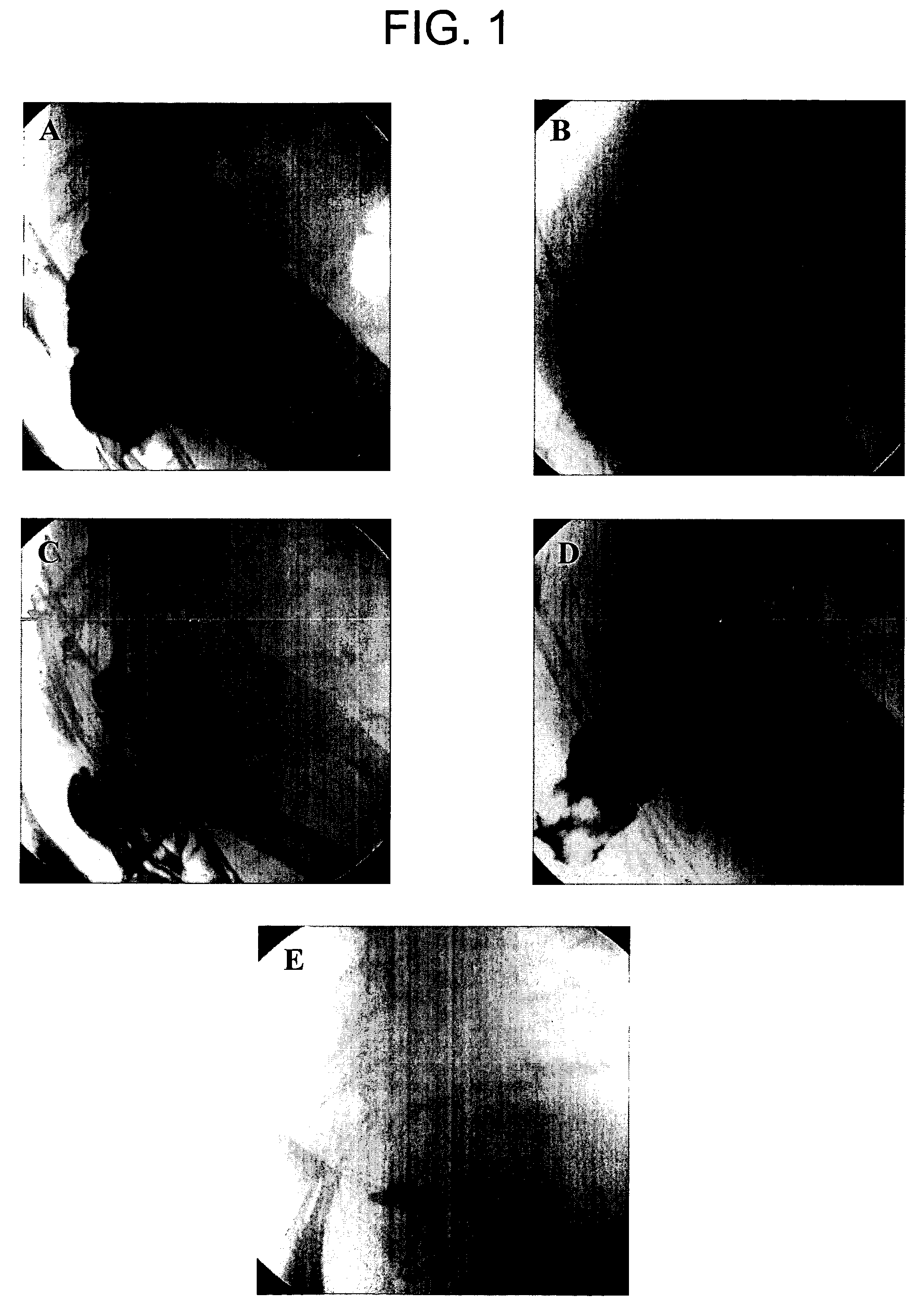Patents
Literature
34 results about "Neostigmine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Neostigmine, sold under the brand name Prostigmin among others, is a medication used to treat myasthenia gravis, Ogilvie syndrome, and urinary retention without the presence of a blockage. It is also used together with atropine to end the effects of neuromuscular blocking medication of the non-depolarizing type. It is given by injection either into a vein, muscle, or under the skin. After injection effects are generally greatest within 30 minutes and last up to 4 hours.
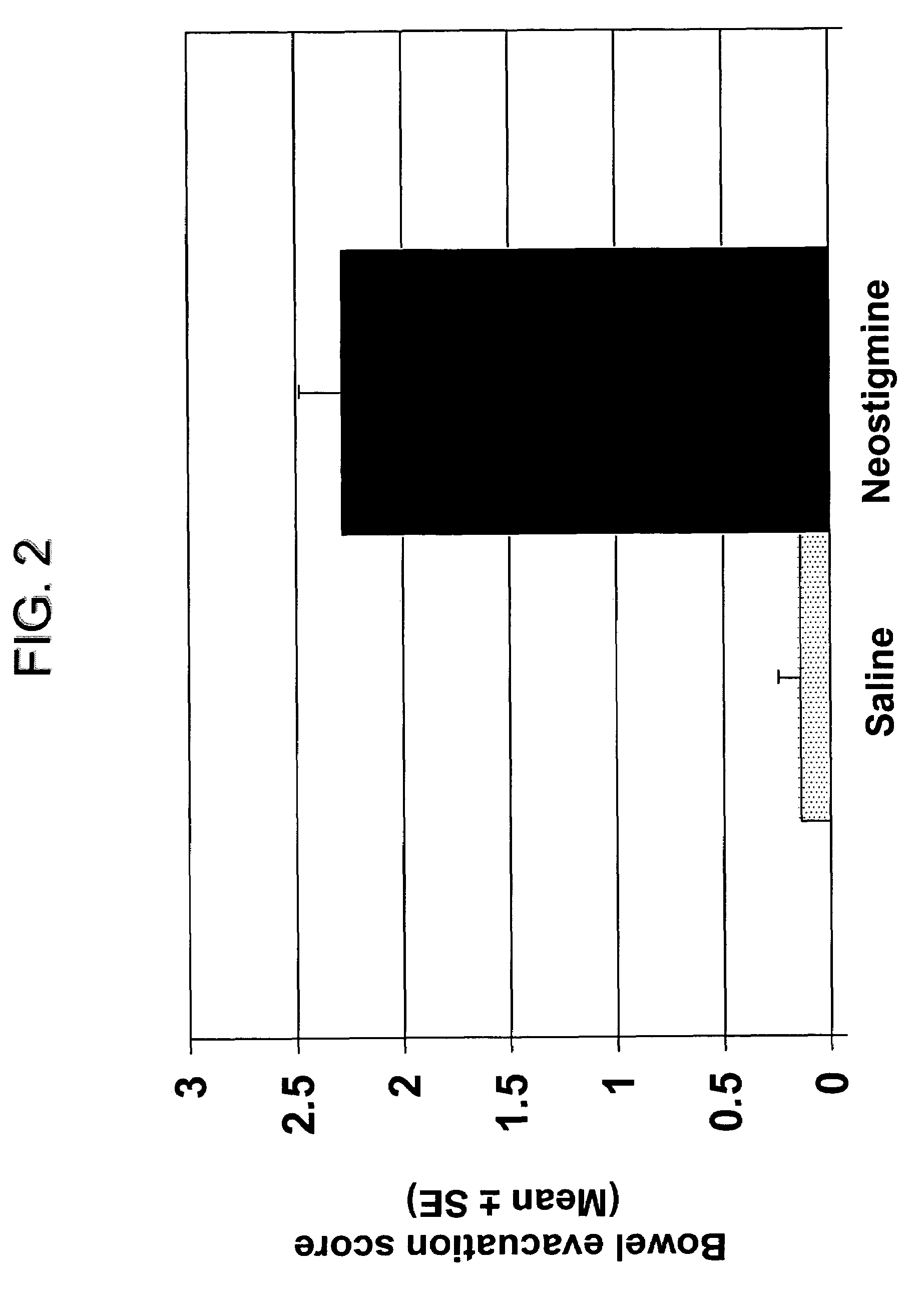
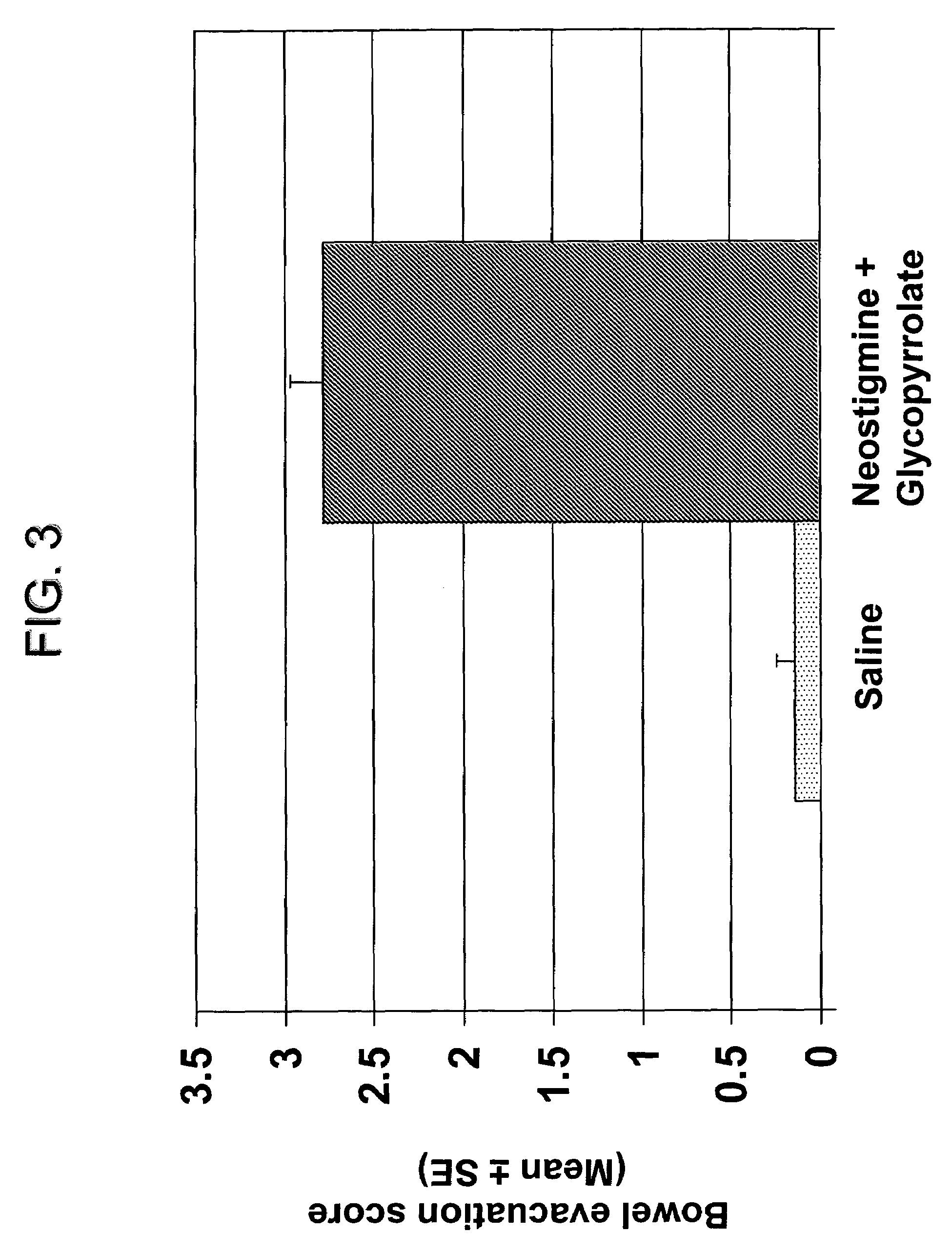
Compositions and methods for bowel care in individuals with chronic intestinal pseudo-obstruction
ActiveUS7635709B2Shorten the construction periodDifficult to administerBiocideAmine active ingredientsBowel careSide effect
The present disclosure provides compositions and methods for on-going bowel care for persons with chronic intestinal pseudo-obstruction. The compositions and methods can be administered in a non-clinical setting. The compositions comprise acetylcholinesterase inhibitors for stimulating motility of the bowel in combination with anti-cholinergic agents to counteract the potentially dangerous cardiac side effects of the acetylcholinesterase inhibitor. In some examples, the acetylcholinesterase inhibitor, neostigmine, and the anti-cholinergic agent, glycopyrrolate, are combined in a pharmaceutical composition. Certain examples also provide the frequency and duration of administration of the disclosed drug combinations.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Cholinesterase Inhibitors In Liposomes And Their Production And Use
InactiveUS20080031935A1Avoid intakeImprove bioavailabilityBiocideNervous disorderEnantiomerRivastigmine
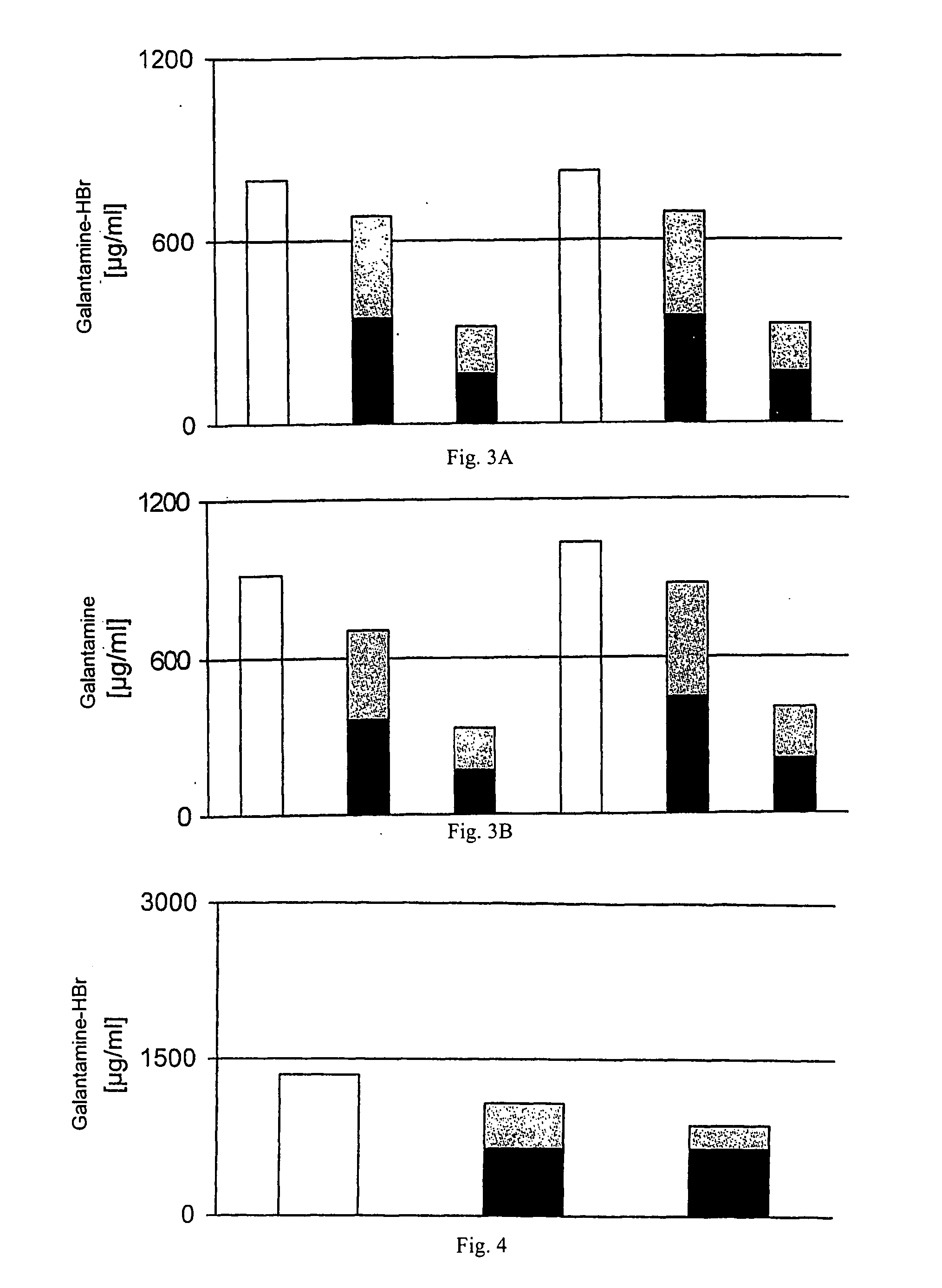
The invention relates to a pharmaceutical composition based on an active ingredient that is enclosed in liposomes for topical, transdermal application. The interior of said liposomes comprises an acidic, aqueous medium containing at least one cholinesterase inhibitor, preferably from the group containing