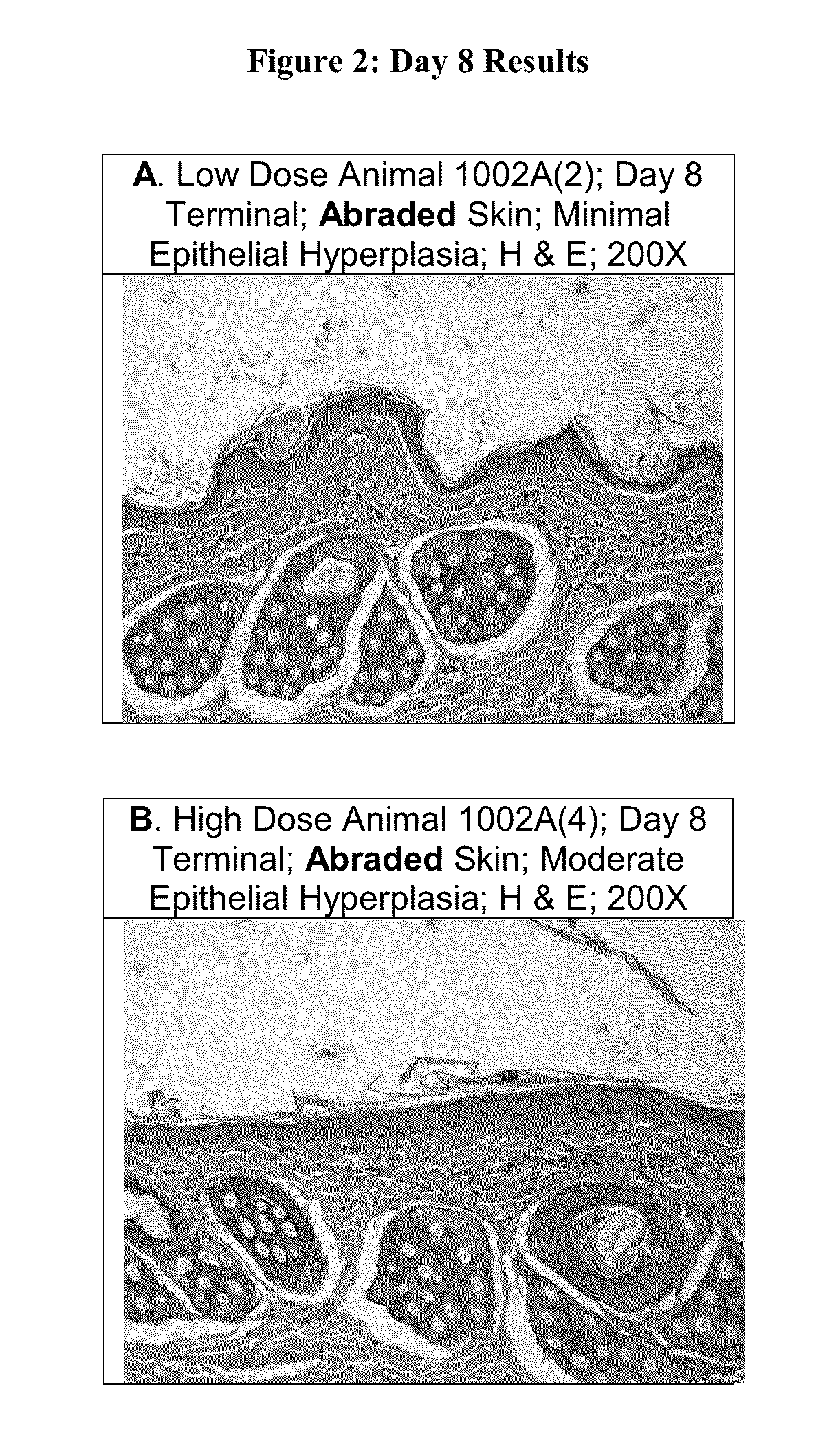
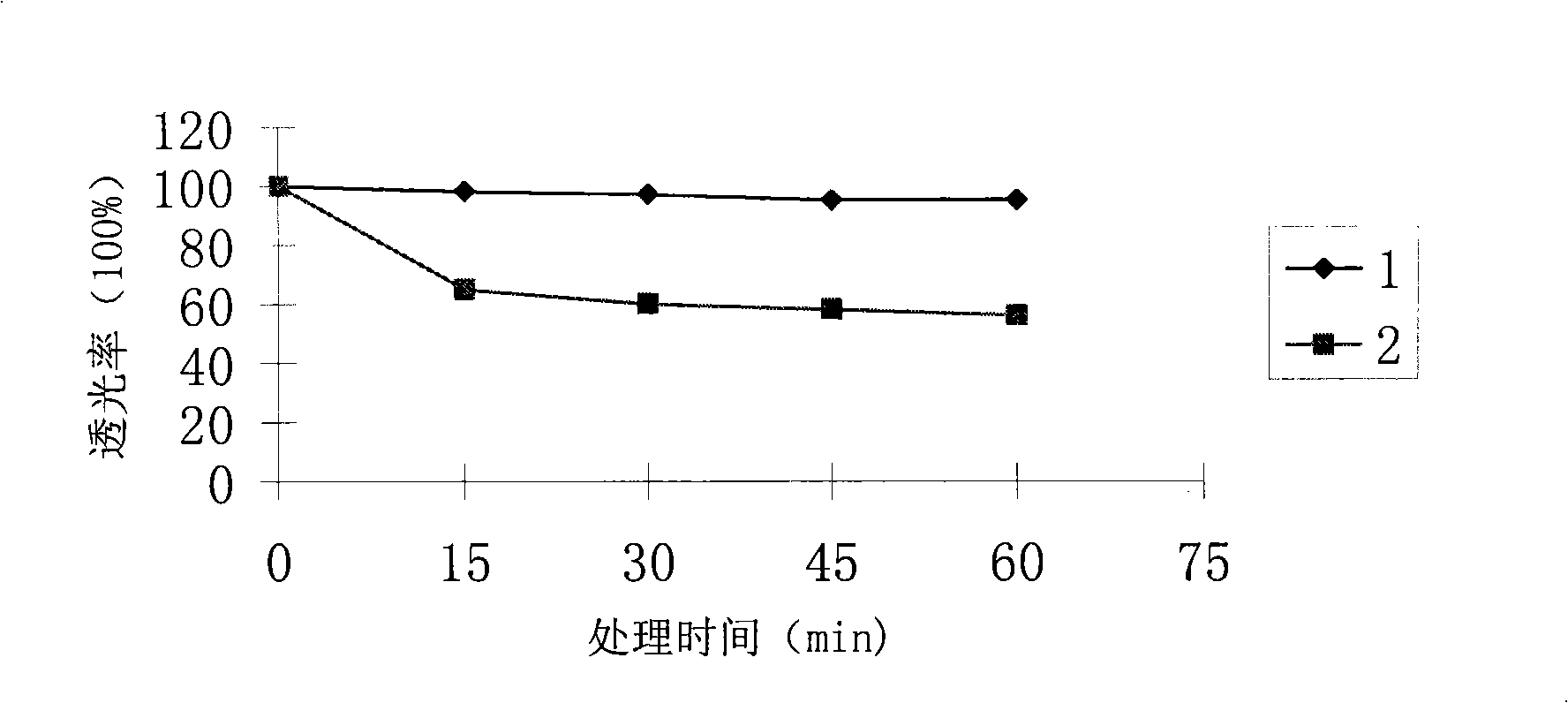
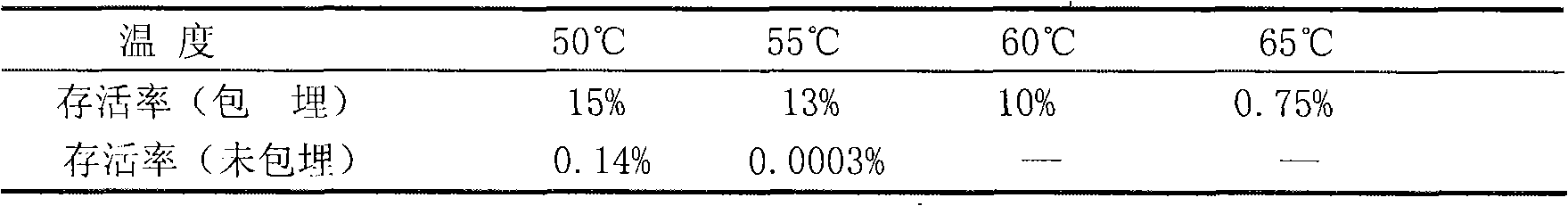
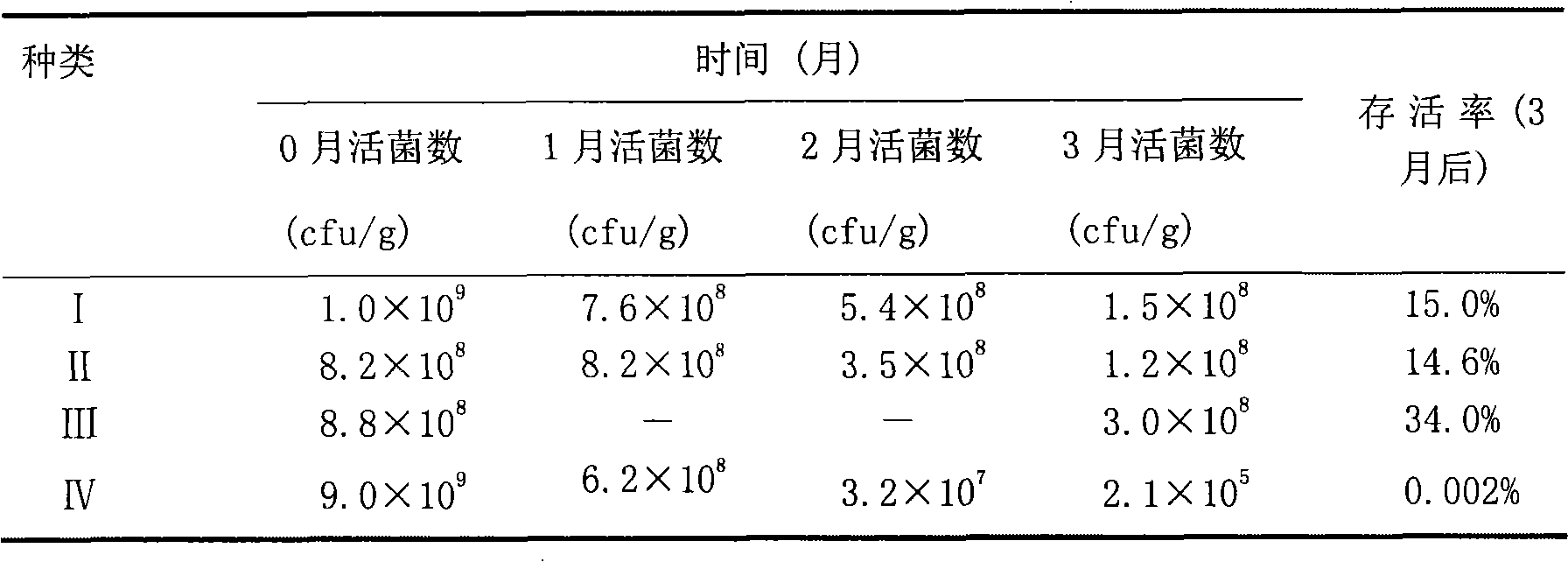
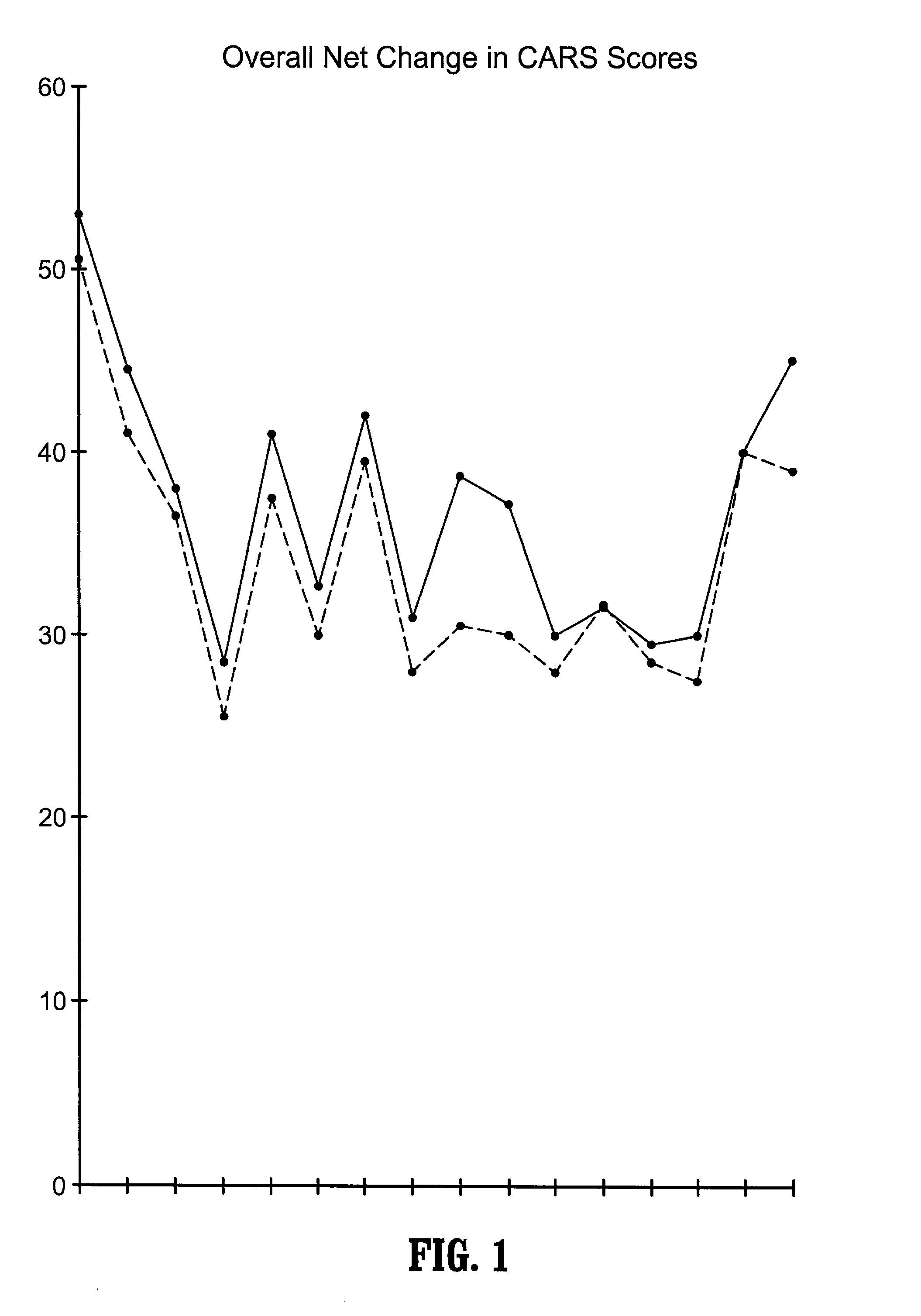Patents
Literature
995 results about "Digestive enzyme" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Digestive enzymes are a group of enzymes that break down polymeric macromolecules into their smaller building blocks, in order to facilitate their absorption by the body. Digestive enzymes are found in the digestive tracts of animals (including humans) and in the tracts of carnivorous plants, where they aid in the digestion of food, as well as inside cells, especially in their lysosomes, where they function to maintain cellular survival. Digestive enzymes of diverse specificities are found in the saliva secreted by the salivary glands, in the secretions of cells lining the stomach, in the pancreatic juice secreted by pancreatic exocrine cells, and in the secretions of cells lining the small and large intestines.
