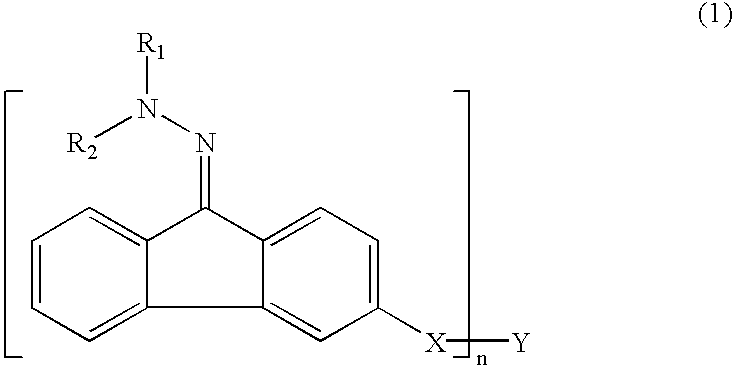
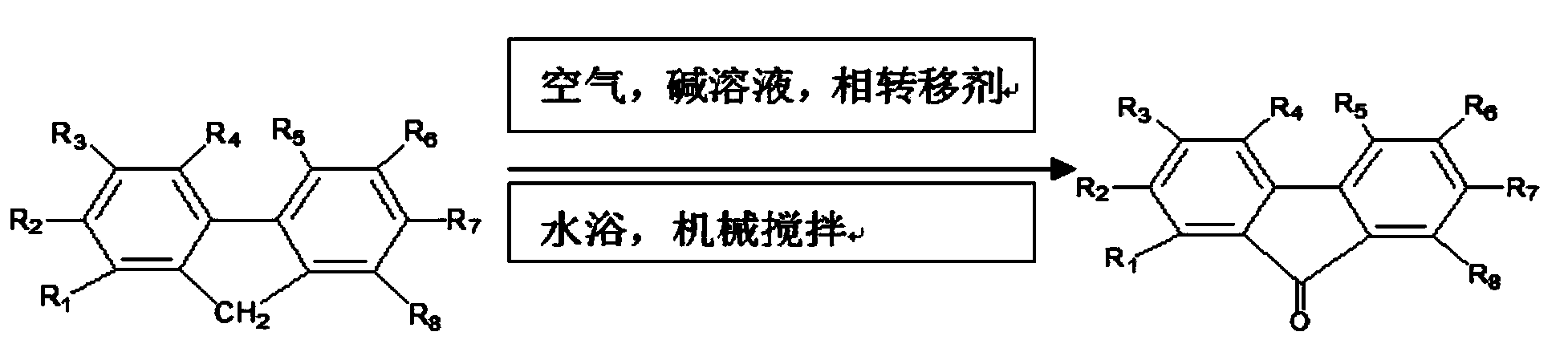
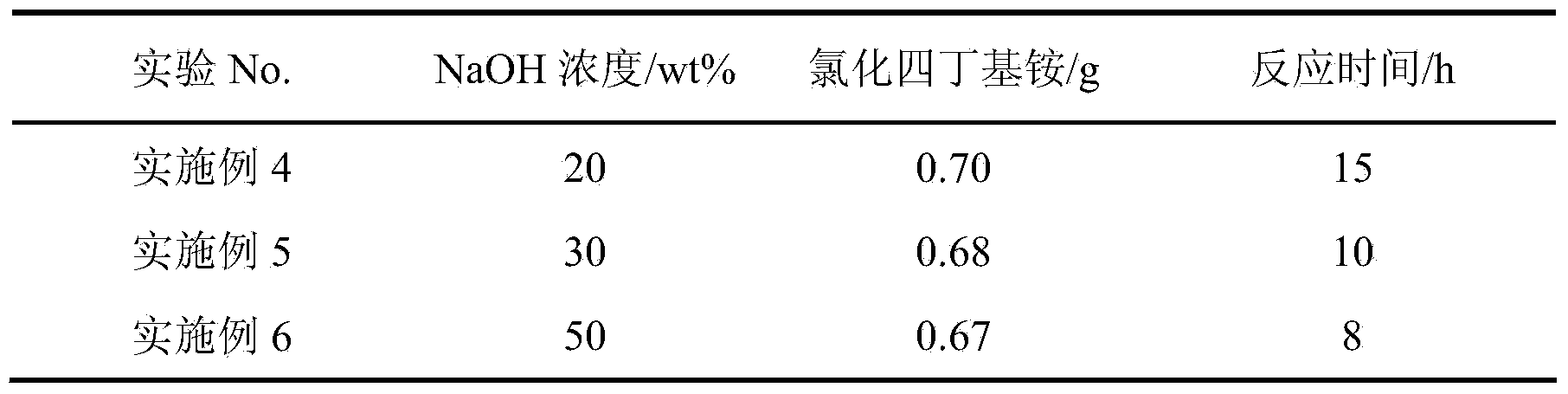
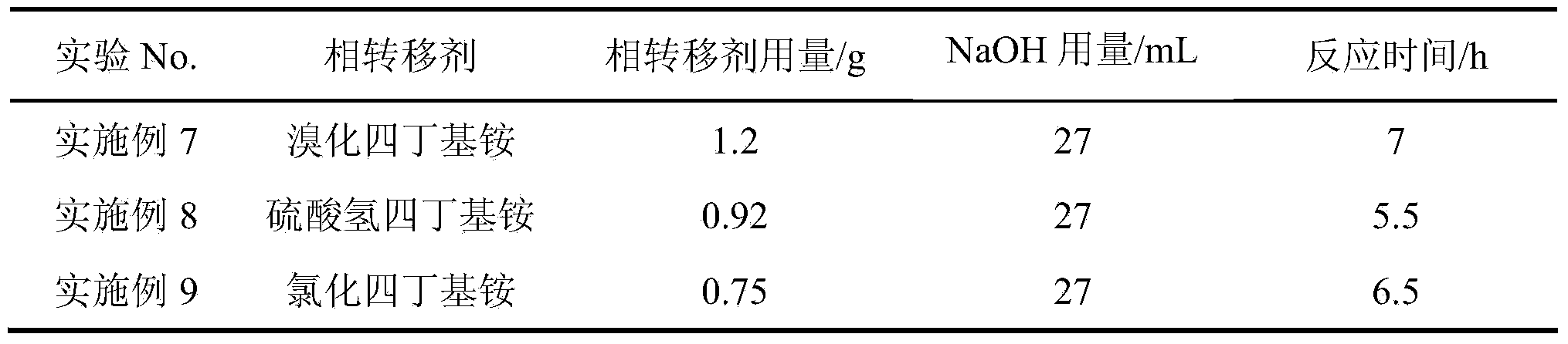
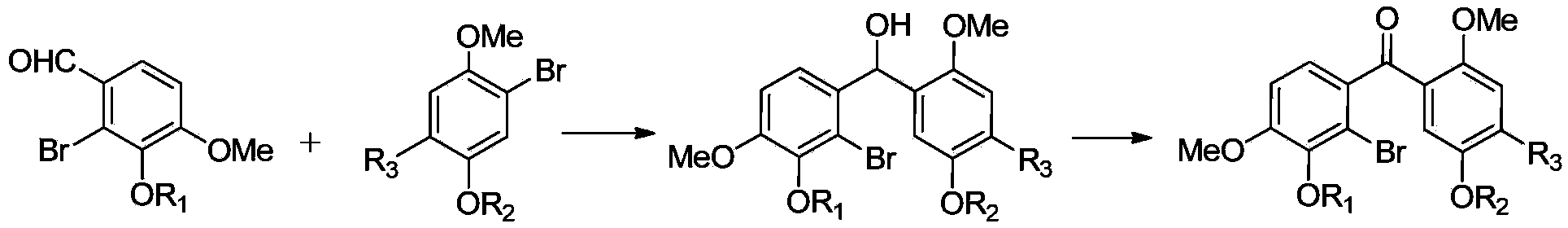
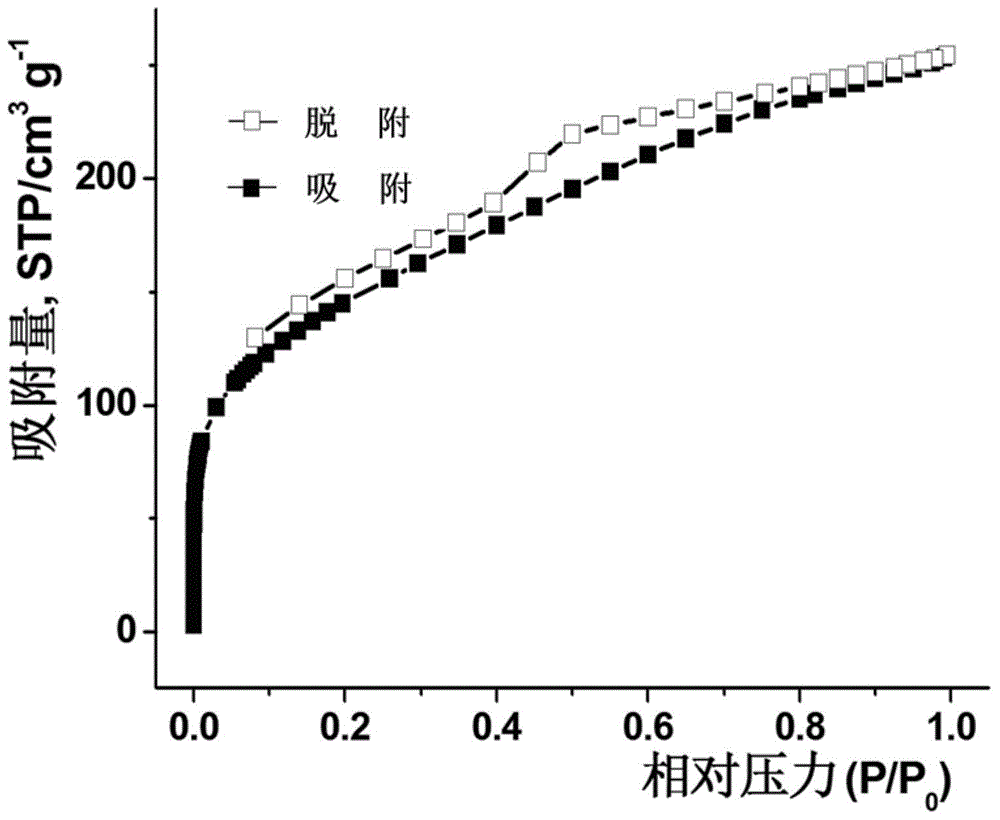
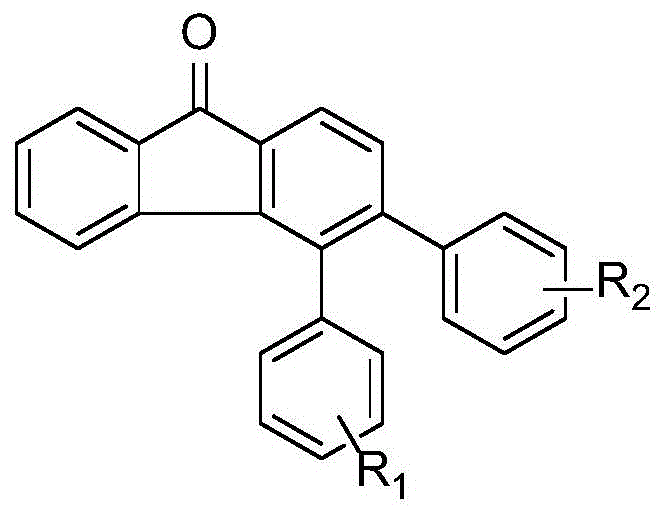
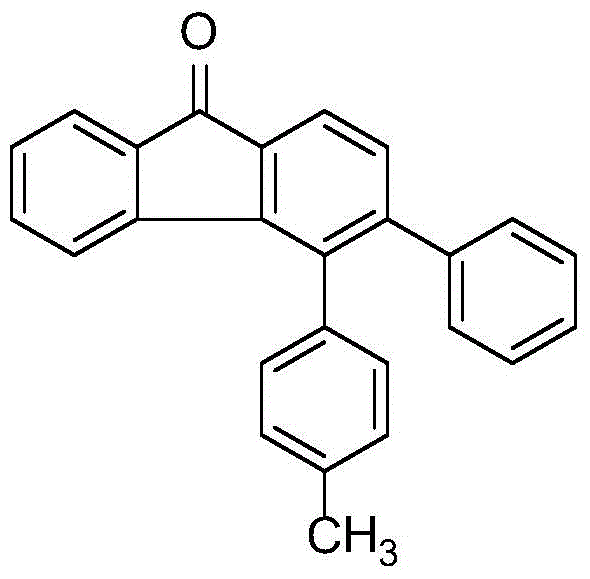
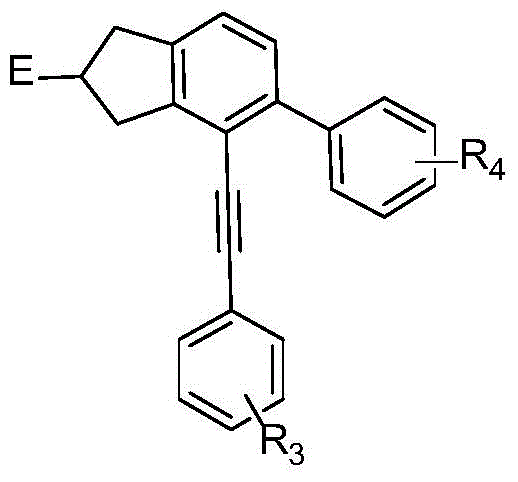
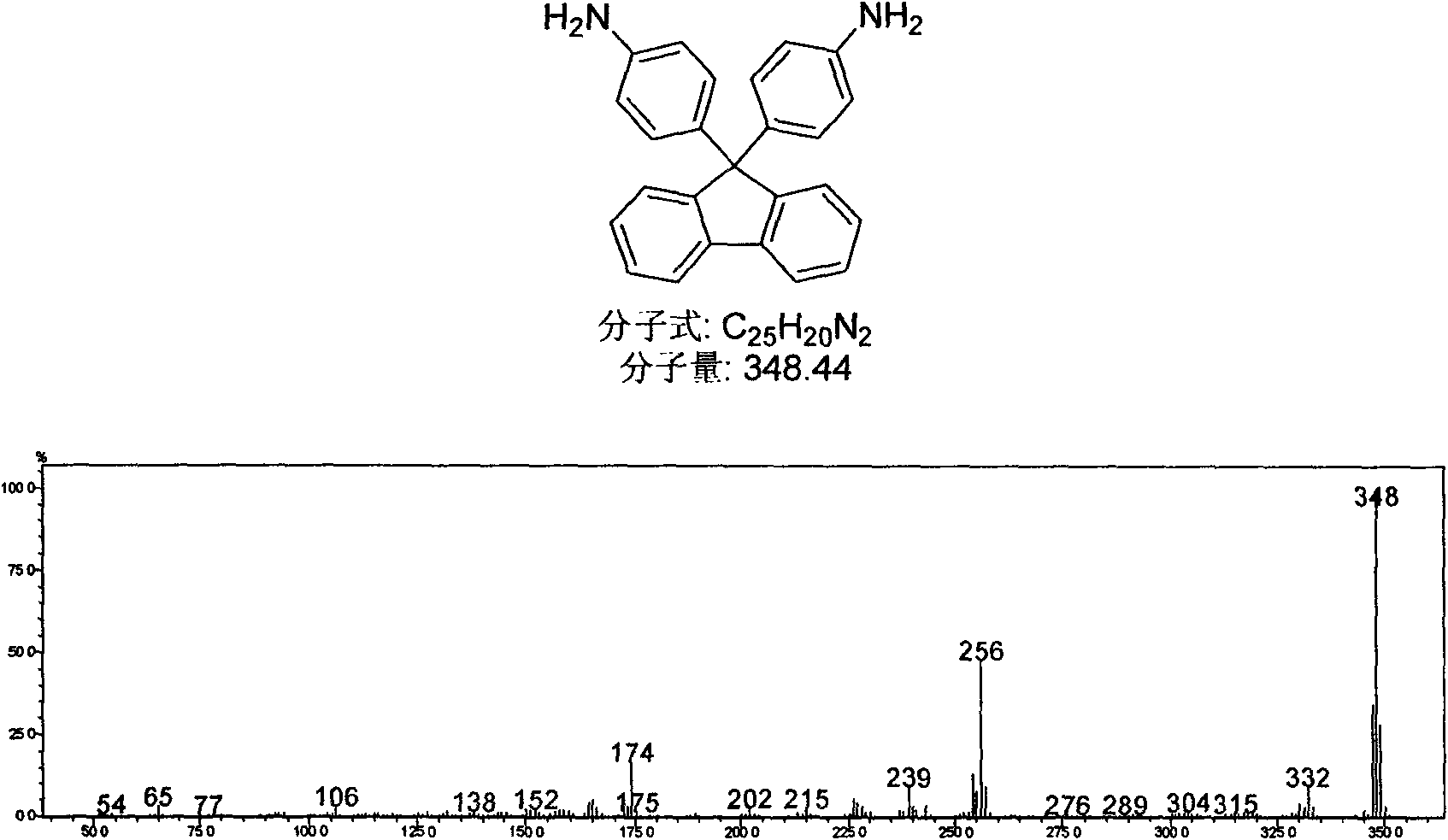
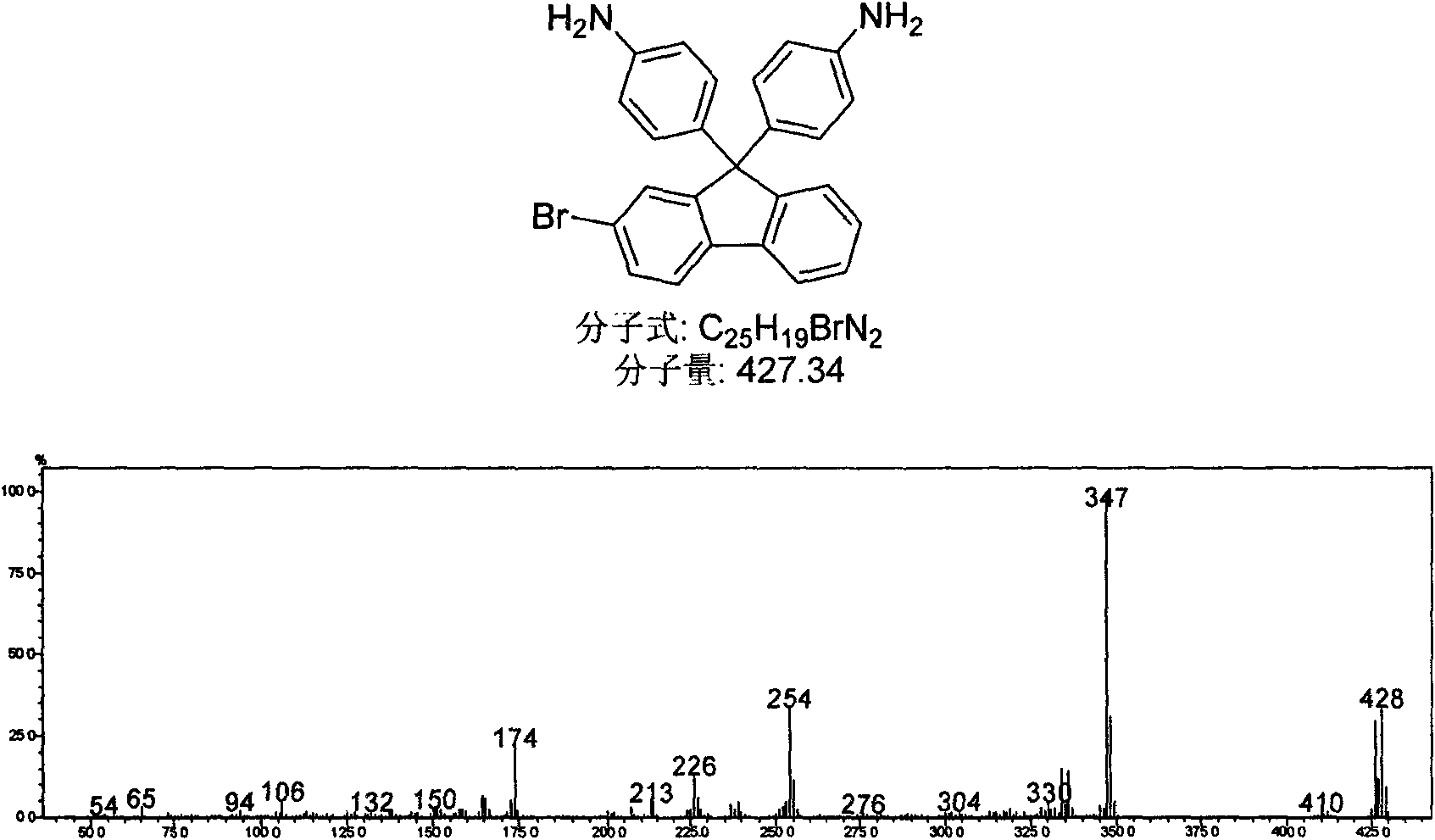