donepezil, rivastigmine, galantamine, physostigmine, heptylphysostigmine, phenserine, tolserine, cymserine, thiatolserine, thiacymserine, neostigmine, huperzine, tacrine, metrifonate and dichlorvos, or an enantiomer or derivative of at least one of said compounds. In addition, the invention relates to a method for producing said composition, optionally in a sterile form and also to the use of the liposomes charged with the active ingredient in various galenic formulations for topical, transdermal application with a depot effect in the epidermis, for the prophylaxis and / or treatment of cutaneous neuropathic pain or the loss of cutaneous sensory function as a result of neuropathy.
Owner:SANOCHEMIA PHARMA AG
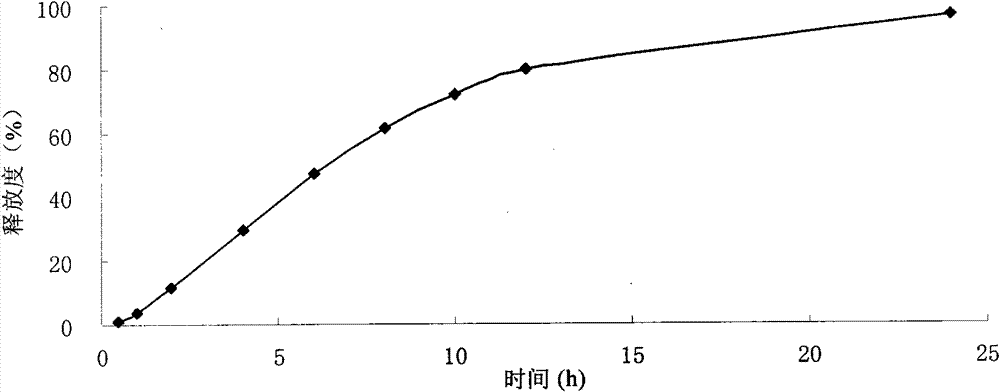
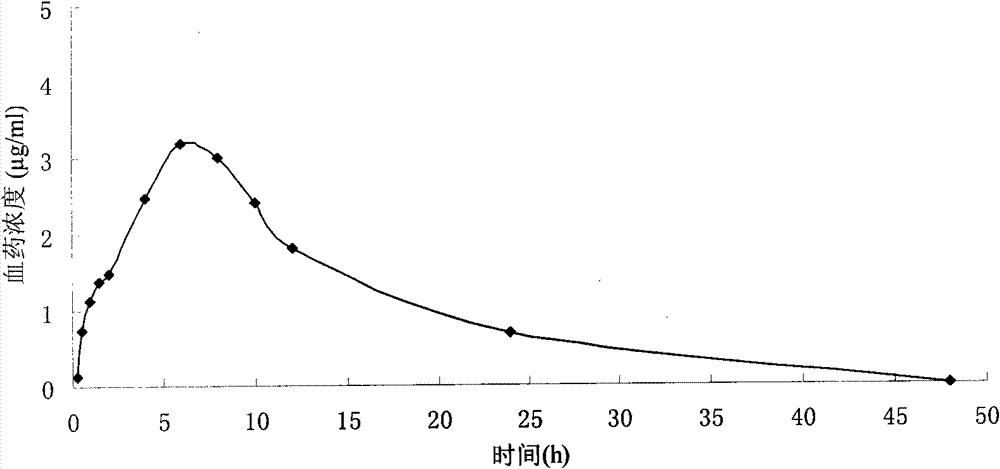
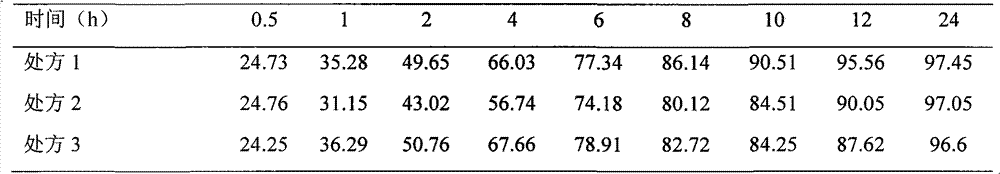
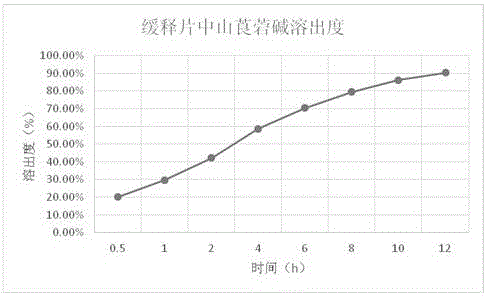
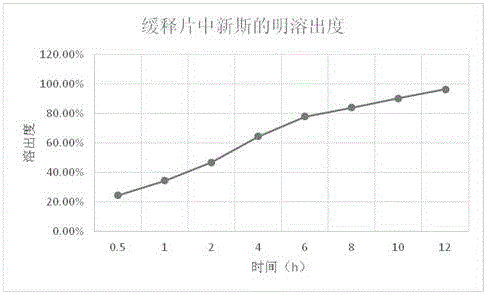
Neostigmine bromide sustained-release tablet and preparation method thereof
InactiveCN102258492AImprove stabilityAvoid instabilityDigestive systemMuscular disorderSustained Release TabletSide effect
The present invention belongs to the technical field of medicinal preparation. The invention discloses a formula of a neostigmine bromide slow release preparation which can be taken once every day for treating myasthemia gravis, functional flatulence after being performed an operation and urinary retention, as well as its preparation method. The preparation is disclosed as a slow release tablet form composed of a skeleton core containing neostigmine bromide and the slow release preparation and a coating. The neostigmine bromide slow release preparation of the present invention is capable of overcoming the disadvantage of present medicament common tablets in the market, slowly releasing to keep stable blood and medicine concentration, acting for a longer period, possessing low toxicity andside effect and conveniently taking the preparation, and the slow release preparation keeps effective blood and medicine concentration for a long time, reduces the medicine taking frequency, raises the compliance of the patients and reduces the side-effect due to over peak concentration. The invention has the advantages of simple preparation technology, low cost, easy control and easy industrial production.
