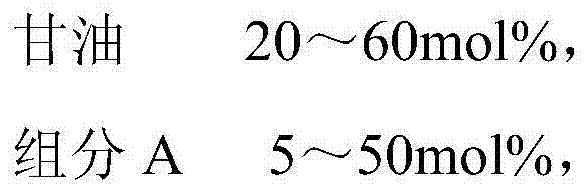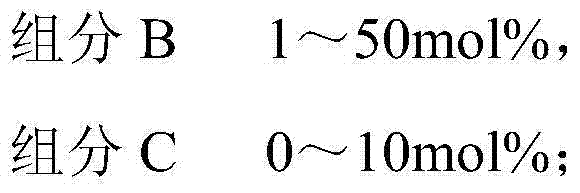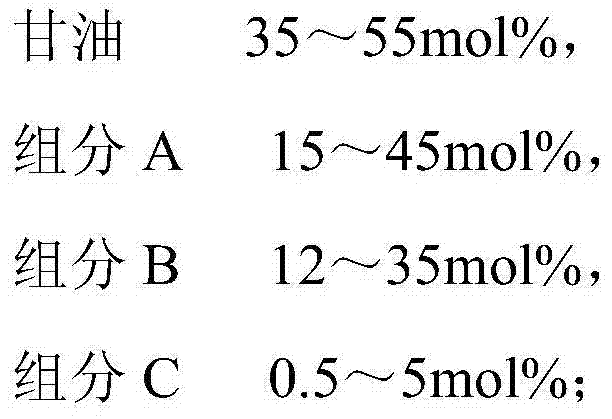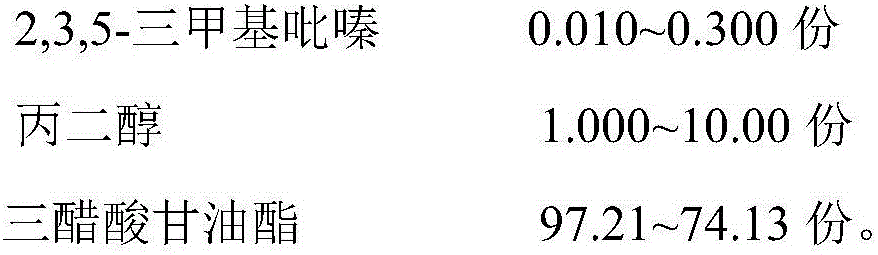Patents
Literature
80 results about "Ethyl caprylate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
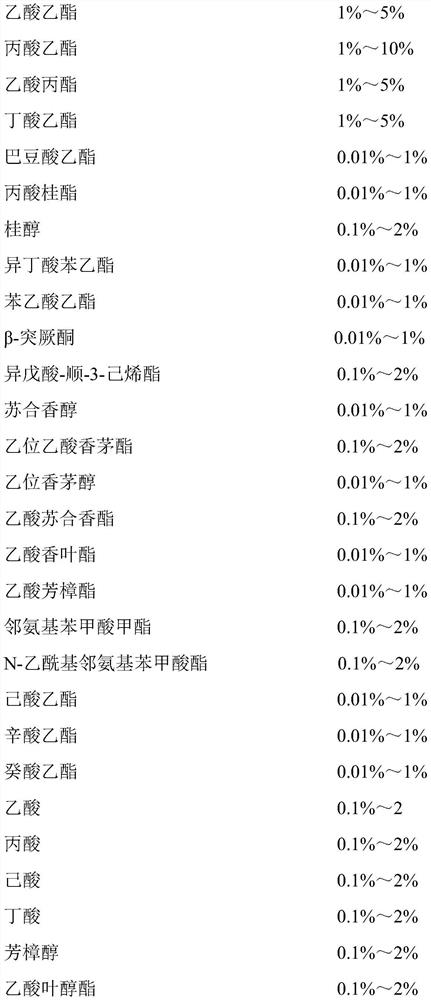
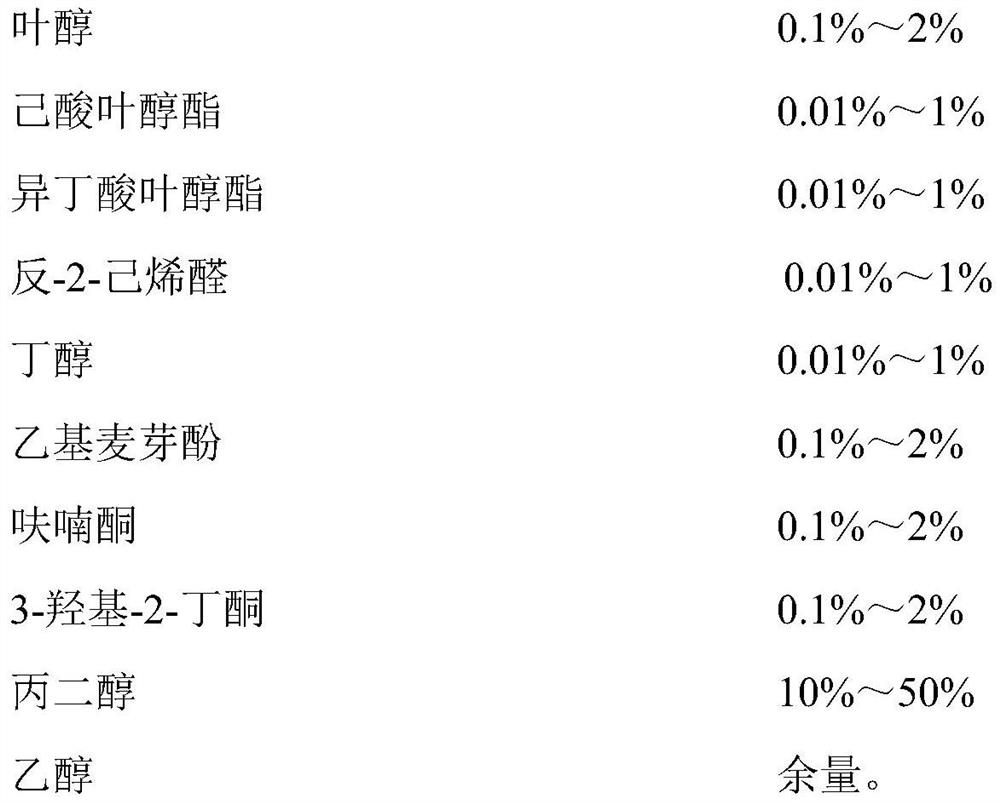
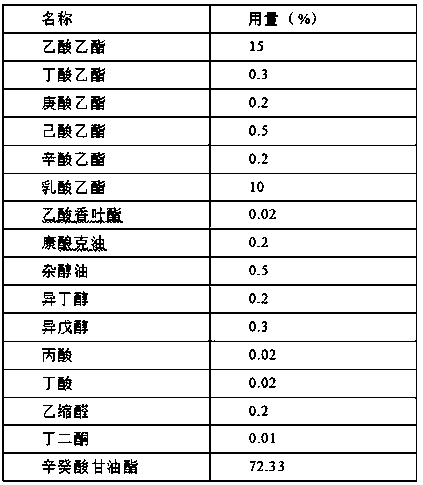
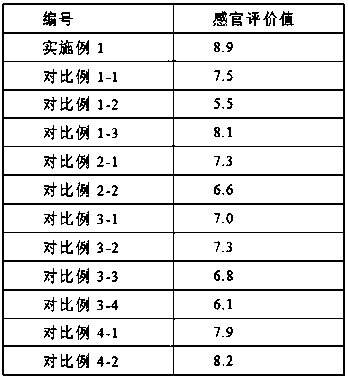
Ethyl octanoate, also known as ethyl caprylate, is a fatty acid ester formed from caprylic acid and ethanol. It has the semi-developed formula of CH 3 (CH 2 ) 6 COOCH 2 CH 3 , and is used in food industries as a flavoring and in the perfume industry as a scent additive.
Milk powder essence
The invention relates to a formulation for milk pulverous flavor and a preparation method thereof. The flavor mainly comprises powdered glucose, ethyl maltol, gamma-Nonanolactone, delta dodecalactone, vanillin, ethyl vanillin, ethyl caprylate, gamma-Heptalactone and other components, and has sterling full-bodied roast powdered milk aroma, delicious mouthfeel and pure flavor. In addition, the flavor has the advantages of high temperature resistance, long flavor lasting time and so on. The formulation and the preparation method are widely applied to food industry, and ensure that products keep sterling natural milk flavor.
Owner:广东雅和生物科技有限公司
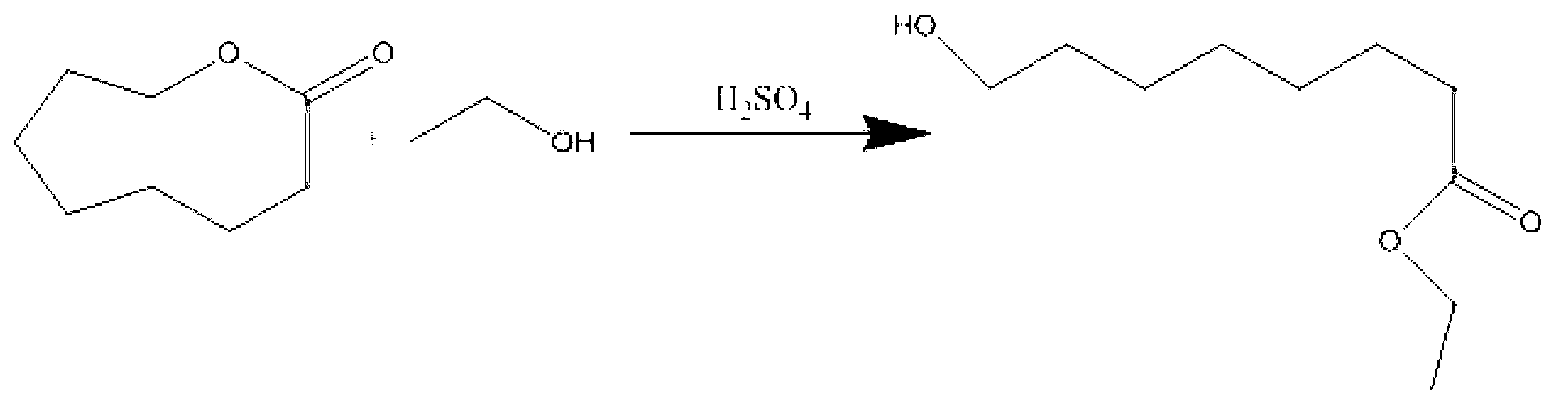
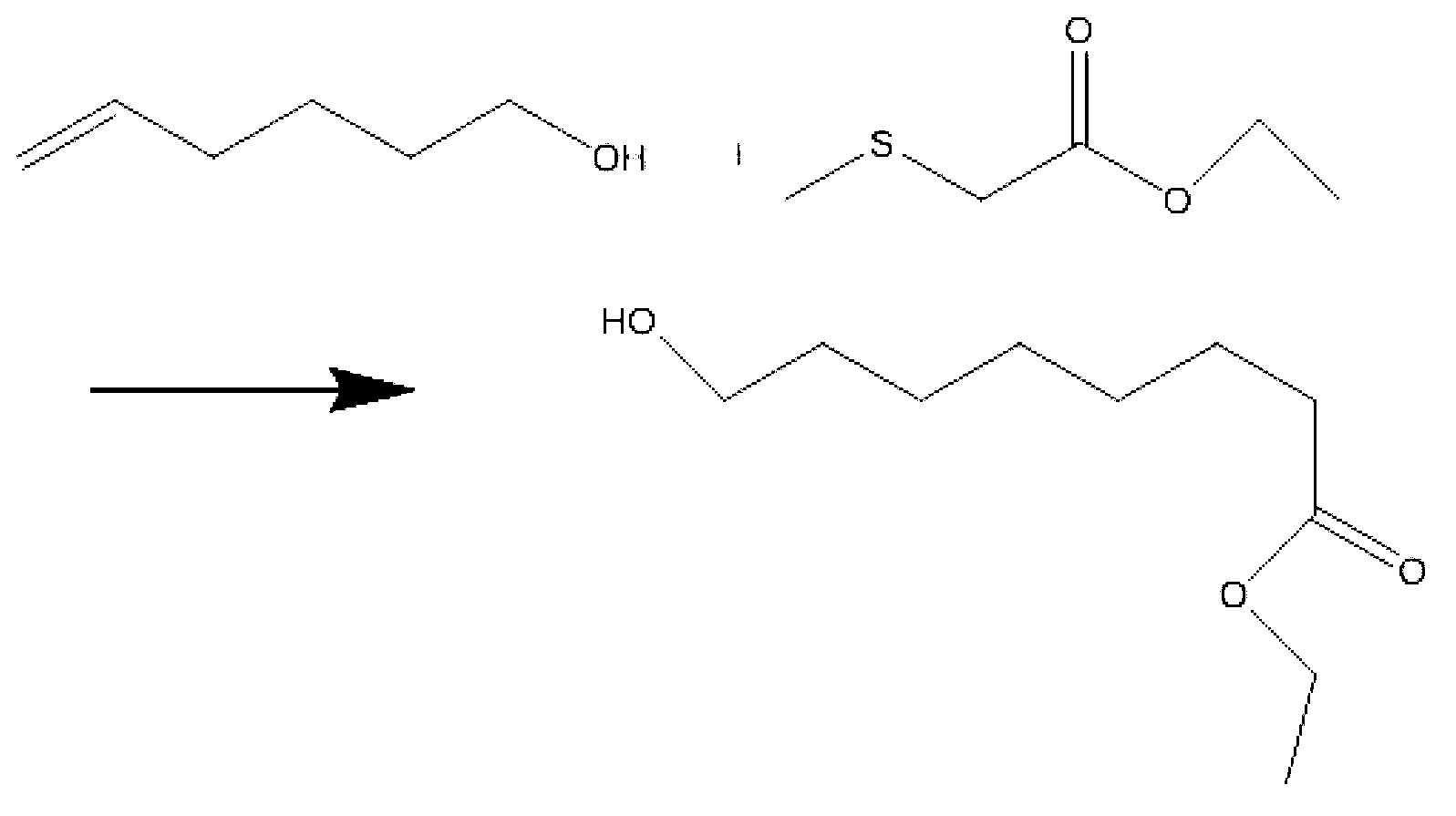
Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate
ActiveCN108689876AReduce usageLow toxicityOrganic compound preparationCarboxylic acid amide separation/purificationEthyl chloroformateCarbonyldiimidazole
The invention discloses a preparation method of a pharmaceutical intermediate, i.e., sodium 8-[(2-hydroxybenzoyl) amino] octanoate. The preparation method comprises the steps of enabling salicylamide,used as a raw material, to react with N' N-carbonyl diimidazole so as to generate an intermediate, i.e., 2H-benzo[e][1,3]oxazine-2, 4 (3H)-dione; enabling the intermediate to react with 8-ethyl bromooctanoate to obtain 8-(2, 4-dicarbonyl-2H-benzo[e][1, 3]oxazine-3(4H)-yl) ethyl caprylate; hydrolyzing by using sodium hydroxide, and then acidizing to obtain 8-[(2-hydroxybenzoyl) amino] caprylic acid; then, enabling the 8-[(2-hydroxybenzoyl) amino] caprylic acid to react with sodium hydroxide to obtain the final product, i.e., the sodium 8-[(2-hydroxybenzoyl) amino] octanoate. The preparation method avoids the use of a genotoxic raw material-ethyl chloroformate, is low in reaction energy consumption, less in by-products and high in yield, greatly lowers the production cost, and is simple inprocess and suitable for industrial production.
