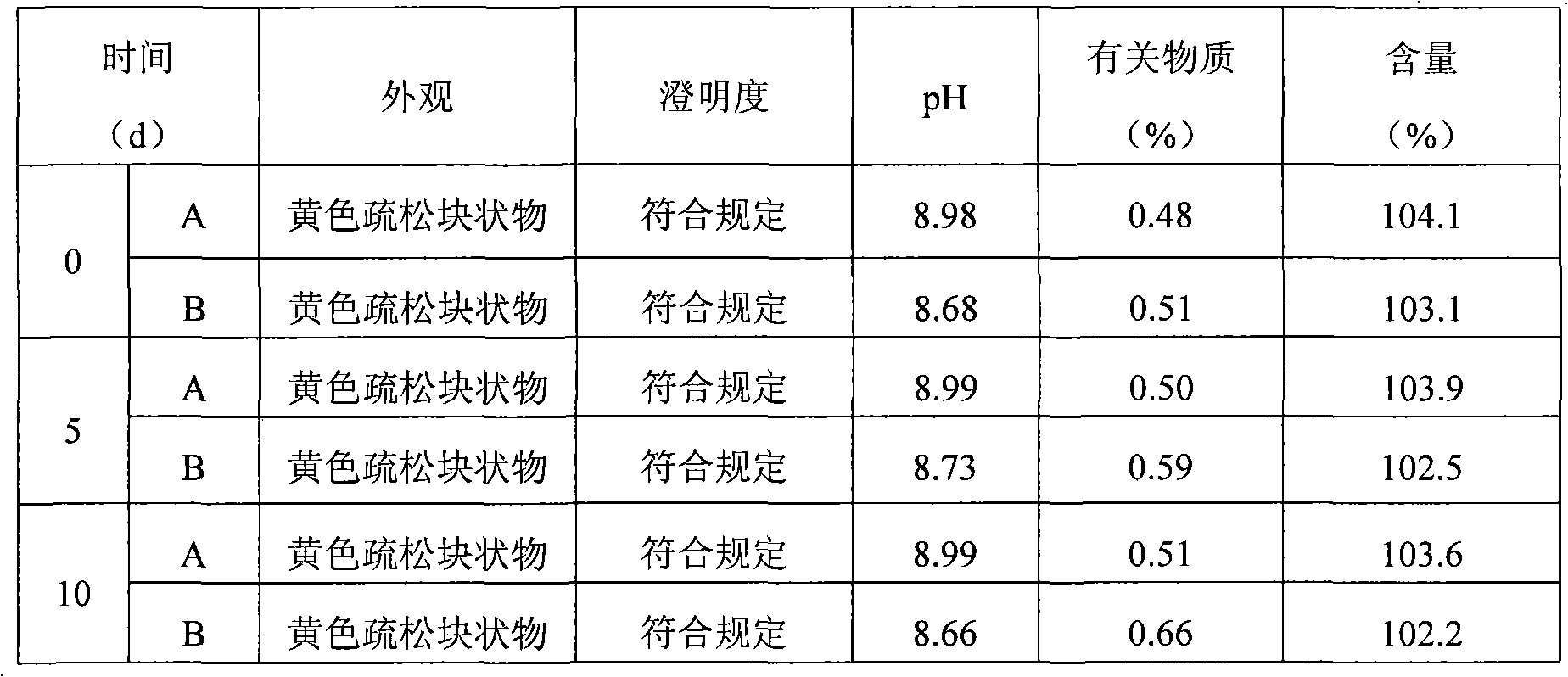
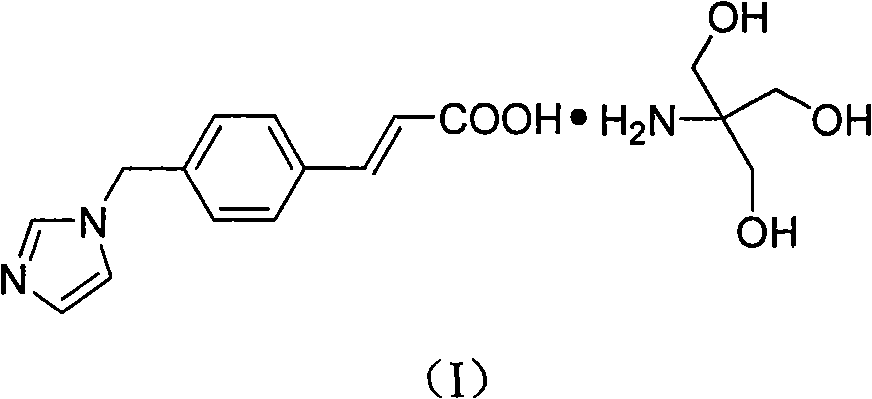
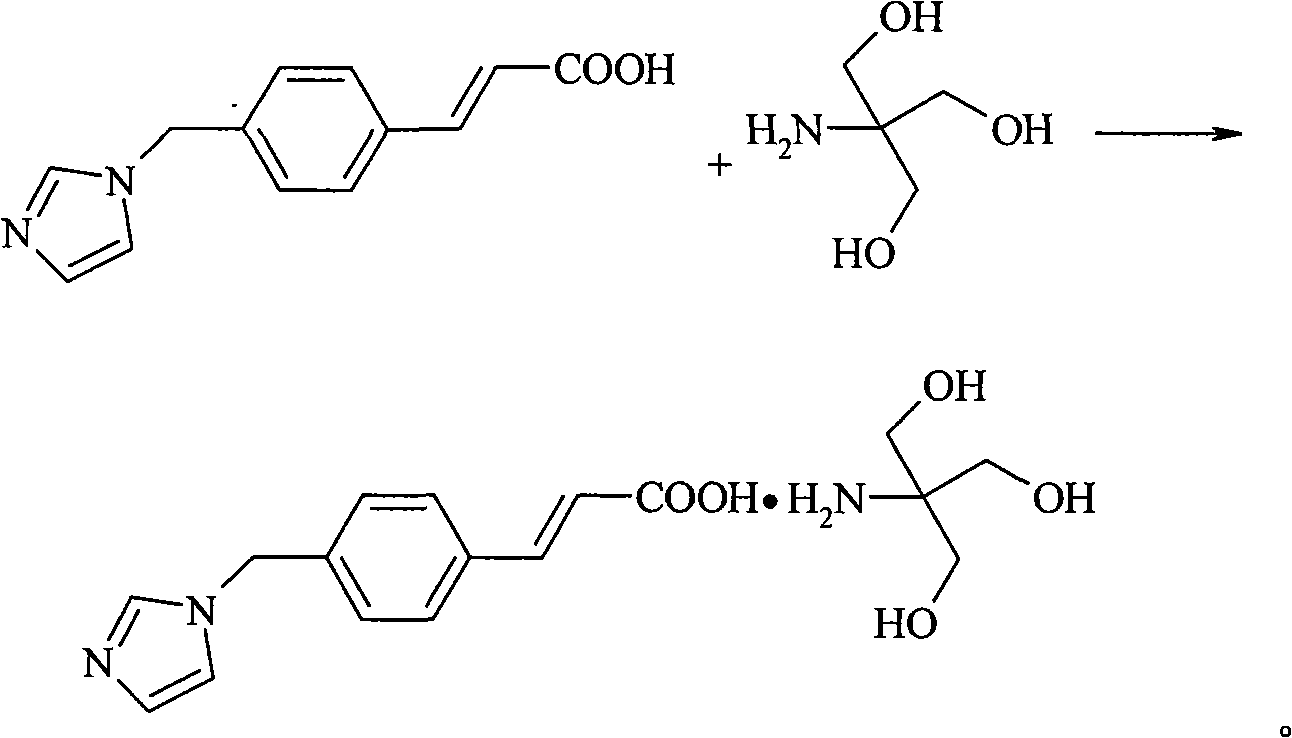
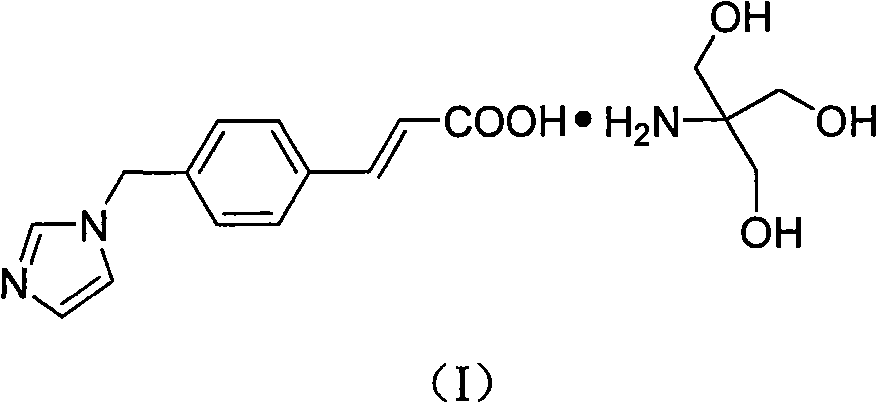
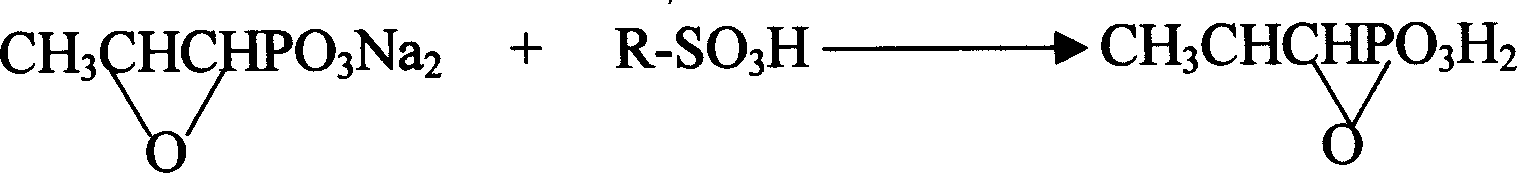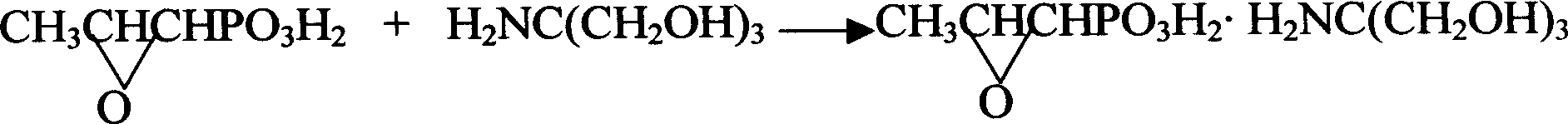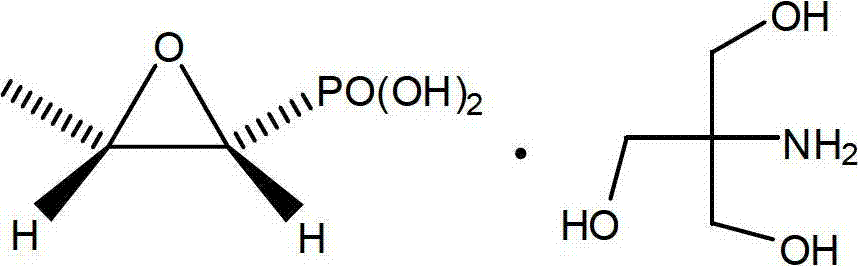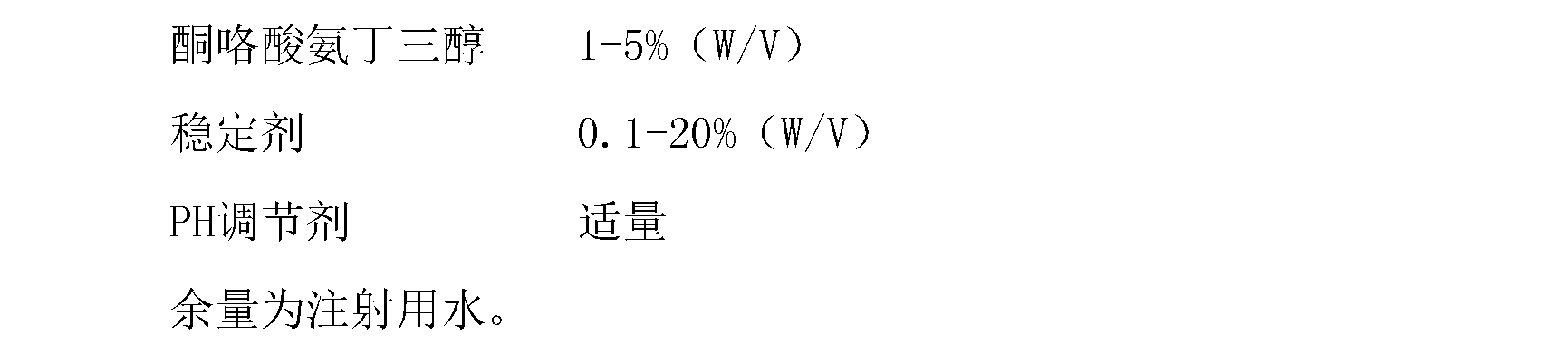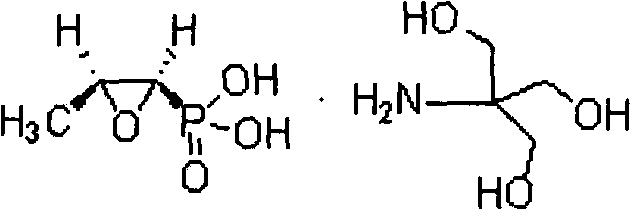Patents
Literature
250 results about "Tromethamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A member of the pyrrolo-pyrrole group of non-steroidal anti-inflammatory drugs (NSAID) with analgesic, anti-inflammatory, and anti-pyretic properties. Tromethamine inhibits both isoforms of cyclooxygenases (COX1 and COX2), thereby blocking the conversion of arachidonic acid to pro-inflammatory pro-prostaglandins. When inhibiting COX2, tromethamine may be effective in relieving pain and inflammation; when inhibiting COX1, this agent may produce unacceptable gastrointestinal side effects.
