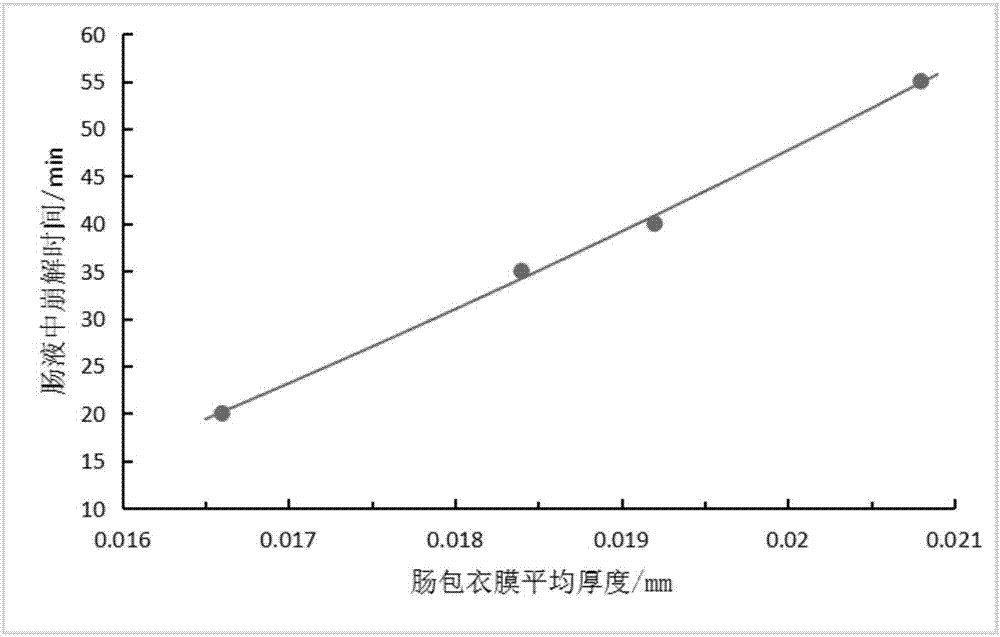
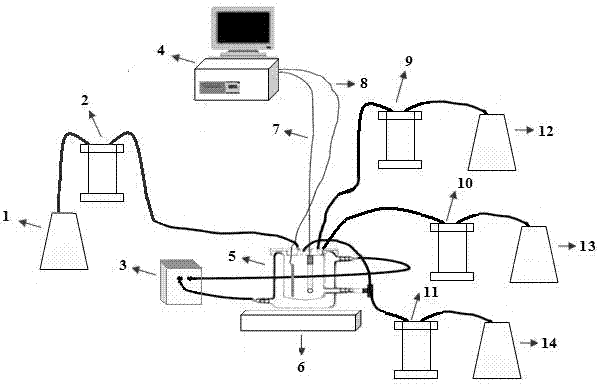
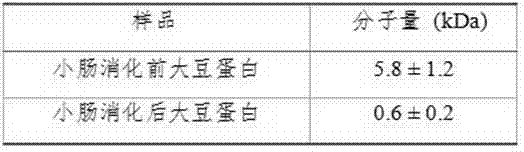
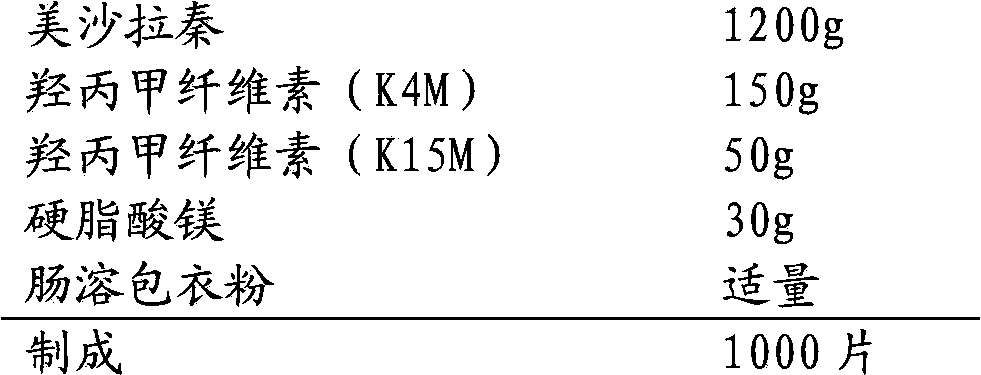Patents
Literature
140 results about "Intestinal fluid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interstitial fluid, also known as tissue fluid, is a liquid — made mostly of water — that fills up the space between the cells of most organisms, including human beings.
Pharmaceutical composition comprising cyclosporin solid-state microemulsion
InactiveUS6306434B1Easy to controlMaintaining blood concentrationPowder deliveryCyclic peptide ingredientsIntestinal fluidBlood concentration
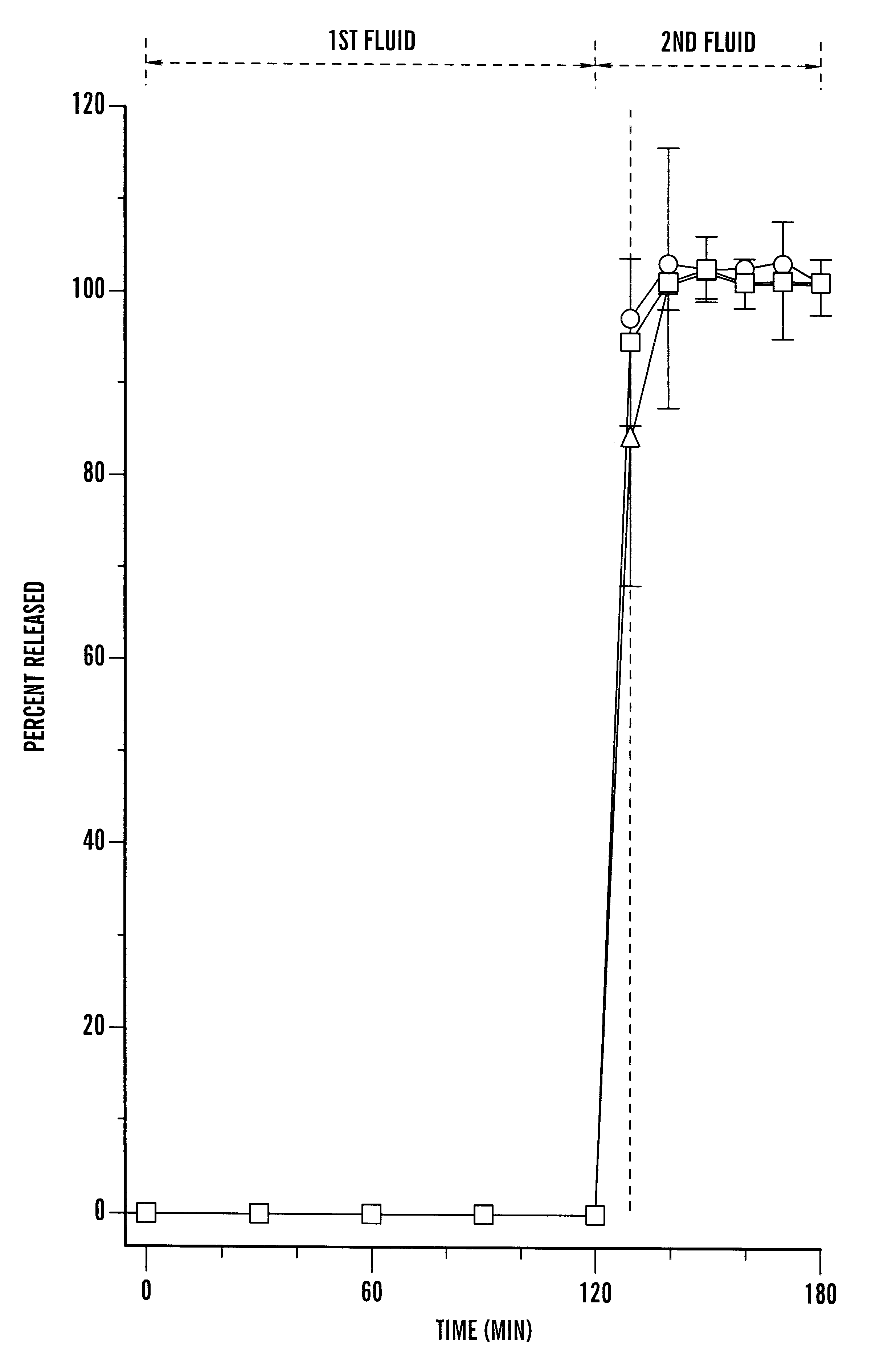
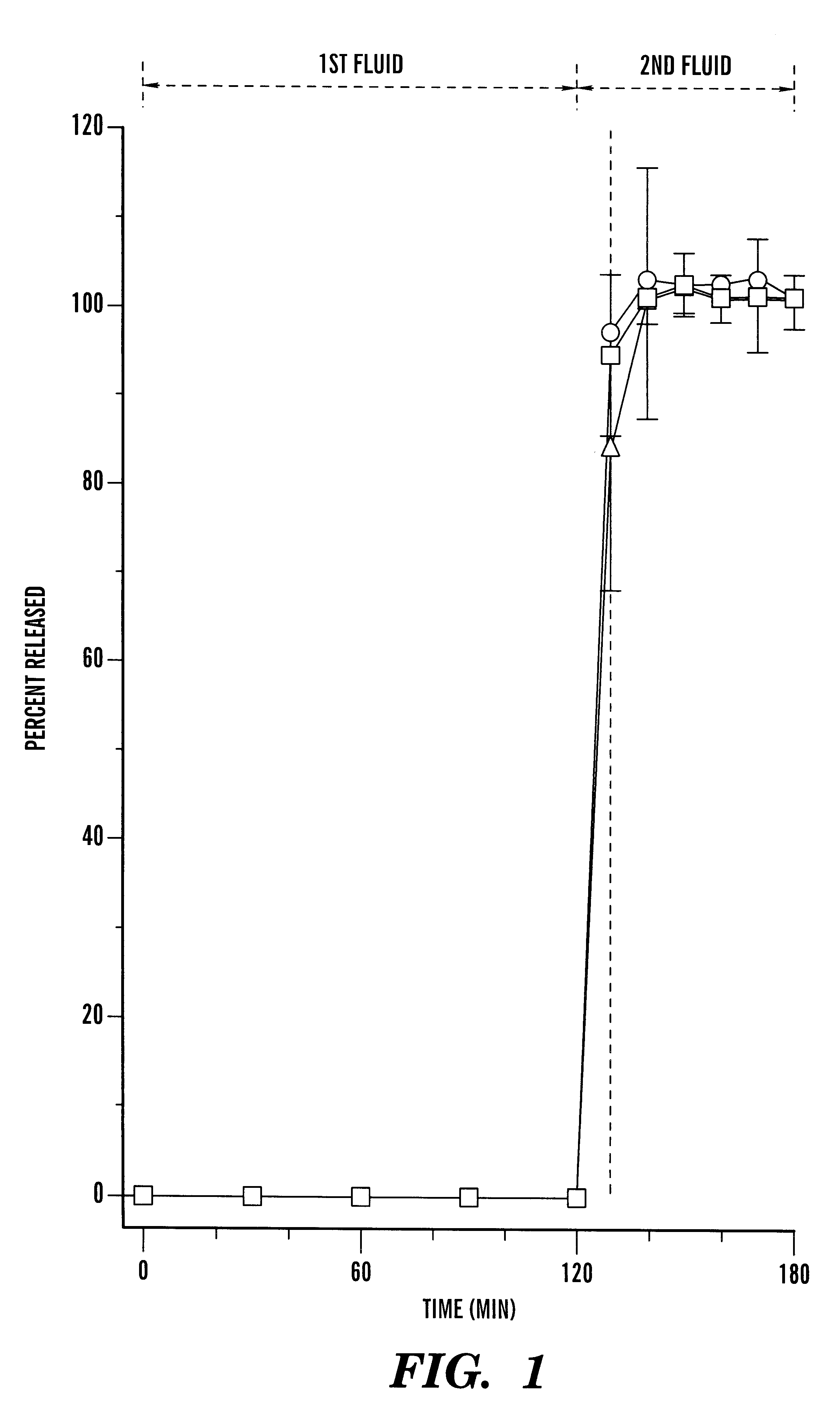
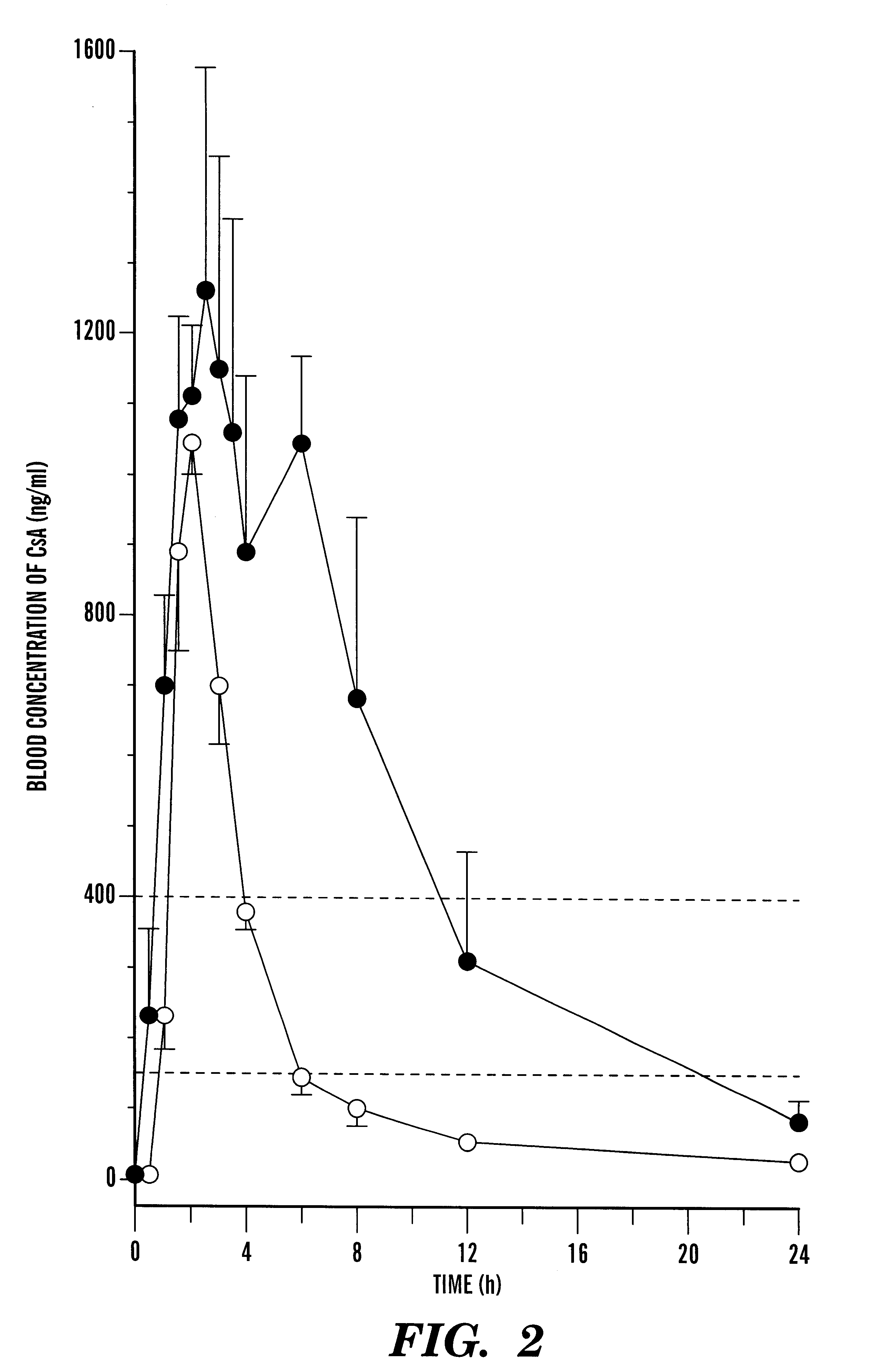
A pharmaceutical composition comprising a cyclosporin solid-state microemulsion is disclosed. In a preferred embodiment, the composition comprises a cyclosporin microemulsion dispersed in an enteric carrier. The composition does not dissolve in external phases such as artificial gastric fluid, but dissolves rapidly in artificial intestinal fluid, whereby it releases the cyclosporin microemulsion, providing rapid delivery of cyclosporin. The composition effectively maintains a therapeutic blood concentration of cyclosporin with once a day dosing, providing for convenience of administration and avoiding adverse effects induced by increasing peak blood cyclosporin concentrations associated with conventional cyclosporin formulations.
Owner:CHONG KUN DANG PHARMA CORP
Microencapsulated and controlled-release formulations of isoflavone from enriched fractions of soy and other plants
InactiveUS6890561B1Facilitate user complianceMaintain activityPowder deliveryOrganic active ingredientsAdditive ingredientBULK ACTIVE INGREDIENT
There is provided an orally-administrable formulation for the controlled release or stable storage of a granulated isoflavone-enriched fraction or mixture of such fractions, comprising at least one granulated isoflavone-enriched fraction and at least one carrier, diluent or excipient therefor. Preferably, the formulation is characterized in that the total in vitro dissolution time of said formulation required for release of 75% of the active ingredients available from the formulation is between about 4 and about 18 hours, as determined by the U.S.P. XXIII paddle method at a paddle speed of 75 rpm, using simulated intestinal fluid without the digestive enzymes normally found in intestinal fluid, at pH 6.8, and a temperature of 37° C. A process for the preparation of such a formulation is also provided.
Owner:LYCORED BIO
Formulations for a tight junction effector
ActiveUS20070196501A1Stable in gastric fluidAntibacterial agentsBiocideIntestinal fluidSmall intestine
Enteric compositions comprising one or more tight junction agonists and / or one or more tight junction antagonists are provided. Compositions of the invention may comprise a delayed-release coating disposed over a tight junction agonist and / or tight junction antagonist layer which may be disposed over an inert core. Delayed-release coatings may be substantially stable in gastric fluid and substantially unstable in intestinal fluid, thus providing for substantial release of the tight junction agonist and / or antagonist from the composition in the duodenum or jejunum of the small intestine.
Owner:ALBA THERAPEUTICS CORP
Feed-use high-activity lactobacillus solid preparation and preparation method thereof
ActiveCN103392911AMorphological stabilityEffective protectionAnimal feeding stuffIntestinal fluidHigh humidity
The invention discloses a feed-use high-activity lactobacillus solid preparation and a preparation method thereof. The feed-use high-activity lactobacillus solid preparation comprises growing beneficial lactobacillus bacterium mud, a protection formula and a basic formula. The invention also provides the preparation method of the feed-use high-activity lactobacillus solid preparation, lactobacillus can be protected through synergistic effects of components in the protection formula and the basic formula, and the living bacterium survival rate in the whole process can be improved. The lactobacillus in the prepared feed-use high-activity lactobacillus solid preparation has improved high temperature and high humidity resistances, has a high survival rate in a simulated intestinal fluid, and a substantial improved animal application effect.