Owner:江苏东南纳米材料有限公司
Lactic acid-tolerant ester-producing pichia pastoris
ActiveCN107287127ARapid growthImprove adaptabilityFungiMicroorganism based processesPichia pastorisEthyl phenylacetate
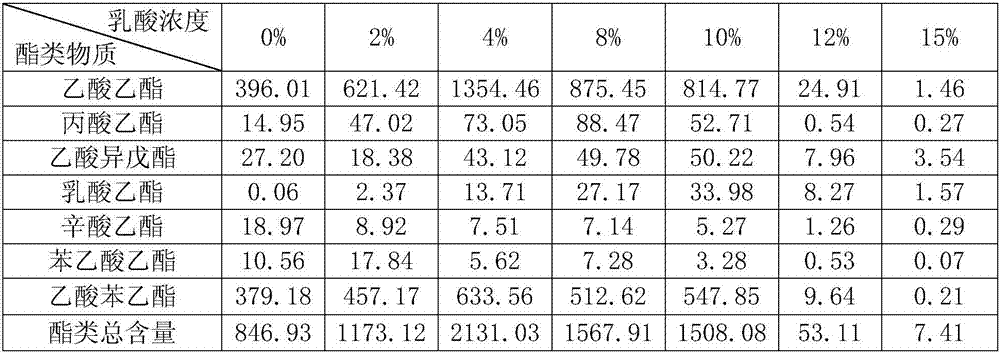
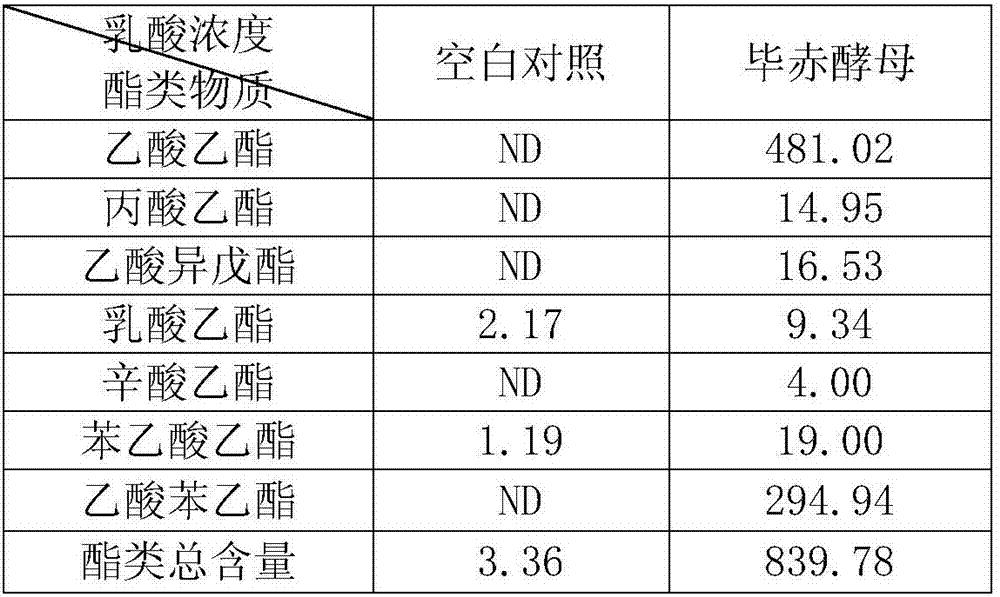
The invention discloses a lactic acid-tolerant ester-producing pichia pastoris, and belongs to the technical field of a bioengineering technology and a brewing biotechnology. The strain is preserved at the General Microbiology Center of the China Committee for Culture Collection of Microorganisms on April 24, 2017, the classification is named pichia kudriavzevii (Pichia kudriavzevii) and the preservation number is CGMCC No.14068. The pichia pastoris is high in environmental tolerance, is capable of tolerating 0-15% lactic acid, is capable of metabolizing to generate multiple ethyl ester volatile flavor substances, such as ethyl acetate, ethyl propionate, ethyl caprylate, phenethyl acetate and ethyl phenylacetate in the environment of 0-12% lactic acid, and is a brewing functional strain with excellent performance.
Owner:KWEICHOW MOUTAI COMPANY
Fatty glyceride as well as preparation method and application thereof
ActiveCN103772196AEasy to useEasy to operateCosmetic preparationsHair cosmeticsOctanoic AcidsGlycerol
The invention belongs to the technical field of fine chemical engineering, and discloses fatty glyceride as well as a preparation method and an application thereof. The fatty glyceride is prepared from the following components by mole percent: 20-60mol% of glycerinum, 5-50mol% of component A, 1-50mol% of component B and 0-10mol% of component C, wherein the component A is octanoic acid, methyl caprylate or ethyl caprylate, the component B is decanoic acid, methyl decanoate or ethyl decanoate and the component C is saturated fatty acid, fatty acid methyl ester or fatty acid ethyl ester with 12-16 carbon atoms. The preparation method comprises the following steps: carrying out a reaction on the components and a catalyst at a normal pressure at 130-180 DEG C for 1-6 hours in nitrogen; then, carrying out a decompression reaction at 130-210 DEG C for 1-6 hours and dehydrating to obtain the fatty glyceride. The fatty glyceride obtained has an extremely good synergic tackifying effect and good foaming characteristic and foaming behavior and can be used as a thickener for synthesis of daily detergent products and cosmetics.
Owner:广州星业科技股份有限公司
Preparation method of R-lipoic acid tromethamine salt
InactiveCN105622571AEmission reductionReduce process stepsOrganic chemistryOrganic solventFiltration
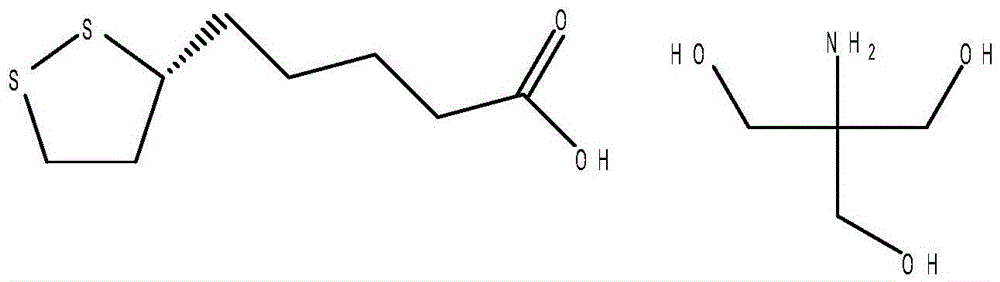
The invention discloses a preparation method of R-lipoic acid tromethamine salt and belongs to the field of organic medicinal chemistry. The method includes the steps that (S)-6,8-dichloro ethyl caprylate and sulphur are put into a reaction vessel, the temperature is raised, a cyclization reaction is carried out, the temperature is preserved, extraction is carried out with a first organic solvent, concentration is carried out, and cyclization liquid is obtained; then, a hydrolysis reaction is carried out, cooling is carried out, and hydrolysis liquid is obtained; a second organic solvent is added into the hydrolysis liquid, the pH value is regulated, extraction is carried out, an obtained organic layer is washed with water to be neutral, the second organic solvent is removed at reduced pressure, and an initial product is obtained; mixed liquor is added into the initial product, the temperature is raised, a first filter aid is added, stirring adsorption is carried out, filtration is carried out, and light yellow liquid is obtained; cooling is carried out to separate out crystals, and R-lipoic acid is obtained; R-lipoic acid is dissolved, trihydroxymethyl aminomethane is added, the temperature is raised for solution, a second filter aid is added for filtration, and light yellow liquid is obtained; cooling is carried out to separate out crystals, centrifugal drying is carried out, and the finished product is obtained. According to the method, few steps are needed, efficiency is high, energy is saved, and waste discharge is reduced.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
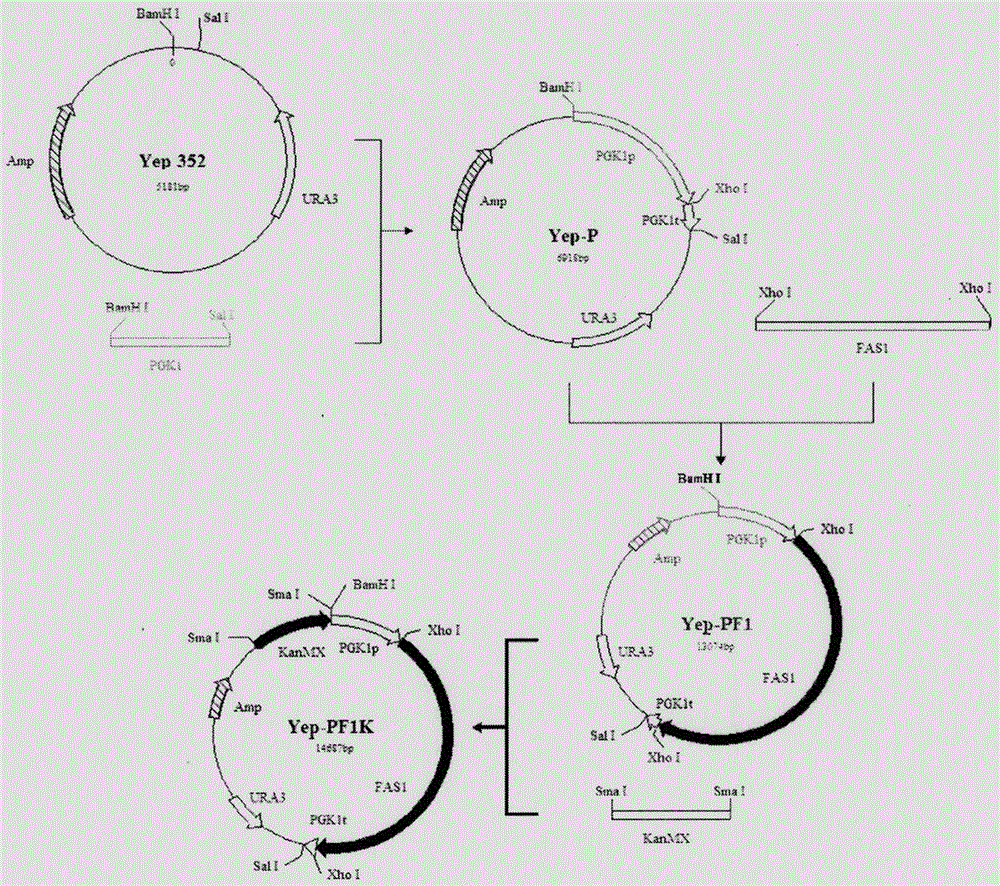
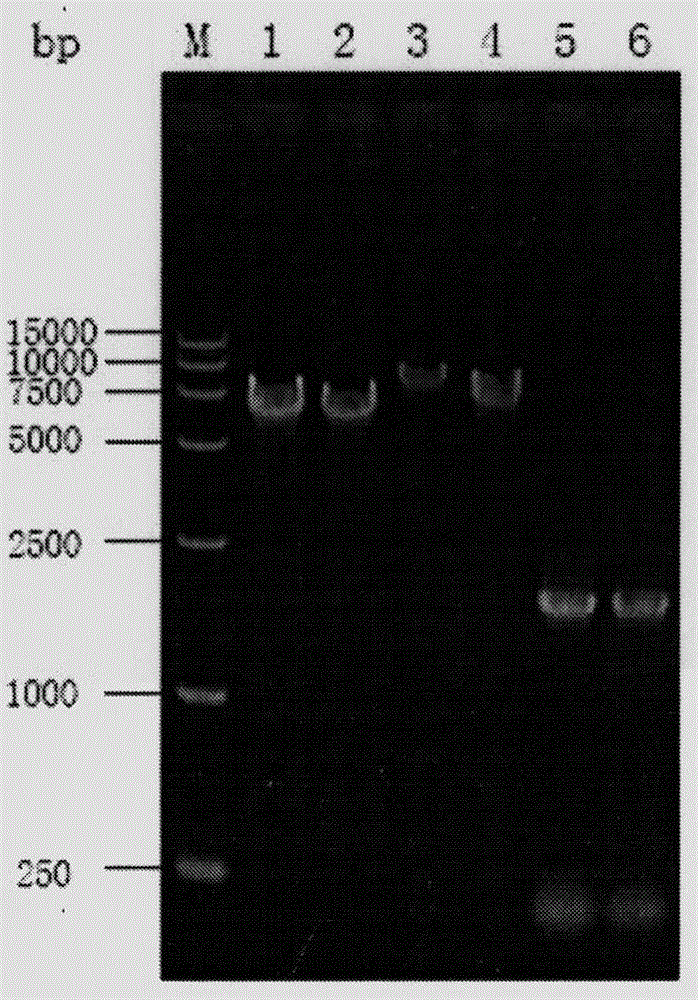
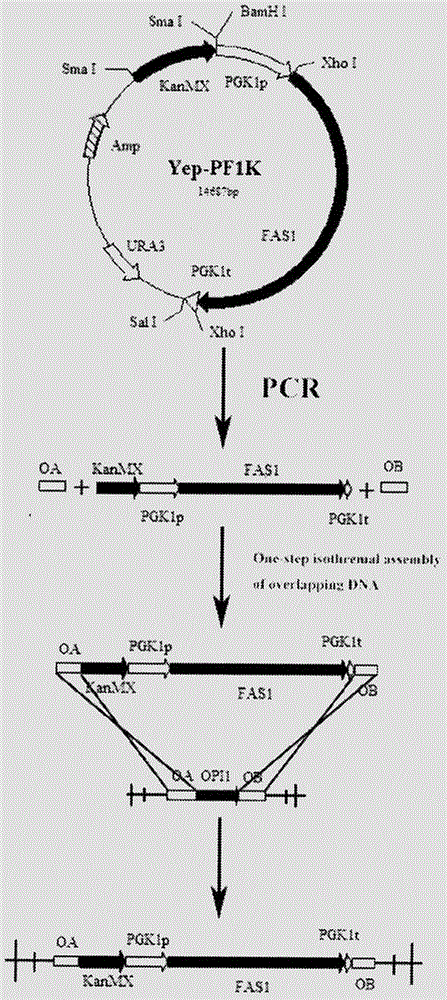
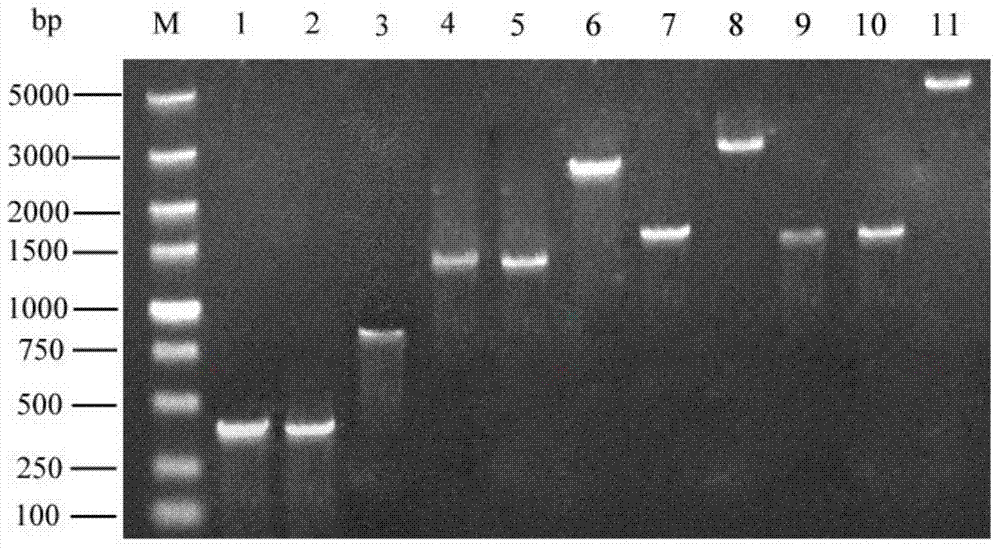
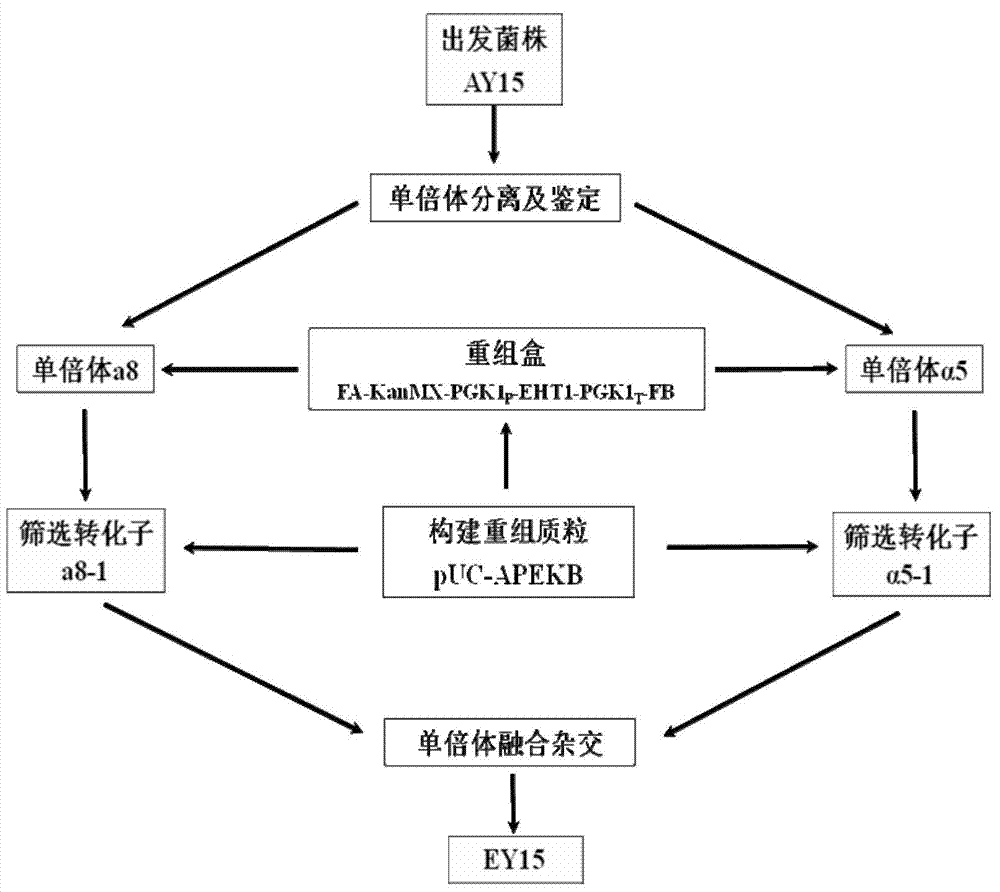
Saccharomyces cerevisiae strain with high yield of flavor ethyl ester and construction method of saccharomyces cerevisiae strain
The invention provides a saccharomyces cerevisiae strain with high yield of flavor ethyl ester and a construction method of the saccharomyces cerevisiae strain. An encoding gene FAS1 of a strong promoter PGK1 over-expressed fatty acid synthetase beta subunit is selected, an inositol-mediated controlling gene OPI1 is knocked out, and the saccharomyces cerevisiae strain with high yield of flavor ethyl ester is obtained. Compared with original strains, the strain has the following advantages under the condition that other fermentation performance of the strain is basically not affected: the content of ethyl acetate reaches 52.49 mg / L and is increased by 2.61 times than that of the original strains after 5 days of simulated liquid-state liquor fermentation of a corn material; the content of medium-chain fatty acid ethyl ester ethyl caproate, the content of ethyl caprylate, the content of ethyl caprate and the content of ethyl laurate are increased by 1.21 times, 2.66 times, 2.53 times and 1.27 times respectively than those of the original strains; the content of long-chain fatty acid ethyl ester ethyl myristate and the content of ethyl palmitate are increased by 1 time and 56.7% respectively.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Anti-microbial fermenting bed padding for pig
The invention discloses an anti-microbial fermenting bed padding for a pig, which is prepared from the following raw materials in parts by weight: 36 to 44 parts of straw ash, 3 to 4 parts of fermenting bed EM stock culture, 20 to 23 parts of lime powder, 3 to 4 parts of green tea powder, 4 to 5 parts of glossy privet fruit, 17 to 20 parts of watermelon peel, 0.5 to 0.6 parts of ethyl caprylate, 37 to 43 parts of corn straw, 26 to 28 parts of beer yeast paste, 1 to 2 parts of proteinase preparation, 0.8 to 0.9 parts of cysteine hydrochloride, 1.4 to 1.6 parts of thiamine, 25 to 29 parts of vanilla pod, a proper amount of 45% ethanol solution, and a proper amount of water. According to the anti-microbial fermenting bed padding for the pig, a large number of pathogenic bacteria are killed through a pre-fermentation mode and the addition of the lime powder, the content of the beneficial bacterium in the padding is improved through the introduction of the fermenting bed EM stock culture, the living space of pathogenic microorganisms is reduced by utilizing the balanced growth of the microorganisms, the negative effect on the fermenting bed body is not caused, the bactericidal effect on the fermenting bed is realized, and the morbidity rate of the pig is reduced.