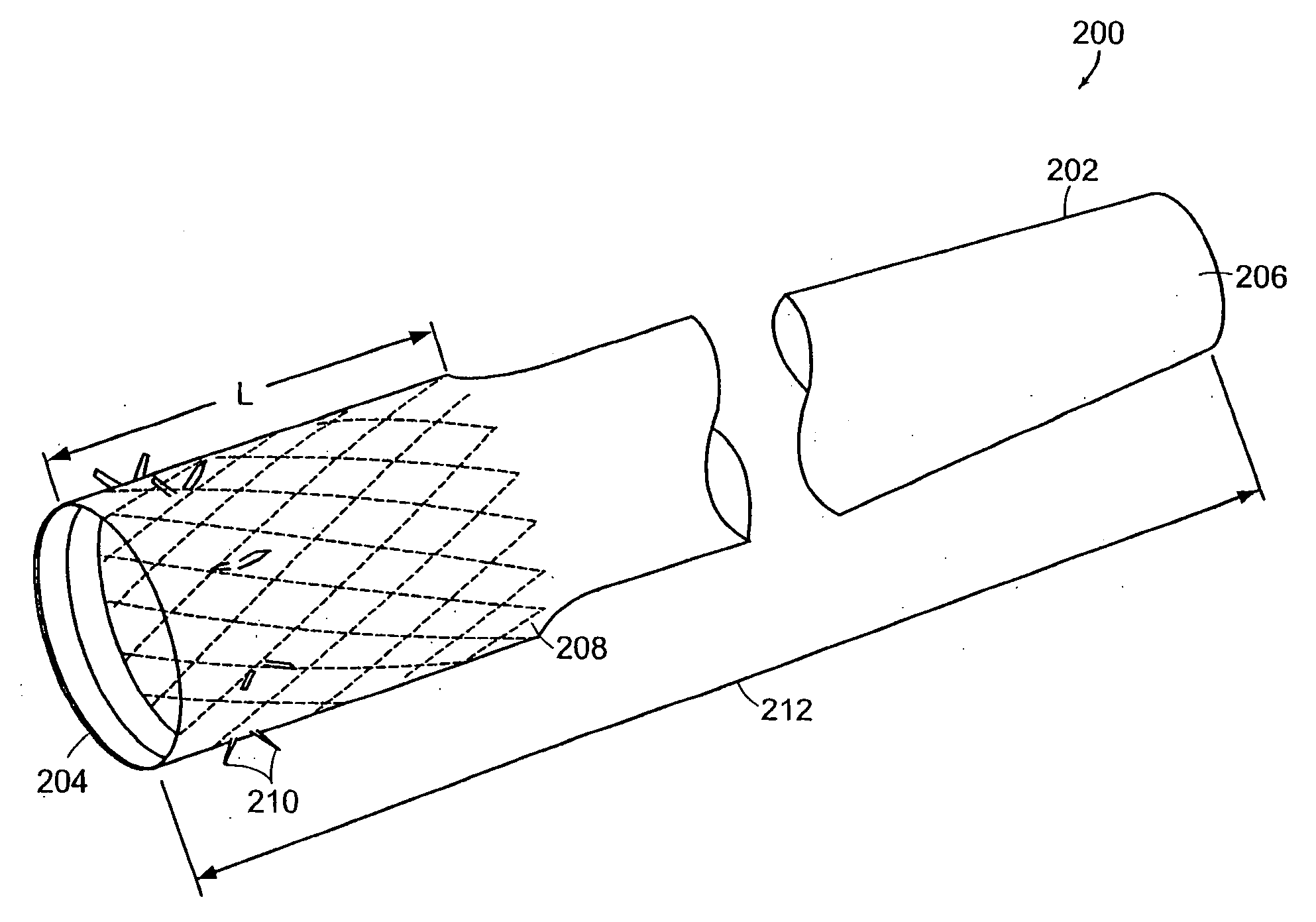
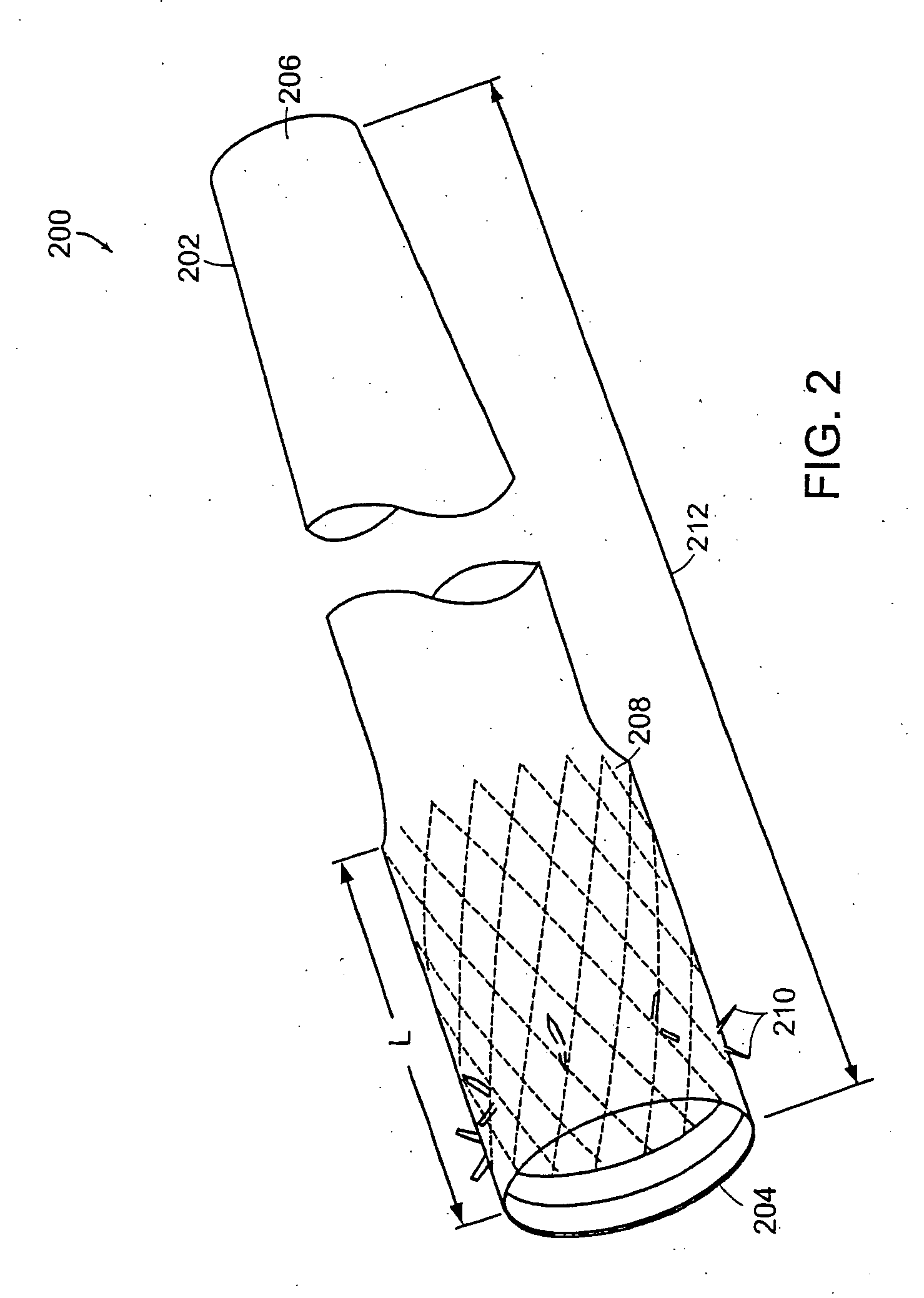
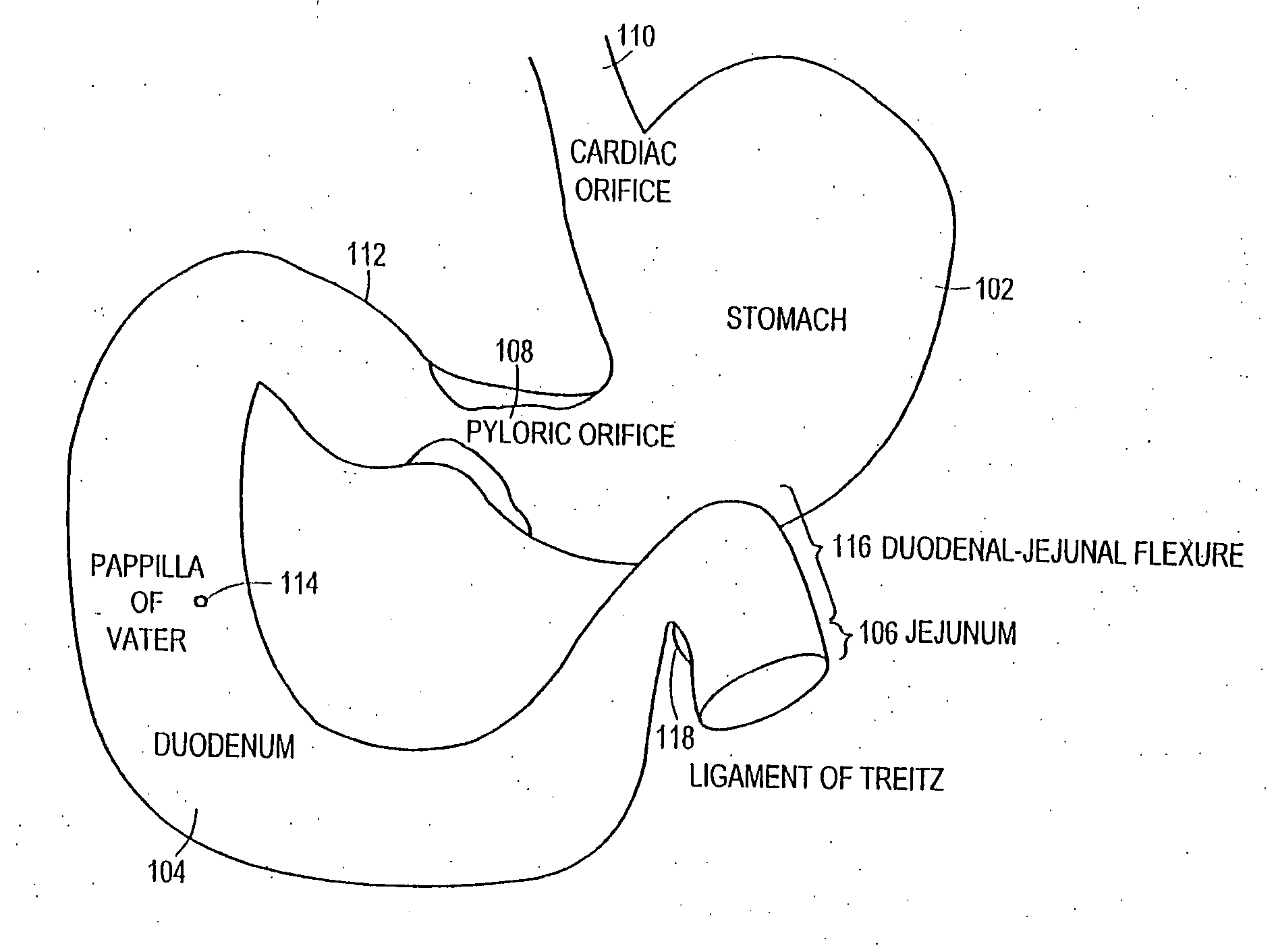
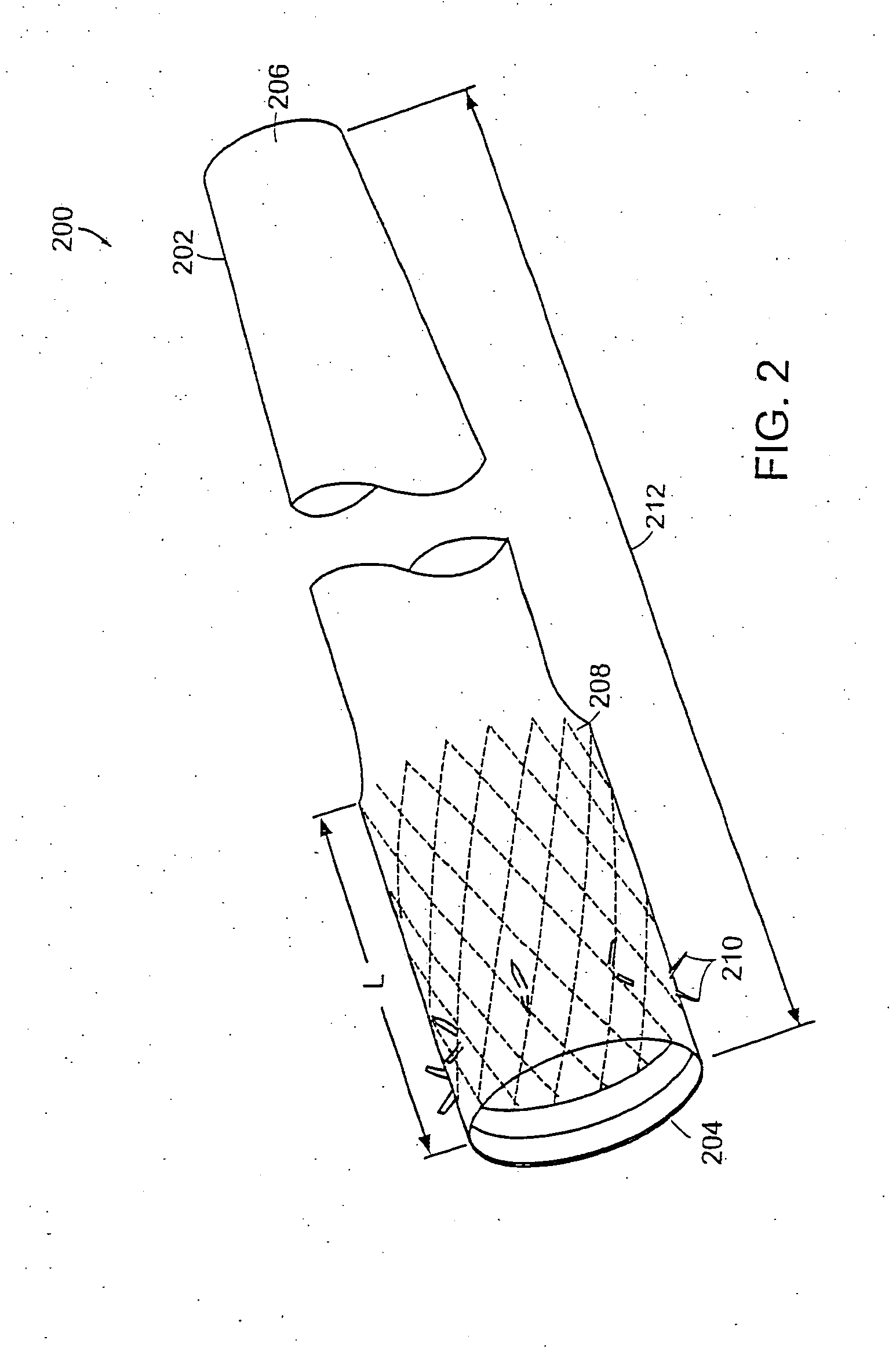
Bariatric sleeve
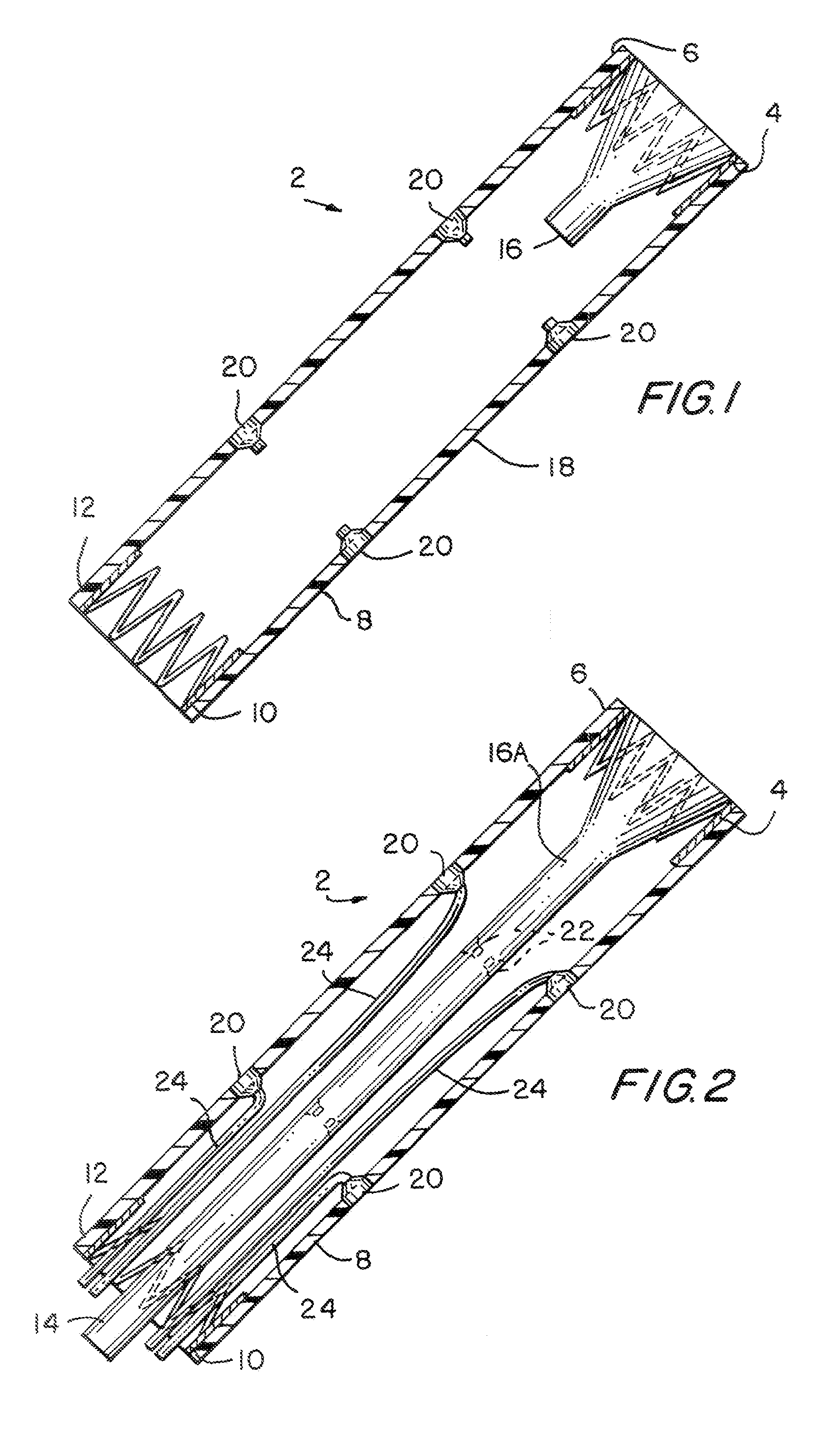
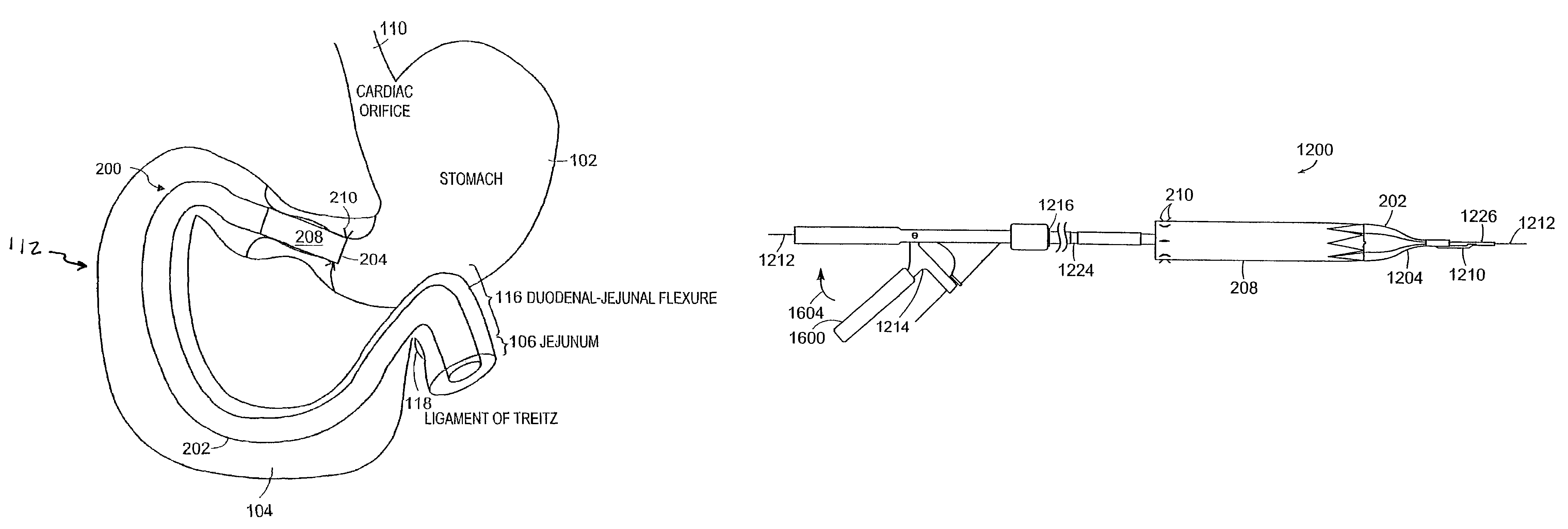
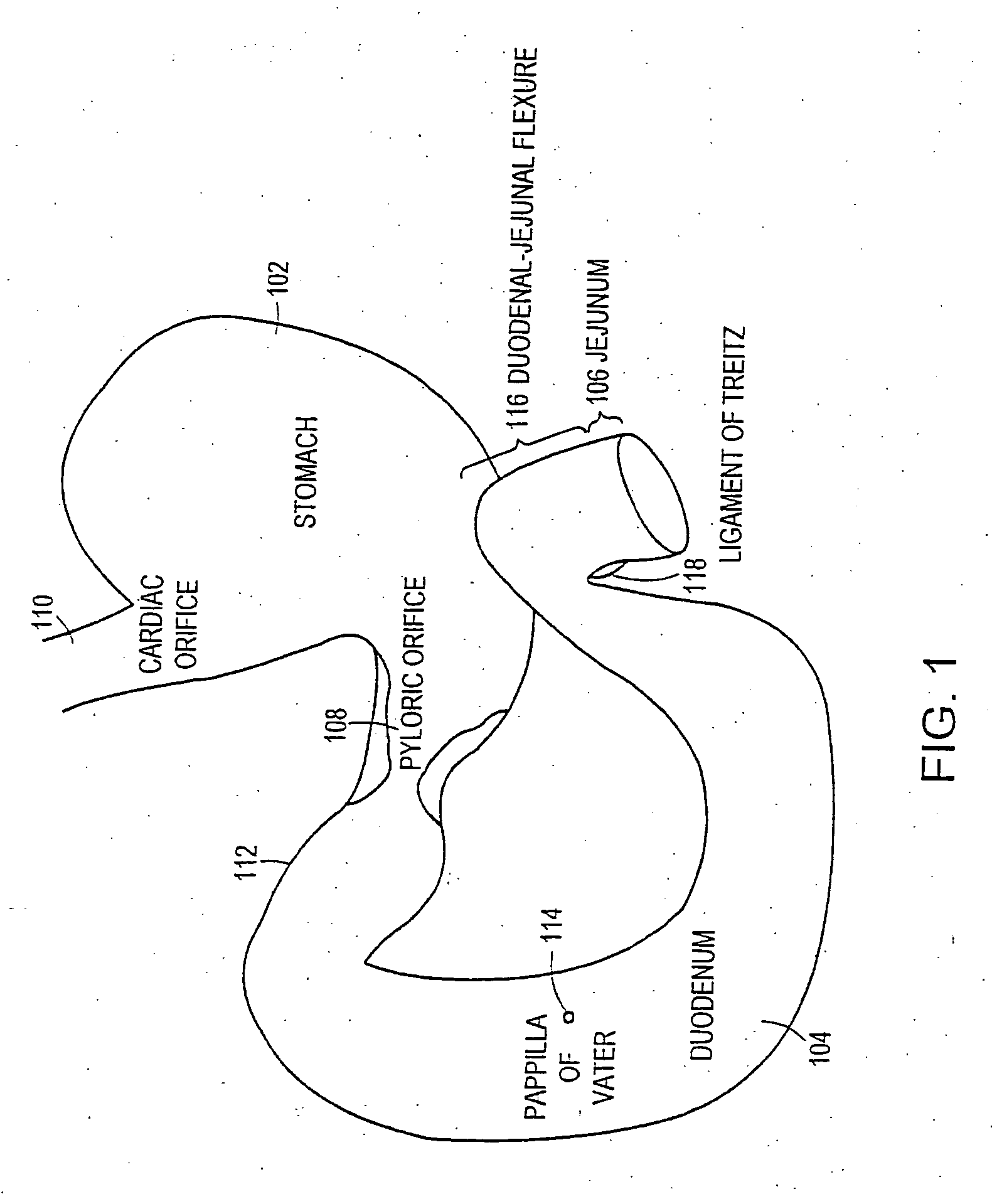
InactiveUS20060161265A1Increased axial stabilityLess movementSuture equipmentsStentsIntestinal structureGastrointestinal device
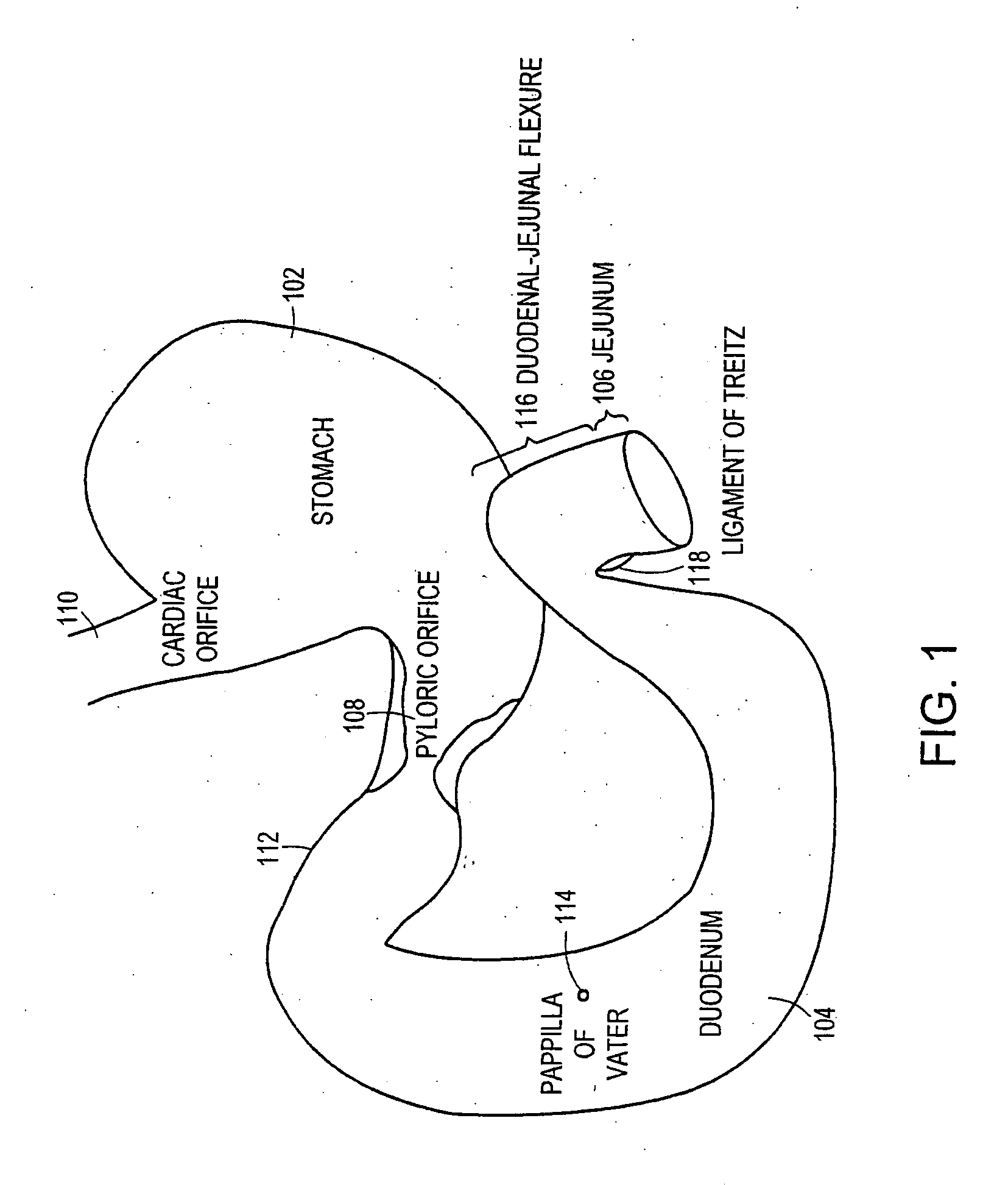
Method and apparatus for limiting absorption of food products in specific parts of the digestive system is presented. A gastrointestinal implant device is anchored in the stomach and extends beyond the ligament of Treitz. All food exiting the stomach is funneled through the device. The gastrointestinal device includes an anchor for anchoring the device to the stomach and a flexible sleeve. When implanted within the intestine, the sleeve can limit the absorption of nutrients, delay the mixing of chyme with digestive enzymes, altering hormonal triggers, providing negative feedback, and combinations thereof. The anchor is collapsible for endoscopic delivery and removal.
Owner:GI DYNAMICS
Endoscopic gastric bypass
An endoscopic device separates ingested food from gastric fluids or gastric fluids and digestive enzymes, to treat obesity. In a particular embodiment a gastric bypass stent comprises a tubular member and two or more stent members defining a lumen. The tubular member has a substantially liquid impervious coating or covering and one or more lateral openings to permit one-way liquid flow.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Methods of treatment using a bariatric sleeve
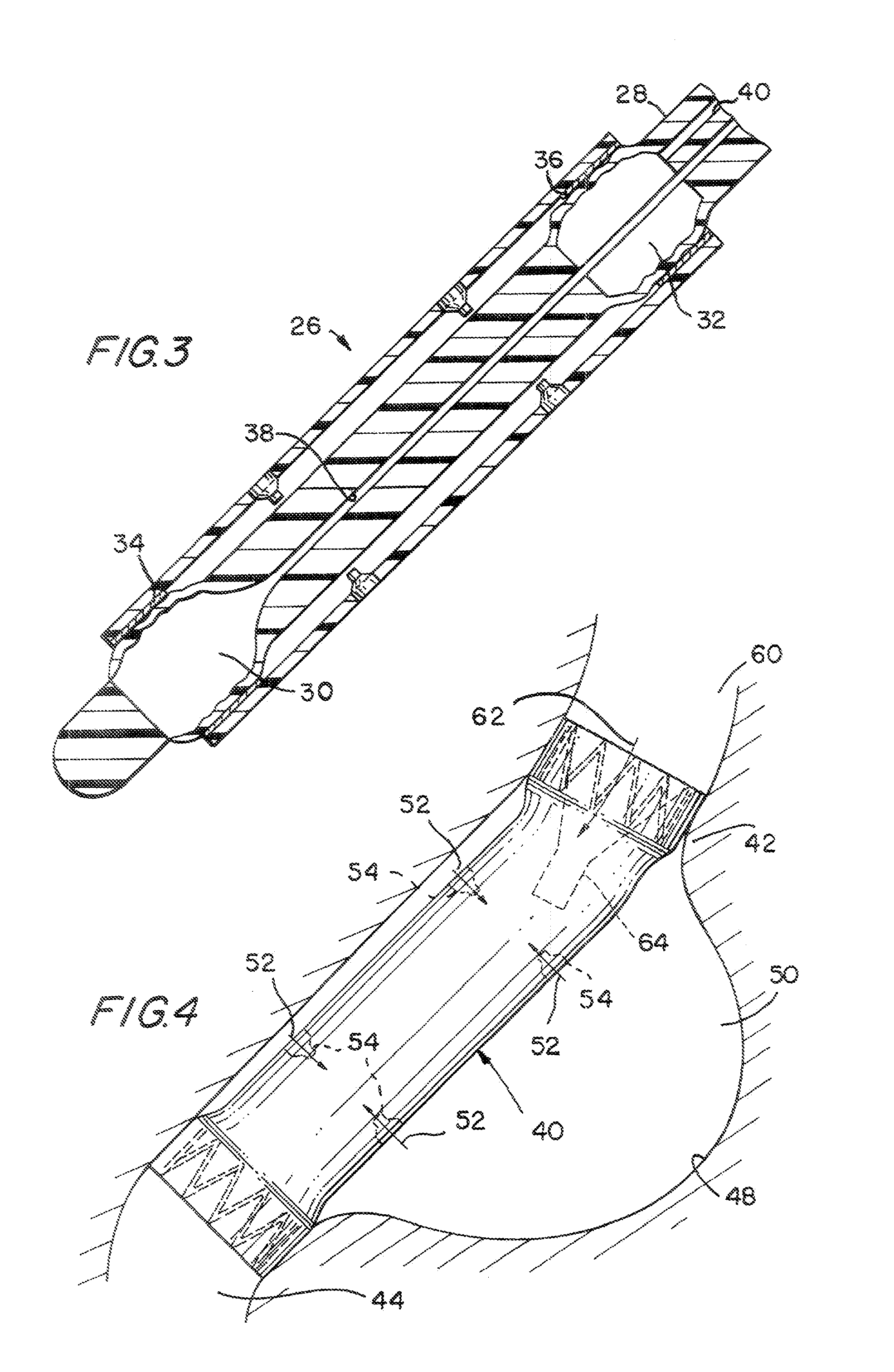
ActiveUS7695446B2Promote healingControlled absorptionSuture equipmentsStentsDiseaseIntestinal structure
Methods of treatment using a gastrointestinal implant device removably anchored within an animal's gastrointestinal tract. For example, the implant device includes a collapsible anchor for anchoring the device coupled to a proximal end of a flexible sleeve. The implant device can be anchored within the stomach, within the pyloric orifice, and / or distal