Patents
Literature
30 results about "Gastric ph" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gastric acid secretion inhibiting composition
InactiveUS20080031941A1Quick and lasting reliefAntibacterial agentsBiocideHelicobacter pyloriGastric ph
An oral pharmaceutical dosage form comprises pharmacologically effective amounts of an acid susceptible proton pump inhibitor or a salt thereof, an H2 receptor antagonist or a salt thereof and a pharmaceutically acceptable carrier. The dosage form is capable of raising gastric pH to above 4 within two hours after administration and to keep it at that level for at least 4 hours. Also disclosed is a method of manufacture of the dosage form, its use in treating dyspepsia and infection by Helicobacter pylori, and a method of treating disorders associated with gastric acid secretion.
Owner:OREXO AB
Protease composition and method for treating a digestive disorder
ActiveUS20040191237A1Prevent and reduce riskImproving enzymaticBiocidePeptide/protein ingredientsDietary supplementGastric ph
There is provided methods, kits, combinations, and compositions comprising a Aspergillus oryzae protease enzyme, a Bacillus subtilis protease enzyme, and a Aspergillus niger lipase enzyme for treating a digestive disorder in a subject in need thereof. The methods, kits, combinations, and compositions may also be used along with an agent (or combination of agents) for raising the gastric pH, a digestive enzyme useful in enhancing digestive activity, a dietary supplement, or a pharmaceutical agent.
Owner:NAT ENZYME CO LLC
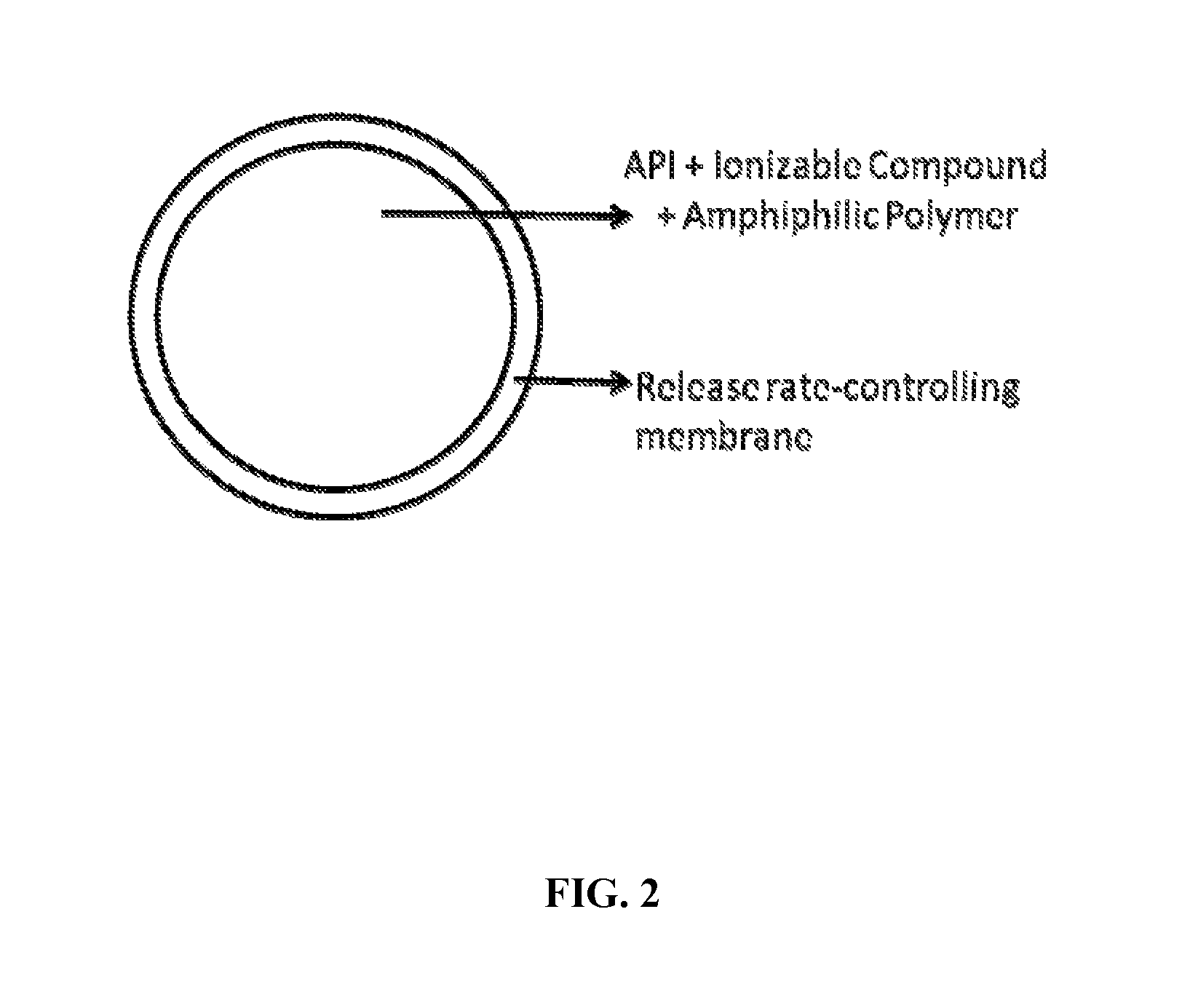
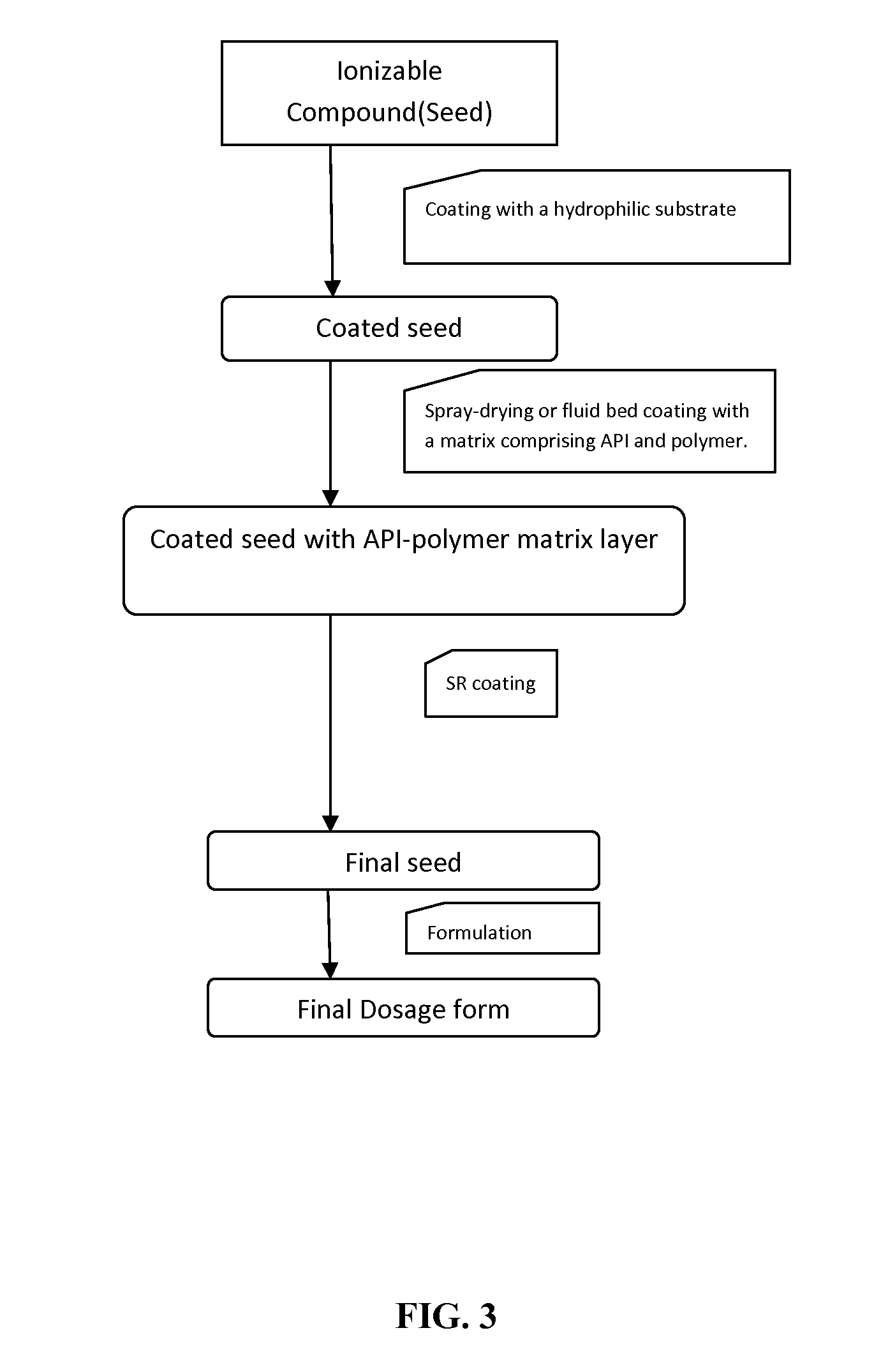
Extended-release oral dosage forms for poorly soluble amine drugs
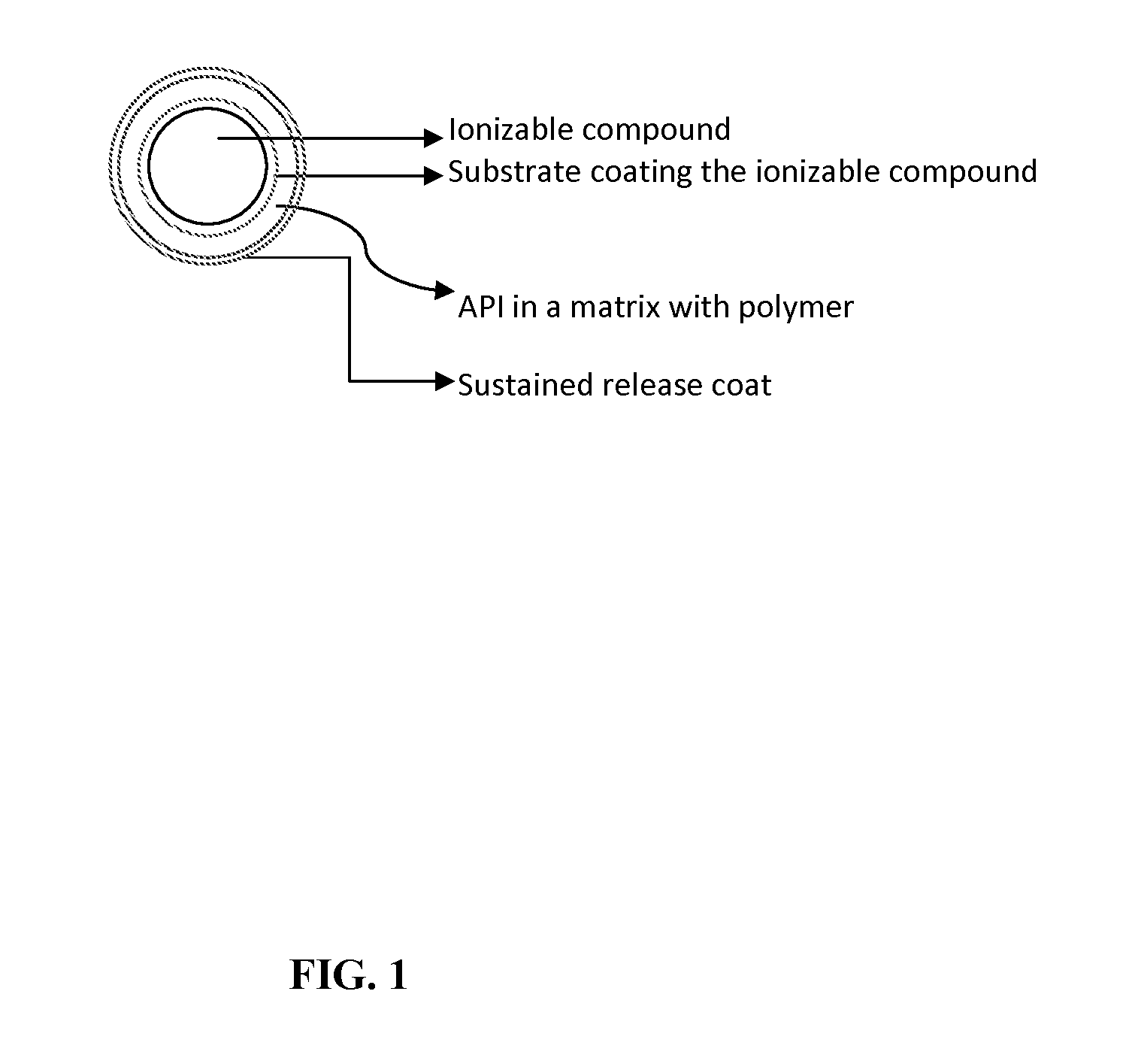
Oral dosage forms for poorly soluble amine drugs are provided. Such dosage forms include an ionizable compound such as an organic acid, an amphiphilic polymer and a release rate-controlling membrane. Such dosage forms allow for the consistent release of the active agent in both gastric pH conditions and in the intestine. Methods of making such dosage forms are also provided.
