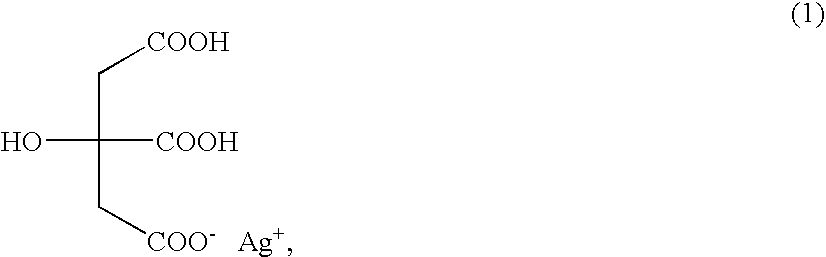Patents
Literature
2775 results about "CITRATE ESTER" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate.
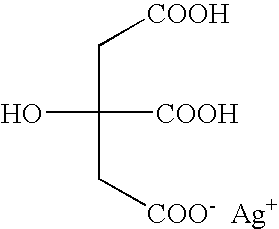
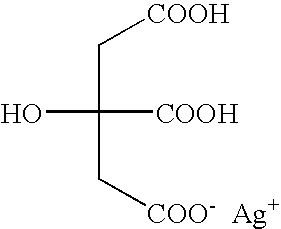
Silver dihydrogen citrate compositions
Personal care and home care compositions are disclosed, which include silver dihydrogen citrate. The inventive compositions advantageously take the form of suspensions, pastes, liquids and gels. The inventive compositions also optionally comprise additional ingredients, and are therefore suitable for a wide variety of personal, home and industrial care purposes.
Owner:PURE BIOSCI
Well treatment fluid compositions and methods for their use
InactiveUS6983801B2High viscosityIncrease volumeFluid removalFlushingAlkaline earth metalCarboxylic acid
Owner:COMBINED SYST +1
Nutritional potato chips and preparation method thereof
InactiveCN103535664AAdd flavorFull of nutritionFood ingredient functionsFood preparationBiotechnologyAdditive ingredient
The invention discloses nutritional potato chips and a preparation method thereof. The nutritional potato chips are characterized by being prepared from the following raw materials in parts by weight: 90-100 parts of potatoes, 25-30 parts of rye flour, 3-4 parts of salt, 4-5 parts of oligosaccharide, 1-2 parts of zinc citrate, 8-10 parts of lily mayonnaise, 10-11 parts of almond, 11-12 parts of peanut, 12-13 parts of sesame, 1-2 parts of burdock, 1.5-1.8 parts of lophatherum gracile, 0.8-1.5 parts of tangerine peel, 1.5-2 parts of cassia seed, 1-1.5 parts of angelica, 2-2.5 parts of broadbean flower, 2-3 parts of apple blossom, 2-3 parts of stellaria media and 3-5 parts of rapeseed oil. The nutritional potato chips have superior flavor, are crisp, refreshing, scientific in proportion of all the ingredients and rich in nutrition, and have the effects of dispelling wind and heat, regulating qi, strengthening spleen, drying dampness, eliminating phlegm, replenishing blood, improving eyesight, cooling blood, detoxifying, maintaining beauty and keeping young.
Owner:ANHUI HUAIYUAN XINTAI CEREAL & OIL
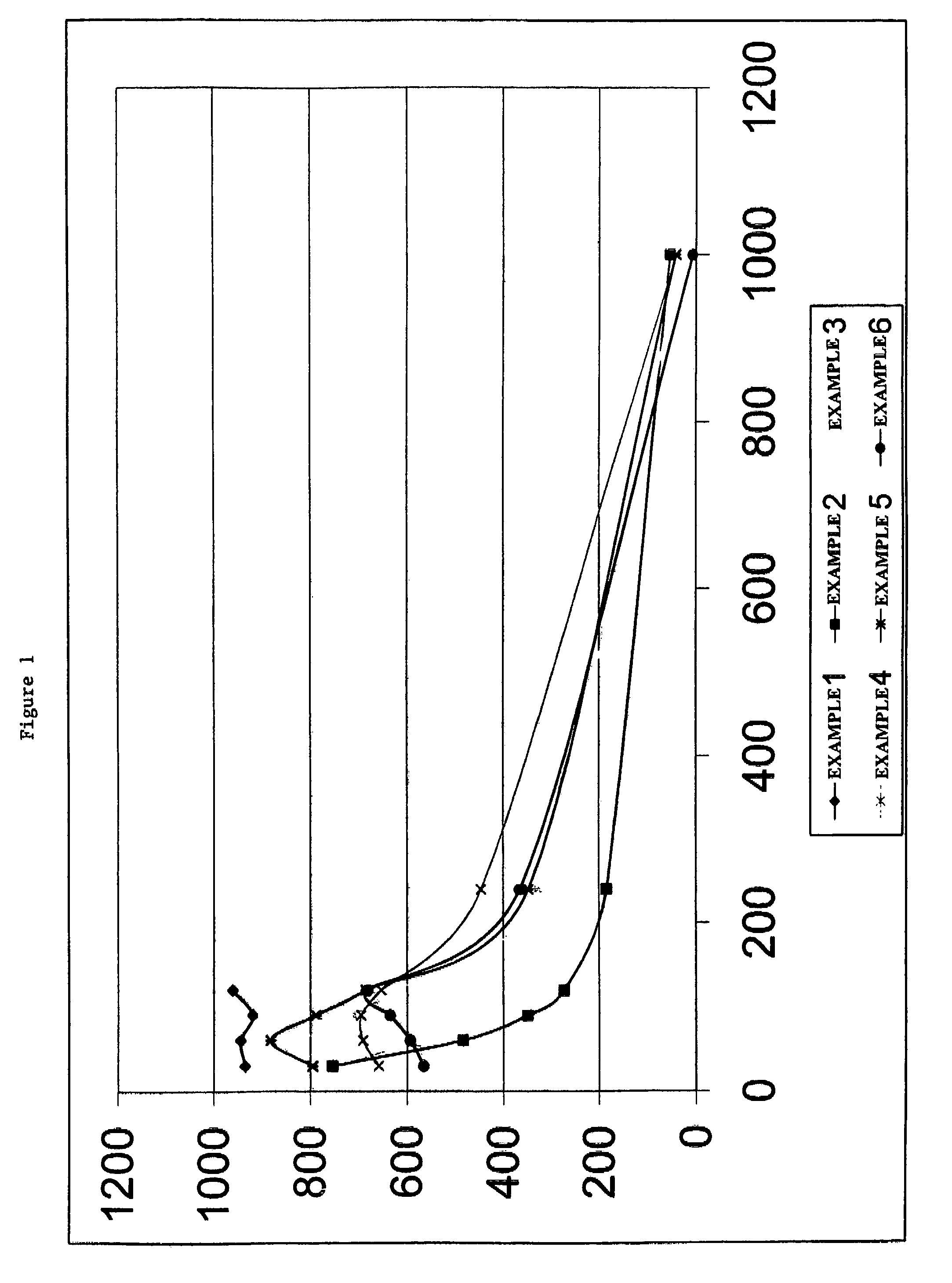
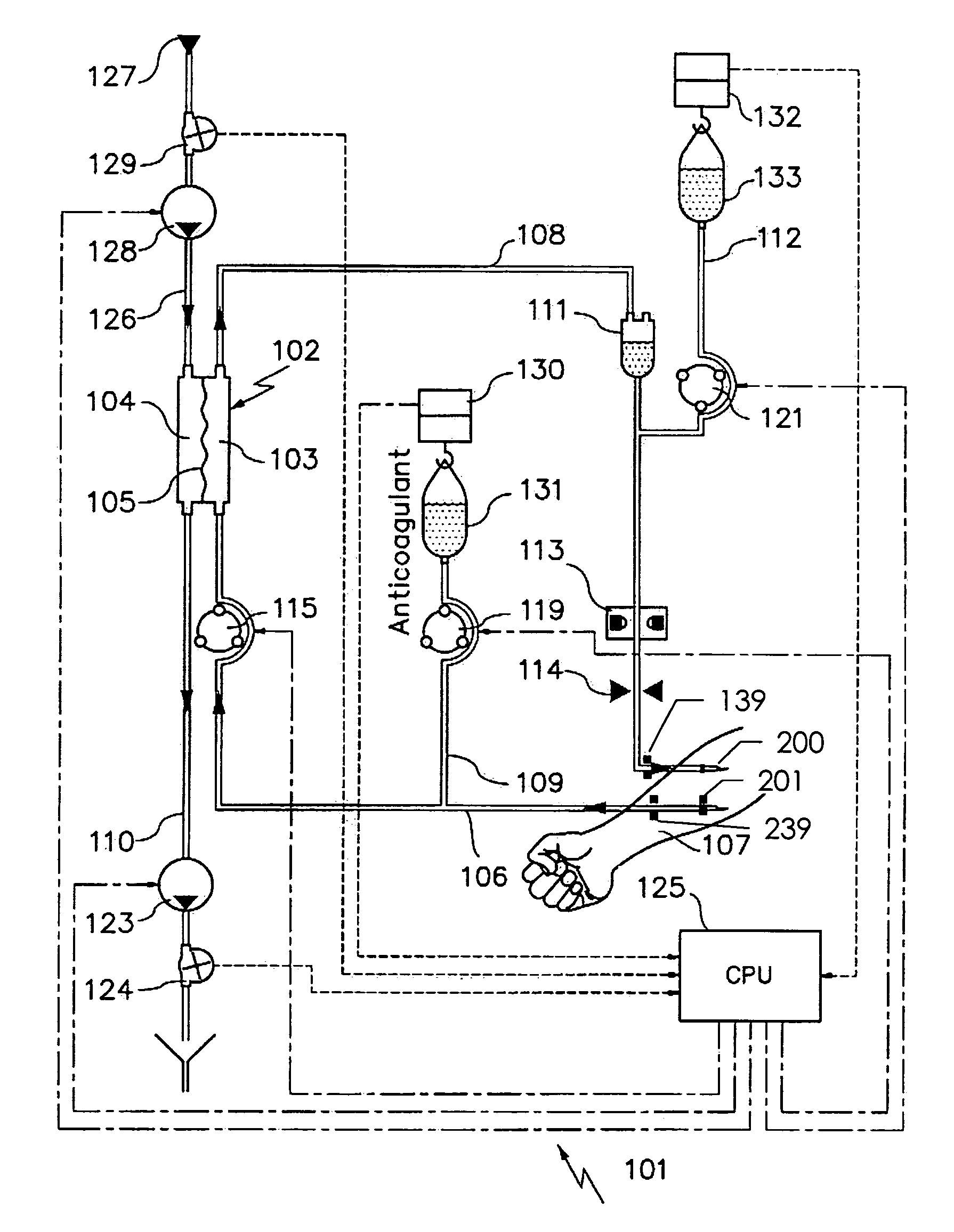
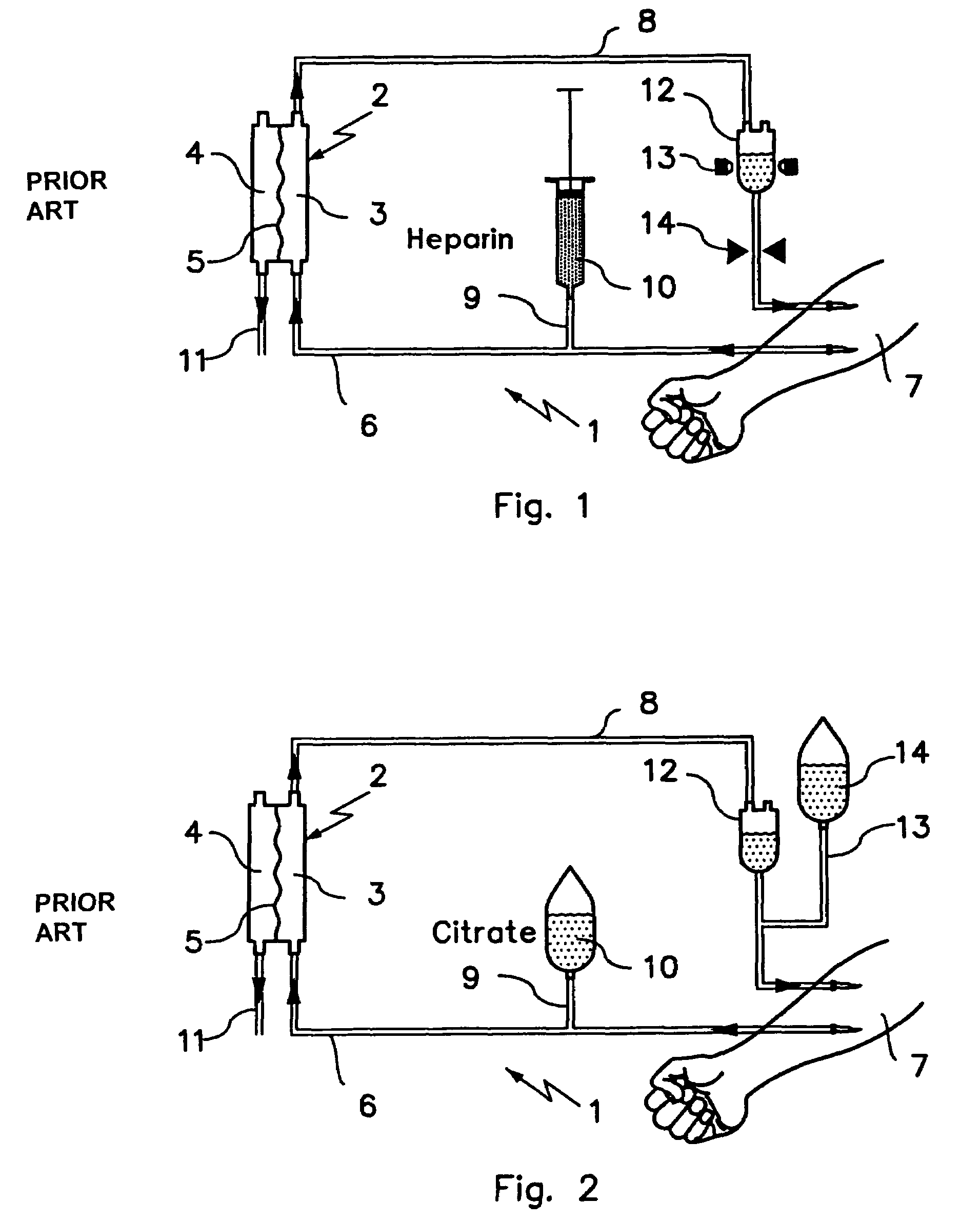
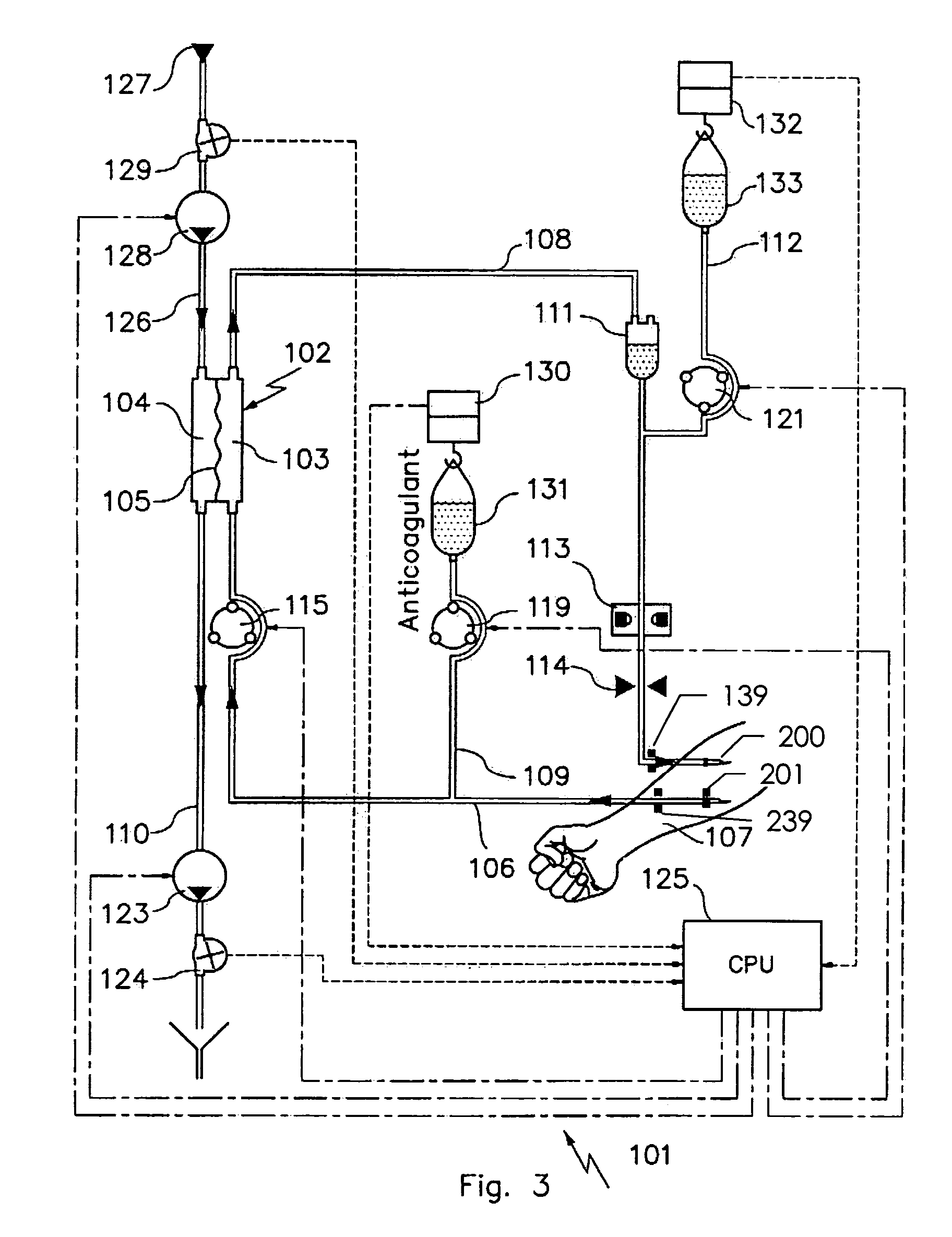
Device and process for extracorporeal treatment by citrate anticoagulant
ActiveUS7351218B2Inhibit blood coagulationSimple structureOther blood circulation devicesHaemofiltrationExtracorporeal circulationVein
A device for treating blood by extracorporeal circulation comprising a filter having a first chamber and a second chamber separated by a semi-permeable membrane. The device has an arterial line connected to the first chamber of the filter, a venous line issuing from the first chamber of the filter, a channel used for pre-infusion of a substance for regional anticoagulation and linked to the arterial line, a purge channel issuing from the second chamber of the filter, and an air separator on the venous line. The device also includes a line for post-infusion of a solution at least partially re-establishing the ionic equilibrium of the blood downstream of the air separator, the post-infusion line being configured immediately upstream of an air detector.