New product and use and manufacture thereof
InactiveUS20070269386A1Improve buffering effectBiocideNervous disorderCrohn's diseaseNicotine dependence
A pharmaceutical oral formulation for delivering nicotine in any form to a subject by transmucosal uptake in the oral cavity comprising nicotine in any form, wherein said oral formulation is buffered with at least trometamol. Also contemplated is a method for the oral delivery of nicotine in any form, a method for the reduction of the urge to smoke or use tobacco as well as methods for manufacturing the oral formulation, the use of the oral formulation for obtaining transmucosal uptake of nicotine in the oral cavity of a subject, and use of nicotine for the production of an oral formulation for the treatment of a disease selected from the group consisting of tobacco or nicotine dependence, Alzheimer's disease, Crohn's disease, Parkinson's disease, Tourette's syndrome, ulcerous colitis and post-smoking-cessation weight control.
Owner:MCNEIL AB
Ophthalmic composition
ActiveUS20120095097A1Stabilizing vitaminGood effectBiocideSenses disorderOptometryPOLYOXYETHYLENE ETHER
Disclosed is an ophthalmic composition containing (A) not less than 50,000 units / 100 mL of vitamin A, (B) not less than 0.4 W / V % of a polyoxyethylene polyoxypropylene glycol, and (C) trometamol.
Owner:LION CORP
Sacubitril derivatives and medicine compositions, preparation methods and application thereof
ActiveCN105693543AEasy to prepareCrystal form controllableAmino compound purification/separationOrganic compound preparationEthylenediamineArginine
The invention provides sacubitril derivatives and medicine compositions, preparation methods and application thereof and belongs to the fields of medicine compounds and preparation thereof. The sacubitril derivatives comprise sacubitril lithium salt, sacubitril kali salt, sacubitril magnesium salt, sacubitril calcium salt, sacubitril strontium salt, sacubitril zinc salt, sacubitril ferric salt, sacubitril ammonium salt, sacubitril diethylamine salt, sacubitril ethylenediamine salt, sacubitril piperazine salt, sacubitril N-(2-ethoxyl)-pyrrolidine salt, sacubitril choline salt, sacubitril cholamine salt, sacubitril diethanol amine salt, sacubitril triethanolamine salt, sacubitril tromethamine salt, sacubitril meglumine salt, sacubitril diisopropylamine salt, sacubitril tert-butylamine salt, sacubitril N, N'-bis-benzyl ethylenediamine salt, sacubitril L-lysine salt, sacubitril L-arginine salt or sacubitril L-histidine salt.
Owner:SICHUAN HAISCO PHARMA CO LTD
Micromolecular donkey-hide gelatin essence anti-aging facial mask
InactiveCN108785241AStable moisturizingFade fine linesCosmetic preparationsToilet preparationsWrinkle skinChondrus crispus extract
The invention relates to a micromolecular donkey-hide gelatin essence anti-aging facial mask, which contains the following components: glycerin, methylpropanediol, glyceryl polyether-26, 1,2-hexanediol, p-hydroxyacetophenone, butanediol, PEG-60 hydrogenated castor oil, panthenol, xanthan gum, allantoin, carbomer, glycerin polyacrylate, tromethamine, sodium hyaluronate, EDTA disodium, glycerin acrylate / acrylic copolymer, phenoxyethanol, propylene glycol, proplis extract, a PVM / MA copolymer, dipotassium glycyrrhizinate, essence, silk amino acids, beta-glucan, BAMBUSA VULGARIS WATER bambusa vulgaris water, hydrolyzed collagen, rice extract, glycine ussuriensis seed extract, sesame seed extract, chondrus crispus extract, ethylhexylglycerin, polysorbates-20, potassium chloride, locust bean gum,dipotassium phosphate, sodium citrate, hydrolyzed hyaluronic acid, glucose, calcium lactate, glucomannan, sodium acetylated hyaluronate and acetyl hexapeptide-8. The micromolecular donkey-hide gelatin essence anti-aging facial mask has the efficacies of supplementing skin nutrition, moisturizing, removing wrinkles and resisting senility.
Owner:SHAN DONG DONG E E JIAO
Lornoxicam freeze-dried injection and preparation method thereof
ActiveCN101327193AHigh clarityGood formabilityPowder deliveryOrganic active ingredientsMedicineFreeze-drying
The invention relates to lomoxicam freeze-dried powder injection and a preparation method thereof. The freeze-dried powder injection comprises omoxicam, mannite, tromethamine, EDTA and pH regulator. The dosage of the mannite is 3.5 to 8.5 g of mannite per gram of lomoxicam, and the dosage of the EDTA is 0.015 to 0.025g of EDTA per gram of lomoxicam. The invention adopts EDTA to replace EDTA-disodium salt and selects the dosages of the mannite and EDTA so that the clarity of the reconstituted obtained freeze-dried powder is greatly improved, and the formability is good; in addition, the invention adopts two pH regulators, in the process, one pH regulator is used to regulate the solution to a pH range, then the other pH regulator is used to regulate the solution to another pH range, and the freeze drying technology is controlled strictly. The prepared freeze-dried powder injection has great improvement of stability and favorable resolubity.
