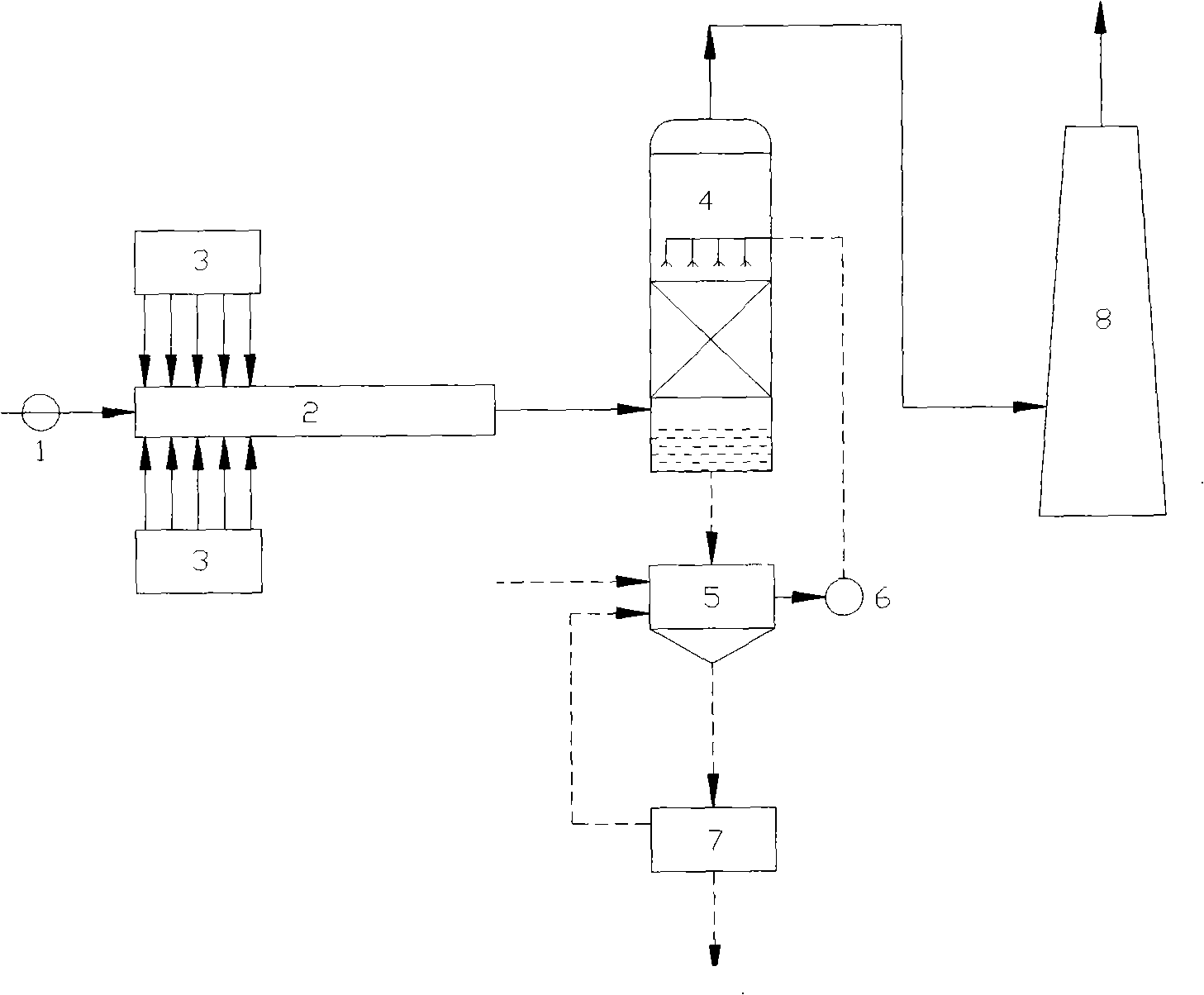Patents
Literature
3132 results about "Nitrite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The nitrite ion, which has the chemical formula NO⁻₂, is a symmetric anion with equal N–O bond lengths. Upon protonation, the unstable weak acid nitrous acid is produced. Nitrite can be oxidized or reduced, with the product somewhat dependent on the oxidizing/reducing agent and its strength. The nitrite ion is an ambidentate ligand, and is known to bond to metal centers in at least five different ways. Nitrite is also important in biochemistry as a source of the potent vasodilator nitric oxide. In organic chemistry the NO⁻₂ group is present in nitrous acid esters and nitro compounds. Nitrite (mostly sodium nitrite) is also used in the food production industry for curing meat.
Process for the removal of nitrogen compounds from a fluid stream
ActiveUS7205448B2Increase capacityReduce accumulationMolecular sieve catalystsHydrocarbonsMolecular sieveNitrite
At lower temperatures an acidic molecular sieve adsorbent preferentially adsorbs water and basic organic nitrogen compounds over weakly basic organic nitrogen compounds such as nitrites. Elevated temperatures improve the capacity of acidic molecular sieve adsorbents to adsorb nitrites in the presence of water.
Owner:UOP LLC
Preparation method of ticagrelor
ActiveCN102675321AHigh yieldReduce manufacturing costOrganic chemistryBulk chemical productionNitriteTicagrelor
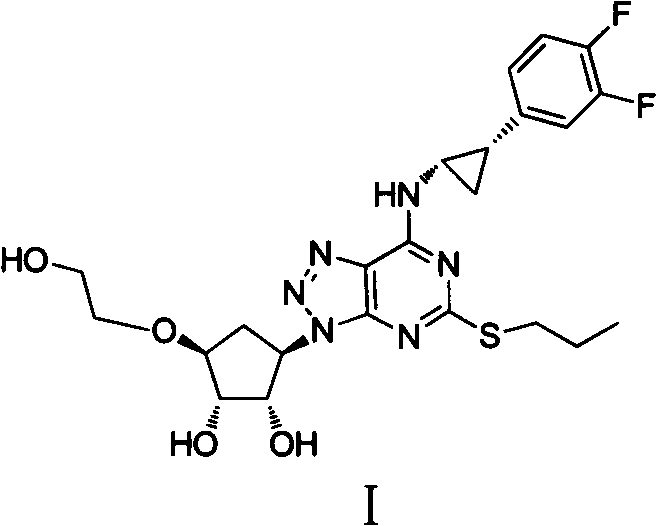
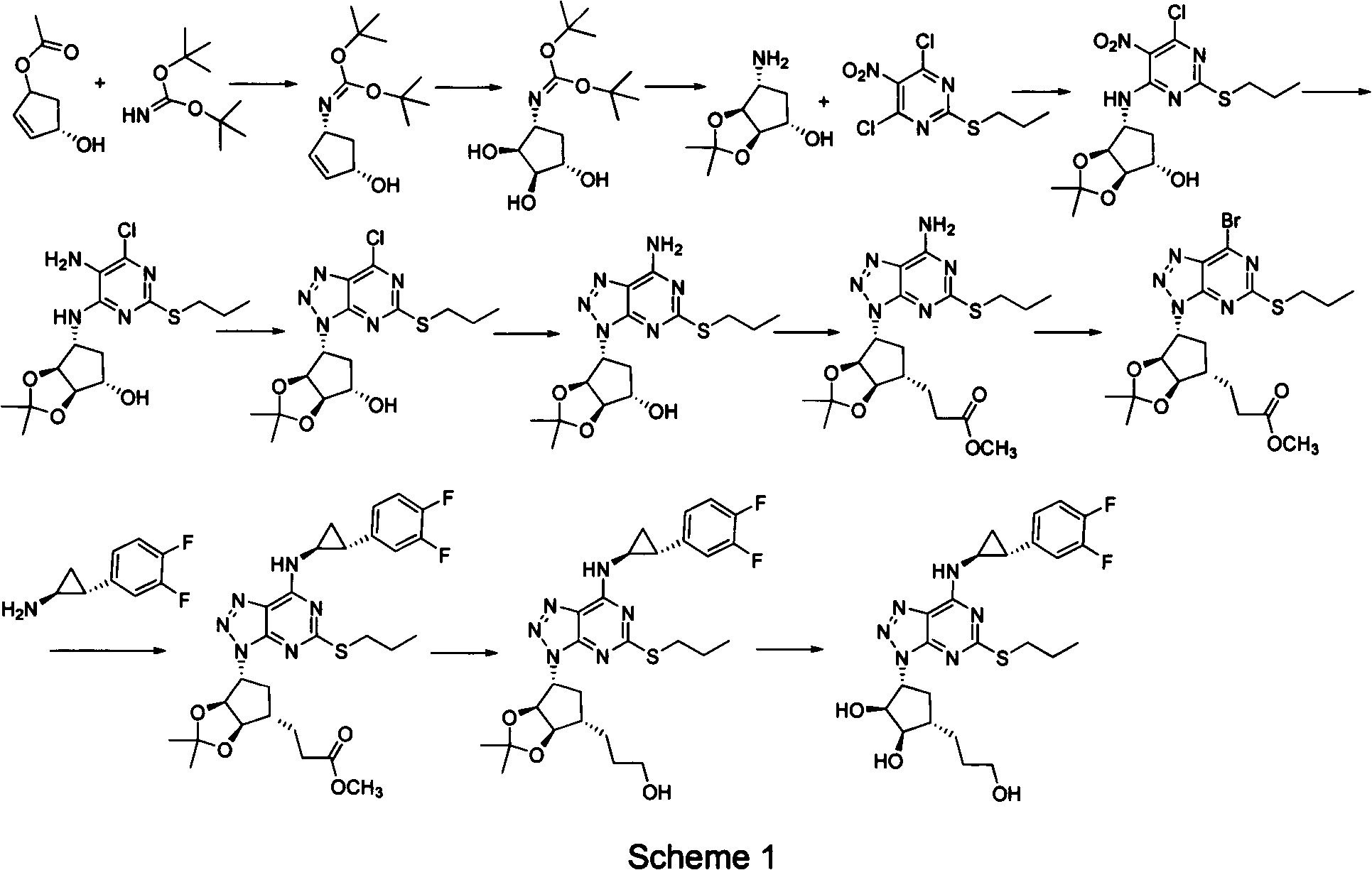
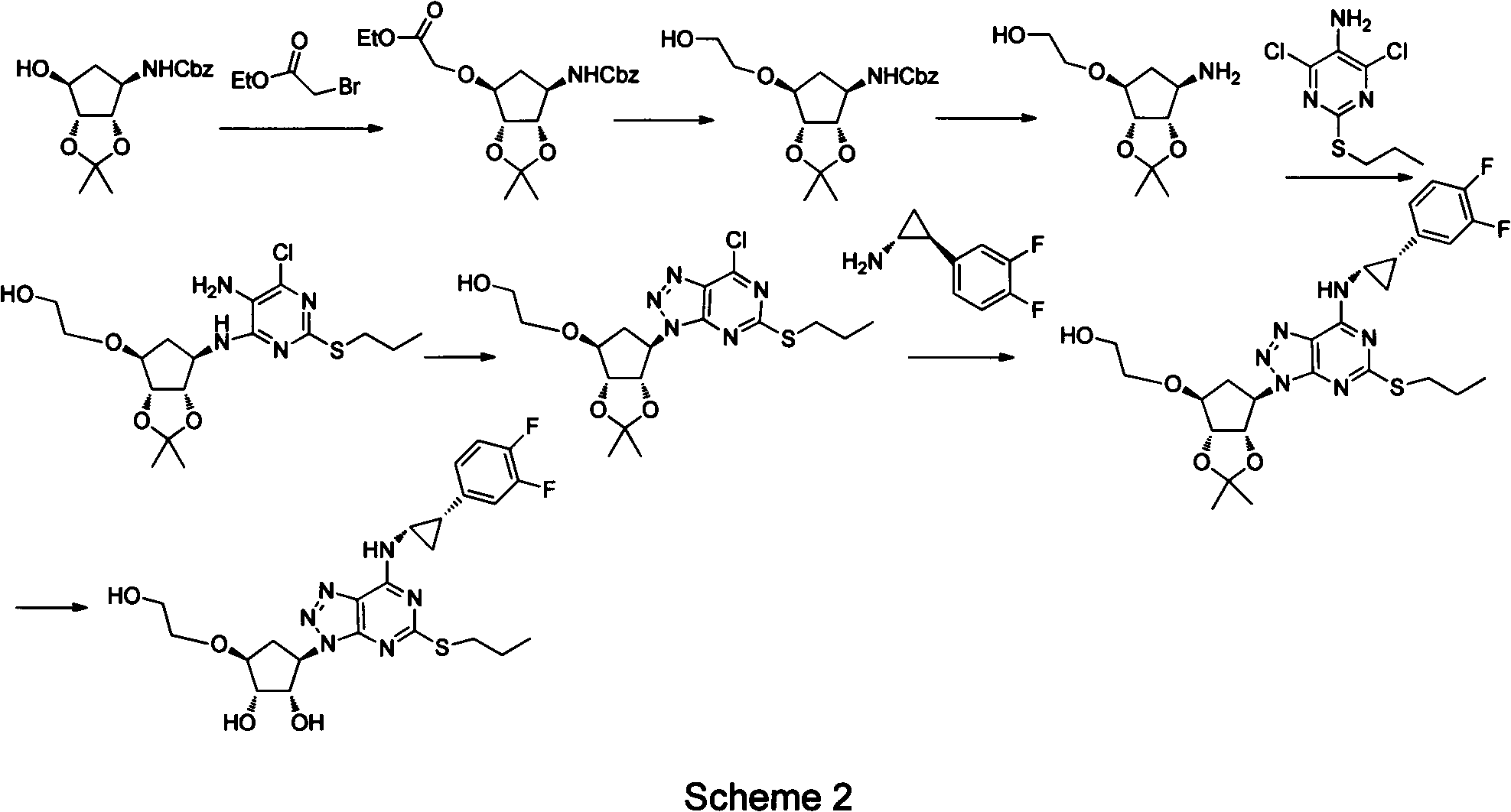
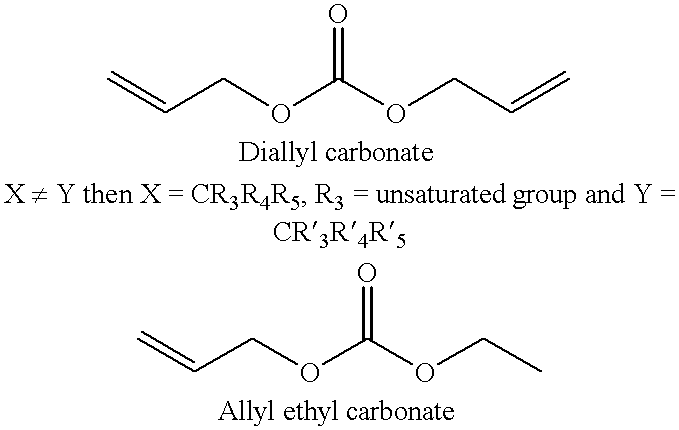
The invention provides a preparation method of ticagrelor, belonging to the technical field of medicine manufacturing. According to the method, a compound VII is taken as a raw material, and the method comprises the steps of: carrying out a nucleophilic substitution reaction on the raw material to obtain a compound VI; hydrogenating the VI, removing carbamazepine (Cbz) protection to obtain a compound V; carrying out a reaction on the V and 4, 6-dichloro-2-(allyl sulfide)-5-amio-pyrimidine to obtain a compound IV; carrying out a reaction on the IV and nitrite of alkali metal to obtain a compound III; carrying out a reaction on the III and (1R, 2S)-2-(3, 4-difluoro phenyl) cyclopropylamine to obtain a compound II; and finally, removing protecting group of the II to obtain a compound I.
Owner:SHANGHAI HAOYUAN CHEMEXPRESS
Process for preparing alpha -hydroxy acids using microorganism and novel microorganism
