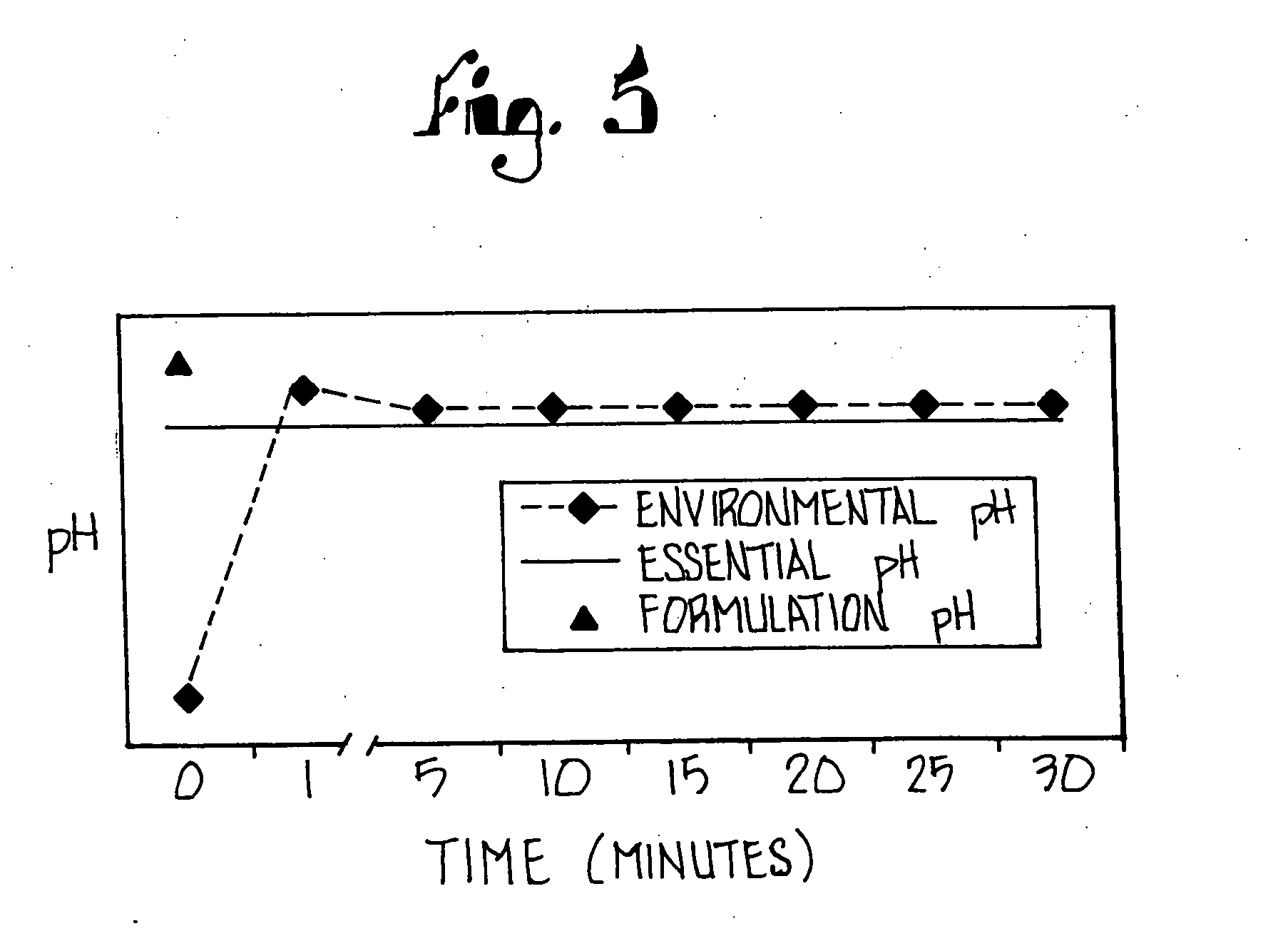
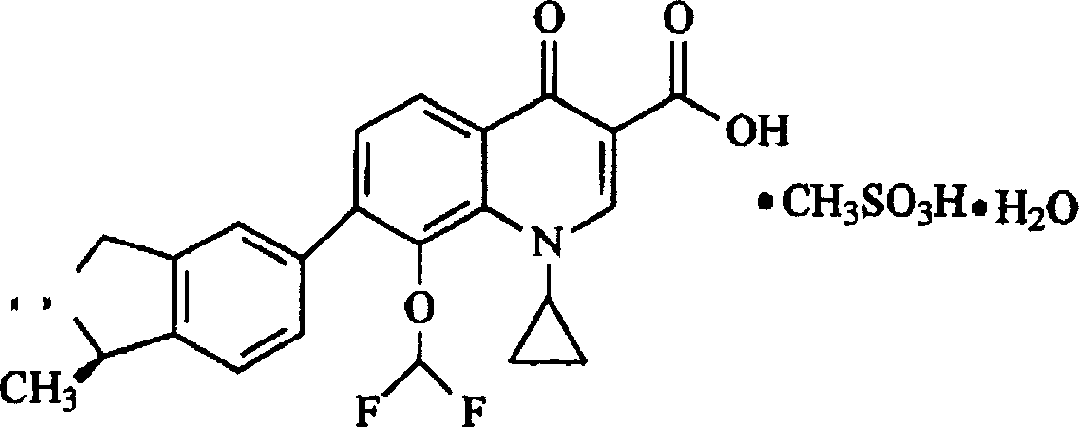
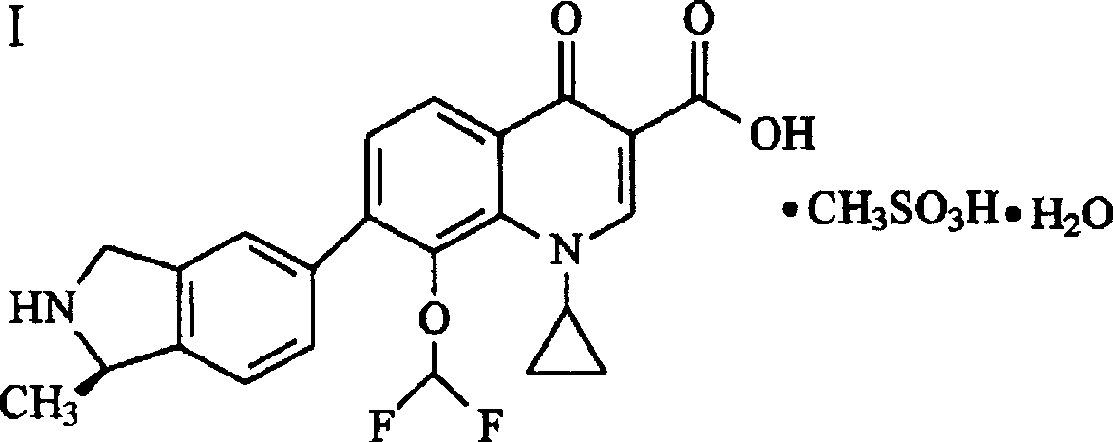
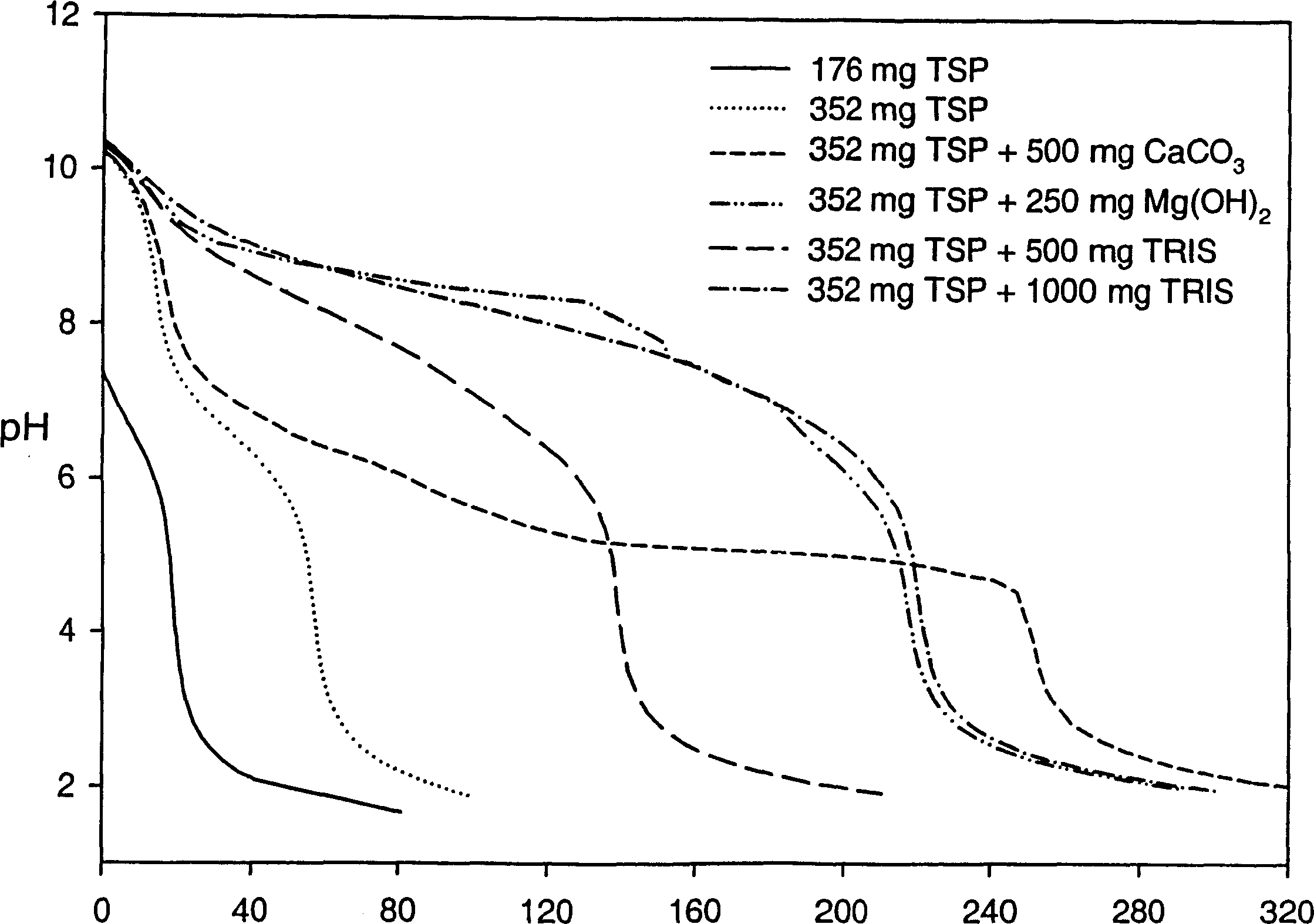
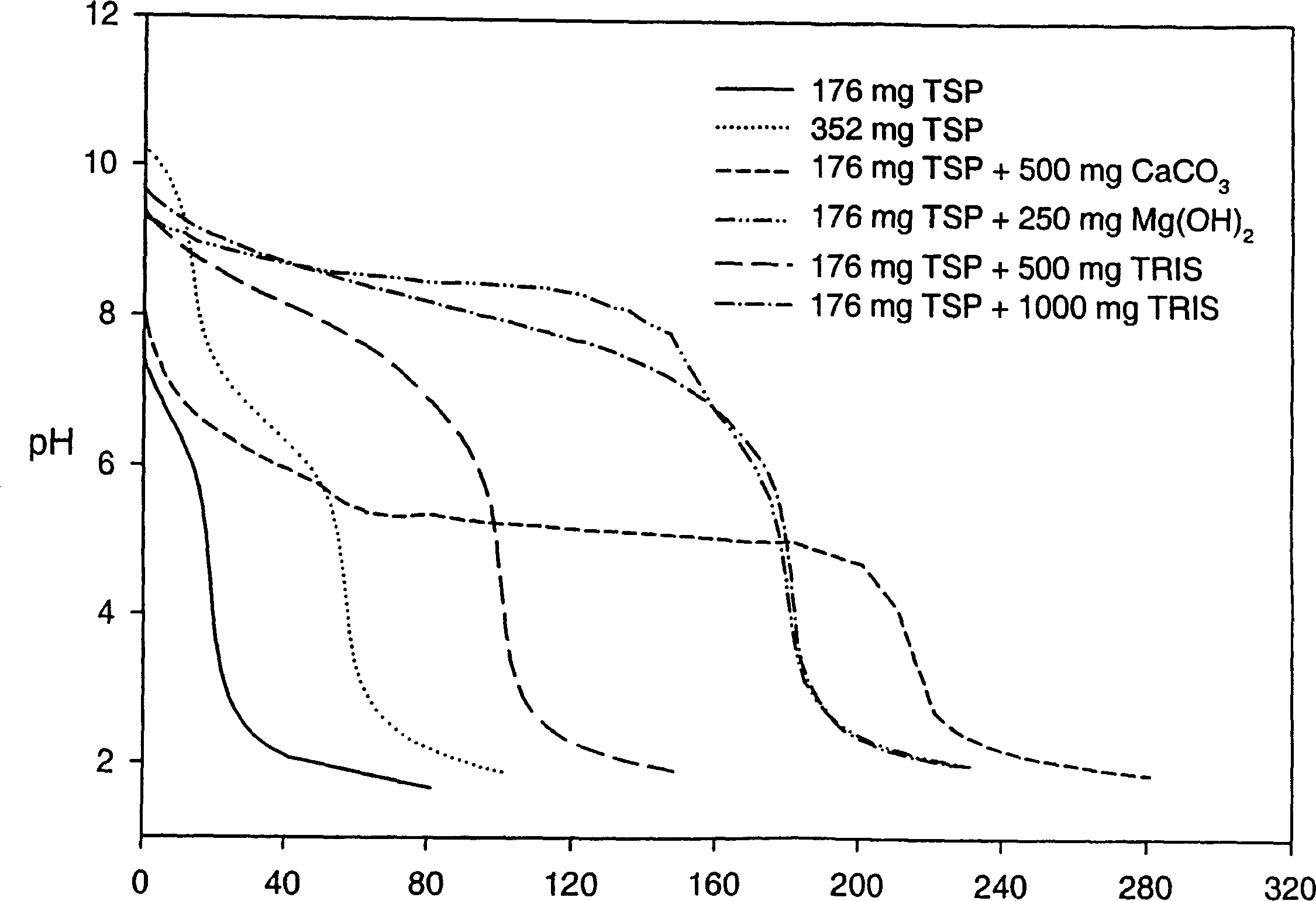
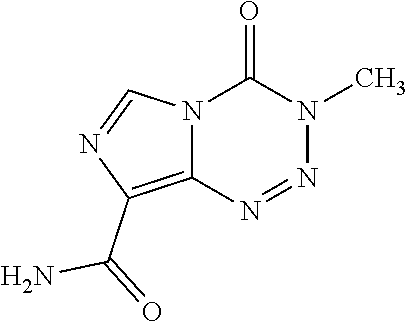
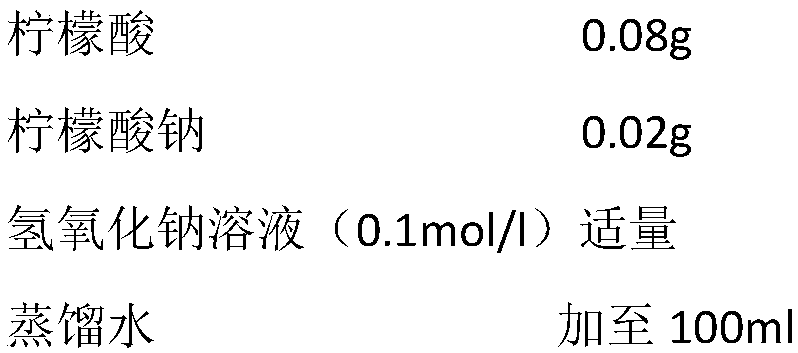
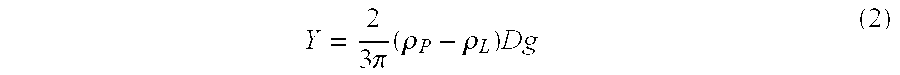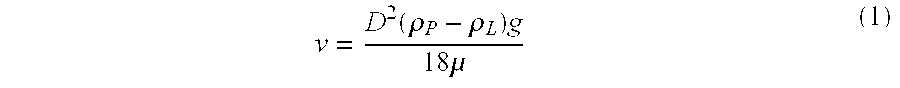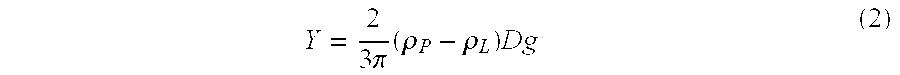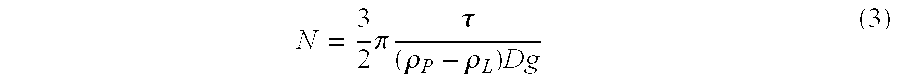Patents
Literature
118 results about "Oral suspensions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
: a suspension consisting of undissolved particles of one or more medicinal agents mixed with a liquid vehicle for oral administration.
Azithromycin dosage forms with reduced side effects
ActiveUS6984403B2Reduce gastrointestinal side effectsAntibacterial agentsPowder deliveryOral suspensionsGLYCERYL MONOBEHENATE
The present invention is related to an oral dosage form comprising an effective amount of an alkalizing agent and an azithromycin multiparticulate wherein said multiparticulate comprises azithromycin, a glyceride which comprises glyceryl monobehenate, glyceryl dibehenate, glyceryl tribehenate, or a mixture thereof and a poloxamer. Typically, the oral dosage form includes any suitable oral dosing means such as a powder for oral suspension, a unit dose packet or sachet, a tablet or a capsule.
Owner:PFIZER INC
Oral suspension of pharmaceutical substance
InactiveUS6184220B1Low viscosityHigh viscosityDispersion deliverySolution deliveryOral suspensionsMeloxicam
The present invention relates to orally administered suspensions of pharmaceutical active substances of the NSAID type, particularly the antirheumatic agent Meloxicam, which are stabilized by the addition of small amounts of highly dispersed silicon dioxide using high shear forces and adding small amounts of hydrophilic polymers to form a three-dimensional siloid structure, and a process for the preparation thereof.
Owner:BOEHRINGER INGELHEIM PHARM KG
Sustained release preparations
Disclosed are sustained release drug particles suitable for forming sustained release oral pharmaceutical compositions. The sustained release drug particles comprise a drug-ion exchange resin complex and a water-permeable, diffusion barrier surrounding at least a portion of the drug-ion exchange resin complex. The diffusion barrier comprises a film-forming polymer and is free or contains no substantial traces of organic solvent. Also disclosed are oral pharmaceutical compositions, for example, oral suspensions, comprising the sustained release drug particles, a method for the controlled administration of a drug to a patient, and a method for manufacturing the sustained release drug particles. The method of manufacturing involves the use of an aqueous coating composition comprising a water-permeable film-forming polymer such as ethylcellulose.
Owner:MALLINCKRODT INC
Oral suspension formulation
An aqueous pharmaceutical suspension for oral administration of a drug, which suspension maintains its content uniformity for prolonged period.
