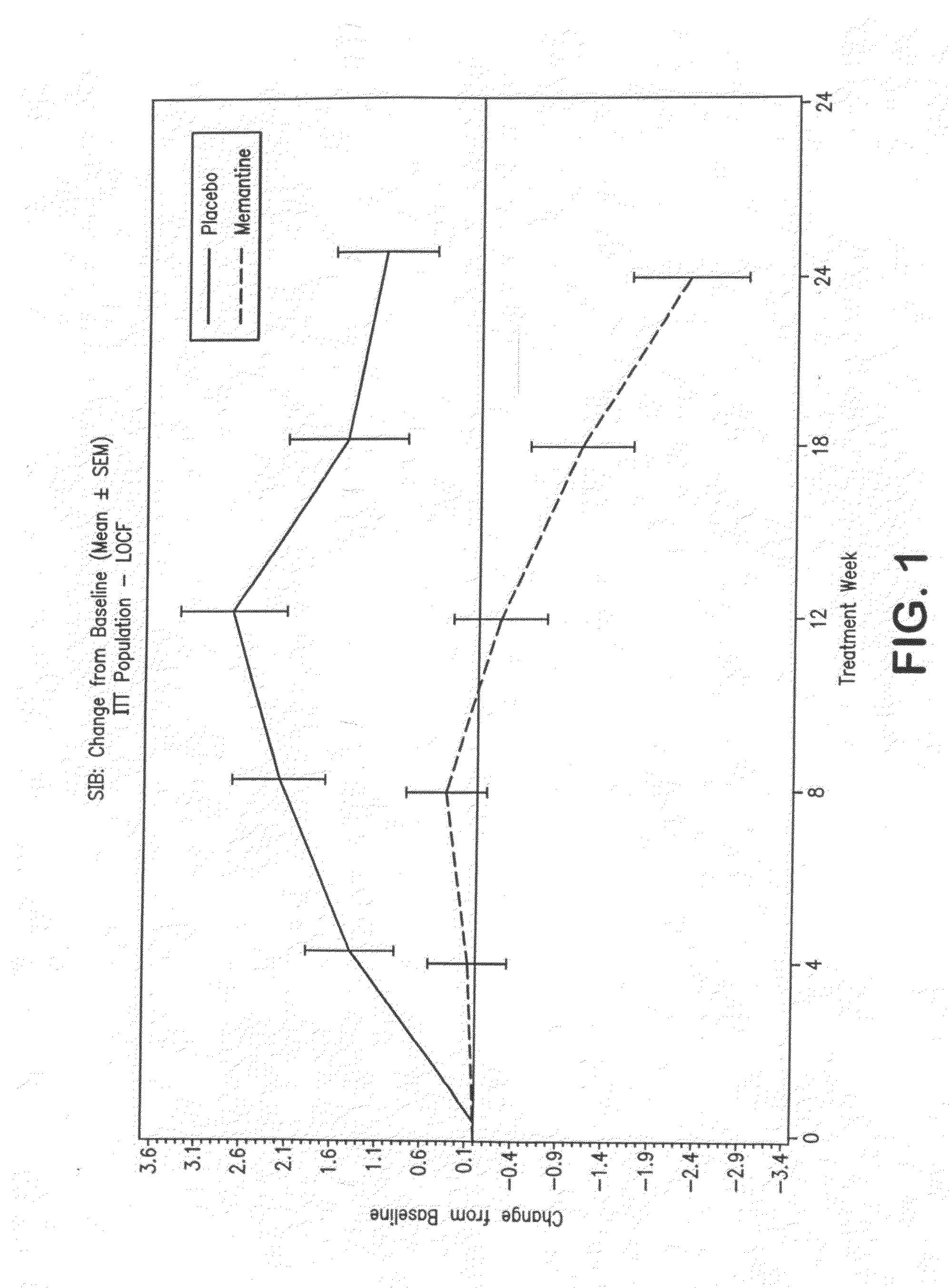
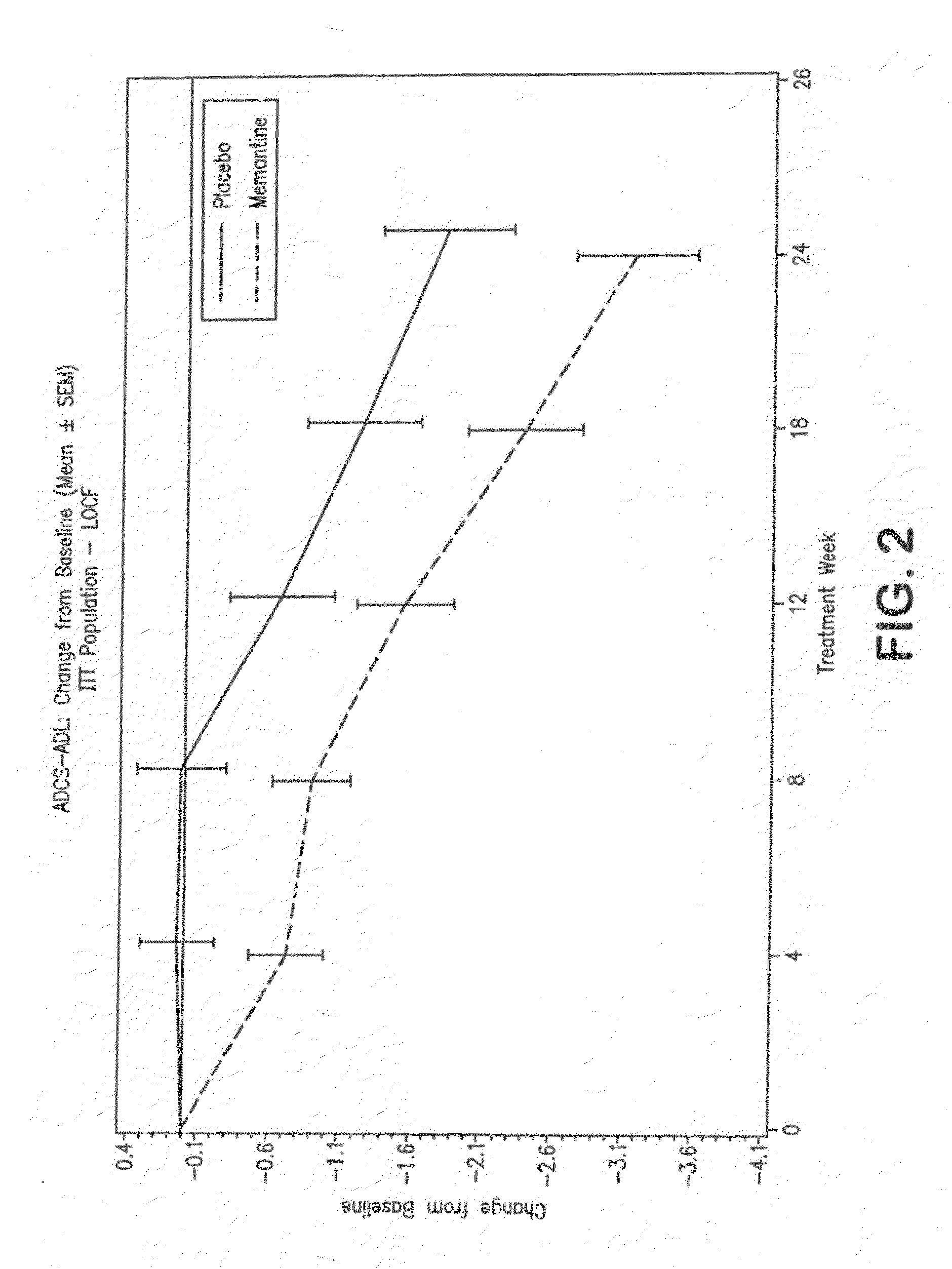
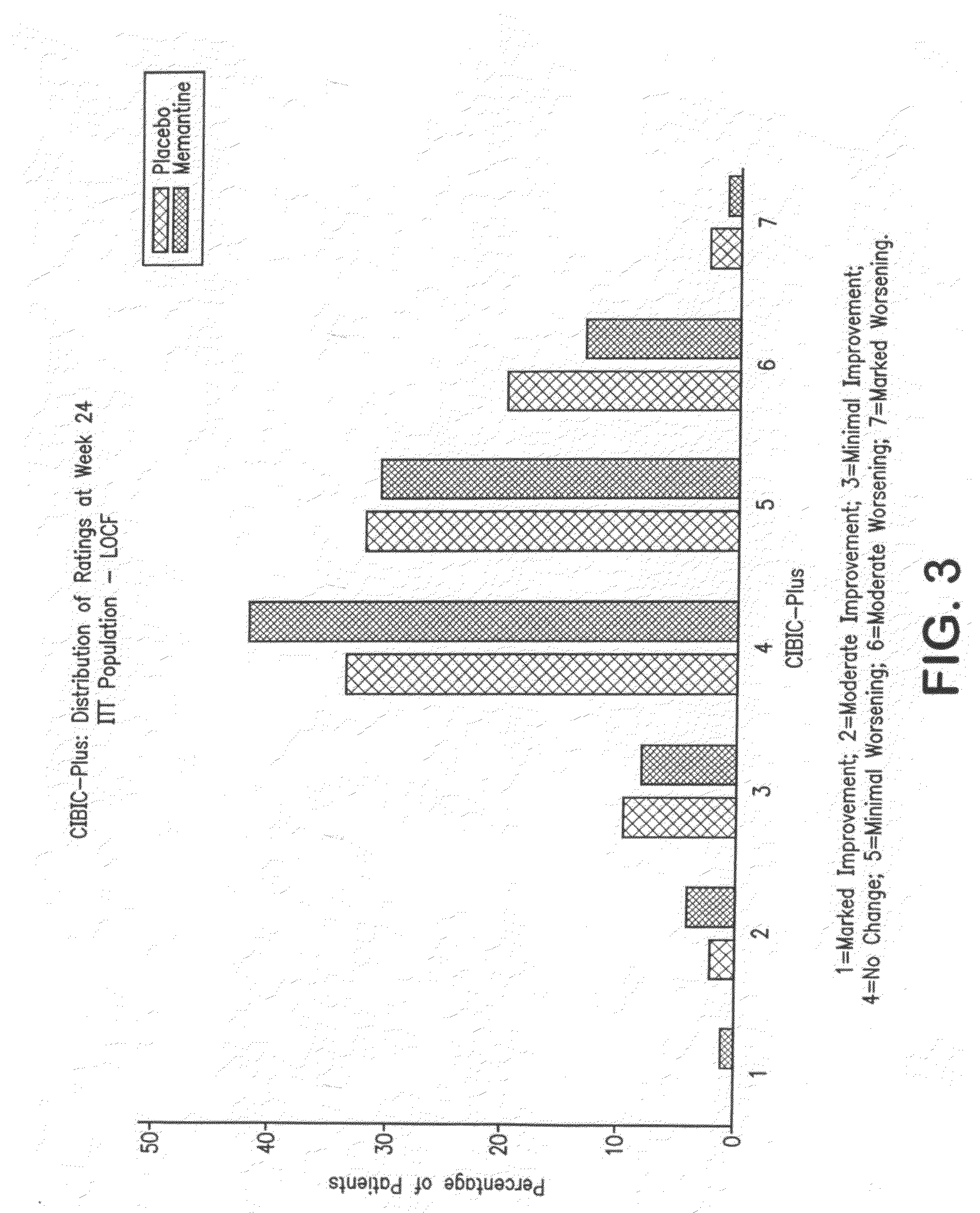
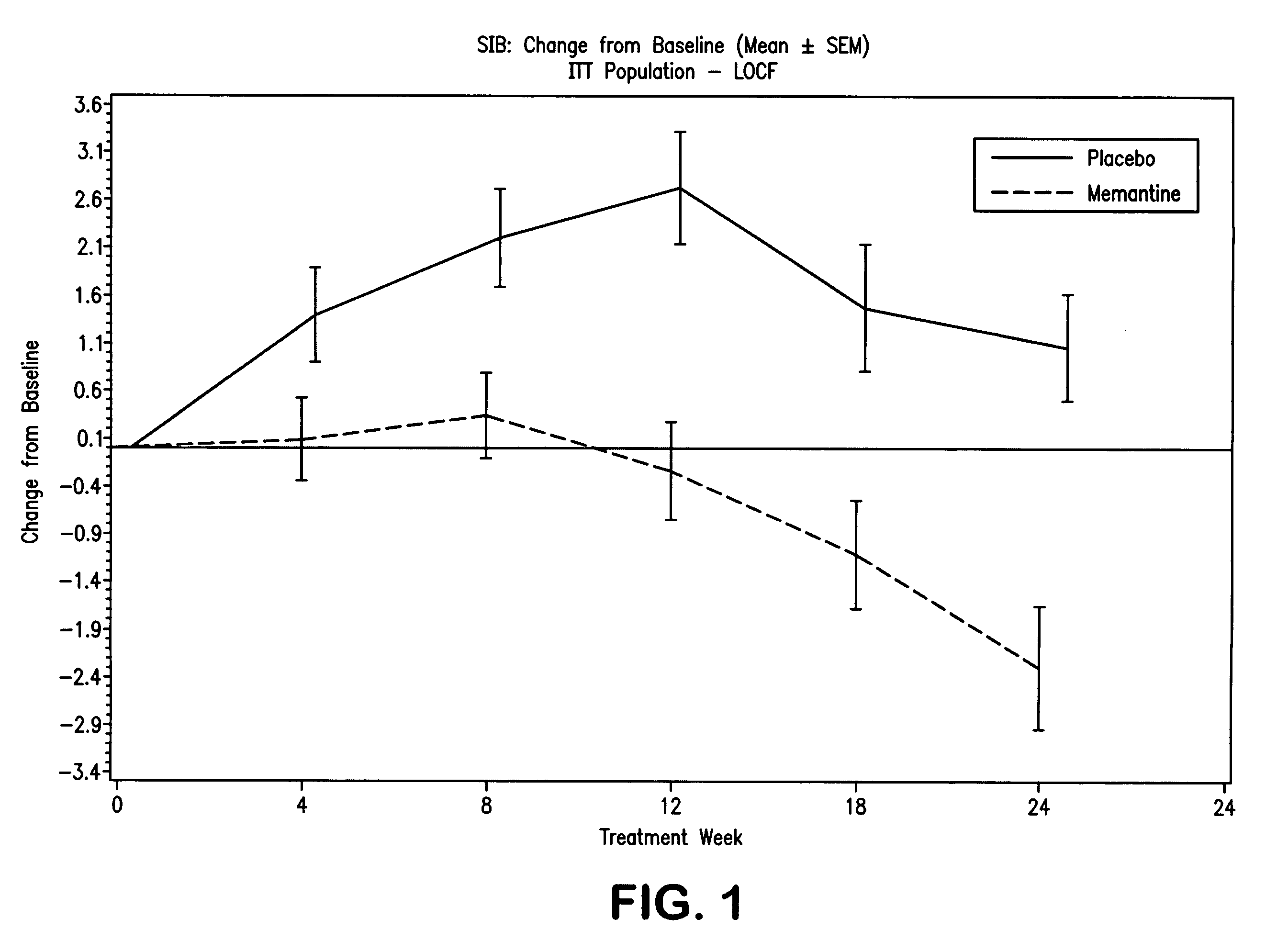
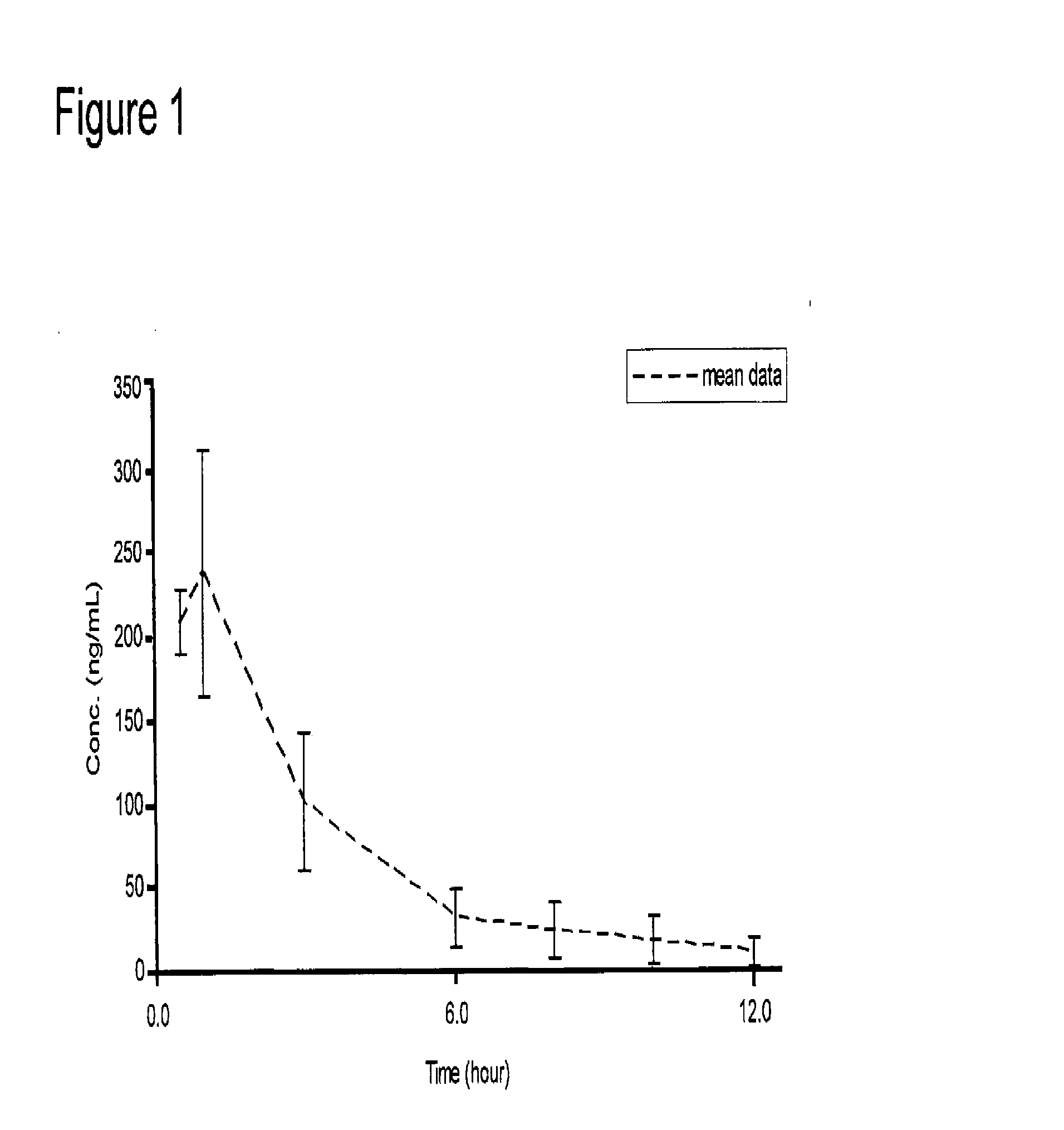
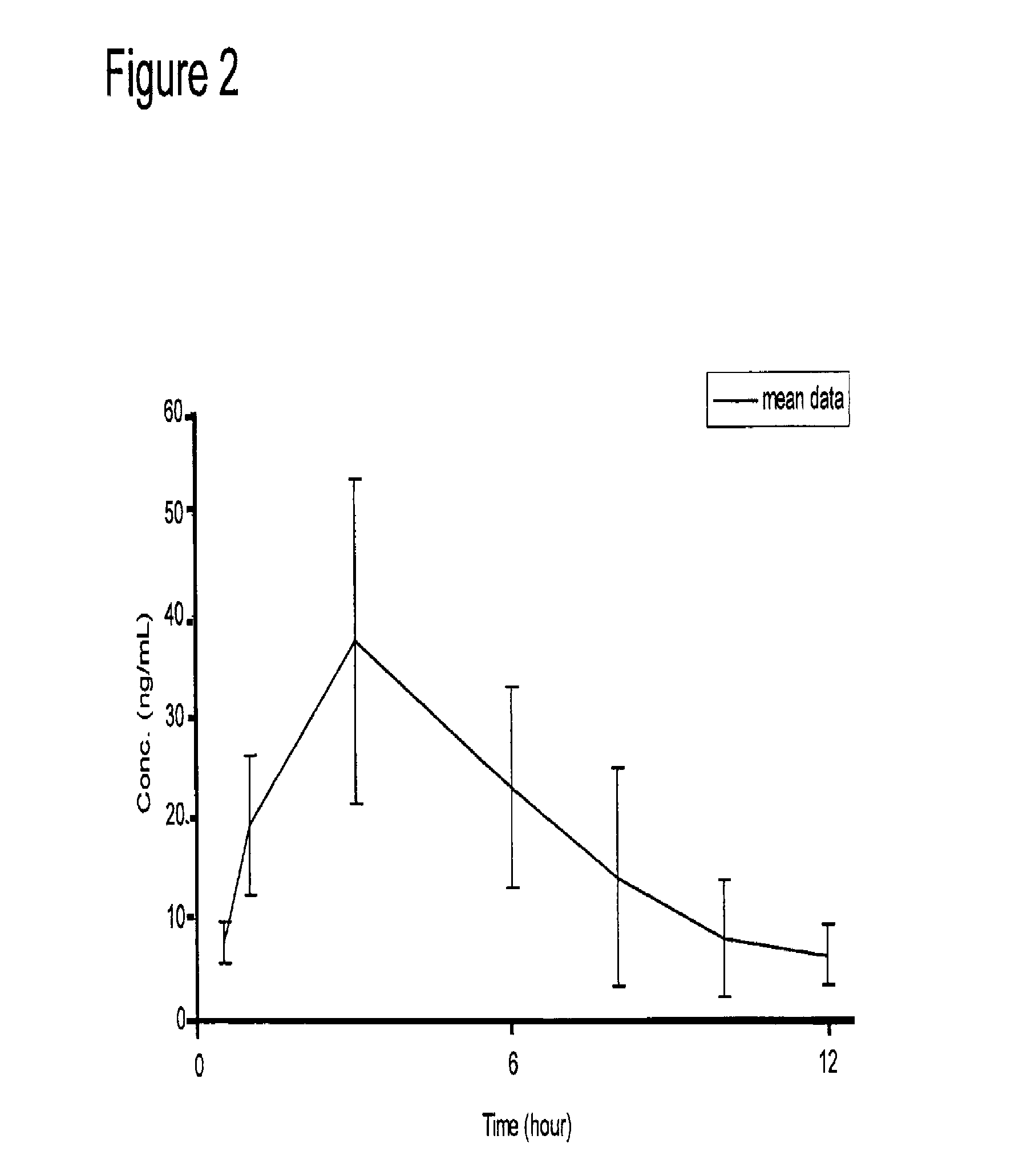
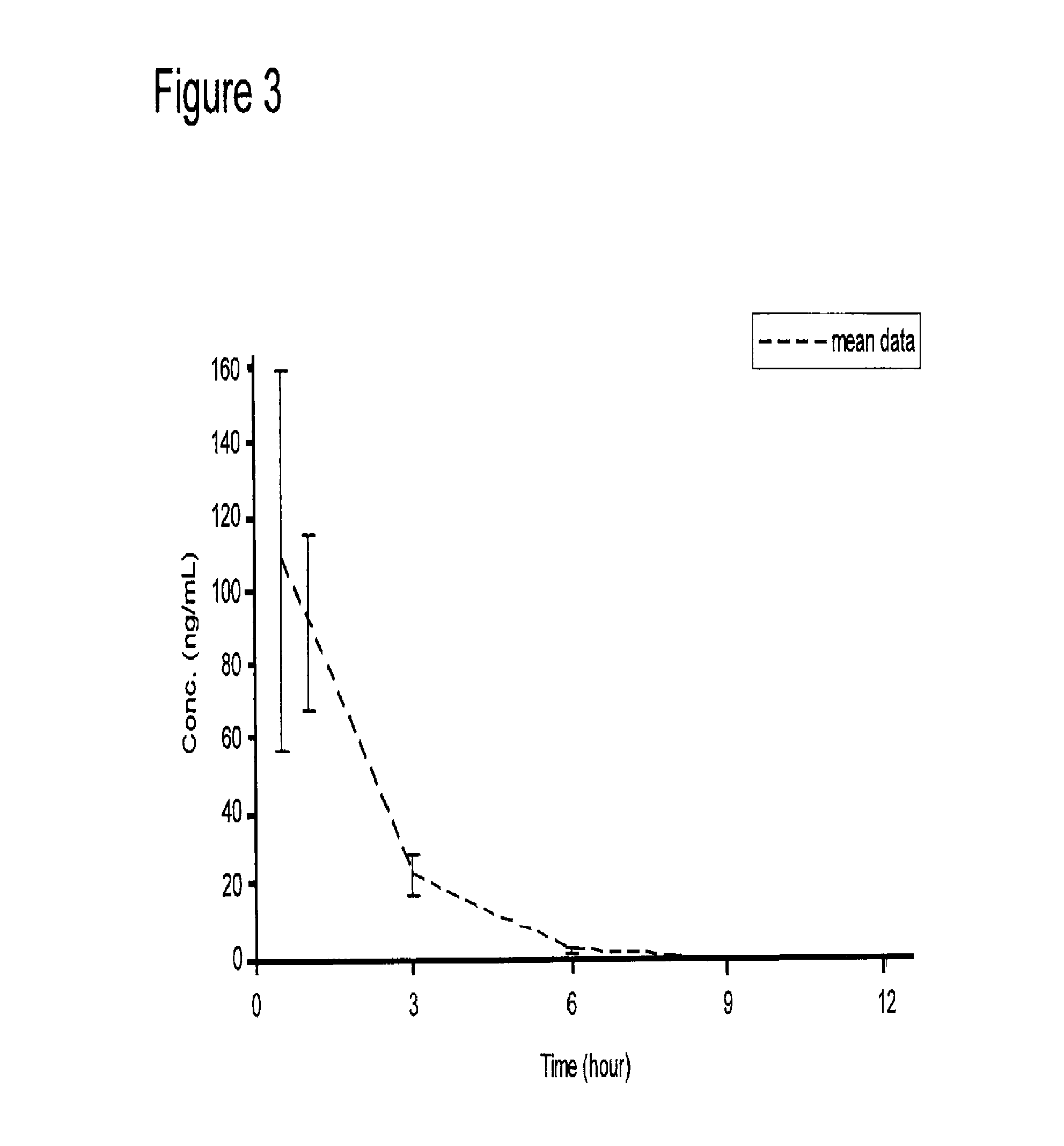
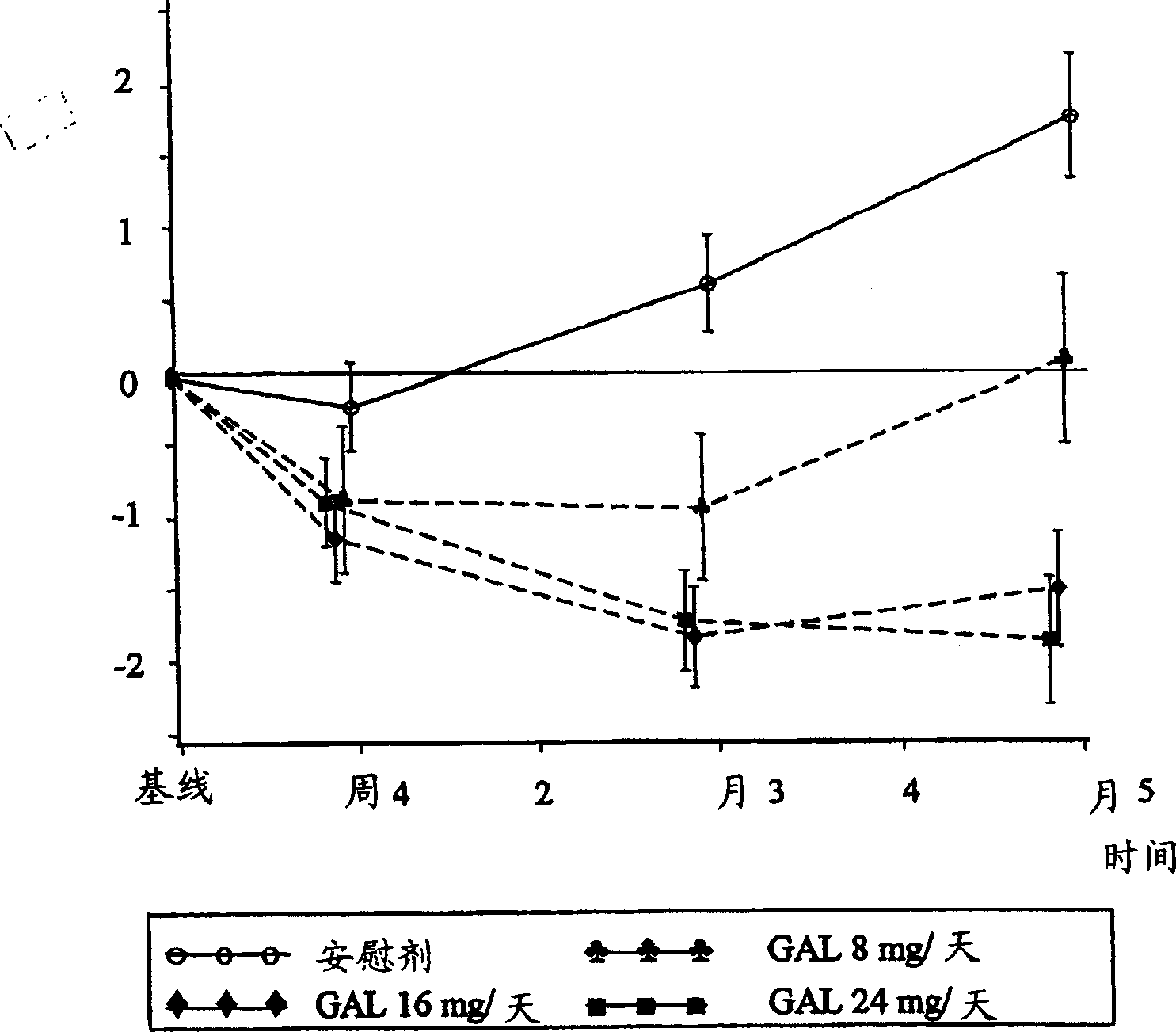
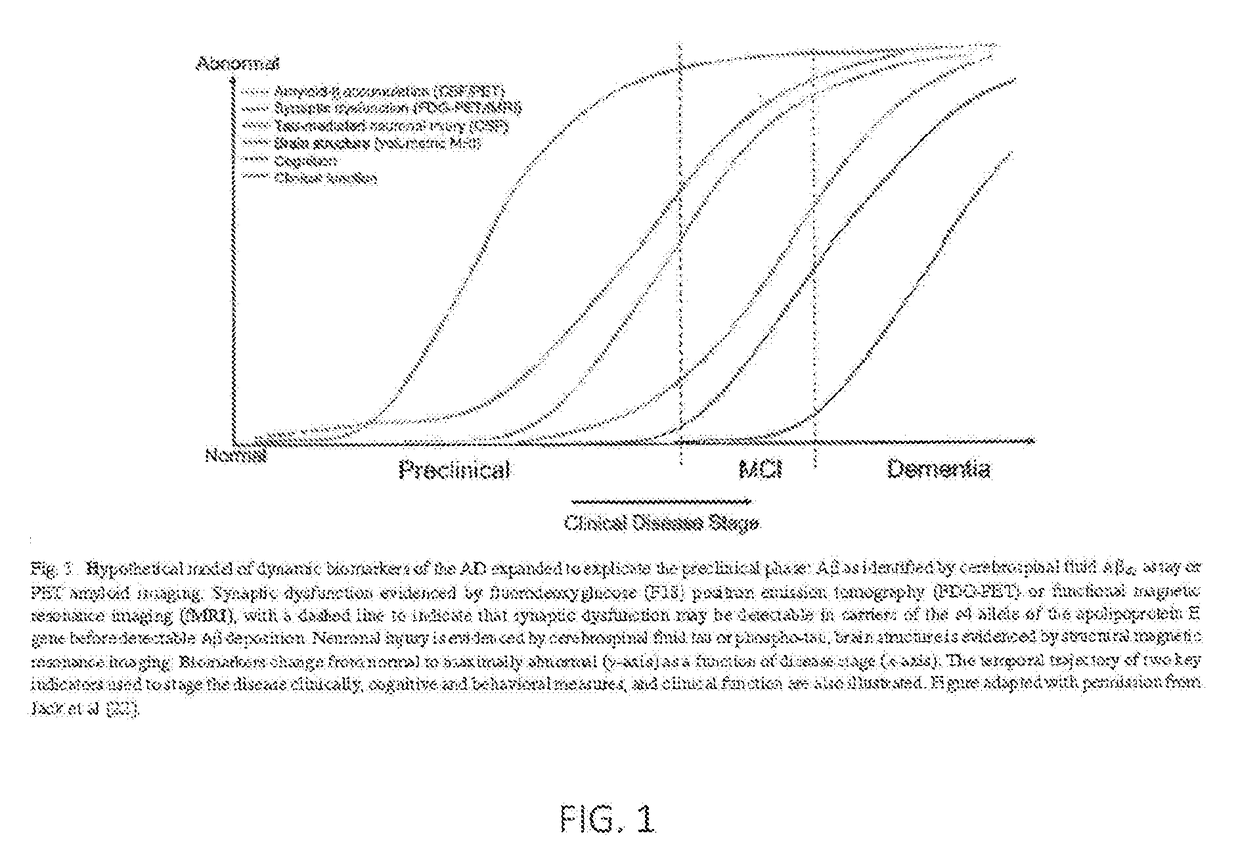
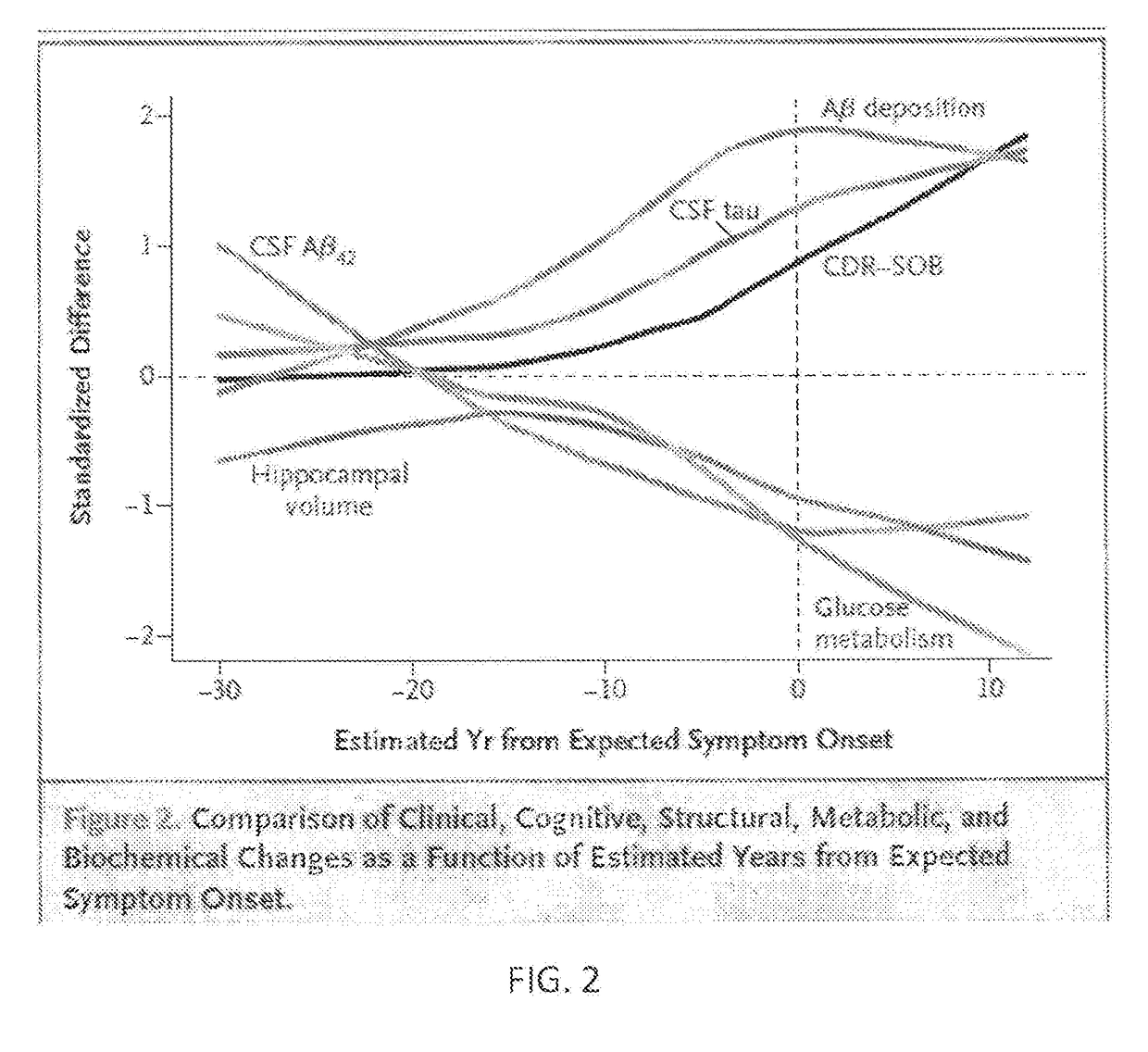
Patents
Literature
70 results about "Galantamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Galantamine is used to treat mild to moderate confusion (dementia) related to Alzheimer's disease.
Galantamine formulations
Galantamine formulations, including sustained-release and fast dissolve formulations, are described.
Owner:ACTAVIS GRP PTC EHF
Compositions and methods using acetylcholinesterase (ACE) inhibitors to treat central nervous system (CNS) disorders in mammals
InactiveUS20060003989A1Reduce deliveryImprove solubilityBiocideNervous disorderSolubilitySide effect
Methods and compositions of the invention employ acetylcholinesterase (ACE) inhibitors to prevent and treat diseases and other disorders of the central nervous system (CNS), including Alzheimer's disease. ACE inhibitors are administered for targeted delivery to the CNS, for example by intranasal delivery. The methods and compositions of the present invention yield therapeutic concentrations of ACE inhibitors in a CNS tissue or compartment without the attendant disadvantages, risks and side effects of oral or injection delivery. Exemplary ACE inhibitors for use within the invention include galantamine and various salts and derivatives of galantamine. Carboxylate salts of galantamine (e.g., galantamine gluconate, galantamine lactate, galantamine citrate and galantamine glucarate) described herein exhibit a significant increase in solubility compared to other forms of galantamine, such as galantamine hydrobromide.