Owner:ANHUI WANLI ECOLOGICAL LANDSCAPE
Formula of fresh and sweet tobacco flavoring essence
ActiveCN102304427AGreat tasteAdd natural sweetnessTobacco preparationEssential-oils/perfumesKetoneFurfural
The invention discloses a formula of fresh and sweet tobacco flavoring essence. According to the formula, the fresh and sweet tobacco flavoring essence consists of the following components in percentage by mass: 0.01 to 1.05 percent of ethyl caprylate, 0.1 to 0.3 percent of damascenone, 0.2 to 0.8 percent of isovaleraldehyde propylene glycol acetal, 0.2 to 0.5 percent of ethyl laurate, 0.05 to 0.15 percent of caproic acid, 0.08 to 0.2 percent of 5-methylfurfurol, 0.3 to 0.8 percent of octoic acid, 0.1 to 0.4 percent of 6-methyl-3,5-heptadiene-2-ketone, 0.5 to 1.2 percent of dihydro-beta-irisone, 0.5 to 1.5 percent of phenethyl ethanol, 1 to 2.5 percent of furfural, 25 to 40 percent of propylene glycol and 50 to 70 percent of edible ethanol. By the formula, tobacco produces fresh and sweetfragrance, the tobacco fragrance can be increased and the taste of the tobacco can be improved, so that the taste of the tobacco is improved, the natural sweet fragrance of the tobacco is enhanced and dry, astringent and bitter mouthfeel of customers is improved and requirements of the customers are met.
Owner:GUANGZHOU AOJIAN PERFUME
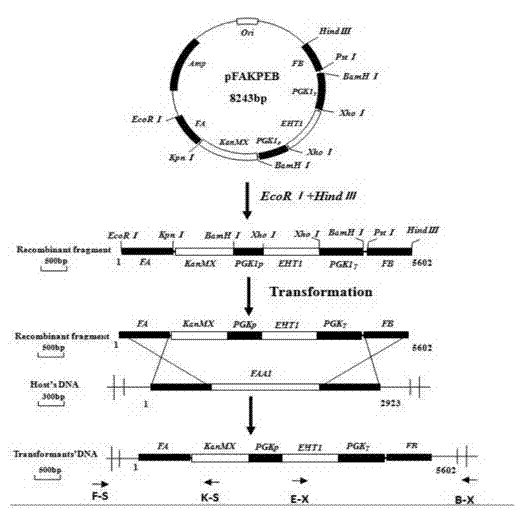
Saccharomyces cerevisiae engineering bacterium for highly yielding medium-chain fatty acid ethyl ester as well as construction method thereof
ActiveCN103571764AIncrease contentFungiMicroorganism based processesFatty acid activating enzymeBacterial strain
The invention provides a saccharomyces cerevisiae engineering bacterium for highly yielding medium-chain fatty acid ethyl ester. The saccharomyces cerevisiae engineering bacterium is realized by selecting a strong promoter PGK1 (Phosphoglycerate kinase 1) for overexpression coding of an EHT1 (Ethanol Hexanoyl Transferase 1) gene of alcohol acyltransferase and knocking out a gene FFA1 (Free Fatty Acid Receptor 1) of an exogenous fatty acid activating enzyme. The preservation number is CGMCC (China General Microbiological Culture Collection Center) No.7937. Under the condition that other fermenting properties are not affected, compared with a parent bacterial strain, the content of ethyl hexanoate can be improved to 2.23mg / L after simulating fermentation of corn raw material liquid white spirit for 15 days, wherein the content is 2.75 times the original bacteria. The contents of ethyl caprylate and ethyl caprate are respectively improved by 52% and 62%. After fermentation for 30 days, the contents of ethyl hexanoate, ethyl caprylate and ethyl caprate are respectively improved by 120%, 16.2% and 16.7%. After simulating fermentation of corn raw material liquid white spirit for 15 days, the content of ethyl hexanoate can be improved to 2.83mg / L which is 2.8 times the original bacteria, and the contents of ethyl caprylate and ethyl caprate are respectively improved by 43.3% and 40.9%.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method for treating addition wastewater in production process of lipoic acid
ActiveCN102531299AHigh yieldEmission reductionGeneral water supply conservationWater/sewage treatment bu osmosis/dialysisChemical oxygen demandWhole body
The invention discloses a method for treating addition wastewater in the production process of lipoic acid, which includes the following steps: adding a certain amount of ammonium sulfate and sodium sulfate into the lipoic acid addition wastewater, performing the reaction to generate aluminium ammonium sulfate, and carrying out crystallization, filtration and recrystallization to obtain the finished product of aluminium ammonium sulfate; and dividing filtrate into concentrated solution and permeate by nanofiltration, extracting adipic acid monoethyl ester and 8-chlorine-6-carbonyl ethyl caprylate from the concentrated solution by dichloroethane and toluene, carrying out reverse osmosis desalination on the extracted concentrated solution and the permeate and then carrying out biochemical treatment to discharge with standard level, wherein CODcr (Chemical Oxygen Demand) is less than or equal to 1,000. According to the invention, a reaction crystallization / nanofiltration / reverse osmosis coupling technology is adopted to carry out comprehensive treatment and resource utilization on the addition wastewater, the treatment process and an original production process are circulated into one whole body, and the treatment efficiency is high; the method can be used for effectively recovering aluminium salt and organic intermediates in the addition wastewater; a byproduct, i.e. aluminium ammonium sulfate with content higher than or equal to 99.0 percent, is obtained, and the method has the advantage on the aspect of production cost.
Owner:JIANGSU TOHOPE PHARMA +1
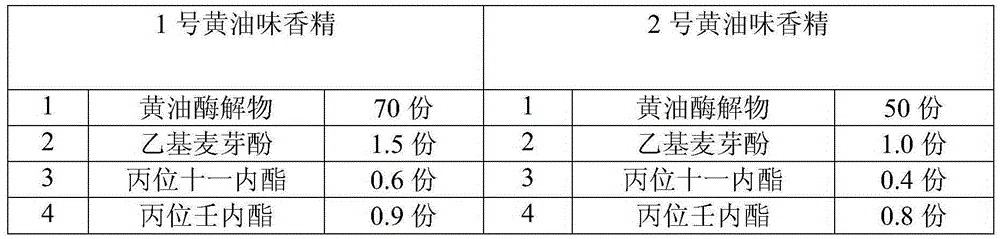
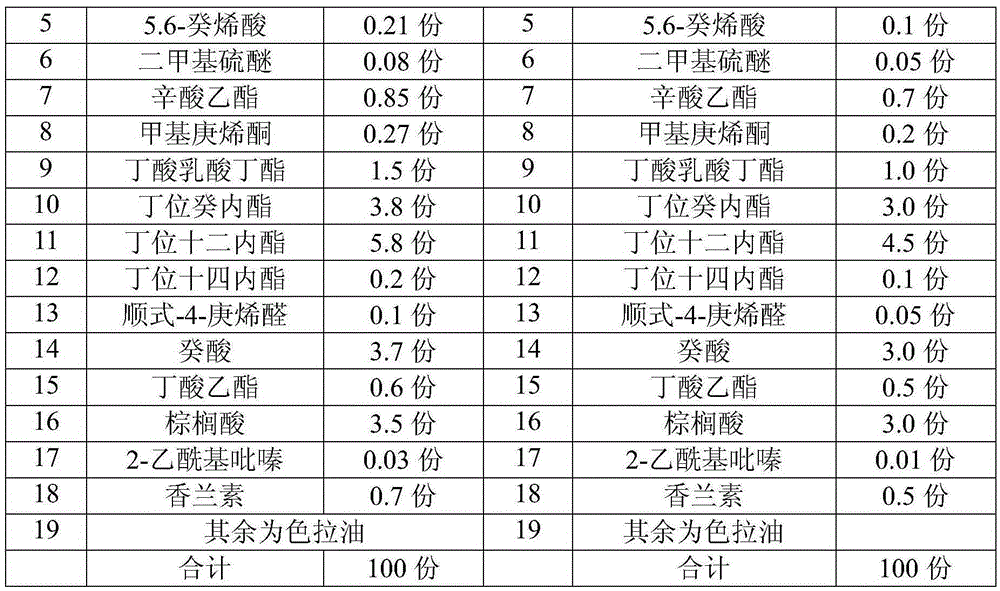
Essence with taste of butter
The invention relates to essence with taste of butter. The essence with the taste of butter comprises the following components in parts by mass: 50-70 parts of butter zymolyte, 1-1.5 parts of ethyl maltol, 0.5-0.6 part of undecalactone gamma, 0.8-1 part of gamma-nonanolactone, 0.1-0.3 part of 5.6-caproleic acid, 0.05-0.1 part of dimethyl sulfide, 0.7-1 part of ethyl caprylate, 0.2-0.4 part of methyl heptenone, 1-1.5 parts of butyric butyl lactate, 3-4 parts of 5-decanolide, 5-6 parts of delta-dodecalactone, 0.1-0.3 part of delta-tetradecalactone, 0.05-0.15 part of cis-4-heptene aldehyde, 3-4 parts of capric acid, 0.5-0.6 part of ethyl butyrate, 3-4 parts of palmitic acid, 0.05-0.1 part of 2-acetylpyrazine, 0.5-1 part of vanillin, and the balance being salad oil. The essence with the taste of butter disclosed by the invention has mellow, rich, comfortable and elegant fragrance in mouth feel, pure and natural milk taste, and plump and rich afteraste, and the temperature resistance of the essence can be permanent.