Owner:HAINAN JINRUI PHARMA CO LTD
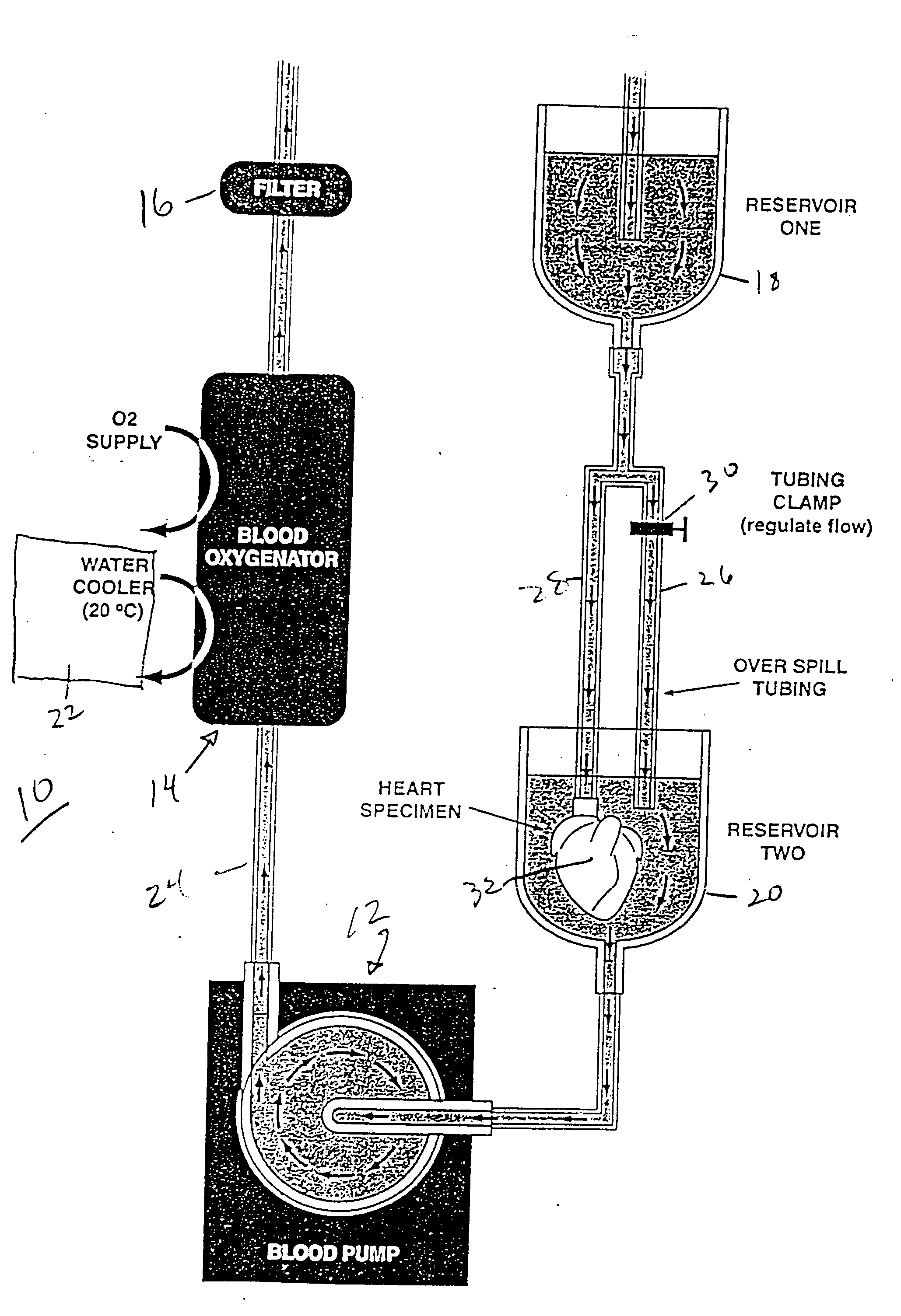
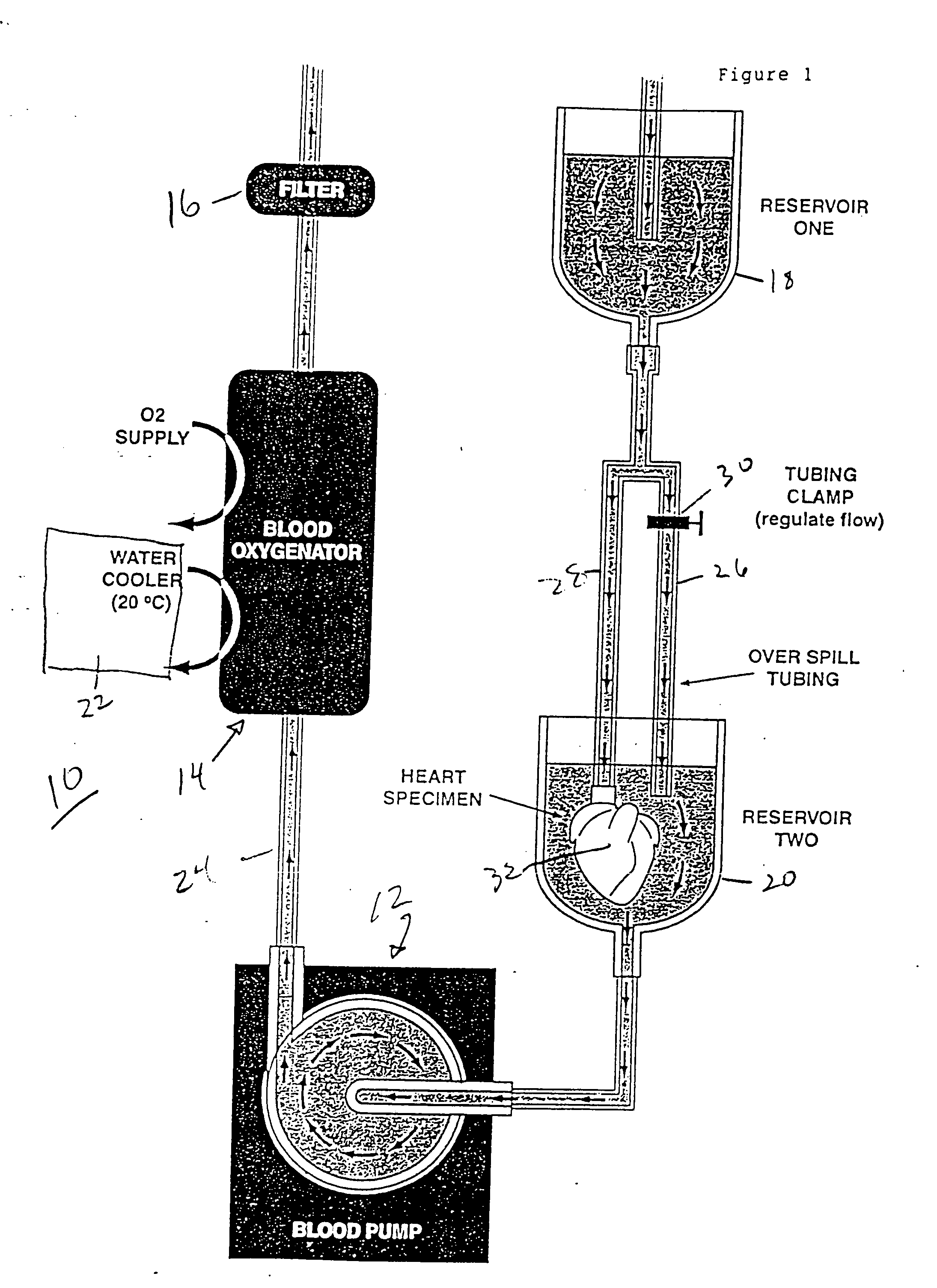
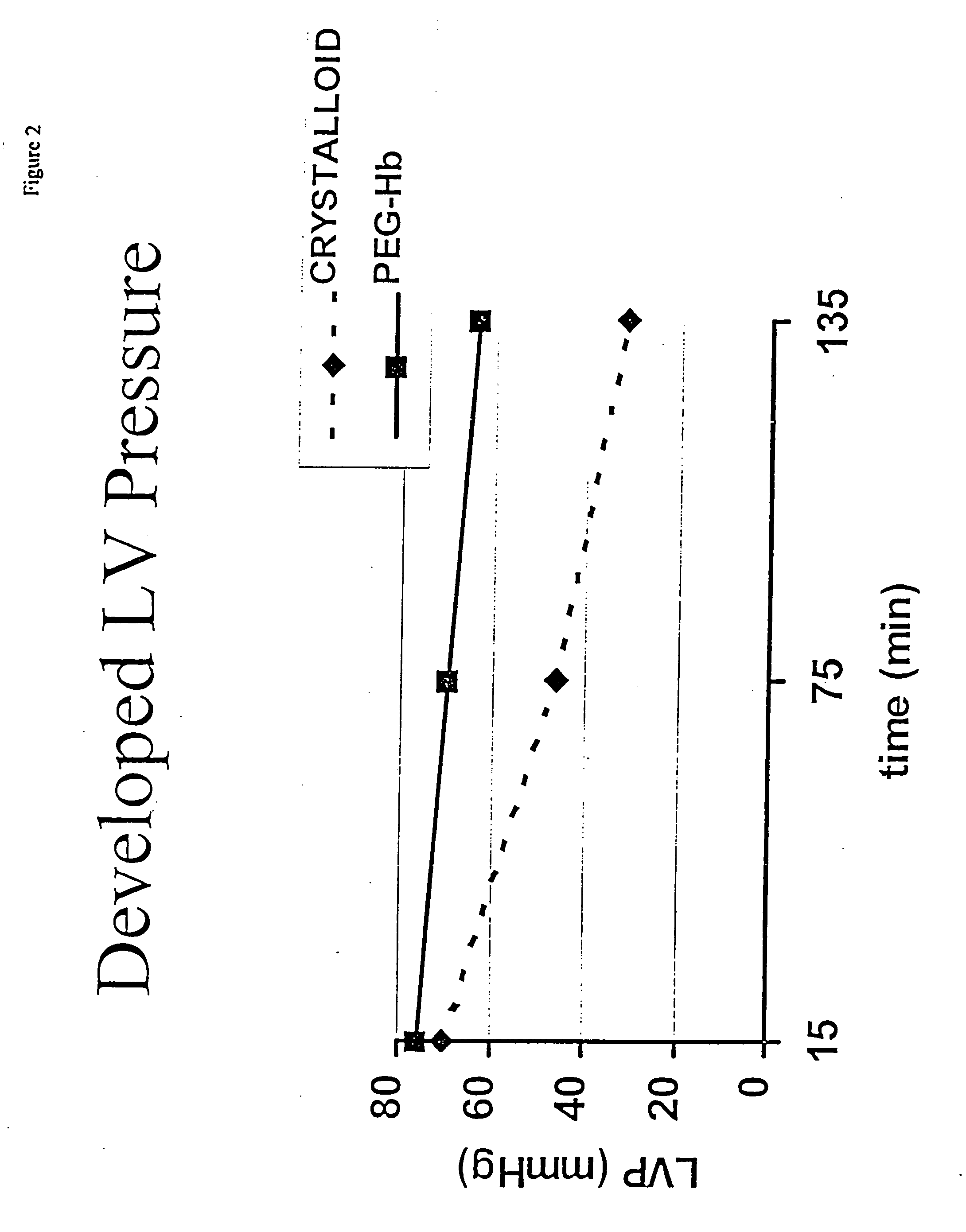
Continuous cardiac perfusion preservation with PEG-HB for improved hypothermic storage
InactiveUS20070243518A1Extended storage timeEasy to storeDead animal preservationHuman albuminPotassium
Efforts to extend myocardial preservation for transplantation by perfusion with prior crystalloid based solutions have been limited by edema and compromised function. Hypothermic perfusion preservation with a polyethylene glycol (PEG) conjugated hemoglobin solution extends preservation times. The polyethylene glycol (PEG) conjugated hemoglobin solution comprises PEG-Hb, and at least one of the constituents selected from the group of human albumin, dextrose, heparin sodium, lidocaine HCl, MgSO4, KCl, CaCl2, Tromethamine (THAM) solution, NaCl, NaHCO3, and Na2HPO4 / NaH2PO4. Comparison of cardiac function after continuous perfusion using a hypocalcemic normokalemic crystalloid perfusate is made with and without the addition of PEG-Hemoglobin (Hb).
Owner:RGT UNIV OF CALIFORNIA
Whole blood genomic DNA high-flux plate type extracting kit and extracting method
InactiveCN106636064AImprove throughputHigh-throughput Whole Blood DNA ExtractionDNA preparationMagnetic beadChelex 100
Owner:LUOYANG G N T BIOLOGICAL TECH CO LTD
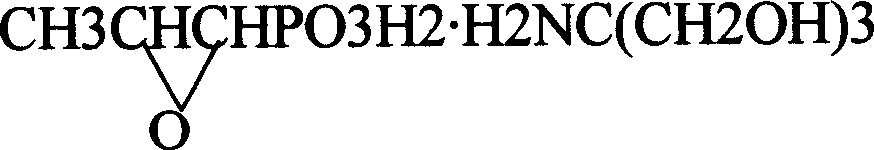
Novel pulveres fosfomycin trometamol synthetic method
InactiveCN1544440ASolve the problem of rework and reuseIncrease production capacityGroup 5/15 element organic compoundsOrganic acidPhosphonomycin
The invention provides a process for synthesizing pulveres fosfomycin trometamol by using phosphonomycin sodium or neutral phosphonomycin sodium containing small amount of organic acid as raw material through the steps of, subjecting 10-16 times of type H cationic ion-exchange resin to exchange reaction in methanol solution at low-temperature, with the methanol solution acting as eluent, thus preparing the methanol solution containing fosfomycin acid, neutralizing the methanol solution with right amount of tromethamine or fosfomycin methanol tromethamine salt so as to obtain the methanol solution of pulveres fosfomycin trometamol, and the end product of the methanol solution of pulveres fosfomycin trometamol is obtained from the methanol solution of pulveres fosfomycin trometamol from decolorizing, filtering, condensing, extracting, filtering and drying.
