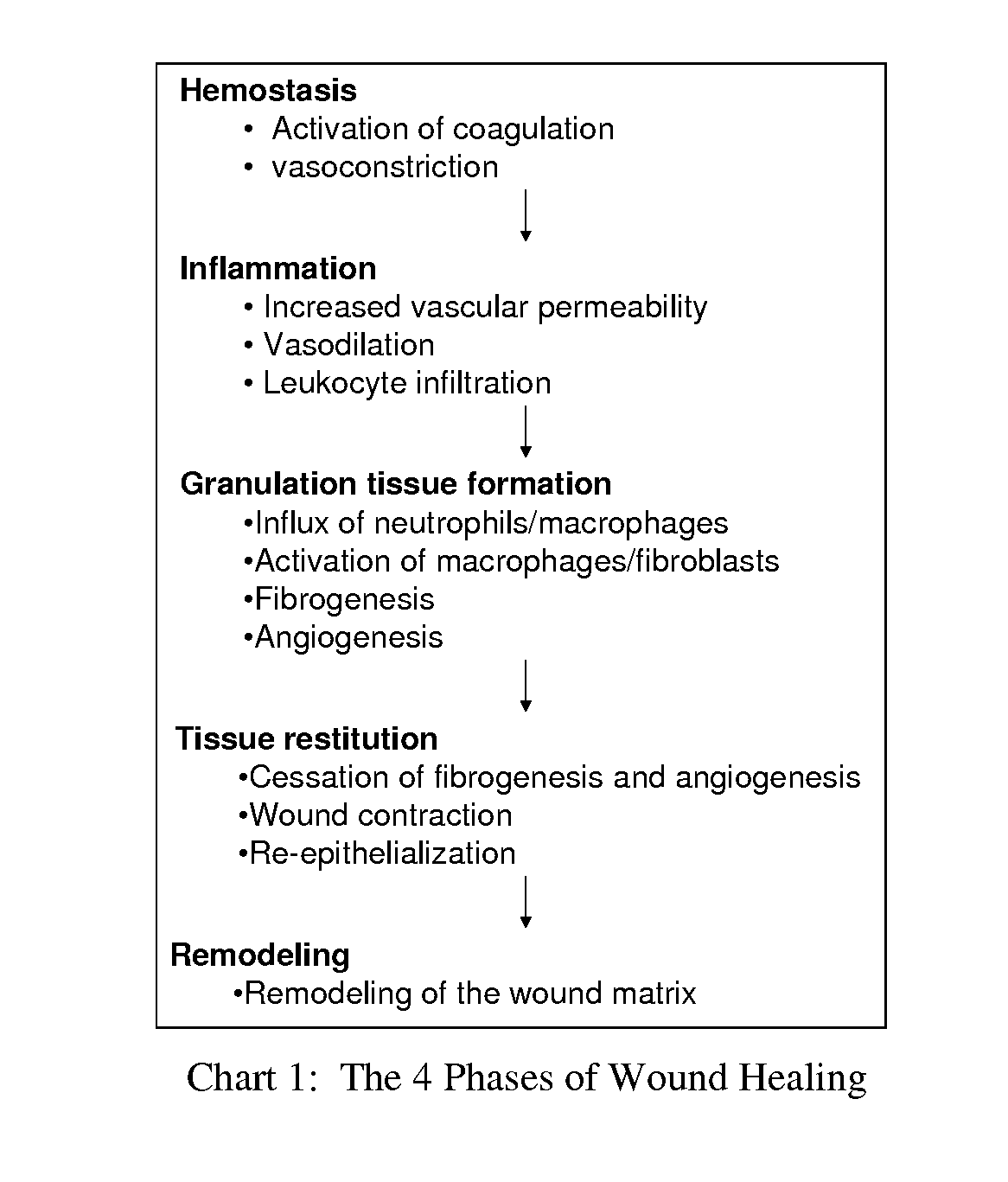
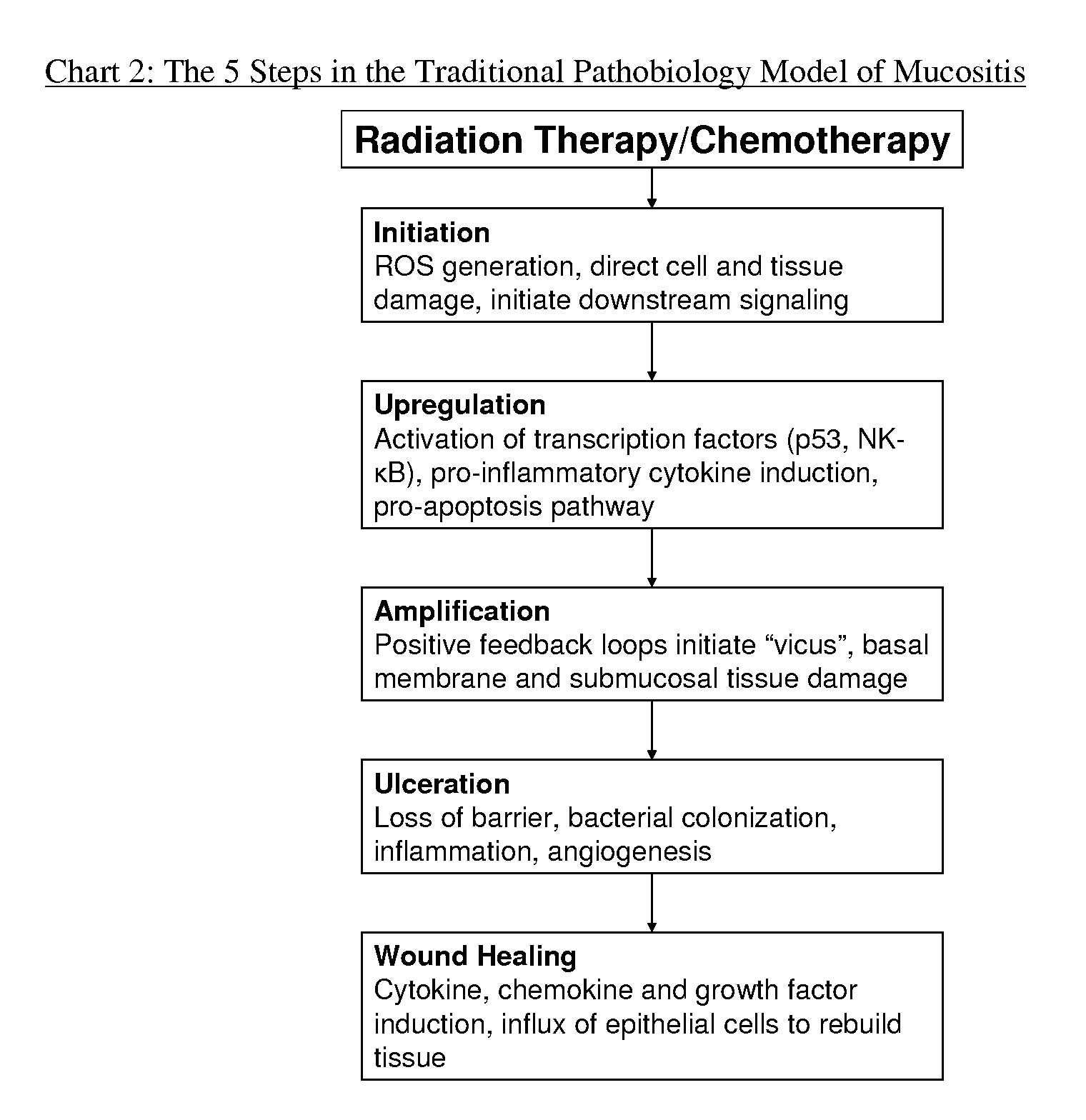
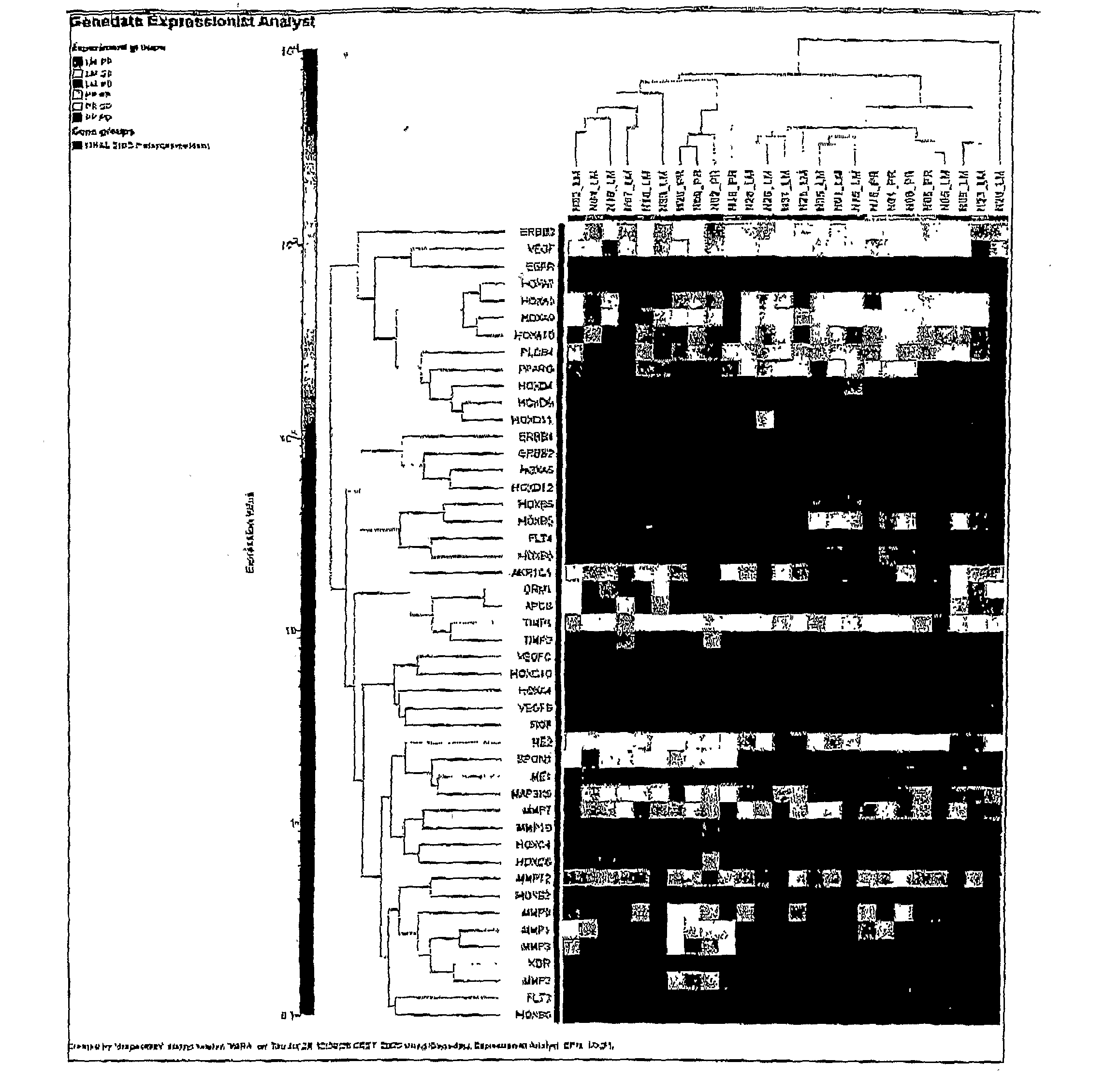
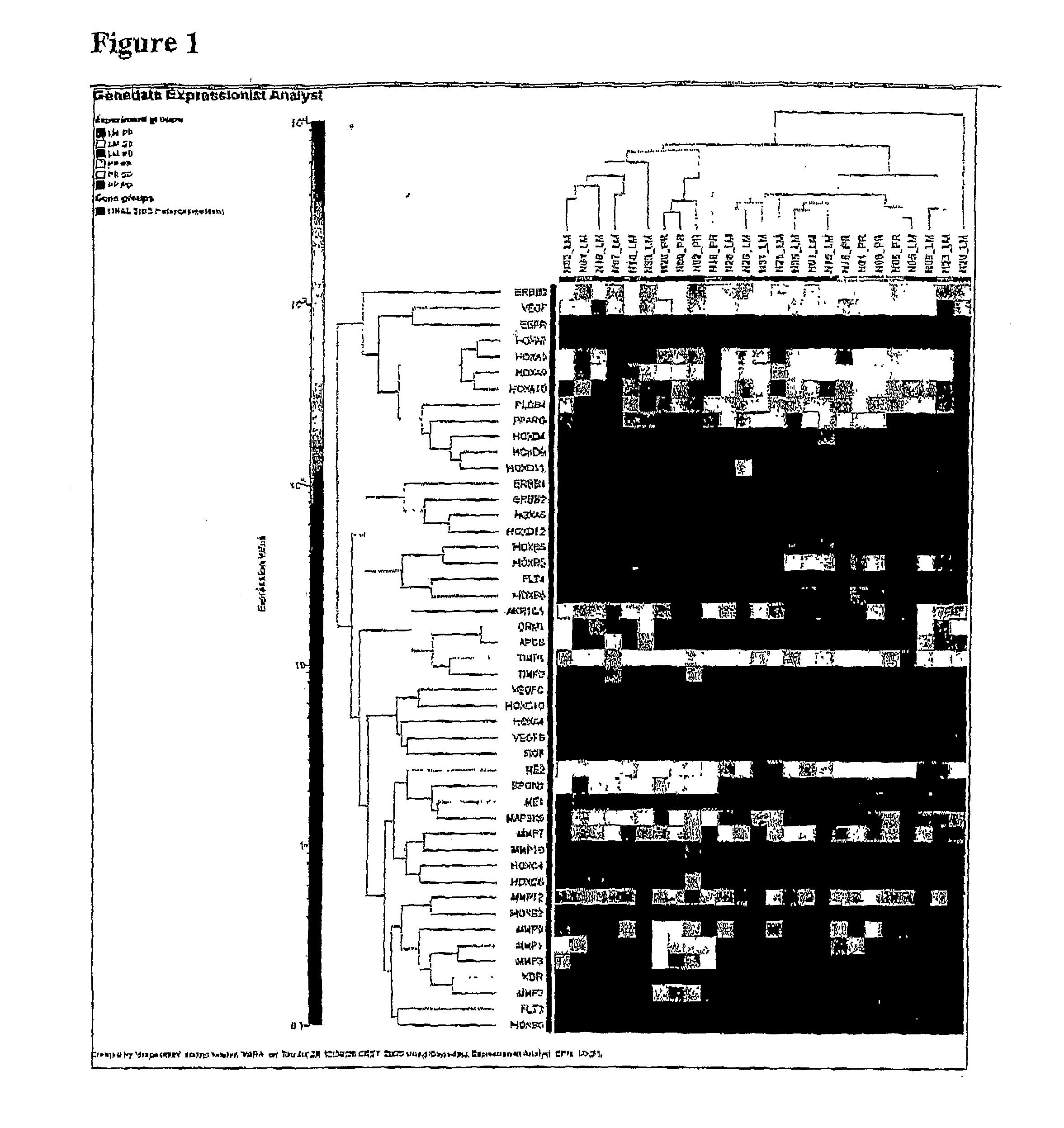
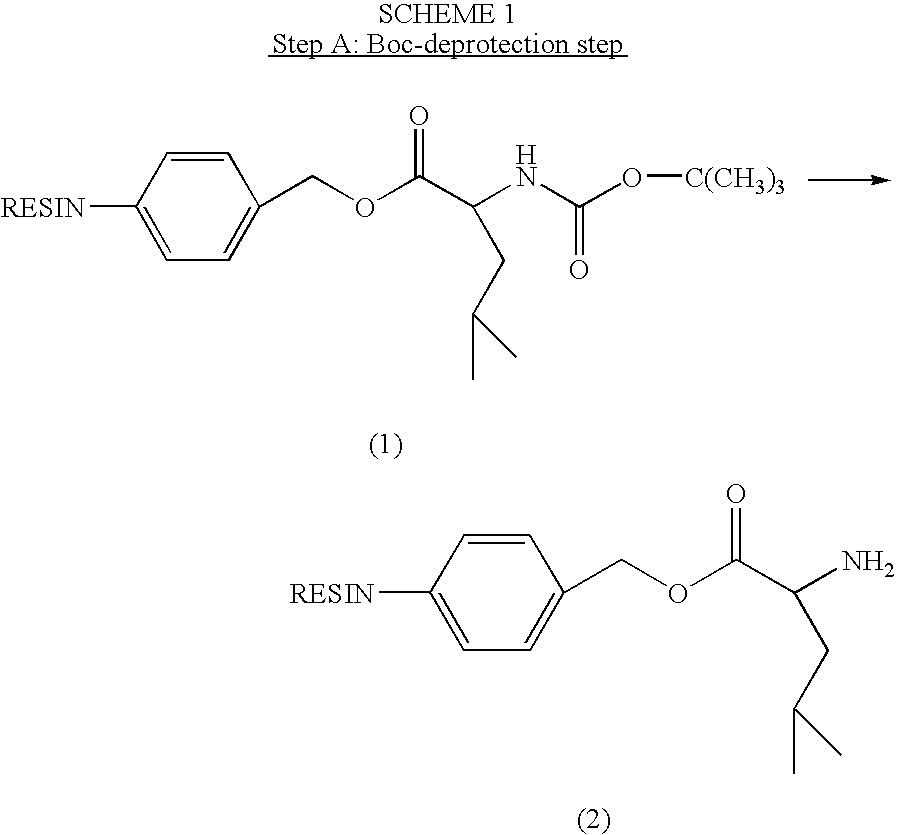
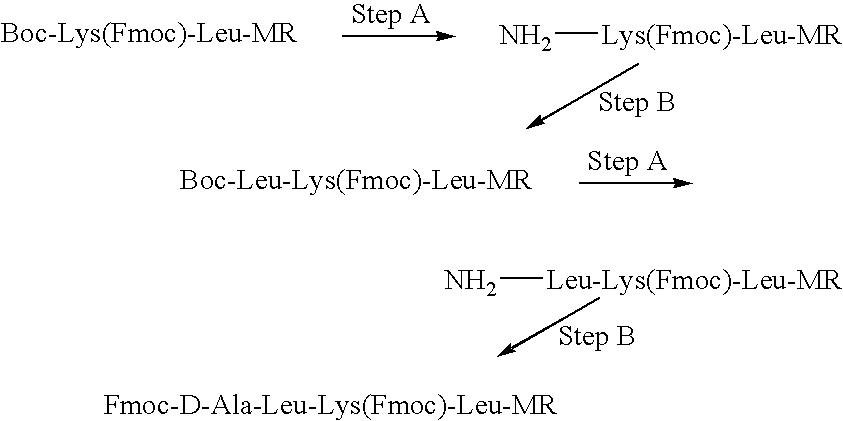
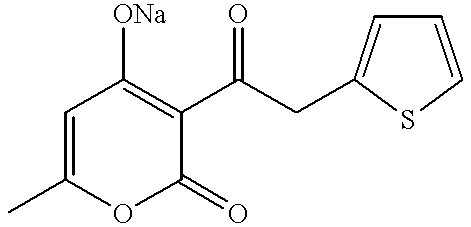
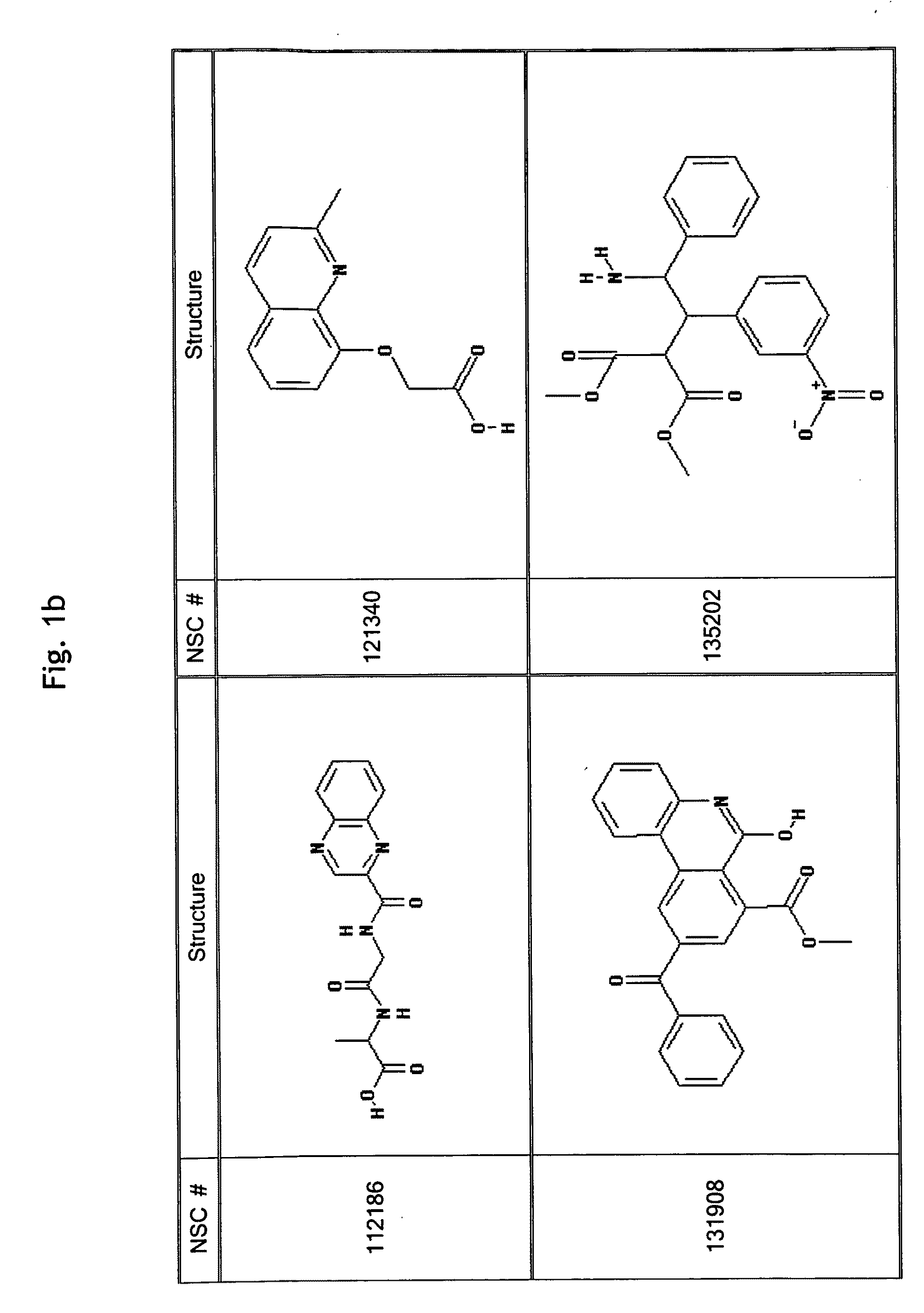
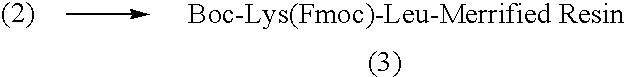Patents
Literature
517 results about "Cancer chemotherapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chemotherapy (also called chemo) is a type of cancer treatment that uses drugs to kill cancer cells. ... Chemotherapy works by stopping or slowing the growth of cancer cells, which grow and divide quickly.
