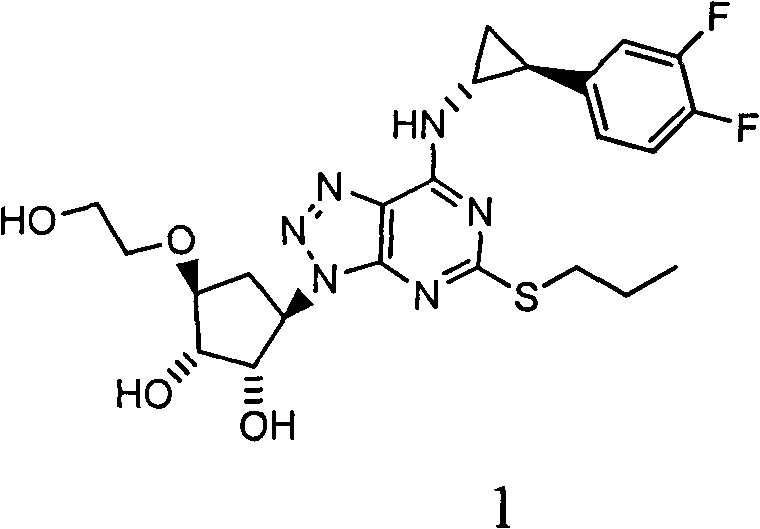
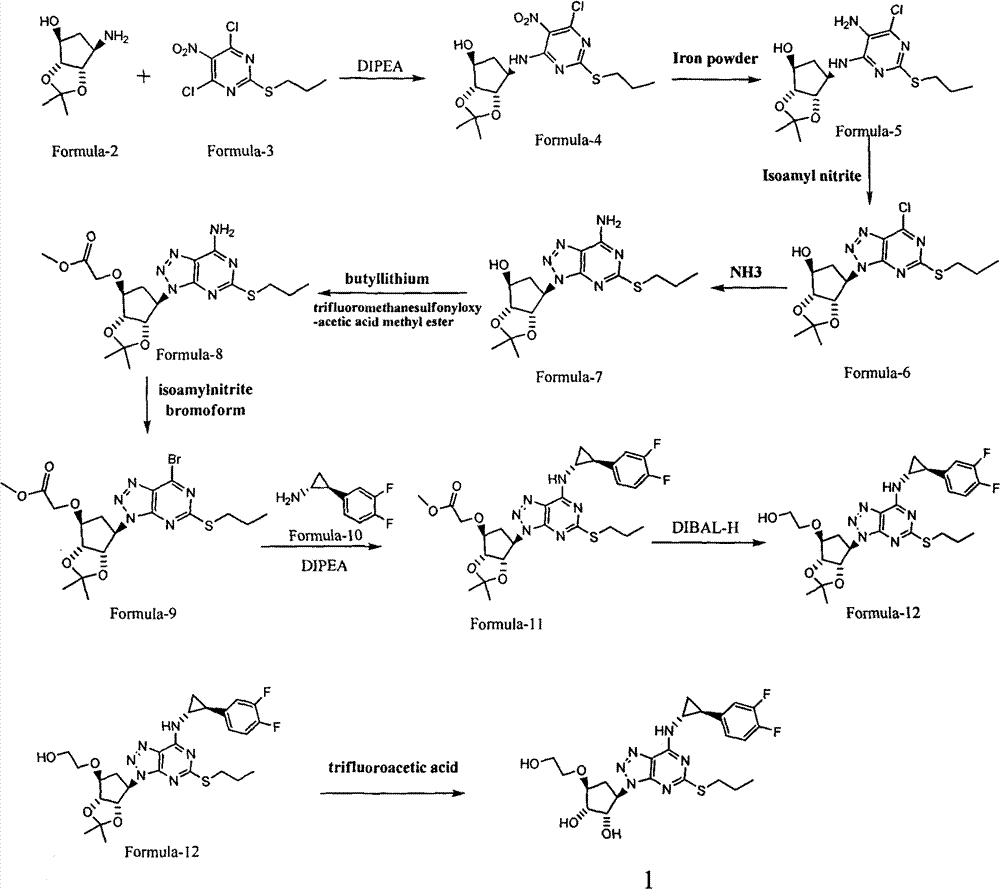
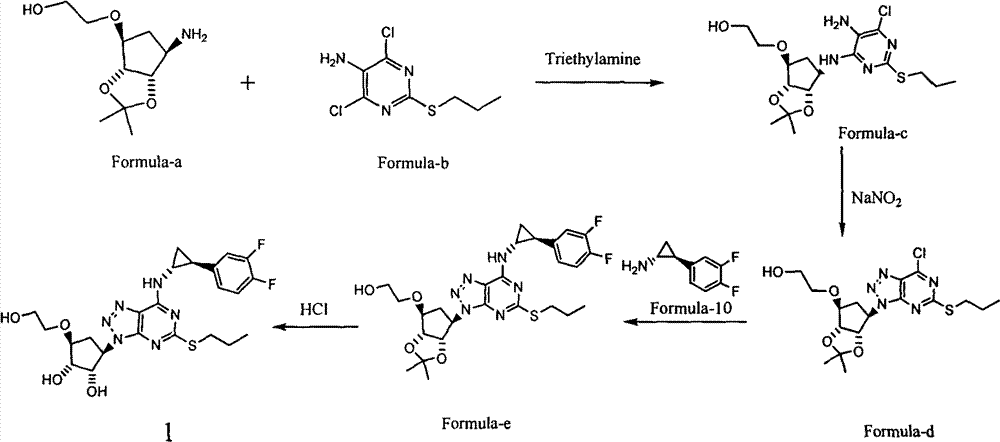
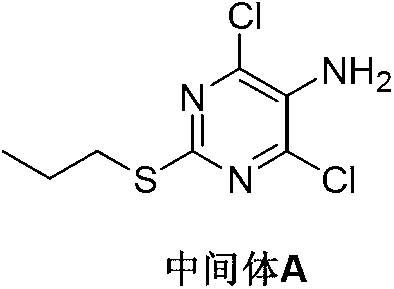
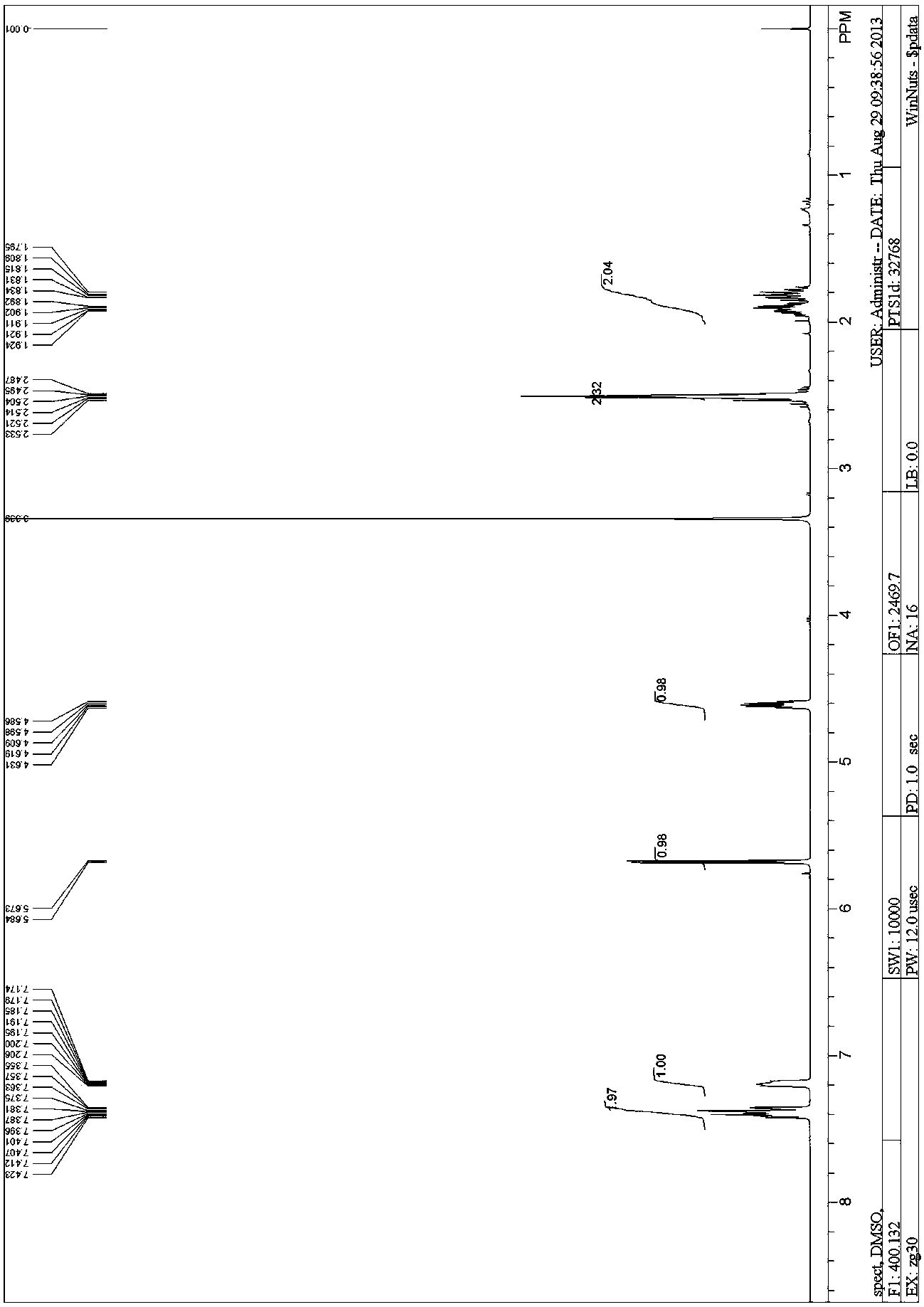
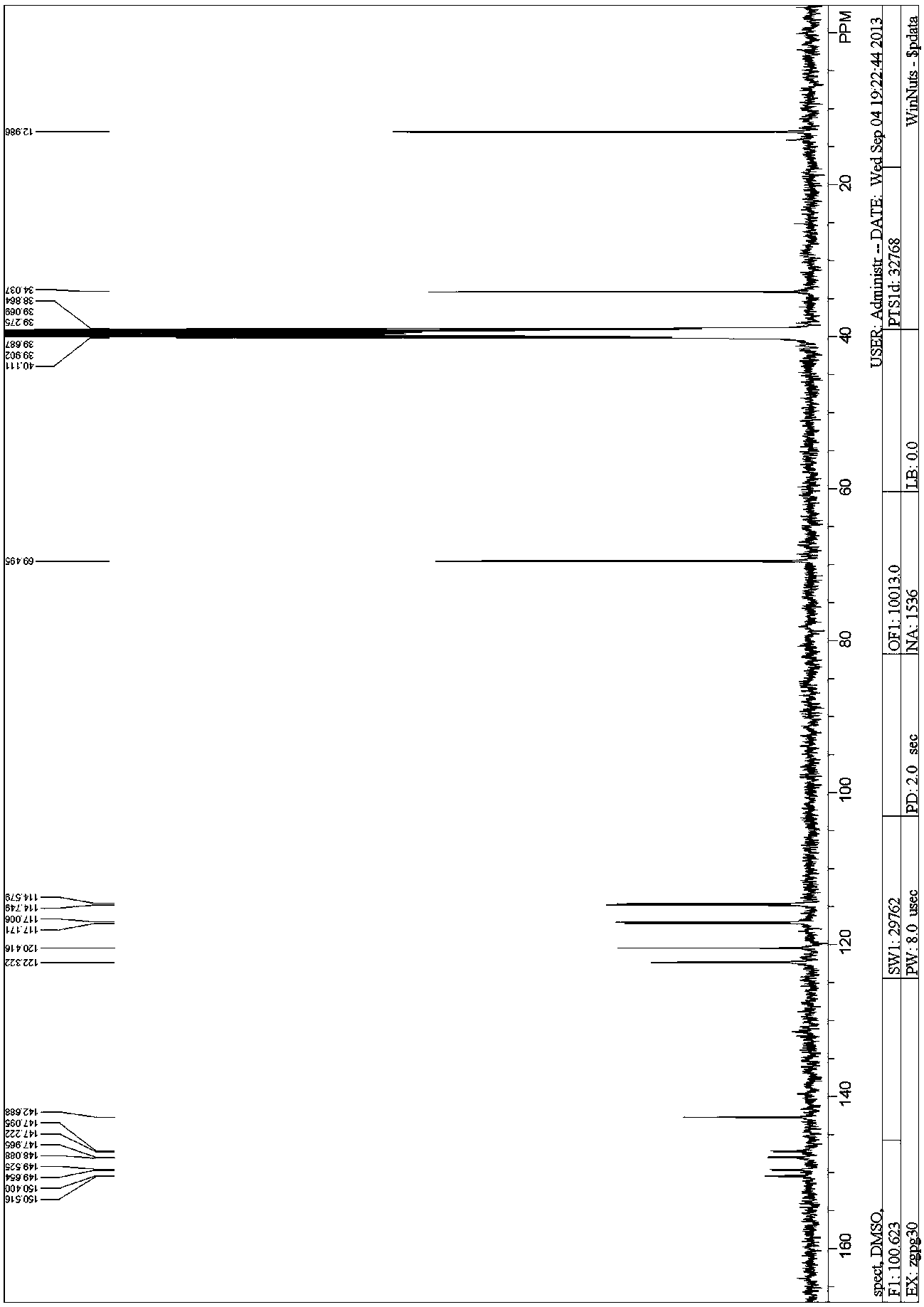
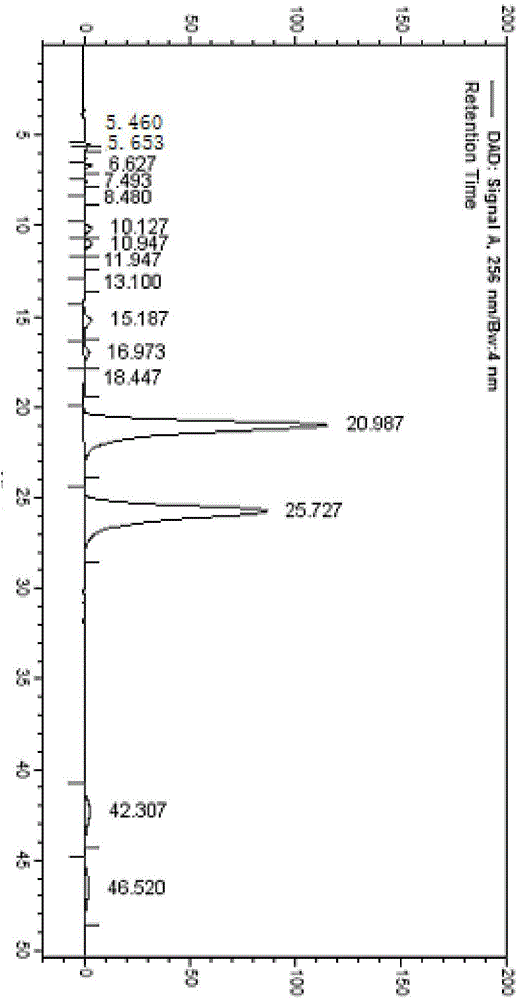
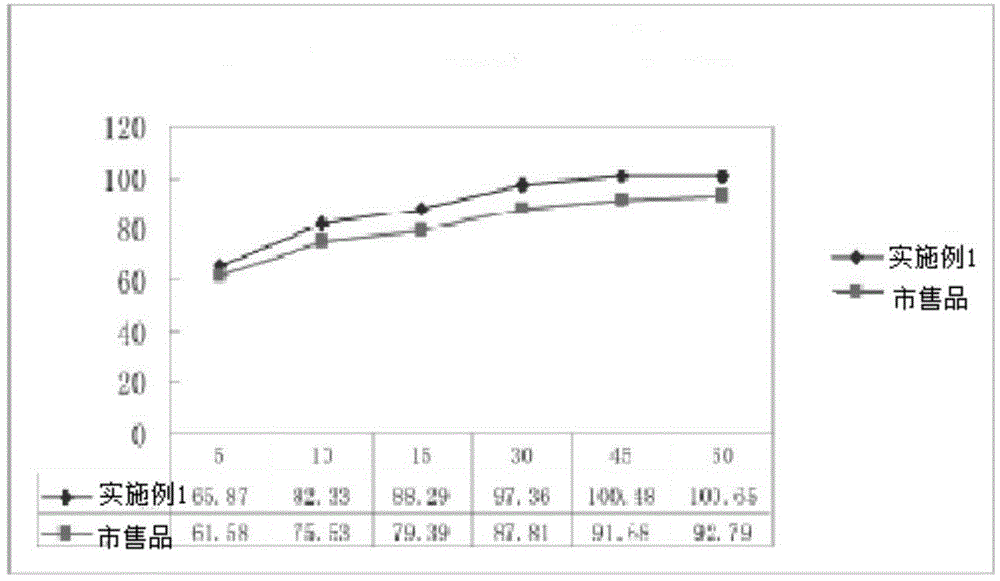
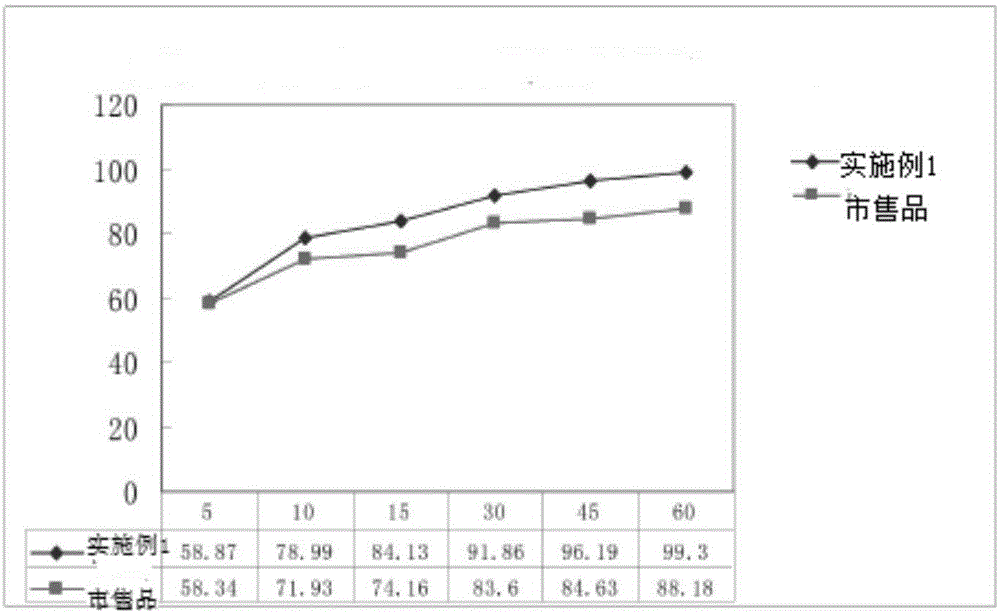
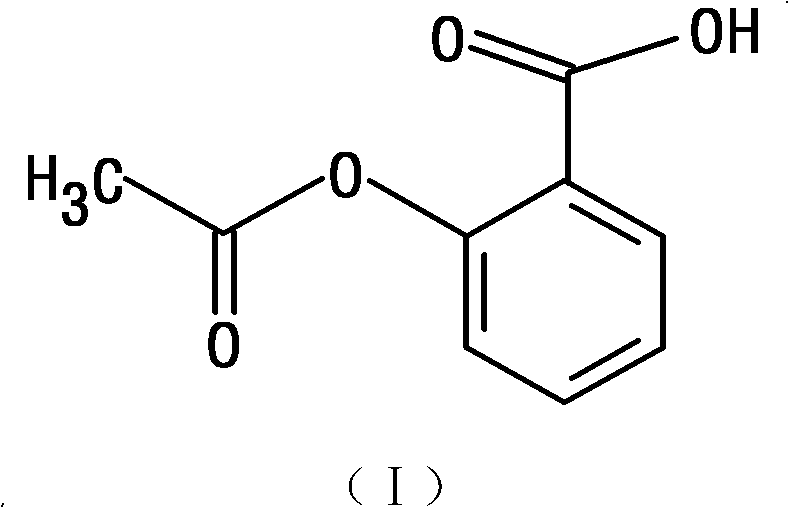
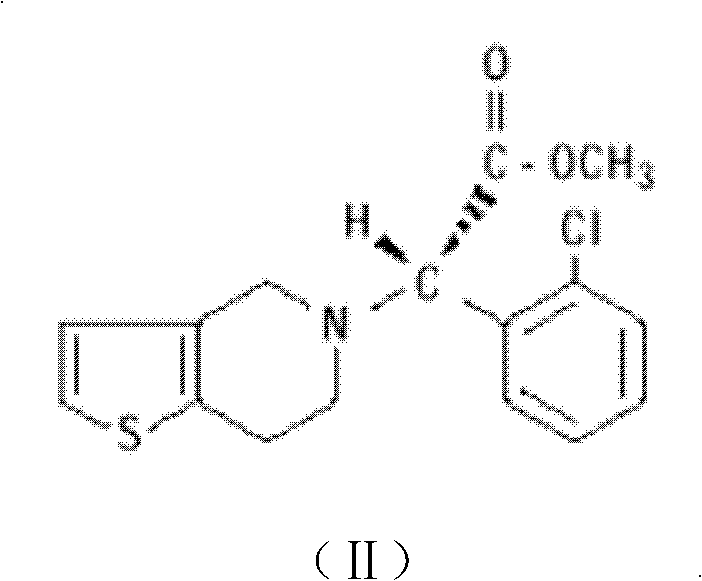
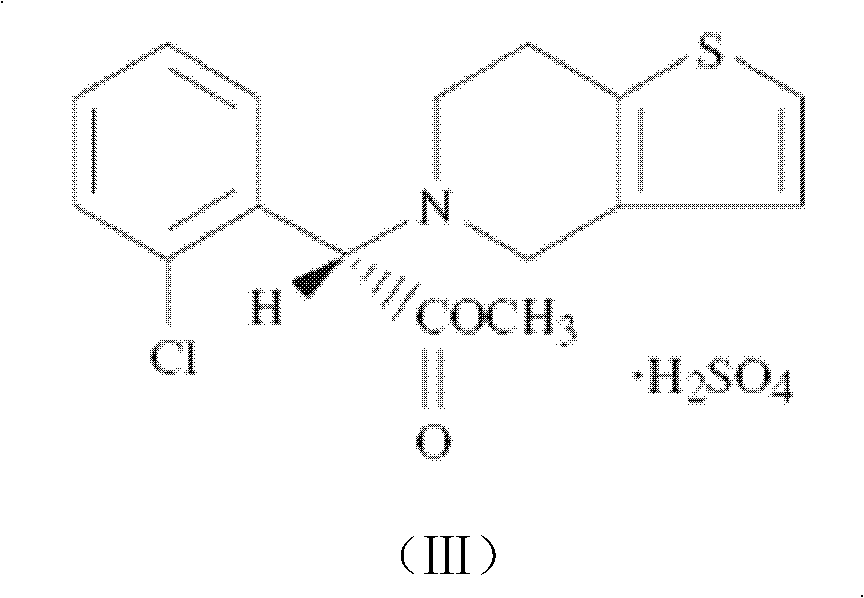
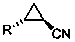Patents
Literature
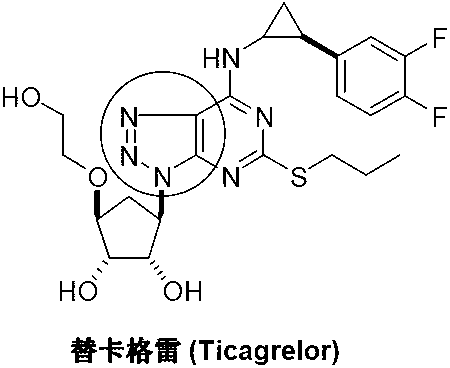
338 results about "Ticagrelor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
