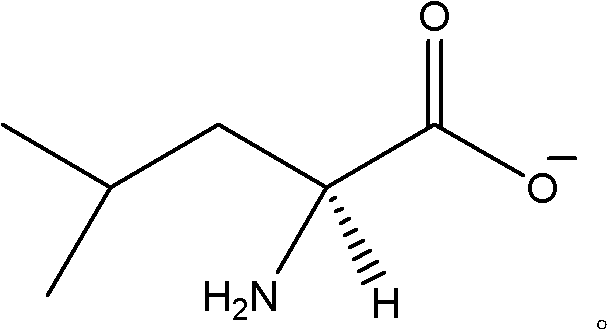
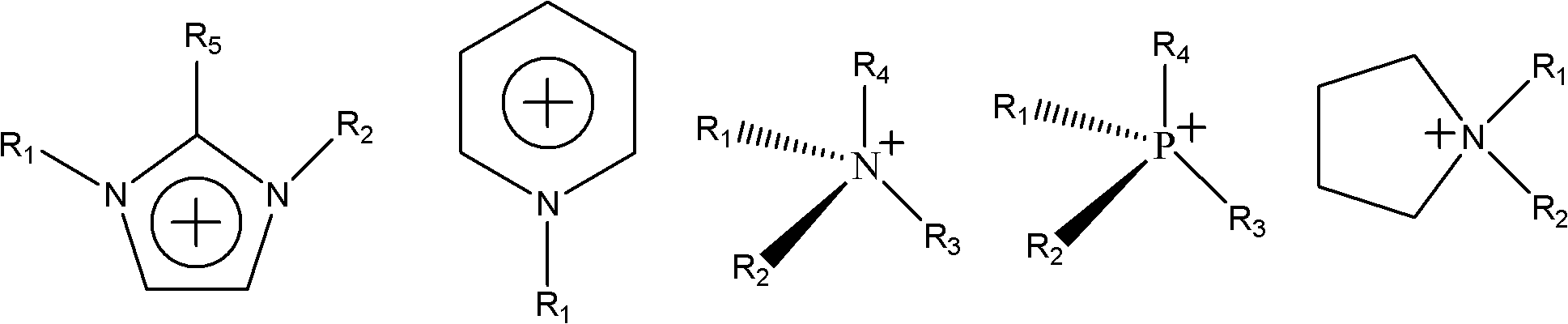
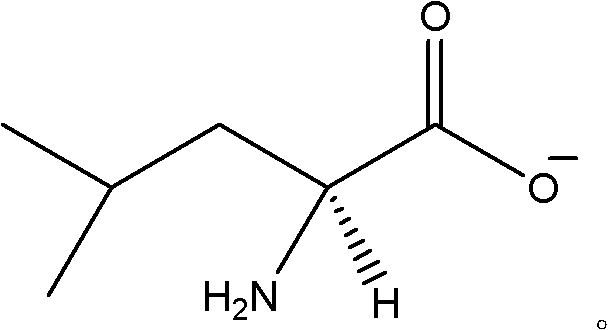
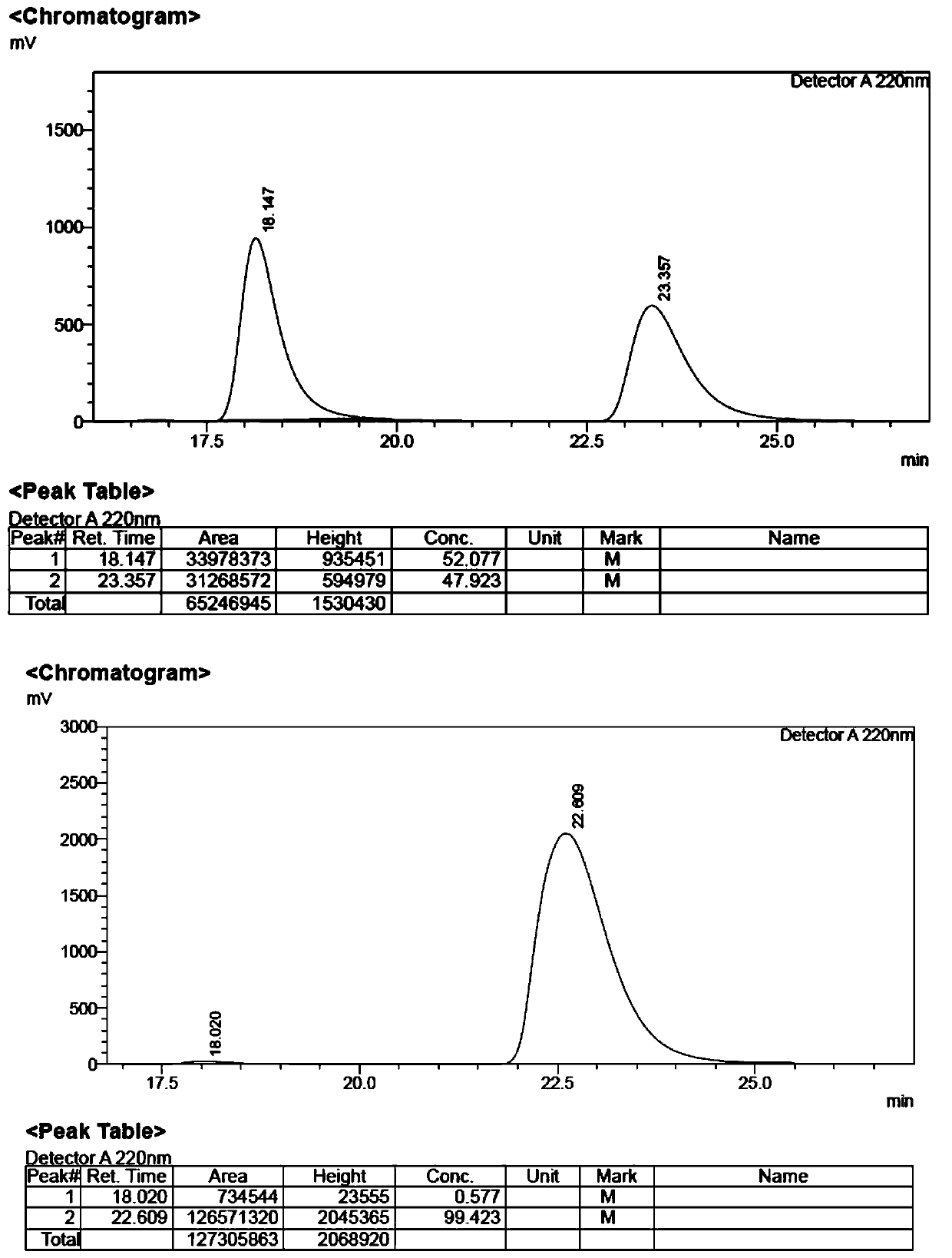
Patents
Literature
133 results about "Chiral induction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
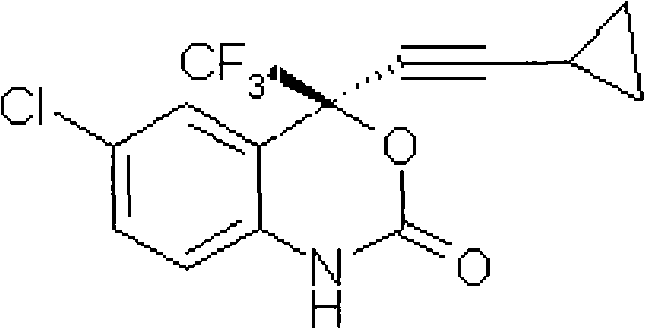
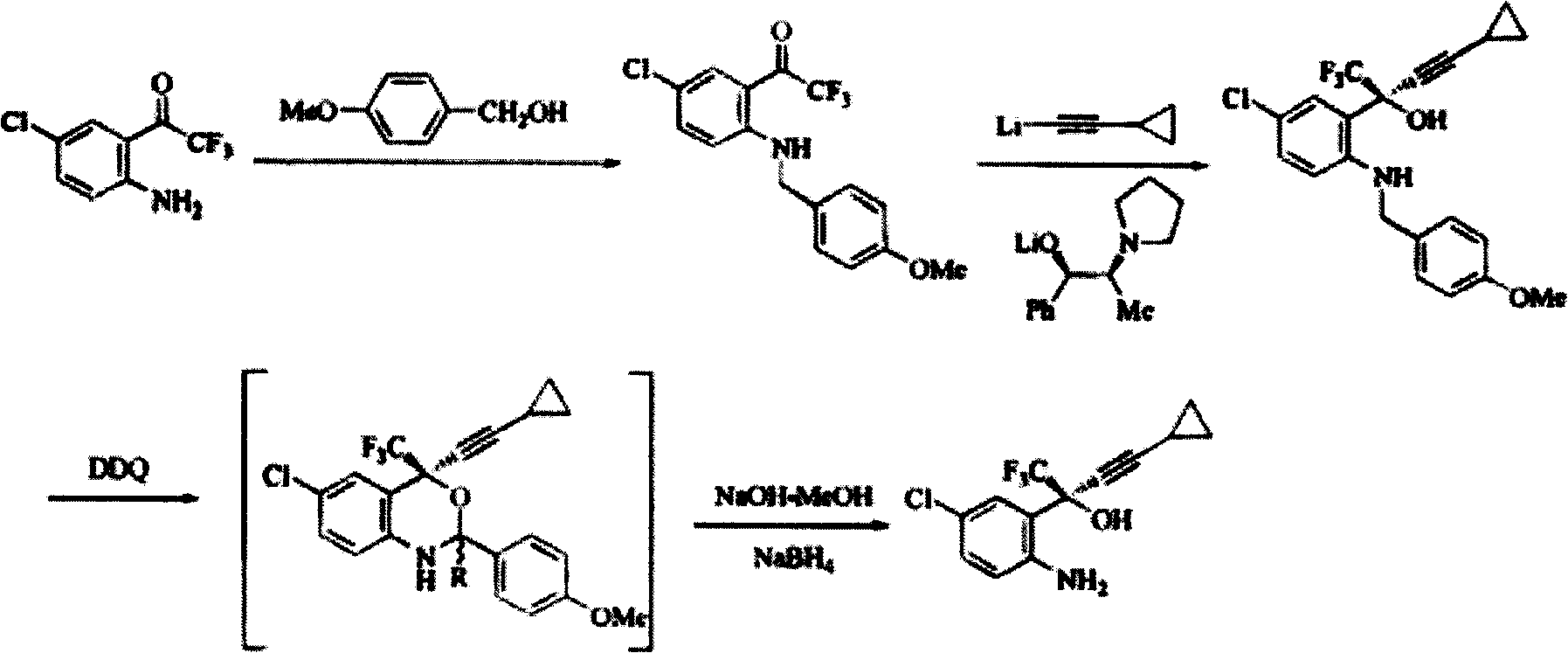
Method for preparing chiral cylopropyl acetenyl tertiary alcohol compound
InactiveCN101786959ALow costSuitable for industrial productionOrganic compound preparationAmino-hyroxy compound preparationTrifluorideButyl lithium
The invention discloses a method for preparing a chiral cylopropyl acetenyl tertiary alcohol compound.The steps of the method are as follows: (a) cylopropyl ethylnen or other derivatives react with a chiral induction reagent, a chirality auxiliary reagent and zinc halide in an organic solvent under the action of an alkaline reagent so as to obtain a zinc complex; (b) addition reaction is carried out between the obtained zinc complex and 5-chlorin-2-amino trifluoro-benzophenone; (c) (S)-2-amino-5-chlorin-alpha-cylopropyl ethylnen-alpha-benzyl alcohol trifluoride is collected from reaction liquid. Compared with butyl lithium and diethylzinc, the alkaline reagent adopted in the invention is safer; in addition, the cheap zinc halide is used as a ligand, which greatly reduces the cost. Therefore, the method is suitable for industrial production.
Owner:SHANGHAI ACEBRIGHT PHARMA GRP +1
Chiral ionic liquid and its preparing method
InactiveCN1749249ALarge structural diversity and designabilitySimple processGroup 5/15 element organic compoundsHalogenIon
The present invention is chiral ionic liquid and its preparation process, and features that inside solvent capable of dissolving ionic liquid and incapable of dissolving haloid generated in the double decomposition reaction, chiral lactate and ionic liquid with halogen ion as anion react to obtain chiral ionic liquid with structural general expression [C]+[A]- and comprising cation [C]+ and chiral lactate radical anion [A]-. Compared with available technology, the present invention has the advantages of simple preparation process and easy realization, and the prepared chiral ionic liquid has the advantages of ionic liquid being non-volatile, non-toxic, wide liquid state temperature range, etc. and the advantages of chiral matter with high selectivity, chrial induction effect, etc. and is expected to be used as chiral catalyst, chiral solvent and other green chemical material.