to the pylorus and extended into the duodenum. All partially-digested food, or chyme, exiting the stomach is funneled through the device. Methods of treatment include treating obesity by one or more of: limiting the absorption of nutrients within the duodenum; delaying the mixing of chyme with digestive enzymes; alter hormonal triggers; and providing negative feedback. Alternatively or in addition, the desired result includes treating a diseases, such as diabetes, or temporarily shielding a portion of the intestine to promote healing within the intestine.
Owner:GI DYNAMICS INC
Methods and compositions for the treatment of pancreatitis
InactiveUS6843998B1Prevents and reduces extent of fusionInhibition formationBacterial antigen ingredientsAntibody mimetics/scaffoldsProtein translocationDigestive enzyme
Methods and compositions for the treatment of acute pancreatitis in a mammal. Particular compositions comprise a binding element, a translocation element, and a therapeutic element able to prevent accumulation of digestive enzymes within the pancreas.
Owner:ALLERGAN INC
Endoscopic Gastric Bypass
An endoscopic device separates ingested food from gastric fluids or gastric fluids and digestive enzymes, to treat obesity. In a particular embodiment a gastric bypass stent comprises a tubular member and two or more stent members defining a lumen. The tubular member has a substantially liquid impervious coating or covering and one or more lateral openings to permit one-way liquid flow.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Stable digestive enzyme compositions
InactiveUS20090117180A1Minimal loss of activityComposition is stablePowder deliveryHydrolasesDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 9% or less or 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 25%, about 20%, about 15% or about 10% after six months of accelerated stability testing and the titer level of a viral contaminant present in the pancreatin is at least about 1000 times less than the titer level of the viral contaminant present in a preparation from which the pancreatin is obtained.
Owner:APTALIS PHARMA
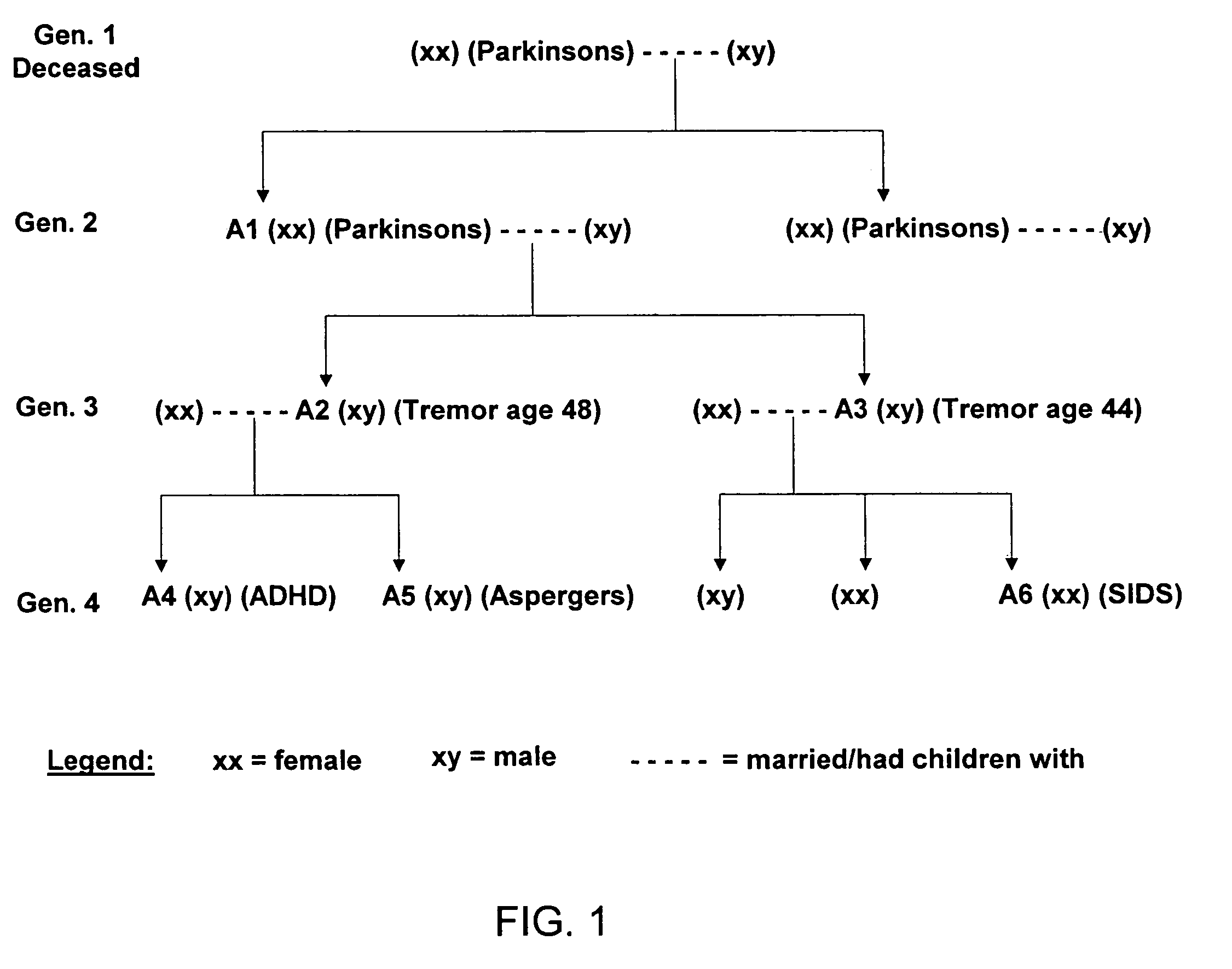