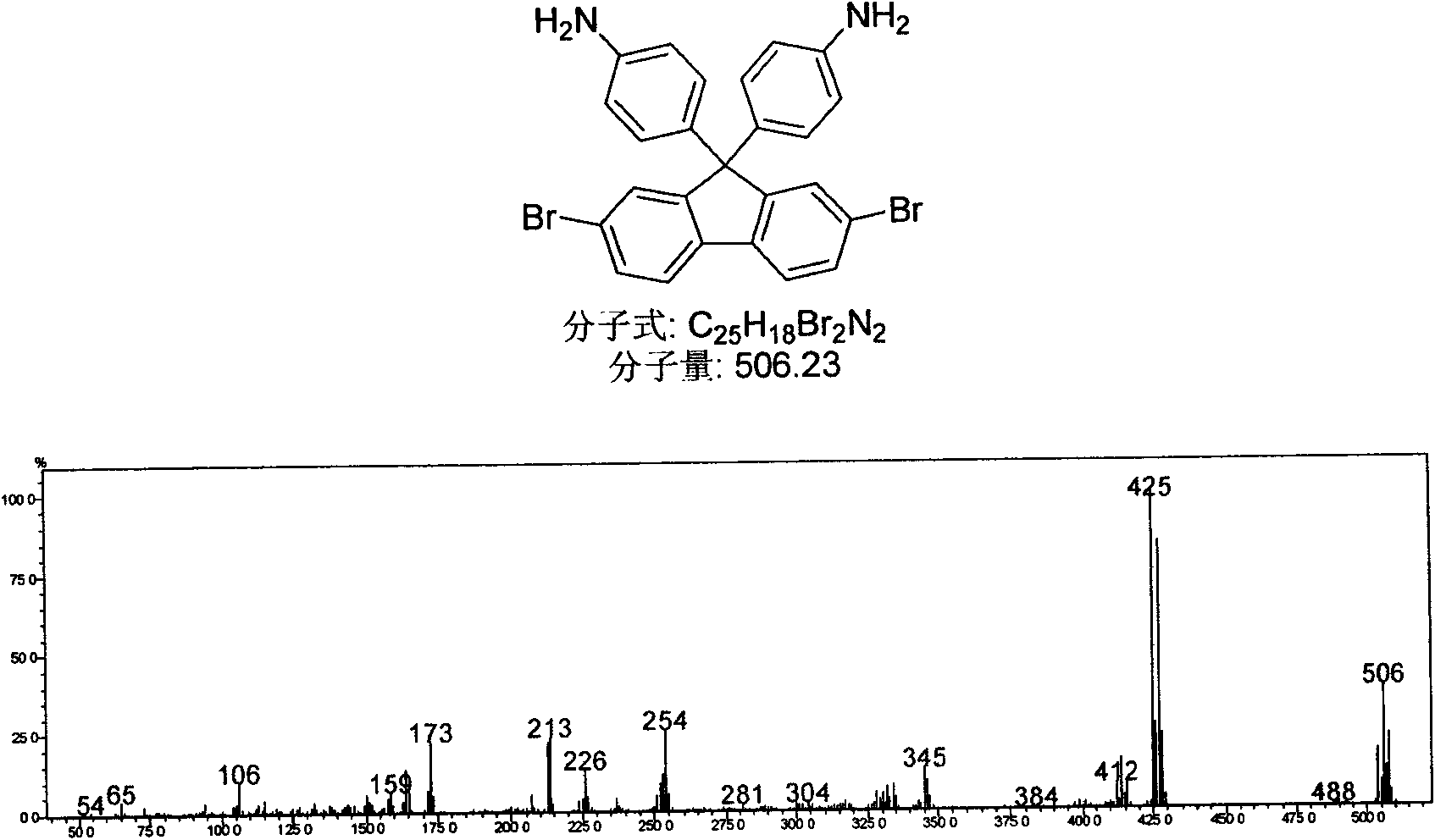
Patents
Literature
320 results about "Fluorenone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fluorenone is an aromatic organic compound with the chemical formula C₁₃H₈O. It is used to make antimalaria drugs. It can be synthesised from fluorene with the addition of glacial acetic acid and sodium hypochlorite solution, undergoing an oxidation reaction. It is bright fluorescent yellow in color and is a solid at room temperature.
