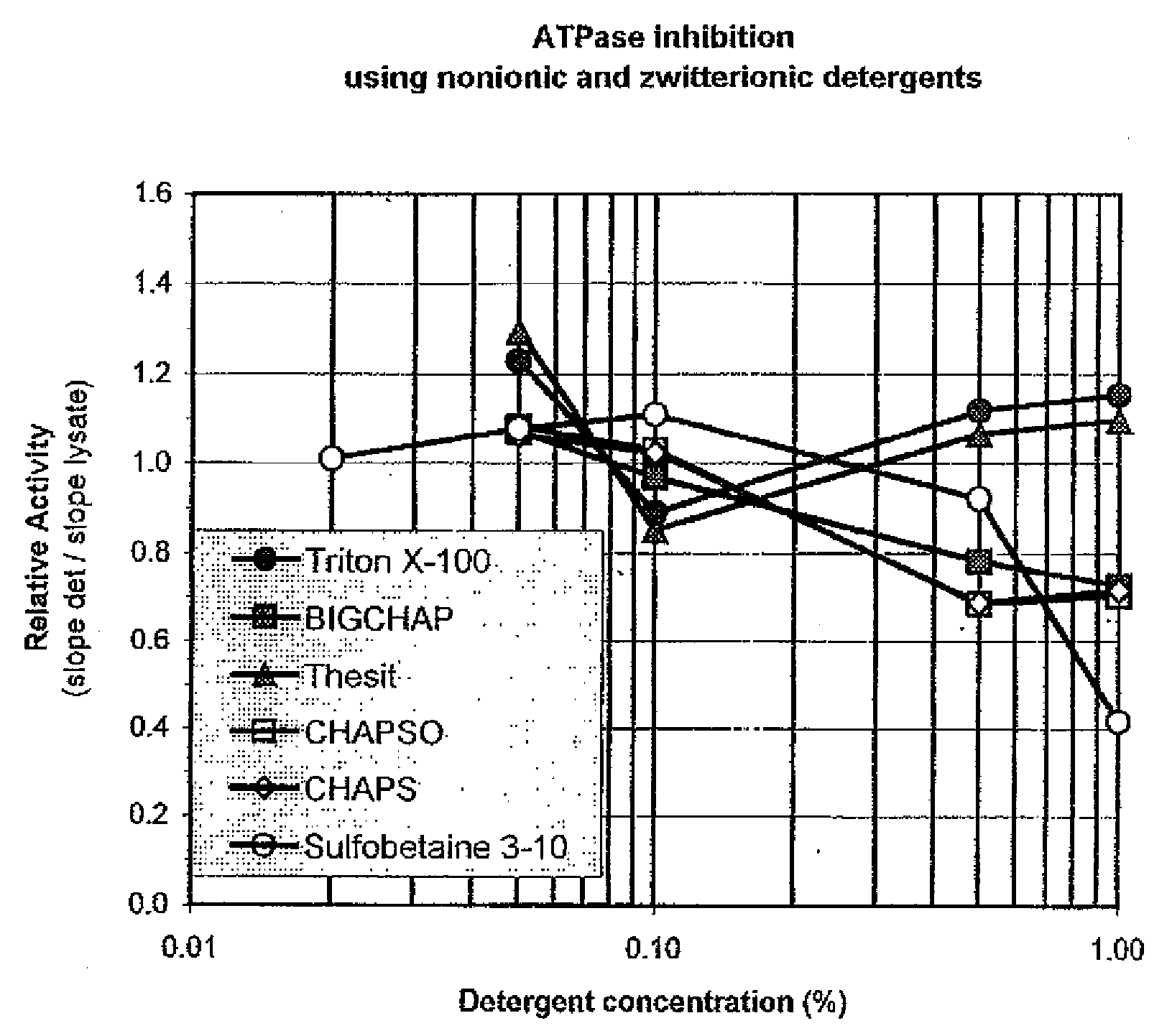
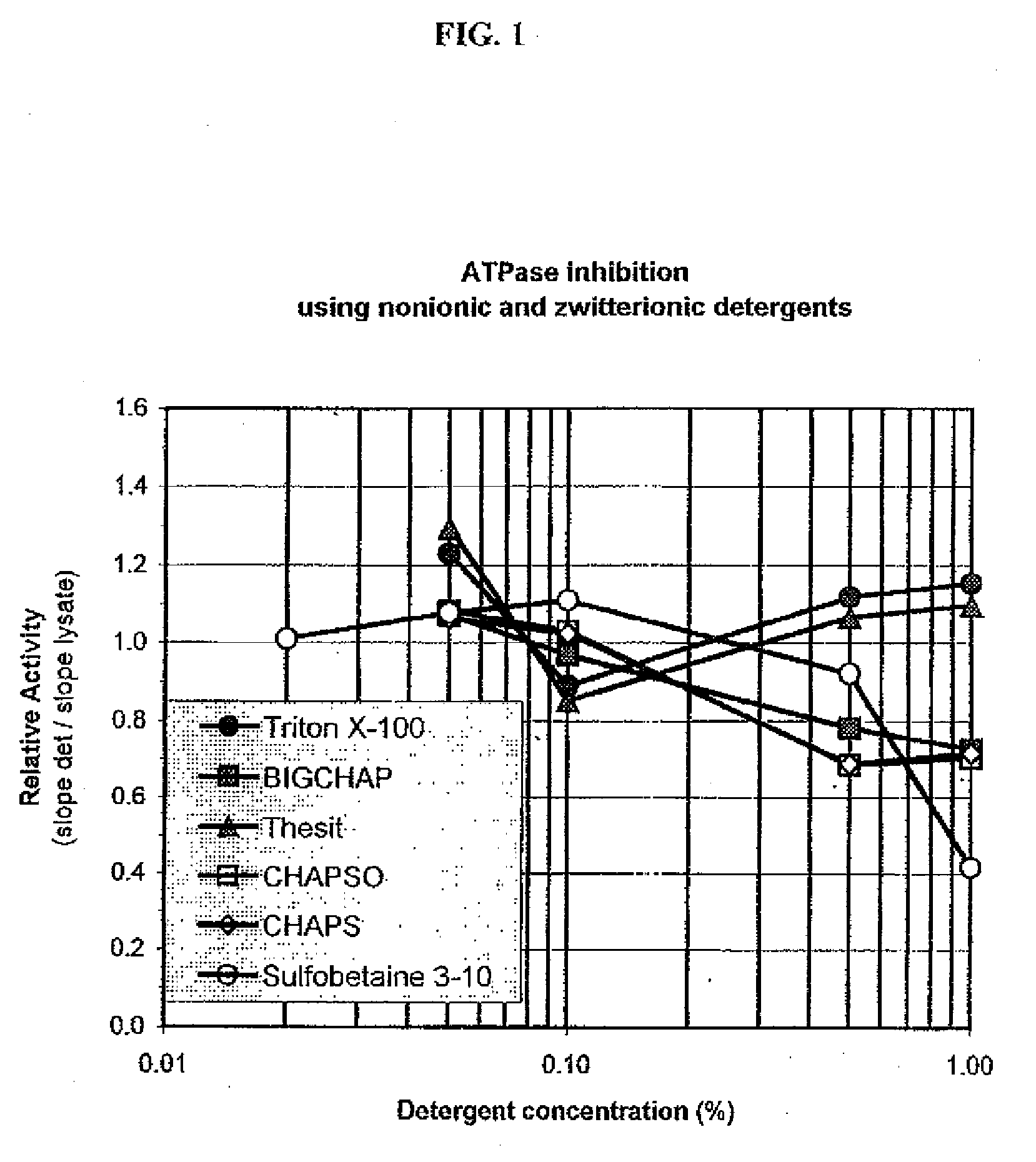
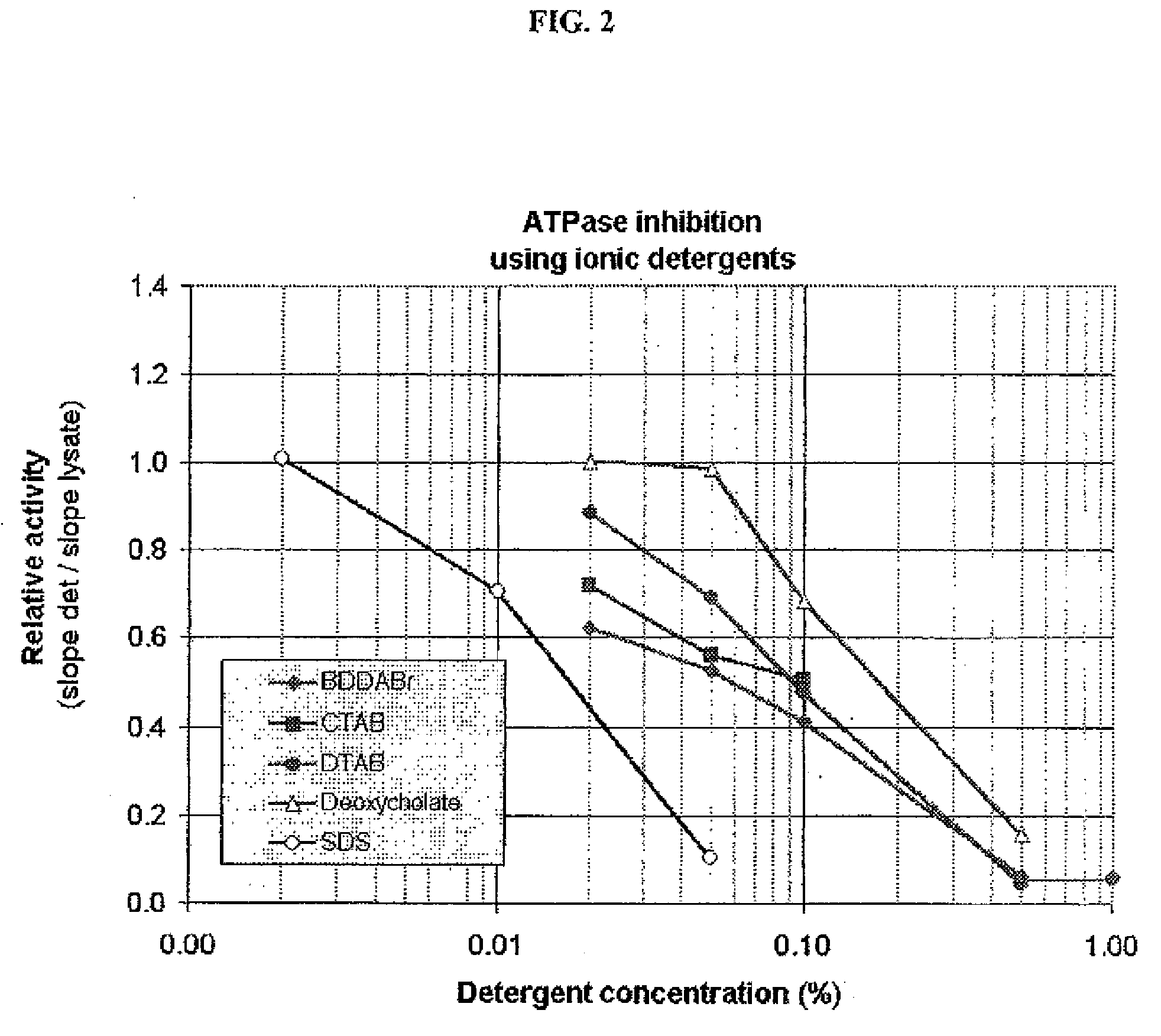
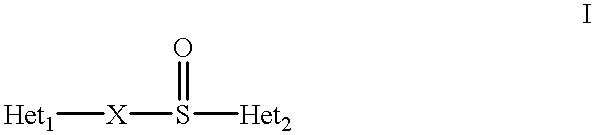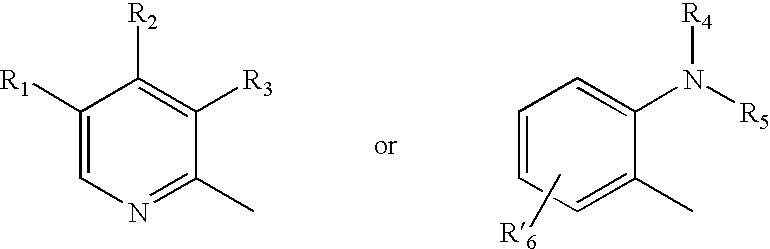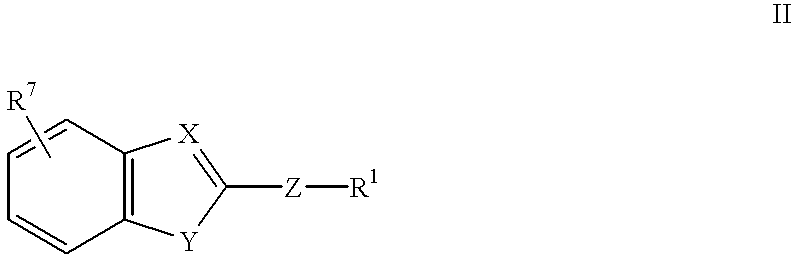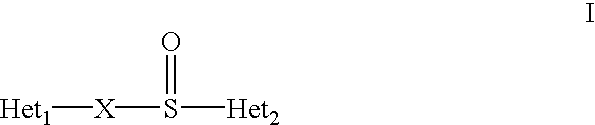Patents
Literature
284 results about "ATPase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
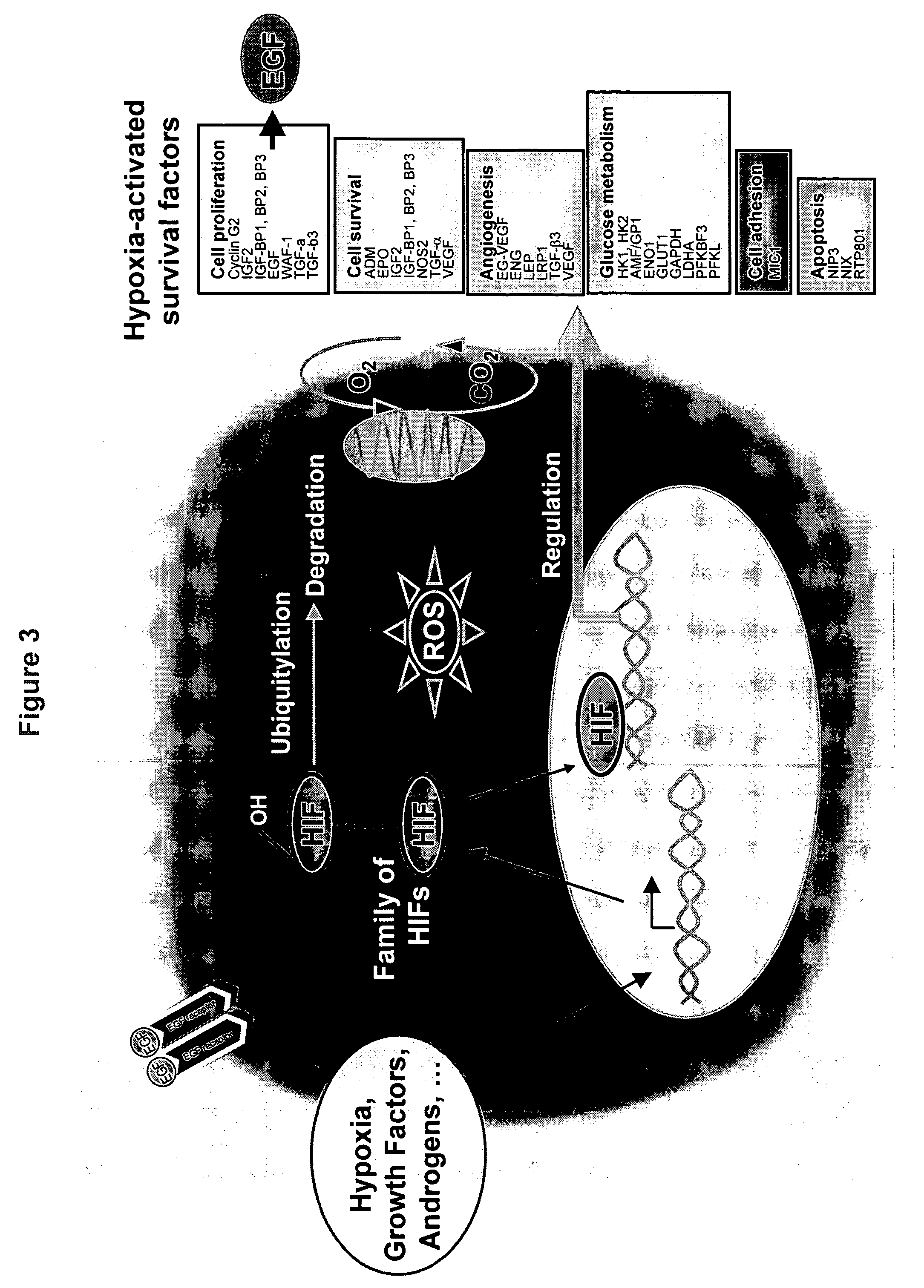
ATPases (EC 3.6.1.3, adenylpyrophosphatase, ATP monophosphatase, triphosphatase, SV40 T-antigen, adenosine 5'-triphosphatase, ATP hydrolase, complex V (mitochondrial electron transport), (Ca²⁺ + Mg²⁺)-ATPase, HCO₃⁻-ATPase, adenosine triphosphatase) are a class of enzymes that catalyze the decomposition of ATP into ADP and a free phosphate ion or the inverse reaction. This dephosphorylation reaction releases energy, which the enzyme (in most cases) harnesses to drive other chemical reactions that would not otherwise occur. This process is widely used in all known forms of life.
