Patents
Literature
2298 results about "Intestinal structure" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
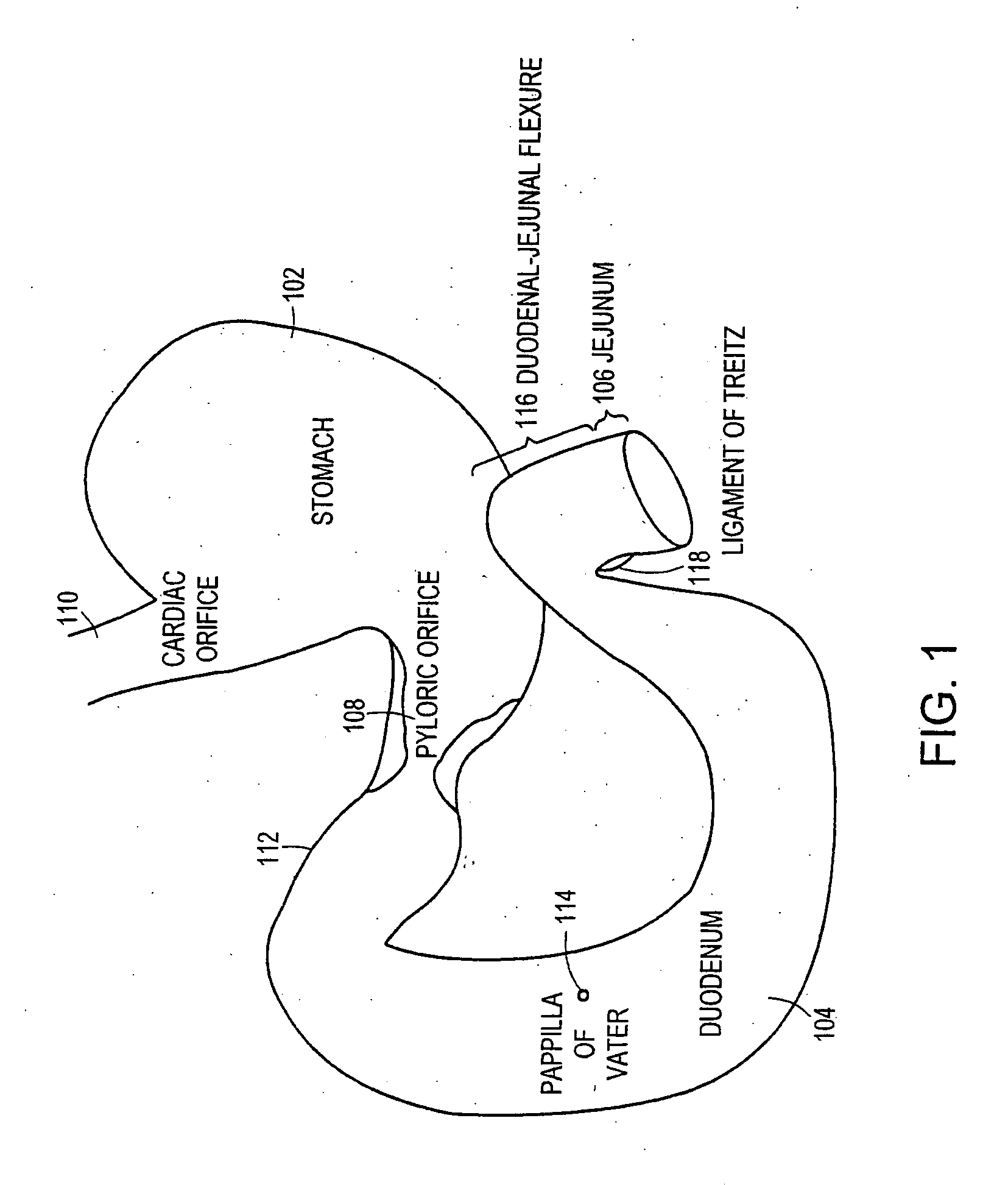
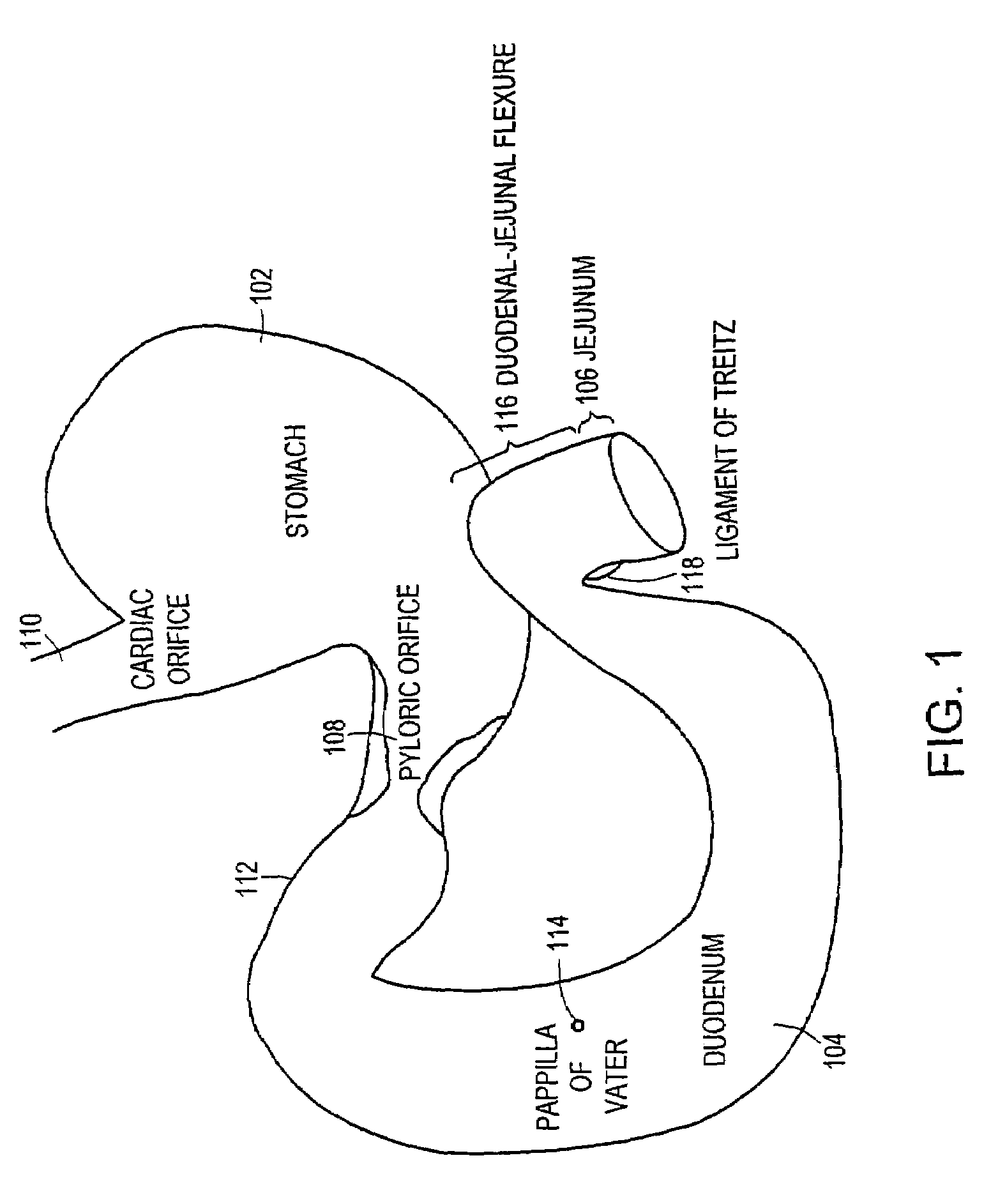
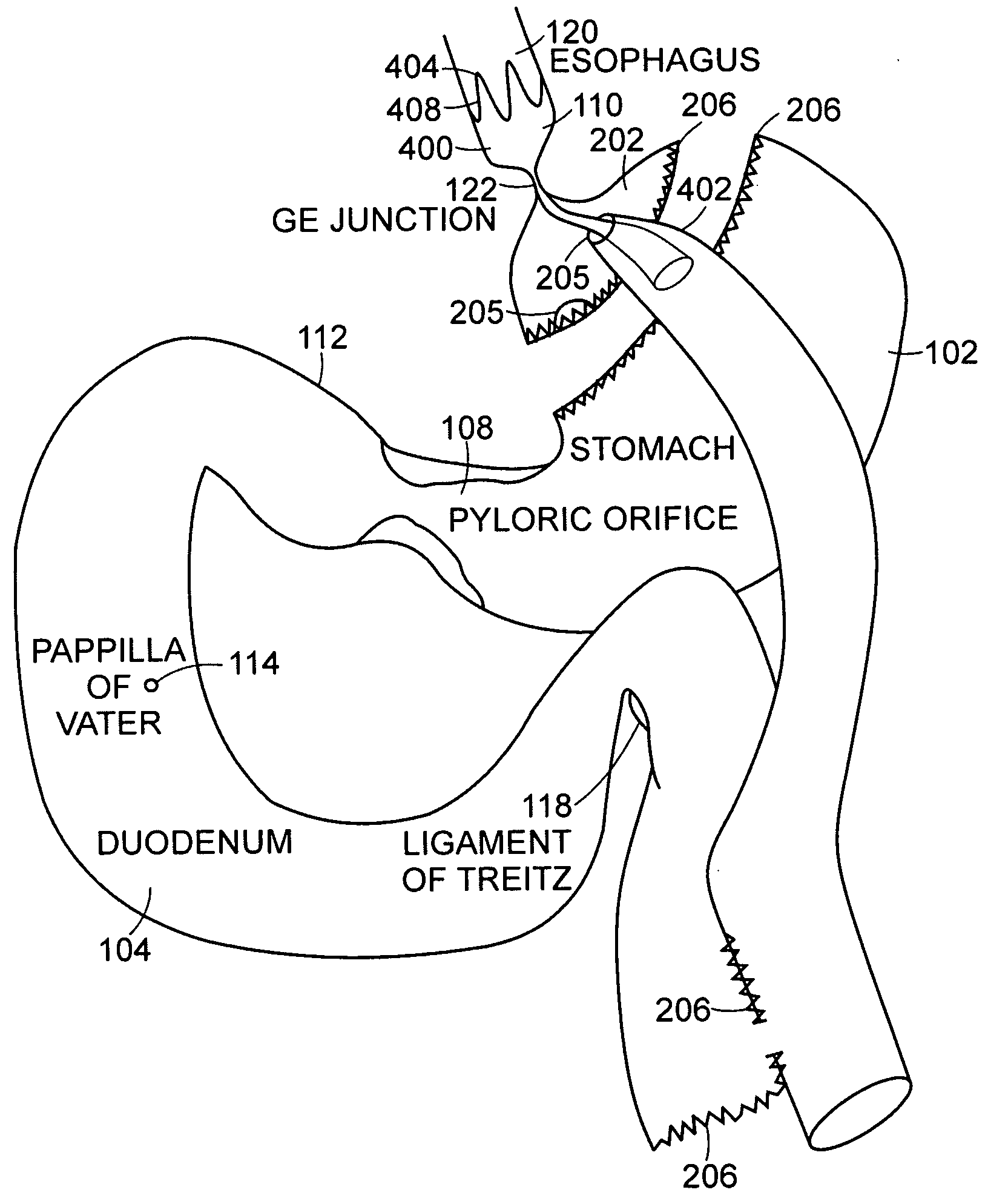
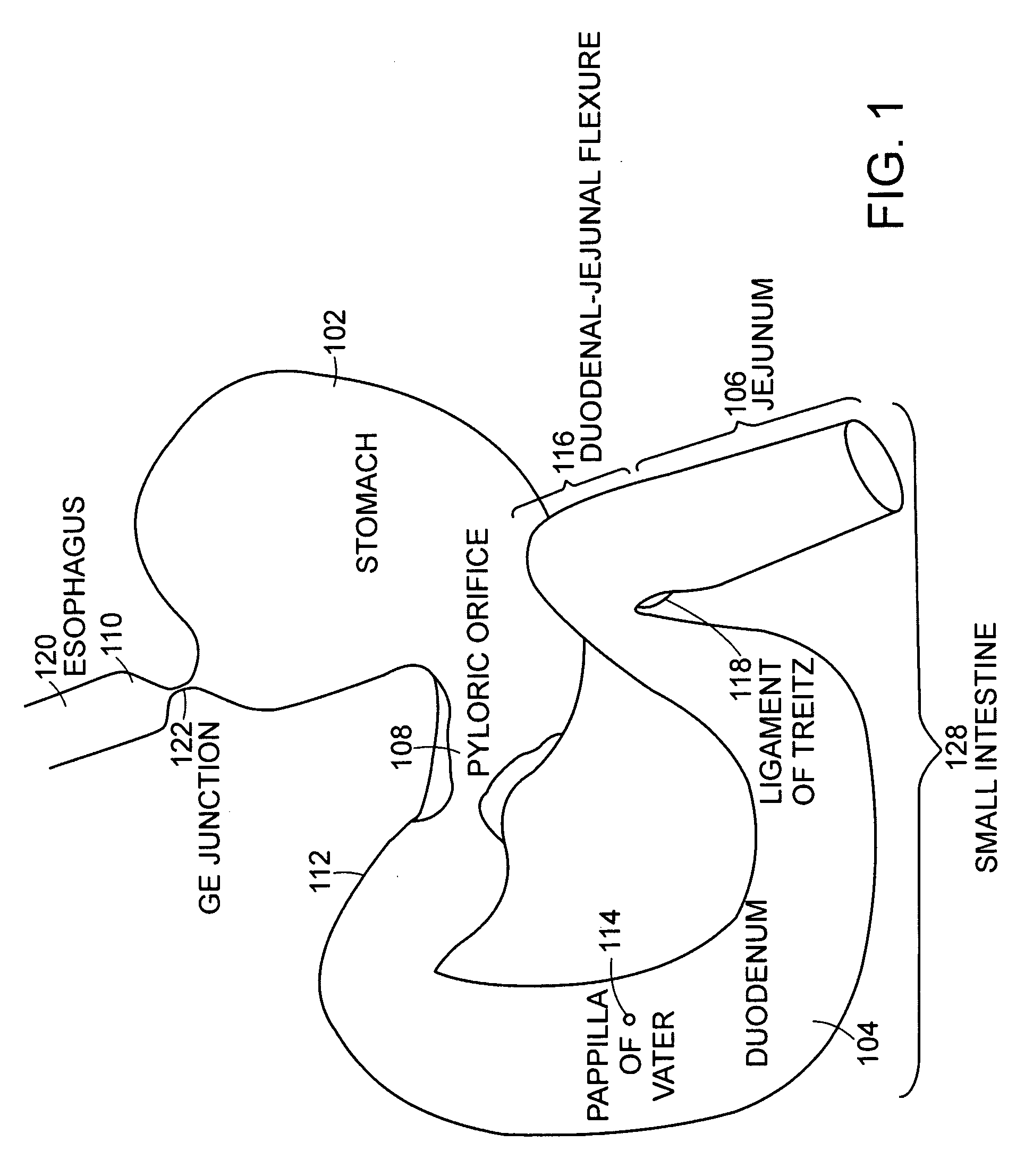
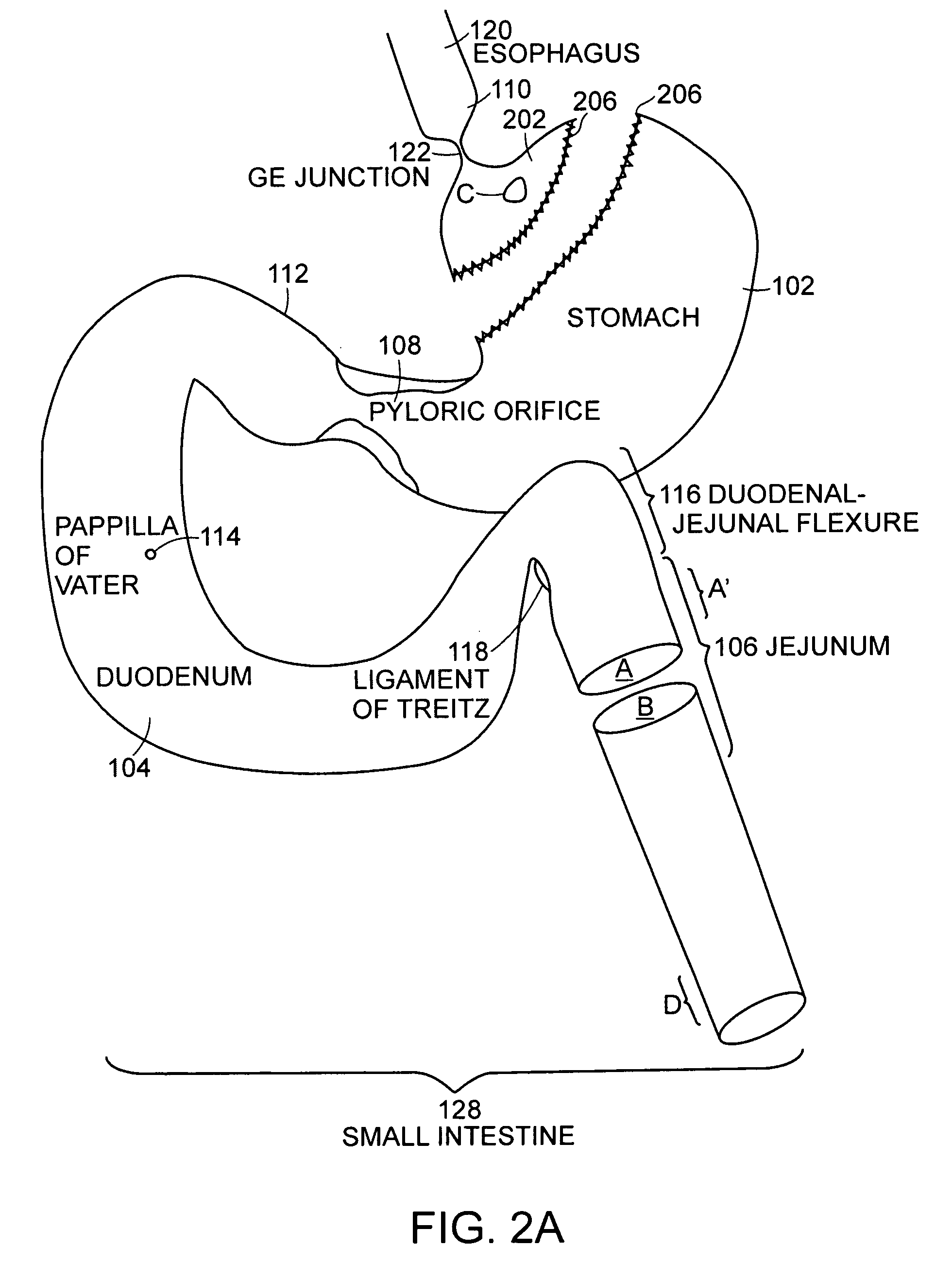
Small intestine. The small intestine begins at the duodenum and is a tubular structure, usually between 6 and 7 m long. Its mucosal area in an adult human is about 30 m2. Its main function is to absorb the products of digestion (including carbohydrates, proteins, lipids, and vitamins) into the bloodstream.