Owner:CHONGQING MEDICAL UNIVERSITY
Chinese medicinal composition for treating irritable bowel syndrome and its preparation method
ActiveCN1654063AAnthropod material medical ingredientsDigestive systemIntestinal structureCyclodextrin
The Chinese medicine composition for treating intestinal irritable syndrome is prepared with gallnut, nutmeg, red ginseng, deglued antler powder, dark plum and myrobalan fruit as material and through the technological process including water extraction, extracting volatile oil, inclusion with cyclodextrin, grinding into fine powder and other steps. The pharmacological experiments show that the medicine of the present invention can inhibit the motion of small intestine, resist the sthenic motion of small intestine caused by Neostigmine, resist diarrhea caused by rhubarb, promote the absorption of water inside intestine caused by mannitol, etc. and has relatively high anti-diarrhea effect and high safety.
Owner:GUIZHOU LIANSHENG PHARMA
Neostigmine bromide muhivescular liposome and preparation method thereof
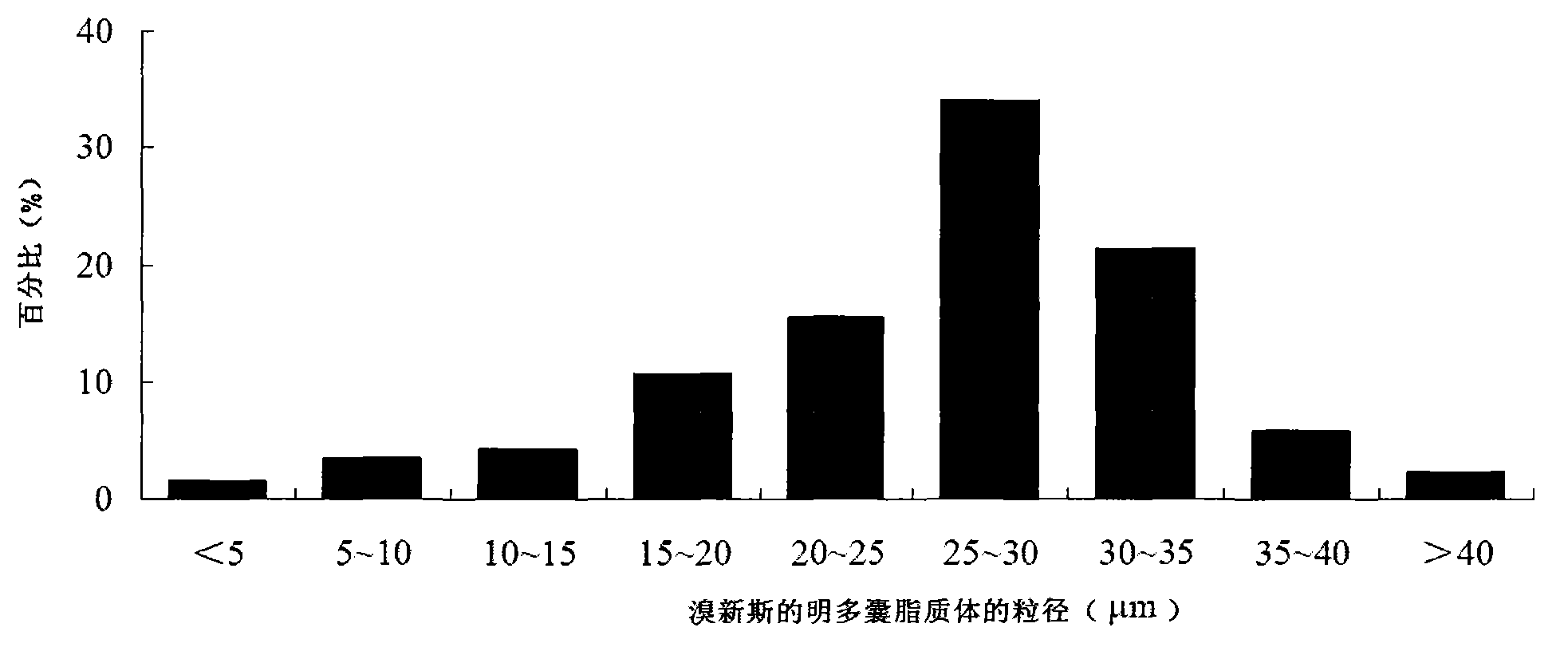
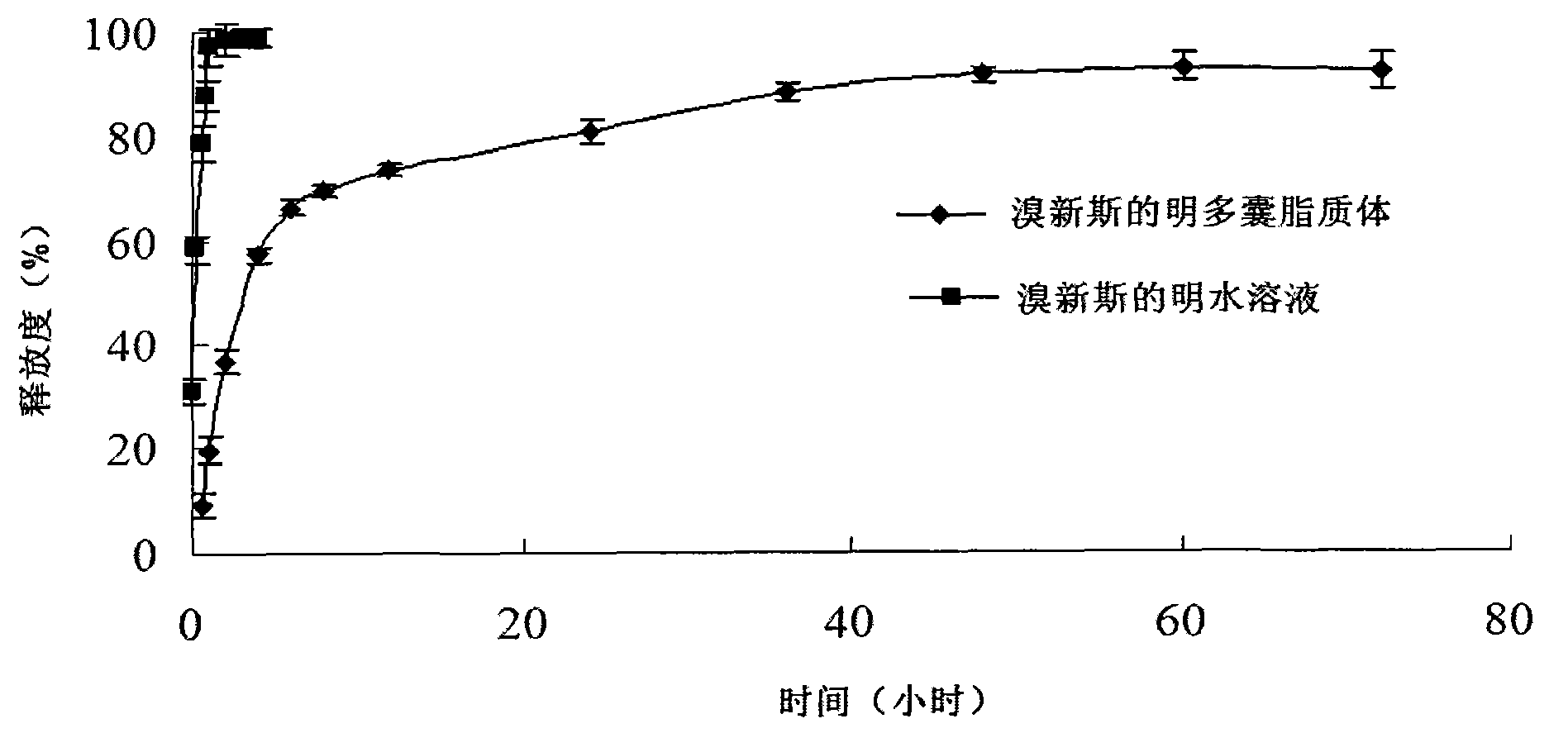
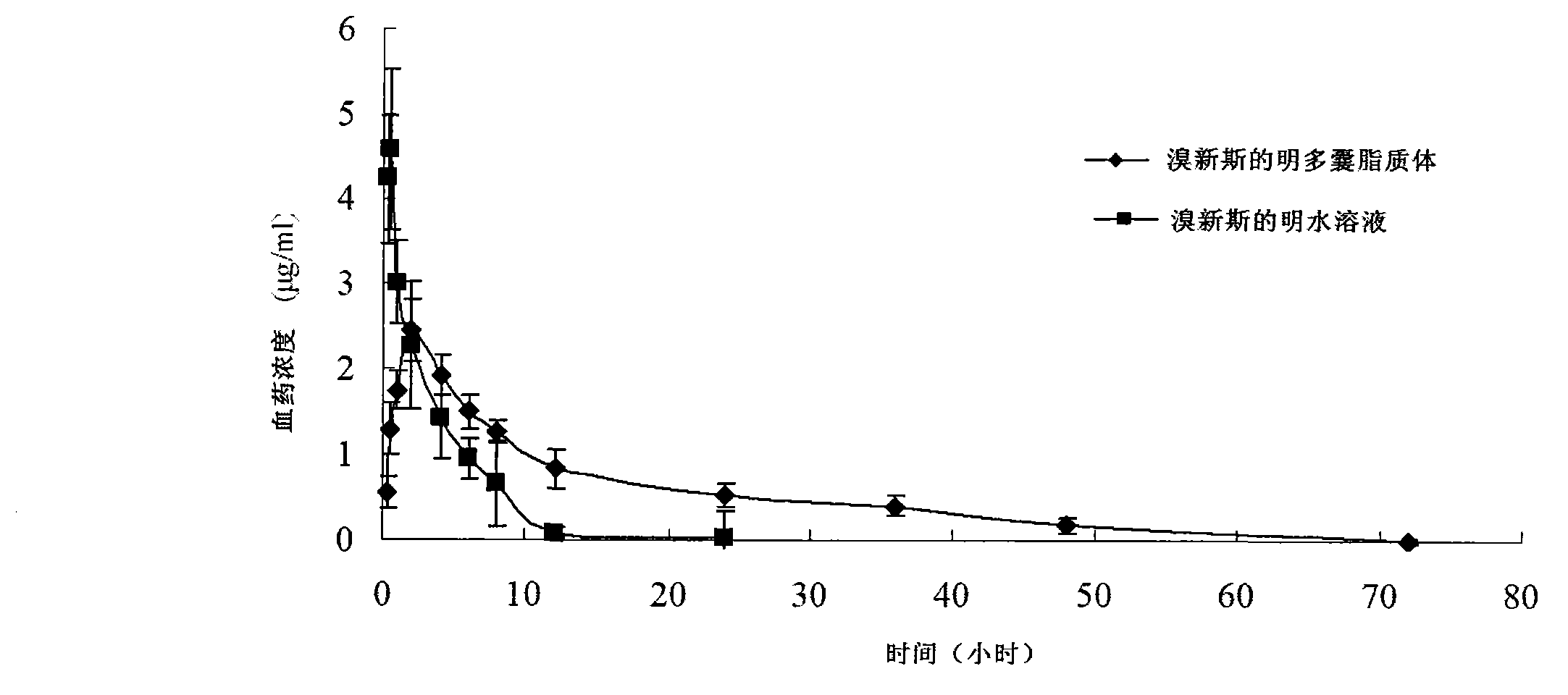
ActiveCN103191059AThe sustained release effect in the body is obviousTo achieve the purpose of sustained releaseDigestive systemMuscular disorderMedicineMultivesicular liposomes
The invention belongs to the field of a pharmaceutic preparation, and relates to a neostigmine bromide muhivescular liposome formula and a preparation method thereof. The neostigmine bromide muhivescular liposome prepared by the method can achieve slow release of a medicine, and prolongs the medicine effect time and the effective medicine duration.
