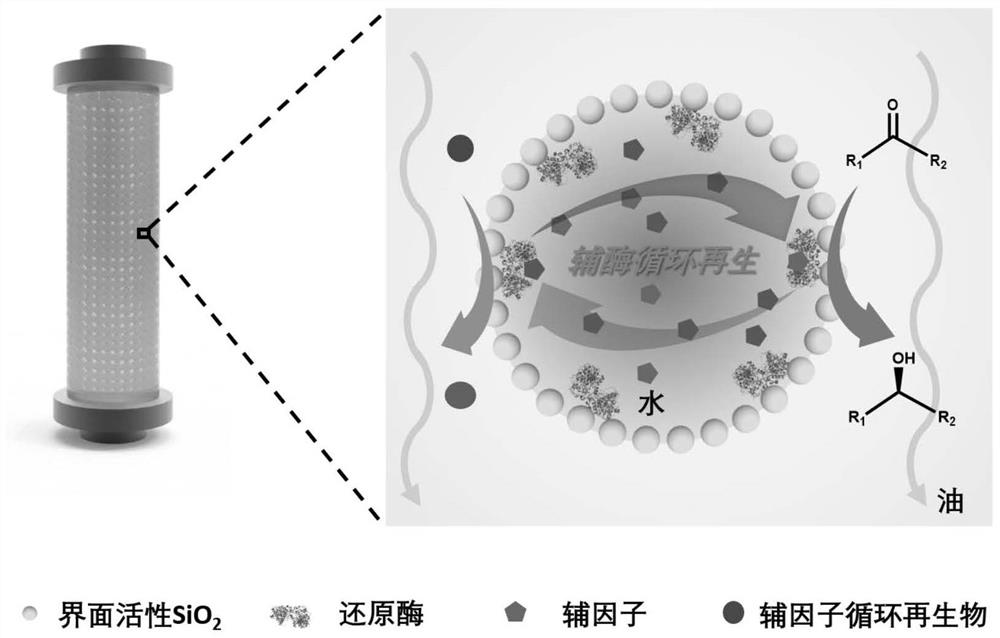
Method for preparing chiral alcohol through ketoreductase continuous reaction
A reductase and chemical reaction technology, applied in the field of enzyme catalysis, can solve problems such as difficulty in continuous and industrialization, cofactor cannot be efficiently recycled and regenerated in the preparation process, ketone reductase activity loss, etc., and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A method for preparing chiral alcohol by continuous reaction of ketoreductase, comprising the steps of:
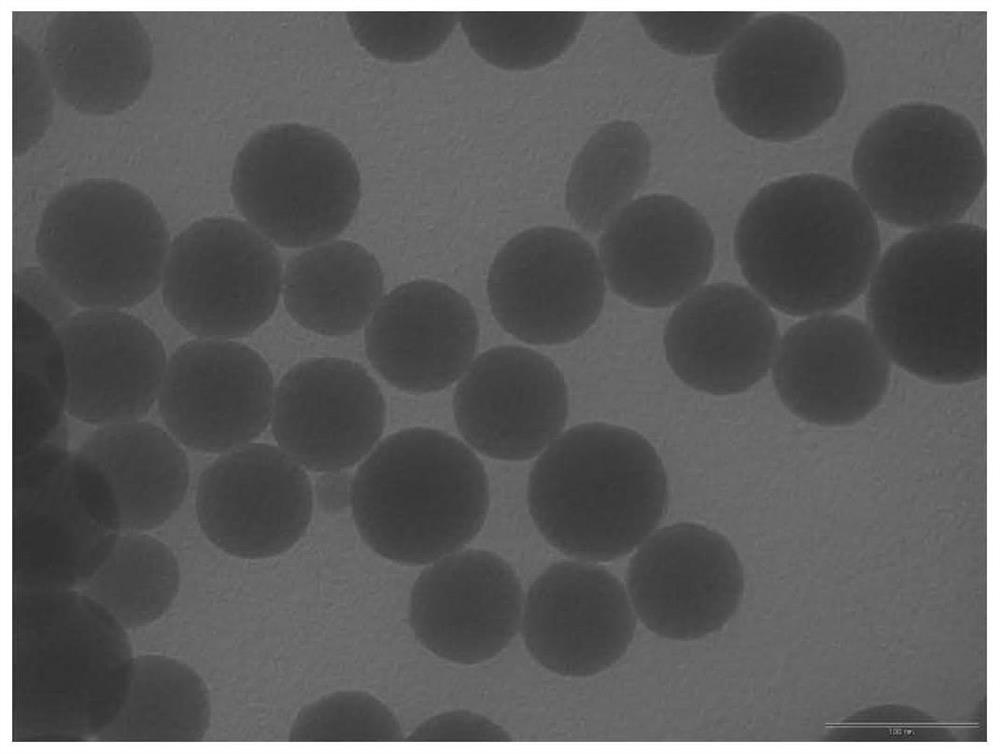
[0033] Step 1: 1.0 g of SiO with a particle size of 60 nm was ultrasonically 2 Disperse the nanoparticles into 12mL of toluene, add 4.5mmol octyltrimethoxysilane and 4.5mmol triethylamine, stir and reflux for 4h at 110°C under nitrogen protection, and centrifuge the above mixed system after cooling. Wash with toluene 3-5 times and dry to obtain interface active nano-SiO 2 (Looks like figure 2 shown);
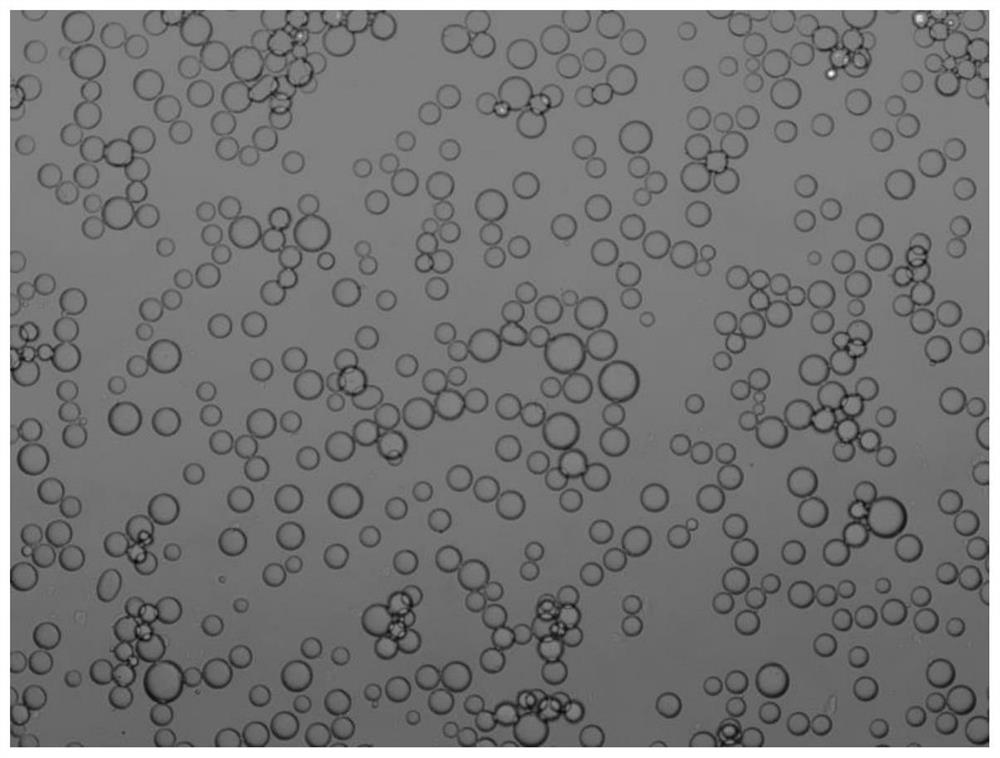
[0034] Step 2: Mix 0.36mL of ES-KRED-113 ketoreductase solution, 0.223mg of NADP + Add it to 3.2mL100mM PBS buffer solution, and mix uniformly by magnetic stirring to obtain an aqueous phase system; then add 0.072g of interface-active SiO 2 Ultrasonic dispersion was obtained in 25mL n-heptane to obtain an oil phase system; the oil-water two-phase system was mixed and stirred at a high speed of 5000rpm to form a Pickering emulsion of immobilized ketone reductase a...
Embodiment 2
[0037] A method for preparing chiral alcohol by continuous reaction of ketoreductase, comprising the steps of:
[0038] Step 1: 1.0 g of SiO with a particle size of 60 nm was ultrasonically 2 Disperse nanoparticles into 15mL toluene, add 4.5mmol dichlorodimethylsilane and 2.25mmol n-hexylamine, stir and reflux for 3 hours at 120°C under nitrogen protection, and centrifuge the above mixed system after cooling, and the obtained solid is washed with toluene Wash 3-5 times and dry to obtain interface active nano-SiO 2 ;
[0039]Step 2: Mix 0.72mL of ES-KRED-115 ketoreductase solution, 1mg NADP + Added to 6.4mL of 100mM PBS buffer solution, mixed uniformly by magnetic stirring to obtain an aqueous phase system; then 0.316g of interface-active SiO 2 Ultrasonic dispersion in 16mL of toluene to obtain an oil phase system; the oil-water two-phase system was mixed and stirred at a high speed of 10,000rpm to form a Pickering emulsion with immobilized ketone reductase and cofactor;
...
example 3
[0042] A method for preparing chiral alcohol by continuous reaction of ketoreductase, comprising the steps of:
[0043] Step 1: 1.2 g of SiO with a particle size of 60 nm was ultrasonically 2 Disperse the nanoparticles into 20mL toluene, add 1.5mmol dichlorodimethylsilane and 4.5mmol n-hexylamine, stir and reflux for 5h at 60°C under nitrogen protection, and centrifuge the above mixed system after cooling, and the obtained solid is washed with toluene Wash 3-5 times and dry to obtain interface active nano-SiO 2 ;
[0044] Step 2: Mix 0.05mL of ES-KRED-172 ketoreductase solution, 0.112mg NADP + Add it to 3.2mL100mM PBS buffer solution, and mix it uniformly by magnetic stirring to obtain an aqueous phase system; then add 0.158g of interface-active SiO 2 Ultrasonic dispersion in 15mL of ethyl acetate to obtain an oil phase system; the oil-water two-phase system was mixed and stirred at a high speed of 8000rpm to form a Pickering emulsion with immobilized ketone reductase and c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com