Owner:ABON PHARMA
Pharmaceutical composition for improving palatability of drugs and process for preparation thereof
InactiveUS20060141053A1Reduce the amount requiredSuppress bitternessBiocidePowder deliveryLipid formationGastric ph
The present invention discloses compositions, comprising a lipid-polymer matrix to mask the bitter or unpleasant taste of the medicament. The lipid or a blend of lipids, are used in combination with the pH dependent polymer where the said polymer is acid soluble or swellable The process for the preparation of taste masked pharmaceutical compositions of the bitter drugs comprising the said lipid-polymer compositions are disclosed. The concomitant use of the acid soluble polymer, which remains collapsed at the pH of saliva, inhibits the release of drug at that pH and hence they further help in bitterness inhibition. The said compositions deliver substantial amount of the bitter drug immediately at the gastric pH with improved palatability.
Owner:COUNCIL OF SCI & IND RES
Montmorillonite loaded calcium hydroxide antacid
ActiveCN103961368AReduce solubilityWeaken Alkaline StrengthDigestive systemPharmaceutical non-active ingredientsGastric phBULK ACTIVE INGREDIENT
The invention aims to provide a montmorillonite loaded calcium hydroxide antacid which uses montmorillonite loaded calcium hydroxide as the antacid on the basis of acid activation of montmorillonite loaded calcium hydroxide, and solves the conventional problem caused by direct contact of active components of the antacid and the gastric mucosa, wherein the montmorillonite has a gastric mucosa protection function simultaneously. The montmorillonite loaded calcium hydroxide antacid is characterized in that the antacid takes medicinal montmorillonite as the raw material, the montmorillonite is taken as a carrier after acid activation, loaded calcium hydroxide serves as an active ingredient, fatty acid is adopted for surface modification, the carrier of the obtained antacid is stable and insoluble, calcium hydroxide serving as the active ingredient can be continuously and firmly loaded on the carrier, the alkaline intensity is weakened, excessive taking of the antacid cannot cause too high gastric pH, and further, the carrier improves the dispersibility of calcium hydroxide serving as the active ingredient and forms a protective barrier, so that mucosa stimulation is avoided.
Owner:GUANGXI UNIV
Oral Medicament Based on a Proton Pump Inhibitor
InactiveUS20100068291A1Extends bioabsorption timeIncrease valueBiocideDigestive systemCo administrationImmediate release
The invention relates to oral medicaments having a modified release of proton pump inhibitors (PPI's) that are, in particular, useful in preventing and treating gastrointestinal disorders. The aim of the invention is to provide a novel oral medicament based on PPI's ideally having all or some of the following characteristics: a) quickly providing relief to the patient by increasing the gastric pH after oral administration of the medicament; b) accelerating the recovery of patients while maintaining this increase in the gastric pH for as long as possible after oral administration of the medicament and, in particular, during the night; c) improving the observance of the treatment and the comfort of the patient by taking the medicament once daily. To this end, the microcapsules of the invention, preferably non-enteric, are constituted of PPI microparticles coated with ethyl cellulose, an ammonio methacrylate copolymer (Eudragit® RL 100), polyvinylpyrrolidone, castor oil and polyoxyethylenated hydrogenated castor oil (40). This medicament is designed so that after its ingestion for a once daily administration, it makes it possible to maintain, from the first day of treatment onward, an average gastric pH, between 0 and 24 h, of greater than or equal to the average gastric pH between 0 and 24 h obtained by an enteric oral medicament having a reference* immediate release, administered under the same conditions. The invention also relates to these microcapsules per se.
Owner:FLAMEL TECHNOLOGIES
NSAID Dose Unit Formulations with H2-Receptor Antagonists and Methods of Use
The present invention generally relates to pharmaceutical unit dosage forms of NSAIDs and H2-receptor antagonists, in which the H2-receptor antagonist is formulated so as to be released in a sustained manner over a predetermined period of time so as to maintain gastric pH above a desired level for a duration of time. The NSAID may then be formulated for immediate release. The pharmaceutical unit dosage forms may be administered to subjects susceptible to the development of NSAID induced gastric and / or duodenal ulcers, as the sustained release H2-receptor antagonist is formulated so as to maintain the gastric environment above the pH levels where NSAID-induced ulceration typically occurs.
Owner:HORIZON PHARMA USA
Coated acidifier and preparation method thereof
InactiveCN102524533AReduce acid contentGuaranteed nutritional valueAnimal feeding stuffWeight gainingNutritive values
The invention relates to a coated acidifier and a preparation method thereof. The coated acidifier is made by mixing acids with an emulsifier, adding a solution of compound shell material and spray-drying. During the manufacturing process of the coated acidifier, no carrier additive for adsorbing acids is added, so that the acid content of the coated acidifier is increased. Owing to encapsulationof the compound shell material, the coated acidifier does not contact or react with vitamins and other nutrients in feed, so as to effectively ensure nutritive value of the feed. The coated acidifieris fully released when reaching the stomach of a suckling piglet, so as to achieve the effect of reducing gastric pH. Therefore, the coated acidifier overcomes the problem that a liquid acidifier is inconvenient to use and hard to preserve. Besides, the total acid content of the coated acidifier is higher than that of other coated acidifiers. The coated acidifier is very suitable for being used in the weaning period and within six weeks after weaning of sucking pigs in sucking pig breeding industry, and can effectively maintain low pH value of gastric juice and greatly increase the average daily weight gain of sucking pigs and the feed utilization efficiency.