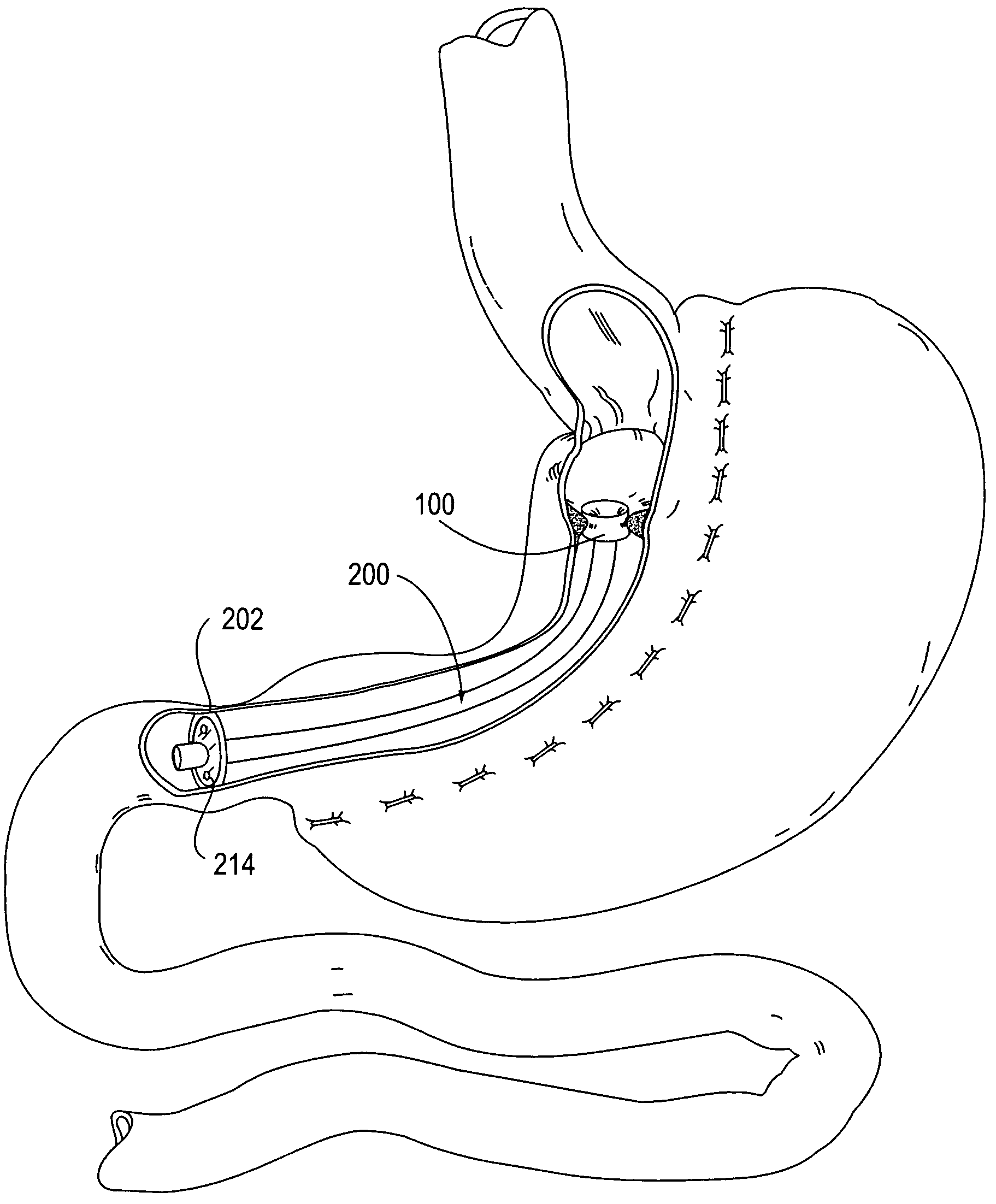
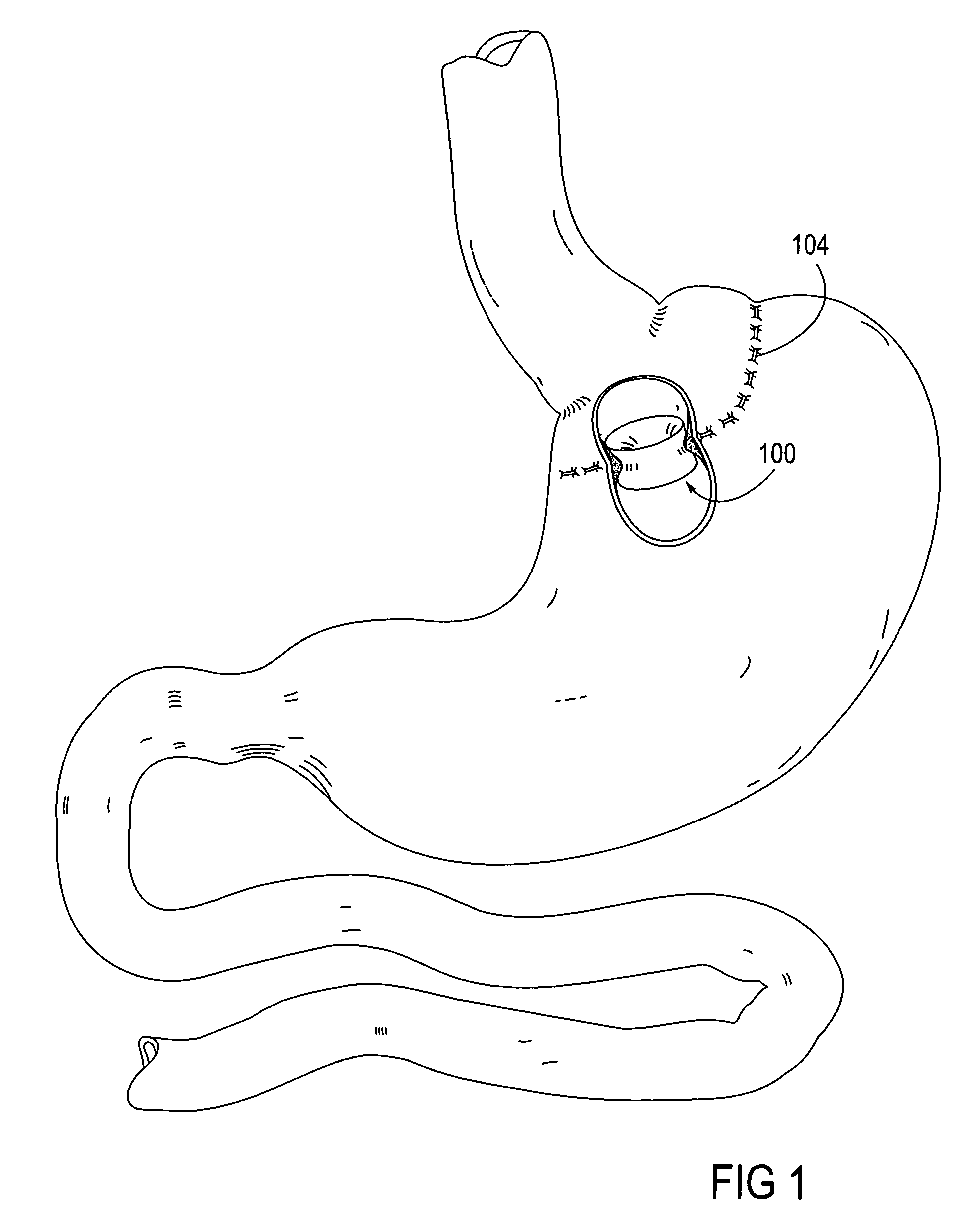
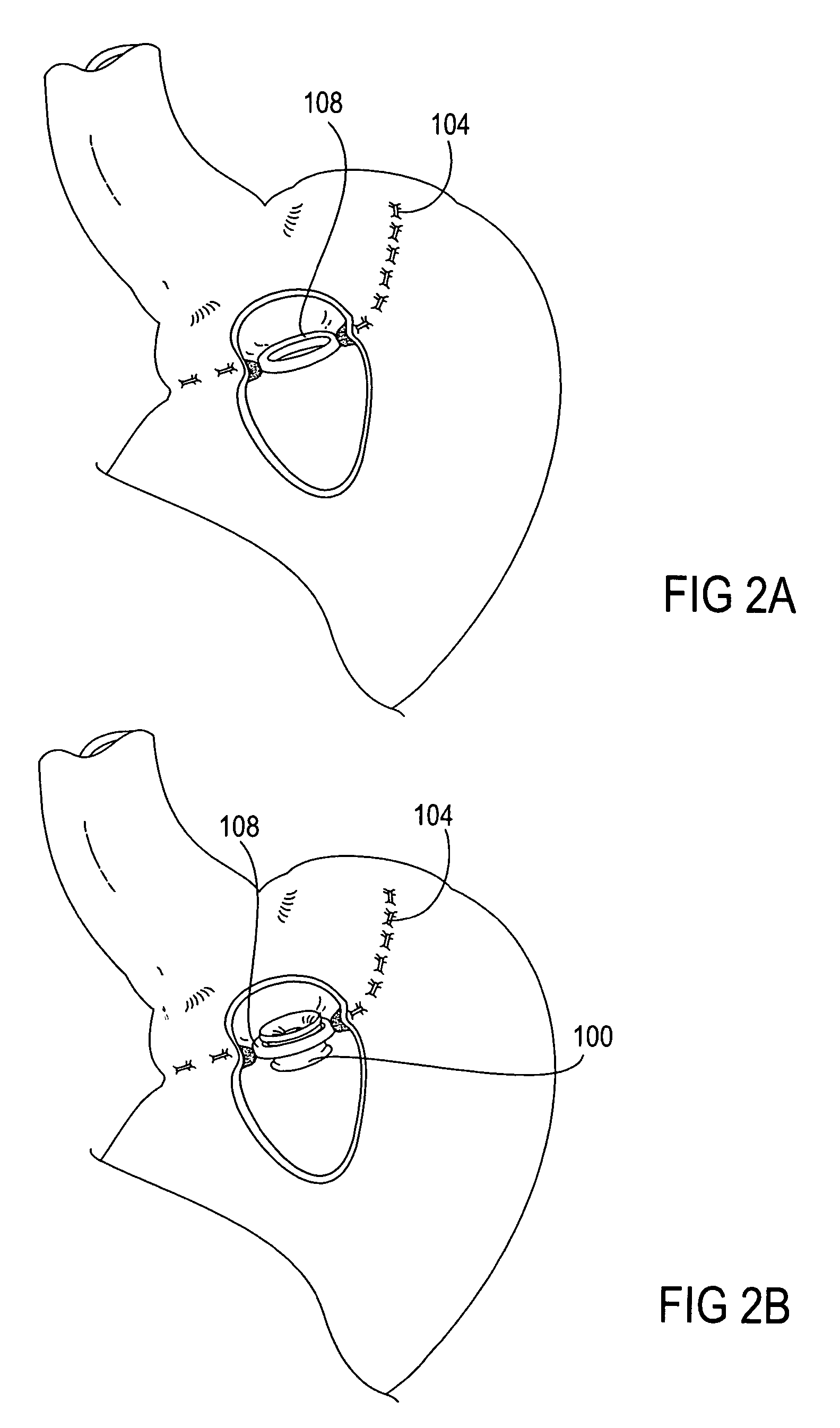
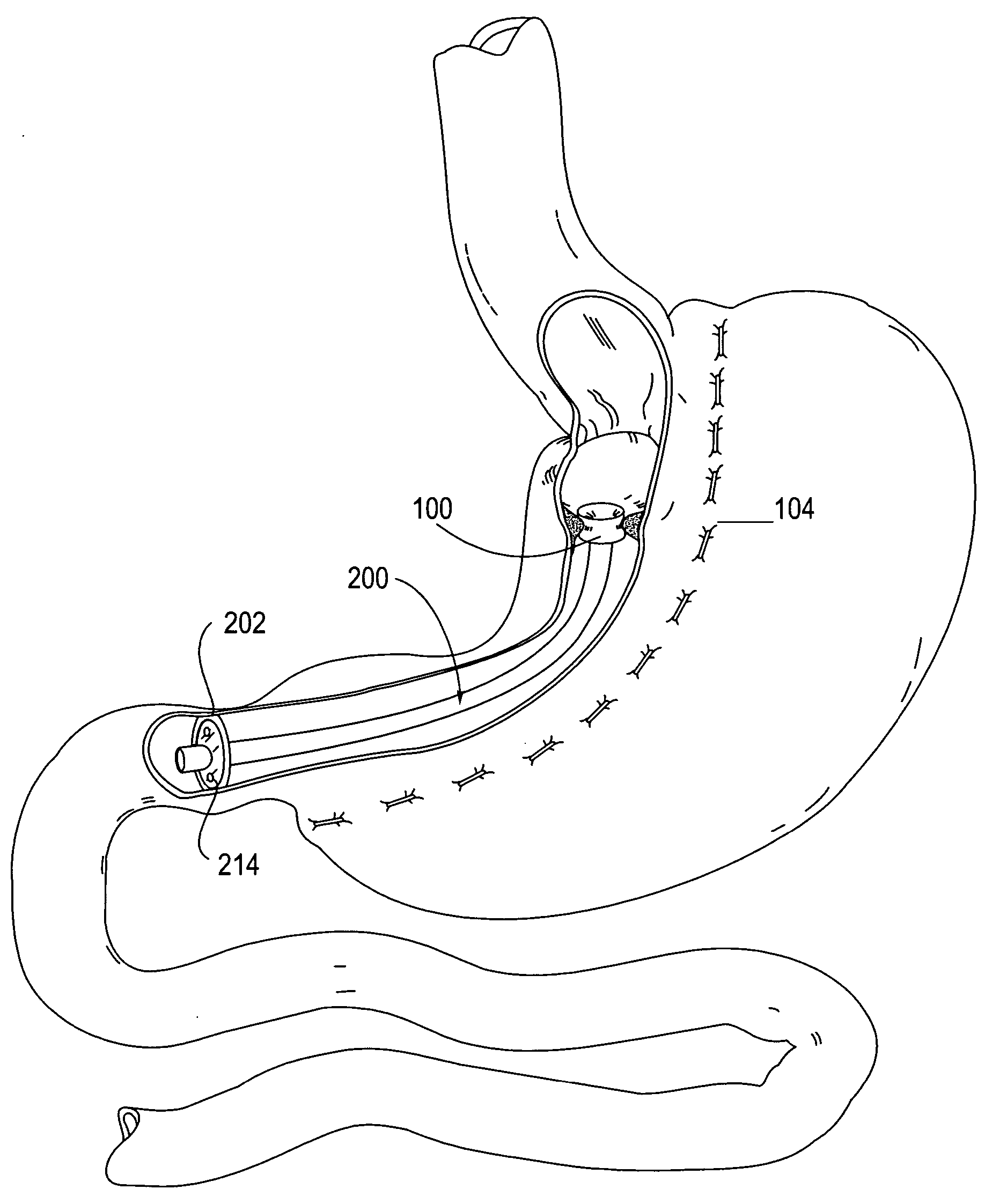
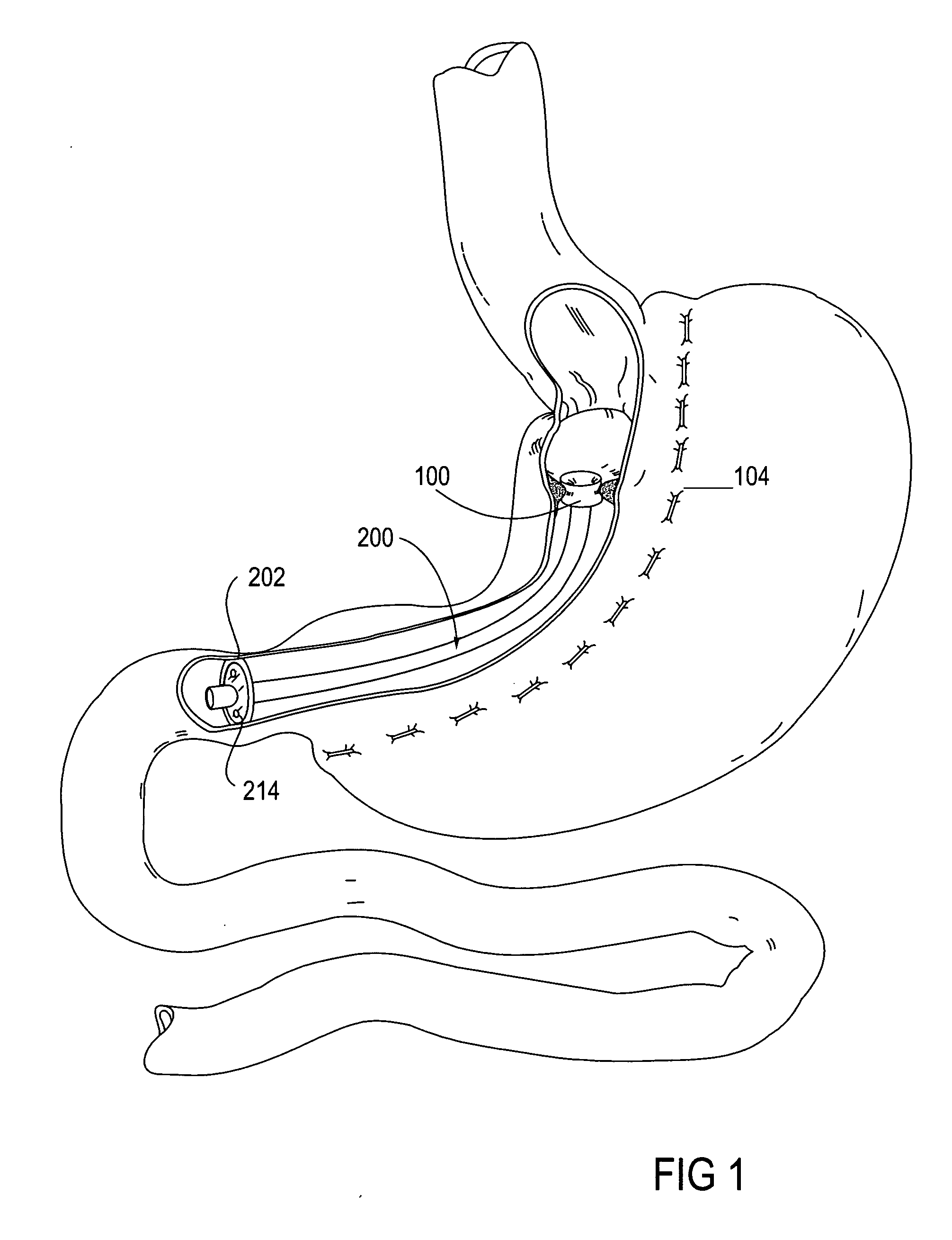
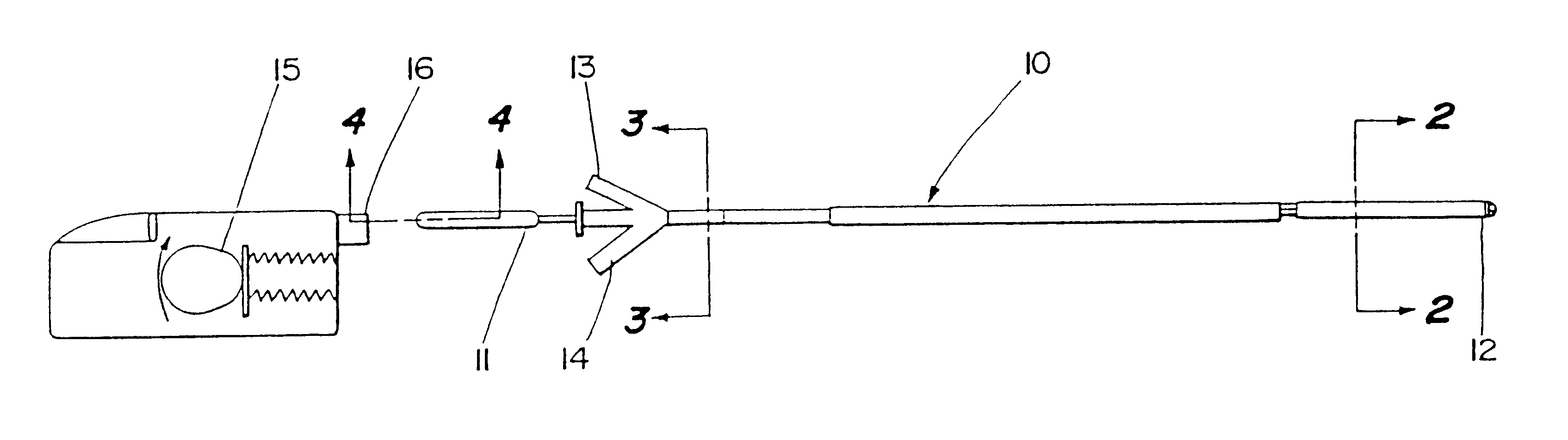
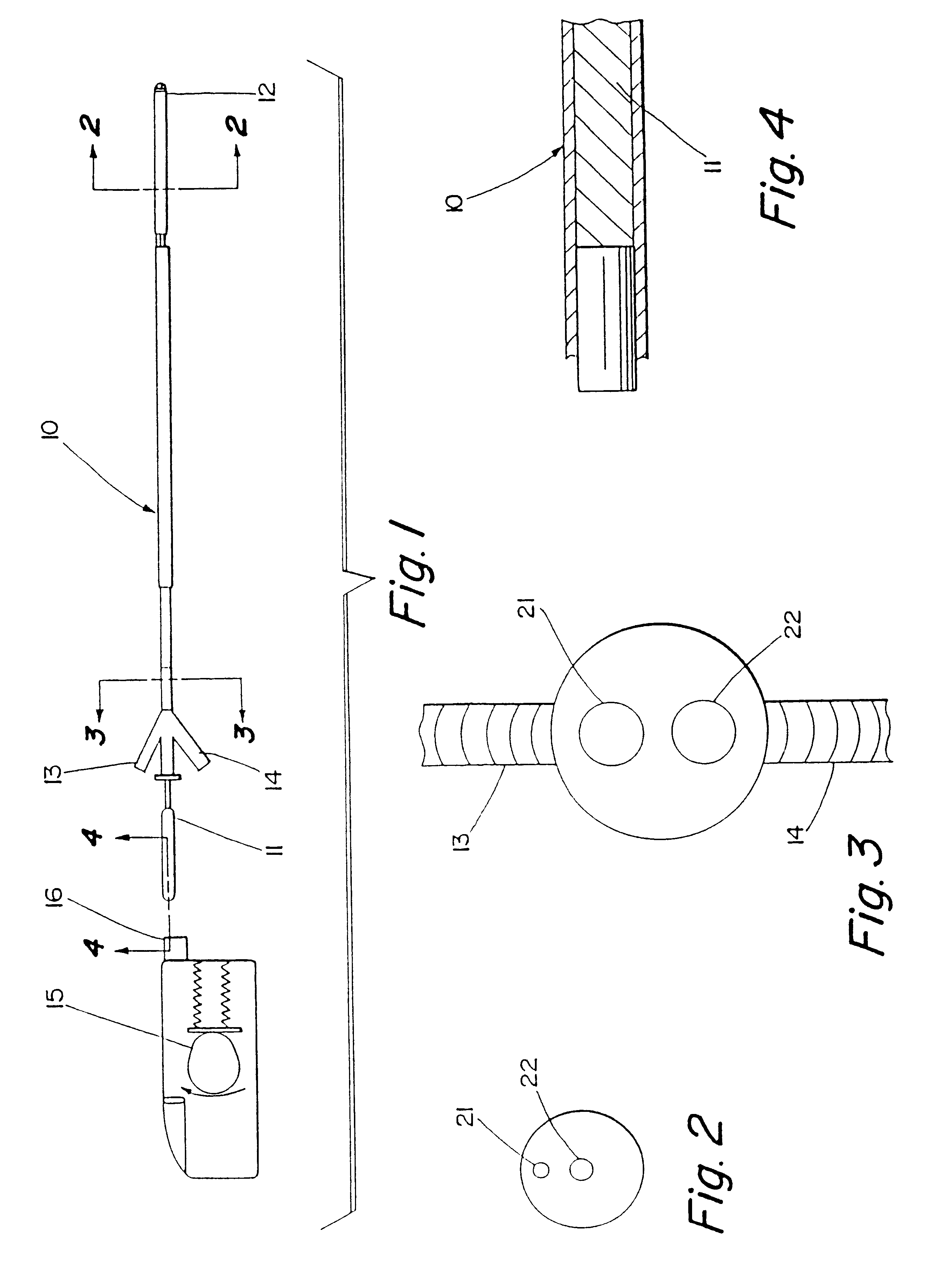
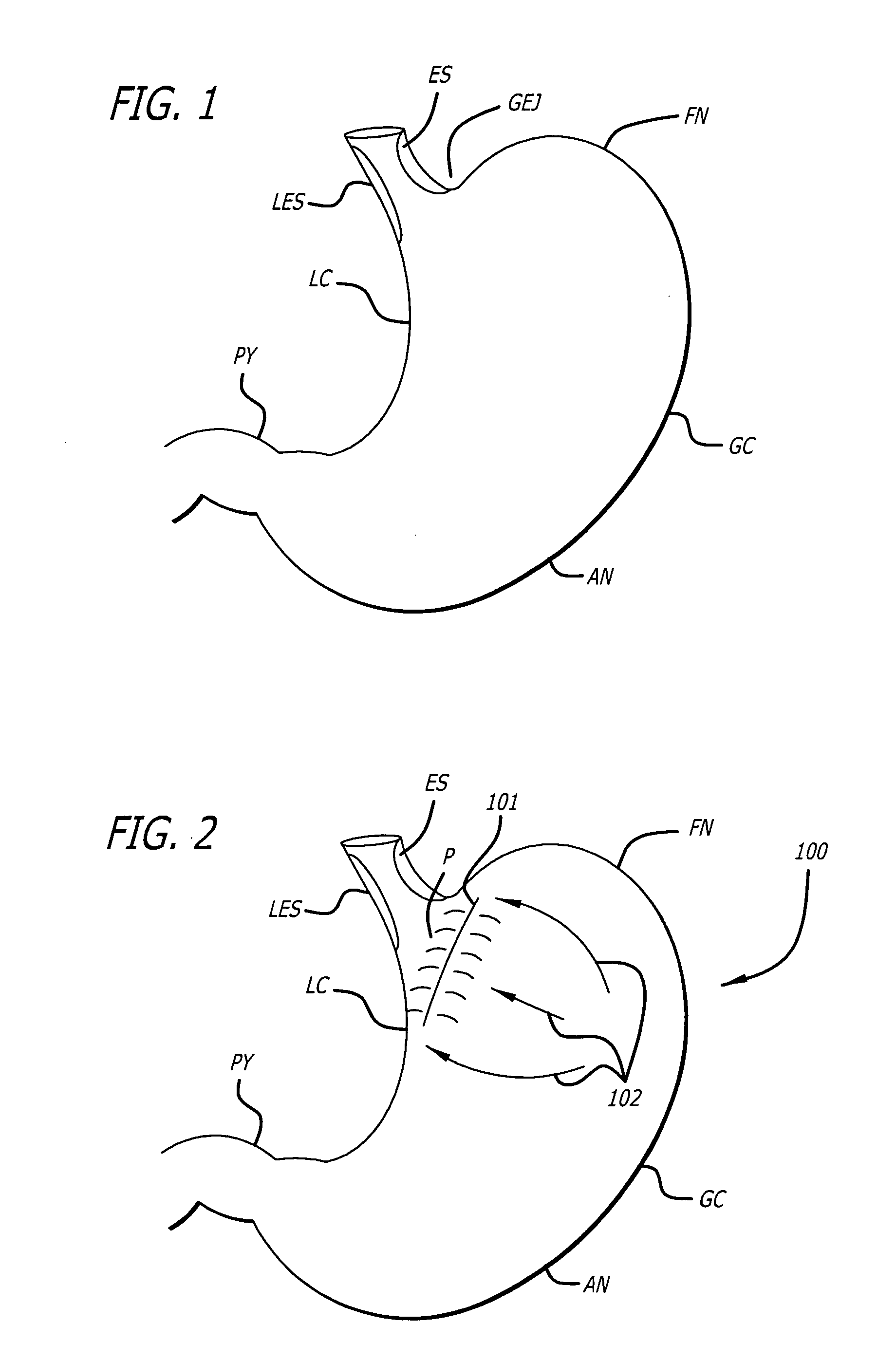
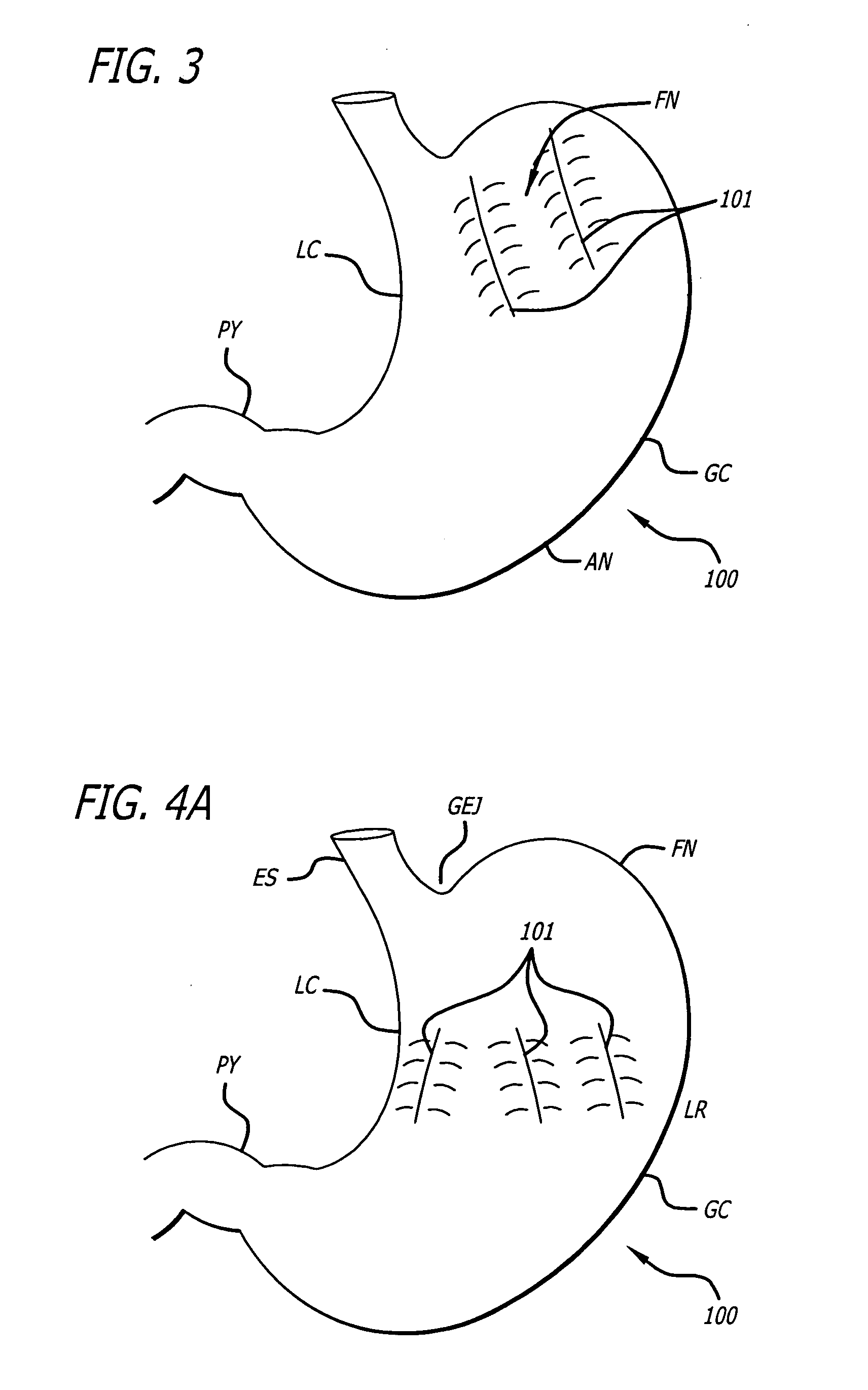
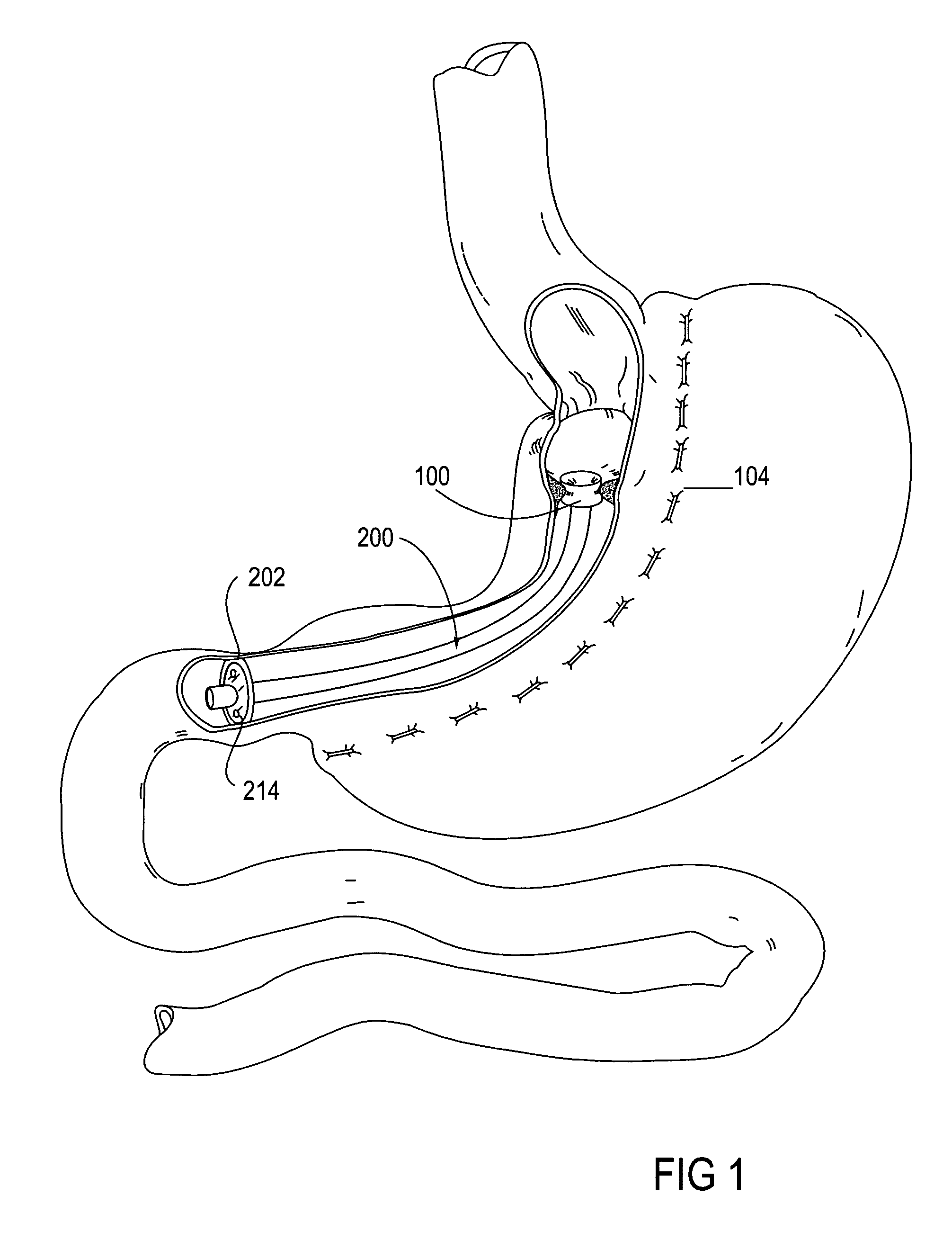
Apparatus and methods for treatment of morbid obesity
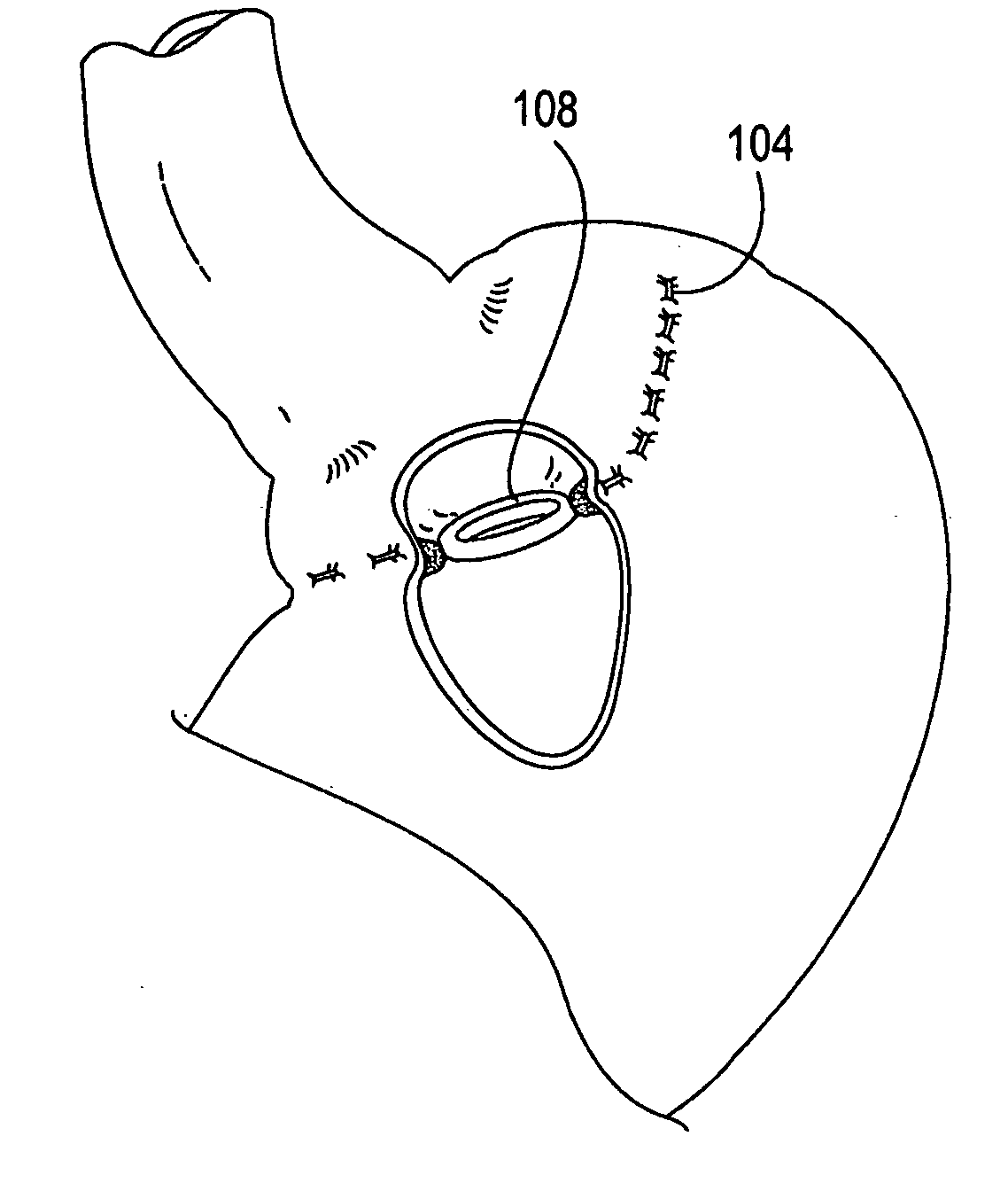
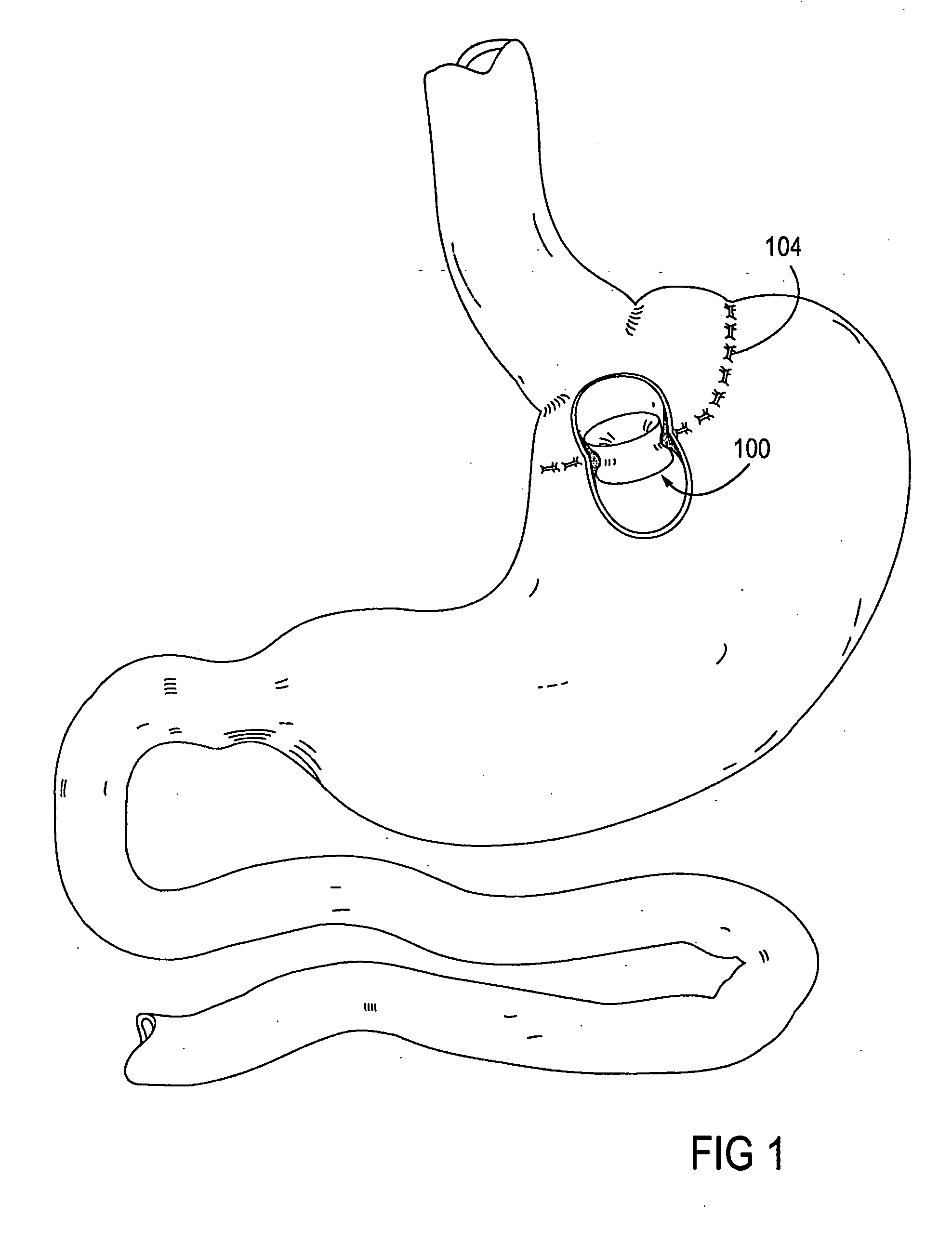
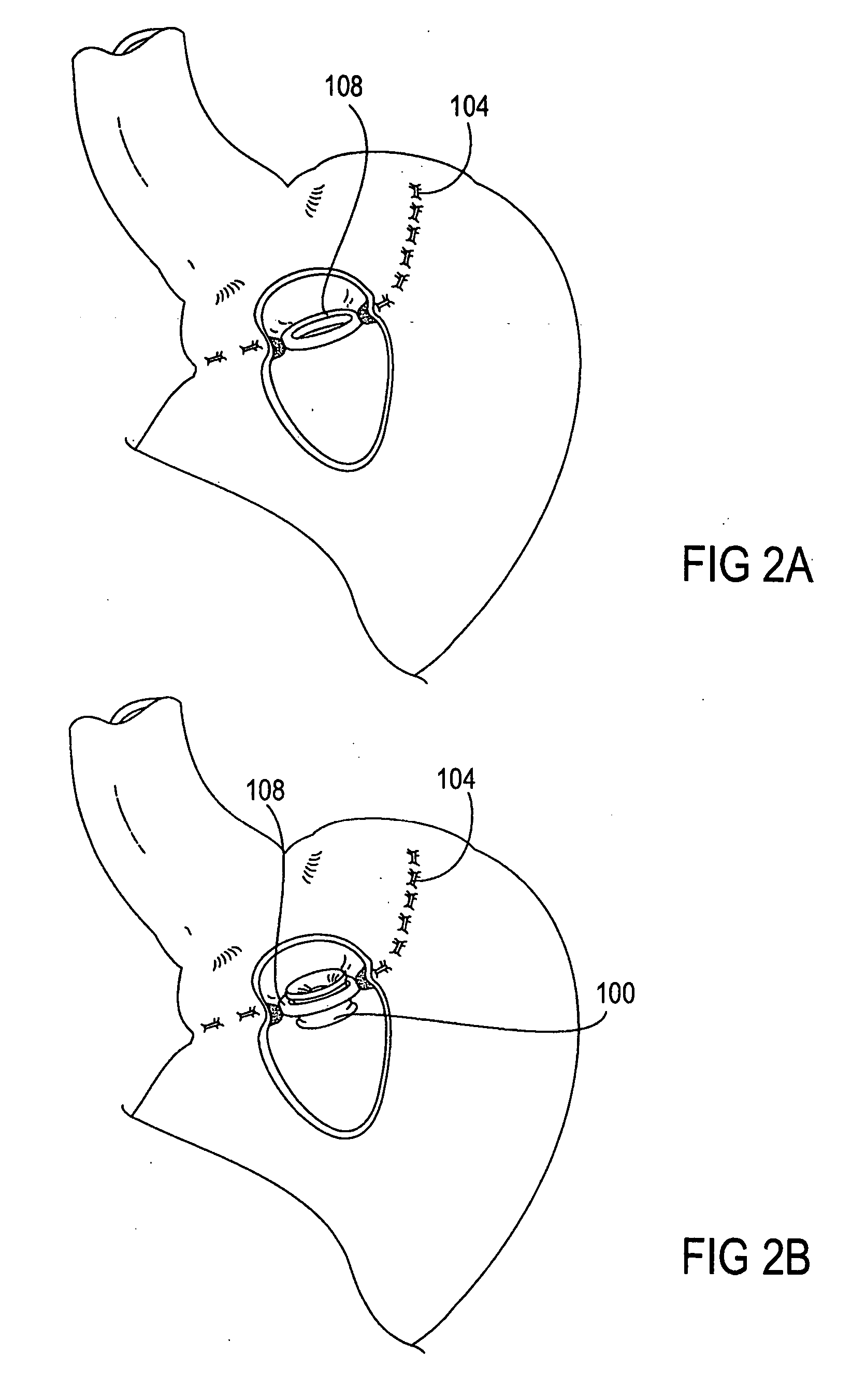
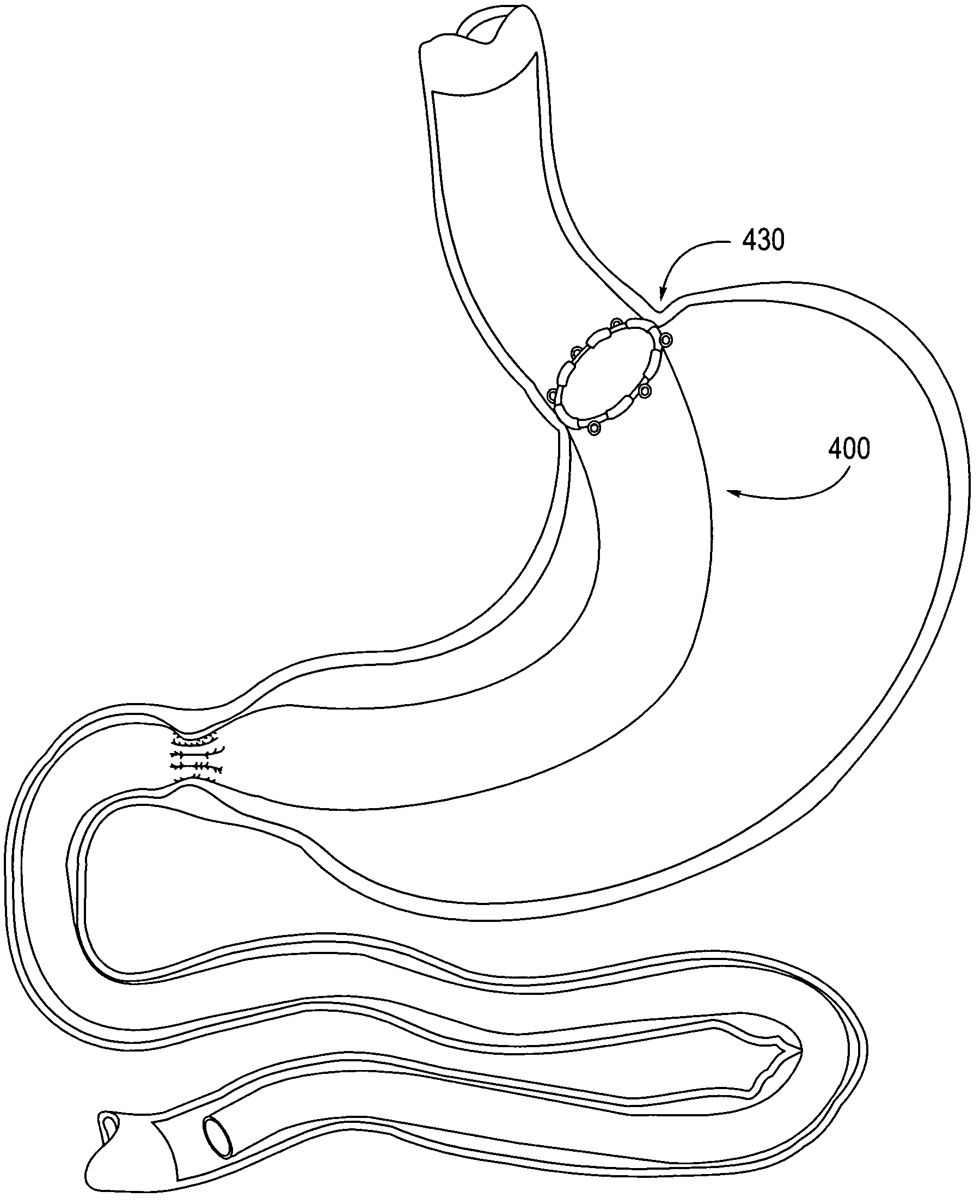
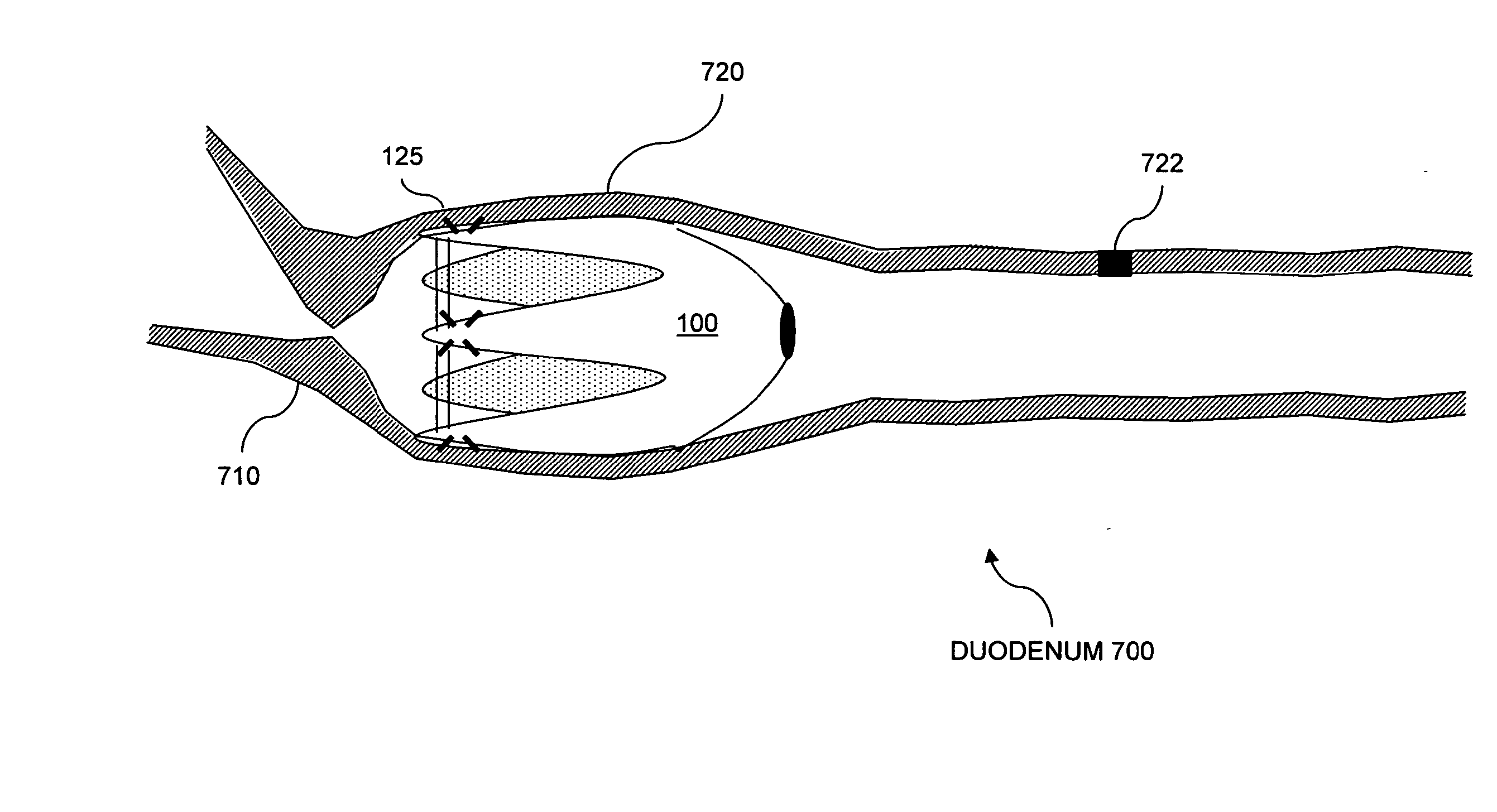
