Patents
Literature
7825 results about "Green tea" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ultra-high fiber supplement and method of reducing weight cardiovascular risks and ingested toxins.
Owner:SMALL GIANT
Compositions containing green tea catechins and one or more polyvalent mineral cations
InactiveUS20050003068A1Promotes cardiovascular healthLower blood pressurePre-extraction tea treatmentTea extractionTurbidityGreen tea
Owner:KELLOGG NORTH AMERICA
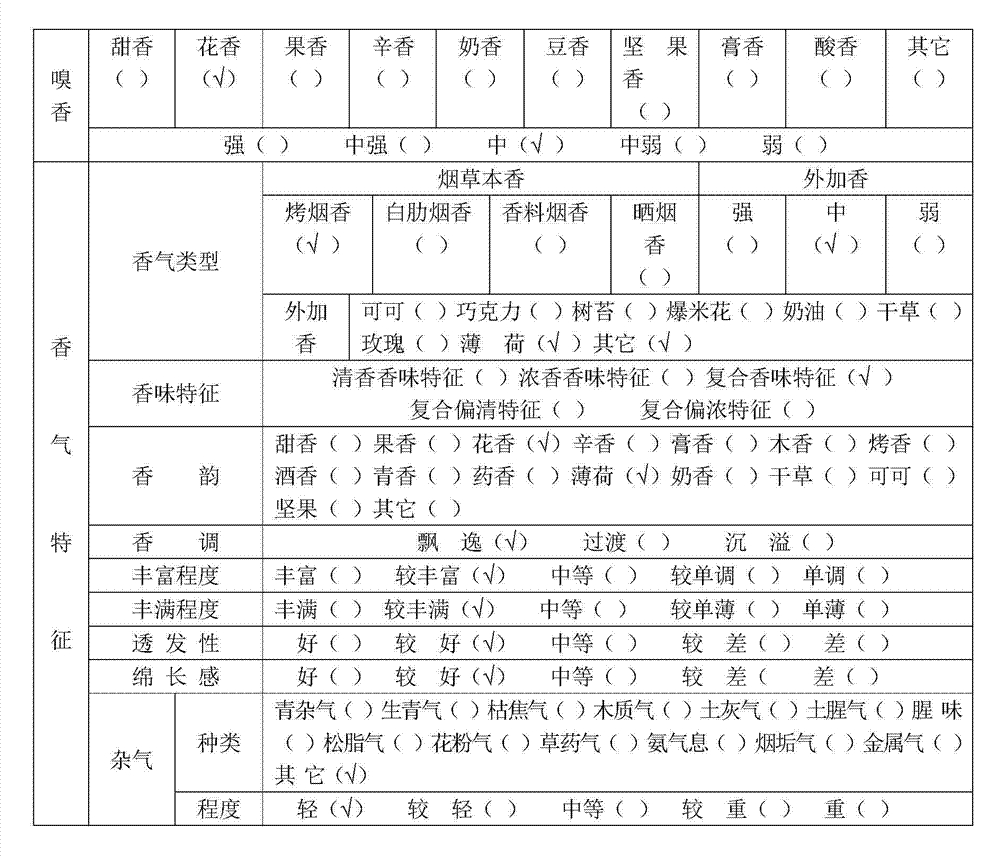
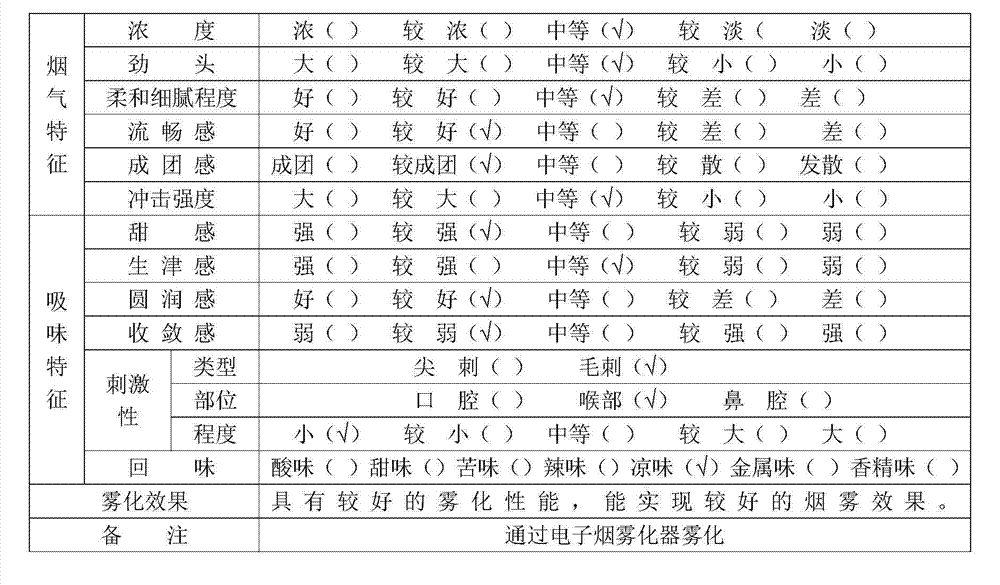
Light-fragrance electronic cigarette atomized smoke solution and preparation method thereof
The invention discloses light-fragrance electronic cigarette atomized smoke solution and a preparation method thereof. The atomized smoke solution comprises the following components in percentage by weight: 30-45 percent of propylene glycol, 20-35 percent of glycerol, 5-15 percent of deionized water, 5-15 percent of alcohol, 15-45 percent of traditional Chinese medicine liquid, 2-5 percent of tobacco flavor, 2-6 percent of tobacco extract and 0.1-2 percent of forming agent, wherein the traditional Chinese medicine liquid is extraction liquid which is obtained by extracting traditional Chinese medicine mixture through water distillation or alcohol extraction, and the traditional Chinese medicine mixture consists of tendrilleaf fritillary bulb, mint leaf, loquat leaf, dendranthema indicum, violet, sweet-scented osmanthus and green tea. The light-fragrance electronic cigarette atomized smoke solution not only can meet the smoking demands of smokers, but also can effectively reduce irritation of smoke to tracheas, bronchi, throats and lungs of bodies, and has the efficacies of clearing heat, moistening lungs, arresting coughs, expelling phlegm, refreshing and restoring consciousness, regulating qi-flowing, resolving stagnation and the like.
Owner:HUBEI CHINA TOBACCO IND +1
Tea beverage powder with function of relieving fatigue and preparation method of tea beverage powder
InactiveCN104222398ARelieve physical fatigueRelieve eye fatigueTea extractionLiver functionsNutrient solution
The invention discloses a tea beverage powder with the function of relieving fatigue and a preparation method of the tea beverage powder. The tea beverage powder is prepared from the raw materials in parts by weight: 15-25 parts of purple sweet potato, 10-20 parts of cranberry, 8-12 parts of dark green tea, 8-15 parts of nonfat yoghourt, 6-10 parts of spina date seeds, 10-14 parts of citrus limonum, 8-12 parts of wheat sprouts, 1-3 parts of Caragana sinica Rehd, 2-4 parts of cape jasmine flower, 1-2 parts of Eucommia ulmoides male flower, 2-3 parts of peanut coats, 2-3 parts of rhizoma polygonati, 2-3 parts of astragalus membranaceus, 1-3 parts of silkworm pupa protein, 8-15 parts of maple sugar, 1-2 parts of cocoa powder, 4-6 parts of ginger powder, an appropriate amount of sour plum vinegar and 8-12 parts of nutrient solution. The tea beverage powder with the function of relieving fatigue, which is prepared by adopting the preparation method, is rich and balanced in nutrients, convenient to store and transport and safe and sanitary to drink, can be drunk while brewing with mellow tea flavor and sweet mouthfeel, has the capabilities of relieving physical fatigue and visual fatigue and contributing to improving the memory and the liver function, and can be used for improving the physique and enhancing resistance to diseases after long-term drinking.
