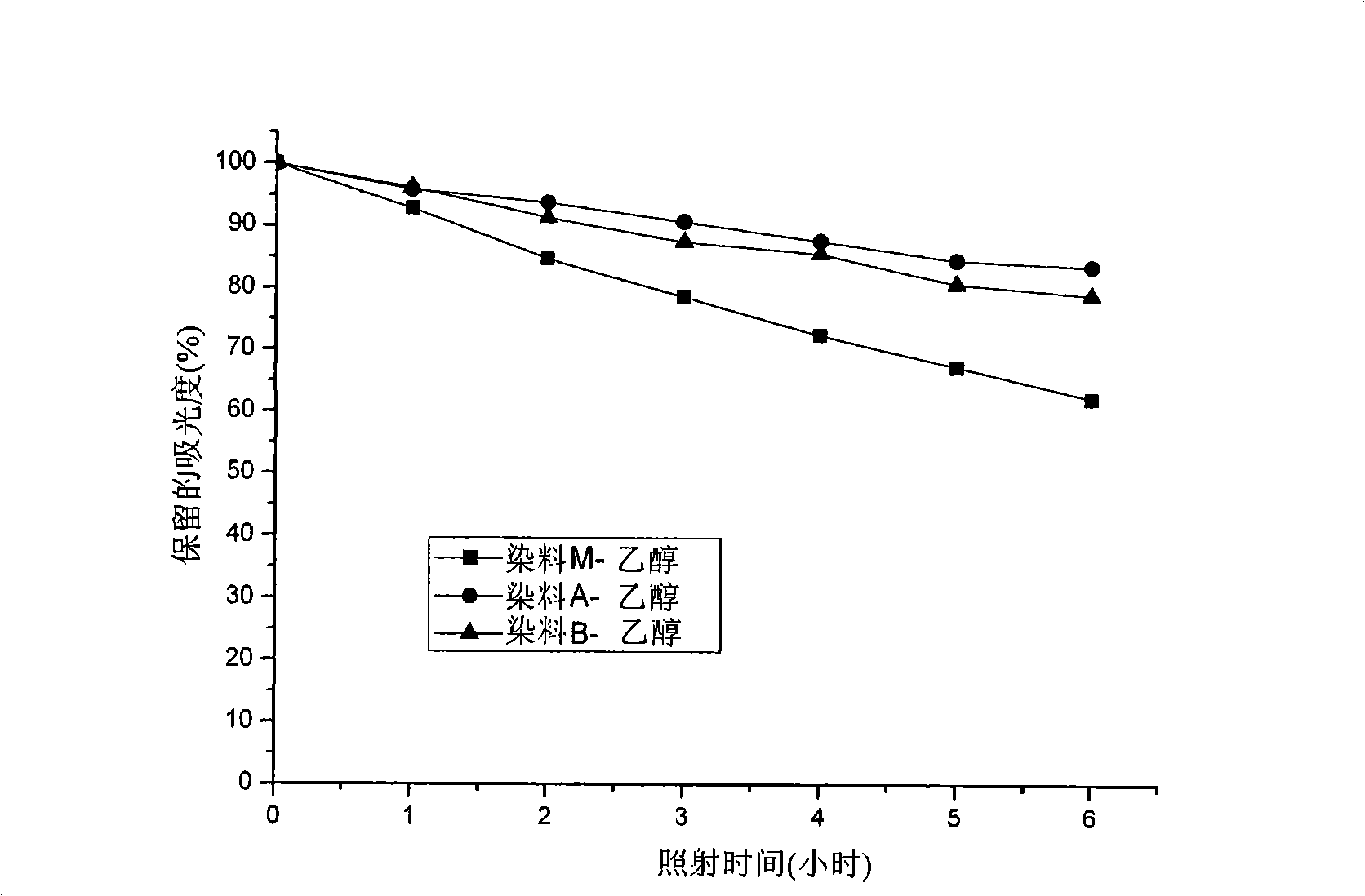
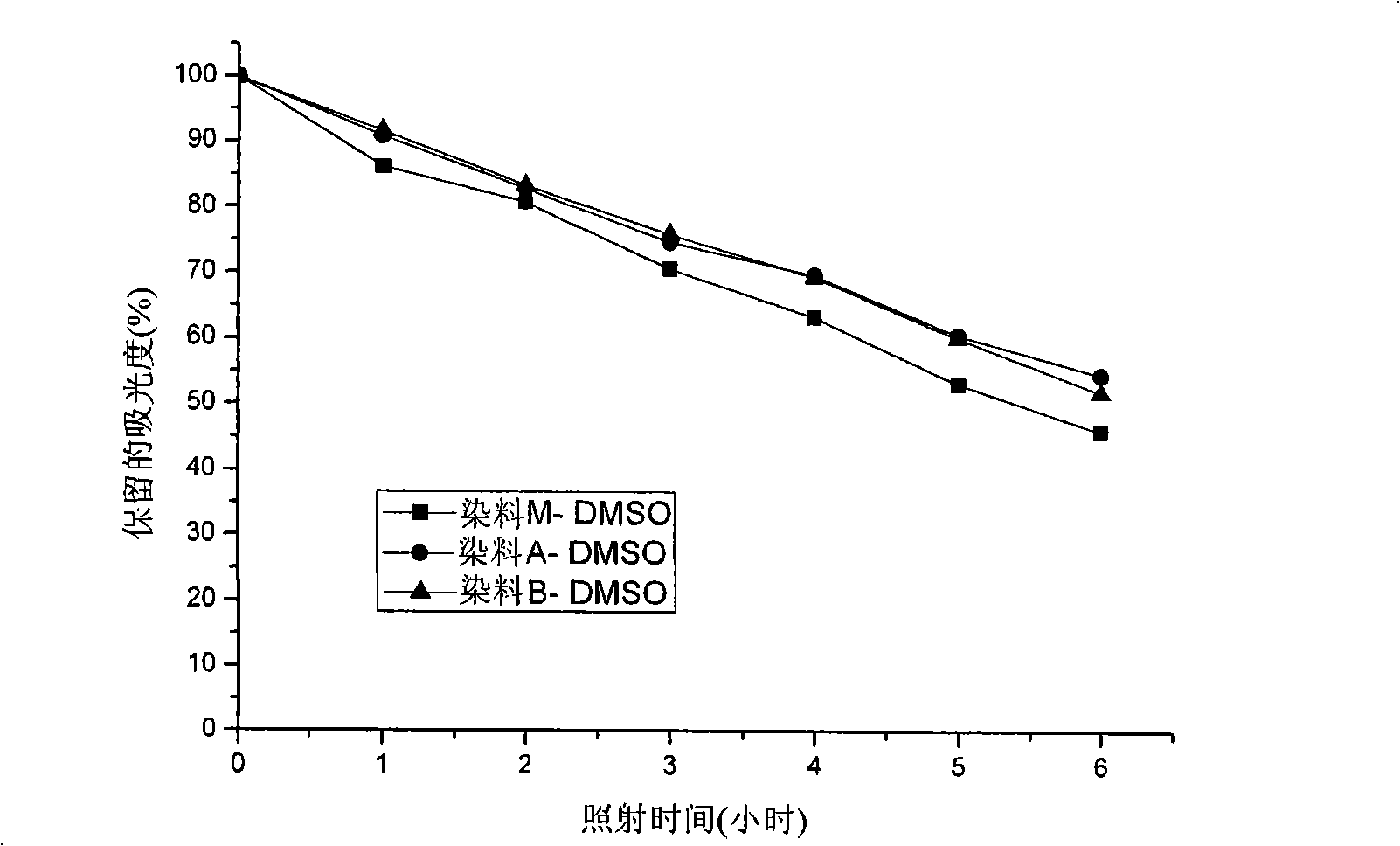
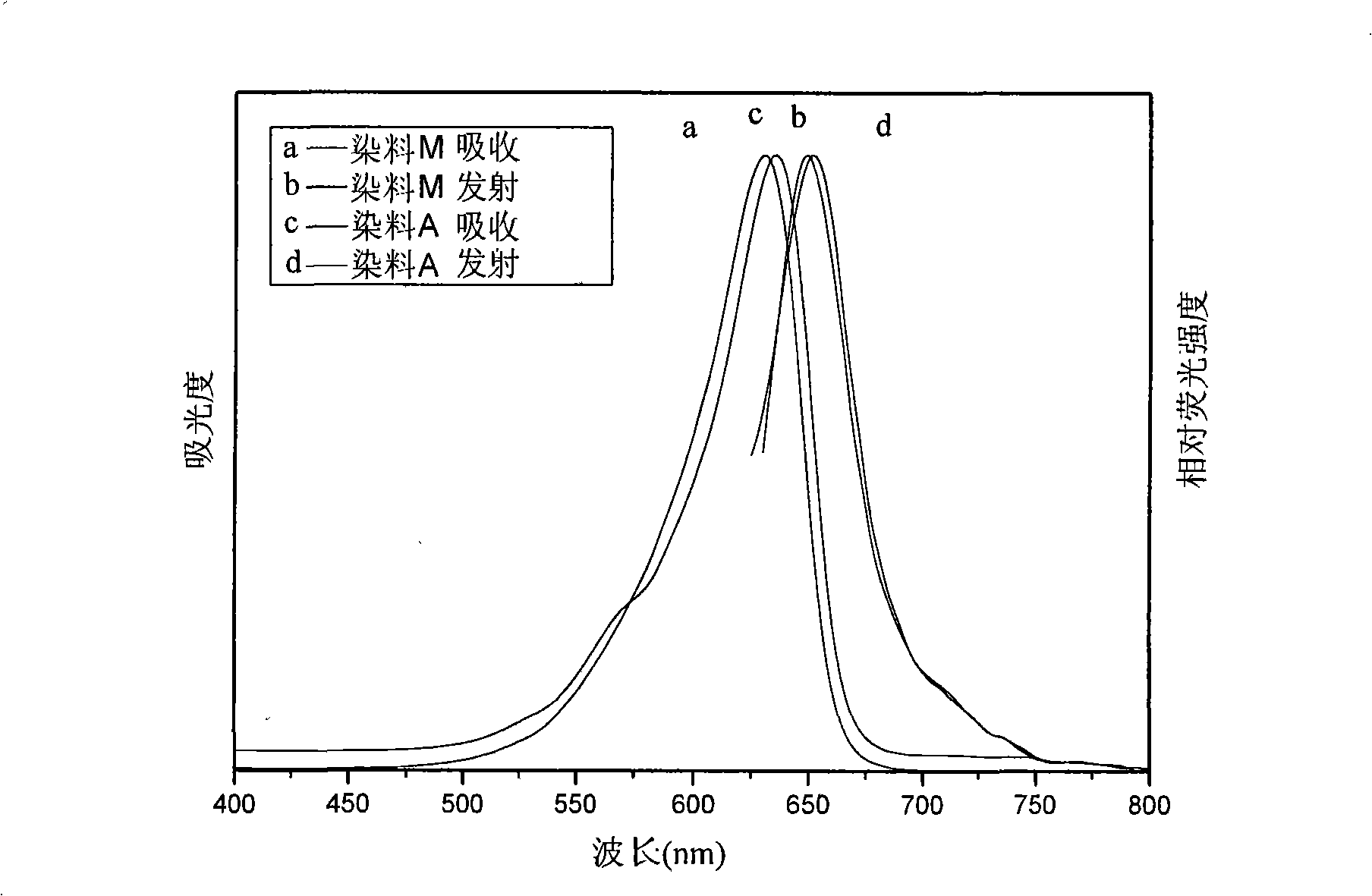
Unsymmetrical cyanines fluorochrome
A technology of fluorescent dyes and dyes, which is applied in the direction of organic dyes, methine/polymethine dyes, analytical materials, etc., and can solve the problems of expensive and high equipment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Synthesis of intermediate 1-benzyl-2-methylbenzothiazole quaternary ammonium salt
[0093] Add 20mmol of 2-methylbenzothiazole and 22mmol of benzyl bromide into a 50ml round bottom flask containing 20ml of toluene under argon protection. The reaction was stopped by heating to reflux for 24 hours. After the mixture was cooled, the precipitate was filtered and the filter cake was washed with ether. After drying, a pale pink solid powder was obtained with a crude yield of 58%.
Embodiment 2
[0095] Preparation of Compound A
[0096]
