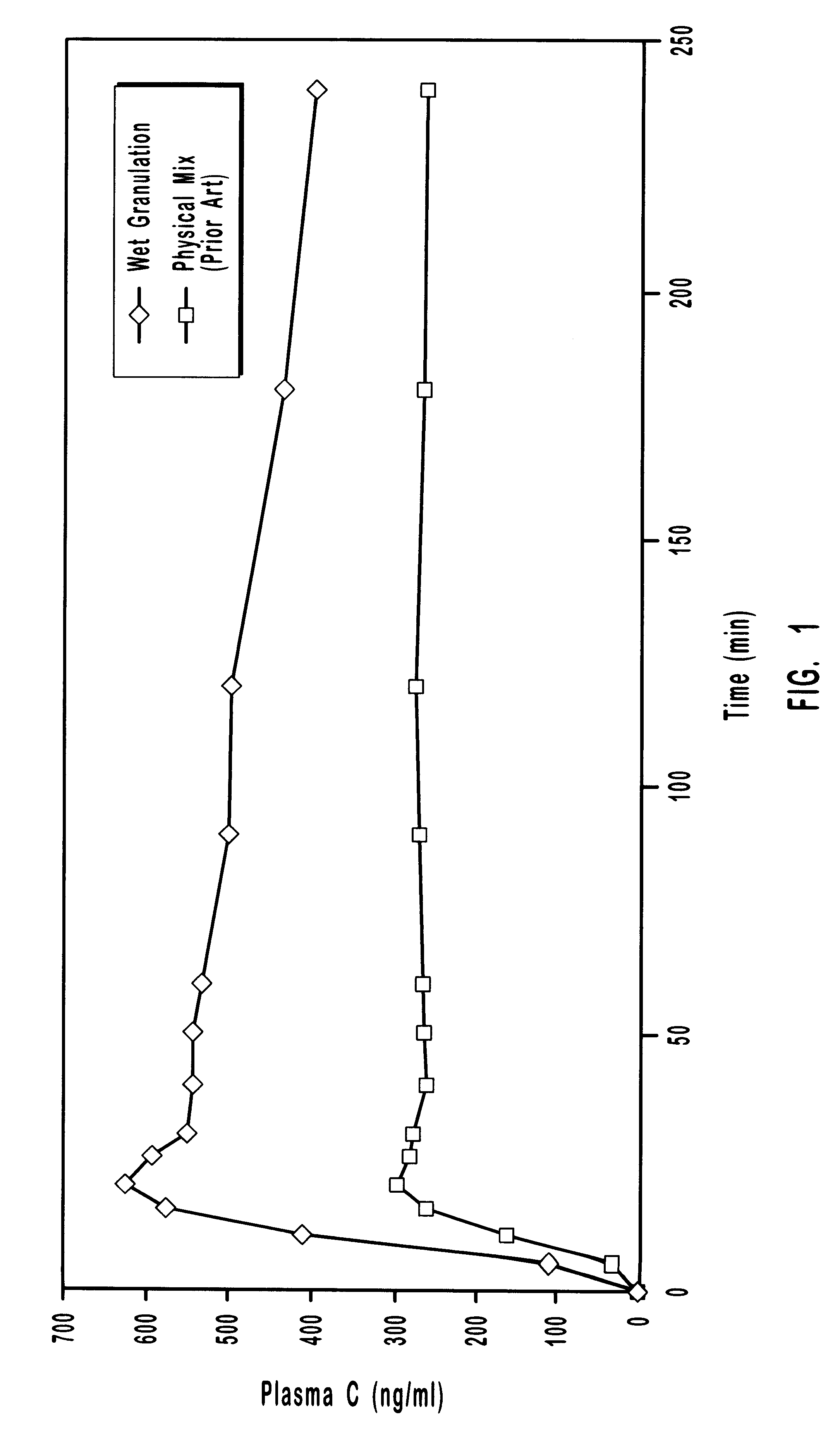
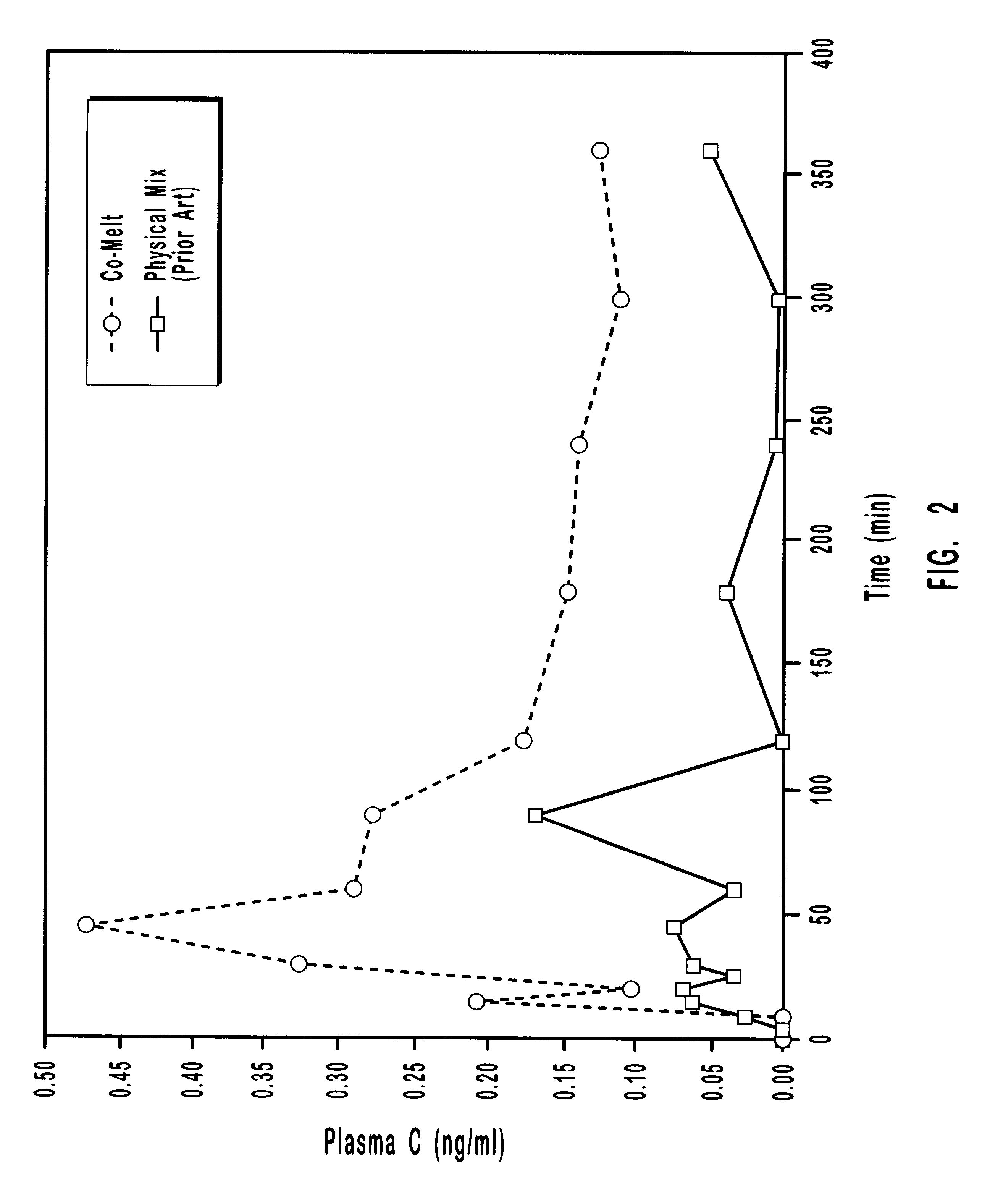
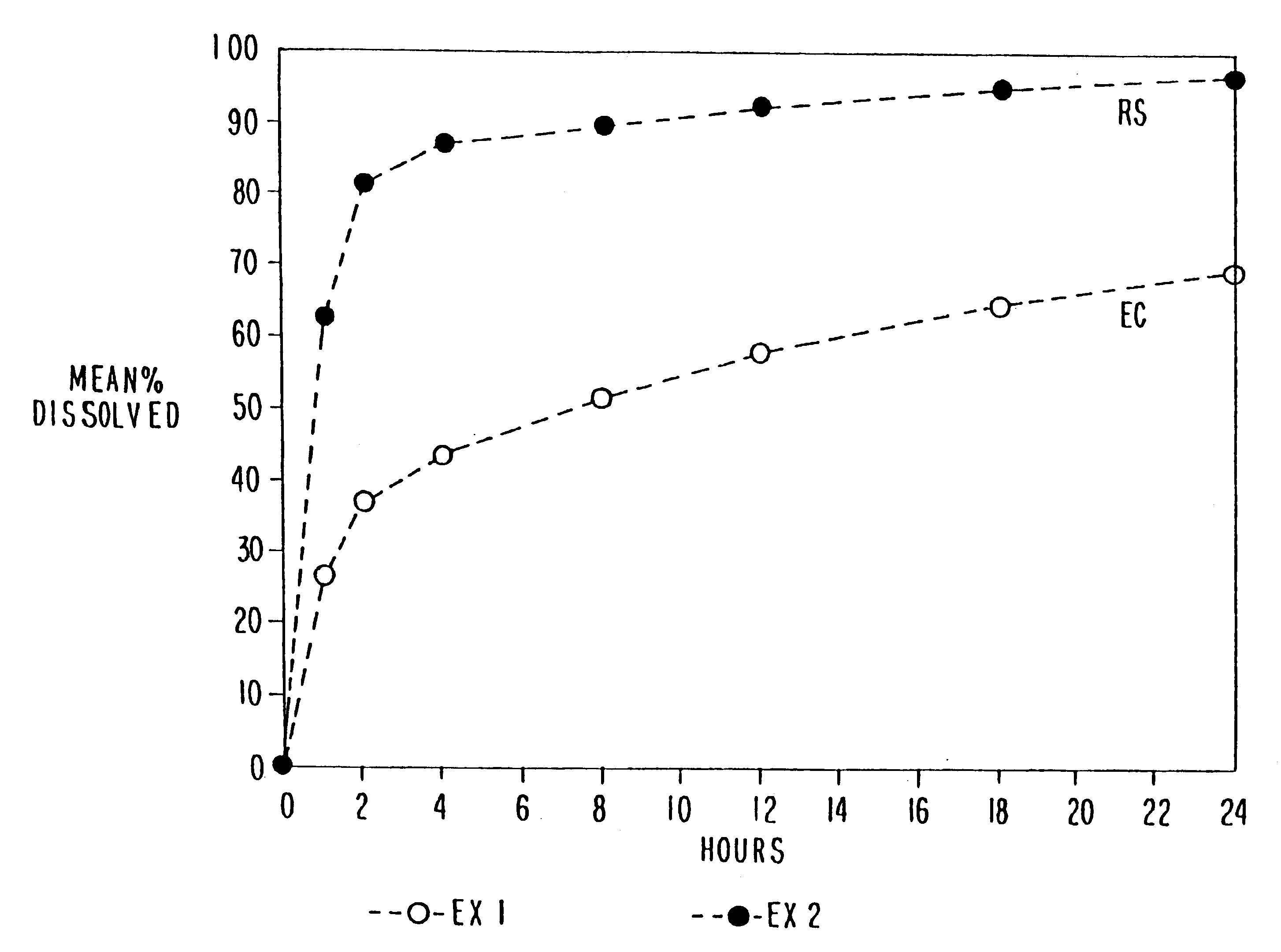
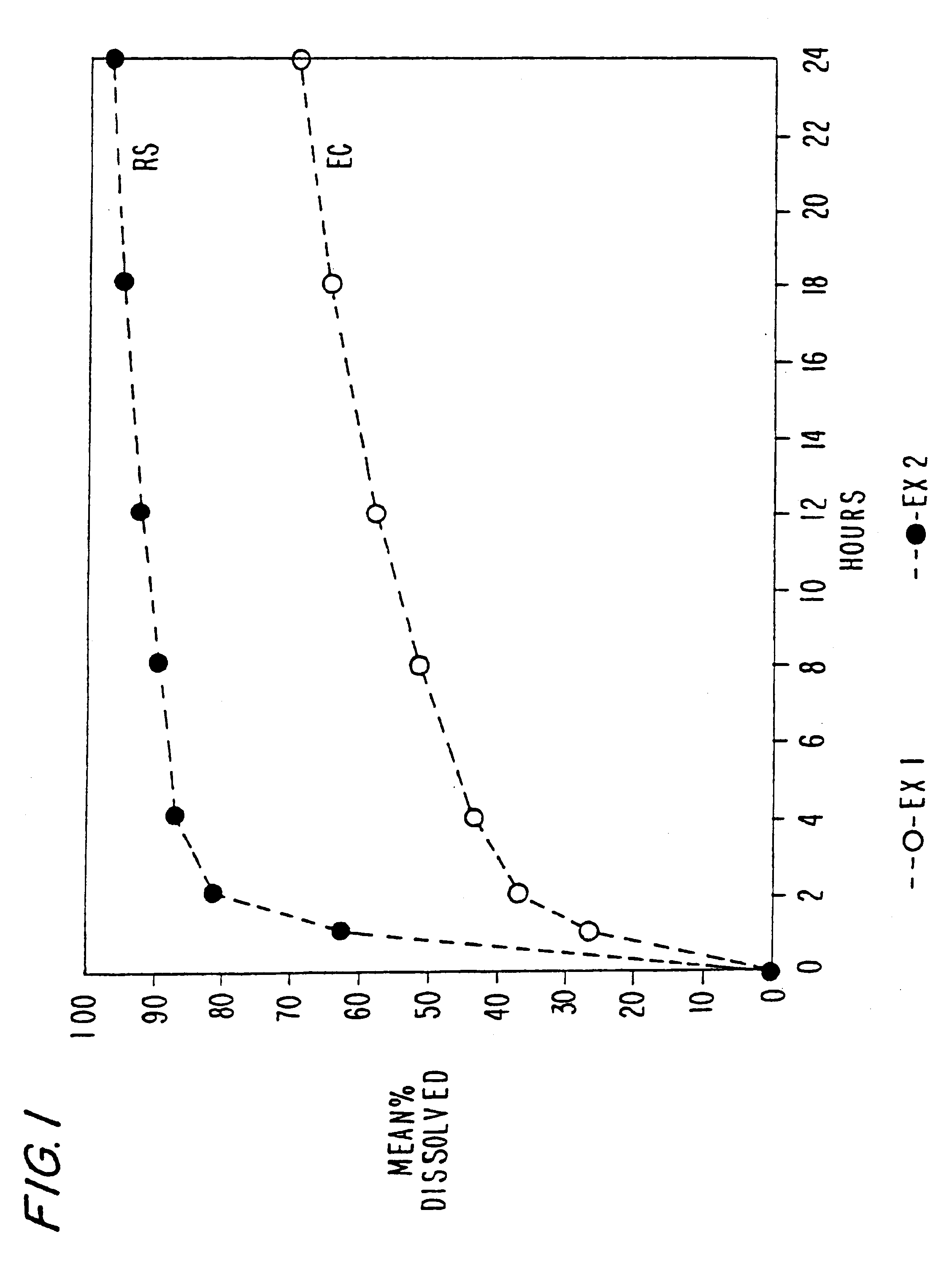
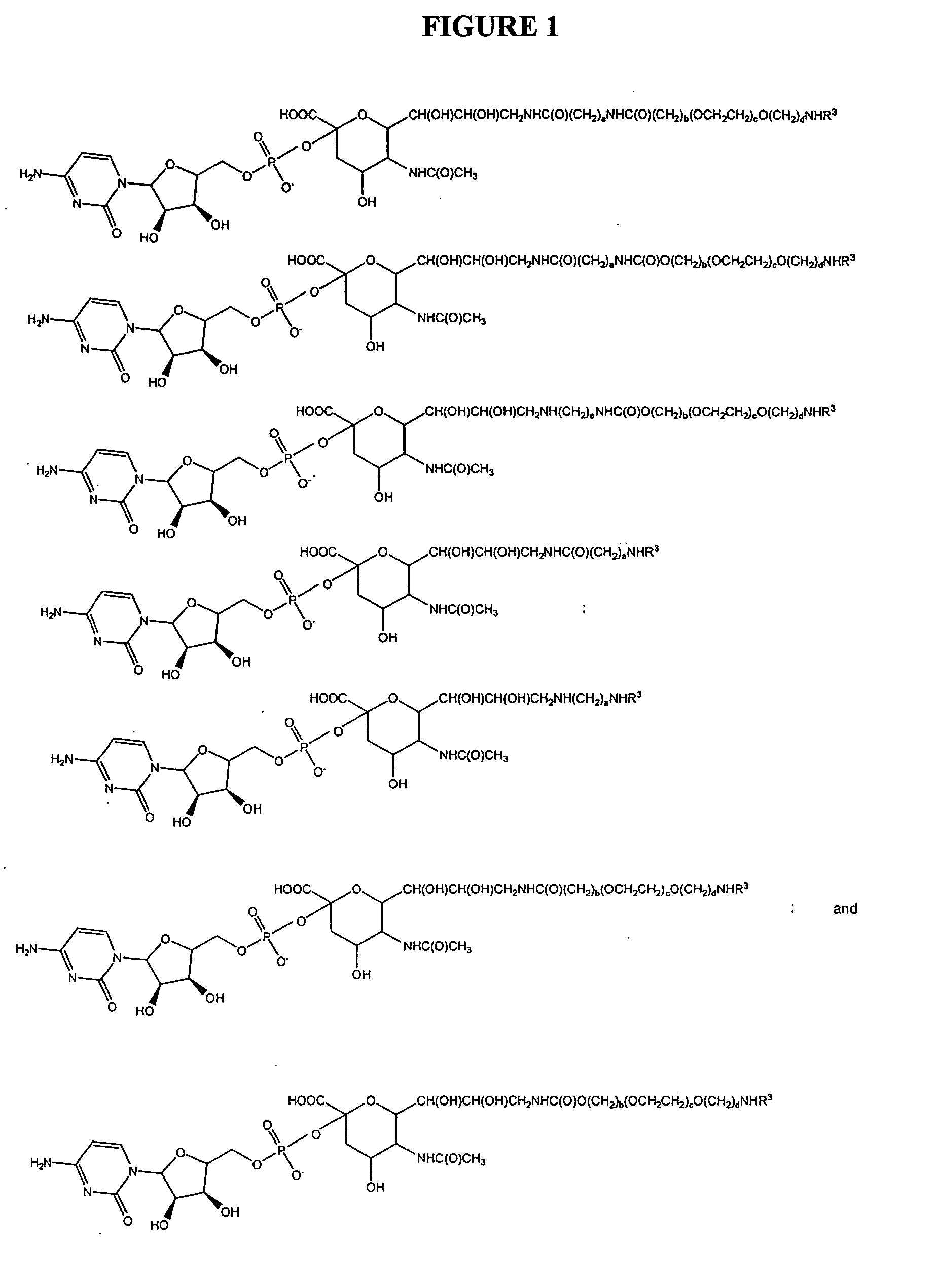
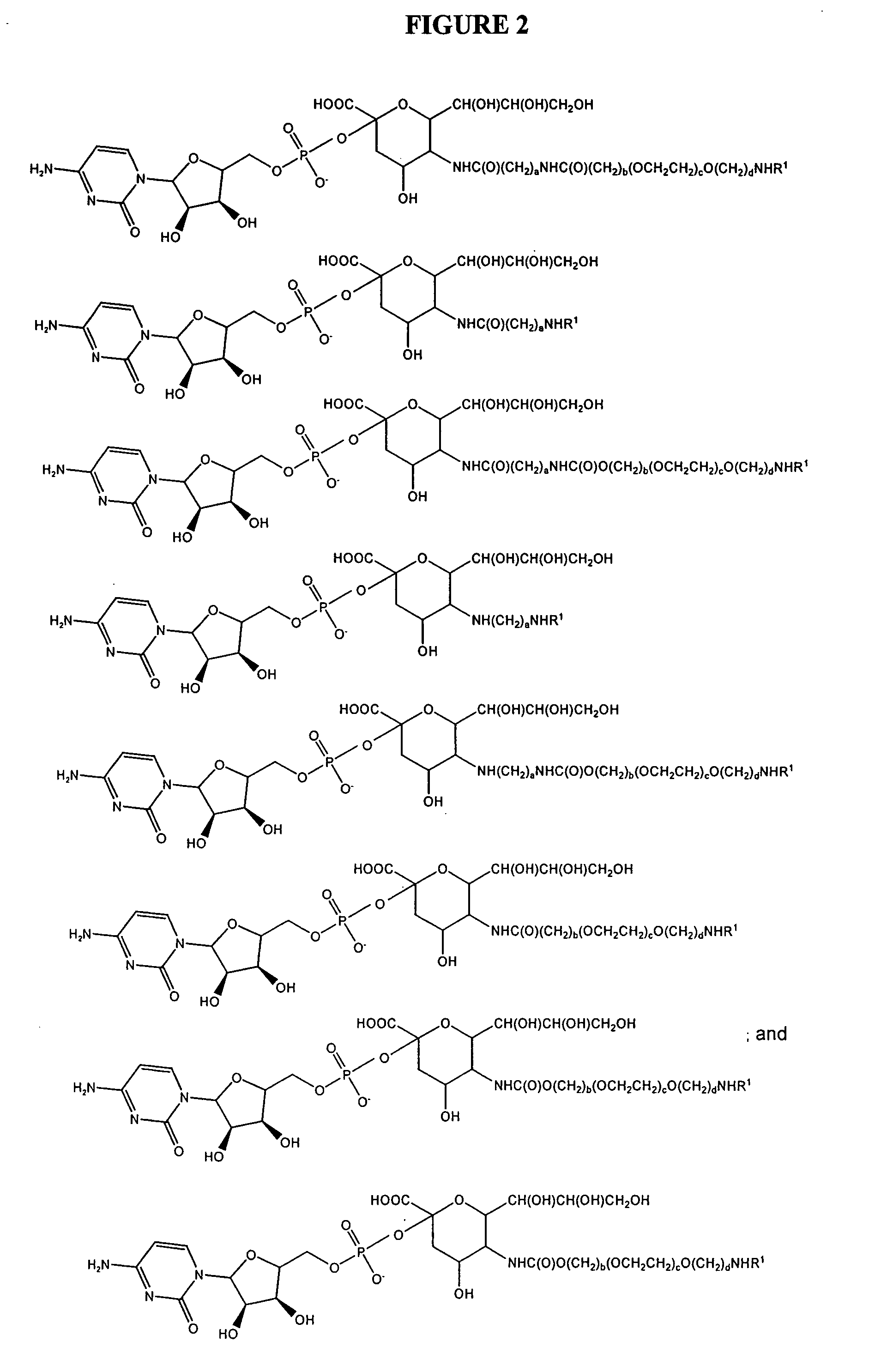
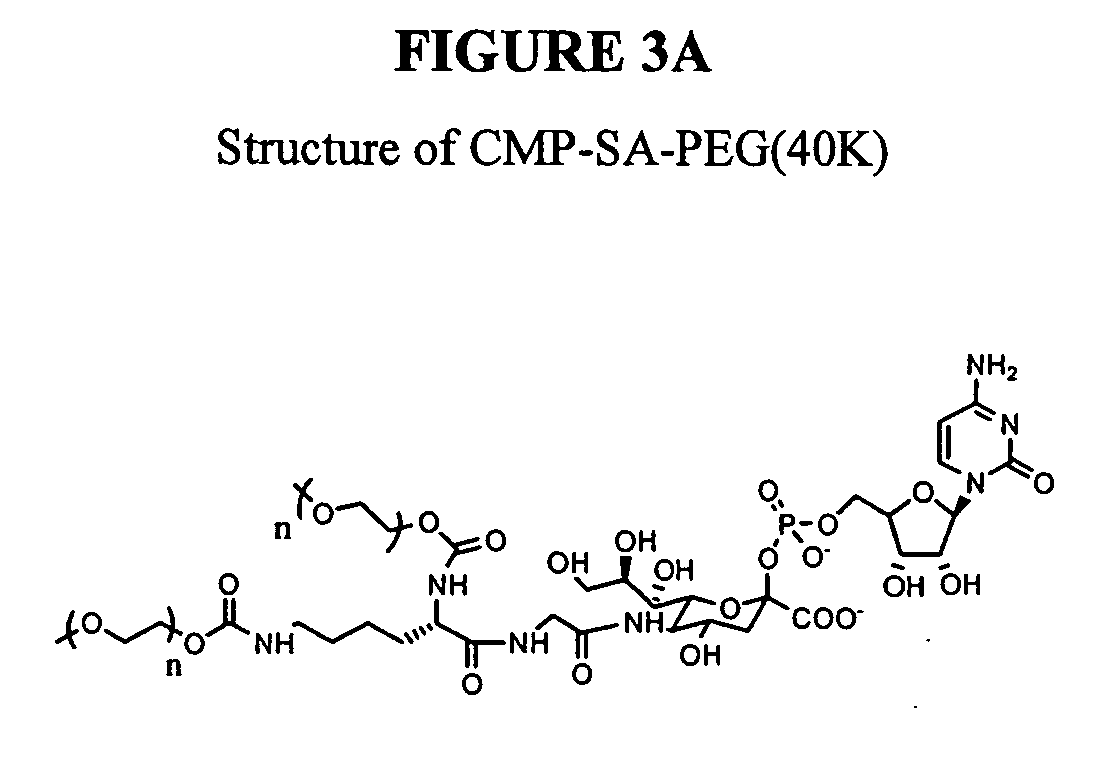
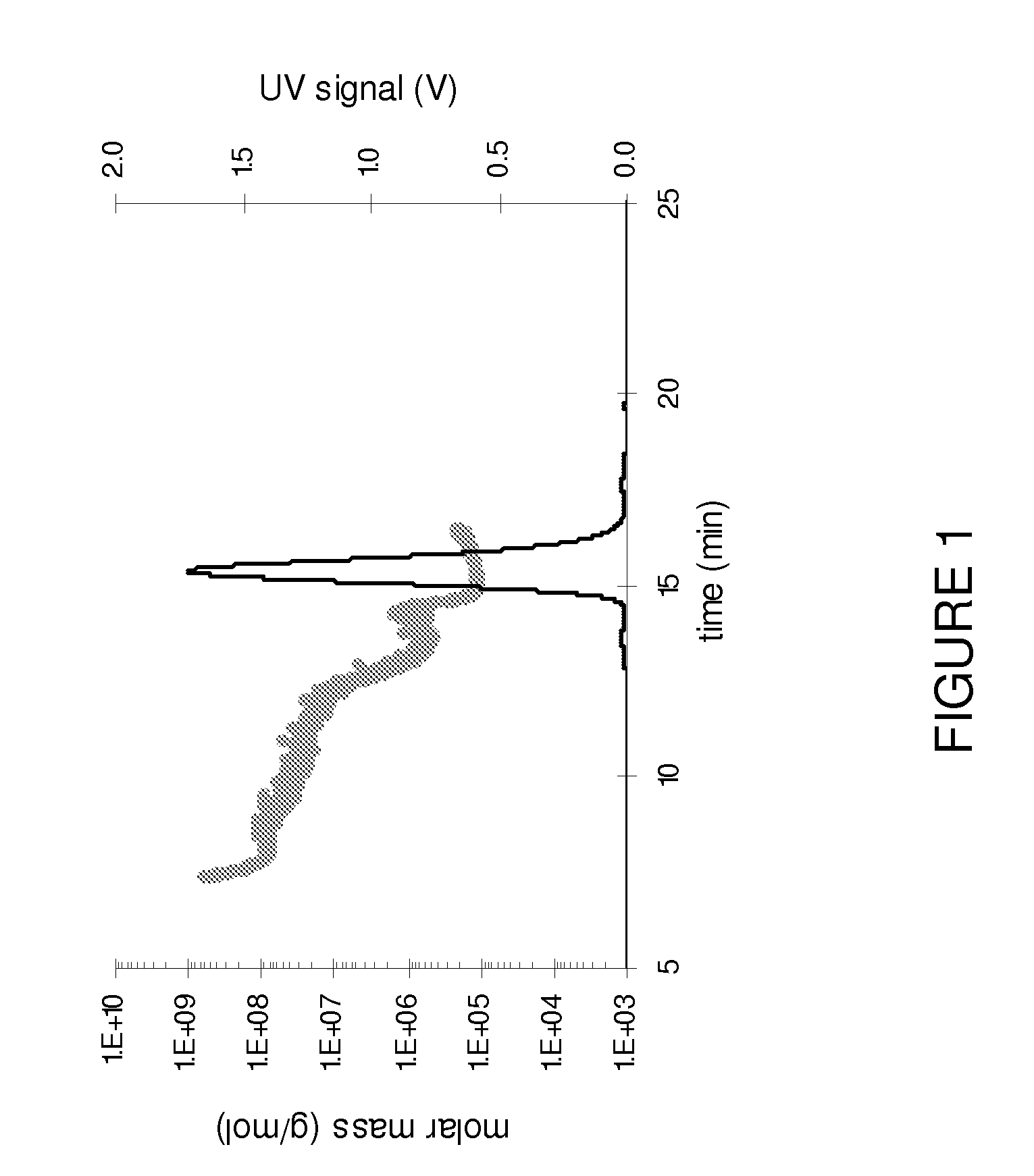
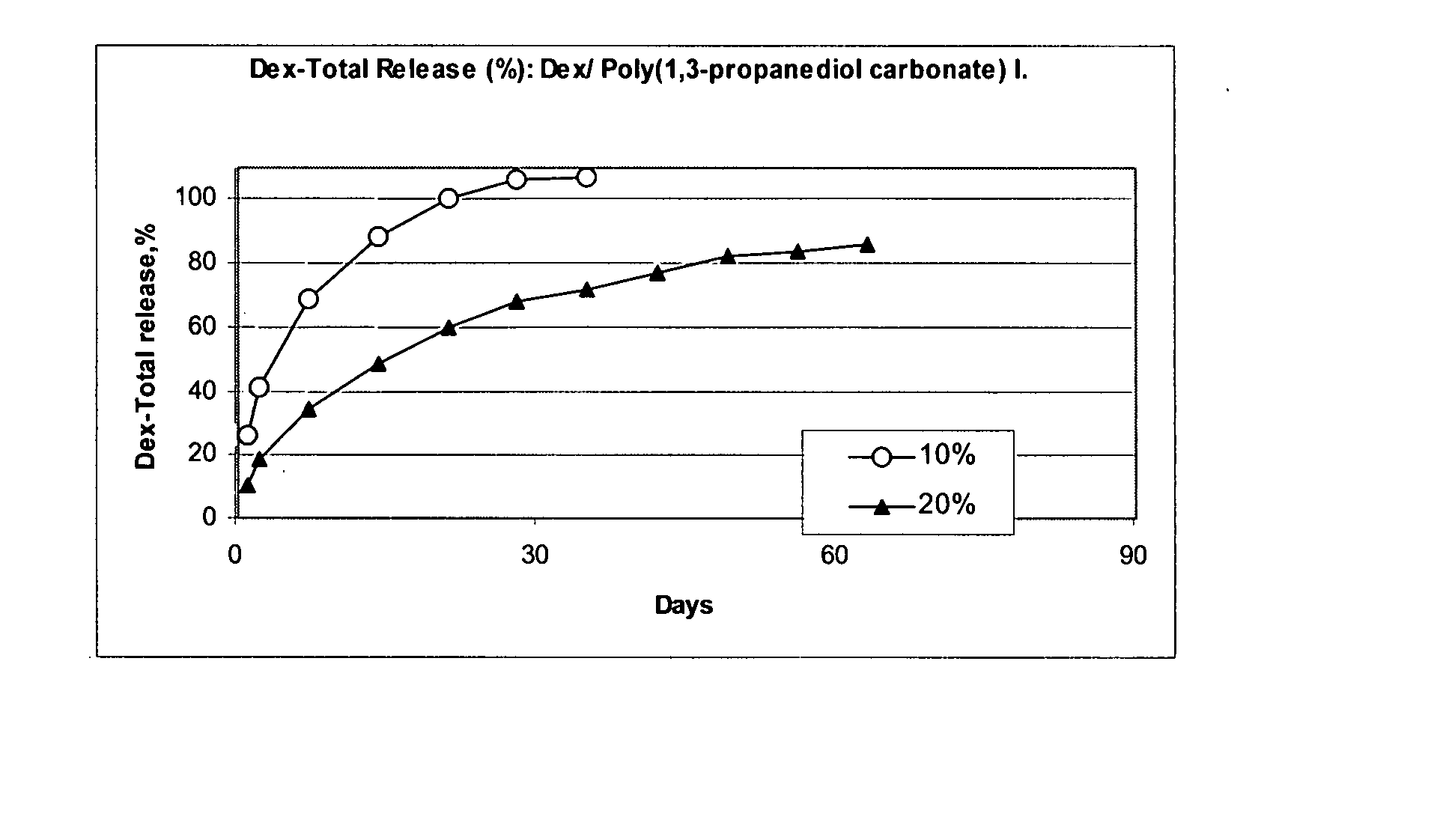
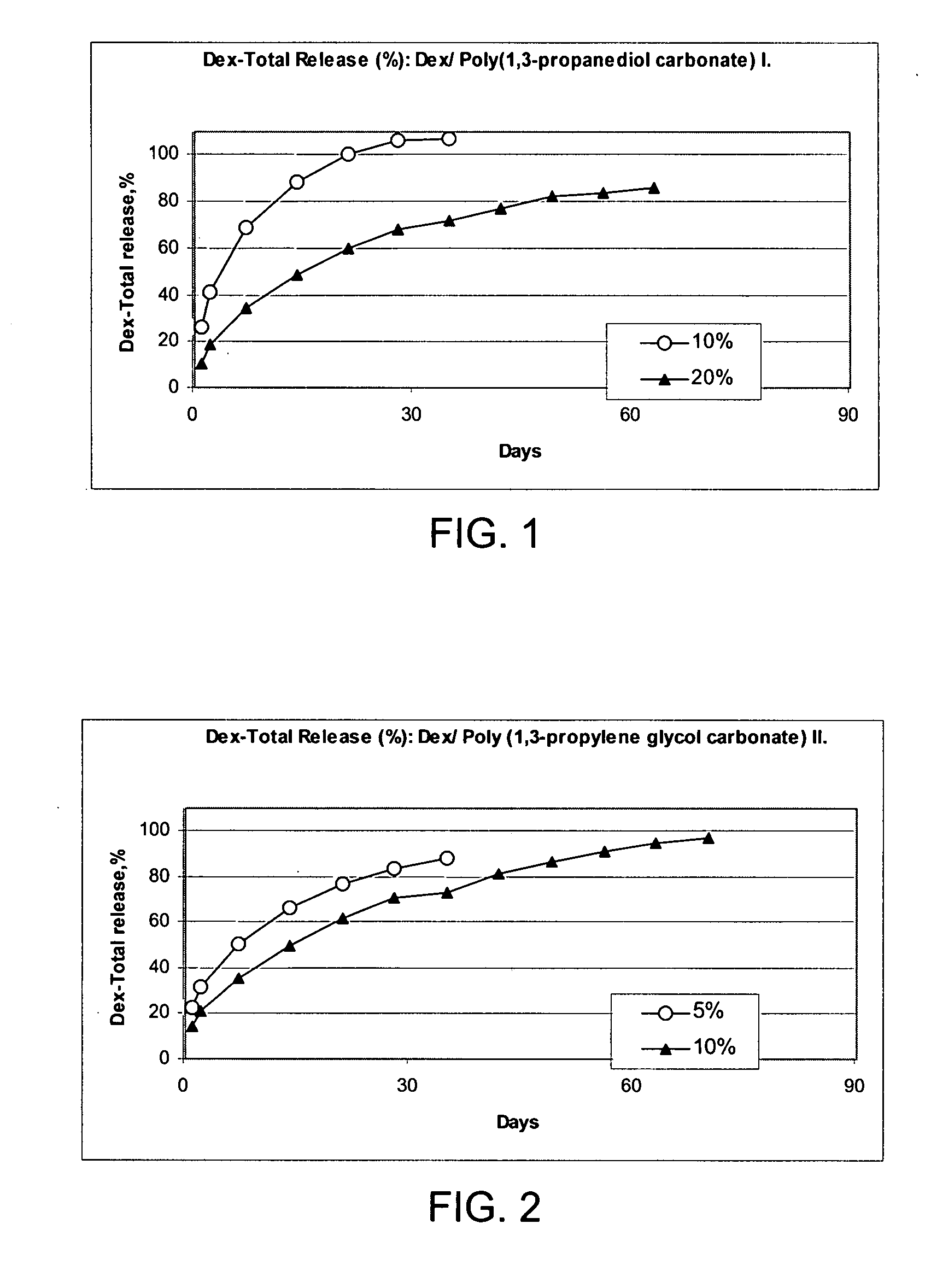
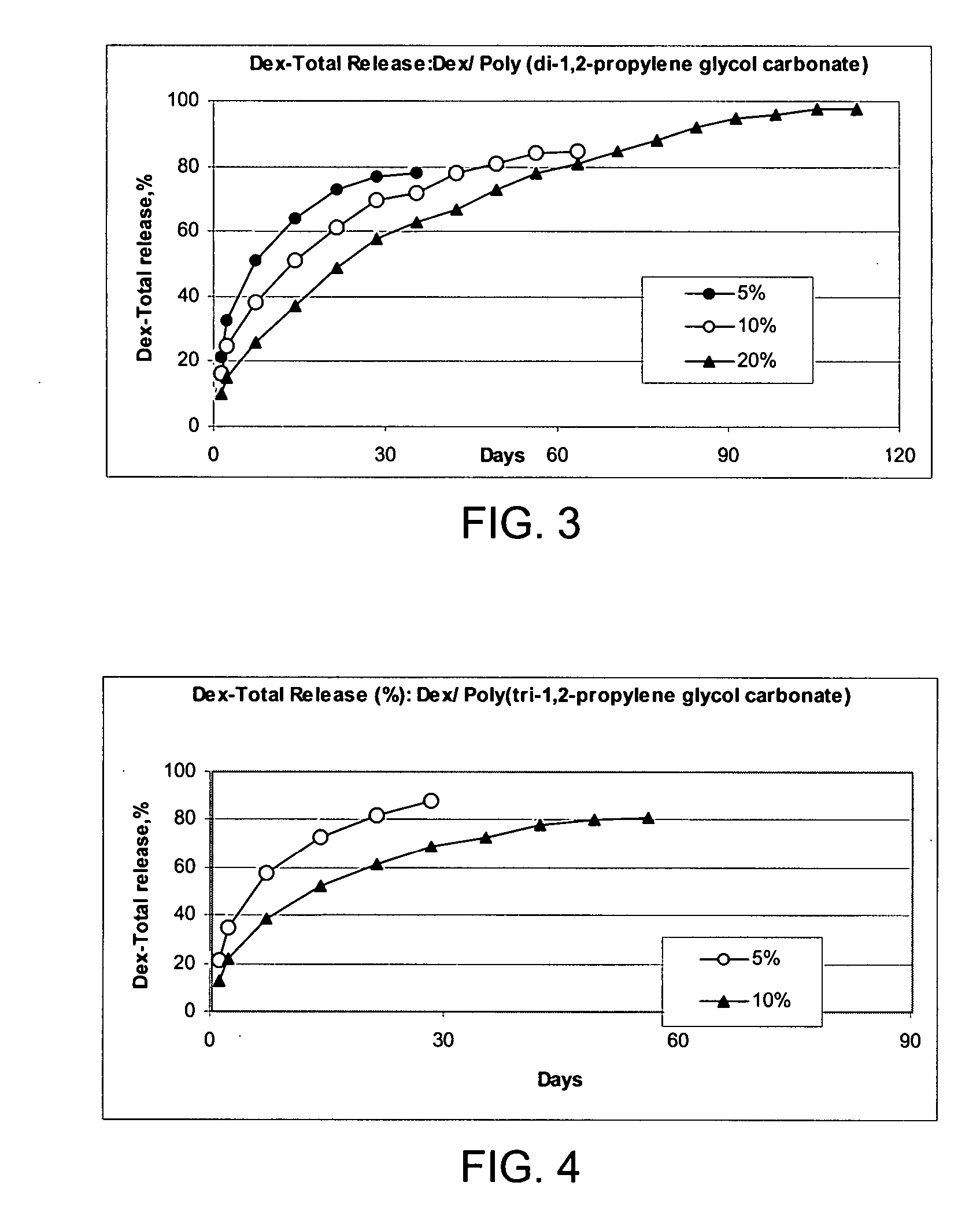
Patents
Literature
9217 results about "Pharmaceutical formulation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmaceutical formulation, in pharmaceutics, is the process in which different chemical substances, including the