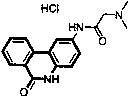
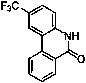
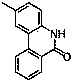
Patents
Literature
111 results about "Phenanthridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
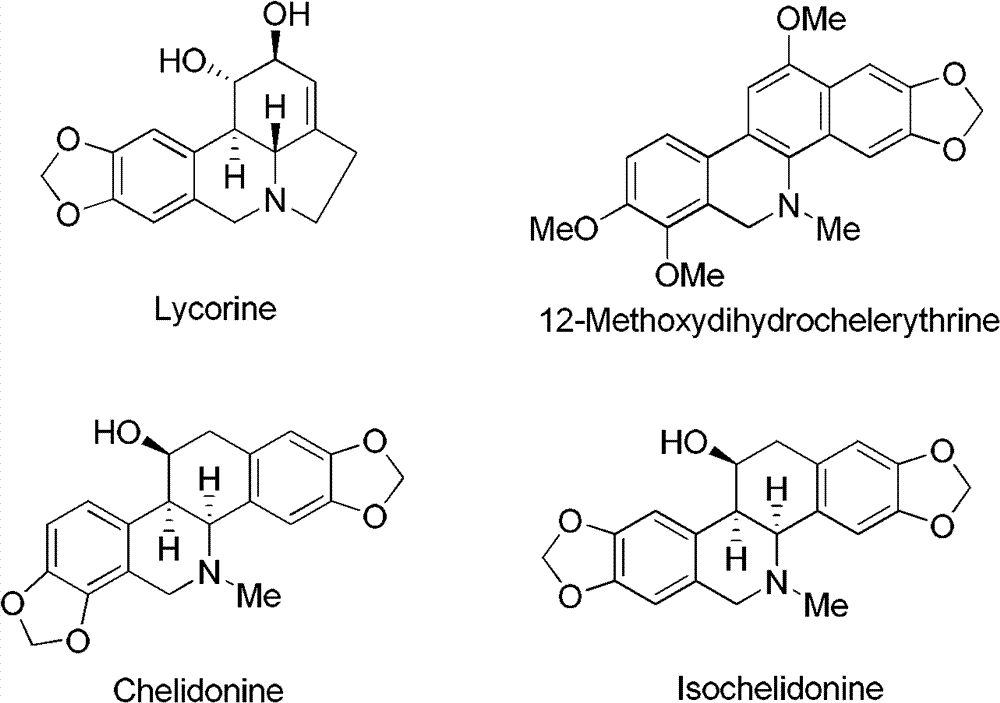
Phenanthridine is a nitrogen heterocyclic compound that is the basis of DNA-binding fluorescent dyes through intercalation. Examples of such dyes are ethidium bromide and propidium iodide. Acridine is an isomer of phenanthridine.
Preparation method for phenanthridine derivative
InactiveCN102827077AStrong designabilityHigh yieldGroup 5/15 element organic compoundsOrganic solventPalladium catalyst
The invention discloses a preparation method for a phenanthridine derivative. The preparation method comprises the following steps of: adding a catalyst, an oxidant, acid, a 2-phenylaniline compound and an olefin compound into organic solvent; after reaction, carrying out post treatment to obtain the phenanthridine derivative, wherein the catalyst is a bivalent palladium catalyst. According to the preparation method for the phenanthridine derivative disclosed by the invention, the adoption of reagents with high toxicity and high corrosiveness is avoided. The preparation method has the advantages of environment friendliness, easiness in operation, higher yield and simplicity and convenience in post treatment. In addition, a substrate obtained by the method is strong in designability and can be used for designing to synthesize a compound with the required structure according to actual requirements, and thus stronger practicability is realized.
Owner:ZHEJIANG UNIV
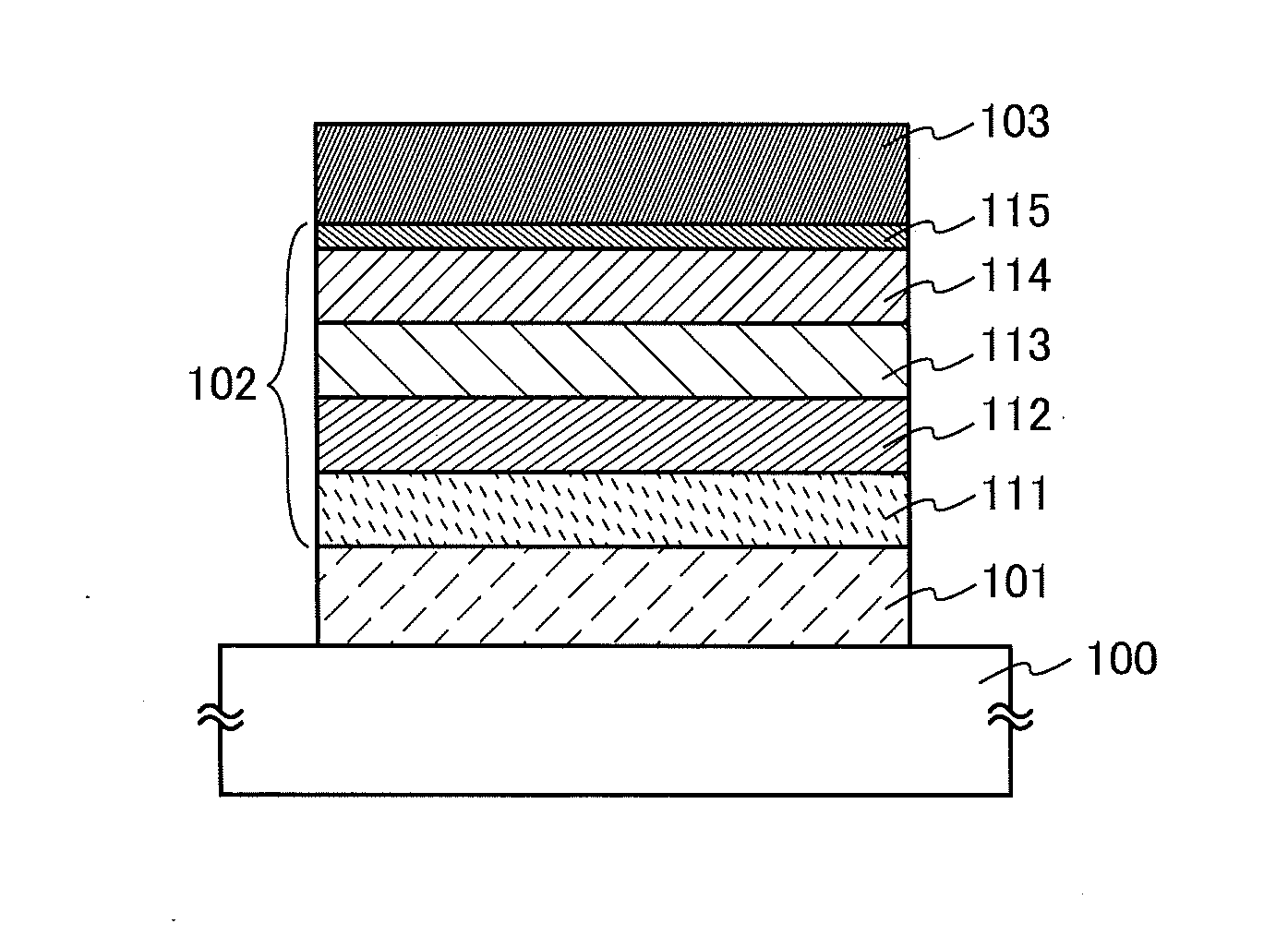
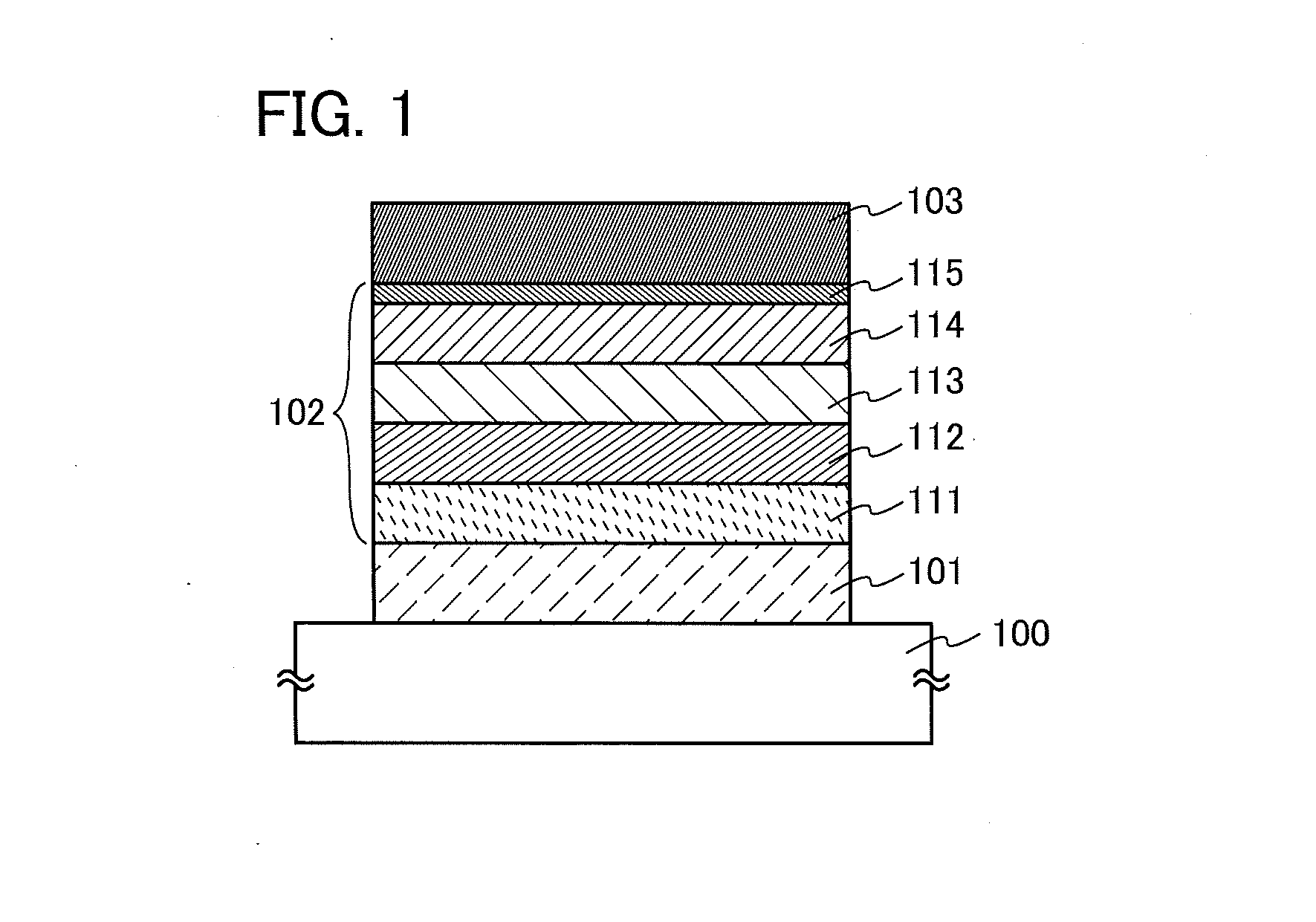
