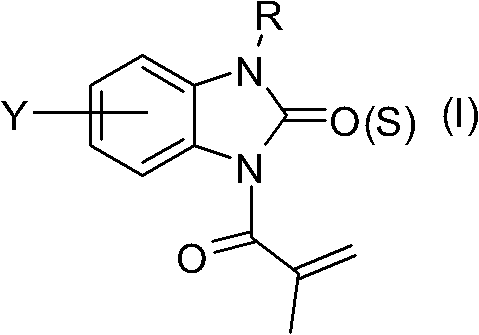
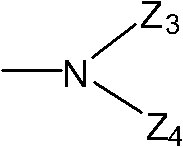
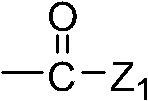Methylacryloyl-benzimidazole (sulfur) ketone derivative and application of serving as antibacterial agent thereof
A technology of methacryloyl benzimidazole and benzoyl, which is applied in the field of antibacterial drugs and new methacryloyl benzimidazolone derivatives, which can solve the antibacterial activity of benzimidazolone derivatives. There are few reports And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0152] The following specific preparation examples and biological examples help to understand the present invention, but do not limit the content of the present invention.
[0153] The following preparation examples 1-18 further illustrate the preparation method of the compound of general formula (I) and the preparation of the present application, but are not limited thereto.
Embodiment 1
[0155] Synthesis of 3-methacryloyl-1H-benzimidazol-2(3H)-one (compound number: IA-01)
[0156]
[0157] Add 1.08g (10mmol) of intermediate I-1, 0.72g (12mmol) of urea, and 10mL of xylene into a 50mL pear-shaped flask, heat to reflux for 4h, cool to room temperature, add ethyl acetate / water (volume ratio 2 / 1) 30 mL, stirred for 10 min, transferred to a separatory funnel for standing and layered, the aqueous phase was extracted once with 10 mL of ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain 1.86 g of intermediate 1-II, Yield 92.1%;
[0158] Weigh 1.62g (8mmol) of dry intermediate 1-II, put 10mL of anhydrous pyridine in a 100mL pear-shaped flask, add 1.04g of methacryloyl chloride (10mmol, dissolved in 5mL of dichloromethane) dropwise under ice-cooling, add After completion, slowly rise to room temperature, continue to stir for 15 minutes, raise the temperature to 60° C. and continue the...
Embodiment 2
[0161] Synthesis of 3-methacryloyl-1H-benzimidazole-2(3H)-thione (compound number: Ia-01)
[0162]
[0163] Except that urea was replaced by thiourea, the rest of the reagents and synthesis steps were exactly the same as in Example 1 to obtain the title compound Ia-01 with a total yield of 52.6%.
[0164] Compound Ia-01: light yellow powder, melting point 163.5-165.2°C. Mass spectrometry (electrospray, negative ion mode) m / z217 (M-H) - . 1 H NMR (CDCl 3 , 500MHz, δ, ppm): 2.16(s, 3H, CH 3 ), 5.60 (s, 1H, C=CH 2 ), 5.63 (s, 1H, C=CH 2 ), 6.85(t, J=8.0Hz, 1H), 6.98(t, J=8.0Hz, 1H), 7.15(d, J=8.0Hz, 1H), 7.66(d, J=8.0Hz, 1H), 5.82(s, 1H, NH). 13 C NMR (CDCl 3 , 125MHz, δ, ppm): 19.6, 109.5, 111.6, 114.7, 122.8, 124.9, 125.7, 131.7, 140.4, 165.9, 172.3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com