Owner:安徽中粮生化格拉特乳酸有限公司
Oral submicron particle delivery system for proteins and process for its production
InactiveUS20100143484A1Powder deliveryPeptide/protein ingredientsGastrointestinal absorptionEnzymatic degradation
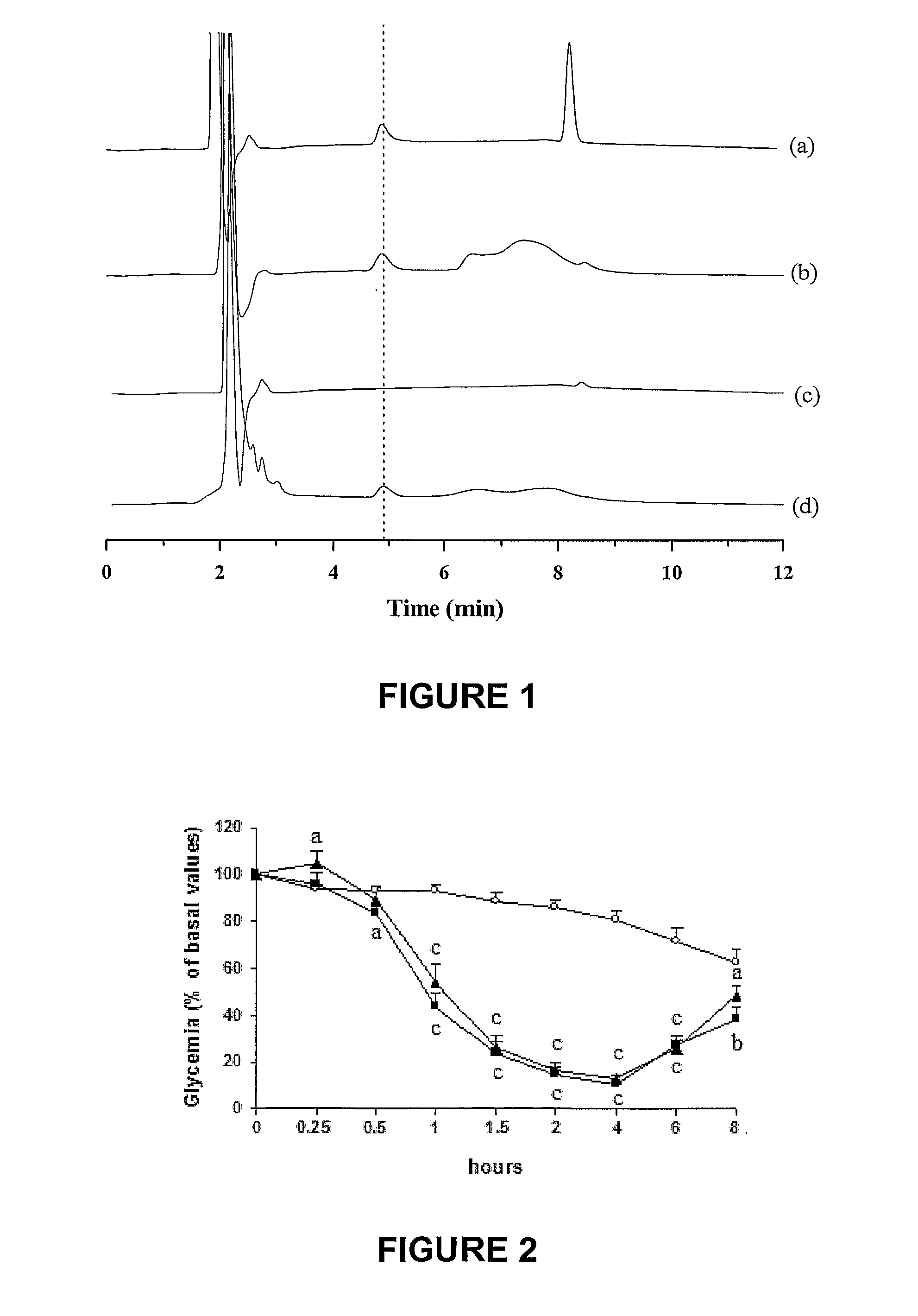
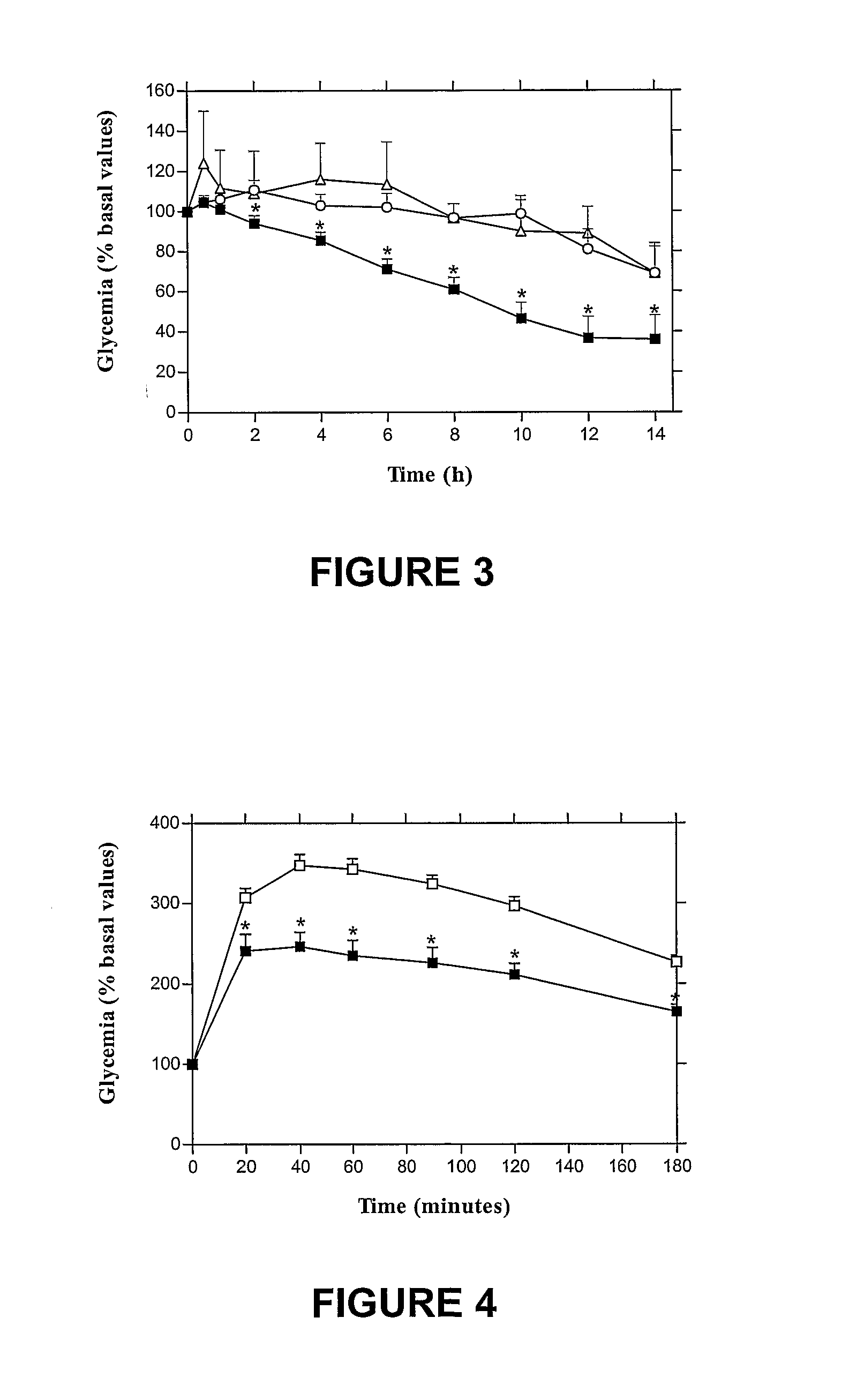
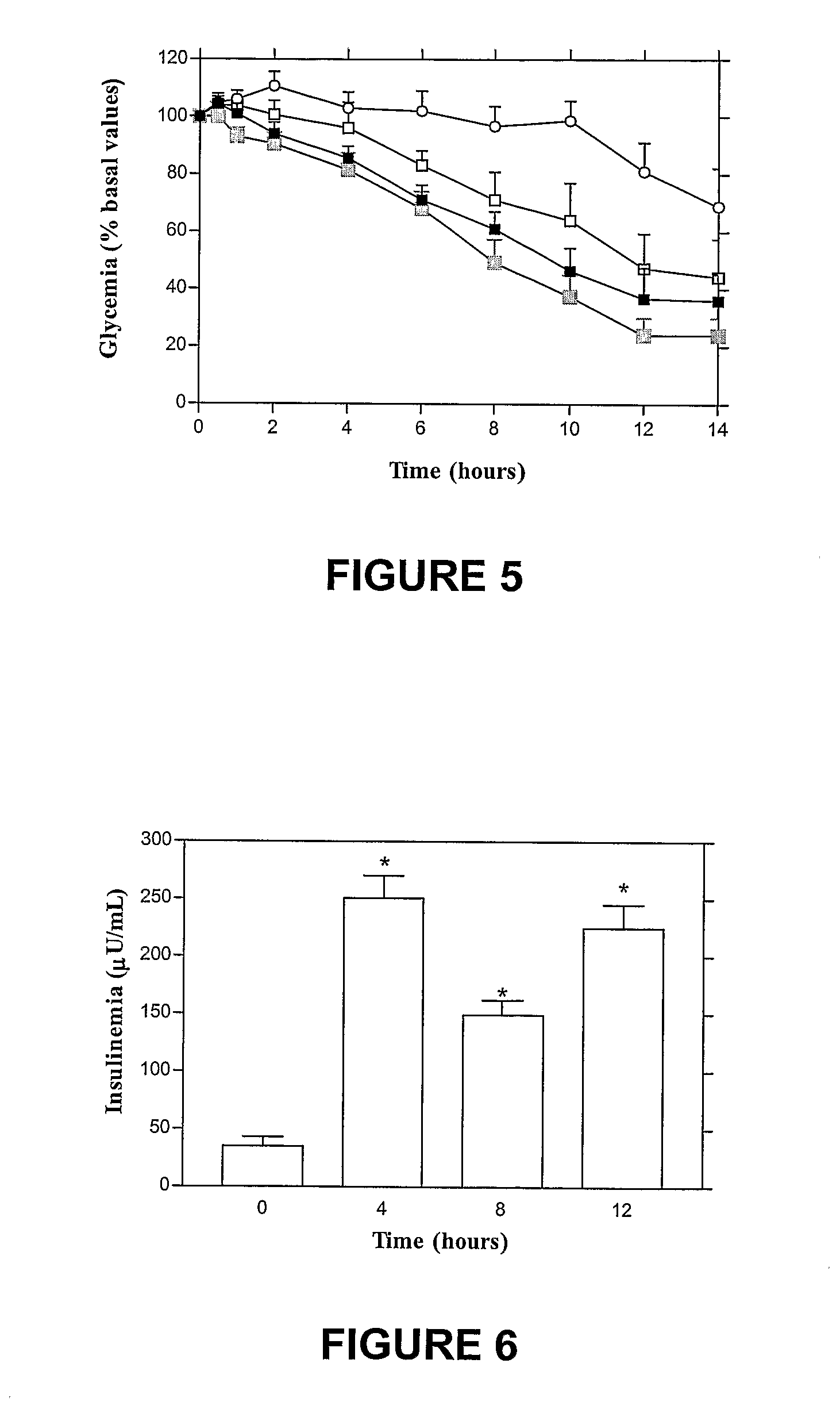
The invention provides a novel submicron system for the oral administration of proteins. An effective oral carrier for proteins should shield its content against the gastrointestinal tract proteases and be capable of facilitating the uptake of the protein drug across the gastrointestinal epithelium. The present invention relates to production of gelled particles which comprises a protein drug susceptible to enzymatic degradation by enzymes and acid conditions in the stomach, a polymeric matrix which undergoes precipitation-swelling process, and two-layer-coating materials which are themselves capable of enhancing absorption of said drug across the intestinal mucosal tissues and of inbihiting degradation of said drug by gastric enzymes. Insulin-loaded particles with appropriate submicron size for gastrointestinal absorption were made of natural occurring polymers by emulsification-based method and proved to be gastric pH and protease protective. Effects on glycemia were observed during 14 h after their oral single administration to rats, achieving 42% of pharmacological activity compared to subcutaneous administration. Postprandial rise in blood glucose was suppressed and insulinemia levels increased by a factor of seven. The relative oral bioavailability of insulin calculated over 8 h by comparison with a subcutaneous injection of free insulin was 34%.