Compositions and methods for the treatment of cancer
InactiveUS20020128228A1Reducing and avoiding adverse effectImprove toleranceBiocideAnimal repellantsIntestinal structureCancer prevention
This invention relates to compositions comprising temozolomide and thalidomide which can be used in the treatment or prevention of cancer, in particular malignant melanoma, cancer of the skin, subcutaneous tissue, lymph nodes, brain, lung, liver, bone, intestine, colon, heart, pancreas, adrenals, kidney, prostate, breast, colorectal, or a combination thereof. A particular composition comprises temozolomide, or a pharmaceutically acceptable salt, solvate, or clathrate thereof, and thalidomide, or a pharmaceutically acceptable salt, solvate, or clathrate thereof. The invention also relates to methods of treating or preventing cancer, in particular malignant melanoma, cancer of the skin, subcutaneous tissue, lymph nodes, brain, lung, liver, bone, intestine, colon, heart, pancreas, adrenals, kidney, prostate, breast, colorectal, or a combination thereof, which comprise the administration of temozolomide and thalidomide and another anti-cancer drug to a patient in need of such treatment or prevention. The invention further relates to methods of reducing or avoiding adverse side effects associated with the administration of cancer chemotherapy or radiation therapy which comprise the administration of temozolomide and thalidomide to a patient in need of such reduction or avoidance.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Compositions and methods for mucositis and oncology therapies
In alternative embodiments, this invention provides compositions and methods for treating cancer or any condition caused by dysfunctional cells, side effects from treatments for cancer or any condition caused by dysfunctional cells, e.g., mucositis therapies (e.g., for oral mucositis; digestive mucositis; esophageal mucositis; intestinal mucositis). In alternative embodiments, the invention provides cytoprotection products that may be used either alone or in combination with other medical therapies such as cancer chemotherapies and radiation therapies.
Owner:VICUS THERAPEUTICS
Methods and Kits for the Prediction of Therapeutic Success, Recurrence Free and Overall Survival in Cancer Therapies
InactiveUS20080305962A1Address bad outcomesLess aggressiveMicrobiological testing/measurementLibrary screeningTherapeutic antibodyAnticancer chemotherapy
The invention provides novel compositions, methods and uses, for the prediction, diagnosis, prognosis, prevention and treatment of malignant neoplasia and cancer. The invention further relates to genes that are differentially expressed in tissue of cancer patients versus those of normal “healthy” tissue. Differentially expressed genes for the identification of patients which are likely to respond to chemotherapy are also provided. The present invention relates to methods for prognosis the prediction of therapeutic success in cancer therapy. In a preferred embodiment of the invention it relates to methods for prediction of therapeutic success of combinations of signal transduction inhibitors, therapeutic antibodies, radio- and chemotherapy. The methods of the invention are based on determination of expression levels of 48 human genes which are differentially expressed prior to the onset of anti-cancer chemotherapy. The methods and compositions of the invention are most useful in the investigation of advanced colorectal cancer, but are useful in the investigation of other types of cancer and therapies as well.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Topical vasoconstrictor preparations and methods for protecting cells during cancer chemotherapy and radiotherapy
ActiveUS20070077219A1Reduce and preferably prevent oral mucositisReduces and completely prevents oral mucositisBiocideCosmetic preparationsVasoconstrictor AgentsDermatology
Vasoconstrictors are administered topically to provide protection against the adverse effects, e.g., alopecia, mucositis or dermatitis, induced by chemotherapy or radiotherapy. Appropriate dosages and formulations of topical vasoconstrictors are provided. Methods for the use of such compositions are also provided.
Owner:WISCONSIN ALUMNI RES FOUND
Prodrugs activated by plasmin and their use in cancer chemotherapy
InactiveUS7402556B2Low toxicityHigh specificity of actionSugar derivativesTetrapeptide ingredientsPeptide substrateOligopeptide
The product of the invention is a modified form of a therapeutic agent and comprises a therapeutic agent, an oligopeptide having a plasmin peptide substrate of 2-4 amino acids and mono- or di-peptide linkage, a stabilizing group and, optionally, a linker group. The prodrug is cleavable by plasmin. Also disclosed are methods of making and using the prodrug compounds.
Owner:MEDAREX LLC
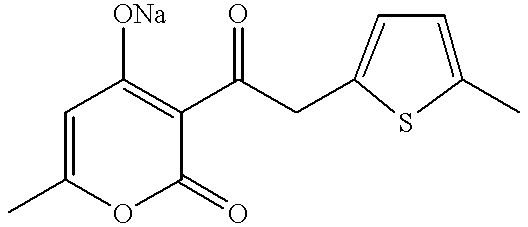
Ketone derivatives and medical application thereof
The present invention relates to ketone derivatives represented by the following formula and medical agents containing the ketone derivatives or pharmacologically acceptable salts thereof as an active ingredient, and in particular, relates to a hematopoietic agent; it is shown that the present invention increases blood cells, such as platelets, white blood cells, and red blood cells, and is effective in preventing and treating cytopenia caused by cancer chemotherapy, radiation therapy, and the like.
Owner:TORAY IND INC
Fatty acid treatment
InactiveUS6407075B1Eliminate side effectsBiocideCarbohydrate active ingredientsAnticancer chemotherapySide effect
The use in preparation of a medicament for treating and preventing the side effects of anti-cancer chemotherapy of a polyunsaturated fatty acid with a carbon chain length of 14 to 26 and with 2 to 6 double bonds in the molecule in cis or trans configuration, and a method of such treatment or prevention wherein said fatty acid is used as an active.
Owner:LUITPOLD PHARMA INC
Amino thiol compounds and compositions for use in conjunction with cancer therapy
ActiveUS20050101676A1Reducing and preventing hair lossImprove toleranceBiocideOrganic chemistrySide effectThiol
The invention provides novel polyamine and amino thiol compounds and pharmaceutical compositions for administration in conjunction with cancer chemotherapy or radiation therapy. The compounds are administered locally to provide protection against the adverse side-effects of chemotherapy or radiation therapy, such as alopecia, mucositis and dermatitis. Pharmaceutical preparations comprising one or more chemoprotective polyamines or amino thiols formulated for topical or local delivery to epithelial or mucosal cells are disclosed. Methods of administering the pharmaceutical preparations are also disclosed.
Owner:WISCONSIN ALUMNI RES FOUND
Ondansetron orally disintegrating tablets
InactiveUS7390503B1Safe and effective absorptionImprove bioavailabilityPowder deliveryPill deliveryWater dispersibleOrally disintegrating tablet
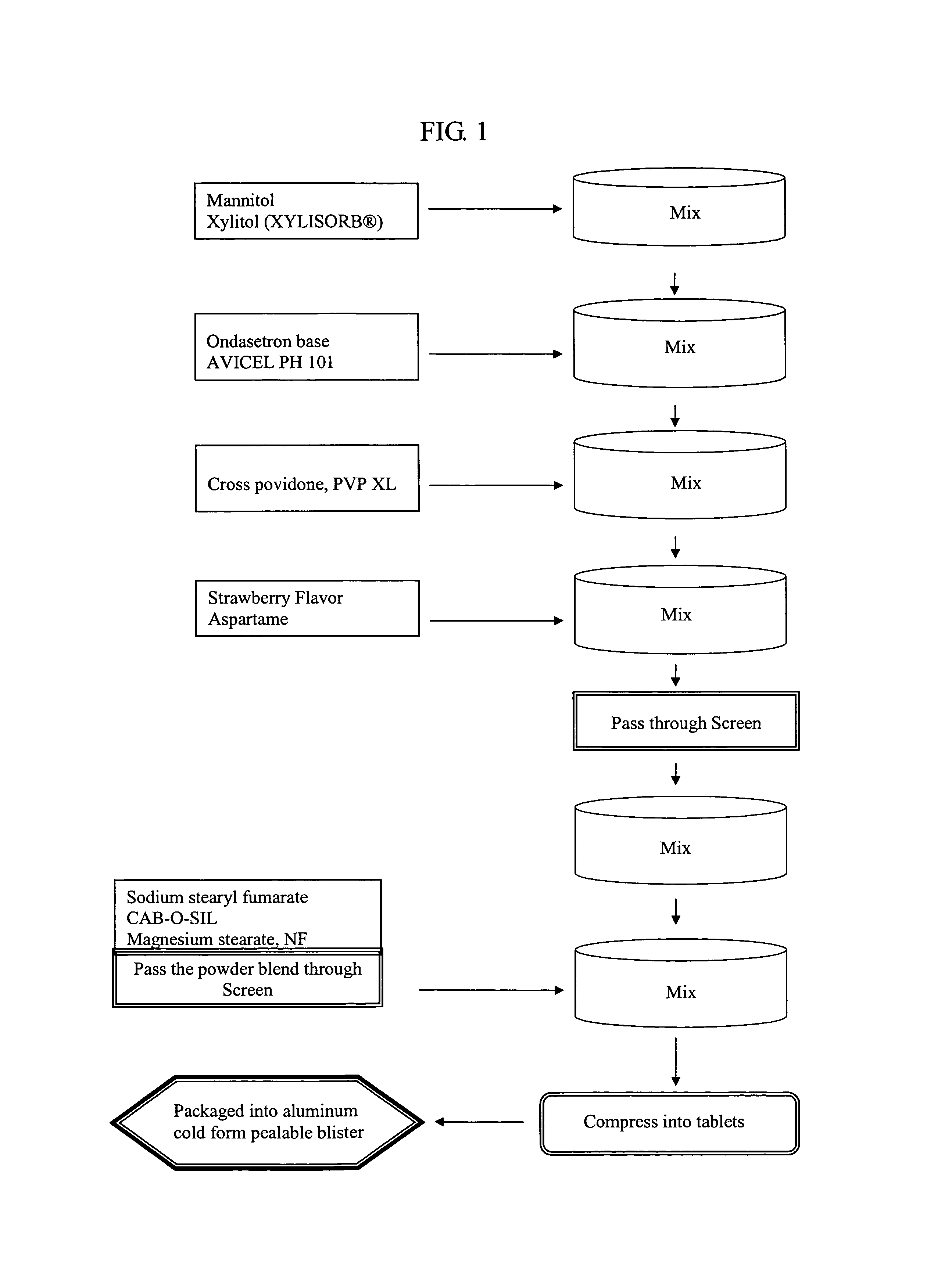
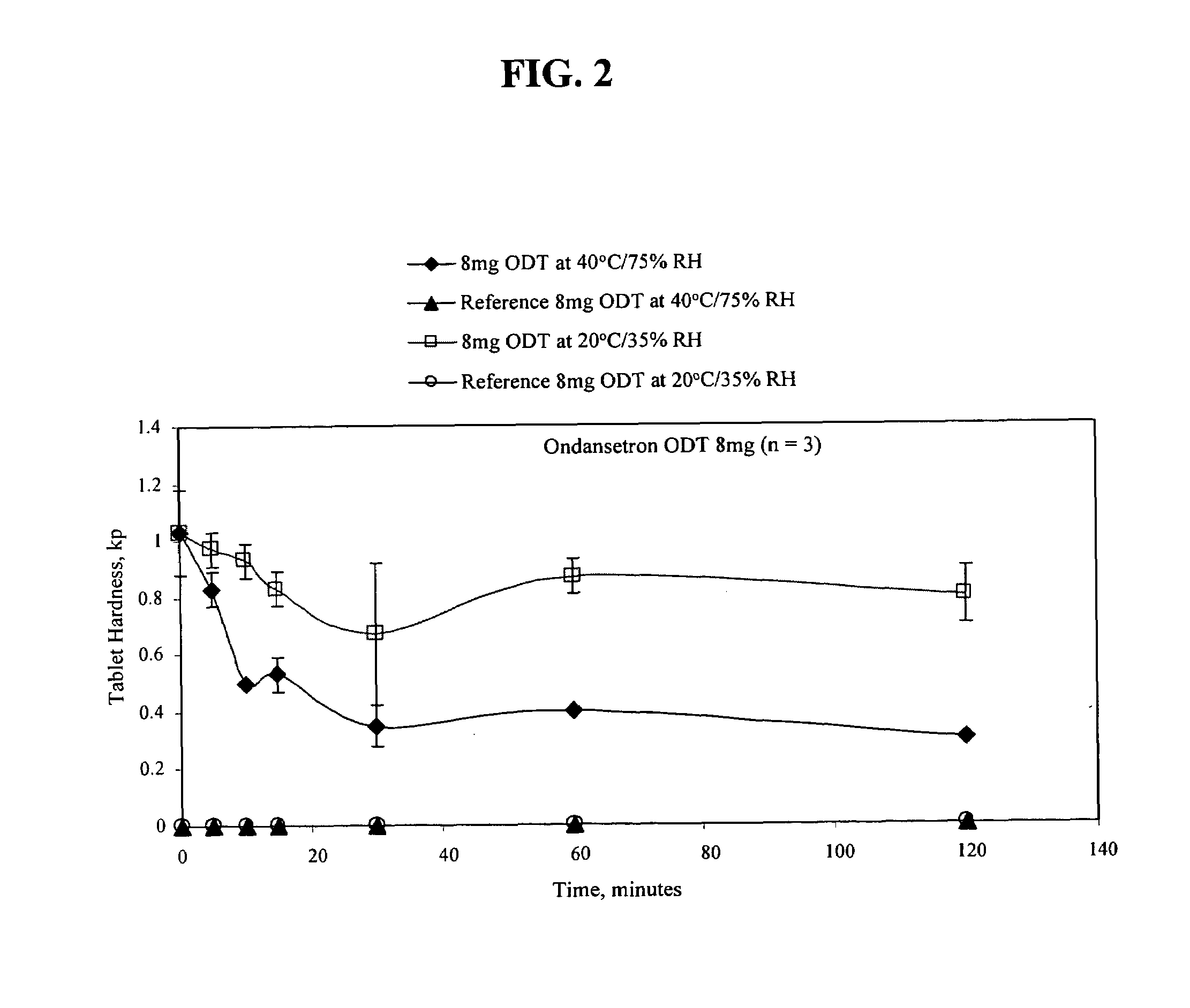
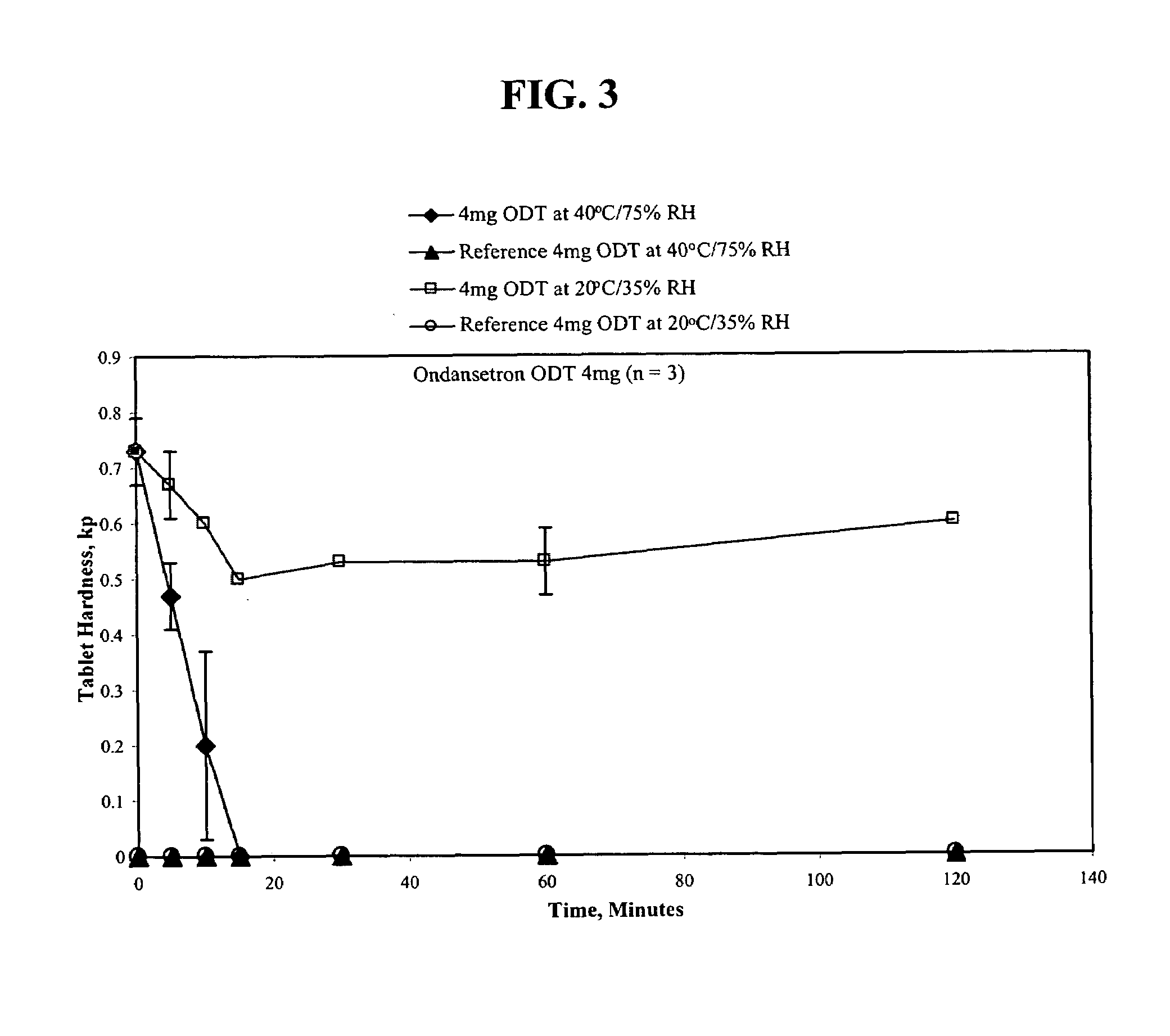
An ondansetron solid orally disintegrating dosage form for oral administration having at least one first water-dispersible component or water-insoluble cellulose derivative, a component having a —CHOH functional group, a disintegrating agent and at least one lubricant is provided. The dosage form can comprise ondansetron, a hydrophilic polymer such as microcrystalline cellulose, a component having a —CHOH functional group such as mannitol or xylitol and a disintegrating agent such as crospovidone. The lubricant may be a mixture of magnesium stearate, sodium stearyl fumarate and colloidal silicon dioxide. The present invention provides a non-effervescent tablet comprising the ondansetron dosage form. Another aspect of the invention is the treatment of emesis such as nausea and vomiting caused by cancer chemotherapy and radiation by the administration of the ondansetron formulation of the present composition. Finally, a process of forming an ondansetron disintegrating tablet using the ondansetron dosage form is disclosed.
