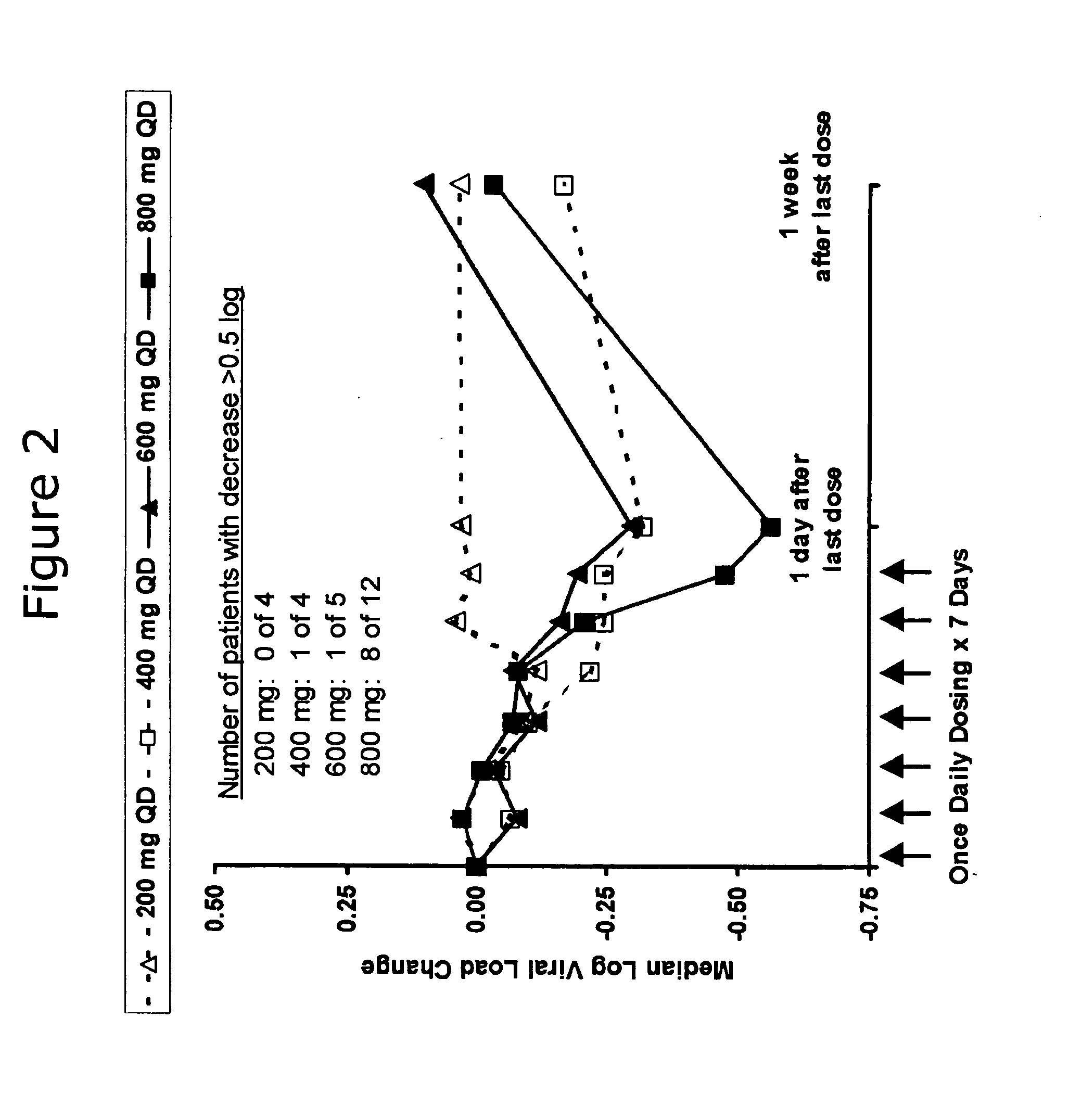
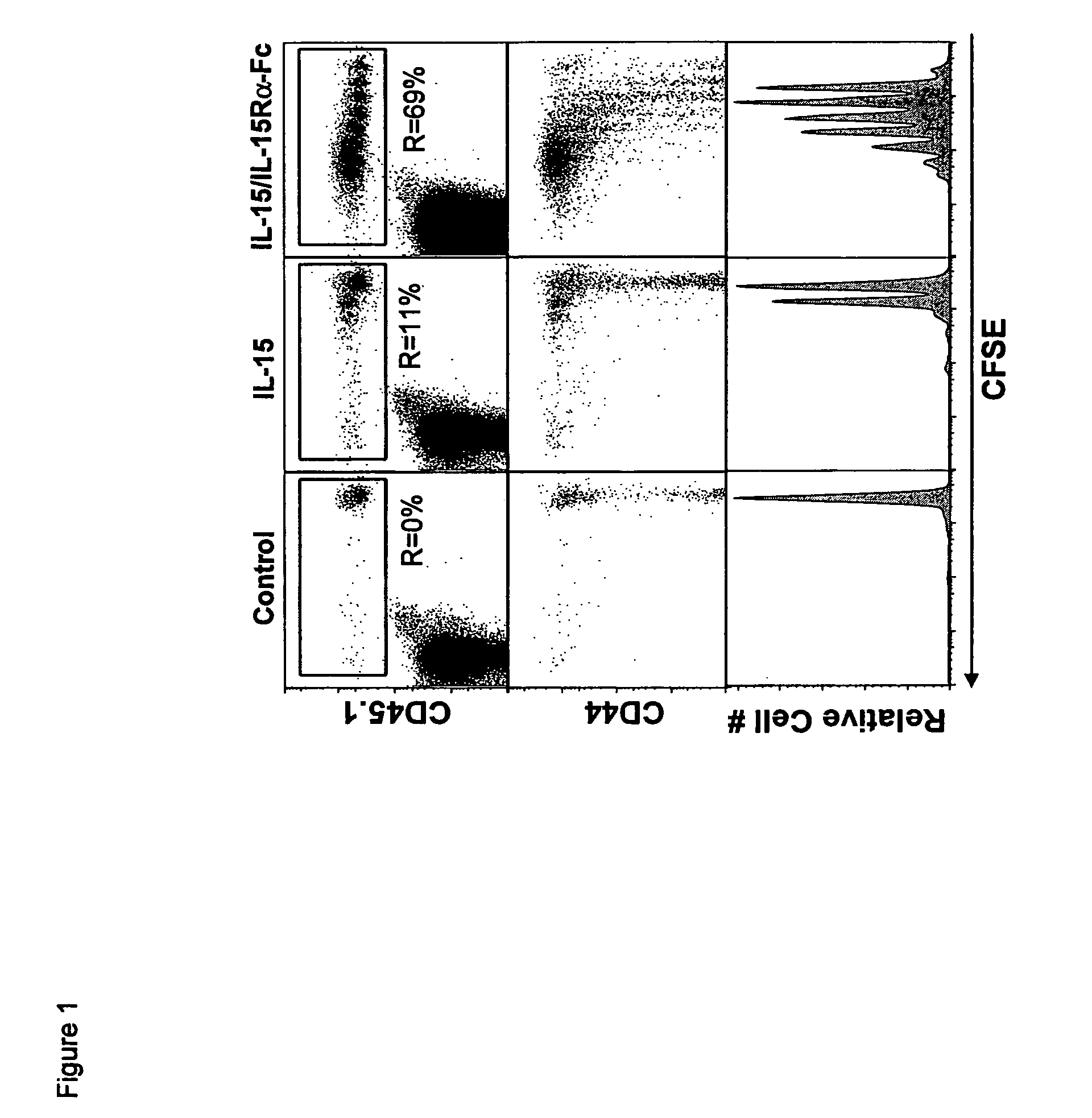
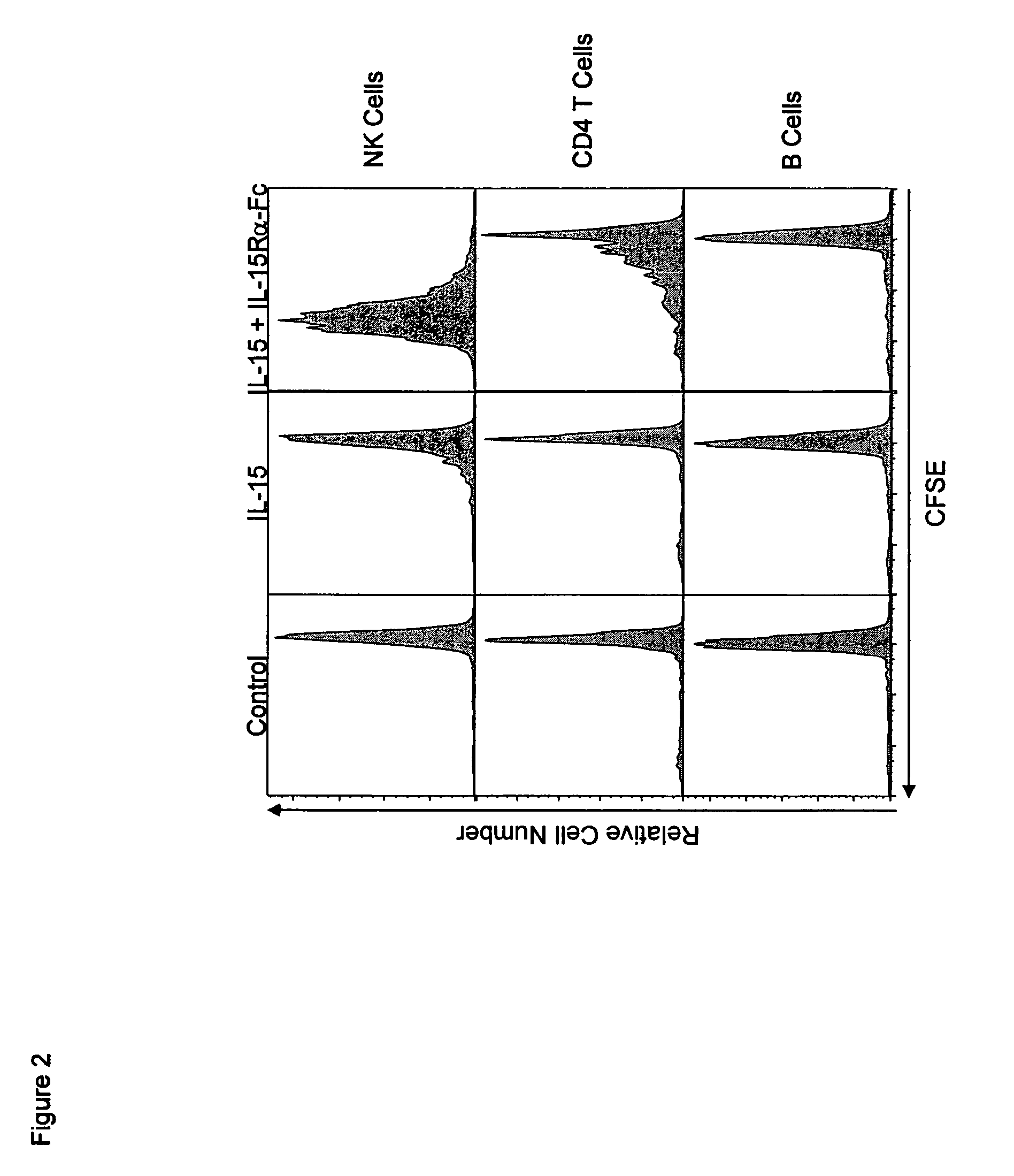
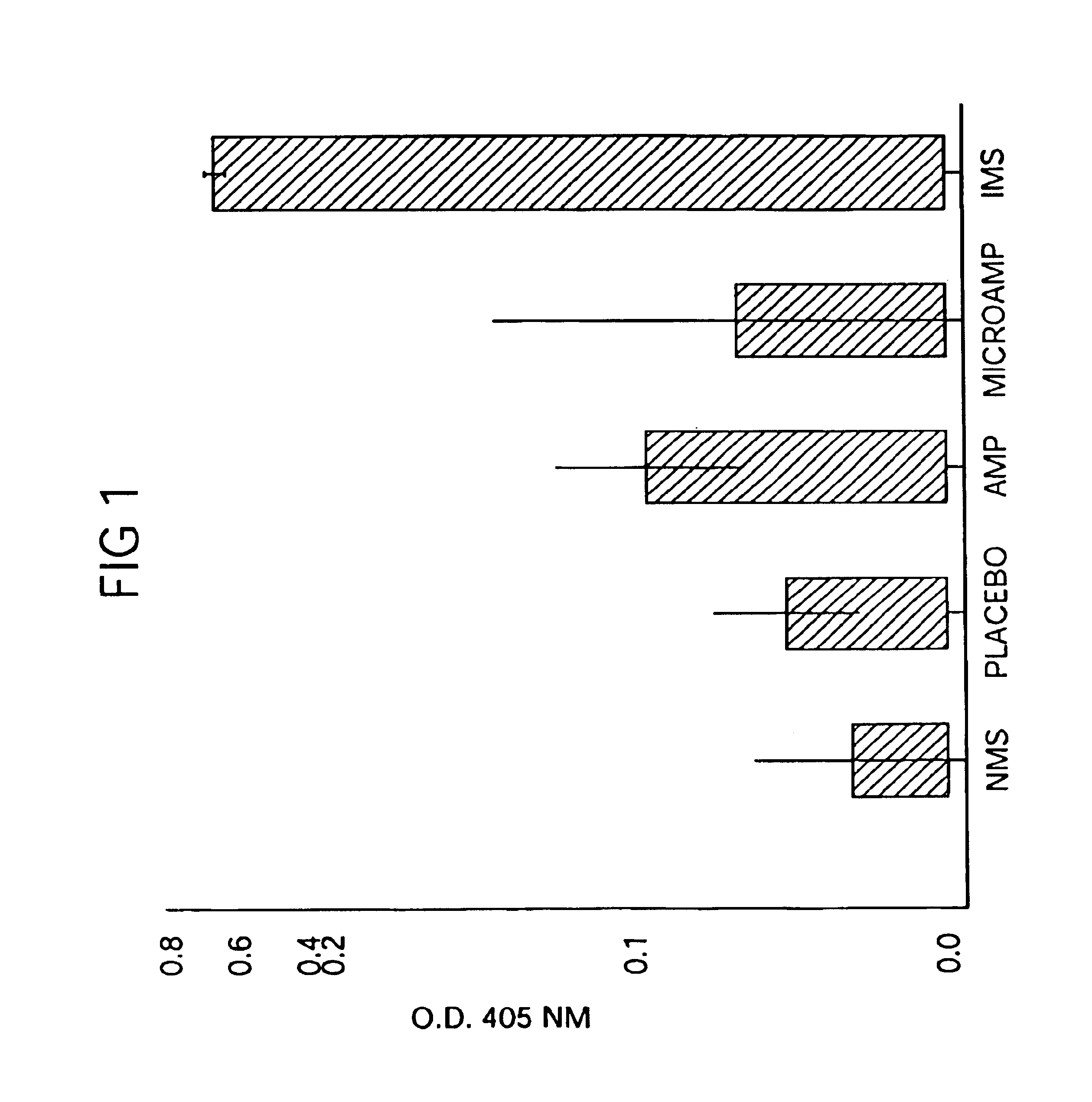
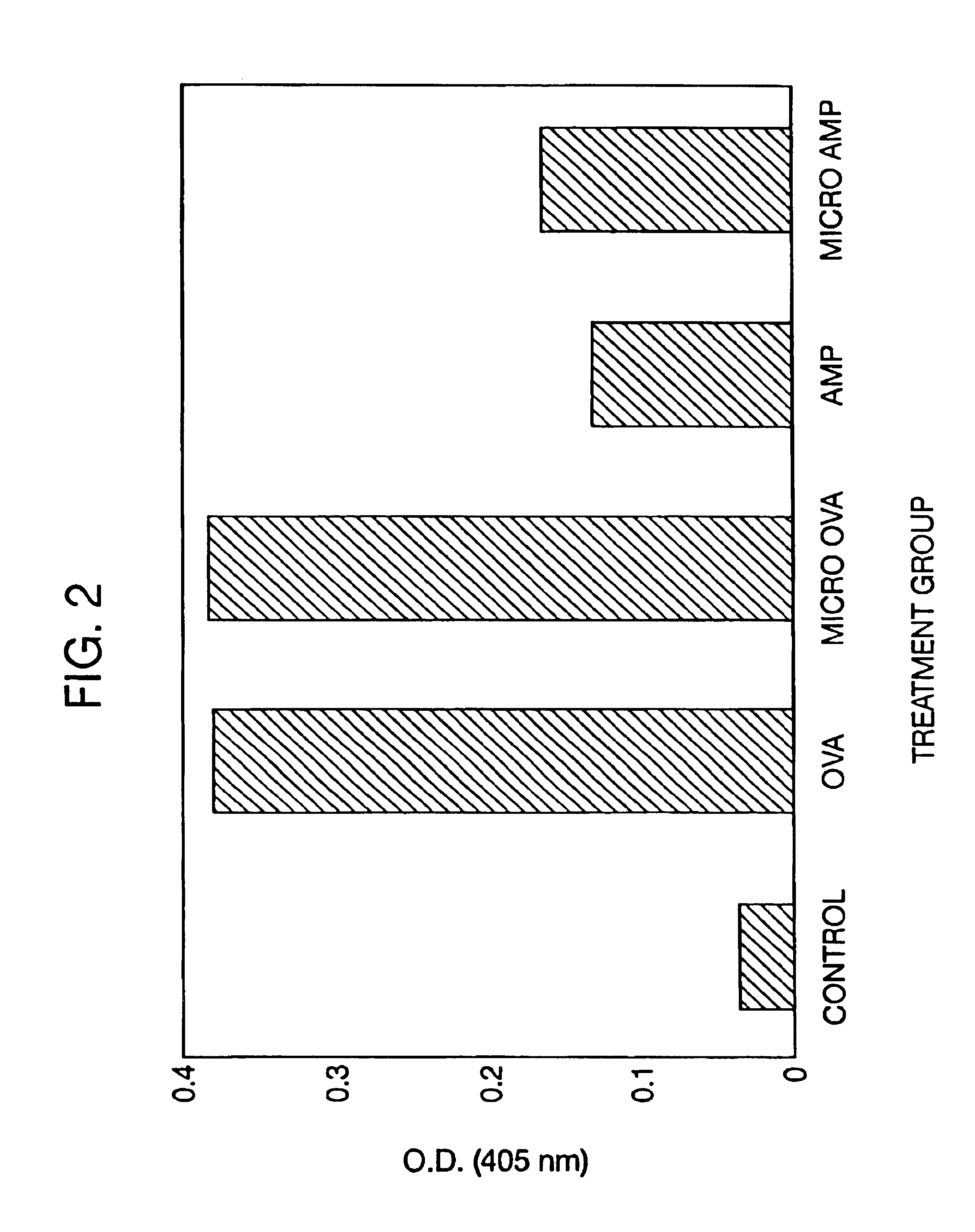
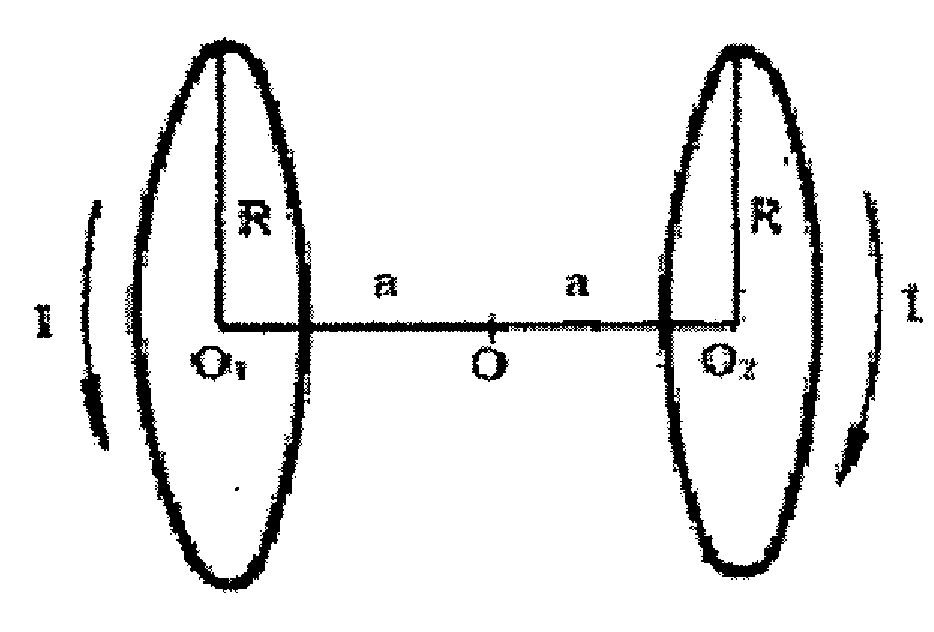
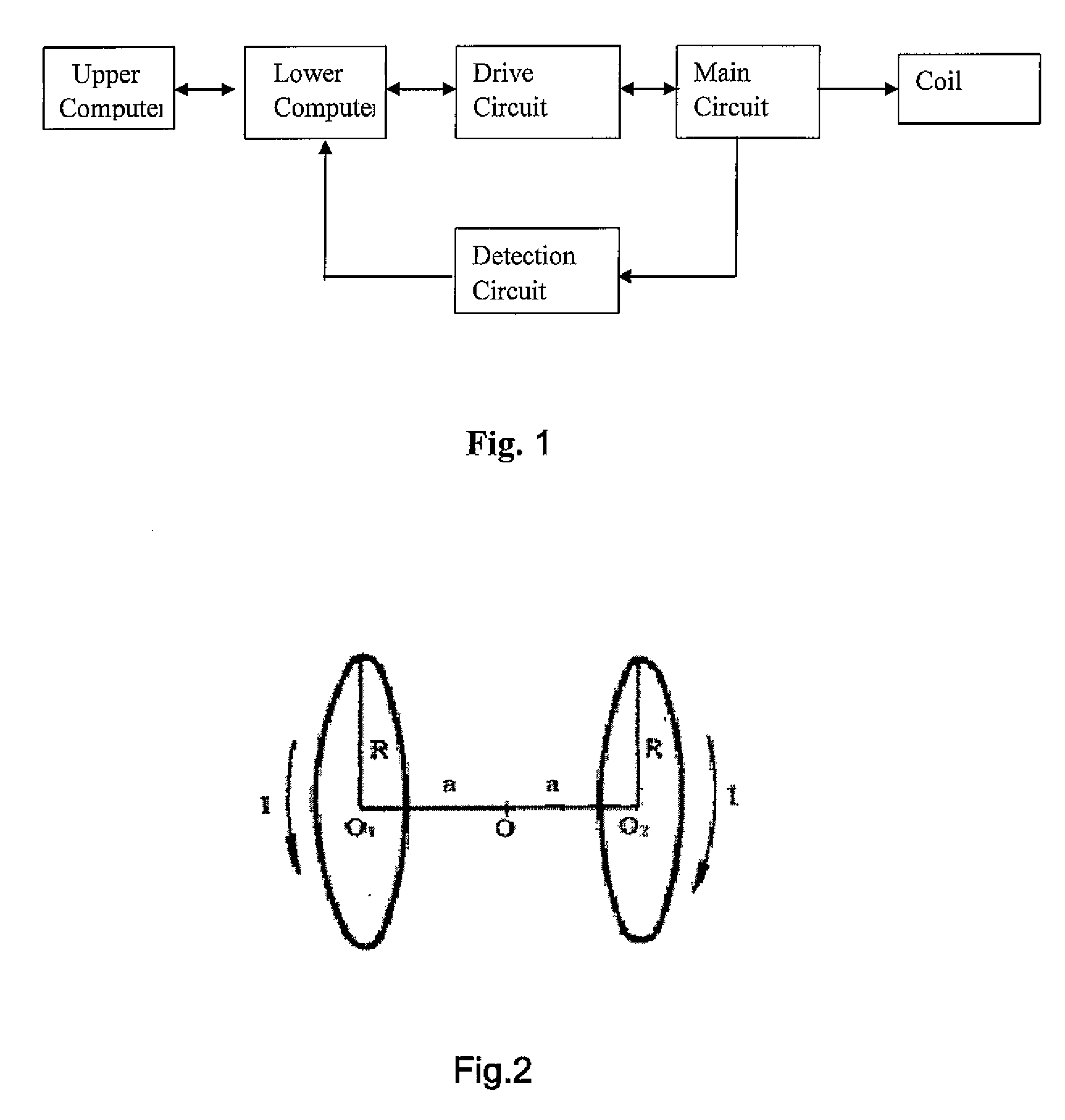
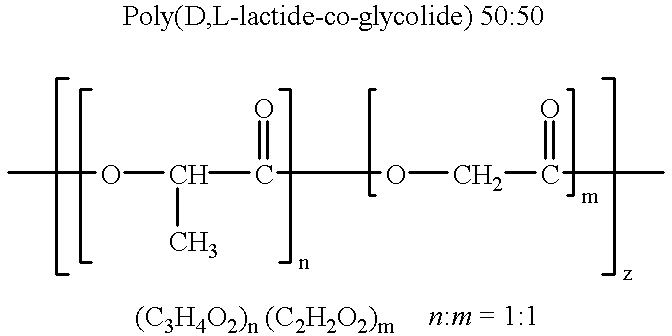Patents
Literature
30830results about How to "Good treatment effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
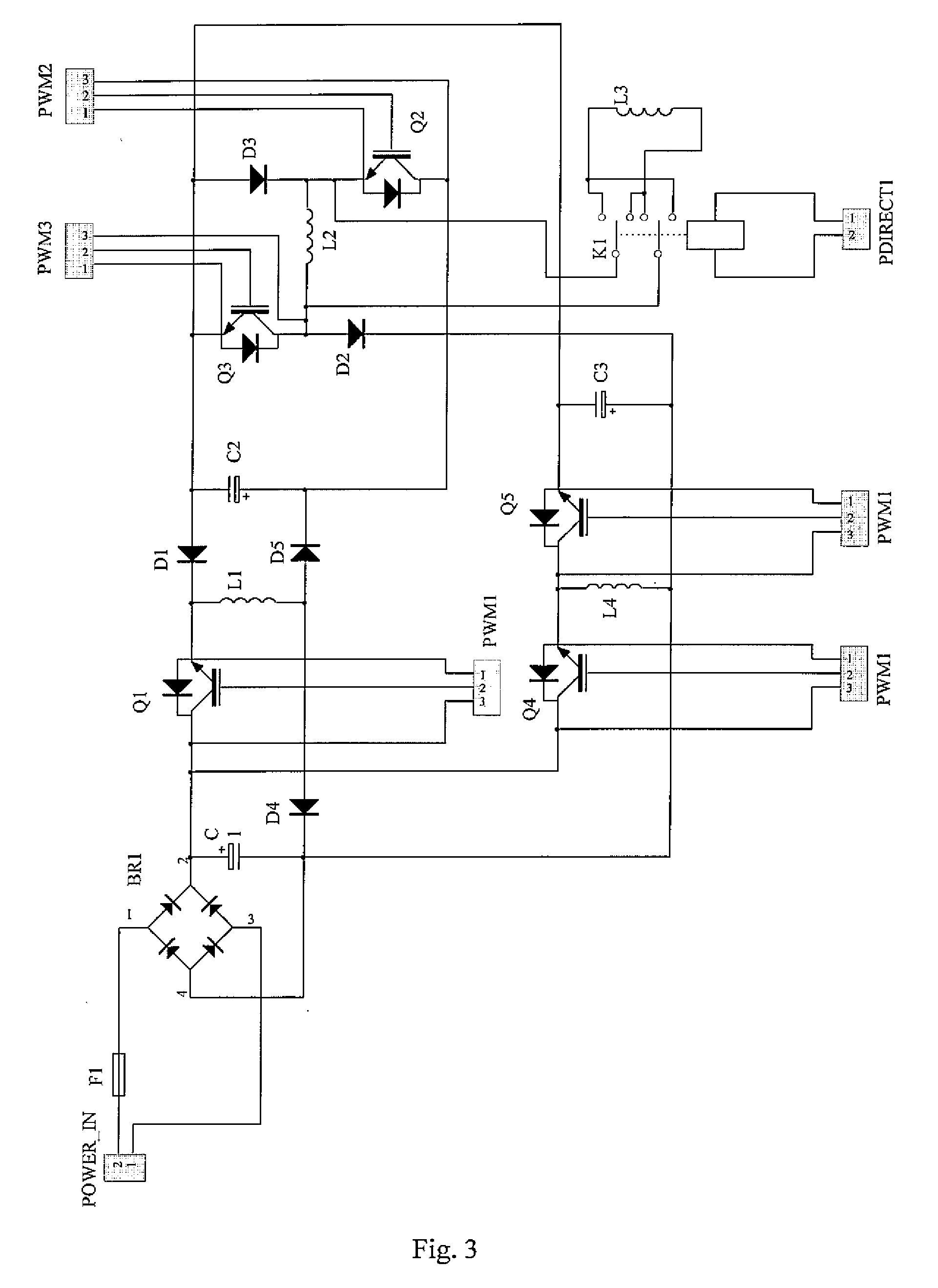
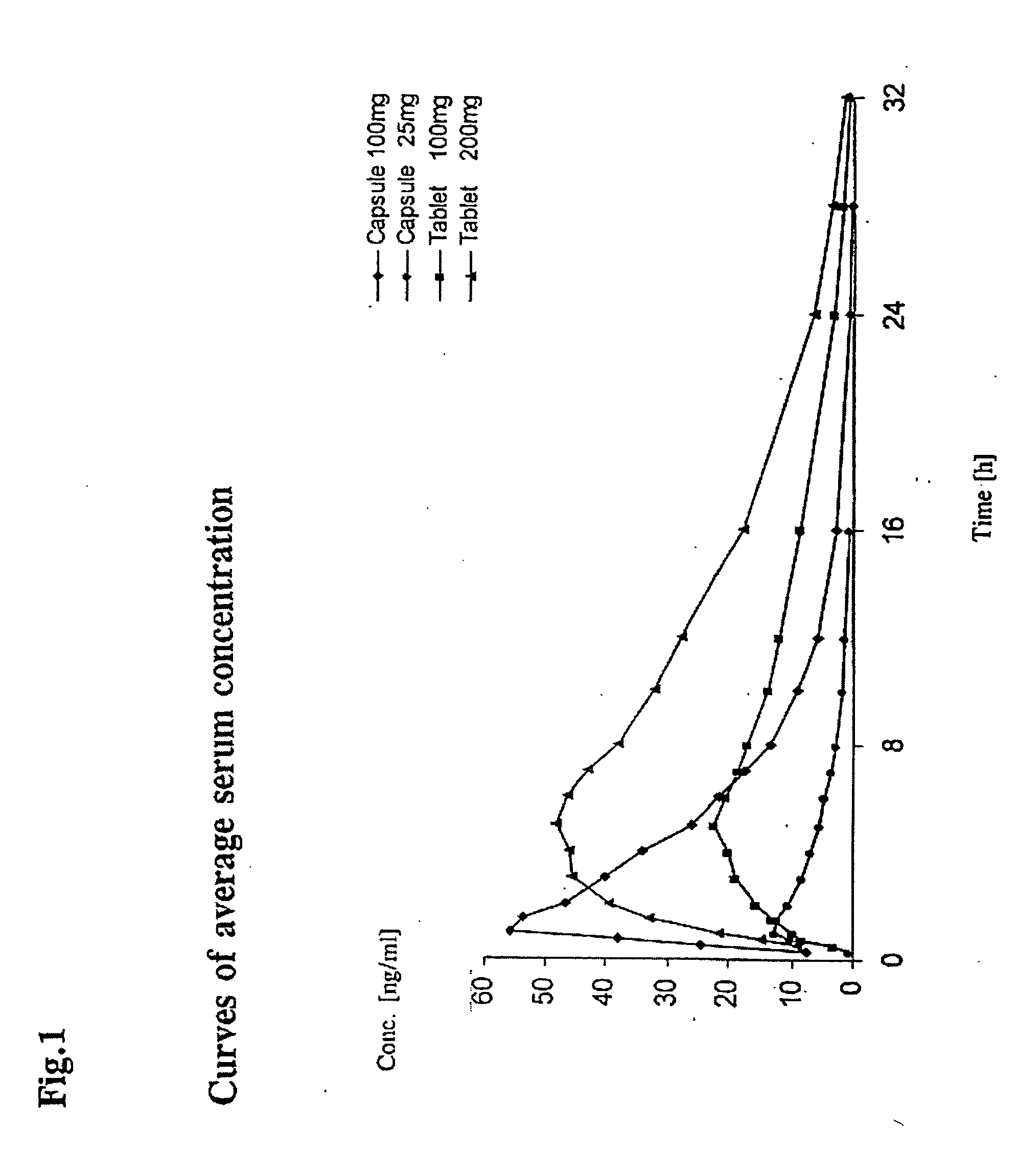
Method of producing a sustained-release preparation
InactiveUS6197350B1Reduce the number of stepsSuitable for industrializationPowder deliveryPeptide/protein ingredientsBlood concentrationOrganic solvent
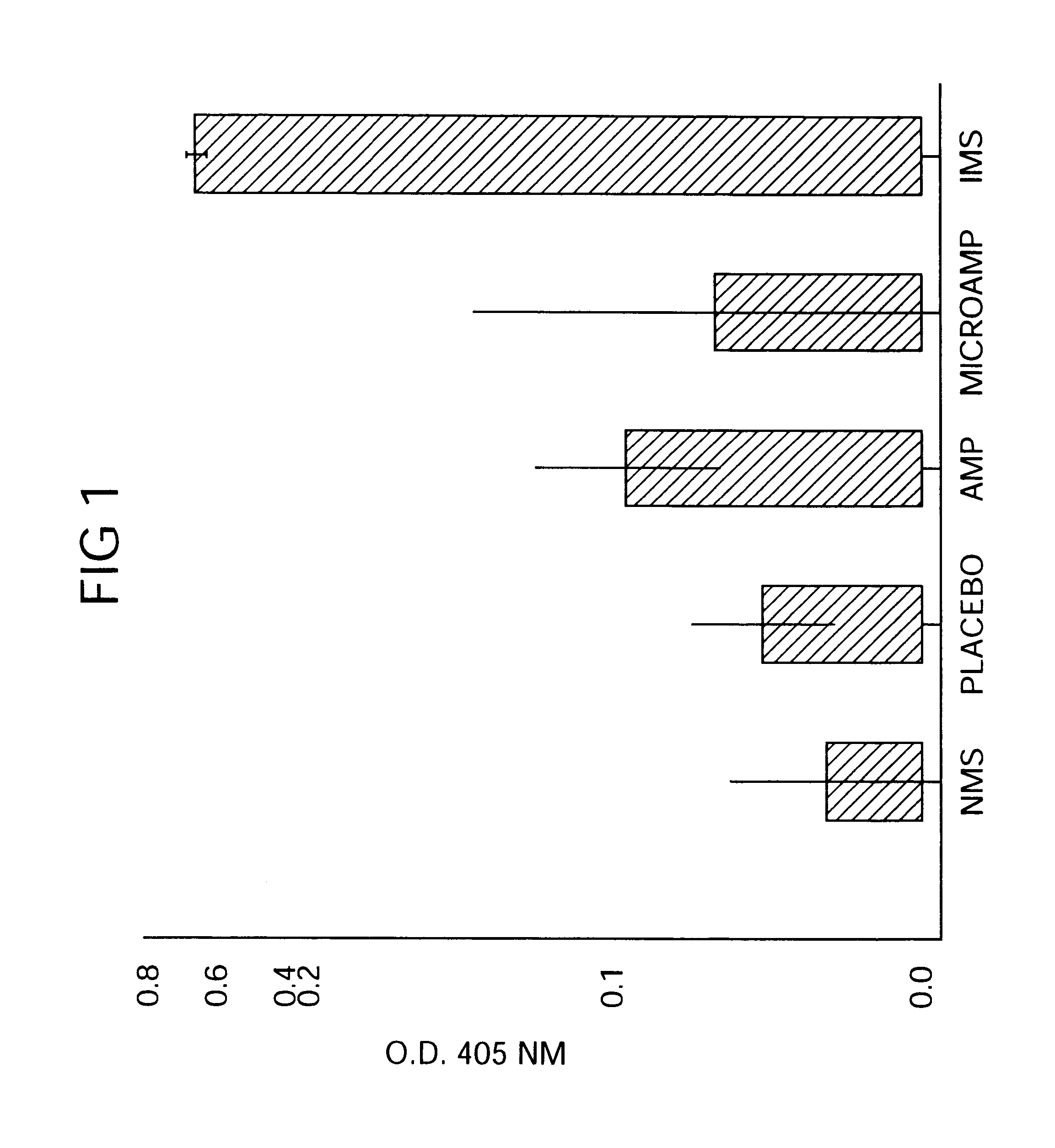
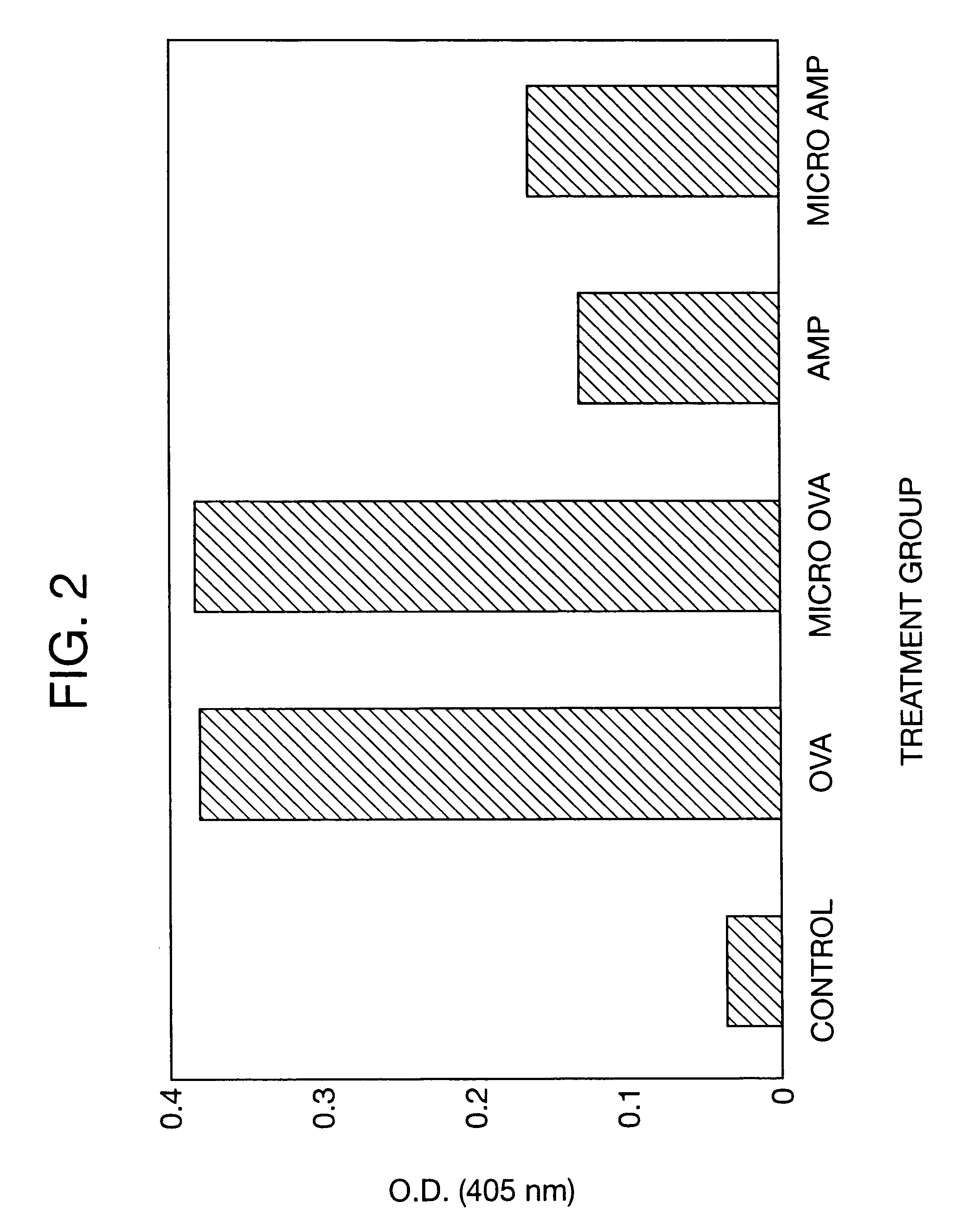