Owner:NASTECH PHARMA
Combination therapy using 1-aminocyclohexane derivatives and acetylcholinesterase inhibitors
InactiveUS20100227852A1Avoid damageReduce riskBiocideNervous disorderCholinesterase inhibitionRivastigmine
Owner:MERZ PHARMA GMBH & CO KGAA
Combination therapy using 1-aminocyclohexane derivatives and acetylcholinesterase and inhibitors
InactiveUS20090124659A1Avoid damageReduce riskBiocideNervous disorderCholinesterase inhibitionDepressant
The invention relates to a novel drug combination therapy useful in the treatment of dementia comprising administering an 1-aminocyclohexane derivative such as memantine or neramexane and an acetylcholinesterase inhibitor (AChEI) such as galantamine, tacrine, donepezil, or rivastigmine.
Owner:MERZ PHARMA GMBH & CO KGAA
Cholinergic enhancers with improved blood-brain barrier permeability for the treatment of diseases accompanied by cognitive impairment
InactiveUS20090253654A1Improve efficacyLow peripheral side effectBiocideGroup 5/15 element organic compoundsChemical structureChemical synthesis
The present invention refers to compounds that, in addition to enhancing the sensitivity to acetylcholine and choline, and their exogenous agonists, of neuronal cholinergic receptors and / or acting as cholinesterase inhibitors and / or neuroprotective agents, have enhanced blood-brain barrier permeability in comparison to their parent compounds. The compounds are derived (either formally by their chemical structure or directly by chemical synthesis) from natural compounds belonging to the class of amaryllidaceae alkaloids