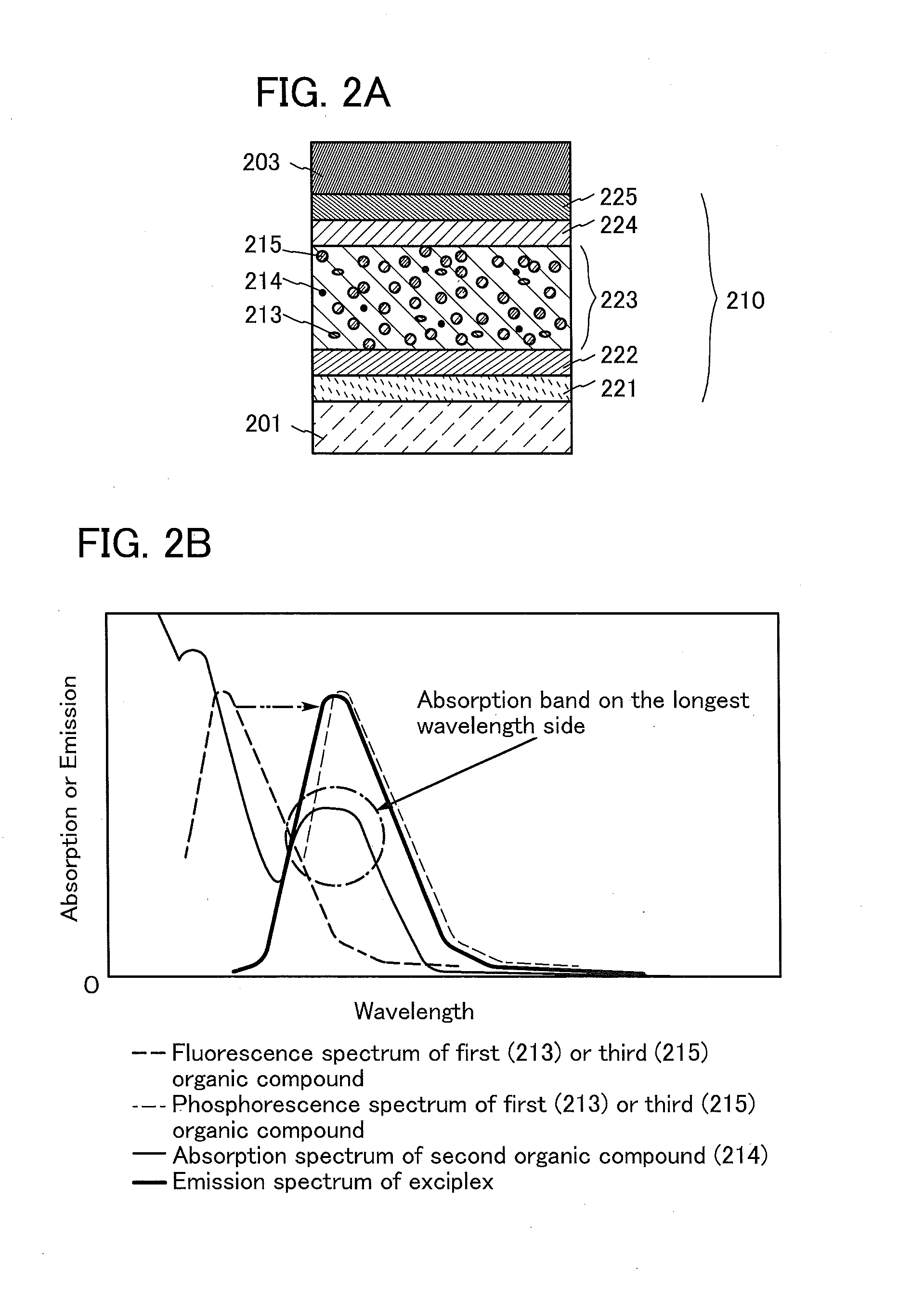
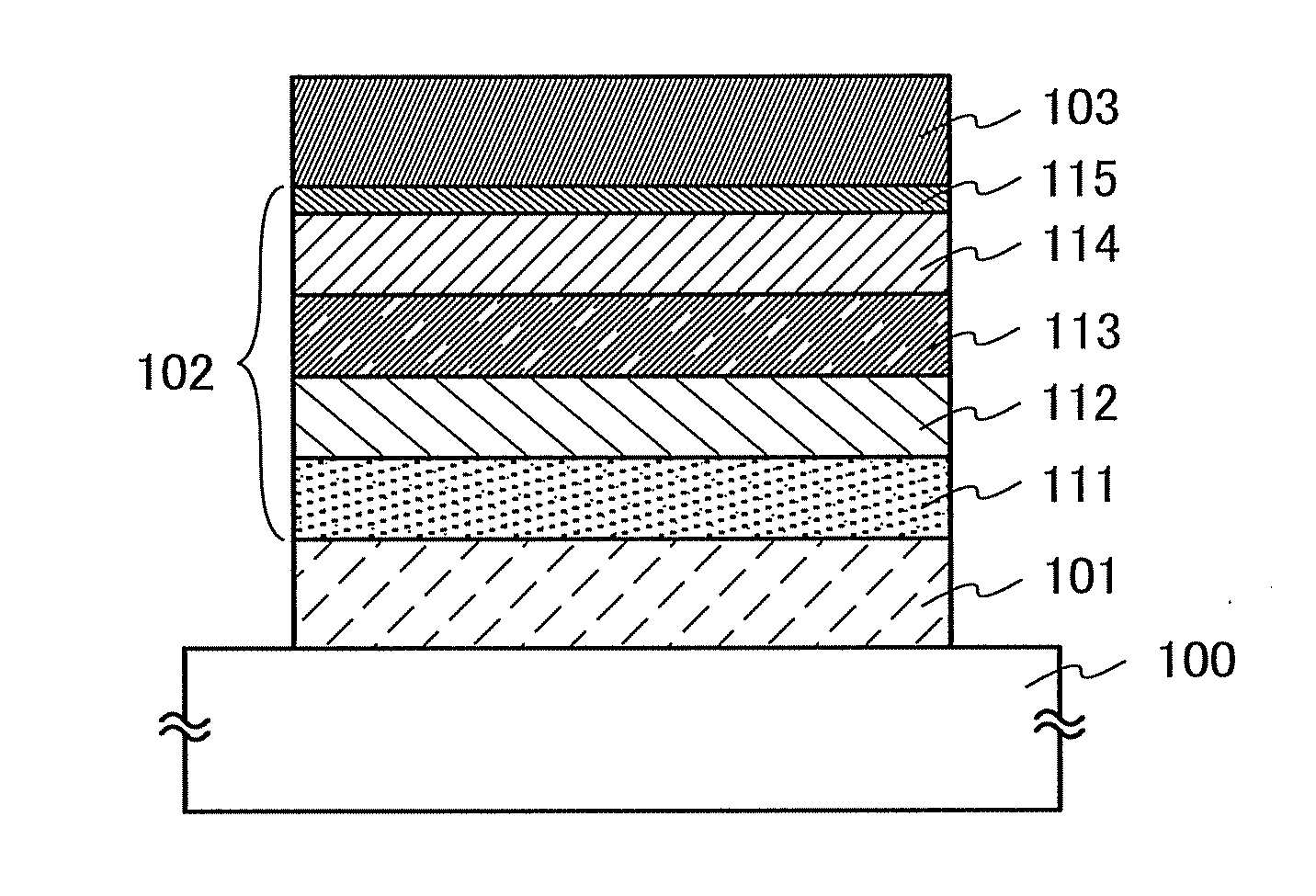
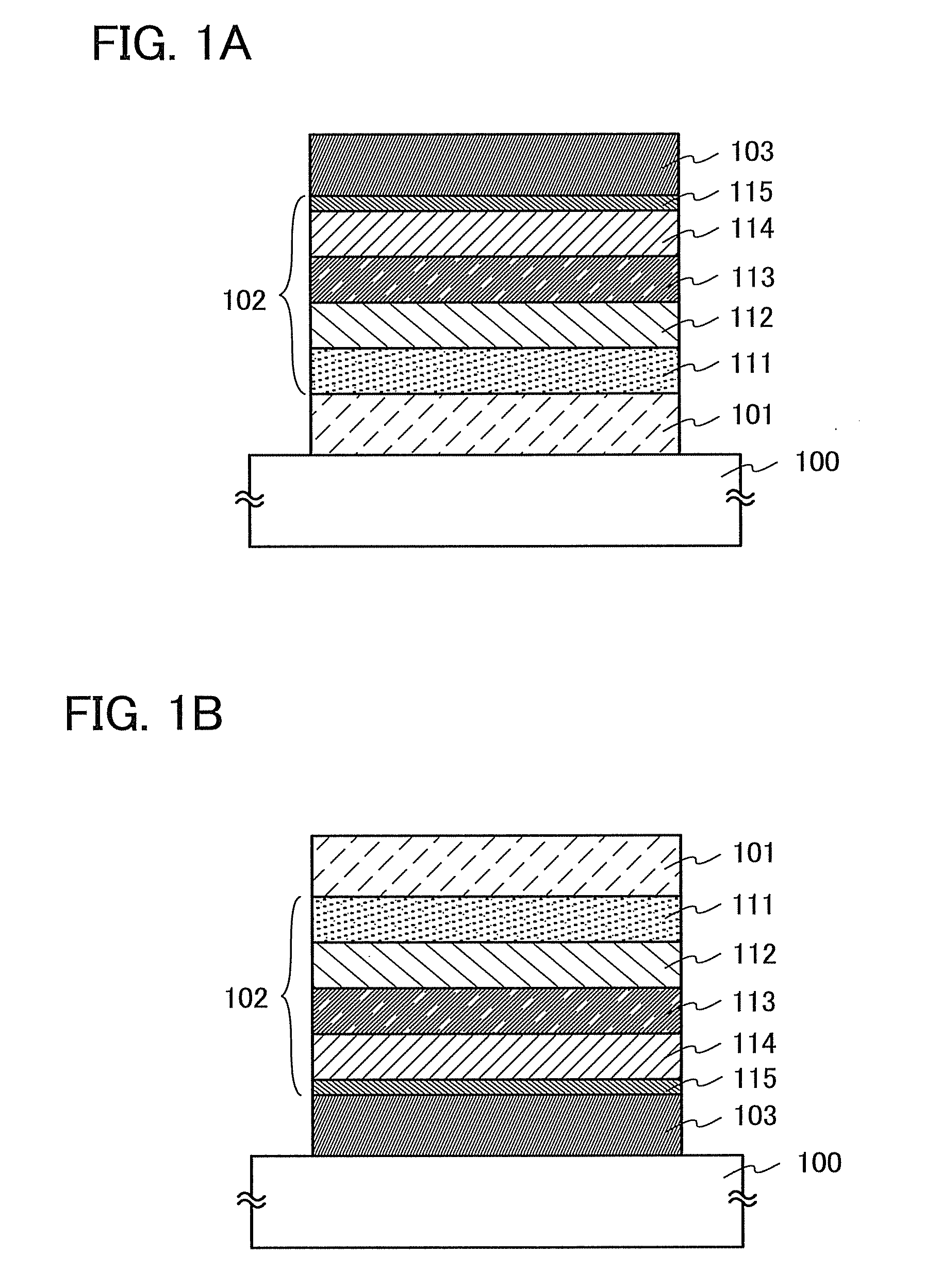
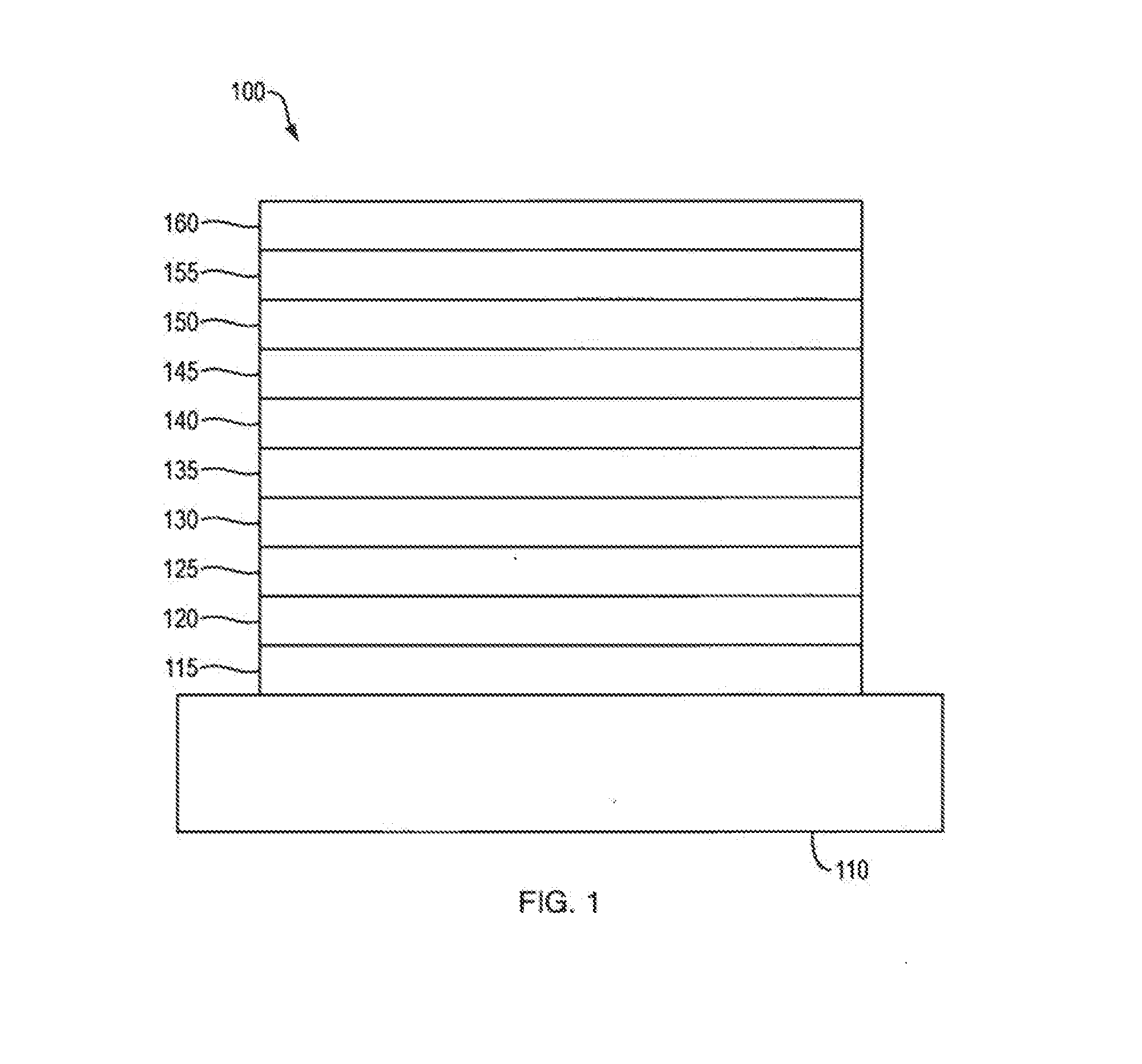
Organic Compound, Light-Emitting Element, Light-Emitting Device, Electronic Device, and Lighting Device
InactiveUS20140014930A1Excellent characteristicsImprove efficiencyOrganic chemistrySolid-state devicesSimple Organic CompoundsDibenzofuran
A novel organic compound with which the emission characteristics, emission efficiency, and reliability of a light-emitting element can be improved is provided. The organic compound has an imidazo[1,2-f]phenanthridine skeleton and a dibenzothiophene skeleton or a dibenzofuran skeleton bonded through an arylene group. The light-emitting element including the organic compound in a light-emitting layer shows high efficiency and low power consumption.
Owner:SEMICON ENERGY LAB CO LTD
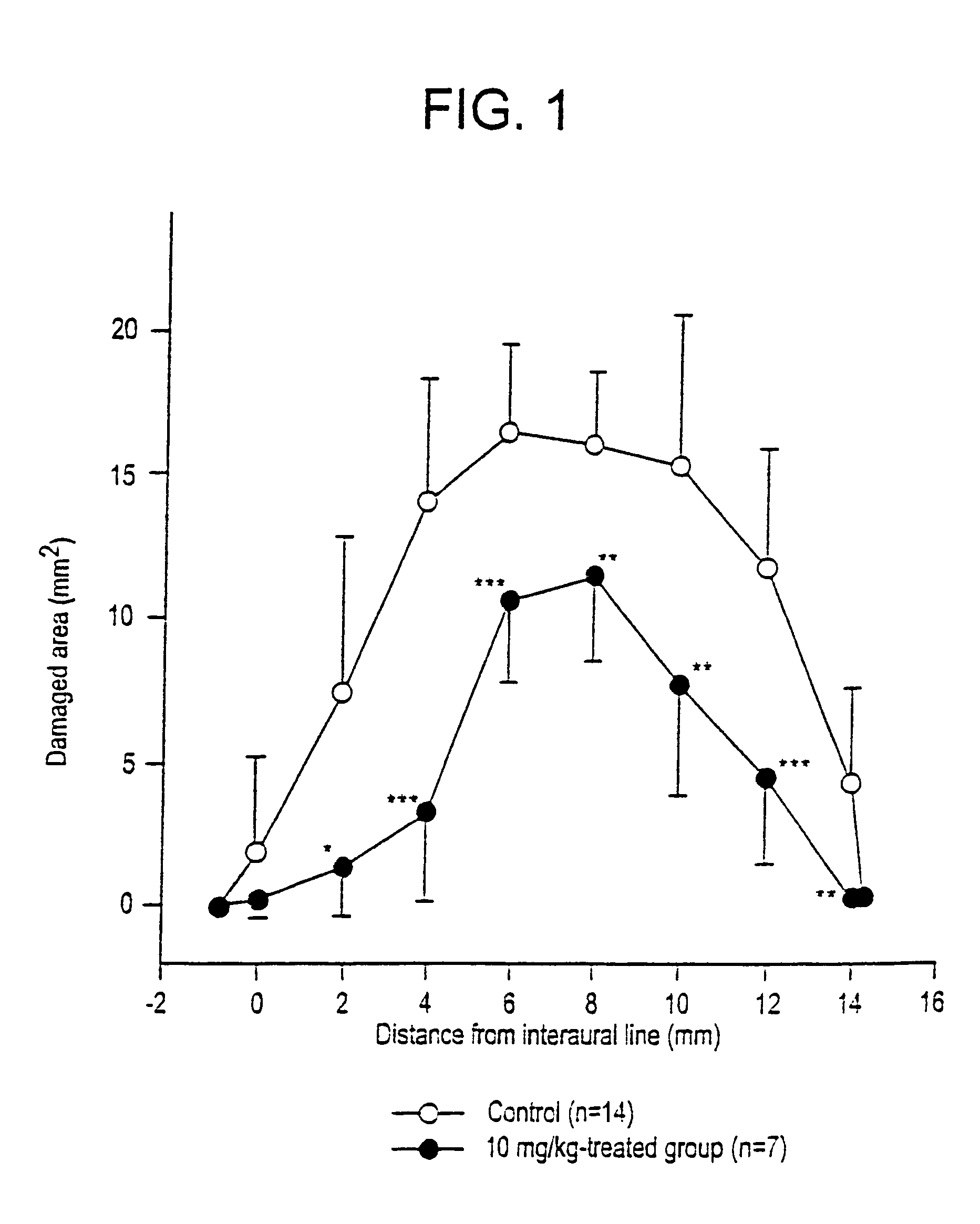
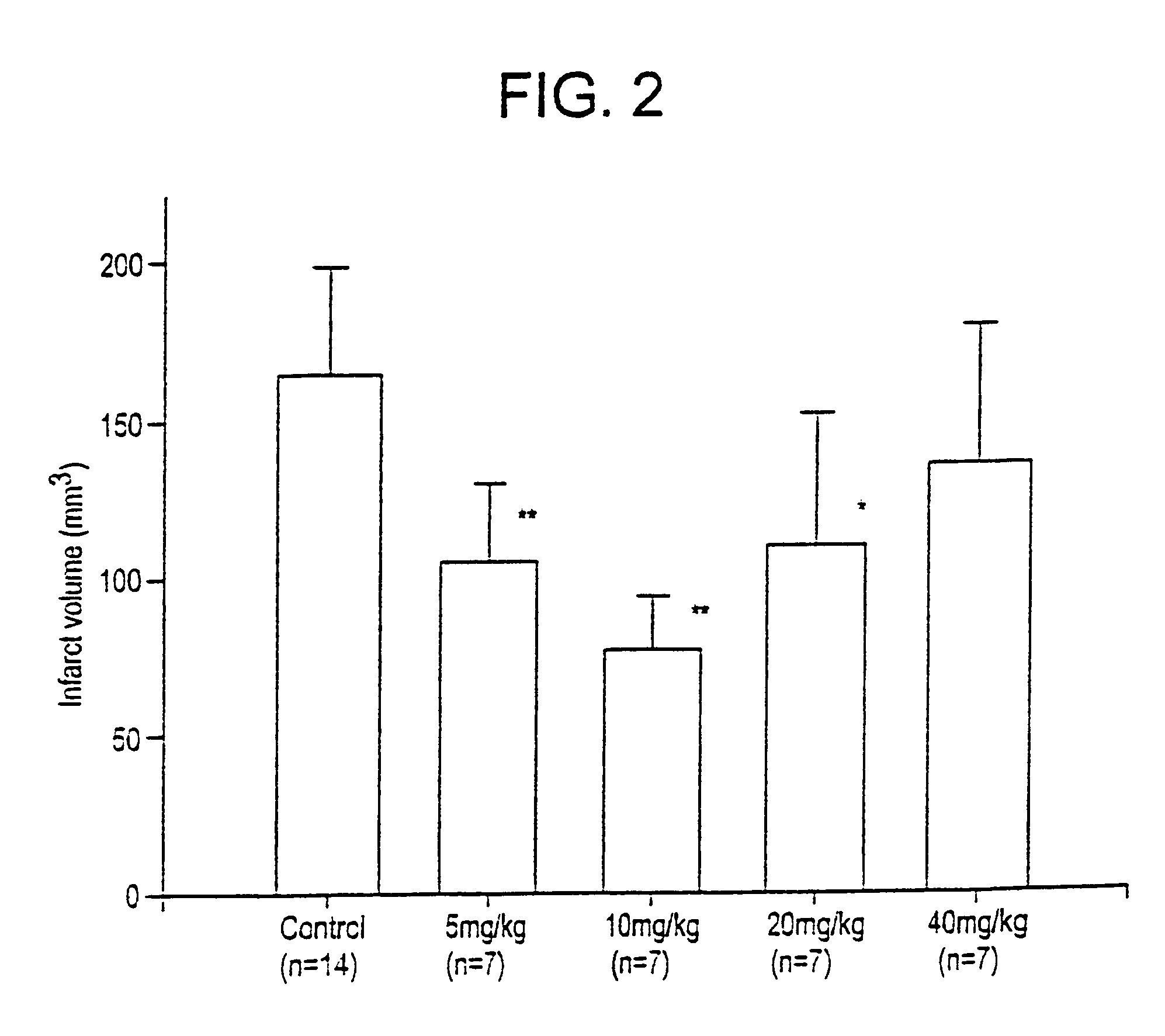
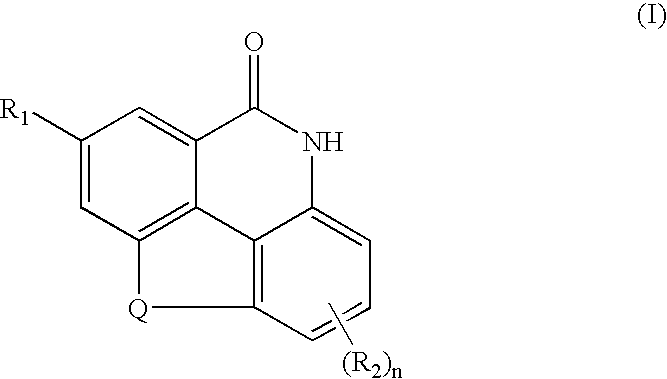
Benzo [c] phenanthridines as antimicrobial agents
ActiveUS20120022061A1Reduce polymerizationAntibacterial agentsBiocideCell divisionBenzo(c)phenanthrene
The present invention provides compounds of formula I: formula (I) wherein X1-X4 and R1-R12 have any of the values defined in the specification, as well as salts and prodrugs thereof, which inhibit major molecular mechanisms associated with bacterial cell division and proliferation so as to be useful for the treatment and / or prevention of bacterial infections. The invention also provides compositions comprising these compounds as well as methods for using these compounds to inhibit bacterial cell division and proliferation and to treat bacterial infections.