Owner:NORTHEAST PHARMA GRP
Gargle for relieving pain for oral inflammation disease
InactiveCN101559077ANot pollutedGuaranteed chemical stabilityInorganic boron active ingredientsHydroxy compound active ingredientsDiseaseSodium bicarbonate
The invention belongs to the technical field of medicine, in particular to a drug composition of gargle which contains sodium dichlorophenolate and can relieve pain and be anti-inflammatory for the oral inflammation disease; wherein the drug composition comprises the following components by percentage: 0.10 to 30 percent of sodium dichlorophenolate hydroxypropyl-Beta-cyclodextrin inclusion (equal to 0.065 to 0.20 percent of concentration of sodium dichlorophenolate), 0.01 to 0.10 percent of lidocaine hydrochloride, 0.10 to 5.0 percent of borax, 0.1 to 2.0 percent of boric acid, 0.1 to 10 percent of glycerin, 0.10 to 5.0 percent of sodium bicarbonate, 0.001 to 0.10 percent of vitamin B12, 0.02 to 1.0 percent of tromethamine, 0.01 to 5.0 percent of sucralose, 0.10 to 5.0 percent of peppermint essence and 0.01 to 1.0 percent of sodium benzoate; a proper amount of pharmaceutical caramel color and water for injection is added till to reach the needed weight / volume concentration. All the above components are weighted by weight / volume percentage; the aqueous solution of the composition has the pH value of 6.5 to 9.0. The composition has little thrill to oral mucosa, stable storage for long time and good biological tolerance and has fast and long-lasting curative effect for relieving pain and being anti-inflammatory to the oral inflammation disease.
Owner:官培龙 +1
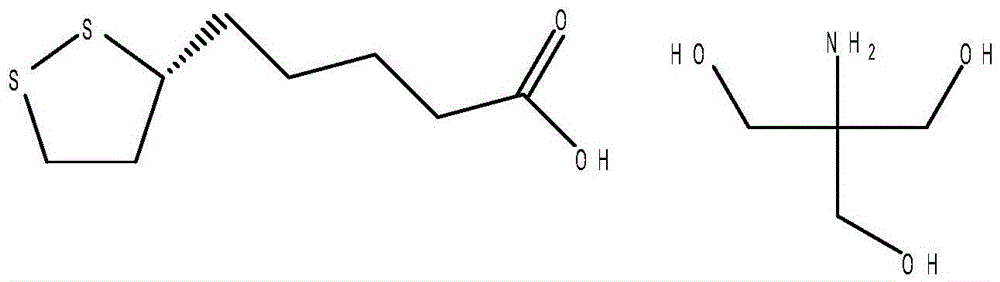
Preparation method of R-lipoic acid tromethamine salt
InactiveCN105622571AEmission reductionReduce process stepsOrganic chemistryOrganic solventFiltration
The invention discloses a preparation method of R-lipoic acid tromethamine salt and belongs to the field of organic medicinal chemistry. The method includes the steps that (S)-6,8-dichloro ethyl caprylate and sulphur are put into a reaction vessel, the temperature is raised, a cyclization reaction is carried out, the temperature is preserved, extraction is carried out with a first organic solvent, concentration is carried out, and cyclization liquid is obtained; then, a hydrolysis reaction is carried out, cooling is carried out, and hydrolysis liquid is obtained; a second organic solvent is added into the hydrolysis liquid, the pH value is regulated, extraction is carried out, an obtained organic layer is washed with water to be neutral, the second organic solvent is removed at reduced pressure, and an initial product is obtained; mixed liquor is added into the initial product, the temperature is raised, a first filter aid is added, stirring adsorption is carried out, filtration is carried out, and light yellow liquid is obtained; cooling is carried out to separate out crystals, and R-lipoic acid is obtained; R-lipoic acid is dissolved, trihydroxymethyl aminomethane is added, the temperature is raised for solution, a second filter aid is added for filtration, and light yellow liquid is obtained; cooling is carried out to separate out crystals, centrifugal drying is carried out, and the finished product is obtained. According to the method, few steps are needed, efficiency is high, energy is saved, and waste discharge is reduced.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
pharmaceutical composition
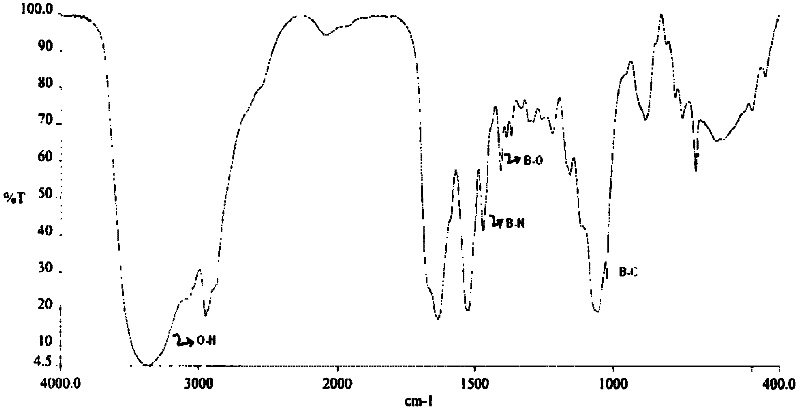
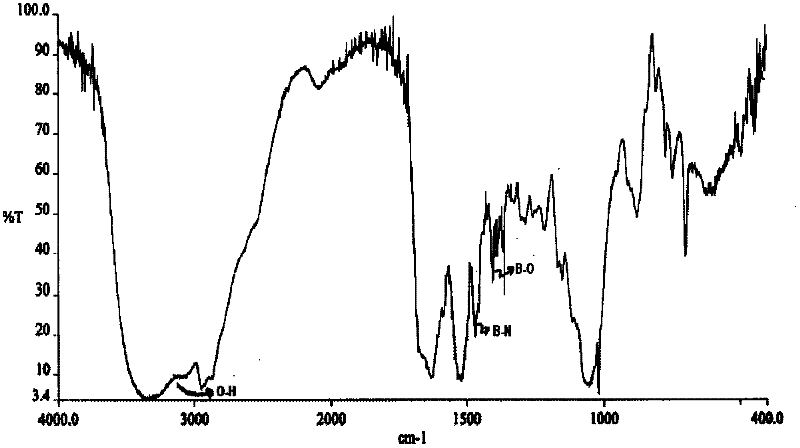
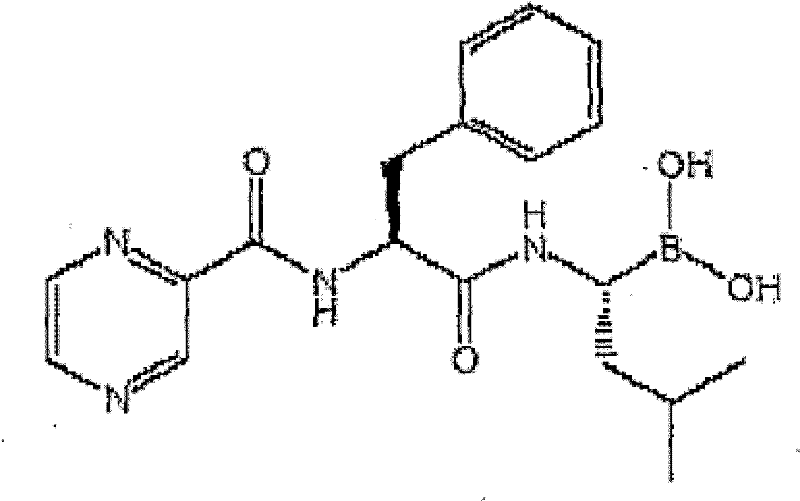
The present invention relates to a parenteral pharmaceutical composition comprising therapeutically effective amounts of N-(2-yrazine) carbonyl-L-phenylalanine-L-leucine boronic acid or its salts or its derivatives and tromethamine wherein the composition is stable.
Owner:SUN PHARMA INDS
Method for improving the oral administration of alpha-lipoic acid
InactiveUS20070196442A1Reducing esophageal irritationGood water solubilityBiocideFood ingredient as antioxidantSolubilityOral medication
The invention provides a method for reducing esophageal irritation associated with alpha-lipoic acid upon oral administration through the use of the trometamol salt of alpha-lipoic acid. The present invention also provides for the increased solubility of alpha-lipoic acid through the use of the trometamol salt of alpha-lipoic acid. The trometamol salt of alpha-lipoic acid, as provided by the present invention can be used as a substitute for regular, non-salt forms alpha-lipoic acid in dietary supplement compositions.
Owner:MULTI FORMULATIONS LTD
Method for preparing ketorolac tromethamine
ActiveCN101143865AThe synthetic route is simpleReduce pollutionOrganic chemistryKetorolac TromethamineNon steroidal anti inflammatory
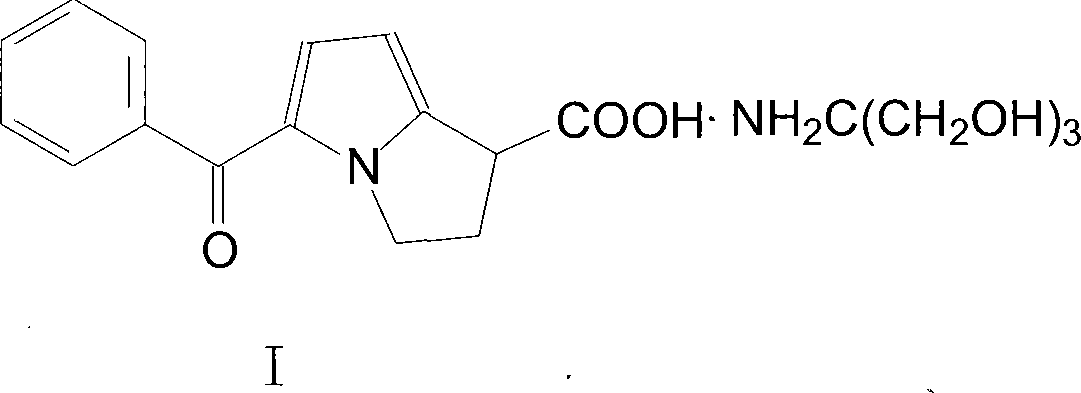
The present invention relates to a non-steroidal anti-inflammatory drug of ketorolac tromethamine salt, which has the functions of strong acesodyne, moderate anti-inflammation and antipyresis. The present invention applies a synthesis route preparing the compound ketorolac tromethamine salt, the technique applies an one-pot method, the reaction process from 2-benzoyl pyrrole to the ketorolac tromethamine salt can be continuously carried out, extraction and purification are not needed, materials can be easily obtained, labor intensity is reduced, the operational environment is improved, the cost of industrialized production is greatly reduced, environmental pollution is reduced, the loss of materials and products is reduced, and the yield rate is increased.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation method of fosfomycin amine salt
ActiveCN102807586ALow costReduce energy consumptionGroup 5/15 element organic compoundsOrganic solventIon exchange
The invention discloses a preparation method of fosfomycin amine salt, which comprises the following steps of: in organic solvent, enabling fosfomycin phenylethylamine salt to react with isocyanate, isothiocyanate, ketene, dipolymer of isocyanate, dipolymer of isothiocyanate or dipolymer of ketene, and tromethamine; or in the organic solvent, enabling fosfomycin bistromethamine to react with isocyanate, isothiocyanate, ketene, dipolymer of isocyanate, dipolymer of isothiocyanate or dipolymer of ketene. The preparation method provided by the invention has the advantages that the fosfomycin amine salt can be prepared in one step only, a low-temperature ion exchange column is not required, the yield is high, the cost is low, the waste gas, waste water and waste residue are less, the reaction conditions are moderate, the energy consumption is low, the operation is convenient to conduct and the method is suitable for industrial production.