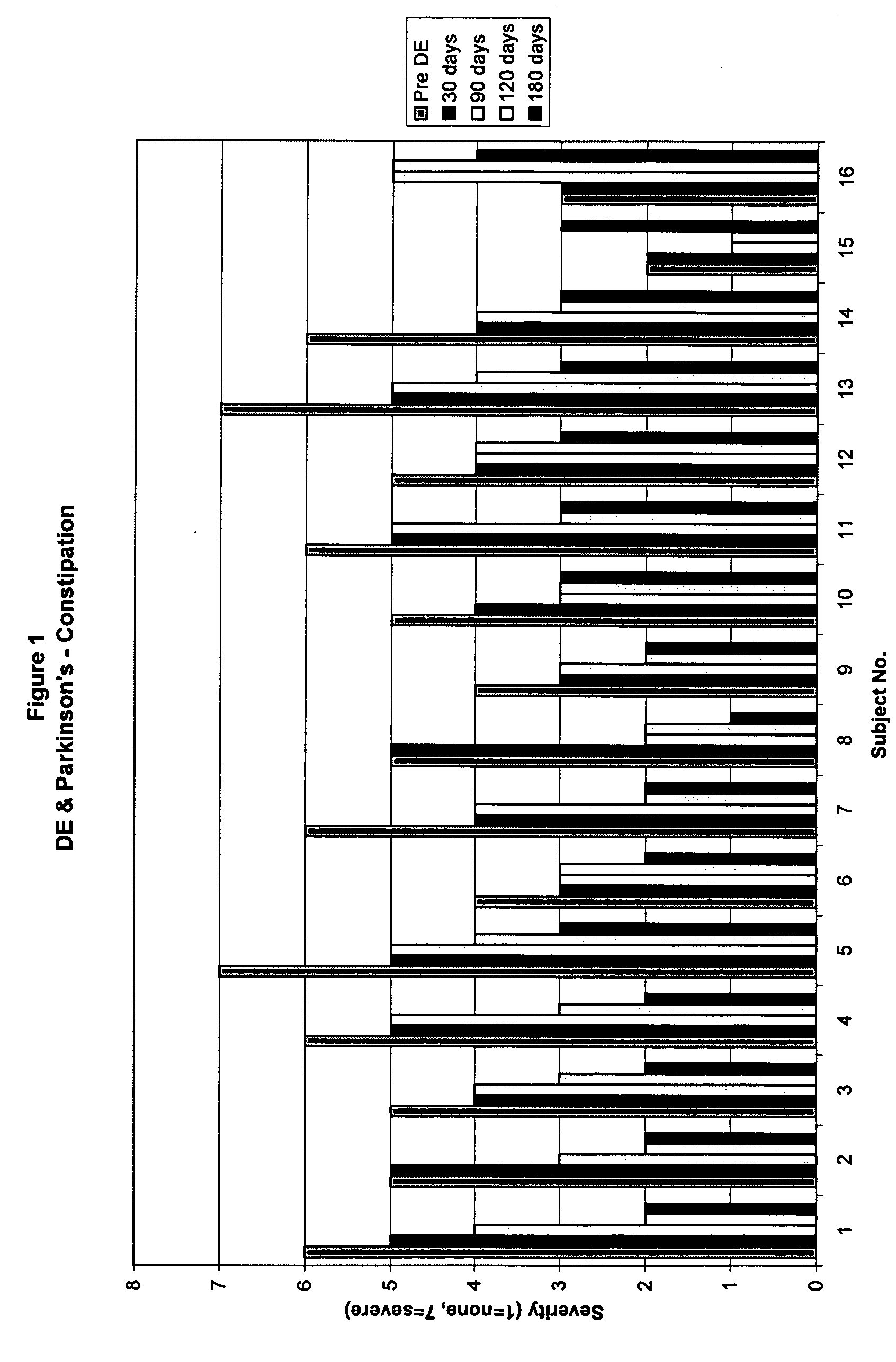
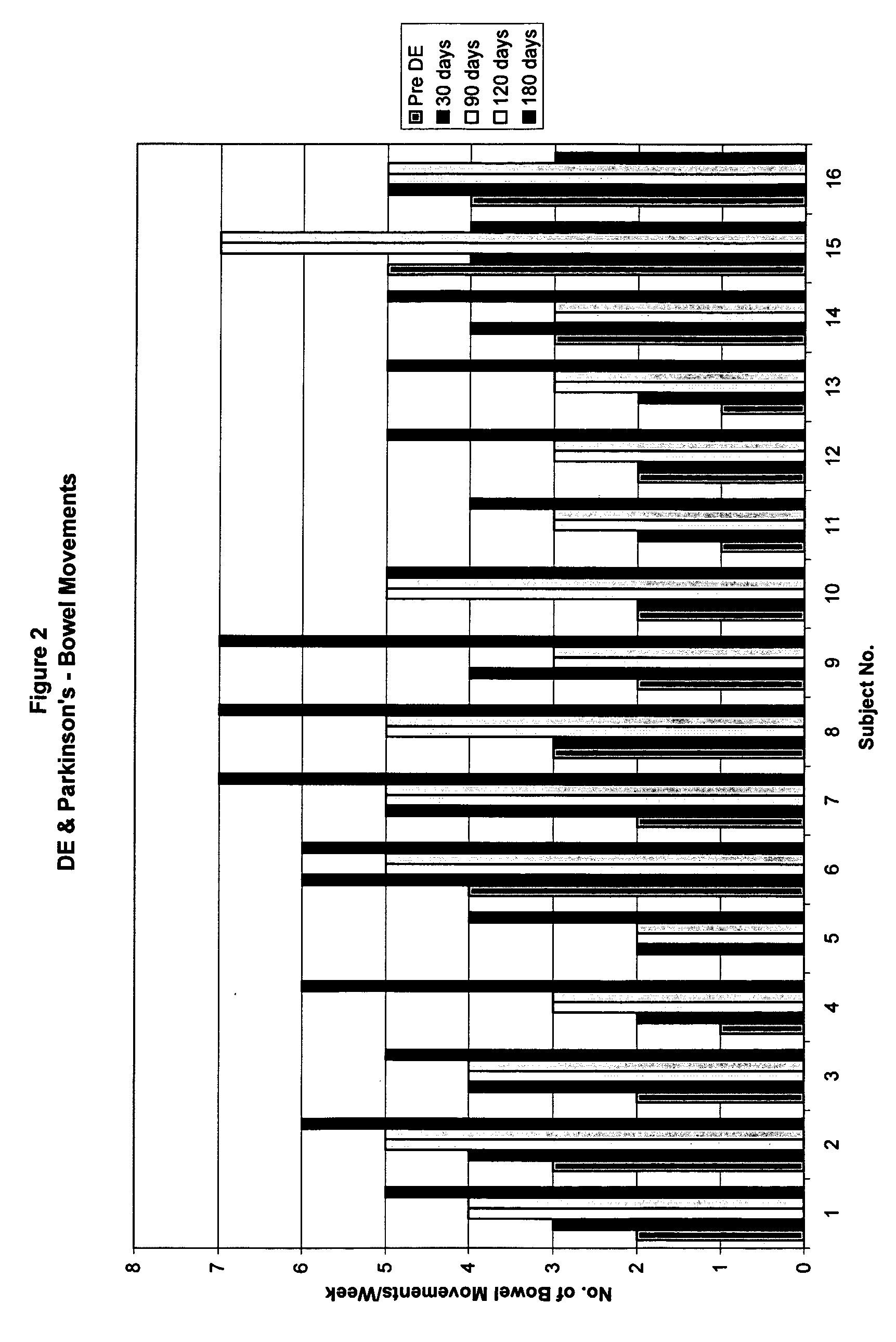
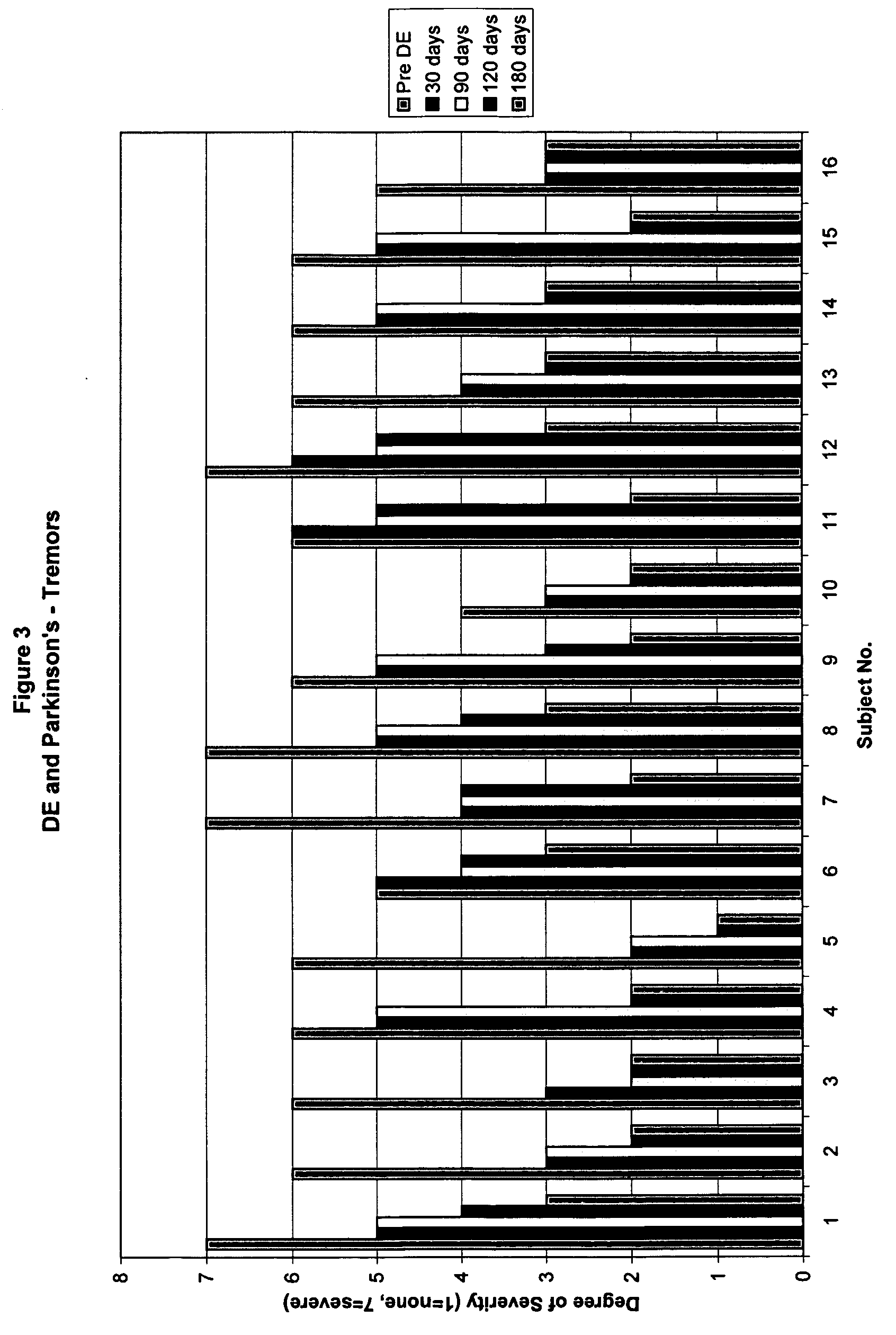
Method of treating and diagnosing parkinsons disease and related dysautonomic disorders
InactiveUS20070053895A1Symptoms improvedNervous disorderPeptide/protein ingredientsAutonomic bladder dysfunctionPsychiatry
A method for treating a Parkinson's patient with digestive / pancreatic enzymes involves administering an effective amount of digestive / pancreatic enzymes to an individual having the disorder in order to improve a symptom of the disorder. In addition, a method is provided for determining whether an individual has, or may develop, Parkinson's disease or related dysautonomic disorders and for determining whether an individual will benefit from the administration of pancreatic / digestive enzymes to treat the dysautonomic disorder.
Owner:CUREMARK
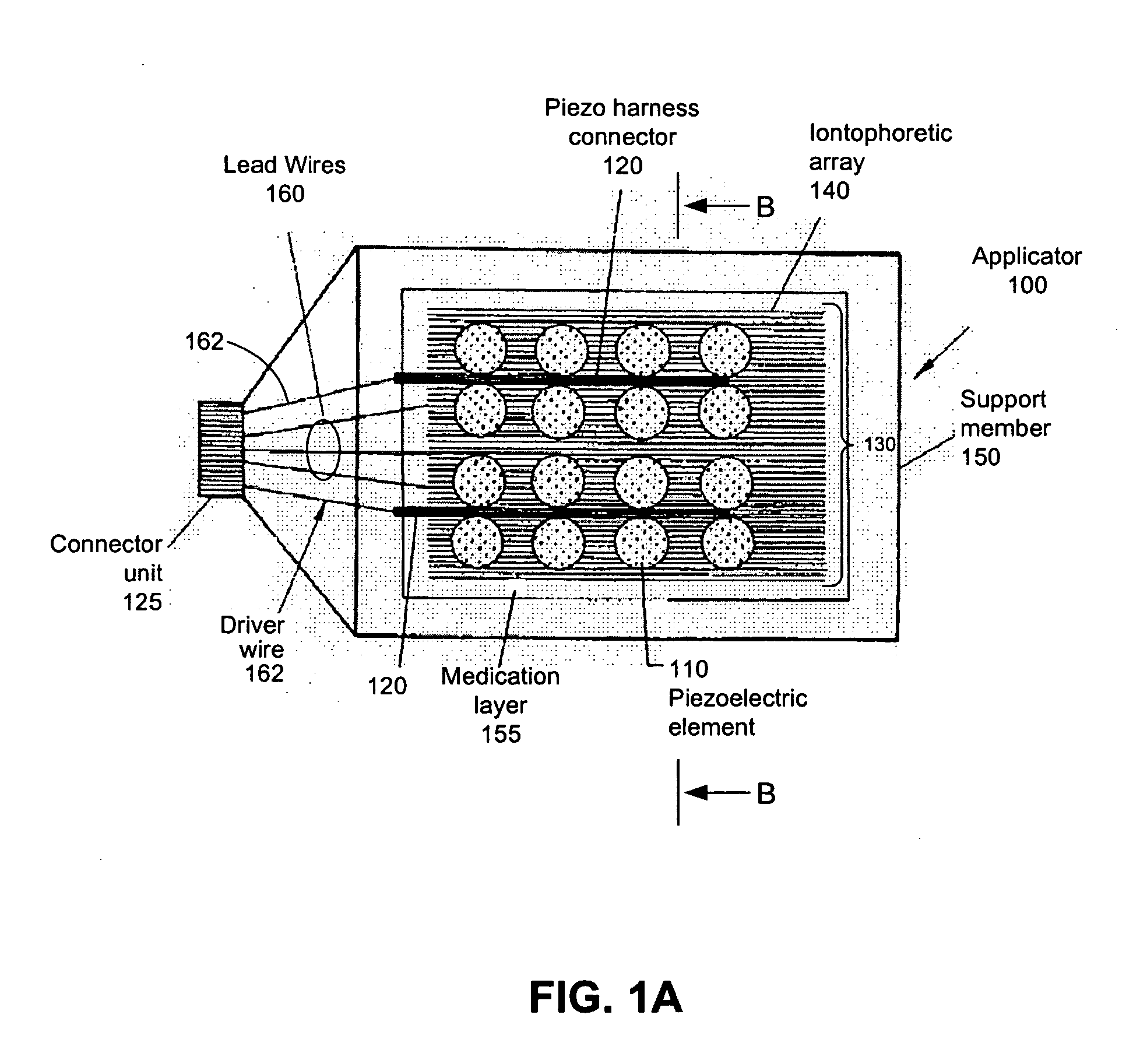
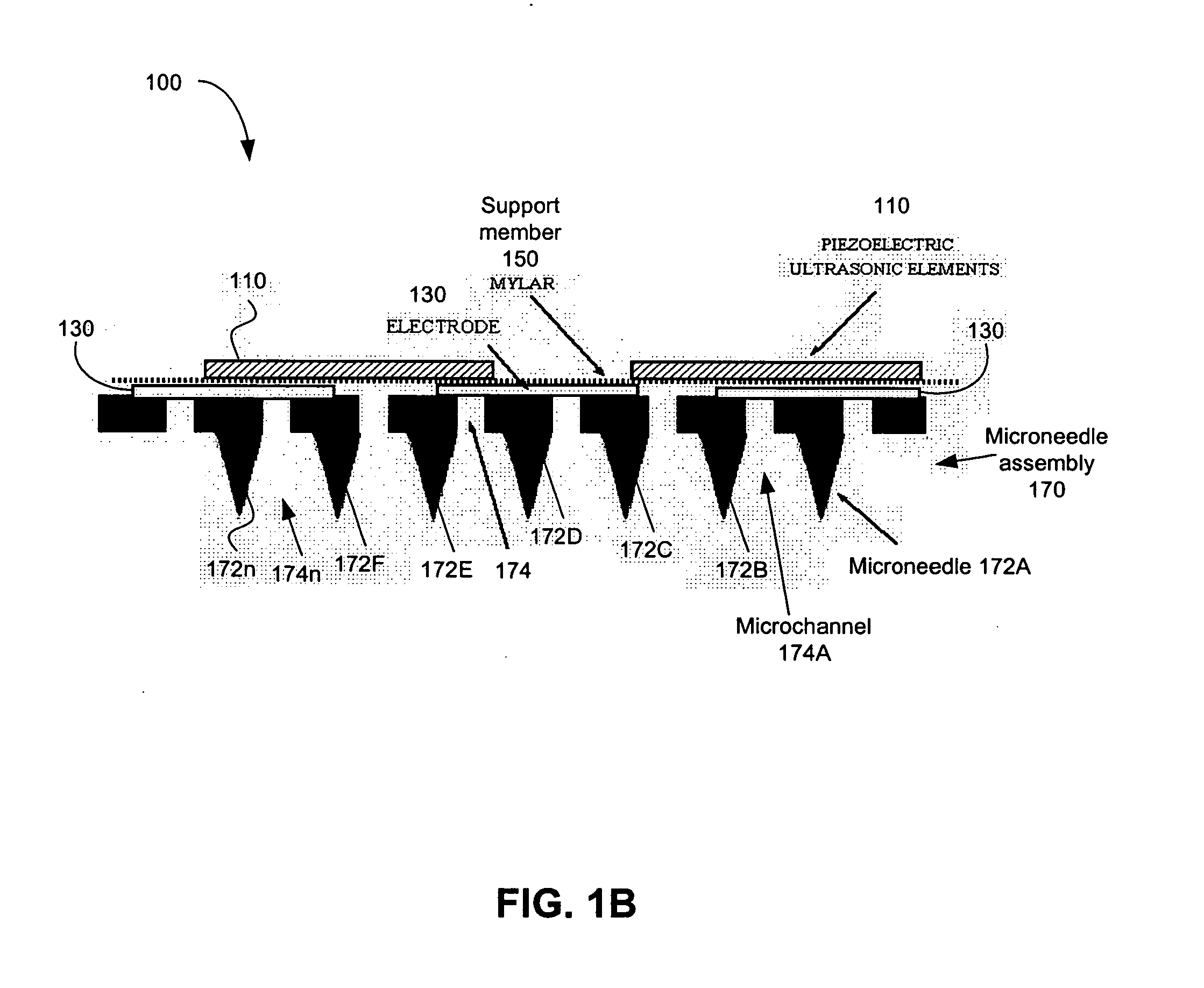
Iontosonic-microneedle applicator apparatus and methods
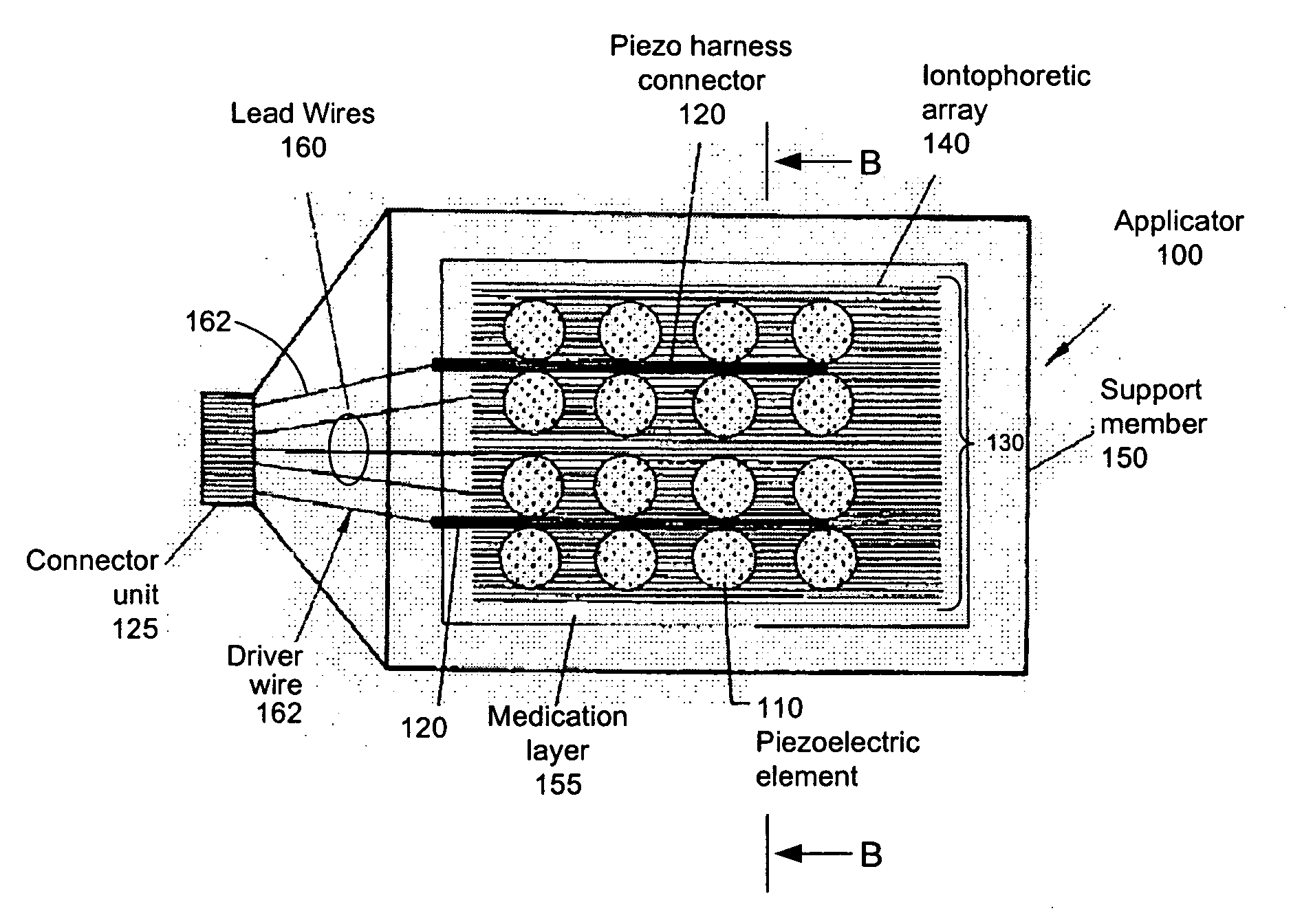
Novel multichannel ionosonic devices with microneedle arrays incorporated into the devices for transdermal and intradermal delivery are described. In an embodiment, the ionosonic device includes a multichannel ionophoretic driver used in combination with a multichannel dispersion electrode integrated with ultrasonic elements and microneedle array elements mounted on a single application electrode. The ionosonic-microneedle transdermal device can be configured in a variety of shapes and structural flexibility for the treatment of skin and systemic disorders through the intradermal and transdermal delivery of one or more of a variety of therapeutic and modulating agents. Because of enhanced transdermal penetration this device offers the transdermal delivery of therapeutic peptides is getting closer to reality. The devices described herein deliver the therapeutic agents to the targeted diseased area as well as systemic levels obviating the need for oral ingestion, the associated side effects and as in the case of peptides bypasses the hydrolyzing digestive enzymes that make such agents ineffective when taken by mouth.