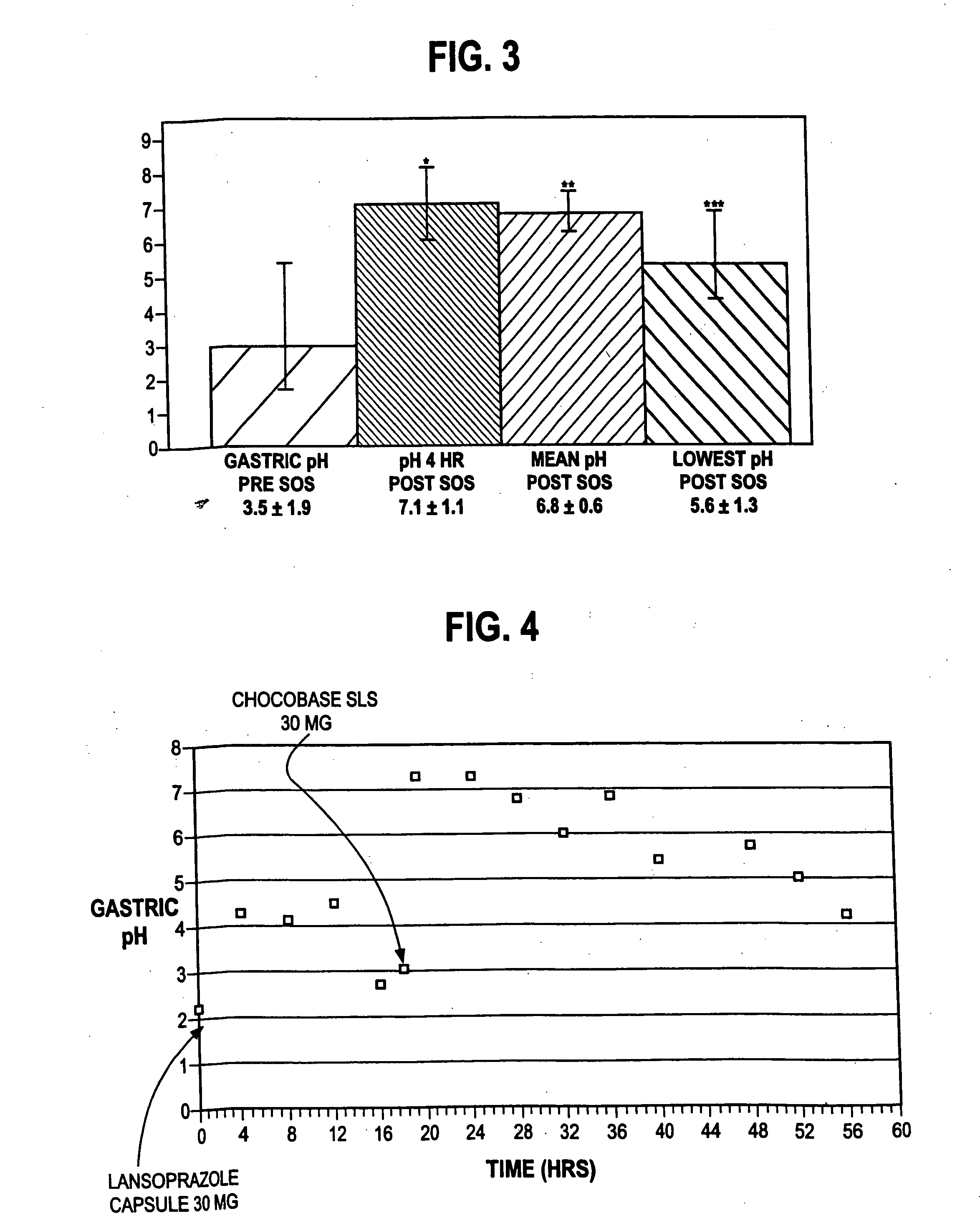
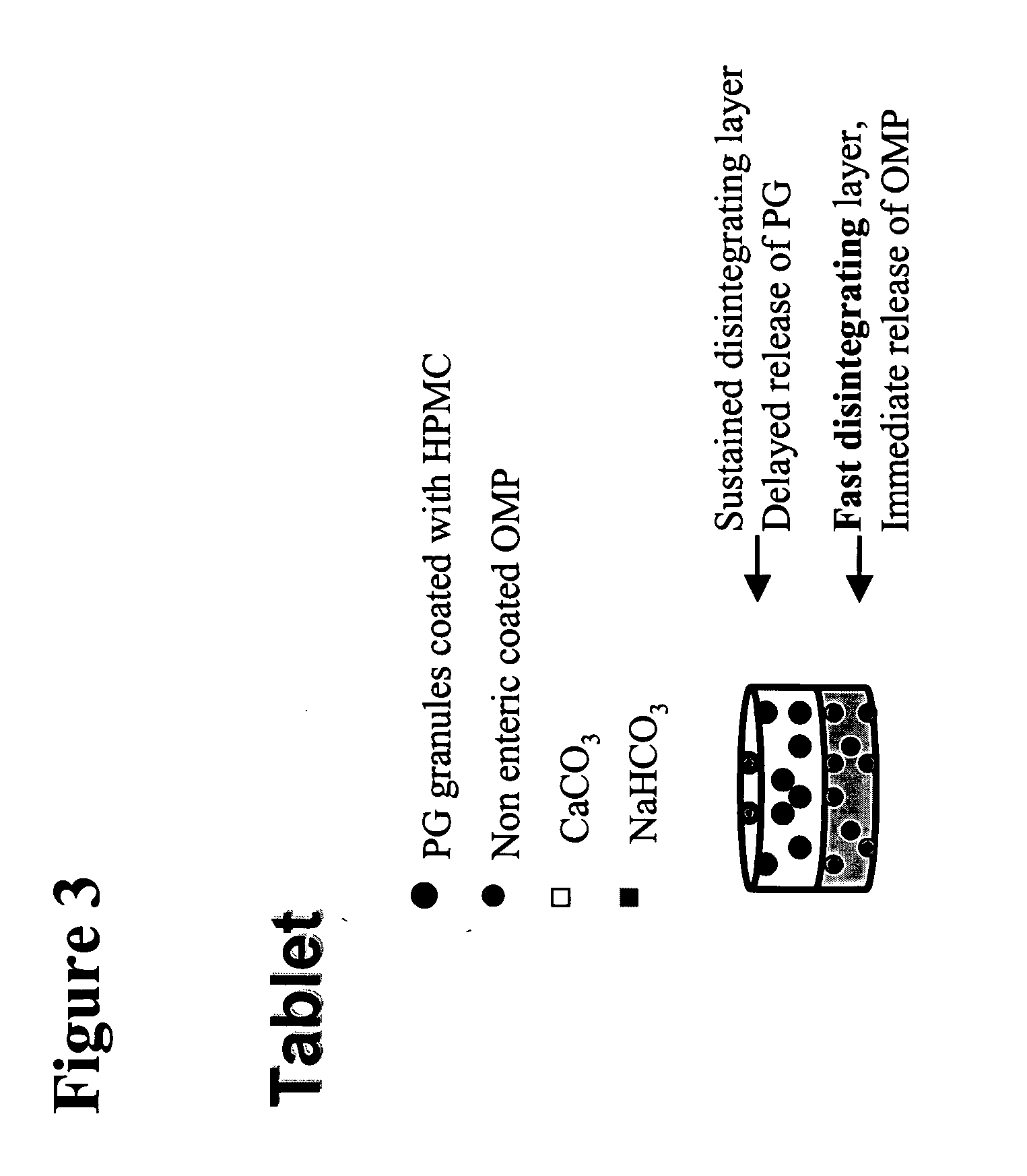
Oral pharmaceutical pulsed release dosage form
An enteric coated pharmaceutical dosage form comprising an H+,K+-ATPase inhibitor is disclosed. The dosage form comprises at least two portions of the H+,K+- ATPase inhibitor to be released in at least two consecutive pulses. The dosage form has at least one fraction with a pulsed delayed release and another fraction with instant release of the H+,K+-ATPase inhibitor. The portions are released in time by from 0.5 and up to 12 hours interval, preferably by from 0.5 and up to 8 hours, and more preferably by from 0.5 and up to 4 hours interval. The dosage form is intended for once daily administration.
Owner:ASTRAZENECA AB
RNAi-mediated inhibition of ocular targets
InactiveUS20060172965A1Lower eye pressureOrganic active ingredientsSenses disorderATPaseOpen angle glaucoma
RNA interference is provided for inhibition of ocular hypertension target mRNA expression for lowering elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Ocular hypertension targets include carbonic anhydrase II, IV, and XII; β1- and β2 adrenergic receptors; acetylcholinesterase; Na+ / K+-ATPase; and Na—K-2Cl cotransporter. Ocular hypertension is treated by administering interfering RNAs of the present invention.
Owner:NOVARTIS AG
Use of Na*/K*-ATPase inhibitors and antagonists thereof
InactiveUS20060135443A1Decreased heart rateGood blood pressureBiocideOrganic active ingredientsNon cancerATPase
The reagent, pharmaceutical formulation, kit, and methods of the invention provides a new approach for treating hypoxia-related pathological conditions, such as Alzheimer's Disease, and those involving excessive angiogenesis, especially those non-cancer pathological conditions. The invention provides the use of Na+ / K+-ATPase inhibitors, such as cardiac glycosides (e.g. ouabain and proscillaridin, etc.), either alone or in combination with other standard therapeutic agents for treating such conditions. The invention also relates to the use of cardiac glycoside inhibitors / antagonists as reagents, pharmaceutical formulations, or in kits and methods for treating conditions arising from excessive amount of cardiac glycosides, including all symptoms of digitalis poisoning, depression, hypertension, etc. The pharmaceutical formulation of the invention may be delivered to a patient either systemically or locally, or both. The pharmaceutical formulations of the invention may be delivered either in one dose, or continuously over a sustained period of time using, for example, sustained drug delivery devices.
Owner:BIONAUT PHARMA
RNAi-mediated inhibition of ocular hypertension targets
InactiveUS20060172963A1Lower eye pressureOrganic active ingredientsSenses disorderIntra ocular pressureATPase
RNA interference is provided for inhibition of ocular hypertension target mRNA expression for lowering elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Ocular hypertension targets include carbonic anhydrase II, IV, and XII; β1- and β2 adrenergic receptors; acetylcholinesterase; Na+ / K+-ATPase; and Na—K-2Cl cotransporter. Ocular hypertension is treated by administering interfering RNAs of the present invention.
Owner:ARROWHEAD RES CORP +1
Oral pharmaceutical extended release dosage form
An enteric coated pharmaceutical extended release dosage form of a H+, K+-ATPase inhibitor giving an extended plasma concentration profile of a H+, K+-ATPase inhibitor. The extended plasma profile is obtained by a pharmaceutical composition which comprises a core material of a hydrophilic or hydrophobic matrix, and the H+, K+-ATPase inhibitor and optionally pharmaceutically acceptable excipients. The dosage form may be administered once daily.
Owner:ASTRAZENECA AB
Combinatorial chemotherapy treatment using Na+/K+ ATPase inhibitors
InactiveUS20060135441A1Reduced responseGood curative effectBiocideOrganic active ingredientsATPaseChemotherapeutic drugs
The reagent, pharmaceutical formulation, kit, and methods of the invention provides a new approach to alleviate or eliminate certain negative effects associated with the use of certain cancer treatment agents (e.g. chemotherapy therapeutics, etc.) or regimens (e.g. radio therapies, etc.), including stimulation of the hypoxic stress response in tumor cells.
Owner:BIONAUT PHARMA
Method for detection of ATP
InactiveUS7083911B2Improve stabilityFacilitate many ATP detectionMicrobiological testing/measurementOxidoreductasesATPaseViable cell
Owner:PROMEGA
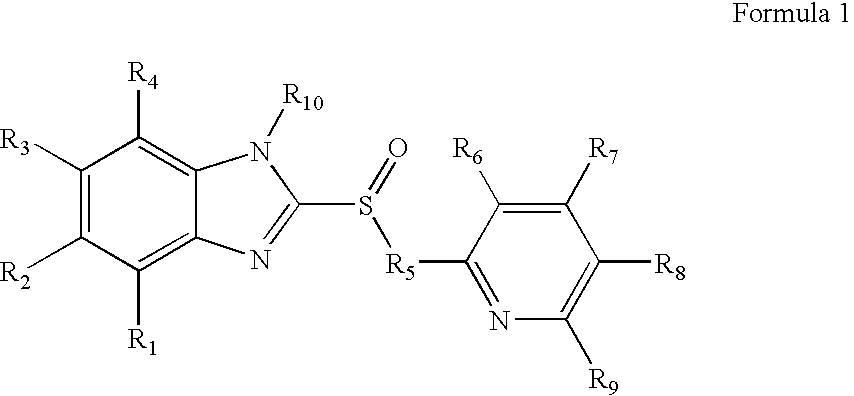
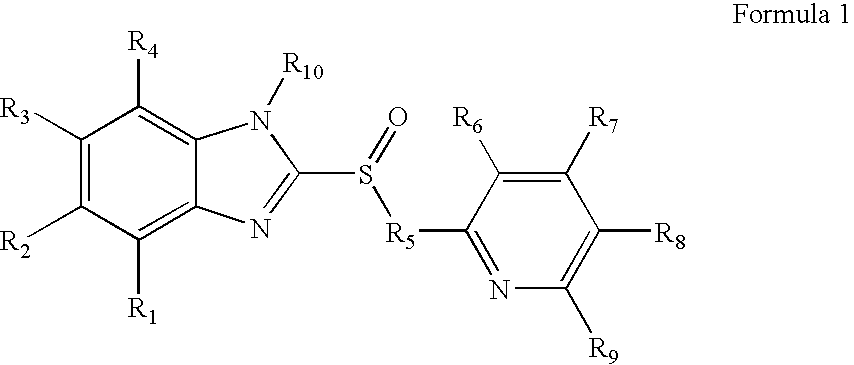
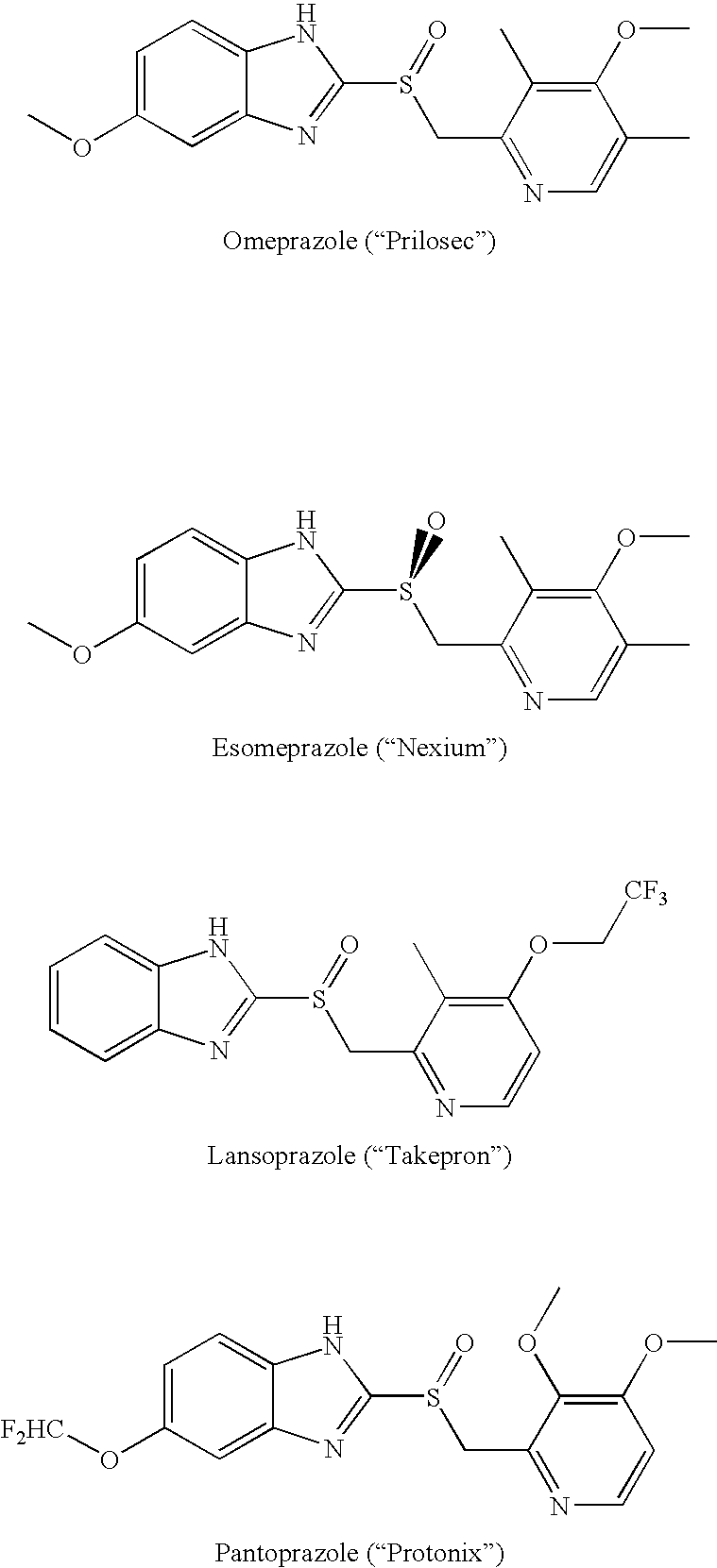
Novel substituted benzimidazole dosage forms and method of using same
InactiveUS20050042304A1Easy to prepareImprove pharmacological activityPowder deliveryBiocideATPaseOral suspensions
A method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor (PPI) in a pharmaceutically acceptable carrier. The present invention provides an oral solution / suspension comprising a proton pump inhibitor and at least one buffering agent. The PPI can be any substituted benzimidazole compound having H+,K+-ATPase inhibiting activity and being unstable to acid. Omeprazole and lansoprazole are the preferred PPIs for use in oral suspensions in concentrations of at least greater than 1.2 mg / ml and 0.3 mg, respectively. The liquid oral compositions can be further comprised of parietal cell activators, anti-foaming agents and / or flavoring agents. The inventive compositions can alternatively be formulated as a powder, tablet, suspension tablet, chewable tablet, capsule, effervescent powder, effervescent tablet, pellets and granules. Such dosage forms are advantageously devoid of any enteric coating or delayed or sustained-release delivery mechanisms, and comprise a PPI and at least one buffering agent to protect the PPI against acid degradation. Similar to the liquid dosage form, the dry forms can further include anti-foaming agents, parietal cell activators and flavoring agents. Kits utilizing the inventive dry dosage forms are also disclosed herein to provide for the easy preparation of a liquid composition from the dry forms. In accordance with the present invention, there is further provided a method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor in a pharmaceutically acceptable carrier and at least one buffering agent wherein the administering step comprises providing a patient with a single dose of the composition without requiring further administering of the buffering agent. Additionally, the present invention relates to a method for enhancing the pharmacological activity of an intravenously administered proton pump inhibitor in which at least one parietal cell activator is orally administered to the patient before, during or after the intravenous administration of the proton pump inhibitor.
