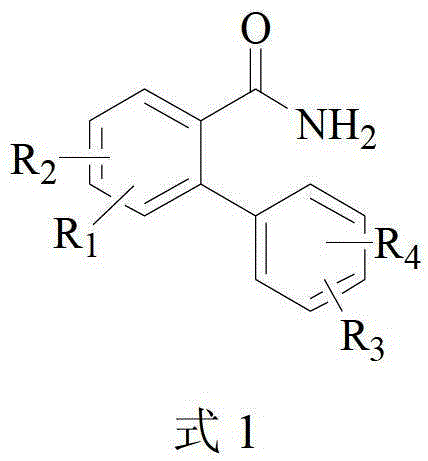
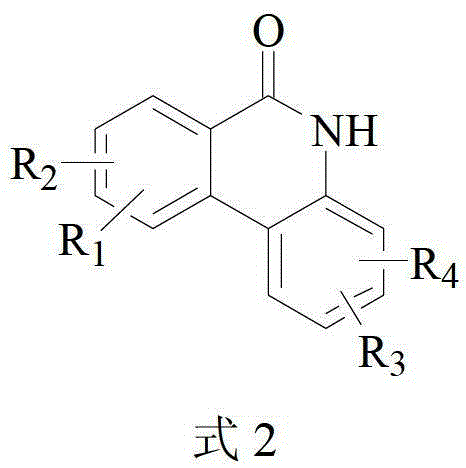
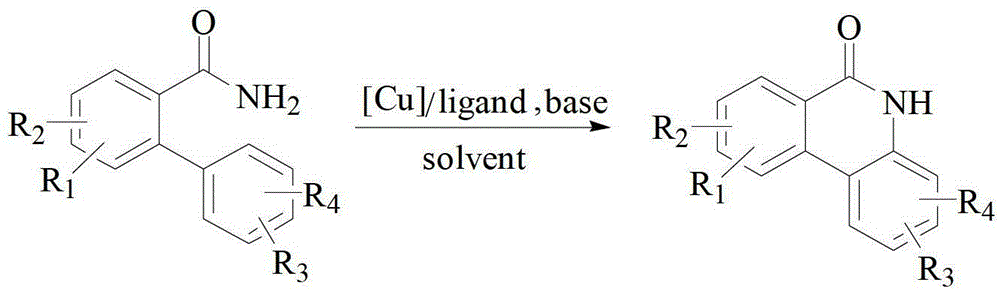Method for catalytically preparing 6(5H)-phenanthridine ketone by copper component
A copper compound, a catalytic preparation technology, applied in the direction of organic chemistry, etc., to achieve the effect of facilitating the development of industrial production, broad application prospects, and simple recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the synthesis of 6 (5H)-phenanthridone
[0027] Substrate 197mg (1mmol) 2-phenylbenzamide, catalyst 19mg (0.1mmol) CuI, ligand 52mg (0.2mmol) PPh 3 , base 224mg (2mmol) KO-tBu, and solvent 3ml o-xylene were added to a 30ml sealed tube in an air environment. Then the sealed tube was placed in an oil bath at 120°C for 12 h. After the reaction, the reaction liquid was cooled to room temperature, 80 ml of saturated brine was added, and then extracted three times with 100 ml of ethyl acetate, and the organic layers were combined. The organic layer was dried with anhydrous sodium sulfate, filtered, and the filtrate was distilled under reduced pressure and separated by silica gel column chromatography (ethyl acetate / petroleum ether: 1 / 2 as eluent) to obtain 175.5 mg of white solid with a yield of 90%.
[0028] 1 H NMR (400MHz, DMSO-d 6 ):δ11.69(s,1H),8.52(d,J=8.0Hz,1H),8.40(d,J=8.0Hz,1H),8.83(d,J=8.0Hz,1H),7.86(t ,J=7.6Hz,1H),7.65(t,J=7.6Hz,1H),7.50(t,J=7.6H...
Embodiment 2
[0031] Embodiment 2: the synthesis of 3,8-dimethyl-6 (5H)-phenanthridinone
[0032] Substrate 225mg (1mmol) 4,4′-dimethyl-[1,1′-biphenyl]-2-amide, catalyst 19mg (0.1mmol) CuI, ligand 52mg (0.2mmol) PPh 3 , base 224mg (2mmol) KO-tBu, and solvent 3ml o-xylene were added to a 30ml sealed tube in an air environment. Then the sealed tube was placed in an oil bath at 120°C for 12 h. After the reaction, the reaction liquid was cooled to room temperature, 80 ml of saturated brine was added, and then extracted three times with 100 ml of ethyl acetate, and the organic layers were combined. The organic layer was dried with anhydrous sodium sulfate, filtered, and the filtrate was distilled under reduced pressure and separated by silica gel column chromatography (ethyl acetate / petroleum ether: 1 / 2 as eluent) to obtain 174.0 mg of white solid with a yield of 78%.
[0033] 1 H NMR (400MHz, DMSO-d 6 ):δ11.54(s,1H),8.33(d,J=8.0Hz,1H),8.21(d,J=8.0Hz,1H),8.10(s,1H),7.64(d,J=8.0Hz ,1H),7.14(...
Embodiment 3
[0036] Embodiment 3: the synthesis of 8-methoxy-3-methyl-6 (5H)-phenanthridinone
[0037] Substrate 241mg (1mmol) 4-methoxy-4′-methyl-[1,1′-biphenyl]-2-amide, catalyst 19mg (0.1mmol) CuI, ligand 52mg (0.2mmol) PPh 3 , base 224mg (2mmol) KO-tBu, and solvent 3ml o-xylene were added to a 30ml sealed tube in an air environment. Then the sealed tube was placed in an oil bath at 120°C for 12 h. After the reaction, the reaction liquid was cooled to room temperature, 80 ml of saturated brine was added, and then extracted three times with 100 ml of ethyl acetate, and the organic layers were combined. The organic layer was dried with anhydrous sodium sulfate, filtered, and the filtrate was distilled under reduced pressure and separated by silica gel column chromatography (ethyl acetate / petroleum ether: 1 / 2 as eluent) to obtain 203.2 mg of white solid with a yield of 85%.
[0038] 1 H NMR (400MHz, DMSO-d 6 ):δ11.65(s,1H),8.39(d,J=8.0Hz,1H),8.18(d,J=8.0Hz,1H),7.73(s,1H),7.42(d,J=8.8Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com