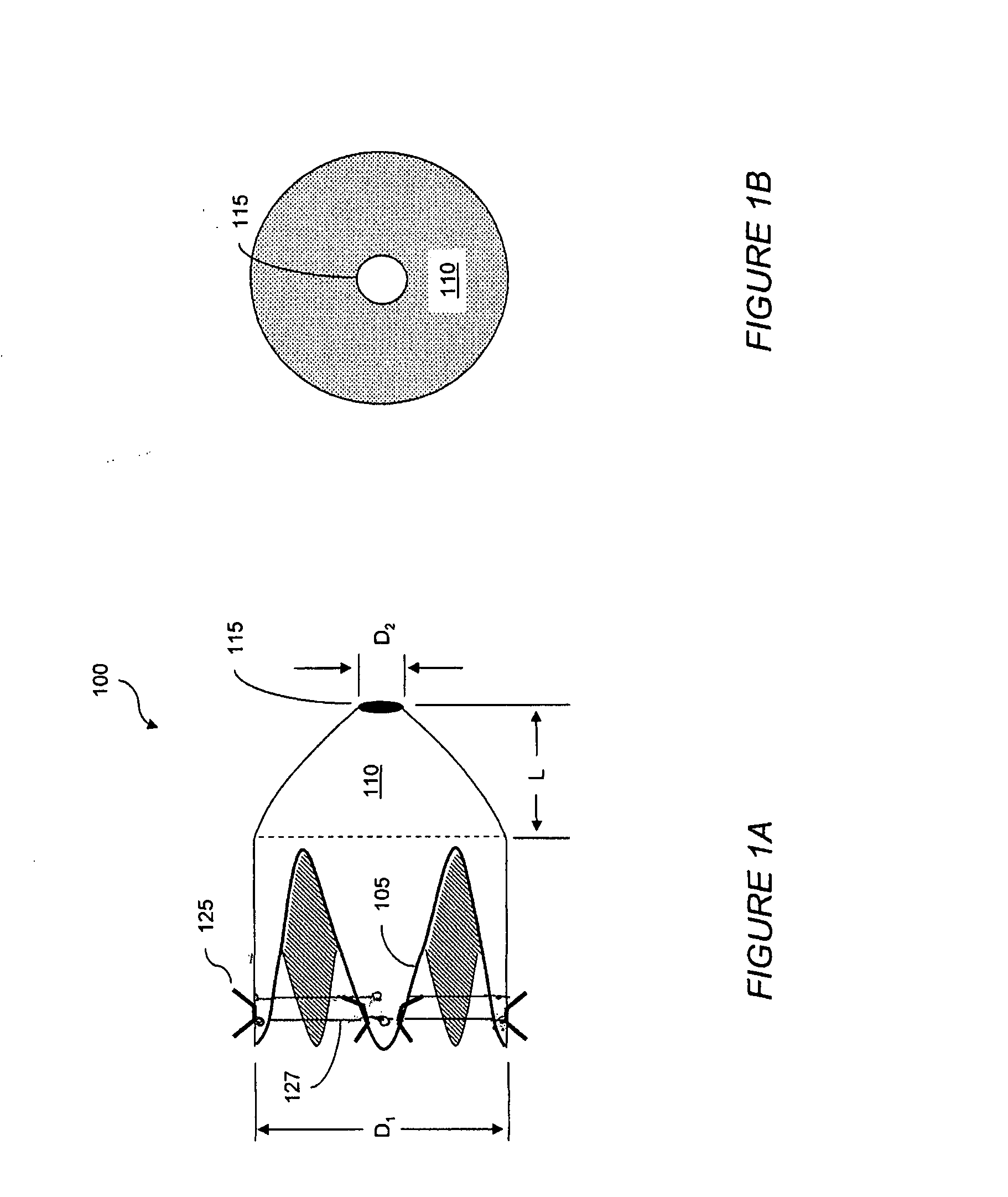
Apparatus and methods are described for treatment of morbid obesity using minimally invasive techniques. The apparatus includes a system of components that may be used separately or in combination for effectively reducing stomach volume, bypassing a portion of the stomach and / or small intestines, reducing nutrient absorption in the stomach and / or small intestines and / or depositing minimally or undigested food farther than normal into the intestines, thereby stimulating intestinal responses. The components described include an artificial stoma device, a gastric sleeve device, an intestinal sleeve device and a combined gastrointestinal sleeve device.
