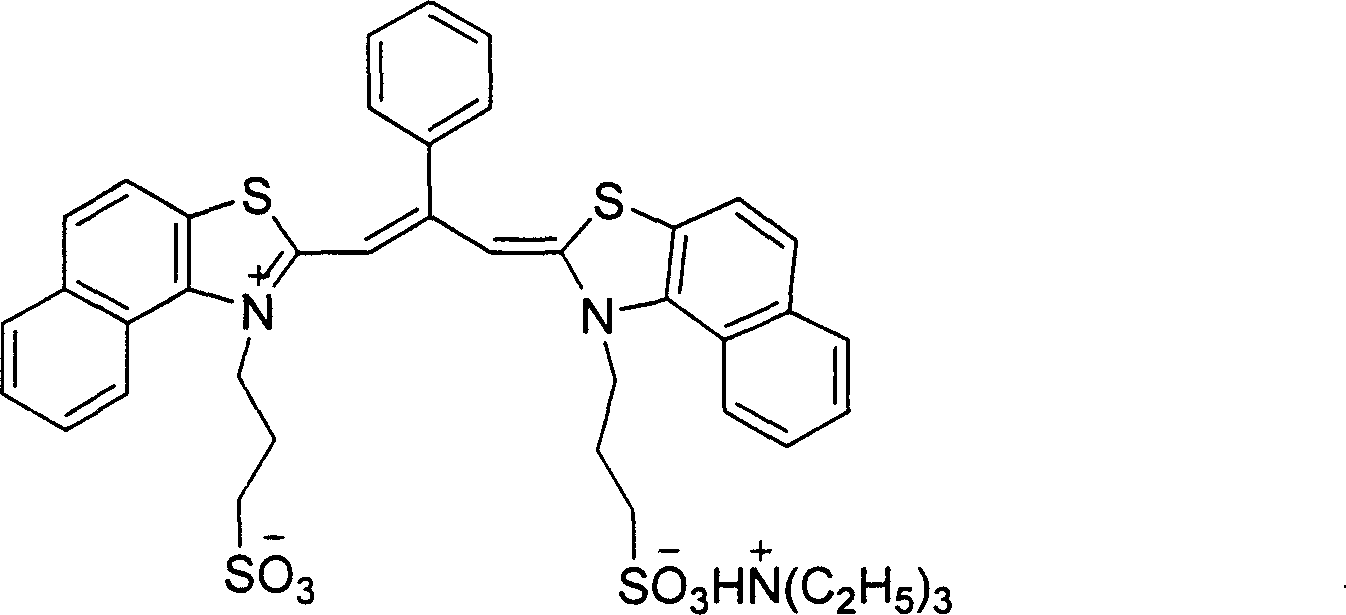
Method for conducting chiral induction to non-chiral cyanine dye aggregate using human serum protein
A technology of serum protein and chiral induction, which is applied in the direction of serum albumin, animal/human protein, albumin peptide, etc., to achieve the effect of shortening the reaction time and increasing the action rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
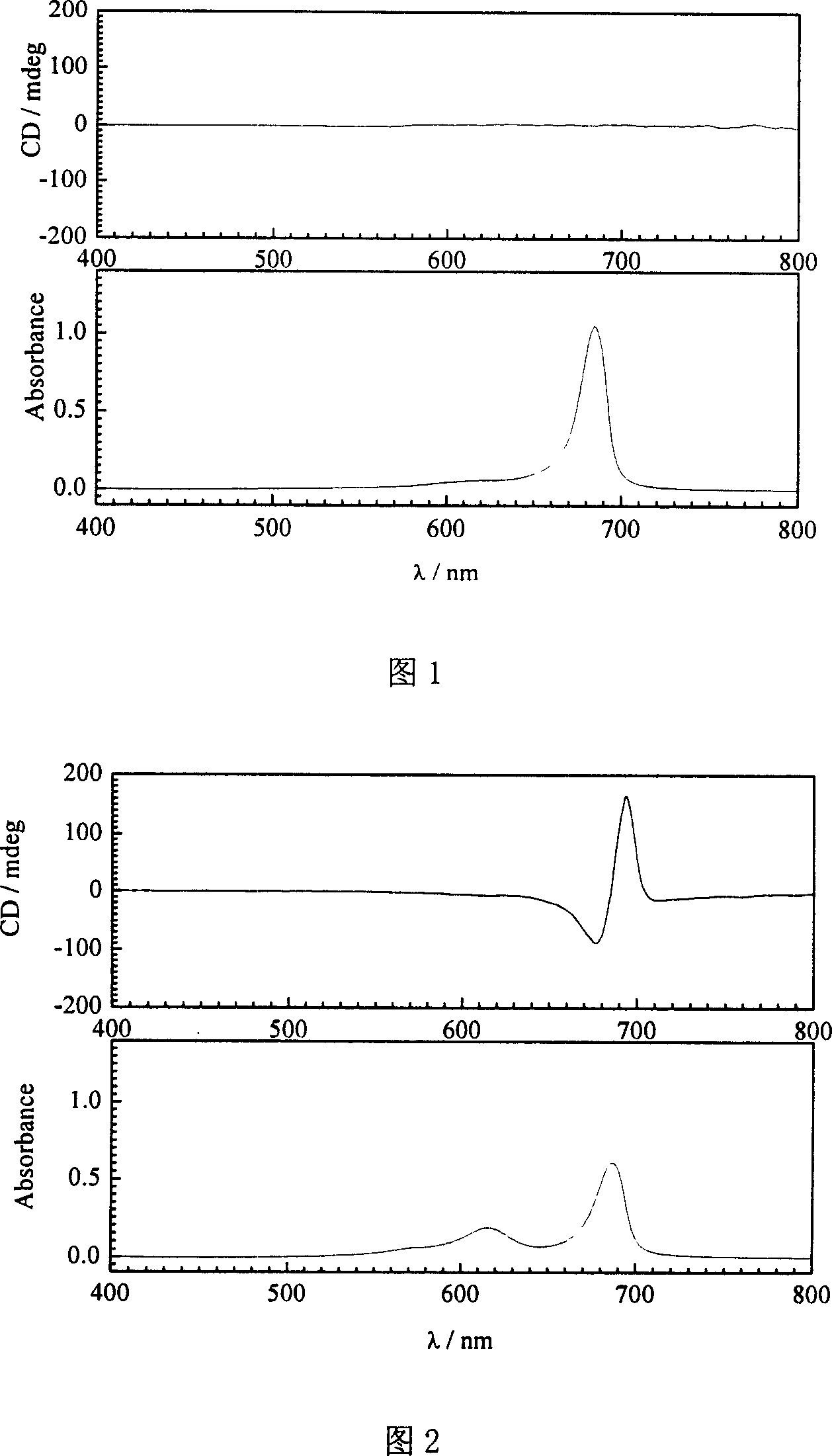
[0025] Add 0.5ml 80.0μM phosphate buffer solution (pH8.0) of achiral cyanine dye aggregates to a 10.00ml volumetric flask, then add 0.4ml 50.0μM phosphate buffer solution (pH8.0) of human serum albumin, in the mixture The molar concentration ratio of the human serum protein to the achiral cyanine dye aggregate is 0.5:1; then the mixed solution is diluted with phosphate buffer solution to a concentration of 5 μM of the achiral cyanine dye aggregate; after the mixed solution is shaken, After reacting in the dark for 12 hours, the induced cyanine dye aggregates were obtained. After UV-Vis absorption spectrum and circular dichroism analysis, as shown in Figure 2, the absorption peak (686nm) of the dye aggregates on the absorption spectrum decreased and appeared in the short wave direction. The characteristic peak (615nm) of the dye monomer, the signal of the aggregate appears on the circular dichroism spectrum, indicating that the dye aggregate produces photoactivity under the acti...
Embodiment 2
[0029] Add 0.5ml 80.0μM phosphate buffer solution (pH6.0) of achiral cyanine dye aggregates to a 10.00ml volumetric flask, then add 1.0ml 50.0μM phosphate buffer solution (pH6.0) of human serum albumin, in the mixture The molar concentration ratio of the human serum protein to the achiral cyanine dye aggregate is 1.125:1; then the mixed solution is diluted with phosphate buffer solution to a concentration of 5 μM of the achiral cyanine dye aggregate; after the mixed solution is shaken, After reacting in the dark for 9 hours, the induced cyanine dye aggregates were obtained. After UV-Vis absorption spectrum and circular dichroism analysis, the absorption peak (686nm) of the dye aggregates in the absorption spectrum decreased, and the characteristic peak of the dye monomer appeared in the short wave direction. (615nm), the signals of aggregates appear on the circular dichroism spectrum, indicating that the dye aggregates produce photoactivity under the action of human serum album...