A method of producing sustained-release microcapsules which comprises dispersing a physiologically active polypeptide into a solution of a biodegradable polymer and zinc oxide in an organic solvent, followed by removing the organic solvent; which provides a sustained-release preparation showing a high entrapment ratio of the physiologically active polypeptide and its constant high blood concentration levels over a long period of time.
Owner:TAKEDA PHARMA CO LTD
Implantable article and method
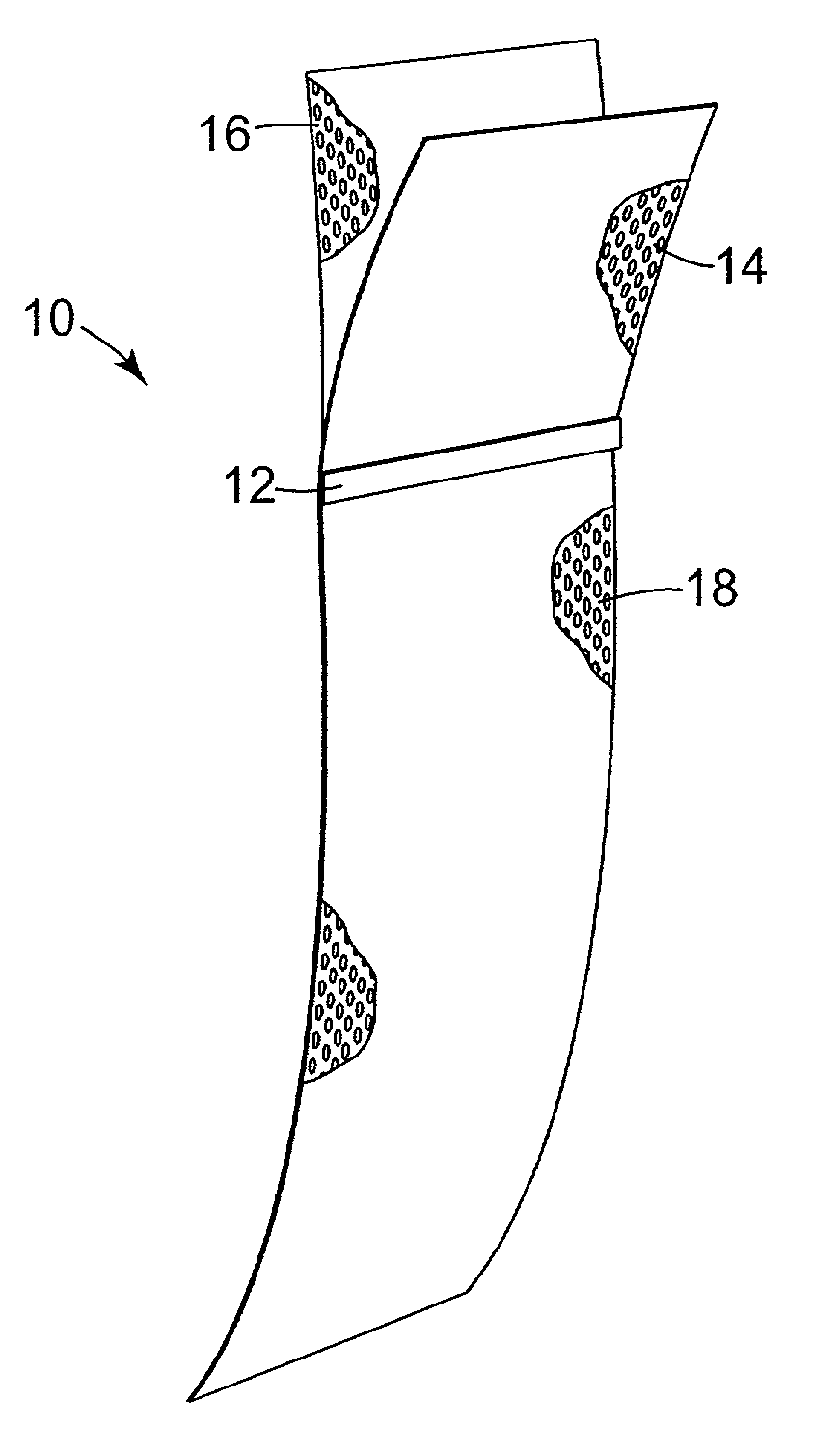
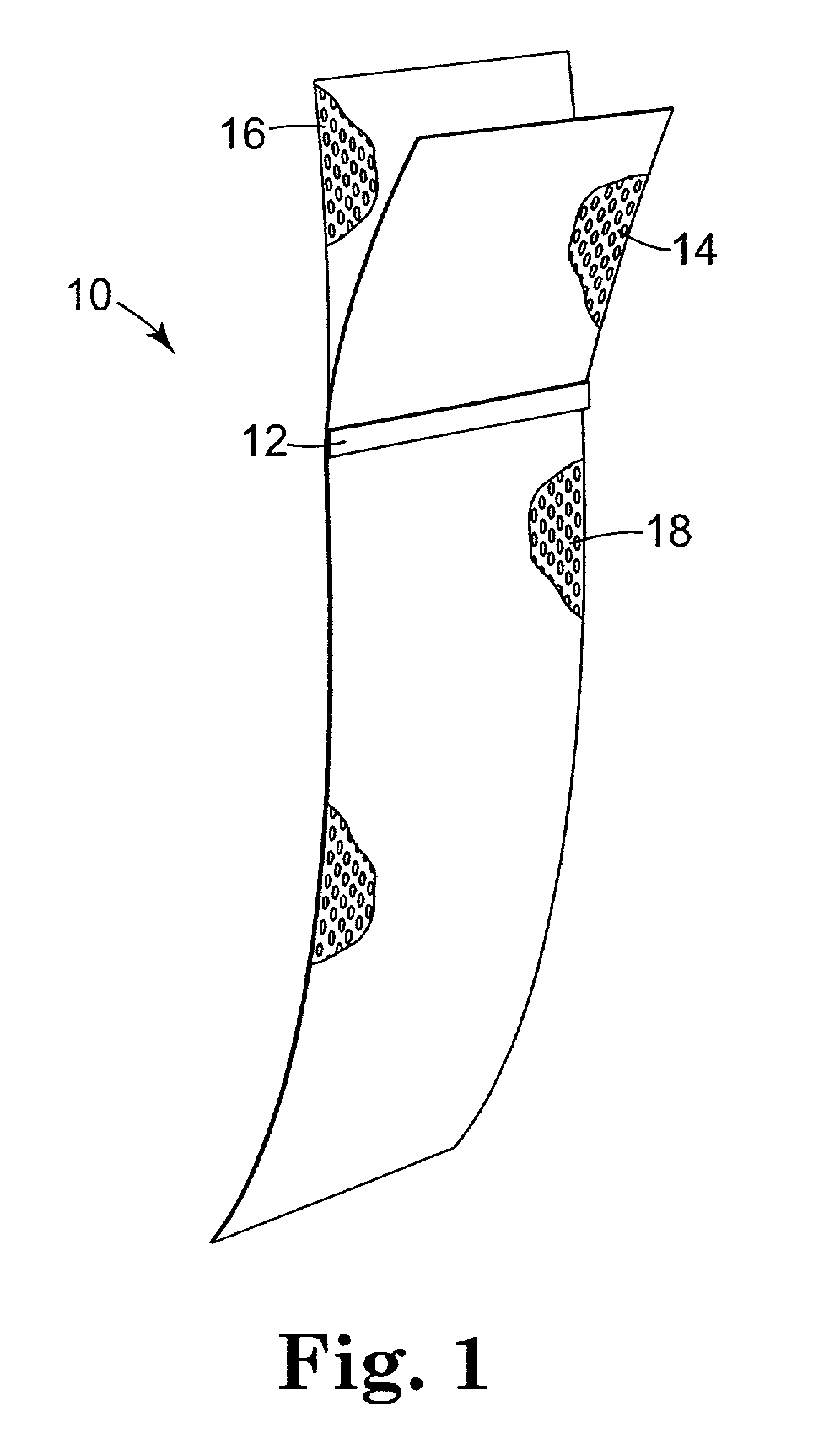
InactiveUS20020028980A1Good treatment effectEncourage tissue ingrowthSuture equipmentsAnti-incontinence devicesDiseaseVaginal vault
An implantable article and method are disclosed for treating pelvic floor disorders such as vaginal vault prolase. A surgical kit useful for performing a surgical procedure such as a sacral colpopexy is also described.