Owner:合肥瑾翔医药科技有限公司
Ultra-high fiber supplement and method of reducing weight, cardiovascular risks and ingested toxins
An improved ultra-high fiber supplement that promotes satiety, caloric reduction, and weight loss. The supplement comprises guar, oat, psyllium, locust bean gum, pectin, green tea, multi-anthocyanadins, pyridoxine, and folic acid. The supplement can exist as a liquid, semi-solid, or solid comestible. It improves cardiovascular health and reduces cardiovascular inflammation and the risk of heart disease. The addition of antioxidants, including green tea, improves weight loss, and general and cardiovascular health. Also it reduces serum lipoprotein oxidation and risk of free-radical related diseases. Consumption of the supplement aids in reducing absorption and assimilation of ingested toxins. A method of providing an ultra-high fiber comestible that is highly palatable and can be used to supplement nutrition and to manage and prevent diet-related diseases is disclosed. Further embodiments increase fiber and other nutrients in the diet and helps manage and prevent all diet-related diseases.
Owner:SMALL GIANT
Electronic cigarette liquid rich in plant flavone
ActiveCN104068467AMeeting smoking needsWill not affect healthTobacco treatmentNicotiana tabacumPropolis
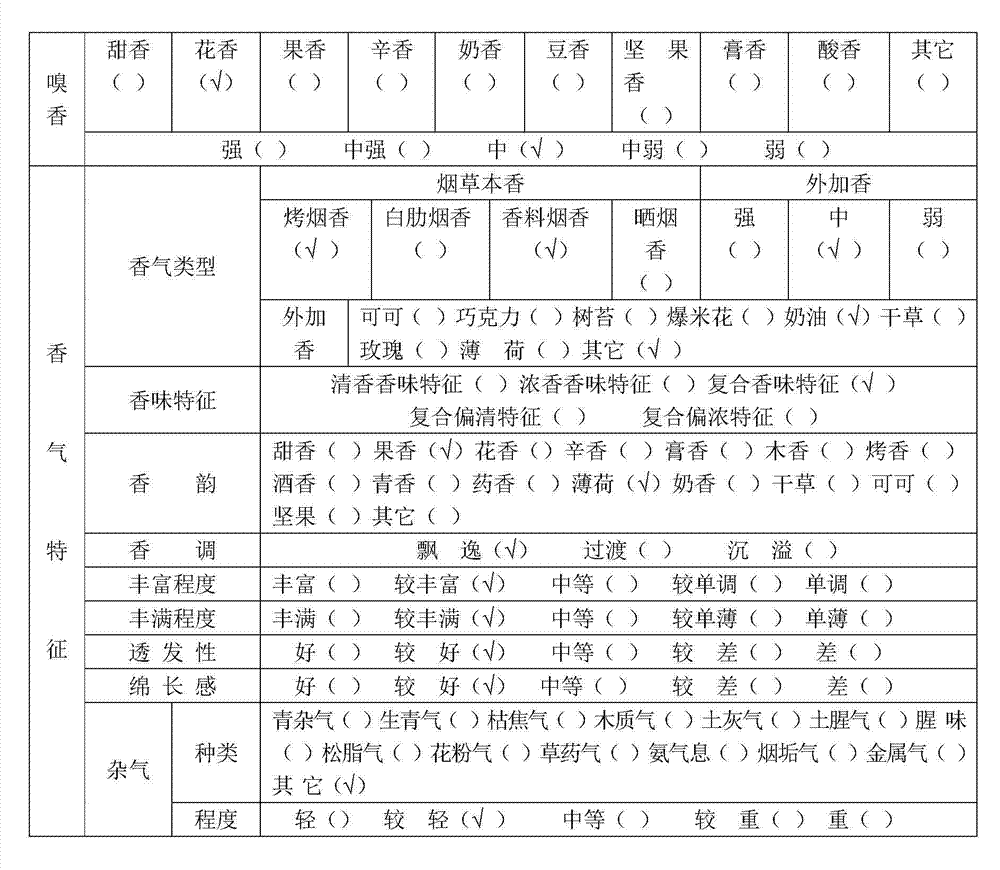
The invention discloses an electronic cigarette liquid rich in plant flavone. The electronic cigarette liquid comprises the following raw materials in percentage by mass: 50-70% of propylene glycol, 5-10% of glycerinum, 5-15% of deionized water, 5-15% of natural flavone extract, 4-8% of tobacco extract, 2-6% of tobacco essence and 2-6% of thickener, wherein the natural flavone extract is the extract obtained by distilling by vapour or extracting a plant mixture by ethyl alcohol, and the total flavonoid content is 20-50% by total flavonoid content; the plant mixture is composed of chuzhou chrysanthemum, iris tectorum roots, propolis, orange peel, grape, peanut coat, lily, radix puerariae, scutellaria