Method of purifying aromatic dicarboxylic acids
InactiveUS6265608B1MinimizationReduce usageOrganic compound preparationCarboxylic compound separation/purificationPalladium catalystCarboxylic acid
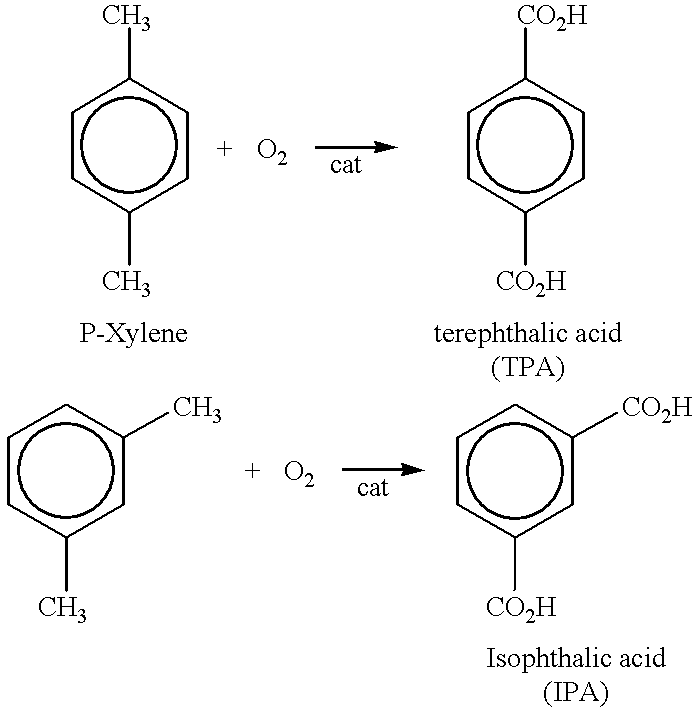
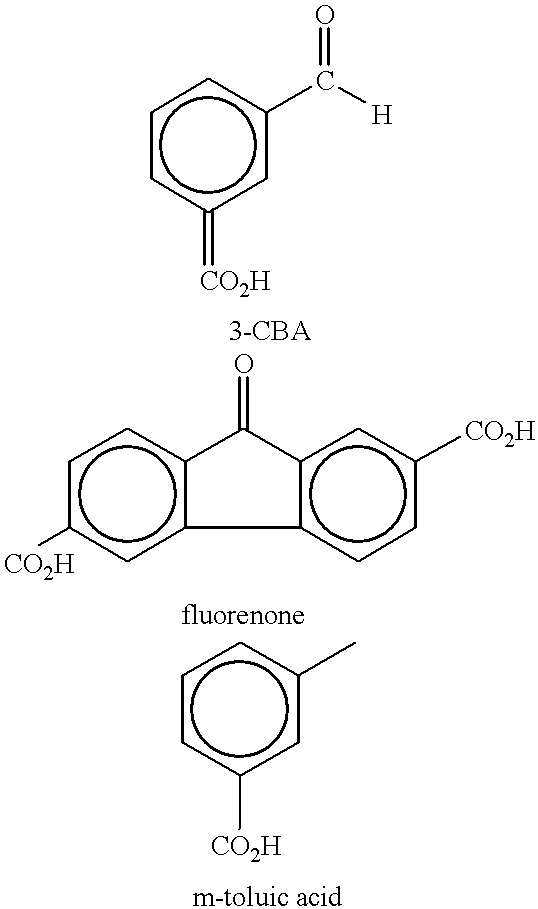
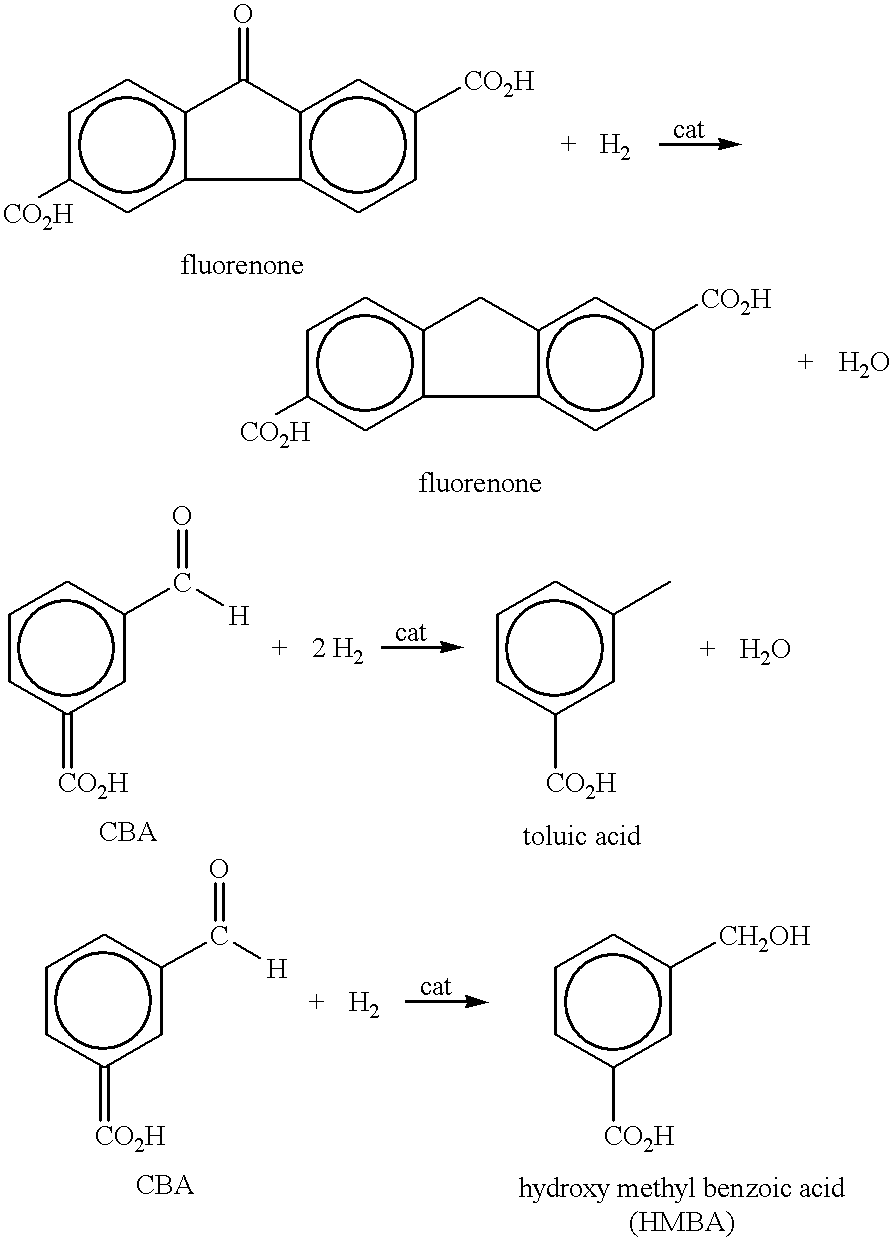
An aromatic dicarboxylic acid is purified by oxidizing m-xylene or p-xylene to produce crude isophthalic acid or crude terephthalic acid, respectively. The products of the oxidizing step are hydrogenated in the presence of a palladium catalyst. Carbon monoxide is introduced during the hydrogenation step. The palladium catalyst is provided on a carbon substrate. The products of the oxidizing step are dissolved in a solvent, which may be water, prior to the hydrogenation step. The products of the oxidizing step may be dissolved at an elevated temperature, above the normal boiling point of the solvent. The oxidation step produces isophthalic acid, 3-carboxybenzaldehyde and fluorenones in the case of oxidizing m-xylene and produces terephthalic acid, 4-carboxybenzaldehyde and fluorenones in the case of oxidizing p-xylene. It may be helpful to monitor the disappearance of 3-carboxybenzaldehyde in the case of oxidizing m-xylene and 4-carboxybenzaldehyde in the case of oxidizing pxylene, and reducing the amount of carbon monoxide when the rate of disappearance is below a predetermined minimum. After the hydrogenation step, the isophthalic acid or terephthalic acid may be crystallized. The carbon monoxide may be maintained at a concentration of 100 to 500 ppm based on added hydrogen and carbon monoxide. Other aromatic dicarboxylic acids may also purified by this procedure.
Owner:GRUPO PETROTEMEX DE C V
Method for producing 9-fluorenone
ActiveCN102020543ALow costEasy to getOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDistillationFluorenone
The invention relates to a method for producing 9-fluorenone, comprising the following steps of: (1) feeding industrial fluorine as a raw material and methylbenzene as a solvent into a reaction kettle according to the weight ratio of 1:(6-8) with quaternary ammonium salt as a catalyst; heating the mixture to 40-80DEG C; adding the catalyst after the raw material is completely dissolved at the addition of 5-20 percent of industrial fluorine; introducing the air according to 2-3m<3> / h of each kilo of industrial fluorine and reacting under the condition of 40-80DEG C for 2-5h; (2) after the reaction is ended, pouring a reactant into a distillation kettle for distillation at normal temperature, recovering the solvent and obtaining an industrial fluorine crude product from the bottom of the kettle; and (3) recrystallizing the crude product with the solvent to obtain fluorenone with the content of 99 percent. The method has the advantages of simple process flow, fewer reactants and low cost, is easy to control and can be widely used for producing the fluorenone; and the finished products prepared by the method have favorable quality.
Owner:鞍钢化学科技有限公司
Method for oxidizing fluorene to 9-fluorenone
ActiveCN1754867AIncrease profitHigh yieldOrganic compound preparationCarbonyl compound preparationWastewaterOxygen
The invention discloses a method to prepare 9-fluorenone with fluorene. Wherein, using dimethyl sulfoxide as solvent, sodium hydroxide as catalyst, oxygen as oxidant and tower filling reactor; cooling and filtering the reacted liquid to obtain 93% coarse fluorenone; distilling liquid and recycling 94% solvent and some coarse fluorenone; refining with oriented crystallization method and obtaining yellow piece 99.8% fluorenone. This method is simple, needs short reaction time, produces no waste water, and convenient to produce in industry.
Owner:SHANGHAI HUAYI ENERGY CHEM
Preparation method of 9-fluorenone
InactiveCN102391087AEasy to handleReduce pollutionOrganic compound preparationCarbonyl compound preparationDistillationGas phase