Owner:宁波威龙香精香料有限公司
Preparation method of Korean pickled vegetable essence
ActiveCN110140935AFragrance aroma is pure and fullIncrease the fragranceClimate change adaptationAcidic food ingredientsPropanoic acidAllyl thiocyanate
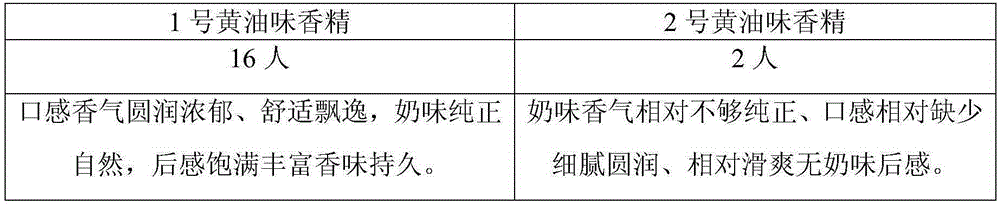
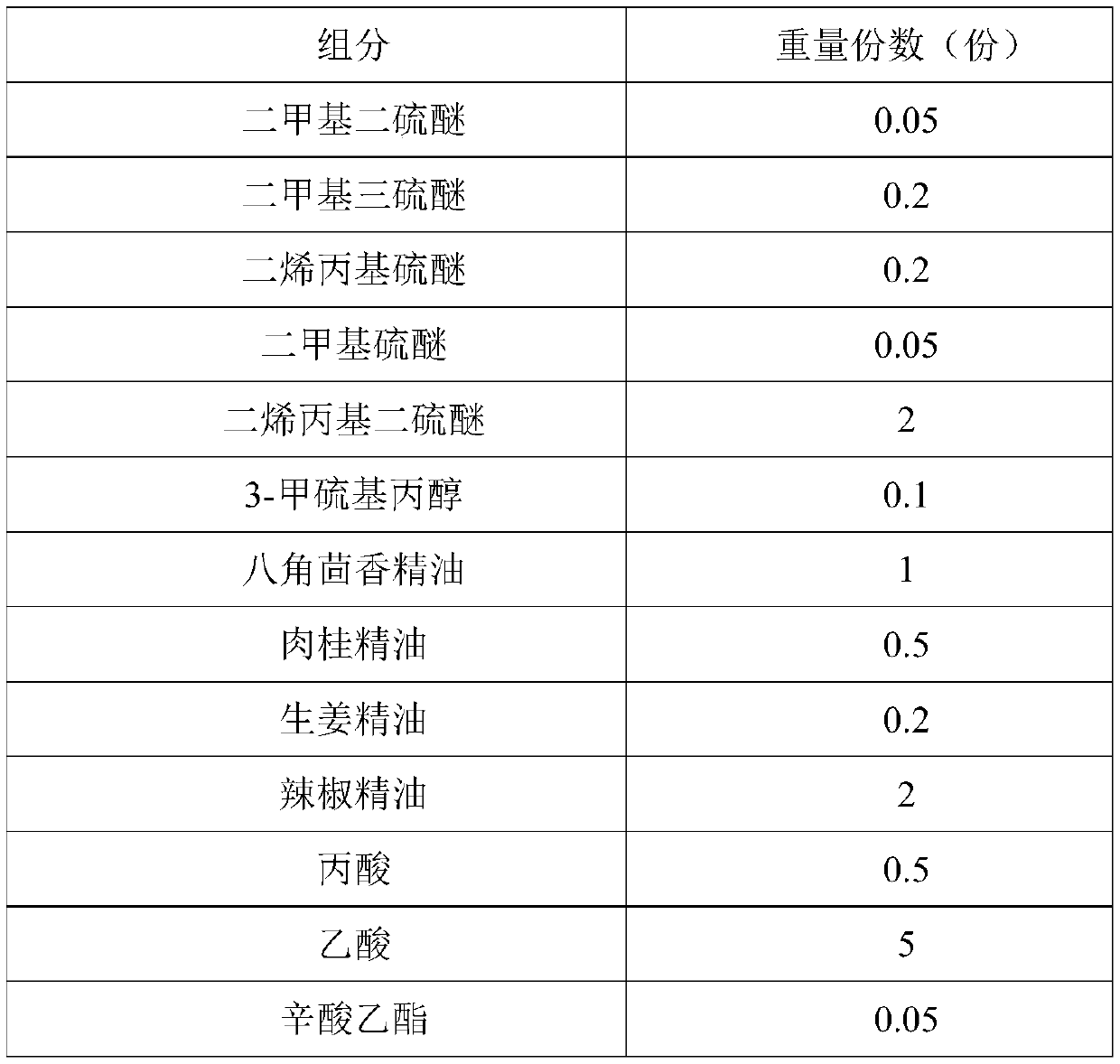
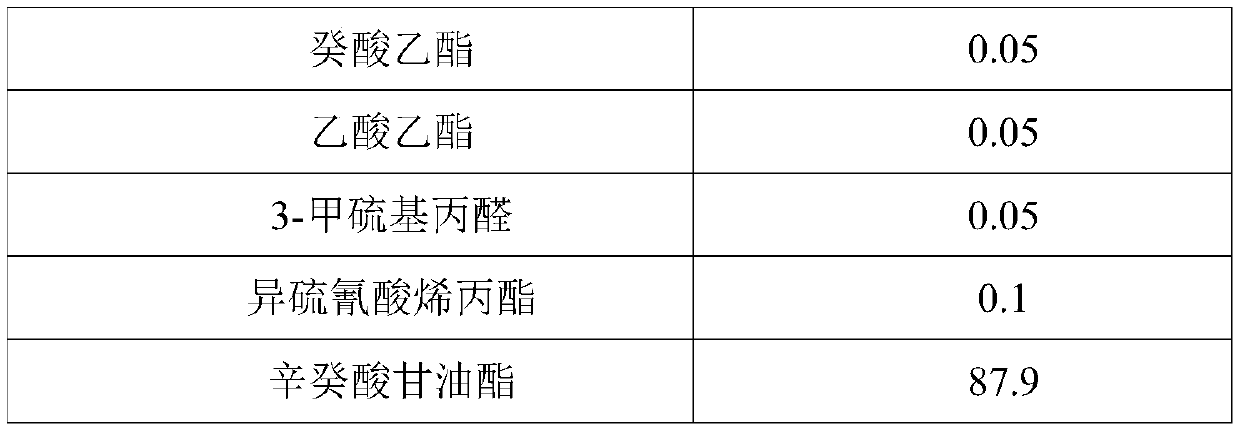
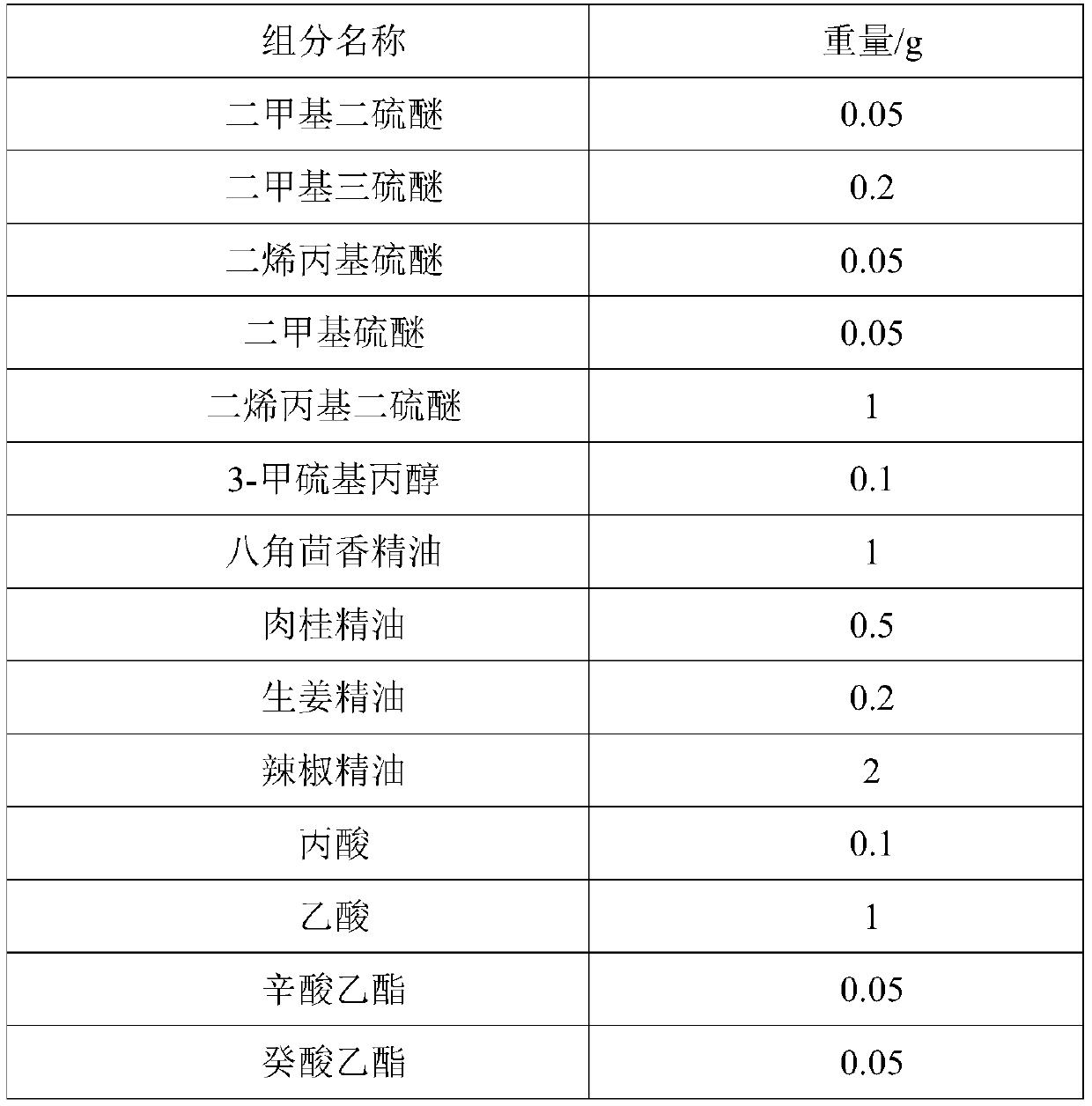
The invention provides a preparation method of Korean pickled vegetable essence. The disclosed Korean pickled vegetable essence comprises the ingredients of Caprylic capric triglycerride,, dimethyl disulfide, dimethyl trisulfide, diallyl sulfide, dimethyl sulfide, diallyl disulfide, 3-methylthiopropanol,, star anise essential oil, cinnamon essential oil, fresh ginger essential oil, chili pepper essential oil, propanoic acid, acetic acid, ethyl caprylate, ethyl caprate, ethyl acetate, 3-methylthiopropyl aldehyde and allyl isothiocyanate. The prepared pickled vegetable essence has the characteristics of being real and natural in fragrance, rich in mouth feel and stable in flavor, the appetence degree of a consumer is increased, and the Korean pickled vegetable essence can be well applied tothe field of foods of leisure puffed foods and the like.