Owner:ACAD OF NAT FOOD & STRATEGIC RESERVES ADMINISTRATION
Lactobacillus plantarum with inhibitory activity to alpha-glucosidase
ActiveCN107502575APromote growthHigh activityBacteriaMetabolism disorderDihydrogen oxideGastric fluid
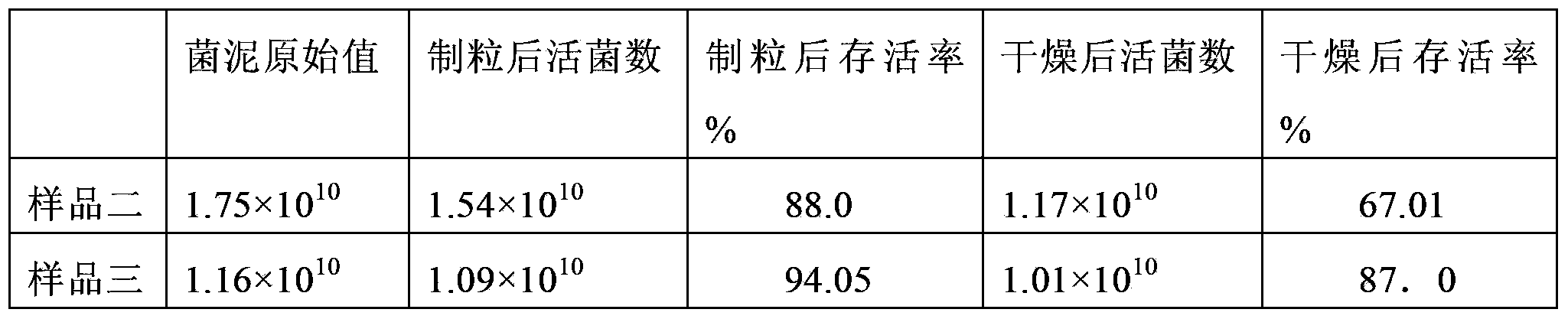
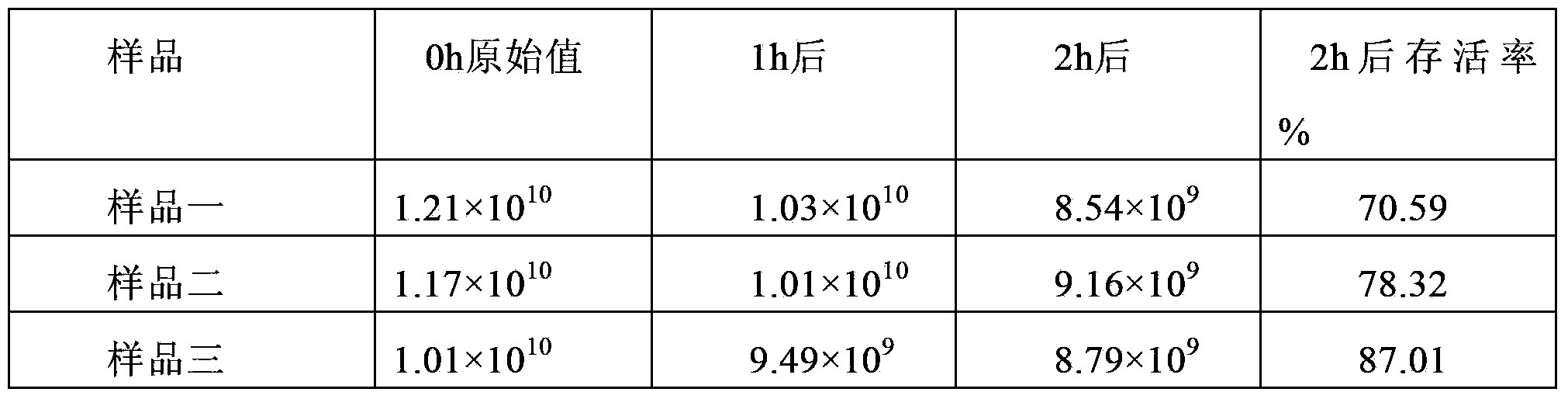
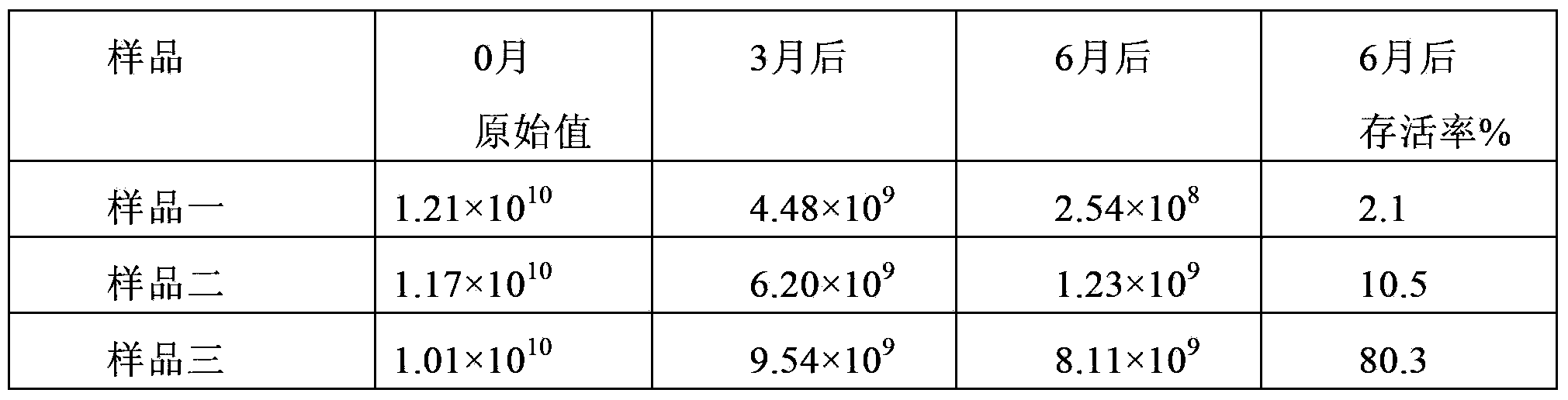
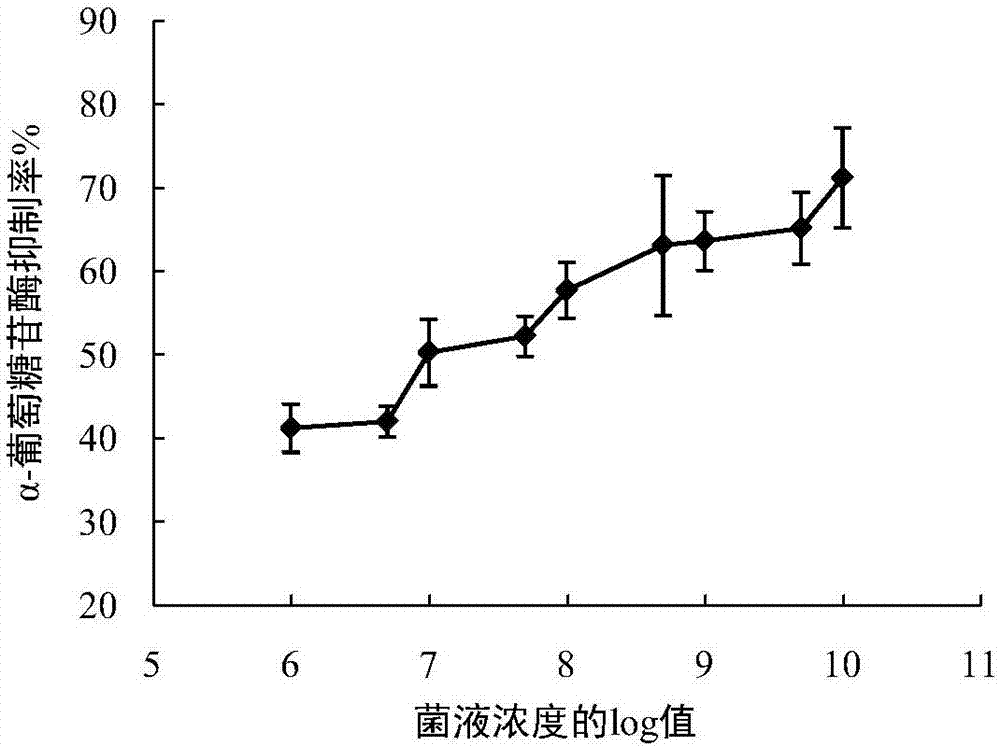
The invention discloses lactobacillus plantarum with the inhibitory activity to alpha-glucosidase. The lactobacillus plantarum is classified and named as Lactobacillus plantarum, is preserved on August 29, 2017 in China General Microbiological Culture Collection Center and has a preservation number of CGMCC NO.14573. By screening, the lactobacillus plantarum has in-vitro inhibitory activity to alpha-glucosidase; the survival rate of the lactobacillus plantarum reaches up to 71.04% under an acid condition that the pH value is 2.0 and is 77.14% in 2.0% cholate deionized water, the tolerances of the lactobacillus plantarum to artificially simulated saliva and intestinal fluid are higher than 90%, the tolerance of the lactobacillus plantarum to gastric fluid reaches 52.49%, and after artificially simulated fluid is sequentially digested, the survival rate reaches 88.27%; the lactobacillus plantarum has good effects on the prevention and treatment of diabetes mellitus type 2; the lactobacillus plantarum is added with a protection agent so as to prepare freeze-drying battier powder with a high viable count, and the viable count reaches up to 87% of the viable count before freeze-drying; and besides, the lactobacillus plantarum further has a good fermentation effect.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
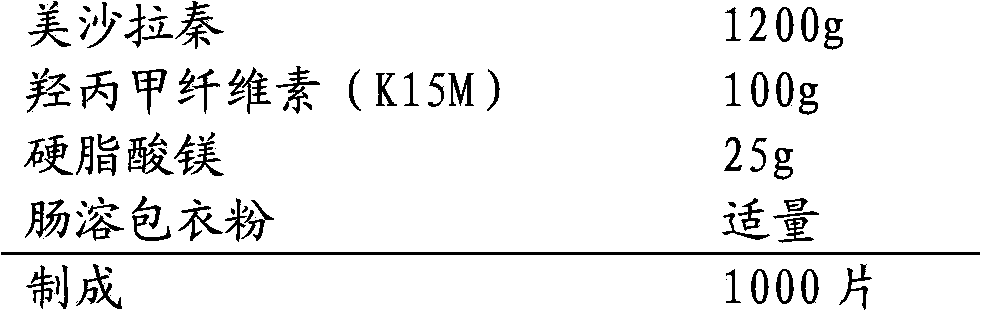
Mesalazine oral controlled release medicine composition
The invention relates to a mesalazine oral colon-targeted sustained release medicine composition which is characterized by containing: (a) a sustained release table core containing mesalazine or medicinal salts or solvates thereof and hydrophilic stroma, wherein the mesalazine or the medicine salts thereof are dispersed in the hydrophilic stroma; and (b) a coating wrapped outside the table core and containing acid-resistant materials; wherein the one-hour release of the composition in simulated intestinal fluid with the pH of 7.2 is less than 20 percent, the four-hour release thereof is 30-60 percent, and the eight-hour release thereof is greater than 70 percent. The composition has simple manufacturing process and low cost, slowly releases the mesalazine in small intestines and colons, and achieves the colon-targeted medicine delivery once a day and the local curative effect.
Owner:CHONGQING PHARMA RES INST
Method for preparing Lactobacillus casei microcapsule
InactiveCN101724622AImprove survival rateRich varietyMilk preparationMetabolism disorderIon exchangeStrong acids
The invention relates to a method for preparing a Lactobacillus casei microcapsule. 2-6 percent of microporous starch solution and 2-6 percent of sodium alginate solution are respectively taken as inner and outer wall materials of the microcapsule; the characteristic that small holes overspread on the surface of the microporous is utilized to absorb a bacteria solution; then sodium alginate and calcium chloride are utilized to generate ion exchange to form a sodium alginate coating; and double layer embedment is carried out on Lactobacillus casei. After the Lactobacillus casei microcapsule is dried at the temperature of 2-6 DEG C for 36-72h, the obtained microcapsule has stronger acid resistance and cholate resistance and high storage stability, i.e. the microcapsule is not dissolved in simulated gastric fluid basically and does not leak but is immediately disintegrated in simulated intestinal fluid; after the Lactobacillus casei microcapsule is treated in 0.3 percent of cholate solution for 1h, the thalli survival rate is 88 percent, and the number of viable bacteria is increased to 1.24*109cfu / g from 5.80*109cfu / g after the Lactobacillus casei microcapsule is stored at the temperature of 4 DEG C for 1 month. The invention greatly increases the survival rate of Lactobacillus casei in products and the use ratio of Probiotics and also enriches the variety of the Probiotics microcapsule.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Treatment of allergic conditions
Orally administered sodium cromoglycate has been found to be effective in the treatment of allergic conditions such as asthma, general food allergies, ulcerative colitis, atopic eczema, chronic urticaria and irritable bowel syndrome if it is presented such that the sodium cromoglycate becomes bioavailable within 10 minutes of exposure to intestinal fluid. The sodium cromoglycate may be presented as enteric-coated tablets or individually enteric-coated pellets or microgranules packaged with disintegrant in a ratio of at least 1.2:1 disintegrant:sodium cromoglycate (w:w). Optionally, the patients are first selected to have a total serum IgE level of at least 150 iu / ml.
Owner:THORNTON & ROSS
Pharmaceutical formulation with enhanced solubility for the delivery of corticosteroids
InactiveUS20080081070A1Improve solubilityPrecision releaseOrganic active ingredientsBiocidePorositySolubility
Formulations have been developed to improve the solubility of corticosteroids such as fluticasone proprionate in a composition designed to achieve localized release of the drug in the small intestine and / or colon. In one embodiment, solid dispersions of fluticasone are prepared wherein the drug is blended with or coated onto a highly water soluble substrate such as nonpareil (sugar beads) then coated with a layer of polymer soluble in small intestinal fluid, then coated with an enteric coating. The inner polymer layer controls release of the drug, and the enteric coating, a pH sensitive polymer that is broken down in the ileum and colon, controls localized release of drug at various sites within the gastrointestinal tract. The multilayer pharmaceutical composition can be in the form of pellets, tablets compressed from pellets or pellets packed into capsules. The release profile of the drug can be manipulated by (1) altering size or shape (i.e., surface area) and solubility of the inert substrate; (2) the ratio of drug to polymer, the polymer composition and solubility, the porosity of the polymer; (3) the drug form (i.e., free base or salt, or which salt); and the thickness and / or surface area of the drug / polymer and / or enteric coating. In a preferred embodiment, the composition is administered orally. This may also be packaged to provide for an escalating or tapering dosage.