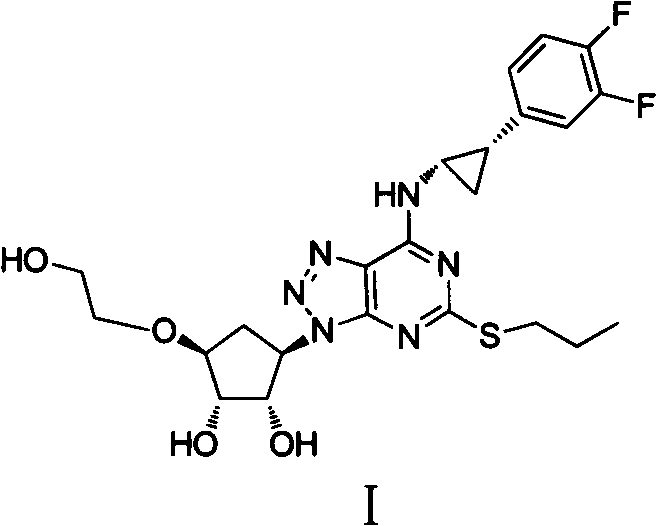
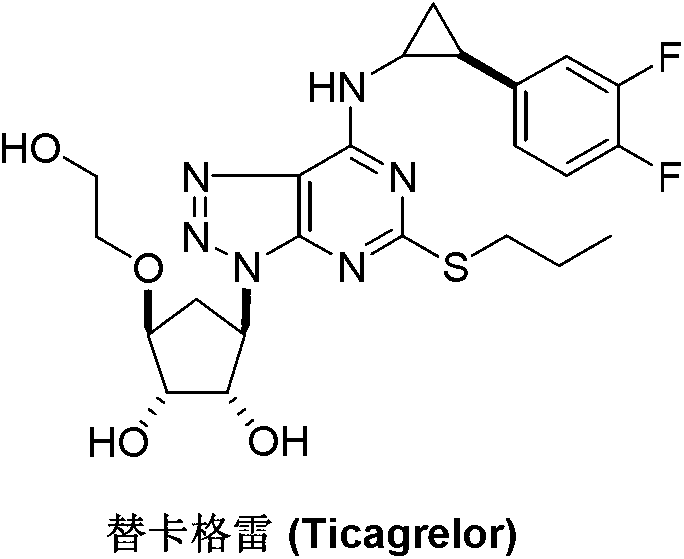
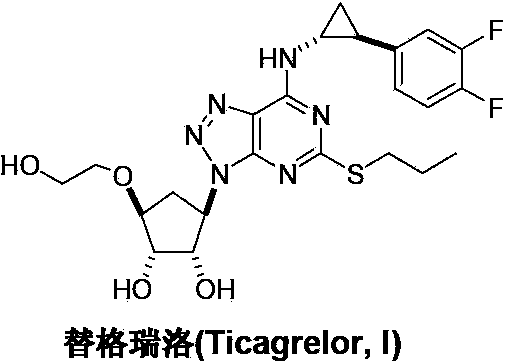
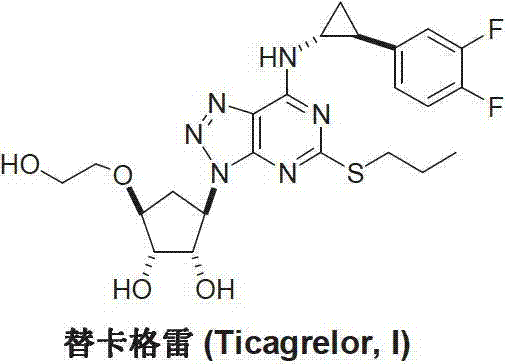
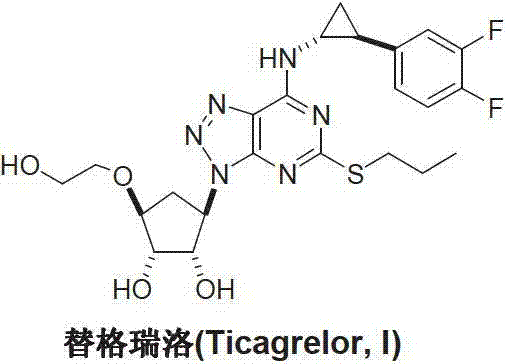
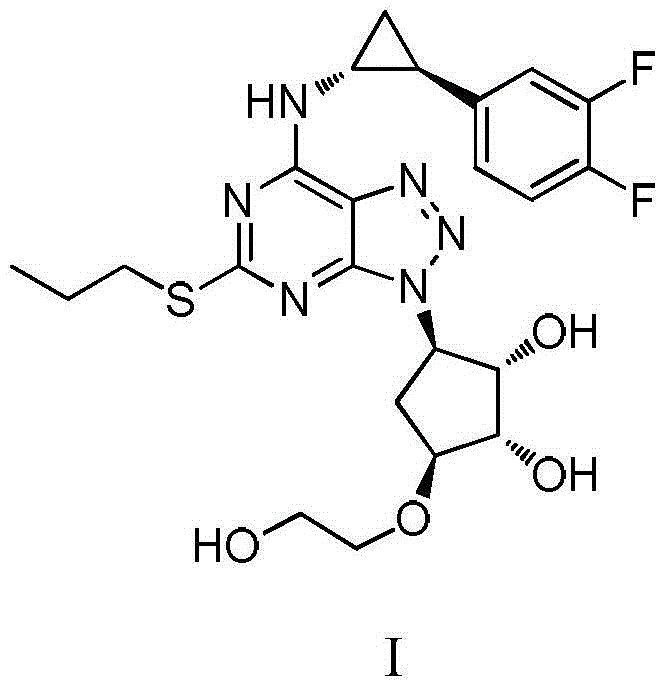
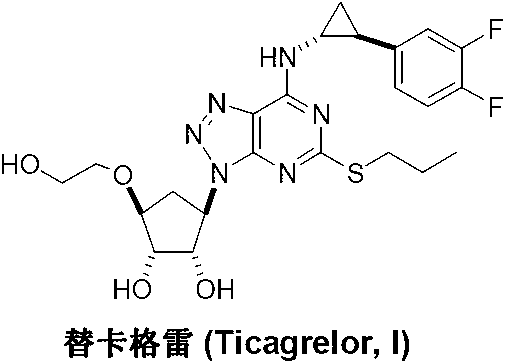
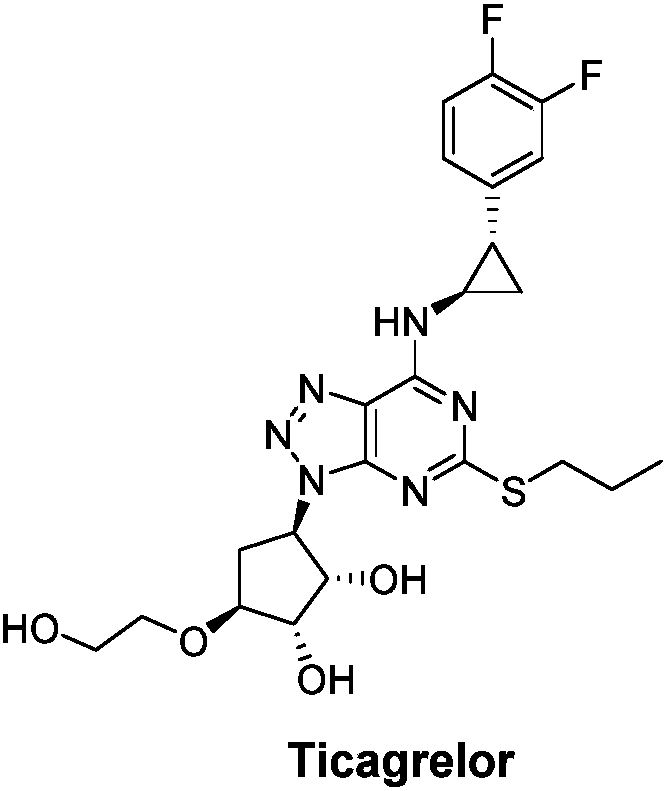
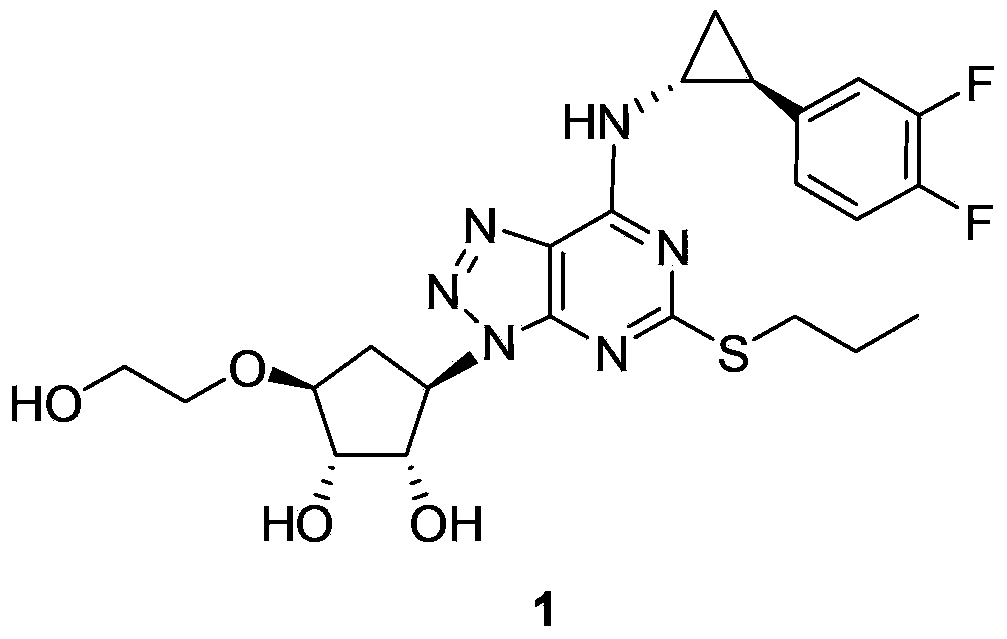
Ticagrelor is used along with low-dose aspirin to help prevent heart attack and stroke in people with heart problems (such as unstable angina, previous heart attack). It may also be used to prevent heart attack or stroke after certain heart surgeries (such as stent placement, coronary artery bypass graft-CABG, or angioplasty).