e.g., galantamine, narwedine and lycoramine, or from metabolites of said compounds. The compounds of the present invention can either interact as such with their target molecules, or they can act as “pro-drugs”, in the sense that after reaching their target regions in the body they are converted by hydrolysis or enzymatic attack to the original parent compound and react as such with their target molecules, or both. The compounds of this invention may be used as medicaments.
Owner:GALANTOS PHARMA
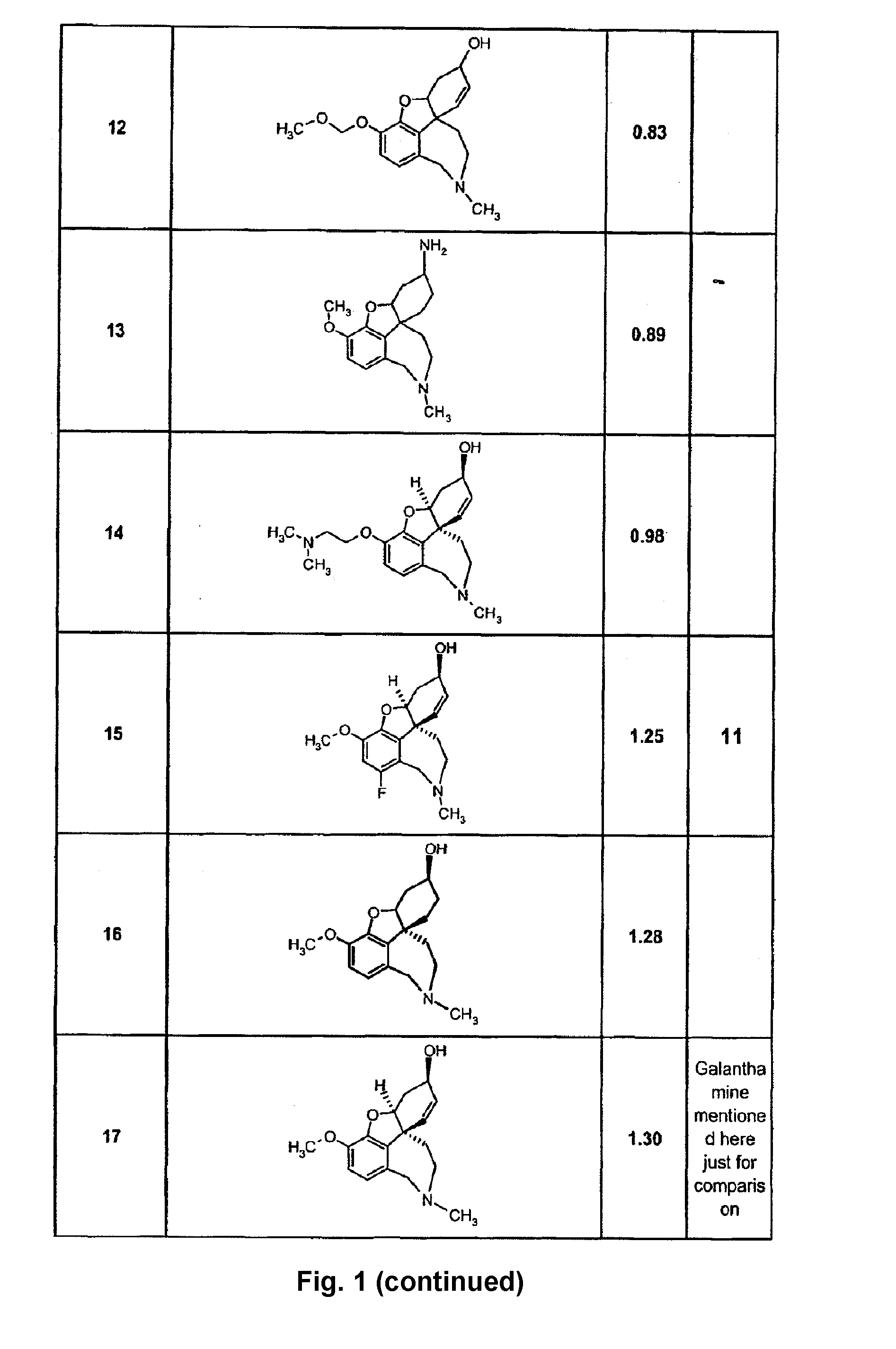
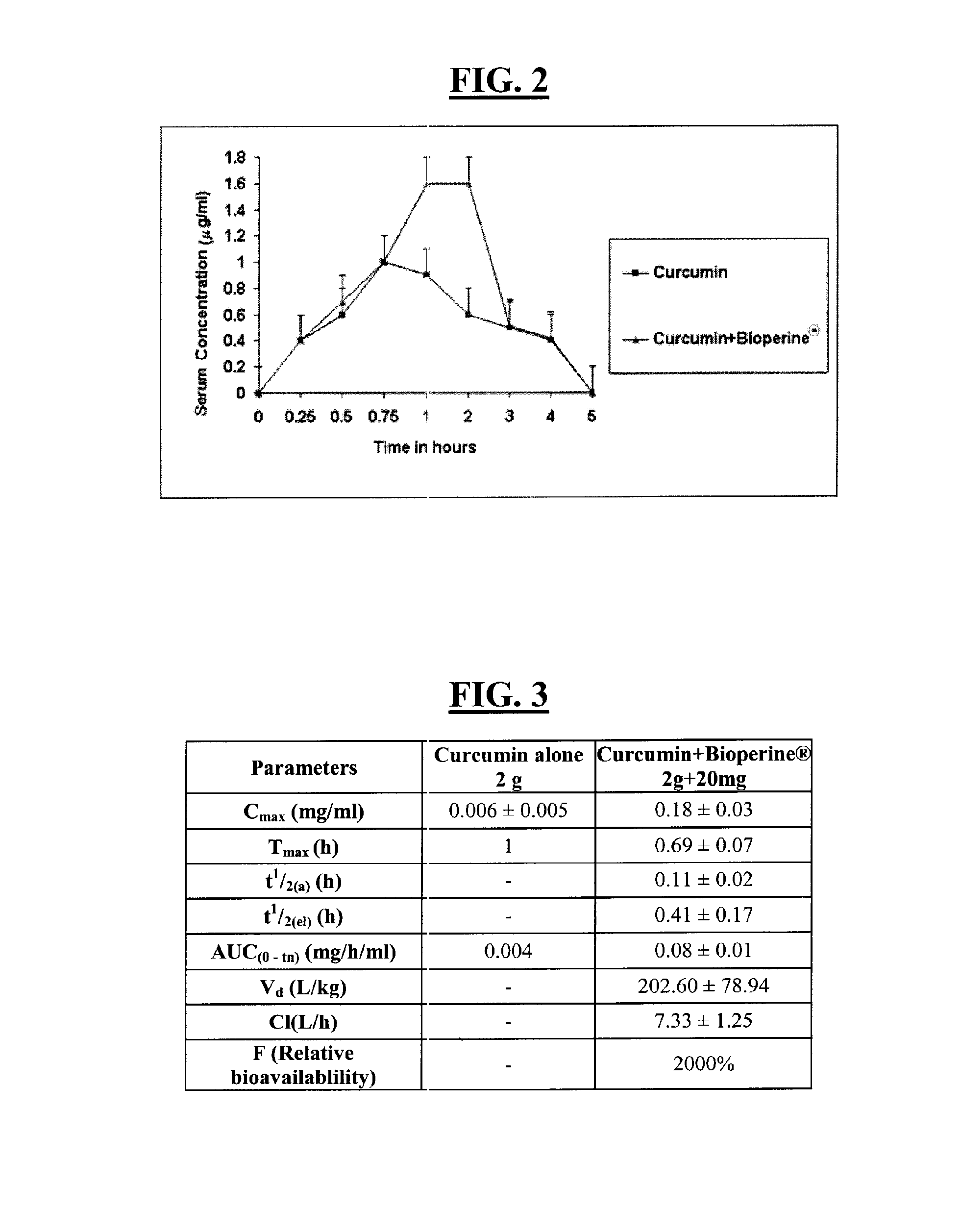
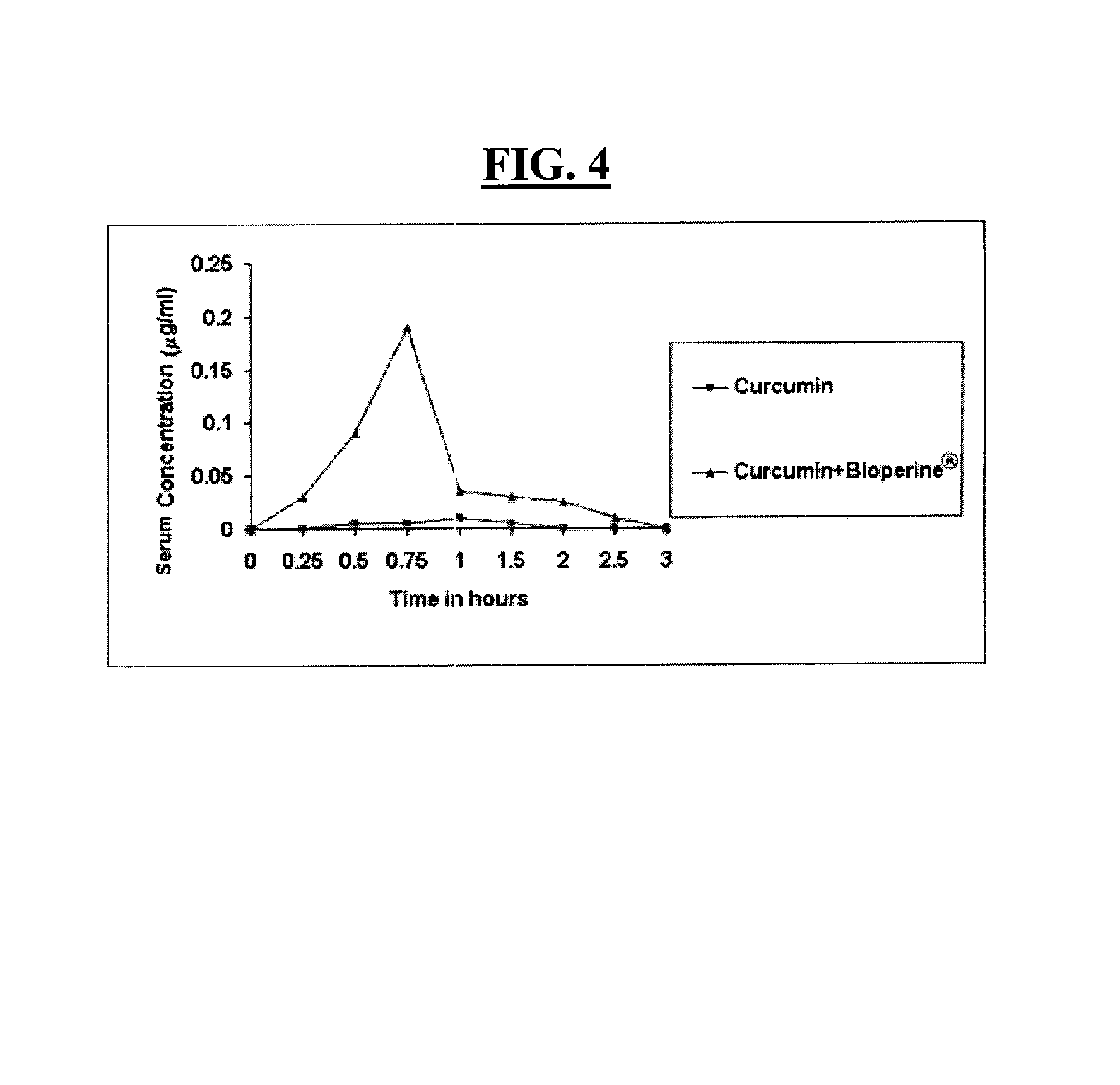
Composition to retard the onset of symptoms of alzheimer's disease
A composition and a method for using the composition to delay the onset of the symptoms of Alzheimer's disease in humans, comprising curcumin, piperine, oleic acid, oleanolic acid, ursolic acid, galantamine, and huperzine A, among other compounds. Curcumin is an antioxidant, while galantamine and huperzine A inhibit the activity of acetylcholinesterase in the brain. Piperine and oleic acid increase the bioavailability and gastrointestinal absorption of curcumin, galantamine, huperzine A, and other nutrients.
Owner:CONSEAL INT
Cholinesterase Inhibitors In Liposomes And Their Production And Use
InactiveUS20080031935A1Avoid intakeImprove bioavailabilityBiocideNervous disorderEnantiomerRivastigmine
The invention relates to a pharmaceutical composition based on an active ingredient that is enclosed in liposomes for topical, transdermal application. The interior of said liposomes comprises an acidic, aqueous medium containing at least one cholinesterase inhibitor, preferably from the group containing