Owner:ASTORA WOMENS HEALTH
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Apparatus and method for disrupting subcutaneous structures
InactiveUS20070060989A1Improve breathabilitySelective disruptionUltrasound therapyElectrotherapyDiseaseCellulite
Methods and apparatus are provided for disruption / destruction of subcutaneous structures in a mammalian body for the treatment of skin irregularities, and other disorders such as excess adipose tissue, cellulite, and scarring. Devices and methods include energy mediated applicators, microneedles, catheters and subcutaneous treatment devices for applying a treatment non-invasively through the skin, less invasively through the skin, or minimally invasively via a subcutaneous approach. Various agents to assist or enhance the procedures are also disclosed.
Owner:THE FOUNDRY INC
Implantable article and method
InactiveUS6592515B2Alleviate challengeGood treatment effectSuture equipmentsAnti-incontinence devicesDiseaseVaginal vault
An implantable article and method are disclosed for treating pelvic floor disorders such as vaginal vault prolase. A surgical kit useful for performing a surgical procedure such as a sacral colpopexy is also described.
Owner:ASTORA WOMENS HEALTH
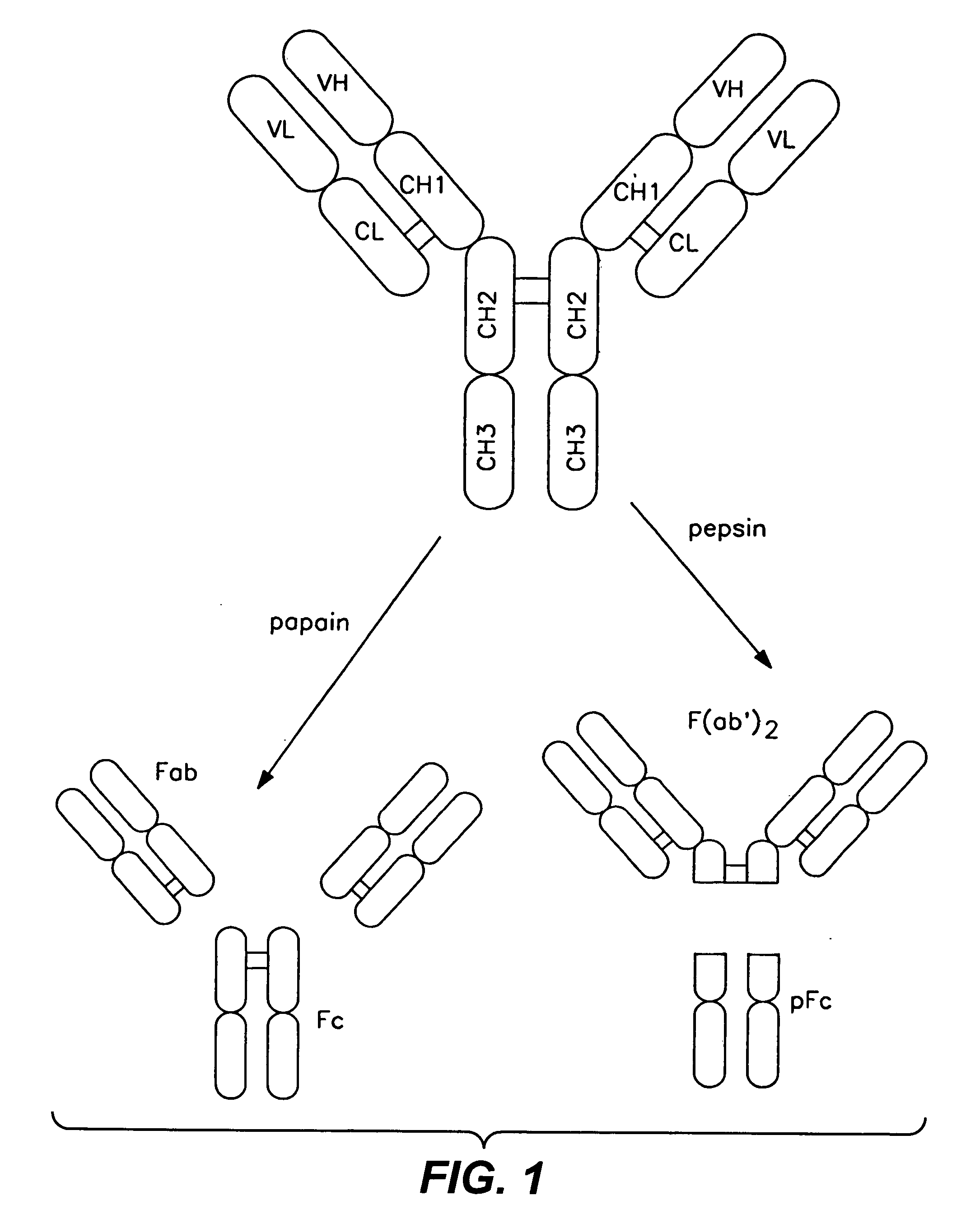
Polypeptide variants with altered effector function
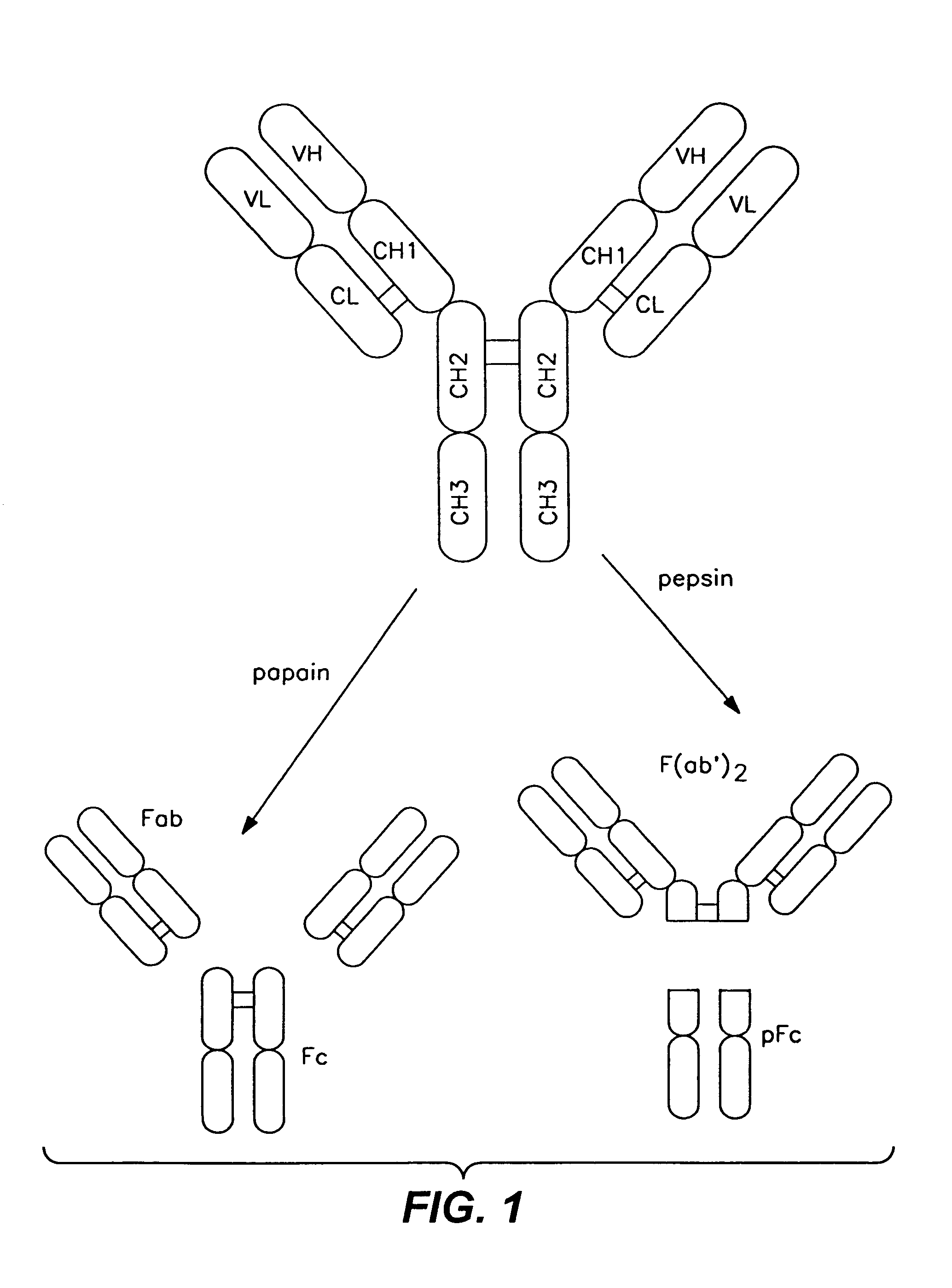
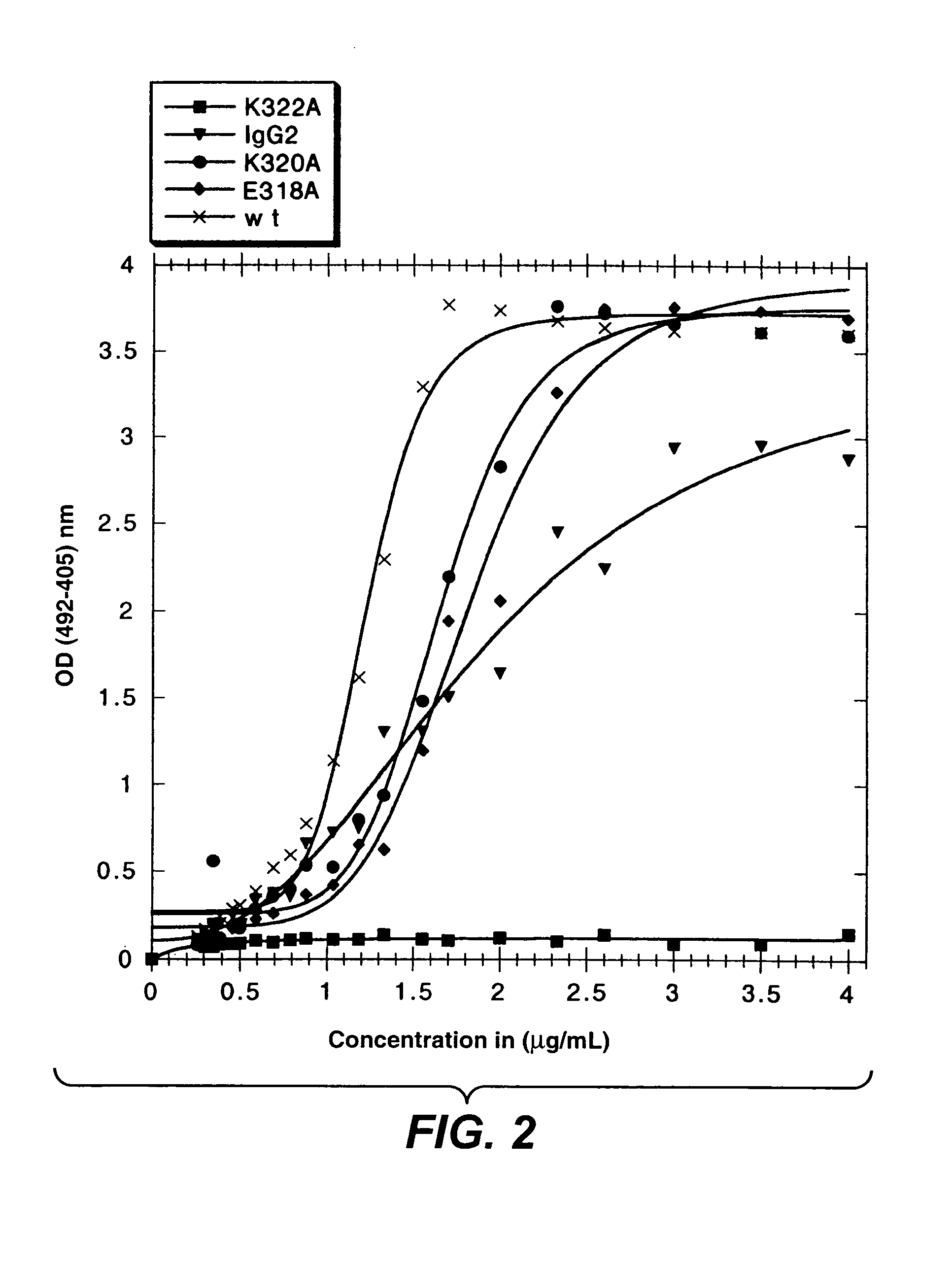
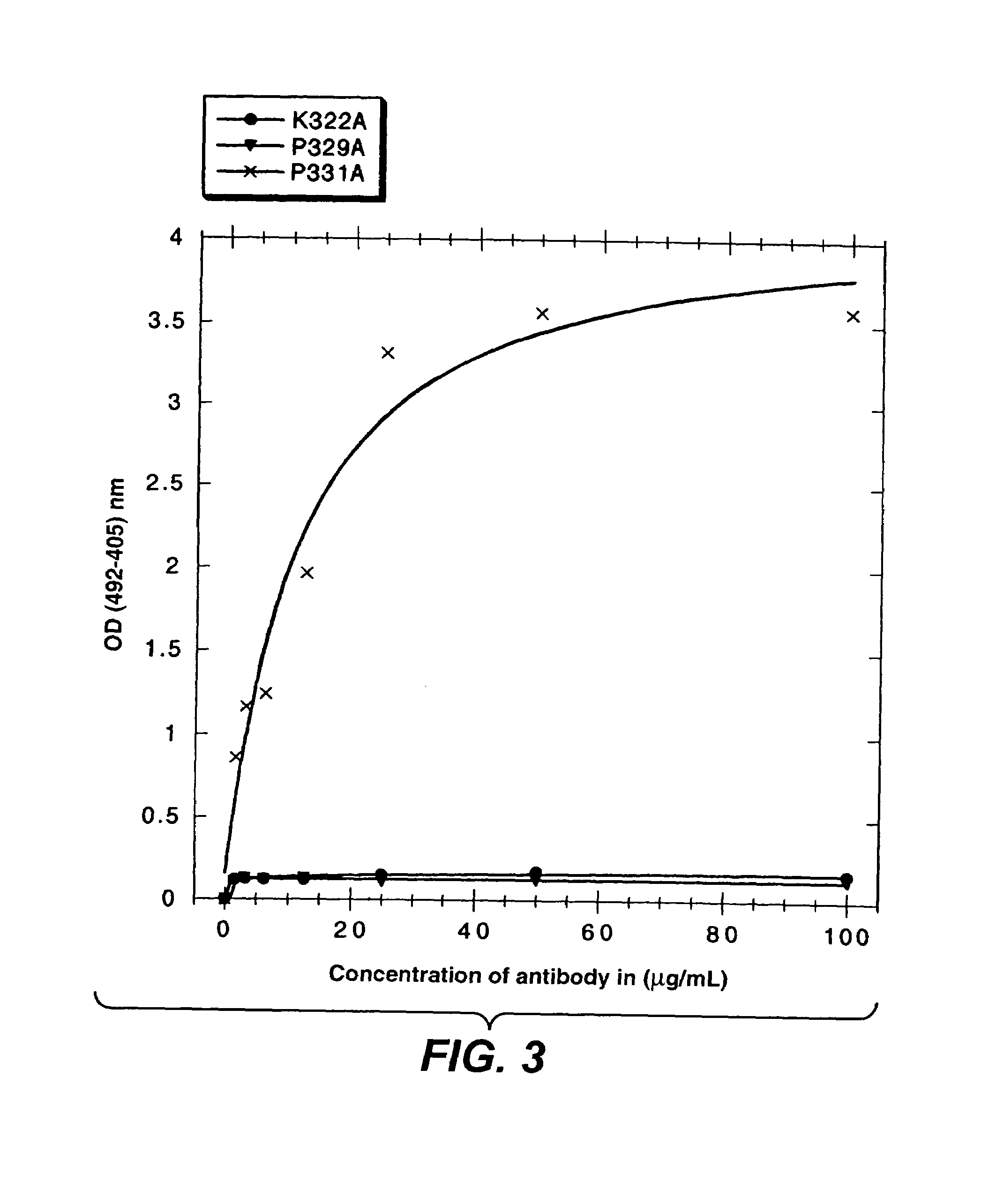
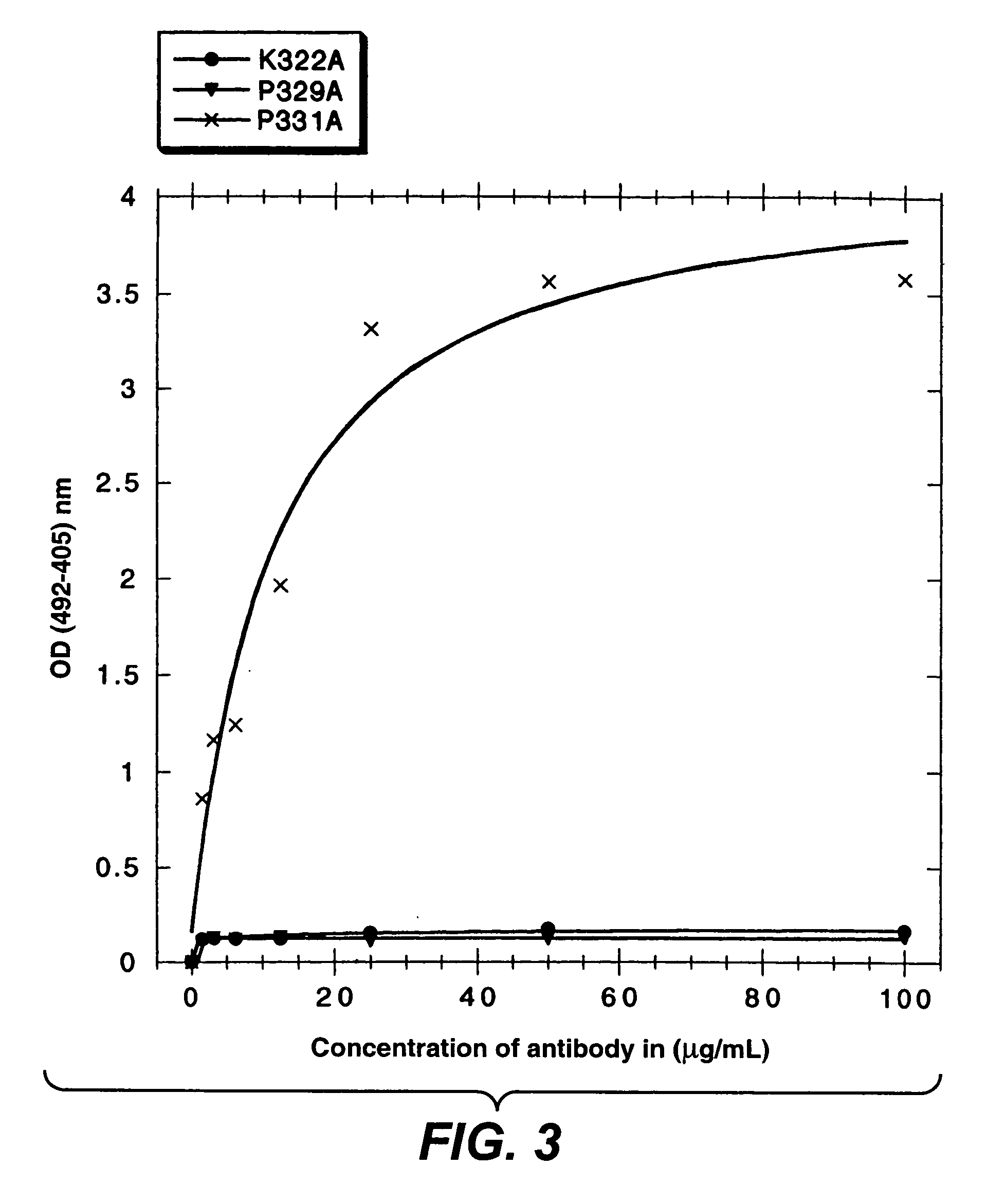
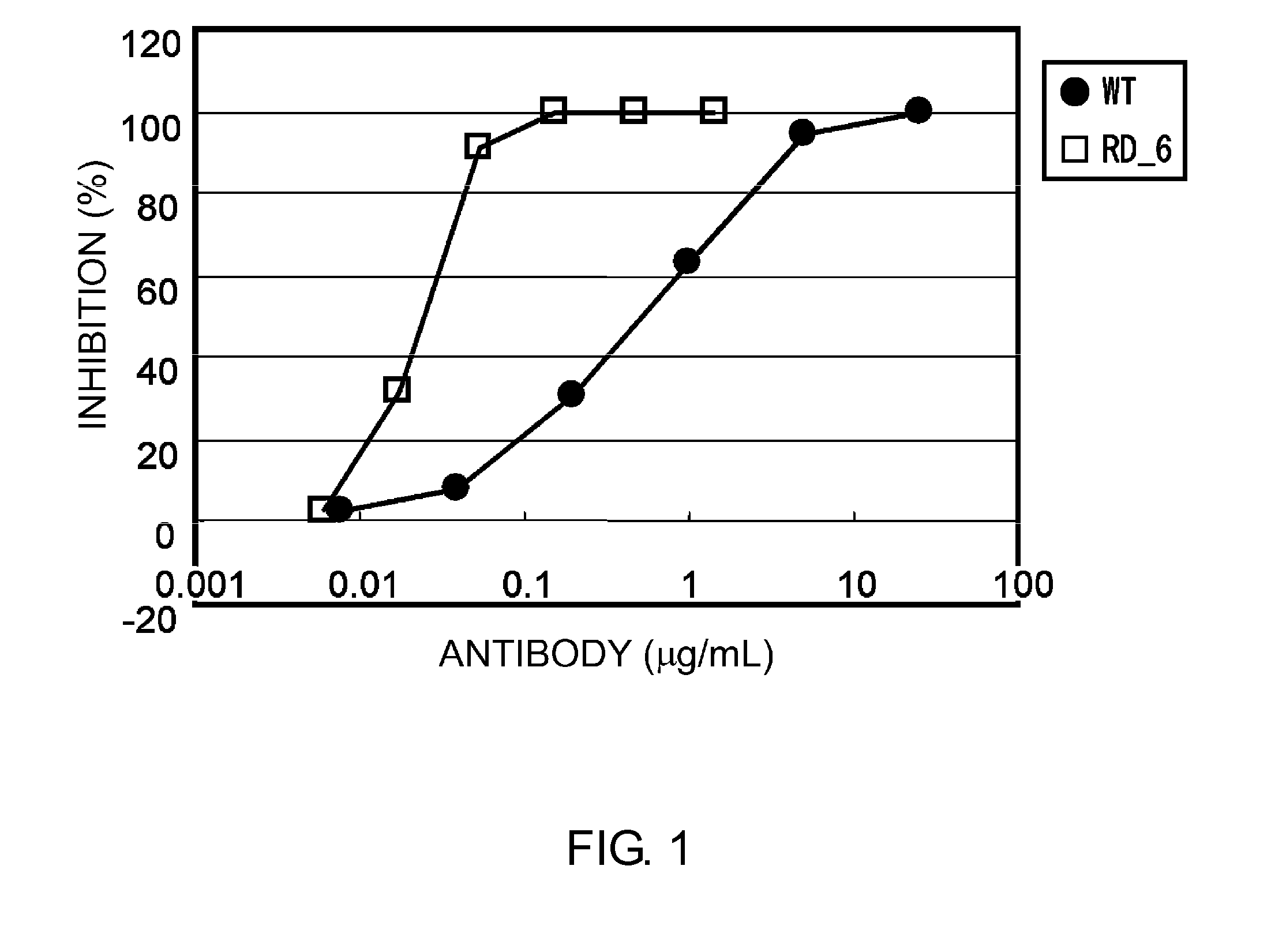
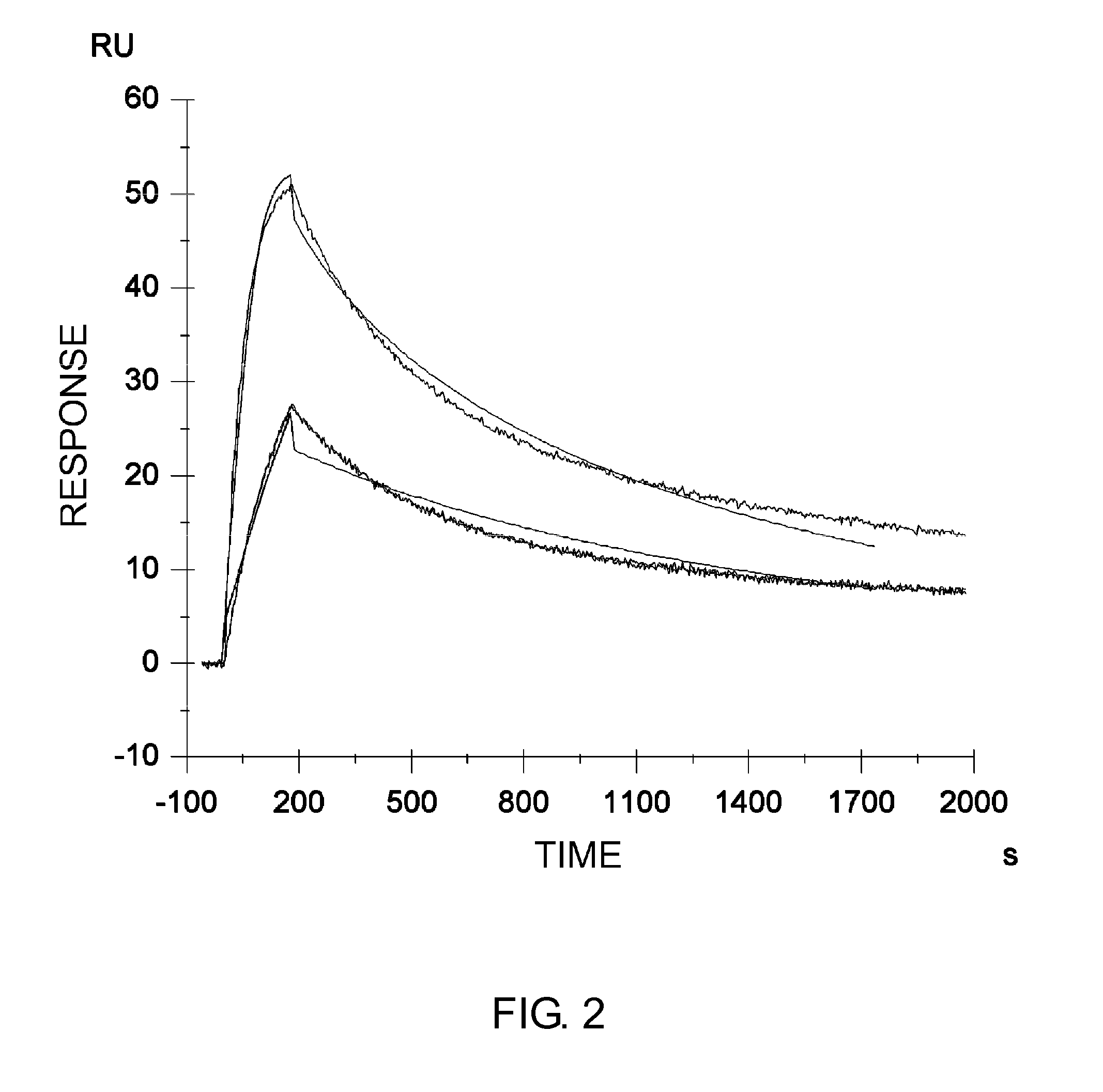
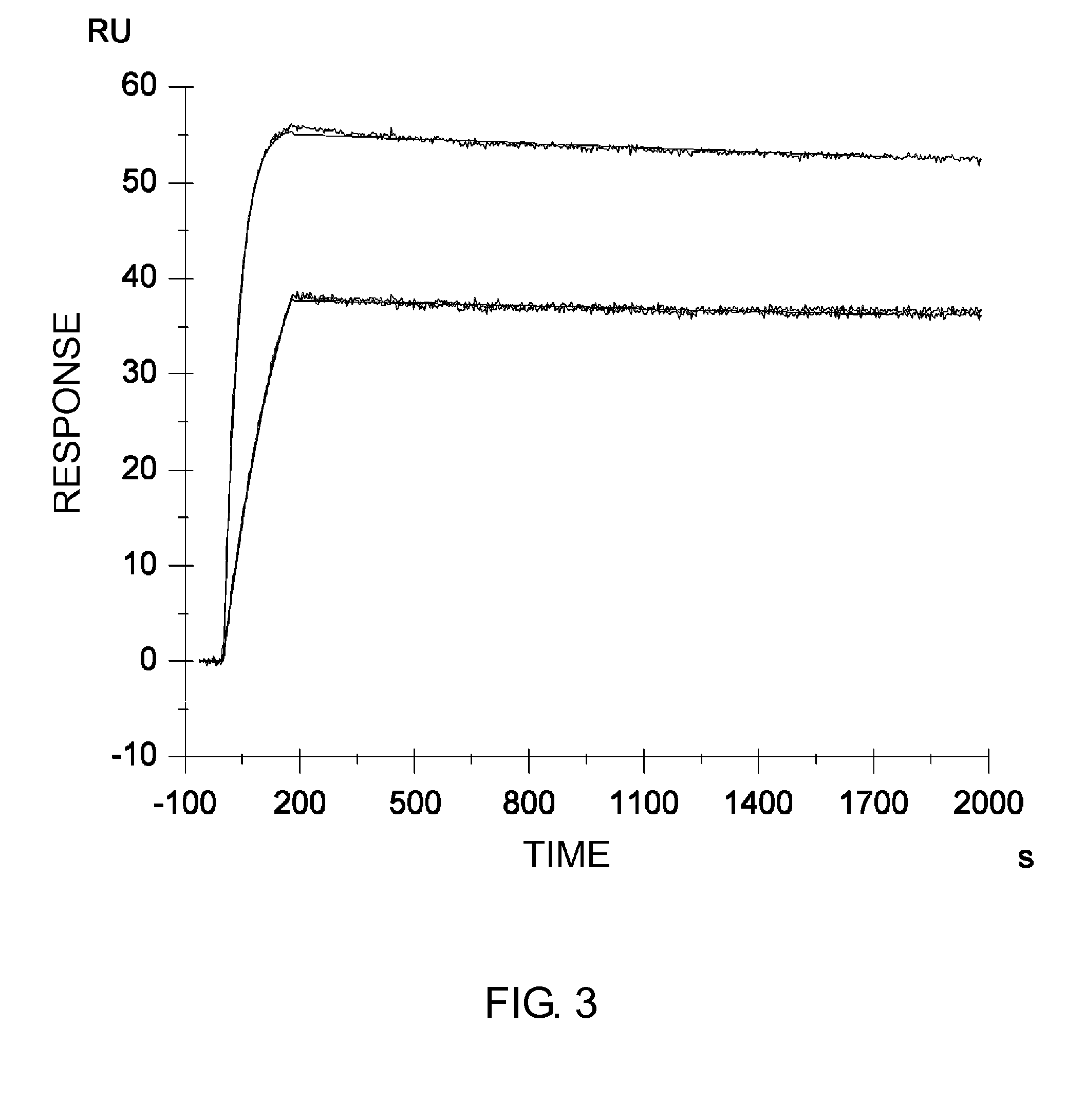
InactiveUS7183387B1Improve bindingAltered affinityAntibody mimetics/scaffoldsImmunoglobulins against growth factorsAmino acidEffector functions
Owner:GENENTECH INC
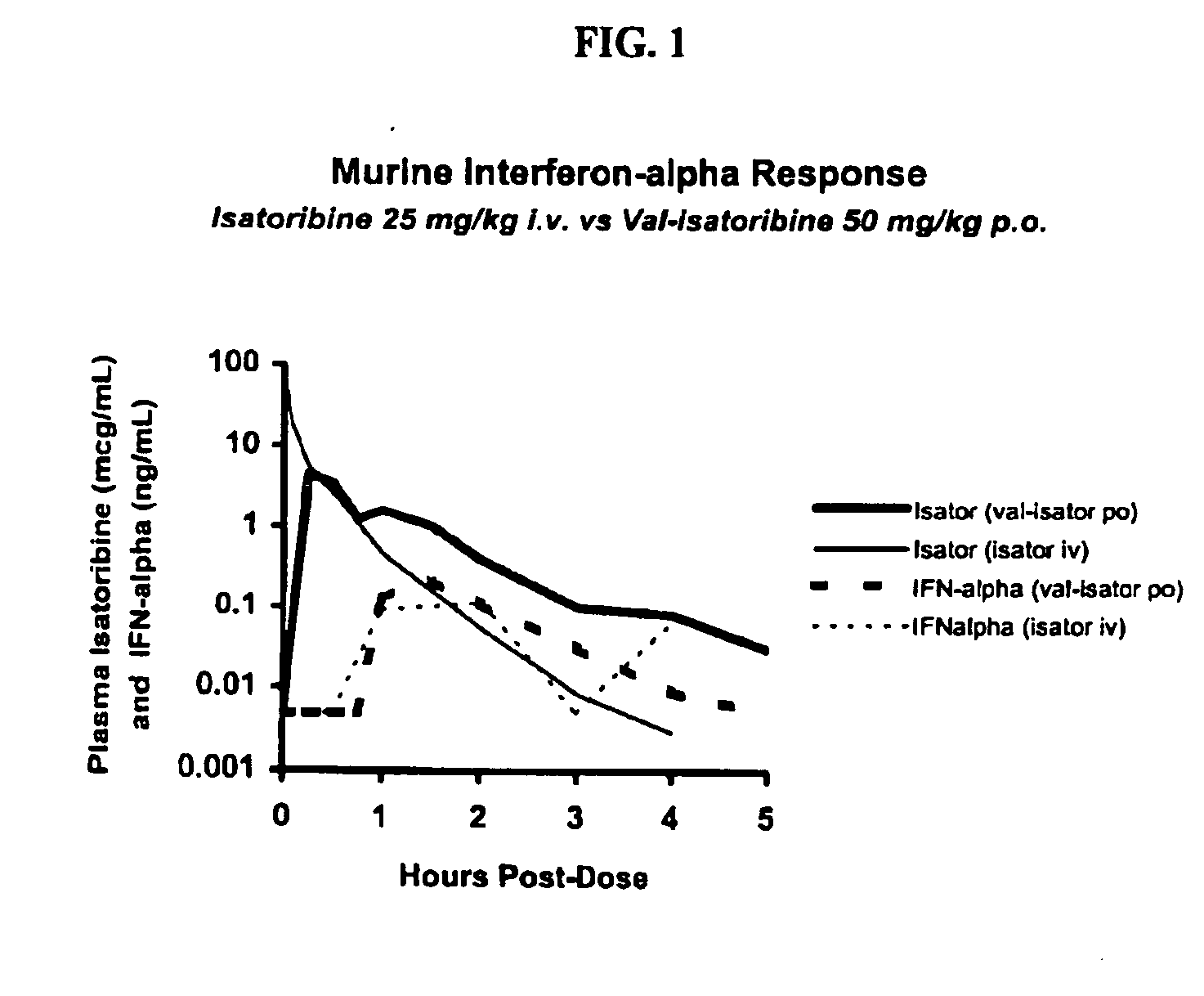