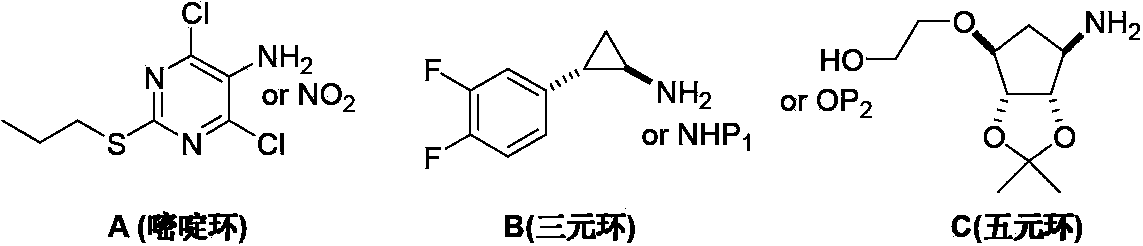
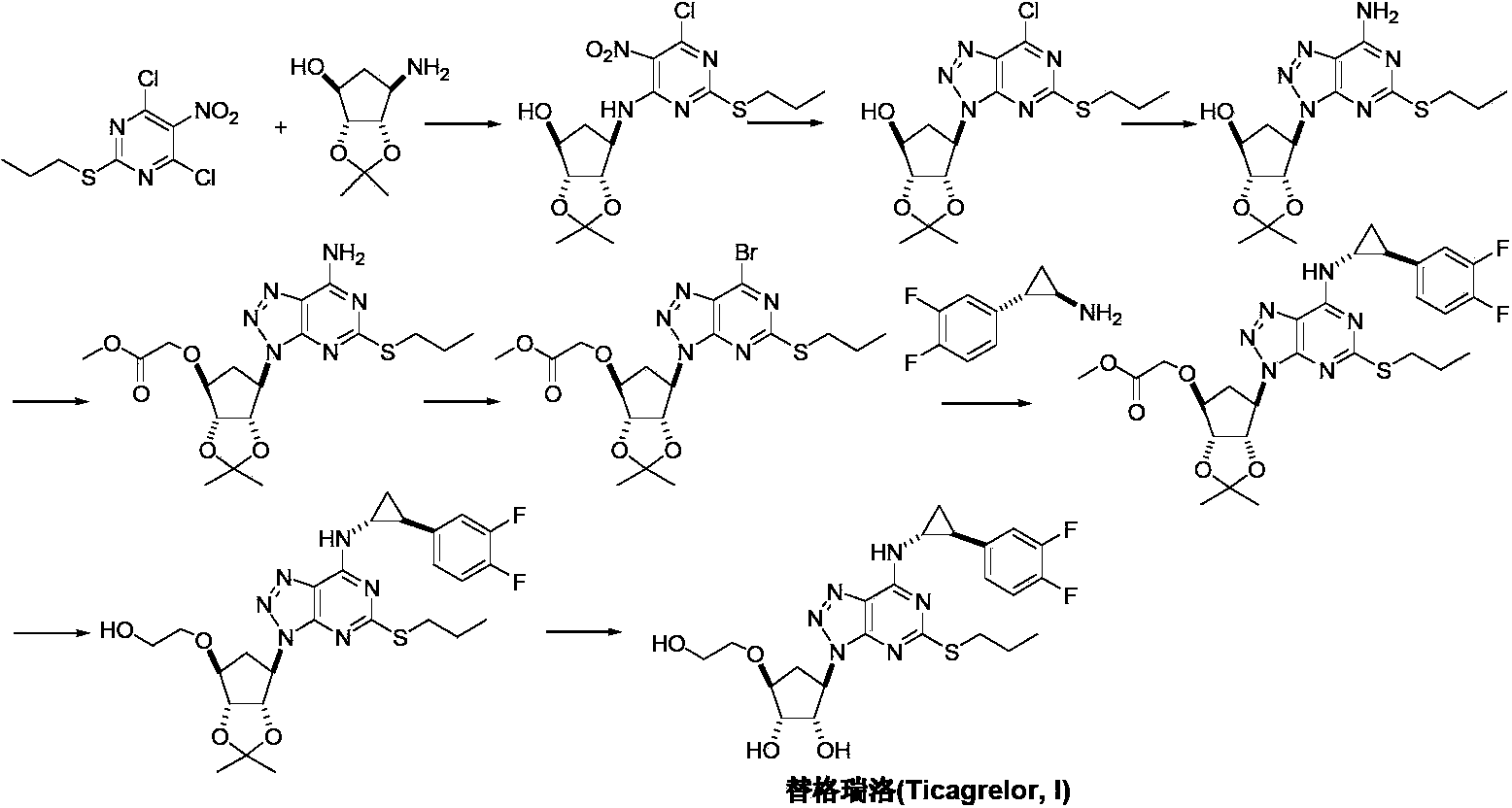
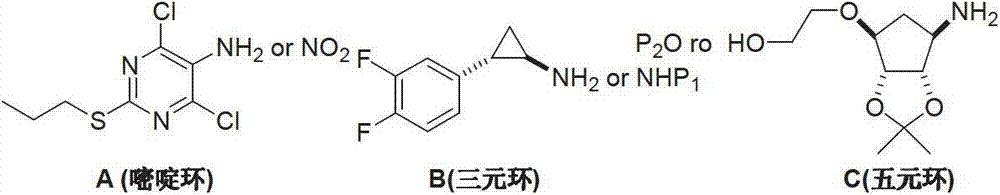
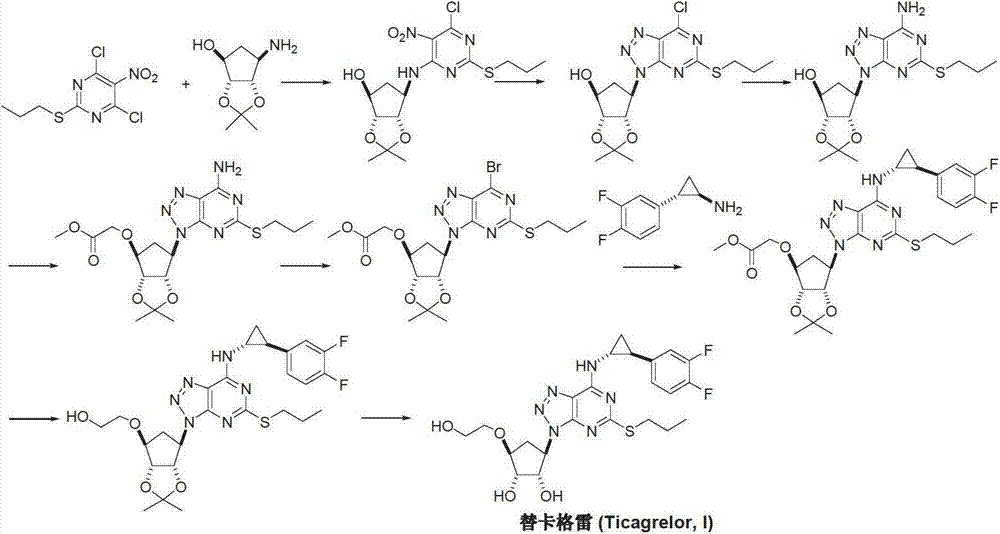
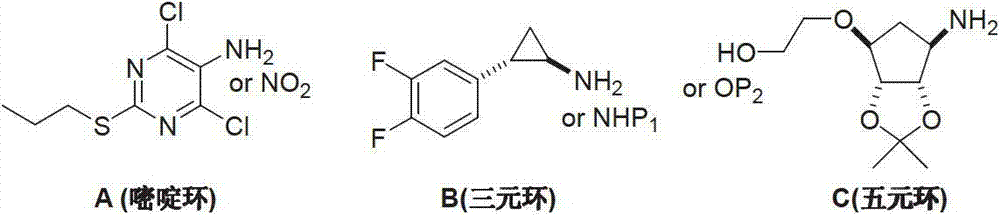
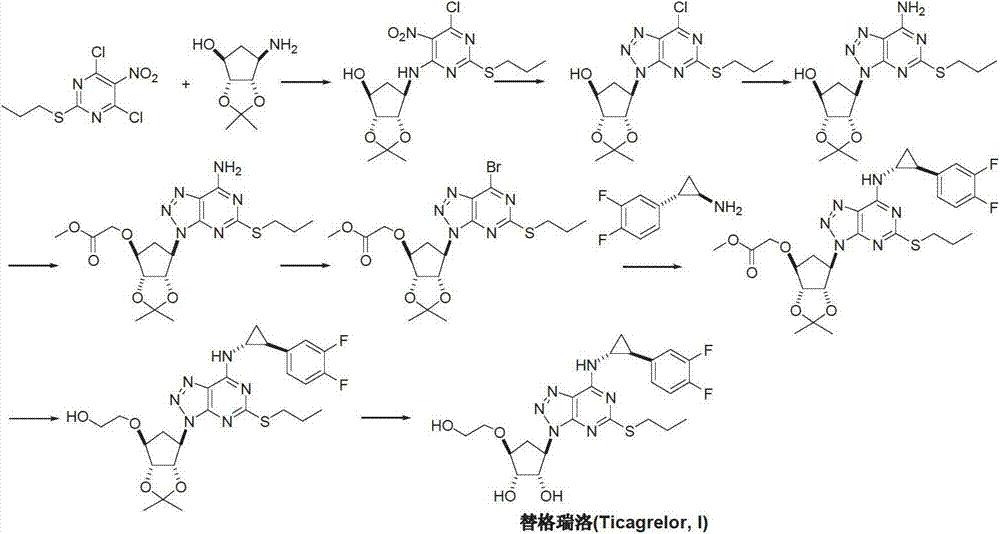
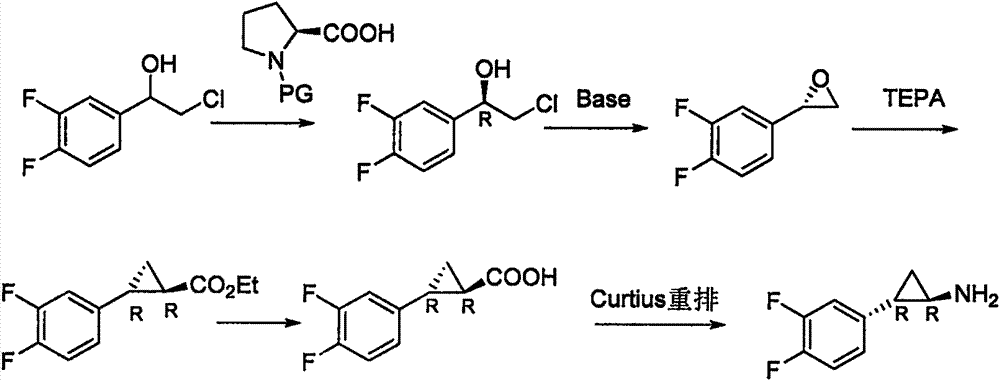
Preparation method of ticagrelor
ActiveCN102675321AHigh yieldReduce manufacturing costOrganic chemistryBulk chemical productionNitriteTicagrelor
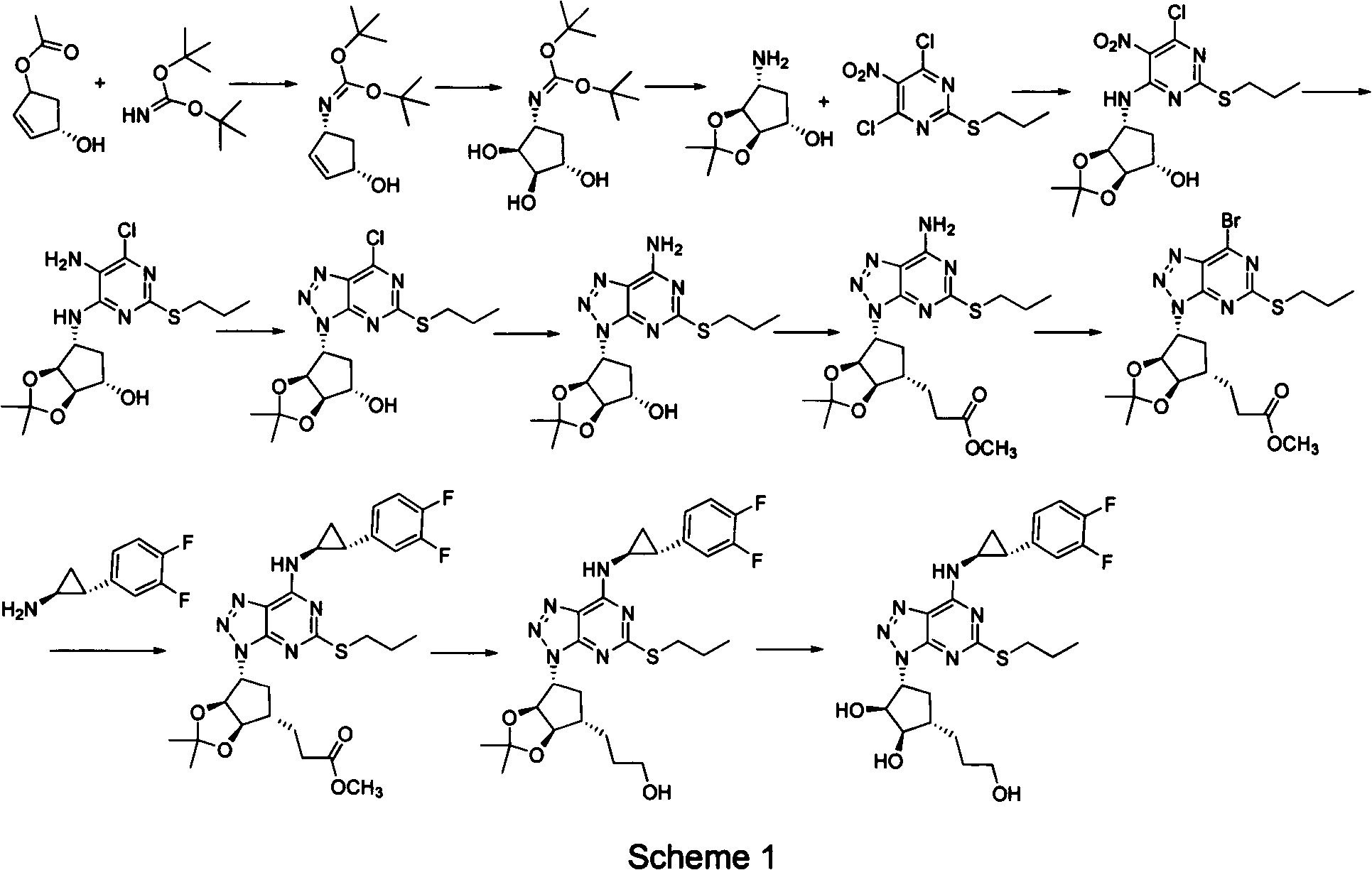
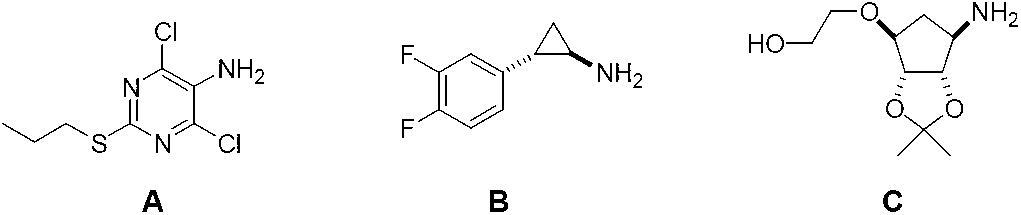
The invention provides a preparation method of ticagrelor, belonging to the technical field of medicine manufacturing. According to the method, a compound VII is taken as a raw material, and the method comprises the steps of: carrying out a nucleophilic substitution reaction on the raw material to obtain a compound VI; hydrogenating the VI, removing carbamazepine (Cbz) protection to obtain a compound V; carrying out a reaction on the V and 4, 6-dichloro-2-(allyl sulfide)-5-amio-pyrimidine to obtain a compound IV; carrying out a reaction on the IV and nitrite of alkali metal to obtain a compound III; carrying out a reaction on the III and (1R, 2S)-2-(3, 4-difluoro phenyl) cyclopropylamine to obtain a compound II; and finally, removing protecting group of the II to obtain a compound I.