Owner:MIT
Endoscopic gastric bypass
An endoscopic device separates ingested food from gastric fluids or gastric fluids and digestive enzymes, to treat obesity. In a particular embodiment a gastric bypass stent comprises a tubular member and two or more stent members defining a lumen. The tubular member has a substantially liquid impervious coating or covering and one or more lateral openings to permit one-way liquid flow.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Stable pancreatic enzyme compositions
ActiveUS20080274174A1Stable digestive enzyme compositionThe dosage form is stablePeptide/protein ingredientsHydrolasesDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 15% after six months of accelerated stability testing.
Owner:SOC DES PROD NESTLE SA
Pharmaceutical preparations for attention deficit disorder, attention deficit hyperactivity disorder and other associated disorders
InactiveUS20070116695A1Well formedFormulation stabilityBiocidePeptide/protein ingredientsChymotrypsinAttention deficits
A pharmaceutical preparation for the treatment of attention deficit disorders combines a therapeutically effective amount of digestive enzymes, such as chymotrypsin, and medication used to treat attention deficit disorders, such as Ritalin®, Concert®, Adderall® and Strattera®. The preparation may be in the form of a tablet, capsule or time released formula in order to reduce the amount of pills per dosage. The pharmaceutical preparation ameliorates the symptoms of the attention deficit disorder. The preparation has a stabilizing matrix containing a solidified microcrystalline cellulose which captures and protects therapeutically effective amounts of digestive enzyme particles within the stabilizing matrix.
Owner:CUREMARK
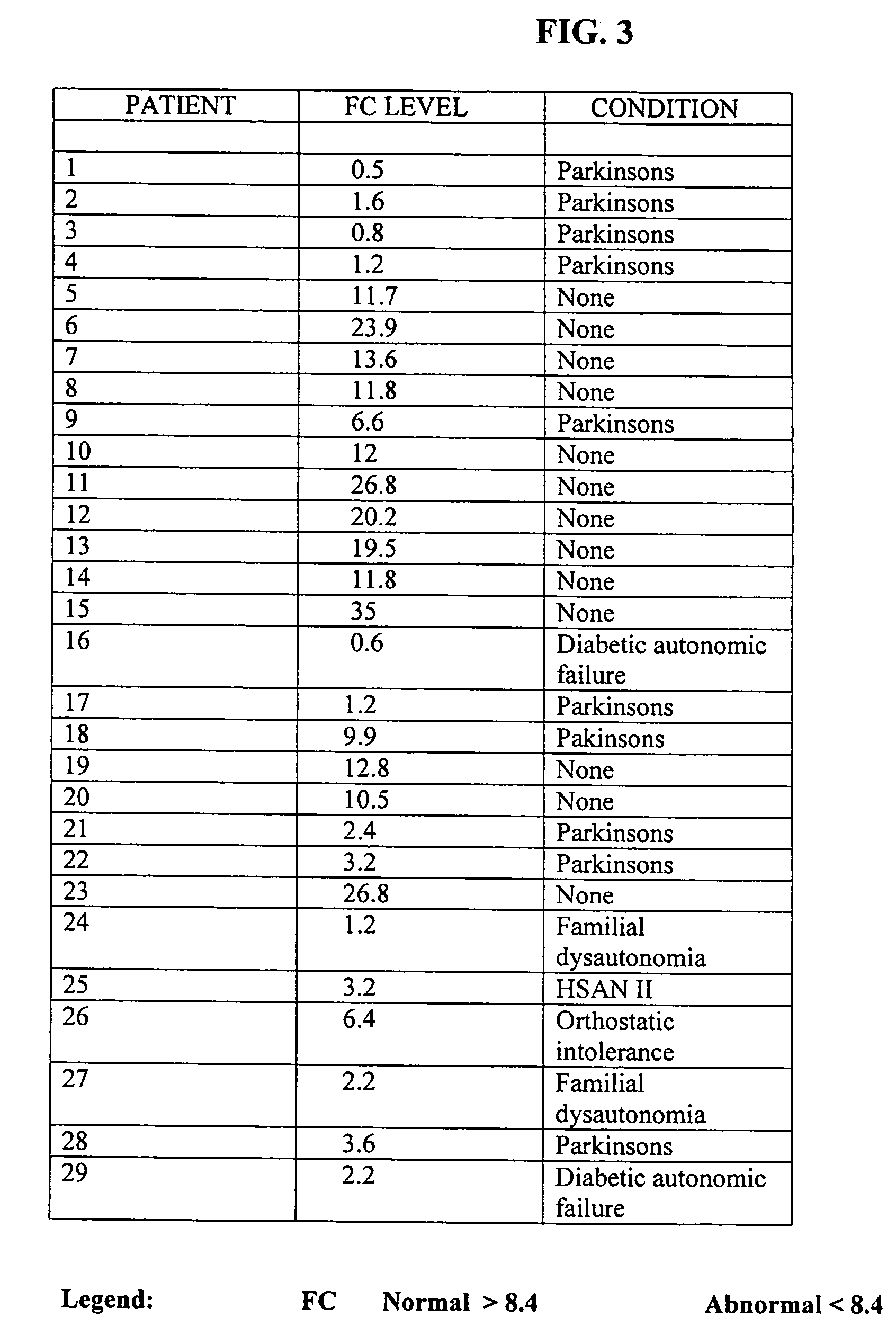
Methods for diagnosing and treating dysautonomia and other dysautonomic conditions
Methods for aiding in the diagnosis of dysautonomic disorders and dysautonomic conditions and methods for treating individuals diagnosed as having a dysautonomic disorder or a dysautonomic condition. In one aspect, a diagnosis method comprising analyzing a stool sample of an individual for the presence of a biological marker wherein the quantity of the biological marker is an indication of whether the individual has, or can develop, a dysautonomic disorder or dysautonomic condition, as well as a therapeutic method for treating a dysautonomic disorder or dysautonomic condition by administration of, e.g., secretin, neuropeptides, peptides and / or digestive enzymes.
Owner:CUREMARK
Enzyme composition for improving food digestion
InactiveUS20080199448A1Improving food absorptionPromote digestionOrganic active ingredientsPeptide/protein ingredientsProteinase activityAdditive ingredient
An orally administered composition for improving food absorption and digestion contains therapeutically effective dosages of digestive enzymes and L-glutamine as active ingredients. The digestive enzymes include at least one each of a lipase, a protease, and an amylase, and at least a portion of each of these enzymes is enteric coated.
Owner:NUTRI-HEALTH SUPPLEMENTS LLC
Methods of producing stable pancreatic enzyme compositions
ActiveUS20080279953A1Minimal loss of activityComposition is stablePowder deliveryPeptide/protein ingredientsDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 15% after six months of accelerated stability testing.