Owner:UNIVE DE COIMBRA
Lipoic acid pellets
Lipoic acid pellets are described, obtained from inert cores externally coated with lipoic acid. The so obtained active cores are coated with a first layer of insulating polymeric material and then with a polymeric coat that is insoluble at the gastric pH. Pellet are then formulated pharmaceutically, for instance in jelly capsules or controlled release capsules or as oral suspensions, dispersible powders, sachets, etc.
Owner:ADARE PHARM SRL +1
Algae-based gastric pH response disintegrative empty capsule and preparation method thereof
InactiveCN104224747ANot easy to disintegrateCharge stabilityPharmaceutical non-active ingredientsCapsule deliveryGastric phMoisture
The invention relates to an algae-based gastric pH response disintegrative empty capsule, wherein an empty capsule body comprises the following materials in percentage by weight: 60-90% of sodium alginate, 0.3-12% of a gel, 0.01-2.5% of a setting agent, 0.02-5% of a plasticizer, 0.02-4% of a moisturizer, 0.1-5% of a pore forming disintegrating agent, 0-0.5% of a surfactant and the balance of de-ionized water. The empty capsule disclosed by the invention, which is mainly prepared from polysaccharide substances extracted from algae, has such characteristics as being stable to charge, not easy to absorb moisture under normal room temperature and humidity, and not easy to grow microorganisms through a scientific formula. Cutting leftovers of the capsule can be recycled, and zero pollution discharge is realized in a production process. The pore forming disintegrating agent contained in the empty capsule disclosed by the invention is easy to dissolve or swell in body liquid at pH value of 0.9-1.5 so that the empty capsule has a disintegrating property matched with a pH physiological property of a gastric area, thus enhancing administration precision to the gastric area in clinic.
Owner:ZHEJIANG WANLI UNIV
Protease composition and method for treating a digestive disorder
ActiveUS7067124B2Promote digestionPoor digestionBiocidePeptide/protein ingredientsDiseaseDietary supplement
There is provided methods, kits, combinations, and compositions comprising a Aspergillus oryzae protease enzyme, a Bacillus subtilis protease enzyme, and a Aspergillus niger lipase enzyme for treating a digestive disorder in a subject in need thereof. The methods, kits, combinations, and compositions may also be used along with an agent (or combination of agents) for raising the gastric pH, a digestive enzyme useful in enhancing digestive activity, a dietary supplement, or a pharmaceutical agent.