active drug, are combined to produce a final medicinal product. The word formulation is often used in a way that includes dosage form.
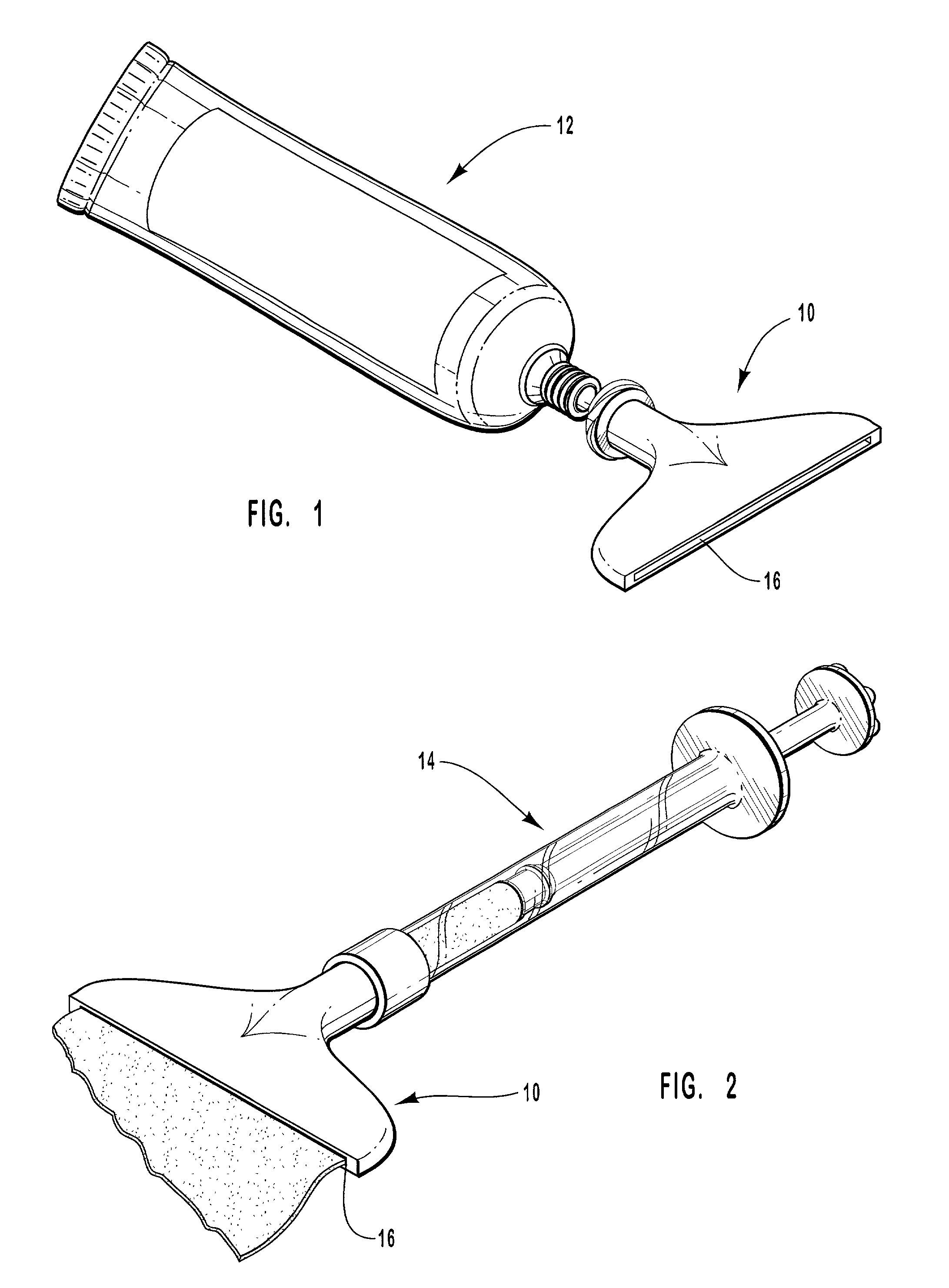
Hand applicator of encapsulated liquids
InactiveUS7419321B2Fast deliveryApply evenlyBathroom accessoriesMedical applicatorsHazardous substancePharmaceutical formulation
A hand applicator of encapsulated liquids, such as cosmetic, hygienic, and pharmaceutical formulations or household chemicals, of different viscosity. When the user squeezes the applicator in her hand, the capsule inside it ruptures, possibly with a clap, and releases a liquid that after being collected by the drain passes through the perforated dissector to get evenly distributed in the absorber and then evenly applied on skin or other surfaces. Inert and impermeable capsules ensure stability of stored liquid formulations. Pressurized capsules are easily tactilely located and crashed. A capsule may be permanently or elastically fixed within the applicator. The applicator may include several capsules that may contain different liquids. A refillable applicator can be refilled with new capsules and used more than once. A transparent impermeable back side the applicator enhances hygiene and creates visibility of the capsule. An impervious glove-applicator for applying hazardous substances is disclosed.