Owner:NORTHEAST PHARMA GRP
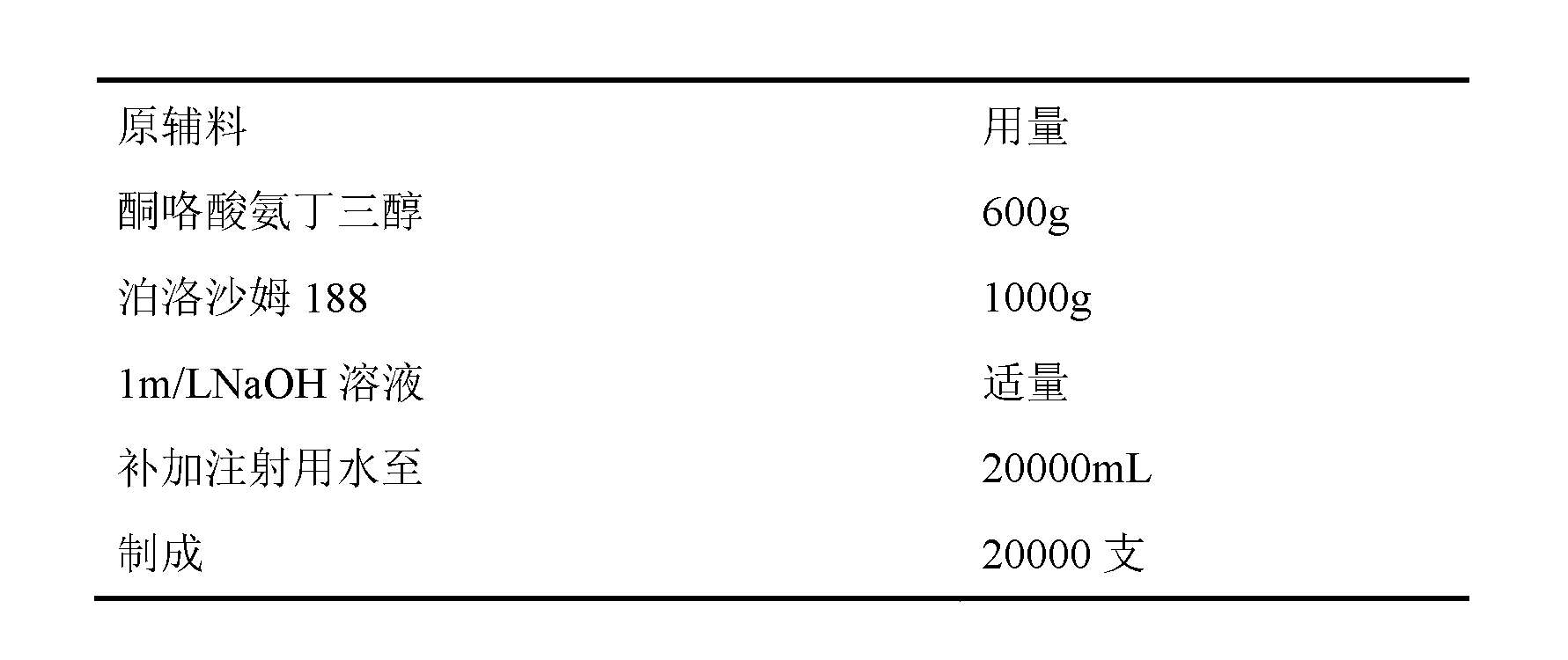
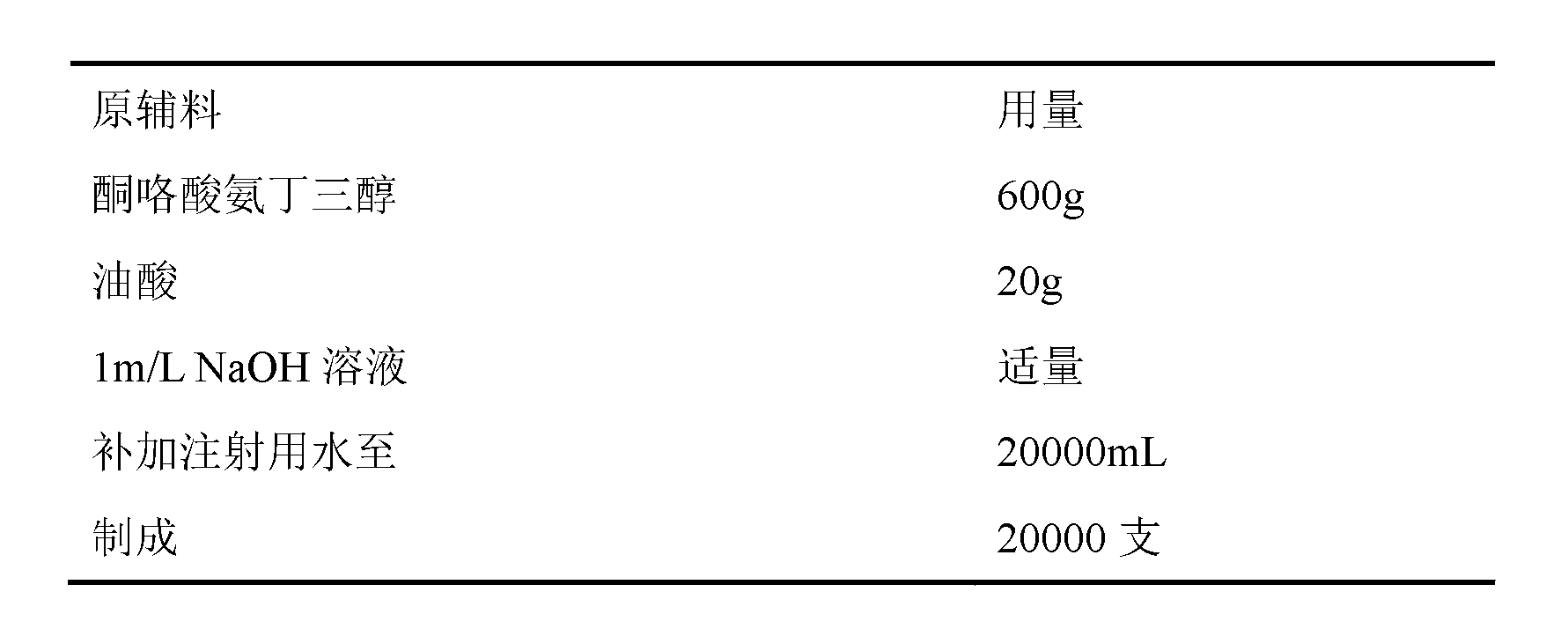
Ketorolac tromethamine injection
InactiveCN102846542ASolve easy discolorationConvenient amountOrganic active ingredientsAntipyreticUse medicationIrritation
The invention discloses a prescription of a ketorolac tromethamine injection and a preparation method. The injection provided by the invention can not only effectively solve the problem that the existing ketorolac tromethamine injection containing ethanol causes irritation while being injected and improve the safety of the drugs and the compliance of the drugs, but also completely avoid the white points caused by the traditional technology after sterilization treatment, and thus the ketorolac tromethamine injection is good in stability, high in safety, reliable in quality and significant in efficacy.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Method for synthesizing 1,2,4-butanetriol
InactiveCN101333151AAvoid introducingNo pollution in the processPreparation by oxygen reductionEpoxideTromethamine
The invention provides a synthesis method for 1,2,4-tromethamine, comprising the following steps: the mixed solution of butene diol, tungsten acid and N-methyl morpholine, and epoxidizing agent are mixed through a mixer which is provided with two channel nozzles and arranged at the top part of a reactor, and then enter the reactor for reaction; the material in the reactor is sent to a nozzle at the top part of the reactor through a circulating pump for recycling use to collect epoxide; the epoxidizing agent is selected from oxygen-rich gases; in the presence of catalysts, the epoxide and ethanol go through hydrogenation reaction, then the 1,2,4-tromethamine is collected. The invention adopts oxygen-rich gas as epoxidizing agent and avoids the introduction of unnecessary media in the reaction system through the pre-mixing in a mixer provided with two channel nozzles; besides, the method of the invention has easily-controlled reaction temperature, less equipment investment and mild operating conditions, which does not use the traditional mercury catalyst and has no pollution to the environment; the reaction yield of the method can reach more than 95%, so the method is easy in industrialized implementation.