Owner:SHANGHAI HAOYUAN CHEMEXPRESS
Novel intermediate of ticagrelor and method for preparing ticagrelor
ActiveCN102731467ASimple processHigh yieldGroup 4/14 element organic compoundsBulk chemical productionTicagrelorProtecting group
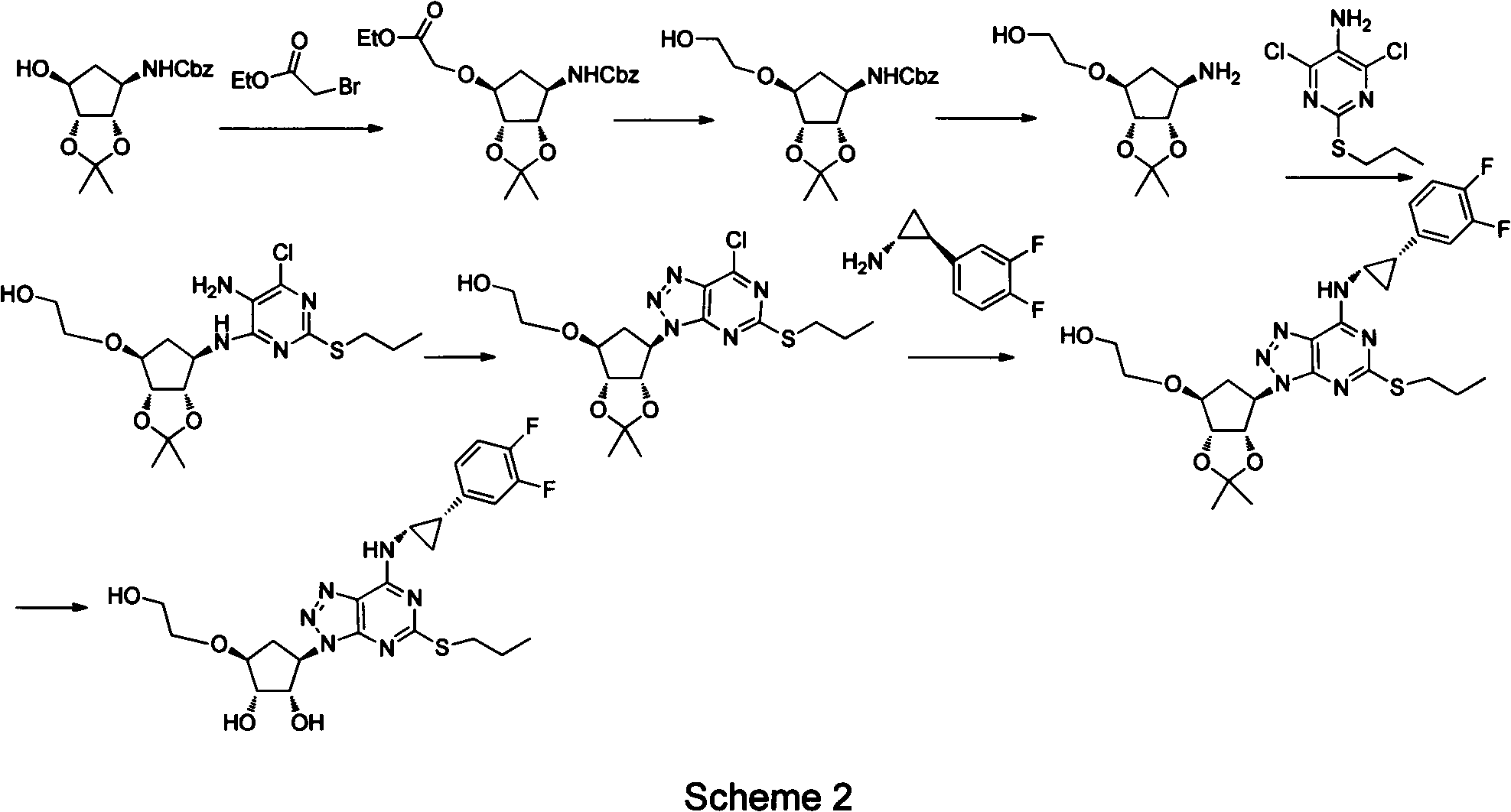
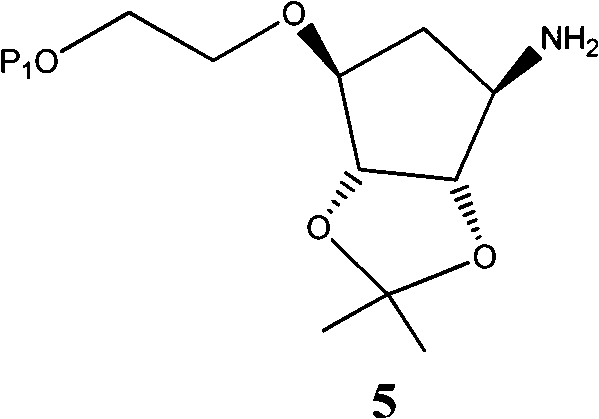
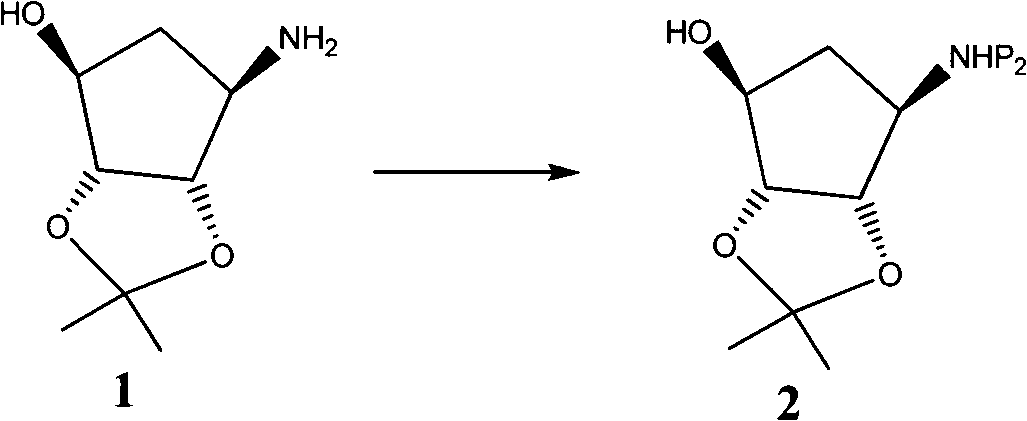
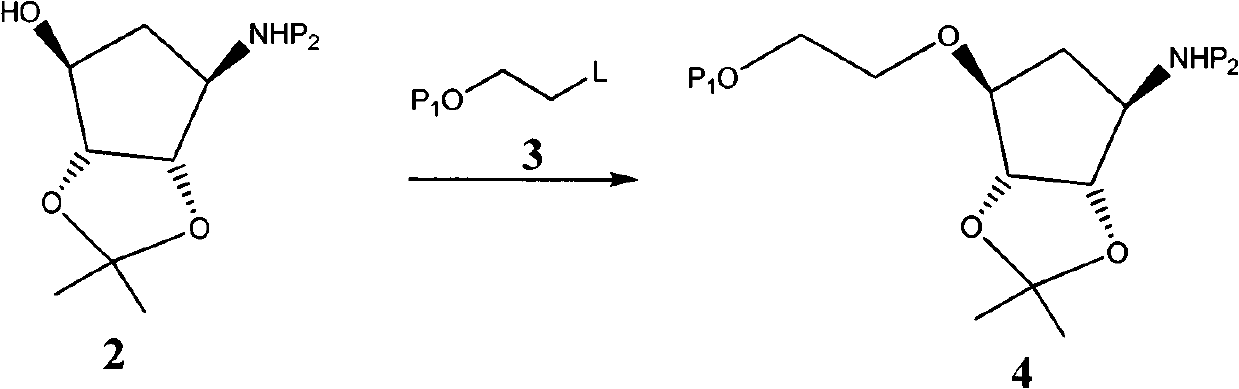
The invention provides a novel intermediate of ticagrelor or its salt. The novel intermediate is shown in the formula 5. In the formula 5, P1 represents a hydroxyl protecting group. The invention also provides a method for preparing ticagrelor from the novel intermediate shown in the formula 5. The method for preparing ticagrelor from the novel intermediate shown in the formula 5 has the advantages of simple processes, high yield and less three wastes, and can satisfy the demand of industrial production.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
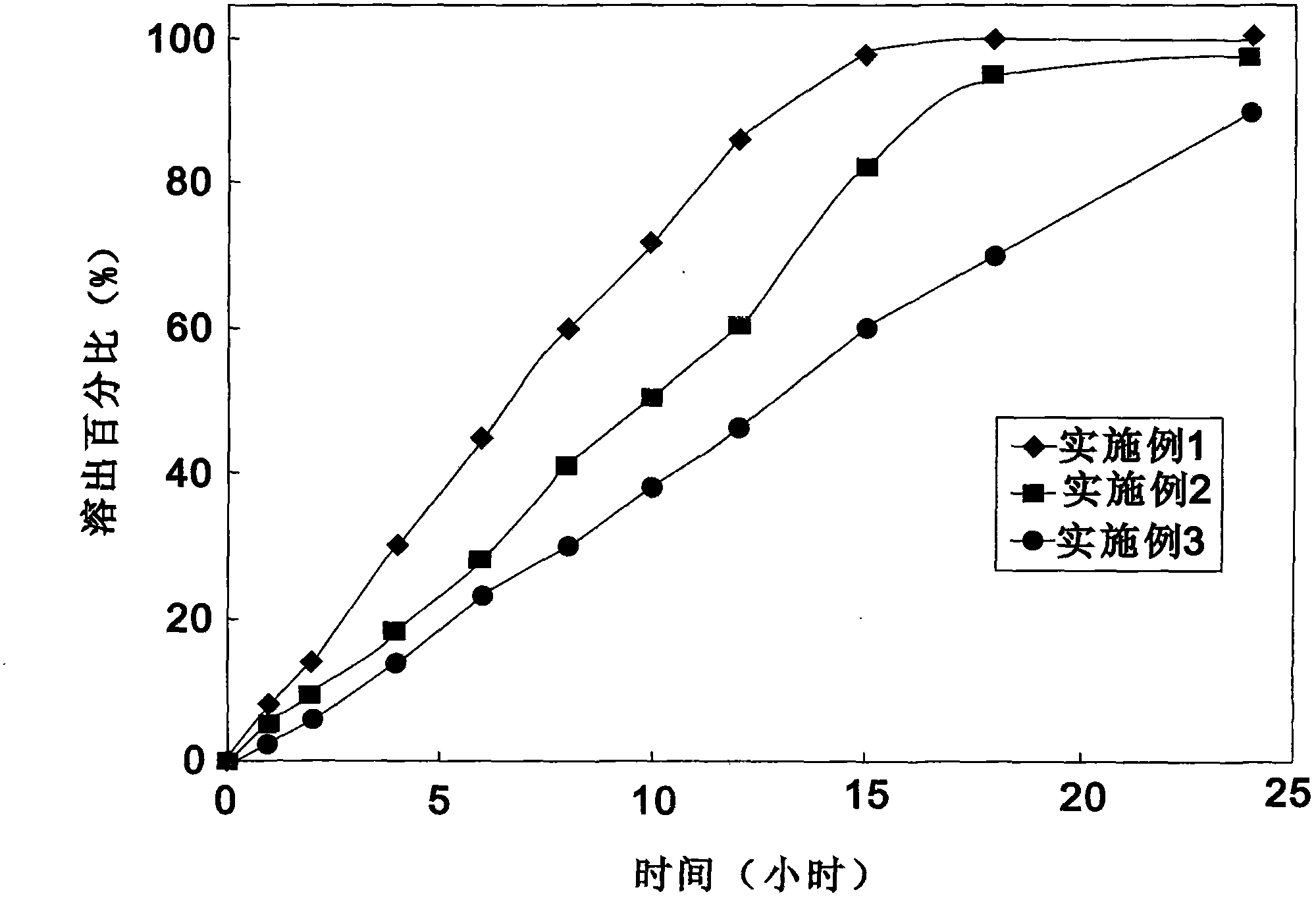
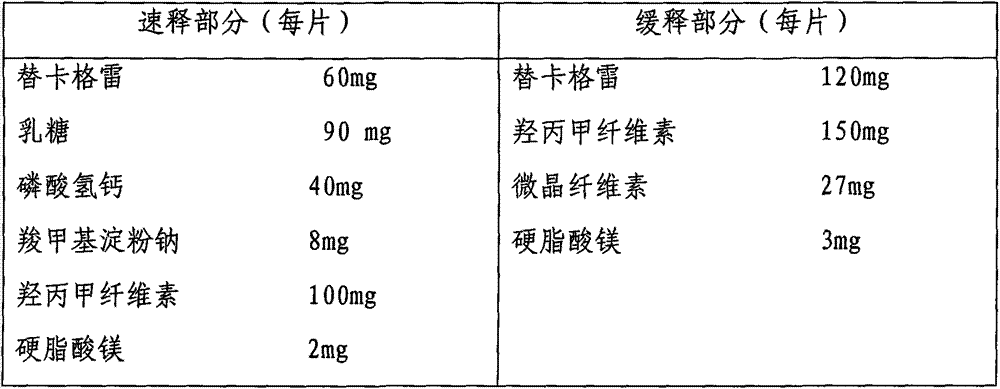
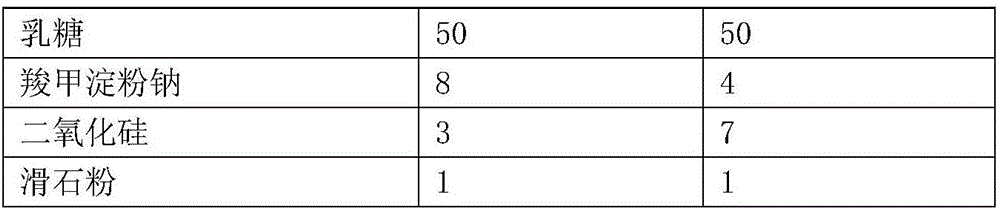
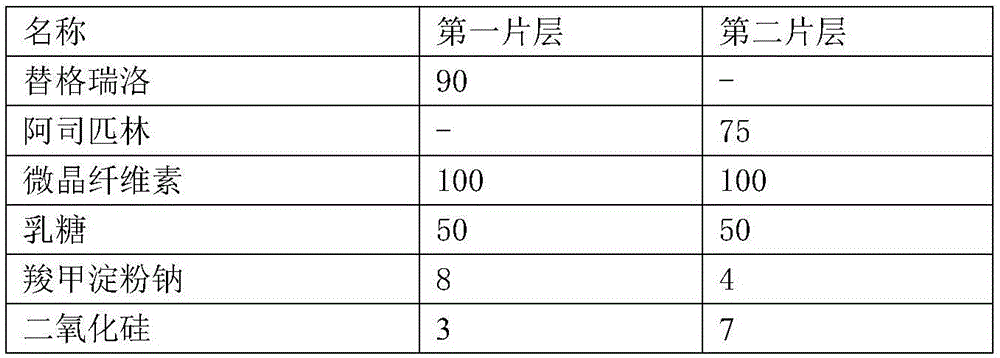
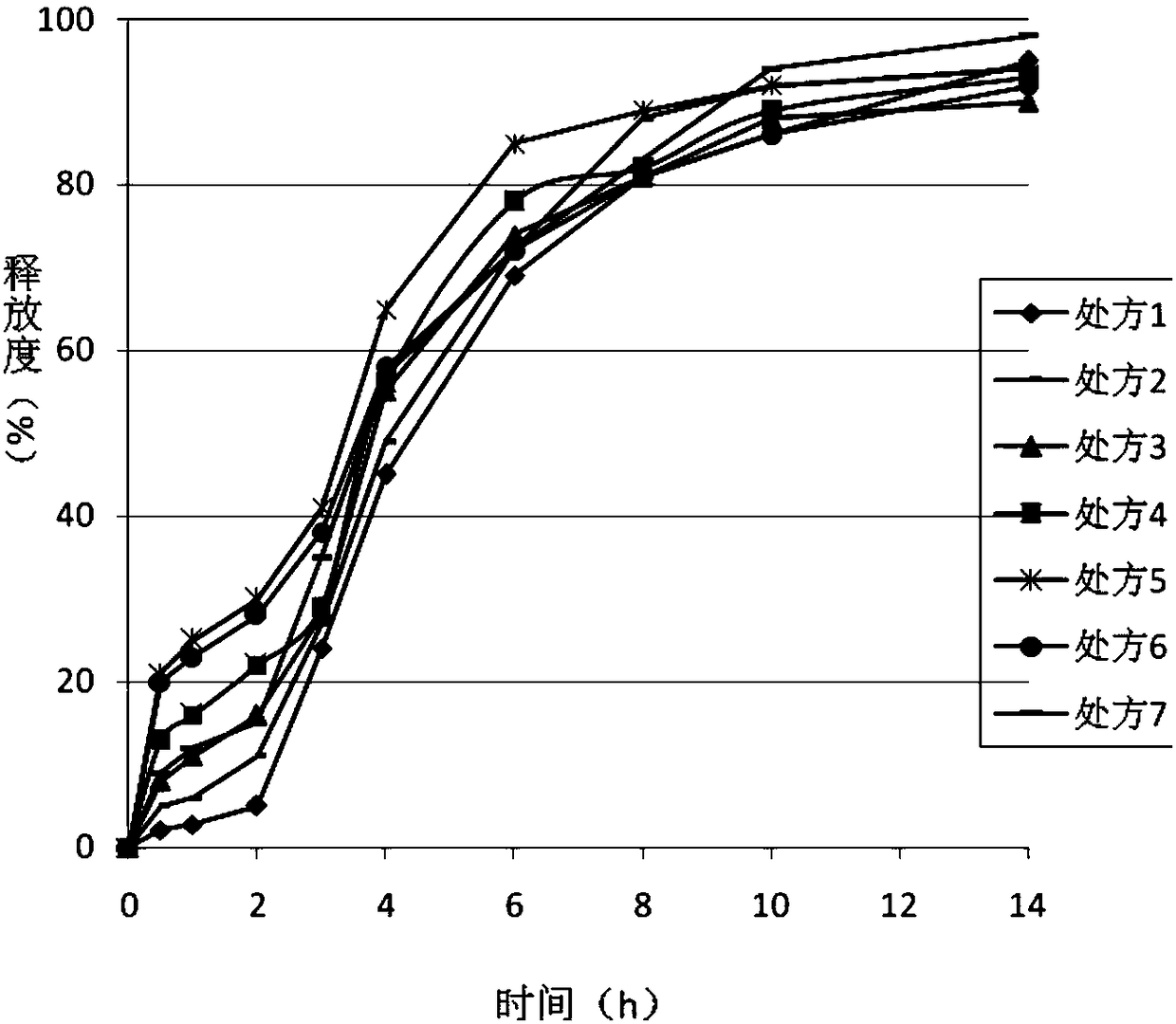
Ticagrelor sustained-release tablet system and preparation method thereof
InactiveCN102657629AMaintain blood levelsOrganic active ingredientsPharmaceutical delivery mechanismTicagrelorPediatrics
The invention provides a ticagrelor sustained-release tablet system and a preparation method of the ticagrelor sustained-release tablet system. The preparation method comprises the following steps of: firstly uniformly mixing 10-60% of ticagrelor, 5-60% of a filler and 5-60% of high molecular polymer in percentage by weight, adding a granulating solution to granulate; fully drying the obtained granules at a temperature of 50-60 DEG C, uniformly mixing the sieved granules with 0.25-10% of a lubricant and / or 0-10% of a flow aid, carrying out tabletting to obtain the ticagrelor matrix type sustained-release tablets, wherein the granulating solution is preferably water, an alcohol-water solution or absolute ethyl alcohol; the granule size of the ticagrelor is below 100 micrometers; and the content of the ticagrelor is 50-300mg in the preparation process. The ticagrelor matrix type sustained-release tablet system provided by the invention has the advantages that a patient can take the ticagrelor once a day to ensure that the drug dependence of the patient can be improved and the risk of myocardial infarction or apoplexy caused by acute thrombosis due to a dose of the ticagrelor missing of the patient is reduced.