Owner:ZHEJIANG UNIV
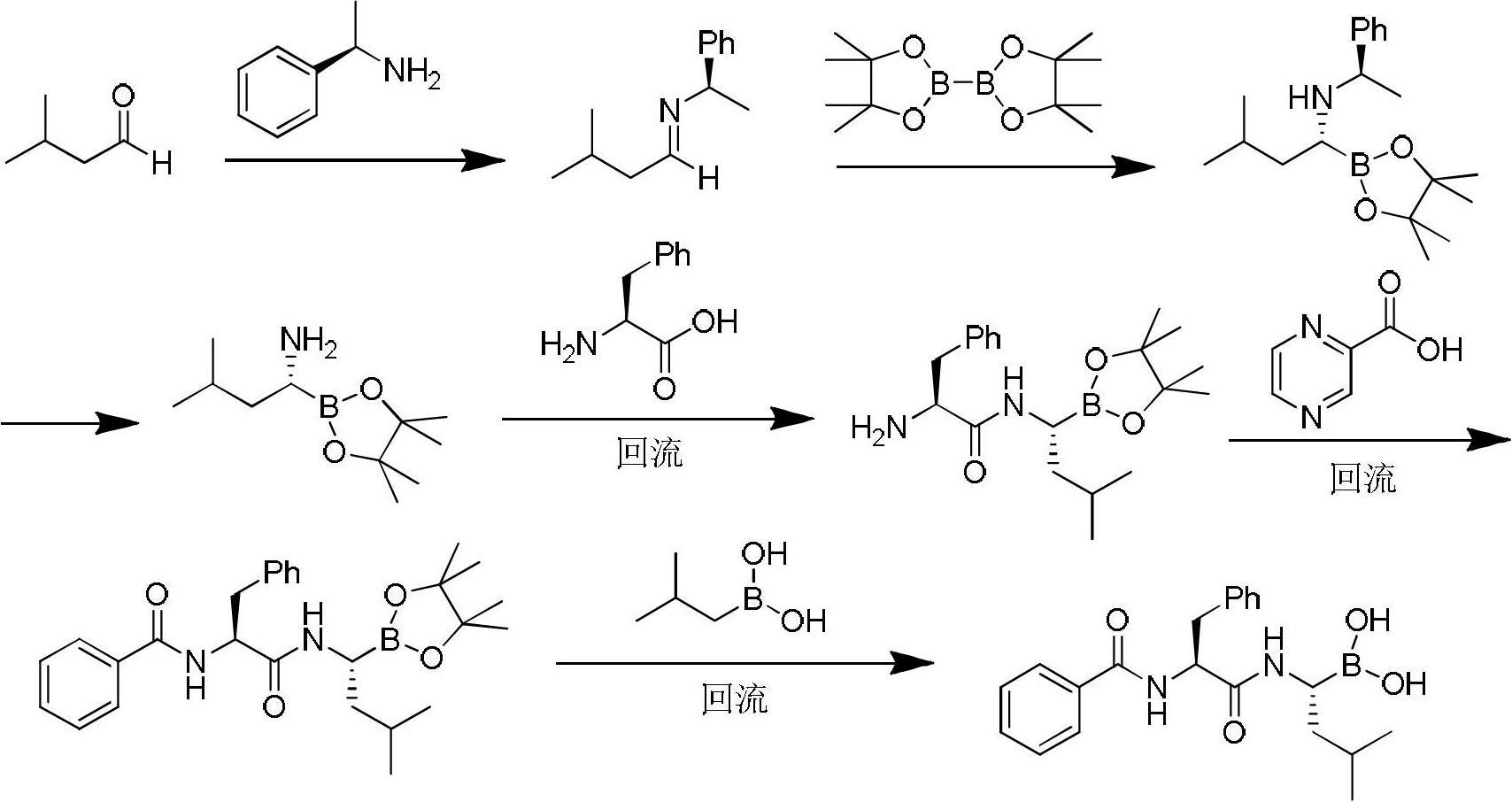
Synthetic method of bortezomib
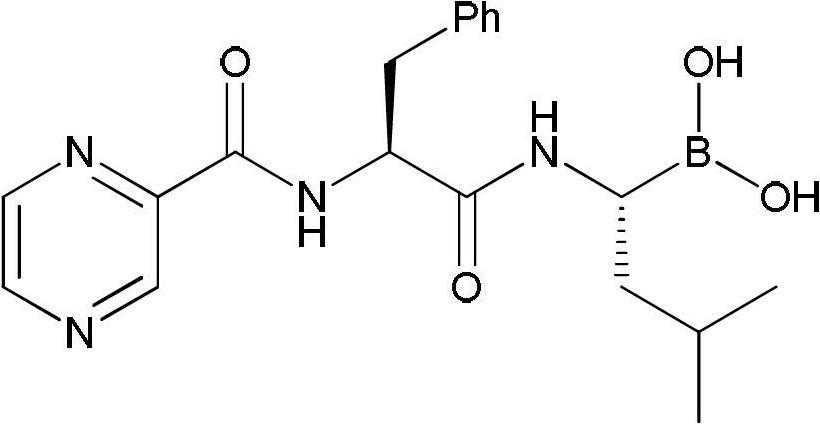
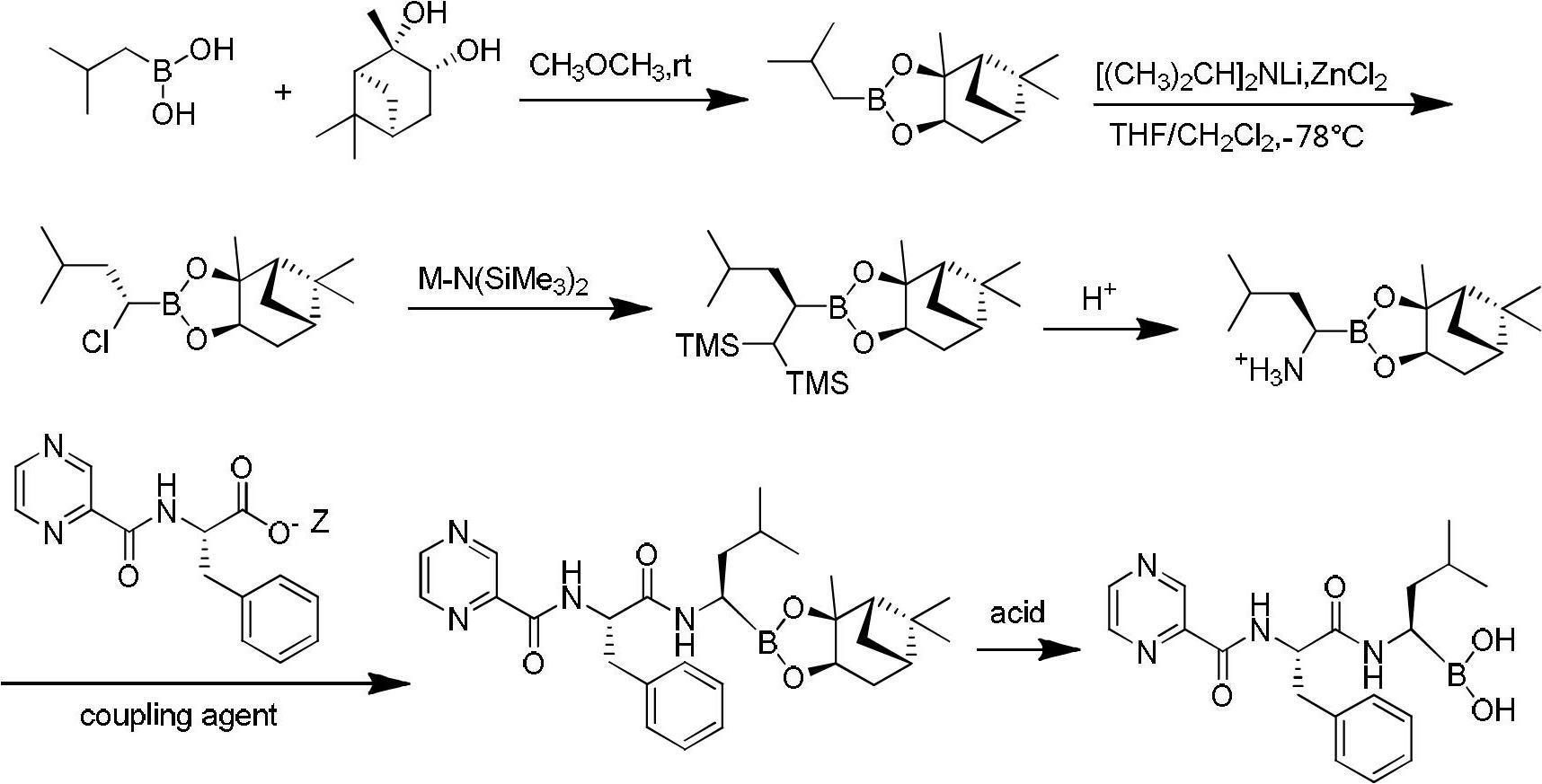
The invention discloses a synthetic method of bortezomib, which comprises the following steps of: taking isovaleraldehyde as an initial raw material, taking (R)-methylpropane-2-sulfinamide as a chiral reagent, generating (R,E)-2-methyl-N-(3-methyl butylidene) propane-2-sulfinamide by a condensation and dehydration reaction, then carrying out a nucleophilic addition reaction with pinacol diboron so as to generate (R)-1-N-methylpropane sulfinyl-3-methyl butane-1-pinacol borate ester, afterwards hydrolyzing under an acidic condition so as to obtain pinacol-(R)-1-amino-3-methyl butane-1-borate ester hydrochloride, then reacting with (S)-3-phenyl-2-(pyrazine-2-formamido) propionic acid under the existence of a coupling agent and also hydrolyzing under the action of isobutyl borate so as to generate a final product of the bortezomib. According to the synthetic method of the bortezomib, the (R)-methylpropane-2-sulfinamide which is easy to obtain is used as the chiral induction reagent, so that an obtained intermediate enantiomorph has higher purity, and a bulk drug which is finally obtained has better quality.
Owner:HEFEI UNIV OF TECH
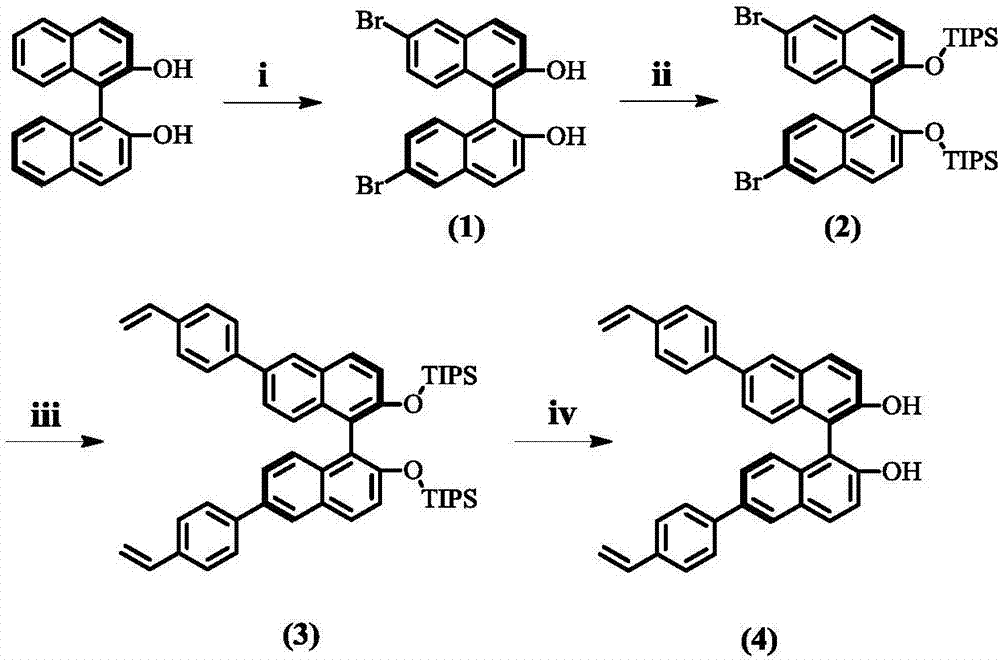
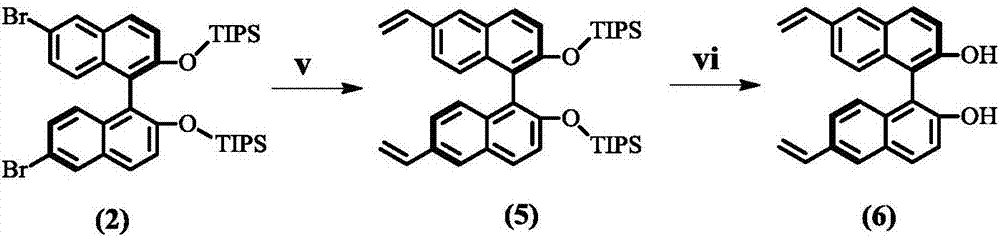
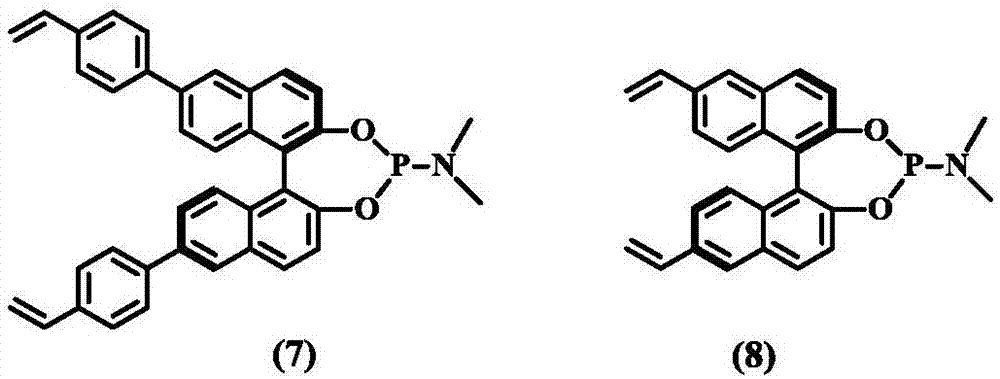
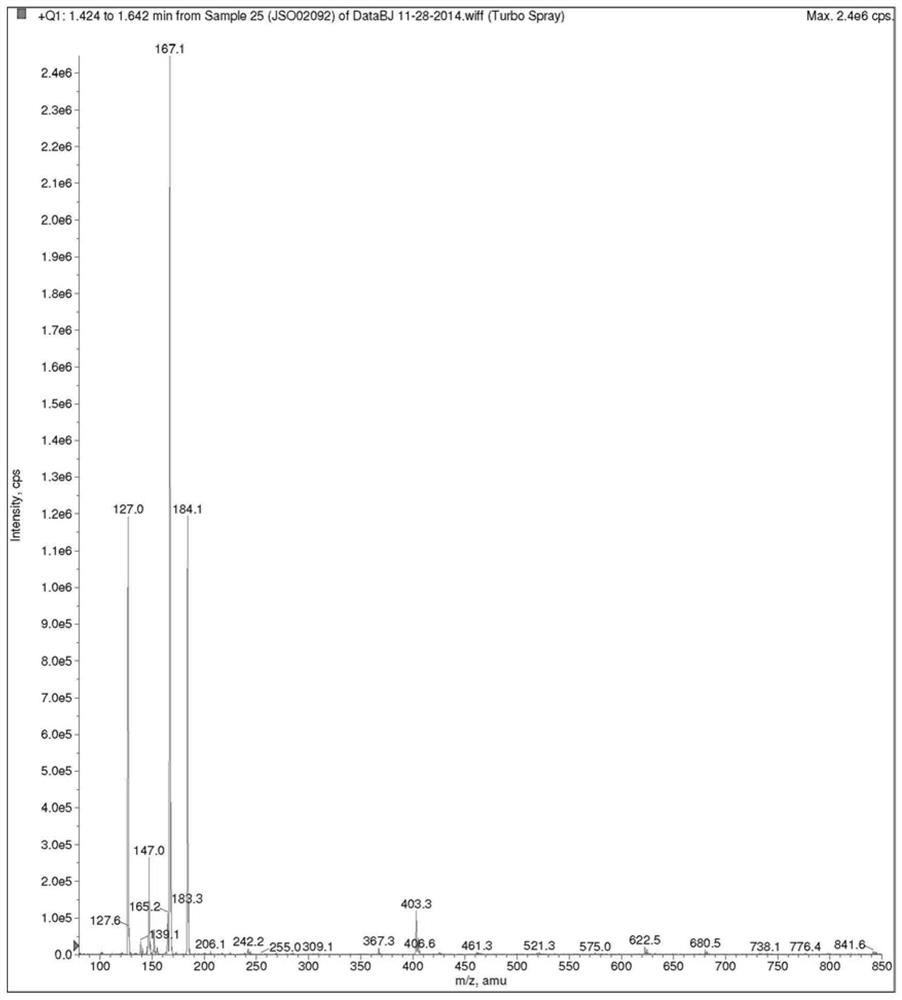
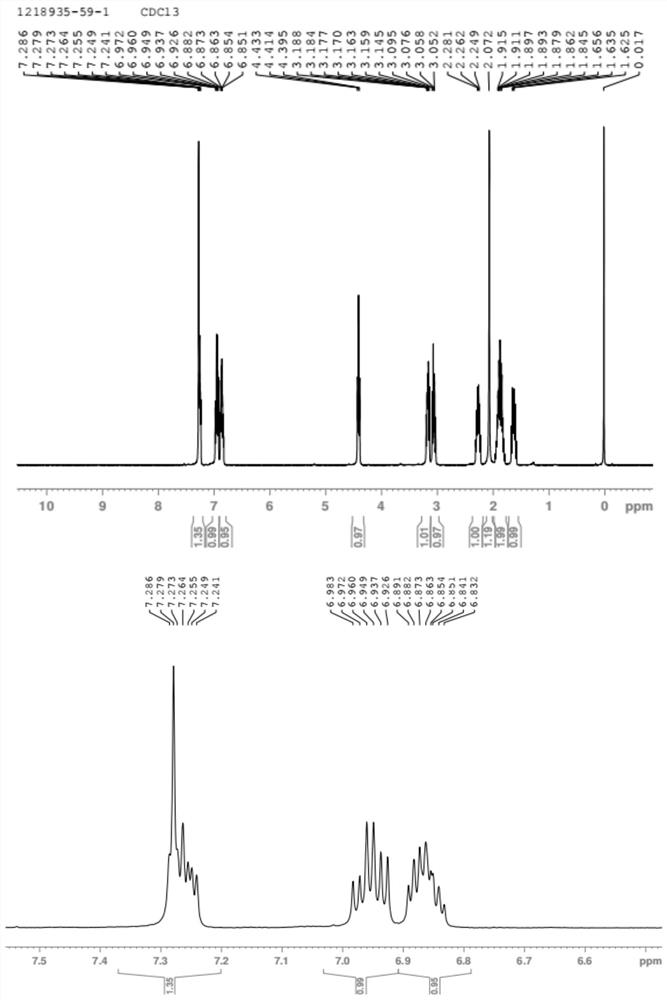
Preparation method of (S, S)-8H-6H-pyrrolo [3, 4-b] pyridine
ActiveCN103030638ASimple methodHigh yieldOrganic chemistryBulk chemical productionHigh pressureBenzylamine
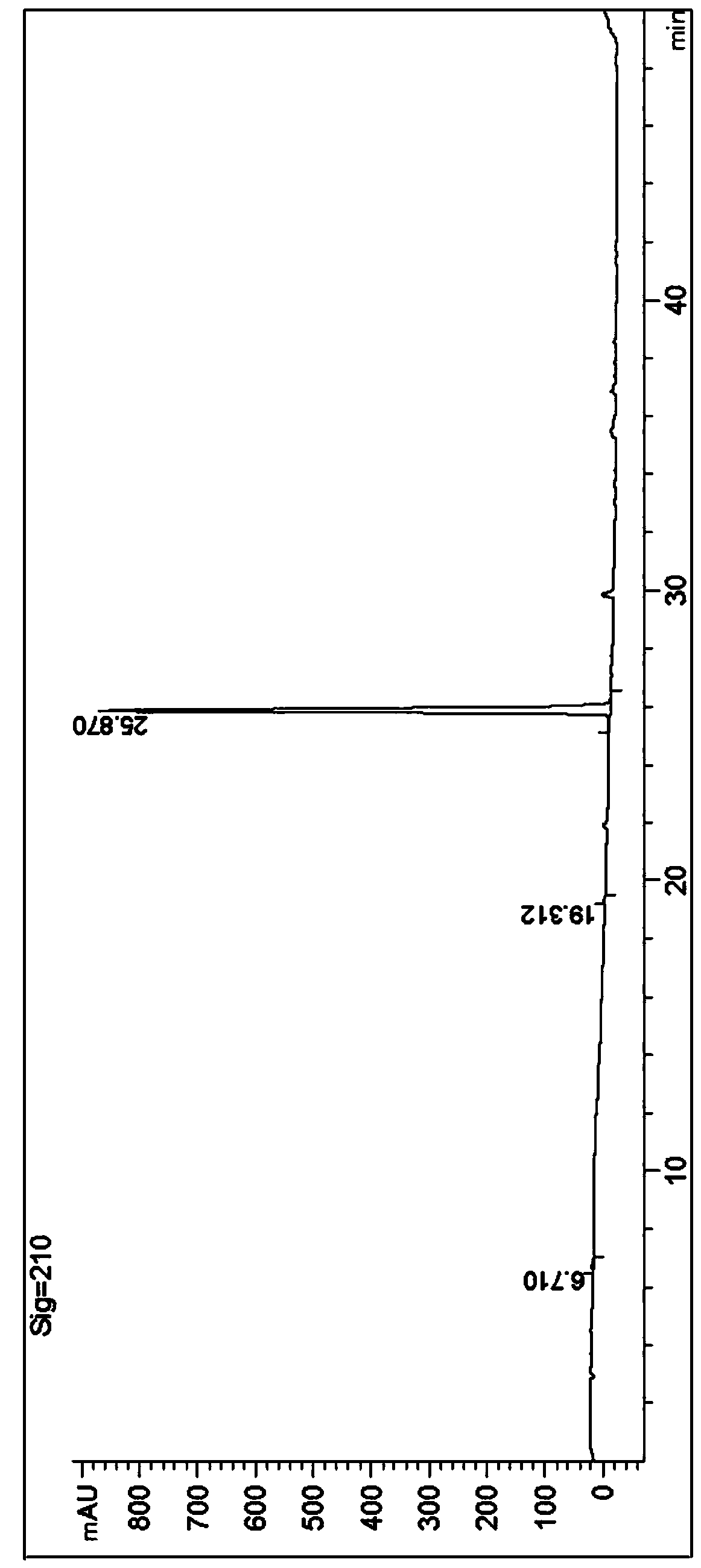
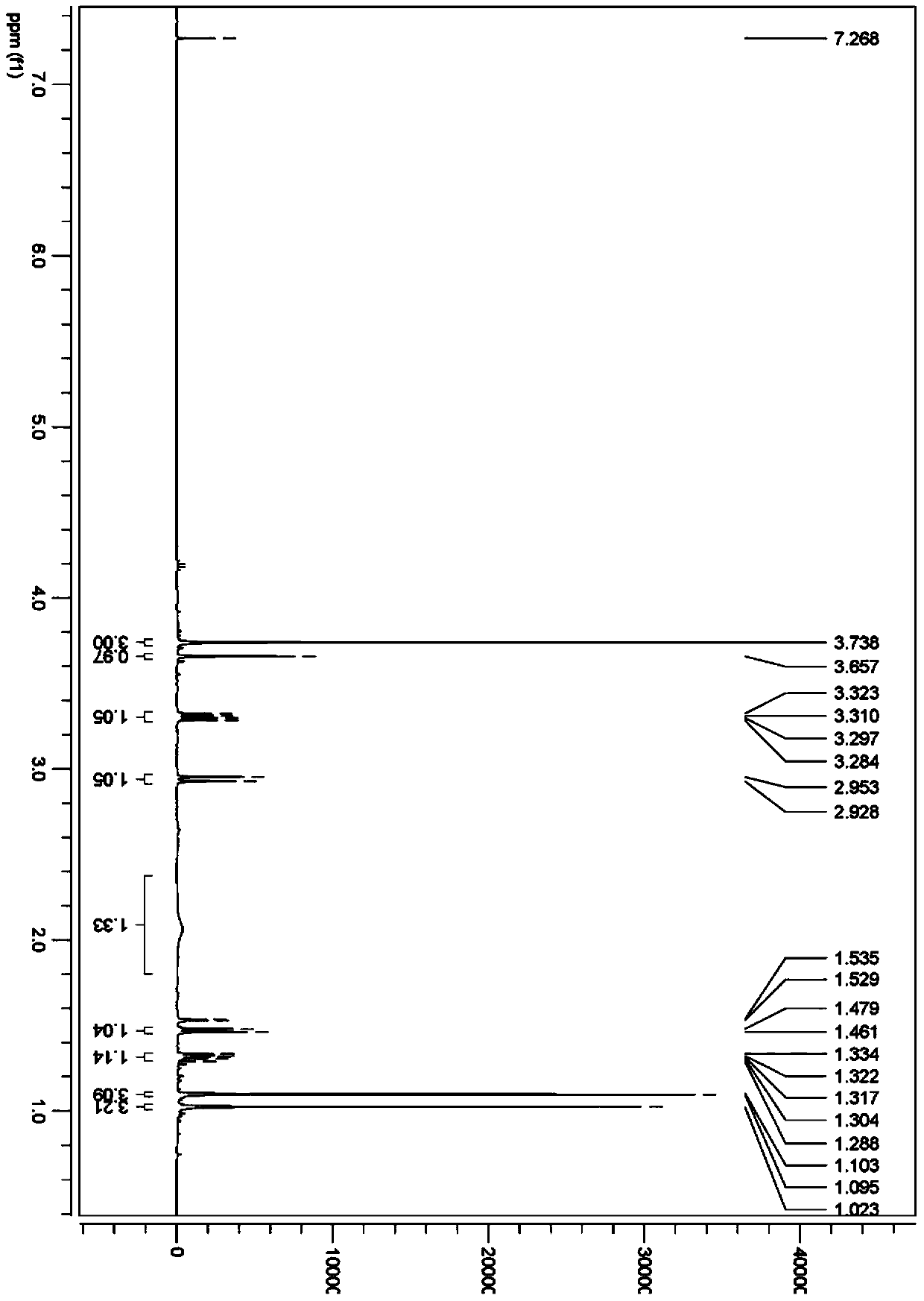
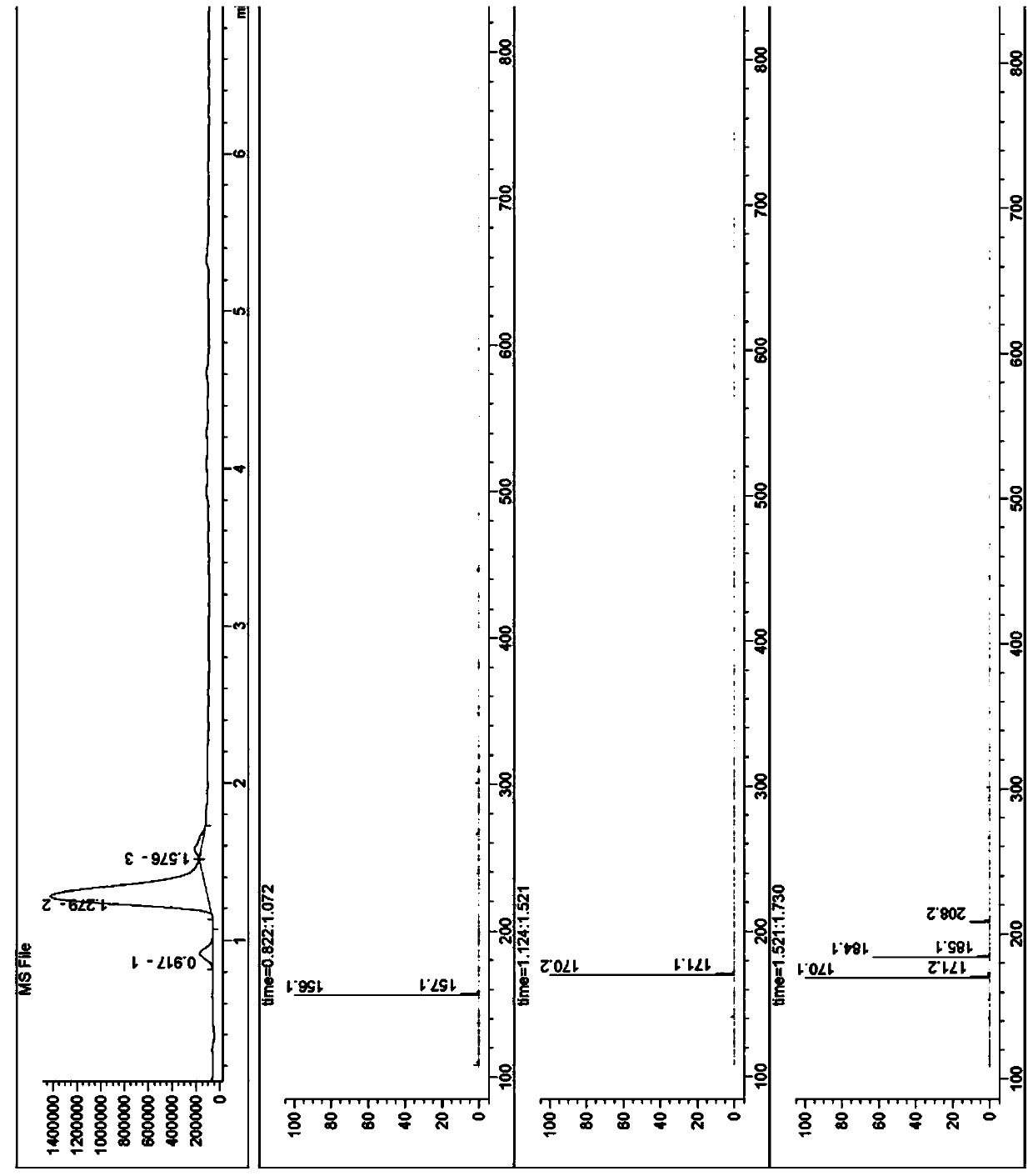
The invention discloses a method for enantioselectively synthetizing (S, S)-8H-6H-pyrrolo [3,4-b] pyridine by using (R)-2-phenylglycinol as a chiral inducing reagent. The method comprises the following steps: by taking (R)-2-phenylglycinol as a raw material, carrying out one-step Michael addition reaction and ester exchange reaction to synthetize a intermediate 2; carrying out condensation on the intermediate 2 and acryloyl chloride to obtain a double-ring intermediate 3; carrying out hydrogenation reduction to obtain an intermediate 4 in high stereoselectivity; reacting the intermediate 4 with benzylamine through one-step amine-ester exchange to obtain an intermediate 5; hydrolyzing the intermediate 5 to obtain an intermediate 6; and carrying out ring closing reaction, reducing, removing protecting group on nitrogen to obtain a final product II. The method has the characteristics of favorable stereoselectivity and high yield, and is simple to operate; compared with the traditional method, for no need of hydrogenating and reducing pyridine ring under high pressure, no need of resolution and the like, the preparation method disclosed by the invention is low in cost, safe and reliable, the yield can achieve 20%-30%; and the method is suitable for industrial production.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Synthetic method of boceprevir intermediate