Owner:TERESCHOUK MISHA
Oral transmucosal drug dosage using solid solution
InactiveUS6264981B1High dissolution rateEasy to usePharmaceutical non-active ingredientsPill deliverySolid solutionPharmaceutical formulation
The present invention is directed toward formulation and method for oral transmucosal delivery of a pharmaceutical. The invention provides a drug formulation comprising a solid pharmaceutical agent in solid solution with a dissolution agent. The formulation is administered into a patient's oral cavity, delivering the pharmaceutical agent by absorption through a patient's oral mucosal tissue. The formulation and method provide for improved oral mucosal delivery of the pharmaceutical agent.
Owner:CEPHALON INC
Melt-extruded orally administrable opioid formulations
InactiveUS6261599B1Sustained effectSlow and control releaseBiocideOrganic active ingredientsMelt extrusionDosage form
Bioavailable sustained release oral opioid analgesic dosage forms, comprising a plurality of multiparticulates produced via melt extrusion techniques disclosed.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Compositions for nasal drug delivery, methods of making same, and methods of removing residual solvent from pharmaceutical preparations
InactiveUS6391452B1Taller in heightSlow down the mixing speedLiquid surface applicatorsPeptide/protein ingredientsNasal cavityNose
The present invention relates to pharmaceutical compositions for delivery of drugs intended to reside in the nose, compositions for nasal administration of drugs, e.g., antiviral agents, and particularly antiviral agents comprising the human major rhinovirus receptor, also known as intercellular adhesion molecule-1 (ICAM-1); to methods of making said nasal drug compositions, and to an improved process for the removal of residual solvent from pharmaceutical matrices.
Owner:BAYER PHARMA CORP
Pharmaceutical formulation containing opioid agonist,opioid antagonist and gelling agent
InactiveUS20030068371A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Glycopegylated erythropoietin
InactiveUS20050143292A1Improved pharmacokinetic propertiesCost-effectiveSugar derivativesPeptide/protein ingredientsDiseaseSugar moiety
The present invention provides conjugates between erythropoietin and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
Stable high protein concentration formulations of human Anti-tnf-alpha-antibodies
InactiveUS20100278822A1Suitable viscosityIncrease concentrationAntibacterial agentsSenses disorderHigh concentrationPolyol
The invention provides a liquid pharmaceutical formulation which does not include NaCl and comprises more than 20 mg of a polyol and at least about 100 mg / mL of a human anti-TNF-alpha antibody, or antigen-binding portion thereof. The invention provides a high concentration antibody formulation having long-term stability and advantageous characteristics for subcutaneous administration.
Owner:ABBVIE BIOTECHNOLOGY LTD
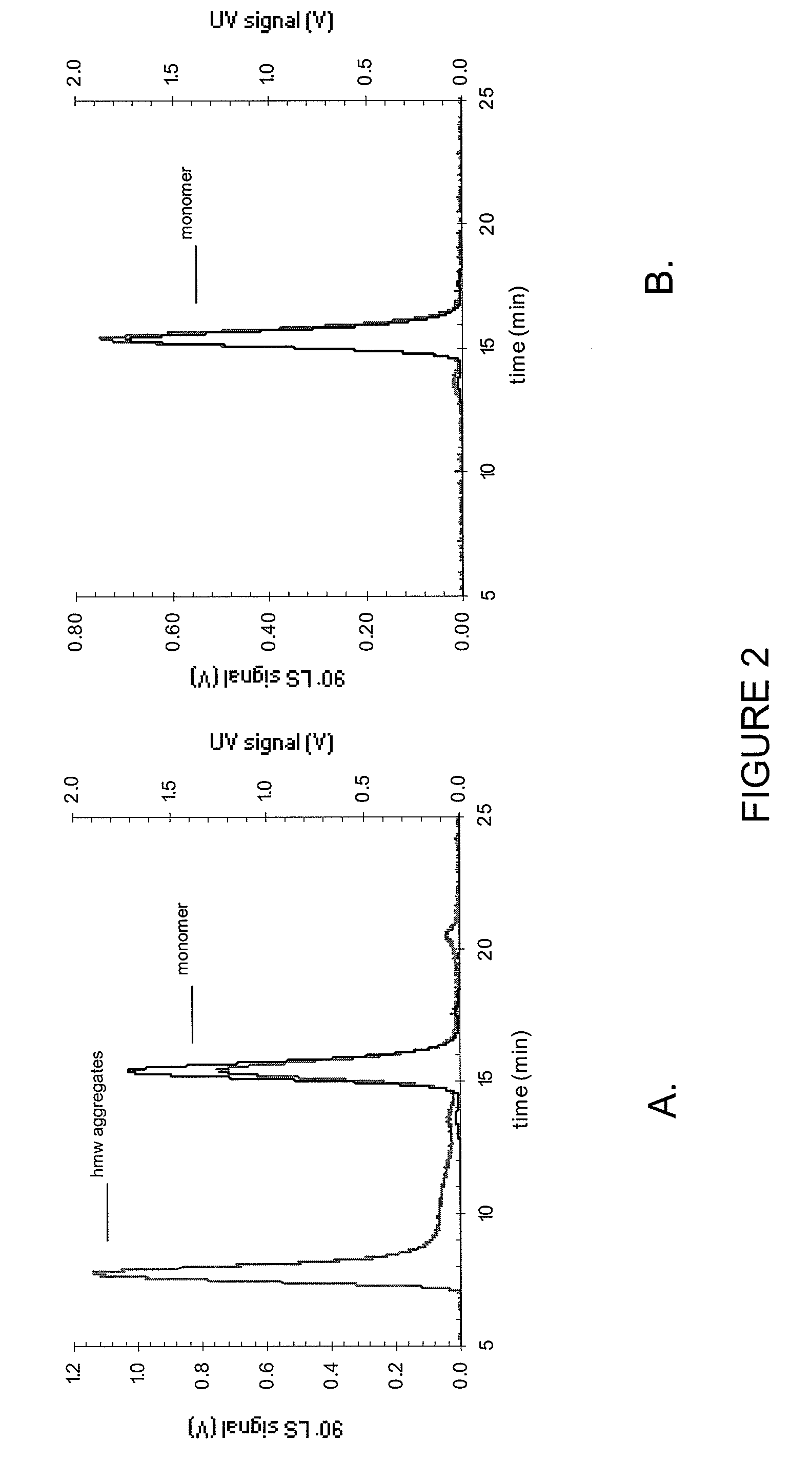
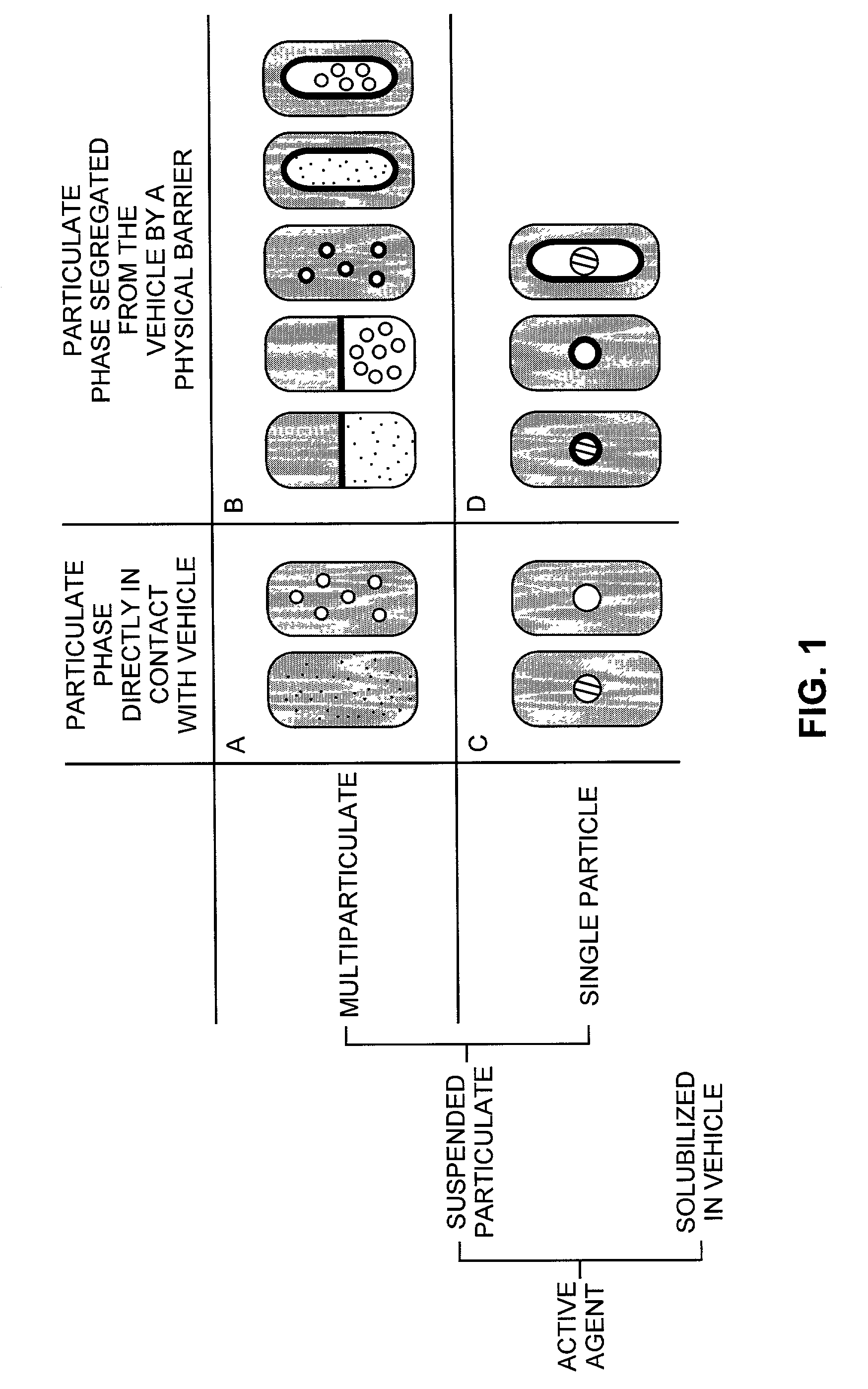
Pharmaceutical formulations and systems for improved absorption and multistage release of active agents
InactiveUS7374779B2Improve bioavailabilityLow variabilityPowder deliveryOrganic active ingredientsActive agentFast release
The present invention pertains to pharmaceutical formulations and systems for delivery of active agents, wherein a first fraction of an active agent is suspended in a vehicle and a second fraction of active agent is solubilized in the vehicle, with the suspended fraction representing about 5 wt. % to about 80 wt. % of the active agent and the second fraction representing about 20 wt. % to about 95 wt. % of the active agent. One or more additional active agents, which may be fully solubilized, partially solubilized, or suspended, may also be present. The first and second fractions of the active agent may or may not have different release profiles. Generally, a significant fraction of the solubilized drug will release rapidly, providing for rapid onset, while the suspended drug may be formulated for delayed and / or sustained release.
Owner:LIPOCINE
Combination Products
InactiveUS20080020018A1Sufficient reliefExtended stayAntibacterial agentsOrganic active ingredientsMethyl xanthineBULK ACTIVE INGREDIENT
A pharmaceutical formulation comprises a plurality of seamless minicapsules having a diameter of from 0.5 mm to 5 mm, at least some of the minicapsules containing a methyxanthine as one active ingredient, and at least some of the minicapsules containing a corticosteriod as another active ingredient.
Owner:SIGMOID PHARM LIMITED
Compositions and methods of delivery of pharmacological agents
InactiveUS20050004002A1Reducing one or more side effectsInhibiting oxidation in the pharmaceutical compositionAntibacterial agentsOrganic active ingredientsSide effectPharmaceutical formulation
The present invention relates to a pharmaceutical composition comprising a pharmaceutical agent and a pharmaceutically acceptable carrier, which carrier comprises a protein, for example, human serum albumin and / or deferoxamine. The human serum albumin is present in an amount effective to reduce one or more side effects associated with administration of the pharmaceutical composition. The invention also provides methods for reducing one or more side effects of administration of the pharmaceutical composition, methods for inhibiting microbial growth and oxidation in the pharmaceutical composition, and methods for enhancing transport and binding of a pharmaceutical agent to a cell.