Owner:NAT ENZYME CO INC
Dietary fiber supplements for appetite suppression
InactiveUS20140087056A1Increase satietyEnhanced inhibitory effectOrganic active ingredientsDispersion deliveryDietary supplementGastric ph
The present invention provides a composition of dietary fiber supplements for satiety enhancement and appetite suppression in treatment of overweight, obesity, or eating disorders. The composition of the dietary fiber supplements consist of at least one cationic polymer and at least one anionic polymer. The cationic polymers and the anionic polymers in the composition can be dissolved or dispersed in an aqueous solution. When the pH of the aqueous solution is lowered, such as the solution is ingested into the stomach with a gastric pH environment or the aqueous solution is added with some acidifying agents, the aqueous solution will turn into a gel. The formation of a gel from the aqueous solution after it being ingested into the stomach before a meal would provide a satiety enhancement and appetite suppression effect.
Owner:YAN GUANG
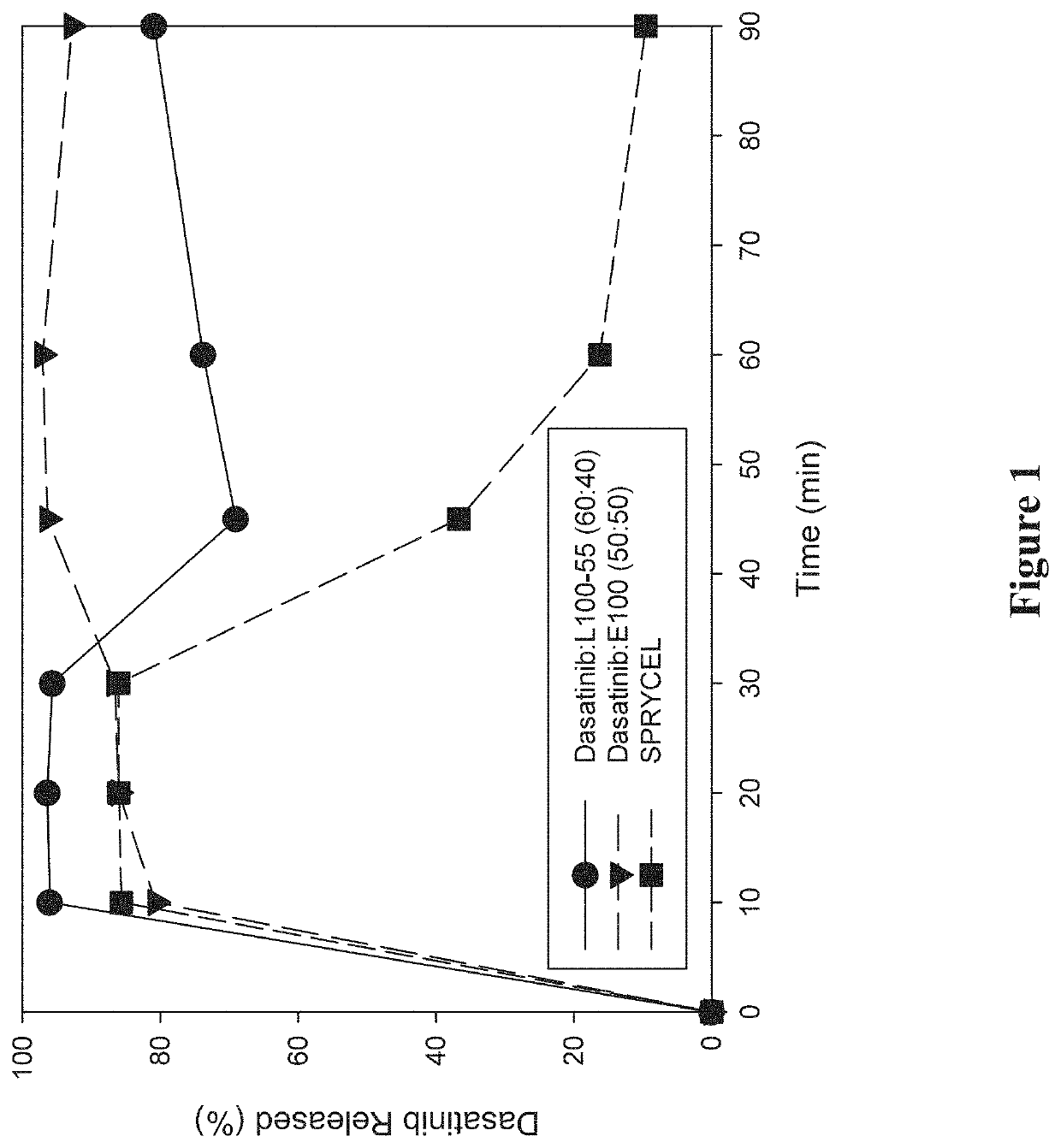
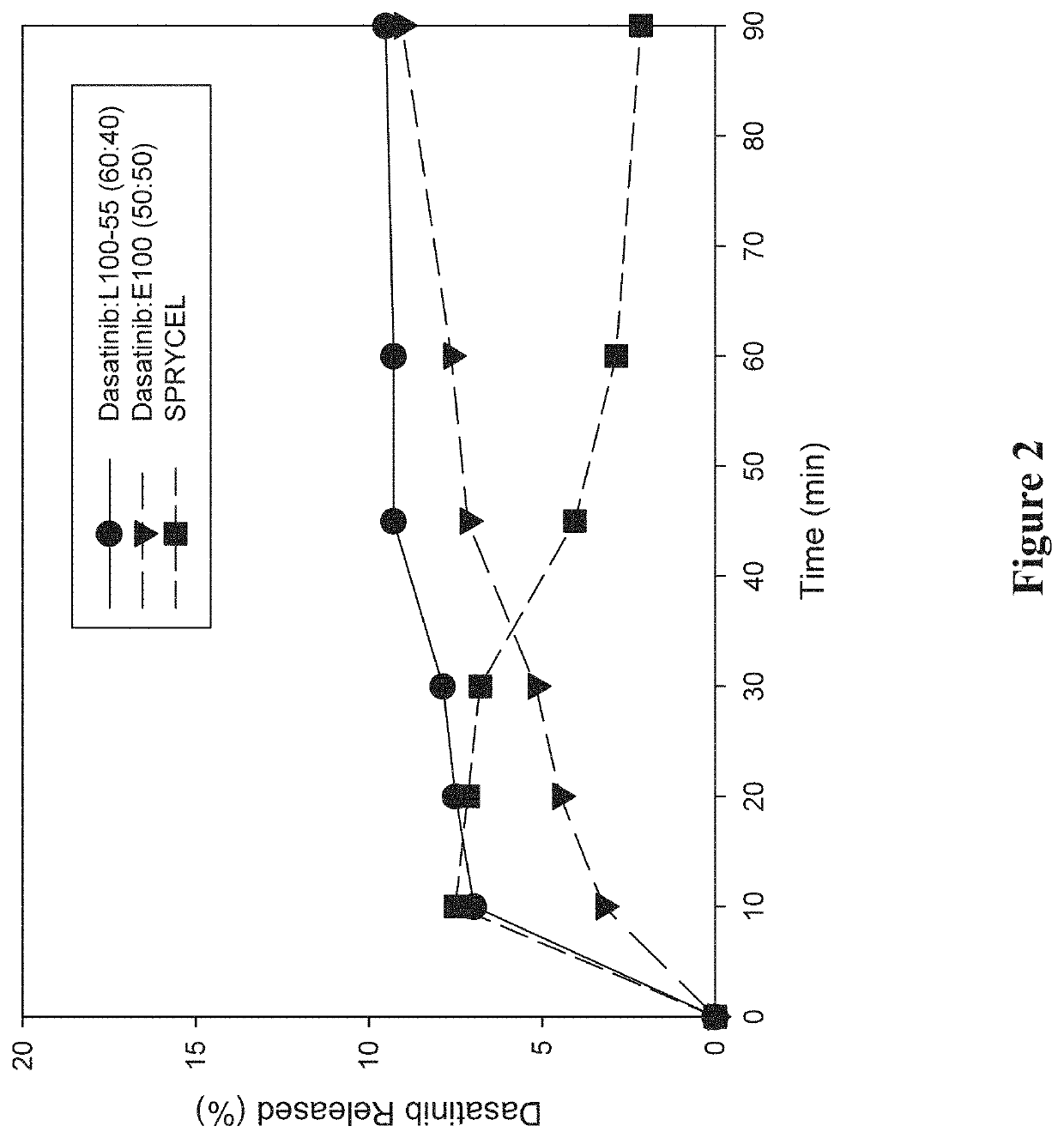
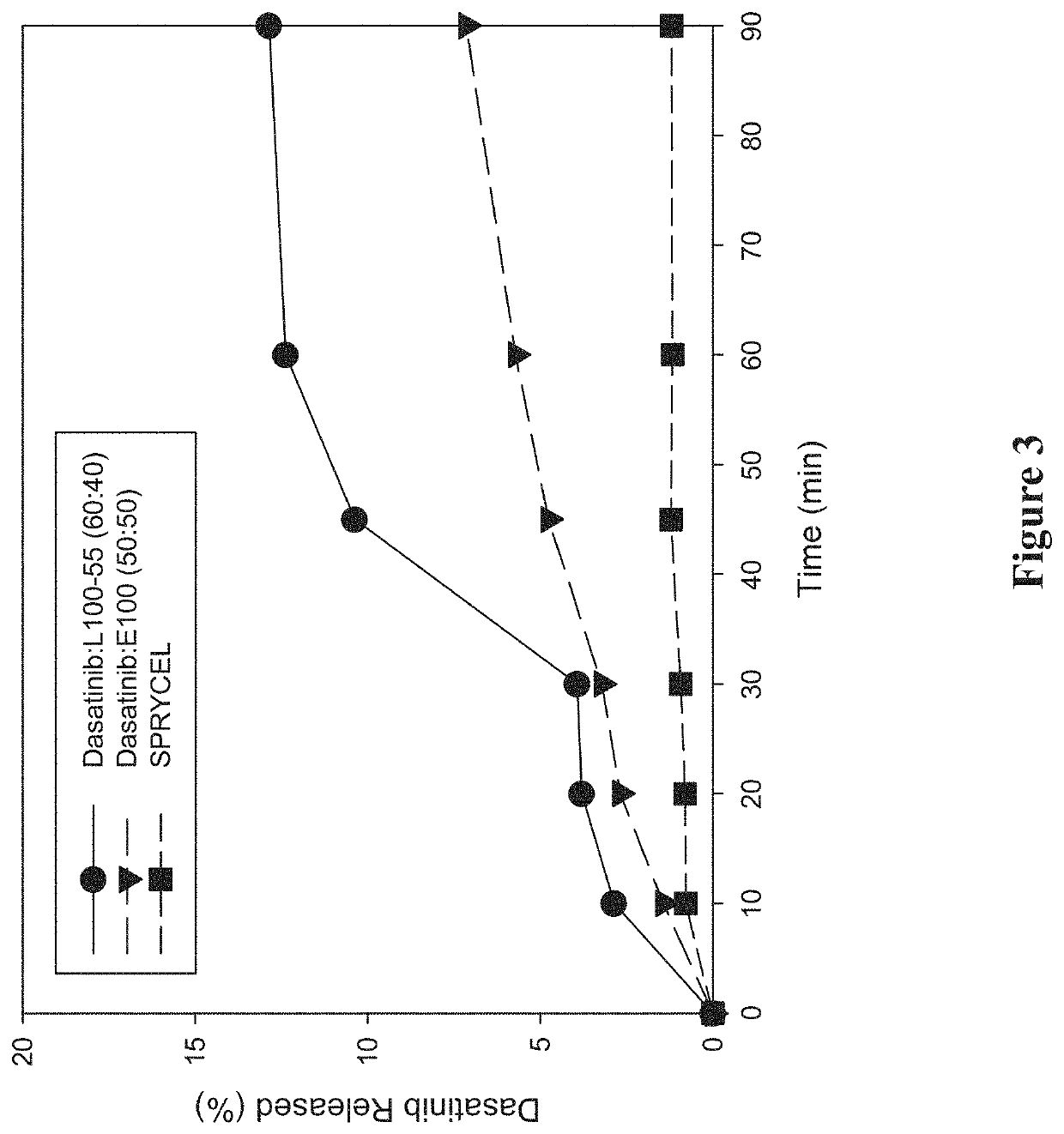
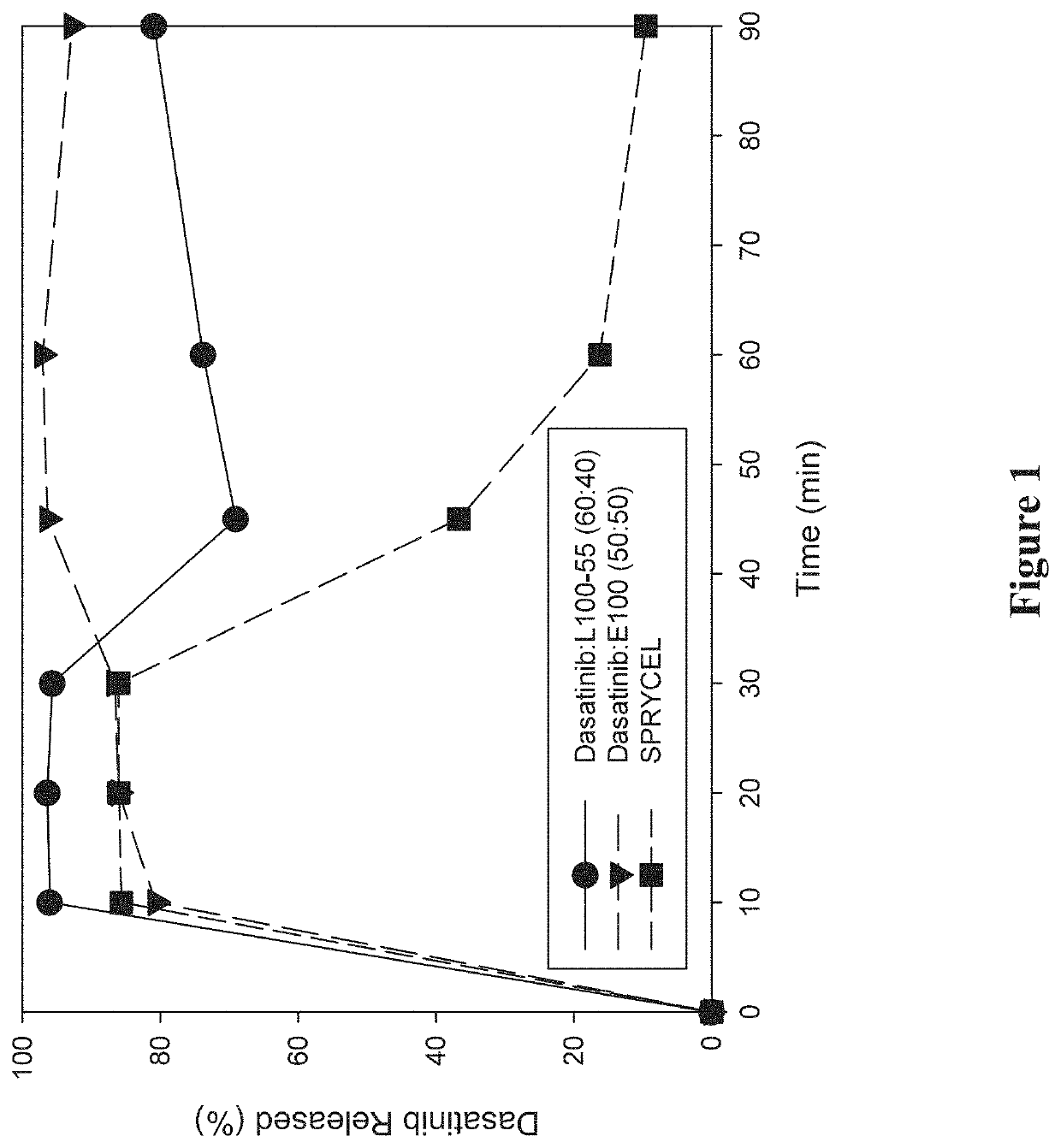
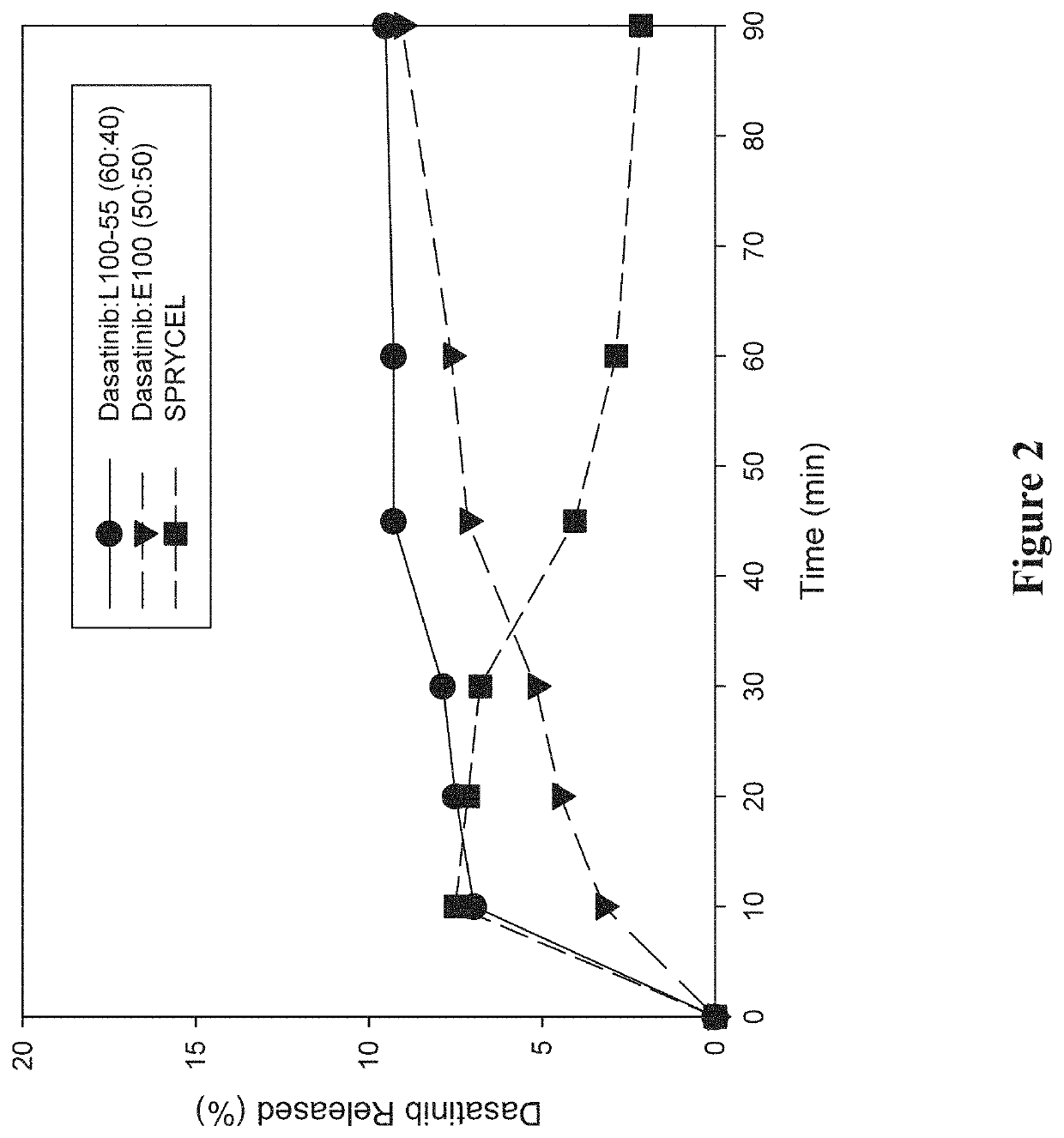
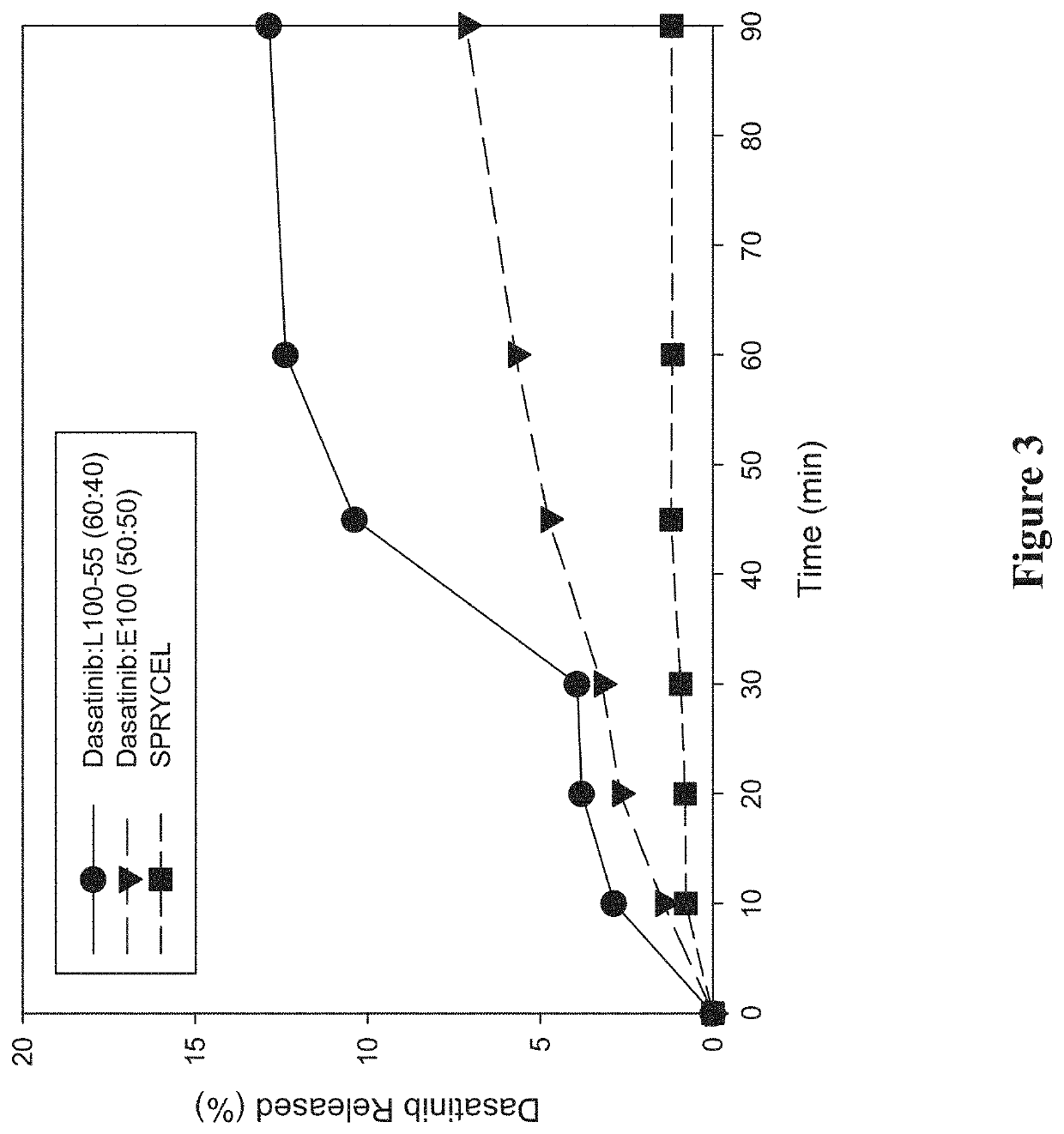
Amorphous solid dispersions of dasatinib and uses thereof
ActiveUS20210236489A1Reduced inter-subject variability variabilityReduced variability within-subject variabilityPowder deliveryOrganic active ingredientsDiseasePTK Inhibitors
Amorphous solid dispersions and pharmaceutical compositions of the protein kinase inhibitor dasatinib. The pharmaceutical compositions may be used in methods of treating a proliferative disorder such as cancer, or in methods of delivering dasatinib to patients without regard to whether the patient is concurrently administered a gastric acid-reducing agent, or without regard to whether the patient has an elevated gastric pH. The compositions may be particularly suitable for patients afflicted by achlorhydria or hypochlorhydria, or Helicobacter pylori infection.
Owner:NANOCOPOEIA
Pharmaceutical composition for improving palatability of drugs and process for preparation thereof
InactiveCN1953734AReduce the amount requiredQuick releaseAntibacterial agentsDispersion deliverySialic acidBitter tastes