Owner:SOC DES PROD NESTLE SA
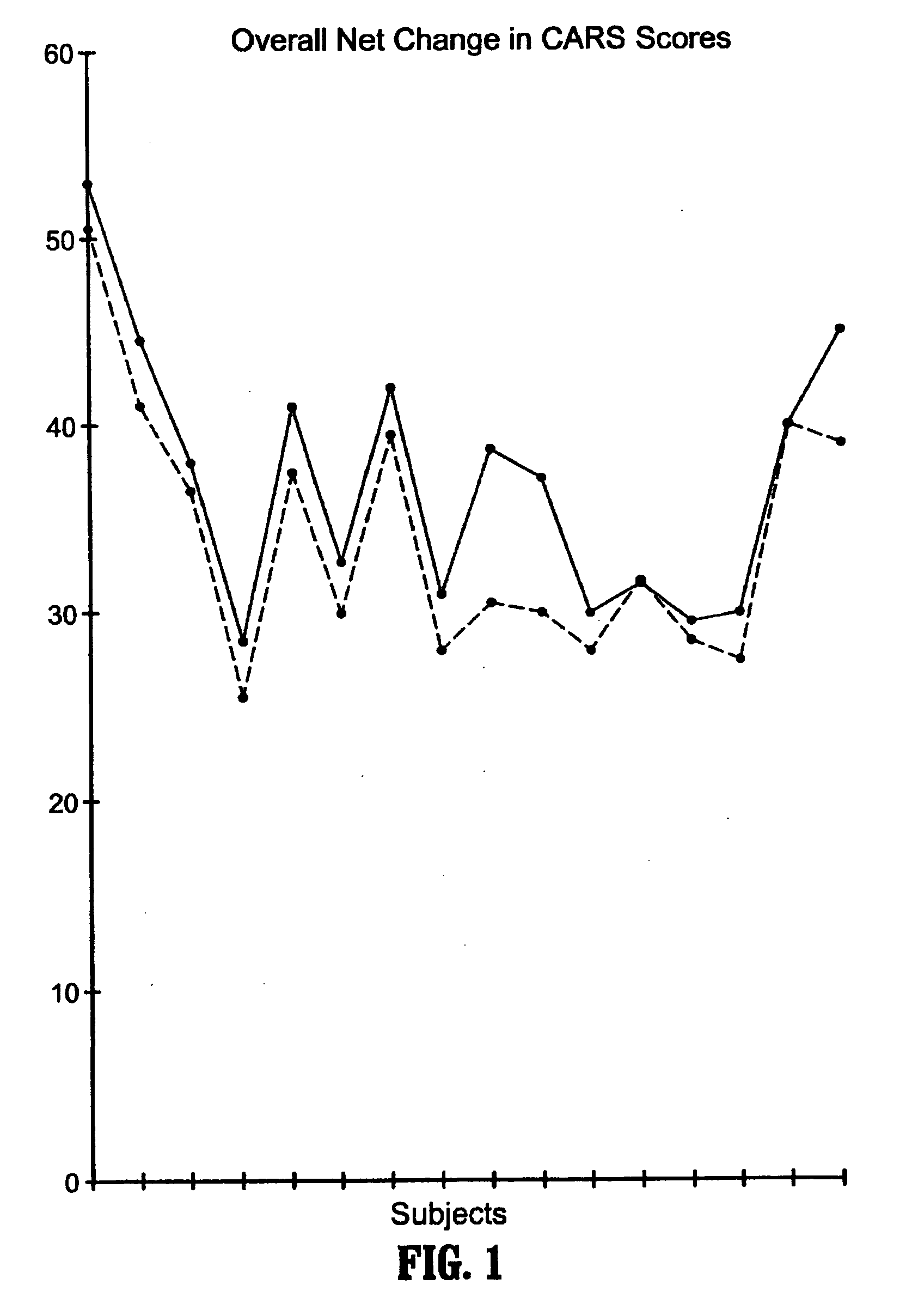
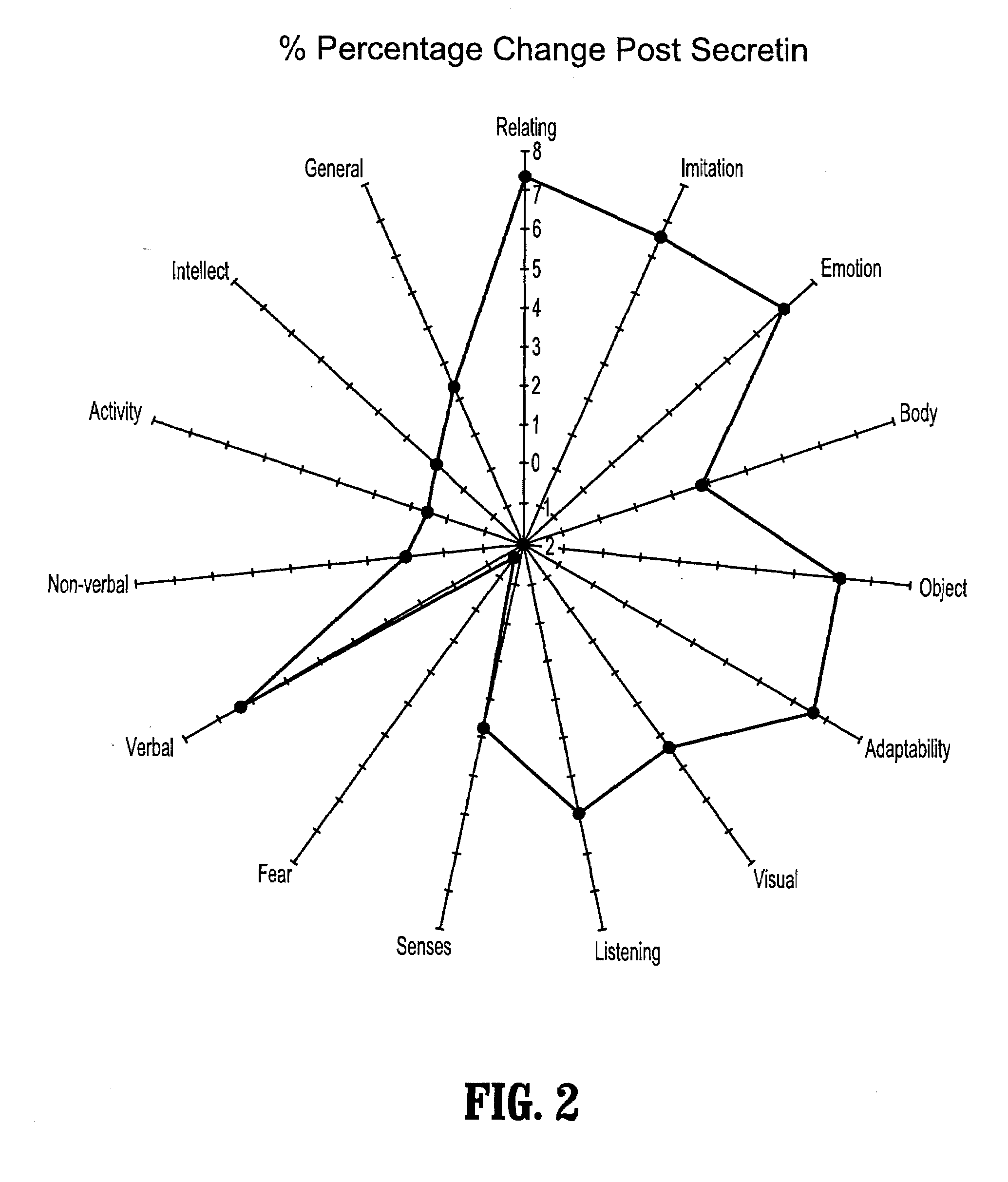
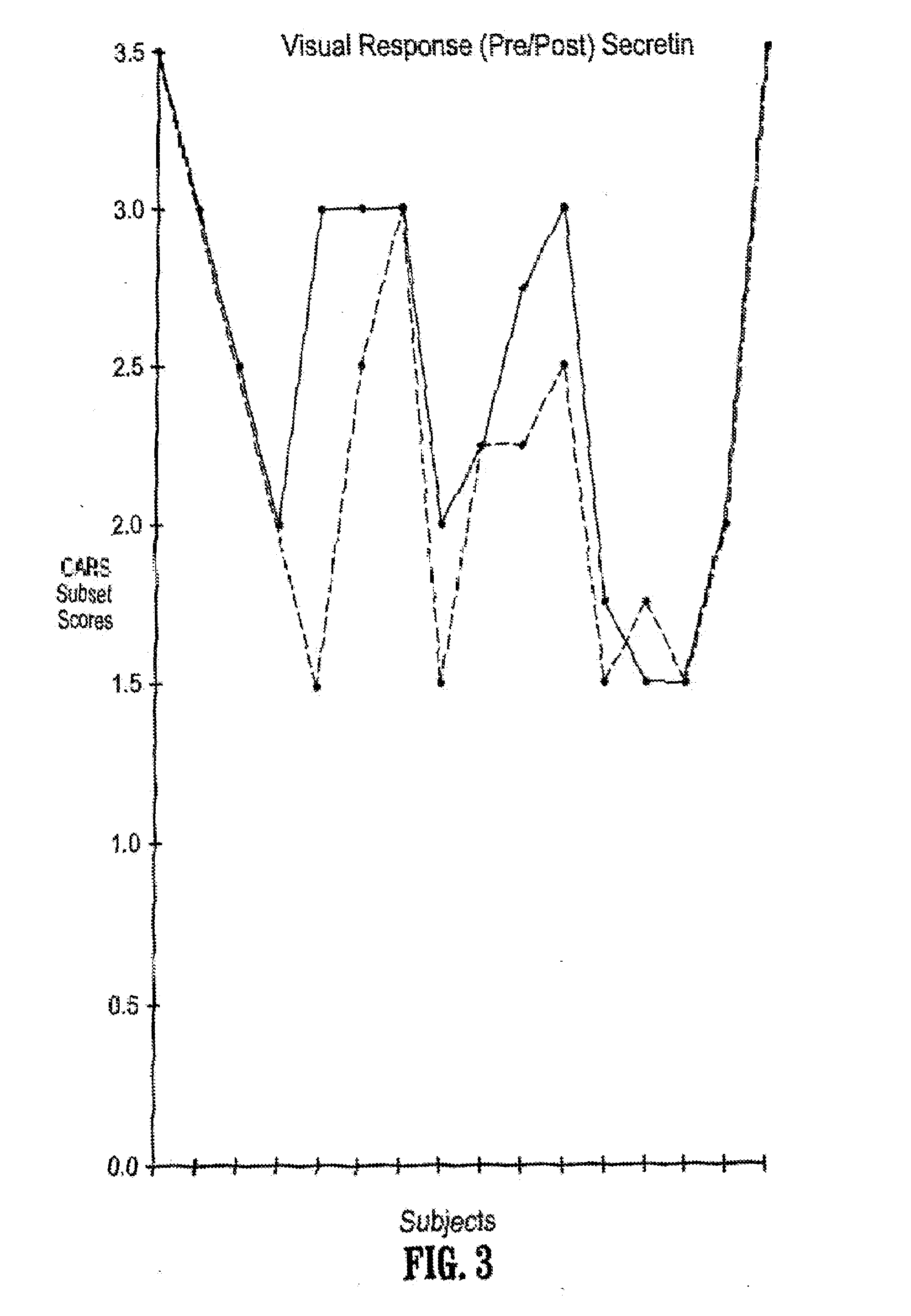
Methods for treating pervasive development disorders
InactiveUS20060183180A1Symptoms improvedPromote digestionNervous disorderPeptide/protein ingredientsDiseasePervasive developmental disorder
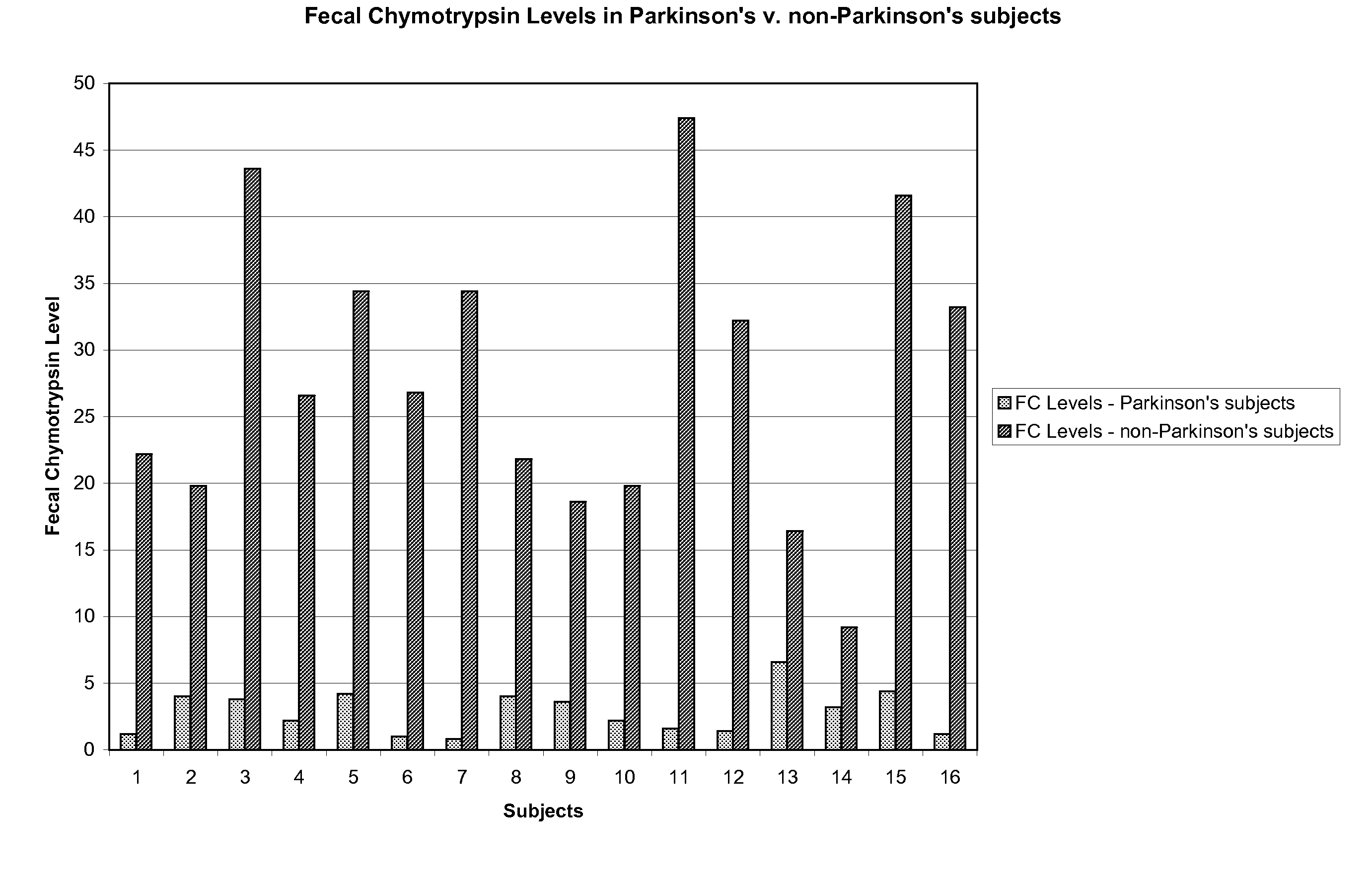
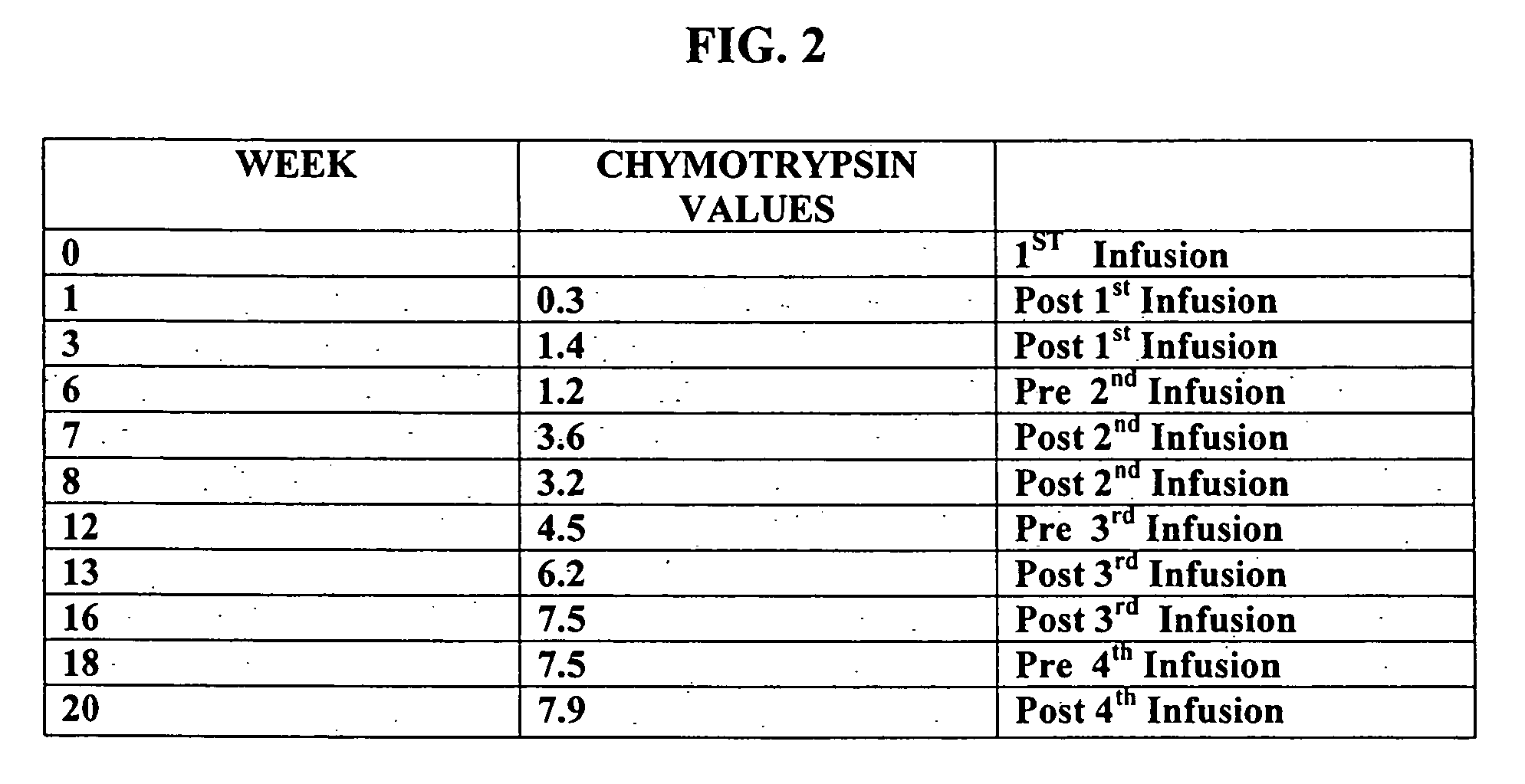
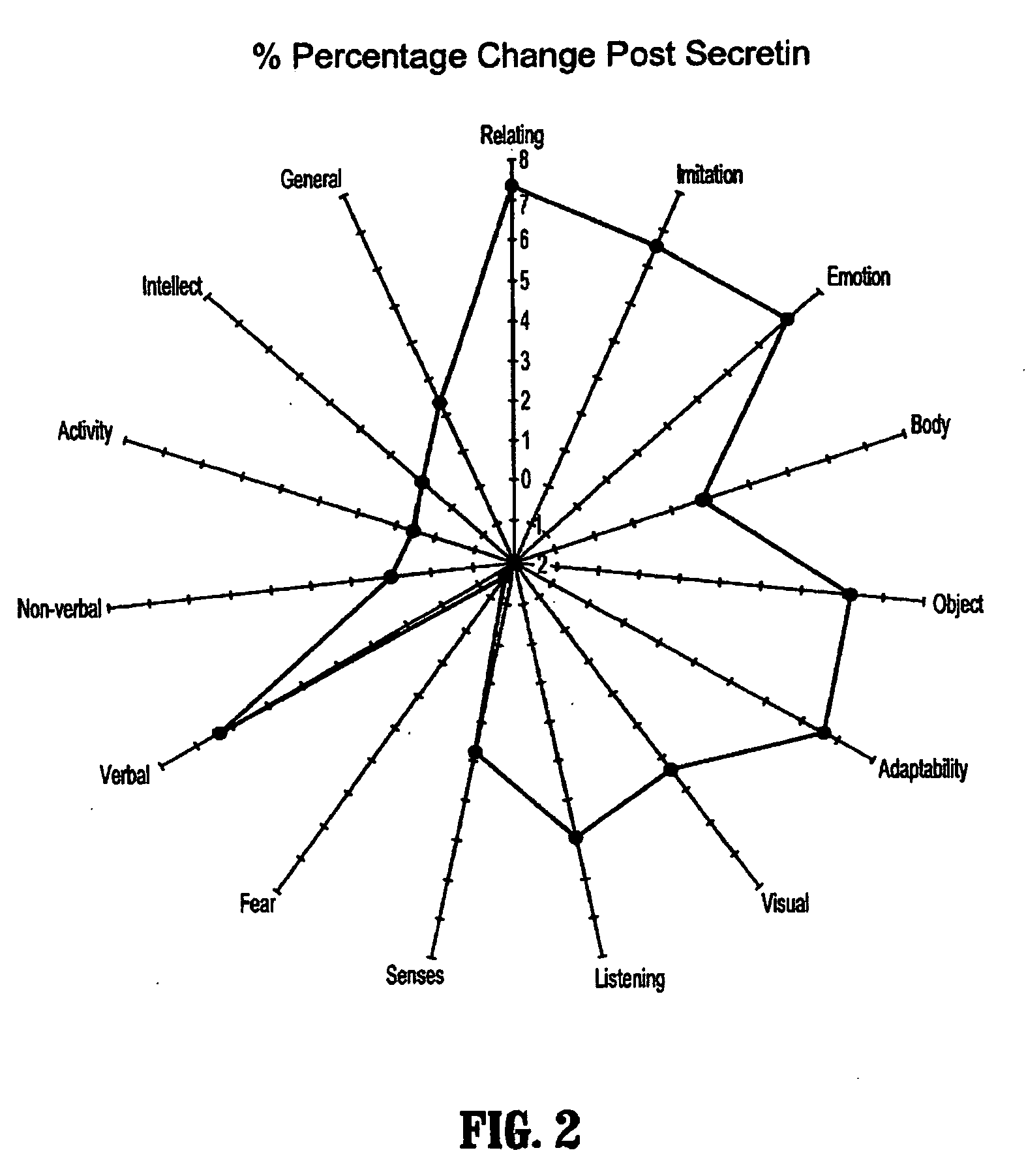
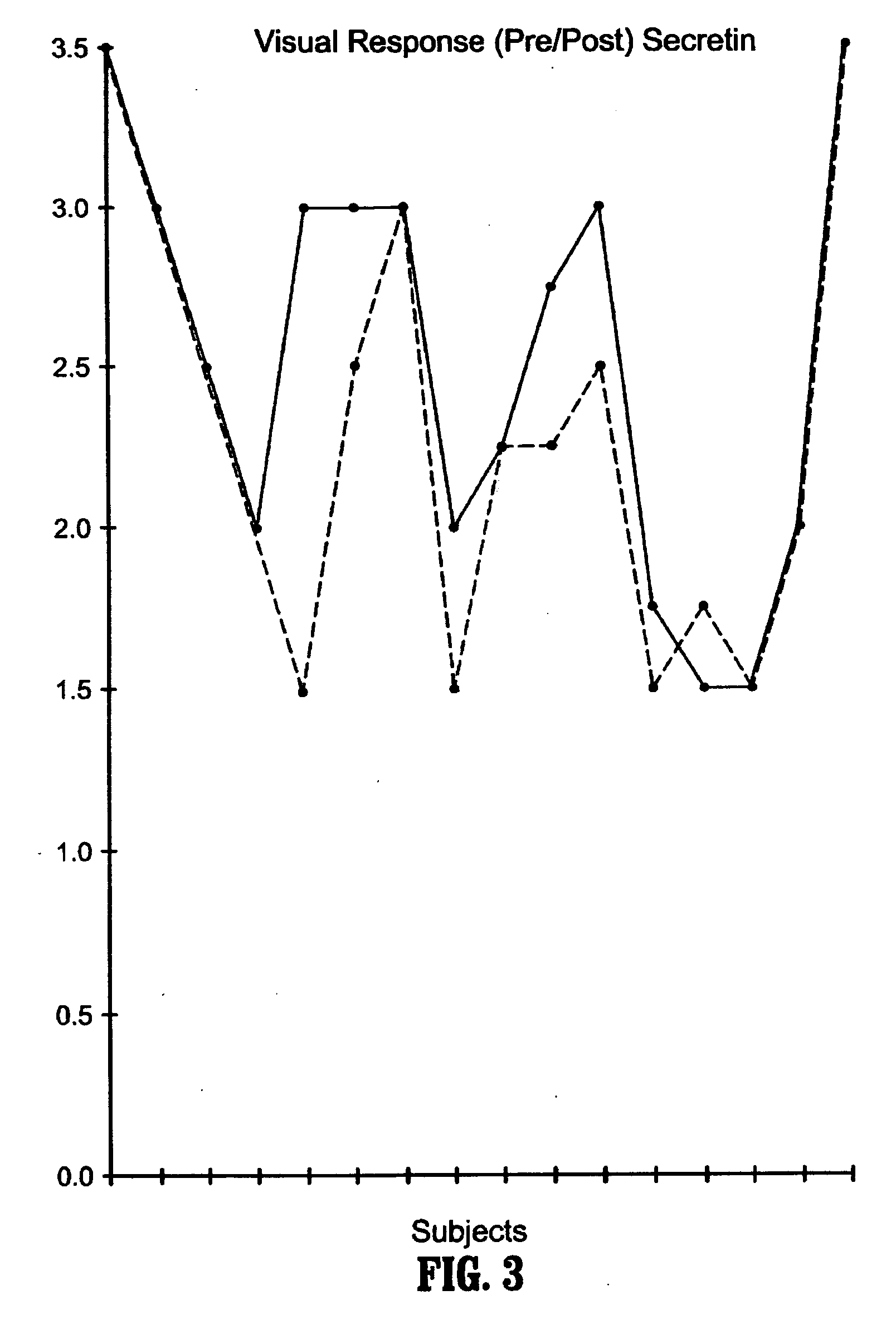
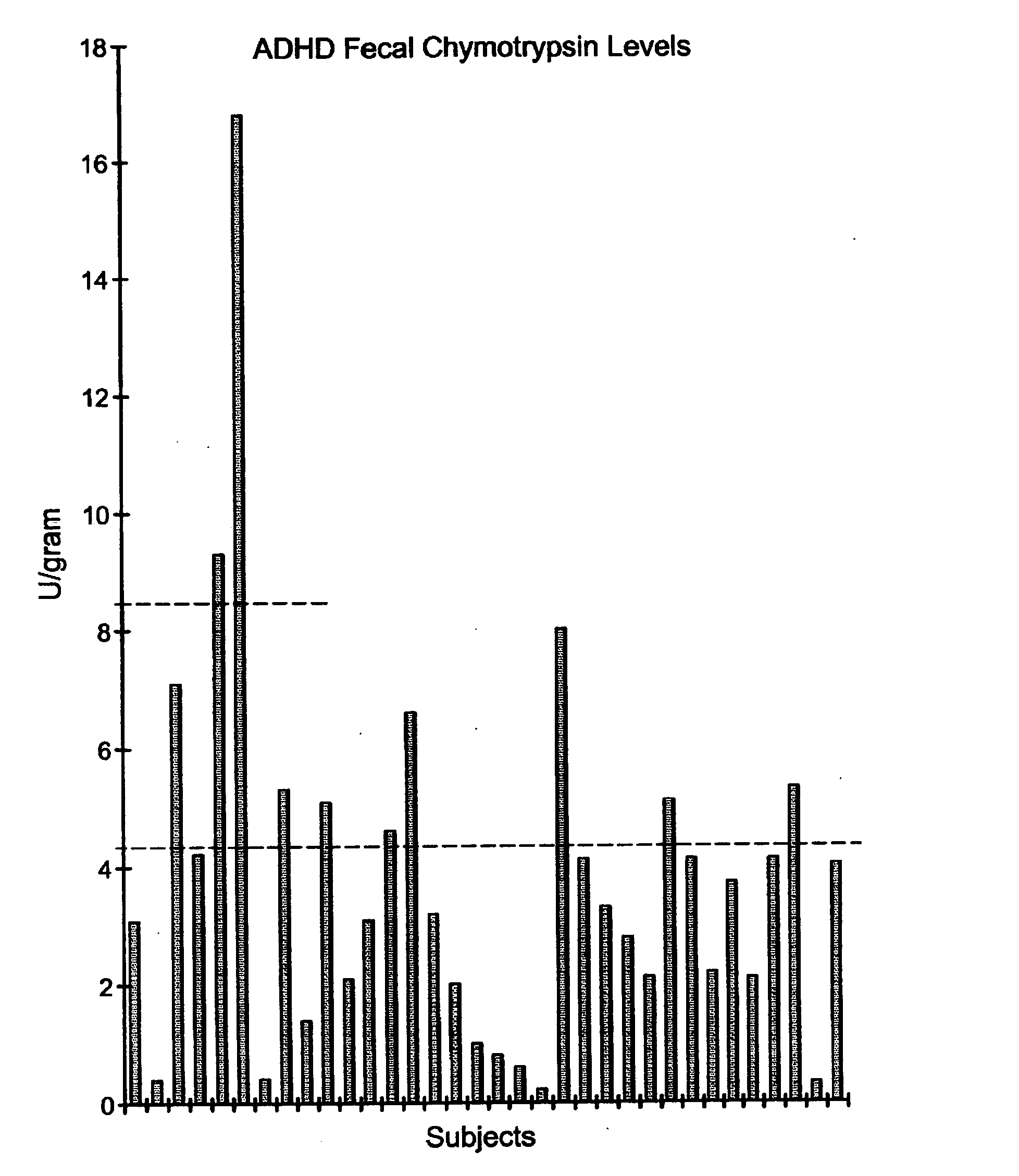
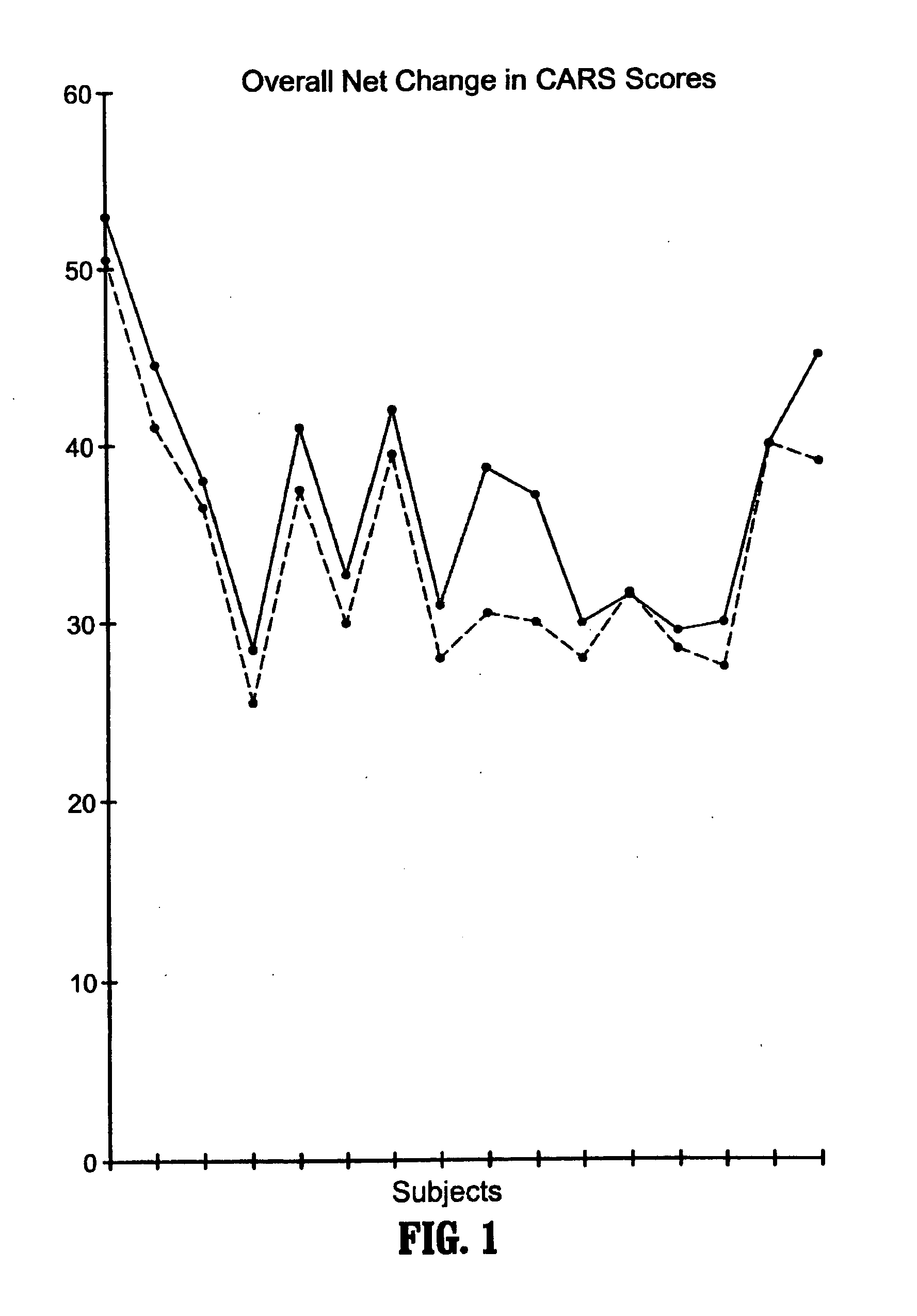
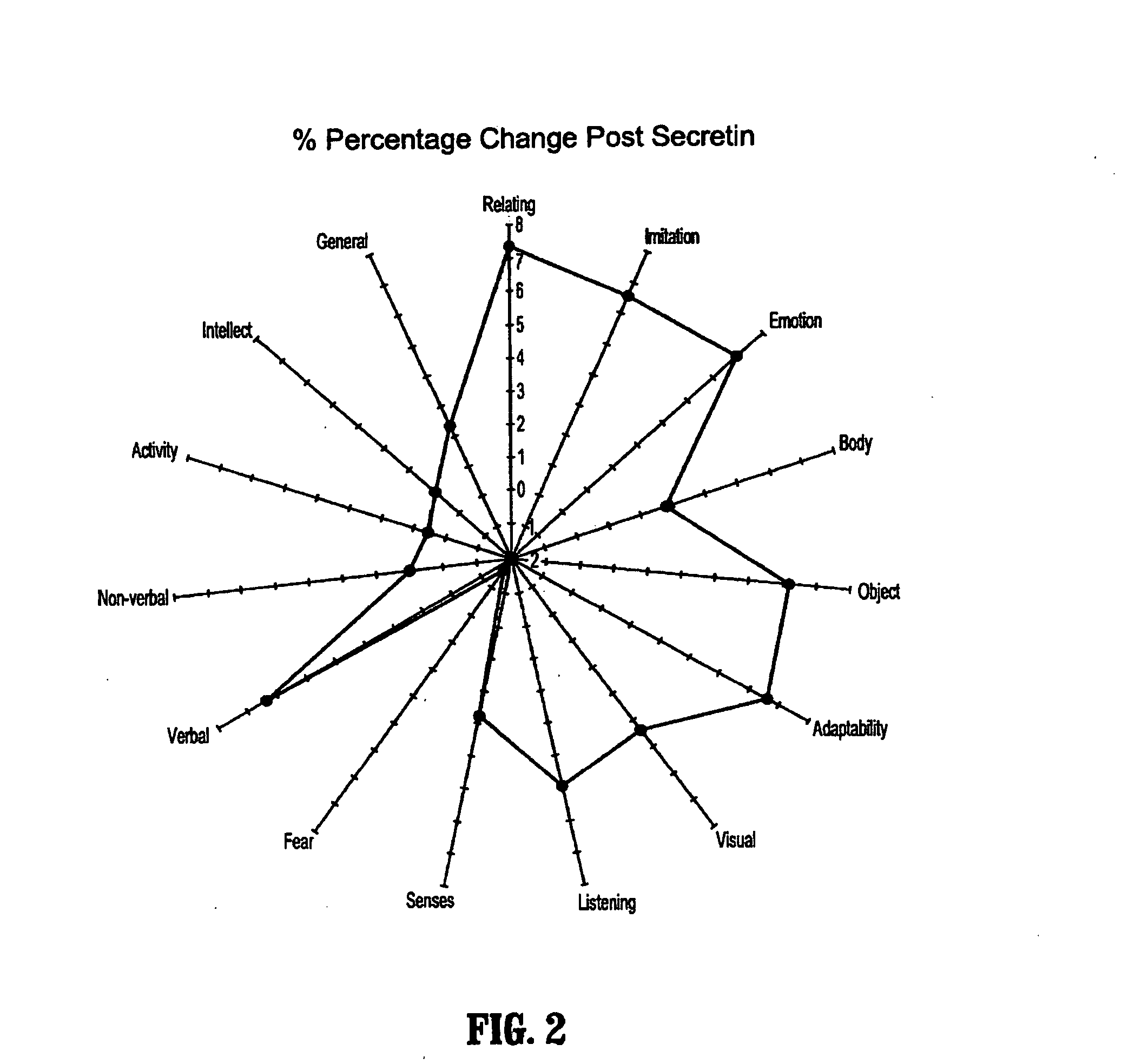
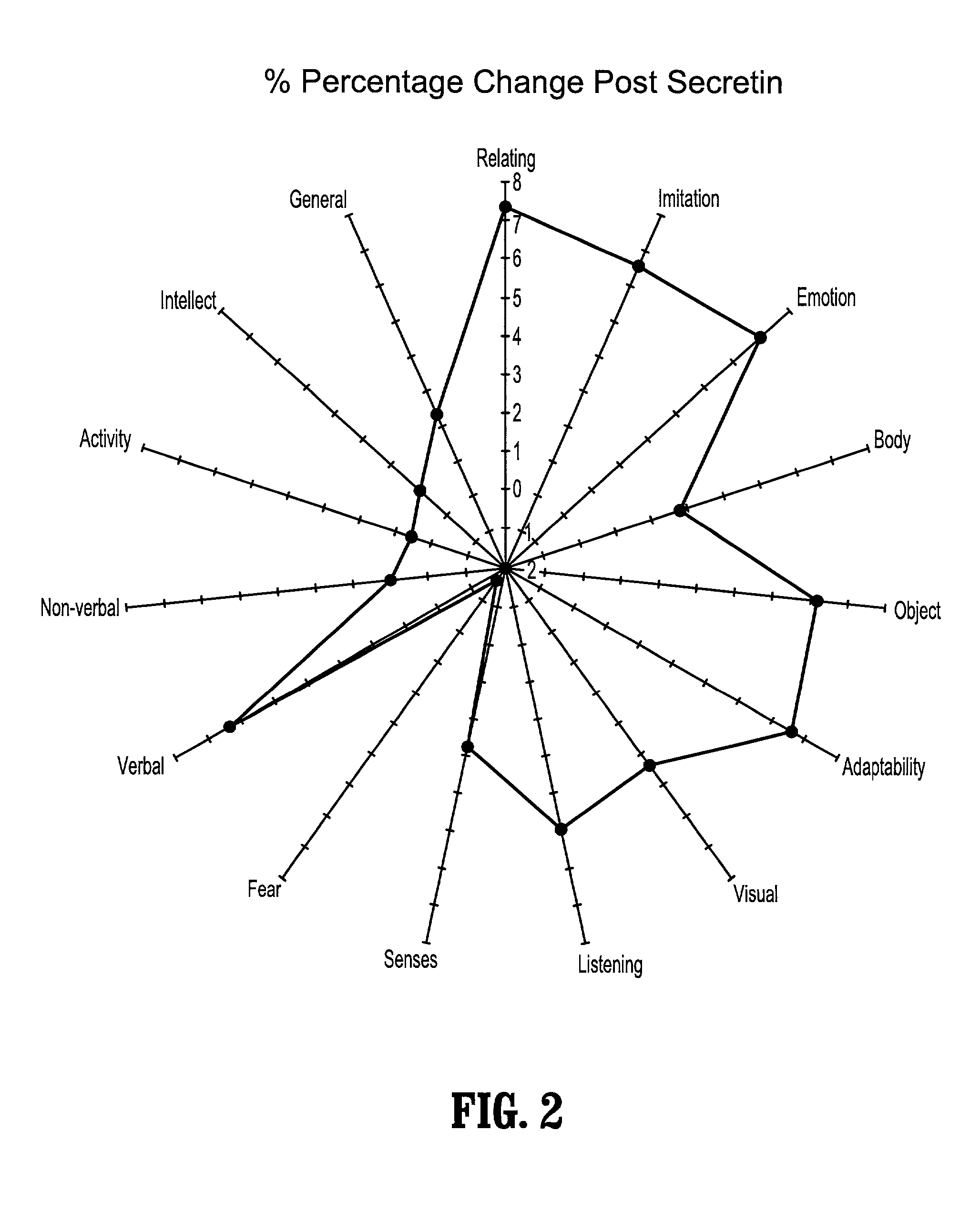
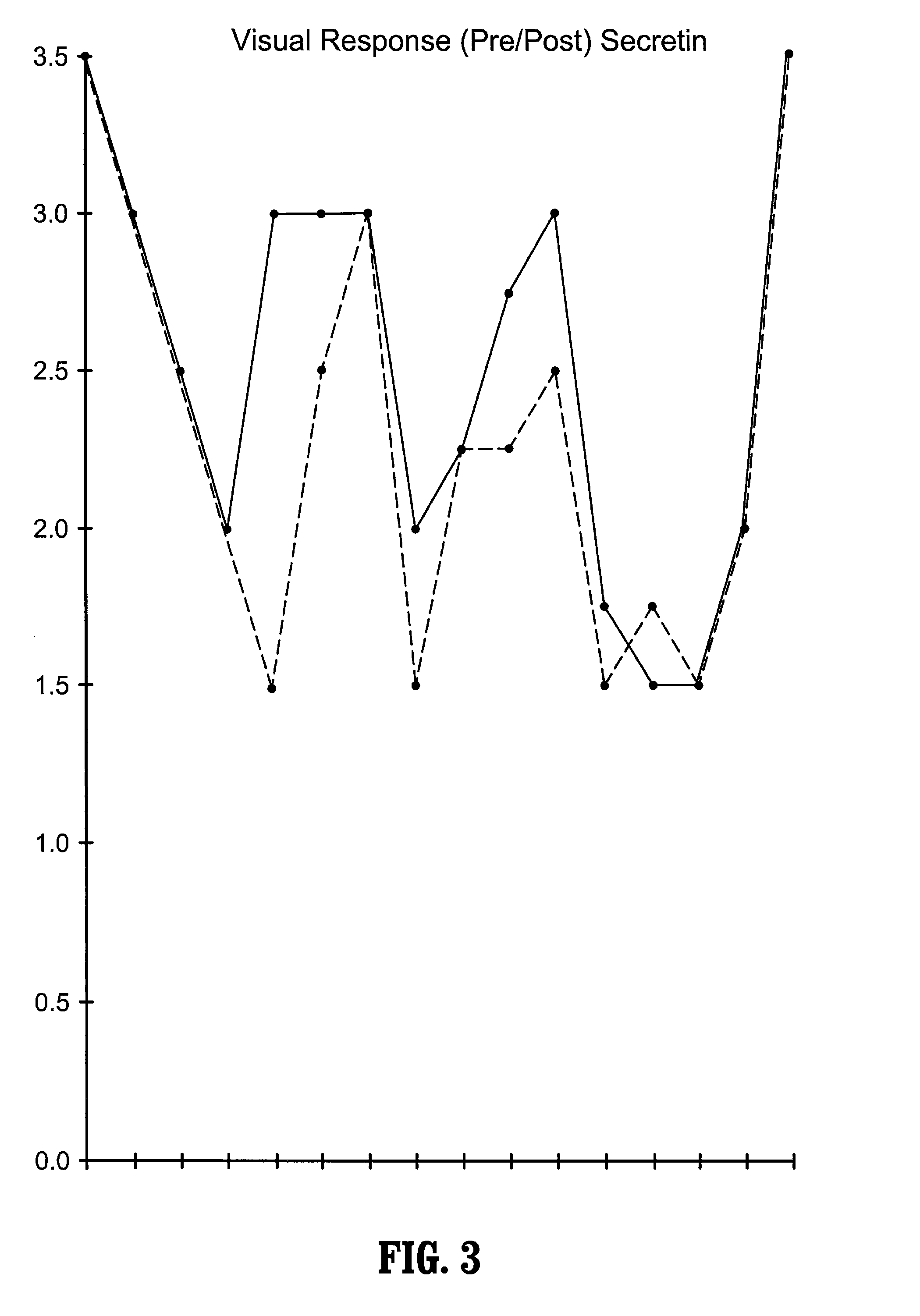
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with i PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
Methods for treating pervasive development disorders
InactiveUS20060182728A1Symptoms improvedPromote digestionNervous disorderPeptide/protein ingredientsDiseasePervasive developmental disorder
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with i PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
Methods of treating pervasive development disorders
InactiveUS20020090653A1Symptoms improvedPromote digestionPeptide/protein ingredientsMicrobiological testing/measurementDiseaseFeces
A method of utilizing the chymotrypsin level of an individual as a measure of the success of secretin, other neuropeptides, and peptides or digestive enzyme administration to such individuals, and in particular, as a prognosticative of potential secretin, other neuropeptides, peptides, and digestive enzyme administration for persons having ADD, ADHD, Autism and other PDD related disorders. In one aspect, a method for determining the efficacy of secretin, other neuropeptides, peptides, or digestive enzymes for the treatment of an individual diagnosed with a pervasive developmental disorder (PDD) comprises obtaining a sample of feces from an individual, determining a quantitative level of chymotrypsin present in the sample, and correlating the quantitative level of chymotrypsin determined to be present in the sample with the PDD to determine the efficacy of treating the individual with secretin, other neuropeptides, peptides, or digestive enzyme administration. In another aspect, a therapeutic method for treating an individual diagnosed with a PDD pervasive developmental disorder comprises determining the efficacy of secretin, other neuropeptides, peptides, and digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering secretin, other neuropeptides, peptides, or digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level.
Owner:CUREMARK
Methods of treating and diagnosing parkinsons disease and related dysautonomic disorders
InactiveUS20080152637A1Nervous disorderPeptide/protein ingredientsAutonomic bladder dysfunctionPsychiatry
A method for treating a Parkinson's patient with digestive / pancreatic enzymes involves administering an effective amount of digestive / pancreatic enzymes to an individual having the disorder in order to improve a symptom of the disorder. In addition, a method is provided for determining whether an individual has, or may develop, Parkinson's disease or related dysautonomic disorders and for determining whether an individual will benefit from the administration of pancreatic / digestive enzymes to treat the dysautonomic disorder.