Owner:AURIGA LAB +1
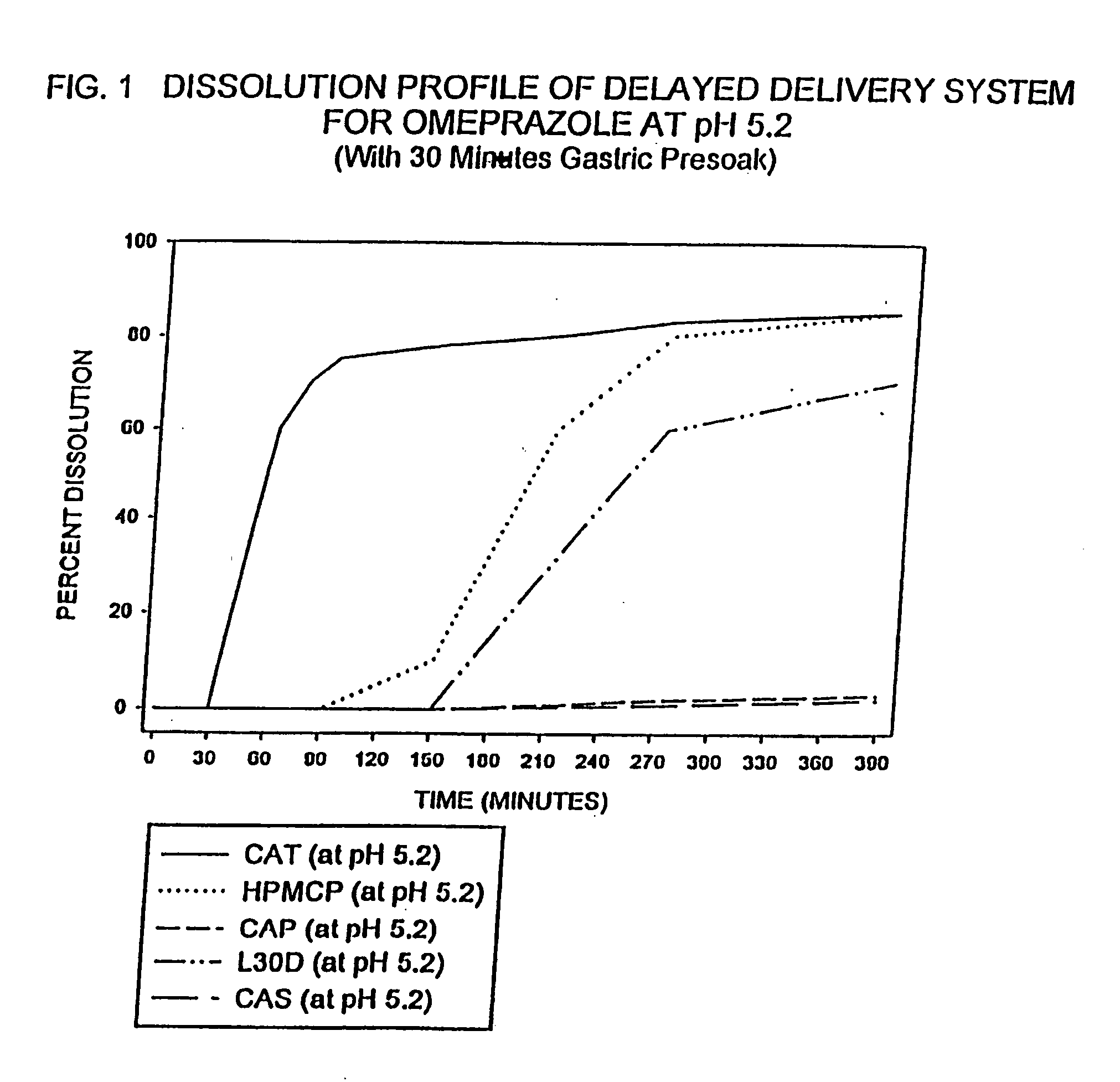
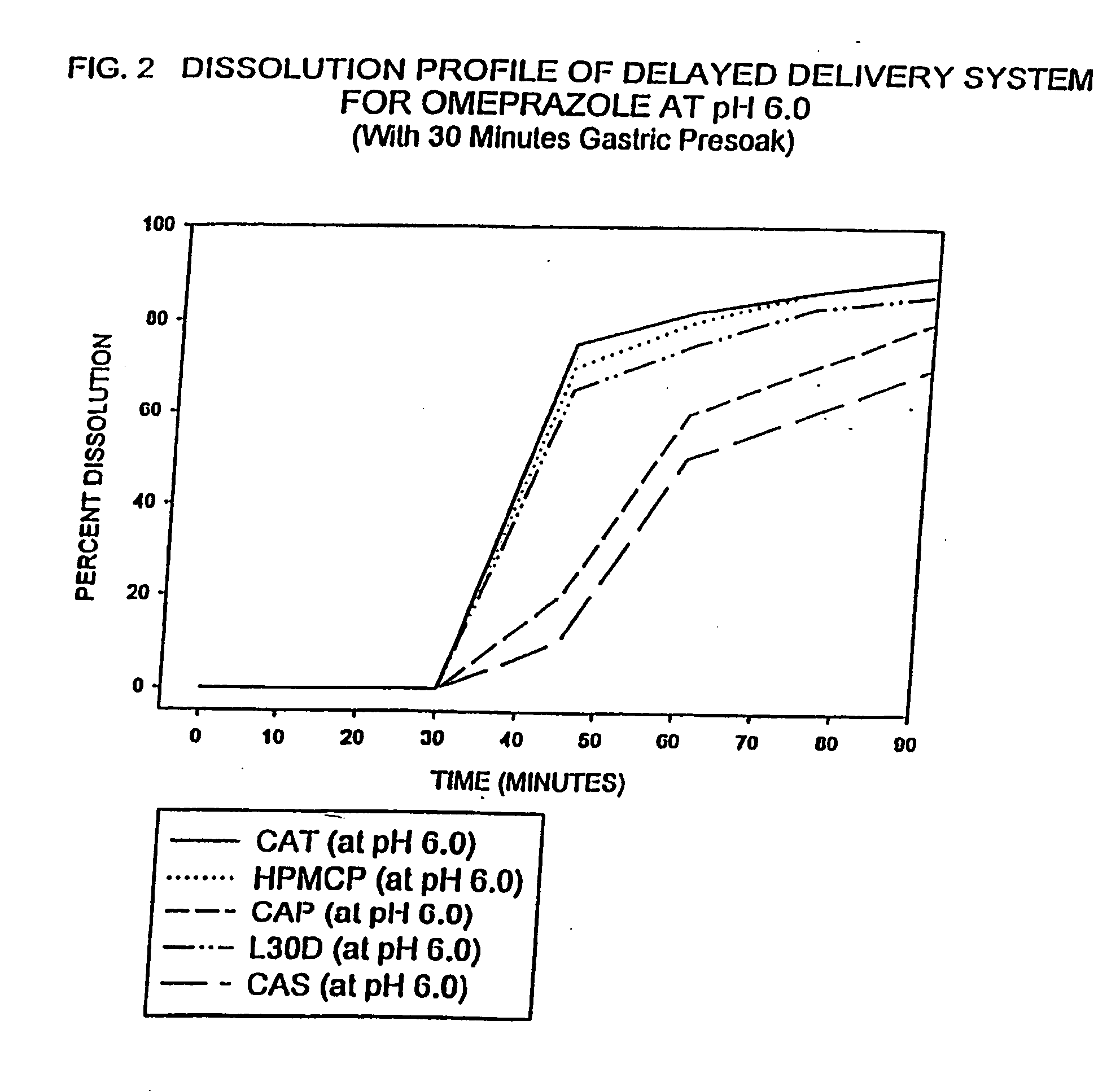
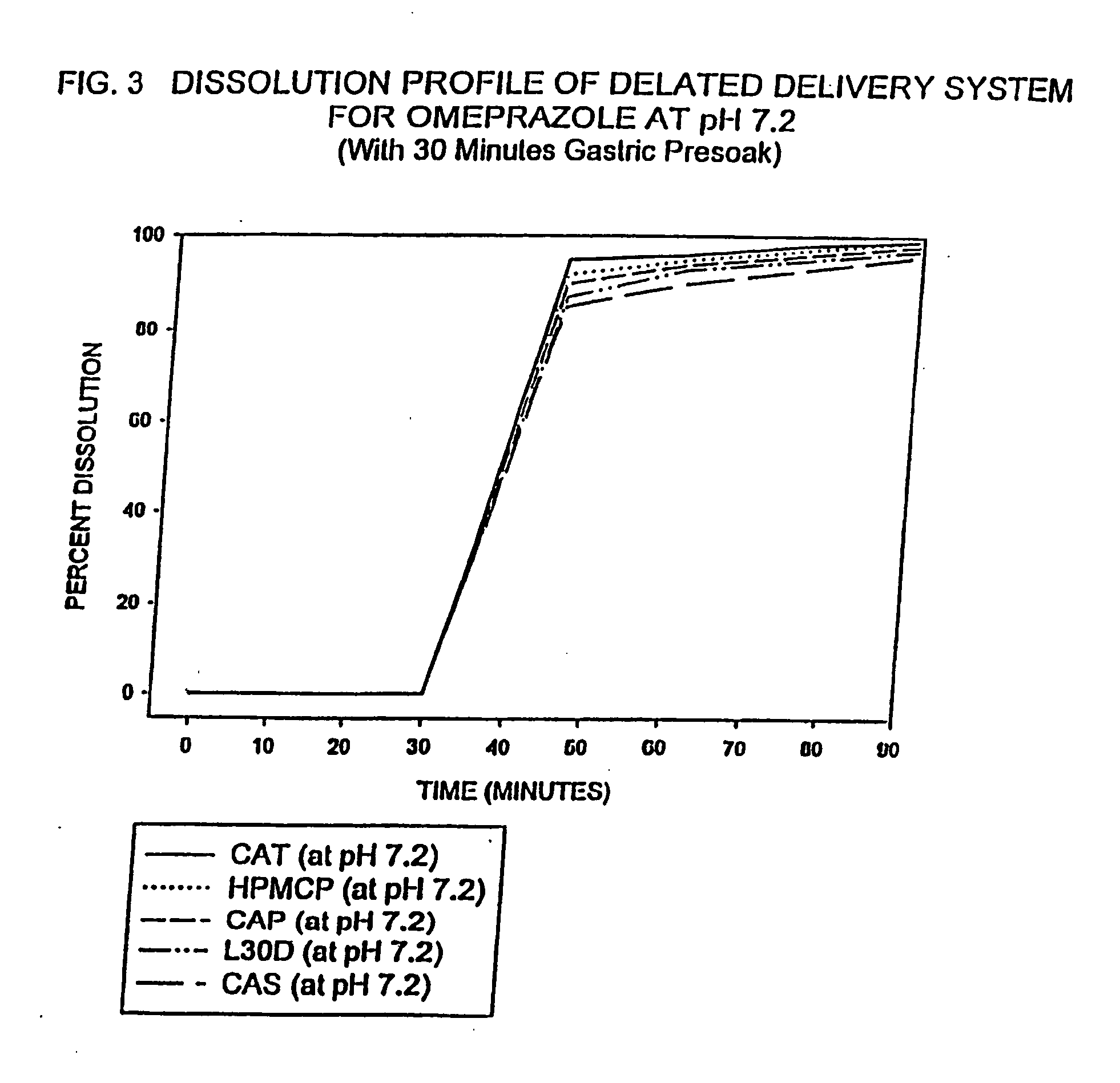
Delayed delivery system for acid-sensitive drugs
InactiveUS20050244497A1High cohesivenessImprove integrityGranular deliveryMicrocapsulesIntestinal fluidGastric fluid
The present invention relates to a delayed release drug delivery system containing omeprazole capable of site-specific delivery and pulsatile (bolus) kinetics for once-a-day dosage comprised of an alkaline core structure sequentially layered with suspensions of omeprazole; a separation barrier; and an enteric barrier. The separation barrier is coated with a pH-dependent enteric membrane, which is relatively insoluble in gastric fluid but rapidly to immediately soluble in intestinal fluid, whereby the drug is released in a pulsatile manner in the proximal segment of the gastrointestinal tract.
Owner:WOCKHARDT LTD
Enteric soluble hollow capsule and preparation method thereof
ActiveCN106924211AImprove toleranceImprove toughnessPharmaceutical non-active ingredientsCapsule deliveryGellan gumIntestinal fluid
The invention discloses an enteric soluble hollow capsule and a preparation method thereof. The outer surface of a capsule body of the hollow capsule is covered with an enteric coating; the capsule body consists of hydroxypropyl methylcellulose, gel and water; the enteric coating consists of low-acyl gellan gum, a curing agent and water; the hollow capsules comprise the following components in percentage by weight: 70-80% of hydroxypropyl methylcellulose, 2-5% of gel, 10.5-14% of low-acyl gellan gum, 0.05-2% of a curing agent and the balance of water. The enteric soluble hollow capsule has the characteristics of being stably maintained and not disintegrated within 2 hours or even longer in simulated gastric fluid, and disintegrated within 1 hour in simulated intestinal fluid, and provisions of Chinese Pharmacopoeia on enteric soluble hollow capsules can be met; the enteric soluble hollow capsule is prepared by using a process of two times of gum dipping, one time of calcification and 1 time of drying molding, the preparation method is simple and easy to operate, and particularly no organic solvent or preservative is used in the preparation process, so that the enteric soluble hollow capsule is safe and reliable and environment-friendly, and current hollow capsule development tendency is met.
Owner:青岛蓝谷药业有限公司
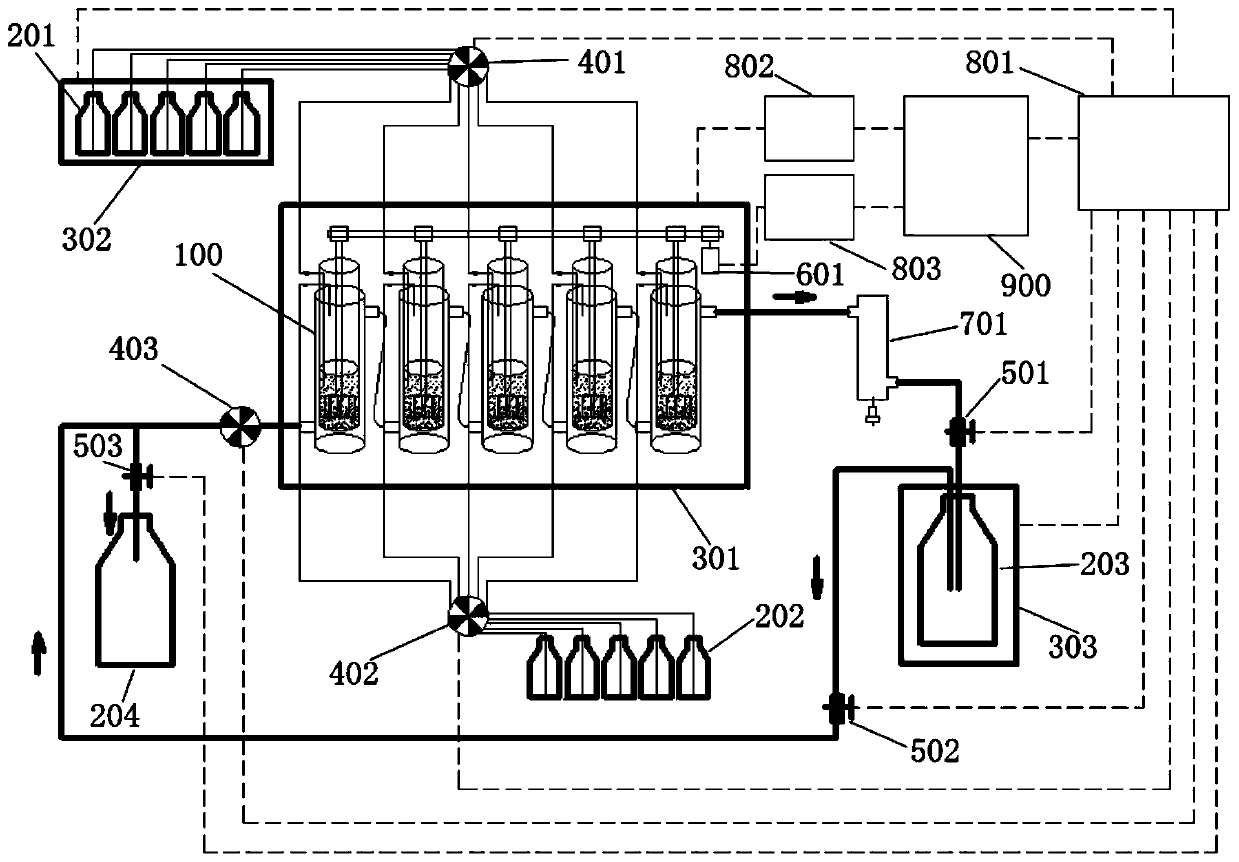
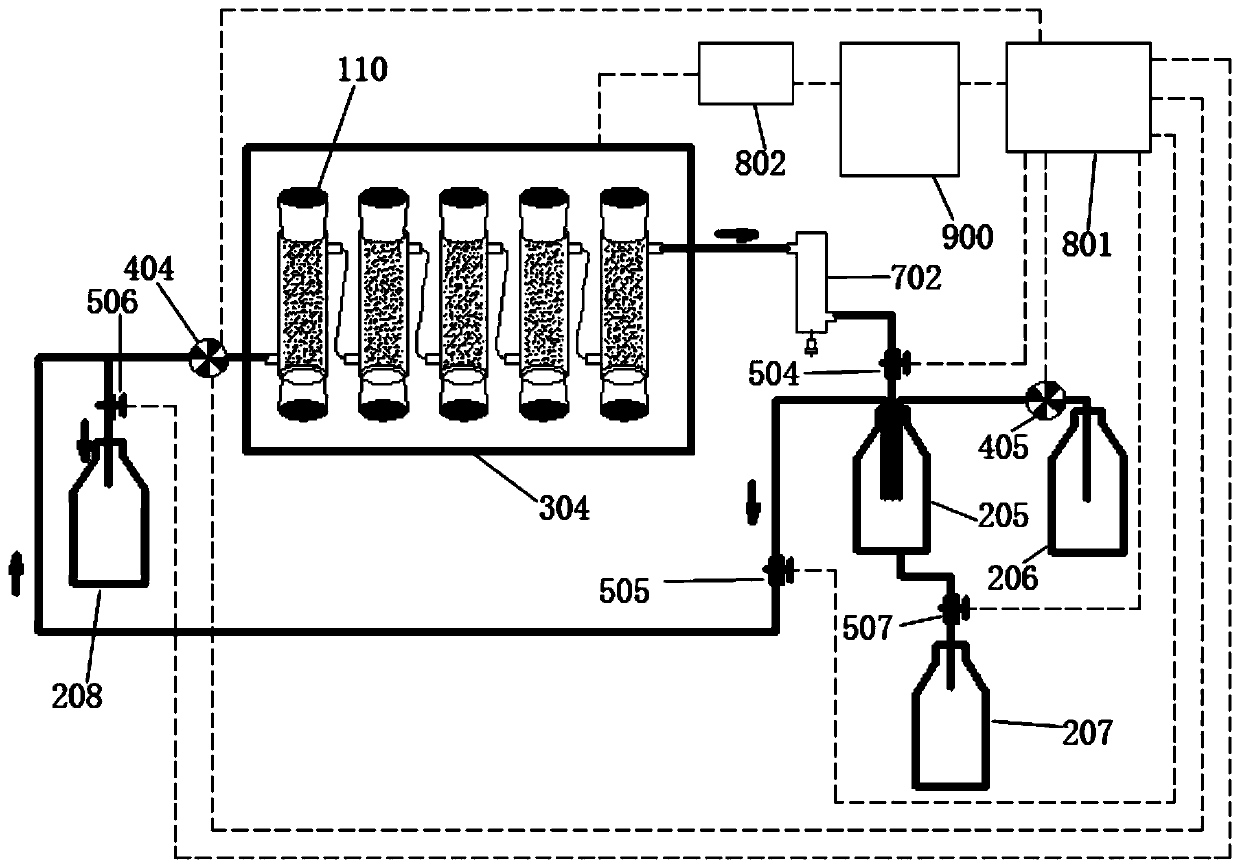
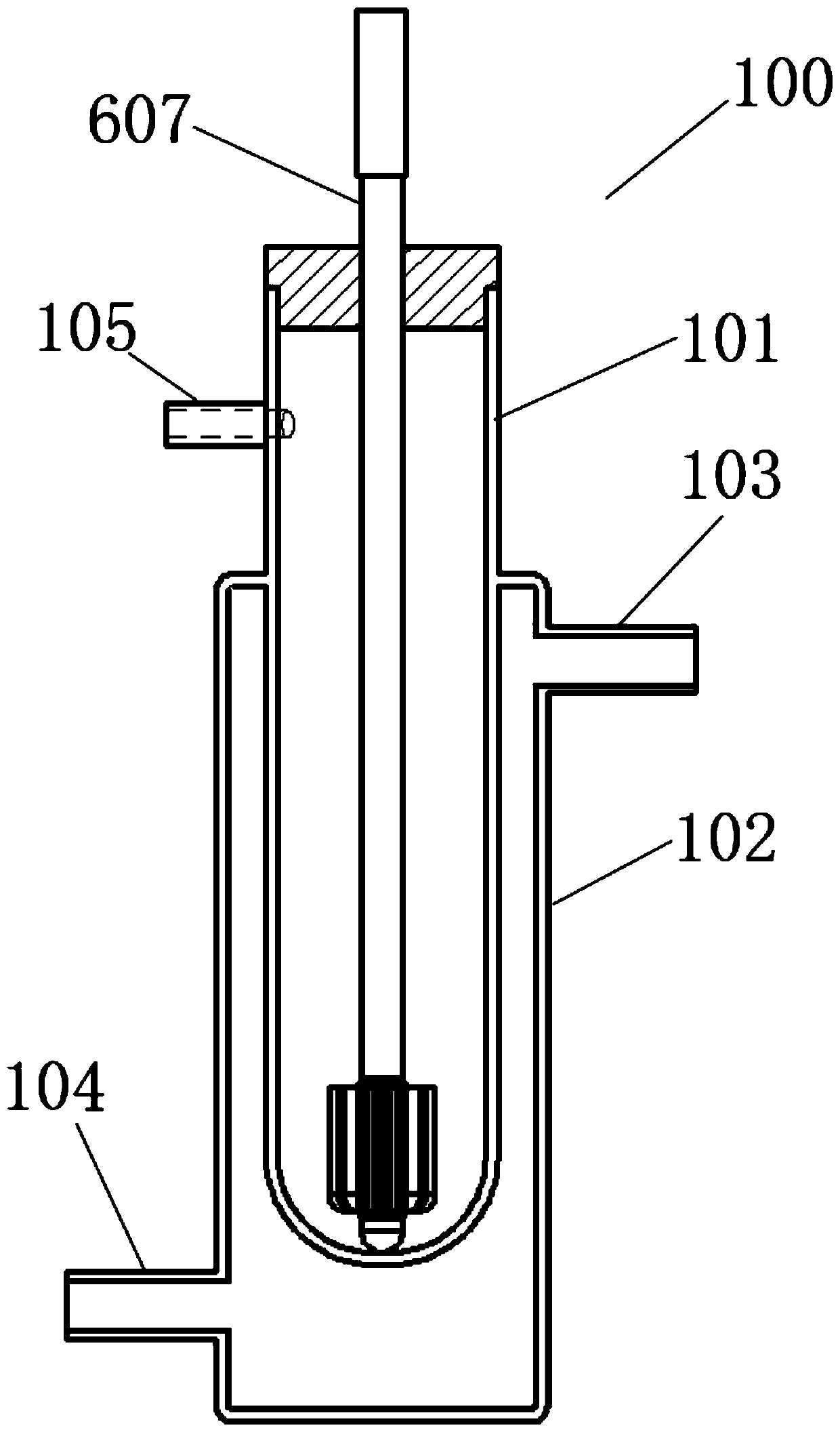
Device for simulation of digestion in small intestine and use method thereof
The invention discloses a device for simulation of digestion in small intestine and a use method thereof. The device comprises a small intestine reactor, a stirrer, a water-bath control device, a computer monitoring system and a small intestine evacuation pump. A small intestinal fluid is added into the small intestine reactor, a solution or a dispersion liquid to be detected is added into the small intestine reactor, a simulator simulates small intestine peristalsis, the water-bath control device controls a temperature in the small intestine reactor, the computer monitoring system monitors an internal temperature, a pH value and digestion time of the small intestine reactor, the small intestine evacuation pump simulates small intestine evacuation, the digested product is collected, pancreatin and trypsin activity is eliminated, the digestion simulation process is repeated three times, and a digestion case of the material to be detected is detected. The device realizes first simulation of specific and detailed digestion in small intestine, realizes observation of digestion of various active materials or nutrients in the human small intestine, carries out simulation in a simulation environment designed according to a human physiological environment, has high conformity to the small intestine digestion and has high accuracy in simulation environment control.