Owner:SHANGHAI XUKAI SCI TECH DEV
Ibuprofen medicinal composition
ActiveCN101978945AGood water solubilityImprove stabilityOrganic active ingredientsAntipyreticSodium lactateArginine
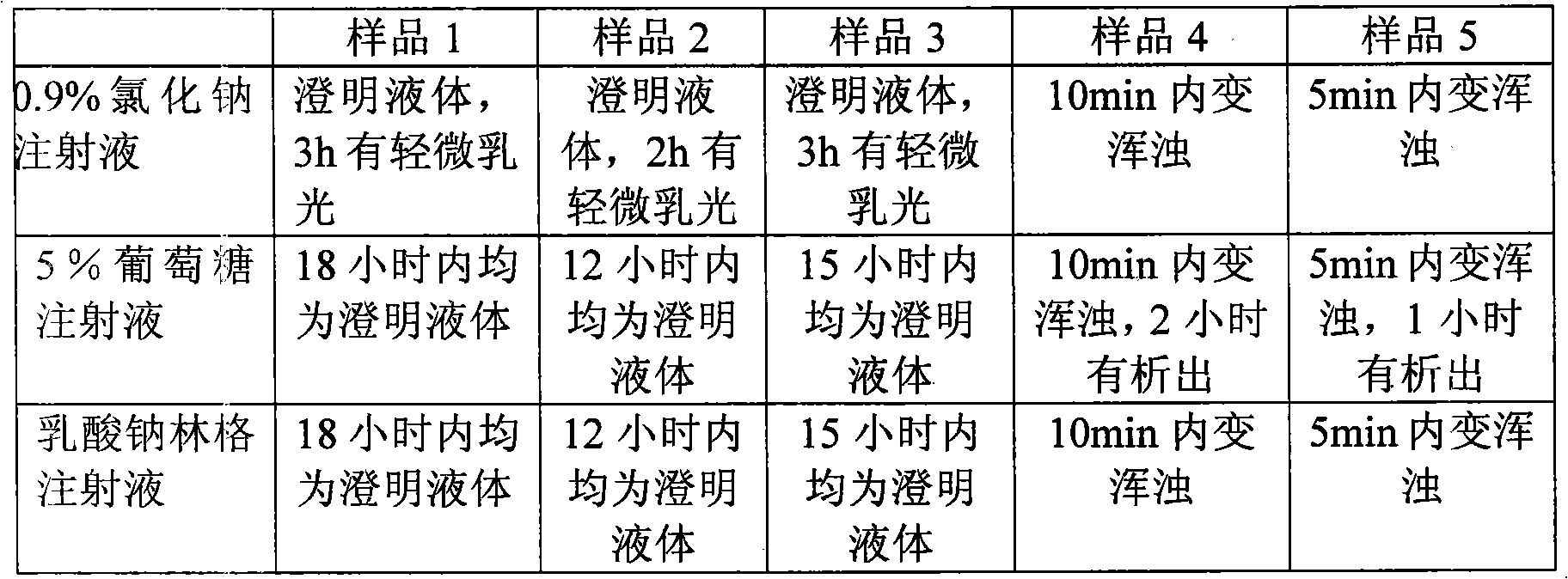
The invention discloses an ibuprofen medicinal composition for injection. By adding tromethamine into solution of ibuprofen and arginine, the stability of the ibuprofen medicinal composition in clinical application is improved. Experiments prove that: after being diluted by the sodium chloride injection or glucose injection or sodium lactate Ringers injection, the composition solution can be stably stored for at least 12 hours.
Owner:茂裕环保科技南通有限公司
Pemetrexed salt and preparation method thereof
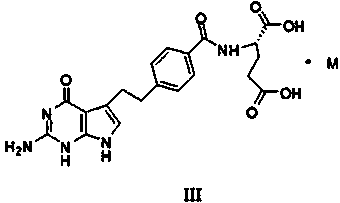
The invention discloses a pemetrexed salt and a preparation method and use thereof, the pemetrexed salt can be dipotassium salt, arginine salt, heme-L-lysinate salt and tromethamine salt of the pemetrexed, and the pemetrexed salt can be used for treatment of various cancers such as mesothelioma, lung cancer and the like.
Owner:CHONGQING PHARMA RES INST
Salts with CRTH2 antagonist activity
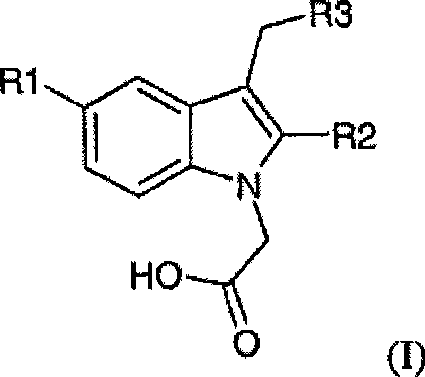
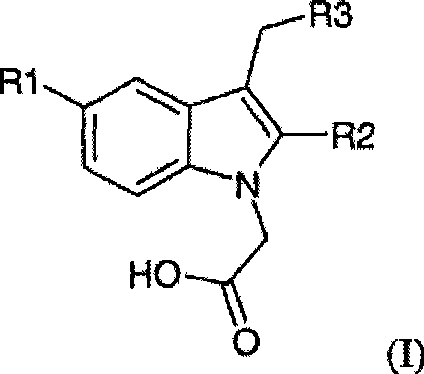
The potassium, sodium, ammonium, lysine, diethylamine, tromethamine (TRIS), piperazine, ethylenediamine and ethanolamine salt of a compound of general formula (I): wherein R<1> is halo or cyano; R<2> is C1-C4 alkyl; and R<3> is quinolyl or phenyl substituted with methane sulfonyl; can be synthesised by a novel method and are substantially more soluble than the parent free acids in a range of solvents.
Owner:OXAGEN
Injection octreotide acetate lyophilized composition and preparation method thereof
ActiveCN103932996AQuality improvementReduce typesPowder deliveryPeptide/protein ingredientsOctreotide acetateTromethamine
The invention discloses an injection octreotide acetate lyophilized composition and a preparation method thereof. The lyophilized composition comprises octreotide acetate, tromethamine and maleic acid. The preparation method comprises the following steps: weighing octreotide acetate and tromethamine, adding into injection water which is cooled to 10-20 DEG C, and stirring to dissolve; regulating the pH of the liquid medicine to be 4.0-5.0 with maleic acid, filtering, filling and lyophilizing. Compared with the prior art, the lyophilized composition is stable in quality, few in auxiliary varieties and simple in preparation process.
Owner:南京易亨制药有限公司
Blood ammonia reagent kit for enzyme detection
The reagent kit for enzyme detection of blood ammonia consists of tromethamine in 50-150 mmol, Tween-20 in 0.1-2 ml, sodium azide om 0.5-2 g, lactose in 5-50 g, glutamate dehydrogenase in 500-1500 u, alpha-ketoglutaric acid in 2-20 mmol, sodium acetonate in 1-10 mmol, and reducing nicotin-amide-adenine dinuclectide phosphate in 0.1-0.5 mmol. After regulating pH to optimal value of 8.0, the components are dissolved, packed and freeze dried and may be cold stored for two years. After being re-dissolved, the components are stabilized at room temperature for 1 week and cold stored to stabilize for 1 month. The reagent kit has powerful interference resistance, medium hemolysis and chylemia, blood sugar below 20 mmol / L, bilirubin below 0.1 mmol / L, enzyme activity loss less than 5 %, MADPH loss less than 10 %, linearity reaching 0.2 mmol / L, CV inside batch of 3.8 % and CV between batches of 5.1 %, and recovering rate of 96.1 %.
Owner:HARBIN MEDICAL UNIVERSITY
Moisture-retaining body cream
InactiveCN105496832AGood sedative and astringent effectGood soothing and anti-inflammatoryCosmetic preparationsToilet preparationsGlycolic acidArginine
The invention discloses a moisture-retaining body cream. The moisture-retaining body cream employs tromethamine to adjust the acid-base property of a body, utilizes glycolic acid to exert exfoliating and whitening effect on the skin, has good moisture-retaining and moisture-supplementing effect due to usage of hyaluronic acid and arginine, exerts good calming and astringing effect on the skin in virtue of sesame flower extract and has good soothing and anti-inflammation effect on the effect as sensitive plant extract and cactus flower extract are used. The moisture-retaining body cream is especially applicable to skin moistening in autumn and winter.
Owner:杨丽莉
Ozagrel tromethamine, compound, preparation method and application thereof
ActiveCN101659640AGood water solubilityExcellent long-term storage stabilityOrganic active ingredientsOrganic chemistryConvulsionSolubility
The invention provides an ozagrel salt which is stable, has functions of inhibiting platelet aggregation and removing blood-vessel convulsion, has good water solubility, and is prepared into a compound suitable for clinical application. The new compound is ozagrel tromethamine; and the ozagrel and the tromethamine are prepared by reaction in a solvent. The compound has good water solubility, strong and long-term stability, and the functions of inhibiting platelet aggregation and removing blood-vessel convulsion.
Owner:徐华
Pharmaceutical composition of ibuprofen for injection
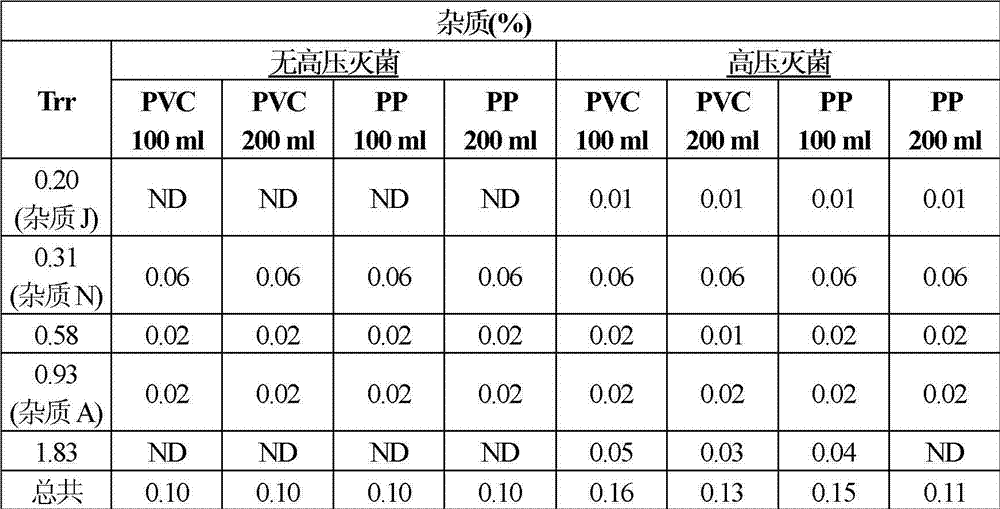
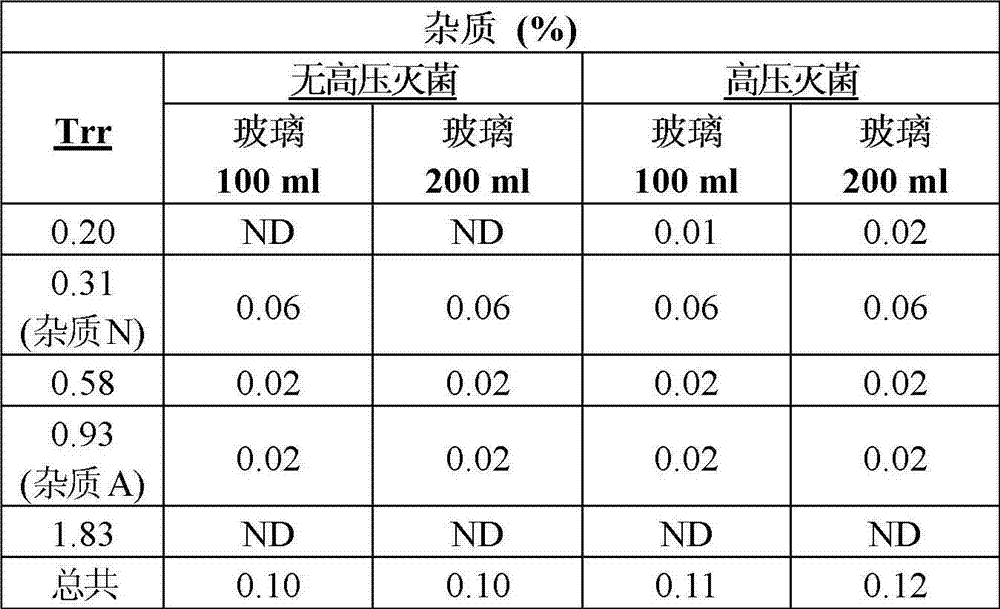
Pharmaceutical composition of ibuprofen for injection that comprises an aqueous solution of ibuprofen and trometamol. These compositions display a minimal loss of active principle and acceptable increase of impurities after autoclaving, properties that have been demonstrated in various types of containers, such as containers made of plastics such as polypropylene, PVC and polyethylene, as well as in glass containers. These compositions, after undergoing autoclaving, still comply with all the relevant technical specifications of the European Pharmacopoeia and of the USP.