ActiveCN103435532ARaw materials are cheap and easy to getReduce energy consumptionOrganic chemistryBulk chemical productionReaction conditions1-Naphthylamine
The invention relates to a synthetic method of a boceprevir intermediate, namely (1R, 2S, 5S)-6, 6-dimethyl-3-aza-bicyclo-[3. 1. 0] hexane-2-carboxylic acid methyl ester hydrochloride, belonging to the technical field of drug synthesis. The synthetic method solves the problems of high cost, complex reaction, low yield, and the like of the synthesis of the boceprevir intermediate in the prior art. The synthetic method comprises the following steps of carrying out amino protection on 6, 6-dimethyl-3-aza-bicyclo-[3. 1. 0] hexane hydrochloride which is taken as an original raw material; then reacting 6, 6-dimethyl-3-aza-bicyclo-[3. 1. 0] hexane hydrochloride with 1, 2, 3, 4-tetralin-1-naphthylamine for 3-4 hours at 30-35 DEG C under the action of a hydrogen drawing reagent by taking 4, 4'-difluoro benzophenone as a chiral inductive agent; finally removing an amino protecting group, and adding acid to form salt to directly obtain a final product. The synthetic method disclosed by the invention has the advantages of low cost, simple reaction condition, few reaction steps, short time and high purity and yield of the final product, namely the boceprevir intermediate.
Owner:SUZHOU UUGENE BIOPHARMA
Chiral quaternary phosphonium salt phase transfer catalyst, preparation method and application thereof
ActiveCN109718851ASimple structureStrong controllabilityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsSolubilityPhosphonium salt
The invention provides a chiral quaternary phosphonium salt phase transfer catalyst, a preparation method and application thereof. The catalyst is prepared from natural amino acid as the starting rawmaterial, has wide raw material sources, and is simple in synthesis. The catalyst has a plurality of modification sites, structural diversity and strong controllability, also has multiple hydrogen bond donor sites, has strong chiral induction catalytic ability, at the same time has stability to air and water, is easy to store, has good water solubility, is environment-friendly, and has strong practicability and industrial application value.
Owner:四川六泰科技有限公司
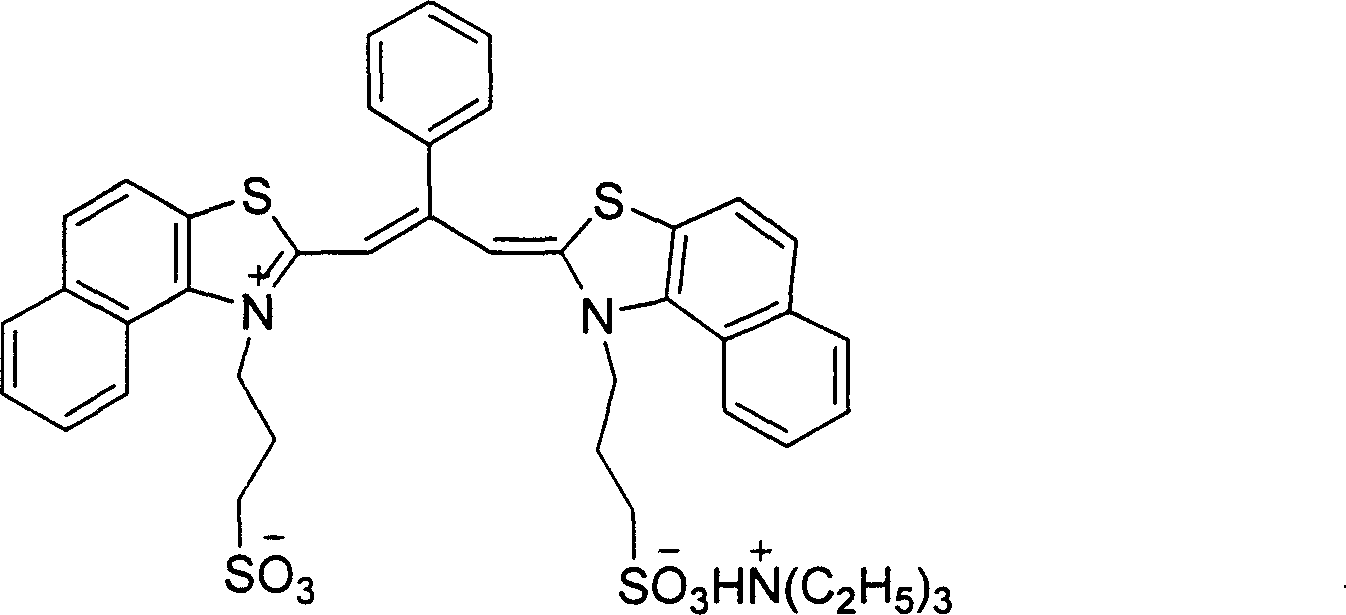
Method for conducting chiral induction to non-chiral cyanine dye aggregate using human serum protein
InactiveCN101024730AControl chiral inversionChirality increaseMethine/polymethine dyesSerum albuminCyaninePhosphoric acid
The invention relates to a method to take chiral induction to non-chiral cyanine dye aggregate by using human albumin that includes the following steps: dissolving non-chiral cyanine dye and human albumin aggregate into phosphoric acid to form 80um and 50um solution, mixing the solution to make the mol ratio to 0.5-5:1; diluting the phosphoric acid solution to 5um, taking non-light reaction for 9-15 hours to gain cyanine dye aggregate, taking analysis by ultraviolet visible absorption spectrum and circle dichromatism spectral, the aggregate has chirality and chirality reversing. The invention could adjust the optical rotation of dye aggregate solution.