PCT No. PCT / JP97 / 00578 Sec. 371 Date Aug. 11, 1998 Sec. 102(e) Date Aug. 11, 1998 PCT Filed Feb. 27, 1997 PCT Pub. No. WO97 / 32030 PCT Pub. Date Sep. 4, 1997A process for preparing alpha -hydroxy acids represented by the general formula (II): RCH(OH)COOH (wherein R represents a hydrogen atom, an optionally substituted C1-C6 alkyl group, an optionally substituted C2-C6 alkenyl group, an optionally substituted C1-C6 alkoxy group, an optionally substituted aryl group, an optionally substituted aryloxy group, or an optionally substituted heterocyclic group) by allowing a microorganism to act on alpha -hydroxy nitriles (I): RCH(OH)CN (wherein R is as defined above) to hydrolyze and convert the alpha -hydroxy nitrites to alpha -hydroxy acids (II), wherein the alpha -hydroxy acids (II) are produced and accumulated in an aqueous solvent by a microorganism having the concentration resistance to the alpha -hydroxy nitrites (I) and / or alpha -hydroxy acids (II) and durability preferably in the presence of a cyanide, and harvested. According to this process, the use of the microorganism having the concentration resistance to the alpha -hydroxy nitriles (I) and / or alpha -hydroxy acids (II) and durability high enough to permit the activity to persist for a long period of time enables alpha -hydroxy acids (II) to be accumulated in high concentrations and cell bodies to be repeatedly used, and hence enables alpha -hydroxy acids (II) to be efficiently prepared. The addition of a cyanide to the reaction system results in more efficient preparation of alpha -hydroxy acids (II).
Owner:NIPPON SODA CO LTD
Emulsification type metal cutting liquor composition
InactiveCN101240218AImprove the lubrication effectImprove cooling effectAdditivesBase-materialsPhenolCutting fluid
Disclosed is an emulsifying metal-cutting-fluid composition comprising base oil or oily agent, mixed alcohol-amine, anionic surfactant, nonionic surfactant, antirust agent, copper alloy corrosion inhibitor, preservative and the like. The invention has a strong general usability, suitable for metal processing, particularly aluminum alloy metal processing with advantages of excellent lubricity, corrosion resistance, a low cost and being free of toxic or harmful substances such as nitrites and phenols, so as to keep the environment and operators away from harmfulness.
Owner:河北九熙新材料科技有限公司
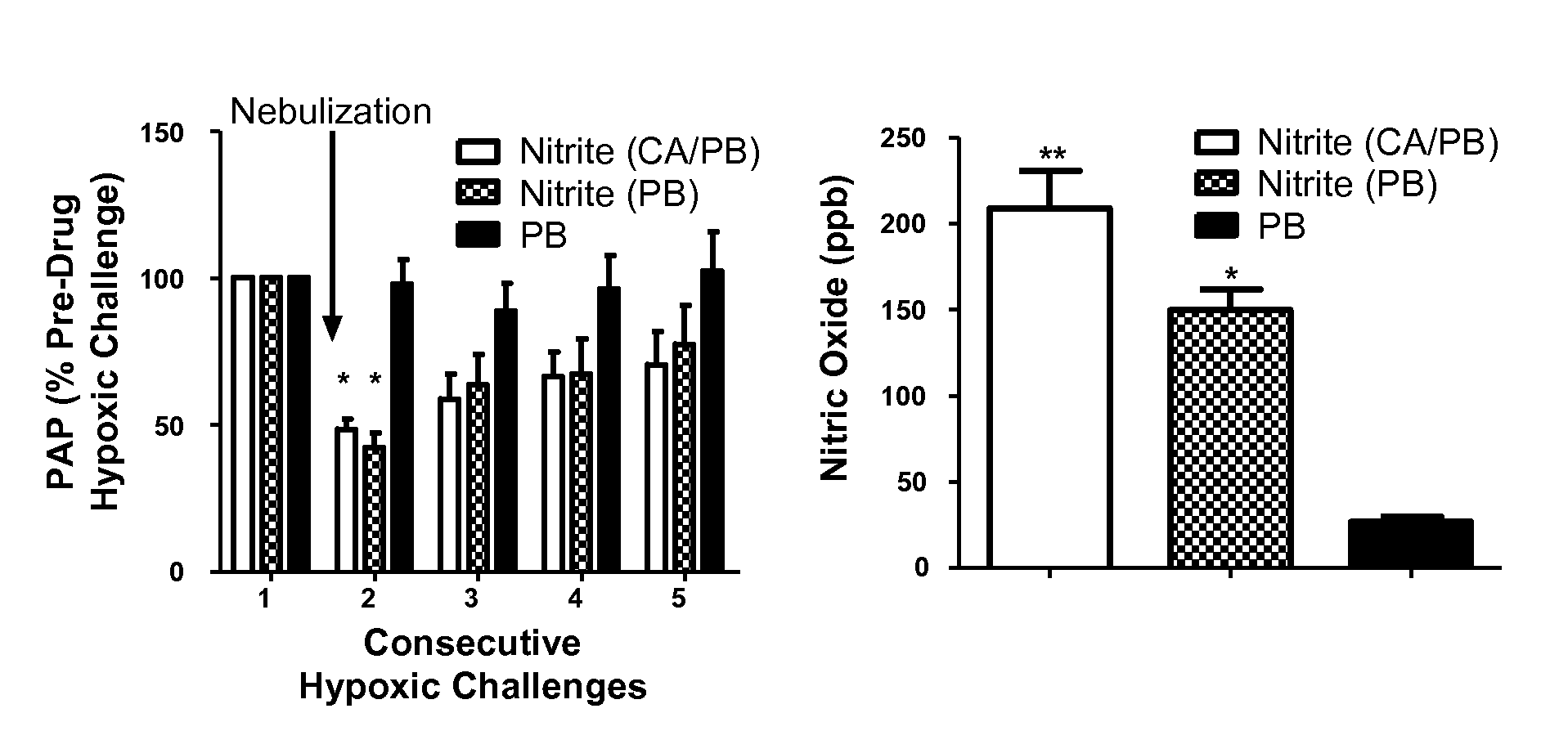
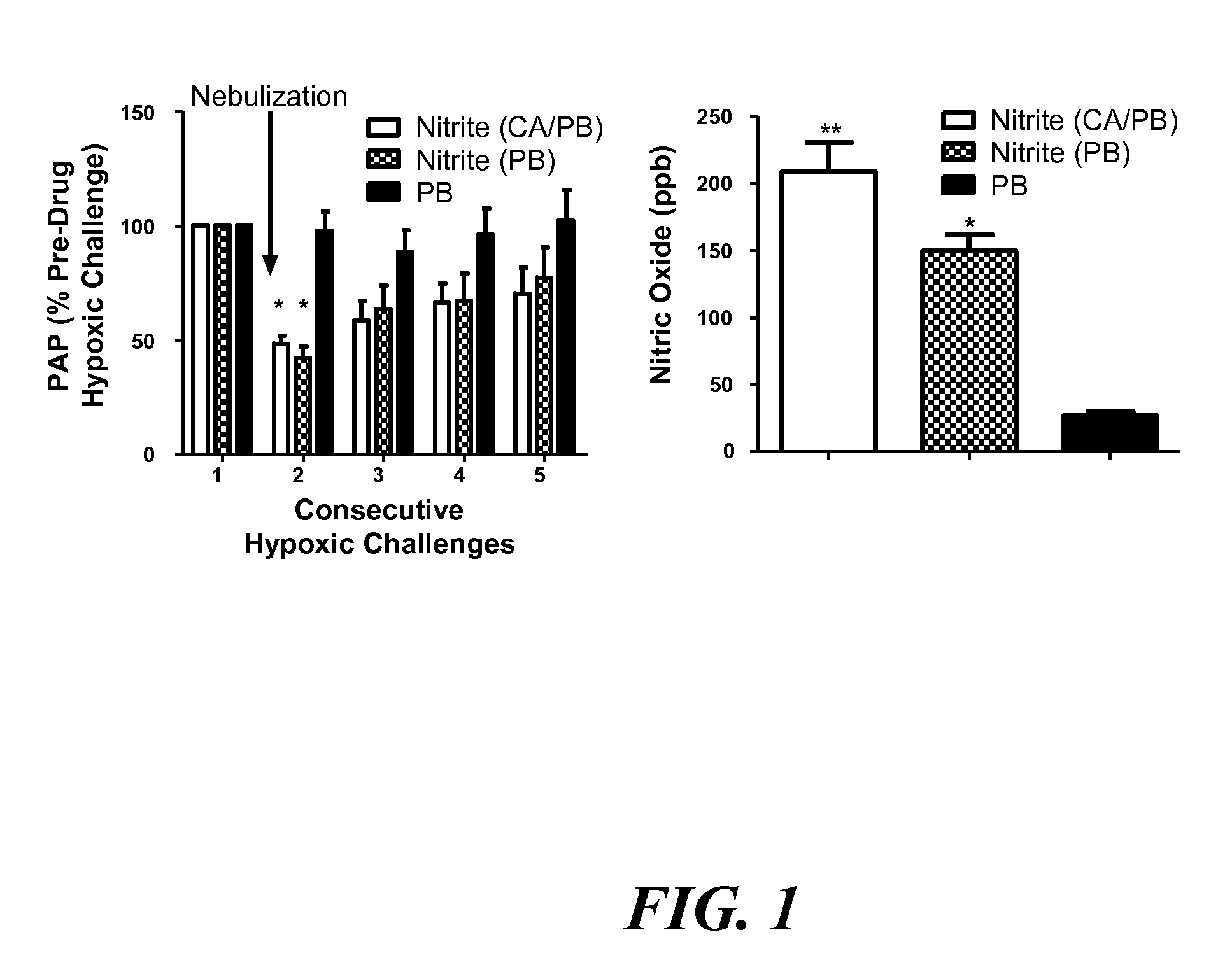
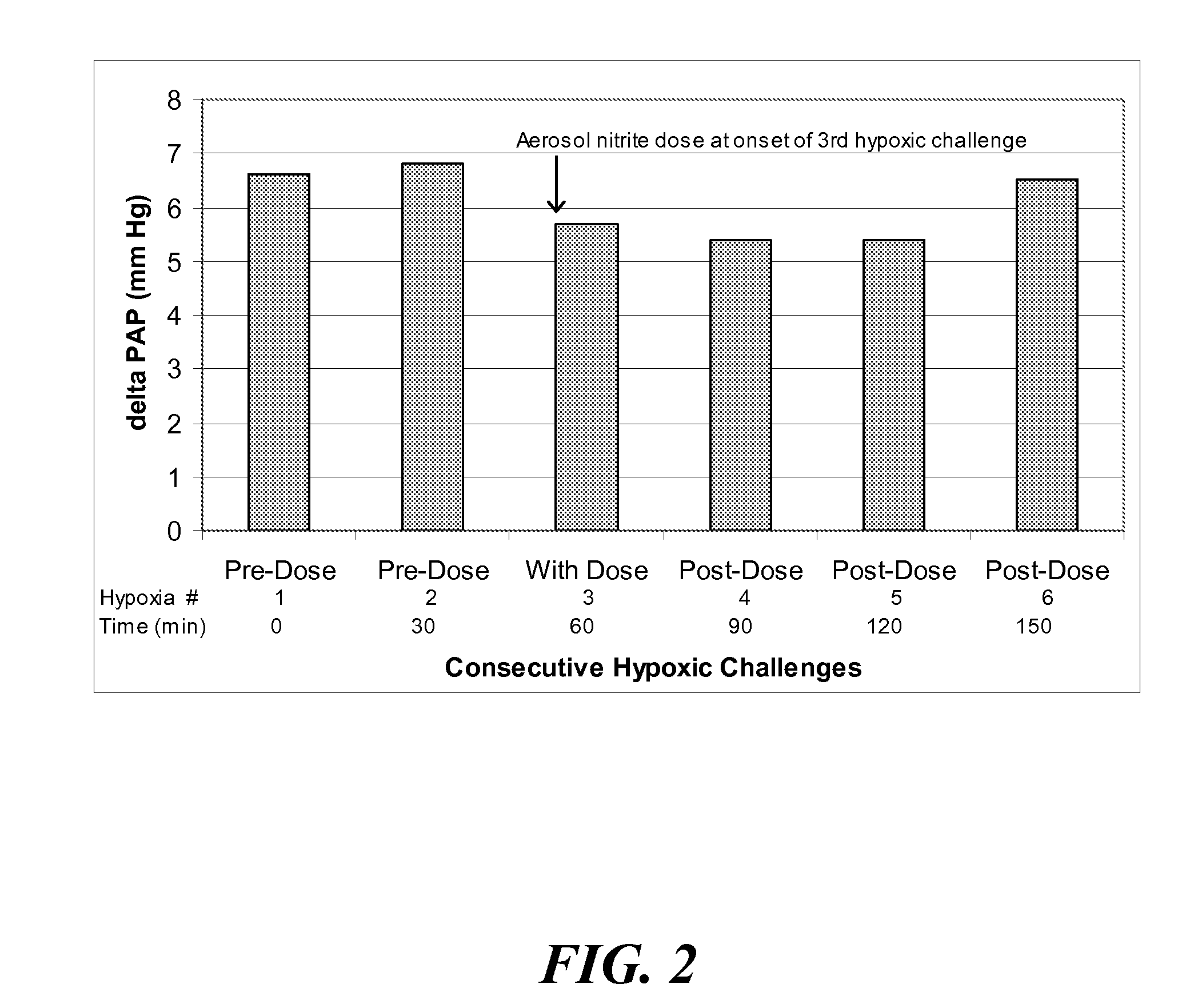
Aerosolized nitrite and nitric oxide -donating compounds and uses thereof
Disclosed herein are formulations of nitrite, nitrite salt, or nitrite- or nitric oxide-producing compounds suitable for aerosolization and use of such formulations for aerosol administration of nitrite, nitrite salt, or nitrite- or nitric oxide-donating compounds for the treatment of pulmonary arterial hypertension, intra-nasal or pulmonary bacterial infections, or to treat or prevent ischemic reperfusion injury of the heart, brain and organs involved in transplantation. In particular, inhaled nitrite, nitrite salt, or nitrite- or nitric oxide-donating compound specifically formulated and delivered to the respiratory tract for the indications is described. Compositions include all formulations, kits, and device combinations described herein. Methods include inhalation procedures and manufacturing processes for production and use of the compositions described.