Owner:江西省华宝孔雀食品科技发展有限公司
Synthesis method of 8-hydroxyl ethyl caprylate
ActiveCN103232345AConvenient sourceLow costOrganic compound preparationCarboxylic acid esters preparationSynthesis methodsCatalytic effect
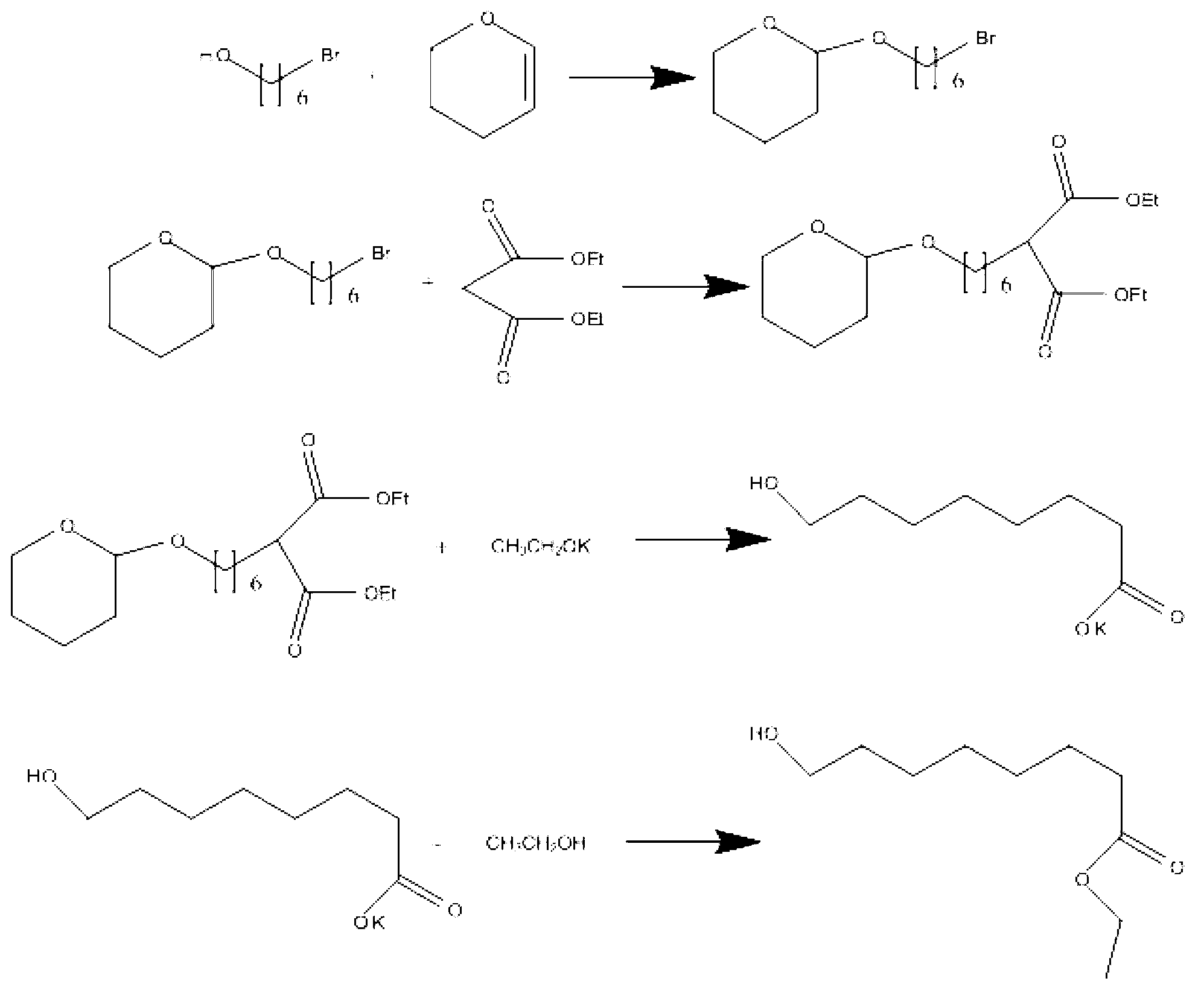
The invention relates to the synthesis technical field of medical intermediates and discloses a synthesis method of 8-hydroxyl ethyl caprylate. The synthesis method comprises the following steps of: dissolving 6-bromohexanol and diethyl malonate in an azeotropic organic solvent, heating to have reaction under alkaline catalysis to generate an intermediate which is 2-(6-hydroxyl hexyl) diethyl malonate, wherein the molar ratio of 6-bromohexanol to diethyl malonate to alkali is 1:0.95-1.05:1.37-2.73; and dissolving the intermediate in a polar aprotic solvent and water, heating to having reaction under catalytic effect of sodium halide to generate 8-hydroxyl ethyl caprylate, wherein the molar ratio of the intermediate to sodium halide is 1:1-3. According to the technical scheme provided by the invention, the process line is simple and the cost of raw materials is low; therefore and the method is favorable to industrial production.
Owner:JIANGSU BOTAI PHARMA COMPANY
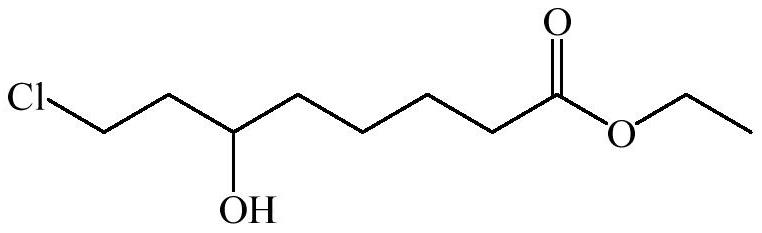
Synthetic method of 6-hydroxy-8-chloro ethyl caprylate, 6, 8-dichloro ethyl caprylate and lipoic acid
PendingCN114149324AHigh activityHigh yieldOrganic compound preparationCarboxylic acid esters preparationLithium chloridePtru catalyst
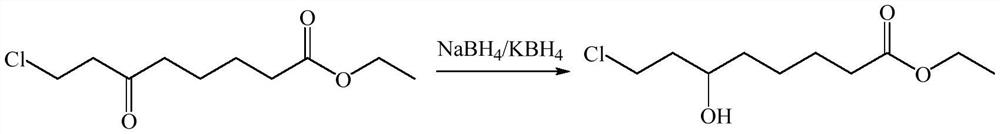
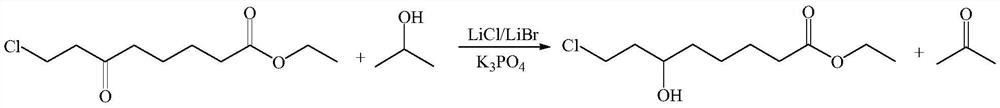
The invention belongs to the field of organic synthesis, and relates to a synthesis method of 6-hydroxy-8-chloro ethyl caprylate, 6, 8-dichloro ethyl caprylate and lipoic acid. The synthesis method of 6-hydroxy-8-chloro ethyl caprylate comprises the following steps: in the presence of a catalyst, 6-oxo-8-chloro ethyl caprylate is converted into 6-hydroxy-8-chloro ethyl caprylate by adopting MPV reduction reaction, the catalyst is composed of a catalyst I and a catalyst II, the catalyst I is lithium chloride and / or lithium bromide, the catalyst II is potassium phosphate, and the catalyst II is potassium chloride and / or lithium bromide. The molar ratio of the catalyst I to the catalyst II is 1: (1-20). According to the method, the MPV reduction reaction is co-catalyzed by adopting two types of inorganic salts, namely lithium chloride / lithium bromide and potassium phosphate, so that the activity of the MPV reduction reaction can be improved, the yield of the obtained product is up to 80% or above, the limitation that the existing MPV reduction reaction condition is not suitable for a beta-position chloro carbonyl compound, namely ethyl 6-oxo-8-chloro caprylate, is perfectly solved, and the method is suitable for industrial production. The reaction has the advantages of low cost, easy operation, safe reducing agent and the like.
Owner:XIAMEN KINGDOMWAY VI TAMIN INC +1
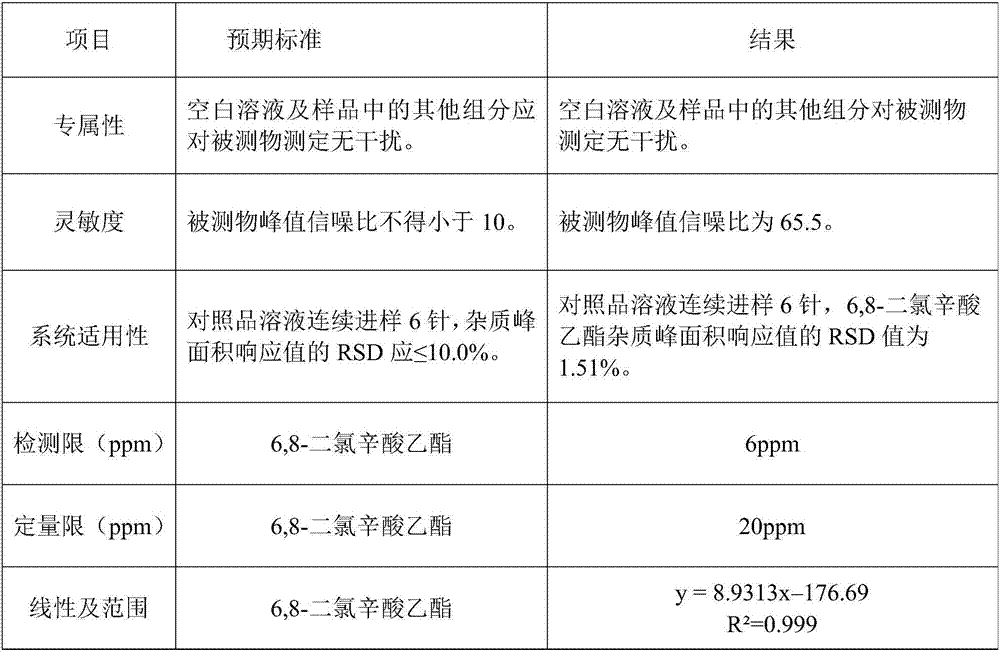
Analysis method for genotoxic impurity 6,8-dichloro ethyl caprylate in lipoic acid
The invention discloses an analysis method for genotoxic impurity 6,8-dichloro ethyl caprylate in lipoic acid. A gas chromatography and mass spectrum coupling technology is adopted to detect the 6,8-dichloro ethyl caprylate in zinc sulfate sample solution; the detection condition of the gas chromatography is that a chromatographic column is DB-WAX 30m*0.25mm*0.25mu m, a stationary phase is polyethylene glycol, and carrier gas is high-purity nitrogen; a column temperature is that an initial temperature is 100 DEG C and is maintained for two minutes, then, the temperature is raised to 230 DEG Cat a rate of 20 DEG C per minute, and the temperature of 230 DEG C is maintained for 5min; a mass spectrum condition that ion source voltage is -70eV, an ion source temperature is 230 DEG C, the temperature of a four-level rod is 150 DEG C, and the temperature of an interface is 280 DEG C. The analysis method has the advantages of high analysis speed and high sensitivity.
Owner:SHANDONG ANALYSIS & TEST CENT
Edible mango essence and preparation method thereof
The invention discloses edible mango essence. A formula comprises the following materials in parts by weight: 1.5 to 2.5 parts of vanillin, 20 to 45 parts of ethyl maltol, 800 to 900 parts of propylene glycol, 1 to 2 parts of delta-decalactone, 1 to 3 parts of gamma-decalactone, 1 to 3 parts of ethyl caprylate, 1 to 4 parts of ethyl hexanoate, 0.1 to 2 parts of 4-hydroxy-3-methoxybenzyl, 1 to 5 parts of methyl dihydrojasmonate, 1 to 3 parts of allyl hexanoate, 1 to 5 parts of caproic acid, 1 to 4 parts of ocimene, 1 to 5 parts of styralyl acetate, 0.2 to 4 parts of dimethyl sulfide, and 3 to 7parts of octanoic acid. As various fragrances or additives are adopted, the mango essence is vivid in flavor and soft and full in fragrance, has very strong nature feeling, is beneficial to improvement of mango fragrance of products, can be widely applied to various food requiring adding fragrance, and is low in production cost.
Owner:广州四季风食品科技有限公司
Camellia essence for cigarette blast beads, blast beads prepared from camellia essence and cigarettes
InactiveCN111621362AEase of comfortImprove comfortTobacco smoke filtersEssential-oils/perfumesPhenethyl acetateLinalool
The invention relates to camellia essence for cigarette blast beads, blast beads prepared from the camellia essence and cigarettes, and belongs to the technical field of tobacco essence. The essence comprises a camellia extract, ethyl vanillin, an oak moss concrete, 2-methylbutyric acid, ethyl caprylate, 2-methylbutyrate-2-phenethyl ester, 2, 3, 5-trimethylpyrazine, sweet orange oil terpene, phenethyl acetate, caprolactone, phenylethyl alcohol, isoamyl acetate, germacrene D, linalool, acetic acid, 2-pentanol, palmitic acid, benzyl benzoate, ethyl isovalerate, damascenone, isovaleric acid, acetophenone, ethyl caprate, and the like. The blast beads can improve the cigarette smoking comfort, improve the cigarette smoking sweetness, reduce the stabbing and choking feeling in cigarette smoke and reduce the stabbing feeling of the tongue surface, the flower fragrance and the cigarette fragrance are good in coordination, and the blast beads have high application value and popularization significance.
Owner:CHINA TOBACCO YUNNAN IND
Banana essence formula for dairy product
The invention discloses a banana essence formula for a dairy product. The banana essence is mainly prepared from the following components: cream esterified esters, isoamyl acetate, isoamyl isovalerate, vanillin, isoamyl butyrate, ethyl caproate, ethyl butyrate, ethyl caprylate, citral and heliotropin. The banana essence prepared by the formula has the obvious advantages that by utilizing the cream esterified esters which are natural and have strong binding capability with the dairy product as a raw material, on one hand, the natural feeling of the banana essence can be strengthened, and on the other hand, the banana essence can be well bonded with the dairy product, so that the problem that fragrance is wafted to be separated from the theme of a beverage is solved.
Owner:王婧婧
Crab-flavored essence, as well as preparation method and application thereof
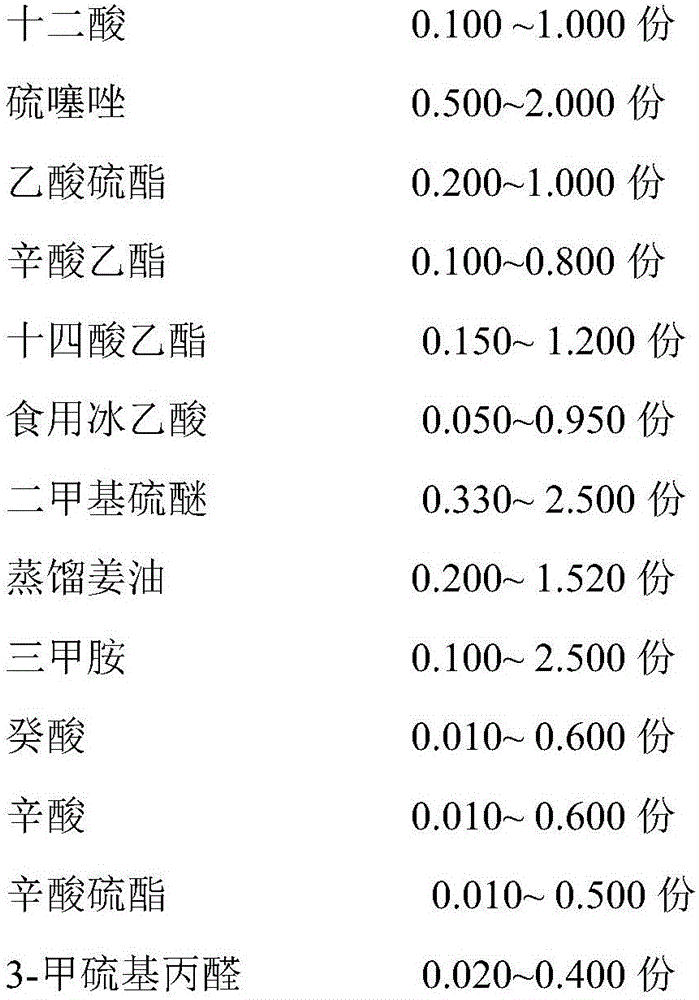
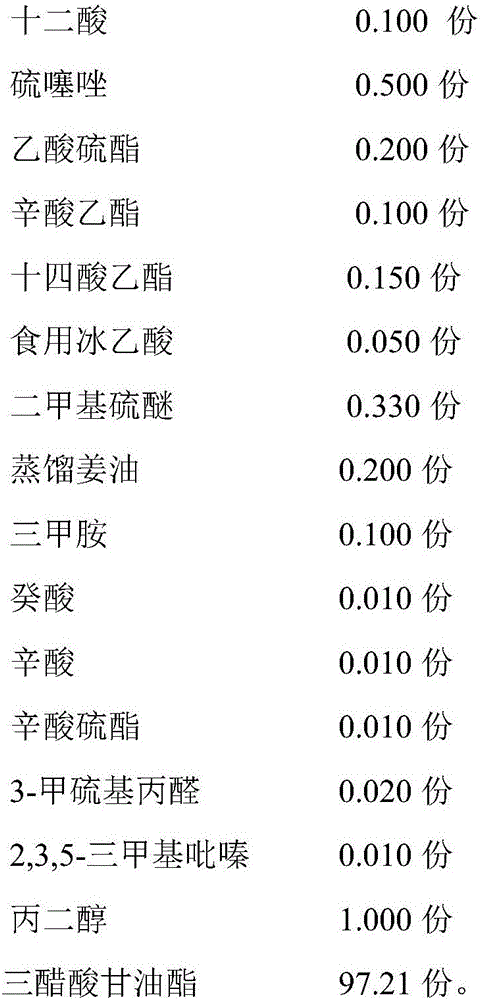
The invention discloses a crab essence and its preparation method and application. The crab essence contains dodecanoic acid, thiothiazole, thioacetate, ethyl octanoate, ethyl myristate, edible glacial acetic acid, dimethyl sulfide, Distilled ginger oil, trimethylamine, capric acid, caprylic acid, caprylic acid thioester, 3-methylthiopropanal, 2,3,5-trimethylpyrazine, propylene glycol and glyceryl triacetate, the present invention has developed a specific formula Crab flavor, just a small amount, has the effect of strong crab meat, good fresh and sweet taste, and high fidelity, and is loved by a wide range of consumers; and even after high-temperature roasting, frying, and steaming, it will not affect the effect of crab flavor. It shows that the crab essence of the present invention has the properties of high temperature resistance and stable flavor. The crab essence of the invention can well supplement the flavor loss of the recombined crab meat due to thermal processing, enhance the sweetness and authenticity of the crab meat-flavored meatballs, and improve consumers' liking.
