Production method of insulin oral sustained-release preparation
A technology of oral preparations and insulin, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of subcutaneous fat atrophy, inconvenience and pain of patients, and achieve mild conditions and form Controllable appearance and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
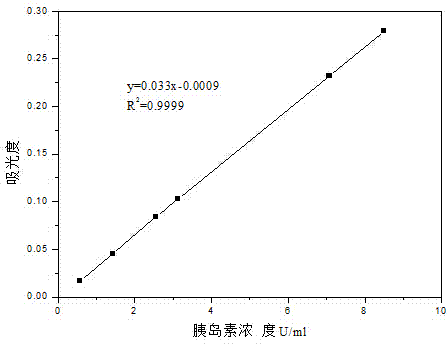
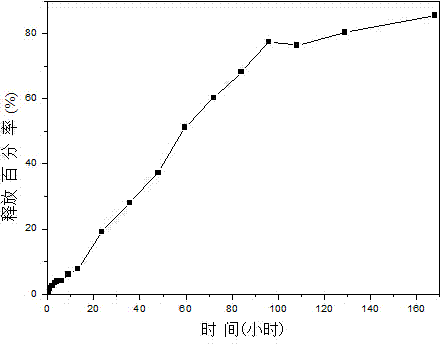
[0027] 1. Preparation process
[0028] 1. Synthesis of PEGylated Chitosan
[0029] Weigh 1g CS (CS is chitosan, molecular weight 500000), dissolve it in 6 ml acetic acid, the volume concentration (v / v) of acetic acid is 2%, dilute it with 20 ml TEMED HCL, add it into a three-necked flask, and measure the pH The value is about 5, then add a certain amount of EDC and NHS, after 30 minutes, weigh mPEG-COOH according to the molar ratio of mPEG-COOH:CS=1:1, add it to a three-necked flask, keep the constant temperature water bath at 35°C, and stir for 36 hours. pH 4-6 was controlled during the reaction.
[0030] The obtained crude product was placed in a dialysis bag for dialysis for 4 days, and freeze-dried to obtain PEGylated chitosan.
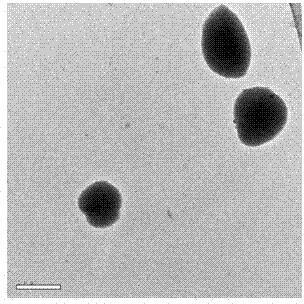
[0031] 2. Preparation of Insulin-Loaded PEGylated Chitosan Microspheres
[0032] (1) Take a certain amount of PEG-CS (polyethylene glycolated chitosan) and TPP (sodium tripolyphosphate) and dissolve them in distilled water respectively to obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com