Owner:RUTGERS THE STATE UNIV
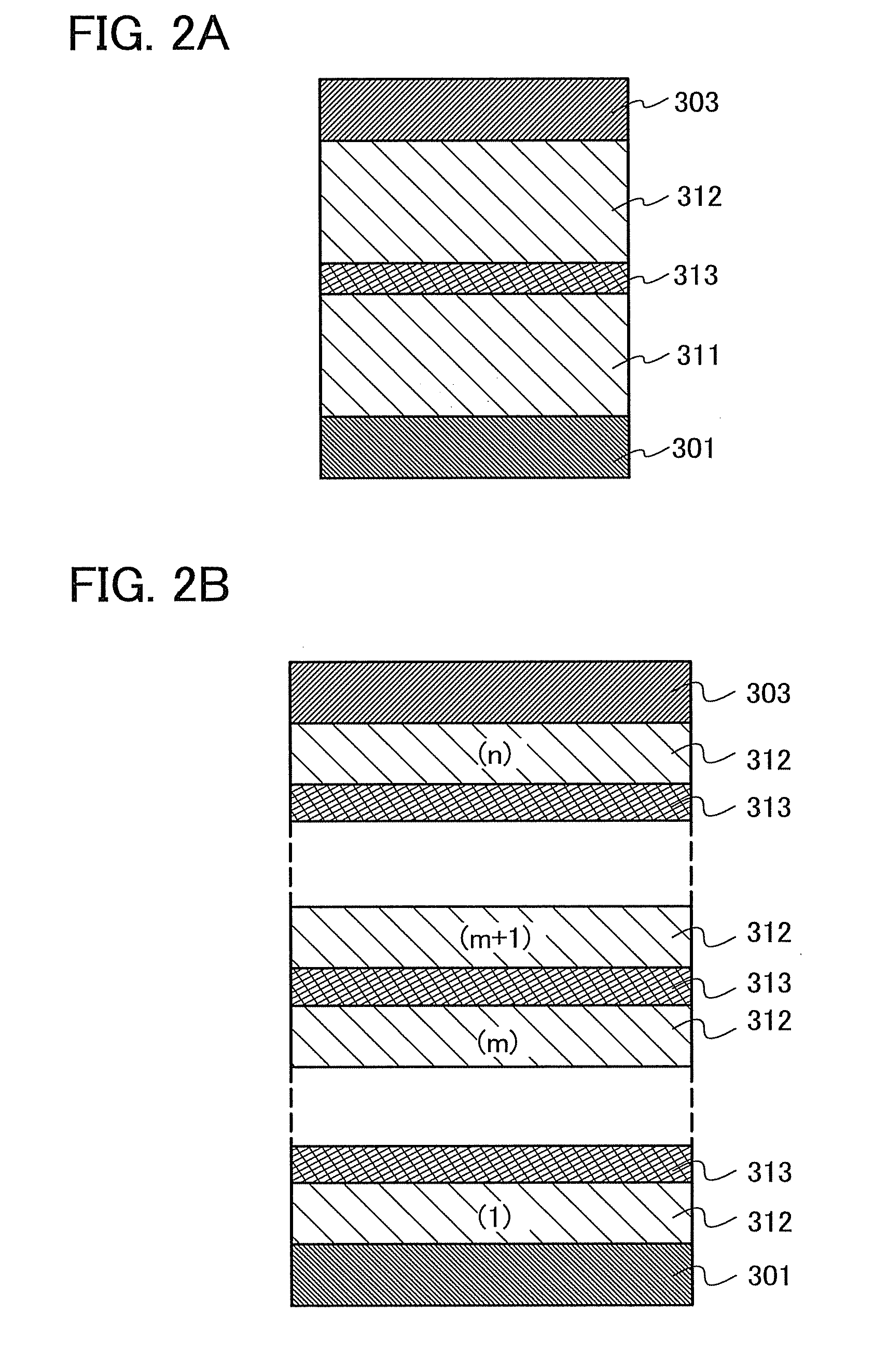
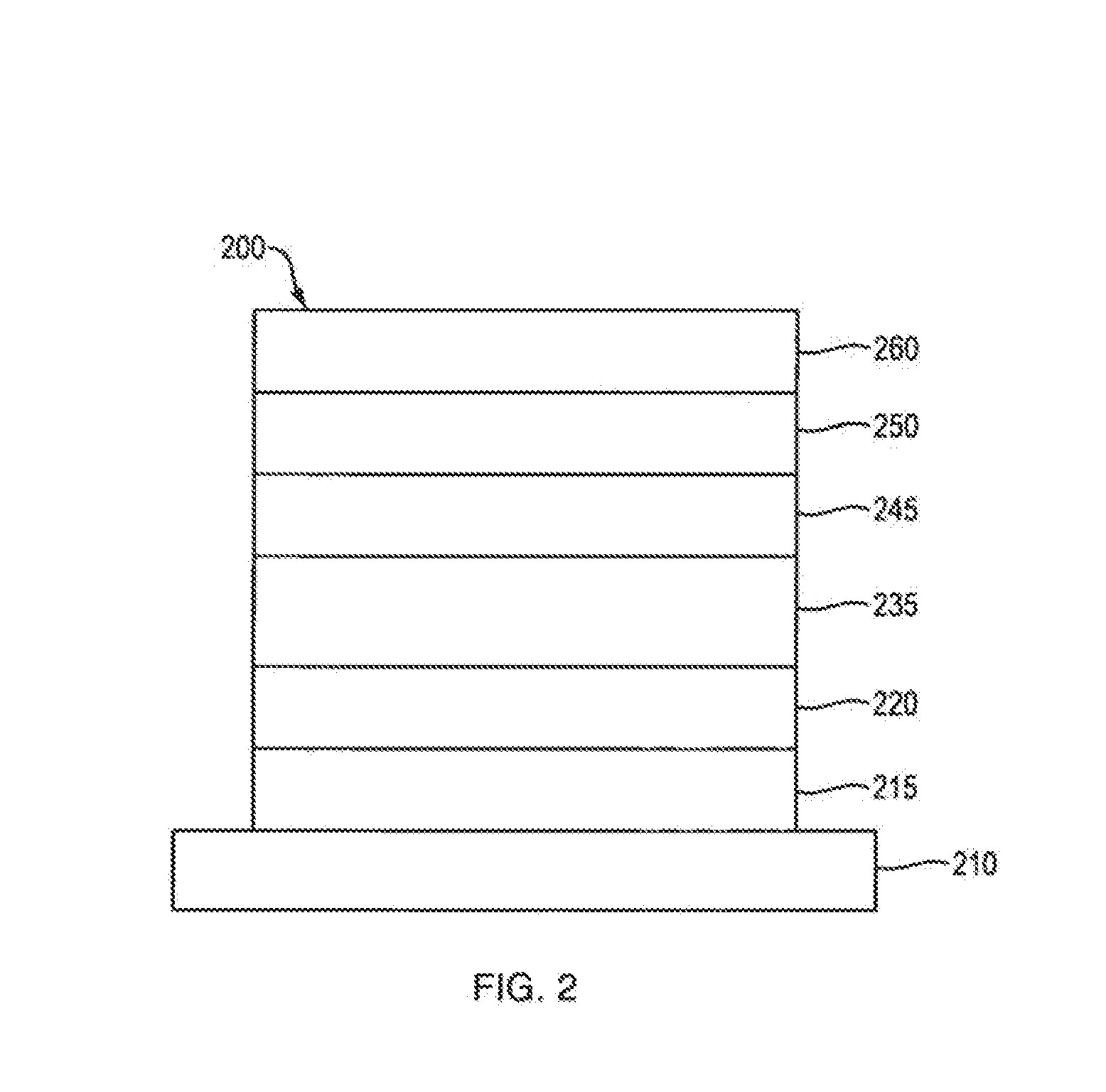
Triazole Derivative, Heterocyclic Compound, Light-Emitting Element, Light-Emitting Device, Electronic Device and Lighting Device
ActiveUS20120025697A1Excellent hole injectionHigh triplet excitation energyOrganic chemistryDischarge tube luminescnet screensIsoquinolineQuinoline
A substance that facilitates hole injection and has high triplet excitation energy is provided. A light-emitting element having high emission efficiency is provided. A light-emitting element driven with a low voltage is provided. Or a light-emitting element having a long lifetime is provided. Provided is a light-emitting element including a triazolo[4,3-f]phenanthridine derivative or a triazolo[3,4-a]isoquinoline derivative. Provided is a triazolo[4,3-f]phenanthridine and triazolo[3,4-a]isoquinoline derivatives, which are novel and can be used for the light-emitting element.
Owner:SEMICON ENERGY LAB CO LTD
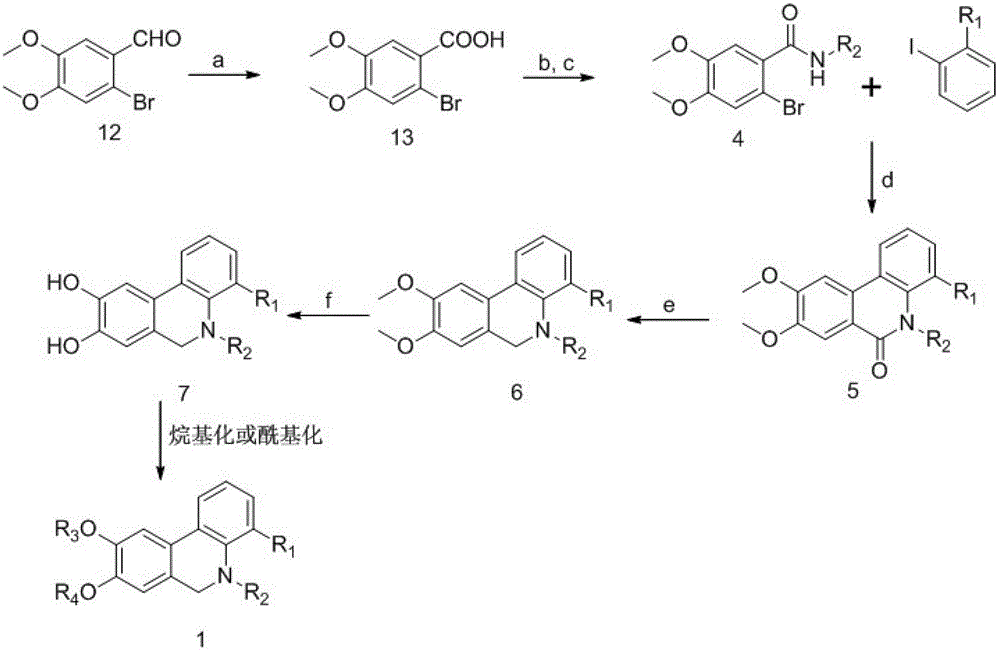
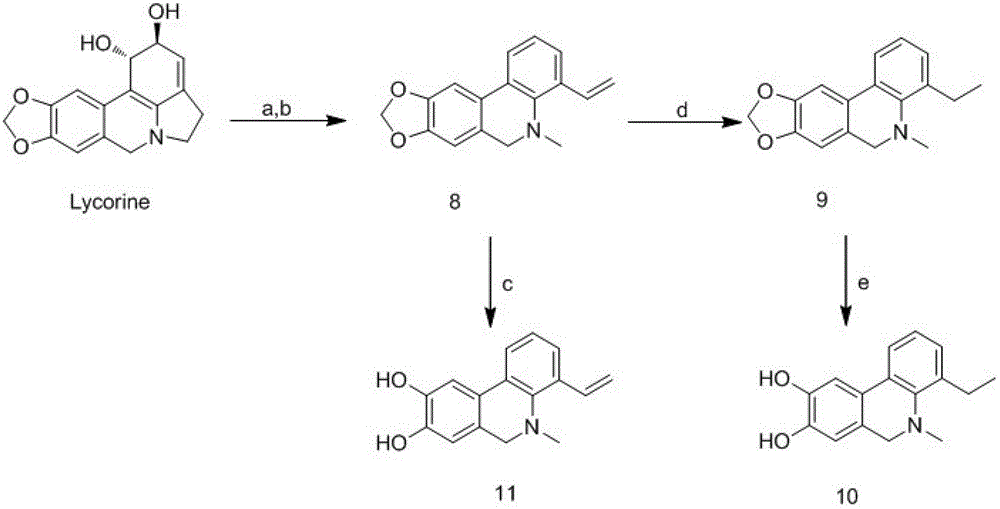
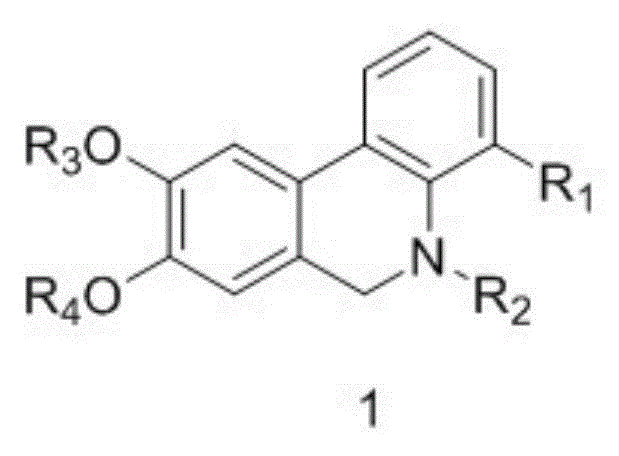
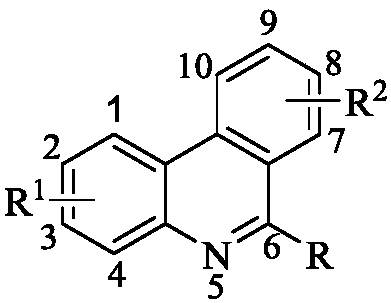
Phenanthridine derivative as well as medicinal composition, preparation method and application thereof
The invention provides a phenanthridine compound with an effect of resisting hepatitis B and hepatitis C, a medicinal composition taking the same as an active medicine component, a preparation method and applications of the phenanthridine compound in preparing a medicine for resisting hepatitis C viruses, and preparing an anti-virus medicine. According to the invention, the activity of benzylphenethylamine alkaloid in resisting various viruses is discovered in a process of studying a plant anti-virus natural product, and an obvious effect in resisting viruses of hepatitis B and hepatitis C of a phenanthridine derivative is discovered through structure modification, structure-function relationship and structure optimization.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI +1
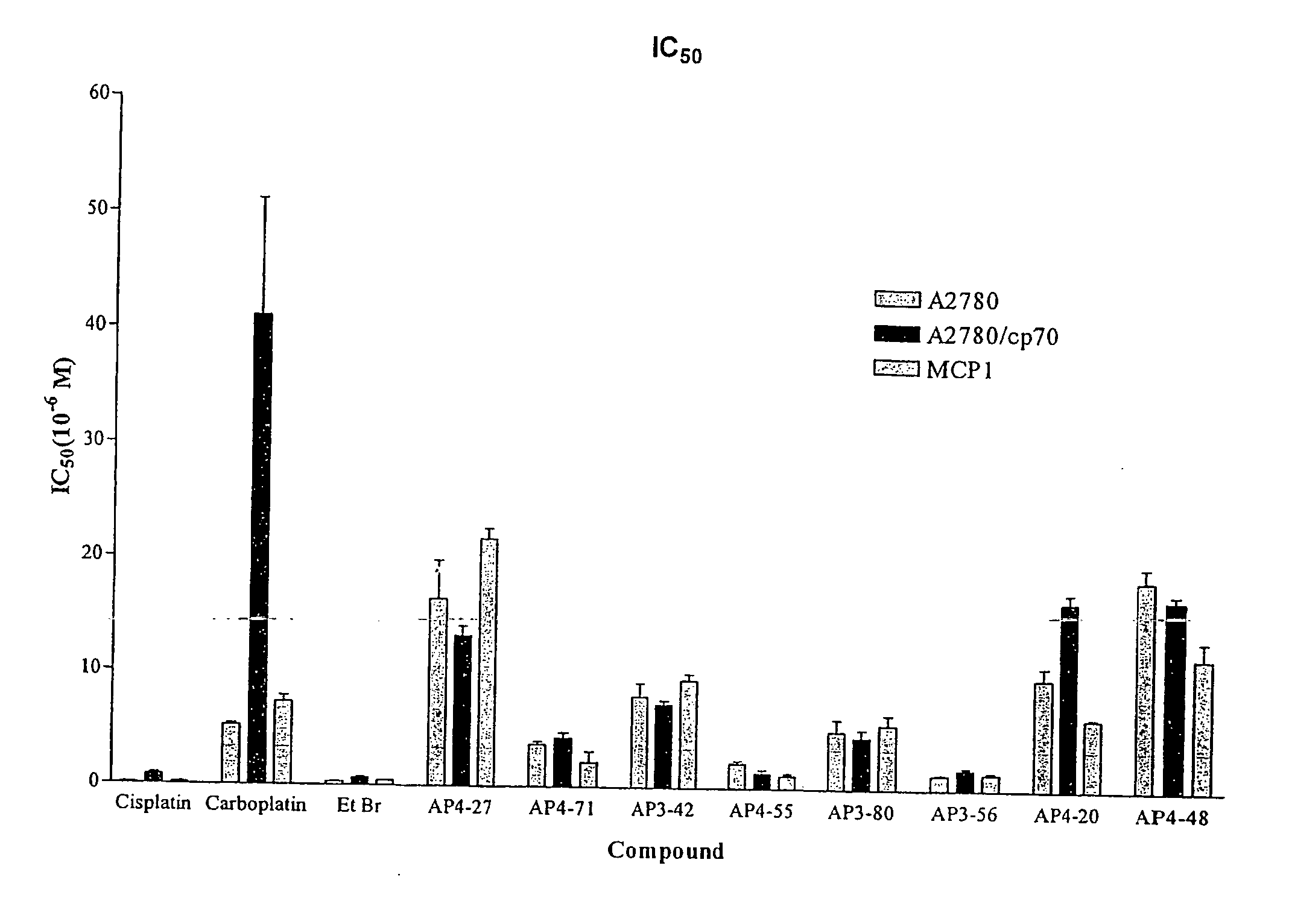
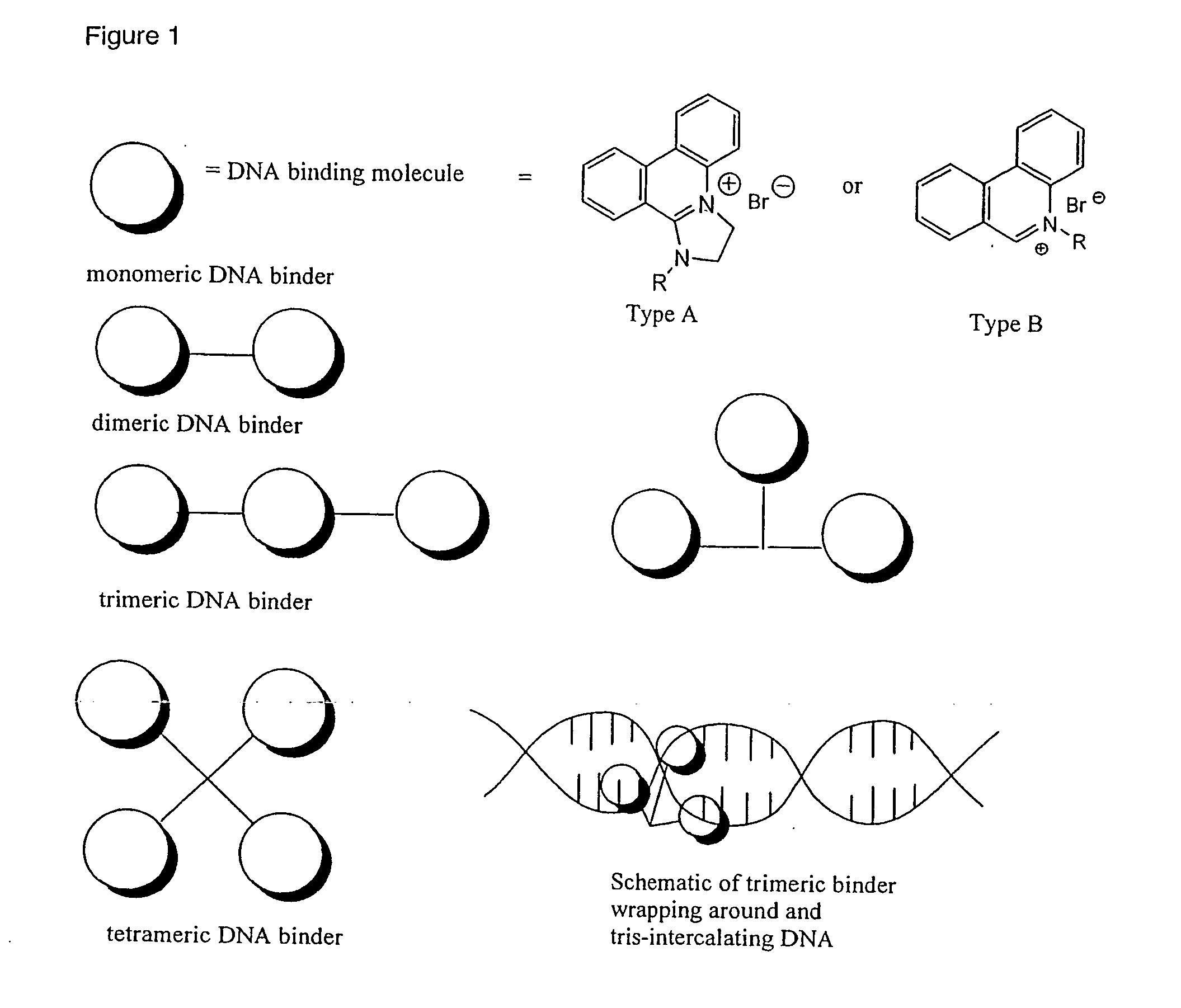
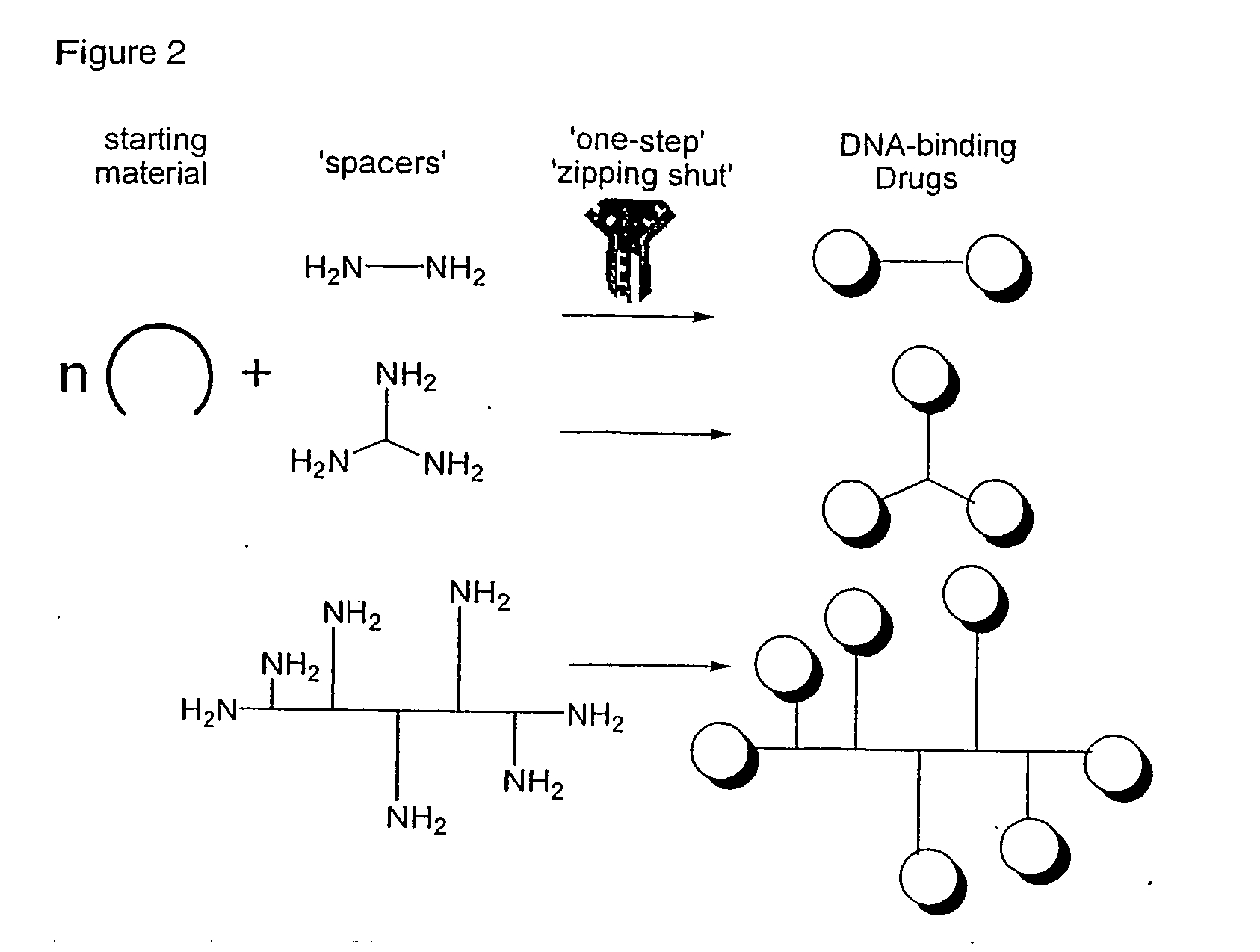
Phenanthridinium Derivatives as Dna Binding Agents
New classes of heterocyclic aromatic cationic compounds are disclosed, and in particular new classes of phenanthridinium derivatives, most notably dihydro-imidazo-phenanthridinium (DIP) compounds. These findings are based on the reaction of the middle b ring of a phenanthridinium core with primary amines to form DIP compounds (Formula A) or secondary amines to form 2-aminoalkyl phenanthridinium derivatives (Formula B). These reactions can also be applied to other classes of starting compounds which comprise a 6-membered ring aromatic heterocycle having a ring nitrogen and at least one alpha hydrogen atom which can be reacted with a primary or secondary amine.