Owner:GAMBRO LUNDIA AB
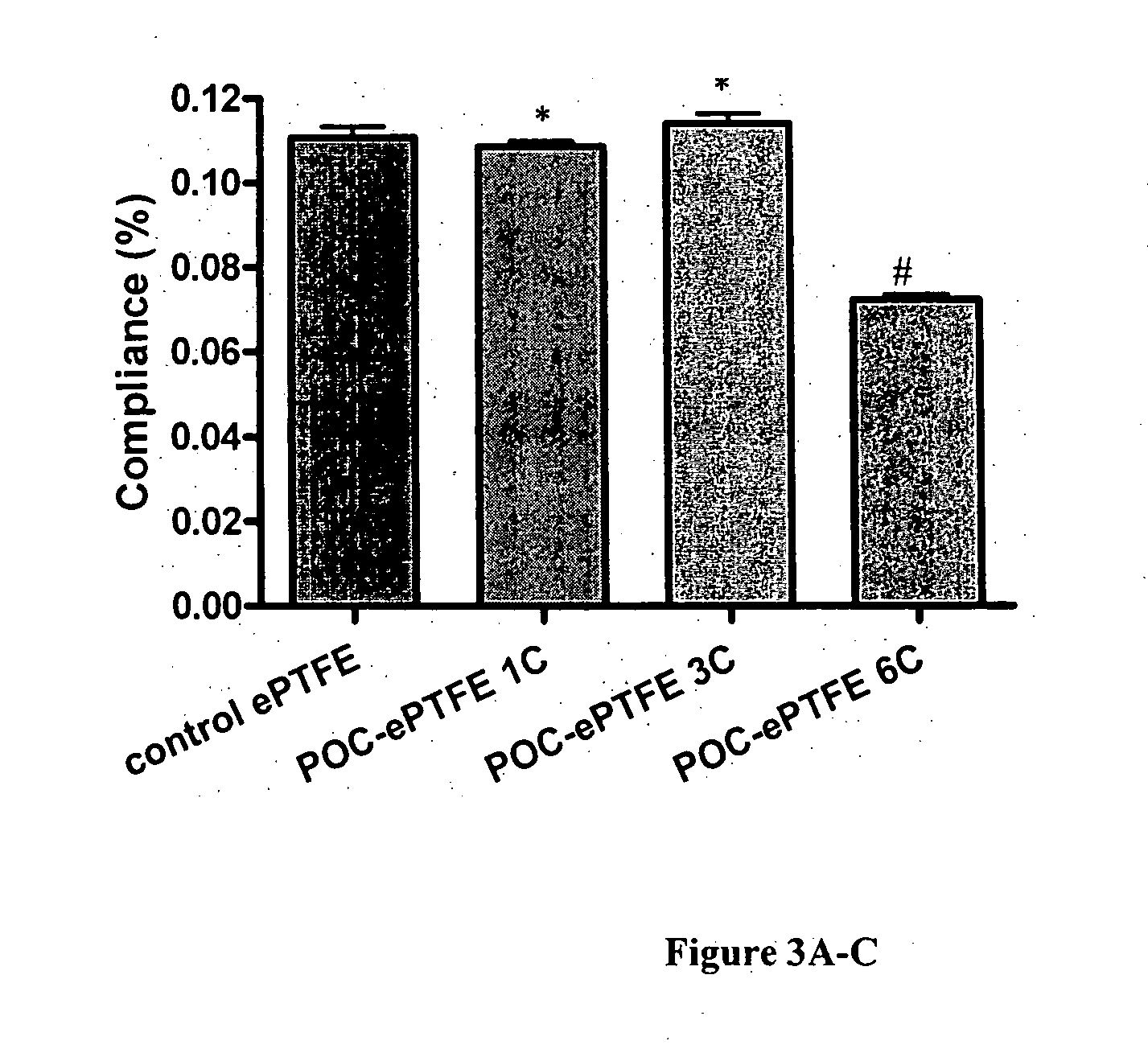
Biodegradable nanocomposites with enhance mechanical properties for soft tissue
InactiveUS20070071790A1Pharmaceutical delivery mechanismPharmaceutical non-active ingredientsMechanical propertyNanocomposite
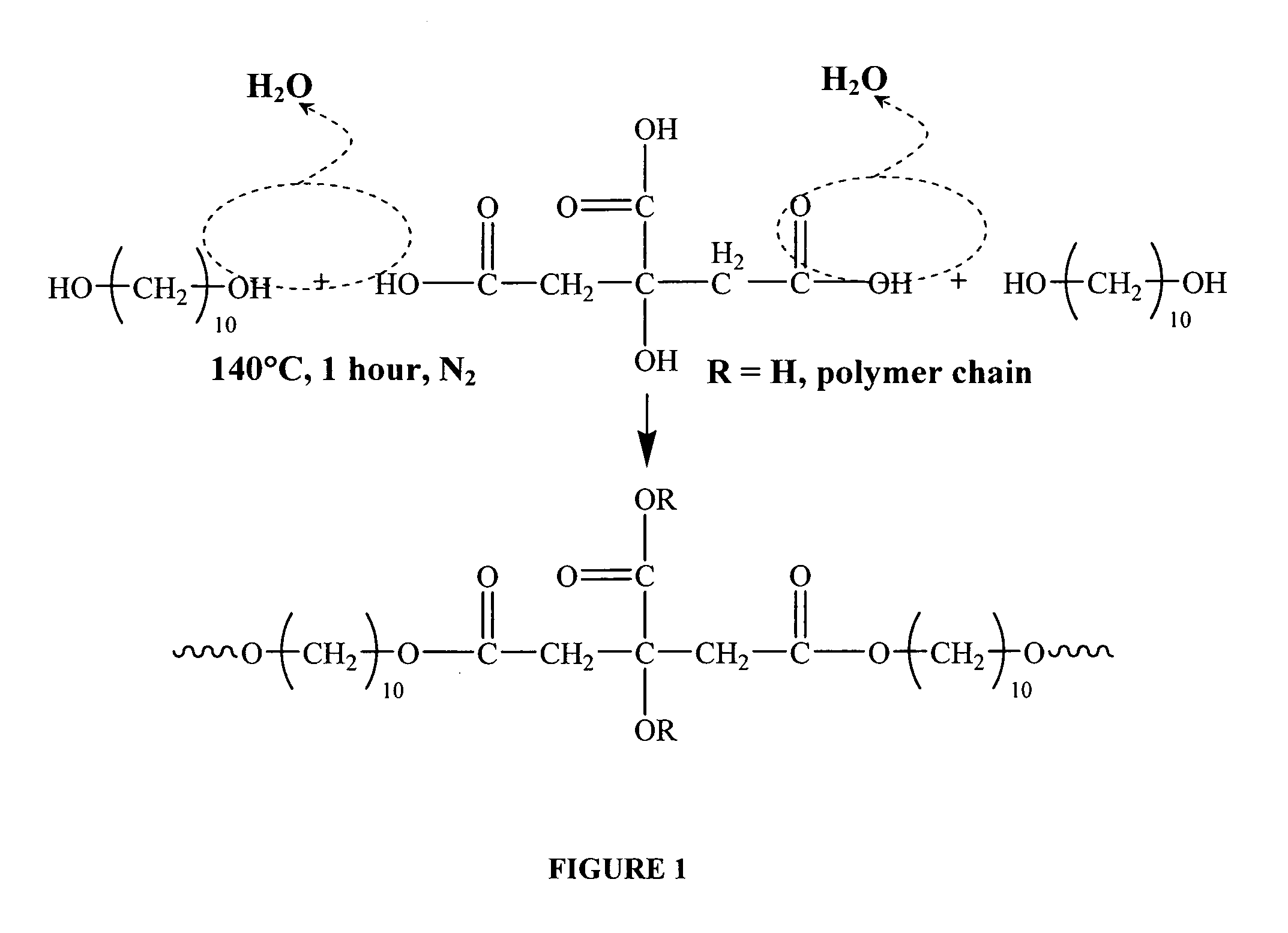
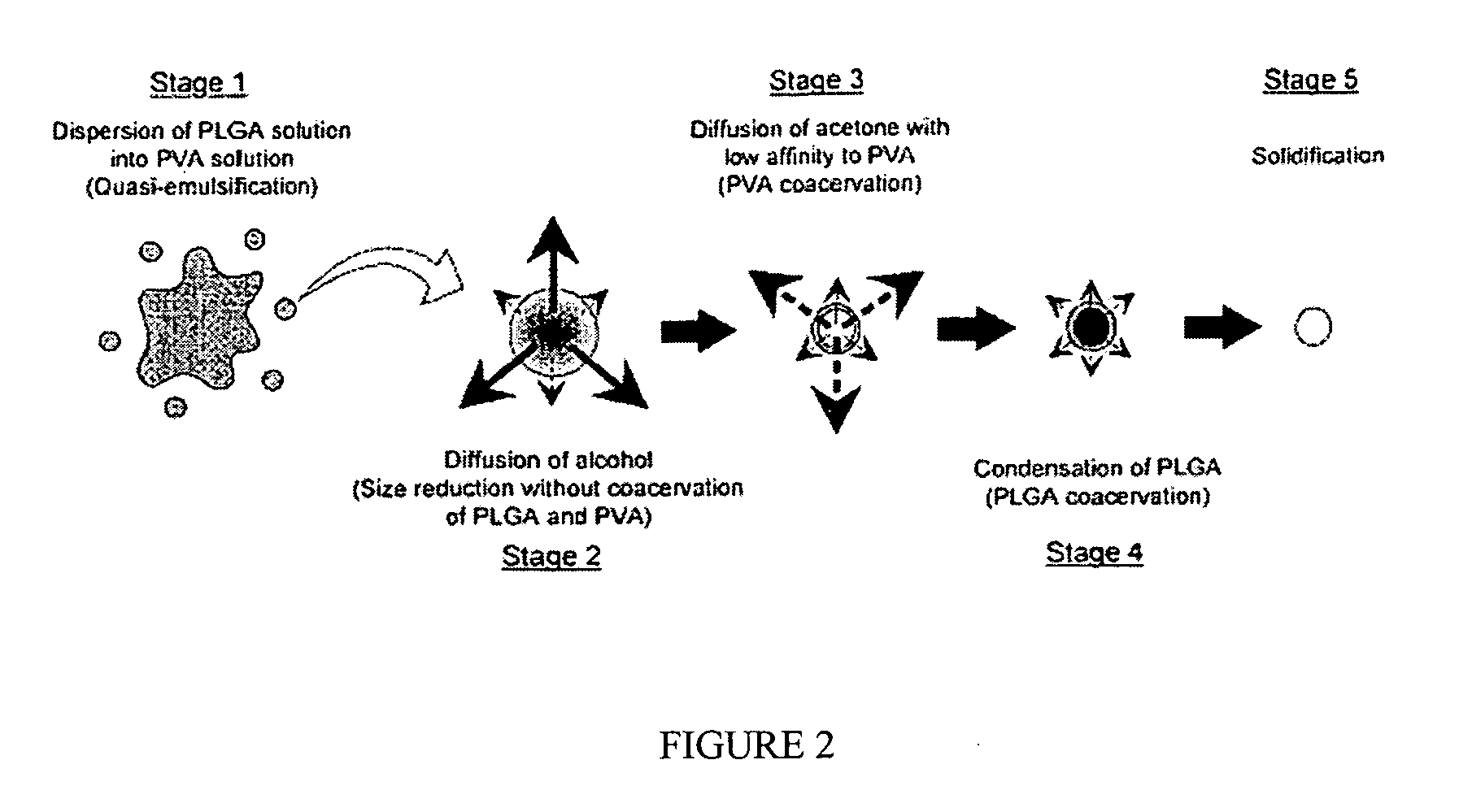
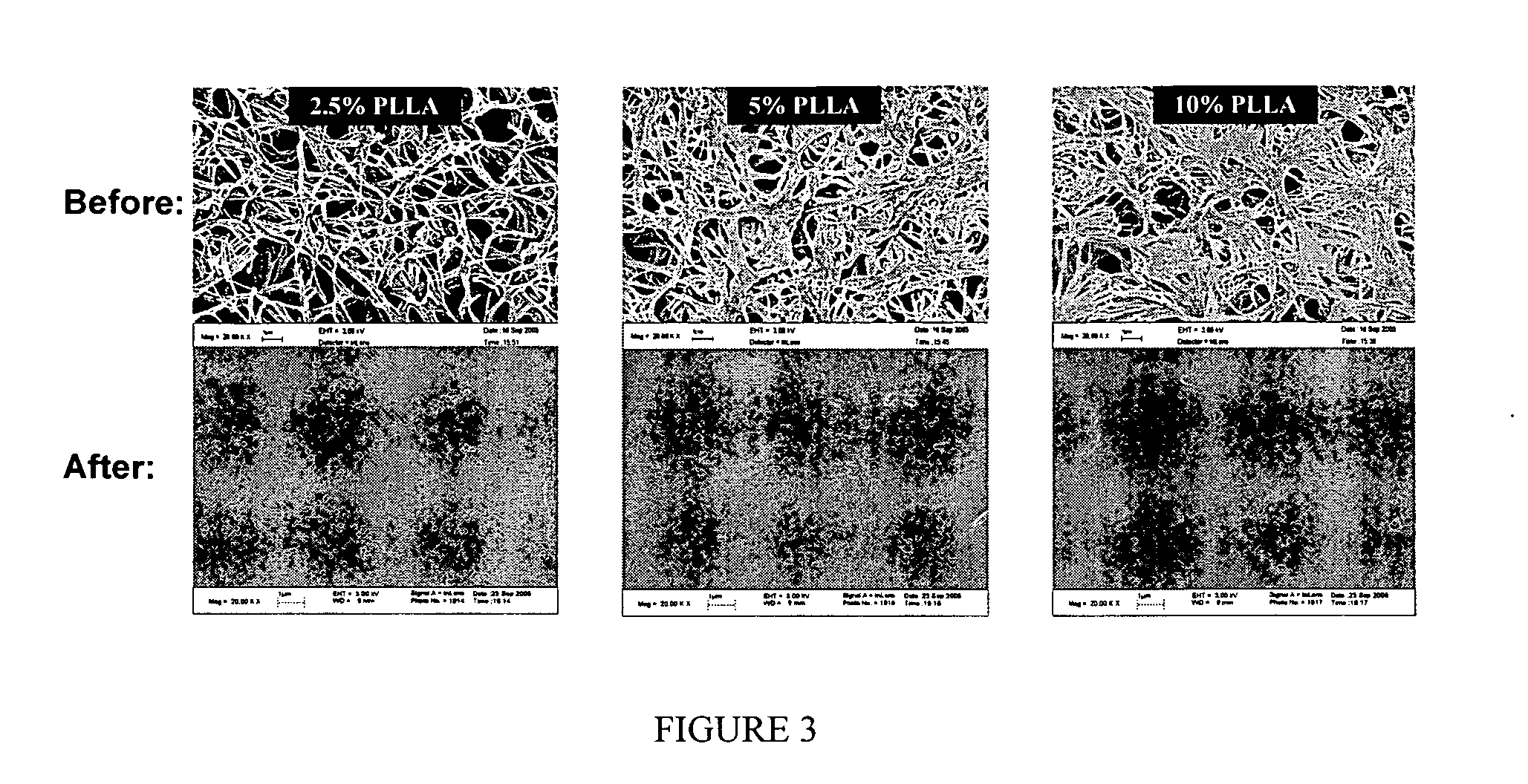
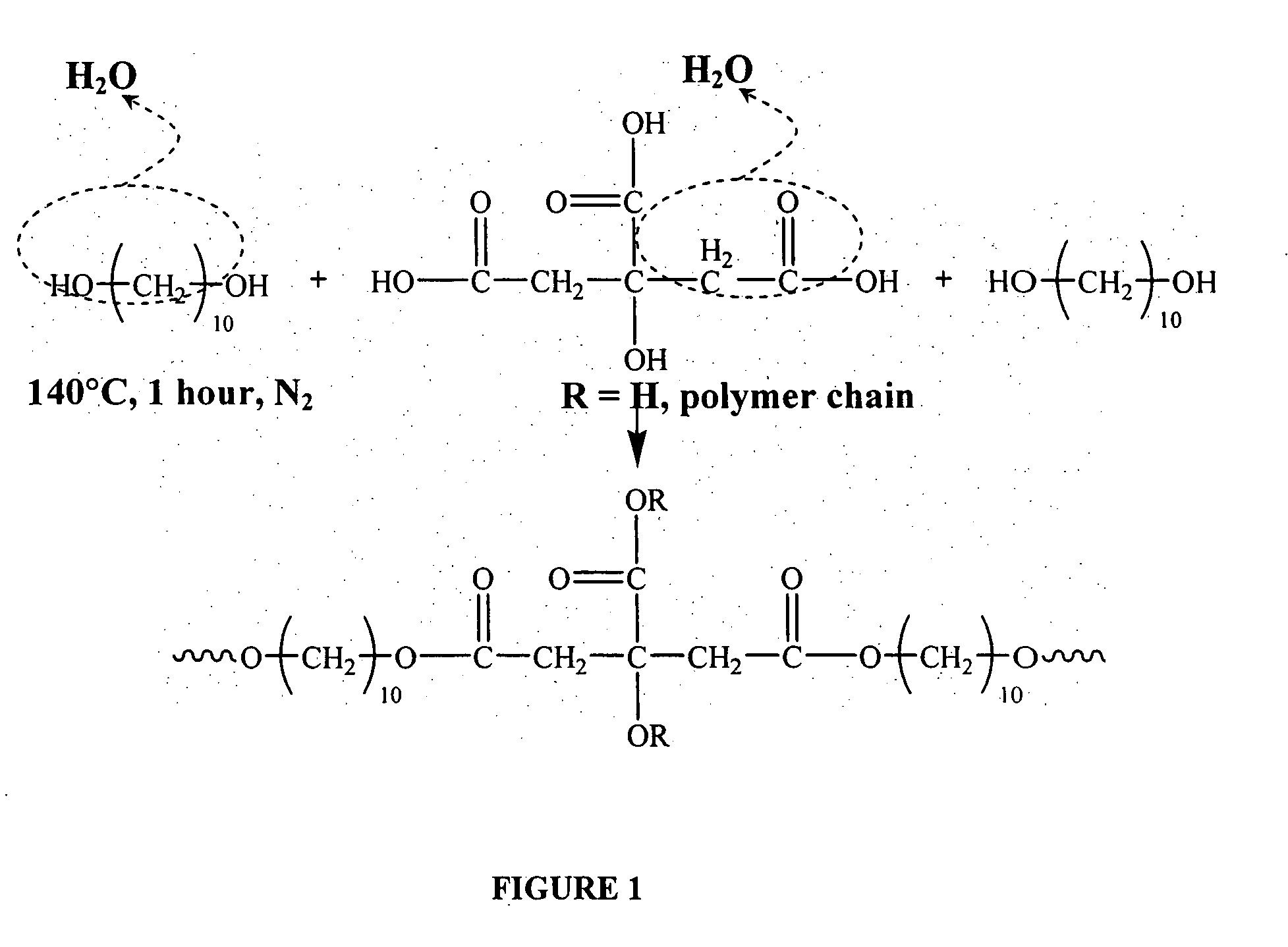
The present invention is directed to a novel poly(diol citrates)-based nanocomposite materials created using completely biodegradable and biocompatible polymers that may be used in tissue engineering. More specifically, the specification describes methods and compositions for making and using nanocomposites comprised of citric acid copolymers and polymers including but not limited to poly(L-lactic acid) (PLLA) and poly(lactic-co-glycolic acid) (PLGA).
Owner:NORTHWESTERN UNIV
Citrate-based dialysate chemical formulations
The present invention constitutes dialysate formulations that are suitable for use in preparing dialysate solutions for use in batch and / or proportioning systems and for improving dialysis efficiency by reducing or preventing clotting of the dialysis flow paths. The dialysate chemical formulations for one batch of dialysate comprise an acid concentrate stored in a first vessel, and a citrate-containing bicarbonate concentrate stored in a second vessel. The contents of the first and second vessels are emptied into a dialysate preparation tank and mixed with water to form a batch quantity of dialysate solution. Alternately, a dry acid and / or a dry citrate-containing base concentrates are dissolved separately in measured quantities of water to form liquid concentrates which are then used in conjunction with a proportioning machine to generate on-line a final dialysis solution stream.
Owner:HHD LLC A DELAWARE LLC +2
Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs
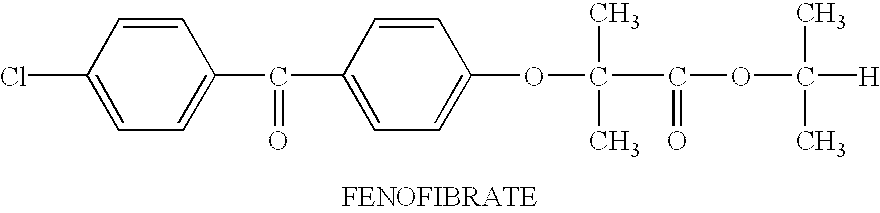
Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs, particularly fenofibrate, are provided. The compositions comprise a therapeutically effective amount of an active agent and a solubilizer. The solubilizer is selected to effectively solubilize active agent in the composition. The solubilizers employed as part of the invention include: a vitamin E substance; monohydric alcohol esters such as trialkyl citrates, lactones and lower alcohol fatty acid esters; nitrogen-containing solvents; phospholipids; glycerol acetates such as acetin, diacetin and triacetin; glycerol fatty acid esters such as mono-, di- and triglycerides and acetylated mono- and diglycerides; propylene glycol esters; ethylene glycol esters; and combinations thereof. The pharmaceutical dosage forms contain the compositions in a suitable dosage form unit such as a capsule. Methods of treating patients comprising administering the compositions are also provided.