Owner:UNIVERSITY OF MISSOURI
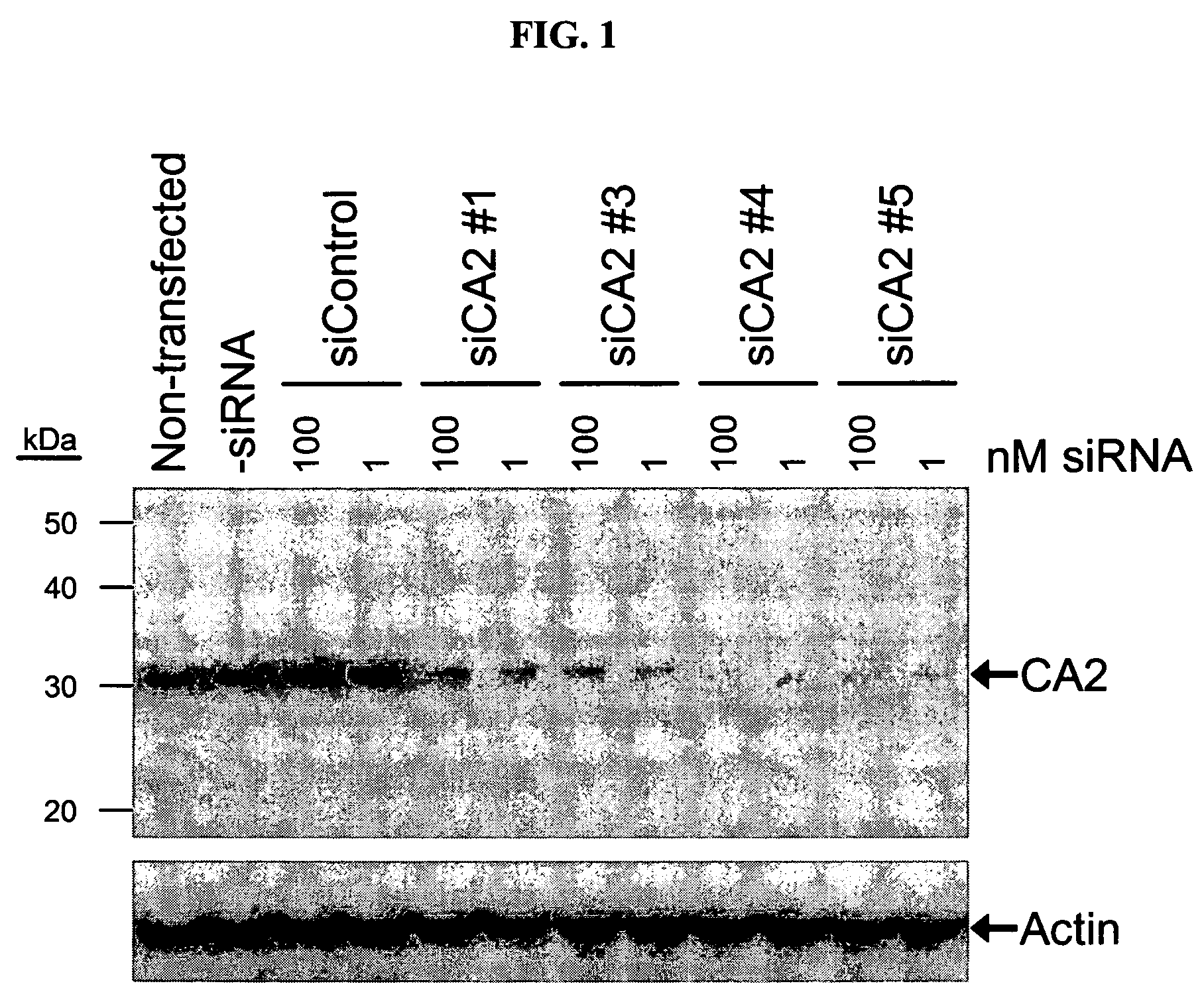
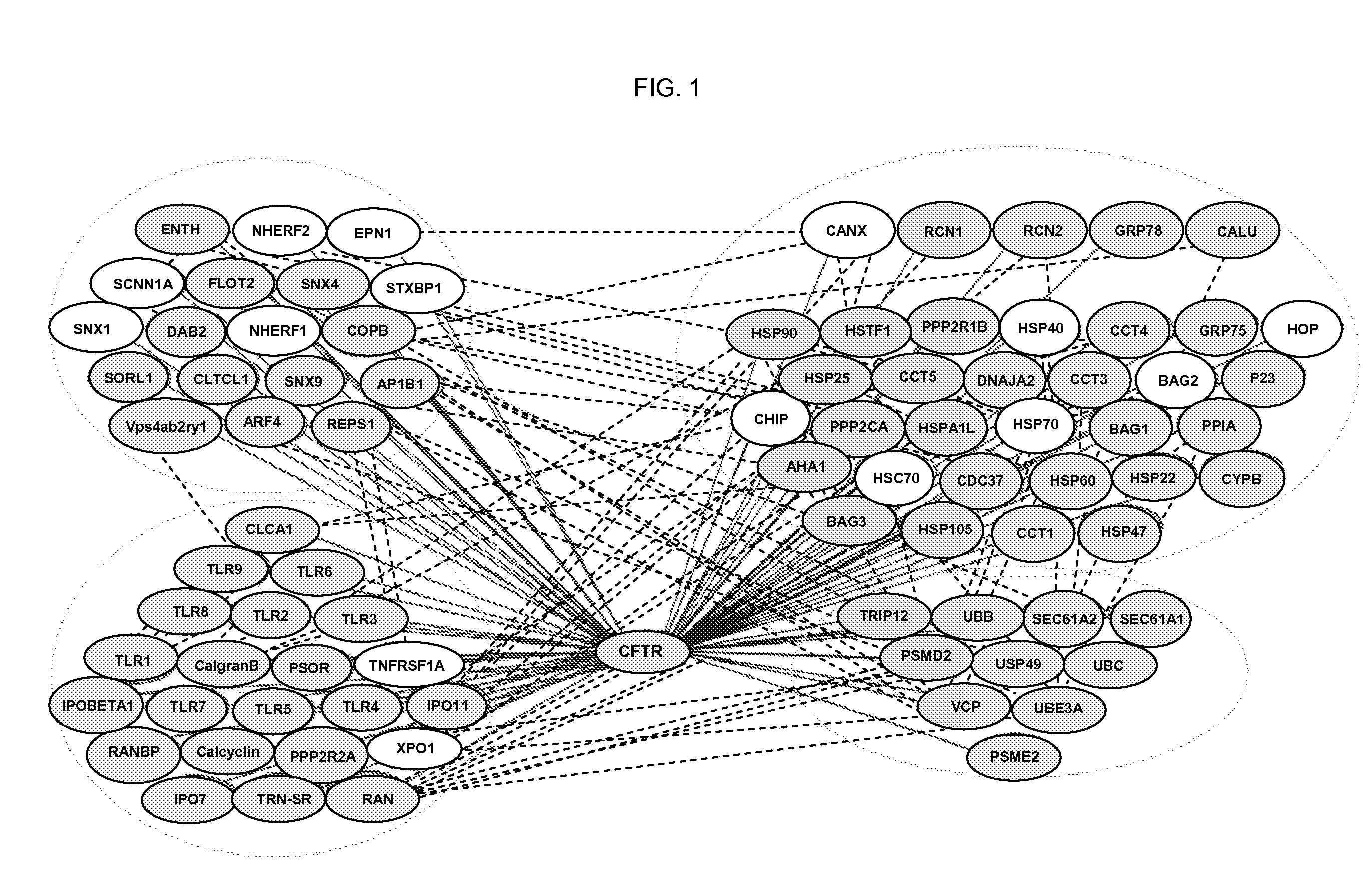
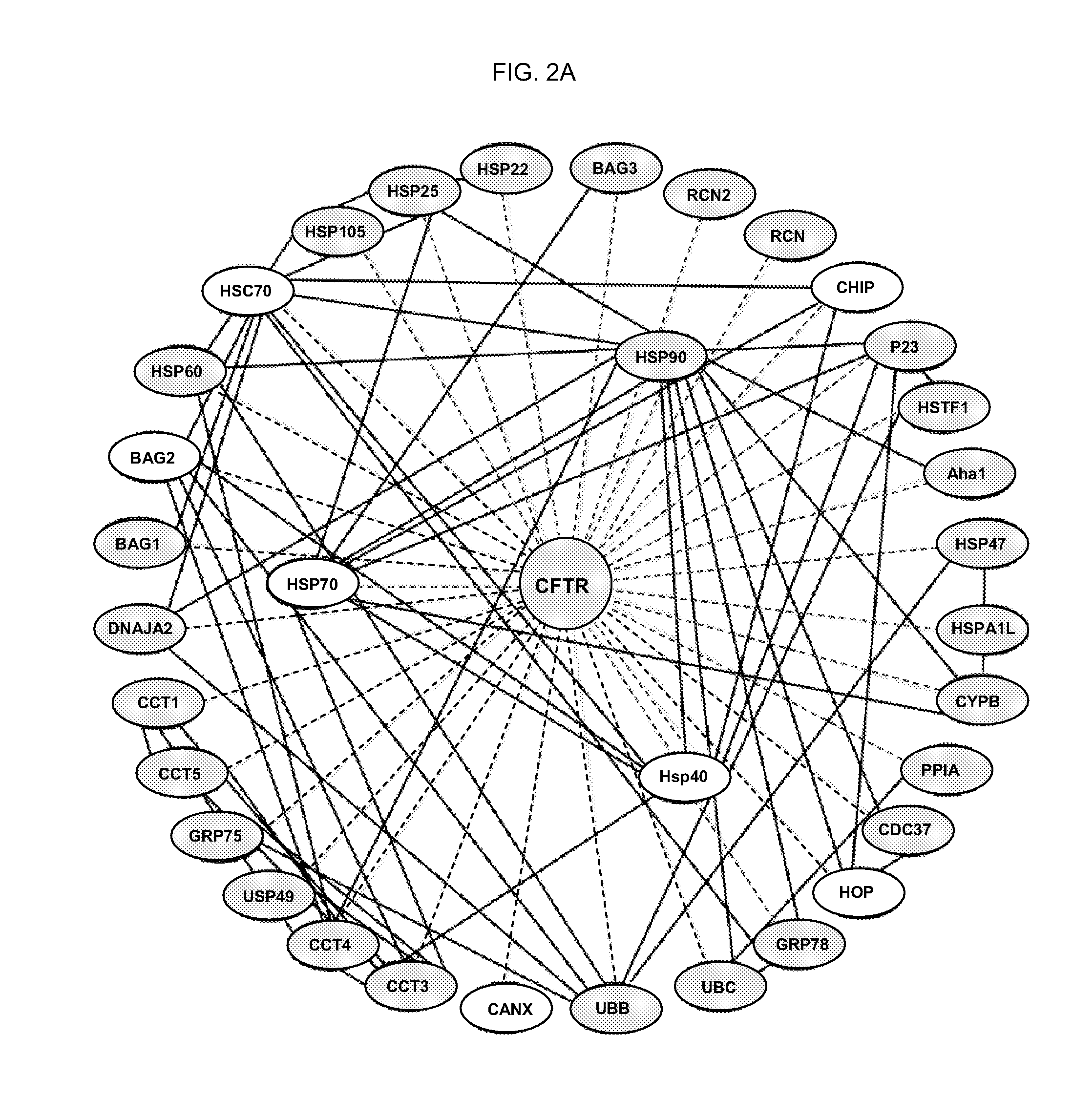
Markers for colorectal cancer
InactiveUS20060188883A1Reduce and eliminate biological activityReduce expressionMicrobiological testing/measurementDisease diagnosisCancer cellBifunctional
Provided are previously uncharacterised markers of cancers, for example colorectal cancers, and uses of these as diagnostic and prognostic markers of cancers, and in particular colorectal cancers. The markers are SEQ ID NO: 1—hnRNP-K; SEQ ID NO:2—HMG-1; SEQ ID NO:3—proteasome subunit alpha type 1; SEQ ID NO:4—bifunctional purine biosynthesis protein; SEQ ID NO:5—ST11; SEQ ID NO:6—annex in IV; SEQ ID NO:7—60 kDa heat shock protein; SEQ ID NO:8—T complex protein 1 beta subunit; SEQ ID NO:9—T complex protein 1 epsilon subunit; SEQ ID NO: 10—mortalin; and SEQ ID NO: 11—TER-ATPase. The invention further provides related methods and materials for the use of the markers in therapeutic intervention in colorectal and other cancers e.g. to specifically target neoplastic cells without causing significant toxicity in healthy tissues, and to provide methods for the evaluation of the ability of candidate therapeutic compounds to modulate the biological activity of cancerous cells from the colon, rectum and other tissues.