The invention relates to the technical field of production of an aromatic compound 9-fluorenone, in particular to a preparation method of the 9-fluorenone. The preparation method includes the steps as follows: 1) taking industrial fluorene, benzene series solvent, sodium hydroxide and quaternary ammonium salt, and adding the four components in a four-mouth bottle; 2) stirring the mixture in normal pressure, increasing the temperature to above 90 DEG C, reacting, and introducing air for oxidization; 3) conducting reduced-pressure distillation and allowing concentrate to crystallize, thus obtaining yellow fluorenone crystals; and 4) washing the fluorenone crystals for one time and then drying the fluorenone crystals, thus obtaining the 9-fluorenone product. The benzene series solvent is toluene or dimethylbenzene. Compared with the prior art, the preparation method has the benefits as follows: 1) the reaction temperature is low, the operation is simple and convenient and the reaction conditions are moderate; 2) the cheap sodium hydroxide is taken as a catalyst, so the cost is low; 3) the benzene series solvent can be recycled by reduced-pressure distillation, washing water is little in quantity, treatment is easy and pollution to environment is small; and 4) the gas chromatographic purity of the product is higher than 99.2%, the yield is higher than 86.7% and the production requirements are met.
Owner:SINOSTEEL ANSHAN RES INST OF THERMO ENERGY CO LTD
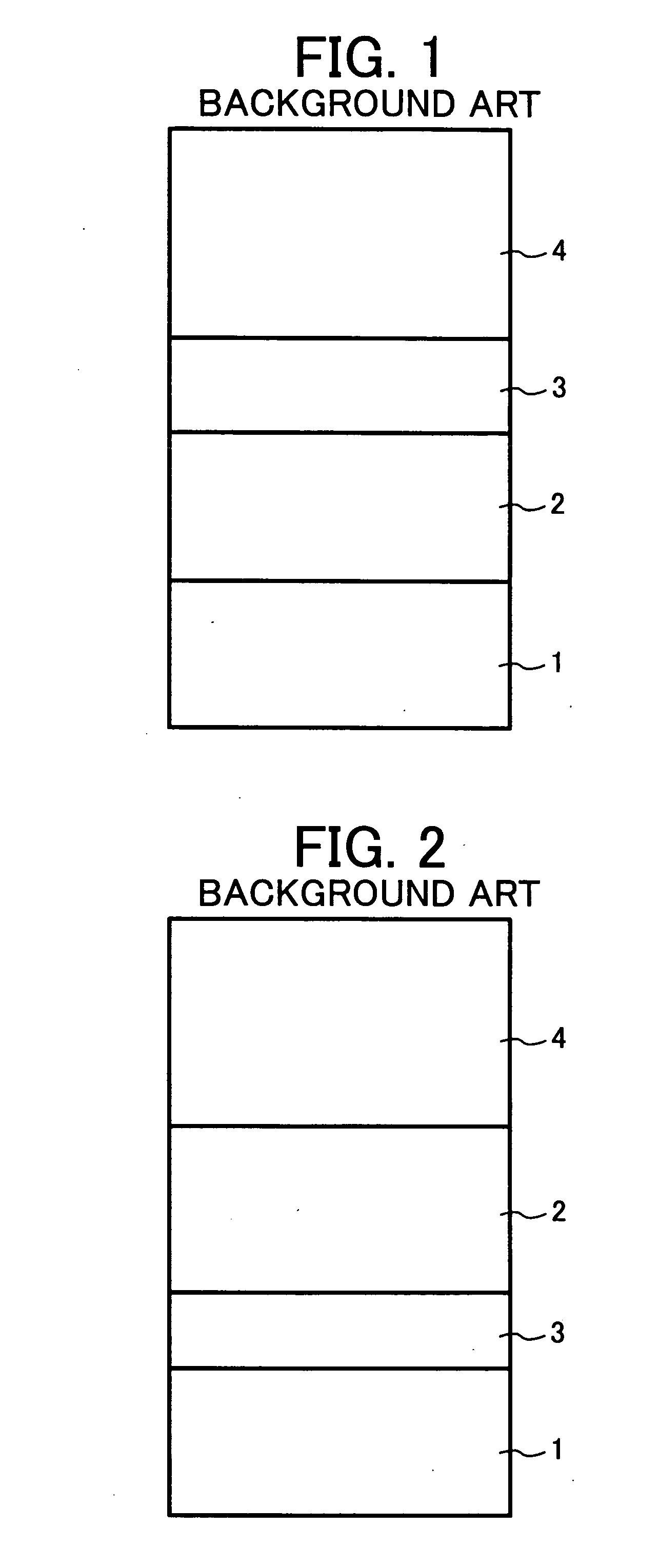
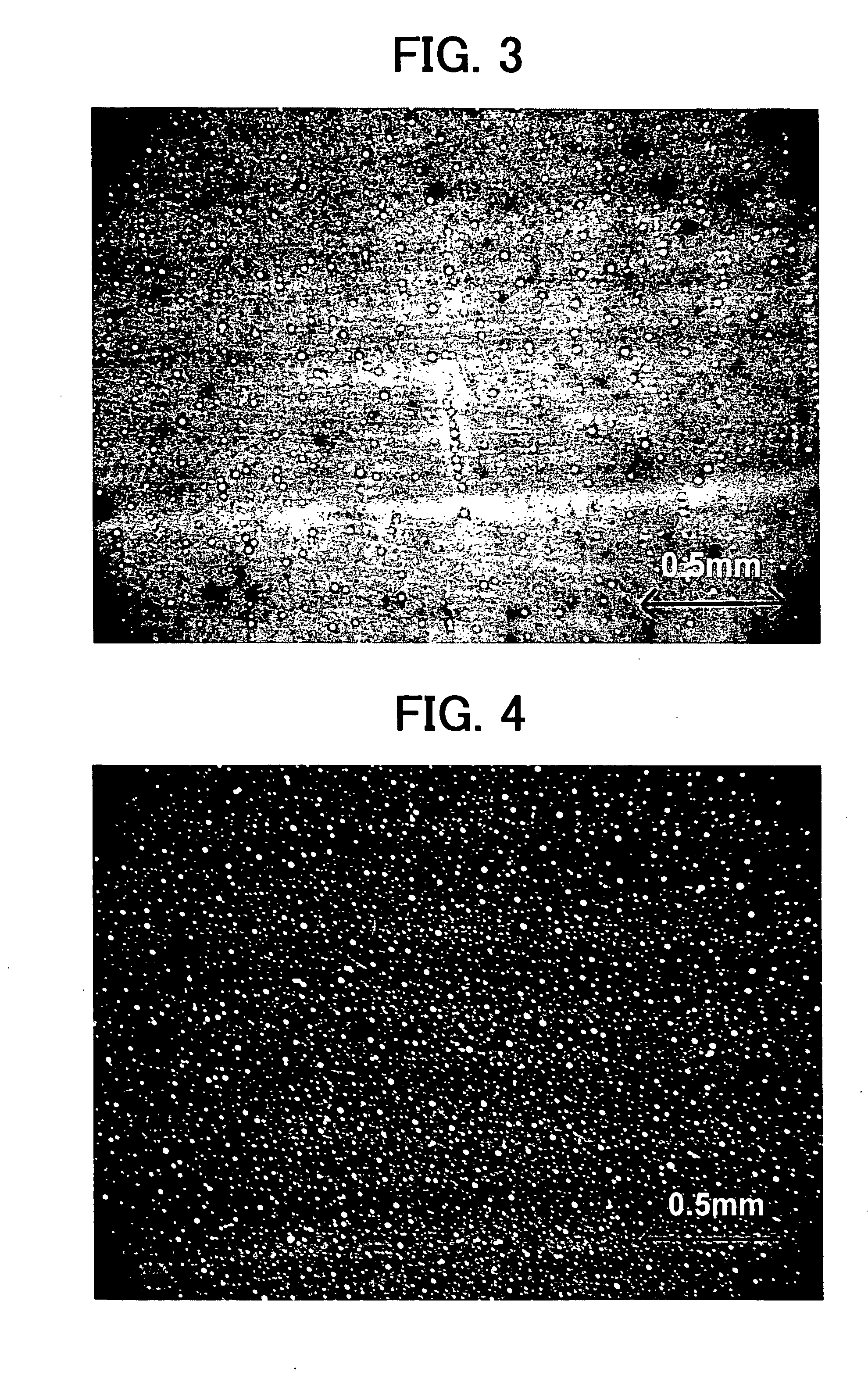
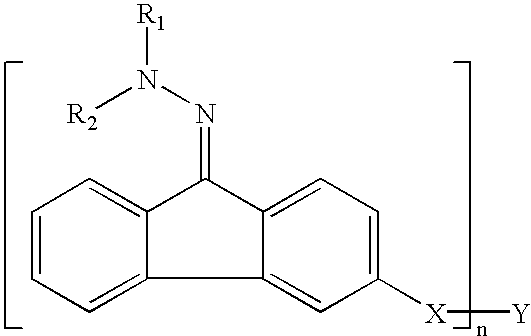
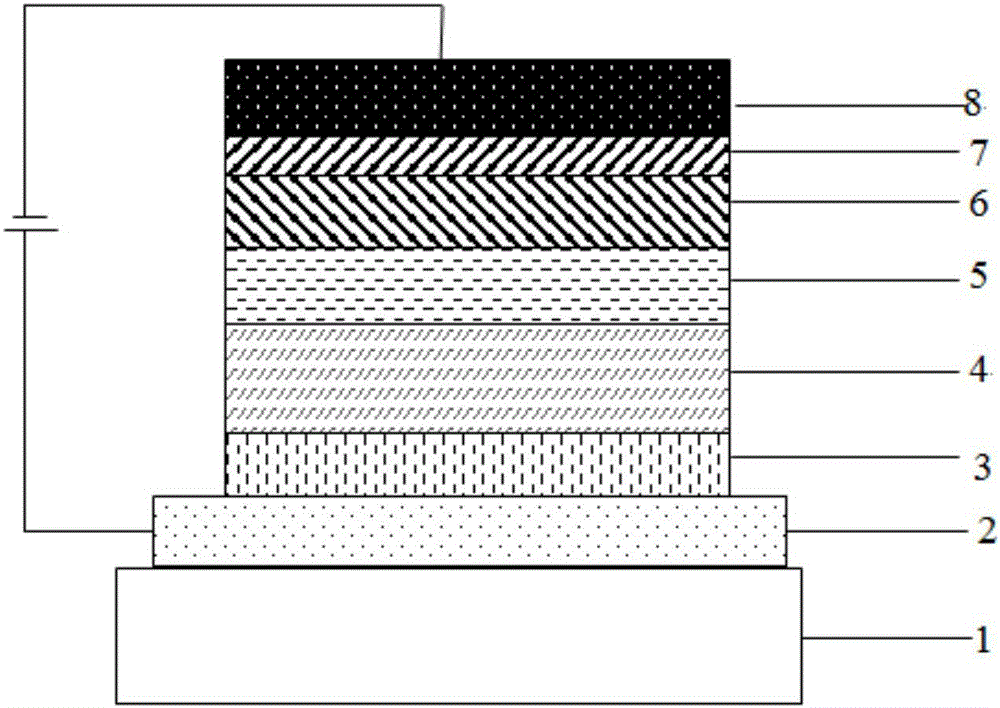
Electrophotographic photoreceptor, and image forming apparatus and process cartridge using the electrophotographic photoreceptor
InactiveUS20060008719A1High quality imagingDifficult problemElectrographic process apparatusCorona dischargeImage formationPore diameter
A photoreceptor including at least an electroconductive substrate, and a charge blocking layer, a moiré preventing layer, and a photosensitive layer, which are located overlying the electroconductive substrate in this order, wherein the photosensitive layer includes an azo pigment having a fluorenone skeleton. The photosensitive layer is preferably prepared by coating a coating liquid including a dispersion which is prepared by dispersing the azo pigment in a solvent to an extent such that the average particle diameter of the azo pigment is not greater than 0.3 μm and the standard deviation of the particle diameter is not greater than 0.2 μm, followed by filtering with a filter having an effective pore diameter not greater than 5 μm. An image forming apparatus and a process cartridge including the photoreceptor.
Owner:RICOH KK
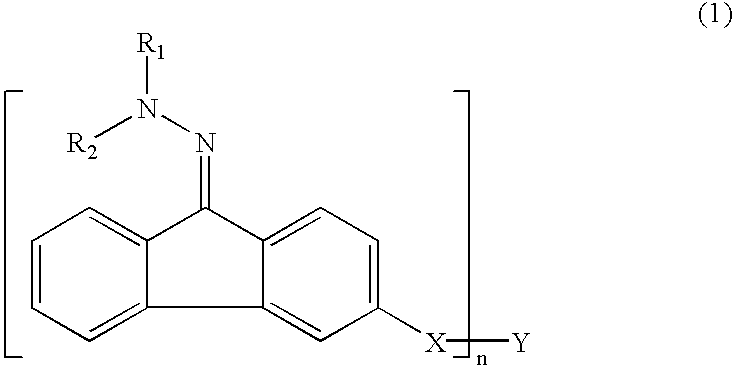
Electrophotographic organophotoreceptors with novel charge transport materials
InactiveUS6905804B2High quality imagingQuality improvementOrganic chemistryOrganic compound preparationArylHydrazone
Owner:S PRINTING SOLUTION CO LTD
Novel polymer composition and method of making the same
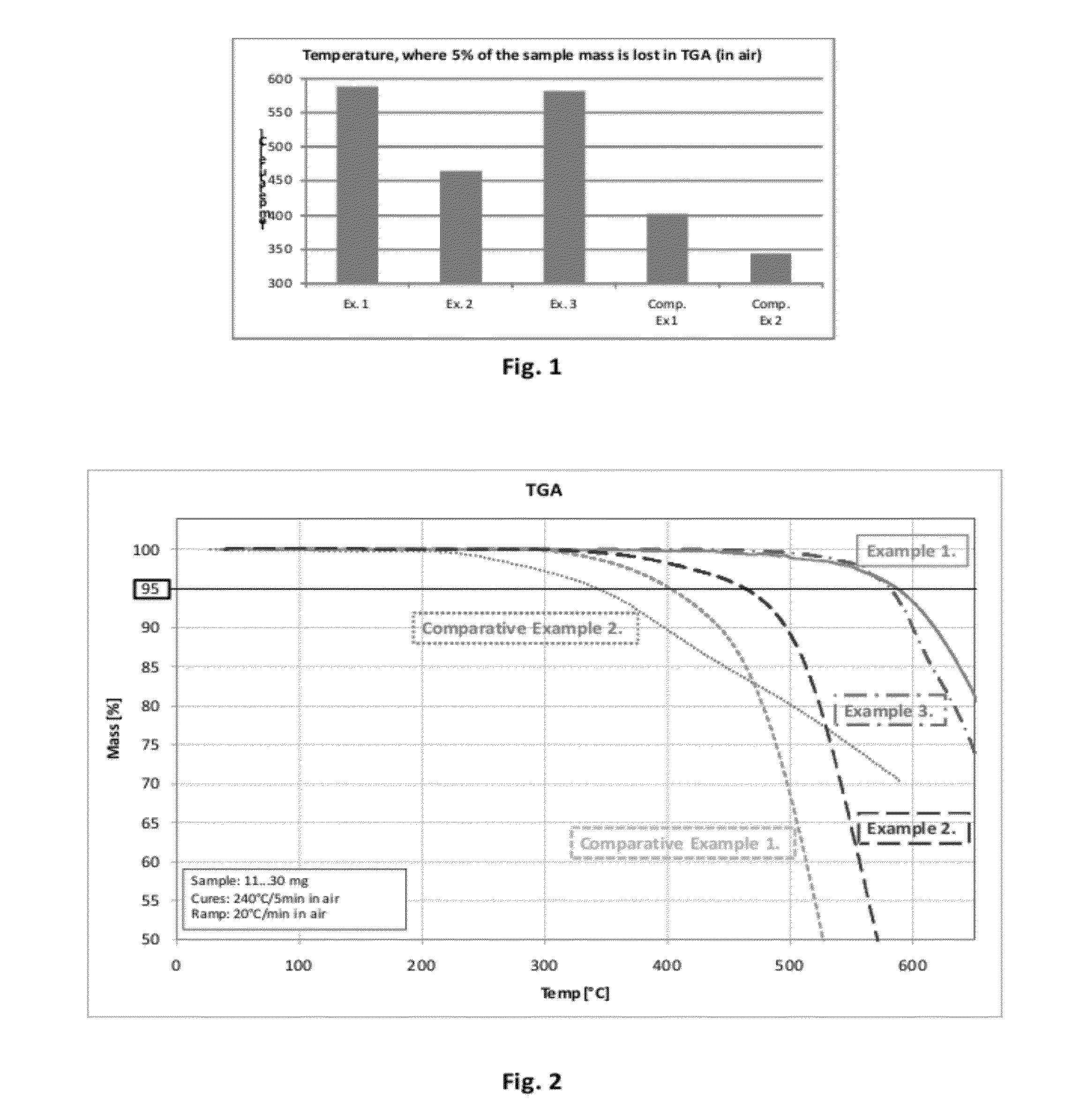
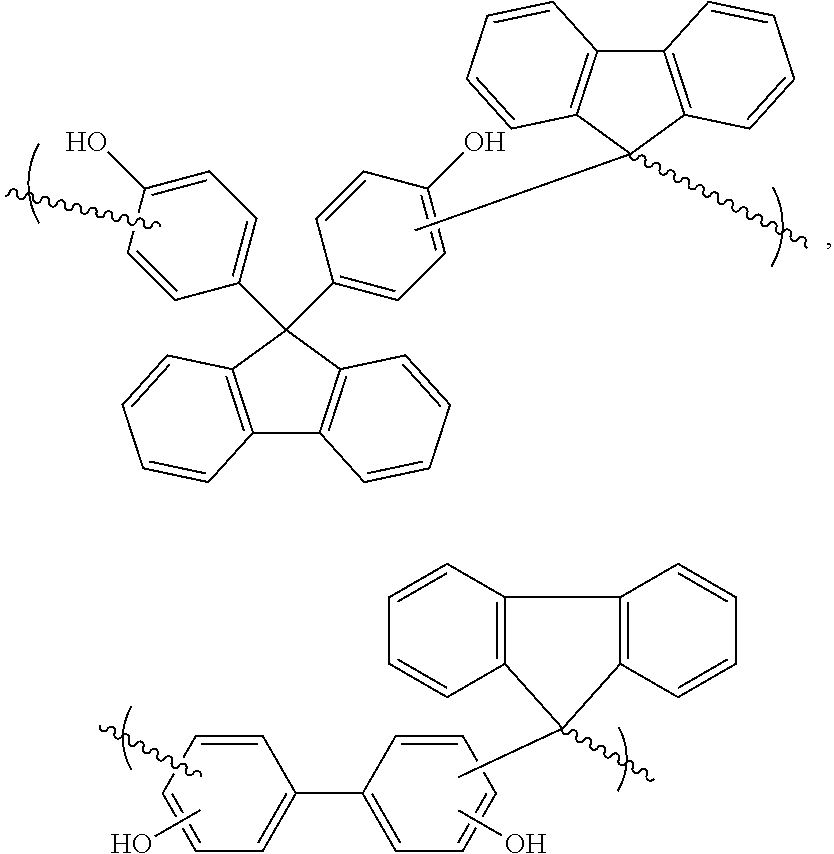
ActiveUS20120322010A1Excellent oxidative thermal stabilityHigh carbon contentImpression capsPlastic/resin/waxes insulatorsPolymer scienceHigh carbon
A novel novolac prepared by acid catalyzed condensation between biphenols or bisphenofluorenes and fluorenone is presented. The polymers exhibit excellent oxidative thermal stability and high carbon content, suitable for dielectric, etch stop applications as spin-on material.
Owner:SILECS OY
Method for preparing 9-fluorenone via four-phase transfer catalysis
ActiveCN103435463AImprove utilization efficiencyAdjustment and PurityOrganic compound preparationCarbonyl compound preparationQuaternary ammonium cationDissolution
The invention discloses a method for preparing 9-fluorenone via four-phase transfer catalysis. At a lower temperature, alkali is taken as a catalyst, quaternary ammonium salt serves as a phase transfer agent, fluorene can be excessive and reacts with oxygen-containing gas in xylene in states of dissolution and suspension, and high-purity 9-fluorenone is directly obtained, a right reaction condition is selected, and the transformation rate of the fluorene can reach 100 percent. According to the invention, the recovered alkali and the dissolvent do not need to be treated complexly and particularly, reuse can be realized, and industrialization is facilitated; in addition, mixed xylene and water are taken as the dissolvent, the oxidation of the fluorene as the reaction condition of the 9-fluorenone, the selection of the catalyst alkali and phase transfer agent, and the reuse of the dissolvent and the alkali are researched, as a result, the conditions of high transmission rate of fluorene and high selective transmission of the 9-fluorenone are obtained, and a more complete compounding process is disclosed.