Owner:ABRAXIS BIOSCI LLC
Dosage form comprising liquid formulation
InactiveUS6174547B1Improve oral bioavailabilityImprove bioavailabilityCapsule deliveryEmulsion deliveryPharmaceutical formulationDosage form
Owner:ENCINAL PHARMA INVESTMENTS
Pharmaceutical formulation containing opioid agonist, opioid antagonist and gelling agent
InactiveUS7842307B2Reducing abuse potential of dosage formLower potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
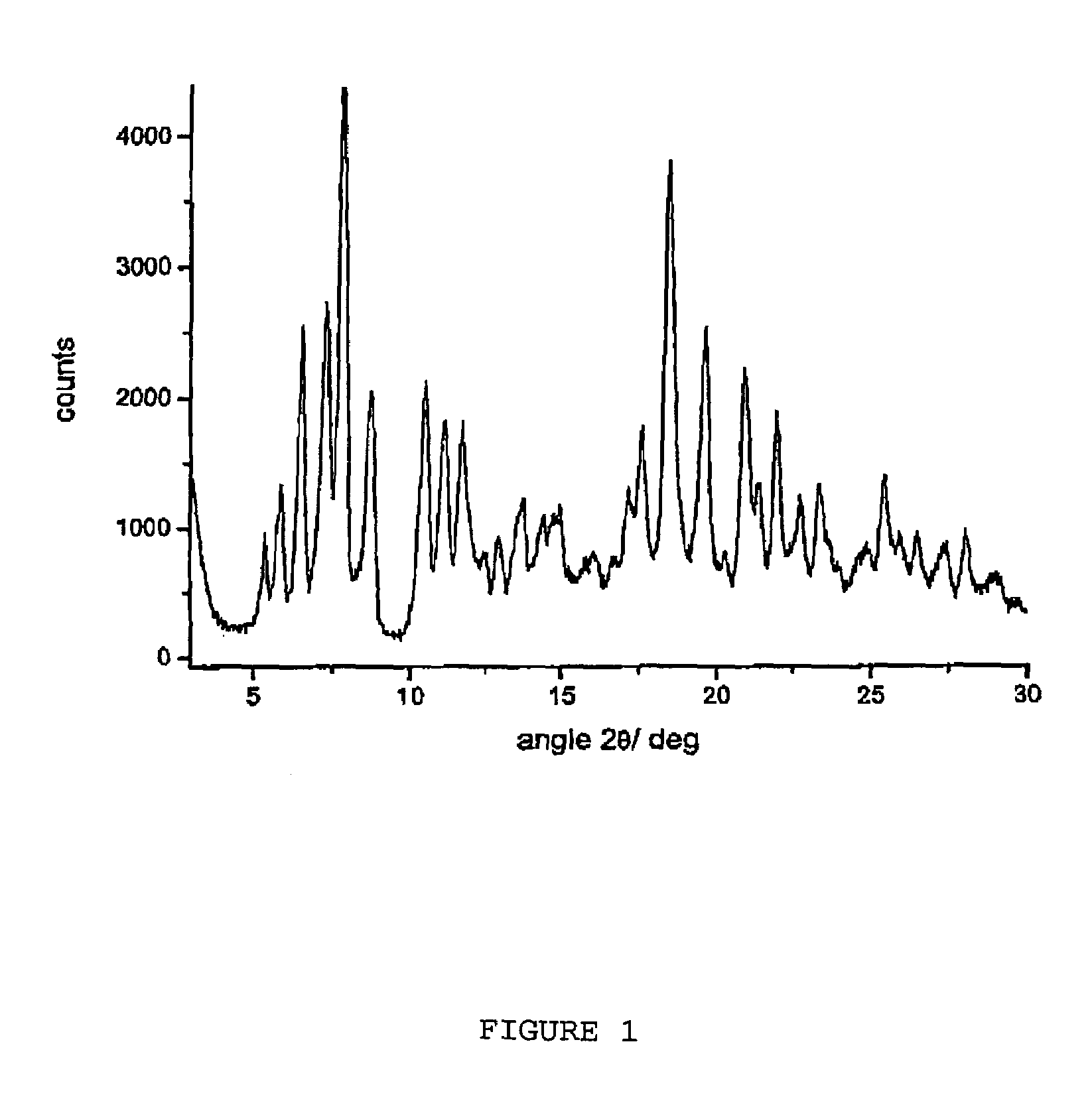
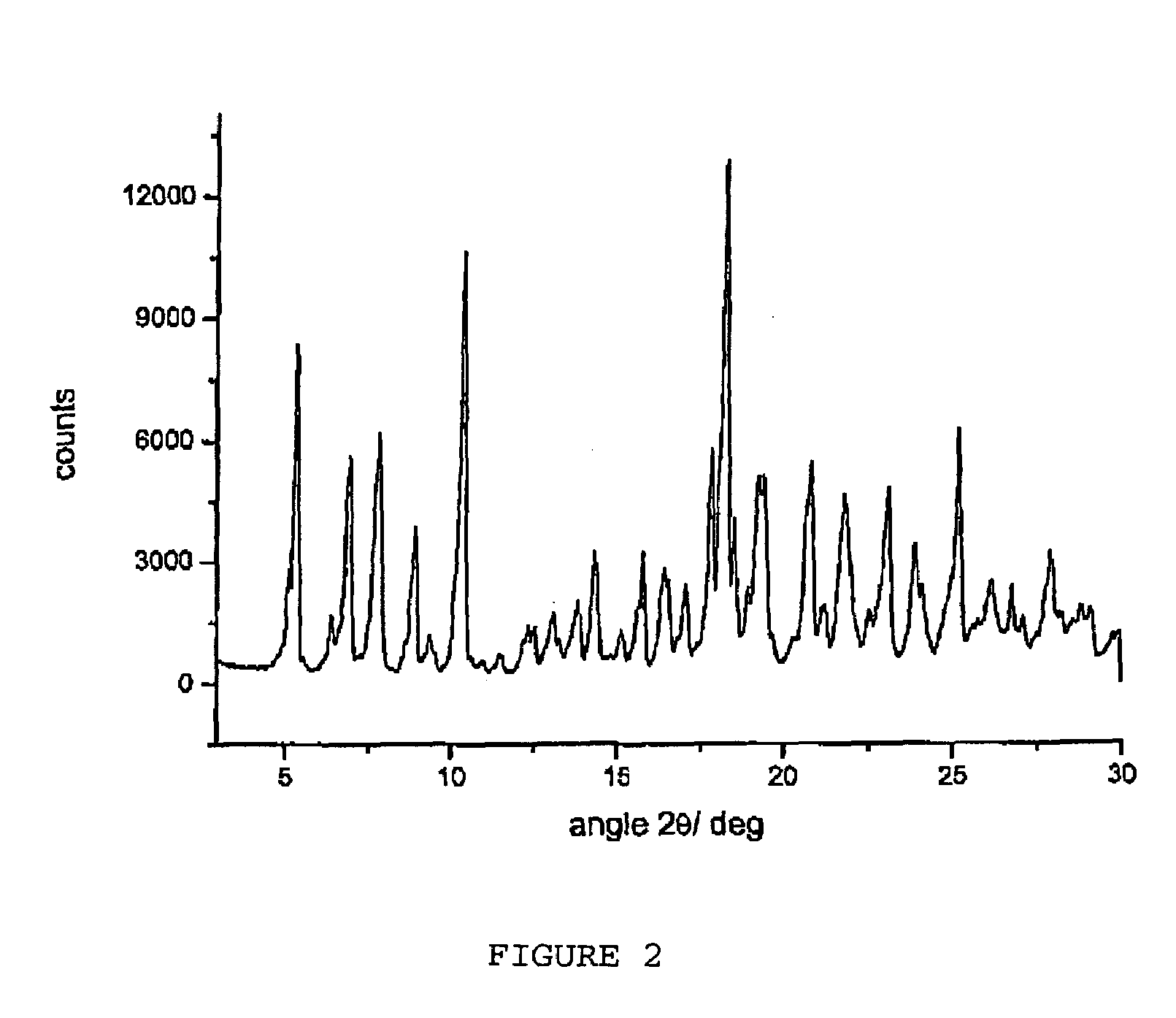
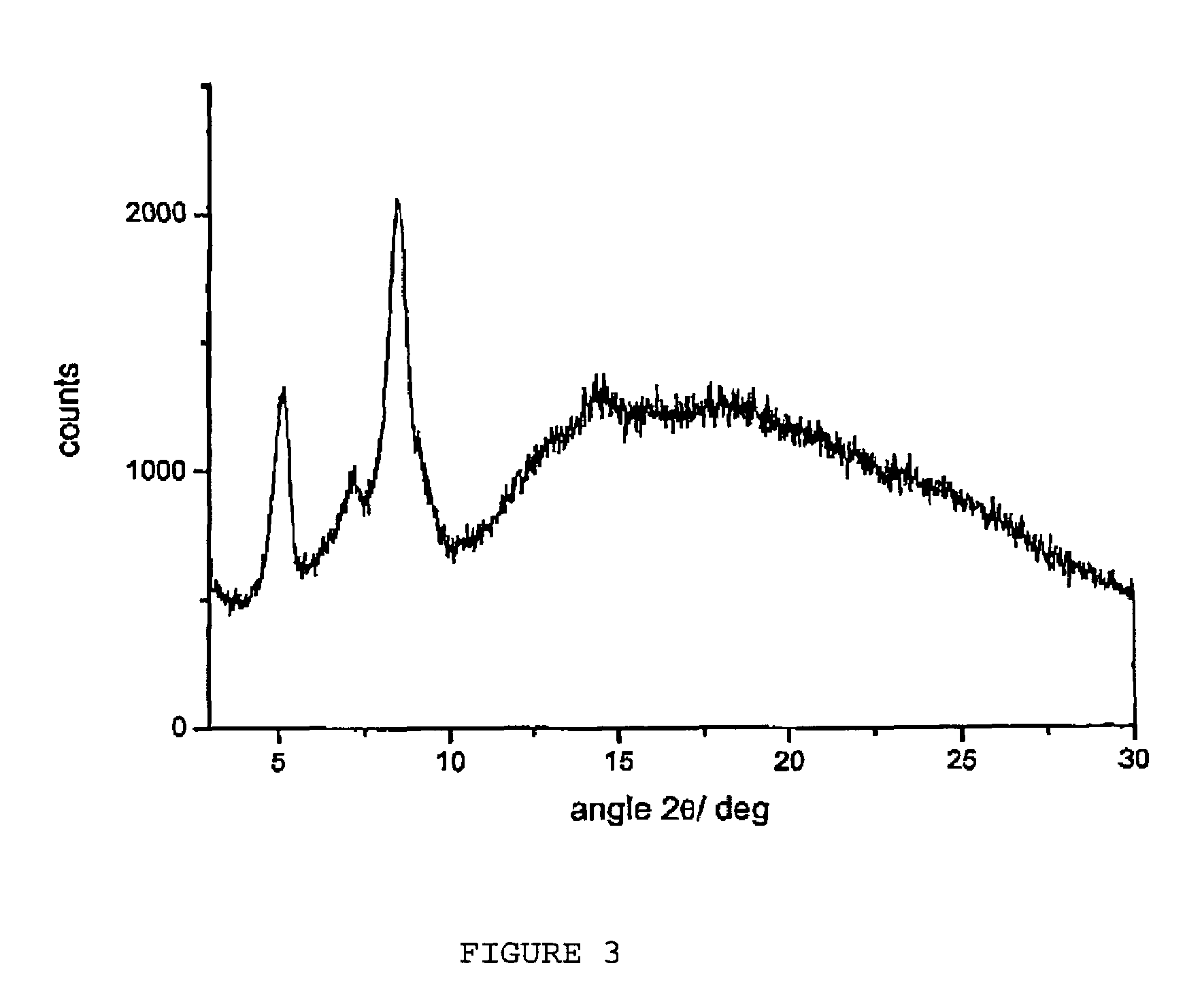
Polymorphous forms of rifaximin, processes for their production and use thereof in medicinal preparations
Crystalline polymorphous forms of the rifaximin (INN) antibiotic named rifaximin α and rifaximin β, and a poorly crystalline form named rifaximin γ have been discovered. These forms are useful in the production of medicinal preparations for oral and topical use and can be obtained by means of a crystallization process carried out by hot-dissolving the raw rifaximin in ethyl alcohol and by causing the crystallization of the product by the addition of water at a determinate temperature and for a determinate period of time. The crystallization is followed by drying carried out under controlled conditions until a specific water content is reached in the end product.
Owner:ALFASIGMA SPA
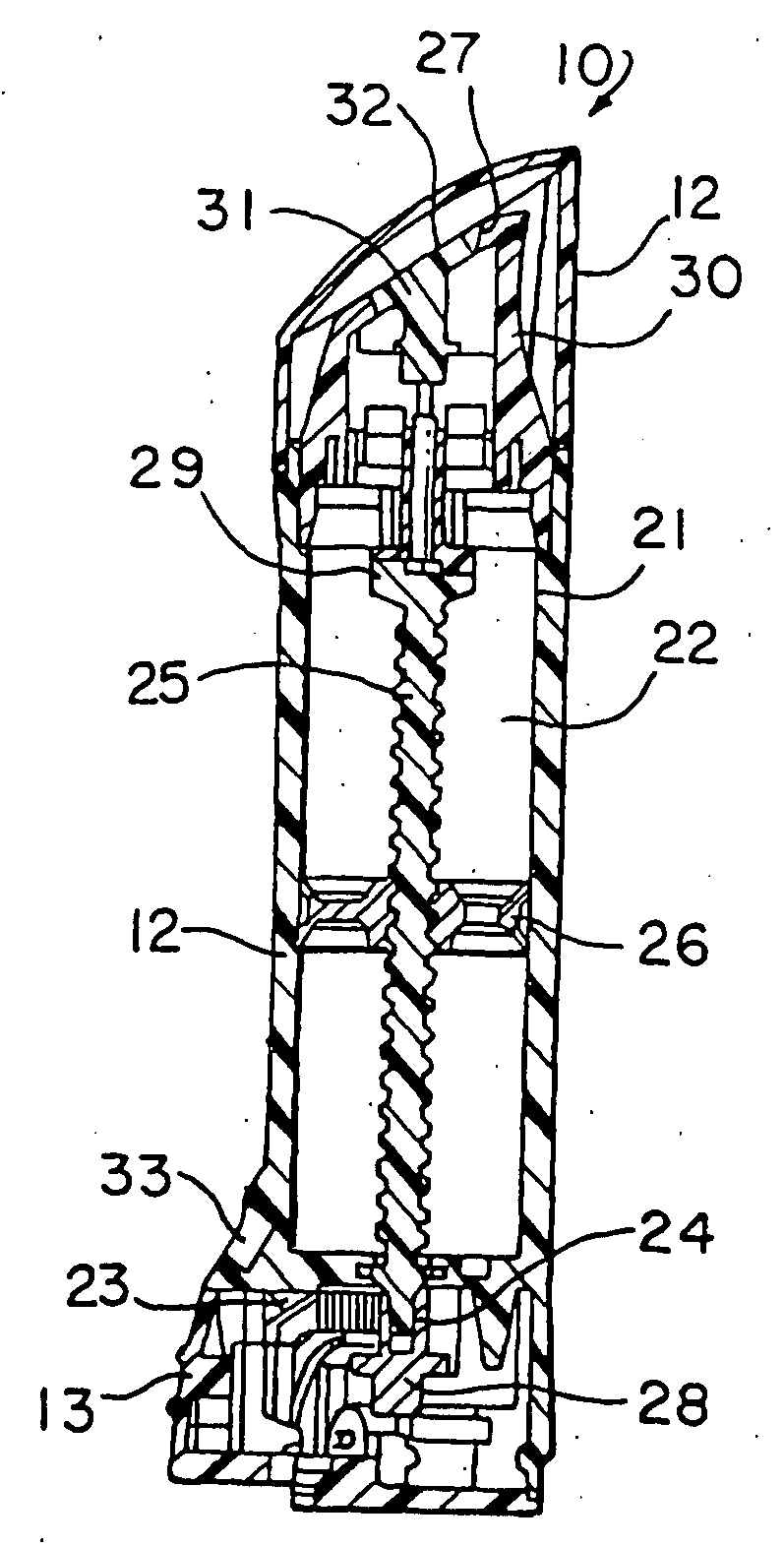
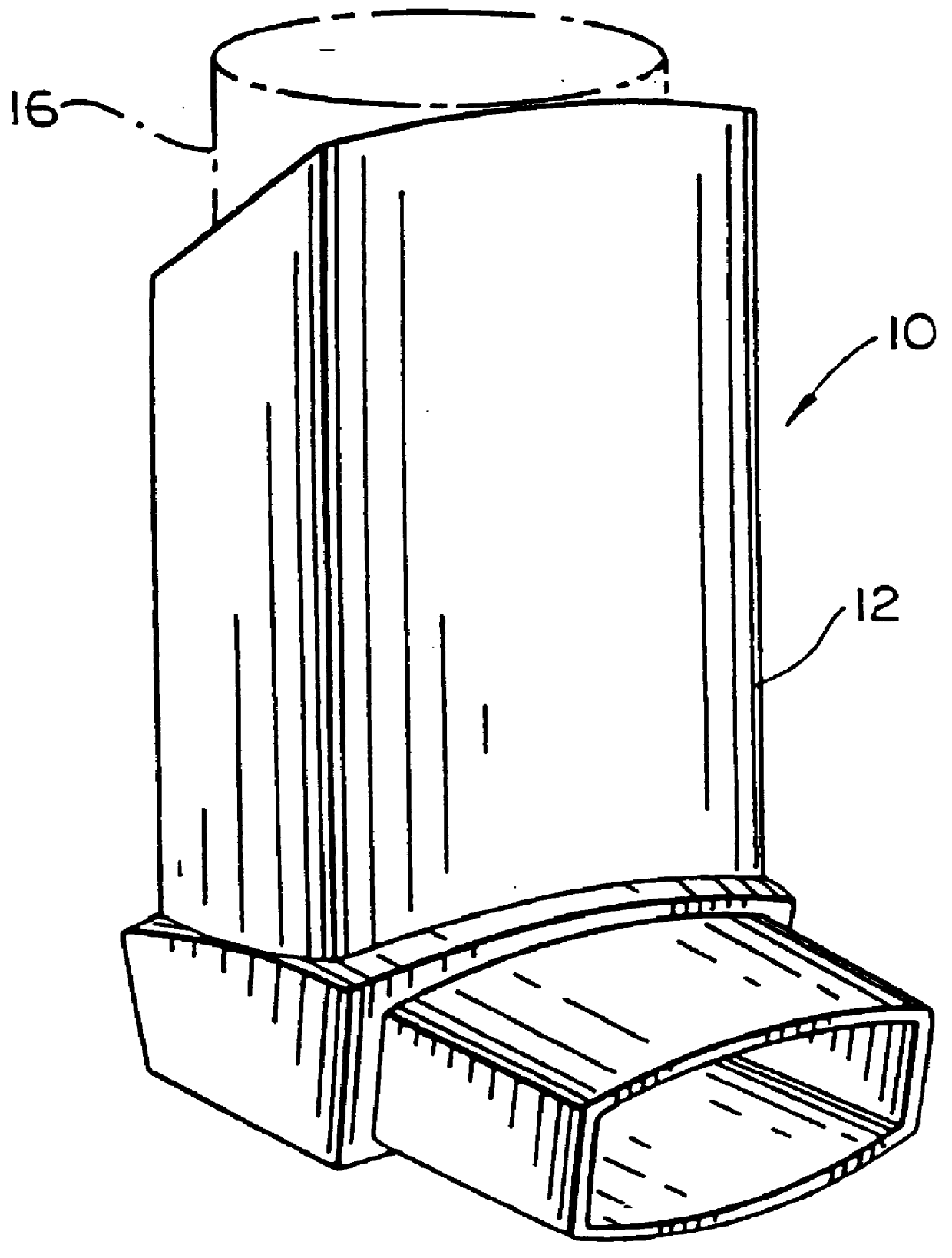
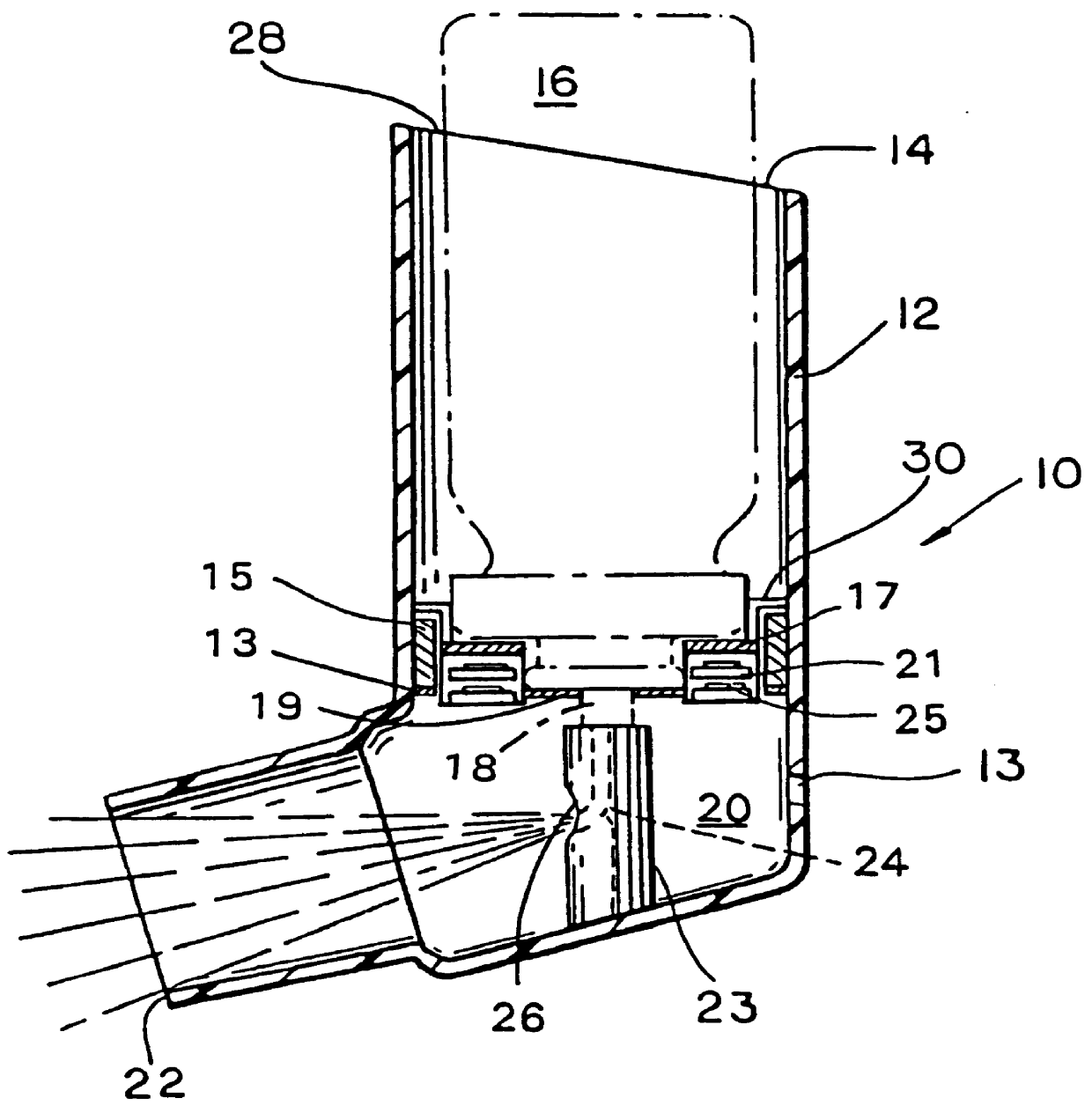
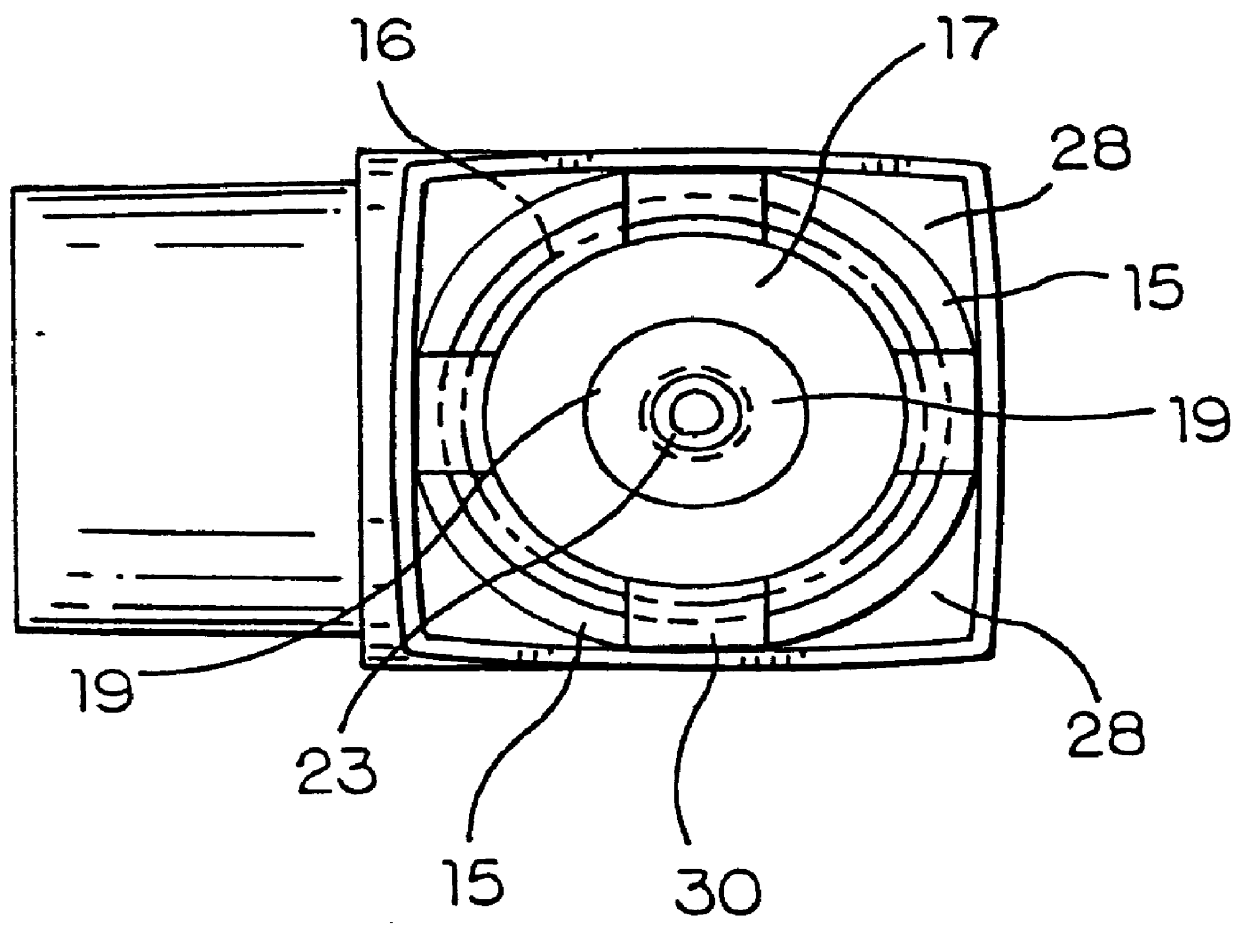
Topical administration device
Disclosed is a dosing device for topically administering a pharmaceutical formulation to a skin of a mammal, the device comprising a housing capable of holding at least one unit dose of a pharmaceutical formulation comprising a drug and a carrier; an applicator adapted for topically administering a unit dose of the pharmaceutical formulation directly onto the skin; and an actuator, wherein upon actuation, the device is capable of metering a unit dose of the pharmaceutical formulation external to the device, from the housing to the applicator; and methods thereof.
Owner:PHARMAKODEX LTD
Pharmaceutical formulation containing opioid agonist, opioid antagonist and bittering agent
InactiveUS7144587B2Reducing abuse potential of dosage formLower potentialPowder deliveryPill deliveryOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and a bittering agent in an effective amount to impart a bitter taste to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Conveniently implantable sustained release drug compositions
ActiveUS20060073182A1Economical and practical and efficientEasy to produceBiocideOrganic active ingredientsDiseaseSustained release drug
This invention provides for biocompatible and biodegradable syringeable liquid, implantable solid, and injectable gel pharmaceutical formulations useful for the treatment of systemic and local disease states.
Owner:RAMSCOR
Pharmaceutical compositions for buccal and pulmonary application
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in mixed micellar form are disclosed. The mixed micelles are formed from an alkali metal alkyl sulfate, and at least three different micelle-forming compounds as described in the specification. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed. A preferred method for administering the present composition is through the buccal region of the mouth.