Owner:VALENTX
Gastrointestinal sleeve device and methods for treatment of morbid obesity
InactiveUS20050049718A1Effectively reducing stomach volumeStimulating intestinal responseMedical devicesTubular organ implantsIntestinal structureMorbid obesity
Apparatus and methods are described for treatment of morbid obesity using minimally invasive techniques. The apparatus includes a system of components that may be used separately or in combination for effectively reducing stomach volume, bypassing a portion of the stomach and / or small intestines, reducing nutrient absorption in the stomach and / or small intestines and / or depositing minimally or undigested food farther than normal into the intestines, thereby stimulating intestinal responses. The components described include a gastric sleeve device, an intestinal sleeve device, and a combined gastrointestinal sleeve device.
Owner:VALENTX
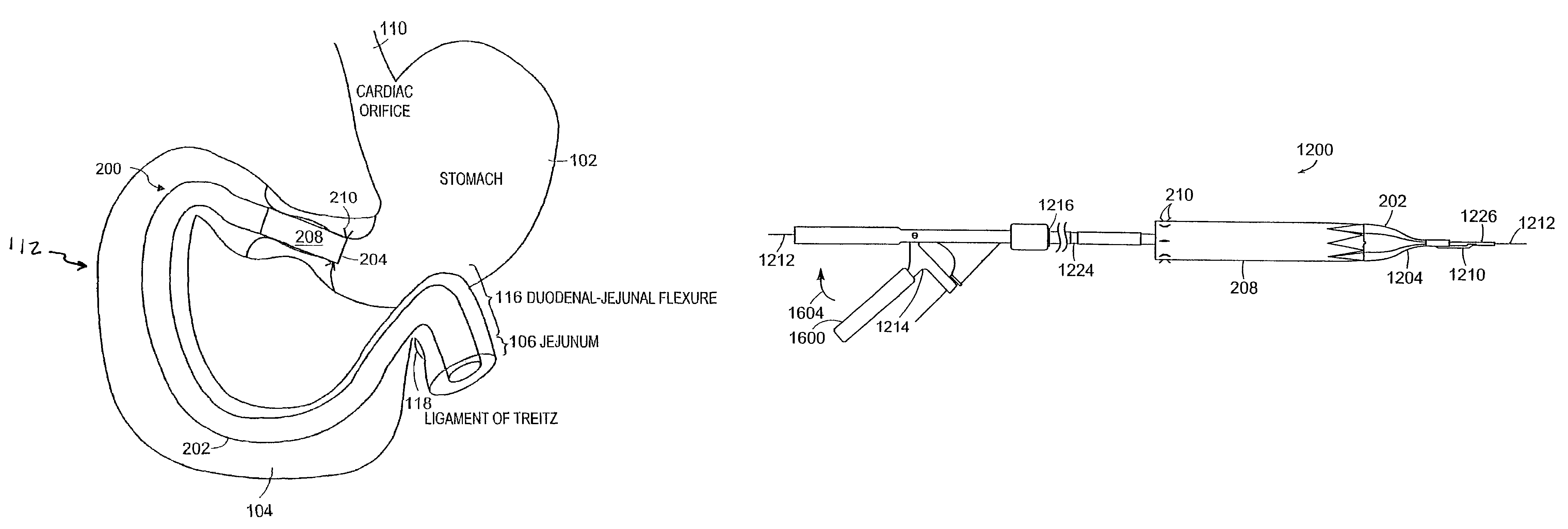
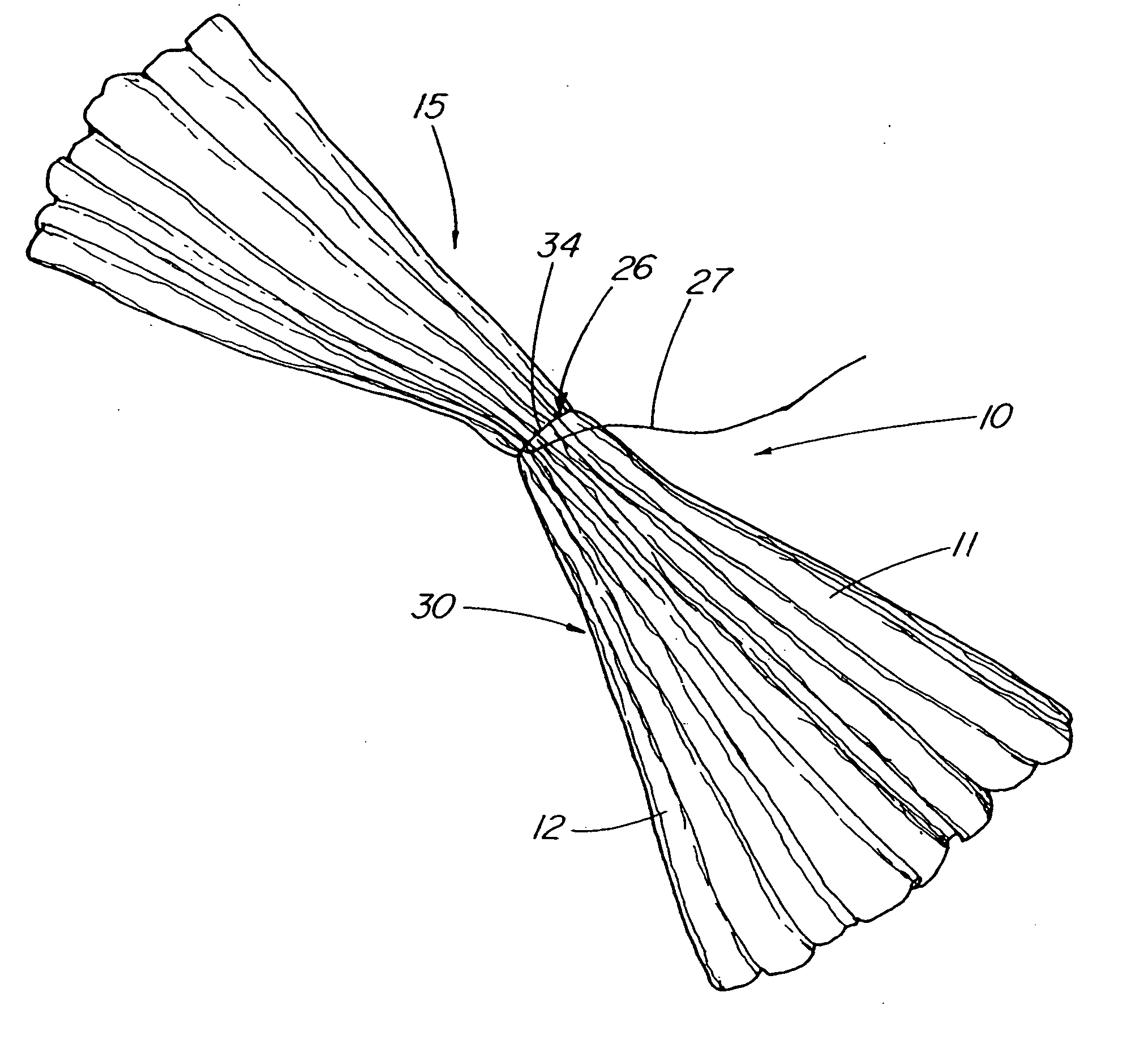
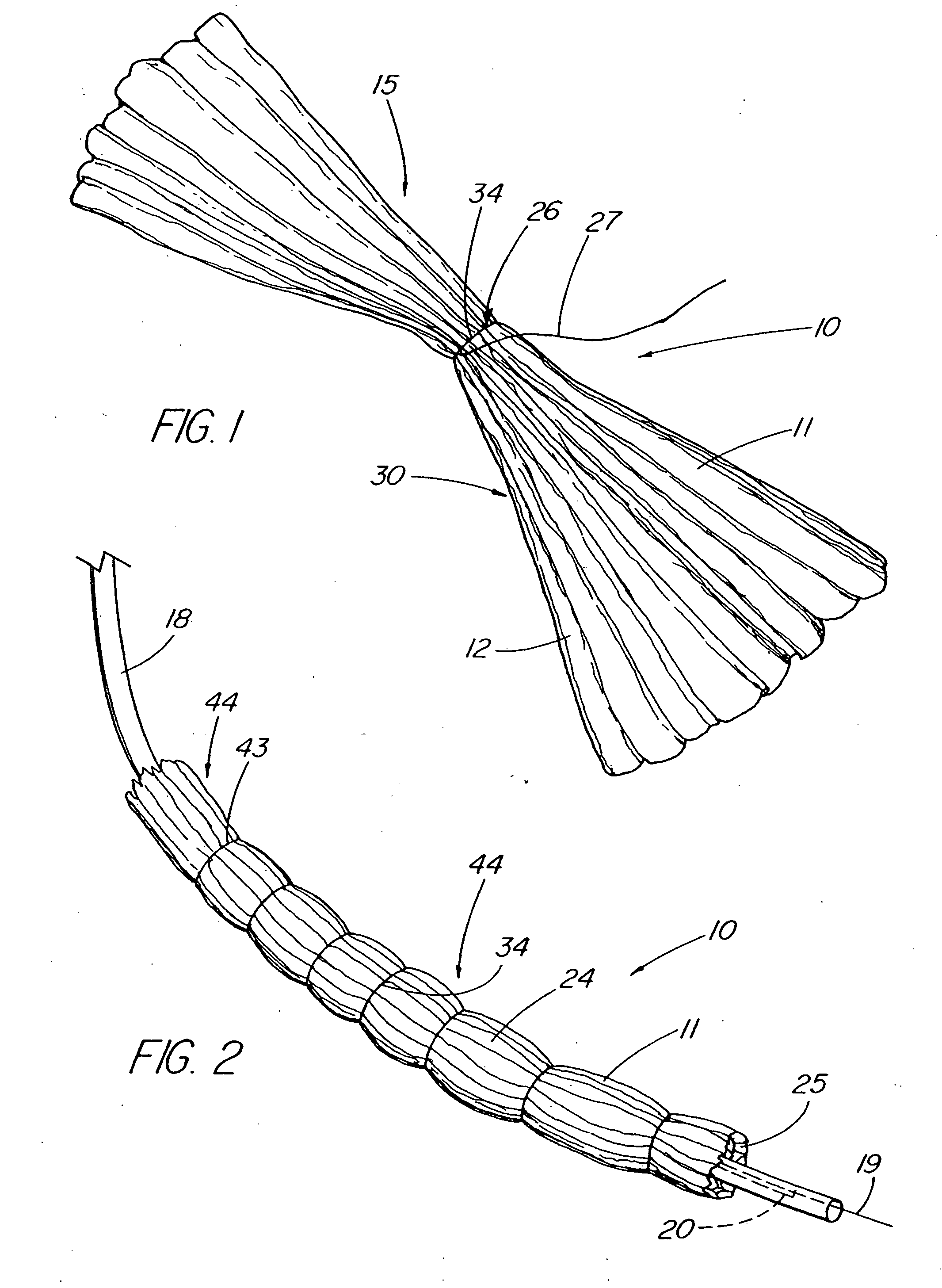
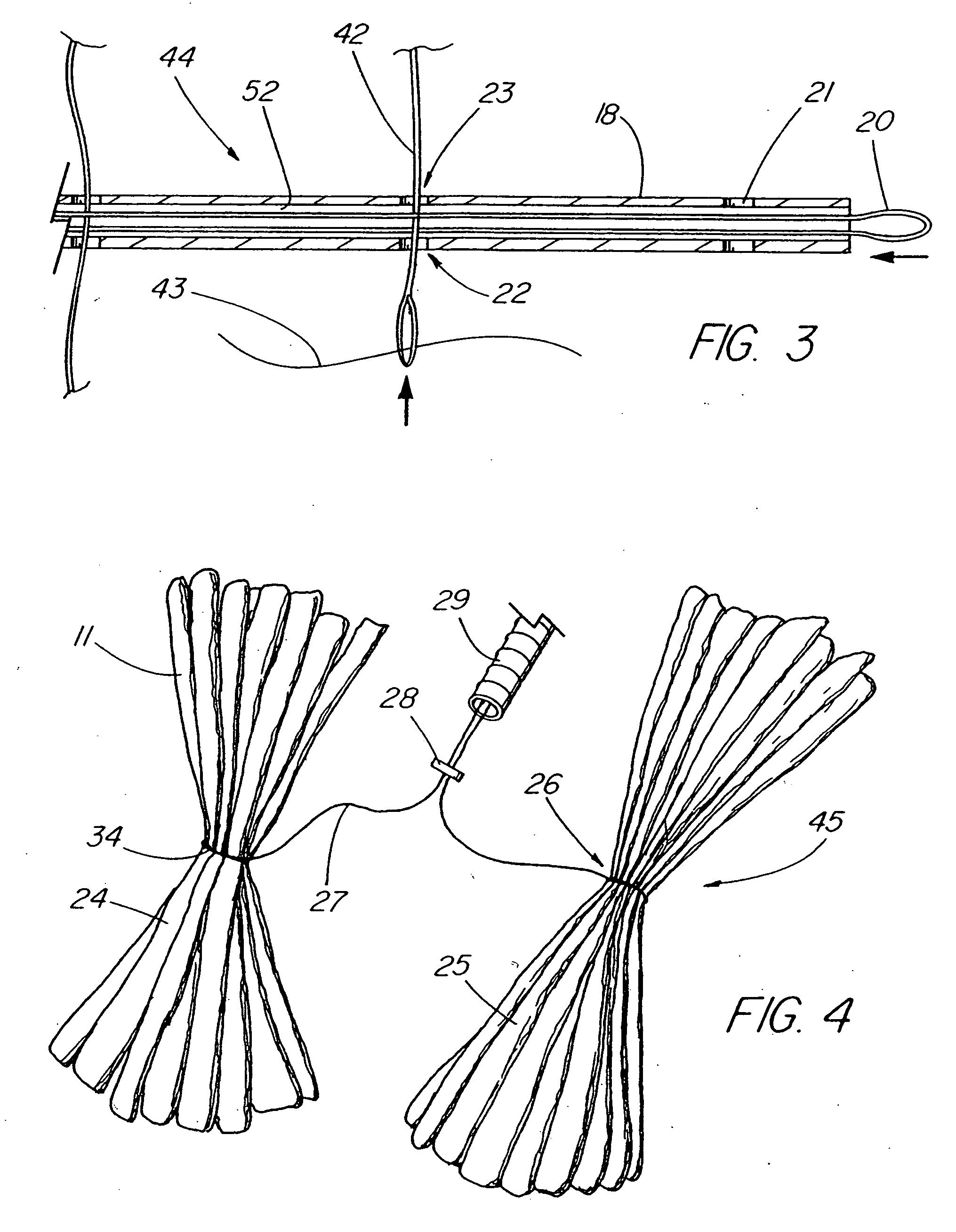
Methods and apparatus for anchoring within the gastrointestinal tract
ActiveUS20050125020A1Minimize traumaLarge caliberSuture equipmentsStentsIntestinal structureMedical device
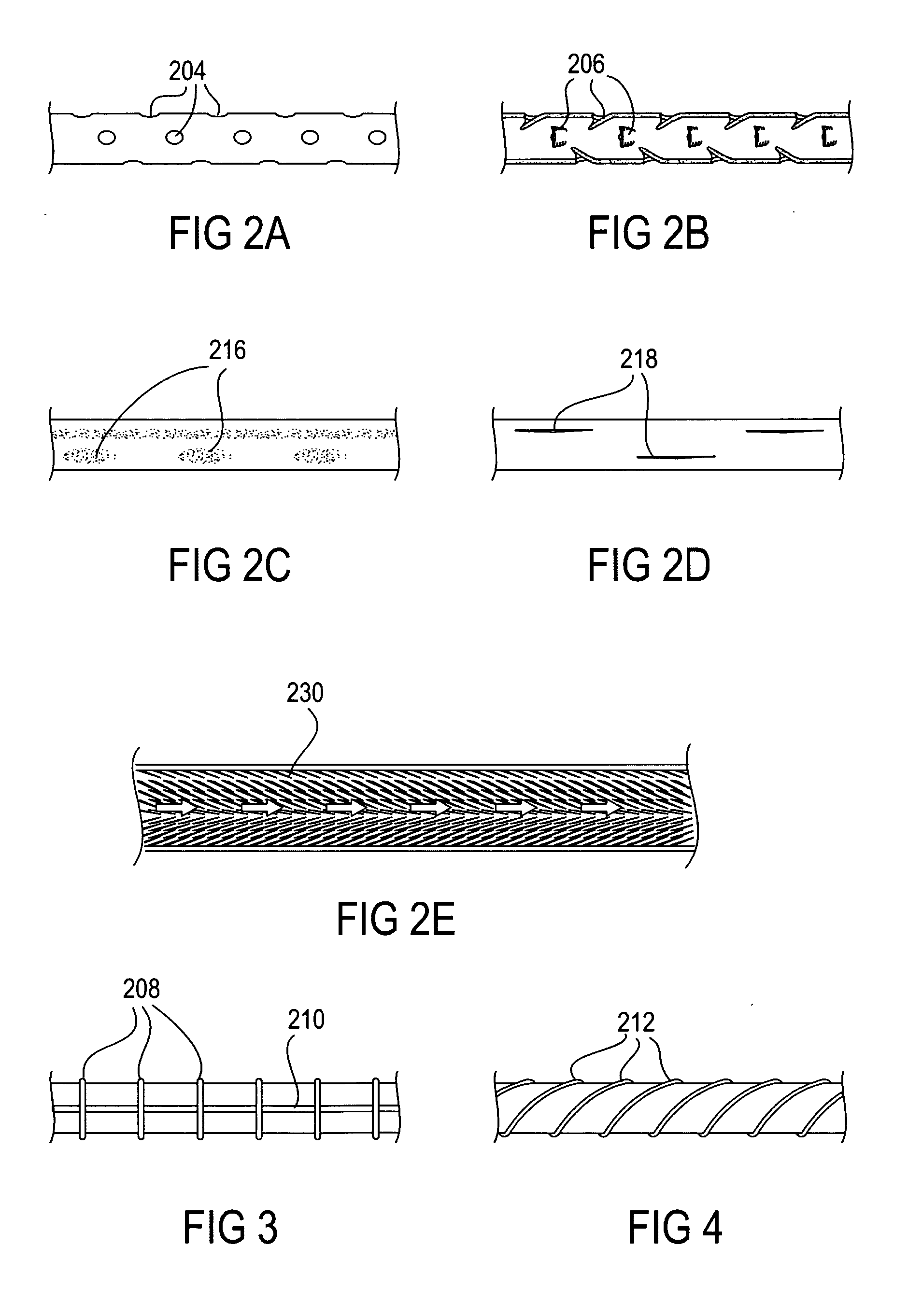
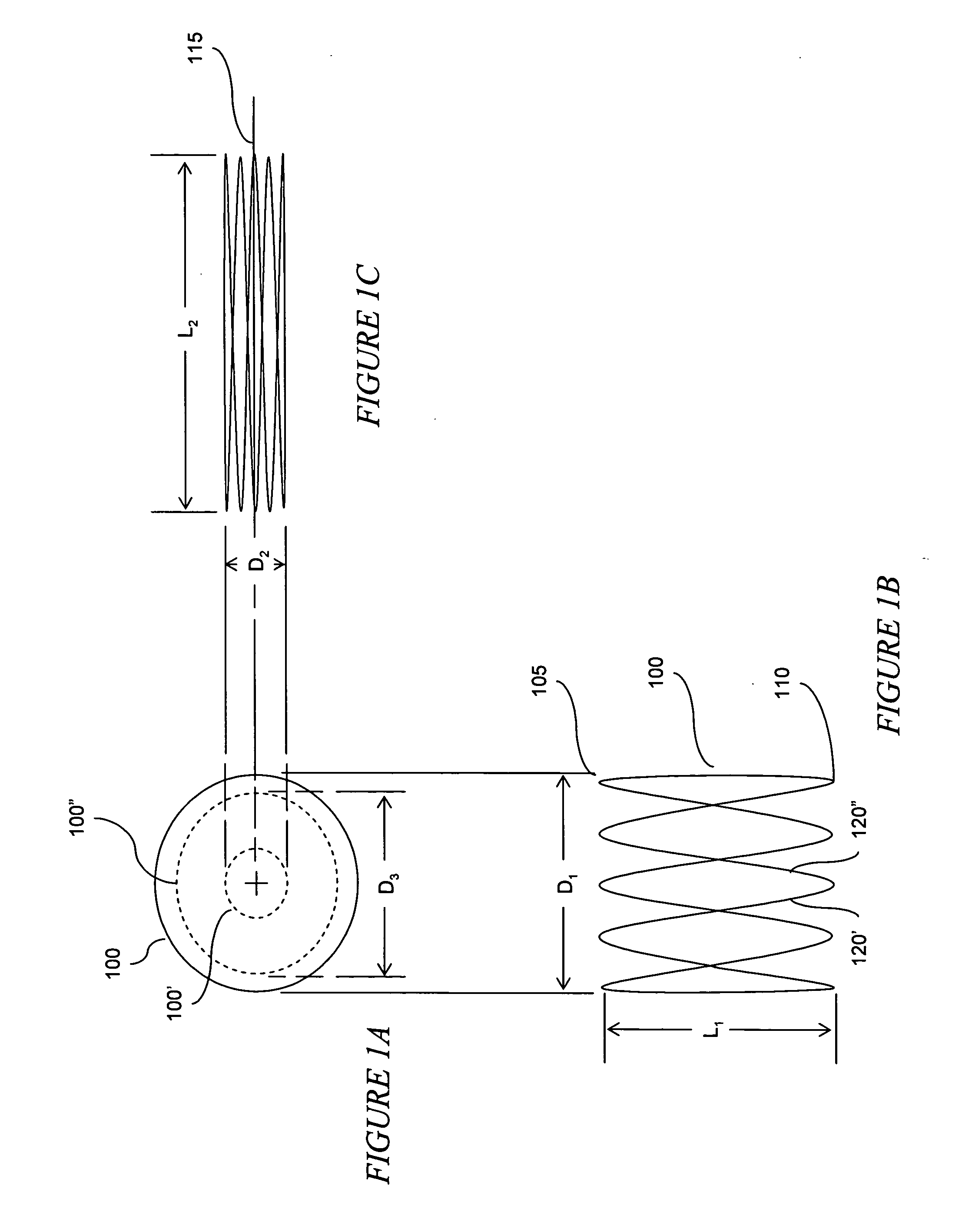
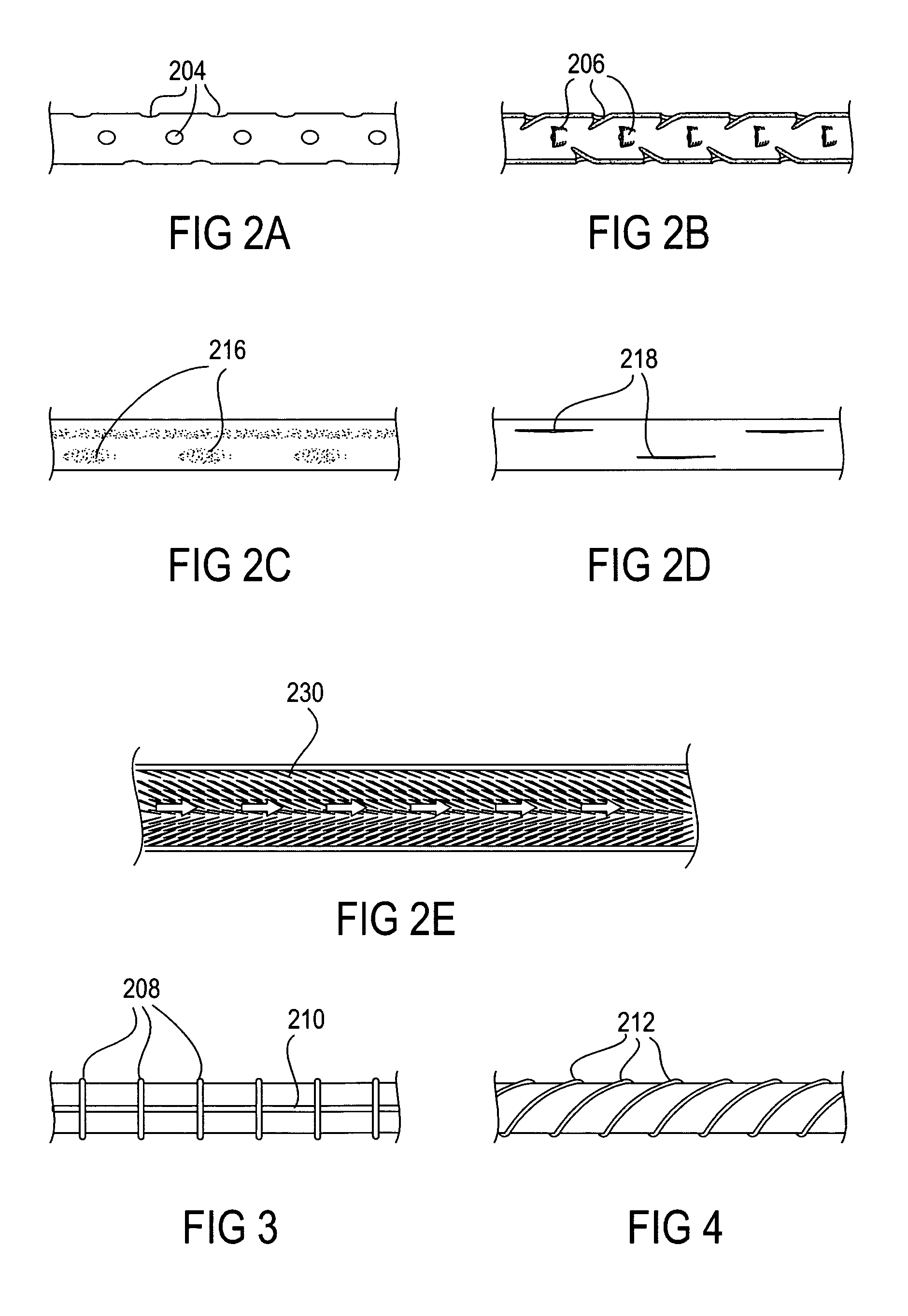
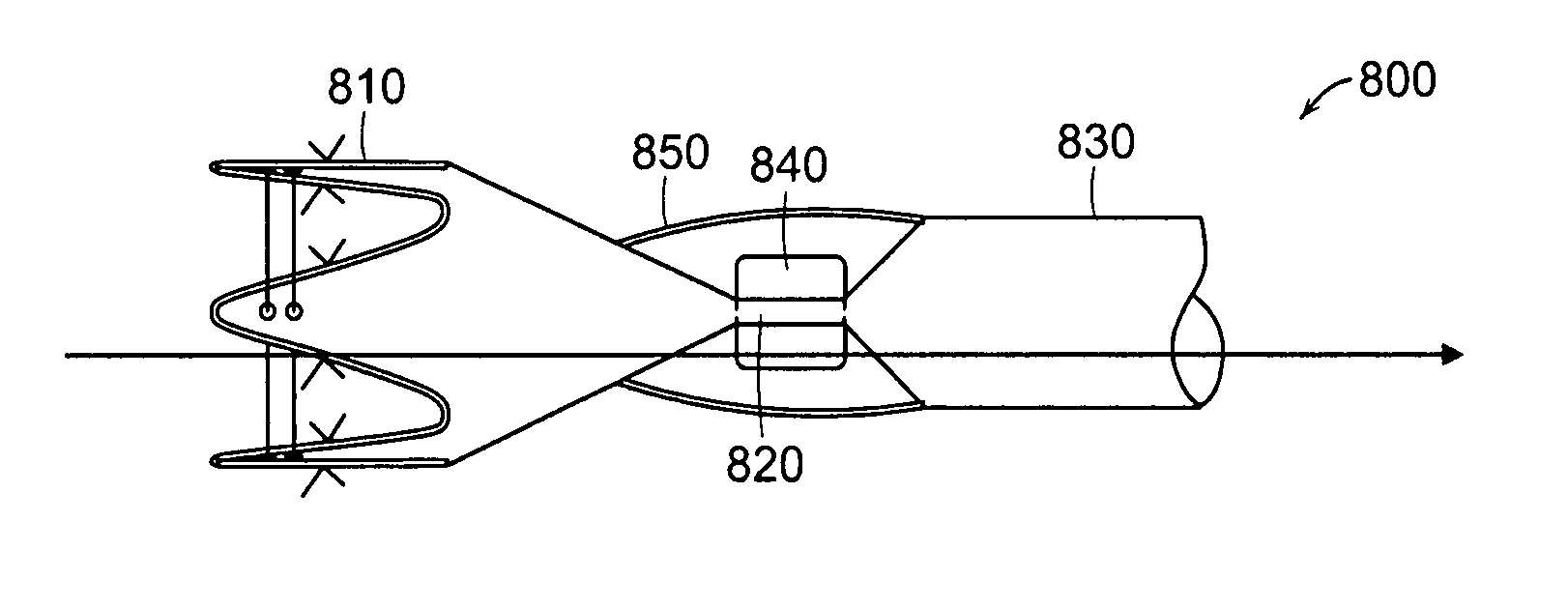
The present invention relates to an anchor configured for minimally-invasive implantation and sized to remain securely positioned within at least a portion of the gastrointestinal tract of an animal. The anchor includes a radial spring formed from an elongated resilient member shaped into an annular wave pattern about a central axis. The anchor defines a central lumen and provides an outward radial force, while allowing for substantial flexure about its perimeter. The anchor is generally removable, but can include fasteners, such as barbs, to further secure it to the surrounding anatomy. In some embodiments, the anchor includes a connector coupling a fixed portion to a removable portion. Further, the anchor can be used to secure a medical device within the body, such as a flexible sleeve within the intestine.