Owner:LIPOCINE
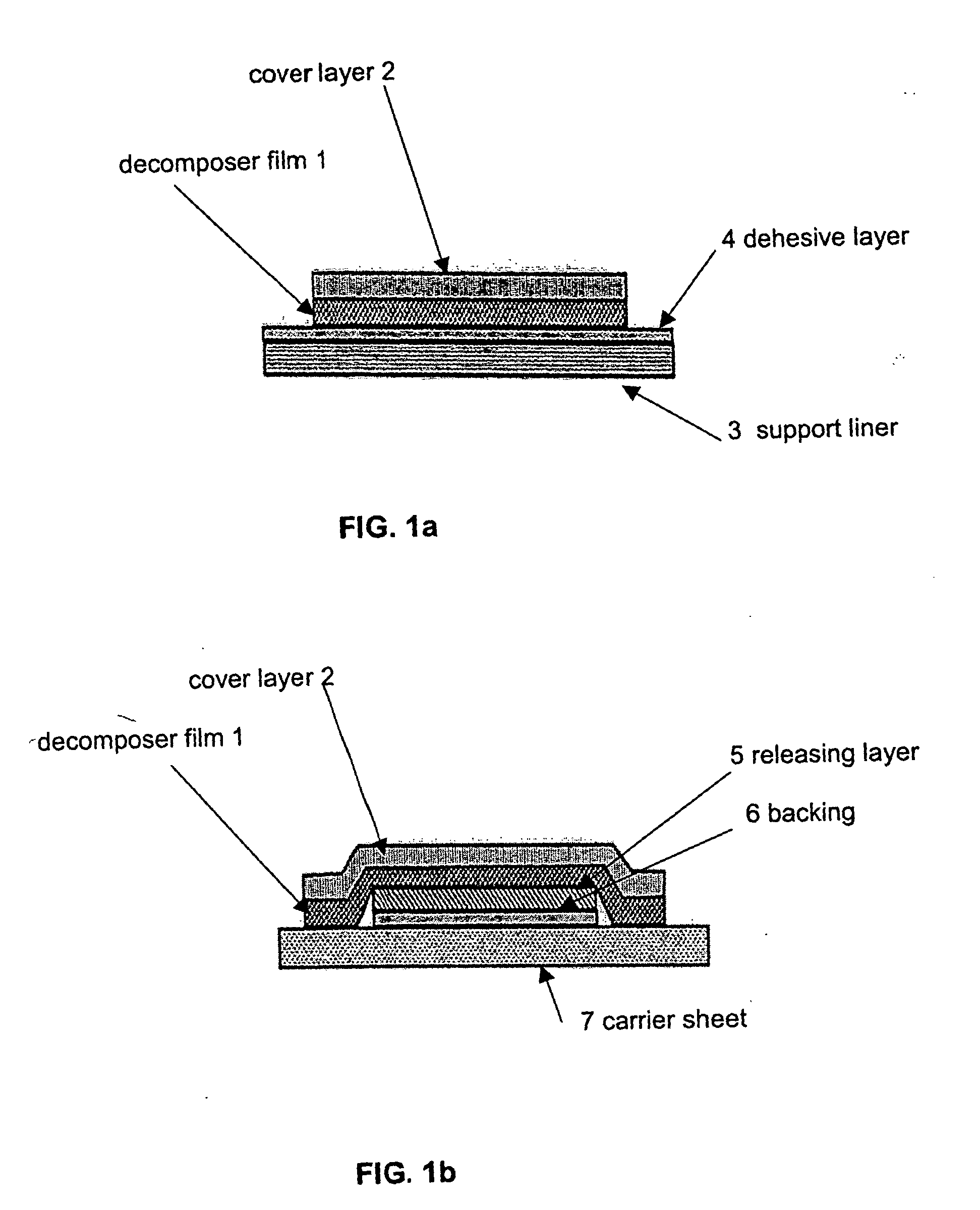
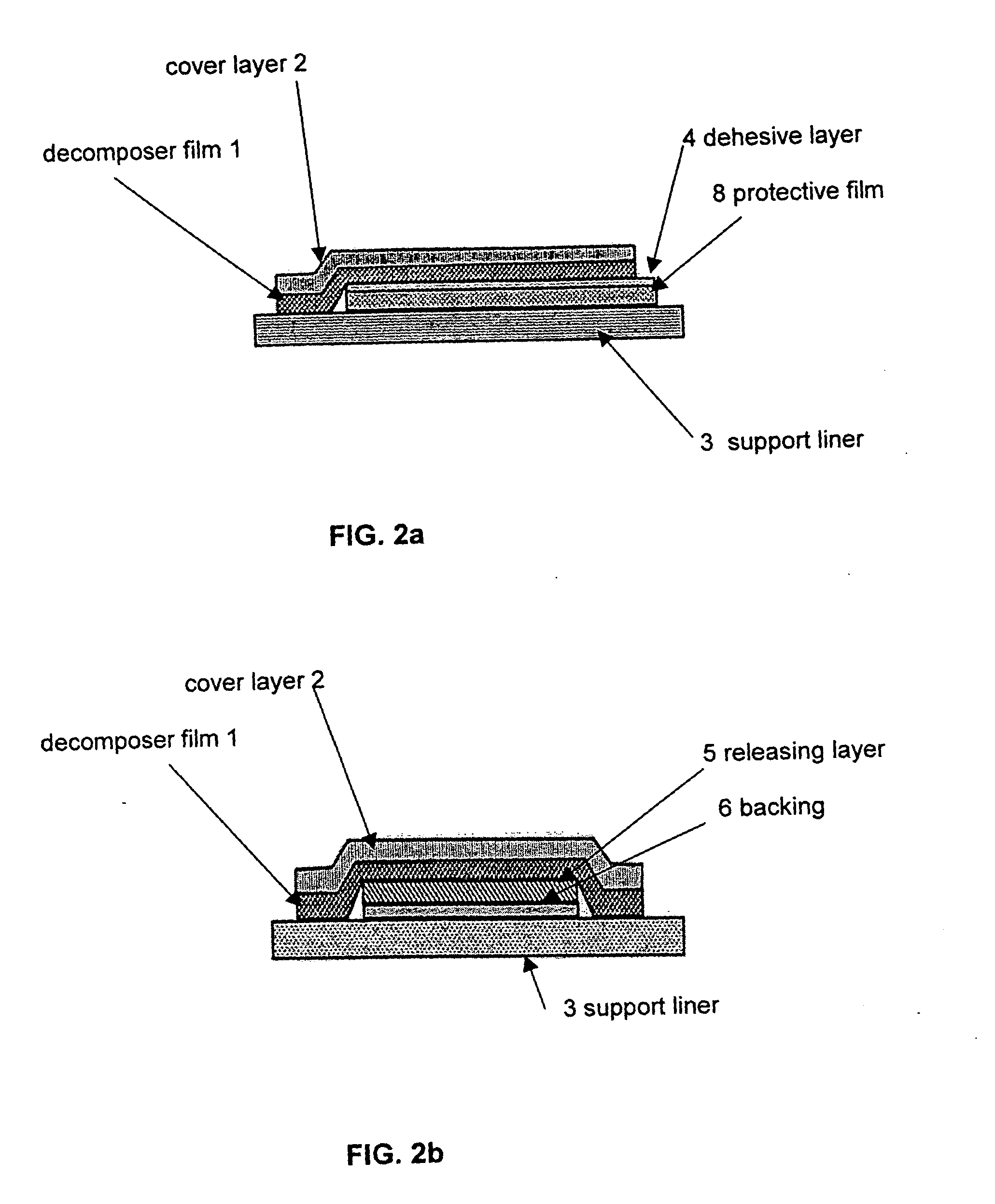
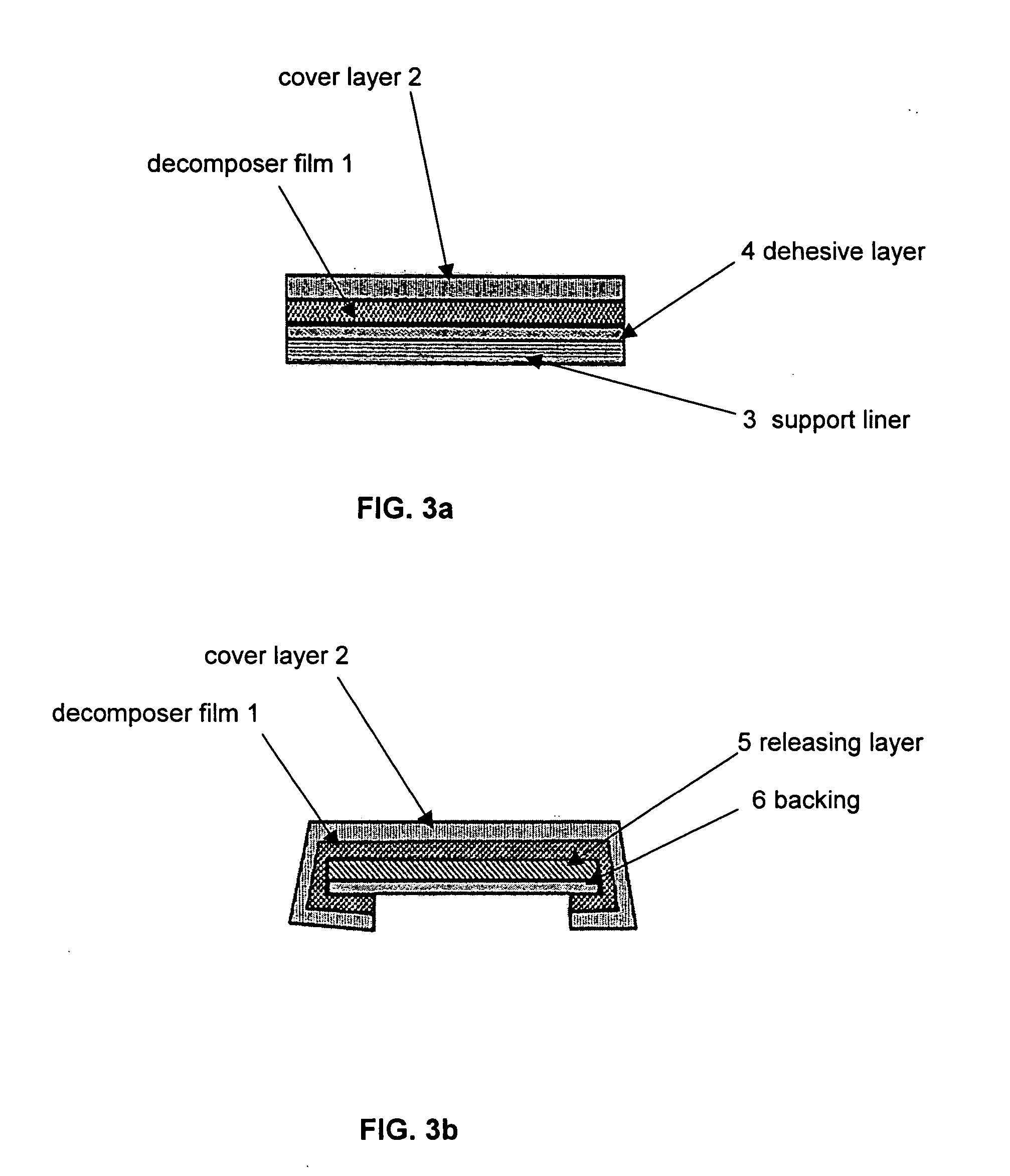
Decomposer film for transdermal patches
InactiveUS20070014839A1Increased internal surface areaAccelerate decomposition reactionOrganic active ingredientsAntipyreticTransdermal patchPolyvinyl alcohol
The decomposer film product has a polymeric decomposer layer, a cover layer for protecting the decomposer film from the surroundings and a releasable support liner, which is removed prior to use. The polymeric decomposer film contains a water-soluble or water-insoluble polymeric adhesive material and a decomposition accelerator, which acts to decompose an effective ingredient, such as a steroid hormone, of a worn or unused transderamal patch, when the effective ingredient releasing layer of the patch adheres to the polymer film, so that the pharmaceutical effective ingredient comes into contact with the decomposition accelerator by diffusion. The decomposition accelerator includes a chemically oxidizing substance, preferably urea peroxide, manganese (III) acetate or iron (III) citrate. The water-insoluble polymeric adhesive material is preferably an acrylates adhesive. The water-soluble polymeric adhesive material is preferably polyvinyl alcohol, polyvinyl pyrrolidone, a cellulose derivative or a polyacrylic acid.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Catheter assembly with bactericidal effect
InactiveUS20060240069A1Reduce decreaseSimple procedureBiocidePharmaceutical containersUrinary catheterOrganic acid
A use in a medical device of at least one salt of organic acid(s), and preferably a benzoate or a sorbate, as an antimicrobial agent is disclosed, and in particular for the manufacturing of an antimicrobial coating for a medical device for the prevention of bacterial infection. This is very useful in medical devices having a hydrophilic coating, such hydrophilic urinary catheters. It is further preferred that the pH of the hydrophilic coating is controlled to be in the range 4.0-8.0, and preferably in the range 5.0-6.0. It is also preferred that the pH of the hydrophilic coating is controlled to be below 7.0. The pH of the hydrophilic coating could be controlled by means of a pH buffer, and preferably a citrate or phosphate buffer. Specifically, the provision of the salt of organic acid in combination with a pH buffer has proven surprisingly efficient for inhibition of bacterial growth, and for prevention of bacterial infections.
Owner:ASTRA TECH SE
Oral fast dissolving films for erectile dysfunction bioactive agents
InactiveUS20090047330A1Improved ease of handlingIncrease usageBiocideAnimal repellantsVardenafilActive agent
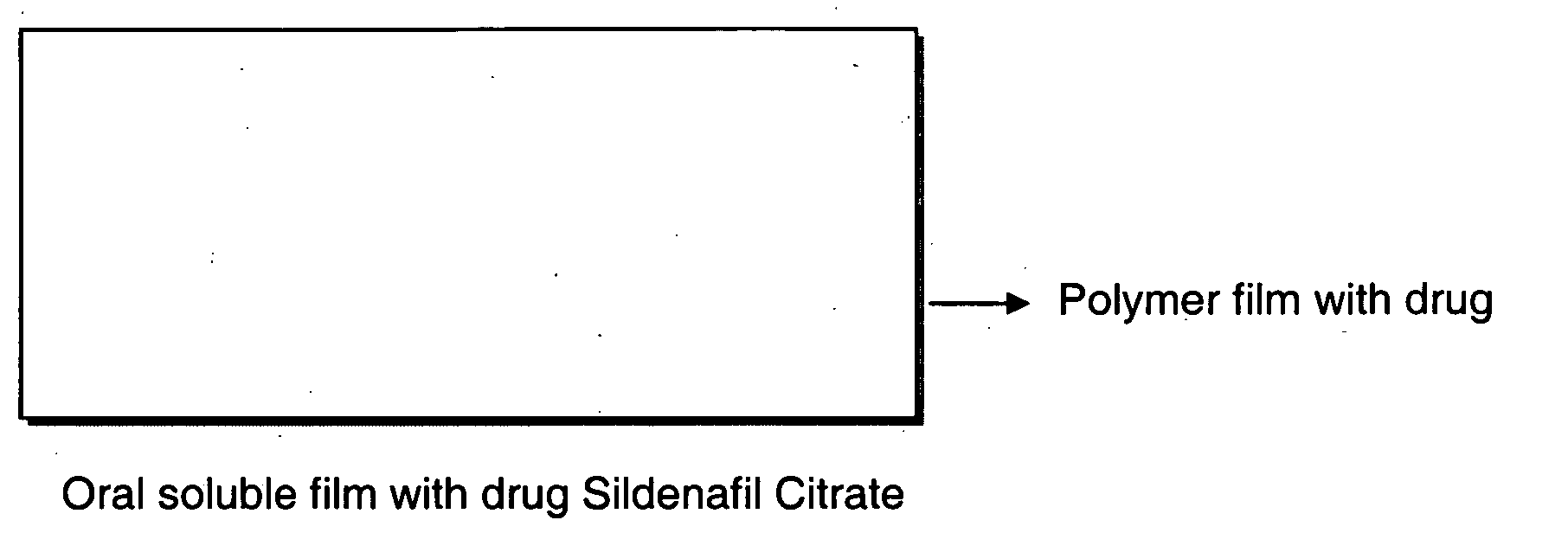
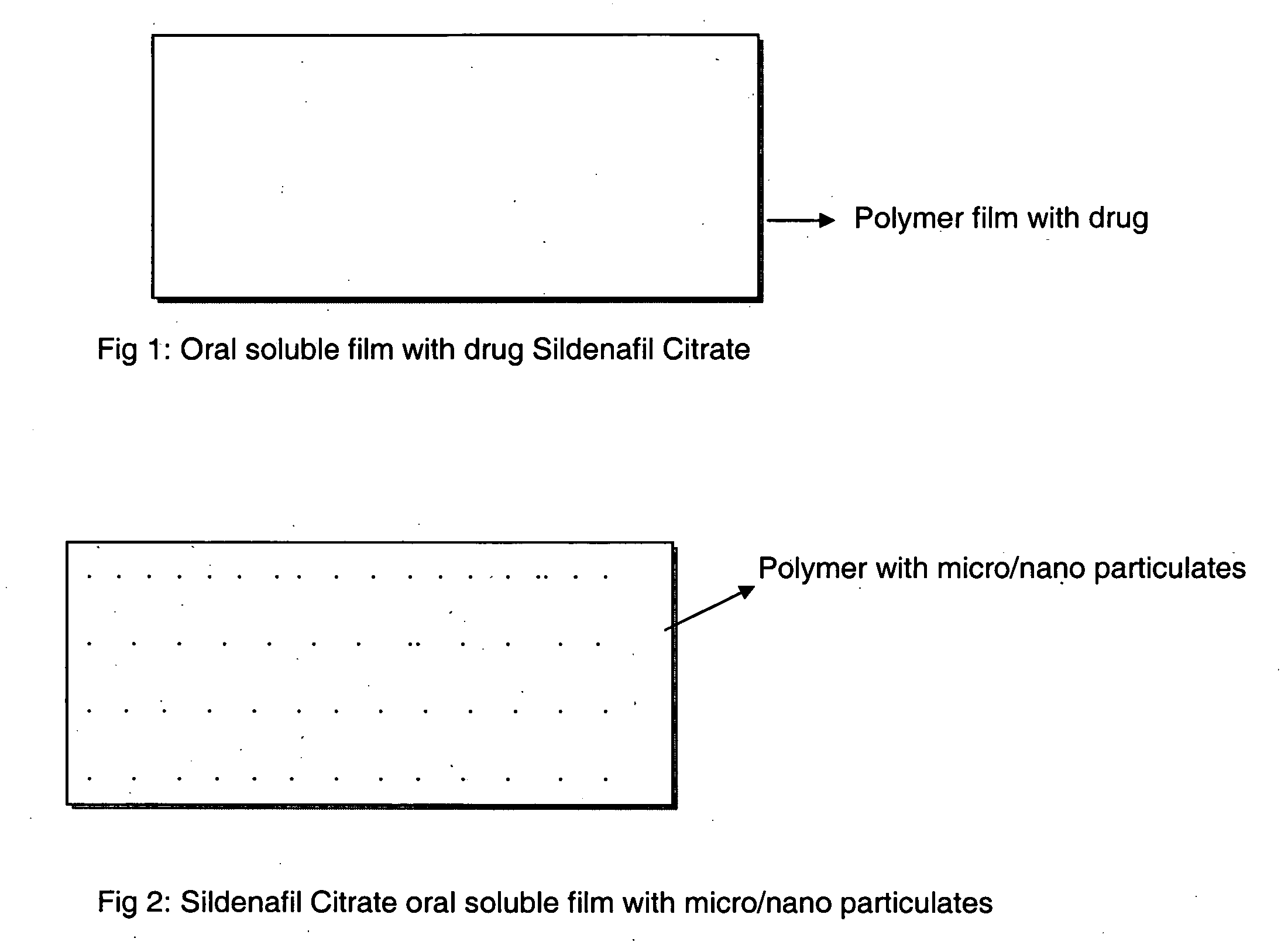
A novel edible polymer based film dosage form manufactured using natural, synthetic, semisynthetic, pharmaceutically acceptable polymers addressing the issues of swallowing difficulties (Dysphagia and Dynaphagia), of tablet or capsule dosage forms and handling and storage difficulties associated with liquid dosage forms, that also includes materials such as emulsifying agents, suspending agents, buffering agents, effervescence agents, colorants, flavorants, sweeteners and specified amounts of bioactive agents, for erectile dysfunction. A flexible film dosage form containing sildenafil citrate, tadalafil or Vardenafil is presented. The film system is enabled to be used in various applications such as oral, mucosal and external environments.