Owner:INST OF CHEM CHINESE ACAD OF SCI
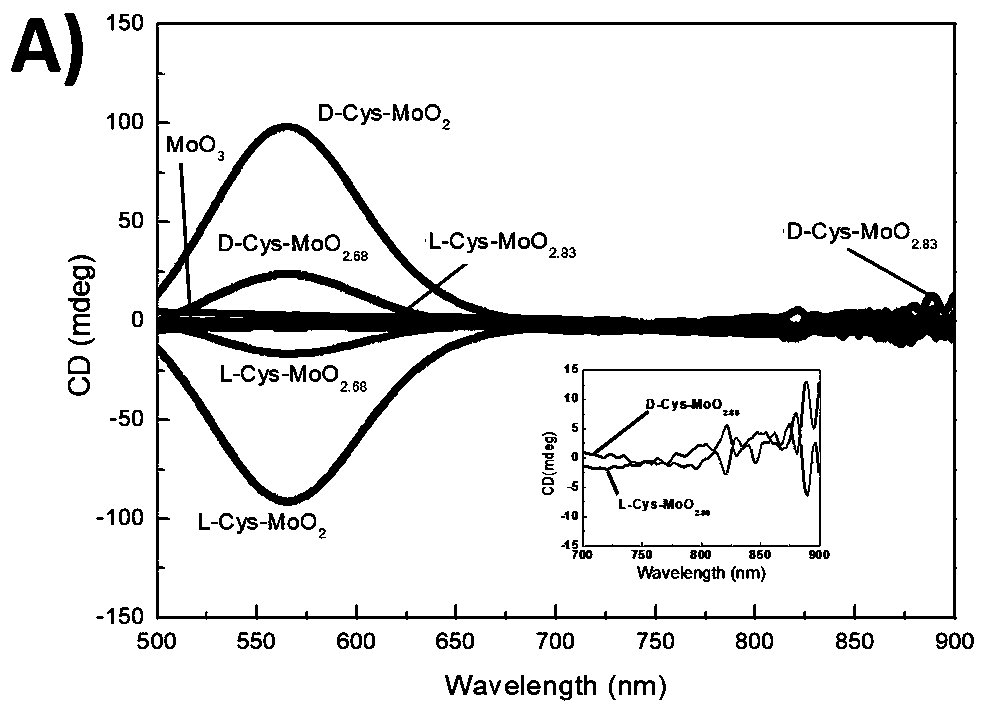
Preparation method of chiral light-controlled oxygen-deletion molybdenum oxide nanometer particles and purpose of chiral light-controlled oxygen-deletion molybdenum oxide nanometer particles to photothermal therapy of tumors
ActiveCN110639015AEasy to operateMild conditionsMaterial nanotechnologyEnergy modified materialsMolybdenum trioxideTumor cells
The invention discloses a preparation method and purpose of chiral light-controlled reduction-state molybdenum oxide nanometer particles having the purpose of targeting optothermal therapy of tumor cells. On the basis of molybdenum trioxide as mother liquid, a chiral enhanced double-channel oxygen-deletion molybdenum oxide nanometer material is innovatively obtained through a chiral induction effect; and through comparing variables of dosage of organic molecules, a combination manner and the like, an optothermal therapy reagent based on a chiral molybdenum oxide nanometer material is developed. The operating method is simple and convenient, and raw materials are cheap and easy to obtain. A chiral semiconductor nanometer material has highly chiral absorption on circular polarization visiblelight laser and near-infrared laser, and can generate high temperature to reach the optothermal therapy effects. A thinking is widened for the application of chiral nanometer materials to the optothermal therapy of the tumors, and the optothermal therapy cost of the cancer can be reduced.
Owner:中国医科大学
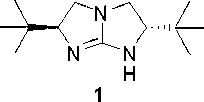
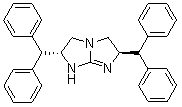
Chiral five-membered bicyclic guanidine compound, preparation method and application thereof
ActiveCN102718768ANovel structureGood catalytic performance for asymmetric reactionsOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsSide chainCombinatorial chemistry
The invention discloses a chiral five-membered bicyclic guanidine compound, a preparation method and an application thereof. The chiral five-membered bicyclic guanidine compound is shown as Formula (I). The preparation method can be obtained by referring to existing synthetic routes of the chiral five-membered bicyclic guanidine compound and performing proper changes. The two side chains of the chiral five-membered bicyclic guanidine compound are diphenylmethyl, so that a larger chiral space is formed. Moreover, phenyl may form Pi-Pi overlap with a substrate containing conjugated groups so as to improve the effects of chiral induction. Therefore, more substrates as catalysts can be applied to the compound of Formula (I), and the chiral five-membered bicyclic guanidine compound of the invention is better than the existing chiral five-membered bicyclic guanidine compound as for catalytic performance.
Owner:RAFFLES PHAMRMATECH CO LTD
Chiral poly (3,4-ethylenedioxythiophene) derivative monomer, polymer and preparation method thereof
InactiveCN102391284AWide range of usesImprove performanceElectrolysis componentsOrganic chemistryChromatographic separationElectrolysis
The invention discloses a chiral poly (3,4-ethylenedioxythiophene) derivative monomer, a polymer and a preparation method thereof. The method comprises the following steps of: obtaining the chiral monomer EDOT-Boc-Ala by using 3,4-dibromothiophene as a raw material and adopting six-step reaction, and performing direct deposition to obtain a polymer poly (EDOT-Boc-Ala) film on a platinum electrode by using dichloromethane as an electrolysis solution, using tetrabutyl ammonium tetrafluoroborate as an electrolyte and adopting an electrochemical method. The specific rotation of the prepared monomer is [alpha] 20.0=-30.77 degrees, and the specific rotation of the polymer is [alpha] 20.0=-191.08 degrees; and the monomer can be dissolved into solvents such as acetone, dimethyl sulfoxide and the like, so the monomer can be applied in the field of chromatographic separation of chiral inducers, chiral catalysts, chiral medicament enantiomer and the like.
Owner:JIANGXI SCI & TECH NORMAL UNIV
New composite method for triptolide
ActiveCN101638426AMethod route shortEasy to operateOrganic-compounds/hydrides/coordination-complexes catalystsSteroidsChemical synthesisComposite field
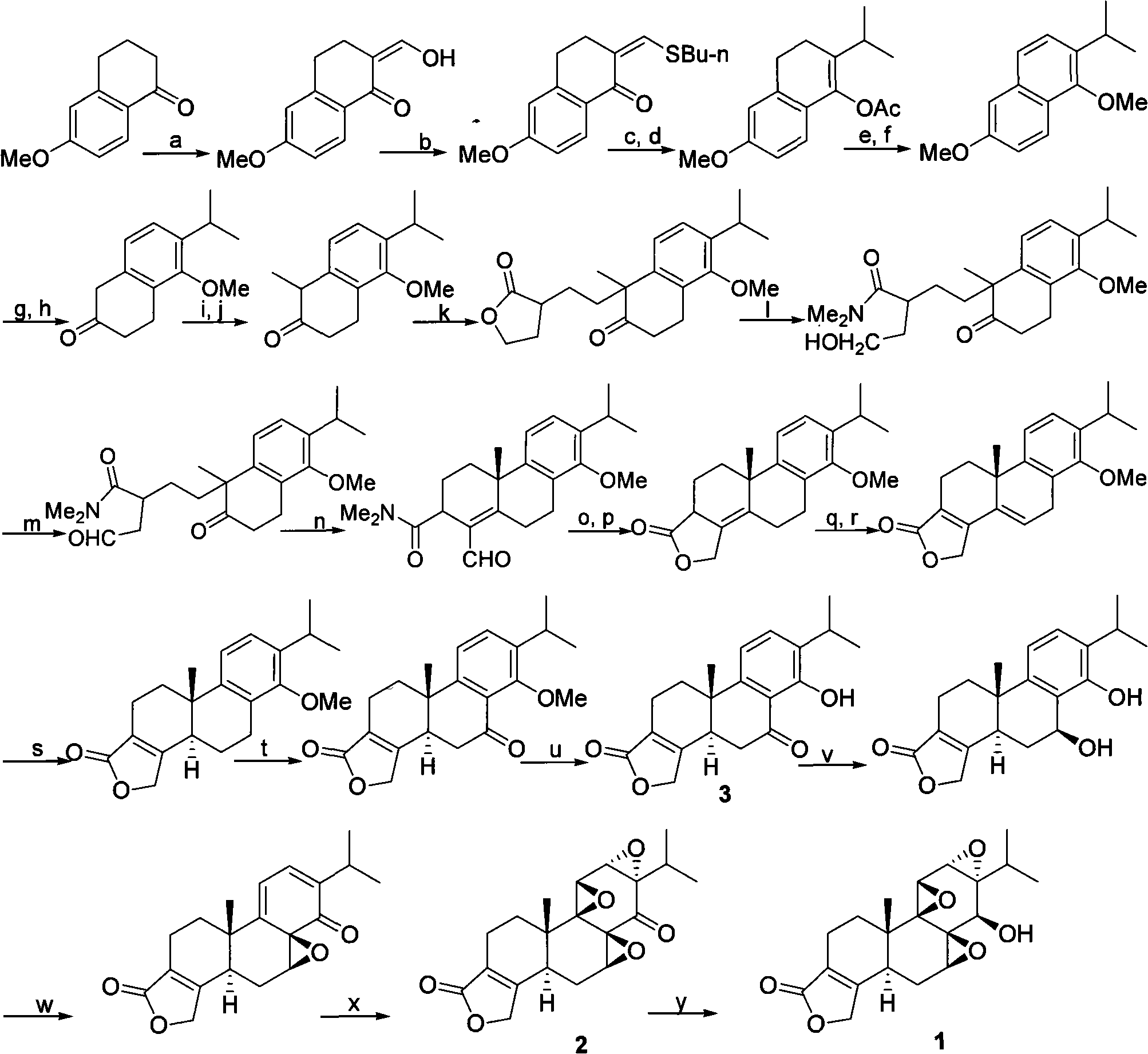
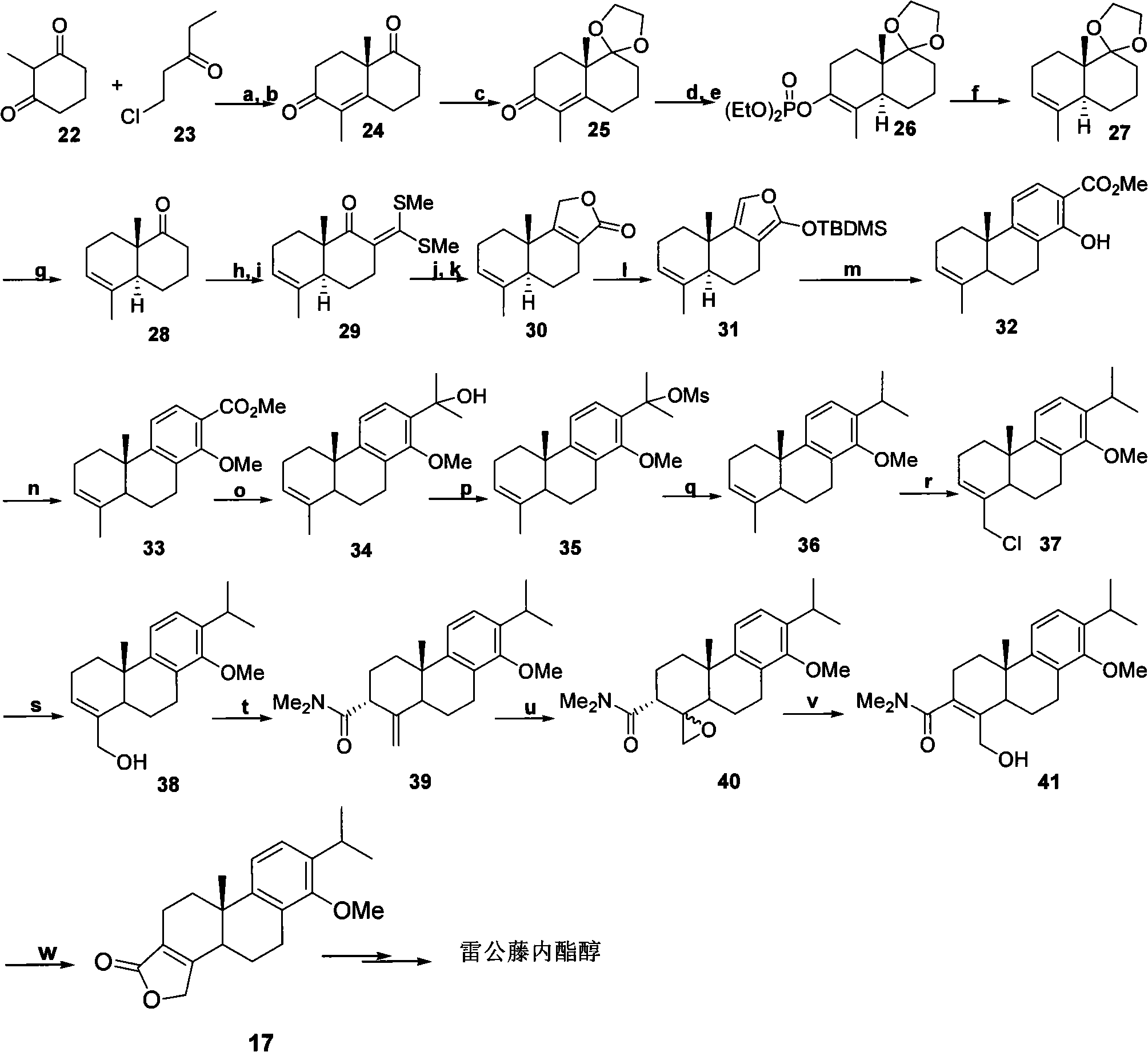
The invention provides a new and effective method for asymmetrically compositing triptolide, belonging to the chemical composite field. The method takes 5-methoxyl-2-tetralone and 2-(2-iodine ethyl)-1, 4-butyrolactone as initial materials and composites the triptolide by chiral induction. The method is brief and convenient in operation and has high yield. As the reaction condition is mild, the composite method is easily industrialized and is further used for large-scale synthesis of target compounds.
Owner:SHANGHAI HAOYUAN CHEMEXPRESS
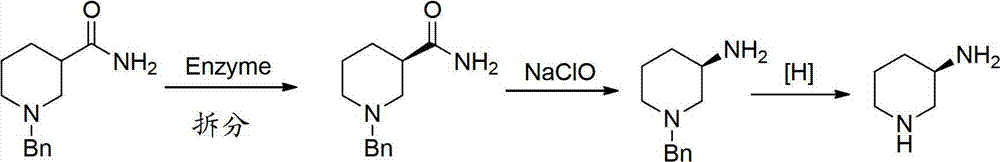
Asymmetric syntheses method and correlated intermediate of (R)-3-aminopiperidine (I)
ActiveCN103588699AHigh enantiopurityEasy to operateOrganic chemistryBulk chemical production1-phenylethanamineSynthesis methods
The invention relates to an asymmetric syntheses method of (R)-3-aminopiperidine (I). The method comprises: reducing a formula (III) compound to obtain a formula (II) compound, and then removing a chiral prosthetic group and an amino protective group from the formula (II) compound to obtain (R)-3-aminopiperidine (I), wherein R is the amino protective group, and specially is C1-4 alkoxycarbonyl or benzyl removable by hydrolysis or hydrogenation. Preferably, the formula (III) compound is obtained by performing a dehydration reaction on 3-piperidone and a chiral amine (R)-1-phenylethylamine. The invention also relates to a new compound (II). The asymmetric syntheses method of (R)-3-aminopiperidine (I) is reasonable in technology and concise in route, the needed product with relatively high ee value is obtained by utilizing chiral induction, the raw material is cheap and does not need resolving, no waste isomer is discharged, and the asymmetric synthesis method is applicable to large-scale industrial production.