baicalensis, roxburgh rose and green tea. The electronic cigarette liquid disclosed by the invention is capable of meeting the smoking needs of smokers; meanwhile, the rich plant flavone has the effects of resisting oxidization, preventing aging, and adjusting immunity, and also has a healthcare effect on heart and cerebral vessels to a certain extent.
Owner:CHINA TOBACCO ANHUI IND CO LTD
Nutraceutical composition and method of use for treatment / prevention of cancer
ActiveUS20100209388A1Easy to understandReduce weightOrganic active ingredientsBiocideMyrrh1,4-Benzoquinone
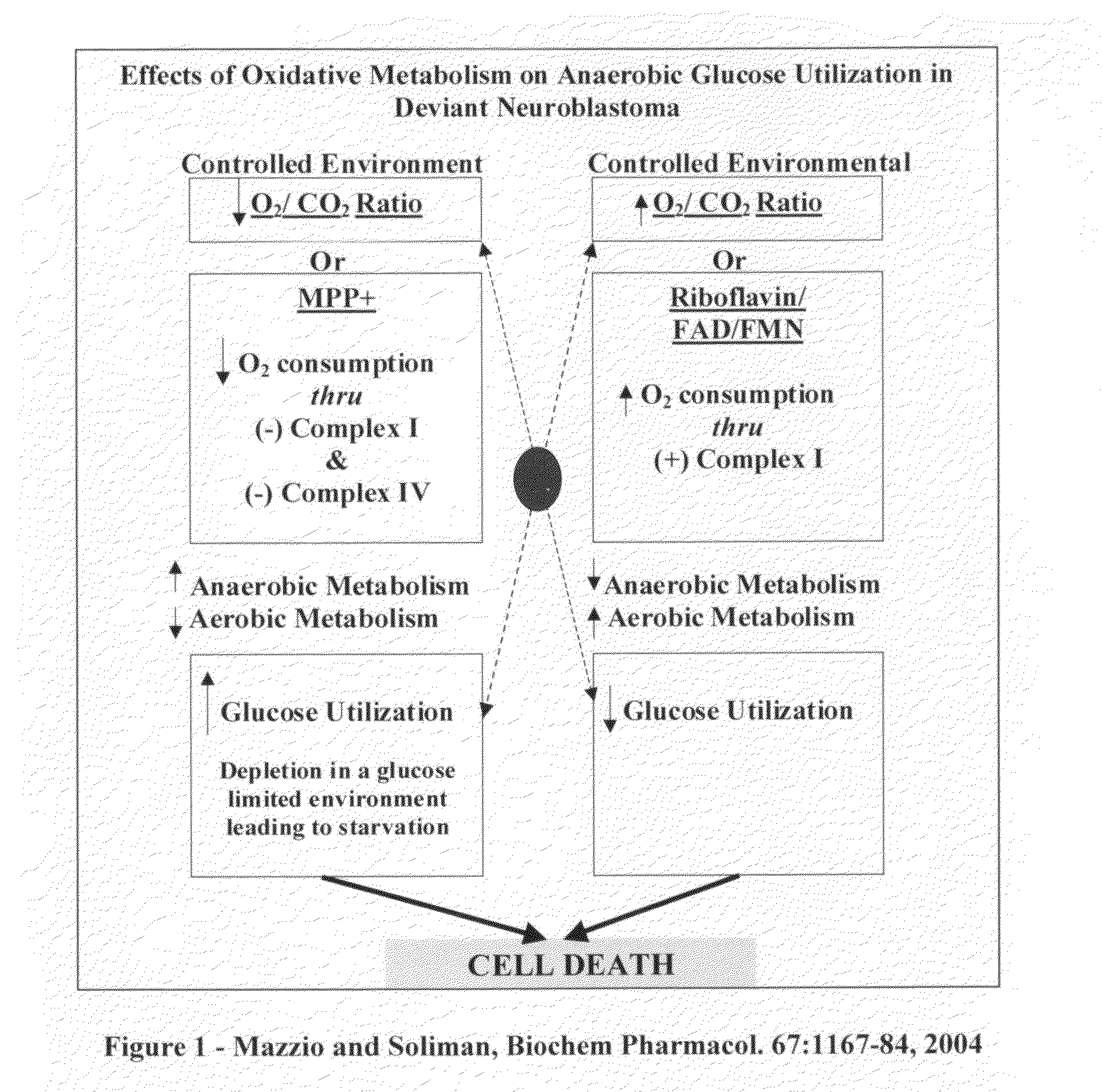
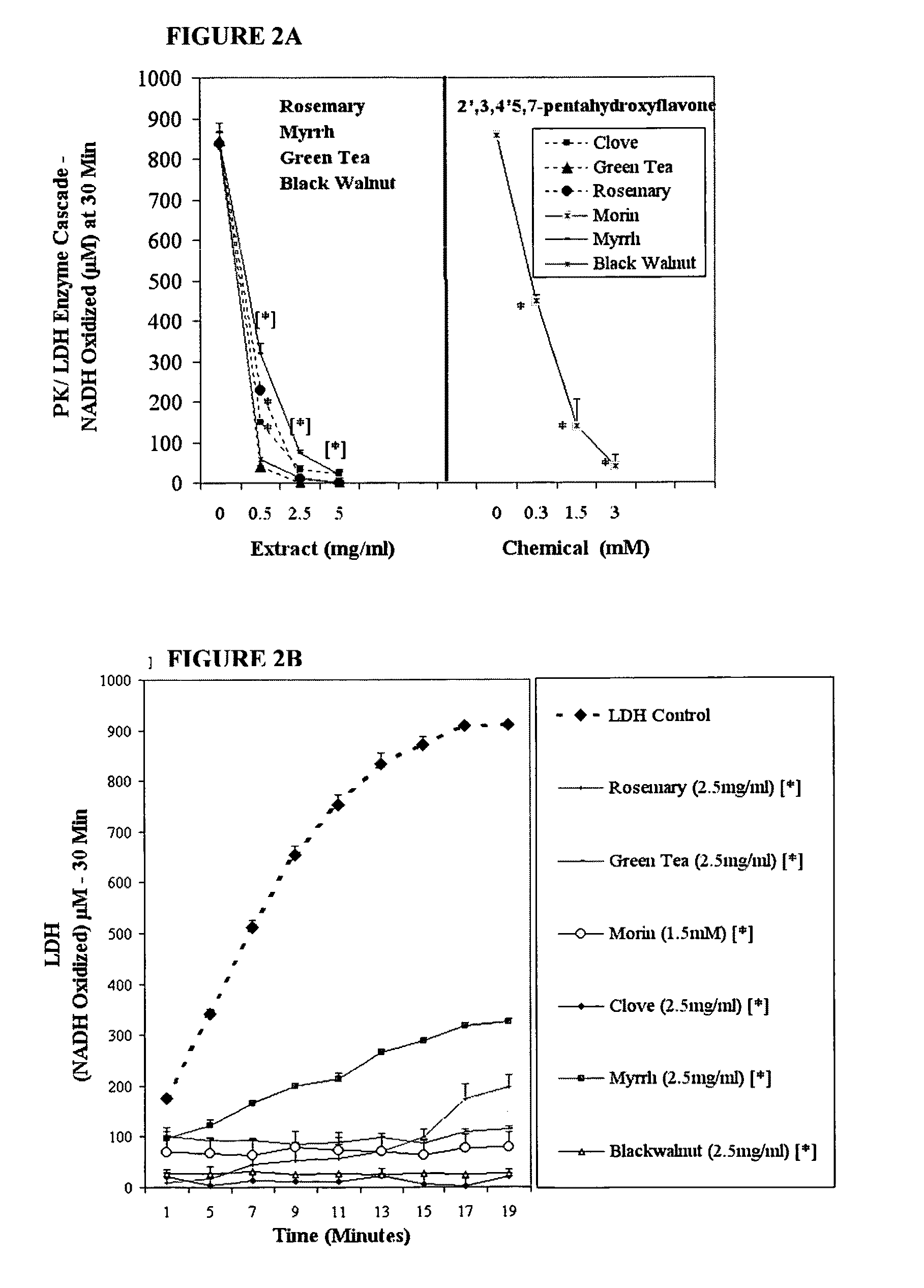
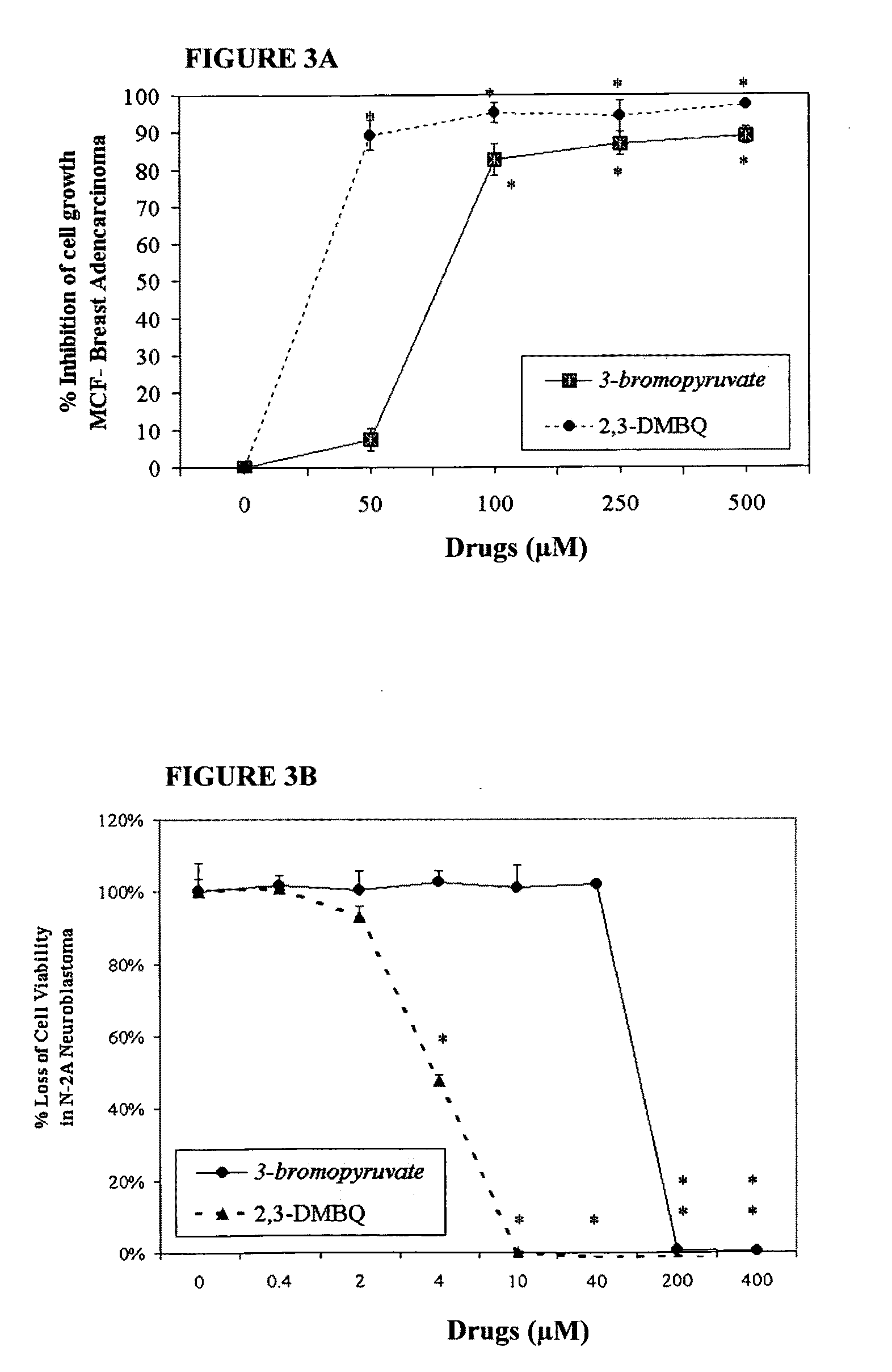
The invention describes a nutraceutical composition and method for preventing / treating cancer comprised of A) quinones (2,3-dimethoxy-5-methyl-1,4-benzoquinone, thymoquinone, tocopherolquinone) B) compounds capable of maximizing oxidative mitochondrial function preferably riboflavin, FAD, FMN, 6,7-Dimethyl-8-(1-D-ribityl)lumazine, ribitol, 5,6-dimethylbenzimidazole, tetrahydrobiopterin, vitamin B1, lipoic acid, biotin, vitamin B6, vitamin B12, folate, B3, C and pantothenate C) lactic acid dehydrogenase inhibitors; 2′,3,4′5,7-pentahydroxyflavone, epigallocatechin gallate, quercetin, citric acid, rosemary, black walnut, clove, nutmeg, licorice root, coriander, cinnamon, ginger root, myrrh gum and green tea D) alkalizing agents: aloe vera, chlorella, wheat grass, apple cider vinegar, burdock root, kudzu root, alfalfa, barley grass, spirulina, parsley leaf, calcium, magnesium, potassium or bicarbonate salts E) potent tumoricidal herbs; gromwell root, wild yam, beth root, teasel root, balm of gilead bud, frankincense, bakuchi seed, dichroa root, kochia seed, kanta kari, sweet myrrh, galbanum, garcinia fruit, mace, white sage and tumoricial plant derived constituents: gambogic acid, shikonin, diosmin or boswellic acid F) an antiproliferative herb (speranskia or goldenseal) and G) a pharmaceutically acceptable carrier.