Owner:BANGALORE RAMESH
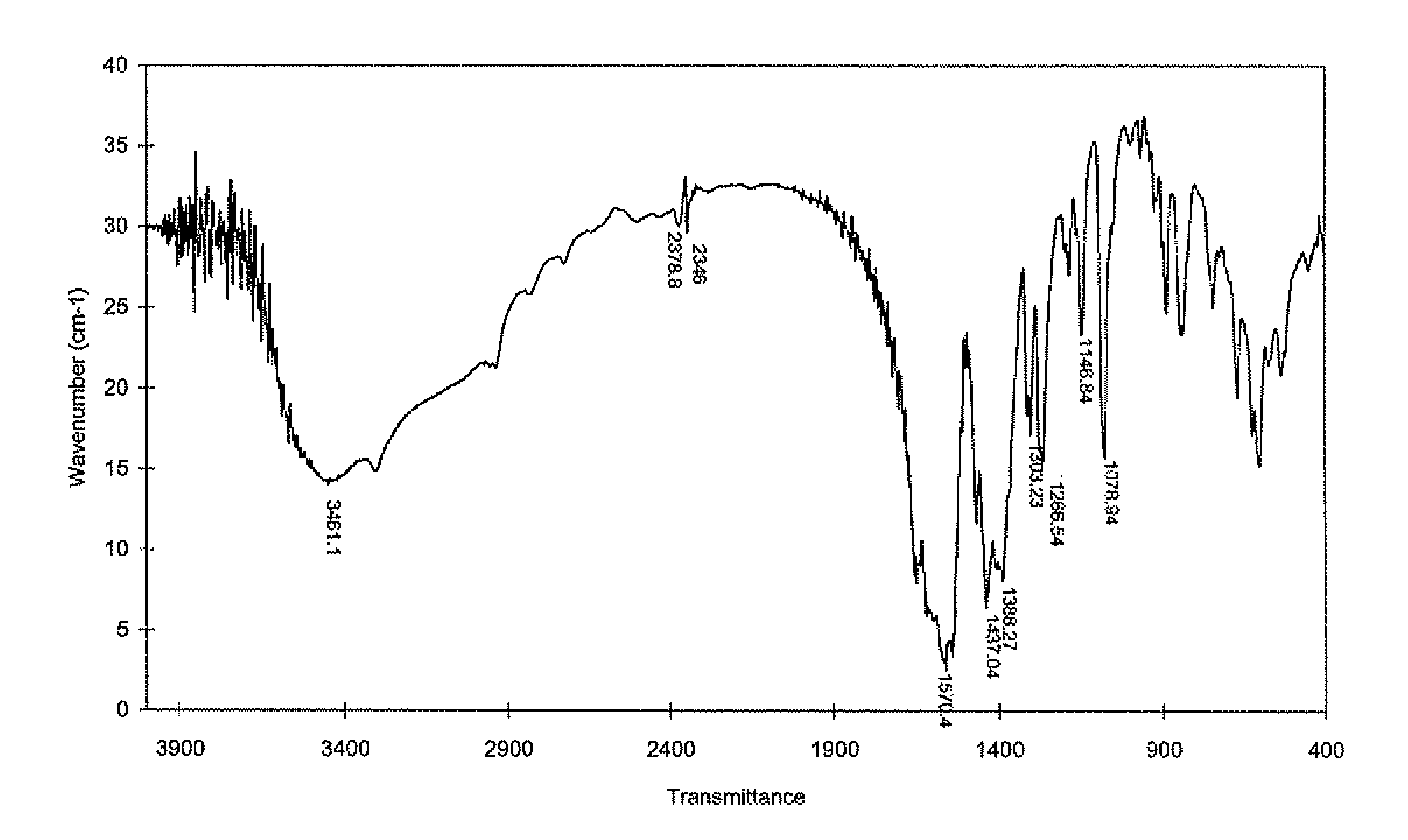
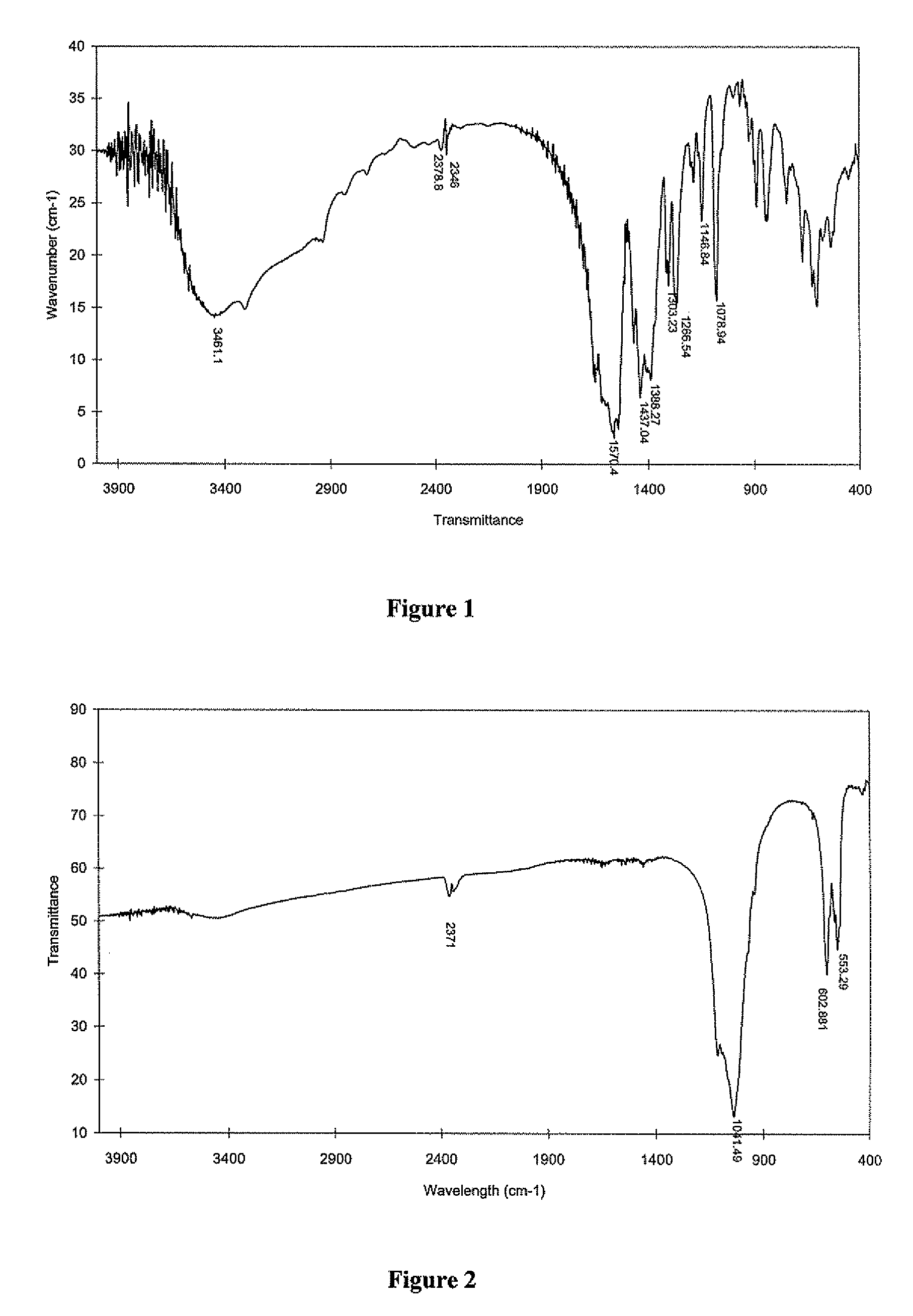
Functionalizing implantable devices with a poly (diol co-citrate) polymer
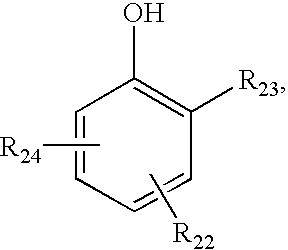
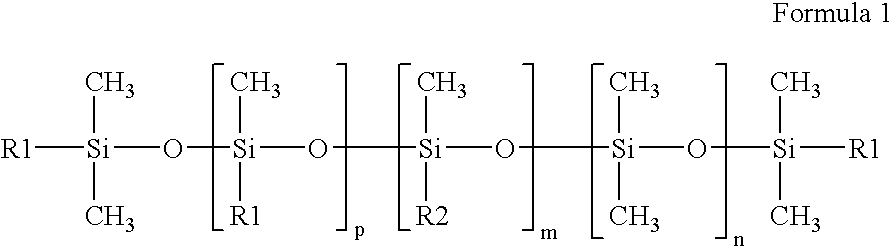
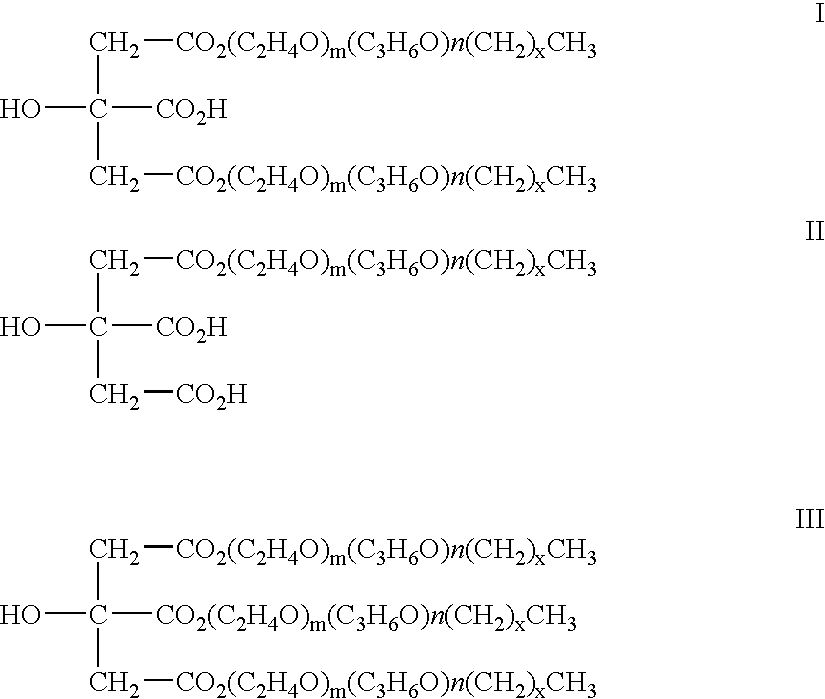
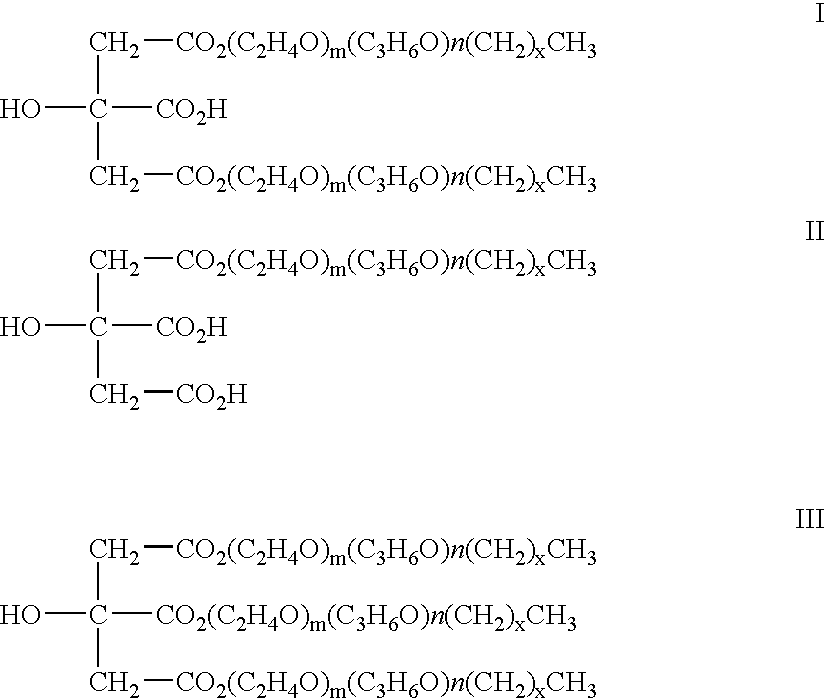
InactiveUS20070208420A1Reduce thrombosisLess prone to thrombosisAntibacterial agentsPretreated surfacesCITRATE ESTERDiol
The present invention is directed to a novel poly(diol citrates)-based coating for implantable devices. More specifically, the specification describes methods and compositions for making and using implantable devices coated with citric acid copolymers or citric acid copolymers impregnated with therapeutic compositions and / or cells.
Owner:NORTHWESTERN UNIV
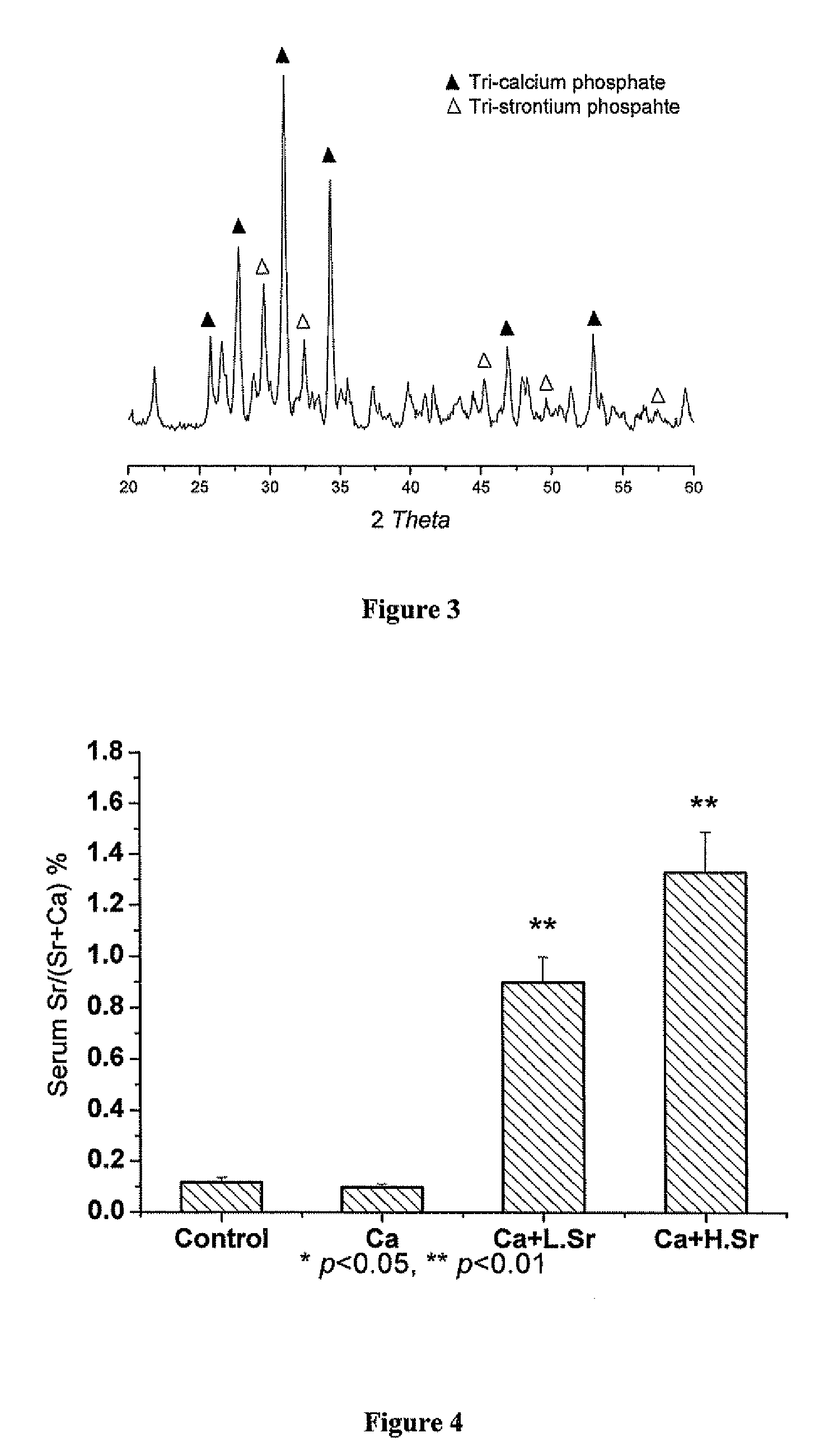
Strontium fortified calcium nano-and microparticle compositions and methods of making and using thereof
InactiveUS20080317807A1Good biocompatibilityPrevent looseningHeavy metal active ingredientsBiocideDiseasePhosphate
Compositions containing strontium fortified calcium nanoparticles and / or microparticles, and methods of making and using thereof are described herein. The strontium fortified calcium compounds contain calcium ions, calcium atoms, strontium ions, strontium atoms, and combinations thereof and one or more anions. Exemplary anions include, but are not limited to, citrate, phosphate, carbonate, and combinations thereof. The particles can be formulated for enteral or parenteral administration by incorporating the particles into a pharmaceutically carrier. The compositions can further contain one or more active agents useful for bone diseases or disorders, such as vitamin D, growth factors, and combinations thereof. The compositions can be used to treat or prevent one or more bone diseases or disorders of the bone, such as osteoporosis. Alternatively, the particles can be coated onto a substrate, such as the surface of an implant. The coatings can be used to improved biocompatibility of the implant, prevent loosening of the implant, reducing leaching of metal ions from metallic implants, and reduce corrosion. The coatings can be applied to the substrate using a variety of techniques well known in the art. In one embodiment, the coating