Owner:BARR LAB
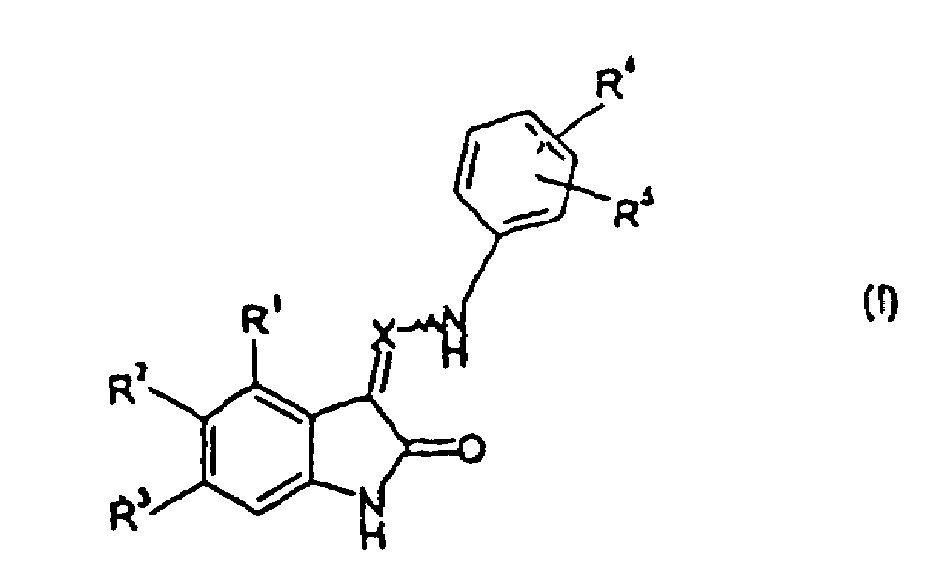
Substituted di-hydroxyl-indol derivatives as protein tyrosine kinase and as protein serine/threenine kinase inhibitors
Owner:GLAXO GRP LTD
Formulation of amino acids and riboflavin useful to reduce toxic effects of cytotoxic chemotherapy
Pharmaceutical compositions effective in alleviating or reducing the effects of fatigue and weakness associated with cancer and cancer chemotherapy are disclosed. The pharmaceutical compositions of the present invention comprise riboflavin, effectors of the urea cycle in free form or pharmacologically acceptable salts thereof, and amino acids selected from the groups of essential and non-essential amino acids, in free form or pharmaceutically acceptable salts thereof, suitably combined with appropriate carriers, diluents, or excipients. Also disclosed are methods of alleviating or reducing the effects of fatigue and weakness associated with cancer and cancer chemotherapy by administration of pharmaceutical compositions of the present invention.
Owner:BURZYNSKI STANISLAW R
Nano-carrier particle controllable in drug release and preparation method thereof
InactiveCN103007290AControl releaseSolve the problem of early leakagePowder deliveryOrganic active ingredientsSide effectMesoporous silica
Owner:SOUTHEAST UNIV
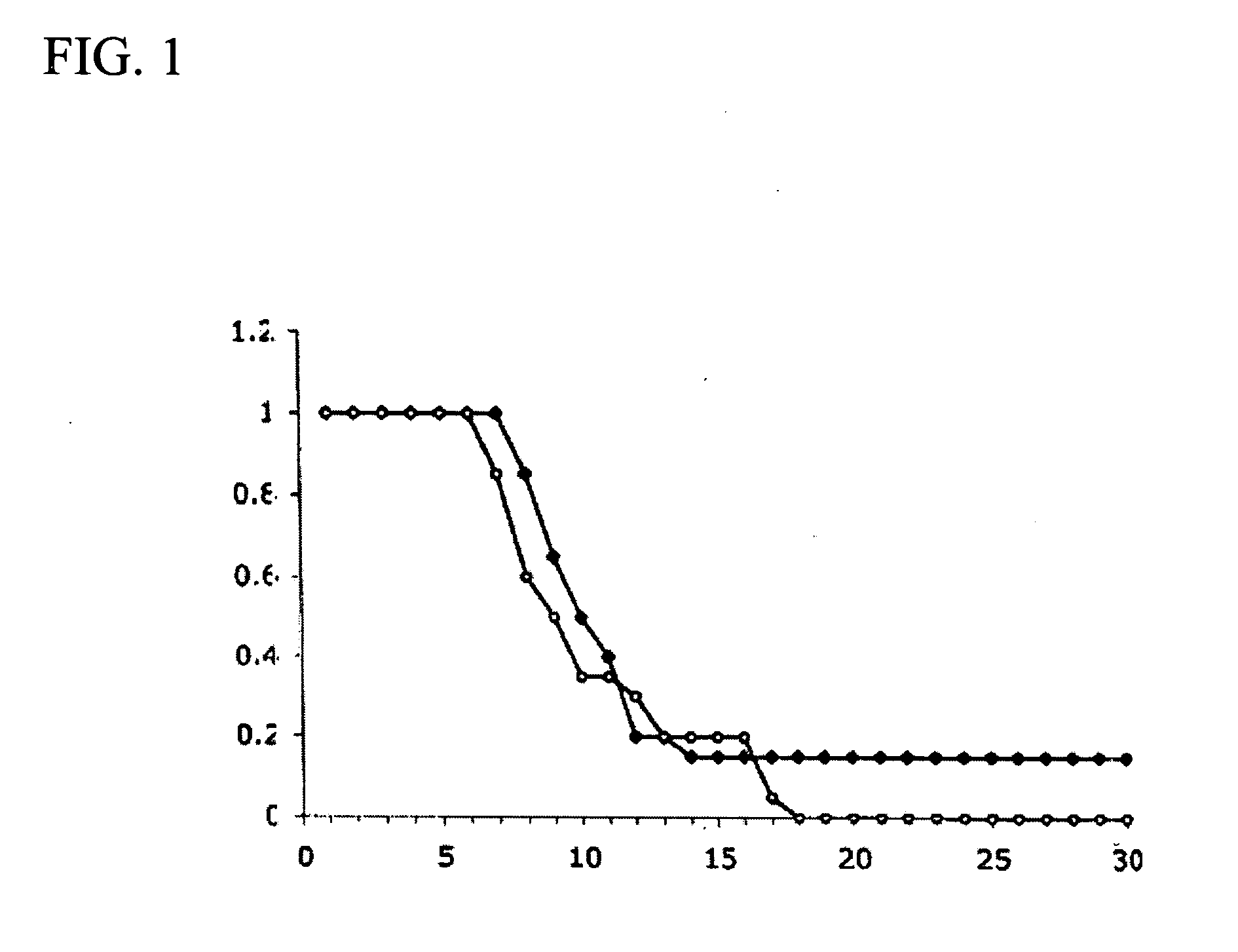
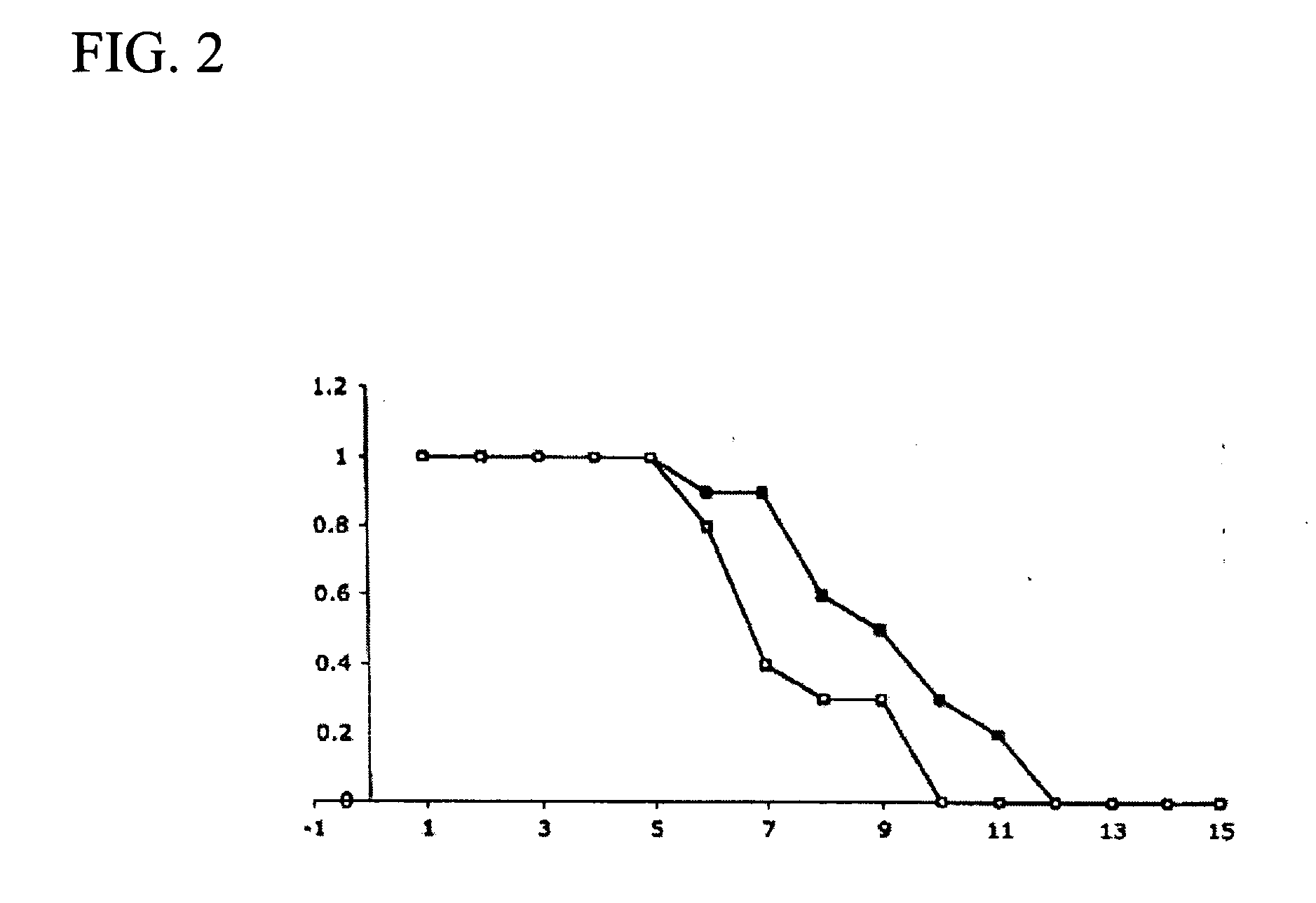
Oral composition containing Ectoine and hyaluronic acid and application of oral composition
ActiveCN110151594AGood moisturizing effectSolve functionCosmetic preparationsHydroxy compound active ingredientsMedical equipmentOral ulcers
The invention discloses an oral composition containing Ectoine and hyaluronic acid and application of the oral composition. The oral composition is prepared from, by mass, 0.1-5% of high molecular weight hyaluronic acid or salt thereof, 0.1-5% of low molecular weight hyaluronic acid or salt thereof, 0.01-1% of tea polyphenol, 1-3% of xylitol, 0.1-1% of Ectoine and the balance water, and the totalpercentage is 100%. Accordingly, the Ectoine, different molecular weights of hyaluronic acid or salt thereof, tea polyphenol and xylitol are compounded, the bio-safety is high, the synergetic effect is achieved, the effects of nursing wound surfaces, diminishing inflammation, promoting healing and the like are achieved, and the oral composition is applicable to oral care application such as oral care after cancer chemotherapy and oral ulcer care and can also be applied to the field of medical products, medical equipment and disinfection products.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD +1