Administration of TLR7 ligands and prodrugs thereof for treatment of infection by hepatitis C virus
InactiveUS20050054590A1Reduce sensitivityAvoid spreadingBiocideDigestive systemHepatitis c viralSide effect
This invention relates to methods for treating or preventing hepatitis C virus infections in mammals using Toll-Like Receptor (TLR)7 ligands and prodrugs thereof. More particularly, this invention relates to methods of orally administering a therapeutically effective amount of one or more prodrugs of TLR7 ligands for the treatment or prevention of hepatitis C viral infection. Oral administration of these TLR7 immunomodulating ligands and prodrugs thereof to a mammal provides therapeutically effective amounts and reduced undesirable side effects.
Owner:ANDADYS PHARMA INC
Compositions and methods for immunomodulation in an organism using IL-15 and soluble IL-15Ra
ActiveUS8124084B2Extended half-lifeImprove bioavailabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsBiological bodyVaccination
The present invention relates to a therapeutic polypeptide and methods for its creation and use for modulating an immune response in a host organism in need thereof. In particular, the invention relates to the administration to an organism in need thereof, of an effective amount of a pre-coupled polypeptide complex comprising a lymphokine polypeptide portion, for example IL-15 (SEQ ID NO: 5, 6), IL-2 (SEQ ID NO: 10, 12) or combinations of both, and an interleukin receptor polypeptide portion, for example IL-15Ra (SEQ ID NO: 7, 8), IL-2Ra (SEQ ID NO: 9, 11) or combinations of both, for augmenting the immune system in, for example, cancer, SCID, AIDS, or vaccination; or inhibiting the immune system in, for example, rheumatoid arthritis, or Lupus. The therapeutic complex of the invention surprisingly demonstrates increased half-life, and efficacy in vivo.