Owner:UNILAB PHARMATECH
Novel substituted benzimidazole dosage forms and method of using same
InactiveUS20050042304A1Easy to prepareImprove pharmacological activityPowder deliveryBiocideATPaseOral suspensions
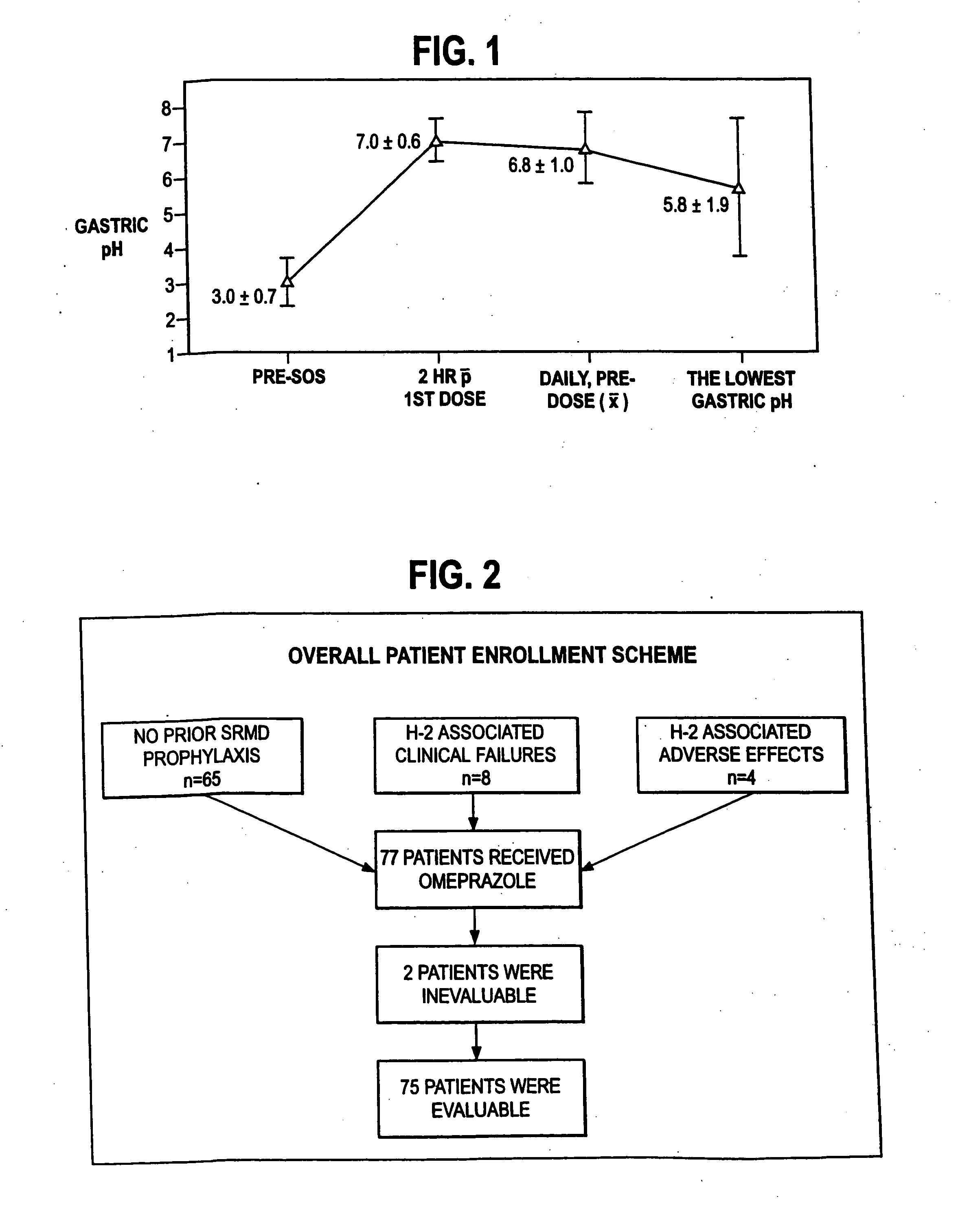
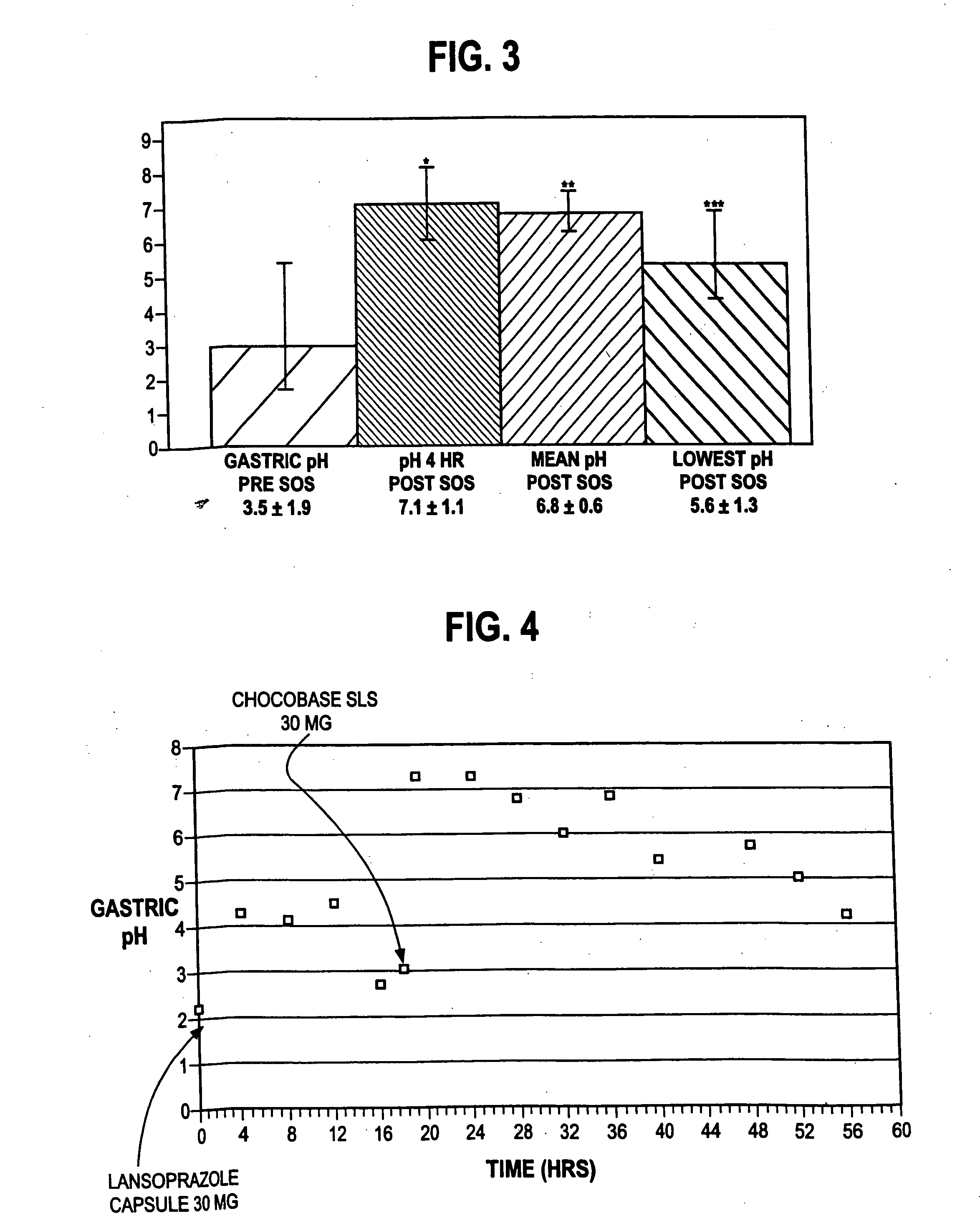
A method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor (PPI) in a pharmaceutically acceptable carrier. The present invention provides an oral solution / suspension comprising a proton pump inhibitor and at least one buffering agent. The PPI can be any substituted benzimidazole compound having H+,K+-ATPase inhibiting activity and being unstable to acid. Omeprazole and lansoprazole are the preferred PPIs for use in oral suspensions in concentrations of at least greater than 1.2 mg / ml and 0.3 mg, respectively. The liquid oral compositions can be further comprised of parietal cell activators, anti-foaming agents and / or flavoring agents. The inventive compositions can alternatively be formulated as a powder, tablet, suspension tablet, chewable tablet, capsule, effervescent powder, effervescent tablet, pellets and granules. Such dosage forms are advantageously devoid of any enteric coating or delayed or sustained-release delivery mechanisms, and comprise a PPI and at least one buffering agent to protect the PPI against acid degradation. Similar to the liquid dosage form, the dry forms can further include anti-foaming agents, parietal cell activators and flavoring agents. Kits utilizing the inventive dry dosage forms are also disclosed herein to provide for the easy preparation of a liquid composition from the dry forms. In accordance with the present invention, there is further provided a method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor in a pharmaceutically acceptable carrier and at least one buffering agent wherein the administering step comprises providing a patient with a single dose of the composition without requiring further administering of the buffering agent. Additionally, the present invention relates to a method for enhancing the pharmacological activity of an intravenously administered proton pump inhibitor in which at least one parietal cell activator is orally administered to the patient before, during or after the intravenous administration of the proton pump inhibitor.
Owner:UNIVERSITY OF MISSOURI
Oral suspension of prednisolone acetate
The present invention relates to novel oral suspension formulation comprising prednisolone acetate, a pharmaceutically acceptable vehicle and a thickening agent. The present invention further provides a method of treating patients in need of prednisolone with the novel formulation.
Owner:TARO PHARMA
Oral suspension formulation
InactiveUS20030191192A1Grittiness decreaseImprove bioavailabilityAntibacterial agentsBiocideOral suspensionsOral medication
An aqueous pharmaceutical suspension for oral administration of a drug, which suspension maintains its content uniformity for prolonged period.