Pyridyl-substituted porphyrin compounds and methods of use thereof
InactiveUS20060003982A1Extended half-lifeAntibacterial agentsOrganic active ingredientsSexual impotenceReperfusion injury
The present invention relates to Pyridyl-Substituted Porphyrin Compounds, compositions comprising an effective amount of a Pyridyl-Substituted Porphyrin Compound and methods for treating or preventing injury due to exposure to a reactive species, erectile dysfunction due to surgery, lung disease, hyperoxia, neurodegenerative disease, liver disease, myocardial damage during cardioplegia, an inflammatory condition, a reperfusion injury, an ischemic condition, a cardiovascular disease, diabetes, a diabetic complication, cancer, a side effect of cancer chemotherapy, or a radiation-induced injury, or to prolong the half-life of an oxidation-prone compound, comprising administering to a subject in need thereof an effective amount of a Pyridyl-Substituted Porphyrin Compound.
Owner:INOTECK PHARMA CORP
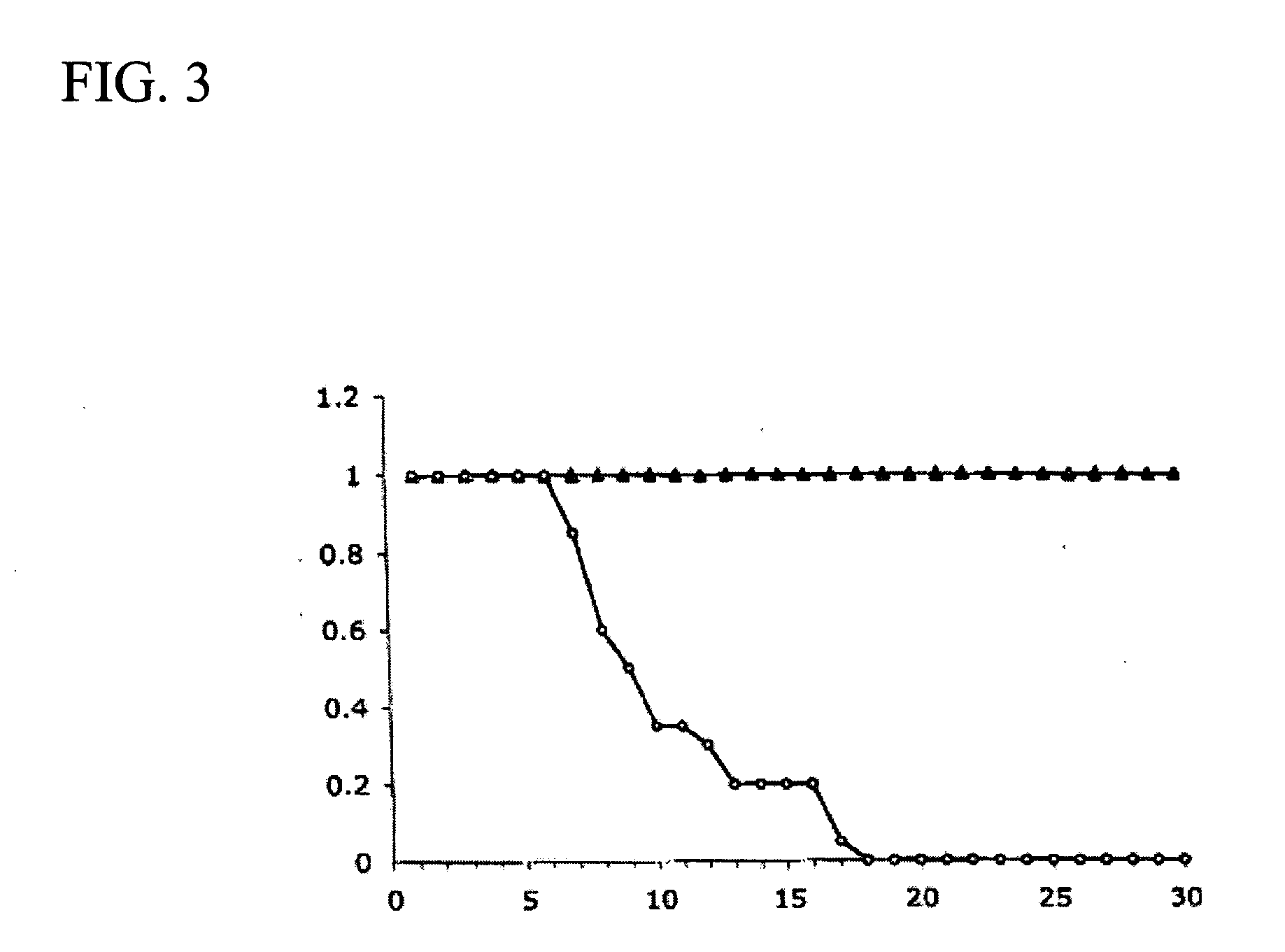
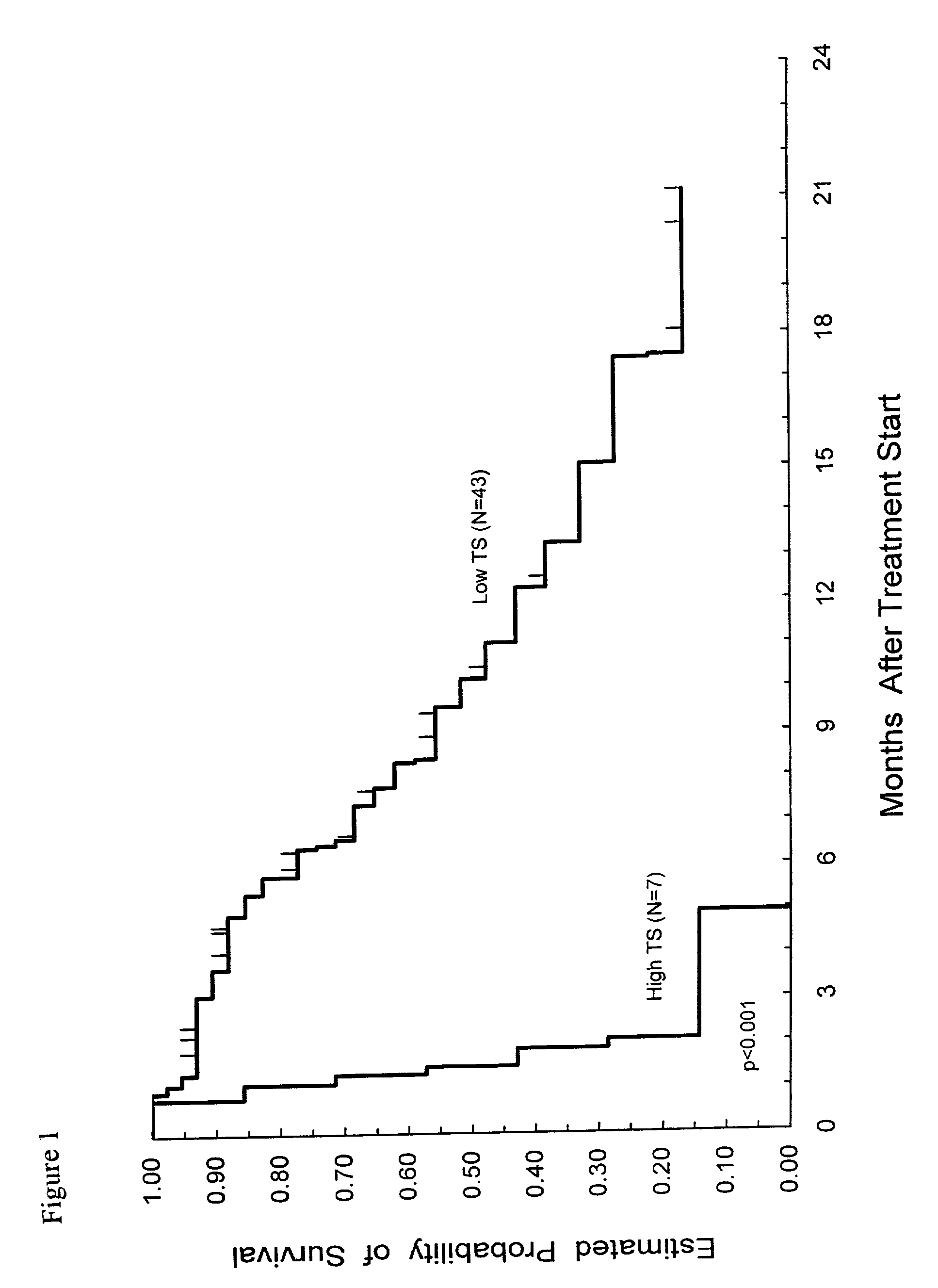
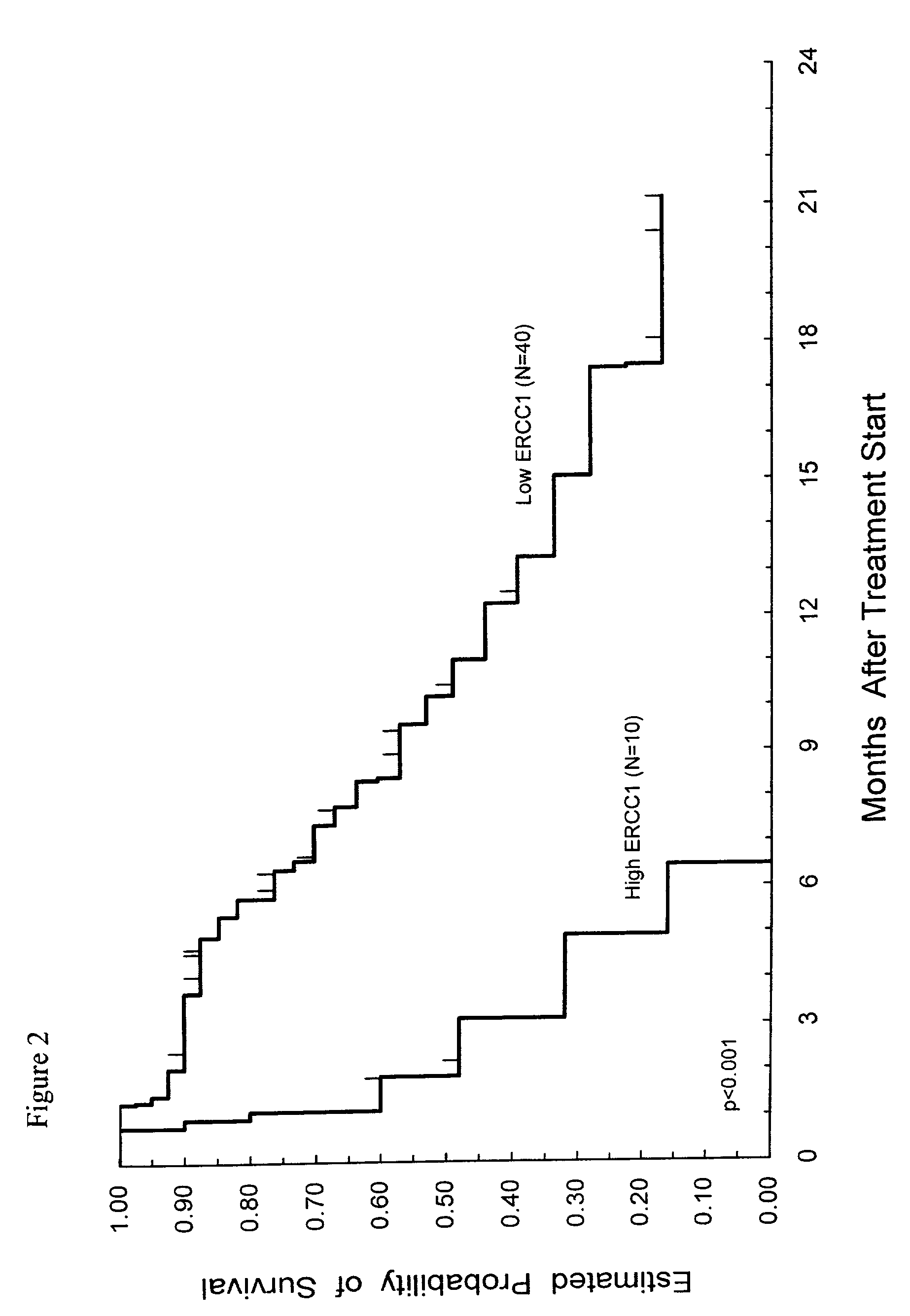
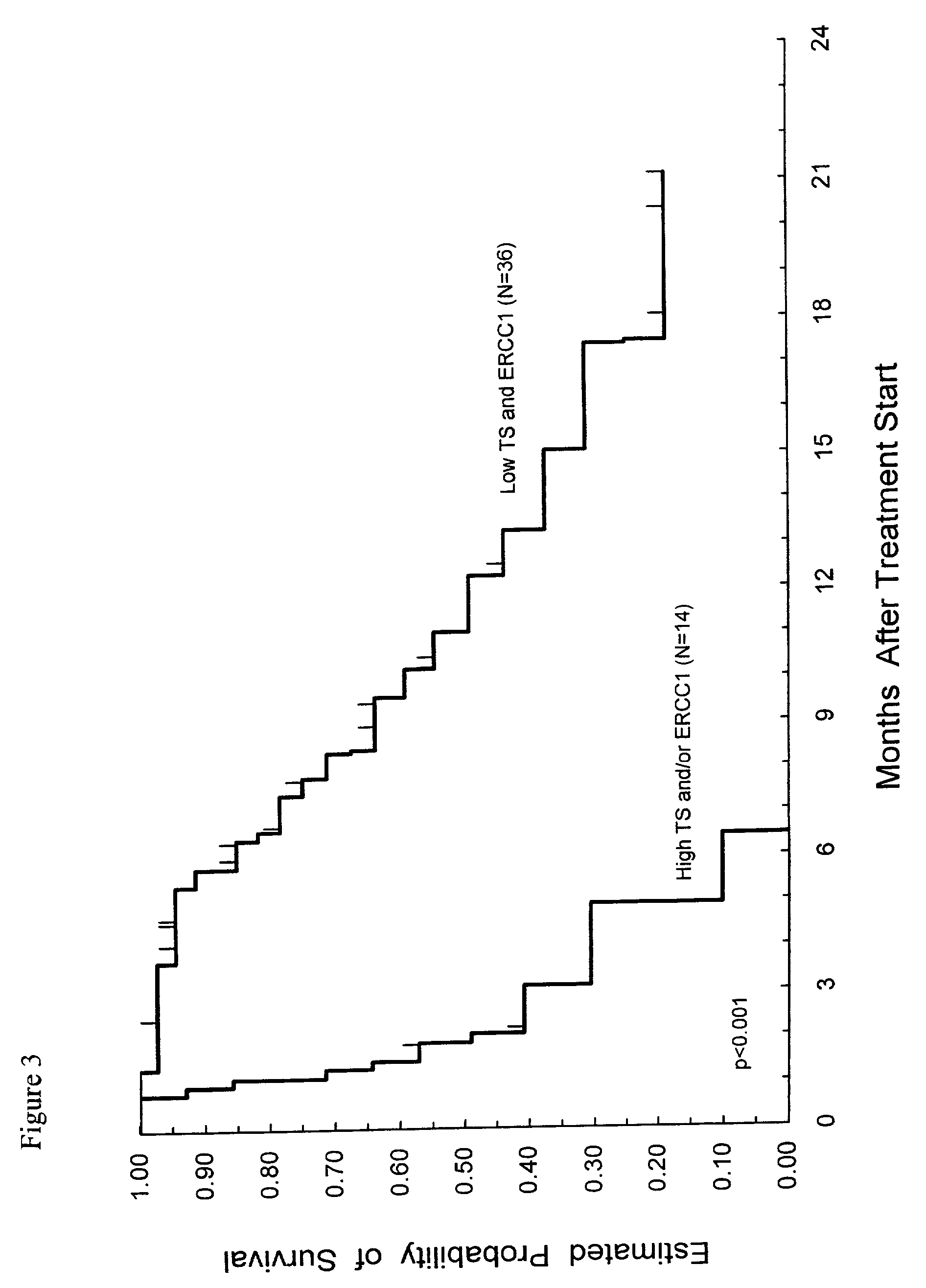
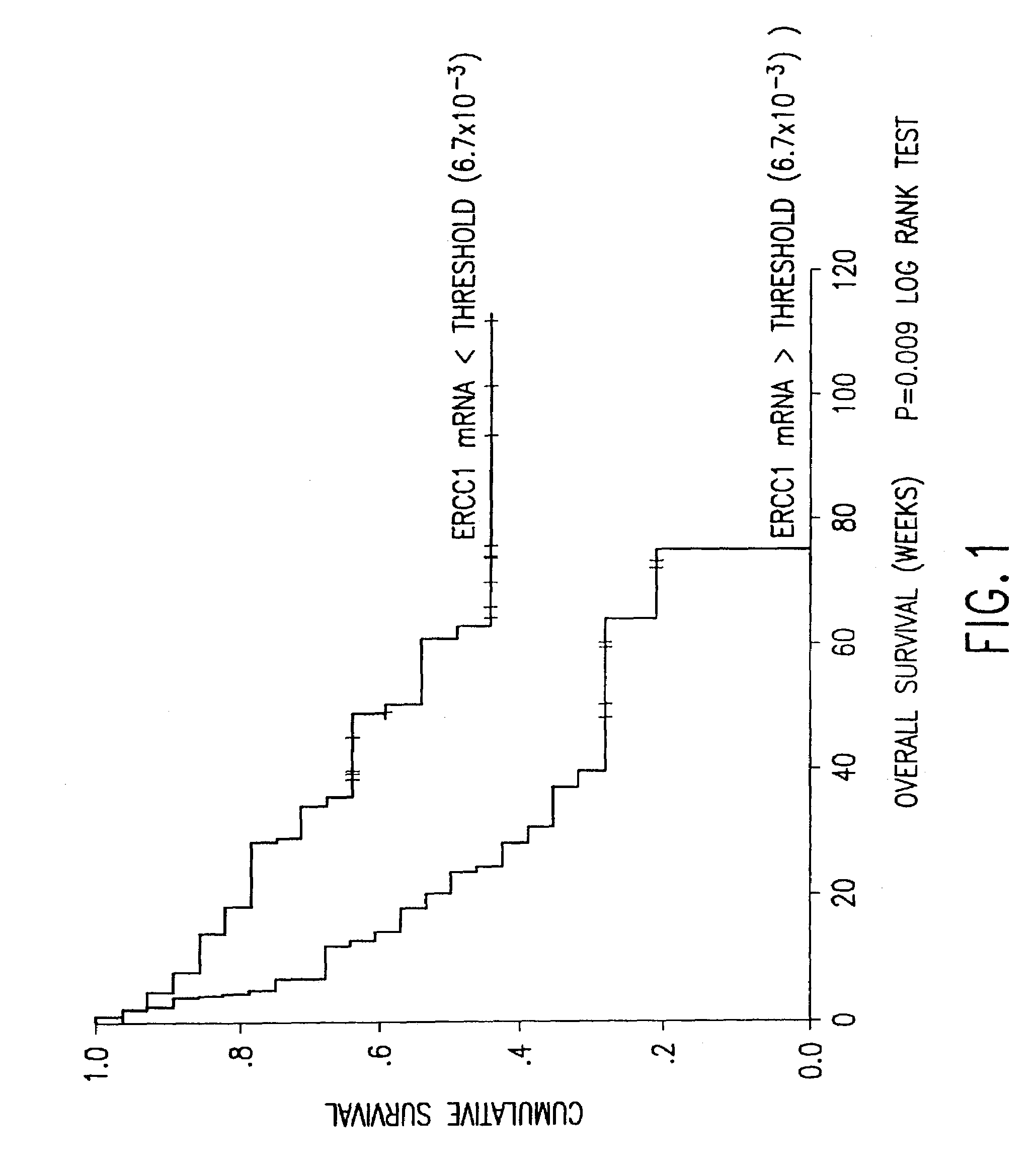
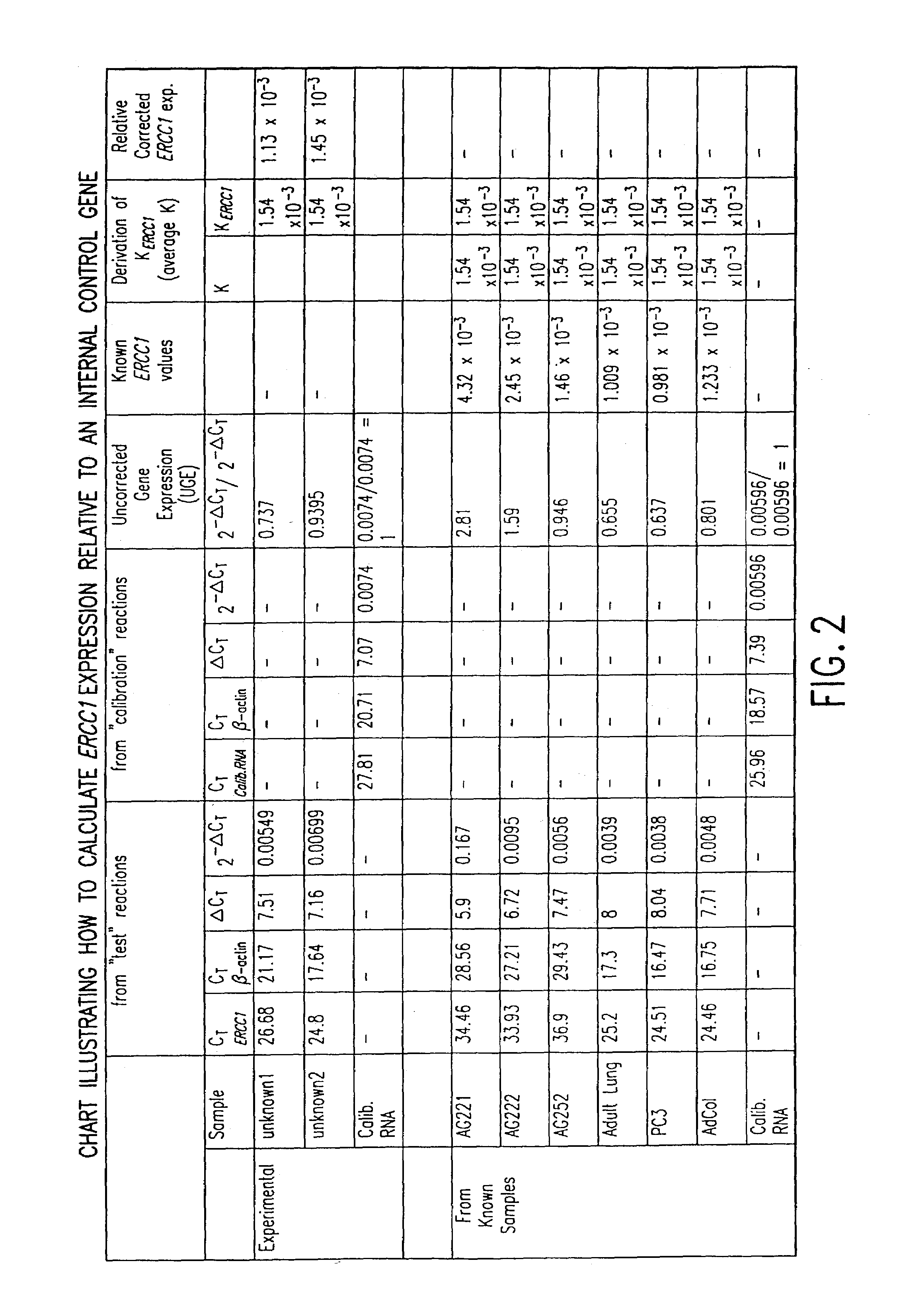
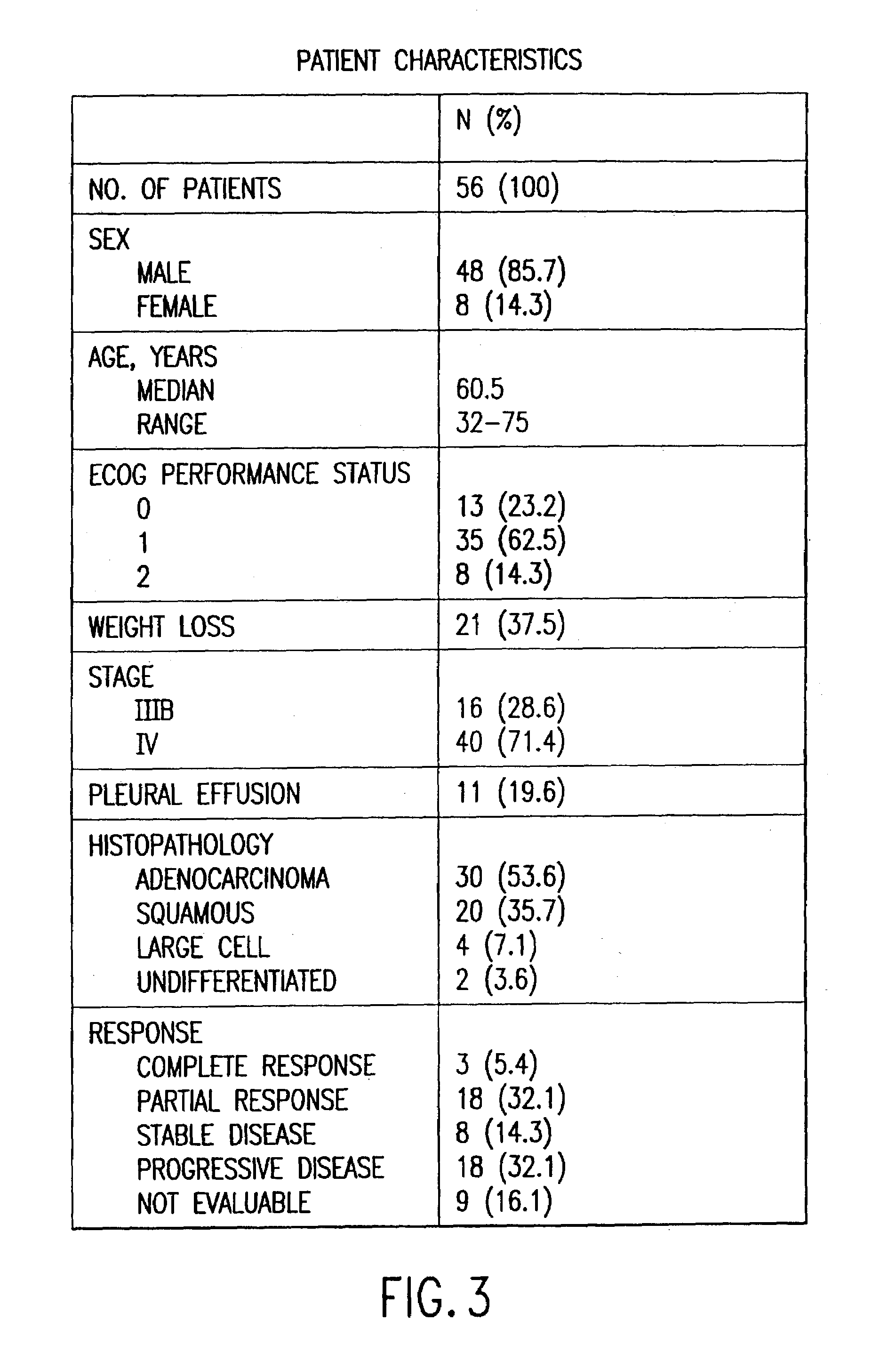
Method of determining a chemotherapeutic regimen based on ERCC1 and TS expression
InactiveUS7049059B2Improve responseMicrobiological testing/measurementRecombinant DNA-technologyAbnormal tissue growthRegimen
The present invention relates to prognostic methods which are useful in medicine, particularly cancer chemotherapy. The object of the invention to provide a method for assessing TS and / or ERCC1 expression levels in fixed or fixed and paraffin embedded tissues and prognosticate the probable resistance of a patient's tumor to treatment with 5-FU and oxaliplatin-based therapies by examination of the amount of TS and / or ERCC1 mRNA in a patient's tumor cells and comparing it to a predetermined threshold expression level for those genes. More specifically, the invention provides to oligonucleotide primer pairs ERCC1 and TS and methods comprising their use for detecting levels of ERCC1 and TS mRNA, respectively.