Owner:NANCHANG UNIV
Program-controlled pig bionic digestion system and method using program-controlled pig bionic digestion system to quickly determining digestible energy value of pig feed
ActiveCN110057964AReal Digestion ApproachingConsistent activityChemical methods analysisGastric digestionAnimal science
The invention discloses a program-controlled pig bionic digestion system and a method using the program-controlled pig bionic digestion system to quickly determining a digestible energy value of pig feed. The method comprises the following steps that a feed sample is pulverized and sieved through a standard sieve; a gastric buffer solution and an intestinal buffer solution are prepared; simulatedgastric juice, simulated small intestinal juice and simulated large intestinal juice are prepared; the pulverized feed sample is filled into a simulated digester of the program-controlled pig bionic digestion system, and the simulated gastric juice is added in sequence through computer program control to simulate gastric digestion; after the intestinal buffer solution is added, the simulated smallintestinal juice is added to simulate small intestinal digestion; the simulated large intestinal juice is added to simulate large intestinal digestion; inactivation is carried out after the digestionis completed; and then cleaning is carried out, after undigested residues are obtained, a total energy value of the residues and the feed sample is tested, and the digestible energy value of the feedis calculated. The method is simple, high in precision, low in implementation cost, and the digestible energy value of the pig feed can be accurately determined within 72 hours.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
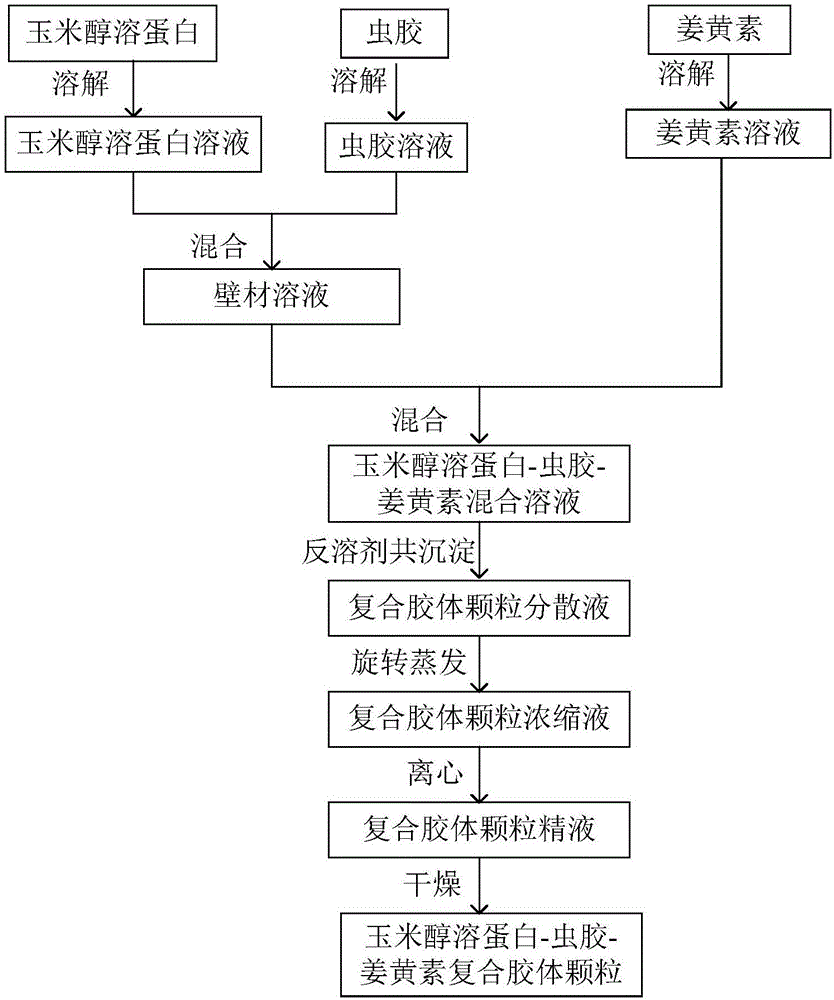
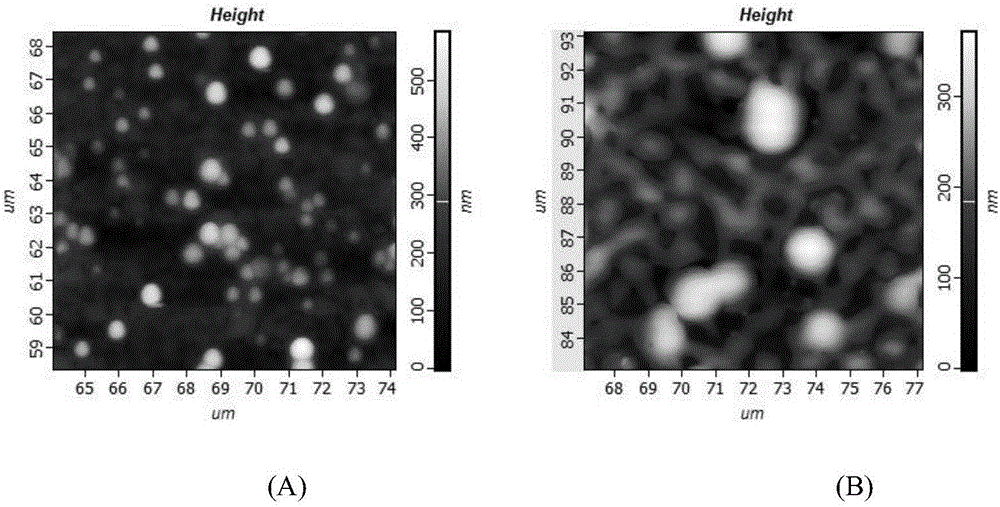
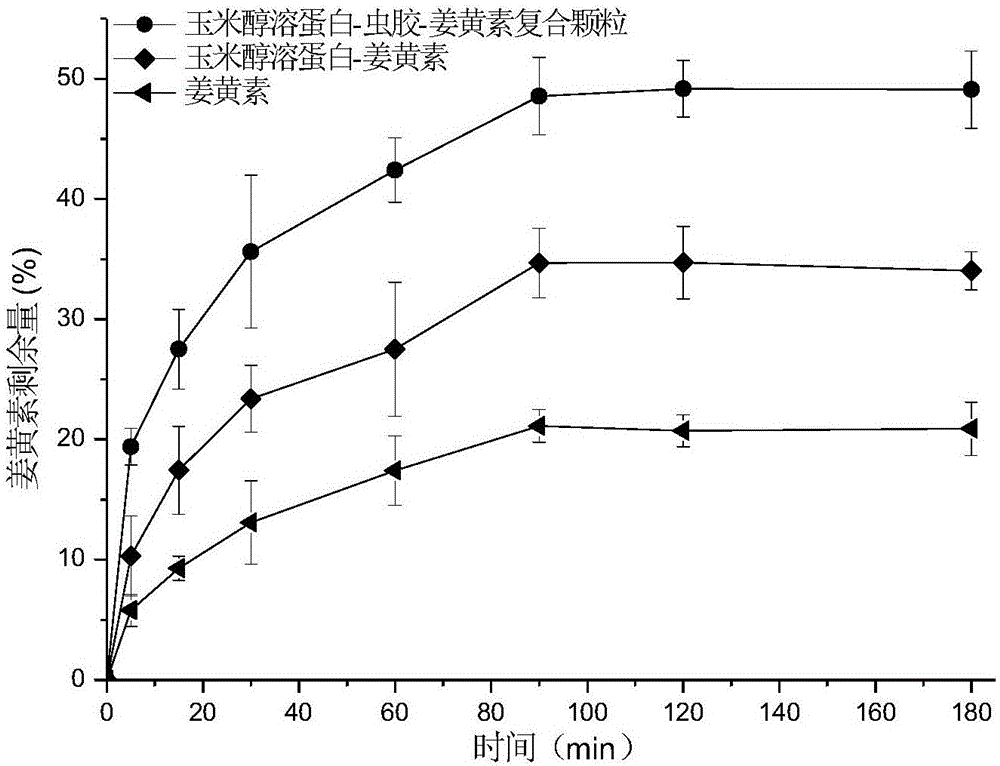
Corn prolamine-shellac-curcumin compound colloidal particles and preparation method thereof
InactiveCN106822035AAntioxidantImprove bioavailabilityAntipyreticAnalgesicsIntestinal fluidAqueous solubility
The invention discloses corn prolamine-shellac-curcumin compound colloidal particles and a preparation method thereof and belongs to the technical field of preparation of the compound colloidal particles. The compound colloidal particles take corn prolamine and shellac as wall materials and take curcumin as a core material, and are prepared by adopting an anti-solvent co-precipitation method; the mass ratio of the corn prolamine to the shellac ranges from (5 to 1) to (1 to 2) and the mass ratio of the wall materials to the curcumin ranges from (10 to 1) to (1 to 2); the corn prolamine, the shellac and the curcumin are combined through hydrogen bonds and a hydrophobic interaction effect. According to the compound colloidal particles provided by the invention, the embedding rate on the curcumin is more than 90 percent; the water solubility of the curcumin is extremely increased and the light degradation and thermal degradation speeds of the curcumin are effectively reduced; the bioavailability of the curcumin is remarkably improved, controlled release of the curcumin is realized to a certain extent and a releasing speed of the curcumin in intestinal fluid is reduced; According to the corn prolamine-shellac-curcumin compound colloidal particles provided by the embodiment of the invention, a new concept and a new way can be provided for a steady state of functional factors.
Owner:CHINA AGRI UNIV
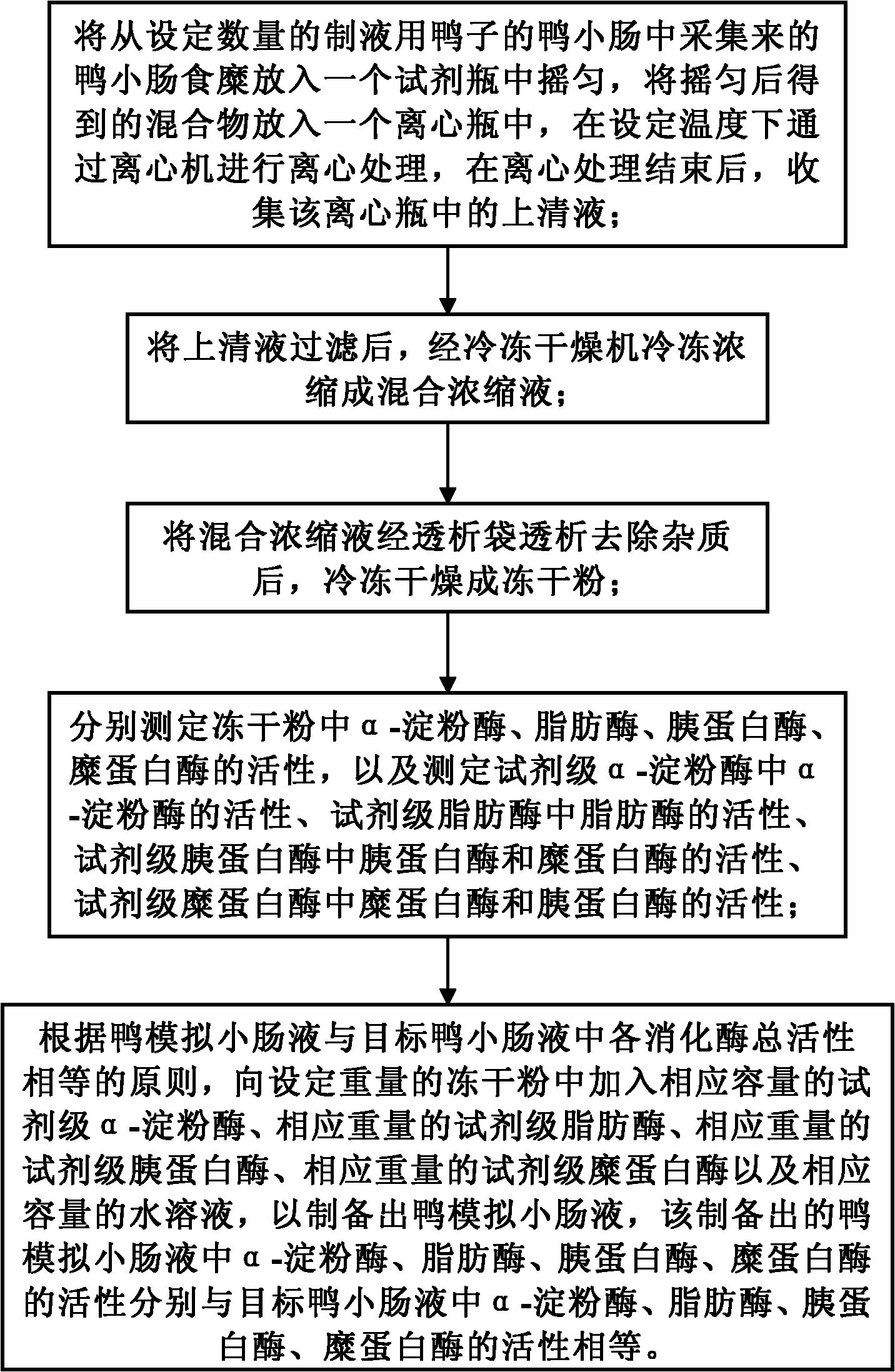
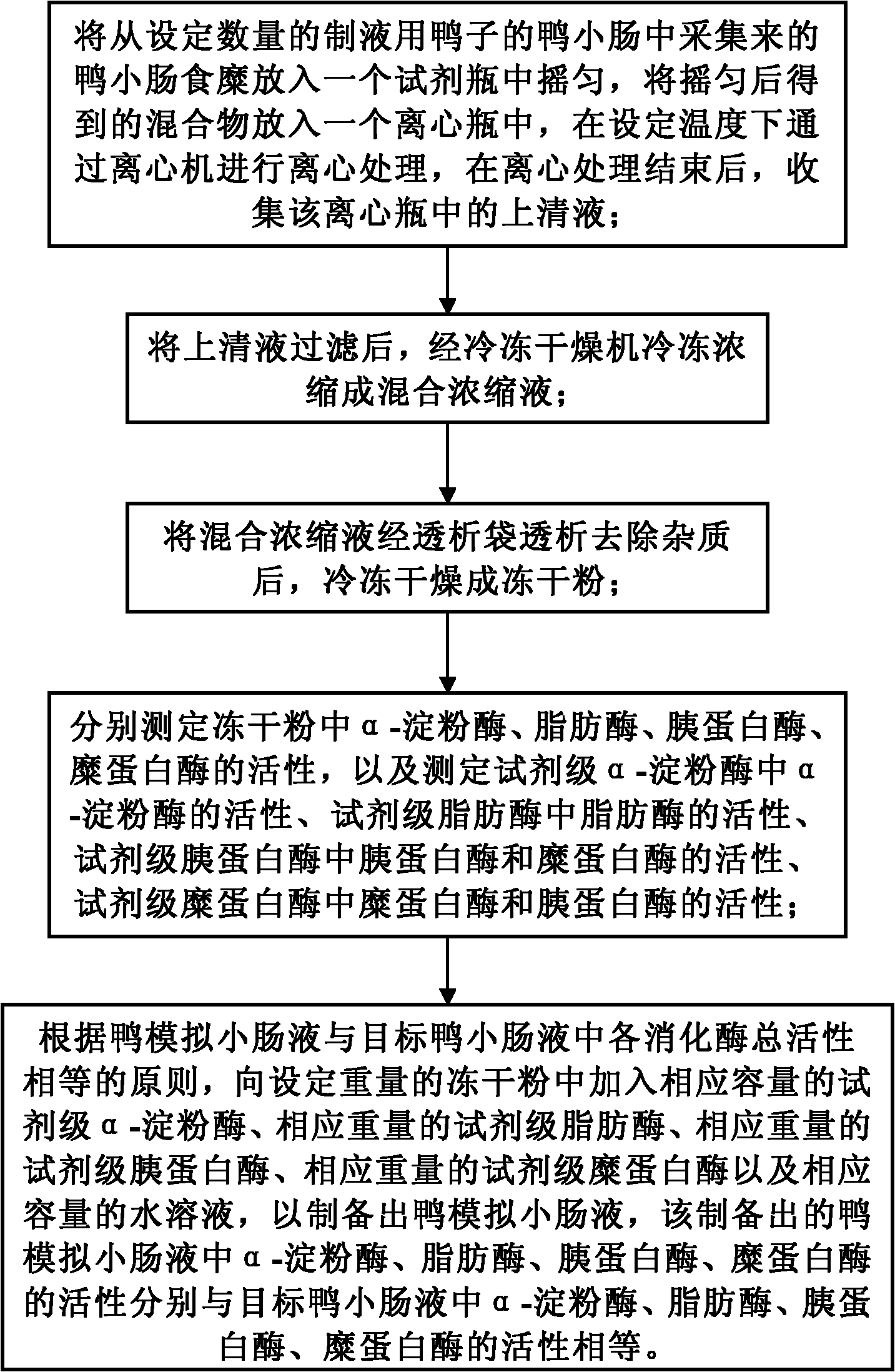
Preparation method of duck simulated intestinal fluid
InactiveCN102313794AReduce manufacturing costLow implementation costTesting foodIntestinal fluidFreeze-drying
The invention discloses a preparation method of a duck simulated intestinal fluid. The method comprises the following steps of: putting collected duck intestinal chyme in a reagent bottle to shake uniformly, putting in a centrifuge bottle to perform centrifuging treatment, and collecting a supernatant fluid; filtering the supernatant fluid, freezing and concentrating into a mixed concentrated liquid; after dialyzing the mixed concentrated liquid and removing impurities, freezing and drying into lyophiled powder; measuring the activities of alpha-amylase, lipase, trypsin and chymotrypsin in the lyophiled powder, and measuring the activities of corresponding digestive enzymes in the reagent level alpha-amylase, lipase, trypsin and chymotrypsin; and according to the principal that the gross activities of various digestive enzymes in the duck simulated intestinal fluid and the targeted duck intestinal fluid are equal, adding corresponding capacity reagent level alpha-amylase, corresponding weight reagent level lipase, corresponding weight reagent level trypsin, corresponding weight reagent level chymotrypsin and a corresponding capacity water solution in the lyophiled powder with a set weight. The method can be carried out in a low-cost way, and can also be used for preparing the duck simulated intestinal fluid basically homologous with four digestive enzymes in a real duck body.