Owner:FLORIDA A&M UNIVERSITY
Green tea formulations and methods of preparation
ActiveUS20050287278A1Large doseWell received naturalTea extractionTea substituesIntracellular substanceFood flavor
Green tea formulations and methods for the preparation thereof are disclosed and described. Generally speaking, the method of preparation includes the mixing of fresh tea leaves in an amount of cold water, followed by pulverization of the leaves to release their intracellular material from the cells of the green tea leaves into the water and form an aqueous extract component. The remaining cellular material forms a leaf residue component which is removed from the mixture. Once the leaf residue is removed, the aqueous extract component is collected and may be dried or further processed to produce a final tea extract that has good natural color, robust natural flavor, and pleasant organoleptic properties, which also is high in polyphenol content, and may be used for various purposes such as the creation of a green tea beverage.
Owner:XEL HERBACEUTICALS INC
Methods and compositions for modulating hair growth or regrowth
InactiveUS20070036742A1Inhibit synthesisIncreased vascularizationBiocideCosmetic preparationsAstaxanthinRed Clover
The present invention relates to compositions and methods for modulating hair growth or regrowth. The compositions of the present invention comprise extracts of one or more of the following: Boswellia serrata, Undaria pinnatifida, green tea (e.g., Camellia sinensis), shiso, Pureraria mirifica, luteolin (e.g. Perilla ocymoides leaf extract), astilbin, vitamin E, amentoflavone, tetrahydropiperine, licochalcone, astaxanthin, red clover, Brassica juncea, unfermented green rooibos, enzyme CoQ10, salvia, ximenynic acid, hops oleoresin, apple, soy, saw palmetto, or ellagic extract, or any derivative thereof. In particular, the compositions and methods of the present invention can be used to stimulate or increase hair growth and / or prevent or slow the loss of hair by having one or more of the following functions: (a) inhibiting synthesis of DHT; (b) inhibiting proteasomal activity; (c) inhibiting IL-1 activity; (d) increasing vascularization; (e) increasing expression of vascular endothelial growth factor; (f) increasing expression of keratinocyte growth factor; (g) inhibiting inflammation; or (h) acting as an antibacterial.
Owner:ACCESS BUSINESS GRP INT LLC
Method for planting organic tea tree
Owner:桂平市西山茶场
Tea jelly sweet
The invention discloses a tea jelly sweet which is prepared from raw material in the formula in parts by weight: 4-10 parts of functional composition, 30-60 parts of maltitol, 30-40 parts of starch syrup, 3.5-7 parts of plural gel, 0.1-10 parts of lecithin, 0.1-3 parts of hydrogenated vegetable oil, 1-3 parts of peppermint oil and 20-40 parts of water, wherein the functional composition comprises one or several of ultra-micropowder or extractions or original materials of pu-erh tea, black tea, green tea, Oolong tea, white tea, yellow tea and dark tea and other functional factors; and the plural gel comprises modified starch, gelatine, agar and carrageenan. The invention has the characteristics of defined functions, attractive appearance, natural aroma, good taste and the like, has no artificially-synthesized pigment and essence and sweetner causing decayed tooth and is suitable for groups to eat for a long term so as to improve the sub-health state.
Owner:云南龙润茶业集团有限公司
Nutritional balanced vegetarian diet and preparing method thereof
InactiveCN102763799AGood for healthEnhance anti-aging and life extensionFood preparationBiotechnologyYeast Proteins
The invention relates to a nutritional balanced vegetarian diet which is characterized by comprising components A, B and C. The component A comprises brown rice, fragrant rice, black rice, cooked brown rice, glutinous rice, oats, sorghum, millet, corn, soybeans, black beans, red beans, white beans, mung beans, peanuts, sesame seeds, barley, apple, banana, mango, orange, strawberry, tomato, carrot, pumpkin, celery, konjac, broccoli, cabbage, mushroom, undaria pinnatifida, seaweed, lily, green tea and pueraria. The component B comprises lecithin, oligosaccharide alcohol prebiotics, and rice bran. The component C comprises nutritional yeast and probiotics. Compared with the prior art, the nutritional balanced vegetarian diet provides all the essential nutrients to the human body.
Owner:NINGBO YUFANGTANG BIOTECH
Materials and Methods for Treating Conditions Associated with Pathogenic Biofilm
ActiveUS20140037688A1Improve efficacyImprove securityAntibacterial agentsCosmetic preparationsMicroorganismAdditive ingredient
The subject invention provides materials and methods that effectively support innate immunity and / or disperse pathogenic biofilms using readily available, nontoxic, natural substances, while supporting restoration of normal microbiotic homeostasis. In one embodiment, the subject invention provides anti-biofilm compositions comprising one or more probiotic organisms, anti-microbial honey, and other ingredients such as prebiotic compounds, other hive products, green tea derivatives, other plant derivatives, and vitamin D3.
Owner:QUORUM INNOVATIONS
Satiety promoting beverage and use in a diet to moderate food consumption
InactiveUS20060188548A1Encourage their consumptionIncrease volumeBiocideMilk preparationFiberRegimen
A satiety promoting beverage and a weight control regimen using it are described comprising: fiber, protein, sweetener and preferably calcium and a catechin, such as purified epigallocatechin gallate derived from green tea. The ingredients are employed in amounts sufficient that a single serving provides a feeling of satiety, both initially and after a period of at least one hour. In a preferred form, a serving of the beverage will comprise fiber in an amount sufficient to supply from 10 to 30% of the DV for fiber; milk protein in an amount sufficient to supply from 4 to 10 grams protein; calcium in an amount sufficient to supply from 10 to 40% of the DV for calcium; a low calorie sweetener and, preferably, the catechin in an amount sufficient to supply from 2 to 4 cup equivalents. The ingredients are homogenized and packaged to provide a smooth textured beverage with a creamy mouthfeel and from about 50 to about 150 calories per serving of from about 4 to 12 ounces. A Fullness Index of greater than 4, and preferably 5 is evidence of both very high volume to calorie and (fiber plus protein) to calorie ratios.
Owner:LIGHTFULL FOODS INC A DELAWARE
Black carp feed and its preparation method
InactiveCN103494014AMeet the needs of growthPromote growthFood processingClimate change adaptationAnimal sciencePollen
The invention discloses a black carp feed and its preparation method, and the black carp feed comprises the following raw materials: by weight, 12-14 parts of freshwater shrimp powder, 30-34 parts of sorghum flour, 12-14 parts of soybean meal, 7-9 parts of sweet potato powder, 3-4 parts of loach powder, 4-5 parts of corn protein powder, 2-3 parts of tenebrio molitor, 1-2 parts of salt, 1-2 parts of jasmine flower powder, 2-3 parts of green tea powder, 2-3 parts of violet pollen, 1-2 parts of clove, 2-3 parts of roughhaired holly root, 1-2 parts of galium aparine and 3-4 parts of a feeding promoting agent; and the black carp feed is scientific and reasonable in formulation, can meet the growth needs of black carp in every stage, effectively improves the meat quality and nutritional value of the black carp, promotes the growth of the black carp, facilitates management, is convenient to prepare, and effectively controls the feed cost.