Owner:CHONGQING MEDICAL UNIVERSITY
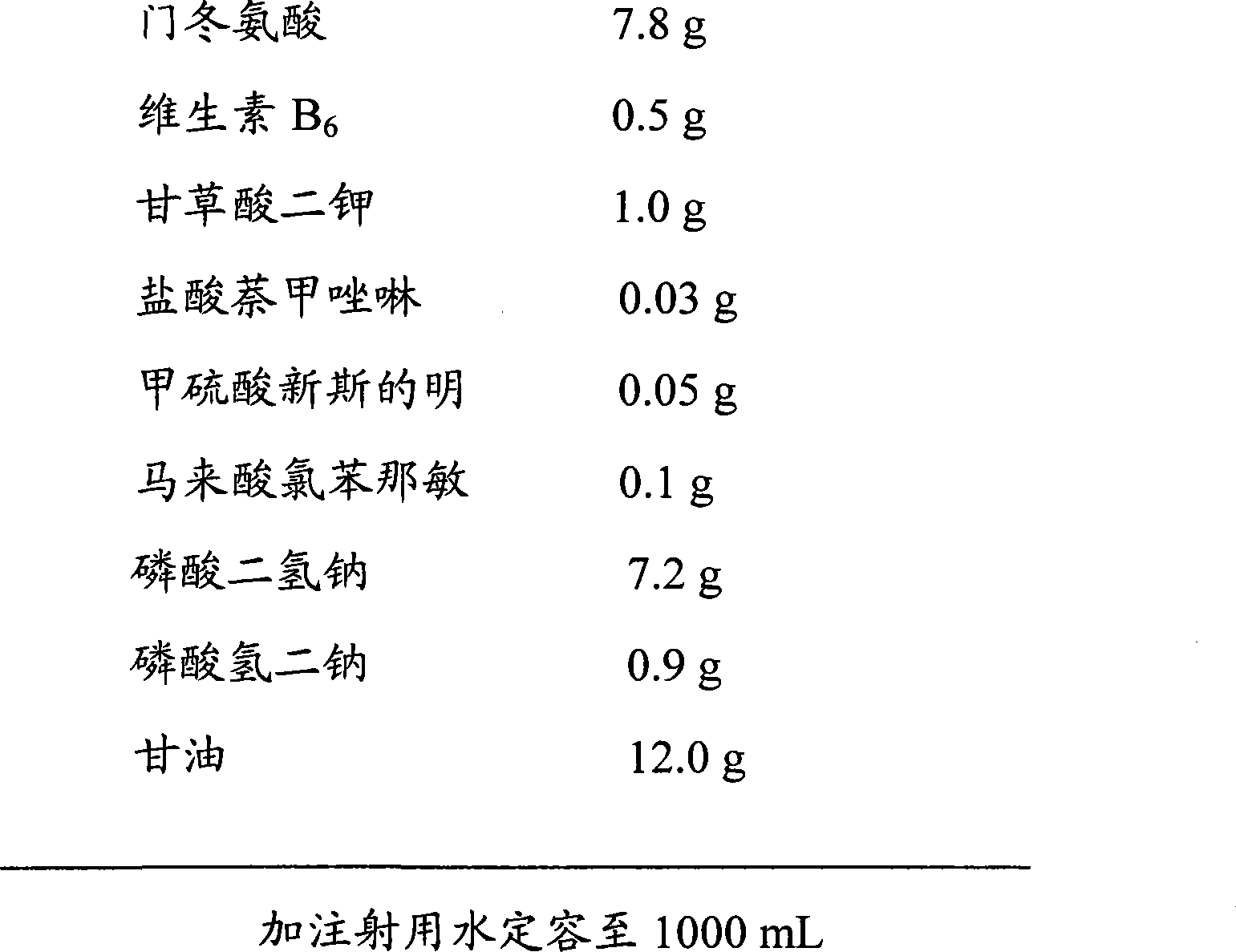
Composite aspartate, vitamin B6 and dipotassium glycyrrhetate eye drops without bacteria inhibitor and preparation method thereof
InactiveCN101455634AAvoid side effectsAvoid potential dangerSenses disorderPharmaceutical delivery mechanismMedicineDipotassium Glycyrrhizate
The present invention discloses compound aspartate, vitamin B6 and dipotassium glycyrrhetate eye drops which do not contain bacteriostat and a preparing method thereof, wherein the compound aspartate, vitamin B6 and dipotassium glycyrrhetate eye drops comprises aspartic acid, vitamin B6, dipotassium glycyrrhetate, naphazoline hydrochloride, neostigmine methysulfate, chlorpheniramine maleate, pH modifying agent, isoosmotic agent, stabilizing agent, thickening agent, algefacient, etc. The preparing method adopts an aseptic manipulation filling technique. Furthermore the eye drops of the invention adopt a disposable single-dose independent packaging. The sterilized performance of product is guaranteed. The product is safer, more reliable, easier and more sanitary.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Neostigmine methylsulfate injection and preparation method thereof
InactiveCN107115284AReduce visible foreign matterGuarantee product qualityMuscular disorderPharmaceutical delivery mechanismForeign matterCarboxylic acid
The invention discloses a neostigmine methyl sulfate injection and a preparation method thereof. It includes the following steps: ① Add water for injection and mix 225.0g of neostigmine methyl sulfate in the preparation tank, stir to dissolve, dilute to the full amount with water for injection, and stir evenly; ② Use Suzhou sand filter rods, 0.45 μm and 0.22 μm polyethylene sulfate. Filter back through the ethersulfone microporous membrane until it becomes clear; ③ Take samples to test the intermediate content and pH value. If the intermediate content is in the range of 95.0 to 105.0% and the pH is in the range of 5.2 to 6.8, the medicinal solution is qualified. Filter the medicinal solution. into the liquid storage tank; ④ The liquid in the liquid storage tank is filtered through two 0.22 μm polyethersulfone microporous membranes. After sampling for visible foreign matter and passing the inspection, it is input into the filling buffer tank for potting; ⑤ Potting; ⑥ Destroy Bacteria: 100℃, 30 minutes. The preparation method of the present invention adopts secondary sterilization filtration, which reduces visible foreign matter in the medicine, makes the whole process sterile, and ensures product quality.
Owner:SHANGHAI XINYI JINZHU PHARMA
Neostigmine bromide composition freeze-dried tablet and preparation method thereof
The invention provides a neostigmine bromide composition freeze-dried tablet and a preparation method thereof and relates to the technical field of medicines and medicine production. The neostigmine bromide composition freeze-dried tablet is prepared from the following components including neostigmine bromide, starch and sucrose. According to the neostigmine bromide composition freeze-dried tablet provided by the invention, with the starch and the sucrose as auxiliary materials, heating process treatment is carried out on common corn starch, so that the actions of adhesion and disintegration of the starch in a tablet and the formability of the tablet are improved, and the neostigmine bromide composition freeze-dried tablet only needs two of the starch and the sucrose serving as the auxiliary materials. The neostigmine bromide composition freeze-dried tablet is prepared by adopting the freeze drying technology with twice cooling and twice temperature raising, wherein with twice cooling and twice temperature raising, the formability of the tablet is good, the dissolution of the tablet is improved, and thus the bioavailability of the tablet is improved. With the adoption of the neostigmine bromide composition freeze-dried tablet, the disadvantages of the common neostigmine bromide tablet are overcome, the species and the dosages of the auxiliary materials in the neostigmine bromide tablet are reduced, the dissolution of the tablet is large, the bioavailability of the tablet is high, and the curative effect and the safety of clinical medication are ensured.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Degradable slow-release invisible eye mask capable of eliminating eye fatigue and preparation method of degradable slow-release invisible eye mask
PendingCN111956791AEliminate dryness and discomfortEliminate eye fatigue and degrade dryness and discomfortSenses disorderHydroxy compound active ingredientsChlorobenzeneDisodium Edetate
The invention discloses a degradable slow-release invisible eye mask capable of eliminating eye fatigue and a preparation method of the degradable slow-release invisible eye mask, and relates to the technical field of invisible eye masks. Per each 10ml, the invisible eye mask comprises the following raw material components: 200mg-300mg of collagen peptide, 0.8-1.2mg of hyaluronic acid, 4-6mg of vitamin B6, 1.8-2.2mg of vitamin B12, 9-11 mg of dipotassium glycyrrhizinate, 0.18-0.22mg of napthoxylin hydrochloride, 0.28-0.32mg of neostigmine methylsulfate, 0.8-1.2mg of chlorpheniramine maleate, 80-150mg of glycerinum, 7-9mg of dexpanthenol, 4-6mg of menthol, 5-7mg of polysorbate 80, 5-7mg of borneol, 3-5mg of edetate disodium, 2-4mg of mint, 2-4mg of aminocaproic acid, 1-3mg of ethanol, 9-11mg of sodium chloride, 2-4 mg of benzalkonium chloride solution, and 3-5 mg of chlorobutanol solution; the mass fraction of the benzalkonium chloride solution is 0.01%; and the mass fraction of the chlorobutanol solution is 0.2%.