Owner:SHANGHAI PUYI CHEM CO LTD
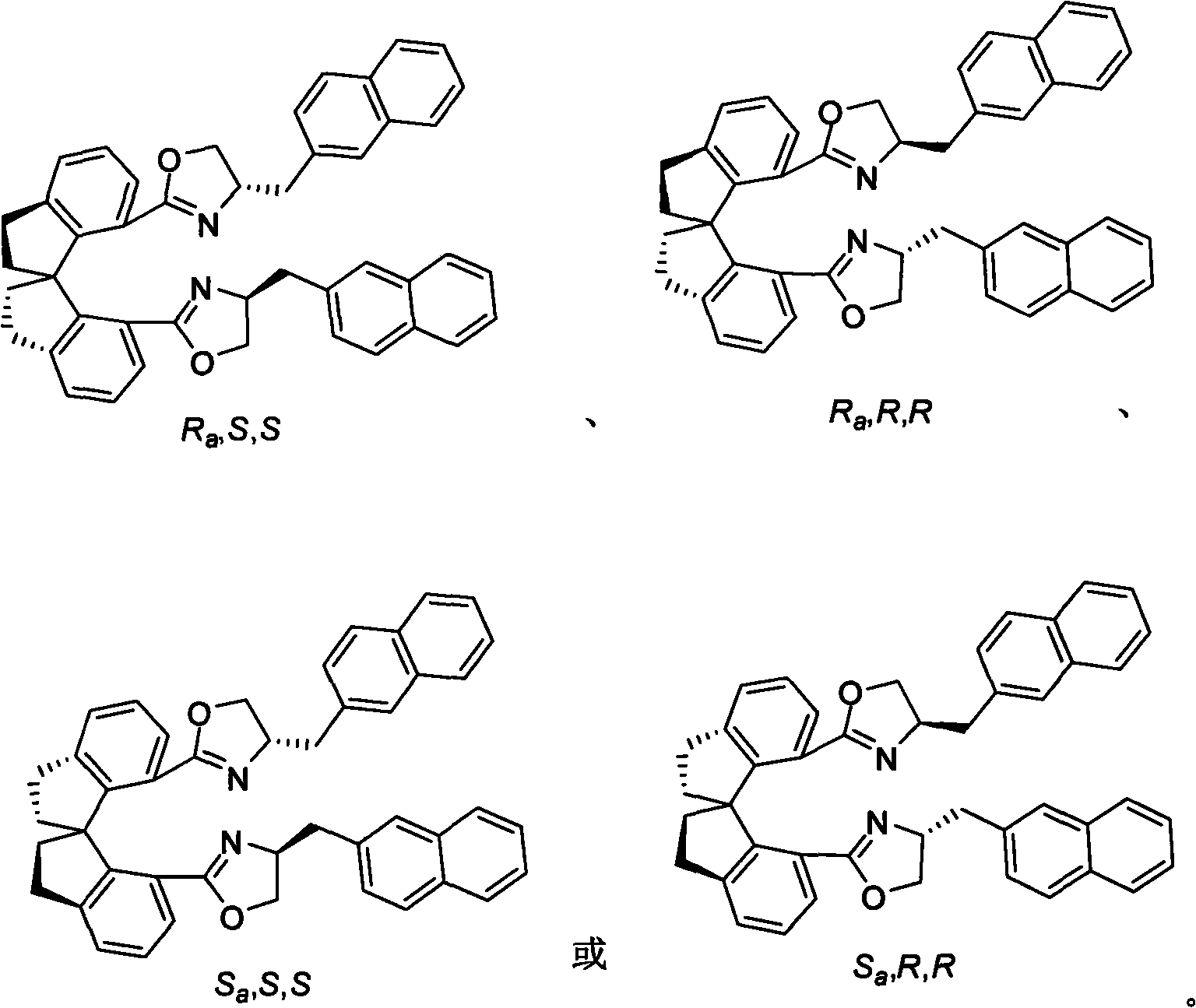
Beta-naphthyl methyl substituted spiral bisoxazoline ligand, synthetic method and application thereof
ActiveCN101671313AOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesRegioselectivityDrug biological activity
The invention relates to a beta-naphthyl methyl substituted spiral bisoxazoline ligand, a synthetic method and an application. The ligand has the advantages of simple synthetic method, mild conditionand applicability for industrialization production. The ligand has a spiral framework and a feature of a plurality of chiral centers, therefore the ligand has high catalytic activity and chiral induction effect and strong adjustment capacity in an asymmetric catalytic reaction of transition metals. Pyrrolidine derivants with various optical activities are prepared through high region selectivity and high enantioselectivity from allenoic and o-iodoaniline by the catalysis of the ligand. With the important biological activity, the pyrrolidine derivant has a better prospect of industry and medicine applications.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Chiral leucine ion liquid
InactiveCN101967126AIncrease freedomHave diversityOrganic compound preparationGroup 5/15 element organic compoundsLiquid temperatureAmmonium compounds
The invention discloses chiral leucine ion liquid. The chiral leucine ion liquid consists of a cation [C]<+> and a chiral leucine ion [A]<-> and has a structural formula, namely [C]<+> [A]<->, wherein the [A]<-> is a chiral L-leucine anion or a chiral D-isoleucine ion; and the cation [C]<+> is imidazoles, an N-alkyl pyridine cation, a quaternary ammonium compound cation, a quaternary phosphonium salt cation or an N,N-dialkyl pyridine cation. The invention provides ion liquid with chiral position on the cation and the cation has more structure and property design freedom degrees, so the chiral leucine ion liquid has structural diversity and designability. The chiral leucine ion liquid of the invention has the advantages of ion liquid, such as nearly nonvolatile property, no toxicity, wide liquid temperature range and the like, and has the chiral characteristics of chiral substances, such as high selectively, chiral induction effect and the like.
Owner:IRICO
Method for synthesizing amino acids with large steric hindrance and oligopeptides through palladium catalytic carboxylic acid guided arylation
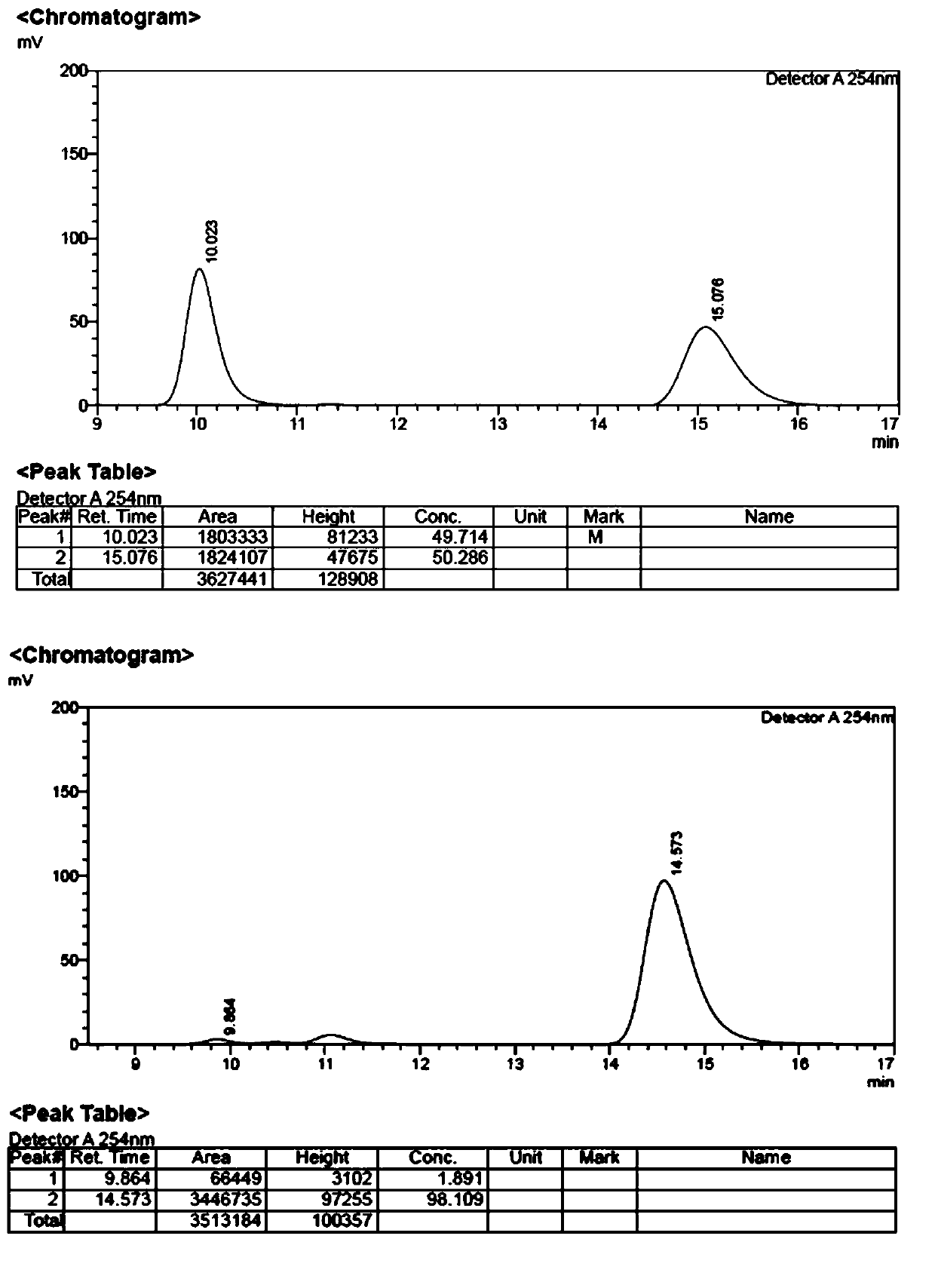
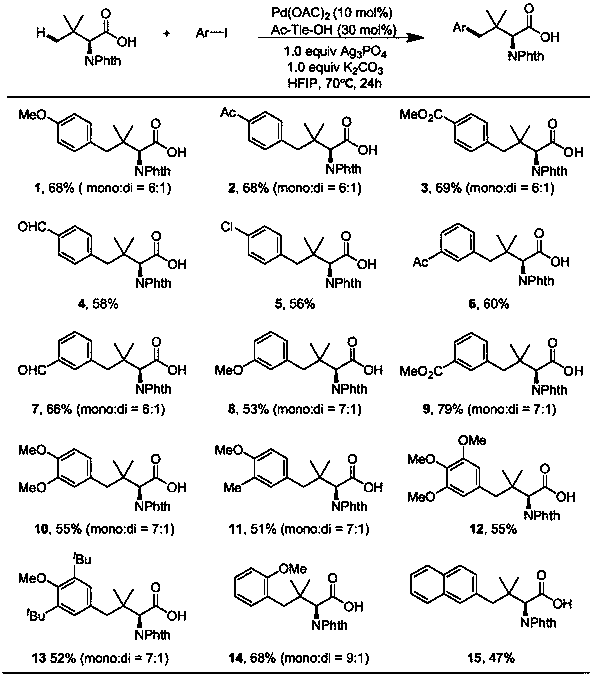
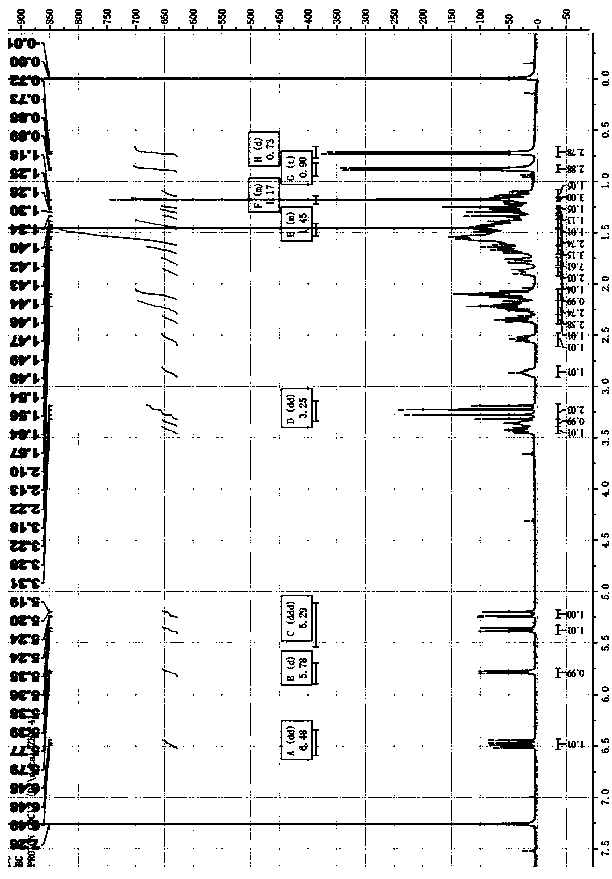
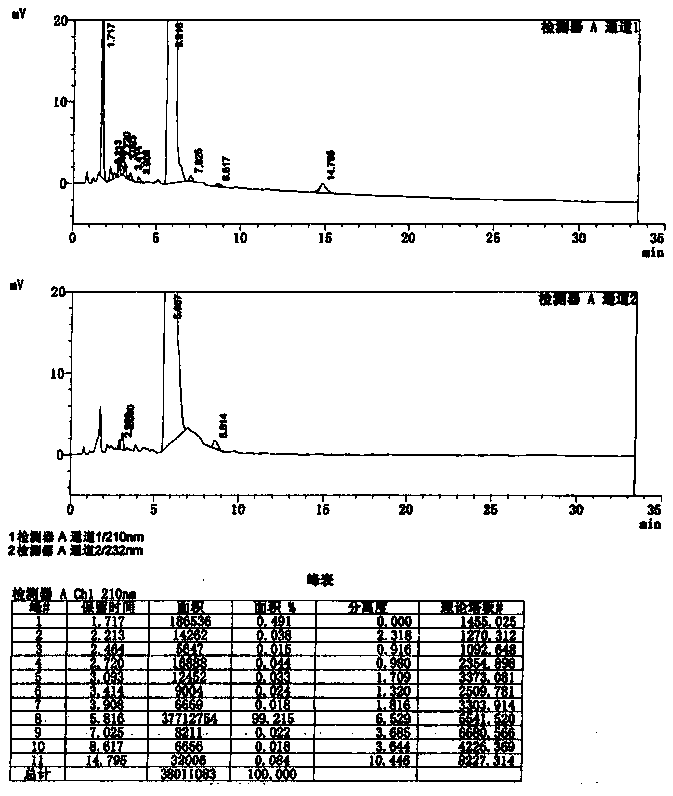
The invention discloses a method for synthesizing amino acids with large steric hindrance and oligopeptides through palladium catalytic carboxylic acid guided arylation. According to the method, a substrate is not racemized in a reaction process, so that the product can be directly used as a ligand to participate in an asymmetric catalytic reaction; the palladium catalytic arylation reaction is characterized in that double-protection L-tert-leucine and an oligopeptide compound containing L-tert-leucine are used as substrates, aryl iodide is used as an arylation reagent, and aryl is introducedto the gamma position of an amino acid to synthesize an amino acid with large steric hindrance and a polypeptide, wherein the reaction is carried out in a hexafluoroisopropanol solvent, palladium acetate is used as a metal catalyst, acetyl tert-leucine is used as an auxiliary agent, and silver phosphate and potassium carbonate are used as additives; and with the method, a variety of branched chainlarge steric hindrance amino acids and oligopeptide compounds can be efficiently synthesized, the compounds are chiral ligands with characteristics of high activity and high selectivity, and the ligands show high chiral induction in applications.
Owner:ZHEJIANG UNIV
Intermediate compound of Lefamulin and application of intermediate compound in preparation of Lefamulin
ActiveCN111170893AOvercoming conversion rateOvercome costsCarbamic acid derivatives preparationOrganic compound preparationBiochemical engineeringEconomic benefits
The invention discloses an intermediate compound of Lefamulin and application of the intermediate compound in preparation of Lefamulin, and aims to solve the technical problems of high preparation cost and low yield of Lefamulin in the prior art. The invention provides theintermediate compound and a preparation method thereof, andapplication of the intermediate compound in preparation of Lefamulinor a pharmaceutical intermediate related to Lefamulin. The method overcomes the defects that chiral oxidation lacks chiral induction in the production process in the prior art, the conversion rate ofa target compound is very low, the cost is very high, and industrial production is difficult; during industrial application, the production cost can be effectively reduced, generation of three wastescan be effectively controlled, and remarkable economic benefits and social benefits are achieved.
Owner:郑州依米花手性药物研究有限公司
Chiral silicon dioxide fiber-reinforced foamed polypropylene composite material
ActiveCN110305412AImprove mechanical propertiesReduce orientationLinear alkylbenzeneInjection moulding
The invention relates to a chiral silicon dioxide fiber-reinforced foamed polypropylene composite material which comprises a polypropylene resin, talcum powder, a chiral silicon dioxide fiber, a surfactant solution, a micro-pore foaming agent and a phase-change material microcapsule and an aid, wherein the surfactant solution is a linear sodium alkyl benzene sulfonate solution. Due to adoption ofthe chiral silicon dioxide fiber, the mechanical properties of the material can be improved; the chiral silicon dioxide fiber has chiral induction under the action of hydrogen bonds among molecules, orientation of polypropylene molecular chain fragments can be reduced, the polypropylene molecular chain fragments can be regularly arranged, and thus the mechanical properties of a polypropylene injection molding finished product can be improved; meanwhile, due to a chiral spiral structure of the chiral silicon dioxide fiber, and through chiral spiral induction on polypropylene, winding of molecular chains can be intensified, and the mechanical properties of the polypropylene injection molding finished product can be further improved; and due to adoption of the chiral silicon dioxide fiber, mechanical properties of a polypropylene material and an injection molding part thereof can be effectively improved.
Owner:SUZHOU RUNJIA ENGINEER PLASTIC
Process for preparing deoxycholic acid
The invention discloses a process for preparing deoxycholic acid. The process comprises the following steps: (1) by taking chenodeoxycholic acid as a raw material, adding a proper solvent; (2) adding a reagent and a catalyst, and selectively generating a derivative together with 3alpha-hydroxyl; (3) adding a chiral inducer, an asymmetric synthesis catalyst, tertiary butanol or octyl pentanol, filling hydrogen and pressurizing, converting 7alpha-hydroxyl in the chenodeoxycholic acid into 7beta-hydroxyl through one step under radiation conditions, thereby generating a coarse product of chenodeoxycholic acid; (4) washing the coarse product of chenodeoxycholic acid, filtering and removing impurities; (5) selecting a corresponding solvent, and extracting to remove the unreacted chenodeoxycholic acid and other impurities; and (6) drying the chenodeoxycholic acid, and collecting and packaging to obtain a finished product. The process flow is simple, the equipment investment is low, fewer chemical reagents are used, the conversion rate is high, raw materials are fully utilized, the production efficiency is improved, the production cost and the energy consumption are greatly reduced, and the process is suitable for industrial production.