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Artificially simulated pig stomach and intestine digestive fluid, preparation method and application of digestive fluid
ActiveCN104297372AScientific in vitro evaluationObjective in vitro evaluationComponent separationMycotoxinSorbent
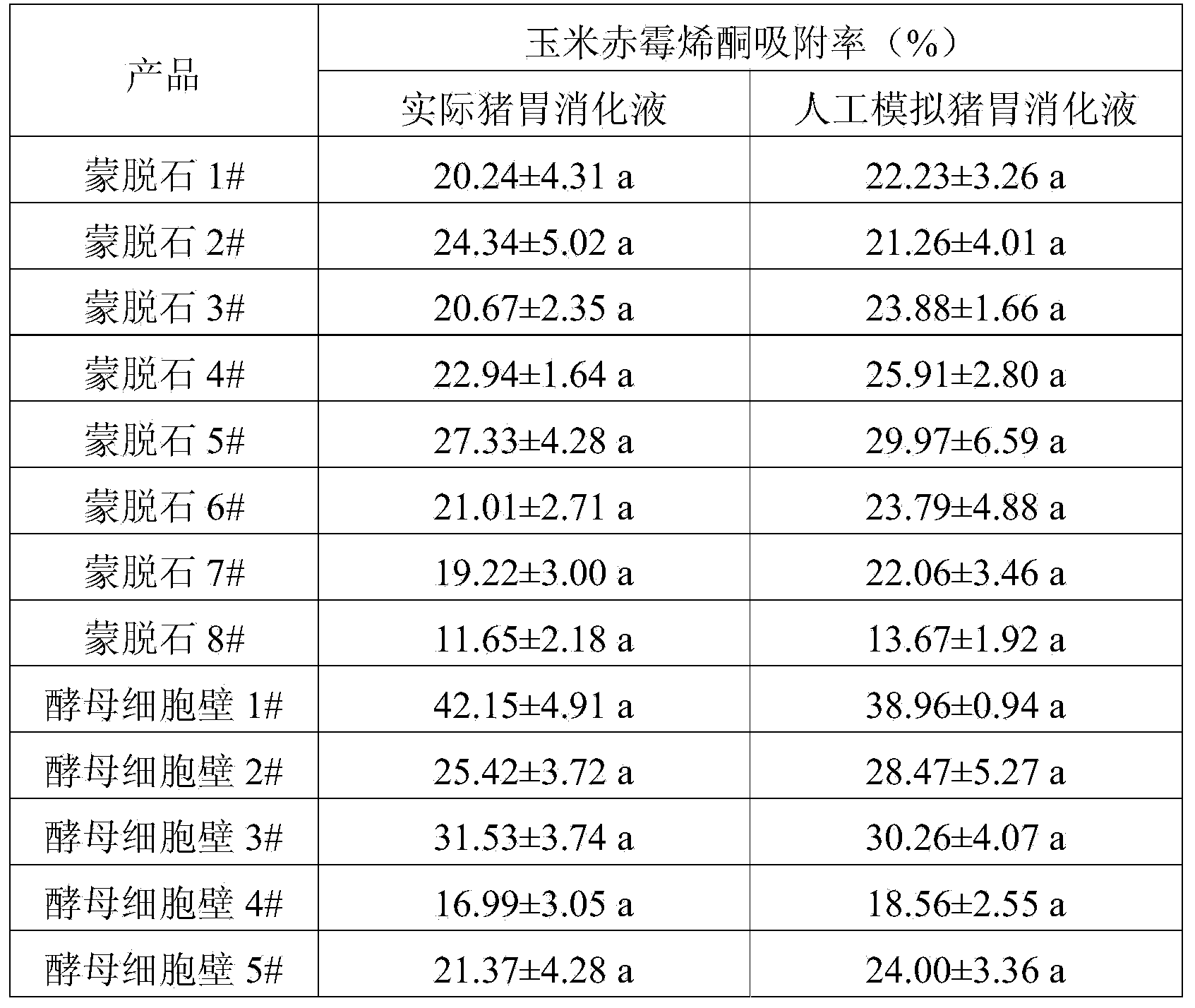
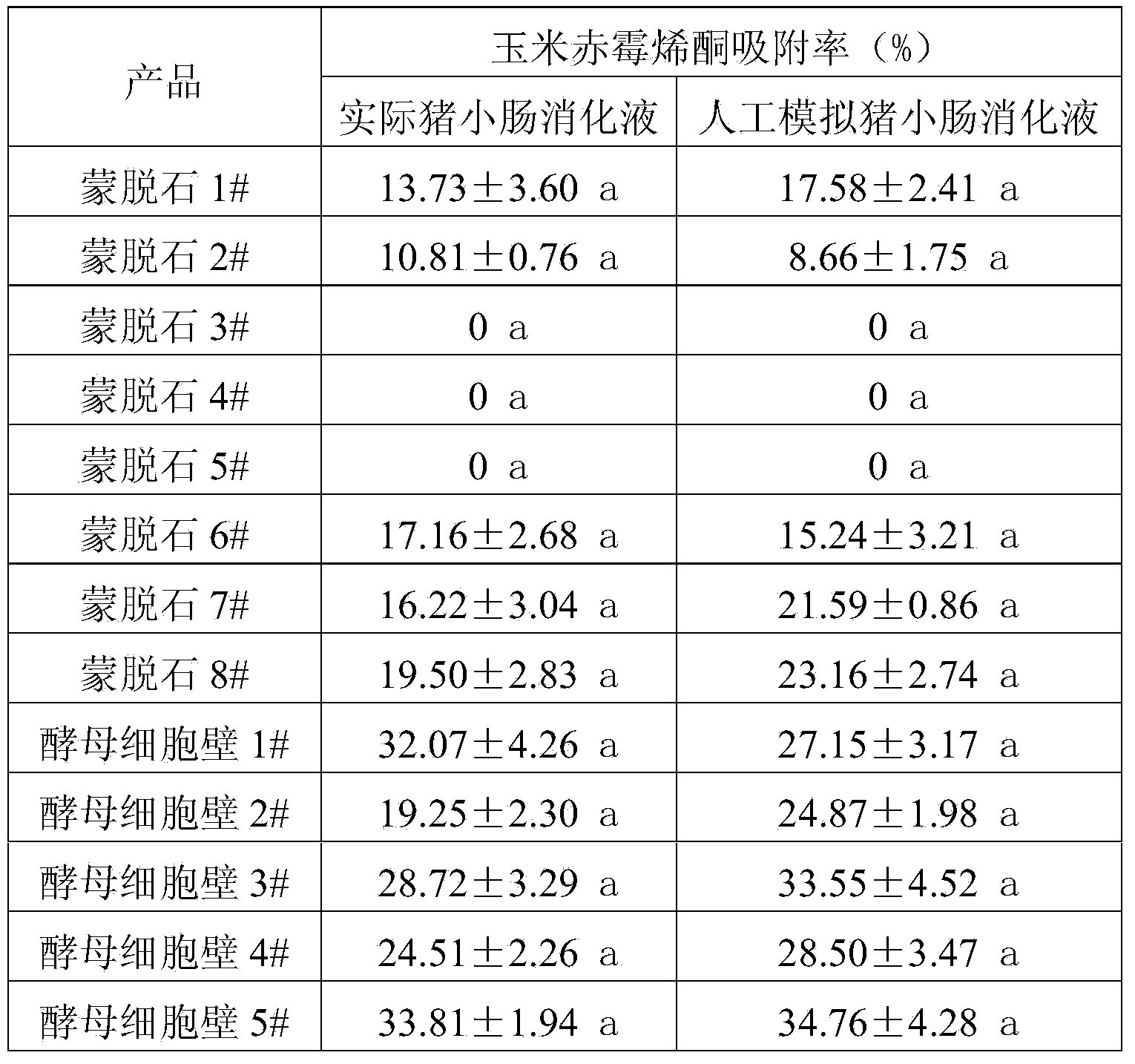
The invention discloses an artificially simulated pig stomach and intestine digestive fluid, a preparation method and application of the digestive fluid. The preparation method comprises the following steps: mixing pig compound feed and artificial gastric fluid according to a ratio of 1g to (3-3.5mL), adjusting the pH to be 1.5-2.0, oscillating at 39+ / -1 DEG C for 1-2 hours, cooling to 4+ / -1 DEG C, centrifuging 8000-9000g at 4+ / -1 DEG C for 5-10 minutes, and taking liquid supernatant as the artificially simulated pig stomach digestive fluid; and adding artificial intestinal fluid which is 3 times of the volume of a sediment obtained by centrifuging, adjusting the pH to be 6.8+ / -0.2, oscillating at 39+ / -1 DEG C for 1-2 hours, cooling to 4+ / -1 DEG C, centrifuging 18000-20000g at 4+ / -1 DEG C for 5-10 minutes, and taking liquid supernatant as the artificially simulated pig intestine digestive fluid. According to the artificially simulated pig stomach and intestine digestive fluid, the conditions such as the pH value and the temperature are consistent with the condition of a pig gastrointestinal tract; by virtue of digestion of special feed, the soluble components in the feed are introduced and are relatively consistent with the component of the pig digestive fluid, in the actual production; the scientific, objective and accurate in-vitro evaluation is carried out on adsorption rate of a mycotoxin absorbent in the pig; the method is simple and quick.
Owner:INST OF QUALITY STANDARD & TESTING TECH FOR AGRO PROD OF CAAS
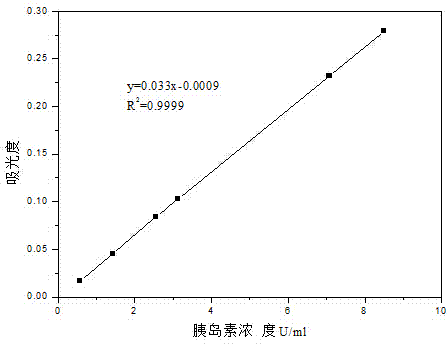
Production method of insulin oral sustained-release preparation
ActiveCN104491844AStable oral administrationEffective encapsulationPeptide/protein ingredientsPharmaceutical non-active ingredientsBiocompatibility TestingDrug biological activity
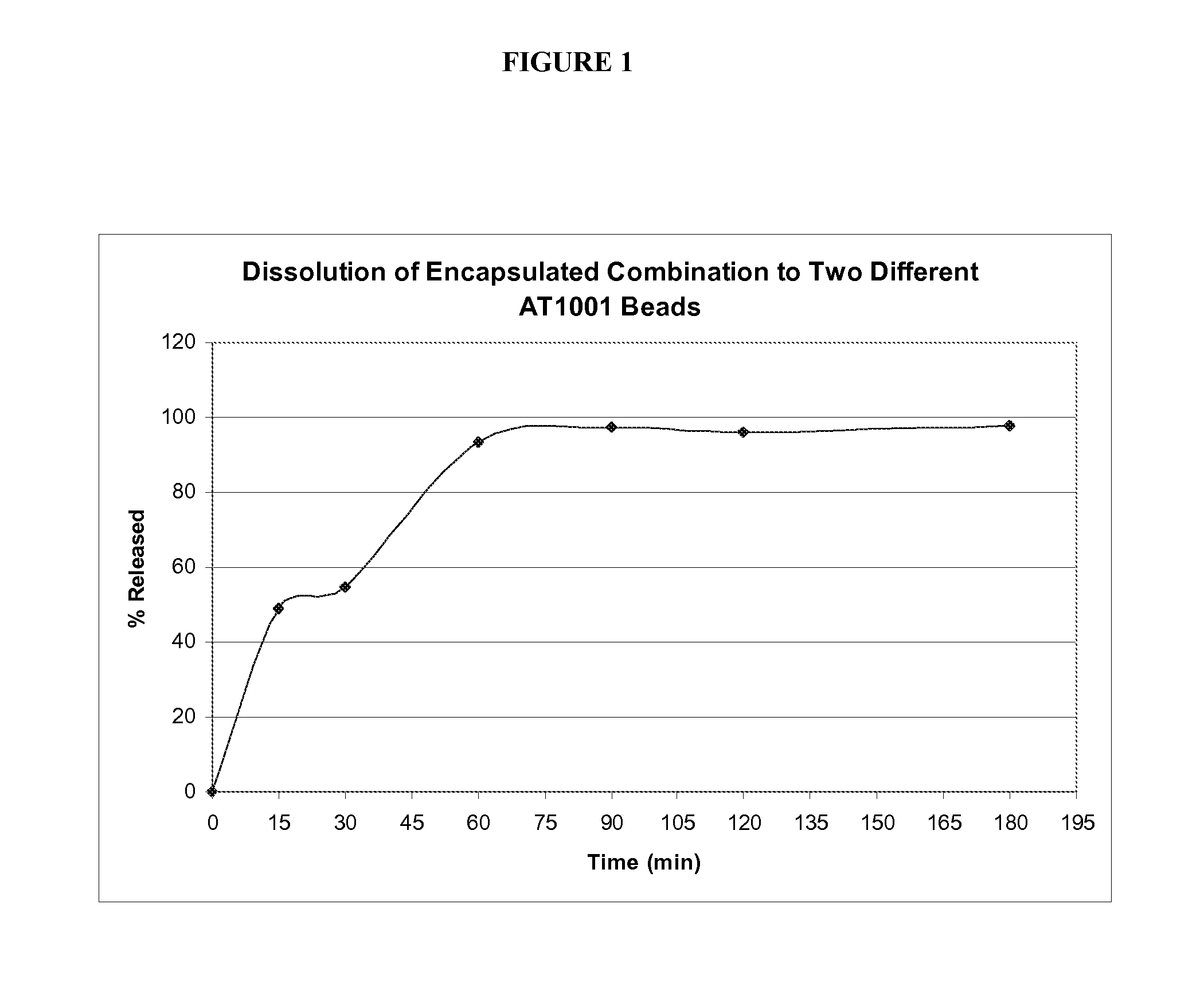
The invention provides a production method of an insulin oral sustained-release preparation and belongs to the technical field of biological medicines. According to the production method, pegylated chitosan (PEG-CS) is taken as an encapsulating material and insulin-loaded pegylated chitosan microspheres are prepared by use of an ionic gel method; in other words, firstly, the PEG-CS solution of a certain concentration is mixed with an insulin solution, low-concentration sodium tripolyphosphate (TPP) of a low concentration is added dropwise under magnetic stirring, the pH is regulated to 5.5 by use of hydrochloric acid, after the reaction is completed, the suspension is centrifuged and then the microspheres are just collected. The production method is simple and mild in conditions; the obtained microspheres are good in biocompatibility, can be stably released in a simulated intestinal fluid for 7 days, and have a sustained-release function; besides, the biological activity of the medicine can be guaranteed, and the oral medication of the insulin is expected to be realized.
Owner:YANGZHOU UNIV
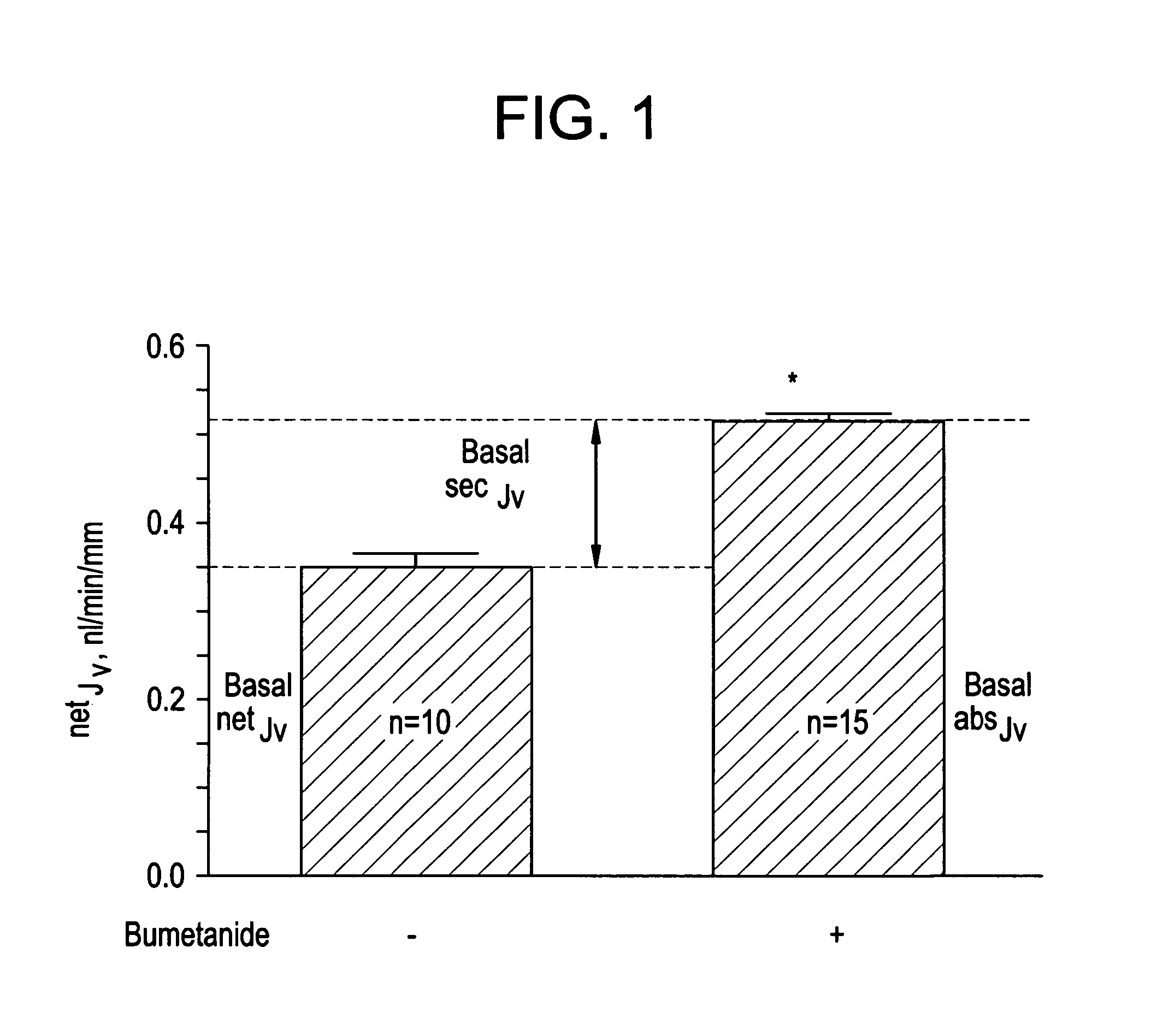
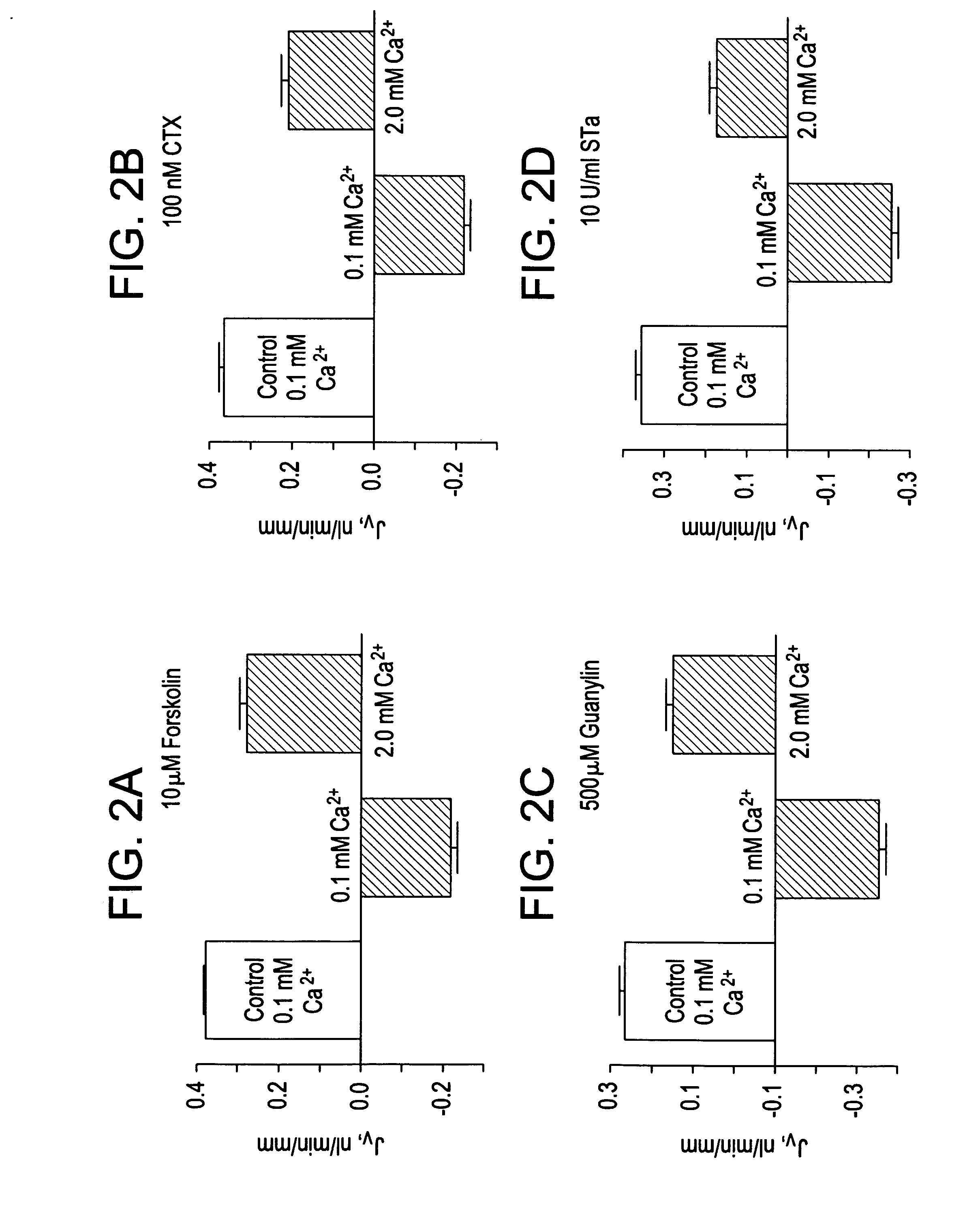
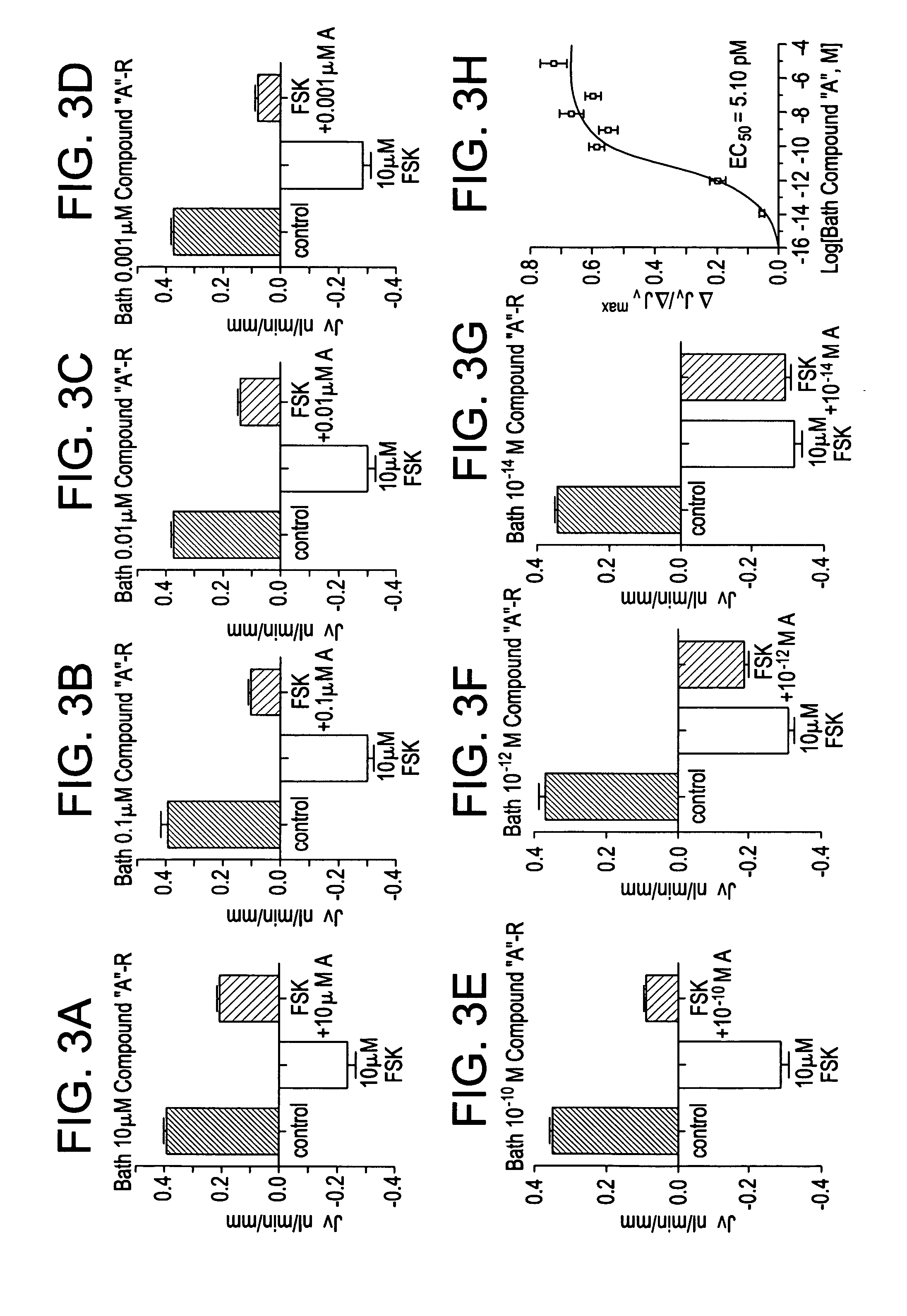
Methods of modulating intestinal fluid balance
ActiveUS20070060625A1Modulating absorptionModulating intestinal fluid secretionBiocidePeptide/protein ingredientsBalance disturbancesIntestinal fluid
The present invention relates to methods for treating or preventing intestinal fluid balance disorders and modulating intestinal fluid secretion and absorption using calcimimetics and calcilytics.