Owner:HEFEI QIANXI MOUNTAIN VILLA AGRI ECOLOGICAL PARK
Use of polyphenols to treat skin conditions
InactiveUS20030175234A1Reduce skin of skin of skinReduce surfaceBiocideCosmetic preparationsWrinkle skinHypopigmentation
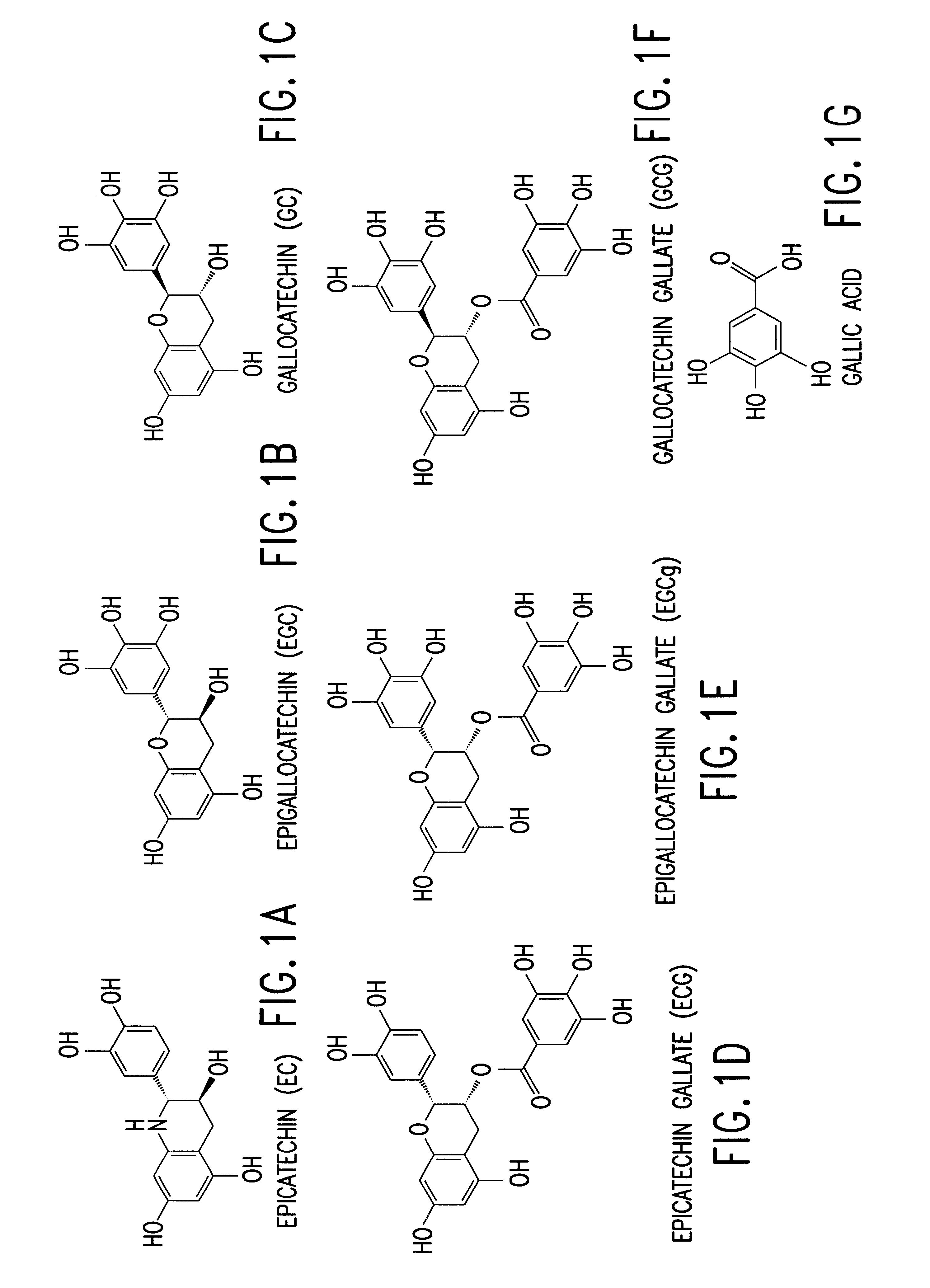
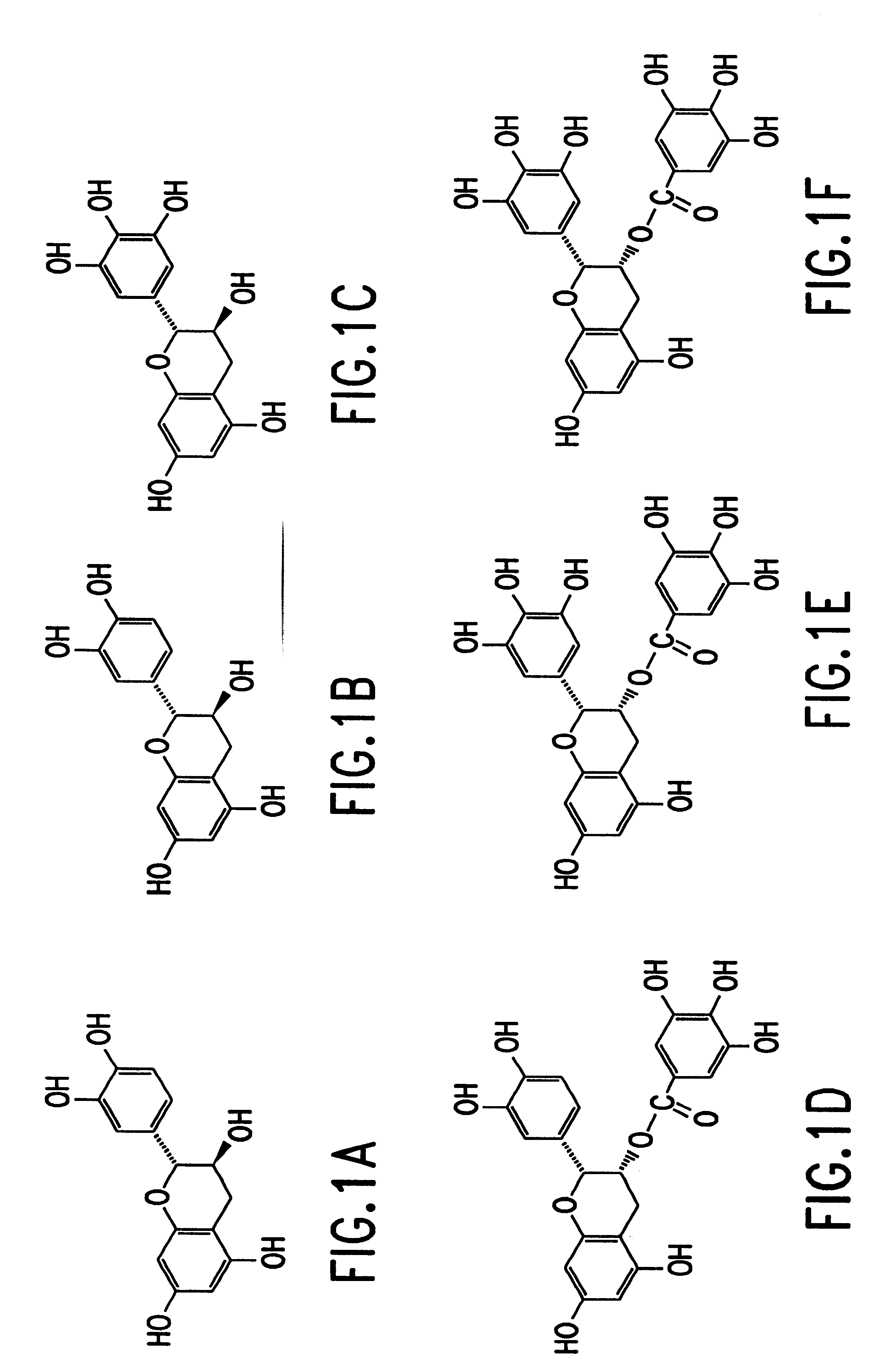
A composition is provided to treat and / or prevent for treating skin conditions including fine lines and wrinkles, acne rosacea, surface irregularities of the skin and hyper-pigmentation melasma of the skin, which includes use of a topically applied composition such as an ointment, lotion, cream, serum, gel or pad applied formulation, containing an effective amount of polyphenols, such as green tea based polyphenols, including but not limited to catechins, such as epigallocatechin gallate (EGCG), epigallocatechin (EGC), epicathechin 3-gallate (ECG) and epicatechin (EC). The formulation is preferably composed of 90 percent polyphenol isolates, derived from green tea with potent antioxidant properties, to assist in minimizing free-radical induced skin damage.