Owner:SHENZHEN HUALIKANG BIOLOGICAL MEDICINE
Novel preparation method of antithrombosis medicine
The invention provides a novel preparation method which is easily industrially achieved and is simple and convenient to carry out and is established under a new intermediate. The invention relates to a novel preparation method of micromolecule anticoagulant Ticagrelor, and synchronously relates to an intermediate body for synthesizing the Ticagrelor and a preparation method of the intermediate. With the adoption of the synthesis method provided by the invention, the side reaction in the reaction process can be effectively reduced, the purity of the intermediate is improved, and the purifying way of the intermediate is simplified.
Owner:CHANGZHOU PHARMA FACTORY
Preparation method of Ticagrelor intermediate 4,6-dichloro-2-(pyridinecarboxylic)-5- aminopyrimidine
InactiveCN103130726AEase of industrial productionPromote the development of economy and technologyOrganic chemistryTicagrelorSodium sulfite
The invention discloses a preparation method of Ticagrelor intermediate 4,6-dichloro-2-(pyridinecarboxylic)-5- aminopyrimidine. The preparation method of the Ticagrelor intermediate 4,6-dichloro-2-(pyridinecarboxylic)-5- aminopyrimidine includes the following steps: using 4,6- dichloro-2-nitro-2-(pyridinecarboxylic) pyrimidine(V), enabling the nitro of a molecular structure to be reduced to amino through the reduction reaction of reducing agent sodium hydrosulfite, and obtaining the Ticagrelor intermediate 4,6-dichloro-2-(pyridinecarboxylic)-5- aminopyrimidine. The preparation method of the Ticagrelor intermediate 4,6-dichloro-2-(pyridinecarboxylic)-5- aminopyrimidine is easy, convenient to implement, economical and environment-friendly, beneficial to industrial production and capable of facilitating the development of economic technology of the bulk drug.
Owner:许学农
Preparation method of Ticagrelor intermediate
ActiveCN102796007AEasy to prepareEasy to operatePreparation by rearrangement reactionsChemical synthesisTicagrelor
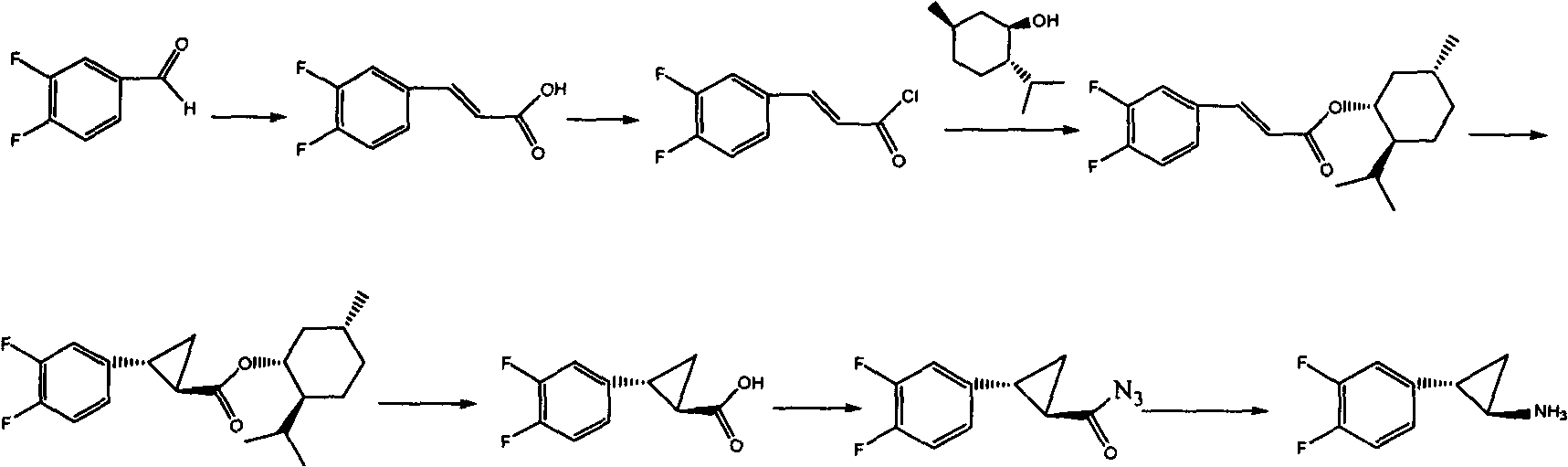
The invention relates to the medicine chemical synthesis field, and especially discloses a preparation method of a Ticagrelor intermediate. The preparation method comprises the following steps: 1) taking 3,4-difluorobenzaldehyde (I) as an initial raw material, reacting with a phosphorus ylide material liquid to obtain (E)-3-(3,4-difluorophenyl)-2-acrylic acid ester (II); 2) performing a Simons-Smith asymmetric cyproteronethe reaction on the (E)-3-(3,4-difluorophenyl)-2-acrylic acid ester (II) to obtain trans-(1R,2R)-2-(3,4-difluorophenyl) cyclopropanecarboxylic acid ester (III); 3) performing aminolysis on the trans-(1R,2R)-2-(3,4-difluorophenyl) cyclopropanecarboxylic acid ester to obtain trans-(1R,2R)-2-(3,4-difluorophenyl)cyclopropanecarboxamide (IV); and 4) performing a Huffman rearrangement reaction on the trans-(1R,2R)-2-(3,4-difluorophenyl)cyclopropanecarboxamide (IV) to obatain the Ticagrelor intermediate (V). The method of the invention has the advantages of simple process, convenient operation, mild reaction condition and easy control, low cost and easy acquisition of raw material, high product yield and product purity, and is adapted to large scale industrial production.
Owner:JINAN RUIFENG PHARMA +2
Method for preparing ticagrelor
ActiveCN103360396AReduce usageEasy to prepareOrganic chemistryBulk chemical productionAcetic acidTicagrelor
The invention discloses a method for preparing ticagrelor, which comprises the following steps of by taking 1-[(3aR,4S,6R,6aS)-[[2,2- dimethyl-tetralin-4H-cyclopentadiene-1,3-dioxo heterocyclelane-4-oxygroup] ethyl acetate-6-base]-5-amidogen-4-formamido-1,2,3- triazole(II) as the raw material, sequentially carrying out cyclizing, replacement, condensation and reduction reactions, and removing the protecting group, so as to prepare the ticagrelor. The method is concise, environment-friendly and economic, has high chemical and chirality purity, and provides the new preparation method of ticagrelor industrialized production.
Owner:铜陵尚东高新科创有限公司
Method for preparing ticagrelor key intermediate and racemate thereof and special intermediate for implementing method
ActiveCN103508899ALow priceEasy to getCarboxylic acid nitrile preparationOrganic compound preparationAfter treatmentTicagrelor
The invention discloses a method for preparing a ticagrelor key intermediate VII and a racemate thereof. The method comprises the following steps: by using a compound V or a racemate thereof as a raw material, performing acidic hydrolysis to obtain a compound VI or a racemate thereof; and performing Curtis rearrangement to obtain a compound VII or a racemate thereof. According to the ticagrelor key intermediate and the racemate thereof prepared by the method, the adopted initial raw materials are low in price and readily available, the requirements of reaction conditions on solvents are low, the operation is safe, simple and convenient, and the method is environment-friendly; moreover, when the ticagrelor key intermediate and the racemate thereof are prepared by adopting a special intermediate, the after-treatment is simple and convenient, and the large-scale production is more easily realized.
Owner:KAIYUAN HENGTAI PHARMA
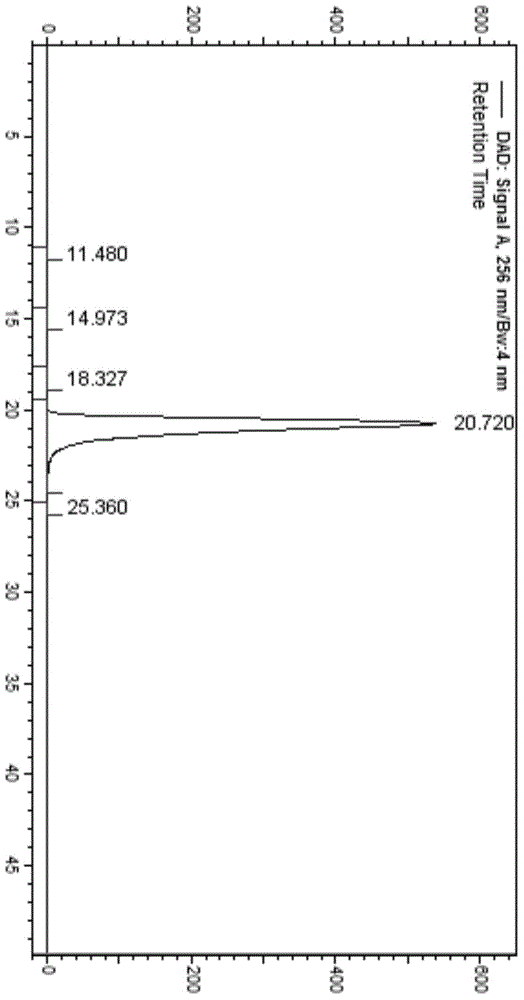
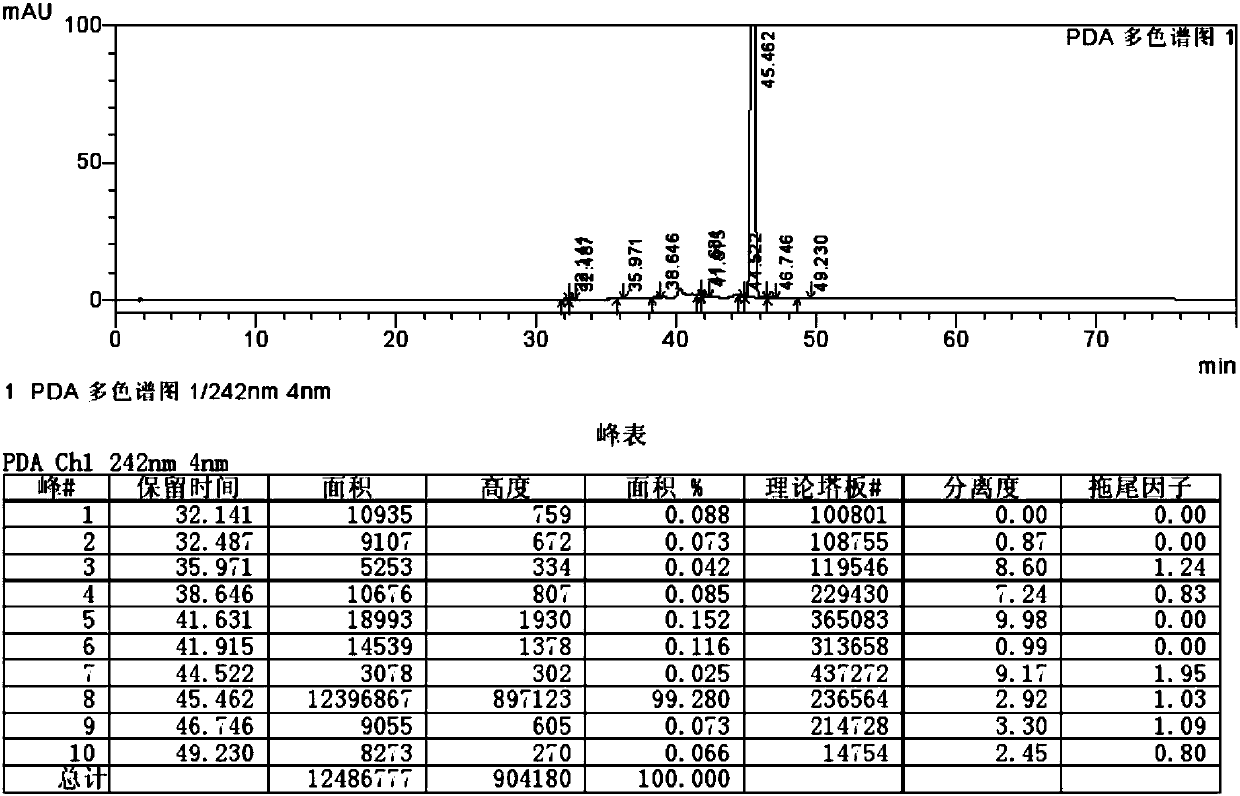
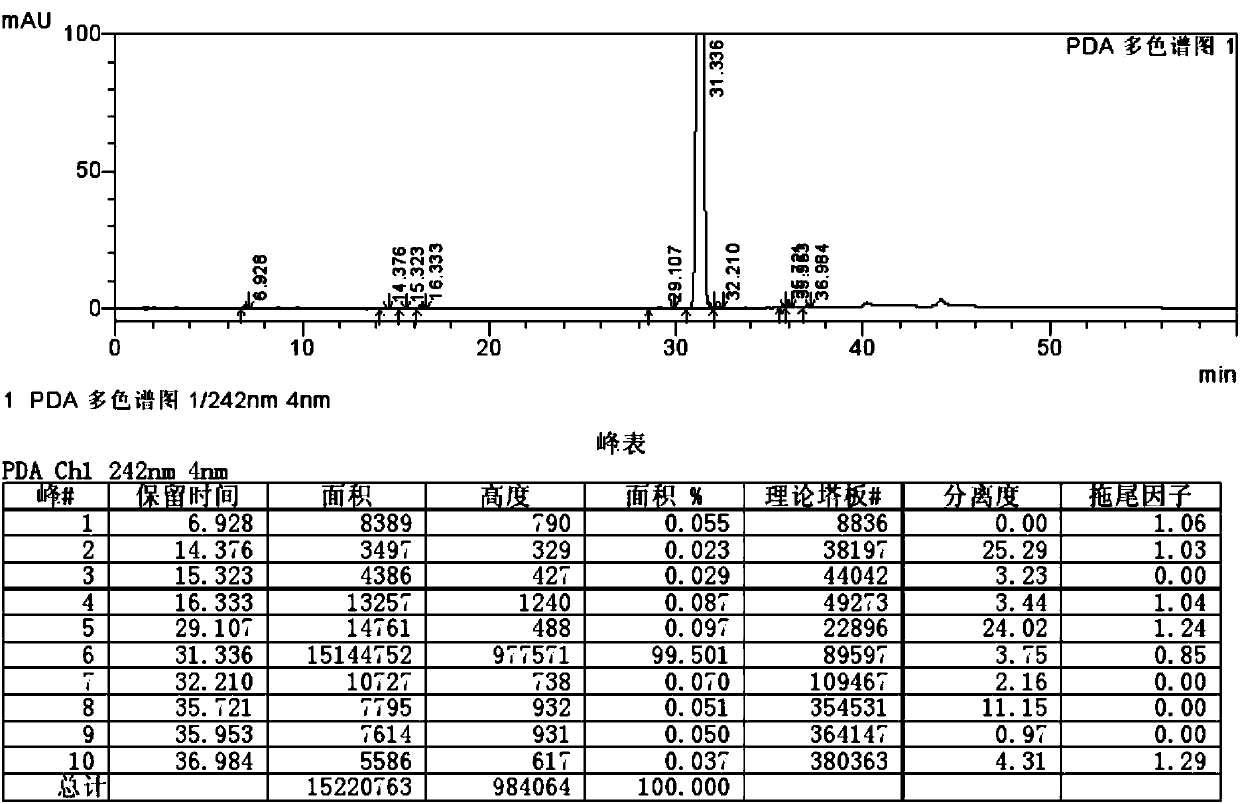
Method for separating and measuring ticagrelor and optical isomer of ticagrelor
The invention belongs to the field of analytical chemistry and in particular relates to a method for separating and measuring ticagrelor and a diastereoisomer of the ticagrelor. The method is characterized in that a chiral HPLC (High Performance Liquid Chromatography) column taking polysaccharide derivative as a filler is used and a mixed solution of lower paraffin hydrocarbon and low alcohol is used as a moving phase; according to the separating and detecting method, the ticagrelor and the diastereoisomer of the ticagrelor are effectively separated, and the mass of the ticagrelor can be effectively controlled. The method can be used for separating and detecting the ticagrelor and the diastereoisomer of the ticagrelor simply, rapidly and accurately.