Owner:HANNFAMA LAB LLC
Sub-muscular essence and preparation method thereof
ActiveCN110693807AEasy to synthesizeAchieve fixCosmetic preparationsToilet preparationsGlycerolAcetophenone
The invention discloses a sub-muscular essence and a preparation method thereof, belongs to the technical field of skin care products, and solves the problem an anti-aging function cannot be effectively achieved in the prior art. The sub-muscular essence is prepared from a group A, a group B, a group C, a group D and the balance water, the group A comprises, in weight percent, 1-4% of glycerin polyether-26, 1-4% of 1, 3-propylene glycol, 1-4% of moisturizing agents A, 1-4% of moisturizing agents B, 1-4% of conditioners A, 1-4% of solvents A, 0.4-2% of 1, 2-hexylene glycol, 0.4-1% of p-hydroxyacetophenone, 0.05-0.5% of ammonium acrylate dimethyl taurine / VP copolymers, 0.03-0.5% of xanthan gum and 0.05-0.15% of EDTA (ethylenediamine tetraacetic acid) disodium salt, the group B comprises, inweight percent, 1-6% of butylene glycol and 0.05-0.4% of carbomer, the group B comprises, in weight percent, 0.05-0.4% of tromethamine, and a group D comprises active substances. According to the sub-muscular essence, excitation of self-activating repair is matched with enhancement of skin barriers, so that overall anti-aging effects are achieved.
Owner:浙江英树生物科技有限公司
Application of tromethamine in drugs for treating hyperuricemia and related disease
InactiveCN105853401APromote dissolution and excretionStone treatment or preventionOrganic active ingredientsSkeletal disorderSodium bicarbonateDisease
The invention discloses application of tromethamine in drugs for treating hyperuricemia and related disease. The tromethamine can serve as a drug in application of treating hyperuricemia, urinary system uric acid calculi and gout, an adult takes 1-7 g of the dose every day, and the medication method is intravenous drip. The tromethamine is used for promoting uric acid to dissolve and discharge, no inorganic ions exist because the tromethamine is organic amine alkali, the inhibiting effect of the inorganic ions can be avoided or weakened, the effect of the tromethamine for promoting uric acid to dissolve and discharge is better than that of sodium bicarbonate, and the tromethamine can be used for treating hyperuricemia, urinary system uric acid calculi and gout. Besides, the tromethamine is a foreign substance, an organic body cannot produce the tromethamine substance, and accordingly, the concentration of the tromethamine in the body is better to control compared with the sodium bicarbonate in the medication process.
Owner:YUNNAN UNIV OF TRADITIONAL CHINESE MEDICINE
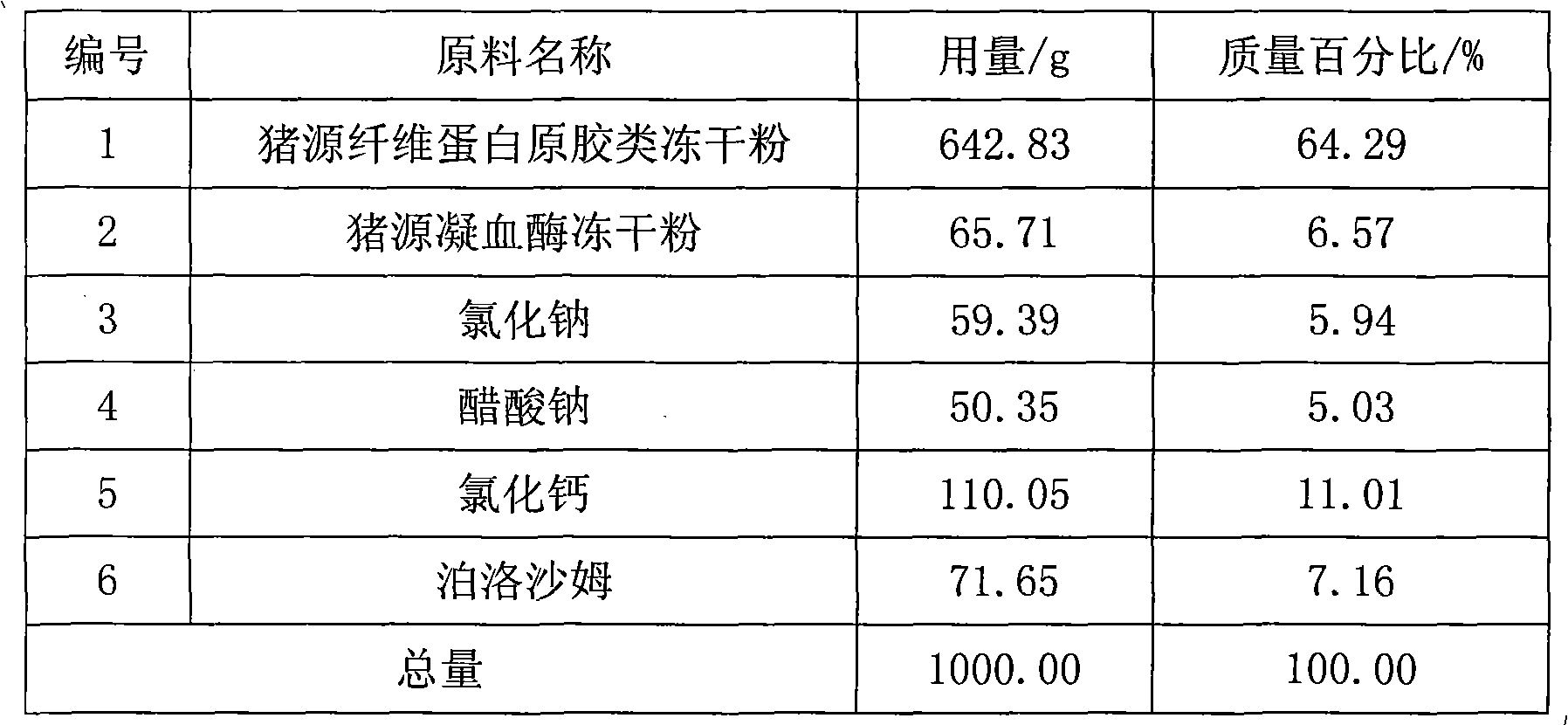
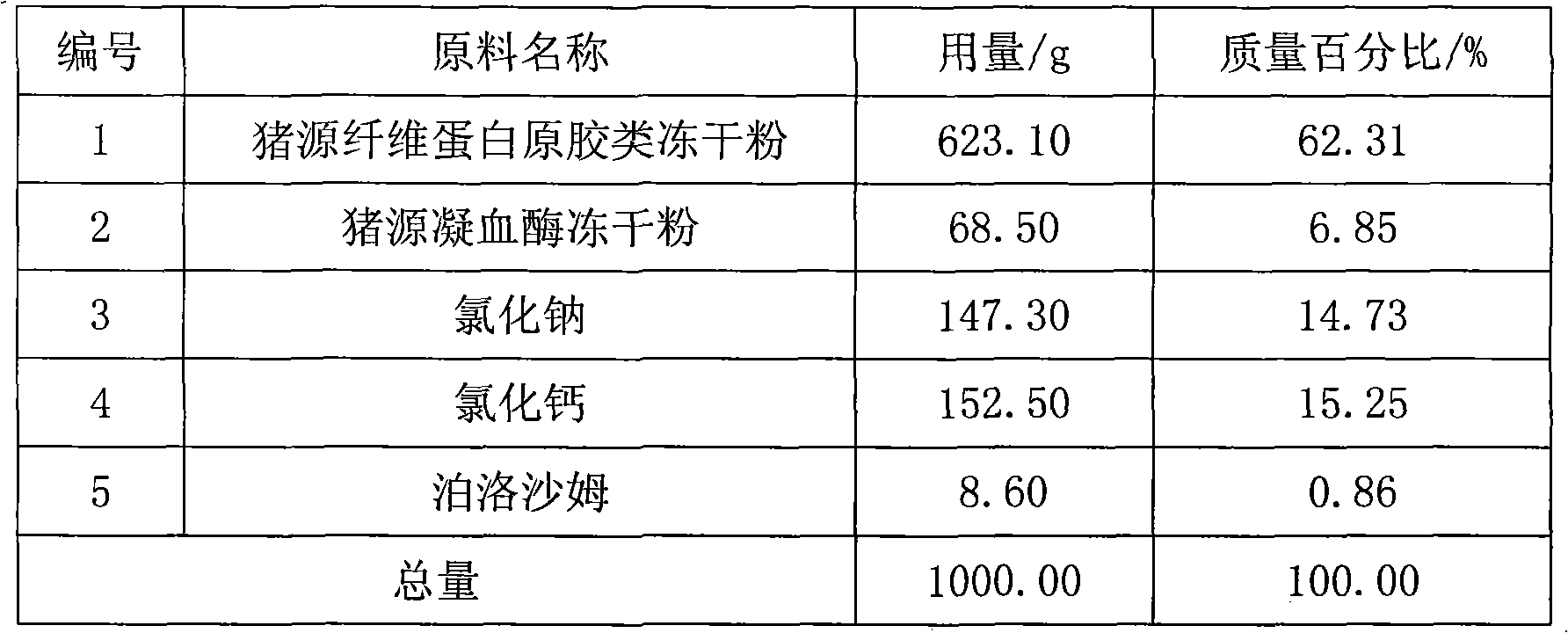
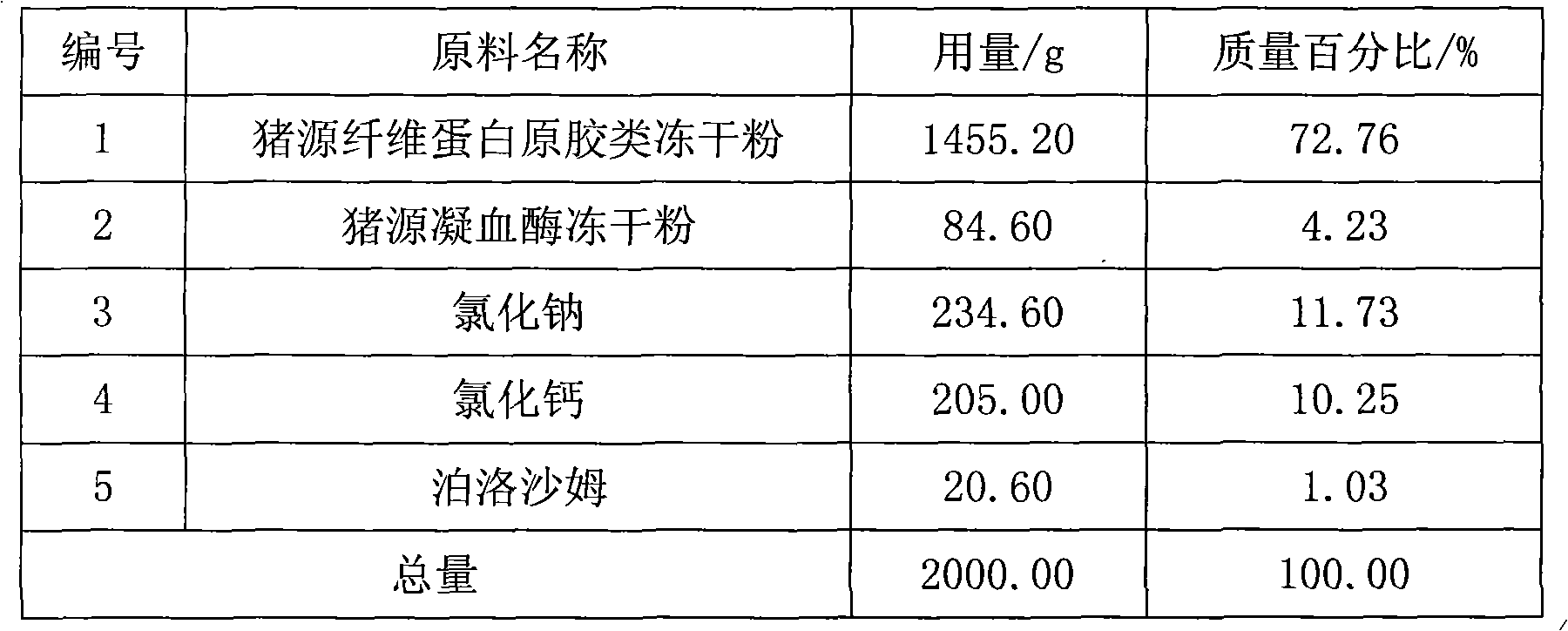
Swine fibrin glue powder inhalation and preparation method and application thereof
ActiveCN101890181AReasonable compositionSimple preparation processPeptide/protein ingredientsAerosol deliverySodium acetateFibrin glue
The invention relates to a swine fibrin glue powder inhalation and a preparation method and application thereof. The swine fibrin glue powder inhalation comprises swine fibrin original gum lyophilized powder, swine thrombin lyophilized powder, sodium chloride and / or sodium acetate, calcium chloride and / or tromethamine and a physiologically acceptable antistatic agent. The preparation method comprises the following steps of: grinding the components into powder with suitable particle size, then uniformly mixing the powder and filling the mixture. The product is stable and convenient to carry and store and can be directly used without preparation. The swine fibrin glue preparation is sprayed on a wound surface in a powder shape, saves time for operation and wound packing, brings great convenience, has a simple production process and is safe and environmentally-friendly.