Owner:HARBIN INST OF TECH
D-biotin purification process
The invention discloses a purification method of d-biotin, while prior art can not remove I-biotin to produce disqualified d-biotin product. The invention uses the recrystallization of the mixture solvent of lower alkyl alcohol and glacial acetic acid to purify d-biotin with foreigner materials, to remove foreigner materials as bis-benzyl biotin and single-benzyl biotin. And when the optical rotation of the product in procedure step is disqualified, in water and lower alkyl alcohol, the invention arranges chiral induction resolving agent I-arginine and d-biotin with disqualified optical rotation to be mixed, dissolved, frozen, crystallized and filtered, to remove foreigner material as I-biotin, heats and dissolves the filter cake via water or lower alkyl alcohol, adjusts pH value via diluted acid until 2-5, freezes and crystallizes, filters to obtain the filter cake as d-biotin pure product. The invention precipitates solid d-biotin and leaves I-biotin in filter liquor, to effectively remove I-biotin and confirm the quality of medical d-biotin.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
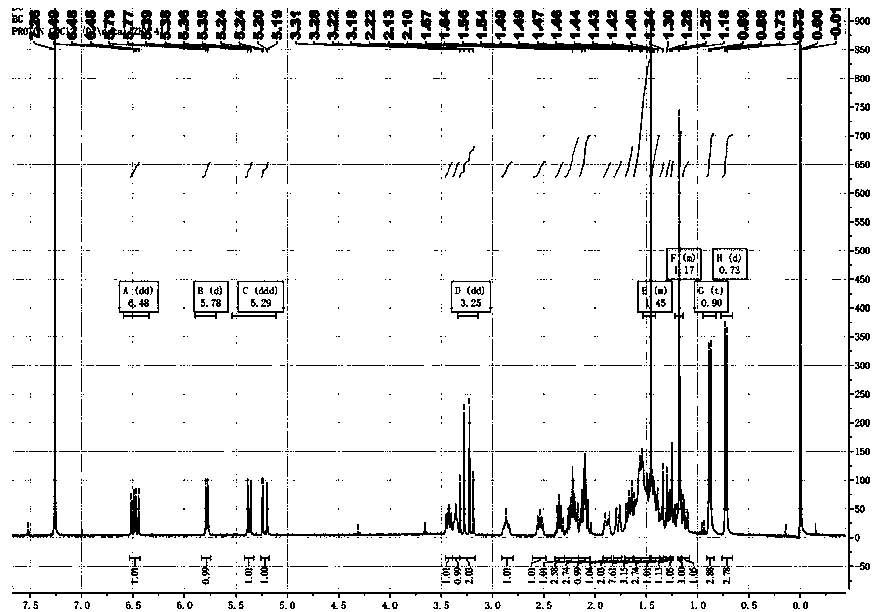
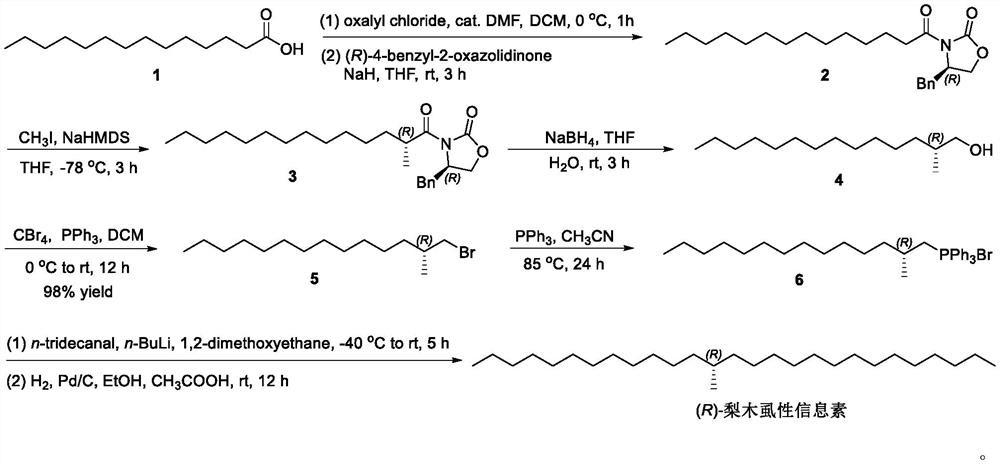
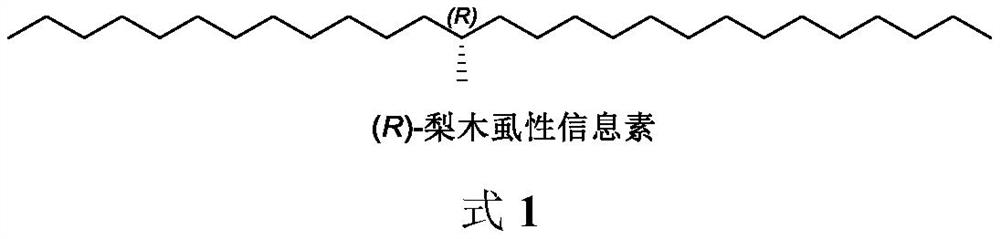
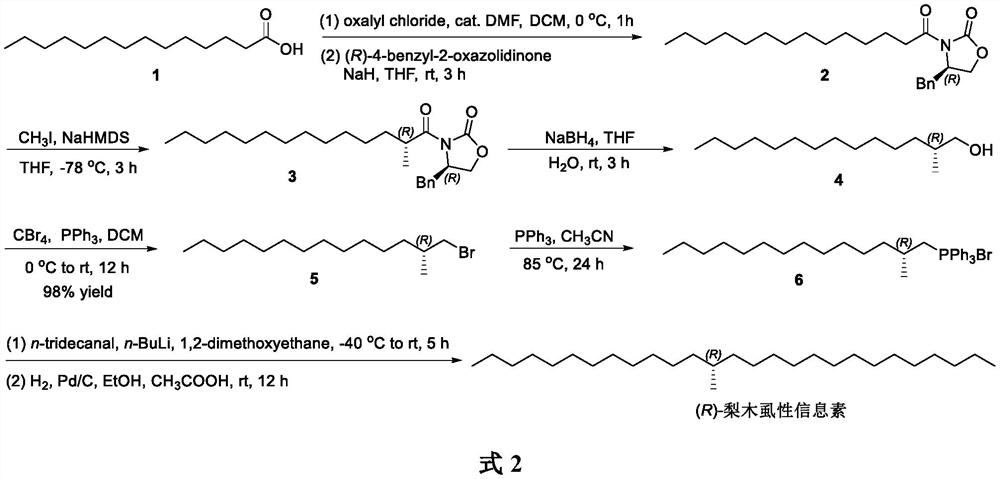
Method for synthesizing (R)-cacopsylla pyricola sex pheromone
InactiveCN113004111AOrganic compound preparationOrganic chemistry methodsPhosphonium saltHeptadecane
The invention belongs to the technical field of green pesticides, and discloses a novel method for synthesizing (R)-cacopsylla pyricola sex pheromone. The method comprises the steps: by taking n-tetradecanoic acid 1 as an initial raw material, firstly reacting with oxalyl chloride to generate n-tetradecanoyl chloride, and then reacting with (R)-4-benzyl-2-oxazolidinone to generate oxazolidinone amide 2; reacting with CH3I in the presence of NaHMDS to generate oxazolidinone amide 3 with one or more methyl groups, and reducing the oxazolidinone amide 3 into chiral alcohol 4 through sodium borohydride; carrying out bromination, and reacting with PPh3 to generate quaternary phosphonium salt 6; and finally carrying out Wittig reaction and catalytic hydrogenation to prepare the (R)-cacopsylla pyricola sex pheromone, namely (R)-13-methylheptacosanoic acid. The chiral methyl is constructed by using an Evans chiral induction method, and the method has the advantages of simple synthetic route, mild reaction conditions, easiness in amplification and the like.
Owner:CHINA AGRI UNIV
Ionic compound containing chiral amine-thiourea (urea) and its preparation method and application
ActiveCN101143862AChiralReduce dosageOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesOrganic solventThiourea
The invention relates to an ion type compound containing a chiral amine-thiourea (urea) structure, the general formula is A plus B minus , wherein, the structure of A plus is shown as the formular (I), and the structure of B minus is shown as the formular (V). In a preparation method, the bromine salt of A plus and the sodium salt of B minus are resolved in organic solvent, under the room temperature, stiring continues untile the reaction is sufficient, and therefore the ion type compound containing a chiral amine-thiourea (urea) structure is produced. The substance of the invention can be used as not only a catalyst in an organic asymmetric reaction but also a chiral shift reagent in the chiral identification. The substance of the invention has a good chiral inducement property and can be used as a chiral reagent, a catalyst and a chiral material in the fields of organic synthesis, materials, etc.
Owner:ZHEJIANG UNIV OF TECH
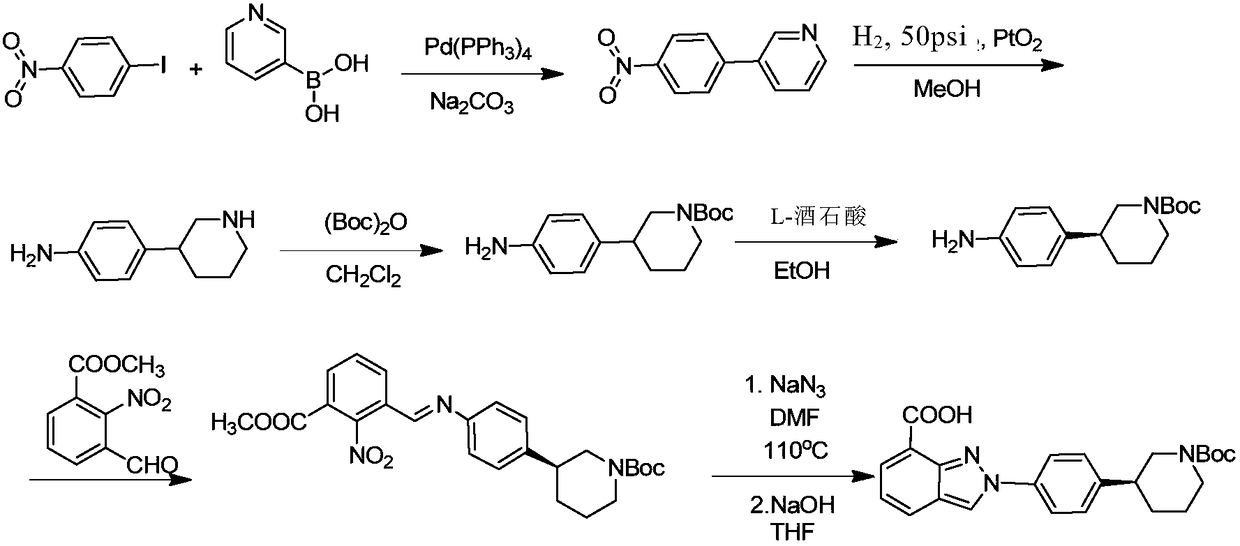
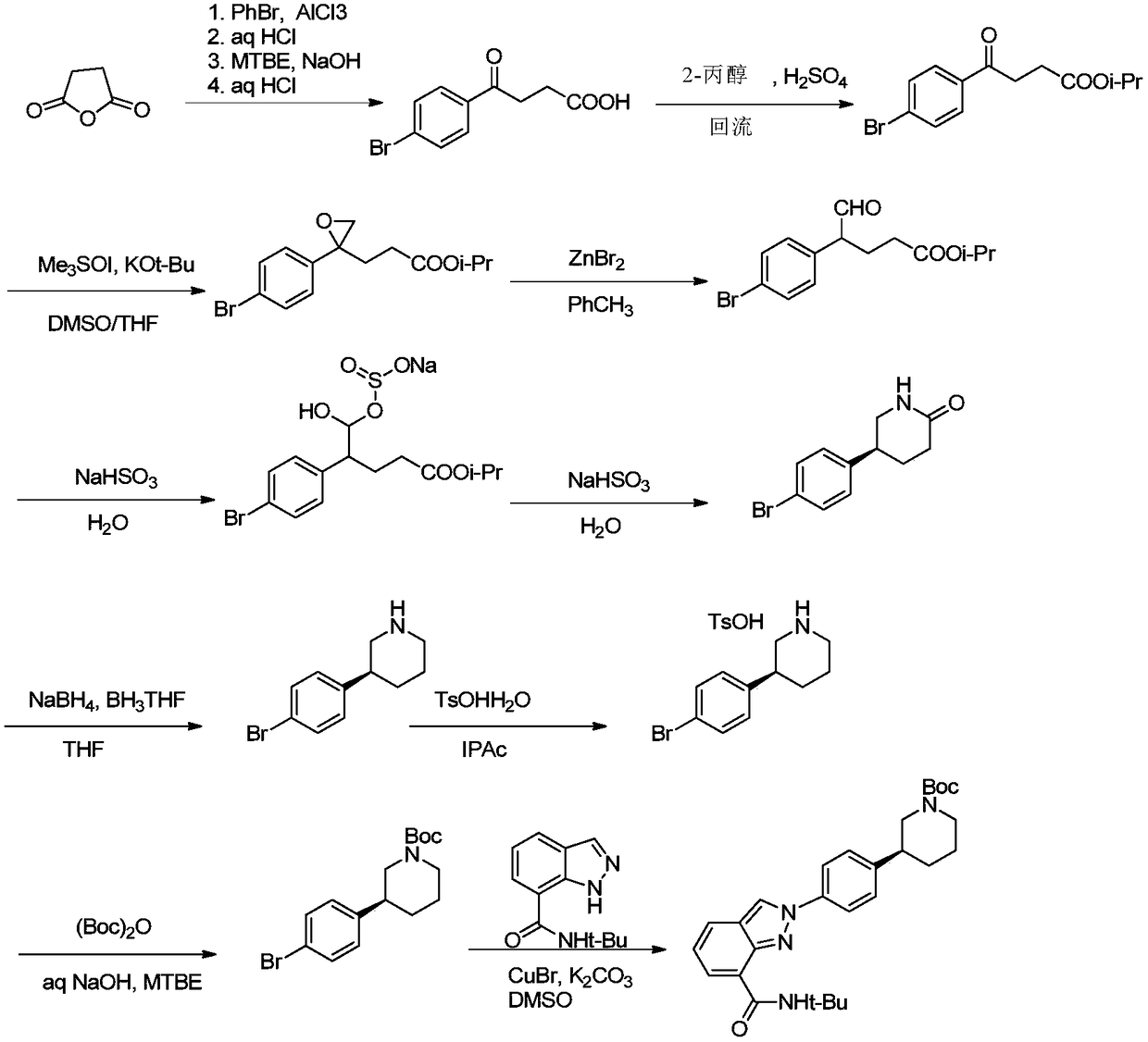
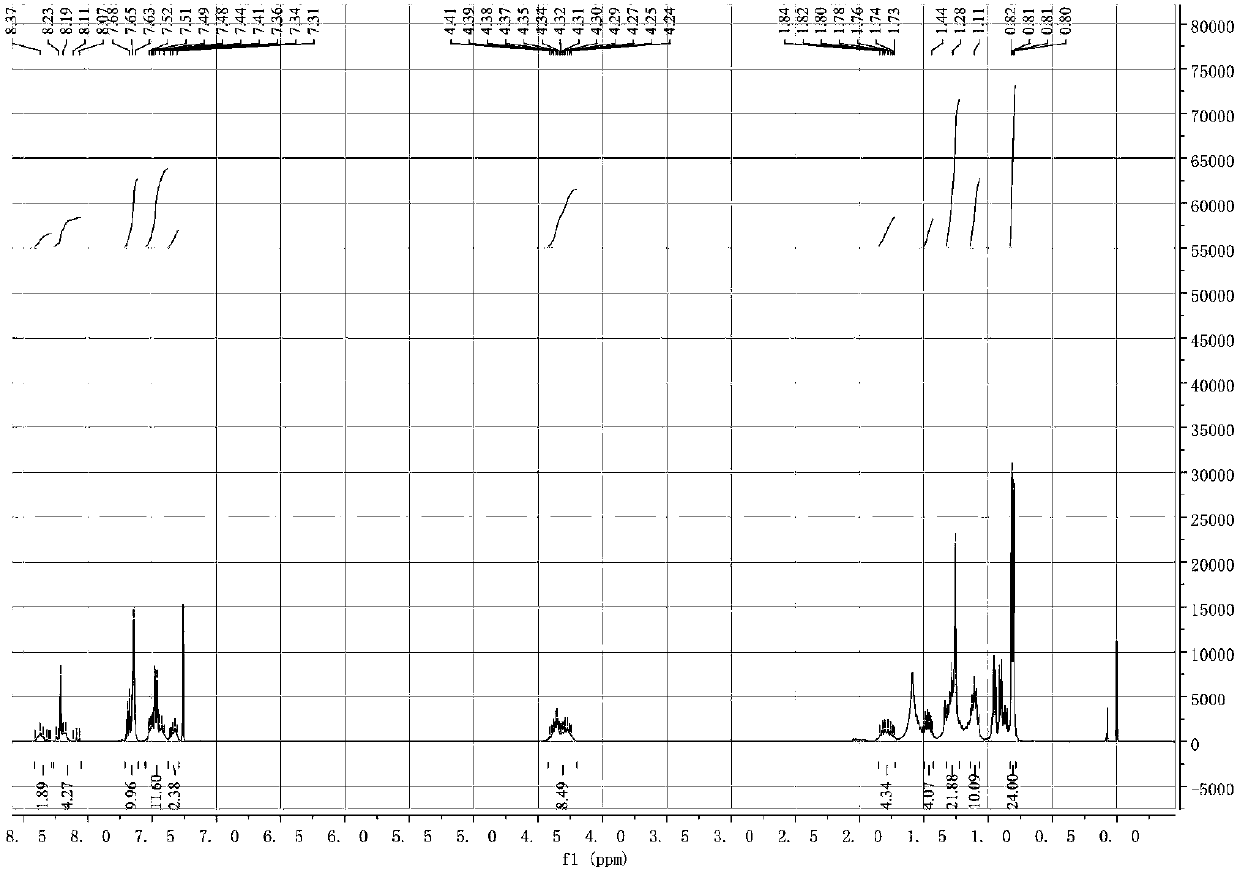
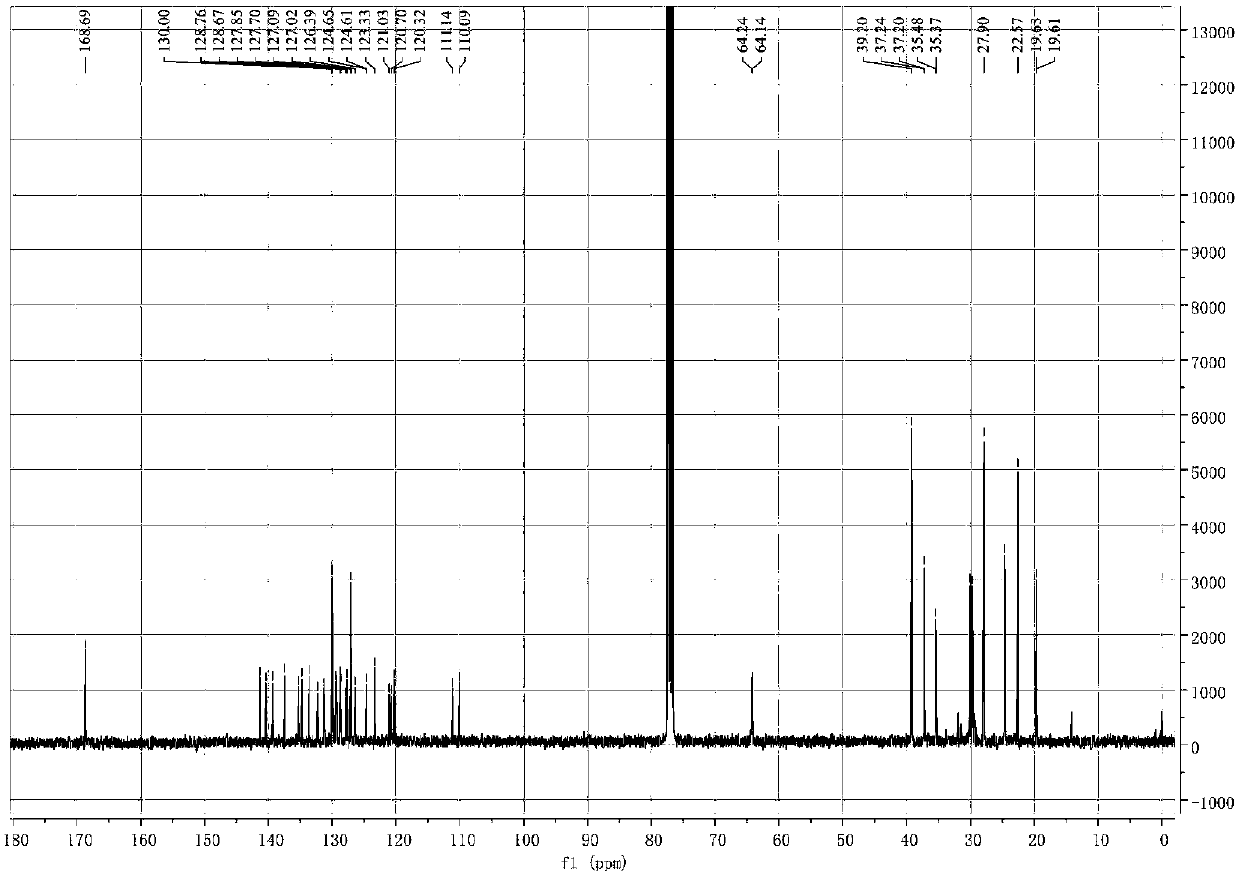