Owner:THE UNIV COURT OF THE UNIV OF GLASGOW
Substituted 4,9-dihydrocyclopenta{imn}phenanthridine-5-ones derivatives thereof and their uses
InactiveUS7157452B2Treat and prevent and ameliorate effectExtend lifespan and proliferative capacity of cellBiocideOrganic chemistryPerylene derivativesPharmaceutical Substances
Owner:EISAI INC
New application of benzo [C] phenanthridine and protopine in producing overriding resistant bacterium medicament
InactiveCN101195627AEnhanced inhibitory effectHigh activityAntibacterial agentsOrganic active ingredientsBenzo(c)phenanthreneDouble bond
The invention relates to a new application of benzo [C] phenanthridine as formula I and original tropine alkaloid for preparing anti-drug-resistant bacterial drug, wherein R1-R10, R12-R15 are hydrogen and hydroxyl groups, cycloalkyl or alkyl groups with 1-12 carbon atoms, alkox or acyloxy groups, benzyloxy, chlorine or other halide atoms, amido, methylol, aldehydo, aldehydo, acetonyl, carboxy group, mesyloxy, 4-methyl-benzene sulfonyl oxygen group, aryl sulfonyl oxygen group, biphenyl phosphine acyloxy and -OCONH2, R11 is hydrogen, methyl or oxygen atom, R5 and R6 (or R15 of the formula I), R14 and R15 are formed with double bonds, R12 or R13 and nearby N atoms are formed with double bonds, N atom can be tertiary or quaternary N type, the substituents of nearby carbons of R1-R10 can have dioxolane structure. The inventive alkaloid can effectively restrain and kill drug-resistant bacterials as MRSA and ESBLs.
Owner:成都军区昆明总医院
Method for synthesizing dihydrogen phenanthridine
InactiveCN102952073ARaw materials are easy to getFew reaction stepsOrganic chemistryMetallolePhenanthridine
The invention relates to a method for synthesizing dihydrogen phenanthridine. According to the invention, transition metals [Ru (II), Rh (I), and Ir (I)] are adopted as catalyst for realizing phenanthridine catalytic hydrogenation. Also, according to the invention, 9,10-dihydrogen phenanthridine in-situ regeneration is applied in asymmetric transfer hydrogenation of unsaturated imine. Only a catalytic amount of phenanthridine is needed to be added, and chiral amine can be synthesized with high enantioselectivity.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for catalytically preparing 6(5H)-phenanthridine ketone by copper component
InactiveCN102977017AEasy to recycleConducive to the development of industrial productionOrganic chemistryKetoneCopper
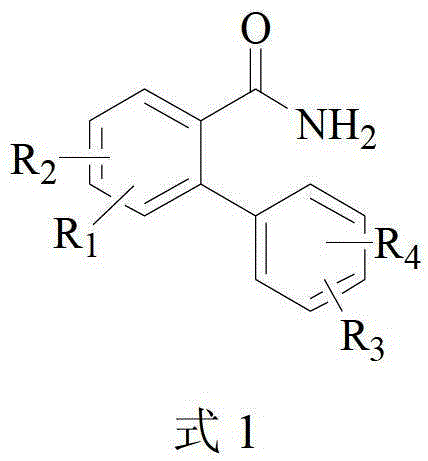
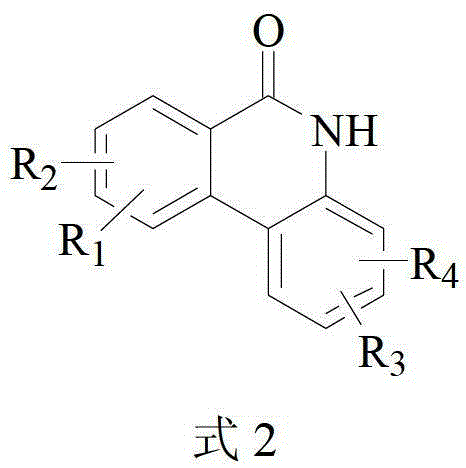
The invention relates to a method for catalytically preparing 6(5H)-phenanthridine ketone by a copper component. The method comprises the step of catalyzing a 2-phenyl benzamide derivative to carry out N-arylated reaction to generate a 6(5H)-phenanthridine ketone derivative by the copper component under the coordination of a ligand in an air environment or airtight environment. The method can be carried out in the air environment, and the catalyst used is simple and easy to obtain and high in reaction yield. The method provided by the invention is beneficial for industrial production.
Owner:HUNAN UNIV
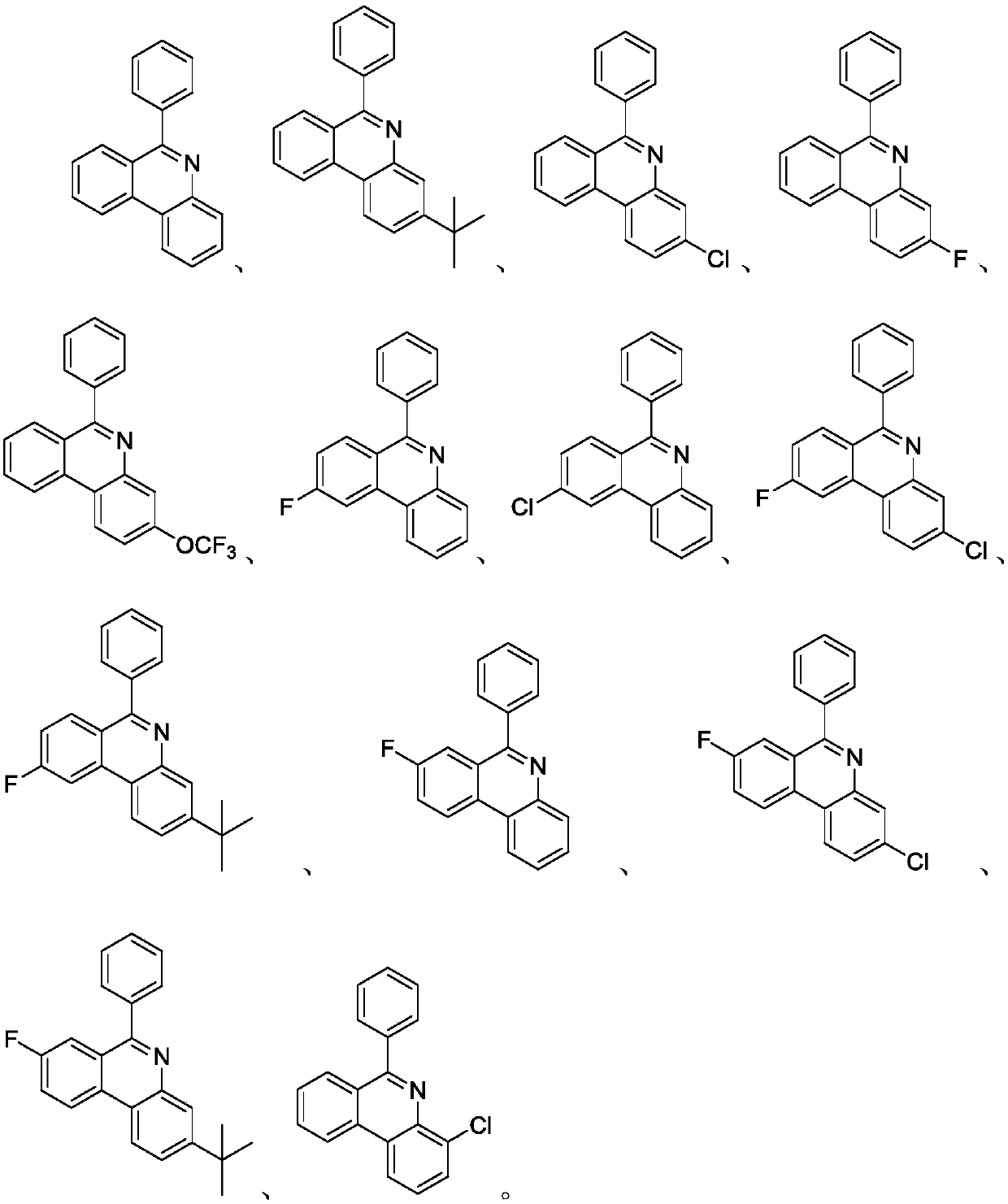
Preparation method of phenanthridine heterocyclic compounds
ActiveCN108299296AMild reaction conditionsEasy to controlOrganic chemistryChemical reactionClean energy
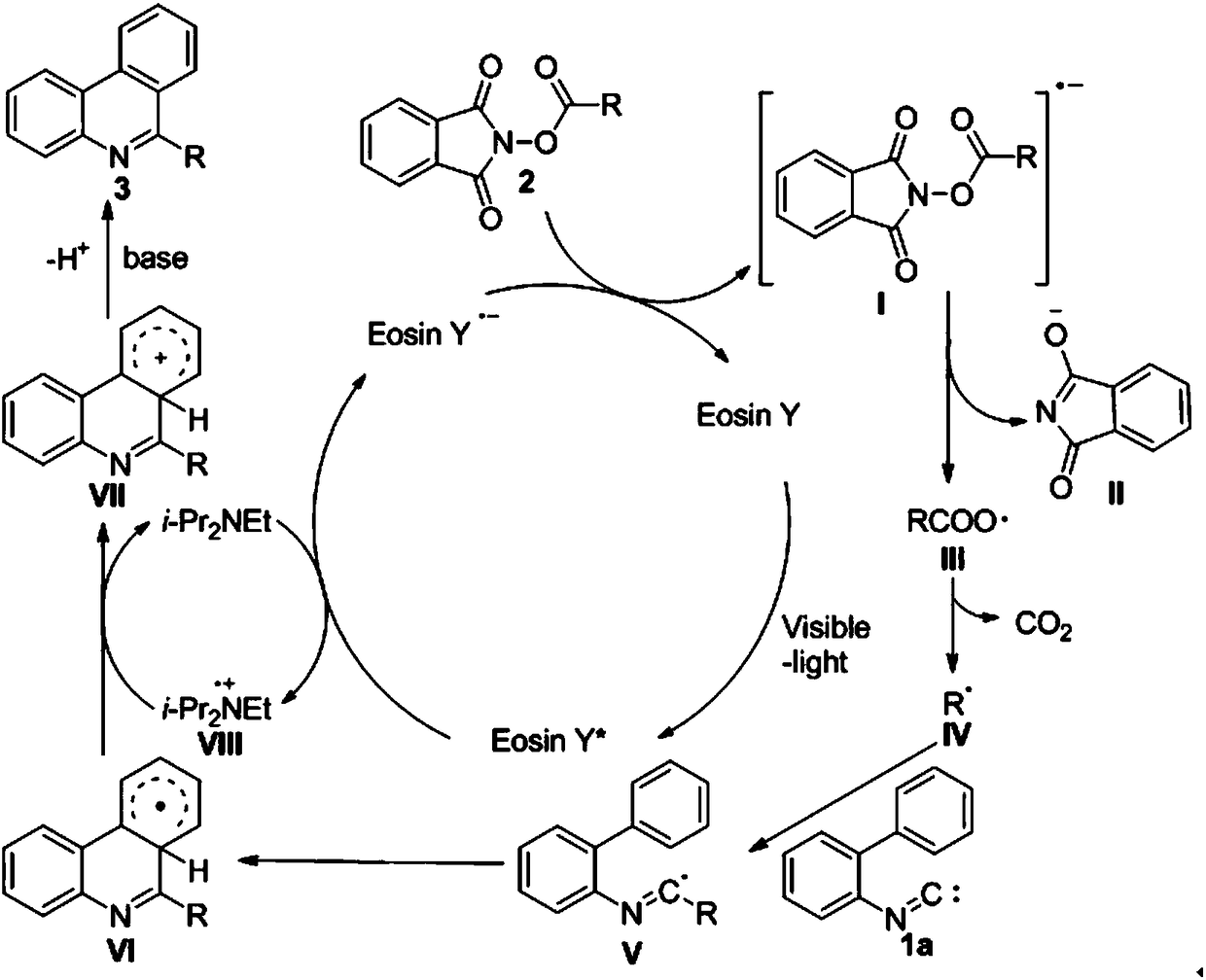
The invention discloses a preparation method of phenanthridine heterocyclic compounds. With isonitrile and N-(acyloxy)phthalimide as raw materials, under the action of an organic small molecular catalyst, a reductive quenching agent and an alkali are added, a reaction is performed for 20-24 h under irradiation of white light, and thus the phenanthridine heterocyclic compounds are prepared. The preparation method is a visible light induced photochemical reaction, gets rid of harsh requirements of a traditional ultraviolet photochemical reaction on equipment, and can be effectively realized by using cheap household fluorescent lamps, LED lamps and inexhaustible clean energy sources (sun light). In addition, the visible light induced photochemical reaction usually has the advantages of mild reaction conditions, easy control, high efficiency and high yield of products. Therefore, the preparation method attracts extensive attention of synthesis chemists in recent years, and is developed into the key point and hot point of current researches.
Owner:NANJING AGRICULTURAL UNIVERSITY
Pseudobase benzo[c]phenantridines with improved efficacy, stability and safety
Pseudobase benzo[c]phenanthridines and the pharmaceutically acceptable salts thereof of Formula Iare provided herein. The variables R, R1, R2, R3, and R4 are defined herein. Certain pseudobase benzo[c]phenanthridines provided herein act as prodrugs, targeting the parent benzo[c]phenanthridinium alkaloid to hydrophilic or hydrophobic regions in the body. Pharmaceutical compositions comprising a pseudobase benzo[c]phenanthridine and a carrier, excipient, or diluent are provided herein. Methods of treating or preventing microbial, fungal and or viral infections and methods of treating diseases and disorders responsive to protein kinase C modulation, topoisomerase I, and / or topoisomerase II modulation are also provided.
Owner:YALE UNIV
Crosslinkable host materials
InactiveCN106715420AEasy to moveImprove efficiencyGroup 5/15 element organic compoundsFinal product manufacturePyridazinePhenanthroline
The invention relates to a crosslinkable organic molecule having a structure of the formula (1) and to the use thereof, wherein Ar is independently of one another, an unsaturated or aromatic carbo- or heterocyclic unit with 5 to 30 ring atoms, selected from the group consisting of naphthalene, anthracene, phenanthrene, pyrene, dihydropyrene, chrysene, perylene, fluoranthene, benzanthracene, tetracene, pentacene, benzpyrene, furan, benzofuran, isobenzofuran, thiophene, benzothiophene, isobenzothiophene, dibenzothiophene, pyrrole, indole, isoindole, carbazole, pyridine, quinoline, isoquinoline, acridine, phenanthridine, benzo-5,6-quinoline, benzo-6,7-quinoline, benzo-7,8-quinoline, phenothiazine, phenoxazine, pyrazole, indazole, imidazole, benzimidazol, naphthimidazole, phenanthrimidazole, pyridimidazole, pyrazine-imidazole, quinoxalinimidazole, oxazole, benzoxazole, naphthoxazole, anthroxazole, phenanthroxazole, isoxazole, isothiazole, 1,3-thiazole, benzothiazole, pyridazine, benzopyridazine, pyrimidine, benzpyrimidine, quinoxaline, pyrazine, phenazine, naphthyridine, azacarbazole, benzocarboline, phenanthroline, 1,2,3-triazole, 1,2,4-triazole, benzotriazole, 1,2,3-oxadiazole, 1,2,4-oxadiazole, 1,2,5-oxadiazole, 1,3,4-oxadiazole, 1,2,3-thiadiazole, 1,2,4-thiadiazole, 1,2,5-thiadiazole, 1,3,4-thiadiazole, 1,3,5-triazine, 1,2,4-triazine, 1,2,3-triazine, tetrazole, 1,2,3,4- oxatriazole, 1,2,3,4-oxatriazole, 1,2,4,5-tetrazine, 1,2,3,4-tetrazine, 1,2,3,5-tetrazin, purine, pteridine, indolizine, benzothiadiazole, indenocarbazole, indenofluorene, spirobifluorene, and indolocarbazole; D1 is a donor group having a structure of the formula (1a); and D2 is a donor group having a structure of the formula (1b).