is applied using electrophoretic deposition. The use of nano- and / or microparticles that provide high surface area helps to improve interfacial strength between the coating and the implant, which allows for the use of lower sintering temperatures. Lowering sintering temperatures minimizes or prevents thermal decomposition of the coating material and / or degradation of the implant material.
Owner:THE UNIVERSITY OF HONG KONG
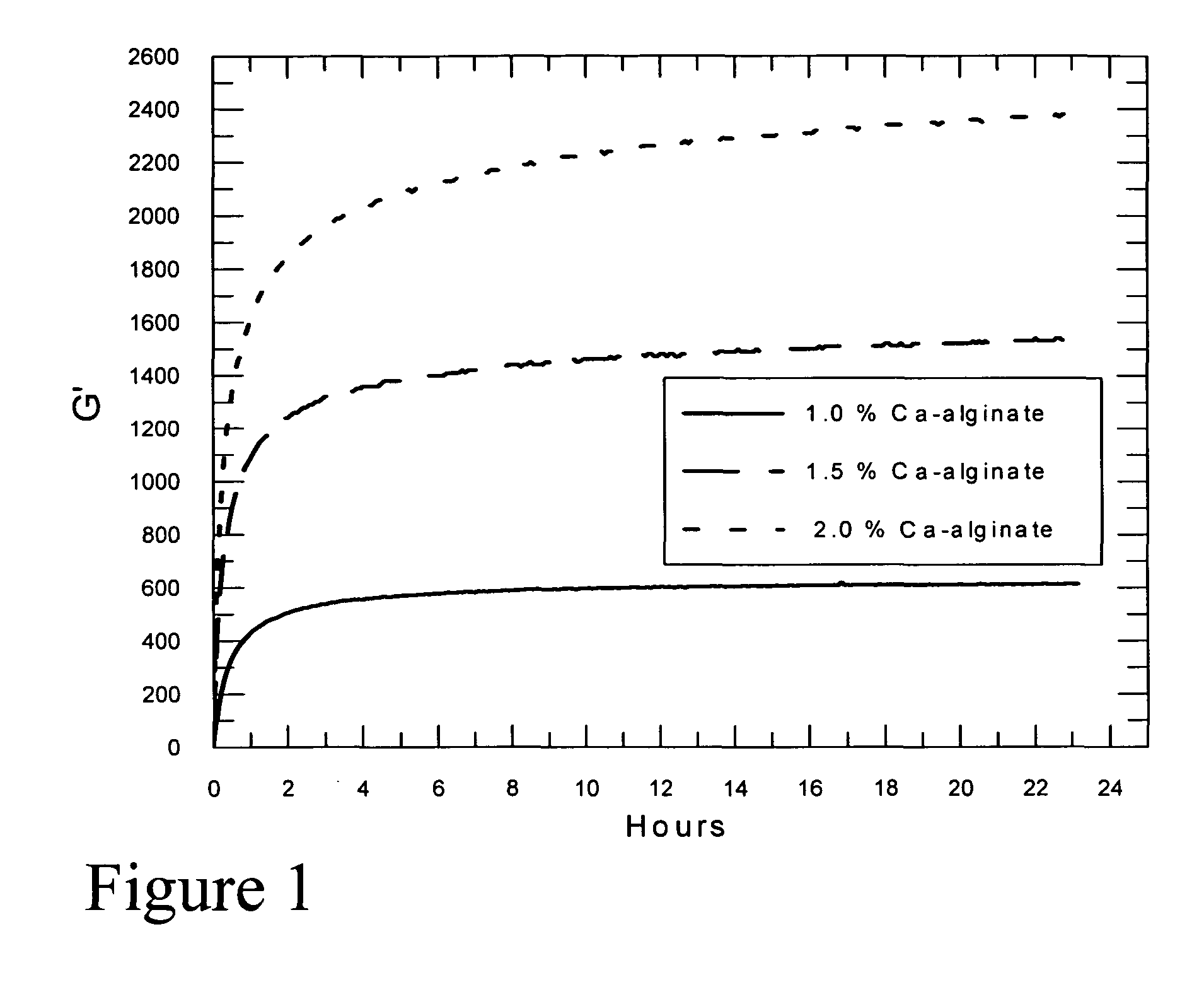
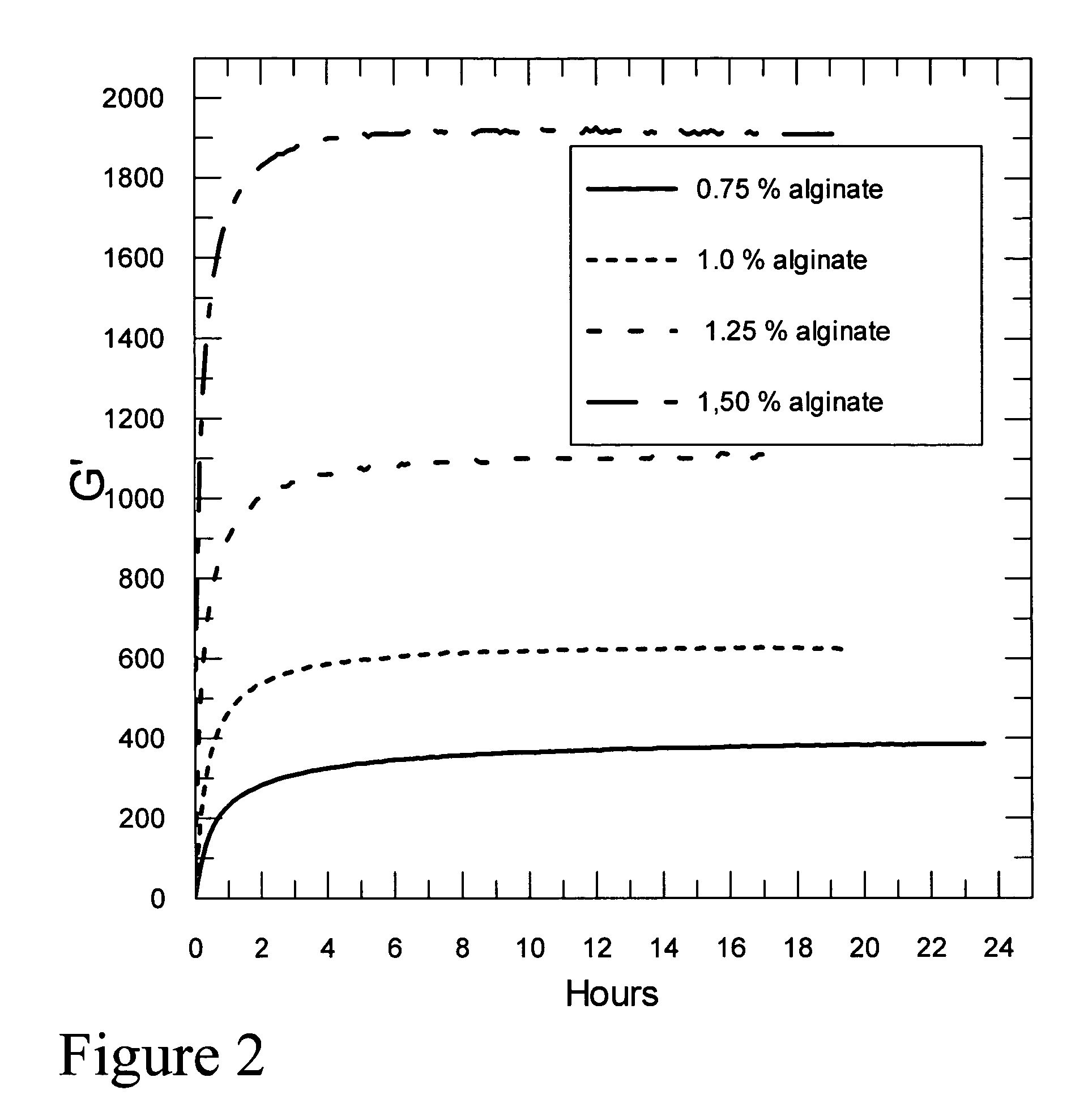
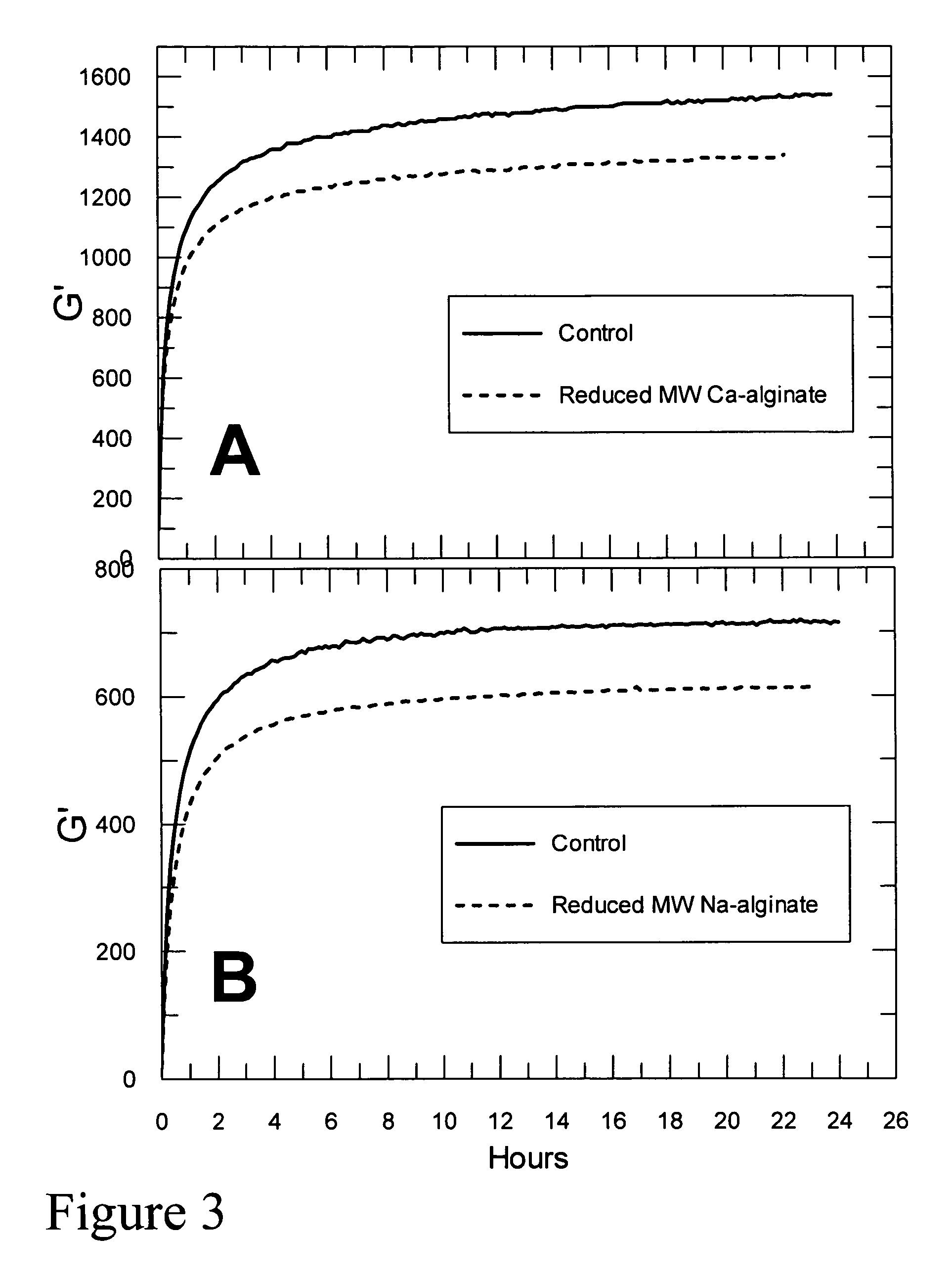
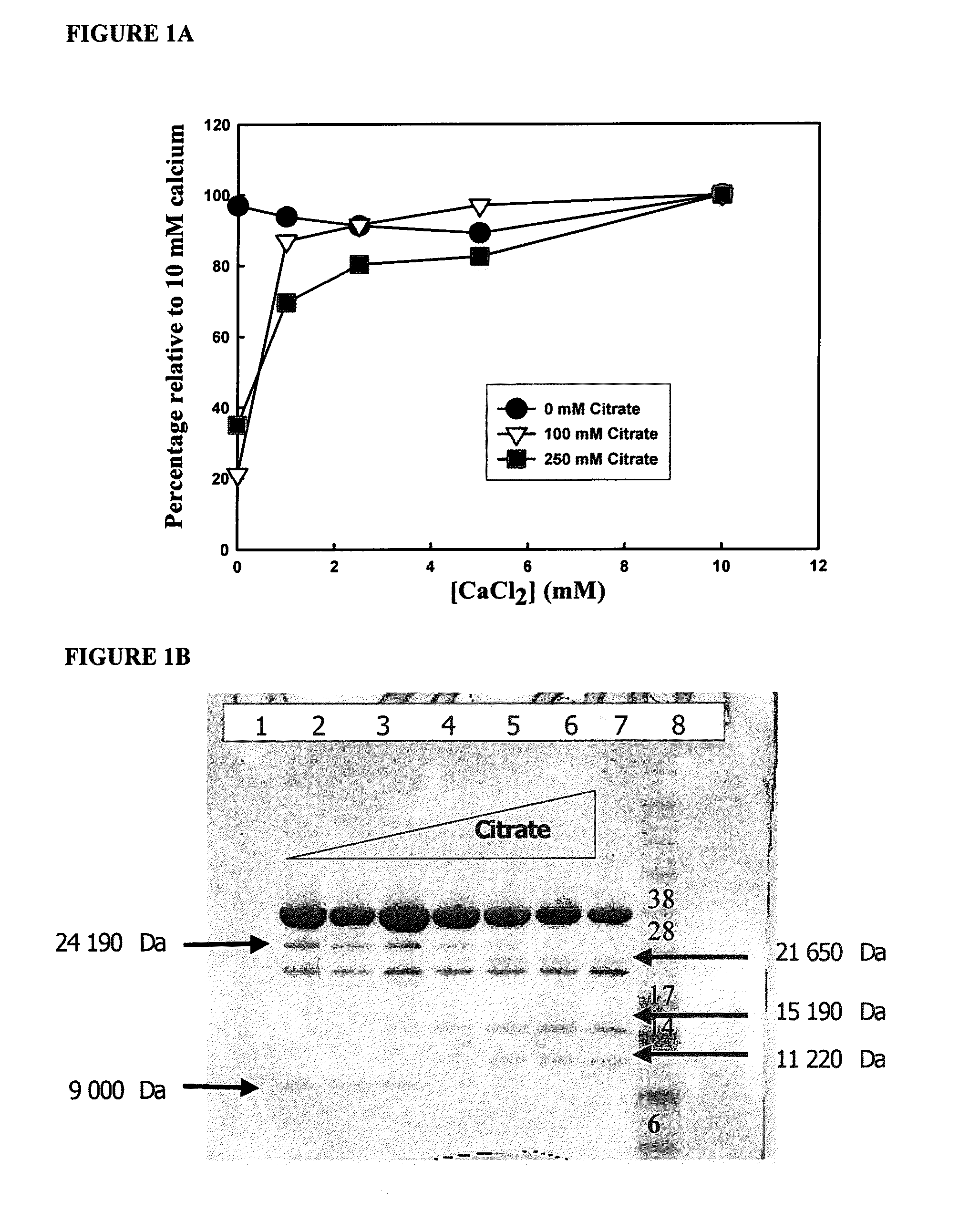
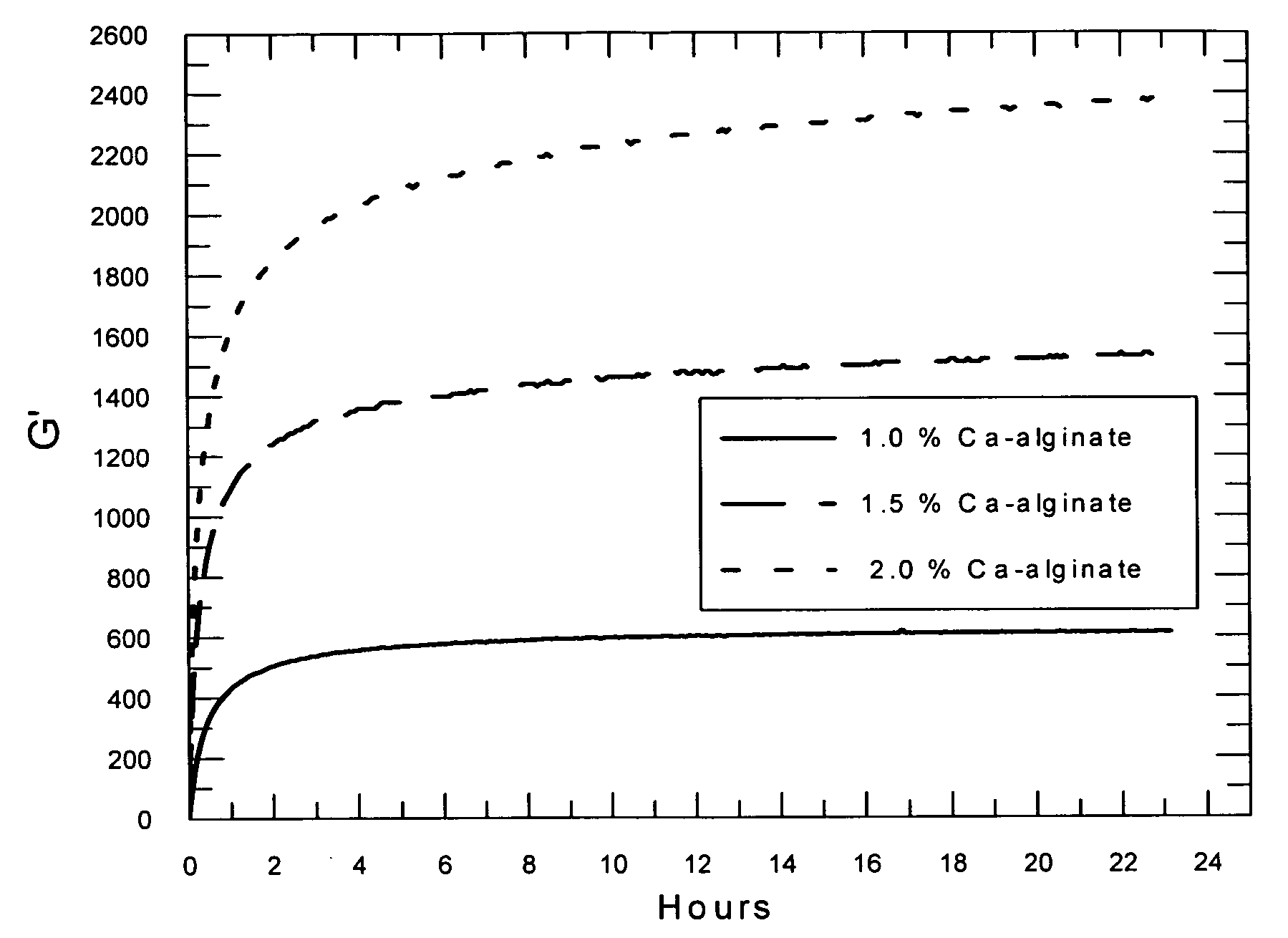
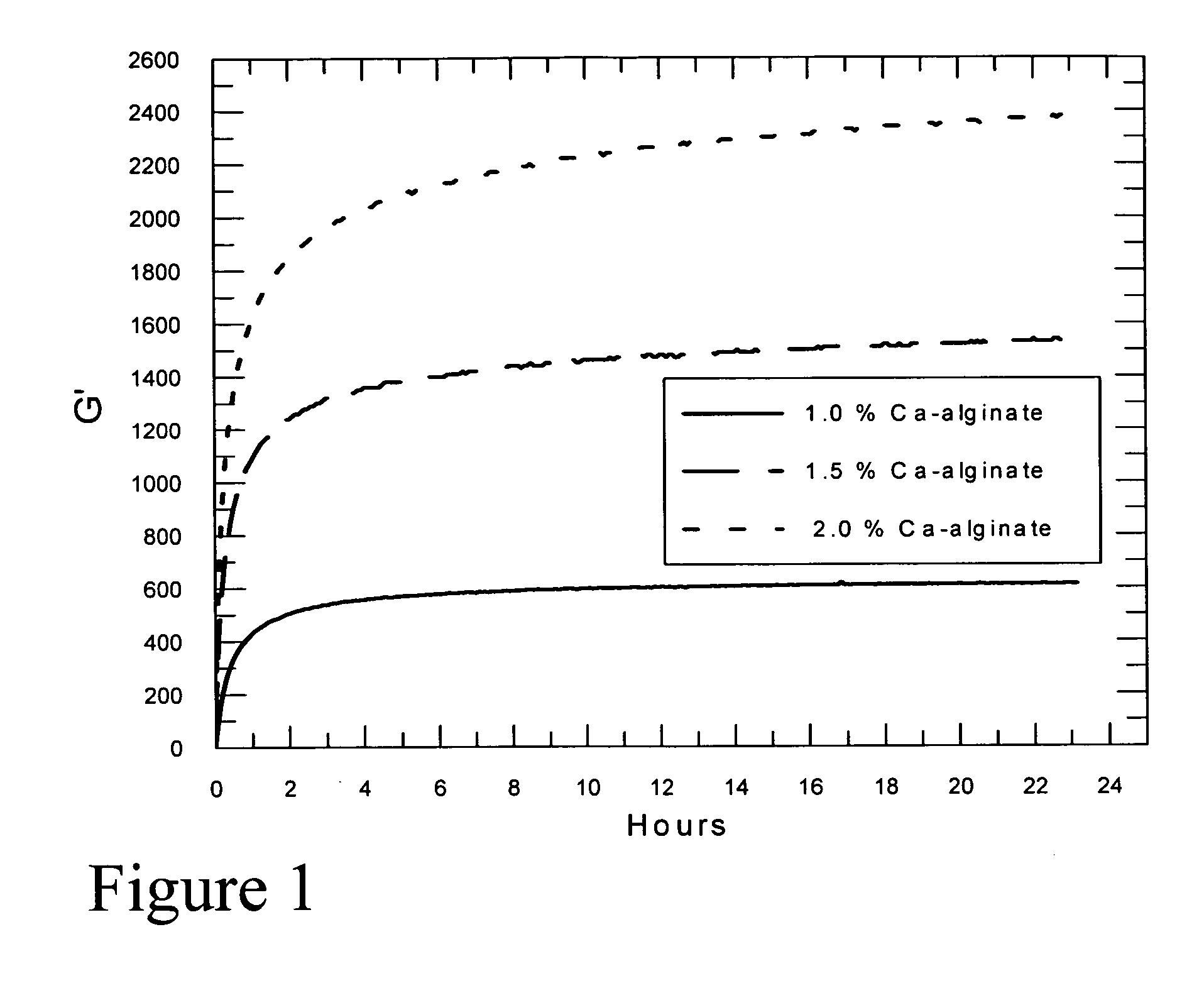
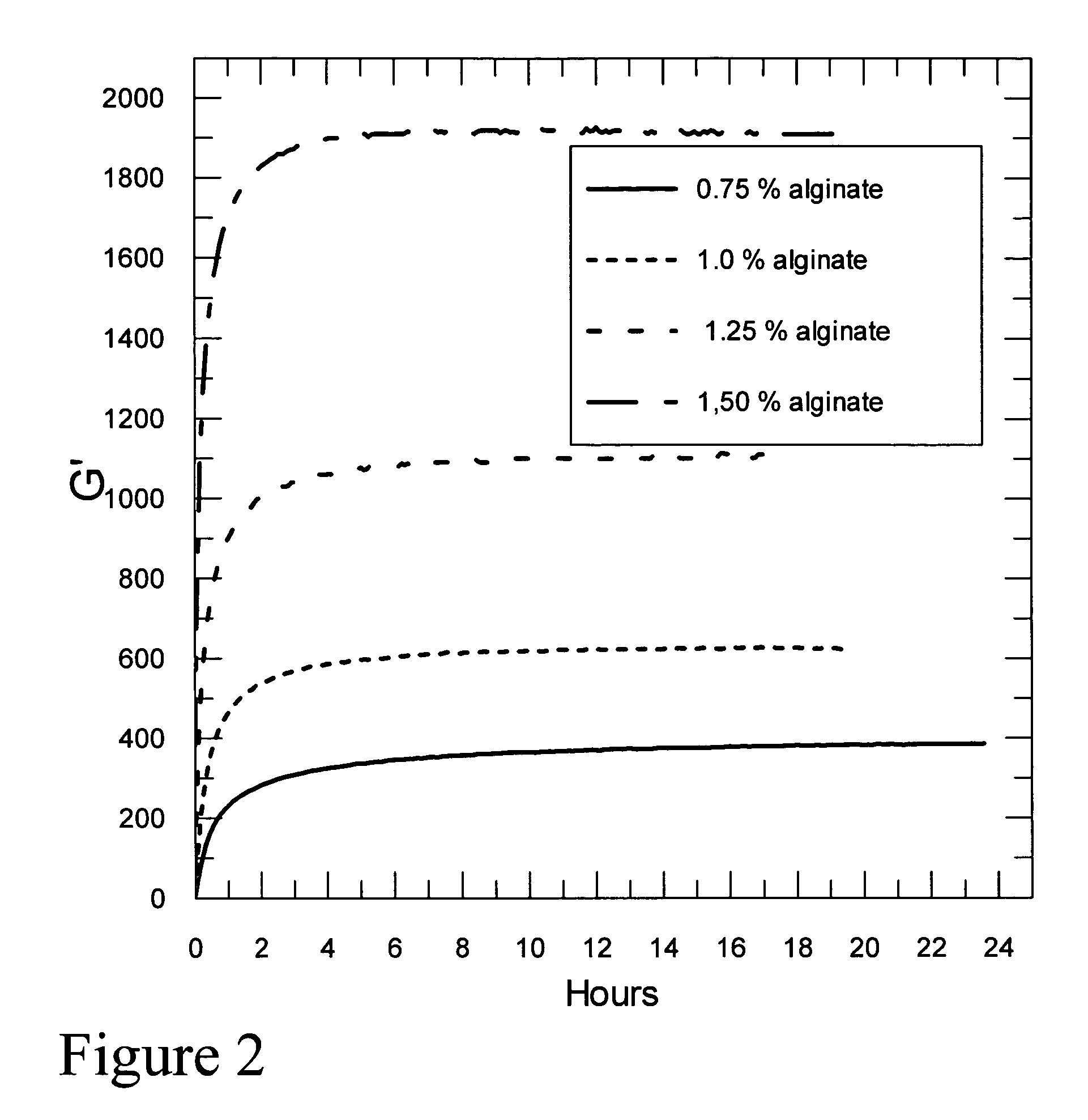
Self-gelling alginate systems and uses thereof
Kits and compositions for producing an alginate gel are disclosed. The kits and compositions comprise soluble alginate and insoluble alginate / gelling ion particles. Methods for dispensing a self-gelling alginate dispersion are disclosed. The methods comprise forming a dispersion of insoluble alginate / gelling ion particles in a solution containing soluble alginate, and dispensing the dispersion whereby the dispersion forms an alginate gel matrix. The methods may include dispensing the dispersion into the body of an individual. An alginate gel having a thickness of greater than 5 mm and a homogenous alginate matrix network and homogenous alginate gels free of one or more of: sulfates citrates, phosphates, lactatates, EDTA or lipids are disclosed. Implantable devices comprising a homogenous alginate gel coating are disclosed. Methods of improving the viability of pancreatic islets, or other cellular aggregates or tissue, following isolation and during storage and transport are disclosed.
Owner:FMC BIOPOLYMER AS
Use and production of citrate-stable neutral metalloproteases
The present invention provides methods and compositions comprising at least one neutral metalloprotease enzyme that has improved stability in the presence of a metal chelator. In some embodiments, the neutral metalloprotease finds use in cleaning and other applications comprising citrate. In some particularly preferred embodiments, the present invention provides methods and compositions comprising variant neutral metalloprotease(s) engineered to resist citrate-induced autolysis.