Owner:SUNSHINE LAKE PHARM CO LTD
Preparation method of ticagrelor
ActiveCN103288836AEase of industrial productionPromote the development of economy and technologyOrganic chemistryTicagrelorAmination
The invention discloses a preparation method of ticagrelor. The preparation method comprises the following steps of: carrying out a cyclization reaction between 5-amino-1,4-di-substituted-1,2,3-triazole (II) and a dialkyl carbonate (III), thereby obtaining 9-substituted-2,6-dihydroxy-8-azaguanine (IV), chlorinating the intermediate (IV) to obtain 9-substituted-2,6-dichloro-8-azaguanine (V), carrying out an amination reaction between the intermediate (V) and trans-(1R, 2S)-2-(3,4-difluorophenyl) cyclopropylamine (VI) to generate 9-substituted-6-amino substituent-2-chloro-8-azaguanine (VII), and carrying out a propylthiolation reaction between the intermediate (VII) and propanethiol (VIII) to obtain the ticagrelor (I). The preparation method disclosed by the invention is simple in process, and high in chemical and chiral purity, and provides a new preparation way for industrial production of the ticagrelor.
Owner:鄄城县人民医院
Preparation method of ticagrelor
ActiveCN103288837AReduce usageThe preparation process is convenientOrganic chemistryBulk chemical productionTicagrelorProtecting group
The invention discloses a preparation method of ticagrelor. The preparation method comprises the following steps of: carrying out a cyclization reaction between 5-amino-1,4-di-substituted-1,2,3-triazole (II) and a sulfur-containing cyclizing agent (III), thereby obtaining 9-substituted-2-sulfo-6-oxo-8-azaguanine (IV), carrying out a substitution reaction between the intermediate (IV) and halogenated propane (V) to generate 9-substituted-2-propylthiol-6-oxo-8-azaguanine (VI), carrying out condensation between the intermediate (VI) and trans-(1R,2S)-2-(3,4-difluorophenyl) cyclopropylamine (VII) to generate 9-substituted-6-amino substituent-2-propylthiol-8-azaguanine (VIII), and depriving an idene acetone protecting group from the intermediate (VIII) to obtain the ticagrelor (I). The preparation method disclosed by the invention is simple in process, economic and environment-friendly, and high in chemical and chiral purity, and provides a new preparation way for industrial production of the ticagrelor.
Owner:铜陵三十九度创客公园管理有限公司
Preparation method, detection method and application for ticagrelor-related substances
ActiveCN105237540ARaise quality standardsAvoid product qualityOrganic chemistryComponent separationTicagrelorQuality control
The invention provides a preparation method and detection method for oxides of ticagrelor, i.e., (1S,2S,3R,5S)-3-[7-{[(1R,2S)-2-(3.4-difluorophenyl)cyclopropyl]amino}-5-(propylthionyl)-3H-[1,2,3]triazolo-[4,5-d]pyrimidine-3-yl]-5-(2-hydroxyethoxyl)cyclopentane-1,2-diol and (1S,2S,3R,5S)-3-[7-{[(1R,2S)-2-(3.4-difluorophenyl)cyclopropyl]amino}-5-(propylthioacyl)-3H-[1,2,3]triazolo-[4,5-d]pyrimidine-3-yl]-5-(2-hydroxyethoxyl)cyclopentane-1,2-diol, and application of the oxides in the quality control research of ticagrelor.
Owner:NANJING CHIA TAI TIANQING PHARMA
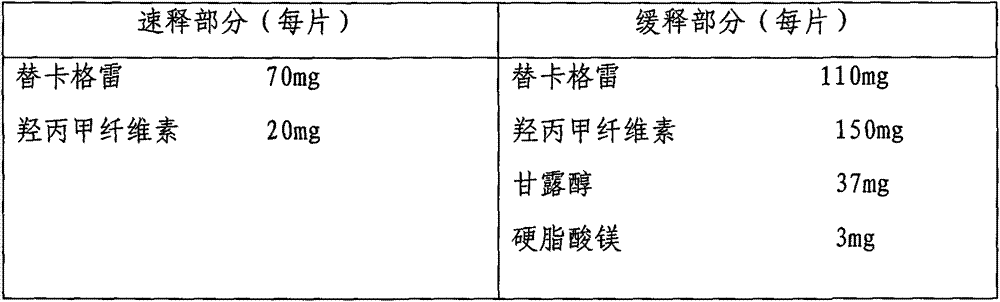
Slow/controlled-release preparation of ticagrelor
InactiveCN103860504AImprove complianceGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismTicagrelorThrombus
The invention provides a slow / controlled-release preparation of ticagrelor. The slow / controlled-release preparation of ticagrelor is an oral drug. The slow / controlled-release preparation contains ticagrelor or its pharmaceutically acceptable salt. A mass percent of ticagrelor to controlled-release accessory materials is in a range of 1: 0.2 to 1: 20 and preferably, the mass percent is in a range of 1: 0.1 to 1: 10, and a proper amount of other accessory materials are used. The slow / controlled-release base comprises one or more of cellulose, cellulose derivatives, alginate, starch, starch derivatives, polypropylene resins, carboxyvinyl polymers and other controlled-release accessory materials. Compared with a fast-release preparation, the slow / controlled-release preparation of ticagrelor provides a ticagrelor slow / controlled-release preparation system, can be eaten by a patient once each day, can change drug compliance of patients, can reduce the risk of myocardial infarction or stroke caused by acute thrombosis caused by missing of ticagrelor, and provides the easily-prepared slow / controlled-release preparation of ticagrelor or its pharmaceutically acceptable salt.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Micronization and crystal form of ticagrelor and preparation method and pharmaceutical application of crystal form of ticagrelor
InactiveCN104650091AImprove wettabilityImprove hydrophilicityOrganic active ingredientsOrganic chemistry methodsTicagrelorOrganosolv
The invention relates to a micronized substance and a micronized crystal form of a water-insoluble compound ticagrelor and further relates to a preparation method and pharmaceutical application of the crystal form of ticagrelor. The preparation method comprises the steps of completely dissolving ticagrelor with a proper organic solvent, adding the solution to a water-containing solvent, stirring to immediately separate ticagrelor out and disperse ticagrelor into the water-containing solvent, filtering, separating solids, and drying. The micronized ticagrelor has excellent water wettability and water dispersibility, the powder stacking density and the powder fluidity of the ticagrelor are well improved, and a pharmaceutical preparation utilizing ticagrelor as a active ingredient has important significance in the aspects of improving the dissolution rate of the pharmaceutical preparation, improving the uniformity of pharmaceutical preparation and improving the quality standard, absorption and utilization.
Owner:北京创世晟源生物科技有限公司
Method for preparing ticagrelor key intermediate
InactiveCN106279095AReduce pressure on environmental protectionSuitable for large-scale industrial productionOrganic chemistryBulk chemical productionChemical synthesisPalladium on carbon
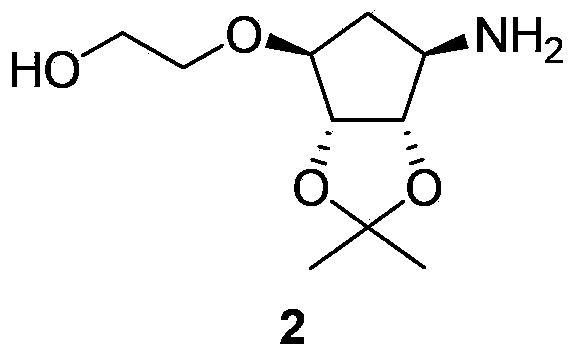
The invention relates to a chemical synthesis method of ticagrelor key intermediate 2-[[(3aR, 4S, 6R, 6aS)-6-aminotetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxolane-4-yl] oxy]ethanol (a key intermediate A). The method comprises the following steps: taking D-ribose as a raw material, and carrying out ten chemical reaction steps of 1-locus methylation and 2,3-loci isopropylidene protection, 4-locus derivatization, iodination, furan ring-opening, hydroxylamine reaction, palladium on carbon catalytic hydrogenation, amino Cbz protection, hydroxy protection, sodium borohydride reduction ester, Cbz removal protection and the like, thereby obtaining the key intermediate A. The raw materials are cheap and readily available, the preparation process is high in operability, steps of optical resolution, chiral induction and the like are avoided, the total yield is relatively high, and the product quality is better; particularly due to the use of sodium borohydride reduction ester, the preparation cost of ticagrelor is greatly reduced; and the method is suitable for large-scale industrial production.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
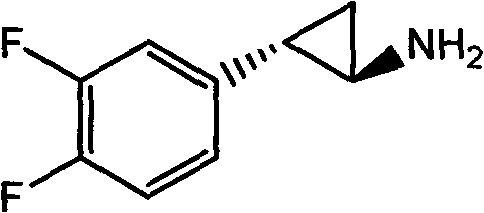
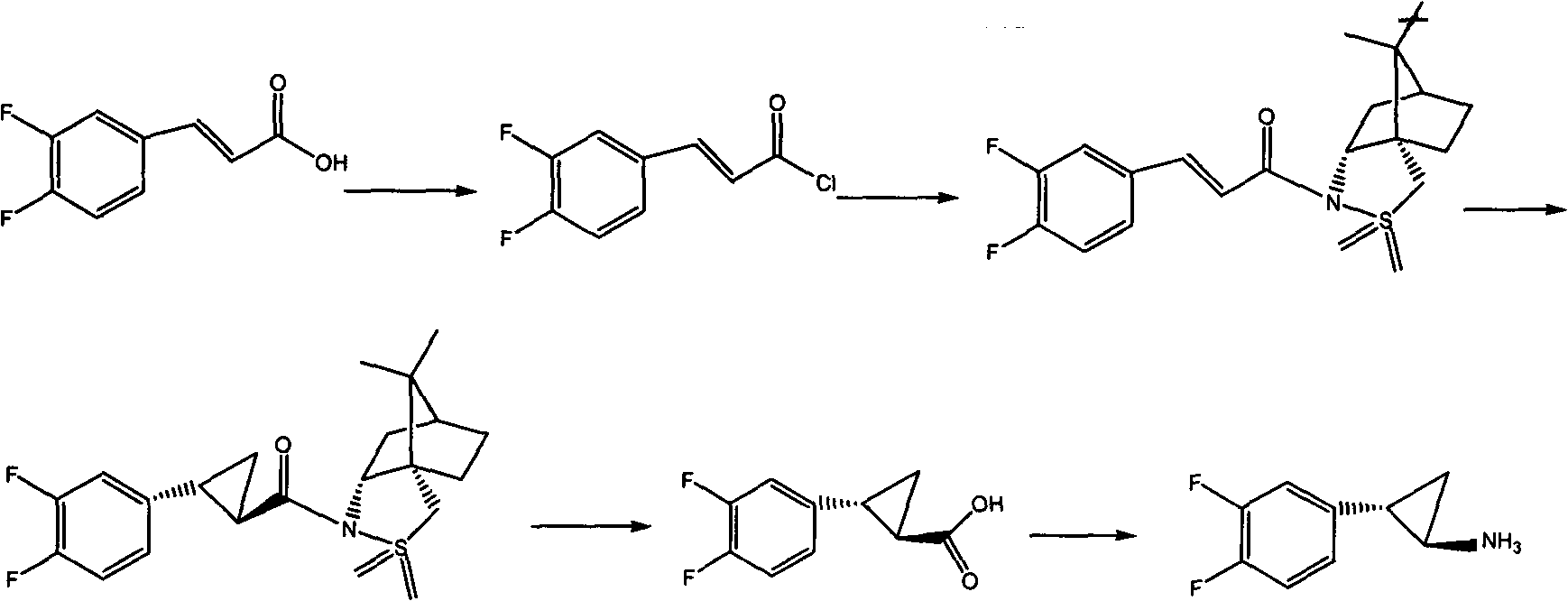
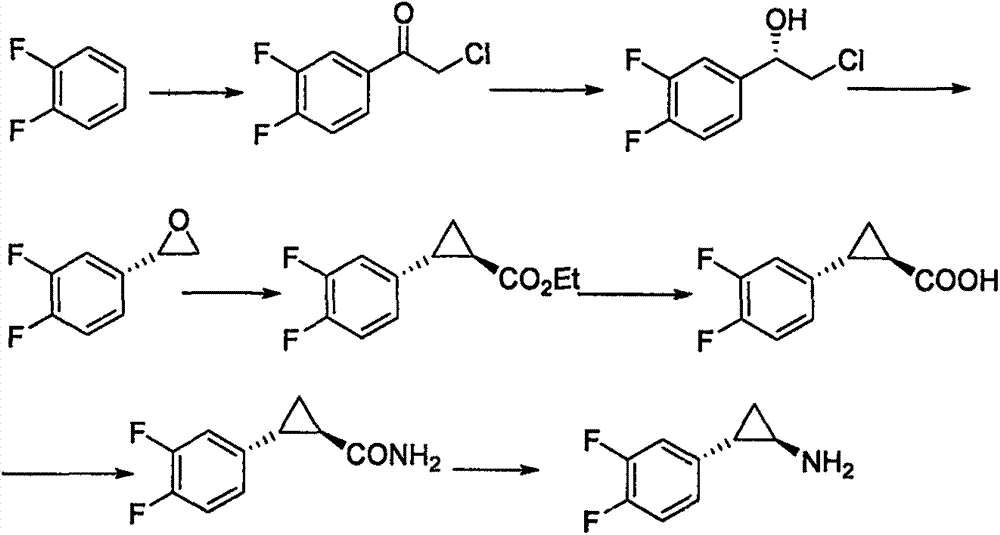
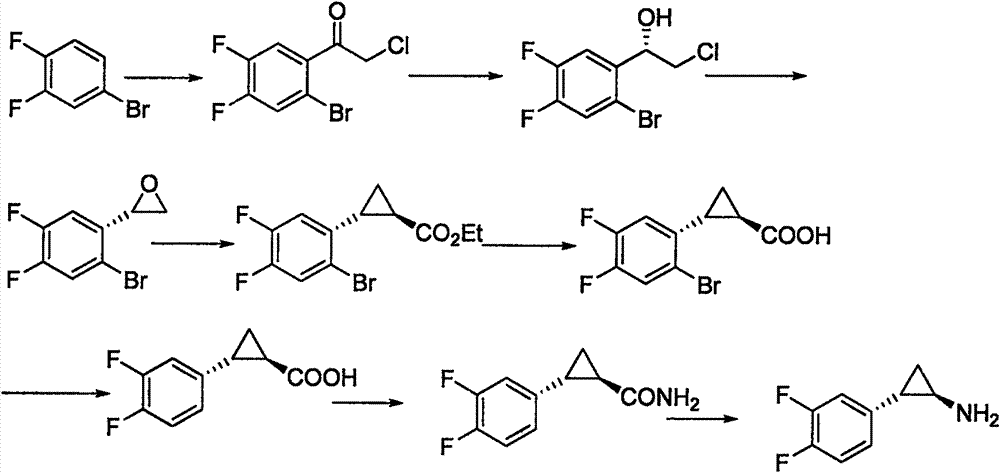
Chemical method used for preparing aromatic cyclopropanecarbonitrile and cyclopropylamine
The invention relates to a method for preparing trans-aryl cyclopropanecarbonitrile with a structure shown in a formula (IV) in the specification through reaction between aryl substituted ethylene oxide and cyan substituted phosphate and further relates to a method for preparing cyclopropylamine from trans-aryl cyclopropanecarbonitrile. Trans-aryl cyclopropanecarbonitrile and cyclopropylamine are used for preparing drugs, especially ticagrelor.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
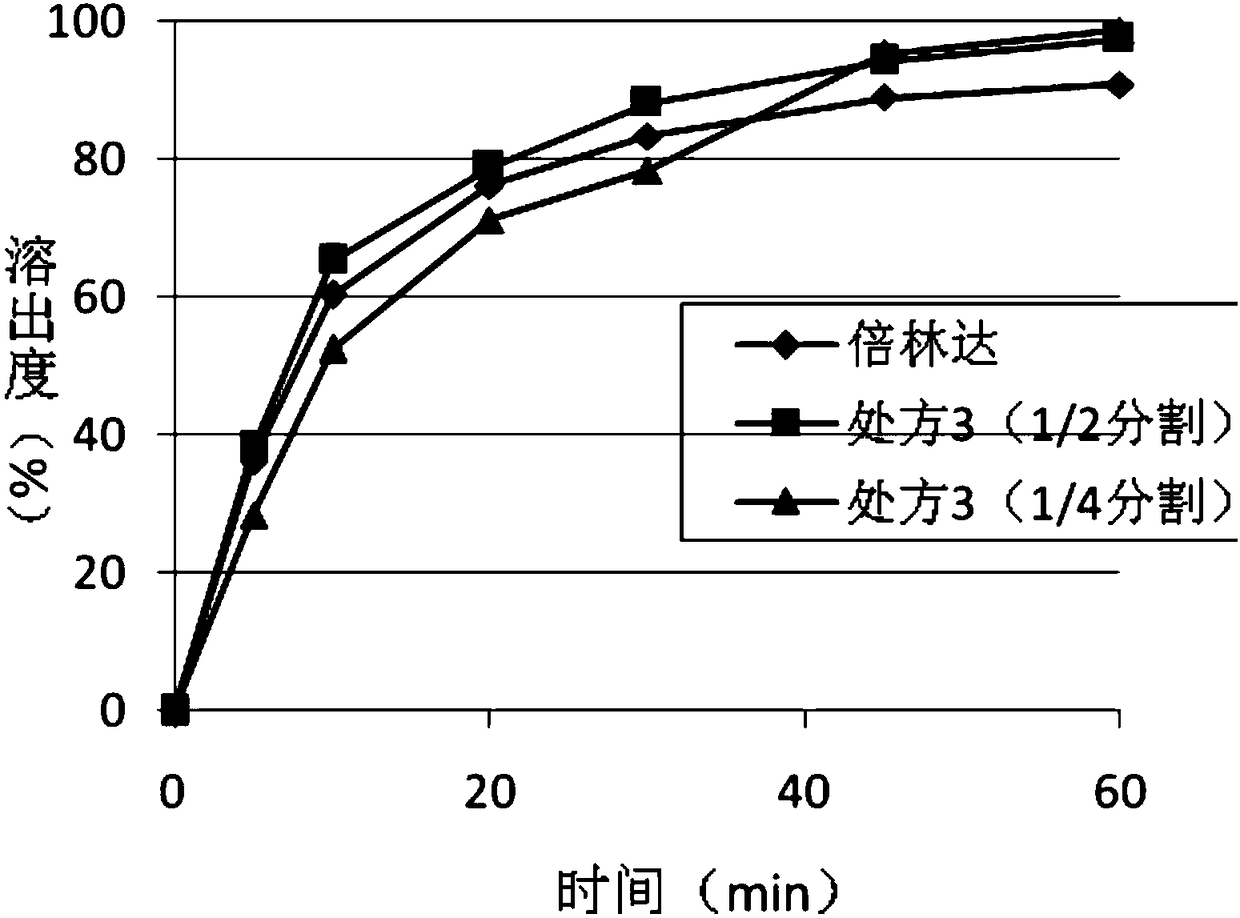
Ticagrelor tablets and preparation method thereof
ActiveCN104523640AIncrease dissolution ratePromote absorptionOrganic active ingredientsPharmaceutical non-active ingredientsMANNITOL/SORBITOLTicagrelor
The invention discloses ticagrelor tablets and a preparation method thereof and belongs to the technical field of medicines. According to the ticagrelor tablets disclosed by the invention, mannitol, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, croscarmellose sodium and magnesium stearate are selected as auxiliary materials and are compounded with the raw material medicine ticagrelor tablets so as to obtain the ticagrelor tablets, and each auxiliary material and the raw material medicine are synergetic in the defined amount range, so that the dissolution rate of the prepared ticagrelor tablets is higher than that of the conventional commercially available tablets. Moreover, the ticagrelor tablets have the same dissolution behavior as the commercially available medicines, the ticagrelor tablets have good absorption effects, and the bioavailability of the ticagrelor tablets is improved. The ticagrelor tablets disclosed by the invention are small in impurity content and are stable in performance under high-temperature illumination conditions. The method for preparing the ticagrelor tablets disclosed by the invention is simple in process flow, easy to operate and implement and suitable for industrial popularization and application.