Owner:TOPIX PHARMA INC
Production method for improving summer green tea quality
A production method for improving quality of summer green tea comprises picking summer fresh tea, spreading at 18-22deg.C for 20-25h, evaporation deenzyming under 0.03kg / m2-0.05kg / m2 for 40-60s, cooling to normal temperature, sprinkling tannase and / or cellulase aqueous solution 0.5-1.5wt. parts for per 45 fresh tea, twisting, unblocking, oven drying, and parching, wherein the enzyme liquid contains tannase and cellulase equal to 15000 u / g tannase powder and cellulase powder 50mg.The method is suitable for improving quality of summer tea with two or three leaves and a bud. The method reduces the content of ester-catechin in summer tea, increases the content of amino acid and soluble sugar, reduces astringency of the tea, and improves the taste of tea, thereby making full use of material of summer tea and improving effectiveness of operations of tea garden.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Roasted famous high-quality green tea and production process thereof
InactiveCN102669313AGuaranteed qualityPromote hydrolysisPre-extraction tea treatmentTemperature controlGreen tea
The invention relates to roasted famous high-quality green tea and a production process thereof, belongs to the technical field of tea processing, and provides a production process of roasted famous high-quality green tea to effectively improve quality of the green tea. The production process includes steps of fresh tea leaf fixation, shaping and drying. Fresh tea leaves are further cured before fixation by placing the fresh tea leaves in a precision temperature control device at the set temperature of 35DEG C to 36 DEG C for 2-4 hours of curing.
Owner:中国测试技术研究院生物研究所
Green tea processing method for reducing bitterness of autumn tea and summer tea
ActiveCN102940053AReduce bitternessFull-bodied taste qualityPre-extraction tea treatmentGreen teaTea leaf
The invention discloses a green tea processing method for reducing bitterness of autumn tea and summer tea, comprising the following steps:1) selecting tea leaves: selecting fresh tea leaves of autumn and summer and grading; 2) withering: spreading the fresh tea leaves for withering, wherein the water content of the withered leaves is 70-75%; 3) conducting fine manipulation: carrying out fine manipulation on the withered leaves to obtain fine-manipulated leaves, wherein fine manipulation comprises rotating and cooling which are alternately and repeatedly; 4) de-enzyming: de-enzyming the fine-manipulated leaves to obtain de-enzymed leaves; 5) dampening: dampening the de-enzymed leaves; 6) rolling: rolling the dampened leaves; 7) drying: using a gross fire to dry the rolled leaves until the water content is 10-20%, then using a complete fire to dry, wherein the water content of the dried leaves is 4-6%. According to the invention, by using the fresh tea leaves and optimizing the processing method, the produced green tea can reduce the bitterness of tea, thus fragrance of green tea with natural fragrance of flower is formed, and the taste quality is thick and mellow.
Owner:婺源县聚芳永茶业有限公司
Hubei Chinese flowering crabapple tea and preparation method and application of extractive thereof
ActiveCN101199301ATake advantage ofPre-extraction tea treatmentMetabolism disorderAntioxidantChinese flowering crabapple