[0097] In 60ml of acetic acid, 10mmol of 1-benzyl-2-methylbenzothiazole quaternary ammonium salt and 30mmol of N,N'-diphenylformamidine were heated and stirred on an oil bath at 90°C for 1.5 hours. The red oil obtained by the reaction was suspended and washed 3 times with petroleum ether to remove acetic acid. Then add a certain amount of ether to precipitate an orange solid powder, filter and dry. The crude product was separated through a silica gel column, and the yellow component was collected with the eluent dichloromethane:methanol=100:3, and the yield was 42%. 4 mmol of 1-benzyl-4-methylquinoline quaternary ammonium salt and 10 ml of pyridine were added thereto, and heated and stirred on an oil bath at 90° C. for 1.5 hours. The reaction solution was poured into ether, and small dark purple particles were precipitated, which were filtered and dried. The dye was separated by silica gel column, and the eluent was dichlorom...
Embodiment 3
[0100] Preparation of Compound B
[0101]
[0102] In 60ml of acetic acid, heat and stir 10mmol 1-(4-fluoro)-benzyl-2-methylbenzothiazole quaternary ammonium salt and 30mmol N,N'-diphenylformamidine on an oil bath at 90°C for 1.5 hours . The red oil obtained by the reaction was suspended and washed 3 times with petroleum ether to remove acetic acid. Then add a certain amount of ether to precipitate an orange solid powder, filter and dry. The crude product was separated by a silica gel column, and the yellow component was collected with the eluent dichloromethane:methanol=100:3, and the yield was 47%. 5 mmol of 1-(4-fluoro)-benzyl-4-methylquinoline quaternary ammonium salt and 10 ml of pyridine were added thereto, and heated and stirred on an oil bath at 90° C. for 1.5 hours. The reaction solution was poured into ether, and small dark purple particles were precipitated, which were filtered and dried. The dye was separated by silica gel column, and the eluent was dichlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com