Owner:GI DYNAMICS
Bariatric sleeve
InactiveUS20060161265A1Increased axial stabilityLess movementSuture equipmentsStentsIntestinal structureGastrointestinal device
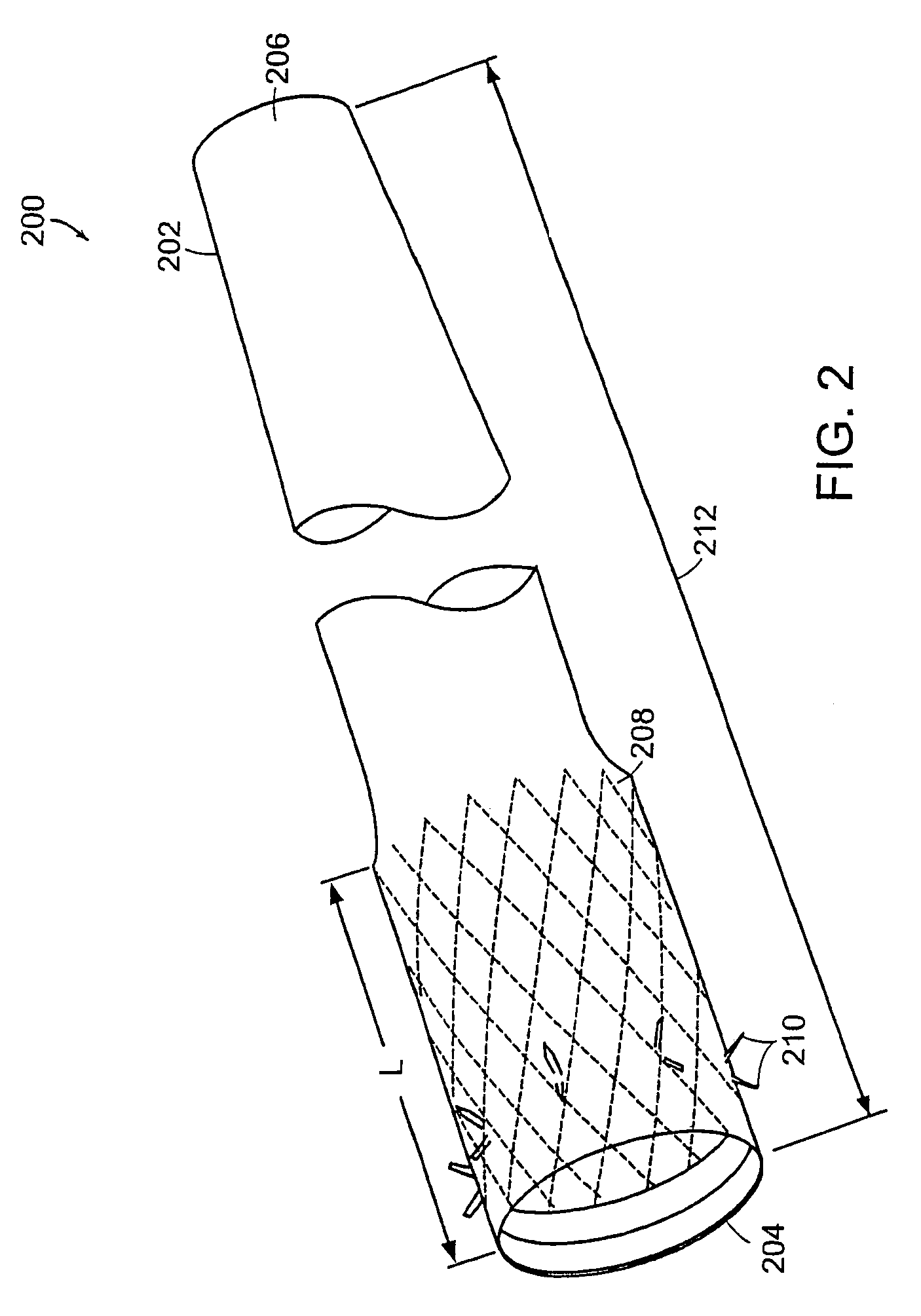
Method and apparatus for limiting absorption of food products in specific parts of the digestive system is presented. A gastrointestinal implant device is anchored in the stomach and extends beyond the ligament of Treitz. All food exiting the stomach is funneled through the device. The gastrointestinal device includes an anchor for anchoring the device to the stomach and a flexible sleeve. When implanted within the intestine, the sleeve can limit the absorption of nutrients, delay the mixing of chyme with digestive enzymes, altering hormonal triggers, providing negative feedback, and combinations thereof. The anchor is collapsible for endoscopic delivery and removal.
Owner:GI DYNAMICS
Gastrointestinal sleeve device and methods for treatment of morbid obesity
InactiveUS20050240279A1Effectively reducing stomach volumeStimulating intestinal responseTubular organ implantsNon-surgical orthopedic devicesIntestinal structureStoma
Apparatus and methods are described for treatment of morbid obesity using minimally invasive techniques. The apparatus includes a system of components that may be used separately or in combination for effectively reducing stomach volume, bypassing a portion of the stomach and / or small intestines, reducing nutrient absorption in the stomach and / or small intestines and / or depositing minimally or undigested food farther than normal into the intestines, thereby stimulating intestinal responses. The components described include an artificial stoma device, a gastric sleeve device, an intestinal sleeve device and a combined gastrointestinal sleeve device.
Owner:VALENTX
System and Method for Treating Nausea and Vomiting by Vagus Nerve Stimulation
ActiveUS20080208266A1Relieve nauseaReduce vomitingInternal electrodesImplantable neurostimulatorsIntestinal structureAbdomen
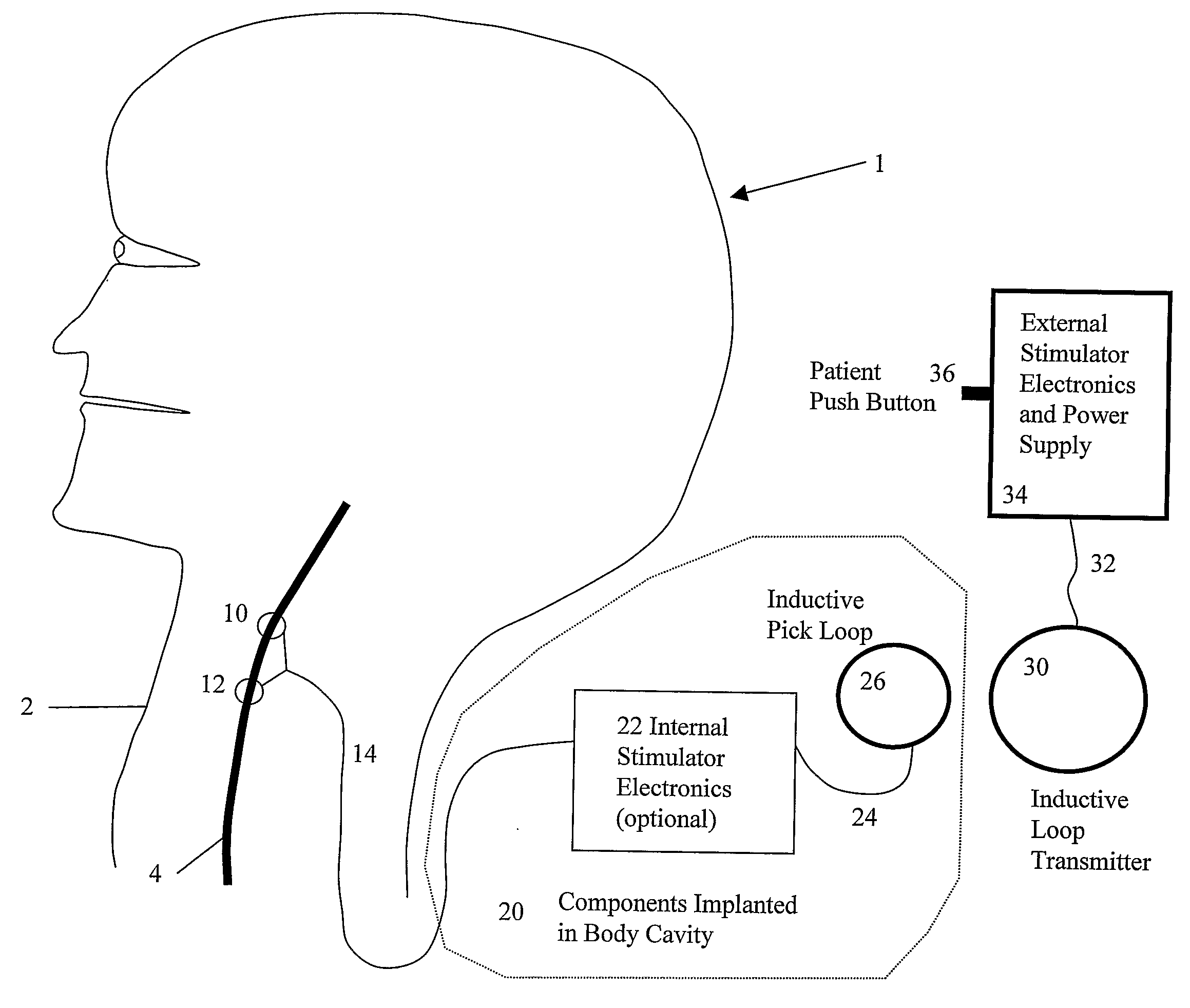
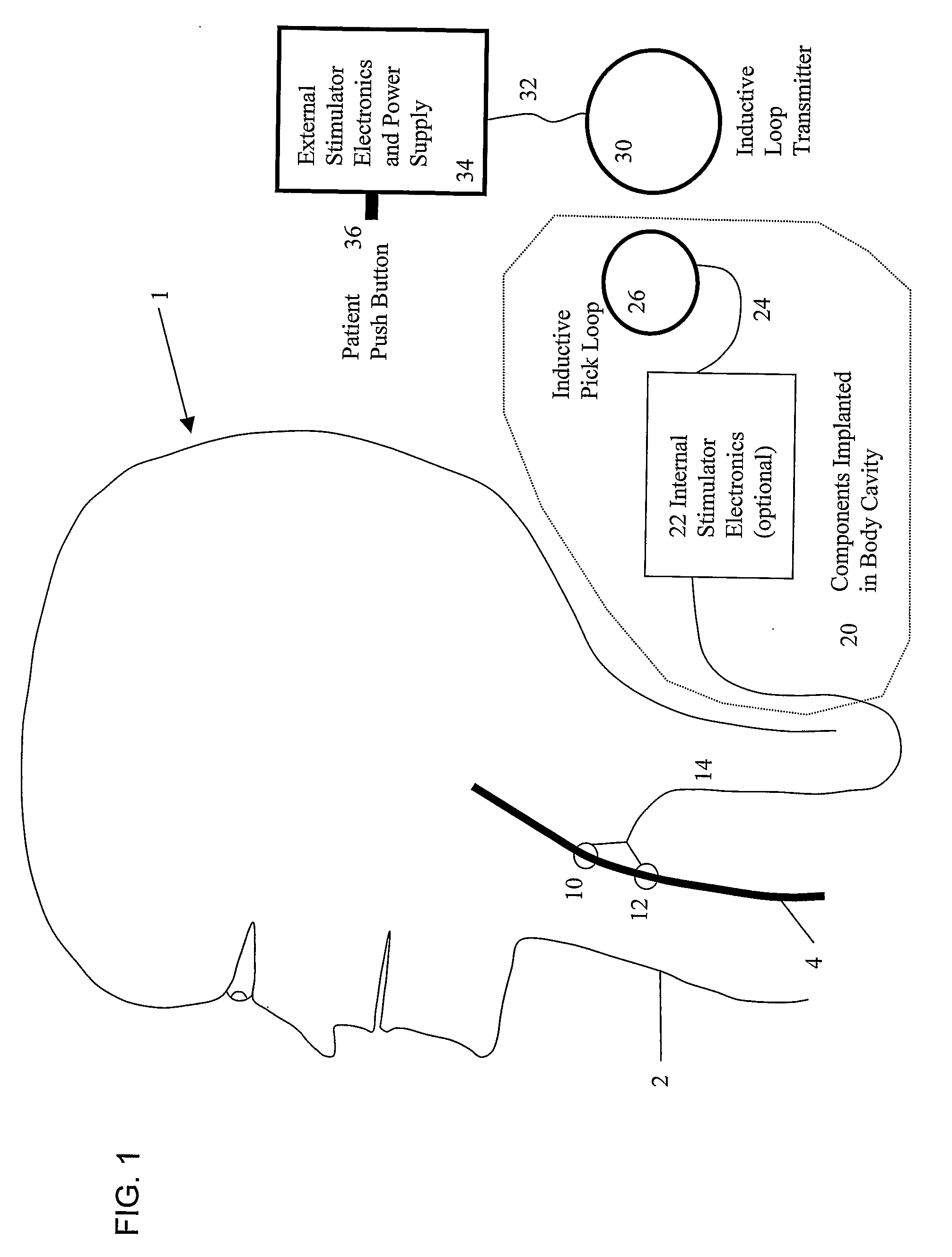
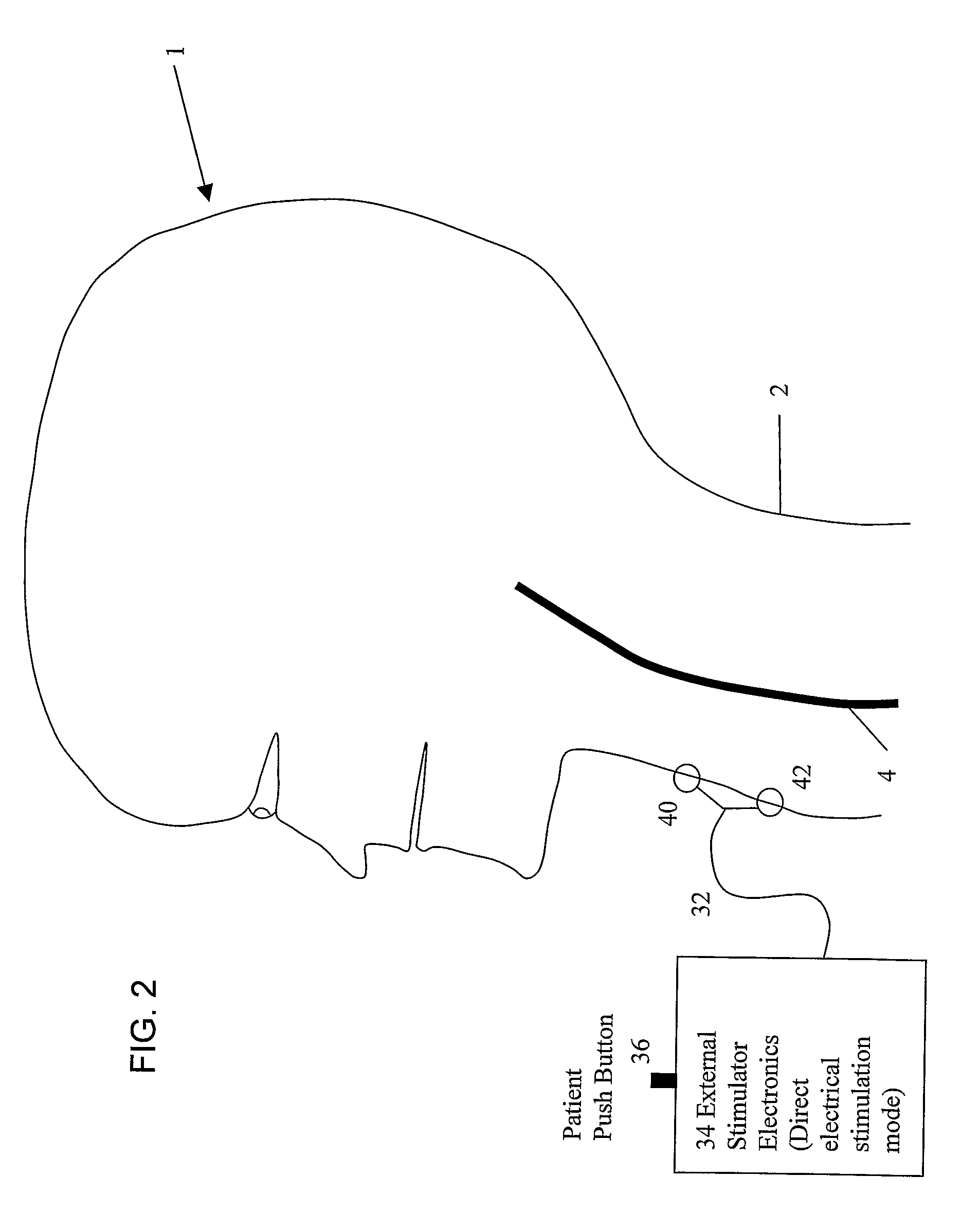
A system and method for treating nausea and vomiting are provided, including one or more electrodes (10, 12) applied on or under the skin, the electrodes being connected to an external current source (34). The electrodes can be implanted under the skin and connect to internal stimulator electronics (22), which can form a magnetic inductive link to the external current source (34). Alternatively, the electrodes can be placed on the skin and directly linked by wires to the external current source. As a further alternative, the vagus nerve can be directly stimulated in the neck, or the esophagus, stomach, duodenum, or intestines can be directly stimulated by magnetic stimulation. The electrodes can stimulate the vagus nerve in the neck to reduce nausea and vomiting, or can be arranged near the chest or abdomen, so as to stimulate the esophagus, stomach, duodenum or intestines. Because the current source is provided outside the body, it is not necessary to implant batteries or another power supply in the body.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
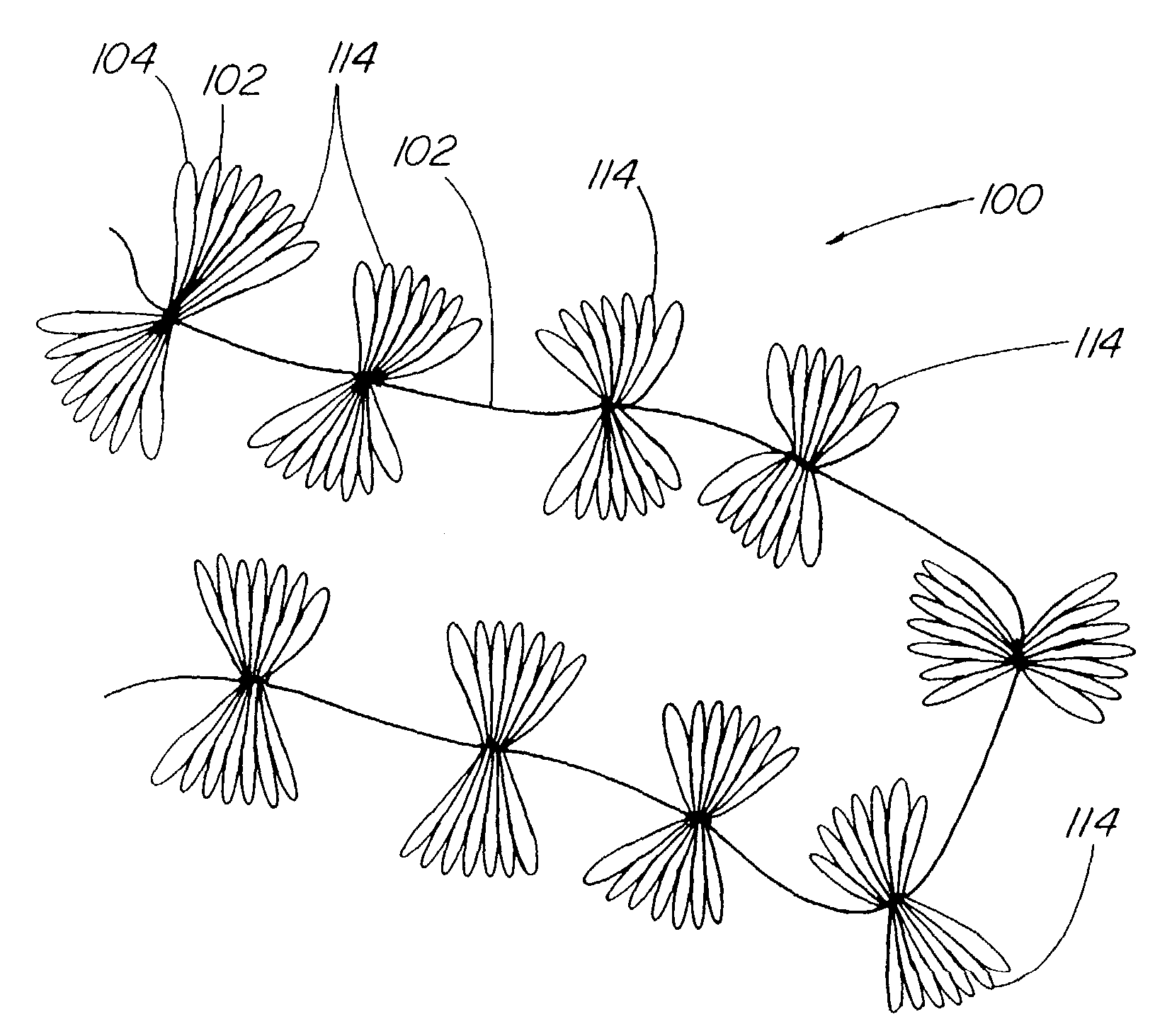
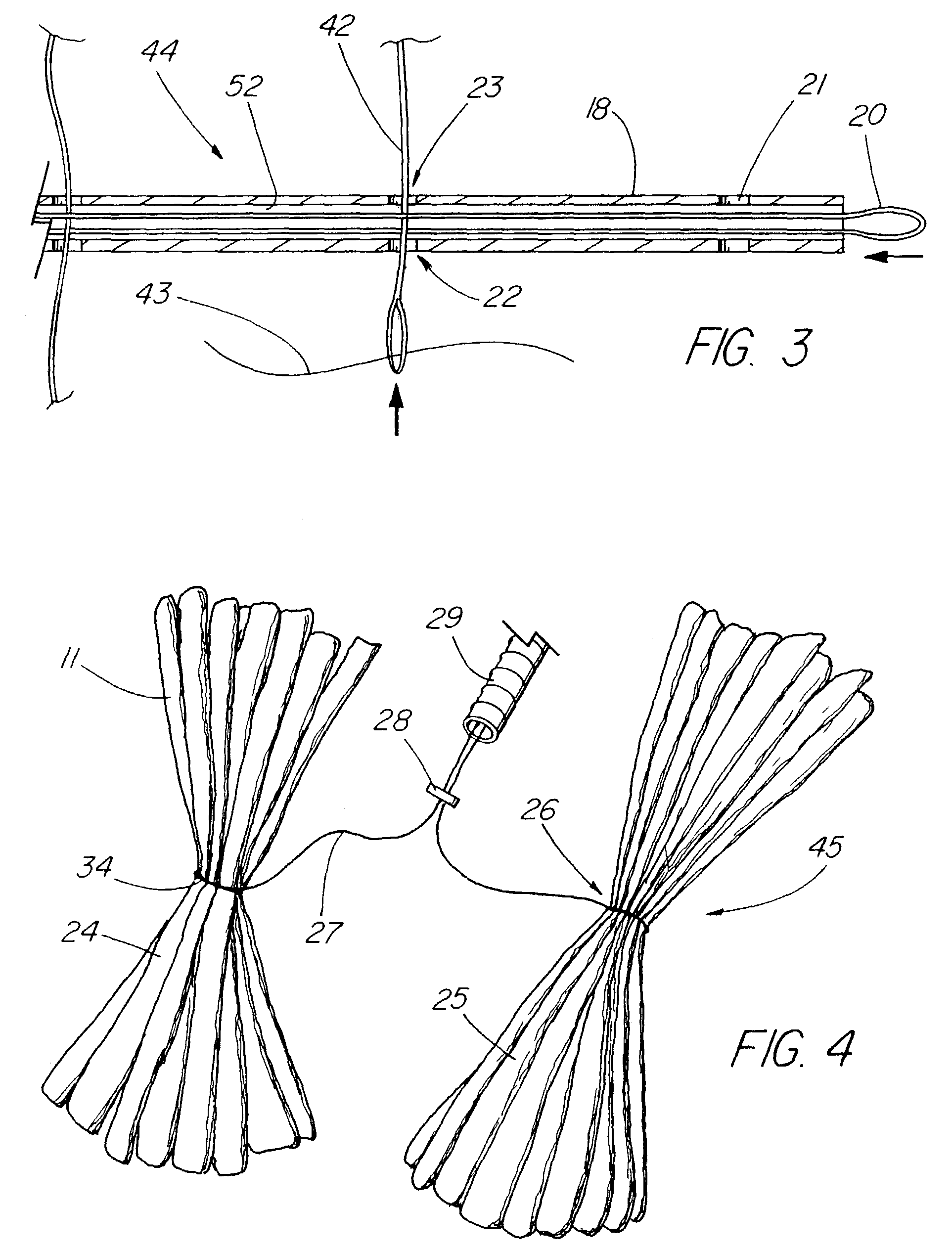
Suture method
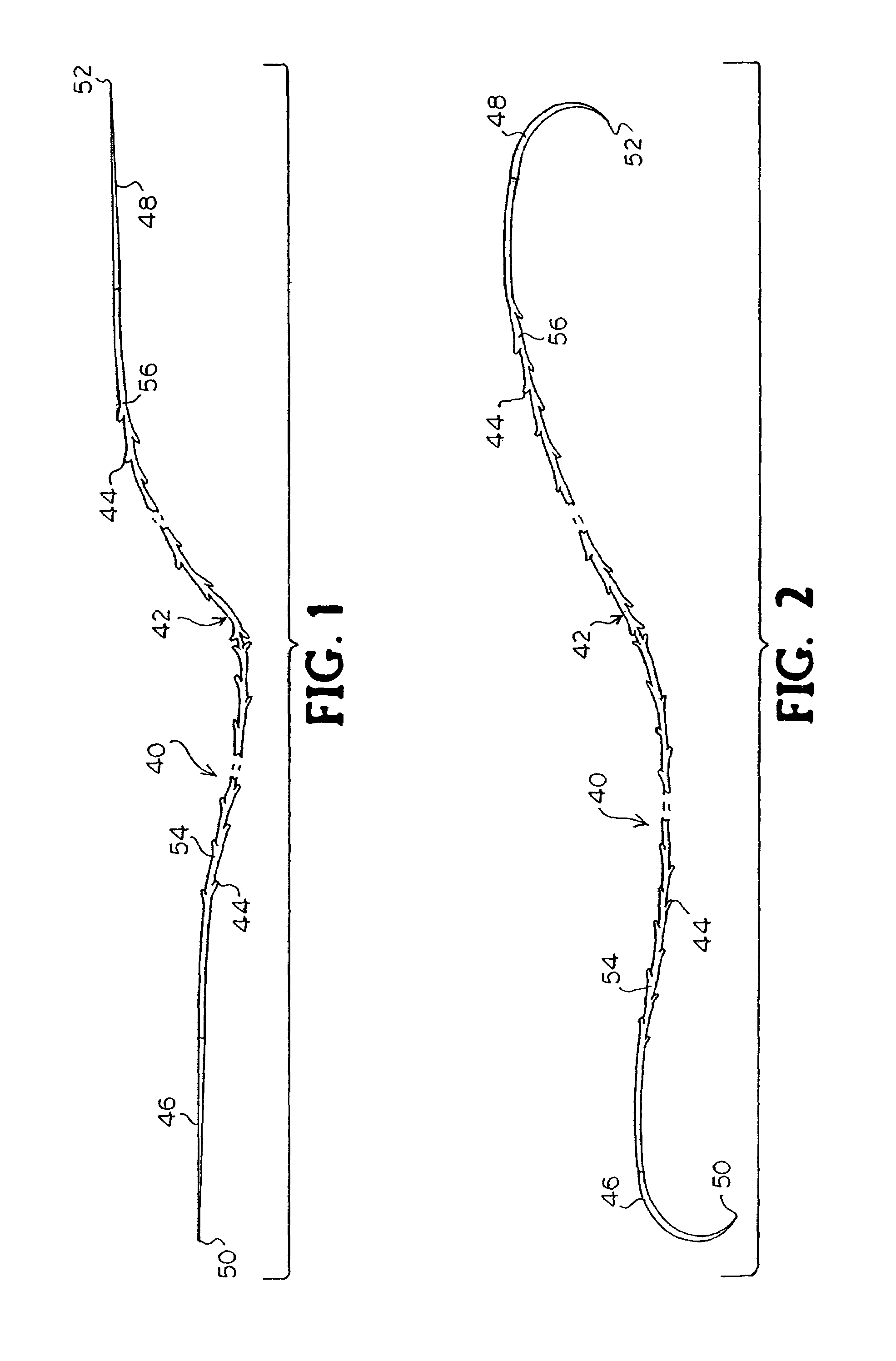
A method for joining and holding closed a wound in bodily tissue, fastening junctions of wounds, tying off wounds, joining a foreign element to tissue, and altering the position of tissue using a barbed suture including sharp pointed ends. Each end of the suture includes barbs on that permit movement in an opposing direction to the barbs on the other end of the suture. This two-way barbed suture is used by the method of the present invention in applications including abdominal surgeries such as a Nissen fundoplication, laparoscopic uses such as stabilizing a bowel structure and performing a closure of a cystostomy, liver to bowel anastomosis, closure of an orifice of a Zenker's Diverticulum, endoscopic uses such as closure of ulcerative lesions or and post-procedural tissue defects, bladder wound closure, valve replacement surgery, device attachment, cosmetic surgery, and blood vessel wound closure.