The invention discloses a preparation method and the application of Hubei crabapple tea and the extract, which comprises the preparation method of Hubei crabapple black tea, green tea, brick tea, concentrated slices and pigment as well as the processing method of bottled Hubei crabapple beverage and the application of the Hubei crabapple tea in blood sugar and blood fat lowering drugs. The invention gets a plurality of Hubei crabapple products and fully utilizes the characteristics of the Hubei crabapple of itself, so the invention is also well applied in the blood sugar and blood fat lowering drugs as well as in antioxidant.
Owner:YICHANG HUMANWELL PHARMA +1
Green tea beverage, and its prepn. method
InactiveCN1718030AIncrease nutritional contentImprove retentionTea extractionNutritive valuesGreen tea
A green tea beverage is prepared from the fresh organic green tea leaves through breaking, squeezing to obtain tea juice, filtering, fine filtering by membrane, mixing it with water in ratio of 1:(15-25),membrane filtering for removing bacteria, and aseptic canning. Its advantages are unique fragrance and high nutritive value.
Owner:YUNNAN LANCANGJIANG BEER ENTERPRISE GRP CO LTD
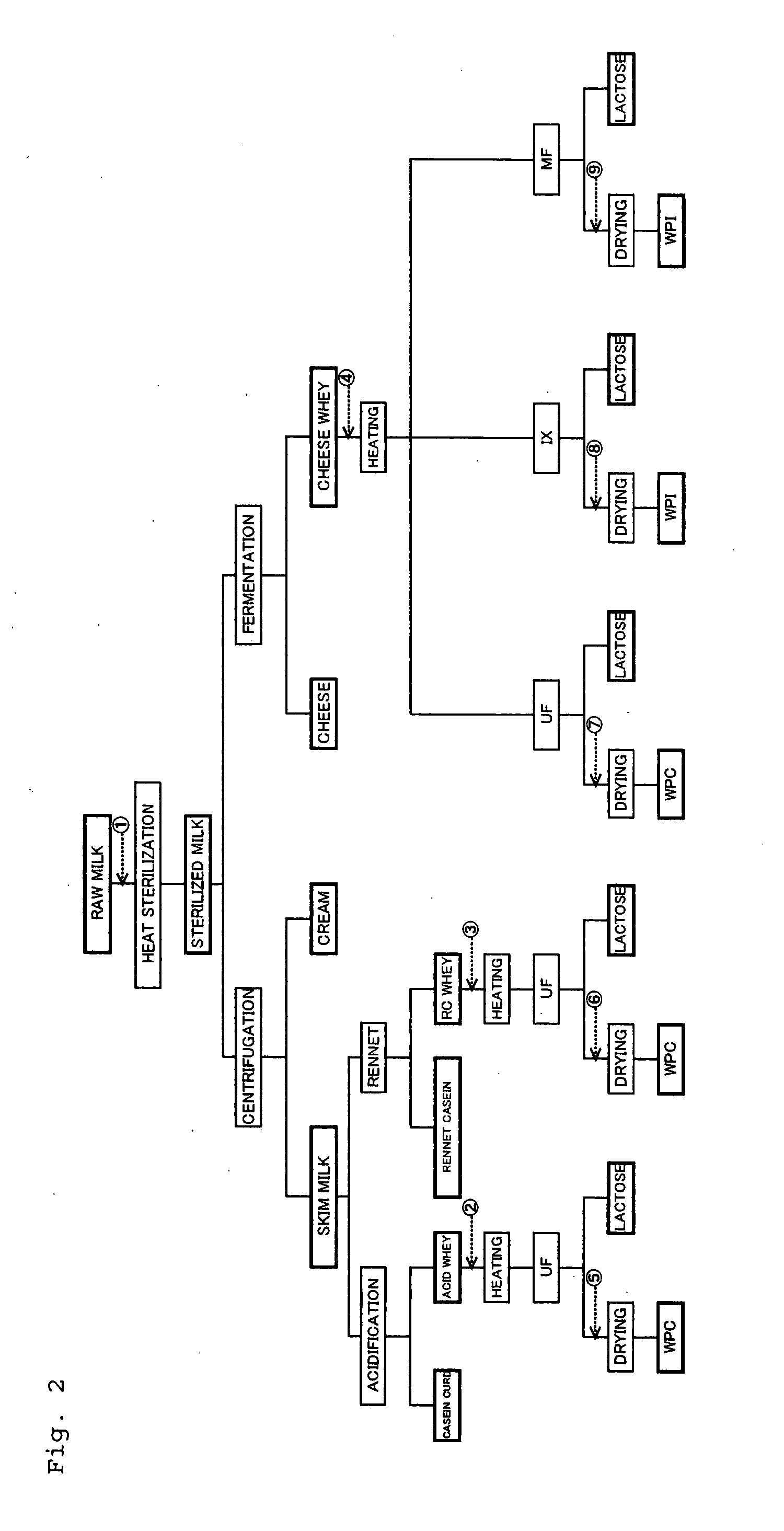
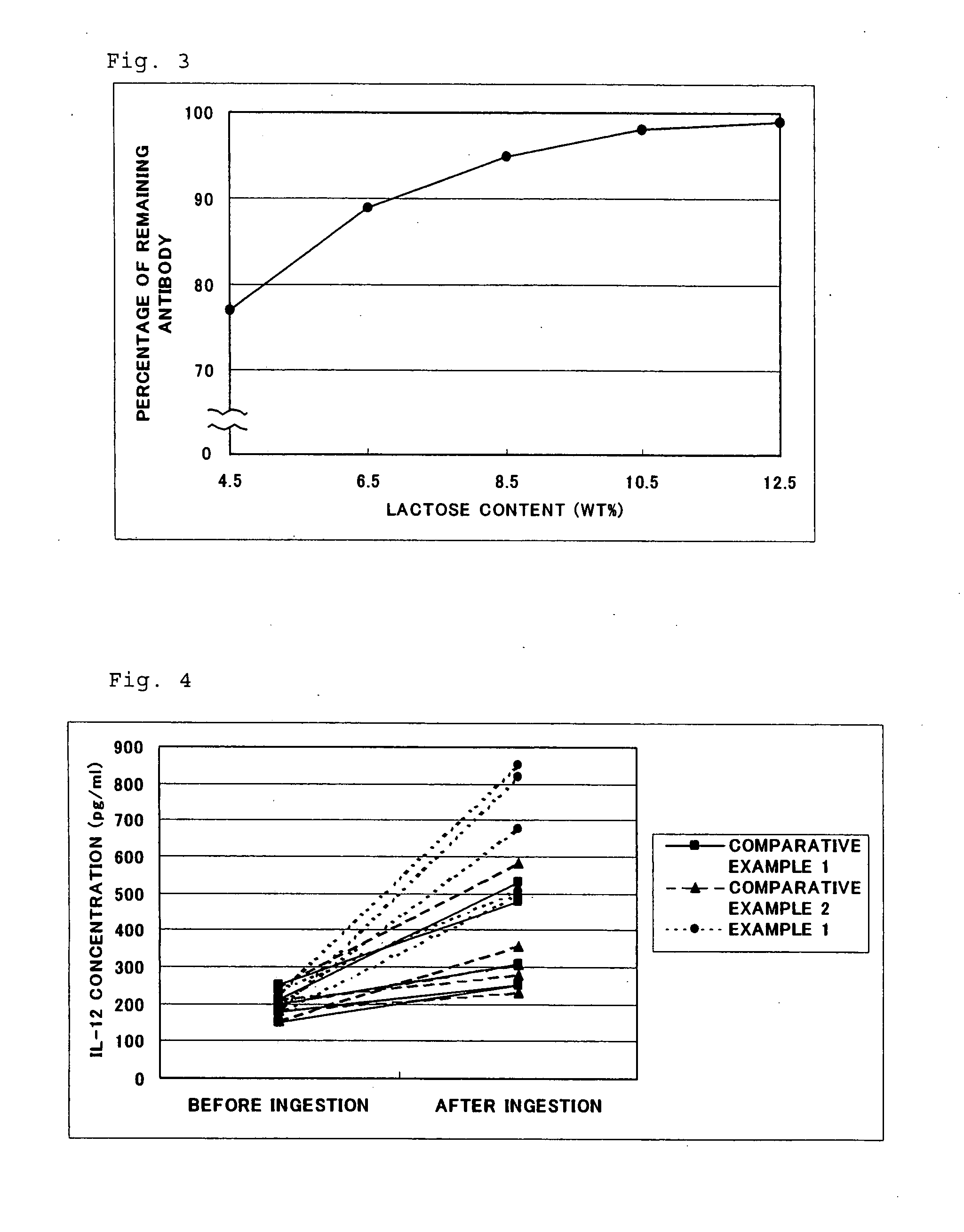
Functional Composition Or Food Comprising Whey Protein, Antibody Derived From Milk Or Antibody
There is provided a functional composition or food comprising whey protein, an antibody derived from milk or the other antibody; and a whey protein food comprising whey protein and glucide, cocoa powder, powdery an unsolidifiable and insoluble substance, green tea, aloe, turmeric, pumpkin, red grape juice, tomato, cranberry, raspberry, blueberry or strawberry powder.
Owner:ASAMA CHEM +1
Extracts and Methods Comprising Green Tea Species
InactiveUS20080113044A1Great beneficial activityBiocideTea extractionGreen teaEpigallo-catechin gallate
Owner:HERBALSCI SINGAPORE PTE
Instant pu'er tea and its preparation method
The invention discloses a rapid-soluble Pu'er tea and preparing method to accelerate body to release energy, which comprises the following steps: blending Pu'er tea and ripe tea ( or black tea and green tea) according to certain proportion; grinding roughly; extracting through water; condensing; drying; adding beta-cyclodextrin; recycling aged perfume benzine ingredient through steam distilling method or super-critical extracting method; packing the benzine and rapid-soluble tea powder together; adding isomaltose hypgather, degrading factor and flavoring factor.
Owner:YUNNAN LONGRUN TEA TECH
Method for processing green tea
InactiveCN101427714AReduced quality impactImprove integrityPre-extraction tea treatmentFlavorDrying time
The invention discloses a green-tea processing method, comprising: fresh leaves withering; de-enzyming, rolling, early drying, uniformly drying, fully drying at 80-100 degree for 10-15 min; the leaves image being changed from tender green to greenish black and the hand being not punctured with dehydration ratio of 30-35%; fully sweating the tea leaves after cooling' shaping in a double-pan roasting machine at 80-100degree and the early-drying temperature being from low to high when the leaf-feeding amount is major pan and the early-drying time being 40-45 minutes; instant scattering and cooling after early shaping and then continuously shaping the cooled leaves in the double-pan roasting machine for 50-60minitue at 60-80degree; cooling when the tea leaves are smooth and 75% dry; drying; sieving and classifying. The green-tea processing method can increase the tea leaves color, aroma, liquor color, flavor and integrity degree of leaf-base and produce high-grade tea leaves using common material.