Owner:AUVATION +2
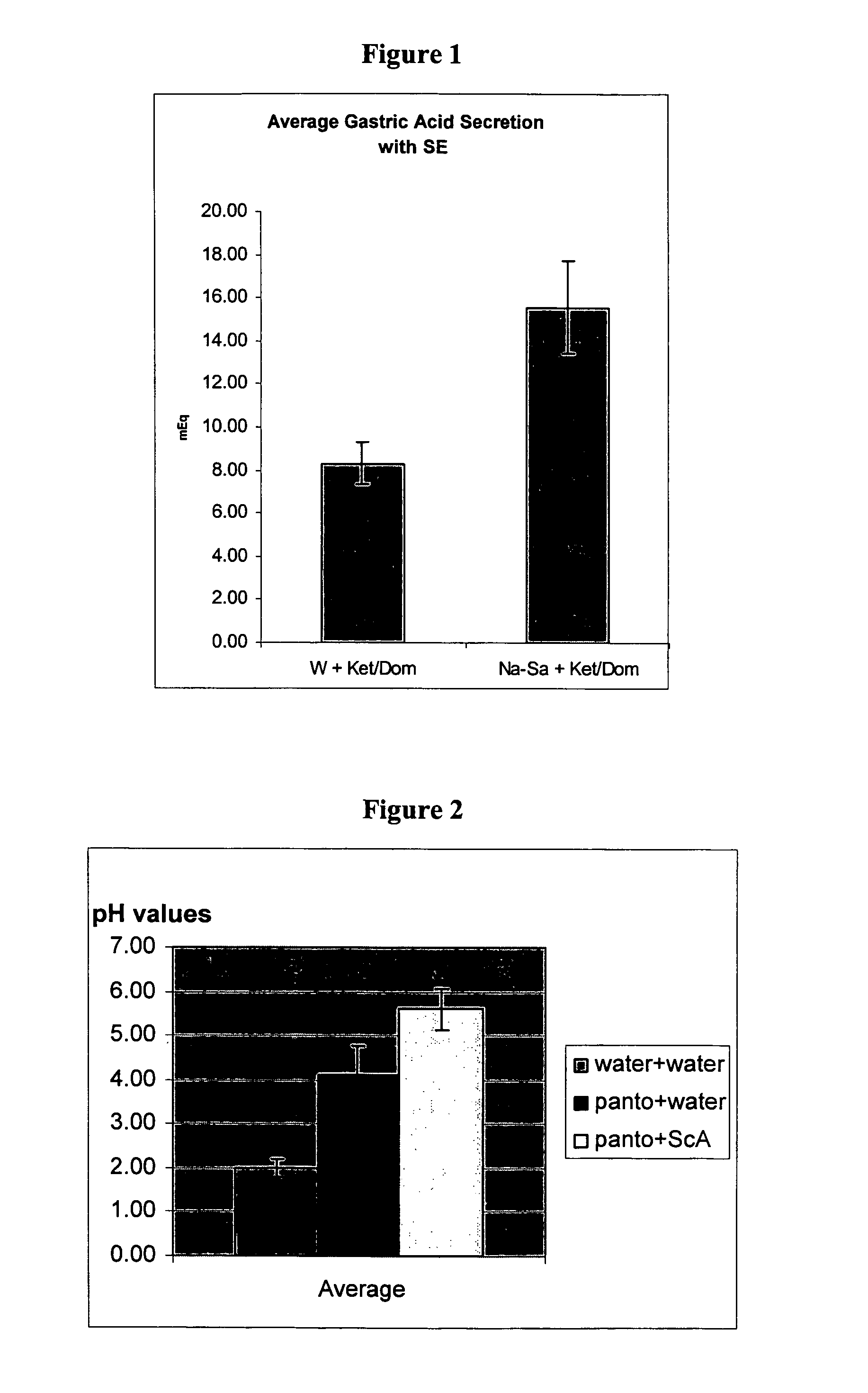
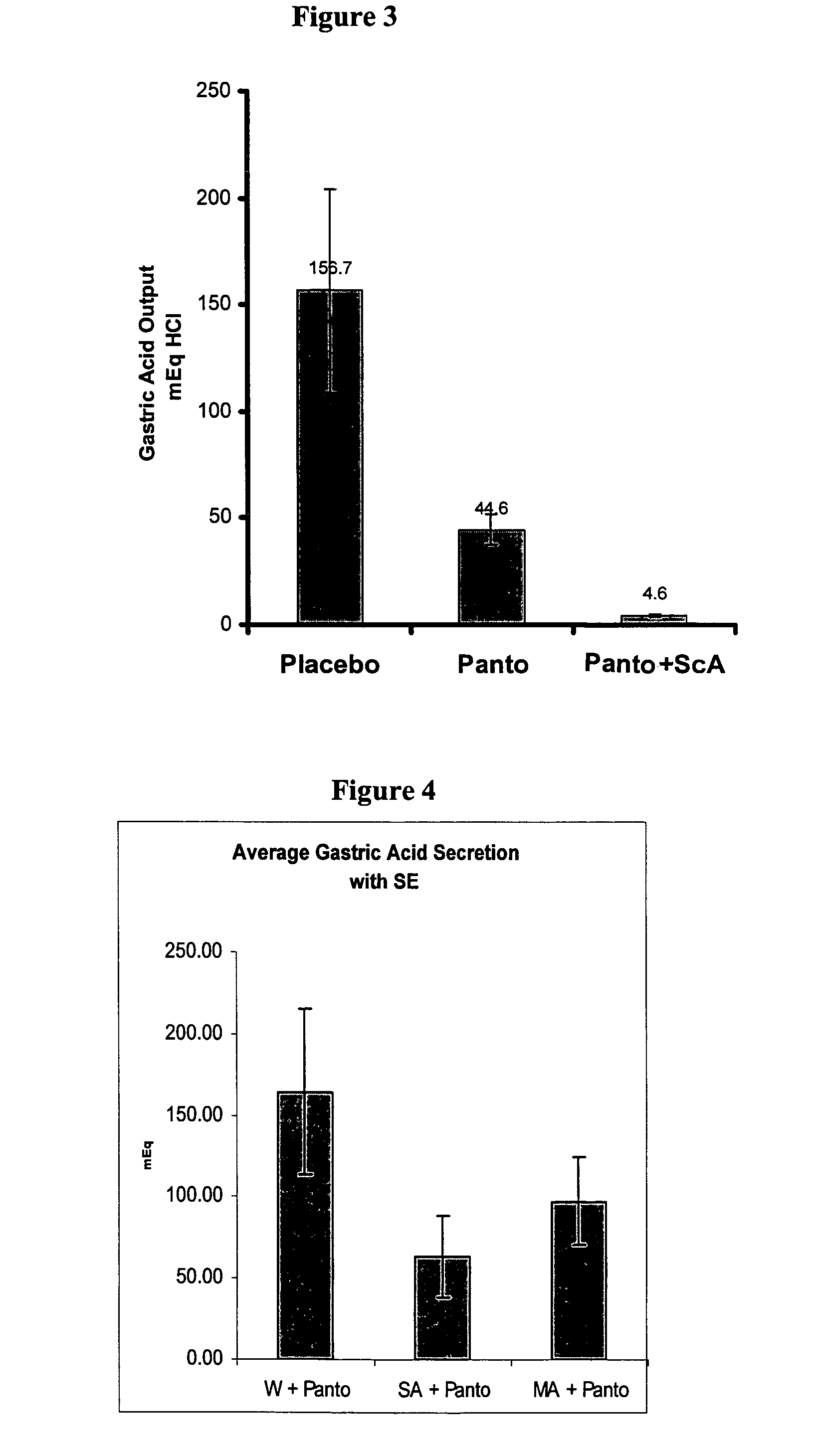
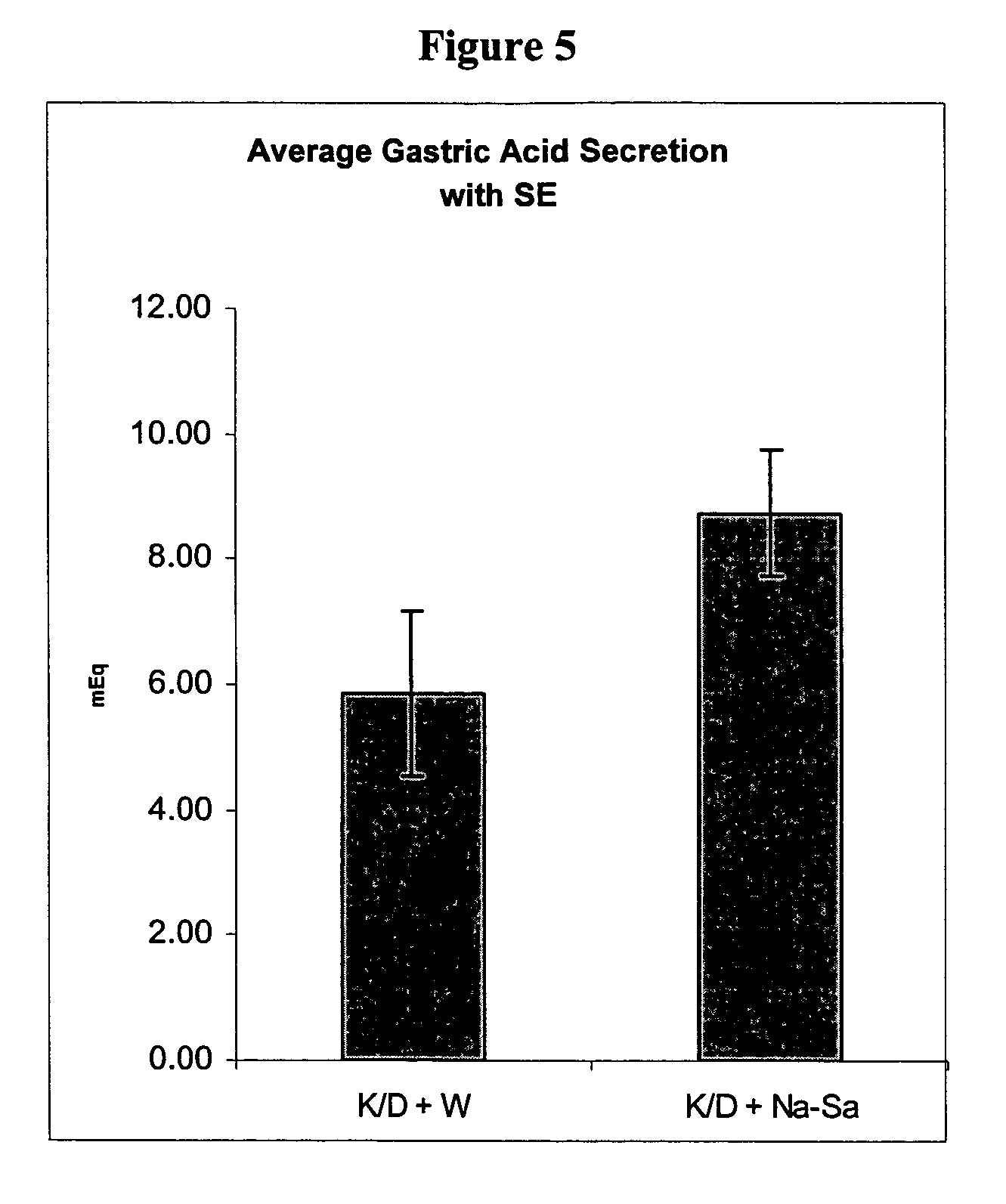
Composition and methods for inhibiting gastric acid secretion
The present invention is related to oral compositions comprising an irreversible gastric H+ / K+-ATPase proton pump inhibitor (PPI) as a gastric acid secretion inhibitor and succinc acid as a parietal cell activator in the gastric lumen. The compositions of the present invention are capable of enhancing the anti-acid activity of PPI in the stomach. The present invention further relates to a method of using such compositions to reduce gastric acid secretion in a mammal.
Owner:VECTA
RNAi-mediated inhibition of ocular targets
RNA interference is provided for inhibition of ocular hypertension target mRNA expression for lowering elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Ocular hypertension targets include carbonic anhydrase II, IV, and XII; β1- and β2 adrenergic receptors; acetylcholinesterase; Na+ / K+-ATPase; and Na—K-2Cl cotransporter. Ocular hypertension is treated by administering interfering RNAs of the present invention.
Owner:NOVARTIS AG
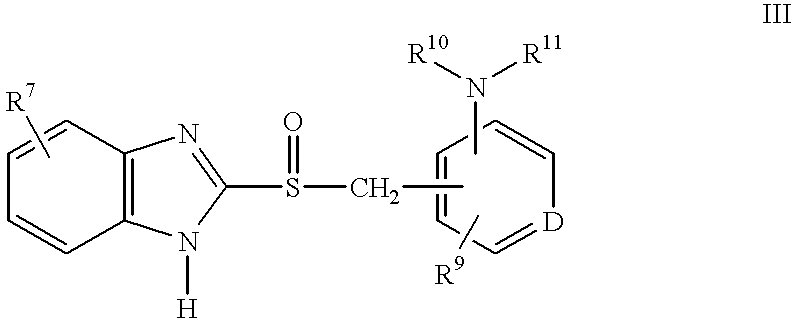
Method of using (H+/K+) ATPase inhibitors as antiviral agents
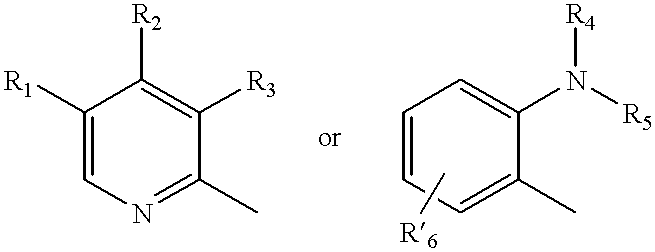
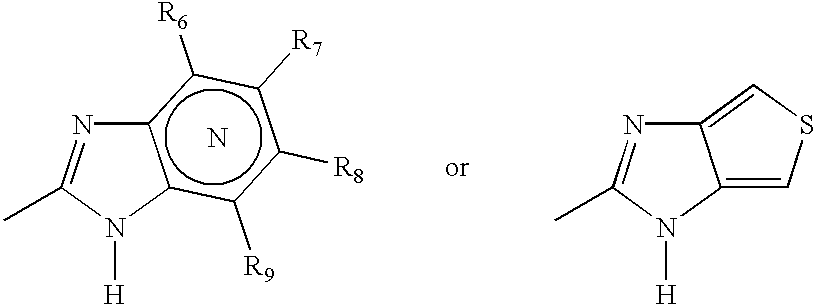
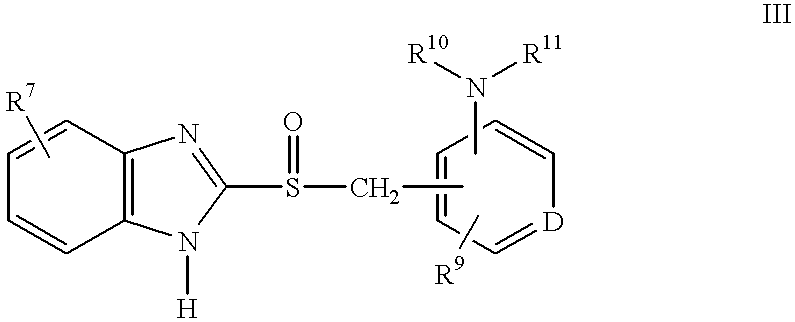
A class of compounds which are (H+ / K+)ATPase inhibitors can be used for the treatment of viral infections. Compounds of particular interest are defined by Formula III: [0001]wherein D is N or CH; wherein R7 is one or more radicals selected from hydrido, alkoxy, amino, cyano, nitro, hydroxyl, alkyl, halo, haloalkyl, carboxyl, alkanoyl, nitro, amino, alkylamino, amide, alkylamide, alkoxycarbonyl, alkylthio, alkylsulfinyl and alkylsulfonyl; wherein R9 is one or more radicals selected from hydrido, alkoxy, amino, alkyl, halo, cyano, nitro, hydroxyl, haloalkyl, carboxyl, alkanoyl, nitro, amine, alkylamine, dialkylamine, amide, alkylamide, alkoxycarbonyl, alkylthio, alkylsulfinyl and alkylsulfonyl; and wherein R10 and R11 are independently selected from hydrido and alkyl; or a pharmaceutically acceptable salt thereof.