Owner:BAOSHUN TECH CO LTD +1
Substituted benzophenone and preparation method thereof
InactiveCN103524320AOvercome the shortcomings that cannot be widely usedEasy to operateGroup 4/14 element organic compoundsOrganic compound preparationDiphenylmethanolPyridinium
The invention discloses substituted benzophenone and a preparation method thereof. The substituted benzophenone has a benzophenone structure, can be used as a hydrogen abstraction type photoinitiator, can be widely applied to ultraviolet light curing and other fields, and can also be used as a key intermediate to synthesize a poly-substituted fluorenone type compound. The preparation method of the substituted benzophenone, provided by the invention, is simple to operate, toxic substances are not used, an intermediate compound B and an intermediate compound A are ingeniously utilized to construct a benzhydrol structure through Grignard reaction, and the compound with the benzophenone structure is further obtained by oxidation with pyridinium chlorodichromate; the defect that synthesis methods of benzophenone type compounds in the prior art can not be widely applied is overcome.
Owner:XI AN JIAOTONG UNIV
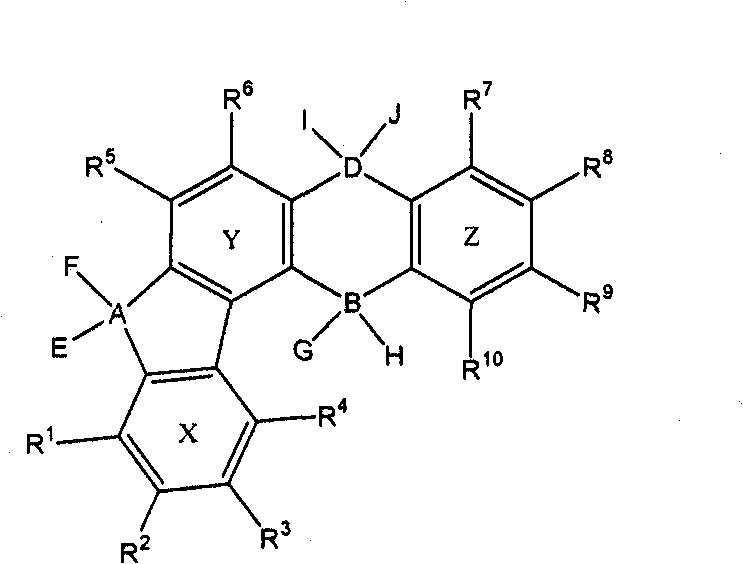
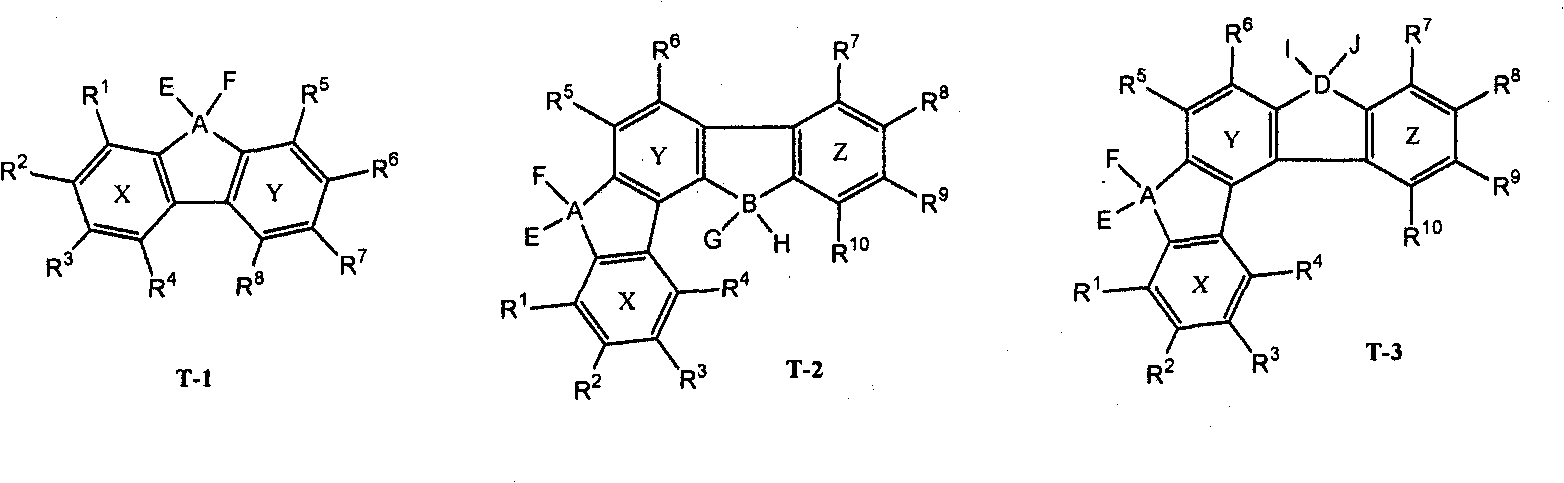
Synthesis method of indenofluorene derivatives, isotruxene and mono-substituted isotruxene derivatives
InactiveCN102627522AOrganic compound preparationHydrocarbon from oxygen organic compoundsEthyl groupKetone
The invention discloses a synthesis method of indenofluorene derivatives, isotruxene and mono-substituted isotruxene derivatives. The synthesis method of indenofluorene derivatives is characterized in that ester-group-containing compounds are formed through Diels-Alder reaction of methyl propiolate and indenocyclopentadienone, ethyl substituted indenofluorene derivatives are formed through hydrolysis, acid catalytic ring closing, carbonyl reduction and introduction of ethyl onto methylene, diketone compounds produced after ring closing react with lithium salt of 4, 4'-di-tert-butyl-2-brominated biphenyl, and then acidification and ring closing are conducted to obtain indenofluorene derivatives with spirofluorene. The synthesis method of isotruxene is characterized in that 1, 4-diphenyl-2, 3-di (carbalkoxy) fluorenone products are formed through the Diels-Alder reaction of indenocyclopentadienone and dimethyl acetylenedicarboxylate, isotruxene ketone is obtained through hydrolysis and acid catalytic ring closing and finally isotruxene is obtained; dibenzyl alcohol products are formed through the Diels-Alder reaction of 1, 4-butynediol and indenocyclopentadienone, and isotruxene is obtained through acetone protection, carbonyl reduction, acetone removal and polyphosphoric acid (PPA) ring closing; and oriented oxy substituted products, i.e. isotruxene ketone which is derived from isotruxene with methylene at a No.5 position being substituted, and corresponding diethyl substituted products are additionally obtained. The indenofluorene derivatives, the isotruxene and the mono-substituted isotruxene derivatives disclosed by the invention can be used in the field of organic electroluminescence and organic micro-molecule solar cells.
Owner:EAST CHINA NORMAL UNIV
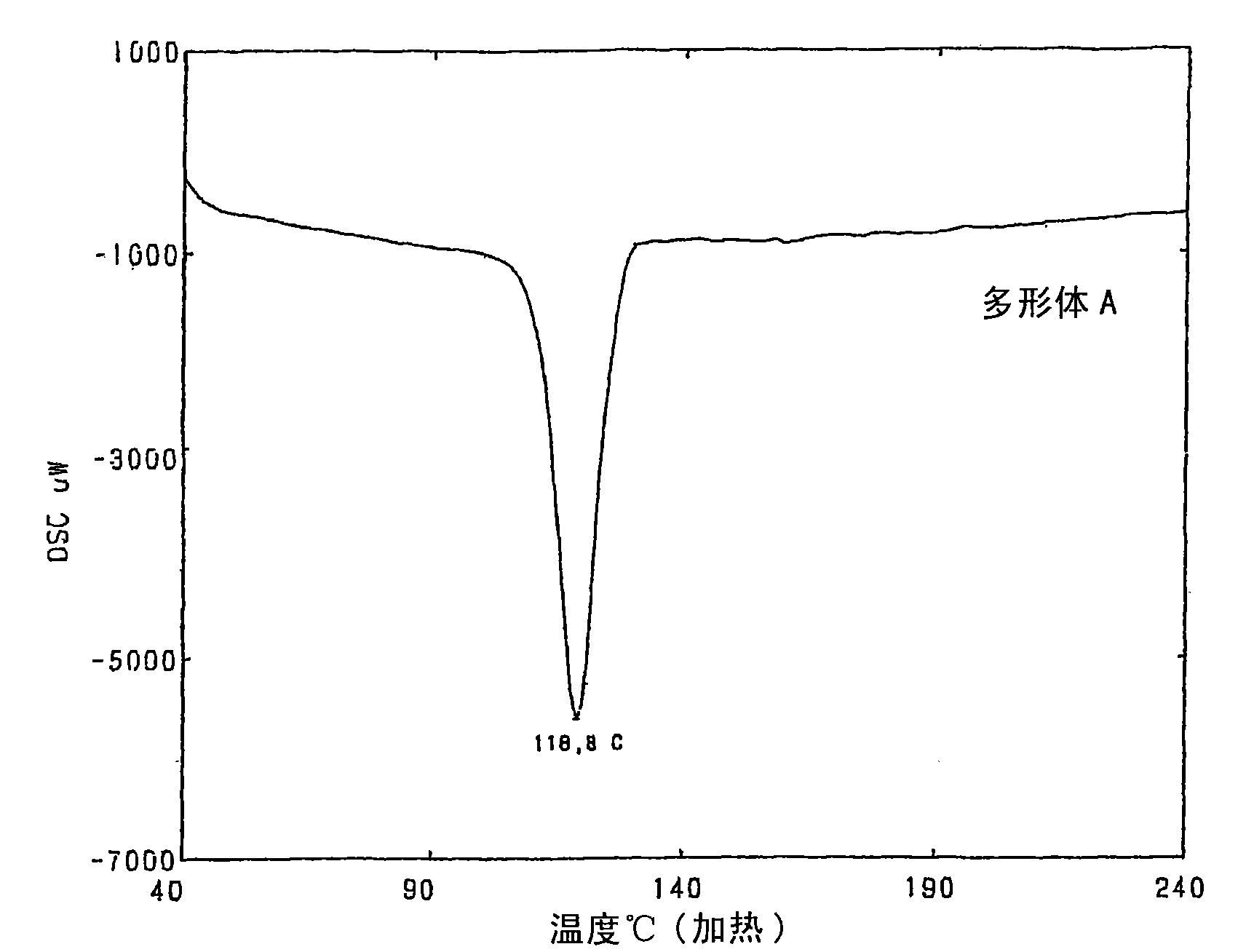
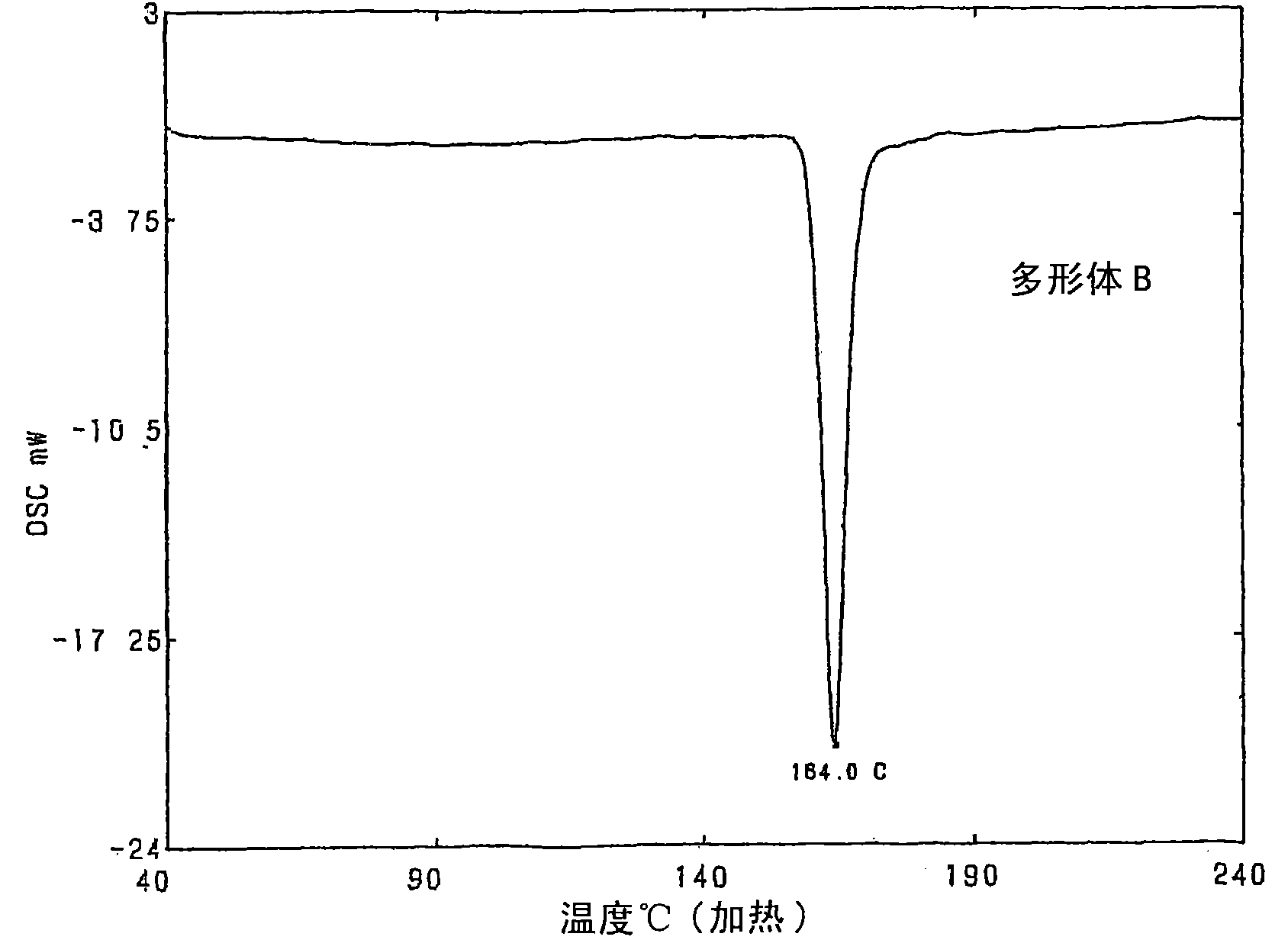
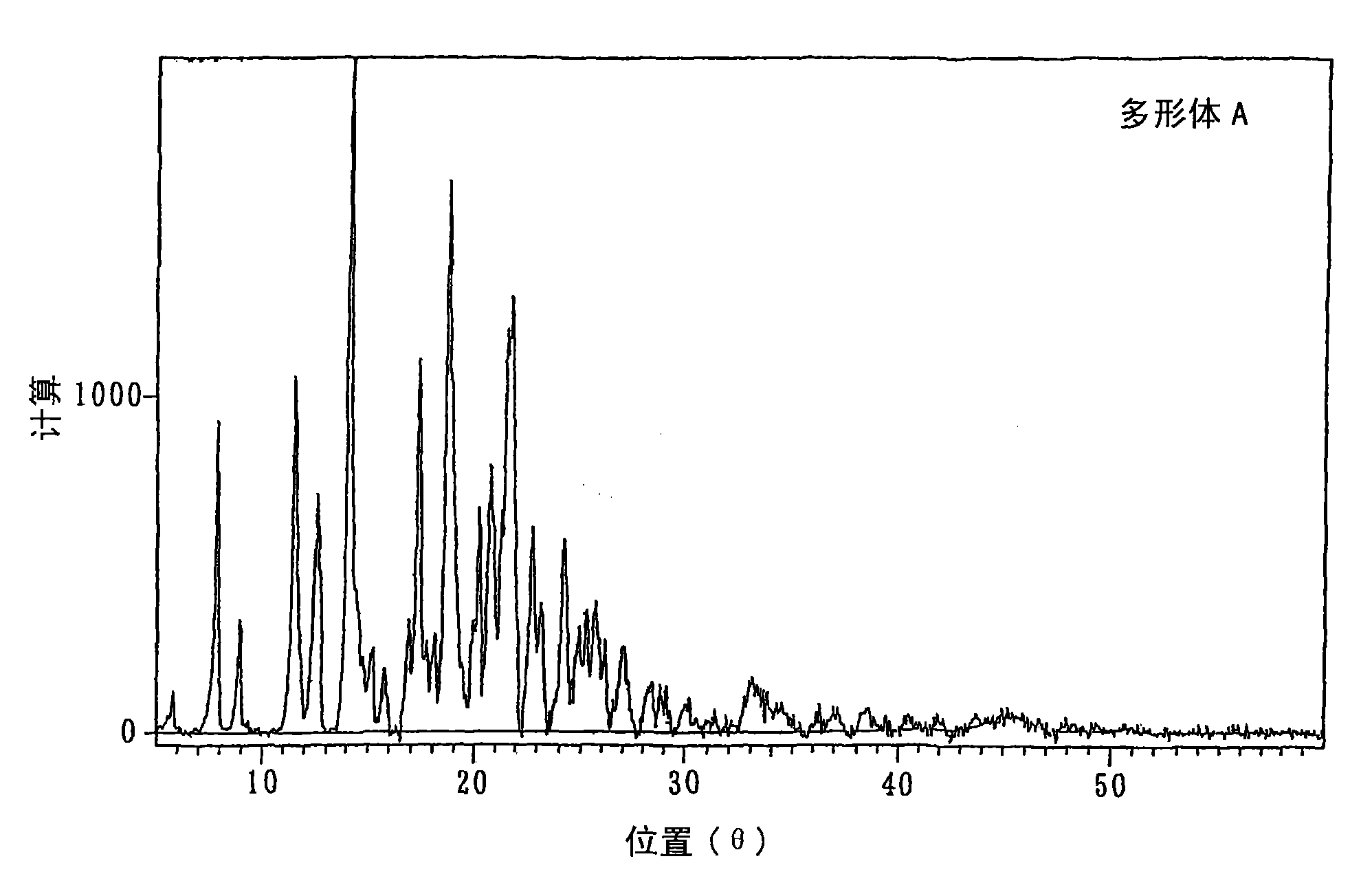
Crystalline polymorph of fluorene derivative and process for production thereof
ActiveCN101657406AEther separation/purificationOrganic compound preparationKetone solventsHeteropoly acid
Disclosed is a process for producing a crystal polymorph of 9,9-bis(4-(2-hydroxyethoxy)phenyl)fluorene, which comprises the steps of: reacting fluorenone with 2-phenoxyethanol in the presence of a heteropoly acid; causing the crystallization of 9,9-bis(4-(2-hydroxyethoxy)phenyl)fluorene from the reaction mixture at a temperature lower than 50 DEG C to produce a crude product of 9,9-bis(4-(2-hydroxyethoxy)phenyl)fluorene; dissolving the crude product in at least one solvent selected from the group consisting of an aromatic hydrocarbon solvent, a ketone solvent and an ester solvent; and causing the crystallization of 9,9-bis(4-(2-hydroxyethoxy)phenyl)fluorene from the reaction mixture at a temperature of 50 DEG C or higher.
Owner:TAOKA CHEM COMPANY
Preparation method for 9-fluorenone
ActiveCN103435456AReduce energy consumptionImprove conversion rateOrganic compound preparationCarbonyl compound preparationNitrogenous heterocyclic compoundQuinoline
The invention discloses a preparation method for 9-fluorenone. According to the method, industrial fluorene (the purity is more than 95 percent) is taken as a raw material, alkali is taken as a catalyst, a heterocyclic compound containing nitrogen and water are used as solvent and quaternary ammonium salt is taken as a phase transfer agent; under the lower temperature and stirring condition, fluorene and gas containing oxide react and the fluorene is converted into 9-fluorenone; by selecting proper reaction conditions, the conversion rate of the fluorene can reach 100 percent. The recovered alkali and solvent in the invention can be recycled without a special complex treatment process and benefit the industrialization. According to the preparation method disclosed by the invention, quinoline and isoquinoline as well as a mixer of the quinoline and the isoquinoline are taken as the solvent and the reaction condition that the fluorene is oxidized into the 9-fluorenone, the selection of the alkali as the catalyst and the phase transfer agent and the recycling of the solvent and the alkali are researched to obtain the conditions that the fluorene is converted into the 9-fluorenone at high conversion rate and high selectivity; and a complete synthetic process is provided.
Owner:BAOSHUN TECH CO LTD +1