Owner:UNIV OF CONNECTICUT
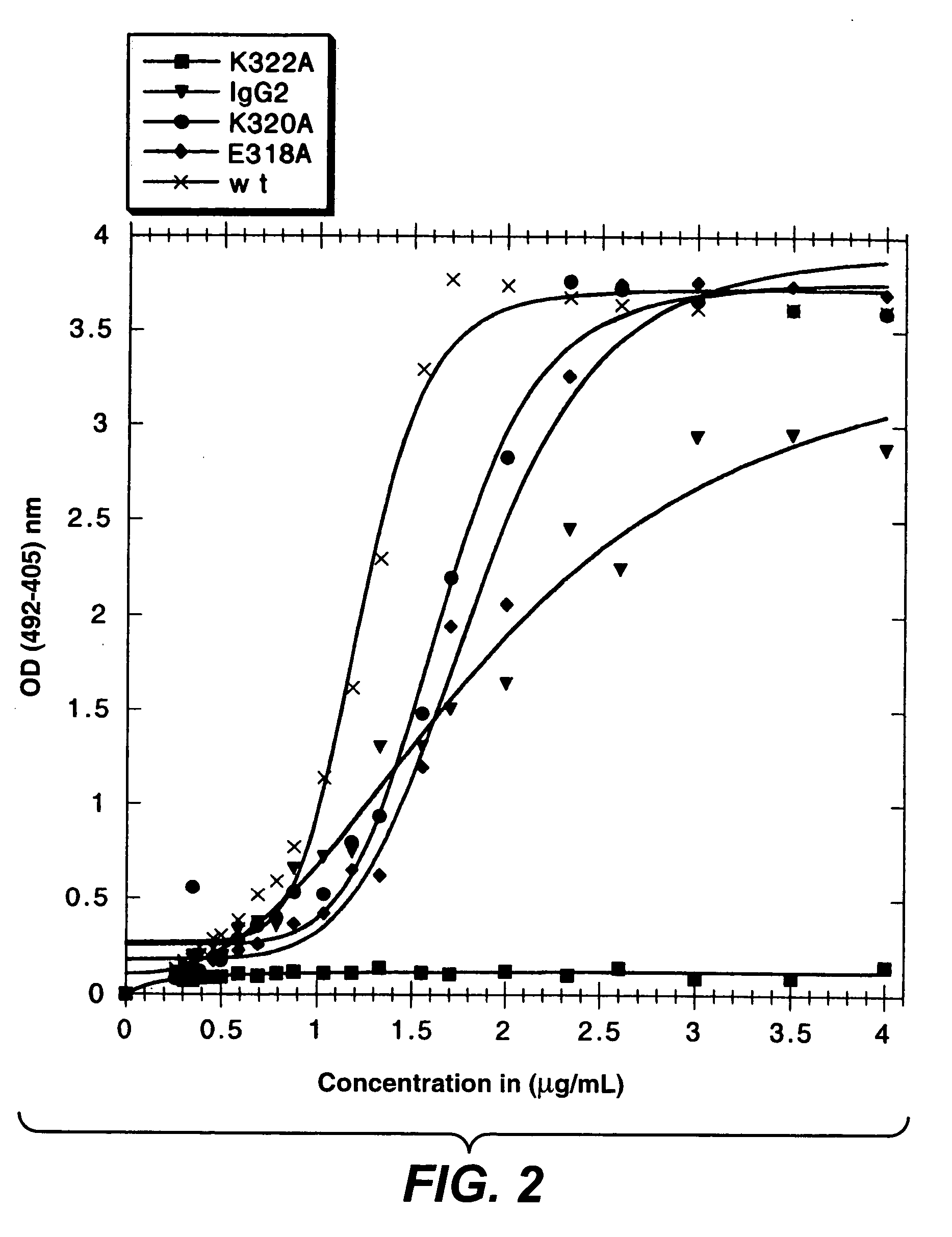
Recombinant antibody composition
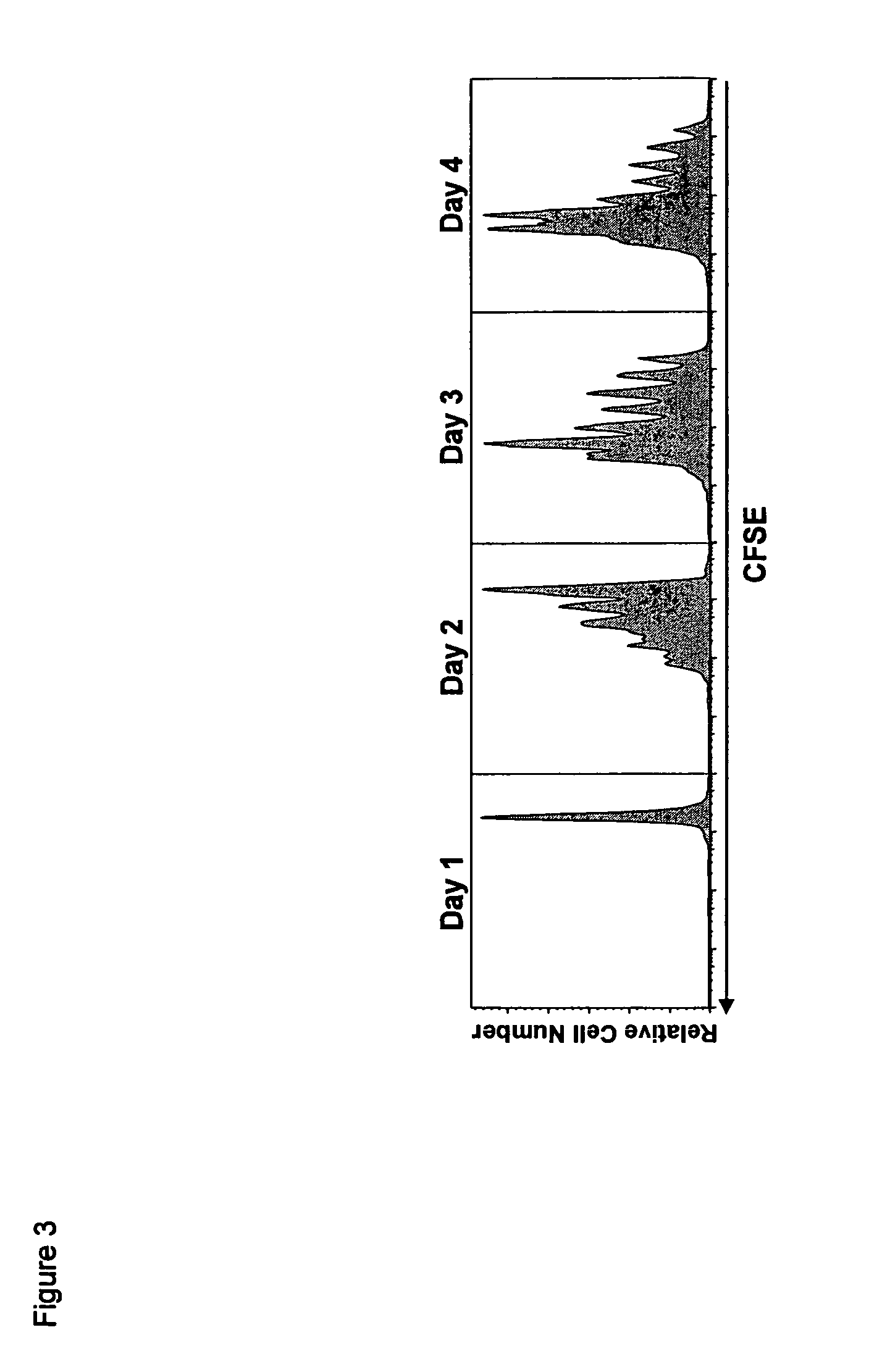
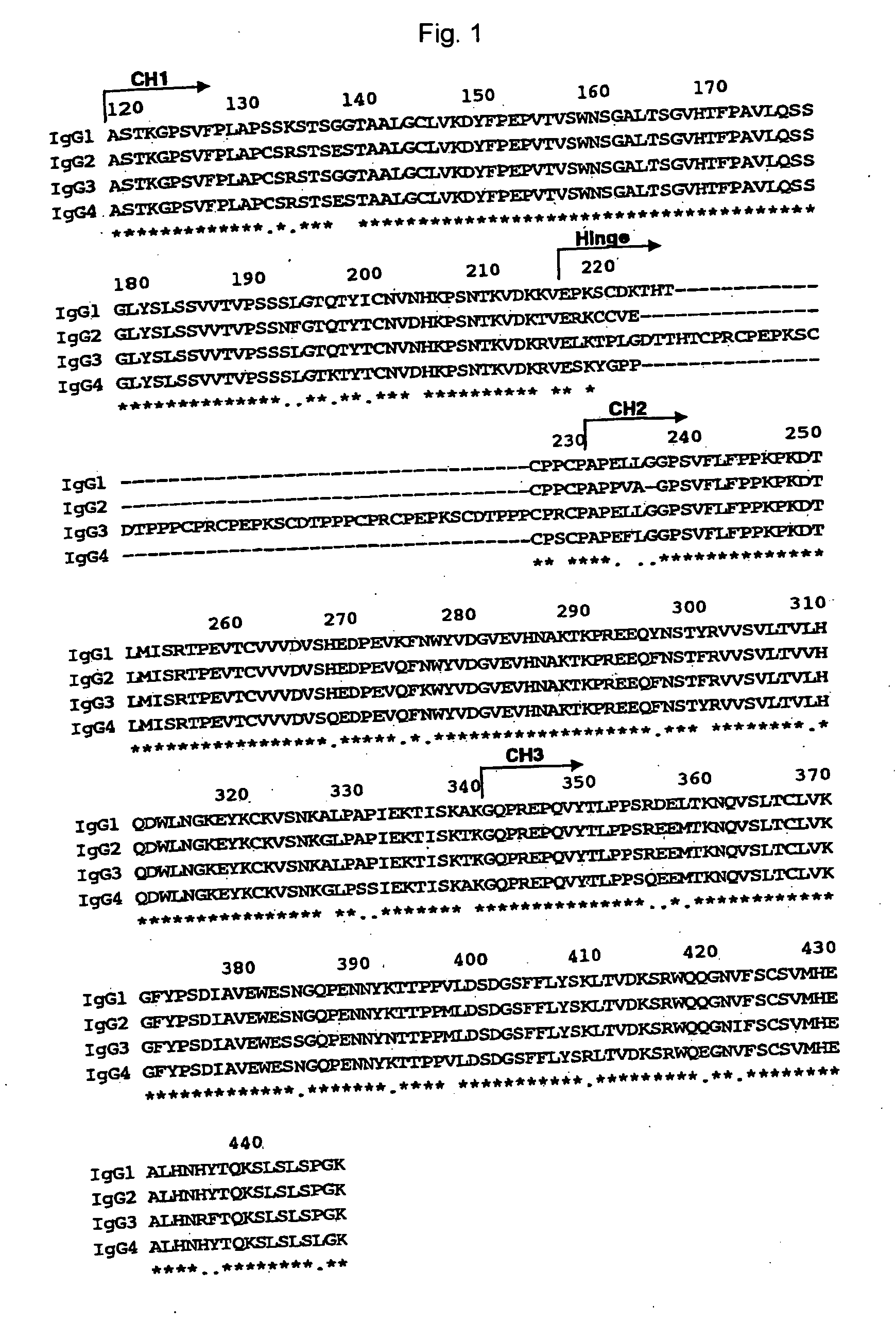
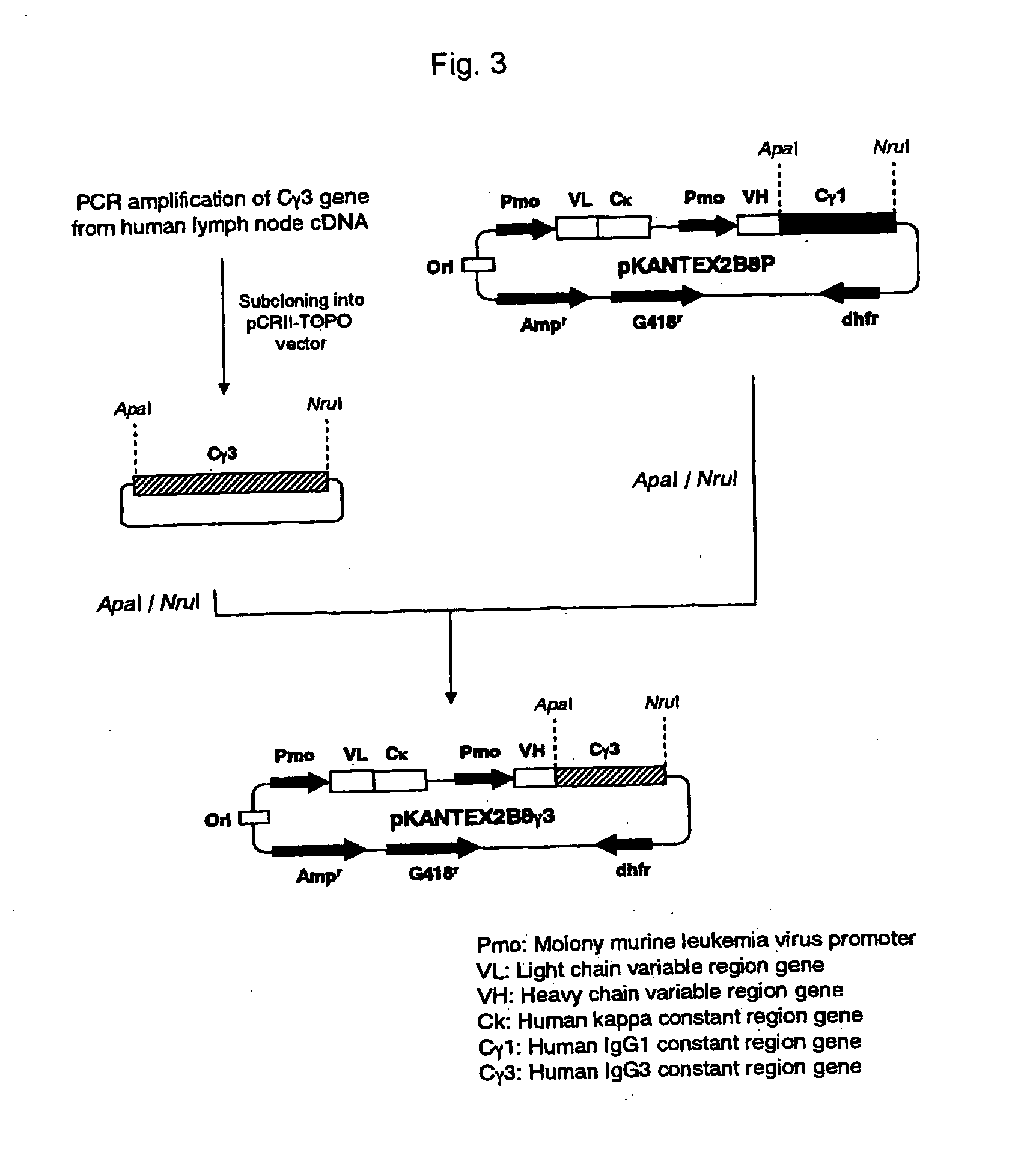
ActiveUS20070148165A1Enhanced effector functionGood treatment effectImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsA-DNABULK ACTIVE INGREDIENT
The present invention relates to a recombinant antibody composition having higher complement-dependent cytotoxic activity than a human IgG1 antibody and a human IgG3 antibody, wherein a polypeptide comprising a CH2 domain in the Fc region of a human IgG1 antibody is replaced by a polypeptide comprising an amino acid sequence which corresponds to the same position of a human IgG3 antibody indicated by the EU index as in Kabat, et al.; a DNA encoding the antibody molecule or a heavy chain constant region of the antibody molecule contained in the recombinant antibody composition; a transformant obtainable by introducing the recombinant vector into a host cell; a process for producing the recombinant antibody composition using the transformant; and a medicament comprising the recombinant antibody composition as an active ingredient.
Owner:KYOWA HAKKO KIRIN CO LTD
Dicarboxylic acid foamable vehicle and pharmaceutical compositions thereof
ActiveUS20080044444A1Convenient vehicle for topical deliveryGood treatment effectPowder deliveryBiocideDicarboxylic acidCarboxylic acid
The present invention teaches a foamable pharmaceutical carrier comprising a benefit agent, selected from the group consisting of a dicarboxylic acid and a dicarboxylic acid ester; a stabilizer selected from the group consisting of at least one surface-active agent; at least one polymeric agent and mixtures thereof; a solvent selected from the group consisting of water, a hydrophilic solvent, a hydrophobic solvent, a potent solvent, a polar solvent, a silicone, an emollient, and mixtures thereof, wherein the benefit agent, stabilizer and solvent are selected to provide a composition that is substantially resistant to aging and to phase separation and or can substantially stabilize other active ingredients. The invention further relates to a foamable composition further containing a liquefied hydrocarbon gas propellant.
Owner:VYNE THERAPEUTICS INC
Multi-channel and multi dimensional system and method
InactiveUS20070156179A1Good treatment effectElectrotherapyArtificial respirationElectrical resistance and conductanceElectricity
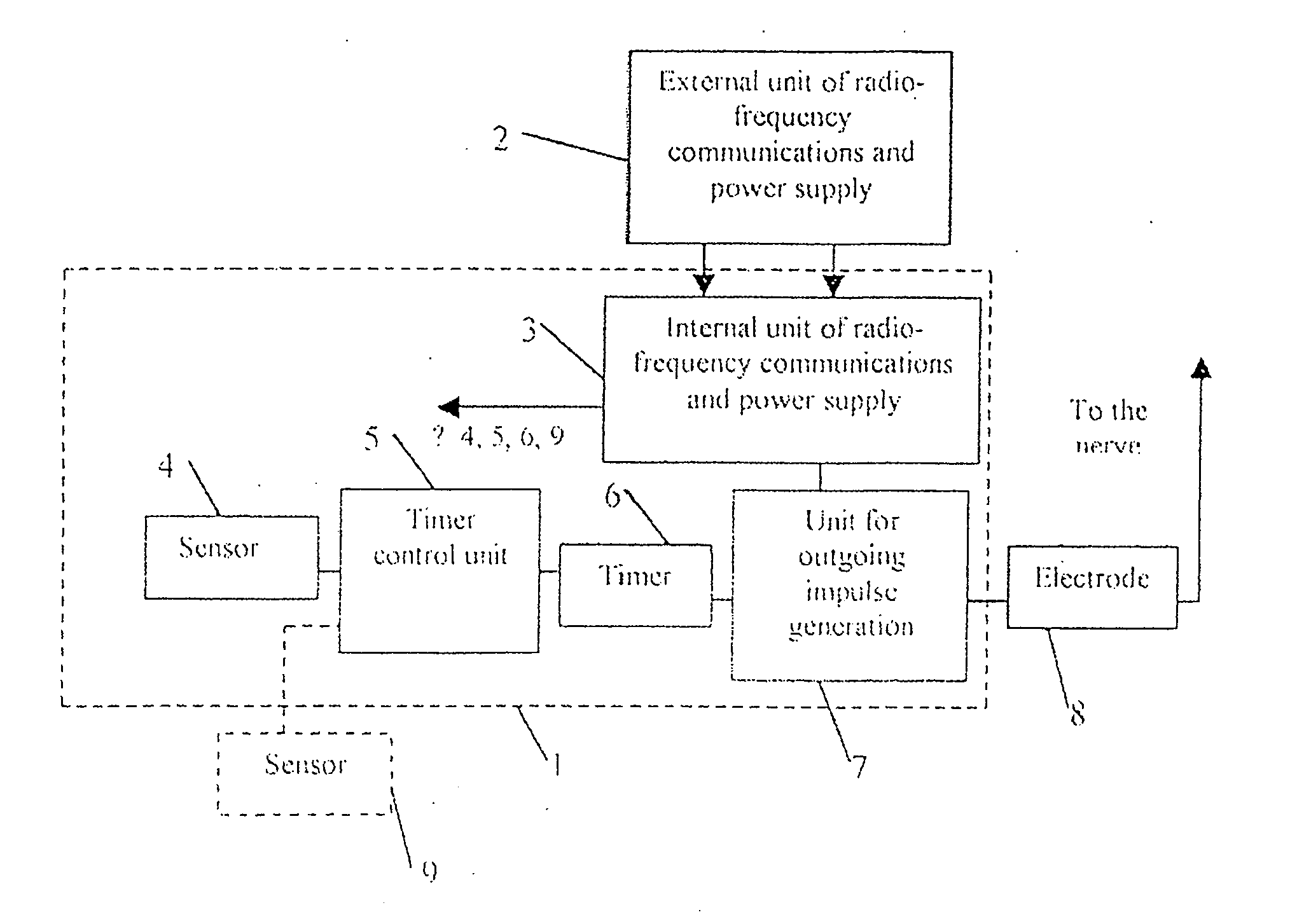
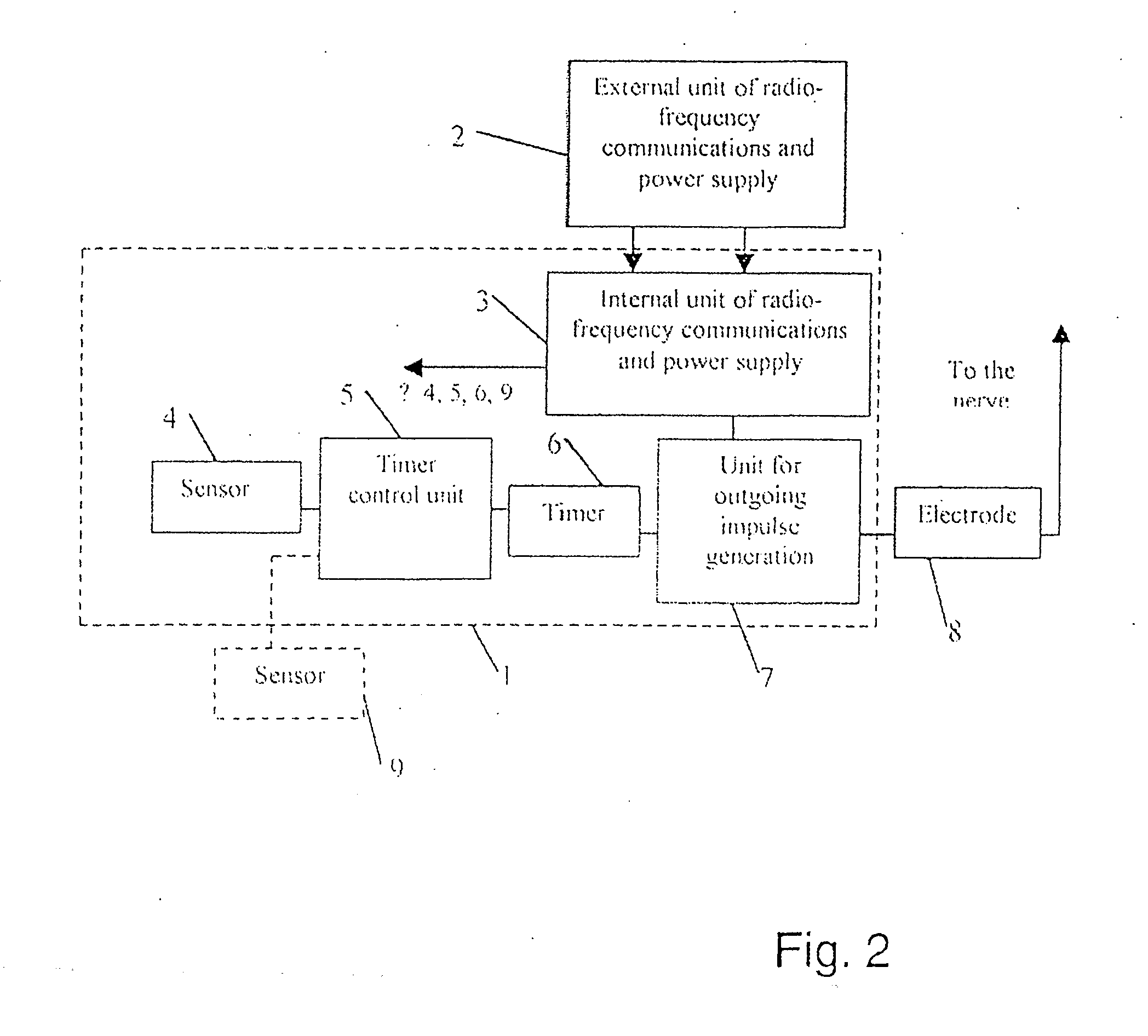
An implanted system for treatment of human diseases by electric stimulation and / or electric blocking of the body tissues, comprising sensor and / or biosensor means for measuring variables in the body, processor means connected to the sensors and biosensors for processing the measured variables and for deciding in real time whether to apply an electric signal to the body tissues, and electrode means implanted at predefined locations and connected to the processor means, for applying the stimulation and / or electric blocking signals to the body tissues. A method for treatment of human diseases using an implanted system by electric stimulation and / or electric blocking of the body tissues, comprising: A. measuring variables in the body using implanted sensor and / or biosensor means; B. processing the measured variables for deciding in real time whether to apply an electric signal to the body tissues; C. applying the stimulation and / or electric blocking signals to the body tissues.
Owner:S E KARASHUROV
Therapeutic treatment and prevention of infections with a bioactive material(s) encapuslated within a biodegradable-bio-compatable polymeric matrix
InactiveUS6902743B1Induce productionSustained release of active agent over timePowder deliveryPeptide/protein ingredientsTherapeutic treatmentActive agent
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer having a molar composition of lactide / glycolide from 90 / 10 to 40 / 60, which may contain a pharmaceutically-acceptable adjuvant, as a blend of uncapped free carboxyl end group and end-capped forms ranging to ratios from 100 / 0 to 1 / 99.