Owner:GENEREX PHARMA INC +1
Spontaneous emulsions containing cyclosporine
A pharmaceutical composition contains cyclosporine as the active ingredient. More specifically, the composition is an orally administered pharmaceutical formulation in the form of a spontaneous emulsion comprising cyclosporine, ethanol ethyl oleate and polyoxyethylene glycerol trioleate. A method for preparing an orally administered pharmaceutical composition involves first dissolving cyclosporine in ethanol. Polyoxyethylene glycerol trioleate and an oil component are then added, mixed and diluted in an aqueous media to form a spontaneous emulsion.
Owner:WOCKHARDT EU OPERATIONS SWISS
Method and package for storing a pressurized container containing a drug
Owner:GLAXO SMITHKLINE LLC
Conveniently implantable sustained release drug compositions
InactiveUS20080038316A1Economical and practical and efficientEasy to produceBiocideOrganic active ingredientsDiseaseSustained release drug
This invention provides for biocompatible and biodegradable syringeable liquid, implantable solid, and injectable gel pharmaceutical formulations useful for the treatment of systemic and local disease states.
Owner:RAMSCOR
Methods and apparatus for drug delivery involving phase changing formulations
InactiveUS20020004063A1Cleanly peeled from the skinMaintenance of such surfaceSalicyclic acid active ingredientsAnaestheticsPharmaceutical formulationSoft solids
This invention relates to an apparatus and method of drug delivery on a human body surface. The formulation comprises a drug, a conversion agent capable of converting the formulation from a less solid phase to a coherent, soft, solid phase, and a vehicle medium or carrier for the drug and conversion agent. The drug formulation is applied to this human body surface in its less than solid phase and is subsequently converted to a soft solid phase while the drug is being delivered through the human body surface. After delivery of the drug is complete, the soft solid formulation can be removed or peeled from the body surface as a coherent solid formulation. The drug formulation provides control over drug delivery rates and allows the formulation to be removed without leaving a messy, residual formulation on the body surface.
Owner:CRESCITA THERAPEUTICS INC
Apparatus and formulations for suprachoroidal drug delivery
InactiveUS20070202186A1Avoid traumaMinimally-invasive deliveryBiocidePowder deliveryPosterior regionPharmaceutical formulation
Drug formulations, devices and methods are provided to deliver biologically active substances to the eye. The formulations are delivered into scleral tissues adjacent to or into the suprachoroidal space without damage to the underlying choroid. One class of formulations is provided wherein the formulation is localized in the suprachoroidal space near the region into which it is administered. Another class of formulations is provided wherein the formulation can migrate to another region of the suprachoroidal space, thus allowing an injection in the anterior region of the eye in order to treat the posterior region.