Owner:UNILAB PHARMATECH
Ordered particle structures and methods of making same
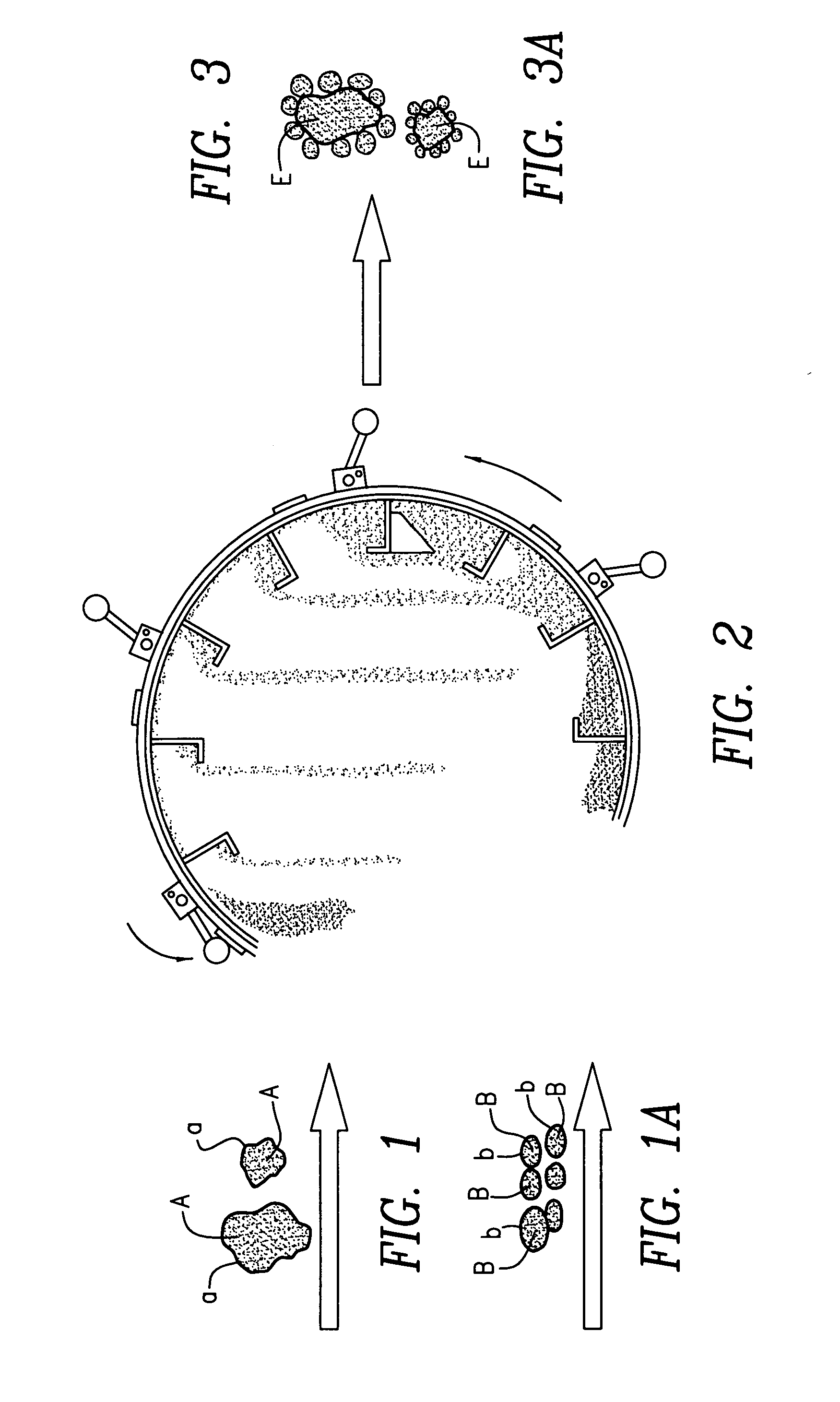
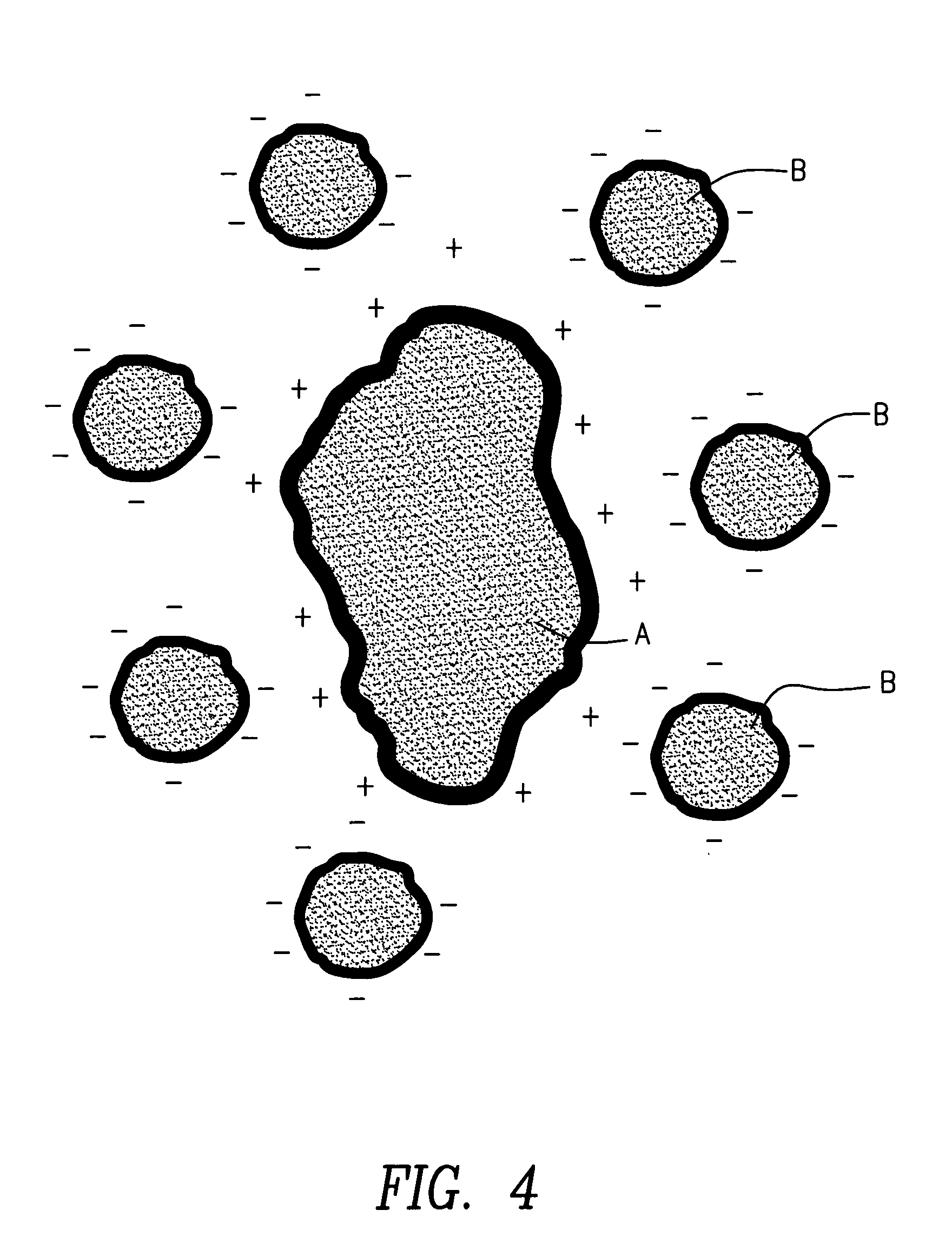
InactiveUS20050228075A1Increase loadCost effectiveSolid fuelsExplosive working-up apparatusOral suspensionsEnergetics
Techniques and methods of formation of ordered mixtures of particles by “clustering”. Clustering comprises local “structuring” consisting of a large “host” and smaller “guest” particles by various techniques. Small amounts of polymer are coated onto solid particles by various means. In one embodiment, an ordered mixture is created wherein the material that is of lesser quantity is of small particle size (the “B” particles) and the “A” particles are of larger size. The “B” particles are then coated onto a single A particle. By creating this ordered structure, each composite particle has the proper or stoichiometric amount of all ingredients. This dry composite material produced is appropriately used in various applications such as pharmaceutical formulations in the form of tablets, capsules, oral suspensions, inhalant, parenteral formulations and the like; energetics manufacture such as but not limited to explosives, propellants and pyrotechnics; agricultural products including but not limited to fertilizers, herbicides and pesticides; nutritional supplements and the like.
Owner:NEW JERSEY INSTITUTE OF TECHNOLOGY
Stable non-dihydrate azithromycin oral suspensions
This invention relates to a powder for oral suspension, and an oral suspension made therefrom, which comprises a n-propanol solvate of non-dihydrate azithromycin and an azithromycin conversion stabilizing excipient, wherein said excipient reduces the conversion of the form of azithromycin, when placed in suspension, to another form of azithromycin.This invention also relates to an oral suspension which comprises a n-propanol solvate of non-dihydrate azithromycin and an aqueous vehicle.
Owner:PFIZER INC +1
Azathioprine Oral Suspensions and Methods of Use
Compositions of azathioprine oral suspensions are disclosed. Disclosed azathioprine oral suspensions may be used to administer azathioprine to subjects such as children and geriatric patients that may have difficulty in swallowing solid dosage forms. The disclosed azathioprine oral suspension may be used for treating autoimmune diseases such as rheumatoid arthritis, pemphigus, Behcet's disease, autoimmune hepatitis, and inflammatory bowel disease, among others. According to an embodiment, an aqueous or non-aqueous vehicle may be used for azathioprine oral suspension. According to a different embodiment, sugar free azathioprine oral suspension may also be produced.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Palatable oral suspension and method
InactiveUS7175856B2Reduce and minimize solubilityReduces and masks bitter tasteAntibacterial agentsBiocideOral suspensionsSolubility
A drug formulation in the form of a dry powder is provided which when mixed with water forms a palatable oral suspension substantially free of bitter taste, the dry powder being formed of a drug, preferably des-quinolone, which in solution has a bitter taste, and a pH modifying agent which is preferably an alkaline material such as L-arginine, where upon mixing the dry-powder in water causes the drug to have reduced solubility or precipitate in-situ to form a palatable oral suspension essentially free of bitter taste.An oral suspension, methods for making same and a method for masking the bitter taste of drugs employing one or more pH modifying agents are also provided.
Owner:TOYAMA CHEM CO LTD
Oral suspension of prednisolone acetate
Owner:TARO PHARMA
Azithromycin dosage forms with reduced side effects
ActiveUS20050123627A1Reduce gastrointestinal side effectsAntibacterial agentsBiocideOral suspensionsGLYCERYL MONOBEHENATE
An oral dosage form comprising azithromycin and an effective amount of an alkalizing agent. Preferably, said oral dosage form comprises an effective amount of an alkalizing agent and an azithromycin multiparticulate wherein said multiparticulate comprises azithromycin, a mixture of glyceryl monobehenate, glyceryl dibehenate and glyceryl tribehenate, and a poloxamer. Typically, the oral dosage form includes any suitable oral dosing means such as a powder for oral suspension, a unit dose packet or sachet, a tablet or a capsule. Additionally disclosed is an oral suspension comprising azithromycin, an effective amount of an alkalizing agent and a vehicle. Preferably, the azithromycin is in multiparticulate form wherein said multiparticulate comprises azithromycin, a mixture of glyceryl monobehenate, glyceryl dibehenate and glyceryl tribehenate, and a poloxamer. Also disclosed is a method for reducing gastrointestinal side effects, associated with administering azithromycin to a mammal, comprising contiguously administering azithromycin and an effective amount of alkalizing agent to said mammal wherein the frequency of gastrointestinal side effects is lower than that experienced by administering an equal dose of azithromycin without said alkalizing agent. Further disclosed is a method of treating a bacterial or protozoal infection in a mammal in need thereof comprising contiguously administering to said mammal a single dose of an oral dosage form wherein said oral dosage form comprises azithromycin and an effective amount of an alkalizing agent. Additionally disclosed are azithromycin multiparticulates comprising azithromycin, a surfactant; and a pharmaceutically acceptable carrier.