Owner:CANCER GENETICS
Method of treating psoriasis, arthritis and reducing the toxicity of cancer chemotherapy
The present invention is a method of treating a person who has psoriasis or arthritis or reducing the toxicity of cancer chemotherapy which comprises administering to the patient an anti-psoriasis effective amount of an oxazolidinone, preferably (S)-N-[[3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide.
Owner:PHARMACIA & UPJOHN CO
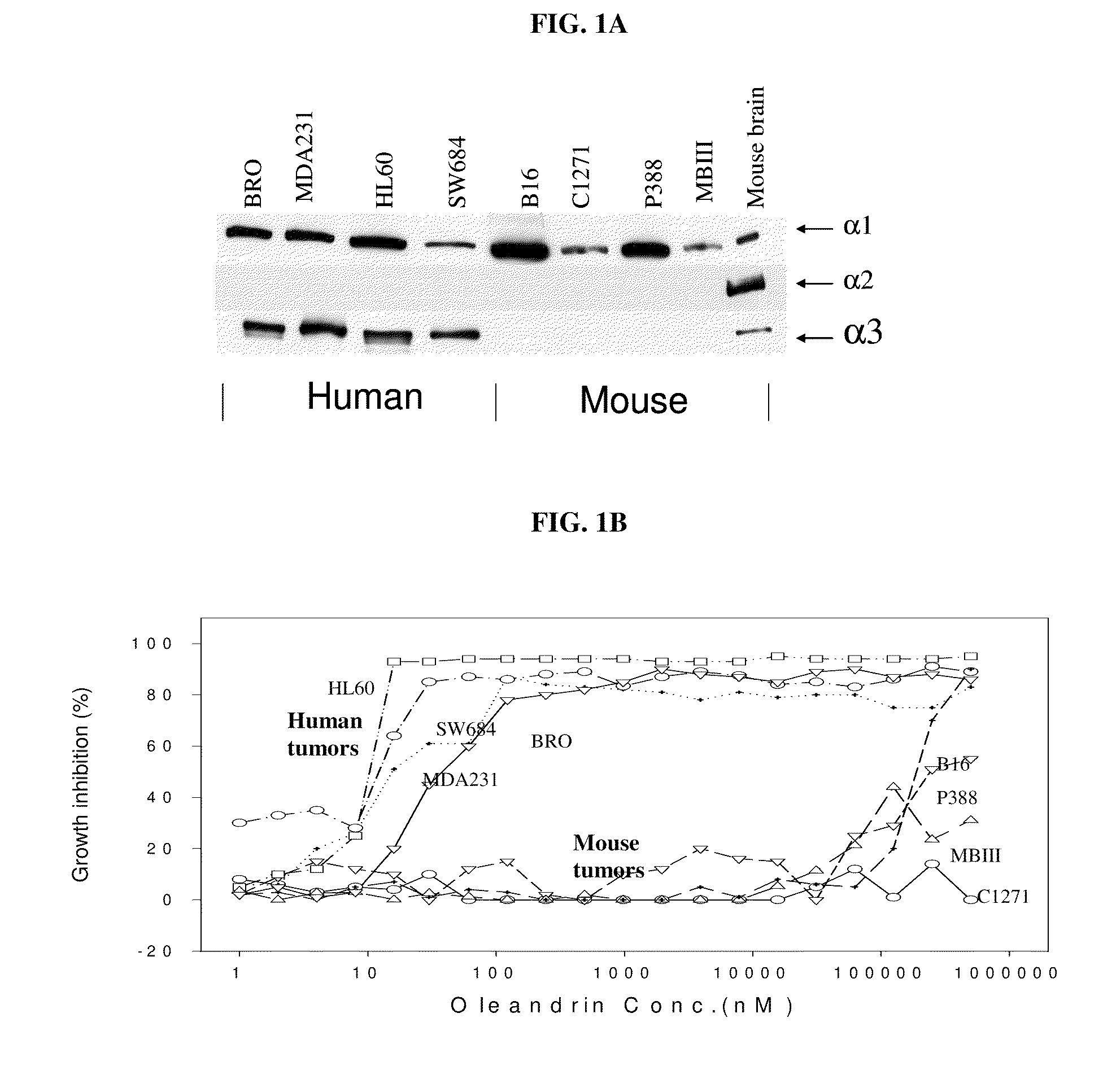
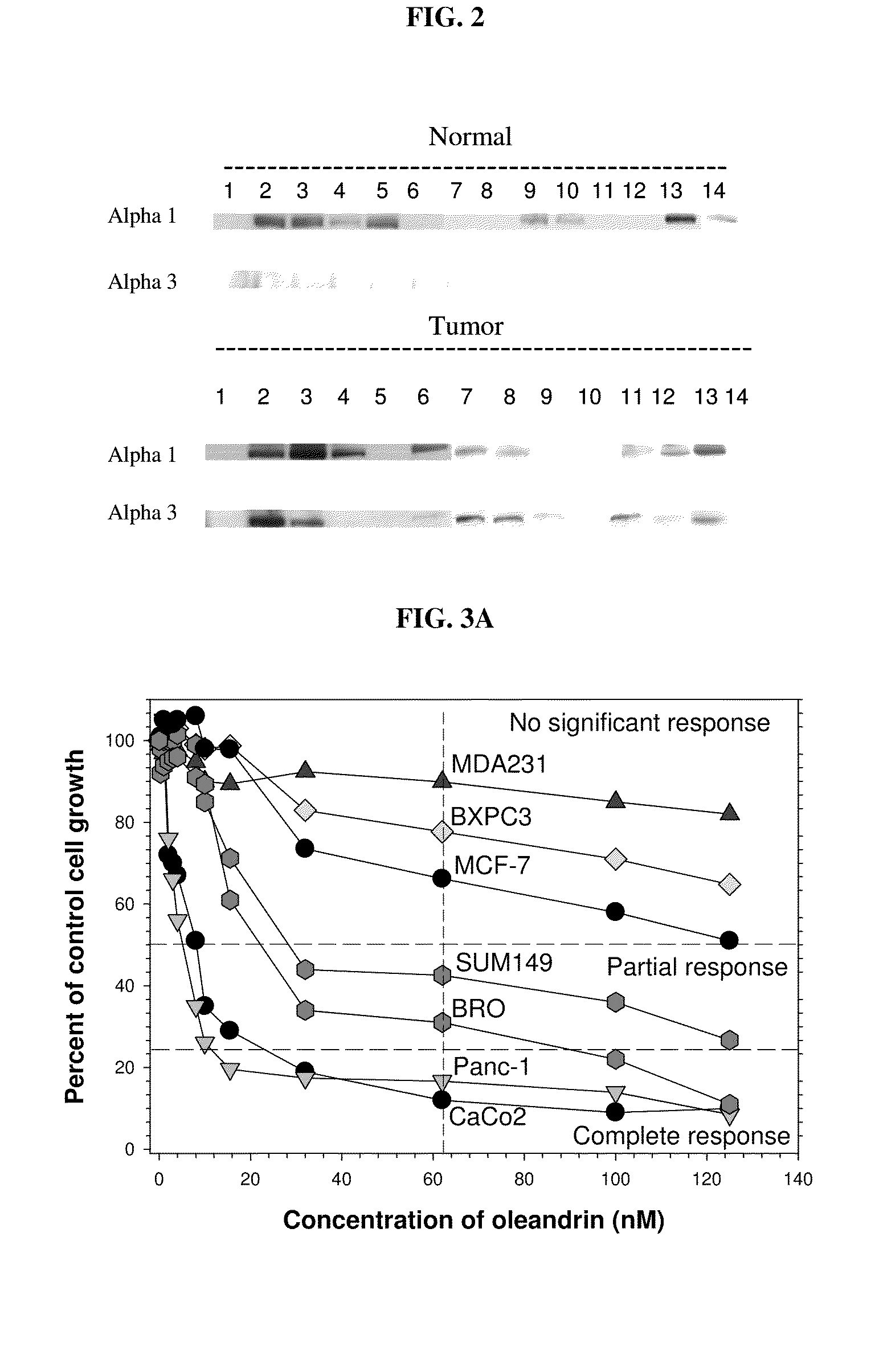
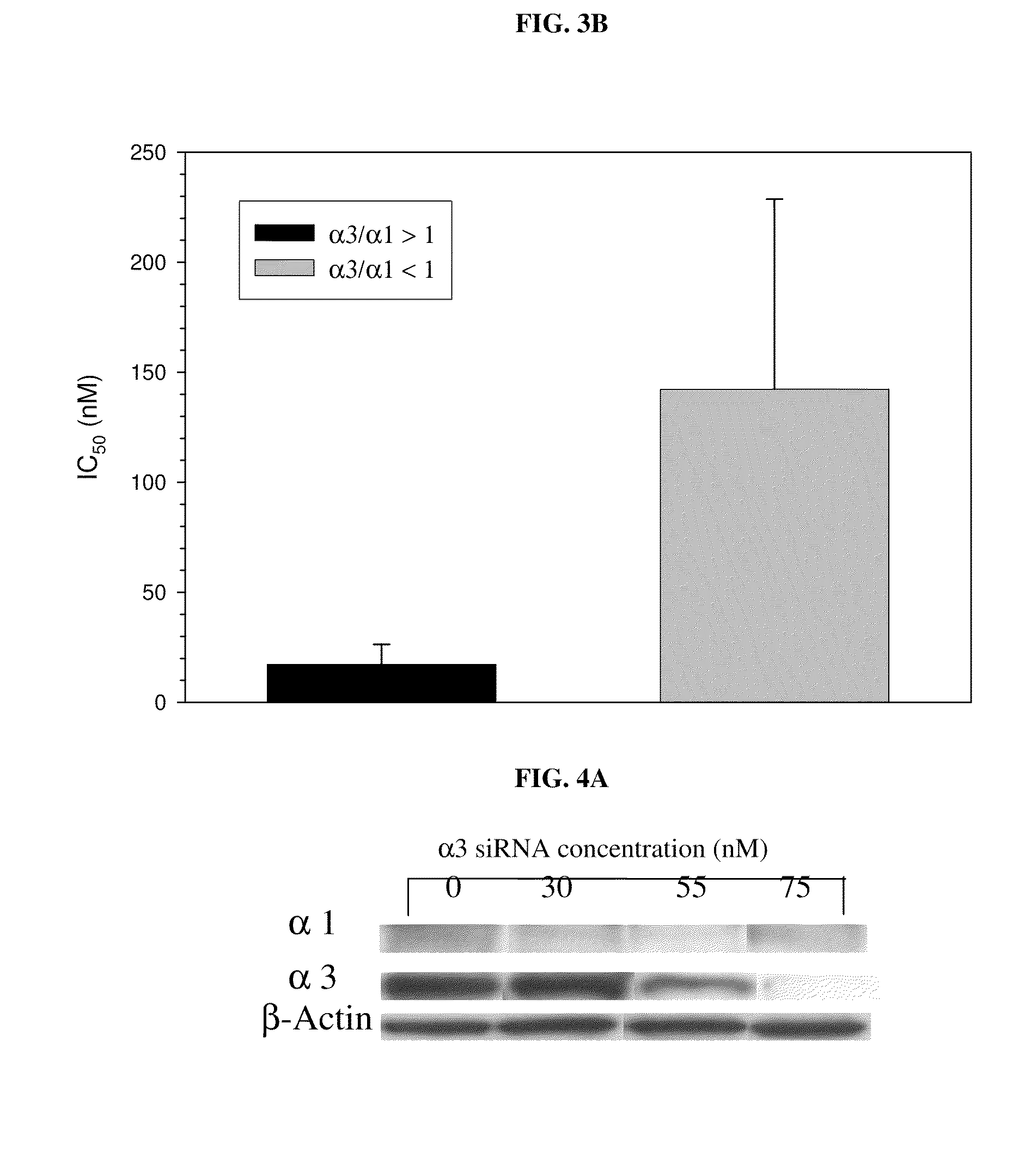
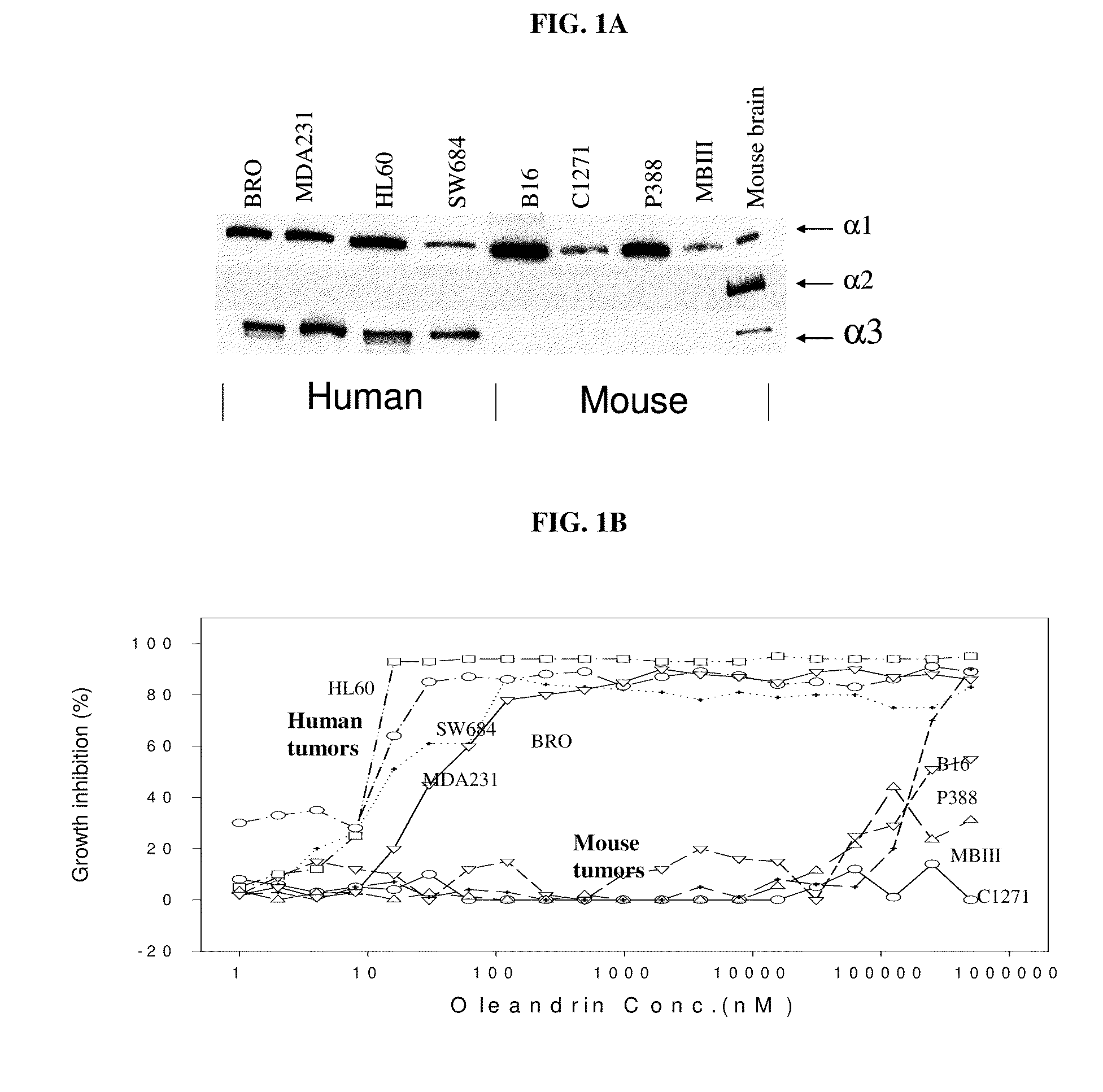
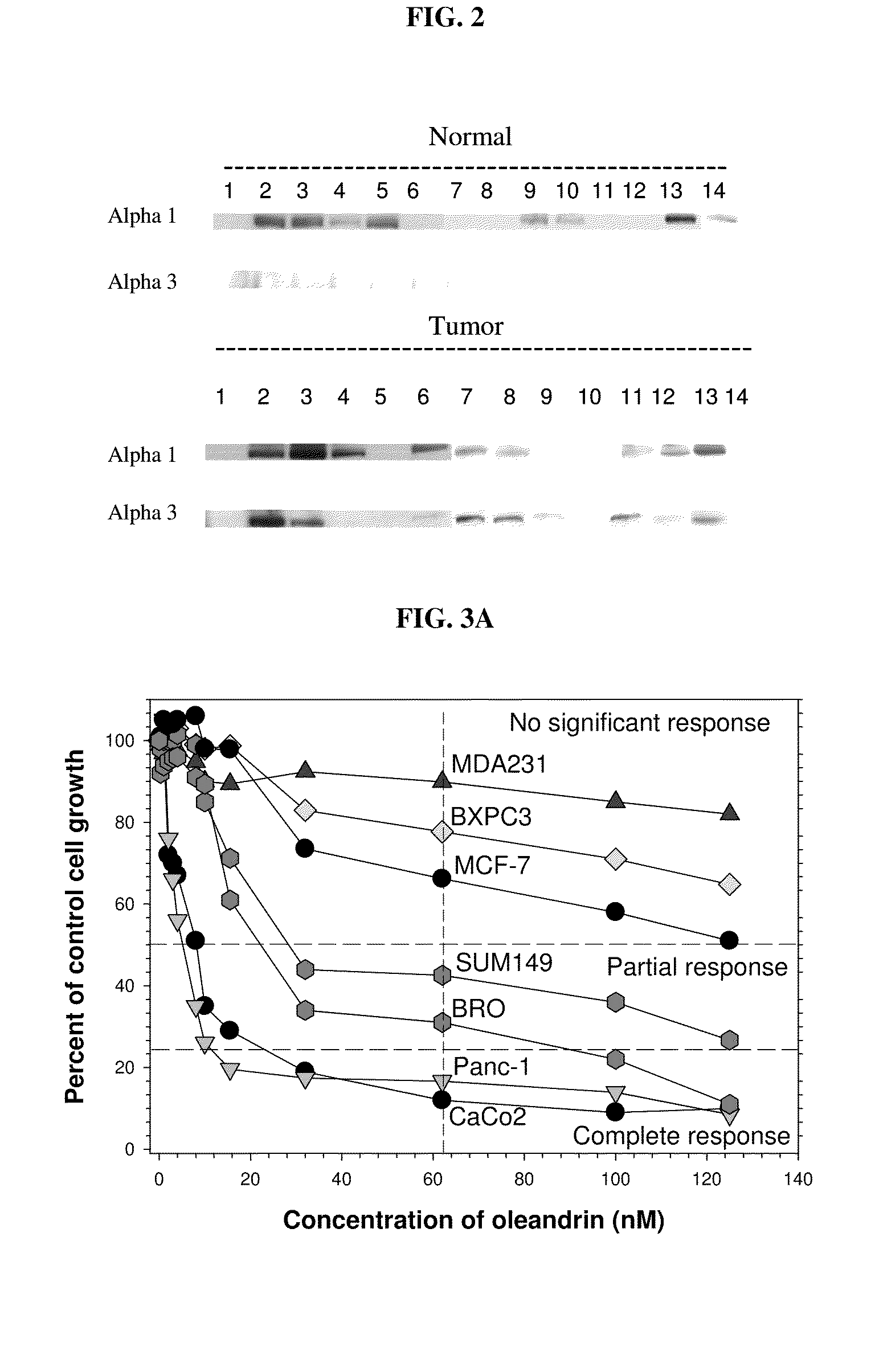
Method of determining the probability of a therapeutic response in cancer chemotherapy with cardiac glycoside
A prognostic assay and kit and method of use thereof are provided. The kit and assay are used to determine the likelihood of a diseased cell or tissue having a therapeutic response to treatment with a cardiac glycoside in a disease having an etiology associated with excessive cell proliferation. The kit and assay are used to determine the ratio of isoforms of the α subunit of Na, K-ATPase obtained from the diseased cell or tissue. The kit can be used to predict the therapeutic responsiveness of cancer or tumor in a subject to treatment with a cardiac glycoside. The kit and assay can be incorporated in a method of treating a disease or disorder having an etiology associated with excessive cell proliferation with a composition comprising a cardiac glycoside.