Owner:江西省华宝孔雀食品科技发展有限公司
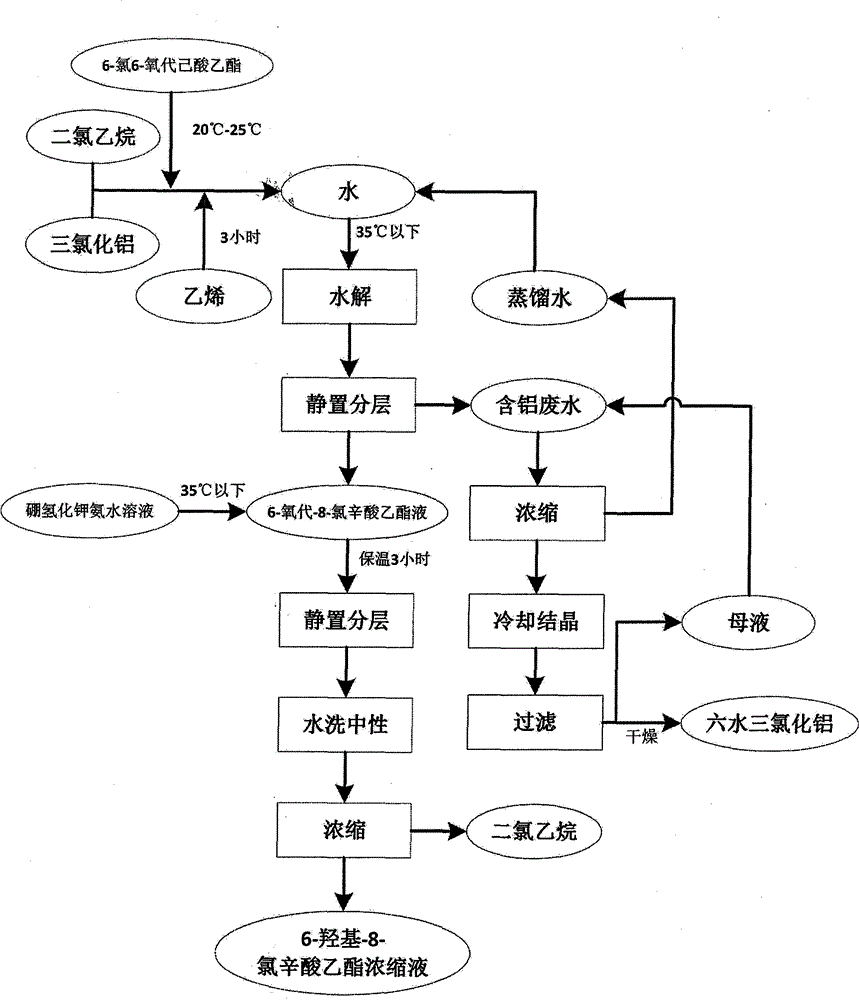
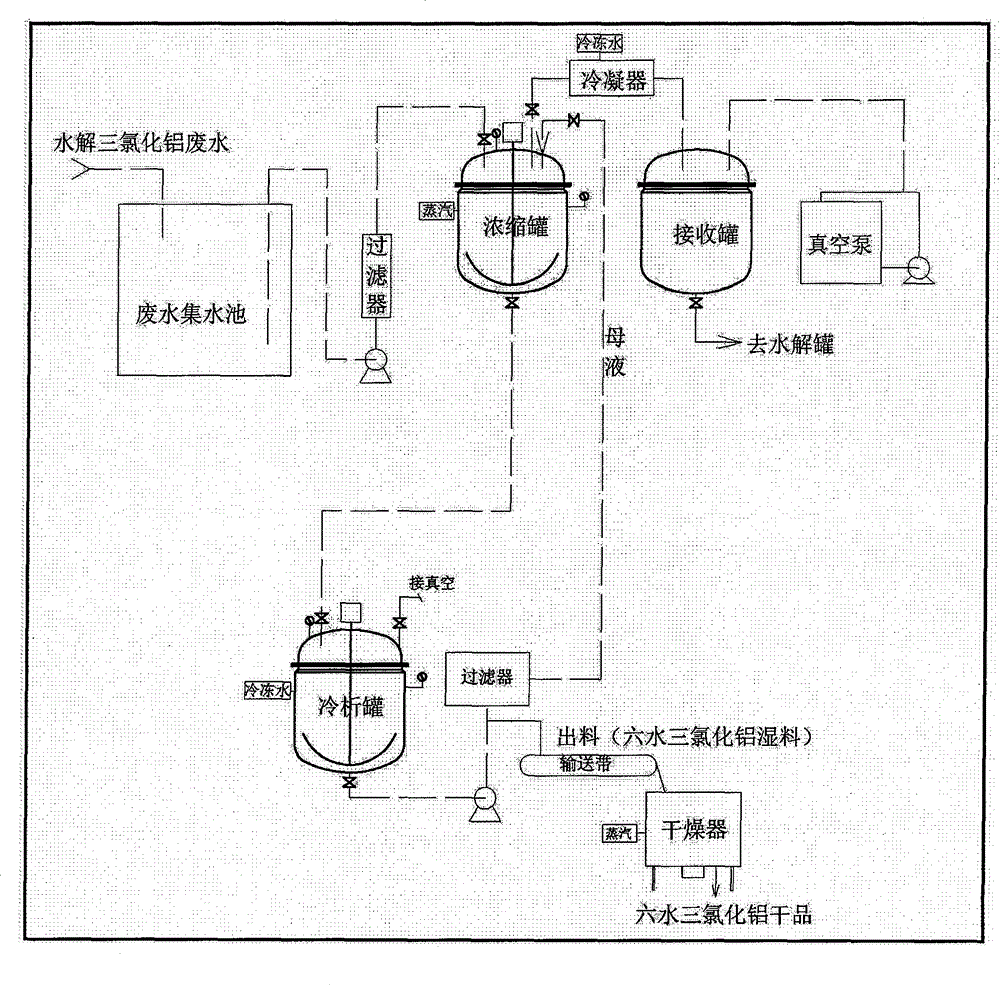
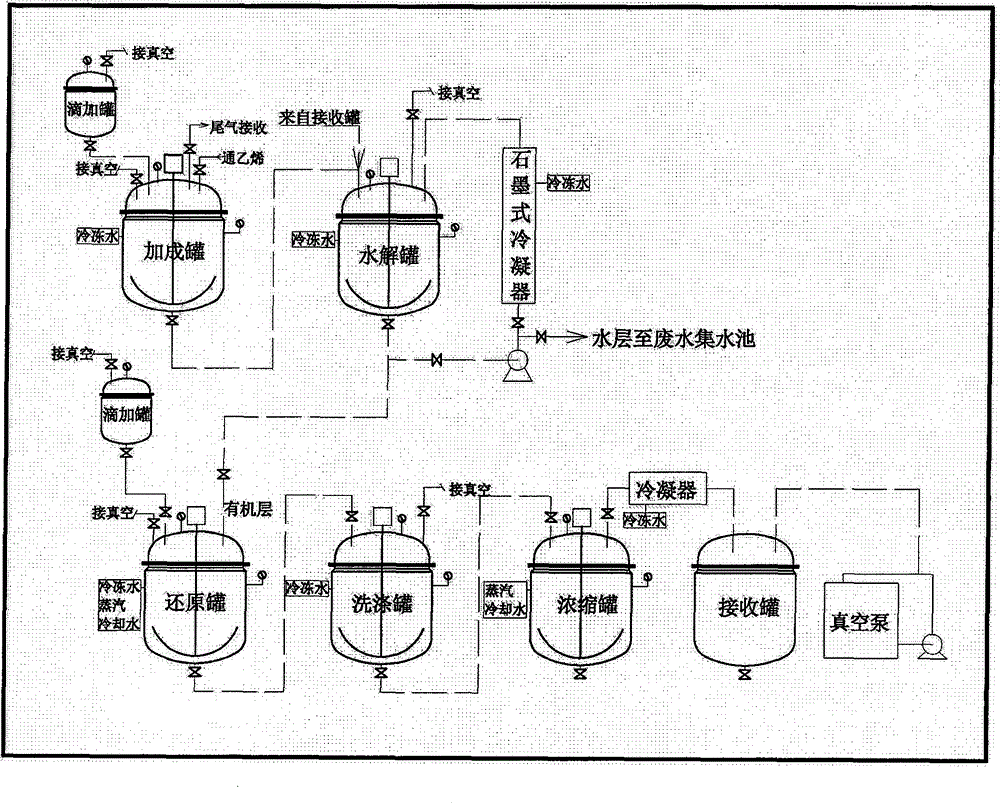
Method for treating aluminum-containing wastewater in preparation process of 6-oxo-8-chloro ethyl caprylate
InactiveCN104671570AAvoid direct dischargeSave waterOrganic compound preparationCarboxylic acid esters preparationEthyl ChlorideOxygen
The invention relates to a method for treating aluminum-containing wastewater in a preparation process of 6-oxo-8-chloro ethyl caprylate, and belongs to the field of industrial wastewater treatment and green production. The technical problem to be solved in the invention is to provide the method for treating aluminum-containing wastewater in a preparation process of 6-oxo-8-chloro ethyl caprylate. The method comprises the following steps: by taking aluminum trichloride as a catalyst, performing addition reaction on 6-oxo-6-oxo ethyl caproate and ethylene in a dichloroethane solution, and hydrolyzing the aluminum trichloride to prepare a target product. According to the technical scheme adopted by the invention, compared with the prior art, direction wastewater emission is avoided, and aluminum trichloride hexahydrate solids can be recycled, so that the method is very remarkable in saving water resources and achieving environmental friendliness.