Owner:AMGEN INC +2
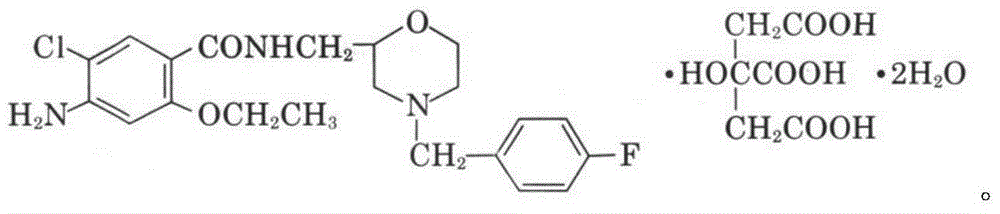
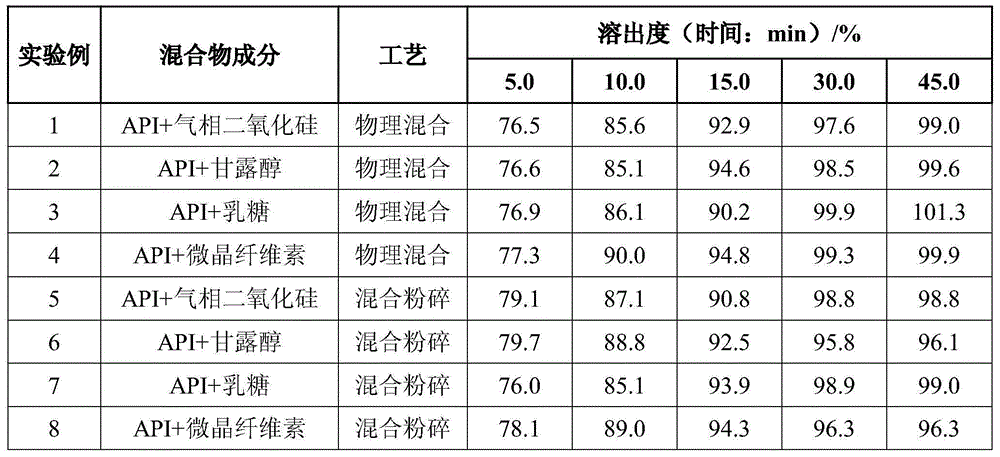
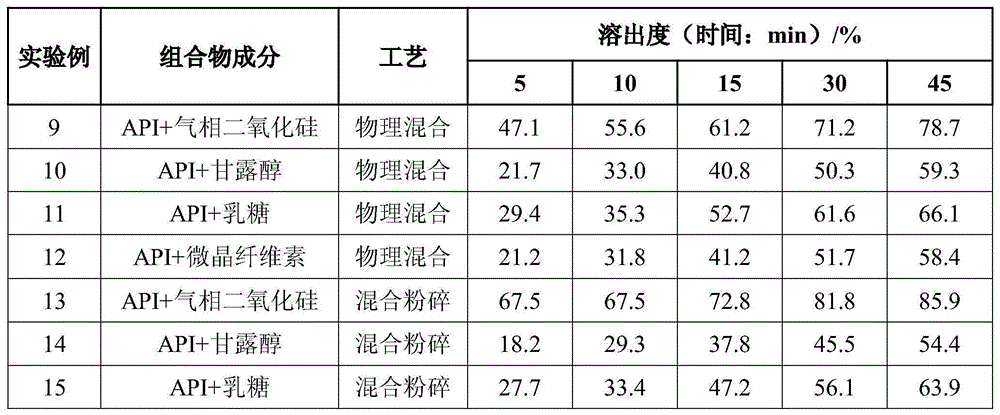
Rapidly-dissolving mosapride citrate composition
ActiveCN105769872AReduce manufacturing costImprove securityOrganic active ingredientsDigestive systemAdditive ingredientAdhesive
The invention provides a rapidly-dissolving mosapride citrate composition, which comprises mosapride citrate and fumed silica. Mosapride citrate and fumed silica are mixed and crushed in a crushing machine, wherein mass ratio of mosapride citrate to fumed silica is 1:0.2-1.2; and particle size D50 of the mosapride citrate is less than or equal to 3.0 microns. The composition also contains one or more ingredients selected from a filler, a disintegrating agent, an adhesive, a lubricant, a flow aid and a flavouring agent. By the use of the mosapride citrate composition, dissolution rate of mosapride citrate in a buffer solution (a simulated intestinal fluid) with pH 6.8 is greatly raised, and medication effectiveness is raised for achlorhydria patients. The preparation technology is simple and is easy to operate. Any organic reagents are not used, and hot and humid process is not required. Instability of mosapride citrate in a hot and humid environment is avoided. The composition of the invention is suitable for industrial production.
Owner:CHENGDU KANGHONG PHARMA GRP
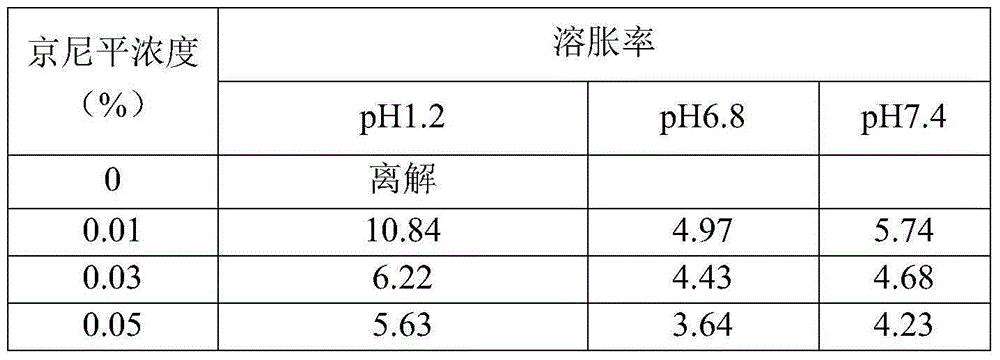
Intestinal tract targeting and pH-sensitive complex coacervation microcapsule transmission system and preparation method and application thereof
ActiveCN105011343AFree from destructionAchieve targeted releaseFood shapingFood ingredient as encapsulating agentIntestinal fluidCentrifugation
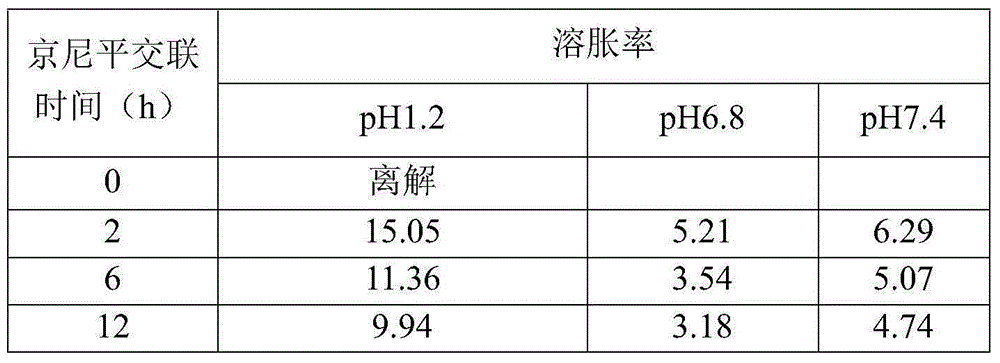
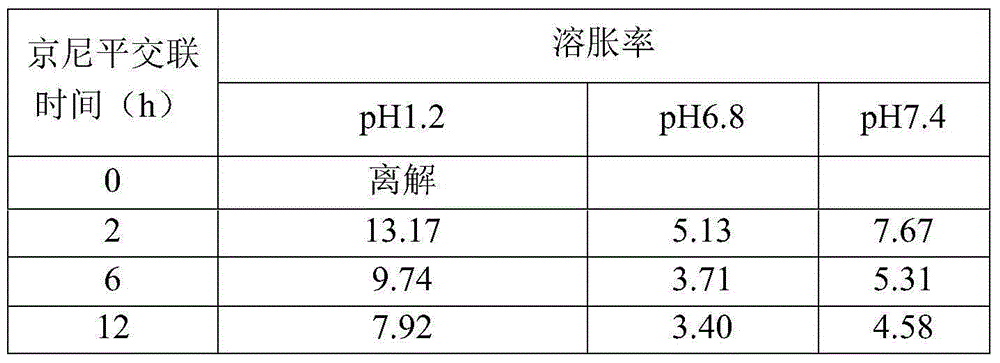
The present invention provides an intestinal tract targeting and pH-sensitive complex coacervation microcapsule transmission system and a preparation method and an application thereof. Carboxymethyl chitosan and arabic gum are polycations and polyanions to constitute a complex coacervationsystem. By adjusting pH value, the carboxymethyl chitosan and the arabic gum are subjected to complex coacervation reaction, and the complex coacervation phase is collected by centrifugation, crosslinked by genipin, and freeze-dried to obtain the intestinal tract targeting and pH-sensitive complex coacervation microcapsule transmission system. The intestinal tract targeting and pH-sensitive complex coacervation microcapsule transmission system can be used either for embedding water-soluble functional components, but also for embedding fat-soluble functional components, can protect core materials against damage caused by strongly acidic gastric environment, safely delivery the core materials to the intestinal tracts to conduct targeted releases, and expand the practical application field of the complex coacervation microencapsulation technology. The intestinal tract targeting and pH-sensitive complex coacervation microcapsule transmission system does not dissociate in a simulated gastric fluid and swells in a simulated intestinal fluid, and has a strong stability, and the process is simple, safe, efficient, and easy for mass production.
Owner:QINGDAO AGRI UNIV
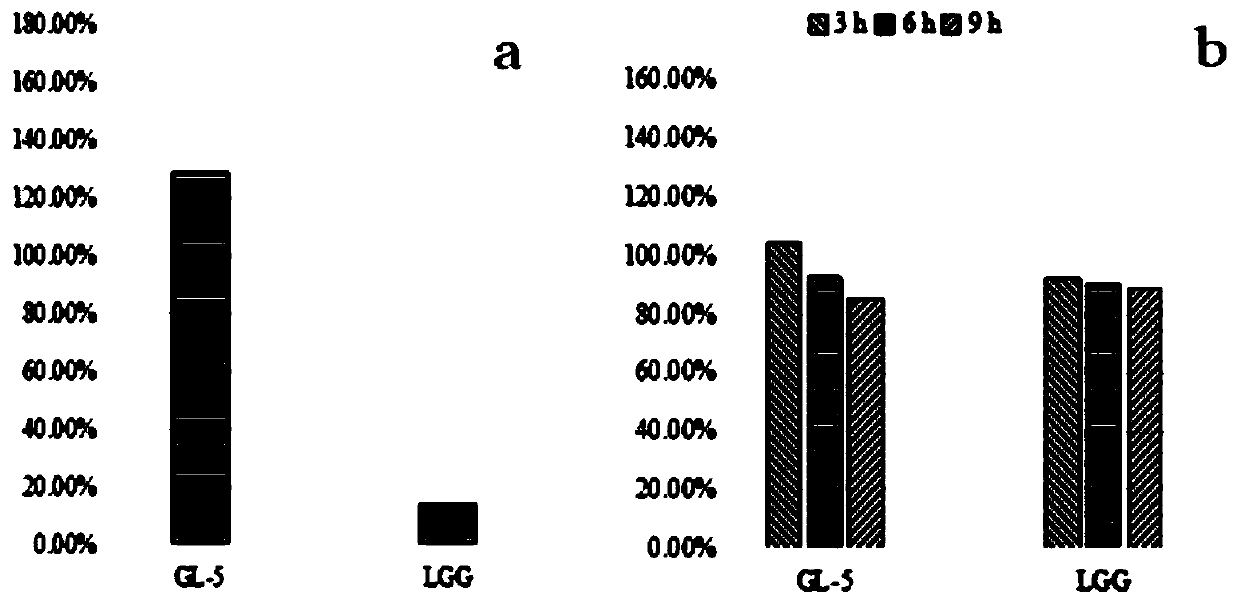
Lactobacillus plantarum GL-5 with oxidation resisting activity and application thereof
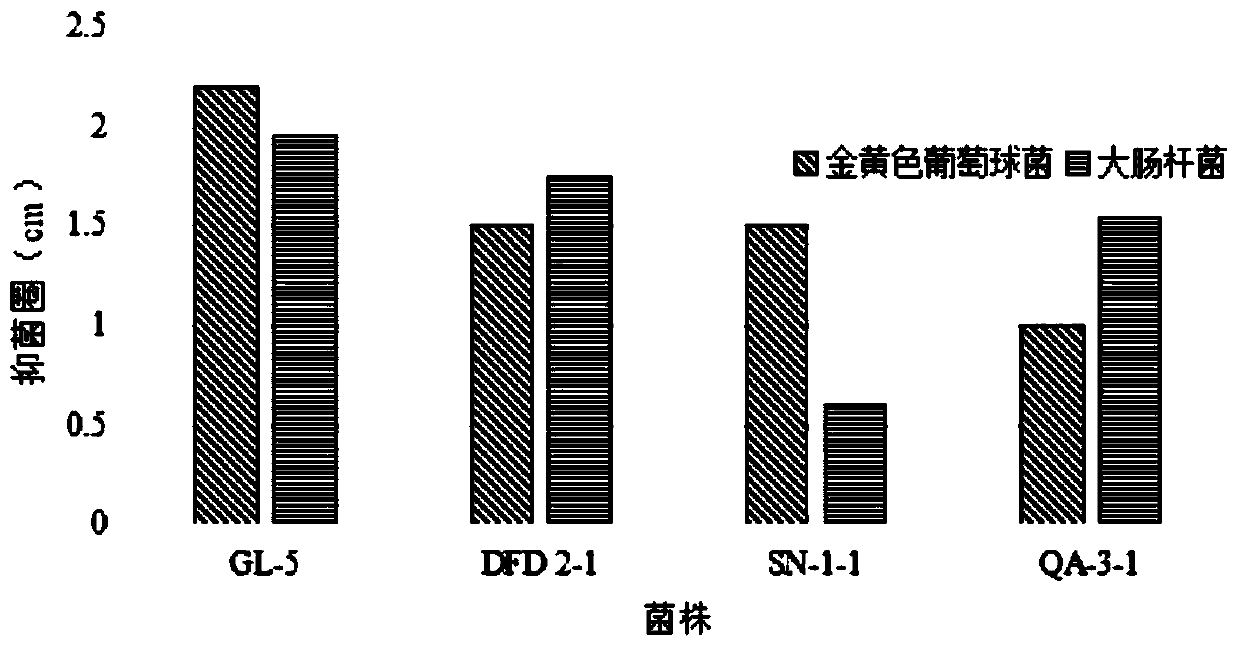
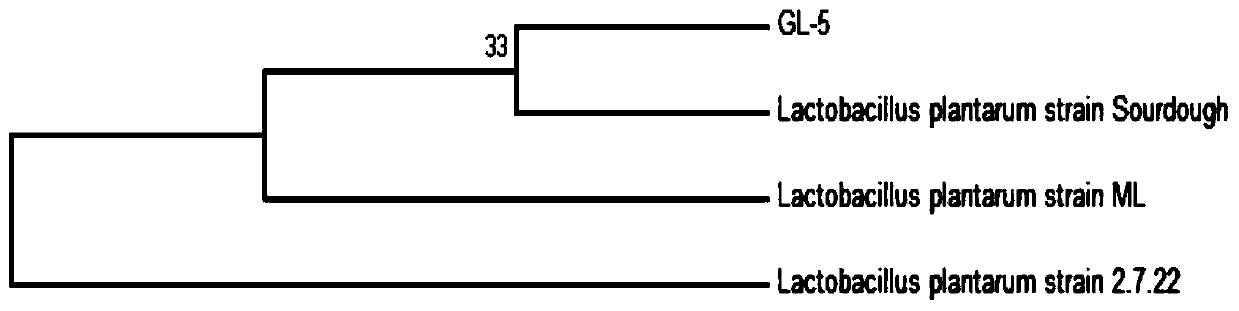
ActiveCN111304117AHigh antibacterial activityImproves antioxidant activityAntibacterial agentsBacteriaBiotechnologyEscherichia coli
The invention belongs to the technical field of microbes and particularly relates to lactobacillus plantarum GL-5 with oxidation resisting activity and application thereof. The lactobacillus plantarumGL-5 with oxidation resisting activity disclosed by the invention is screened out from a home-made fermented dairy product of a herdsman's house of a Qinghai Guoluo region with an altitude of 4,000mor more, is collected in China General Microbiological Culture Collection Center of China Committee for Culture Collection of Microorganisms on November, 21, 2019 and has an accession number of CGMCCNo. 18988. The lactobacillus plantarum GL-5 has a 16S rRNA sequence shown in SEQ ID NO: 1. The lactobacillus plantarum GL-5 provided by the invention has relatively good bacteriostatic activity, and activity of enteropathogenic bacteria, i.e., staphylococcus aureus and escherichia coli can be remarkably inhibited; the lactobacillus plantarum GL-5 provided by the invention has relatively high oxidation resisting activity and can remarkably tolerate catalase; and the lactobacillus plantarum GL-5 provided by the invention has relatively high gastrointestinal fluid tolerance and cholate tolerance,and the survival rate of the lactobacillus plantarum GL-5 after culture in simulated gastric fluid and intestinal fluid is remarkably higher than that of lactobacillus rhamnosus LGG.