Owner:康洛信(广东)生物科技有限公司
Aspartic vitamin eye drops and preparation method thereof
ActiveCN101129392ASimple preparation processImprove yieldSenses disorderPharmaceutical delivery mechanismMentholBenzalkonium chloride
The invention discloses a compound asparticitin gutta and making method, which comprises the following parts: aspartic acid, vitamin B6, glycyrrhizin dipotassium, naphazoline hydrochloride, neostigmine methylsulfate, maleic chlorobenzenenamin, polysorbate 80, sodium chloride, menthol, benzachloridamine, sodium hydroxide solution and injecting water. The invention paves basis for scaling manufacturing of the compound asparticitin gutta further, which improves the productivity with stable content of maleic chlorobenzenenamin.
Owner:江西闪亮制药有限公司
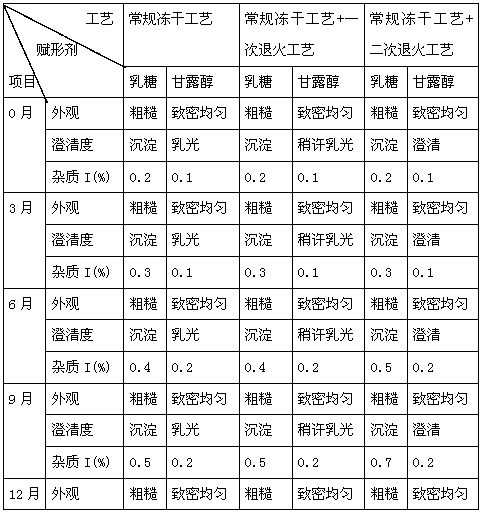
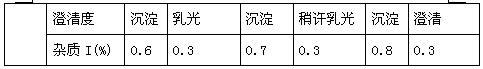
Neostigmine methylsulfate frozen-dried preparation for injection and preparation method of neostigmine methylsulfate frozen-dried preparation
ActiveCN110585143AHemolysis NoneAgglutination NonePowder deliveryDrying solid materials without heatFreeze-dryingMedicine
The invention relates to a neostigmine methylsulfate frozen-dried preparation for injection and a preparation method of the neostigmine methylsulfate frozen-dried preparation. Neostigmine methylsulfate injection is instable when meeting light and heat, and a mainly degradation impurity namely 3-hydroxy tricresylamine neostigmine methylsulfate has a genotoxicity warning structure. In order to improve stability, the inventor improves the preparation formulation, and the frozen-dried preparation is prepared. Through recipe screening and process improvement, the frozen-dried preparation product isobtained. The obtained product is good in appearance, good in redissolution properties and good in clarity, and the main degradation impurity namely 3-hydroxy tricresylamine neostigmine methylsulfateis greatly reduced, so that the product safety and stability are improved.
Owner:JIANGSU JIUXU PHARMA +1
Aspartic vitamin eye drops and preparation method thereof
ActiveCN100586440CSimple preparation processImprove yieldSenses disorderPharmaceutical delivery mechanismMentholBenzalkonium chloride
The invention discloses a compound asparticitin gutta and making method, which comprises the following parts: aspartic acid, vitamin B6, glycyrrhizin dipotassium, naphazoline hydrochloride, neostigmine methylsulfate, maleic chlorobenzenenamin, polysorbate 80, sodium chloride, menthol, benzachloridamine, sodium hydroxide solution and injecting water. The invention paves basis for scaling manufacturing of the compound asparticitin gutta further, which improves the productivity with stable content of maleic chlorobenzenenamin.
Owner:江西闪亮制药有限公司
Chinese patent medicine oral preparation promoting recovery of postoperative gastrointestinal motility
InactiveCN107970429APromote functional recoveryShort course of treatmentDispersion deliveryDigestive systemPatient needRegimen
The invention discloses a Chinese patent medicine oral preparation promoting recovery of postoperative gastrointestinal motility. The Chinese patent medicine oral preparation is prepared from poria cocos, rhizoma atractylodis macrocephalae, rhizoma alismatis, rhizoma zingiberis, rhizoma zingiberis recens, rhizoma pinelliae, cortex magnoliae officinalis, liquorice root, fructus amomi, and pericarpium citri reticulatae through decocting and concentrating, wherein 20g of fructus amomi is used and 50g of pericarpium citri reticulatae is used. In treatment, a patient takes no food and no water andreduce pressure of the stomach and intestinal tract constantly, thereby enabling the stomach to rest thoroughly; the patient needs to lie with face downward and raise the lower part of the body, so asto eliminate pressure on the horizontal part of the dodecadactylon, further, 0.5mg of neostigmine is injected into muscle for six times each day; fluid infusion for maintenance, aqueous electrolyte is corrected and acid-base balance is kept, enough heat, protein, vitamin and trace element are supplemented, and negative nitrogen balance is corrected; the preparation is decocted and concentrated to350-500ml according to the quantity, the patient takes three preparations each day for three times through a gastric canal or an oral route, and the postoperative gastrointestinal motility function can be recovered after the patient takes 50 preparations consecutively. In terms of treatment of postoperative gastroparesis, the Chinese patent medicine oral preparation has the characteristics of having definite curative effect, being economical, helping recovering overall function of the patient, having no side effects, and having shorter treatment course.
Owner:黄志
Preparation of honokiol ophthalmic medicine and application of honokiol ophthalmic medicine in treatment of fungal keratitis
PendingCN114869885AEfficient killingReduce inflammationSenses disorderAntimycoticsVitamin b6Tissue repair
The invention provides preparation of honokiol ophthalmic medicine and application of honokiol ophthalmic medicine in treatment of fungal keratitis, and relates to the technical field of medicine preparation. According to the preparation method of the ophthalmic medicine based on honokiol, honokiol eye drops are prepared, specifically, the honokiol eye drops comprise 130 [mu] g / mL of L-potassium aspartate, 6.5 [mu] g / mL of vitamin B6, 0.39 [mu] g / mL of naphazolin hydrochloride, 0.65 [mu] g / mL of neostigmine methyl sulfate, 0.16 [mu] g / mL of L-menthol and 8 [mu] g / mL of honokiol, and a substrate component is a sterile injection containing 5-10% of hydroxyethyl cellulose. The honokiol ophthalmic medicine disclosed by the invention takes honokiol as an effective component, can effectively kill pathogenic fungi of fungal keratitis as a medicine for treating fungal keratitis, does not damage corneal epithelium to generate a bad effect, does not cause toxic damage to ocular surface and promote tissue repair when being reasonably used, and has the advantages of simple preparation process, low cost and the like. The prognosis of a fungal keratitis patient is greatly facilitated.
Owner:战璐
Cholinesterase inhibitors in liposomes and their production and use
Owner:SANOCHEMIA PHARMA AG
Chinese medicinal composition for treating irritable bowel syndrome and its preparation method
The Chinese medicine composition for treating intestinal irritable syndrome is prepared with gallnut, nutmeg, red ginseng, deglued antler powder, dark plum and myrobalan fruit as material and through the technological process including water extraction, extracting volatile oil, inclusion with cyclodextrin, grinding into fine powder and other steps. The pharmacological experiments show that the medicine of the present invention can inhibit the motion of small intestine, resist the sthenic motion of small intestine caused by Neostigmine, resist diarrhea caused by rhubarb, promote the absorption of water inside intestine caused by mannitol, etc. and has relatively high anti-diarrhea effect and high safety.
Owner:GUIZHOU LIANSHENG PHARMA
Pharmaceutical compositions and methods utilizing neostigmine and an NK-1 antagonist for treating myasthenia gravis
The present invention describes the use of a NK1-antagonist, in constant combination with neostigmine, to facilitate the treatment of a patient suffering from myasthenia gravis by providing a therapeutically effective neostigmine bromide or methylsulfate daily dose without the dose-limiting gastrointestinal adverse effects.
Owner:DAS MG INC
Neostigmine combination and compositions
InactiveUS20200000756A1Preventing and attenuating dose-limiting gastrointestinal adverse effectMuscular disorderPharmaceutical delivery mechanismSide effectMetaclazepam
The present invention describes the use of a 5HT3-antagonist, in combination with neostigmine, to facilitate the treatment of a patient suffering from myasthenia gravis or other myasthenic syndromes by providing a therapeutically effective neostigmine bromide or methylsulfate daily dose that attenuates or even abrogates the dose-limiting gastrointestinal adverse effects of neostigmine.