Owner:AIRES PHARMA
Non-phosphorus compound scale and corrosion inhibitor for treatment of circulating cooling water
ActiveCN1621362AEasy to useIncrease the concentration factorScale removal and water softeningPhosphateTungstate
The composite phosphate-free scale inhibiting corrosion inhibitor for treating circular cooling water consists of scale inhibitor and corrosion inhibitor. The scale inhibitor consists of one or several of PASP, PVA, oxidized starch, polyacrylic acid, acrylic acid / acrylate copolymer and acrylic acid / acrylate copolymer with sulfo radical. The corrosion inhibitor consists of one or several of sodium salt / potassium salt / ammonium salt of organic salt, sodium / potassium / ammonium borate, nitrous organic matter, soluble molybdenate, soluble tungstate, soluble nitrate, soluble nitrite and soluble zinc salt. The composite scale inhibiting corrosion inhibitor has excellent scale inhibiting and corrosion inhibiting performance, is environment friendly, and is especially the treatment of hard circulation water with high calcium and high alkali content.
Owner:BEIJING YANHUA XINGYE TECH DEV +1
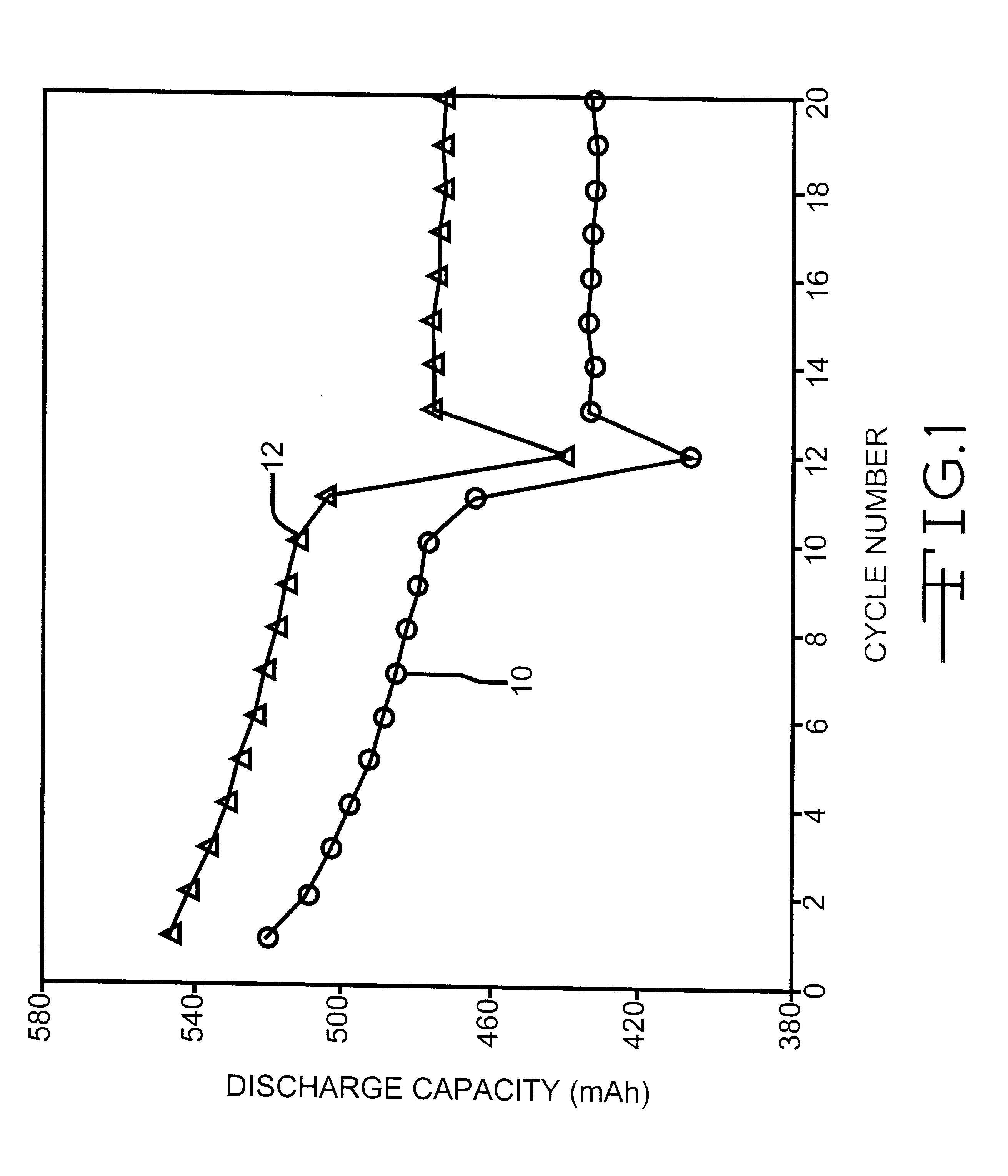
Nitrite additives for nonaqueous electrolyte rechargeable electrochemical cells
InactiveUS6210839B1Improve oxidation stabilityImprove dynamic stabilityOrganic electrolyte cellsSolid electrolyte cellsNitritePhysical chemistry
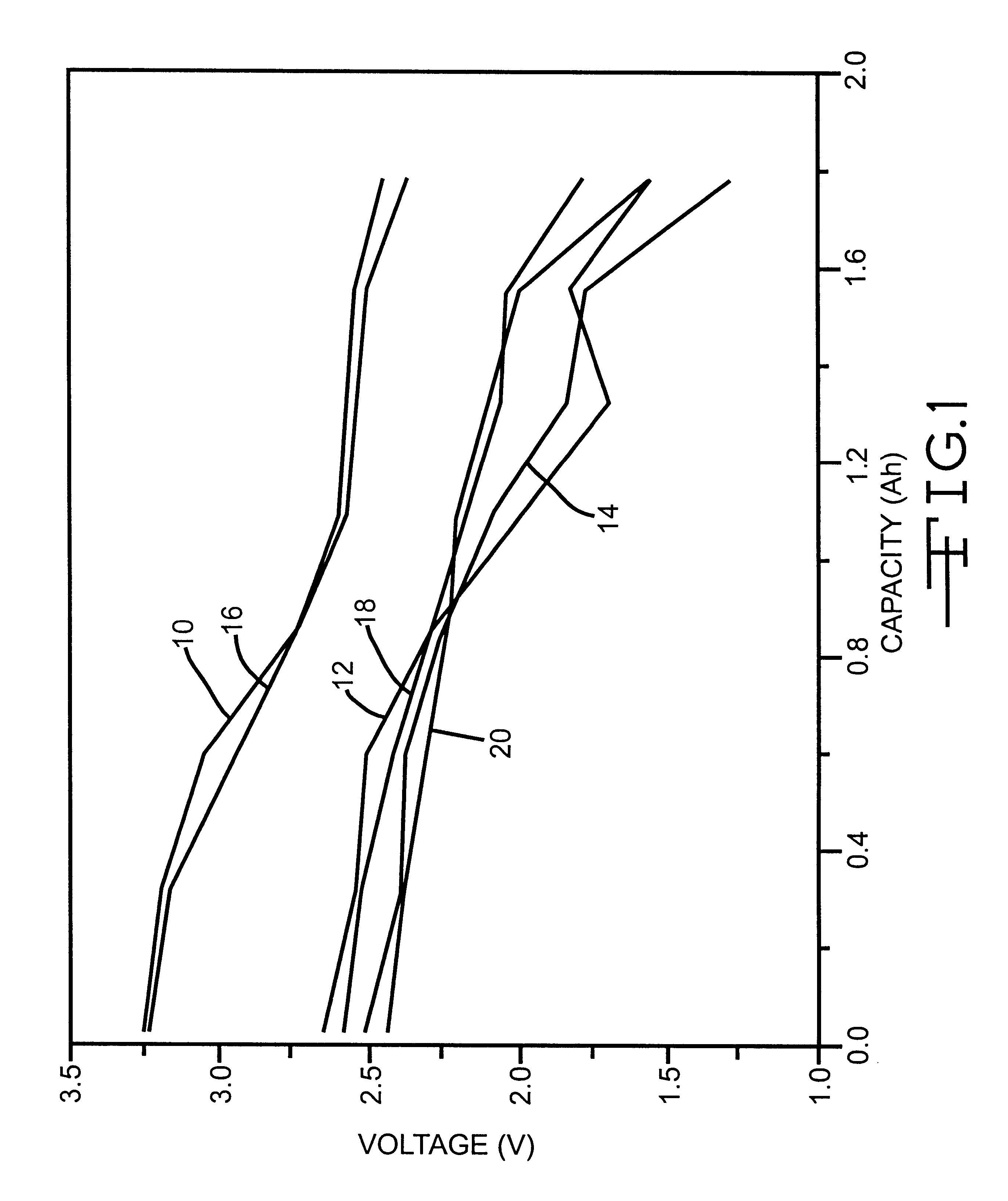
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one nitrite additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an alkyl nitrite compound.
Owner:WILSON GREATBATCH LTD
Gas generants comprising transition metal nitrite complexes
InactiveUS6077371AMetal azide explosive compositionsAlkali metal salt explosive compositionsNitriteHydrazine compound
High nitrogen gas generant compositions, useful for inflating passenger restraint gas inflator bags, comprise a nitrogen rich coordination compound selected from coordination complexes comprised of anionic nitro and nitrito ligands coordinated with a transitional metal template, and nonmetallic or nonmetallic / metallic cations. The gas generant compositions generate relatively more gas and less solids, and are safer than known gas generant compositions. Certain gas generant compositions ignite at lower autoignition temperatures thereby facilitating the use of an aluminum or light weight metal pressure vessel. Other gas generants self-deflagrate eliminating the need for other constituents in the composition. Novel methods for the synthesis of nonmetal derivative coordination complexes, guanidine and hydrazine for example, are also presented.
Owner:AUTOMOTIVE SYST LAB +1
Sodium ion secondary battery
ActiveUS20070218361A1High densityNon-aqueous electrolyte accumulatorsSolid electrolyte cellsCyclic etherManganese