Owner:PFIZER INC
Palatable oral suspension and method
A drug formulation in the form of a dry powder is provided which when mixed with water forms a palatable oral suspension substantially free of bitter taste, the dry powder being formed of a drug, preferably des-quinolone, which in solution has a bitter taste, and a pH modifying agent which is preferably an alkaline material such as L-arginine, where upon mixing the dry-powder in water causes the drug to have reduced solubility or precipitate in-situ to form a palatable oral suspension essentially free of bitter taste. An oral suspension, methods for making same and a method for masking the bitter taste of drugs employing one or more pH modifying agents are also provided.
Owner:TOYAMA CHEM CO LTD
Antibiotic compositions
An oral suspension comprising (a) an antibiotic composition which comprises coated micropellets and optionally one or more excipients, (b) additional excipients, and (c) a solvent, wherein said coated micropellets comprise (i) a core comprising at least one antibiotic; (ii) an inner coating comprising at least one cellulose polymer which is not an enteric coating polymer; and (iii) an outer coating comprising at least one enteric coating polymer, wherein said coated micropellets have a mean particle size of about 100 μm to about 650 μm. The oral suspension of the invention is characterized by a lack of bitter taste.
Owner:SANDOZ AG
Oral lurasidone suspension and preparation method thereof
InactiveCN104606133AQuality improvementGreat tasteOrganic active ingredientsNervous disorderOral suspensionsPreservative
The invention discloses an oral lurasidone suspension and a preparation method thereof. The lurasidone suspension is a pharmaceutical composition which comprises lurasidone or salt thereof, a suspending aid, a wetting agent, a pH regulator, a preservative, a sweetening agent and a corrigent, wherein the particle size range of the lurasidone or salt thereof is 0.1-20 microns. The prepared oral suspension has the characteristics of being stable in quality and favorable in taste, facilitates dose distribution, and is beneficial to the acceptance of schizophrenia patients, simple in preparation method and suitable for industrial production.
Owner:AVENTIS PHARMA HAINAN
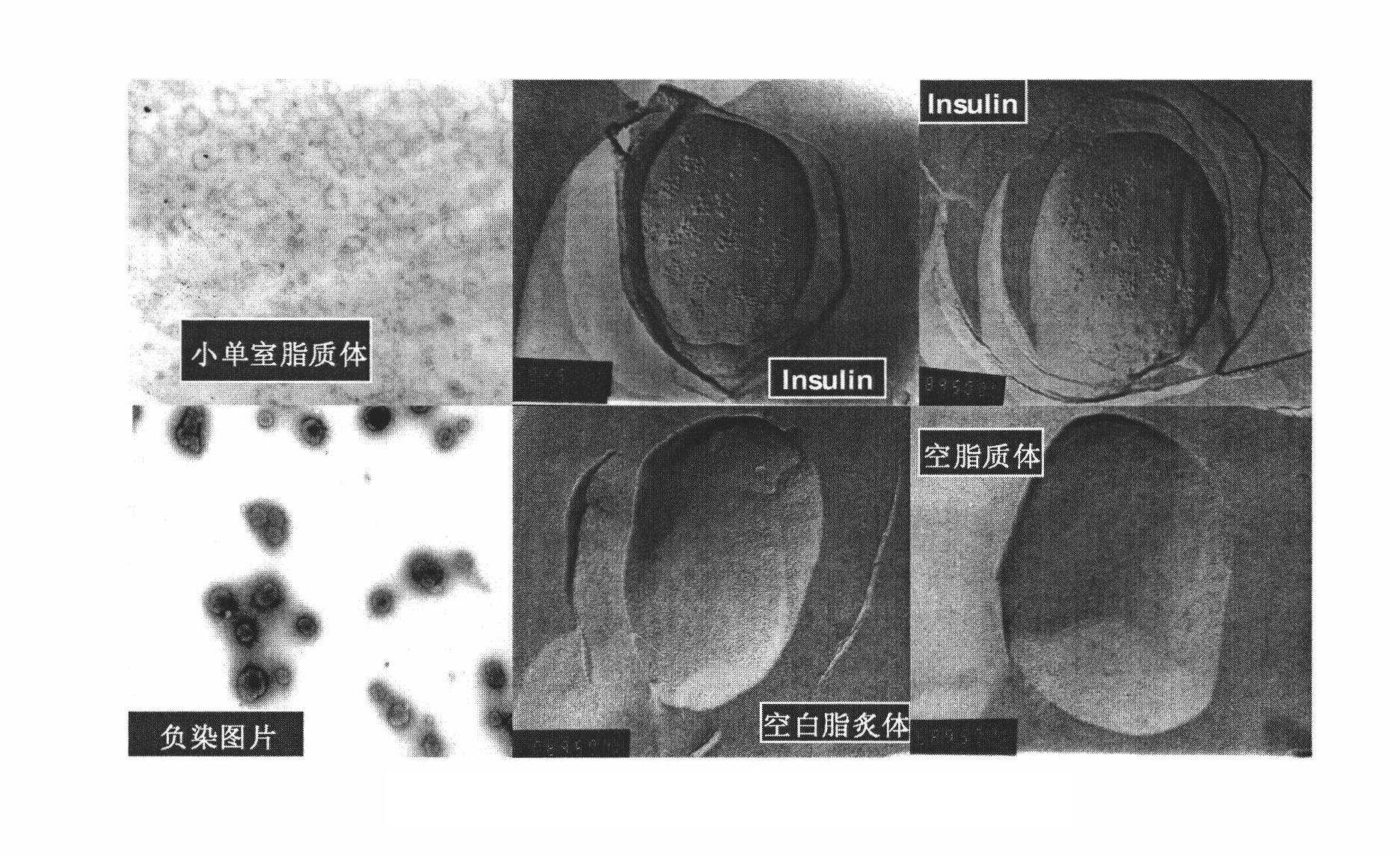
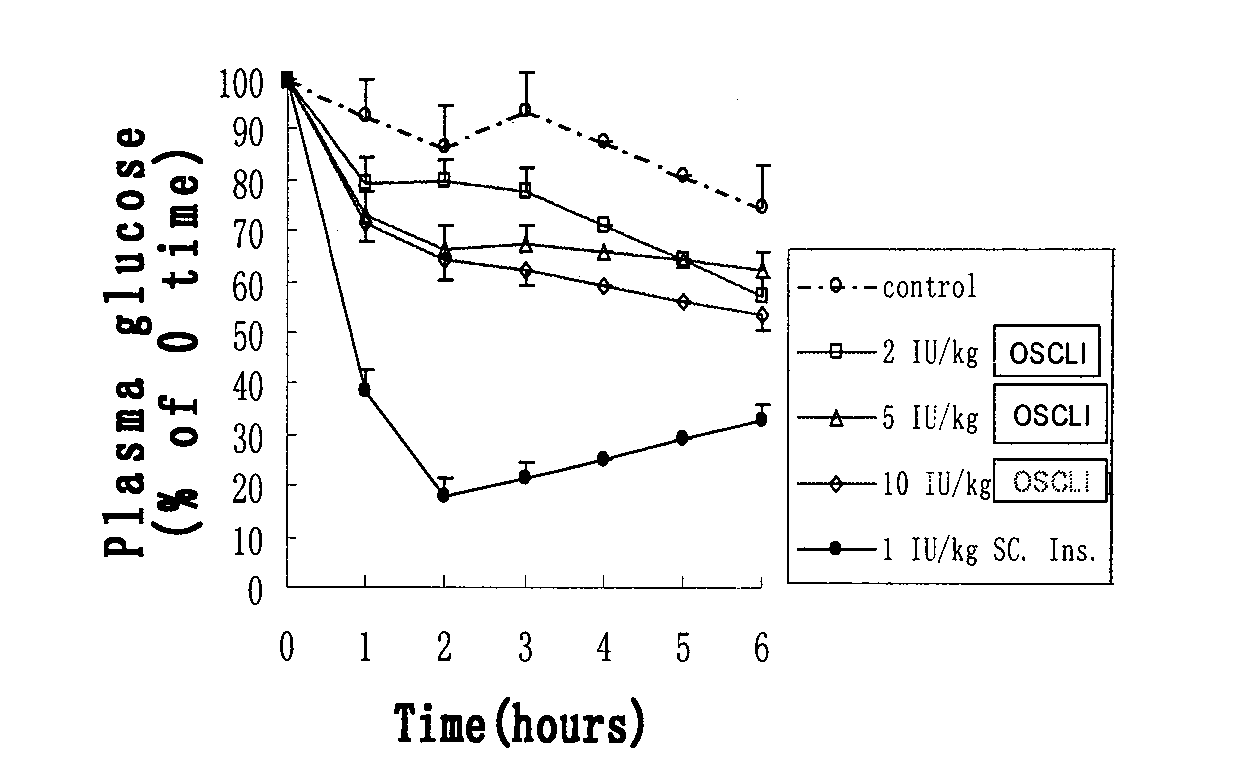
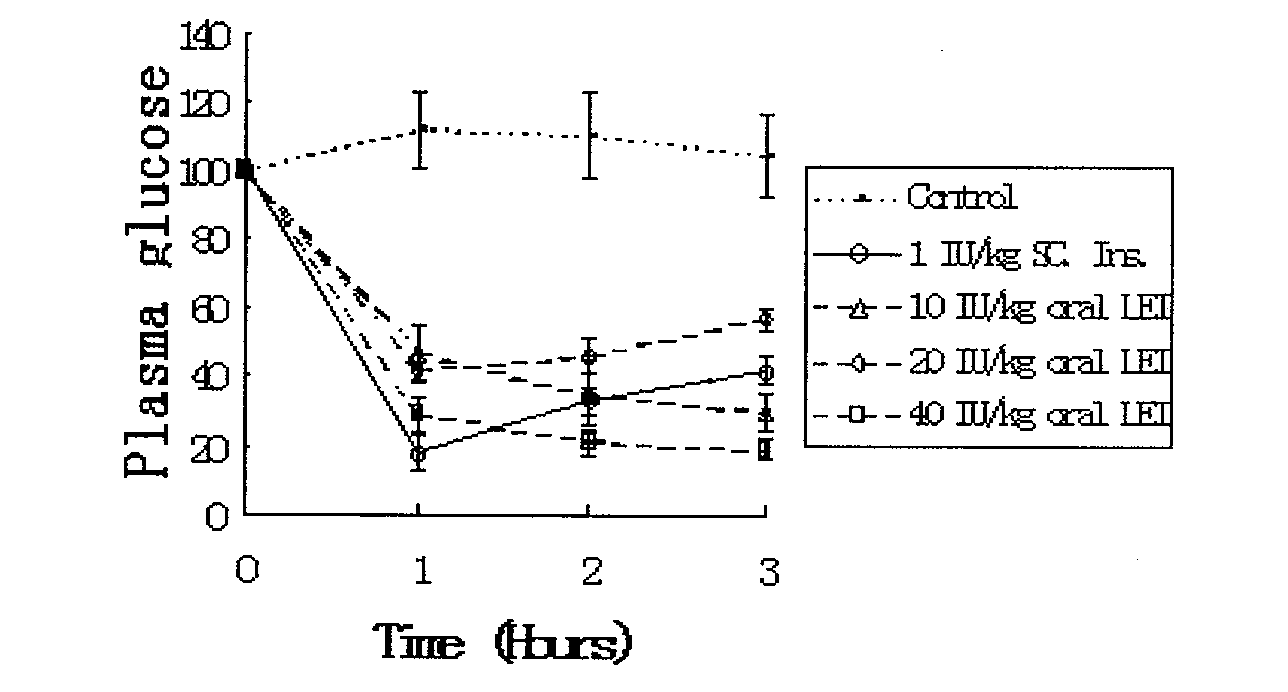
Oral suspension of liposome-encapsulated insulin lyophilized preparation and preparation process thereof
InactiveCN102144968AMaintain a healthy weightGood comprehensive curative effectPeptide/protein ingredientsMetabolism disorderOral suspensionsLiver functions
The invention relates to an oral suspension of a liposome-encapsulated insulin lyophilized preparation, named as O-SCULI. The O-SCULI contains lecithin, cholesterol, polyglycol aliphatic acid ester, vitamin E, insulin, water, sodium chloride and phosphate buffer. The invention also provides a two-step process of the O-SCULI, comprising (1) a dewatering / moistening control process: preparing a liposome-encapsulated insulin lyophilized preparation and SCLI so that the encapsulation rate of the insulin is increased to 80 percent by liposome amalgamation; and (2) a respective constant volume / stepped liquefaction process: preparing the SCLI into an O-SCULI oral suspension, preventing the SCLI liposome from leaking and keeping the constant encapsulation rate of the insulin. After a patient acutely takes the O-SCULI, the liposome insulin effectively enters the intestine-hepatic portal vein, the liver and the blood circulation to reduce blood sugar, blood fatty acids and blood ketone with high utilization degree. After a patient continuously orallly takes the oral suspension, the saccharification of hematoglobin, triglyceride and, aminotransferase and the oxidative stress are reduced, the structural damage of liver cells is recovered, and the comprehensive curative effects of diabetes treatment by using various liver functions are improved. The invention also opens up a new path for non-injection administration of active polypeptide protein, such as cytochrome C, albumin, interferon, and the like.