Owner:CUREMARK
Microencapsulated and controlled-release formulations of isoflavone from enriched fractions of soy and other plants
InactiveUS6890561B1Facilitate user complianceMaintain activityPowder deliveryOrganic active ingredientsAdditive ingredientBULK ACTIVE INGREDIENT
There is provided an orally-administrable formulation for the controlled release or stable storage of a granulated isoflavone-enriched fraction or mixture of such fractions, comprising at least one granulated isoflavone-enriched fraction and at least one carrier, diluent or excipient therefor. Preferably, the formulation is characterized in that the total in vitro dissolution time of said formulation required for release of 75% of the active ingredients available from the formulation is between about 4 and about 18 hours, as determined by the U.S.P. XXIII paddle method at a paddle speed of 75 rpm, using simulated intestinal fluid without the digestive enzymes normally found in intestinal fluid, at pH 6.8, and a temperature of 37° C. A process for the preparation of such a formulation is also provided.
Owner:LYCORED BIO
Alleviation of non-specific binding in microarray assays
InactiveUS7258990B2Bioreactor/fermenter combinationsBiological substance pretreatmentsLysosomeSolid particle
A post-incubation treatment is employed to effectively remove targets, such as proteins / protein complexes, or other label-bearing moieties that may non-specifically bind to a microarray substrate during a binding assay. Following incubation, a one-step wash is carried out with a liquid containing digester, e.g., a digestive enzyme (protease) or lysosome, which is effective to remove non-specifically bound targets or at least labeled portions of such targets from the substrate. Proteases are bound to or coated onto large molecules or onto solid particles of such a size such that they are prohibited from entering the porous surfaces of 3-D hydrogel microspots and are unable to reach and digest labeled target-probe complexes that are disposed within such porous hydrogel microspots. Digested segments of such protein which contain labels (or of essentially the entire protein) are carried away in the wash liquid and thus are not present to create background noise during imaging.
Owner:BIOCEPT INC
Bariatric sleeve
InactiveUS20090240340A1Limit absorptionReducing hormone triggersSuture equipmentsStentsGastrointestinal deviceIntestinal structure
Method and apparatus for limiting absorption of food products in specific parts of the digestive system is presented. A gastrointestinal implant device is anchored in the stomach and extends beyond the ligament of Treitz. All food exiting the stomach is funneled through the device. The gastrointestinal device includes an anchor for anchoring the device to the stomach and a flexible sleeve. When implanted within the intestine, the sleeve can limit the absorption of nutrients, delay the mixing of chyme with digestive enzymes, altering hormonal triggers, providing negative feedback, and combinations thereof. The anchor is collapsible for endoscopic delivery and removal.