Embodiment 3
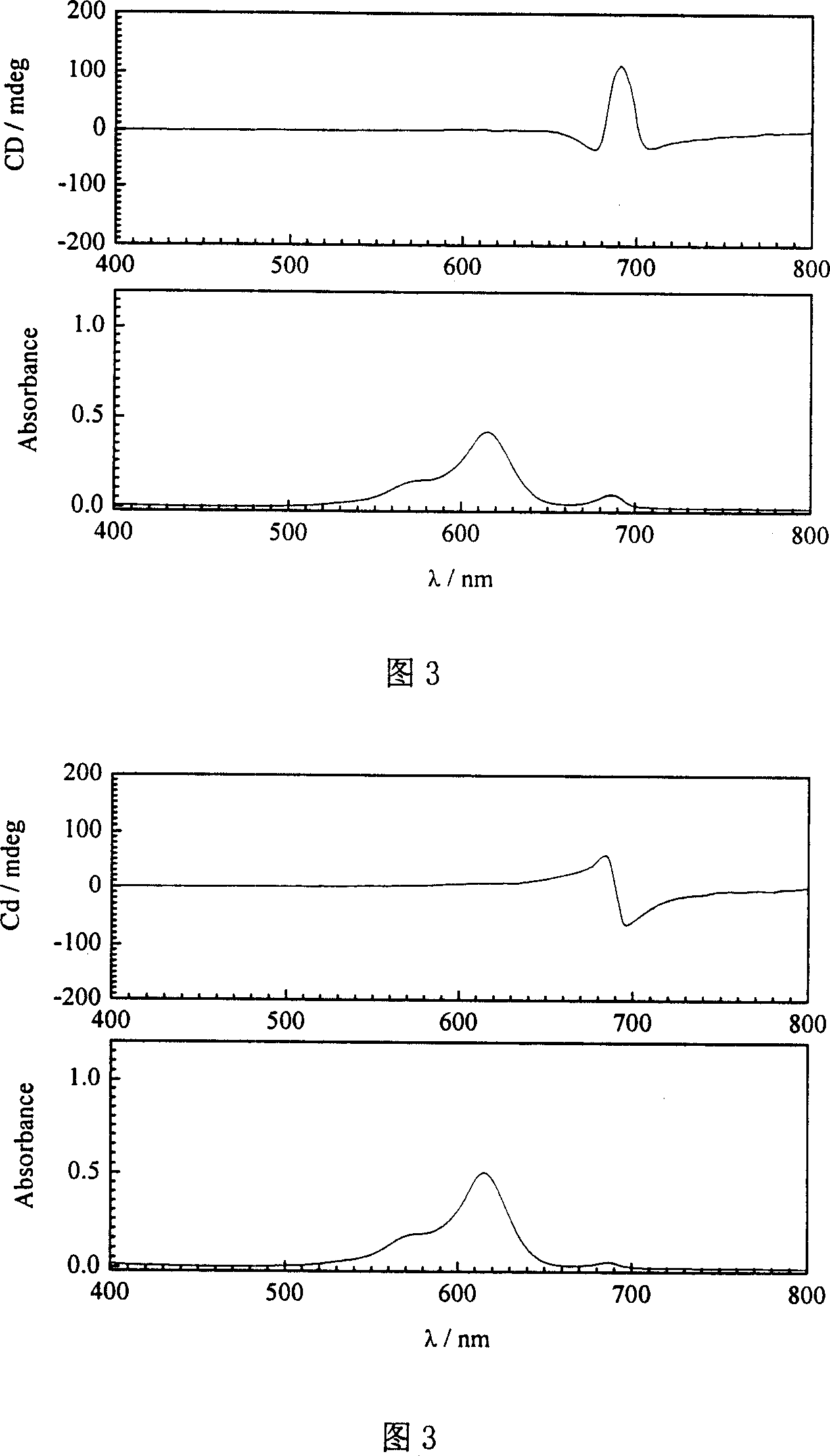
[0031] Add 0.5ml 80.0μM phosphate buffer solution (pH7.0) of achiral cyanine dye aggregates in a 10.00ml volumetric flask, then add 1.6ml 50.0μM phosphate buffer solution (pH7.0) of human serum albumin, the mixture The molar concentration ratio of the human serum protein to the achiral cyanine dye aggregate is 2:1; then the mixed solution is diluted with phosphate buffer solution to a concentration of 5 μM of the achiral cyanine dye aggregate; after the mixed solution is shaken well, After 15 hours of light-shielding reaction, the induced cyanine dye aggregates were obtained. After UV-Vis absorption spectrum and circular dichroism analysis, as shown in Figure 3, the absorption peak (686nm) of the dye aggregates on the absorption spectrum was significantly reduced. A strong characteristic peak (615nm) of the dye monomer appears, and aggregate signals appear on the circular dichroism spectrum, indicating that the dye aggregate produces photoactivity under the action of human seru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com