The positive electrode active material of a positive electrode includes a sodium-containing transition metal oxide (NaaLibMxO2±α). The M includes at least two of manganese (Mn), iron (Fe), cobalt (Co), and nickel (Ni). For a negative electrode, a sodium metal or a metal that forms an alloy with sodium is used. A non-aqueous electrolyte produced by dissolving an electrolytic salt (sodium salt) in a non-aqueous solvent is used. Examples of the non-aqueous solvent may include a cyclic carbonate, a chain carbonate, esters, cyclic ethers, chain ethers, nitrites, amides and a combination thereof.
Owner:SANYO ELECTRIC CO LTD
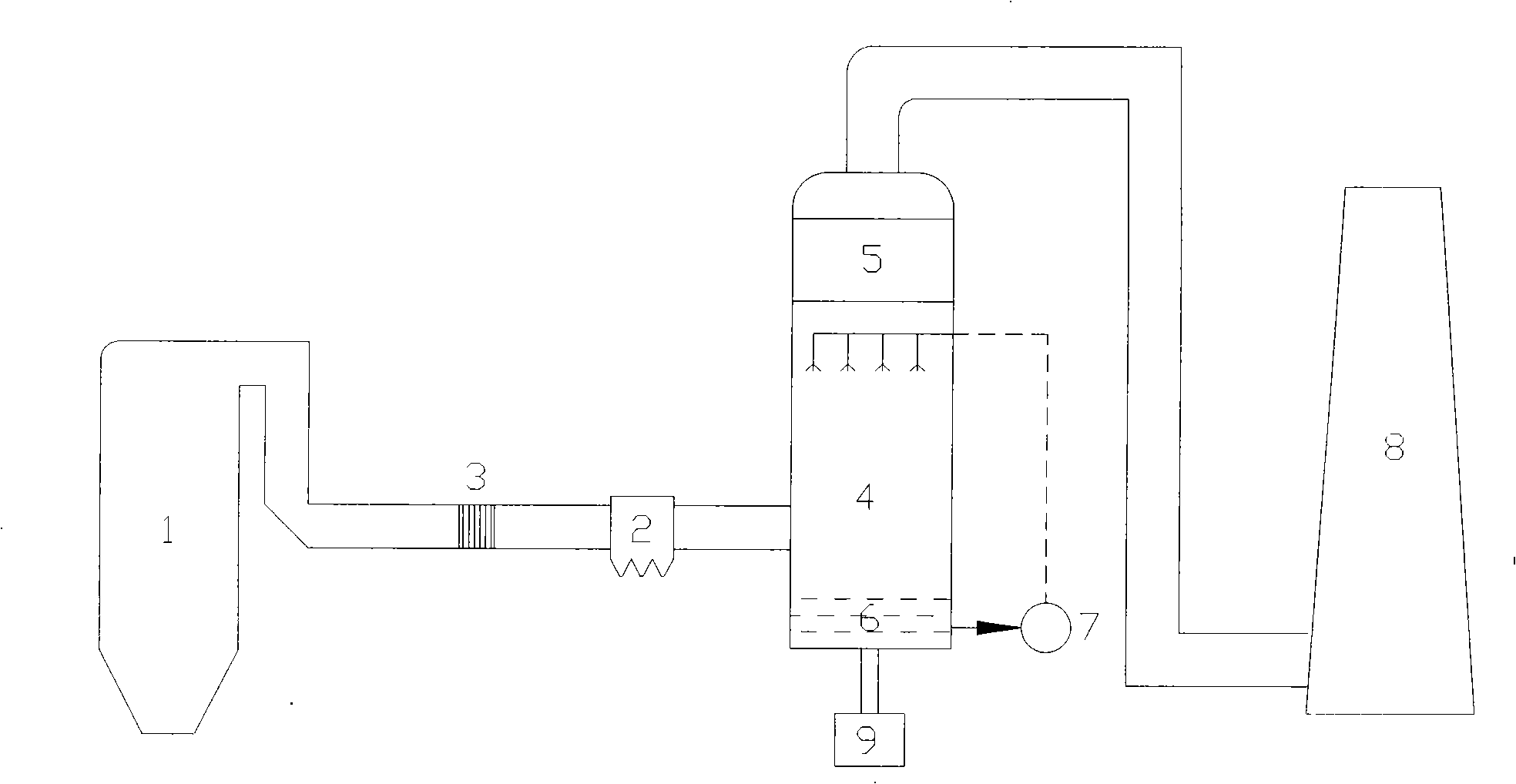
Liquid-phase oxidation-absorption two-stage wet method flue-gas denitration technique
ActiveCN101385942ALow investment costLow running costDispersed particle separationPartial oxidationGas phase
The invention discloses a wet method smoke gas denitration technology of two segments of liquid phase oxidation and absorption, which adopts solution or mixtures of one or more of potassium permanganate, sodium chlorite, sodium hypochlorite, calcium hypochlorite, oxyful and chlorine dioxide as oxidizing agents to ensure nitrogen oxide in smoke gas contact and react with the oxidizing agents. After the nitrogen oxide is partially oxidized into nitrogen dioxide, oxidized nitrogen oxide in the smoke gas is absorbed by alkali liquid to generate corresponding nitrite. The technology adopts liquid phase oxidation to replace gas phase oxidation so as to reduce the investment and the running cost, simplify the technical process and the system structure, and enhance the operability. Compared with a method that oxidation and absorption are simultaneously carried out in the liquid phase, the two-segment technology not only can increase the removal efficiency, avoid secondary pollution caused by incomplete absorption of NO2, but also can achieve the purposes of selectively generating and recovering the nitrite in the absorption stage by controlling the oxidation degree of the oxidation stage.
Owner:ZHEJIANG TIANLAN ENVIRONMENTAL PROTECTION TECH
Concrete super instant coagulant
The invention discloses a concrete early-strength agent, which is characterized in comprising the following components according to weight percentage: inorganic salt early-strength component 35-55 percent, organic early-strength component 5-10 percent, water reducing component 15-25 percent, wherein the inorganic salt early-strength component is prepared by at least two among sulfate, carbonate, nitrate, and nitrite; the organic early-strength component selects any one among calcium formate, sodium acetate, calcium oxalate, triethanolamine, tri-iso-propanolamine and carbamide; the water reducing component selects one among naphthalenesulfuric acid type, melamine type and polycarboxylate type. The invention makes the concrete be coagulated and hardened rapidly under the condition of low temperature, so that the early strength of the concrete is greatly improved as well as later strength is ensured.