donepezil, rivastigmine, galantamine, physostigmine, heptylphysostigmine, phenserine, tolserine, cymserine, thiatolserine, thiacymserine, neostigmine, huperzine, tacrine, metrifonate and dichlorvos, or an enantiomer or derivative of at least one of said compounds. In addition, the invention relates to a method for producing said composition, optionally in a sterile form and also to the use of the liposomes charged with the active ingredient in various galenic formulations for topical, transdermal application with a depot effect in the epidermis, for the prophylaxis and / or treatment of cutaneous neuropathic pain or the loss of cutaneous sensory function as a result of neuropathy.
Owner:SANOCHEMIA PHARMA AG
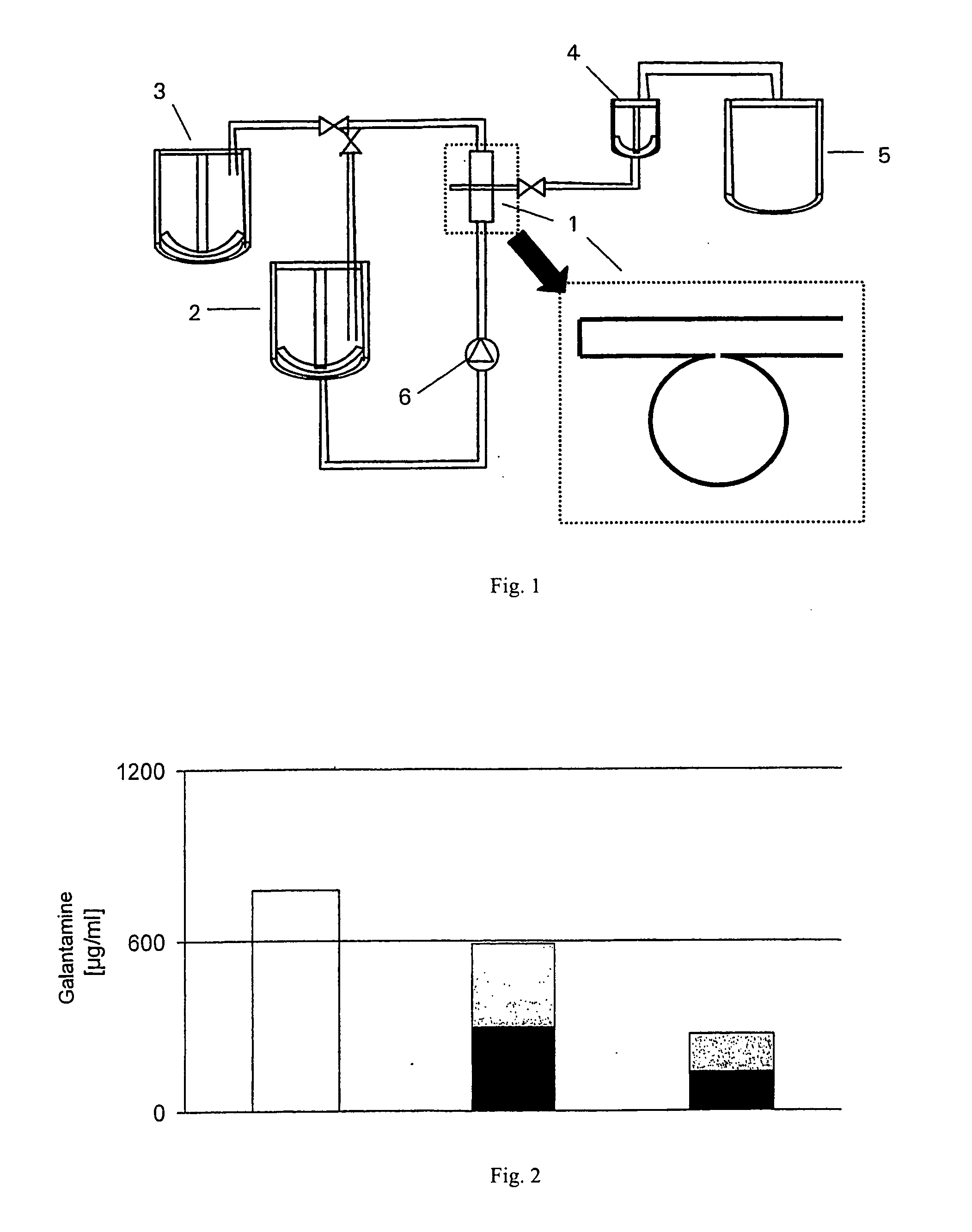
Controlled-release galantamine formulations
Controlled-release galantamine formulations, including controlled-release particles, pellets, granules, and spheres are described. Controlled-release particles, pellets, granules, and spheres with immediate release top-coat are also described. Method of preparing such formulations and method of treating a variety of disorders are also disclosed.
Owner:ACTAVIS GRP PTC EHF
Pharmaceutical compositions and methods for treating Alzheimer's disease
A pharmaceutical composition containing the therapeutically active ingredients of azelastine or a pharmaceutically acceptable salt of azelastine and donepezil or rivastigmine or galantamine or a pharmaceutically acceptable salt of thereof is disclosed. A method of using the pharmaceutical composition for treating patients suffering from mental, behavioral, cognitive disorders is also disclosed.
Owner:LA PHARMATECH INC
Galantamine formulations
Galantamine formulations substantially free of microcrystalline cellulose, lactose, and / or starch are described.