Owner:PHARMACIA CORP
Pancreatic cancer treatment using Na+/K+ ATPase inhibitors
InactiveUS20070105790A1Patient compliance is goodKept lowBiocideCarbohydrate active ingredientsATPaseAglycone
The reagent, pharmaceutical formulation, kit, and methods of the invention provides a new approach for treating pancreatic cancers. The invention provides the use of Na+e / K+-ATPase inhibitors, such as cardiac glycosides (e.g. ouabain and proscillaridin, etc.), either alone or in combination with other standard therapeutic agents (chemo- or radio-therapies, etc.) for treating pancreatic cancers. The subject Na+ / K+-ATPase inhibitors, such as cardiac glycosides, including bufadieneolides or their corresponding aglycones (e.g., proscillaridin, scillaren, and scillarenin, etc.), especially in oral formulations and / or solid dosage forms containing more than 1 mg of active ingredients.
Owner:BIONAUT PHARMA
Formulation of substituted benzimidazoles
The present invention relates to stable liquid formulations that comprise a water free or almost water free, polyethylene glycol solution of sodium or potassium salt of a H<+>, K<+>-ATPase inhibitor of Formula I or a sodium or potassium salt of one single enantiomer thereof. Alternatively, the sodium or potassium salt of the H<+>, K<+>-ATPase inhibitor may be formed in situ in the polyethylene glycol solution by adding sodium or potassium hydroxide together and the active compound. The invention is also directed to the preparation of the claimed formulation, use of the stable liquid formulations in medicine and in the treatment of gastrointestinal diseases.
Owner:ASTRAZENECA AB
Treatment of Protein Misfolding
InactiveUS20080014191A1Improve biological activityBlock functional activityBiocideOrganic active ingredientsATPaseMedicine
Owner:THE SCRIPPS RES INST
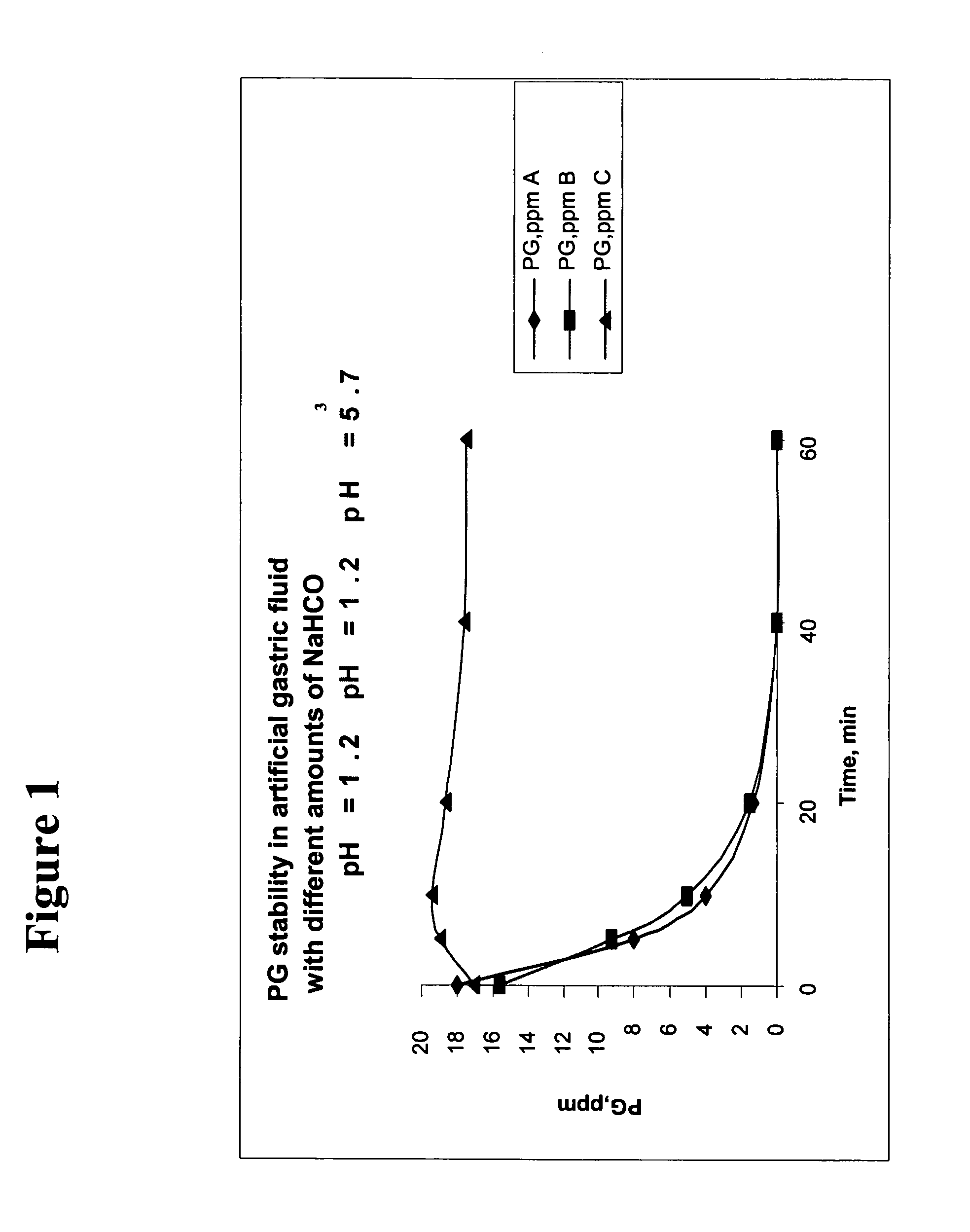
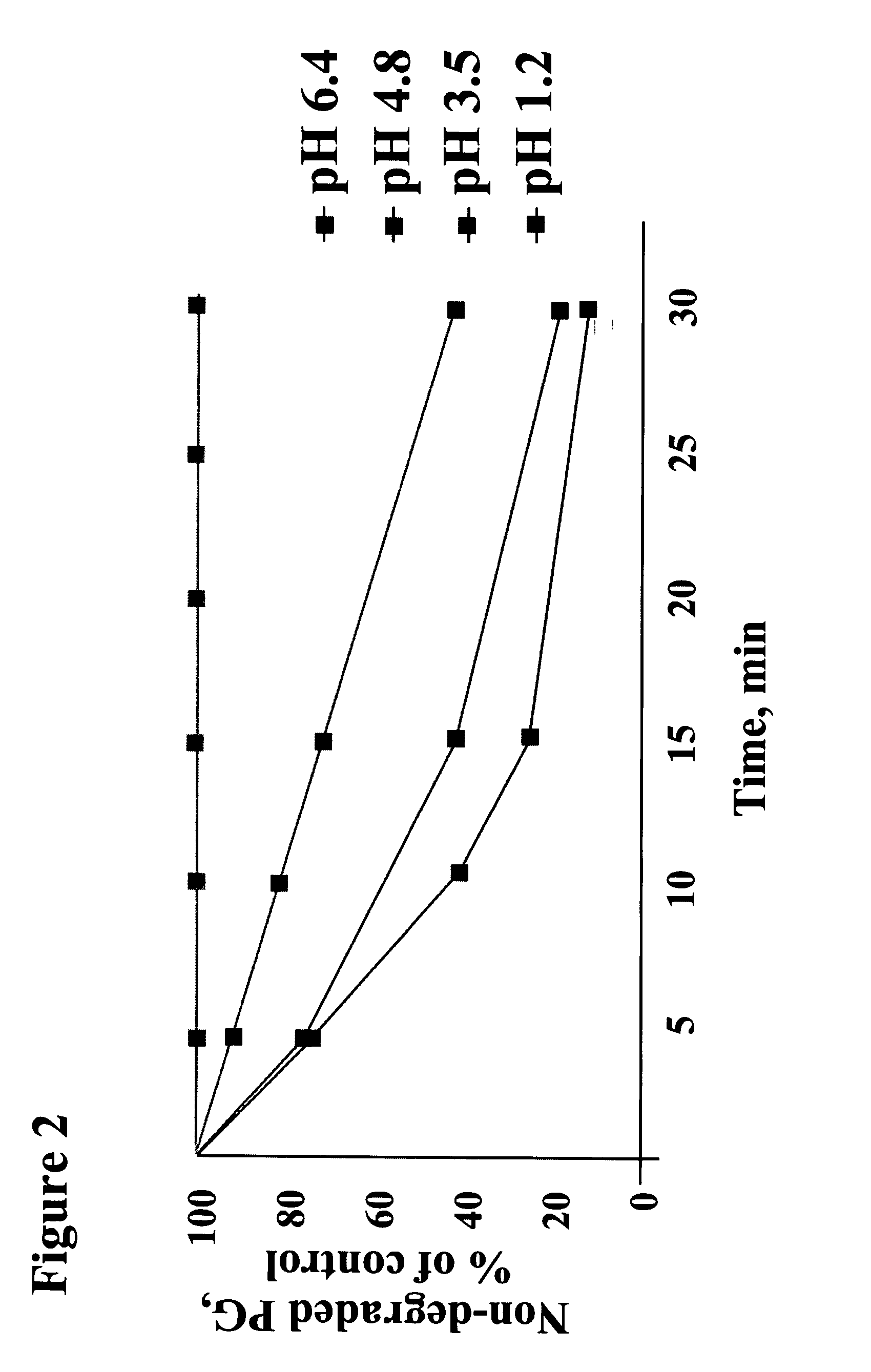
Compositions and methods for treating pathologies that necessitate suppression of gastric acid secretion
InactiveUS20060135406A1Rapid onsetEnhanced inhibitory effectBiocidePeptide/protein ingredientsATPasePentagastrin stimulation test
The present invention is related to novel oral compositions comprising an irreversible gastric H+ / K+-ATPase proton pump inhibitor (PPI) as a gastric acid secretion inhibitor, pentagastrin (PG) or a PG analogue as an activator of parietal cells in the gastric lumen. In a preferred embodiment, the composition further comprises at least one agent that preserves the availability of PG in the gastric fluids, thus enabling PG to act locally in the stomach. Unexpectedly, the compositions of the present invention exhibit anti-acid activity locally in the stomach that is meal-independent, exhibit fast onset and prolonged inhibition of acid secretion.
Owner:VECTA
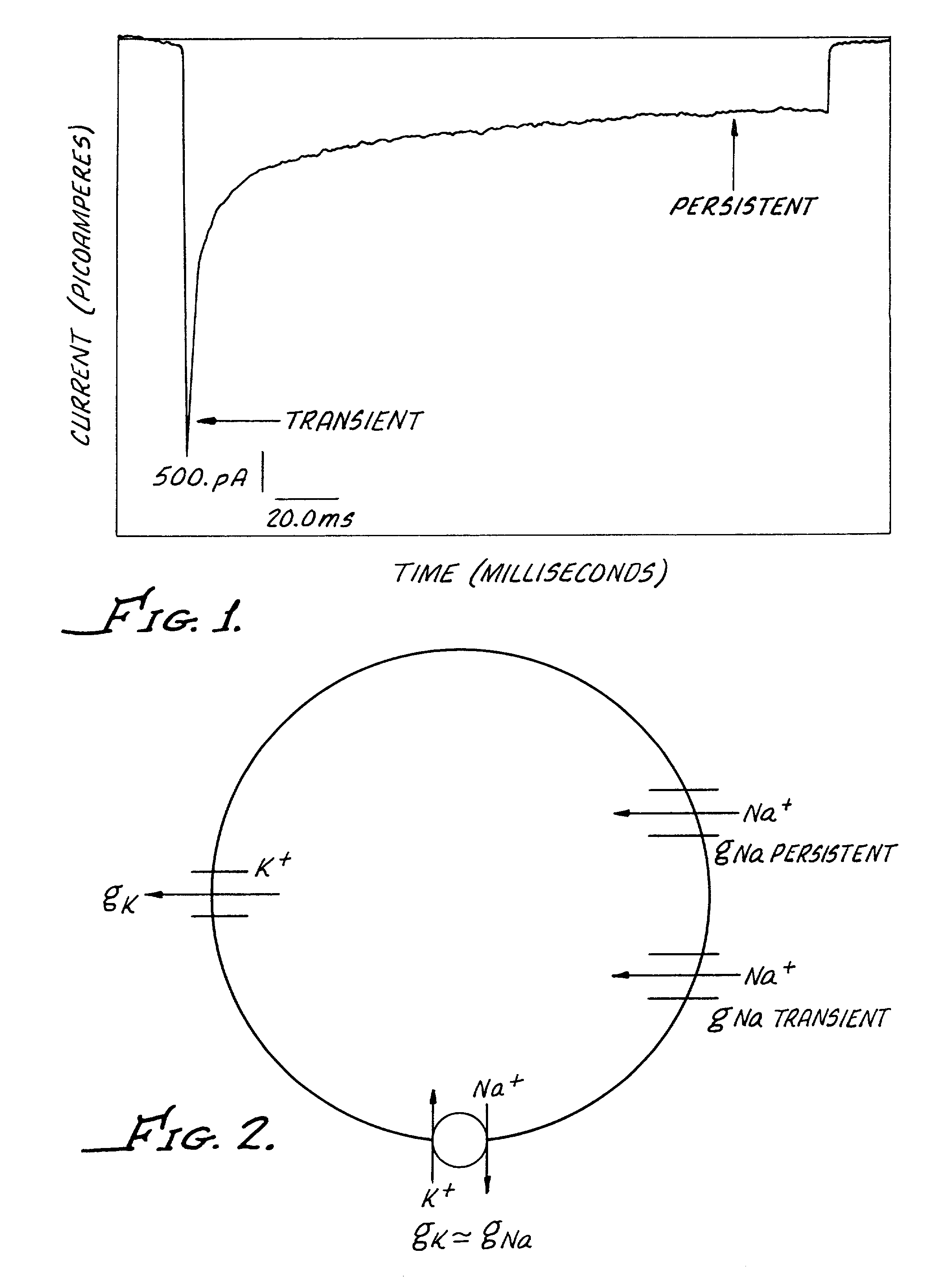
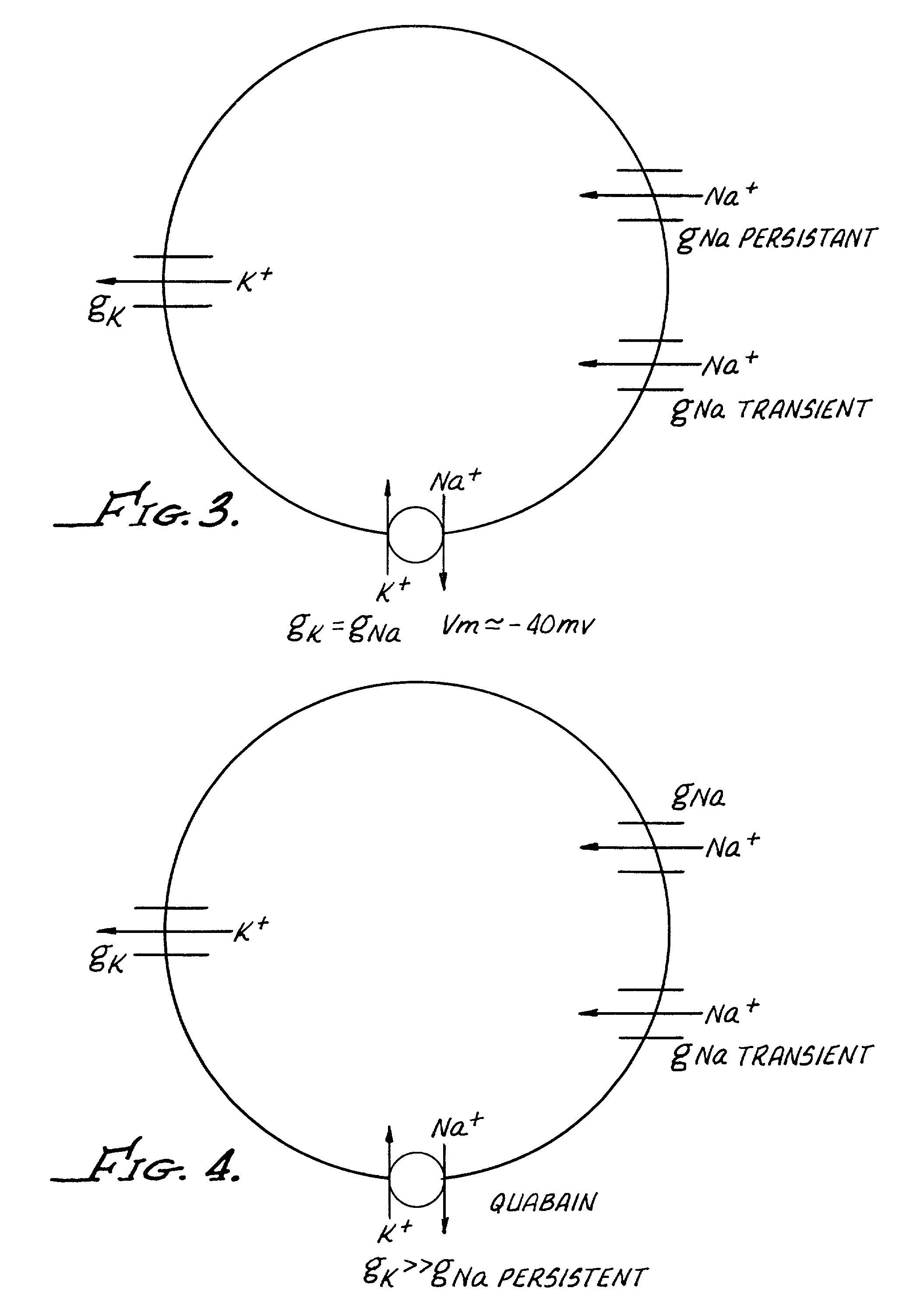
High-throughput screen for identifying channel blockers that selectively distinguish transient from persistent sodium channels
A method for identifying a Na+ channel blocker, including providing a cell containing a Na+ channel, demonstrating both a transient and a persistent current. The cell includes a potassium (K+) channel and a Na / K ATPase (Na+ pump). A fluorescent dye is disposed into the well. The fluorescent dye is sensitive to change in cell membrane potential in order to enable optical measurement of cell membrane potential. A Na+ channel blocker, to be identified, is added to the well and a stimulating current is passed through the cell in an amount sufficient to generate an action potential before and after the addition of the Na+ channel blocker. Thereafter, a change in cell membrane potential is optically measured.