Owner:CLEARSIDE BIOMEDICAL
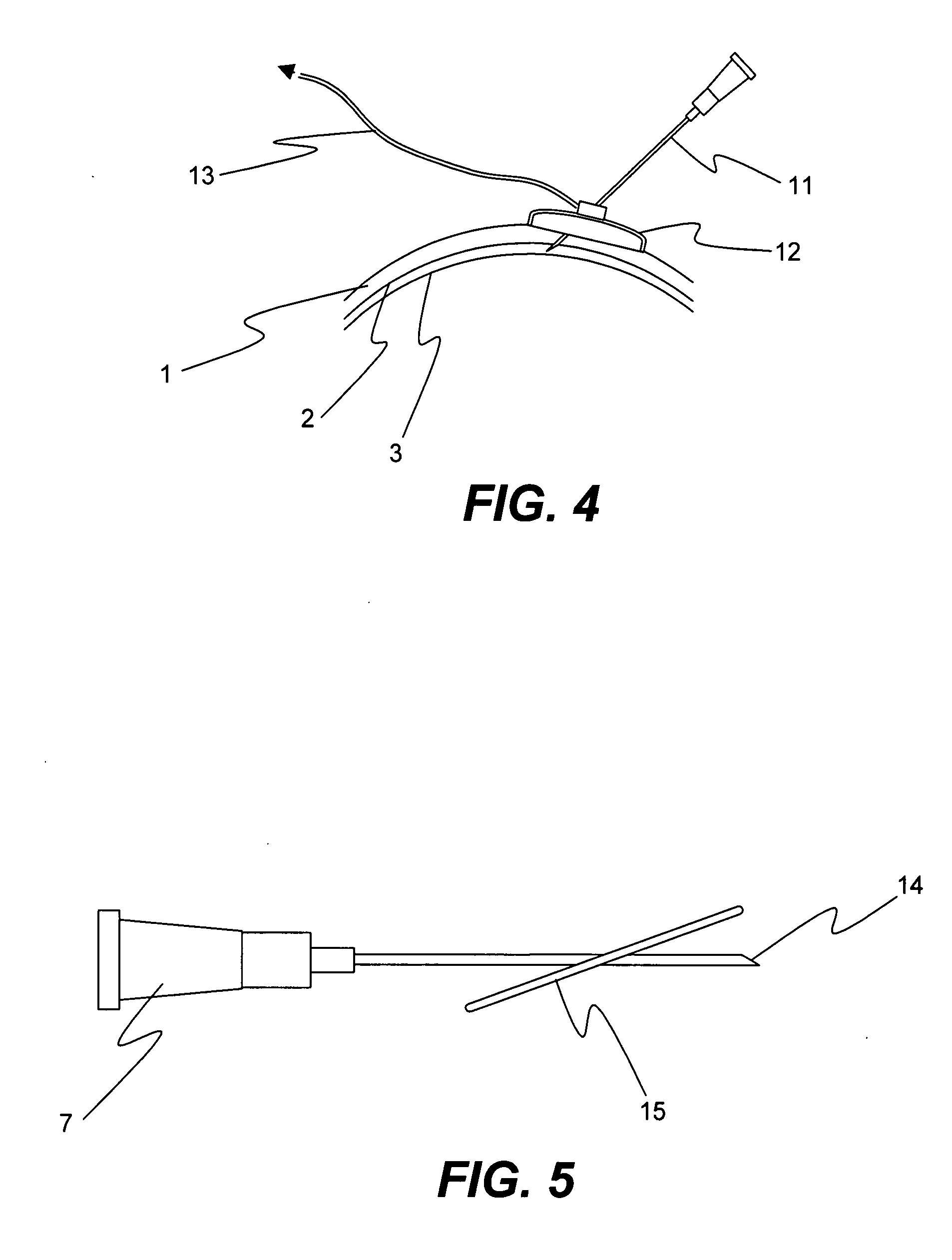
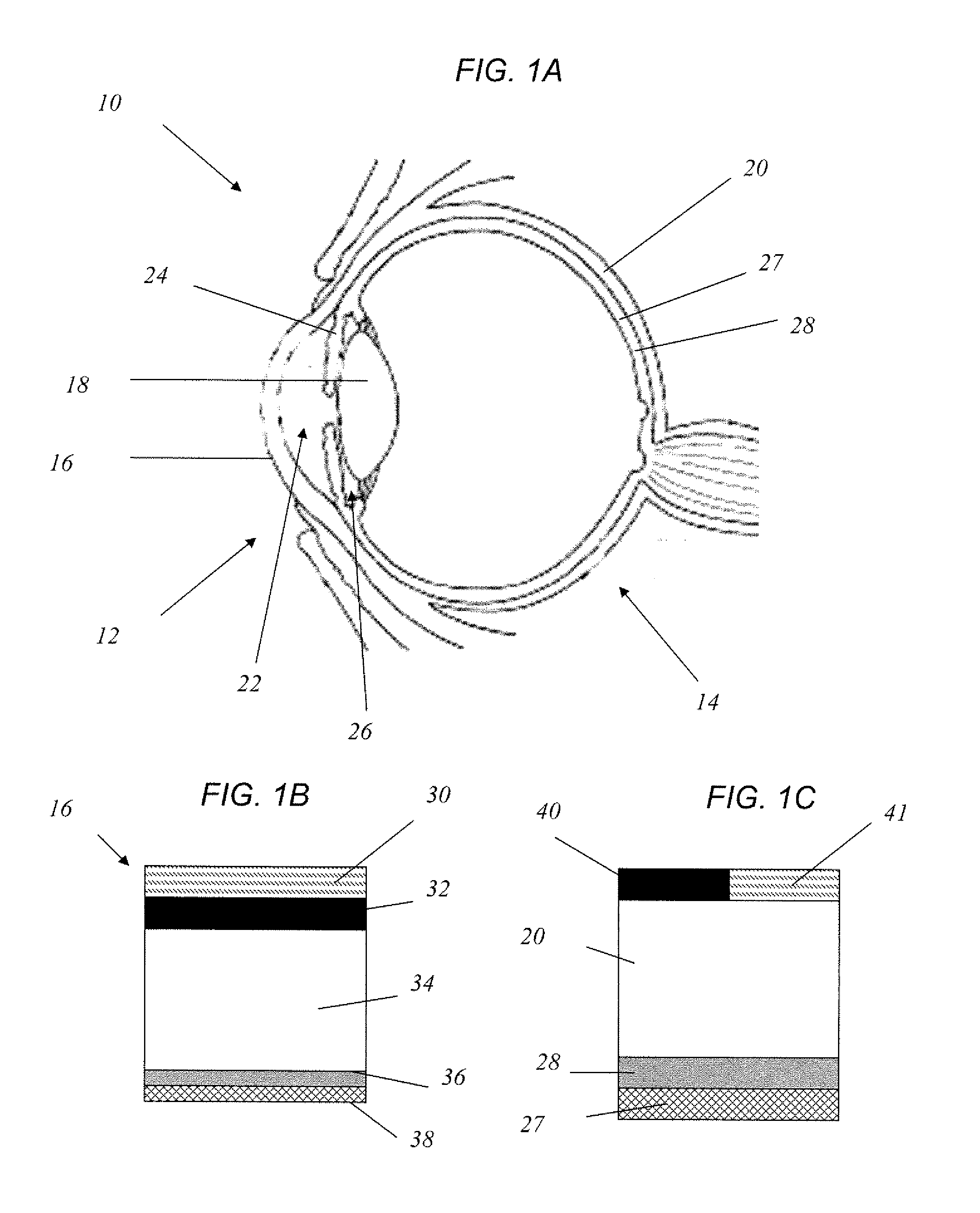
Method for drug delivery to ocular tissue using microneedle
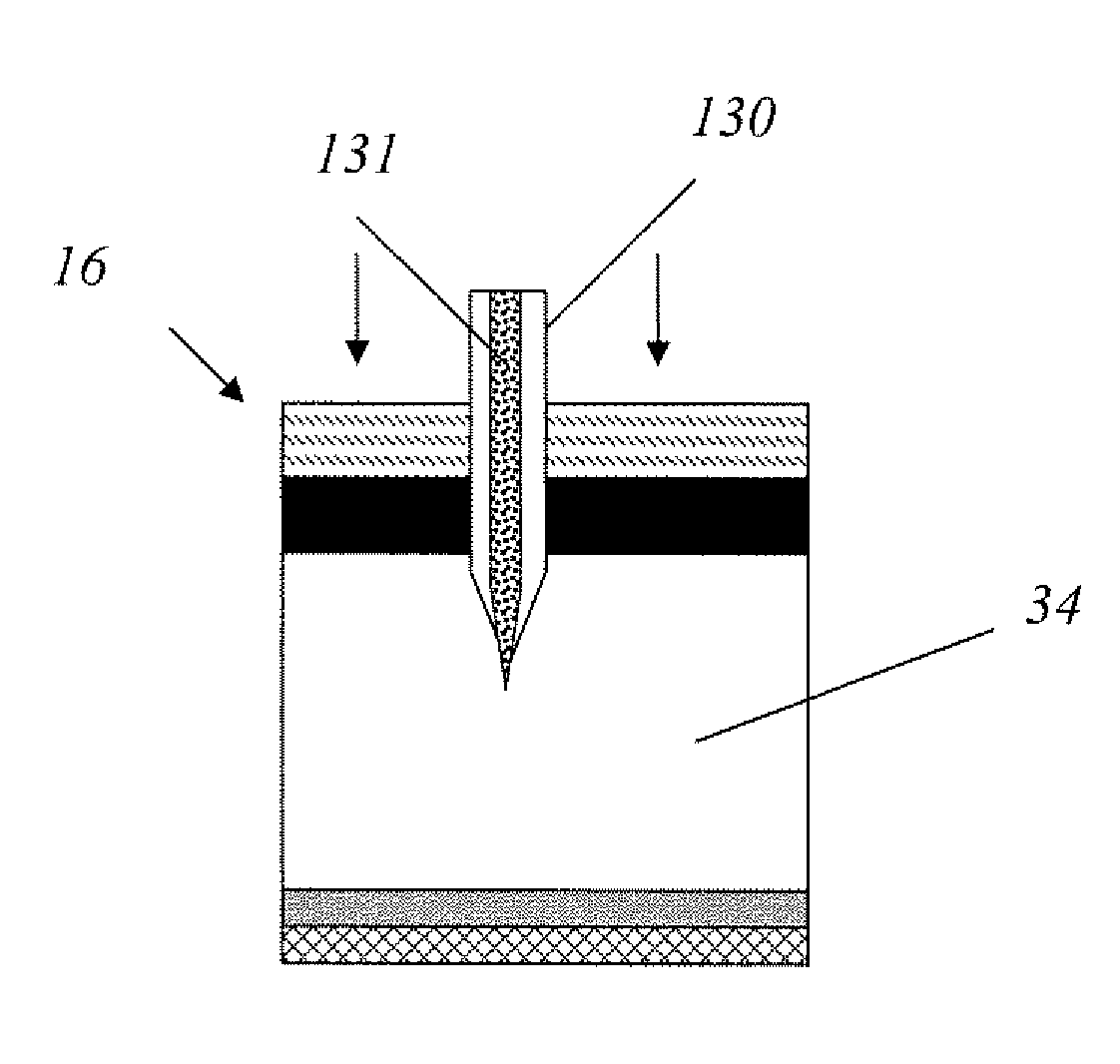
Methods and devices are provided for administering a drug to a patient's eye. The methods include (a) inserting a hollow microneedle into the sclera or corneal stroma without penetrating across the sclera or corneal stroma; and (b) infusing a fluid drug formulation through the microneedle and into the sclera or cornea. It further may include partially retracting the microneedle before infusion to enhance delivery. Alternatively, the methods may include (a) inserting a solid microneedle into the sclera or corneal stroma without penetrating across the sclera or corneal stroma, wherein the solid microneedle comprises a first quantity of a drug formulation and inserting causes the solid microneedle to form a pocket in the sclera or corneal stroma; and (b) releasing the drug formulation into the pocket to form a drug depot, whereby a drug is released from the depot. The methods and devices may include an array of multiple microneedles.
Owner:GEORGIA TECH RES CORP +1
Metered dose inhaler agitator
A metered dose inhaler including a mechanism for agitating the medicament formulation prior to its separation in a measured dose, for administration to a mammal, including a human. The separated dose is a homogeneous mixture of prescribed medicine in a fluid carrier.
Owner:KOS LIFE SCI
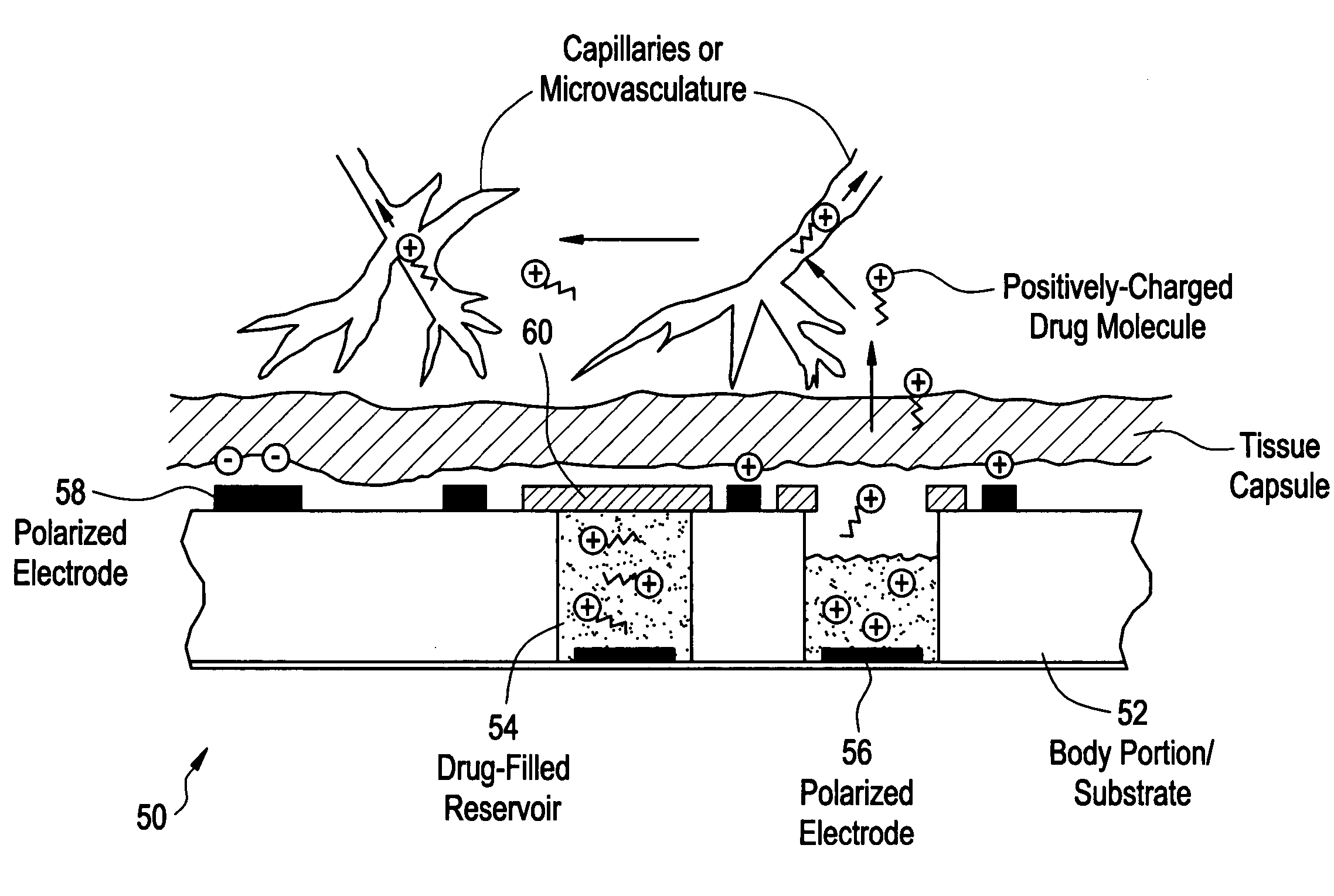
Devices and methods for measuring and enhancing drug or analyte transport to/from medical implant
InactiveUS20050267440A1Easy to transportEfficient propulsionElectrocardiographyAntipyreticControlled drugsAnalyte
Methods and devices are provided for enhancing mass transport through any fibrous tissue capsule that may form around an implanted medical device following implantation. Methods and devices are also provided to enhance vascularization around the implanted device, which also will aid in mass transport to / from the device. The device preferably comprises multiple reservoirs containing (i) a drug formulation for short- or long-term, controlled drug delivery, (ii) sensors for sensing an analyte in the patient, or (iii) a combination thereof.