Owner:GUANGZHOU BIOSEAL BIOTECH
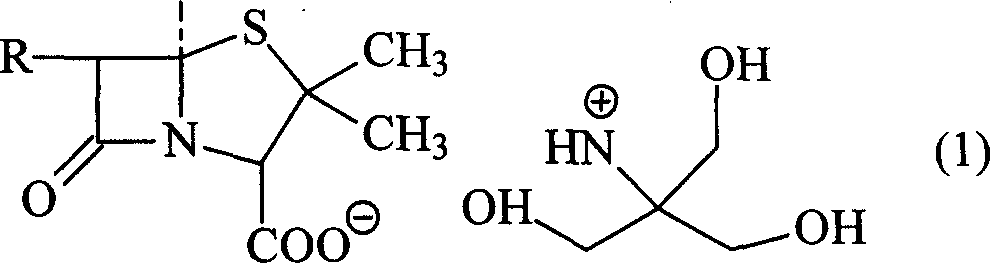
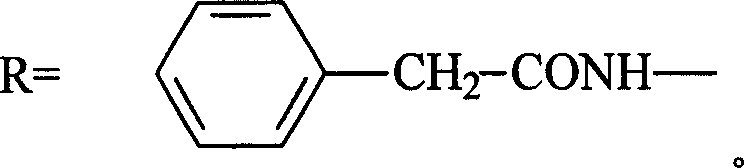
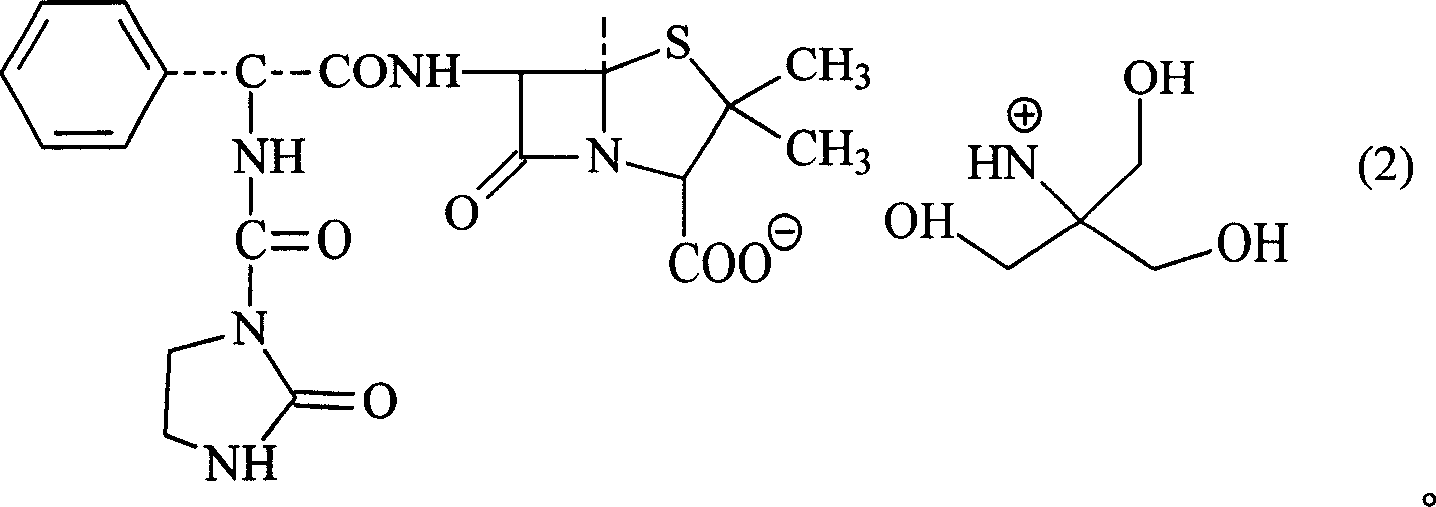
Trometamol salt in compound of cillin category, and preparation method
InactiveCN101003539AReduce intakeAvoid the risk of hypernatremiaAntibacterial agentsOrganic chemistryFlucloxacillinFloxacillin
This invention discloses trometamol salts of cillin compounds, or their hydrates that can be used to treat bacillosis. As hown in formula 1, the salts comprise trometamol salts of azlocillin, amoxicillin, ampicillin, oxazacillin, furbucillin, cloxacillin, mezlocillin, mecillinam, piperacillin, ticarcillin, floxacillin, hetacillin, cloxacillin and penicillin. This invention provides drug compositions with the salts shown in formula 1 as the active components, and their application in drugs for treating bacillosis.
Owner:GUANGDONG ZHONGKE DRUG R&D
Synthetic method of water-solubility biocompatibility monodisperse spherical gold nanometer crystals
Provided is a synthetic method of water-solubility biocompatibility monodisperse spherical gold nanometer crystals. The synthetic method of the water-solubility biocompatibility monodisperse spherical gold nanometer crystals comprises the following steps (1) using ultra-pure water to prepare a sodium citrate solution, a chloroauric acid solution, a silver nitrate solution and a glutathione solution or a tromethamine solution; (2) mixing the ultra-pure water, the sodium citrate solution, the chloroauric acid solution and the silver nitrate solution together to form a premixed solution, adding the glutathione solution or the tromethamine solution into the premixed solution to form a mixed solution; when the silver nitrate solution and the ultra-pure water are not used, solutions does not need to be mixed; (3) rapidly injecting the mixed solution into the boiling ultra-pure water which is used in a reaction, or directly injecting the solutions into the boiling ultra-pure water which is used in the reaction; and (4) carrying out heating reflux on the mixture, and naturally cooling extraction to room temperature. The synthetic method of the water-solubility biocompatibility monodisperse spherical gold nanometer crystals has the advantages of being simple in operation, good in repeatability, capable of obtaining the water-solubility biocompatibility monodisperse spherical gold nanometer crystals which are high in quality and can not be obtained in other existing methods.
Owner:SHANDONG UNIV
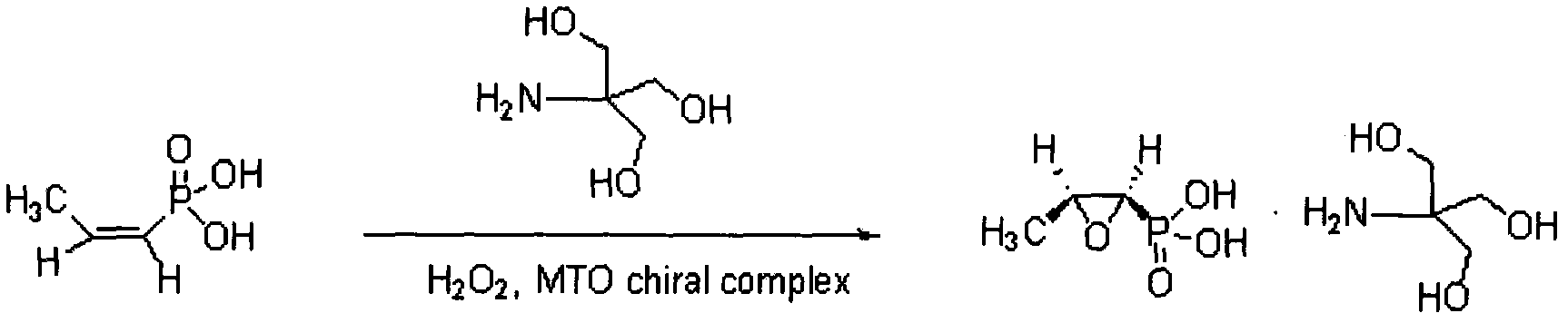
New method for synthesizing fosfomycin trometamol
InactiveCN102659842ARaw materials are cheap and easy to getLow toxicityOrganic compound preparationGroup 5/15 element organic compoundsPhosphonomycinO-Phosphoric Acid
The invention relates to a new method for synthesizing fosfomycin trometamol, which comprises the following steps: directly oxidizing under the catalysis of a chiral ligand through maleic phosphoric acid, obtaining optically-pure free fosfomycin acid and adding an equivalent of tromethamine for neutral reaction to obtain fosfomycin trometamol.
Owner:FARMASINO PHARMA ANHUI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com