Owner:LANZHOU UNIVERSITY
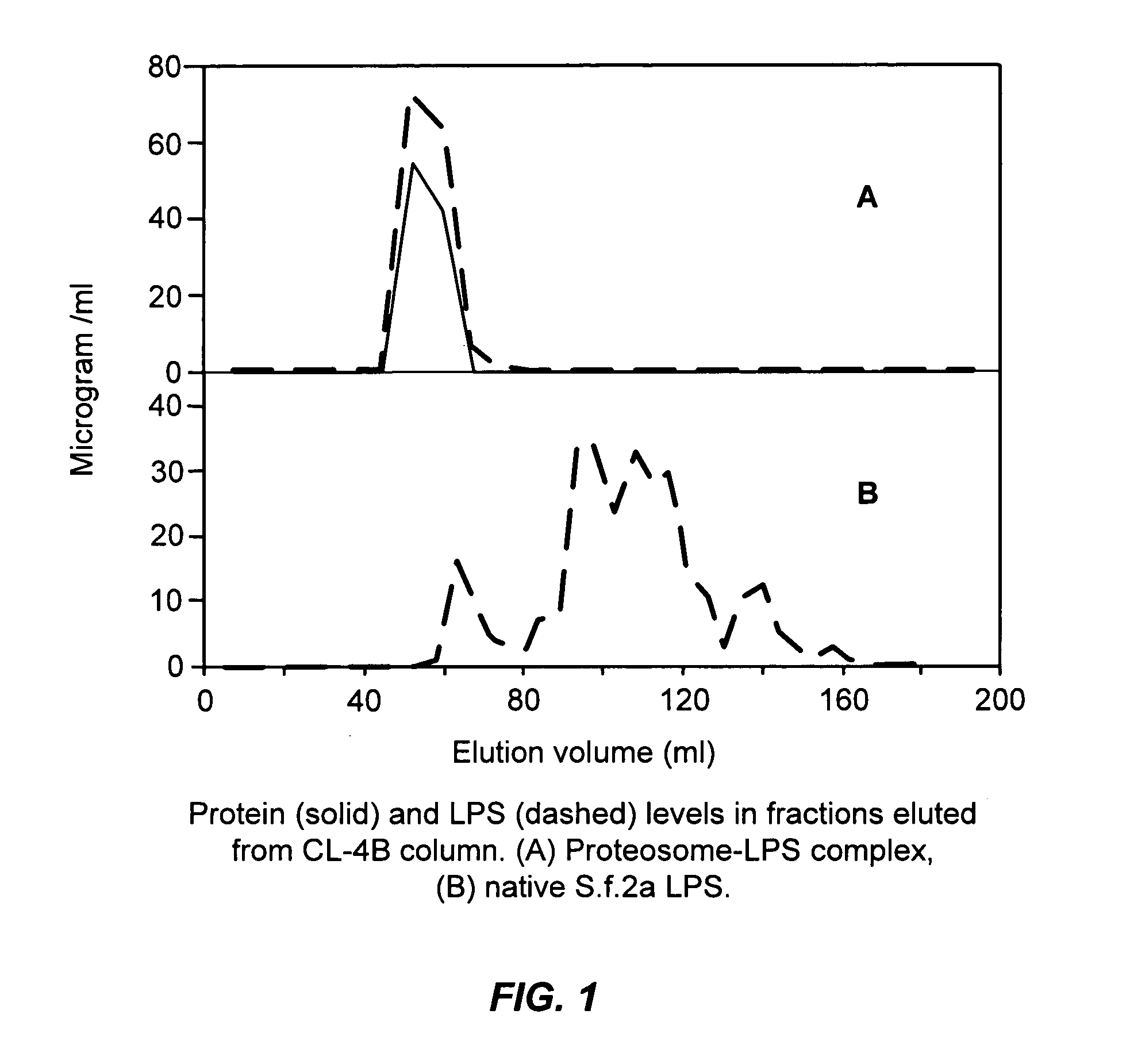
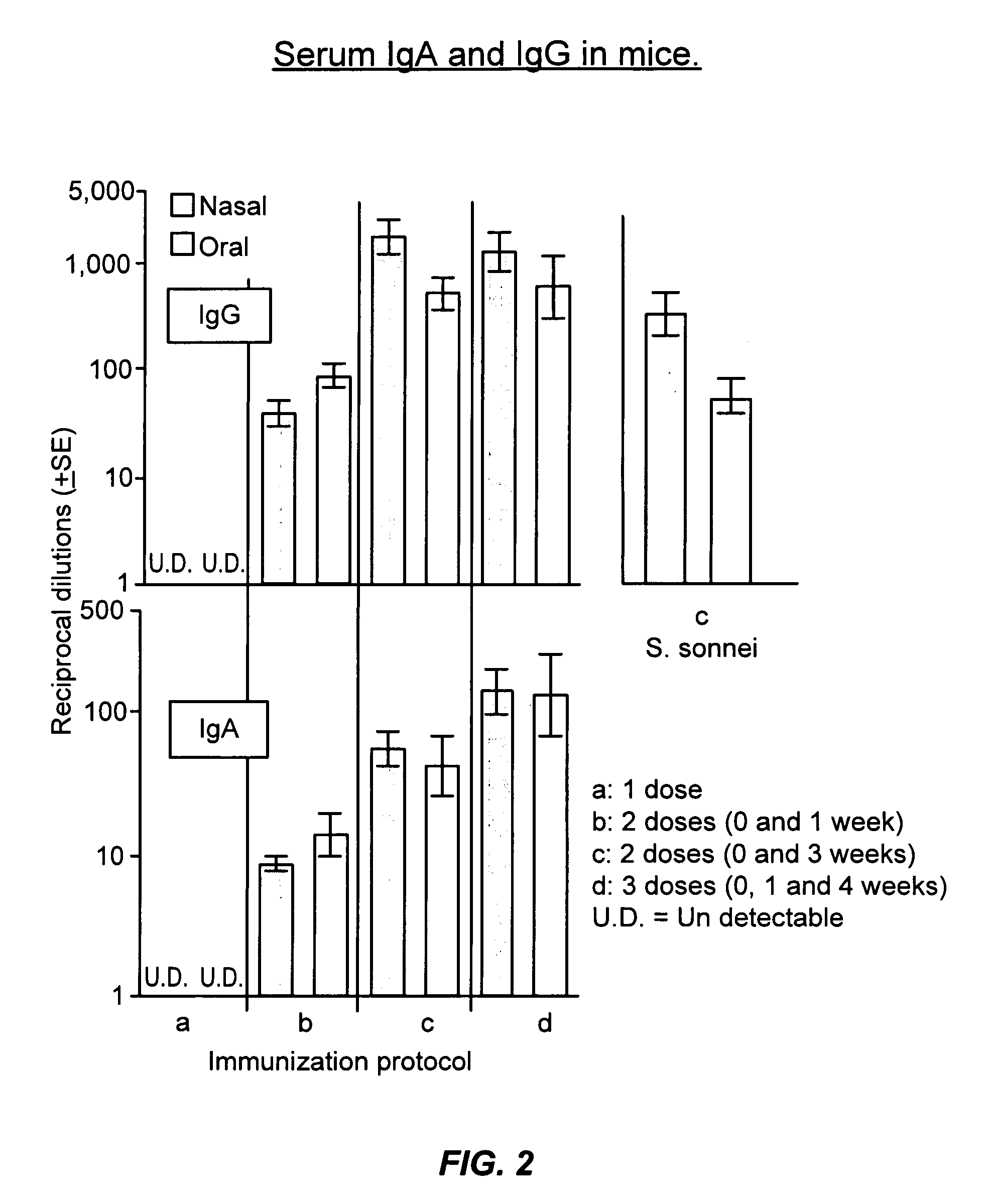
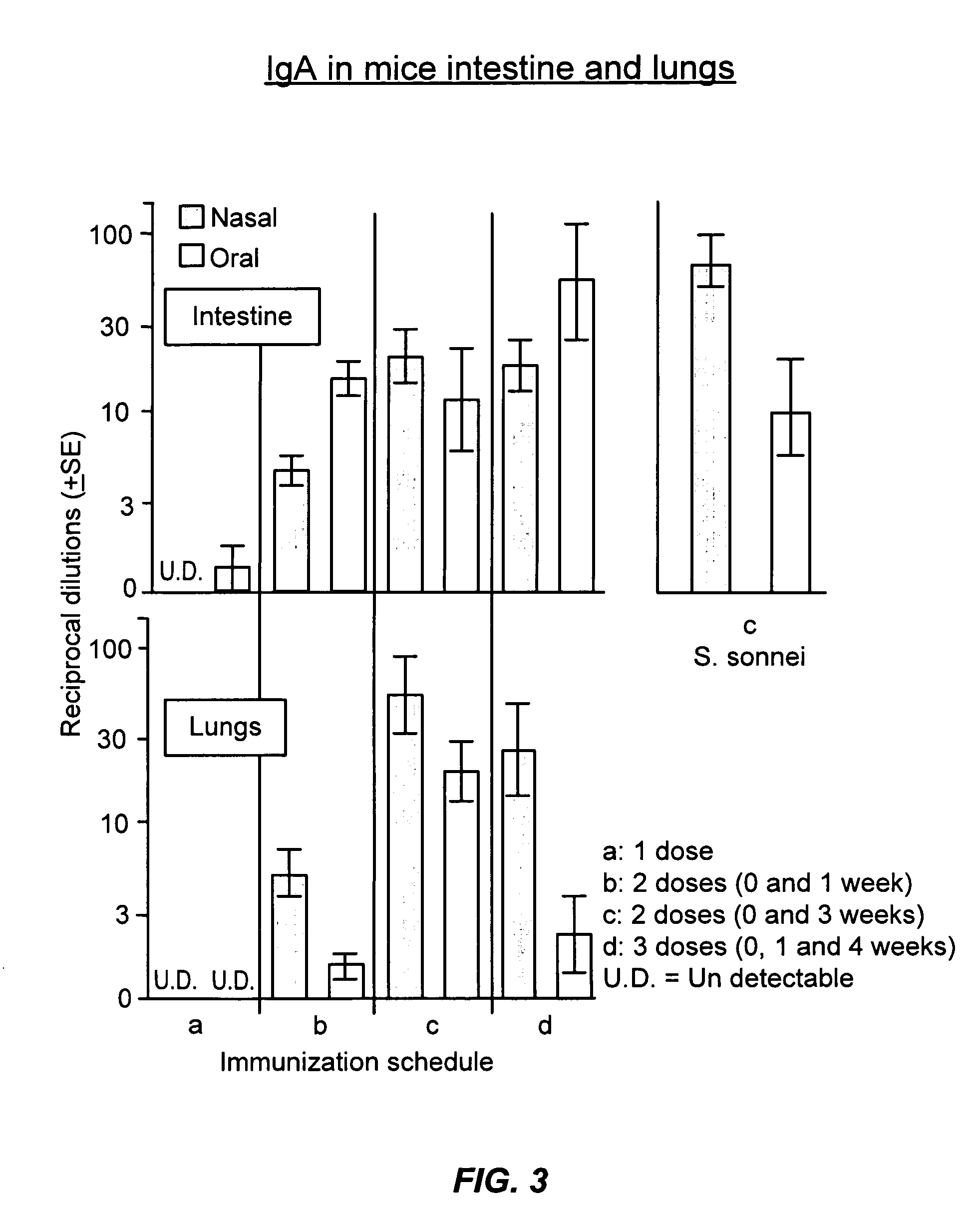
Oral or intranasal vaccines using hydrophobic complexes having proteosomes and lipopolysaccharides
InactiveUS6803042B2Improving immunogenicityImprove immunityAntibacterial agentsBacterial antigen ingredientsSerum igeIntestinal fluid
An immunogenic complex, essentially consisting of neisserial outer membrane protein proteosomes hydrophobically complexed to native purified bacterial lipopolysaccharide and formulated in accordance with the current invention for mucosal delivery such as via the oral or intranasal route is used as a vaccine. Specifically, a vaccine using shigella lipopolysaccharides complexed to proteosomes for such mucosal administration induces IgG and IgA antibodies in sera and in respiratory and intestinal fluids. Furthermore, such antibodies are associated with protection against shigella infection and these vaccines are herein demonstrated to protect against mucosal infection with shigella.