Owner:ETHICON INC
Resistive anti-obesity devices
ActiveUS20060161139A1Improve overall senseReduce food consumptionSurgeryOesophagiIntestinal structureGastric emptying
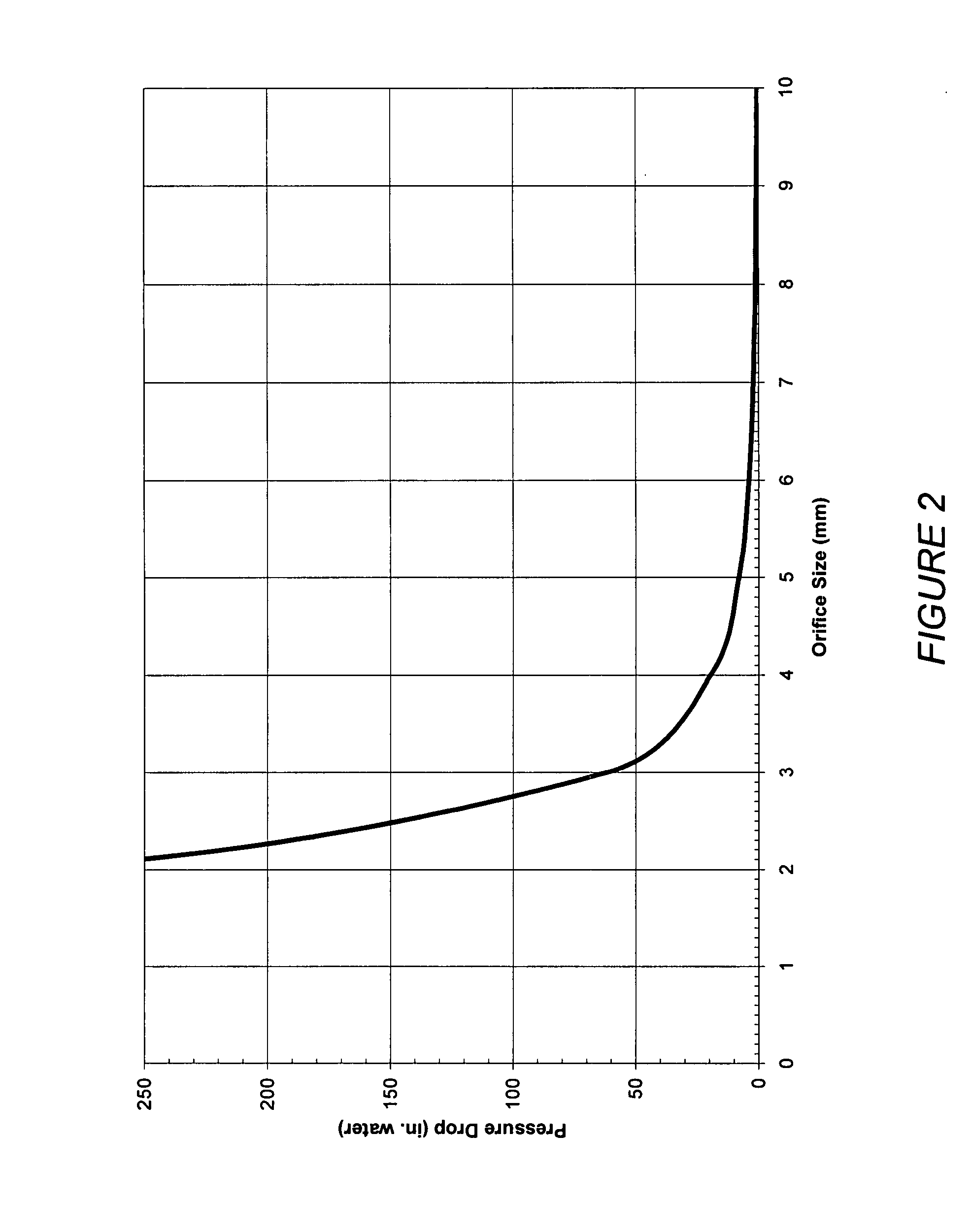
A patient is provided with an increased sense of satiety by increasing resistance to the outflow of food from the stomach and through the intestines. Stomach emptying may be slowed with devices implantable within the gastrointestinal tract below the stomach. Implants are preferably removable and can include artificial strictures that may be adjustable to vary the rate of stomach emptying. Slowing gastric emptying may induce satiety for a longer period and may therefore reduce food consumption. Many of the embodiments include intestinal liners or sleeves, but they need not. The resistor concept may be applied to a simple anchor and resistor without a long liner.
Owner:GI DYNAMICS
Motion catheter
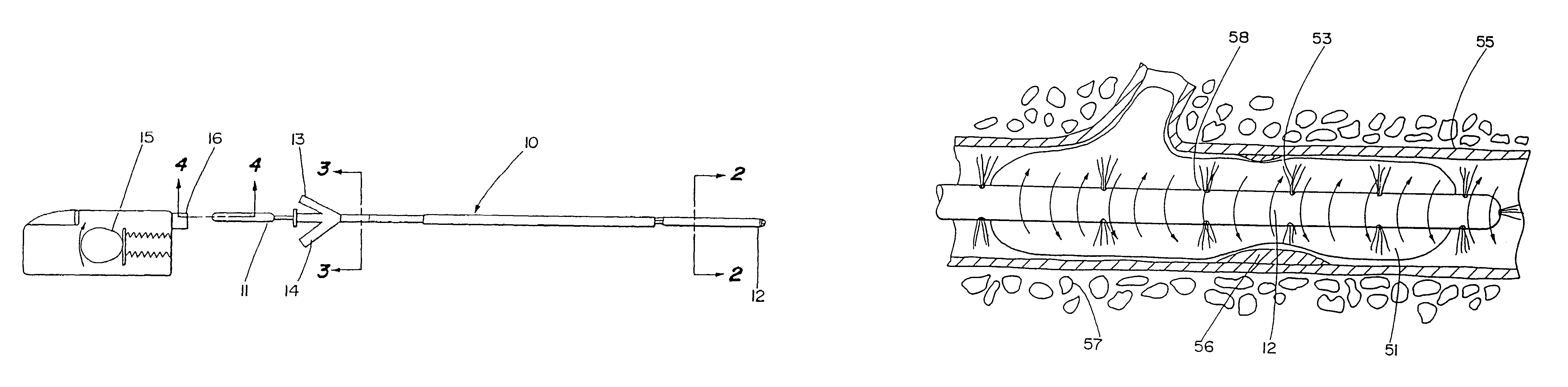
The present invention describes a catheter suitable for introduction into a tubular tissue for dissolving blockages in such tissue. The catheter is particularly useful for removing thrombi within blood vessels. In accordance with the preferred embodiments, a combination of vibrating motion and injection of a lysing agent is utilized to break up blockages in vessels. The vessels may be veins, arteries, ducts, intestines, or any lumen within the body that may become blocked from the material that flows through it. As a particular example, dissolution of vascular thrombi is facilitated by advancing a catheter through the occluded vessel, the catheter causing a vibrating, stirring action in and around the thrombus usually in combination with the dispensing of a thrombolytic agent such as urokinase into the thrombus. The catheter has an inflatable or expandable member near the distal tip which, when inflated or expanded, prevents the passage of dislodged thrombus around the catheter. The dislodged portions of thrombus are directed through a perfusion channel in the catheter, where they are removed by filtration means housed within the perfusion channel before the blood exits the tip of the catheter. Catheters that allow both frequency (1-1000 Hz) vibratory motion and delivery of such agents to a blockage and a method for using such catheters are disclosed.
Owner:TYCO HEALTHCARE GRP LP
Method and devices for modifying the function of a body organ
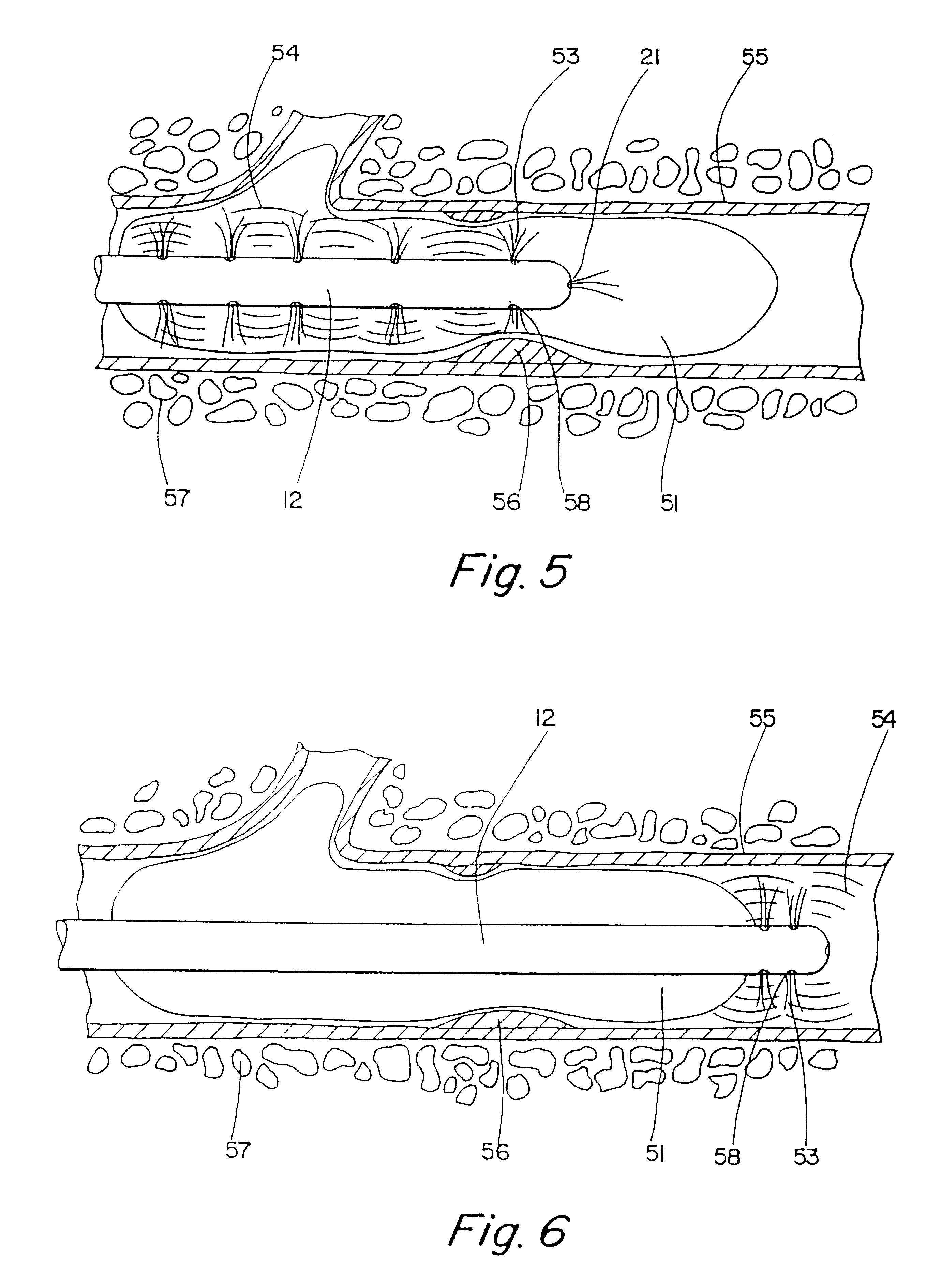
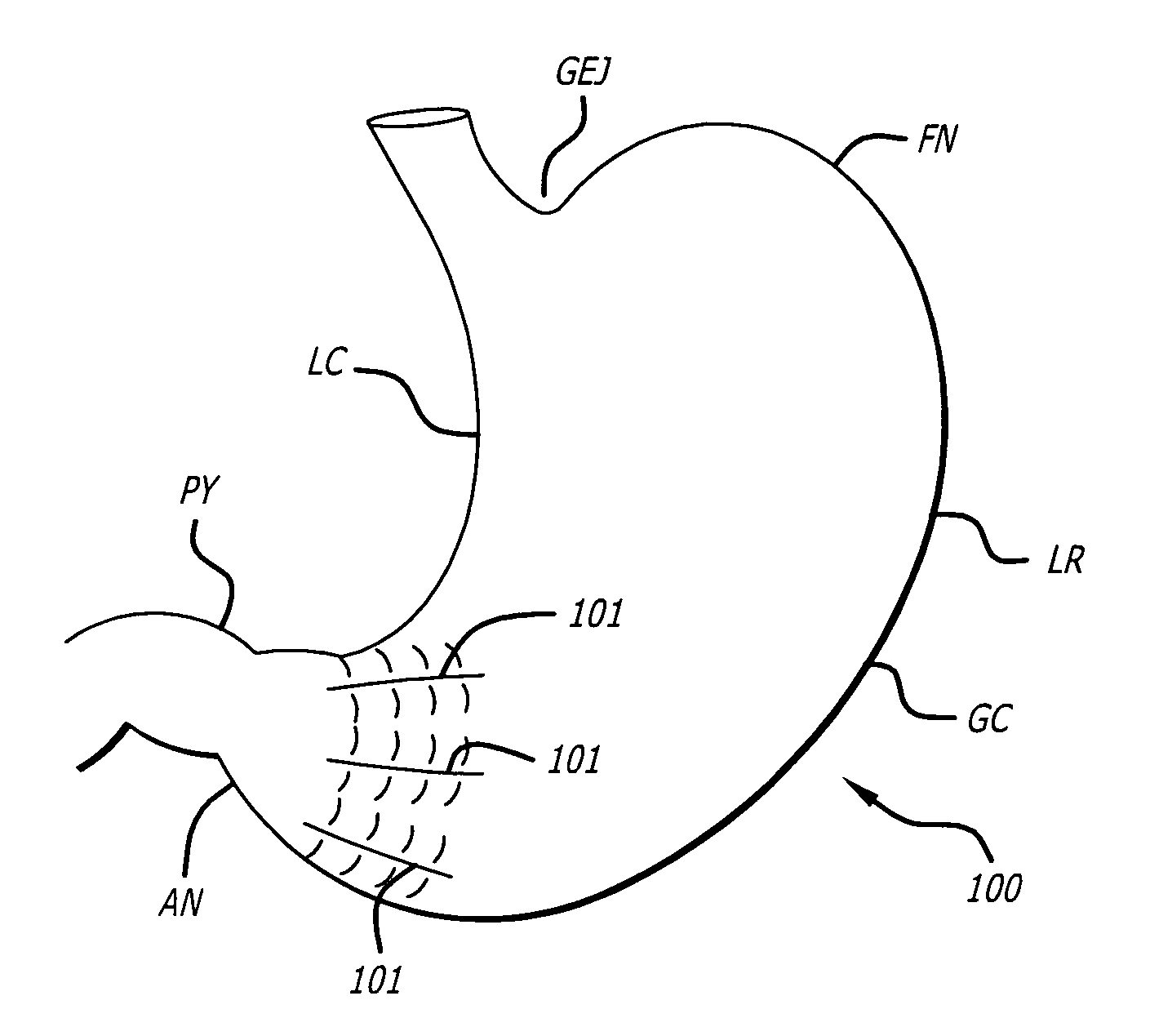
InactiveUS7175638B2Prevent shortening and unrollingMinimize distensionSuture equipmentsNon-surgical orthopedic devicesBody organsIntestinal structure
Methods and devices for partitioning or plicating a region of a hollow body organ are described herein. These methods and devices relate generally to medical apparatus and methods and more particularly to devices and methods for affecting a change in the function of a hollow body organ, particularly a stomach, intestine or gastrointestinal tract. These changes can include reducing the volume capacity of the hollow body organ, disrupting or altering the normal function of the organ, functionally excluding certain sections of the organ either by affixing adjacent tissue or excising certain regions, or affecting or correcting the response of the organ to naturally occurring stimuli, such as ingestion.