Polymer composition and method of making the same
ActiveUS8952121B2Excellent oxidative thermal stabilityHigh carbon contentImpression capsPlastic/resin/waxes insulatorsPolymer scienceHigh carbon
A novel novolac prepared by acid catalyzed condensation between biphenols or bisphenofluorenes and fluorenone is presented. The polymers exhibit excellent oxidative thermal stability and high carbon content, suitable for dielectric, etch stop applications as spin-on material.
Owner:SILECS OY
Noble metal/TiO2-C catalyst and preparation method thereof
ActiveCN102476051AHigh compressive strengthMesopore richOrganic compound preparationCarboxylic compound preparationAnthranilFluorenone
The invention discloses a noble metal / TiO2-C catalyst for removing p-carboxybenzaldehyde, 2,6-dicarboxyl anthraquinone, 2,6-dicarboxyl fluorenone, and other colored impurities from crude p-phthalic acid. The noble metal / TiO2-C catalyst of the present invention can be used for the hydrogenation refinement process of the crude p-phthalic acid. With the noble metal / TiO2-C catalyst of the present invention, the contents of the p-carboxybenzaldehyde, the 2,6-dicarboxyl anthraquinone, the 2,6-dicarboxyl fluorenone, and other colored impurities can be significantly reduced.
Owner:CHINA PETROLEUM & CHEM CORP +1
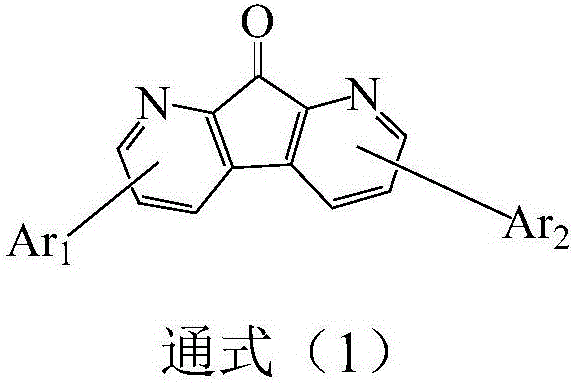
Compound based on 1,8-diazafluoren-9-one and application of compound
ActiveCN106188037AGood film formingHigh fluorescence quantum efficiencyOrganic chemistrySolid-state devicesTM compoundCrystallinity
The invention discloses a compound based on 1,8-diazafluoren-9-one and an application of the compound. According to the compound, the 1,8-diazafluoren-9-one is used as a mother nuclide, and two aromatic heterocyclic groups are connected to two sides of the 1,8-diazafluoren-9-one, so that crystallinity of molecules is destroyed, aggregation between the molecules is avoided, and good film forming performance is obtained. The compound disclosed by the invention is used as a luminescent layer material to be applied to an organic electroluminescent device, and the organic electroluminescent device applying the compound has favorable photoelectric properties and can meet requirements of panel manufacturing enterprises.
Owner:JIANGSU SUNERA TECH CO LTD
Bisphenol monomer containing bi-tert-butyl and fluorenyl structure, and preparation method and application thereof
ActiveCN103274908AThe synthetic route is simpleHigh yieldOrganic chemistryOrganic compound preparationPolymer scienceTert butyl phenol
The invention relates to a bisphenol monomer containing a bi-tert-butyl and fluorenyl structure. The bisphenol monomer is 9, 9-bi(4-hydroxyl-3-tert-butyl phenyl) fluorine. The monomer is simple in a synthetic route, high in yield, easy to purify, stable at the room temperature and can be used for preparing fluorine-contained polyarylether. The bisphenol monomer containing the bi-tert-butyl and fluorenyl structure is prepared by the following steps of: reacting reactants 2-tert butyl phenol and 9-fluorenone in the presence of an acid catalyst, transferring a product into a mixed solvent of ethanol and water, carrying out suction filtering, and further recrystallizing to obtain the target product. The bisphenol monomer containing the bi-tert-butyl and fluorenyl structure is used for preparing the fluorine-contained polyarylether. The prepared polyarylether has good dissolving film forming property, excellent thermal property and low dielectric constant.
Owner:HUAWEI TEHCHNOLOGIES CO LTD
Process for the catalytic synthesis of bisphenol fluorene by using concentrated sulphuric acid
InactiveCN101735020AReduce dosageAvoid wastingOrganic chemistryOrganic compound preparationPropanoic acidSoftened water
The invention relates to a process for the catalytic synthesis of bisphenol fluorene by using concentrated sulphuric acid. Concentrated sulphuric acid is taken as catalyst, beta-mercapto propionic acid is taken as cocatalyst, phenol is taken as reactant and solvent, reaction with fluorenone is fully carried out under mild condition, one organic polar solvent and water are added, so as to cause unreacted phenol and product are layered with concentrated sulphuric acid; water phase is removed, the obtained organic phase is washed by alkaline solution and softened water step by step, condition is controlled and reduced pressure distillation is carried out to obtain the organic polar solvent and phenol respectively, temperature reduction is carried out, then recrystallization solvent is added for recrystallization, thus obtaining bisphenol fluorine white solid the purity of which is more than 99.0%. The process has low reaction temperature, short time, high productivity more than 90% and high purity more than 99.0%; besides, the used catalyst concentrated sulphuric acid and cocatalyst beta-mercapto propionic acid are low in cost, concentrated sulphuric acid consumption is low, phenol is easy to recycled, recovery rate reaches 80%, and environmental pollution is less, thus the method is applicable to industrialized production.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Compound based on monosubstituent-9-fluorenone and application thereof
ActiveCN106220645ADestroy crystallinityInhibit aggregationOrganic chemistrySolid-state devicesFluorenoneCrystallinity
The invention discloses a compound based on monosubstituent-9-fluorenone and application thereof. The compound uses monosubstituent-9-fluorenone as a mother nucleus and allows a single side to be connected with a heteroaromatic group, so crystallinity of molecules is destroyed, aggregation of molecules is prevented, and good film forming ability is obtained. The compound is used as a luminescent layer material for an organic light-emitting diode and has good photoelectric performance.