The present invention discloses compositions, comprising a lipid-polymer matrix to mask the bitter or unpleasant taste of the medicament. The lipid or a blend of lipids, are used in combination with the pH dependent polymer where the said polymer is acid soluble or swellable. The process for the preparation of taste masked pharmaceutical compositions of the bitter drugs comprising the said lipid-polymer compositions are disclosed. The concomitant use of the acid soluble polymer, which remains collapsed at the pH of saliva, inhibits the release of drug at that pH and hence they further help in bitterness inhibition. The said compositions deliver substantial amount of the bitter drug immediately at the gastric pH with improved palatability.
Owner:COUNCIL OF SCI & IND RES
ORAL PHARMACEUTICAL FOR BASED ON AT LEAST ONE ACTIVE PRINCIPLE WHOSE SOLUBILITY VARIES AS A FUNCTION OF THE GASTRIC pH CONDITIONS
InactiveUS20110159088A1Eliminate and reduce erratic natureExtend posting timeBiocideNervous disorderSolubilityControl release
The field of the present invention is that of oral pharmaceutical forms of at least one active principle AP whose solubility varies greatly as a function of the gastric pH, and also treatments and administration methods relating thereto.The invention relates to the use, in an oral pharmaceutical form comprising AP, of a coating or of a matrix including the said AP and allowing the controlled release of the said AP, in order for this form administered orally to a sample of individuals to lead, irrespective of the fed or fasted state of the individuals, to a reduction in the inter- and / or intra-individual standard deviation of the Cmax, which makes it possible to ensure lower variability of the efficacy and of the therapeutic safety of the pharmaceutical form, compared with an immediate-release AP pharmaceutical form administered to this same sample of individuals, at the same dose.
Owner:FLAMEL TECHNOLOGIES
Amorphous solid dispersions of dasatinib and uses thereof
Amorphous solid dispersions and pharmaceutical compositions of the protein kinase inhibitor dasatinib. The pharmaceutical compositions may be used in methods of treating a proliferative disorder such as cancer, or in methods of delivering dasatinib to patients without regard to whether the patient is concurrently administered a gastric acid-reducing agent, or without regard to whether the patient has an elevated gastric pH. The compositions may be particularly suitable for patients afflicted by achlorhydria or hypochlorhydria, or Helicobacter pylori infection.
