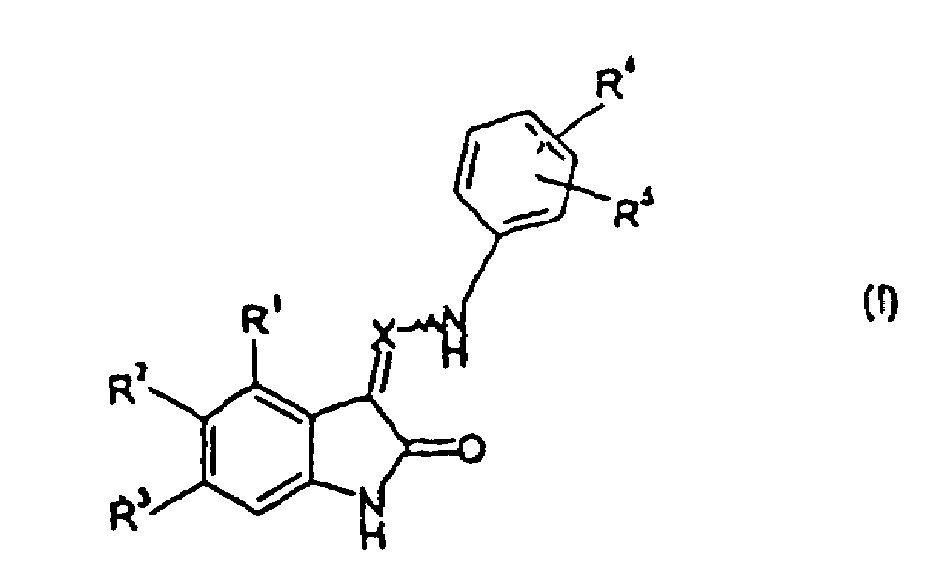
Substituted di-hydroxyl-indol derivatives as protein tyrosine kinase and as protein serine/threenine kinase inhibitors
A technology of hydroxyl and biological hydrolysis, which is applied in the direction of medical preparations containing active ingredients, anti-toxic agents, anti-inflammatory agents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 17
[0324] Example 17: 4-[N'-(4-isopropoxy-2-oxo-1,2-dihydro-indole-3-ylidene)-hydrazino]-benzenesulfonamide
[0325] A solution of 3.78 g (25.0 mmol) of 3-isopropoxyaniline and di-tert-butyl dicarboxylate in 25 ml of THF was heated to reflux for 2 hours. The solution was cooled to ambient temperature and the solvent was removed in vacuo. The residue was dissolved in 100 mL of EtOAc and the solution was washed with 3 50 mL portions of 0.5M citric acid and 50 mL of brine. Dilute the solution with MgSO 4 Drying and removal of solvent in vacuo afforded N-(tert-butoxy-carbonyl)-3-isopropoxyaniline (5.75 g, 92%) as a white solid: mp 79-81°C; 1 H NMR (DMSO-d 6 ): δ1.21 (d, J=6.0Hz, 6H), 1.43 (s, 9H), 4.46 (septet, J=6Hz, 1H), 6.47 (dd, J=2. 1, 8.1Hz, 1H), 6.94(d, J=8.1Hz, 1H), 7.0-7.1(m, 2H), 9.23(s, 1H); APCl-MS m / z 274(M+Na) + . At -78°C, add 15ml (25mmol) 1.7M tert-butyllithium hexane to a solution of 2.5g (10mmol) N-(tert-butoxycarbonyl)-3-isopropoxyaniline in 15ml anhydrous...
Embodiment 27
[0330] Example 27: Methyl 2-oxo-3-(4-sulfamoyl-phenylamino-methylene)-2,3-dihydro-1H-indole-5-carboxylate (Z-isomer body)
[0331] Under stirring, a solution of 2.66 g (20.0 mmol) of ethyl (methylthio)acetate dissolved in 200 ml of dichloromethane was cooled to -70°C and 2.7 g (20.0 mmol) of sulfonyl chloride was added. The reaction was stirred at -70°C for 30 minutes and 3.0 g (20 mol) of methyl 4-aminobenzoate and 4.3 g (20 mmol) of Proton Sponge were added dropwise within 1 hour 250 mL of dichloromethane solution, the resulting pink slurry was treated with a 2.3 g (23 mmol) portion of TEA and the solution was allowed to warm to room temperature. The solution was washed with three 250ml portions of water, MgSO 4 Drying and concentration gave an oil. This was chromatographed on silica gel eluting with hexane:EtOAc (1:1) to afford 2.0 g (42% yield) of 3-methylthio-2-oxo-2,3-dihydro- Methyl 1H-indole-5-carboxylate:
[0332] 1 H NMR (DMSO-d 6 ): δ1.97(s, 3H), 3.35(s, 3H...
Embodiment 99
[0339] Example 99: C-{4-[N'-(5-Hydroxy-4,6-dimethyl-2-oxo-1,2-dioxin(3-ylidene)hydrazino]phenyl} -N-Methylmethanesulfonamide
[0340] 4,6-Dimethyl-5-hydroxy-1H-2,3-dione was prepared from 3,5-dimethyl-4-hydroxyaniline according to method A: 1 H NMR (DMSO-d 6 ): δ2.17(s, 3H), 2.30(s, 3H), 6.45(s, 1H), 8.29(s, 1H), 10.65(s, 1H); ES1-MS m / z 190(M-H) - . 100mg (0.52mmol) of 4,6-dimethyl-5-hydroxy-1H-indole-2,3-dione and 144mg (0.57mmol) of C-(4-hydrazinophenyl)-N- A mixture of methylmethanesulfonamide hydrochloride in 5 ml EtOH was heated to 80°C for 1 hour. After cooling, add 10ml H 2O and the solid was collected by vacuum filtration and dried in a vacuum oven at 60 °C to afford the title compound (79 mg, 79%) as a yellow solid; mp 252-255 °C; 1 H NMR (DMSO-d 6 ): δ2.16(s, 3H), 2.44(s, 3H) 2.52(d, J=4.9Hz, 3H), 4.25(s, 2H), 6.47(s, 1H ), 6.84(q, J=4.9Hz, 1H), 7.28-7.34(m, 4H), 7.92(s, 1H), 10.69(s 1H), 12.87 (s, 1H); APCl-MS m / z 411 (M+Na) + . Elemental analysis, calc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com