Owner:DAS MG INC
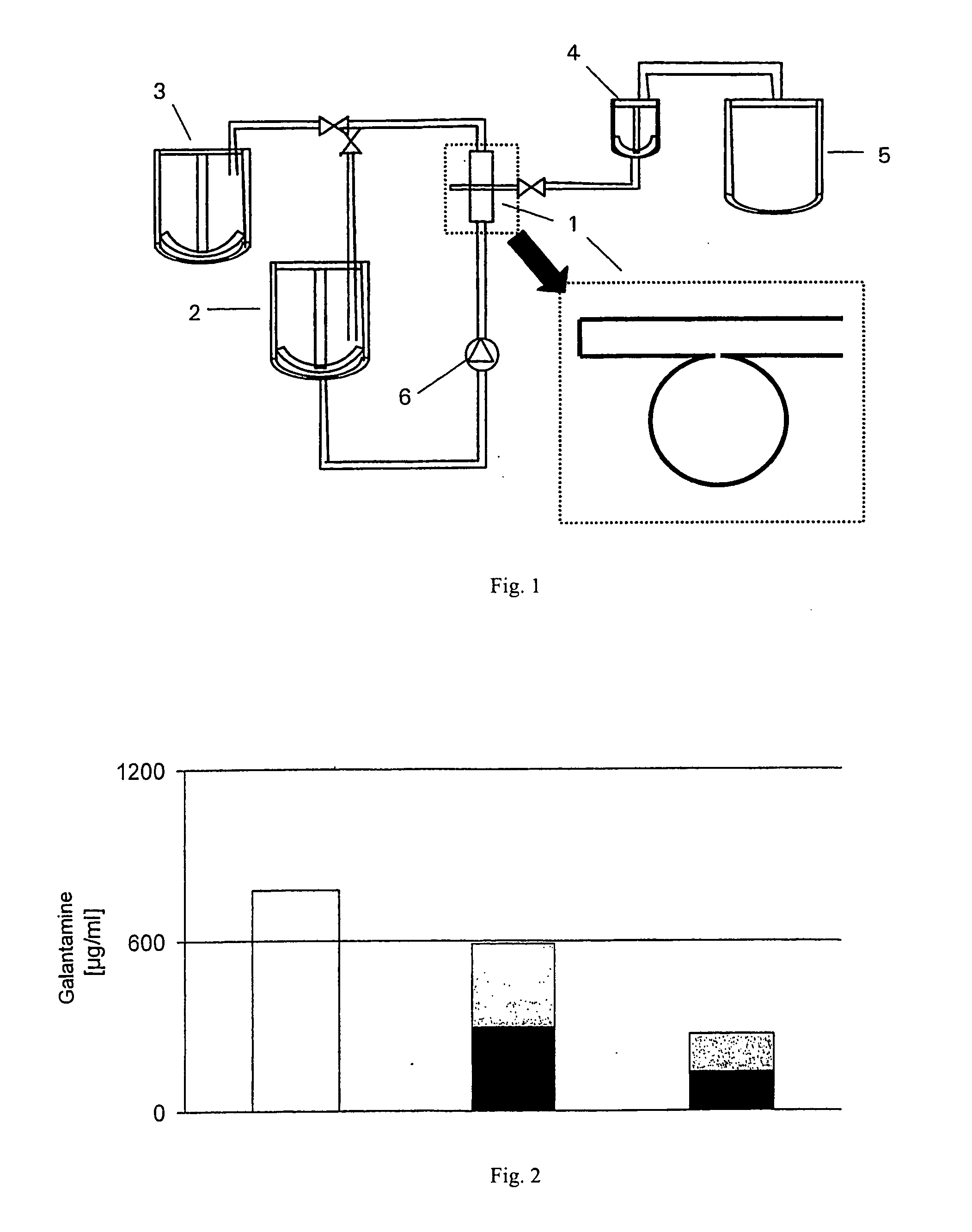
Novel preparation of neostigmine and derivatives thereof
PendingCN114010575AHigh absolute bioavailabilityExcellent absolute bioavailabilityDigestive systemMuscular disorderParoxysmal AFUterus
The invention provides a novel preparation of neostigmine and derivatives thereof. The preparation comprises a sublingual mucosa preparation, a nasal mucosa preparation, a rectum mucosa preparation, a vagina and uterus mucosa preparation and an oral buccal mucosa preparation which take neostigmine or neostigmine derivatives as active ingredients. The preparation is administered through any mucosa pathway of oral buccal mucosa, sublingual mucosa, nasal mucosa, rectum mucosa or vagina and uterus mucosa. The preparation has a great clinical application value in preparation of drugs for treating myasthenia gravis, postoperative abdominal distension and urinary retention, supraventricular paroxysmal tachycardia and detoxification in the case of excessive tupistrine.
Owner:江苏谛奇医药科技有限公司
Traditional Chinese medicine composition for abdominal enhancement as well as preparation method and application of traditional Chinese medicine composition
PendingCN114767812AInhibition of peristalsisSuppress spasmsDispersion deliveryDigestive systemWolfiporia extensaIntestinal peristalsis
The invention provides a traditional Chinese medicine composition for abdominal enhancement and a preparation method and application thereof, and relates to the technical field of traditional Chinese medicine compositions. The traditional Chinese medicine composition for abdominal enhancement comprises the following raw materials in parts by weight: 10-30 parts of codonopsis pilosula, 10-30 parts of white wood, 10-30 parts of poria cocos, 10-25 parts of radix aucklandiae, 10-30 parts of fructus amomi and 10-30 parts of pericarpium citri reticulatae. The traditional Chinese medicine composition prepared by the invention inhibits gastric emptying and small intestine peristalsis of normal animals, can resist hyperfunction of gastrointestinal motility caused by neostigmine or acetylcholine, and inhibits intestinal spasm caused by histamine and barium chloride. The traditional Chinese medicine composition prepared by the invention not only can inhibit gastrointestinal tract peristalsis, but also can enable gastrointestinal tracts to be in a full state, and also has the efficacy of protecting intestines and stomach, and a patient is treated and protected while being subjected to CT abdominal enhancement.
Owner:泸州市中医医院
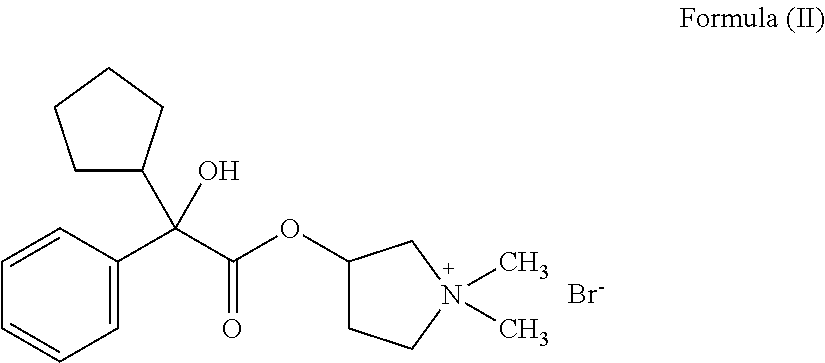
Ready-to-use injectable pharmaceutical compositions comprising neostigmine and glycopyrrolate
ActiveUS11110054B2Pharmaceutical delivery mechanismPharmaceutical non-active ingredientsCarboxylic acidPharmaceutical medicine
The present invention provides stable, ready-to-use injectable pharmaceutical compositions, comprising the combination of neostigmine, glycopyrrolate, a stabilizing amount of one or more aminopolycarboxylic acids, and a pharmaceutically acceptable liquid vehicle. Other aspects of the invention relate to methods for making such compositions and methods of using such compositions for reversing the effects of non-depolarizing neuromuscular blocking agents. Preferably, the composition comprises neostigmine methylsulfate, glycopyrronium bromide, ethylenediaminetetraacetic acid (EDTA) and a pharmaceutically acceptable liquid vehicle, and is provided in a pre-filled, ready-to-use sealed container, such as a pre-filled syringe, suitable for intravenous administration.
Owner:SLAYBACK PHARMA LLC
Maxillofacial medicament paste for treating snore and sleep apnea syndrome
InactiveCN101721393ANon-traumaticSleep does not affectRespiratory disorderEster active ingredientsNasal cavityEphedrine
Owner:江继平
Application of compound anisodamine injection in aspect of treating scalds
PendingCN114533732AReduce releaseReduce mortalityPharmaceutical delivery mechanismEster active ingredientsInflammatory factorsL-Hyoscyamine
The invention relates to the technical field of scald treatment, compound anisodamine is composed of a neostigmine injection and an anisodamine injection according to the proportion of 1: 500, and the proportion has good effects on hemorrhagic shock, allergic shock and increase of tolerance of mice to lethal dose endotoxin in the previous research of the unit. The pharmacological basis of the compound preparation is as follows: neostigmine is a reversible cholinesterase (AChE) inhibitor, temporarily inhibits cholinesterase activity, and increases the amount of acetylcholine (ACh) in vivo to excite an M receptor and an N receptor; a large dose of anisodamine has the effects of improving microcirculation and blocking M receptors, blocking of the M receptors enables N receptors to be more fully excited, in the excited N receptors and subtypes of the N receptors, after alpha 7 is excited, release of inflammatory factors can be reduced, and therefore it is proved that the compound anisodamine injection can remarkably reduce the mortality rate of serious scalds, and the compound anisodamine injection can be used for treating the severe scalds. The survival time after injury is prolonged.
Owner:范德里希(上海)生物科技有限公司
Chinese and Western compound medicine for treating progressive spinal muscular atrophy and preparing method thereof
InactiveCN106177629AGood treatment effectImprove medicinal effectNervous disorderMuscular disorderLiver and kidneyPotentilla anserina
The invention discloses Chinese and Western compound medicine for treating progressive spinal muscular atrophy and a preparing method thereof, and belongs to the technical field of medicine. The medicine is prepared from large-leaved chrysosplenium, sweet pomegranate, semen coicis, pine needles, herba epimedii, ficus lyrata, heterophyllous wing seedtreeroot, nude fern herb, silverweed cinquefoil root, David's deerhorn, cajeput, radix achyranthis bidentatae, radix asparagi, tarenna attenuata, elemental diet, ambenonium chloride, piribedil and neostigmine. The adopted Chinese and Western medicine combined treatment method has a better effect than that of just using traditional Chinese medicine or Western medicine, with the treatment rule of nourishing liver and kidney, strengthening the muscles and bones, dispelling wind and removing obstruction in the meridians, nourishing heart, tonifying qi and moistening and promoting granulation, the traditional Chinese medicine components and Western medicine effects are improved with the maximum efficiency, complementation and promotion functions are brought into play, the medicinal effect is improved, central nervous systems of nerves, internal secretion and immunity of the organism are stimulated to carry out stress response, and the Chinese and Western compound medicine has good treat effects on muscular atrophy, skin numb, myasthenia, periodic paralysis and myodystrophy caused by progressive spinal muscular atrophy.