Owner:NANOCOPOEIA
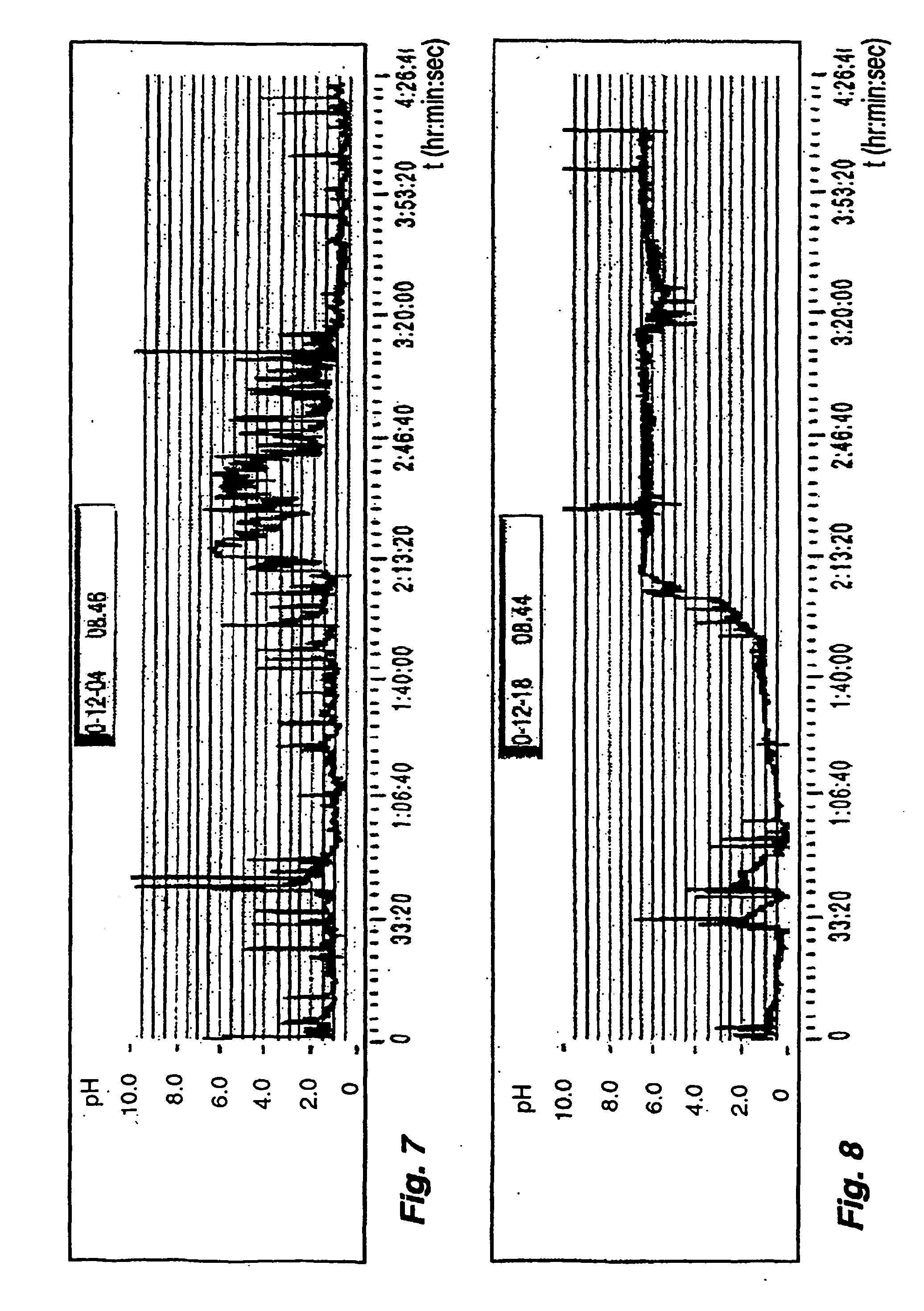
Method for processing dynamic monitoring data of upper gastrointestinal tract pH value
InactiveCN106236107AVisual display of monitoring resultsDiagnostic signal processingCatheterProcess dynamicsUpper gastrointestinal
The invention provides a method for processing dynamic monitoring data of upper gastrointestinal tract pH value. A pH electrode probe dynamically and continuously measures gastric pH value within 24 hours and dynamically and continuously measures esophageal pH value within 48 hours, a microprocessor stores the data of the measured gastric pH value and esophageal pH value respectively, the data of the gastric pH value and esophageal pH value stored in the microprocessor are transmitted to a computer system for processing and analyzing, and a medical statistic chart, pH profile and related clinical pH monitoring reports for the gastric and esophageal pH values are printed out. The method enables detection for the gastric pH value, esophageal pH value and relative pH values and enables the detected gastric pH value, esophageal pH value and relative pH values to be stored, processed and analyzed before the medical statistical chart, pH profile and related clinical pH monitoring reports for the gastric and esophageal pH values are printed out, and it is possible to visually display the monitoring results.
Owner:HEFEI KAILI PHOTOELECTRIC TECH
Rumen pH meter
ActiveCN102621187AFacilitate early diagnosisTake precautions in advanceMaterial analysis by electric/magnetic meansGastric fluidGastric ph
The invention belongs to the technical field of animal husbandry machinery, and relates to a rumen pH meter. The rumen pH meter comprises a pH meter probe, a guide tube, a pH display, a controller and a clamp. In the pH meter probe, a pH meter electrode is arranged on an electrode support, the electrode support is fixedly connected outside an electrode casing at the window of the electrode casing, and during the process that the pH meter probe is inserted into the rumen through an oral cavity and an esophagus of a ruminant, in order to avoid contamination of saliva and esophagus mucus during insertion process, the pH meter probe is closed, and the pH meter electrode is sealed. When the rumen pH meter probe works, the pH meter probe is open to expose the window of the electrode casing, and the pH meter electrode is exposed in a gastric fluid environment to measure the pH of rumen fluid in the rumen. The rumen pH meter provided by the invention can conveniently and rapidly measure the pH value of the rumen, has low cost and high practicability, has good effects in early diagnosis and prevention in advance of ruminal acidosis, and is suitable for monitoring the gastric pH value in the rumen of the ruminant during scientific research and production.
Owner:CHINA AGRI UNIV
Pharmaceutical composition for improving palatability of drugs and process for preparation thereof
InactiveUS7378109B2Improve palatabilityReduce the amount requiredBiocidePowder deliveryLipid formationGastric ph
The present invention discloses compositions, comprising a lipid-polymer matrix to mask the bitter or unpleasant taste of the medicament. The lipid or a blend of lipids, are used in combination with the pH dependent polymer where the said polymer is acid soluble or swellable The process for the preparation of taste masked pharmaceutical compositions of the bitter drugs comprising the said lipid-polymer compositions are disclosed. The concomitant use of the acid soluble polymer, which remains collapsed at the pH of saliva, inhibits the release of drug at that pH and hence they further help in bitterness inhibition. The said compositions deliver substantial amount of the bitter drug immediately at the gastric pH with improved palatability.
Owner:COUNCIL OF SCI & IND RES
Rumen pH meter
ActiveCN102621187BFacilitate early diagnosisEasy to measureMaterial analysis by electric/magnetic meansGastric fluidGastric ph
The invention belongs to the technical field of animal husbandry machinery, and relates to a rumen pH meter. The rumen pH meter comprises a pH meter probe, a guide tube, a pH display, a controller and a clamp. In the pH meter probe, a pH meter electrode is arranged on an electrode support, the electrode support is fixedly connected outside an electrode casing at the window of the electrode casing, and during the process that the pH meter probe is inserted into the rumen through an oral cavity and an esophagus of a ruminant, in order to avoid contamination of saliva and esophagus mucus during insertion process, the pH meter probe is closed, and the pH meter electrode is sealed. When the rumen pH meter probe works, the pH meter probe is open to expose the window of the electrode casing, and the pH meter electrode is exposed in a gastric fluid environment to measure the pH of rumen fluid in the rumen. The rumen pH meter provided by the invention can conveniently and rapidly measure the pH value of the rumen, has low cost and high practicability, has good effects in early diagnosis and prevention in advance of ruminal acidosis, and is suitable for monitoring the gastric pH value in the rumen of the ruminant during scientific research and production.
Owner:CHINA AGRI UNIV
Dietary fiber supplements for appetite suppression
InactiveUS8993539B2Enhanced inhibitory effectBiocideDispersion deliveryDietary fiberDietary supplement
The present invention provides a composition of dietary fiber supplements for satiety enhancement and appetite suppression in treatment of overweight, obesity, or eating disorders. The composition of the dietary fiber supplements consist of at least one cationic polymer and at least one anionic polymer. The cationic polymers and the anionic polymers in the composition can be dissolved or dispersed in an aqueous solution. When the pH of the aqueous solution is lowered, such as the solution is ingested into the stomach with a gastric pH environment or the aqueous solution is added with some acidifying agents, the aqueous solution will turn into a gel. The formation of a gel from the aqueous solution after it being ingested into the stomach before a meal would provide a satiety enhancement and appetite suppression effect.
Owner:YAN GUANG
Double-layer tablet comprising proton pump inhibitor
The invention provides a double-layer tablet comprising a proton pump inhibitor. The double-layer tablet includes a fast-release tablet layer, a sustained-release tablet and a pharmaceutically acceptable excipient. The sustained release tablet layer contains a pharmacologically effective amount of the proton pump inhibitor or its salt; the a fast-release tablet layer contains an H2 receptor antagonist or its salt; and the pharmacologically acceptable excipient includes one or more of a filler, an adhesive, a lubricant and a surface active agent. In the invention, the H2 receptor antagonist as the fast release part reduces the secretion of gastric acid, and the proton pump inhibitor as the sustained-release part continuously suppresses the secretion of gastric acid, thereby effectively controlling the gastric pH value in a long time, prolonging the action time of drug and promoting the cure of diseases related to excessive acid.
Owner:上海中邦斯瑞生物药业技术有限公司
Pharmaceutical composition for improving palatability of drugs and process for preparation thereof
InactiveCN100548270CReduce the amount requiredQuick releaseAntibacterial agentsDispersion deliverySialic acidBitter tastes
The present invention discloses compositions, comprising a lipid-polymer matrix to mask the bitter or unpleasant taste of the medicament. The lipid or a blend of lipids, are used in combination with the pH dependent polymer where the said polymer is acid soluble or swellable. The process for the preparation of taste masked pharmaceutical compositions of the bitter drugs comprising the said lipid-polymer compositions are disclosed. The concomitant use of the acid soluble polymer, which remains collapsed at the pH of saliva, inhibits the release of drug at that pH and hence they further help in bitterness inhibition. The said compositions deliver substantial amount of the bitter drug immediately at the gastric pH with improved palatability.