Owner:ZHONGYIFENG CONSTR GRP +1
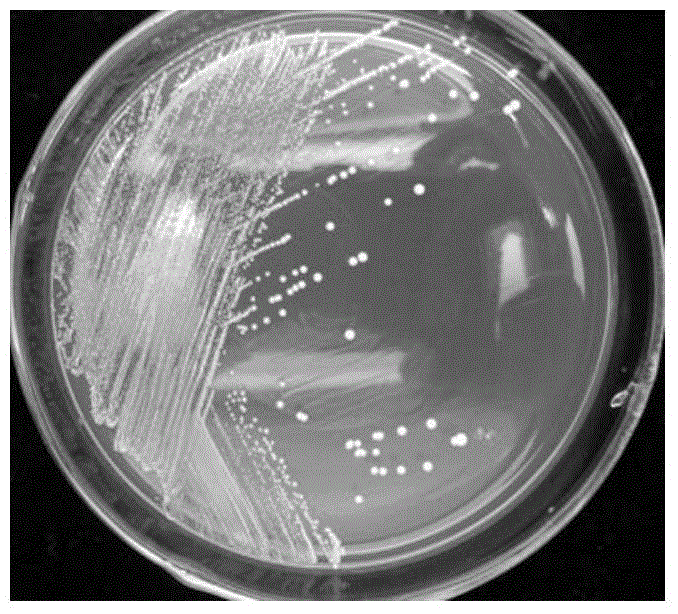
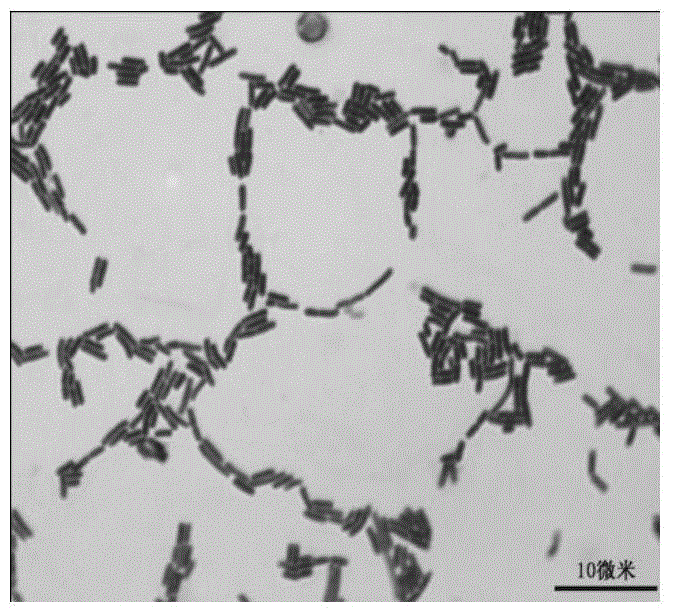
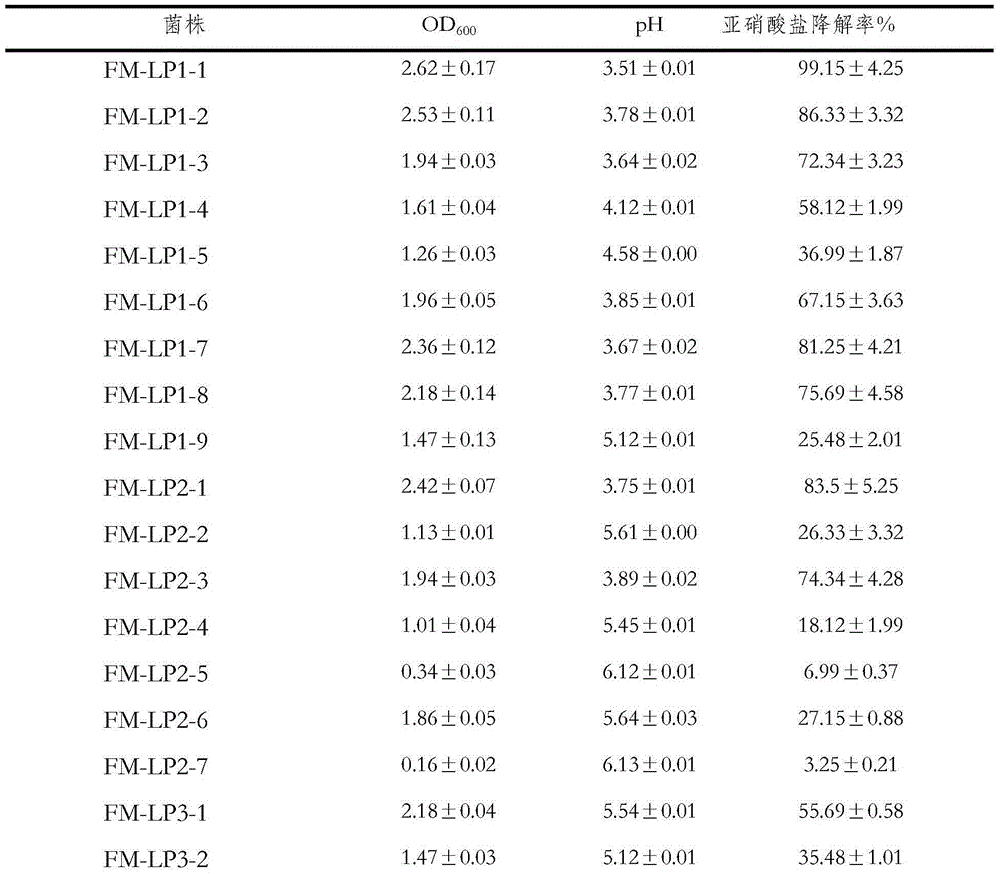
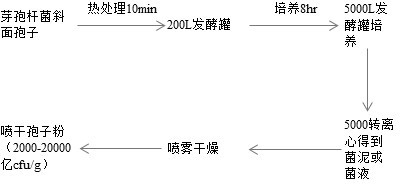
Lactobacillus plantarum strain having functions of effectively degrading nitrite and strongly producing acid and application of lactobacillus plantarum strain
ActiveCN104531578AHigh ability to degrade nitriteEfficient ability to degrade nitriteBacteriaLactobacillusMicroorganismNitrite
The invention relates to a lactobacillus plantarum strain having functions of effectively degrading nitrite and strongly producing acid and an application of the lactobacillus plantarum strain, and belongs to the technical field of bioengineering. The lactobacillus plantarum strain having the functions of effectively degrading nitrite and strongly producing acid is selected from conventional fermented pickled vegetables in Shenyang peasant families, wherein the strain is named FM-LP1-1, identified to be lactobacillus plantarum, and the strain is preserved in China General Microbiological Culture Collection Center with the preservation number of CGMCC No. 9488 on August 4, 2014. By taking FM-LP1-1 as a fermenting agent for fermenting vegetables, the content of nitrate in the fermented vegetable products is effectively reduced, the fermenting duration of the vegetables is shortened and the fermenting flavor of the vegetables is enhanced; and the strain disclosed by the invention is broad in application prospect.
Owner:NANJING CUIERSHUANG VEGETABLE FOOD
Process for the preparation of a catalyst component and components therefrom obtained
The invention provides a solid catalyst component for the polymeriztion of olefins comprising a Ti compound and an electron donor (ED) selected from alcohol, ketones, amines, amides, nitrites, alkoxysilanes, aliphatic ethers, and esters of aliphatic carboxylic acids supported on Mg dichloride in which the ED / Ti molar ratio ranges from 1.5 to 3.5 and the Mg / Ti molar ratio is higher than 5.5. The invention also provides a catalyst for the polymerization of olefins using the solid catalyst component and a process for the (co)polymerization of olefins CH2=CHR.
Owner:BASSELL POLIOLEFINE ITAL SRL
Flue gas catalytic oxidation denitration technique and catalyst thereof
ActiveCN101352645ALow costImprove efficiencyDispersed particle separationCatalyst activation/preparationNitriteResource utilization
The invention provides a smoke catalysis and oxidation denitration process which takes a catalyst using TiO2 or ZrO2-TiO2 as a carrier and Co as active ingredient, uses the oxygen contained in the smoke to oxidate the NO as NO2 which is easy to be dissolved in water, utilizes the alkali solution to absorb the NO2 and remove the NOx. The process of the invention has high denitration efficiency and low cost, can selectively recover the nitrite in the denitration outgrowth and realize the resource utilization of the outcome after controlling the content of the NO2 in the oxidated smoke.
Owner:ZHEJIANG TIANLAN ENVIRONMENTAL PROTECTION TECH
Phosphorus-free water-based metal cleaning agent
The invention relates to a phosphorus-free metal cleaning agent comprising the components of, by weight, 2-20% of a cleaning aid, 1-20% of a surfactant, 1-10% of a chelating agent, 1-20% of an emulsifier, 1-10% of an antirust agent, 0.2-4% of a copper alloy corrosion inhibitor, 1-10% of a penetration agent, 1-6% of a solubilizing agent, 0.1-0.6% of a defoaming agent, and balance of water. The cleaning agent provided by the invention has excellent cleaning capacity, long rust proof period, low foam, long service life, and the like. The cleaning agent is suitable for ferrous metal and non-ferrous metal processing industries. The agent has the advantages of no volatilization, no irritation, no toxic or harmful substance such as nitrite, no damage to health, and no environment pollution.
Owner:SHENYANG PARKERIZING
Induction plasma synthesis of nanopowders
ActiveUS20070029291A1Tight controlEasy to controlMaterial nanotechnologyOxygen/ozone/oxide/hydroxideIodideInduction plasma technology
A process and apparatus for synthesizing a nanopowder is presented. In particular, a process for the synthesis of nanopowders of various materials such as metals, alloys, ceramics and composites by induction plasma technology, using organometallic compounds, chlorides, bromides, fluorides, iodides, nitrites, nitrates, oxalates and carbonates as precursors is disclosed. The process comprises feeding a reactant material into a plasma torch in which is generated a plasma flow having a temperature sufficiently high to yield a superheated vapour of the material; transporting said vapour by means of the plasma flow into a quenching zone; injecting a cold quench gas into the plasma flow in the quenching zone to form a renewable gaseous cold front; and forming a nanopowder at the interface between the renewable gaseous cold front and the plasma flow.
Owner:TEKNA PLASMA SYST INC
Method of stabilizing human eye tissue by reaction with nitrite and related agents such as nitro compounds
A method for stabilizing collagenous eye tissues by nitrite and nitroalcohol treatment. The topical stiffening agent contains sodium nitrite or a nitroalcohol in a buffered balanced salt solution and can be applied to the surface of the eye on a daily basis for a prolonged period. Application of the solution results in progressive stabilization of the corneal and scleral tissues through non-enzymatic cross-linking of collagen fibers. The compounds can penetrate into the corneal stroma without the need to remove the corneal epithelium. In addition, ultraviolet light is not needed to activate the cross-linking process. The resulting stabilization of corneal and scleral tissues can prevent future alterations in corneal curvature and has utility in diseases such as keratoconus, keratectasia, progressive myopia, and glaucoma.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Leaven for producing sour and sweet cabbage and method for producing the same
The invention relates to a dry powder ready-to-use starter and a preparation method for the starter. The dry powder ready-to-use starter consists of the compound lactic acid powder made from lactobacillus plantarum 550 and lactobacillus buchneri 225, the nutritional agents promoting the lactobasillus to grow or perfume selectively added. The lactobacillus plantarum powder and the lactobacillus buchneri powder are prepared by liquefying, saccharifying, inoculating, fermenting, centrifugating, spraying and drying the corn powder. The compound lactobacillus powder is prepared by mixing the lactobacillus plantarum powder with the lactobacillus buchneri powder by a weight ratio of the lactobacillus plantarum powder to the lactobacillus buchneri powder ranging from 65 to 30: 70 to 35. When the infusing is carried out, the compound lactobacillus powder is dissolved into the fermentation broth by the cold boiling water and the salt, the sugar, the peptone and the perfume are added to the fermentation broth to make the pickle. The pickle products can be obtained by infusing the fresh vegetables in the pickle for 18 to 24 hours. With easy storage, convenient carrier and use, quick and simple preparation, the starter mannufactures the pickle which not only reaches the flavor and quality of the traditional products, but also effectively lowers the formation of the nitrites.