Owner:PHOENIX BIOTECH INC
N-benzyl substituted pyridyl porphyrin compounds and methods of use thereof
InactiveUS20070072825A1Extended half-lifeAvoid adjustmentAntibacterial agentsBiocideSide effectReperfusion injury
The present invention relates to N-Benzyl-Substituted Pyridyl Porphyrin Compounds, compositions comprising an effective amount of an N-Benzyl-Substituted Pyridyl Porphyrin Compound and methods for treating or preventing injury due to exposure to a reactive species, erectile dysfunction, urinary incontinence, lung disease, hyperoxia, neurodegenerative disease, liver disease, myocardial damage during cardioplegia, an inflammatory condition, a reperfusion injury, an ischemic condition, a cardiovascular disease, diabetes, a diabetic complication, cancer, a side effect of cancer chemotherapy, or a radiation-induced injury, and methods for prolonging the half-life of an oxidation-prone compound, comprising administering to a subject in need thereof an effective amount of an N-Benzyl-Substituted Pyridyl Porphyrin Compound.
Owner:INOTECK PHARMA CORP
GPCR Ligands Identified by Computational Modeling
Disclosed are pharmacophores for developing and screening compounds having G-protein-coupled receptor antagonist activity, including LPA1, LPA2, LPA3 and S1P antagonists. These compositions have therapeutic benefit in the fields of cancer chemotherapy, cardiovascular disease prevention, and fertility protective agents during radiation and chemotherapy.
Owner:PARRILL BAKER ABBY L +3
Method of determining a chemotherapeutic regimen based on ERCC1 expression
The present invention relates to prognostic methods which are useful in medicine, particularly cancer chemotherapy. The object of the invention to provide a method for assessing ERCC1 expression levels in fixed or fixed and paraffin embedded tissues and determine a platinum-based chemotherapy by examination of the amount of ERCC1 mRNA in a patient's tumor cells and comparing it to a predetermined threshold expression level. More specifically, the invention provides to oligonucleotide primer pair ERCC1 and methods comprising their use for detecting levels of ERCC1 mRNA.
Owner:CANCER GENETICS
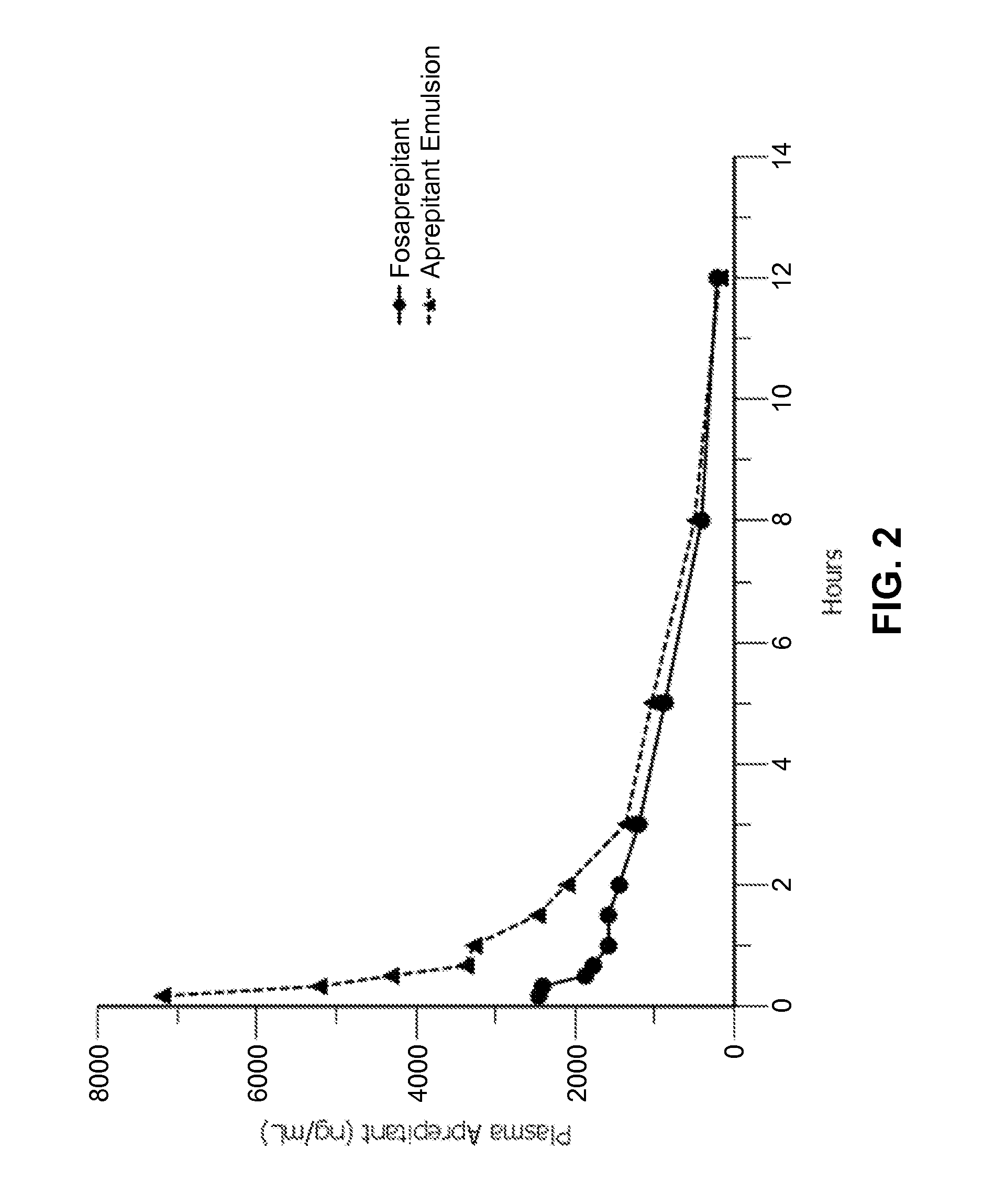
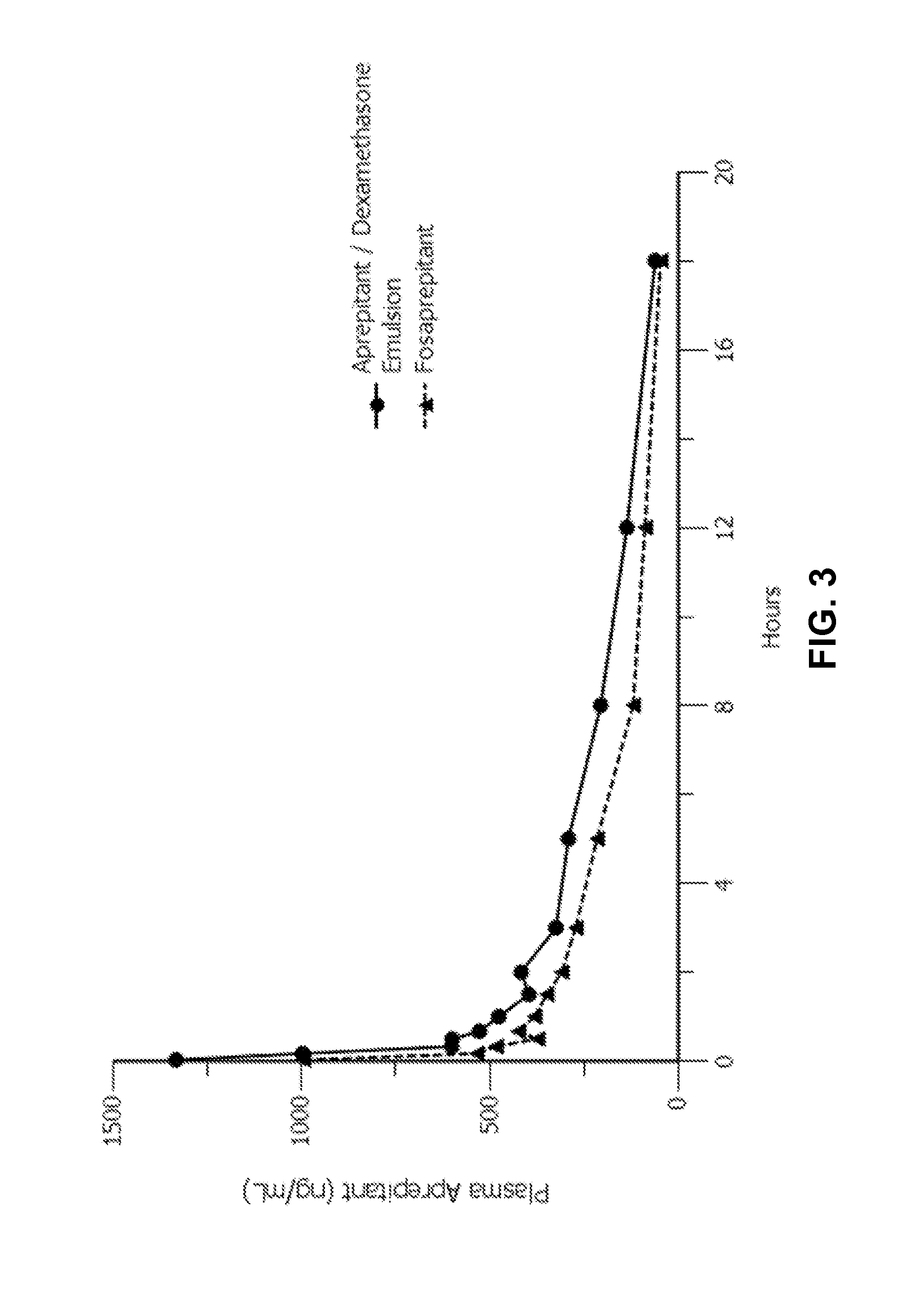
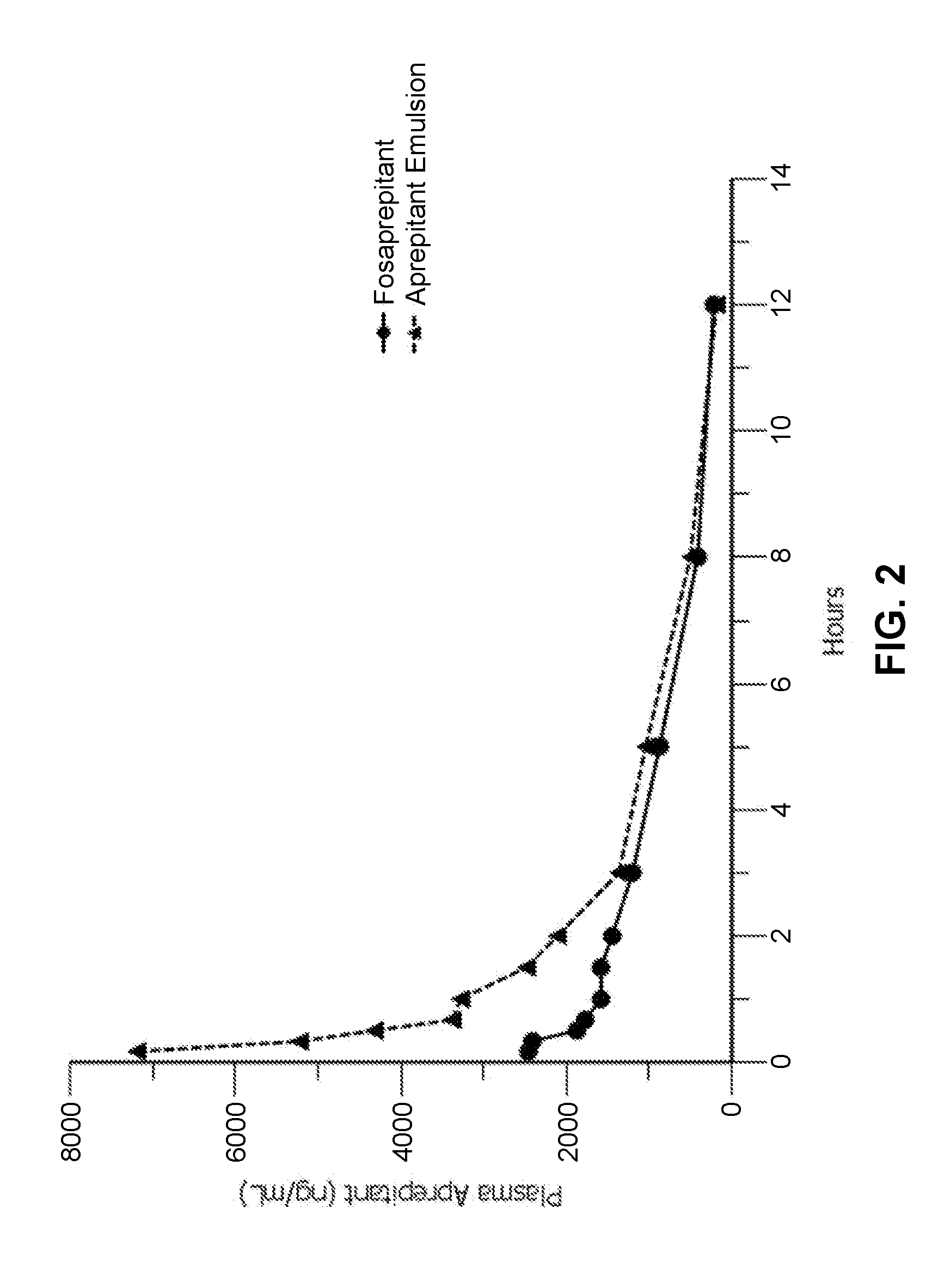
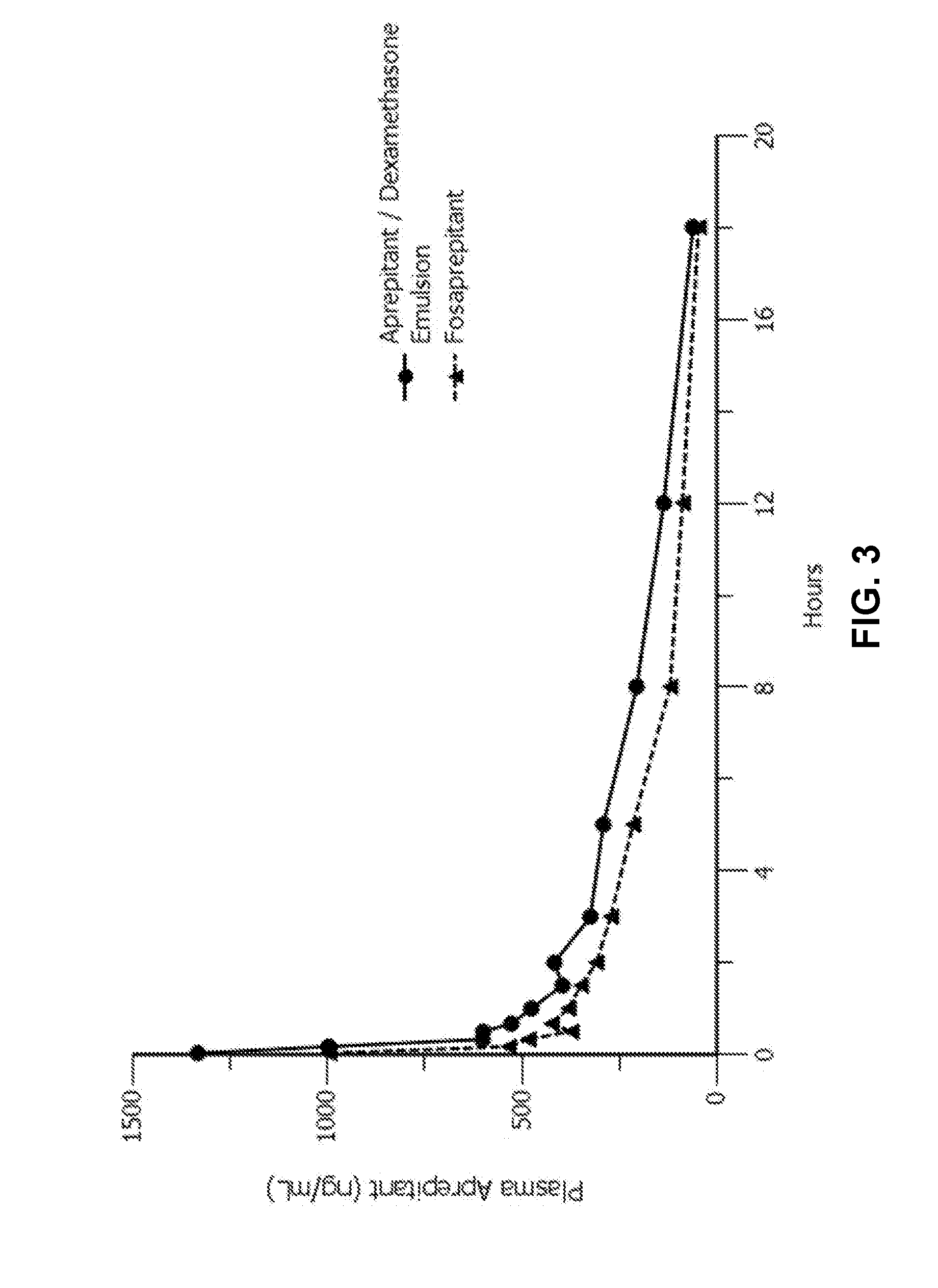
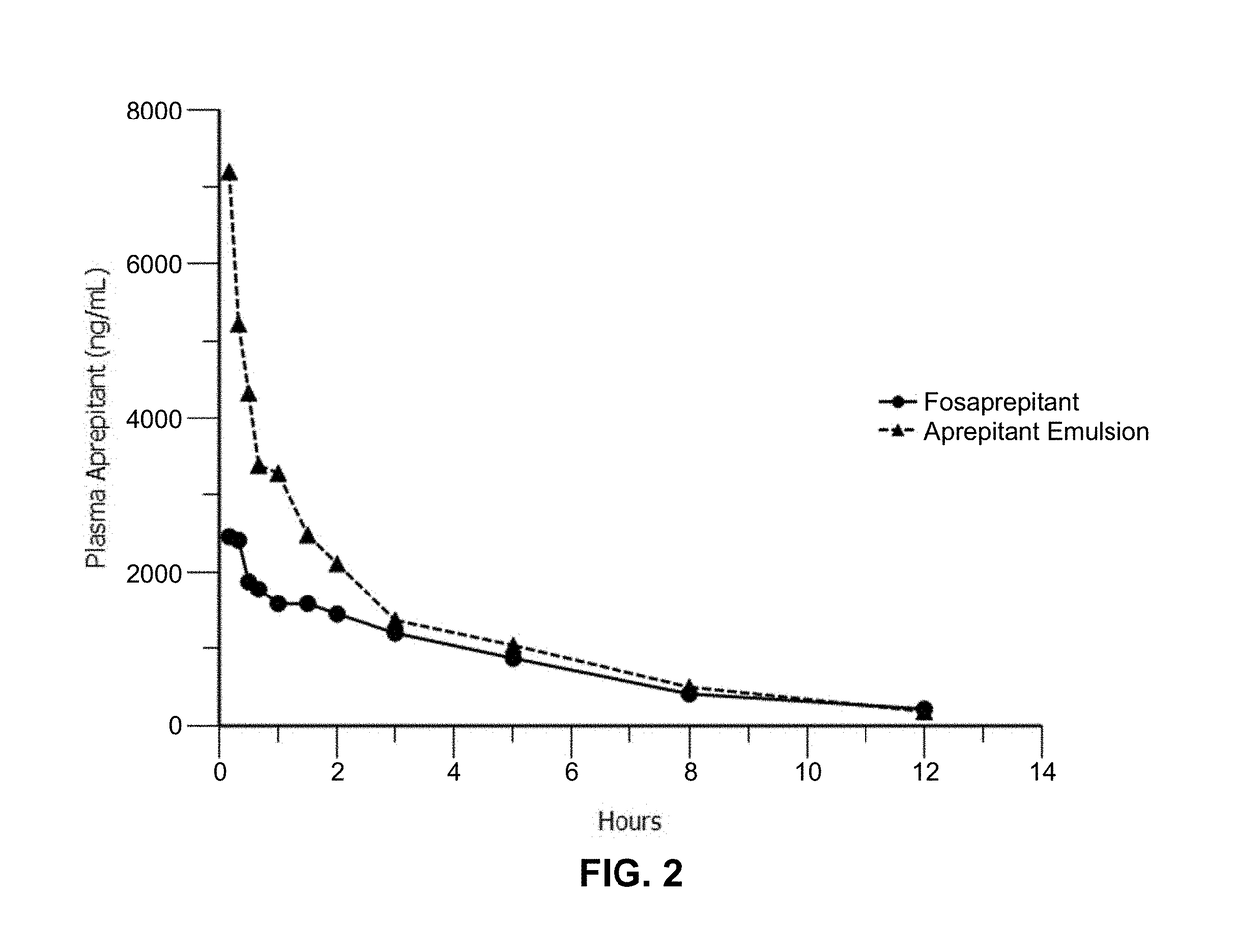
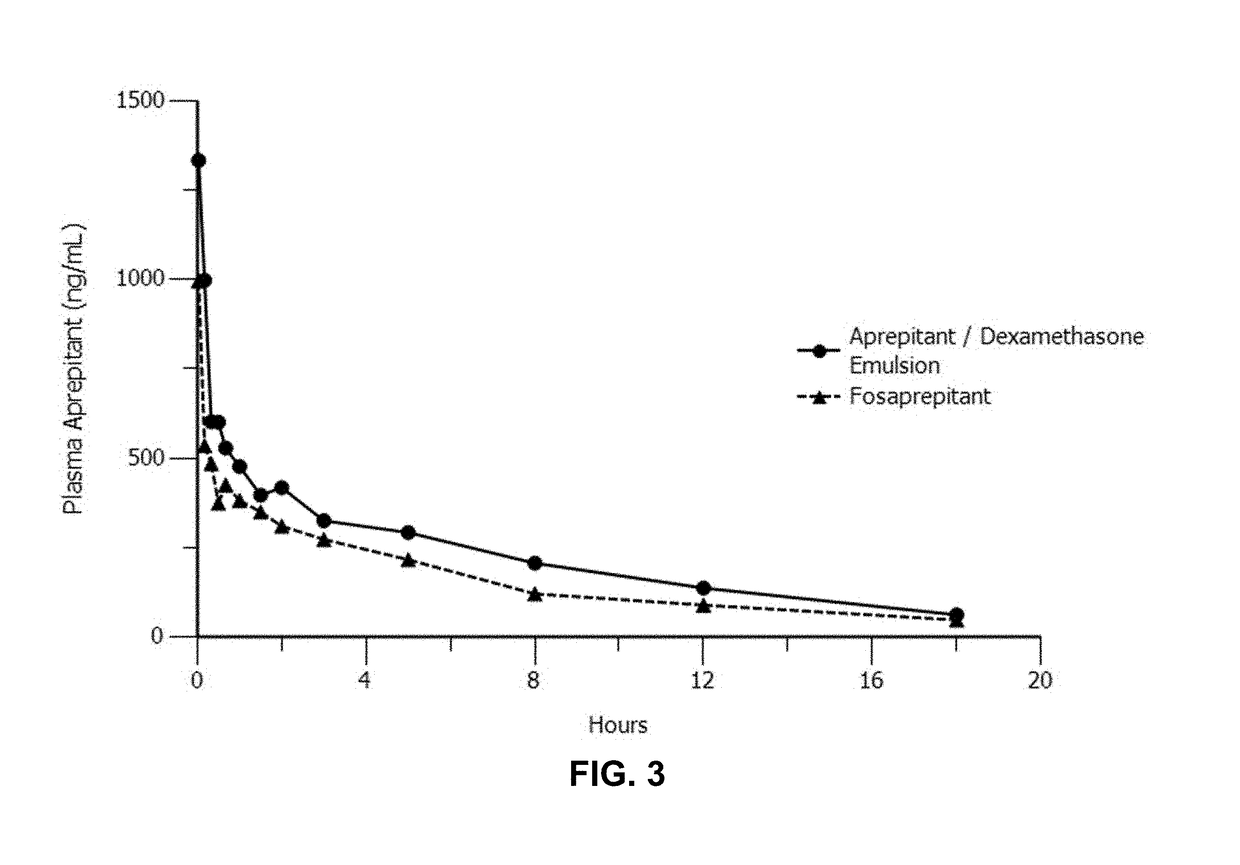
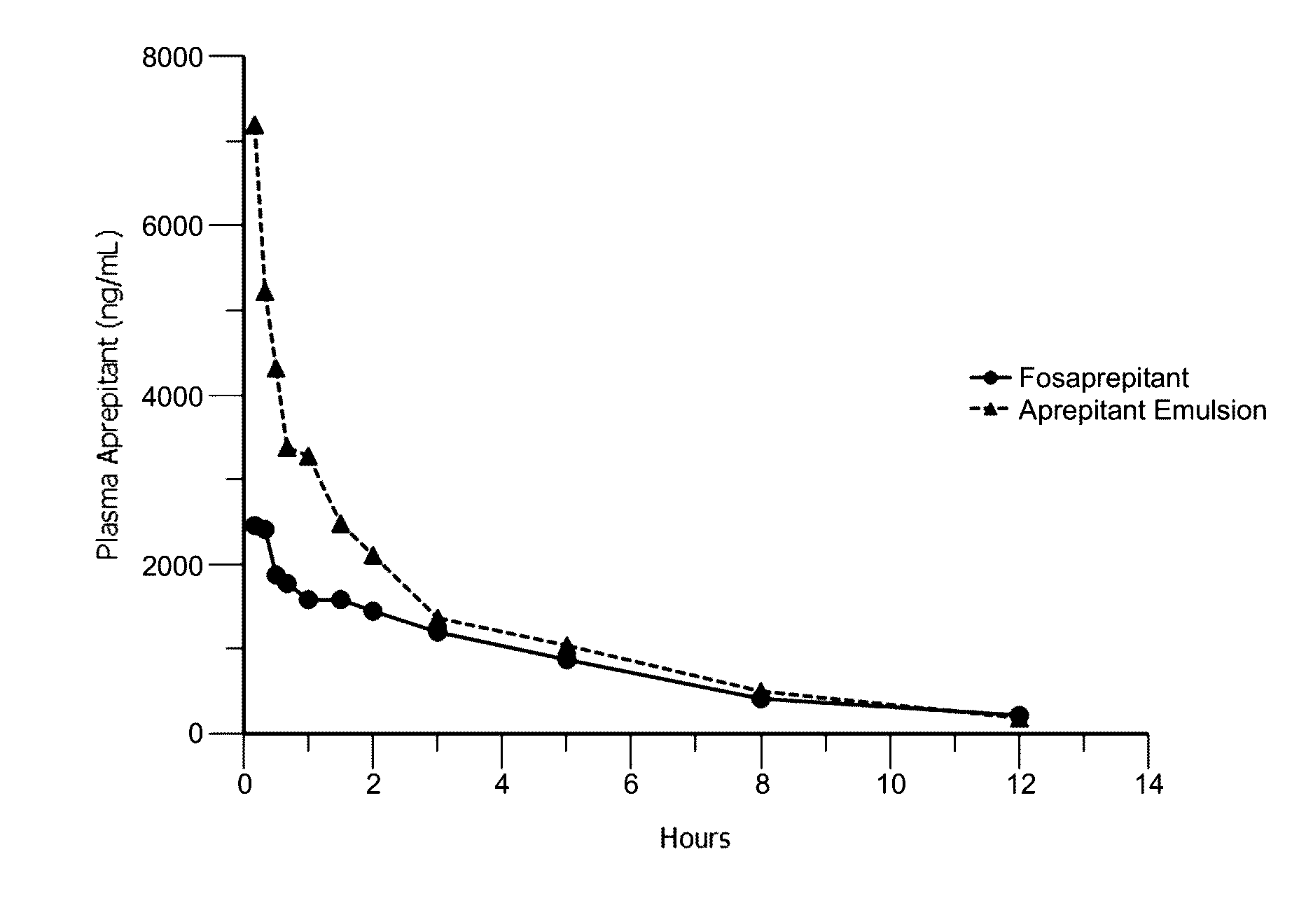
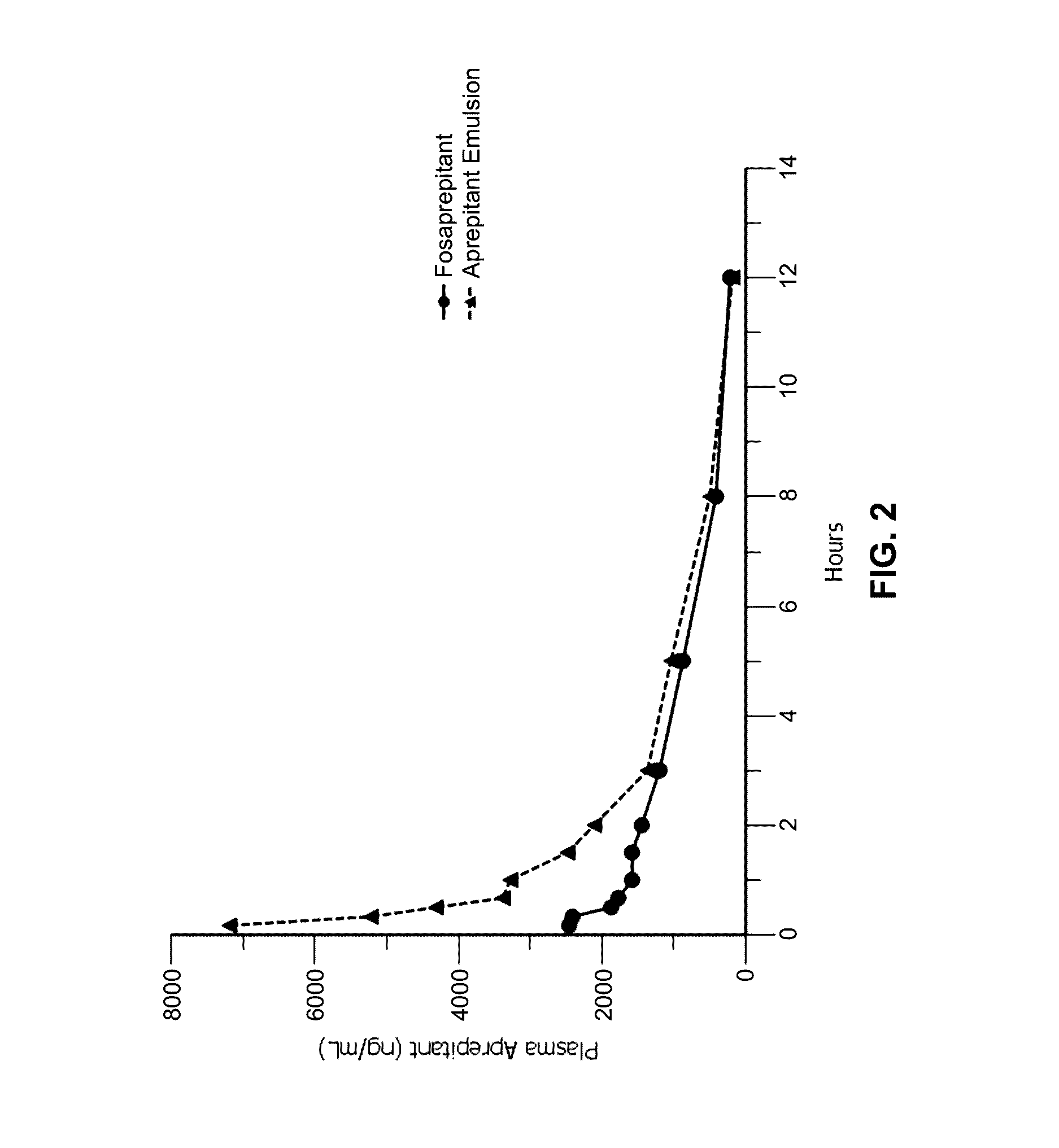
Emulsion formulations of aprepitant
ActiveUS20160082013A1Organic active ingredientsDigestive systemOral treatmentPharmaceutical formulation
Disclosed herein are novel pharmaceutical formulations of aprepitant suitable for parenteral administration including intravenous administration. Also included are formulations including both aprepitant and dexamethasone sodium phosphate. The pharmaceutical formulations are stable oil-in-water emulsions for non-oral treatment of emesis and are particularly useful for treatment of subjects undergoing highly emetogenic cancer chemotherapy.
Owner:HERON THERAPEUTICS
Cyclic ketone derivatives and their medical applications
The present invention relates to cyclic ketones represented by the following formulaand to drugs in which an effective component is such a cyclic ketone or a pharmacologically acceptable salt thereof.The cyclic ketones of the present invention encourage the production of blood platelets, leukocytes and erythrocytes, and can be employed in the prevention or treatment of cytopaenia brought about by cancer chemotherapy, radiotherapy or drug therapy, or by immunological abnormality, anaemia and the like.
Owner:TORAY IND INC
Method of Determining the Probability of a Therapeutic Response in Cancer Chemotherapy With Cardiac Glycoside
ActiveUS20100317541A1Sensitivity and therapeutic responsivenessRaise the ratioOrganic active ingredientsSenses disorderAssayIV Chemotherapy
A prognostic assay and kit and method of use thereof are provided. The kit and assay are used to determine the likelihood of a diseased cell or tissue having a therapeutic response to treatment with a cardiac glycoside in a disease having an etiology associated with excessive cell proliferation. The kit and assay are used to determine the ratio of isoforms of the α subunit of Na, K-ATPase obtained from the diseased cell or tissue. The kit can be used to predict the therapeutic responsiveness of cancer or tumor in a subject to treatment with a cardiac glycoside. The kit and assay can be incorporated in a method of treating a disease or disorder having an etiology associated with excessive cell proliferation with a composition comprising a cardiac glycoside.