Owner:COUNCIL OF SCI & IND RES
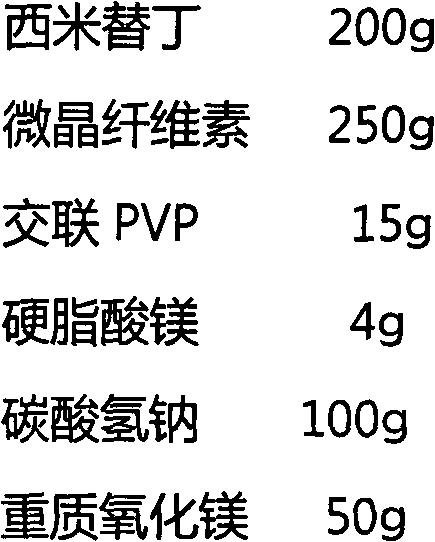
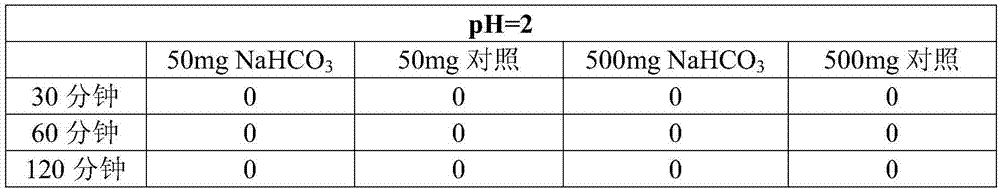
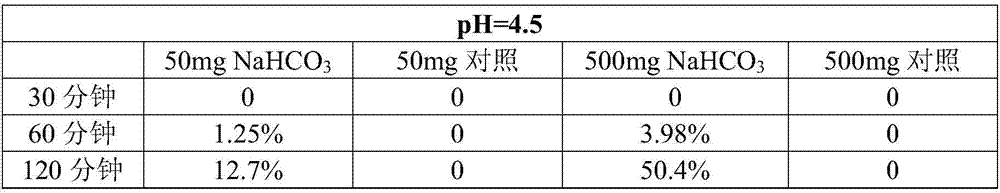
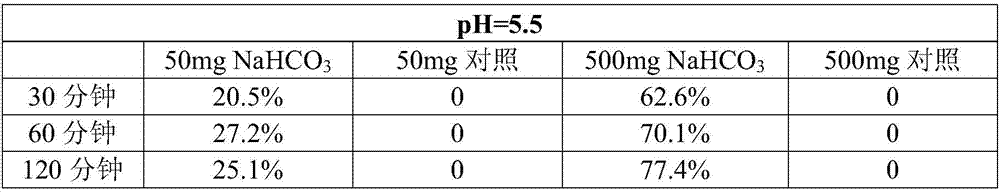
A kind of intragastric ph detection micropill and its application
InactiveCN104940961BAccurately judge pHHigh clinical application valueRadioactive preparation carriers13c labelMedicine
The invention relates to a micropill for detecting pH in the stomach and its application. Specifically, a kind of intragastric pH detection pellet is provided, the pellet core of the intragastric pH detection pellet contains 13C-labeled NaHCO3, and the coating of the intragastric pH detection pellet is a pH-dependent coating It also provides an intragastric pH detection capsule; and provides the application of the intragastric pH detection pellets and capsules in the preparation of medicines for detecting intragastric pH. The invention has the advantages of: it can accurately determine the pH in the stomach of the subject; the patient has good tolerance and can be tested repeatedly; it is safe and reliable; the operation is simple, and the subject only needs to take the gastric pH detection capsule of the present invention before exhaling The test can be completed; the price is cheap.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
A wireless detection system for gastric pH based on edible and digestible materials
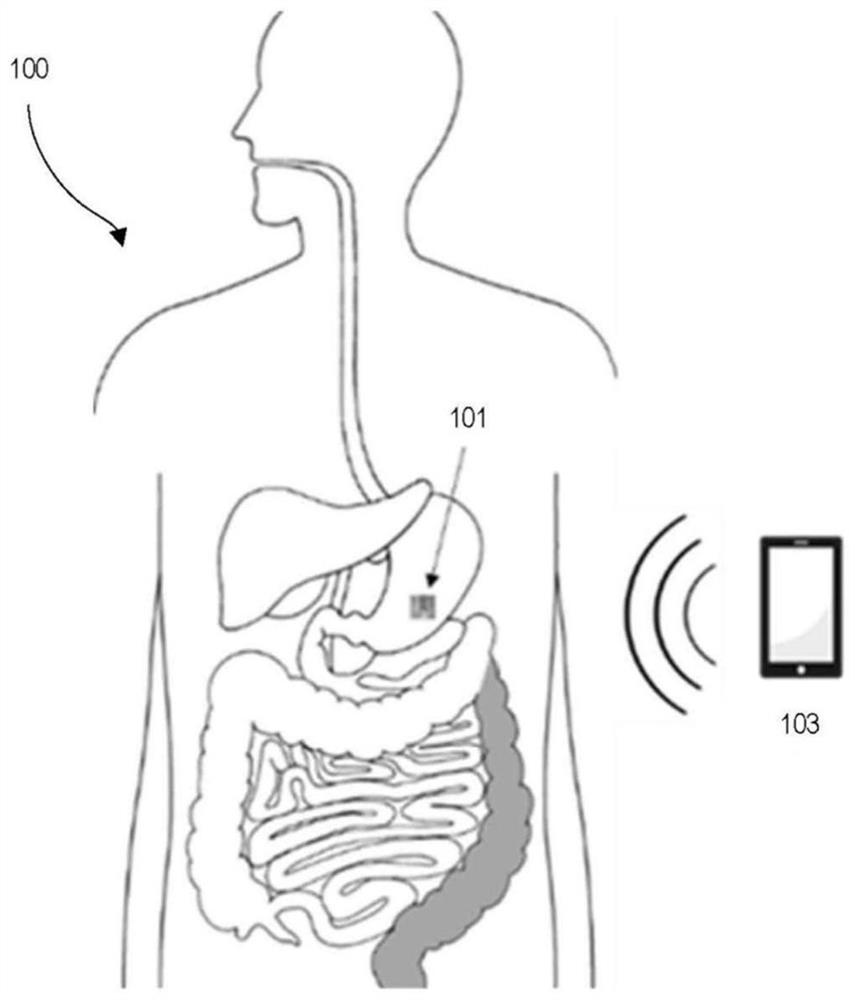
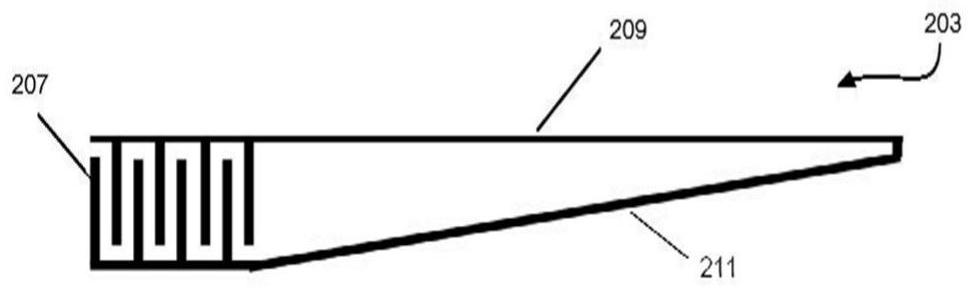
ActiveCN111601518BMaterial analysis using sonic/ultrasonic/infrasonic wavesPattern printingStomach partEngineering
The invention discloses a gastric pH wireless detection system prepared based on edible and digestible materials. The system includes a planar structure rolled into a cylindrical form, and a circuit pattern is printed on the surface of the planar structure. The circuit pattern includes a plurality of interdigital electrodes and an antenna part. The planar structure was rolled into a cylindrical form such that the interdigitated electrodes were on the outermost layer of the rolled planar structure. The rolled planar structure makes the antenna part in the circuit pattern into a coil shape.
Owner:梅奥医学教育研究基金会 +1
Gastric acid secretion inhibiting composition
InactiveUS20040131674A1Quick and lasting reliefAntibacterial agentsBiocideHelicobacter pyloriGastric secretion
An oral pharmaceutical dosage form comprises pharmacologically effective amounts of an acid susceptible proton pump inhibitor or a salt thereof, an H2 receptor antagonist or a salt thereof and a pharmaceutically acceptable carrier. The dosage form is capable of raising gastric pH to above 4 within two hours after administration and to keep it at that level for at least 4 hours. Also disclosed is a method of manufacture of the dosage form, its use in treating dyspepsia and infection by Helicobacter pylori, and a method of treating disorders associated with gastric acid secretion.
Owner:OREXO AB
Guanfacine sustained-release tablet and preparation method thereof
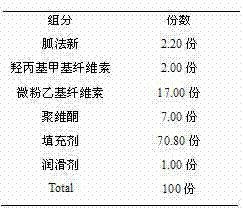
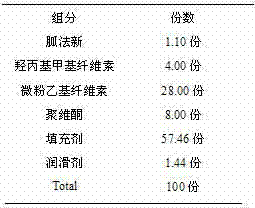
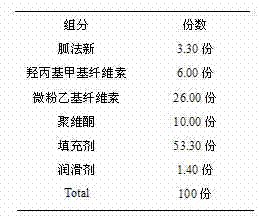
InactiveCN102525983ASimple preparation processUniform and slow drug releaseOrganic active ingredientsNervous disorderCelluloseProlonged-release tablet
The invention discloses a guanfacine sustained-release tablet and a preparation method thereof. The sustained-release tablet contains pharmaceutically-acceptable carriers and guanfacine or a pharmaceutically-acceptable salt, wherein the sustained-release tablet contains 1 to 5 parts of guanfacine, 12 to 30 parts of ethyl cellulose micropowder, 1 to 6 parts of hydroxypropyl methylcellulose, 5 to 10 parts of polyvinylpyrrolidone, 48 to 71 parts of fillers and 0.9 to 1.5 parts of lubricants. Compared with the prior art, the preparation method has a simple process, and the guanfacine sustained-release tablet can uniformly and sustainedly release guanfacine in the gastrointestinal tract regardless of gastric pH and can play a more effective role in blood sugar reduction.
Owner:北京万全阳光医药科技有限公司
Coated acidifier and preparation method thereof
InactiveCN102524533BReduce acid contentGuaranteed nutritional valueAnimal feeding stuffWeight gainingNutritive values
The invention relates to a coated acidifier and a preparation method thereof. The coated acidifier is made by mixing acids with an emulsifier, adding a solution of compound shell material and spray-drying. During the manufacturing process of the coated acidifier, no carrier additive for adsorbing acids is added, so that the acid content of the coated acidifier is increased. Owing to encapsulationof the compound shell material, the coated acidifier does not contact or react with vitamins and other nutrients in feed, so as to effectively ensure nutritive value of the feed. The coated acidifieris fully released when reaching the stomach of a suckling piglet, so as to achieve the effect of reducing gastric pH. Therefore, the coated acidifier overcomes the problem that a liquid acidifier is inconvenient to use and hard to preserve. Besides, the total acid content of the coated acidifier is higher than that of other coated acidifiers. The coated acidifier is very suitable for being used in the weaning period and within six weeks after weaning of sucking pigs in sucking pig breeding industry, and can effectively maintain low pH value of gastric juice and greatly increase the average daily weight gain of sucking pigs and the feed utilization efficiency.
Owner:安徽中粮生化格拉特乳酸有限公司
Lipoic acid pellets
Owner:ADARE PHARM SRL +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com