Owner:ARMY UNITED STATES GOVERNMENT AS REPRESENTED BY THE SEC OF THE
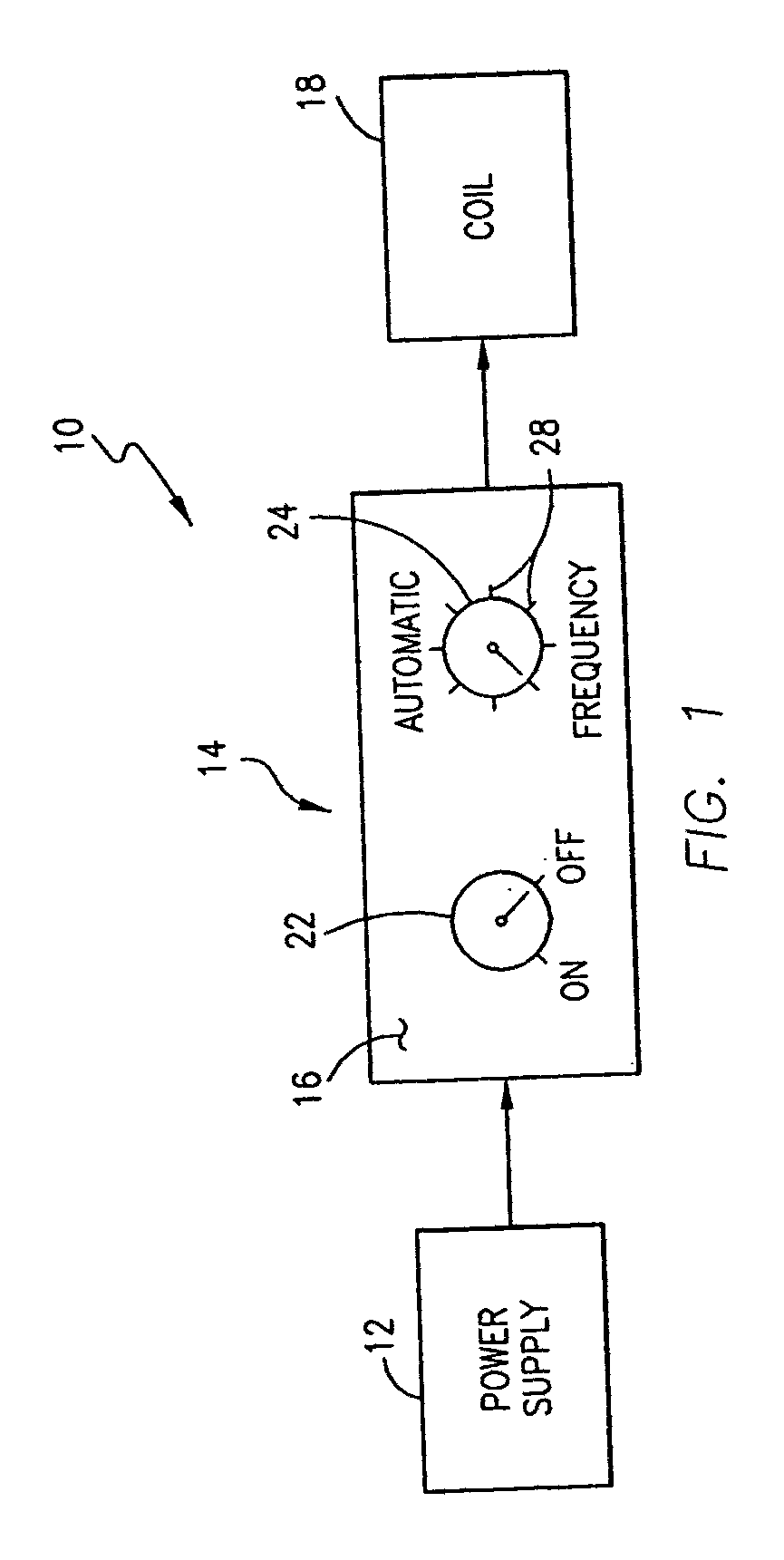
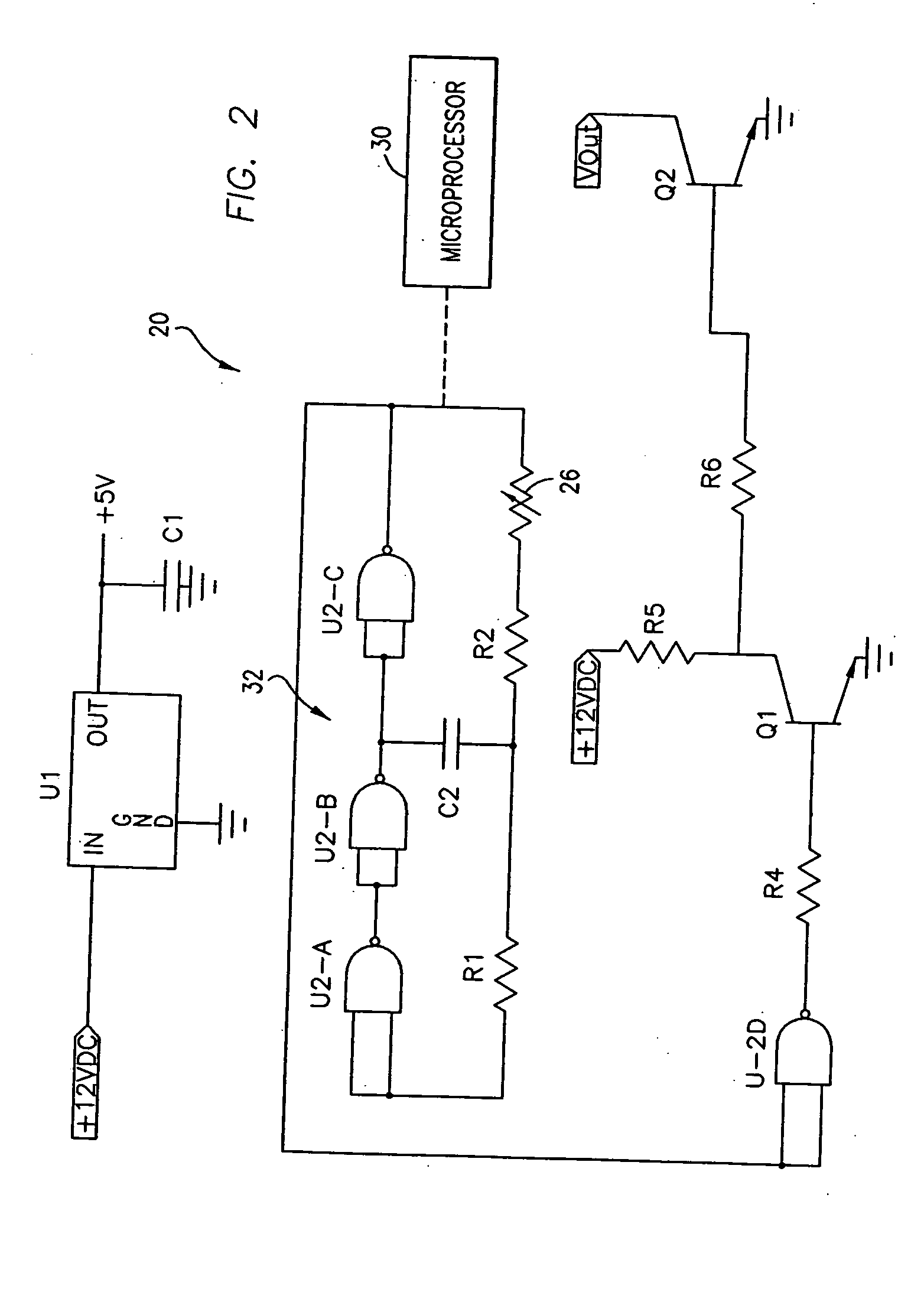
Magnetic Stimulating Circuit For Nervous Centralis System Apparatus, Purpose, and Method Thereof
ActiveUS20080200749A1High frequencyImprove brain functionElectrotherapyMagnetotherapy using coils/electromagnetsWide areaDisease
A magnetic stimulation apparatus for central nervous system and circuit thereof and use of the apparatus and method of using the apparatus are shown. Controlling circuit design and outputting wave form signal to a drive power supply circuit enables the drive power supply circuit to output current of corresponding wave form to coils, and by means of the design of the shape, number of turns, size, interval of the coils, generates within a certain region inside the coils a desired time-variant magnetic field which is then applied to the brain of an animal or a human being so that the central nervous system can receive a wide area synergy magnetic stimulation with a precise wave form, high frequency or a combination of a plurality of frequency components, thus achieving the treatment of nervous and psychiatric diseases or brain function improvement in combination with behavior guidance, thought guidance, or psychological guidance.
Owner:ZHENG YUNFENG
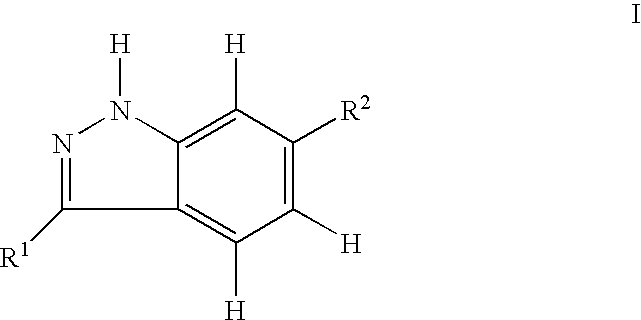
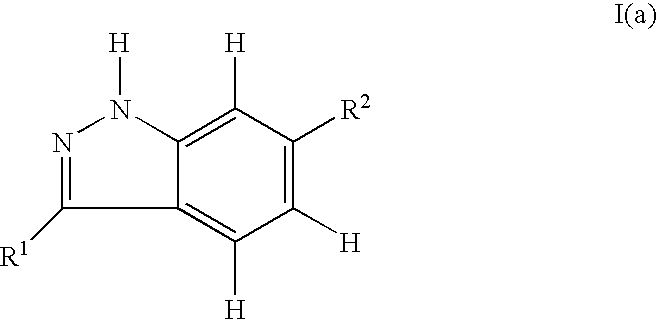
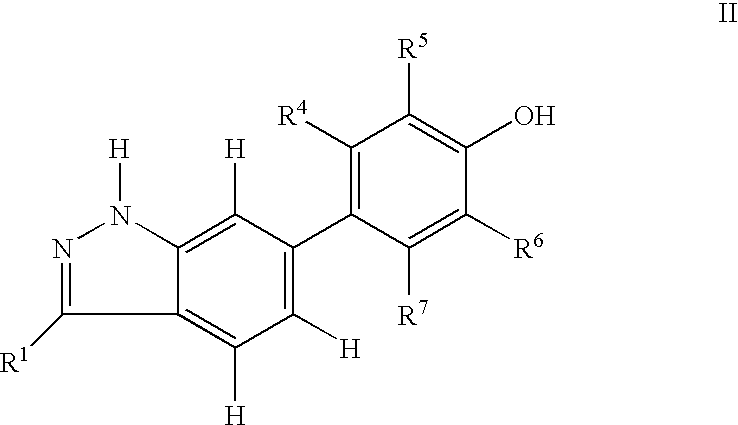
Indazole compounds and pharmaceutical compositions for inhibiting protein kinases, and methods for their use
InactiveUS6884890B2Improve anti-tumor effectGood treatment effectBiocideOrganic chemistryDiabetic retinopathyProtein kinase domain
Indazole compounds that modulate and / or inhibit the activity of certain protein kinases are described. These compounds and pharmaceutical compositions containing them are capable of mediating tyrosine kinase signal transduction and thereby modulate and / or inhibit unwanted cell proliferation. The invention is also directed to the therapeutic or prophylactic use of pharmaceutical compositions containing such compounds, and to methods of treating cancer and other disease states associated with unwanted angiogenesis and / or cellular proliferation, such as diabetic retinopathy, neovascular glaucoma, rheumatoid arthritis, and psoriasis, by administering effective amounts of such compounds.
Owner:AGOURON PHARMA INC
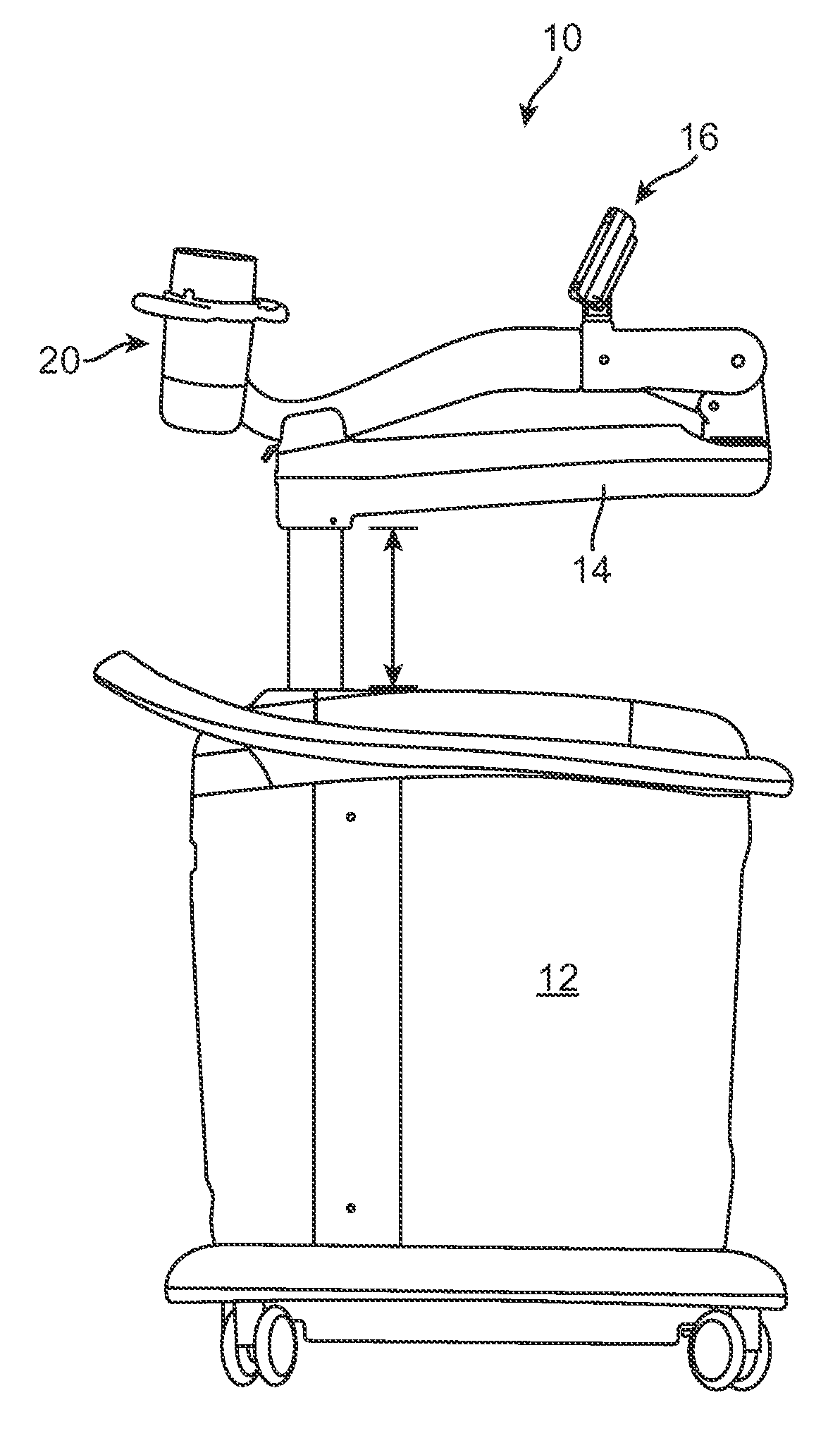
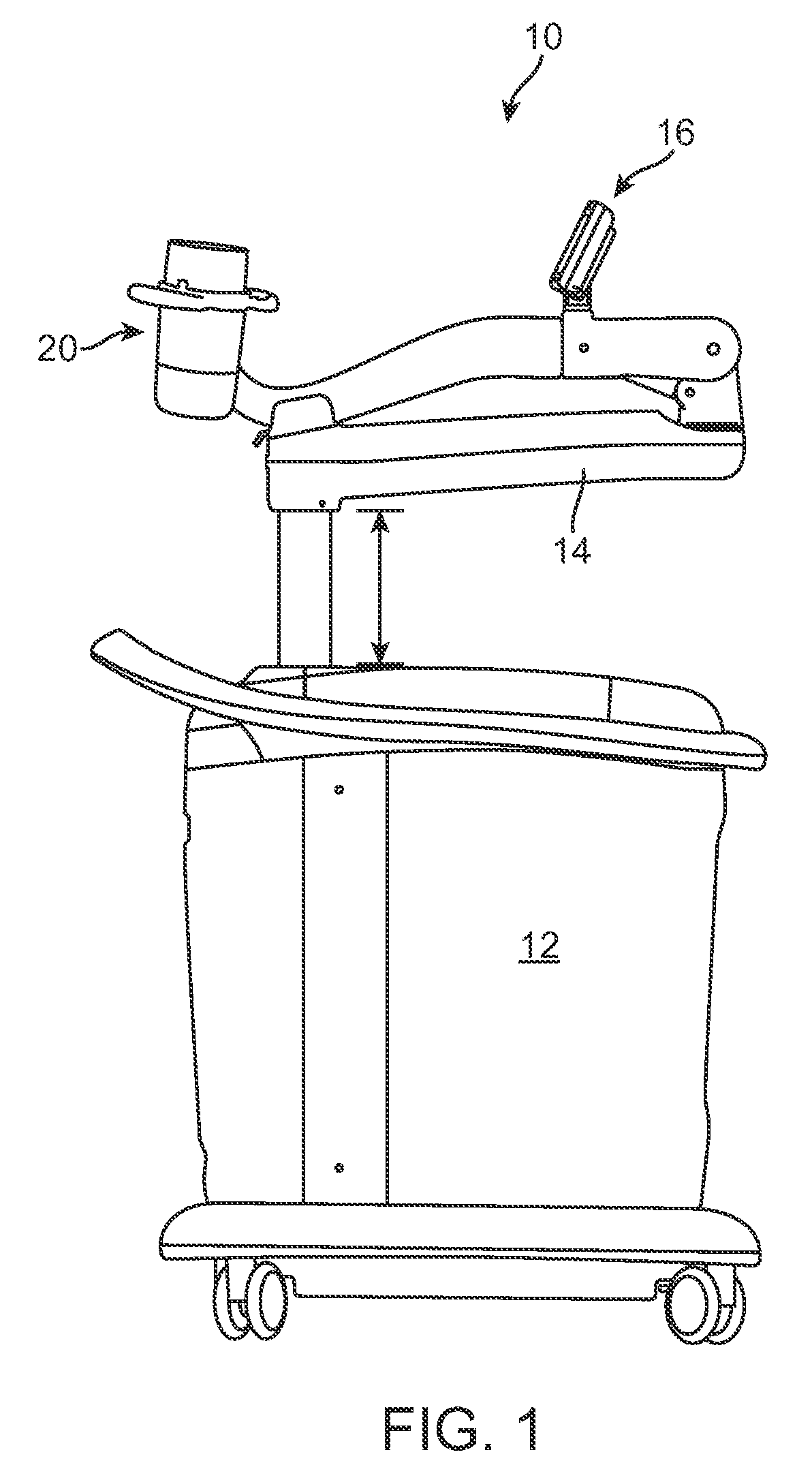
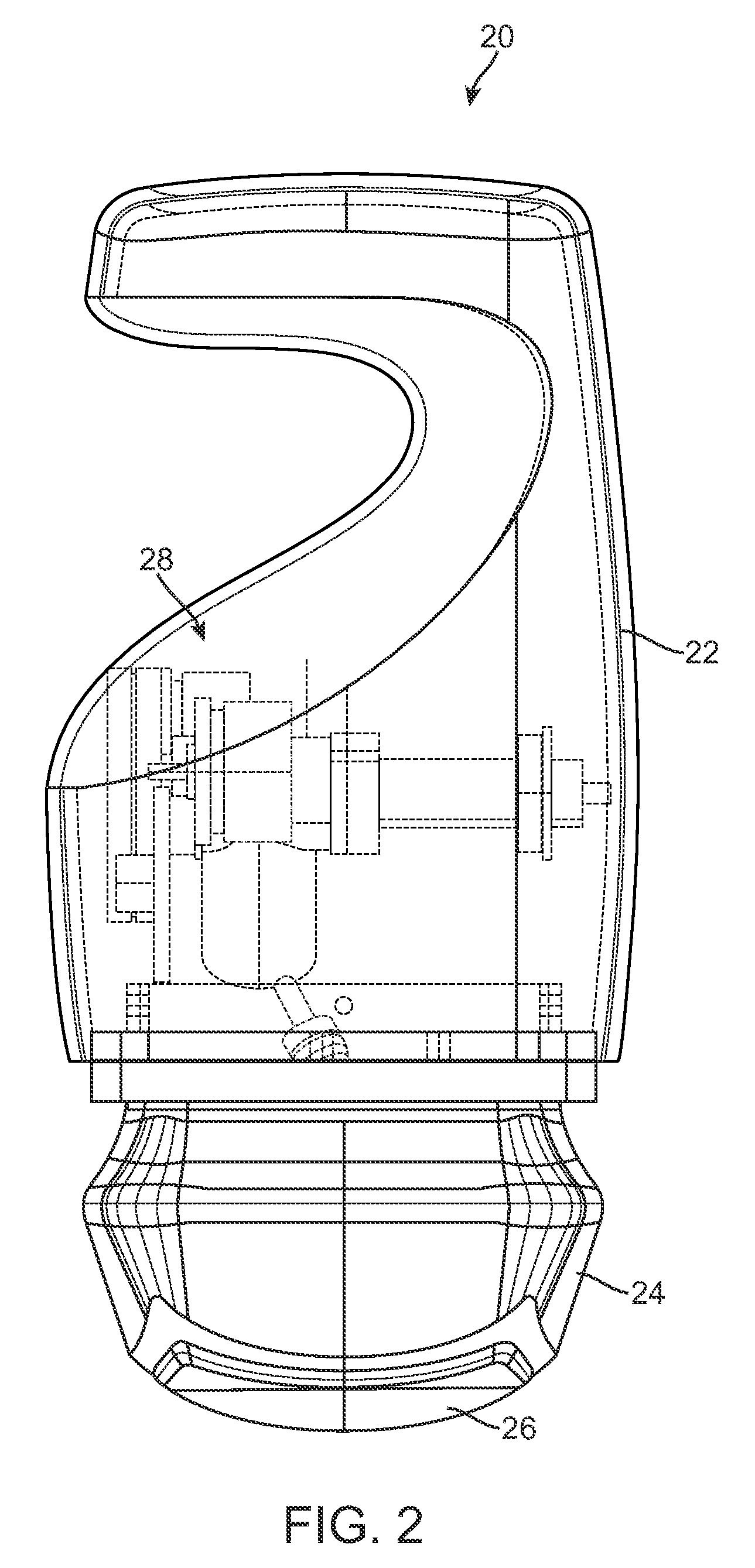
Therapy head for use with an ultrasound system
ActiveUS20090171252A1Improve accuracyGood treatment effectUltrasound therapyDiagnosticsEnergy applicatorControl arm
Therapy heads and related medical systems having an actuation assembly for controlling the position / orientation of a directional energy applicator in at least two planes are disclosed. A therapy head includes an enclosure, a partition separating a lower compartment from an upper compartment, an aperture in the partition, a control arm extending through the aperture, an actuation assembly positioned within the upper compartment, and a directional energy applicator positioned in the lower compartment for transmitting energy through a window. The control arm includes an upper end disposed within the upper compartment and a lower end disposed within the lower compartment. The actuation assembly is coupled with the upper end of the control arm such that the control arm is movable by the actuation assembly in at least two planes. The directional energy applicator is coupled with the lower end of the control arm.