Owner:JIANGSU TOHOPE PHARMA
Sodium 8-(2-hydroxylbenzamido)caprylate and preparation method therefor
InactiveCN111978193ADoes not affect yieldGood for stirring reactionOrganic compound preparationCarboxylic acid amide separation/purificationSalicylic acidAmidogen
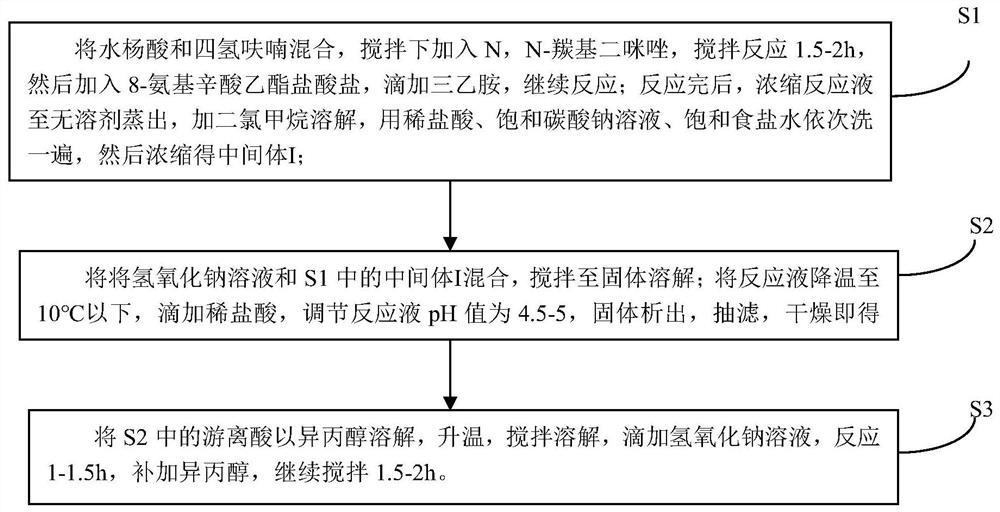
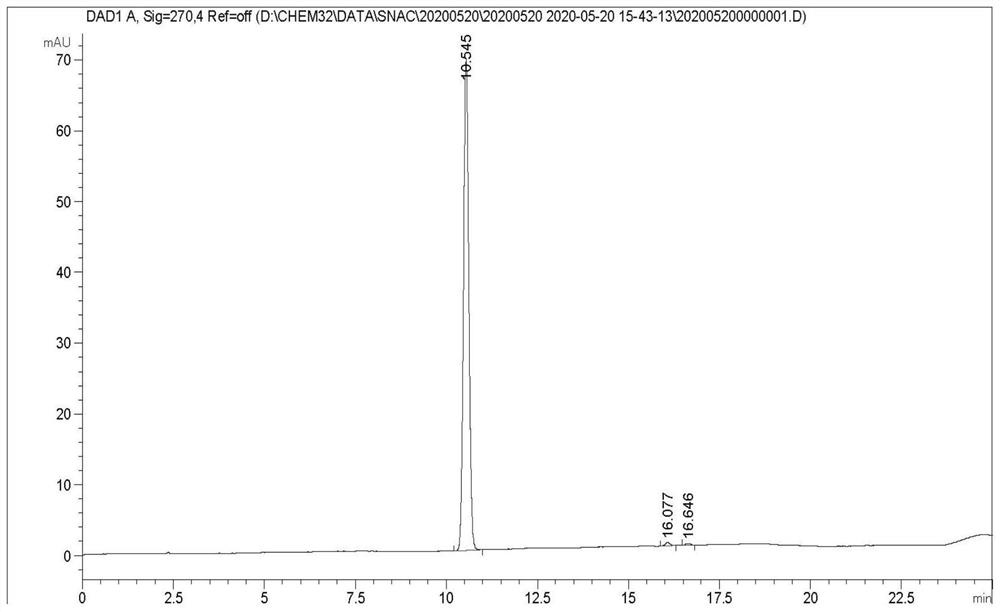
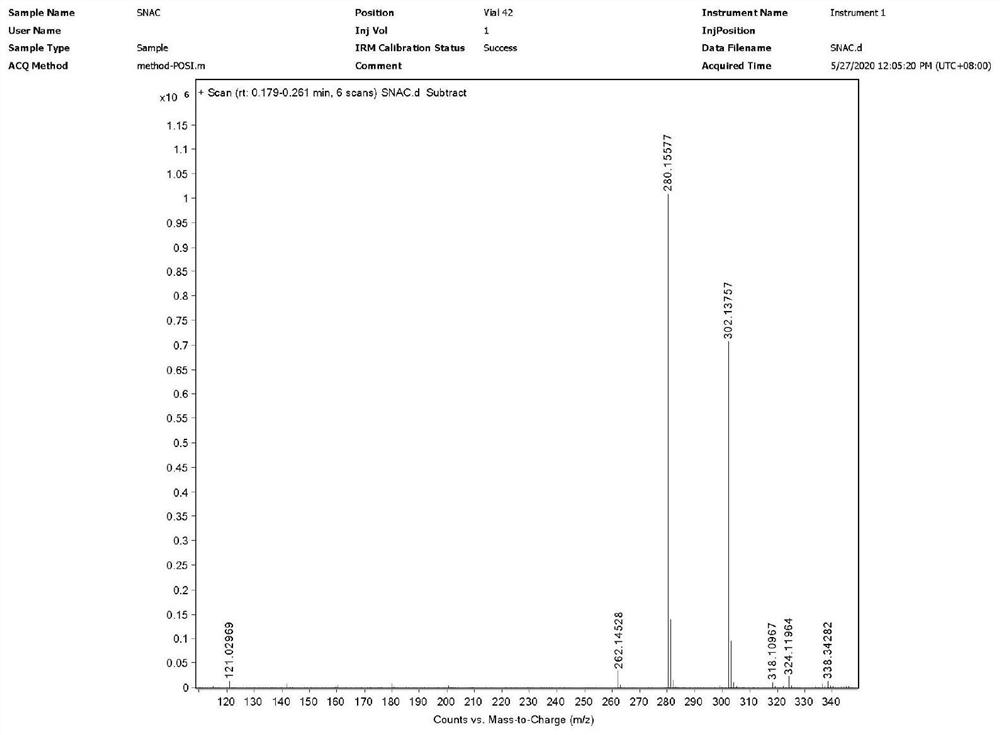
The invention discloses sodium 8-(2-hydroxylbenzamido)caprylate and a preparation method therefor and belongs to the field of preparation of compounds. A key of the technical scheme of the invention is as follows: the preparation method comprises the steps: mixing salicylic acid with tetrahydrofuran, adding N,N-carbonyl diimidazole, adding 8-amino ethyl caprylate hydrochloride, and dropwise addingtriethylamine; carrying out a concentrating reaction solution until no solvent is distilled off, adding dichloromethane for dissolving, and carrying out washing once separately with diluted hydrochloric acid, a saturated sodium carbonate solution and a saturated saline solution, so as to obtain an intermediate I; mixing a sodium hydroxide solution with the intermediate I, and carrying out stirring until solids are dissolved; cooling the temperature of a reaction solution to 10 DEG C or below, dropwise adding diluted hydrochloric acid, adjusting a pH value of a reaction solution to 4.5 to 5, and carrying out solid precipitation, so as to obtain free acids; and dissolving the free acids with isopropanol, dropwise adding a sodium hydroxide solution, carrying out a reaction for 1 to 1.5 hours, supplementing isopropanol, and continuing to carry out stirring for 1.5 to 2 hours. The method has the advantages that steps are few, the yield of each step is high, the product purity is good, impurities are more easily controlled, and raw materials are more readily available.
Owner:无锡紫杉药业股份有限公司
Volatile attractant applied to citrus fruit flies and application thereof
The invention provides a volatile attractant applied to citrus fruit flies. The volatile attractant is prepared from the following components: ethyl propanoate, ethyl caprylate and Z,3-hexenyl acetateaccording to a mass ratio of (1-8) to (1-8) to (1-8). When the attractant is used, the attractant is added into a carrier or is placed in a monitoring, trapping and killing device used for attractingand killing the citrus fruit flies; the device comprises a main body, an upper insertion part and a lower insertion part; the attractant is placed in the main body; a contact insecticide is distributed on the outer surface of the main body. The attractant is composed of a series of volatile substances included in fruits, is easily available in raw materials, simple in formula and convenient to prepare, and can achieve an excellent effect of attracting mature citrus fruit flies and especially mature mated citrus fruit flies.
Owner:NANJING SINO GREEN BIOTECH +1
Watermelon essence for oil-based ink and preparation method of watermelon essence
InactiveCN105132174AIncrease added valueEasy to useInksEssential-oils/perfumesSolubilityPhenethyl acetate
The invention relates to a watermelon essence for oil-based ink. The watermelon essence is prepared from watermelon essence, maltodextrin and starch sodium octenylsuccinate, wherein the watermelon essence is prepared from leaf alcohol, hexenyl acetate, ligustral, phenyl acetaldehyde dimethyl acetal, methyl heptenone, trans-2-hexenal, nonadienaldehyde, melonal, ethyl caprylate, ethyl isovalerate, hexyl acetate, allyl cyclohexanepropionate, benzyl alcohol, linalool, citronellol, geraniol, Alpha terpneol, phenethyl alcohol, hydroxycitronellal, lilial, heliotropin, citral, dimethylbenzylcarbinyl acetate, vanillin, alpha-damascenone, isomethyl ionone, verdyl acetate, linalyl acetate, linalyl acetate, styralyl acetate, phenethyl acetate, phenethyl isobutyrate, phenoxyethyl isobutyrate, terpinyl acetate, n-pentyl salicylate, benzyl benzoate, benzyl salicylate and Brazil orange oil. The invention also provides a preparation method for the watermelon essence for the oil-based ink. According to the watermelon essence and the preparation method, the fragrance retention effect and the oil solubility of the essence are improved.
Owner:SHANGHAI INST OF TECH
Lasting mango essence and preparation method thereof
InactiveCN108606300ALong lasting flavorImproves flavor persistenceFood scienceEthyl butyrateGamma-nonalactone
Belonging to the technical field of preparation of edible essence, the invention discloses a lasting mango essence and a preparation method thereof. The mango essence comprises the following raw materials: propylene glycol, dimethyl sulfide, gamma-decalactone, glyceryl triacetate, gamma-caprylolactone, nerol, 8-mercaptomenthone, nerolyl acetate, ethyl butyrate, alpha-pinene, beta-pinene, geraniol,hexanoic acid, leaf aldehyde, propyl acetate, butyric acid, delta-decalactone, ethyl 2-methylbutyrate, propyl butyrate, ocimene, terpinolene, butyl acetate, ethyl caprylate, gamma-nonalactone, xanthan gum, casein and sodium alginate. The mango essence is prepared by stepwise mixing, stirring and other steps. By adopting xanthan gum, casein and sodium alginate as the reinforcing system, the flavorpersistence of the mango essence is improved.
Owner:长沙小新新能源科技有限公司
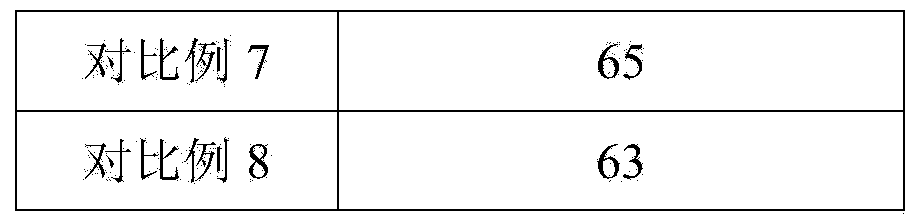
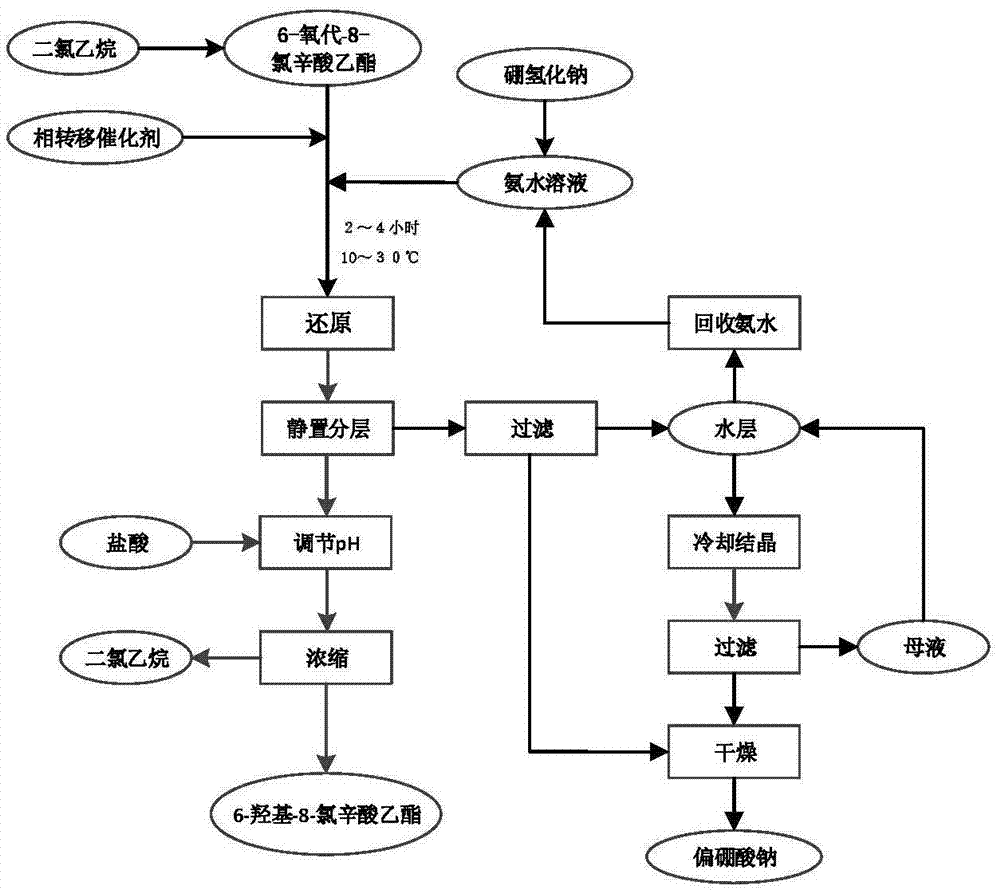
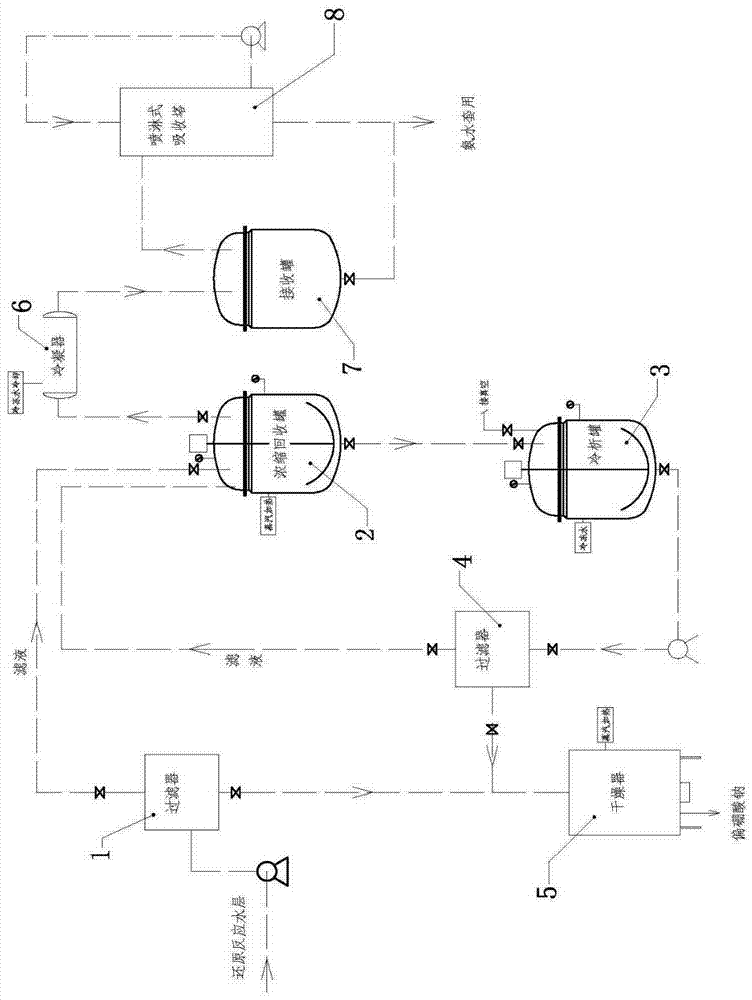
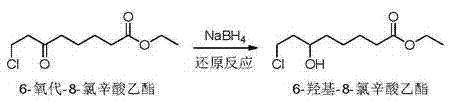
6-hydroxy-8-chloro ethyl caprylate preparation method thereof
ActiveCN106966901AReduce usageAvoid direct dischargeOrganic compound preparationCarboxylic acid esters preparationOrganic solventWastewater
The present invention discloses a preparation method of a lipoic acid key intermediate 6-hydroxy-8-chloro ethyl caprylate having a structure formula represented by a formula (I), wherein 6-oxo-8-chloro ethyl caprylate having a structure represented by a formula (II) is adopted as a starting raw material and is reduced to prepare the 6-hydroxy-8-chloro ethyl caprylate. Compared to the preparation method in the prior art, the preparation method of the present invention mainly has the following advantages that the synthesis steps are simple, the use of the organic solvent is reduced, the direction discharging of the wastewater is avoided, the generated sodium metaborate is easily separated and cannot react with other substances, the sodium metaborate solid is successfully recovered, and the ammonia water is recovered and utilized; particularly by recovering the sodium metaborate, the possibility is created for the recycling of the boron element through the sodium borohydride preparation with the further industrial recovery of the boron element; and the whole process meets the green synthesis and cleaning production technology requirement, and is suitable for the large-scale industrial production. The formulas (I) and (II) are defined in the specification.