Owner:ACTAVIS GRP PTC EHF
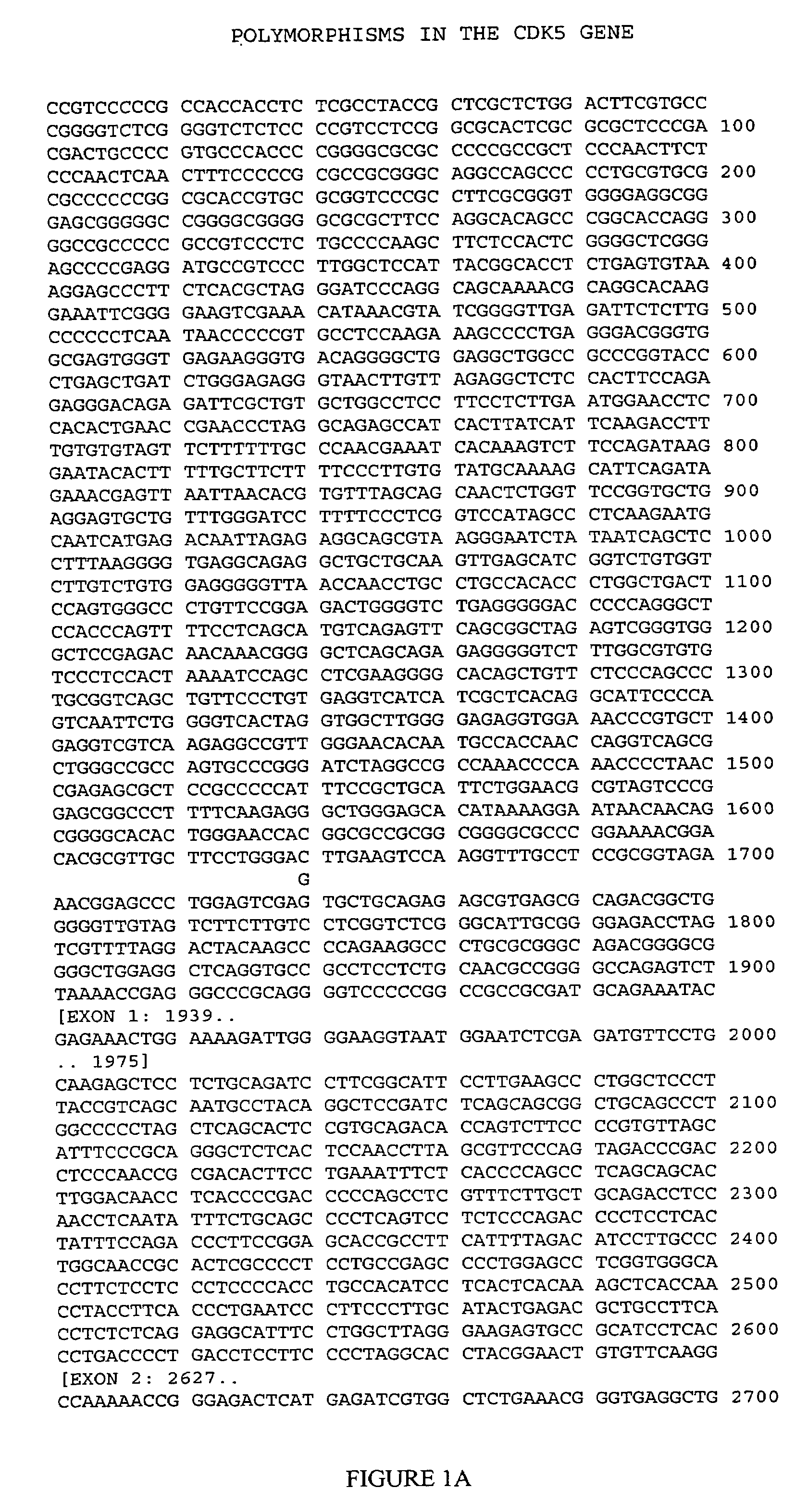
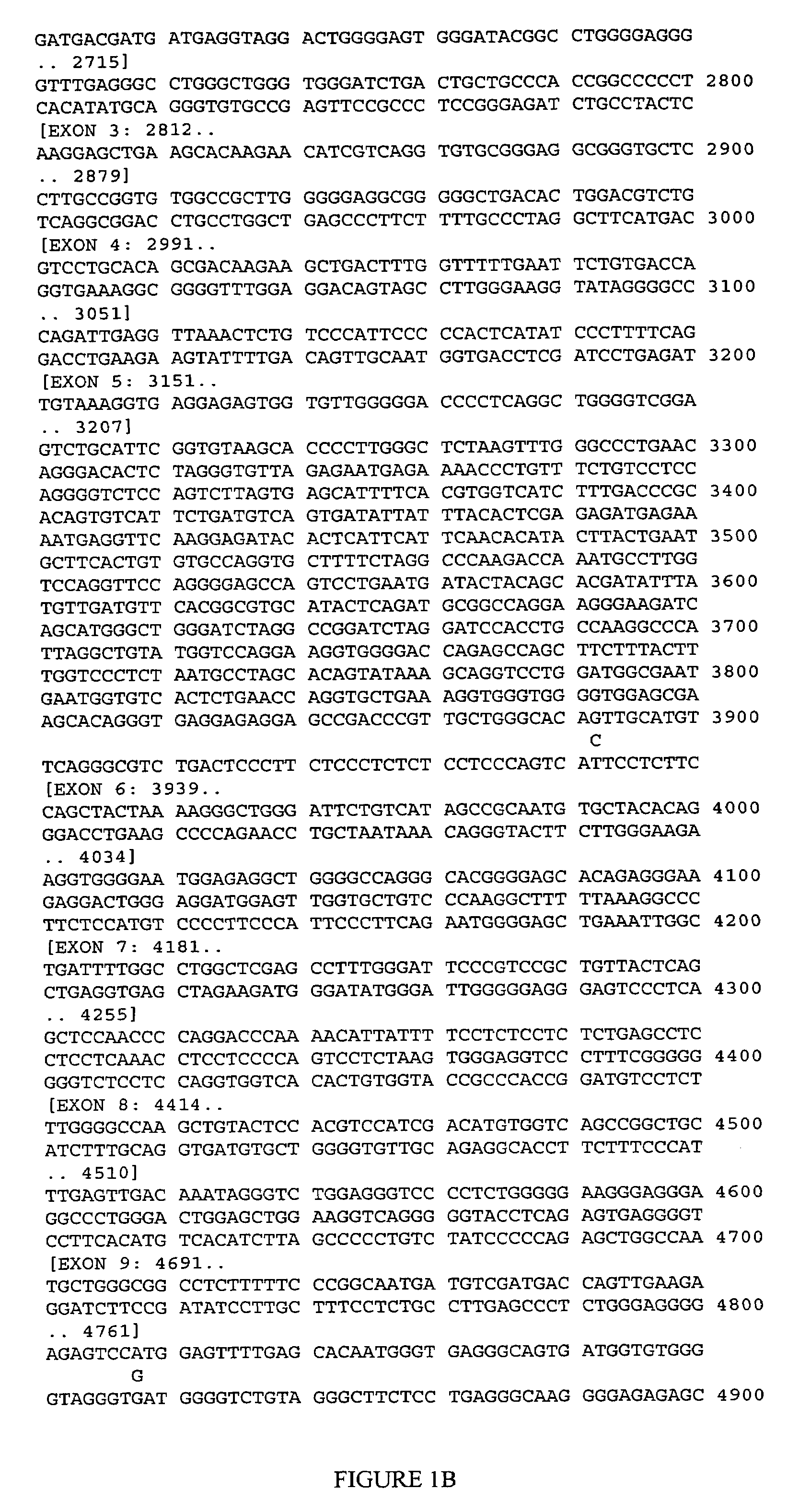
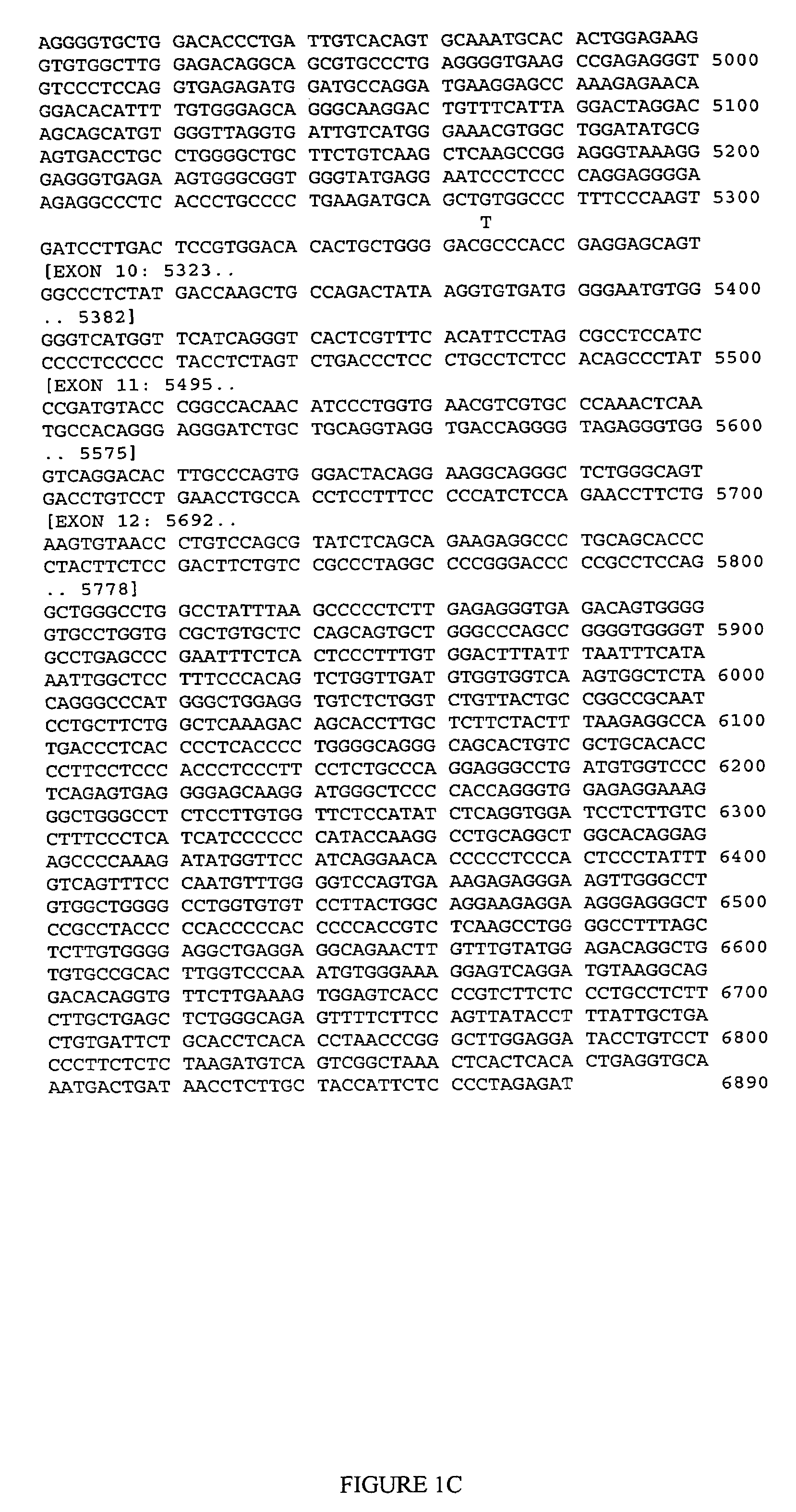
CDK5 genetic markers associated with galantamine response
InactiveUS20050255494A1Improve cognitive functionBioreactor/fermenter combinationsBiological substance pretreatmentsHaplotypeCognitive response
Haplotypes in the CDK5 gene associated with cognitive response to galantamine treatment are disclosed. Compositions and methods for detecting and using these CDK5 haplotypes in a variety of clinical applications are disclosed. Such applications include articles of manufacture comprising galantamine or derivatives thereof that are approved for treating patients having one of these CDK5 haplotypes, methods and kits for predicting the response of an individual to galantamine based upon his / her haplotype profile, and methods for treating Alzheimer's patients based upon their haplotype profile.
Owner:PGXHEALTH
Cholinergic enhancers with improved blood-brain barrier permeability for the treatment of diseases accompanied by cognitive impairment
ActiveUS20130210808A1Improve efficacyEliminate side effectsBiocideGroup 5/15 element organic compoundsChemical synthesisChemical structure
The present invention refers to compounds that, in addition to enhancing the sensitivity to acetylcholine and choline, and their exogenous agonists, of neuronal cholinergic receptors and / or acting as cholinesterase inhibitors and / or neuroprotective agents, have enhanced blood-brain barrier permeability in comparison to their parent compounds. The compounds are derived (either formally by their chemical structure or directly by chemical synthesis) from natural compounds belonging to the class of amaryllidaceae alkaloids e.g., galantamine, narwedine and lycoramine, or from metabolites of said compounds. The compounds of the present invention can either interact as such with their target molecules, or they can act as “pro-drugs”, in the sense that after reaching their target regions in the body they are converted by hydrolysis or enzymatic attack to the original parent compound and react as such with their target molecules, or both. The compounds of this invention may be used as medicaments.
Owner:NEURODYN LIFE SCI
Galantamine tablet formulation
The present invention relates to a direct compression tablet formulation comprising, as the active ingredient, galantamine, and more specifically, galantamine hydrobromide along with a process of making the same. The tablet formulation is a direct compression tablet and has excellent content uniformity and dissolution properties.
Owner:ROXANE LAB
Application of puerarin or medicinal composition containing puerarin in preparation of medicament with effect of preventing or treating senile dementia
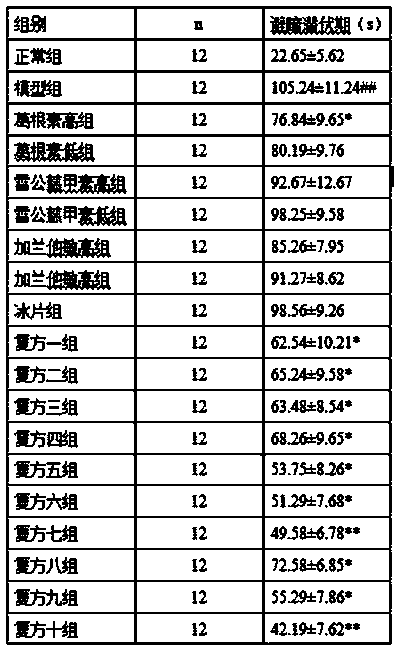
ActiveCN103520147AAvoid depositionDelay disease progressionNervous disorderHydroxy compound active ingredientsSide effectMechanism of action
The invention discloses application of puerarin or a medicinal composition containing the puerarin in preparation of a medicament with an effect of preventing or treating senile dementia. The puerarin can be singly used as a medicament or can be used together with other senile dementia medicaments with different mechanisms of action when used for treating or preventing the senile dementia of people or mammals, wherein the puerarin not only can reduce side or toxic effects of medicaments but also can achieve an effective of collaborative treatment when used for treating the senile dementia together with a medicinal composition consisting of triptolide, galantamine and borneol. The puerarin can significantly inhibit the deposition of beta-amyloid, and has a definite curative effect and small side effects when used for preventing and treating the senile dementia, so that the puerarin has a wide medical application prospect.
Owner:INFINITUS (CHINA) CO LTD
CDK5 genetic markers associated with galantamine response
InactiveUS7250258B2Bioreactor/fermenter combinationsBiological substance pretreatmentsHaplotypeCognitive response
Haplotypes in the CDK5 gene associated with cognitive response to galantamine treatment are disclosed. Compositions and methods for detecting and using these CDK5 haplotypes in a variety of clinical applications are disclosed. Such applications include articles of manufacture comprising galantamine or derivatives thereof that are approved for treating patients having one of these CDK5 haplotypes, methods and kits for predicting the response of an individual to galantamine based upon his / her haplotype profile, and methods for treating Alzheimer's patients based upon their haplotype profile.
Owner:PGXHEALTH
CHRNA2 genetic markers associated with galantamine response
InactiveUS20050048543A1Improve cognitive functionNervous disorderMicrobiological testing/measurementHaplotypeCognitive response
Haplotypes in the CHRNA2 gene associated with cognitive response to galantamine treatment are disclosed. Compositions and methods for detecting and using these CHRNA2 haplotypes in a variety of clinical applications are disclosed. Such applications include articles of manufacture comprising galantamine or derivatives thereof that are approved for treating patients having one of these CHRNA2 haplotypes, methods and kits for predicting the response of an individual to galantamine based upon his / her haplotype profile, and methods for treating Alzheimer's patients based upon their haplotype profile.
Owner:PGXHEALTH
EPHX2 Genetic markers associated with galantamine
InactiveUS20050250118A1Improve cognitive functionSugar derivativesMicrobiological testing/measurementEPHX2 geneHaplotype
Haplotypes in the EPHX2 gene associated with cognitive response to galantamine treatment are disclosed. Compositions and methods for detecting and using these EPHX2 haplotypes in a variety of clinical applications are disclosed. Such applications include articles of manufacture comprising galantamine or derivatives thereof that are approved for treating patients having one of these EPHX2 haplotypes, methods and kits for predicting the response of an individual to galantamine based upon his / her haplotype profile, and methods for treating Alzheimer's patients based upon their haplotype profile.