Owner:HENAN RUNHONG PHARMA
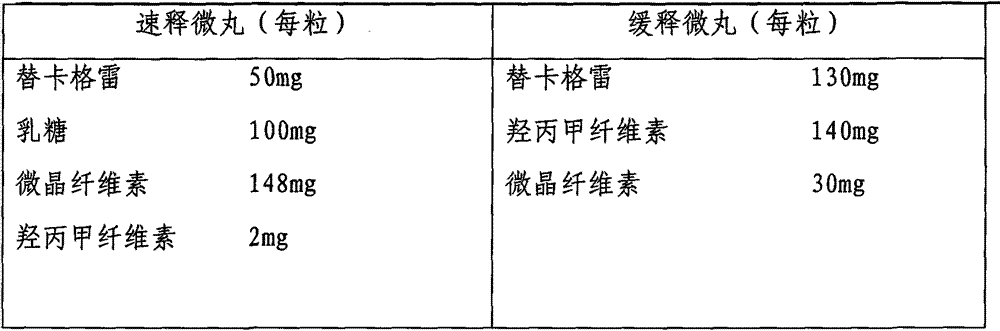
Ticagrelor sustained-release preparation
ActiveCN103520164AOrganic active ingredientsGranular deliveryImmediate releaseSustained Release Capsule
The invention relates to a sustained-release preparation composed of ticagrelor, a pharmaceutically acceptable sustained-release material, and other pharmaceutically acceptable auxiliary materials. The sustained-release preparation has an immediate-release part and a sustained-release part. The preparation can be double-part tablets obtained by compression by using a double-part tabletting machine, or tablets with the sustained-release medicine as a tablet core and the immediate-release medicine as outer coating, or sustained-release capsules composed of the immediate-release part and the sustained-release part. With the sustained-release preparation provided by the invention, medicine effect is fast, and medicine effective concentration can be maintained for a long time. Therefore, an ideal treatment effect can be provided.
Owner:BEIJING KANG LISHENG PHARMA TECH DEV
Oral enteric preparation containing Grel drugs and aspirin
The invention relates to a novel oral enteric preparation which is composed of 0.1-1000mg of Grel drugs, or medically acceptable salts, ester or derivatives, 37.5-325mg of aspirin and at least one medically acceptable load, wherein the Grel drugs, or medically acceptable salts, esters or derivatives are clopidogrel, prasugrel, brilinta, sarpogrelate, ozagrel, anagrelide, pamicogrel, or medically acceptable salts, ester or derivatives, preferably, clopidogrel sulfate. The oral enteric preparation is used for curing acute coronary syndrome (ACS), angor pectoris, stroke, myocardial infarction or cardia cerebrovascular diseases of patients. According to the oral enteric preparation, adverse reactions such as functional gastrointestinal disorders, nausea, vomit, gastritis, concealed hemorrhage, ulcer exacerbation and gastrointestinal bleeding caused by strong stimulus of aspirin to stomach can be avoided.
Owner:王定豪
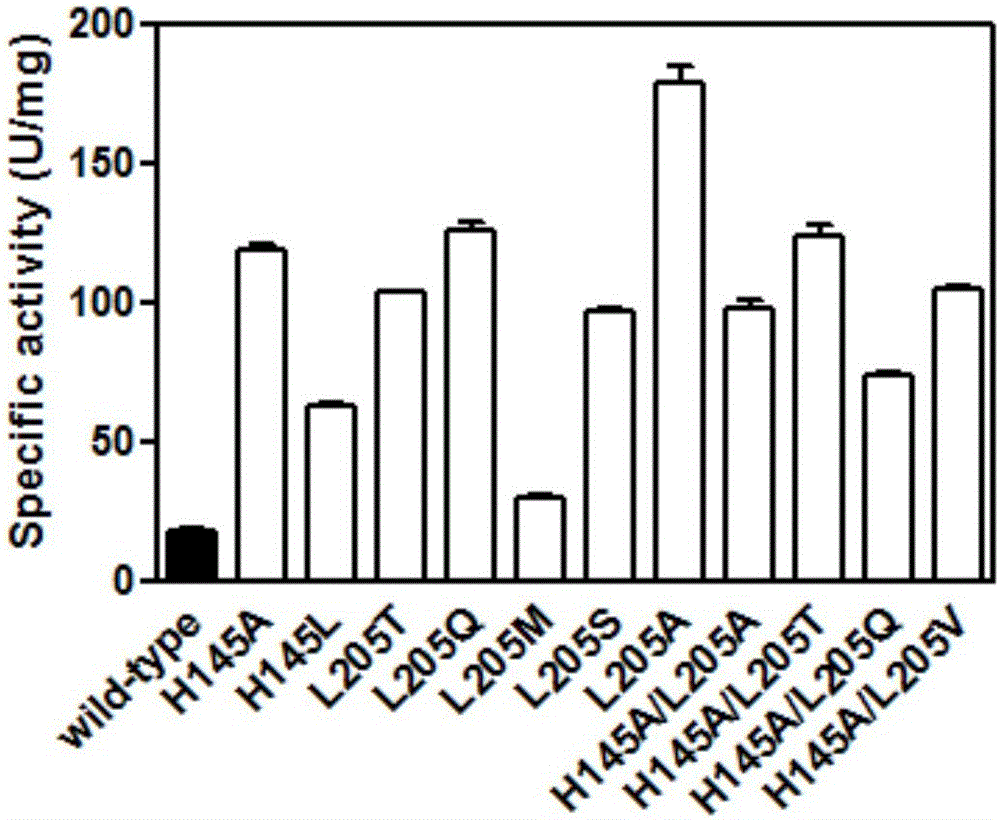
Carbonyl reductase ChKRED20 mutant and application thereof
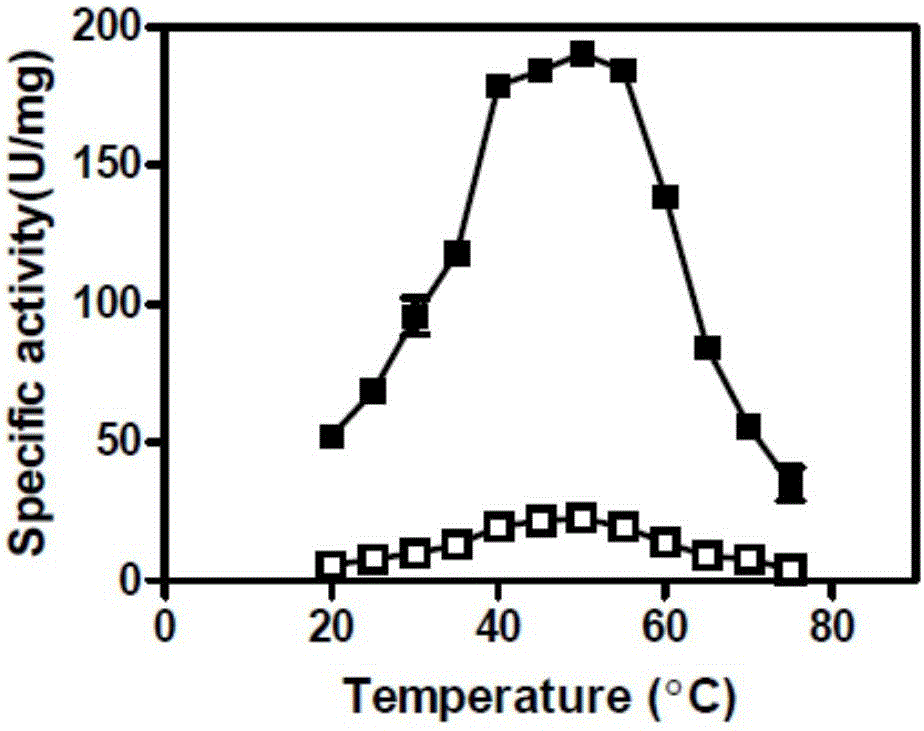
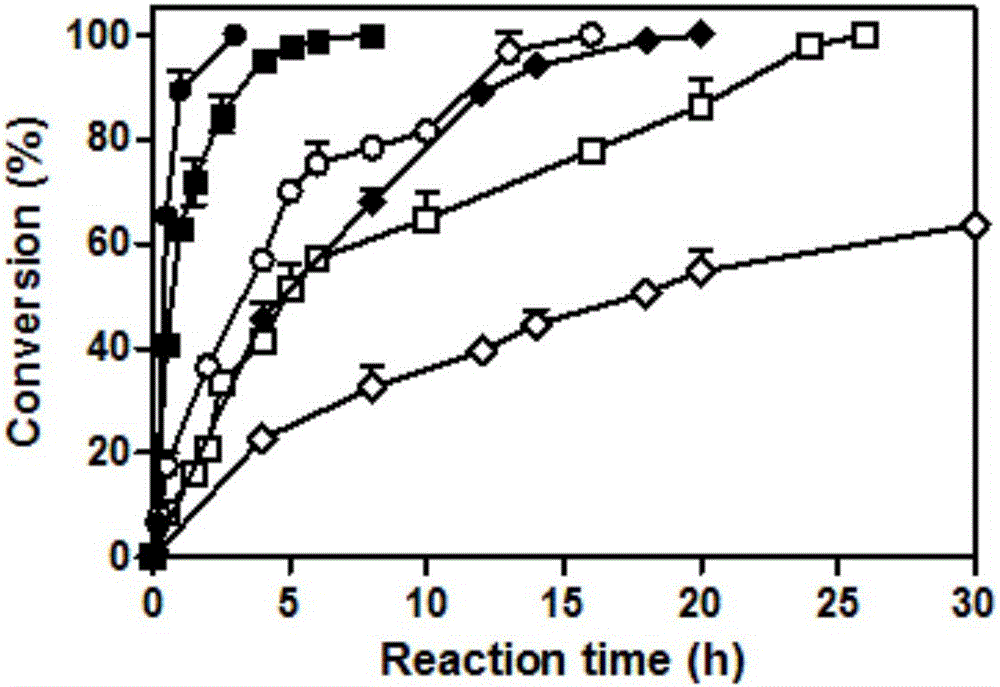
ActiveCN106047828AQuick responseHigh space-time efficiencyOxidoreductasesFermentationTicagrelorGene engineering
The invention belongs to the technical field of gene engineering and enzyme engineering, and particularly relates to a carbonyl reductase mutant and application thereof. The carbonyl reductase ChKRED20 can realize asymmetric reduction of 2-chloro-1-(3,4-difluoro-phenyl)-ethanone to obtain a Ticagrelor intermediate (S)-2-chloro-1-(3,4-difluorophenyl)ethanol (the e. e. value is larger than 99%), and the error-prone PCR technology and the single-point saturation mutagenesis technology are utilized to realize orthogenesis of enzyme molecules to obtain 11 mutants of which the enzyme activities are improved by 1.6-10 times, so that the application potential in biocatalysis is shown.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
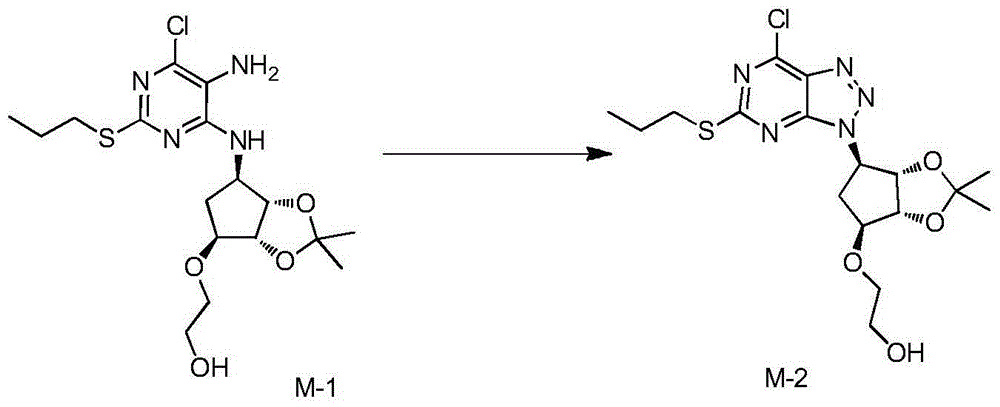
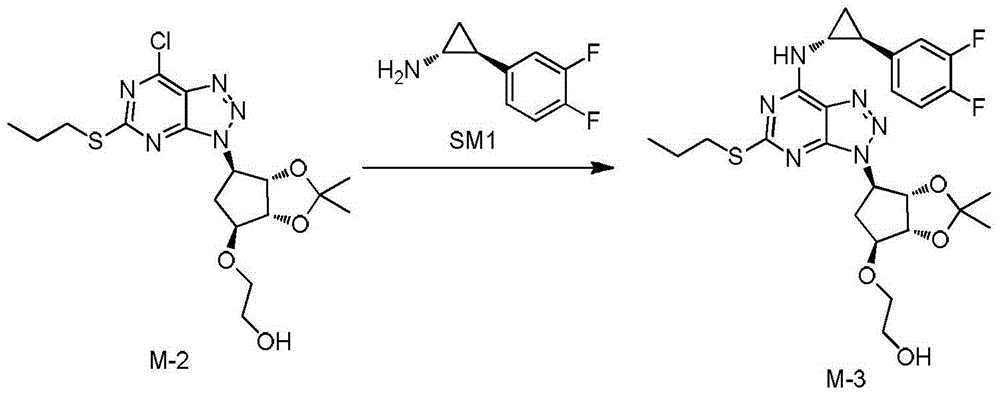
Preparation method of Ticagrelor
A preparation method of Ticagrelor comprises the following steps: (1) dissolving a compound shown in formula M-1 into methylbenzene and acetic acid, dropwise adding an aqueous solution of sodium nitrite, and keeping the temperature at 30DEG C or below to obtain a compound shown in formula M-2; (2) performing acetonitrile beating treatment on a compound shown in formula SM1, adding the treated compound shown in formula SM1 into an aqueous solution of potassium carbonate, under the protection of inert gas, adding the aqueous solution of potassium carbonate into the compound shown in formula M-2, which is obtained in step (1), and performing a condensation reaction to obtain a compound shown in formula M-3; (3) under the protection of inert gas, hydrolyzing the compound shown in formula M-3, which is obtained in step (2), into a mixed solution of concentrated hydrochloric acid and methyl alcohol to obtain coarse Ticagrelor shown in formula TGRL; and (4) recrystallizing the coarse Ticagrelor obtained in step (3) with ethyl acetate-isooctane to obtain high-purity Ticagrelor. Ticagrelor prepared according to the preparation method provided by the invention is up to the purity above 99.50% and contains 0.1wt% of TGRL-A to TGRL-D and less than 0.5wt% of total impurities.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Preparation method of ticagrelor
The invention discloses a preparation method of ticagrelor. The preparation method comprises the following steps of: carrying out cyclization reaction between 5-amido-1,4-bis-substituent group-1,2,3-triazole (II) and a sulfur-containing cyclizing agent (III) to obtain 9-substitution-2-sulfo-6-oxo-8-azapurine (IV); carrying out substitution reaction between an intermediate (IV) and halogenated propane (V) to generate 9-substitution-2-propyl sulfydryl-6-oxo-8-azapurine (VI); chloridizing an intermediate (VI), and carrying out amination reaction between the intermediate (VI) and trans-(1R, 2S)-2-(3,4-difluorophenyl) rolicyprine (VII) to generate 9-substitution-6-amido substituendum-2-propyl sulfydryl-8-azapurine (VIII); and removing the propylidene acetonyl out of an intermediate (VIII) to obtain ticagrelor (I). The preparation method has the advantages of simple process and high chemical and chiral purity and provides a new preparation way for the industrialization of the ticagrelor.