Owner:王延林
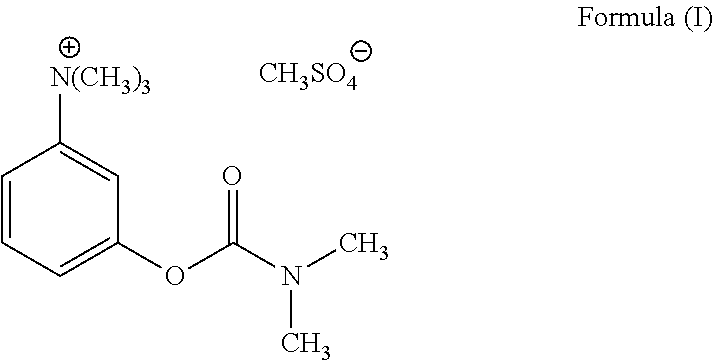
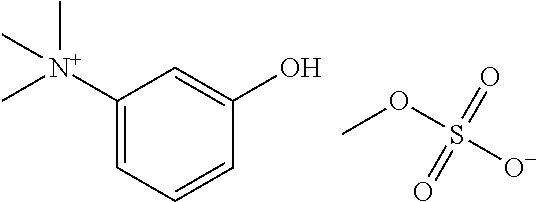
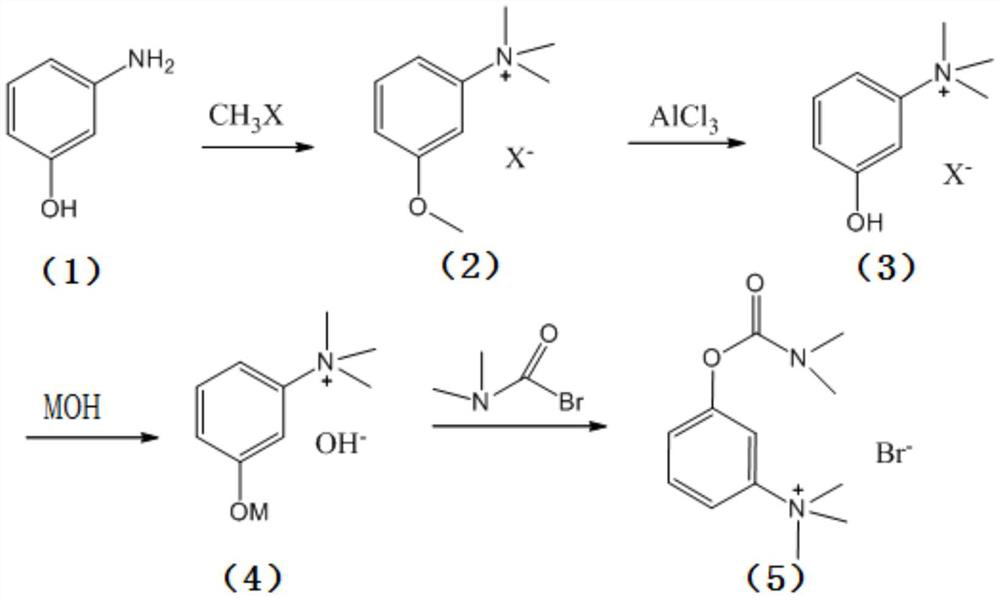
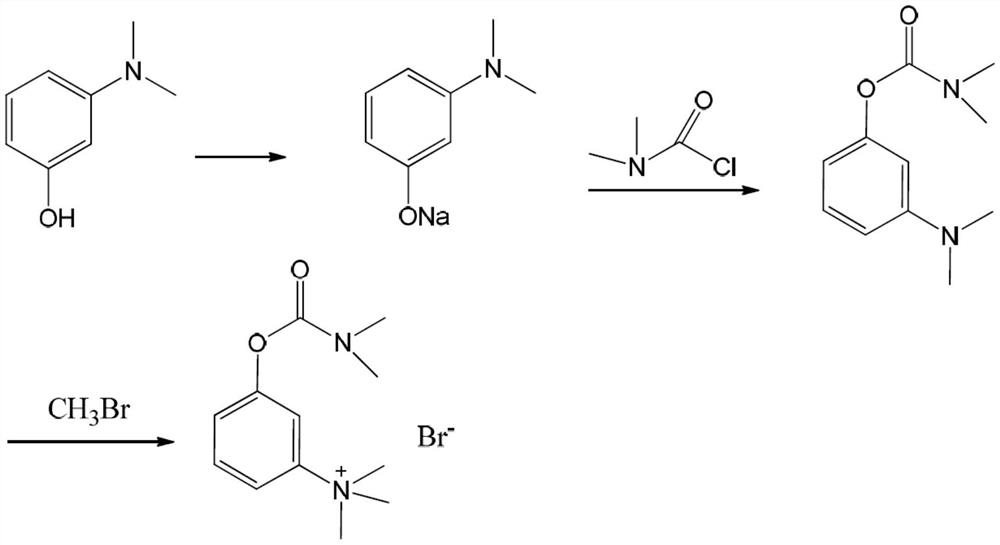
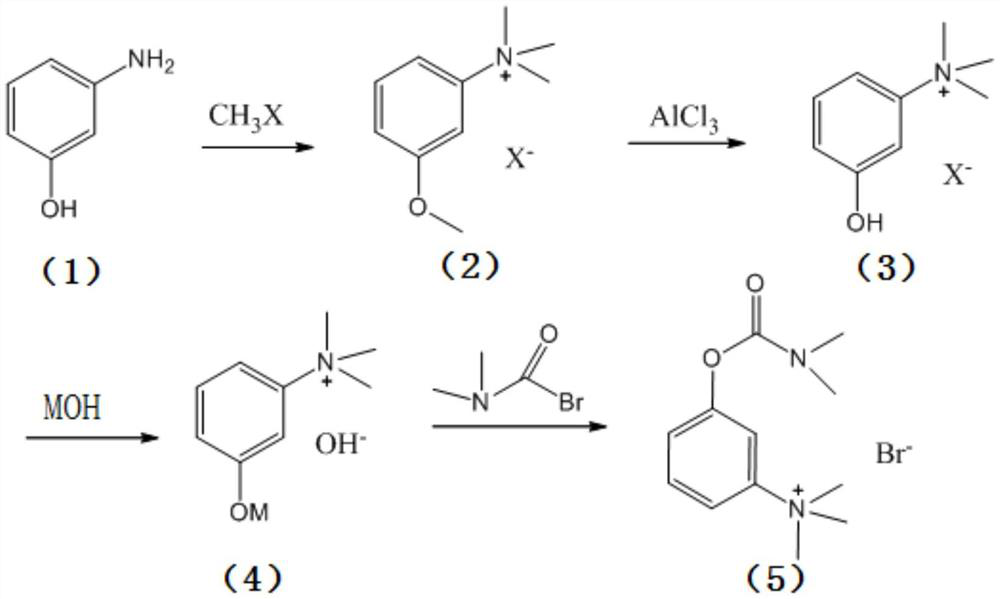
Synthesis method of neostigmine bromide
ActiveCN113277961AEasy to buySufficient sourceCarbamic acid derivatives preparationOrganic compound preparationM-aminophenolEther
The invention relates to the technical field of medicine synthesis, in particular to a synthesis method of neostigmine bromide. The synthesis method comprises the following steps of 1) selecting m-aminophenol of a formula (1) to react with halogenated methane to obtain halogenated quaternary ammonium salt methyl ether of a formula (2), 2) adding a catalyst into the halogenated quaternary ammonium salt methyl ether in the formula (2), and removing methyl ether to obtain m-phenolic hydroxyl quaternary ammonium salt in a formula (3), 3) forming a compound shown in a formula (4) from the m-phenolic hydroxyl quaternary ammonium salt shown in the formula (3) under a strong alkali condition, and 4) carrying out esterification reaction on the compound in the formula (4) and dimethyl carbamoyl bromide to obtain neostigmine bromide in a formula (5). According to the method, the m-aminophenol is adopted as the starting material, the price of the m-aminophenol is only one hundred of that of m-dimethylaminophenol, the cost of the neostigmine bromide can be greatly reduced when the m-aminophenol is used for synthesizing the neostigmine bromide, and mass production can be achieved.
Owner:深圳市新浩瑞医药科技有限公司
Traditional Chinese medicinal composition for preventing and treating postpartum urinary retention as well as preparation method and application thereof
ActiveCN109675008AGood prevention effectDecreased urinary retentionPharmaceutical delivery mechanismSexual disorderSide effectBleeding postpartum
The invention belongs to the technical field of traditional Chinese medicine preparation, and relates to a traditional Chinese medicinal composition for preventing and treating postpartum urinary retention as well as a preparation method and application thereof. The traditional Chinese medicinal composition is prepared from the following components in parts by weight: 3 to 30 parts of rhizoma zingiberis recens, 3 to 30 parts of fistular onion stalk, 0.5 to 5 parts of fine salt, 2 to 30 parts of raw radix astragali powder, 2 to 30 parts of poria cocos powder and 2 to 30 parts of motherwort herbpowder. Through half an hour of umbilical application, the traditional Chinese medicinal composition has a significant effect on preventing postpartum urinary retention, and greatly reduces the incidence of urinary retention. The traditional Chinese medicinal composition has a remarkable prevention effect, and the therapeutic effect is more obvious than that of neostigmine and other Western medicines. The traditional Chinese medicinal composition has no obvious toxic and side effects, is economical, practical and convenient to operate, shortens the hospitalization time of patients, can reducethe occurrence of post-operative urinary tract infection and postpartum hemorrhage, provides reliable clinical evidence for the popularization of application of traditional Chinese medicine to Shenque acupoint on umbilicus, and has clinical and basic medical institution popularization and application value.
Owner:王华英
Compound anisodamine neostigmine sustained-release tablet and preparation method thereof
ActiveCN105943537BSmooth releaseImprove complianceAntipyreticAnalgesicsSustained Release TabletSide effect
The invention discloses a sustained-release tablet prepared by compounding anisodamine and neostigmine and a preparation method of the sustained-release tablet. The sustained-release tablet is prepared from the following components in parts by weight: 10-20 parts of the anisodamine, 0.01-1 part of the neostigmine, 5-50 parts of sustained-release matrix materials, 0.3-5 parts of a lubricant, 1-20 parts of an adhesive, 1-15 parts of a diluting agent, 1-50 parts of a disintegrating agents and 1-50 parts of a filling agent. The sustained-release tablet provided by the invention is smooth and steady in release; by taking the sustained-release tablet provided by the invention, dosing times are reduced, and cting time is increased; the compliance of a patient is improved; side effects caused by an overhigh peak concentrationh are lowered; the sustained-release tablet is simple in preparation process, relatively low in cost, easy to control and suitable for large-scale industrial production, and has relatively high application value.