Owner:DANISCO US INC
Silver dihydrogen citrate compositions
Personal care and home care compositions are disclosed, which include silver dihydrogen citrate. The inventive compositions advantageously take the form of suspensions, pastes, liquids and gels. The inventive compositions also optionally comprise additional ingredients, and are therefore suitable for a wide variety of personal, home and industrial care purposes.
Owner:PURE BIOSCI
Self-gelling alginate systems and uses thereof
ActiveUS20060159823A1Improve survivabilityBiocideOrganic active ingredientsLipid formationCITRATE ESTER
Kits and compositions for producing an alginate gel are disclosed. The kits and compositions comprise soluble alginate and insoluble alginate / gelling ion particles. Methods for dispensing a self-gelling alginate dispersion are disclosed. The methods comprise forming a dispersion of insoluble alginate / gelling ion particles in a solution containing soluble alginate, and dispensing the dispersion whereby the dispersion forms an alginate gel matrix. The methods may include dispensing the dispersion into the body of an individual. An alginate gel having a thickness of greater than 5 mm and a homogenous alginate matrix network and homogenous alginate gels free of one or more of: sulfates citrates, phosphates, lactatates, EDTA or lipids are disclosed. Implantable devices comprising a homogenous alginate gel coating are disclosed. Methods of improving the viability of pancreatic islets, or other cellular aggregates or tissue, following isolation and during storage and transport are disclosed.
Owner:FMC BIOPOLYMER AS
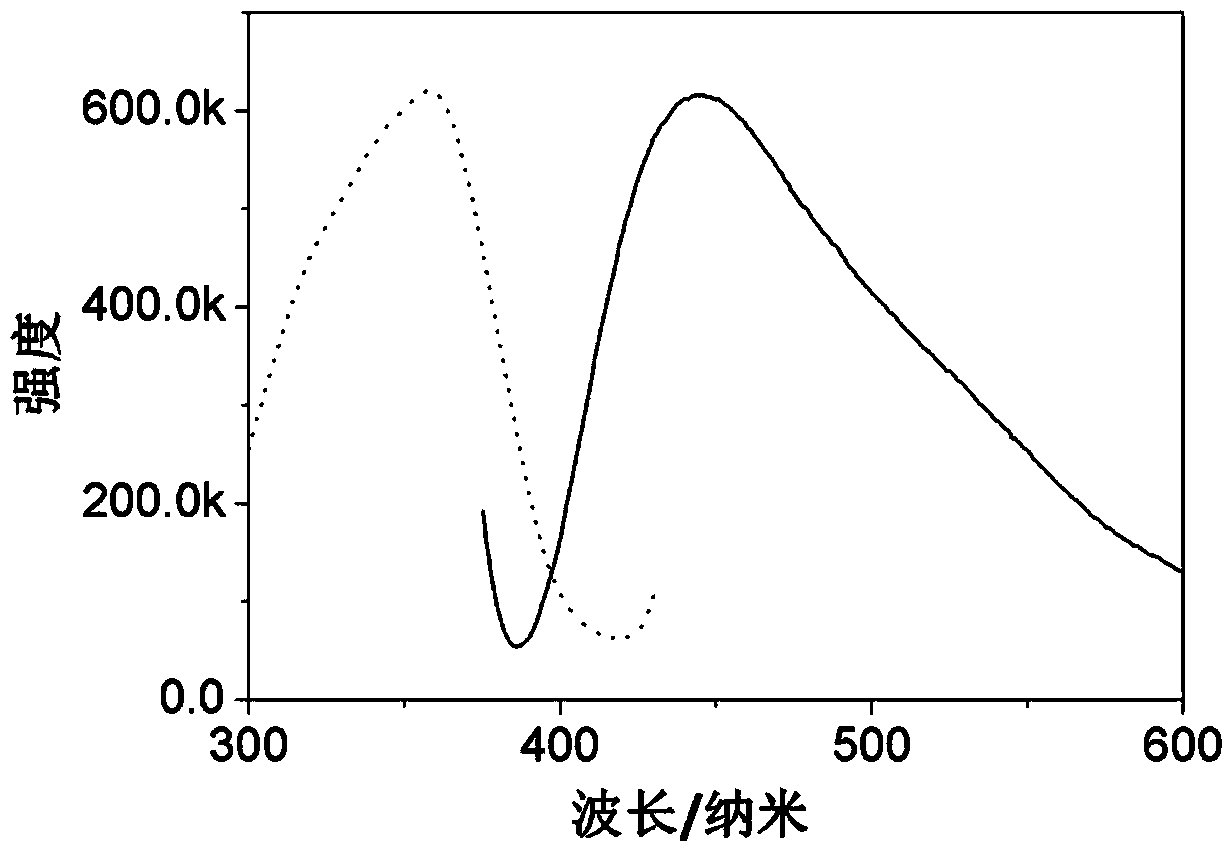
Preparation method of carbon quantum dots with adjustable fluorescence colors
InactiveCN103663412AHigh yieldImprove quantum efficiencyNano-carbonFluorescence/phosphorescenceUltraviolet lightsTumor cells
The present invention relates to a preparation method of carbon quantum dots with adjustable fluorescence colors, and belongs to the technical field of nanometer materials. According to method, citric acid or a citrate is adopted as a carbon source, a nitrogen-containing compound is adopted as a nitridation agent, hydrogen peroxide is adopted as an oxidant, a hydrothermal synthesis method is adopted to obtain an aqueous solution of carbon quantum dots emitting blue or green fluorescence under ultraviolet light excitation, reaction conditions are easily controlled, and the method is suitable for scale production. The prepared carbon quantum dots have advantages of adjustable fluorescence color, high yield, high quantum efficiency, good result reproducibility and the like, wherein the product can be directly used for tumor cell labeling and live cell imaging labeling. According to the present invention, only the one reactant is required, the raw materials are easily-available and non-toxic, the production process does not require special protection, the reaction condition is easily controlled, and the obtained carbon quantum dots have advantages of high yield, high quantum efficiency, good result reproducibility and the like; and the method has characteristics of high yield, simple preparation process, low cost, easy scale production and the like.
Owner:UNIVERSITY OF CHINESE ACADEMY OF SCIENCES
Preparation method of biological preservative
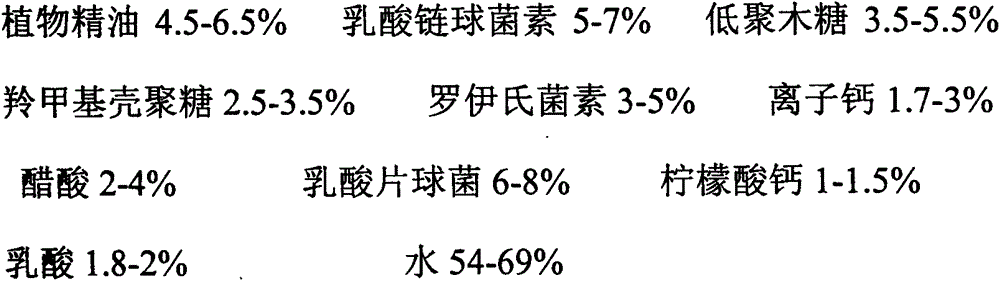
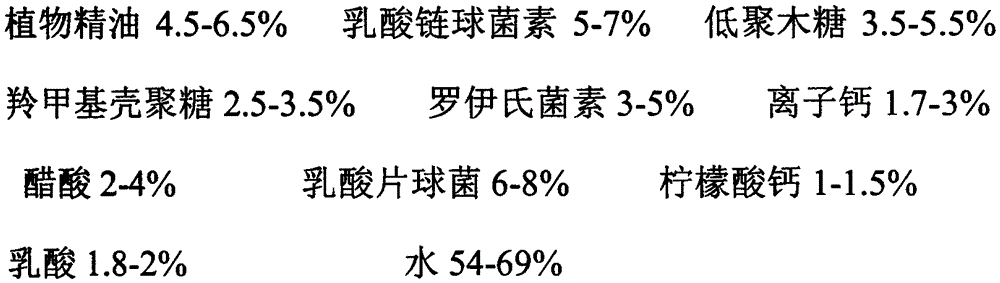
InactiveCN105614644AGrowth inhibitionDoes not affect increaseClimate change adaptationFood preservationBacteroidesNormal growth
The invention relates to a preparation method of a biological preservative. As known to all, along with the rapid development of the society, chemical preservatives have been used in food, have strong impact on food safety and endanger the health of consumers, therefore, the use of preservatives causes great concern of government departments and consumers. According to the biological preservative, natural plants and microbial antibiotic substances are used as base materials, for example, the biological preservative is prepared by mixture of carboxymethyl chitosan, xylooligosaccharide, nisin from lactic streptococci, acetic acid, lactobacillus reuteri, pediococcus acidilactici, calcium citrate, ionized calcium, lactic acid, plant essential oil and water. The biological preservative can inhibit growth of harmful microorganisms by antagonistic and competitive inhibition effects among different microorganisms and produce extracted and separated components of antibacterial active ingredients having antifungal and antibacterial functions in normal growth and metabolism processes, thereby achieving antiseptic and fresh-keeping effects.
Owner:长沙满旺生物工程有限公司
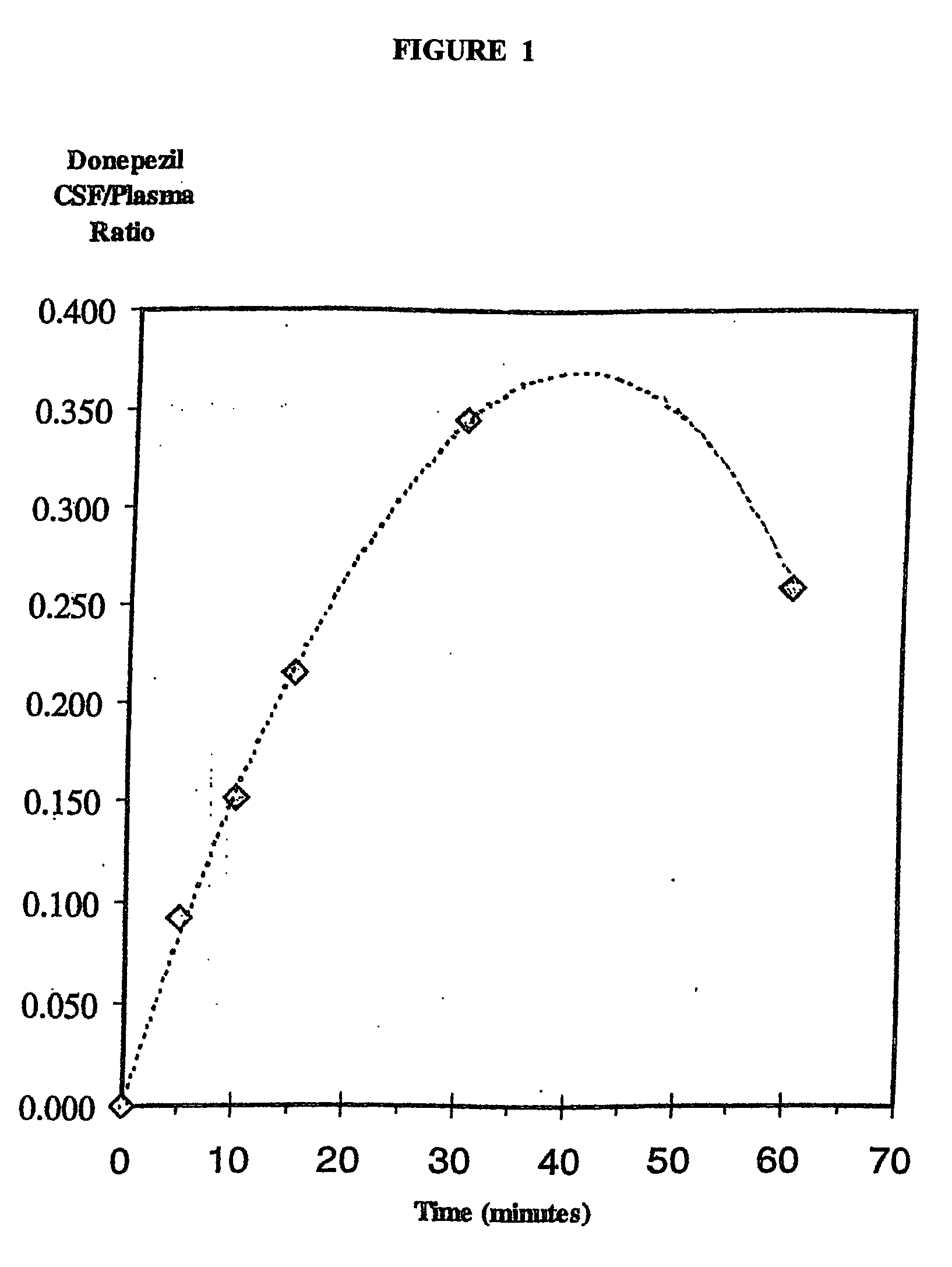
Compositions and methods using acetylcholinesterase (ACE) inhibitors to treat central nervous system (CNS) disorders in mammals