Owner:贵州贵茶(集团)有限公司
Tea catechin formulations and processes for making same
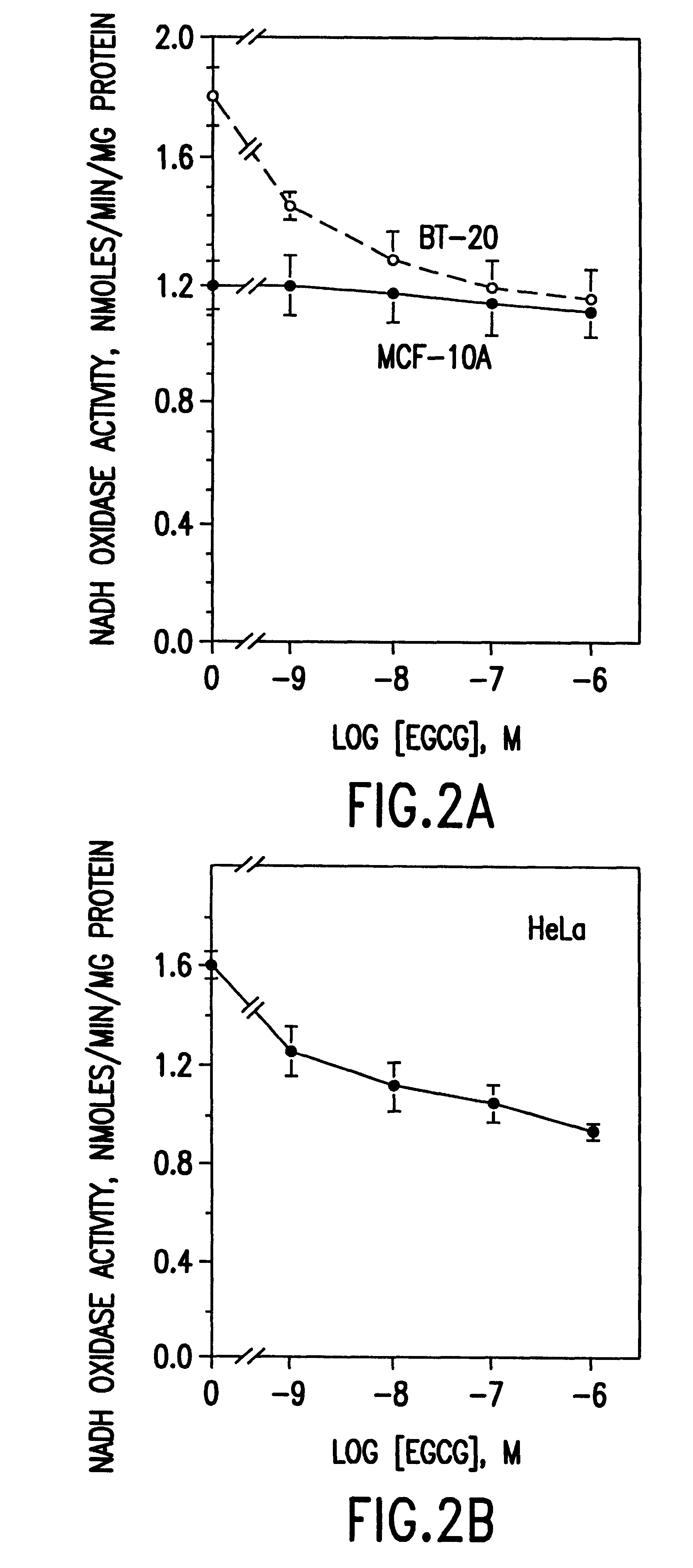
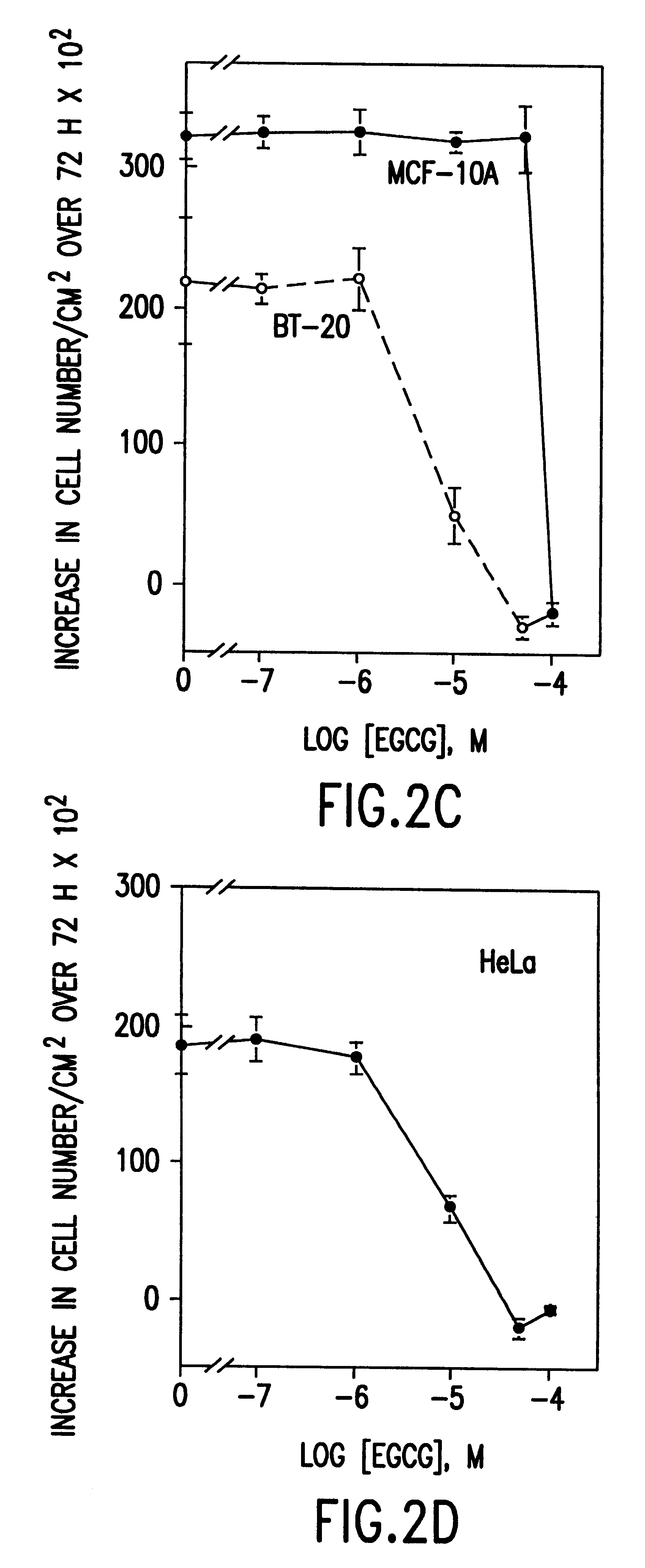
InactiveUS6428818B1Inhibit activity of specificMinimal levelBiocideHydroxy compound active ingredientsSolid tumorGreen tea
The invention described herein encompasses methods and compositions of treating cancer or solid tumors comprising the administration of a therapeutically effective amount of catechins, a group of polyphenols found in green tea, to a mammal in need of such therapy. Compositions of catechins include but not limited to, epigallocatechin gallate (EGCg), epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC). The unique compositions of the invention contain various combinations of the catechins with reduced levels of EGCg, alone or in combination with each other or other therapeutic agents and are used to treat primary and metastatic cancers in humans. The invention also encompasses the varying modes of administration of the therapeutic compounds.
Owner:PURDUE RES FOUND INC +1
Method for production of organic green-tea
InactiveCN100998357AImprove insulation effectImprove heat utilizationPre-extraction tea treatmentTea flavoringGreen teaEpigallo-catechin gallate
A process for preparing the organic green tea based on Huang-mountain Maofeng tea includes such steps as spreading fresh tea leaves for drying in the air, heating, shaping, fastly baking for fixating color, parching for fixating shape, improving smell and refining. The details about the step of improving its smell are also disclosed. Its product is good in color, taste and smell.
Owner:黄山光明茶业有限公司
Tea catechins as cancer specific proliferation inhibitors
InactiveUS6410061B1Good treatment effectReduce adverse effectsBiocideHydroxy compound active ingredientsSolid tumorGreen tea
The invention described herein encompasses a methods and compositions of treating cancer or solid tumors comprising the administration of a therapeutically effective amount of catechins, a group of polyphenols found in green tea, to a mammal in need of such therapy. Compositions of catechins include but not limited to, epigallocatechin gallate (EGCg), epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC). The unique compositions of the invention contain various combinations of the catechins, alone or in combination with each other or other therapeutic agents and are used to treat primary and metastatic cancers in humans. The invention also encompasses the varying modes of administration of the therapeutic compounds.
Owner:PURDUE RES FOUND INC
Ligaloes tea and its preparing process
ActiveCN101095438AEasy to brewGreat tastePre-extraction tea treatmentClimate change adaptationGreen teaEpigallo-catechin gallate
The invention relates to a kind of combae tea and the method for preparing the same, which comprises following steps: (1)picking fresh leaves, (2)airing, (3)water-removing leaves, (4)sun drying leaves, (5)cutting leaves, (6)mixing old and mature leaves with young leaves, (7)perfuming leaves, (8)packing, (9)getting finished product. The invention is characterized in that the old and mature leaves are mixed with fresh leaves with a certain proportion, the packing is much easier by using machine after cut, the taste of tea is good, and no additive is added during production process. It is a organic green tea product.
Owner:汪科元
Method for processing cured meat
The invention discloses a method for processing cured meat, which mainly comprises the following steps: a, selecting materials, refrigerating and slitting; b, smearing spice, kneading and standing; c, cleaning; d, airing and hanging; and e, smoke curing. The method for processing the cured meat has the following characteristics: firstly, the selected spice is prepared by mixing green tea, wild pepper, anise, tsaoko, geranium, ganoderma lucidum, patchouli and cumin, the selection of the spice is scientific and the using amount is reasonable, wherein the green tea component not only can inhibit the generation of nitrite but also can inhibit the absorption of human large intestines on fat so that the cured meat reduces the burden of the human intestines and stomach caused by the fat at the same time of having unique fragrance; and secondly, the method strictly controls various process indexes during processing, and adopts active carbon to absorb benzopyrene in fume mist at a smoke curing stage so that the nitrite residue in a cured meat product is little, the benzopyrene content is low, and the cancer risk of the human body caused by the salt-cured product is further reduced.
Owner:巫溪县李大姐肉食品有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com