Owner:ALLERGAN INC
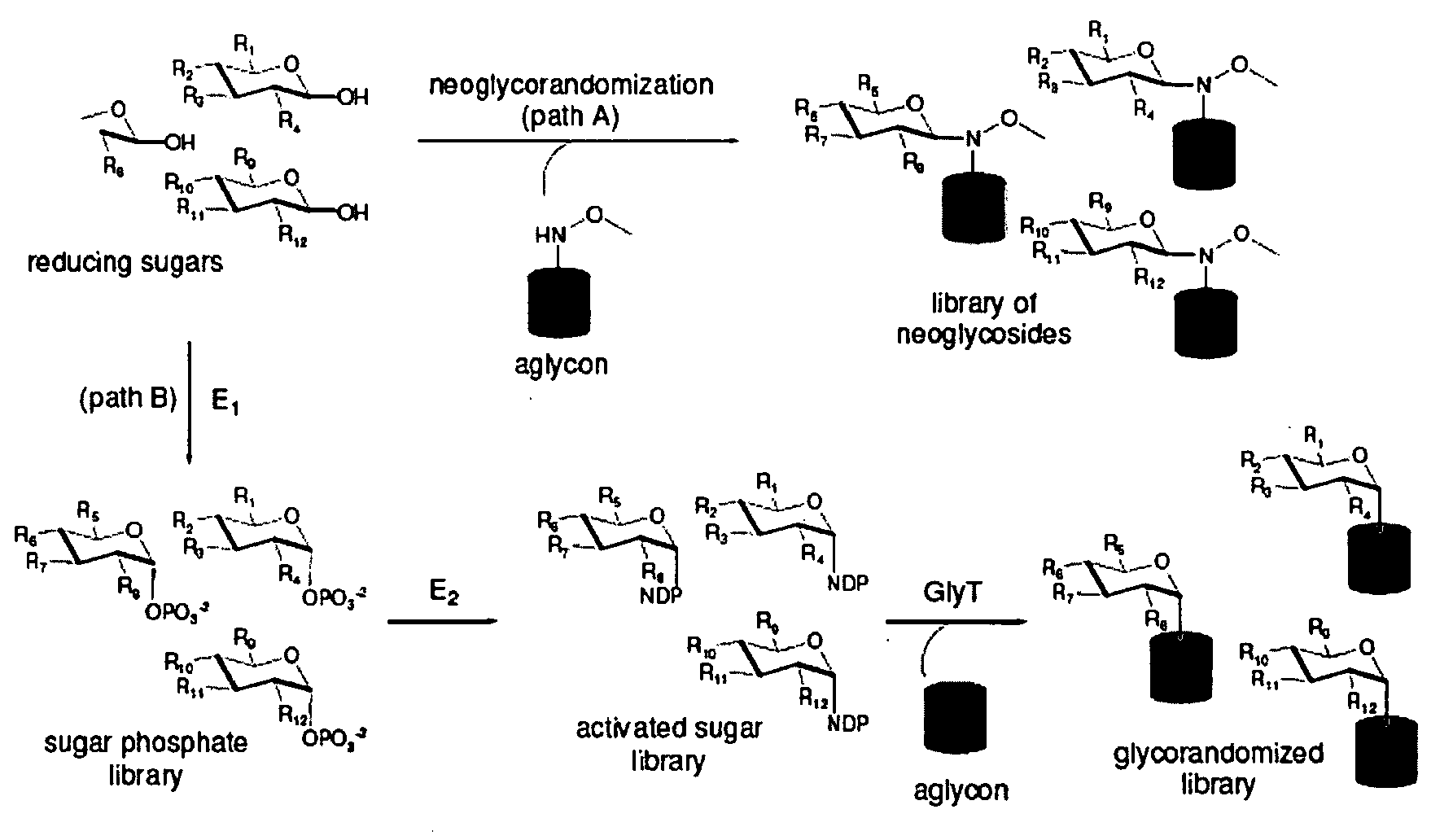
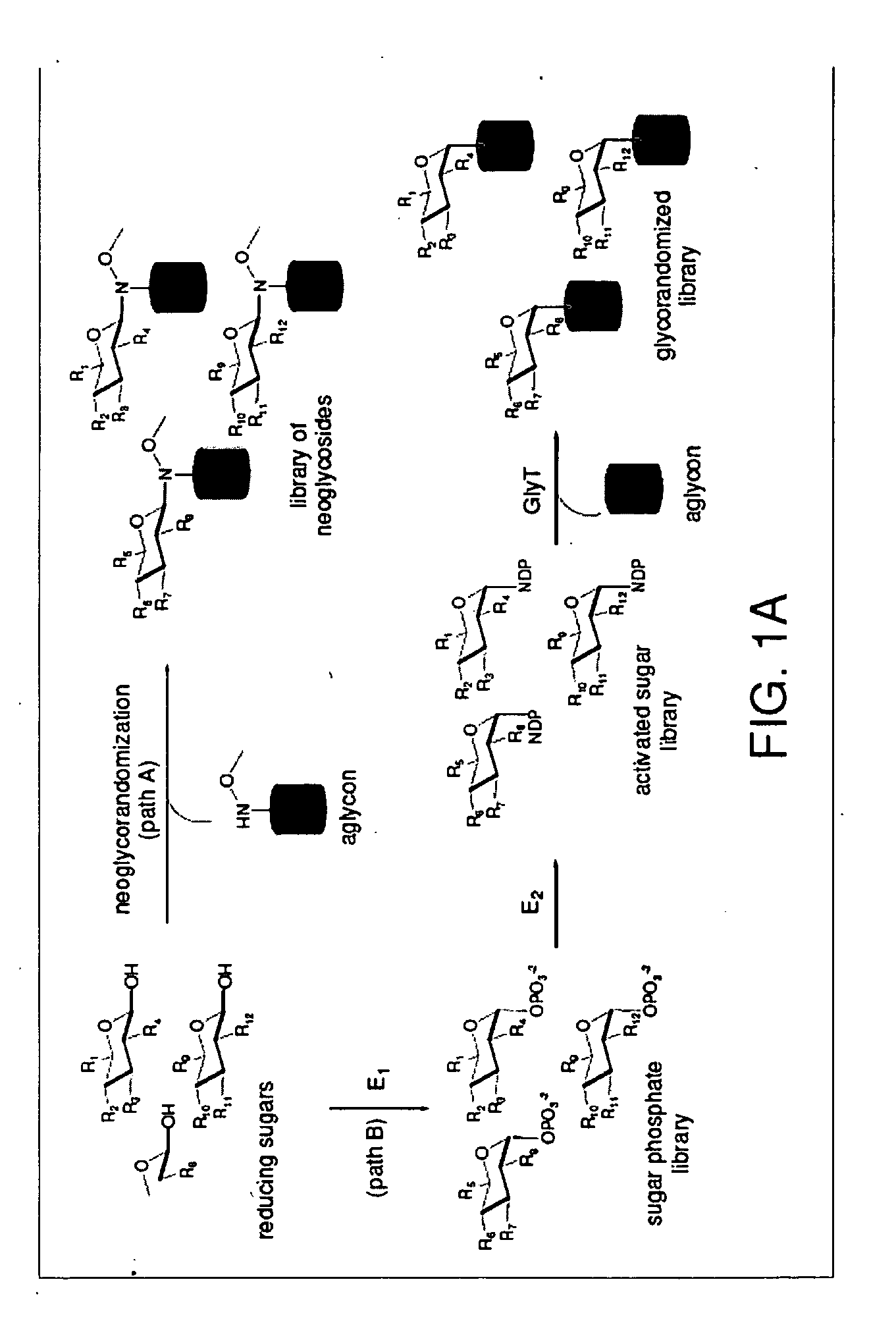
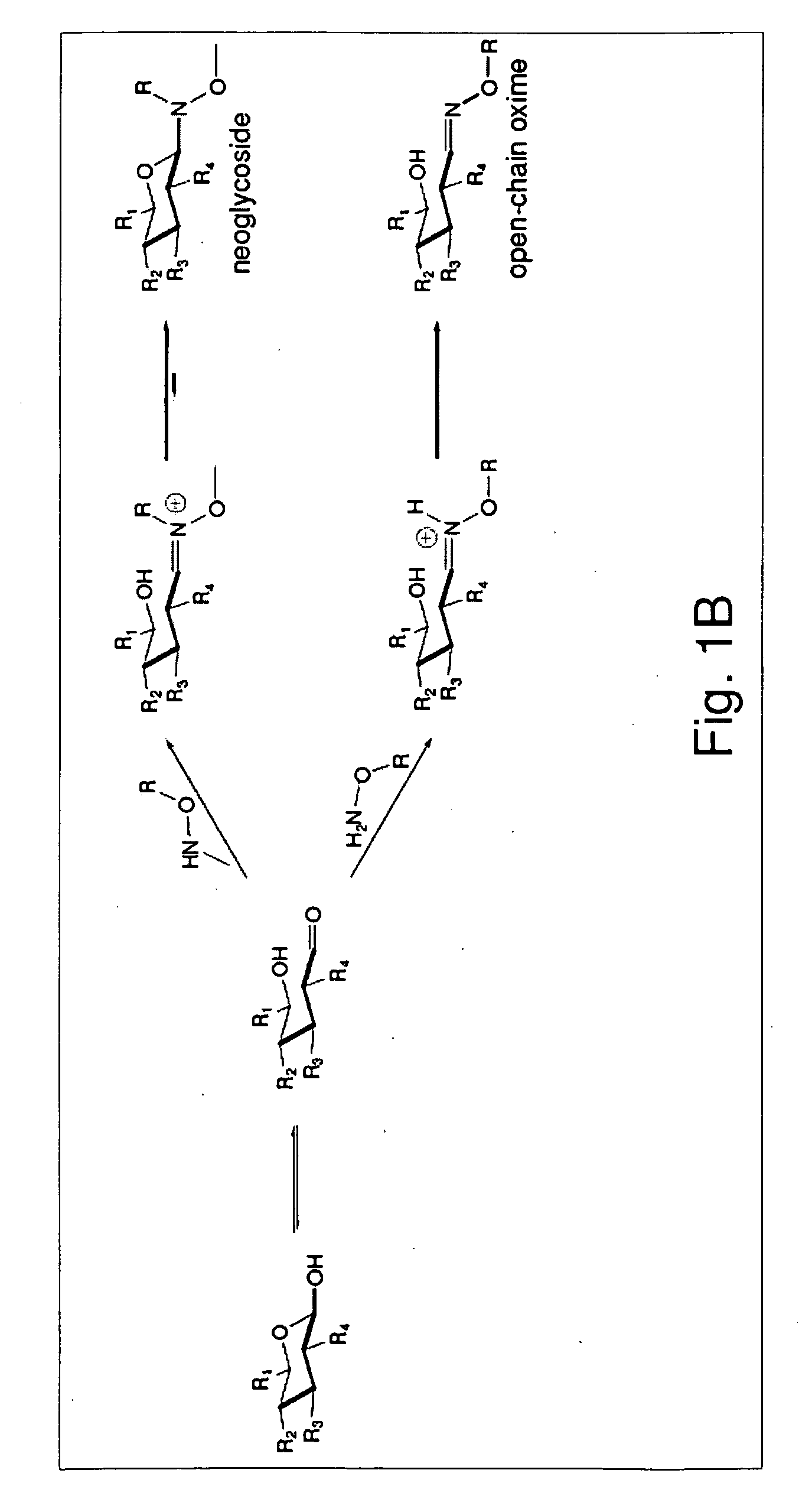
Neoglycorandomization and digitoxin analogs
ActiveUS20090075842A1Enhance desirable propertyEliminate side effectsBiocideSugar derivativesATPaseSide effect
The present invention provides methods of producing libraries of compounds with enhanced desirable properties and diminished side effects as well as the compounds produced by the methods. In preferred embodiments, methods of the present invention use a universal chemical glycosylation method that employs reducing sugars and requires no protection or activation. In a preferred embodiment, the invention provides a library of neoglycoside digitoxin analogs that includes compounds with significantly enhanced cytotoxic potency toward human cancer cells and tumor-specificity, but are less potent Na+ / K+-ATPase inhibitors in a human cell line than digitoxin.
Owner:WISCONSIN ALUMNI RES FOUND
Treatment of refractory cancers using Na+/K+-ATPase inhibitors
InactiveUS20080027010A1Improve stress responseGood tumor growthBiocideOrganic active ingredientsATPaseAglycone
The reagent, pharmaceutical formulation, kit, and methods of the invention provides a new approach to treat refractory cancers using Na+ / K+-ATPase inhibitors, such as cardiac glycosides, including bufadienolides or their corresponding aglycones (e.g., proscillaridin, scillaren, and scillarenin, etc.), especially in oral formulations and / or solid dosage forms containing more than 1 mg of active ingredients.