Method of synthesizing (S)-3-(4-bromophenyl)-piperidine or salt thereof with chiral induction
ActiveCN109456253ARaw materials are cheap and easy to getSimple and fast operationOrganic chemistryDiastereomerCrystallization
The invention relates to a method of synthesizing (S)-3-(4-bromophenyl)-piperidine or salt thereof with chiral induction. Particularly, the method comprises the following steps of by taking methyl ortho-4-bromophenylacetate and (S)-(-)-2-methyl-2-propanesulfinamide as starting materials, sequentially performing reactions including condensation, replacement, reduction, ring closure, removal of chiral induced groups and the like, and the (S)-3-(4-bromophenyl)-piperidine or the salt thereof can be obtained, wherein after the reduction reaction, a high-purity single diastereoisomer can be obtainedwith recrystallization; and after the ring closure reaction, a high-purity single diastereoisomer can be further obtained with recrystallization. The method provided by the invention has the advantages of cheap and available raw material, simple operation, high yield, and low cost and is suitable for industrial production.
Owner:SHANGHAI VASTPRO TECH DEV CO LTD
Preparation method of intelligent regulation circular polarization fluorescent perylene derivative photoelectric material
InactiveCN107602391AThe synthesis method is simpleManipulableOrganic compound preparationCarboxylic acid esters preparationFluorescenceSide chain
The invention discloses an intelligent regulation circular polarization fluorescent perylene derivative photoelectric material, which has the following chemical structural formula defined in the specification, wherein the substituted functional group R is any one of the following structures in the structural formula, R1 is a C1-C12 linear chain or branched chain alkyl or alkoxy chain, and *1 or *2or* 3 is a coupling position. According to the present invention, the chromophore using perylene as the core and having the side chain of the chiral inducing functional group and other chromophores are subjected to the Suzuki coupling reaction to construct a class of the photoelectric materials with the circular polarization fluorescence, wherein the class of the CPL materials have the followingadvantages that the intelligent CPL signal regulation is achieved in the case of the external stimuli response, the CPL luminescence of the single molecule and the dimer is achieved by regulating theconcentration in the good solvent tetrahydrofuran, the aggregation-induced CPL intelligent regulation is achieved in the tetrahydrofuran-water system, and especially the un-annealed film has a good CPL signal.
Owner:NANJING UNIV OF POSTS & TELECOMM
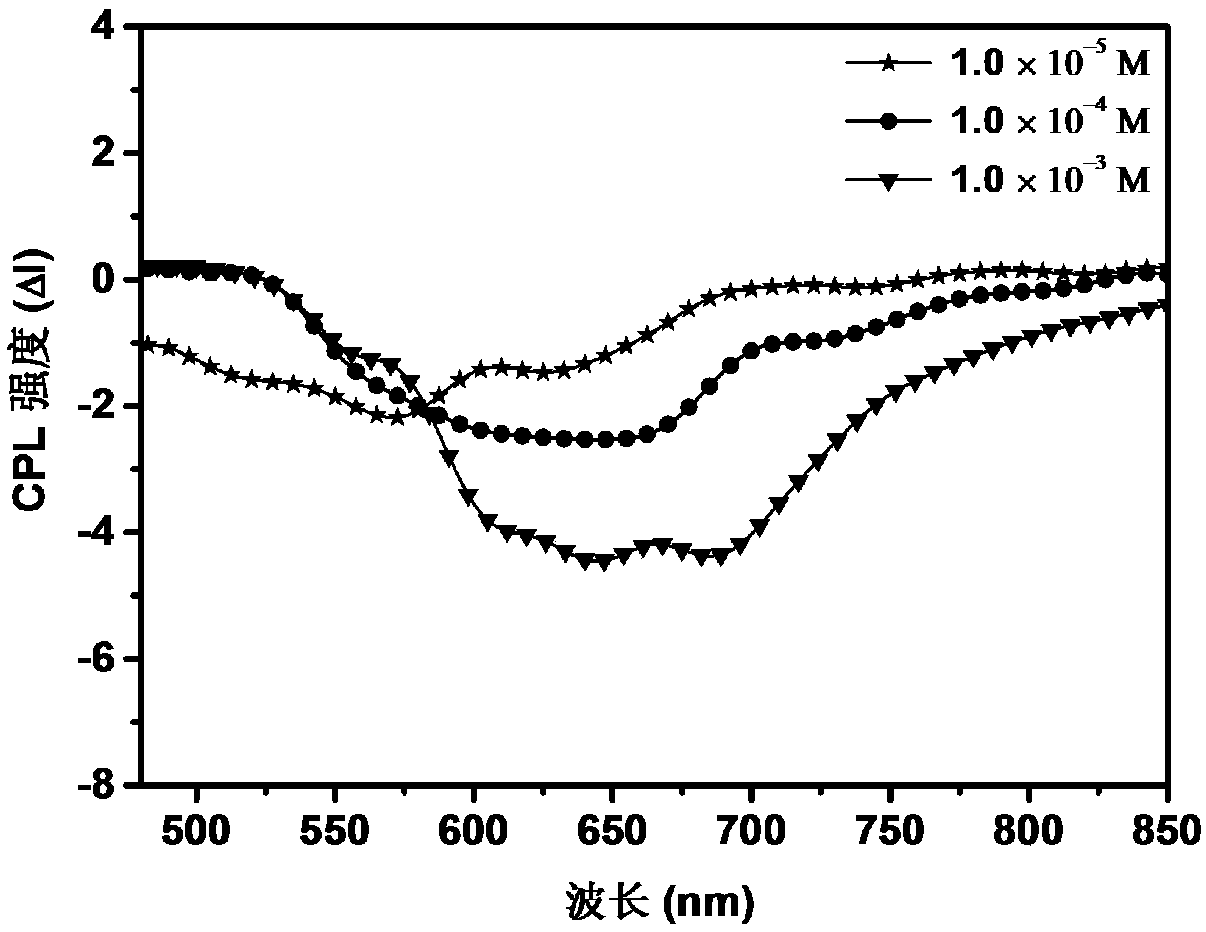
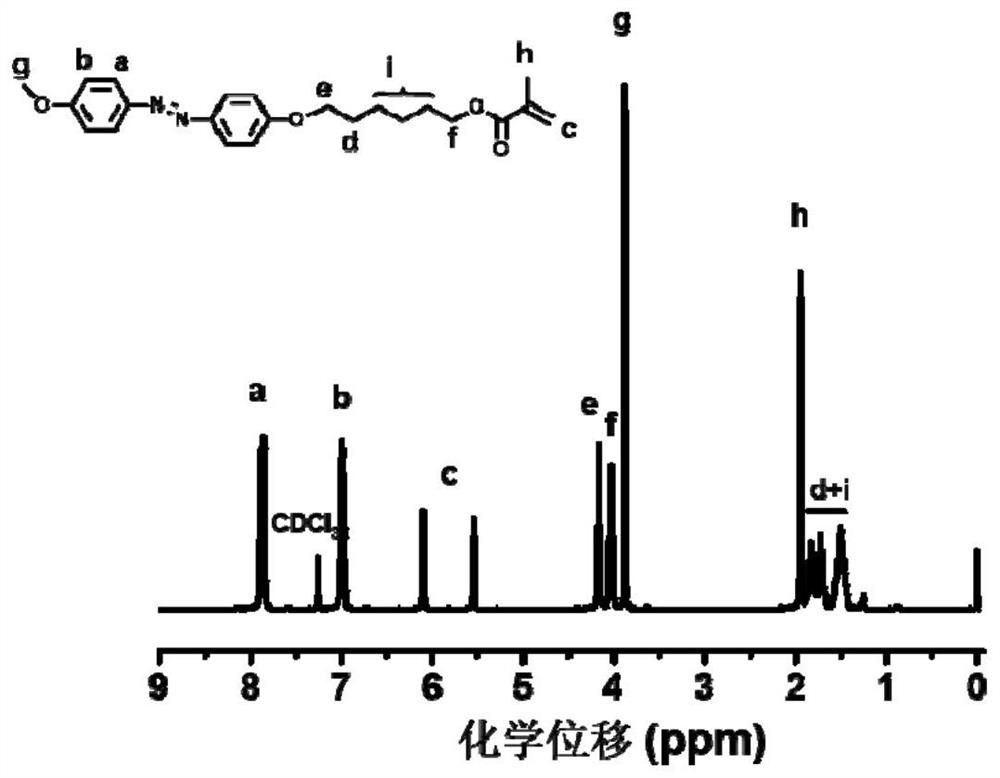
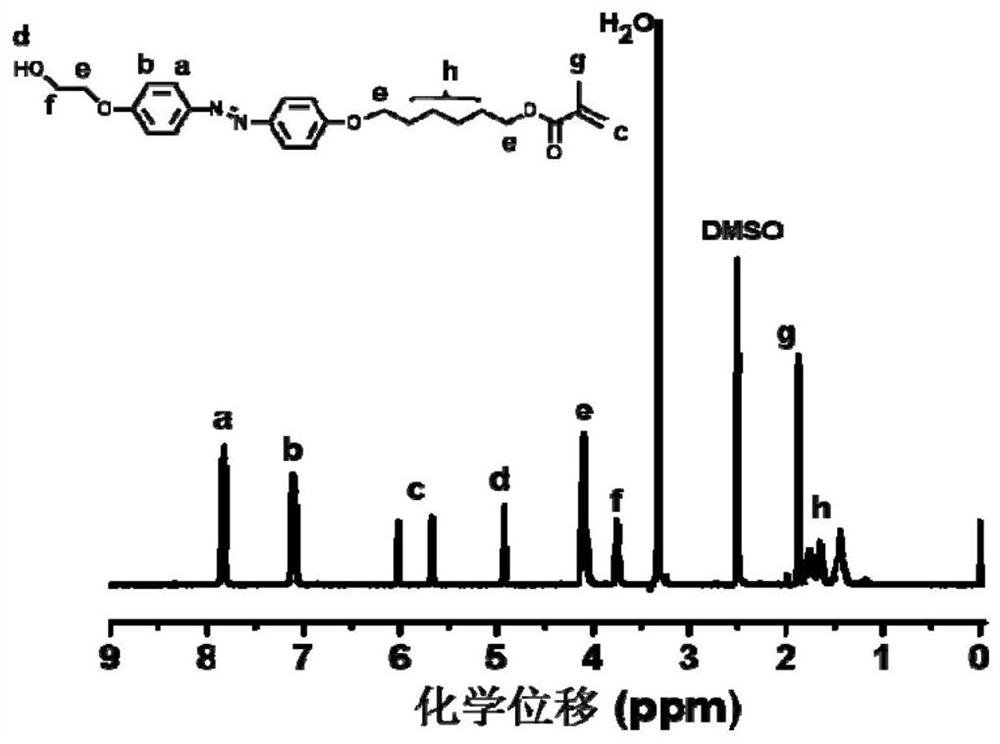
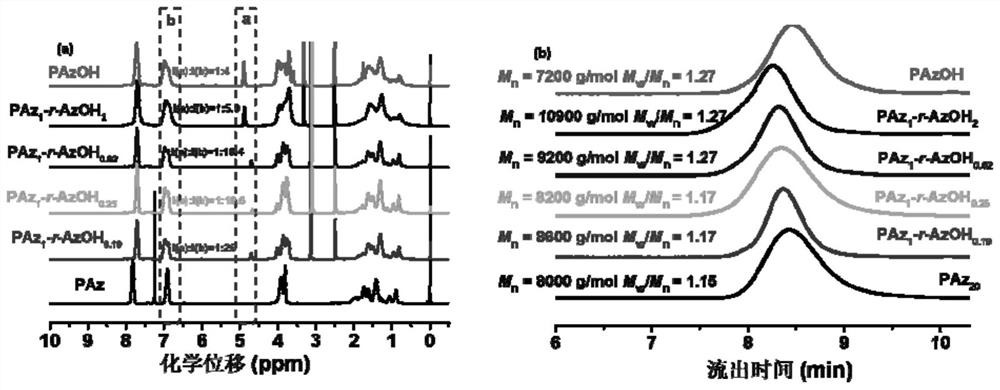
Azobenzene polymer as well as preparation method and application thereof
The invention discloses an azobenzene polymer as well as a preparation method and application thereof. An azobenzene polymer is prepared from monomers Az, AzOH, an RAFT reagent and an initiator; the azobenzene polymer is prepared into a film; and chiral reagent induction is carried out to obtain a chiral azobenzene polymer film. The method comprises the following steps: firstly, synthesizing an azobenzene random copolymer with hydroxyl at the tail end of a side chain through a series of organic synthesis reactions and RAFT polymerization, and carrying out detailed investigation on the molecular weight and the liquid crystal performance of the polymer by utilizing characterization means such as nuclear magnetism, GPC, DSC, POM and XRD; and then preparing the polymer film in a spin-coating manner. Chiral limonene steam can be selected to carry out chiral induction on the polymer film prepared by the method to obtain an optically active polymer film, and the optically active polymer filmcan be further cross-linked to obtain a cross-linked film.