Owner:SAMSUNG DISPLAY CO LTD
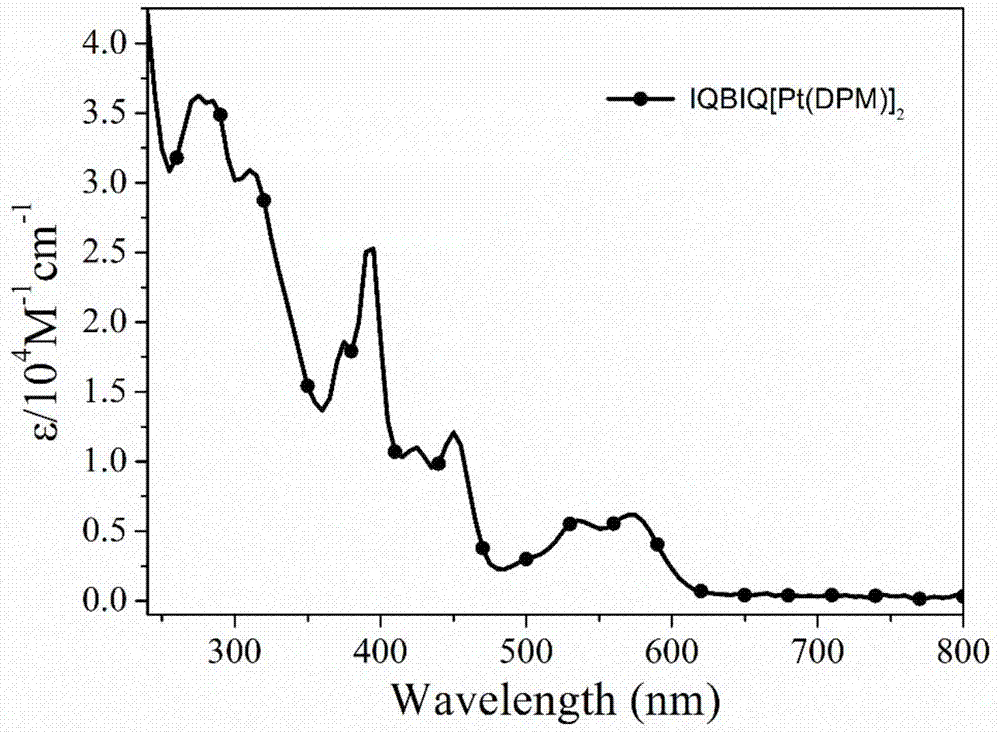
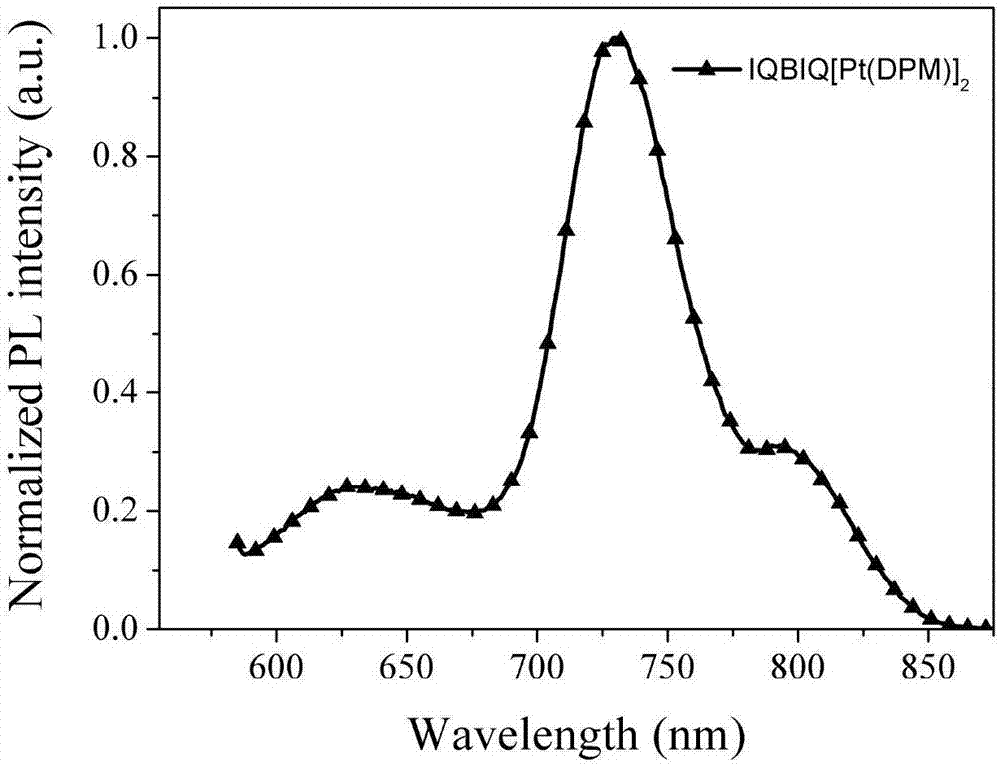
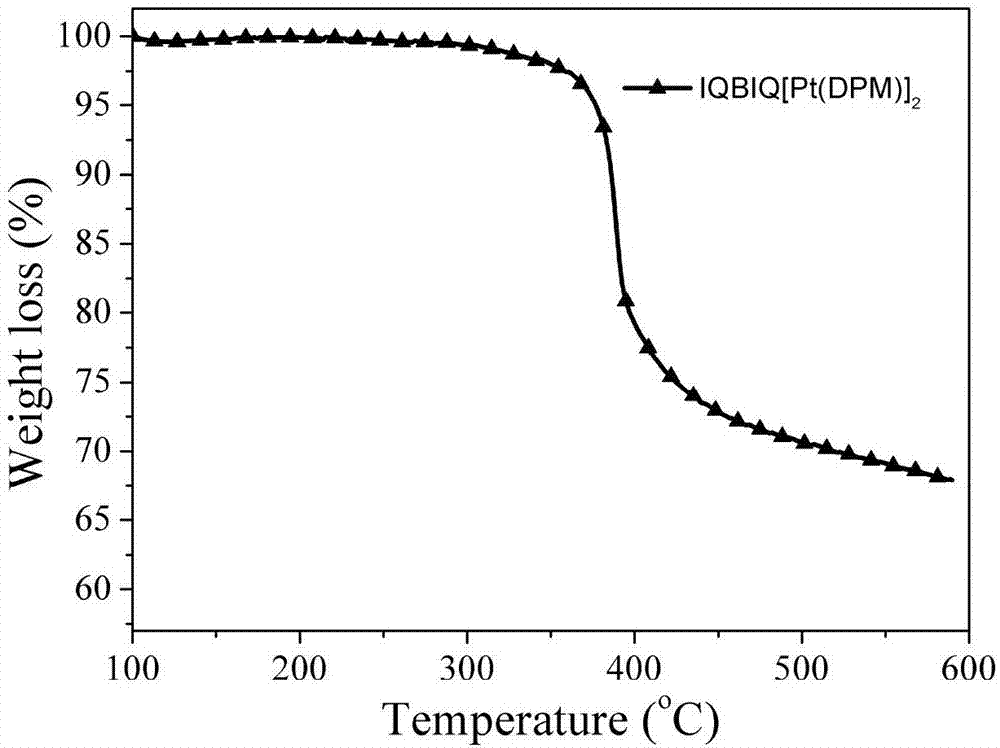
Synthesis and application of dinuclear ring metal platinum (II) complex near-infrared electrophosphorescent material containing different conjugated bridges
InactiveCN107400147AImprove the rigidity of the structureEnhanced spin couplingSolid-state devicesPlatinum organic compoundsRigid structureLight-emitting diode
The invention discloses a dinuclear ring metal platinum (II) complex near-infrared electrophosphorescent material containing different conjugated bridges, and application of the electrophosphorescent material to an organic electroluminescent diode. The dinuclear ring metal platinum complex near-infrared electrophosphorescent material uses different conjugated rigid structures (benzene, 1,4-difluorobenzene, carbazole, fluorene and pyrene) as luminous kernel donor (D) units, and uses nitrogen-containing dentate conjugate ligands (isoquinoline, anthraquinoline and phenanthridine) as receptor (A) units for building the ring metal platinum complex near-infrared electrophosphorescent material using A-D-A structure as a main ligand, using 2,2,6,6-tetramethyl-3,5-heptadione as an auxiliary ligand and using platinum ions. The organic electrophosphorescent luminous device is prepared by using the near-infrared electrophosphorescent material as a luminous layer doping agent and using m-MTDATA:CBP as a body material. The device is a near-infrared electrophosphorescent luminous device with the maximum emitting peak being 709nm, the outer quantum efficiency being 3.97 percent and the maximum radiation degree being 2354nW.Sr<-1>.cm<-2>.
Owner:CHANGZHOU UNIV
Organic electroluminescent materials and devices
Imidazophenanthridine ligands and metal complexes are provided. The compounds exhibit improved stability through a linking substitution that links a nitrogen bonded carbon of an imidizole ring to a carbon on the adjacent fused aryl ring. The compounds may be used in organic light emitting devices, particularly as emissive dopants, providing devices with improved efficiency, stability, and manufacturing. In particular, the compounds provided herein may be used in blue devices having high efficiency.
Owner:UNIVERSAL DISPLAY
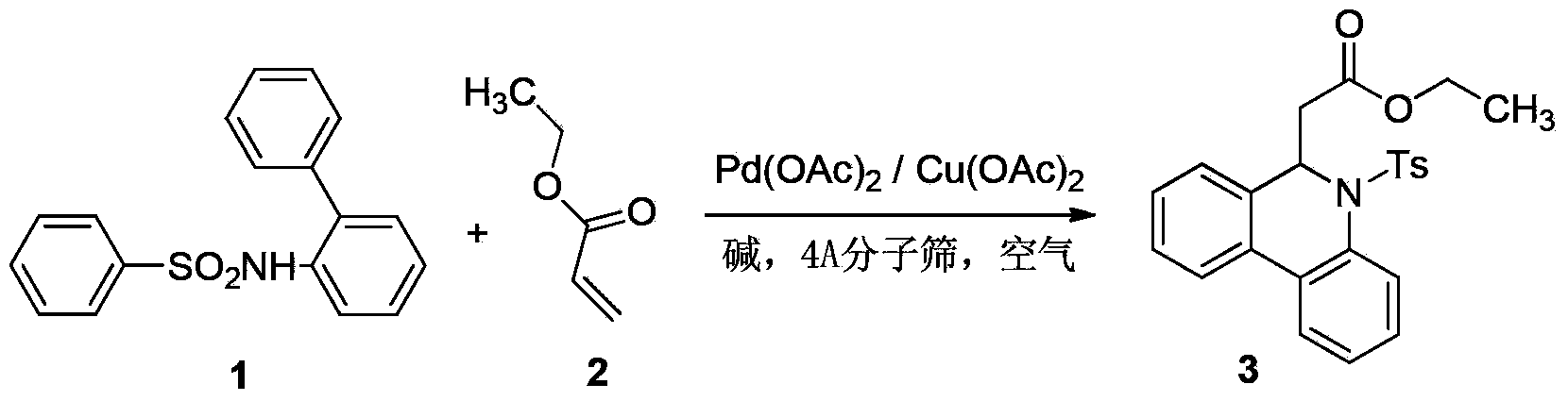
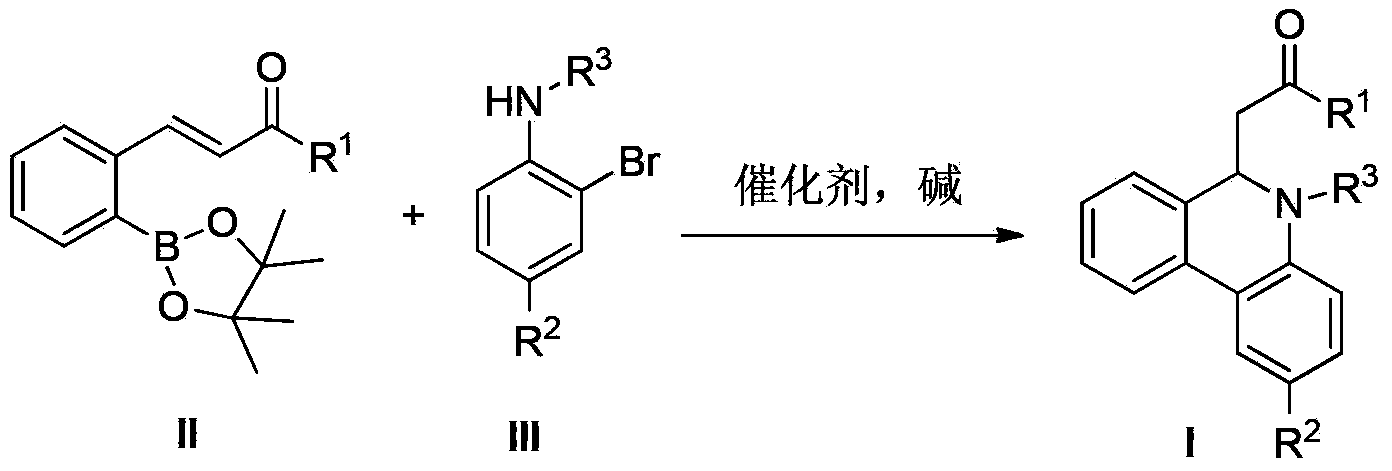
Preparation method of 6-substitute-5,6-dihydro phenanthridine derivative
The invention relates to the field of organic synthesis, and particularly relates to a preparation method of a 6-substitute-5,6-dihydro phenanthridine derivative. The preparation method disclosed by the invention comprises the following steps of: carrying out Suzuki-Miyaura reaction and Michael addition reaction on phenylboronic acid ester II and an o-bromoaniline derivative III which used as raw materials in the presence of a palladium catalyst in a one-pot manner. The method has the advantages of easily available raw material, wide substrate application range, high yield, and the like.
Owner:CHINA PHARM UNIV
Organic electroluminescent compound, organic electroluminescence device and application thereof
ActiveCN108101896AReduce the driving voltageExtend your lifeOrganic chemistrySolid-state devicesAnilinePhenyl group
The invention provides an organic electroluminescent compound, an organic electroluminescence device and application thereof. The structure of the organic electroluminescent compound is described in the description, wherein R1 and R4 are respectively independent hydrogen, heavy hydrogen, linear or branched chain alkyls of C1-C20, and or one of phenyl, anilino, diphenylamino, 2-phenyl-3-amino-pyridyl, 3-amino-dipyridyl, 2-phenyl-1-naphthylamine, 2-amino-dinaphthyl, 2-phenyl-1-amino-phenanthryl, 3-amino-diphenanthryl, 2-phenyl-1-amino-anthranyl, 2-amino-dianthranyl, phenanthridinyl, biphenyl, pyridyl, pyrimidinyl or triazinyl; R2 and R3 are respectively independent hydrogen, heavy hydrogen, linear or branched chain alkyls of C1-C20, or phenyl, pyridyl, naphthyl, phenanthryl, anthryl, phenanthridine, biphenyl, pyridyl, pyrimidinyl and triazinyl; l, m, n and o are integers of 0-4.
Owner:NANJING TOPTO MATERIALS CO LTD
Organic semiconductor materials containing 2,9-dialkyl-6-alkoxy phenanthridine unit and preparation method and application thereof
ActiveCN105860032AThe synthetic route is simpleEasy to purifySolid-state devicesSemiconductor/solid-state device manufacturingIsomerizationMetal catalyst
The invention discloses organic semiconductor materials containing 2,9-dialkyl-6-alkoxy phenanthridine and a preparation method and an application thereof. The organic semiconductor materials are prepared by carrying out a reaction of 2,9-dialkyl-6-alkoxy phenanthridine halogenated derivatives and a monomer containing an aromatic group structure under a metal catalyst, wherein the aromatic group and a 2,9-dialkyl-6-alkoxy phenanthridine unit are connected in a conjugate manner. The invention discloses a method for synthesis of 2,9-dialkyl-6-alkoxy phenanthridine by a phenanthridone isomerization reaction; a series of organic semiconductor materials containing the 2,9-dialkyl-6-alkoxy phenanthridine unit are synthesized and are applied in the field of organic optoelectronic devices, and solar cell devices with high open circuit voltage and deep blue polymer blue light devices are obtained.
Owner:SOUTH CHINA UNIV OF TECH
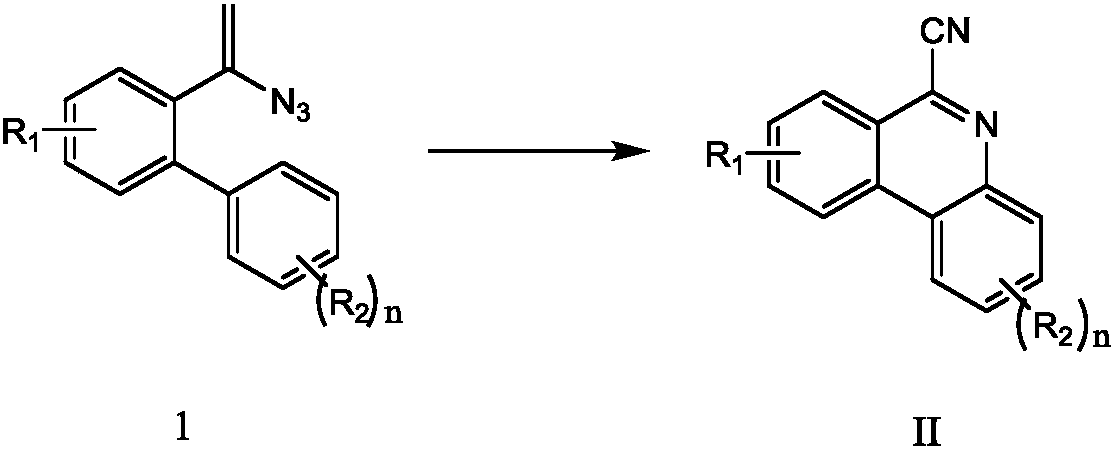
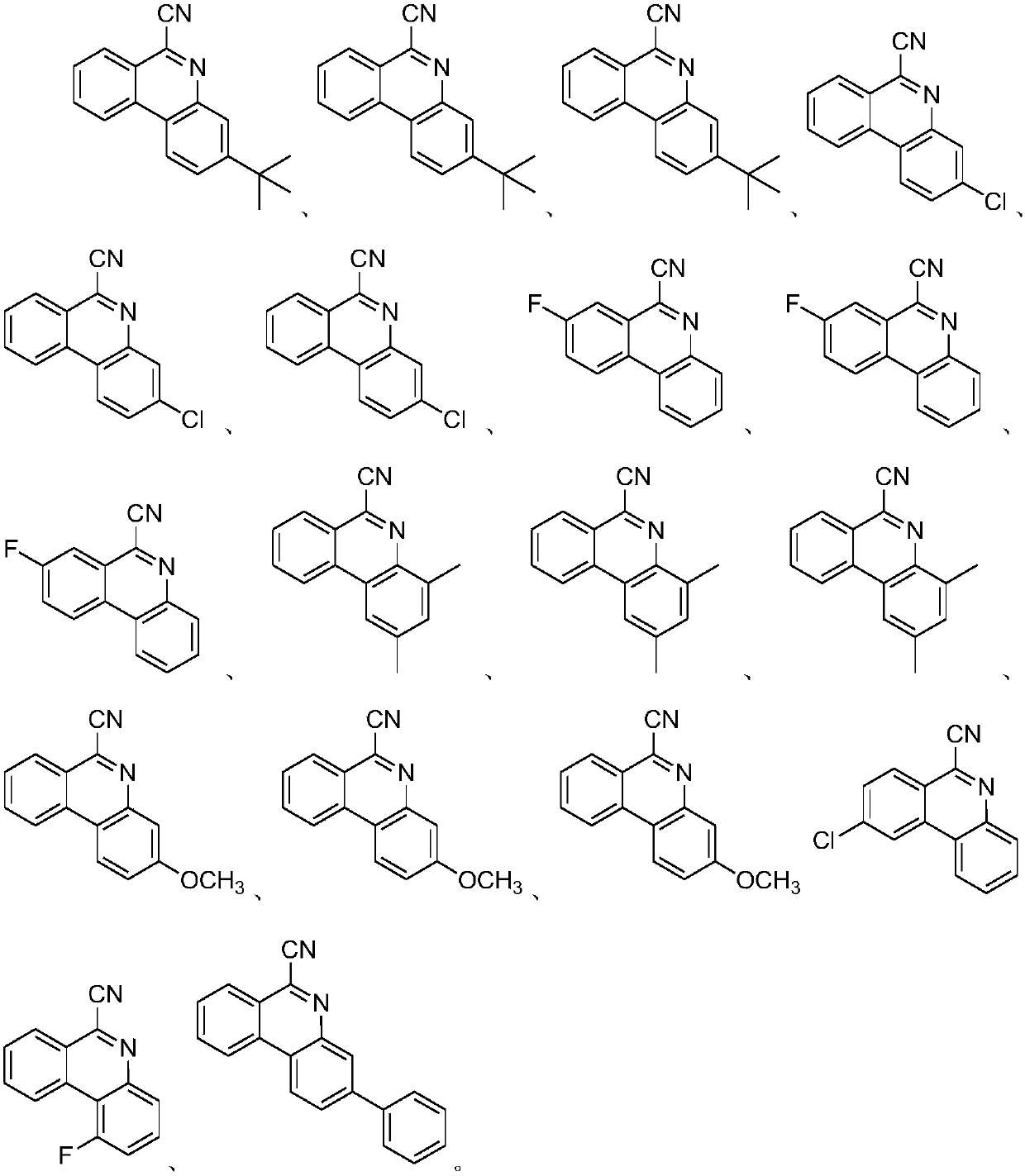
Method for synthesizing 6-cyano phenanthridine compound
ActiveCN107641100AGood substrate adaptabilityAchieve aromatization/cyanationOrganic chemistrySodium azideTert butyl
The invention relates to a method for preparing 6-cyano phenanthridine and derivatives thereof. The method comprises the steps of adding an azido-terminal-substituted olefin compound represented by aformula I shown in the description, a nitrogen source, a catalyst, an oxidant and an organic solvent into a pressure-proof reactor, carrying out a full reaction at the temperature of 30 DEG C to 60 DEG C, and subjecting a reaction solution to separation and purification, thereby obtaining the 6-cyano phenanthridine represented by a formula II shown in the description and the derivatives thereof, wherein the nitrogen source comprises sodium azide, the catalyst comprises copper powder, cuprous bromide, copper sulfate, copper chloride, copper acetate or cuprous oxide, R1 and R2 in the formulae Iand II are separately the same, R1 is H, F or Cl, R2 is H, or H is monosubstituted or polysubstituted by methyl, chlorine, fluorine, phenyl, tert-butyl or methoxy. Compared with the prior art, the method has the advantages that the method is safe and environmentally friendly, no waste gas and waste water is produced, the substrate adaptability is good, all kinds of substituents can be aromatized / cyanated, the reaction conditions are mild, and the reaction steps are simple.