Owner:PHOENIX BIOTECH INC
Amino thiol compounds and compositions for use in conjunction with cancer therapy
The invention provides novel polyamine and amino thiol compounds and pharmaceutical compositions for administration in conjunction with cancer chemotherapy or radiation therapy. The compounds are administered locally to provide protection against the adverse side-effects of chemotherapy or radiation therapy, such as alopecia, mucositis and dermatitis. Pharmaceutical preparations comprising one or more chemoprotective polyamines or amino thiols formulated for topical or local delivery to epithelial or mucosal cells are disclosed. Methods of administering the pharmaceutical preparations are also disclosed.
Owner:WISCONSIN ALUMNI RES FOUND
Emulsion formulations of aprepitant
Disclosed herein are novel pharmaceutical formulations of aprepitant suitable for parenteral administration including intravenous administration. Also included are formulations including both aprepitant and dexamethasone sodium phosphate. The pharmaceutical formulations are stable oil-in-water emulsions for non-oral treatment of emesis and are particularly useful for treatment of subjects undergoing highly emetogenic cancer chemotherapy.
Owner:HERON THERAPEUTICS
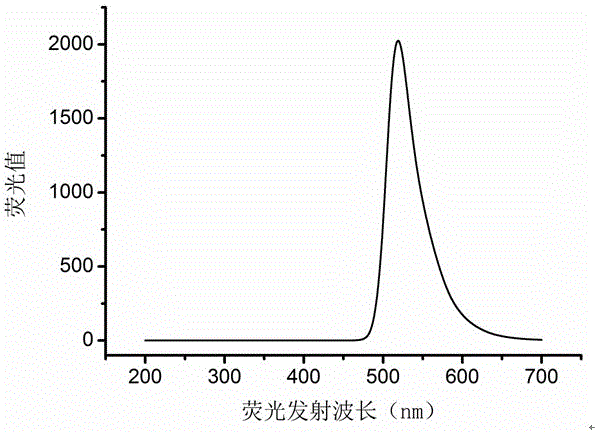
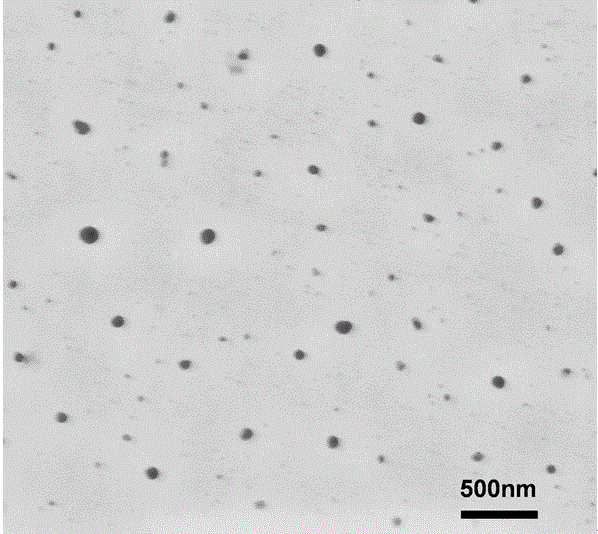
Preparation method of star polymer-based drug carrier material with fluorescence labeling and temperature responsiveness
ActiveCN106589270AWide variety of sourcesThe synthesis method is simpleNanoopticsPharmaceutical non-active ingredientsPolyesterHydrophilic monomer
The invention belongs to the field of macromolecular materials and biomedical engineering, and particularly relates to a preparation method of a star polymer-based drug carrier material with fluorescence labeling and temperature responsiveness. The preparation method comprises the following steps: carrying out ring opening polymerization on a cyclo-carbonate monomer serving as a raw material by using a star initiator to prepare a star polyester macromolecular material, and carrying out reaction on the star polyester macromolecular material and 2-bromoisobutyryl bromine to prepare a star macromolecular initiator; introducing a hydrophilic monomer with temperature responsiveness into the macromolecular initiator through atom transfer radical polymerizationto prepare an amphipathic segmented copolymer with temperature responsiveness, and introducing a methacrylic acid hydroxyethyl monomer through the atom transfer radical polymerization to provide a hydroxyl; and preparing the star polymer-based drug carrier material with fluorescence labeling and temperature responsiveness by the chemical reaction between the hydroxyl and fluorescent small molecules. The material can be self-assembled into a fluorescence labeled nano drug carrier micelle in an aqueous solution, and has good application prospects in the fields of cancer chemotherapy, drug transportation, distribution and monitoring and the like.
Owner:TONGJI UNIV
Emulsion formulations of an nk-1 receptor antagonist and uses thereof
Disclosed herein are novel pharmaceutical formulations of a neurokinin-1 (NK-1) receptor antagonist suitable for parenteral administration including intravenous administration. Also included are formulations including both the NK-1 receptor antagonist and dexamethasone sodium phosphate. The pharmaceutical formulations are stable oil-in-water emulsions for non-oral treatment of emesis and are particularly useful for treatment of subjects undergoing highly emetogenic cancer chemotherapy.
Owner:HERON THERAPEUTICS
Emulsion formulations of aprepitant
Disclosed herein are novel pharmaceutical formulations of aprepitant suitable for parenteral administration including intravenous administration. Also included are formulations including both aprepitant and dexamethasone sodium phosphate. The pharmaceutical formulations are stable oil-in-water emulsions for non-oral treatment of emesis and are particularly useful for treatment of subjects undergoing highly emetogenic cancer chemotherapy.