Owner:SOLTA MEDICAL
Radiotherapy apparatus
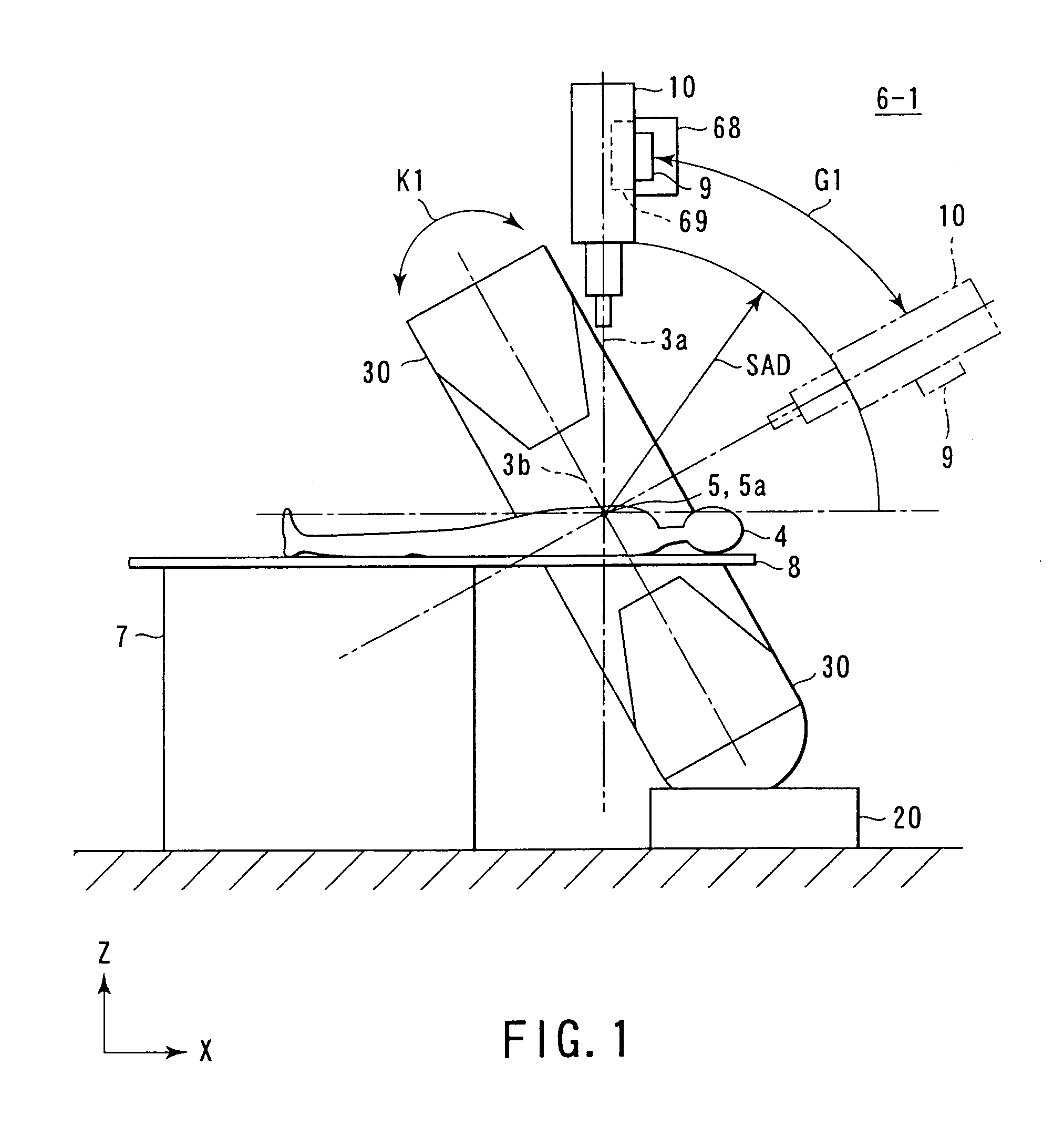
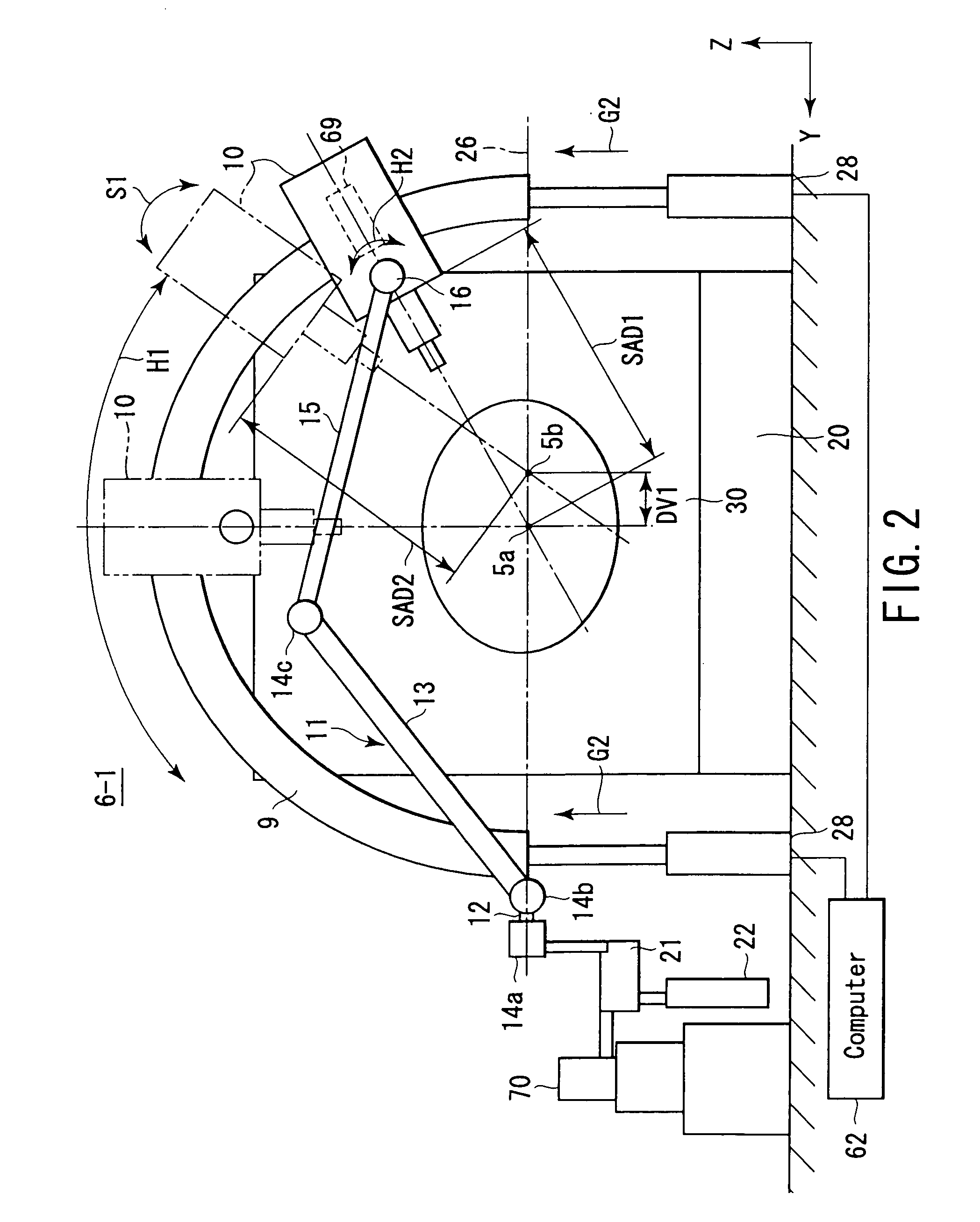
InactiveUS6977987B2High therapeutic performanceGood treatment effectDiagnosticsSurgeryEngineeringWaveguide
A radiotherapy apparatus comprising an irradiation head having a linear accelerator and an intra-head waveguide unit whose one end portion is electromagnetically connected to the linear accelerator, a supporting moving mechanism which supports and moves the irradiation head on predetermined first spherical coordinates, a microwave oscillator which generates microwaves to be supplied to the irradiation head, and which is placed in a stationary position, a fixed waveguide unit having one end portion electromagnetically connected to the microwave oscillator, and the other end portion positioned on the supporting moving mechanism, and a moving waveguide unit having one end portion electromagnetically connected to the other end portion of the fixed waveguide unit positioned on the supporting moving mechanism.
Owner:HITACHI LTD
Polypeptide variants with altered effector function
InactiveUS20070009523A1Improve bindingAltered affinityAntibody mimetics/scaffoldsImmunoglobulins against growth factorsAmino acidMolecular biology
The present invention concerns polypeptides comprising a variant Fc region. More particularly, the present invention concerns Fc region-containing polypeptides that have altered effector function as a consequence of one or more amino acid modifications in the Fc region thereof.
Owner:GENENTECH INC
Anti-IL-6 Receptor Antibody
InactiveUS20110245473A1Enhanced antigen-neutralizing activity and pharmacokineticsGood treatment effectCompound screeningApoptosis detectionHigh concentrationHinge region
The present inventors succeeded in discovering specific amino acid mutations in the variable region, framework region, and constant region of TOCILIZUMAB, and this enables to reduce immunogenicity risk and the heterogeneity originated from disulfide bonds in the hinge region, as well as to improve antigen binding activity, pharmacokinetics, stability under acidic conditions, and stability in high concentration preparations.
Owner:CHUGAI PHARMA CO LTD
Non-invasive neuro stimulation system
A device (10, 50, 60, 70, 80, 90) is used to apply an electric pulse or spike to a patient to treat the patient. The device can have a series of preset treatments programmed therein. A user can select a treatment from menus displayed on a display (100). The impedance of the skin and underlying tissue to be treated can be measured prior to the treatment to locate active areas on the skin for treatment. The impedance measurement can be made at a sufficiently low level to avoid treatment of the patient that could cause a change in the impedance. A phase detector can be used to isolate the capacitance value in the impedance. The charge delivered to the patient can be measured and the device can adjust the charge as the skin impedance varies during treatment to deliver uniform charges to the skin. A variety of probes can be used with the device, with the device automatically detecting the type of probe attached. Multiple electrodes can be used on the probe, which allows the active areas in contact with the probe to be identified prior to treatment to allow the treatment to concentrate on the active areas.
Owner:HTK ENTERPRISES INC
Method and apparatus for the treatment of physical and mental disorders with low frequency, low flux density magnetic fields
ActiveUS20050182287A1Limited extentTreatment safetyElectrotherapyMagnetotherapy using coils/electromagnetsMicrocontrollerPulsed DC
A method and apparatus for generating electromagnetic fields for healing. A device preferably includes a microcontroller and associated memory, a wire coil in electrical communication with a driving circuit that is controlled by the microcontroller in accordance with a program stored in the associated memory, wherein the driving circuit is effective to produce a pulsed DC output having a frequency in the range of about 0-45 Hz, more preferably in the range of 0.5-14.1 Hz and most preferably around 9.6 Hz. A user interface is provided for selecting one of a plurality of modes of operation and a port (e.g., a USB port) is provided to allow the program stored in the associated memory to be modified by way of a computer, memory card or the Internet. In another embodiment, the apparatus takes the form of a medallion that can be worn around a user's neck or strategically placed on a user's body or embedded in other user hardware such as a combat or racing helmet.
Owner:BECKER PAUL F
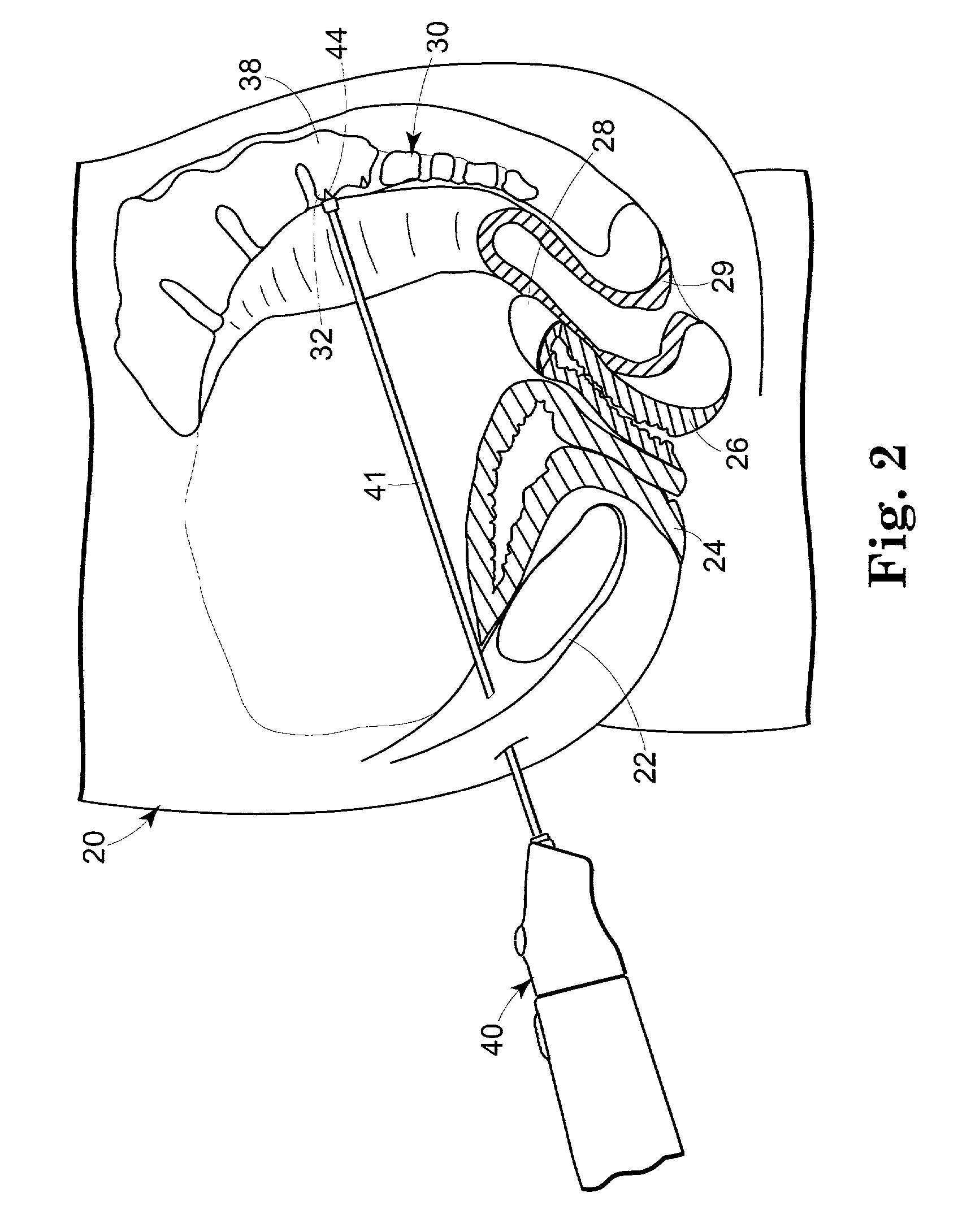
Compositions and methods for augmentation or repair of intervertebral discs
InactiveUS20050118228A1Enhance the imageGood treatment effectBiocideBone implantStem Cell IsolationIntervertebral disc
A method of augmenting and / or repairing an intervertebral disc by administering stem cell material into the disc. The stem cells may be undifferentiated cells, or they may be cells that have differentiated and have subsequently been dedifferentiated. The stem cells may be induced to express at least one characteristic of human intervertebral disc cells, such as fibroblast cells, chondrocyte cells, or notochordal cells, by exposing them to agents and / or environments calculated to induce the desired differentiation. In some embodiments, the stem cell material may be provided in conjunction with a collagen-based material, which may be a collagen-rich lattice or particles of collagen material. The stem cell material may be provided as a stem cell isolate, which may be substantially free of non-stem cell material. Other therapeutic agents may be administered with the stem cell material.
Owner:SDGI HLDG
Phenylepherine containing dosage form
ActiveUS20060057205A1Good treatment effectRelieve symptomsBiocidePill deliveryNorphenylephrineBlood plasma
A pharmaceutical dosage form which comprises phenylepherine or a pharmaceutically acceptable salt thereof and a second drug. The dosage form provides a plasma concentration within the therapeutic range of the second drug over a period which is coextensive with at least about 70% of the period over which the dosage form provides a plasma concentration within the therapeutic range of phenylepherine. This abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:CAPELLON PHARMA LLC
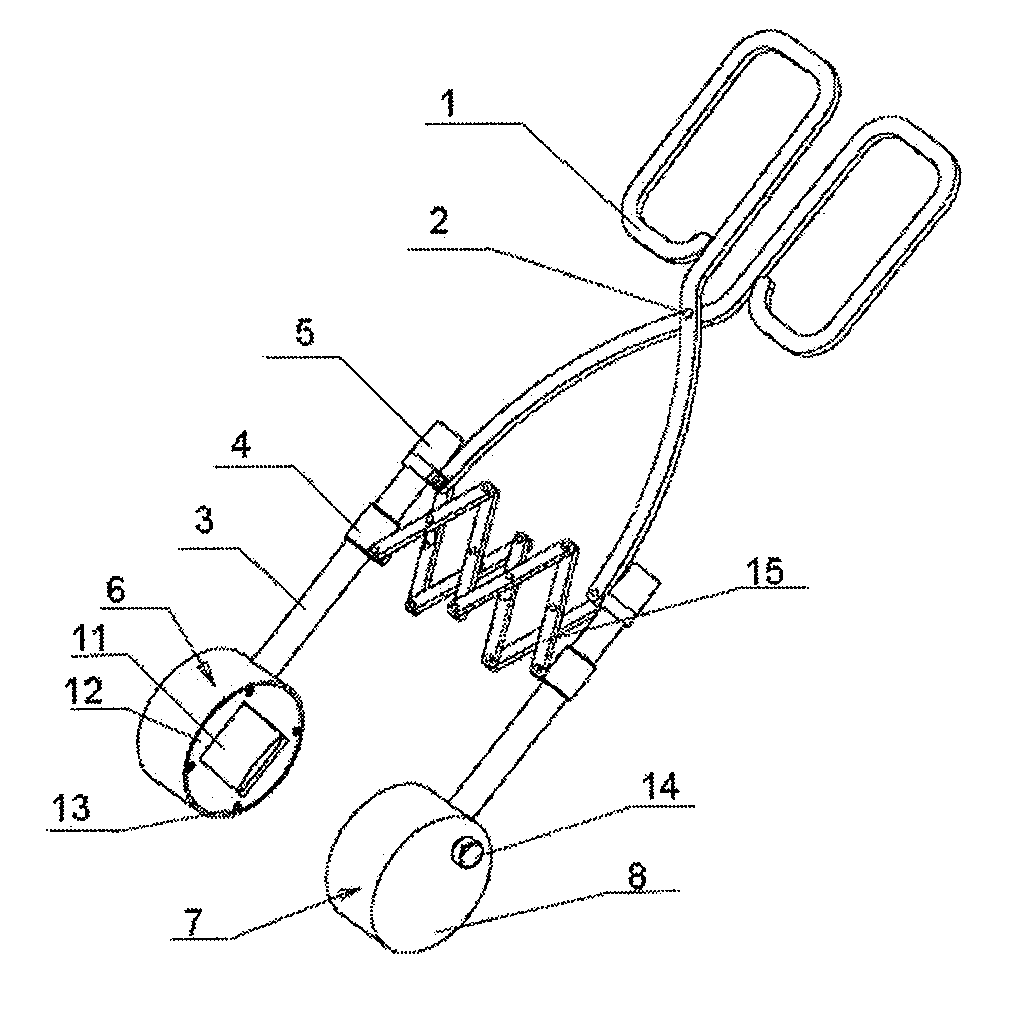
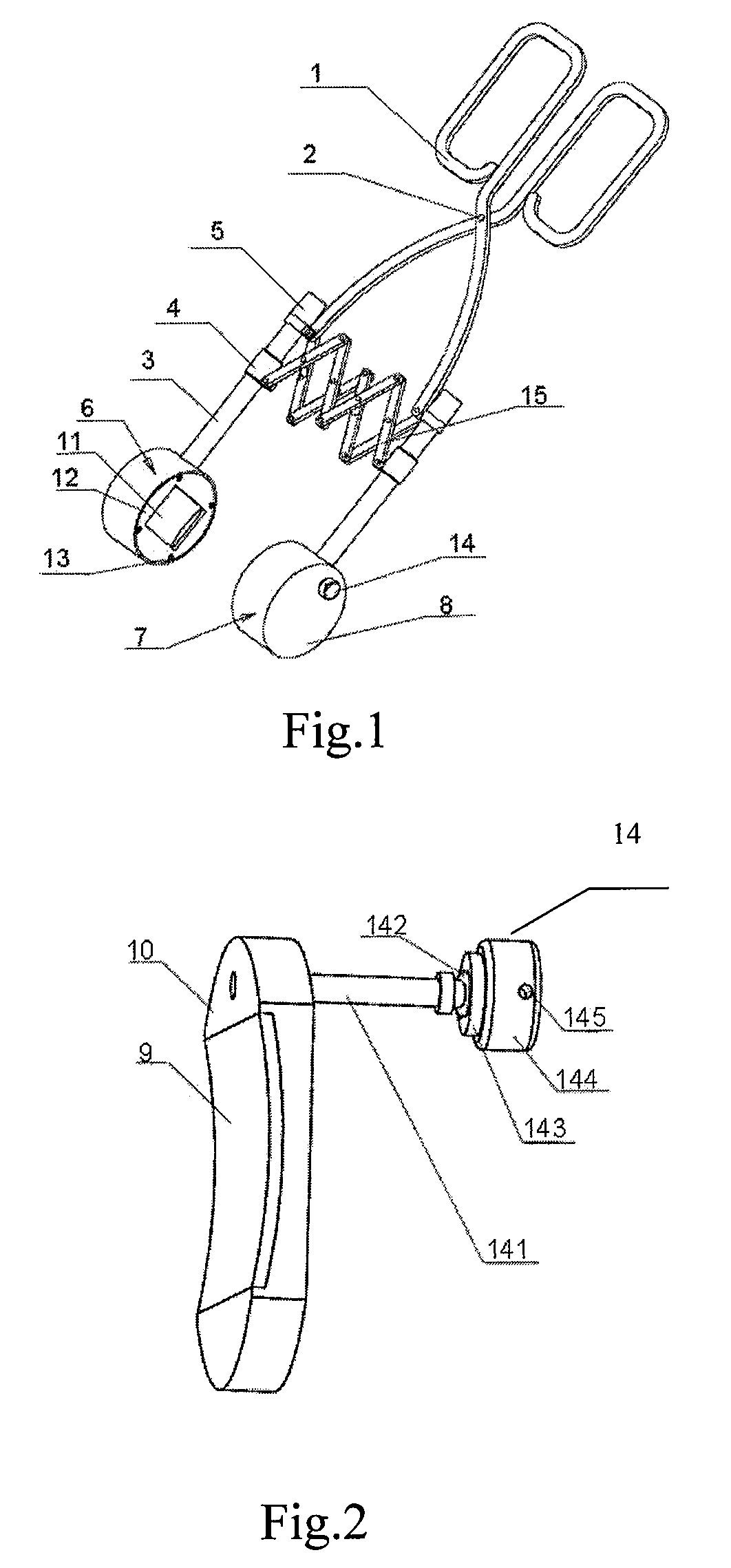
Ultrasound treatment clamp
ActiveUS8282581B2Easy to operateQuickly causingUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyDiseaseMedicine
The present invention relates to an ultrasound treatment clamp. The ultrasound treatment clamp comprises ultrasound therapy applicators and handles connected to the ultrasound therapy applicators. The handles are clamp-shaped. The two ultrasound therapy applicators with their central axes overlapping each other are mounted face to face on the two clamps of the clamp-shaped handles respectively. A parallel moving mechanism for keeping the two ultrasound therapy applicators in parallel when moving along with clamps is connected between the two clamps. The present invention has a compact structure, a convenient operation, a low treatment cost, a capability of quickly causing a coagulative necrosis of the diseased part. Furthermore, the present invention has an abroad use in treating many kinds of diseases.