Owner:刘树森
Composition and preparation method and preparation thereof
InactiveCN110812365AImprove stabilityControlled release rateOrganic active ingredientsSenses disorderOral suspensionsCaplet Dosage Form
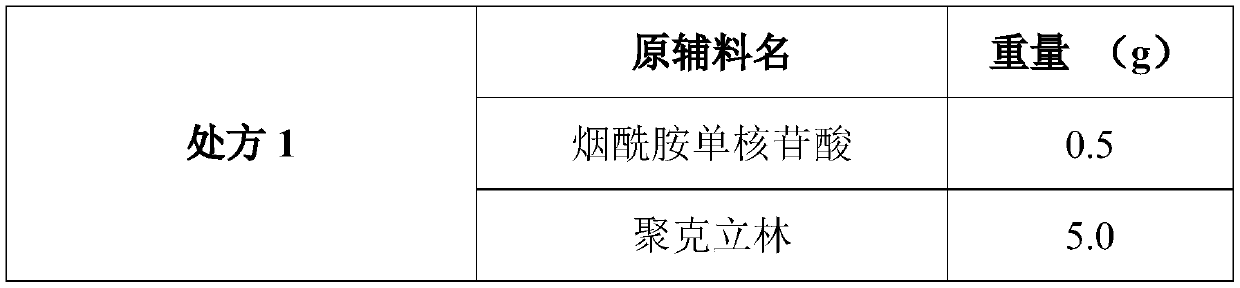
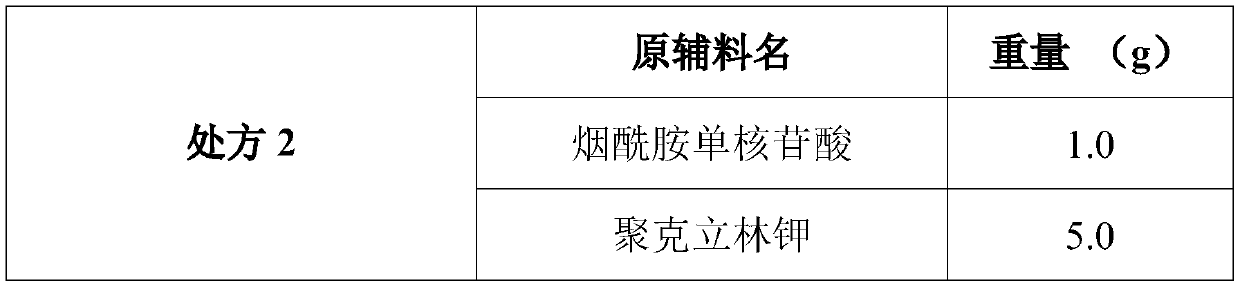
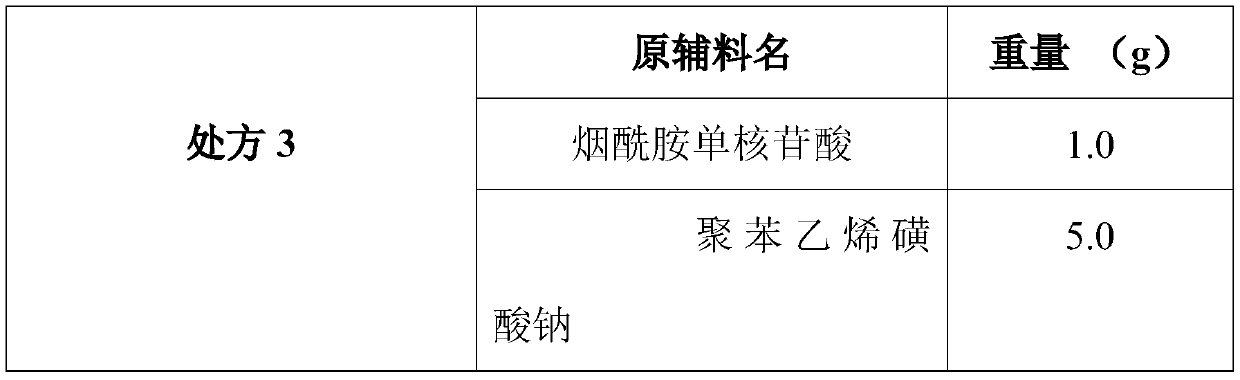
The invention discloses a composition as well as a preparation method and a preparation thereof. The composition of the present invention includes nicotinamide mononucleotide, an ion exchange resin, acoating material, and a plasticizer. The preparation method comprises the following steps: preparing the drug-loaded resin particles, and coating the drug-loaded resin particles. The coated drug-loaded resin particles are mixed with other auxiliary materials to prepare corresponding dosage forms, such as oral suspension, tablets, capsules, granules, cream, ointment, facial masks and the like. Thecomposition is simple in production process, easy to amplify and produce, capable of effectively improving the stability of nicotinamide mononucleotide, accurate in dosage, lasting in effect and stable in curative effect.
Owner:明特奇点医疗科技(北京)有限公司
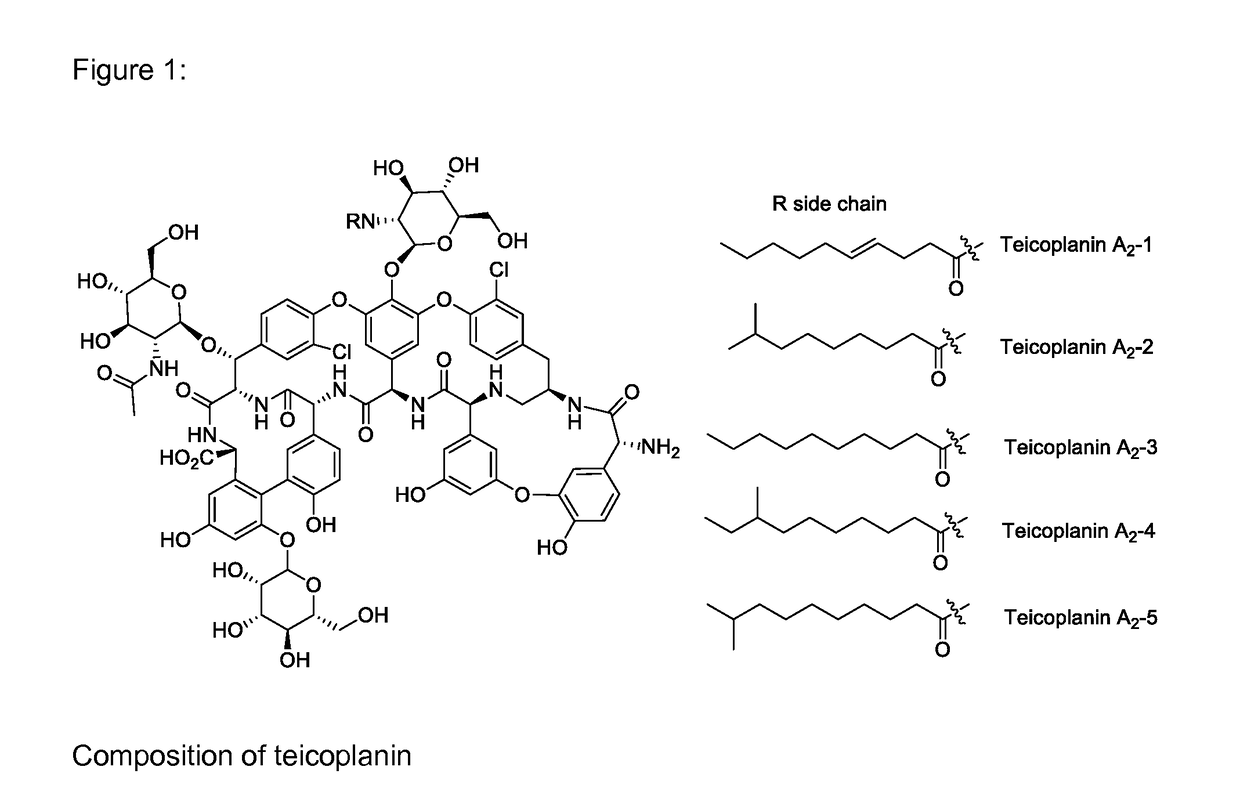
Methods for producing an oralsuspension of teichoplanin or teichoplanin analogs
InactiveUS20170065671A1Great tasteImprove stabilityPowder deliveryDispersion deliveryOralSuspensionChemistry
A stable suspension of teicoplanin and one or more pharmaceutically acceptable additives and method for preparation wherein the teicoplanin is mixed with an aqueous or non aqueous suspension base and pharmaceutically acceptable additives and wherein the teicoplanin is milled to obtain a homogeneously dispersed suspension.
