Bisphenol monomer containing bi-tert-butyl and fluorenyl structure, and preparation method and application thereof
A technology of bis-tert-butyl and tert-butyl phenyl, which is applied to bisphenol monomer and its preparation and application fields, can solve problems such as unfavorable industrialized practical application, and achieve excellent thermal and mechanical properties, high yield, and ease of use. The effect of purification and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
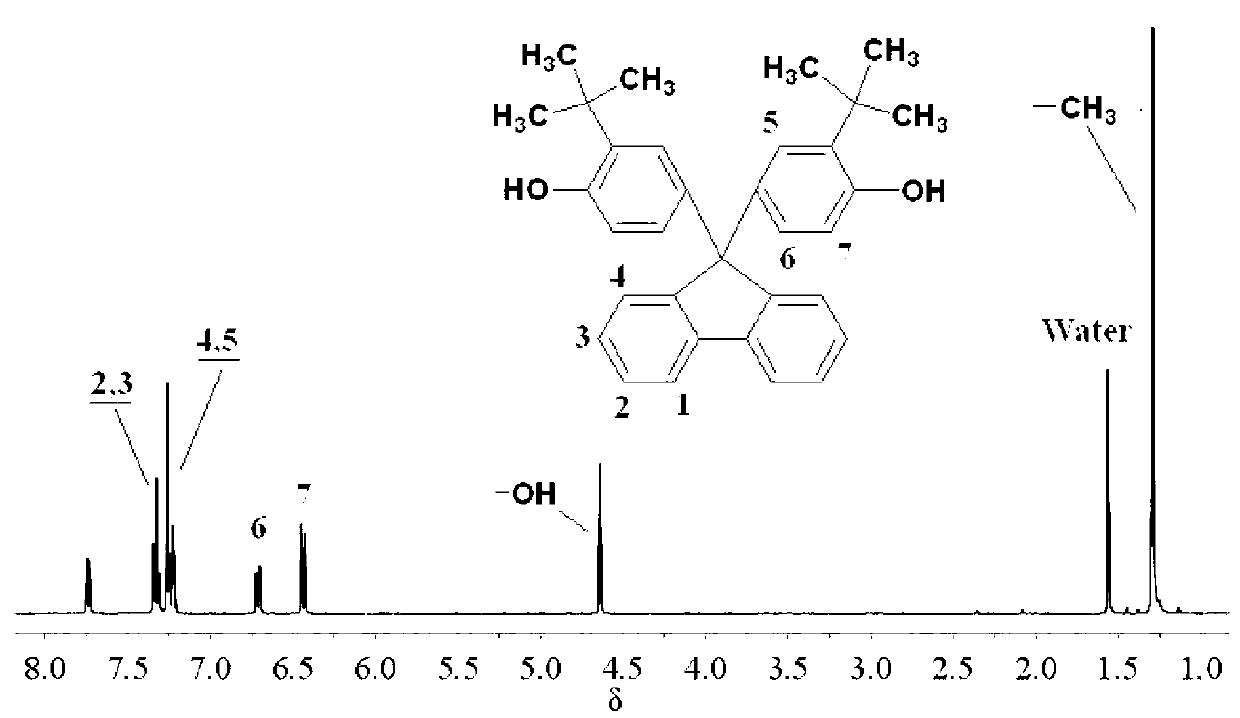
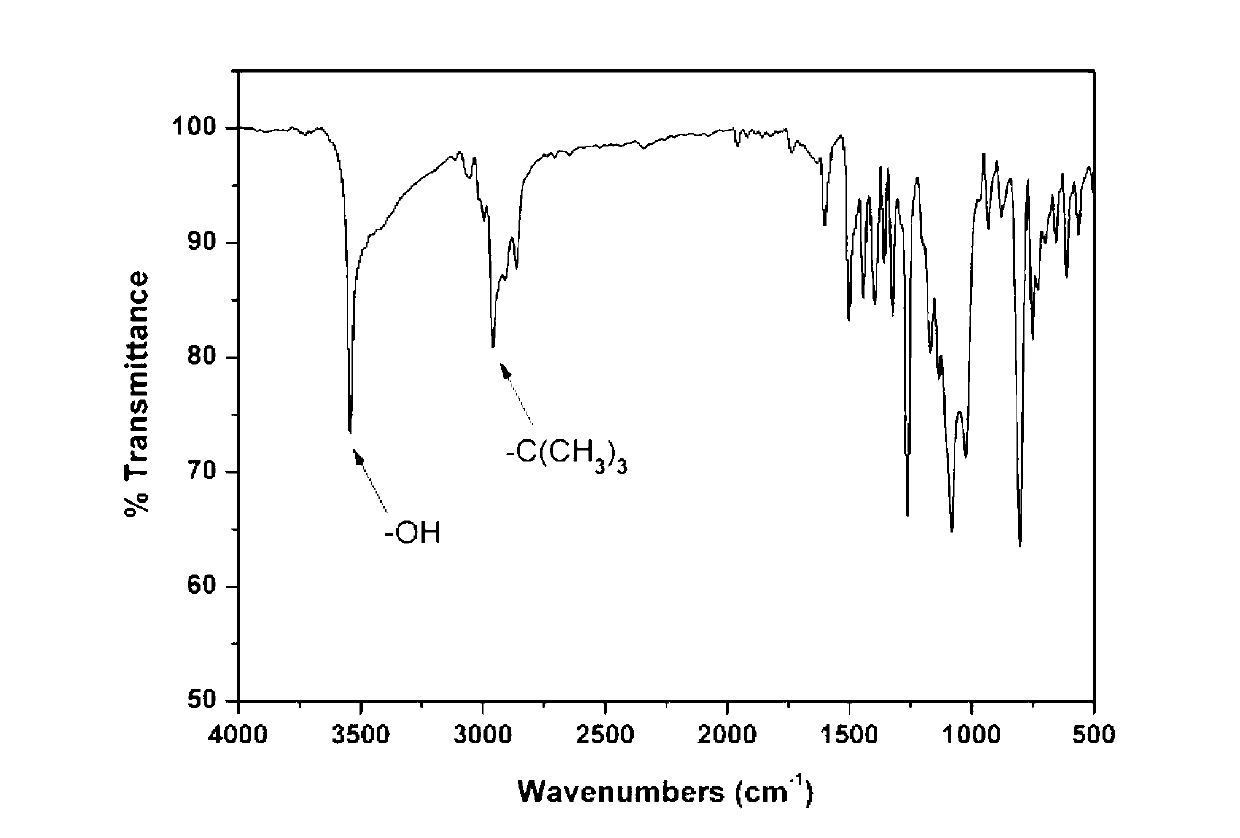
[0023] Preparation of 9,9-bis(4-hydroxy-3-tert-butylphenyl)fluorene:
[0024] Under nitrogen protection, 18.02g (0.10mol) 9-fluorenone and 45.07g (0.3mol) 2-tert-butylphenol were added to a 250ml three-necked flask, stirred at room temperature until 9-fluorenone was dissolved, and then respectively Slowly add 1g (0.01mol) of 98% concentrated sulfuric acid and 0.11g (0.001mol) of 3-mercaptopropionic acid, react at 30-40°C for 0.5h, continue to react at 50-60°C for 4h, and transfer the reaction product to ethanol and In a mixed solvent of water (the volume ratio of ethanol and water is 5:1), fully stir, precipitate, filter, and further use toluene to recrystallize to obtain a white powdery solid, that is, bis-tert-butyl and fluorenyl-containing bis-tert-butyl Phenolic monomer, 9,9-bis(4-hydroxy-3-tert-butylphenyl)fluorene.