Owner:JIANGSU SUNERA TECH CO LTD
Synthesis of 2-aminobiphenyl compounds
InactiveCN101367736AAtom utilization is highMild reaction conditionsOrganic compound preparationAmino compound preparationSynthesis methodsFluorenone
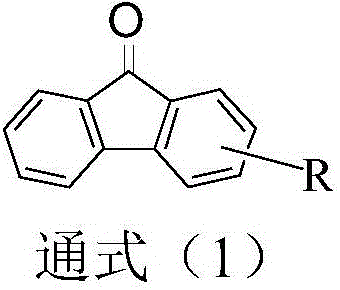
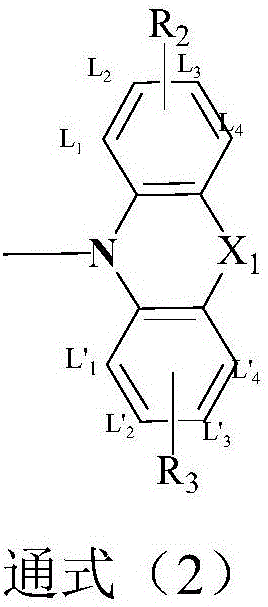
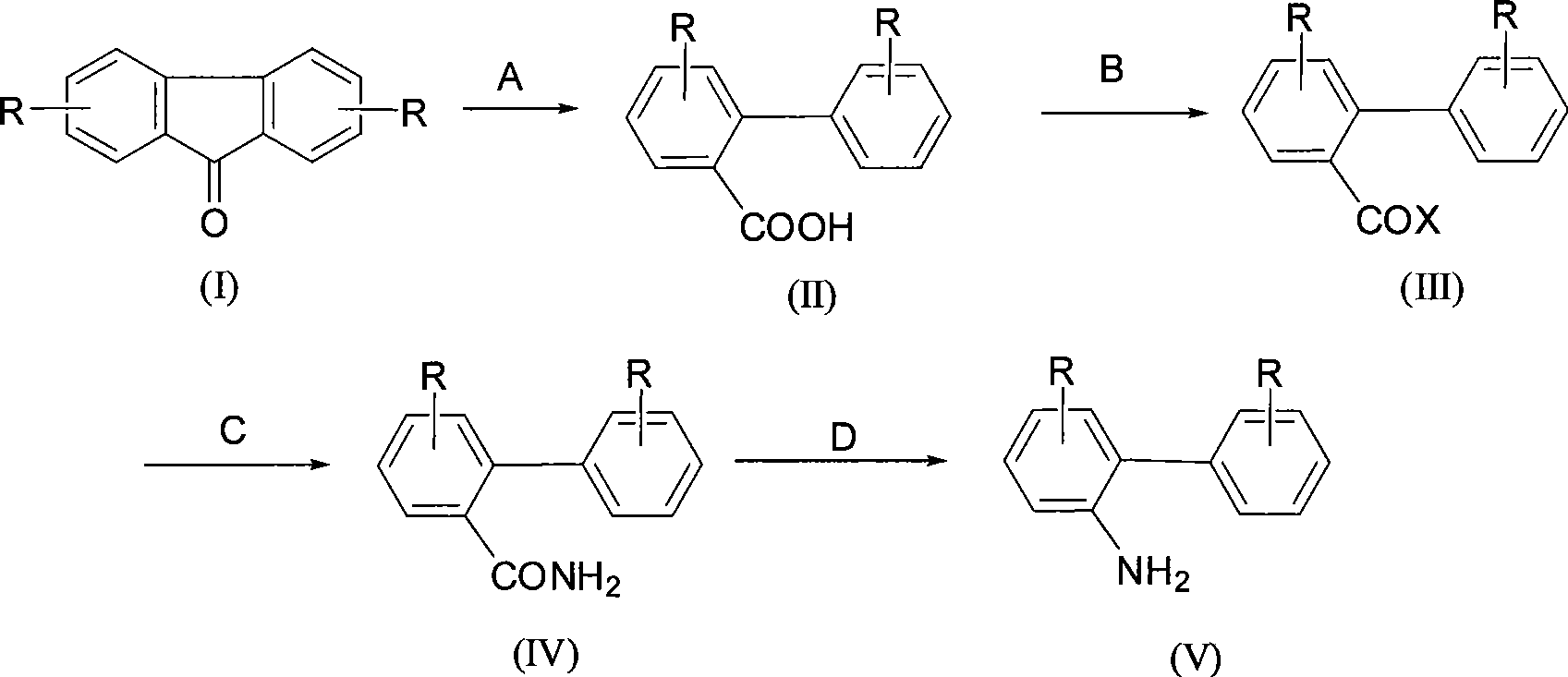
The present invention relates to a synthesis method of 2-phenylaniline compounds as shown in the formula (V). The synthesis method comprises the following steps: substituted 9-fluorenone as shown in the formula (I) reacts under alkaline conditions to prepare a compound (II); the compound (II) and acylation agent perform acyl halogenation reaction to prepare a compound (III); the compound (III) performs ammoniation reaction to prepare a compound (IV); and the compound (IV) performs Hofmann degradation reaction to prepare the compound (V). The present invention discloses a novel synthesis method of the 2-phenylaniline compounds, and the present invention has the advantages of mild reaction conditions, easy operation, high utilization rate of atoms, high reaction yield, low production cost, suitability for industrial production, high implementation value and the socioeconomic benefits.
Owner:ZHEJIANG UNIV OF TECH
Top-bottom asymmetrical tert-butyl spirobifluorene compound
InactiveCN102617466ABroaden the synthesis pathThe synthetic route is simpleOrganic chemistrySolid-state devicesAlkaneSolubility
The invention relates to a top-bottom asymmetrical tert-butyl spirobifluorene compound. The compound of the invention has a structural formula as represented by (I), and in the structural formula, R1 can be any one of various electron-withdrawing groups and electron-donating groups, such as a variety of alkyl groups, substituted aryl groups, substituted heterocyclic radicals, substituted alkylene groups, substituted amino groups, chlorine, bromine and iodine, except tert-butyl groups. According to the invention, cheap and easily available 4,4'-di-tert-butyl-biphenyl is used a raw material and reacts with 2,7-biX-fluorenone (wherein X represents substituents) through bromination, X substituents on a fluorene ring are converted into a variety of functional groups, and other steps are also carried out so as to obtain the compound (I). The top-bottom asymmetrical tert-butyl spirobifluorene compound in the invention has the characteristics of low cost, a simple synthesis route, high yield, easiness in industrial production, a special spiro-conjugation effect, good dissolvability, high thermal stability and a wide applicability in the fields of organic light-emitting displays, organic non-linear materials, fluorescent probes and the like.
Owner:SHANGHAI NORMAL UNIVERSITY
Preparation method of POSS (polyhedral oligomeric silsesquioxane)-based porous material capable of simultaneously improving porosity and carbon dioxide storage volume
ActiveCN104558016ALarge specific surface areaHigh pore volumeSilicon organic compoundsOther chemical processesPorosityOrganic acid
The invention relates to a preparation method of a POSS (polyhedral oligomeric silsesquioxane)-based porous material capable of simultaneously improving porosity and carbon dioxide storage volume. The preparation method comprises the following steps: (1) dispersing the POSS-based porous material in an organic solvent, adding a primary amine compound and an organic acid catalyst, and carrying out reflux reaction under a stirring condition for 12-48 hours, wherein the POSS-based porous material comprises a POSS unit and a fluorenone unit, and the primary amine compound at least comprises two amino groups; and (2) after reaction, filtering, washing the filter cake and drying in vacuum to obtain the material. Compared with a raw material POSS-based porous material, the POSS-based porous material prepared by the method has preferable porous performance and carbon dioxide storage performance, and the BET specific surface area, pore volume and carbon dioxide storage volume are improved to a certain extent.
Owner:SHANDONG UNIV
Fluorenone derivative, preparation method of fluorenone derivative and redox method of synthetic fluorenone
ActiveCN104370724AOxygen-containing compound preparationOrganic compound preparationOxidation-Reduction AgentRedox
The invention relates to a fluorenone derivative, a preparation method of the fluorenone derivative and a redox method of synthetic fluorenone. The preparation method includes a, performing precursor synthesis; b, performing target product synthesis; c, performing purifying. The fluorenone derivative is obtained by the redox method of four alkyne and polyenynes and serves as non-silver sensitive material applied widely. The fluorenone serving as a medical intermediate is used for synthesis of various drugs.
Owner:ANHUI NORMAL UNIV
Oxidation process for aromatic compound
InactiveUS7488843B1Avoid the needOrganic compound preparationCarbonyl compound preparation by oxidationBenzoic acidOrganic solvent
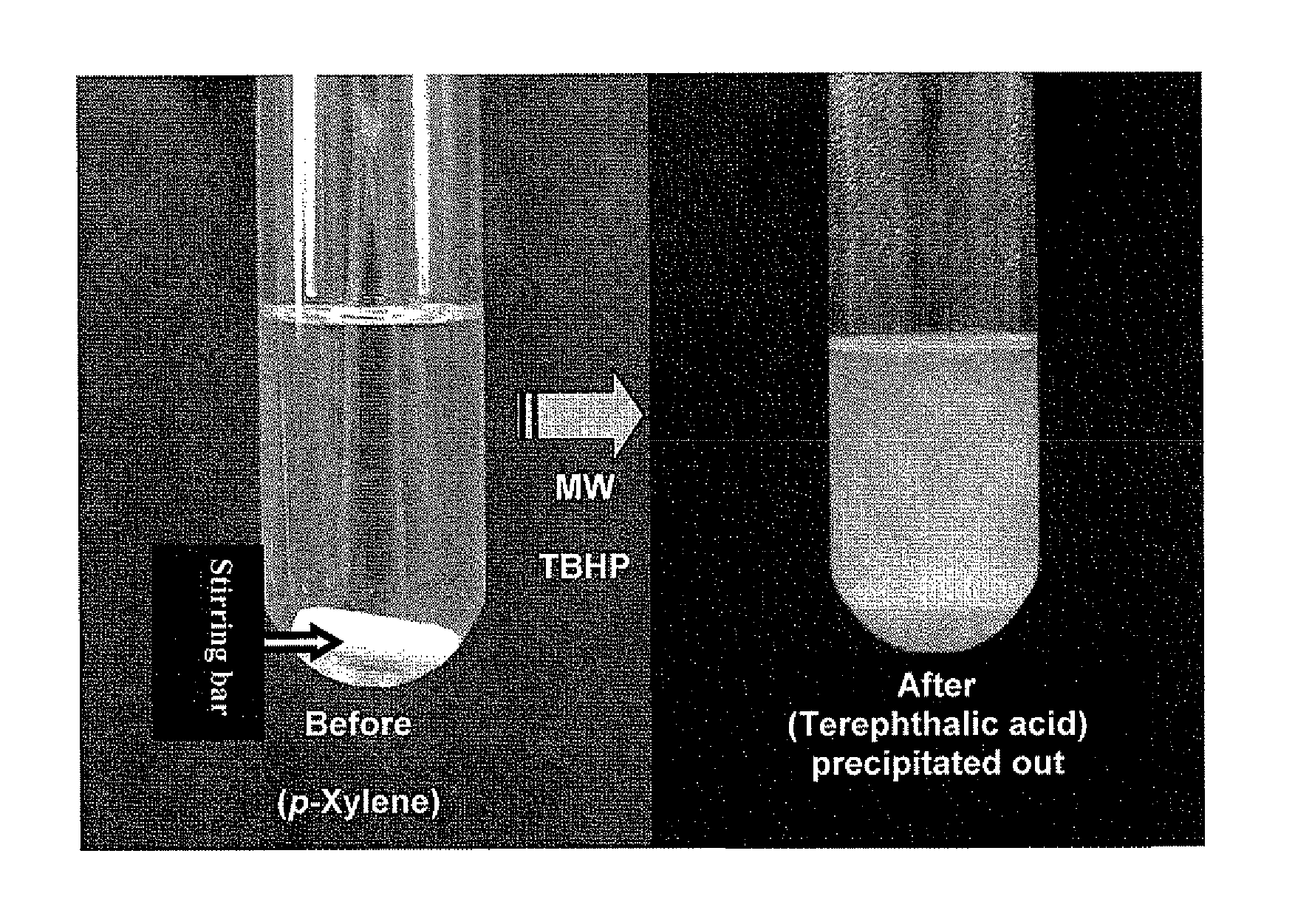
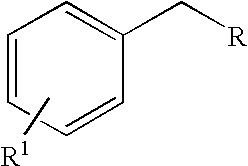
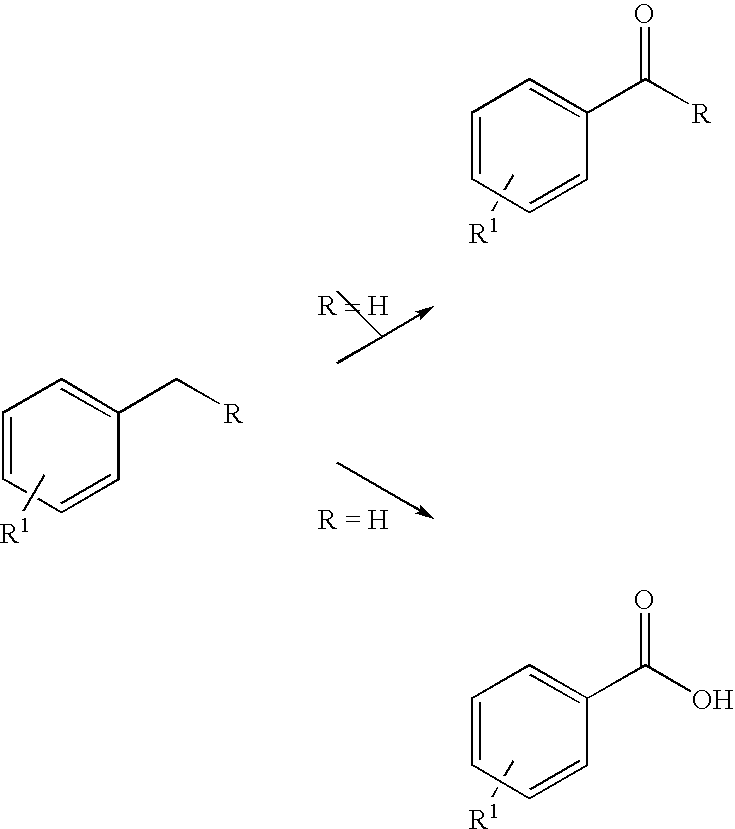
Methylene substituted aromatic compounds can be oxidized using t-butyl hydroperoxide and microwave radiation. In particular, xylenes give primarily phthalic acids, while toluene gives benzoic acid. Other methylene substituted aromatic compounds where the methylene group is not part of a methyl group give ketones, rather than acids. For example, fluorene gives fluorenone. The process avoids the need for the presence of metals such as in catalysts or in oxidizing agents, and can be carried out using water rather than an organic solvent. Thus the process can conform with the ideals of green chemistry.
Owner:HONG KONG BAPTIST UNIV
Method for preparing 9-fluorenone through using industrial fluorene
ActiveCN103467264AReduce energy consumptionOrganic compound preparationChemical recyclingSolventFluorenone
A method for preparing 9-fluorenone through using industrial fluorene is characterized in that a raw material industrial fluorene (having a purity of above 95%) reacts with an oxygen-containing gas at a low temperature under stirring in a solvent comprising toluene and water under the action of an alkali as a catalyst and a quaternary ammonium salt as a phase transfer agent to form 9-fluorenone. The fluorene conversion rate can reach 100% under appropriate reaction conditions. The alkali and solvent recovered in the invention can be recycled without special complex treatment processes, so it is beneficial for industrialization; and in the invention, the recycle of the catalyst alkali, the phase transfer agent quaternary ammonium salt and the solvent, a recrystallization technology and the like are researched, pure fluorenone is obtained, and the complete preparation method of the 9-fluorenone is provided.