Owner:MAHER ILLYA KEITH
Omeprazole enteric dry suspension and preparation method thereof
InactiveCN105726512AEvenly distributedLess prone to burstOrganic active ingredientsDispersion deliveryOral suspensionsMicrosphere
The invention provides an omeprazole enteric dry suspension and a preparation method thereof, and belongs to the technical field of medicine.The enteric dry suspension is prepared from, by weight, 40%-49% of omeprazole enteric microspheres, 0%-1% of wetting agent, 45%-58% of flavoring agent and 1%-5% of suspending aid.According to the omeprazole enteric dry suspension and the preparation method thereof, a hot-melting spraying method is adopted to prepare omeprazole and a sustained-release skeleton material into sustained-release microspheres A, the sustained-release microspheres A are each sequentially wrapped with an isolation coating layer and an enteric coating layer, the wrapped microspheres A are mixed with the flavoring agent, the wetting agent and the suspending aid, and then the omeprazole enteric dry suspension is obtained; the prepared omeprazole enteric dry suspension is distributed uniformly in the intestinal tract and is not prone to cause initial burst release; compared with tablets and capsules, the omeprazole enteric dry suspension is good in stability, fractional doses are convenient, an oral suspension is formed by adding water to the dry suspension to enable the dry suspension to be dissolved, absorption is good, the efficacy is quick to achieve, the bioavailability is good, the mouthfeel is good, the dry suspension is particularly suitable for patients with dysphagia, specially for child patients and elderly patients to take, compliance of the patients is improved, and the preparation method is simple and suitable for industrial production and application.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
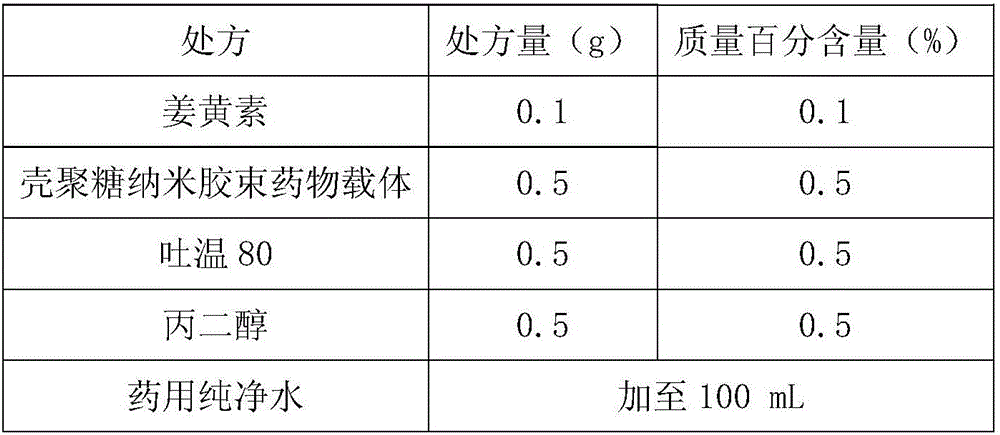
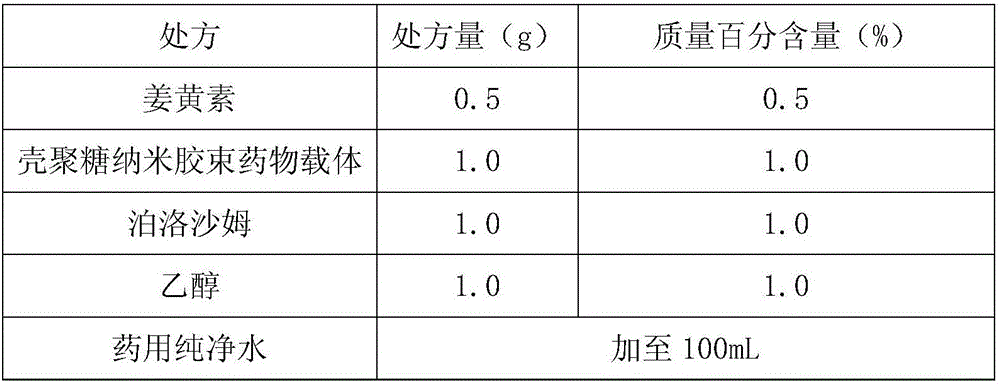
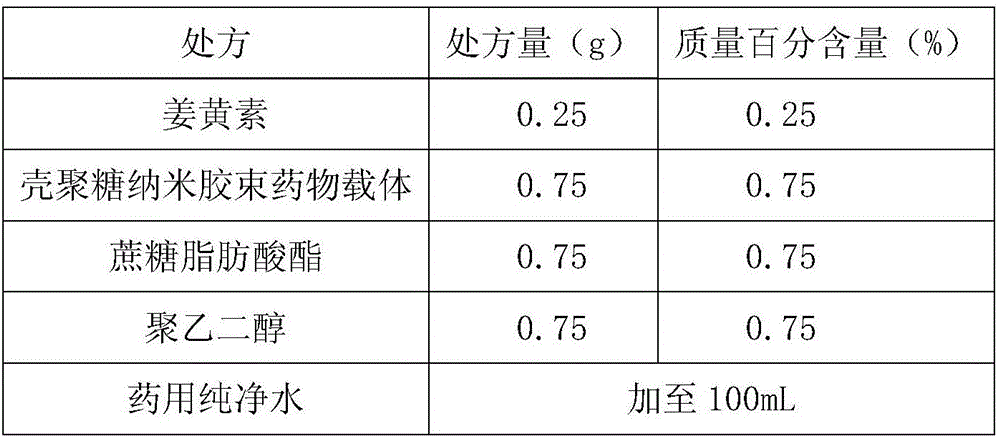
Curcumin nano-micelle oral suspension, gel and application thereof
InactiveCN106619511AImprove bioavailabilityImprove stabilityAntibacterial agentsDispersion deliveryOral suspensionsSecondary hyperlipidemia
The invention provides curcumin nano-micelle oral suspension. The curcumin nano-micelle oral suspension comprises the following active ingredients: curcumin, a chitosan nano-micelle drug carrier, a surfactant, a cosurfactant and purified water. The invention further provides curcumin nano-micelle gel. The curcumin nano-micelle oral suspension and the curcumin nano-micelle gel have good drug stability, safety and effectiveness, and can effectively treat diseases such as hyperlipidemia, sinusitis, gingivitis and cervicitis.
Owner:GUANGZHOU BAIEN BIOTECH CO LTD
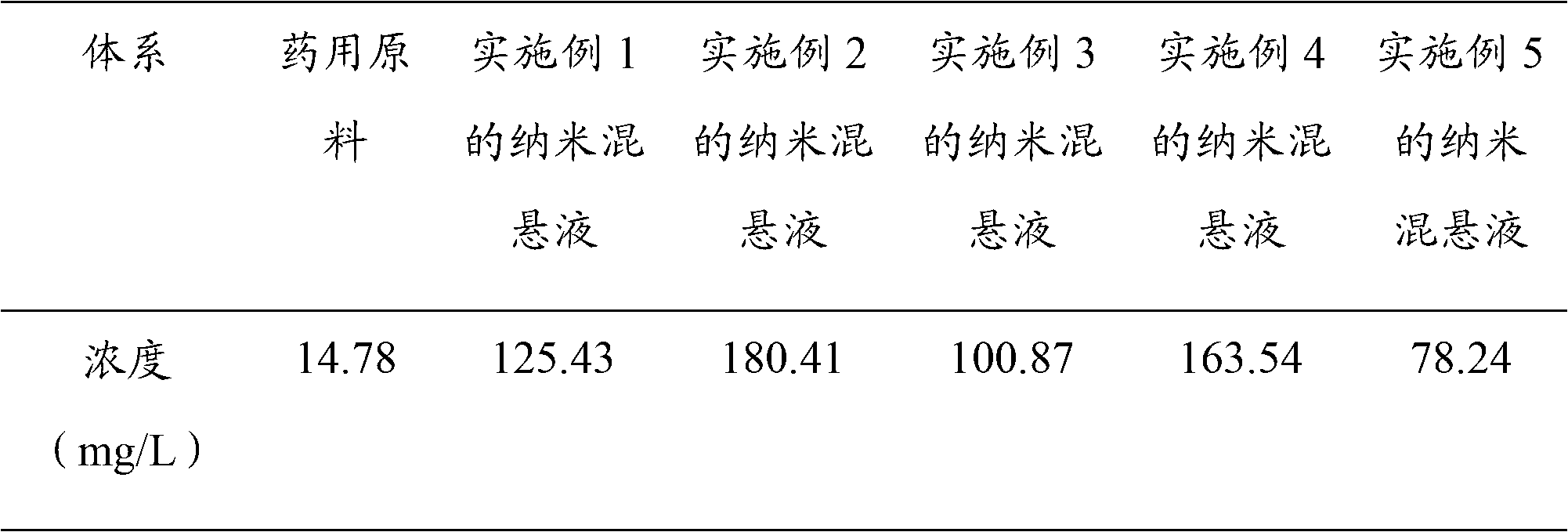
Scutellarin nanosuspension and preparation method thereof
InactiveCN103006556AImprove solubilityImprove oral bioavailabilityOrganic active ingredientsSolution deliveryOral suspensionsSolubility
The invention belongs to the technical field of pharmaceutical preparations, and relates to a scutellarin nanosuspension and a preparation method thereof. The scutellarin nanosuspension is prepared from a stabilizer and scutellarin in a weight ratio of (1:1)-(10:1). The preparation method is implemented through dissolving the scutellarin into an alkaline solution and then adding the obtained mixture into an acid solution containing the stabilizer, so that due to the solubility difference of the scutellarin in the acid solution and the alkaline solution, the scutellarin is oversaturated and then crystallized by separating; and carrying out homogenization on the obtained product by using a high-pressure micro jet homogenizer so as to obtain the scutellarin nanosuspension, wherein the particle size of the scutellarin nanosuspension is 100-500 nm, and the polydispersity index is 0.1-0.5. The scutellarin nanosuspension and preparation method thereof disclosed by the invention have the significant advantages that the nanosuspension improves the solubility of the scutellarin and increases the oral bioavailability of the scutellarin; the scutellarin nanosuspension can be solidified through freeze drying or spray drying, and applied to dosage forms such as tablets, capsules, granules, oral suspensions and the like; and the scutellarin nanosuspension is simple in preparation process, and convenient for industrial production.