Owner:SICHUAN GAOFUJI BIOLOGICAL TECH
Denitrifying strain with nitrifying function, strain-containing water body improver of multiple active microorganisms and preparation method of water body improver
ActiveCN102492642AStable survivalImprove survivabilityBacteriaMicroorganism based processesBacillus licheniformisBacillus megaterium
The invention discloses a denitrifying strain with nitrifying function, a strain-containing water body improver of multiple active microorganisms and a preparation method of the water body improver, belonging to the technical fields of aquaculture and water body improvement. The strain disclosed by the invention is conserved in the China center for type culture collection (CCTCC), the CCTCC NO isM2011443; and the water body improver of the active microorganisms which is based on the strain comprises multiple strains such as Bacillus subtilis, Bacillus licheniformis, Bacillus pumilus, Bacillus cereus, Bacillus megaterium, Rhodopeudomonas palustris, Arthrobacter globiformis, Rhodotorula rubra and Lactobacillus plantarum. The strain is used to perform liquid fermentation and obtain fermentation liquor of which number of live bacteria is 0.5-3*10<10>cfu / mL, bacterial powder of which the number of live bacteria is 0.2-2*10<12>cfu / mL is further prepared; and the bacterial powder is used along with a diluent to prepare a liquid or powdery water body improver, wherein the effective live bacteria of the water body improver is 0.1-3*10<10>cfu / mL(or cfu / g). The water body improver has the function of reducing the contents of ammonia nitrogen, nitrites and nitrates in the culture water body; and the water body improver is suitable for different culture water bodies and has the function of improving the water quality.
Owner:JIANGSU DAYOU BIOLOGICAL DEVAL +1
Wet flue gas denitration technique for nitrite recovery
ActiveCN101352644AHigh economic valueIncrease profitDispersed particle separationAir quality improvementNitriteResource utilization
The invention discloses a wet-method smoke denitration process used for recovering nitrite, comprising the steps as follows: hydrogen peroxide or ozone is taken as oxidant; the oxidant is uniformly sprayed into smoke disposed by pre-dedusting and desulfurizing so as to carry out gas oxidation reaction and lead the NO in the smoke to be oxidated as NO2; subsequently, alkali liquid is taken as absorbent so as to absorb the mixture of the oxidated NO and NO2 and generate the nitrite; the absorbent is concentrated after absorbing the NO and NO2 and cooled and crystallized; after centrifuging separation, the nitrite crystal is gained. The process of the invention can effectively remove the NOx in the smoke, gains the nitrite with high economic values at the same time, and realizes the resource utilization of denitration outgrowth.
Owner:ZHEJIANG TIANLAN ENVIRONMENTAL PROTECTION TECH
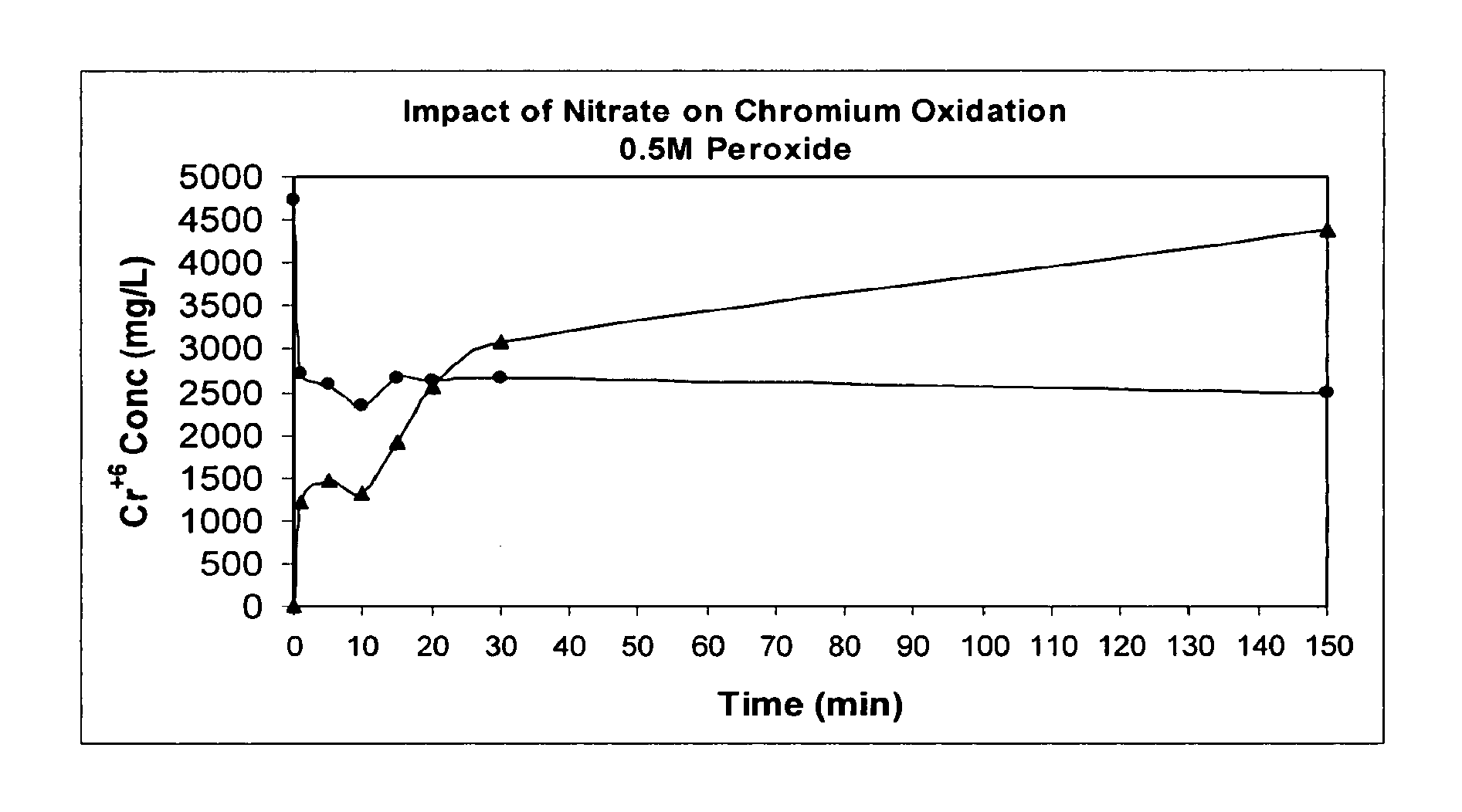
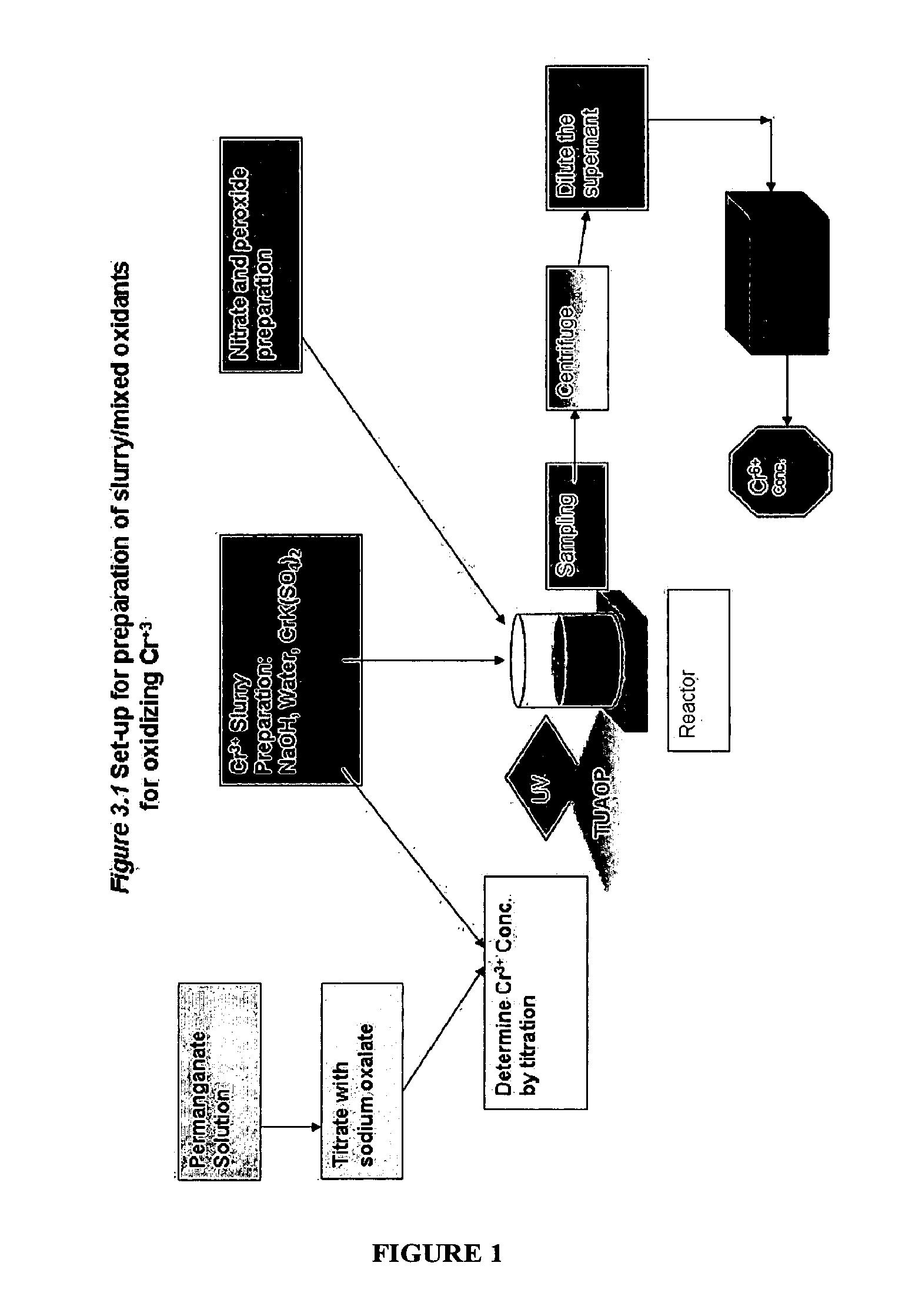
Oxidative Treatment Method
InactiveUS20100320156A1Quickly and efficiently decomposeEffective treatmentWater/sewage treatment by irradiationDecorative surface effectsElectrolysisWaste stream
The present invention provides a method for oxidizing a substance (e.g., in a waste stream, drinking water, a paper pulp slurry, or on a surface), which uses free radicals and reactive species generated from multiple oxidants. The method comprises combining peroxynitrite or peroxynitrous acid and at least one additional oxidizing agent for a period of time sufficient to oxidize the substance of interest. The peroxynitrite or peroxynitrous acid preferably is formed by irradiation of nitrate ion and / or nitric acid (e.g., with UV or gamma rays). The yield of free radicals and reactive species, which are the intermediate species that perform the oxidation may be increased by addition of a catalysts, electromagnetic radiation, sonic waves, and / or electrolysis.