Owner:ETHICON ENDO SURGERY INC
Intragastric device for treating obesity
An apparatus and method comprising at least one intragastric member or artificial bezoar made of a digestive-resistant or substantially indigestible material that is introduced into a gastric lumen of a mammal for the treatment of obesity. The intragastric member or artificial bezoar is typically at inserted into the gastric lumen in a partially compacted configuration, whereby it is then manipulated into, or allowed to assume, a second expanded configuration sufficiently large to remain within the reservoir of the stomach during normal activities and not be passed through the pylorus into the intestines. In animals, the present invention has been found to be effective in achieving weight loss over a several month period, while being easy to place and retrieve.
Owner:COOK MEDICAL TECH LLC
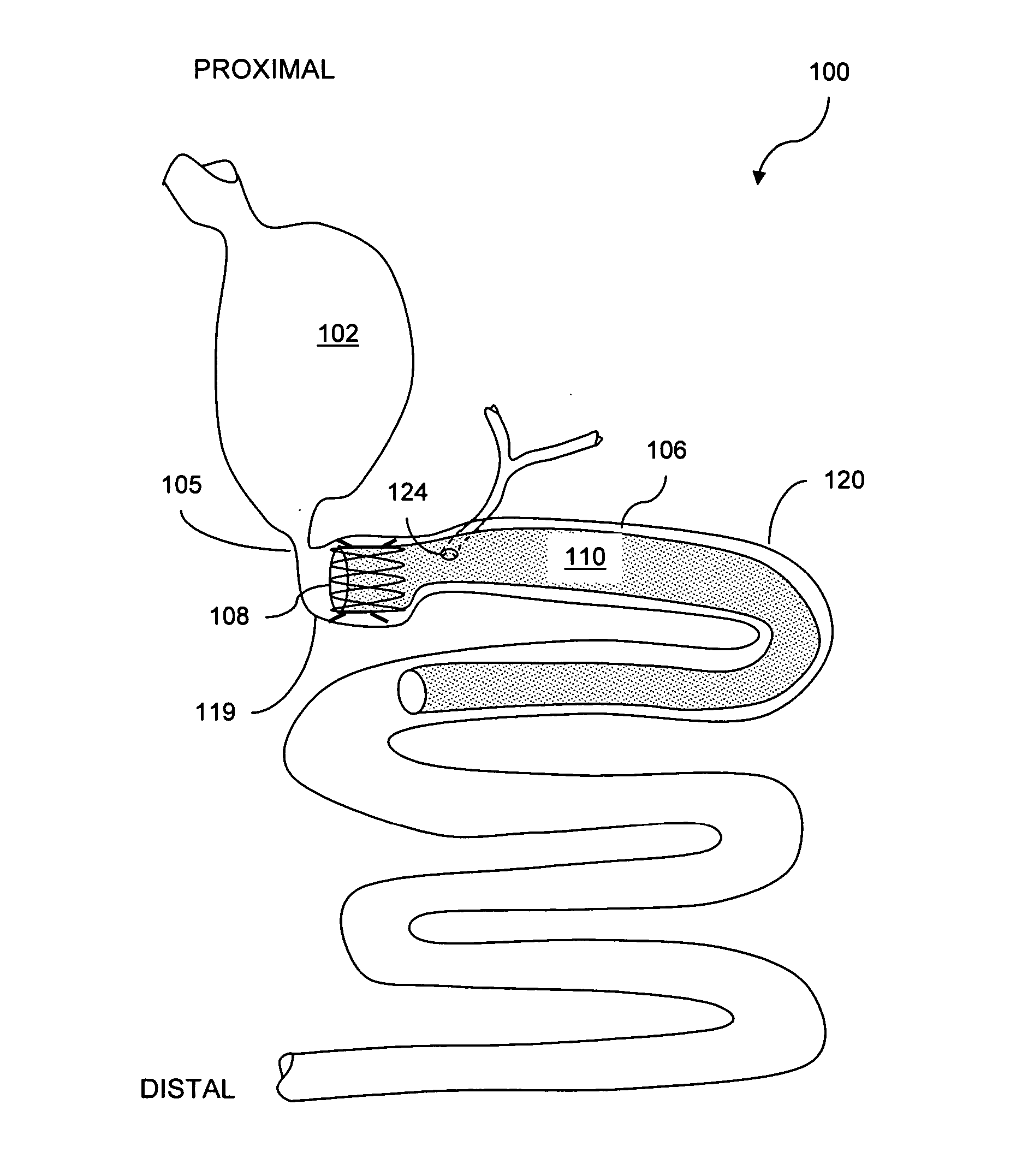
Methods of treatment using a bariatric sleeve
ActiveUS7695446B2Promote healingControlled absorptionSuture equipmentsStentsDiseaseIntestinal structure
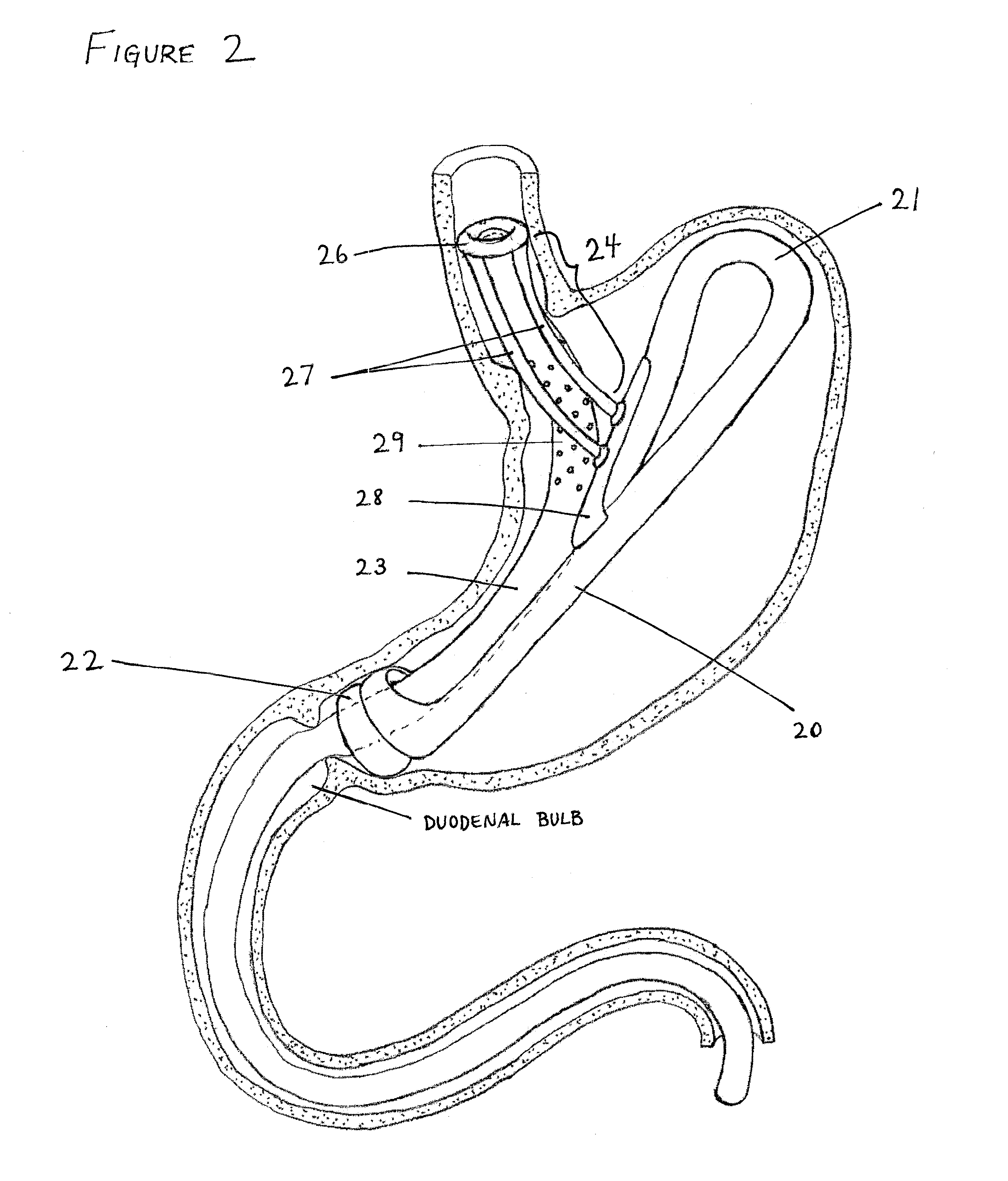
Methods of treatment using a gastrointestinal implant device removably anchored within an animal's gastrointestinal tract. For example, the implant device includes a collapsible anchor for anchoring the device coupled to a proximal end of a flexible sleeve. The implant device can be anchored within the stomach, within the pyloric orifice, and / or distal to the pylorus and extended into the duodenum. All partially-digested food, or chyme, exiting the stomach is funneled through the device. Methods of treatment include treating obesity by one or more of: limiting the absorption of nutrients within the duodenum; delaying the mixing of chyme with digestive enzymes; alter hormonal triggers; and providing negative feedback. Alternatively or in addition, the desired result includes treating a diseases, such as diabetes, or temporarily shielding a portion of the intestine to promote healing within the intestine.
Owner:GI DYNAMICS INC
Microparticles for Oral Delivery
The invention provides microbeads containing oil-associated biologically active compounds and methods for their manufacture and use. The microbeads consist of a soluble complex of non-digestible polymer and emulsifier with oil-associated biologically active compounds embedded in a matrix of digestible polymer. The disclosed microbead complex protects the biologically active compounds, such as vitamins, fish oil and carotenoids, from oxidation, taste and odor degradation. The disclosed microbeads also provide protection from the stomach digestive distraction, and allows for the delivery of the biologically active compounds in the intestine.
Owner:INTERVET INC
Gastrointestinal sleeve device and methods for treatment of morbid obesity
InactiveUS7794447B2Reduce volumeReduce absorptionMedical devicesTubular organ implantsIntestinal structureSmall intestine
Owner:VALENTX
Devices and methods for disruption and removal of luminal occlusions
InactiveUS7618434B2Effective disruptionQuantity minimizationCannulasDilatorsIntestinal structureUrethra
The subject invention pertains to an elastic sheath, device, and methods for disrupting and / or removing occlusive material from lumens, particularly biological lumens, such as the vasculature, ureter, urethra, fallopian tubes, bile duct, intestines, and the like. The subject invention provides for effective disruption and removal of occlusive material, such as a thrombus, from the body lumen with minimal risk of injury to the lumen wall. Advantageously, the invention can be used to achieve a high degree of removal while minimizing the amount of occlusive material that is released into the body lumen. The subject invention further pertains to methods for disrupting and removing occlusive material from a biological lumen. In another aspect, the present invention concerns a device useful as an in vitro model of luminal occlusion and methods for using the device to test the efficacy of devices and methods for treating luminal occlusions.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Compositions and methods for the treatment of cancer
InactiveUS20020128228A1Reducing and avoiding adverse effectImprove toleranceBiocideAnimal repellantsIntestinal structureCancer prevention
This invention relates to compositions comprising temozolomide and thalidomide which can be used in the treatment or prevention of cancer, in particular malignant melanoma, cancer of the skin, subcutaneous tissue, lymph nodes, brain, lung, liver, bone, intestine, colon, heart, pancreas, adrenals, kidney, prostate, breast, colorectal, or a combination thereof. A particular composition comprises temozolomide, or a pharmaceutically acceptable salt, solvate, or clathrate thereof, and thalidomide, or a pharmaceutically acceptable salt, solvate, or clathrate thereof. The invention also relates to methods of treating or preventing cancer, in particular malignant melanoma, cancer of the skin, subcutaneous tissue, lymph nodes, brain, lung, liver, bone, intestine, colon, heart, pancreas, adrenals, kidney, prostate, breast, colorectal, or a combination thereof, which comprise the administration of temozolomide and thalidomide and another anti-cancer drug to a patient in need of such treatment or prevention. The invention further relates to methods of reducing or avoiding adverse side effects associated with the administration of cancer chemotherapy or radiation therapy which comprise the administration of temozolomide and thalidomide to a patient in need of such reduction or avoidance.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Use of a gastrointestinal sleeve to treat bariatric surgery fistulas and leaks
ActiveUS20080208357A1Minimize traumaCurved bigOesophagiObesity treatmentIntestinal structureDamages tissue
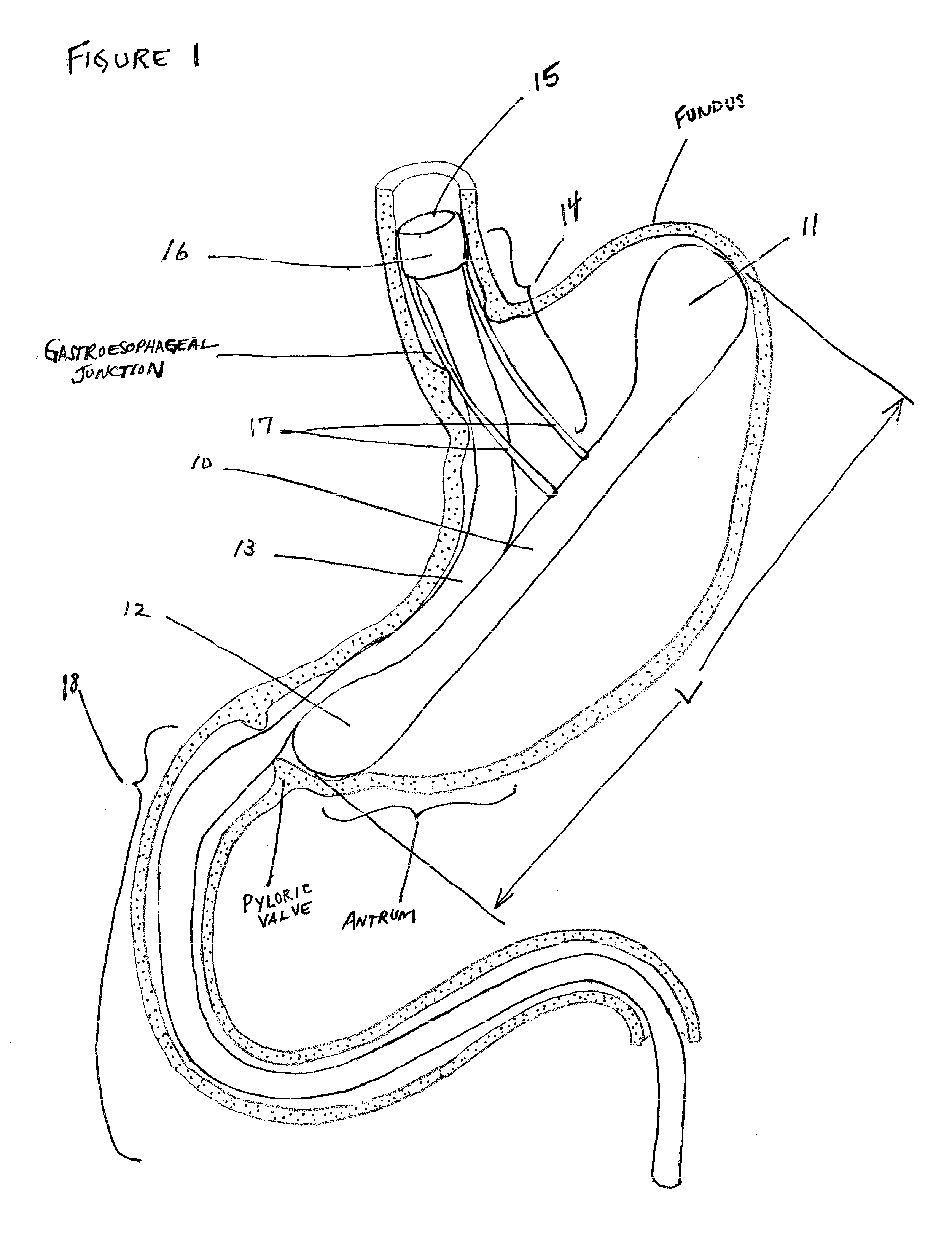
Method for treating a Roux-en-Y patient having fistulas and leaks as a result of bariatric surgery. A gastrointestinal implant device is anchored in the esophagus and extends through a stomach pouch into an intestine anastomosed to the stomach pouch to prevent fistulas and other damaged tissue from making contact with food and fluids entering the esophagus. The gastrointestinal implant device includes an unsupported flexible sleeve and an anchor coupled to a proximal portion of the sleeve. The flexible sleeve is open at both ends, and adapted to extend below a jejunum. The anchor is adapted to be retained within the esophagus, preferably just above the gastroesophageal (GE) Junction. The anchor can include a stent such as a wave anchor and is collapsible for catheter-based delivery and removal.
Owner:GI DYNAMICS
Intragastric Implant Devices
InactiveUS20100049224A1Avoid luminal blockageStress minimizationMedical devicesCatheterIntestinal structurePylorus
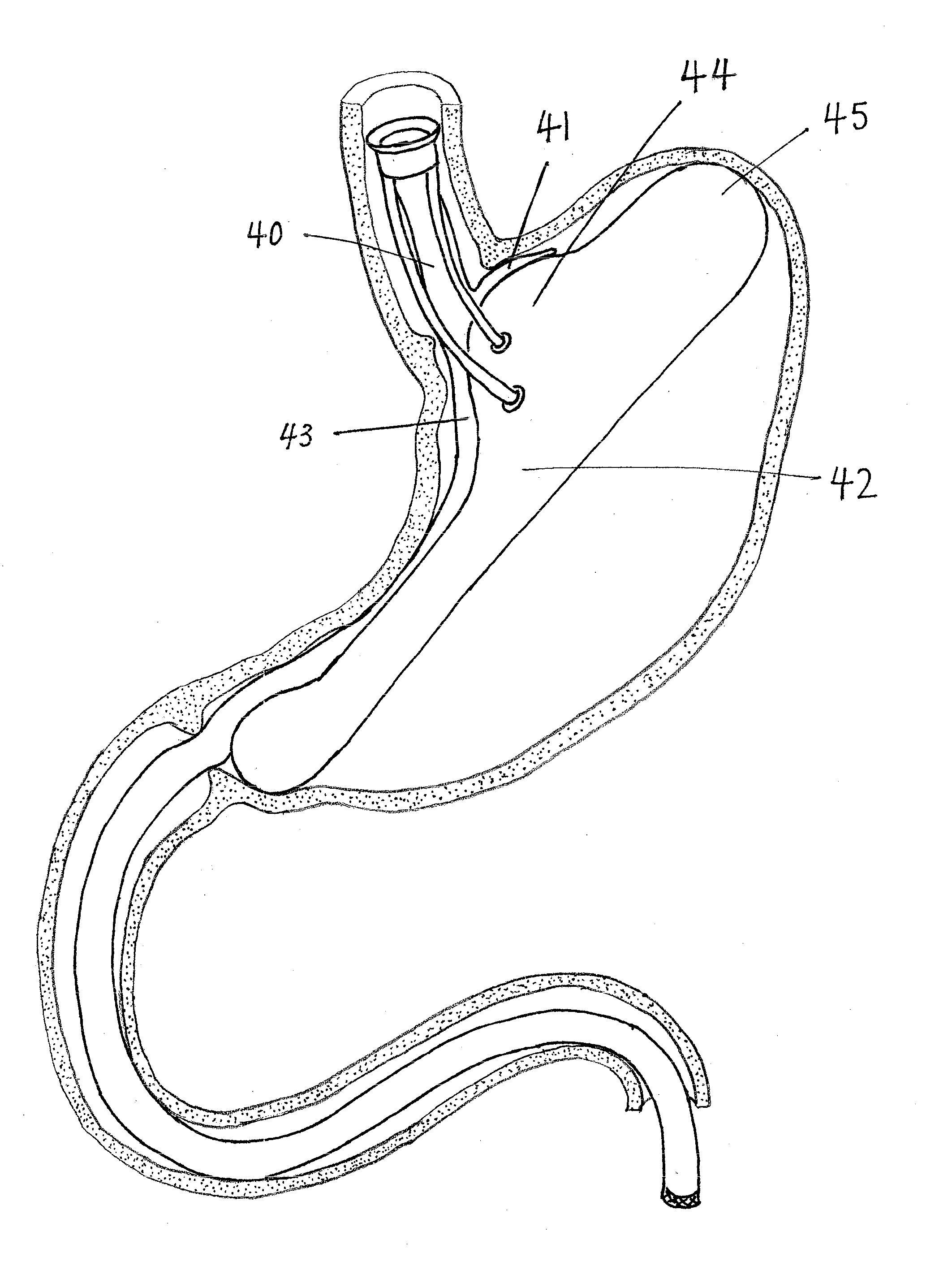
An intragastric implant comprises an anchor and a therapeutic device or a diagnostic device. The anchor is adapted to extend between the fundus and the pyloric valve of a stomach, to be retained without attachment to the stomach wall, and to anchor the device within the stomach with a relatively stable position and orientation. The therapeutic or diagnostic device is adapted to extend from the esophagus or stomach to the intestines or stomach. The therapeutic or diagnostic device, when extending into the esophagus, will be slidably received through the gastroesophageal junction and, when extending into the intestines, will be slidably received in the pyloric valve.
Owner:IBIS MEDICAL
Resistive anti-obesity devices
ActiveUS7819836B2Control outflowSurgical instrument detailsIntravenous devicesIntestinal structureGastric emptying
A patient is provided with an increased sense of satiety by increasing resistance to the outflow of food from the stomach and through the intestines. Stomach emptying may be slowed with devices implantable within the gastrointestinal tract below the stomach. Implants are preferably removable and can include artificial strictures or apertures that may be adjustable or elastic to vary the rate of stomach emptying. Slowing gastric emptying may induce satiety for a longer period and may therefore reduce food consumption. Many of the embodiments include intestinal sleeves or sleeves, but they need not. The resistor concept may be applied to a simple anchor and resistor without a long sleeve.