Owner:HERON THERAPEUTICS
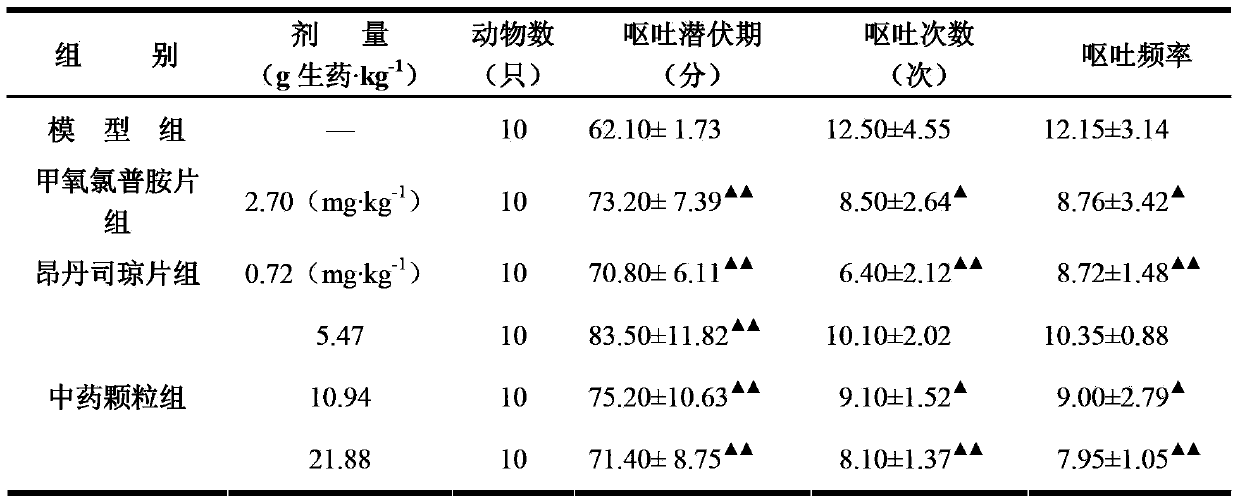
Traditional Chinese medicine granule for treating side effects on alimentary canal after cancer chemotherapy and preparation method thereof
InactiveCN103623338ARegulatory immune abnormalitiesImprove immunityDigestive systemGranular deliveryAdjuvantSide effect
The objective of the invention is to provide a traditional Chinese medicine granule for treating side effects on an alimentary canal after cancer chemotherapy and a preparation method thereof. The invention is characterized in that the granule is prepared from an active component, a binder and pharmaceutically acceptable adjuvant, and the active component is prepared from the traditional Chinese medicines consisting of 600 to 800 g of milkvetch root, 250 to 350 g of dwarf lilyturf tuber, 150 to 250 g of red ginseng, 150 to 250 g of white atractylodes rhizome fried with bran, 150 to 250 g of Poria cocos, 200 to 300 g of licorice, 200 to 300 g of radix scrophulariae, 200 to 300 g of Chinese angelica, 250 to 350 g of dark plum, 250 to 350 g of prepared pinellia tuber, 200 to 300 g of cablin patchouli herb, 200 to 300 g of dried orange peel and 200 to 300 g of curcuma rhizome. The granule is applicable to cancer patients who vomit or retch because of damage of both qi and yin and rising of stomach qi resulting from damage of the spleen and the stomach by anti-radiogenic cancer and chemotherapy drugs, and the granule can mitigate symptoms like no appetite, tiredness, hypodynamia, a red or light red tongue with little fluid and weak pulse.
Owner:辽宁省中医药研究院
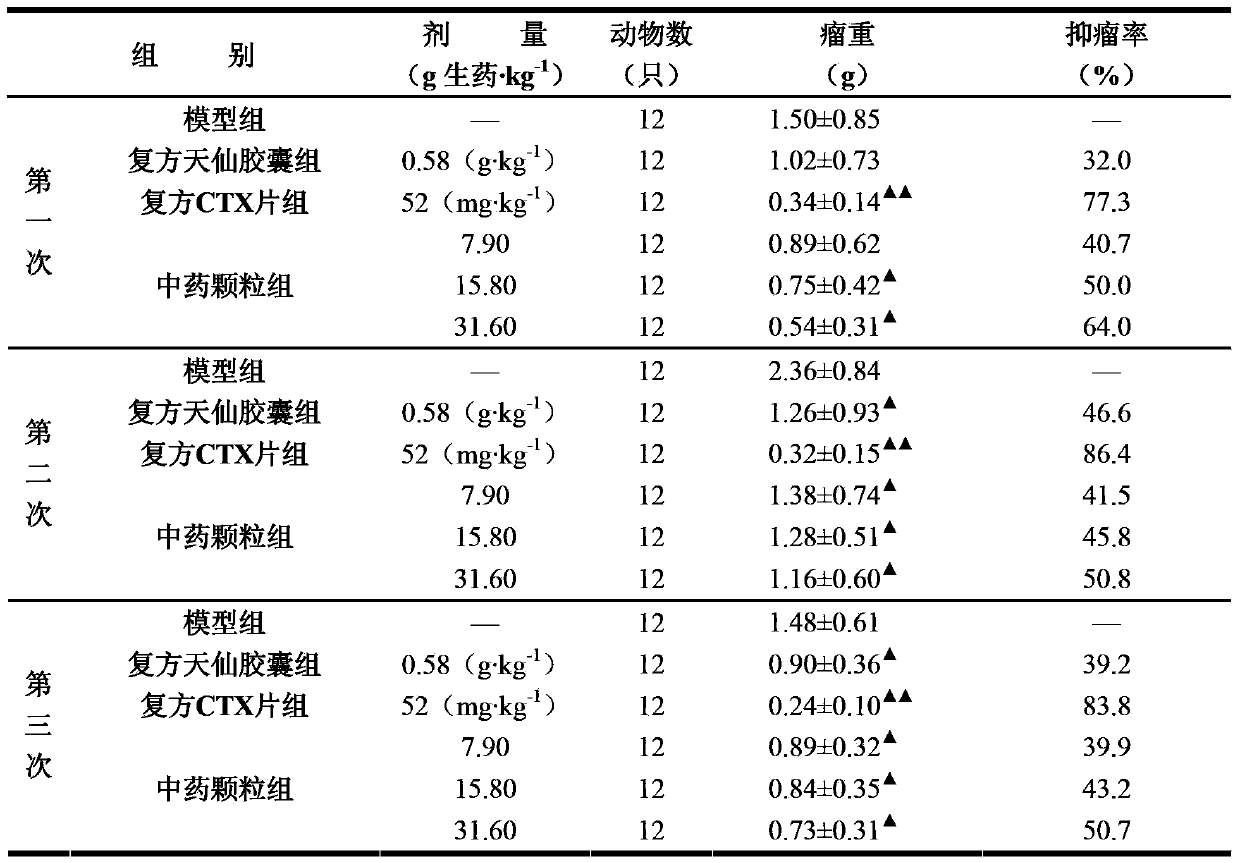
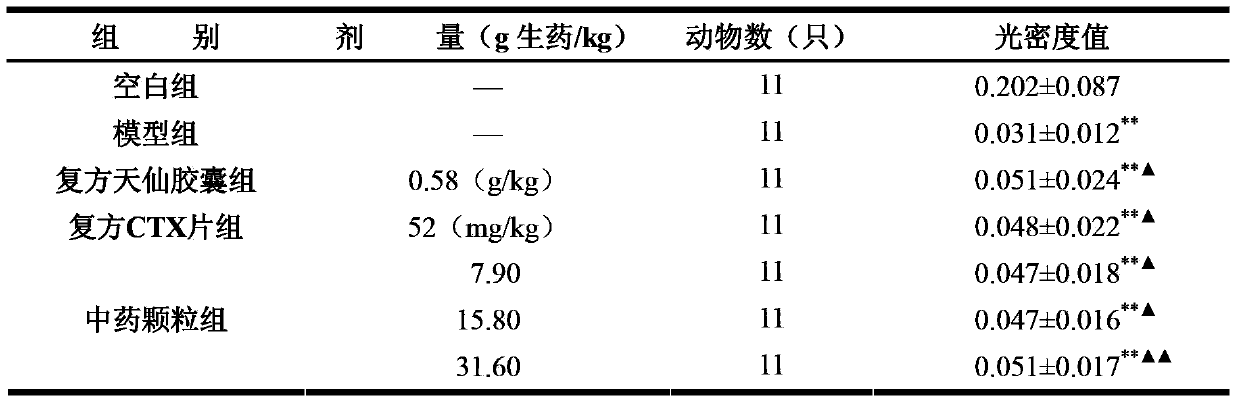
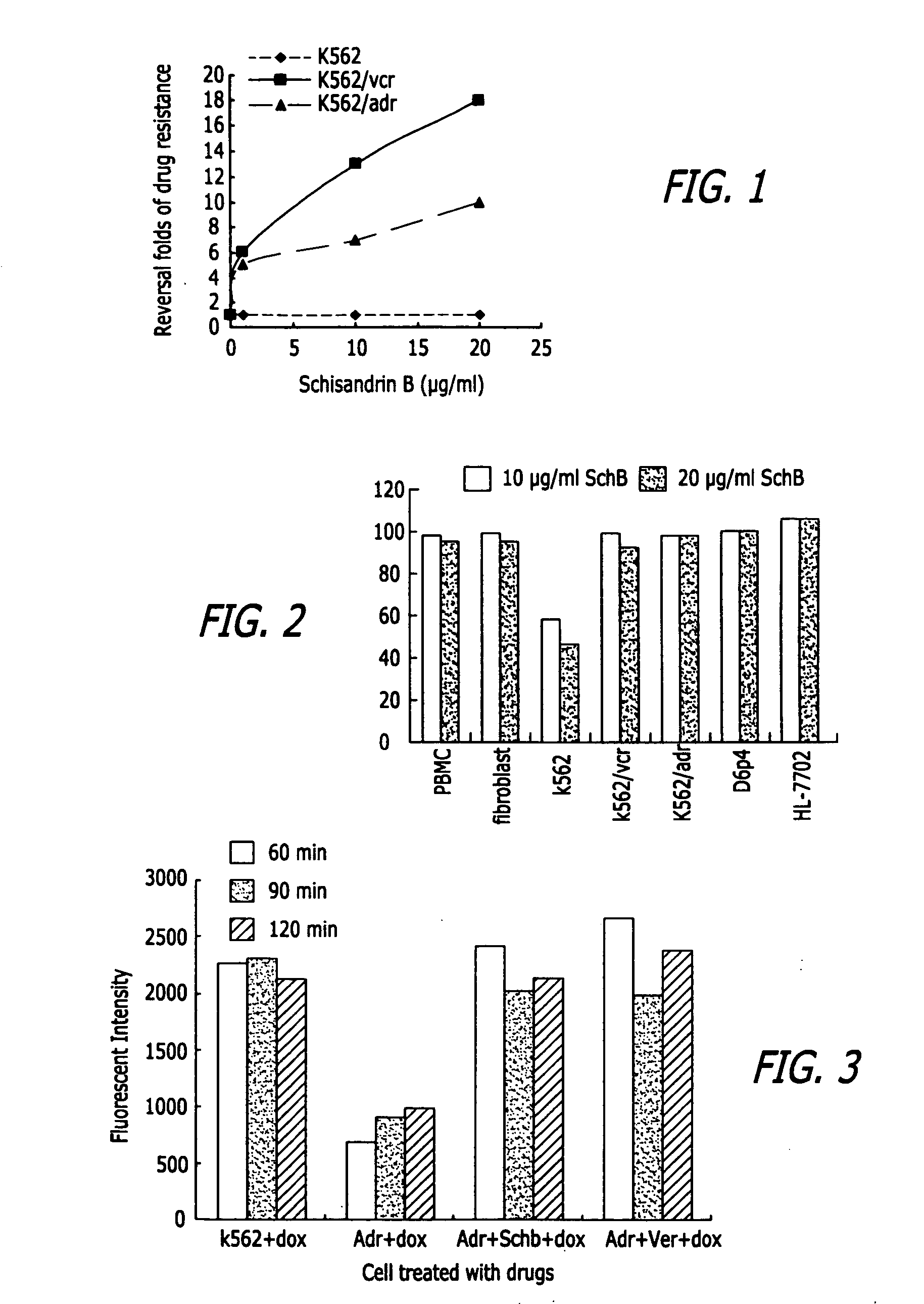
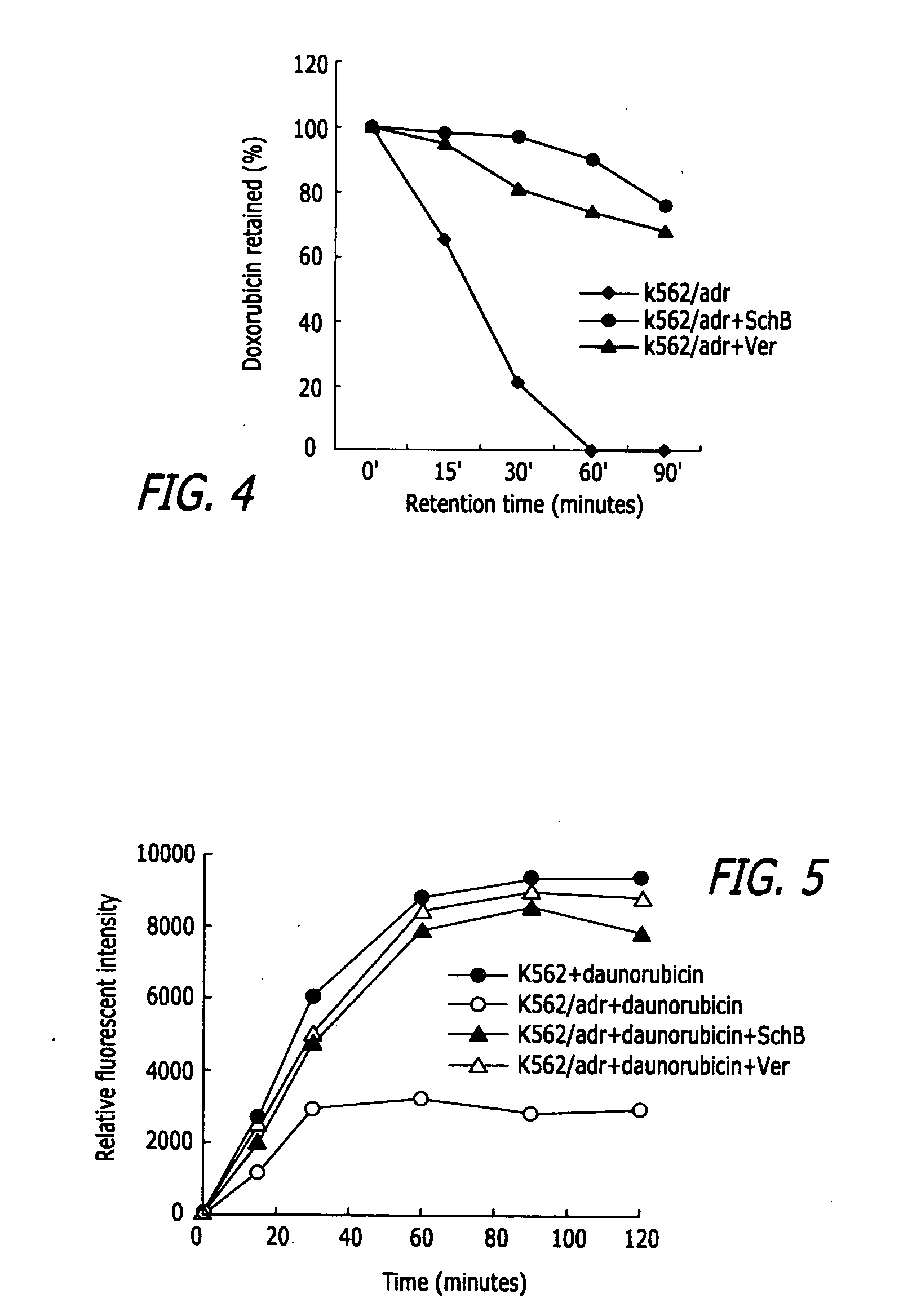
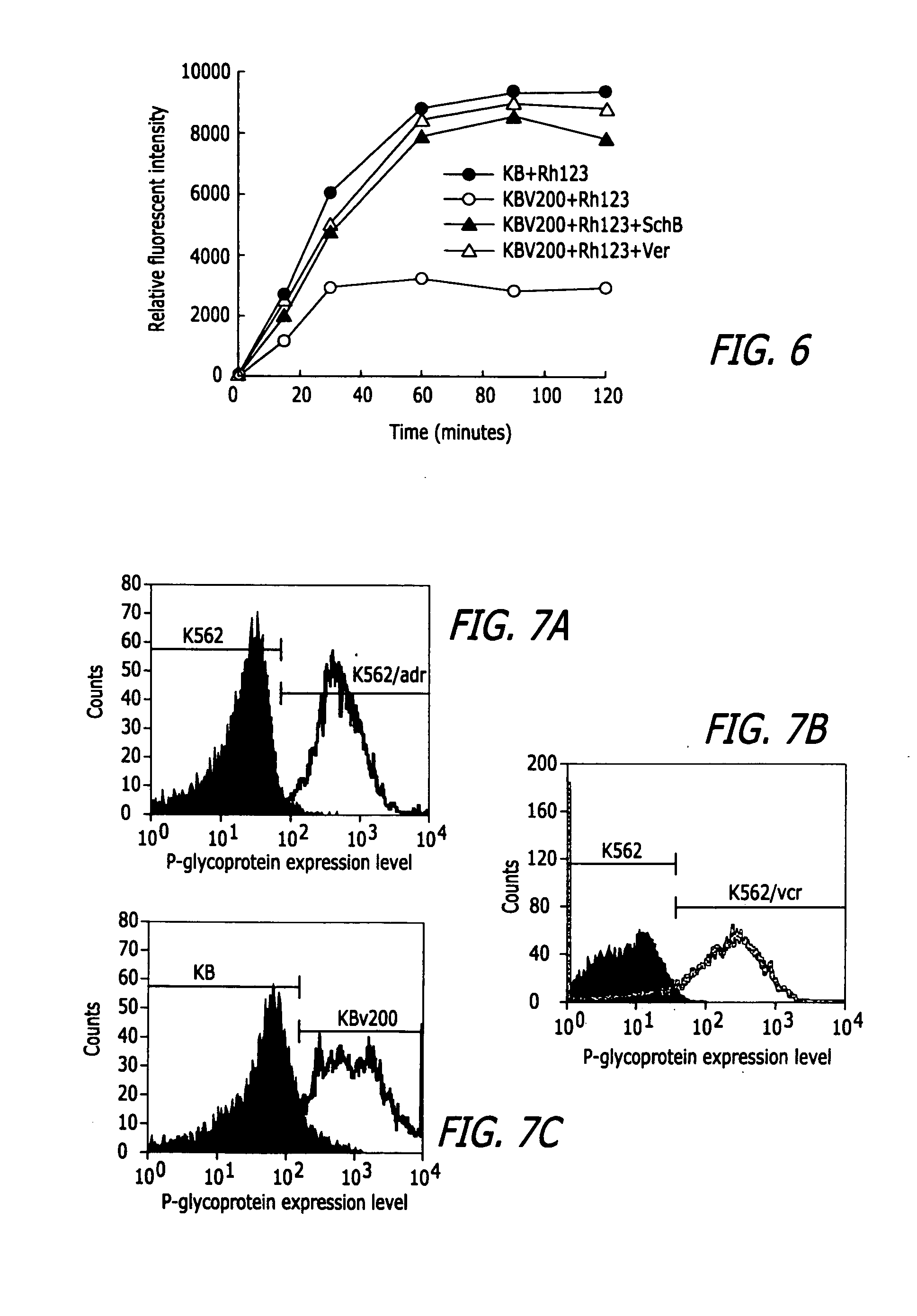
Methods of application of Schisandrin B in the preparation of anticancer medications
InactiveUS20050119337A1Inhibits drug transport function of P-glycoproteinHigh activityBiocideHeavy metal active ingredientsHuman cancerCancer cell
Methods of application of Schisandrin B in the preparation of anticancer medications, and particularly for the preparation of medications for the treatment of multidrug resistant (MDR) cancer. The compound of Schisandrin B effectively reverses MDR cancer in combination with other anticancer chemotherapeutic agents. Schisandrin B reverses MDR cancer by inhibiting the drug efflux activity of P-glycoprotein, indicating its significance in clinical applications. Although it is of low toxicity, Schisandrin B is cytotoxic to human cancer cells, revealing its application in cancer chemotherapy. It is emphasized that this abstract is provided to comply with the rules requiring an abstract that will allow a searcher or other reader to quickly ascertain the subject matter of the technical disclosure. It is submitted with the understanding that it will not be used to interpret or limit the scope or meaning of the claims.
Owner:NINGBO INNOPHARMA TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com