Owner:PGXHEALTH
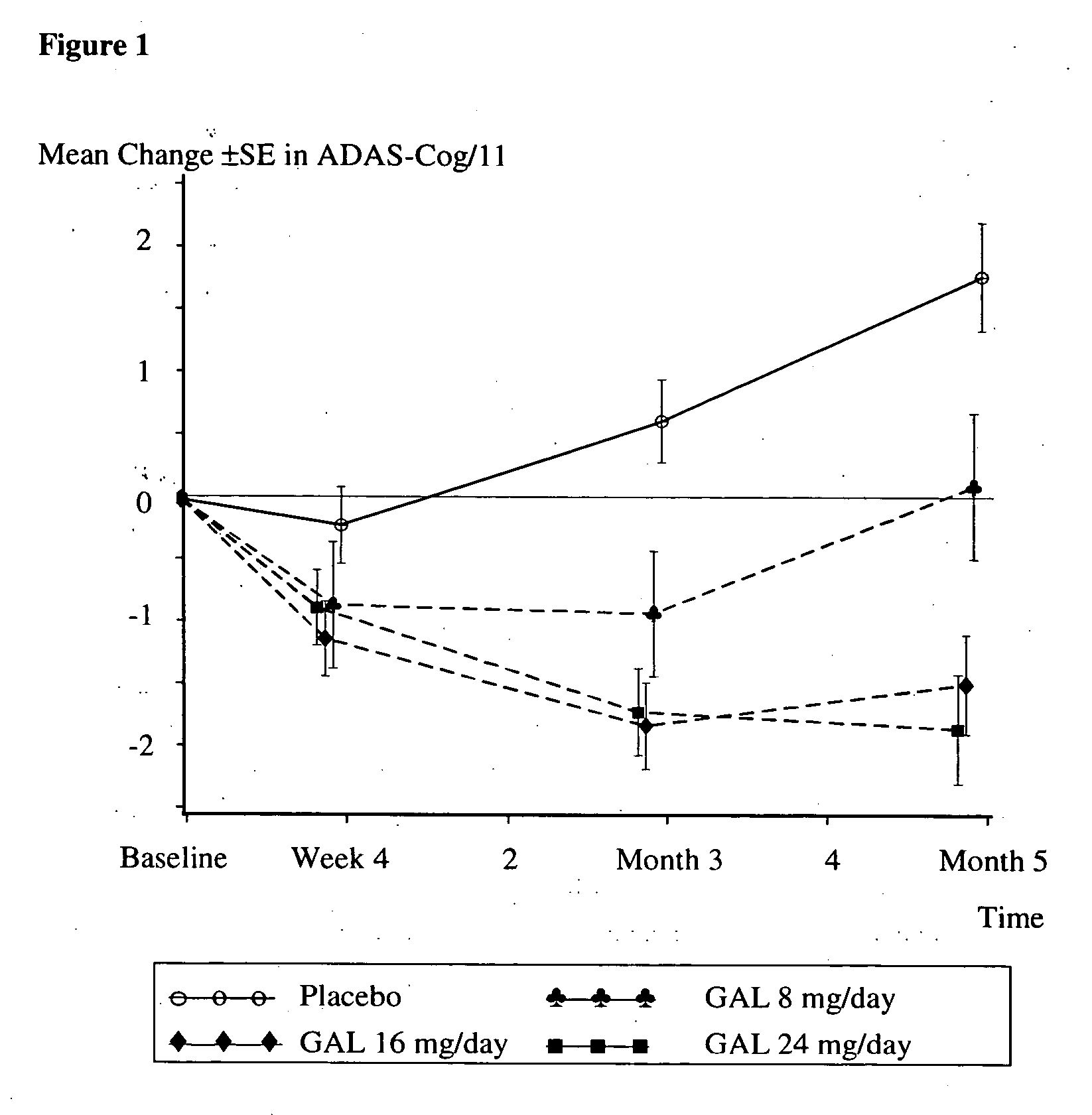
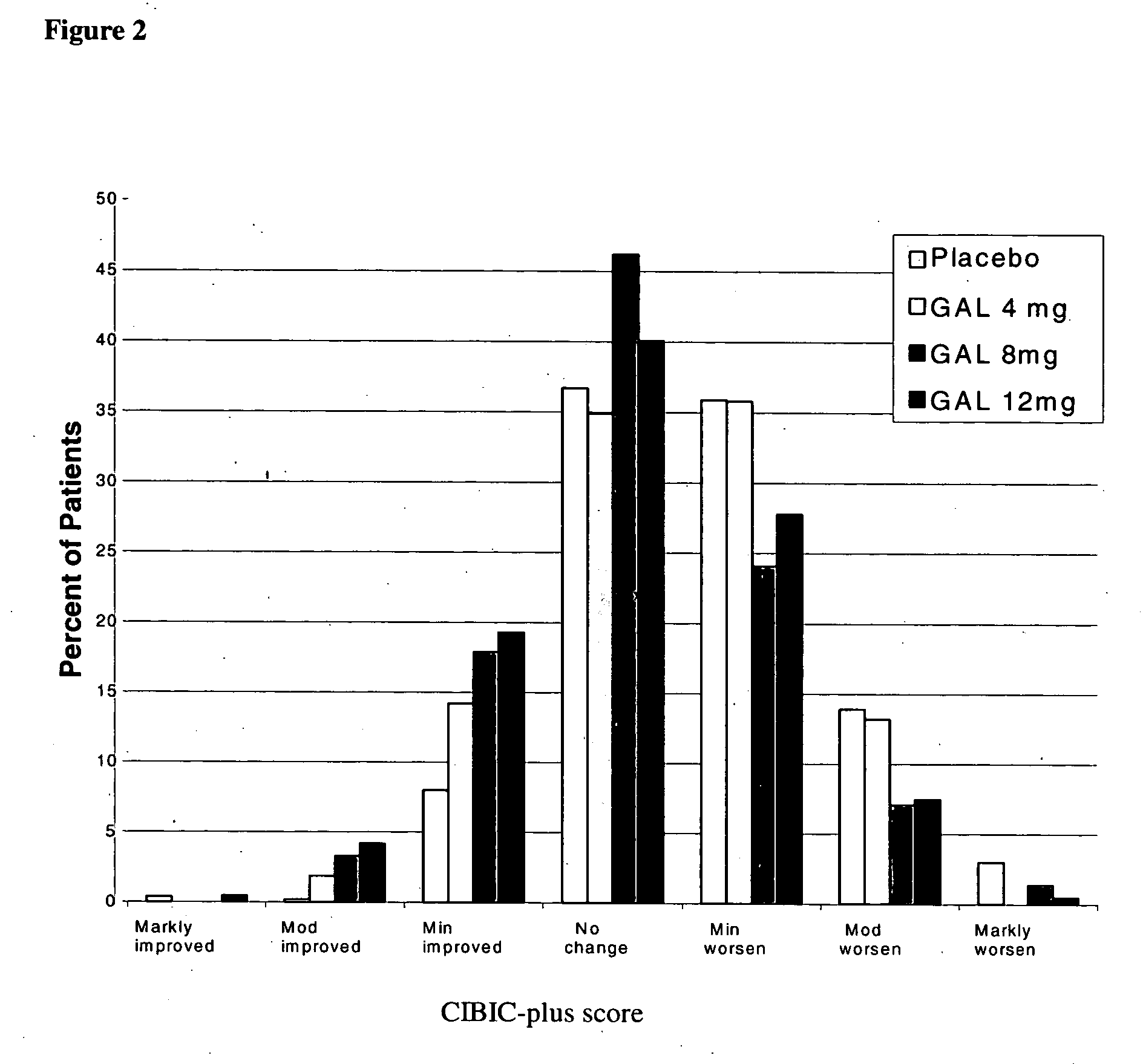
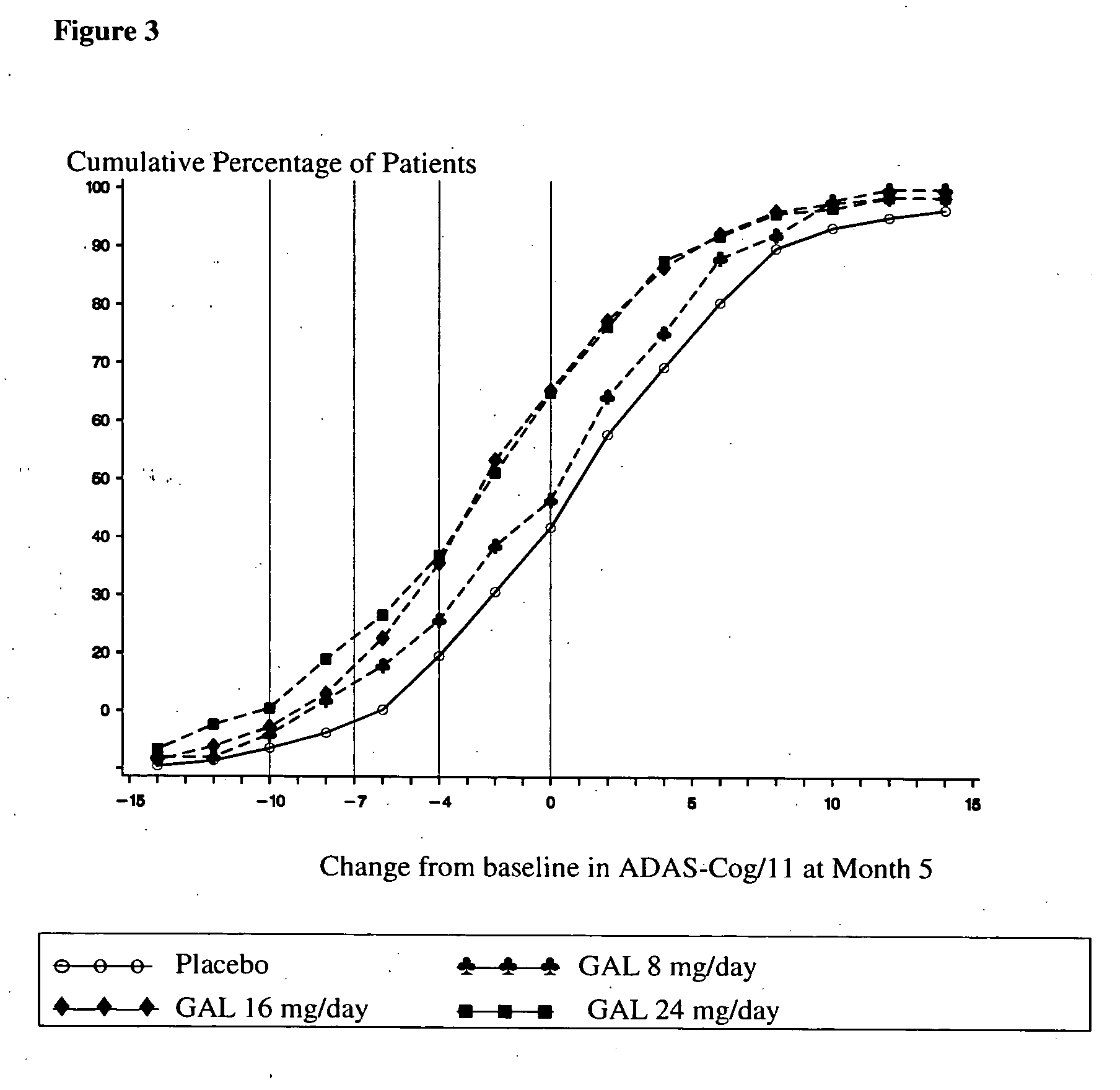
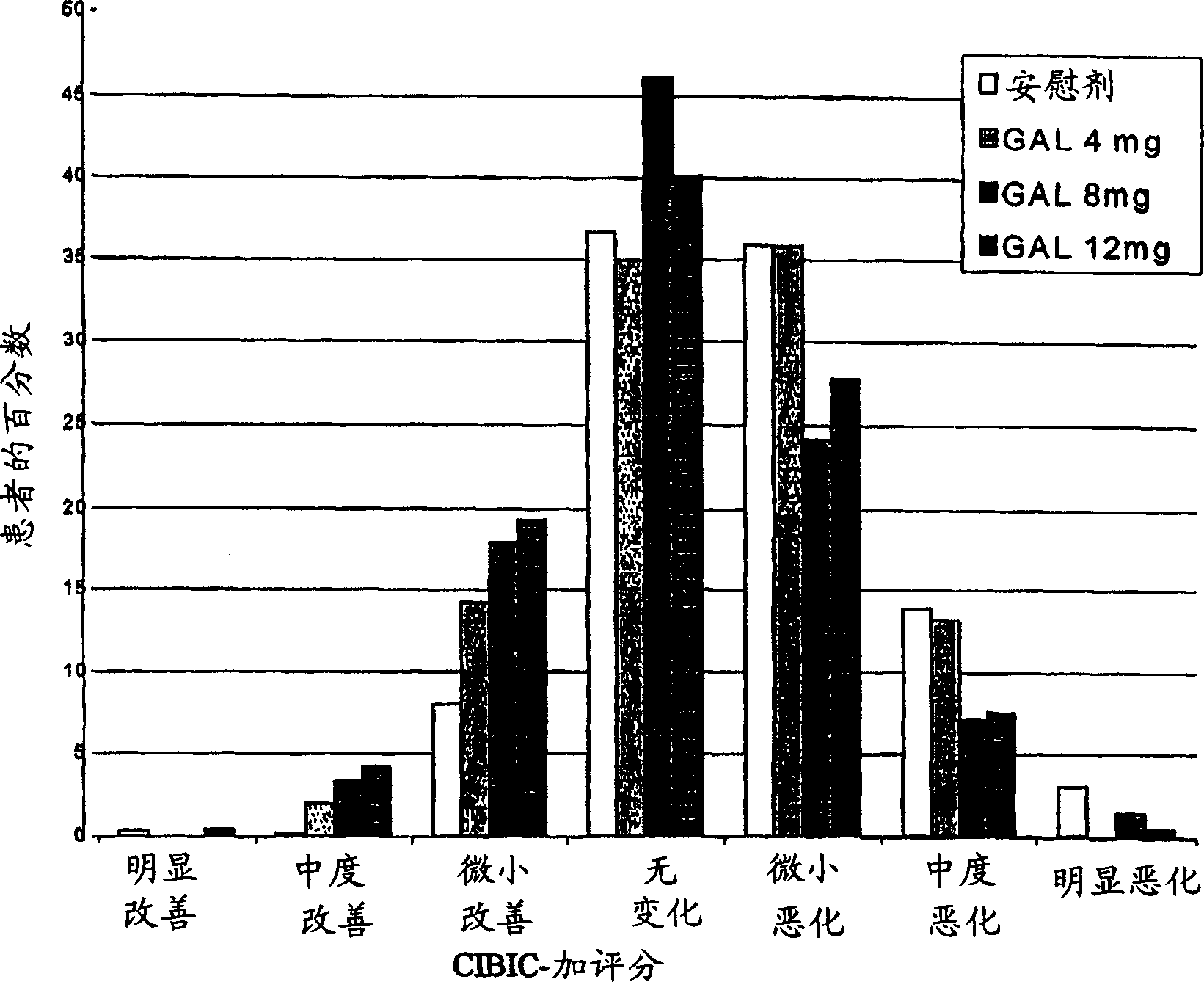
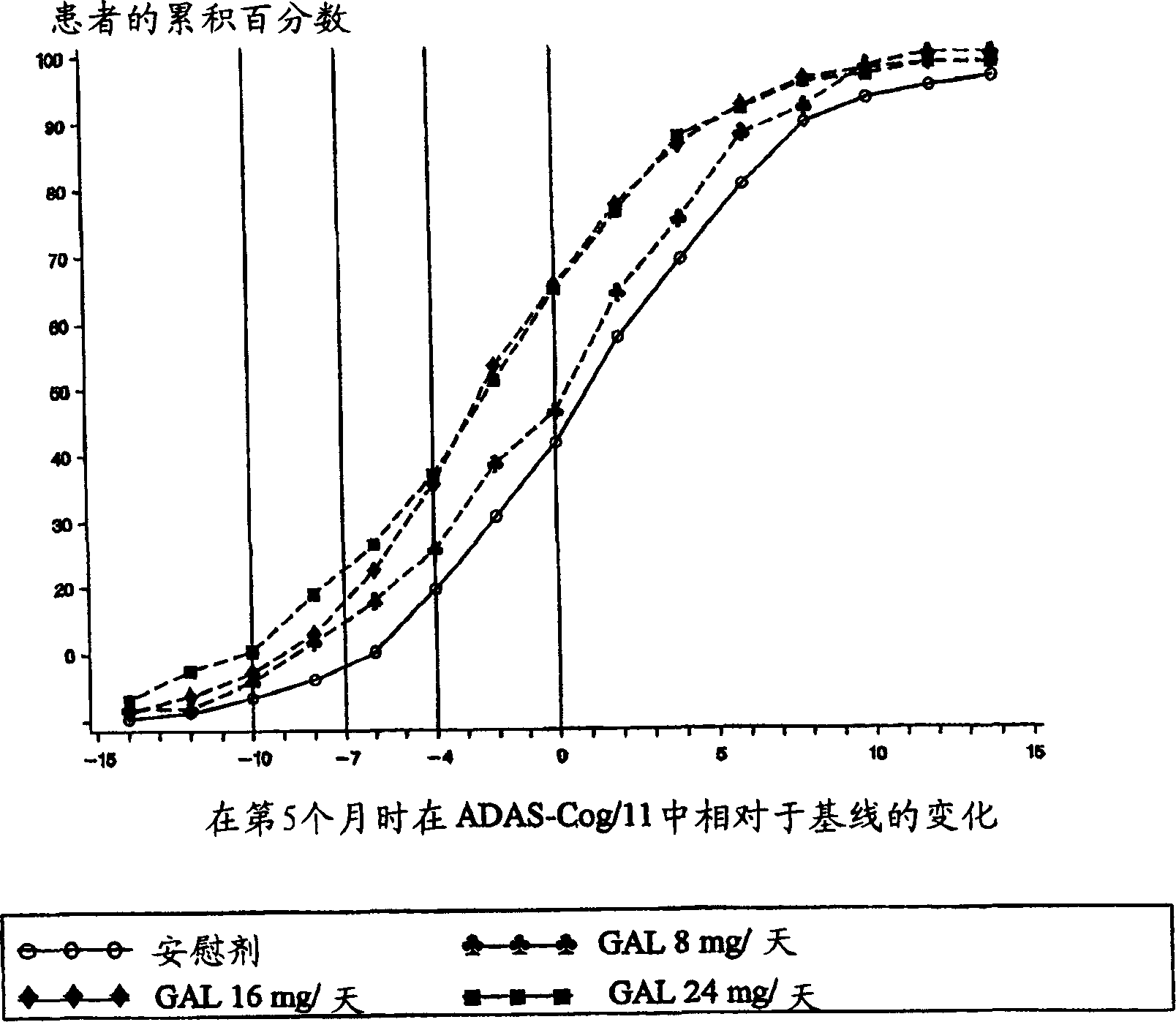
Use of galantamine for the treatment of neuropsychiatric behaviour associated with Alzheimer's disease
Galantamine has be used in the treatment of a number of chronic diseases. Galantamine has been found to be safe and effective in the treatment of Alzheimer's disease. Neuropsychiatric disorders are often associated with Alzheimer's disease. It is demonstrated that galantamine is also effective in reducing or stabilizing the incidence of neuropsychiatric behaviour seen in Alzheimer's patients.