Owner:BIONAUT PHARMA
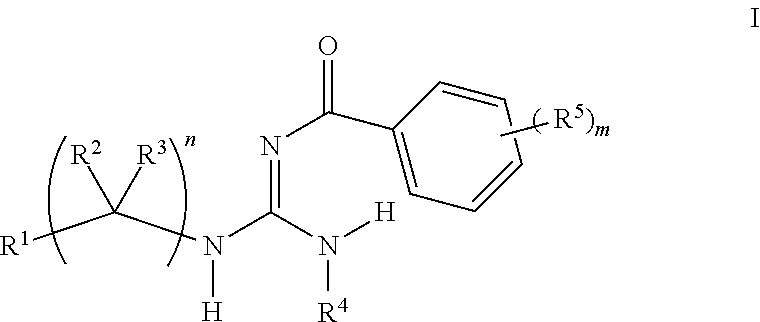
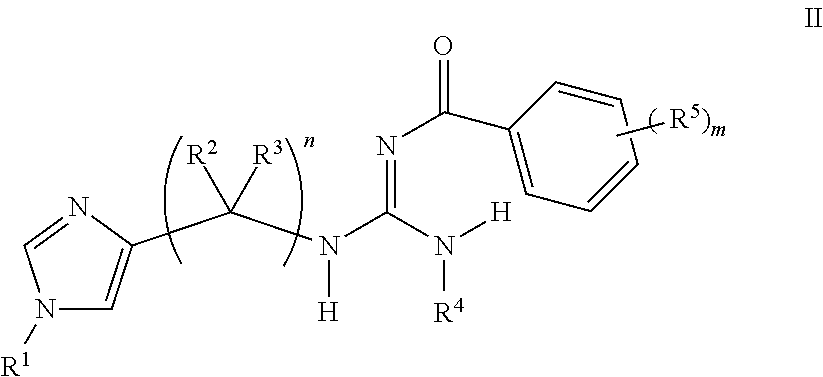
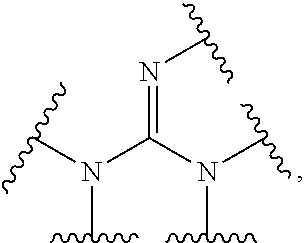
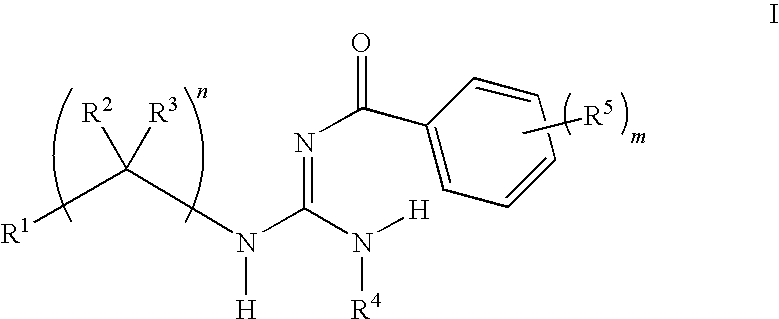
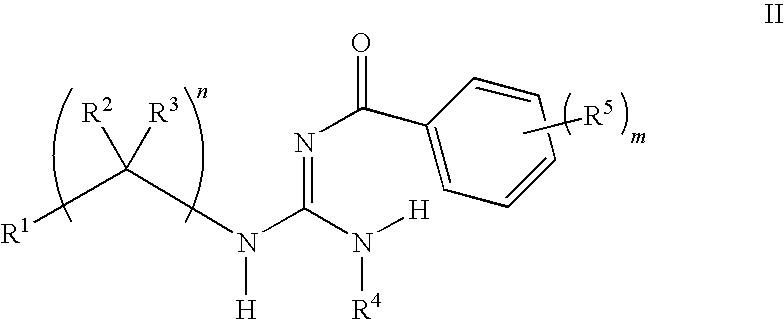
Aryl guanidine F1F0-ATPase inhibitors and related methods
Owner:RGT UNIV OF MICHIGAN
Treatment of refractory cancers using NA+/K+ ATPase inhibitors
InactiveUS20060135468A1Effective treatmentBiocideHeavy metal active ingredientsATPasePharmaceutical formulation
The reagent, pharmaceutical formulation, kit, and methods of the invention provides a new approach to treat refractory cancers using Na+ / K+-ATPase inhibitors, such as cardiac glycosides (e.g. ouabain or proscillaridin, etc.).
Owner:BIONAUT PHARMA
Compositions and methods for treatment of neurogenerative diseases
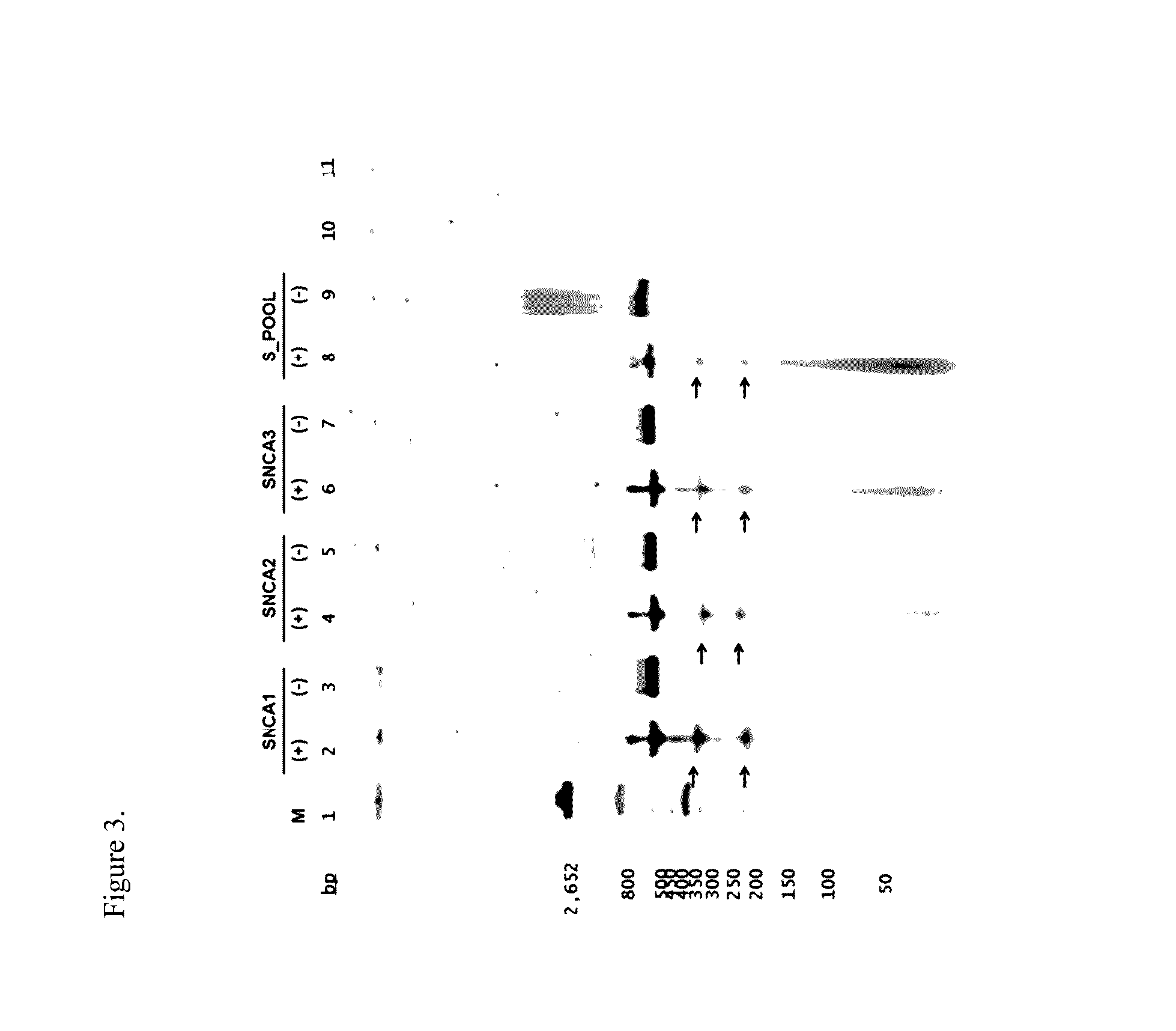
Medical compositions and methods of treating or preventing neurodegeneration in a human suffering from or that is at risk of or susceptible to neurodegeneration or cellular dysfunction associated with expression or impaired cellular function of a neuronal protein encoded by one or more genes that code for alpha-synuclein (SNCA), Parkin RBR E3 ubiquitin protein ligase, (PARK2), Leucine-rich repeat kinase 2 (LRRK2), PTEN-induced putative kinase / (PINK1), Daisuke-Junko 1, (DJ-1) and ATPase type 13A2 (ATP13A2), are disclosed. Methods of treatment for these disorders is also provided, comprising administering a vector into a cell, wherein the vector facilitates expression of a molecular component that alters one of the aforementioned genes in the cell or expression of the gene in the cell, the gene being implicated in an etiology of the neurological deficit.
Owner:FLYNN ALEXANDER C
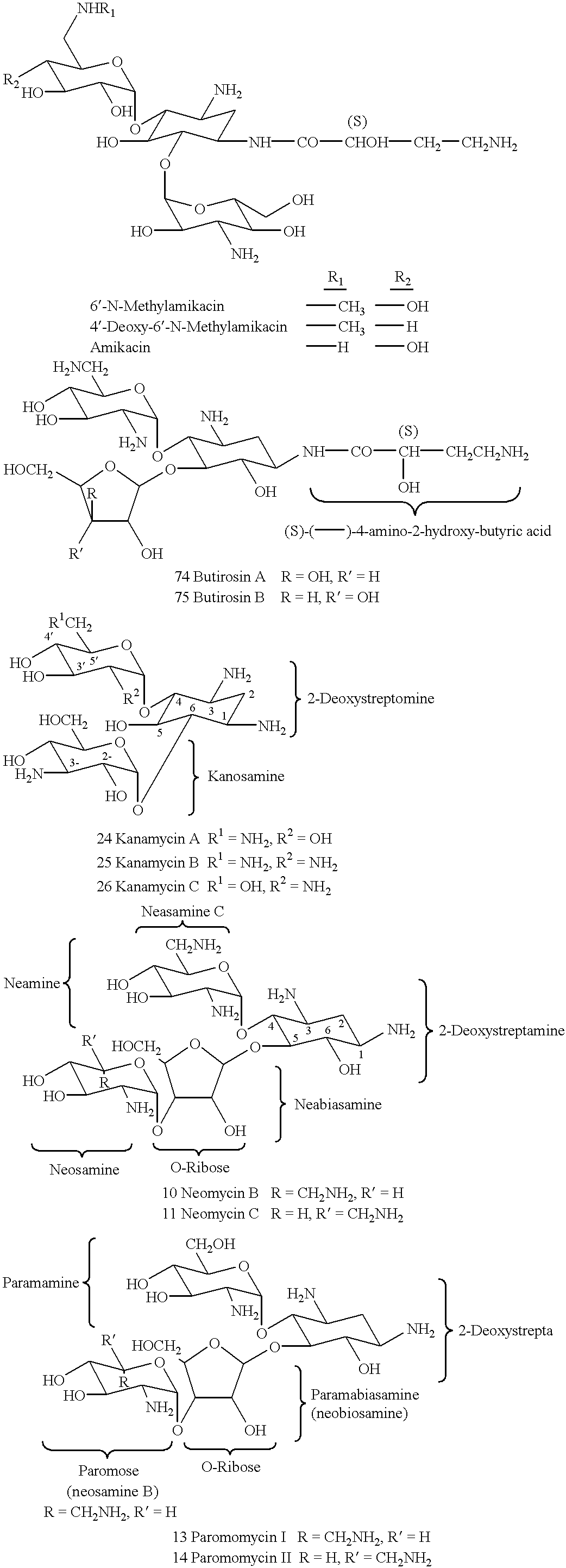
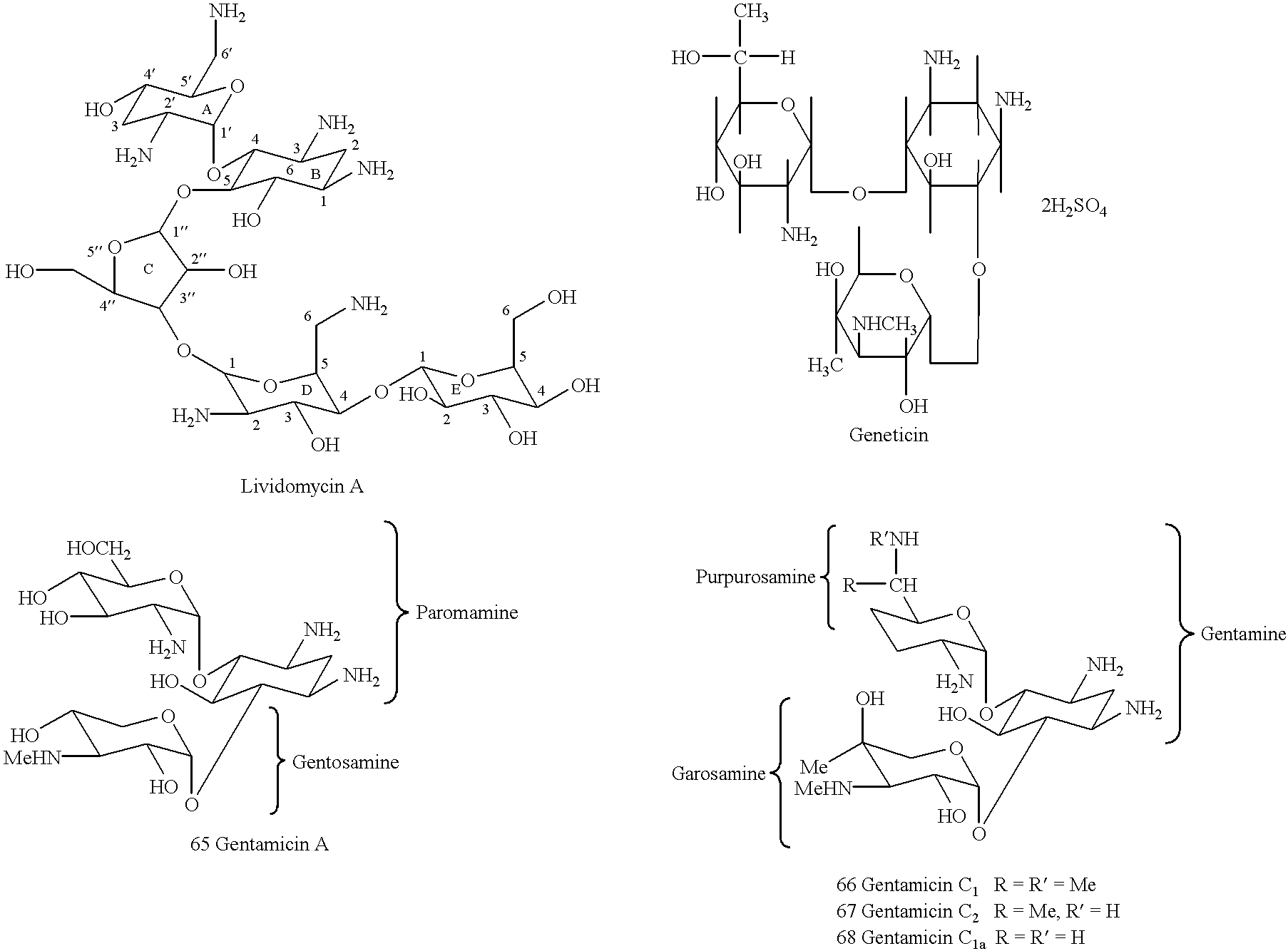
Methods and compositions for targeting DNA metabolic processes using aminoglycoside derivatives
Protein targets for disease intervention through inhibition of nucleic acid metabolism are disclosed. Novel polypeptides for one such target, DNA-dependent ATPase A, and novel polynucleotides encoding DNA-dependent ATPase A are disclosed. Phosphoaminoglycoside compounds which act on such protein targets to inhibit nucleic acid metabolism. In addition, screening assays for identifying compounds that inhibit nucleic acid-dependent ATPase activity, including, but not limited to, DNA-dependent ATPase A, are disclosed. Such compounds are useful in the treatment of diseases, including but not limited to cancer and infectious disease, through disruption of nucleic acid metabolism and induction of apoptosis. Moreover, methods for prevention and treatment of diseases including, but not limited to cancer and infectious disease are disclosed.
Owner:VIRGINIA UNIV OF THE +1
Yeast fermentation small-molecular recombinant fibronectin protein peptide and preparation method and application thereof
ActiveCN110204608AImprove heat resistanceHigh degree of glycosylationCosmetic preparationsPeptide/protein ingredientsATPaseBinding domain
The invention discloses a yeast fermentation small-molecular recombinant fibronectin protein peptide comprising at least one sodium-potassium ATPase beta subunit binding domain, and the sodium-potassium ATPase beta subunit binding domain has the amino acid sequence shown in SEQ ID NO:2. The invention also discloses a preparation method of the yeast fermentation small-molecular recombinant fibronectin protein peptide and an application of the yeast fermentation small-molecular recombinant fibronectin protein peptide. The yeast fermentation small-molecular recombinant fibronectin protein peptidecan be effectively absorbed by skin and has excellent healing and repairing effects on traumatic skin lesions or subcutaneous lesions with intact keratin.