InactiveUS20060003989A1Reduce deliveryImprove solubilityBiocideNervous disorderSolubilitySide effect
Methods and compositions of the invention employ acetylcholinesterase (ACE) inhibitors to prevent and treat diseases and other disorders of the central nervous system (CNS), including Alzheimer's disease. ACE inhibitors are administered for targeted delivery to the CNS, for example by intranasal delivery. The methods and compositions of the present invention yield therapeutic concentrations of ACE inhibitors in a CNS tissue or compartment without the attendant disadvantages, risks and side effects of oral or injection delivery. Exemplary ACE inhibitors for use within the invention include galantamine and various salts and derivatives of galantamine. Carboxylate salts of galantamine (e.g., galantamine gluconate, galantamine lactate, galantamine citrate and galantamine glucarate) described herein exhibit a significant increase in solubility compared to other forms of galantamine, such as galantamine hydrobromide.
Owner:NASTECH PHARMA
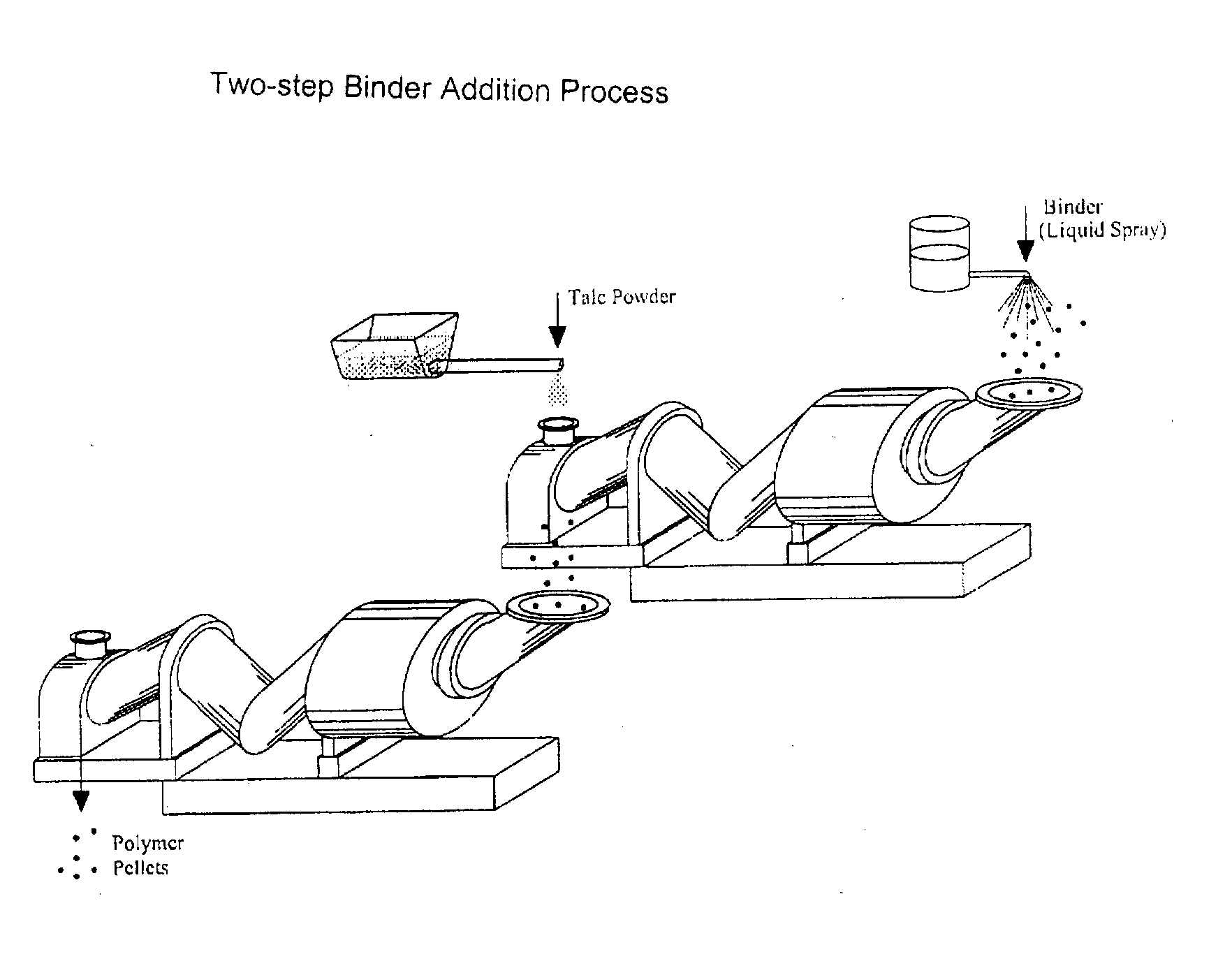
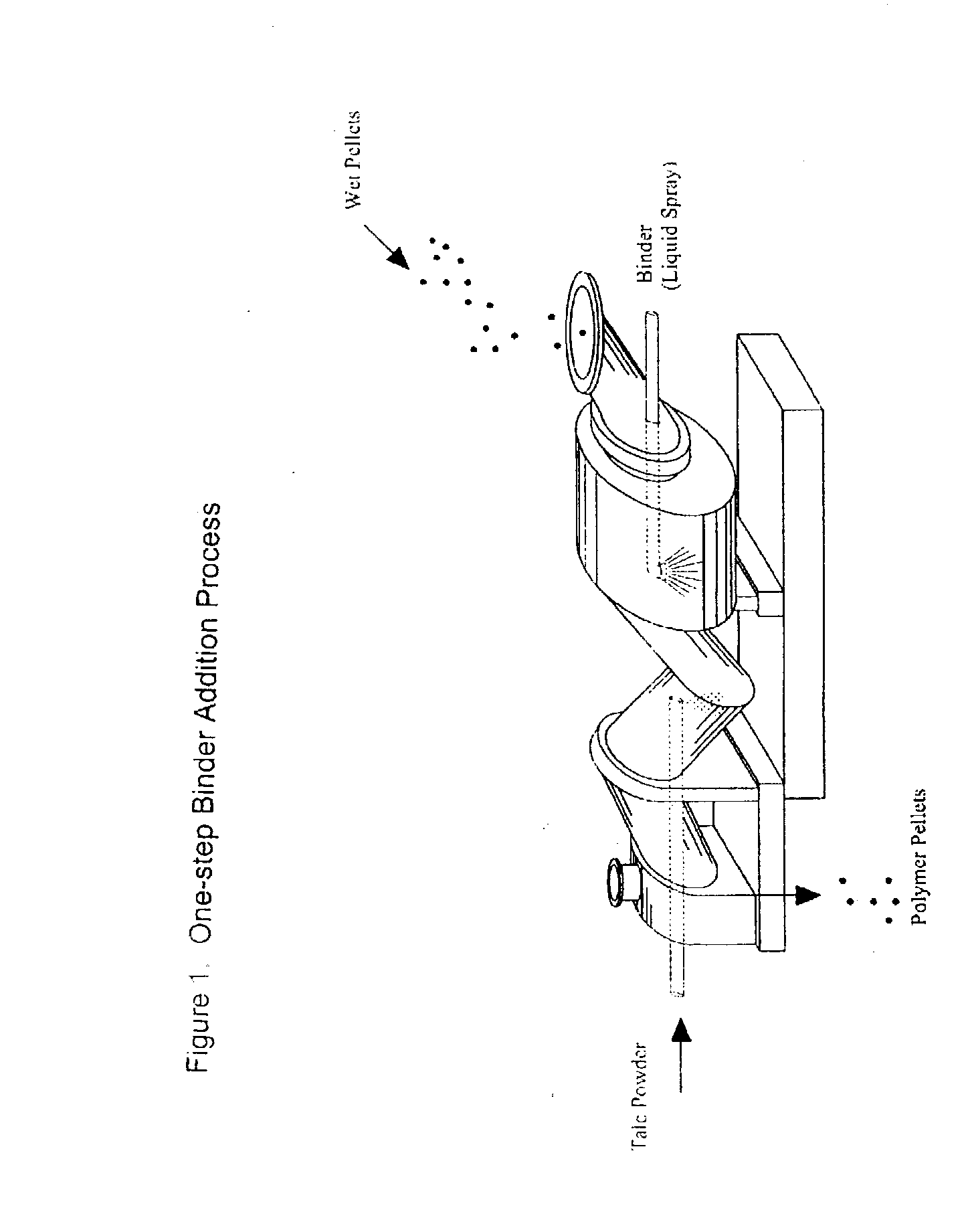
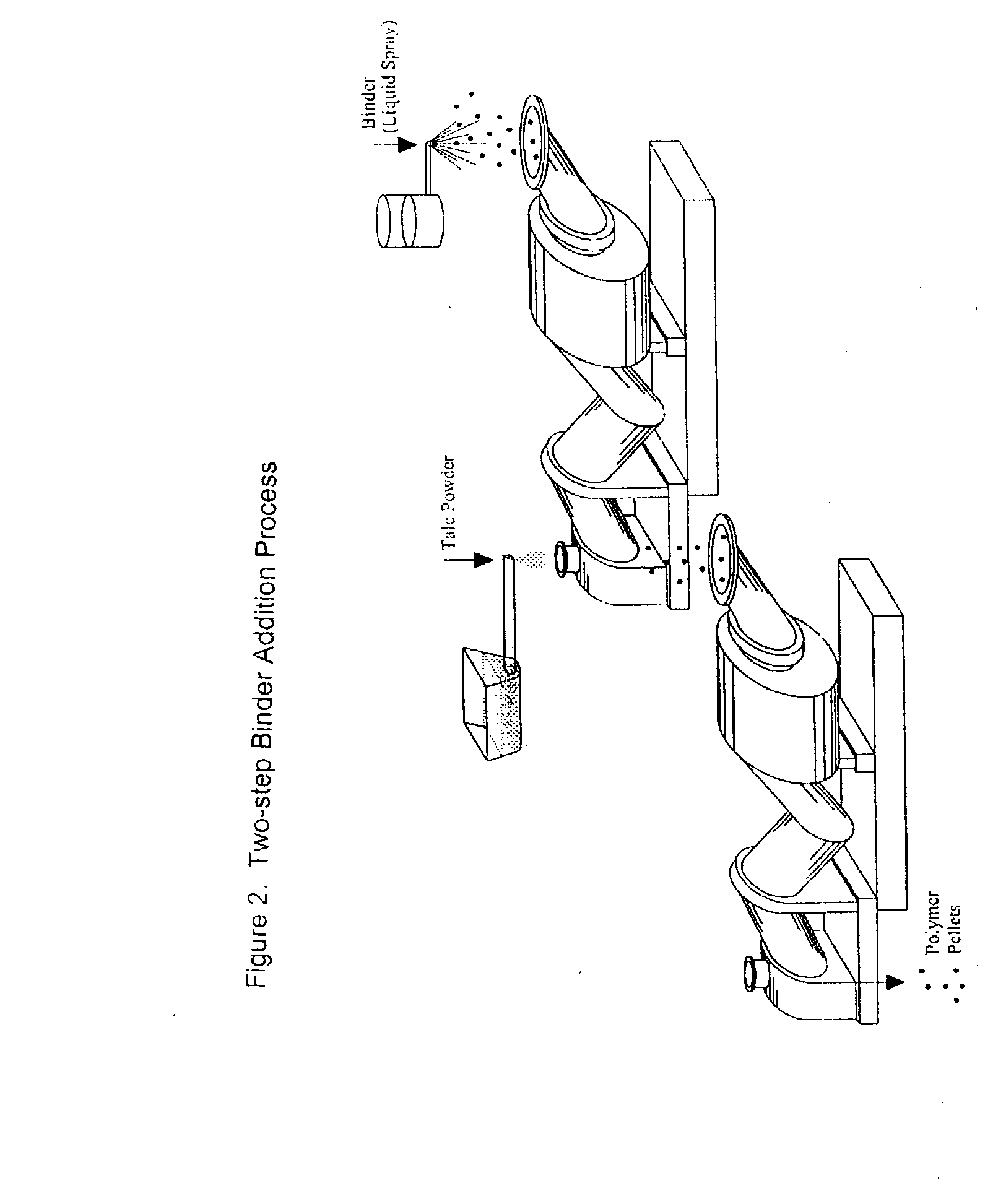
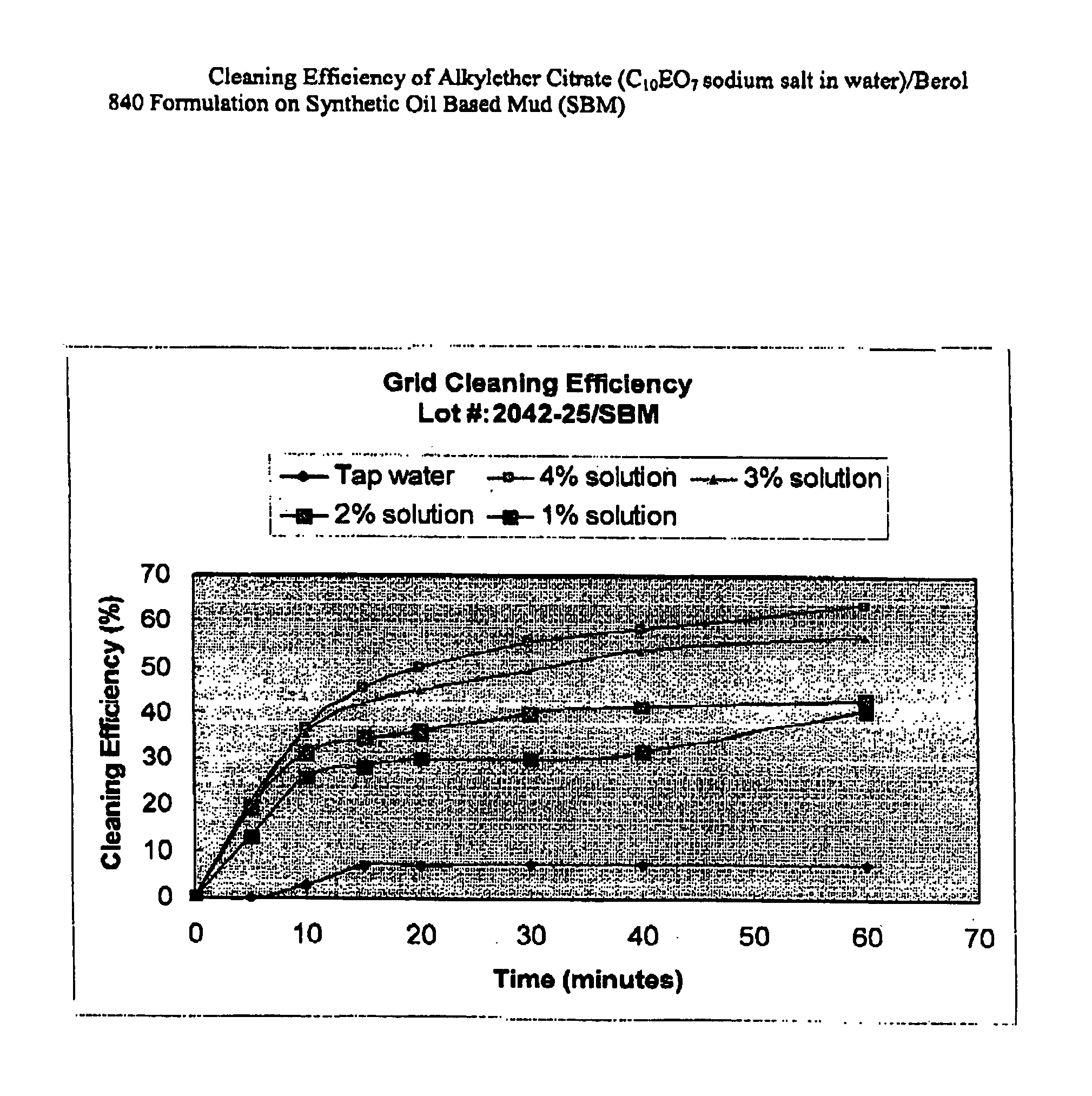
Process of Coating Tacky and Soft Polymer Pellets
InactiveUS20040209082A1Easy downstream handlingAvoid stickingConfectioneryChewing gumPolymer scienceMonoglyceride
An improved process of coating tacky or soft polymer pellets to maintain a free-flowing property, uses a liquid binder, in conjunction with an anti-tack or partitioning powder such as talc to prevent aggregation during storage. The binder is a non-volatile material such as an oil or plasticizer including triglycerides, mono- / di-glycerides, acetylated mono- / di-glycerides, fatty acids, epoxidized triglycerides, phthalates, benzoates, sebacates, lactates, citrates, mineral oils etc. Applications of this process include chewing gum bases, hot-melt adhesives, sealants, rubber masterbatches, powdered rubber, and other soft and tacky polymer materials.
Owner:WM WRIGLEY JR CO
Ultrafine lead oxide prepared by using waste lead plaster and preparation method thereof
InactiveCN103374657AReduce energy consumptionSimple ingredientsReclaiming serviceable partsLead oxidesFiltrationTwo step
The invention discloses an ultrafine lead oxide prepared by using a waste lead plaster and a preparation method thereof. The preparation method comprises the following steps of: carrying out desulphurization process by mixing the waste lead plaster with an aqueous solution containing a composite desulfurizer for reaction; carrying out filtration to remove the desulphurization filtering solution to obtain the desulfurated lead plaster (filter residue); carrying out a leaching and crystal transformation process by adding a citric acid solution and a reducing agent into the desulfurated lead plaster obtained in the process, and carrying out filtration, washing, and drying to obtain the lead citrate after the desulfurated lead plaster reacts with the citric acid solution; carrying out a roasting process by roasting the lead citrate to obtain the ultrafine lead oxide. According to the preparation method disclosed by the invention, the ultrafine lead oxide is prepared from the waste lead storage lead plaster; a two-step leaching process is adopted; the filtering solution is simple in ingredient and can be recycled; a side product is recycled from the desulphurization solution. The preparation method disclosed by the invention is low in energy consumption, simple in equipment, high in lead recycling rate, and high in ultrafine lead product quality, and has the characteristics of good resource recycling effect, environmentally-friendly and pollution-free production process, and capability of clean production.
Owner:湖北金洋冶金股份有限公司 +1
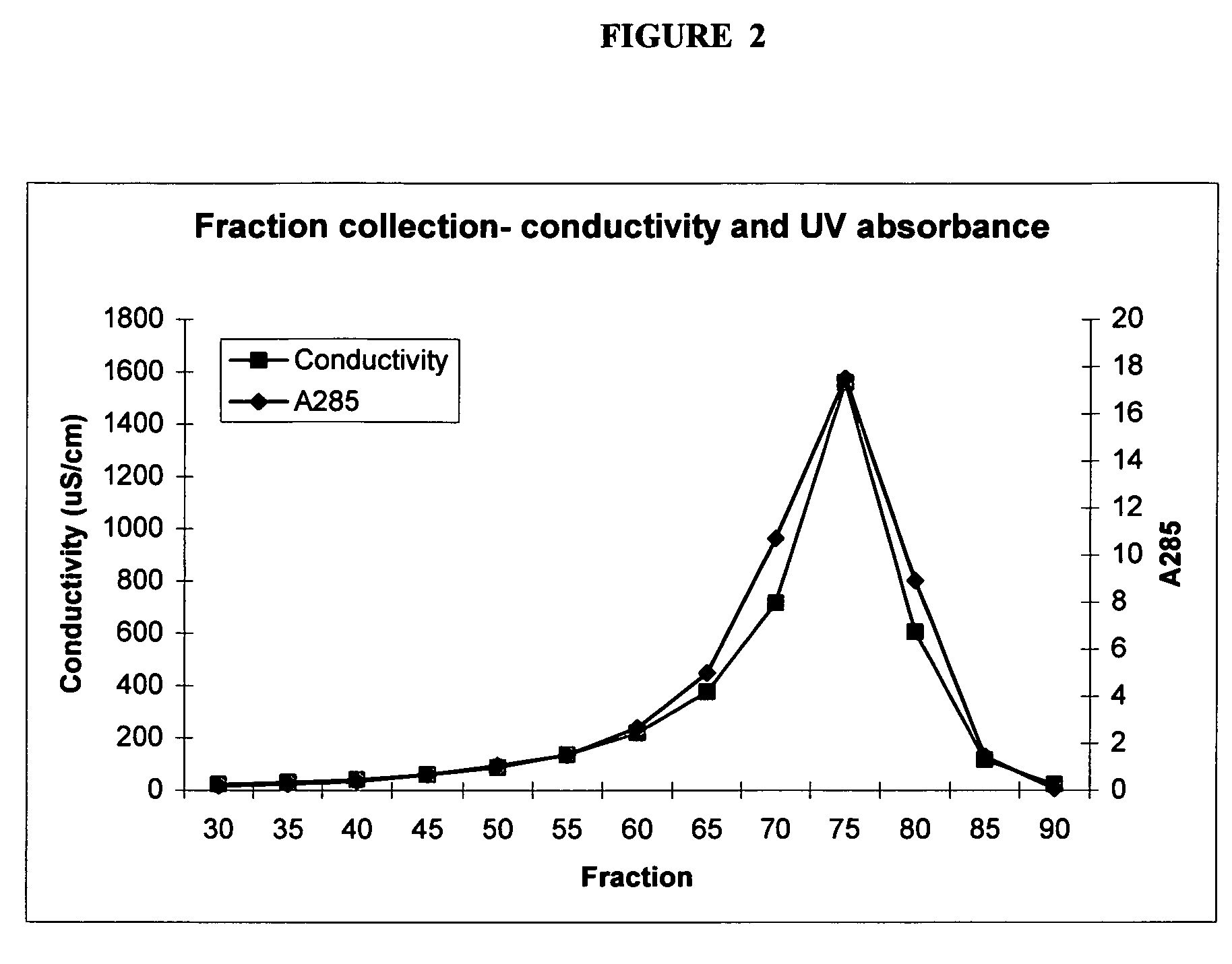
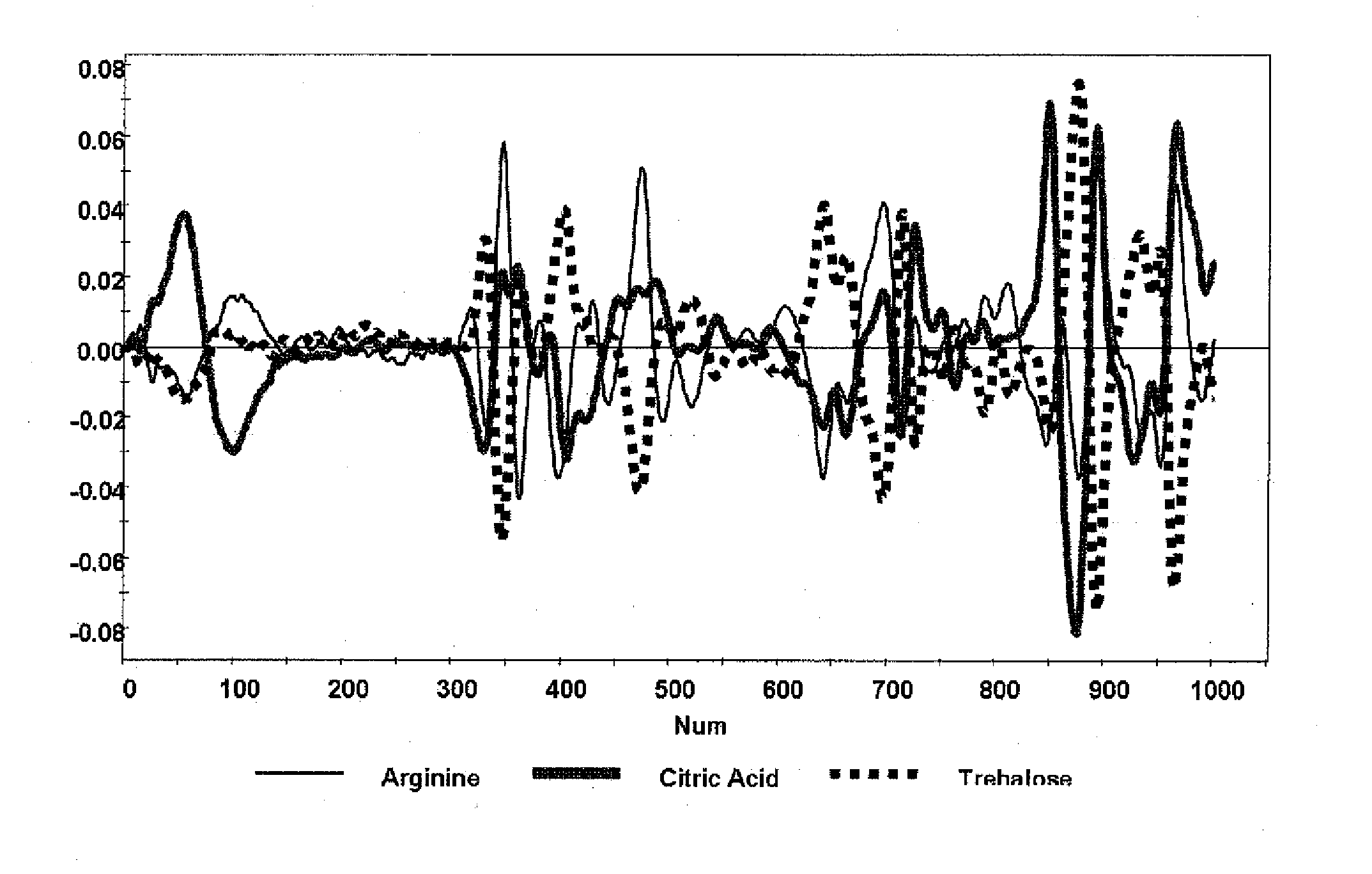
Raman spectroscopy for bioprocess operations
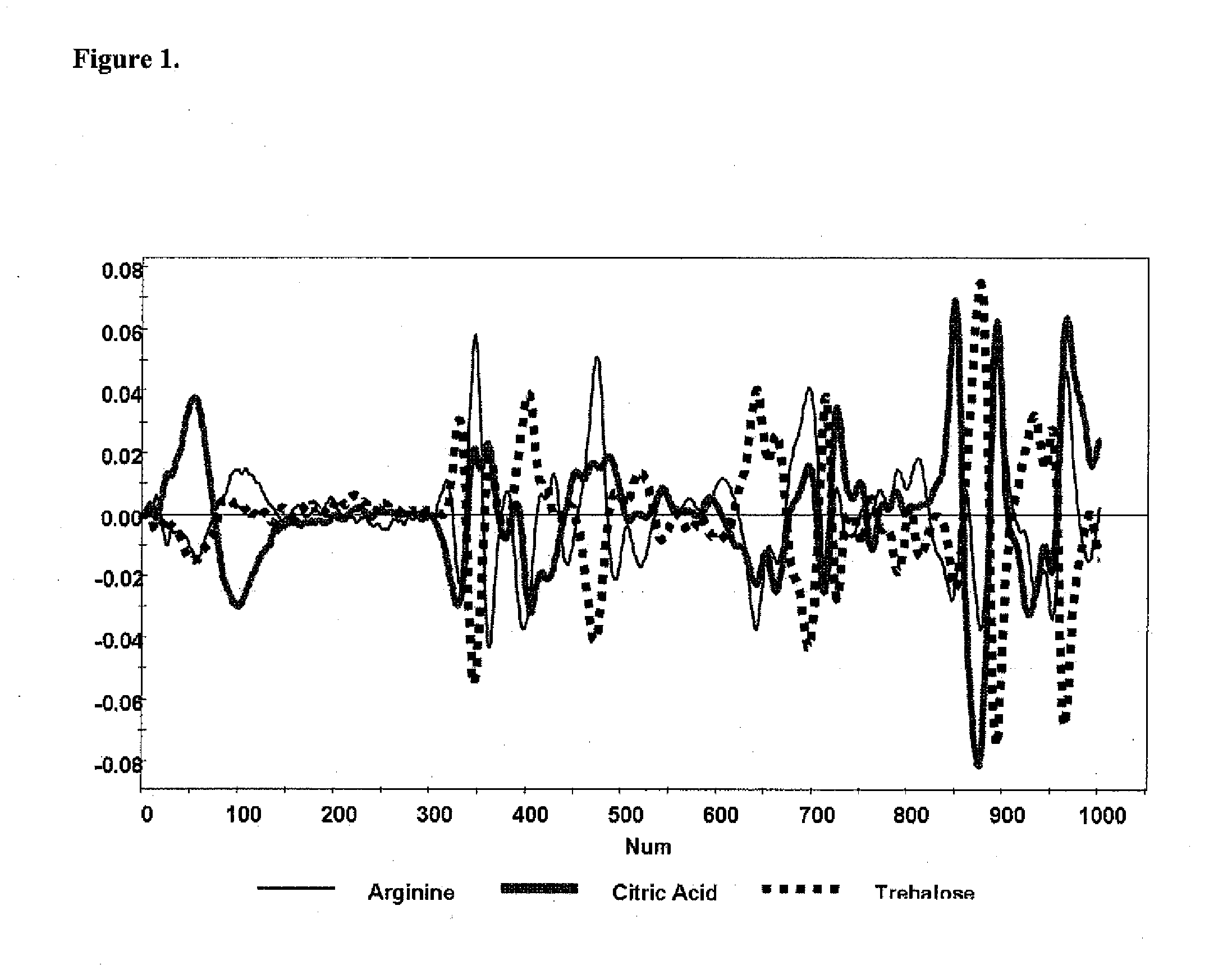
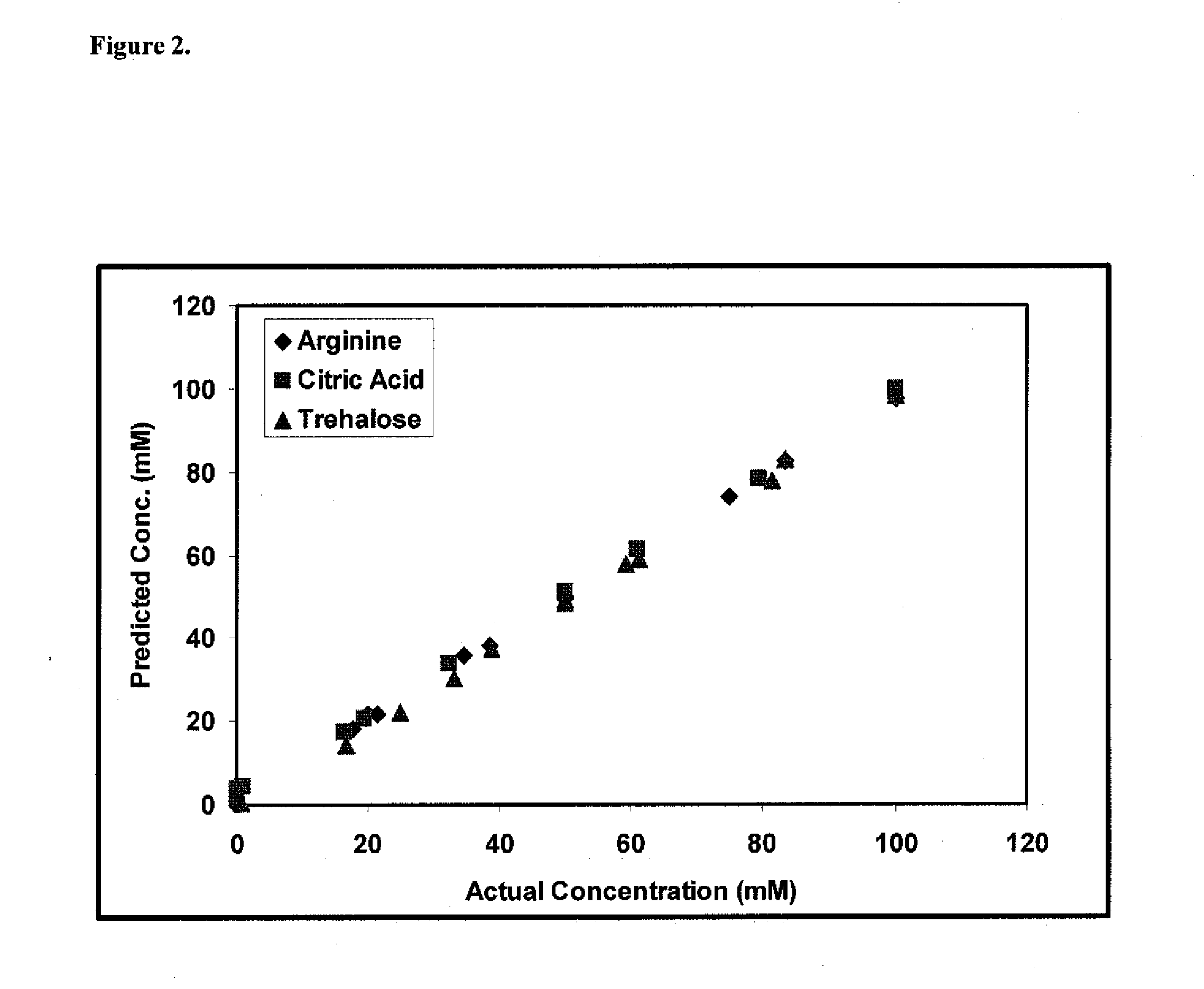
A method of characterizing a multi-component mixture for use in a bioprocess operation that includes providing a multi-component mixture standard with pre-determined amounts of known components; performing a Raman Spectroscopy analysis on the multi-component mixture standard; providing a multi-component test mixture from the bioprocess operation; performing a Raman Spectroscopy analysis on the multi-component test mixture; and comparing the analysis of the multi-component mixture standard and the multi-component test mixture to characterize the multi-component test mixture. In one embodiment, the multi-component mixture standard and the multi-component test mixture both comprise one or more of, at least two, at least three of, or each of, a polysaccharide (e.g. sucrose or mannitol), an amino acid (e.g., L-arginine, L-histidine or L-ornithine), a surfactant (e.g. polysorbate 80) and a pH buffer (e.g., a citrate formulation).