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC
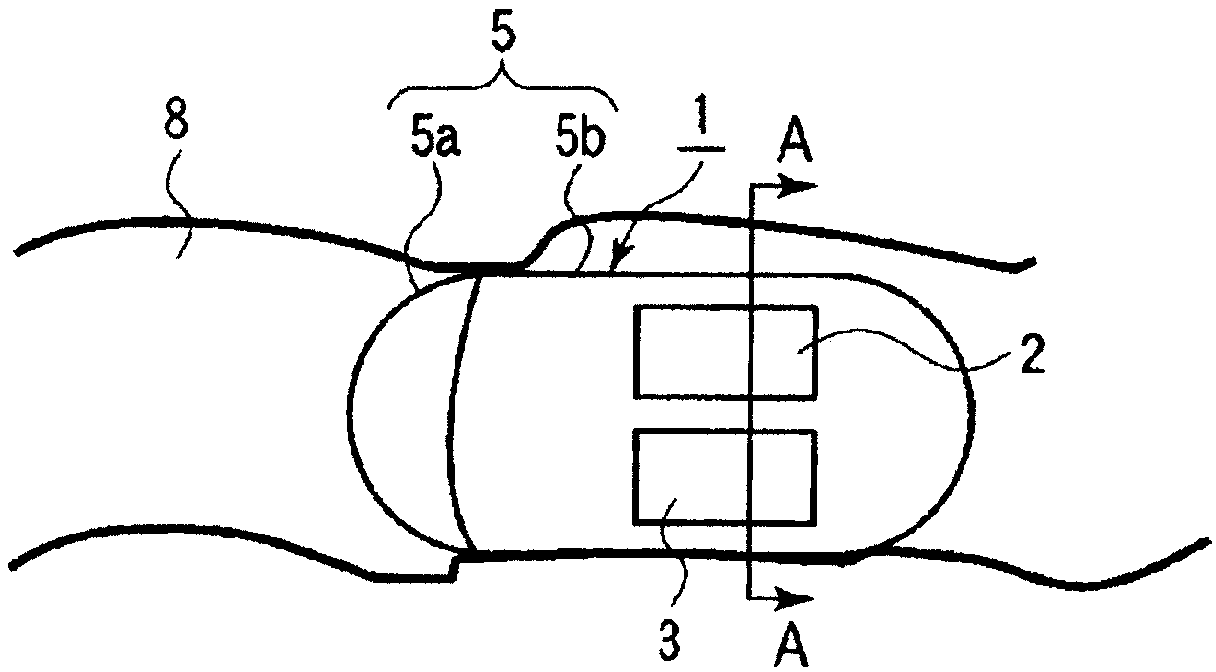
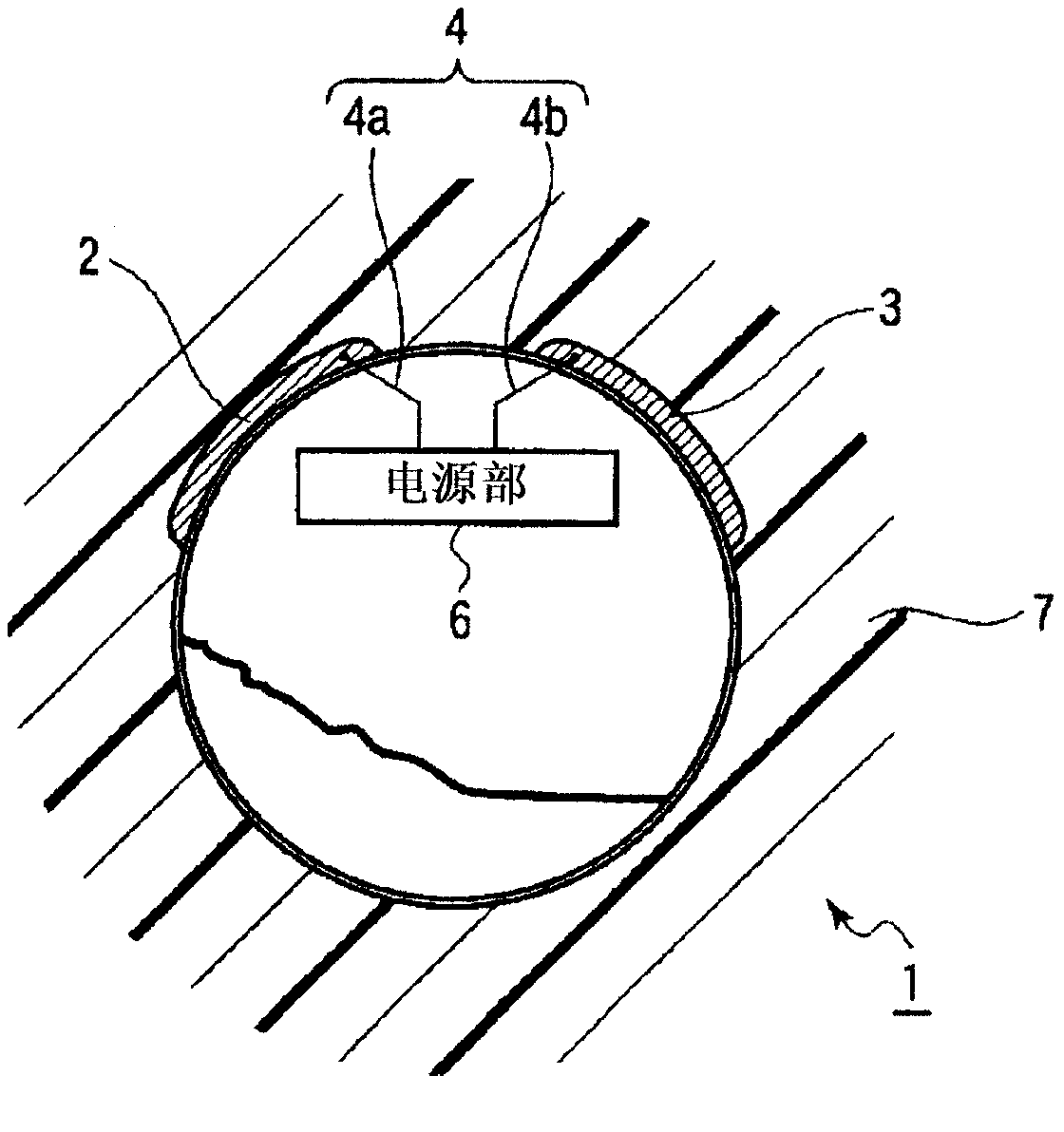
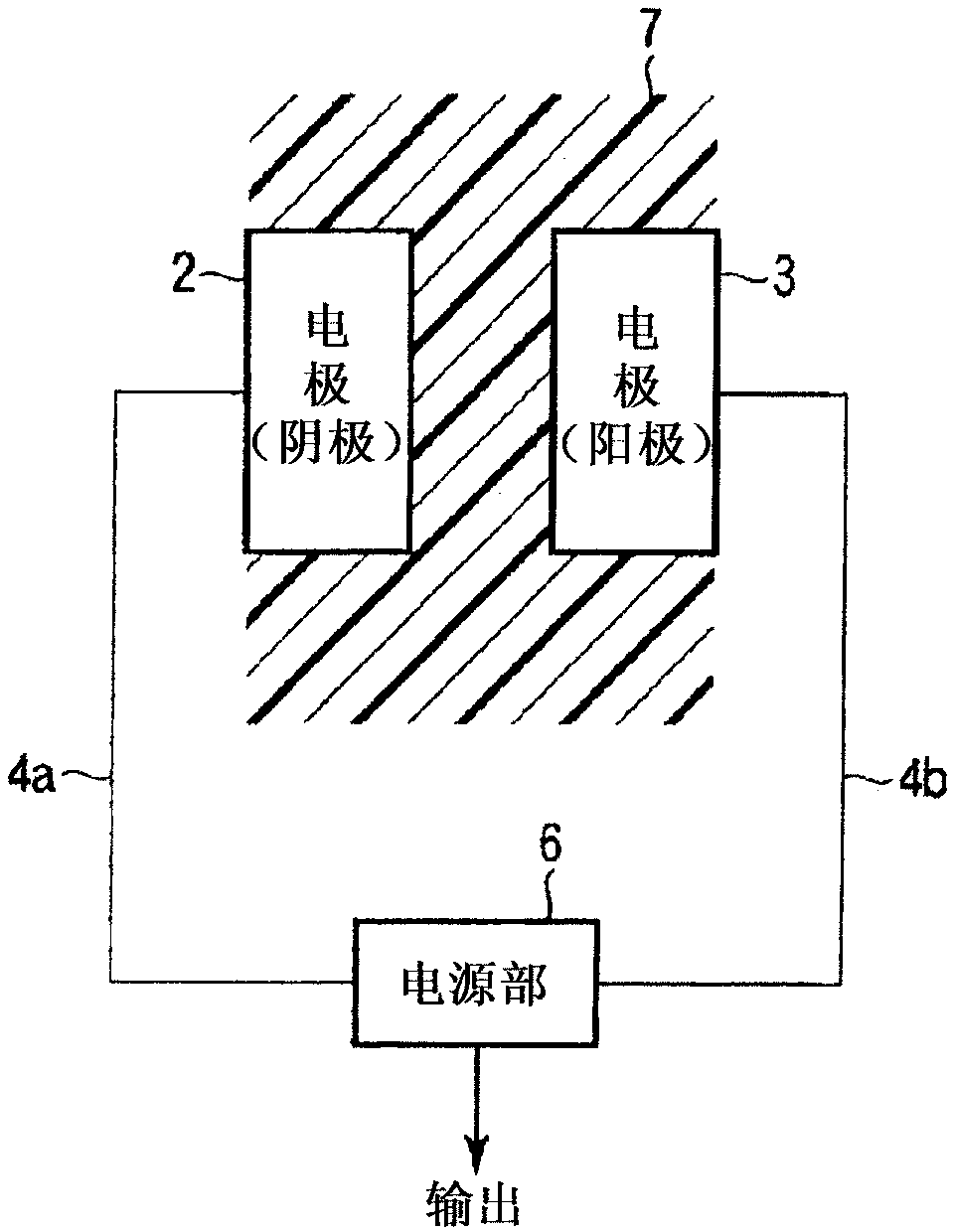
Power supply system and medical capsule device mounted with same
Provided is a power generation system that does not restrict patient's area of activity, is harmless to a living body, and can obtain a sufficient amount of electric power in the stomach or bowel, and also is provided a disposable medical capsule device that is mounted with the power generation system and does not need to be collected after being used. The power generation system is mounted in the capsule device, includes at least two electrodes or a pair of electrodes consisting of an aluminum electrode and a catalyst-supported carbon electrode which are provided on the outer wall surface of the capsule main body, is immersed in an electrolyte solution consisting of stomach fluid or bowel fluid to generate electricity, and supplies the power supply to a constituent region in the capsule device.
Owner:OLYMPUS CORP
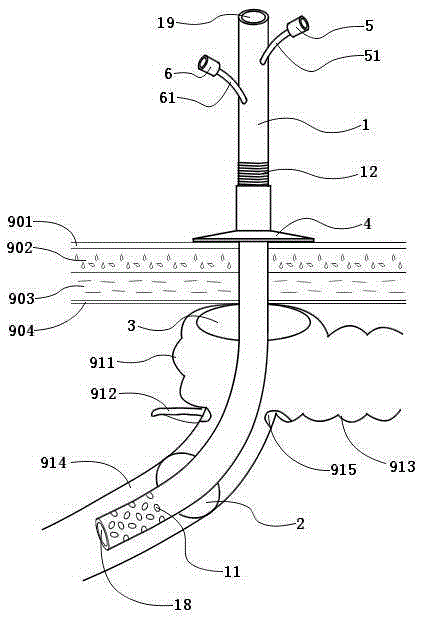
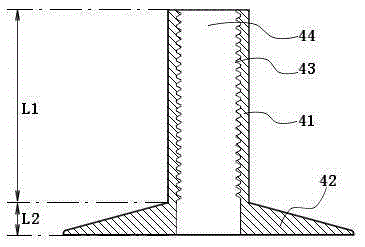
Double-balloon intestine fistulization catheter for preventing anastomotic fistula after colorectal surgery
InactiveCN105251099APrevent extravasationPrevent slippageBalloon catheterIntestinal fluidAbdominal cavity
The invention discloses a double-balloon intestine fistulization catheter for preventing anastomotic fistula after a colorectal surgery. The double-balloon intestine fistulization catheter comprises a catheter body, a first balloon, a second balloon, a threaded compressed tablet, a first water injection opening and a second water injection opening, wherein the first balloon and the second balloon are two hollow balloons which sleeve the catheter body and are made of an elastic material; the first balloon is close to the head end; the threaded compressed tablet sleeves the part between the second balloon and the tail end; a side hole is formed in the wall, between the first balloon and the head end, of the catheter body; the first water injection opening and the second water injection opening are positioned at the tail end of the catheter body, and are respectively communicated with the first balloon and the second balloon. According to the invention, when the catheter is inserted into the intestinal tract after an intestinal surgery, the circulation of the small intestine is blocked after the first balloon is injected with water to enable intestinal fluid to be led out of the body through the head end and side hole of the catheter; after being injected with water, the second balloon and the threaded compressed tablet positioned outside the body are coordinated to fix the catheter to prevent slipping or shifting of the catheter, and at the same time, the intestinal wall of the cecum is clung to and adhered with the abdominal wall to prevent intestinal fluid from leaking outwards to the abdominal cavity.
Owner:NANJING DRUM TOWER HOSPITAL
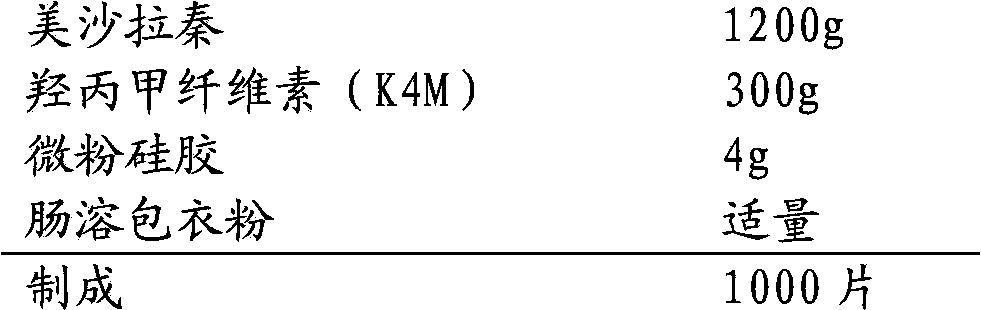
Mesalazine enteric-coated sustained release tablet
InactiveCN103565766AImprove uniformityEasy to prepareOrganic active ingredientsDigestive systemSustained Release TabletIntestinal fluid
The invention discloses a mesalazine enteric-coated sustained release tablet. The mesalazine enteric-coated sustained release tablet is characterized by consisting of the following components in a unit dose: 1.2g of mesalazine, 0.1-0.3g of a sustained release material, 0.004.0.03g of a lubricant and 0.02-0.1g of enteric-coated powder. The release of the mesalazine enteric-coated sustained release tablet in one hour in simulated intestinal fluid of which the pH value is 7.2 is less than 30%, the release in four hours is not more than 70%, and the release in eight hours exceeds 80%.
Owner:四川健能制药有限公司
Oral or intranasal vaccines using hydrophobic complexes having proteosomes and lipopolysaccharides
An immunogenic complex, essentially consisting of neisserial outer membrane protein proteosomes hydrophobically complexed to native purified bacterial lipopolysaccaride and formulated in accordance with the current invention for mucosal delivery such as via the oral or intranasal route is used as a vaccine. Specifically, a vaccine using shigella lipopolysaccharides complexed to proteosomes for such mucosal administration induces IgG and IgA antibodies in sera and in respiratory and intestinal fluids. Furthermore, such antibodies are associated with protection against shigella infection and these vaccines are herein demonstrated to protect against mucosal infection with shigella.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Oral or intranasal vaccines using hydrophobic complexes having proteosomes and lipopolysaccharides
InactiveUS20020037295A1Promote intestinal absorptionAvoid contactAntibacterial agentsBacterial antigen ingredientsSerum igeIntestinal fluid
An immunogenic complex, essentially consisting of neisserial outer membrane protein proteosomes hydrophobically complexed to native purified bacterial lipopolysaccharide and formulated in accordance with the current invention for mucosal delivery such as via the oral or intranasal route is used as a vaccine. Specifically, a vaccine using shigella lipopolysaccharides complexed to proteosomes for such mucosal administration induces IgG and IgA antibodies in sera and in respiratory and intestinal fluids. Furthermore, such antibodies are associated with protection against shigella infection and these vaccines are herein demonstrated to protect against mucosal infection with shigella.
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC
Bifidobacterium lactis capable of preventing osteoporosis and application of bifidobacterium lactis capable of preventing osteoporosis
ActiveCN110964656AResistant to gastric acidResistant to intestinal fluidsMilk preparationBacteriaOsteocyteBifidobacterium lactis
The invention provides bifidobacterium lactis (Bifidobacterium lactis) capable of preventing osteoporosis and an application of the bifidobacterium lactis capable of preventing osteoporosis, and belongs to the technical field of microorganisms. The bifidobacterium lactis provided by the invention has properties of being resistant to gastric acid and intestinal juice, when the bifidobacterium lactis is treated for 30min in gastric acid liquid having pH being 2.5, the survival rate of viable bacteria is 62% or above, when the bifidobacterium lactis is treated for 2 hours, the survival rate of the viable bacteria is 61% or above, and when the bifidobacterium lactis is treated for 2 hours in small intestine liquid having pH being 6.8, the survival rate of the viable bacteria is 70% or above. The bifidobacterium lactis can significantly reduce loss of bone mass caused by lack of estrogen, promote blood calcium and blood phosphorus, restrain the number of in vivo osteoclasts, restrain the absorption effects of the in vivo osteoclasts on bones, and increase the level of protein through promoting the expression protein of bone synthesis and metabolism correlation factor genes so as to promote formation of new bones. The strain disclosed by the invention can be used for preparing foods and the like capable of preventing osteoporosis, and has great application prospects.
Owner:INNER MONGOLIA YILI INDUSTRIAL GROUP CO LTD
Hydrogel for assisting in controlling diet and preparation method and application thereof
ActiveCN112262984AIncrease satietyImprove solubilityAcidic food ingredientsFood ingredient as gelling agentCelluloseWater soluble polysaccharides
The invention discloses hydrogel for assisting in controlling diet and a preparation method and application thereof, and belongs to the technical field of medical assistance. The hydrogel with pH sensitivity is prepared by taking sodium carboxymethylcellulose, water-soluble polysaccharide macromolecules and polybasic carboxylic acid as raw materials and performing cross-linking reaction; the obtained hydrogel can swell in acidic gastric juice and neutral small intestine juice and can scatter and dissolve in alkaline colon juice; and by controlling the using amount of the polybasic carboxylic acid and adopting the non-ionic water-soluble polysaccharide macromolecules, the obtained hydrogel has a higher swelling ratio in a stomach acidic environment. The hydrogel disclosed by the invention can be used for preparing hydrogel capsules for assisting in controlling the diet, is safe and harmless to a human body, has very good satiety and does not influence the absorption of the human body tonutrients.
Owner:吴良平
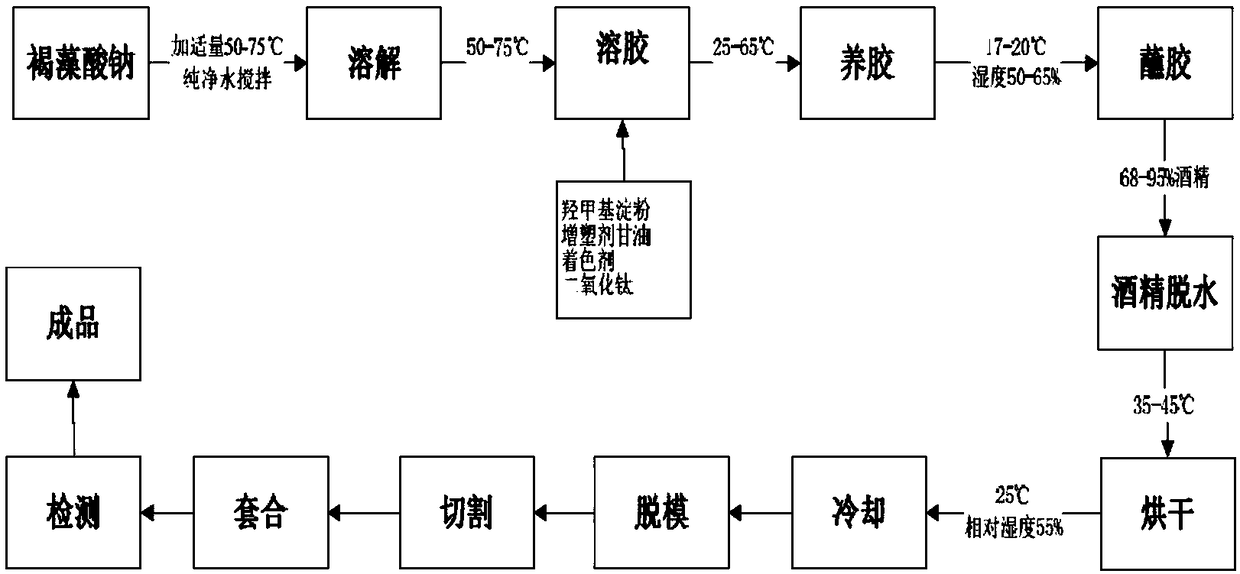
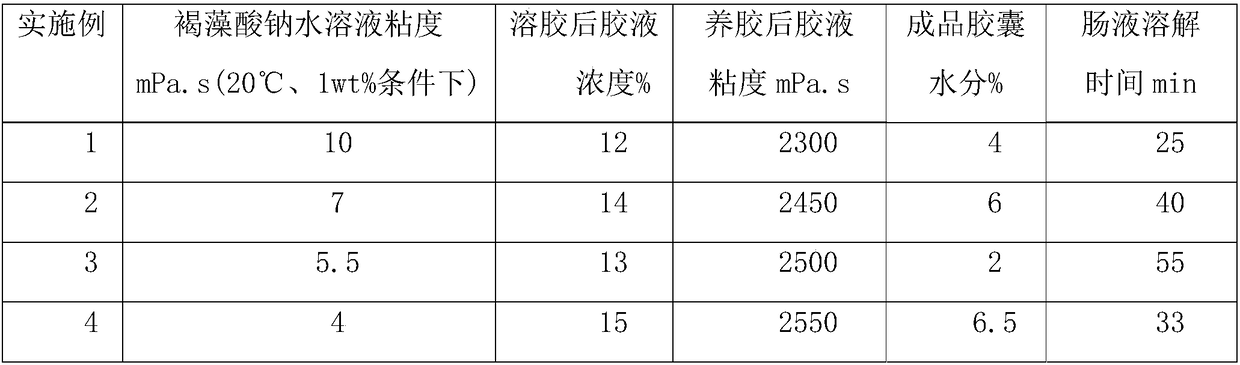
Enteric algal polysaccharide vacant capsule and preparation method thereof
InactiveCN109157528AChange disintegrationControl release speedInorganic non-active ingredientsCapsule deliveryIrritationDrug release
The invention provides an enteric algal polysaccharide vacant capsule and a preparation method thereof. The enteric algal polysaccharide vacant capsule is prepared from the following raw materials: sodium alginate, hydroxymethyl starch, a plasticizer, a colorant and titanium dioxide. Meanwhile, the invention also discloses a preparation method of the enteric algal polysaccharide vacant capsule. The enteric algal polysaccharide vacant capsule disclosed by the invention has the benefits of reasonable matching, and simple and feasible preparation method; a main material of a finished capsule produced by the invention is sodium alginate; in a simulated gastric environment, sodium alginate easily forms hydrophilic colloid which is not easily digested, has high tolerance of acid, and changes thedisintegrating property of the capsule, so that the capsule can be kept in the stomach for 2 hours without disintegration, the drug release speed is effectively controlled, and the irritation of a drug to the stomach is reduced; however, the capsule can be all disintegrated in the simulated intestinal fluid environment within 1 hour, which shows that the capsule can be used for efficacy release of specific drugs in the intestine and has a good application prospect in the drug release aspect.
Owner:青岛聚大洋藻业集团有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com