Owner:无锡圆道医药科技研究院有限公司
Neostigmine bromide slow release preparation and its preparation method
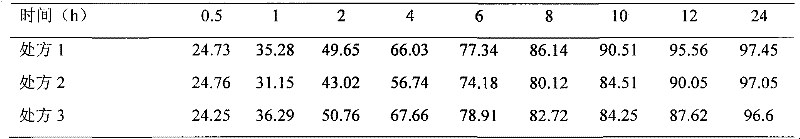
InactiveCN102258492BImprove stabilityAvoid instabilityDigestive systemMuscular disorderSide effectNeostigmine
The present invention belongs to the technical field of medicinal preparation. The invention discloses a formula of a neostigmine bromide slow release preparation which can be taken once every day for treating myasthemia gravis, functional flatulence after being performed an operation and urinary retention, as well as its preparation method. The preparation is disclosed as a slow release tablet form composed of a skeleton core containing neostigmine bromide and the slow release preparation and a coating. The neostigmine bromide slow release preparation of the present invention is capable of overcoming the disadvantage of present medicament common tablets in the market, slowly releasing to keep stable blood and medicine concentration, acting for a longer period, possessing low toxicity andside effect and conveniently taking the preparation, and the slow release preparation keeps effective blood and medicine concentration for a long time, reduces the medicine taking frequency, raises the compliance of the patients and reduces the side-effect due to over peak concentration. The invention has the advantages of simple preparation technology, low cost, easy control and easy industrial production.
Owner:CHONGQING MEDICAL UNIVERSITY
Medicine for treating colonitis
PendingCN110742889AOvercome the shortcomings of large side effects of single applicationThe synthesis method is simple and easy to obtainDigestive systemEster active ingredientsSide effectPharmaceutical drug
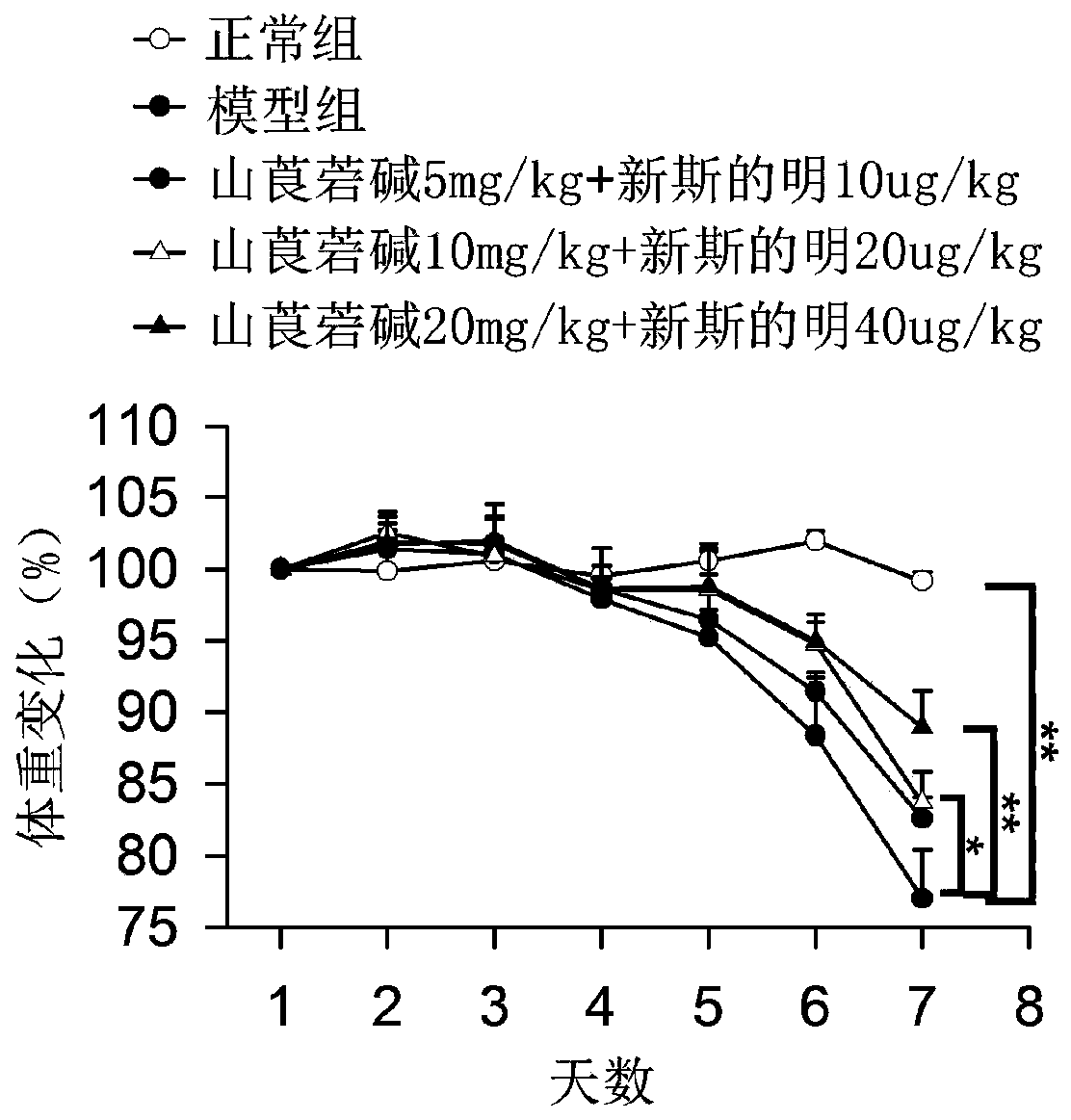
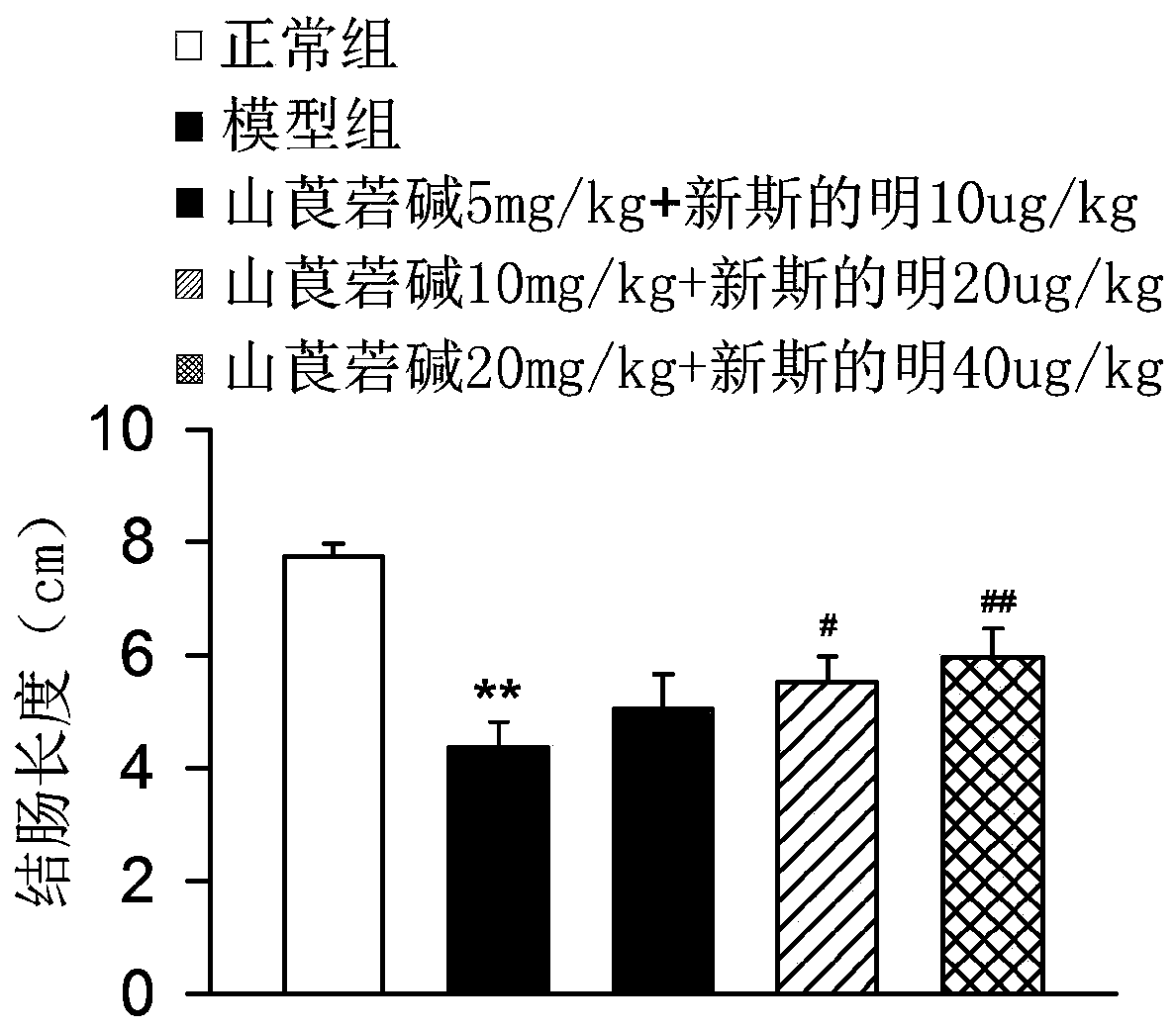
The invention discloses a medicine for treating colonitis, and belongs to the technical field of medicines. The invention discloses the medicine for treating colonitis. According to the medicine, themass ratio of racanisodamine and neostigmine is 500:1; and the racanisodamine is used as a traditional medicine, is simple and convenient in synthetic method, is easy to get, and is combined with theneostigmine for application so as to be improved in therapeutical effect and reduced in toxic and side effects, so that the defect of severe side effects caused by the independent application of a medicine is overcome. The medicine for treating colonitis provided by the invention provides a new means for the clinic treatment of colonitis.
Owner:镇江星盘商务咨询有限公司
Compound anisodamine and neostigmine sustained-release tablet and preparation method thereof
ActiveCN105943537ASmooth releaseImprove complianceAntipyreticAnalgesicsSustained Release TabletSide effect
The invention discloses a sustained-release tablet prepared by compounding anisodamine and neostigmine and a preparation method of the sustained-release tablet. The sustained-release tablet is prepared from the following components in parts by weight: 10-20 parts of the anisodamine, 0.01-1 part of the neostigmine, 5-50 parts of sustained-release matrix materials, 0.3-5 parts of a lubricant, 1-20 parts of an adhesive, 1-15 parts of a diluting agent, 1-50 parts of a disintegrating agents and 1-50 parts of a filling agent. The sustained-release tablet provided by the invention is smooth and steady in release; by taking the sustained-release tablet provided by the invention, dosing times are reduced, and cting time is increased; the compliance of a patient is improved; side effects caused by an overhigh peak concentrationh are lowered; the sustained-release tablet is simple in preparation process, relatively low in cost, easy to control and suitable for large-scale industrial production, and has relatively high application value.
Owner:无锡圆道医药科技研究院有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com