ceramic membrane, operating pressure, feed liquid temperature, and the molecular weight cutoff of the hollow ceramic membrane, the heparin fragment with a reasonable molecular weight distribution range is effectively separated.
Owner:BEIJING GUANHONG TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com