Owner:PARYS WIM LOUIS JULIEN +1
LRPAP1 genetic markers associated with galantamine
InactiveUS20050260613A1Improve cognitive functionMicrobiological testing/measurementFermentationMedicineHaplotype
Haplotypes in the LRPAP1 gene associated with cognitive response to galantamine treatment are disclosed. Compositions and methods for detecting and using these LRPAP1 haplotypes in a variety of clinical applications are disclosed. Such applications include articles of manufacture comprising galantamine or derivatives thereof that are approved for treating patients having one of these LRPAP1 haplotypes, methods and kits for predicting the response of an individual to galantamine based upon his / her haplotype profile, and methods for treating Alzheimer's patients based upon their haplotype profile.
Owner:PGXHEALTH
Galantamine clearance of amyloid-beta
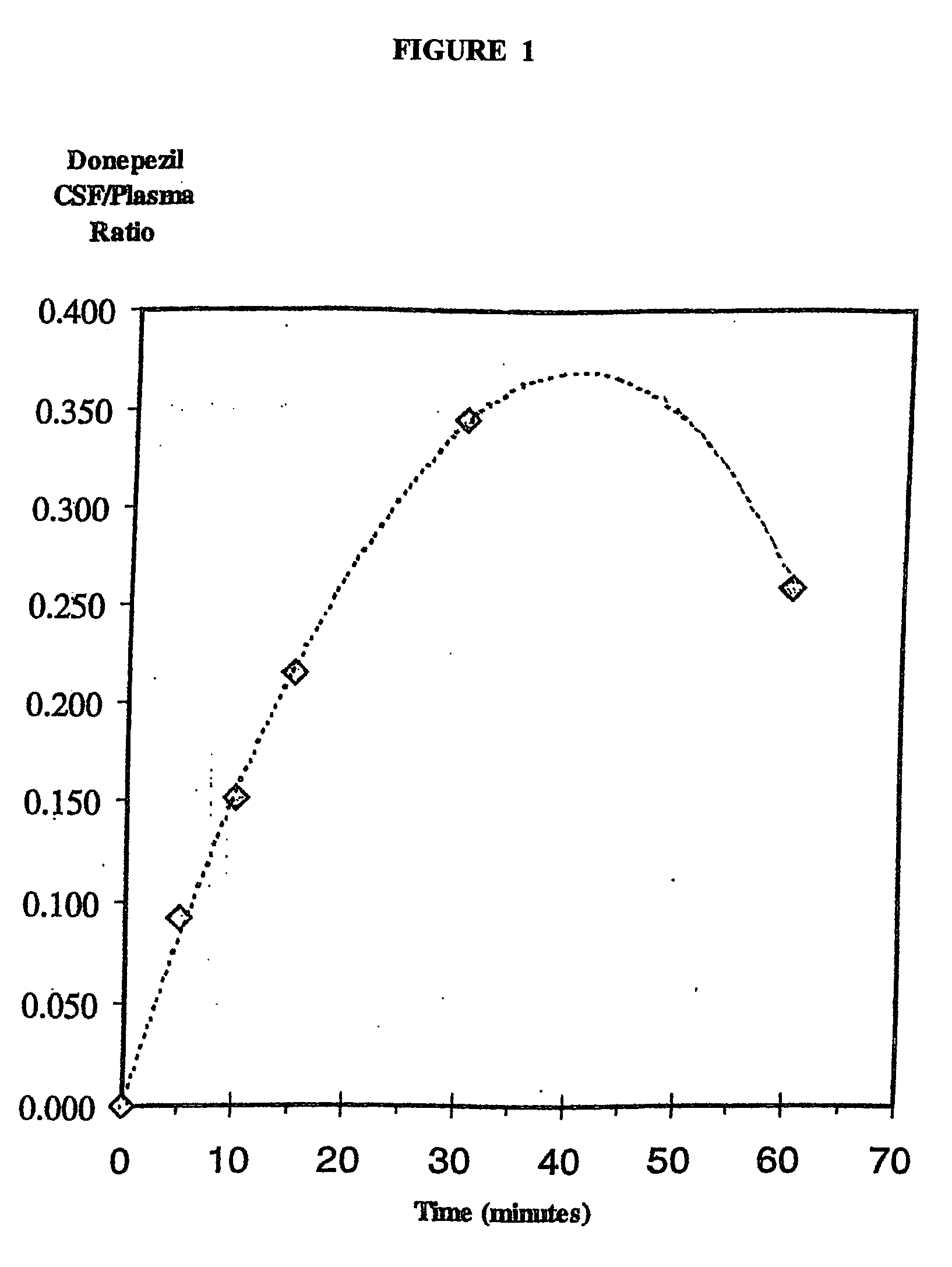
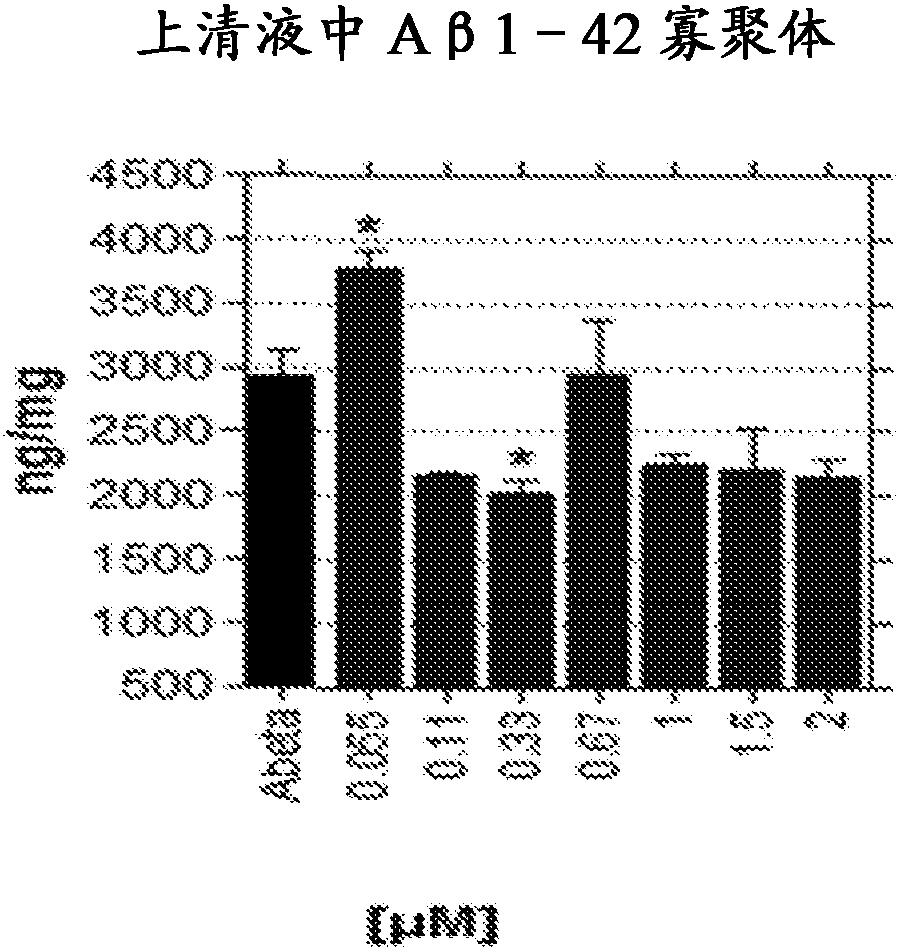
Galantamine and its pharmaceutically acceptable salts are of use in treating persons meeting criteria for having a risk of developing Alzheimer's type dementia, before dementia occurs by reducing thedecline of A[beta] amyloid in CSF or the increase in cortical beta amyloid, in order to delay cognitive decline.
Owner:SYNAPTEC DEV
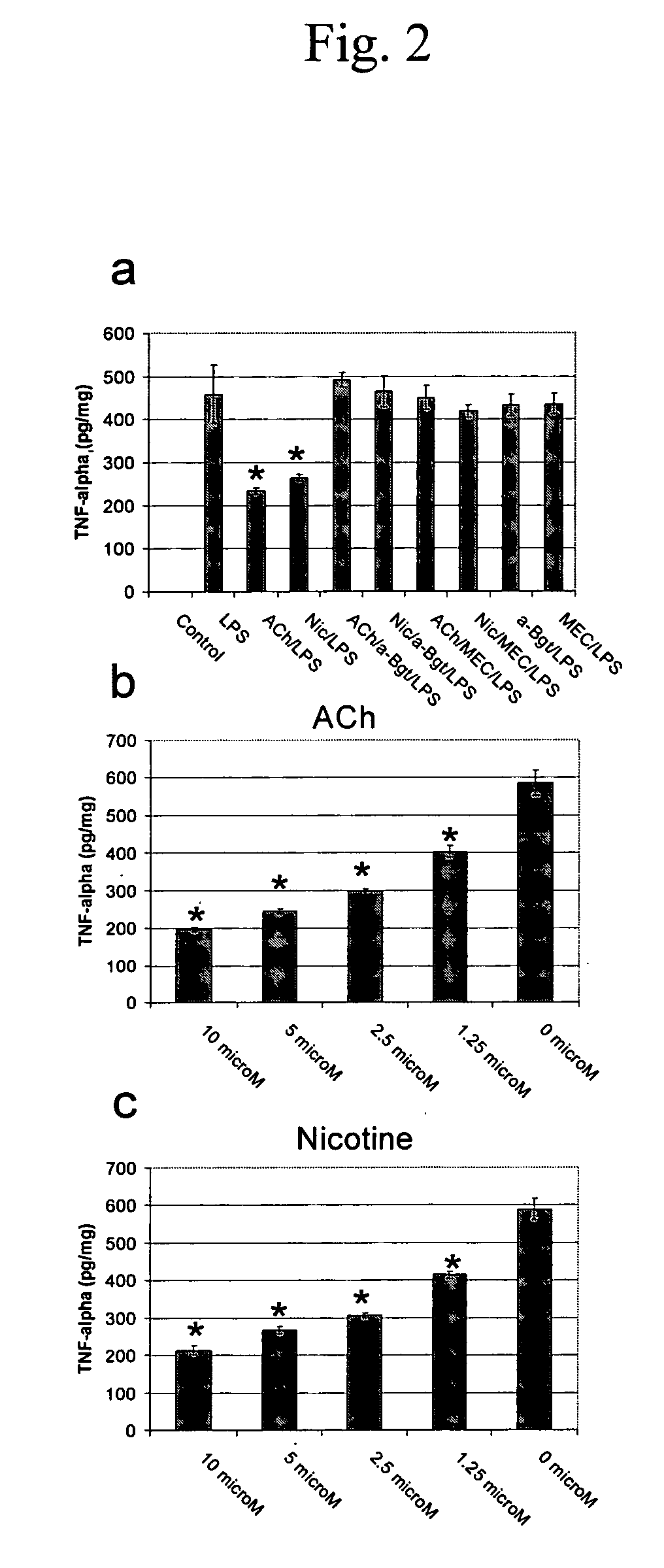
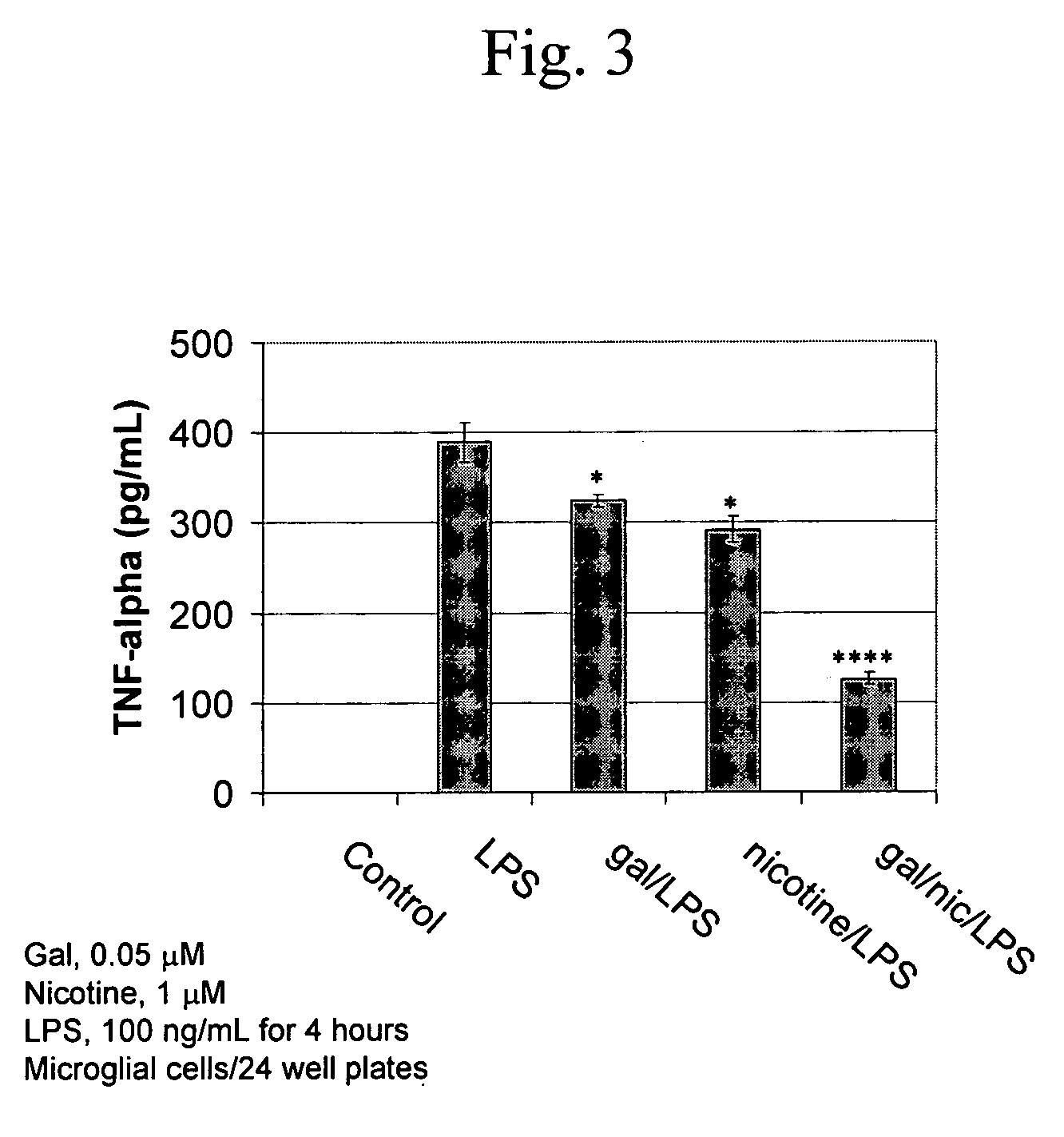
Modulation of Microglial by Nicotinic Medications
InactiveUS20060223790A1Reduce riskReduce aggregationBiocideAnimal repellantsNeuro-degenerative diseaseBiological activation
A method of treating a subject suffering from a neurodegenerative disease by modulating microglial activation with a therapeutically effective amount of a cholinergic agonist and a cholinesterase inhibitor. In one embodiment of the invention, the cholinergic agonist is nicotine and the cholinesterase is galantamine (a relatively weak acetylcholinesterase inhibitor and a potent allosteric potentiating ligand of nAChRs)
Owner:UNIV OF SOUTH FLORIDA
Galantamine amino acid and peptide prodrugs and uses thereof
InactiveUS20120184532A1Reducing adverse gastrointestinal (GI) side effectsMinimizing gastrointestinal side effectBiocideNervous disorderBenzoic acidSide effect
Owner:SHIRE PLC
Reagent and kit for enriching breast cancer stem cells from breast cancer cell strains
InactiveCN106635997AIncrease contentFormation period is shortCulture processCell culture mediaSulfite saltMicrosphere
The invention discloses a reagent and kit for enriching breast cancer stem cells from breast cancer cell strains. The reagent includes galantamine and lycorine as a screening agent. The breast cancer cell strains are MCF-7 breast cancer cell strains. The kit comprises a double-antibody, galantamine and lycorine as a screening agent, a screening-agent-dissolving auxiliary agent, sodium sulfite as an anti-oxidant, a recombinant human epidermal growth factor (EFG) which is a conventional growth factor, bovine serum albumin (BSA), insulin and B27. The provided reagent can enrich breast cancer stem cells from human breast cancer MCF-7 cell strains in a convenient, fast and efficient way, the cell spheroids are short in forming period, and the content of cancer cells and SP cells marked by CD44<+>CD24<- / low> molecules in microsphere cells is high. The developed kit can improve the efficiency for enriching cancer stem cells by science researchers.