Owner:ZHEJIANG UNIV OF TECH
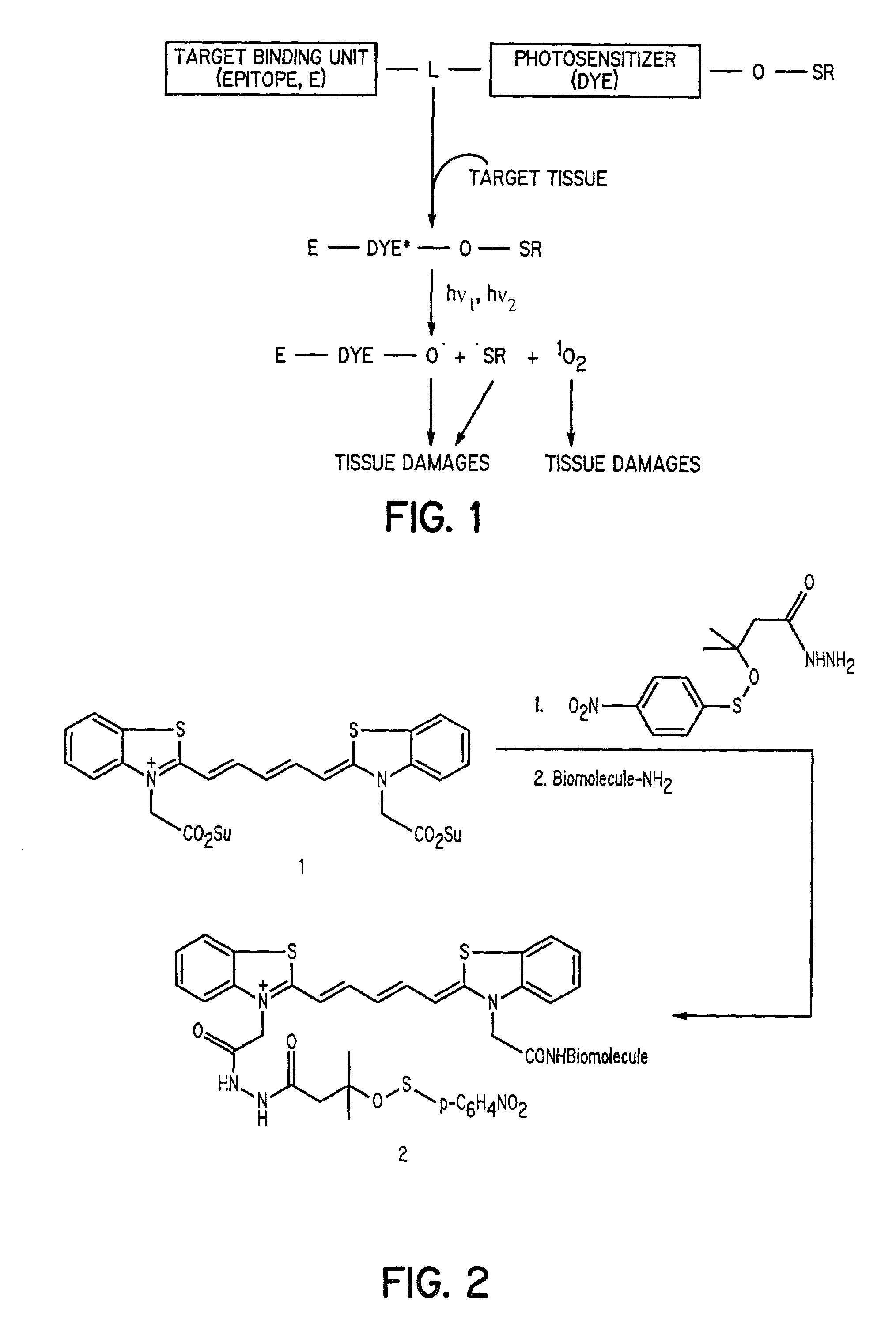
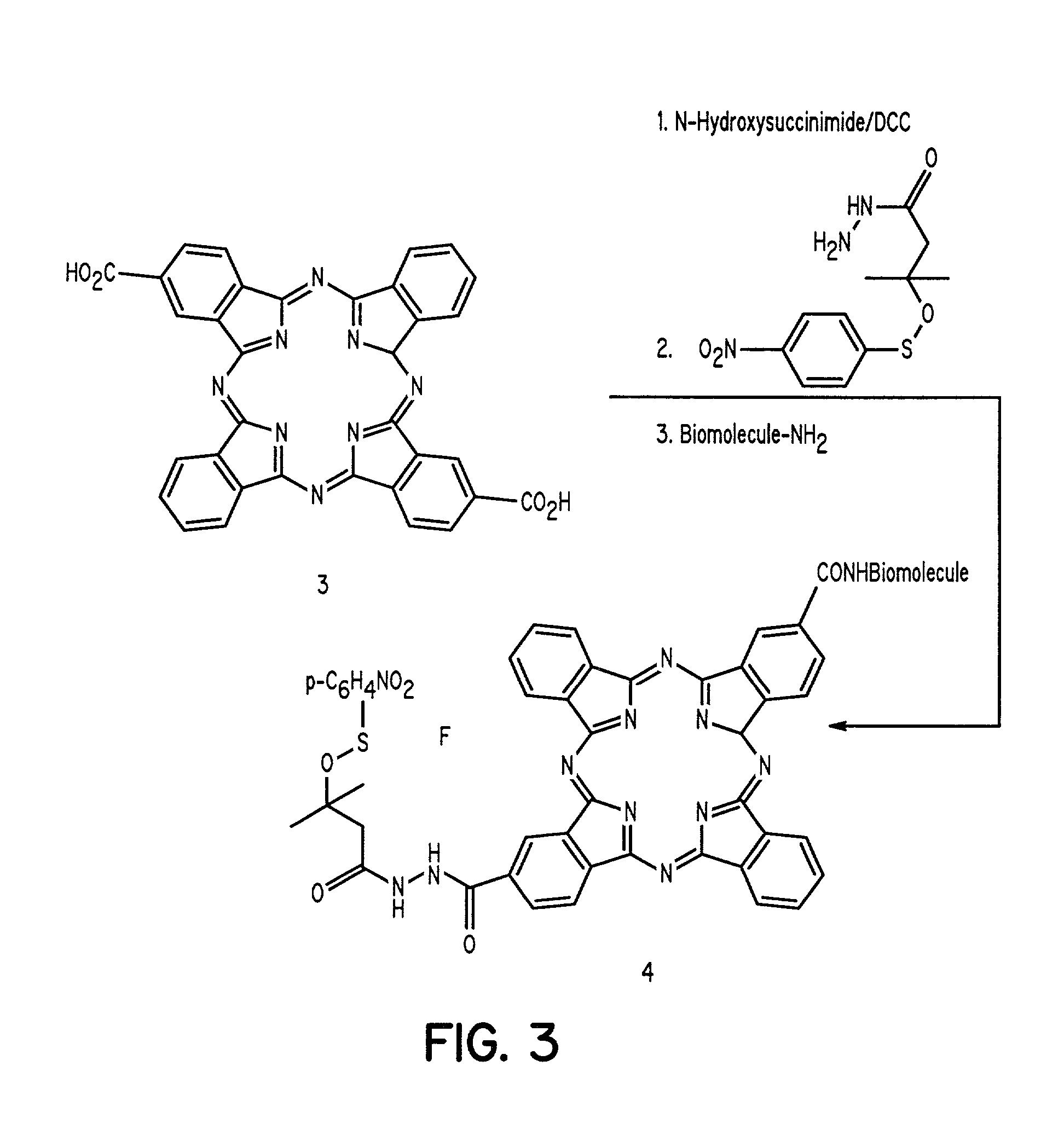
Cyanine-sulfenates for dual phototherapy
Dye-sulfenate derivatives and their bioconjugates for dual phototherapy of tumors and other lesions. The compounds comprise sulfenates having the formula, t,0080where E is selected from the group consisting of somatostatin receptor binding molecules, heat sensitive bacterioendotoxin receptor binding molecules, neurotensin receptor binding molecules, bombesin receptor binding molecules, cholecystekinin receptor binding molecules, steroid receptor binding molecules, and carbohydrate receptor binding molecules, and dihydoxyindolecarboxylic acid; L and X are independently selected from the group consisting of —(R5)NOC—, —(R5)NOCCH2O—, —(R5)NOCCH2CH2O—, —OCN(R5)—, —HNC(═S)NH—, and HNC(═O)NH—; DYE is an aromatic or a heteroaromatic radical derived from the group consisting of cyanines, indocyanines, phthalocyanines, rhodamines, phenoxazines, phenothiazines, phenoselenazines, fluoresceins, porphyrins, benzoporphyrins, squaraines, corrins, croconiums, azo dyes, methine dyes, indolenium dyes, crellins, and hypocrellins; R1 to R5 are independently selected from the group comprising hydrogen, C1-C10 alkyl, C5-C10 aryl, C1-C10 polyhydroxyalkyl, and C1-C10 polyalkoxyalkyl; and Ar is an aromatic or heteroaromatic radical derived from the group consisting of benzenes, naphthalenes, naphthoquinones, diphenylmethanes, fluorenes, anthracenes, anthraquinones, phenanthrenes, tetracenes, naphthacenediones, pyridines, quinolines, isoquinolines, indoles, isoindoles, pyrroles, imidiazoles, oxazoles, thiazoles, pyrazoles, pyrazines, purines, benzimidazoles, furans, benzofurans, dibenzofurans, carbazoles, acridines, acridones, phenanthridines, thiophenes, benzothiophenes, dibenzothiophenes, xanthenes, xanthones, flavones, coumarins, and anthacylines. The compounds are designed to produce both Type 1 and Type 2 phototherapeutic effects at once using a dual wavelength light source that will produce singlet oxygen and free radicals at the lesion of interest.
Owner:MEDIBEACON
Preparation method of 6-H-phenanthridine compounds by one-pot process
InactiveCN105153032AHigh yieldRaw materials are easy to getOrganic chemistryBulk chemical productionChemical reactionPtru catalyst
The invention discloses a preparation method of 6-H-phenanthridine compounds by a one-pot process, which comprises the following steps: adding reactants N-protected arylamine and bromobenzyl bromide compounds into a solvent, adding a metal palladium catalyst, a ligand and an alkali, carrying out chemical reaction under heating and inert gas protective conditions, and after the reaction finishes, carrying out after-treatment to obtain the compound pure products. By using the simple accessible N-protected arylamine and the commercial accessible bromobenzyl bromide compounds as the raw materials, one-pot three-step cascade reaction is performed to obtain the 6-H-phenanthridine compounds. The method is simple to operate, and has the characteristics of simple after-treatment, higher yield and the like.
Owner:ZUNYI MEDICAL UNIVERSITY
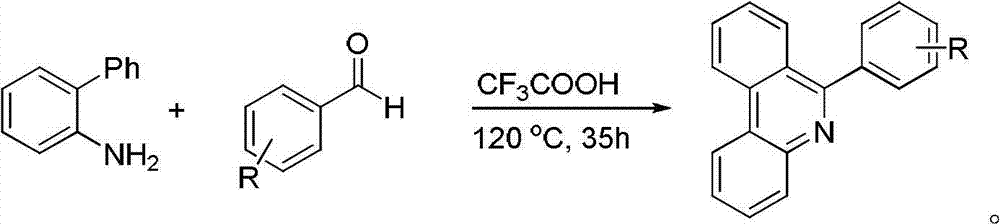
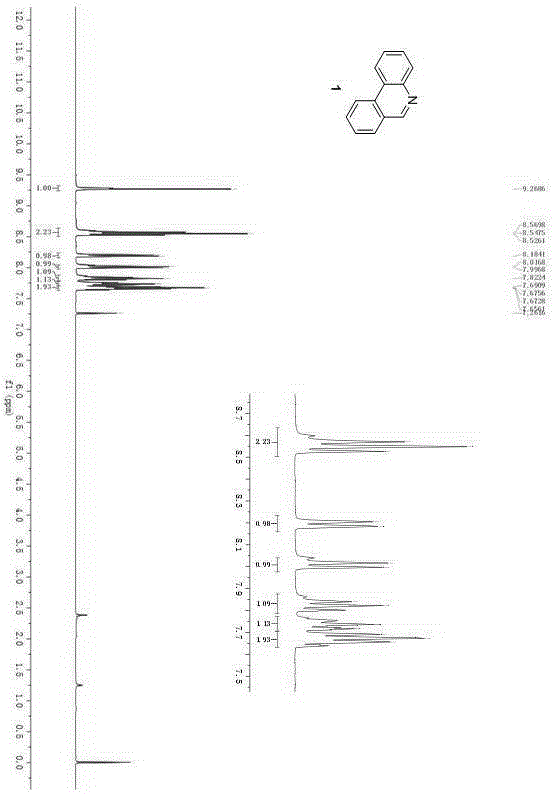
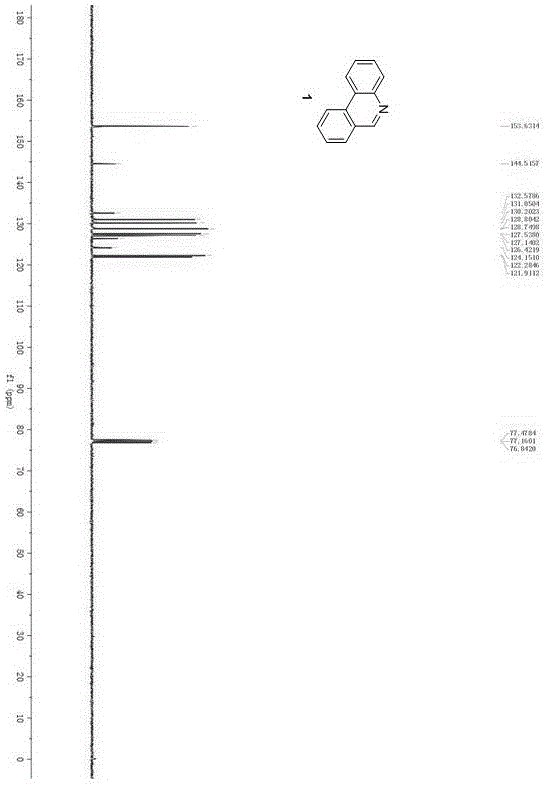
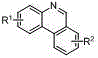
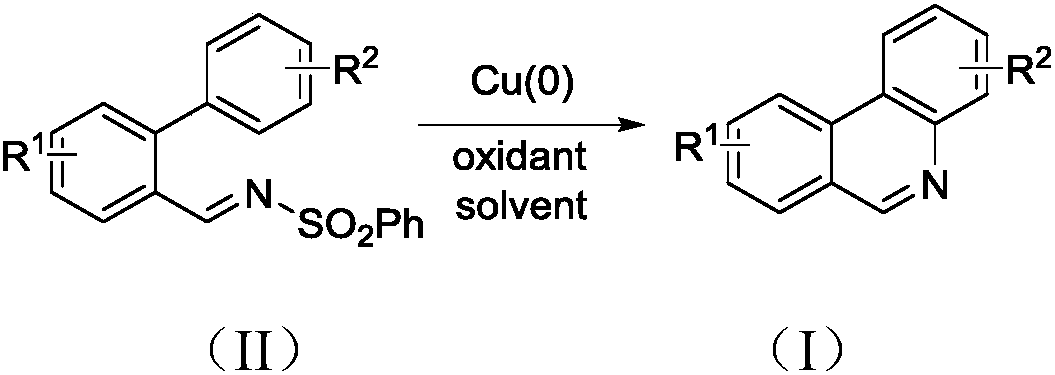
Synthesis method of phenanthridine and derivative of phenanthridine
InactiveCN107778239AReduce consumptionRaw materials are easy to obtainOrganic chemistryOrganic solventSynthesis methods
The invention discloses a synthesis method of phenanthridine as shown in a formula (I) and a derivative of the phenanthridine. In the synthesis method, o-arylphenylsulfimide shown as a formula (II) isused as a raw material and reacts in an organic solvent under the action of a [Cu] / Selectfluor catalyst to obtain a corresponding target product (I). The synthesis method disclosed by the invention has the advantages of cheap and easily available and low-toxicity catalyst, environmental friendliness, mild reaction conditions, high universality of functional group and easiness and convenience in operation. The formulas (I) and (II) are shown in the description.