Owner:MICROCHIPS INC
Methods for making pharmaceutical formulations comprising microparticles with improved dispersibility, suspendability or wettability
InactiveUS20050079138A1Good dispersibilityImproved suspendabilityPowder deliveryGranulation by liquid drop formationPowder mixtureMicroparticle
Methods are provided for making a dry powder blend pharmaceutical formulation, comprising the steps of: (a) providing microparticles which comprise a pharmaceutical agent; (b) blending the microparticles with at least one excipient in the form of particles to form a powder blend; and (c) jet milling the powder blend to form a dry powder blend pharmaceutical formulation having improved dispersibility, suspendability, or wettability as compared to the microparticles of step (a) or the powder blend of step (b). The method can further include dispersing the dry powder blend pharmaceutical formulation in a liquid pharmaceutically acceptable vehicle to make an formulation suitable for injection. Alternatively, the method can further include processing the dry powder blend pharmaceutical formulation into a solid oral dosage form. In one embodiment, the microparticles of step (a) are formed by a solvent precipitation or crystallization process.
Owner:ACUSPHERE INC
Methods for using tetanus toxin for beneficial purposes in animals (mammals)
Methods of using tetanus toxin to modulate or control neural functions or nonneural cellular activities at selected sites in animals, particularly in mammals, and more particularly in humans, are provided. Pharmaceutical formulations to modulate neural functions or non-neural cellular activities of an animal at selected sites in animals, particularly in mammals, and more particularly in humans are also provided. Uses of tetanus toxin in preparation of medicaments for methods of treating clinical disorders or symptoms of animals, particularly mammals and more particularly humans are also provided.
Owner:SANDERS
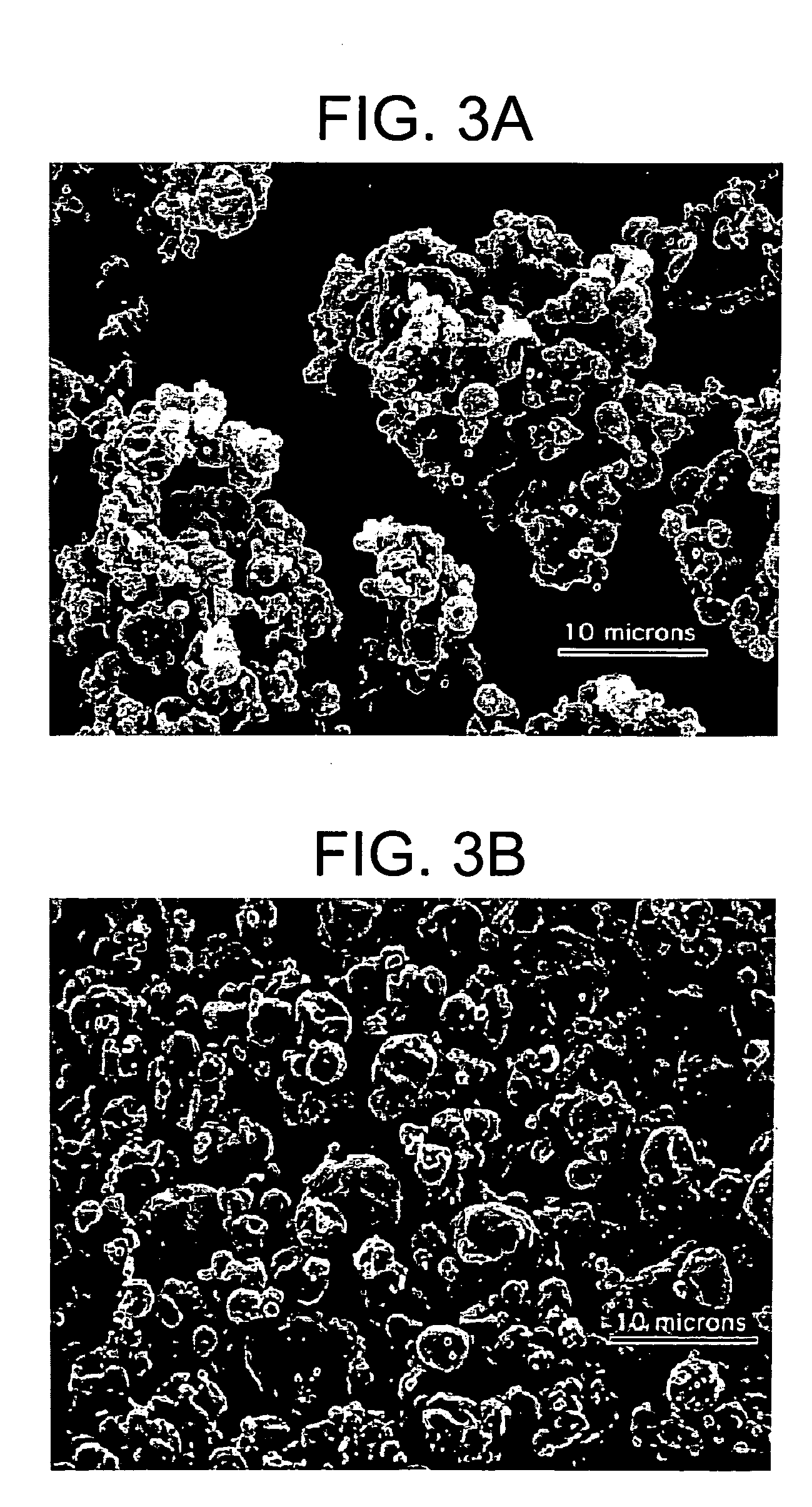
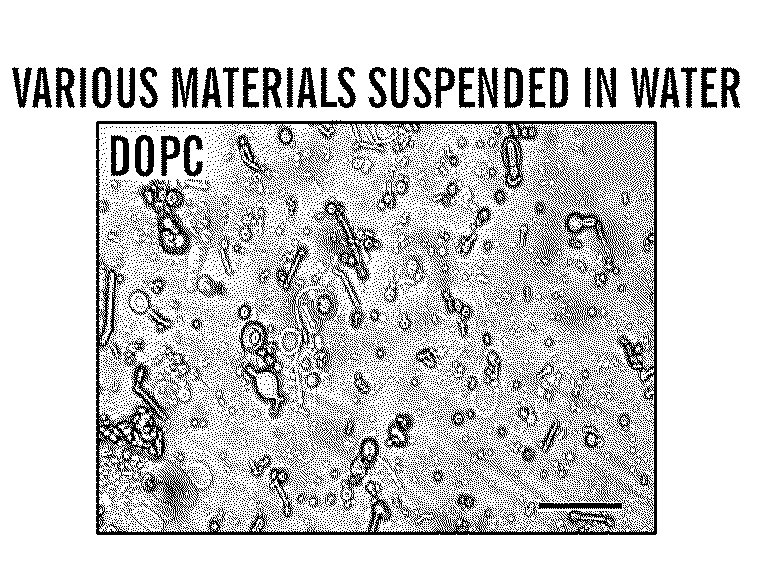
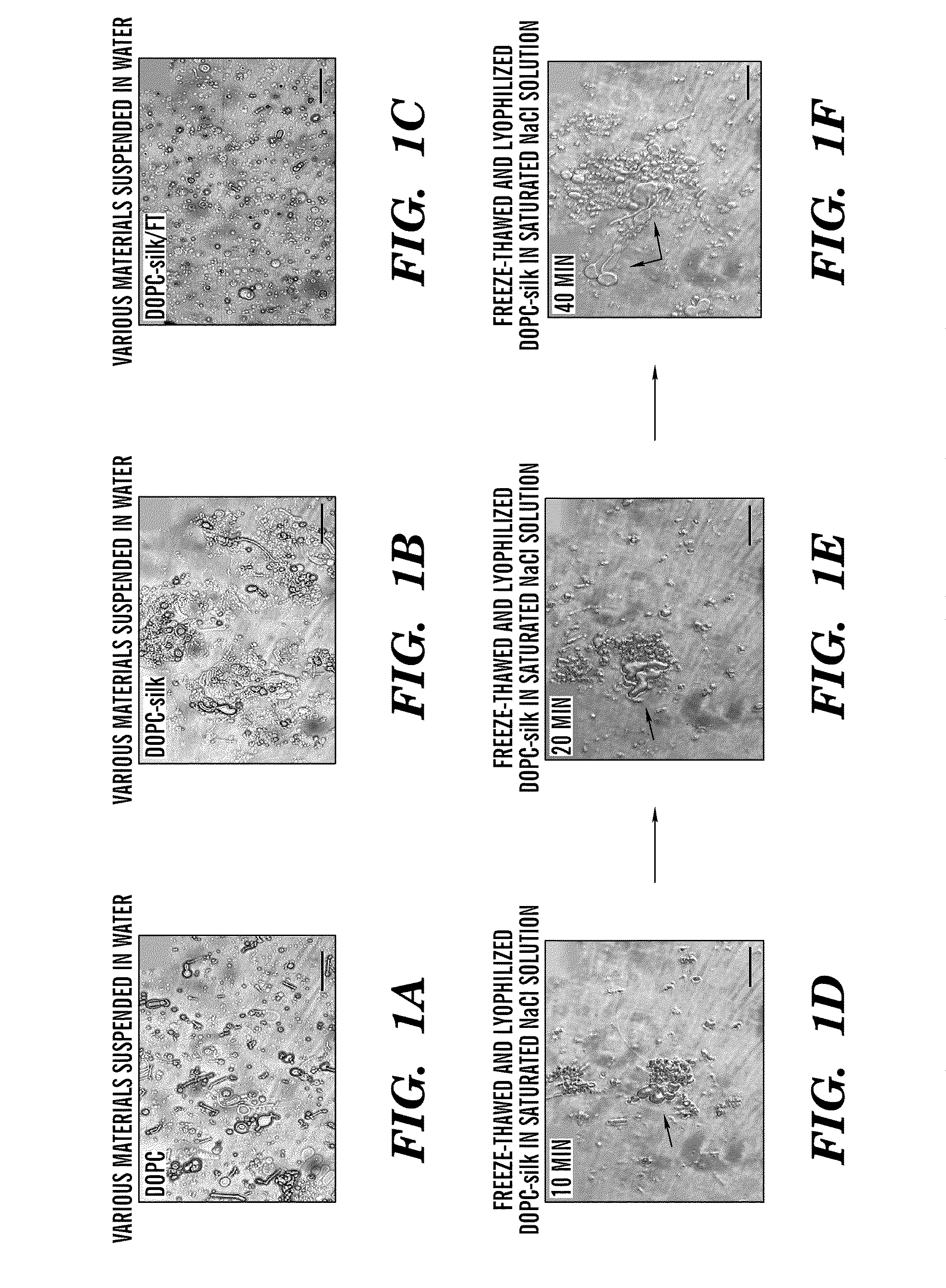
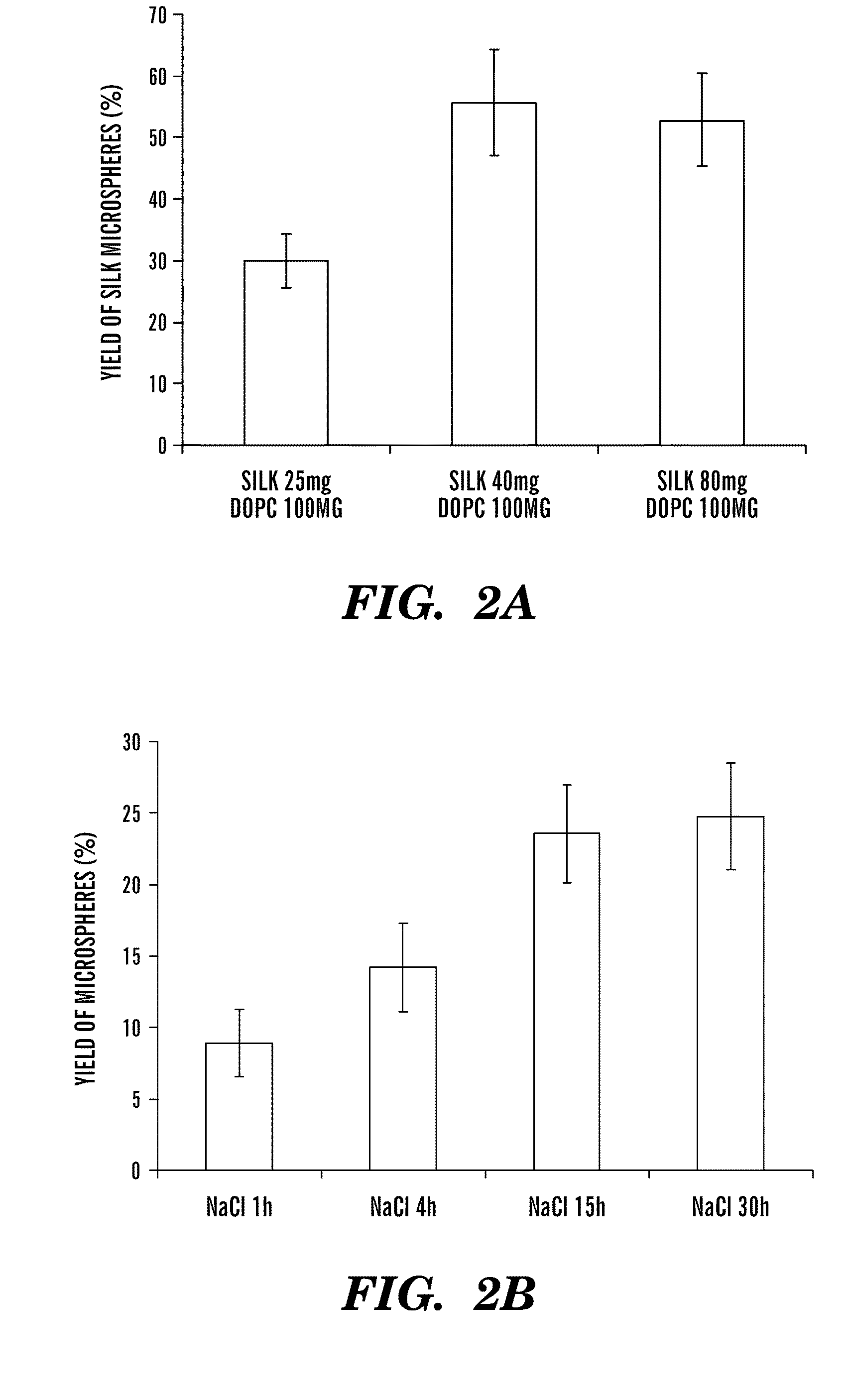
Silk microspheres for encapsulation and controlled release
A method was developed to prepare silk fibroin microspheres using lipid vesicles as templates to efficiently load therapeutic agents in active form for controlled release. The lipids are subsequently removed through the use of a dehydration agent, such as methanol or sodium chloride, resulting in β-sheet structure dominant silk microsphere structures having about 2 μm in diameter. The therapeutic agent can be entrapped in the silk microspheres and used in pharmaceutical formulations for controlled-release treatments.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Methods for preparing coated drug particles and pharmaceutical formulations thereof
Disclosed are methods using pulsed laser ablation to prepare coated drug particles of uniform size and thickness. The coated drug particles ranged in size from several nanometers to several millimeters in diameter size, and were coated with organic polymer particle having average diameter sizes from about 1 to 50 nm. In illustrative embodiments, coated drug particles or drug delivery particles are disclosed comprising a biodegradable or biocompatible polymer coating having controlled thickness and controlled coating uniformity, that offer superior pharmaceutical properties for controlled delivery and increased bioavailability.
Owner:FLORIDA UNIV PF +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com