Owner:CHONGQING HAIFU (HIFU) TECHNOLOGY CO LTD
Directed delivery of agents to neural anatomy
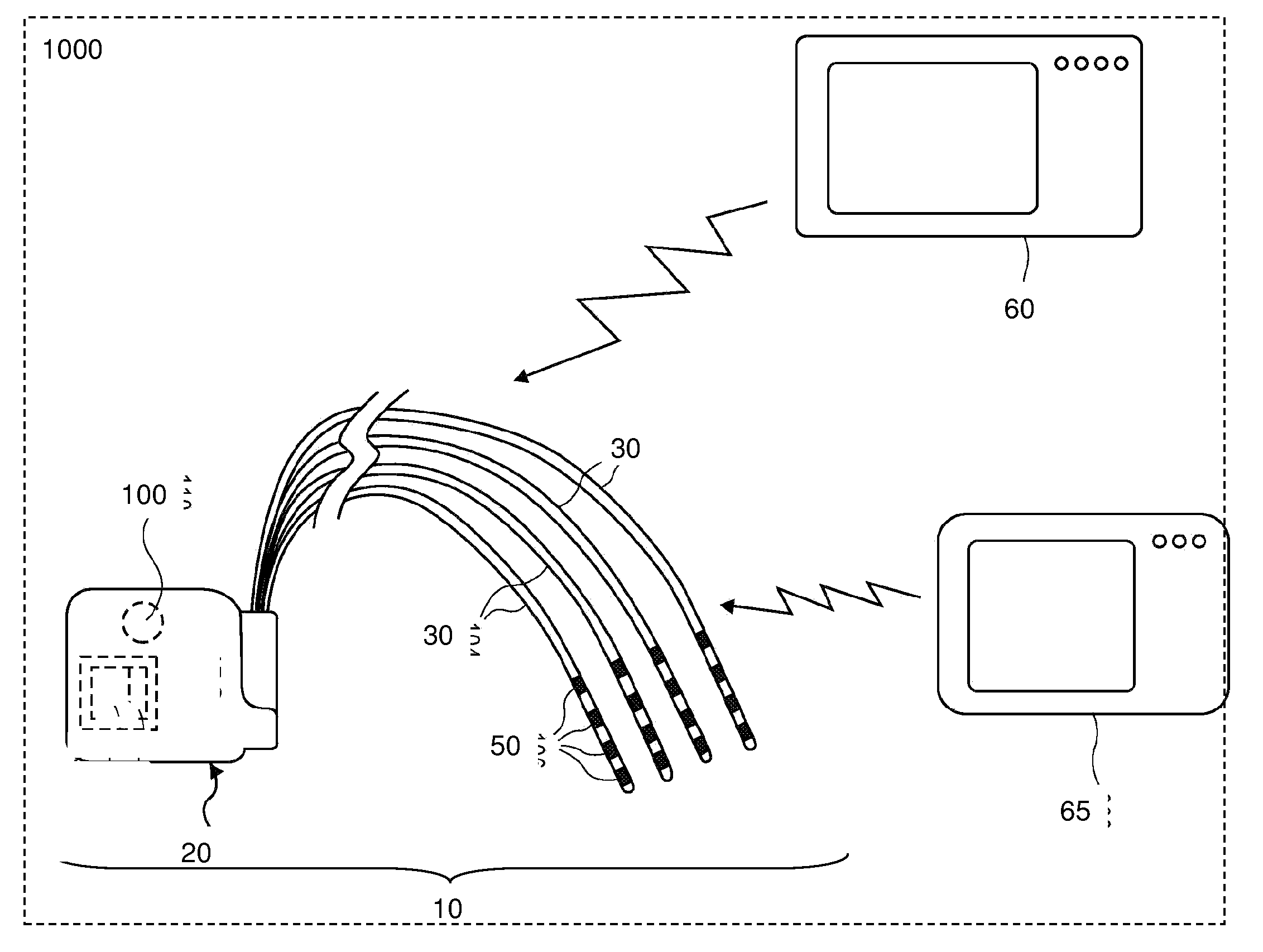
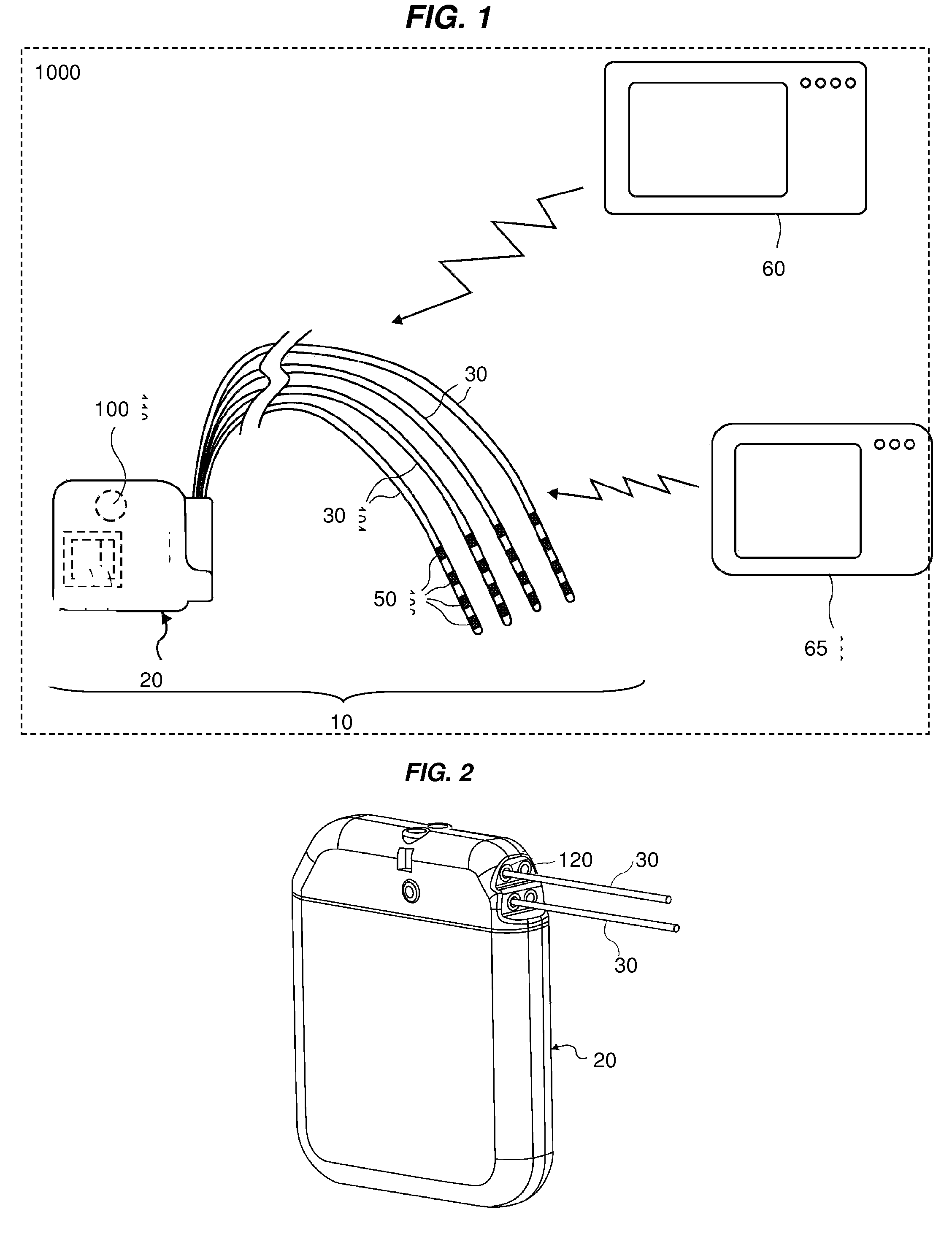
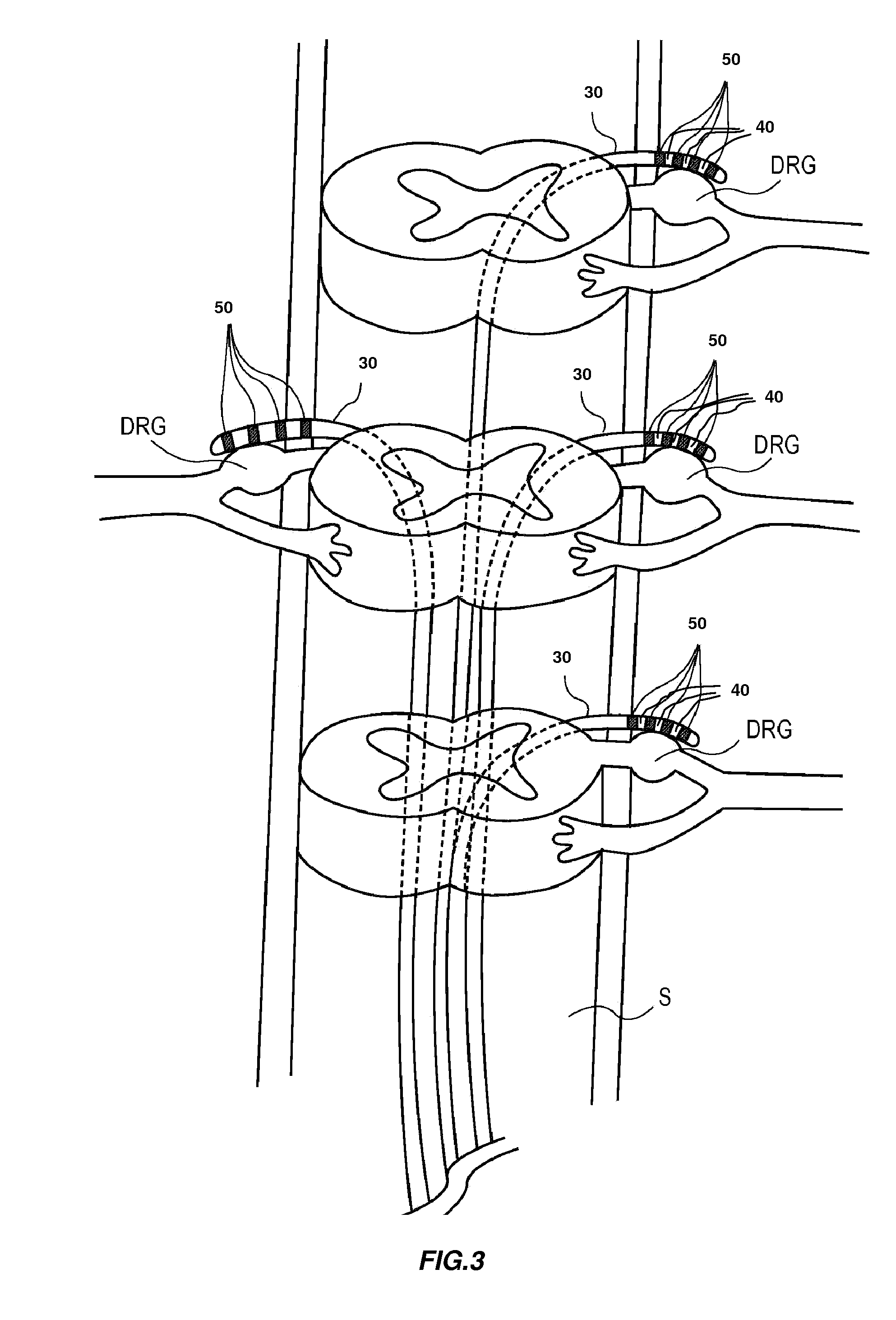
InactiveUS20120310140A1Effective pain managementAdequate pain reliefSpinal electrodesPharmaceutical delivery mechanismDiseaseAutomatic control
The present invention is directed generally to systems, devices and methods for direct delivery of agents, e.g., pharmaceutical agents, to target spinal and neuronal anatomies, e.g., the dorsal root ganglia (DRG), for the treatment of various disorders, particularly pain and pain related disorders, such as chronic itch, sensory disorders, multiple sclerosis, post-herpetic neuralgia and the like. The system, devices and methods of the invention encompass the agents to be delivered to the target anatomy alone or in combination with electrical stimulation. The delivery device and systems and methods as disclosed herein place the distal end of the delivery element, which comprises at least one agent delivery structure, and optionally at least one electrode, in close proximity, or in contact with or next to the target spinal anatomy, e.g., DRG. A variety of agents can be delivered using the device, including sodium channel blockers, biologics, neuroinflammatory modulators, toxins etc., to selectively neuromodulate the neurons. Agent delivery and / or electrical stimulation can be automated and / or can be controlled automatically or by a pre-determined program, or by a patient control pump (PCA).
Owner:ST JUDE MEDICAL LUXEMBOURG HLDG SMI S A R L SJM LUX SMI
Therapeutic Cosmetic Dispensing Device
InactiveUS20080014011A1Small sizeAccurate distanceElectrotherapyWriting implementsElectricityPharmaceutical formulation
A cosmetic dispensing device with a reservoir to hold a supply of a cosmetic or pharmaceutical preparation. The preparation can be extruded from the reservoir to the surface of the device. The device further has a stimulator built-in. This stimulator supplies light, vibration, or electricity or any combination thereof to the user as the preparation is applied to the skin.
Owner:ROSSEN JOEL STEPHEN
Oral pharmaceutical pulsed release dosage form
An enteric coated pharmaceutical dosage form comprising an H+,K+-ATPase inhibitor is disclosed. The dosage form comprises at least two portions of the H+,K+- ATPase inhibitor to be released in at least two consecutive pulses. The dosage form has at least one fraction with a pulsed delayed release and another fraction with instant release of the H+,K+-ATPase inhibitor. The portions are released in time by from 0.5 and up to 12 hours interval, preferably by from 0.5 and up to 8 hours, and more preferably by from 0.5 and up to 4 hours interval. The dosage form is intended for once daily administration.
Owner:ASTRAZENECA AB
Methods and devices for the treatment of ocular diseases in human subjects
InactiveUS20150258120A1Reduce in quantityReduce severityOrganic active ingredientsPowder deliveryDiseaseMicroparticle
Methods and devices are provided for targeted non-surgical administration of a drug formulation to the suprachoroidal space (SCS) of the eye of a human subject for the treatment of a posterior ocular disorder or a choroidal malady. In one embodiment, the method comprises inserting a hollow microneedle into the eye at an insertion site and infusing a drug formulation through the inserted microneedle and into the suprachoroidal space of the eye, wherein the infused drug formulation flows within the suprachoroidal space away from the insertion site during the infusion. In one embodiment, the fluid drug formulation comprises drug nanoparticles or microparticles.
Owner:CLEARSIDE BIOMEDICAL
Humanized Fc.gamma.RIIB-Specific Antibodies and Methods of Use Thereof
InactiveUS20080044417A1Good curative effectEnhanced effector functionDisease diagnosisTissue cultureFc(alpha) receptorFc receptor
The present invention relates to humanized FcγRIIB antibodies, fragments, and variants thereof that bind human FcγRIIB with a greater affinity than said antibody binds FcγRIIA. The invention encompasses the use of the humanized antibodies of the invention for the treatment of any disease related to loss of balance of Fc receptor mediated signaling, such as cancer, autoimmune and inflammatory disease. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the humanized antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing the efficacy of a vaccine composition by administering the humanized antibodies of the invention. The invention encompasses methods for treating an autoimmune disease and methods for elimination of cancer cells that express FcγRIIB.
Owner:MACROGENICS INC
Delayed release pharmaceutical composition containing 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol
InactiveUS20050058706A1Good repeatabilityGood treatment effectOrganic active ingredientsBiocideHydrophobic polymerBULK ACTIVE INGREDIENT
A pharmaceutical formulation for delayed release of the active ingredient 3-(3-dimethylamino-1-ethyl-2-methylpropyl)phenol or a pharmaceutically acceptable salt thereof in a matrix containing between 1 and 80 wt. % of at least one pharmaceutically acceptable hydrophilic or hydrophobic polymer as a matrix forming agent and exhibiting in vivo the following release rate: 3 to 35% by weight (based on 100% by weight active ingredient) 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 0.5 hours; 5 to 50% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 1 hour; 10 to 75% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 2 hours; 15 to 82% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 3 hours; 30 to 97% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 6 hours; more than 50% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 12 hours; more than 70% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 18 hours, and more than 80% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 24 hours.
Owner:GRUNENTHAL GMBH
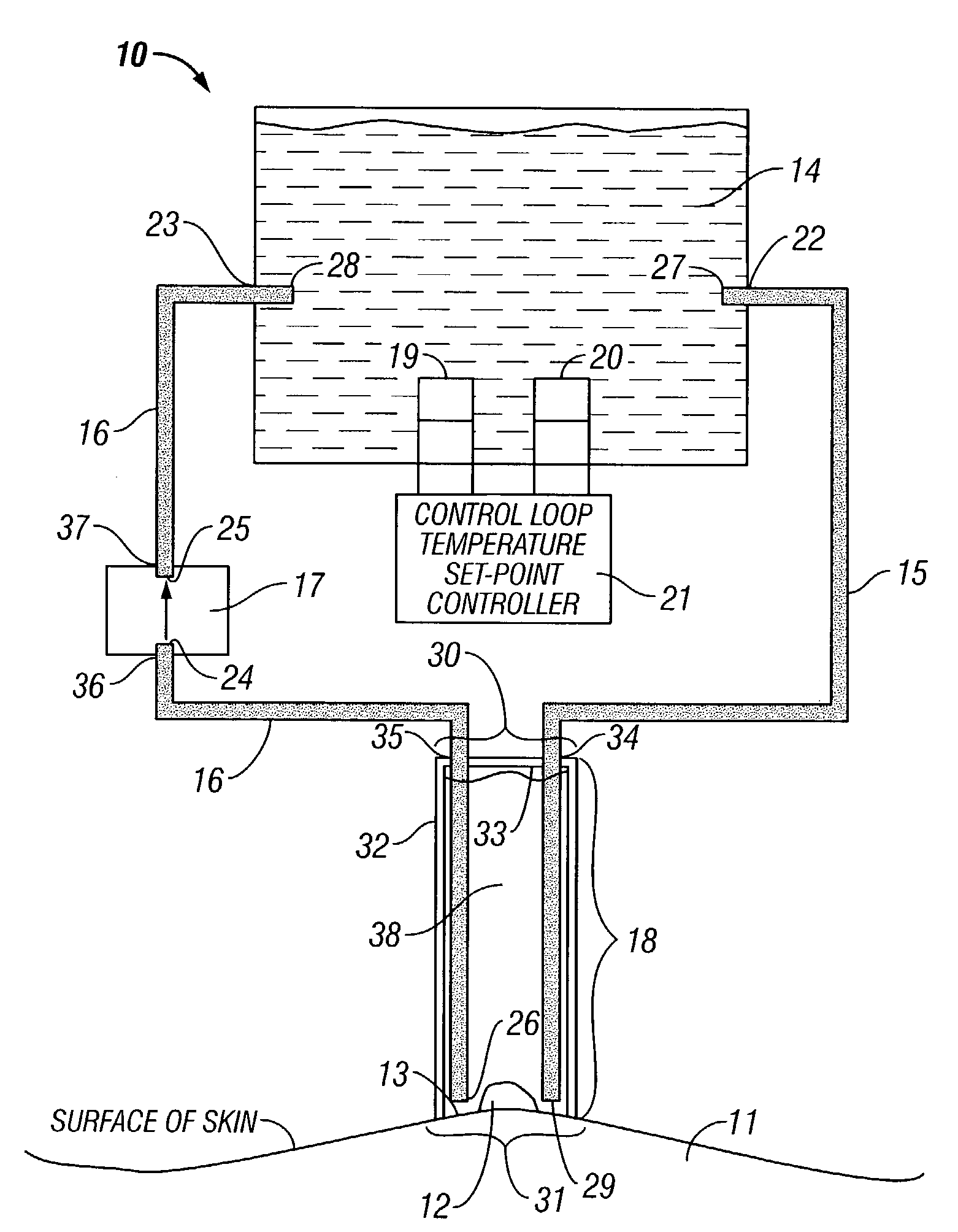
Localized liquid therapy and thermotherapy device
InactiveUS7422576B2Fast circulationGood treatment effectDiagnosticsSurgeryWater basedTherapeutic effect
A device for directly applying thermotherapeutic liquid to an area upon the surface of an afflicted patient, and methods of use thereof, are described. In particular a device for applying water-based liquid at a therapeutic temperature directly to an afflicted area in order to create a localized hyperthermia, is presented. The afflicted area may be either on the skin of the patient, or subcutaneous. The device is also effective for disinfection, irrigation, lavage, and the like, when employing a suitable solution. The liquid may also have a mild oxidizing effect, which, if greater upon afflicted than upon non-afflicted cells, would enhance the therapeutic effect in conjunction with the therapy herein described.
Owner:KCI LICENSING INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com