Owner:JIANGSU TOHOPE PHARMA
Kyoho grape essence as well as preparation method and application thereof
The invention discloses a Kyoho grape essence, which is prepared from ethyl acetate, ethyl propionate, propyl acetate, ethyl butyrate, ethyl crotonate, cinnamyl propionate, cinnamyl alcohol, phenethylisobutyrate, ethyl phenylacetate, beta-damascenone, cis-3-hexenyl isovalerate, storax alcohol, beta-citronellol acetate, beta-citronellol, styrallyl acetate, beta-geranyl acetate, beta-linalyl acetate and methyl anthranilate, N-acetyl anthranilate, ethyl caproate, ethyl caprylate, ethyl caprate, acetic acid, propionic acid, hexanoic acid, butyric acid, linalool, leaf alcohol acetate, leaf alcohol, hexanoic acid leaf alcohol ester, isobutyrate leaf alcohol ester, trans-2-hexenal, butanol, ethyl maltol, furanone, 3-hydroxy-2-butanone, ethanol and propylene glycol. The Kyoho grape essence disclosed by the invention is mellow and full in grape characteristic aroma and strong in natural feeling, and can enable a product to have natural Kyoho grape aroma and taste after being added into foods such as beverages, jam and pastries as a food additive.
Owner:CHINA TOBACCO YUNNAN IND
Wine essence for tobacco for supplementing tobacco wine aroma and method
ActiveCN109971542AImprove stabilityProlong fragrance timeEssential-oils/perfumesBulk chemical productionIsobutanolSucrose
The invention relates to a wine essence for tobacco for supplementing tobacco wine aroma and a method, and belongs to the technical field of essences and fragrances. The wine essencefor the tobacco ismainly composed of ethyl acetate, ethyl butyrate, ethyl heptanoate, ethyl hexanoate, ethyl caprylate, ethyl lactate, geranyl acetate, Cognac oil, fusel oil, isobutanol, isoamyl alcohol, propionic acid, butyric acid, acetal, butanedione, caprylic capric triglycerride, Fenjiu essence extracts, sucrose fatty acid ester and polyoxyl-40-stearate. Accordingly, sucrose fatty acid ester and polyoxyl-40-stearate are added in the formula, an emulsifying system is formed through high speed stirring at 1,000-3,000r / min, and the longer fragrance retention time is achieved. Accordingly, by taking a clear flavor baijiu aroma compound in China as a basis, the self-made Fenjiu essence extracts are combined, more natural local wine essence for tobacco is prepared, the tobacco wine aroma is supplemented, and the fresh, sweet, moisturizing, comfortable and long flavor absorption feeling of cigarettes is improved.
Owner:山西昆明烟草有限责任公司 +1
Ester-producing saccharomycetes and application thereof in sour meat production
ActiveCN112940951APrevent fat oxidationImprove qualityFungiClimate change adaptationBiotechnologyPropanoic acid
The invention provides a saccharomycetes LXPSC1, the preservation number of which is CGMCC No. 21289. The invention also provides a sour meat food prepared based on the saccharomycetes and a preparation method of the sour meat food. The preparation method of the sour meat food specifically comprises the following steps that: meat and saccharomycetes LXPSC1 are proportioned according to a proportion of 1g: (7-9) logCFU; and a proper amount of pickling materials and mixing materials are added. The saccharomyces cerevisiae LXPSC1 can inhibit oxidation of acid meat lipid and reduce the content of biogenic amine, so that the safety of the product is improved; besides, the contents of flavor amino acids and esters in the product are increased, and the contents of ethyl caprylate, ethyl caprate, ethyl propionate, ethyl palmitate, ethyl hexanoate and ethyl butyrate are respectively 1.42 times, 2.74 times, 7.49 times, 12.73 times, 4.20 times and 4.00 times of those of sour meat prepared in the prior art, so that the sensory quality such as smell, taste and the like of the sour meat can be effectively improved.
Owner:DALIAN POLYTECHNIC UNIVERSITY
Saccharomyces cerevisiae highly producing C6-C10 ethyl esters and construction method and purpose of saccharomyces cerevisiae
ActiveCN110804561AHigh yieldGood yieldFungiMicroorganism based processesEthyl groupAcyl CoA dehydrogenase
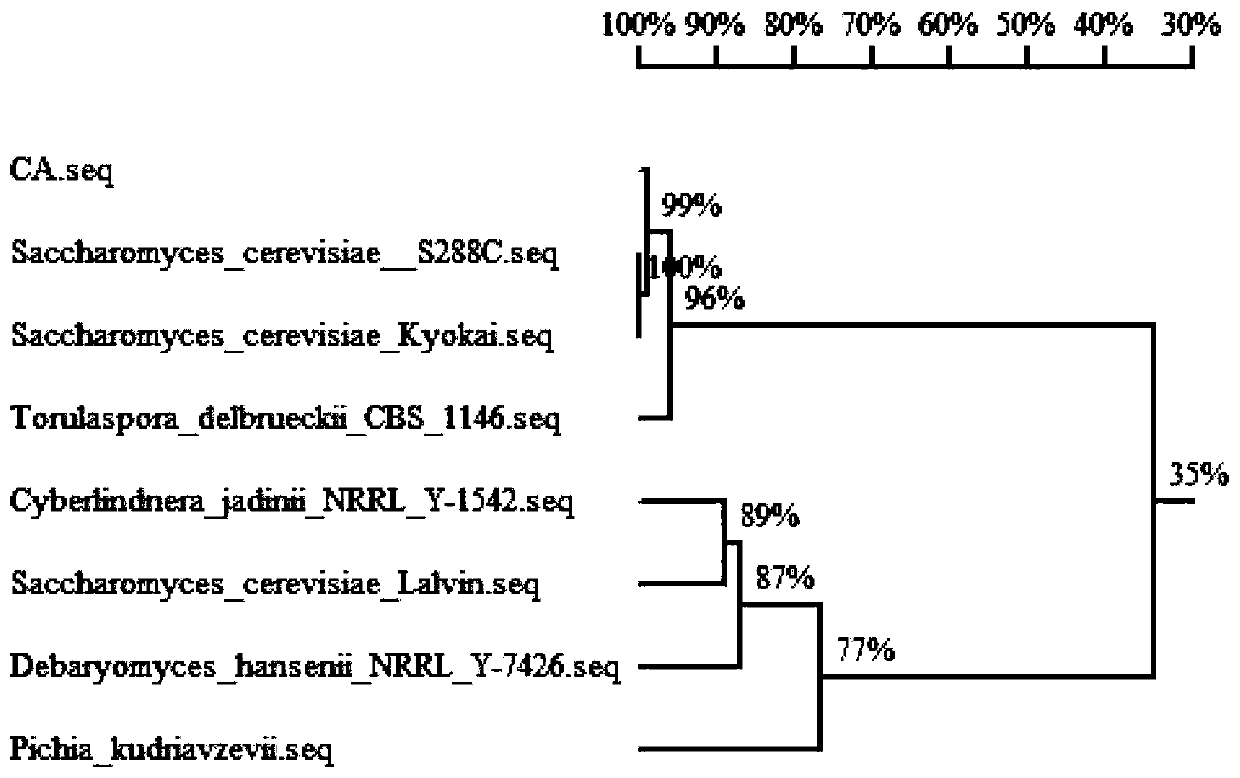
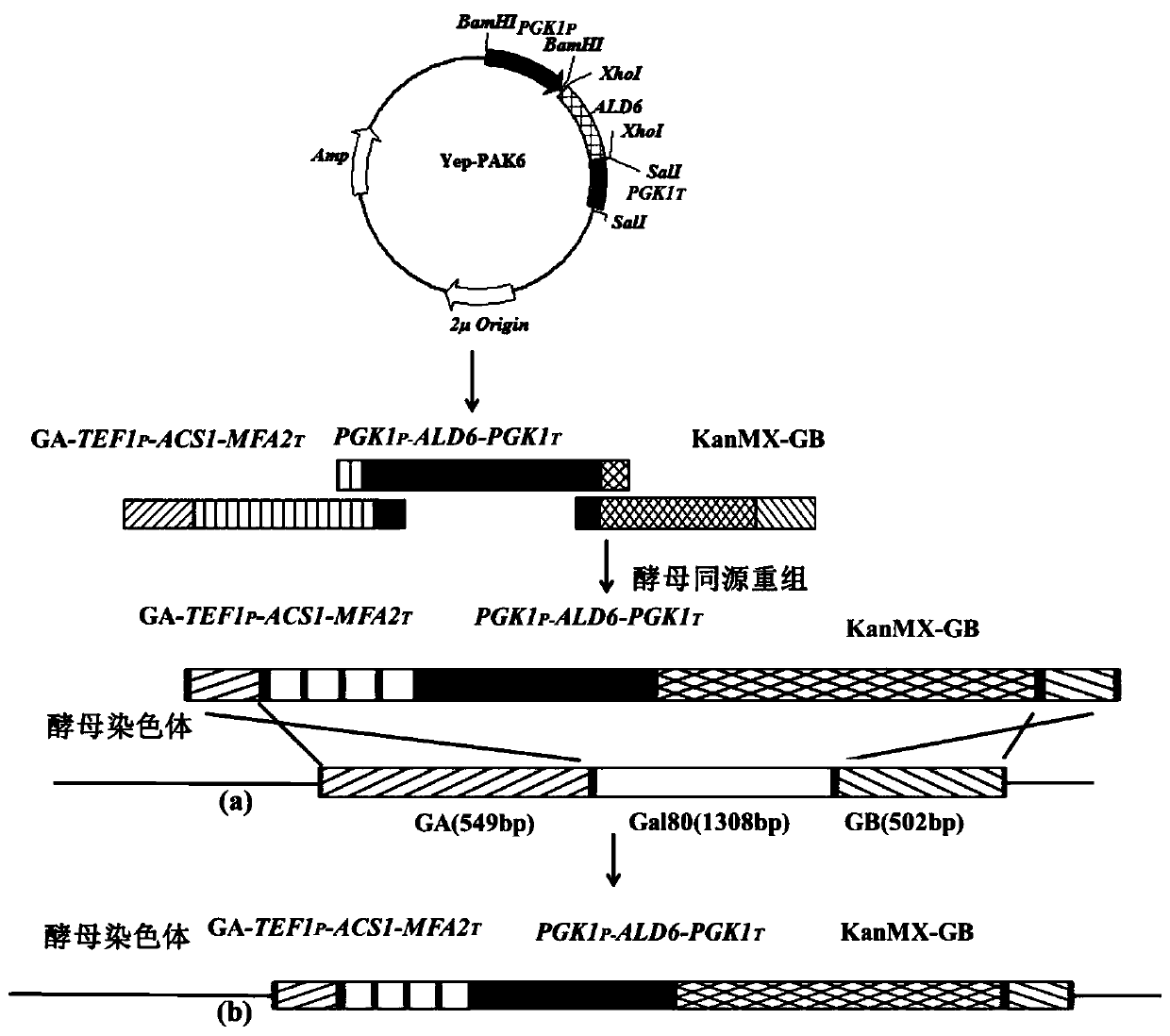
The invention discloses saccharomyces cerevisiae highly producing C6-C10 ethyl esters and a construction method and purpose of the saccharomyces cerevisiae, and belongs to the technical field of bioengineering. Through overexpressing acetaldehyde dehydrogenase ALD6 and acetyl-CoA synthase ASC1 in an original strain, synthesis of acetyl-CoA is strengthened, through overexpressing acetyl-CoA carboxylase ACC1**, malonyl CoA is strengthened, through overexpressing fatty acid synthetase FAS1 and fatty acid synthetase FAS2, medium-chain acyl-CoA is strengthened, more metabolism flows flow to medium-chain acyl groups CoA, alcohol acyl-transferase genes SAAT in strawberries are further exogenously introduced, and a reformed strain C-ald6acs1A*F1F2S is obtained. Under the condition of fermentation,the yield of ethyl caproate is 7.53mg / L which is 2.72 times of that of an original strain C-ald6acs1A*F1F2, and 26.89 times of that (only 0.27mg / L) of an original strain CA, the yield of ethyl caprylate is 13.65mg / L which is 9.11 times of that of the original strain CA, the yield of ethyl decanoate is 13.89mg / L which is 7.27 times of that of the original strain, and the saccharomyces cerevisiae has potential application prospects in improving flavor of wine and improving the quality of the wine.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Scorch-aroma essence for cigarettes and application thereof
PendingCN111187666AImprove aroma qualityIncrease the amount of aromaTobacco treatmentEssential-oils/perfumesBiotechnologyFuran
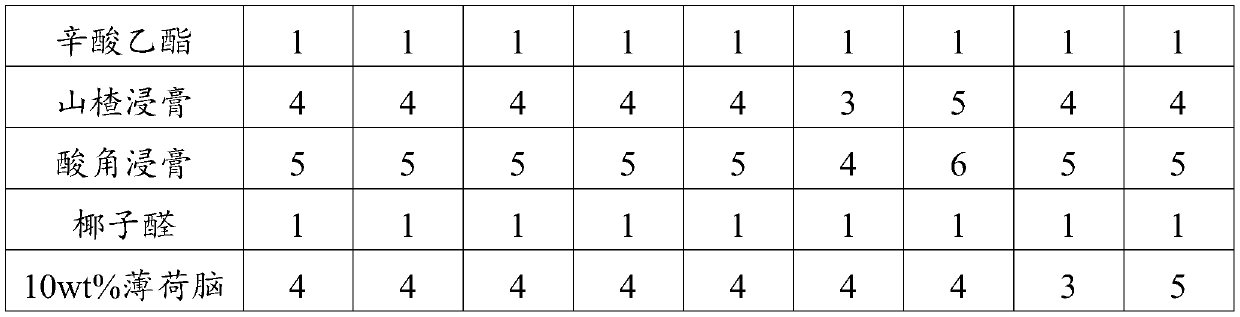
The invention provides scorch-aroma essence for cigarettes and application of the scorch-aroma essence, belonging to the technical field of tobaccos. According to the invention, a rye extract, methylcyclopentenolone, a Maillard reactant, a malt extract, 2-acetylfuran, methyl maltol and furanone function as monarch substances, and jointly reflect the dominant effect of scorch aroma; vanillin, perillartine, a cocoa extract and a Yuyan extract function as minister substances and assist in the embodiment of the scorch aroma; ethyl caprylate, a hawthorn extract and a tamarind fruit extract functionas assistant substances and play a supporting role; coconut aldehyde and menthol function as adjuvant substances and produce overall coordination effect to enable the whole essence to be completely and consistently fused.
Owner:广州科百欣香料有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate](https://images-eureka.patsnap.com/patent_img/dedce3e0-89a9-4c1f-8423-a087da736200/HDA0001711615060000011.png)
![Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate](https://images-eureka.patsnap.com/patent_img/dedce3e0-89a9-4c1f-8423-a087da736200/HDA0001711615060000021.png)
![Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate](https://images-eureka.patsnap.com/patent_img/dedce3e0-89a9-4c1f-8423-a087da736200/HDA0001711615060000022.png)