Owner:SUZHOU UNIV
Method for constructing porous polymer on the basis of pure chiral molecules of 1, 1 '-bi-2-naphthol
ActiveCN106939059AGood chiral induction environmentLarge specific surface areaGroup 5/15 element organic compoundsSynthesis methods2-Naphthol
Owner:ZHEJIANG UNIV
Synthesis method of (R)-2-(2, 5-difluorophenyl) pyrrolidine
The invention belongs to the technical field of chemical pharmacy, and relates to a synthetic method of (R)-2-(2, 5-difluorophenyl)pyrrolidine. The synthesis method comprises the following steps: (1) carrying out dehydration condensation reaction on a compound as shown in a formula 1 and a chiral induction reagent (R) tert-butylsulfinamide to obtain a compound as shown in a formula 2; (2) carrying out addition reaction on the compound in the formula 2 and a Grignard reagent to obtain a compound in a formula 3; (3) carrying out a reduction / cyclization reaction on the compound shown in the formula 3, trifluoroacetic acid and triethylsilane to obtain a compound shown in a formula 4; (4) splitting the compound shown in the formula 4 by (D)-malic acid to obtain the (R)-2-(2, 5-difluorophenyl) pyrrolidine*D malate compound with EE being greater than 98% shown in the formula 5; and (5) dissociating the compound in the formula 5 with a sodium hydroxide solution to obtain (R)-2-(2, 5-difluorophenyl) pyrrolidine. By utilizing the synthetic method of (R)-2-(2,5-difluorophenyl)pyrrolidine, the cost is low, the optical purity is high, the subsequent separation process is simplified, the raw materials are easy to obtain, the process conditions are mild, and the synthetic method is suitable for synthesizing (R)-2-(2, 5-difluorophenyl) pyrrolidine in large-scale production.
Owner:北京蓝博特科技有限公司
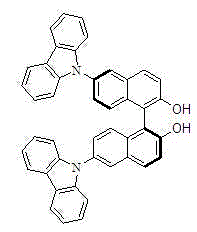
Chiral 6, 6'-2 carbazole base binaphthol
A chiral binaphthol and chiral ligand adopting the chiral binaphthol as a framework show good chiral induction capability in unsymmetrical catalysis and obtain high enantioselectivity in a plurality of catalytic reactions. A membrane reactor can recycle homogeneous phase catalysts, and for expensige homogeneous phase chiral catalysts, recycling the chiral catalysts has more important values. The invention discloses a chiral compound 6,6'-2 carbazole base binaphthol derived from binaphthol with large molecular dimensions, and the 6,6'-2 carbazole base binaphthol is hopeful for being used as the chiral ligand of the membrane reactor. Chiral 6, 6'-2 carbazole base binaphthol is obtained by synthetizing 6,6'-carbazole base binaphthol, carbazole, palladium and alkali at a next step. The chiral compound can serve as the chiral ligand and used for unsymmetrical catalytic addition reaction of aryl acetylene and aromatic aldehyde.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
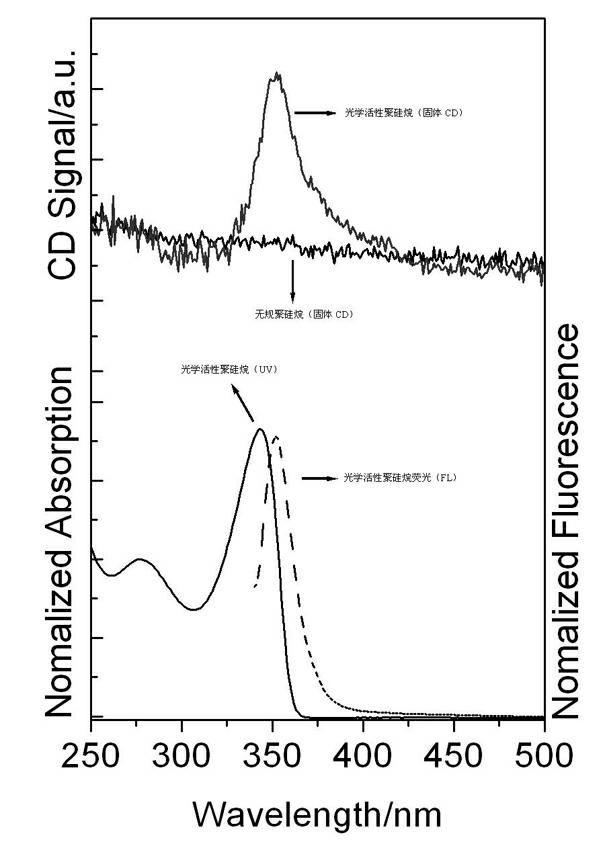
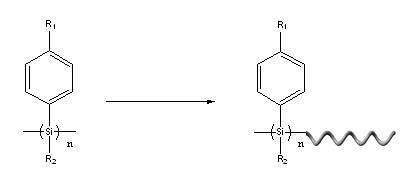
Method for preparing optically active poly(alkyl-aryl) silane
The invention relates to an organic high molecular compound, in particular to a method for preparing optically active poly(alkyl-aryl) silane. As the traditional method for synthesizing optically active poly(alkyl-aryl) silane is extremely limited, the invention provides a novel method for preparing optically active poly(alkyl-aryl) silane. Polysilane which is not optically active is used as a raw material in an organic solvent A, alkyl lithium is a reactant, chiral diamine is used as a chiral inductor, and saturated ammonium chloride is used for quenching reaction to prepare the optically active poly(alkyl-aryl) silane. The preparing method has the advantages of convenience for operation and low cost.
Owner:HANGZHOU NORMAL UNIVERSITY
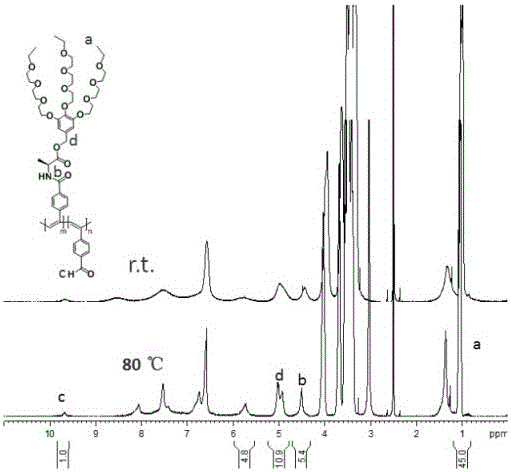
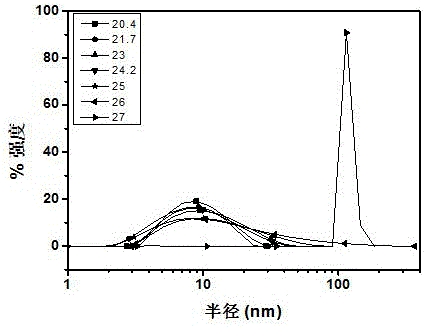
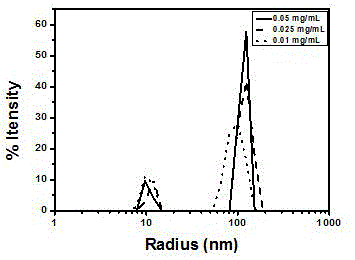
Alkoxy ether thermosensitive chiral polymer nano-microspheres and preparation method thereof
The invention relates to alkoxy ether thermosensitive chiral polymer nano-microspheres and a preparation method thereof. The nano-microspheres are prepared by carrying out copolymerization reaction on alkoxy ether dendronized macromonomer with chiral induction primitive and p-alkynyl benzaldehyde to obtain a copolymer, and cross-linking the copolymer by using adipic dihydrazide as a cross-linking agent to form the alkoxy ether chiral polymer nano-microspheres, wherein the mol ratio of the aldehyde group in the copolymer to the cross-linking agent is 2:1. The nano-microspheres have good dispersibility in water solution and excellent thermosensitive behavior, and by adjusting temperature, concentration and molecular weight, chiral nano-microspheres with different particle sizes can be prepared. Simultaneously, compared with variable helical conformation of copolymers, the conformation of the obtained nano-microspheres is stable and single. The nano-microspheres are constructed by cross-linking a dynamic bond with a thermosensitive polymer, chiral transference of the chiral induction primitive provides a theoretical basis, and the nano-microspheres are of important significance in fields of realizing selectivity, identification or function control of some protein and amino acid, medicine release, envelop and the like.
Owner:SHANGHAI UNIV
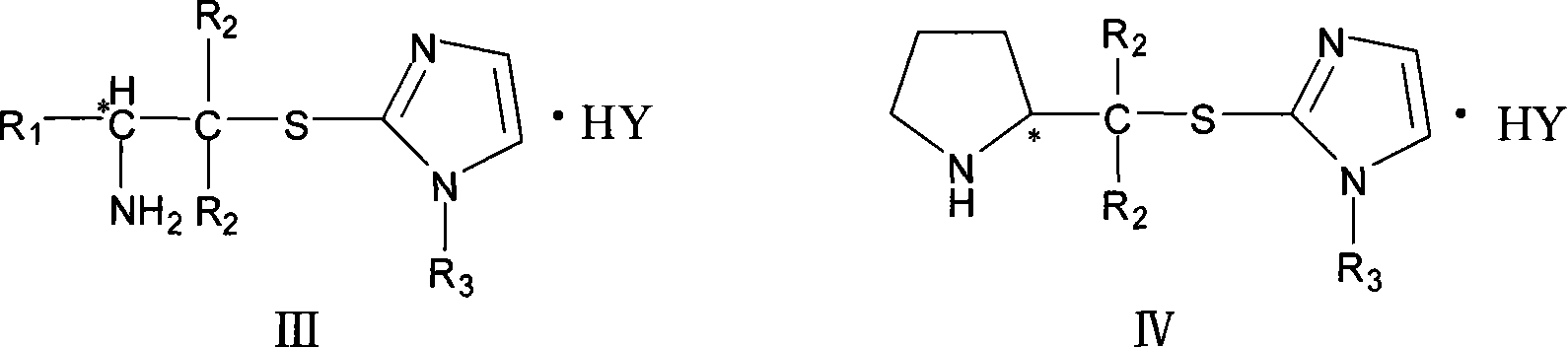
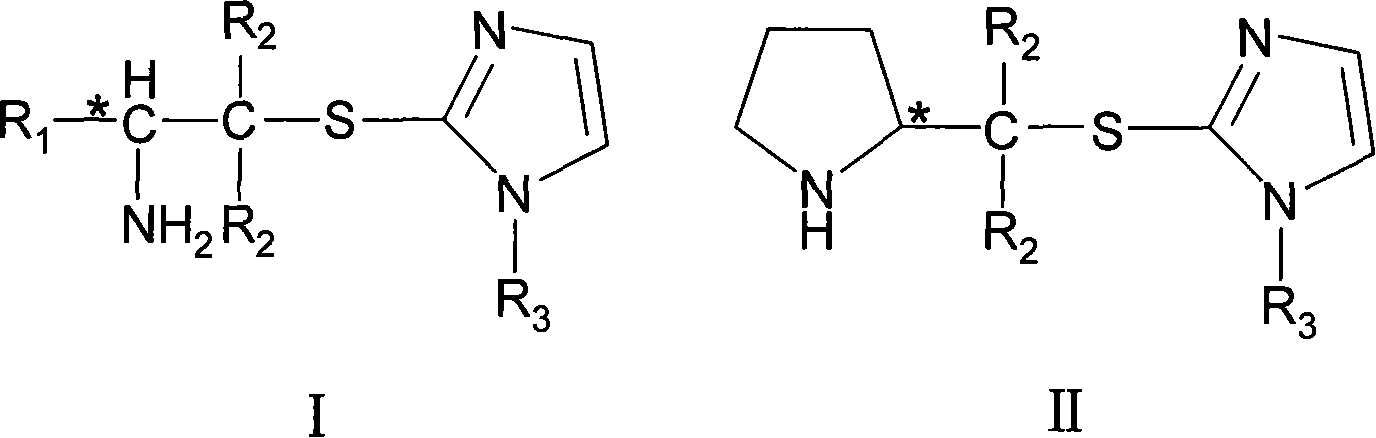
Chirality amine protonic acid salt containing imidazole sulfur ether structure and preparation method and usage thereof
ActiveCN101041637AChiralGood chiral induction propertiesOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesOrganic solventSulfur Ethers
The invention discloses a making method chiral amine protonic acid salt with general formula as III and IV, which comprises the following steps: dissolving chiral amine with imidazole sulfide structure as general formula I or II in the organic solvent; adding the solution of protonic acid HY into chiral amine solution; stirring to react completely; removing solvent; obtaining the product as chiral agent, catalyst and chiral material or ionic liquid.
Owner:ZHEJIANG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
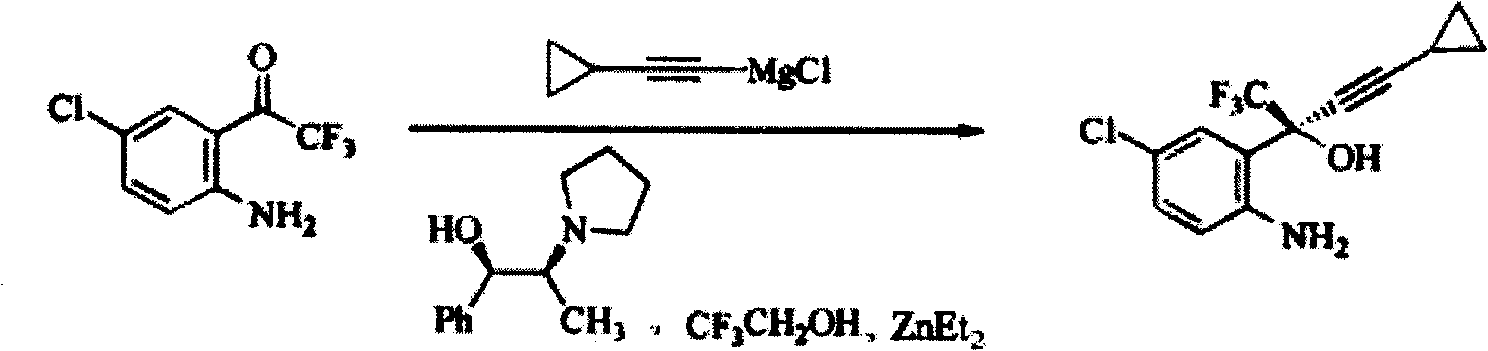
![Preparation method of (S, S)-8H-6H-pyrrolo [3, 4-b] pyridine Preparation method of (S, S)-8H-6H-pyrrolo [3, 4-b] pyridine](https://images-eureka.patsnap.com/patent_img/5bddebff-bd5c-4554-be69-d72e3113a71c/BDA00002489042500061.png)
![Preparation method of (S, S)-8H-6H-pyrrolo [3, 4-b] pyridine Preparation method of (S, S)-8H-6H-pyrrolo [3, 4-b] pyridine](https://images-eureka.patsnap.com/patent_img/5bddebff-bd5c-4554-be69-d72e3113a71c/BDA00002489042500062.png)
![Preparation method of (S, S)-8H-6H-pyrrolo [3, 4-b] pyridine Preparation method of (S, S)-8H-6H-pyrrolo [3, 4-b] pyridine](https://images-eureka.patsnap.com/patent_img/5bddebff-bd5c-4554-be69-d72e3113a71c/BDA00002489042500071.png)