Owner:TULANE EDUCATIONAL FUND
Process and apparatus for the removal of nitrogen compounds from a fluid stream
InactiveUS6894201B1Increase capacityImprove adsorption capacityHydrocarbonsAdsorption purification/separationAlkyl transferMolecular sieve
Disclosed is a process and apparatus for removing nitrogen compounds from an alkylation substrate such as benzene. A conventional adsorbent bed can be used to adsorb basic organic nitrogen compounds and a hot adsorbent bed of acidic molecular sieve can adsorb the weakly basic nitrogen compounds such as nitrites. Water facilitates the adsorption of the weakly basic nitrogen compounds. Running an alkylation substrate stream from a fractionation column of elevated temperature and suitable water concentration to the hot adsorbent bed may be advantageous.
Owner:UOP LLC
Organic nitrite additives for nonaqueous electrolyte in alkali metal electrochemical cells
InactiveUS6027827AGood charge and discharge cycleImprove efficiencyElectrotherapyPrimary cell maintainance/servicingPermittivitySolvent
Owner:WILSON GREATBATCH LTD
Alkali metal electrochemical cell having an improved cathode activated with a nonaqueous electrolyte having a carbonate additive
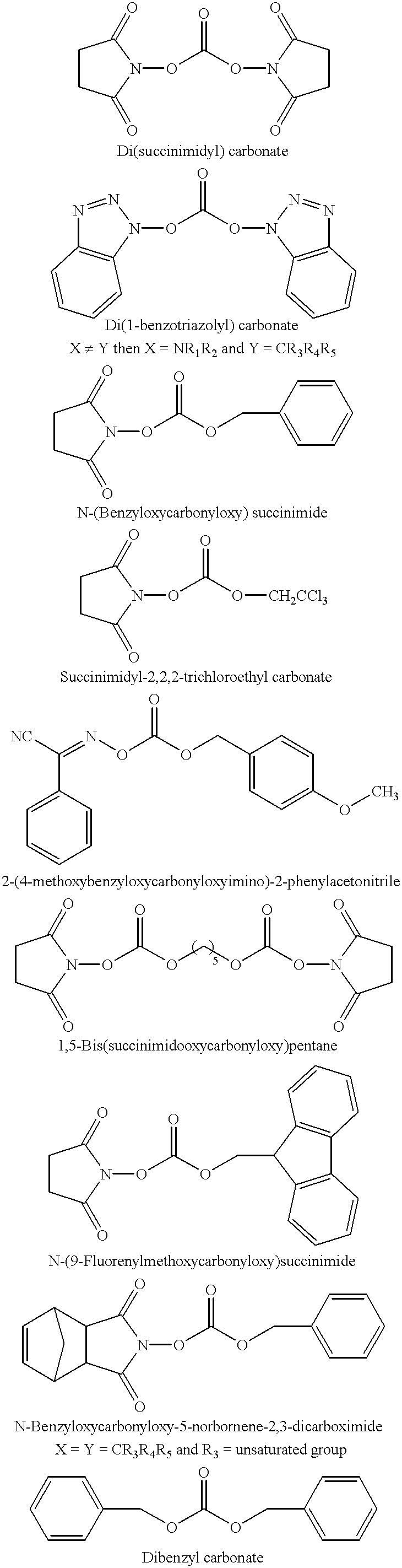
The present invention is directed to an unexpected benefit in a lithium cell derived from using a combination of silver vanadium oxide prepared in a temperature range of about 450° C. to about 500° C. activated with a nonaqueous electrolyte having a passivation inhibitor additive selected from a nitrite, a nitrate, a carbonate, a dicarbonate, a phosphonate, a phosphate, a sulfate and hydrogen fluoride, and mixtures thereof. The benefits include additional battery life resulting from a reduction in voltage delay and RDC build-up. A preferred electrolyte is 1M LiAsF6 in a 50:50 mixture, by volume, of PC and DME having dibenzyl carbonate added therein.
Owner:WILSON GREATBATCH LTD
Heterotrophic nitrification microbial preparation, cultivation method and use thereof
ActiveCN101302485AWide range of substratesEasy to trainImmobilised enzymesBacteriaHigh concentrationMicroorganism
The invention belongs to the environmental microorganism field, and in particular relates to a heterotrophic nitrification microorganism agent, a method for cultivating the same, and a use of the same in culture wastewater treatment. The microorganism agent contains Stenotrophomonas maltophilias strain DN1.1 and Pseudomonas putida strain DN1.2 which have the preservation registration numbers respectively as CCTCC M 207074 and CCTCC M 207075. The microorganism agent can effectively remove ammonia nitrogen and total nitrogen in a water body, accumulates no nitrite or nitrate during denitrification, and can simultaneously remove CODCr in organic wastewater, which is applicable to the treatment of high-concentration culture wastewater. The use of the microorganism agent in treating culture wastewater is simple in process and stable in effect.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Novel mesoporous material for absorbing granule phase substance, coke tar, phenol and amine nitrite in mainstream flue gas of tobacco
InactiveCN101433818ANo change in microscopic morphologyNo change in macroscopic appearanceTobacco smoke filtersSilicon compoundsParticulatesToxicant
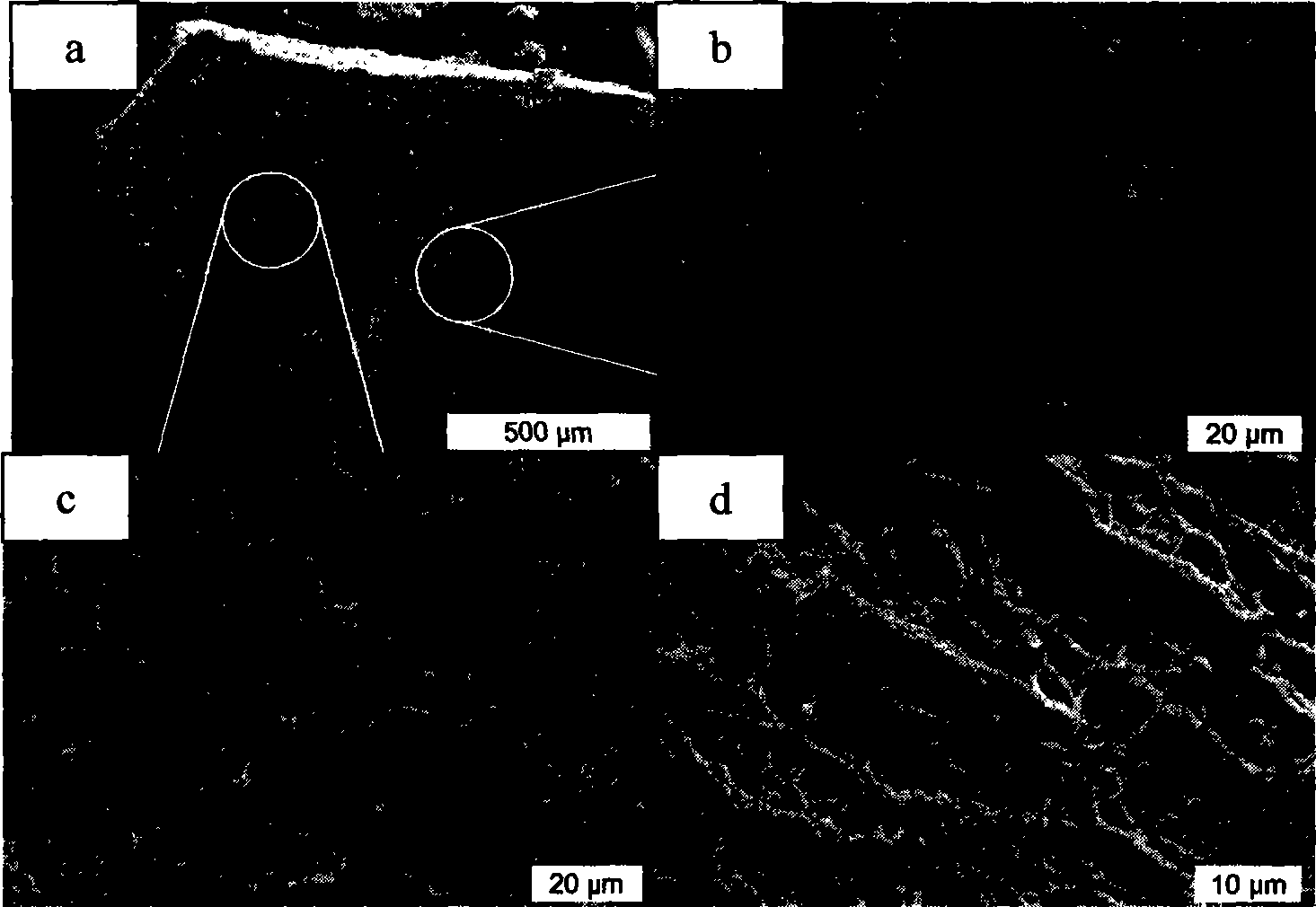
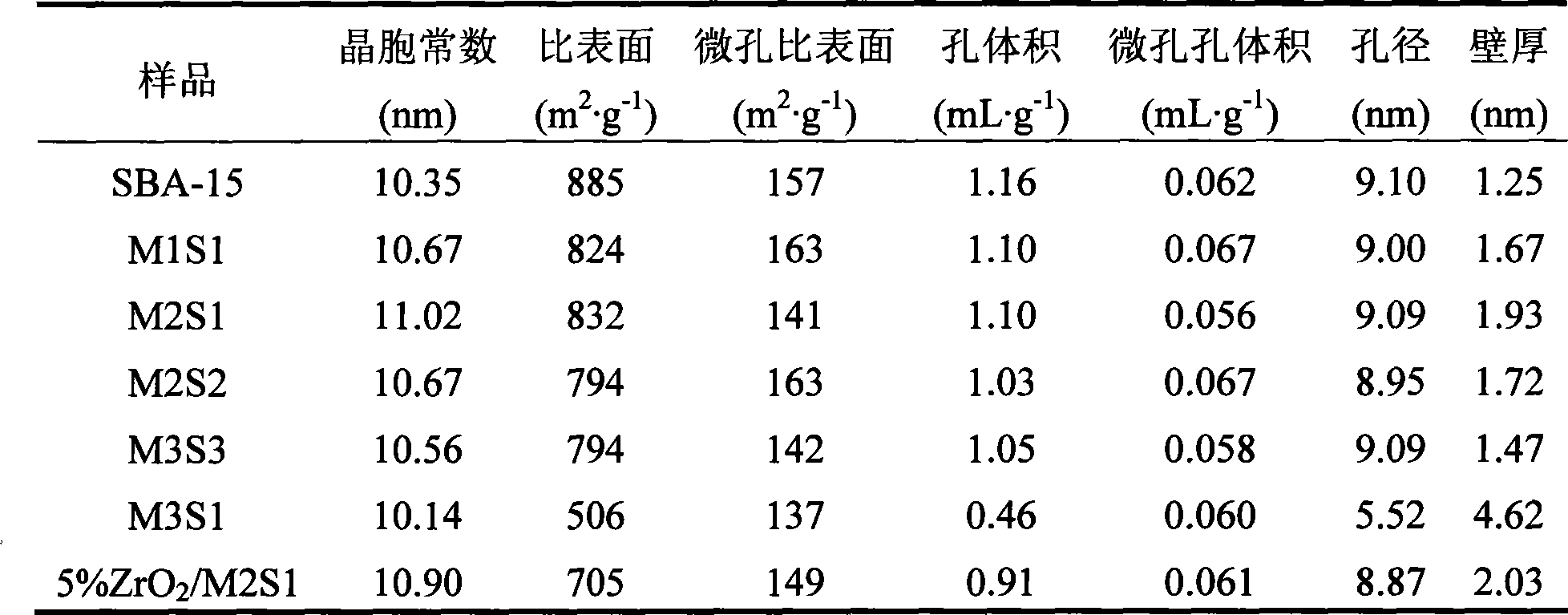
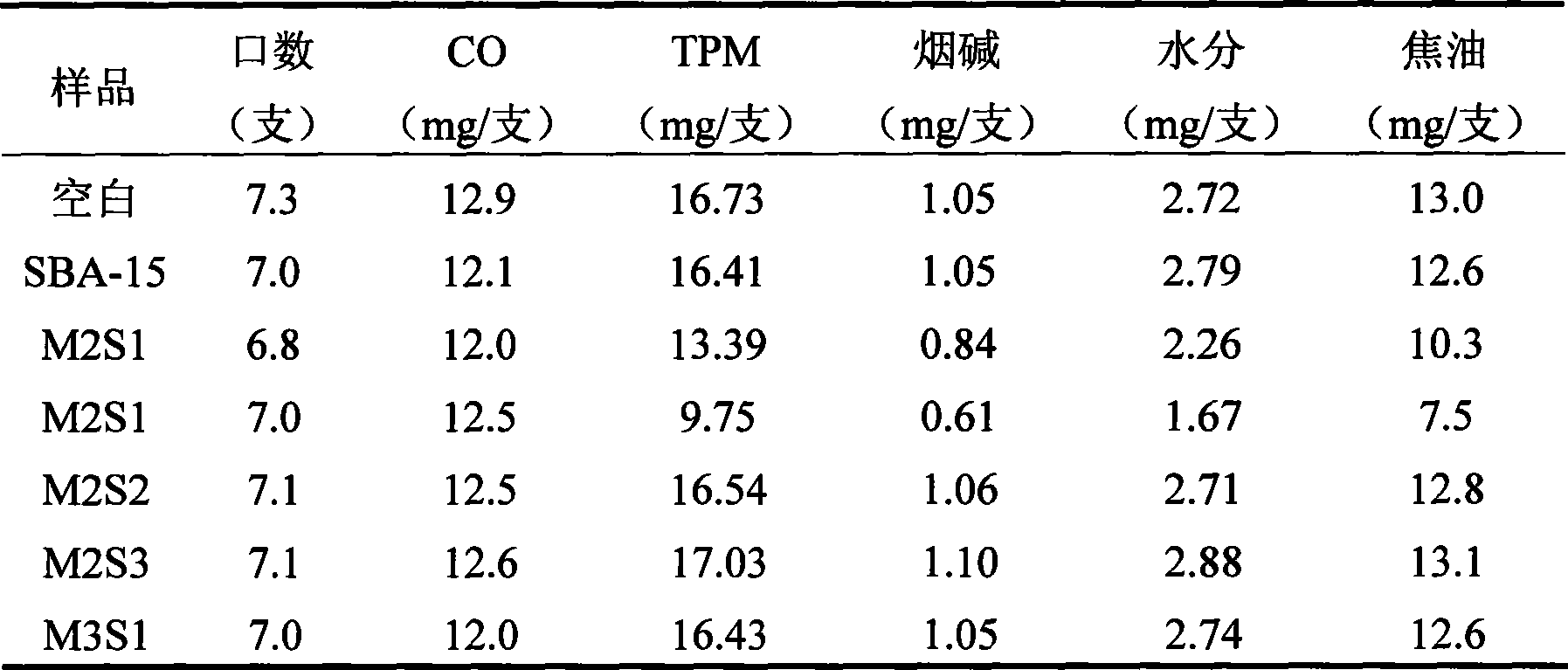
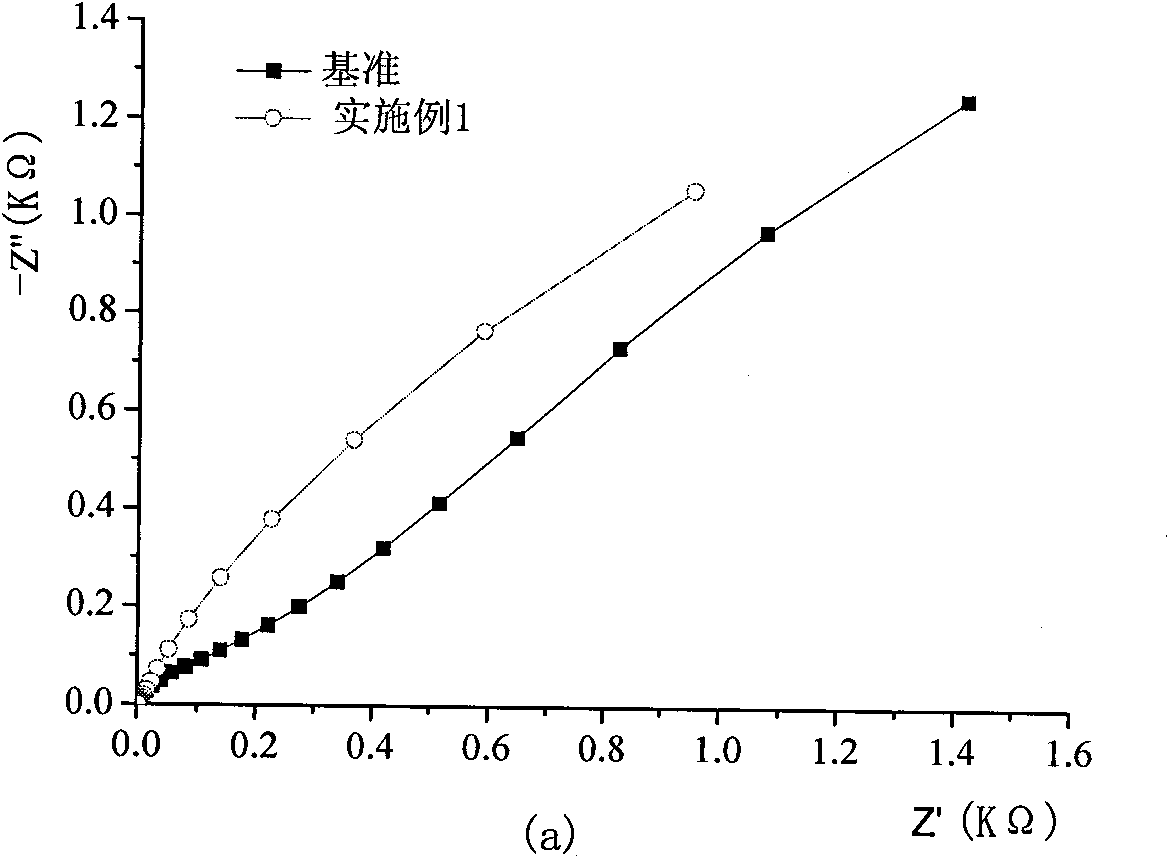
The invention discloses a novel mesoporous material for adsorbing tar, phenol and special nitrosamine in tobacco and so on, in mainstream smoke of the tobacco. The mesoporous material with high adsorption property is used as an addition material for filter tips of cigarettes; and the mesoporous material is a mesoporous molecular sieve with a three-dimensional mesh microstructure and monolithic appearance such as SBA-15,or is a SBA-15 mesoporous molecular sieve material which is plated with a liquid film or is modified by metal oxides such as zirconia and is provided with the three-dimensional mesh microstructure and the monolithic appearance. The invention adopts a more convenient and effective method to develop the SBA-15 mesoporous molecular sieve material with the three-dimensional mesh microstructure and the monolithic appearance and modify the surface / a surface layer, has good performance of intercepting environmental toxins such as interception particulate phase matters and phenol in smoke, has the characteristics of saving energy, saving time, and reducing environmental pollution during the preparation, can also reduce cost, and has remarkable economic benefit and social benefit.