Owner:ZHEJIANG UNIV OF TECH
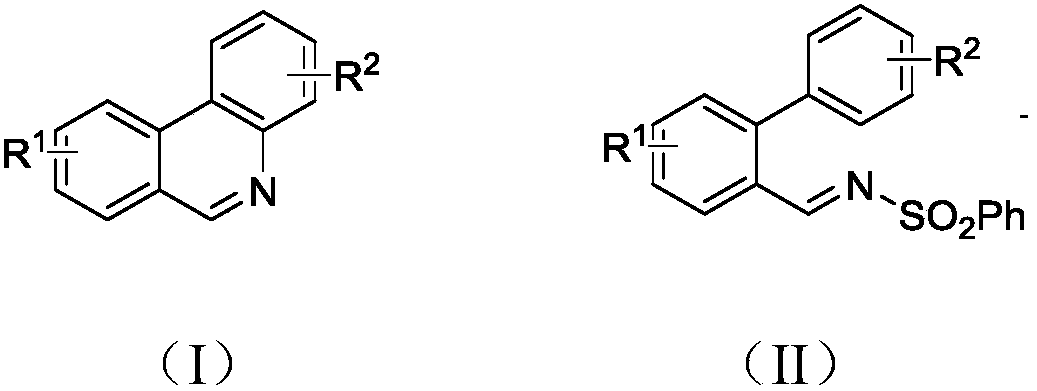
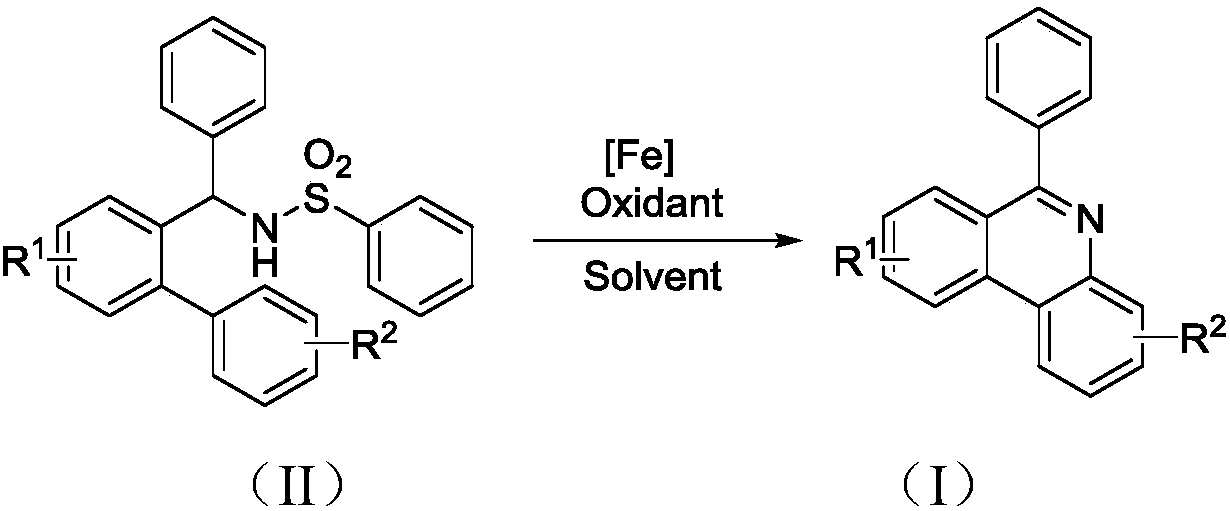
Synthesis method of 6-substituted phenanthridine compound
InactiveCN107793358AReduce consumptionRaw materials are easy to obtainOrganic chemistryOrganic solventSynthesis methods
The invention discloses a synthesis method of a 6-substituted phenanthridine compound represented by a formula (I), wherein a N-[1-phenyl-1'-biphenyl]benzenesulfonamide compound represented by a formula (II) is used as a raw materials and is subjected to a reaction in an organic solvent under the action of a [Fe] / Selectfluor catalyst to prepare a corresponding target product represented by the formula (I). According to the present invention, the synthesis method has advantages of environmental friendliness, mild reaction conditions, good functional group popularity, easy operation and the like, and uses the catalyst with advantages of low cost, easy availability and low toxicity. The formulas (I) and (II) are defined in the specification.
Owner:ZHEJIANG UNIV OF TECH
Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device
The invention discloses a method for continuously preparing a dihydrobenzo [j] phenanthridine compound containing a trifluoromethyl functional group by using a micro-channel reaction device, which comprises the following steps: (1) dissolving a 1,7-eneyne compound and alkali in a proper solvent to obtain a material I; (2) dissolving a trifluoromethyl reagent and a photocatalyst in a proper solventto obtain a material II; (3) respectively pumping the material I and the material II into a micro-channel reaction device, fully mixing, and carrying out a photocatalytic trifluoromethylation reaction to obtain a reaction solution; and (4) quenching the reaction liquid, adding a corresponding organic solvent for extraction, collecting an organic phase, drying, concentrating and recrystallizing toobtain a target product. The micro-channel reaction device is used for preparing the 1,7-eneyne trifluoromethylation product, the reaction conditions are milder, the reaction rate can be effectivelycontrolled, the reaction time is shortened, continuous production is achieved, side reactions are reduced, the maximum product yield can reach 99.3%, the amplification effect is basically avoided, andindustrial amplification is facilitated.
Owner:NANJING UNIV OF TECH
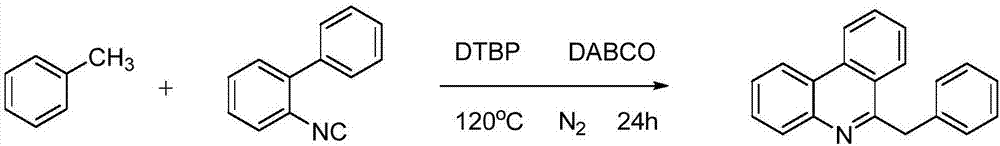
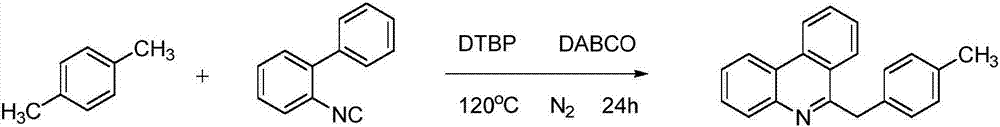
Synthetic method of 6-benzyl phenanthridine compounds
ActiveCN107235900APost-processing is simpleSimple and fast operationOrganic chemistryHydrogenHigh pressure
The invention discloses a synthetic method of 6-benzyl phenanthridine compounds. A reaction equation is as follows: formula (shown in the description), wherein R represents hydrogen, fluorine, chlorine, methyl, methoxyl or a naphthalene ring. The synthetic method has the beneficial effects that (1) the operation of the preparation process is simple and convenient, and the post-treatment of obtained products is easy; (2) high temperature and high pressure conditions are not required, and reaction conditions are mild; (3) a catalyst is not required, and the synthetic cost is lowered; (4) functional groups of a reaction substrate are high in tolerance, and the substrate is wide in range and easy to prepare; (5) an oxidant is cheap, easily available and relatively economic; (6) the reaction is efficient and high in yield; and (7) the synthetic method is pollution-free and environment-friendly.
Owner:WENZHOU UNIVERSITY
Pseudobase benzo[c]phenanthridines with improved efficacy, stability, and safety
Pseudobase benzo[c]phenanthridines and the pharmaceutically acceptable salts thereof of Formula I are provided herein. The variables R, R1, R2, R3, and R4 are defined herein. Certain pseudobase benzo[c]phenanthridines provided herein act as prodrugs, targeting the parent benzo[c]phenanthridinium alkaloid to hydrophilic or hydrophobic regions in the body. Pharmaceutical compositions comprising a pseudobase benzo[c]phenanthridine and a carrier, excipient, or diluent are provided herein. Methods of treating or preventing microbial, fungal and or viral infections and methods of treating diseases and disorders responsive to protein kinase C modulation, topoisomerase I, and / or topoisomerase II modulation are also provided.
Owner:YALE UNIV
Synthetic method for (1,2,3-triazolyl)[1, 5-f]phenanthridine-10-ethyl carboxylate compound
InactiveCN107216326AImprove compatibilitySimple starting materialOrganic chemistryAcetic acidOrganic synthesis
The invention discloses a synthetic method for a (1,2,3-triazolyl)[1, 5-f]phenanthridine-10-ethyl carboxylate compound, belonging to the technical field of organic synthesis. According to main points of a technical scheme in the invention, a reaction equation in the synthesis process of the method is as described in the specification. The method is mild in reaction conditions and simple in process; starting raw materials are noncyclic compounds like iodomethane, 4-azidoethyl acetoacetate, alkyne-terminated compounds and 1,2-allenyl ketones which are convenient to prepare or easy to purchase; the synthetic method can simultaneously construct three rings in a phenanthridine ring; and the finally synthesized compound is easy to separate and purify.
Owner:HENAN POLYTECHNIC UNIV
Environment-friendly synthetic method of 6(5H)-phenanthridine derivative
InactiveCN108707111ARaw materials are easy to getEasy to operateOrganic chemistryCatalytic oxidationOxygen
The invention discloses an environment-friendly synthetic method of a 6(5H)-phenanthridine derivative. The method specifically comprises the step of carrying out catalyzed oxidation by virtue of metalcobalt, so as to realize the high-selectivity and high-yield preparation of the 6(5H)-phenanthridine derivative, wherein N-([1,1'-biphenyl]-2-yl)pyridine amide is take as the raw material, azodiformate is taken as a carbonyl source for replacing poisonous and harmful carbon monoxide, and oxygen is taken as an oxidant. The environment-friendly synthetic method has the characteristics that the rawmaterial is easily available, the operation is simple and convenient, the chemical selectivity and the regional selectivity are high, and the like; and the environment-friendly synthetic method has relatively high implement values and social and economic values.
Owner:ZHEJIANG UNIV OF TECH
Organic electroluminescent compound and organic electroluminescent device
ActiveUS20180105534A1Improve performanceReduce the driving voltageOrganic chemistrySolid-state devicesAnilinePhenyl group