Owner:MELLGEN SHENZHEN BIOTECHNOLOGY CO LTD
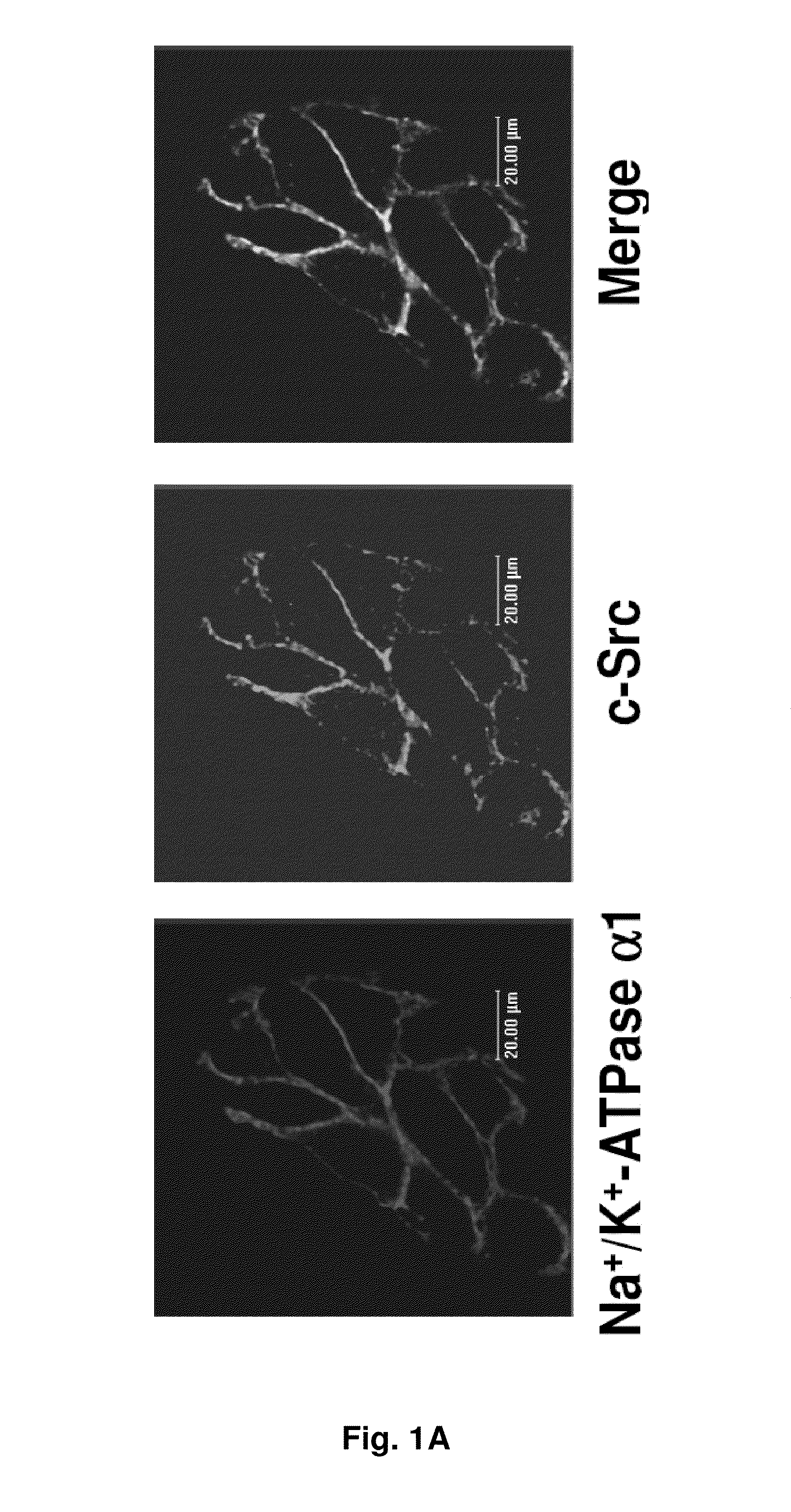
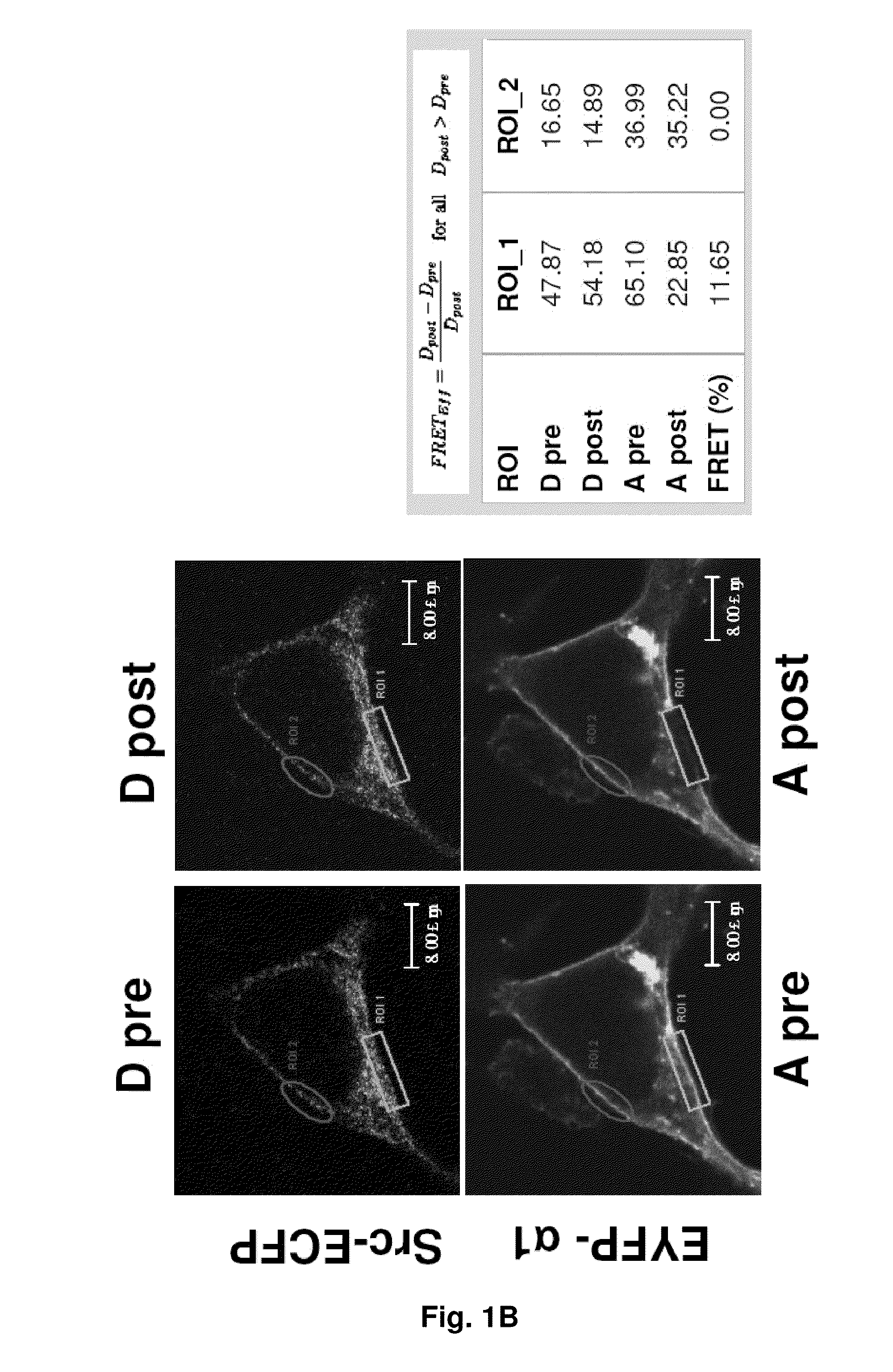
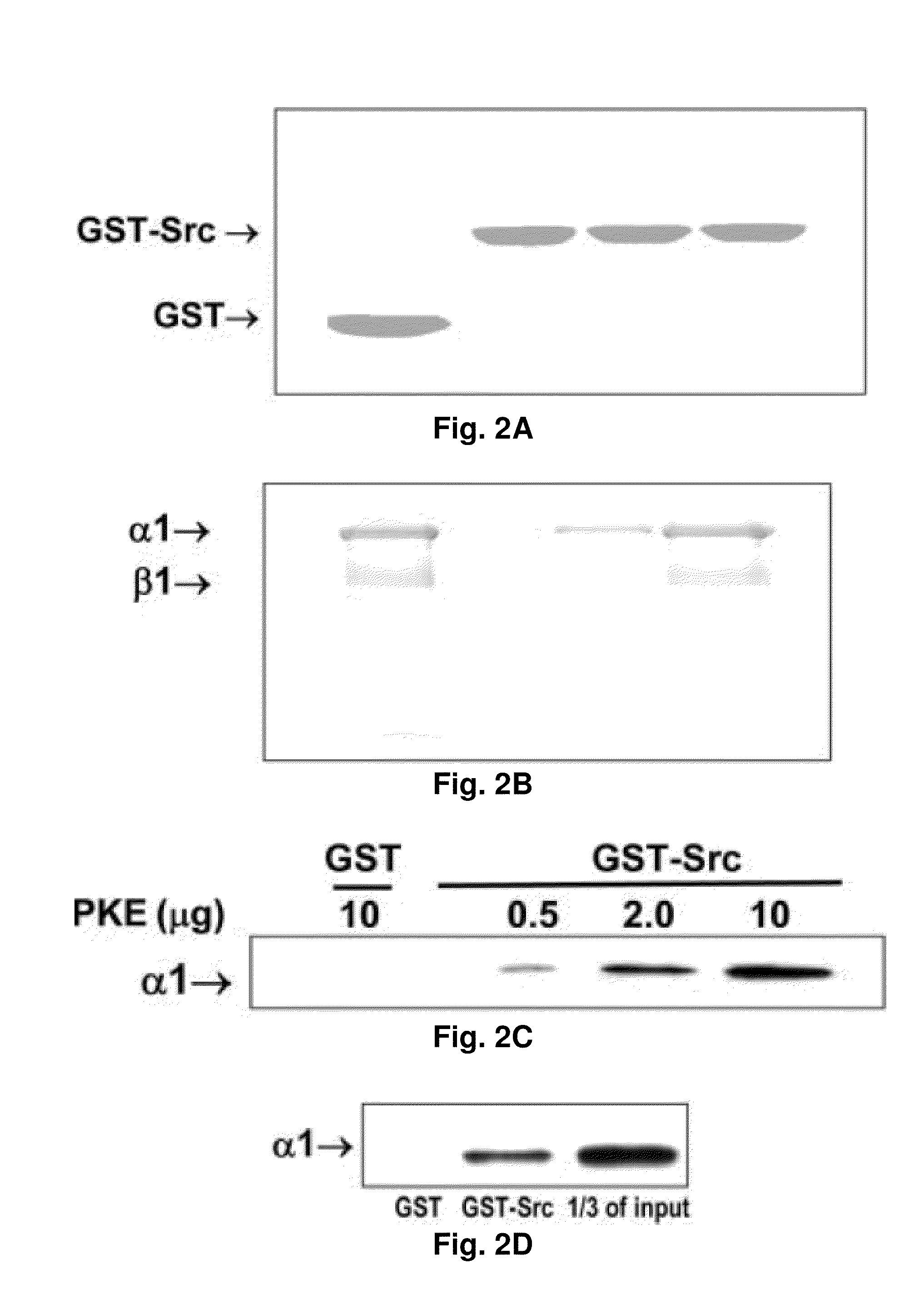
NA+K+-ATPase-Specific Peptide Inhibitors/Activators of SRC and SRC Family Kinases
ActiveUS20100056446A1Reduce expressionSilencing expressionPeptide/protein ingredientsHydrolasesATPaseCardiotonic steroid
A method for regulating Src and its downstream signaling pathway which includes binding between Src and Na+ / K+-ATPase is disclosed. The Na+ / K+-ATPase / Src complex is a functional receptor for cardiotonic steroids such as ouabain. Also disclosed are Src inhibitors or activators which include either Na+ / K+-ATPase or Src that interfere with the interaction between the Na / K-ATPase and Src, act via a different mechanism from ATP analogues, and is pathway (Na+ / K+-ATPase) specific.
Owner:UNIVERSITY OF TOLEDO
Mammalian dihydroouabain-like factor and therapeutic compositions
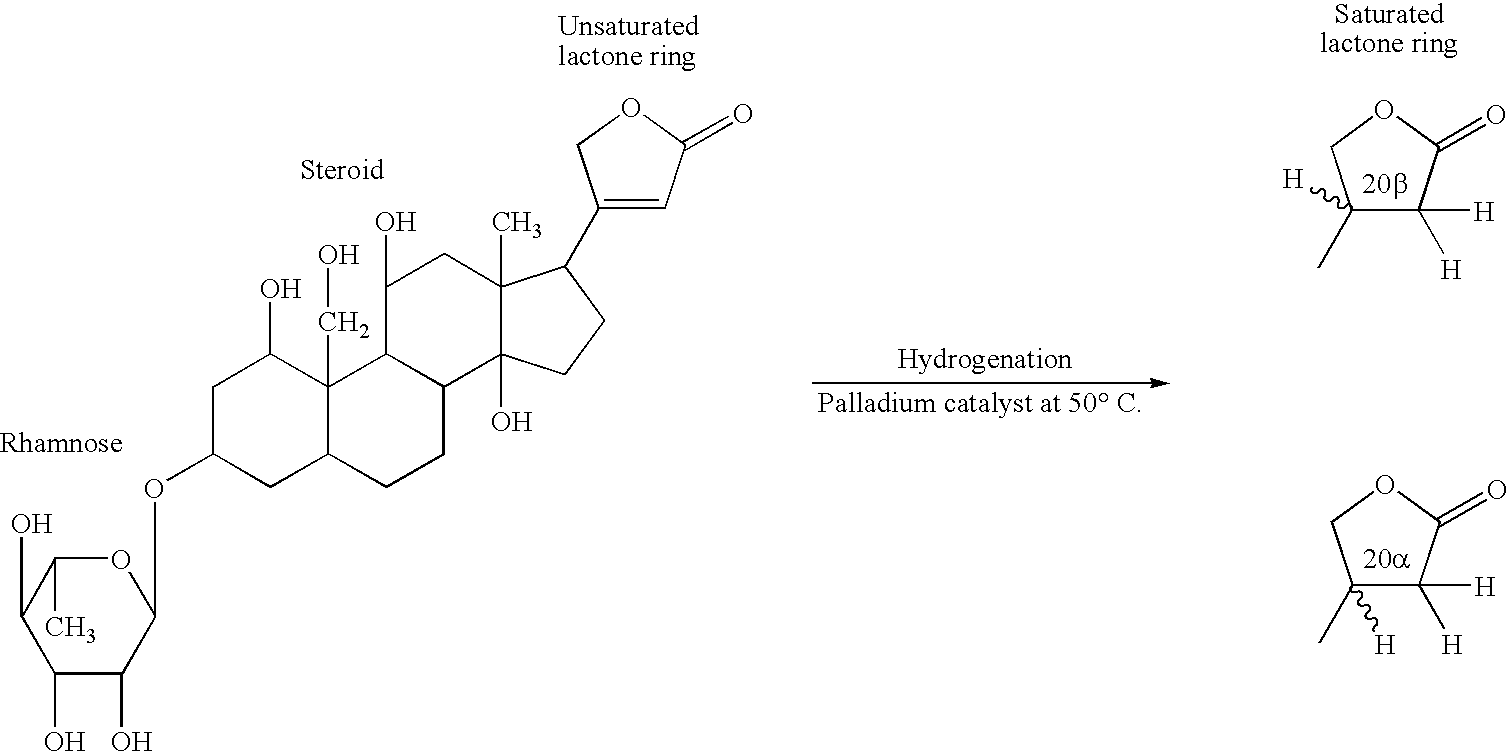
A novel mammalian dihydroouabain-like factor is disclosed which substantially fails to cross-react with mammalian ouabain-like factor (OLF) for binding to anti-OLF antibody, but cross-reacts with plant-related dihydroouabain (dho) for binding to anti-dho antibody, has maximal u.v. absorbance at 196 nm, has a non-peptidic, non-lipidic chemical structure and a fully hydrogenated lactone ring, has a concentration-dependent Na<+>,K<+>-ATPase (sodium pump) catalytic inhibitory activity which is 10-fold lower than OLF and 3-fold higher than plant-related dihydroouabain, and a high pressure liquid chromatography elution time about the same as dho. This factor is useful for therapy for congestive heart failure. An antibody and antibody fragments having affinity for mammalian Dh-OLF but not for OLF, and diagnostic and therapeutic methods comprise the antibody and means for quantifying the antibody and are useful for treating a condition caused by high level of OLF or Dh-OLF. Two isomers of plant-related dihydroouabain have been isolated. These compositions and methods are suitable for characterizing a variety of diseases and conditions associated with reduced sodium pump activity.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Method and composition for preventing and treating viral infections
ActiveUS20160367517A1Reducing replication ratePreserving cellular integrityOrganic active ingredientsAntiviralsATPasePenetration enhancer
A method and composition for treating viral infections using a combination of naturally occurring compounds is provided. The method includes administering to a patient at risk of or diagnosed with a viral infection a composition including therapeutically effective amounts of a helicase ATPase inhibitor, a sialidase enzyme inhibitor, and ICAM-1 inhibitor which each also down regulate the immune response. The composition may further include a permeation enhancer.
Owner:GLOBAL BIOLIFE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com