Owner:BAOSHUN TECH CO LTD +1
Diarylfluorene intermediate preparation method
InactiveCN101643381AReduce manufacturing costHydrocarbonsHalogenated hydrocarbon preparationOrganic laserOrganic synthesis
The invention provides a diarylfluorene intermediate preparation method and relates to the application field of fine organic synthesis and photovaltaic material. Diarylfluorene can be used as an important intermediate in the material fields such as organic electroluminescence, photovoltaic cells, organic electrical storage, organic non-linear optics, chemical sensor and biosensor, organic laser and the like. The preparation method of diarylfluorene intermediate comprises the following two specific steps: (1) using arylamine, arylamine hydrochloride and fluorenone or the derivative of fluorenone to react under heat condition, alkalifying, stirring, filtrating and washing to obtain high purity intermediate product; (2) adding the intermediate product in dichloromethane to react with alkyl nitrite on the condition of refluxing and finally obtaining the high purity target product through recrystallization. The diarylfluorene intermediate preparation method has the advantage that (1) two-step method is adopted to prepare diarylfluorene intermediate, the raw material is cheap, the steps are simple, the yield is high and the product is easy to purify; (2) the method has mass production value and is environmentally friendly.
Owner:NANJING UNIV OF POSTS & TELECOMM
Clean, environmentally-friendly and economical bisphenol fluorene synthesizing method
ActiveCN102795970AFew reaction stepsEasy to operateOrganic chemistryOrganic compound preparationPtru catalystPropanoic acid
The invention provides a clean, environmentally-friendly and economic bisphenol fluorene synthesizing method, and relates to the field of preparation and organic synthesis of bisphenol fluorene monomers. The method comprises the following steps of: (1) preparation: synthesizing bisphenol fluorene by using cheap 9-fluorenone serving as a starting raw material and phenol in the presence of an acid catalyst and a thiohydracrylic acid cocatalyst; and (2) post treatment: adding a weak base salt to neutralize the catalyst, adding an organic solvent for dissolving, filtering, collecting an organic phase, and reclaiming the solvent and the redundant phenol under reduced pressure. The method is few in reaction steps and easy to operate, the reaction time is shortened, the production efficiency is greatly improved, and industrialized production is facilitated.
Owner:山东益华生物科技有限公司
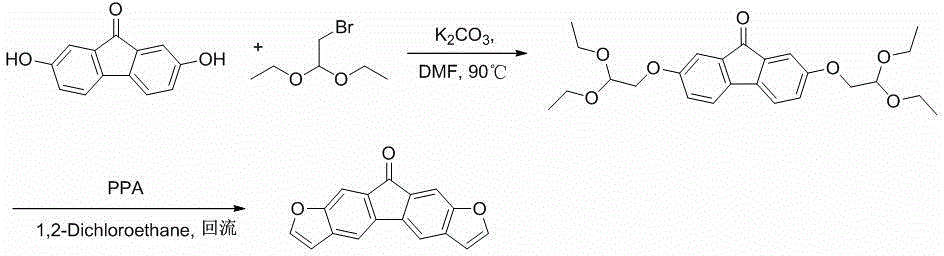
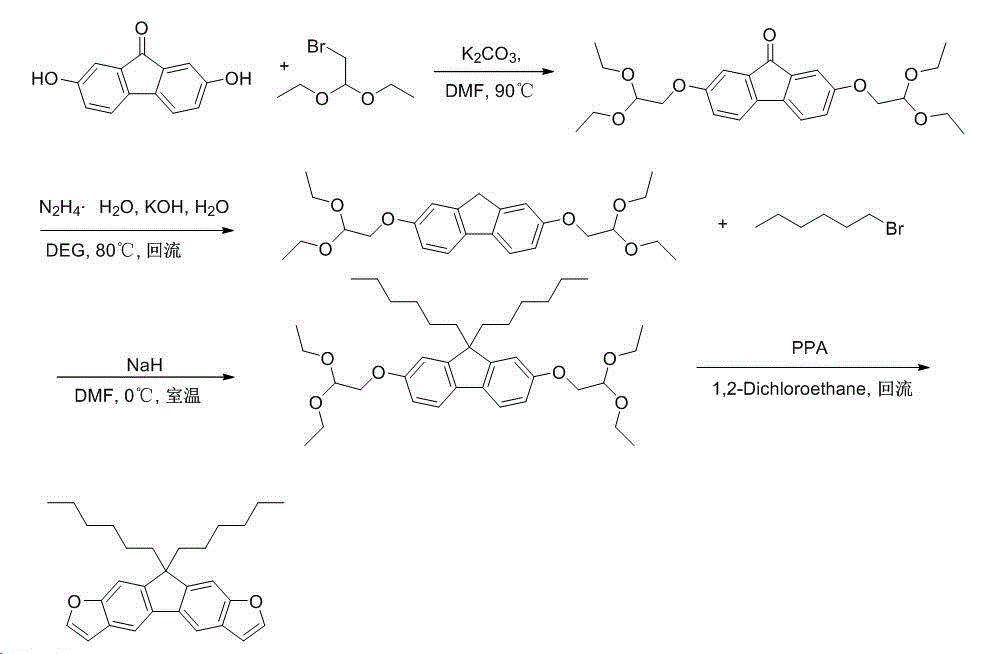
Double-furofluorenone as well as derivative and preparation method thereof
InactiveCN104788466AEasy to makeEasy post-processingOrganic chemistryLuminescent compositionsOrganic field-effect transistorBromine
The invention discloses double-furofluorenone and a derivative thereof. The double-furofluorenone adopts the structure as shown in the specification. The preparation method comprises the following steps: by taking 2,7-dihydroxy fluorenone as a raw material, enabling 2,7-dihydroxy fluorenone to react with bromo-acetadehyde diethylacetal so as to obtain ether, and performing direct ring closure by using polyphosphoric acid so as to obtain double-furofluorenone; by taking 2,7-dihydroxy fluorenone as a raw material, enabling 2,7-dihydroxy fluorenone to react with bromo-acetadehyde diethylacetal so as to obtain ether, reducing fluorenone by using a Huang-ming-long method, further enabling reaction with 1-bromohexane under the action of sodium hydride, and finally performing polyphosphoric acid ring closure, thereby obtaining 9,9-dihexyl double-furofluorenone. Due to the adoption of the compound disclosed by the invention, novel option can be provided for a predecessor of an organic field effect crystal tube material, and the range of an organic electroluminescence material is widened.
Owner:EAST CHINA NORMAL UNIV
Oxidation preparation method for 9- fluorenone compound from fluorine compound
InactiveCN1962597AReduce pollutionMild reaction conditionsOrganic compound preparationCarbonyl compound preparationPotassium hydroxideFluorenone
The invention discloses a preparing method of 9-fluorenone compound through oxidizing fluorene compound, which comprises the following steps: dissolving fluorene compound in the tetrahydrofuran; adding potassium hydroxide; setting the weight rate of fluorene compound and tetrahydrofuran at 1:4-6 with the molar rate of fluorene compound and potassium hydroxide at 1:0.5-2.5; stirring under normal temperature and pressure; oxidizing in the air; reacting for 1-8h; filtering; distilling; washing; drying; obtaining the product with producing rate at 98-99% and purity at 99-99.5%.
Owner:SHANXI UNIV
Pretreatment method for fluorenone production wastewater
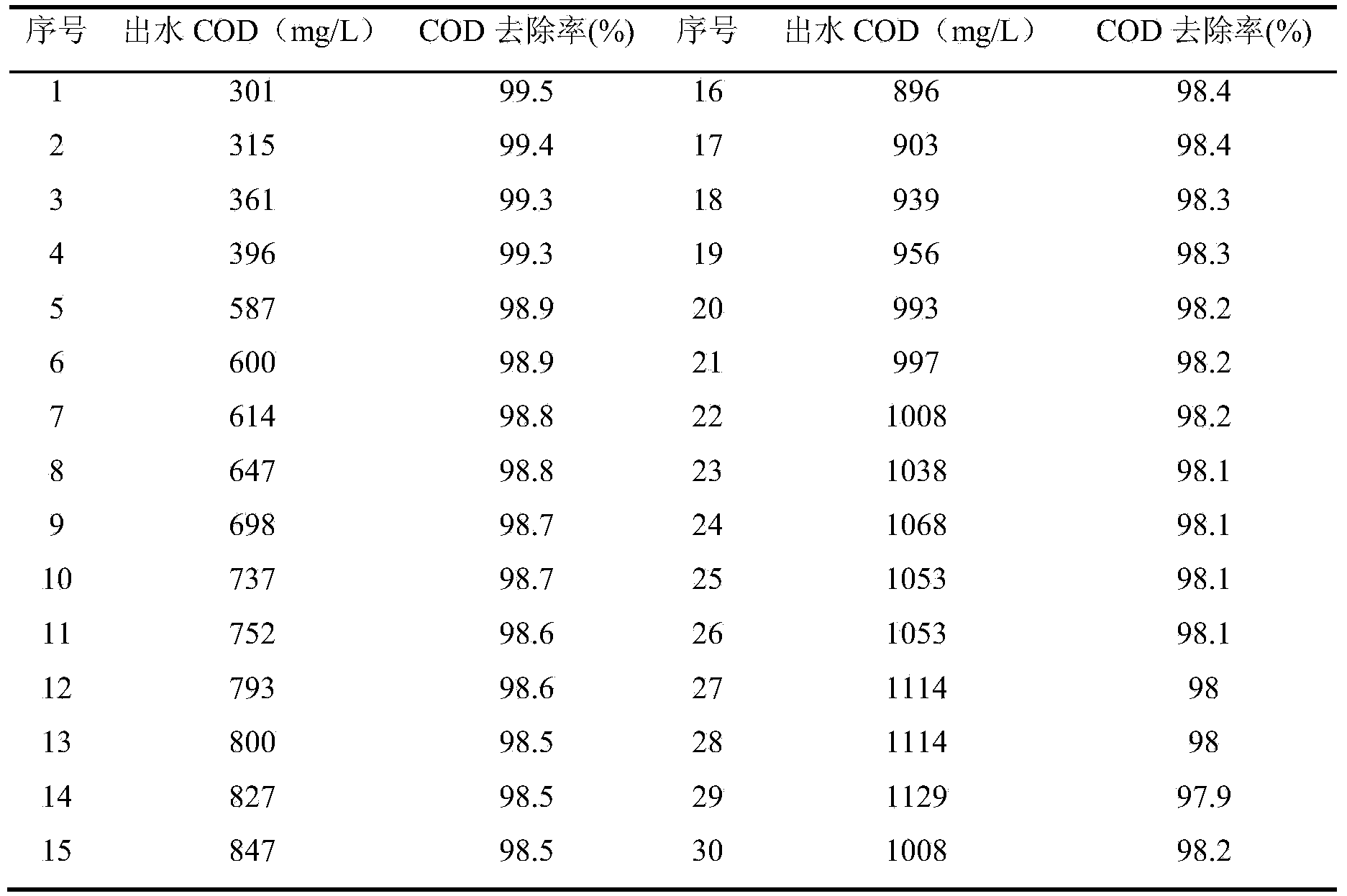
ActiveCN103819024AImprove biodegradabilityImprove removal efficiencyMultistage water/sewage treatmentChemical industryPretreatment method
The invention belongs to the technical field of treatment of wastewater from the chemical industry and particularly relates to a pretreatment method for fluorenone production wastewater. The pretreatment method is characterized by comprising the two steps of acidification and oxidation by activated carbon and Fenton's reagent. Specially, the pretreatment method comprises the following steps: (1) acidification: adding industrial sulfuric acid to the fluorenone production wastewater to adjust the pH to 1-3, stirring, and standing for layering; (2) oxidation by activated carbon and Fenton's reagent: simultaneously adding H2O2, FeSO4.7H2O and activated carbon to the wastewater subject to acidification, and conducting reactions under the condition of 35-55 DEG C for 30-120 min. Adsorption, oxidation and regeneration of activated carbon all occur in the step (2). Compared with the prior art, the pretreatment method has the benefits as follows: the pollutant removal efficiency for the fluorenone production wastewater is high; the COD removal rate can reach more than 97%; the biodegradability of the wastewater is significantly improved; the B / C is increased to more than 0.4 from less than 0.2. Therefore, the pretreatment method is of great significance for the sustainable development of fluorenone production enterprises and other chemical production enterprises.
Owner:SINOSTEEL ANSHAN RES INST OF THERMO ENERGY CO LTD
Novel donor-acceptor fluorene scaffolds : a process and uses thereof
The present invention relates to novel donor-acceptor fluorene compounds, which can be used as for the fabrication of electroluminescent devices, and a process of preparing said novel compounds. More particularly, the present invention relates to amine donor and nitrile / ester acceptor fluorenes, fluorenones their pi-conjugated systems and related compounds, processes for preparing the said compounds including oxidation of fluorenes to corresponding fluorenones and their use in preparing organic electronic devices such as organic light emitting diodes (OLEDs), photovoltaic / solar cell, field effect transistors and other useful electroluminescent devices. The compounds are prepared by reacting 2H-pyran-2-ones in isolated or rigid conformations with cyclic ketones containing methylene carbonyl moiety in the presence of a base in- an organic solvent. The present invention also relates to a new concept and approach to overcome the problem of 'Green emission defect' in 9-unsubstituted fluorene-based organic light emitting diodes which occurrs due to the conversion of fluorenes to fluorenones that show emission mainly in green-yellow region. In the present invention we have placed donor-acceptor substituents in such a way that donor-acceptor fluorenones show emission in the blue region (instead of green-yellow region) thus improving the blue colour purity and overcoming the problem of green emission defect.
Owner:COUNCIL OF SCI & IND RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com