[0025] The yield is 83% (obtained by the ratio of the mass of bisphenol monomer obtained in practice to the mass of bisphenol monomer obtained in theory), ...
Embodiment 2
[0034] Preparation of 9,9-bis(4-hydroxy-3-tert-butylphenyl)fluorene:
[0035] Under nitrogen protection, 25.23g (0.14mol) 9-fluorenone and 45.07g (0.3mol) 2-tert-butylphenol were added to a 250ml three-necked flask, stirred at room temperature until 9-fluorenone was dissolved, and then respectively Slowly add 4.2g (0.042mol) of 98% concentrated sulfuric acid and 0.44g (0.0042mol) 3-mercaptopropionic acid, react at 30-40°C for 0.5h, continue to react at 50-60°C for 6h, and transfer the reaction product into a mixed solvent of ethanol and water (the volume ratio of ethanol and water is 2:1), fully stirred, precipitated and filtered, and further recrystallized with toluene to obtain a white powdery solid, that is, bis-tert-butyl and fluorenyl structure Bisphenol monomer 9,9-bis(4-hydroxy-3-tert-butylphenyl)fluorene.
[0036] The yield is 81% (obtained by the ratio of the mass of bisphenol monomer obtained in practice to the mass of bisphenol monomer obtained in theory),
[0037...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com