Owner:鄄城县人民医院
Ticagrelor impurity preparation method
PendingCN109553622AShort synthetic routeSimple and fast operationOrganic chemistryCouplingTicagrelor
The invention discloses a ticagrelor impurity preparation method, wherein 4,6-dichloro-2-(propylthio)-5-aminopyrimidine and (1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine(R)-mandelate are used as starting raw materials, and are subjected to a nucleophilic substitution reaction under alkaline conditions, a diazotization ring-closing reaction is performed to prepare a triazole intermediate, the triazole intermediate and 2-(((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH- cyclopentyl[d][1,3]dioxa-4-yl)oxy)ethanol L-(+)-tartrate are subjected to C-N coupling to obtain a ticagrelor impurity E,and the impurity E is hydrolyzed under acidic conditions to remove acetonylidene protection so as to obtain an impurity A. According to the present invention, the preparation method has characteristics of short synthesis route, simple operation and high product purity, and the obtained target product can be used as the impurity reference substance for controlling the purity of the ticagrelor raw materials or preparations.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Preparation method for ticagrelor intermediate and mandelate thereof
PendingCN106906249AWide variety of sourcesHigh yieldOrganic compound preparationCarboxylic acid esters preparationTicagrelorWastewater
The invention discloses a synthetic method for an intermediate compound (I) of ticagrelor and a mandelate compound(VI) thereof. Asymmetric reduction is carried out by the enzymic method; the synthetic method has the advantages of simple operation, mild reaction condition, little pollution, high yield of the product, good optical purity, thus the synthetic method is suitable for large-scale production; the energy consumption and the discharge of organic waste-water are greatly reduced, and requirements of large-scale industrial production are met well.
Owner:LIAONING TIANHUA CHEM
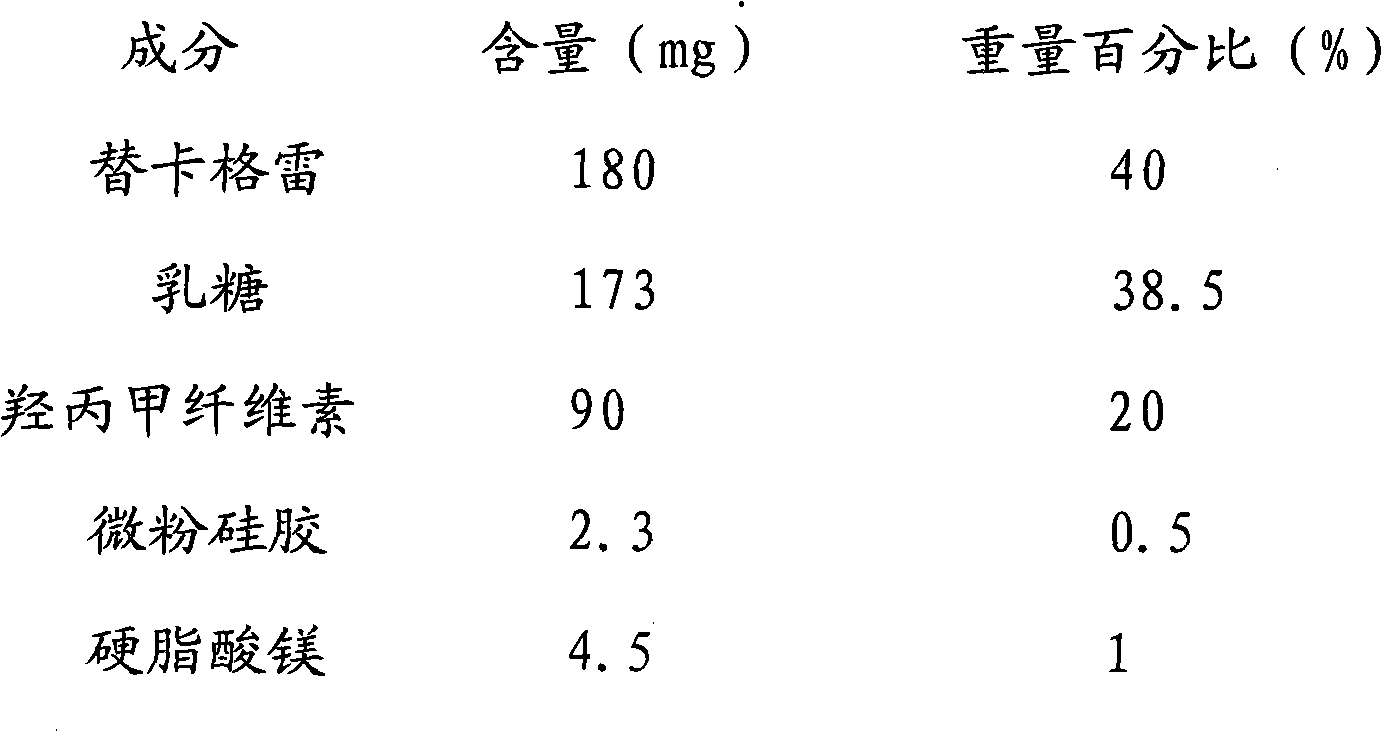
Ticagrelor pharmaceutical composition and preparation method thereof
ActiveCN104414989AStable and controllable qualityGood control effectOrganic active ingredientsBlood disorderTreatment effectTicagrelor
The invention relates to a tablet composition of anticoagulation medicament ticagrelor and a preparation method of the tablet composition. The tablet composition comprises ticagrelor, a filling agent, a disintegrating agent, a lubricating agent and / or an adhesive. The tablet composition is characterized in that a tablet is prepared by virtue of a direct compression method or a dry granulation compression method. The preparation method has the beneficial effects that the operation steps are simple, the controllability of process parameters is good, and the process repeatability is good; meanwhile, the crystal type transform and impurity increase which can be caused in damp and hot processes of ticagrelor are avoided; the prepared ticagrelor tablet has a similar in vitro dissolution behavior with a primarily researched preparation. Therefore, the ticagrelor tablet is stable and controllable in quality and good in treatment effect and safety.
Owner:SICHUAN HAISCO PHARMA CO LTD
5-amino-1,4-disubstituent-1,2,3-triazole and preparation method thereof
ActiveCN103304545AEasy to prepareThe reaction conditions are mild and easy to controlOrganic chemistryTicagrelorCombinatorial chemistry
The invention discloses a 5-amino-1,4-disubstituent-1,2,3-triazole and a preparation method thereof. The compound (I) is a key intermediate for preparing medicines with a 1,2,3-triazole structure, such as ticagrelor. The preparation method for 5-amino-1,4-disubstituent-1,2,3-triazole comprises the following step of: performing cyclization reaction on an azide (II) with a cyano derivative (III) to obtain 5-amino-1,4-disubstituent-1,2,3-triazole. The preparation method is easily-available in raw materials, moderate in conditions, and high in yield.
Owner:鄄城县人民医院
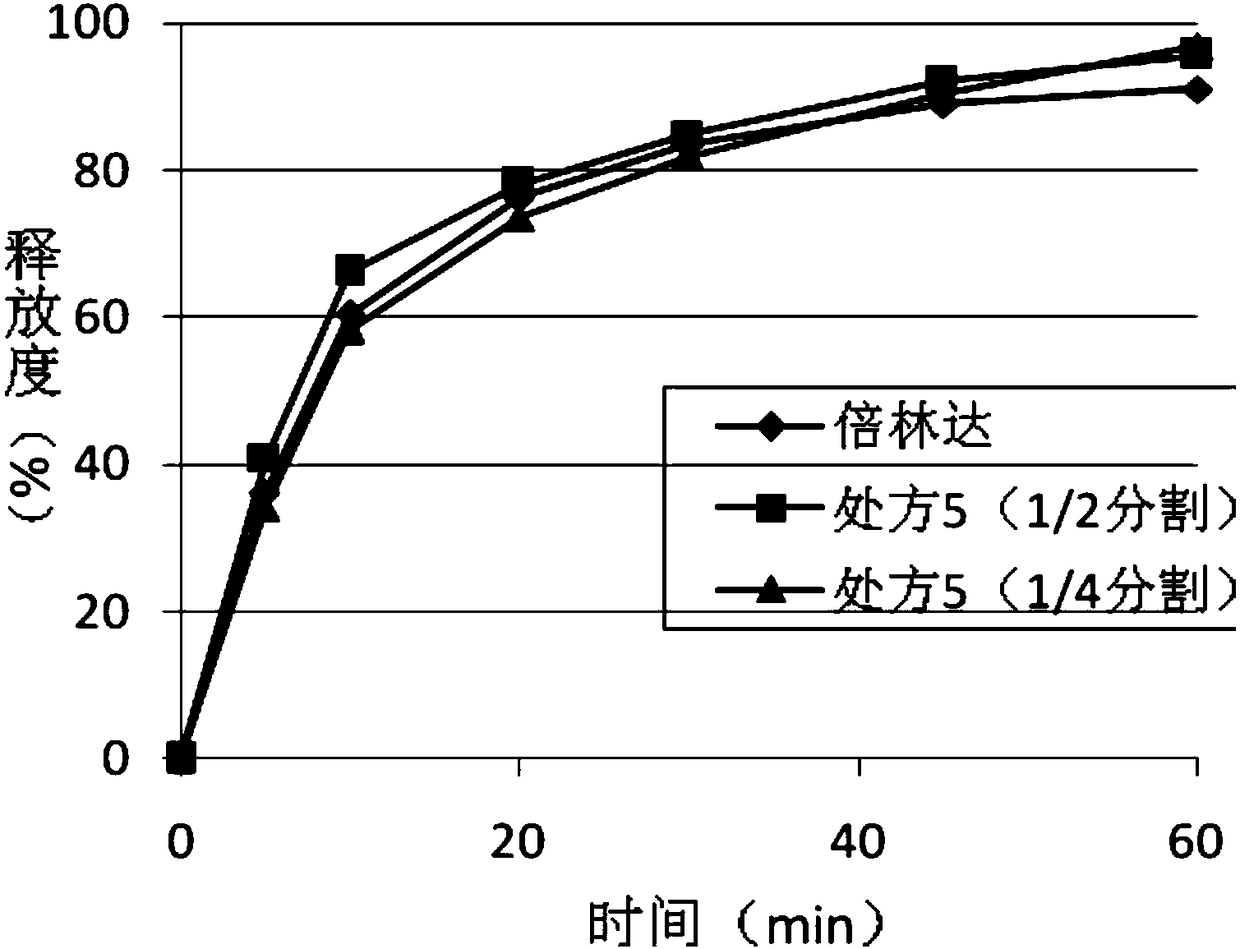
Ticagrelor pharmaceutical composition and preparing method thereof
ActiveCN105998026AImprove complianceQuick effectOrganic active ingredientsInorganic non-active ingredientsPatient complianceTicagrelor
The invention relates to a sustained-release preparation composition of an anticoagulant drug ticagrelor (also called brilinta) and a preparing method thereof. The composition comprises ticagrelor and other pharmaceutical accessories. The composition is characterized in that the composition can be quick in acting, also can ensure persistent effectivity within 24 hours, and further can reduce Cmax based on guarantee of the effective plasma concentration, thereby improving patient compliance and reducing drug safety problem in the premise without reduction of curative effect.
Owner:SICHUAN HAISCO PHARMA CO LTD
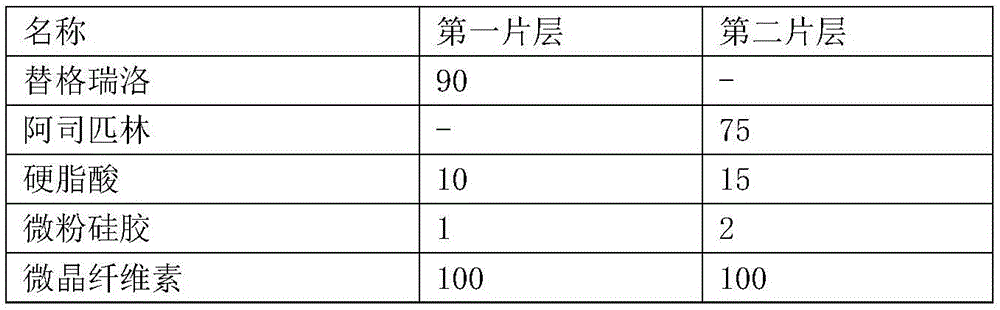
Ticagrelor and aspirin compound tablet and preparation method thereof
ActiveCN106619549AGood anti-adhesionReduce generationOrganic active ingredientsPharmaceutical non-active ingredientsAspirinTicagrelor
The invention provides a ticagrelor and aspirin compound tablet and a preparation method thereof. The ticagrelor and aspirin compound tablet is prepared from an anti-sticking agent formed by stearic acid and aerosil in a mass ratio of (5-30) to (1-3). The ticagrelor and aspirin compound tablet has high stability, is easy to store, is less in adhesion between the tablet and a tableting device in a tableting process, and has a high tableting success rate.
Owner:SUZHOU SIXTH PHARMA PLANT OF JIANGSU WUZHONG PHARMA GROUP
Preparation method of ticagrelor intermediate
InactiveCN103351372AReduce unit operationsAtom economy is highOrganic chemistryBulk chemical productionTicagrelorReaction step
The invention discloses a preparation method of a ticagrelor intermediate. The preparation method comprises the following steps: (1) reacting a compound in formula (8) with a compound in formula (9), so as to obtain a compound in formula (10); (2) carrying out ester group reduction on the compound in formula (10), so as to obtain a compound in formula (11); (3) desorbing benzyl protection of the compound in formula (11), so as to obtain a compound in formula (2). A synthetic method and a path provided by the invention have the advantages of short reaction step, high atom economy and low cost, and are environmentally friendly and suitable for industrial production. General formulas are as follows (as shown in the specification).
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD +1
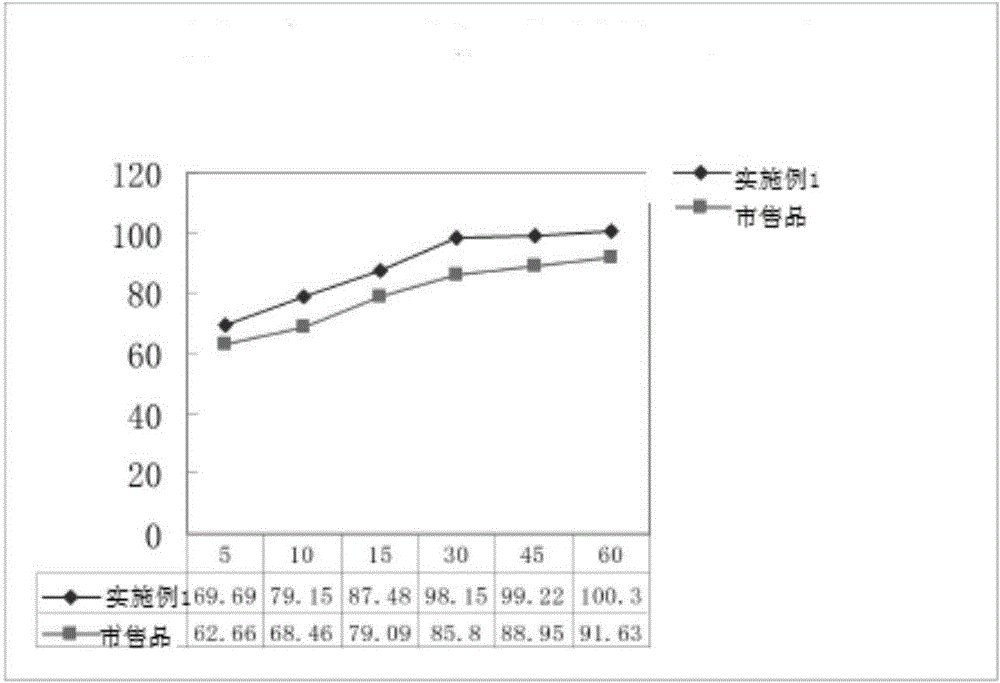
Slow-release preparation of ticagrelor
InactiveCN108210498ATo meet the need for the first loading doseAvoid wastingOrganic active ingredientsPill deliveryOral medicationTicagrelor
The invention relates to a slow-release preparation of ticagrelor. Specifically, the slow-release preparation of the ticagrelor, provided by the invention, is suitable for being orally taken one timeevery day; meanwhile, a rapid drug releasing effect can be realized after tablets are damaged. The releasing tablets which are administrated one time every day can reduce the number of times of oral administration, so that the oral drug taking compliance of patients can be improved and risks that myocardial infarction or stroke is caused when acute thrombus formation is caused by the fact that thepatients forget to orally take the ticagrelor are reduced; meanwhile, when the tablets provided by the invention are orally taken for the first time, the tablets can be opened to meet the requirementof giving a load dosage to the patients, so as to rapidly take effect.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com