Owner:ABBVIE INC
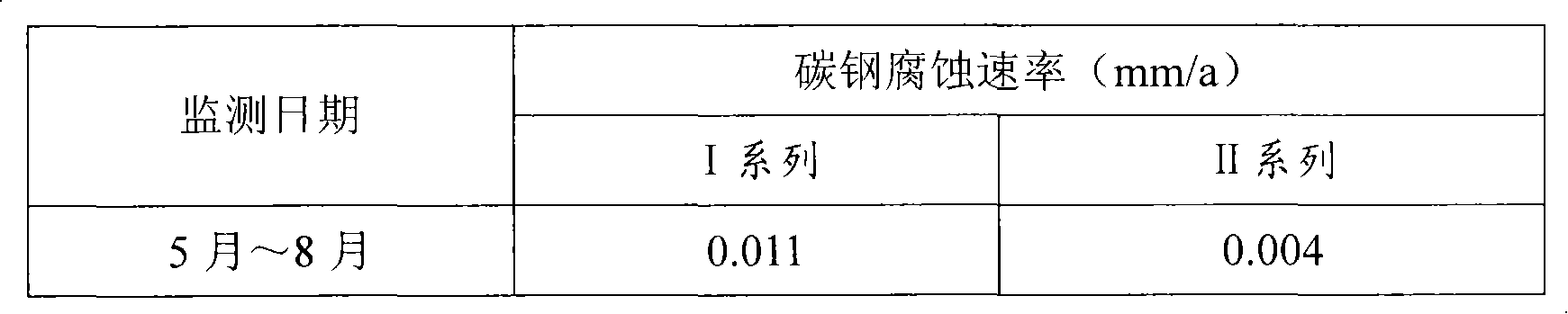
Phosphorus-free corrosion and scale inhibitor
InactiveCN101607763AReduce dosagePlay a role in corrosion inhibitionTreatment using complexing/solubilising chemicalsChelationPrecipitation types
The invention relates to a phosphorus-free corrosion and scale inhibitor, which is prepared from sodium molybdate, zinc salt, citrate, triethanolamine, benzotriazole (BTA), polyaspartic acid (PASP), polyepoxysuccinic acid (PESA), AA / AMPS terpolymer, solid alkali and water. The phosphorus-free corrosion and scale inhibitor inhibits corrosion of metals by forming oxidization type and precipitation type films on the surfaces of the metals, has the effect of inhibiting scale through chelation and dispersive action on salts causing scale in cooling water, is non-toxic and phosphorus-free, has easily biodegradable major organic compositions, does not cause environmental pollution and is not limited by phosphorus in emission.
Owner:SHANGHAI WEILAI ENTERPRISE
Antibacterial composition and method of production
An electrolysis method is described for generating an aqueous solution of copper citrate that has bacteriocidal activity against methicillin resistant Staphylococcus aureus (MRSA) bacteria. Gram positive bacteria are known to be relatively more sensitive to the bacteriocidal activities of copper ions than are Gram negative bacteria. Situations exist in which a disinfectant that is relatively more toxic for Gram positive bacteria will be advantageous over a more broadly active disinfectant, such as that provided by most other disinfectants. In particular, a disinfectant that is relatively selective for Gram positive bacteria could help preserve various non-pathogenic Gram negative microbial populations. The residual Gram negative bacteria can potentially compete with, and thereby lessen the chances of the reintroduction of pathogenic Gram positive bacteria, such as MRSA, Streptococcus, Clostridium difficile and Listeria monocytogenes.
Owner:MI HOPE
Biological preparation capable of preventing and treating cruciferae club root and use thereof
InactiveCN101416641AGood control effectSolve prevention and control problemsBiocideBacteriaSucroseRussulaceae
The invention relates to a biological agent against crucifer club root and application thereof, belonging to the technical field of bio-pesticide. The strain for production is Bacillus subtilis XF-1, whose preserving number is CGMCC NO.2357. The stain has the characteristics as follows: (1) the primary colony on the LB culture substrate is white and round having a wet surface; the later colony is light yellow having uneven edge with dry and crimple surface; observed from the microscope, the strain is short-bar shaped and movable with spore, peritricha and dimension of 0.7 - 0.8 * 2.0-2.4 mum; (2) the strain is Gram-positive and aerobic and makes use of glycogen, sugar, citrate, gelatin hydrolysate, starch and casein, but does not make use of cellulose, tyrosine and catalase positive; (3) the stain has the function of sterilization, disease prevention, and yield improvement. The embodiment of the invention is as follows: using the test tube of Bacillus subtilis XF-1 stain, shake cultivation, and culture solution for fermentation to prepare biological agent, then applying the biological agent to the rhizosphere soil of crucifer crops, thereby having good effect in preventing and treating, and simple production.
Owner:YUNNAN AGRICULTURAL UNIVERSITY
Compositions for microbial and chemical protection
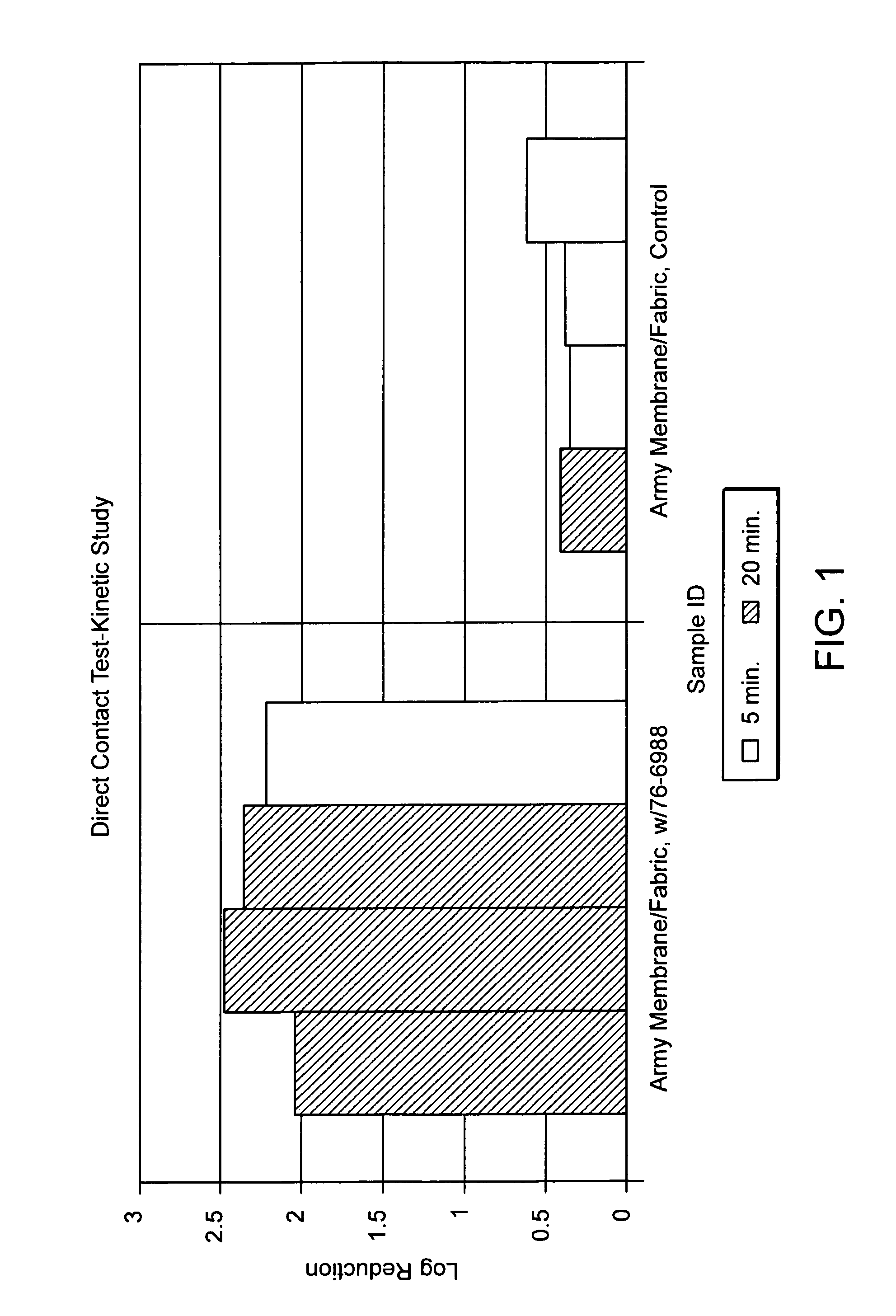
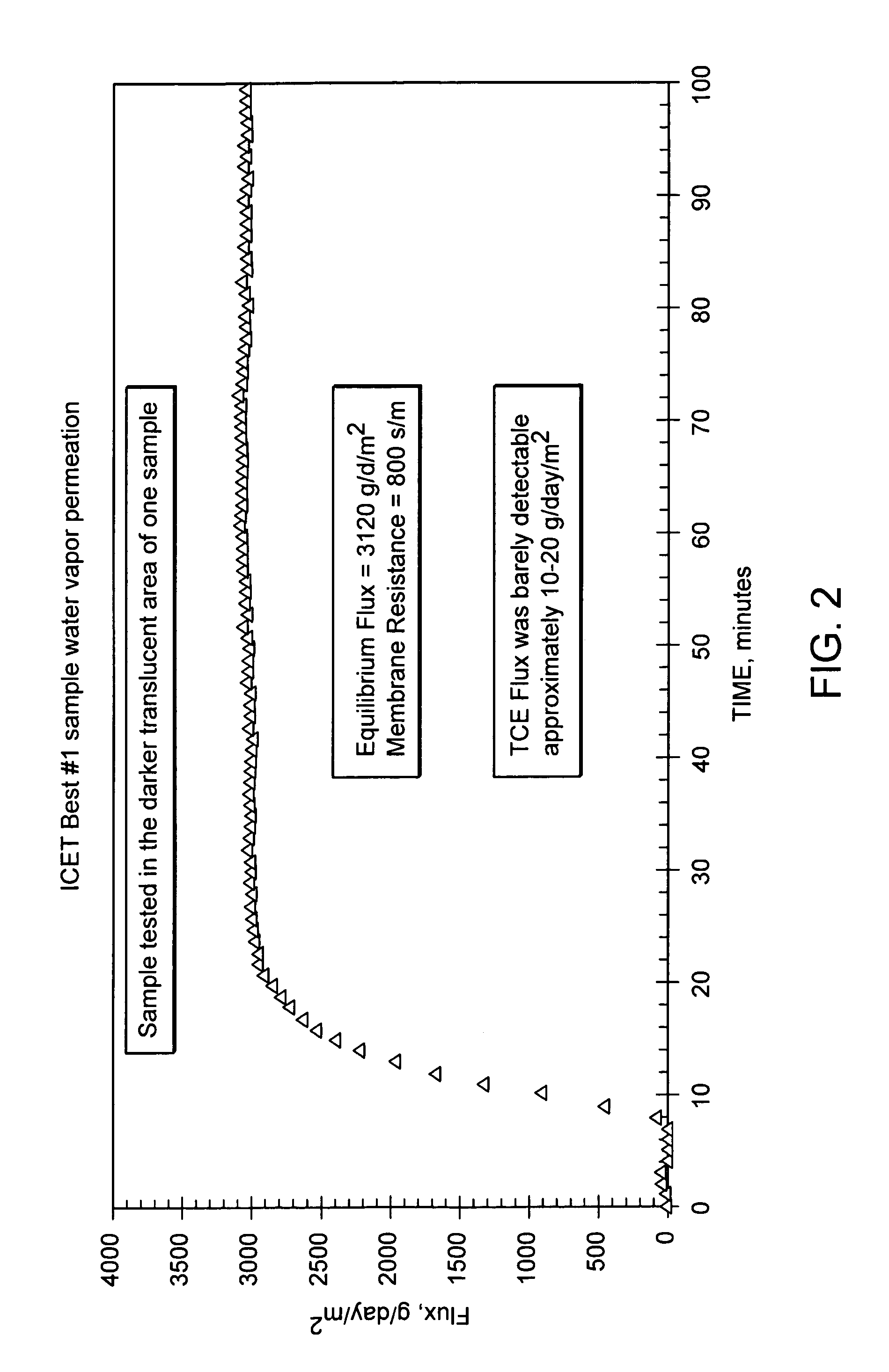
An antimicrobial and chemical deactivating composition for use in a liquid medium or for incorporation into a coating, structural plastic materials, thin microporous membranes, textiles and sponges. The composition includes macrosize or submicron particles of silver, platinum with silver and their salts with parabens, oxide, salicylate, acetate, citrate, benzoate and phosphate along with copper and zinc salts of the same.
Owner:ICET
Silver dihydrogen citrate compositions comprising a second antimicrobial agent
Compositions comprising silver dihydrogen citrate in combination with a second antimicrobial compound are provided. The second antimicrobial compound may be quaternary ammonia, an oxidizer or a halogen species, such as chlorine, bromine or iodine. Methods of using the antimicrobial composition provide superior antimicrobial effects.
Owner:PURE BIOSCI
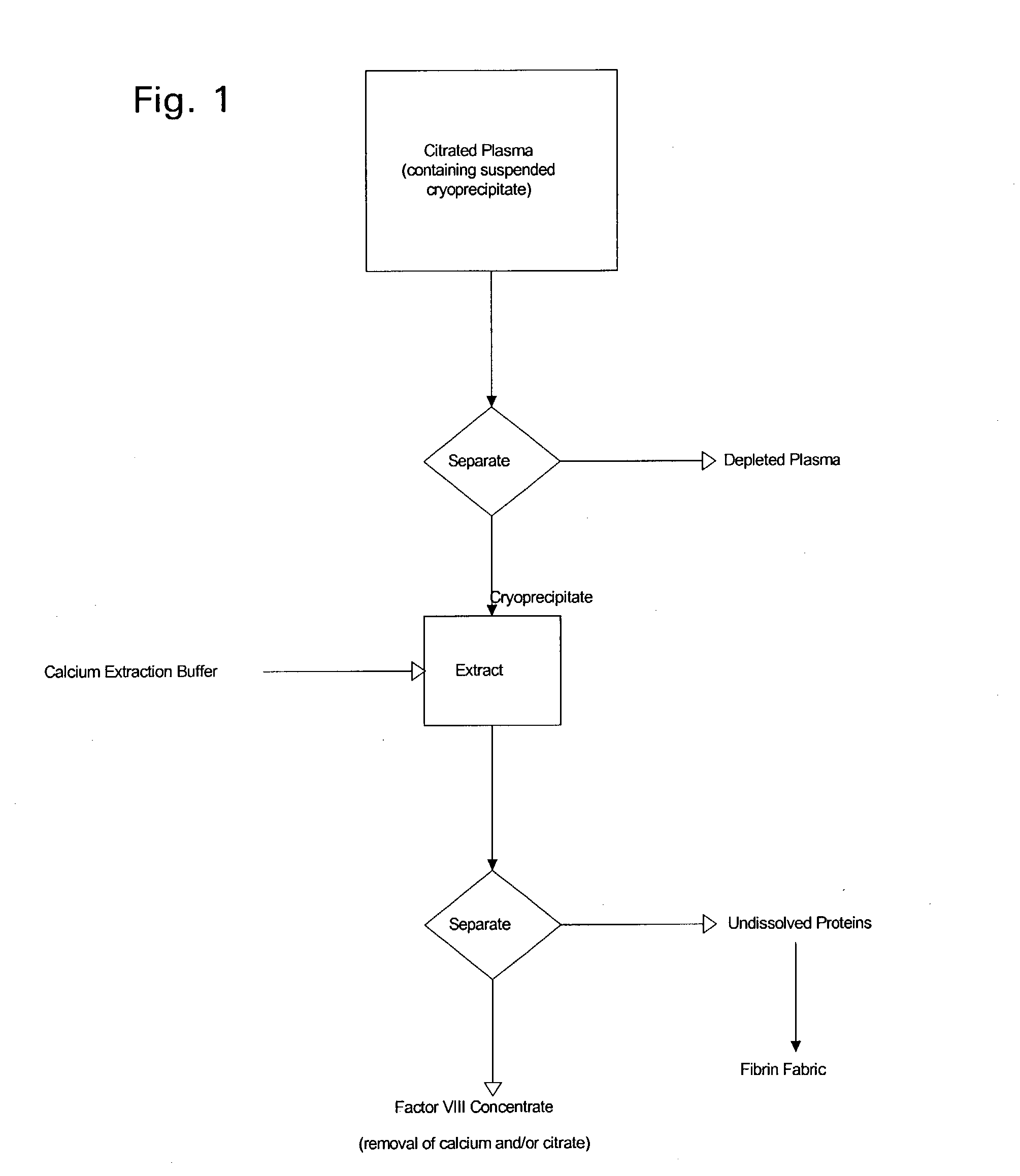
Enhanced production of blood clotting factors and fibrin fabric
The blood collection, processing and transfer by separation of discrete components containing additional citrate (at least about trisodium citrate 9% w / v) in one or other of collection or processing bag provides for enhanced yield and purity of cryoprecipitate. Inhibiting the activation or denaturation of blood components including blood cells and plasma proteins and with the removal of the activated and denatured components thereby improving safety and efficacy of end products. The inventive process is particularly suited to an improved extraction process to yield concentrated clotting factors from single donors or limited pools without use of chromatography. Following extraction the remaining cryoprecipitate can advantageously be formed into a fibrin fabric used in surgeries and in the treatment of wounds
Owner:SHANBROM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com