Owner:南京千年健干细胞基因工程有限公司
Use of galantamine for treatment of neuropsychiatric behaviour associated with alzheimer's disease
Galantamine has been used in the treatment of a number of chronic diseases. Galantamine has been found to be safe and effective in the treatment of Alzheimer's disease. Neuropsychiatric disorders are often associated with Alzheimer's disease. It is demonstrated that galantamine is also effective in reducing or stabilizing the incidence of neuropsychiatric behaviour seen in Alzheimer's patients.
Owner:JANSSEN PHARMA NV
Pharmaceutical compositions and methods for treating parkinson's and huntington's disease
A pharmaceutical composition containing the therapeutically active ingredients of azelastine or a pharmaceutically acceptable salt of azelastine and donepezil or rivastigmine or galantamine or a pharmaceutically acceptable salt of thereof is disclosed. A method of using the pharmaceutical composition for treating patients suffering from mental, behavioral, cognitive disorders is also disclosed.
Owner:LA PHARMATECH INC
Method of Treating Organophosphorous Poisoning
A method of treating organophosphorous (OP) poisoning comprising administering to a mammal at risk for OP poisoning an OP poisoning-inhibiting amount of galantamine.
Owner:UNIV OF MARYLAND BALTIMORE +1
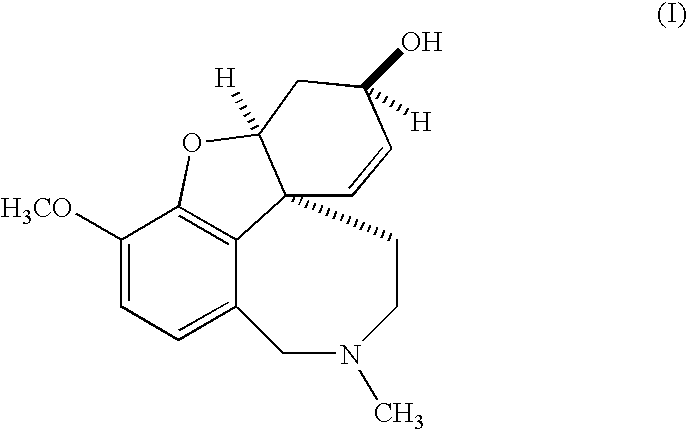
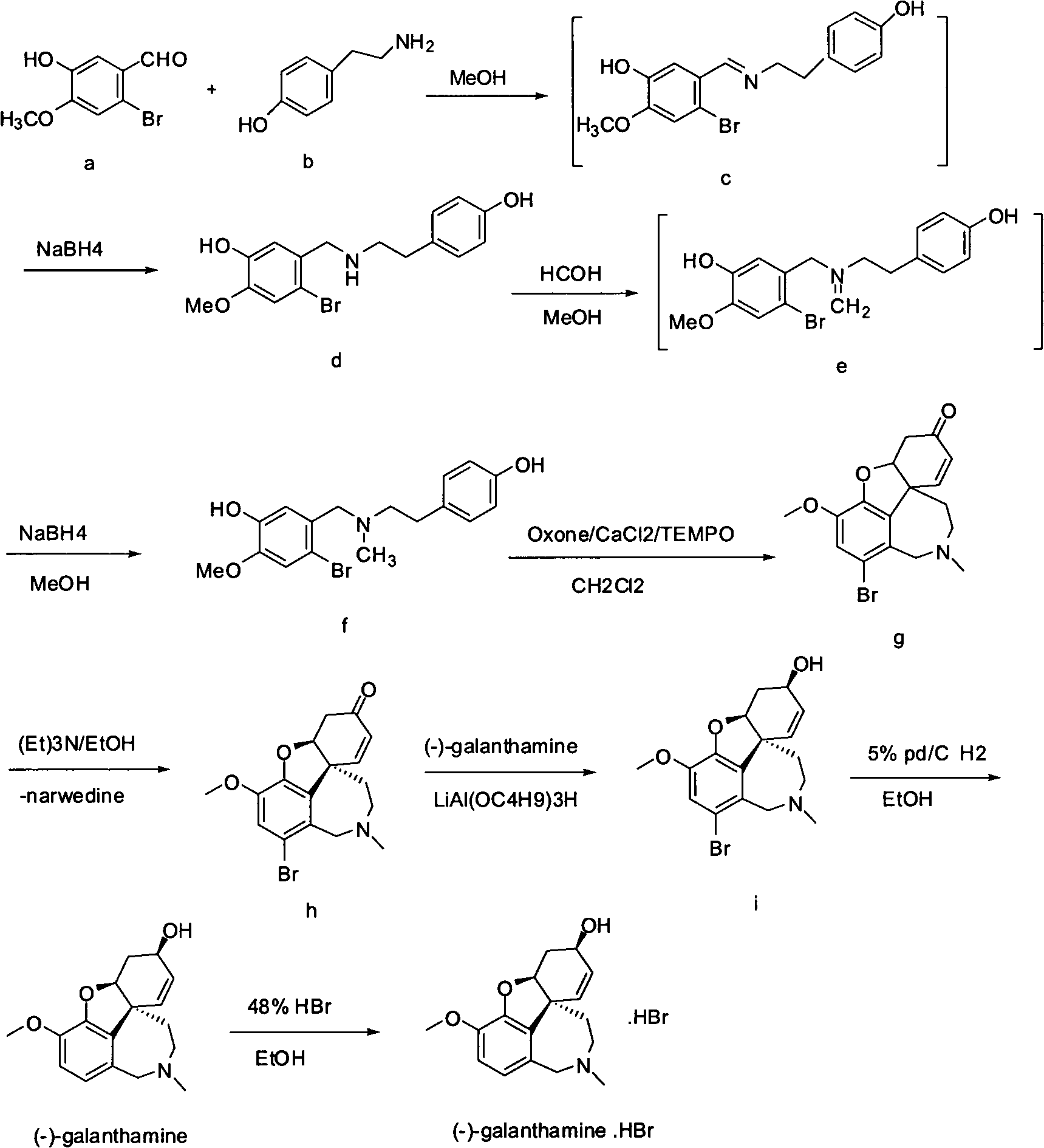
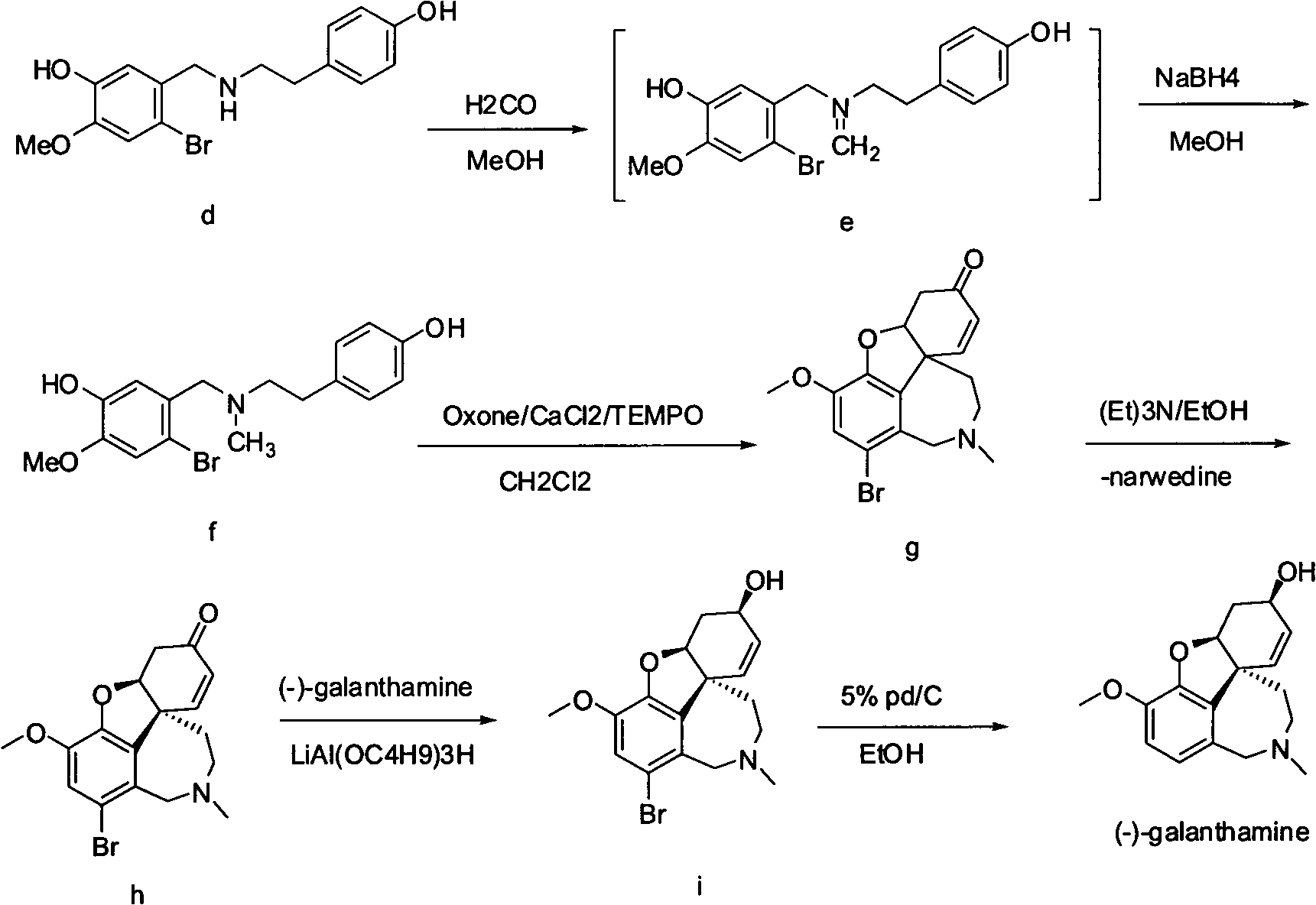
Synthesis method of galanthamine
The invention provides a new method for the preparation of (-)-galanthamine. The method includes the following steps: preparing N-methyl-N-(4-hydroxyl phenethyl)-3-hydroxyl-4-methoxyl-6-bretylium (f) by condensation of N-(4-hydroxyl phenethyl)-3-hydroxyl-4-methoxyl-6-bretylium (d) as a starting material and formaldehyde, then preparing bromo-narwedine (g) by catalytic oxidation using an oxidizing agent (Oxone / CaCl2 / TEMPO) and a phase-transfer catalyst, transforming the bromo-narwedine to levo bromo-narwedine (h) by Shieh method, obtaining bromo-(-)-galantamine (i) by asymmetric reduction, and then obtaining the (-)-galanthamine by hydrogenated debromination.
Owner:TIANJIN HAIGELI TECH DEV
Compound medicinal composition containing acetylcholinesterase inhibitor and metformin
ActiveCN104623671BNervous disorderHeterocyclic compound active ingredientsCholinesterase inhibitionBULK ACTIVE INGREDIENT
The invention belongs to the technical field of medicine, and specifically relates to a compound medicinal composition with acetylcholinesterase inhibitors such as donepezil, galantamine or huperzine A and metformin as active ingredients, a preparation method thereof and a therapeutic use thereof. Pharmacodynamic tests show that the compound pharmaceutical composition of the present invention can be used for the prevention and treatment of Alzheimer's dementia, and the combined use of the compound medicine has a synergistic effect in curative effect.
Owner:南京千手共创生物科技有限公司 +1
Method for detecting galantamine in lycoris aurea
ActiveCN108717002AShorten the timeHigh sensitivityPreparing sample for investigationMaterial electrochemical variablesScreen printingDifferential pulse voltammetry
The invention discloses a method for detecting galantamine in lycoris aurea, and relates to plant sample handling, preparation of a nanogold printing electrode sensor and electrochemical detection. According to the method, the nanogold-modified printing electrode sensor is adopted to fully absorb in a sample extracting solution, then the nanogold printing electrode sensor is subjected to differential pulse voltammetry scanning to measure current values at oxidation peaks, within a detectable range, the content of the galantamine in the lycoris aurea absorbed by the nanogold printing electrodesensor is in a linear relation with the current intensities at the oxidation peaks, and according to a working curve, the content of the galantamine in the detected lycoris aurea can be calculated. The method has the advantages that the nanotechnology and the silk-screen printing technology are combined by the prepared nanogold printing electrode sensor, sensitivity, in galantamine concentration detection, of the sensor can be improved remarkably, and the minimum limit of detection of the sensor can reach to 0.013 microns.
Owner:上海联星医药科技有限公司
Galantamine clearance of amyloid ß
PendingUS20180200259A1Lower Level RequirementsDelaying the onset of such dementiaNervous disorderHeterocyclic compound active ingredientsCerebrospinal fluidAβ amyloid
Galantamine and its pharmaceutically acceptable salts are of use in treating persons meeting criteria for having a risk of developing Alzheimer's type dementia, before dementia occurs by reducing the decline of Aβ amyloid in CSF or the increase in cortical beta amyloid, in order to delay cognitive decline.
Owner:SYNAPTEC DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com