Owner:MACAU UNIV OF SCI & TECH
Avian decoquinate oral suspension and preparation method thereof
InactiveCN102008435AOvercome the shortcomings of uneven mixing and insufficient absorptionEasy to useSolution deliveryAntiparasitic agentsOral suspensionsOrganic solvent
The invention relates to an avian decoquinate oral suspension and a preparation method thereof, belonging to the technical field of veterinary drug preparations. The avian decoquinate oral suspension comprises the following components per 100ml: 5-15g of decoquinate, 0.5-10g of surfactant, 0.1-2g of binding agent, 0.1-2g of stabilizer and 100ml of volume-metered solvent. The avian decoquinate oral suspension overcomes the defects of nonuniform stirring of premixed preparation and insufficient absorption, and can be clinically used only by dilution so that the use method is simple and convenient; and in addition, the organic solvent of the avian decoquinate oral suspension is low in content, the cost of unit preparation is effectively reduced, and the problems of high preparation cost and difficult popularization and application of the traditional preparation are solved.
Owner:QILU ANIMAL HEALTH PROD
Oral suspension comprising telmisartan
ActiveUS20140364473A1Improve subjective overall impressionIncrease contact timeOrganic active ingredientsBiocideOral suspensionsAlcohol sugars
A pharmaceutical solution with a pH value of 10 or higher contains an angiotensin II receptor antagonist, where one or more sugar alcohols are present up to a total concentration of 40 wt. % to 70 wt. %.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Azithromycin dosage forms with reduced side effects
An oral dosage form comprising azithromycin and an effective amount of a basifying agent. Preferably, the oral dosage form comprises an effective amount of a basifying agent and azithromycin multiparticulates, wherein the multiparticulates comprise azithromycin, a mixture of glyceryl monobehenate, glyceryl dibehenate, and glyceryl tribehenate, and porol sham. Typically, such oral dosage forms include any form suitable for oral administration such as powders for oral suspensions, unit dose sachets or sachets, tablets or capsules. Also disclosed is an oral suspension comprising azithromycin, an effective amount of a basifying agent, and a vehicle. Preferably, the azithromycin is in the form of multiparticulates, wherein the multiparticulates comprise azithromycin, a mixture of glyceryl monobehenate, glyceryl dibehenate and glyceryl tribehenate, and a poloxamer. Also disclosed is a method of reducing gastrointestinal side effects associated with administration of azithromycin to a mammal, comprising continuously administering to said mammal azithromycin and an effective amount of an alkalizing agent, wherein the incidence of gastrointestinal side effects is lower than that of an equivalent dose of azithromycin Incidence without administration of the basifying agent. Also disclosed is a method of treating a mammal in need against a bacterial or protozoan infection comprising sequentially administering to said mammal a single oral dosage form, wherein said oral dosage form comprises azithromycin and an effective amount of a basifying agent. Also disclosed are azithromycin multiparticulates comprising azithromycin, a surfactant; and a pharmaceutically acceptable carrier.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Lipoic acid pellets
Lipoic acid pellets are described, obtained from inert cores externally coated with lipoic acid. The so obtained active cores are coated with a first layer of insulating polymeric material and then with a polymeric coat that is insoluble at the gastric pH. Pellet are then formulated pharmaceutically, for instance in jelly capsules or controlled release capsules or as oral suspensions, dispersible powders, sachets, etc.
Owner:ADARE PHARM SRL +1
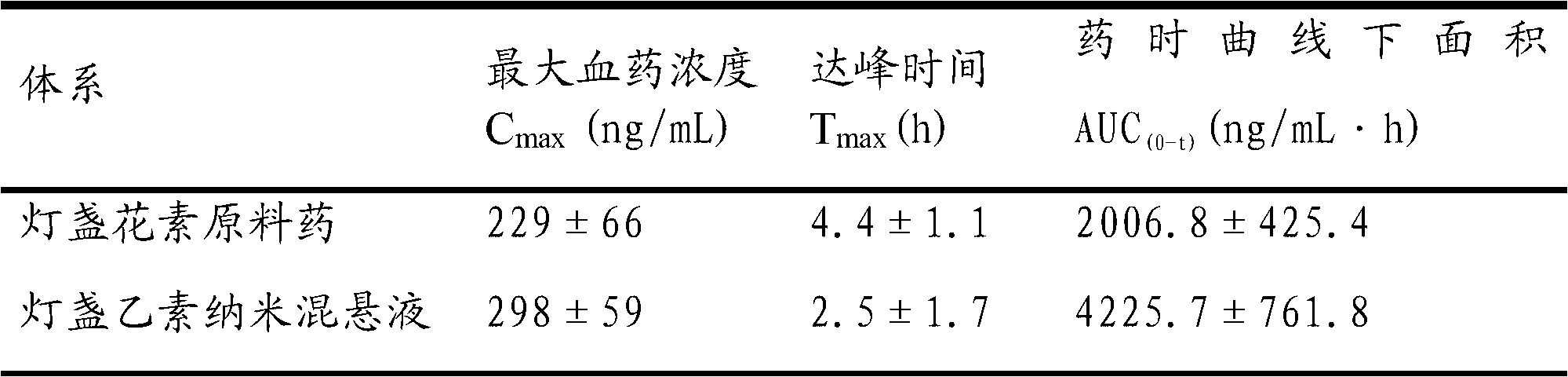
Stable non-dihydrate azithromycin oral suspensions
InactiveCN1822858ALow viscosityHigh viscosityOrganic active ingredientsMacromolecular non-active ingredientsOral suspensionsOralSuspension
This invention relates to a powder for oral suspension, and an oral suspension made there from, which comprises non-dihydrate azithromycin and an azithromycin conversion stabilizing excipient, wherein said excipient reduces the conversion of the form of azithromycin, when placed in suspension, to another form of azithromycin. This invention further relates to a method for reducing the conversion of a form of non-dihydrate azithromycin, in an oral suspension, by adding a surface tension reducing excipient that reduces the surface tension of the aqueous vehicle. Furthermore, this invention relates to a method for reducing the conversion of a non-dihydrate azithromycin, in an unflavored oral suspension, by raising the viscosity of the oral suspension, and in a flavored oral suspension by lowering the viscosity of the oral suspension.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Pharmaceutical composition of oral suspension of anti-neoplastic alkylating agents
ActiveUS11147810B2Improve palatabilityImprove stabilityOrganic active ingredientsDispersion deliveryAlkylating antineoplastic agentOral suspensions
The present invention provides pharmaceutical composition of antineoplastic alkylating agent in oral suspension dosage form. The oral suspension composition comprises of alkylating agent with other pharmaceutical excipients such as vehicle, preservative, antioxidant, suspending agent, surfactant, sweetener and flavouring agent. The present invention is an oral suspension having improved stability and palatability. The present invention also provides oral solution with flavor that has masked bitter taste of the drug. Further, the present invention also provides a process for preparation thereof.
Owner:FTF PHARMA LTD
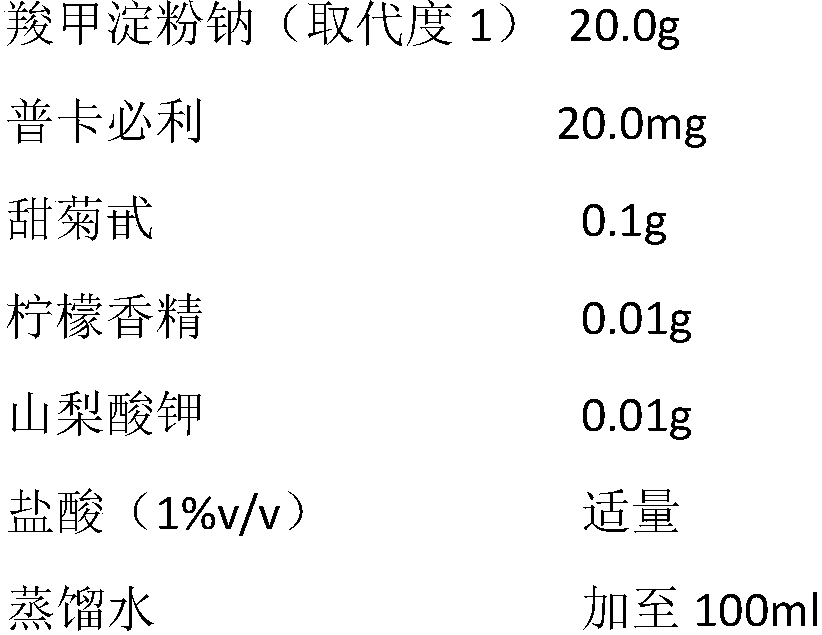
Pharmaceutical composition for treating constipation and application thereof
InactiveCN108904808AIncrease the average daily defecation volumeIncrease the number ofElcosanoid active ingredientsPeptide/protein ingredientsOral suspensionsMotility
The invention discloses the combined application of a pharmaceutical composition composed of water-soluble starch salt modified by carboxylic acid groups and gastrointestinal motility promoting drugsor secretion promoting drugs in the prevention and treatment of constipation, the pharmaceutical composition can be suitable for various functional constipation, especially has good curative effects on old age refractory constipation, chronic constipation, habitual constipation, drug-induced constipation and the like, has slight adverse reactions, and can be suitable for various crowds. The representative drug of the water-soluble starch salt modified by the carboxylic acid groups is sodium carboxymethyl starch, and the gastrointestinal motility promoting drugs or secretion promoting drugs areselected from dopamine receptor antagonist, 5-HT4 receptor agonist, motilin receptor agonist, chloridion channel activator, guanylate cyclase agonist. The two components are combined according to a suitable weight ratio and can be prepared into any oral pharmaceutical dosage form including oral solution, oral suspension, granule, powder, tablet, capsule and the like.
Owner:XIAN LIBANG PHARMA TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com