Owner:GI DYNAMICS
Sucking pig compound feed and preparation method thereof
InactiveCN102370102AReduce Nutritional StressEnsure overall economic benefitAnimal feeding stuffAccessory food factorsWeaningInstability
The invention relates to a sucking pig compound feed and a preparation method thereof. The feed comprises the following raw materials in parts by weight: 30-90 parts of vegetable feed raw material, 25-80 parts of vegetable puffed feed, 5-25 parts of fermentation bean pulp, 2-10 parts of fish meal, 6-30 parts of dairy product, 1-4 parts of oil, 0.2-3 parts of amino acid, 0.01-0.05 part of liquid additive and 1-3 parts of solid additive. The preparation method of the feed provided by the invention can ensure the best use of the nutrient components of different materials, can overcome the instability of enzyme agents and microbial ecological agents caused by heating, and can effectively eliminate the indigestion caused by hyposecretion of digestive enzymes before and after the weaning of thesucking pig and improve the intestinal canal health as well as the pig immunity. Through the sucking pig compound feed provided by the invention, the problem of weaning stress of the sucking pig is solved; and compared with the common piglet feed, the morbidity is greatly reduced, the feed intake and daily gain are increased, and the material-meat ratio, morbidity and mortality are reduced.
Owner:湖南普爱生物饲料有限公司
Systems and methods for delivery of biologically active agents
InactiveUS20100196445A1Efficient deliveryPeptide/protein ingredientsMicroneedlesCell-Extracellular MatrixActive agent
The invention provides methods and devices for the delivery of therapeutic or biologically active agents to tissue, for example by facilitating the transport of said agents through the skin of a human or animal. Therapeutic agents include various botulinum neurotoxin (BoNT) and other biologically active agents, which can be delivered across the skin and into the dermis using multiple strategies including microneedle drug delivery, transport moieties, or penetration enhancers such as extracellular matrix-digestive enzymes.
Owner:THE SCRIPPS RES INST +1
Stable low digestive enzyme content formulation
ActiveUS20120201875A1Good content uniformityMinimal loss of enzymatic activityPeptide/protein ingredientsMetabolism disorderMedicineDigestive enzyme
The present invention is directed to a pharmaceutical composition or dosage form having a stable, low (diluted) digestive enzyme content comprising at least one digestive enzyme and at least one carrier, or a dosage form thereof. The invention is also directed to a process of preparation of the composition or the dosage form. In addition the invention is directed to the treatment and prevention of disorders or conditions associated with a digestive enzyme deficiency in a patient in need thereof, comprising administering to said patient a pharmaceutically acceptable amount of the composition having a stable low digestive enzyme content or dosage form thereof.
Owner:SOC DES PROD NESTLE SA
Enzyme compositions and use thereof for wound healing
InactiveUS20130202581A1Avoid re-openingGreater re-growth of hairPeptide/protein ingredientsDermatological disorderWhite blood cellHypersensitive response
Compositions and methods of using the compositions for wound healing are provided. The compositions include one or more digestive enzymes, for example, one or more protease, lipases, and amylases. The compositions can be formulated as topical pharmaceutical compositions and can be used for faster healing through stimulation of epidermal cells in the absence of scarring. The compositions may deposit a short term fibrosis and help prevent re-opening of wounds. The compositions may improve recruitment of white blood cells, thereby inducing or enhancing growth factor and immune system activation via an enzyme antibiotic effect. The compositions may enhance the epidermal integrity beyond that of the normal physiological restorative process. Application of the compositions may result in greater re-growth of hair on regions of wounds healed with enzyme and reduced alopecia. The compositions may be administered without causing allergic reactions and without causing biological damage or burns.
Owner:GALENAGEN LLC
Lactobacillus helveticus microcapsule, preparation and use thereof
InactiveCN101323850AOvercoming the problem of low survival rate of freeze-dryingImprove efficiencyMilk preparationMetabolism disorderFreeze-dryingDietary supplement
The invention provides a lactobacillus helveticus microcapsule as well as the preparation and application thereof, pertaining to the embedding technology of lactobacillus helveticus and aiming at microencapsulating the lactobacillus helveticus so as to solve the problems of low embedding efficiency and embedding yield in single-layer or double-layer embedment. The technical proposal adopted by the invention is that isolated soy protein, microporous starch and sodium alginate are respectively taken as the first, the second and the third layers of wall materials for microencapsulation; the three-layer embedment is carried out to the lactobacillus helveticus for the first time, wherein, in the process of preparing bacterial suspension, an orthogonal optimization protective agent is adopted to improve the livability after freeze drying. The microcapsule of the invention has strong heat resistance and acid resistance, solves the inactivation problem caused by gastric acid, digestive enzyme and antibiotic, etc. to active bacillus, is capable of releasing when reaching large intestine, and balances intestinal flora. The reusability of fermented milk is good. The lactobacillus helveticus used by the invention enriches the variety of probiotic bacteria microcapsule and is a good dietary supplement.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method for separating mesenchymal stem cells from placenta
ActiveCN102676451AHigh purityEasy to operateSkeletal/connective tissue cellsEmbryonic cellsFiltrationMesenchymal stem cell
The invention relates to a method for separating mesenchymal stem cells from placenta. The method comprises the following steps: (a) taking placental cotyledon, and fully washing by using a phosphate buffer solution (PBS) to remove residual blood from the placenta; (b) cutting the placental cotyledon into blocks, adding a PBS containing tissue digestive enzyme, and incubating and digesting at 37DEG C; (c) filtering the tissue blocks by using a copper net, and grinding if necessary to promote filtration; (d) centrifuging the collected filtrate, separating mononuclear cells, suspending the obtained cells by using a mesenchymal stem cell (MSC) culture medium, and culturing in a 5 percent CO2 incubator at 37DEG C; and (e) after the dispersed cells form clones, selecting the clone cells, respectively culturing by using an MSC culture medium, and after the cells are fused, performing digestion and passage by using pancreatin to obtain the mesenchymal stem cells of the placenta. By the method, high purity mesenchymal stem cells of the placenta can be obtained.
Owner:BOYALIFE
Methods of treating pervasive development disorders
InactiveUS20080219966A1Symptoms improvedPromote digestionNervous disorderPeptide/protein ingredientsPervasive developmental disorderMethylphenidate
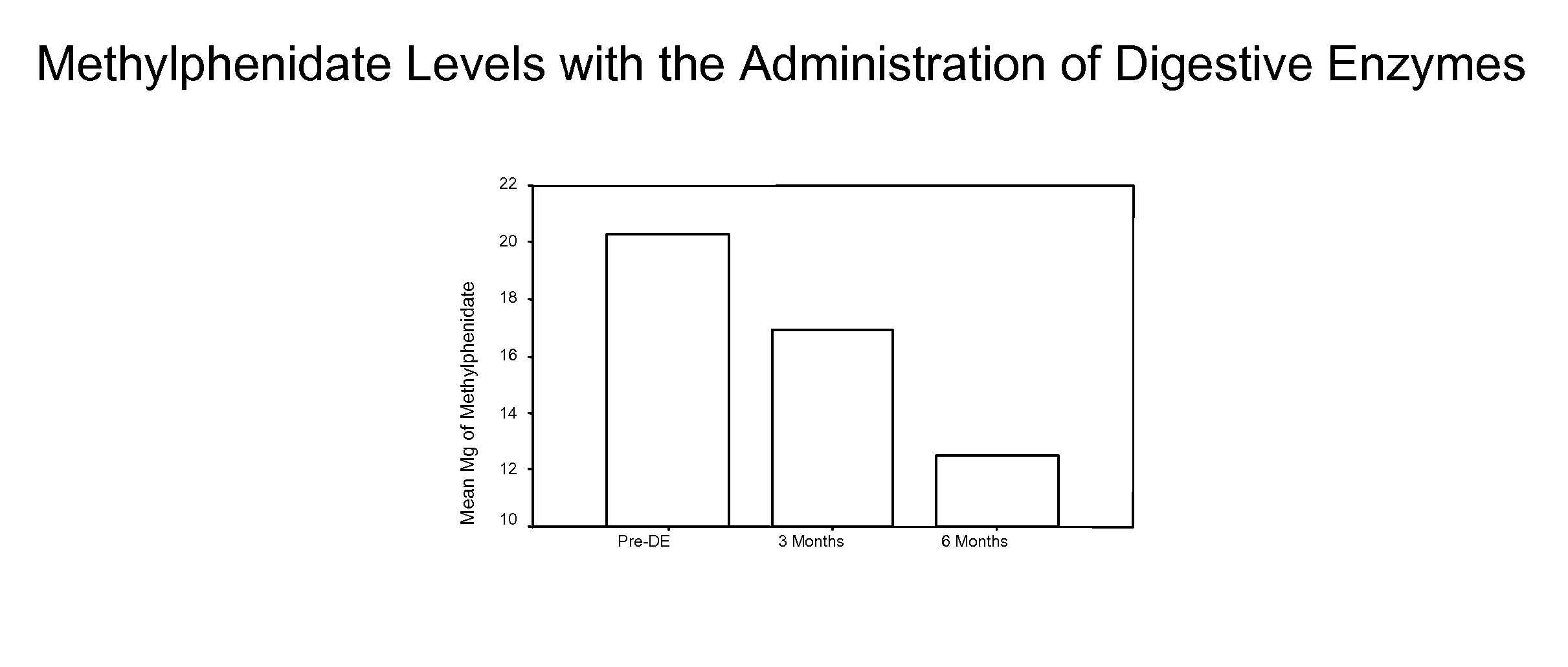
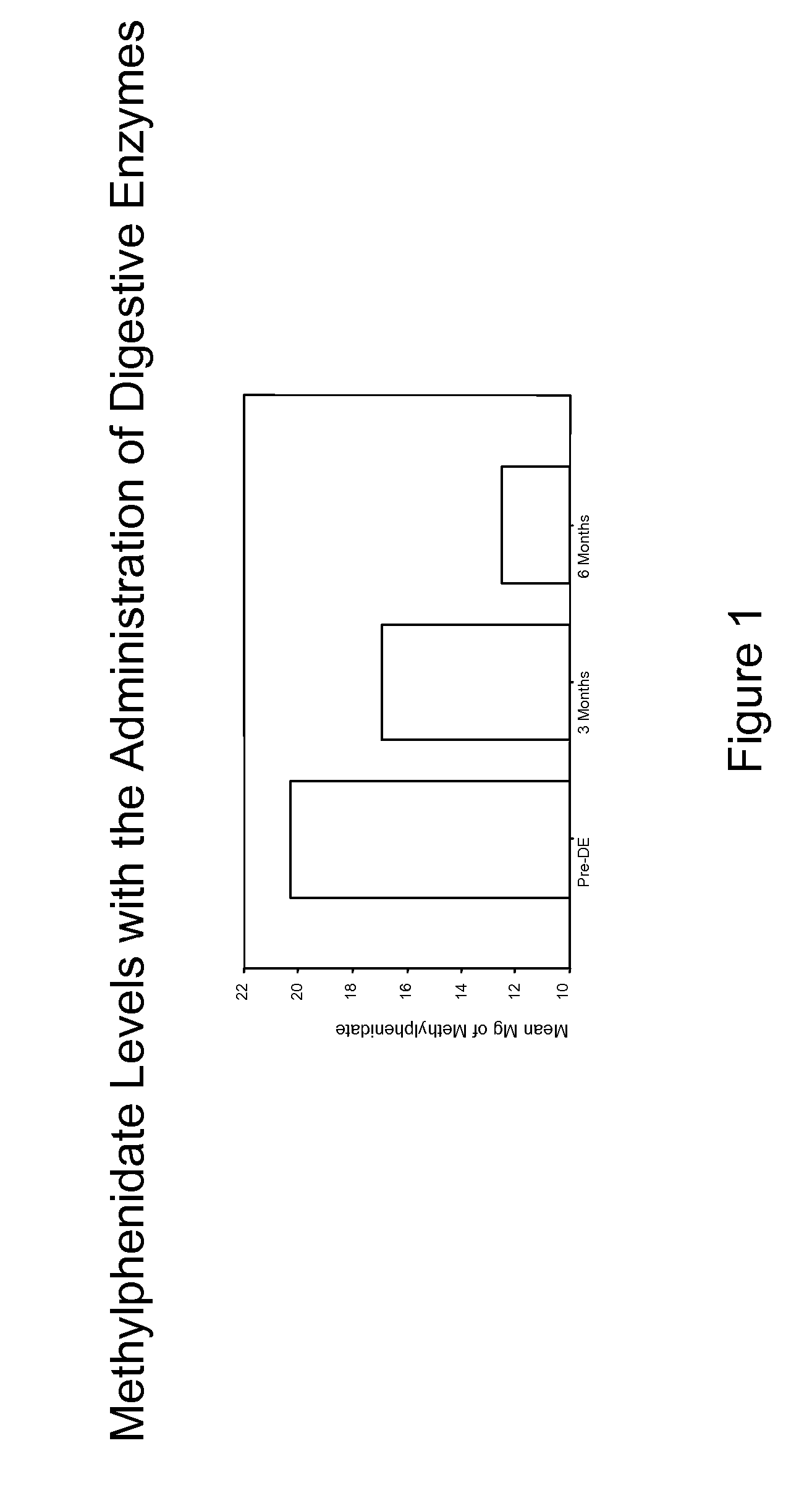
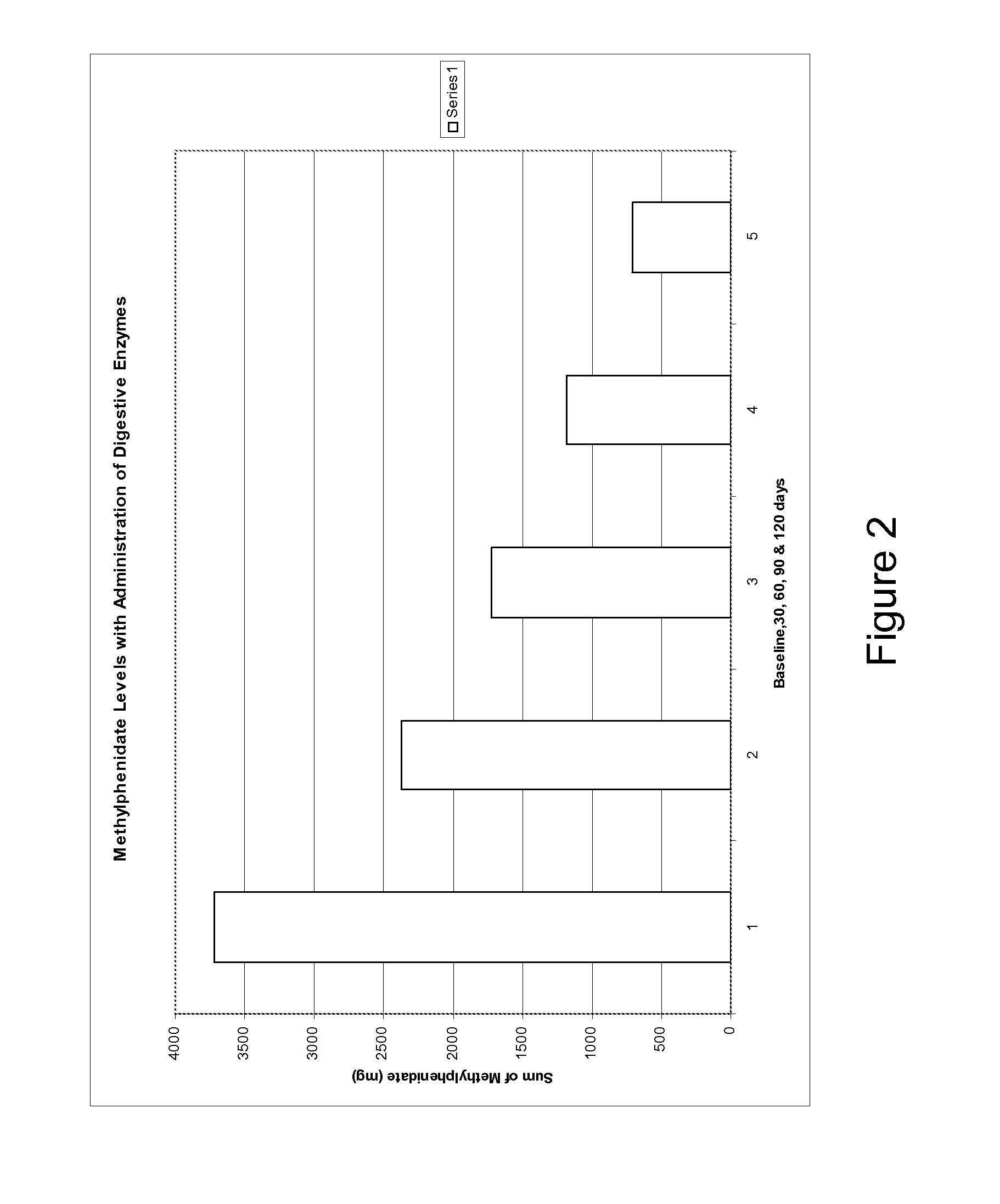
A therapeutic method for treating an individual diagnosed with PDD pervasive developmental disorder comprises determining the efficacy of digestive enzyme administration for the treatment of the individual based on a measure of the individual's chymotrypsin level, and administering digestive enzymes to the individual based on the determination of the measure of the individual's chymotrypsin level. A method for reducing the amount of methylphenidate (Ritalin) being taken by an individual with attention deficit disorder (ADD) or attention deficit hyperactivity disorder (ADHD) by administering a therapeutic amount of digestive enzymes is also provided.
Owner:CUREMARK
Method for the treatment of the symptoms of drug and alcohol addiction
A therapeutic agent for the treatment of the symptoms of addiction and the method for preparing the therapeutic agent is disclosed. The therapeutic agent is a stable pharmaceutical preparation containing, but not limited to, digestive / pancreatic enzymes. The therapeutic agent may be manufactured by a variety of encapsulation technologies. Delivery of the therapeutic agent may be made orally, through injection, by adherence of a medicated patch or other method. Further, a method of using of a biomarker, the presence of chymotrypsin in the gastrointestinal tract to determine the presence of symptoms of addiction, and the likelihood of relapsing into addiction is disclosed.
Owner:CUREMARK
Composite biological feed additive agent for fattening early weaning mutton sheep
InactiveCN101485402AImprove developmentIncrease weightAnimal feeding stuffEcological environmentMicrobial agent
The invention relates to an early weaning meat sheep fattening compound biological feed additive, which belongs to the technical field of animals feeding by feed. The feed additive comprises prebiotics consisting of candida utilis, bacillus subtilis and a lactobacillus plantarum microbial agent, non-starch polysaccharide enzyme containing cellulase, xylanase, beta-dextranase, beta-mannase and pectinase and a yeast culture formed by using saccharomyces cerevisiae to completely ferment bean pulp. The additive is specially used for fattening period ration of the early weaning(the weaning lunar age is less than or equal to 2 ages of the moon) meat sheep, and the adding percentage for the full-mixing ration of the fast-fattening meat sheep in 3 to 5 ages of the moon is 1 percent. The additive can provide functional nutrients such as digestive enzyme, B group vitamins and growth promoting factors, and the like which are inadequately produced and insufficient for the early weaning baby sheep and simultaneously contains probiotics for adjusting the micro-ecological environment in intestinal canals, thereby improving the immunity and the healthy level of the baby meat sheep; and after weaning, the daily gaining in weight is more than 10 percent in the prior period and later period of fattening, and the economic benefits are obviously improved.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com