Owner:GI DYNAMICS
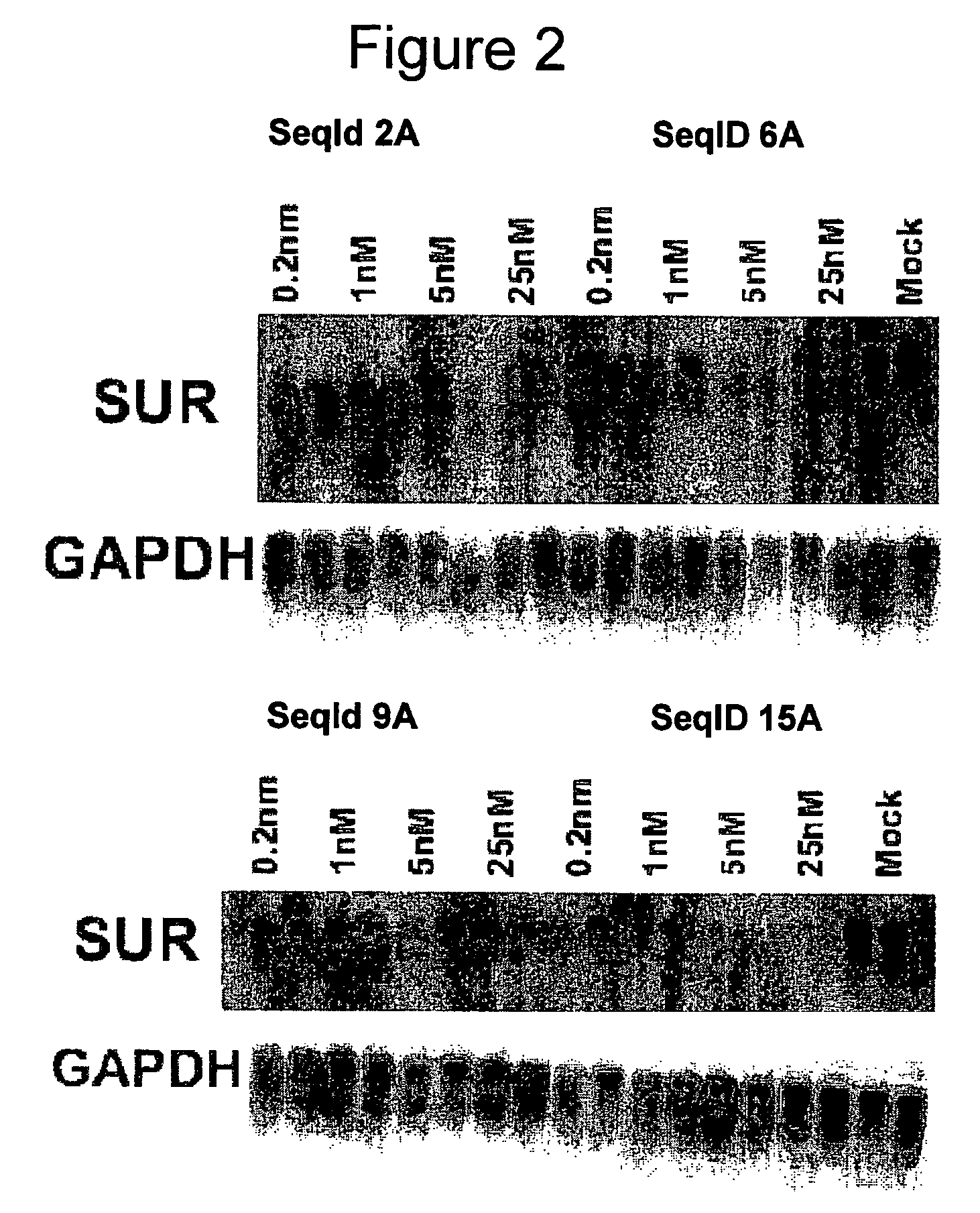
Oligomeric compounds for the modulation of survivin expression
Oligonucleotides directed against the survivin gene are provided for modulating the expression of survivin. The compositions comprise oligonucleotides, particularly antisense oligonucleotides, targeted to nucleic acids encoding the survivin. Methods of using these compounds for modulation of survivin expression and for the treatment of diseases associated with either overexpression of survivin, expression of mutated survivin or both are provided. Examples of diseases are cancer such as lung, breast, colon, prostate, pancreas, lung, liver, thyroid, kidney, brain, testes, stomach, intestine, bowel, spinal cord, sinuses, bladder, urinary tract or ovaries cancers. The oligonucleotides may be composed of deoxyribonucleosides or a nucleic acid analogue such as for example locked nucleic acid or a combination thereof.
Owner:ENZON PHARM INC
Resistive anti-obesity devices
ActiveUS7771382B2Improve overall senseImprove the immunityOesophagiIntravenous devicesIntestinal structureGastric emptying
A patient is provided with an increased sense of satiety by increasing resistance to the outflow of food from the stomach and through the intestines. Stomach emptying may be slowed with devices implantable within the gastrointestinal tract below the stomach. Implants are preferably removable and can include artificial strictures that may be adjustable to vary the rate of stomach emptying. Slowing gastric emptying may induce satiety for a longer period and may therefore reduce food consumption. Many of the embodiments include intestinal liners or sleeves, but they need not. The resistor concept may be applied to a simple anchor and resistor without a long liner.
Owner:GI DYNAMICS
Resistive anti-obesity devices
ActiveUS20080071383A1Improve overall senseReduce food consumptionSurgical instrument detailsIntravenous devicesIntestinal structureGastric emptying
A patient is provided with an increased sense of satiety by increasing resistance to the outflow of food from the stomach and through the intestines. Stomach emptying may be slowed with devices implantable within the gastrointestinal tract below the stomach. Implants are preferably removable and can include artificial strictures or apertures that may be adjustable or elastic to vary the rate of stomach emptying. Slowing gastric emptying may induce satiety for a longer period and may therefore reduce food consumption. Many of the embodiments include intestinal sleeves or sleeves, but they need not. The resistor concept may be applied to a simple anchor and resistor without a long sleeve.
Owner:GI DYNAMICS
Thrombolysis device
A catheter suitable for dissolving blockages in tubular tissue provides a combination of low frequency (1–100 Hz) vibratory motion and injection of a lysing agent. The tubular tissue may be veins, arteries, ducts, intestines, or any other blocked body lumen. For vascular thrombi, the catheter may induce a vibrating, stirring action in and around the thrombus in combination with dispensing of a thrombolytic agent, such as urokinase, into the thrombus. An inflatable or expandable member may be provided near a distal tip of the catheter to prevent release of dislodged thrombus.
Owner:TYCO HEALTHCARE GRP LP
Composite probiotics micro-ecological formulation and preparation method
InactiveCN101496822AEnsure balancePromotes the detoxification processBacteria material medical ingredientsDigestive systemDiseaseChronic diarrhea
The invention relates to a composite probiotic bacterium microecological preparation and a preparation method thereof. The preparation is prepared by mixing 1.0 to 20 percent of composite microbial bacterium powder, 5.0 to 50 percent of composite oligosaccharide, 30 to 80 percent of auxiliary protective carrier and 0.1 to 0.2 percent of edible essence. The composite probiotic bacterium microecological preparation is prepared from a beneficial microbial preparation and a microbial growth-promoting factor, namely the oligosaccharide, through special combination and technological recombination. The composite probiotic bacterium microecological preparation has the advantages that beneficial active bacteria and the oligosaccharide promote each other, so that the composite probiotic bacterium microecological preparation has the advantages of obviously improving the microecological environment of the whole intestine and contributing to the digestion, preventing and treating intestine diseases, treating diarrhea, chronic diarrhea, diarrhea which can not be cured by antibiotic, constipation, dyspepsia and abdominal distension which are caused by intestine dysbacteriosis, recovering the intestine flora balance, protecting the liver, reinforcing the functions of detoxication and toxin expulsion of the liver, reinforcing the disease prevention and resistance such as the resistance of human body, and the like.
Owner:上海谱莱生物技术有限公司
Hernial prosthesis for intraprosthetic fixation
An implantable hernial prosthesis having top and bottom layers and a central sleeve to facilitate manual expansion and placement of the prosthesis within an incision in a patient. The top layer and a bottom layer are secured together with at least one seam at the perimeter of the prosthesis. The top layer is made of a synthetic mesh, preferably polypropylene mesh, to promote incorporation into the abdominal wall, and the bottom layer is made of the same material or in some cases of a mechanical barrier to prevent adhesions to the intestine. The top layer is provided with a central sleeve to introduce one or two fingers to expand the prosthesis in place, and also to introduce an articulated hernial stapler to secure the mesh into the abdominal wall.
Owner:ALVARADO ALFREDO
Integrated body fluid collection and analysis device with sample transfer component
A single, integrated device in which a body fluid (e.g., blood) of a human or animal can be both collected and analyzed easily and without risk of contamination is disclosed. The collection portion and analysis portion of the device are permanently joined to permit movement of small quantities of body fluid under controlled conditions, to minimize any waste of the body fluid, to ensure that no contamination reaches the main body fluid volume, and to create a permanent physical record of the results of the analysis in association with the fluid sample itself: A wide variety of different body fluid components which may be indicative of various diseases, dysfunctions and abnormalities of the human or animal or the body fluid itself can be tested for. The device includes a container for collecting human or animal body fluid, one or more testing chambers containing one or more analysis units activated by body fluid; a transfer pump or vacuum assembly to transfer one or more samples into the analysis units; and one-way valves or the equivalent to prevent any portion of the withdrawn sample from being returned to the collection container. The body fluid acted upon may be blood, blood plasma, urine, bile, pleural fluid, ascites fluid, stomach or intestine fluid, colostrom, milk or lymph.
Owner:AALTO SCI
Treatment of inflammatory conditions of the intestine
Owner:CSL BEHRING AG
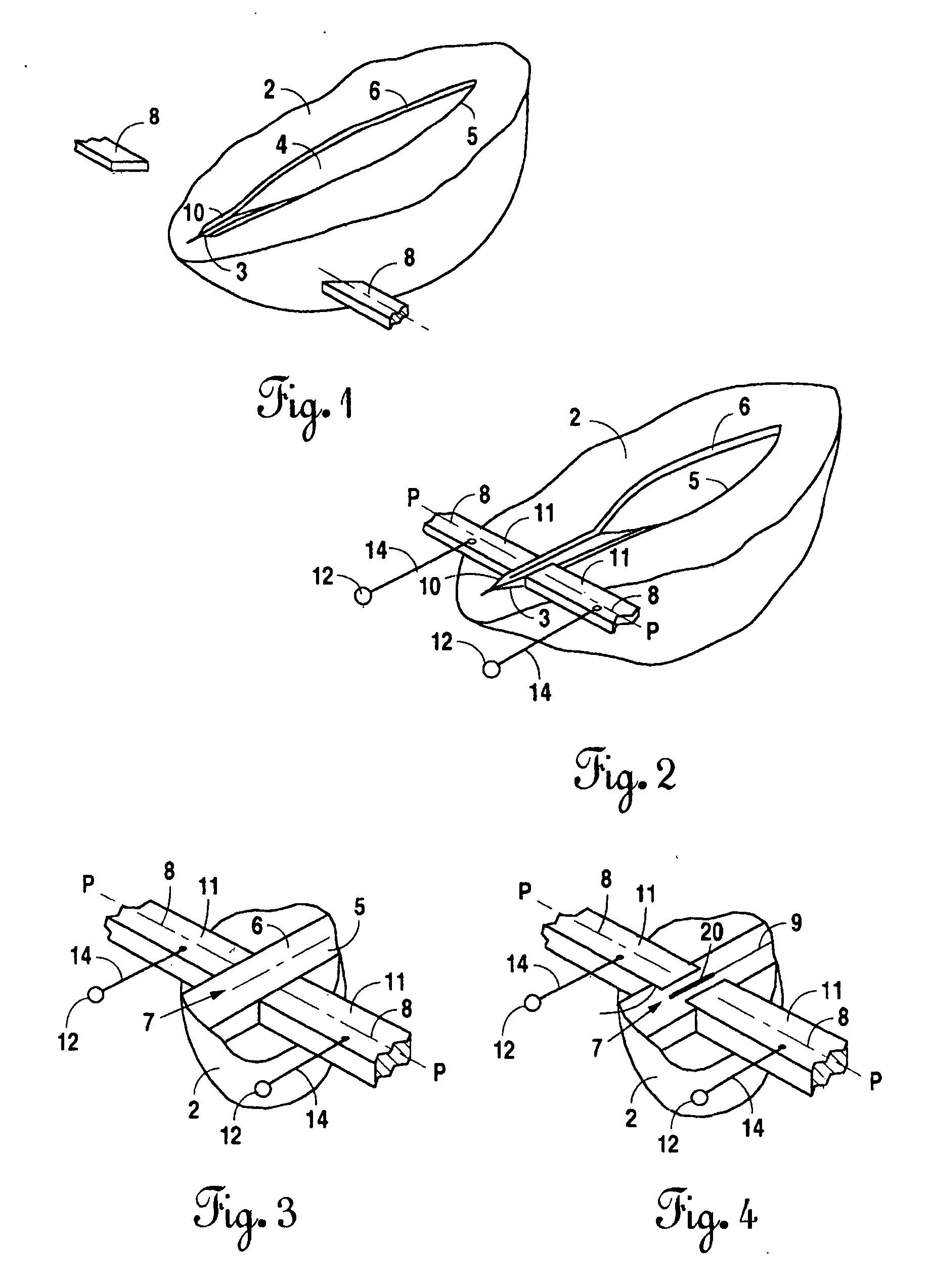
Instrument and method for the end-to-end reconnection of intestinal tissues
InactiveUS20070276363A1Easy to useEasy to cleanDiagnosticsSurgical instruments for heatingIntestinal structureAbdominal cavity
The current methods for reconnecting intestinal tissues, suturing, or metallic staples, can result in a leaky anastomosis that may result in post-operative infections in the patient's abdominal cavity. By utilizing electrical current to bond or weld the intestinal tissue, this potential for leaky anastomosis can be significantly reduced. This invention provides tools and processes that allow electrical tissue bonding to be used on a hollow tissue, such as an intestine. This invention also discloses a means for further reducing the inherent problem of tissue sticking to the electrodes, by introducing a superior electrode design that uses a composite material, copper-molybdenum (CuMo).
Owner:LIVE TISSUE CONNECT +2
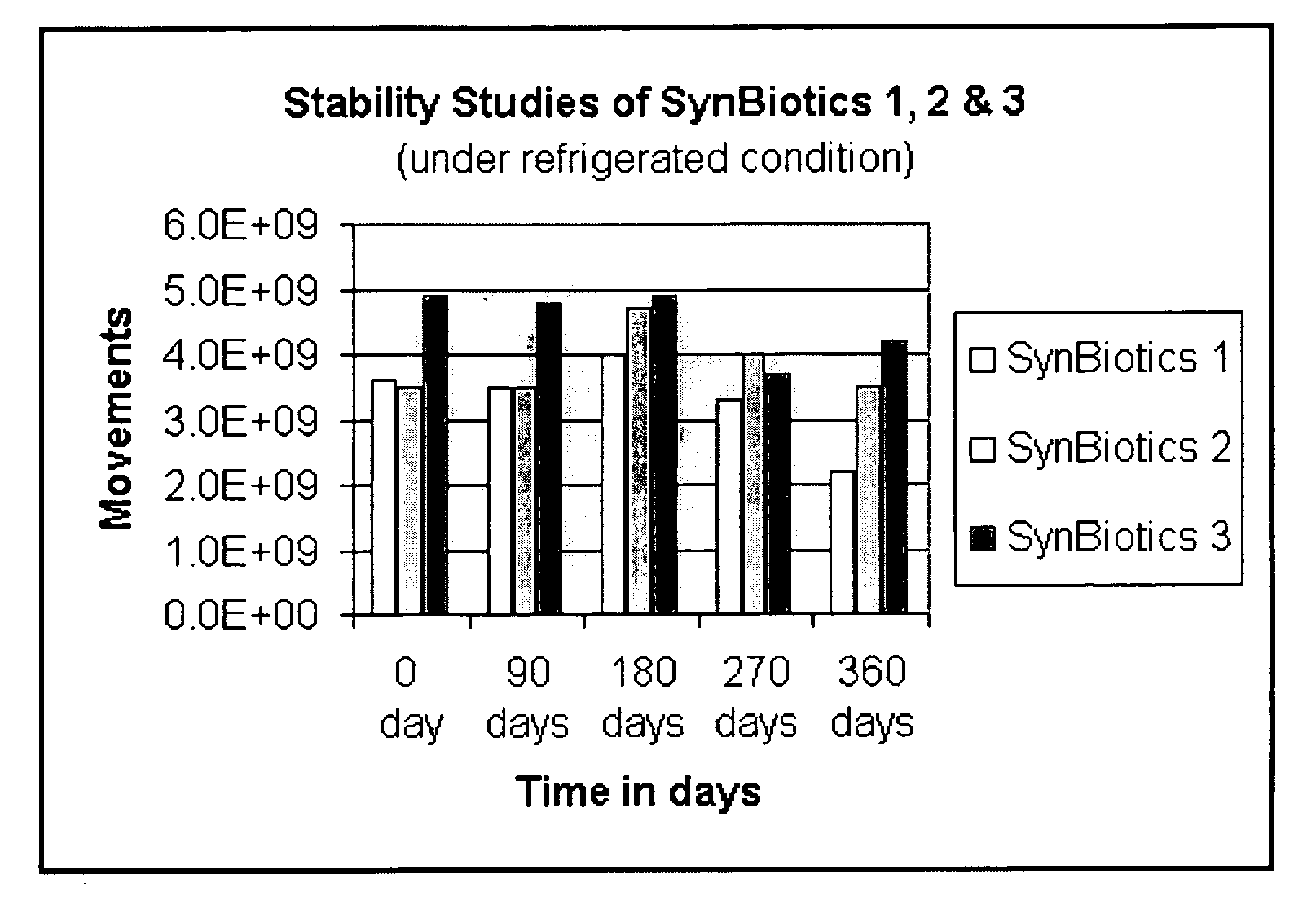
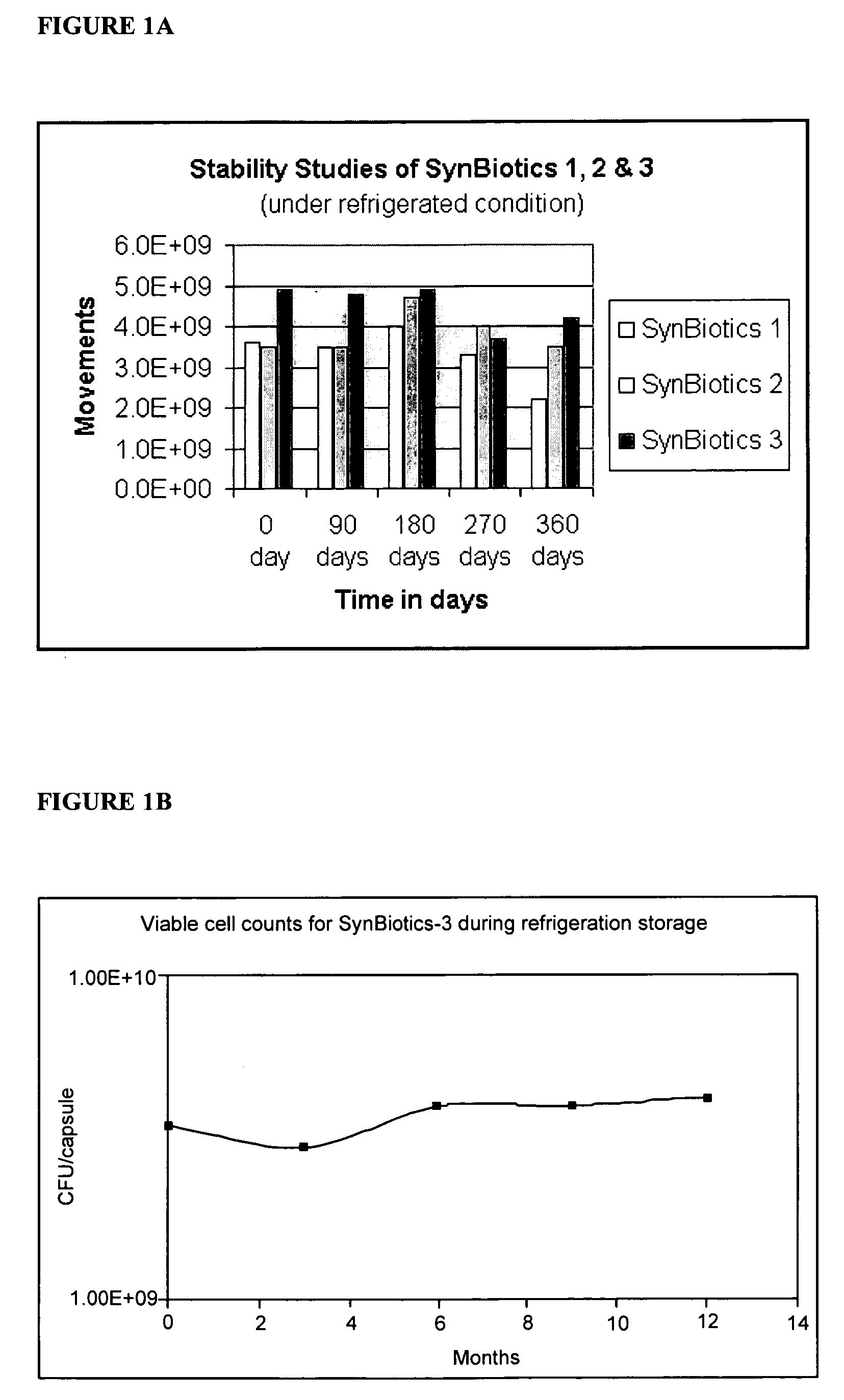
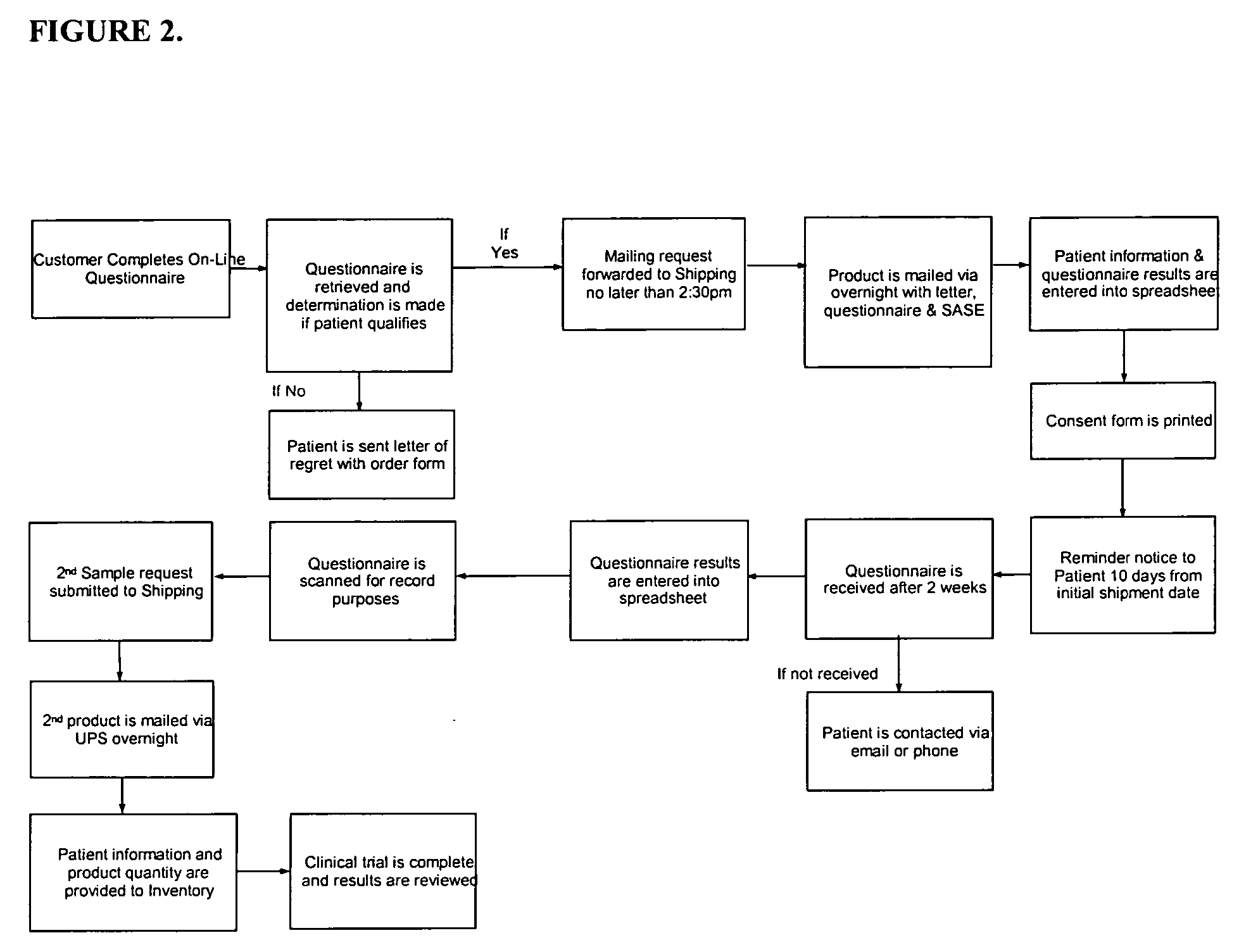
Synbiotics
InactiveUS20060093592A1Enhance immune functionImprove Gut HealthAntibacterial agentsBiocideIntestinal structureSynbiotics
A novel product comprising a mixture of probiotic and prebiotic ingredients for the treatment of IBS, IBD, Crohn's disease, antibiotic induced diarrhea and other bowel disorders is described herein. Stabilized rice bran derivatives, including stabilized rice bran, RiSolubles, RiceMucil, and Cea100, are used as the source of prebiotics. The prebiotic source is not only rich in fructo-oligosaccharides, but also has potent antioxidants and phytonutrients for intestinal health and the proliferation of bifido-bacteria in the intestines. The probiotics used are different combinations and concentrations of Lactobacilli species, depending on the specific gastrointestinal disease which is targeted. When refrigerated, the products are shelf-stable (95-99%) for at least one year.
Owner:NUTRACEA
Intragastric device for treating obesity
InactiveUS20060155311A1Small volumeEasy to placeStentsNon-surgical orthopedic devicesIntestinal structurePylorus
An apparatus and method comprising at least one intragastric member or artificial bezoar made of a digestive-resistant or substantially indigestible material that is introduced into a gastric lumen of a mammal for the treatment of obesity. The intragastric member or artificial bezoar is typically at inserted into the gastric lumen in a partially compacted configuration, whereby it is then manipulated into, or allowed to assume, a second expanded configuration sufficiently large to remain within the reservoir of the stomach during normal activities and not be passed through the pylorus into the intestines. In animals, the present invention has been found to be effective in achieving weight loss over a several month period, while being easy to place and retrieve.
Owner:HASHIBA KIYOSHI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com