The present invention provides an organic electroluminescent compound and an organic electroluminescent device using the organic electroluminescent compound, the compound has the following structural formula:wherein R1, R2 and R4 are, each independently, selected from a group consisting of a hydrogen atom, a C1-C20 linear or branched alkyl group, a substituted or unsubstituted N-(phenylmethyl)imino group, a phenyl group, phenylamine, diphenylamine, phenyl pyridinylamine, bipyridinylamine, phenyl naphthylamine, binaphthylamine, phenyl phenanthrylamine, biphenanthrylamine, phenyl anthrylamine, bianthrylamine, phenanthridine, biphenyl, a pyridyl group, a pyrimidinyl group, a quinolinyl group and a triazinyl group; R3 is selected from a group consisting of hydrogen atom, a C1-C10 linear or branched alkyl group, a substituted or unsubstituted N-(phenylmethyl)imino group, a phenyl group, phenylamine, diphenylamine, phenyl pyridinylamine, bipyridinylamine, phenyl naphthylamine, binaphthylamine, phenyl phenanthrylamine, biphenanthrylamine, phenyl anthrylamine, bianthrylamine, phenanthridine, biphenyl, a pyridyl group, a pyrimidinyl group, a quinolinyl group and a triazinyl group.
Owner:NANJING TOPTO MATERIALS CO LTD
Phenanthridine compound and synthesis method thereof
The invention discloses a phenanthridine compound and a synthesis method thereof. An aromatic amine compound and a diazocarbonyl compound are added into a solvent, reacting is carried out in an oxygenenvironment, and then separating and purifying are carried out to obtain the phenanthridine compound. The invention provides the method for synthesizing the phenanthridine compound by taking the aromatic amine and the diazocarbonyl compound as raw materials for the first time. The method is simple and efficient, does not need metal catalysts, and has wide applicability of substrates.
Owner:SHAANXI UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Benzo [c] phenanthridines as antimicrobial agents Benzo [c] phenanthridines as antimicrobial agents](https://images-eureka.patsnap.com/patent_img/764a375c-7353-41c5-a263-27ec9326a293/US20120022061A1-20120126-D00000.png)
![Benzo [c] phenanthridines as antimicrobial agents Benzo [c] phenanthridines as antimicrobial agents](https://images-eureka.patsnap.com/patent_img/764a375c-7353-41c5-a263-27ec9326a293/US20120022061A1-20120126-D00001.png)
![Benzo [c] phenanthridines as antimicrobial agents Benzo [c] phenanthridines as antimicrobial agents](https://images-eureka.patsnap.com/patent_img/764a375c-7353-41c5-a263-27ec9326a293/US20120022061A1-20120126-D00002.png)
![New application of benzo [C] phenanthridine and protopine in producing overriding resistant bacterium medicament New application of benzo [C] phenanthridine and protopine in producing overriding resistant bacterium medicament](https://images-eureka.patsnap.com/patent_img/1952f98d-dba1-4c2a-9709-072b88d30fd4/s2007100664977e00011.PNG)
![New application of benzo [C] phenanthridine and protopine in producing overriding resistant bacterium medicament New application of benzo [C] phenanthridine and protopine in producing overriding resistant bacterium medicament](https://images-eureka.patsnap.com/patent_img/1952f98d-dba1-4c2a-9709-072b88d30fd4/s2007100664977c00011.PNG)
![New application of benzo [C] phenanthridine and protopine in producing overriding resistant bacterium medicament New application of benzo [C] phenanthridine and protopine in producing overriding resistant bacterium medicament](https://images-eureka.patsnap.com/patent_img/1952f98d-dba1-4c2a-9709-072b88d30fd4/s2007100664977d00021.PNG)
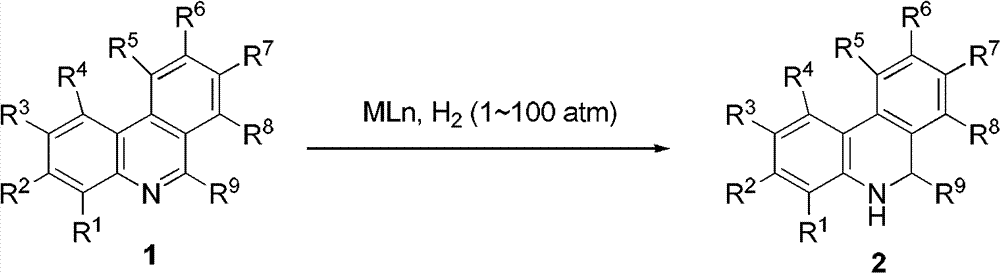
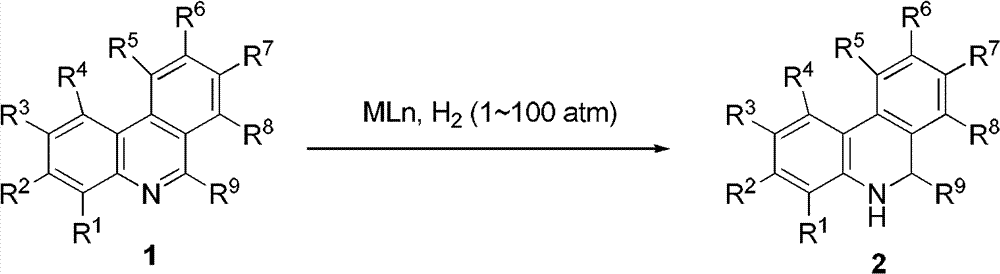
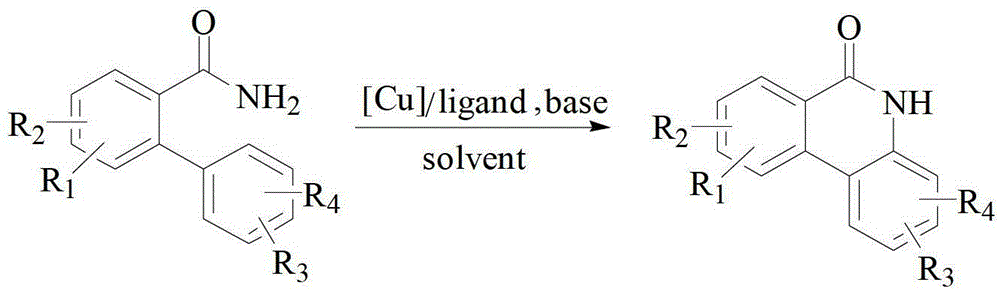
![Pseudobase benzo[c]phenantridines with improved efficacy, stability and safety Pseudobase benzo[c]phenantridines with improved efficacy, stability and safety](https://images-eureka.patsnap.com/patent_img/42cd5dc9-1921-4b8d-aca8-55bee39c3718/US20100256164A1-20101007-C00001.png)
![Pseudobase benzo[c]phenantridines with improved efficacy, stability and safety Pseudobase benzo[c]phenantridines with improved efficacy, stability and safety](https://images-eureka.patsnap.com/patent_img/42cd5dc9-1921-4b8d-aca8-55bee39c3718/US20100256164A1-20101007-C00002.png)
![Pseudobase benzo[c]phenantridines with improved efficacy, stability and safety Pseudobase benzo[c]phenantridines with improved efficacy, stability and safety](https://images-eureka.patsnap.com/patent_img/42cd5dc9-1921-4b8d-aca8-55bee39c3718/US20100256164A1-20101007-C00003.png)
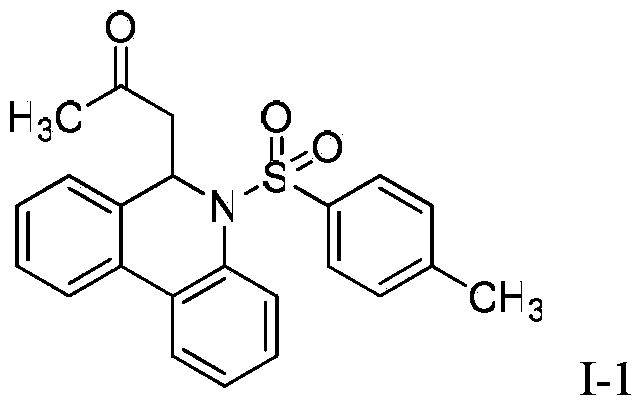
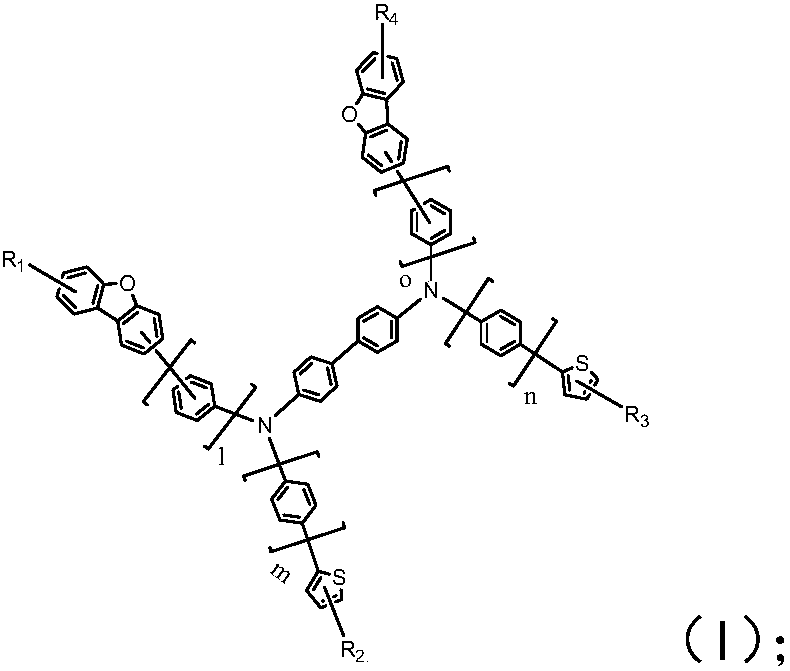
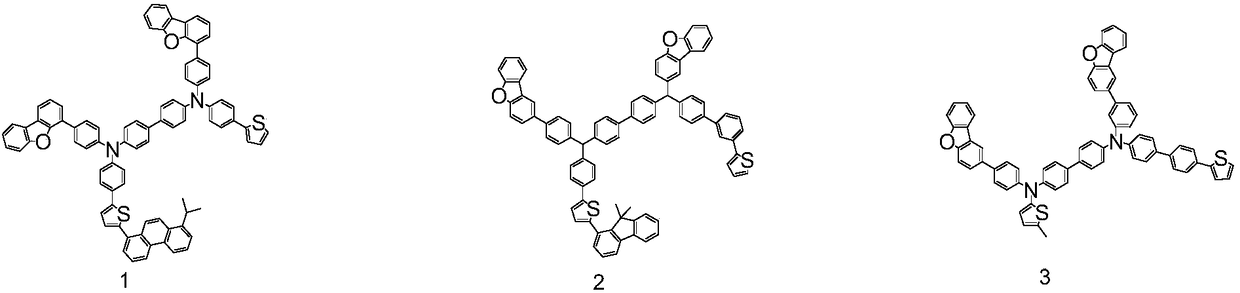
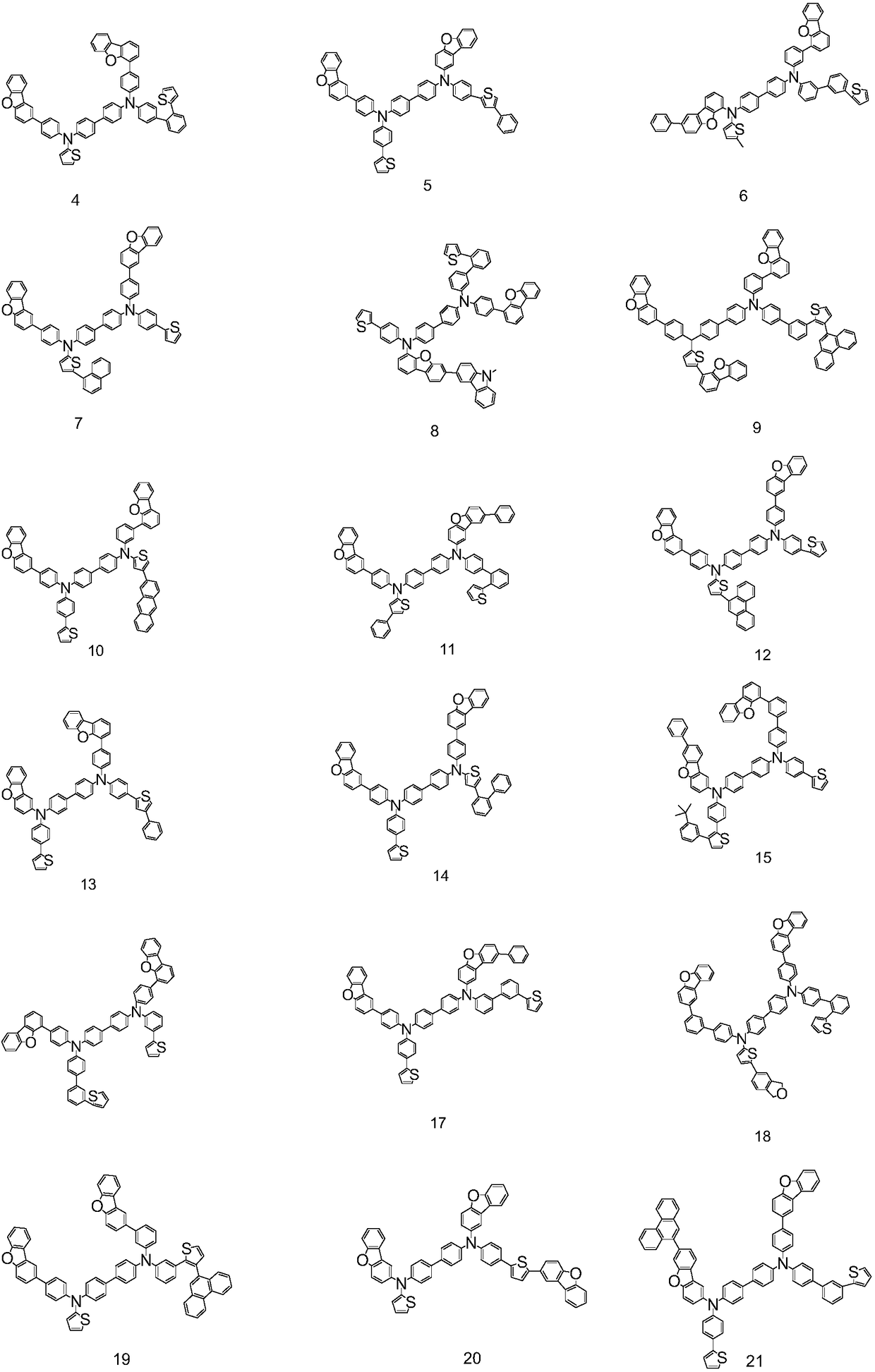
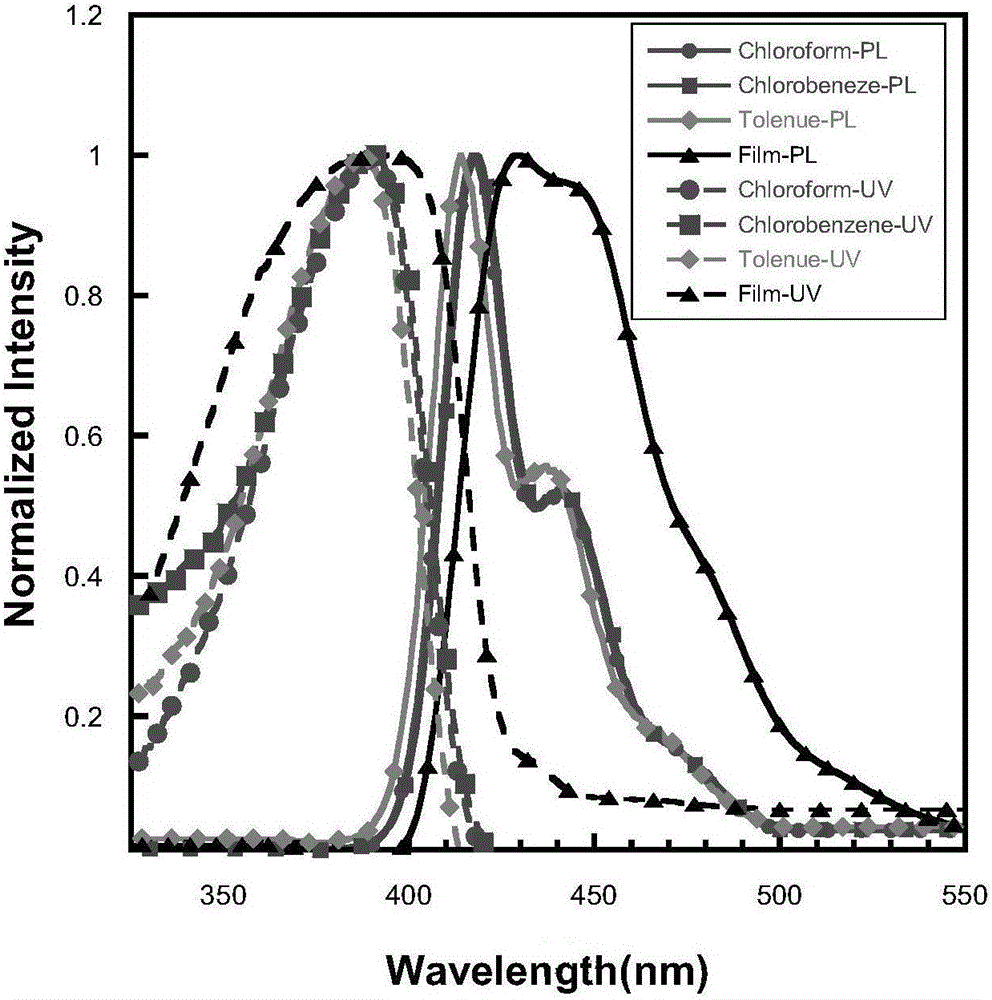
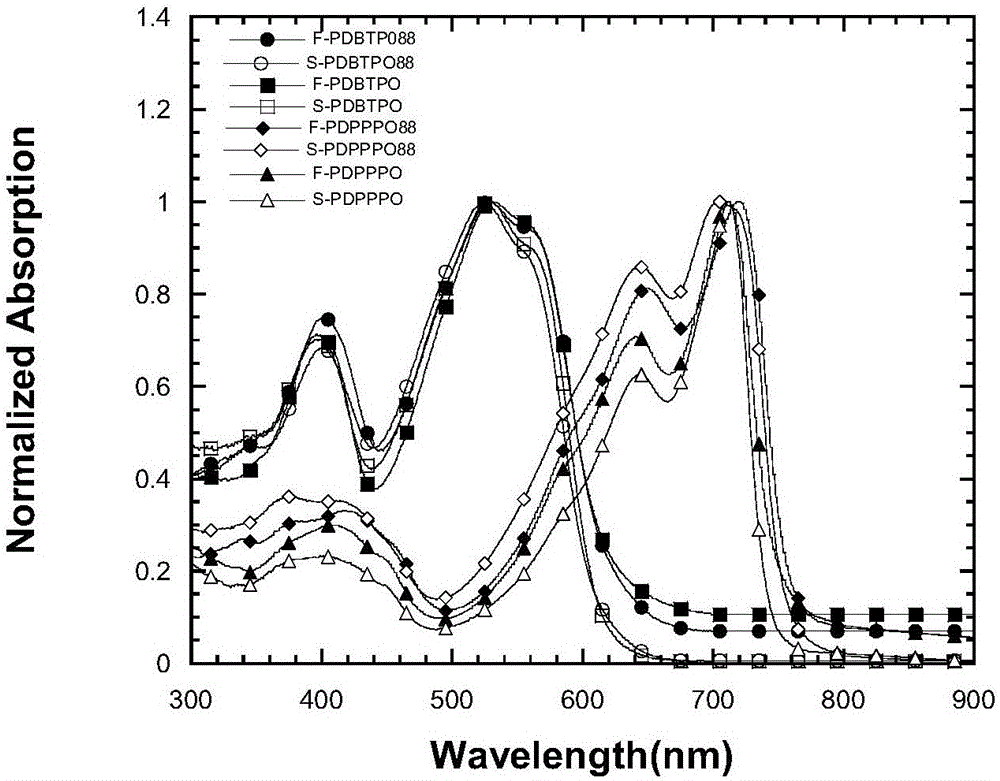
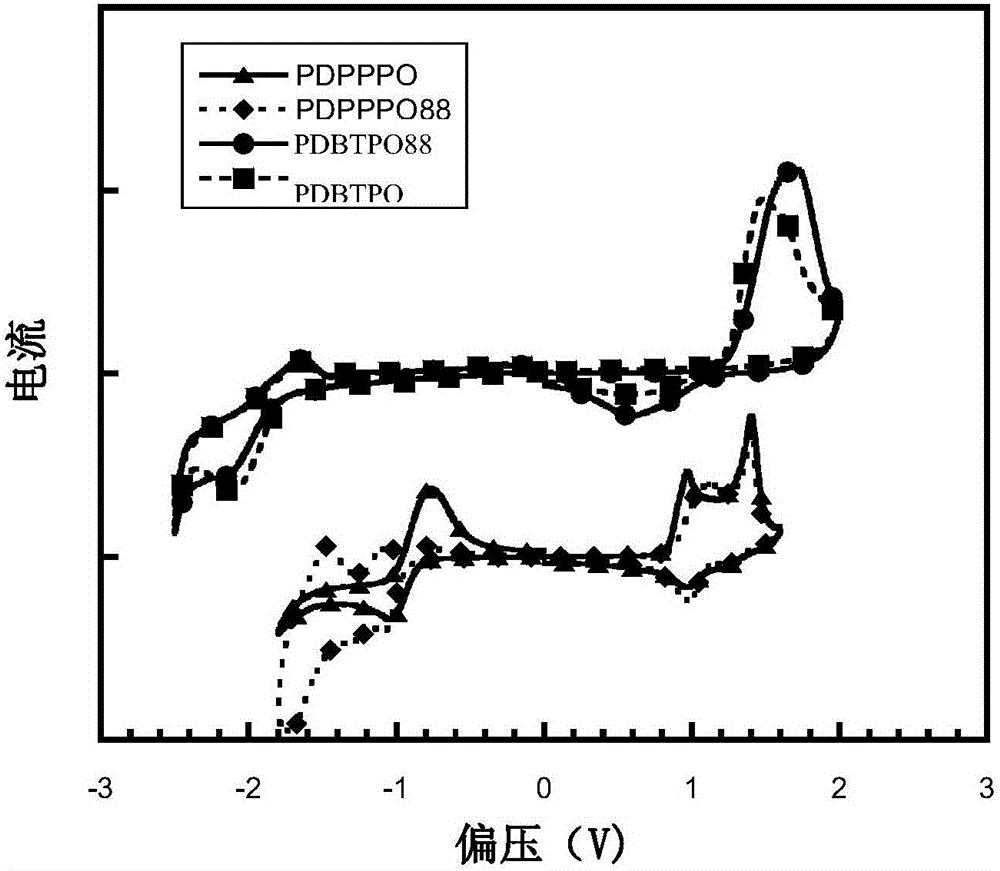
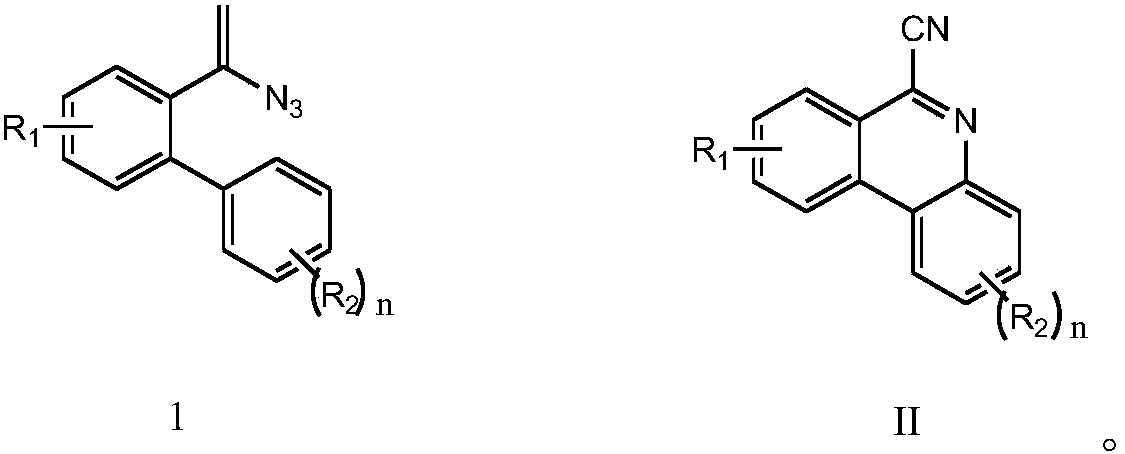

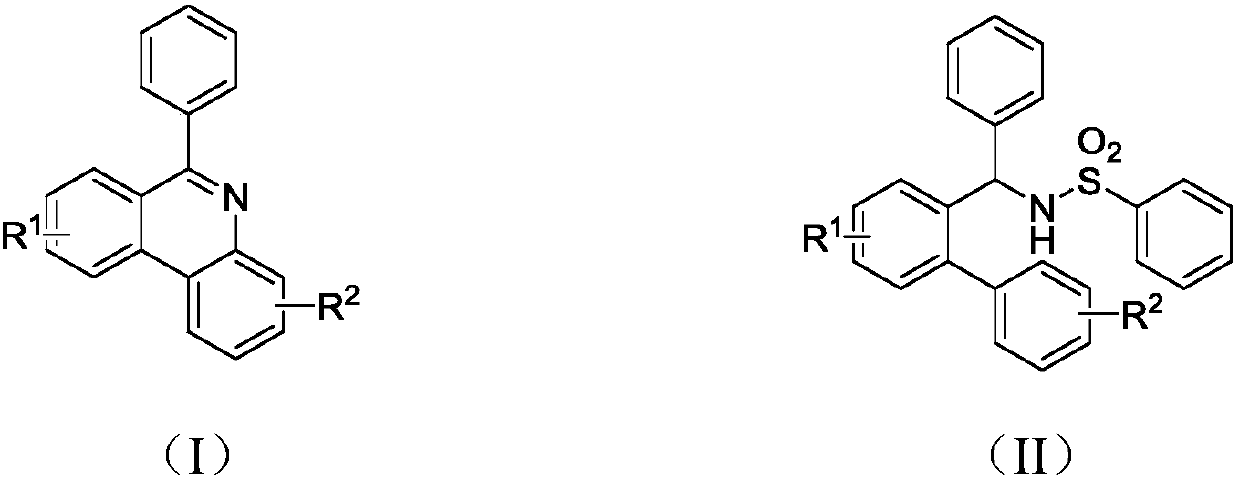
![Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device](https://images-eureka.patsnap.com/patent_img/c79237f6-fdc7-4c52-9239-219f382fae14/HDA0002486394790000011.png)
![Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device](https://images-eureka.patsnap.com/patent_img/c79237f6-fdc7-4c52-9239-219f382fae14/HDA0002486394790000012.png)
![Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device Method for continuously preparing dihydrobenzo [j] phenanthridine compound containing trifluoromethyl functional group by using micro-channel reaction device](https://images-eureka.patsnap.com/patent_img/c79237f6-fdc7-4c52-9239-219f382fae14/HDA0002486394790000021.png)

![Pseudobase benzo[c]phenanthridines with improved efficacy, stability, and safety Pseudobase benzo[c]phenanthridines with improved efficacy, stability, and safety](https://images-eureka.patsnap.com/patent_img/02256eab-15dd-48db-a501-5e8860657fe1/US20080076781A1-20080327-C00001.png)
![Pseudobase benzo[c]phenanthridines with improved efficacy, stability, and safety Pseudobase benzo[c]phenanthridines with improved efficacy, stability, and safety](https://images-eureka.patsnap.com/patent_img/02256eab-15dd-48db-a501-5e8860657fe1/US20080076781A1-20080327-C00002.png)
![Pseudobase benzo[c]phenanthridines with improved efficacy, stability, and safety Pseudobase benzo[c]phenanthridines with improved efficacy, stability, and safety](https://images-eureka.patsnap.com/patent_img/02256eab-15dd-48db-a501-5e8860657fe1/US20080076781A1-20080327-C00003.png)
![Synthetic method for (1,2,3-triazolyl)[1, 5-f]phenanthridine-10-ethyl carboxylate compound Synthetic method for (1,2,3-triazolyl)[1, 5-f]phenanthridine-10-ethyl carboxylate compound](https://images-eureka.patsnap.com/patent_img/3e7532c4-ef12-4abe-8d10-602002597d9f/BDA0001331704530000011.png)
![Synthetic method for (1,2,3-triazolyl)[1, 5-f]phenanthridine-10-ethyl carboxylate compound Synthetic method for (1,2,3-triazolyl)[1, 5-f]phenanthridine-10-ethyl carboxylate compound](https://images-eureka.patsnap.com/patent_img/3e7532c4-ef12-4abe-8d10-602002597d9f/BDA0001331704530000031.png)
![Synthetic method for (1,2,3-triazolyl)[1, 5-f]phenanthridine-10-ethyl carboxylate compound Synthetic method for (1,2,3-triazolyl)[1, 5-f]phenanthridine-10-ethyl carboxylate compound](https://images-eureka.patsnap.com/patent_img/3e7532c4-ef12-4abe-8d10-602002597d9f/BDA0001331704530000041.png)