Owner:NANJING UNIV
Migration-type organic reinforced concrete anti-corrosion admixture
The invention relates to a migration-type organic reinforced concrete anti-corrosion admixture which is prepared by mixing organic carboxylic acid and organic amine in the following percentage by weight: 40-80% of the organic carboxylic acid and 20-60% of the organic amine; adding the mixture into water; heating to 60-70 DEG C; and stirring the mixture for reaction for 1-2 hours to obtain the migration-type organic reinforced concrete anti-corrosion admixture. The anti-corrosion admixture does not contain nitrite and inorganic base and can prevent damage on reinforcing steel bar passive films caused by chloride ions, thus preventing corrosion.
Owner:JIANGSU SUBOTE MATERIAL +1
Dumbbell-like nanoparticles and a process of forming the same
InactiveUS20060053971A1Simple processGood signalMaterial nanotechnologyNanomagnetismAlkaneMetallic materials
Dumbbell-shaped or flower-shaped nanoparticles and a process of forming the same, wherein the process comprises forming a mixture of a nanoparticle with a precursor in a first solvent, wherein the nanoparticle comprises a hydrophobic outer coating; heating the mixture; cooling the mixture to room temperature; modifying the hydrophobic outer coating into a hydrophilic outer coating; precipitating a solid product from the mixture, and dispersing the product in a second solvent. The nanoparticles comprise any of a semiconducting, magnetic, and noble metallic material, wherein the nanoparticles comprise a first portion comprising any of PbSe, PbS, CdSe, CdS, ZnS, Au, Ag, Pd, and Pt, and wherein the precursor comprises any of a cationic, neutral or particulate Au, Ag, Pd, Pt, or transition metal (Fe, Co, Ni) precursors of Fe(CO)5, Co(CO)8, Ni(CO)4 or their analogues. The first and second solvents comprise any of alkanes, arenes, ethers, nitrites, ketones, and chlorinated hydrocarbons.
Owner:IBM CORP +1
Stabilized organoborane polymerization initiators and polymerizable compositions
ActiveUS20050004332A1High strengthImprove relationshipLayered productsOrganic-compounds/hydrides/coordination-complexes catalystsNitriteHydroxy compound
The invention is polymerizable compositions comprising in one part an organoboron compound capable of forming a free radical generating species and a stabilizing amount of one or more compounds comprising a dihydrocarbyl hydroxyl amine, an alicyclic hydroxyl amine, or a nitrite oxide of a dihydrocarbyl hydroxyl amine or an alicyclic hydroxyl amine, and in the second part one or more compounds capable of free radical polymerization.
Owner:DOW GLOBAL TECH LLC
Bamboo leaf antioxide and use thereof
InactiveCN1528197AWide variety of sourcesMild flavorAntibacterial agentsFood ingredient as antioxidantNitriteWater soluble
The present invention discloses a bamboo leaf antioxidant material (AOB) and its application. AOB is a yellow or brownish yellow powder or granules obtained from bamboo leaf, its main antioxidant component includes flavone, lactone and phenolic acid compound, the total flavone glucoside content determined by colourimetry is greater than or equal to 30%, total lactone content is greater than or equal to 15% and phenolic acid content is greater than or equal to 7.5%. It not only can be block the chain reaction of automatic oxidation of fat, but also can chelate trasition state metal ion, at the same time can be used as first-grade and second-grade antioxidant to produce action, and can effectively remove nitrite and can block synthesis of nitrosamine, and has the actions of resisting bacteria, inhibiting fungi, deodorization and increasing perfume, so that it has extensive application.
Owner:ZHEJIANG UNIV HANGZHOU LEAF BIO TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com